- 1Xiangya Nursing School, Central South University, Changsha, China
- 2Health Management Medicine Center, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 3Nursing Department, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 4Department of Geo-Informatics, Central South University, Changsha, China
- 5School of Automation, Central South University, Changsha, China
- 6College of Nursing and Public Health, Adelphi University, Garden City, NY, United States
- 7Department of Clinical Psychology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
Background: Periodontitis is a prevalent chronic inflammatory non-infectious condition, primarily induced by subgingival bacteria. The aim of this study was to investigate the interaction between body roundness index (BRI) and TyG index on periodontitis and to explore whether the connection was associated with sex.
Study design: Cross-sectional study.
Methods: This cross-sectional study included 261,454 participants between 2017 and 2024. The associations of the TyG index and BRI with periodontitis risk were investigated via logistic regression analysis and restricted cubic splines. Subgroup analysis was used to explore potential differences. A sensitivity analysis was conducted using multivariate regression to evaluate the associations between TyG-related indicators and periodontitis.
Results: Among the 261,454 participants, 40,991 individuals were diagnosed with periodontitis. Individuals with high TyG and high BRI (TyG > 8.60 and BRI > 3.19) had the highest risk of developing periodontitis, suggesting a synergistic effect. Next, we multiplied these two metrics to establish TyG-BRI. TyG-BRI was nonlinearly positively correlated with the risk of periodontitis, with the highest quartile having a more significant effect on the risk of periodontitis compared to the lowest quartile (OR = 1.47; 95% CI: 1.40, 1.53). The TyG-BRI index had a significant effect on periodontitis in those less than 60 years of age, female, and non-smokers, and this effect was particularly prominent in women aged 30–50 years. Sensitivity analysis showed that the associations between the correlation indices (TyG index, BRI, and TyG-BRI) and periodontitis are statistically significant.
Conclusion: Overall, the TyG-BRI index can be used as a predictor of periodontitis risk. The link was strongest in individuals under 60 years, women, and non-smokers, suggesting potential roles of age-related metabolic changes, estrogen, and smoking-induced systemic inflammation in modulating this relationship. In the future, this mechanism needs to be further verified in combination with the levels of sex hormones.
Introduction
Periodontitis is a prevalent chronic inflammatory non-infectious condition, primarily induced by subgingival bacteria (1). It is marked by the progressive degradation of periodontal tissues, evidenced by the creation of periodontal pockets, loss of alveolar bone, and hemorrhaging gums (2). The prevalence of periodontitis in China varies from 1% to 69% and escalates progressively with age (3, 4). Furthermore, the prevalence of periodontitis in China is substantial and on the rise (5). This scenario underscores the critical public health issue of periodontitis and the pressing necessity for enhanced research and focus. The global frequency of periodontitis is notably high, with severe instances accounting for 11.2% (6). Periodontitis results in tooth loss, malnutrition, and several health complications (7). Consequently, it is crucial to acknowledge the elevated prevalence of periodontitis, particularly in China, to enhance public health.
Furthermore, periodontitis is significantly linked to a heightened risk of chronic conditions, including metabolic syndrome and diabetes, with insulin resistance (IR) potentially serving as a critical element in this relationship (8–10). Traditional methods of measuring IR are intricate; therefore, the researchers devised more straightforward assessment tools, such as the triglyceride glucose (TyG) index (11). The TyG index, which estimates IR by measuring triglyceride (TG) and fasting blood glucose levels, has been shown to be significantly associated with periodontitis risk (12). According to research, being overweight may make the dysregulation of periodontal microbiota worse by increasing the release of inflammatory mediators and affecting insulin resistance (13–15). To measure fat distribution more comprehensively, Thomas et al. proposed a new indicator, the body roundness index (BRI) (16). Unlike waist-to-height ratio (WHtR), the BRI ellipsoid model can more accurately reflect the geometric changes of visceral fat accumulation. Studies have shown that BRI is superior to other traditional anthropometric measures in predicting the risk of a variety of clinical endpoints, such as cardiometabolic disease, cancer, and death (17–19). Simultaneously, BRI is intricately associated with insulin resistance (20). However, at present, for the synergy between BRI and TyG indices, further verification and in-depth exploration are still needed.
Recent research has shown that there is an independent association between a single component and its combined components of gingival bleeding (BOP) in women and metabolic syndrome (21). This discovery strongly supports the necessity of further exploring the correlation between biological sex and periodontitis. Sex is also a key factor in obesity outcomes. Compared with men, women are more often obese but have fewer metabolic complications (22). Central obesity in women has the strongest correlation with CRP, while the fat percentage of systemic inflammation in men is a stronger predictor (23). The basic aspects of metabolic homeostasis are regulated differently in men and women, and may affect the risks of obesity and insulin resistance, both of which are risk factors for periodontitis (24). Whether the TyG-BRI index has different effects in different genders still needs further exploration. In view of the above situation, it is very necessary to conduct subgroup discussions on the association between the TyG-BRI index and periodontitis.
Therefore, this study aims to investigate the synergistic effect of TyG and BRI on periodontitis risk, develop a composite index to explore their integrated relevance, and identify subgroup-specific associations that may inform targeted screening strategies.
Methods
Study population and design
This research utilized data from the database of the Third Xiangya Hospital at Central South University. The database is a cross-sectional survey administered by the Xiangya Third Hospital Center of Central South University. The Ethics Committee of the Third Xiangya Hospital approved the survey (No: 25118), and informed consent was acquired. The data collection for this study comprised two components: laboratory testing and the completion of a health assessment questionnaire via the website1. All blood sample collections strictly followed the Chinese “WS/T2252002” standard. After the subjects fasted for ≥8 h, venous blood was collected using vacuum blood collection tubes. Blood glucose was detected by the hexokinase method (in compliance with WS/T 352–2011), and triglyceride was detected by the glycerophosphate oxidase-peroxidase method (in compliance with WS/T 356–2011). The testing equipment is [Mindray BS-2000M fully automatic Biochemical Analyzer]. Each batch of testing includes quality control products provided by the National Health Commission Clinical Laboratory Center (batch Number: GBW09193). Quality control was strictly observed throughout the process. Data was collected by qualified and experienced medical staff in accordance with standards. The data was rechecked by two people and de-identified. Figure 1 illustrates the flow chart for the inclusion and exclusion of participants. We excluded (1) individuals who did not receive an oral examination (n = 107,015); (2) participants younger than 20 (n = 1,007); (3) participants with malignancy (n = 2,163); (4) participants with incomplete data on pertinent variables (TyG index, BRI) (n = 6,756); (5) participants with significantly impaired liver (transaminase elevation > 5 times the upper limit of normal) (n = 544). The conclusive sample of participants incorporated in this investigation comprised 261,454 individuals.
