Abstract
Background:
Arsenic (As), a class I carcinogen, affected 200 million people globally either through consumption of contaminated groundwater or food crops especially rice, leading to acute or chronic health issues including fatigue, respiratory diseases, liver fibrosis, and cancer.
Research gap:
For reclamation, majority of the efforts focused on single application of a particular amendment in reducing As levels in rice ecosystems.
Methodology:
This particular article comprehensively studied package of those amendments being used in reducing the bioaccumulation of As.
Results:
Consortia based package involving Si-rich agro-wastes (intact waste, compost, ash etc.) and agriculturally important microbes have the potential to reduce translocation of As to the above ground biomass by various mechanisms viz., competitive inhibition of transporters, iron plaque formation, anti-oxidant defense system, microbial oxidation etc. Rice straw compost (RSC) and husk composts (RHC) which are rich sources of Si (7–10%), Fe (700–900 ppm), Zn (40–60 ppm) and P (0.35–0.5%) have been explored owing the ability of Si and P to hinder the uptake of highly toxic As (III) and As (V) within plants by competitively inhibiting LSi1 and LSi2 for Si, and Pht4 and Pht8 transporters for P uptake with additional Fe released from amendments can form Fe-plaques that might work like As filters. Agro-wastes combined with silicate solubilizing bacteria significantly reduced As loading in final produce (25–52%), thereby reducing dietary exposure (ADI) even up to one third compared to control.
Conclusion:
This comprehensive review on understanding and validation of the mechanism provides a valuable insight in formulating a feasible As toxicity management strategy.
1 Introduction
Generation of agro-wastes is a ground reality by default with the extensive growth of agricultural productivity. The world population has been increased from 2.49 billion in 1950 to 8.19 billion in 2025. It is predicted to reach 9 billion by 2050 and to 11 billion by 2,100, respectively (1). Therefore, future food security poses a significant issue. There has been a dramatic increase in crop and livestock production to meet the intensive demands of a growing population, which has led to the formation of agro-wastes (2). Rapid population growth, economic prosperity, and an increase in agro-wastes production capacities have all been witnessed in Africa, China, and India within the past century (1). India produces around 850 Mt of agro-wastes annually which makes it the second largest producer of agro-wastes after China. Among the total agro-wastes generated by India larger portion are coming from paddy straw (130 Mt) (3). India's food grain production rose by 6% to a record 353.2 million ton (Mt) in the 2024–25 crop year (July–June) compared to previous year because of a significant rise in rice, wheat, pulses and oilseed output. This huge amount of production results in huge amount of waste materials. Additionally, there are public health concerns regarding the air pollution caused by the practice of burning rice residue, often known as parali (4). Greenhouse gases (GHGs) such as carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) are produced when agricultural residue is not disposed of properly and are harmful to both humans and the environment (5). Whether it is waste material or a huge resource that is the main concern. The effective utilization of this vast volume of agricultural waste as a resource rather than a liability holds immense potential for advancing sustainable agriculture and contributing to societal wellbeing. Utilization or conversion of this huge resource is a tremendous challenge with a resultant impairment of natural resources due to unsustainable practices. The substantial generation of agro-wastes facilitates the reduction of heavy metal contamination in plants due to its intrinsic makeup. This review concentrates on arsenic among all heavy metals. Agro-wastes, particularly rice straw, rice husk, maize cob and sugarcane bagasse, possess a substantial amount of silicon. Utilizing this concentrated silica through the incorporation of agro-wastes into the soil might diminish arsenic bioaccumulation and enhance plant resilience against diverse biotic and abiotic stressors.
Several management strategies were proposed to maintain soil As bioavailability and grain As content below the recommended limits (6) as arsenic has impacted 200 million individuals worldwide from the ingestion of contaminated groundwater or food crops, particularly rice (7), resulting in acute or chronic health complications such as weakness, respiratory ailments, liver fibrosis, and cancer (8–11). However, majority of the efforts focused on single application of a particular amendment. Among different physical, chemical and biological remediation options implied, application of silicon (Si) emerged as a potential strategy in reducing the As load in grains (12, 13). Si and As(III) share the same transporters (Lsi1 and Lsi2) for their uptake and movement within the plant. Si can competitively inhibit the transporters and reduce the uptake of As. Application of Si can enhance the iron plaque formation and also reduce the conversion of short-range order ferrihydrite to goethite or siderite or other crystalline compounds of iron oxides or hydroxides present in Fe-plaque. Application of Si facilitate rhizosphere oxygenation by enhancing the radial oxygen loss which in turn induces microbial oxidation of Fe2+ to Fe3+ leads to more formation of Fe-plaque around the roots (14). This Fe-plaque has the potential of trapping As by adsorption or co-precipitation mechanism (15). A lot of studies described that application of inorganic silica sources (CaSiO3, NaSiO3, Si nano-particles etc.) reduced mobility of As from soil to plant. But exploring Si-based agro-wastes as a potential source of Si is rare and not exclusively studied. Moreover, these wastes are a rich source of iron, zinc, carbon, cellulose, lignin, and various inorganic or organic chemicals. These compounds play a specific role in restricting arsenic absorption.
Co-application of these Si-rich agro-wastes with silicate solubilizing microbes (SSM) can open a new path in reducing the As loading in final produce, thereby reducing dietary exposure up to one third compared to control. Si is abundantly available in earth crust (27.06% by weight) but often insufficiently available for crops, as plants generally uptake Si as monosilicic acid (H4SiO4). Higher plants especially rice removes Si rapidly, requires its supplementation. Although aqua soluble silica fertilizers like CaSiO3, NaSiO3, Si nano-particles provides large amount of Si, it can present a cost challenge for conventional agricultural practices. There is rare occurrence of negative effect of Si-fertilizer application (16). In light of high cost of inorganic Si-fertilizers, there is a much need of thinking viable, sustainable alternative strategies to address the issue of remediating As bioaccumulation. Application of Si-rich agro-wastes already resulted in a reduction of 20–40% of As concentration in rice grains (10). The potential of resistant SSM presents a practical, ecological, sustainable, and economical method to increase Si availability for crops by affecting the complex process of Si cycling. The solubilization of silica has been enhanced by SSM by many methods, including the formation of organic and inorganic acids, extracellular polysaccharides, ligands, or via nucleophilic assault. The microorganisms facilitate the solubilization of potassium (K) and Si, rendering them a viable alternative for bio-fertilization and potentially reducing reliance on synthetic fertilizers. The function of As-resistant silicate solubilizing bacteria (SSB) in reducing As uptake by rice necessitates further exploration, despite a rather comprehensive understanding of the role of bacteria associated with rice in the solubilization of silicate minerals (17, 18). New insights into the complexities of As absorption, dispersion, and the potential impact of Si highlight the importance of this characteristic. The dual influence of Si on As accumulation in rice may be amplified by As-resistant SSB, according to recent results. Implementing SSB-inoculum into simple hydroponic systems reduced As uptake by rice plants. This was achieved by increasing the availability of Si and encouraging root-based competition between As and Si for aquaporin transporters (19). Research conducted by Bist et al. (20) concluded that the silicate-solubilizing Bacillus amyloliquefaciens effectively reduced As levels in rice grains.
This review paper examines the integration of SSM and Si-rich agro-wastes to evaluate their dual efficacy in mitigating As levels in the final product. It highlights the potential of SSM and Si-rich agro-wastes, either individually or in conjunction, as a cost-effective and environmentally sustainable alternative to commercially available Si fertilizers. This comprehensive review on understanding and validation of the mechanism provides a valuable insight in formulating a feasible As toxicity management strategy.
2 Arsenic contamination and food security
Arsenic, a toxic metalloid naturally present in the Earth's crust, has become an increasingly significant threat to agriculture due to anthropogenic sources such as the use of As pesticides, mining activities, and irrigation with As-contaminated groundwater (21). One of the most critical pathways through which As impacts human health indirectly is by altering the nutritional quality of crops (22). This degradation begins at the soil-root interface, where As disrupts nutrient uptake, mobility, and assimilation, leading to deficiencies in essential macro- and micro-nutrients in edible plant parts. This section reviews in detail how As interferes with nutrient acquisition and the resulting effects on crop nutritional profiles.
2.1 Arsenic speciation and its interaction with nutrient pathway into plants
Arsenic exists primarily in two inorganic forms in the soil: arsenate (As5+) and arsenite (As3+), with methylated organic forms like monomethylarsonic acid and dimethylarsinic acid found to a lesser extent (23). In aerobic soils, arsenate (As5+) predominates and structurally and chemically mimics phosphate (), allowing it to compete for absorption via phosphate transporters in root cells (PHT1 family). This phosphate pathway mimicry leads to a physiological phosphorus deficiency even in P-sufficient soils (24). Under anaerobic circumstances, such as inundated paddy fields, As3+ emerges as the predominant species and infiltrates plant roots via nodulin 26-like intrinsic protein aquaporin channels (25). This absorption pathway indicates that As directly disrupts the transport and bioavailability of key nutrients, starting with phosphorus and extending to others via various indirect and regulatory processes (Table 1).
Table 1
| Nutrient interaction | Mechanism of As interaction with the nutrient | Implication on plant health | Implication on human health | References |
|---|---|---|---|---|
| Nitrogen (N) | Arsenic suppresses nitrate transporters (notably NRT1.1 and NRT2.1 in cereals), decreases nitrate reductase and nitrite reductase activities by altering their gene expression and promoting reactive oxygen species (ROS) accumulation and interferes with ammonium incorporation into amino acids. Furthermore, As interferes with the incorporation of ammonium into amino acids via glutamine synthetase and glutamate synthase, leading to reduced pools of glutamine and glutamate—precursors for the biosynthesis of all other amino acids. | Reduced concentrations of total nitrogen, free amino acids, and protein in consumable tissues, impaired amino acid and protein biosynthesis. | Populations consuming these crops may experience reduced dietary protein intake leading to protein malnutrition, impaired growth, weakened immunity, and lowered nutritional status. | (124, 255–257) |
| Phosphorus (P) | In aerobic soils, arsenate (As5+) predominates and structurally and chemically mimics phosphate (−), and competes for phosphate transporters (PHT1 family); disrupts ATP formation by substituting phosphate, creating unstable ADP-As intermediates, which decompose rapidly and dissipate cellular energy. | Impaired cellular energy metabolism, reduced active nutrient transport and metabolic activity. | Low phosphorus in foods can increase risks of bone and dental problems, poor energy metabolism, and general weakness, particularly among groups like children, pregnant women, and those with limited dietary diversity | (43) |
| Iron (Fe) | Arsenic downregulates iron transporter genes (IRT1, FRO2), causes oxidative stress that mobilizes iron, depleting Fe in edible parts. | As induced chlorosis in fully developed young leaves, lower iron content in edible parts (such as grains, fruits, and vegetables), directly reducing their nutritional value. | Iron-deficiency anemia (fatigue, weakened immunity, developmental and cognitive problems in children), greater susceptibility to arsenic toxicity, which impacts the skin, cardiovascular system, neurological function, and increases cancer risk | (258) |
| Zinc (Zn) | Zn uptake is inhibited through both competitive interactions at root uptake sites and indirect effects on membrane permeability. Zinc deficiency due to As has been associated with reduced activity of carbonic anhydrase and superoxide dismutase enzymes essential for crop health and nutritional density | Lower Zn concentration in straw, roots, grains; increased ROS, DNA modification, reduced growth hormone efficiency like auxins, gibberellins, and carotenoids. | Frequent illness or infection, slow wound healing, reduced DNA synthesis and neurotransmission, hair loss, skin rashes, white spots on nails | (259) |
| Manganese (Mn) | As interferes with Mn acquisition by disrupting Mn transporter expression and root oxidation capacity, which is essential for converting Mn+2 into absorbable forms | Damaged chloroplast structure, lowers chlorophyll content, reduces net photosynthesis, and decreases soluble sugar concentrations | Reduced fertility, impaired bone development, and metabolic disturbances, neurodegenerative disorder | (260) |
| Calcium (Ca) | Specific Ca2+ signals are generally detected by various Ca2+ sensors such as CALCIUM-DEPENDENT PROTEIN KINASES (CPKs), CALMODULIN (CaM), CALCINEURIN B-LIKE PROTEINS (CBLs), CALMODULIN-LIKE PROTEINS, and their interacting kinases, called CBL INTERACTING PROTEIN KINASES (CIPKs). These sensors then translate the signals into metabolic and transcriptional responses. In response to arsenic stress, the differential expression of CaMs indicates a potential role for Ca2+-dependent signaling in the arsenic tolerance mechanisms of plants. In this context, Calcium-Dependent Protein Kinases (CPKs) are key regulatory proteins that typically play a role in decoding Ca2+ signals triggered by As stress. | As stress causes cytosolic acidification, disrupting calcium signaling pathways and causing cellular leakage of Ca2+ ions which ultimately reduces cell wall integrity and cell membrane stability | Weak, brittle bones with risk of osteopenia and osteoporosis; muscle cramps, spasms, twitching; dental issues like enamel weakening; potential cardiovascular effects. | (44, 261) |
| Magnesium (Mg) | As reduces Mg availability through altered transporter function and membrane fluidity. Although docking interaction studies between 60CE protein with both Mg2+ and As3+ showed a better link with As3+ via hydrogen bond, it can damage the plant more effectively with Mg deficiency. | Reduced photosynthetic efficiency and nutritional development | Abnormal heart rhythm (arrhythmia), palpitations, and increased risk of cardiac arrest, obesity, insulin resistance, metabolic syndrome, and type 2 diabetes. | (26) |
Interaction of arsenic with nutrients and its implication on plant and human health.
The competitive interaction between arsenic and essential nutrients—especially phosphorus, nitrogen, and iron—not only hampers plant health and productivity but also diminishes the nutritional quality of food crops. These interactions are critical in arsenic-exposed regions, where targeted nutrient management could mitigate arsenic toxicity and improve food safety.
2.2 Disruption of root architecture
Roots are the initial organs that interact with metals and metalloids in the soil; hence, various morphological modifications of root tissues may be anticipated (Figure 1). As toxicity causes morphological changes in root systems, including reduced root length, branching, and surface area. These alterations limit the physical capacity of the root to explore soil nutrients, thereby compounding the problem of nutrient deficiency (26). Water lettuce (Pistia stratoides) exhibited root loss with exposure to As (27). Talukdar (28) observed a threefold and two and a half-fold decrease in root length and root dry weight, respectively, in seedlings of Phaseolus vulgaris. The application of As led to a notable brown discoloration of the roots, accompanied by a reduction in the development of lateral roots. The presence of As at a concentration of 2 mg L−1 led to the total eradication of lateral roots, leaving merely a few lateral root primordia in the cortex (29). The number of lateral roots decreased, becoming concentrated in the basal region of the roots, alongside a darkening of the roots in soybean (Glycine max) plants subjected to As treatment (30).
Figure 1
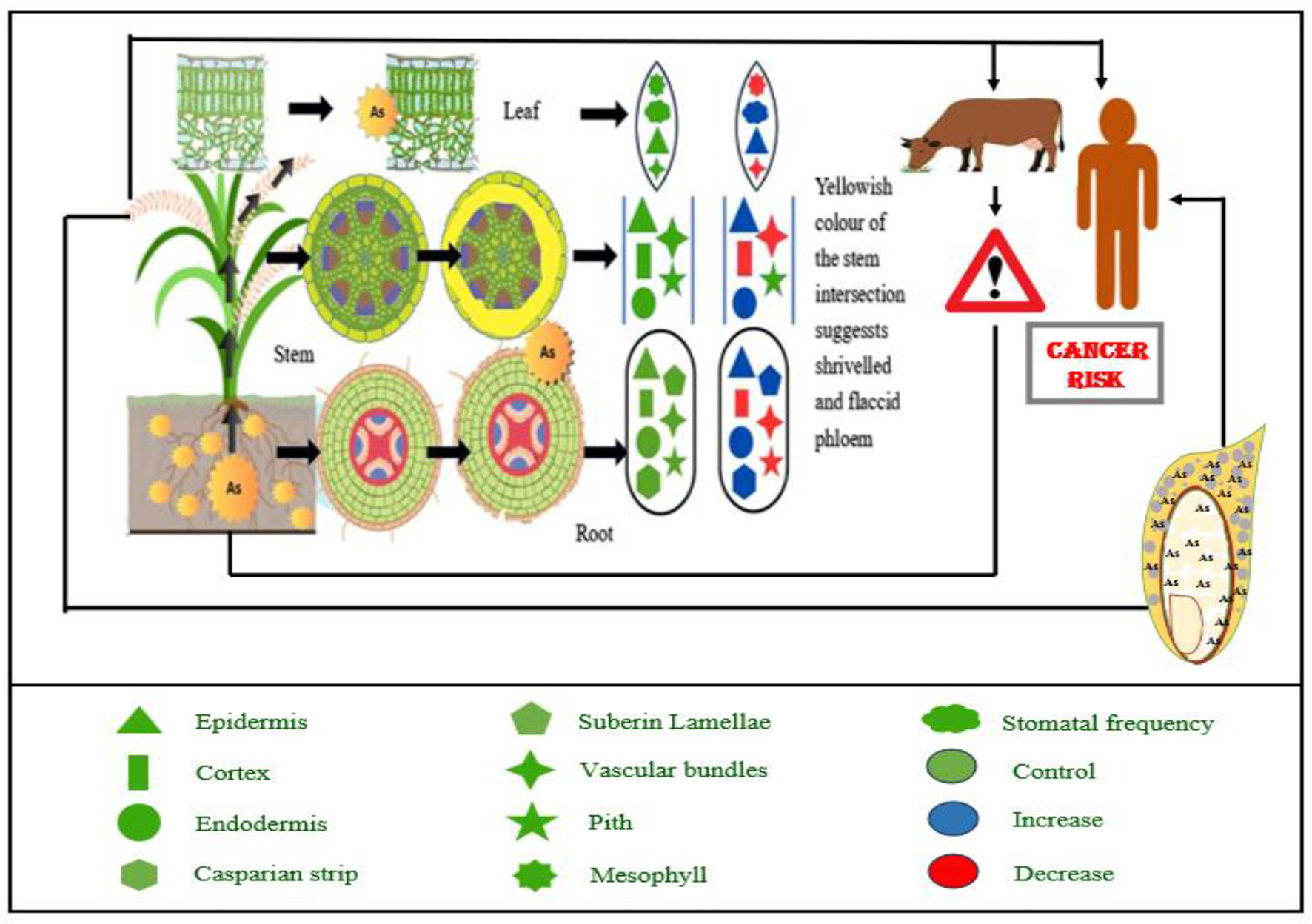
Arsenic contamination impacts food security and increases cancer risk for humans and animals by altering nutrient mobilization from soil to plants and affecting the structural organization of various plant parts. Abbreviation: As, Arsenic.
Although the root apical meristem, safeguarded by the root cap, is pivotal in influencing subsequent root growth, anatomy, morphology, and functionality (31, 32), it is the initial segment of the root that directly encounters toxic soil conditions and is consequently vulnerable to As exposure. Exposed roots often preserved the functioning of the root apical meristem concerning cellular division. The genotoxic effects of As on onion root growth were described by Gupta et al. (33). This was demonstrated by the increased frequency of micronuclei inside the intermediate phase of root meristem cells. There have been cases when arsenic's negative impacts on tap root lateral root primordia's growth and development have led to an increase in their activation along the tap root axis, which in turn has changed the root morphology. Arsenic and cadmium, according to Ronzan et al. (34), both facilitated the growth of lateral roots, which were associated with altered and weakened meristem organization. In addition, the uneven creation of the quiescent center and aberrant cell divisions in the root apical meristem prevented the emergence of several lateral root primordia from the tap root. These changes may subsequently lead to various anatomical alterations in older tissues (35).
The rhizodermis is the first root tissue affected by arsenic (As) contamination, disrupting water and nutrient uptake (35). Arsenic alters root hair development (36), often reducing or eliminating root hairs in species like Phaseolus aureus (37) and P. vulgaris (28), while Pteris vittata, a known As hyperaccumulator, shows minimal morphological changes (38). Cortical tissues—exo-, meso-, and endodermis—exhibit significant damage under As exposure (35), including cell disintegration, reduced parenchyma thickness (30), and dark deposit accumulation, as observed in Glycine max and Cajanus cajan (29). Structural changes also affect the central cylinder and vascular tissues. Brassica juncea showed increased cylinder diameter, while B. oleracea showed a decrease (39). As toxicity caused xylem deformation and vascular tissue destruction in P. vulgaris and C. cajan. Notably, dark deposits in vascular tissues were more pronounced under As(III) than As(V) (30). These findings highlight the species-specific morphological responses and the detrimental impact of arsenic on root structure and function (Figure 1).
2.3 Changes in stem tissue anatomy
The stem is the part of the plant organ that links the roots with the primary photosynthetic organs, which are the leaves. One of the primary roles of the stem is to support and transport nutrients to leaves and blossoms. Metals and metalloids are conveyed to aerial organs via vascular tissues; hence, the vasculature and its environs are often the locus of notable morphological modifications within stem tissues (35). Sclerenchymatous cells next to the phloem become desiccated and limp after As exposure, which impedes water transport and causes abnormalities in the phloem cells of the stem (40). The introduction of As led to the formation of crystals and druses within the epidermal layer, vascular bundles, cortex, and pith region of the stem (28).
2.4 Modifications in leaf tissue anatomy
The leaf functions as the central organ of photosynthesis, an essential process that generates the energy required to maintain physiological functions throughout all plant tissues. The predominant approach employed by many plants involves limiting the absorption and movement of heavy metals and metalloids to aerial structures, thus protecting photosynthetically active tissues from the adverse impacts of these toxic elements (35). Numerous findings indicate that leaf thickness has diminished as a result of the inclusion of metalloids. This was noticed as a result of the presence of As (39, 41). The narrowing of xylem channels (Figure 1) in the leaves of various plant species due to As exposure has been documented (41, 42).
2.5 Impact on plant metabolism
2.5.1 Impact on photosynthesis
According to various research, As accumulation greatly hinders photosynthesis process (43, 44) (Table 2; Figure 2). According to the mainstream view, the previously described inhibition is linked to ROS accumulation which is caused by As and their discrepant effect on basic photosynthetic mechanism. Kalita et al. (45) posited that, contrary to conventional understanding, oxidative stress resulted from the suppression of photosynthesis at lethal concentrations of As. Accumulation leads to a substantial decrease in chlorophyll concentration (46, 47). While As has a greater impact on chlorophyll synthesis, it has a smaller effect on the degradation of carotenoid pigments, which is linked to the reduction of chlorophyll in As-grown plants (48, 49).
Table 2
| Impact on plant metabolism | Observed effect under As stress | Proposed mechanism | References | |
|---|---|---|---|---|
| Photosynthetic components | Chlorophyll a (Chl a) | Decline in Chl a concentration and the inhibition or diminished availability of precursors such as d-aminolevulinic acid | Suppression of d-aminolevulinic acid dehydrogenase activity, elevated activity of chlorophyllase, Mg2+ replacement by As(III) in tetrapyrrole ring | (262, 263) |
| Chlorophyll b (Chl b) | Inhibition of activity | Oxidative damage, reduction in Chl a content | (48) | |
| Carotenoids | Variable: decrease or increase | Inhibition of precursor synthesis/ROS-induced non-enzymatic antioxidant response | (264–266) | |
| PS II | Reaction center inactivation, lowered plastoquinone reduction, decreased OJIP kinetics including Fv/F0 values | Oxidative damage to thylakoid proteins and D1 protein turnover, blocking effect on the donor end of PS II | (48, 267–269) | |
| Dark reaction | Minimal impact | Primary target is light reaction components | (270) | |
| Protein metabolism | Reduction in total protein content, enzyme inhibition, protein carbonylation | As binding to sulfhydryl groups; inhibition of nitrate/nitrite reductases; ROS-induced oxidation of amino acid residues | (53, 59) | |
| Lipid metabolism | Lipid peroxidation, membrane damage, altered lipid biosynthesis gene expression | ROS-induced peroxidation; altered expression of lipid synthesis genes; cytotoxic radical production | (53, 56) | |
| Carbohydrate metabolism | Decrease in reducing and non-reducing sugars; inhibition of starch-degrading enzymes | Suppression of sucrose synthesis; inhibition of starch phosphorylase, α- and β-amylase; altered hexose monophosphate pathway | (50, 51) | |
Effects of arsenic phytotoxicity on plant metabolism.
