- 1ICAR-Central Agroforestry Research Institute, Jhansi, Uttar Pradesh, India
- 2R and D, Indofil Industry Ltd., Thane West, Maharashtra, India
- 3Bundelkhand University, Jhansi, Uttar Pradesh, India
- 4ICAR-Indian Institute of Soil and Water Conservation, Regional Center-Chandigarh, Chandigarh, India
Introduction: Manila tamarind is an underutilized and multipurpose crop with considerable value for food, fodder, fuel, and green manuring. Its hardy nature, drought tolerance, and diverse applications make it a promising climate-smart crop for agroforestry systems, especially in the drylands of the Bundelkhand region. However, research on its antioxidant potential and phytochemical composition has been largely neglected.
Methods: To address this gap, the present study was conducted to assess the antioxidant content and identify health-related phytochemicals in the arils of 15 different Manila tamarind accessions, which were collected locally from the Bundelkhand region. Antioxidant analysis (DDPH, ABTS, Metal Chelating), phenol, flavonol, and anthocyanin were estimated as per standard procedures, whereas the phytochemicals were estimated through UHPLC-Q-TOF-MS analysis.
Results: The results revealed significant variation in vitamin content (vitamin A: 0.18–0.28 mg/100 g, thiamin: 0.18–0.24 mg/100 g, riboflavin: 0.11–0.18 mg/100 g, vitamin C: 122–139 mg/100 g, and b-carotenoid equivalent 11.4–19.6 mg/100 g) and antioxidant activity across various assays, including DPPH (65.58–282.44 mg/ml), ABTS (117.80–508 IC50 mg/ml), metal chelating capacity (167.41–376.06 IC50 mg/ml), total phenolic content (0.019–0.174 mg GAE/g fresh weight), and total flavonol content (0.0042–0.0088 mg QE/g fresh weight). Anthocyanin levels ranged from 0.126 to 0.262 mg TAC/g fresh weight. Phytochemical profiling led to the identification of 144 compounds, which were classified into 43 biological function categories. The predominant compounds exhibited antioxidant, anti-inflammatory, anticancer, neuroprotective, antifungal, antibacterial, antimicrobial, antiviral, anti-tumor, analgesic, cardioprotective, and antidiabetic properties, highlighting the crop's immense potential for health and nutritional applications.
Conclusion: The primary aim of this study was to evaluate the potential of this plant, and the findings provide strong evidence that this plant possesses significant bioactive compounds, suggesting its potential use in combating a range of infectious diseases. In addition to this, the findings of this study are valuable for selecting superior parent lines to enhance desirable traits in future Manila tamarind breeding programs.
1 Introduction
Manila tamarind (Pithecellobium dulce), also known as sweet tamarind, is a member of the Leguminosae family and belongs to the genus Pithecellobium, which comprises 18 species. This versatile tree is known by various names across different languages and regions, reflecting its wide distribution and cultural significance. Some of these are known by various names in different languages, such as Monkey Pod (English), Vilayati Imli, Jangal Jalebi, Singri, Dakhani Babul (Hindi), Kodukkappuli (Tamil), Vilayati Chinch (Marathi), Me Keo, Keo Tay (Vietnamese), Asam Koranji (Indonesian), and Makham Thet (Thai). Manila tamarind is a fast-growing, underutilized tree species that is hardy, evergreen, highly drought-tolerant, offers significant nutritional benefits, and plays a vital role in improving food security and reducing poverty among communities living in challenging agro-climatic conditions (1). It is remarkably adaptable and capable of thriving in nutritionally poor and environmentally challenging conditions. The species grows well across a wide range of soil types, including sandy, loamy, and clayey soils, as well as acidic, neutral, alkaline, and even saline environments (2). Manila tamarind can grow at elevations up to 1,550 meters and withstand extreme temperatures, tolerating conditions as high as 48 °C in arid, hot tropical, and subtropical regions. Its adaptability also extends to varying rainfall regimes—it thrives in areas receiving annual precipitation between 700 and 1,800 mm and can survive in regions with as little as 250 mm of rainfall (2, 3). The seeds and pods of Manila tamarind are highly nutritious, with the aril, edible pulp, being particularly rich in essential nutrients. It contains moisture (75.8–77.8 g), energy (78.8 kcal), ash (0.6%), protein (12.47–23.3 g), fat (0.4–0.5 g), carbohydrates (18.2–76.87 g), fiber (1.1–1.3 g), calcium (13–21 mg), phosphorus (42–58 mg), iron (0.5–1.1 mg), sodium (3.7–19 mg), potassium (222–377 mg), magnesium (40 mg), and copper (13.8–33.0 mg) per 100 g of aril (4–6). As a leguminous species, Manila tamarind has nitrogen-fixing properties that enhance soil fertility. Its fast growth, spiny structure, and dense branching make it an excellent choice for use as a bio-fence against wild and stray animals. Additionally, the wood is valued for furniture-making and tool construction, serves as a good source of firewood due to its high calorific value, and is widely employed in windbreaks and shelterbelts (7).
Since ancient times, human civilizations have relied on plants for the development of therapeutic agents. The traditional use of natural products in treating various ailments underscores the importance of exploring plant-based sources for novel pharmacological compounds (8). The therapeutic potential of medicinal plants is primarily attributed to the diversity and complexity of their phytochemical constituents, which exert a wide range of physiological effects on the human body (9). Consequently, phytochemical screening plays a crucial role in identifying these bioactive compounds, laying the groundwork for the discovery and development of modern medicines (10).
There has been a significant rise in microbial resistance to synthetic drugs, coupled with a decline in the development of new antimicrobial agents (3). In response to this growing challenge, attention has increasingly shifted toward the discovery of novel, effective, and affordable therapeutic alternatives, particularly for combating microbial infections prevalent in underdeveloped and developing countries, where infectious diseases account for nearly 50% of the mortality rate (3). Phytochemicals, the secondary metabolites produced through diverse plant metabolic pathways, have emerged as promising candidates for drug development due to their potent antimicrobial properties and natural origin. In light of the escalating threat posed by multidrug-resistant microbes, there is a growing emphasis in modern medicine on the urgent need to identify and develop innovative phytochemicals from natural sources. These compounds hold significant potential to provide effective and sustainable solutions to combat antimicrobial resistance and improve human health outcomes.
Furthermore, cancer remains a leading global health challenge, with ~20 million new cases and 10 million cancer-related deaths recorded in 2020 alone (4). The most common cancer types include lung, breast, colorectal, prostate, and gastric cancers. This is projected to increase by 47% by 2040, particularly in low- and middle-income countries, due to demographic shifts, urbanization, and limited access to early diagnosis and preventive healthcare (5). Despite the traditional use of Manila tamarind for its nutritional and therapeutic properties, there is a notable lack of comprehensive scientific studies focusing on the antioxidant capacity, phytochemical composition, anthocyanin concentration, and vitamin content of its aril. While various plant-based foods have been extensively analyzed for their bioactive compounds, Manila tamarind remains underexplored, particularly for its potential health-promoting properties using modern analytical methodologies. This gap hinders the full utilization and recognition of the species in functional food and nutraceutical development. Hence, identifying accessions with superior yield and biochemical traits forms the foundation for developing high-yielding, nutritionally enhanced cultivars (6–8). Therefore, the present investigation aims to systematically evaluate its antioxidant activity, anthocyanin content, vitamin profile, and overall phytochemical composition using advanced analytical techniques.
2 Materials and methods
The field survey for the collection of different Manila tamarind accessions was carried out in the three villages (Bhojla, Karari, and Simardha) of Jhansi district of Uttar Pradesh. Jhansi is the heart of the Bundelkhand region (23°10′-26°30′N and 78°20′-81°40′E), which has a semi-arid type climate with a moisture deficiency index varying from 40 to 60. A total of 15 Manila tamarind (MT) accessions (MT-1 to MT-15) were collected, and dried Manila tamarind pods were initially cleaned with tap water, followed by a rinse with distilled water to remove surface impurities. The pods were then air-dried at room temperature. After drying, the pods were manually crushed to separate the aril from the seeds. The aril (seed-free pulp) was ground into a coarse powder using a mortar and pestle. The resulting powder was stored in sealed containers under dry conditions and later used for further nutraceutical analyses, including antioxidant activity, mineral content, and vitamin composition.
2.1 Sample extraction
Methanolic extracts of Manila tamarind aril were prepared using the cold maceration technique, as described by Omaye et al. (11). Precisely, 50 g of coarsely powdered aril was placed in stoppered containers containing 250 mL of methanol. The mixture was kept at room temperature for 72 h with frequent shaking to facilitate the extraction of soluble phytochemicals. Following maceration, the mixture was filtered through Whatman No. 1 filter paper (125 mm) to obtain a clear filtrate. The filtrate was then concentrated using a rotary evaporator—aqueous extracts at 100 °C, and methanolic extracts at 78 °C—until the volume was reduced to one-fourth of the original. The concentrated extracts were reconstituted in an appropriate volume of solvent to achieve the desired concentration and stored in desiccators until further use.
2.2 Antioxidant analysis
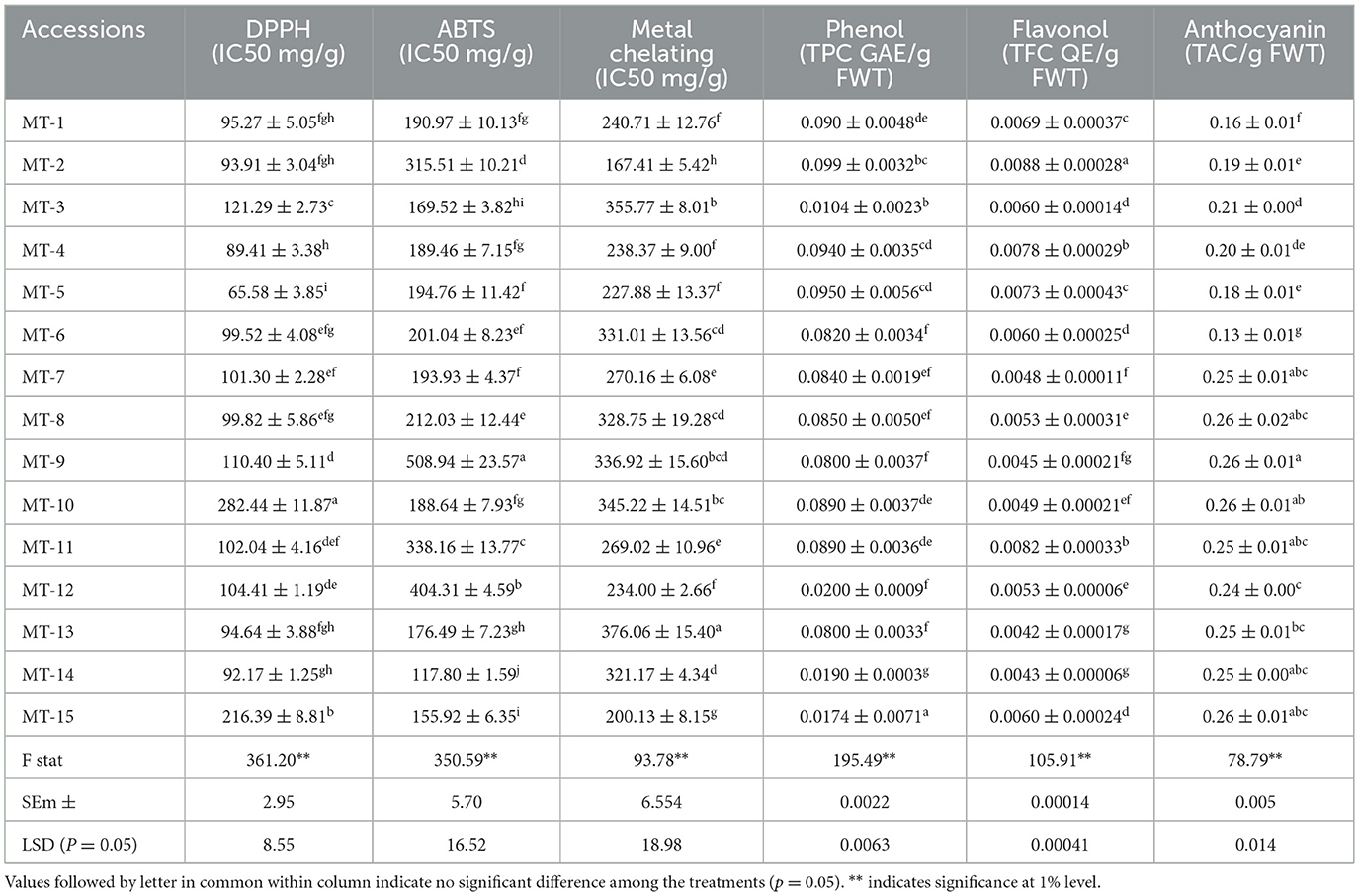
Antioxidant analysis of 15 Manila tamarind accessions was conducted using three different methods: DPPH (2,2-Diphenyl-1-picrylhydrazyl) (12, 13), ABTS (22′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) (14), and metal chelating activity (MCA) (15). The results of DPPH, ABTS, and metal chelating assays were expressed in terms of IC50 (mg/ml), indicating the concentration required to inhibit 50% of free radicals or metal ions.