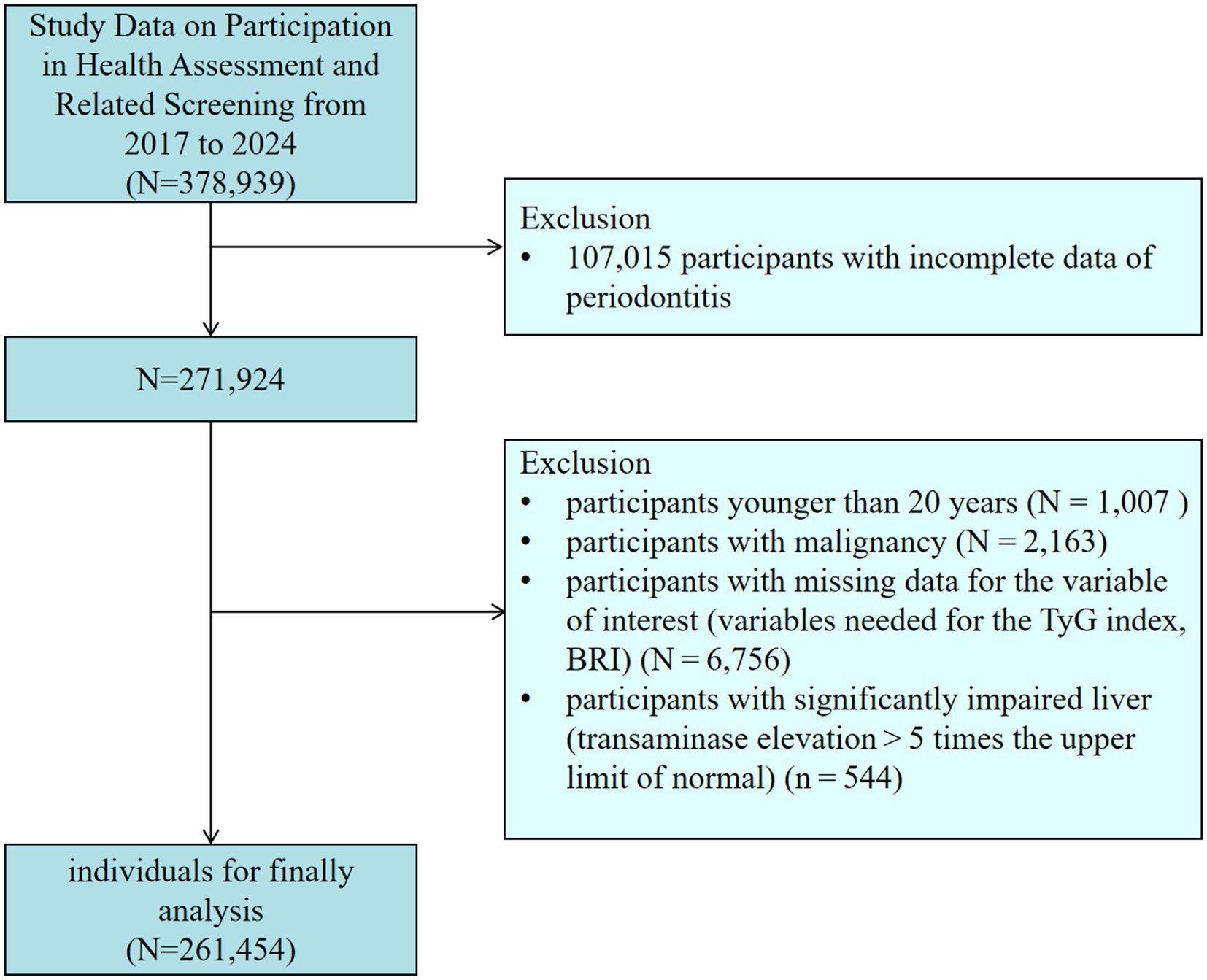
Figure 1. Flowchart of participants in this study. BRI, body roundness index; TyG, triglyceride-glucose.
Assessment of periodontitis
Full dental examination (excluding third molar) by a trained professional. Measurements were taken at 6 sites on each tooth. The case definition for a patient with periodontitis is defined as interdental clinical attachment loss detectable at ≥ 2 non-adjacent teeth, or buccal or oral clinical attachment loss ≥ 3 mm with pocketing ≥ 3 mm detectable at ≥ 2 teeth (25). After a series of assessments, individuals without periodontitis were assigned to the non-periodontitis group.
The calculation of insulin resistance indices and obesity-related parameters
TyG index = Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2] (26);
The Body Roundness Index (BRI) was calculated using the formula: BRI = 364.2–365.5 × √(1 – [waist circumference in centimeters/2π]2/[0.5 × height in centimeters]2) (16). Waist circumference and height were measured at the examination centers. WHtR was determined by WC (cm)/height (cm) (27).
Definitions of covariates
The covariates included age, sex, knowledge, body mass index (BMI) and some laboratory findings. Sex was specifically classified as male or female. Age was divided into two groups with a threshold of 60. The knowledge variable, which indicates whether participants were actively acquiring information about periodontitis, was categorized into two levels: “No” and “Yes.” BMI “<25” was considered within the normal weight range, while BMI “≥25” was defined as overweight/obese (28). Laboratory parameters included: systolic blood pressure (SBP), diastolic blood pressure (DBP), total serum protein (TP), serum albumin (ALB), serum globulin (GLB), blood uric acid (BUA), Total Bilirubin (TBIL), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C).
Statistical analysis
The study used R software (4.3.1) for data processing and statistical analysis. Categorical variables were described using frequencies (n) and percentages (%), and chi-square tests were used for between-group comparisons. Used column-wise median imputation to preserve original distribution. Since none of the continuous variables in this study conformed to normal distribution, they were expressed using medians and interquartile range (IQRs), and intergroup comparisons were made using the Mann–Whitney U test.
Logistic regression analysis was employed to investigate the associations of the TyG index and BRI with periodontitis. The covariate selection strategy aimed to control confounding effects by integrating theoretical frameworks with univariate statistical screening. Specifically, all measured covariates first underwent univariate logistic regression, and variables with p < 0.05 were shortlisted into a candidate pool. Final covariates for Model 2 were determined by synthesizing literature-supported biological plausibility and collinearity analysis results. Model 1 adjusted for age, sex, diabetes (29), and high-density lipoprotein-cholesterol (30). Model 2 adjusted for Model 1 plus Knowledge (31), BMI ≥ 25 (32), systolic blood pressure (33), diastolic blood pressure (33), blood uric acid (34), low-density lipoprotein-cholesterol (30), smoking (35), alcohol consumption (36). To assess multicollinearity in the multivariate logistic regression model, we calculated both tolerance and variance inflation factors (VIF). Variables with VIF > 5 (tolerance < 0.1) were excluded to ensure no significant collinearity among predictors. A restricted cubic spline model was used to determine the potential nonlinear associations of the TyG index and BRI with periodontitis. We explored whether there was a synergistic effect of the TyG index and BRI on periodontitis. Significance was set at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***). The classification accuracy of TyG-related indices was assessed through the implementation of a receiver operating characteristic (ROC) framework. The discriminative ability of these indices was quantified by calculating the area under the curve (AUC) metrics, with 1,000 bootstrap-resampled iterations employed to enhance the robustness of the estimates. Multiple subgroup analyses were performed to assess the robustness of the results of the correlation of TyG-BRI with periodontitis. To evaluate the robustness of the research results, a sensitivity analysis was conducted using the Chinese version of the BMI cut-off value (BMI ≥ 24) (37).
