- 1School of Life Sciences, Jiangsu University, Zhenjiang, China
- 2Zhenjiang Fengzhou Zhiyu Biotechnology Co., Ltd., Zhenjiang, China
- 3School of Grain Science and Technology, Jiangsu University of Science and Technology, Zhenjiang, China
Marine microalgae have emerged as a sustainable and renewable feedstock for lipid production, offering significant potential to address environmental challenges and feed resource scarcity. This review provides a comprehensive analysis of marine microalgae-based lipid production, integrating insights from biosynthesis, technological advancements, and practical applications. First, we elucidate lipid accumulation process in marine microalgae, focusing on metabolic regulation, environmental stressors, and pharmaceutical functions. Next, this paper critically evaluate cutting-edge innovations in marine microalgae cultivation strategies, such as culture medium alternative, two-stage cultivation model, and microalgal cells immobilization. Last, the review highlights diverse applications of microalgal lipids in feed production for aquatic animals, livestock and poultry. Specific effects of dietary supplementation of microalgal lipid on the growth performance, health status and meat quality of animals are summarized. This review also assesses the technical challenges and practical viability of marine microalgae-based lipid production. Accordingly, some potential solutions which will promote the wide application of microalgal lipid in aquaculture and livestock/poultry farming are proposed. It is expected that this review can help researchers gain a more comprehensive understanding of marine microalgal lipids and encourage them to find out more actionable strategies to maximize the ecological and economic potential of marine microalgal lipids.
1 Introduction
As one of the essential raw materials in animal feed, lipids play a key role in aquaculture and livestock/poultry farming (1–3). Dietary intake of unsaturated fatty acids are favorable to the health status of animals (4–6). In the past, the production of lipids as an additive in animal feed relied excessively on reduction fishery, which yields fish oil containing long-chain polyunsaturated fatty acid (PUFA). Besides, traditional agriculture, particularly the cultivation of soybean, peanut, oil palm, and sunflower, could provides vegetable oil (7–10). However, global ocean pollution and soil degradation are constraining the sustainable development of lipid production reliant on reduction fishery and traditional agriculture (11, 12). Additionally, the expansion of oil crop cultivation competes with staple food production, creating potential food security risks and threatening the stability of human society (13, 14). Recently, aforementioned problems are intensifying the search for an alternative lipid resource, which can support the sustainable development of aquaculture and livestock/poultry farming.
Among the emerging bio-based technologies, marine microalgae have garnered significant attention as a sustainable and eco-friendly platform to produce a variety of bio-products, including lipids (15, 16). In the feed or food industry, lipids containing functional components, such as PUFAs, natural antioxidants, and functional pigments, are widely used. These lipids have more beneficial effects on the health of animals or humans and possess higher market value. Therefore, lipids containing functional components have garnered increasing attention in both scientific research and industrial sectors. Marine microalgae, a diverse group of photosynthetic microorganisms naturally living in ocean and coastal regions, exhibit unparalleled advantages over terrestrial crops (e.g., soybean, peanut, sunflower, etc.) for the production of lipids (17). Firstly, marine microalgae have much higher growth rate and shorter cultivation period than terrestrial oil crops. It was reported that the cultivation period of marine microalgae ranged between 10 and 20 days while that of soybean could reach several months (18–20). According to the experimental results in laboratory research, compared to terrestrial oil crops, microalgae demonstrate 10–20 times higher oil yield per unit area, making them a scalable solution for large-scale lipid production. Secondly, cultivation of marine microalgae for lipid production does not intensively compete with traditional agriculture for arable land, posing no threat to the food security of human society. Thirdly, some marine microalgae contain much more PUFA, such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and α-linolenic acid (ALA), which are of importance to the health status and growth performance of animals (21, 22). For example, dietary intake of PUFA could scavenge the reactive oxygen species in cells and improve the immunity of animals. In recent years, as the functional benefits of marine microalgal lipids become increasingly recognized, feed industry, which traditionally focused on fish oil, have expanded their microalgal lipid product offerings, leading to a burgeoning array of health-promoting microalgal lipid.
In fact, as early as several decades ago, researchers had reported the presence of abundant lipids in marine microalgae. However, in the field of science and technology, the transition from laboratory research to industrial implementation requires a holistic understanding of the technological and socioeconomic factors (23, 24). Key knowledge gaps persist in elucidating the regulatory mechanisms of lipid metabolism under stress conditions, optimizing large-scale cultivation systems, and developing high-value bio-products using microalgal lipids. Hence, it was not until recent technological advancements that microalgal lipid production gradually transitioned from theoretical potential to practical reality, now actively contributing to the development of the bio-based economy (25, 26). In the past several years, researchers conducted extensive studies in the field of microalgal lipid production, with the primary focuses covering lipid synthesis, microalgae cultivation, and practical application of microalgal lipids. Particularly, as carbon neutrality is becoming a necessary condition of the achievement of Paris Agreement temperature goals, carbon bio-sequestration by employing microalgae lipid production has garnered growing attention in recent research (27–29). According to the data, soy oil production in 2015 was 48.8 million ton, which accounted for 29% of global vegetable oil production (30). Hence, it was estimated that 168.3 million ton vegetable oil was produced in 2015. Given the present states of technology and production capability, microalgal lipids remain unable to fully substitute fish oil or vegetable oils as principal lipid sources. Given the advantages of marine microalgae in synthesizing PUFA, they are primarily positioned as a lipid production platform rather than mere substitutes for vegetable oil.
This review provides a comprehensive analysis of the current state of marine microalgae-based lipid production, with a focus on three interconnected themes: (1) the metabolic pathways and environmental triggers governing lipid biosynthesis in marine microalgae, (2) cutting-edge technological innovations for marine microalgae cultivation, and (3) practical applications of marine microalgal lipids as feed additives in the aquaculture and the livestock and poultry farming. It should also be noted that despite the technological advances, commercialization of marine microalgal lipids faces multifaceted challenges, including safety risks of biomass, poor digestibility and palatability, and uncertainty of new models. By summarizing recent research advances and identifying persistent challenges, this work aims to guide future research and industrial efforts toward realizing the full potential of marine microalgae as a sustainable lipid source in the bio-based economy. Compared with publications which mainly focused on either microalgal lipid synthesis, cultivation techniques, or feed production technology, this review provides a comprehensive analysis of the aforementioned aspects from an industry chain perspective. Through integrating unsaturated fatty acids induction, microalgae-based wastewater remediation, and dietary supplementation of microalgal lipids, This review presents to readers the application of microalgae in PUFA production, wastewater treatment, and animal farming across the integrated industrial chain. In the view of the present authors, the main innovations of this review include: (1) discussing emerging technologies for marine microalgae-based lipid production from the perspective of industrial chain development; (2) examining the advantages of microalgae lipid as a carbon capture carrier for wastewater remediation; and (3) providing a comprehensive analysis of the applications of marine microalgae lipid in aquaculture and livestock farming.
2 Lipid production in marine microalgae
In the field of fundamental research, scientists primarily focus on three key aspects: the synthesis of lipid and lipid-soluble components, the relationship between lipid synthesis and carbon bio-sequestration, and environmental factors influencing microalgal lipid synthesis.
2.1 Synthesis of lipid and lipid-soluble components
2.1.1 Lipid and polyunsaturated fatty acid
As one of the critical cellular constituents, lipids serve fundamental metabolic functions in microalgal metabolism. Accumulation of lipid, which contains more energy than starch and protein, as a major survival strategy favoring the microalgal cells exposed to cold environment has recently been demonstrated (31). Also, lipid degradation is essential for microalgae to remodel membrane lipids and mobilize storage lipids, enabling survival and growth during environmental fluctuations (32).
In fact, although microalgae from both marine environment and freshwater environment contain PUFA, marine microalgae are more widely employed as the producers of lipids. Main reasons for this phenomenon include the vast marine cultivation environment, high PUFA content, and diverse algal species in ocean. Firstly, the rapid growth of global population has led to increasing pressure on terrestrial resources, making freshwater microalgae cultivation on land potentially competitive with traditional agriculture. In contrast, the vast marine environment offers sustainably developed space for microalgae cultivation. This is one of the key reasons for utilizing marine microalgae as a bio-carrier for lipid production. Secondly, marine microalgae have higher PUFA content and lipid productivity. According to the survey of 19 brackish and marine microalgae, average lipid content of dry weight was around 22% and some microalgae even contained up to 40.6% lipid content (33). In marine microalgae, lipid synthesis, starting from carbon fixation via the Calvin cycle or glycolysis, primarily occurs in chloroplasts and endoplasmic reticulum. In addition, compared with freshwater microalgae, marine microalgae contain much higher content of long-chain PUFA, such as ARA (Arachidonic acid), DHA and EPA (34, 35). In nature, enrichment of long-chain PUFA in lipid can be regarded as a self-protection mechanism of marine microalgae in low-temperature environment. The comparatively lower freezing point of PUFA enhances the cold tolerance of marine microalgae by maintaining the membrane fluidity and improving their survival rates in low-temperature environments. Thirdly, marine microalgae encompass a much greater diversity of species than freshwater microalgae, thus offering a broader selection for industrial applications. Considering the advantages of marine microalgae in the production of lipids, in this review paper,.
Mechanism study has revealed that the biosynthesis of PUFA in marine microalgae initiates with the elongation and desaturation of precursor fatty acids, primarily starting from saturated fatty acids, such as palmitic acid (36–38). Two groups of membrane-bound enzymes, elongases and desaturases, are driving elongation and desaturation, respectively (39). Notably, final forms of fatty acids by the end of elongation and desaturation process not only influenced by genetic expression, but also regulated by environmental factors (40). In addition, polyketide synthase (PKS) pathway, which is an oxygen-independent pathway widely documented in Dinophytes or Thraustochytrids, also exist to produce PUFA (41). The elucidation of multiple metabolic routes has bolstered the practical feasibility of metabolic engineering approaches for boosting lipid biosynthesis in microalgae.
In addition to environmental factors and metabolic pathways, advances in research techniques have progressively identified key regulatory genes controlling microalgal lipid synthesis (38, 42). However, given the stringent regulatory constraints on the application of genetic modification techniques, environmental factors are still the major factors that can be adjusted to enhance the PUFA biosynthesis in marine microalgae.
2.1.2 Lipid-soluble components
Lipids derived from some marine microalgae not only have higher concentrations of PUFA, but also contain more anti-oxidants, such as zeaxanthin, carotene, lutein and fucoxanthin (22, 43, 44). Recently, some lipid-soluble components derived from marine microalgae have been scientifically validated as essential for the growth and health of humans and animals.
As one of the typical lipid-soluble components, astaxanthin with superior antioxidant capacity has been intensively studied to reveal its biosynthesis pathway. Glyceraldehyde 3-phosphate is produced in microalgal cells through glycolysis or the Calvin cycle, and then converted to isopentenyl pyrophosphate, which is the precursor of β-carotene (45). Astaxanthin synthesis pathway various in different microalgal species and the intermediates of synthesis process include echinenone, canthaxanthin, adonirubin, zeaxanthin, and adonixanthin. Finally, a portion of the synthesized astaxanthin is combined with fatty acids to form astaxanthin esters, which can be used as food or feed additive in downstream industry (46, 47).
Pharmaceutical functions of some lipid-soluble components in marine microalgae have been intensively studied. For example, it was reported that some antioxidants, such as β-carotene and astaxanthin, dissolved in lipids can effectively eliminate free radicals and reduce oxidative stress within cells. Theoretically, supplementation of these antioxidants in animal feeds could prevent the peroxidation in cells and delay the cellular aging. In addition, a couple of indirect pharmaceutical functions of lipid-soluble components were reported. For example, astaxanthin from marine microalgae can be added in feeds to increase the growth rate of aquatic organisms, thus preventing the use of synthetically produced hormone. Different from the synthetic chemicals, natural astaxanthin at a reasonable addition level in feed has no negative effects on the health status of aquatic animals. Accordingly, dietary supplementation of astaxanthin can enhance the growth of aquatic animals and improve their survival rate, performing an indirect pharmaceutical function.
2.2 Carbon bio-sequestration
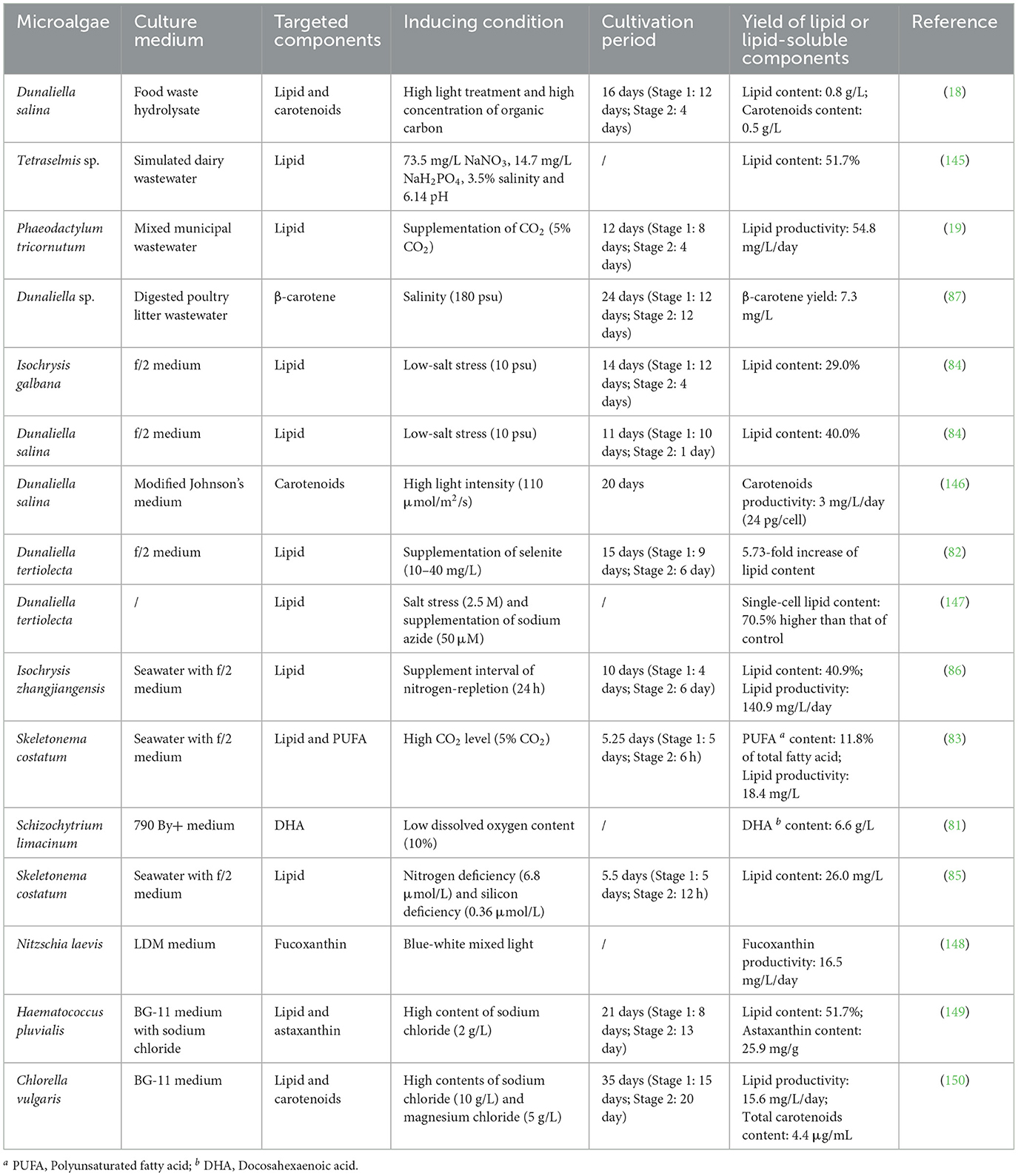
Lipids, particularly fatty acids with a long carbon skeleton, serve as excellent carriers of carbon element, playing a key role in carbon bio-sequestration. Percentage of carbon element in microalgal compounds can be estimated according to the equation as follows:
where PC is the percentage of carbon element; NC refers to the number of carbon atom in certain microalgal compounds; M is the relative molecular weight.