Figure 2
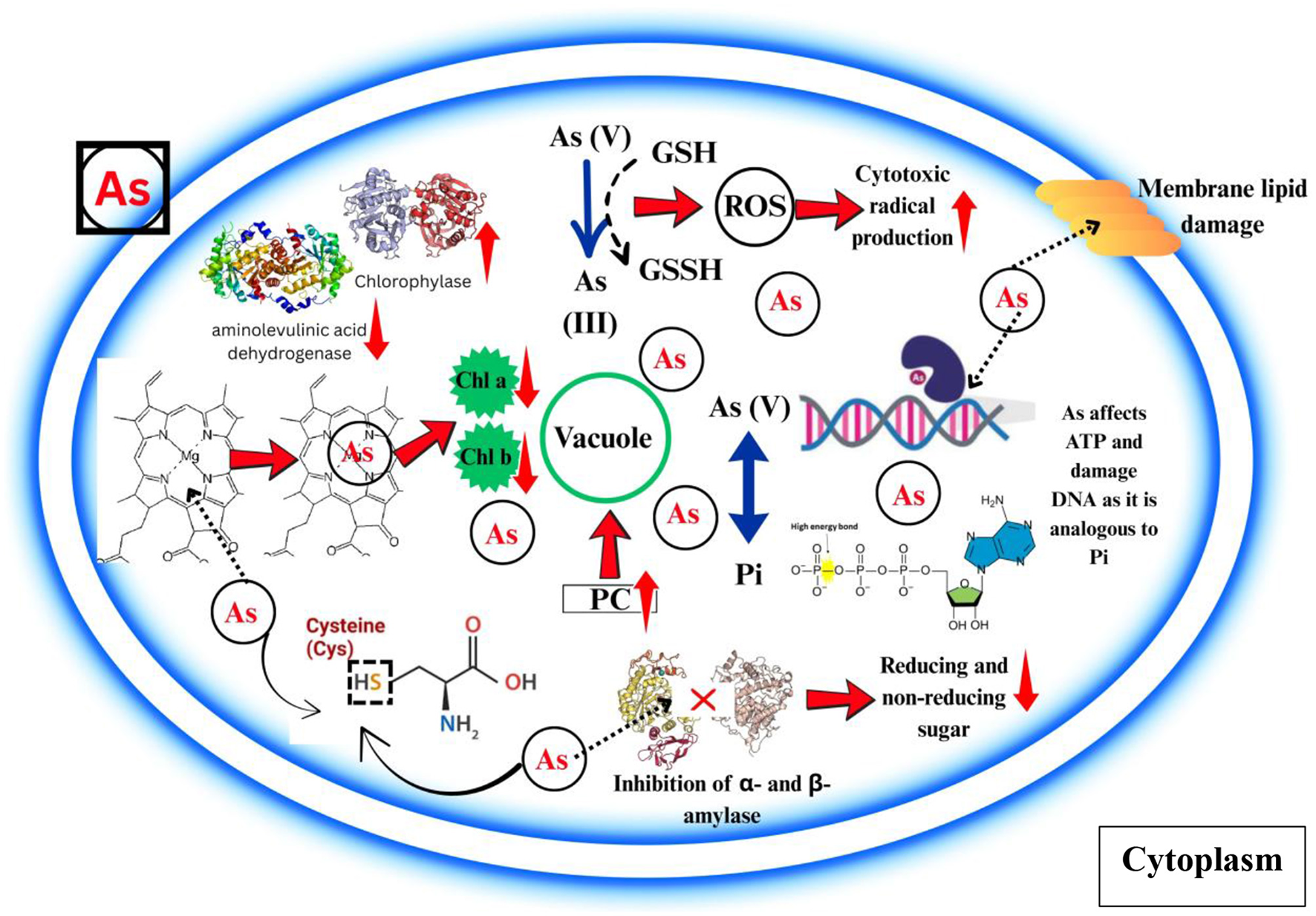
The effect of arsenic phytotoxicity on photosynthetic pigments, protein, carbohydrate and lipid molecules. It is shown how arsenic can compete with Pi in the metabolic processes that require Pi. Abbreviations: Arsenic (As), Inorganic arsenic [As (III)], Arsenite [As(III)], Arsenate [As(V)], Phosphorus (Pi), Reactive oxygen species (ROS), Glutathione (GSH), Oxidized glutathione (GSSH), Phytochelatin (PC), Adenosine triphosphate (ATP), Deoxyribonucleic acid (DNA).
2.5.2 Impact on protein, lipid, and carbohydrate metabolism
The presence of As adversely impacts the metabolic processes (Table 2; Figure 2) of vital carbohydrates, such as sugars and starches. The incorporation of As in Oryza sativa led to a decrease in both reducing (hexoses) and non-reducing (sucrose) sugars in the shoots (50), suggesting a suppression of sucrose synthesis in comparison to hexose monophosphate. Its phytotoxicity was enhanced because it significantly suppressed the functions of enzymes that break down starch, namely starch phosphorylase and α- and β-amylase. On the other hand, when stress was applied to Oryza sativa and Phaseolus aureus seedlings, it increased starch phosphorylase activity, leading to higher levels of soluble sugars (51).
The stress induced by As leads to lipid oxidation, a process considered significantly harmful to plants. Cellular electrolyte leakage and membrane degradation were significantly enhanced in several plant species that were subjected to As stress (52–54). According to Clemens and Ma (55), the peroxidation of lipid molecules within cellular and organelle membranes is influenced by the elevated level of ROS caused by As. In the end, the cytotoxic radicals that are mediated by lipids damage the functionality of cells or tissues. It has been discovered that As exposure alters the mechanism for lipid synthesis. Significant changes in the expression of 59 genes associated with lipid formation were seen in a comparative transcriptome analysis of rice following exposure to As(III) treatment (56). Despite evidence that As affects genes involved in lipid formation, studies elucidating how As affects plant lipid levels are scarce. The strong binding of inorganic As compounds to sulfhydryl groups in proteins causes damage to plant cell membranes and eventual cell death, significantly interfering with plant metabolism. The total protein content in plants is reduced when As is present (53). The external introduction of As impeded the activity of nitrate and nitrite reductase, enzymes integral to the reduction of protein concentrations in plants. The disintegration of proteins into individual amino acids is primarily facilitated by proteases and peptidases. A reduction in exposure leads to diminished protease levels, subsequently hindering the growth and development of plants (57). The trivalent form of As can bind directly to the sulfhydryl groups of proteins and obstruct several biological pathways; in contrast, the pentavalent form acts as a phosphate analog and disrupts phosphorylation activities (58). According to Fedorova et al. (59), proteins undergo carbonylation changes due to an overabundance of ROS produced by As stress. Proteins incorporate carbonyl (C=O) groups either directly or indirectly via interactions with reactive carbonyl species or the oxidation of certain amino acids (60). When their side chains are oxidized, some amino acids that are known to be proteinogenic—including arginine, histidine, lysine, proline, threonine, and tryptophan—are able to form carbonyl groups. Biomolecular impairment, increased toxicity, and the induction of apoptotic cell death are caused by the increased presence of carbonyl compounds, which are a result of reactive carbonylated species and their interactions with nucleophilic substrates (61).
2.6 Impact on soil microbial activity
Arsenic (As) contamination in soils presents a substantial risk to the ecological viability of agroecosystems by adversely affecting microbial populations, enzymatic activity, and nutrient cycling. These biological disruptions impair soil health and diminish plant productivity, thereby jeopardizing long-term food and nutritional security. Li et al. (62) documented a significant alteration in microbial community composition due to As stress, characterized by a rise in Gemmatimonadota and a decrease in Bacteroidota and Nitrospirota. In the arsenic-contaminated soils of the Bengal Delta Plain, significant alteration of microbial species including Alpha-, Beta-, and Gamma-proteobacteria, Actinobacteria, and Acidobacteria was reported (63). These groupings, functionally associated with soil nutrients such as nitrogen, potassium, phosphate, and iron, exhibited a negative correlation with increasing arsenic levels. Evaluations of microbial activity using basal respiration, substrate-induced respiration (SIR), and fluorescein diacetate (FDA) hydrolysis demonstrate persistent declines in arsenic-contaminated soils. Ghosh et al. (64) documented an elevation in the microbial metabolic quotient (qCO2), signifying increased respiratory stress in relation to microbial biomass carbon. The decline in FDA hydrolysis was ascribed to the inhibited production of hydrolyzing enzymes (protease, lipase, esterase) and diminished fluorescein absorption and release in microbial cells (65). Soil enzyme activities—specifically β-glucosidase, arylsulfatase, urease, and both acid and alkaline phosphatase—diminish markedly with elevated labile As concentrations. Bhattacharyya et al. (66) exhibited significant negative associations between these enzymatic activities and exchangeable or water-soluble arsenic components. The activity of alkaline phosphatase is notably sensitive because of the structural resemblance between As(V) and phosphate, resulting in competitive inhibition (67). Nonetheless, urease exhibited merely a 33–38% decrease, suggesting a diminished direct reliance on arsenic concentrations (68). Environmental variables additionally influence these consequences. Enzyme activities were significantly inhibited under anaerobic circumstances, like those in paddy fields, compared to aerobic soils, owing to microbial sensitivity to oxygen (69). Anaerobic respiration with low molecular weight organic acids (e.g., acetate, formate) facilitates arsenic desorption and impairs enzymatic activity (70). The microbial reduction of iron oxyhydroxides increases arsenic solubility at low redox potential, intensifying enzyme inhibition, particularly for glucosidase (68).
Soil microbial communities demonstrate differing tolerances to arsenic species. Guan et al. (71) discovered that As(III)-tolerant bacteria and actinomycetes are present in lesser quantities than their As(V)-tolerant equivalents, but fungus exhibited comparable resistance to both As(III) and As(V), indicating superior fungal resilience. Despite these detrimental impacts, certain microbes possess arsenic-detoxifying abilities, such as As(V) reduction, As(III) oxidation, methylation, or sequestration in biomass (Section 3.2). These groups can reduce arsenic mobility and bioavailability, indirectly reducing plant uptake of As. Harnessing such microbial processes—either naturally occurring or through bioaugmentation—can complement other remediation strategies.
As both microbial processes and soil amendments can influence arsenic speciation and mobility, integrating microbial remediation with silicon (Si) supplementation offers a synergistic approach. While Si reduces arsenic bioavailability through adsorption, precipitation, and competition with phosphate uptake, beneficial microbes can further enhance this effect by immobilizing or transforming arsenic into less bioavailable forms. Together, they form a dual strategy for mitigating As bioaccumulation in crops.
3 Role of Si in mitigating As bioaccumulation
Silicon, although not essential for plant growth, confers numerous physiological benefits and interact in the soil environment through several mechanisms, primarily affecting bioavailability of As and its uptake by plants.
3.1 Si-mediated iron plaque–As interaction in plants
The interaction between Si, iron (Fe) plaques, and As at the root–soil interface is a critical process influencing As uptake and toxicity in wetland crops, especially rice (Figure 3). Rice cultivated in inundated soil conditions, along with other aquatic flora, develops a Fe plaque on the root surfaces as a result of pronounced redox gradients from the roots to the reduced bulk soil. The diminished bulk soil is defined by the reductive dissolution of iron oxide minerals, leading to elevated Fe(II) concentrations in the soil solution (25, 72). Oxygen escaping from the expanded gas cavities of aerenchyma tissue into the rhizosphere, known as radial oxygen loss, significantly influences the redox chemistry in close proximity to the root (73, 74). The oxic rhizosphere facilitates the fast oxidation of porewater Fe(II) to insoluble Fe(III) precipitates on the exterior of roots, predominantly at root tips and lateral root junctions (75, 76). Figure 4 depicts the sequential formation of Fe plaque in distinct stages. This Fe plaque is predominantly made up of the Fe oxyhydroxides ferrihydrite [Fe(OH)3·nH2O], lepidocrocite (γ-FeOOH), and goethite (α-FeOOH) (77, 78). The elevated zero charge potential of FeOx (>7) facilitates the formation of robust inner-sphere adsorption complexes with various anions and promotes adsorption at edge and corner sites (79). Porewater containing arsenate [As(V), H3AsO4] and arsenite [As(III), H3AsO3] exhibits significant monodentate or bidentate complexation with iron plaque (80). Ferrihydrite is a highly reactive mineral that initially predominates in the rhizosphere but can subsequently convert into the more crystalline forms of lepidocrocite and goethite over time. Anions adhere to the edge and corner sites of FeOx, with ferrihydrite exhibiting a greater abundance of the more robust edge sites compared to the other two (81).
Under dynamic redox conditions, As(V) or As(III) can be immobilized through coprecipitation with Fe oxides during Fe(II) oxidation and Fe(III) hydrolysis (82). Arsenic becomes structurally incorporated within the Fe oxide matrix as it forms:
Figure 3
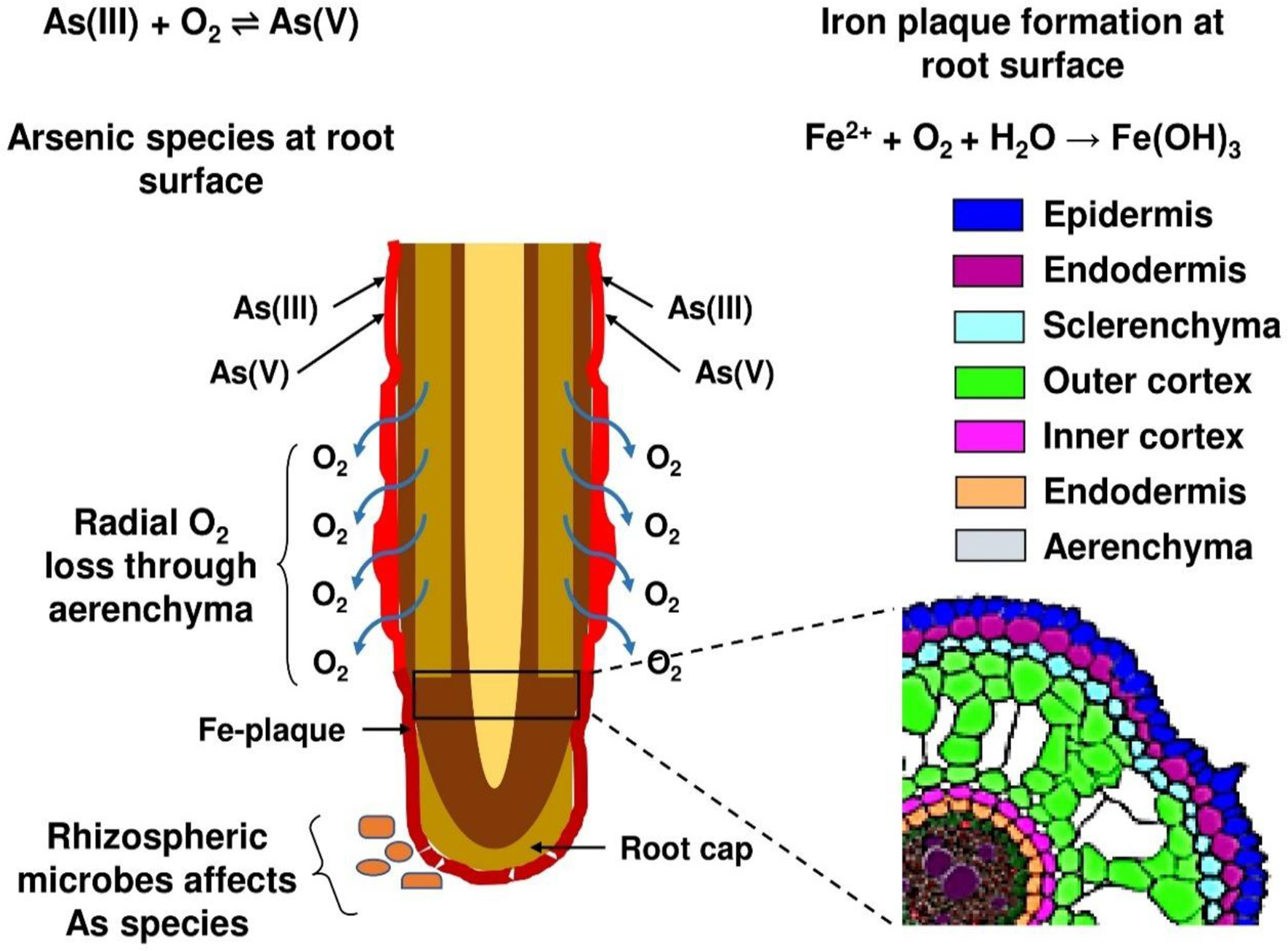
The influence of Fe plaque formation on rice root surface on As availability.
Figure 4
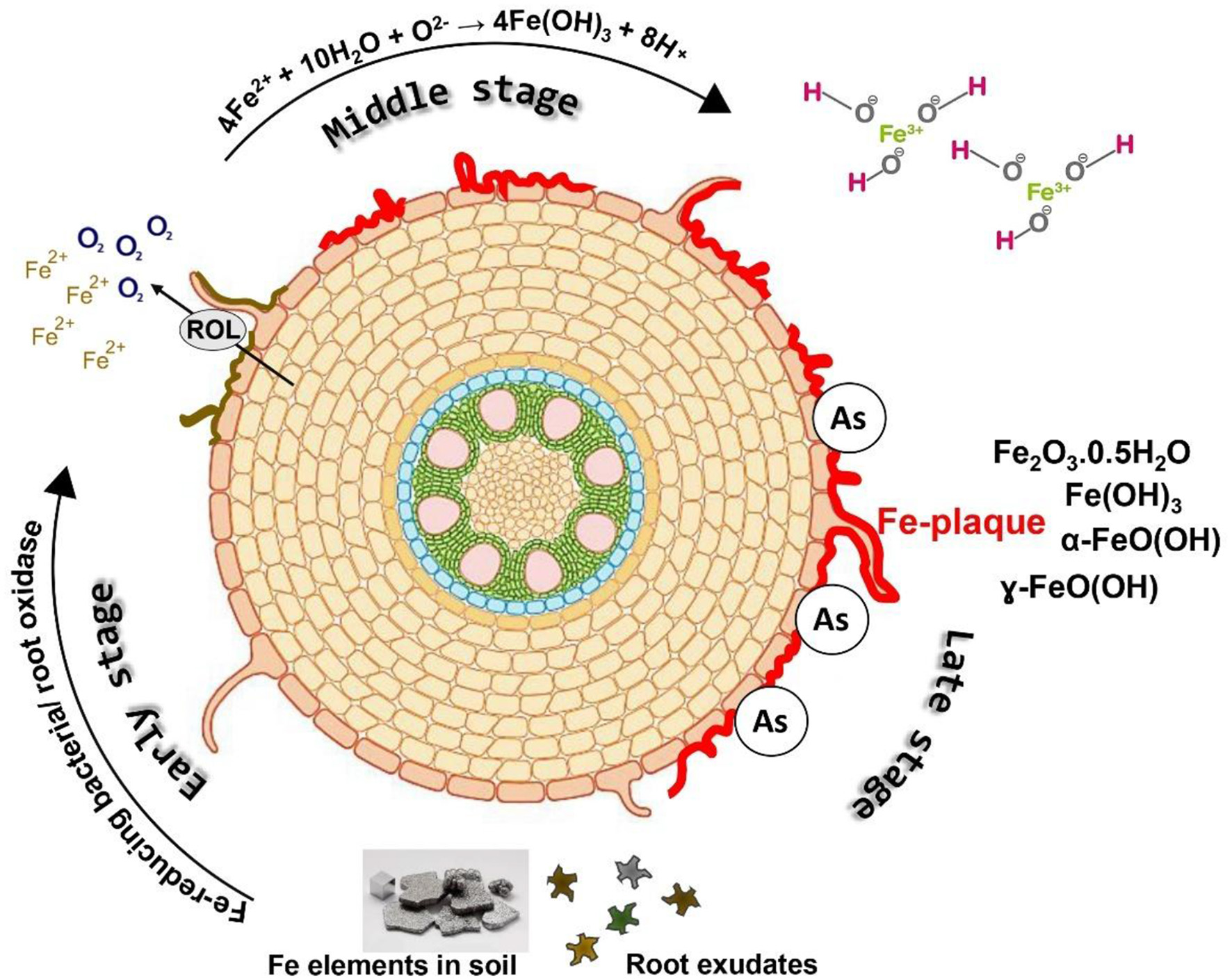
The formation process of Fe plaque (IP) through various oxidation-reduction processes occurring outside the root.
Si can be adsorbed onto or co-precipitated with Fe oxides during plaque formation. The incorporation of Si into Fe oxides interferes with their structural ordering due to steric hindrance and disruption of Fe–O–Fe bonding, which alters nucleation and crystal growth kinetics (83). Si incorporation has a retarding effect on Fe oxide crystallization by binding to surface hydroxyl groups and blocking reactive sites necessary for phase transformation. This results in the stabilization of poorly crystalline phases like ferrihydrite over more crystalline forms like goethite or hematite, both under pure mineral systems and in rice root experiments (78, 84). The resulting plaques exhibit higher specific surface areas, greater sorption capacities for metals like arsenic (As), and altered redox reactivity. Hence, elevated concentrations of Si in porewater may enhance the retention of As by promoting the formation of ferrihydrite-dominated Fe plaques (85). Moreover, Si nutrition benefits rice plants growth and improves oxygen secretion ability of the roots, maintaining an oxic microenvironment for plaque formation strength and silicate anions compete with arsenite for sorption sites, thereby increasing As mobility in the (86). But under Si-rich flooded condition, reduction of arsenate to arsenite decreases its adsorption rhizosphere (85, 87).
Gu et al. (88) observed that Fe content in amorphous fraction of plaque (AIP) was higher than the crystalline fraction (CIP) and further increased (40.8–205.8% in AIP and 2.9–187.9% in CIP) after supplying Si-rich rice husk ash (RHA). Compared with non-RHA addition, the As contents in the AIP and CIP increased by 22.4–235.6% and 51.5%, respectively, with HA supplication at low-concentration single As stress. The application of HA reduced As contents in the shoots and roots by 31.9–42.8% and 9.9–17.9%, respectively at single As stress. Jiang et al. (89) reported an increased Fe and As content in plaque by 9.4–53.7% and 28.0–33.1%, respectively, after application of 0.5–2.0% RHA. Compared to no-RHA treatments, 0.5–2.0% RHA treatments significantly reduced the As contents in stem, leaves and roots by 50.0–78.8%, 16.8–82.8% and 14.9–38.1%, respectively. 2.0% RHA application decreased inorganic As content in brown rice by 30.8% compared to no-RHA treatment. Khanam et al. (10) showed co-application rice straw compost (RSC) and SSB resulted in the maximum Fe plaque formation with a concentration of 3,140 mg kg−1, followed by the sole RSC (2,911 mg kg−1), which were significantly higher than the control (2,321 mg kg−1). Leksungnoen et al. (90) found that Si-rich RHA (0.64% w/w) almost doubled that As concentration in Fe plaque compared to untreated plots and plaque As was higher that compared to RHB.
3.2 Microbe mediated immobilization of As
A variety of bacteria associated with the rice rhizosphere can play a role in the biotransformation of As (As) by oxidizing As(III), reducing As(V), methylating As(III), and respiring As(V) (91). Microorganisms containing As functional genes, including arsenite oxidase, arsenate reductase, respiratory arsenate reductase and arsenite methyltransferase play a key role in regulating the speciation and mobility of As in paddy soil as shown in Figure 5 (92). The oxidation and methylation of As(III) are recognized as natural detoxification pathways of the As biotransformation cycle in the paddy rice system (91, 93).
Figure 5
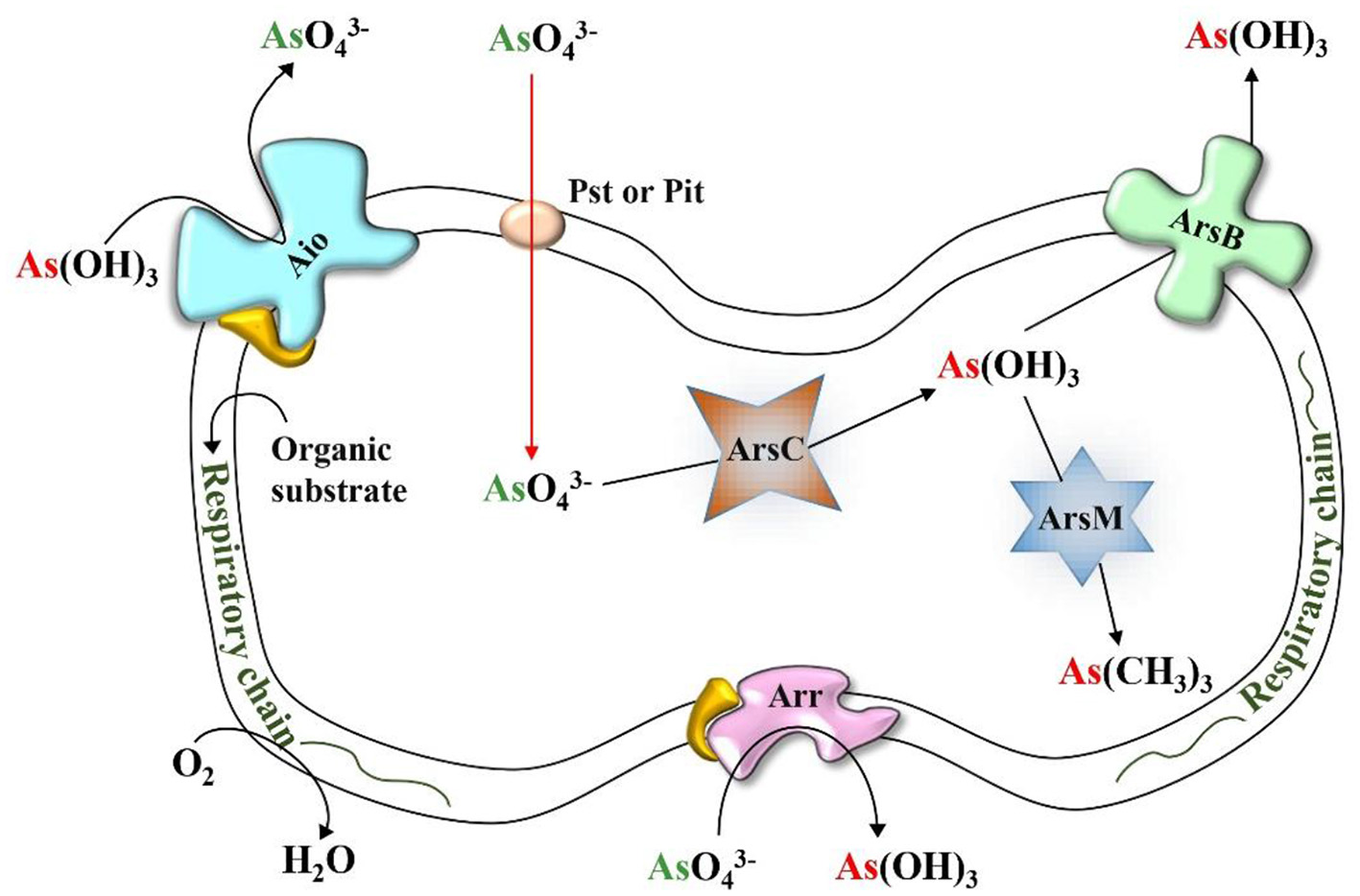
Cellular locations and functioning of microbial enzymes involved in As immobilization.
