- 1The First Hospital of Hunan University of Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2Key Laboratory of Dryness Syndrome in Chinese Medicine, Ministry of Education, Ningxia Medical University, Yinchuan, Ningxia, China
- 3Department of Chinese Medical Gastrointestinal, The Affiliated TCM Hospital of Ningxia Medical University, Wuzhong, China
- 4Department of Clinical Medicine, Ningxia Medical University, Yinchuan, Ningxia, China
- 5Department of Pharmacy, Ningxia Medical University, Yinchuan, Ningxia, China
- 6Department of Pharmacy, General Hospital of Ningxia Medical University, Yinchuan, China
Platycodon grandiflorum (Jacq.) A. D C. (P. grandiflorum), as a medicinal and edible plant, is widely distributed in China. In terms of Chinese medical theories, Traditional Chinese Medicine (TCM) believes that the root of P. grandiflorum has the functions of relieving sore throat, eliminating phlegm, and draining pus, and is widely used in lung diseases and respiratory system disorders. Modern pharmacological studies have shown that Platycodon grandiflorum saponin, flavonoids, polysaccharides, and phenolic acids are the main active components of P. grandiflorum, which have significant antitussive, expectorant, anti-tumor, anti-oxidation and hypoglycemic effects. In recent years, an increasing number of research evidence has demonstrated the anticancer potential of P. grandiflorum. In this study, we firstly identified the anti-tumor active components of P. grandiflorum (platycodin D, P. grandiflorum polysaccharides, lobetyolin, luteolin, and apigenin) as well as their mechanism of action in inhibiting cancer cell proliferation (inducing apoptosis, blocking the cell cycle and inhibiting tumor metastasis). Additionally, we investigated the detoxification and synergy-enhancing effects of these active components when combined with chemotherapeutic drugs, as well as the improvement of their drug delivery and the bioavailability through nano-delivery systems, which hold great anticancer potential for clinical application. However, research on the anti-tumor effects of P. grandiflorum extract and its active components still lacks large-scale clinical trials. Finally, we further summarized the applications of P. grandiflorum in preventive healthcare, emphasizing its dual value in the pharmaceutical and food industries. This summary provides a scientific basis for further research on its anti-tumor properties and highlights its potential development in modern preventive healthcare.
1 Introduction
Malignant tumors are a class of diseases with complex pathogenesis, poor prognosis, and high morbidity and mortality rates. According to World Health Organization (WHO) reports, 17.5 million cancer patients deaths and 27 million new cancer patients will happen annually by 2050 (1). Nowadays, traditional treatment methods still form the backbone of clinical tumor treatment, including surgery, radiotherapy, and chemotherapy. However, these methods often cannot avoid affecting normal tissues-thus having the characteristic of “non-specificity” (2). In addition, targeted therapy for tumors is a precise treatment method that selectively targets specific receptors on tumor cells. However, targeted therapy also encounters challenges, including tumor cell resistance, high costs, a diversity of therapeutic targets, and potential side effects (3). Therefore, continued research and development of new treatment methods are crucial to improve treatment effectiveness and safety.
Traditional Chinese medicine (TCM), as one of the effective means of prevention and treatment of malignant tumors, has achieved significant efficacy in tumor treatment. Active natural compounds derived from traditional Chinese herbs play a crucial role in cancer treatment by producing anti-cancer effects, effectively improving clinical effects, and reducing the toxic side effects associated with chemotherapy and radiation therapy. According to the literature, natural products have a more diverse structure and can effectively exert specific biological functions. For example, they mainly regulate the body’s internal environment to inhibit tumor growth, reduce tumor metastasis, and modulate immune responses (4). Statistics show that a significant proportion of FDA-approved anti-cancer drugs are derived from natural plants. This makes natural compounds promising and practical alternatives for cost-effective, highly effective, and safer cancer treatments and prevention. However, currently only 2.5–5 million plant species have been thoroughly studied, which means there is still enormous potential to be explored in the development of anti-cancer therapies based on natural plants.
Platycodon grandiflorum (Jacq.) A. D C. (P. grandiflorum), known as “Jiegeng” or “Lingdanghua” (in China), “Doraji” (in Korea) and “Kikyo” (in Japan), is a dried root of the Platycodon genus, which attributes to the Campanulaceae family (5). The Campanulaceae family has about 60–70 genera, and P. grandiflorum is the only genus in this family (6). P. grandiflorum is primarily found in the northeastern, northern, and central regions of China, along with Korea, Japan, the Russian Far East, and southeastern Siberia (7). According to a record of TCM dated 2000 years ago, P. grandiflorum was first mentioned in “Shennong Bencao Jing” (a famous monograph of TCM). The ancient book stated that P. grandiflorum had a slightly warm and pungent taste, and it was used to treat chest and hypochondriac pain, fullness in the abdomen, faint intestinal sounds, and palpitations caused by fear. Subsequently, throughout different historical periods, medical scholars have consistently described the taste and efficacy of P. grandiflorum. “Mingyi Bie Lu,” “Xinxiu Bencao,” “Kaibao Bencao,” “Tangye Bencao,” “Jingyue Quanshu,” and “Bencao Gangmu” all described P. grandiflorum having a bitter and slightly warm taste, which can relieve sore throat and help to evacuate pus. Since the Qing Dynasty, people have commonly used P. grandiflorum to make pickled vegetables in northeastern of China. Therefore, P. grandiflorum is a medicinal and edible species, which has great potential in the development and application of medicine, health care products, dietary supplements and food (8).
Currently, the rhizome of P. grandiflorum has been widely used in TCM with remarkable ability to promote lung function, ease sore throat, eliminate phlegm and pus, as determined by the Committee for the Pharmacopoeia of PR China (2020). Modern pharmacological studies have shown that the medicinal component of P. grandiflorum is its root, which has various pharmacological effects such as cough suppression (9), immune stimulation (10), anti-inflammatory activity (11), anti-oxidant activity, and anti-tumor activity (12). According to the literature, an increasing number of research evidence has demonstrated the anticancer potential of P. grandiflorum. However, there is a notable absence of comprehensive reports detailing the anti-tumor effects of P. grandiflorum. It has been shown that the active ingredients of P. grandiflorum, such as saponins, flavonoids and polysaccharides play important roles in inhibiting tumor cell proliferation, inducing cell apoptosis and cell autophagy, inhibiting the invasion and migration of tumor cells, regulating immune function, inhibiting tumor angiogenesis (13).
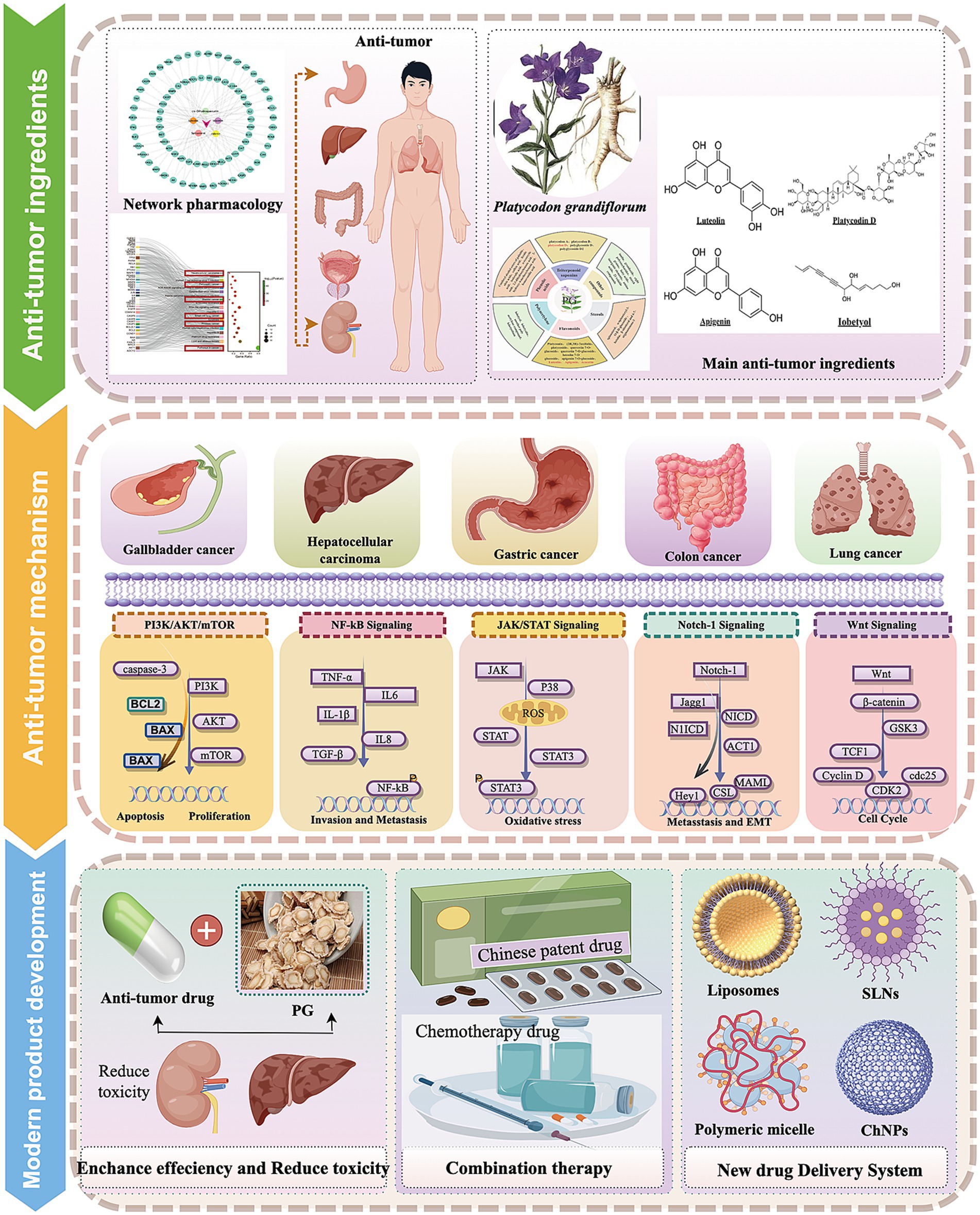
Before writing this review, we reviewed the literature search in PubMed,1 Google Scholar,2 ScienceDirect,3 Web of Science,4 and other databases using the keywords of “Platycodon grandiflorum” AND (“anti-tumor” OR “anti-cancer”) and “Platycodin D” AND (“anti-tumor” OR “anti-cancer”) from the past 5 years. Fortunately, two reviews attracted our attention, primarily focusing on summarizing the botanical description, distribution, traditional uses, chemical composition, and pharmacological effects of P. grandiflorum (5, 14). However, due to the over-comprehensive describe the P. grandiflorum efficacy, only a brief summary on the anti-tumor potential of P. grandiflorum provided without a comprehensive discussion on the antitumor mechanism. Consequently, to address this research gap, we conducted a comprehensive and systematic review in the present study. The purpose of this review is to systematically clarify the anti-tumor potential of P. grandiflorum, including its anti-tumor components (platycodin D, P. grandiflorum polysaccharides, luteolin, apigenin, and lobetyolin), mechanisms, application prospects, and preventive healthcare, with the following research objectives and implementation processes, shown as in graphical abstract.
2 Network pharmacological analysis of P. grandiflorum
The active ingredients of P. grandiflorum were analyzed by TCMSP database5 (accessed May 10, 2025) using the screening criteria of Drug-Like (DL) ≥ 0.18 and Oral Bioavailability (OB) ≥ 0.3. The active components were obtained and their corresponding ID, Molecule Name, OB and DL values were displayed in Supplementary Table 1. The targets of P. grandiflorum’s active ingredients were predicted by TCMSP database, and the targets were corrected by UniProt database6 (accessed May 12, 2025) and converted to gene targets. Additionally, SwissTargetPrediction database7 (accessed May 12, 2025) was incorporated to further supplement the active ingredients of P. grandiflorum and their corresponding gene targets. We performed Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis through the Metascape database8 (accessed May 12, 2025), the pathway information was obtained and their corresponding Description, Gene Ratio, gene ID, and Count were displayed in Supplementary Table 2. Next, we visualized the result through the Wei Shengxin platform.9 Based on the screening criteria, a total of five active ingredients and 95 targets of P. grandiflorum were obtained in the TCMSP database. After importing into Cytoscape3.8 (The Cytoscape Consortium, United States), the “target-ingredient” network was constructed in Figure 1A. Then, these drug targets were imported into the Metascape database. The results indicated that among the top 20 signaling pathways enriched in P. grandiflorum drug targets, six pathways were primarily associated with cancer, including hepatocellular cancer, pancreatic cancer, bladder cancer, small cell lung cancer, prostate cancer, pathways in cancer, etc. (Figure 1B). Therefore, based on the network pharmacology results, we have reason to believe that P. grandiflorum has widely anti-tumor effects.