As shown in Table 1, percentages of carbon element in lipid compounds are high, falling in a scope of 69.8%−80.5%. Particularly, percentages (75.0%−80.5%) of carbon element in the common microalgal fatty acids are much higher. By contrast, percentages of carbon element in amino acids and polysaccharide are lower. For example, percentages of carbon element in cysteine and starch are only 29.8% and 44.4%, respectively (Table 1). On an equal mass basis, lipid-rich marine microalgae capture and sequester more carbon compared to those rich in protein or polysaccharides. Therefore, lipid-rich marine microalgae can be regarded as a promising carbon sink, capturing both carbon-containing organics in culture medium and atmospheric CO2.
2.3 Environmental factors
Environmental factors which can significantly influence the lipid metabolism in marine microalgae include the nutrient profile of culture medium, microalgae cultivation temperature, and illumination characteristics.
2.3.1 Nutrient profile of culture medium
Since nitrogen and phosphorus assimilation is closely related to protein synthesis and carbon metabolism, adjustment of nutrient concentration in culture medium can change the trophic mode, particularly the synthesis of protein and lipid in marine microalgae. Past studies confirmed that through conducting nitrogen or phosphorus starvation, lipid content in marine microalgae could be improved (18, 48). In addition, the type of organic carbon can influence the lipid content in microalgae. For example, when the C/N ratio in culture medium was set as 15:1, microalgae supplied with acetate as the carbon source contained 18.2% lipid in dry biomass while those supplied with dextrose only contained 8.7% lipid (49).
In recent years, researchers have conducted more in-depth mechanistic studies to elucidate the effect of nutrient-deficiency on lipid composition and relevant metabolism in marine microalgae (50, 51). In the study of marine microalga, Diacronema lutheri, grown in nitrogen-deficiency environment, it was observed that the neutral lipid content increased significantly while glycolipid, phospholipid, and betaine lipid content decreased during the 11-day cultivation period (52). Therefore, nutrient stress not only affects total lipid content, but also modify the lipid composition. Additionally, although both nitrogen deficiency and phosphorus deficiency can enhance the lipid synthesis in marine microalgae, it was observed phosphorus starvation caused enlargement of cell size and increased carbon content of Tisochrysis lutea while nitrogen starvation had not similar effect on the cell size (50). The enlargement of cell size may be related to perturbation in the progression of cell-cycle (50, 53). In the future, with the elucidation of more lipid metabolism mechanisms, researchers will be able to more effectively improve the lipid quality of marine microalgae through nitrogen and phosphorus deficiencies.
In a real-world application, negative effect of nutrient deficiency on microalgae biomass production, which has no direct relation with lipid content but determines lipid productivity and lipid yield, should not be neglected. It was reported that with the application of nitrogen depletion, biomass productivity of marine microalga, Dunaliella salina, dropped dramatically (54). As a consequence, although nitrogen deficiency improved lipid content in Dunaliella salina. from ~26% to ~48%, lipid productivity decreased slightly (54). Negative effects of nitrogen deficiency on the growth rates of marine microalgae, Dunaliella tertiolecta and Thalassiosira pseudonana, were also reported (55).
2.3.2 Cultivation temperature
Lipid content in marine microalgae is negatively correlated with temperature increase. In the study of growing marine microalga, Chaetoceros sp., at different temperatures, when the temperature increased from 25 °C to 40 °C, lipid content of Chaetoceros sp. Decreased from 20.4% to 8.0% and lipid productivity dropped from 66.7 to 15.9 mg/L/day (56). Negative effects of temperature increase on lipid content were also observed in the culture of many other marine microalgae, such as Tetraselmis suecica, Nannochloropsis sp., and Porosira glacialis (56, 57). Significantly, low temperature impairs microalgal growth kinetics, leading to detrimental effects on overall lipid productivity and lipid yield. This necessitates a strategic equilibrium between cellular lipid content and total lipid output in the psychrophilic cultivation systems.
Not only lipid content, but also fatty acid composition in microalgal lipid is impacted by the cultivation temperature. In general, the fatty acids of marine microalgae grown at lower temperature exhibit a high degree of unsaturation. When the cultivation temperature increased from 8 °C to 26 °C, percentage of PUFA in the lipid of Nannochloropsis salina decreased from 14.50% to 9.48% while percentage of saturated fatty acid (SFA) increased from 33.9% to 46.7% (58). It was observed that with the increase of temperature from 8 °C to 24 °C, percentage of PUFA significantly dropped from 40.1% to 6.4% and percentage of EPA decreased from 34.8% to 3.5%, confirming the positive contribution of low temperature to PUFA synthesis (59). The main reason for this phenomenon is that to maintain their membrane fluidity at lower temperature, marine microalgae incorporate higher levels of PUFA in membrane lipids (60, 61). It should be noted that there are exceptional cases in which the percentage of PUFA demonstrates a positive correlation with temperature elevation. For example, the increase of temperature from 8 °C to 20 °C resulted in the increase of PUFA percentage of Nannochloropsis oculata from 17.5% to 26.9% while did not caused significant improvement of SFA percentage (58). Therefore, in the practical application, the relationship between PUFA synthesis and temperature in different marine microalgal species requires case-by-case analysis.
2.3.3 Illumination characteristics
A variety of illumination characteristics, such as light wavelength, light intensity and photoperiod, exert a critical influence on lipid synthesis of marine microalgae. Illumination could determine lipid accumulation through impacting the photosynthesis of microalgae (62). Firstly, specific spectral irradiation can significantly augment lipid synthesis and accumulation in marine microalgae. Compared to red light and white light, blue light has been demonstrated as a superior light source in stimulating marine microalgae to produce elevated levels of lipids (63). Moreover, it was observed that green light of light-emitting diode (LED) has obvious advantages over blue light and red light for lipid accumulation in four marine microalgae, Phaeodactylum tricornutum, Isochrysis galbana, Nannochloropsis salina, and Nannochloropsis oceanica (64). Green light of LED also significantly improved the percentage of UFA in the aforementioned microalgal strains from 14.3%−21.6% to 23.4%−38.1% (64). Secondly, lipid content in marine microalgae can be improved by optimizing light intensity. During the cultivation of marine diatom, Amphiprora sp., increase of light intensity from 6 to 24 μmol/m2/s resulted in the improvement of lipid content from 16.5% to 52.5% while excessive light intensity (>24 μmol/m2/s) reduced lipid content (65). Similarly, lipid contents in Isochrysis galbana, Nannochloropsis oculata, and Dunaliella salina reached peak values when the light intensity increased to 150 μmol/m2/s (66). Thirdly, photoperiod, which can change the photosynthesis of microalgal cells, is another important factor influencing the lipid synthesis of marine microalgae. According to the experimental results, the maximum total lipid content (31.3%) in Nannochloropsis sp. was obtained when microalgae were exposed to 18 h light and 6 h dark while 24:0 light/dark regime and 12:12 light/dark regime resulted in lower lipid content (67). It was reported that when photoperiod were 12:12 light/dark, 24:0 light/dark, and 0:24 light/dark, lipid contents of Pavlova lutheri were 35%, 30%, and 15%, respectively (68). Hence, neither constant illumination nor complete darkness has been demonstrated to be conducive to lipid biosynthesis and accumulation in marine microalgae. It is also noteworthy that percentage of PUFA in the lipid of marine microalgae could be improved by the optimized photoperiod (69).
In culture medium supplied with organic carbon as the major carbon source, illumination has no obvious effect on lipid synthesis in microalgae. As reported by Silaban et al., lipid contents in microalgae supplied with acetate and dextrose had not significant difference (49). The main reason for this phenomenon is that microalgae are prone to utilize organic carbon for cellular metabolism and lipid synthesis if there are sufficient organic carbon in culture medium. Under this situation, the role of photosynthesis-driven inorganic carbon uptake in the lipid synthesis process will be diminished. Accordingly, supply of illumination did not significantly improve lipid content in microalgae (49).
3 Technological advancements in microalgae-based lipid production
To bridge the gap between lab-scale innovation and industrial deployment, concerted efforts have been dedicated to three key technological dimensions: cost mitigation strategies for large-scale production, innovation of cultivation models for lipid productivity improvement, and streamlining cultivation protocols to improve process scalability.
3.1 Wastewater-based microalgae cultivation
3.1.1 Selection of wastewater for marine microalgae cultivation
The cost of artificial culture medium accounts for 30%−45% of the total microalgae cultivation cost. By contrast, some wastewater enriched with nitrogen, phosphorus, metal ions, and organic carbon, which are essential nutrients to microalgae growth, poses a serious threat to the environment. Under this situation, the use of wastewater to replace artificial culture medium for microalgae cultivation not only reduces the cost of microalgal biomass, but also yields profound environmental sustainability impacts.
To our knowledge, wastewater used to cultivate lipid-rich marine microalgae as animal feed additives must meet specific standards. Otherwise, serious environmental contamination and health risk may be caused (70, 71). Firstly, wastewater should contain no toxic compounds, such as heavy metals, toxic chemicals, and excessive ammonia. On one hand, toxic compounds may limit the growth of marine microalgae or even result in the failure of microalgae-based lipid production. For example, when the initial free ammonia concentration in culture medium reached 13.3 mM, the growth of marine microalga, Chlorella vulgaris, was inhibited (72). On the other hand, heavy metals are absorbed by microalgae, threatening the health of consumers through the bio-accumulation in food chain. It was reported that due to the functional groups (e.g., hydroxyl, carboxyl, methylene groups, etc.) on the surface of microalgal cells, heavy metal ions with positive changes can be adsorbed, resulting in the contamination of microalgal biomass (73). Therefore, to rule out the negative effects of toxic compounds on the utilization of microalgal biomass as animal feed additives, the safety of wastewater should be controlled strictly. Secondly, wastewater with balanced nutrient profile can be employed for marine microalgae cultivation. Since the low ratio of N/C is favorable to the accumulation of lipid in algal cells, wastewater with higher total organic carbon (TOC) concentration should be used for marine microalgae-based lipid production (74). It should also be noted that digestibility of organic carbon in wastewater should be taken into consideration. Thirdly, wastewater should be obtained at a low cost for marine microalgae cultivation, thus enhancing the market competitiveness of microalgal lipid products. For example, microalgae cultivation plant can be co-established with food processing factory, which produces a huge amount of organics-rich wastewater. Compared with ex-situ treatment, in-situ treatment of wastewater by microalgae cultivation can significantly reduce the wastewater transportation cost.
3.1.2 Wastewater pretreatment for microalgae cultivation
Up to now, food processing wastewater and agricultural wastewater with high safety level have been widely regarded as a potential artificial medium alternative for marine microalgae cultivation (75–77). To put the concept of growing marine microalgae in wastewater into practice, a couple of technical problems, such as low salinity, growth-limiting factors, nutrient deficiency, should be addressed.
Different from freshwater microalgae, marine microalgae naturally live in seawater environment with high salinity. However, most wastewater from food industry and agriculture does not contain sufficient salt for the survival of marine microalgae. For example, in the study of Wang et al., salinity of seawater and artificial culture medium reached 2.7% while that of wastewater was 0% (19). As a result, although wastewater contained more nutrients, particularly total nitrogen (TN), total phosphorus (TP), and chemical oxygen demand (COD), than artificial culture medium, biomass yield of marine diatom Phaeodactylum tricornutum grown in wastewater was only around 0.25 g/L (19). Accordingly, microalgae-based nutrient removal in wastewater was negatively impacted. In a real-world application, sea salt or seawater could be mixed with wastewater to create a comfortable environment for marine microalgae (19, 34).
In addition to the mixture with sea salt or seawater, dilution is widely applied to pretreat wastewater before the inoculation of marine microalgae. Reyimu et al. discovered that specific growth rates of Tetraselmis suecica in original wastewater and 25% wastewater were 0.1488 and 0.4778 d−1, respectively (78). Positive effect of wastewater dilution was also observed in the cultivation of Nannochloropsis oculata, of which the specific growth rate was highest in the 75% diluted wastewater (78). The main reason for this phenomenon is that dilution can reduce the concentrations of some growth-limiting factors, such as high turbidity, ammonia toxicity, high osmotic pressure, and so on. For example, high ammonia concentration (>0.725 mM) in wastewater has inhibitory effects on microalgae of Diatomophyceae (79). Compared with freshwater microalgae, marine diatoms are more sensitive to high concentration of ammonia in culture medium (79). Therefore, appropriate dilution, which could reduce the concentration of ammonia, is necessary to be applied for wastewater pretreatment.
Balancing nutrient profile is an effective way to alleviate the deficiency of nutrient in some wastewater, enhancing microalgae growth and wastewater remediation. As mentioned above, low ratio of N/C in culture medium or wastewater is favorable to the lipid accumulation in microalgal cells. Ge et al. increased the adding amount of leftover dough hydrolysates from 0 to 50.0 mg/L, resulting in a significant improvement of lipid yield from 54.6 to 2,436.0 mg/L and DHA yield from 3.8 to 341.3 mg/L (35). In this case, starch-rich leftover dough hydrolysates with very low N/C ratio modified nutrient profile of the culture medium, providing marine microalgae with more organic carbon for lipid synthesis.