2.3 Phenol, flavonol, and anthocyanin content
Total phenolic and flavonol contents were measured as TPC (mg/g fresh weight tissue, FWT), while anthocyanin content (16) was expressed as total anthocyanin content (TAC, mg/g FWT). Among the Manila tamarind accession, total phenolic content (TPC) (17) and total flavonol content (TFC) (18) were calculated as per the standard method. Anthocyanin content in Manila tamarind samples was estimated using the pH differential method, as described by Wallace and Giusti (16). In this technique, aril samples are first homogenized and extracted with an acidified solvent—typically methanol or ethanol, containing 0.1% hydrochloric acid (HCl)—to stabilize the anthocyanins. The extract is then filtered or centrifuged to remove any solid residues. Two aliquots of the clarified extract are prepared: one is diluted with a buffer at pH 1.0, and the other with a buffer at pH 4.5.
Absorbance readings are recorded at 520 nm, where anthocyanins exhibit peak absorbance, and at 700 nm to correct for haze or turbidity, using a UV-vis spectrophotometer. The difference in absorbance between the two pH conditions is used to calculate anthocyanin concentration. Results are typically expressed as cyanidin-3-glucoside equivalents (mg/g fresh weight), based on a standard formula that incorporates molecular weight, molar extinction coefficient, path length, and dilution factors.
The absorbance (A) is calculated using the following formula:
The monomeric anthocyanin pigment concentration (expressed as cyanidin-3-glucoside equivalents) is then calculated by:
where:
• MW = molecular weight of cyanidin-3-glucoside (449.2 g/mol),
• DF = dilution factor,
• ε = molar extinction coefficient (26,900 L·mol−1·cm−1 for cyanidin-3-glucoside),
• l = path length of cuvette (1 cm).
2.4 Vitamin content analysis
Fresh and ripe pods of Manila tamarind were collected to estimate different vitamin contents, including vitamin A, thiamin, riboflavin, vitamin C, and β-carotenoid equivalents. The values were expressed in mg/100 g. The details of the methodology are described in the following sections.
2.4.1 Vitamin A
The vitamin-A content was estimated among Manila tamarind accessions using a colorimetric method as per the method suggested by Rodriguez-Amaya and Kimura (9). The sample is first saponified using alcoholic potassium hydroxide (KOH) to release retinol from esterified forms. The unsaponifiable matter, including retinol, is extracted using petroleum ether. After evaporation and redissolution in chloroform, trichloroacetic acid (TCA) is added, leading to the development of a blue color that is measured at 620 nm using a spectrophotometer (9).
2.4.2 Thiamin (vitamin B1)
Thiamin in Manila tamarind accessions was estimated using the fluorometric thiochrome method (10). The sample undergoes acid hydrolysis with 0.1N hydrochloric acid, followed by enzymatic digestion, often using takadiastase, to release thiamin. It is then oxidized with alkaline potassium ferricyanide to form thiochrome, a fluorescent compound. Thiochrome is extracted into isobutanol, and its fluorescence is measured with excitation at 366 nm and emission at 435 nm.
2.4.3 Riboflavin (vitamin B2)
Riboflavin in Manila tamarind accessions was estimated using a fluorometric method that involved both acid hydrolysis and enzymatic digestion for vitamin extraction, followed by fluorometric analysis (19). Approximately 5 g of the sample were homogenized and hydrolyzed with 50 mL of 0.1 N hydrochloric acid by heating in a boiling water bath for 30 min to break protein–vitamin complexes and release bound riboflavin. After cooling, the pH was adjusted to around 4.5 using sodium acetate buffer, and enzymatic digestion was carried out by adding takadiastase enzyme, allowing the mixture to incubate at 37 °C for 2 h to further liberate riboflavin. Following digestion, the mixture was filtered, and the filtrate was subjected to fluorometric analysis. Riboflavin's natural fluorescence was measured using a fluorometer set at an excitation wavelength of 450 nm and an emission wavelength of 520 nm. Quantification was done by comparing sample fluorescence to a standard riboflavin curve prepared under identical conditions.
2.4.4 Vitamin C
The estimation of vitamin C (ascorbic acid) was performed using the 2,6-dichlorophenolindophenol (DCPIP) titration method (11). In this method, approximately 5 g of the sample was homogenized in 50 mL of a 3% metaphosphoric acid solution, which serves to precipitate proteins and prevent oxidative degradation of ascorbic acid by stabilizing it in the acidic medium. The homogenate is filtered through Whatman No. 1 filter paper to obtain a clear extract. An aliquot (usually 10 ml) of the filtrate is then titrated against a freshly prepared standard DCPIP dye solution of known concentration. The dye is reduced by ascorbic acid, leading to a color change from blue to colorless; the endpoint of the titration is marked by the appearance of a light pink color that persists for at least 15 s, indicating that all the ascorbic acid has been oxidized. The amount of DCPIP used is directly proportional to the amount of vitamin C in the sample. A standard curve using known concentrations of ascorbic acid is used for calibration to calculate the vitamin C content, usually expressed in mg per 100 g of sample.
2.4.5 β-carotene equivalents estimation
The β-carotene equivalents in Manila tamarind samples were estimated using a colorimetric method involving extraction with organic solvents followed by spectrophotometric analysis, as described by Rodriguez-Amaya and Kimura (9). In this method, approximately 5 g of a finely homogenized sample was extracted with cold acetone to solubilize carotenoids, including β-carotene. The extraction is carried out under low-light conditions to prevent degradation, and the extract is filtered through filter paper. The acetone extract is then transferred to a separating funnel containing petroleum ether (or hexane), and the carotenoids are partitioned into the non-polar solvent. The aqueous phase is discarded, and the ether layer is washed several times with distilled water to remove any residual acetone and polar impurities. The ether phase, now containing the β-carotene, is collected, and its absorbance is measured at 450 nm using a UV–vis spectrophotometer. The β-carotene content is then quantified by comparing the absorbance to a standard calibration curve prepared with known concentrations of pure β-carotene, and results are expressed as β-carotene equivalents (mg/100 g sample).
2.5 Phytochemical profiling of Manila tamarind accessions
For the phytochemical profiling of Manila tamarind (Pithecellobium dulce), samples were analyzed using a Vanquish UHPLC (ultra-high-performance liquid chromatography) system coupled with a Q Exactive™ quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) on the IITB_QE-PC platform. Extracts were filtered through a 0.22 μm membrane filter before injection. Separation was carried out on a Hypersil GOLD C18 column (100 × 2.1 mm, 1.9 μm particle size) maintained at 40 °C. The mobile phases used were (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. A gradient elution was employed as follows: 0–2 min: 5% B; 2–20 min: 5% B; 20–25 min: linear increase to 95% B; 25–26 min: hold at 95% B; 26–30 min: 5% B; and 30–35 min: re-equilibrate to 5% B. The flow rate was maintained at 3 μL/min, and the injected sample volume was 5 μL. The highest flow rate for ramp-up and down was set at 6.0 ml/min2, with pressure ranging from 0 to 1,034 bar. The mass spectrometer operated in positive electrospray ionization (ESI+) mode with the following parameters: spray voltage 3.5 kV, capillary temperature 320 °C, sheath gas flow 35 units, auxiliary gas flow 10 units, and S-lens RF level 50. Full scan MS data were acquired over an m/z range of 100–1,000 at a resolution of 70,000 (FWHM at m/z 200) with an automatic gain control (AGC) target of 1e6. Data-dependent MS/MS acquisition was enabled to fragment the top 5 most intense ions per scan cycle. The identification process comprised several critical steps, including library matching, feature recognition, elemental composition analysis, background subtraction using blank samples, retention time alignment, and fragmentation search (FISh) scoring with a threshold above 40. Phytochemicals present in the aril of Manila tamarind were primarily identified by comparing MS/MS spectra with the mzCloud database, while unmatched signals were further analyzed and cross-referenced using the ChemSpider database for confirmation (12).
2.6 Statistical analysis
The data on antioxidants, anthocyanins, and vitamins of 15 Manila tamarind accessions were analyzed as per the procedure of the analysis of variance (ANOVA) for the completely randomized block design (CRBD), and significance was tested at 5% level (13). The differences between mean values were also determined using Duncan's multiple range test at a 5% significance level.
3 Results and discussion
3.1 Antioxidant analysis
Antioxidants are vital to human health as they neutralize free radicals—unstable molecules that can damage cells, proteins, and DNA through oxidative stress. By mitigating this damage, antioxidants help reduce the risk of chronic conditions, including cardiovascular disease, cancer, diabetes, and neurodegenerative disorders such as Alzheimer's disease. Antioxidants also support immune function, reduce inflammation, and slow down the aging process by preserving skin and tissue health (14). Considering this, we estimated the antioxidant activity of 15 Manila tamarind accessions using multiple assays, including DPPH, ABTS, metal chelating activity, total phenolic content, and flavonol content. All the results were statistically significant (p < 0.05) and are summarized in Table 1. The DPPH radical scavenging activity (IC50) ranged from 65.58 mg/g (MT-5) to 282.44 mg/g (MT-10), with an average of 117.91 mg/g, indicating a broad spectrum of antioxidant capacities of these accessions. Significantly lower IC50 values of MT-5 suggested stronger antioxidant activity and higher potency. Similarly, ABTS assay exhibited maximum antioxidant activity in MT-14 (117.80 IC50 mg/g) while minimum in MT-9 (508.94 IC50 mg/g) with an overall mean of 237.17 IC50 mg/g. The MC assay unveiled MT-2 with a significantly higher value (167.41 IC50 mg/g), followed by MT-15 (200.13 IC50 mg/g) and MT-13 with the lowest (376.06 IC50 mg/g) antioxidant activity. The study showed that Manila tamarind has the ability to chelate metals, which may function as a protective mechanism against oxidative damage brought on by metal-catalyzed degradation processes (15).
