- 1College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, China
- 2Shaanxi Province Key Laboratory of Bio-Resources, Shaanxi University of Technology, Hanzhong, China
- 3Qinba Mountain Area Collaborative Innovation Center of Bioresources Comprehensive Development, Shaanxi University of Technology, Hanzhong, China
- 4Qinba State Key Laboratory of Biological Resources and Ecological Environment (Incubation), Shaanxi University of Technology, Hanzhong, China
- 5Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 6Department of Medical Pharmacology, Faculty of Medicine, Atatürk University, Erzurum, Türkiye
Skin aging is a gradual physiological process influenced by both intrinsic and environmental factors and is characterized by the appearance of wrinkles, pigmentation, reduced elasticity, dryness, and vascular changes. In recent years, interest in the use of natural bioactive compounds to mitigate skin aging has increased, reflecting the global shift toward safer, sustainable, and health-conscious skincare solutions. Proanthocyanidins (PCs), a class of polyphenolic compounds derived from plant sources, exhibit strong antioxidant, anti-inflammatory, and antipigmentation properties. These compounds have considerable potential for enhancing the structure and function of aged skin by reducing oxidative stress, promoting collagen and elastin synthesis, alleviating the skin inflammatory response, and inhibiting pigmentation. Despite their promising therapeutic value, the efficacy of PCs can be compromised by their environmental instability and low bioavailability. Advances in encapsulation techniques and combination formulations have shown promise in enhancing the stability and delivery efficiency of PCs, thereby improving their performance in antiaging applications. In conclusion, PCs offer a scientifically grounded and sustainable approach for addressing skin aging. Their integration into dermatological products represents an innovative and eco-conscious strategy for developing next-generation skincare solutions with broad health and consumer benefits.
1 Introduction
Skin aging is a complex phenomenon resulting from the interplay of internal (genetic) and external (environmental) factors. It can be categorized into two distinct types: endogenous and exogenous skin aging (1, 2). Endogenous skin aging is influenced by genetic factors and progresses gradually with advancing age. Its characteristic manifestations include a decline in overall skin texture, the appearance of fine and evenly distributed wrinkles, a slightly dull complexion, and an absence of prominent pigmentation spots or erythema. In contrast, exogenous skin aging, on the other hand, is associated with detrimental external environmental factors and poor lifestyle habits, such as ultraviolet (UV) radiation exposure, air pollution, sleep deprivation, and smoking. Among these factors, UV radiation stands out as the most significant contributor to this form of aging; it is commonly referred to as photoaging of the skin. The typical manifestations of photoaged skin include a leather-like appearance in areas exposed to UV light, characterized by dense localized wrinkles along with common pigmentation spots and erythema (3, 4). UVA (320–400 nm) possesses substantial penetration capability, allowing it to directly reach the dermis and induce the production of reactive oxygen species (ROS) (5). Conversely, UVB (275–320 nm) primarily accumulates in the epidermis and is recognized as the principal pathogenic factor responsible for sunburn erythema, hence referred to as the “erythema effect” of UV radiation (6). In contrast to UVA and UVB rays, most UVC (230–275 nm) wavelengths are absorbed by the surface of the ozone layer before it reaches the Earth’s surface (7).
Compared with endogenous aging, skin aging induced by UV exposure is substantially more severe. Endogenous aging typically involves progressive degeneration characterized by gradual degradation of the extracellular matrix without causing pronounced visible damage. Conversely, photoaging resulting from prolonged UV exposure leads to extensive collagen degradation and the denaturation of elastin, which promotes acute damage to the extracellular matrix and results in substantial harm. Furthermore, UV radiation induces excessive ROS within skin tissues, facilitating local capillary dilation and triggering an inflammatory response that manifests as redness, swelling, and burning sensations on the skin (8). Additionally, UV radiation can increase melanocyte activity, leading to excessive melanin production and accumulation. This process results in pigmentation changes on sun-exposed areas of the skin, giving rise to pigmented spots (9, 10). Skin aging profoundly impacts individuals’ appearance and causes significant physical and psychological distress. Consequently, research focused on preventing and treating skin aging has garnered considerable attention from scientific researchers (11).
Currently, in addition to maintaining a healthy lifestyle, cultivating a positive mindset, and utilizing skincare products tailored to individual needs, the prevention and treatment methods for natural skin aging primarily encompass medical aesthetic interventions along with the administration of metformin (12, 13) and doxycycline (DOX) (14). The strategies for preventing and treating skin photoaging mainly involve sun protection measures alongside topical medications such as retinoic acid (15), chloroacetic acid (16), and 5-fluorouracil (17). Moreover, medical aesthetic techniques are employed within these treatments. While medical aesthetic approaches yield rapid results in the short term, they also carry certain risks. These risks include but are not limited to local skin infections at injection sites, induration, ecchymosis, vascular embolism, nerve damage, fat embolism, allergic reactions, and other complications—potentially resulting in unnatural facial expressions. Adverse reactions associated with pharmacological treatments for skin aging can be severe and may present pronounced side effects. Consequently, it is essential to explore new anti-skin aging products.
Compared with traditional pharmaceuticals used to combat skin aging, natural bioactive substances have emerged as promising alternatives because of their reduced side effects and diverse biological activities (18, 19). Natural bioactive compounds such as retinoids, peptides and antioxidants (such as vitamin C) and PCs play beneficial roles in the prevention and treatment of skin aging. Retinoids promote keratin renewal, stimulate collagen production, and inhibit its degradation (20, 21). Peptides serve as antiaging agents primarily by increasing the levels of collagen, elastin, and hyaluronic acid, thereby enhancing the elasticity and firmness of the skin (22). Vitamin C is a well-known antioxidant that effectively neutralizes free radicals, mitigates oxidative stress damage to cells, and acts as an essential cofactor in collagen synthesis. It promotes collagen production while inhibiting melanin formation, thus reducing pigmentation and increasing the degree of complexion (23). However, all the aforementioned drugs or ingredients have varying degrees of side effects, which can not only impact therapeutic efficacy but also lead to adverse reactions within the human body. Retinoids are known for their high irritability to the skin, which may result in adverse reactions such as dryness and peeling (20, 24). The effectiveness of peptides tends to be relatively slow; furthermore, they require specific concentrations and careful formulation design for optimal results (22). Vitamin C is prone to oxidation, which can diminish its efficacy. Additionally, high concentrations of vitamin C may cause irritation both on the skin surface and in the gastric mucosa (23, 25).
PCs are internationally recognized as effective natural polyphenolic antioxidants with notable properties. In comparison with other antioxidants, PCs are widely available from various sources at low cost while maintaining a relatively high safety profile—making them suitable for long-term consumption or application (26). PCs possess a robust capacity to neutralize free radicals effectively while inhibiting ROS formation (27). Compared with retinoids, peptides, and vitamin C, PCs exhibit unique antiaging potential that manifests not only through the enhancement of glycolipid metabolism and microvascular perfusion—achieved by inhibiting adipogenesis and improving mitochondrial function, respectively (28, 29)—but also by mitigating oxidative stress-induced damage to skin cells via the elimination of free radicals. This protective action safeguards both the structure and function of skin cells. Furthermore, PCs can inhibit the release of inflammatory factors, thereby alleviating the inflammatory response in the skin and consequently slowing its aging process (30, 31).
The reparative effects of PCs on aging skin have garnered significant attention in recent years. It exerts beneficial effects through various mechanisms: inhibiting the oxidative stress associated with aging skin; enhancing the collagen and elastin contents; alleviating inflammatory responses; and reducing the prevalence of pigmentation issues in aged skin (Figure 1). This paper reviews the structure and stability of PCs as well as their underlying mechanisms contributing to their ability to repair aging skin. Furthermore, strategies aimed at enhancing the stability and bioavailability of these products are discussed, providing novel insights for research focused on utilizing PCs to combat skin aging.

Figure 1. The beneficial effects of PCs on the repair of aging skin. Four main effects include inhibiting oxidative stress, increasing collagen and elastin content, alleviating the inflammation, and inhibiting pigmentation. ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; NF-κB, nuclear transcription factor-κB; TGF-β, transforming growth factor-β.
2 Resources, structure and stability of PCs
Fruits and seeds containing PCs include berries (e.g., grapes, blueberries, blackberries, cranberries), nuts (e.g., almonds, hazelnuts), as well as apples, hawthorns, cocoa beans, black beans, red beans, black-skinned peanuts, and red-skinned peanuts. Grape seeds are indeed one of the richest natural sources of PCs among known plant species, with a content exceeding 95% on the basis of dry weight, which is significantly greater than that found in other commonly known plants. PCs have been isolated and purified from raw materials via techniques such as supercritical CO₂ extraction, solvent extraction, column chromatography, high-speed countercurrent chromatography, membrane separation, and crystallization or recrystallization.
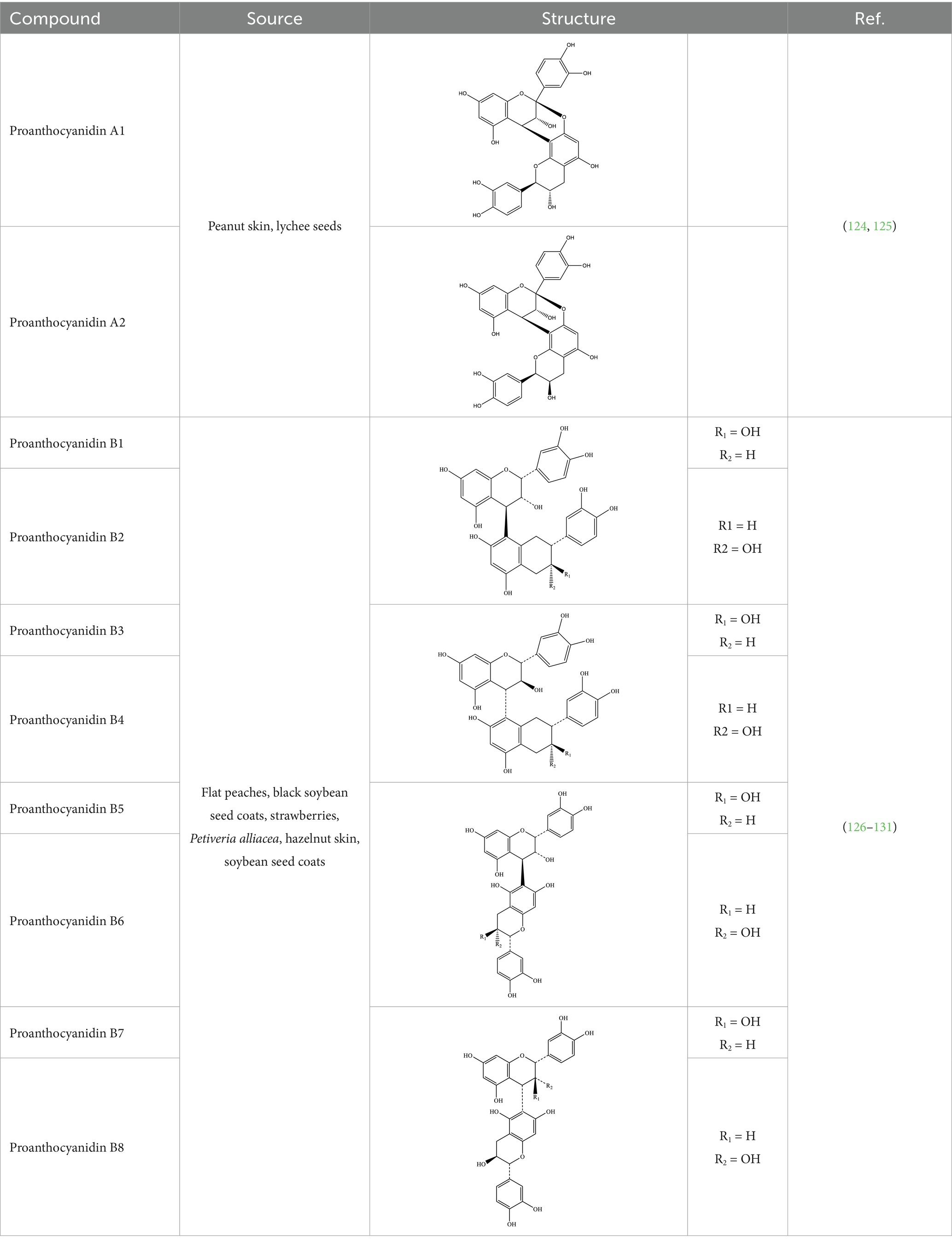
PCs, commonly known as condensed tannins, are polymers characterized by both low and high molecular weights. It is formed through the linkage of multiple hydroxylated flavane-3-alcohol units via carbon–carbon bonds. The monomers that constitute these polymers encompass four distinct structural types: (+)-catechin (designated C), (−)-epicatechin (designated EC), (+)-catechin gallate (designated CG), and (−)-epicatechin gallate (designated ECG) (32), as illustrated in Figure 2. The simplest form of PCs consists of catechin (C) or epicatechin (EC) monomers, which can undergo polymerization to yield dimers, trimers, tetramers, and so forth, extending up to decamers. PCs within the range of 2 °C to 4 °C are typically classified as oligomeric PCs on the basis of the degree of polymerization; those above 4 °C are categorized as polymeric PCs (33). PCs are further divided into two categories: Type A and Type B. Type A PCs comprise trimers (such as PCs A1--A2) that contain double bonds—specifically, a C=C double bond and a C=O double bond—as illustrated in Table 1. This type is found in select plants such as peanuts and lychees (34–36). Currently, the structures of eight type B dimers have been successfully isolated and elucidated; they are designated B1 through B8 (37), as presented in Table 1. The primary structural distinction among these dimers lies in their varying carbon connection sites. Dimers B1 to B4 are linked via the C4--C8 site, whereas dimers B5 to B8 connect through the C4--C6 site (38).