3.1.3 Microalgae-based lipid production and nutrient removal
In terms of microalgae growth and lipid production, some wastewater can be much more promising nutrient source than artificial culture medium. Malibari et al. compared the growth of five marine microalgal species in artificial medium and shrimp farm wastewater, discovering that this wastewater was more favorable to the lipid accumulation in algal cells (80). In this study, total lipid contents of Chlorella sp., Dunaliella sp., Navicula sp., and Tetraselmis sp. grown in artificial medium (f/2 medium) were only 6.3, 35.3, 19.3, and 762.3 pg/cell, respectively. However, when these marine microalgae were cultivated in shrimp farm wastewater, their total lipid contents reached 968.0, 238.3, 613.0, and 4667.7 pg/cell. Compared with f/2 medium, wastewater improved the total lipid of Chlorella sp., Dunaliella sp., Navicula sp., and Tetraselmis sp. by 152.7, 5.8, 30.8, and 5.1 times, respectively.
As shown in Table 2, marine microalgae removed over 50% nitrogen and phosphorus in food processing wastewater and agricultural wastewater, demonstrating the practical feasibility of integrating microalgae cultivation with wastewater remediation. For example, Dunaliella salina even removed 72.5% COD, 84.8% nitrogen, and 80.5% phosphorus in saline food industry wastewater during 15-day period (77). In the view of the present authors, this is attributed to the high growth rate of marine microalgae and the strong biodegradability of food processing wastewater and agricultural wastewater.
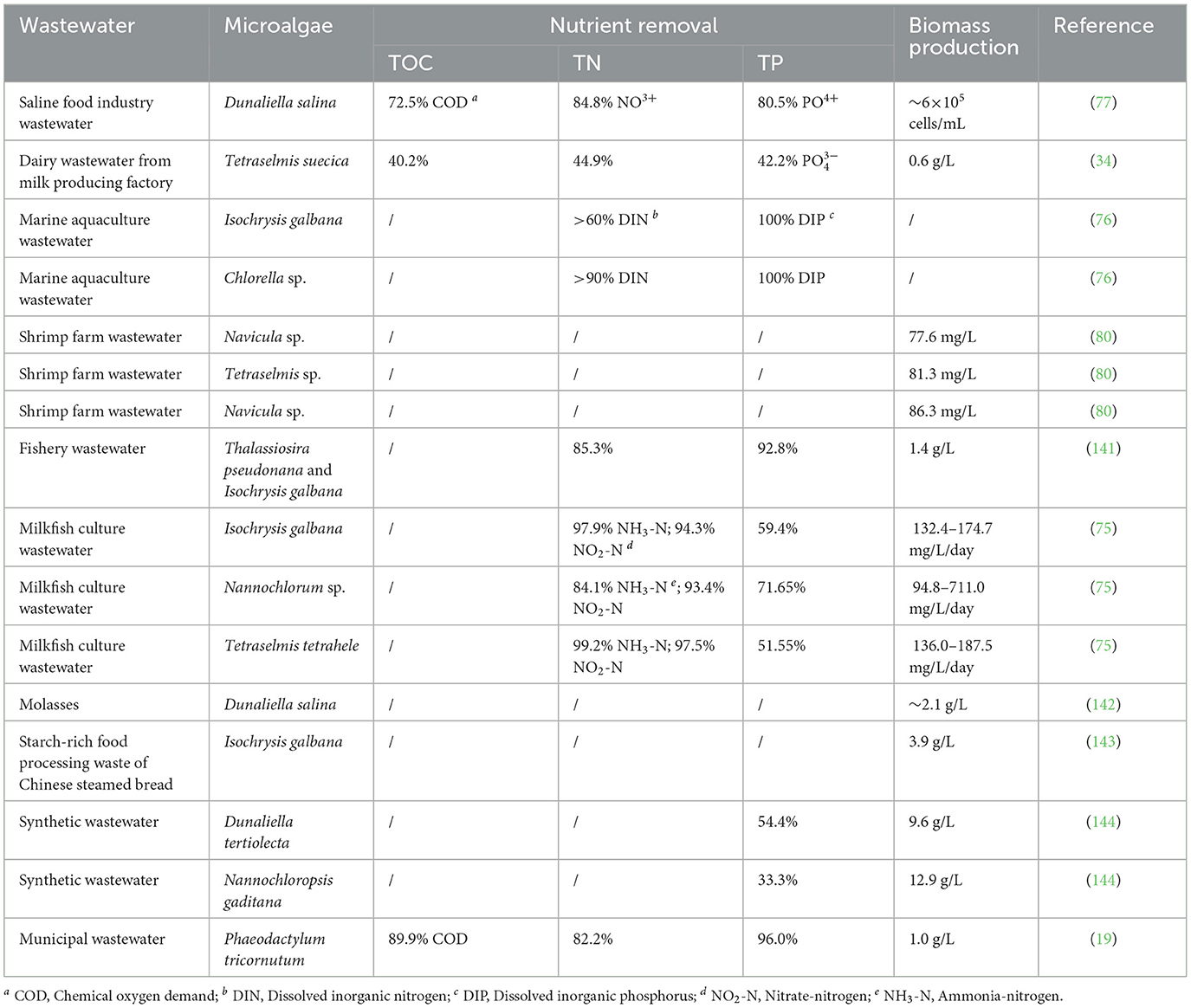
Table 2. Cultivation of marine microalgae in wastewater for nutrient removal and biomass production.
Considering the aforementioned economic and ecological benefits of wastewater-based marine microalgae growth, some nutrient-rich wastewater without toxic risks can be exploited as a cost-saving and effective alternative of artificial culture medium for marine microalgae cultivation and lipid production.
3.2 Advanced cultivation models
3.2.1 Two-stage cultivation of microalgae
Under conventional approaches, the synthesis of PUFA-rich lipids in marine microalgae is induced by applying stress conditions, such as low temperature or nitrogen deficiency, while the synthesis of carotenoid in marine microalgae is normally enhanced through high-salinity stress or intensive illumination (Table 3). However, the aforementioned environmental factors enhance the accumulation of lipid or lipid-soluble components in marine microalgae at the expense of biomass production (45). For example, low content of dissolved oxygen (DO) enhanced DHA synthesis in marine microalga, Schizochytrium limacinum. However, the cultures of Schizochytrium limacinum controlled at 50% DO saturation produced a cell density of 181 million cells/mL while the cultures with 10% DO produced only 98.4 million cells/mL (81).
In fact, the high accumulation of PUFA and other antioxidative components in marine microalgae under stress conditions can be considered a self-protection mechanism. For example, at low temperature, to prevent the solidification of membrane lipids, microalgae cells tend to synthesize PUFA to lower the solidification point of lipids, thereby enhancing their survival rate. Meanwhile, due to the negative effects of low temperature, the proliferation metabolism of microalgae cells will be constrained, leading to a decrease in biomass productivity. Due to the self-protection mechanism under environmental stress, marine microalgae achieved high accumulation of PUFA and other antioxidative components at the expense of biomass production. To attenuate the negative effects of the environmental stress on marine microalgae, a two-stage cultivation model has been developed. In the first stage, marine microalgae are cultivated in a comfortable environment without any stress conditions. During this period, although microalgae do not rapidly accumulate lipids or lipid-soluble components, their growth rate can be maintained at a relatively high level, resulting high biomass productivity and yield. In the second stage, stress conditions are applied to induce the synthesis of lipid or lipid-soluble components in marine microalgae. Microalgae growth is limited while the contents of lipid or lipid-soluble components could be significantly improved. Hence, the second stage can be regarded as a process of adding values of the microalgal lipids.
Recent studies primarily focused on exploring the technical advantages of two-stage cultivation over single-stage cultivation, optimizing novel inducing conditions for marine microalgae, and investigating the practical application of two-stage cultivation in real-world production (Table 3). Firstly, previous study compared the applications of single-stage cultivation and two-stage cultivation for lipid production, discovering that with the supplementation of selenite at a density of 20 mg/L, lipid content of Dunaliella tertiolecta in single-stage cultivation increased 2.39-fold while that in tow-stage cultivation increased 5.73-fold (82). The advantage of two-stage cultivation over single-stage cultivation was also reported in the study of growing Skeletonema costatum for PUFA production (83). In the study of Ra et al. (84), the transfer of Dunaliella salina from Stage 1 to Stage 2 resulted in a rapid increase of lipid content (40.0%). It should be noted that dry cell weights of marine microalgae in Stage 2 were not negatively impacted by the inducing conditions (84). These discoveries confirmed that two-stage cultivation model can realize the high accumulation of lipids, PUFA, and lipid-soluble components in marine microalgae without compromising the microalgae biomass production. Secondly, a couple of innovative inducing conditions have been developed to cultivate marine microalgae for lipid and lipid-soluble components production. For example, silicon deficiency was adopted to induce the lipid synthesis in Skeletonema costatum, resulting in an increase of lipid productivity by 113.7% compared with the control group without stress condition (85). Besides, supplement interval of nitrogen-repletion was adjusted to enhance lipid synthesis in Isochrysis zhangjiangensis (86). Thirdly, two-stage cultivation model has progressively transitioned from laboratory research to industrial-scale application. This novel model was implemented in the integrated marine microalgae cultivation and wastewater (food waste hydrolysate, digested poultry litter wastewater, mixed municipal wastewater, etc.) remediation (18, 19, 87). It was discovered that compared with the lipid productivity of marine microalgae in Stage 1, that of marine microalgae in Stage 2 was improved dramatically, reaching 54.8 mg/L/day (19). These achievements confirmed the practical feasibility of employing marine microalgae to produce lipid by two-stage cultivation model in the industry.
It is noteworthy that some inducing conditions are unfavorable to the cellular metabolisms of marine microalgae. It was discovered that selenite concentrations ranging from 2.5 to 20.0 mg/L caused the lipid peroxidation, reflected by the thiobarbituric acid reactive substances (TBARS) content, in Dunaliella tertiolecta during both single-stage and two-stage cultivation models, emphasizing the selenite-induced oxidative stress accompanied by the increased lipid accumulation in microalgal cells (82). Besides, excessive low temperature, which enhances the synthesis of PUFA in marine microalgae, may lead to the formation of intracellular ice crystals, thereby causing a certain degree of damage to the physical structure of marine microalgal cells (88). Therefore, in practical applications of two-stage cultivation model, efforts should focus on minimizing the negative impacts of inducing conditions on cellular metabolism and cellular structure of marine microalgae.
3.2.2 Immobilization of marine microalgae
It was estimated that microalgae harvesting process could account for 20%−30% of total cost (89). Immobilization technology has been regarded as an effective approach to simplifying microalgae harvesting process and reducing biomass production cost. Generally, innovative microalgae immobilization technologies intensively studied in recent years can be classified into three major categories, namely alginate-based immobilization, rotating algal biofilm, and filamentous fungal pelletization.
Firstly, alginate is a kind of polymer matrix, which can form beads ranging from hundreds of micrometers to millimeters in size and be cross-linked with microalgal cells for immobilization (90). In practical applications, large-sized alginate bead with unicellular marine microalgae are harvested efficiently by simple filtration. Up to now, alginate has been successfully employed for the immobilization of a variety of marine microalgae, such as Nannochloropsis sp., Phaeodactylum tricornutum, Chaetoceros gracilis, Thalassiosira weissflogii, Cylindrotheca sp., and so on (90–92). It is noteworthy that alginate-based immobilization could maintain the high growth rate of marine microalgae and improve the nutritional value of microalgal lipid. It was reported that in alginate bead with the optimized compositions, specific growth rates of Phaeodactylum tricornutum reached around 1.2 day−1 (90). It was also discovered the enhancement in DHA (0.7%), EPA (7.7%), and PUFA (11.1%) due to immobilization of marine microalgae in alginate beads (91). Promisingly, this immobilization technology has been successfully adopted for wastewater remediation, provides opportunities for coupling the immobilized production of microalgal lipids with nutrient recovery from wastewater (92). In the downstream application, sodium alginate is regarded as a feed additive (maximum concentration: 30,000 mg/kg feed) safe for consumers and environments (93).
Secondly, rotating algal biofilm refers to a new microalgae cultivation designed to make microalgae attached on the substratum, which is normally made of cotton materials (cotton duct, cotton rag, cotton denim, cotton corduroy, etc.) and lignocellulosic materials (pine sawdust, rice husk, oak sawdust, sugarcane bagasse, etc.) (94, 95). Based on the types of intercellular forces, formation of microalgal biofilm on attachment substrate is consisted of two main steps, namely initial adherence by physical adsorption and biofilm thickening by microbial extracellular polymeric substances (96, 97). In the first step, attachment of microalgae occurs when the repulsive electrostatic interaction is overcome by the attractive van der Waals and acid-base interactions (97). In the second step, microalgae and other microorganisms (bacteria, fungi, etc.) attached on the surface can secret extracellular polymeric substances (EPS), which act as “glue” to increase the intercellular adhesion and accelerate the process of biofilm thickening. With the formation of mature microalgal biofilm, a complex ecosystem with various synergistic relationships, such as pH balancing and intercellular exchange of O2 and CO2, gradually takes shape. By the end of microalgae cultivation on biofilm, biomass can be directly harvested using scrappers, while the residual microalgae on the substratum surface remain viable for continued growth, enabling continuous microalgae production. According to the survey, biomass productivity of microalgae on biofilm could reach 1.3–10.9 g/m2/day (97).
Thirdly, filamentous fungi-based pelletization is another potential method to realize the microalgae immobilization. Due to the fungal filamentous structure, which provides numerous binding site for microalgal cells, microalgae can be attached on the surface of fungal pellets. According to the immobilization process, this model can be classified into two categories, fungal pellet-assisted microalgae immobilization (FPA) and fungal spore-assisted microalgae immobilization (FSA) (98). In the model of FPA, filamentous fungi pelletize prior to their application in microalgae adsorption and immobilization. In the model of FSA, fungal spores are inoculated with microalgae together and then form spherical pellets with microalgae embedded within. Adsorption efficiency of microalgae in both FPA and PSA models could be higher than 90% (98, 99). In practical applications, by the end of microalgae cultivation, microalgal-fungal pellets ranging from millimeters to centimeters in size are harvested by filtration or sedimentation. In addition to the microalgae attachment on fungal pellets, the selection of filamentous fungi for microalgae immobilization has also become a key focus of current research. Recently, some edible filamentous fungi, such as Rhizopus oligosporus, Aspergillus oryzae, Neurospora intermedia, and Monascus purpureus, with nutritional components have been selected to form pellets. With the application of edible fungi to immobilize microalgae, the harvested biomass can be directly use as raw material for animal feed production. Particularly, in this technical route, some edible filamentous fungi not only provide attachment carrier, but also contain protein, lipid, and various natural pigments and antioxidants. For instance, Aspergillus oryzae, which has been successfully utilized for microalgae immobilization, contain kojic acid, γ-aminobutyric acid, chitin-glucan complexes, and other components favorable to the health of animals (99, 100). Therefore, employment of filamentous fungi for microalgae immobilization is considered as a novel technology with the potential to upgrade microalgae cultivation models.