The archetypal aio system, the aioBA operon, was first identified and completely sequenced from the β-proteobacteria Herminiimonas arsenicoxydans. It encodes arsenite oxidase (Aio), comprising two subunits: AioA, the large molybdopterin-containing catalytic unit, and AioB, a small Rieske [2Fe-2S] cluster protein (94). Aio catalyzes the oxidation of As(III) to As(V) through four sequential electron transfer steps (95). Stopped-flow spectroscopy and isothermal titration calorimetry revealed that As(III) binds near a funnel-shaped cavity of AioA, where polar residues coordinate it via the molybdopterin cofactor. The bound As(III) donates electrons to the Mo(VI) center, reducing it to Mo(IV) while being oxidized to As(V) at rates exceeding 4,000 s−1. Electrons are then rapidly transferred from Mo to the Rieske centers. The final, rate-limiting step involves electron transfer from the AioB Rieske cluster to the terminal electron acceptor, cytochrome c, completing the catalytic cycle (96).
Microbial methylation, or biomethylation, refers to the biological transformation of metals and metalloids into volatile and nonvolatile methylated compounds with the help of methyltransferase enzyme (97). First identified in fungi, this process is crucial for As detoxification and its environmental cycling. The arsM gene enables microbes to methylate and resist As toxicity (98). The most widely accepted pathway, proposed by Challenger et al. (99, 100), involves initial reduction of As(V) to As(III), followed by two successive enzyme-mediated reductions. Each reduced As(III) intermediate undergoes methylation, ultimately forming trimethylarsine (Figure 4). S-adenosylmethionine (SAM) serves as the primary methyl group donor, though some anaerobic bacteria may use methylcobalamin (97).
Microorganisms reduce As(V) via two distinct pathways: the first involves cytoplasmic arsenate reductases encoded by the ars operon, and the second utilizes dissimilatory or respiratory arsenate reduction mediated by the arr gene cluster (Figure 4) (101). Serendipitously, ars genes were originally discovered during studies on antibiotic resistance in Staphylococcus aureus, not through direct investigation of arsenic resistance. Each gene in the ars operon contributes uniquely to arsenic detoxification: arsR encodes a transcriptional repressor of the SmtB/ArsR family (102); arsA encodes an ATPase that, along with ArsB, forms an ATP-dependent As(III) efflux pump (103); arsD encodes a metallochaperone that binds As(III) and transfers it to the ArsAB pump (104); and arsC encodes a cytoplasmic arsenate reductase, converting As(V) to As(III) (105). Alternatively, the arr operon, first characterized in Shewanella sp. ANA-3, encodes respiratory arsenate reductase ArrAB. The ArrA subunit, a large protein containing a bis-molybdopterin guanine dinucleotide cofactor and a [4Fe−4S] cluster, catalyzes As(V) reduction. ArrB, the smaller subunit, harbors four [4Fe−4S] clusters that facilitate electron transfer. This system enables anaerobic respiration using As(V) as a terminal electron acceptor, contributing to arsenic cycling under anoxic conditions (106).
The abundance of these As functional genes is generally dependent on the bacterial community structure that can be evaluated based on the diversity of 16S rRNA genes. The incorporation of Si-rich agro-wastes amendments, such as rice straw and rice husk, enhances soil organic matter and reduces soil redox potential, thereby directly affecting the soil microbiota (107). Porewater inorganic As(III) levels can be increased by elevated organic matter in two ways: first, by enriching an anaerobic microbial community that may be pivotal in As methylation; and second, by increasing the activity of Fe-reducers and As-reducers. Amendments high in Si had different effects on the total microbial population and the specific group of microbes that methylated As (108). Elevated calcium from calcium silicate treatments enhanced carbon storage in the first year, leading to carbon release in the second year, which may have influenced the distribution of both 16S rRNA and arsM genes. Modifications to the arsM community composition may have been impacted by reduced porewater redox potentials caused by rice husk amendment. In their study, Das et al. (109) found that indica rice grains had a 28% reduction in As and in Japonica rice grains a 30% reduction after being treated with slag-based Si. Additionally, the application of this Si increased the number of bacteria that were As-resistant and arsenite-oxidizing, which helped the soil naturally attenuate the As. Herath et al. (92) examined three different types of modified rice husk biochar (RHBC): unmodified RHBC, Si-modified RHBC, and nano-montmorillonite clay modified RHBC. The results showed that Si-RHBC significantly raised the number of bacteria (16S rRNA gene) and doubled the number of aioA gene copies compared to RHBC, which was already 25% higher than the control. The arrA, arsC, and arsM gene copy numbers were somewhat upregulated with Si-RHBC, but this effect did not reach statistical significance. The results suggest that bacteria in paddy soil that are connected with the aioA gene may help with the anaerobic oxidation of As(III) to As(V). Soil treated with Si-RHBC also showed a marked decrease in the relative abundance of Fe-reducing bacteria, particularly Bacillus and Geobacter. This suggests that the decreased abundance of these bacteria in paddy soil leads to a drop in the dissolution of As(III) from iron oxide minerals. In their study, Gao et al. (86) showed that reducing bacteria, Anaeromyxobacter and Geobacteraceae, and levels of As(III) and Fe in the rhizoplane were significantly increased by adding Si. This, in turn, inhibited the uptake of As(III) into roots.
3.3 Competition for transport pathways in plants
Si is an essential element in the soil and crust of the earth, but only 0.1 to 0.6% is soluble (110). Plants absorb Si as ionized Si(OH)3O and silicic acid. Si and As, specifically arsenite, As(III), exhibit striking chemical similarities under soil solution conditions. Both exist as uncharged molecules at typical pH ranges: H4SiO4 and H3AsO3 (arsenous acid) (111). Due to this similarity, plants, particularly rice (Oryza sativa), inadvertently take up As(III) using the same transporter systems that are primarily involved in Si uptake (112). The two major transporters identified for this dual uptake mechanism are Low silicon 1 (Lsi1) and Low silicon 2 (Lsi2). Both transporters play complementary roles in Si transport, yet they differ in their structure, localization, transport mechanisms, and energy dependence.
Lsi1, a member of the NIP subfamily of aquaporins within the major intrinsic protein (MIP) superfamily, is a passive channel facilitating silicic acid influx via facilitated diffusion, characterized by ar/R selectivity filters and NPA motifs (113, 114). In contrast, Lsi2 is not an aquaporin but a secondary active efflux transporter likely driven by a proton gradient, functioning as a putative anion transporter (115, 116). Unlike Lsi1, structure of Lsi2 remains less defined, though its functional role is critical for Si translocation.
Both Lsi1 and Lsi2 are polarly localized in the plasma membranes of root cells but on opposite sides. Lsi1 is localized on the distal (outer) side of both exodermis and endodermis cells as shown in Figure 6, facilitating the influx of silicic acid from the soil into root cortical cells (113, 117). On the other hand, Lsi2 is localized on the proximal (inner) side of the same cells, promoting the efflux of silicic acid from the root cells into the stele (Figure 6), enabling xylem loading and translocation to the shoot (115). Lsi1 operates via passive transport, relying solely on the concentration gradient of silicic acid. It does not require energy input in the form of ATP or electrochemical gradients. This aligns with its role as a bidirectional channel that can facilitate both influx and efflux depending on substrate concentration (118). Whereas, Lsi2 functions via an active transport mechanism, coupling the efflux of silicic acid with the inward movement of protons. This energy-dependent process enables Lsi2 to transport silicic acid against its concentration gradient, a necessary step to move Si from root cortical cells into the xylem (115).
Figure 6
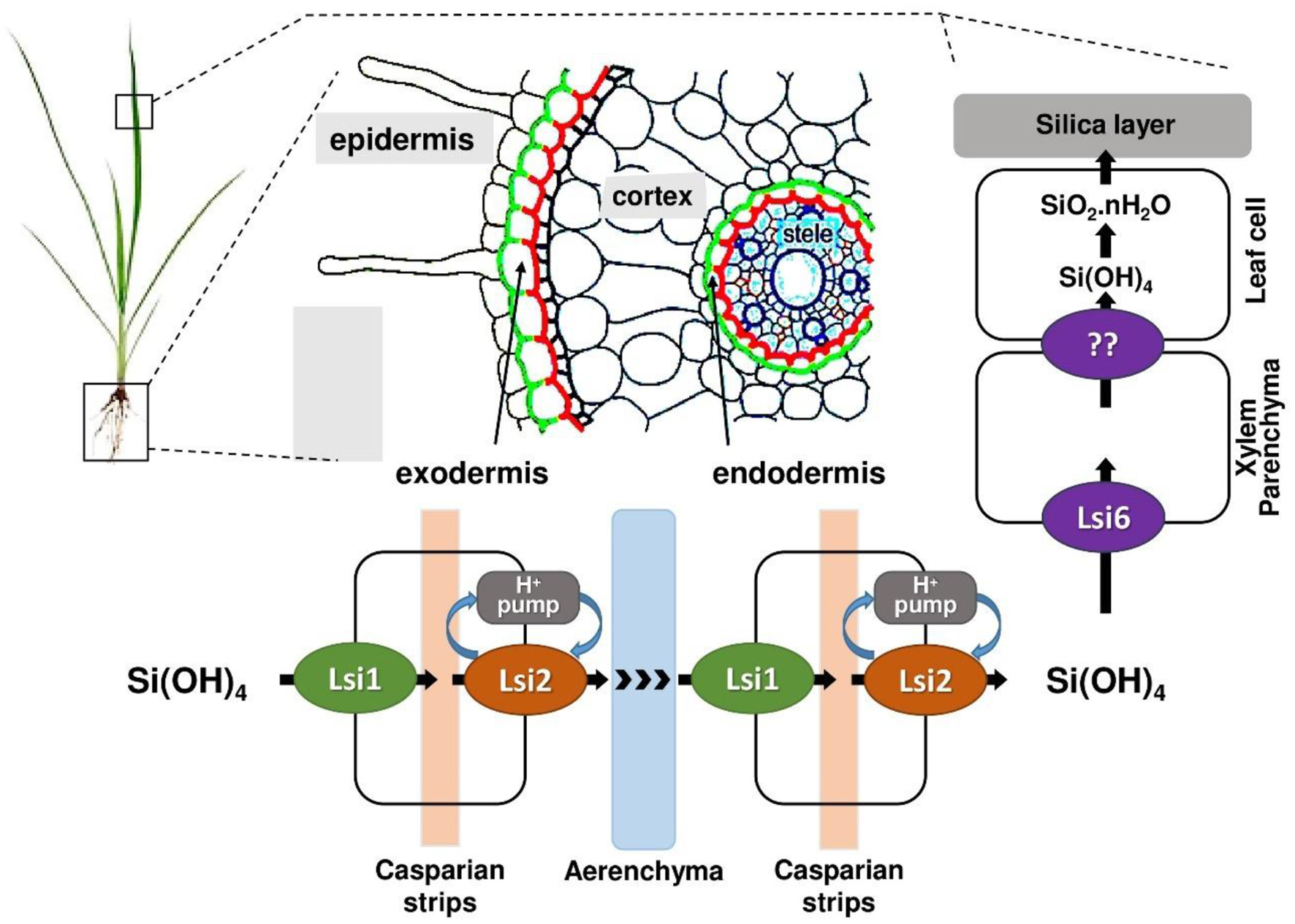
The existing Si transport model in rice roots. Lsi1 is expressed at the distal end, while Lsi2 is expressed at the proximal end.
Subsequent to absorption, over 95% of Si is swiftly translocated to the xylem by both Lsi2 and Lsi3 in rice. Lsi3, a homolog of Lsi2, is situated in the root pericycle cells and helps in xylem loading of Si (119). The unloading of Si from the xylem into leaf is facilitated by Lsi6, a homolog of Lsi1. Lsi6 is positioned in a polar manner on the adaxial side of the xylem parenchyma cells within the leaf sheaths and leaf blades (120, 121). Basically, Lsi6 and Lsi3 play a role in distributing silicon within the plant, including loading Si into the xylem and unloading it in specific tissues like leaf sheaths. Each plant contains specialized transporters for the uptake and accumulation of Si in various sections, such as OsLsi (Rice), TaLsi (Wheat), and ZmLsi (Maize), as indicated in Table 3.
Table 3
| Transporter/aquaporin | Plant species | Specific genes | Type and expression sites | Functional significance | References |
|---|---|---|---|---|---|
| Lsi1 | Oryza sativa | OsLsi1 | Influx; basal roots | Facilitates passive transport of silicic acid [Si(OH)4] into root cells following the concentration gradient; first step in Si uptake; mutations in Lsi1 severely reduce Si accumulation, leading to weaker stress tolerance and lower yield stability. | (113, 271) |
| Hordeum vulgare | HvLsi1 | Influx; basal roots | (272) | ||
| Triticum aesativum | TaLsi1 | Influx; roots | (273, 274) | ||
| Zea mays | ZmLsi1 | Influx; roots | (275, 276) | ||
| Sorghum bicolor | SbLsi1 | Influx; roots | (277) | ||
| Cucurbita moschata | CmLsi1 | Influx; roots and shoots | (114) | ||
| Solanum lycopersicum | SlLsi1 | Influx; root | (278) | ||
| Cucumis sativus | CsLsi1 | Influx; root tips | (279) | ||
| Lsi2 | Oryza sativa | OsLsi2 | Efflux; main and lateral roots (not in root hairs) | Actively exports Si from root cells into the apoplast toward the xylem, working in tandem with Lsi1 to achieve directional Si transport; Essential for loading Si into xylem; disruption of Lsi2 leads to Si retention in root tissues and impaired long-distance transport. | (115, 280) |
| Hordeum vulgare | HvLsi2 | Efflux; basal roots | (272, 275) | ||
| Zea mays | ZmLsi2 | Efflux; basal roots | (275, 276) | ||
| Cucurbita moschata | CmLsi2 | Efflux; roots and shoots | (114) | ||
| Equisetum arvense | EaLsi2 | Efflux; roots and shoots | (281) | ||
| Lsi3 | Oryza sativa | OsLsi3 | Influx; Panicles | Facilitates unloading of Si into xylem transfer cells in upper nodes to ensure distribution to panicles and flag leaves; Regulates partitioning of Si to developing reproductive organs | (282) |
| Lsi6 | Oryza sativa | OsLsi6 | Influx; leaves | Mediates inter-vascular transfer and redistribution of Si within shoots, particularly toward developing tissues; Critical for optimizing Si allocation within shoots, ensuring enhanced stress resistance | (120) |
| Hordeum vulgare | HvLsi6 | Influx; leaf blade and sheaths | (283) | ||
| Zea mays | ZmLsi6 | Influx; leaf blade and sheaths | (275) | ||
| Aquaporins like MIP, NIP etc. | Equisetum arvense | EaNIP3;1 | Influx; roots and shoots | Provide a structural and evolutionary framework for specialized channels such as Lsi1 and Lsi6, which evolved for Si transport; Key mediators of Si homeostasis, influencing plant stress adaptation, detoxification of arsenite, and efficient nutrient management. | (284) |
| Glycine max | GmNIP2 | Influx; roots and shoots | (285) |
Literature survey of Si specific genes and transporters or sub families aquaporins of various plants.
These transporters, primarily evolved for Si uptake, inadvertently become conduits for a toxic metalloid. This functional convergence presents a critical interface in As-contaminated environments, especially in paddy fields where anaerobic conditions favor the prevalence of As(III). The dual uptake mechanism is not merely a biochemical or physiological curiosity but a pressing agronomic challenge, as it tightly links beneficial and toxic element transport. Advances in protein modeling and transporter engineering have opened new avenues to selectively modify Lsi1 pore architecture and selectivity filters (e.g., ar/R and NPA motifs) to discriminate between silicic acid and arsenous acid. This possibility was largely unexplored until recent structural insights emerged from high-resolution cryo-EM and in silico mutagenesis studies (121). The potential to reengineer Si transporters to reduce As permeability while maintaining Si uptake marks a paradigm shift in plant nutrient and stress management strategies. Moreover, limited information exists on how transporter expression is modulated under simultaneous Si deficiency and As stress, or how root exudates and rhizospheric microbiota influence transporter functionality. These unexplored areas represent novel frontiers to enhance our understanding of Si-As dynamics.
Boorboori et al. (122) elucidated the mechanisms of Lsi1 regulating Si uptake, which influences As accumulation in rice seedlings. They discovered that the Lsi1 overexpression line (LE-OE) exhibited a superior capacity for Si absorption under hydroponic conditions compared to the wild type (LE-WT). Furthermore, the addition of Si to the LE-OE rice lines possessing the Lsi1 gene conferred enhanced As resistance relative to the LE-WT line. Khan and Gupta (123) demonstrated that compared to the control and Si treatments, the As(III)+Si treatment increased the expression levels of the OsLsi1, OsLsi2, and OsLsi6 genes involved in transporting As(III), but this increase was less pronounced than in treatments where As(III) was used alone.
3.4 As tolerance through improved antioxidant defense system and reduced uptake
A surplus of ROS, such as superoxide radicals (O2+·), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH), is produced when toxic substances are present in plants, leading to oxidative stress (124). In the presence of ROS, various physiological processes are disrupted, including lipid peroxidation, protein oxidation, DNA damage, and the eventual stunting of plant development (125). Under As stress, Si supplementation dramatically boosts the activities of key antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD) and glutathione reductase (GR), as well as important non-enzymatic antioxidants like cysteine, ascorbic acid (AsA), and glutathione (GSH). Additionally, Si can induce heavy metal co-precipitation by surface adsorption by Si-rich tissues and thicken the cell wall, both of which impede heavy metal transport. Cui et al. (126) observed that treatment with SiO2 NPs could maintain the integrity of the cell, increase the thickness of the cell wall (77.4%) and the ratio of As in the pectin (19.6%). In addition, the pectin content, cation exchange capacity (CEC) and pectin methylesterase (PME) activity were also increased in the SiO2 NPs-pretreated cells, leading to a decreased degree of pectin methylesterification and an improved mechanical force of the cell walls. Silica-rich tissues (phytoliths) in rice can incorporate trace amounts of As, either through physical entrapment or surface adsorption (127).
Tripathi et al. (128) showed Si treatment enhanced SOD, GR and APX activities in rice plants exposed to As, resulting in lower ROS accumulation. Boorboori et al. (129) also found addition Si during As exposure significantly increased SOD, CAT, APX and POD activity and decreased MDA content in two different cultivars of rice. Geng et al. (130) observed that application of sodium silicate @ 168 mg L−1 increased SOD, CAT and POD activities along with elevated GSH and AsA contents implied the active involvement of ROS scavenging and played, at least in part, to Si-mediated alleviation of organoarsenic arsanilic acid (ASA) toxicity in rice. Li et al. (131) demonstrated As content in wheat shoots and grains decreased with the addition of Si-rich materials and maximum reduction of 16.2% and 17.8% in shoots and grains, respectively, was observed in rice husk biochar+2 g kg−1 bentonite treatment compared to control. Activity of GSH and AsA significantly increased with application of Si-rich materials with subsequent decrement in MDA content. However, As content in subcellular fractions of wheat shoots displayed no significant change after the Si-rich material addition. More similar studies have been summarized in Table 4.
Table 4
| Crop | Application rate of Si | Results | References |
|---|---|---|---|
| Rice | 1 mM silicic acid | Reduced H2O2, malondialdehyde (MDA) content and EC by 24.78–34.78%, 20.0% and 32.92–37.79%, respectively and increased SOD, CAT, APX and POD activity by 36.89–68.89%, 135.58%, 59.36–66.77% and 48.69–53.59%, respectively, in two different rice cultivars | (286) |
| Rice | Silicic acid @ 0, 0.5 and 1.0 mM | Decreased O2−·, H2O2, electrolyte leakage (EC) and MDA content by 11–16%, 9–10%, 13–17% and 13–18%, respectively | (128) |
| Maize | Si and biochar @ 100 mg kg−1 and 50 g kg−1, respectively | Combined application of Si and biochar significantly enhanced the antioxidant activities (SOD, POD, CAT, and APX) by 34.72, 23.12, 24.49, and 35.29%, respectively | (287) |
| Wheat | 1.0 mM H4O4Si | CAT, POD, and GR activities significantly increased in roots under Si supplementation in As-stressed plants. In shoots, application of Si showed a significant increase in CAT activity compared with As stress. | (288) |
| Date palm | 1 mM Na2SiO3 | Enhanced accumulation of polyphenols (48%) and increased antioxidant activities (POD: 50%, PPO: 75%, GSH: 26.1%, CAT: 51%) resulted in a significant decrease in superoxide anion (O2+· : 58%) and lipid peroxidation (MDA: 1.7-fold) | (289) |
| Brassica juncea | SiO2 NPs @ 200 ppm | Significant reduction in oxidative stress markers, with H2O2 and MDA levels decreasing by 41% and 39%, respectively, and increased activities of antioxidant enzymes activity by 84% (SOD), 73% (POX), and 69% (CAT) along with 27% (proline content) | (290) |
Impact of Si application on antioxidant defense mechanisms under As stress.
4 Agro-wastes
Agricultural wastes are residual byproducts from crop cultivation and initial processing of agricultural produce, including vegetables, fruits, dairy, meat, and poultry (132). These wastes encompass non-edible materials such as crop residues, forest litter, animal manure, and chemical remnants from fertilizers and pesticides (133). Generated through activities like seed production and livestock management, agro-waste poses serious environmental concerns, particularly when openly burned, contributing to air pollution and health risks (134). Post-harvest waste accounts for nearly 80% of total agricultural biomass, with burning still widely practiced. In India, Punjab, Maharashtra, and Gujarat are the leading states where extra residue is incinerated (Figure 7). Sustainable management requires conservation, recycling, and reuse strategies (135). Agro-wastes are categorized as field residues (e.g., stalks, stems), process residues (e.g., husk, bagasse, molasses), and commercial byproducts such as orange peel and oil cakes (136). Annually, millions of tons of agro-waste are generated worldwide, with over 90% in low-income nations being incinerated or discarded in open spaces, exacerbating environmental deterioration (137). Asian nations lead in the production of crop residues, particularly from silica-dense grains. India produces over 500 million tons of agricultural waste each year, contributing to a worldwide total of almost 1 billion tons (138). Due to escalating population pressures and food demand, nations such as India and China are encountering growing leftover surpluses. While usage and surplus fractions vary by crop type the surplus crop residue (Table 5) is improbable to meet potential demands; nevertheless, high-resolution spatio-temporal biomass availability may assist in overcoming current challenges in crop residue utilization (139).