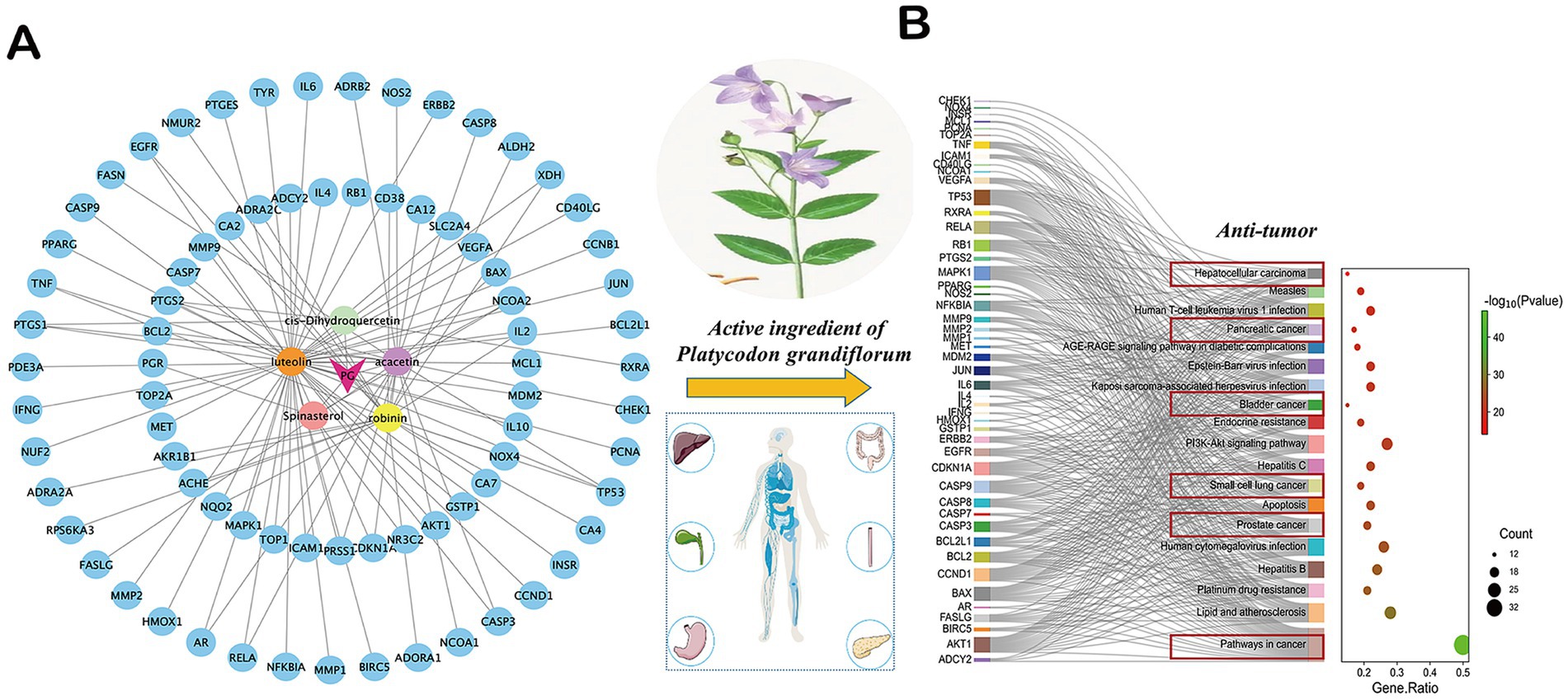
Figure 1. Network pharmacological analysis of related active ingredients, targets, and pathways in P. grandiflorus. (A) Network diagram of active ingredients and targets in P. grandiflorus. (B) The KEGG pathway of active ingredients from P. grandiflorus anti-tumors. The red box represents the type of tumor treatment.
3 Main active ingredients of P. grandiflorum
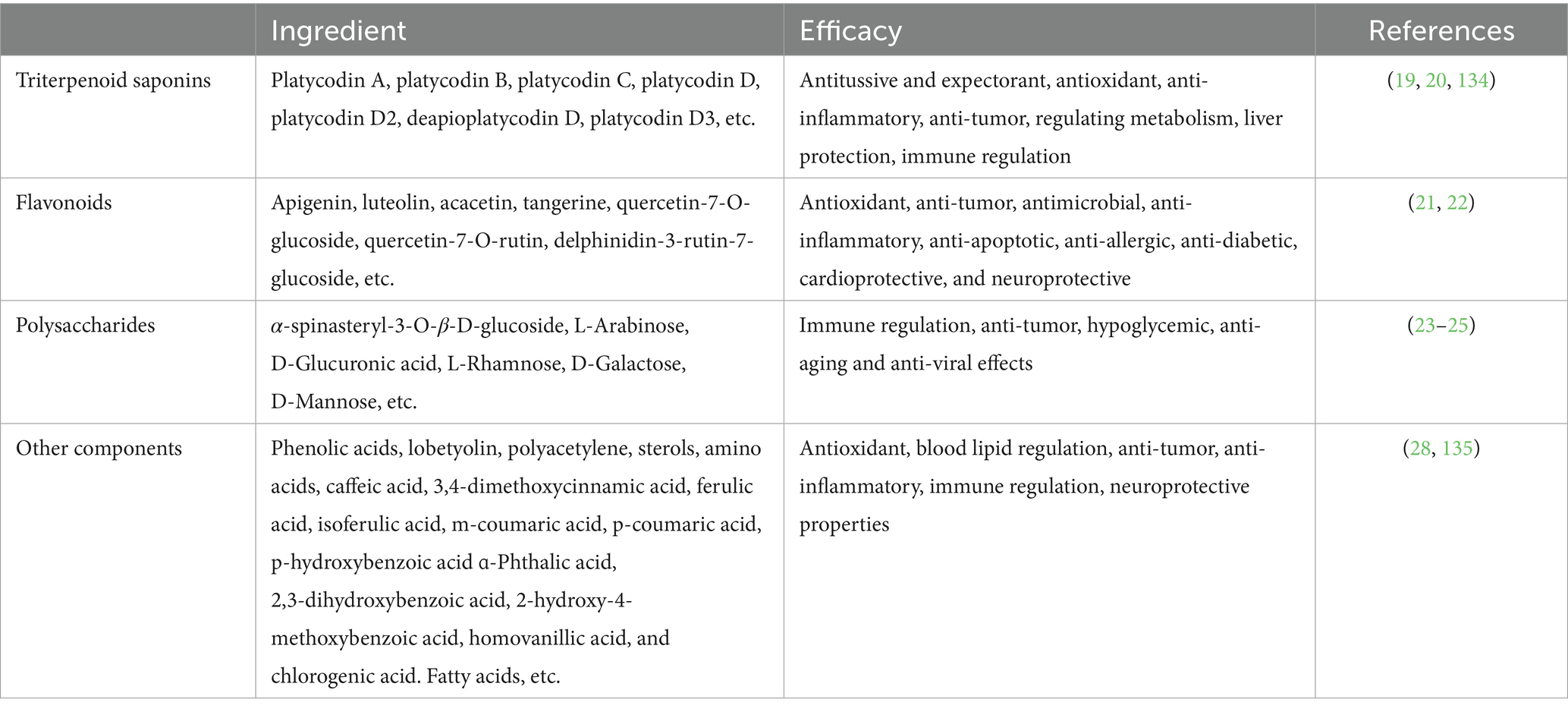
The roots of P. grandiflorum contain high levels of carbohydrates (90%), protein (2.4%), fat (0.1%), ash (1.5%), and approximately 2% triterpene saponins (15). Over the past several decades, at least 100 compounds have been isolated from P. grandiflorum, including steroidal saponins, flavonoids, polysaccharide, phenolic acids, polyacetylenes, sterols, fatty oils, fatty acids, various amino acids, and vitamins, etc. (16, 17). The variety of natural chemical components makes the pharmacological applications of P. grandiflorum very broad. The P. grandiflorum main active components (Table 1) and chemical structures with potential medicinal functions (Figure 2).
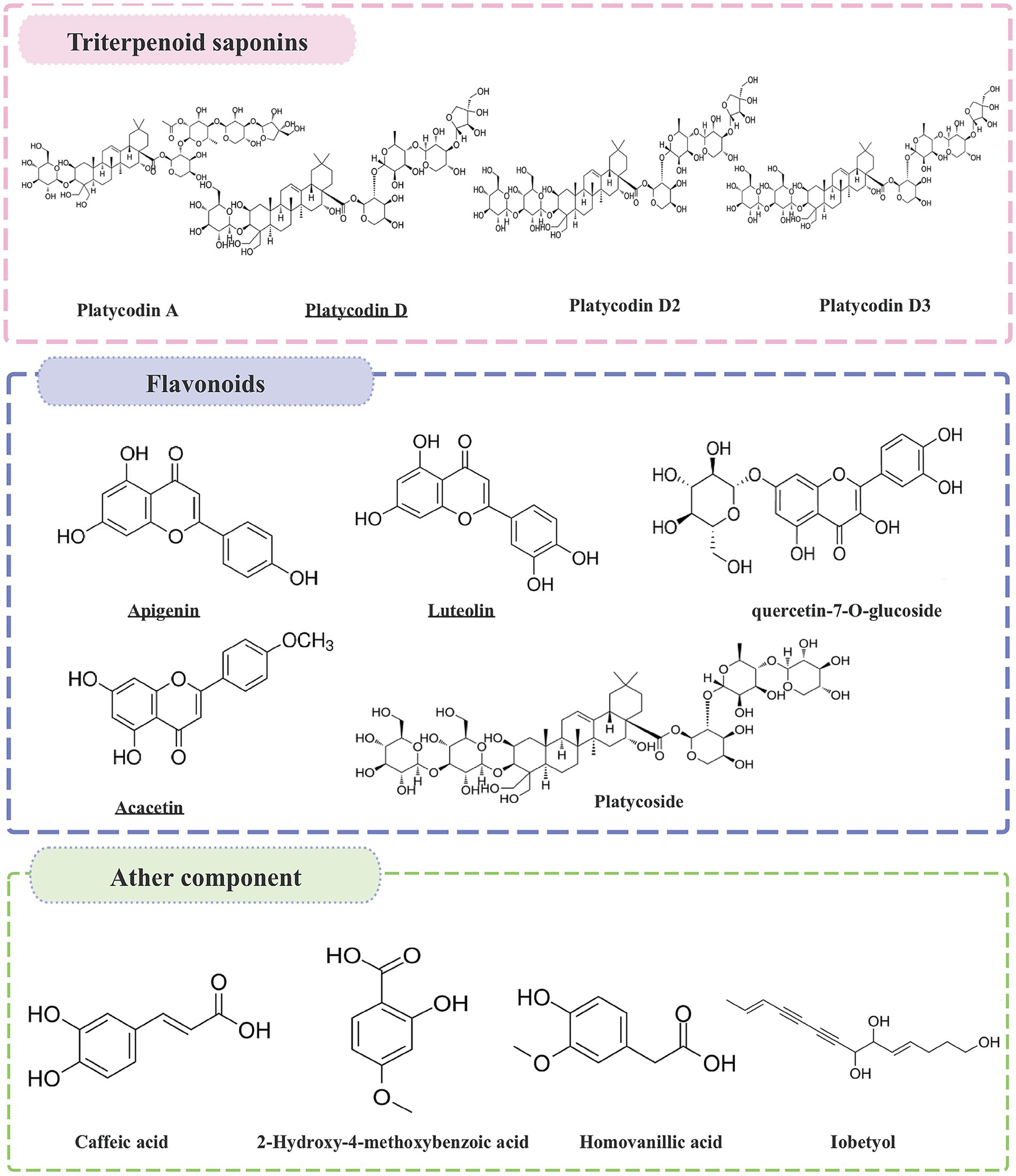
Figure 2. The chemical structures of the active ingredients in P. grandiflorus with potential medicinal functions.
First of all, triterpene saponins are a class of compounds found in many plants. Platycodin refers to a variety of purified cholane-type triterpene saponins with diverse pharmacological activities present in the roots of P. grandiflorum (18). There are numerous types of Platycodin, including platycodin A, platycodin B, platycodin D, Polygala D, Polygala D2, etc. (19). Among all the saponin components, platycodin D is the first saponin isolated from P. grandiflorum, and is recognized as one of the most potent pharmacological components (15). It has been reported to have antitussive, anti-inflammatory, anti-tumor, regulating metabolism effects (20). Subsequently, Flavonoids are primarily found in the aboveground parts of P. grandiflorum and can be classified into flavonoids, dihydroflavonoids, and flavonoid glycosides. To date, more than 10 flavonoid components have been isolated from the aboveground parts (21), including apigenin, luteolin, acacetin, tangerine, quercetin-7-O-glucoside, quercetin-7-O-rutin, delphinidin-3-rutin-7-glucoside and others. Among these components, apigenin and luteolin are the main constituents (22). According to relevant studies, luteolin glycosidic acid and luteolin 7-glucoside exhibit antioxidant, anti-tumor, antimicrobial, anti-inflammatory, anti-apoptotic, anti-allergic, and neuroprotective properties (23). P. grandiflorum polysaccharide is an important active ingredient in P. grandiflorum (24). Polysaccharide is composed of fructose, mannose, xylose, and arabinose; mannose is the main sugar component (25). Currently, polysaccharides with known structures have been identified, such as the eustoma polysaccharides GF2 ~ GF9. They have significant pharmacological effects in enhancing immune function, anti-tumor, hypoglycemic, anti-aging and anti-viral effects, which are well documented in the China Pharmacopoeia (26). Park et al. (27) found that P. grandiflorum polysaccharide can effectively inhibit the autophagic damage induced by chromium (VI) [Cr(VI)] to the mitochondria of DF-1 cells. In addition to saponins, flavonoids, and polysaccharides, P. grandiflorum also contains active ingredients such as phenolic acids, lobetyolin, polyacetylene, sterols, and amino acids. Lee et al. (28) isolated two phenolic compounds from the roots of P. grandiflorum, namely coniferyl palmitate and coniferyl oleate.
4 Extraction process and detection method of antitumor components from P. grandiflorum
In recent years, the extraction of active components from natural plants using safe, eco-friendly, and efficient methods has gained significant popularity. The extraction methods of natural compounds mainly include water extraction, alcohol extraction, cold immersion extraction, ultrasonic extraction and supercritical extraction, among which organic solvent extraction method is the most widely used method. In addition, many determination techniques have been developed for plant extracts (29). Among these determination methods, high-performance liquid chromatography (HPLC) is the most widely employed, and it is characterized by high sensitivity, excellent repeatability, and straightforward standardization (30).