Results
The characteristics of study participants
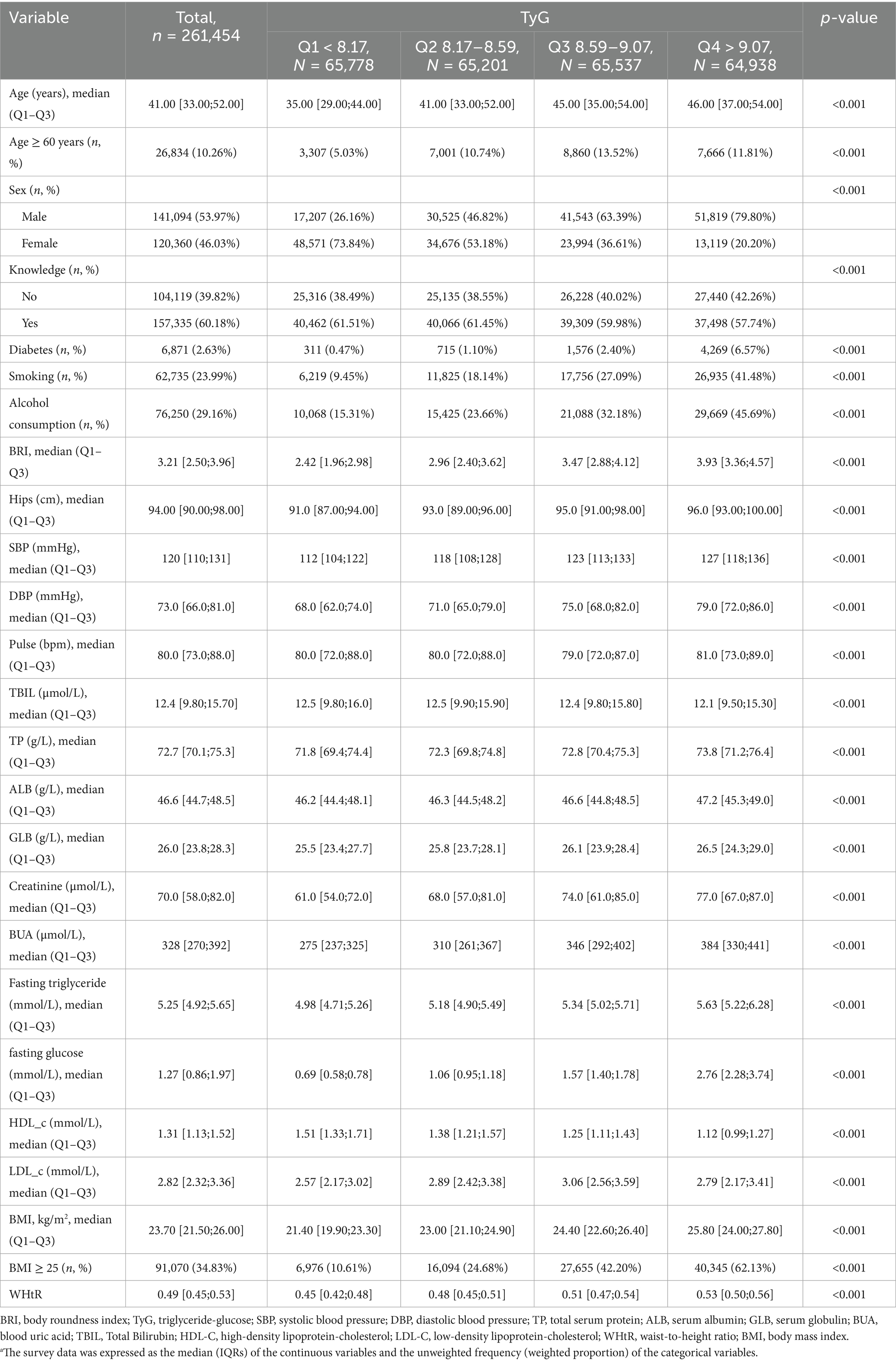
Table 1 shows the baseline characteristics of the 261,454 study individuals according to TyG index quartiles. Among all participants, 40,991 individuals were diagnosed with periodontitis (15.68%). 10.3% of the participants were older than 60 years of age, and 54.0% were male in the gender distribution. Individuals in the higher quartile of the TyG index were older, tended to be male, and were more likely to be smokers. They were more likely to have diabetes than those in the lower TyG index quartile. Supplementary Table 1 summarizes the baseline characteristics of study individuals according to BRI quartiles.
Association between the TyG index and periodontitis
The results of the univariate logistic regression analyses are shown in Supplementary Figure 1. The risk of developing periodontitis increased with age, and men, smokers, and diabetics were more likely to develop periodontitis. A positive understanding of periodontitis was a relevant protective factor. Before conducting logistic regression, we performed a multicollinearity analysis on the independent variables in the model, showing that VIF < 5, suggesting that there was no significant multicollinearity in our model (Supplementary Table 2). As shown in Supplementary Table 3, independent of covariate adjustment, the risk of periodontitis increased with increasing TyG index (p < 0.001). In the fully adjusted model (model 2), TyG index (OR = 1.08; 95% CI: 1.06, 1.10; p < 0.001) was considered a risk factor for periodontitis. Restricted cubic spline analysis showed a significant positive linear relationship between TyG index and periodontitis. The risk of developing periodontitis was significantly higher when the TyG index exceeded 8.60 (Figure 2A).
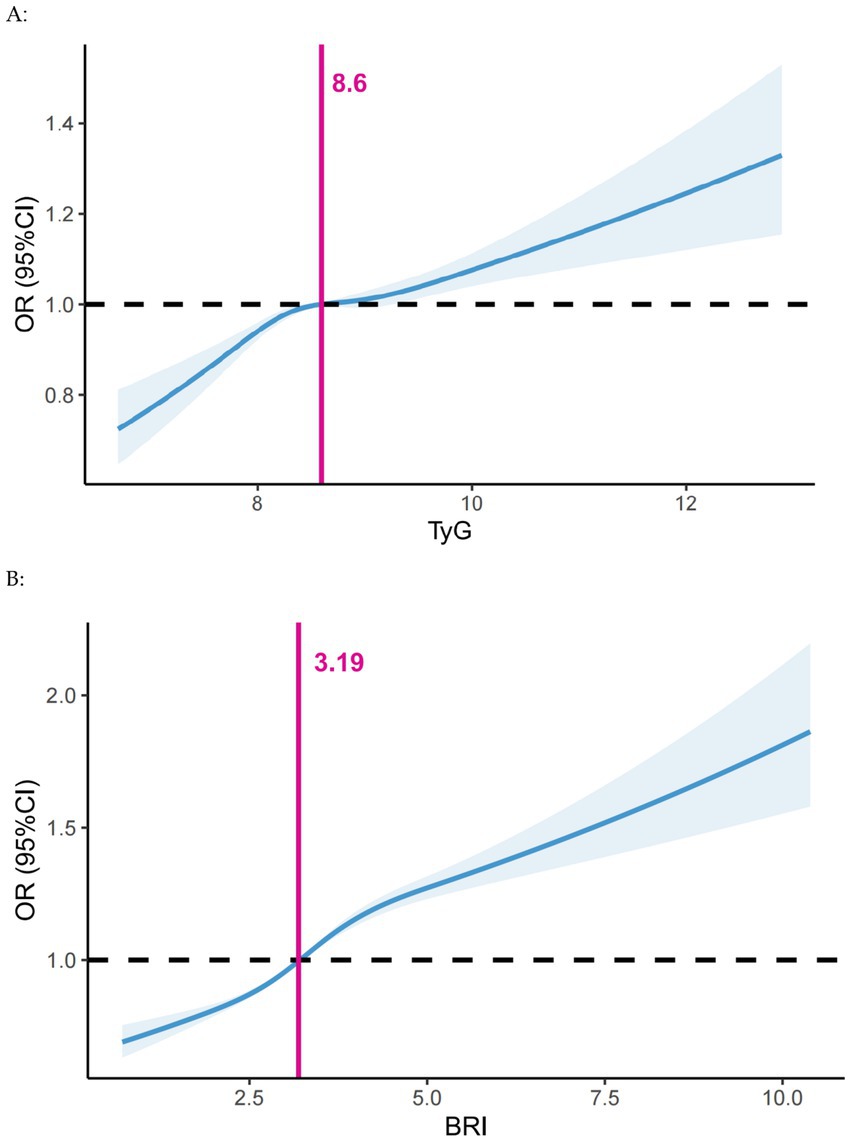
Figure 2. Dose–response associations of (A) the TyG index and (B) BRI with periodontitis assessed by restricted cubic spline model. Adjusted for Model 2 in the logistic analysis. BRI, body roundness index; CI, confidence interval; OR, Odds Ratio; TyG, triglyceride-glucose.