Figure 7
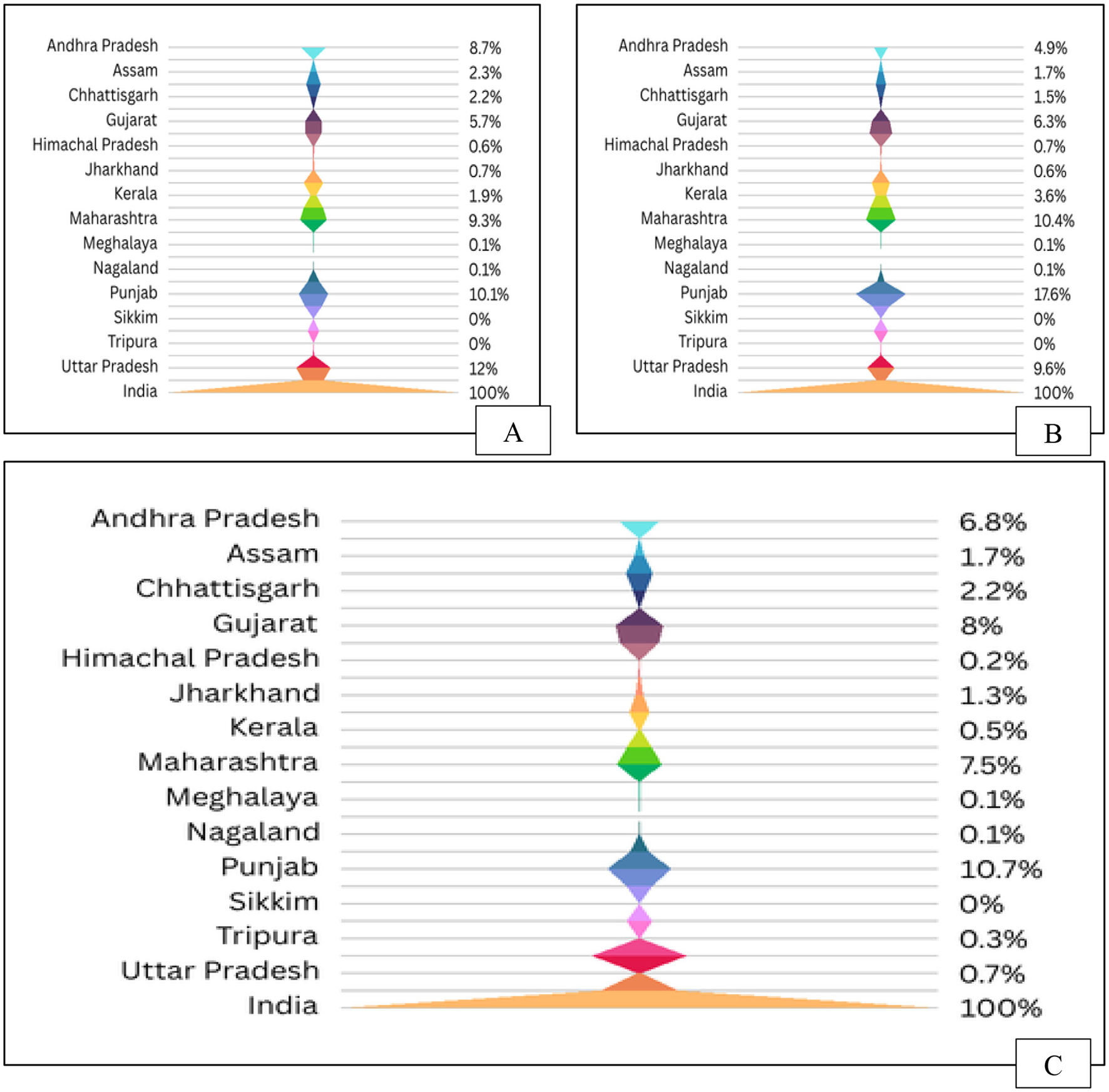
Crop residue dynamics in India: (A) State-wise residue generation, (B) Residue surplus availability, and (C) Residue burning status.
Table 5
| Country | Gross residue generation (MT yr−1) | Surplus (MT yr−1) |
|---|---|---|
| India | 500 | 140 |
| 912 | 300 | |
| 682.6 | 178 | |
| 683 | – | |
| Bangladesh | 72 | – |
| 99.6 | 24.3 | |
| Indonesia | 55 | – |
| Myanmar | 19 | – |
| Afghanistan | 9.7 | 2.2 |
| Bhutan | 0.4 | 0.1 |
| Nepal | 22.8 | 6.3 |
| Pakistan | 122.8 | 37.3 |
| Sri Lanka | 4.7 | 1.3 |
| China | 1,039.5 | – |
| USA | 488 | – |
| World | 2,445.2 | – |
| 3,758 | – |
Global estimates of crop residue generation and surplus availability (MT yr−1) across selected countries [adopted from Sen et al. (291)].
4.1 Si rich agro-wastes
Agricultural waste generation has increased steadily, driven largely by population growth, making it essential for environmental agencies to adopt strategies that minimize waste production. Recycling agro-wastes offers an effective means to reduce their adverse impacts on the environment and human health (140). Recent research focuses on using silica-rich waste materials to mitigate As bioaccumulation in plants (Table 6). Although Si is the most abundant element, its concentration in agro-wastes is lower than in primary minerals. Nonetheless, the vast quantities of agro-waste generated globally present a promising source for Si extraction (141). Alternative silica sources currently explored worldwide include rice husk, wheat husk, palm oil fuel ash, Miscanthus ash, e-waste, coal ash, reed ash, sedge ash, Carex riparia, sugarcane bagasse, bamboo leaves, natural clay, and ore tailings.
Table 6
| Raw material | Mechanism of arsenic mitigation | Effectiveness/efficiency (%) | Microbial mediation role | References |
|---|---|---|---|---|
| Rice husk | Enhanced Si release to pore-water, Fe-plaque formation | 25–50 | Increased activity of soil microbes that express the As methyltransferase gene, arsM | (244) |
| Rice husk and husk ash | The competitive interactions between Si and As for plant uptake and sorption | 36–58 | (245) | |
| Rice husk | Significantly more ferrihydrite and less goethite, thereby more As(III) associated with Fe-plaque | ~40 | (292) | |
| Rice husk biochar and husk ash | 20–24 | (90) | ||
| Rice husk and husk ash | Increase in the mole ratio of porewater Si/As, indicating an elevated pool of dissolved Si to compete with As for root uptake by their shared transporters | ~50 | (249) | |
| Fe-modified rice hull biochar | Decreased As/Fe ratio in root plaque | 37–79 | Reduced abundance of Fe(III) reducing bacteria by 24–64% | (250) |
| Rice straw biochar | Increased As solubilization in the porewater, functional groups of biochar capable of immobilizing As | 41.4–57.5 | (251) | |
| Paddy straw compost with SSB | Reduced bioavailable As, higher Fe-plaque formation and presence of As uptake transporters in rice roots | 34.2–53.2 | SSB improved solubilization of Si from straw compost than its sole applicaiton | (10) |
| Charred rice husk | Increased the fraction of ferrihydrite in the root plaques | 70.6 | Increased the copy number of arsM in paddy soil, suggesting an increased capacity for arsenite methylation | (78) |
Effectiveness of Si-rich agro-wastes and their derivatives in reducing As bio-accumulation in final produce.
4.1.1 Rice husk
Rice is one of the most widely cultivated crops globally, with production surpassing 756 million tons in 2020. Milling generates approximately 20% of this yield as rice husk, a major by-product (142). Commonly discarded or used as fuel during parboiling, rice husk contains high levels of organic compounds such as lignin and cellulose, along with significant mineral content, particularly silica (143, 144). The high silica content has attracted interest for environmental applications, notably in reducing arsenic (As) toxicity in soils and plants. Rice husk ash (RHA), produced by combustion, typically contains 87–99% silica, depending on husk origin and burning conditions (143, 145). Quality and composition of RHA are influenced by soil type, climate, cultivation methods, and pre-treatment. RHA is characterized by high ash content compared to other biomass fuels, with properties such as high porosity, low bulk density, and large surface area, making it highly suitable for adsorption processes, including As mitigation. Silica in RHA occurs in both amorphous and crystalline polymorphs like, quartz, cristobalite, and tridymite, whose proportions depend on combustion temperature and treatment parameters (146). The crystalline structure formed is contingent upon the combustion temperature and treatment parameters. The extraction of amorphous silica generally entails acid leaching, succeeded by burning or pyrolysis to eliminate organic material and produce high-purity silica. This technique guarantees the synthesis of silica customized for catalytic, adsorption, and other sophisticated material applications (147).
The structural differences between amorphous and crystalline polymorphs influence adsorption affinity and binding mechanisms for As species [both As(III) and As(V)] in soil–water systems (148). Amorphous silica typically has a much higher specific surface area and more silanol (Si–OH) groups than crystalline quartz, enhancing As adsorption through ligand exchange or hydrogen bonding (149). The density and reactivity of Si–OH groups vary with polymorph type and surface treatment. More reactive surfaces (common in amorphous forms) facilitate stronger chemisorption of arsenate and arsenite ions. Crystalline silica is generally less reactive due to lower surface hydroxyl density, resulting in weaker As retention, unless weathering or surface functionalization creates active sites. Also, the point of zero charge (PZC) of different silica polymorphs influences As speciation and binding. For example, at pH above the PZC, surfaces become negatively charged, reducing electrostatic attraction for arsenate but allowing specific adsorption via inner-sphere complexes (150).
Utilizing RHA as a silica source enhances the value of an agricultural byproduct while fostering environmentally sustainable practices. The capacity to regulate silica polymorph formation by temperature and pre-treatment presents opportunities for specific applications in environmental research, such as arsenic remediation, water purification, and nanomaterial synthesis (151).
4.1.2 Rice straw
Rice straw, a significant agricultural by-product, is produced in excess of 700 million tons each year after the rice harvest (152). Worldwide, around 20% of rice straw is employed, with more than 100 million tons incinerated each year (153), resulting in significant environmental and health issues, especially in nations such as India (154, 155). Rice straw possesses various potential applications, including animal feed, mushroom growing, energy generation, biochar, bioethanol, and biogas production; nevertheless, its elevated silica content constitutes a significant constraint. rice requires a significant amount of silica (10–12% of dry matter) (156) for mechanical strength and resistance to biotic and abiotic stressors (157). Silica exists in the dry matter of straw, predominantly as phytoliths, which enhance the plant's structural integrity (158). These silica-rich structures are integrated within the lignocellulosic matrix of the straw, consisting of cellulose (32–47%), hemicellulose (19–27%), and lignin (5–24%) (159, 160). Although it poses a hindrance to its application in certain sectors, the silica present in rice straw has significant environmental advantages. The integration of rice straw into soil using rice straw-based composites (RSBC) facilitates gradual Si release (159), hence augmenting nutrient availability, promoting plant development, and enhancing stress resilience, particularly in Si-deficient paddy fields (161). This promotes sustainable agriculture and aids in the attainment of Sustainable Development Goals (SDGs). Rice straw is a hybrid nanocomposite composed of cellulose and silica (SiO2), wherein silica nanoparticles serve as reinforcing agents within the plant's cellular matrix (162). Studies demonstrate that the majority of silica in straw is present in an amorphous state, predominantly located on the external surfaces of the sheath and stem (162). The incorporation of rice straw into circular bioeconomy methods, specifically for sustainable silica recovery and reuse, offers a practical approach to managing agricultural waste, mitigating environmental effect, and fostering resource-efficient farming systems (163).
4.1.3 Sugarcane bagasse
Sugarcane (Saccharum officinarum) is crucial to the economics of numerous developing countries because of its importance in worldwide sugar production (164). Presently, Brazil is the foremost producer, accounting for approximately 36% of global output (165). Nonetheless, sugarcane processing produces substantial quantities of byproducts, including bagasse, straw, and cane tops (166), which present environmental disposal difficulties. Sugarcane bagasse is a viable feedstock for reducing arsenic (As) translocation in plants, owing to its availability, affordability, and substantial silica (Si) concentration. The buildup of silica in sugarcane is contingent upon the availability of silicon in the soil, which is absorbed by the roots in the form of silicic acid, thereafter transported, and deposited as amorphous silica throughout the plant tissues via transpiration. The silica concentration in sugarcane bagasse fluctuates according to species, soil conditions, fertilizer use, and growing methods. Sugarcane bagasse ash (SCBA) has both amorphous and crystalline silica, including quartz and cristobalite (167), with quartz occasionally included through sand adherence during harvesting (168). SCBA provides a sustainable alternative for silica production, facilitating waste valorization and circular economy frameworks. Enhancing recovery techniques, including response surface approach, guarantees high-purity silica (169) appropriate for diverse industrial applications while promoting ecologically sustainable resource management.
4.1.4 Wheat husk
Wheat husk serves as a significant by-product in the wheat production process, with estimates indicating that around 1.5 tons of wheat husk are generated as solid waste for every ton of wheat produced (170). Conversely, wheat husk has frequently been incinerated or utilized as livestock feed and fertilizer. Consequently, the ash generated from burning wheat husk (WHA) can lead to significant environmental issues due to the emission of substantial amounts of harmful pollutants. To mitigate this significant environmental issue, studies have been undertaken regarding the utilization of WHA as a renewable, cost-effective, and environmentally friendly source of amorphous silica, considering the high silica content found in WHA (171). The wheat husk primarily consists of cellulose (23–42% by weight), hemicellulose (18–21% by weight), lignin (14–28% by weight), and starch (9–19% by weight); lignin renders it a possible source of silica/lignin hybrid minerals (172). Various researchers conducted analyses on the elemental silica content, determining it to be approximately 2.1% (weight basis) to 2.57% (weight basis). Sodium silicate is a compound that serves as a precursor to Si. Its extraction from ashes presents an alternative method, as traditional production processes demand significant energy, typically sourced from quartz sand combined with sodium carbonate at 1,300 °C (173). Biosilica-based materials derived from wheat waste may serve as secondary products that enhance the value of agricultural crops. Furthermore, silica with varying properties, such as nano silica and meso/macro porous silica, can be efficiently produced from wheat husk tailored to its specific application (174). The ash content of the wheat husk and spike exceeds 20%, comprising 86% SiO2, which is ascribed to the type of fertilization applied (172). Terzioglu et al. (175) determined that an ashing temperature of 1,000 °C yields the highest SiO2 content; however, this temperature cannot be regarded as the optimal ashing temperature due to the irrecoverable structure of silica (cristobalite). Wheat husk phytoliths are spherical (14–22 μm diameter) and oblong (18–40 μm length, 12–18 μm width) in epidermal cells and consist of a silica shell and the plant cell's organism core (176). Wheat husk possesses a higher concentration of surface Si, rendering it a more viable Si source for the remediation of As toxicity (138).
4.1.5 Bamboo leaf
Bamboo is one of the most important non-wood forest products worldwide, valued for its rapid growth and diversity, particularly in subtropical regions of Asia, Africa, and Latin America (145). It is widely used in construction, household items, pulp, paper, textiles, and handicrafts. However, only about 40% of harvested bamboo is effectively utilized, with 50–80% discarded as agro-industrial waste (177). While bamboo stalks are the primary raw material, leaves are generally treated as waste. These leaves can be used as a fuel source, producing considerable quantities of bamboo leaf ash (145). Although agro-wastes like rice husk, corn cob, and sugarcane bagasse are well-known silica sources, bamboo leaves remain underutilized, despite being an abundant, low-cost, and commercially untapped source of high silica content. The ash from bamboo leaves contains a significant silica content, ranging from approximately 75.90% to 82.86%, as indicated by Olawale (178). Setiadji et al. (179) successfully extracted 81.76% pure amorphous silica from bamboo leaf ash using an alkaline solvent.
4.1.6 Corn cob
Corn cobs, an agricultural byproduct of maize—a major grain crop cultivated globally—are composed primarily of cellulose and lignin, with notable mineral content including silicon (Si, 0.133 wt%) (180, 181). Upon combustion, corn cob ash (CCA) contains over 60% silica by mass along with trace metallic elements (182). Produced as a fine powder, CCA requires no further grinding, making it a highly cost-effective raw material for silicate, silica, and silica nanoparticle production (183). While corn cobs have been extensively studied for uses such as enzyme production, protein extraction, adsorbents, fuels, and cement manufacturing, limited research has explored CCA for silica extraction and applications. Chanadee and Chaiyarat (184) demonstrated that sweet corn cobs (Zea mays saccharata L.) yield optimal silica powder at a combustion temperature of 600 °C. XRD analysis confirmed its amorphous structure, FTIR identified silanol and siloxane functional groups, and XRF revealed a silica content of 46.9% (185). These findings highlight CCA as a promising, low-cost, and underutilized silica source for industrial and environmental applications.
4.1.7 Reed ash
Phragmites australis (Cav) Trin. ex Steud, commonly known as common reed, is a native perennial plant found in wetlands globally, primarily utilized as a domestic fodder (186). It can be utilized for several applications, including paper manufacture, construction materials, feed, phytoremediation, electricity generation, energy supply, and bioethanol. Aquatic common reed significantly contributes to aquatic habitats by serving as a natural cleanser through its phytoremediating properties and mitigating river erosion (82). Currently, the common reed is recognized as a significant environmental issue, as its adaptability to various environments obstructs the growth of other ecologically vital plant species. Notwithstanding the various applications of reed, it has been utilized in certain regions globally as a financially sustainable biomass for energy generation, as noted by Kobbing et al. (187). Subsequently, the incineration of common reed for energy generation results in the formation of common reed ash (CRA) as the primary by-product (188). CRA possesses a significant SiO2 content and offers a distinctive opportunity to serve as a cost-effective and plentiful source of amorphous silica (145) for the environmentally conscious mitigation of As toxicity.
5 Microbial mediated solubilization of Si
Although constituting 27% of the Earth's crust and ranking as the second most abundant element, the limited solubility of most Si forms inhibits their absorption by plants (189). Si exhibits a notable affinity for oxygen; consequently, it predominantly occurs in nature as silicates (SiO3), a form that is not readily absorbable by plants (17). Aluminosilicates, ferromagnesian silicates, silicon dioxides, amorphous silica, clay, feldspar, and mica are all examples of compounds that fall under the umbrella term “silicates.” Other silicates contain iron, calcium, sodium, potassium, or sodium, and ferromagnesian silicates include amphiboles, olivine, and pyroxenes. Silicas make up more than 90% of the Earth's crust and are present in substantial amounts in sedimentary, igneous, and metamorphic rocks as well. Depending on the soil's pH levels, Si can also appear as silicic acid (190). The release of Si into the soil by weathering or dissolution is necessary for plant uptake (191). Along with water, plants absorb orthosilicic acid, a soluble form of Si. According to Klotzbücher et al. (192), monosilicic acid is produced when soil nutrients are depleted, Si-containing minerals weather, and irrigation is used. Si fertilizers, in contrast to more conventional fertilizers, are expensive and scarce, making them out of reach for most farmers. Hence, Si fertilizers are rarely used, especially in developing countries (17). Reusing materials with Si concentrations from mining, agriculture, and construction and demolition can lead to the production of silicate fertilizers with long-term economic viability (193). Thakral et al. (194) reported that the concentration of Si in the soil solution is significantly affected by the solubilities of both primary and secondary minerals. Soil applications involving biochemical and physicochemical treatments can speed up the solubilization of these chemicals, with microbial activity being the most important factor in biochemical action (17).
Microorganisms are recognized for their ability to breakdown and mobilize minerals in the soil (195). Numerous investigations have established that microorganisms isolated from silicate mineral surfaces weather various silicates (196, 197). This signifies the crucial function of silicate-solubilizing microorganisms (SSM) as biofertilizers in the solubilization of silicates and phosphates (198, 199). Microorganisms are prevalent in soils, although only a limited subset is capable of solubilizing insoluble silicates. Plants and microflora are known to generate chelating ligands, modify soil physical properties, and influence the dissolution and mobilization of soil silicate minerals (199). Among microorganisms, plant-associated bacteria, fungi, actinomycetes have been documented to facilitate the dissolution of silicates and expedite the release of Si into the plant-soil system through bio-weathering processes.
5.1 Silicate solubilizing bacteria (SSB)
Microorganisms such as Burkholderia, Bacillus, Pseudomonas, and Enterobacter have been documented to solubilize various types of silicates, including magnesium silicate, quartz, feldspar, and other insoluble silicates (Table 7). SSB is primarily located in soil, water, sediment, mineral ore, weathered rocks, and the rhizosphere of plants, where it plays a crucial role in regulating the biogeochemical cycle of Si (200). Vasanthi et al. (201) indicated that a considerable amount of SSB linked with phyto-sil, muscovite, and calcium aluminosilicate suggests that these minerals preserve them from their natural sources of extraction. The clarification provided indicates that the ratio of SSB associated with a mineral does not align with its silica concentration. For instance, muscovite, which contains 21% silica, displayed a higher proportion of SSB compared to phyto-sil, which has 78% silica. This observation contrasts with quartz at 98%, talc at 54%, and feldspar at 45% silica. The findings clearly demonstrate a notable difference in the overall bacterial presence within soil or silicate minerals compared to the SSB.
Table 7
| Sl. no. | Agro-ecology | Silicate solubilizing bacteria | Plant cultivar | Source of isolation | Medium of isolation | Source of silicate used for isolation/characterization | Focused area of interest | References |
|---|---|---|---|---|---|---|---|---|
| Daegu, a city of Gyeongbuk Province, Republic of Korea | Burkholderia eburnea | Oryza sativa L. cv. Dongjin | Rice rhizosphere | Silicate medium | Magnesium trisilicate | Silicate solubilization, IAA production, ↑ plant growth, ↑ Sie uptake and deposition | (18) | |
| Institute of Natural Sciences and Mathematics, Ural Federal University, Ekaterinburg, Russia | Bacillus Species | Brassica juncea (L.) | Clay substarte | Zak-Alexandrov medium | Sodium silicate | Structural and functional parameter of photosynthetic apparatus | (293) | |
| Microbiology and Environment Laboratory, the Indonesian Research Institute for Biotechnology and Bioindustry, Bogor | Burkholderia cenocepacia KTG, Aeromonas punctata RJM3020 and Burkholderia vietnamiensi ZEO3 | _ | Sandy soil | Bunk and Rovira medium | Magnesium trisilicate and quartz | Production of citric, acetic and oxalic acid; ↑ solubilization of silica | (294) | |
| Teaching Farm of Fujian Agriculture and Forestry University, Fuzhou, China | Aeromonas, Bacillus, Cellvibrio, Ensifer, Flavobacterium, Microbacterium, Paracoccus, Pseudomonas, Rhizobium, and Streptomyces | Zea mays L. cv. Yuebai | Earthworm gut and surrounding soil | Orthoclase feldspar | Aleksandrov's medium | Silicate weathering and availability to plants | (295) | |
| Division of Microbial Technology, CSIR-National Botanical Research Institute, Lucknow, India | Pseudomonas and Bacillus (Sphingobacterium sp., B. amyloliquefaciens) | Oryza sativa cv. Jayanti | Rhizospere | Magnesium trisilicate, talc, and feldspar | Si solubilizing media (NBRISSM) containing feldspar as silicate | ↑ Si uptake, ↓ disease severity, and antioxidative enzyme activities | (242) | |
| Gyeongbuk, South Korea | Enterobacter ludwigii | Rice mutant Waito-C and rice cultivar ‘Hwayoungbyeo | Paddy soil and forest soil samples | Magnesium trisilicate | Glucose agar medium | Potential Si and phosphate bio-fertilizer | (296) | |
| Bacillus mucilaginosus | Rhizosphere soil | Magnesium trisilicate | Bunt and Rovira medium | (297) | ||||
| Puducherry, India | Bacillus flexus, B. mucilaginosus, B. megaterium and Pseudomonas fluorescens | _ | Soil samples from red soil, plantation soil, sea sand, pond sediment, sea water | Magnesium trisilicate, feldspar, calcium aluminosilicate, sodium aluminosilicate, talc, muscovite, illite and quartz | Bunt and Rovira medium | The dissolution of silica in solution functions as a nutrition for living organisms. | (201) | |
| Longshan (Nanjing, China) | Rhizobium tropici | _ | Weathered rocks | Feldspar and biotite | Solid K-limited medium (KLM) | ↑ Si and K concentrations | (298) | |
| Besut, Terengganu, Malaysia | Serratia marcescens and Pseudomonas aeruginosa | Oryza sativa var MR219 | Rhizosphere soil | Magnesium trisilicate | Magnesium trisilicate media | ↓ chemical application in rice sheath blight | (49) | |
| Fujian Agriculture and Forestry University, Fuzhou, China | Kosakonia sp. | Zea mays L. | Bryophyte Hypnum plumaeforme rhizoids | Feldspar and silica | Aleksandrov medium | Si availability in the soil, Si uptake and plant growth | (203) | |
| ICAR- IRRI farm, Rajendranagar, Hyderabad, Telangana, India | Rhizobium sp. | Oryza sativa genotype BPT 5204 | Rhizopshere soil | Bentonite, calcium aluminio silicate, fuller's earth, kaolin, magnesium trisilicate, potassium alumino silicate and quartz | Bunt and Rovira medium | ↑ Rhizosphere available Si concentration. | (224) | |
| Chinese Academy of Agricultural Sciences | Bacillus mucilaginosus | _ | Chinese Academy of Agricultural Sciences | Mica and feldspar | Silicate medium | Decomposition of silicate minerals by the becterium | (299) |
Silicate solubilizing bacteria isolated from different cultivars and their role.
The rhizosphere of crop plants such as rice has been extensively studied for the isolation of SSB due to the significant Si need and uptake by rice plants. Comparable initiatives have been implemented with numerous other Si-rich accumulator plant species (200). Tropical forests, especially bamboo forests, are recognized as significant sources of soluble Si in rivers. This is mostly attributable to the significant accumulation of Si in bamboo leaves, perhaps due to the activities of soil-Si bacteria. Nevertheless, minimal attempts have been made to discover SSB inside bamboo rhizosphere or forest environments. Recent reports indicate Si buildup in 456 distinct plant species cultivated under same soil conditions (202). Hu et al. (203) identified a Kosakonia genus SSB from the rhizomes of Hypnum plumaeforme, which promotes Si absorption and accumulation in maize, hence increasing growth. The investigation of SSBs offers a cost-efficient and eco-friendly approach to augmenting plant nutrition in Si, phosphorus, and potassium, consequently raising agricultural yields (201, 204).
5.2 Silicate solubilizing fungi (SSF)
The majority of the literature is based on the population and variety of SSB, whereas SSF has been minimally investigated. The fungal species Aspergillus niger, Trichoderma sp., Beauveria caledonica, and Serpula himantioides have been examined for their ability to solubilize silicates (205). Two SSF isolated from soil were screened and identified as Penicillium limosum and Bipolaris sorokiniana (206).