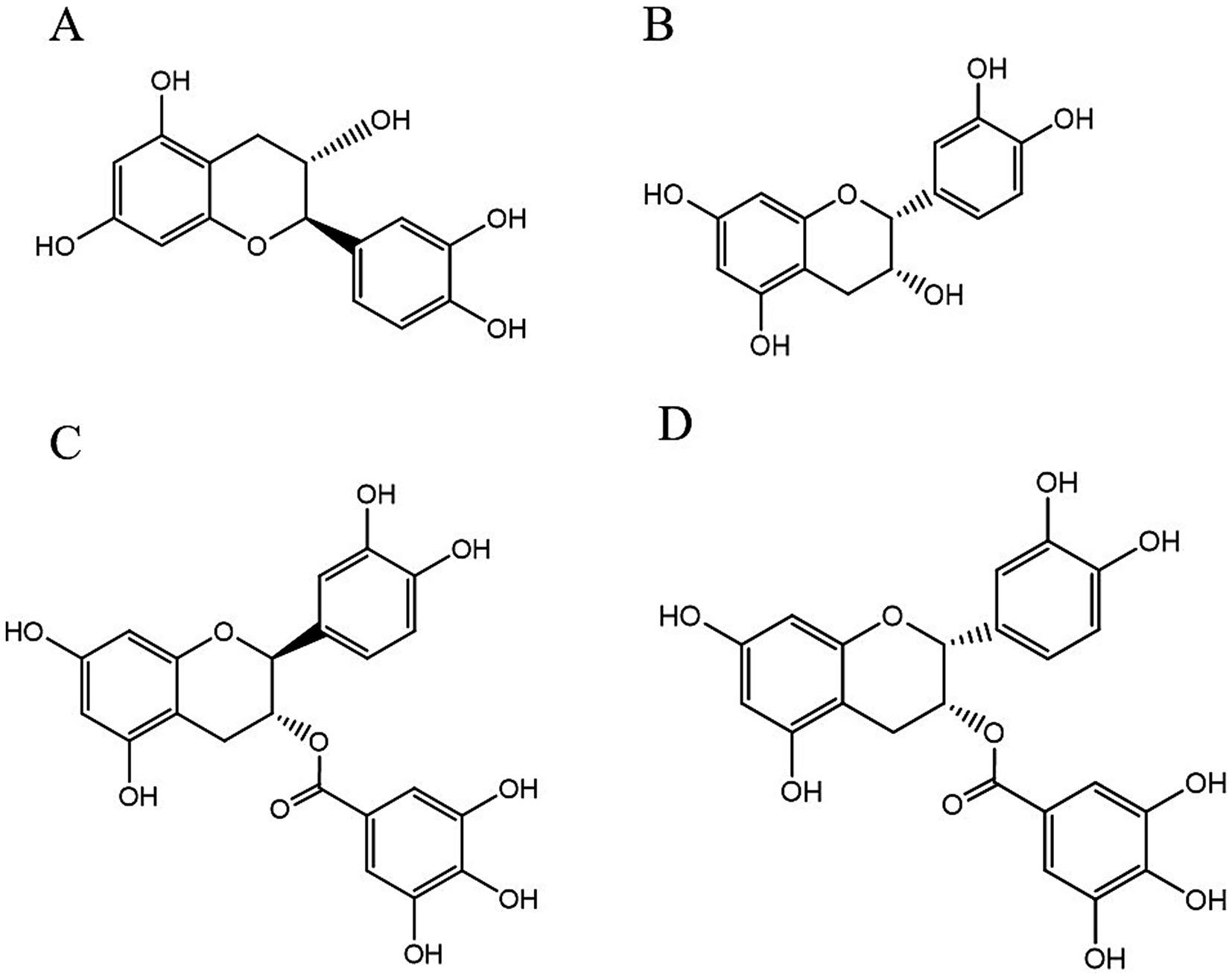
Figure 2. The structural formulas of different types of monomers that make up PCs. Each structure is a flavonoid compound, with variations in hydroxyl group arrangements and benzene rings. The compounds display different stereochemical configurations, indicated by solid and dashed wedges. (A) (+) -catechin (C), (B) (−) -epicatechin (EC), (C) (+) -catechin gallic acid (CG), (D) (−) -epicatechin gallic acid (ECG).
PCs are recognized for their instability, and environmental factors such as pH, temperature, and light play a significant role in influencing their stability (39). The stability of cocoa bean PCs is optimal within a neutral to weakly acidic environment. In contrast, under alkaline conditions, the B-ring hydroxyl group of PCs becomes vulnerable to oxidation, resulting in a decrease in their antioxidant activity (40). In experiments involving purple sweet potato PCs treated with citric acid–phosphate buffer solutions at various pH values (3.0, 5.0, and 7.0), at pH 3.0, purple sweet potato PCs exhibited relative stability characterized by a slow degradation rate. However, at pH 7.0, the phenolic hydroxyl group (-OH) within the PCs molecule undergoes oxidation to form a quinone structure; this transformation diminishes both its thermal stability and reactivity (41). Elevated temperatures further exacerbate the degradation of PCs (42). For example, when PCs purified from black carrots, elderberries, and strawberries were subjected to heating at 95 °C for 6 hours at pH 3.5, there was an observable lightening of sample color along with the formation of degradation products such as chalketonoside, phenolic acids, and maroumarin. Although these newly formed degradation products exhibited some antioxidant capacity, they were insufficient to compensate for the loss of activity associated with PCs (43).
The drying process of blueberry pulp containing PCs at various temperatures (175 °C, 200 °C, and 225 °C) leads to continuous degradation of the compound. Notably, the rate of degradation increases with increasing temperature, whereas the antioxidant activity correspondingly decreases with both elevated temperature and prolonged exposure time. These findings indicate that high-temperature dry heat treatment adversely affects the stability of PCs and consequently reduces their bioavailability (44). When the storage temperature exceeds 35 °C, the degradation rate of PCs in blueberries significantly increases, indicating that elevated temperatures can compromise the stability of PCs in these fruits. Therefore, low-temperature storage is essential for preserving the PCs content (45). Additionally, during storage and transportation, PCs are inevitably subjected to degradation caused by light exposure. When the duration of exposure exceeds 96 h, a marked increase in the degradation rate of PCs occurs (46). Consequently, to maintain the activity of PCs, it is imperative to sustain an acidic environment throughout processing, storage, and transportation; maintain temperatures below 65 °C; and minimize light exposure (47).
In addition, processing technology significantly impacts the stability of PCs in food (39). Research has shown that the PCs content in processed blackcurrant products, such as juice and jam, can decrease by more than 90% compared with that in fresh fruits. This finding indicates that traditional processing methods for juice and jam may lead to a substantial loss of PCs from blackcurrants (48). Cocoa powder is recognized for its high PCs content. During the fermentation process of cocoa powder, hydrolases secreted by microorganisms—such as lactic acid bacteria and yeast—including glycosidases and esterases—can cleave the glycosidic or ester bonds within PCs. This degradation results in the formation of small-molecule phenolic acids or oligomers. Furthermore, oxidases produced by these microorganisms, such as polyphenol oxidase, may catalyze the oxidation of PCs into quinone compounds. Consequently, this process reduces its antioxidant activity and affects its bioaccessibility (49). Extrusion treatment has been shown to significantly increase the levels of PCs monomers and dimers present in grape seeds and skins. However, it simultaneously reduces the total PCs content in grape skins and residues by 18 to 53%. These findings demonstrate that while food processing can release PCs by disrupting the food matrix, it may also induce alterations in their chemical structure, which could compromise their stability and subsequently affect their bioavailability (50).
3 Repair mechanism of PCs in skin aging
Natural skin aging is a physiological decline characterized by the deterioration of bodily functions and metabolism, which is largely irreversible. In contrast, photoaging refers to pathological damage induced by UV radiation. Certain symptoms associated with photoaging can be alleviated through pharmacological interventions and stringent sun protection measures aimed at repairing photodamage. Currently, pharmacological treatments focus primarily on addressing skin photoaging resulting from UV exposure. However, relatively few studies have investigated skin aging caused by other factors. Research has demonstrated that PCs treatments can inhibit oxidative stress in aging skin, increase the collagen and elastin contents within aged dermal layers, mitigate inflammatory responses related to skin aging, and suppress pigmentation changes in aged skin. Collectively, these actions contribute to the reparative effect of PCs in aging skin.
3.1 PCs inhibit oxidative stress in aging skin
Excessive accumulation of ROS in the body is a primary factor contributing to oxidative stress in the skin (51). ROS are a group of highly reactive molecules containing oxygen, including superoxide radicals (O2-), hydroxyl radicals (OḤ), and hydrogen peroxide (H₂O₂) (52). In naturally aging skin, ROS predominantly originate from mitochondria, while intracellular enzyme systems also generate ROS during catalytic reactions (53). In photoaged skin, UV radiation interacts with oxygen molecules within the body, leading to the generation and accumulation of ROS (54). The presence of ROS can initiate lipid peroxidation, which represents one of the critical mechanisms underlying cell membrane damage and is closely associated with cellular senescence (55).
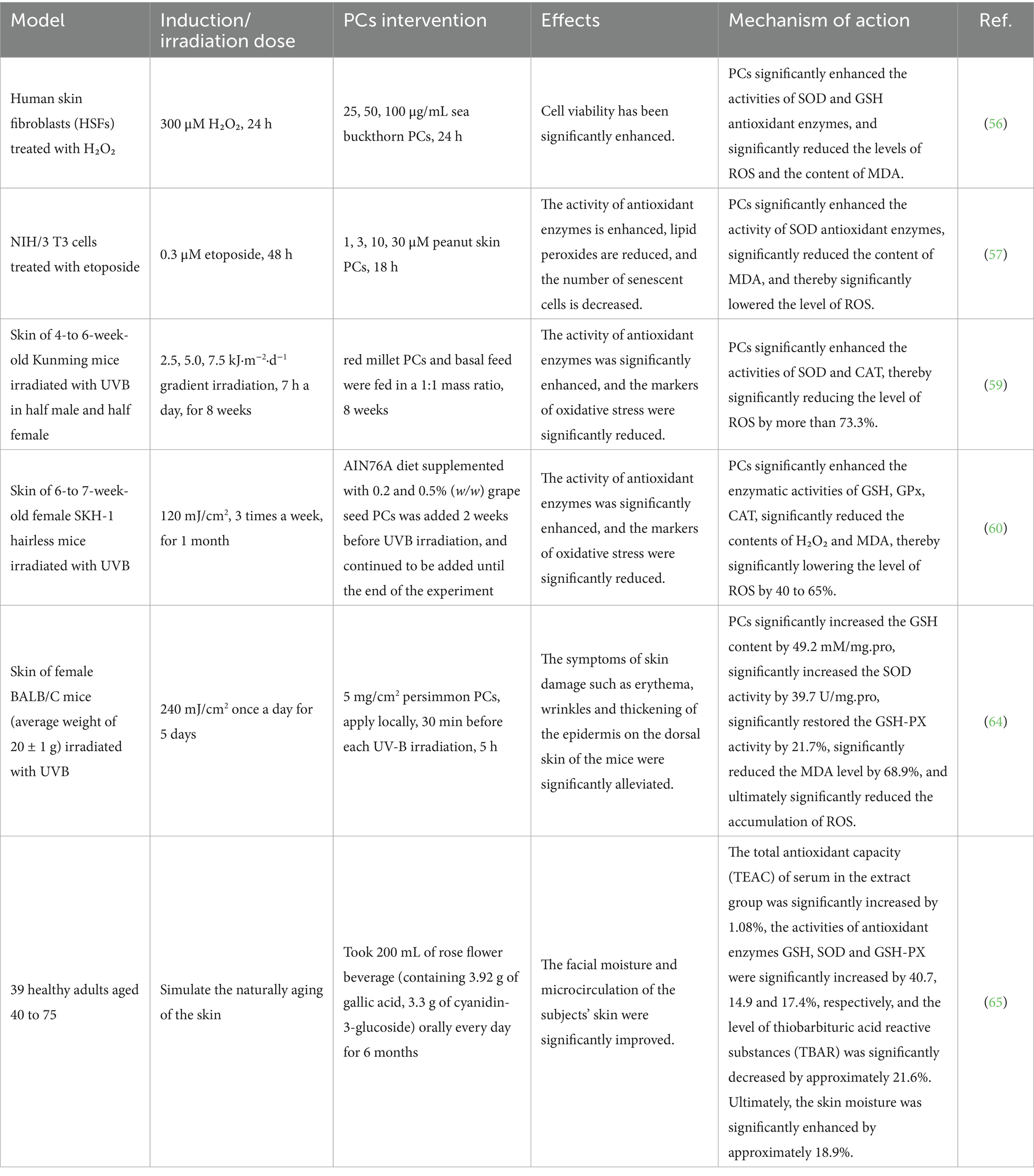
As bioactive substances, the prominent characteristic of PCs is their capacity to neutralize ROS and mitigate the production of oxidative stress products, thereby decelerating the process of skin aging. In experiments involving H₂O₂-treated human skin fibroblasts, treatment with sea buckthorn PCs significantly decreased ROS levels and notably restored the morphology of senescent cells while enhancing cellular activity. This intervention effectively alleviated cellular senescence and delayed the progression of skin aging (56). Li et al. (57) induced senescence in rat adrenal pheochromocytoma cell lines and mouse embryonic fibroblast lines via the use of rapamycin and etoposide, followed by treatment with peanut skin PCs. The findings revealed that peanut skin PCs decreased the ROS levels in these two cell lines. Furthermore, it diminished the proportion of senescent cells while restoring their proliferative capacity, thus effectively mitigating cellular senescence. Additionally, PCs have been shown to inhibit lipid peroxidation caused by ROS, further suppressing skin aging. Anshu Mittal et al. (58) investigated the effects of grape seed PCs on photoaging in hairless SKH-1 mice exposed to UVB radiation. The results indicated that dietary supplementation with 0.2 and 0.5% grape seed PCs significantly inhibited the formation of lipid peroxides induced by UVB exposure and reduced ROS levels in a dose-dependent manner in these murine models. These findings suggest that grape seed PCs may delay the aging process of skin cells by decreasing the generation of lipid peroxidation products such as malondialdehyde (MDA), which are associated with UVB-induced ROS. Li et al. (59) investigated the effects of red millet PCs on aging model mice subjected to UVB radiation. The results demonstrated that PCs significantly enhanced the activity of antioxidant enzymes in these aging model mice while reducing MDA levels, thereby highlighting their potential in combating skin photoaging. Sharma et al. (60) induced photoaging in SKH-1 hairless mouse skin through UVB exposure and subsequently administered diets containing 0.2 and 0.5% (w/w) grape seed PCs. The results revealed a significant reduction in ROS, H₂O₂, and MDA levels within the mouse skin, suggesting that grape seed PCs could effectively increase the antioxidant capacity of photoaged skin and delay the aging process.