Association between the BRI and periodontitis
Supplementary Table 4 illustrates that the risk of periodontitis escalated with a rising BRI (p < 0.001), independent of covariate adjustments. In the fully adjusted model (model 2), BRI (OR = 1.15; 95% CI: 1.13, 1.16; p < 0.001) was a risk factor for periodontitis. Restricted cubic spline analysis revealed a significant positive linear relationship between BRI and periodontitis. The risk of periodontitis was elevated when the BRI index surpassed 3.19 (Figure 2B).
The synergistic effect of the TyG index and BRI on periodontitis
The joint effect of BRI and TyG index on the risk of periodontitis was assessed. The results showed that the interaction term between BRI and TyG index was statistically significant (p < 0.001). Individuals with high TyG and high BRI values (TyG > 8.60 and BRI > 3.19) had a higher risk of developing periodontitis than individuals with isolated high BRI values (BRI alone > 3.19) or isolated high TyG values (TyG > 8.60 only) and low TyG values (TyG < 8.60 and BRI < 3.19) (Supplementary Figure 2 and Supplementary Table 5). These results suggest that TyG index and BRI have a synergistic effect on periodontitis.
Construction and analysis of TyG-BRI
Considering the synergistic effect of the TyG index and the BRI on the risk of periodontitis, we multiplied these two indices to create the TyG-BRI. When analyzing the TyG-BRI as a continuous variable, the dose–response analysis showed a significant increase in the risk of periodontitis when the TyG-BRI exceeded 27.83 (Figure 3). In the fully adjusted model 2, TyG-BRI was categorised according to a threshold of 27.83 and was identified as a risk factor for periodontitis (OR = 1.21; 95% CI: 1.18, 1.24; P < 0.001) (Supplementary Table 6).
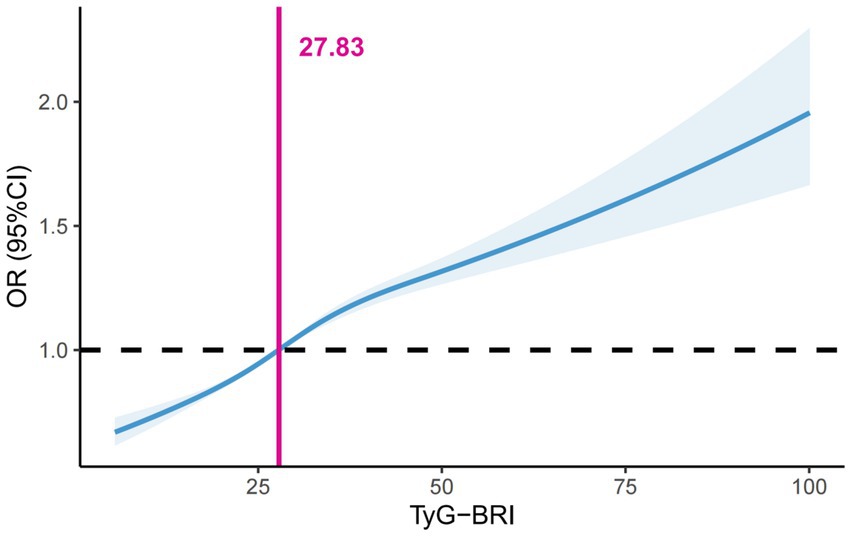
Figure 3. Dose–response associations of the TyG-BRI index with periodontitis assessed by restricted cubic spline model. BRI, body roundness index; TyG, triglyceride-glucose.
The receiver operating characteristic analysis for evaluating the TyG index and its obesity complex derivatives on the risk of periodontitis demonstrated a significant discriminatory ability. The predictive validity of TyG-BRI was evaluated, yielding an AUC of 0.5541 (95% CI: 0.5511–0.5571), demonstrating slightly higher discriminative ability than TyG-WHtR (AUC = 0.5533, 95% CI: 0.5503–0.5562), TyG (AUC = 0.5396, 95% CI: 0.5366–0.5426), BRI (AUC = 0.5529, 95% CI: 0.5499–0.5559), and WHtR (AUC = 0.5527, 95% CI: 0.5497–0.5557). DeLong’s test further validated the statistical significance of AUC differences. Compared with TyG, BRI, and WHtR, TyG-BRI showed significantly higher discriminative ability (all p < 0.001). The AUC of TyG-BRI was marginally higher than that of TyG-WHtR (p = 0.017), indicating a modest yet statistically significant improvement in predictive validity.
Further subgroup analyses were performed to explore whether TyG-BRI had a different effect in different genders. The results demonstrated that the association between TyG-BRI and periodontitis was significantly stronger in participants under 60 years of age, females, and non-smokers (Figure 4A). The interaction terms for age and TyG-BRI (p < 0.001), gender and TyG-BRI (p < 0.001), and smoking status and TyG-BRI (p < 0.001) were all statistically significant. To gain more insight into the role of the TyG-BRI index in the female population, an age-stratified analysis was performed on females (Figure 4B). The results of the analysis showed that the effect of the TyG-BRI index on periodontitis was significant in women aged 30–50 years.