5.3 Mechanism involved in silicate solubilizing activity observed in microbes
The extraction of microbiological nutrients from insoluble silicates depends on a conventional geochemical process known as bio-weathering (200). In this process, living things break down soil minerals and bring them to the surface. A wide variety of saprophytic bacteria, actinomycetes, and fungi are the principal agents of bioweathering. The growth of plants is supported by these bacteria because they dissolve important nutrients for plant-soil interactions. Plants are able to absorb and use newly formed nutrients because bio-weathering is the main process that transforms polymerized silica into monomeric forms (207). The bond strength between Si and its neighboring components determines how easily the bonded Si can be released from the framework (208). An example of a material that shows resistance to dissolving even when subjected to high temperatures and pressures is SiO2 polymers (82). Materials such as quartz, silica, and phytoliths can only be dissolved through proton action and mineral-bound cation exchange, while metal-bound silicates require a coordinated shift in pH and ligand attack (200, 209). Some bacterial species may have varying solubilization capacities depending on the mineral supply. Biogenic materials like siliceous earth, diatomaceous earth, rice straw, and rice husk, insoluble inorganic silicates of potassium, magnesium, and aluminum, and silicate minerals like biotite and feldspar are all potential sources of soluble silica that these bacteria can release (17).
In the most fundamental concept of silicate solubilization, bacteria employ a number of mechanisms to facilitate a multi-step process. According to many studies (200, 210–212), the process begins by replacing protons on the mineral surface with charged cations such as K+, Na+, and Ca2+. Then, hydrolysis occurs and the silica species is detached from the framework. In order for microbes to break down and dissolve silicates, they are thought to employ a number of interconnected processes (Figure 8), such as (i) lowering the pH through the production of inorganic and organic acids, (ii) synthesis of chelating metabolites, and (iii) engaging in nucleophilic attack and exchange reactions (213). The primary mechanism observed is the acidolysis occurring in the vicinity of microorganisms (214).
Figure 8
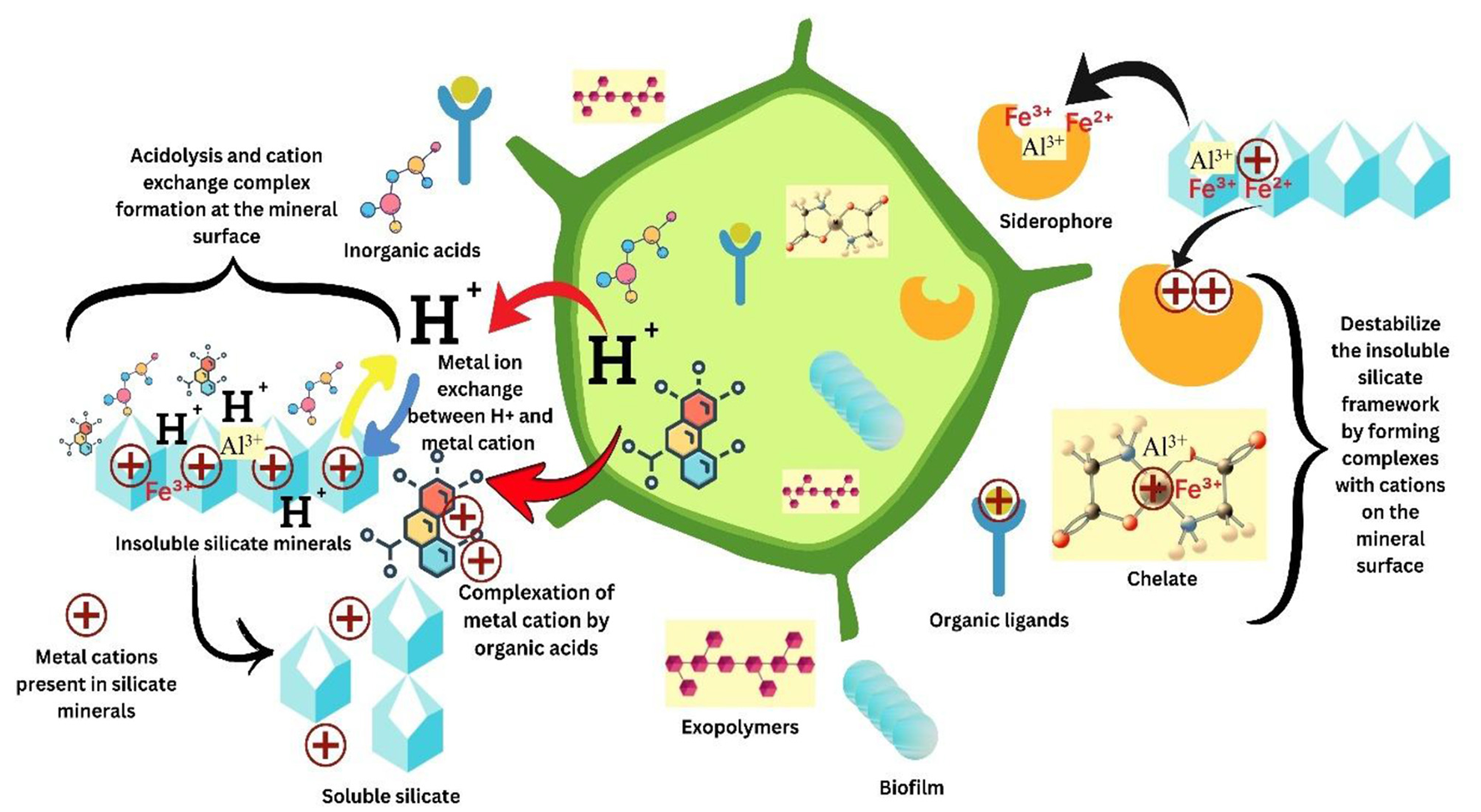
The molecular mechanisms behind the silicate solubilising activity reported in bacteria. The silicate solubilisation process is facilitated by several processes, including the reduction of pH through the production of organic and inorganic acids, the displacement of charged particles at mineral surfaces, and the synthesis of microbial metabolites, enzymes, and exopolymers.
5.3.1 Changes in pH due to organic acid production
An important geochemical phenomenon, the interaction between organic acids and mineral surfaces has been studied extensively for decades, especially LMOAs (215). LMOAs are frequently present in rhizosphere soils, particularly in the layer immediately adjacent to the soil-root contact (216). The breakdown of plant roots, fungal remnants, and other organic components mostly produces these acids (217). Lazo et al. (218) found that organic acids and their anions can accelerate mineral weathering. Kong et al. (216) suggested that organic acids can form complexes with soil elements due to the presence of carbonyl and hydroxyl functional groups. According to Drever and Stillings (219) and Lazo et al. (218), there are three primary processes that impact mineral weathering caused by organic acids: (a) changes in solution ion speciation, (b) adjustments in solution saturation relative to the mineral, and (c) disturbance of the dissolution reaction from equilibrium. Casey et al. (220) described mineral bio weathering as an acid-base process involving bridging oxygens that occurs when hydroxyl or hydrogen ions are adsorbed onto the surface of minerals. Consequently, the amount of hydroxyl ions and protons on the surface plays a pivotal role in the release of Si. In the microenvironment, that microbes create surrounding a mineral, protons and other organic and inorganic compounds are secreted, which aid in the breakdown of silicates (200). As a consequence of released H+ exchanging cations within the silicate framework, a cation exchange complex might form on the surface of the material. Acidolysis is further expedited when bacteria release both organic and inorganic acids (221). A high proton content and an acidic environment make cation replacement easier (222, 223). A change in the dissolution rate, away from equilibrium, causes silicates to dissolve more quickly in an acidic environment (200). The diversity of microbes determines the specific organic acid that is emitted. There are a number of organic acids that have been found to dissolve silicates in media that contain quartz, feldspar, and magnesium trisilicates as sources of Si. These acids include maleic, succinic, fumaric, gluconic, tartaric, and hydroxy propionic acids (201, 224). In addition to the organic acids generated by bacteria, the breakdown of organic matter generates NH3, H2S, and CO2. These byproducts are easily bio-converted into inorganic acids by microorganisms such as Thiobacillus, Nitrosomonas, and Nitrobacter (200). It has been suggested that the creation of ammonia and amines might cause an increase in the pH of the surrounding environment, which in turn affects silicates. This suggests that the production of alkali could be a way for silicates to be solubilized (225). According to Kutuzova (225) and Rajabipour et al. (226), silicates can be influenced by changes in environmental pH caused by the generation of ammonia and amines, suggesting that alkali production could be a way for silicates to be solubilized. In a study conducted by Sheng et al. (227), it was found that Bacillus globisporus Q12, a type of bacteria that can dissolve silicates, was able to dissolve K and Si in silicate minerals like muscovite, biotite, and feldspar. The researchers found that organic acids, specifically acetic and gluconic acids, were the most effective in this process. In a different study, Sheng and He (228) found that SSB-driven illite and feldspar help produce organic acids like malic, tartaric, gluconic, citric, oxalic, succinic, and 2-ketogluconic acids. When it comes to solubilizing potassium or silicates, tartaric acid is by far the most common agent. The local environment and ionic composition can be altered by microbial deposition near silicate sources; however, our understanding of the consequences of ionic strength is lacking on-exchange reactions taking place on mineral surfaces might be hindered by elevated ionic strength (229). Speciation on the surface is affected by changes in ionic strength because of the double layer effect. When the ionic strength increases, the surface charge becomes more positively charged at pH levels below pHzpc and more negatively charged at pH levels above pHzpc. As a result, it speeds up the breakdown process (230).
5.3.2 Synthesis of chelating metabolites
In addition to acidifying and improving the solubility of silicates, Organic and inorganic acids can protonate and hydrolyze them, while concurrently complexing with the cationic components of silicates, making them possible chelating agents (201). Microbial metabolites such as extracellular enzymes, siderophores and other reaction byproducts play a significant role in silicate dissolution. These microbially excreted metabolites possess metal complexing properties that can bind with aluminum and iron in silicates, eventually destabilizing the silicate framework, thereby increasing the solubility of silicates. Drever and Stillings (219) reported the formation of oxalate complex due to the reaction of oxalic acids with Fe and Al. This in turn reduces the chemical activity of the cations in the silicate framework. The dissolution of silicates resulting from the production of keto-gluconic acid by bacteria, which complexes and chelates with metals, has also been reported (201, 231).
Siderophores are low molecular weight organic chelators characterized by a high and specific affinity for Fe (III). Siderophore biosynthesis is regulated by iron concentrations, and siderophores facilitate iron uptake in microbial cells (232). Bacteria, such as cyanobacteria, fungi, and plants that utilize phytosiderophores, synthesize siderophores in environments with low Fe3+ concentrations (17). Siderophores produced by SSB can solubilize Si by extracting iron from silicate minerals, as evidenced in hornblende degradation (233, 234). Phosphate-solubilizing bacteria (PSB) can also solubilize silicates via siderophores, potentially affecting the solubilization of Si and phosphorus from rocks (235).
5.3.3 Nucleophilic attack and exchange reactions
Stumm (230) found that when ligands are included in the coordination sphere of metal ions, the reactivity of the other molecules of coordinated H2O is enhanced. In general, the coordinated ligand's σ electron-donating, nucleophilic capacity causes the water exchange rate to increase. The water exchange rate is increased by several orders of magnitude when an OH− ion attacks a hexa-coordinated aquo metal ion nucleophilically (230). This means that the surface functional groups have been deprotonated, which increases the reactivity of the –Si–O-bond. The dissolution process at the surface of the mineral, which is aided by OH-bonding, deprotonation, or ligand complexation, is known as depolymerization, or the dissociation of a Si–O–Si link. Surface hydroxyl group replacement ligands can bind nucleophilically with metal ions in the surface lattice to form surface crystalline bonds. Dicarboxylic acids, hydroxy carboxylates, diphenols, EDTA, and NTA are ligands that contain functional groups with two or more donor atoms; these ligands can form bi- or multi- dentate mononuclear surface chelates, which are very efficient. The surface lattice is negatively charged and surface protonation is enhanced by the presence of certain ligands. As the surface concentration of ligand increases, so does the rate of ligand-assisted dissolution. The surface metal centers can be released into solution more easily when a bi- or multi- dentate ligand coordinates inside a mononuclear inner-sphere surface complex, which aids in ligand-facilitated dissolution (236). Factors of paramount importance include the surface chelate size and the quantity of donor atoms coordinating to a particular surface metal center. When it comes to improving the rate of dissolution of Al-minerals, Furrer and Stumm (237) reported that the five-membered surface chelate ring of oxalate is better than the six-membered rings of salicylate and malonate, as well as the seven-membered rings of succinate and phthalate. Monodentate organic surface complexes have a negligible effect on the dissolution of σ-Al2O3. Complex formers generally form rather weak surface complexes on silica surfaces; nevertheless, the nucleophilic citrate and oxalate enhance the dissolution rate of quartz (238).
5.3.4 Exopolymers and enzymes
Silicate-solubilizing bacteria generate extracellular proteins and polysaccharides that create biofilms surrounding their colonies (239). These biofilms facilitate microbial adhesion to mineral surfaces and affect mineral dissolution. They establish a micro-environment that minimizes the loss of protons, ligands, and organic acids (200). Biofilms possess water retention properties, hence promoting mineral weathering. Elements of the bacterial cell membrane, including lipopolysaccharides, peptidoglycan, and teichoic acids, can interact with silicate ions for solubilization (240). Engineered gluconic acid synthesis and excellent dissolving of poorly soluble calcium phosphates were achieved by cloning the gabY gene from Pseudomonas cepacia. This process shed light on the genetic principles of mineral solubilization (241). When it comes to solubilizing Si, Bist et al. (242) have shown that acidic phosphatase activity and organic acid generation are functionally related. New developments in high-throughput whole genome sequencing have made it possible to identify the genes that play a role in the metabolic pathways of acids, exopolymers, membrane transporters, and silicate-solubilizing ligands (200). For weathering of silicate minerals, these phenomena can be studied in greater detail.
6 Methods of Si-rich agro-wastes applications
Si-rich agro-waste, derived from crop residues such as rice husk, rice straw, wheat straw, sugarcane bagasse, corn stover etc. provides a sustainable source of plant-available Si to enhance crop productivity and stress tolerance (243). The effectiveness of these residues depends not only on their Si content but also on the application method, which influences Si solubilization, nutrient dynamics, and interaction with soil contaminants such as As.
6.1 Direct soil incorporation of raw biomass
Unprocessed crop residues are chopped and incorporated into soil before planting. Their subsequent decomposition releases soluble Si (H4SiO4) via microbial mineralization (191). Si accumulates in the rhizosphere, enhancing uptake by plant roots. This process although enhances soil organic carbon and microbial activity, the release of Si is slow and initial nitrogen immobilization during decomposition may occur, therefore, suitable for long-duration crops. Seyfferth et al. (244) observed that rice husk incorporation to soil (1% w/w) decreased grain As by 25–50% and straw As by at least 50%, and increased straw and husk Si by 25–60% without affecting yield in three different rice cultivars. Mamud et al. (245) conducted a study in Meghna Estuarine floodplain of Bangladesh which is known for its As laden groundwater and found out that in the Pleistocene terrace soils, fresh rice husk (1% w/w) reduced As in grain, husk, and straw by 36–40%, 36–41%, and 42–45%, respectively and in the Holocene floodplain soils, by 39–45%, 55–58%, and 50–51%, respectively.
6.2 Application of agrowaste in combusted form
Depending on the process of combustion two types of amendment can be derived from agrowastes. Open-air burning or controlled combustion of biomass in presence of oxygen at higher temperature produces ash such as RHA or sugarcane bagasse ash, can be applied directly to the field (246). Ash contains amorphous silica which dissolves in water to release monosilicic acid (173). Ash can also co-precipitate As and bind other heavy metals. Biomass, when pyrolyzed at controlled temperature (350–600 °C) and oxygen environment, produces biochar. Biochar retains Si in a reactive form and serves as a slow-release Si source (247). It also improves cation exchange capacity and As adsorption due to its porous structure. Several studies show the advantage of ash and biochar over direct incorporation of residue. Penido et al. (248) observed that both RHA and rice straw ash (RSA) amended soils had low As levels at or less than 0.2 μM L−1. Soils supplemented with fresh husk (FH), whether whole or powdered, exhibited marginally increased solution-phase Concentrations varied from 0.2 to less than 0.6 μM L−1 for FH whole and from 0.2 to 0.5 μM L−1 for FH powder amended soils. The solution phase exhibited concentrations approximately nine times greater in fresh straw amended soils compared to those amended with FH, RSA, or RHA, ranging from 1.0 to 1.8 μM L−1. Leksungnoen et al. (90) evaluated biochar and ash formed from Si-rich rice husk and showed that rice husk biochar and RHA (64% w/w) effectively reduced inorganic As buildup in rice grain to 0.27–0.29 mg kg−1, representing a 20–24% reduction compared to the control. Moreover, RHA substantially reduced grain-As(V) concentrations. Wang et al. (249) documented a 15.9–40.5% reduction in pore water As from tillering to harvest of rice, attributed to the application of Si-rich RHA in comparison to rice husk. The sequestration of As in the soil solid phase and root plaque rose by 8.0% and 26.9% with the application of RHA, likely due to the co-precipitation of iron and As facilitated by the liming effect of RHA, which was associated with a significant reduction in As transit. The inorganic As content in white rice diminished from 0.36 mg kg−1 in the control group to 0.24 mg kg−1 with rice husk and 0.17 mg kg−1 with Si-rich RHA, underscoring the efficacy of Si-rich RHA compared to rice husk. Kumarathilaka et al. (250) found that iron-modified Si-rich rice hull biochar (Fe-RBC) under intermittent flooding reduced As buildup in rice roots, shoots, husks, and unpolished grains by 62%, 37%, 79%, and 59%, respectively, in comparison to the standard flooded treatment. Limmer et al. (78) found a reduced concentration of straw and root As from 0.65 and 11.2 mg kg−1 in husk treated plants to 0.57 and 7.4 mg kg−1, respectively in charred rice husk treated plants. Maximum reduction (70.6%) in dimethylarsinic acid content in panicles was found in high-Si rice straw biochar applied pots followed by low-Si rice straw biochar applied pots (60.2% reduction) as compared to control (251).
6.3 Composted or co-composted Si-rich biomass
Crop residues are composted alone or co-composted with nitrogen-rich material (e.g., animal manure) to produce stable organic fertilizer enriched with Si. Microbial culture like PSB, SSB, and potash mobilizing bacteria can also be added for faster release of nutrients. Composting enhances Si bioavailability by degrading the phytolith matrix and increasing microbial solubilization (252). Khanam et al. (10) observed rice straw compost (RSC) significantly reduced (32.5% reduction) bioavailable As (NaHCO3 extractable) content compared to other amendments. The combination of SSB+RSC caused a further reduction by 38.7% in soil. The application of SSB+RSC resulted in a greater reduction in roots, shoot, and grains with the value of 49.4%, 34.2%, and 53.2%, respectively. The SSB+RSC treatment resulted in the highest transfer rates of As from soil to root and from shoot to grain were found to be the lowest (3.4 and 0.16, respectively) with SSB+RSC followed by RSC (4.0, 0.20). Yamaguchi et al. (253) reported that in lime + 2,250 g m−2 rice compost applied site, the pseudototal As concentration in the soils after 97 cycles of rice cultivation was approximately 60% of that in the plots without annual compost application. As concentration in the shoots and panicles of rice plants was consistently lowest in the lime + 2,250 g m−2 rice compost applied plots for last 6 years (92nd to 97th cropping).
6.4 Extraction of silica from agrowaste
Silica (SiO2) is well known as a precursor for many applied forms of Si like calcium silicate, sodium silicate, silicic acid, silica nanoparticles (SiO2 NPs), silica gel etc. These materials can be directly incorporated by soil applied or foliar spray and get rapidly absorbed through roots or stomata. Production of SiO2 from agricultural wastes can be accomplished in three different ways: chemical treatment, thermal treatment, or microbiological treatment (254). Different extraction methods with their final product has been depicted in Table 8.
Table 8
| Raw material | Extraction method | Extraction condition | Product | Average silica particle size | Silica purity (wt %) | References |
|---|---|---|---|---|---|---|
| Rice husk | Hydrothermal extraction | Ethanol 180 °C, 0.1 MPa, 24 h | Amorphous silica | 101 m2 g−1 of specific surface area | (300) | |
| Sorghum husk | Hydrothermal extraction | 1 M HCl, 120 °C, 0.1 MPa, 2 h | Amorphous silica | spherical, saddle, and dumbbell shape | (301) | |
| Rice husk | Combustion in muffle furnace | 600 °C, 2 h | Amorphous silica | 0.50–0.70 μm | 95.77 | (144) |
| Rice straw | Combustion | 500 °C, 8 h | Amorphous silica | 72.60 | (302) | |
| Coconut husk | 5N H2SO4 treatment, Combustion | 700 °C, 3 h | Crystalline silica | 91.76 | (303) | |
| Pine cone | 3M H2SO4, Thermal decomposition | 600 °C | SiO2 NPs | 37 nm | (304) | |
| Rice husk ash | Acid precipitation method | HCl washing before extraction, 60 °C | Amorphous silica | 0.50–0.70 μm | 99.2% | (144) |
| Rice husk ash | Acid precipitation technique | 80 °C | Amorphous silica | 10–15 nm | 98.9% | (305) |
| Paddy straw | Acid precipitation | Acid wash, 37 °C | Nano-silica | 15–20 nm | (306) | |
| Wheat straw | Leaching in a 10% (v/v) HNO3 and calcination | 4:1 (v:v) mixture of nitric and sulphuric acid washing, 400–700 °C | Amorphous hydrated silica | 75–320 nm | (307) | |
| Sugarcane bagasse (SCB), corn stalk (CS), and rice husk (RH) | Calcination in a Thermolyne muffle furnace | 1M HCl washing, SCB was calcined at 950 °C, 4 h, CS at 550 °C, 4.5 h, and RH at 500 °C, 4 h | Crystalline SiO2 for SCB and CS and amorphous for RH | 25.0, 6.84 and 3.79nm for SCB, CS and RH, respectively | 30.21, 29.51 and 31.4% for SCB, CS and RH, respectively | (308, 314) |
| Olive stone | Alkali leaching process | 10% HCl wash, Ambient temp | Crystalline silica | 15–68 nm | (309) | |
| Olive stones | Alkali leaching extraction method | Acid wash, 900 °C | Crystalline silica | 15–68 nm | (310) | |
| Cassava periderm | Sol–gel method | 0.1 M HCl, 700 °C | Silica nanoparticles | 62.69 nm | (311) | |
| Teff straw | Sol–Gel method | Acid wash, 900 °C | Biosilica | >99% | (312) | |
| Palm kernel shell ash | Sol–gel method | 750 °C | Amorphous silica nanoparticle | 50–98 nm | (313) |
7 Conclusion
The increasing generation of agro-wastes, necessitated by the need to sustain a rapidly expanding global population, poses both challenges and opportunities for sustainable agricultural management. The improper disposal of these wastes, particularly through residue burning, has considerable environmental and public health consequences. At the same time, these wastes represent a valuable, underutilized resource for improving soil fertility, particularly in regions facing heavy metal and metalloid contamination such as arsenic (As) which poses significant risks to soil health and food security, especially in the rice-cultivated areas of the Ganges-Brahmaputra-Meghna plain. Arsenic not only threatens human health but also disrupts the uptake of essential plant nutrients, diminishing crop quality and exacerbating malnutrition risks.
Various remediation strategies have been explored to address these challenges, including physical, chemical, and biological approaches. This review highlights the potential of silicon (Si) and silicate-solubilizing microorganisms (SSM) in mitigating As toxicity. Si and arsenite (AsIII) utilize the same uptake transporters (Lsi1 and Lsi2), enabling Si to competitively inhibit As absorption in plants. Additionally, Si application promotes the formation of iron plaques around roots, serving as a barrier to As translocation by adsorbing or co-precipitating As in the rhizosphere. However, low solubility of Si in neutral soil makes it difficult to lessen the toxicity and buildup of As. One potential strategy is to employ consortia of silicate-solubilizing microorganisms (SSM) and agro-wastes that are rich in Si. Soil fertility is improved, the biogeochemical Si cycle is optimized, and optimal orthosilicic acid concentrations are maintained by these bio-fertilizers; as a result, agriculture can thrive even when As is present. No matter how high the As levels are, SSM tolerant to As toxicity can still promote rice development since they dissolve silicates and also increase the solubilization of phosphate and potassium. Also, agricultural residues that are rich in silicates can be bio-converted or decomposed into a bioavailable Si form more quickly with the help of SSM. This consortia based application not only mitigates As toxicity but also enhances plant resilience to biotic and abiotic stresses, and decreases dependence on expensive inorganic Si fertilizers.
The combination of SSM and Si-rich agro-wastes presents a sustainable, cost-effective, and environmentally friendly alternative to traditional remediation methods. This approach recognizes agro-wastes as a resource instead of a disposal issue, aligning with circular economy principles and enhancing environmental sustainability and public health protection.