PCs can also stimulate key antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). The synthesis of GSH-Px and glutathione reductase (GSH-Rd), along with the increase in their activities, contributes to a reduction in MDA levels. This process ultimately increases the antioxidant capacity of the skin and plays a significant role in combating skin aging. Experimental results from Hwang et al. (61) evaluated the antioxidant effects of proanthocyanidin oligomers on human retinal pigment epithelial cells and demonstrated that these oligomers significantly increased SOD and GSH-Px activities within the cells, indicating their substantial antioxidant activity. Li et al. (62) established a natural aging model in mice through a high-fat diet and subsequently treated them with PCs (200 mg/kg in the diet) extracted from lotus seed skins. Their findings revealed that PCs markedly increased both GSH-Px and SOD activities in the mouse liver while simultaneously reducing MDA levels. These findings suggest that PCs derived from lotus seed skins possess strong antioxidant properties that are capable of delaying skin aging. Chen et al. (63) investigated the effects of lotus seed PCs on UV radiation-induced damage in human skin fibroblast epithelial cells. They reported that treatment with lotus seed PCs led to a reduction in the amount of ROS generated by UV exposure, an increase in the intracellular SOD level, and decreased MDA production, thereby mitigating oxidative stress-related damage to the skin. Shi et al. (64) investigated the effects of persimmon PCs on in vitro injury to human keratinocyte HaCaT cells induced by UVB radiation. These findings demonstrated that persimmon PCs significantly elevated the levels of antioxidant enzymes, including SOD and GSH-Px, while concurrently inhibiting the production of ROS and MDA. Consequently, this treatment effectively alleviated the oxidative stress response in these cells. Additionally, in SKH hairless mice exhibiting skin photoaging due to UVB radiation, dietary inclusion of 0.2 and 0.5% grape seed PCs was found to increase the levels of antioxidant enzymes such as GSH-Px. These findings suggest that grape seed PCs can substantially reduce oxidative stress resulting from UVB exposure while providing protective effects against skin damage caused by UVB radiation (58). Furthermore, a clinical trial involving 39 healthy adults who consumed roselle beverages rich in polyphenols and PCs reported significant increases in the serum levels of SOD and GSH-Px and reduced GSH-Rd, following the daily intake of 200 mL of the beverages (containing 3.92 g of gallic acid and 3.3 g anthocyanins of cyanidin-3-glucoside) for six months. Concurrently, there was also a significant increase in facial skin moisture content among the participants. These findings indicate that roselle beverages contribute to increased antioxidant capacity and improve the skin condition of the participants (65).
In conclusion, the aforementioned research indicates that PCs can mitigate oxidative stress associated with aging skin by neutralizing ROS accumulation, suppressing lipid peroxidation caused by ROS, and enhancing overall antioxidant capacity, as shown in Table 2.
3.2 PCs increase the content of collagen and elastin in aging skin
ROS generated by skin aging can facilitate the degradation of collagen and elastin through the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, thereby contributing to the process of skin aging. ROS activate receptor tyrosine kinases (RTKs) via oxidative modification, which in turn leads to the activation of MAPKs (66). The MAPK family comprises extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK). ERK promotes the expression of the oncogenic factor c-Fos, whereas both p38 and JNK are involved in activating the transcription factor c-Jun (67, 68). C-Jun dimerizes with c-Fos to form activator protein-1 (AP-1) (69). AP-1 subsequently enhances the upregulation of MMPs such as MMP-1, MMP-3, and MMP-8 (70). The expression of matrix metalloproteinases (MMPs) can be inhibited by tissue inhibitors of metalloproteinase-1 (TIMP-1) but activated by epidermal growth factor receptors (EGFRs) and cytokine receptors. These enzymes play crucial roles in degrading collagen within the extracellular matrix, ultimately leading to structural damage in the skin (71–73).
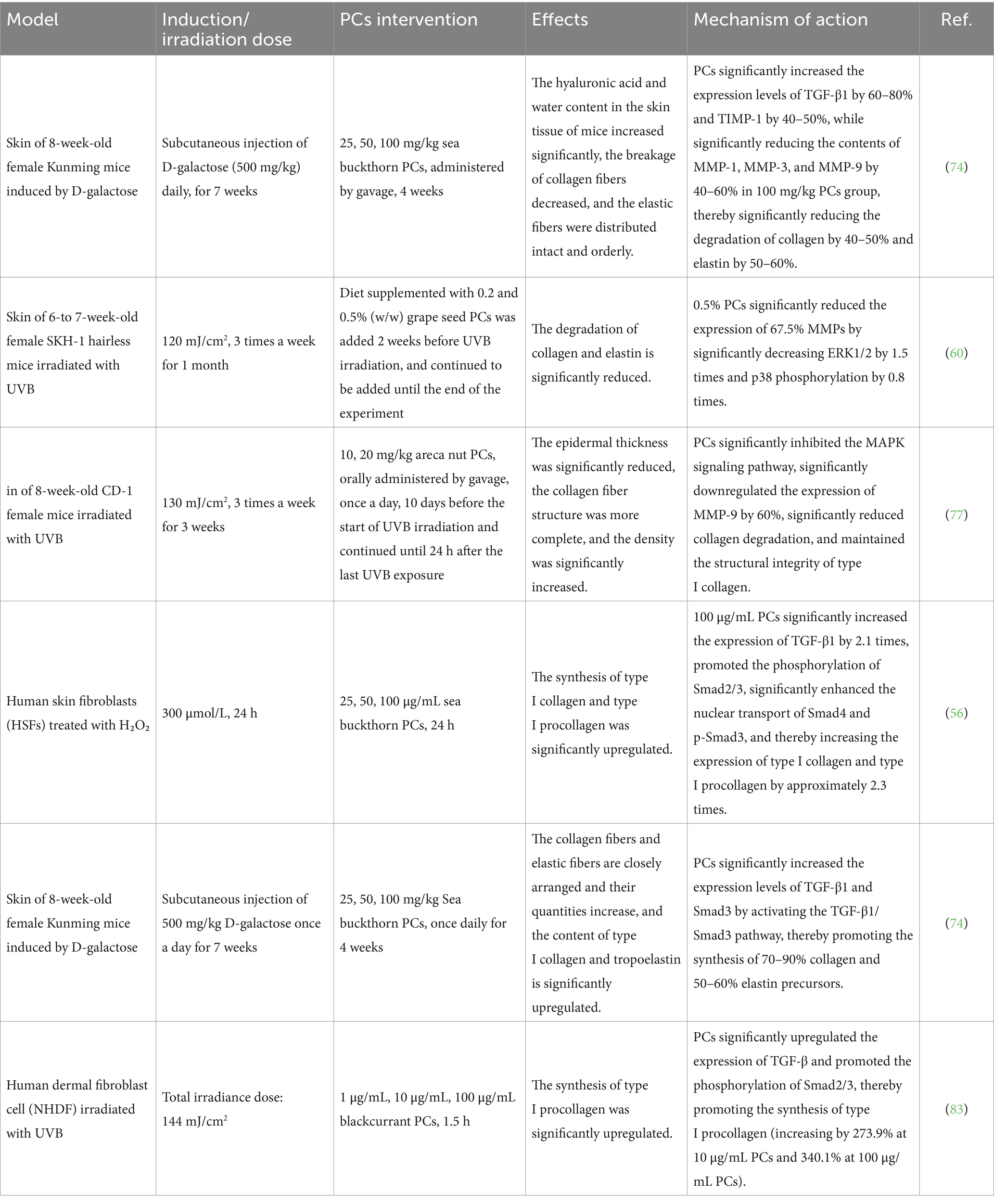
PCs inhibit the MAPK signaling pathway, thereby suppressing the expression of MMPs and increasing the levels of tissue inhibitors of TIMP-1. This mechanism ultimately prevents collagen and elastin degradation in the skin, promoting the repair of aging skin. Liu et al. (74) investigated the ability of sea buckthorn PCs to repair D-galactose-induced skin aging in mice and reported that they slowed collagen and elastin degradation by increasing TIMP-1 levels in skin tissue while simultaneously inhibiting MMP-1, MMP-3, and MMP-9 expression, thereby improving the conditions associated with skin aging. Michael et al. (75) examined the effects of various concentrations of low-bush blueberry proanthocyanidin enrichment on DU145 human prostate cancer cells. The results demonstrated that this enrichment induced TIMP-1 activity in a dose-dependent manner, leading to reduced MMP expression.
Additionally, findings revealed that grape seed extract inhibited the phosphorylation of proteins such as ERK, JNK, and P38 in human epidermal keratinocytes subjected to UVB irradiation, suggesting its ability to suppress the MAPK signaling pathway and consequently reduce UVB-induced collagen degradation (60, 76). Weng et al. (77) reported a decrease in the gene expression of MMP-2 and MMP-9 alongside an increase in TIMP1 expression in murine skin exposed to UVB radiation when 20 mg/kg areca nut PCs were orally administered to mice, indicating their ability to mitigate collagen degradation and slow photoaging effects on the skin through the inhibition of the MAPK signaling pathway. Shi et al. (64) investigated the effects of persimmon PCs on the photoaging of mouse skin exposed to UVB radiation. Research has shown that external application of 5 mg/cm2 can reduce the gene expression of MMP-1 and MMP-8, indicating that persimmon PCs may alleviate the expression of the MMP by inhibiting the MAPK signaling pathway, thereby potentially reducing collagen degradation and slowing the photoaging process in the skin. Moreover, UV radiation and ROS can inhibit collagen synthesis by suppressing the TGF-β/Smad signaling pathway (78). In fibroblasts, TGF-β1 binds to its specific receptor complexes—TβR I (the receptor for TGF-β type I) and TβR II (the receptor for TGF-β type II). Activation occurs when TβR II phosphorylates TβR I, which subsequently rephosphorylates Smad2/3. Phosphorylated Smad2/3 forms complexes with Smad4, a transcription factor that regulates type I collagen synthesis. Upon entering the nucleus, Smad4 promotes collagen synthesis through transcriptional regulation (79, 80). However, exposure to excessive UVA irradiation results in the upregulation of Smad7 in fibroblasts. This protein subsequently interacts with TβR I to prevent the activation of Smad2/3, thereby inhibiting collagen synthesis (81).
