- 1Department of Community Nutrition, Faculty of Human Ecology, IPB University, Bogor, Indonesia
- 2Food Technology Program, Sahid University, Jakarta Selatan, Indonesia
- 3State Islamic University of Sunan Kalijaga (UIN Sunan Kalijaga), Yogyakarta, Indonesia
- 4Master of Basic Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
- 5Medical Research Center of Indonesia, Surabaya, Indonesia
Vitamin D deficiency is a global health concern, and fortifying widely consumed staples offers a scalable solution. Granulated sugar, accessible across socio-economic groups, shows promise as a novel vehicle for cholecalciferol delivery. Evidence indicates that vitamin D3 stability can be maintained through encapsulation and protective packaging, with over 90% potency retained under proper storage. Fortified sugar demonstrates good bioavailability, achieving absorption comparable to dairy products, and could increase serum 25(OH)D by 10–20 nmol/L at the population level. While offering low-cost impact, fortification requires careful dosing, quality control, and public education to mitigate risks of hypervitaminosis and prevent misperceptions that sugar itself is a health food.
1 Introduction
Food fortification is a widely recognized public health strategy aimed at enriching staple foods with essential nutrients to address widespread nutrient deficiencies (1–3). It offers a cost-efficient solution to target populations with poor adherence to supplementation. Given its broad consumption across all age and income groups, granulated sugar has been proposed as a potential vehicle for vitamin D fortification (4–7). Vitamin D plays a vital role in maintaining bone integrity and supporting immune function, yet its deficiency remains prevalent in many populations worldwide (8, 9). Adequate vitamin D levels help suppress excessive inflammation, promote regulatory T-cell development, and reduce the risk of autoimmune diseases such as multiple sclerosis, type 1 diabetes, and rheumatoid arthritis (10, 11). Current recommendations suggest maintaining serum 25-hydroxyvitamin D levels of at least 30 ng/mL (75 nmol/L), ideally 40–60 ng/mL (100–150 nmol/L), through diet, supplementation, and sunlight exposure to optimize bone and immune health (8, 12). Although clinical studies have not yet provided direct evidence on the health effects of vitamin D-fortified sugar, findings from other fortified food products indicate that, if properly implemented, sugar fortification may offer similar benefits (13–15). Before such an intervention can be effectively implemented, two key factors must be carefully evaluated: (1) the chemical stability of vitamin D when incorporated into sugar during storage and food processing, and (2) the biological and public health implications of consuming vitamin D-fortified sugar, including the nutrient's bioavailability and any potential health benefits or risks. This report explores these critical considerations using current scientific data and evidence.
2 Vitamin D stability in sugar
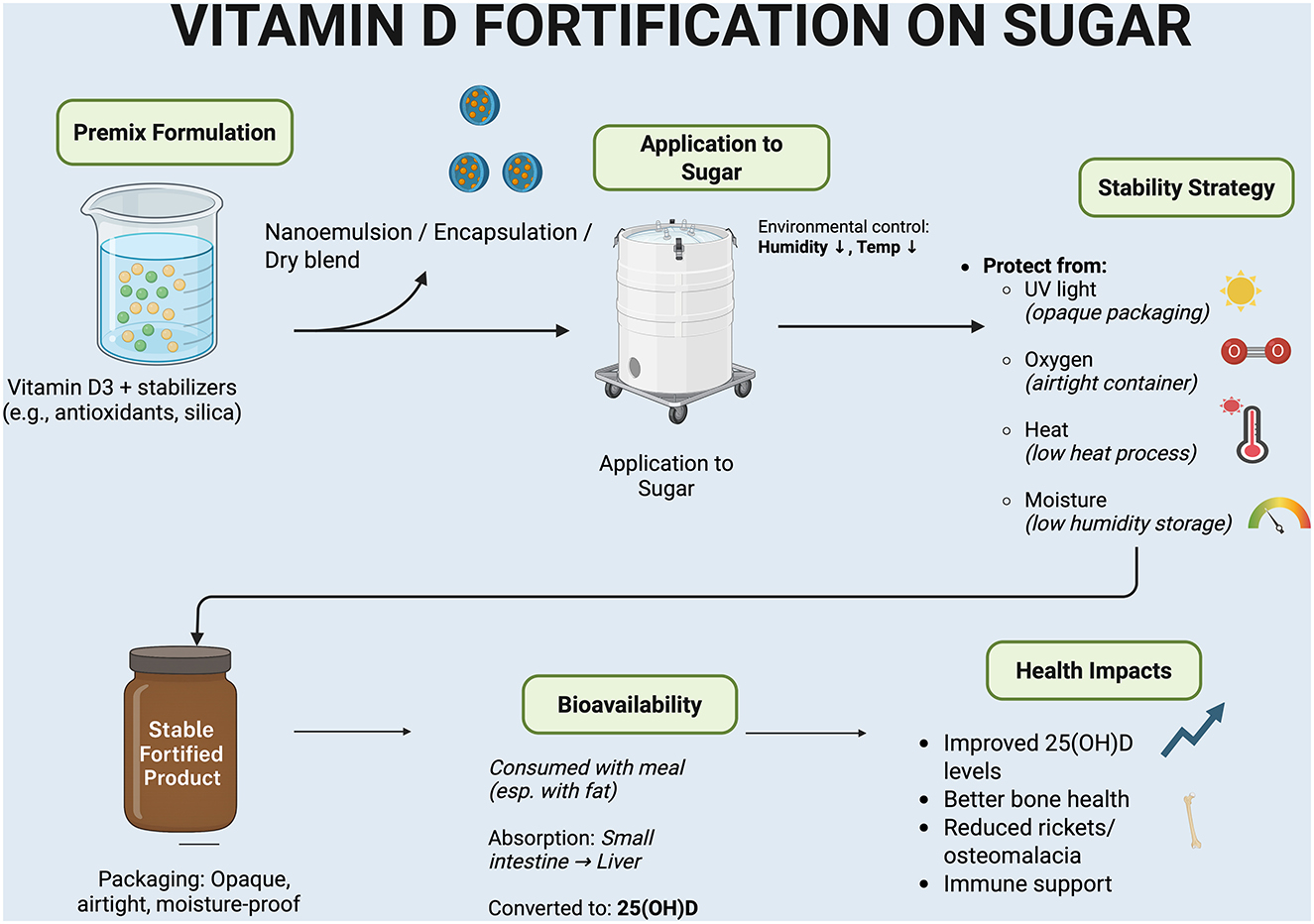