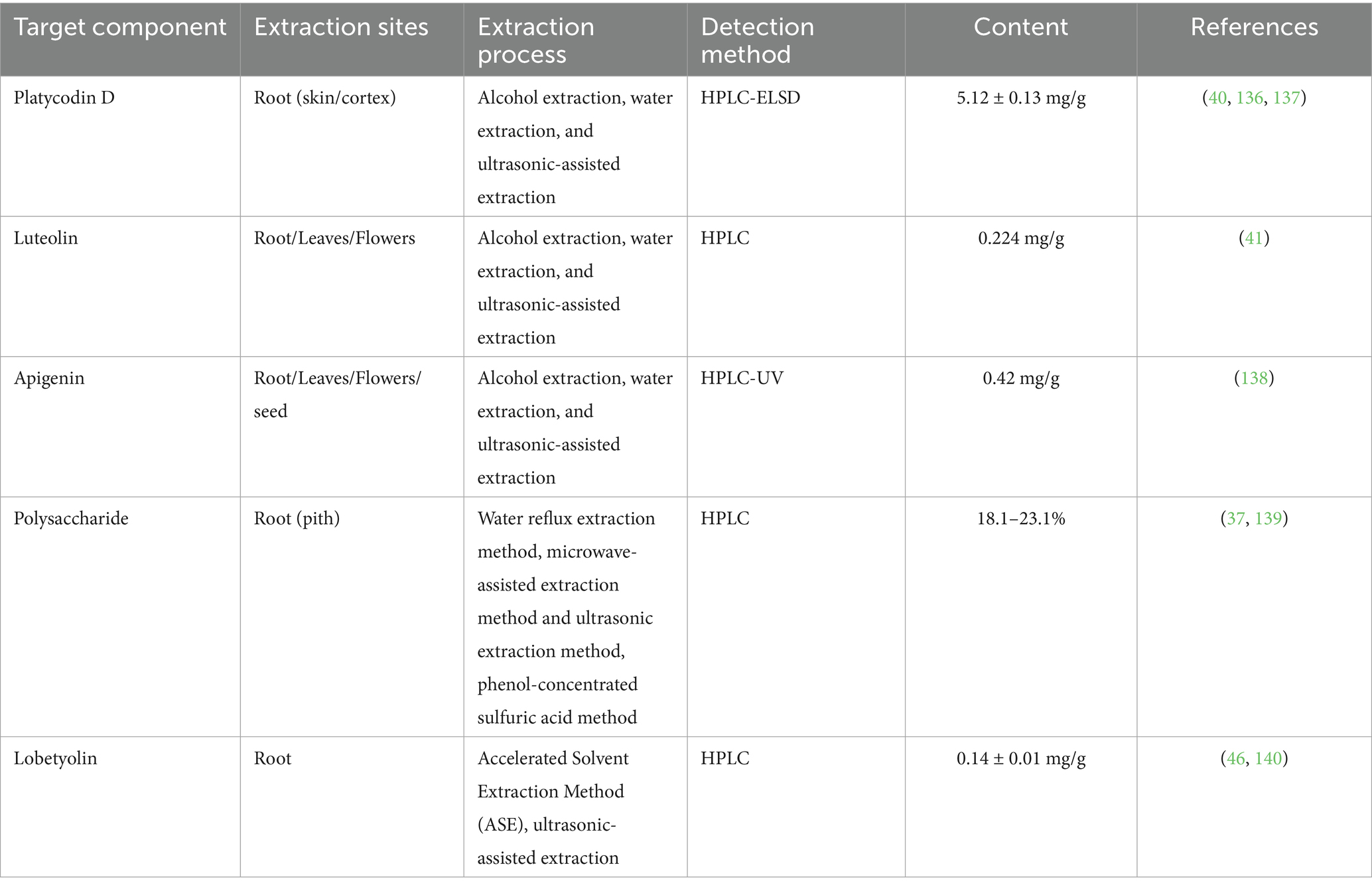
In this review, we focused primarily on the anti-tumor active components and extraction methods of P. grandifloram. P. grandiflorum, as a plant with both medicinal and edible properties, contains various anti-tumor active components-including platycodin D (31), luteolin, apigenin (32, 33), polysaccharides (34) and lobetyolin (35). Currently, the extraction methods and processes for these components have been extensively studied (36, 37). Jingyin et al. (38) determined that the content of platycodin D has been recognized as the major active compound in P. grandiflorum roots than that of leaves. Kwon et al. (39, 40) used HPLC-ELSD to determine that the content of platycodin D in P. grandiflorum root varies depending on three processing methods: alcohol extraction, water extraction, and ultrasonic-assisted extraction. In addition, luteolin and apigenin, as flavonoids, are mainly found in the above-ground parts of the P. grandiflorum. Zhou et al. (41) used ultrasonic extraction and HPLC simultaneous determined the contents of luteolin and apigenin in P. grandiflorum.
Currently, the extraction of polysaccharides has attracted significant attention. Zhang et al. (42) used water reflux extraction and ultrasonic extraction method to extract polysaccharides of P. grandiflorum, among them, the water reflux extraction efficiency is as high as 44%. In addition, there are other methods for polysaccharides. Zhang et al. (43) determined the content of polysaccharides in the roots of P. grandiflorum from China’s three major production areas via the phenol-concentrated sulfuric acid method, and reported that the content ranged from 18.1 to 23.1% (44). Meanwhile, Yin et al. (45) used ultrasonic extraction and HPLC determined the contents of platycodin D and lobetyolin from P. grandiflorum (The content of platycodin D was 5.12 ± 0.13 mg/g, and the yield of lobetyolin was 0.14 ± 0.01 mg/g). Since lobetyolin is mainly found in the root of the P. grandiflorum, its extraction usually uses the root as the raw material. Chen et al. (46) employed accelerated solvent extraction methods and used HPLC to detect content level of lobetyolin. In conclusion, we summarized the extraction sites, extraction processes and detection methods of these anti-tumor components in P. grandiflorum, as shown in Table 2.
5 Anti-cancer effects and underlying mechanisms
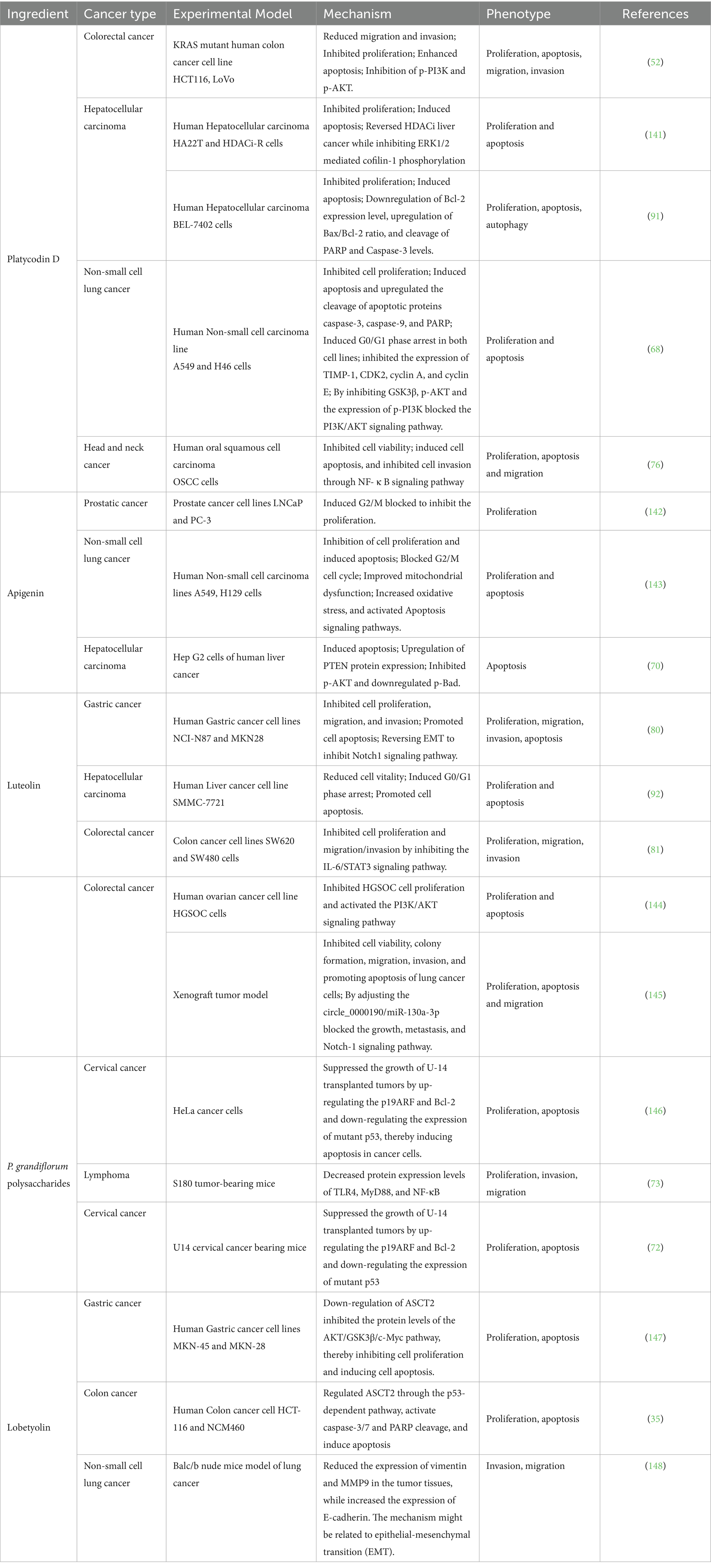
In the anti-cancer research, Platycodin D is the main active component of P. grandiflorum, which has been the most extensively studied (47); followed by flavonoids and polysaccharides, which have also received significant research attention in anti-cancer studies. In addition, the network pharmacological analysis and literature retrieval of P. grandiflorum extracts and active components have been demonstrated which can inhibit multiple types of cancer (Colon cancer, gallbladder cancer, stomach cancer, hepatoma carcinoma cell lung cancer, etc.) and have anti-tumor activity by inhibiting several signaling pathways, including PI3K/AKT/mTOR, MAPK, JNK/STAT3, Wnt, Notch-1, and NF-κB signaling pathways, etc. Further research findings have indicated that P. grandiflorum has promising therapeutical potential for cancer treatment. It inhibits cancer cell proliferation, induces cancer cell apoptosis, suppresses cancer cell migration and invasion, inhibits angiogenesis, and regulates autophagy, etc. (Table 3).
5.1 Inhibiting cell proliferation and blocking cell cycle
Excessive cell proliferation is one of the key indicators of cancer development, and cancer cells possess the capability to evade apoptosis through the activation of survival support signals, which stands as a significant characteristic. The PI3K/AKT signaling pathway is commonly overexpressed in a variety of human cancers (48). The c-Myc transcription factors play an essential role in normal, untransformed cells, regulating proliferation, differentiation, cell growth, and apoptosis (49). Cancer cells often escape terminal differentiation by increasing the c-Myc expression, which is an effective inhibitor of differentiation in numerous cell lines (50). In gastric cancer, platycodin D exerts its anti-cancer effect by promoting c-Myc protein ubiquitination and degradation (51). Liu et al. (52) found that platycodin D can suppresses tumor cell growth in vitro and vivo by down-regulating the p-PI3K and p-AKT expression in HCT116 cells. Zhou et al. (53) found that the water extract of P. grandiflorum inhibits the PI3K/Akt signaling pathway, thereby reducing the apoptosis of lung cells and the infiltration of inflammatory cells in mice induced by lipopolysaccharide.
In addition, abnormal cell cycle progression is also one of the basic mechanisms of tumor proliferation (54). Cyclin B1 is a crucial constituent of the cyclin family, which can activate specific cyclin dependent kinases (CDKs) required for cell cycle progression (55). Activated Cyclin B1 can promote cells from the G2 phase to the M phase, and initiate mitotic progression (56). In the study, Lee et al. (57) found that apigenin can trigger G2/M cell cycle arrest, inhibit the expression of Cyclin B1, Cdc2, and Cdc25c, thereby inhibiting the proliferation of HCT116 colon cancer cells. In their study, Ujiki et al. (58) discovered that the growth of pancreatic cancer cells is hindered by apigenin due to its ability to suppress the G2/M cell cycle and decreased the activity of CyclinB1-Cdc2. In ovarian cancer, luteolin induced cell apoptosis and arrested cell cycle in G2/M by reducing the expression of VRK1 and activating the p53 signaling pathway, and inhibited HGSOC cell proliferation (59). P53 is a transcription factor responsible for inhibiting tumor growth. Its main anti-tumor mechanisms include inhibiting cell proliferation by blocking the cell cycle and promoting cell apoptosis (60). In the study of esophageal cancer, luteolin can inhibit the proliferation and growth of EC1, EC9706, KYSE30, and KYSE450 cells; Up-regulation of the expression of cell cycle inhibitory proteins p21 and p53 inhibits ESCC cells proliferation (61). He et al. (62) evaluated the tumor growth inhibitory effects of two P. grandiflorum polysaccharides (PRP1 and PRP2) isolated and purified from hot water extraction on liver cancer. PRP1 inhibited the proliferation of HepG2 cells by down-regulating miR-21 and activating PTEN. Cai et al. (63) extracted and purified polysaccharides from P. grandiflorum and conducted in vitro cell experiments. The results confirmed that P. grandiflorum polysaccharides promote apoptosis in liver cancer cells by enhancing the expression of LINC01554 (a long non-coding RNA), thereby inhibiting the progression of liver cancer.
5.2 Inducing tumor cell apoptosis
Apoptosis is a cell suicide plan executed by caspases, which is crucial for maintaining tissue homeostasis. Impaired apoptosis is now known to be a key step in cancer development. Whether a cell is alive or dead is largely determined by the anti-apoptotic modulators of the Bcl-2 family, and the release of cytochrome C into the cytoplasm is a crucial event in mitochondrial apoptosis, and it is regulated by the Bcl-2 protein family. Therefore, Bcl-2 plays a crucial role in intrinsic apoptosis by regulating mitochondrial membranes (64). In bladder cancer, platycodin D can increase the expression of Caspase-9, Caspase-8, Caspase-3 and p53 in tumor tissue, and promote cancer cell apoptosis by reducing the expression of Bcl-2 (64). Zeng et al. (65) demonstrated that platycodin D upregulated the expression of cytochrome C by regulating Bcl-2 in gallbladder cancer, further validating the endogenous cell apoptosis mechanism induced by platycodin D. In addition, the anti-apoptosis mechanism of platycodin D has been confirmed in many cancers, such as breast cancer (50), colon cancer (52), gallbladder cancer (66), prostate cancer (67) and non-small cell lung cancer (68). In gastric cancer, a research has shown that luteolin can suppress the proliferation of MKN45 cells, induce apoptosis, lower the mitochondrial membrane potential, and increase the expression of BAX, Caspase3, and cytochrome C, and reduce the expression of Bcl-2 and p-AKT (69). As is well known, triggering tumor cell apoptosis is related to various signaling pathways (PI3K/AKT/mTOR, MAPK, JAK/STAT3, and NF-κB, etc.). In liver cancer research, apigenin can induce the expression of PTEN protein in human liver cancer cells, though inhibiting PI3K/AKT signaling and reducing the level of phosphorylated Bad protein, thereby achieving the effect of inducing liver cancer cell apoptosis (70); apigenin has also been proven to have therapeutic effects on cancer cells. Apigenin induces autophagy by inhibiting the activity of key molecules p-AKT and p-mTOR in gastric cancer cells, thereby enhancing the apoptotic ability of gastric cancer cells (71).
Lu et al. (72) used the polysaccharides extracted from dried P. grandiflorum root to conduct in vitro studies. They found that the P. grandiflorum polysaccharides could significantly inhibit the growth of HeLa cancer cells. Moreover, they could also suppress the growth of U-14 transplanted tumors by up-regulating the p19ARF and Bcl-2 and down-regulating the expression of mutant p53, thereby inducing apoptosis in cancer cells. Wang et al. (73) administered S180 tumor-bearing mice with P. grandiflorum polysaccharides by gavage. They found that the lymphocyte proliferation index significantly increased, while the protein expression levels of TLR4, MyD88, and NF-κB significantly decreased. This might be related to the inhibition of tumor growth and the improvement of the body’s immune function.
