- 1Cadre Health Care Office, Central Hospital of Zibo, Zibo, Shandong, China
- 2Department of Otolaryngology, Central Hospital of Zibo, Zibo, Shandong, China
- 3Department of Internal Medicine, Zibo Maternal and Child Health Hospital, Zibo, Shandong, China
1 Introduction
The global metabolic disorder, type 2 diabetes mellitus (T2DM), significantly increases the risk of cognitive dysfunction, including Alzheimer's disease (AD) and mild cognitive impairment (MCI). Evidence shows that there is the same pathophysiological mechanism between T2DM and neurodegeneration (1–4). The Mediterranean diet (MedDiet)—a dietary pattern rich in fruits, vegetables, whole grains, nuts, fish, and olive oil, with low saturated fat intake—may improve cognitive dysfunction of T2DM. However, challenges such as the lack of a unified MedDiet definition and compliance standards make translational research difficult to operate. This opinion article summarizes the current understanding of the pathological mechanisms that link T2DM with cognitive dysfunction and evaluates the protective effects of the MedDiet. The aim is to inform therapeutic strategies that could delay or prevent cognitive decline associated with T2DM. Additionally, it analyzes the potential challenges related to implementing the MedDiet in the context of T2DM-associated cognitive impairment.
2 Pathophysiological links between type 2 diabetes mellitus and cognitive dysfunction
2.1 Insulin resistance and brain insulin signaling
Peripheral insulin resistance (IR) in T2DM disrupts central insulin signaling through various mechanisms, leading to neurodegeneration. Hyperinsulinemia, a characteristic feature of T2DM, reduces insulin passing through the blood–brain barrier (BBB) by downregulating insulin receptors (5). This process diminishes insulin availability in critical brain regions, such as the hippocampus and cortex (6). Decreased insulin signaling impedes the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, which is crucial for neuronal survival, synaptic plasticity, and glucose metabolism (7). Inhibition of PI3K/AKT activity triggers the activation of glycogen synthase kinase-3β (GSK-3β), resulting in tau hyperphosphorylation and the formation of neurofibrillary tangles—a pathological marker of AD (8, 9). Meanwhile, the decrease in insulin-degrading enzyme (IDE) activity due to IR can diminish amyloid-β (Aβ) clearance, accelerating the deposition of Aβ plaques (10). Aβ aggregates further exacerbate insulin resistance by binding to neuronal insulin receptors and activating pro-inflammatory pathways, forming a vicious cycle.
Chronic peripheral insulin resistance can also induce neuroinflammation by causing systemic metabolic disorders. Elevated levels of free fatty acids and cytokines, such as TNF-α and IL-6, activate microglia and astrocytes, causing oxidative stress and permanent synaptic damage (11). Inflammatory mediators disrupt the integrity of the BBB, allowing peripheral inflammatory molecules to penetrate the brain and exacerbate neuronal inflammation. In addition, advanced glycation end products (AGEs) caused by hyperglycemia interact with AGE receptors on neurons, triggering NF-κB activation and further enhancing the production of Aβ and tau pathology (12–14). Neuroimaging studies have shown that the utilization rate of glucose in the brain of patients with T2DM is reduced and the hippocampus is atrophied (15), which correlates with impairments in memory and executive function. These findings highlight that peripheral insulin resistance plays an important role in the dysfunction of brain insulin signaling and AD-like changes in T2DM.
2.2 Dyslipidemia and lipid metabolism dysregulation
Dyslipidemia, also known as lipid metabolism disorders, involves imbalances in cholesterol, triglycerides, and other lipid components. These imbalances are closely linked to cardiovascular diseases, obesity, and metabolic syndrome. The process of lipid metabolism changes with age (16).