From a chemical standpoint, vitamin D exhibits relative stability under controlled conditions; however, it is susceptible to degradation when exposed to certain environmental stressors. Among the various forms of vitamin D (cholecalciferol, calcifediol, and calcitriol), only cholecalciferol is recommended for use in fortification and supplementation due to its superior stability, potency, and resistance to degradation during cooking and baking compared to others (16). Granulated sugar, due to its chemically inert nature, does not directly interact with vitamin D. As a result, the stability of vitamin D in fortified sugar largely depends on how the fortification is carried out and the conditions under which the product is stored. Scientific studies have identified several environmental factors that can reduce the integrity of vitamin D, including ultraviolet (UV) light exposure, air oxidation, elevated temperatures, and high humidity levels (17). Moreover, acidic conditions may induce structural changes in the vitamin molecule, potentially affecting its efficacy. The following section outlines the primary factors influencing the stability of vitamin D in sugar and other dry food matrices.
2.1 Ultraviolet light (UV)
Ultraviolet rays can damage vitamin D through photodegradation (photo-oxidation) to suprasterol I, superasterol II, and 5,6-transvitamin D3 with a quantum yield of 0.42 ± 0.1 (18). When vitamin D-fortified sugar is stored in clear or translucent containers exposed to sunlight or intense artificial light, the vitamin content can deteriorate significantly (19). This vulnerability has been observed in studies on vitamin D-fortified dairy products, where exposure to light caused a notable reduction in vitamin D levels. As a preventive measure, the use of opaque or UV-blocking packaging is strongly recommended for fortified food products (20). The same principle applies to vitamin D-enriched sugar. To preserve the vitamin's stability, it should be stored in sealed and dark containers that minimize light exposure during transportation and storage.
2.2 Oxygen and air exposure
Exposure to oxygen accelerates oxidative degradation of vitamin D, which is especially vulnerable in dry sugar lacking a lipid matrix (21). Airtight packaging and antioxidant incorporation can help protect stability and extend shelf life during storage and distribution (22, 23).
2.3 High temperature
Vitamin D is relatively heat-stable up to ~200 °C, but prolonged or high-temperature exposure accelerates degradation through isomerization (19). In fortified bread, 170 °C baking retains ~85% of vitamin D, while 200 °C reduces retention to 40–65% (21). For sugar fortification, low-temperature mixing preserves stability, though cooking or caramelization can cause partial losses. In the context of sugar, fortification is usually done through mixing at low temperatures; however, if fortified sugar is used in cooking (e.g., for caramelization or baking), a portion of vitamin D may be lost due to high heat.
2.4 Moisture
Moisture strongly influences vitamin D stability in dry food systems. Granulated sugar's low water activity helps limit oxygen exposure and preserve vitamin D, but high humidity causes clumping and accelerates degradation. Data from fortified wheat flour show that vitamin D3's half-life is about 173 days under dry conditions (ap 0.33, 25 °C), falling to 116 days in high humidity (ap 0.93) and just 63 days at 45 °C (21). These findings underscore the importance of maintaining low humidity and cool storage to prolong vitamin D stability in fortified sugar (21, 24).