3.2 Phenol, flavonol, and anthocyanin content
Manila tamarind accessions exhibited a low range of phenol and flavonol content in the arils of the pods. Table 1 shows the values of total phenolic content (TPC) ranging from 0.019 mg Gallic Acid Equivalent (GAE)/g FWT (MT-14) to 0.174 mg GAE/g FWT (MT-12), with a mean value of 0.089 mg GAE/g FWT. The total flavonol content (TFC) ranged between 0.0042 mg QE/g FWT (MT-13) and 0.0088 mg QE/g FWT (MT-2), with a mean of 0.0060 mg QE/g FWT. Phenolic compounds are well-documented for their therapeutic potential in managing various human health disorders, including hypertension, metabolic syndromes, inflammatory conditions, and neurodegenerative diseases. Their efficacy is primarily attributed to their ability to inhibit key enzymes involved in the progression of these conditions (17). Among them, flavonoids—a major subclass of phenolics—demonstrate a broad spectrum of biological activities, such as antiviral, anticancer, antioxidant, and anti-inflammatory effects. Additionally, they possess cardioprotective and neuroprotective properties, contributing significantly to disease prevention and overall health maintenance (18). Consequently, extensive screening of additional Manila tamarind accessions is warranted to identify genotypes with superior phenol and flavonol content. Such efforts could support the development of functional foods aimed at reducing disease incidence in human populations. The findings of the present study highlight the considerable phytochemical and antioxidant diversity among Manila tamarind accessions, offering valuable potential for breeding programs focused on enhancing nutritional and therapeutic value. Anthocyanins and other dietary bioactive compounds contribute significantly to long-term health and wellbeing through their diverse biological activities. Regular consumption of colorful fruits and vegetables, rich in natural sources of these compounds, is an essential component of a balanced diet and has been associated with a reduced risk of various chronic diseases (16). Total anthocyanin content (TAC) in different Manila tamarind accessions (Table 1) indicated anthocyanin content ranged from 0.126 (MT-6) to 0.262 TAC mg/g FWT (MT-9), with a mean of 0.22 mg/g FWT. Superior accessions such as MT-9 and MT-10, with higher TAC, may serve as promising candidates for nutraceutical applications (20).
3.3 Vitamins and pigments
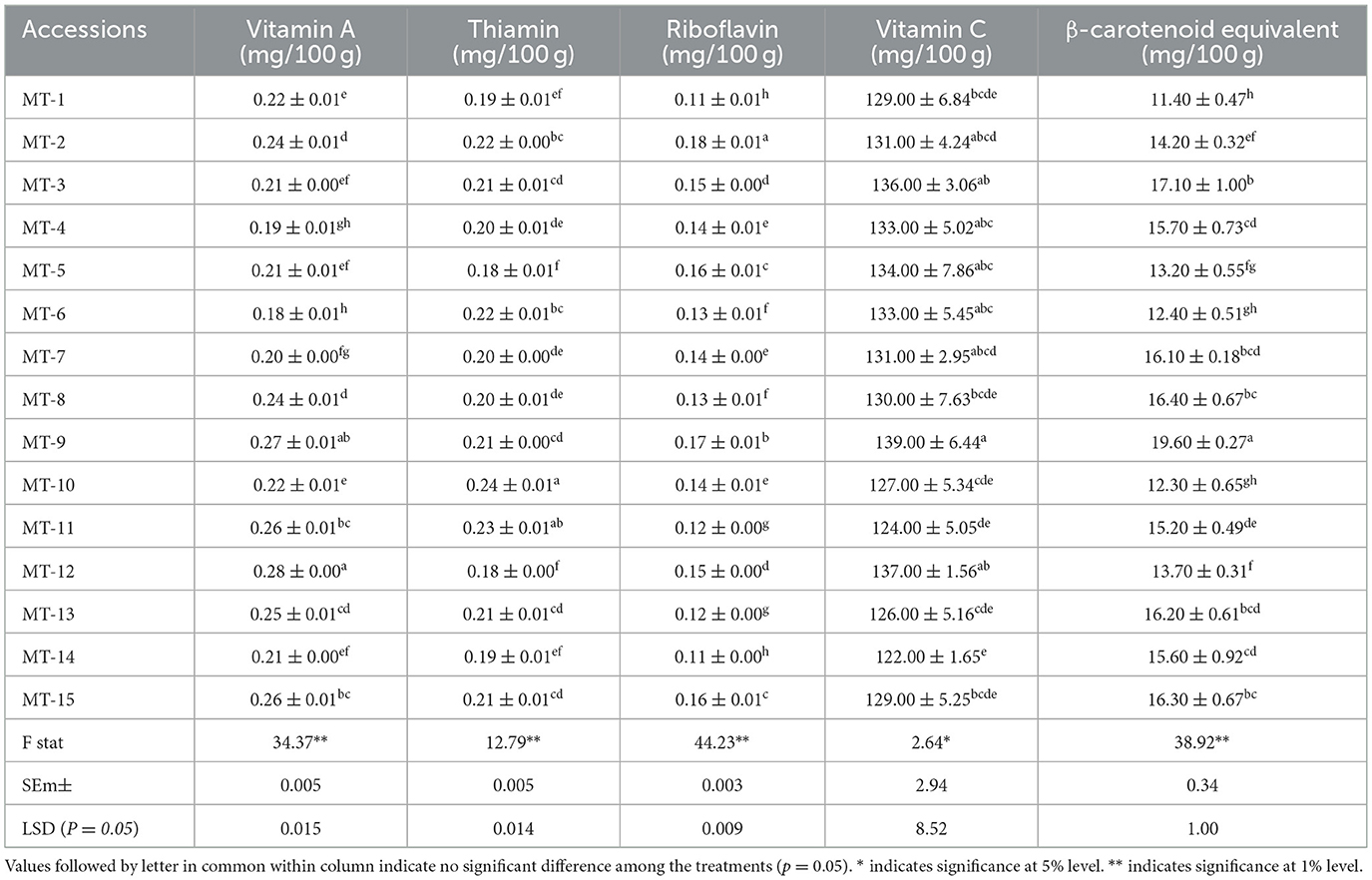
Vitamin profiling of 15 Manila tamarind accessions revealed considerable variability in the content of vitamin A (mg/100 g), thiamin (mg/100 g), riboflavin (mg/100 g), vitamin C (mg/100 g), and β-Carotenoid equivalents (mg/100 g) (Table 2). The vitamin A ranged from 0.18 (MT-6) to 0.28 mg/100 g (MT-12), thiamin ranged from 0.18 mg (MT-5 and MT-12) to 0.24 mg (MT-10), riboflavin ranged from 0.11 mg (MT-1, MT-14) and 0.18 mg (MT-2), vitamin C from 122 mg (MT-14) to 139 mg (MT-9), and β-carotenoid equivalents ranged from 11.4 mg (MT-1) to 19.6 mg (MT-9).
The highest vitamin A content of accession MT-12 indicated its potential therapeutic use in supporting vision and immune functions. These values align with previous studies indicating that legumes and tropical fruits are effective sources of vitamin A (21). Thiamin, essential for carbohydrate metabolism, was found in appreciable amounts in accessions MT-10, MT-11, and MT-2, suggesting their potential utility in dietary interventions aimed at improving thiamin intake. Riboflavin, which plays a key role in energy production and cellular function (22), showed relatively low variability across accessions, indicating possible genetic stability of this trait. Vitamin C, a potent antioxidant essential for collagen synthesis and immune defense, was present in significant quantities. With a daily recommended intake of 90 mg for men and 75 mg for women, the vitamin C content in Manila tamarind was approximately 1.6 times higher than the recommended daily allowance, suggesting that regular consumption could readily meet and exceed daily requirements. The observed vitamin C levels were notably higher than those reported for many commonly consumed fruits, positioning Manila tamarind as a rich natural source of ascorbic acid (23). Similarly, β-carotenoids, which serve both as precursors to vitamin A and as antioxidants (24), were found in higher concentrations in accessions MT-9, MT-3, and MT-8. Given the recommended daily intake of β-carotene (6–15 mg/day for adults and adolescents), the required amount can be fulfilled by consuming approximately 80–90 g of Manila tamarind. Among all accessions, MT-9 exhibited the highest levels of both vitamin C and β-carotenoids. Accessions MT-9, MT-12, and MT-2 consistently recorded elevated levels of multiple vitamins and carotenoids, marking them as promising candidates for use in nutritional improvement programs and the development of functional foods.
3.4 Phytochemical profiling
A total of 144 phytochemicals were identified in Manila tamarind, comprising 117 compounds detected in positive ionization mode and 27 in negative ionization mode. These phytochemicals were functionally classified based on their biological activities and grouped into 43 distinct categories (Figure 1, Table 3). LC-MS chromatographic profiling of the methanolic pulp extract revealed a diverse and complex phytochemical landscape. The total ion chromatogram (TIC) demonstrated elution of various compounds within a 0–35 min retention time (RT) window (Figure 2). The positive ionization mode spectrum exhibited the prominent peaks at RT 1.44, 3.80, 6.39, 10.26, 12.14, and a cluster of intense peaks between 22.64 and 24.00 min. In contrast, negative ion mode revealed sharper and more intense peaks at RT 1.30, 10.26, 13.22, 15.26, and 22.64 min. Notably, a strong and consistent peak at RT 22.64 min was observed in both modes, suggesting the presence of a key compound detectable across polarities, indicative of its abundance or unique ionization behavior.
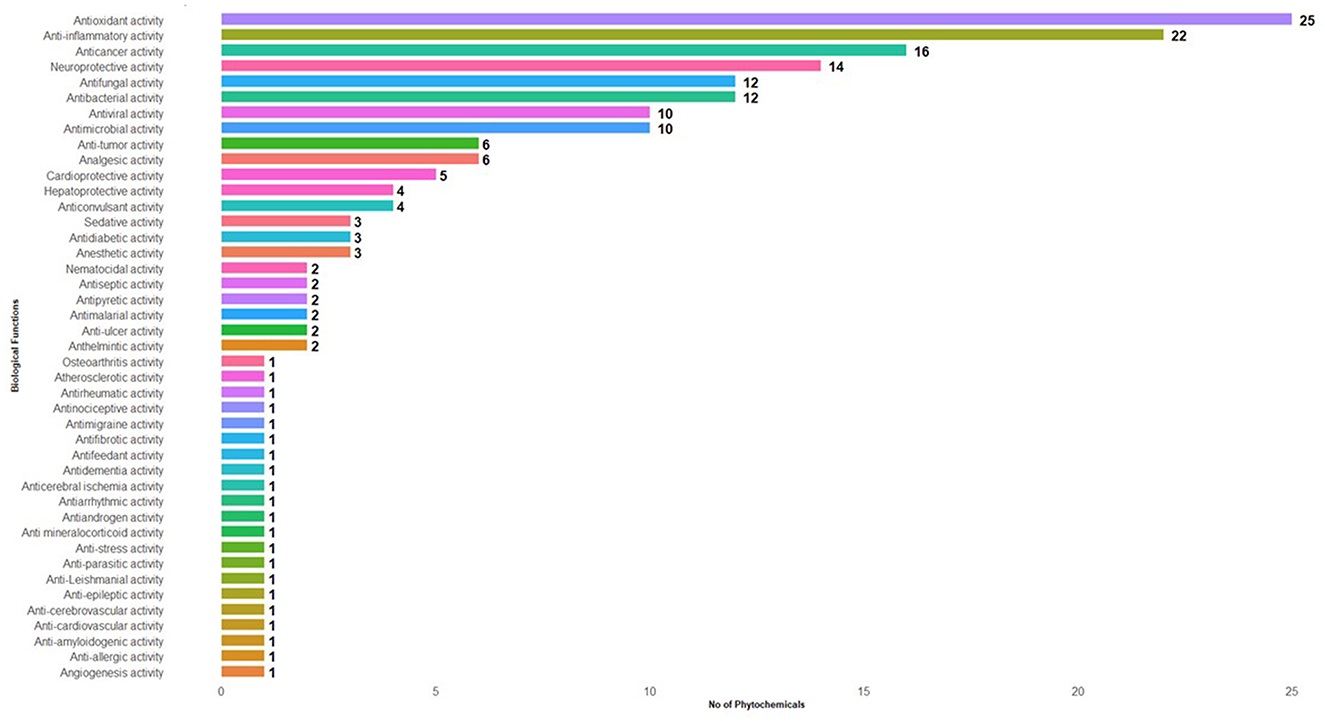
Figure 1. List of biological functions and the number of phytochemical compounds found in the aril of Mania tamarind.