The key subtype of dyslipidemia, dysregulation of cholesterol metabolism, and the synergistic effect of the ApoE4 allele, the main genetic risk factor of AD, exacerbate AD-related pathological changes. ApoE4 reduces the efficiency of cholesterol transport in the brain, decreasing the clearance of Aβ peptides, while dysregulated cholesterol metabolism promotes the abnormal processing of the amyloid precursor protein (APP), increasing Aβ production (17, 18). The above dual mechanisms amplify Aβ deposition, while cholesterol imbalance activates kinases such as GSK-3β, driving tau hyperphosphorylation and the formation of neurofibrillary tangles (19). ApoE4 accelerates neuronal damage and further exacerbates tau pathology by disrupting microtubule stability and reducing tau degradation. In patients with T2DM, chronic hyperglycemia and IR will destroy brain lipid homeostasis and aggravate the imbalance of cholesterol metabolism. The metabolic stress associated with T2DM impairs ApoE-mediated clearance of Aβ, which synergizes with the intrinsic clearance deficiency of ApoE4 to accelerate the accumulation of Aβ. Meanwhile, hyperglycemia and abnormal lipid metabolism enhance tau phosphorylation, while ApoE4 enhances tau pathology by altering intracellular signaling. These interactions collectively elevate the dementia risk in T2DM patients carrying ApoE4 far beyond that of individuals with either condition alone.
2.3 Vascular injury and cerebral hypoperfusion
Vascular injury and cerebral hypoperfusion are critical contributors to cognitive dysfunction in patients with T2DM, with chronic hyperglycemia playing a central role in these pathological processes. Prolonged hyperglycemia directly damages vascular endothelial cells, the protective barrier between blood and vessel walls, by activating the TLR4 signaling pathway. This activation promotes the release of pro-inflammatory cytokines (e.g., IL-6 and TNF-α) and triggers oxidative stress through excessive production of reactive oxygen species (ROS) (20, 21). Endothelial damage disrupts the balance between the anticoagulant and procoagulant functions of endothelial cells, leading to platelet aggregation, lipid deposition, and the formation of atherosclerotic plaques. Consequently, vascular stenosis and stiffening occur, which reduces cerebral blood flow and impairs brain perfusion.
Cerebral blood insufficiency reduces the supply of nutrients and oxygen to neurons, particularly affecting microvascular networks that are critical for maintaining cognitive function (22). Microvascular complications, including diabetic retinopathy (a marker of cerebral microvascular injury), are strongly associated with more severe cognitive decline (23). Research has shown that extensive microvascular damage can affect synaptic plasticity and neuronal metabolism (24). Additionally, chronic hyperglycemia-induced endothelial dysfunction weakens the BBB, increasing the risk of cerebral edema, hemorrhage, and the accumulation of neurotoxic substances such as β-amyloid, which further exacerbates neuronal damage.
Beyond microvascular injury, large-vessel atherosclerosis, such as carotid artery stenosis, significantly impacts cognitive networks by altering cerebral hemodynamics. Studies have shown that the severity of carotid stenosis in patients with T2DM correlates with the extent of cognitive impairment, with severe stenosis doubling the risk of cognitive decline (25). Systemic atherosclerosis also accelerates the progression of cognitive deficits, as a decrease in blood flow in key brain areas, such as the left wedge and superior occipital gyrus, will damage visual processing, memory, and attention, which are domains critical for maintaining cognitive function. Asymptomatic cerebral infarction and microbleeds can further impair cognitive networks, particularly in the domains of attention and processing speed (26). Collectively, these vascular pathologies create a vicious cycle: hyperglycemia leads to endothelial injury and hypoperfusion, which in turn exacerbates metabolic dysregulation and accelerates cognitive decline in patients with T2DM.