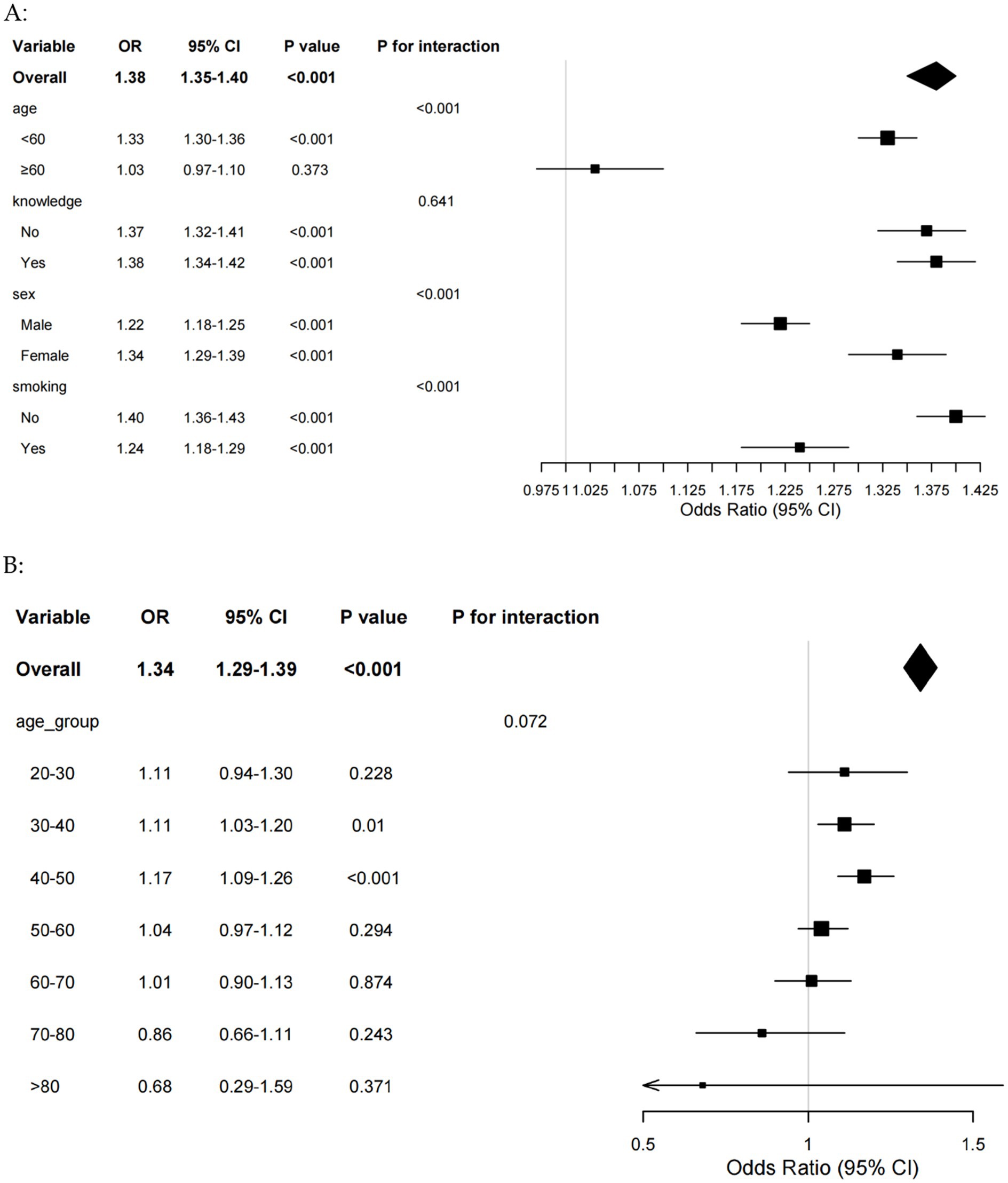
Figure 4. (A) Forest plot of subgroup analyses of TyG-BRI for periodontitis risk. (B) Forest plot of age subgroup analyses of TyG-BRI for periodontitis risk in female. BRI, body roundness index; TyG, triglyceride-glucose.
To evaluate the robustness of the research results, a sensitivity analysis was conducted using the Chinese version of the BMI cut-off value (BMI ≥ 24). In all multivariate adjusted models analyzed continuously and categorically (based on quartiles), the associations between the correlation indices (TyG index, TyG-BRI, BRI) and periodontitis remained statistically significant (Supplementary Table 7).
Discussion
This extensive cross-sectional study of the Chinese population explores the correlation between the BRI, TyG index, and the risk of periodontitis. The primary findings were as follows: (1) BRI, an essential indicator of central obesity, significantly increased the risk of periodontitis and functioned as an independent risk factor for the disease; (2) A synergistic interaction between BRI and the TyG index was observed among participants, collectively influencing the risk of periodontitis; (3) The TyG-BRI index had a significant effect on periodontitis in those less than 60 years of age, female, and non-smokers, and this effect was particularly prominent in women aged 30–50 years. Therefore, it is essential to acknowledge the influence of TyG-BRI index on the risk of periodontitis. Especially for women between the ages of 30 and 50, it is important to pay close attention to the TyG-BRI index. Meanwhile, through the TyG-BRI index, some interesting phenomena were found in this study.
The BRI index is a new validated index for detecting central obesity, which is important for the prevention of periodontitis and other chronic diseases (17, 38). Central obesity may lead to osteoporosis and interfere with osteoblast differentiation, which in turn increases the risk of periodontitis (39, 40). However, the BRI as a continuous indicator does not have clear cut-off values. This study employed restricted cubic spline analysis, which revealed a cutoff value of 3.19 for the nonlinear relationship between BRI and periodontitis, aligning with the cutoff value of BRI in other disease risks (41). Fat loss may have a significant therapeutic effect on the aforementioned diseases, such as dietary interventions, exercise interventions, and health education (42, 43). And acquisition of relevant knowledge was also a protective factor for reducing the risk of periodontitis. This implies that improving individuals’ knowledge and health literacy is equally crucial for effective obesity control and prevention of periodontitis.
Moreover, this study found that BRI and TyG exhibit synergistic effects in the development and progression of periodontitis. These two metrics reflect the metabolic status of the body from different perspectives, which together influence the risk of periodontitis (44, 45). Previous studies have also shown that the higher the TyG index, the greater the risk of periodontitis (46, 47). Restricted cubic spline analysis identified a TyG index threshold of 8.60, indicating that values ≥8.6 may reflect metabolic dysfunction potentially aggravating periodontal inflammation. Chronic inflammation from central obesity reduces insulin sensitivity and disrupts the oral immune microenvironment, thereby worsening periodontitis severity (13, 48). However, it has been shown that there may be a complex interaction between visceral adiposity and TyG, and that this interaction may manifest differently in different diseases (49). This phenomenon may be intricately linked to the metabolic pathways by which BRI and TyG index influence the risk of periodontitis (50). While single clinical indices offer advantages in practicality and interpretability, our study highlights the value of TyG-BRI, as evidenced by TyG-BRI’s statistically significant (though marginally higher) AUC compared to single indices. While the incremental improvements in AUC may appear modest, such exploratory research is crucial for advancing our understanding of optimal risk stratification strategies. When evaluating the clinical utility of a novel biomarker or risk factor, its potential should not be summarily dismissed solely on account of a suboptimal AUC value. This tension between simplicity and comprehensiveness embodies the core challenge of clinical biomarker research: balancing translational feasibility with mechanistic insight. Our findings underscore that such explorations, while requiring rigorous validation, are essential for advancing risk assessment frameworks beyond single-dimensional metrics. Therefore, the joint indicator BRI-TyG not only reveals the interaction between BRI and TyG, but also helps us to more accurately stratify the at-risk population and identify those individuals who are at high risk.