2.5 Acidic conditions
Although sugar is chemically neutral (pH ~7), vitamin D is sensitive to acidic environments, where it can undergo acid-catalyzed isomerization into biologically inactive forms. This effect is illustrated in vitamin D2-fortified rye bread, which, due to its lower pH, retained only ~73% of its vitamin D after baking compared to ~85% in less acidic wheat bread. The loss is attributed to the conversion of vitamin D2 into isomers like isotachysterol (21). While pure sugar does not pose an acid risk, interactions with acidic components such as fruit juices or sour food matrices could subtly reduce vitamin D's stability during use.
The mechanism of Vitamin D degradation have been identified in some pathways, which are photo-oxidation, free radical oxidation, and acid-induced isomerization (21, 25). Photo-oxidation is triggered when vitamin D absorbs light energy, leading to the formation of oxidized products. Free radical oxidation typically involves oxygen and trace metals, which break down the vitamin's side chain (21, 26). Acid-catalyzed isomerization alters the structure of the vitamin's double bonds, producing inactive forms like lumisterol or tachysterol. While these degradation products are non-toxic, they no longer retain biological activity. There is no report about sugar itself chemically react with vitamin D or accelerate its breakdown. Instead, environmental factors (light, oxygen, heat, and acidity) are the main contributors to instability. In this context, sugar serves as a relatively safe carrier, provided that storage conditions are well controlled.
The Strategies to Enhance Stability of vitamin D in fortified sugar, careful attention must be paid to processing methods and packaging by the manufacturer. Common fortification techniques involve incorporating vitamin D into a premix, which is then coated onto sugar crystals using surface coating or encapsulation technologies. Encapsulation is embedding vitamin D in microcapsules or nanoparticles offers added protection against oxygen and light exposure. Reviews suggest that encapsulation methods, along with using vitamin D3 (which is more stable than D2), improve retention in food matrices (27). Mixed micelles and nanoemulsions, especially those stabilized by surfactants such as Tween 80 and lecithin, help preserve vitamin D against degradation caused by temperature changes, light, and oxidation, maintaining over 70% retention after 1 month of storage at 4 °C (28–30). Water-dispersible formulations of vitamin D have also been developed to enhance uniformity and stability during mixing.
Packaging plays a critical role. Fortified sugar should be stored in airtight, opaque containers and kept in cool, dry environments (23). Studies have shown that with proper formulation and packaging, vitamin D3 can retain over 90% of its potency during storage in fortified products such as UHT milk, yogurt, and packaged juice over several weeks (19). In summary, vitamin D remains stable in fortified sugar when environmental stressors are controlled. No scientific evidence indicates that sugar alone degrades vitamin D; instability arises primarily from exposure to light, oxygen, heat, or acidic conditions. With optimized processing and storage, vitamin D-fortified sugar can maintain its nutritional value throughout distribution and shelf life.
3 The effect of fortifying sugar with vitamin D on health
3.1 Bioavailability of vitamin D in sugar
A key question is whether vitamin D added to sugar remains bioavailable upon consumption. In general, vitamin D fortification in foods has demonstrated good bioavailability, meaning the vitamin can be effectively released from the food matrix and absorbed in the small intestine (21). Studies have consistently shown that vitamin D3 from fortified products like milk (31), cheese (32–36), yogurt (37), bread (38) and orange juice (39) has comparable bioavailability to that from supplements. There is no evidence suggesting that sugar interferes with or deactivates vitamin D. When fortified sugar is consumed (e.g., dissolved in beverages or incorporated into recipes), the vitamin dissolves alongside the food or drink and is absorbed, particularly when dietary fats are present.
Since vitamin D is fat-soluble, optimal absorption occurs when consumed with fat. Pure granulated sugar contains no fat; thus, in extreme cases such as ingesting fortified sugar alone absorption may be suboptimal (40, 41). However, in real-world scenarios, sugar is typically consumed with other ingredients (e.g., milk in tea or desserts), which often provide enough fat to aid vitamin D absorption (20, 41). Even small amounts of fat can facilitate uptake in the gut. Research supports that lipid-based food matrices, such as dairy products, enhance both the stability and absorption of vitamin D. Bread fortified with vitamin D has shown potential to elevate serum 25(OH)D levels and reduce parathyroid hormone concentrations; however, additional research is required to validate its efficacy in the prevention or treatment of vitamin D deficiency (38). Therefore, vitamin D in fortified sugar remains bioavailable, especially when part of a balanced diet containing fats. Once absorbed, its metabolic fate is identical to other sources converted to 25(OH)D in the liver and utilized by the body as normal (13, 41).