3.3 Selection standards of marine microalgae species
There are countless species of marine microalgae, but only a limited number are suitable for industrial production of lipids. In the view of the present authors, to put the aforementioned technologies for microalgal lipids production into practice, microalgal species, which are crucial for lipid productivity, cost, and market value, should be selected properly. Factors that should be taken into consideration mainly include the content of target components, robustness to harsh environment, and presence of toxic components. Firstly, marine microalgal species with high contents of PUFA, astaxanthin, β-carotene or other lipid-soluble components should be screened and then applied for industrialization. Up to now, marine microalgal species, which have been widely employed for lipid production in the industry, include Dunaliella salina, Nannochloropsis sp., Schizochytrium sp., and so on. It should also be noted that in the foreseeable future, with the advancement of technologies, more and more marine microalgal species will be available for lipid production. For example, in recent years, synthetic biology technologies have been widely adopted to regulate the lipid synthetic pathways in microalgal cells, thus improving the productivity or lipid-soluble components (101). Secondly, the selected marine microalgal species should be robust to the harsh environment. Different from artificial culture medium, wastewater may have some growth-liming factors, such as low pH value, high turbidity, and unbalanced nutrient profile. Only by selecting marine microalgal species that are tolerant to harsh conditions can they be cultivated in wastewater to achieve the recovery of nutrients from the wastewater. Thirdly, marine microalgae used as animal feed ingredient should no contain any toxic components, which not only threaten the health of animals, but also accumulate in the food chain. For instance, intake of microcystins, which are commonly found in some marine algae, may result in the gastrointestinal symptoms of mammals (102). In this case, if marine microalgae containing toxins are selected as the producers of lipids for animal farming, the growth of animals may severely challenged.
4 Practical applications of microalgal lipids
Practical application of microalgal lipids in downstream industries is is a critical factor in determining both the economic viability and industrial scalability of microalgae-based oil production. Various solutions, such as biofuel production, edible oil, and animal feed additives, were proposed for the industrial application of plant-based and microalgae-based oil (103, 104). In the practice, fluctuations of global crude oil prices limits the development of microalgae-based biofuel and low consumer acceptance of marine microalgal lipids as the alternative of traditional edible oils (e.g., soybean oil, peanut oil, and palm oil) results in the low consumption of microalgal lipids in the food sector. Therefore, exploitation of microalgal lipid as animal feed additive is playing an increasingly important role in the marine microalgae industry.
4.1 Use of microalgae lipid for aquaculture
4.1.1 Inclusion of marine microalgae in aquafeed
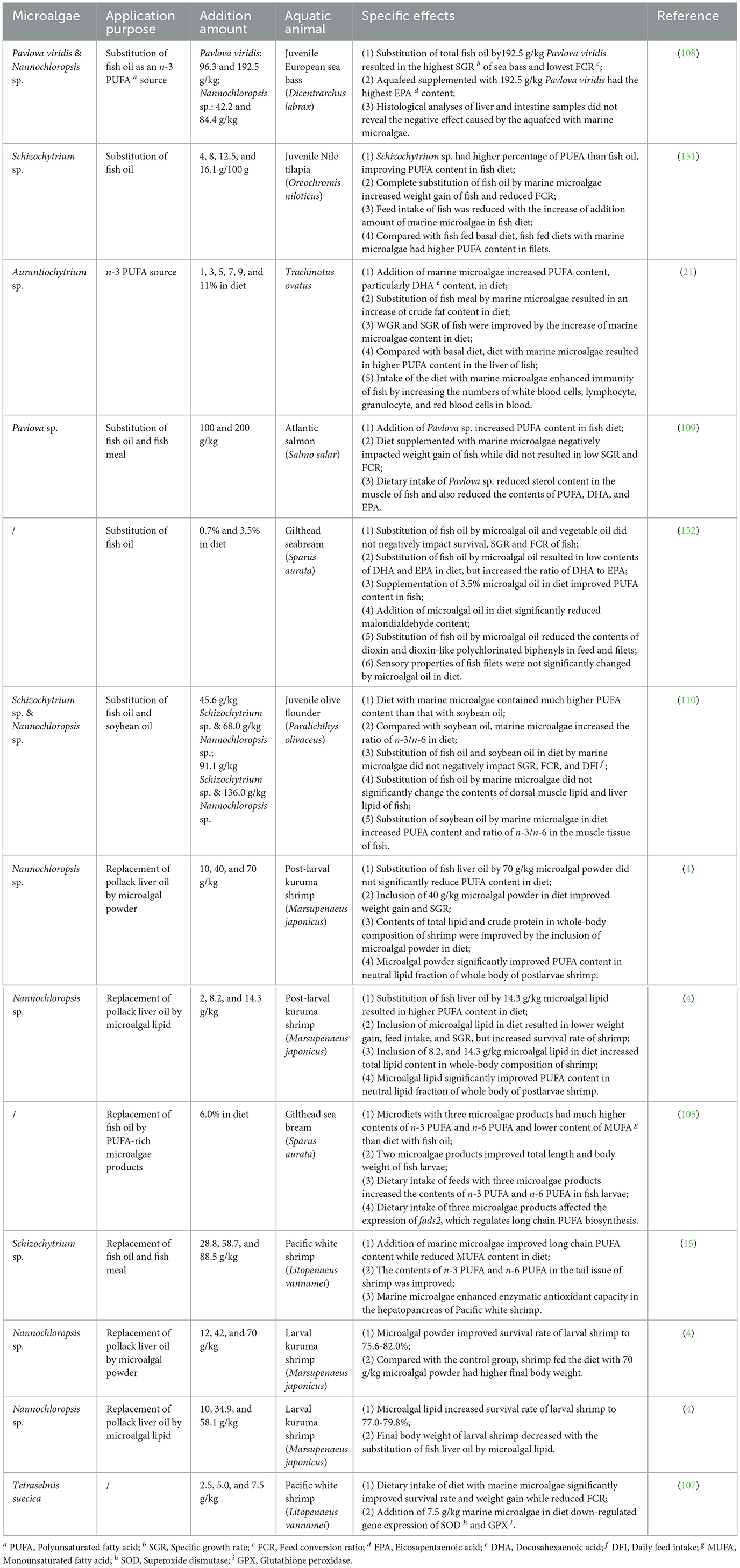
In recent years, substitution of fish oil or vegetable oil by microalgal lipid for aquafeed production is being intensively studied in both academics and industry. This phenomenon is attributed to the recession of reduction fishery, supply chain stability, high nutritional value of microalgal lipid, and land use efficiency. Firstly, fish oil is primarily derived from wild-caught fish, which has been heavily exploited and overfished in some marine regions. Particularly, overfishing will disrupt marine food webs and result in unbalanced ecological system. Secondly, fisheries harvesting is seasonal while microalgae production is less affected by seasons. Accordingly, supply chain of microalgal lipid for aquafeed use is more stable than that of fish oil. Thirdly, compared with vegetable oil, microalgal lipid contains more long-chain PUFA, which may enhance the immune response and improve the health status of aquatic animals. Fourthly, microalgae cultivation can be conducted in non-arable land for lipid production. From the perspective of agricultural sustainability, microalgal oil holds greater advantages over vegetable oil.
Marine microalgae can be added into aquafeed in the form of extracted lipid, biomass powder, or commercial bio-product to substitute fish oil or vegetable oil (Table 4). For example, lipid was extracted from Nannochloropsis sp. and then added in aquafeed to replace pollack liver oil (4). However, in the practical application, extraction process not only leads to lipid loss, but also increases the overall cost of aquafeed production. In most cases, therefore, marine microalgae are added into aquafeed in the form of biomass powder. Normally, omniferous and herbivorous fish could efficiently digest microalgae-based diet and absorb nutrients from microalgae. However, it should also be noted that cellulose, pectin, and other compounds with low digestibility in microalgae biomass may negatively impact the digestive metabolism of carnivorous fish and increase feed conversion ratio (FCR). Recently, with the commercial value of microalgae being increasingly explored, lipid-rich microalgae bio-products are being developed and utilized as an oil source in aquafeed. Three commercial bio-products, ALL G RICH, DHA GOLD, and MO060, made in USA, Switzerland, and France, respectively, were employed to completely replace sardine oil in the diet of gilthead sea bream (105).
In the view of the present authors, to select the most appropriate form of microalgal lipid for aquafeed production, a couple of critical factors, including economic viability, operational efficiency in feed manufacturing processes, and digestive adaptability, should be taken into consideration. It should also be noted that due to the diverse compounds in microalgae, the addition of biomass powder in aquafeed could not only replace fish oil or vegetable oil, but also substitute other nutrients. For example, in the experiment of culturing pacific white shrimp, supplementation of Nannochloropsis sp. meal in diet not only reduced the inclusion levels of cod liver oil and soy oil, but also lowered the contents of casein, corn starch, vitamins and so on (106).
4.1.2 Growth performance and health status of aquatic animals
The addition of microalgae-derived lipids in feed exerts complex effects on aquatic animals, but overall improves their growth performance. Many studies reported the positive effects of diet containing marine microalgal lipid on the growth rate and weight gain of aquatic animals, such as gilthead sea bream, Pacific white shrimp, and European sea bass (105, 107, 108). As shown in Table 4, not only the extracted lipid, but also biomass powder in aquafeed has been proven to be favorable to the growth of aquatic animals. In the application of microalgae-based aquafeed, inclusion level is an important factor that can determine the specific effects on growth performance of aquatic animals. In the study of culturing Atlantic salmon, compared with the control group, high inclusion levels (100 and 200 g/kg) of marine microalga, Pavlova sp., resulted in lower weight gain (109). Another negative case is that supplementing Nannochloropsis sp. powder in aquafeed resulted in diminished growth performance of postlarvae kuruma shrimps (4). When the inclusion level of biomass powder was 10 and 70 g/kg, weight gain and SGR of shrimp were reduced to 188.2%−226.0% and 2.8%−3.2%/day, respectively (4). In the view of the present authors, adverse effects of biomass powder on the growth of aquatic animals are mainly attributed to the degradation-resistant cell wall and anti-nutritional factors in microalgae. Particularly, this weakness becomes amplified during the aquaculture of carnivorous fish species, which could not efficiently digest cellulose-rich cell wall of microalgae. Therefore, to apply microalgal lipid as an alternative of fish oil or vegetable oil for aquafeed production, pretreatment must be performed to attenuate potential adverse effects of microalgal compounds on aquatic animals.
Positive effects of microalgal lipid or biomass powder on the health status of aquatic animals mainly include the enhancement of immunity systems and improvement of biochemical properties. Firstly, immunity systems of aquatic animals can be adjusted by the dietary intake of microalgal lipid or biomass powder, thus improving the stress resistance and survivability of aquatic in adverse environmental conditions. For example, white blood cells, lymphocyte, granulocyte, and red blood cells of Trachinotus ovatus fed marine microalgae-supplemented diet were improved (21). Besides, the levels of SOD, CPX, and some other antioxidant enzymes in aquatic animals can be impacted by the dietary intake of marine microalgae (107). Secondly, some biochemical parameters, which determine the health status of aquatic animals, are influenced by marine microalgae-supplemented diet. The improvement of PUFA content in fish tissue has been widely documented (Table 4). Also, significant drop of triglyceride concentration in the plasma of olive flounder was reported when soybean oil in diet was replaced by the biomass powder of Schizochytrium sp. and Nannochloropsis sp. (110). In aquaculture practice, the improvement and of biochemical parameters not only benefit the health of aquatic animals, but also enhance the nutritional value of aquatic products. Normally, fish/shrimp products with high level of PUFA are more popular among consumers (111).
4.2 Use of microalgae lipid for livestock and poultry farming
4.2.1 Inclusion of marine microalgae in animal feed
Compared with traditional lipid sources, particularly terrestrial plant oil, marine microalgal lipids contain much higher contents of long-chain PUFA (e.g., EPA, DHA, etc.), of which the bio-accumulation in meat, milk, and egg can improve the nutritional values of food products. It was reported that DHA contents in the total fatty acids of salmon oil and flaxseed oil were 3.7% and 0%, respectively, while DHA content in Schizochytrium sp. reached 29.4% (112). In addition to PUFA, a couple of lipid-soluble components with high nutritional values are included in marine microalgal lipids. For example, lipid-soluble components crucial for animal's health and metabolism, such as rosmarinic acid, cinnamic acid, gallic acid, and caffeic acid, have been identified in marine microalga, Dunaliella salina (113). For example, as a natural additive in animal feed, rosmarinic acid has shown promising results in promoting growth, productive and reproductive performance and improving anti-oxidant status and immunologic indices of livestock (114). Besides, some antioxidants, such as β-carotene, astaxanthin, zeaxanthin, and lutein, are rich in Dunaliella salina lipid (115, 116). Although genetic modification techniques have been applied to induce the synthesis of long-chain PUFA in some terrestrial plant oils, the safety of genetically-modified organism remains unclear. At present, microalgae is still a promising source with high safety level providing long-chain PUFA and other natural antioxidants to livestock and poultry.
Marine microalgae with high lipid contents were applied in a variety of forms to provide nutrition to livestock and poultry. In the experiment of employing Nannochloropsis oceanica to feed lamb, performances of spray-dried biomass, freeze-dried biomass, and microalgal oil were assessed according to the EPA protection (117). The results showed that EPA content in the rumen of lamb fed freeze-dried microalgae was about 50% higher than that of lamb fed spray-dried microalgae and microalgal oil, demonstrating freeze-dried biomass as a natural rumen-protected source of EPA to ruminants (117). It should be noted that in some cases, even when the same pretreatment method is applied to different marine microalgal species, it can result in different fatty acid utilization rates. For example, it was observed that EPA disappearance of freeze-dried Nannochloropsis oceanica and freeze-dried Phaeodactylum tricornutum reached 44% and 83%, respectively, by the end of 24-h in vitro batch incubation with strained rumen fluid of fistulated sheep (118). Therefore, to maximize the efficacy of marine microalgae in livestock and poultry farming, microalgal species and their supplementation forms in animal feed requires focused attention.