PCs can also promote collagen synthesis by activating the TGF-β/Smad signaling pathway, thereby mitigating skin aging. Liu et al. (56) established a natural aging model by treating human skin fibroblasts with varying concentrations of H₂O₂. Following treatment with sea buckthorn PCs, they observed an increase in collagen synthesis through the activation of the TGF-β1/Smad pathway, resulting in notable antiaging effects on the skin. In experiments involving a mouse model of skin aging induced by D-galactose, treatment with higher concentrations of sea buckthorn PCs (50 and 100 mg/kg) led to an increase in TGF-β1 levels within the skin tissue and the upregulation of Smad3 expression. This process promotes collagen and elastin synthesis, augments the content of collagen fibers and elastic fibers in the dermis, and improves signs associated with skin aging (74). Sang et al. (82) investigated the protective effects of PCs extracted from black soybean seed coats on skin fibroblasts and reported that this extract significantly reduced UV radiation-induced apoptosis and intracellular ROS generation while decreasing Smad3 mRNA expression. Additionally, it increased Smad7 mRNA expression to inhibit UV-induced collagen degradation, confirming that PCs enhance skin conditions via activation of the TGF-β/Smad signaling pathway, thus indicating its potential for delaying skin aging. Lu et al. (83) treated human skin fibroblasts exposed to UVB radiation with blackcurrant PCs and reported that this treatment elevated TGF-β expression in cells post-UVB exposure. Concurrently, it facilitated the phosphorylation of Smad2/3 downstream from TGF-β, enhancing transduction within the TGF-β signaling pathway, which subsequently promoted increased collagen content in these cells, thereby achieving significant antiphotoaging effects on the skin.
In conclusion, the findings of the present study indicate that PCs can alleviate the degradation of collagen and elastin by inhibiting the MAPK signaling pathway. Additionally, it promotes collagen synthesis through the activation of the TGF-β/Smad signaling pathway. As a result, this leads to an increase in the collagen and elastin contents in aging skin, thereby slowing the process of skin aging, as shown in Table 3.
3.3 PCs alleviate the inflammatory response in aging skin
The transcription factor nuclear factor-κB (NF-κB) plays a crucial role in regulating a significant number of genes associated with inflammation and immune responses. It exists as a trimer composed of IκB-α, P50, and P65. ROS can phosphorylate and activate IκB kinase (IKK), thereby initiating the NF-κB signaling pathway (84). This activation facilitates the synthesis and secretion of various inflammatory factors by epidermal and dermal cells, including interleukin-1 (IL-1), interleukin-6 (IL-6), cyclooxygenase-2, and tumor necrosis factor-alpha (TNF-α), which collectively induce inflammatory responses (85, 86). Additionally, NF-κB serves as a downstream target within the MAPK pathway. The expression of ERK, p38 MAPK, and AP-1 can increase transcriptional activity within the NF-kB pathway, consequently triggering skin inflammation and accelerating the process of skin aging (87).
PCs have been demonstrated to inhibit the NF-kB signaling pathway, thereby suppressing the production of inflammatory factors associated with this pathway and alleviating the inflammatory response observed in aging skin, as shown in Table 4.
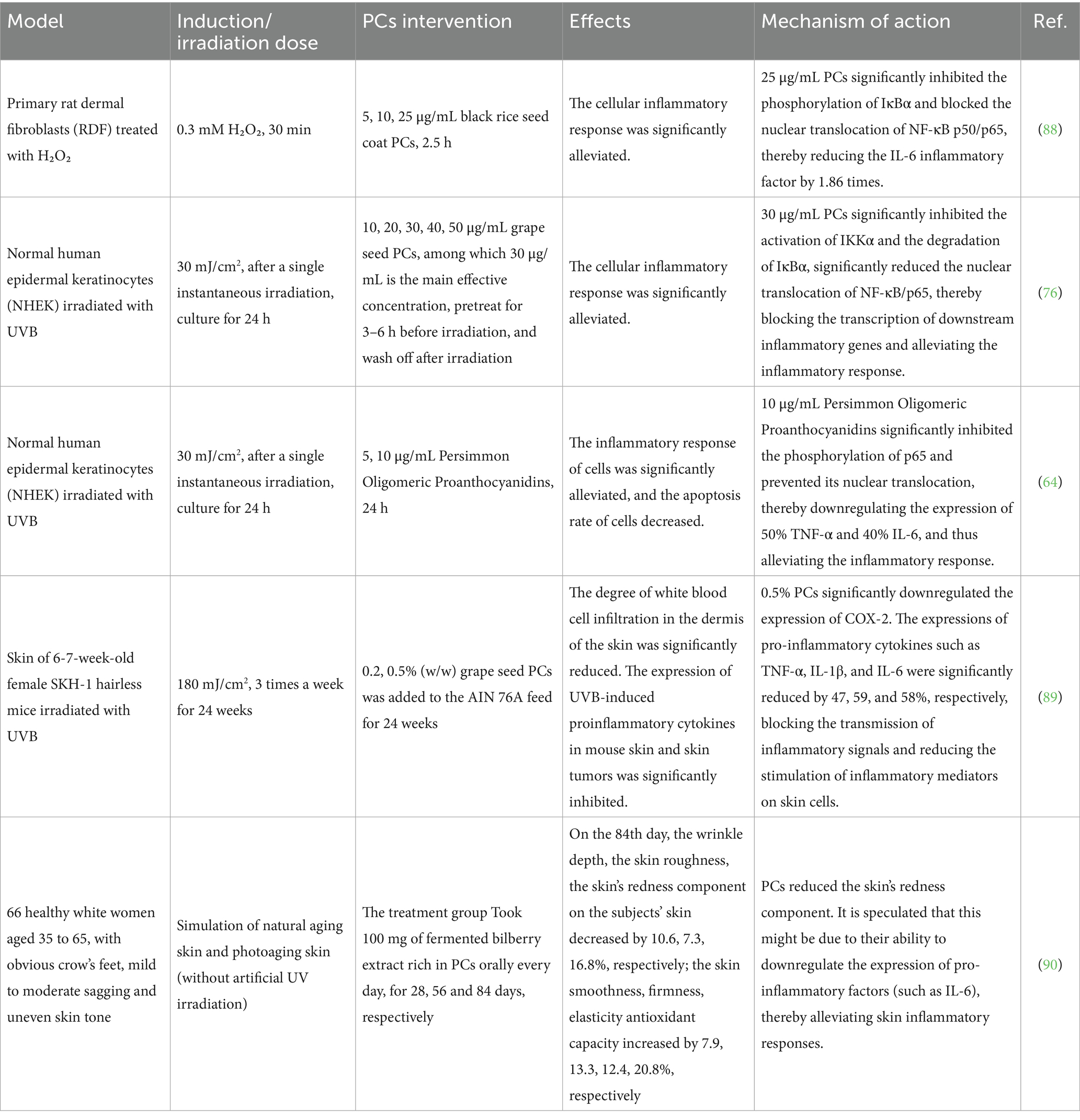
In vitro cell experiments have demonstrated that PCs can alleviate the inflammatory response associated with aging skin. Treatment of H₂O₂-stimulated primary rat skin fibroblasts with PCs extracted from rice inhibited the nuclear translocation of the NF-kBp50/p65 heterodimer within these cells. This inhibition results in reduced inflammatory responses while promoting collagen formation and inhibiting collagen degradation at the cellular level, ultimately contributing to a deceleration of the skin aging process (88). Sudheer et al. (76) reported that grape seed PCs could inhibit phosphorylation events involving ERK, JNK, and p38 in human epidermal keratinocytes following UVB irradiation; they also inhibited both the activation effects and the nuclear translocation of NF-kBp50/p65. These findings suggest that grape seed PCs may effectively reduce UVB-induced skin inflammation through the suppression of NF-kB pathway transcription. Xin et al. (64) demonstrated that persimmon oligoproanthocyanidins mitigate skin inflammatory responses by inhibiting the activation of the NF-kB signaling pathway in UVB-damaged human keratinocyte HaCaT cells and reducing the expression of inflammatory cytokines, including TNF-α and IL-6.
Additionally, in vivo animal experiments and a clinical trial confirmed that PCs can alleviate the inflammatory response associated with aging skin. Sharma et al. (89) administered grape seed PCs of 0.2 and 0.5% (w/w) in the diet to hairless SKH-1 mice exposed to UVB radiation. The experimental findings demonstrated that grape seed PCs inhibited the activation and nuclear translocation of NF-kB while downregulating the expression of proinflammatory cytokines such as TNF-α, IL-1, and IL-6, as well as cyclooxygenase-2. These findings suggest that grape seed PCs exert an antiskin aging effect by inhibiting the NF-kB signaling pathway, thereby mitigating inflammatory responses and reducing skin collagen degradation. Similar outcomes were reported in a study conducted by Anshu et al. (58) where SKH-1 hairless mice were irradiated with UVB and subsequently administered grape seed PCs. The results indicated that grape seed PCs significantly reduced the levels of inflammatory factors such as TNF-α and IL-6 within the skin of these mice. In a clinical trial investigating the antiaging, brightening, and antioxidant effects of fermented bilberry extract (FBE) containing PCs, 66 female participants aged 35 to 65 years who exhibited prominent wrinkles along with mild to moderate skin laxity and uneven skin tone were enrolled. The participants were randomly assigned to either the FBE group receiving 100 mg of fermented bilberry extract daily or the placebo group receiving maltodextrin and magnesium stearate devoid of PCs. These findings revealed that FBE enhanced the antioxidant capacity of the skin while concurrently reducing inflammation (90).
3.4 PCs inhibit pigmentation in aging skin
UV radiation is the primary inducer of melanin production. Prolonged exposure to UV radiation can lead to an increase in the number of melanocytes within the skin, thereby increasing melanin synthesis and resulting in skin pigmentation (9, 91). Specifically, UVA radiation activates opsin receptors in melanocytes, which subsequently promotes melanin synthesis. Conversely, UVB radiation enhances melanin synthesis by facilitating melanocyte differentiation and stimulating keratinocytes to release melanocyte-stimulating hormone (MSH), along with associated immunomodulatory factors and stem cell growth factors (92, 93). Upon exposure to UV radiation, endogenous α-melanocyte stimulating hormone (α-MSH) binds to the melanocortin 1 receptor (MC1R). This interaction activates adenylate cyclase (AC), leading to an increase in intracellular cyclic adenosine monophosphate (cAMP) levels. Subsequently, cAMP activates protein kinase A (PKA), which ultimately mediates the activation of cAMP response element binding protein (CREB) and microphthalmia-associated transcription factor (MITF). These transcription factors increase the expression of Tyrosinase (TYR), a key enzyme involved in melanin synthesis, thereby stimulating melanin production (94, 95).
PCs can inhibit the activity of tyrosinase, leading to a reduction in tyrosine expression and a decrease in melanin synthesis. This process ultimately results in diminished melanin deposition within the body and contributes to the slowing of skin aging. Chong et al. (96) treated thyroxine-induced senescent mice with grape seed PCs and reported that these PCs significantly inhibited tyrosinase activity in the skin cells of these aged mice, thereby suppressing melanin synthesis and effectively alleviating skin pigmentation issues. Matthew et al. (97) demonstrated that tyrosinase activity was markedly reduced when PCs extracted from lychee roots were used, suggesting that these PCs could achieve skin whitening effects by inhibiting tyrosinase activity. Chen et al. (63) explored the inhibitory effect of lotus seed PCs on melanin synthesis in human skin fibroblast epithelial cells exposed to UVB radiation. Their research revealed that lotus seed PCs effectively suppressed the conversion of melanin precursors (such as tyrosine and DOPA) into melanin by inhibiting the activity of tyrosinases, including monophenolase and diphenolase, thus significantly reducing UV-induced pigmentation.
In a clinical trial designed to evaluate the effects of apple extract (AP) supplements containing PCs on skin pigmentation issues induced by UV exposure in healthy women, participants were randomly assigned to three groups: a low-dose AP group (300 mg/day), a high-dose AP group (600 mg/day), and a placebo group. These groups underwent continuous supplementation for 12 weeks. The results indicated that AP administration significantly reduced the melanin content of the skin following UV exposure, confirming its capacity to diminish melanin production through the inhibition of tyrosinase activity (98). In another clinical trial investigating the impact of red wine beverages containing PCs on sunburn and dryness resulting from UV exposure in women, 100 healthy female subjects were randomly allocated into two groups: the test group receiving 200 mg/day of PCs-containing red wine beverages and the control group receiving 200 mg/day of red wine beverages devoid of PCs. After 12 weeks of continuous consumption, measurements revealed a significant reduction in the melanin index at sunspots within the test group, indicating that PCs-containing red wine beverages effectively inhibited tyrosinase activity, leading to decreased melanin production (99).