3.2 Potential health benefits
Enriching sugar with vitamin D holds considerable potential for enhancing public health outcomes, especially in regions where vitamin D deficiency is widespread. Several key benefits include:
3.2.1 Enhancing population-level vitamin D intake and status
Daily integration of vitamin D into sugar could markedly improve intake and reduce deficiency at the population level. Evidence from milk and margarine fortification shows significant rises in serum 25(OH)D—up to 17 nmol/L—and reductions in deficiency prevalence from over 90% to below 2% (42). Properly fortified sugar could achieve similar benefits, lowering risks of rickets, osteomalacia, and supporting overall bone and immune health.
3.2.2 Reaching underserved and at-risk groups
Unlike individual supplementation, which depends on personal awareness and access, food fortification is universal by nature. Sugar is used across all socio-economic strata, meaning this method could reach individuals who may lack the resources or knowledge to obtain vitamin D supplements (43, 44). This is similar to the successful public health strategies that introduced iodine in salt and vitamin A in sugar (45). For instance, in Iran, daily consumption of a traditional drink (doogh) fortified with vitamin D not only raised serum 25(OH)D levels among participants but also improved glycemic control in individuals with type 2 diabetes (21). Such outcomes demonstrate how widely consumed fortified foods or beverages can serve as practical, large-scale health interventions.
3.2.3 Long-term benefits for bone and immune health
Improved vitamin D levels across a population are associated with reduced prevalence of health problems related to its deficiency. Since vitamin D is crucial for calcium absorption, maintaining adequate levels supports bone mineralization, helps prevent osteoporosis, and lowers the risk of fractures in older adults (46–48). Beyond skeletal benefits, vitamin D is recognized for its broader impact on health, including modulation of immune function, reduction of inflammation, and potential protection against chronic diseases like cardiovascular disease, diabetes, autoimmune disorders, and certain cancers (8, 46, 49–51). While sugar fortification does not directly deliver these benefits, the resulting improvement in vitamin D status can indirectly lead to long-term positive health outcomes, such as decreased incidence of rickets, enhanced bone strength, and possibly stronger immune responses. Meta-analyses have indicated that national vitamin D fortification policies are linked with measurable declines in deficiency-related conditions and improvements in health indicators (27).
It is important to emphasize that the success of such an initiative depends on implementing proper fortification levels aligned with daily recommended intakes without surpassing upper safety limits and should be supported by nutrition education campaigns. Fortifying sugar with vitamin D does not make it a therapeutic product, but rather a functional food component aiding in daily micronutrient adequacy (13, 43, 44).
3.3 Potential risks and health perspectives
While fortifying sugar with vitamin D offers potential public health benefits, it also presents several challenges and risks that must be carefully considered:
3.3.1 Risk of vitamin D toxicity (hypervitaminosis D)
Excessive intake of vitamin D can lead to hypercalcemia (elevated calcium levels in the blood) which may cause symptoms like nausea, fatigue, and even kidney damage (52). Although vitamin D toxicity is rare from regular food consumption, errors in the fortification process can be dangerous. A case reported in The Lancet described vitamin D poisoning due to table sugar that had been adulterated with doses thousands of times higher than recommended. Similarly, there was an outbreak of vitamin D toxicity when milk was mistakenly fortified with over 200,000 IU per liter, leading to poisoning in several individuals. These cases highlight the critical importance of quality control in the fortification process. To minimize risk, manufacturers must ensure that added vitamin D levels align with regulatory standards typically a few hundred IU per serving and that the distribution is homogeneous. When properly formulated and controlled, the risk of toxicity is extremely low. For reference, the tolerable upper intake level for vitamin D in adults is about 4,000 IU per day, and fortification levels are generally far below this threshold (53). However, without rigorous quality assurance, mass overdosing remains a serious concern, making strict regulatory oversight essential.
3.3.2 Behavioral side effects (increased sugar consumption)
Fortifying sugar with vitamin D risks misleading consumers into viewing it as a “healthy” product, potentially driving higher intake despite sugar's link to obesity and diabetes. Market trends show micronutrient-enriched sugary foods can encourage excess calories, so any fortification program must be paired with clear public education that the goal is nutrient delivery, not promoting more sugar consumption (17, 54, 55).
3.3.3 Unequal distribution of vitamin D intake
Sugar fortification relies on assumed uniform consumption, but intake varies widely. People with low sugar intake—such as those with diabetes or restrictive diets—may not receive enough vitamin D, while heavy consumers could get disproportionately more. Although overdose risk is low with proper dosing, this uneven distribution highlights the need to pair sugar fortification with complementary strategies to ensure adequate coverage for all groups (43, 56).