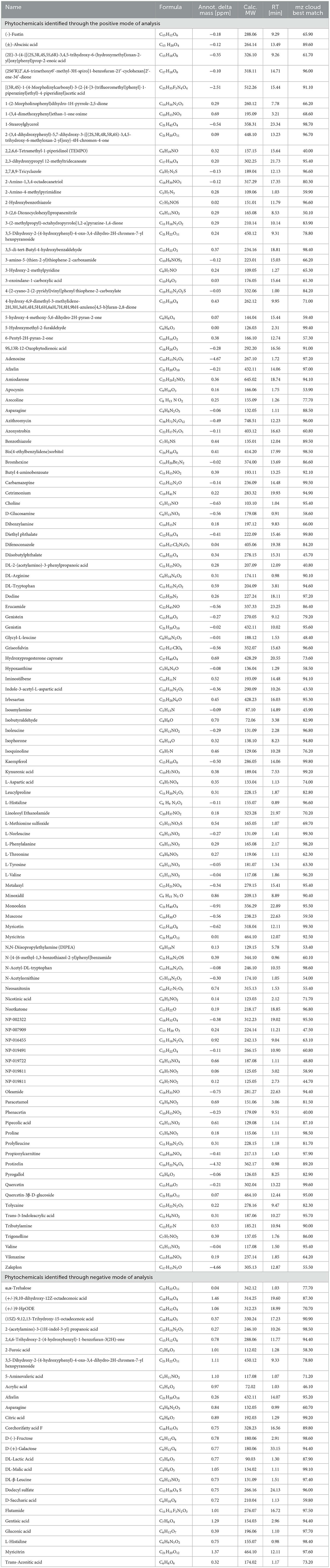
Table 3. Phytochemicals identified in Manila tamarind through the positive and negative mode of analysis.
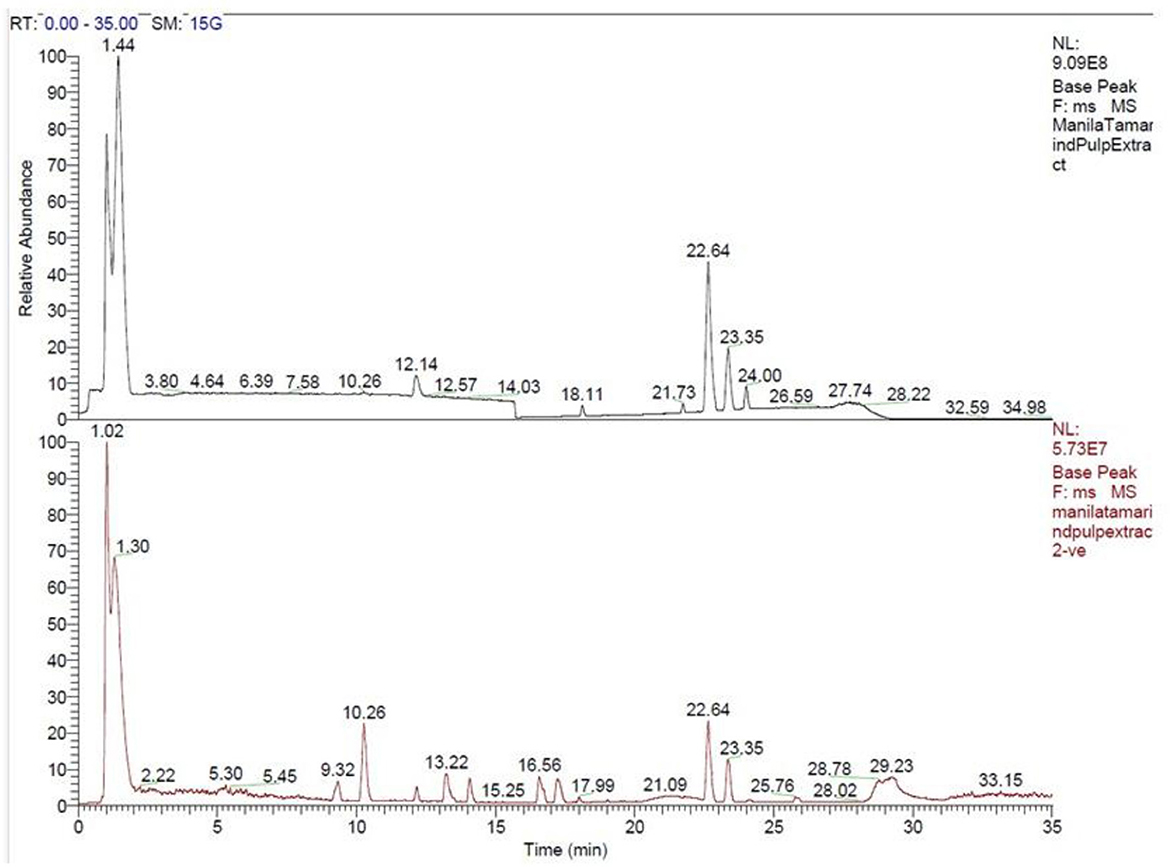
Figure 2. Total ion chromatograms (TIC) of methanolic extract of Manila tamarind pulp in positive (top, black) and negative (bottom, red) ionization modes obtained via LC-MS analysis. The x-axis represents retention time (RT, in minutes) while the y-axis shows relative abundance of ion signals. The presence of the prominent peaks indicates the presence of multiple phytoconstituents in both modes.
Various phytochemicals identified in Manila tamarind (Table 4) were antioxidant activity (25), anti-inflammatory activity (22), anticancer activity (15), neuroprotective activity (13), antifungal activity (11), antibacterial activity (11), antimicrobial activity (10), antiviral activity (10), anti-tumor activity (6), analgesic activity (6), cardio-protective activity (5), hepatoprotective activity (4), anticonvulsant activity (4), antidiabetic activity (3), anesthetic activity (3), sedative activity (3), anti-ulcer activity (2), antiseptic activity (2), antipyretic activity (2), anthelmintic activity (2), antimalarial activity (2), nematocidal activity (2), antimigraine activity (1), antiarrhythmic activity (1), anti-amyloidogenic activity (1), antiandrogen activity (1), antifibrotic activity (1), osteoarthritis activity (1), angiogenesis activity (1), antifeedant activity (1), anti-epileptic activity (1), anticerebral ischemia activity (1), anti-leishmanial activity (1), anti-allergic activity (1), atherosclerotic activity (1), anti-stress activity (1), antirheumatic activity (1), antinociceptive activity (1), anti-cardiovascular activity (1), anti-mineralocorticoid activity (1), anti-parasitic activity (1), antidementia activity (1), and anti-cerebrovascular activity (1). The chemical structures of the representative compounds are illustrated in Figure 3.
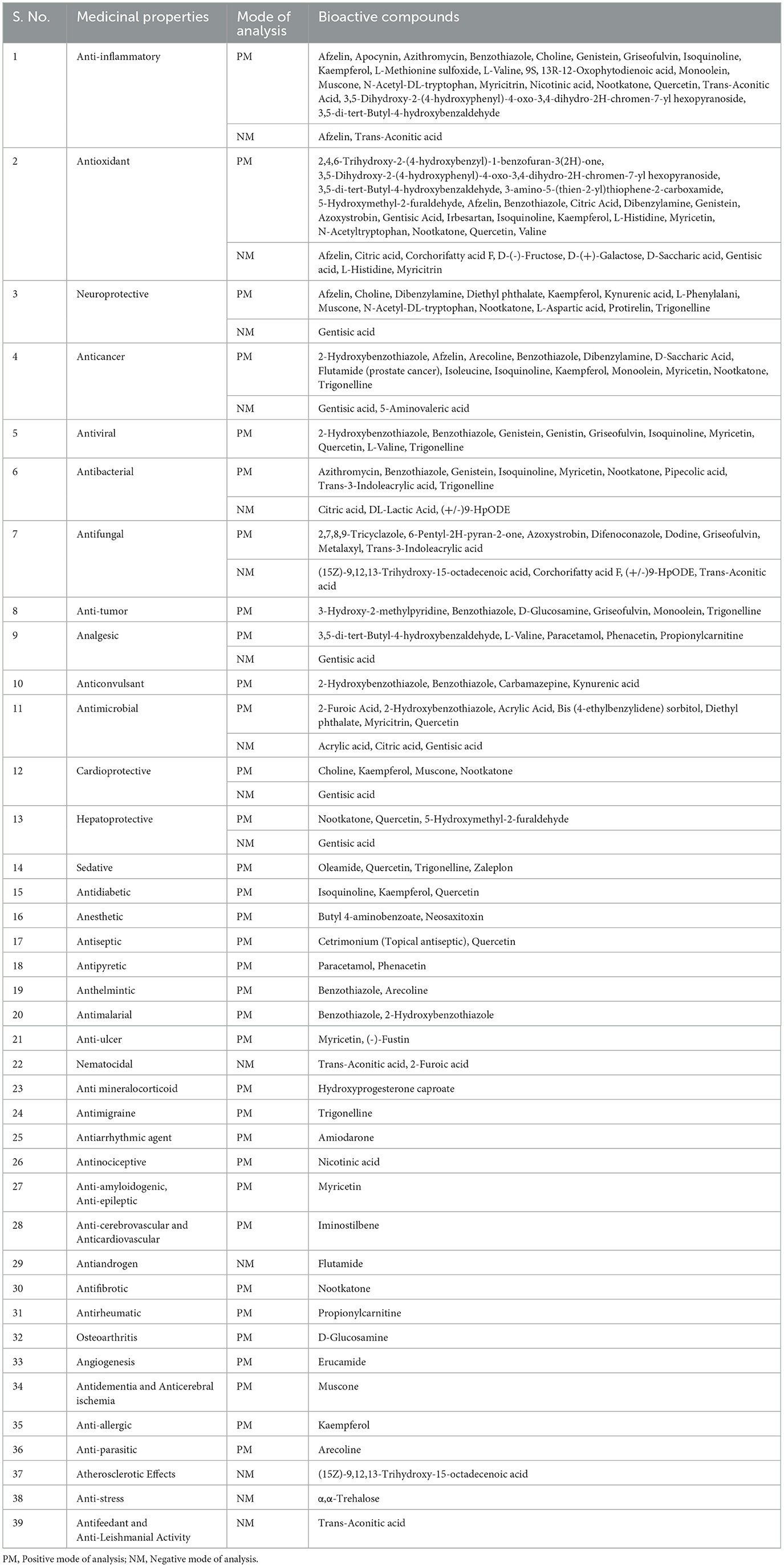
Table 4. Categorization of bioactive compounds (Phytochemicals) identified in Manila tamarind based on medicinal properties.