3 Mechanisms of MedDiet in T2DM-related cognitive dysfunction
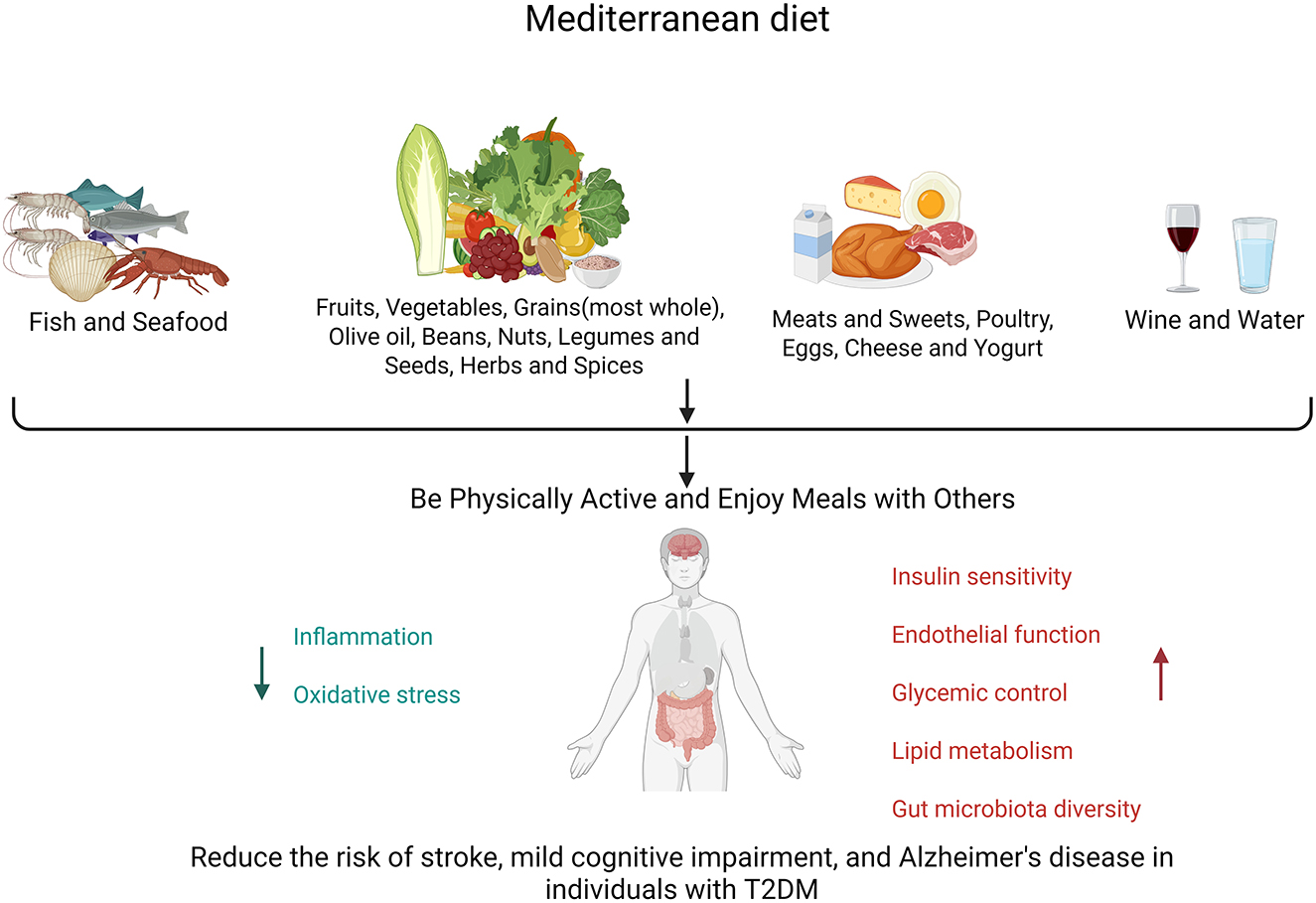
The MedDiet refers to the lifestyle of residents in the Mediterranean region, rather than a simple, strict dietary plan. Its core concepts encompass consuming more legumes, vegetables, fruits, nuts, whole grains, spices, and fish (with olive oil as a key source of fat); consuming fish or other seafood at least twice a week; moderately consuming dairy products and eggs; eating less red meat and desserts; and drinking a small amount of wine. It also encourages people to interact with each other and to savor healthy, fresh foods. This dietary pattern is low in saturated fat, accounting for ~10% of energy intake (27, 28), and is rich in various micronutrient functional components, including vitamins, carotenoids, unsaturated fatty acids, and diverse bioactive plant phenolic compounds with antioxidant and anti-inflammatory effects. These plant-derived phenolic compounds may regulate insulin action and metabolism in insulin-sensitive tissues, exerting effects that prevent or treat insulin resistance and its related diseases (29). Initially, Ancel Keys, an expert in animal physiology and biology, discovered through experiments that Italians had a lower prevalence of heart disease and coronary artery disease (30, 31). However, his views were challenged by other scholars. In response, he conducted the renowned “Seven Countries Study,” which confirmed that the Mediterranean dietary pattern was associated with a lower risk of cardiovascular events (32, 33). It is hypothesized that such effects may contribute to a reduction in the risk of dementia (34–36). Building on his research, the Mediterranean dietary pattern has gradually gained widespread recognition.