3.3.4 Potential interactions with other nutrients
In some cases, fortifying sugar with multiple micronutrients can lead to interactions between vitamins. For example, in certain Latin American countries, sugar is also fortified with vitamin A. If both vitamins are added, their stability in the mixture must be verified. Some studies show that certain vitamins can affect each other's stability when combined. However, reports of significant interactions between vitamin D and other fortifying agents are rare. Vitamin D is generally stable and non-reactive when mixed with other vitamins and minerals. The key is to ensure that the vitamin D premix does not contain compounds that may accelerate degradation (13).
4 Integrative interpretation
Taken together, enriching sugar with vitamin D presents a promising nutritional intervention, provided it is executed with careful planning and rigorous oversight (Figure 1). The stability of vitamin D within sugar can be well maintained during storage, especially when destabilizing factors such as ultraviolet light, air exposure, heat, and humidity are effectively controlled (Figure 1). Sugar itself does not directly degrade vitamin D; in fact, under optimal storage conditions, over 90% of the vitamin can remain intact throughout the product's shelf life. Moreover, vitamin D delivered through fortified sugar remains bioavailable and has been shown to effectively elevate the body's vitamin D status, as evidenced by successful food fortification programs that have significantly increased population serum 25(OH)D levels (13, 18, 20).
On the other hand, safety considerations and public education must not be overlooked. The amount of vitamin D added should be carefully calibrated to ensure it is safe for daily intake across all age groups, with levels set well below toxic thresholds. A monitoring system for periodic testing of vitamin D content in fortified sugar should be established to prevent accidental overdosing. Additionally, it is crucial to communicate to the public that vitamin D-fortified sugar is not a license for excessive sugar consumption; sugar remains a food component that must be consumed in moderation for metabolic health. The primary goal of fortification is to enhance the nutritional value of sugar, not to encourage its increased intake (43, 57, 58).
5 Future directions, safety risks, and economic impact
Vitamin D deficiency remains highly prevalent in Indonesia, and fortification offers strong health and economic returns—up to US$27 gained for every US$1 invested, with European programs showing substantial cost savings. Cholecalciferol (D3) is more effective than ergocalciferol (D2), making it the preferred form for large-scale use. Future research should optimize sugar-based matrices for stability in tropical climates, and conduct long-term trials to evaluate impacts on serum 25(OH)D, fracture risk, and metabolic outcomes, alongside cost-effectiveness analyses across diverse regions. Safety remains critical: intakes above the tolerable upper level (4,000 IU/day) can cause adverse effects, requiring monitoring of serum calcium and vitamin D, particularly in vulnerable groups. Vehicle choice also matters, with sucrose favored over HFCS to avoid additional cardiometabolic risks. By leveraging Indonesia's existing sugar supply chains, fortification could reduce healthcare costs, improve productivity, and lessen the burden of metabolic and skeletal disease at scale.
6 Conclusion
Vitamin D–fortified sugar presents a practical alternative to milk, flour, or oils due to its universal consumption, stability, and ease of distribution. Its crystalline structure enables uniform dispersal of vitamin D, with fewer sensory or rancidity issues compared to oils. However, sugar's caloric load, link to caries and metabolic disease, and risk of vitamin D degradation or over-fortification demand cautious messaging and precise dosing. Future work should test bioavailability in human trials, assess consumer acceptance, evaluate storage stability, and model population-level health impacts. By weighing benefits and risks, this article highlights sugar's potential as a scalable fortification vehicle while guiding research and policy for safe implementation.
Author contributions
HH: Supervision, Investigation, Conceptualization, Validation, Writing – review & editing, Writing – original draft, Methodology, Data curation. RR: Data curation, Investigation, Writing – original draft, Validation, Writing – review & editing, Formal analysis, Supervision. AS: Formal analysis, Data curation, Project administration, Validation, Writing – review & editing, Methodology, Investigation, Writing – original draft, Supervision, Conceptualization. FN: Investigation, Formal analysis, Writing – original draft, Software, Visualization, Project administration, Conceptualization, Methodology, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. We acknowledge the use of AI assistance, specifically ChatGPT, for language refinement and improving the clarity and conciseness of the manuscript. No AI tools were used for data analysis, interpretation, or generating scientific content. All scientific concepts, results, and conclusions were developed and verified by the author(s).