Men have a higher risk of developing periodontitis than women, a phenomenon that has been confirmed in several studies (51). Studies have shown that biological sex differences may influence the pathogenesis of periodontitis through a number of aspects, including gene expression on sex and autosomal chromosomes, the endocrine system, and the immune system (52–57). Nonetheless, there are some paradoxes, such as shown in this study, the effect of TyG-BRI on periodontitis was more pronounced in the female population. These paradoxical phenomena are partly due to differences in the distribution of adipose tissue between the sexes (22). In women, central obesity correlated most strongly with CRP, whereas in men, percent body composition fat was a stronger predictor of systemic inflammation (23). Androgens and estrogens are thought to play a key role in these gender differences and are thought to play a role in lipolysis, muscle metabolism, and satiety (58, 59). Meanwhile, a further subgroup analysis was conducted on the female population, and it was found that the influence of TyG-BRI on periodontitis was significant among women aged 30–50. This may be related to changes in hormone levels in women after the age of 30. Research based on multimodal measurements has identified a set of known and unknown age-related markers, determining that the ages of 30 and 50 are transitional periods of aging for women, during which there are significant changes in their hormone levels (60). The stratification results vary by age group, suggesting that in clinical practice, for the risk assessment and intervention of periodontitis, a one-size-fits-all approach should not be adopted. For females aged 30–50, more attention can be paid to TyG-BRI, and risk screening and early intervention can be carried out.
Although smokers have a higher risk of periodontitis, the effect of TyG-BRI on periodontitis is more pronounced in non-smokers. Smoking weakens the immune defenses of periodontal tissues, making it more difficult for the body to fight off periodontal infections (61). Nicotine may attenuate host immunity in early periodontitis, thereby increasing the risk of periodontitis (62, 63). In addition, there is a significant association between smoking and systemic inflammation (expressed as C-reactive protein levels) (24). Smoking causes vascular endothelial cells to constrict, reducing blood flow to the periodontal tissues, which decreases the number of immune cells and gingival sulcus fluid (64). This mechanism not only affects periodontal health, but may also have an impact on insulin resistance. However, smoking also leads to changes in gonadal hormone levels, particularly reduced androgen levels (65, 66). Considering that androgen levels are positively correlated with indicators of insulin resistance (67), future studies will allow testing this hypothesis and further elucidating the relationship between the relevance of dose-dependent effects of smoking.
Although individuals in the higher quartiles of BRI and TyG indices were older, the effect of TyG-BRI on periodontitis was more pronounced in those younger than 60 years. Aging can cause dysregulation of the inflammatory response, leading to increased expression of pro-inflammatory cytokines, which not only exacerbate local inflammation, but also affect the systemic metabolic state through blood circulation, leading to insulin resistance (68, 69). In younger individuals, metabolic dysregulation likely exerts a more direct impact on periodontal inflammation, whereas in older adults, chronic inflammatory states and multifactorial comorbidities may obscure this relationship (70). Another study also found that the association between TyG index and periodontitis was more pronounced in younger and middle-aged populations (12). Clinically, these results imply that age-stratified risk assessment using TyG-BRI could optimize early intervention strategies, particularly for younger adults.
This study offers significant insights but is not without limitations. Firstly, the observational design restricts a thorough examination of the causal relationships among the TyG index, BRI, and periodontitis, necessitating verification of the clinical practicability and predictive value of TyG-BRI through larger-scale prospective studies. Secondly, despite efforts to adjust for known confounders, eliminating the influence of other potential confounding variables remains challenging. Lastly, the study predominantly involved Chinese participants, which may limit the generalisability of the findings across different ethnic groups.
Conclusion
In conclusion, based on the analysis of data from Chinese adult participants, the BRI and TyG indices not only correlate with the prevalence of periodontitis, but also interact with each other, potentially exacerbating the severity of periodontitis. Notably, our subgroup analysis suggests that TyG-BRI may possess enhanced predictive utility in populations (Female, younger adults, non-smokers), though this finding warrants validation in multiethnic, multicenter cohorts to address generalizability. Age-related metabolic changes, estrogen levels and systemic inflammation caused by smoking may play a potential role in regulating this relationship. While the clinical utility of the TyG-BRI index warrants further exploration and validation, this study provides valuable insights into the risk stratification strategy and lays the foundation for future research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third Xiangya Hospital (Approval no. 25118). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LW: Writing – original draft, Methodology, Data curation. JL: Supervision, Writing – review & editing, Conceptualization. JW: Writing – original draft, Data curation. PL: Writing – original draft, Formal analysis. QZ: Software, Writing – original draft. YW: Methodology, Writing – original draft. HZ: Writing – original draft. XZ: Supervision, Writing – review & editing. SL: Writing – review & editing, Software. JZ: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Hunan Provincial Health High-Level Talents Scientific Research Project (grant number: R2023155) and the Fundamental Research Funds for the Central Universities of Central South University (2025JGB117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1642112/full#supplementary-material
Footnotes
References
1. Eke, PI, Thornton-Evans, GO, Wei, L, Borgnakke, WS, Dye, BA, and Genco, RJ. Periodontitis in US adults: National Health and nutrition examination survey 2009-2014. J Am Dent Assoc. (1939) 149:576–588.e6.
2. Kwon, T, Lamster, IB, and Levin, L. Current concepts in the Management of Periodontitis. Int Dent J. (2021) 71:462–76. doi: 10.1111/idj.12630
3. Jiao, J, Jing, W, Si, Y, Feng, X, Tai, B, Hu, D, et al. The prevalence and severity of periodontal disease in mainland China: data from the fourth National Oral Health Survey (2015-2016). J Clin Periodontol. (2021) 48:168–79. doi: 10.1111/jcpe.13396
4. Wen, X, Li, H, Li, S, Chang, B, Chen, S, Li, H, et al. Associated factors of periodontitis and predicted study among young man in China: a population-based cross-sectional study. BMC Public Health. (2024) 24:1235. doi: 10.1186/s12889-024-18732-2
5. Wang, Y, Wang, Y, Fan, L, and Yu, Y. The burden of severe periodontitis in China from 1990 to 2021, with projections to 2050: a comprehensive analysis from the global burden of disease study 2021. Int Dent J. (2025) 75:32–44. doi: 10.1016/j.identj.2024.12.013
6. Kassebaum, NJ, Bernabé, E, Dahiya, M, Bhandari, B, Murray, CJL, and Marcenes, W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. doi: 10.1177/0022034514552491
7. Shaheen, MY, Al-Zawawi, AS, Divakar, DD, Aldulaijan, HA, and Basudan, AM. Role of chlorhexidine and herbal Oral rinses in managing periodontitis. Int Dent J. (2023) 73:235–42. doi: 10.1016/j.identj.2022.06.027
8. Pirih, FQ, Monajemzadeh, S, Singh, N, Sinacola, RS, Shin, JM, Chen, T, et al. Association between metabolic syndrome and periodontitis: the role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol. (2000) 87:50–75.