5.3 Inhibiting tumor cell migration and invasion
Tumor invasion and migration is a complex, multi-step biochemical process that is considered to be one of the hallmarks of cancer biology. The degradation of the basement membrane and remodeling of the ECM are hallmarks of tumor invasion and metastasis (74). Abnormal expression of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, has been observed in tumors, and their involvement in tumor invasion and metastasis is widely recognized. Lee et al. (75) found that the aqueous extract of P. grandiflorum root inhibits tumor invasion and metastasis. Zhang et al. (76) found that platycodin D hindered NF-κ B regulates the activity of downstream genes of MMP-2 and MMP-9, thereby hindering the migration and invasion of oral squamous cell carcinoma cells. This phenomenon has also been confirmed in gallbladder cancer (66) and prostate cancer (77). In the study of liver cancer, platycodin D effectively hindered the growth of HePG2 and Hep3B cells by significantly suppressing the development of HepG2 cell colonies, and triggering cellular apoptosis, and inhibited TPA induced cell migration and invasion (78). Huang et al. (79) found that luteolin can inhibit the expression of EMT and MMP2, MMP7 and MMP9 in a dose-dependent manner, and inhibit the invasive ability of PANC-1 and SW1990 pancreatic cancer cells. In gastric cancer research, Zhang et al. (80) found that luteolin can significantly suppress the migration and invasion of NCI-N87 and MKN28 cell through the Notch1 pathway, induce cell apoptosis, and reverse the occurrence of EMT. In colorectal cancer, luteolin hindered migration and invasion of SW620 and SW480 cancer cells through inhibiting the STAT3 pathway (81). In non-small cell lung cancer, luteolin inhibited the growth of H460 cells by EGFR/PI3K/AKT pathway and inhibited cell migration by reducing the expression levels of MMP-2 and ICAM-1 (82). Zhang et al. (83) modified the P. grandiflorum polysaccharide (PGP40-1) using the HNO3/Na2SeO3 method to obtain seleniumated P. grandiflorum polysaccharide (Se-PGP40-1). Se-PGP40-1 could inhibit tumor proliferation and migration by inducing cell apoptosis and blocking angiogenesis.
5.4 Inhibition of new vascular growth
The metastasis and recurrence of tumors are closely related to neovascularization, which is a complex physiological process and is considered a marker of cancer progression (74). The VEGF is a highly characteristic factor that promotes endothelial cell growth and has the effect of promoting angiogenesis. The VEGF and IL-8 play crucial roles in promoting tumor angiogenesis by directly enhancing processes like endothelial cell proliferation, adhesion, migration, and angiogenesis. Therefore, developing drugs with high selectivity for VEGF could improve the prognosis of patients with metastatic cancer. The anti-angiogenic effects of platycodin D have recently been studied. Heydarzadeh et al. (84) have shown that platycodin D inhibited HUVEC proliferation, migration, and angiogenesis in a dose-dependent manner. The anti-angiogenic activity can be achieved by inhibiting VEGF induced p-VEGFR-2 and downstream targets. In studies of non-small cell lung cancer, platycodin D has been shown to hinder A549 cell proliferation and trigger apoptosis by modulating the p53/VEGF/MMP2 pathway (85). Meanwhile, in the study of esophageal cancer, apigenin was known as a tumor cell IL-6 transcription inhibitor, which inhibited the transcription and expression of IL-6, thereby inhibiting Eca-109 and Kyse-30 cell proliferation, inducing angiogenesis, and promoting apoptosis of esophageal cancer cell (86).
5.5 Regulating cell autophagy
Autophagy, known as type II programmed cell death, plays a dual role in the development of cancer. First, autophagy can maintain genomic stability and prevent the occurrence, proliferation, invasion, and metastasis of tumors. In addition, autophagy can also serve as a cellular defense mechanism, maintaining functional mitochondria, reducing DNA damage, and thus maintaining tumor development, and even promoting tumor tissue metastasis and drug resistance (87). Research has shown that platycodin D can trigger autophagy in different cancer cells (A549, H358, MCF7, HT29, and HepG2) (88, 89). In non-small cell lung cancer, Zhao et al. (90) found that P. grandiflorum induced autophagy in NCI-H460 and A549 cells by inhibiting the PI3K/AKT/mTOR signaling pathway and activating the JNK/MAPK signaling pathway. In liver cancer, platycodin D has anti-tumor effects of inhibiting BEL-7402 cell proliferation and inducing cell apoptosis. At the same time, it can trigger autophagy by activating the ERK and JNK pathways (91). In liver cancer research, luteolin can reduce the activity of SMMC-7721 cells, induce apoptosis, upregulate Caspase-8, downregulate the expression level of Bcl-2, and promote the transformation of LC-I to LC3-II, increase the expression of Beclin-1, and activate autophagy (92). In gastric cancer research, apigenin inhibited APG induced autophagy through endoplasmic reticulum stress and inhibited HIF-1α inducing endoplasmic reticulum stress and autophagy with Ezh2 to activate AGS tumor cell death (93).
In summary, this study found that P. grandiflorum can inhibit tumor progression through multiple components, multiple targets, and multiple pathways. Based on literature review, among its active components, platycodin D has been the most extensively studied for its anti-tumor effects, with flavonoids (such as apigenin and luteolin) and polysaccharides also playing important roles. Its inhibitory effects have been demonstrated in various tumor cells (e.g., lung cancer cells, hepatoma cells), primarily through the PI3K/AKT signaling pathways. In addition, P. grandiflorum exerts its anti-tumor effects by inhibiting tumor cell proliferation, promoting apoptosis, and preventing metastasis, etc. The anticancer signaling pathways of P. grandiflorum extract are illustrated in Figure 3. Furthermore, given the diverse molecular mechanisms underlying cancer, the mechanisms involved in the tumor-inhibiting process of P. grandiflorum extracts and their active components are shown in Figure 4.
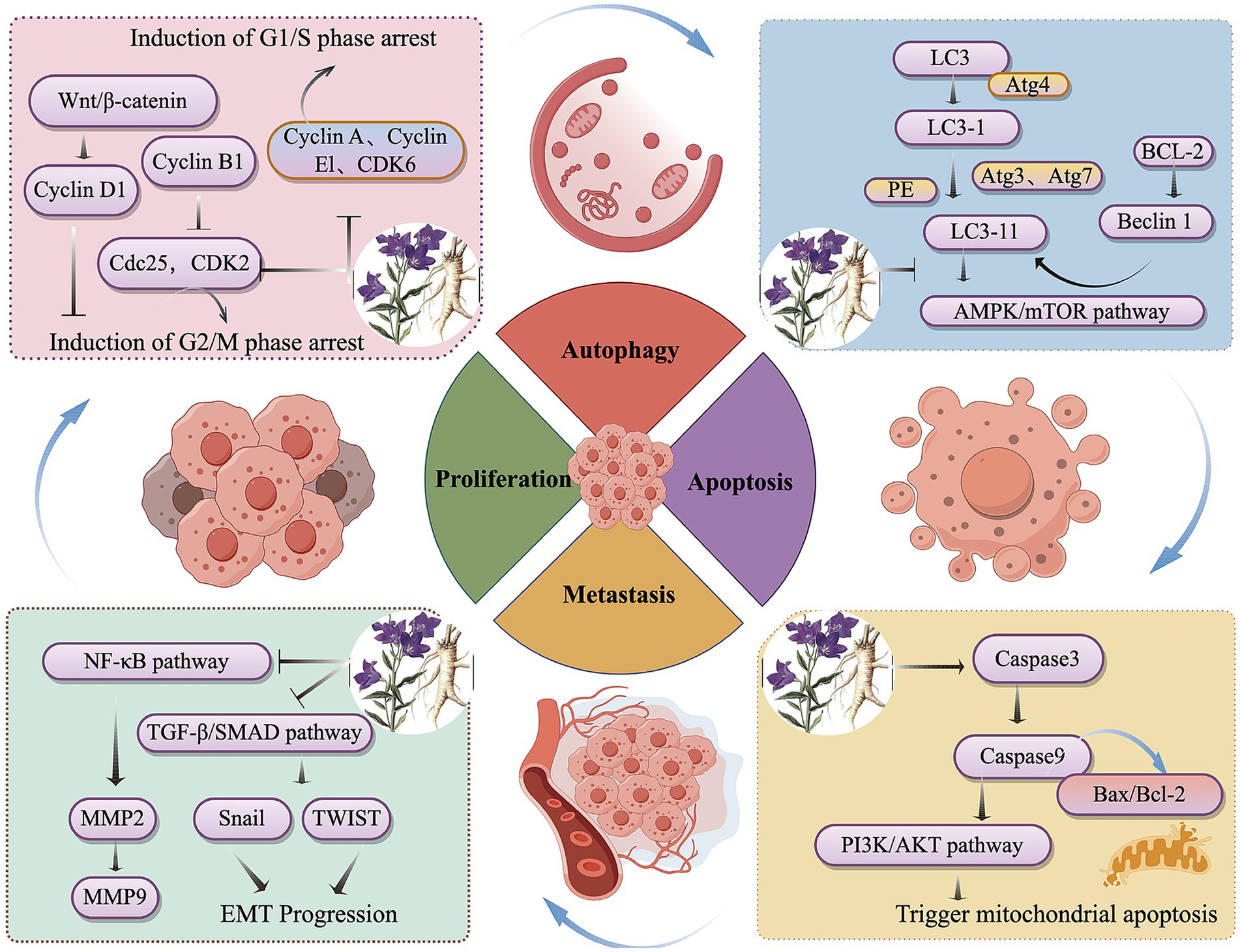
Figure 3. Mechanism of the active of ingredients in P. grandiflorus anti-tumors (by Figdraw, http://www.figdraw.com/).
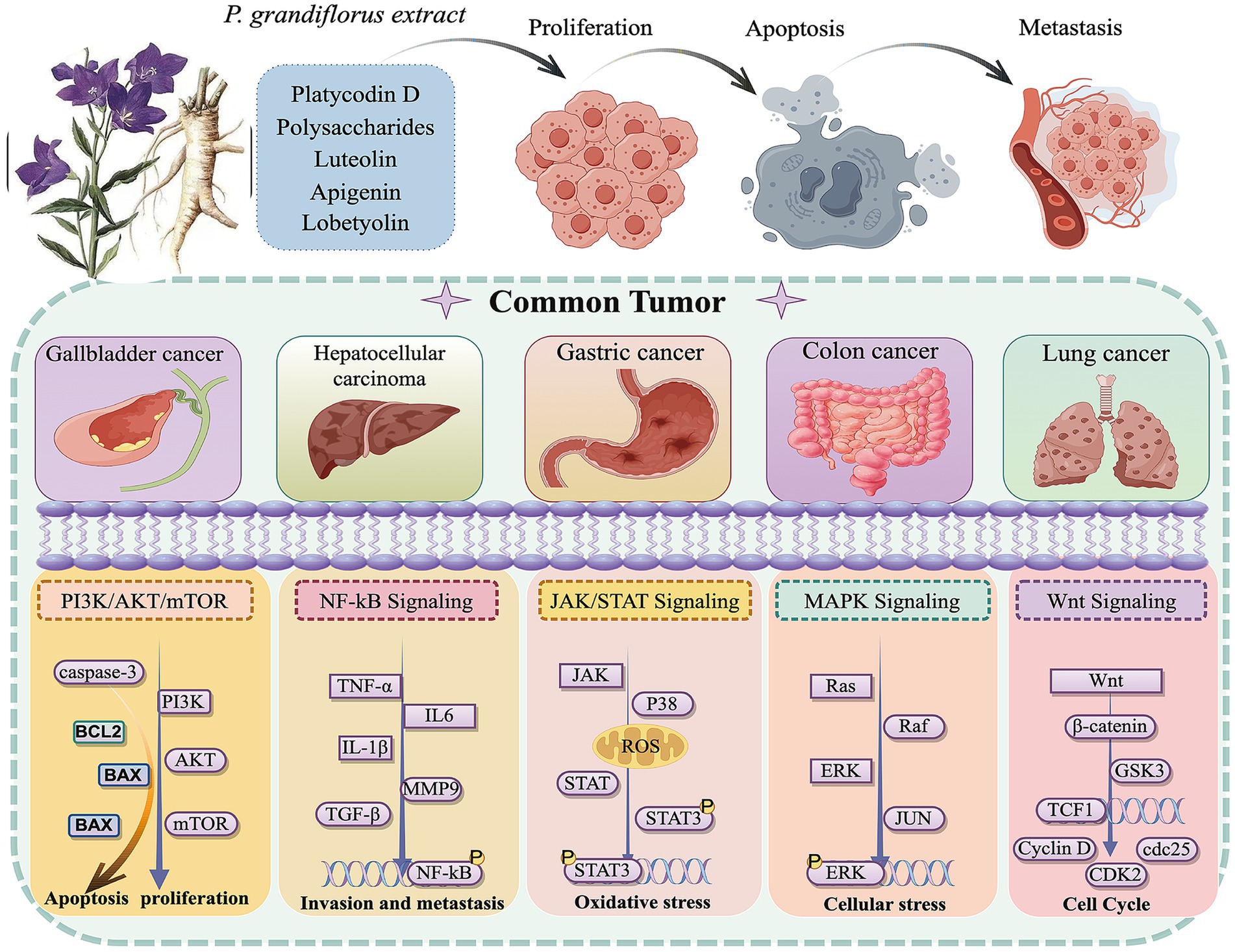
Figure 4. Ṭhe extract of P. grandiflorus inhibits cancers (such as Gallbladder cancer, Hepatocellular cancer, Gastric cancer, Lung cancer) through multiple signaling pathways (by Figdraw, http://www.figdraw.com/).