The MedDiet has been proven to better ameliorate IR in obese individuals, with its induced reductions in insulin levels and other IR markers—such as the homeostatic model assessment (HOMA) index—being early and sustained (37, 38). A meta-analysis encompassing several randomized trials, including the large-scale PREDIMED trial (34), revealed that the MedDiet, when compared to low-fat diets, decreased the risk of stroke (HR = 0.60, 95% CI = 0.45–0.80). Another observational study reported lower incidence rates of Parkinson's and AD among individuals adhering to the MedDiet (39). An observational study that utilized dietary questionnaires to evaluate and quantify dietary adherence among various population groups found that individuals with the highest adherence to the MedDiet had lower incidence rates of MCI and AD, as well as slower rates of cognitive decline (Figure 1), compared to those with poor adherence (40–43). A 4.2-year observational study in Israel found that strict adherence to a MedDiet in patients with type 2 diabetes was associated with slower cognitive decline (44). To further explore the key components of the Mediterranean dietary pattern, scholars conducted a follow-up of ~250,000 participants for 11.4 years and found that consuming 2–4 servings of fish per week and 1–2 servings of fruits per day were associated with a reduced risk of dementia (45).
The primary role of the MedDiet lies in its positive effects on glucose metabolism, including improvements in IR, enhancements in insulin clearance, and the strengthening of β-cell function (46). It counteracts cognitive decline through the antioxidant and anti-inflammatory properties of polyphenols (found in olive oil and berries) and omega-3 fatty acids (present in fish) (47–49). It stabilizes glucose levels, improves lipid profiles (reducing LDL-C while increasing HDL-C) (28), enhances endothelial function for cerebrovascular health (50), and modulates the gut–brain axis through its high fiber content, which fosters beneficial microbiota (51, 52). Cross-sectional studies indicate that high adherence is associated with improved verbal memory in diabetes patients (P = 0.043), whereas the PREDIMED trial confirmed a 30% reduction in diabetes incidence and a slowdown in cognitive decline among high-risk groups (53). When combined with aerobic exercise, it synergistically enhances glycemic control, lipid metabolism, and gut microbiota diversity. Metformin, a GLP-1 receptor agonist, and other hypoglycemic drugs may reduce cognitive dysfunction in T2DM, a finding that warrants further study.
However, the MedDiet currently lacks a standardized definition, adherence criteria, and scoring system. This significantly hampers comparisons between studies and impedes the translation of scientific research into practical recommendations for the general public (54). On the other side of the shield, despite the well-established benefits of the MedDiet, researchers acknowledge that uncertainties persist regarding its implementation in non-Mediterranean regions. Adherence to the MedDiet poses a common challenge, as it is often perceived as difficult to maintain in non-Mediterranean areas (55).
4 Conclusion
T2DM and cognitive dysfunction share common pathophysiological mechanisms. MedDiet's anti-inflammatory, antioxidant, and metabolic regulatory effects provide a promising intervention for patients with cognitive impairment of T2DM. By improving insulin sensitivity, stabilizing glucose levels, optimizing lipid metabolism, and improving cerebrovascular function, MedDiet has been proven to reduce the risk of stroke, MCI, and AD in T2DM patients. Observational studies, such as PREDIMED, have demonstrated their role in slowing cognitive decline, particularly when combined with lifestyle changes including exercise.
There are still challenges regarding MedDiet, including standardization of MedDiet and long-term adherence strategies, especially in non-Mediterranean regions. Research on MedDiet bioactive ingredients for specific pathologies may become the future research direction, and dietary interventions will be included in the personalized management of type 2 diabetes to maximize cognitive protection. In general, these efforts may bridge the gap between basic research and clinical practice and ultimately reduce the burden of cognitive dysfunction associated with T2DM.