9. D’Aiuto, F, Gkranias, N, Bhowruth, D, Khan, T, Orlandi, M, Suvan, J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-Centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. (2018) 6:954–65. doi: 10.1016/S2213-8587(18)30038-X
10. Molina, A, Huck, O, Herrera, D, and Montero, E. The association between respiratory diseases and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2023) 50:842–87. doi: 10.1111/jcpe.13767
11. Lee, J, Kim, B, Kim, W, Ahn, C, Choi, HY, Kim, JG, et al. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U.S. population. Sci Rep. (2021) 11:2366. doi: 10.1038/s41598-021-82053-2
12. Lee, HJ, Lee, JW, Kim, S, and Kwon, YJ. Comparison of the triglyceride glucose index and modified triglyceride glucose indices in assessing periodontitis in Korean adults. J Periodontal Res. (2023) 58:503–10. doi: 10.1111/jre.13108
13. Jepsen, S, Suvan, J, and Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. (2000) 83:125–53.
14. Zeze, T, Shinjo, T, Sato, K, Nishimura, Y, Imagawa, M, Chen, S, et al. Endothelial insulin resistance exacerbates experimental periodontitis. J Dent Res. (2023) 102:1152–61. doi: 10.1177/00220345231181539
15. Thouvenot, K, Turpin, T, Taïlé, J, Clément, K, Meilhac, O, and Gonthier, MP. Links between insulin resistance and periodontal Bacteria: insights on molecular players and therapeutic potential of polyphenols. Biomolecules. (2022) 12:378. doi: 10.3390/biom12030378
16. Thomas, DM, Bredlau, C, Bosy-Westphal, A, Mueller, M, Shen, W, Gallagher, D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. (2013) 21:2264–71. doi: 10.1002/oby.20408
17. Rico-Martín, S, Calderón-García, JF, Sánchez-Rey, P, Franco-Antonio, C, Martínez Alvarez, M, and Sánchez Muñoz-Torrero, JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13023. doi: 10.1111/obr.13023
18. Wu, L, Pu, H, Zhang, M, Hu, H, and Wan, Q. Non-linear relationship between the body roundness index and incident type 2 diabetes in Japan: a secondary retrospective analysis. J Transl Med. (2022) 20:110. doi: 10.1186/s12967-022-03321-x
19. Zhang, X, Ma, N, Lin, Q, Chen, K, Zheng, F, Wu, J, et al. Body roundness index and all-cause mortality among US adults. JAMA Netw Open. (2024) 7:e2415051. doi: 10.1001/jamanetworkopen.2024.15051
20. Yao, F, Cui, J, Shen, Y, Jiang, Y, Li, Y, Liu, X, et al. Evaluating a new obesity indicator for stroke risk prediction: comparative cohort analysis in rural settings of two nations. BMC Public Health. (2024) 24:3301. doi: 10.1186/s12889-024-20631-5
21. Pietropaoli, D, Altamura, S, Ortu, E, Guerrini, L, Pizarro, TT, Ferri, C, et al. Association between metabolic syndrome components and gingival bleeding is women-specific: a nested cross-sectional study. J Transl Med. (2023) 21:252. doi: 10.1186/s12967-023-04072-z
22. Varghese, M, Griffin, C, and Singer, K. The role of sex and sex hormones in regulating obesity-induced inflammation. Adv Exp Med Biol. (2017) 1043:65–86. doi: 10.1007/978-3-319-70178-3_5
23. Valentine, RJ, Vieira, VJ, Woods, JA, and Evans, EM. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause. (2009) 16:84–9. doi: 10.1097/gme.0b013e31817fcb8f
24. Farina, R, Simonelli, A, Tomasi, C, Ioannidou, E, and Trombelli, L. Sexual dimorphism in periodontal inflammation: a cross-sectional study. J Periodontol. (2025) 96:346–54. doi: 10.1002/JPER.24-0466
25. Heitz-Mayfield, LJA. Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol. (2000) 95:10–9.
26. Guerrero-Romero, F, Simental-Mendía, LE, González-Ortiz, M, Martínez-Abundis, E, Ramos-Zavala, MG, Hernández-González, SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
27. Zhang, B, Jiang, D, Ma, H, and Liu, H. Association between triglyceride-glucose index and its obesity indicators with hypertension in postmenopausal women: a cross-sectional study. Front Nutr. (2025) 17:1623697. doi: 10.3389/fnut.2025.1623697
28. Yang, L, Fang, S, Zhang, R, and Xia, R. Associations between different triglyceride glucose index-related obesity indices and periodontitis: results from NHANES 2009-2014. Lipids Health Dis. (2024) 23:213. doi: 10.1186/s12944-024-02192-z
29. Liu, Y, Yu, Y, Nickel, JC, Iwasaki, LR, Duan, P, Simmer-Beck, M, et al. Gender differences in the association of periodontitis and type 2 diabetes. Int Dent J. (2018) 68:433–40. doi: 10.1111/idj.12399
30. Ma, W, Zou, Z, Yang, L, Lin, D, Guo, J, Shan, Z, et al. Exploring the bi-directional relationship between periodontitis and dyslipidemia: a comprehensive systematic review and meta-analysis. BMC Oral Health. (2024) 24:508. doi: 10.1186/s12903-023-03668-7
31. Xu, Y, Wang, M, Bao, L, Cheng, Z, and Li, X. A cross-sectional study based on the comprehensive model of information seeking: which factors influence health information-seeking behavior in patients with periodontitis. BMC Oral Health. (2024) 24:1307. doi: 10.1186/s12903-024-05068-x
32. Jiang, S, Liang, C, Jing, J, Wang, M, Sun, H, and Zuo, B. Insulin resistance as a mediator between obesity and periodontitis risk. BMC Oral Health. (2025) 25:1015. doi: 10.1186/s12903-025-06362-y
33. Simonelli, A, Citterio, F, Falcone, F, D’Aiuto, F, Sforza, NM, Corrao, S, et al. Effect of periodontal treatment on metabolic syndrome parameters: a systematic review. Oral Dis. (2025). doi: 10.1111/odi.70018
34. Bai, J, Zhou, C, Liu, Y, Ding, M, Zhang, Z, Chen, Z, et al. Relationship between serum uric acid levels and periodontitis-a cross-sectional study. PLoS One. (2024) 19:e0310243. doi: 10.1371/journal.pone.0310243
35. Alwithanani, N. Periodontal disease and smoking: systematic review. J Pharm Bioallied Sci. (2023) 15:S64–71. doi: 10.4103/jpbs.jpbs_516_22
36. Gandhi, UH, Benjamin, A, Gajjar, S, Hirani, T, Desai, K, Suhagia, BB, et al. Alcohol and periodontal disease: a narrative review. Cureus. (2024) 16:e62270. doi: 10.7759/cureus.62270
37. Zhou, BF. Cooperative Meta-analysis Group of the Working Group on obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.