6 The effect-enhancing and toxicity-reducing of the active ingredient derived from P. grandiflorum
6.1 The protective effects on the liver and kidneys
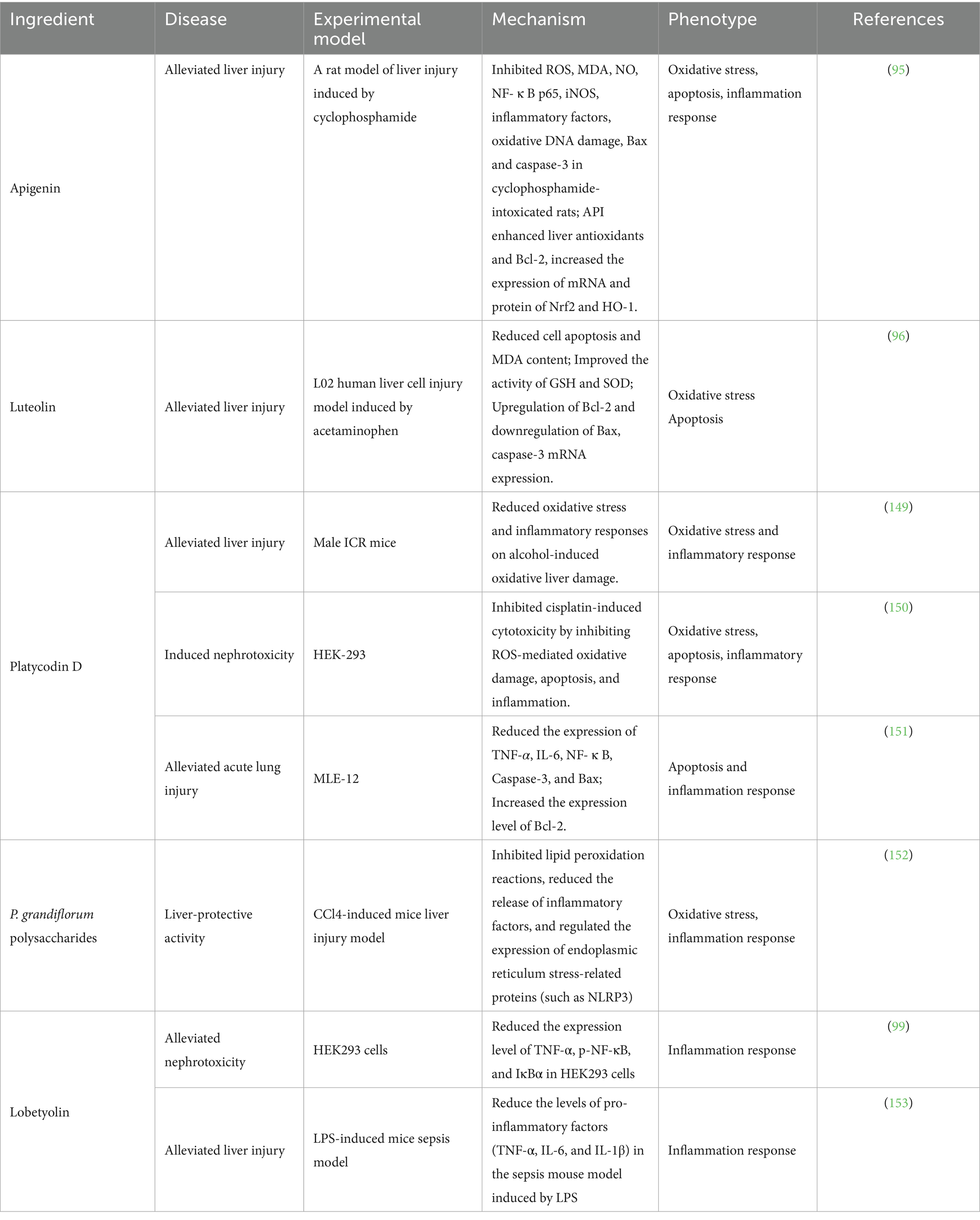
Some active ingredients in P. grandiflorum not only have anti-cancer effects, but also have functions such as protecting the liver and reducing nephrotoxicity. Kim et al. (94) found that the fermentation extract of P. grandiflorum can alleviate acute liver injury induced by endotoxin in mice through reducing liver cell apoptosis and oxidative stress. Platycodin D protected against liver damage from acetaminophen by inhibiting oxidative stress and cell apoptosis. In addition, platycodin D can inhibit cisplatin-induced cell damage through various pathways, reverse cell apoptosis induced by Bax, Bad, Caspase-3, Caspase-9, and modulate PI3K/Akt and ERK/JNK/p38 signaling pathways, providing evidence for reduced cisplatin-induced nephrotoxicity. Apigenin has antioxidant, liver protective, anti-viral, and anti-inflammatory effects. In the treatment of a rat model of liver injury induced by chemotherapy drug cyclophosphamide, it can reduce liver function indicators, prevent liver cell damage, and inhibit ROS, LPO, NF- κ B. Pro-inflammatory mediators and indicators of apoptosis by activating Nrf2 signaling and reducing oxidative damage and inflammatory responses (95). Similarly, luteolin can also have a protective effect on liver cell damage caused by acetaminophen (96). P. grandiflorum polysaccharides also have certain liver-protective activity. Hou et al. (97) prepared nano-selenium saikosaponin complexes using the vitamin C reduction method. They administered the drugs by gavage and compared the protective effects of the nano-selenium saikosaponin complex and saikosaponin on liver-damaged mice. In addition, Liu et al. (98) found that the P. grandiflorum polysaccharides also have anti-inflammatory and antioxidant effects, and can regulate the immune function of the colon. They can alleviate ulcerative colitis caused by DSS through the mesenteric lymph circulation. Hou et al. (99) found that Lobetyolin, a Q-marker isolated from radix of P. grandiflorum, exerted protective effects on cisplatin-induced cytotoxicity in HEK293 cells (Figure 5; Table 4).
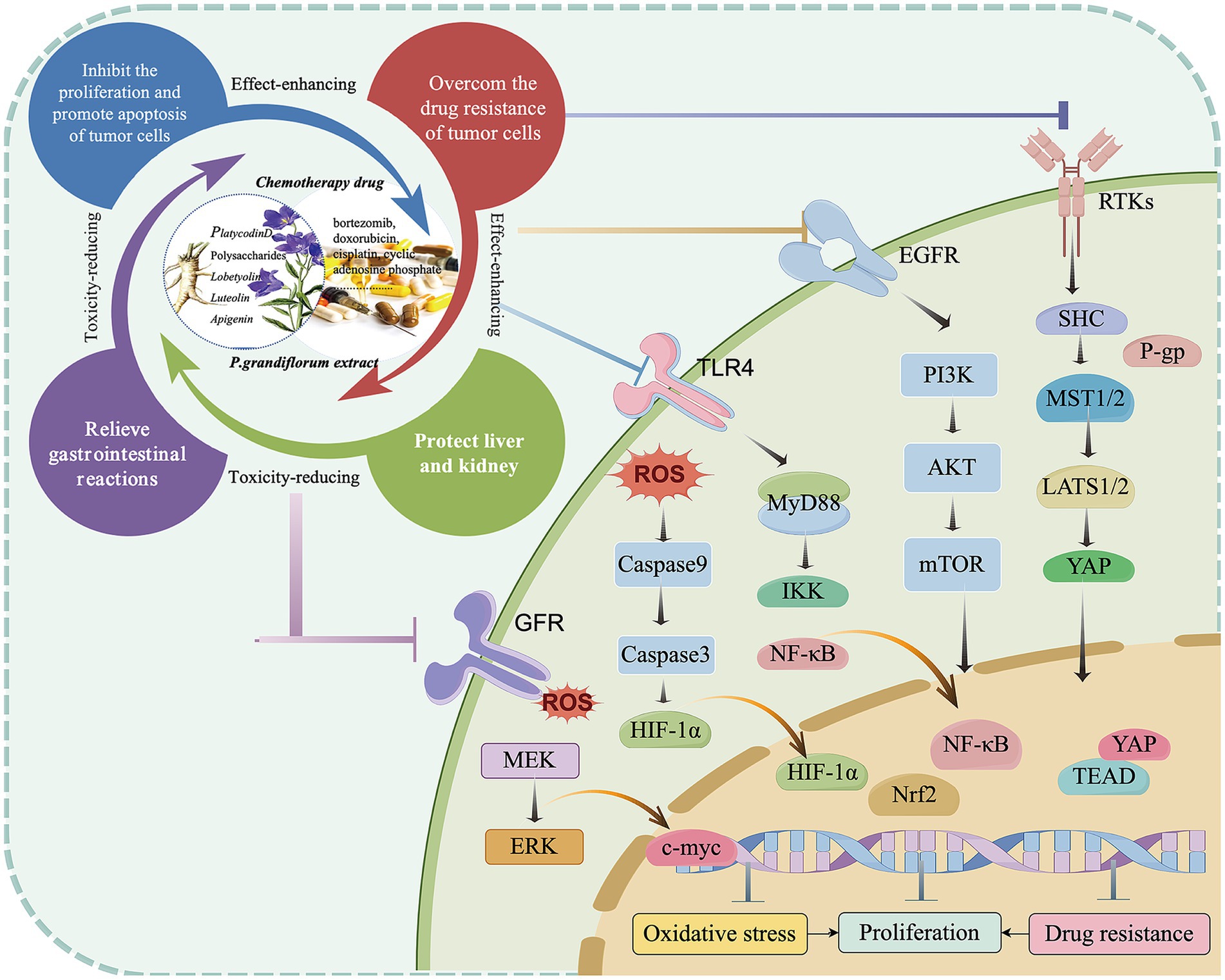
Figure 5. The effect-enhancing and toxicity-reducing molecular mechanism of the active ingredient derived from P. grandiflorus (by Figdraw, http://www.figdraw.com/).
In summary, the primary active components of P. grandiflorum listed in the table—including platycodin D, flavonoids, polysaccharides, and lobetyolin—have demonstrated significant liver and kidney protective effects in both in vitro cell experiments and animal models. Their key mechanism involves regulating key signaling pathways such as PI3K/AKT, ERK/JNK, and NF-κB, thereby inhibiting oxidative stress responses and inflammatory cascades, which contributes to their organ-protective effects. The emphasis of these components is different: platycodin D exhibits liver and kidney protective effects in vitro and in vivo, and also protects against acute lung injury; flavonoids show notable protective effects against acute liver injury in animal experiments; polysaccharides have confirmed liver protective effects in animal studies; and lobetyolin demonstrates liver and kidney protective potential in both in vitro and in vivo models. However, current research is limited to in vitro and animal studies, with no published clinical data available. Due to the absence of human pharmacokinetic information (such as drug concentration distribution in blood and target tissues), clinical efficacy indicators (such as symptom improvement and pathological changes), and safety evaluations (including adverse event incidence and dose tolerance), it is currently impossible to accurately assess the actual efficacy and potential risks of these components in humans. Therefore, their clinical translational value requires further validation through well-designed, standardized clinical trials.
6.2 Combined use of chemotherapeutic drugs
Chinese medicines can significantly enhance sensitivity to chemotherapeutic drugs, enhance tumor suppression and inhibit adverse reactions to chemotherapeutic drugs (100). Currently, there are more studies on Chinese medicine monomers and their active ingredients, and the combination of Chinese medicine with anti-tumor chemotherapeutic drug treatment can achieve significant tumor inhibition, reduce drug resistance, and improve adverse reactions and patients’ quality of life (101). Natural compounds frequently exhibit more diverse “Drug like” and “Biological friendliness” characteristics than pure synthetic compounds, and have enormous potential in future drug research and development. The literature consistently demonstrates that the most valuable aspect of the main active ingredients of P. grandiflorum lies in its ability to exert anti-tumor activity and synergistic effects with various anticancer chemotherapy drugs, while virtually having no cytotoxic effects on normal cells, and also having excellent auxiliary properties. P. grandiflorum has low toxicity and side effects, which is a traditional Chinese herbal medicine with similar medicinal and nutritional uses and has great potential for development. For example, platycodin D is widely used in combination therapy and adjuvant therapy to enhance anticancer and adjuvant activity. Huang et al. (102) found that the synergistic effect of platycodin D and sorafenib inhibited p-AKT, thereby promoting sorafenib induced cell apoptosis and G0/G1 phase cell cycle arrest in the PC3 cell line. In multiple myeloma cell lines, Wu et al. (103) demonstrated that platycodin D and bortezomib (BTZ) also exhibited this synergistic effect, significantly increasing the apoptosis of NCI-H929 and U266B1 cells. In addition, Tan et al. (104) found platycodin D and doxorubicin (DOX) worked synergistically to increase cellular uptake of doxorubicin, enhance its inhibitory effect on cell proliferation, and inhibit DOX anti-inflammatory effect in MCF-7 and MDA-MB-231 cells. Lei et al. (105) discovered that platycodin D, without damaging the membranes of organelles, can promote the escape within the body, thereby reversing the drug resistance of HepG2 cells to chemotherapy drugs. Zhang et al. (106) observed that the aqueous extract of P. grandifloras and platycodin D can affect the Cyclophosphamide (CTX) pharmacokinetic parameters (delays the absorption process of CTX in the body, reduces the peak concentration, and makes the absorption process more gradual). The combination of P. grandiflorum extract or platycodin D has a reducing-toxicity and enhancing-effect significance on CTX. To sum up, these results indicates that platycodin D may enhance efficacy and reduce toxicity by reversing drug resistance and influencing the pharmacokinetics of chemotherapeutic drugs.
In addition to platycodin D, the combination of some flavonoids in P. grandiflorum with doxorubicin can also alter the pharmacokinetics of doxorubicin in vivo. For instance, the combination of apigenin and doxorubicin can hinder the growth of HepG2 cells, increase the G0/G1 phase arrest of liver cancer cells, and increase the apoptosis of hepatocellular carcinoma (107). Similarly, Ahmed et al. (108) found that the combined of luteolin and doxorubicin can enhance the cytotoxicity of breast cancer, inhibit its proliferation and promote apoptosis. Chen et al. (109) found that luteolin increased the sensitivity of cisplatin induced apoptosis in HepG2 cells by upregulating p-JNK protein expression. Overall, studies have shown that the combination of the active ingredients of P. grandiflorum and anti-tumor chemotherapy drugs (bortezomib, doxorubicin, cisplatin, cyclic adenosine phosphate) performed adequately in promoting the uptake of therapeutic drugs, promoting therapeutic efficacy, and reducing the damage of chemotherapy drugs to normal cells. Therefore, the active ingredients of P. grandiflorum can be used as a synergistic herbal medicine for treating tumors and has broad clinical application potential.