8 Future perspective
Future field validation of SSM–Si agro-waste consortia across various soil types and agro-climatic areas is essential. The selection of microbial strains and the formation of consortia are necessary to improve silicate solubilisation, nutrient mobilization, and arsenic mitigation efficiency, alongside the standardization of silicon-rich agro-waste processing to provide uniform bioavailability and scalability for farm-level implementation. Comprehensive Long-term studies evaluating the effects on soil health, carbon sequestration, and agricultural yield in As-contaminated environments are necessary to corroborate laboratory findings. Pathways for the commercialization of cost-effective bioformulations accessible to resource-limited farmers should be established. Farmers will increasingly be able to adopt SSM-enriched agro-waste amendments as a low-cost alternative to conventional silicon fertilizers, thereby enhancing crop yields and nutritional quality even under arsenic-contaminated conditions. The agricultural industry can transform silicon-rich residues into standardized biofertilizer formulations, creating sustainable value chains while simultaneously reducing the harmful practice of residue burning. Policymakers and extension agencies are expected to play a critical role in mainstreaming this approach as a climate-smart, circular agriculture solution, ensuring food security while safeguarding environmental and public health.
Statements
Author contributions
SK: Conceptualization, Methodology, Writing – review & editing, Writing – original draft, Data curation. JG: Conceptualization, Writing – original draft, Data curation. KG: Methodology, Investigation, Writing – original draft. AR: Conceptualization, Investigation, Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Koul B Yakoob M Shah MP . Agricultural waste management strategies for environmental sustainability. Environ Res. (2022) 206:112285. 10.1016/j.envres.2021.112285
2.
Tripathi N Hills CD Singh RS Atkinson CJ . Biomass waste utilisation in low-carbon products: harnessing a major potential resource. NPJ Clim Atmos Sci. (2019) 2:35. 10.1038/s41612-019-0093-5
3.
Singh Y Sidhu HS . Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic plains of India. Proc Indian Natl Sci Acad. (2014) 80:95–114. 10.16943/ptinsa/2014/v80i1/55089
4.
Shyamsundar P Springer NP Tallis H Polasky S Jat ML Sidhu HS et al . Fields on fire: Alternatives to crop residue burning in India. Science. (2019) 365:536–8. 10.1126/science.aaw4085
5.
Kaab A Sharifi M Mobli H Nabavi-Pelesaraei A Chau KW . Combined life cycle assessment and artificial intelligence for prediction of output energy and environmental impacts of sugarcane production. Sci Total Environ. (2019) 664:1005–19. 10.1016/j.scitotenv.2019.02.004
6.
Majumder S Biswas PK Banik P . Impact of water regimes and amendments on inorganic arsenic exposure to rice. Int J Environ Res. (2021) 18:4643. 10.3390/ijerph18094643
7.
Biswas JK Khanam R Srivastava S . Arsenic in rice in a nutshell: tell-tale toxic interplay of chemical offense and biochemical defense. In:AwasthiGSrivastavaSSankhlaMS, editors, Arsenic in Rice. New York: Apple Academic Press (2024). p. 1–25.
8.
Hossain MN Howladar MF Ahammed S Haque MR Khan MI Hasan M et al . Application of multi-indexing approach within a GIS framework to investigate the quality and contamination of ground water in Barisal Sadar, Bangladesh. Heliyon. (2025) 11:e42262. 10.1016/j.heliyon.2025.e42262
9.
Rajan M Karunanidhi D Jaya J Preethi B Subramani T Aravinthasamy P et al . A comprehensive review of human health hazards exposure due to groundwater contamination: a global perspective. Phys Chem Earth A/B/C. (2024) 135:103637. 10.1016/j.pce.2024.103637
10.
Khanam R Nayak AK Kulsum PGPS Mandal J Shahid M Tripathy R et al . Silica sources for arsenic mitigation in rice: machine learning-based predictive modeling and risk assessment. Environ Sci Pollut Res. (2023) 30:113660–73. 10.1007/s11356-023-30339-5
11.
Khanam R Kumar A Nayak AK Shahid M Tripathi R Vijayakumar S et al . Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci Total Environ. (2020) 699:134330. 10.1016/j.scitotenv.2019.134330
12.
Mawia AM Hui S Zhou L Li H Tabassum J Lai C et al . Inorganic arsenic toxicity and alleviation strategies in rice. J Hazard Mater. (2021) 408:124751. 10.1016/j.jhazmat.2020.124751
13.
Pan D Huang G Yi J Cui J Liu C Li F et al . Foliar application of silica nanoparticles alleviates arsenic accumulation in rice grain: co-localization of silicon and arsenic in nodes. Environ Sci Nano. (2022) 9:1271–81. 10.1039/D1EN01132D
14.
Wu C Zou Q Xue SG Pan WS Huang L Hartley W et al . The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL). Environ Pollut. (2016) 212:27–33. 10.1016/j.envpol.2016.01.004
15.
Zandi P Yang J Darma A Bloem E Xia X Wang Y et al . Iron plaque formation characteristics, and its role as a barrier and/or facilitator to heavy metal uptake in hydrophyte rice (Oryza sativa L.). Environ Geochem Health. (2023) 45:525–59. 10.1007/s10653-022-01246-4
16.
Suriyagoda LD Dittert K Lambers H . Arsenic in rice soils and potential agronomic mitigation strategies to reduce arsenic bioavailability: a review. Pedosphere. (2018) 28:363–82. 10.1016/S1002-0160(18)60026-8
17.
Etesami H Jeong BR . Biodissolution of silica by rhizospheric silicate-solubilizing bacteria. In:EtesamiHAl SaeediAHEl-RamadyHFujitaMPessarakliMHossainMA, editors. Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement. Cambridge, MA, USA: Academic Press (2022). p. 265–276. 10.1016/B978-0-323-91225-9.00020-0
18.
Kang SM Waqas M Shahzad R You YH Asaf S Khan MA et al . Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv Dongjin). Soil Sci Plant Nutr. (2017) 63:233–41. 10.1080/00380768.2017.1314829
19.
Etesami H Jeong BR Maathuis FJ Schaller J . Exploring the potential: Can arsenic (As) resistant silicate-solubilizing bacteria manage the dual effects of silicon on As accumulation in rice?Sci. Total Environ. (2023) 903:166870. 10.1016/j.scitotenv.2023.166870
20.
Bist V Anand V Srivastava S Kaur J Naseem M Mishra S et al . Alleviative mechanisms of silicon solubilizing Bacillus amyloliquefaciens mediated diminution of arsenic toxicity in rice. J Hazard Mater. (2022) 428:128170. 10.1016/j.jhazmat.2021.128170
21.
Bhat A Ravi K Tian F Singh B . Arsenic contamination needs serious attention: An opinion and global scenario. Pollutants. (2024) 4:196–211. 10.3390/pollutants4020013
22.
Singh S Yadav R Sharma S Singh AN . Arsenic contamination in the food chain: a threat to food security and human health. J Appl Biol Biotechnol. (2023) 11:24–33. 10.7324/JABB.2023.69922
23.
Kamal MZU Miah MY . Arsenic speciation techniques in soil water and plant: an overview. In:StoytchevaMZlatevR, editors. Arsenic Monitoring, Removal and Remediation. London: IntechOpen (2021). p. 9-40.
24.
Wu J Liang J Björn LO Li J Shu W Wang Y et al . Phosphorus-arsenic interaction in the ‘soil-plant-microbe' system and its influence on arsenic pollution. Sci Total Environ. (2022) 802:149796. 10.1016/j.scitotenv.2021.149796
25.
Zhang Y Fei S Xu Y He Y Zhu Z Liu Y et al . The structure, function and expression analysis of the nodulin 26-like intrinsic protein subfamily of plant aquaporins in tomato. Sci Rep. (2022) 12:9180. 10.1038/s41598-022-13195-0
26.
Chandrakar V Dubey A Keshavkant S . Modulation of arsenic-induced oxidative stress and protein metabolism by diphenyleneiodonium, 24-epibrassinolide and proline in Glycine max L. Acta Bot Croat. (2018) 77:51–61. 10.2478/botcro-2018-0004
27.
Farnese FDS Oliveira JA Lima FS Leão GA Gusman GS Silva LC et al . Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz J Biol. (2014) 74:S103–12. 10.1590/1519-6984.01113
28.
Talukdar D . Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants. (2013) 19:69–79. 10.1007/s12298-012-0140-8
29.
Pita-Barbosa A Gonçalves EC Azevedo AA . Morpho-anatomical and growth alterations induced by arsenic in Cajanus cajan (L.) DC (Fabaceae). Environ Sci Pollut Res. (2015) 22:11265–74. 10.1007/s11356-015-4342-9
30.
Armendariz AL Talano MA Travaglia C Reinoso H Wevar Oller AL Agostini E et al . Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol Biochem. (2016) 98:119–27. 10.1016/j.plaphy.2015.11.021
31.
Shishkova S Rost TL Dubrovsky JG . Determinate root growth and meristem maintenance in angiosperms. Ann Bot. (2008) 101:319–40. 10.1093/aob/mcm251
32.
Rost TL . The organization of roots of dicotyledonous plants and the positions of control points. Ann Bot. (2011) 107:1213–22. 10.1093/aob/mcq229
33.
Gupta K Mishra K Srivastava S Kumar A . Cytotoxic assessment of chromium and arsenic using chromosomal behavior of root meristem in Allium cepa L. Bull Environ Contam Toxicol. (2018) 100:803–8. 10.1007/s00128-018-2344-2
34.
Ronzan M Piacentini D Fattorini L Della Rovere F Eiche E Riemann M et al . Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ Exp Bot. (2018) 151:64–75. 10.1016/j.envexpbot.2018.04.008
35.
Yadav V Arif N Kováč J Singh VP Tripathi DK Chauhan DK et al . Structural modifications of plant organs and tissues by metals and metalloids in the environment: a review. Plant Physiol Biochem. (2021) 159:100–12. 10.1016/j.plaphy.2020.11.047
36.
Kohanová J Martinka M Vaculík M White PJ Hauser MT et al . Root hair abundance impacts cadmium accumulation in Arabidopsis thaliana shoots. Ann Bot. (2018) 122:903–14. 10.1093/aob/mcx220
37.
Singh HP Batish DR Kohli RK Arora K . Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. (2007) 53:65–73. 10.1007/s10725-007-9205-z
38.
Forino LMC Castiglione MR Bartoli G Balestri M Andreucci A Tagliasacchi AM et al . Arsenic-induced morphogenic response in roots of arsenic hyperaccumulator fern Pteris vittata. J Hazard Mater. (2012) 236:271–8. 10.1016/j.jhazmat.2012.07.051
39.
Freitas-Silva L Araújo TO Silva LC Oliveira JA Araujo JM . Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol Environ Saf. (2016) 124:1–9. 10.1016/j.ecoenv.2015.09.028
40.
Kholodova V Volkov K Abdeyeva A Kuznetsov V . Water status in Mesembryanthemum crystallinum under heavy metal stress. Environ Exp Bot. (2011) 71:382–9. 10.1016/j.envexpbot.2011.02.007
41.
Vezza ME Llanes A Travaglia C Agostini E Talano MA . Arsenic stress effects on root water absorption in soybean plants: physiological and morphological aspects. Plant Physiol Biochem. (2018) 123:8–17. 10.1016/j.plaphy.2017.11.020
42.
Gupta P Bhatnagar AK . Spatial distribution of arsenic in different leaf tissues and its effect on structure and development of stomata and trichomes in mung bean. Vigna radiata (L) Wilczek. Environ Exp Bot. (2015) 109:12–22. 10.1016/j.envexpbot.2014.08.001
43.
Chandrakar V Naithani SC Keshavkant S . Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: a review. Biologia. (2016) 71:367–77. 10.1515/biolog-2016-0052
44.
Siddiqui MH Alamri S Khan MN Corpas FJ Al-Amri AA Alsubaie QD et al . Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mater. (2020) 398:122882. 10.1016/j.jhazmat.2020.122882
45.
Kalita J Pradhan AK Shandilya ZM Tanti B . Arsenic stress responses and tolerance in rice: physiological, cellular and molecular approaches. Rice Sci. (2018) 25:235–49. 10.1016/j.rsci.2018.06.007
46.
Gusman GS Oliveira JA Farnese FS Cambraia J . Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol Plant. (2013) 35:1201–9. 10.1007/s11738-012-1159-8
47.
Praveen A Pandey C Panthri M Gupta M . Silicon-mediated genotoxic alterations in Brassica juncea under arsenic stress: a comparative study of biochemical and molecular markers. Pedosphere. (2020) 30:517–27. 10.1016/S1002-0160(17)60435-1
48.
Wang S Zhang D Pan X . Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotoxicol Environ Saf. (2012) 84:104–11. 10.1016/j.ecoenv.2012.06.028
49.
Ng L Anuar S Jong J Elham M . Phytobeneficial and plant growth-promotion properties of silicon-solubilising rhizobacteria on the growth and control of rice sheath blight disease. Asian J Plant Sci. (2016) 15:92–100. 10.3923/ajps.2016.92.100
50.
Jha AB Dubey RS . Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol. (2004) 161:867–72. 10.1016/j.jplph.2004.01.004
51.
Roitsch T González MC . Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. (2004) 9:606–13. 10.1016/j.tplants.2004.10.009
52.
Hartley-Whitaker J Ainsworth G Meharg AA . Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. (2001) 24:713–22. 10.1046/j.0016-8025.2001.00721.x
53.
Singh HP Kaur S Batish DR Sharma VP Sharma N Kohli RK et al . Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide. (2009) 20:289–97. 10.1016/j.niox.2009.02.004
54.
Srivastava M Ma LQ Singh N Singh S . Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot. (2005) 56:1335–42. 10.1093/jxb/eri134
55.
Clemens S Ma JF . Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol. (2016) 67:489–512. 10.1146/annurev-arplant-043015-112301
56.
Yu LJ Luo YF Liao B Xie LJ Chen L Xiao S et al . Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol. (2012) 195:97–112. 10.1111/j.1469-8137.2012.04154.x
57.
Kaur S Singh HP Batish DR Negi A Mahajan P Rana S et al . Arsenic (As) inhibits radicle emergence and elongation in Phaseolus aureus by altering starch-metabolizing enzymes vis-à-vis disruption of oxidative metabolism. Biol Trace Elem Res. (2012) 146:360–8. 10.1007/s12011-011-9258-8
58.
Menezes RA Amaral C Batista-Nascimento L Santos C Ferreira RB Devaux F et al . Contribution of Yap1 towards Saccharomyces cerevisiae adaptation to arsenic-mediated oxidative stress. Biochem J. (2008) 414:301–11. 10.1042/BJ20071537
59.
Fedorova M Bollineni RC Hoffmann R . Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev. (2014) 33:79–97. 10.1002/mas.21381
60.
Zhang Z Guo X Liu Y Ke P Meng Y Gu J et al . PKM2 alleviates mitochondrial oxidative stress and neuronal apoptosis through metabolic and non-metabolic pathways to protect SOD1 mice. Free Radic Biol Med. (2025) 238:1–16. 10.1016/j.freeradbiomed.2025.06.012
61.
Curtis JM Hahn WS Long EK Burrill JS Arriaga EA Bernlohr DA et al . Protein carbonylation and metabolic control systems. Trends Endocrinol Metab. (2012) 23:399–406. 10.1016/j.tem.2012.05.008
62.
Li H Wang Z Feng B Shi J Liao M He K et al . Arsenic stress on soil microbial nutrient metabolism interpreted by microbial utilization of dissolved organic carbon. J Hazard Mater. (2024) 470:134232. 10.1016/j.jhazmat.2024.134232
63.
Bose H Sahu RP Sar P . Impact of arsenic on microbial community structure and their metabolic potential from rice soils of West Bengal, India. Sci Total Environ. (2022) 841:156486. 10.1016/j.scitotenv.2022.156486
64.
Ghosh AK Bhattacharyya P Pal R . Effect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, India. Environ Int. (2004) 30:491–9. 10.1016/j.envint.2003.10.002
65.
Majumdar A Afsal F Pathak SK Upadhayay MK Roychowdhury T Srivastava S et al . Molecular aspects of arsenic responsive microbes in soil-plant-aqueous triphasic systems. In:NiaziNKBibiIAftabT, editors. Global Arsenic Hazard: Ecotoxicology and Remediation.Cham: Springer (2022). p. 291–312. 10.1007/978-3-031-16360-9_14
66.
Bhattacharyya P Tripathy S Kim K Kim SH . Arsenic fractions and enzyme activities in arsenic-contaminated soils by groundwater irrigation in West Bengal. Ecotoxicol Environ Saf. (2008) 71:149–56. 10.1016/j.ecoenv.2007.08.015
67.
Juma NG Tabatabai MA . Effects of trace elements on phosphatase activity in soils. Soil Sci Soc Am J. (1977) 41:343–6. 10.2136/sssaj1977.03615995004100020034x
68.
Majumder S Powell MA Biswas PK Banik P . The impact of arsenic induced stress on soil enzyme activity in different rice agroecosystems. Environ Technol Innov. (2022) 26:102282. 10.1016/j.eti.2022.102282
69.
Tamás MJ Sharma SK Ibstedt S Jacobson T Christen P . Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules. (2014) 4:252–67. 10.3390/biom4010252
70.
Wang X Huang R Li L He S Yan L Wang H et al . Arsenic removal from flooded paddy soil with spontaneous hygrophyte markedly attenuates rice grain arsenic. Environ Int. (2019) 133:105159. 10.1016/j.envint.2019.105159
71.
Guan H Caggìa V Gómez-Chamorro A Fischer D Coll-Crespí M Liu X et al . The effects of soil microbial disturbance and plants on arsenic concentrations and speciation in soil water and soils. Expos Health. (2024) 16:805–20. 10.1007/s12403-023-00593-6
72.
Zhang R Huang B Zeng H Wang X Peng B Yu H et al . Arsenic extraction from seriously contaminated paddy soils with ferrihydrite-loaded sand columns. Chemosphere. (2022) 307:135744. 10.1016/j.chemosphere.2022.135744
73.
Tian X Chai G Lu M Xiao R Xie Q Luo L et al . A new insight into the role of iron plaque in arsenic and cadmium accumulation in rice (Oryza sativa L.) roots. Ecotoxicol Environ Saf. (2023) 254:114714. 10.1016/j.ecoenv.2023.114714
74.
Nauman M Iqbal N Parveen N Jamil HMA Riyazuddin R . Anatomical, morphological, and physiological consequences and adaptations in flood-stressed plants. In:RiyazuddinRGuptaRRamtekePWSayyedR, editors. Plant Flooding. Plant in Challenging Environments. Cham: Springer (2025). p. 3–27. 10.1007/978-3-031-83068-6_1
75.
Irshad MK Noman A Alhaithloul HA Adeel M Rui Y Shah T et al . Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci Total Environ. (2020) 717:137086. 10.1016/j.scitotenv.2020.137086
76.
Limmer MA Evans AE Seyfferth AL . A new method to capture the spatial and temporal heterogeneity of aquatic plant iron root plaque in situ. Environ Sci Technol. (2020) 55:912–8. 10.1021/acs.est.0c05671
77.
Amaral D Lopes G Guilherme LRG Seyfferth AL . A new approach to sampling intact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot As in rice. Environ Sci Technol. (2017) 51:38–45. 10.1021/acs.est.6b03558
78.
Limmer MA Mann J Amaral DC Vargas R Seyfferth AL . Silicon-rich amendments in rice paddies: Effects on arsenic uptake and biogeochemistry. Sci Total Environ. (2018) 624:1360–8. 10.1016/j.scitotenv.2017.12.207
79.
Linam FA Limmer MA Seyfferth AL . Contrasting roles of rice root iron plaque in retention and plant uptake of silicon, phosphorus, arsenic, and selenium in diverse paddy soils. Plant Soil. (2024) 502:397–415. 10.1007/s11104-024-06553-6
80.
Awasthi S Chauhan R Srivastava S Tripathi RD . The journey of arsenic from soil to grain in rice. Front Plant Sci. (2017) 8:1007. 10.3389/fpls.2017.01007
81.
Hiemstra T . Ferrihydrite interaction with silicate and competing oxyanions: geometry and hydrogen bonding of surface species. Geochim Cosmochim Acta. (2018) 238:453–76. 10.1016/j.gca.2018.07.017
82.
Liu X Wang J He Y Li J Tian Q Xu H et al . The redistribution process of As (III) and Fe (II) caused by As/Fe ratio, organic matter, and co-existing ions: Co-precipitation and co oxidation. Ecotoxicol Environ Saf. (2024) 281:116631. 10.1016/j.ecoenv.2024.116631
83.
Dixit S Hering JG . Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol. (2003) 37:4182–9. 10.1021/es030309t
84.
Seyfferth AL Limmer M Wu W . Si and water management drives changes in Fe and Mn pools that affect As cycling and uptake in rice. Soil Syst. (2019) 3:58. 10.3390/soilsystems3030058
85.
Limmer MA Thomas J Seyfferth AL . The effect of silicon on the kinetics of rice root iron plaque formation. Plant Soil. (2022) 477:171–81. 10.1007/s11104-022-05414-4
86.
Gao Z Chen H Zhang X Xiao Z Fan X Yin C et al . Silicon enhances abundances of reducing microbes in rhizoplane and decreases arsenite uptake by rice (Oryza sativa L.). Environ Pollut. (2022) 306:119405. 10.1016/j.envpol.2022.119405
87.
Luxton TP Eick MJ Rimstidt DJ . The role of silicate in the adsorption/desorption of arsenite on goethite. Chem Geol. (2008) 252:125–35. 10.1016/j.chemgeo.2008.01.022
88.
Gu JF Yi XT Ouyang K Li Q You P Zhou R et al . Rich-silicon rice husk ash increases iron plaque formation and decreases cadmium and arsenic accumulation in rice seedlings. Chemosphere. (2024) 364:143239. 10.1016/j.chemosphere.2024.143239
89.
Jiang Y Liu Y Yi X Zeng P Liao B Zhou H et al . Regulation of rhizosphere microenvironment by rice husk ash for reducing the accumulation of cadmium and arsenic in rice. J Environ Sci. (2024) 136:1–10. 10.1016/j.jes.2022.09.005
90.
Leksungnoen P Wisawapipat W Ketrot D Aramrak S Nookabkaew S Rangkadilok N et al . Biochar and ash derived from silicon-rich rice husk decrease inorganic arsenic species in rice grain. Sci Total Environ. (2019) 684:360–70. 10.1016/j.scitotenv.2019.05.247
91.
Zhang J Zhao S Xu Y Zhou W Huang K Tang Z et al . Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils. Environ Sci Technol. (2017) 51:4377–86. 10.1021/acs.est.6b06255
92.
Herath I Zhao FJ Bundschuh J Wang P Wang J Ok YS et al . Microbe mediated immobilization of arsenic in the rice rhizosphere after incorporation of silica impregnated biochar composites. J Hazard Mater. (2020) 398:123096. 10.1016/j.jhazmat.2020.123096
93.
Gu Y Van Nostrand JD Wu L He Z Qin Y Zhao FJ et al . Bacterial community and arsenic functional genes diversity in arsenic contaminated soils from different geographic locations. PLoS ONE. (2017) 12:e0176696. 10.1371/journal.pone.0176696
94.
Muller D Lievremont D Simeonova DD Hubert JC Lett MC . Arsenite oxidase aox genes from a metal-resistant β-proteobacterium. J Bacteriol. (2003) 185:135–41. 10.1128/JB.185.1.135-141.2003
95.
Warelow TP Oke M Schoepp-Cothenet B Dahl JU Bruselat N Sivalingam GN et al . The respiratory arsenite oxidase: structure and the role of residues surrounding the rieske cluster. PLoS ONE. (2013) 8:e72535. 10.1371/journal.pone.0072535
96.
Watson C Niks D Hille R Vieira M Schoepp-Cothenet B Marques AT et al . Electron transfer through arsenite oxidase: Insights into Rieske interaction with cytochrome c. Biochim Biophys Acta Bioenerg. (2017) 1858:865–72. 10.1016/j.bbabio.2017.08.003
97.
Bentley R Chasteen TG . Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev. (2002) 66:250–71. 10.1128/MMBR.66.2.250-271.2002
98.
Ye J Rensing C Rosen BP Zhu YG . Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. (2012) 17:155–62. 10.1016/j.tplants.2011.12.003
99.
Challenger F Higginbottom C Ellis L . The formation of organo-metalloidal compounds by microorganisms. Part I Trimethylarsine and dimethylethylarsine. J Chem Soc. (1933) 95–101. 10.1039/jr9330000095
100.
Cullen WR . Chemical mechanism of arsenic biomethylation. Chem Res Toxicol. (2014) 27:457–61. 10.1021/tx400441h
101.
William VU Magpantay HD . Arsenic and microorganisms: genes, molecular mechanisms, and recent advances in microbial arsenic bioremediation. Microorganisms. (2024) 12:74. 10.3390/microorganisms12010074
102.
Busenlehner LS Pennella MA Giedroc DP . The SmtB/ArsR family of metalloregulatory transcriptional repressors: structural insights into prokaryotic metal resistance. FEMS Microbial Rev. (2003) 27:131–43. 10.1016/S0168-6445(03)00054-8
103.
Meng YL Liu Z Rosen BP . As (III) and Sb (III) uptake by GlpF and efflux by ArsB in Escherichia coli. J Biol Chem. (2004) 279:18334–41. 10.1074/jbc.M400037200
104.