Figure 3. Structures of identified bioactive compounds and their structures observed in the aril of Manila tamarind.
Early detection, improved treatment options, and preventive measures, such as healthy diets, remain critical strategies to combat this growing health challenge (4, 5). In Manila tamarind, 16 anti-cancerous compounds (Table 4) were identified through positive mode (2-hydroxybenzothiazole, afzelin, arecoline, benzothiazole, dibenzylamine, d-saccharic acid, flutamide (prostate cancer), isoleucine, isoquinoline, kaempferol, monoolein, myricetin, nootkatone, trigonelline) and negative mode of analysis (gentisic acid and 5-aminovaleric acid). These identified phytochemicals control different types of cancer such as breast cancer (2-hydroxybenzothiazole, afzelin, benzothiazole, kaempferol, myricetin, and trigonelline), colon cancer (D-saccharic acid, afzelin, myricetin, nootkatone, and gentisic acid), prostate cancer (flutamide), liver cancer (nootkatone and trigonelline), leukemia (isoquinoline derivatives), and pancreatic cancer (kaempferol and myricetin).
Consuming a diet rich in antioxidants from fruits, vegetables, and phytochemicals has been linked to a lower risk of many serious health conditions, such as cancer (14), cardiovascular diseases (25), diabetes mellitus (26), neurodegenerative diseases such as Alzheimer's, Parkinson's (27), inflammatory diseases (28), aging (29), kidney disorders (30), liver diseases (31), skin disorders (32), and respiratory diseases, e.g., COPD and asthma (33).
3.4.1 Alkaloids
Alkaloids are a diverse group of nitrogen-containing secondary metabolites found in plants, fungi, and certain animal species, which are known for their potent pharmacological properties. These compounds play a critical role in the development of modern pharmaceuticals, ranging from analgesics to chemotherapeutic agents (34). In Manila tamarind, we identified 18 alkaloid compounds, viz., arecoline, isoquinoline, azithromycin, muscone, trigonelline, isoamylamine, azoxystrobin, L-tyrosine, L-histidine, L-phenylalanine, L-norleucine, DL-tryptophan, N-acetyl-DL-tryptophan, DL-arginine, proline, propionylcarnitine, leucylproline, and prolylleucine, which are often used for several health benefits (Tables 4, 5). Historically, alkaloids like morphine and its derivative codeine have been essential in pain management, while quinine revolutionized malaria treatment and offers anti-inflammatory effects (35). Quinine was the first effective treatment for malaria and is used in the treatment of resistant strains of Plasmodium falciparum, and also possesses analgesic and anti-inflammatory properties (36). Caffeine, a methylxanthine alkaloid, stimulates the central nervous system and is linked to reduced risks of Parkinson's and Alzheimer's disease (37). Despite its addictive nature, nicotine from Nicotiana tabacum shows neuroprotective potential and is used in smoking cessation therapies (38). Ephedrine has long been used as a bronchodilator and nasal decongestant. It stimulates adrenergic receptors, leading to increased heart rate and bronchial relaxation (39). Atropine, used in clinical settings to dilate pupils, treat bradycardia, and counteract the effects of organophosphate poisoning (40). Reserpine was among the first alkaloids used to manage hypertension and certain psychiatric disorders. It depletes catecholamines and serotonin from central and peripheral neurons, which explains its tranquilizing effect (41). Berberine, a protoberberine alkaloid found in Berberis species, has gained attention for its antimicrobial, anti-inflammatory, and antidiabetic properties. It has been shown to lower blood glucose and cholesterol levels, making it a promising agent for metabolic syndrome (42). In oncology, vincristine and vinblastine, derived from the Catharanthus roseus plant, are widely used chemotherapeutic agents. These vinca alkaloids inhibit mitosis by binding to tubulin and are essential in treating leukemias, lymphomas, and other cancers (43). Though strychnine, an alkaloid from Strychnos nux-vomica, is primarily known for its toxicity, it has historically been used in small doses as a stimulant and performance enhancer. Its mechanism involves the inhibition of glycine receptors, which affects motor neurons (44). Yohimbine, derived from Pausinystalia yohimbe, has been used for erectile dysfunction and is being studied for potential benefits in weight loss and anxiety treatment (45). The presence of these bioactive alkaloids in Manila tamarind highlights their significant therapeutic potential. Continued research is warranted to better understand their mechanisms of action and broaden their applications in clinical medicine.
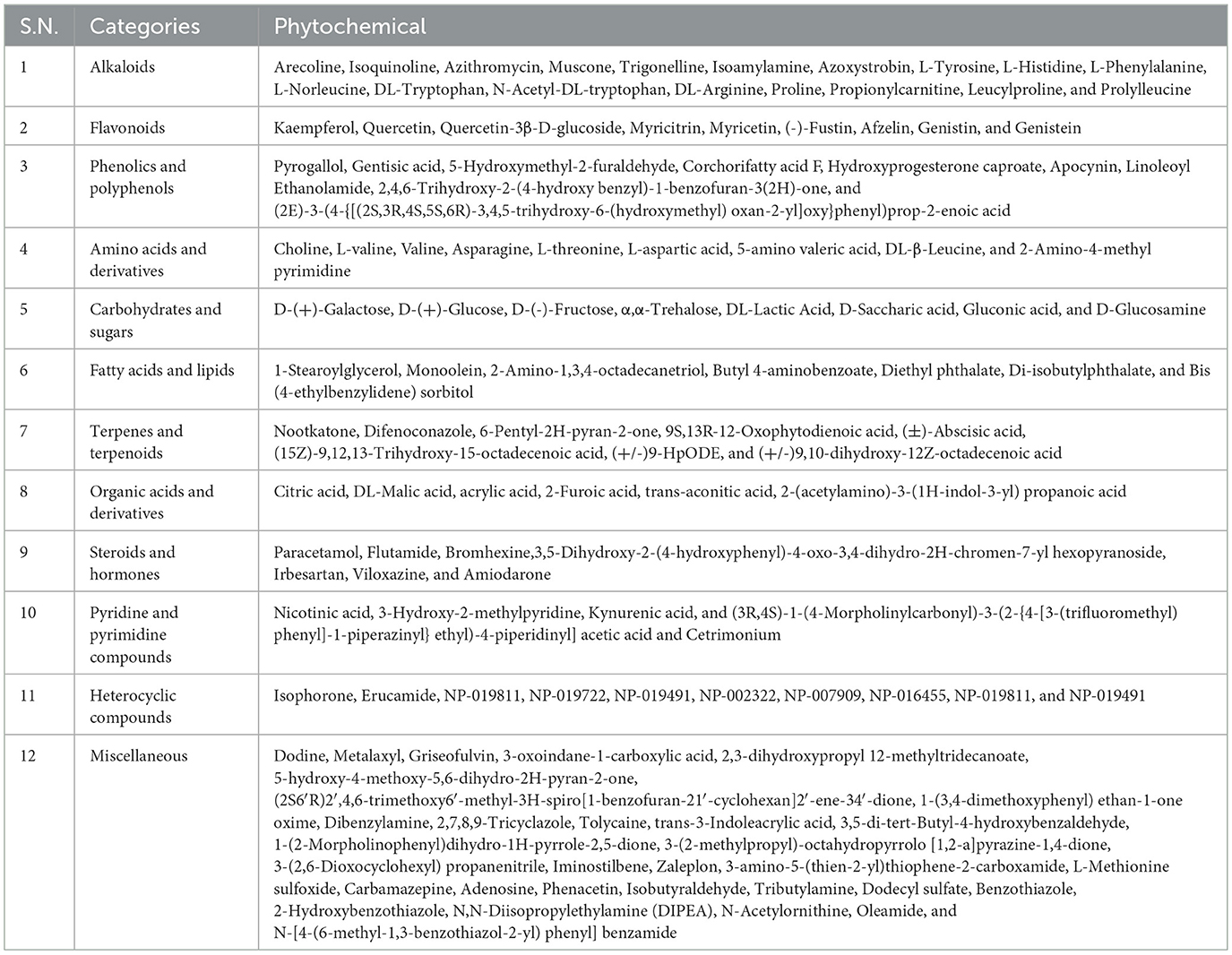
Table 5. Categorization of phytochemicals of different Manila tamarind accessions based on the classes.
3.4.2 Flavonoids
Flavonoids are another prevalent class of polyphenolic compounds found in fruits, vegetables, and medicinal plants, renowned for their antioxidant, anti-inflammatory, anticancer, and cardioprotective properties. The phytochemical screening resulted in nine flavonoid compounds (kaempferol, quercetin, quercetin-3β-D-glucoside, myricitrin, myricetin, (-)-fustin, afzelin, genistin, and genistein) in the aril of Manila tamarind (Table 5). Among them, kaempferol and quercetin have been extensively studied for their health-promoting effects. Kaempferol exhibits strong antioxidant and anti-inflammatory activities and has been shown to inhibit cancer cell proliferation and induce apoptosis, particularly in breast and liver cancer models (46). Quercetin, another abundant flavonol, contributes to cardiovascular health by reducing oxidative stress, lowering blood pressure, and improving endothelial function (47). Its glycosylated derivative, quercetin-3β-D-glucoside, shows significant bioavailability and exhibits similar biological activities, including anti-diabetic and neuroprotective effects (48). Myricetin and its glycoside myricitrin also exhibit significant bioactivity. Myricetin has demonstrated anticancer effects via modulation of key signaling pathways such as PI3K/Akt and MAPK, while myricitrin exerts hepatoprotective and anti-inflammatory actions (49). The flavonol (-)-fustin, found primarily in Rhus species, has antioxidant and antidiabetic effects, attributed to its ability to inhibit aldose reductase and prevent lipid peroxidation (50). Similarly, afzelin, a kaempferol glycoside, has shown anti-inflammatory, anti-allergic, and anticancer properties through the inhibition of mast cell degranulation and NF-κB activation (51). Isoflavones such as genistin and genistein, predominantly found in soy products, have been widely explored for their phytoestrogenic activity. Genistein, in particular, exhibits potent anticancer, antioxidant, and osteoprotective properties and is being investigated for its role in hormone-related cancers and osteoporosis prevention (52). Genistin, the glycosylated form of genistein, is more water-soluble and converts to the active aglycone in the gut, making it effective in delivering systemic benefits, including cardiovascular and bone health support (53). Collectively, the flavonoids identified in Manila tamarind present diverse therapeutic potential and are promising candidates for natural drug development and preventive healthcare. Further studies are needed to optimize their bioavailability and substantiate their clinical efficacy.