7 New drug delivery systems anti-tumor-nanotechnology
The natural extracts of P. grandiflorum (such as platycodin D, flavonoids, and polysaccharides) exhibit significant antitumor activity. However, these active components generally suffer from low water solubility, and the amount that enters the bloodstream via traditional administration routes is insufficient—this not only impairs their oral bioavailability but also restricts their clinical application (110). Therefore, improving the bioavailability of the active components from P. grandiflorum is crucial for the prevention and treatment of cancer. Studies have shown that nanodelivery systems can significantly enhance therapeutic efficacy by prolonging drug circulation time, reducing side effects, improving bioavailability, and achieving tissue targeting (111). In recently years, the widely studied nanoparticulate drug delivery systems mainly include solid lipid nanoparticles, liposomes, chitosan nanoparticles, polymeric micelles, hydrogels, etc. (112). The differences in their key properties are determined by carrier materials, structural design, and functional positioning.
Liposomes research is the most extensive, which exhibit advantages such as low toxicity, high targeting ability, high biodegradability, and ease of functionalization. These properties enable them to facilitate the targeted delivery of the drugs they carry, thereby exerting unique advantages in tumor therapy (113). Among them, Solid lipid nanoparticles (SLNs) have a wide range of applications in drug delivery systems, which can be used to encapsulate drugs and achieve targeted delivery to improve drug efficacy and reduce adverse reactions (114). Polymer nanoparticles are mainly used for the surface modification of small molecules like RNA and proteins, with the aim of enhancing the targeting ability of antitumor drugs (115). Hydrogels are composed of hydrophilic polymers and are similar to the extracellular matrix, possessing good biocompatibility. They can be modified through processes that prevent the degradation of biomolecules during delivery while maintaining the sustained release of drugs (116). Chitosan nanoparticles have good biocompatibility and safety. They are suitable for oral administration, capable of overcoming the intestinal barrier, and exhibit sustained and controlled release properties. Furthermore, they have no restrictions on gene size and are simple to prepare (117). Currently, Conjugated nanodelivery systems, such as polymer-lipid hybrid nanoparticles (PLHNPs), not only have strong stability and high drug-loading capacity but also possess long circulation time and excellent biodegradability (118). In addition to the aforementioned organic nanoparticles, inorganic nanoparticles (such as silica, iron oxide, gold nanoparticles, and selenium nanoparticles, etc.) have attracted considerable attention in cancer treatment. They can be used both as carriers for drug delivery and as active pharmaceutical ingredients themselves, exhibiting great potential and safety in the field of medical applications (119).
Notably, the new drug delivery system has significantly improved the anti-tumor activity of P. grandiflorum extract. Consequently, the integration of P. grandiflorum extract with nanotechnology held considerable promise for the future development of clinical anti-tumor drugs (as shown in Figure 6). Firstly, the combination of platycodin D and liposomes nanoparticles becomes an effective drug delivery method and effectively improves drug stability, bioavailability, targeting of platycodin D and prolong its circulating time in vivo. Kim et al. (120) demonstrated that the combination of platycodin D and liposome nanotechnology enables the delivery of therapeutic cargoes to cells, offers enhanced protection for drugs during the delivery process, and exhibits no hepatotoxicity. In colorectal cancer, we developed platycodin D2 and liposomes as a tumor-targeting therapy. The platycodin D2-liposomes treatment demonstrated a successful tumor-targeting ability in the colorectal cancer xenografts, in which PCD2 not only exerted a potent antitumor effect by inducing apoptotic cell death and but also functioned as a liposome membrane stabilizer (121). Luteolin, as one of the flavonoids in P. grandiflorum. Cao et al. (122) developed luteolin-loaded nanoliposomes that target the PG-1 receptor on the surface of liver cancer cells, enhancing drug accumulation at the tumor site. Meanwhile, these nanoliposomes increase the expression of lactate dehydrogenase (LDH), disrupting tumor growth pathways and thereby addressing the compound’s low oral bioavailability. Adel et al. (123) found that apigenin bound liposomes enhanced the bioavailability and chemical stability of apigenin in breast cancer, thereby enhancing the anti-breast cancer effect. In the xenograft tumor models of human colorectal cancer HCT-15 and HT-2 cells (124), Apigenin liposomes not only enhance the apoptosis-inducing effect on tumor cells, but also improve the hemocompatibility and cytocompatibility with normal fibroblasts, thereby reducing drug toxicity. In addition to liposome nanoparticles, chitosan nanoparticles (ChNPs) can also introduce chemotherapy drugs into cells, increase their activity, and release encapsulated compounds over time (125). In liver cancer, Mabrouk et al. (126) first added apigenin to chitosan to increase its hydrophobicity, and then coated the chitosan surface with folate albumin to increase its stability and bioavailability. Shi et al. (127) found an oral herbal system-nanoparticle mixture (HNS), which is formed through self-association of P. grandiflorum-Curcuma zedoaria, and combined with lipid-polymer nanoparticles (LPNs). HNS induced cell apoptosis and regulated the expression of MMP-9 and TGF-β1, thereby reducing lung metastasis in 4 T1 tumor-bearing mice and altering the tumor microenvironment. In addition, more and more evidence indicated (128) that P. grandiflorum polysaccharides can be used as a stabilizer to modify nano-selenium particles, thereby enhancing their stability in solution. The polysaccharide PGP-AE can inhibit gastric cancer cells in vitro, but its anti-tumor effect in vivo is not significant; while PGP90 needs to be combined with nano-selenium to significantly enhance its activity, indicating that nano-modification is crucial for the efficacy of the drug.
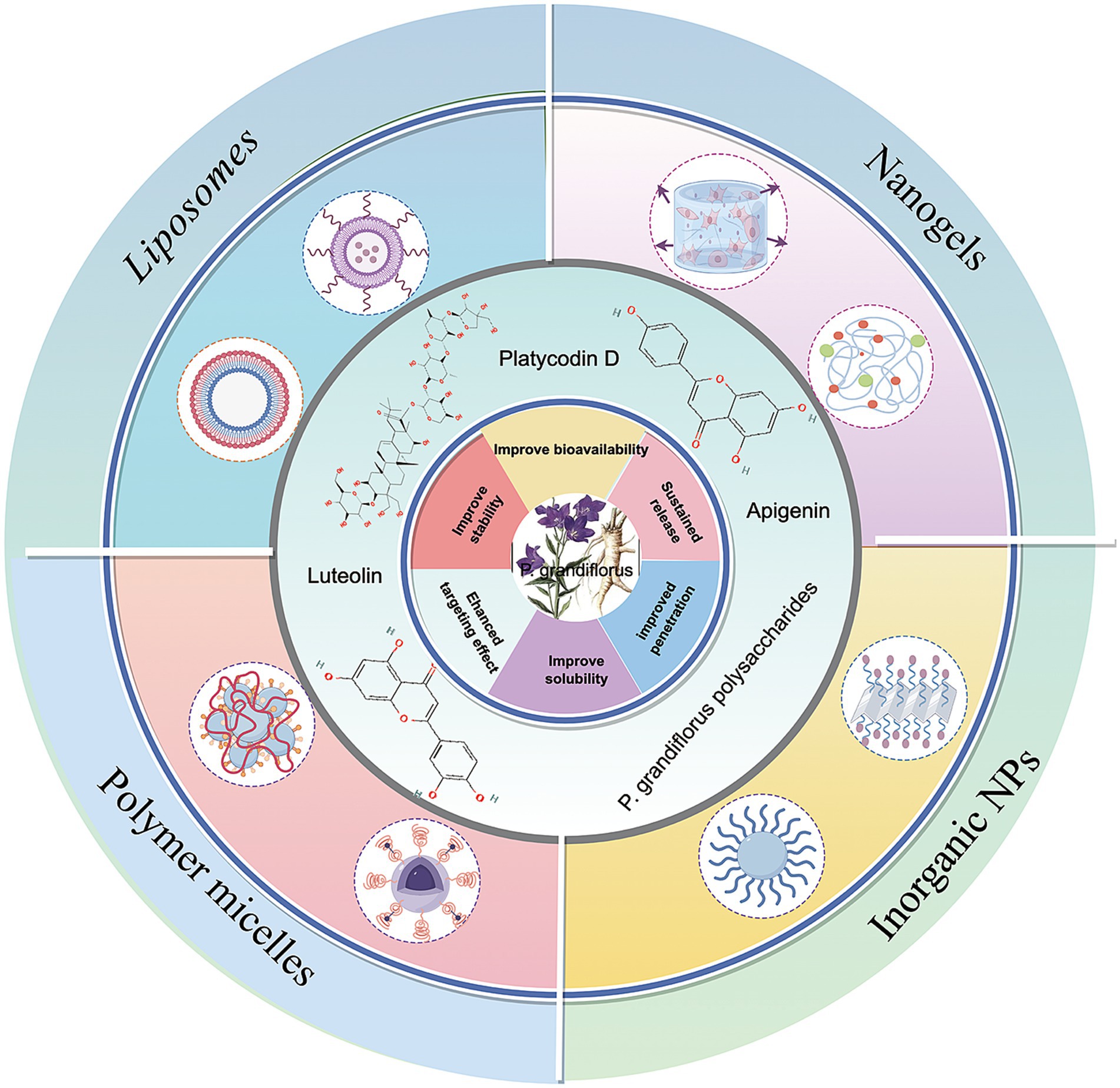
Figure 6. Application of P. grandiflorus extract in nanoparticle drug delivery system (by Figdraw, http://www.figdraw.com/).
P. grandiflorum extract combined with nanoparticles have been widely studied in cancer treatment. In conclusion, platycodin D mainly exerts its effects by combining with liposomes, while flavonoid components mostly enhance antitumor efficacy with the help of solid nanoparticles, liposomes, and chitosan nanocarriers. In addition, polymer-based nano-delivery systems are often combined with polysaccharide components, which can significantly improve anti-cancer activity. It can be seen that nanopharmaceutical delivery systems address issues such as low solubility and poor bioavailability of the active components from P. grandiflorum, significantly optimizing them in vivo pharmacokinetic and pharmacodynamic properties, and providing a feasible technical pathway for the clinical translation of these active components.
8 Homology of medicine and food: preventive healthcare
8.1 Clinical trials
In the past decade, researchers have discovered the new pharmacological potential of platycodin in the treatment of chronic conditions, including hyperlipidemia, hypertension, diabetes and obesity (129). P. grandiflorum, as a homologous plant of medicine and food, not only exhibits anti-tumor potential, which also plays many important roles in prevention and healthcare (15). We performed a search using the keywords “Platycodon grandiflorum” and “preventive healthcare” to identify relevant clinical trials on the International Clinical Trials Registry Platform10 (accessed June 12, 2025). The search results indicated (130) that P. grandiflorum extract (GCWB107) can reduce blood lipids in patients with obesity (trial registration number: NCT04023864). Another study discovered (131) that P. grandiflorum extract can enhance human immune function (trial registration number: KCT0005945). In addition, P. grandiflorum extract mixture also can effectively improve oxidative stress (trial registration number: KCT0007161).
These preliminary trials indicate safety, but efficacy data are limited. In addition, P. grandiflorum oral medicine may have certain potential adverse reactions, with common ones including gastrointestinal discomfort and allergic reactions; high-dose use or long-term administration may also cause liver damage. In terms of drug-nutrient interactions, concurrent use of these preparations with anticoagulant drugs may increase the risk of bleeding, while co-administration with gastrointestinal drugs can affect the absorption of their own active ingredients, resulting in absorption disorders (132). Therefore, further clinical verification of the efficacy of P. grandiflorum is required. Only through clinical trials can the optimal dosage (suitable for specific populations) and administration method be determined, and its side effects and potential risks be comprehensively evaluated. This process ensures that the drug data are thoroughly validated and comply with research and regulatory standards.
8.2 Preventive healthcare product
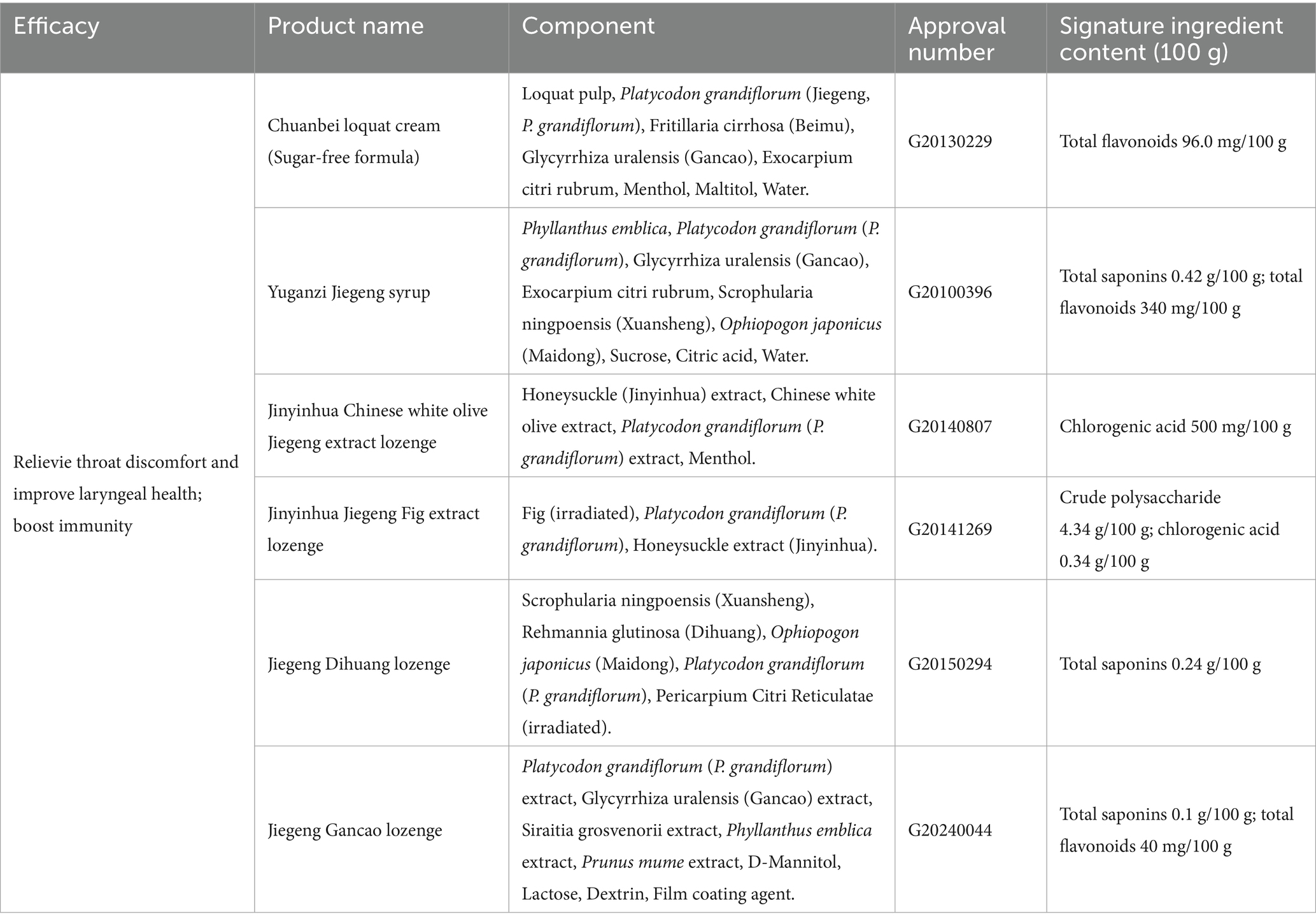
P. grandiflorum is integrated into daily therapeutic or preventive medicine on the basis of its properties as both a drug and a food. This practice not only improves the edible value of P. grandiflorum, but also maximizes the medicinal value of P. grandiflorum. Currently, there are numerous health products containing P. grandiflorum as a primary ingredient in treatments and preventive medications available on the market. A search of the Special Food Information Query Platform11 (accessed June 22, 2025) of China’s State Administration for Market Regulation reveals that there are currently 11 health products with a “National Food Health Note” on the market. The P. grandiflorum-based health products are recognized not only for their ability to alleviate throat discomfort and enhance laryngeal health but also for their role in boosting immunity, as shown in Table 5. However, P. grandiflorum preparations still face challenges in regulation and dose standardization. Specifically, natural medicine complex composition—containing saponins, polysaccharides, flavonoids, and other active substances—further exacerbates difficulties in standardization; meanwhile, variations in raw materials (e.g., variety, origin) and dosage forms can cause fluctuations in the content and absorption efficiency of active ingredients, rendering precise dose control difficult (133). Therefore, to ensure the quality of health products, it is essential to emphasize clinical validation and strengthen regulatory oversight of products classified under “food-medicine homology.