Lin YF Yang J Rosen BP . ArsD residues Cys12, Cys13, and Cys18 form an As (III)-binding site required for arsenic metallochaperone activity. J Biol Chem. (2007) 282:16783–91. 10.1074/jbc.M700886200
105.
Kaur S Kamli MR Ali A . Diversity of arsenate reductase genes (arsC genes) from arsenic-resistant environmental isolates of E. coli. Curr Microbiol. (2009) 59:288–94. 10.1007/s00284-009-9432-9
106.
Duval S Ducluzeau AL Nitschke W Schoepp-Cothenet B . Enzyme phylogenies as markers for the oxidation state of the environment: the case of respiratory arsenate reductase and related enzymes. BMC Evol Biol. (2008) 8:206. 10.1186/1471-2148-8-206
107.
Wang HY Chen P Zhu YG Cen K Sun GX . Simultaneous adsorption and immobilization of As and Cd by birnessite-loaded biochar in water and soil. Environ Sci Pollut Res. (2019) 26:8575–84. 10.1007/s11356-019-04315-x
108.
Dykes GE Limmer MA Seyfferth AL . Silicon-rich soil amendments impact microbial community composition and the composition of arsM bearing microbes. Plant Soil. (2021) 468:147–64. 10.1007/s11104-021-05103-8
109.
Das S Hwang HY Song HJ Cho SR Van Nostrand JD Kim PJ et al . Soil microbial response to silicate fertilization reduces bioavailable arsenic in contaminated paddies. Soil Biol Biochem. (2021) 159:108307. 10.1016/j.soilbio.2021.108307
110.
Boorboori MR Gao Y Wang H Fang C . Usage of Si, P, Se, and Ca decrease arsenic concentration/toxicity in rice, a review. Appl Sci. (2021) 11:8090. 10.3390/app11178090
111.
Pandey A Wu LB Murugaiyan V Schaaf G Ali J Frei M et al . Differential effects of arsenite and arsenate on rice (Oryza sativa) plants differing in glutathione S-transferase gene expression. Environ Sci Pollut Res. (2023) 30:92268–81. 10.1007/s11356-023-28833-x
112.
Roy A Datta SP Barman M Golui D Bhattacharyya S Meena MC et al . Co-application of silicate and low-arsenic-accumulating rice cultivars efficiently reduces human exposure to arsenic—a case study from West Bengal, India. Toxics. (2023) 11:64. 10.3390/toxics11010064
113.
Ma JF Tamai K Yamaji N Mitani N Konishi S Katsuhara M et al . A silicon transporter in rice. Nature. (2006) 440:688–91. 10.1038/nature04590
114.
Mitani-Ueno N Yamaji N Zhao FJ Ma JF . The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot. (2011) 62:4391–8. 10.1093/jxb/err158
115.
Ma JF Yamaji N Mitani N Tamai K Konishi S Fujiwara T et al . An efflux transporter of silicon in rice. Nature. (2007) 448:209–12. 10.1038/nature05964
116.
Mitani-Ueno N Ma JF . Linking transport system of silicon with its accumulation in different plant species. Soil Sci Plant Nutr. (2021) 67:10–7. 10.1080/00380768.2020.1845972
117.
En Y Yamaji N Ma JF . Linking root morphology and anatomy with transporters for mineral element uptake in plants. Plant Soil. (2023) 484:1–12. 10.1007/s11104-022-05692-y
118.
Ma JF Yamaji N . Functions and transport of silicon in plants. Cell Mol Life Sci. (2008) 65:3049–57. 10.1007/s00018-008-7580-x
119.
Huang S Yamaji N Sakurai G Mitani-Ueno N Konishi N Ma JF . A pericycle-localized silicon transporter for efficient xylem loading in rice. New Phytol. (2022) 234:197–208. 10.1111/nph.17959
120.
Yamaji N Mitani N Ma JF . A transporter regulating silicon distribution in rice shoots. Plant Cell. (2008) 20:1381–9. 10.1105/tpc.108.059311
121.
Mitani-Ueno N Yamaji N Huang S Yoshioka Y Miyaji T Ma JF et al . A silicon transporter gene required for healthy growth of rice on land. Nat Commun. (2023) 14:6522. 10.1038/s41467-023-42180-y
122.
Boorboori MR Lin W Jiao Y Fang C . Silicon modulates molecular and physiological activities in Lsi1 transgenic and wild lemont rice seedlings under arsenic stress. Agronomy. (2021) 11:1532. 10.3390/agronomy11081532
123.
Khan E Gupta M . Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep. (2018) 8:10301. 10.1038/s41598-018-28712-3
124.
Nahar K Rhaman MS Parvin K Bardhan K Marques DN García-Caparrós P et al . Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses. (2022) 2:179–209. 10.3390/stresses2020013
125.
Afzal S Abdul Manap AS Attiq A Albokhadaim I Kandeel M Alhojaily SM et al . From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol. (2023) 14:1269581. 10.3389/fphar.2023.1269581
126.
Cui J Li Y Jin Q Li F . Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ Sci Nano. (2020) 7:162–71. 10.1039/C9EN01035A
127.
Nguyen MN Dam TT Nguyen AT Nguyen AM Nguyen LN Duong LT et al . Arsenic in rice straw phytoliths: Encapsulation and release properties. Appl Geochem. (2021) 127:104907. 10.1016/j.apgeochem.2021.104907
128.
Tripathi P Tripathi RD Singh RP Dwivedi S Goutam D Shri M et al . Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol. Eng. (2013) 52:96–103. 10.1016/j.ecoleng.2012.12.057
129.
Boorboori MR Lin W Zhan W Fang C . The role of silicon to increase arsenic tolerance in rice (Oryza sativa L.) seedlings by reinforcing anti-oxidative defense. Bioagro. (2020) 32:159–168.
130.
Geng A Wang X Wu L Wang F Wu Z Yang H et al . Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicol Environ Saf. (2018) 158:266–73. 10.1016/j.ecoenv.2018.03.050
131.
Li Z Yuan Y Xiang L Su Q Liu Z Wu W et al . Silicon-rich biochar detoxify multiple heavy metals in wheat by regulating oxidative stress and subcellular distribution of heavy metal. Sustainability. (2022) 14:16417. 10.3390/su142416417
132.
El-Ramady H Brevik EC Bayoumi Y Shalaby TA El-Mahrouk ME Taha N et al . An overview of agro-waste management in light of the water-energy-waste nexus. Sustainability. (2022) 14:15717. 10.3390/su142315717
133.
Tu Nguyen M Binh Nguyen T Khoi Dang K Luu T Hung Thach P Lan Phuong Nguyen K et al . “Current and potential uses of agricultural by-products and waste in main food sectors in Vietnam—A circular economy perspective. In:RenJZhangL, editors. Circular Economy and Waste Valorisation: Theory and Practice from an International Perspective. Cham: Springer (2022). p. 131–51.
134.
Rani GM Pathania D Umapathi R Rustagi S Huh YS Gupta VK et al . Agro-waste to sustainable energy: a green strategy of converting agricultural waste to nano-enabled energy applications. Sci Total Environ. (2023) 875:162667. 10.1016/j.scitotenv.2023.162667
135.
Hall R Topham E João E . Environmental impact assessment for the decommissioning of offshore wind farms. Renew Sustain Energy Rev. (2022) 165:112580. 10.1016/j.rser.2022.112580
136.
Sadh PK Duhan S Duhan JS . Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioproc. (2018) 5:1–15. 10.1186/s40643-017-0187-z
137.
UNEP . Solid Waste Management. UNEP—UN Environment Programme. (2020) Available online at: https://www.unenvironment.org/explore-topics/resourceefficiency/what-we-do/cities/solid-waste-management (Accessed May 10, 2020).
138.
Barani S Sebastian SP Dhevagi P Prasanthrajan M Suganthi A . Synthesis of silica nanoparticles (SiNPs) from agro-wastes for removal of heavy metals from an aqueous medium–a mini review. Green Chem Lett Rev. (2024) 17:2422416. 10.1080/17518253.2024.2422416
139.
Kapoor TS Navinya C Anurag G Lokhande P Rathi S Goel A et al . Reassessing the availability of crop residue as a bioenergy resource in India: a field-survey based study. J Environ Manage. (2023) 341:118055. 10.1016/j.jenvman.2023.118055
140.
Costa JAS Sarmento VH Romão LP Paranhos CM . Synthesis of functionalized mesoporous material from rice husk ash and its application in the removal of the polycyclic aromatic hydrocarbons. Environ Sci Pollut Res. (2019) 26:25476–90. 10.1007/s11356-019-05852-1
141.
Etesami H . Enhancing soil microbiome resilience: the mitigating role of silicon against environmental stresses. Front Agron. (2024) 6:1465165. 10.3389/fagro.2024.1465165
142.
Sohrabnezhad S Mooshangaie SD . In situ fabrication of n-type Ag/AgBr nanoparticles in MCM-41 with rice husk (RH/MCM-41) composite for the removal of Eriochrome Black-T. Mater Sci Eng B. (2019) 240:16–22. 10.1016/j.mseb.2019.01.007
143.
Chen P Bie H Bie R . Leaching characteristics and kinetics of the metal impurities present in rice husk during pretreatment for the production of nanosilica particles. Korean J Chem Eng. (2018) 35:1911–8. 10.1007/s11814-018-0103-z
144.
Bakar RA Yahya R Gan SN . Production of high purity amorphous silica from rice husk. Procedia Chem. (2016) 19:189–95. 10.1016/j.proche.2016.03.092
145.
Costa JAS Paranhos CM . Systematic evaluation of amorphous silica production from rice husk ashes. J Clean Prod. (2018) 192:688–97. 10.1016/j.jclepro.2018.05.028
146.
Dhaneswara D Fatriansyah JF Situmorang FW Haqoh AN . Synthesis of amorphous silica from rice husk ash: comparing HCl and CH3COOH acidification methods and various alkaline concentrations. Synthesis. (2020) 11:200–8. 10.14716/ijtech.v11i1.3335
147.
Umeda J Kondoh K . High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J Mater Sci. (2008) 43:7084–90. 10.1007/s10853-008-3060-9
148.
Ige AO Ogunsile BO Ore OT Olawade DB . Adsorption of congo red from aqueous solution using rice husk, calcined kaolin clay, and microwaved rice husk clay hybrid. Discov Chem. (2024) 1:9. 10.1007/s44371-024-00010-0
149.
Pavan C Santalucia R Leinardi R Fabbiani M Yakoub Y Uwambayinema F et al . Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc Natl Acad Sci. (2020) 117:27836–46. 10.1073/pnas.2008006117
150.
Angotzi MS Mameli V Cara C Borchert KB Steinbach C Boldt R et al . Meso-and macroporous silica-based arsenic adsorbents: effect of pore size, nature of the active phase, and silicon release. Nanoscale adv. (2021) 3:6100–13. 10.1039/D1NA00487E
151.
Guo W Li G Zheng Y Li K . Nano-silica extracted from rice husk and its application in acetic acid steam reforming. RSC Adv. (2021) 11:34915–22. 10.1039/D1RA05255A
152.
Bhattacharyya P Bisen J Bhaduri D Priyadarsini S Munda S Chakraborti M et al . Turn the wheel from waste to wealth: economic and environmental gain of sustainable rice straw management practices over field burning in reference to India. Sci Total Environ. (2021) 775:145896. 10.1016/j.scitotenv.2021.145896
153.
Singh J Bhattu M Liew RK Verma M Brar SK Bechelany M et al . Transforming rice straw waste into biochar for advanced water treatment and soil amendment applications. Environ Technol Innov. (2024) 32:103932. 10.1016/j.eti.2024.103932
154.
FAOSTAT . Crops and Livestock Products. (2020) Available at: http://www.fao.org/faostat/en/#data/QCL (Accessed May 10, 2020).
155.
Singh R Srivastava M Shukla A . Environmental sustainability of bioethanol production from rice straw in India: a review. Renew Sustain Energy Rev. (2016) 54:202–16. 10.1016/j.rser.2015.10.005
156.
Van Soest PJ . Rice straw, the role of silica and treatments to improve quality. Anim Feed Sci Technol. (2006) 130:137–71. 10.1016/j.anifeedsci.2006.01.023
157.
Ma JF Yamaji N . A cooperative system of silicon transport in plants. Trends Plant Sci. (2015) 20:435–42. 10.1016/j.tplants.2015.04.007
158.
Singh Y Sharma S Kumar U Sihag P Balyan P Singh KP et al . Strategies for economic utilization of rice straw residues into value-added by-products and prevention of environmental pollution. Sci Total Environ. (2024) 906:167714. 10.1016/j.scitotenv.2023.167714
159.
Li Z Delvaux B . Phytolith-rich biochar: a potential Si fertilizer in desilicated soils. GCB Bioenergy. (2019) 11:1264–82. 10.1111/gcbb.12635
160.
Xing S Zhang G Chen S Zhang N Wang C . Response of soil erosion resistance to straw incorporation amount in the black soil region of Northeast China. J Environ Manage. (2024) 357:120801. 10.1016/j.jenvman.2024.120801
161.
Schaller J Kleber M Puppe D Stein M Sommer M Rillig MC et al . The importance of reactive silica for maintaining soil health. Plant Soil. (2025). In press. 10.1007/s11104-025-07299-5
162.
Pan M Du J Gan X Lü X . Distribution and ultrastructure of silica on rice straw surface. Trans Chin Soc Agric Eng. (2016) 32:309–14. 10.11975/j.issn.1002-6819.2016.04.044
163.
Uda MA Gopinath SC Hashim U Uda MA Hulwani Ibrahim N Parmin NA et al . Simple and green approach strategy to synthesis graphene using rice straw ash. IOP Conf Ser Mater Sci Eng. (2020) 864:012181. 10.1088/1757-899X/864/1/012181
164.
Ruggeri G Corsi S . An analysis of the Fairtrade cane sugar small producer organizations network. J Clean Prod. (2019) 240:118191. 10.1016/j.jclepro.2019.118191
165.
Zanchet A Demori R De Sousa FDB Ornaghi HL Schiavo LSA Scuracchio CH . Sugar cane as an alternative green activator to conventional vulcanization additives in natural rubber compounds: thermal degradation study. J Clean Prod. (2019) 207:248–60. 10.1016/j.jclepro.2018.09.203
166.
Freitas JV Bilatto S Squinca P Pinto AS Brondi MG Bondancia TJ et al . Sugarcane biorefineries: potential opportunities towards shifting from wastes to products. Ind Crops Prod. (2021) 172:114057. 10.1016/j.indcrop.2021.114057
167.
Chindaprasirt P Rattanasak U . Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci Rep. (2020) 10:9890. 10.1038/s41598-020-66885-y
168.
Negrão DR Driemeier C . Fate of silica phytoliths in the industrial crushing of sugarcane stalks. Ind Crops Prod. (2022) 185:115132. 10.1016/j.indcrop.2022.115132
169.
Zewide YT Yemata TA Ayalew AA Kedir HJ Tadesse AA Fekad AY et al . Application of response surface methodology (RSM) for experimental optimization in biogenic silica extraction from rice husk and straw ash. Sci Rep. (2025) 15:132. 10.1038/s41598-024-83724-6
170.
Bangar SP Chaudhary V Kajla P Balakrishnan G Phimolsiripol Y . Strategies for upcycling food waste in the food production and supply chain. Trends Food Sci Technol. (2024) 143:104314. 10.1016/j.tifs.2023.104314
171.
Adeleye SO Adeleke AA Nzerem P Ikubanni PP Salihu A Olosho AI et al . Characterization of wheat husk ASH and calcined eggshell as potential glass former. In: 2023 2nd International Conference on Multidisciplinary Engineering and Applied Science (ICMEAS).New York: IEEE (2023). p. 1–4.
172.
Hernández-Martínez D Leyva-Verduzco AA Rodríguez-Félix F Acosta-Elías M Wong-Corral FJ . Obtaining and characterization of silicon (Si) from wheat husk ash for its possible application in solar cells. J Clean Prod. (2020) 271:122698. 10.1016/j.jclepro.2020.122698
173.
Hamidu I Afotey B Kwakye-Awuah B Annang DA . Synthesis of silica and silicon from rice husk feedstock: a review. Heliyon. (2025) 11:e42491. 10.1016/j.heliyon.2025.e42491
174.
Terzioglu P Yücel S Kuş Ç . Review on a novel biosilica source for production of advanced silica-based materials: Wheat husk. Asia-Pac J Chem Eng. (2019) 14:e2262. 10.1002/apj.2262
175.
Terzioglu P Yücel S Rababah T Özçimen D . Characterization of wheat hull and wheat hull ash as a potential source of SiO2. BioResources. (2013) 8:4406–20. 10.15376/biores.8.3.4406-4420
176.
Chen H Wang F Zhang C Shi Y Jin G Yuan S et al . Preparation of nano-silica materials: The concept from wheat straw. J Non-Cryst Solids. (2010) 356:2781–5. 10.1016/j.jnoncrysol.2010.09.051
177.
Ge X Cao Y Zhou B Wang X Yang Z Li MH et al . Biochar addition increases subsurface soil microbial biomass but has limited effects on soil CO2 emissions in subtropical moso bamboo plantations. Appl Soil Ecol. (2019) 142:155–65. 10.1016/j.apsoil.2019.04.021
178.
Olawale O . Bamboo leaves as an alternative source for silica in ceramics using Box Benhken design. Sci Afr. (2020) 8:e00418. 10.1016/j.sciaf.2020.e00418
179.
Setiadji S Sundari CDD Lala E Nurbaeti DF Novianti I Suhendar D et al . The increased use value of bamboo leaves as silica source for t-type zeolite synthesis. In MATEC Web Conf. (2018) 197:05003. 10.1051/matecconf/201819705003
180.
Pinto J Paiva A Varum H Costa A Cruz D Pereira S et al . Corn's cob as a potential ecological thermal insulation material. Energy Build. (2011) 43:1985–90. 10.1016/j.enbuild.2011.04.004
181.
Velmurugan P Shim J Lee KJ Cho M Lim SS Seo SK et al . Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J Ind Eng Chem. (2015) 29:298–303. 10.1016/j.jiec.2015.04.009
182.
Prempeh CO Formann S Hartmann I Nelles M . An improved method for the production of biogenic silica from cornhusk using sol–gel polymeric route. Biomass Convers Biorefin. (2024) 14:28701–11. 10.1007/s13399-022-03615-6
183.
Okeke FO Ahmed A Imam A Hassanin H . A review of corncob-based building materials as a sustainable solution for the building and construction industry. Hybrid Adv. (2024) 6:100269. 10.1016/j.hybadv.2024.100269
184.
Chanadee T Chaiyarat S . Preparation and characterization of low cost silica powder from sweet corn cobs (Zea mays saccharata L.). J Mater Environ Sci. (2016) 7:2369–74.
185.
Alam K Biswas DR Bhattacharyya R Das D Suman A Das TK et al . Recycling of silicon-rich agro-wastes by their combined application with phosphate solubilizing microbe to solubilize the native soil phosphorus in a sub-tropical Alfisol. J Environ Manage. (2022) 318:115559. 10.1016/j.jenvman.2022.115559
186.
CíŽková H Kučera T Poulin B Květ J . Ecological basis of ecosystem services and management of wetlands dominated by common reed (Phragmites australis): European perspective. Diversity. (2023) 15:629. 10.3390/d15050629
187.
Köbbing JF Patuzzi F Baratieri M Beckmann V Thevs N Zerbe S et al . Economic evaluation of common reed potential for energy production: a case study in Wuliangsuhai Lake (Inner Mongolia, China). Biomass Bioenergy. (2014) 70:315–29. 10.1016/j.biombioe.2014.08.002
188.
Azizi SN Ghasemi S Rangriz-Rostami O . Synthesis of MCM-41 nanoparticles from stem of common reed ash silica and their application as substrate in electrooxidation of methanol. Bull Mater Sci. (2018) 41:88. 10.1007/s12034-018-1580-8
189.
Hameed MA Bashir H Ghafoor N Rashid A Nazir MJ Ali M et al . Silicon and sustainable agriculture: strengthening soil-plant synergy for food security. Plant Environ. (2024) 5:35–54.10.54219/plantenviron.05.01.2024.100
190.
Jiménez-Vázquez A Jaimes-López R Morales-Bautista CM Pérez-Rodríguez S Gochi-Ponce Y Estudillo-Wong LA et al . Catalytic applications of natural iron oxides and hydroxides: a review. Catalysts. (2025) 15:236. 10.3390/catal15030236
191.
Cruz JA Tubana BS Fultz LM Dalen MS Ham JH . Identification and profiling of silicate-solubilizing bacteria for plant growth-promoting traits and rhizosphere competence. Rhizosphere. (2022) 23:100566. 10.1016/j.rhisph.2022.100566
192.
Klotzbücher T Marxen A Vetterlein D Schneiker J Türke M Van Sinh N et al . Plant-available silicon in paddy soils as a key factor for sustainable rice production in Southeast Asia. Basic Appl Ecol. (2015) 16:665–73. 10.1016/j.baae.2014.08.002
193.
Ostapchuk GO . Eco-friendly synthesis of silica nanoparticles and their applications. Mater Res Found. (2024) 169:34. 10.21741/9781644903261-1
194.
Thakral V Raturi G Sudhakaran S Mandlik R Sharma Y Shivaraj SM et al . Silicon, a quasi-essential element: availability in soil, fertilizer regime, optimum dosage, and uptake in plants. Plant Physiol Biochem. (2024) 208:108459. 10.1016/j.plaphy.2024.108459
195.
Khan N Siddiqui MH Ahmad S Ahmad MM Siddiqui S . New insights in enhancing the phosphorus use efficiency using phosphate-solubilizing microorganisms and their role in cropping system. Geomicrobiol J. (2024) 41:485–95. 10.1080/01490451.2024.2331111
196.
Swoboda P Döring TF Hamer M . Remineralizing soils? The agricultural usage of silicate rock powders: a review. Sci Total Environ. (2022) 807:150976. 10.1016/j.scitotenv.2021.150976
197.
Etesami H Schaller J . Improving phosphorus availability to rice through silicon management in paddy soils: a review of the role of silicate-solubilizing bacteria. Rhizosphere. (2023) 27:100749. 10.1016/j.rhisph.2023.100749
198.
Chen W Yang F Zhang L Wang J . Organic acid secretion and phosphate solubilizing efficiency of Pseudomonas sp. PSB12: effects of phosphorus forms and carbon sources. Geomicrobiol J. (2016) 33:870–7. 10.1080/01490451.2015.1123329
199.
Madhupriyaa D Baskar M Sherene Jenita Rajammal T Kuppusamy S Rathika S Umamaheswari T et al . Efficacy of chelated micronutrients in plant nutrition. Commun. Soil Sci. Plant Anal. (2024) 55:3609–37. 10.1080/00103624.2024.2397019
200.
Raturi G Sharma Y Rana V Thakral V Myaka B Salvi P et al . Exploration of silicate solubilizing bacteria for sustainable agriculture and silicon biogeochemical cycle. Plant Physiol Biochem. (2021) 166:827–38. 10.1016/j.plaphy.2021.06.039
201.
Vasanthi N Saleena LM Raj SA . Silica solubilization potential of certain bacterial species in the presence of different silicate minerals. Silicon. (2018) 10:267–75. 10.1007/s12633-016-9438-4
202.
Deshmukh R Sonah H Belanger RR . New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J Exp Bot. (2020) 71:6775–88. 10.1093/jxb/eraa342
203.
Hu L Xu C Wang J Chen D Zeng R Song Y et al . Application of bryophyte rhizoid-associated bacteria increases silicon accumulation and growth in maize (Zea mays L.) seedlings. Appl Ecol Environ Res. (2019) 17:13423–33. 10.15666/aeer/1706_1342313433
204.
Wang C Zhang C Xie Z Wang D Meng Y Sun Y et al . Engineered silicate-solubilizing bacterial community alleviates nutrient stress in field-grown maize by enhancing silicon uptake and optimizing rhizosphere microecology. Field Crops Res. (2025) 326:109827. 10.1016/j.fcr.2025.109827
205.
Wei Z Liang X Pendlowski H Hillier S Suntornvongsagul K Sihanonth P et al . Fungal biotransformation of zinc silicate and sulfide mineral ores. Environ Microbiol. (2013) 15:2173–86. 10.1111/1462-2920.12089
206.
Nitu M Prabhjot K Daljeet K Jitender S Sunita D . Isolation of silicate solubilising microbes (SSM) from soil and water samples as potential components of biofertilizers. Res J Chem Environ. (2024) 28:7. 10.25303/287rjce0940100
207.
Hutchens E Valsami-Jones E McEldowney S Gaze W McLean J . The role of heterotrophic bacteria in feldspar dissolution–an experimental approach. Miner Mag. (2003) 67:1157–70. 10.1180/0026461036760155
208.
Sun Y Qian G Pang S Guo J Wang D Wang Z et al . Element partitioning and stabilization for impurities removal between liquid silicon and silicate melts: Ab initio insights into electronic structure. J Mol Liq. (2024) 400:124566. 10.1016/j.molliq.2024.124566
209.
Alam MS Wu Y Cheng T . Silicate minerals as a source of arsenic contamination in groundwater. Water Air Soil Pollut. (2014) 225:1–15. 10.1007/s11270-014-2201-9
210.