Author contributions
CZ: Writing – original draft. QZ: Writing – review & editing. FL: Writing – review & editing. GQ: Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. (2020) 8:535–45. doi: 10.1016/S2213-8587(20)30118-2
2. Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 diabetes mellitus and Alzheimer's disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. (2018) 19:3306. doi: 10.3390/ijms19113306
3. Chen W, Huang Q, Lazdon EK, Gomes A, Wong M, Stephens E, et al. Loss of insulin signaling in astrocytes exacerbates Alzheimer-like phenotypes in a 5xFAD mouse model. Proc Natl Acad Sci USA. (2023) 120:e2220684120. doi: 10.1073/pnas.2220684120
4. Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. (2019) 55:100944. doi: 10.1016/j.arr.2019.100944
5. Kciuk M, Kruczkowska W, Gałeziewska J, Wanke K, Kałuzińska-Kołat Ż, Aleksandrowicz M, et al. Alzheimer's disease as type 3 diabetes: understanding the link and implications. Int J Mol Sci. (2024) 25:11955. doi: 10.3390/ijms252211955
6. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet. (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
7. Campbell IH, Campbell H, Smith DJ. Insulin signaling as a therapeutic mechanism of lithium in bipolar disorder. Transl Psychiatry. (2022) 12:350. doi: 10.1038/s41398-022-02122-6
8. Kitagishi Y, Nakanishi A, Ogura Y, Matsuda S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer's disease. Alzheimers Res Ther. (2014) 6:35. doi: 10.1186/alzrt265
9. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koeing AM, Wang HY, Ahima RX, et al. Brain insulin resistance in type 2 diabetes and Alzheimer's disease: concepts and conundrums. Nat Rev Neurol. (2018) 14:168–81. doi: 10.1038/nrneurol.2017.185
10. Kumari S, Dhapola R, Reddy DH. Apoptosis in Alzheimer's disease: insight into the signaling pathways and therapeutic avenues. Apoptosis. (2023) 28:943–57. doi: 10.1007/s10495-023-01848-y
11. Mruczyk K, Cisek-Wozniak A, Molska M, Skoczek-Rubińska A. The role of inflammatory markers in linking metabolic syndrome to cognitive decline in middle-aged women: a focus on TNF-α and IL-6. Metabolites. (2025) 15:186. doi: 10.3390/metabo15030186
12. Almutary AG, Begum MY, Kyada AK, Gupta S, Jyothi SR, Chaudhari K, et al. Inflammatory signaling pathways in Alzheimer's disease: mechanistic insights and possible therapeutic interventions. Ageing Res Rev. (2025) 104:102548. doi: 10.1016/j.arr.2024.102548
13. Ying C, Li Y, Wu S, Gao L, Zhu Y, Qian Y, et al. MKK3 K329 mutation attenuates diabetes-associated cognitive dysfunction by blocking the MKK3-RAGE interaction and inhibiting neuroinflammation. Aging Dis. (2024) 16:598–618. doi: 10.14336/AD.2024.0222
14. Zhang H, Wei W, Zhao M, Ma L, Jiang X, Pei H, et al. Interaction between Aβ and tau in the pathogenesis of Alzheimer's disease. Int J Biol Sci. (2021) 17:2181–92. doi: 10.7150/ijbs.57078
15. Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. (2015) 48:195–204. doi: 10.1016/j.bbi.2015.03.015
16. Shen X, Wang C, Zhou X, Zhou W, Hornburg D, Wu S, et al. Nonlinear dynamics of multi-omics profiles during human aging. Nat Aging. (2024) 4:1619–34. doi: 10.1038/s43587-024-00692-2
17. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J. (2019) 40:2813–24. doi: 10.1093/eurheartj/ehz402
18. Ehtewish H, Arredouani A, El-Agnaf O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int J Mol Sci. (2022) 23:6144. doi: 10.3390/ijms23116144
19. Zhang Y, Zhang Y, Aman Y, Ng CT, Chau WH, Zhang Z, et al. Amyloid-β toxicity modulates tau phosphorylation through the PAX6 signalling pathway. Brain. (2021) 144:2759–70. doi: 10.1093/brain/awab134