4.2.2 Growth performance of livestock and poultry
As shown in Table 5, marine microalgae have been widely adopted for livestock and poultry farming, exerting favorable effects on animal's growth performance and health status. Firstly, dietary supplementation of marine microalgal lipid or microalgal biomass powder can improve the growth rate of livestock and poultry and reduce FCR. In the study of adding DHA-rich Schizochytrium limacinum in feed for lamb feeding, the increase of total weight gain from 9.2 to 11.9 kg was observed during the 7-week experiment (119). Simultaneous increases were also observed in the following parameters, daily gain, growth rate, and feed efficiency (119). Promoting effects of lipid-rich marine microalgae on animals were also reported in the studies of supplementing Dunaliella salina for quail feeding and adding Schizochytrium sp. for broiler feeding (112, 113). Secondly, immune response of animals can be enhanced by the addition of marine microalgae in feed. The experiment, which supplemented Nannochloropsis oculata in Barki sheep's diet, revealed the up-regulated expression pattern of immune and antioxidant markers in ewes post-lambing and their newly born lambs (120). Thirdly, health status of livestock and poultry fed marine microalgae can be improved through the optimization of blood physicochemical parameters, improvement of PUFA in retinal tissues, and so on (2, 121). In the study of feeding broiler with Dunaliella salina and Spirulina sp., the contents of total cholesterol, low-density lipoprotein, and malondialdehyde in blood samples were reduced, reflecting the improvement of health status of broiler (2). The increase of EPA content was observed in retinal tissues of lamb fed Nannochloropsis oceanica (121).
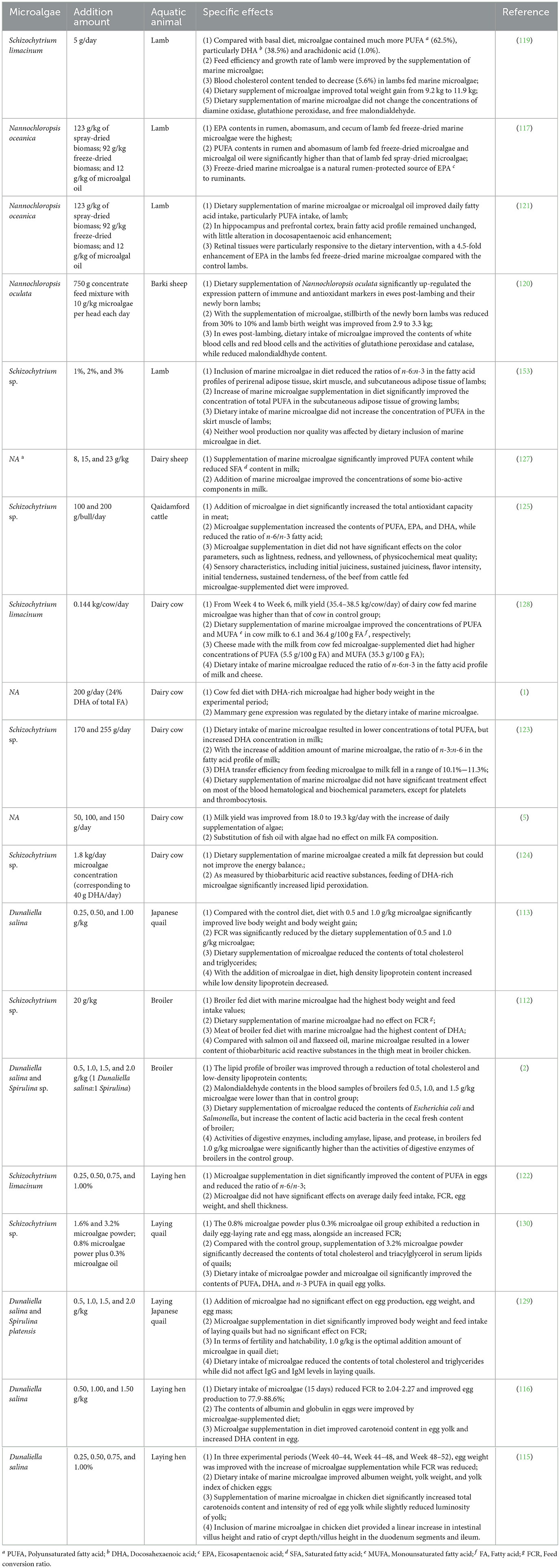
Table 5. Application of marine microalgal lipid as feed ingredient for livestock and poultry farming.
Although dietary intake of marine microalgae or microalgal lipid could improve growth performance and health status of livestock and poultry, some exceptional cases should not be neglected. For example, in the study of adding DHA-rich Schizochytrium limacinum for laying hen feeding, significant effects of microalgae supplementation on FCR and daily feed intake were not observed (122). Additionally, in the experiment of using Schizochytrium sp., for cow feeding, significant treatment effect on most of the blood hematological and biochemical parameters were not reported (123). In practical applications, if marine microalgae or microalgal lipids are added without producing any significant effects on animal's growth and health, this would increase animal farming costs and complicate feed production processes.
It should also be noted that dietary intake of marine microalgae even have negative effects on the growth or health status of animals sometimes. For example, it was reported that lipid peroxidation in the plasma of cow fed PUFA-rich Schizochytrium sp. was more serious than that of cow fed basal diet. This phenomenon is probably attributed to the high content of PUFA with the dietary intake of marine microalgae (124). Therefore, the comprehensive impacts of incorporating marine microalgae or microalgal lipids into animal feed should undergo thorough evaluation.
4.2.3 Quality of meat, dairy products and poultry eggs
Due to the enrichment of unsaturated fatty acids and lipid-soluble components in marine microalgal lipids, dietary intake of marine microalgae can change the physicochemical and sensory properties of meat. Previous study reported the improvement of initial juiciness, sustained juiciness, flavor intensity, initial tenderness, and sustained tenderness of the beef from cattle fed Schizochytrium-supplemented diet (125). In addition, when the supplementation of marine microalgae in animal feed increased from 0 to 200 g/bull/day, the contents of PUFA, including DHA, EPA, and α-Linolenic acid (ALA), in fresh meat were significantly improved (125). Carotenoids from microalgae added into feed could also accumulate in the meat of animals (126). Compared with conventional meat products, those enriched with high-value components or possessing superior sensory characteristics demonstrate significantly higher consumer preference in the market.
PUFA and lipid-soluble components not only exert influence on meat quality, but also transfer from marine microalgae to milk through animal's metabolisms. Compared with the milk (5.5 g/100 g fatty acid methyl esters and 4.1) from ewes fed basal diet, that from ewes fed diet with 23 g/kg marine microalgae contained significantly higher PUFA content (8.9 g/100 g fatty acid methyl esters and n-6:n-3 ratio of 4.2) and lower ratio of n-6:n-3 fatty acid (2.1) (127). In the processing of using Schizochytrium-based diet to feed dairy cows, 10.1%−11.3% DHA in microalgae was transferred into the milk (123). In addition to milk, dairy products made with milk can be impacted by the animal feed supplemented with marine microalgae or microalgal lipids. It was reported that PUFA content and ratio of n-6:n-3 fatty acid in the cheese made with milk from diary cow fed DHA-rich Schizochytrium limacinum were 5.5 g/100g of total fatty acid and 6.9, respectively (128). By contrast, in the the cheese made with milk in control group, PUFA content was only 3.9 g/100g of total fatty acid and ratio of n-6:n-3 fatty acid reached as high as 8.1 (128).
In the studies of supplementing marine microalgae for poultry farming, the improvements of laying rate and poultry eggs' quality were widely reported (Table 5). Fertility and hatchability of laying quails fed diet with 1 g/kg microalgae reached 96.7% and 73.3%, respectively, which were much higher than those in control group (129). In addition, dietary supplementation of Dunaliella salina increased albumen weight, yolk weight, and yolk index of chicken eggs (115). Intensity of red of egg yolk was also improved, showing the influence of natural pigments in marine microalgae on the egg quality (115). The increases of carotenoid content and DHA content were observed in the eggs of laying hens fed Dunaliella salina-supplemented diet (116). Enrichment of PUFA in egg yolks was also observed in another study which supplemented Schizochytrium sp. and its lipid in diet for laying quails feeding (130).
Dietary intake of dairy products and eggs with high nutritional values mentioned above will finally impact the health of consumers. For example, in the context of carotenoids' unique antioxidative properties, dietary carotenoid intake has beneficial effect on the treatment of some widespread modern civilization diseases, such as cardiovascular, cancer, or photosensitivity disorders (131). In addition, the importance of long-chain PUFA intake has been intensively reported in patients with major depressive and bipolar disorders, amyotrophic lateral sclerosis, Parkinson's disease, and Alzheimer's disease (132). Therefore, supplementation of microalgal lipid in animal feed can indirectly exert a positive impact on consumer health by increasing the antioxidant content in dairy, meat, and egg products.
5 Challenges and prospects
5.1 Challenges
Groundbreaking advancements in three pivotal domains, fundamental research on marine microalgal lipid metabolism, applied research on lipid-rich marine microalgae cultivation, and industrialization research on lipid utilization in aquaculture and livestock/poultry farming, have paved the way for the comprehensive utilization of marine microalgal lipid. However, there are still some challenges that merit the attentions of researchers and policy-makers. Due to the existing challenges, substitution of fish oil or vegetable oil by microalgal lipid as an ingredient of animal feed remains far from industrial-scale application. Therefore, researchers should abandon overly optimistic attitudes and address existing technical challenges, further advancing the application of microalgal lipids in the feed industry through innovation.
5.1.1 Safety risks of marine microalgal lipid from wastewater
As discussed above, wastewater, particularly food processing wastewater and agricultural wastewater, can be exploited as an alternative of artificial culture medium to cultivate marine microalgae for lipid production. However, potential safety risks of marine microalgal lipid produced in wastewater limit the use of lipid in downstream industry. Firstly, due to the presence of various negatively charged functional groups on microalgal cell surfaces, marine microalgae exhibit a strong capacity for metal ion adsorption and enrichment (133, 134). For example, in the culture medium with 4 and 8 mg/dm3 chromium and 5 and 10 mg/dm3 plumbum, three marine microalgae, Amphora coffaeiformis, Navicula salinicola, and Dunaliella salina, isolated from Tunisian coasts absorbed around 90% chromium and Dunaliella salina absorbed over 90% plumbum (135). Although food processing wastewater and agricultural wastewater might contain trace amounts of heavy metals, their bio-accumulation by marine microalgae can still adversely affect the safety of microalgal lipid products, ultimately compromising consumer health through the food chain (136, 137). Secondly, rapid proliferation of airborne pathogenic microorganisms in the nutrient-rich wastewater during marine microalgae cultivation is also one of the primary limiting factors constraining the utilization of wastewater as a culture medium alternative. Therefore, enhancing the safety of marine microalgae biomass harvested from wastewater will play a pivotal role in enabling the widespread application of this novel model.
5.1.2 Poor digestibility and palatability of marine microalgae
The cell walls of most marine microalgae contain various components that are difficult to digest, which results in poor digestibility and absorption of marine microalgae products by carnivorous fish. In aquaculture practice, to attenuate the negative effects of microalgae-supplemented diets on aquatic animals' digestive metabolism, inclusion ratios of marine microalgae biomass in aquafeed were controlled at a low level. In some publications, inclusion ratios of marine microalgae biomass in aquafeed for Trachinotus ovatus, Paralichthys olivaceus, and Marsupenaeus japonicus were lower than 10% (4, 21, 110). By contract, excessive addition of marine microalgae biomass in aquafeed not only negatively affects the digestion of aquatic animals, but also results in the nutrient wastage in marine microalgae.
Some marine microalgae have off-flavors that compromise feed palatability, thereby diminishing the feed intake of animals, particularly the animals which do not feed on marine microalgae in the natural environment. It was discovered that the excessive supplementation of microalgae biomass in aquafeed resulted in the decrease of feed intake of Micropterus salmoides (138). In addition to the natural off-flavors, deterioration of marine microalgae during the transportation, processing, and storage could also result in the poor palatability of microalgae-supplemented feed. For example, in the process aquafeed extrusion, high temperature and pressure may accelerate the peroxidation of PUFA in microalgal lipid and intensify the rancid odor of lipid (139).
5.1.3 Uncertainty of applying new models for marine microalgae cultivation
Rotating algal biofilm and filamentous fungal pelletization mentioned above have been widely adopted to immobilize freshwater microalgae, however, their applications in the immobilization and cultivation of marine microalgae remains highly uncertain.
Performance of marine microalgae and bacteria/fungi in EPS secretion, which determines the formation of biofilm, under high-salinity conditions has not been fully studied. As an extreme environmental condition, high salinity may negatively impact microbial survival and metabolism, thereby suppressing the formation of microalgal biofilms. In addition, high salinity exhibits significant corrosiveness, imposing stringent requirements on mechanical strength and corrosion resistance of the attachment substratum. Consequently, traditional rotating algal biofilm employed for freshwater microalgae immobilization may require structural and material modifications to adapt them for the immobilized cultivation of marine microalgae. Up to now, few studies have evaluated the performance of rotating algal biofilm in the immobilized cultivation of marine microalgae.
Most filamentous fungi were employed to immobilize freshwater microalgae through pelletization. Only few studies have evaluate the feasibility of using fungal pellets for marine microalgae immobilization. For example, co-agitation of Aspergillus oryzae with marine Tetraselmis subcordiformis resulted in the immobilization of 99.5% marine microalgae through adsorption onto mycelial surface (100). However, performance of immobilized marine microalgae on fungal pellets in biomass production and lipid accumulation has been rarely studied. Compared with freshwater microalgae, marine microalgae have totally different culturing conditions. High salinity may negatively impact the metabolisms of filamentous fungi or the surface charge of fungal pellets. Therefore, effects of environmental conditions (e.g., high salinity, low temperature, etc.) that promote marine microalgae growth and lipid accumulation on the metabolic activities of filamentous fungi and the adsorption characteristics of fungal pellets require further investigation.
5.2 Prospects
Based on the discussion of this review, marine microalgae lipid holds significant application potential in fields such as carbon capture, feed nutrition, and animal health from the perspective of industrial chain development. Firstly, this review highlights the application prospects of marine microalgal lipid synthesis in the field of carbon emission reduction at an industrial scale, demonstrating its significant importance for achieving carbon neutrality goals. This aspect has often been overlooked. Secondly, this review comprehensively discusses the production conditions of marine microalgal lipids and their applications in the animal feed industry, providing readers with a more intuitive understanding from an industrial chain perspective. In contrast, previous papers have typically focused on only one aspect, seldom analyzing and discussing the topic from a full industrial chain perspective. Thirdly, this review explores the impact of marine microalgal lipid on animal health, which holds significance for achieving sustainable development in aquaculture and livestock farming industries. In summary, by examining the production and utilization of marine microalgal lipid from a full industrial chain perspective, this review presents the tremendous potential of the marine microalgae-based lipid industry to readers.