Oter C Gokkus K Gur M Butun V . Polymeric adsorbent for the effective removal of toxic dyes from aqueous solutions: Equilibrium, kinetic, and thermodynamic modeling. ChemistrySelect. (2024) 9:e202403526. 10.1002/slct.202403526
211.
Helgeson HC Murphy WM Aagaard P . Thermodynamic and kinetic constraints on reaction rates among minerals and aqueous solutions. II Rate constants, effective surface area, and the hydrolysis of feldspar. Geochim Cosmochim Acta. (1984) 48:2405–32. 10.1016/0016-7037(84)90294-1
212.
Wild B Bas-Lorillot J Daval D . Effect of pH and temperature on olivine dissolution anisotropy. Geochim Cosmochim Acta. (2025) 405:55–65. 10.1016/j.gca.2025.08.004
213.
Li GL Zhou CH Fiore S Yu WH . Interactions between microorganisms and clay minerals: New insights and broader applications. Appl Clay Sci. (2019) 177:91–113. 10.1016/j.clay.2019.04.025
214.
Jongmans AG Van Breemen N Lundström U Van Hees PAW Finlay RD Srinivasan M et al . Rock-eating fungi. Nature. (1997) 389:682–3. 10.1038/39493
215.
Wei W Zhang X Cui J Wei Z . Interaction between low molecular weight organic acids and hydroxyapatite with different degrees of crystallinity. Colloids Surf A Physicochem Eng Asp. (2011) 392:67–75. 10.1016/j.colsurfa.2011.09.034
216.
Kong M Huang L Li L Zhang Z Zheng S Wang MK et al . Effects of oxalic and citric acids on three clay minerals after incubation. Appl Clay Sci. (2014) 99:207–14. 10.1016/j.clay.2014.06.035
217.
Wang X Li Q Hu H Zhang T Zhou Y . Dissolution of kaolinite induced by citric, oxalic, and malic acids. J Colloid Interface Sci. (2005) 290:481–8. 10.1016/j.jcis.2005.04.066
218.
Lazo DE Dyer LG Alorro RD . Silicate, phosphate and carbonate mineral dissolution behaviour in the presence of organic acids: a review. Miner Eng. (2017) 100:115–23. 10.1016/j.mineng.2016.10.013
219.
Drever JI Stillings LL . The role of organic acids in mineral weathering. Colloids Surf A Physicochem Eng Asp. (1997) 120:167–81. 10.1016/S0927-7757(96)03720-X
220.
Casey W Bunker B Hochella M White A . Mineral-water interface geochemistry. Rev Mineral. (1990) 23:397–426. 10.1515/9781501509131-014
221.
Salek SS Kleerebezem R Jonkers HM Voncken JHL Van Loosdrecht MCM . Determining the impacts of fermentative bacteria on wollastonite dissolution kinetics. Appl Microbiol Biotechnol. (2013) 97:2743–52. 10.1007/s00253-012-4590-2
222.
Rozendal RA Hamelers HV Buisman CJ . Effects of membrane cation transport on pH and microbial fuel cell performance. Environ Sci Technol. (2006) 40:5206–11. 10.1021/es060387r
223.
Yuan J Appel J Gutekunst K Lai B Krömer JO . Molecular dynamics of photosynthetic electron flow in a biophotovoltaic system. Environ Sci Ecotechnol. (2025) 23:100519. 10.1016/j.ese.2024.100519
224.
Chandrakala C Voleti S Bandeppa S Kumar NS Latha P . Silicate solubilization and plant growth promoting potential of Rhizobium sp. isolated from rice rhizosphere. Silicon. (2019) 11:2895–906. 10.1007/s12633-019-0079-2
225.
Kutuzova RS . Release of silica from minerals as a result of microbial activity. Mikrobiologiya. (1969) 38:596–602.
226.
Rajabipour F Giannini E Dunant C Ideker JH Thomas MD . Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem Concr Res. (2015) 76:130–46. 10.1016/j.cemconres.2015.05.024
227.
Sheng XF Zhao F He LY Qiu G Chen L . Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol. (2008) 54:1064–8. 10.1139/W08-089
228.
Sheng XF He LY . Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can J Microbiol. (2006) 52:66–72. 10.1139/w05-117
229.
Bennett PC Rogers JR Choi WJ Hiebert FK . Silicates, silicate weathering, and microbial ecology. Geomicrobiol J. (2001) 18:3–19. 10.1080/01490450151079734
230.
Stumm W . Reactivity at the mineral-water interface: dissolution and inhibition. Colloids Surf A Physicochem Eng Asp. (1997) 120:143–66. 10.1016/S0927-7757(96)03866-6
231.
Singh S Chhabra R Sharma A Bisht A . Harnessing the power of zinc-solubilizing bacteria: a catalyst for a sustainable agrosystem. Bacteria. (2024) 3:15–29. 10.3390/bacteria3010002
232.
Schalk IJ . Bacterial siderophores: diversity, uptake pathways and applications. Nat Rev Microbiol. (2025) 23:24–40. 10.1038/s41579-024-01090-6
233.
Kalinowski BE Liermann LJ Brantley SL Barnes A Pantano CG . X-ray photoelectron evidence for bacteria-enhanced dissolution of hornblende. Geochim Cosmochim Acta. (2000) 64:1331–43. 10.1016/S0016-7037(99)00371-3
234.
Yadegari AH Etesami H . The multifaceted role of silicon and silicon-solubilizing bacteria in sustainable agriculture. In:de Mello PradoREtesamiHSrivastavaAK, editors. Silicon Advances for Sustainable Agriculture and Human Health: Increased Nutrition and Disease Prevention. Cham: Springer (2024). p. 145–164.
235.
Timofeeva AM Galyamova MR Sedykh SE . Plant growth-promoting soil bacteria: nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants. (2023) 12:4074. 10.3390/plants12244074
236.
Heuer-Jungemann A Feliu N Bakaimi I Hamaly M Alkilany A Chakraborty I et al . The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem Rev. (2019) 119:4819–80. 10.1021/acs.chemrev.8b00733
237.
Furrer G Stumm W . The coordination chemistry of weathering: I. Dissolution kinetics of δ-Al2O3 and BeO. Geochim Cosmochim Acta. (1986) 50:1847–60. 10.1016/0016-7037(86)90243-7
238.
Tian X Sun Y Li H Duan X Zhao Q Ma T et al . Crystallographic texturing of electrodeposits for sustainable Zn anodes. Adv Energy Mater. (2025) 15:2403995. 10.1002/aenm.202403995
239.
Brindavathy R . Silicate minerals induced by microorganisms. In:BerenjianASeifanM, editors. Mineral Formation by Microorganisms: Concepts and Applications.Cham: Springer (2022). p. 125–59.
240.
Yin X Shan J Dou L Cheng Y Liu S Hassan RY et al . Multiple bacteria recognition mechanisms and their applications. Coord Chem Rev. (2024) 517:216025. 10.1016/j.ccr.2024.216025
241.
Bashir Z Hamid B Yatoo AM Nisa M Sultan Z Popescu SM et al . Phosphorus solubilizing microorganisms: An eco-friendly approach for sustainable plant health and bioremediation. J Soil Sci Plant Nutr. (2024) 24:6838–54. 10.1007/s42729-024-02007-1
242.
Bist V Niranjan A Ranjan M Lehri A Seem K Srivastava S et al . Silicon-solubilizing media and its implication for characterization of bacteria to mitigate biotic stress. Front Plant Sci. (2020) 11:28. 10.3389/fpls.2020.00028
243.
Setiawan WK Chiang KY . Crop residues as potential sustainable precursors for developing silica materials: a review. Waste Biomass Valor. (2021) 12:2207–36. 10.1007/s12649-020-01126-x
244.
Seyfferth AL Morris AH Gill R Kearns KA Mann JN Paukett M et al . Soil incorporation of silica-rich rice husk decreases inorganic arsenic in rice grain. J Agric Food Chem. (2016) 64:3760–6. 10.1021/acs.jafc.6b01201
245.
Mamud A Saha B Hossain SA Chowdhury MTA . Alleviation of arsenic accumulation in rice by applying silicon-rich rice husk residues in Bangladesh soil. Bangladesh J Sci Ind Res. (2021) 56:195–206. 10.3329/bjsir.v56i3.55967
246.
Zhai J Burke IT Stewart DI . Beneficial management of biomass combustion ashes. Renew Sustain Energy Rev. (2021) 151:111555. 10.1016/j.rser.2021.111555
247.
Dinh VM Nguyen HT Nguyen AM Tran HT Nguyen DT Duong LH et al . Biochar-phytolith composite from rice straw: a dual-function material for hydrogen sulfide removal and slow-release nutrient delivery. Biomass Bioenergy. (2025) 195:107705. 10.1016/j.biombioe.2025.107705
248.
Penido ES Bennett AJ Hanson TE Seyfferth AL . Biogeochemical impacts of silicon-rich rice residue incorporation into flooded soils: implications for rice nutrition and cycling of arsenic. Plant Soil. (2016) 399:75–87. 10.1007/s11104-015-2682-3
249.
Wang H Wang X Peng B . Using an improved Si-rich husk ash to decrease inorganic arsenic in rice grain. Sci Total Environ. (2022) 803:150102. 10.1016/j.scitotenv.2021.150102
250.
Kumarathilaka P Bundschuh J Seneweera S Marchuk A Ok YS . Iron modification to silicon-rich biochar and alternative water management to decrease arsenic accumulation in rice (Oryza sativa L.). Environ Pollut. (2021) 286:117661. 10.1016/j.envpol.2021.117661
251.
Yang Y Tang Z Gao A Chen C Wang P Zhao FJ et al . Silicon-enriched rice straw biochar and silicon fertilizer mitigate rice straighthead disease by reducing dimethylarsinic acid accumulation. Plant Soil. (2025). In press. 10.1007/s11104-025-07478-4
252.
Xu R Huang J Guo H Wang C Zhan H . Functions of silicon and phytolith in higher plants. Plant Signal Behav. (2023) 18:2198848. 10.1080/15592324.2023.2198848
253.
Yamaguchi N Ando K Daikoku T Suda A Yada S Furuya M et al . How does lime and rice straw compost application affect arsenic uptake in rice after a century of cropping under flooded conditions?. Soil Sci Plant Nutr. (2025) 71:205–14. 10.1080/00380768.2024.2437435
254.
Seghir BB Hemmami H Hocine BME Soumeia Z Sharifi-Rad M Awuchi CG et al . Methods for the preparation of silica and its nanoparticles from different natural sources. Biol Trace Elem Res. (2023) 201:5871–83. 10.1007/s12011-023-03628-w
255.
Xu N Cheng L Kong Y Chen G Zhao L Liu F et al . Functional analyses of the NRT2 family of nitrate transporters in Arabidopsis. Front Plant Sci. (2024) 15:1351998. 10.3389/fpls.2024.1351998
256.
Rajput P Singh A Agrawal S Ghazaryan K Rajput VD Movsesyan H et al . Effects of environmental metal and metalloid pollutants on plants and human health: exploring nano-remediation approach. Stress Biol. (2024) 4:27. 10.1007/s44154-024-00156-y
257.
Masclaux-Daubresse C Reisdorf-Cren M Pageau K Lelandais M Grandjean O Kronenberger J et al . Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. (2006) 140:444–56. 10.1104/pp.105.071910
258.
Nath S Panda P Mishra S Dey M Choudhury S Sahoo L et al . Arsenic stress in rice: redox consequences and regulation by iron. Plant Physiol Biochem. (2014) 80:203–10. 10.1016/j.plaphy.2014.04.013
259.
Kumarathilaka P Seneweera S Ok YS Meharg A Bundschuh J . Arsenic in cooked rice foods: assessing health risks and mitigation options. Environ Int. (2019) 127:584–91. 10.1016/j.envint.2019.04.004
260.
Sinha D Datta S Mishra R Agarwal P Kumari T Adeyemi SB et al . Negative impacts of arsenic on plants and mitigation strategies. Plants. (2023) 12:1815. 10.3390/plants12091815
261.
Faizan M Alam P Iqbal S Waheed Z Eren A Shamsi A et al . Calcium-mediated mitigation strategies and novel approaches to alleviate arsenic induced plant stress. Plant Sci. (2025) 356:112527. 10.1016/j.plantsci.2025.112527
262.
Mishra S Alfeld M Sobotka R Andresen E Falkenberg G Küpper H et al . Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J Exp Bot. (2016) 67:4639–46. 10.1093/jxb/erw238
263.
Sharma P Dubey RS . Lead toxicity in plants. Braz J Plant Physiol. (2005) 17:35–52. 10.1590/S1677-04202005000100004
264.
Coelho DG de Andrade HM Marinato CS Araujo SC de Matos LP da Silva VM et al . Exogenous jasmonic acid enhances oxidative protection of Lemna valdiviana subjected to arsenic. Acta Physiol Plant. (2020) 42:97. 10.1007/s11738-020-03086-0
265.
Joardar S Dewanjee S Bhowmick S Dua TK Das S Saha A et al . Rosmarinic acid attenuates cadmium-induced nephrotoxicity via inhibition of oxidative stress, apoptosis, inflammation and fibrosis. Int J Mol Sci. (2019) 20:2027. 10.3390/ijms20082027
266.
Kumari A Pandey-Rai S . Enhanced arsenic tolerance and secondary metabolism by modulation of gene expression and proteome profile in Artemisia annua L. after application of exogenous salicylic acid. Plant Physiol Biochem. (2018) 132:590–602. 10.1016/j.plaphy.2018.10.010
267.
Wang Y Chai L Yang Z Mubarak H Tang C . Chlorophyll fluorescence in leaves of Ficus tikoua under arsenic stress. Bull Environ Contam Toxicol. (2016) 97:576–81. 10.1007/s00128-016-1905-5
268.
Patel A Tiwari S Prasad SM . Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in Nostoc muscorum. Ecotoxicol Environ Saf. (2018) 157:369–79. 10.1016/j.ecoenv.2018.03.056
269.
Tripathi DK Singh VP Prasad SM Chauhan DK Dubey NK Rai AK et al . Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll fluorescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol Environ Saf. (2015) 113:133–44. 10.1016/j.ecoenv.2014.09.029
270.
Piršelová B Boleček P Gálusová T . Effect of cadmium and arsenic on chlorophyll fluorescence of selected soybean cultivars. Russ J Plant Physiol. (2016) 63:469–73. 10.1134/S1021443716040129
271.
Kim YH Khan AL Kim DH Lee SY Kim KM Waqas M et al . Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. (2014) 14:1–13. 10.1186/1471-2229-14-13
272.
Hosseini SA Maillard A Hajirezaei MR Ali N Schwarzenberg A Jamois F et al . Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency. Front Plant Sci. (2017) 8:1359. 10.3389/fpls.2017.01359
273.
Montpetit J Vivancos J Mitani-Ueno N Yamaji N Rémus-Borel W Belzile F et al . Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol Biol. (2012) 79:35–46. 10.1007/s11103-012-9892-3
274.
Gaur S Kumar J Kumar D Chauhan DK Prasad SM Srivastava PK et al . Fascinating impact of silicon and silicon transporters in plants: a review. Ecotoxicol Environ Saf. (2020) 202:110885. 10.1016/j.ecoenv.2020.110885
275.
Mitani N Chiba Y Yamaji N Ma JF . Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell. (2009) 21:2133–42. 10.1105/tpc.109.067884
276.
Bokor B Vaculík M Slováková L Masarovič D Lux A . Silicon does not always mitigate zinc toxicity in maize. Acta Physiol Plant. (2014) 36:733–43. 10.1007/s11738-013-1451-2
277.
Markovich O Kumar S Cohen D Addadi S Fridman E Elbaum R et al . Silicification in leaves of sorghum mutant with low silicon accumulation. Silicon. (2019) 11:2385–91. 10.1007/s12633-015-9348-x
278.
Sun H Duan Y Mitani-Ueno N Che J Jia J Liu J et al . Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ. (2020) 43:732–44. 10.1111/pce.13679
279.
Sun H Guo J Duan Y Zhang T Huo H Gong H et al . Isolation and functional characterization of CsLsi1, a silicon transporter gene in Cucumis sativus. Physiol Plant. (2017) 159:201–14. 10.1111/ppl.12515
280.
Yamaji N Ma JF . Further characterization of a rice silicon efflux transporter, Lsi2. Soil Sci Plant Nutr. (2011) 57:259–64. 10.1080/00380768.2011.565480
281.
Vivancos J Deshmukh R Grégoire C Rémus-Borel W Belzile F Bélanger RR et al . Identification and characterization of silicon efflux transporters in horsetail (Equisetum arvense). J Plant Physiol. (2016) 200:82–9. 10.1016/j.jplph.2016.06.011
282.
Yamaji N Sakurai G Mitani-Ueno N Ma JF . Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc Natl Acad Sci USA. (2015) 112:11401–6. 10.1073/pnas.1508987112
283.
Yamaji N Chiba Y Mitani-Ueno N Feng Ma J . Functional characterization of a silicon transporter gene implicated in silicon distribution in barley. Plant Physiol. (2012) 160:1491–7. 10.1104/pp.112.204578
284.
Grégoire C Rémus-Borel W Vivancos J Labbé C Belzile F Bélanger RR . Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J. (2012) 72:320–30. 10.1111/j.1365-313X.2012.05082.x
285.
Deshmukh RK Vivancos J Guérin V Sonah H Labbé C Belzile F et al . Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol Biol. (2013) 83:303–15. 10.1007/s11103-013-0087-3
286.
Siddiqui K Babar M Jamail I Musharraf G Galani S . Silicon-mediated arsenic tolerance: restriction of arsenic uptake and modulation of antioxidant defense system in rice seedlings. J Plant Growth Regul. (2025) 44:674–85. 10.1007/s00344-024-11472-y
287.
Sattar A Sher A Abourehab MA Ijaz M Nawaz M Ul-Allah S et al . Application of silicon and biochar alleviates the adversities of arsenic stress in maize by triggering the morpho-physiological and antioxidant defense mechanisms. Front Environ Sci. (2022) 10:979049. 10.3389/fenvs.2022.979049
288.
Hossain MM Khatun MA Haque MN Bari MA Alam MF Mandal A et al . Silicon alleviates arsenic-induced toxicity in wheat through vacuolar sequestration and ROS scavenging. Int J Phytoremediation. (2018) 20:796–804. 10.1080/15226514.2018.1425669
289.
Khan T Bilal S Asaf S Alamri SS Imran M Khan AL et al . Silicon-induced tolerance against arsenic toxicity by activating physiological, anatomical and biochemical regulation in Phoenix dactylifera (date palm). Plants. (2022) 11:2263. 10.3390/plants11172263
290.
Faisal M Bilmez Ozcinar A Karadeniz E Faizan M Sultan H Alatar AA et al . Supplementation of silicon oxide nanoparticles mitigates the damaging effects of arsenic stress on photosynthesis, antioxidant mechanism and nitrogen metabolism in Brassica juncea. Sci Rep. (2025) 15:21476. 10.1038/s41598-025-04553-9
291.
Sen M Roy A Rani K Nalia A Das T Tigga P et al . Crop residue: Status, distribution, management, and agricultural sustainability. In:MeenaVSRakshitAMeenaMDBaslamMRizwanul FattahIMLamSSKabaJS, editors. Waste Management for Sustainable and Restored Agricultural Soil. Cham: Elsevier (2024). 10.1016/B978-0-443-18486-4.00017-8
292.
Teasley WA Limmer MA Seyfferth AL . How rice (Oryza sativa L.) responds to elevated as under different Si-rich soil amendments. Environ Sci Tech. (2017) 51:10335–43. 10.1021/acs.est.7b01740
293.
Maleva M Borisova G Koshcheeva O Sinenko O . Biofertilizer based on silicate solubilizing bacteria improves photosynthetic function of Brassica juncea. AGROFOR Int J. (2017) 2:13–9. 10.7251/AGRENG1703013M
294.
Perkebunan M . Solubilization of silicate from quartz mineral by potential silicate solubilizing bacteria. Menara Perkebunan. (2017) 85:95–104.
295.
Hu L Xia M Lin X Xu C Li W Wang J et al . Earthworm gut bacteria increase silicon bioavailability and acquisition by maize. Soil Biol Biochem. (2018) 125:215–21. 10.1016/j.soilbio.2018.07.015
296.
Lee KE Adhikari A Kang SM You YH Joo GJ Kim JH et al . Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy. (2019) 9:144. 10.3390/agronomy9030144
297.
Vijayapriya M Mahalakshmi S Prabudoss V Pandeeswari N . Natural efficiency of Bacillus mucilaginosus on the solubilization of silicates. J Pharmacogn Phytochem. (2019) 8:549–52.
298.
Wang RR Wang Q He LY Qiu G Sheng XF . Isolation and the interaction between a mineral-weathering Rhizobium tropici Q34 and silicate minerals. World J Microbiol Biotechnol. (2015) 31:747–53. 10.1007/s11274-015-1827-0
299.
Liu W Xu X Wu X Yang Q Luo Y Christie P et al . Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ Geochem Health. (2006) 28:133–40. 10.1007/s10653-005-9022-0
300.
de Cordoba MCF Matos J Montaña R Poon PS Lanfredi S Praxedes FR et al . Sunlight photoactivity of rice husks-derived biogenic silica. Catal Today. (2019) 328:125–35. 10.1016/j.cattod.2018.12.008
301.
Periasamy VS Athinarayanan J Alshatwi AA . Extraction and biocompatibility analysis of silica phytoliths from sorghum husk for three-dimensional cell culture. Process Biochem. (2018) 70:153–9. 10.1016/j.procbio.2018.04.017
302.
Wattanasiriwech S Wattanasiriwech D Svasti J . Production of amorphous silica nanoparticles from rice straw with microbial hydrolysis pretreatment. J Non-Cryst Solids. (2010) 356:1228–32. 10.1016/j.jnoncrysol.2010.04.032
303.
Anuar MF Fen YW Zaid MHM Matori KA Khaidir REM . Synthesis and structural properties of coconut husk as potential silica source. Results Phys. (2018) 11:1–4. 10.1016/j.rinp.2018.08.018
304.
Assefi M Davar F Hadadzadeh H . Green synthesis of nanosilica by thermal decomposition of pine cones and pine needles. Adv Powder Technol. (2015) 26:1583–9. 10.1016/j.apt.2015.09.004
305.
Mor S Manchanda CK Kansal SK Ravindra K . Nanosilica extraction from processed agricultural residue using green technology. J Clean Prod. (2017) 143:1284–90. 10.1016/j.jclepro.2016.11.142
306.
Kauldhar BS Yadav SK . Turning waste to wealth: a direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J Clean Prod. (2018) 194:158–66. 10.1016/j.jclepro.2018.05.136
307.
Ibrahim IA Zikry AAF Sharaf MA . Preparation of spherical silica nanoparticles: Stober silica. J Am Sci. (2010) 6:985–9.
308.
Morales-Paredes CA Rodríguez-Linzán I Saquete MD Luque R Osman SM Boluda-Botella N et al . Silica-derived materials from agro-industrial waste biomass: Characterization and comparative studies. Environ Res. (2023) 231:116002. 10.1016/j.envres.2023.116002
309.
Emamian SA Eskandari-Naddaf H . Effect of porosity on predicting compressive and flexural strength of cement mortar containing micro and nano-silica by ANN and GEP. Constr Build Mater. (2019) 218:8–27. 10.1016/j.conbuildmat.2019.05.092
310.
Batchelor L Loni A Canham LT Hasan M Coffer JL . Manufacture of mesoporous silicon from living plants and agricultural waste: an environmentally friendly and scalable process. Silicon. (2012) 4:259–66. 10.1007/s12633-012-9129-8
311.
Adebisi JA Agunsoye JO Bello SA Ahmed II Ojo OA Hassan SB et al . Potential of producing solar grade silicon nanoparticles from selected agro-wastes: a review. Sol Energy. (2017) 142:68–86. 10.1016/j.solener.2016.12.001
312.
Adam F Chew TS Andas J . A simple template-free sol–gel synthesis of spherical nanosilica from agricultural biomass. J Sol-Gel Sci Technol. (2011) 59:580–3. 10.1007/s10971-011-2531-7
313.
Okoronkwo EA Imoisili PE Olusunle SOO . Extraction and characterization of amorphous silica from corn cob ash by sol-gel method. Chem Mater Res. (2013) 3:68–72.
314.
Bortolotto LT Guzi de Moraes E Paolinelli Shinhe G Falk G Novaes de Oliveira AP . Obtaining biogenic silica from sugarcane bagasse and leaf ash. Waste Biomass Valor. (2021) 12:3205–21. 10.1007/s12649-020-01230-y
Summary
Keywords
arsenic, Si-based agro-wastes, microbes, transporters, dietary exposure
Citation
Koley S, Garg J, Golui K and Rakshit A (2025) Microbially mediated silicon-based agro-wastes: a possible option in reducing bioaccumulation of arsenic. Front. Nutr. 12:1657640. doi: 10.3389/fnut.2025.1657640
Received
01 July 2025
Accepted
26 August 2025
Published
15 September 2025
Volume
12 - 2025
Edited by
Durgesh K. Jaiswal, Graphic Era University, India
Reviewed by
Chaowei Fang, Henan Normal University, China
Frank Mabagala, Tanzania Agricultural Research Institute (TARI), Tanzania
Updates
Copyright
© 2025 Koley, Garg, Golui and Rakshit.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amitava Rakshit amitavar@bhu.ac.in
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.