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Olson R, Gavin-Smith B, Ferraboschi C, Kraemer K. Food fortification: the advantages, disadvantages and lessons from Sight and Life programs. Nutrients. (2021) 13:1118. doi: 10.3390/nu13041118
2. Dwyer JT, Wiemer KL, Dary O, Keen CL, King JC, Miller KB, et al. Fortification and health: challenges and opportunities. Adv Nutr. (2015) 6:124–31. doi: 10.3945/an.114.007443
3. Zamora G, Flores-Urrutia MC, Mayén A-L. Large-scale fortification of condiments and seasonings as a public health strategy: equity considerations for implementation. Ann N Y Acad Sci. (2016) 1379:17–27. doi: 10.1111/nyas.13183
4. Das JK, Salam RA, Mahmood SB, Moin A, Kumar R, Mukhtar K, et al. Food fortification with multiple micronutrients: impact on health outcomes in general population. Cochrane Libr. (2019) 12:CD011400. doi: 10.1002/14651858.CD011400.pub2
5. Keats EC, Neufeld LM, Garrett GS, Mbuya MNN, Bhutta ZA. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: evidence from a systematic review and meta-analysis. Am J Clin Nutr. (2019) 109:1696–708. doi: 10.1093/ajcn/nqz023
6. Duggal M, Sesikeran B, Arlappa N, Nair S, Shekhar V, Sabharwal V. Large-scale staple food fortification as a complementary strategy to address vitamin and mineral vulnerabilities in India: a critical review. Indian J Public Health. (2022) 66:313. doi: 10.4103/ijph.ijph_708_22
7. Prajapati P, Sharma R. Advances in food fortification: ensuring a healthier humanity, types, and analytical methods. Curr Trends Pharm Pharm Chem. (2024) 6:76–83. doi: 10.18231/j.ctppc.2024.021
8. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:2097. doi: 10.3390/nu12072097
9. Khammissa RAG, Fourie J, Motswaledi MH, Ballyram R, Lemmer J, Feller L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. Biomed Res Int. (2018) 2018:1–9. doi: 10.1155/2018/9276380
10. Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. (2017) 7:697. doi: 10.3389/fimmu.2016.00697
11. Gallo D, Baci D, Kustrimovic N, Lanzo N, Patera B, Tanda ML, et al. How does vitamin D affect immune cells crosstalk in autoimmune diseases? Int J Mol Sci. (2023) 24:4689. doi: 10.3390/ijms24054689
12. Sîrbe C, Rednic S, Grama A, Pop TL. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int J Mol Sci. (2022) 23:9784. doi: 10.3390/ijms23179784
13. Alnafisah RY, Alragea AS, Alzamil MK, Alqahtani AS. The impact and efficacy of vitamin D fortification. Nutrients. (2024) 16:4322. doi: 10.3390/nu16244322
14. Al Khalifah R, Alsheikh R, Alnasser Y, Alsheikh R, Alhelali N, Naji A, et al. The impact of vitamin D food fortification and health outcomes in children: a systematic review and meta-regression. Syst Rev. (2020) 9:144. doi: 10.1186/s13643-020-01360-3
15. Nyakundi PN, Némethné Kontár Z, Kovács A, Járomi L, Zand A, Lohner S. Fortification of staple foods for household use with vitamin D: an overview of systematic reviews. Nutrients. (2023) 15:3742. doi: 10.3390/nu15173742
16. Vieth R. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. (2020) 74:1493–7. doi: 10.1038/s41430-020-0697-1
17. Fortified Sugar Market. Available online at: https://www.factmr.com/report/fortified-sugar-market (Accessed July 22, 2025).
18. van Dijk A. Quantum yield for the photo-degradation of vitamin D3. Photochem Photobiol Sci. (2017) 16:690–3. doi: 10.1039/c6pp00393a
19. Ritu G, Gupta A. Fortification of foods with vitamin D in India. Nutrients. (2014) 6:3601–23. doi: 10.3390/nu6093601
20. Jafari T, Askari G, Mirlohi M, Javanmard SH, Faghihimani E, Fallah AA. Stability of vitamin D3 in fortified yoghurt and yoghurt drink (Doogh). Adv Biomed Res. (2016) 5:52. doi: 10.4103/2277-9175.178796
21. Lavelli V, D'Incecco P, Pellegrino L. Vitamin D incorporation in foods: formulation strategies, stability, and bioaccessibility as affected by the food matrix. Foods. (2021) 10:1989. doi: 10.3390/foods10091989
22. Molaveisi M, Shahidi-Noghabi M, Naji-Tabasi S. Vitamin D3-loaded nanophytosomes for enrichment purposes: formulation, structure optimization, and controlled release. J Food Process Eng. (2020) 43:e13560. doi: 10.1111/jfpe.13560