20. Yang Y, Wang Y, Wang Y, Ke T, Zhao L. PCSK9 inhibitor effectively alleviated cognitive dysfunction in a type 2 diabetes mellitus rat model. Peer J. (2024) 12:e17676. doi: 10.7717/peerj.17676
21. Xue P, Long Z, Jiang G, Wang LP, Bian C, Wang Y, et al. The role of LRP1 in Aβ efflux transport across the blood-brain barrier and cognitive dysfunction in diabetes mellitus. Neurochem Int. (2022) 160:105417. doi: 10.1016/j.neuint.2022.105417
22. Poh L, Fann DY, Wong P, Lim HM, Foo SL, Kang SW, et al. AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia. Mol Psychiatry. (2021) 26:4544–60. doi: 10.1038/s41380-020-00971-5
23. Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. (2017) 57:89–107. doi: 10.1016/j.preteyeres.2017.01.001
24. Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. (2008) 371:1800–9. doi: 10.1016/S0140-6736(08)60768-0
25. Gray VL, Goldberg AP, Rogers MW, Anthony L, Terrin ML, Guralnik JM, et al. Asymptomatic carotid stenosis is associated with mobility and cognitive dysfunction and heightens falls in older adults. J Vasc Surg. (2020) 71:1930–7. doi: 10.1016/j.jvs.2019.09.020
26. Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, et al. Arterial stiffness and dementia pathology: atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology. (2018) 90:e1248–56. doi: 10.1212/WNL.0000000000005259
27. Diolintzi A, Panagiotakos DB, Sidossis LS. From Mediterranean diet to Mediterranean lifestyle: a narrative review. Public Health Nutr. (2019) 22:2703–13. doi: 10.1017/S1368980019000612
28. Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
29. Mirabelli M, Chiefari E, Arcidiacono B, Corigliano DM, Brunetti FS Maggisano V, et al. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients. (2020) 12:1066. doi: 10.3390/nu12041066
30. Menotti A, Puddu PE. How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: a 50-year journey. Nutr Metab Cardiovasc Dis. (2015) 25:245–52. doi: 10.1016/j.numecd.2014.12.001
31. Wright CM. Biographical notes on Ancel Keys and Salim Yusuf: origins and significance of the seven countries study and the INTERHEART study. J Clin Lipidol. (2011) 5:434–40. doi: 10.1016/j.jacl.2011.09.003
32. Aboul-Enein BH, Puddy WC, Bernstein J. Ancel Benjamin Keys (1904-2004): His early works and the legacy of the modern Mediterranean diet. J Med Biogr. (2020) 28:139–47. doi: 10.1177/0967772017727696
33. Blackburn H. Invited commentary: 30-year perspective on the seven countries study. Am J Epidemiol. (2017) 185:1143–7. doi: 10.1093/aje/kwx071
34. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
35. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. (2018) 378:2441–2. doi: 10.1056/NEJMoa1200303
36. Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. (2015) 128:229–38. doi: 10.1016/j.amjmed.2014.10.014
37. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkov S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet [published correction appears in N Engl J Med. (2009) 361:2681]. N Engl J Med. (2008) 359:229–41. doi: 10.1056/NEJMoa0708681
38. Greco M, Chiefari E, Montalcini T, Accattato F, Costanzo FS, Pujia A, et al. Early effects of a hypocaloric, Mediterranean diet on laboratory parameters in obese individuals. Mediators Inflamm. (2014) 2014:750860. doi: 10.1155/2014/750860
39. Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. (2008) 337:a1344. doi: 10.1136/bmj.a1344
40. Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer's disease. JAMA. (2009) 302:627–37. doi: 10.1001/jama.2009.1144
41. Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. (2013) 74:580–91. doi: 10.1002/ana.23944
42. Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer's disease risk: a protective diet. Arch Neurol. (2010) 67:699–706. doi: 10.1001/archneurol.2010.84