In addition to the previously mentioned four beneficial effects on aging skin, PCs can also enhance barrier function and hydration. They promote the upregulation of filaggrin and keratin expression. Filaggrin plays a crucial role in lipid synthesis within the stratum corneum of the skin, whereas keratin is essential for the formation of the skin barrier. Consequently, PCs contribute to improved moisture retention in the skin and strengthen its resistance to external stimuli (100, 101). Furthermore, PCs can facilitate cell migration, which is a key aspect of tissue repair. In fibroblasts subjected to oxidative stress from H₂O₂ exposure, treatment with PCs significantly increased cell migration rates. This enhancement enables these cells to resynthesize extracellular matrices such as collagen, thereby directly supporting skin regeneration and repair while delaying collagen imbalance-related skin aging (56, 102).
4 Methods for improving the stability and bioavailability of PCs
PCs exhibit poor stability, which significantly diminishes their bioavailability. To address this limitation, encapsulation techniques can be employed to increase the stability of PCs, thereby improving their bioavailability (103, 104). Encapsulation refers to the process of embedding bioactive compounds within solid particles or liquid vesicles. This technique not only regulates the release of bioactive substances but also masks undesirable odors, stabilizes biological activity, and protects sensitive compounds from degradation (105, 106). Currently, encapsulation is a focal point of research with extensive application potential. It is widely acknowledged as an effective strategy for preserving the stability of bioactive substances and extending the shelf-life of materials that are sensitive to light and heat (107, 108). The primary forms of encapsulation for PCs include microcapsules, liposomes, and nanoparticles, as shown in Table 5.
Microcapsule technology represents an innovative packaging approach that has emerged in recent years. This technique primarily involves the dispersion of small-molecule active compounds, sensitive materials, or volatile substances through physical or chemical methods, followed by the formation of microcapsules encapsulated with protective films (109). First, these microcapsules effectively shield bioactive components from environmental degradation, prolong their stability, and ensure precise delivery to targeted sites. Additionally, they minimize interactions between different ingredients, thereby extending the shelf-life of the product. Owing to these advantages, microcapsule technology has found extensive applications across diverse fields, such as food science, biomedicine, sensing technologies, and textiles (110–112). Moreover, microencapsulation can enhance the light and oxidation resistance of PCs, thereby broadening their potential and application range in functional products (113).
Liposomes play a crucial role in colloidal drug delivery systems, particularly within the fields of food and nutrition. Primarily composed of phospholipids, they can encapsulate water-soluble substances in their internal aqueous phase while accommodating lipophilic compounds within the lipid bilayer, thereby forming liposomes with a well-defined bilayer structure (114, 115). These structures exhibit several advantageous properties, including low toxicity, high plasticity, complete biodegradability, lack of immunogenicity, self-assembly capability, and ease of modification (115). Given that PCs are water soluble, they can be effectively incorporated into the aqueous core of liposomes. This incorporation protects PCs from degradation caused by environmental factors such as light exposure, pH fluctuations, and temperature variations (113).
Nanoparticles have been shown to be an optimal choice for encapsulating phenolic compounds and enhancing their bioavailability. They significantly improve the uptake efficiency of polyphenols by increasing their solubility, facilitating their absorption through endocytosis, and promoting the entrapment or surface adsorption of biomolecules (116). This encapsulation technology not only enhances the solubility and stability of bioactive compounds but also plays a pivotal role in drug delivery systems. The carrier materials used for nanoparticles are typically biodegradable or ion sensitive and exhibit low toxicity, minimal side effects, and controllable release characteristics. Moreover, nanoparticles can traverse intercellular and intertissue gaps to reach target sites directly, thereby further improving the bioavailability of bioactive substances (117–119).
In addition, the intestinal microbiota, dietary fiber and fat, various antioxidants, pH regulation, and low-temperature extraction methods can increase the bioavailability of PCs. PCs operate synergistically with intestinal microorganisms such as Lactobacillus and Bifidobacterium. The metabolism of PCs by these intestinal microorganisms results in the generation of short-chain fatty acids and other secondary metabolites that further augment the antioxidant effects of PCs (120). Moreover, PCs exhibit a synergistic relationship with dietary fiber and fat. When combined with dietary fiber, PCs are protected from degradation by digestive enzymes during digestion; this facilitates their gradual release in the intestines, thereby prolonging their antioxidant activity (121). Additionally, when PCs are paired with other antioxidants, such as vitamin C, they promote preferential oxidation of these antioxidants, which enhances the bioavailability of PCs (122). The stability and bioavailability of PCs in aqueous solutions can be improved by incorporating an acidic buffer to adjust the pH to acidic levels or by avoiding mixing the PCs solution with alkaline substances (46). Furthermore, employing low-temperature extraction techniques for PCs—such as low-temperature prefmentation impregnation and dry ice freezing—can minimize degradation during extraction processes. These approaches collectively improve both the stability and bioavailability of polyphenol compounds (123).
5 Summary and outlook
Skin aging is a multifaceted biological process influenced by various factors. Currently recognized contributors to this phenomenon include increased oxidative stress, elevated expression of MMPs, degradation of collagen and elastin, heightened inflammatory responses, and melanin deposition, among others. The mechanisms through which PCs exert anti-skin aging effects involve multiple targets, levels, and pathways, as shown in Figure 3. PCs can alleviate oxidative stress in aging skin by neutralizing ROS, inhibiting ROS-induced lipid peroxidation, and enhancing the antioxidant capacity of the skin. Furthermore, it reduces the degradation of collagen and elastin in aged skin while promoting collagen synthesis by inhibiting the MAPK pathway and activating the TGF-β/Smad signaling pathway. This dual action results in increased levels of collagen and elastin within aging skin. Additionally, PCs mitigate the inflammatory response associated with skin aging by inhibiting the NF-kB signaling pathway, thereby decreasing the secretion of proinflammatory cytokines. Moreover, it inhibits tyrosinase activity to reduce melanin production in aged skin, effectively suppressing pigmentation.
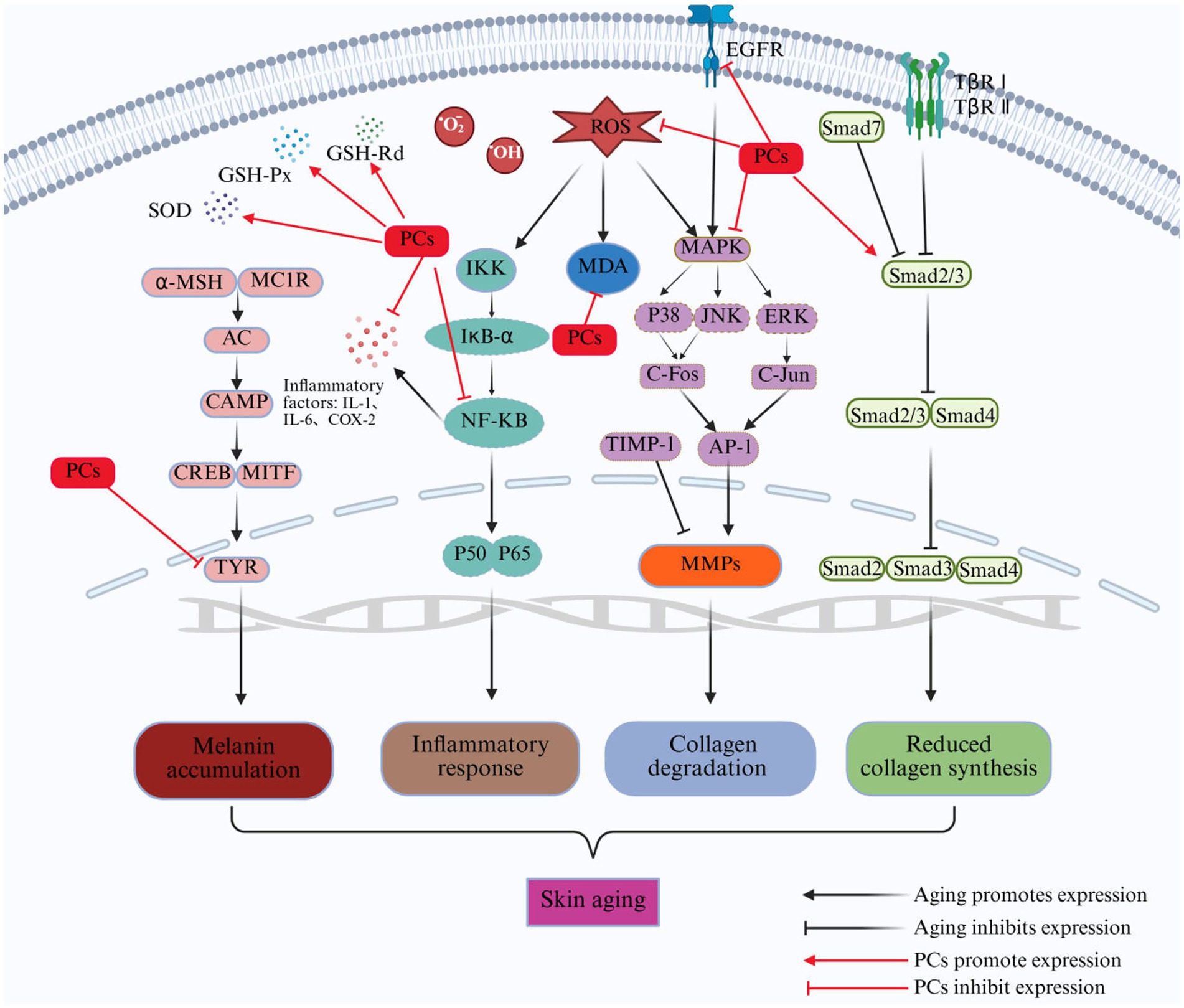
Figure 3. Repair mechanism of PCs in aging skin. ROS, reactive oxygen species; O2−, superoxide radical; OḤ, hydroxyl radical; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, C-Jun N-terminal kinase; AP-1, activator protein-1; IKK, inhibitor of Kappab kinase; IkB-α, NF-kappa-B Inhibitor Alpha; TβR I, transforming growth factor-β receptor I; TβR II, transforming growth factor-β receptor II; Smad, small mothers against decapentaplegic proteins; MMPs, matrix metalloproteinases; α-MSH, α-melanocyte stimulating hormone; MC1R, melanocortin 1 receptor; AC, activate adenylate cyclase; cAMP, cyclic adenosine monophosphate; TYR, tyrosinase; MDA, malondialdehyde; TIMP-1, tissue inhibitor of metalloproteinases 1; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; GSH-Rd, glutathione reductase.
With increasing awareness of public health and ongoing in-depth research on PCs, the development and application of PCs present significant opportunities. PCs not only delay and repair skin aging but also exhibit remarkable efficacy in combating skin cancer, alleviating allergic reactions on the skin, protecting cardiovascular health and cerebrovascular functions, preventing myopia development, and relieving physical fatigue and mental distress, such as depression. However, it is important to acknowledge that there are certain limitations and knowledge gaps in the current research regarding the anti-skin aging effects of PCs. The limitations of existing research are reflected in several key aspects: (1) Lack of clinical research: Most studies investigating the antiaging effects of PCs rely on in vitro cell culture or in vivo animal models. While these models provide valuable preliminary data, they may not fully capture the complexity of human skin physiology and metabolism. Additionally, clinical trials often involve relatively small sample sizes, which limits both the statistical power and generalizability of their findings. (2) Verification is needed for nanoparticles validity: PCs exhibit poor stability under various environmental conditions, such as high temperature, light exposure, and alkalinity. This instability significantly impacts their bioavailability and efficacy. Although packaging techniques have been explored to increase stability, their effectiveness in improving the bioavailability of PCs within the human body remains inadequately understood. (3) Unresolved mechanism underlying photoaged skin repair: Although some mechanisms by which PCs combat skin aging have been partially elucidated, a comprehensive understanding of their molecular and cellular interactions is still lacking. In particular, further detailed investigations into the signaling pathways and gene networks regulated by PCs within human skin are necessary for advancing this field.