9 Summary and future prospects
Cancer is a complex malignancy, not only because of its diverse proliferation and metastasis, but also because cancer cells are highly adaptable. Chinese herbal medicines, as natural compounds, often have diverse chemical structures and biological activities, and are widely studied and applied in the prevention and treatment of multiple tumors. Through an analysis of relevant literature and network pharmacological results, this paper showed that the anti-tumor active ingredients of P. grandiflorum include platycodin D, P. grandiflorum polysaccharides, Lobetyolin, luteolin, and apigenin. In this paper, we investigated the mechanism of P. grandiflorum on cell proliferation, cell cycle, and cell migration based on its active components. It has been found that platycodin D mainly hindered cancer cell proliferation and induced cancer cell apoptosis, which is the main mechanism for producing anti-cancer activity. Apigenin mainly inhibited tumor growth, induced endogenous apoptosis and autophagy, and inhibited angiogenesis as the main mechanism for producing anti-cancer activity. Luteolin and Lobetyolin mainly inhibited the growth, migration and invasion of tumor cells, which were the main mechanisms of tumor activity. P. grandiflorum polysaccharides can stimulate the activity of immune cells and macrophages, enhance the functions of T cells and NK cells, thereby improving the body’s immune surveillance and clearance ability against tumors. Additionally, we explored the application of extracts from P. grandiflorum in modern drug development. These extracts can be combined with certain chemotherapy drugs to target tumor tissues based on their anti-tumor effects, ultimately enhancing efficacy while reducing toxicity. With the emergence of new drug delivery systems, the challenges of low utilization and poor solubility of active ingredients in traditional oral Chinese medicine have been extensively studied. Among them, Nano-delivery systems have the advantages of high bioavailability, strong targeting ability, and good sustained release effect, gradually improving the limitations of P. grandiflorum extract anti-tumor applications. Furthermore, as an herbal medicine, P. grandiflorum has a long history of medicinal and edible use. In recently years, P. grandiflorum also plays many important roles in prevention and health care of cancer. However, there is a lack of large-scale, rigorously designed, and well-implemented clinical trials to confirm the anti-cancer properties of P. grandiflorum. With the continued development of research methods and the deepening of the level of research, it is worth looking forward to the application of the active ingredients of P. grandiflorum in the development of clinical drug formulations with anti-tumor effects. Therefore, in the future, we need to strengthen a series of clinical application research based on sufficient basic research, expanding the scope of clinical application from conventional applications to various fields such as cancer, antiviral agents, vaccine adjuvants, etc.
Author contributions
WL: Writing – original draft. SJ: Visualization, Writing – review & editing. XM: Software, Writing – review & editing. DL: Visualization, Writing – review & editing. YD: Writing – review & editing. ZZ: Software, Writing – review & editing. LY: Writing – review & editing. RY: Conceptualization, Writing – review & editing. YN: Conceptualization, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Ningxia Key Research and Development Program (No.2023BEG02015) and the Characteristic Discipline Project of Ningxia Medical University (TSXK2025007).
Acknowledgments
The authors acknowledge any support given which is not covered by the authors’ contribution or funding sections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1674705/full#supplementary-material
Footnotes
1. ^https://pubmed.ncbi.nlm.nih.gov
2. ^https://scholar.google.com
3. ^https://www.sciencedirect.com
4. ^https://www.webofscience.com
6. ^https://www.uniprot.org/uniprot/
7. ^http://swisstargetprediction.ch/
References
1. Beaumont, JEJ, Beelen, NA, Wieten, L, and Rouschop, KMA. The immunomodulatory role of hypoxic tumor-derived extracellular vesicles. Cancer. (2022) 14:4001. doi: 10.3390/cancers14164001
2. Lu, W, Chen, T, Xiao, D, Qin, X, Chen, Y, and Shi, S. Application and prospects of nucleic acid nanomaterials in tumor therapy. RSC Adv. (2023) 13:26288–301. doi: 10.1039/D3RA04081J
3. Zhou, Y, Farooqi, AA, and Xu, B. Comprehensive review on signaling pathways of dietary saponins in cancer cells suppression. Crit Rev Food Sci Nutr. (2023) 63:4325–50. doi: 10.1080/10408398.2021.2000933
4. Yan, Z, Lai, Z, and Lin, J. Anticancer properties of traditional Chinese medicine. Comb Chem High Throughput Screen. (2017) 20:423–9. doi: 10.2174/1386207320666170116141818
5. Zhang, L, Wang, Y, Yang, D, Zhang, C, Zhang, N, Li, M, et al. Platycodon grandiflorus - an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. (2015) 164:147–61. doi: 10.1016/j.jep.2015.01.052
6. Ma, C-H, Gao, Z-J, Zhang, J-J, Zhang, W, Shao, J-H, Hai, M-R, et al. Candidate genes involved in the biosynthesis of triterpenoid Saponins in Platycodon grandiflorum identified by transcriptome analysis. Front Plant Sci. (2016) 7:673. doi: 10.3389/fpls.2016.00673
7. Kim, Y-A, Oh, S-H, Lee, G-H, Hoa, PT, Jin, SW, Chung, YC, et al. Platycodon grandiflorum-derived saponin attenuates the eccentric exercise-induced muscle damage. Food Chem Toxicol. (2018) 112:150–6. doi: 10.1016/j.fct.2017.12.045
8. Jiang Tao, Z, and Juxiong, LL. Research progress in targeted breeding of medicinal and edible Platycodon grandiflorum. Modern Distance Educ Tradit Chin Med China. (2018) 16:158–60.
9. Lee, S, Han, EH, Lim, M-K, Lee, S-H, Yu, HJ, Lim, YH, et al. Fermented Platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity in vivo. J Med Food. (2020) 23:1060–9. doi: 10.1089/jmf.2019.4595
10. Noh, E-M, Kim, J-M, Lee, HY, Song, H-K, Joung, SO, Yang, HJ, et al. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement Altern Med. (2019) 19:322. doi: 10.1186/s12906-019-2724-0
11. Wang, Y, Zhang, X, Wei, Z, Wang, J, Zhang, Y, Shi, M, et al. Platycodin D suppressed LPS-induced inflammatory response by activating LXRα in LPS-stimulated primary bovine mammary epithelial cells. Eur J Pharmacol. (2017) 814:138–43. doi: 10.1016/j.ejphar.2017.07.037
12. Wang, B, Tu, Y, Zhao, SP, Hao, YH, Liu, JX, Liu, FH, et al. Effect of tea saponins on milk performance, milk fatty acids, and immune function in dairy cow. J Dairy Sci. (2017) 100:8043–52. doi: 10.3168/jds.2016-12425
13. Kiran, A, Altaf, A, Sarwar, M, Malik, A, Maqbool, T, and Ali, Q. Phytochemical profiling and cytotoxic potential of Arnebia nobilis root extracts against hepatocellular carcinoma using in-vitro and in-silico approaches. Sci Rep. (2023) 13:11376. doi: 10.1038/s41598-023-38517-8
14. Zhang, S, Chai, X, Hou, G, Zhao, F, and Meng, Q. Platycodon grandiflorum (Jacq.) a. DC.: a review of phytochemistry, pharmacology, toxicology and traditional use. Phytomedicine. (2022) 106:154422. doi: 10.1016/j.phymed.2022.154422
15. Nyakudya, E, Jeong, JH, Lee, NK, and Jeong, Y-S. Platycosides from the roots of Platycodon grandiflorum and their health benefits. JFN. (2014) 19:59–68. doi: 10.3746/pnf.2014.19.2.059
16. Su, X, Liu, Y, Han, L, Wang, Z, Cao, M, Wu, L, et al. A candidate gene identified in converting platycoside E to platycodin D from Platycodon grandiflorus by transcriptome and main metabolites analysis. Sci Rep. (2021) 11:9810. doi: 10.1038/s41598-021-89294-1
17. Wang, Z, Wang, P, Cao, H, Liu, M, Kong, L, Wang, H, et al. Genome-wide identification of bZIP transcription factors and their expression analysis in Platycodon grandiflorus under abiotic stress. Front Plant Sci. (2024) 15:1403220. doi: 10.3389/fpls.2024.1403220
18. Wang, C, Zhang, N, Wang, Z, Qi, Z, Zhu, H, Zheng, B, et al. Nontargeted Metabolomic analysis of four different parts of Platycodon grandiflorum grown in Northeast China. Molecules. (2017) 22:1280. doi: 10.3390/molecules22081280
19. Luo, Q, Wei, G, Wu, X, Tang, K, Xu, M, Wu, Y, et al. Platycodin D inhibits platelet function and thrombus formation through inducing internalization of platelet glycoprotein receptors. J Transl Med. (2018) 16:311. doi: 10.1186/s12967-018-1688-z
20. Guo, R, Meng, Q, Wang, B, and Li, F. Anti-inflammatory effects of Platycodin D on dextran sulfate sodium (DSS) induced colitis and E. coli lipopolysaccharide (LPS) induced inflammation. Int Immunopharmacol. (2021) 94:107474. doi: 10.1016/j.intimp.2021.107474
21. Shuting, Z, Miaomiao, T, Zheng, X, and Wei, H. The historical evolution, product development, and modern research Progress of Platycodon grandiflorum processing. Specialty Res (2023):1–7. doi: 10.16720/j.cnki.tcyj.2023.018
22. Mazol, I, Gleńsk, M, and Cisowski, W. Polyphenolic compounds from Platycodon grandiflorum a. DC Acta Pol Pharm. (2004) 61:203–8.
23. Huang, L, Kim, M-Y, and Cho, JY. Immunopharmacological activities of Luteolin in chronic diseases. Int J Mol Sci. (2023) 24:2136. doi: 10.3390/ijms24032136
24. Pang, D-J, Huang, C, Chen, M-L, Chen, Y-L, Fu, Y-P, Paulsen, BS, et al. Characterization of inulin-type Fructan from Platycodon grandiflorus and study on its prebiotic and Immunomodulating activity. Molecules. (2019) 24:1199. doi: 10.3390/molecules24071199
25. Liu, Z-J, Wang, Y-L, Li, Q-L, and Yang, L. Improved antimelanogenesis and antioxidant effects of polysaccharide from Cuscuta chinensis lam seeds after enzymatic hydrolysis. Braz J Med Biol Res. (2018) 51:e7256. doi: 10.1590/1414-431x20187256
26. Jun, Z, BoBai, Y, and Yang, HX. Research progress on chemical components and modern pharmacology of Platycodon grandiflorum. J Liaoning Univ Tradit Chin Med. (2019) 21:113–6. doi: 10.13194/j.issn.1673-842x.2019.01.031
27. Park, MJ, Ryu, HS, Kim, JS, Lee, HK, Kang, JS, Yun, J, et al. Platycodon grandiflorum polysaccharide induces dendritic cell maturation via TLR4 signaling. Food Chem Toxicol. (2014) 72:212–20. doi: 10.1016/j.fct.2014.07.011
28. Lee, J-Y, Yoon, J-W, Kim, C-T, and Lim, S-T. Antioxidant activity of phenylpropanoid esters isolated and identified from Platycodon grandiflorum a. DC. Phytochemistry. (2004) 65:3033–9. doi: 10.1016/j.phytochem.2004.08.030
29. Xue, X, Zhao, A, Wang, Y, Ren, H, Su, W, Li, Y, et al. Metabolomics and transcriptomics analyses for characterizing the alkaloid metabolism of Chinese jujube and sour jujube fruits. Front Plant Sci. (2023) 14:1267758. doi: 10.3389/fpls.2023.1267758
30. Shi, X, Li, X, Li, X, He, Z, Chen, X, Song, J, et al. Antibacterial properties of TMA against Escherichia coli and effect of temperature and storage duration on TMA content, lysozyme activity and content in eggs. Foods. (2022) 11:527. doi: 10.3390/foods11040527
31. Choi, YH, Yoo, DS, Cha, M-R, Choi, CW, Kim, YS, Choi, S-U, et al. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J Nat Prod. (2010) 73:1863–7. doi: 10.1021/np100496p
32. Singh Tuli, H, Rath, P, Chauhan, A, Sak, K, Aggarwal, D, Choudhary, R, et al. Luteolin, a potent anticancer compound: from chemistry to cellular interactions and synergetic perspectives. Cancers. (2022) 14:5373. doi: 10.3390/cancers14215373
33. Moslehi, M, Rezaei, S, Talebzadeh, P, Ansari, MJ, Jawad, MA, Jalil, AT, et al. Apigenin in cancer therapy: prevention of genomic instability and anticancer mechanisms. Clin Exp Pharmacol Physiol. (2023) 50:3–18. doi: 10.1111/1440-1681.13725
34. Guifeng, Z, Chuang, L, Guangdong, L, and Rentang, Z. Research Progress on antitumor mechanism and structure-activity relationship of plant polysaccharides. Sci Technol Food Ind. (2023) 44:428–37. doi: 10.13386/j.issn1002-0306.2022050063
35. He, W, Tao, W, Zhang, F, Jie, Q, He, Y, Zhu, W, et al. Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J Cellular Molecular Medi. (2020) 24:3359–69. doi: 10.1111/jcmm.15009
36. Juan, L. Extract process and quality standard of platycodongrandiflorum extract. [Master’s Thesis]. Heifei: Hefei University (2009).