3.4.3 Phenolics and polyphenols
Phenols and polyphenols are plant-derived secondary metabolites with notable anti-inflammatory, anticancer, antioxidant, and antibacterial properties. Phytochemical screening of Manila tamarind aril revealed nine phenolic and polyphenolic compounds (Table 5). Among them, pyrogallol—a trihydroxybenzene derivative—displays both antioxidant and pro-oxidant effects and has shown cytotoxicity against cancer cells via oxidative stress mechanisms (54). Gentisic acid, a type of dihydroxybenzoic acid, is known for its anti-inflammatory and analgesic effects, primarily through modulation of the cyclooxygenase (COX) pathway and neutralization of reactive oxygen species (55). 5-Hydroxymethyl-2-furaldehyde (5-HMF), a degradation product of carbohydrates, exhibits notable antioxidant, anti-sickle cell, and cytoprotective activities. It interacts with hemoglobin to increase oxygen affinity and has demonstrated therapeutic potential in preclinical models of cardiovascular disorders (56).
Corchorifatty acid F, a lesser-known polyunsaturated fatty acid derivative from Corchorus species, has been preliminarily noted for its anti-inflammatory and lipid-modulating potential (57). Hydroxyprogesterone caproate (HPC), a synthetic progestin primarily used to prevent preterm birth, also demonstrates anti-inflammatory activity by engaging glucocorticoid receptor-mediated pathways and regulating cytokine expression (58). Apocynin, a methoxy-substituted catechol, is recognized for its ability to inhibit NADPH oxidase activity, reducing oxidative stress and inflammation, particularly in models of neurodegeneration, cardiovascular disease, and diabetes (59). Linoleoyl ethanolamide (LEA), an endogenous lipid amide, acts as a signaling molecule in energy metabolism and inflammation. It exerts anti-obesity and anti-inflammatory effects, potentially by modulating peroxisome proliferator-activated receptors (PPARs) and inhibiting pro-inflammatory cytokines (60). The compound 2,4,6-trihydroxy-2-(4-hydroxybenzyl)-1-benzofuran-3(2H)-one, structurally related to aurones, has shown strong antioxidant and anti-tyrosinase activity, making it a candidate for skin-whitening and neuroprotective therapies (https://www.pubchem.ncbi.nlm.nih.gov/compound/54378453). The (2E)-3-(4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]oxy}phenyl)prop-2-enoic acid, a glycosylated derivative of p-coumaric acid, combines antioxidant and hepatoprotective properties, and may play a role in modulating glucose and lipid metabolism in metabolic syndrome (61). These phenolic and polyphenolic compounds identified in Manila tamarind collectively demonstrate promising bioactivities that justify further exploration as therapeutic agents or dietary supplements.
3.4.4 Amino acids and derivatives
Amino acids and their derivatives are essential for a wide range of physiological processes, including protein synthesis, neurotransmission, metabolic regulation, and cellular signaling. Phytochemical screening of the aril of Manila tamarind identified nine amino acids and related compounds (Table 5). Among them, choline is an essential nutrient that supports cell membrane integrity and serves as a precursor for the neurotransmitter acetylcholine. It also plays a key role in methyl group metabolism and exhibits both neuroprotective and hepatoprotective properties (62). L-valine and valine, branched-chain amino acids (BCAAs), play critical roles in muscle metabolism and energy production, particularly during exercise and catabolic stress. They enhance muscle repair and reduce exercise-induced fatigue (63). Similarly, asparagine is involved in nitrogen transport and protein glycosylation, and its availability has been linked to cancer cell adaptability and metastasis (64). L-threonine, a key component of mucin proteins, contributes to intestinal health and immune modulation, particularly in neonatal and weaning animals (65). L-aspartic acid functions as both a building block for protein synthesis and a neurotransmitter in the central nervous system (66). 5-Aminovaleric acid, a GABA analog, has shown potential in modulating neuroactive signaling and holds promise for therapeutic intervention in neurodegenerative disorders (67). DL-β-Leucine may exert modulatory effects on neurotransmission and serve as a precursor in synthetic biochemical pathways (68). Finally, 2-Amino-4-methyl pyrimidine is of pharmaceutical interest due to its structural similarity to vitamin B1 (thiamine) and potential antimicrobial and enzyme inhibitory activities (69). Collectively, these amino acids and derivatives contribute to a broad spectrum of metabolic and therapeutic functions, underscoring their significance in nutritional science and biomedical research.
3.4.5 Carbohydrates and sugars
Carbohydrates are essential biomolecules that function as primary energy sources and structural components in living organisms. Phytochemical screening of the aril of Manila tamarind identified eight carbohydrate- and sugar-related compounds (Table 5). Among them, D-(+)-Glucose and D-(-)-Fructose are key monosaccharides involved in glycolysis and energy metabolism, contributing to ATP production and cellular respiration (70). D-(+)-Galactose is vital in the biosynthesis of glycoproteins and glycolipids, particularly in neuronal development (71). α,α-Trehalose, a disaccharide, functions as a stress protectant in organisms by stabilizing proteins and cellular structures under desiccation or thermal stress (72). DL-Lactic acid, a byproduct of anaerobic metabolism, is utilized therapeutically in skin care and wound healing due to its antimicrobial and exfoliating properties (46). D-Saccharic acid has been investigated for its role in detoxification and potential anti-carcinogenic effects through modulation of phase II enzyme activity (73). Gluconic acid is known for its chelating properties and applications in the pharmaceutical and food industries. D-Glucosamine, an amino sugar, is a precursor in glycosaminoglycan synthesis and is widely used for joint health, particularly in osteoarthritis management (74).
3.4.6 Fatty acids and lipids
Fatty acids and lipid derivatives are essential for membrane structure, signal transduction, and energy metabolism. Phytochemical screening of Manila tamarind (Pithecellobium dulce) aril identified seven lipid-related compounds (Table 5). 1-Stearoylglycerol and monoolein, both monoacylglycerols, aid in lipid digestion and are used in drug delivery systems (75). 2-Amino-1,3,4-octadecanetriol, a sphingoid base derivative, supports membrane integrity and apoptosis signaling (76). Butyl 4-aminobenzoate, a lipid-soluble anesthetic, is common in topical applications (77). Diethyl phthalate and di-isobutylphthalate, though industrial plasticizers, are concerning due to their endocrine-disrupting potential (77). In contrast, bis(4-ethylbenzylidene) sorbitol is a non-toxic polymer clarifier with biomedical potential (78). These lipid compounds exhibit diverse roles in physiology and material science.
3.4.7 Terpenes and terpenoids
Terpenes and terpenoids are structurally diverse natural products known for their broad pharmacological activities. Phytochemical screening of the aril of Manila tamarind identified eight terpene- and terpenoid-related compounds (Table 5). Nootkatone, a sesquiterpene, exhibits insecticidal, antimicrobial, and anti-inflammatory properties (79). Difenoconazole, a triazole fungicide with a terpenoid backbone, exhibits potent antifungal activity by inhibiting ergosterol biosynthesis (80). 6-Pentyl-2H-pyran-2-one, a volatile compound from fungi, demonstrates antifungal and plant growth-promoting effects (81). 9S,13R-12-Oxophytodienoic acid and (±)-Abscisic acid are oxylipin derivatives regulating plant stress responses and are now being explored for anti-inflammatory and antitumor activities in humans (82). Hydroxyoctadecenoic acids such as (15Z)-9,12,13-trihydroxy-15-octadecenoic acid, (+/-)9-HpODE, and (+/-)9,10-dihydroxy-12Z-octadecenoic acid are lipid mediators involved in oxidative stress and have implications in cardiovascular and inflammatory diseases (83).
3.4.8 Organic acids and derivatives
Organic acids are integral to cellular metabolism and often function as signaling molecules or therapeutic agents. Phytochemical screening of the aril of Manila tamarind identified six organic acids and derivative-related compounds (Table 5). Citric acid and DL-malic acid are key intermediates of the tricarboxylic acid (TCA) cycle, essential for cellular energy production and carbon metabolism (70). Acrylic acid, while primarily industrial, has antimicrobial properties and is being examined for biopolymer development. 2-Furoic acid, derived from biomass, has been reported for its antioxidant and antimicrobial activities (84). Trans-aconitic acid is an inhibitor of phosphofructokinase and may regulate glycolysis under stress (85). The tryptophan derivative 2-(acetylamino)-3-(1H-indol-3-yl) propanoic acid (N-acetyltryptophan) serves as an antioxidant and stabilizer in therapeutic protein formulations (86).
3.4.9 Steroids and hormone analogs
Steroids and hormone analogs exhibit a broad spectrum of therapeutic actions. The phytochemical screening resulted in seven steroids and hormone-related compounds in the aril of Manila tamarind (Table 5). Paracetamol is a widely used analgesic and antipyretic with hepatic metabolism, while flutamide is an antiandrogen used in prostate cancer therapy (87). Bromhexine serves as a mucolytic agent that enhances pulmonary secretion clearance. 3, 5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl hexopyranoside, a flavonoid glycoside, has antioxidant and estrogenic activity (88). Irbesartan, an angiotensin II receptor blocker, is effective in hypertension and diabetic nephropathy. Viloxazine is a norepinephrine reuptake inhibitor recently approved for ADHD treatment. Amiodarone, a class III antiarrhythmic, modulates potassium and calcium channels, although it poses risks of thyroid and pulmonary toxicity (89). Collectively, these compounds underscore the therapeutic relevance of steroidal and hormone-like constituents present in the plant matrix.
3.4.10 Pyridine and pyrimidine compounds
Pyridine and pyrimidine derivatives are critical scaffolds in drug design due to their bioactivity. The phytochemical screening resulted in seven pyridine and pyrimidine-related compounds in the aril of Manila tamarind (Table 5). Nicotinic acid (vitamin B3) is a precursor of NAD+ and NADP+, crucial in redox reactions and energy metabolism (90). 3-Hydroxy-2-methylpyridine is structurally related to vitamin B6 and exhibits neuroprotective potential. Kynurenic acid, a tryptophan metabolite, acts as a neuroinhibitory agent through NMDA receptor antagonism and is implicated in neurodegenerative disorders like schizophrenia (91). The synthetic compound (3R,4S)-1-(4-Morpholinylcarbonyl)-3-(2-{4-[3-(trifluoromethyl) phenyl]-1-piperazinyl} ethyl)-[4-piperidinyl] acetic acid (a piperidine-based derivative) and cetrimonium, a quaternary ammonium salt, demonstrate antimicrobial and membrane-disrupting properties, making them valuable in antiseptics and pharmaceuticals (92).
3.4.11 Heterocyclic compounds
Heterocyclic compounds are structurally diverse molecules that play vital roles in pharmaceuticals, agrochemicals, and materials science. The phytochemical screening resulted in 10 heterocyclic compounds in the aril of Manila tamarind (Table 5). Isophorone, a cyclic ketone, is widely used as an industrial solvent and as an intermediate in the synthesis of fine chemicals. It has also exhibited antibacterial and antifungal properties (93). Erucamide, though primarily an amide derived from erucic acid, features a heterocyclic moiety in many functional derivatives and is known for its lubricating and anti-blocking effects in polymer films. The NP-coded compounds (NP-019811, NP-019722, and NP-019491) are likely natural product derivatives or synthetic heterocycles identified in screening databases for their bioactivity. These types of compounds often contain nitrogen, oxygen, or sulfur in their ring structures and are known to exhibit a broad spectrum of pharmacological activities, including antimicrobial, anticancer, and neuroactive properties (94). Their structural diversity, including fused ring systems and spirocyclic configurations, makes them valuable for exploring new therapeutic targets and for structure–activity relationship (SAR) studies in drug discovery.