Author contributions
HY: Writing – review & editing, Writing – original draft. JS: Writing – original draft. LH: Writing – original draft, Funding acquisition, Supervision, Writing – review & editing. CA: Writing – original draft. WJ: Supervision, Writing – review & editing, Writing – original draft. AA: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Shaanxi Foreign Expert Service Project (Grant No. 2024WZYBXM-40) and the Innovation Team Support Plan of Shaanxi “Sanqin Scholars” (Shaanxi Group Tongzi (2018) 34).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deng, M, Wang, J, Li, Y, Chen, HX, Tai, M, Deng, L, et al. Impact of polyphenols extracted from tricholoma matsutake on UVB-induced photoaging in mouse skin. J Cosmet Dermatol. (2021) 21:781–93. doi: 10.1111/JOCD.14127
2. Boismal, F, Serror, K, Dobos, G, Zuelgaray, E, Bensussan, A, and Michel, L. Skin aging: pathophysiology and innovative therapies. Med Sci (Paris). (2020) 36:1163–72. doi: 10.1051/medsci/2020232
3. Wei, W, Li, T, Chen, JL, Fan, Z, Gao, F, Yu, ZB, et al. SIRT3/6: an amazing challenge and opportunity in the fight against fibrosis and aging. Cell Mol Life Sci. (2024) 81:69. doi: 10.1007/S00018-023-05093-Z
4. Yu, GT, Ganier, C, Allison, DB, Tchkonia, T, Khosla, S, Kirkland, JL, et al. Mapping epidermal and dermal cellular senescence in human skin aging. Aging Cell. (2024) 24:e14358. doi: 10.1111/ACEL.14358
5. Austin, E, Geisler, AN, Nguyen, J, Kohli, I, Hamzavi, I, Lim, HW, et al. Visible light. Part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. (2021) 84:1219–31. doi: 10.1016/J.JAAD.2021.02.048
6. Mohania, D, Chandel, S, Kumar, P, Verma, V, Digvijay, K, Tripathi, D, et al. Ultraviolet radiations: skin defense-damage mechanism. Adv Exp Med Biol. (2017) 996:71–87. doi: 10.1007/978-3-319-56017-5_7
7. Darice, YW, Ranganath, T, and Andrea, MK. Low-dose, long-wave UV light does not affect gene expression of human mesenchymal stem cells. PLoS One. (2015) 10:e0139307. doi: 10.1371/journal.pone.0139307
8. Liu, YX, Qin, D, Wang, HN, Zhu, Y, Bi, SC, Liu, Y, et al. Effect and mechanism of fish scale extract natural hydrogel on skin protection and cell damage repair after UV irradiation. Colloids Surf B Biointerfaces. (2023) 225:113281. doi: 10.1016/J.COLSURFB.2023.113281
9. Rachmin, I, Stephen, MO, Qing, YW, and David-E, F. Topical treatment strategies to manipulate human skin pigmentation. Adv Drug Deliv Rev. (2020) 153:65–71. doi: 10.1016/j.addr.2020.02.002
10. Orioli, D, and Dellambra, E. Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells. (2018) 7:268. doi: 10.3390/cells7120268
11. He, SY, Cai, J, Jia, TJ, Mao, Z, Zhou, LH, Zhang, XN, et al. New sight of renal toxicity caused by UV-aged polystyrene nanoplastics: induced ferroptosis via adsorption of transferrin. Small. (2024) 20:e2309369. doi: 10.1002/SMLL.202309369
12. Chen, S, Gan, DH, Lin, SX, Zhong, YM, Chen, MJ, Zou, XN, et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics. (2022) 12:2722–40. doi: 10.7150/THNO.71360
13. Yang, YH, Lu, XY, Liu, N, Ma, S, Zhang, H, Zhang, ZY, et al. Metformin decelerates aging clock in male monkeys. Cell. (2024) 187:6358–6378.e29. doi: 10.1016/J.CELL.2024.08.021
14. Wang, M, Zhang, J, Qiu, JP, Ma, X, Xu, CZ, Wu, QH, et al. Doxycycline decelerates aging in progeria mice. Aging Cell. (2024) 23:e14188. doi: 10.1111/ACEL.14188
15. Szymański, Ł, Skopek, R, Palusińska, M, Schenk, T, Stengel, S, Lewicki, S, et al. Retinoic acid and its derivatives in skin. Cells. (2020) 9:2660. doi: 10.3390/CELLS9122660
16. Vellaichamy, G, Kohli, I, Zubair, R, Yin, C, Braunberger, T, Nahhas, AF, et al. An in vivo model of postinflammatory hyperpigmentation and erythema: clinical, colorimetric and molecular characteristics. Br J Dermatol. (2021) 186:508–19. doi: 10.1111/BJD.20804
17. Aggarwal, I, Puyana, C, Chandan, N, Jetter, N, and Tsoukas, M. Field cancerization therapies for the management of actinic keratosis: an updated review. Am J Clin Dermatol. (2024) 25:391–405. doi: 10.1007/S40257-023-00839-8
18. Zhao, Y, Jiang, CQ, Lu, JY, Sun, YH, and Cui, YY. Research progress of proanthocyanidins and anthocyanidins. Phytother Res. (2023) 37:2552–77. doi: 10.1002/PTR.7850
19. Parisi, M, Verrillo, M, Maria-Antonietta, L, Caiazzo, G, Quaranta, M, Scognamiglio, F, et al. Use of natural agents and agrifood wastes for the treatment of skin photoaging. Plants. (2023) 12:840. doi: 10.3390/PLANTS12040840
20. Siddiqui, Z, Zufall, A, Nash, M, Rao, D, Hirani, R, and Russo, M. Comparing tretinoin to other topical therapies in the treatment of skin photoaging: a systematic review[Z]. Am J Clin Dermatol. (2024) 25:873–90. doi: 10.1007/s40257-024-00893-w
21. Szczuraszek, P, Szczuraszek, H, Sałata, P, Paluch, M, Tomkiewicz, M, Tomkiewicz, J, et al. The role of the retinoids in topical treatment of skin diseases the review of the literature. J Educ Health Sport. (2023) 13:309–19. doi: 10.12775/JEHS.2023.13.03.040
22. Zhang, YH, Li, Y, Quan, ZZ, Xiao, P, and Duan, JA. New insights into antioxidant peptides: an overview of efficient screening, evaluation models, molecular mechanisms, and applications. Antioxidants (Basel). (2024) 13:203. doi: 10.3390/antiox13020203
23. Marques, C, Hadjab, F, Porcello, A, Lourenço, K, Scaletta, C, Abdel, P, et al. Mechanistic insights into the multiple functions of niacinamide: therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants (Basel). (2024) 13:425. doi: 10.3390/antiox13040425
24. Zapała, M, Hunek, A, Kaziród, K, Chmielarz, K, Wiśniewska, SJ, Tylutka, K, et al. Use of retinoids in the treatment of skin lesions and prevention of signs of skin aging a systematic review. J Educ Health Sport. (2023) 27:87–107. doi: 10.12775/JEHS.2023.27.01.009
25. Markiewicz, E, Ruth, N, Mammone, T, and Idowu, OC. Investigating the dual functions of butylated hydroxytoluene, vitamin E and vitamin C as antioxidants and anti-glycation agents in vitro: implications for skin health. Int J Cosmet Sci. (2025) 9:1–16. doi: 10.1111/ics.13079
26. Skibska, A, and Perlikowska, R. Signal peptides promising ingredients in cosmetics. Curr Protein Pept Sci. (2021) 22:716–28. doi: 10.2174/1389203722666210812121129
27. Baranowska, M, and Bartoszek, A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Postepy Hig Med Dosw. (2016) 70:1460–8. doi: 10.5604/17322693.1227896
28. Krasutsky, AG, and Aksenov, IV. Anthocyanins as an element of nutritional support for athletes: effects and molecular mechanisms of action. Vopr Pitan. (2024) 93:5–13. doi: 10.33029/0042-8833-2024-93-3-5-13
29. Ockermann, P, Headley, L, Lizio, R, and Hansmann, J. A review of the properties of anthocyanins and their influence on factors affecting cardiometabolic and cognitive health. Nutrients. (2021) 13:2831. doi: 10.3390/nu13082831
30. Huang, R, Fang, W, Xie, XQ, Liu, YT, and Xu, CM. Identification of key astringent compounds in aronia berry juice. Food Chem. (2022) 393:133431. doi: 10.1016/j.foodchem.2022.133431
31. Qi, QQ, Chu, MJ, Yu, XT, Xie, YN, Li, YL, Du, YM, et al. Anthocyanins and Proanthocyanidins: chemical structures, food sources, bioactivities, and product development. Food Rev Intl. (2023) 39:4581–609. doi: 10.1080/87559129.2022.2029479
32. Zhao, J, Wang, J, Chen, Y, and Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. (1999) 20:1737–45. doi: 10.1093/carcin/20.9.1737
33. Huang, Q, Liu, X, Zhao, G, Hu, T, and Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. (2018) 4:137–50. doi: 10.1016/j.aninu.2017.09.004
34. Gong, YH, Fang, F, Zhang, X, Liu, B, Luo, HH, Li, Z, et al. B type and complex A/B type epicatechin trimers isolated from litchi pericarp aqueous extract show high antioxidant and anticancer activity. Int J Mol Sci. (2018) 19:301. doi: 10.3390/ijms19010301
35. Dudek, MK, Gliński, VB, Davey, MH, Sliva, D, Kaźmierski, S, and Gliński, JA. Trimeric and tetrameric A-type procyanidins from peanut skins. J Nat Prod. (2017) 80:415–26. doi: 10.1021/acs.jnatprod.6b00946
36. Zeng, Y, Zhao, L, Wang, K, Renard, CMGC, Carine, LB, Hu, ZY, et al. A-type proanthocyanidins: sources, structure, bioactivity, processing, nutrition, and potential applications. Compr Rev Food Sci Food Saf. (2024) 23:e13352. doi: 10.1111/1541-4337.13352
37. Yang, W, Laaksonen, O, Kallio, H, and Yang, B. Proanthocyanidins in sea buckthorn (hippophaë rhamnoides l.) berries of different origins with special reference to the influence of genetic background and growth location. J Agric Food Chem. (2016) 64:1274–82. doi: 10.1021/acs.jafc.5b05718
38. Fuleki, T, Silva, JM, and Ricardo, D. Catechin and procyanidin composition of seeds from grape cultivars grown in Ontario. J Am Chem Soc. (1997) 45:1156–60. doi: 10.1021/jf960493k
39. Li, ZJ, Xu, YB, Liu, YW, Kong, MR, Wang, JR, Li, YX, et al. Study on the extraction, purification, stability, free radical scavenging kinetics, polymerization degree and characterization of proanthocy-anidins from Pinus koraiensis seed scales. Food Chem. (2024) 454:139776. doi: 10.1016/J.FOODCHEM.2024.139776
40. Toro-uribe, S, López-giraldo, LJ, and Decker, EA. Relationship between the physiochemical properties of cocoa procyanidins and their ability to inhibit lipid oxidation in liposomes. J Agric Food Chem. (2018) 66:4490–502. doi: 10.1021/acs.jafc.8b01074
41. Jiang, T, Mao, Y, Sui, LS, Yang, N, Li, SY, Zhu, ZZ, et al. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. (2018) 274:460–70. doi: 10.1016/j.foodchem.2018.07.141
42. Castilla-ramírez, P, Servin-uribe, RI, Barba-franco, JDJ, and Reynoso-camacho, R. Ethanol and citric acid: an efficient alternative for extracting anthocyanins and proanthocyanidins, and copigments associated with thermostability from Syrah grape pomace. J Food Meas Charact. (2024) 19:1–14. doi: 10.1007/S11694-024-03049-W
43. Sadilova, E, Carle, R, and Stintzing, FC. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol Nutr Food Res. (2007) 51:1461–71. doi: 10.1002/mnfr.200700179
44. Bener, M, Shen, YX, Apak, R, Finley, JW, and Xu, ZM. Release and degradation of anthocyanins and phenolics from blueberry pomace during thermal acid hydrolysis and dry heating. J Agric Food Chem. (2013) 61:6643–9. doi: 10.1021/jf401983c
45. Hao, LC, Xu, WX, and Wu, MH. Dispersive solid phase extraction coupled with chromogenic reaction and UV–Vis spectrophotometry for the investigation of storage stability of proanthocyanidins in blueberry. J Food Meas Charact. (2023) 18:1734–9. doi: 10.1007/S11694-023-02303-X
46. Duan, CC, Xiao, XF, Yu, YH, Xu, MT, Zhang, YP, Liu, XD, et al. In situ Raman characterization of the stability of blueberry anthocyanins in aqueous solutions under perturbations in temperature, UV, pH. Food Chem. (2023) 431:137155. doi: 10.1016/J.FOODCHEM.2023.137155
47. Song, BJ, Sapper, TN, Burtch, CE, Brimmer, K, Goldschmidt, M, and Ferruzzi, MG. Photo and thermodegradation of anthocyanins from grape and purple sweet potato in model beverage systems. J Agric Food Chem. (2013) 61:1364–72. doi: 10.1021/jf3044007
48. Hollands, W, Gary-M, B, Radreau, P, Saha, S, Teucher, B, Richard-N, B, et al. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects. Food Chem. (2008) 108:869–78. doi: 10.1016/j.foodchem.2007.11.052