43. Nucci D, Sommariva A, Degoni LM, Gallo G, Mancarella M, Natarelli F, et al. Association between Mediterranean diet and dementia and Alzheimer's disease: a systematic review with meta-analysis. Aging Clin Exp Res. (2024) 36:77. doi: 10.1007/s40520-024-02718-6
44. Lotan R, Ravona-Springer R, Shakked J, Lin HM, Ouyang Y, Shahar DR, et al. Greater intake of the MEDI diet is associated with better cognitive trajectory in older adults with type 2 diabetes. Diabetes Res Clin Pract. (2022) 190:109989. doi: 10.1016/j.diabres.2022.109989
45. Dobreva I, Marston L, Mukadam N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience. (2022) 44:2541–54. doi: 10.1007/s11357-022-00615-2
46. Tricò D, Moriconi D, Berta R, Baldi S, Quinones-Galvan A, Guiducci L, et al. Effects of low-carbohydrate versus Mediterranean diets on weight loss, glucose metabolism, insulin kinetics and β-cell function in morbidly obese individuals. Nutrients. (2021) 13:1345. doi: 10.3390/nu13041345
47. Filippatos TD, Panagiotakos DB, Georgousopoulou EN, Pitaraki E, Kouli GM, Chrysohoou C, et al. Mediterranean diet and 10-year (2002-2012) incidence of diabetes and cardiovascular disease in participants with prediabetes: the ATTICA study. Rev Diabet Stud. (2016) 13:226–35. doi: 10.1900/RDS.2016.13.226
48. Kastorini CM, Panagiotakos DB, Chrysohoou C, Georgousopoulou E, Georgousopoulou E, Pitaraki E, et al. Metabolic syndrome, adherence to the Mediterranean diet and 10-year cardiovascular disease incidence: the ATTICA study. Atherosclerosis. (2016) 246:87–93. doi: 10.1016/j.atherosclerosis.2015.12.025
49. Shen J, Wilmot KA, Ghasemzadeh N, Molloy DL, Burkman G, Mekonnen G, et al. Mediterranean dietary patterns and cardiovascular health. Annu Rev Nutr. (2015) 35:425–49. doi: 10.1146/annurev-nutr-011215-025104
50. Hoscheidt S, Sanderlin AH, Baker LD, Jung Y, Kockhart S, Kellar D, et al. Mediterranean and Western diet effects on Alzheimer's disease biomarkers, cerebral perfusion, and cognition in mid-life: a randomized trial. Alzheimers Dement. (2022) 18:457–68. doi: 10.1002/alz.12421
51. Solch RJ, Aigbogun JO, Voyiadjis AG, Talkington GM, Darensbourg RM, O'Connell S, et al. Mediterranean diet adherence, gut microbiota, and Alzheimer's or Parkinson's disease risk: a systematic review. J Neurol Sci. (2022) 434:120166. doi: 10.1016/j.jns.2022.120166
52. Calabrese FM, Disciglio V, Franco I, Sorino P, Bonfiglio C, Bianco A, et al. A low glycemic index Mediterranean diet combined with aerobic physical activity rearranges the gut microbiota signature in NAFLD patients. Nutrients. (2022) 14:1773. doi: 10.3390/nu14091773
53. Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. (2013) 84:1318–25. doi: 10.1136/jnnp-2012-304792
54. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. (2019) 124:779–98. doi: 10.1161/CIRCRESAHA.118.313348
Keywords: Mediterranean diet, type 2 diabetes mellitus, cognitive dysfunction, insulin resistance, chronic hyperglycemia, dyslipidemia
Citation: Zheng C, Zhang Q, Liu F and Qiu G (2025) The role of the Mediterranean diet in the treatment of cognitive dysfunction in patients with type 2 diabetes mellitus. Front. Nutr. 12:1654684. doi: 10.3389/fnut.2025.1654684
Received: 26 June 2025; Accepted: 25 July 2025;
Published: 14 August 2025.
Edited by:
Qingqing Yin, Shandong Provincial Hospital Affiliated to Shandong First Medical University, ChinaReviewed by:
Yu Bin, Mianyang Central Hospital, ChinaJingwen Zhang, Affiliated Hospital of Weifang Medical University, China
Copyright © 2025 Zheng, Zhang, Liu and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guihong Qiu, enh5eWdiYkBzaW5hLmNvbQ==
 Chunyan Zheng1
Chunyan Zheng1 Guihong Qiu
Guihong Qiu