3.4.12 Miscellaneous compounds
This category encompasses a broad array of biologically active and industrially relevant compounds that do not fit neatly into traditional classes. Dodine and Metalaxyl are widely used fungicides in agriculture, known for their systemic action and inhibition of nucleic acid synthesis in plant pathogens (95). Griseofulvin, a natural antifungal agent, disrupts microtubule function and is used clinically against dermatophytic infections (96). Various synthetic and semi-synthetic molecules, such as 3-oxoindane-1-carboxylic acid and 3,5-di-tert-butyl-4-hydroxybenzaldehyde, exhibit antioxidant or anti-inflammatory properties and are used as intermediates in medicinal chemistry (97). Zaleplon and Carbamazepine are central nervous system (CNS) active drugs, used as hypnotics and antiepileptics, respectively, highlighting the pharmacological breadth within this group (98). Natural products such as trans-3-indoleacrylic acid and oleamide are associated with neuroactive and anti-inflammatory functions. Surfactants such as Dodecyl sulfate and bases like N, N-Diisopropylethylamine (DIPEA) serve crucial roles in biochemistry and organic synthesis. Additionally, bioactive sulfur- and nitrogen-containing heterocycles such as benzothiazole and 2-hydroxybenzothiazole are investigated for anticancer, antimicrobial, and enzyme-inhibitory activities (99). This chemically eclectic group underscores the importance of structural diversity in modulating biological function and facilitating innovation across pharmacology, agriculture, and industrial chemistry.
4 Conclusion
The increasing global emphasis on medicinally valuable fruit-bearing plants and the continuous pursuit of novel bioactive compounds provided the rationale for investigating the aril of Manila tamarind fruit pods. This study offers scientific validation of the antioxidant potential of the Manila tamarind plant, which is of considerable traditional significance. The analysis unveiled a rich spectrum of bioactive constituents with promising therapeutic applications in treating various human ailments. These findings underscore the Manila tamarind's value as a potent source of nutraceutical and pharmacological agents. To realize its full medicinal and dietary value, future studies should focus on isolating and characterizing key compounds and evaluating their biological activities through in vitro and in vivo models. Advances in metabolomics and molecular docking can further elucidate mechanisms of action and compound interactions. Such efforts could lead to the development of Manila tamarind-based interventions for oxidative stress, inflammation, and metabolic disorders.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://figshare.com/s/15ccc0df782eb51546f5?file=57133391.
Author contributions
AY: Formal analysis, Data curation, Project administration, Visualization, Writing – original draft, Methodology, Validation, Resources, Investigation, Supervision, Software, Conceptualization, Writing – review & editing. SJ: Writing – original draft, Formal analysis, Software, Visualization, Resources, Supervision, Methodology, Writing – review & editing, Data curation, Investigation, Validation. GC: Writing – original draft, Formal analysis, Visualization, Writing – review & editing, Conceptualization, Data curation. AR: Writing – review & editing, Formal analysis, Writing – original draft, Resources, Supervision, Visualization, Conceptualization. RK: Data curation, Writing – review & editing, Writing – original draft, Formal analysis. NK: Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis, Data curation. HA: Data curation, Software, Visualization, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis. SG: Data curation, Formal analysis, Conceptualization, Writing – original draft, Writing – review & editing. AK: Formal analysis, Writing – review & editing, Data curation, Writing – original draft, Conceptualization. PS: Conceptualization, Data curation, Writing – review & editing, Writing – original draft, Formal analysis. RD: Supervision, Writing – original draft, Writing – review & editing, Resources. AA: Writing – review & editing, Supervision, Conceptualization, Writing – original draft, Data curation, Resources. DJ: Visualization, Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to the ICAR and the Director of the Central Agroforestry Research Institute, Jhansi, Uttar Pradesh, for their help and direction.
Conflict of interest
SJ was employed by R and D, Indofil Industry Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh AK, Mishra DS, Krishna H, Yadav V, Yadav LP, Anil GK, et al. Exploring New Frontiers in Multiplication of Underutilized Dryland Fruits: a Review of Recent Developments. Greater Noida: Vegetos (2025). p. 1–9.
2. Brewbaker JL. Pithecellobium dulce sweet and thorny. In: Nitrogen Fixing Tree Association (NFTA) Highlights. Honolulu, HI: University of Hawaii (1992). p. 2. Available online at: https://www.cabidigitallibrary.org/doi/full/10.5555/199406083549.
3. Elisha IL, Botha FS, McGaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. (2017) 17:133. doi: 10.1186/s12906-017-1645-z9.
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.216609.
5. WHO (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed March 6, 2025).
6. Yadav LP, Gangadhara K, Singh AK, Yadav V, Apparao V, Pawar A, et al. Genetic variability, morphological diversity, and antioxidant potential in gynoecious Coccinia accessions: implications for breeding and biofortification. BMC Plant Biol. (2025) 25:844. doi: 10.1186/s12870-025-06335-x
7. Yadav LP, K G, Apparao V., Singh AK, Rane J, Kaushik P, et al. Nutritional, antioxidants and protein profiling of leaves of Moringa oleifera germplasm. South Afr J Botany. (2024) 165:443–54. doi: 10.1016/j.sajb.2024.01.012
8. Yadav LP, Koley TK, Tripathi A, Singh S. Antioxidant potentiality and mineral content of summer season leafy greens: comparison at mature and microgreen stages using chemometric. Agricult Res. (2019) 8:165–75. doi: 10.1007/s40003-018-0378-7
9. Rodriguez-Amaya DB, Kimura M. HarvestPlus Handbook for Carotenoid Analysis [Internet]. Washington, DC: International Food Policy Research Institute (IFPRI) (2004). Available online at: https://www.harvestplus.org/resources/harvestplushandbook-carotenoid-analysis (Accessed April 25, 2025).
10. Okwu DE, Josiah C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr J Biotechnol. (2006) 5:357–61.
11. Omaye ST, David TJ, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. (1979) 1979:62:3–11. doi: 10.1016/0076-6879(79)62181-X
12. Pence HE, Williams A. Chemspider: An online chemical information resource. J Chem Educ. (2010) 87:ed100697w. doi: 10.1021/ed100697w
13. SAS Institute. SAS/STAT 9.2 User's Guide, Second Edition (2016). North Carolina: SAS Institute Inc.
14. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. (2010) 4:118. doi: 10.4103/0973-7847.70902
15. Joel JS, Sheena OE, Martins OE, Onyemauche NSC, Emmanuel AA. Comparative antioxidant capacity of aqueous and ethanol fruit extracts of Tetrapleura tetraptera. J Biol Sci. (2017) 17:185–93. doi: 10.3923/jbs.2017.185.193
17. Rahman MdM, Rahaman MdS, Islam MdR, Rahman F, Mithi FM, Alqahtani T, et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules. (2021) 27:233. doi: 10.3390/molecules27010233
18. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important flavonoids and their role as a therapeutic agent. Molecules. (2020) 25:5243. doi: 10.3390/molecules25225243
19. Ndawula J, Kabasa JD, Byaruhanga YB. Alterations in fruit and vegetable β-carotene and vitamin C content caused by open sun drying, visqueen-covered and polyethylene-covered solar dryers. Afr Health Sci. (2004) 4:125–30.
20. Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In:Wrolstad RE, , editor. Current Protocols in Food Analytical Chemistry. Hoboken, NJ: John Wiley & Sons (2001). p. 1–2.
22. Watanabe F. Vitamin B Sources and bioavailability. Exp Biol Med. (2007) 232:1266–74. doi: 10.3181/0703-MR-67
23. Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. (2000) 20:207–20. doi: 10.1016/S0925-5214(00)00133-2
25. Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. (2012) 70:257–65. doi: 10.1111/j.1753-4887.2012.00476.x
26. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. (2004) 24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78
27. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. (2006) 97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x
28. Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. (2008) 4:89–96.
29. Harman D. The free radical theory of aging. Antioxid Redox Signal. (2003) 5:557–61. doi: 10.1089/152308603770310202
30. Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. (2016) 25:119–46. doi: 10.1089/ars.2016.6665
31. Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. (2000) 21:49–98. doi: 10.1016/S0098-2997(00)00004-2
32. Kumar V, Tanwar N, Goel M, Khan M, Kumar D, Singh G, et al. Antioxidants for skin health. Recent Adv Food Nutr Agric. (2024) 6:15. doi: 10.2174/012772574X311177240710100118
33. Barnes PJ. Oxidative stress in chronic obstructive pulmonary disease. Antioxidants. (2022) 11:965. doi: 10.3390/antiox11050965
34. Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. (2014) 44:377–86. doi: 10.1016/j.ijantimicag.2014.06.001
35. Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used world health organization step III opioids (Buprenorphine, Fentanyl, Hydromorphone, Methadone, Morphine, Oxycodone). Pain Practice. (2008) 8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x
36. Sanders NG, Meyers DJ, Sullivan DJ. Antimalarial efficacy of hydroxyethylapoquinine (SN-119) and its derivatives. Antimicrob Agents Chemother. (2014) 58:820–7. doi: 10.1128/AAC.01704-13
37. Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. (2018) 70:384–411. doi: 10.1124/pr.117.014407
38. Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology. (2014) 76:545–53. doi: 10.1016/j.neuropharm.2013.04.028
39. Chen KK, Schmidt CF. The pharmacology of ephedrine alkaloids. J Pharmacol Exp Therapeut. (1930) 39:361–75.
40. McDonough JH, Shih TM. Atropine and other anticholinergic drugs. In: Chemical Warfare Agents: Toxicology and Treatment. Chichester; West Sussex: John Wiley & Sons Ltd., (2007). p. 287–303. doi: 10.1002/9780470060032.ch14
41. López-Muñoz F BVACCE. Historical approach to reserpine discovery and its introduction in psychiatry. Actas Esp Psiquiatr. (2004) 32:387–95.
42. Hu X, Zhang Y, Xue Y, Zhang Z, Wang J. Berberine is a potential therapeutic agent for metabolic syndrome via brown adipose tissue activation and metabolism regulation. Am J Transl Res. (2018) 10:3322–9.
44. Guo R, Wang T, Zhou G, Xu M, Yu X, Zhang X, et al. Botany, Phytochemistry, pharmacology and toxicity of Strychnos nux-vomica L.: a review. Am J Chin Med. (2018) 46:1–23. doi: 10.1142/S0192415X18500015
45. Cimolai N. An overview of yohimbine in sports medicine. In:Bagchi D, , editor. Sustained Energy for Enhanced Human Functions and Activity [Internet]. London: Elsevier Inc. (2017). p. 251–60.