49. Wallace, TC, and Giusti, MM. Selective removal of the violet color produced by anthocyanins in procyanidin-rich unfermented cocoa extracts. J Food Sci. (2011) 76:C1010–7. doi: 10.1111/j.1750-3841.2011.02322.x
50. Khanal, RC, Howard, LR, and Prior, RL. Procyanidin content of grape seed and pomace, and total anthocyanin content of grape pomace as affected by extrusion processing. J Food Sci. (2009) 74:H174–82. doi: 10.1111/j.1750-3841.2009.01221.x
51. Hajam, YA, Rani, R, Ganie, SY, Sheikh, TA, Javaid, D, Qadri, SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. (2022) 11:552. doi: 10.3390/cells11030552
52. Schweikl, H, Godula, M, Petzel, C, Bolay, C, Hiller, KA, and Buchalla, W. Critical role of superoxide anions and hydroxyl radicals in HEMA-induced apoptosis. Dent Mater. (2017) 33:110–8. doi: 10.1016/j.dental.2016.11.003
53. Kiely, CJ, Pavli, P, and O'Brien, CL. The role of inflammation in temporal shifts in the inflammatory bowel disease mucosal microbiome. Gut Microbes. (2018) 9:1–25. doi: 10.1080/19490976.2018.1448742
54. Hadshiew, IM, Eller, MS, and Gilchrest, BA. Skin aging and photoaging: the role of DNA damage and repair. Dermatitis. (2000) 11:19–25. doi: 10.1016/S1046-199X(00)90028-9
55. Xie, YJ, Wang, J, Li, Z, Luan, YY, Li, MY, Peng, XJ, et al. Damage prevention effect of milk-derived peptides on UVB irradiated human foreskin fibroblasts and regulation of photoaging related indicators. Food Res Int. (2022) 161:111798. doi: 10.1016/J.FOODRES.2022.111798
56. Liu, XY, Xing, Y, Yuen, M, Yuen, T, Yuen, H, and Peng, Q. Anti-aging effect and mechanism of proanthocyanidins extracted from sea buckthorn on hydrogen peroxide-induced aging human skin fibroblasts. Antioxidants. (2022) 11:1900–17. doi: 10.3390/ANTIOX11101900
57. Li, YJ, Lan, X, and Qi, JH. Procyanidin A1 from peanut skin exerts anti-aging effects and attenuates senescence via antioxidative stress and autophagy induction. Antioxidants. (2025) 14:3322. doi: 10.3390/ANTIOX14030322
58. Mittal, A, Craig-A, E, and Santosh-K, K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. (2003) 24:1379–88. doi: 10.1093/carcin/bgg095
59. Li, X, Sheng, JJ, Li, Z, He, YM, Zu, YQ, and Li, Y. Enhanced UV-B radiation induced the proanthocyanidins accumulation in red rice grain of traditional rice cultivars and increased antioxidant capacity in aging mice. Int J Mol Sci. (2023) 24:3397. doi: 10.3390/IJMS24043397
60. Sharma, SD, Meeran, SM, and Katiyar, SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. (2007) 6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661
61. Hwang, JW, Kim, EK, Lee, SJ, Kim, YS, Moon, SH, Jeon, BT, et al. Antioxidant activity and protective effect of anthocyanin oligomers on H₂O₂-triggered G2/M arrest in retinal cells. J Agric Food Chem. (2012) 60:4282–8. doi: 10.1021/jf205321j
62. Li, T, Li, QL, Wu, WG, Li, Y, Hou, DX, Xu, H, et al. Lotus seed skin proanthocyanidin extract exhibits potent antioxidant property via activation of the Nrf2-ARE pathway. Acta Biochim Biophys Sin. (2019) 51:31–40. doi: 10.1093/abbs/gmy148
63. Chen, YS, Zhang, RJ, Xie, BJ, Sun, ZD, and Mcclements, DJ. Lotus seedpod proanthocyanidin-whey protein complexes: impact on physical and chemical stability of β-carotene-nanoemulsions. Food Res Int. (2019) 127:108738. doi: 10.1016/j.foodres.2019.108738
64. Shi, X, Shang, FF, Zhang, YJ, Wang, RF, Jia, YY, and Li, KK. Persimmon oligomeric proanthocyanidins alleviate ultraviolet B-induced skin damage by regulating oxidative stress and inflammatory responses. Free Radic Res. (2020) 54:765–76. doi: 10.1080/10715762.2020.1843651
65. Hui, FC, Yi, RL, You, CS, Yi, CH, Oksana, G, Kamesh, V, et al. Improvement on blood pressure and skin using roselle drink: a clinical trial. J Food Biochem. (2022) 46:e14287. doi: 10.1111/jfbc.14287
66. Toutfaire, M, Bauwens, E, and Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: a notion of mosaic and therapeutic strategies. Biochem Pharmacol. (2017) 142:1–12. doi: 10.1016/j.bcp.2017.04.011
67. Zong, BB, Xiao, Y, Ren, MM, Wang, PY, Fu, SL, and Qiu, YS. Baicalin weakens the por-cine expectinduced inflammatory response in 3D4/21 cells by inhibiting the expression of NF-kB/MAPK signaling pathways and reducing NLRP3 inflammasome activation. Microorganisms. (2023) 11:2126. doi: 10.3390/MICROORGANISMS11082126
68. Kim, MJ, Yang, YJ, Min, GY, Heo, JW, Son, JD, You, YZ, et al. Anti-inflammatory and antioxidant properties of Camellia sinensis L. extract as a potential therapeutic for atopic dermatitis through NF-κB pathway inhibition. Sci Rep. (2025) 15:2371. doi: 10.1038/S41598-025-86678-5
69. Fisher, GJ, Kang, S, Varani, J, Bata-csorgo, Z, Wan, Y, Datta, S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. (2002) 138:1462–70. doi: 10.1001/archderm.138.11.1462
70. Maria, C, and Pidder, JD. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol. (2017) 94:78–82. doi: 10.1016/j.exger.2017.01.009
71. Hsiu, MC, Hsin, CC, Hua, HC, Chien, WC, Ssu, MW, and Kuo, CW. Neonauclea reticulata (Havil.) Merr stimulates skin regeneration after UVB exposure via ROS scavenging and modulation of the MAPK/MMPs/collagen pathway. Evid Based Complement Alternat Med. (2013) 2013:1–9. doi: 10.1155/2013/324864
72. Ji, EP, Hee, BP, Seon, WW, Jae, HJ, and Jae, KH. The protective effect of kaempferia parvifloraextract on UVB-induced skin photoaging in hairless mice. Photodermatol Photoimmunol Photomed. (2014) 30:237–45. doi: 10.1111/phpp.12097
73. Kim, J, Lee, CW, Kim, EK, Lee, SJ, Park, NH, Kim, HS, et al. Inhibition effect of gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J Ethnopharmacol. (2011) 137:427–33. doi: 10.1016/j.jep.2011.04.072
74. Liu, XY, Yuen, M, Yuen, T, Yuen, H, Wang, M, and Peng, Q. Anti-skin aging effect of sea buckthorn proanthocyanidins in D-galactose-induced aging mice. Food Sci Nutr. (2023) 12:1082–94. doi: 10.1002/FSN3.3823
75. Matchett, MD, Mackinnon, SL, Sweeney, MI, Gottschall-pass, KT, and Hurta, RAR. Inhibition of matrix metalloproteinase activity in DU145 human prostate cancer cells by flavonoids from lowbush blue-berry (Vaccinium angustifolium): possible roles for protein kinase C and mitogen-activated protein-kinase-mediated events. J Nutr Biochem. (2005) 17:117–25. doi: 10.1016/j.jnutbio.2005.05.014
76. Mantena, SK, and Katiyar, SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kB signaling in human epidermal keratinocytes. Free Radical Bio Med. (2006) 40:1603–14. doi: 10.1016/j.freeradbiomed.2005.12.032
77. Weng, CL, Chen, CC, Tsou, HH, Liu, TY, and Wang, HT. Areca nut procyanidins prevent ultraviolet light B-induced photoaging via suppression of cyclooxygenase-2 and matrix metalloproteinases in mouse skin. Drug Chem Toxicol. (2019) 45:353–9. doi: 10.1080/01480545.2019.1696813
78. Liu, ZH, Li, YQ, Song, HD, He, J, Li, G, Zheng, YY, et al. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. (2019) 10:6121–34. doi: 10.1039/C9FO00610A
79. Park, B, Hwang, E, Seo, SA, Cho, JG, Yang, JE, and Yi, TH. Eucalyptus globulus extract protects against UVB-induced photoaging by enhancing collagen synthesis via regulation of TGF-β/Smad signals and attenuation of AP-1. Arch Biochem Biophys. (2018) 637:31–9. doi: 10.1016/j.abb.2017.11.007
80. Oh, JH, Kim, J, Karadeniz, F, Kim, HR, Park, SY, Seo, YW, et al. Santamarine shows anti-photoaging properties via inhibition of MAPK/AP-1 and stimulation of TGF-β/Smad signaling in UVA-irradiated HDFs. Molecules. (2021) 26:3585. doi: 10.3390/MOLECULES26123585
81. Liu, XM, Zhang, RZ, Shi, HX, Li, XB, Li, YH, Taha, A, et al. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol Med Rep. (2018) 17:7227–37. doi: 10.3892/mmr.2018.8791
82. Sang, WP, C, J, K, J, J, W, Jun, SK, Bae, KJ, et al. Anthocyanins from black soybean seed coat prevent radiation-induced skin fibrosis by downregulating TGF-β and Smad3 expression. Arch Dermatol Res. (2018) 310:401–12. doi: 10.1007/s00403-018-1827-7
83. Li, L, Hwang, E, Ngo, HTT, Seo, SA, Lin, P, Gao, W, et al. Ribes nigrum L. prevents UVB-mediated Photoaging in human dermal fibroblasts: potential antioxidant and Antiinflammatory activity. Photochem Photobiol. (2018) 94:1032–9. doi: 10.1111/php.12938
84. Cho, JL, Allanson, M, and Reeve, VE. Hypoxia inducible factor-1α contributes to UV radiation-induced inflammation, epidermal hyperplasia and immunosuppression in mice. Photochem Photobiol Sci. (2012) 11:309–17. doi: 10.1039/C1PP05265A
85. Chiu, LY, Wu, NL, Huang, CF, Péter, B, Dai, YS, and Lin, WW. PARP-1 involves in UVB-induced inflammatory response in keratinocytes and skin injury via regulation of ROS-dependent EGFR transactivation and p38 signaling. FASEB J. (2021) 35:e21393. doi: 10.1096/fj.202002285RR
86. Martinez, AY, Amezcua, GE, Davila, RJ, Sandoval, RA, Galicia, MM, Almeida, LM, et al. Pirfenidone protects from UVB-induced photodamage in hairless mice. Molecules. (2023) 28:2929. doi: 10.3390/MOLECULES28072929
87. Yeon, JC, U, Y, Ji, YP, Seong, JK, So, RK, Hee, WL, et al. MHY884, a newly synthesized tyrosinase inhibitor, suppresses UVB-induced activation of NF-κB signaling pathway through the downregulation of oxidative stress. Bioorg Med Chem Lett. (2014) 24:1344–8. doi: 10.1016/j.bmcl.2014.01.040
88. Palungwachira, P, Tancharoen, S, Phruksaniyom, C, Klungsaeng, S, Srichan, R, Kikuchi, K, et al. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxidative Med Cell Longev. (2019) 2019:2089817. doi: 10.1155/2019/2089817
89. Sharma, SD, and Katiyar, SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. (2010) 27:1092–10102. doi: 10.1007/s11095-010-0050-9
90. Nobile, V, Dudonné, S, Kern, C, Roveda, G, and Garcia, C. Antiaging, brightening, and antioxidant efficacy of fermented bilberry extract (vaccinium myrtillus): a randomized, double-blind, placebo-controlled trial. Nutrients. (2024) 16:2203. doi: 10.3390/NU16142203
91. Solano, F. Photoprotection and skin pigmentation: melanin-related molecules and some other new agents obtained from natural sources. Molecules. (2020) 25:1537. doi: 10.3390/molecules25071537
92. de Gruijl, FR. Uv adaptation: pigmentation and protection against overexposure. Exp Dermatol. (2017) 26:557–62. doi: 10.1111/exd.13332
93. Yardman-frank, JM, and Fisher, DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol. (2020) 30:560–71. doi: 10.1111/EXD.14260
94. Thanigaimalai, P, Manoj, M, and J, S-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. (2017) 40:99–115. doi: 10.1016/j.cellsig.2017.09.004
95. Kim, SH, Lee, J, Jung, J, Kim, GH, Yun, CY, Jung, SH, et al. Interruption of p38 MAPK -MSK1-CREB-MITF-M pathway to prevent hyperpigmentation in the skin. Int J Biol Sci. (2024) 20:1688–704. doi: 10.7150/IJBS.93120
96. Yuan, C, Ren, HT, Hu, KX, Chen, LL, Yue, K, He, KM, et al. Effect of proanthocyanidins on cognitive improvement in thyroxin-induced aging mice. Food Funct. (2025) 16:207–18. doi: 10.1039/D4FO03987D
97. Saive, M, Genva, M, Istasse, T, Frederich, M, Maes, C, and Fauconnier, ML. Identification of a Proanthocyanidin from Litchi Chinensis Sonn. Root with anti-Tyrosinase and antioxidant activity. Biomolecules. (2020) 10:1347. doi: 10.3390/biom10091347