38. Zhang, X, Ding, L, Hu, H, He, H, Xiong, Z, and Zhu, X. Associations of body-roundness index and sarcopenia with cardiovascular disease among middle-aged and older adults: findings from CHARLS. J Nutr Health Aging. (2023) 27:953–9. doi: 10.1007/s12603-023-2001-2
39. Wang, ZZ, Ma, GL, Xu, B, Chen, X, Yang, BW, Qin, XK, et al. Association between a body shape index and bone mineral density in middle-aged and elderly adults: a retrospective analysis of NHANES 2005-2018. Front Endocrinol. (2025) 16:1506841. doi: 10.3389/fendo.2025.1506841
40. Guo, Y, Jiang, S, Li, H, Xie, G, Pavel, V, Zhang, Q, et al. Obesity induces osteoimmunology imbalance: molecular mechanisms and clinical implications. Biomed Pharmacother. (2024) 177:117139. doi: 10.1016/j.biopha.2024.117139
41. Wei, C, and Zhang, G. Association between body roundness index (BRI) and gallstones: results of the 2017-2020 national health and nutrition examination survey (NHANES). BMC Gastroenterol. (2024) 24:192. doi: 10.1186/s12876-024-03280-1
42. Bendall, CL, Mayr, HL, Opie, RS, Bes-Rastrollo, M, Itsiopoulos, C, and Thomas, CJ. Central obesity and the mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr. (2018) 58:3070–84. doi: 10.1080/10408398.2017.1351917
43. Al-Zahrani, MS, Borawski, EA, and Bissada, NF. Periodontitis and three health-enhancing behaviors: maintaining normal weight, engaging in recommended level of exercise, and consuming a high-quality diet. J Periodontol. (2005) 76:1362–6. doi: 10.1902/jop.2005.76.8.1362
44. Tahapary, DL, Pratisthita, LB, Fitri, NA, Marcella, C, Wafa, S, Kurniawan, F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581
45. Zhou, C, Zhan, L, Yuan, J, Tong, X, Peng, Y, and Zha, Y. Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients. Eat Weight Disord. (2020) 25:727–34. doi: 10.1007/s40519-019-00678-9
46. Kalhan, AC, Kalhan, TA, Romandini, M, Bitencourt, FV, Cooray, UMP, Leite, FRM, et al. Insulin resistance and periodontitis: mediation by blood pressure. J Periodontal Res. (2025) 60:226–235. doi: 10.1111/jre.13333
47. Ladeira, LLC, Nascimento, GG, Leite, FRM, Alves-Costa, S, Barbosa, JMA, Alves, CMC, et al. Obesity, insulin resistance, caries, and periodontitis: Syndemic framework. Nutrients. (2023) 15:3512. doi: 10.3390/nu15163512
48. Byun, JS, Lee, HY, Tian, J, Moon, JS, Choi, J, Lee, SH, et al. Effect of salivary Exosomal miR-25-3p on periodontitis with insulin resistance. Front Immunol. (2021) 12:775046. doi: 10.3389/fimmu.2021.775046
49. Yang, Y, Li, S, Ren, Q, Qiu, Y, Pan, M, Liu, G, et al. The interaction between triglyceride-glucose index and visceral adiposity in cardiovascular disease risk: findings from a nationwide Chinese cohort. Cardiovasc Diabetol. (2024) 23:427. doi: 10.1186/s12933-024-02518-2
50. Benguigui, C, Bongard, V, Ruidavets, JB, Chamontin, B, Sixou, M, Ferrières, J, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. (2010) 37:601–8. doi: 10.1111/j.1600-051X.2010.01571.x
51. Shiau, HJ, and Reynolds, MA. Sex differences in destructive periodontal disease: exploring the biologic basis. J Periodontol. (2010) 81:1505–17. doi: 10.1902/jop.2010.100045
52. Weiss, LA, Abney, M, Cook, EH, and Ober, C. Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet. (2005) 76:33–41. doi: 10.1086/426697
53. Ober, C, Loisel, DA, and Gilad, Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. (2008) 9:911–22. doi: 10.1038/nrg2415
54. Valerio, MS, Basilakos, DS, Kirkpatrick, JE, Chavez, M, Hathaway-Schrader, J, Herbert, BA, et al. Sex-based differential regulation of bacterial-induced bone resorption. J Periodontal Res. (2017) 52:377–87. doi: 10.1111/jre.12401
55. Adams, M, and Sabaté, J. Sexual dimorphism in cardiovascular disease risk and risk factors among vegetarians: an exploration of the potential mechanisms. Curr Atheroscler Rep. (2019) 21:35. doi: 10.1007/s11883-019-0796-4
56. Shepherd, R, Cheung, AS, Pang, K, Saffery, R, and Novakovic, B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol. (2020) 11:604000. doi: 10.3389/fimmu.2020.604000
57. Edwards, M, Dai, R, and Ahmed, SA. Our environment shapes us: the importance of environment and sex differences in regulation of autoantibody production. Front Immunol. (2018) 9:478. doi: 10.3389/fimmu.2018.00478
58. Ribas, V, Drew, BG, Zhou, Z, Phun, J, Kalajian, NY, Soleymani, T, et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. (2016) 8:334ra54. doi: 10.1126/scitranslmed.aad3815
59. Kautzky-Willer, A, Harreiter, J, and Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. (2016) 37:278–316. doi: 10.1210/er.2015-1137
60. Li, J, Xiong, M, Fu, XH, Fan, Y, Dong, C, Sun, X, et al. Determining a multimodal aging clock in a cohort of Chinese women. Med. (2023) 4:825–848.e13. doi: 10.1016/j.medj.2023.06.010
61. Kanmaz, M, Kanmaz, B, and Buduneli, N. Periodontal treatment outcomes in smokers: a narrative review. Tob Induc Dis. (2021) 19:77. doi: 10.18332/tid/142106
62. Danielsen, B, Manji, F, Nagelkerke, N, Fejerskov, O, and Baelum, V. Effect of cigarette smoking on the transition dynamics in experimental gingivitis. J Clin Periodontol. (1990) 17:159–64. doi: 10.1111/j.1600-051X.1990.tb01080.x
63. Silva, H. Tobacco use and periodontal disease-the role of microvascular dysfunction. Biology. (2021) 10:441. doi: 10.3390/biology10050441
64. Zhang, Y, He, J, He, B, Huang, R, and Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob Induc Dis. (2019) 17:40. doi: 10.18332/tid/106187
65. Flouris, AD, Metsios, GS, Carrillo, AE, Jamurtas, AZ, Gourgoulianis, K, Kiropoulos, T, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med. (2009) 179:1029–33. doi: 10.1164/rccm.200812-1920OC
66. Flouris, AD, Metsios, GS, Jamurtas, AZ, and Koutedakis, Y. Sexual dimorphism in the acute effects of secondhand smoke on thyroid hormone secretion, inflammatory markers and vascular function. Am J Physiol Endocrinol Metab. (2008) 294:E456–62. doi: 10.1152/ajpendo.00699.2007
67. Polak, AM, Adamska, A, Krentowska, A, Łebkowska, A, Hryniewicka, J, Adamski, M, et al. Body composition, serum concentrations of androgens and insulin resistance in different polycystic ovary syndrome phenotypes. J Clin Med. (2020) 9:732. doi: 10.3390/jcm9030732
68. Ebersole, JL, Al-Sabbagh, M, Gonzalez, OA, and Dawson, DR. Ageing effects on humoral immune responses in chronic periodontitis. J Clin Periodontol. (2018) 45:680–92. doi: 10.1111/jcpe.12881
69. Lee, YC, Lee, JW, and Kwon, YJ. Comparison of the triglyceride glucose (TyG) index, triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, and metabolic score for insulin resistance (METS-IR) associated with periodontitis in Korean adults. Ther Adv Chronic Dis. (2022) 13:20406223221122671. doi: 10.1177/20406223221122671
Keywords: body roundness index, triglyceride-glucose index, periodontitis, cross-sectional study, body fat
Citation: Wang L, Liu J, Wang J, Liu P, Zhao Q, Wang Y, Zhao H, Zhu X, Liu S and Zhang J (2025) Joint association of triglyceride glucose index (TyG) and body roundness index with the risk of periodontitis: a cross-sectional study. Front. Nutr. 12:1642112. doi: 10.3389/fnut.2025.1642112
Edited by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandReviewed by:
Monica Tarcea, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaChuan Shao, Chongqing General Hospital, China
Copyright © 2025 Wang, Liu, Wang, Liu, Zhao, Wang, Zhao, Zhu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Liu, Y2h1Y2tsZWpsQDE2My5jb20=; Jinqiang Zhang ODM1MTkxMDQxQHFxLmNvbQ==
 Liang Wang1
Liang Wang1 Jia Liu
Jia Liu Shan Liu
Shan Liu