Some suggestions to solve the aforementioned challenges, promoting the industrial application of marine microalgal lipid in aquaculture and livestock/poultry farming are listed as follows. Firstly, government, which recognizes the dual economic and ecological benefits of using wastewater for marine microalgae cultivation and microalgal lipid production, should develop comprehensive regulatory frameworks governing wastewater selection, pretreatment protocols, and lipid quality control. For example, effluents with high contents of contaminants, such as heavy metals and antibiotic residues, should not be permitted for marine microalgae cultivation, thereby ensuring bio-safety compliance and sustainable lipid production. Secondly, fermentation or enzymatic hydrolysis technology can be adopted to improve digestibility and palatability of marine microalgae-supplemented feeds. Specific enzymes, such as chitinase, lysozyme, pectinase, and sulfatase, have been employed to digest microalgae biomass and degrade algal cell wall (140). In the industrial application, as the cell walls of marine microalgae break down or are disrupted, the components within the cells become more easily absorbed and utilized by animals. It should be noted that the cost of fermentation or enzymatic hydrolysis should be controlled by technological innovation. Otherwise, market acceptance of fermented or hydrolyzed marine microalgae product is low. Thirdly, intensive studies should be conducted to evaluate the survival of filamentous fungi in in marine microalgal cultivation conditions and analyze the relation between filamentous fungi and immobilized marine microalgae in lipid synthesis. Besides, measures can be taken to screen filamentous fungi with high resilience to high-salinity and low-temperature conditions. For example, marine filamentous fungi obtained from polar marine environment can be collected to support the industrial-scale immobilization of marine microalgae for lipid production.
6 Conclusion
Marine microalgae have emerged as a transformative solution for sustainable lipid production, offering a viable alternative to traditional lipid sources, such as soybean, peanut, and reduction fishery. This review underscores the unique advantages of marine microalgae-based lipid production in carbon sequestration toward the goal of carbon neutrality. Biosynthesis of lipid, PUFA, and lipid-soluble components in marine microalgae is intricately regulated by environmental factors, such as nutrient availability, temperature, light conditions, and so on, which can be strategically manipulated to improve the nutritional values of microalgal lipids. Technological advancements, including two-stage cultivation models, wastewater-based microalgae cultivation, and immobilization techniques, demonstrate significant potential to reduce production costs and improve scalability of marine microalgae-based lipid production.
The practical applications of marine microalgal lipids in aquaculture and livestock/poultry farming highlight their role in improving growth performance, immune response, and nutritional value of animal-derived products. However, challenges persist, particularly regarding potential safety risks of microalgal biomass, low digestibility due to resilient cell walls, and uncertainty of applying new technologies for marine microalgae production. Addressing these issues requires robust regulatory frameworks for wastewater selection, technological advancements in enzymatic or fermentation-based pretreatments of biomass, and further research into marine-specific immobilization technologies.
Looking ahead, marine microalgae-based lipid production hold immense promise in contributing to global food security, carbon sequestration, and the transition toward a bio-based circular economy. By overcoming existing technical and socioeconomic barriers, marine microalgae can play a pivotal role in achieving the goals of sustainable development, fostering a resilient agricultural system which harmonizes environmental protection with economic development.
Author contributions
LY: Writing – original draft. BD: Writing – original draft. QL: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Basic Research Program Natural Science Foundation of Jiangsu Province (BK20230665).
Conflict of interest
BD was employed by Zhenjiang Fengzhou Zhiyu Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vahmani P, Glover KE, Fredeen AH. Effects of pasture versus confinement and marine oil supplementation on the expression of genes involved in lipid metabolism in mammary, liver, and adipose tissues of lactating dairy cows. J Dairy Sci. (2014) 97:4174–83. doi: 10.3168/jds.2013-7290
2. Alghamdi MA, Elbaz MI, Ismail IE, Reda FM, Alagawany M, El-Tarabily KA, et al. Dietary supplementation with a mixture of Dunaliella salina and Spirulina enhances broiler performance by improving growth, immunity, digestive enzymes and gut microbiota. Poult Sci. (2024) 103:103337. doi: 10.1016/j.psj.2023.103337
3. Noreen S, Tufail T, Ul Ain HB, Awuchi CG. Pharmacological, nutraceutical, and nutritional properties of flaxseed (Linum usitatissimum): An insight into its functionality and disease mitigation. Food Sci Nutr. (2023) 11:6820–9. doi: 10.1002/fsn3.3662
4. Adissin TOO, Manabu I, Shunsuke K, Saichiro Y, Moss AS, Dossou S. Effects of dietary Nannochloropsis sp. powder and lipids on the growth performance and fatty acid composition of larval and postlarval kuruma shrimp, Marsupenaeus japonicus Aquaculture. Nutrition. (2019) 26:186–200. doi: 10.1111/anu.12980
5. AbuGhazaleh AA, Potu RB, Ibrahim S. Short communication: The effect of substituting fish oil in dairy cow diets with docosahexaenoic acid-micro algae on milk composition and fatty acids profile. J Dairy Sci. (2009) 92:6156–9. doi: 10.3168/jds.2009-2400
6. Adu-Frimpong M, Omari-Siaw E, Mukhtar YM, Xu X, Yu J. Formulation of pomegranate seed oil: a promising approach of improving stability and health-promoting properties. Eur J Lipid Sci Technol. (2018) 120:1800177. doi: 10.1002/ejlt.201800177
7. Xu B, Han J, Zhou S, Wu Q, Ding F. Quality characteristics of wheat germ oil obtained by innovative subcritical butane experimental equipment. J Food Process Eng. (2015) 39:79–87. doi: 10.1111/jfpe.12201
8. Bilal M, Shabbir MA, Xiaobo Z, Arslan M, Usman M, Azam M, et al. Characterization of peanut seed oil of selected varieties and its application in the cereal-based product. J Food Sci Technol. (2020) 57:4044–53. doi: 10.1007/s13197-020-04437-y
9. He W-S, Zhao L, Sui J, Li X, Huang S, Ding H, et al. Enzymatic synthesis of a novel antioxidant Octacosanol lipoate and its antioxidant potency in sunflower oil. J Agric Food Chem. (2024) 72:21781–93. doi: 10.1021/acs.jafc.4c07240
10. Yao-Say Solomon Adade S, Lin H, Jiang H, Haruna SA, Osei Barimah A, Zareef M, et al. Fraud detection in crude palm oil using SERS combined with chemometrics. Food Chem. (2022) 388:132973. doi: 10.1016/j.foodchem.2022.132973
11. Payanthoth NS, Mut NNN, Samanta P, Li G, Jung J. A review of biodegradation and formation of biodegradable microplastics in soil and freshwater environments. Appl Biol Chem. (2024) 67:110. doi: 10.1186/s13765-024-00959-7
12. Lakhiar IA, Yan H, Zhang J, Wang G, Deng S, Bao R, et al. Plastic pollution in agriculture as a threat to food security, the ecosystem, and the environment: an overview. Agronomy. (2024) 14:548. doi: 10.3390/agronomy14030548
13. Knápek J, Králík T, Vávrová K, Valentová M, Horák M, Outrata D. Policy implications of competition between conventional and energy crops. Renew Sustain Energy Rev. (2021) 151:111618. doi: 10.1016/j.rser.2021.111618
14. Darko RO, Yuan S, Hong L, Liu J, Yan H. Irrigation, a productive tool for food security – a review. Acta Agric Scandin. (2015) 66:191–206. doi: 10.1080/09064710.2015.1093654
15. Kumar V, Habte-Tsion H, Allen KM, Bowman BA, Thompson KR, El-Haroun E, et al. Replacement of fish oil with Schizochytriummeal and its impacts on the growth and lipid metabolism of Pacific white shrimp (Litopenaeus vannamei). Aquac Nutr. (2018) 24:1769–81. doi: 10.1111/anu.12816
16. Garcia-Perez P, Cassani L, Garcia-Oliveira P, Xiao J, Simal-Gandara J, Prieto MA, et al. Algal nutraceuticals: a perspective on metabolic diversity, current food applications, and prospects in the field of metabolomics. Food Chem. (2023) 409:135295. doi: 10.1016/j.foodchem.2022.135295
17. Surendhiran D, Li C, Cui H, Lin L. Marine algae as efficacious bioresources housing antimicrobial compounds for preserving foods - a review. Int J Food Microbiol. (2021) 358:109416. doi: 10.1016/j.ijfoodmicro.2021.109416
18. Wang X, Zhang M-M, Liu S-F, Xu R-L, Mou J-H, Qin Z-H, et al. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Conv Manag. (2021) 234:113908. doi: 10.1016/j.enconman.2021.113908
19. Wang X-W, Huang L, Ji P-Y, Chen C-P, Li X-S, Gao Y-H, et al. Using a mixture of wastewater and seawater as the growth medium for wastewater treatment and lipid production by the marine diatom Phaeodactylum tricornutum. Bioresour Technol. (2019) 289:121681. doi: 10.1016/j.biortech.2019.121681
20. Sun W-X, Zhang R-J, Fan J, He Y, Mao X-H. Comprehensive transformative profiling of nutritional and functional constituents during germination of soybean sprouts. J Food Measur Character. (2018) 12:1295–302. doi: 10.1007/s11694-018-9743-2
21. Li S, Wang B, Liu L, Song Y, Lv C, Zhu X, et al. Enhanced growth performance physiological and biochemical indexes of Trachinotus ovatus fed with marine microalgae Aurantiochytrium sp. rich in n-3 polyunsaturated fatty acids. Front Mar Sci. (2021) 7. doi: 10.3389/fmars.2020.609837
22. Silva A, Cassani L, Grosso C, Garcia-Oliveira P, Morais SL, Echave J, et al. Recent advances in biological properties of brown algae-derived compounds for nutraceutical applications. Crit Rev Food Sci Nutr. (2022) 64:1283–311. doi: 10.1080/10408398.2022.2115004
23. Kang W, Lin H, Jiang H, Yao-Say Solomon Adade S, Xue Z, Chen Q. Advanced applications of chemo-responsive dyes based odor imaging technology for fast sensing food quality and safety: a review. Compr Rev Food Sci Food Safety. (2021) 20:5145–72. doi: 10.1111/1541-4337.12823
24. Osae R, Essilfie G, Alolga RN, Akaba S, Song X, Owusu-Ansah P, et al. Application of non-thermal pretreatment techniques on agricultural products prior to drying: a review. J Sci Food Agric. (2020) 100:2585–99. doi: 10.1002/jsfa.10284
25. Chamorro F, Carpena M, Fraga-Corral M, Echave J, Riaz Rajoka MS, Barba FJ, et al. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: a circular economy model. Food Chem. (2022) 370:131315. doi: 10.1016/j.foodchem.2021.131315
26. Greene C, Scott-Buechler C, Hausner A, Johnson Z, Lei XG, Huntley M. Transforming the future of marine aquaculture: a circular economy approach. Oceanography. (2022) 35:26–34. doi: 10.5670/oceanog.2022.213
27. Lu Q, Ma C, Guo L, Lu Y, Li H. Co-fermentation of chlorella vulgaris with oleaginous yeast in starch processing effluent as a carbon-reducing strategy for wastewater treatment and biofuel feedstock production. Fermentation. (2023) 9:476. doi: 10.3390/fermentation9050476
28. Nazir MJ Li G, Nazir MM, Zulfiqar F, Siddique KHM, Iqbal B, et al. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. (2024) 237:105959. doi: 10.1016/j.still.2023.105959
29. Liu X, Chen Y, Liu Y, Wang S, Jin J, Zhao Y, et al. A framework combining century modeling and chronosequences sampling to estimate soil organic carbon stock in an agricultural region with large land use change. Agronomy. (2023) 13:1055. doi: 10.3390/agronomy13041055
30. Mielke T. World markets for vegetable oils and animal fats. In: Biokerosene. Springer (2017). p. 147–188. doi: 10.1007/978-3-662-53065-8_8
31. Goold H, Beisson F, Peltier G, Li-Beisson Y. Microalgal lipid droplets: composition, diversity, biogenesis and functions. Plant Cell Rep. (2014) 34:545–55. doi: 10.1007/s00299-014-1711-7
32. Kong F, Romero IT, Warakanont J, Li-Beisson Y. Lipid catabolism in microalgae. New Phytol. (2018) 218:1340–8. doi: 10.1111/nph.15047
33. Schwenk D, Seppälä J, Spilling K, Virkki A, Tamminen T, Oksman-Caldentey K-M, et al. Lipid content in 19 brackish and marine microalgae: influence of growth phase, salinity and temperature. Aquat Ecol. (2013) 47:415–24. doi: 10.1007/s10452-013-9454-z
34. Daneshvar E, Zarrinmehr MJ, Hashtjin AM, Farhadian O, Bhatnagar A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour Technol. (2018) 268:523–30. doi: 10.1016/j.biortech.2018.08.032
35. Ge F, Song K, Yang Z, Li J, Yan F, Zhang M, et al. Enhancing docosahexaenoic acid production of isochrysis galbana from starch-rich food processing byproducts. Fermentation. (2023) 9:158. doi: 10.3390/fermentation9020158
36. Song L, Wen S, Ye Q, Lou H, Gao Y, Bajpai VK, et al. Advances on delta 5-unsaturated-polymethylene-interrupted fatty acids: Resources, biosynthesis, and benefits. Crit Rev Food Sci Nutr. (2021) 63:767–89. doi: 10.1080/10408398.2021.1953960
37. Ma X-N, Chen T-P, Yang B, Liu J, Chen F. Lipid production from Nannochloropsis. Mar Drugs. (2016) 14:61. doi: 10.3390/md14040061