98. Shoji, T, Masumoto, S, Moriichi, N, Ohtake, Y, and Kanda, T. Administration of Ap-ple polyphenol supplements for skin conditions in healthy women: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2020) 12:1071–1. doi: 10.3390/nu12041071
99. Tsuchiya, T, Fukui, Y, Izumi, R, Numano, K, and Zeida, M. Effects of oligomeric proanthocyanidins (OPCs) of red wine to improve skin whitening and moisturizing in healthy women - a placebo-controlled randomized double-blind parallel group comparative study. Eur Rev Med Pharmacol Sci. (2020) 24:1571–84. doi: 10.26355/eurrev_202002_20215
100. Özdemіr, E, and Öksüz, L. Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis. Arch Microbiol. (2024) 206:410. doi: 10.1007/S00203-024-04134-W
101. Leśniak, W. Dynamics and epigenetics of the epidermal differentiation complex. Epigenomes. (2024) 8:9. doi: 10.3390/EPIGENOMES8010009
102. Zhang, M, Xing, JY, Zhong, YJ, Zhang, TT, Liu, XL, and Xing, DM. Advanced function, design and application of skin substitutes for skin regeneration. Mater Today Bio. (2024) 24:100918. doi: 10.1016/j.mtbio.2023.100918
103. Ghosh, D, and Konishi, T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. (2007) 16:200–8. doi: 10.6133/apjcn.2007.16.2.01
104. Zhang, N, and Jing, P. Anthocyanins in Brassicaceae: composition, stability, bioavailability, and potential health benefits. Crit Rev Food Sci Nutr. (2020) 62:2205–20. doi: 10.1080/10408398.2020.1852170
105. Jiao, G, Peng, XY, Chen, JP, Li, J, and Guo, XL. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocolloid. (2018) 74:23–31. doi: 10.1016/j.foodhyd.2017.07.029
106. Jiao, G, Xue, YY, Shuo, W, Jin, PC, Jin, L, Yue, S, et al. Nanocomplexes composed of chitosan derivatives and β-lactoglobulin as a carrier for anthocyanins: preparation, stability and bioavailability in vitro. Food Res Int. (2019) 116:336–45. doi: 10.1016/j.foodres.2018.08.045
107. Ewelina, P, and Marcin, AK. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for micro-encapsulation of anthocyanins from chokeberry. Int J Biol Macromol. (2019) 129:665–71. doi: 10.1016/j.ijbiomac.2019.02.073
108. Ahmed, KR, Naymul, K, Yang, X, Jia, HX, Hao, XC, Meng, RM, et al. Potential micro−/nano-encapsulation systems for improving stability and bioavailability of anthocyanins: an updated review. Crit Rev Food Sci. (2021) 63:21–4. doi: 10.1080/10408398.2021.1987858
109. Zhang, SH, Jiang, ZY, Wang, XL, Yang, C, and Shi, JF. Facile method to prepare microcapsules inspired by polyphenol chemistry for efficient enzyme immobilization. ACS Appl Mater Interfaces. (2015) 7:19570–8. doi: 10.1021/acsami.5b03823
110. Tarone, AG, Cazarin, CBB, and Junior, MRM. Anthocyanins: new techniques and challenges in microencapsulation. Food Res Int. (2020) 133:109092. doi: 10.1016/j.foodres.2020.109092
111. Salerno, A, Causa, F, Natale, CD, Domingo, C, and Vecchione, R. Editorial: microencapsulation for biomedical applications. Front Bioeng Biotechnol. (2022) 10:891981. doi: 10.3389/FBIOE.2022.891981
112. Timilsena, YP, Akanbi, TOKhalid, N, Adhikari, B, and Barrow, CJ. Complex coacervation: principles, mechanisms and applications in microencapsulation. Int J Biol Macromol. (2018) 121:1276–86. doi: 10.1016/j.ijbiomac.2018.10.144
113. Cheng, YY, Liu, JY, Li, L, Ren, JL, Lu, J, and Luo, FJ. Advances in embedding techniques of anthocyanins: improving stability, bioactivity and bioavailability. Food Chem X. (2023) 20:100983–3. doi: 10.1016/J.FOCHX.2023.100983
114. Ghorbanzade, T, Jafari, SM, Akhavan, S, and Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. (2016) 216:146–52. doi: 10.1016/j.foodchem.2016.08.022
115. Rafiee, Z, Barzegar, M, Sahari, MA, and Maherani, B. Nanoliposomal carriers for improvement the bioavailability of high - valued phenolic compounds of pistachio green hull extract. Food Chem. (2016) 220:115–22. doi: 10.1016/j.foodchem.2016.09.207
116. Liu, ZH, Jiao, YP, Wang, YF, Zhou, CR, and Zhang, ZY. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. (2008) 60:1650–62. doi: 10.1016/j.addr.2008.09.001
117. Jung, T, Kamm, W, Breitenbach, A, Kaiserling, E, Xiao, JX, and Kissel, T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. (2000) 50:147–60. doi: 10.1016/S0939-6411(00)00084-9
118. Balkrishna, A, Bhattacharya, K, Samanta, HS, Tomer, M, and Varshney, A. Advancements in nano-mandoor bhasma: unravelling the particle size-ascorbic acid synergy for enhanced iron bioavailability for anemia treatment. Biol Trace Elem Res. (2024) 203:2320–38. doi: 10.1007/s12011-024-04304-3
119. Ha, ES, Park, H, Jeong, JS, Lee, SK, Kang, HT, Baek, IH, et al. Effect of process parameters on nano-microparticle formation during supercritical antisolvent process using mixed sol-vent: application for enhanced dissolution and oral bioavailability of telmisartan through particle-size control based on experimental design. Pharmaceutics. (2024) 16:1508. doi: 10.3390/PHARMACEUTICS16121508
120. Herrera-rocha, KM, Manjarrez-juanes, MM, Larrosa, M, Barrios-payán, JA, Rocha-guzmán, NE, and Macías-salas, A. The synergistic effect of quince fruit and probiotics (lactobacillus and bifidobacterium) on reducing oxidative stress and inflammation at the intestinal level and improving athletic performance during endurance exercise. Nutrients. (2023) 15:4764. doi: 10.3390/NU15224764
121. Fernandes, A, Mateus, N, and Freitas, VD. Polyphenol-dietary fiber conjugates from fruits and vegetables: nature and biological fate in a food and nutrition perspective. Foods. (2023) 12:1052. doi: 10.3390/FOODS12051052
122. Li, P, Chen, JJ, Guo, CE, Li, WD, and Gao, ZL. Lactobacillus cofermentation of cerasus humilis juice alters chemical properties, enhances antioxidant activity, and improves gut microbiota. Food Funct. (2023) 14:8248–60. doi: 10.1039/D3FO02583G
123. Busse-valverde, M, Gómez-plaza, E, López-roca, JM, Gil-muñoz, R, and Bautista-ortín, AB. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J Agric Food Chem. (2011) 59:5450–5. doi: 10.1021/jf2002188
124. Oldoni, TLC, Melo, PS, Massarioli, AP, Moreno, IAM, Bezerra, RMN, Rosalen, PL, et al. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. (2015) 192:306–12. doi: 10.1016/j.foodchem.2015.07.004
125. Xu, XY, Xie, HH, Wang, YF, and Wei, XY. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J Agric Food Chem. (2010) 58:11667–72. doi: 10.1021/jf1033202
126. Ito, C, Oki, T, Yoshida, T, Nanba, F, Yamada, K, and Toda, T. Characterisation of proanthocyanidins from black soybeans: isolation and characterisation of proanthocyanidin oligomers from black soybean seed coats. Food Chem. (2013) 143:2507–12. doi: 10.1016/j.foodchem.(2013).05.039
127. Li, CY, Xu, YY, Wu, HM, Zhao, RR, Wang, XW, Wang, FF, et al. Flavor characterization of native Xinjiang flat peaches based on constructing aroma fingerprinting and stoichiometry analysis. Foods. (2023) 12:2554. doi: 10.3390/foods12132554
128. Blanch, M, Alvarez, I, Sanchez-ballesta, MT, Escribano, MI, and Merodio, C. Increasing catechin and procyanindin accumulation in high-CO2-treated Fragaria vesca strawberries. J Agric Food Chem. (2012) 60:7489–96. doi: 10.1021/jf301547t
129. Esatbeyoglu, T, Juadjur, A, Wray, V, and Winterhalter, P. Semisynthetic preparation and isolation of dimeric procyanidins B1-B8 from roasted hazelnut skins (Corylus avellana L.) on a large scale using countercurrent chromatography. J Agric Food Chem. (2014) 62:7101–10. doi: 10.1021/jf501312a
130. Navarro, M, Moreira, I, Arnaez, E, Quesada, S, Azofeifa, G, Alvarado, D, et al. Proanthocyanidin characterization, antioxidant and cytotoxic activities of three plants commonly used in traditional medicine in Costa Rica: petiveria alliaceae L., phyllanthus niruri L. and senna reticulata Willd. Plants (Basel, Switz). (2017) 6:50. doi: 10.3390/plants6040050
131. Fan, YF, Hussain, S, Wang, XS, Yang, M, Zhong, XJ, Tao, L, et al. Metabolomic and transcriptomic analyses of flavonoid biosynthesis in different colors of soybean seed coats. Int J Mol Sci. (2024) 26:294–4. doi: 10.3390/IJMS26010294
132. Li, YF, Zhang, H, Zhao, Y, Lv, HX, and Liu, KL. Encapsulation and characterization of proanthocyanidin microcapsules by sodium alginate and carboxymethyl cellulose. Foods. (2024) 13:740. doi: 10.3390/FOODS13050740
133. Yourdkhani, M, Leme-kraus, AA, Aydin, B, Bedran-russo, AK, and White, SR. Encapsulation of grape seed extract in polylactide microcapsules for sustained bioactivity and time-dependent release in dental material applications. Dent Mater. (2017) 33:630–6. doi: 10.1016/j.dental.2017.03.009
134. Chen, J, Fang, WJ, Liu, W, Liu, JH, and Gong, P. Microcapsules and nanoliposomes based strategies to improve the stability of blueberry anthocyanins. Molecules. (2023) 28:7344. doi: 10.3390/MOLECULES28217344
135. Sun, LB, Wang, H, Du, J, Wang, T, and Yu, DY. Ultrasonic-assisted extraction of grape seed procya-nidins, preparation of liposomes, and evaluation of their antioxidant capacity. Ultrason Sonochem. (2024) 105:106856. doi: 10.1016/j.ultsonch.2024.106856
136. Chen, YS, Huang, FH, Mcclements, DJ, Xie, BJ, Sun, ZD, and Deng, QC. Oligomeric procyanidin nanoliposomes prevent melanogenesis and UV radiation-induced skin epithelial cell (HFF-1) damage. Molecules. (2020) 25:1458. doi: 10.3390/molecules25061458
137. Sun, XB, Wu, XJ, Chen, XY, Guo, R, Kou, YX, Li, XJ, et al. Casein-maltodextrin Maillard conjugates encapsulation enhances the antioxidative potential of proanthocyanidins: an in vitro and in vivo evaluation. Food Chem. (2020) 346:128952. doi: 10.1016/j.foodchem.2020.128952
138. Lv, JM, Ismail, BB, Ye, XQ, Zhang, XY, Gu, Y, and Chen, JC. Ultrasonic-assisted nano-encapsulation of kiwi leaves proanthocyanidins in liposome delivery system for enhanced biostability and bioavailability. Food Chem. (2023) 416:135794. doi: 10.1016/J.FOODCHEM.2023.135794
139. Maslizan, M, Haris, MS, Ajat, M, Jamil, SNAM, Azhar, SC, Zahid, NI, et al. Non-lamellar lyotropic liquid crystalline nanoparticles as nanocarriers for enhanced drug encapsulation of atorvastatin calcium and proanthocyanidins. Chem Phys Lipids. (2024) 260:105377. doi: 10.1016/J.CHEMPHYSLIP.2024.105377
140. Alfaro, VE, Esquivel, AD, Madrigal, CS, Krueger, CG, and Reed, JD. Proanthocyanidin-chitosan composite nanoparticles prevent bacterial invasion and colonization of gut epithelial cells by extra-intestinal pathogenic Escherichia coli. Int J Biol Macromol. (2019) 135:630–6. doi: 10.1016/j.ijbiomac.2019.04.170
Keywords: proanthocyanidins (PCs), oxidative stress, collagen and elastin, inflammatory response, skin pigmentation
Citation: Ye H, Sun J, He L, Ai C, Jin W and Abd El-Aty AM (2025) Beneficial effects of proanthocyanidins on skin aging: a review. Front. Nutr. 12:1650328. doi: 10.3389/fnut.2025.1650328
Edited by:
Karolina Wojtunik-Kulesza, Medical University of Lublin, PolandReviewed by:
Sara Luz Morales-Lázaro, National Autonomous University of Mexico, MexicoRogério Saad Vaz, Federal University of Paraná, Brazil
Anna Oniszczuk, Doctoral School of Medical University of Lublin, Poland
Copyright © 2025 Ye, Sun, He, Ai, Jin and Abd El-Aty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin He, aGVsaW5saW4zMTJAMTYzLmNvbQ==; Wengang Jin, amlud2VuZ2FuZ0Bud2FmdS5lZHUuY24=
 Hua Ye1,2
Hua Ye1,2 Jiaqiang Sun
Jiaqiang Sun Linlin He
Linlin He Wengang Jin
Wengang Jin