46. Calderon-Montano MJ, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. (2011) 11:298–344. doi: 10.2174/138955711795305335
47. Li Y, Yao J, Han C, Yang J, Chaudhry M, Wang S, et al. Quercetin, inflammation and immunity. Nutrients. (2016) 8:167. doi: 10.3390/nu8030167
48. Kim Y, Keogh J, Clifton P. Polyphenols and glycemic control. Nutrients. (2016) 8:17. doi: 10.3390/nu8010017
49. Semwal D, Semwal R, Combrinck S, Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. (2016) 8:90. doi: 10.3390/nu8020090
50. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. (2018) 5:93. doi: 10.3390/medicines5030093
51. Zhou Y, Zheng J, Li Y, Xu DP Li S, Chen YM, et al. Natural polyphenols for prevention and treatment of cancer. Nutrients. (2016) 8:515. doi: 10.3390/nu8080515
52. Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, et al. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFκB. FASEB J. (2006) 20:2136–8. doi: 10.1096/fj.05-5522fje
53. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. (2009) 61:598–606. doi: 10.1080/01635580902825639
54. Shirani A, Mirabbasi R, Shabani L, Reiisi S. Pyrogallol acts as a novel anticancer factor to enhance the sensitivity to cisplatin in ovarian cancer cells through inducing miR-15a upregulation. Egypt J Med Human Genet. (2025) 26:15. doi: 10.1186/s43042-025-00647-1
55. Sánchez-Moreno CA, Larrauri J, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. (1999) 32:407–12. doi: 10.1016/S0963-9969(99)00097-6
56. Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, et al. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. (2005) 128:552–61. doi: 10.1111/j.1365-2141.2004.05332.x
57. Yoshikawa M, Murakami T, Shimada H, Yoshizumi S, Saka M, Yamahara J, et al. Medicinal Foodstuffs. XIV On the Bioactive Constituents of Moroheiya (2): New Fatty Acids, Corchorifatty Acids A, B, C, D, E, and F, from the Leaves of Corchorus olitorius L (Tiliaceae): Structures and Inhibitory Effect on NO Production in Mouse Peritoneal Macrophages. Chem Pharm Bull. (1998) 46:1008–14. doi: 10.1248/cpb.46.1008
58. Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 Alpha-Hydroxyprogesterone Caproate. N Engl J Med. (2003) 348:2379–85. doi: 10.1056/NEJMoa035140
59. ‘t Hart BA, Copray S, Philippens I. Apocynin, a low molecular oral treatment for neurodegenerative disease. Biomed Res Int. (2014) 2014:1–6. doi: 10.1155/2014/298020
60. Matias I, Gonthier M, Petrosino S, Docimo L, Capasso R, Hoareau L, et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and β -cells. Br J Pharmacol. (2007) 152:676–90. doi: 10.1038/sj.bjp.0707424
61. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. (2004) 79:727–47. doi: 10.1093/ajcn/79.5.727
62. Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. (2009) 67:615–23. doi: 10.1111/j.1753-4887.2009.00246.x
63. Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, et al. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. (2006) 136:529S−32S. doi: 10.1093/jn/136.2.529S
64. Knott SR V, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. (2018) 554:378–81. doi: 10.1038/nature25465
65. Tang Q, Tan P, Ma N, Ma X. Physiological functions of threonine in animals: beyond nutrition metabolism. Nutrients. (2021) 13:2592. doi: 10.3390/nu13082592
66. Zhang S, Zeng X, Ren M, Mao X, Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. (2017) 8:10. doi: 10.1186/s40104-016-0139-z
67. Gajcy K, Lochynski S, Librowski T. A role of GABA analogues in the treatment of neurological diseases. Curr Med Chem. (2010) 17:2338–47. doi: 10.2174/092986710791698549
68. Wolf S, Becker J, Tsuge Y, Kawaguchi H, Kondo A, Marienhagen J, et al. Advances in metabolic engineering of Corynebacterium glutamicum to produce high-value active ingredients for food, feed, human health, and well-being. Essays Biochem. (2021) 65:197–212. doi: 10.1042/EBC20200134
69. Venugopala KN, Kamat V. Pyrimidines: a new versatile molecule in the drug development field, scope, and future aspects. Pharmaceuticals. (2024) 17:1258. doi: 10.3390/ph17101258
70. Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. New York, NY: WH Freeman. (2017).
71. Conte F, van Buuringen N, Voermans NC, Lefeber DJ. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochimica et Biophysica Acta (BBA) - General Subjects. (2021) 1865:129898. doi: 10.1016/j.bbagen.2021.129898
72. Elbein AD. New insights on trehalose: a multifunctional molecule. Glycobiology. (2003) 13:17R−27. doi: 10.1093/glycob/cwg047
73. Bhattacharya S, Chatterjee S, Manna P, Das J, Ghosh J, Gachhui R, et al. Prophylactic role of D-saccharic acid-1,4-lactone in tertiary butyl hydroperoxide induced cytotoxicity and cell death of murine hepatocytes via mitochondria-dependent pathways. J Biochem Mol Toxicol. (2011) 25:341–54. doi: 10.1002/jbt.20393
74. Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. (2005) 43:187–201. doi: 10.1016/j.fct.2004.11.006
75. Feltes MMC, de Oliveira D, Block JM, Ninow JL. The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food Bioproc Tech. (2013) 6:17–35. doi: 10.1007/s11947-012-0836-3
76. Quinville BM, Deschenes NM, Ryckman AE, Walia JS. A Comprehensive review: sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int J Mol Sci. (2021) 22:5793. doi: 10.3390/ijms22115793
77. Krátký M, Konečná K, Janoušek J, Brablíková M, Jandourek O, Trejtnar F, et al. 4-aminobenzoic acid derivatives: converting folate precursor to antimicrobial and cytotoxic agents. Biomolecules. (2019) 10:9. doi: 10.3390/biom10010009
78. Shepard TA, Delsorbo CR, Louth RM, Walborn JL, Norman DA, Harvey NG, et al. Self-organization and polyolefin nucleation efficacy of 1,3:2,4-di-p-methylbenzylidene sorbitol. J Polym Sci B Polym Phys. (1997) 35:2617–28.
79. Câmara JS, Perestrelo R, Ferreira R, Berenguer C V, Pereira JAM, Castilho PC. Plant-derived terpenoids: a plethora of bioactive compounds with several health functions and industrial applications—a comprehensive overview. Molecules. (2024) 29:3861. doi: 10.3390/molecules29163861
80. Liu R, Li J, Zhang L, Feng T, Zhang Z, Zhang B. Fungicide difenoconazole induced biochemical and developmental toxicity in wheat (Triticum aestivum L). Plants. (2021) 10:2304. doi: 10.3390/plants10112304
81. Garnica-Vergara A, Barrera-Ortiz S, Muñoz-Parra E, Raya-González J, Méndez-Bravo A, Macías-Rodríguez L, et al. The volatile 6-pentyl-2 -pyran-2-one from Trichoderma atroviride regulates, Arabidopsis thaliana, root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE functioning. New Phytol. (2016) 209:1496–512. doi: 10.1111/nph.13725
82. Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. (2010) 61:651–79. doi: 10.1146/annurev-arplant-042809-112122
83. Porter NA, Weber BA, Weenen H, Khan JA. Autoxidation of polyunsaturated lipids. Factors controlling the stereochemistry of product hydroperoxides. J Am Chem Soc. (1980) 102:5597–601. doi: 10.1021/ja00537a032
84. Chaturvedi T, Hulkko LSS, Fredsgaard M, Thomsen MH. Extraction, isolation, and purification of value-added chemicals from lignocellulosic biomass. Processes. (2022) 10:1752. doi: 10.3390/pr10091752
85. Ksiażek E. Citric acid: properties, microbial production, and applications in industries. Molecules. (2023) 29:22. doi: 10.3390/molecules29010022
86. Hogan KL, Leiske D, Salisbury CM. Characterization of N-acetyl-tryptophan degradation in protein therapeutic formulations. J Pharm Sci. (2017) 106:3499–506. doi: 10.1016/j.xphs.2017.08.012
88. Middleton E, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. (1992) 43:1167–79. doi: 10.1016/0006-2952(92)90489-6
89. Vassallo P, Trohman RG. Prescribing amiodarone. JAMA. (2007) 298:1312. doi: 10.1001/jama.298.11.1312
90. Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD + precursor vitamins in human nutrition. Annu Rev Nutr. (2008) 28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443
91. Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. (1993) 52:305–79. doi: 10.1016/S0031-6997(25)00441-7
92. Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. (2005) 99:703–15. doi: 10.1111/j.1365-2672.2005.02664.x
93. Kiran I, Özşen Ö, Çelik T, Ilhan S, Gürsu BY, Demirci F. Microbial transformations of isophorone by Alternaria alternata and Neurospora crassa. Nat Prod Commun. (2013) 8:114. doi: 10.1177/1934578X1300800114
94. Sharma S, Kumar D, Singh G, Monga V, Kumar B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur J Med Chem. (2020) 200:112438. doi: 10.1016/j.ejmech.2020.112438
95. Sukul P, Spiteller M. Metalaxyl: persistence, degradation, metabolism, and analytical methods. Rev Environ Contam Toxicol. (2000) 164:1–26.
96. Aris P, Wei Y, Mohamadzadeh M, Xia X. Griseofulvin: an updated overview of old and current knowledge. Molecules. (2022) 27:7034. doi: 10.3390/molecules27207034
97. Wright A, Crowne R, Hathway D. The metabolism of di-(3,5-di- tert-butyl-4-hydroxybenzyl) ether (Ionox 201) in the rat. Biochem J. (1967) 102:351–61. doi: 10.1042/bj1020351
98. Heydorn WE. Zaleplon - a review of a novel sedative hypnotic used in the treatment of insomnia. Expert Opin Investig Drugs. (2000) 9:841–58. doi: 10.1517/13543784.9.4.841
Keywords: functional foods, Manila tamarind, nutraceuticals, natural antioxidants underutilized, UHPLC-Q-TOF-MS
Citation: Yadav A, Jha S, Choudhary G, Ram A, Kumar R, Kumar N, Anuragi H, Garg S, Kumar A, Singh P, Dwivedi RP, Arunachalam A and Jinger D (2025) Nutritional valorization of Manila tamarind accessions through antioxidant analysis and UHPLC-Q-TOF-MS-based metabolomic profiling. Front. Nutr. 12:1646522. doi: 10.3389/fnut.2025.1646522
Received: 13 June 2025; Accepted: 11 August 2025;
Published: 02 September 2025.
Edited by:
Ram Swaroop Bana, Indian Agricultural Research Institute (ICAR), New Delhi, IndiaReviewed by:
Derya Alkan, Mugla University, TürkiyeP. Janani, Indian Council of Agricultural Research (ICAR), New Delhi, India
Lalu Prasad Yadav, Indian Council of Agricultural Research (ICAR), New Delhi, India
Copyright © 2025 Yadav, Jha, Choudhary, Ram, Kumar, Kumar, Anuragi, Garg, Kumar, Singh, Dwivedi, Arunachalam and Jinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashok Yadav, YXNob2tjYWZyaWhvcnQxQGdtYWlsLmNvbQ==; Girija Choudhary, Z2lyaWphY2hvdWRoYXJ5ODlAZ21haWwuY29t
 Ashok Yadav
Ashok Yadav Suchisree Jha
Suchisree Jha Girija Choudhary
Girija Choudhary Asha Ram
Asha Ram Rajeev Kumar
Rajeev Kumar Naresh Kumar
Naresh Kumar Hirdayesh Anuragi
Hirdayesh Anuragi Sandeep Garg
Sandeep Garg Anil Kumar
Anil Kumar Pradyuman Singh
Pradyuman Singh Raghunandan Prasad Dwivedi1
Raghunandan Prasad Dwivedi1 Ayyanadar Arunachalam
Ayyanadar Arunachalam Dinesh Jinger
Dinesh Jinger