38. Monroig Ó, Shu-Chien AC, Kabeya N, Tocher DR, Castro LFC. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog Lipid Res. (2022) 86:101157. doi: 10.1016/j.plipres.2022.101157
39. Zhou C, Pan W, Peng Q, Chen Y, Zhou T, Wu C, et al. Characteristics of metabolites by seed-specific inhibition of FAD2 in Brassica napus L. J Agric Food Chem. (2021) 69:5452–62. doi: 10.1021/acs.jafc.0c06867
40. Tian L, Chi G, Lin S, Ling X, He N. Marine microorganisms: natural factories for polyunsaturated fatty acid production. Blue Biotechnol. (2024) 1:15. doi: 10.1186/s44315-024-00012-8
41. Remize M, Brunel Y, Silva JL, Berthon J-Y, Filaire E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar Drugs. (2021) 19:113. doi: 10.3390/md19020113
42. Liu B, Benning C. Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol. (2013) 24:300–9. doi: 10.1016/j.copbio.2012.08.008
43. Shao W, Ebaid R, El-Sheekh M, Abomohra A, Eladel H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas y Aceites. (2019) 70:e292. doi: 10.3989/gya.0690181
44. Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. (2014) 160:393–400. doi: 10.1016/j.foodchem.2014.03.087
45. Lu Q, Lu Y. Microalga- and yeast-based astaxanthin production via nutrient recovery from wastewater for aquaculture practice: an emerging technology for sustainable development. J Chem Technol Biotechnol. (2022) 97:3035–48. doi: 10.1002/jctb.7164
46. Gowd V, Xiao J, Wang M, Chen F, Cheng K. Multi-mechanistic antidiabetic potential of astaxanthin: an update on preclinical and clinical evidence. Molec Nutr Food Res. (2021) 65:2100252. doi: 10.1002/mnfr.202100252
47. Zhu Y, Gu Z, Liao Y, Li S, Xue Y, Firempong MA, et al. Improved intestinal absorption and oral bioavailability of astaxanthin using poly (ethylene glycol)-graft-chitosan nanoparticles: preparation, in vitro evaluation, and pharmacokinetics in rats. J Sci Food Agric. (2021) 102:1002–11. doi: 10.1002/jsfa.11435
48. Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH. Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol. (2014) 27:1425–31. doi: 10.1007/s10811-014-0470-8
49. Silaban A, Bai R, Gutierrez-Wing MT, Negulescu II, Rusch KA. Effect of organic carbon, < scp>C < /scp>: < scp>N < /scp> ratio and light on the growth and lipid productivity of microalgae/cyanobacteria coculture. Eng Life Sci. (2013) 14:47–56. doi: 10.1002/elsc.201200219
50. Huang B, Marchand J, Thiriet-Rupert S, Carrier G, Saint-Jean B, Lukomska E, et al. Betaine lipid and neutral lipid production under nitrogen or phosphorus limitation in the marine microalga Tisochrysis lutea (Haptophyta). Algal Res. (2019) 40:101506. doi: 10.1016/j.algal.2019.101506
51. Zienkiewicz K, Du Z-Y, Ma W, Vollheyde K, Benning C. Stress-induced neutral lipid biosynthesis in microalgae — Molecular, cellular and physiological insights. Biochimica et Biophysica Acta. (2016) 1861:1269–81. doi: 10.1016/j.bbalip.2016.02.008
52. Huang B, Mimouni V, Lukomska E, Morant-Manceau A, Bougaran G. Carbon partitioning and lipid remodeling during phosphorus and nitrogen starvation in the marine microalga Diacronema lutheri (Haptophyta). J Phycol. (2020) 56:908–22. doi: 10.1111/jpy.12995
53. Ginzberg MB, Kafri R, Kirschner M. On being the right (cell) size. Science. (2015) 348:6236. doi: 10.1126/science.1245075
54. Gao Y, Yang M, Wang C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol. (2013) 147:484–91. doi: 10.1016/j.biortech.2013.08.066
55. Jiang Y, Yoshida T, Quigg A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol Biochem. (2012) 54:70–7. doi: 10.1016/j.plaphy.2012.02.012
56. Chaisutyakorn P, Praiboon J, Kaewsuralikhit C. The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J Appl Phycol. (2017) 30:37–45. doi: 10.1007/s10811-017-1186-3
57. Svenning JB, Dalheim L, Eilertsen HC, Vasskog T. Temperature dependent growth rate, lipid content and fatty acid composition of the marine cold-water diatom Porosira glacialis. Algal Res. (2019) 37:11–6. doi: 10.1016/j.algal.2018.10.009
58. Aussant J, Guihéneuf F, Stengel DB. Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl Microbiol Biotechnol. (2018) 102:5279–97. doi: 10.1007/s00253-018-9001-x
59. Pasquet V, Ulmann L, Mimouni V, Guihéneuf F, Jacquette B, Morant-Manceau A, et al. Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J Appl Phycol. (2014) 26:2265–71. doi: 10.1007/s10811-014-0252-3
60. Lu Q, Li H, Xiao Y, Liu H. A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res. (2021) 55:102281. doi: 10.1016/j.algal.2021.102281
61. Gao B, Hong J, Chen J, Zhang H, Hu R, Zhang C. The growth, lipid accumulation and adaptation mechanism in response to variation of temperature and nitrogen supply in psychrotrophic filamentous microalga Xanthonema hormidioides (Xanthophyceae). Biotechnol Biofuels Bioprod. (2023) 16:12. doi: 10.1186/s13068-022-02249-0
62. Goold HD, Cuiné S, Légeret B, Liang Y, Brugière S, Auroy P, et al. Saturating light induces sustained accumulation of oil in plastidal lipid droplets in Chlamydomonas reinhardtii. Plant Physiol. (2016) 171:2406–17. doi: 10.1104/pp.16.00718
63. Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol. (2014) 162:38–44. doi: 10.1016/j.biortech.2014.03.113
64. Ra CH, Sirisuk P, Jung J-H, Jeong G-T, Kim S-K. Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess Biosyst Eng. (2017) 41:457–65. doi: 10.1007/s00449-017-1880-1
65. Jayakumar S, Bhuyar P, Pugazhendhi A, Rahim MHA, Maniam GP, Govindan N. Effects of light intensity and nutrients on the lipid content of marine microalga (diatom) Amphiprora sp. for promising biodiesel production. Sci Total Environ. (2021) 768:145471. doi: 10.1016/j.scitotenv.2021.145471
66. Gim GH Ryu J, Kim MJ, Kim PI, Kim SW. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J Industr Microbiol Biotechnol. (2016) 43:605–16. doi: 10.1007/s10295-016-1741-y
67. Wahidin S, Idris A, Shaleh SRM. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol. (2013) 129:7–11. doi: 10.1016/j.biortech.2012.11.032
68. Shah SMU, Che Radziah C, Ibrahim S, Latiff F, Othman MF, Abdullah MA. Effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri. Ann Microbiol. (2013) 64:157–64. doi: 10.1007/s13213-013-0645-6
69. Lim K, Zaleha K. Effect of photoperiod on the cellular fatty acid composition of three tropical marine microalgae. Malaysian J Appl Biol. (2013) 42:41–9.
70. Han Z, Wang N, Zhang H, Yang X. Heavy metal contamination and risk assessment of human exposure near an e-waste processing site. Acta Agric Scandin. (2016) 67:119–25. doi: 10.1080/09064710.2016.1229016
71. Chen A, Liang H, Chen T, Yang W, Ding C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J Soils Sediments. (2015) 16:1352–9. doi: 10.1007/s11368-015-1161-z
72. Goto M, Nagao N, Yusoff FM, Kamarudin MS, Katayama T, Kurosawa N, et al. High ammonia tolerance on growth rate of marine microalga Chlorella vulgaris. J Environ Biol. (2018) 39:843–848. doi: 10.22438/jeb/39/5(SI)/4
73. Faruque MO, Uddin S, Hossain MM, Hossain SMZ, Shafiquzzaman M, Razzak SA. A comprehensive review on microalgae-driven heavy metals removal from industrial wastewater using living and nonliving microalgae. J Hazardous Mater Adv. (2024) 16:100492. doi: 10.1016/j.hazadv.2024.100492
74. Lu Q, Liu H, Sun Y, Li H. Combined zeolite-based ammonia slow-release and algae-yeast consortia to treat piggery wastewater: Improved nitrogen and carbon migration. Bioresour Technol. (2023) 387:129671. doi: 10.1016/j.biortech.2023.129671
75. Piñosa LAG, Apines-Amar MJS. Optimization of stocking density for Isochrysis galbana, Nannochlorum sp., and Tetraselmis tetrahele in the bioremediation of aquaculture wastewater. Aquac Int. (2023) 32:3597–3616. doi: 10.1007/s10499-023-01340-z
76. Qian Z, Na L, Bao-Long W, Tao Z, Peng-Fei M, Wei-Xiao Z, et al. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J Environ Manage. (2022) 320:115673. doi: 10.1016/j.jenvman.2022.115673
77. Balaji E, Swaminathan D, Deepa VS. Assessment of nutrient removal efficiency of marine microalgae Dunaliella salina in saline food industry wastewater. J Environ Nanotechnol. (2024) 13:46–51. doi: 10.13074/jent.2024.06.242598
78. Reyimu Z, ‘̀Ozçimen D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J Clean Prod. (2017) 150:40–6. doi: 10.1016/j.jclepro.2017.02.189
79. Collos Y, Harrison PJ. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull. (2014) 80:8–23. doi: 10.1016/j.marpolbul.2014.01.006
80. Malibari R, Sayegh F, Elazzazy AM, Baeshen MN, Dourou M, Aggelis G. Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea – Jeddah. J Clean Prod. (2018) 198:160–9. doi: 10.1016/j.jclepro.2018.07.037
81. Chi Z, Liu Y, Frear C, Chen S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl Microbiol Biotechnol. (2009) 81:1141–8. doi: 10.1007/s00253-008-1740-7
82. Pere{^c}inec MG, Babić S, {^C}i{^z}mek L, Selmani A, Popović NT, Sikirić MD, et al. Selenite as a lipid inductor in marine microalga Dunaliella tertiolecta: comparison of one-stage and two-stage cultivation strategies. Appl Biochem Biotechnol. (2021) 194:930–49. doi: 10.1007/s12010-021-03659-w
83. Wu M, Gao G, Jian Y, Xu J. High CO 2 increases lipid and polyunsaturated fatty acid productivity of the marine diatom Skeletonema costatum in a two-stage model. J Appl Phycol. (2022) 34:43–50. doi: 10.1007/s10811-021-02619-5
84. Ra CH, Kang C-H, Kim NK, Lee C-G, Kim S-K. Cultivation of four microalgae for biomass and oil production using a two-stage culture strategy with salt stress. Renew Energy. (2015) 80:117–22. doi: 10.1016/j.renene.2015.02.002
85. Gao G, Wu M, Fu Q, Li X, Xu J, A. two-stage model with nitrogen and silicon limitation enhances lipid productivity and biodiesel features of the marine bloom-forming diatom Skeletonema costatum. Bioresour Technol. (2019) 289:121717. doi: 10.1016/j.biortech.2019.121717
86. Feng D, Chen Z, Xue S, Zhang W. Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol. (2011) 102:6710–6. doi: 10.1016/j.biortech.2011.04.006
87. Han T, Lu H, Zhao Y, Xu H, Zhang Y, Li B. Two-step strategy for obtaining dunaliella sp. biomass and β-carotene from anaerobically digested poultry litter wastewater. Int Biodeterior Biodegrad. (2019) 143:104714. doi: 10.1016/j.ibiod.2019.06.002
88. Boroda AV, Aizdaicher NA, Odintsova NA. The influence of ultra-low temperatures on marine microalgal cells. J Appl Phycol. (2013) 26:387–97. doi: 10.1007/s10811-013-0093-5
89. Barros AI, Gonçalves AL, Simões M, Pires JCM. Harvesting techniques applied to microalgae: a review. Renew Sustain Energy Rev. (2015) 41:1489–500. doi: 10.1016/j.rser.2014.09.037
90. Moreira SM, Moreira-Santos M, Guilhermino L, Ribeiro R. Immobilization of the marine microalga Phaeodactylum tricornutum in alginate for in situ experiments: bead stability and suitability. Enzyme Microb Technol. (2006) 38:135–41. doi: 10.1016/j.enzmictec.2005.05.005
91. Tyagi R, Singh PK, Saxena A, Parikh H, Bhattacharjya R, Kaushik N, et al. Evaluating the nutraceutical and antioxidative potential of alginate beads entrapped marine diatoms. South African J Botany. (2025) 180:644–52. doi: 10.1016/j.sajb.2025.03.013
92. Soo C-L, Chen C-A, Bojo O, Hii Y-S. Feasibility of marine microalgae immobilization in alginate bead for marine water treatment: bead stability, cell growth, and ammonia removal. Int J Polym Sci. (2017) 2017:1–7. doi: 10.1155/2017/6951212
93. EFSA EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V, Azimonti G, Bastos MD, Christensen H, Dusemund B, et al. Safety and efficacy of a feed additive consisting of sodium alginate for all animal species (ALGAIA). EFSA J. (2022) 20:e07164. doi: 10.2903/j.efsa.2022.7164
94. Zhang Q, Liu C, Li Y, Yu Z, Chen Z, Ye T, et al. Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol Biofuels. (2017) 10:1–16. doi: 10.1186/s13068-017-0799-8
95. Gross M, Henry W, Michael C, Wen Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol. (2013) 150:195–201. doi: 10.1016/j.biortech.2013.10.016
96. Lu Q, Xiao Y, Lu Y. Employment of algae-based biological soil crust to control desertification for the sustainable development: a mini-review. Algal Res. (2022) 65:102747. doi: 10.1016/j.algal.2022.102747
97. Hu Y, Xiao Y, Liao K, Leng Y, Lu Q. Development of microalgal biofilm for wastewater remediation: from mechanism to practical application. J Chem Technol Biotechnol. (2021) 96:2993–3008. doi: 10.1002/jctb.6850
98. Chen J, Leng L, Ye C, Lu Q, Addy M, Wang J, et al. A comparative study between fungal pellet- and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour Technol. (2018) 259:181–90. doi: 10.1016/j.biortech.2018.03.040
99. Li Z, Xie D, Chi C, Zhou Y, Zheng M, Wang M, et al. Efficient strategy by a newly isolated aspergillus oryzae to harvest chlorella vulgaris MBFJNU-1 from original swine wastewater. ACS Sustain Chem Eng. (2022) 10:5599–610. doi: 10.1021/acssuschemeng.2c00325
100. Tölle M, Kuenz A. Optimizing separation: Utilizing Aspergillus oryzae for bioflocculation of marine Tetraselmis subcordiformis on agricultural residues. J Appl Phycol. (2024) 37:233–45. doi: 10.1007/s10811-024-03400-0