23. Majeed M, Rather MA. Enhancing shelf life and bioavailability of vitamin D through encapsulation: a comprehensive review. Food Biophys. (2025) 20:15. doi: 10.1007/s11483-024-09906-x
24. de Jesus Costa T, Thomazini M, Cristina José J, Peres Brexó R, Martelli-Tosi M, Sílvia Favaro-Trindade C. Impact of plasmolysis process on the enrichment of brewer's spent yeast biomass with vitamin D3 by biosorption followed by spray-drying process. Food Res Int. (2024) 191:114677. doi: 10.1016/j.foodres.2024.114677
25. Mahmoodani F, Perera CO, Fedrizzi B, Abernethy G, Chen H. Degradation studies of cholecalciferol (vitamin D3) using HPLC-DAD, UHPLC-MS/MS and chemical derivatization. Food Chem. (2017) 219:373–81. doi: 10.1016/j.foodchem.2016.09.146
26. Temova Rakuša Ž, Pišlar M, Kristl A, Roškar R. Comprehensive stability study of vitamin D3 in aqueous solutions and liquid commercial products. Pharmaceutics. (2021) 13:617. doi: 10.3390/pharmaceutics13050617
27. Grønborg IM, Tetens I, Christensen T, Andersen EW, Jakobsen J, Kiely M, et al. Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: a randomized controlled trial. Eur J Nutr. (2020) 59:741–53. doi: 10.1007/s00394-019-01941-6
28. Mehmood T, Ahmed A. Tween 80 and soya-lecithin-based food-grade nanoemulsions for the effective delivery of vitamin D. Langmuir. (2020) 36:2886–92. doi: 10.1021/acs.langmuir.9b03944
29. Mulrooney SL, O'Neill GJ, Brougham DF, Lyng JG, O'Riordan D. Improving vitamin D3 stability to environmental and processing stresses using mixed micelles. Food Chem. (2021) 362:130114. doi: 10.1016/j.foodchem.2021.130114
30. Ahmed MZ, Gupta A, Warsi MH, Ali AMA, Hasan N, Ahmad FJ, et al. Nano matrix soft confectionary for oral supplementation of vitamin D: stability and sensory analysis. Gels. (2022) 8:250. doi: 10.3390/gels8050250
31. Salehi S, Sadeghi F, Akhlaghi M, Hanifpour MA, Roshanzamir M. Vitamin D3-fortified milk did not affect glycemic control, lipid profile, and anthropometric measures in patients with type 2 diabetes, a triple-blind randomized clinical trial. Eur J Clin Nutr. (2018) 72:1083–92. doi: 10.1038/s41430-017-0062-1
32. Boivin-Piché J, Vuillemard J-C, St-Gelais D. Technical note: vitamin D-fortified cheddar type cheese produced from concentrated milk. J Dairy Sci. (2016) 99:4140–5. doi: 10.3168/jds.2015-10567
33. Santanatoglia A, Nzekoue FK, Alesi A, Ricciutelli M, Sagratini G, Suo X, et al. Development of innovative vitamin D enrichment designs for two typical Italian fresh cheeses: burrata and giuncata. Molecules. (2023) 28:1049. doi: 10.3390/molecules28031049
34. Nzekoue FK, Alesi A, Vittori S, Sagratini G, Caprioli G. Development of functional whey cheese enriched in vitamin D3: nutritional composition, fortification, analysis, and stability study during cheese processing and storage. Int J Food Sci Nutr. (2021) 72:746–56. doi: 10.1080/09637486.2020.1857711
35. Crevier B, Bélanger G, Vuillemard J-C, St-Gelais D. Short communication: production of cottage cheese fortified with vitamin D. J Dairy Sci. (2017) 100:5212–6. doi: 10.3168/jds.2016-12308
36. Hendy S, Gamal El Din A, Awad R. Production of functional Karish cheese fortified with vitamin D3 in nanoemulsion. Al-Azhar J Agric Res. (2023). doi: 10.21608/ajar.2023.165487.1095
37. Gasparri C, Perna S, Spadaccini D, Alalwan T, Girometta C, Infantino V, et al. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J Dairy Sci. (2019) 102:8587–603. doi: 10.3168/jds.2018-16046
38. Souza SVS, Borges N, Vieira EF. Vitamin d-fortified bread: systematic review of fortification approaches and clinical studies. Food Chem. (2022) 372:131325. doi: 10.1016/j.foodchem.2021.131325
39. Salma Faeza AF, Loh SP, Nurzalinda ZZ, Norhafizah AM, Muhammad Najib MA. Effect of vitamin D3-fortified fruit juice supplementation of 4000 IU daily on the recovery of iron status in childbearing-aged women with marginally low iron stores: Protocol for an 8-week, parallel group, double-blind randomized controlled trial. PLoS ONE. (2022) 17:e0265772. doi: 10.1371/journal.pone.0265772
40. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. (2018) 76:60–76. doi: 10.1093/nutrit/nux034
41. Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. (2015) 55:1193–205. doi: 10.1080/10408398.2012.688897
42. Martucci M, Conte M, Bucci L, Giampieri E, Fabbri C, Palmas MG, et al. Twelve-week daily consumption of ad hoc fortified milk with ω-3, D, and group B vitamins has a positive impact on inflammaging parameters: a randomized cross-over trial. Nutrients. (2020) 12:3580. doi: 10.3390/nu12113580
43. Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, et al. Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol. (2018) 9:373. doi: 10.3389/fendo.2018.00373
44. Niedermaier T, Gredner T, Kuznia S, Schöttker B, Mons U, Lakerveld J, et al. Vitamin D food fortification in European countries: the underused potential to prevent cancer deaths. Eur J Epidemiol. (2022) 37:309–20. doi: 10.1007/s10654-022-00867-4
45. Iwuozor KO, Mbamalu PS, Olaniyi BO, Anyanwu VU, Emenike EC, Adeniyi AG. Fortification of sugar: a call for action. Sugar Tech. (2022) 24:1284–94. doi: 10.1007/s12355-022-01183-7
46. Wimalawansa SJ. Physiology of vitamin D—focusing on disease prevention. Nutrients. (2024) 16:1666. doi: 10.3390/nu16111666
47. Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. (2019) 11:2861. doi: 10.3390/nu11122861
48. LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the Us preventive services task force. Ann Intern Med. (2015) 162:109–22. doi: 10.7326/M14-1659
49. Wang H, Chen W, Li D, Yin X, Zhang X, Olsen N, et al. Vitamin D and chronic diseases. Aging Dis. (2017) 8:346. doi: 10.14336/AD.2016.1021
50. Zmijewski MA. Vitamin D and human health. Int J Mol Sci. (2019) 20:145. doi: 10.3390/ijms20010145
51. Wimalawansa SJ. Physiological basis for using vitamin D to improve health. Biomedicines. (2023) 11:1542. doi: 10.3390/biomedicines11061542
52. Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity–a clinical perspective. Front Endocrinol. (2018) 9:550. doi: 10.3389/fendo.2018.00550
53. EFSA EFSA Panel on Nutrition, Novel Foods, FoodAllergens (NDA), Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst K-I, et al. Scientific opinion on the tolerable upper intake level for vitamin D, including the derivation of a conversion factor for calcidiol monohydrate. EFSA J. (2023) 21:e08145. doi: 10.2903/j.efsa.2023.8145
54. Mayengbam S, Virtanen H, Hittel DS, Elliott C, Reimer RA, Vogel HJ, et al. Metabolic consequences of discretionary fortified beverage consumption containing excessive vitamin B levels in adolescents. PLoS ONE. (2019) 14:e0209913. doi: 10.1371/journal.pone.0209913
55. Ikonen I, Sotgiu F, Aydinli A, Verlegh PWJ. Consumer effects of front-of-package nutrition labeling: an interdisciplinary meta-analysis. J Acad Mark Sci. (2020) 48:360–83. doi: 10.1007/s11747-019-00663-9
56. Hayes A, Cashman KD. Food-based solutions for vitamin D deficiency: putting policy into practice and the key role for research. Proc Nutr Soc. (2017) 76:54–63. doi: 10.1017/S0029665116000756
57. Adebayo FA, Itkonen ST, Öhman T, Kiely M, Cashman KD, Lamberg-Allardt C, et al. Safety of vitamin D food fortification and supplementation: evidence from randomized controlled trials and observational studies. Foods. (2021) 10:3065. doi: 10.3390/foods10123065
Keywords: vitamin D, granulated sugar, food fortification, stability, health, 25-hydroxyvitamin D
Citation: Hardinsyah H, Rahmawati R, Sulaeman A and Nurkolis F (2025) A blueprint for vitamin D fortification in sugar: stability and health impact. Front. Nutr. 12:1671785. doi: 10.3389/fnut.2025.1671785
Received: 23 July 2025; Accepted: 22 August 2025;
Published: 05 September 2025.
Edited by:
Aliyu Idris Muhammad, Bayero University, Kano, NigeriaReviewed by:
Piotr Suski, Medical University of Lublin, PolandCopyright © 2025 Hardinsyah, Rahmawati, Sulaeman and Nurkolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hardinsyah Hardinsyah, aGFyZGluc3lhaEBhcHBzLmlwYi5hYy5pZA==; Fahrul Nurkolis, ZmFocnVsLm51cmtvbGlzLm1haWxAZ21haWwuY29t
 Hardinsyah Hardinsyah
Hardinsyah Hardinsyah Rahmawati Rahmawati
Rahmawati Rahmawati Ahmad Sulaeman
Ahmad Sulaeman Fahrul Nurkolis
Fahrul Nurkolis