101. Rock A, Novoveská L, Green D. Synthetic biology is essential to unlock commercial biofuel production through hyper lipid-producing microalgae: a review. Appl Phycol. (2021) 2:41–59. doi: 10.1080/26388081.2021.1886872
102. McLellan NL, Manderville RA. Toxic mechanisms of microcystins in mammals. Toxicol Res. (2017) 6:391–405. doi: 10.1039/C7TX00043J
103. Pan S, Zabed HM, Wei Y, Qi X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: current status, challenges and future outlook. Ind Crops Prod. (2022) 188:115684. doi: 10.1016/j.indcrop.2022.115684
104. Lu Q, Xiao Y. From manure to high-value fertilizer: the employment of microalgae as a nutrient carrier for sustainable agriculture. Algal Res. (2022) 67:102855. doi: 10.1016/j.algal.2022.102855
105. Carvalho M, Marotta B, Xu H, Geraert P-A, Kaushik S, Montero D, et al. Complete replacement of fish oil by three microalgal products rich in n-3 long-chain polyunsaturated fatty acids in early weaning microdiets for gilthead sea bream (Sparus aurata). Aquaculture. (2022) 558:738354. doi: 10.1016/j.aquaculture.2022.738354
106. Guimarães AM, Guertler C, do Vale Pereira G, da Rosa Coelho J, Costa Rezende P, Nóbrega RO, et al. Nannochloropsis spp. as feed additive for the Pacific white shrimp: effect on midgut microbiology, thermal shock resistance and immunology. Animals. 11:150. doi: 10.3390/ani11010150
107. Sharawy ZZ, Ashour M, Abbas E, Ashry O, Helal M, Nazmi H, et al. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacificwhite shrimp Litopenaeus vannamei. Aquac Res. (2020) 51:2216–28. doi: 10.1111/are.14566
108. Haas S, Bauer JL, Adakli A, Meyer S, Lippemeier S, Schwarz K, et al. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J Appl Phycol. (2015) 28:1011–21. doi: 10.1007/s10811-015-0622-5
109. Wei M, Parrish CC, Guerra NI, Tibbetts SM, Colombo SM. Dietary inclusion of a marine microalgae meal for Atlantic salmon (Salmo salar): impact of Pavlova sp. 459 on growth performance and tissue lipid composition. Aquaculture. (2022) 553:738084. doi: 10.1016/j.aquaculture.2022.738084
110. Qiao H, Wang H, Song Z, Ma J, Li B, Liu X, et al. Effects of dietary fish oil replacement by microalgae raw materials on growth performance, body composition and fatty acid profile of juvenile olive flounder, Paralichthys olivaceus. Aquac Nutr. (2014) 20:646–53. doi: 10.1111/anu.12127
111. Fomena Temgoua NS, Sun Z, Okoye CO, Pan H. Fatty acid profile, physicochemical composition, and sensory properties of atlantic salmon fish (Salmo salar) during different culinary treatments. J Food Qual. (2022) 2022:1–16. doi: 10.1155/2022/7425142
112. Jeon J-J, Kim H-J, Kang H-K, Kim C-H, Kim H-S, Hong E-C, et al. Effects of dietary thraustochytrid Schizochytrium sp. and other omega-3 sources on growth performance, carcass characteristics, and meat quality of broilers. Animals. (2022) 12:1166. doi: 10.3390/ani12091166
113. Alagawany M, Lestingi A, Abdelzaher HA, Elnesr SS, Madkour M, El-Baz FK, et al. Dietary supplementation with Dunaliella salina microalga promotes quail growth by altering lipid profile and immunity. Poult Sci. (2024) 103:103591. doi: 10.1016/j.psj.2024.103591
114. Alagawany M, Abd El-Hack ME, Farag MR, Gopi M, Karthik K, Malik YS, et al. Rosmarinic acid: modes of action, medicinal values and health benefits. Animal Health Res Rev. (2017) 18:167–76. doi: 10.1017/S1466252317000081
115. Fernandes RTV, Gonçalves AA, de Arruda AMV. Production, egg quality, and intestinal morphometry of laying hens fed marine microalga. Rev Brasileira Zootecnia. (2020) 49:e20200011. doi: 10.37496/rbz4920200011
116. Madkour M, Ali SI, Alagawany M, El-Kholy MS, El-Baz FK, Alqhtani AH, et al. Dietary Dunaliella salina microalgae enriches eggs with carotenoids and long-chain omega-3 fatty acids, enhancing the antioxidant and immune responses in heat-stressed laying hens. Front Veter Sci. (2025) 12:1545433. doi: 10.3389/fvets.2025.1545433
117. Vítor ACM, Francisco AE, Silva J, Pinho M, Huws SA, Santos-Silva J, et al. Freeze-dried Nannochloropsis oceanica biomass protects eicosapentaenoic acid (EPA) from metabolization in the rumen of lambs. Sci Rep. (2021) 11:21878. doi: 10.1038/s41598-021-01255-w
118. Alves SP, Mendon{̧c}a SH, Silva JL, Bessa RJB. Nannochloropsis oceanica, a novel natural source of rumen-protected eicosapentaenoic acid (EPA) for ruminants. Sci Rep. (2018) 8:10269. doi: 10.1038/s41598-018-28576-7
119. Sucu E, Udum D, G'́uneş N, Canbolat ‘̀O, Filya İ. Influence of supplementing diet with microalgae (Schizochytrium limacinum) on growth and metabolism in lambs during the summer. Turkish J Veter Animal Sci. (2017) 41:167–74. doi: 10.3906/vet-1606-65
120. El-Sayed A, Ebissy E, Ateya A. Positive impacts of Nannochloropsis oculata supplementation on gene expression of immune and antioxidant markers and metabolic profile of Barki sheep in the transition period and lipogenic effects on progeny. Vet Res Commun. (2024) 48:2207–26. doi: 10.1007/s11259-024-10392-2
121. Vítor ACM, Correia JJ, Alves SP, Bessa RJB. Enrichment of brain n-3 docosapentaenoic acid (DPA) and Retinal n-3 Eicosapentaenoic acid (EPA) in lambs fed Nannochloropsis oceanica microalga. Animals. (2023) 13:828. doi: 10.3390/ani13050828
122. Kiran I, Umbreen H, Nisa MU, Al-Asmari F, Zongo E. Supplementation of laying hens' feed with Schizochytrium powder and its effect on physical and chemical properties of eggs. BMC Veter Res. (2024) 20:1–10. doi: 10.1186/s12917-024-04424-x
123. Liu G, Yu X, Li S, Shao W, Zhang N. Effects of dietary microalgae (Schizochytrium spp.) supplement on milk performance, blood parameters, and milk fatty acid composition in dairy cows. Czech J Animal Sci. (2020) 65:162–171. doi: 10.17221/19/2020-CJAS
124. Wullepit N, Hostens M, Ginneberge C, Fievez V, Opsomer G, Fremaut D, et al. Influence of a marine algae supplementation on the oxidative status of plasma in dairy cows during the periparturient period. Prev Vet Med. (2012) 103:298–303. doi: 10.1016/j.prevetmed.2011.09.007
125. Xu C, Zhang S, Sun B, Xie P, Liu X, Chang L, et al. Dietary supplementation with microalgae (Schizochytrium sp) improves the antioxidant status, fatty acids profiles and volatile compounds of beef. Animals. (2021) 11:3517. doi: 10.3390/ani11123517
126. Martins CF, Ribeiro DM, Costa M, Coelho D, Alfaia CM, Lordelo M, et al. Using microalgae as a sustainable feed resource to enhance quality and nutritional value of pork and poultry meat. Foods. (2021) 10:2933. doi: 10.3390/foods10122933
127. Toral PG, Hervás G, Gómez-Cortés P, Frutos P, Juárez M, de La Fuente MA. Milk fatty acid profile and dairy sheep performance in response to diet supplementation with sunflower oil plus incremental levels of marine algae. J Dairy Sci. (2010) 93:1655–1667. doi: 10.3168/jds.2009-2769
128. Gagliostro GA, Antonacci LE, Pérez CD, Rossetti L, Carabajal A. Improving the quality of milk fatty acid in dairy cows supplemented with soybean oil and DHA-micro algae in a confined production system. Agric Sci. (2018) 09:1115–30. doi: 10.4236/as.2018.99078
129. Abd El-Hack ME, Majrashi KA, Fakiha KG, Roshdy M, Kamal M, Saleh RM, et al. Effects of varying dietary microalgae levels on performance, egg quality, fertility, and blood biochemical parameters of laying Japanese quails (Coturnix coturnix Japonica). Poult Sci. (2024) 103:103454. doi: 10.1016/j.psj.2024.103454
130. Chang Y, Xuan Y, Zhang R, Ding X, Zeng Q, Wang J, et al. Effects of dietary schizochytrium algae as ω-3 PUFA source on the egg-laying quail performance, serum indexes, and egg yolk fatty acids contents. Animals. (2024) 15:21. doi: 10.3390/ani15010021
131. Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. (2014) 6:466–88. doi: 10.3390/nu6020466
132. Zárate R, el Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. (2017) 6:1–19. doi: 10.1186/s40169-017-0153-6
133. Li H, Chen S, Liao K, Lu Q, Zhou W. Microalgae biotechnology as a promising pathway to ecofriendly aquaculture: a state-of-the-art review. J Chem Technol Biotechnol. (2020) 96:837–52. doi: 10.1002/jctb.6624
134. Dineshkumar R, Sowndariya M, Kalaiselvi S, Israth Rehana G, Durai Murugan M, Abraham M, et al. Effective removal of lead (Pb) by natural biosorbent marine microalgae (Dunaliella salina) through batch experiment. Biomass Conv Biorefin. (2022) 14:1847–52. doi: 10.1007/s13399-021-02260-9
135. Elleuch J, Hmani R, Drira M, Michaud P, Fendri I, Abdelkafi S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci Total Environ. (2021) 799:149464. doi: 10.1016/j.scitotenv.2021.149464
136. Wang H, Wang C, Ge B, Zhang X, Zhou C, Yan X, et al. Effect of heavy metals in aquaculture water on the growth of microalgae and their migration mechanism in algae-shellfish system. Chem Eng J. (2023) 473:145274. doi: 10.1016/j.cej.2023.145274
137. Anandkumar A, Li J, Prabakaran K, Xi Jia Z, Leng Z, Nagarajan R, et al. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J Food Compos Anal. (2020) 88:103449. doi: 10.1016/j.jfca.2020.103449
138. Deng Y, Chen F, Liao K, Xiao Y, Chen S, Lu Q, et al. Microalgae for nutrient recycling from food waste to aquaculture as feed substitute: a promising pathway to eco-friendly development. J Chem Technol Biotechnol. (2021) 96:2496–508. doi: 10.1002/jctb.6786
139. Yang P, Wang H, Zhu M, Ma Y. Evaluation of extrusion temperatures, pelleting parameters, and vitamin forms on vitamin stability in feed. Animals. (2020) 10:894. doi: 10.3390/ani10050894
140. Gerken HG, Donohoe B, Knoshaug EP. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta. (2012) 237:239–53. doi: 10.1007/s00425-012-1765-0
141. Wang H, Qi M, Bo Y, Zhou C, Yan X, Wang G, et al. Treatment of fishery wastewater by co-culture of Thalassiosira pseudonana with Isochrysis galbana and evaluation of their active components. Algal Res. (2021) 60:102498. doi: 10.1016/j.algal.2021.102498
142. Gastelum-Franco JJ, Esparza-Leal HM, García-Ulloa M, López-Álvarez ES, Muy-Rangel MD, Pérez-Rubio V, et al. Preliminary evaluation of the green microalga Dunaliella salina as a potential feedstock for biodiesel: effect of molasses on growth and lipid profile. Lat Am J Aquat Res. (2021) 49:763–72. doi: 10.3856/vol49-issue5-fulltext-2755
143. Zheng H, Ge F, Song K, Yang Z, Li J, Yan F, et al. Docosahexaenoic acid production of the marine microalga Isochrysis galbana cultivated on renewable substrates from food processing waste under CO2 enrichment. Sci Total Environ. (2022) 848:157654. doi: 10.1016/j.scitotenv.2022.157654
144. Aravantinou AF, Theodorakopoulos MA, Manariotis ID. Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol. (2013) 147:130–4. doi: 10.1016/j.biortech.2013.08.024
145. Swain P, Tiwari A, Pandey A. Enhanced lipid production in Tetraselmis sp. by two stage process optimization using simulated dairy wastewater as feedstock. Biomass Bioenergy. (2020) 139:105643. doi: 10.1016/j.biombioe.2020.105643
146. Sui Y, Muys M, Van de Waal DB, D'Adamo S, Vermeir P, Fernandes TV, et al. Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour Technol. (2019) 287:121398. doi: 10.1016/j.biortech.2019.121398
147. Chen H-H, Xue L-L, Liang M-H, Jiang J-G. Sodium azide intervention, salinity stress and two-step cultivation of Dunaliella tertiolecta for lipid accumulation. Enzyme Microb Technol. (2019) 127:1–5. doi: 10.1016/j.enzmictec.2019.04.008
148. Lu X, Sun H, Zhao W, Cheng K-W, Chen F, Liu B, et al. Hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar Drugs. (2018) 16:219. doi: 10.3390/md16070219
149. Li Q, You J, Qiao T, Zhong D, Yu X. Sodium chloride stimulates the biomass and astaxanthin production by Haematococcus pluvialis via a two-stage cultivation strategy. Bioresour Technol. (2022) 344:126214. doi: 10.1016/j.biortech.2021.126214
150. Ali HE, El-Fayoumy EA, Rasmy WE, Soliman RM, Abdullah MA. Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J Appl Phycol. (2020) 33:227–239. doi: 10.1007/s10811-020-02308-9
151. Sarker PK, Kapuscinski AR, Lanois AJ, Livesey ED, Bernhard KP, Coley ML. Towards sustainable aquafeeds: complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile nile tilapia (Oreochromis niloticus). PLoS ONE. (2016) 11:e0156684. doi: 10.1371/journal.pone.0156684
152. Santigosa E, Brambilla F, Milanese L. Microalgae oil as an effective alternative source of EPA and DHA for gilthead seabream (Sparus aurata) aquaculture. Animals. (2021) 11:971. doi: 10.3390/ani11040971
Keywords: microalgae, unsaturated fatty acids, cultivation, animal feed, lipid
Citation: Yang L, Duan B and Lu Q (2025) A review of marine microalgae-based lipids production: biosynthesis, technological advancements, and practical applications. Front. Nutr. 12:1664674. doi: 10.3389/fnut.2025.1664674
Received: 12 July 2025; Accepted: 17 October 2025;
Published: 11 November 2025.
Edited by:
Yulia Smyatskaya, Peter the Great St.Petersburg Polytechnic University, RussiaCopyright © 2025 Yang, Duan and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Lu, bHVxaWFuQGp1c3QuZWR1LmNu
 Limin Yang
Limin Yang Bomingxin Duan2
Bomingxin Duan2 Qian Lu
Qian Lu