37. Ping, S, Hui, X, Yanhong, H, Zerun, L, Yupeng, N, Shanshan, W, et al. Review of extraction methods and pharmacological effect of chemical components from Platycodon grandiflorum. China Brewing. (2022) 41:18–23.
38. Jingyin, G, Zibing, L, Peng, Z, Yuqing, Z, Tao, Z, Tingting, B, et al. General situation of research on quality standard of Jiegeng (Platycodon grandiflorum) and its processed products. Chin Arch Tradit Chin Med. (2024) 42:151–155+287. doi: 10.13193/j.issn.1673-7717.2024.05.030
39. Wei, H, Juanmin, L, Lei, G, Haiyan, F, Weiguo, S, Weidong, H, et al. Determination of the content of platycodin D in Platycodon root by HPLC method. Jiangxi. J Tradit Chin Med. (2014) 45:59–61.
40. Kwon, J, Lee, H, Kim, N, Lee, J-H, Woo, MH, Kim, J, et al. Effect of processing method on platycodin D content in Platycodon grandiflorum roots. Arch Pharm Res. (2017) 40:1087–93. doi: 10.1007/s12272-017-0946-6
41. Xiujuan, Z, Xiaoqin, C, Shaungyin, G, Jutao, W, Daiyin, P, and Chuanshan, J. Content determination of Luteolin and Apigenin in Platycodon Grandiflorus by high-performance liquid chromatography. J Anhui Univ Chin Med. (2013) 32:73–5.
42. Lizhen, Z, Zhuang, M, Xinyue, Y, Niu, S, Xing, SS, Rong, W, et al. Optimization of extraction process of Platycodon grandifloras polysaccharide and its Solubilization effect on Platycodin. JShandong Univ Tradit Chin Med. (2024) 48:708–16. doi: 10.16294/j.cnki.1007-659x.2024.06.011
43. Yan, Z, Jianhe, W, Juan, L, Yue, J, Hongliang, J, Kun, S, et al. Analysis and evaluation of nutritional component of Platycodon grandiflorus in three Main producing areas. Modern Chin Med. (2019) 21:194–8. doi: 10.13313/j.issn.1673-4890.20181017002
44. Xinyue, W, Weimin, W, Nan, W, Ling, S, and Rina, W. Research Progress of Platycodon grandiflorum functionality and food development. Food Res Dev. (2022) 43:199–206.
45. Xing, Y. Extraction of platycodn D and lobetyolin fromPlatycodon grandifloras and catalytic conversion. [Master’s Thesis]. Northeast Forestry University (2023).
46. Bao, C, Xinpei, L, Xiaohui, H, Zhiman, L, Wei, L, and Yinshi, S. HPLC method for simultaneous determination of three polyacetylenes in Platycodonis Radix from different habitats. Chinese. J Pharm Anal. (2018) 38:1484–9. doi: 10.16155/j.0254-1793.2018.09.03
47. Yu, JS, and Kim, AK. Platycodin D induces apoptosis in MCF-7 human breast Cancer cells. J Med Food. (2010) 13:298–305. doi: 10.1089/jmf.2009.1226
48. Luo, J, Manning, BD, and Cantley, LC. Targeting the PI3K-Akt pathway in human cancer. Cancer Cell. (2003) 4:257–62. doi: 10.1016/S1535-6108(03)00248-4
49. Dang, CV, and Lewis, BC. Role of oncogenic transcription factor c-Myc in cell cycle regulation, apoptosis and metabolism. J Biomed Sci. (1997) 4:269–78. doi: 10.1007/BF02258350
50. Khan, M, Maryam, A, Zhang, H, Mehmood, T, and Ma, T. Killing cancer with platycodin D through multiple mechanisms. J Cell Mol Med. (2016) 20:389–402. doi: 10.1111/jcmm.12749
51. Xu, Q, Pan, G, Wang, Z, Wang, L, Tang, Y, Dong, J, et al. Platycodin-D exerts its anti-cancer effect by promoting c-Myc protein ubiquitination and degradation in gastric cancer. Front Pharmacol. (2023) 14:1138658. doi: 10.3389/fphar.2023.1138658
52. Liu, Y, Tian, S, Yi, B, Feng, Z, Chu, T, Liu, J, et al. Platycodin D sensitizes KRAS-mutant colorectal cancer cells to cetuximab by inhibiting the PI3K/Akt signaling pathway. Front Oncol. (2022) 12:1046143. doi: 10.3389/fonc.2022.1046143
53. Zhou, Y, Jin, T, Gao, M, Luo, Z, Mutahir, S, Shi, C, et al. Aqueous extract of Platycodon grandiflorus attenuates lipopolysaccharide-induced apoptosis and inflammatory cell infiltration in mouse lungs by inhibiting PI3K/Akt signaling. Chin Med. (2023) 18:36. doi: 10.1186/s13020-023-00721-z
54. Liu, J, Peng, Y, and Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. (2022) 32:30–44. doi: 10.1016/j.tcb.2021.07.001
55. Zhang, H, Zhang, X, Li, X, Meng, W-B, Bai, Z-T, Rui, S-Z, et al. Effect of CCNB1 silencing on cell cycle, senescence, and apoptosis through the p53 signaling pathway in pancreatic cancer. J Cell Physiol. (2018) 234:619–31. doi: 10.1002/jcp.26816
56. Dai, P, Xiong, L, Wei, Y, Wei, X, Zhou, X, Zhao, J, et al. A pancancer analysis of the oncogenic role of cyclin B1 (CCNB1) in human tumors. Sci Rep. (2023) 13:16226. doi: 10.1038/s41598-023-42801-y
57. Lee, Y, Sung, B, Kang, YJ, Kim, DH, Jang, J-Y, Hwang, SY, et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int J Oncol. (2014) 44:1599–606. doi: 10.3892/ijo.2014.2339
58. Ujiki, MB, Ding, X-Z, Salabat, MR, Bentrem, DJ, Golkar, L, Milam, B, et al. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. (2006) 5:76. doi: 10.1186/1476-4598-5-76
59. Chang, X, Tamauchi, S, Yoshida, K, Yoshihara, M, Yokoi, A, Shimizu, Y, et al. Downregulating vaccinia-related kinase 1 by luteolin suppresses ovarian cancer cell proliferation by activating the p53 signaling pathway. Gynecol Oncol. (2023) 173:31–40. doi: 10.1016/j.ygyno.2023.04.003
60. Kanapathipillai, M. Treating p53 mutant aggregation-associated Cancer. Cancers. (2018) 10:154. doi: 10.3390/cancers10060154
61. Chen, P, Zhang, J-Y, Sha, B-B, Ma, Y-E, Hu, T, Ma, Y-C, et al. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget. (2017) 8:27471–80. doi: 10.18632/oncotarget.15832
62. He, J-Q, Zheng, M-X, Ying, H-Z, Zhong, Y-S, Zhang, H-H, Xu, M, et al. PRP1, a heteropolysaccharide from Platycodonis Radix, induced apoptosis of HepG2 cells via regulating miR-21-mediated PI3K/AKT pathway. Int J Biol Macromol. (2020) 158:542–51. doi: 10.1016/j.ijbiomac.2020.04.193
63. Yanwei, C, and Yijiong, Y. Mechanisms of polysaccharides of radix platycodonis in the proliferation and apoptosis of liver cancer cells via LINC01554. Chin J Cell Stem Cell. (2022) 12:14–8.
64. Li, XG, Gao, S, Yang, WS, and Sun, S. Investigation of the inhibitory effect of Platycodin D in human transitional cell carcinoma cell line 5637. Folia Biol. (2021) 67:37–47. doi: 10.14712/fb2021067010037
65. Zeng, C-C, Zhang, C, Yao, J-H, Lai, S-H, Han, B-J, Li, W, et al. Platycodin D induced apoptosis and autophagy in PC-12 cells through mitochondrial dysfunction pathway. Spectrochim Acta A Mol Biomol Spectrosc. (2016) 168:199–205. doi: 10.1016/j.saa.2016.06.005
66. Zhang, X, Zhai, T, Hei, Z, Zhou, D, Jin, L, Han, C, et al. Effects of Platycodin D on apoptosis, migration, invasion and cell cycle arrest of gallbladder cancer cells. Oncol Lett. (2020) 20:1. doi: 10.3892/ol.2020.12174
67. Zhou, R, Lu, Z, Liu, K, Guo, J, Liu, J, Zhou, Y, et al. Platycodin D induces tumor growth arrest by activating FOXO3a expression in prostate cancer in vitro and in vivo. Curr Cancer Drug Targets. (2015) 14:860–71. doi: 10.2174/1568009614666141128104642
68. Seo, Y-S, Kang, O-H, Kong, R, Zhou, T, Kim, S-A, Ryu, S, et al. Polygalacin D induces apoptosis and cell cycle arrest via the PI3K/Akt pathway in non-small cell lung cancer. Oncol Rep. (2018) 39:1702–10. doi: 10.3892/or.2018.6230
69. Yajie, D, Feng, L, Zhaoyan, LI, Yan, X, Nida, C, Guangao, Z, et al. Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism. J Tradit Chin Med. (2023) 43:34–41. doi: 10.19852/j.cnki.jtcm.2023.01.005
70. Youhua, W, Xiaohong, A, Dai Wenxiang, W, Xiaoping, TS, and Hao, J. Apigenin induces apoptosis in human liver cancer cells by upregulating PTEN protein expression. China J Modern Med. (2009) 19:1793–6.
71. Sun Yue, Y, and Kun, YW. Apigenin induces autophagy in gastric cancer SGC-7901 cells and its effect on apoptosis. CarcinogenesisDistortionMutation. (2017) 29:289-294+299.
72. Wenzong, L, Yali, Y, Guangfeng, J, and Chen, Z. Anti-tumor activity of polysaccharides isolated from Radix platycodonis. Northwest Pharm J. (2013) 28:43–5.
73. Wang, J, and Lei, L. The effects of Radix platycodonis polysaccharide on tumor growth and immune function in S180 tumor-bearing mice. Current Immunol. (2021) 41:462–7.
74. Hanahan, D, and Weinberg, RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
75. Lee, KJ, Kim, JY, Choi, JH, Kim, HG, Chung, YC, Roh, SH, et al. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem Toxicol. (2006) 44:1890–6. doi: 10.1016/j.fct.2006.06.009
76. Zhang, Z, Zhao, M, Zheng, W, and Liu, Y. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-κB pathway. J Biochem Mol Toxicol. (2017) 31:9. doi: 10.1002/jbt.21934
77. Lu, Z, Song, W, Zhang, Y, Wu, C, Zhu, M, Wang, H, et al. Combined anti-Cancer effects of Platycodin D and Sorafenib on androgen-independent and PTEN-deficient prostate Cancer. Front Oncol. (2021) 11:648985. doi: 10.3389/fonc.2021.648985
78. Li, T, Xu, W-S, Wu, G-S, Chen, X-P, Wang, Y-T, and Lu, J-J. Platycodin D induces apoptosis, and inhibits adhesion, migration and invasion in HepG2 hepatocellular carcinoma cells. Asian Pac J Cancer Prev. (2014) 15:1745–9. doi: 10.7314/apjcp.2014.15.4.1745
79. Huang, X, Dai, S, Dai, J, Xiao, Y, Bai, Y, Chen, B, et al. Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. Onco Targets Ther. (2015) 8:2989–3001. doi: 10.2147/OTT.S91511
80. Zang, M, Hu, L, Fan, Z, Wang, H, Zhu, Z, Cao, S, et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the notch signaling pathway. J Transl Med. (2017) 15:52. doi: 10.1186/s12967-017-1151-6
81. Jiang, J, Zhu, F, Zhang, H, Sun, T, Fu, F, Chen, X, et al. Luteolin suppresses the growth of colon cancer cells by inhibiting the IL-6/STAT3 signaling pathway. J Gastrointest Oncol. (2022) 13:1722–32. doi: 10.21037/jgo-22-507
82. Lin, H-W, Shen, T-J, Yang, N-C, Wang, M, Hsieh, W-C, Chuang, C-J, et al. Luteolin reduces aqueous extract PM2.5-induced metastatic activity in H460 lung Cancer cells. Int J Med Sci. (2022) 19:1502–9. doi: 10.7150/ijms.73947
83. Zhang, J, Li, Y, Li, Y, Li, Y, Gong, X, Zhou, L, et al. Structure, selenization modification, and antitumor activity of a glucomannan from Platycodon grandiflorum. Int J Biol Macromol. (2022) 220:1345–55. doi: 10.1016/j.ijbiomac.2022.09.029
84. Heydarzadeh, S, Moshtaghie, AA, Daneshpour, M, and Hedayati, M. The effect of Apigenin on Glycometabolism and cell death in an anaplastic thyroid cancer cell line. Toxicol Appl Pharmacol. (2023) 475:116626. doi: 10.1016/j.taap.2023.116626
85. Li, J, Ma, A, Lan, W, and Liu, Q. Platycodon D-induced A549 cell apoptosis through RRM1-regulated p53/VEGF/MMP2 pathway. ACAMC. (2022) 22:2458–67. doi: 10.2174/1871520622666220128095355
86. Ma, J, Pan, Z, Du, H, Chen, X, Zhu, X, Hao, W, et al. Luteolin induces apoptosis by impairing mitochondrial function and targeting the intrinsic apoptosis pathway in gastric cancer cells. Oncol Lett. (2023) 26:327. doi: 10.3892/ol.2023.13913
87. Liu, Z, Qin, G, Yang, J, Wang, W, Zhang, W, Lu, B, et al. Targeting mitochondrial degradation by chimeric autophagy-tethering compounds. Chem Sci. (2023) 14:11192–202. doi: 10.1039/d3sc03600f
88. Lee, SJ, Choi, Y-J, Kim, HI, Moon, HE, Paek, SH, Kim, TY, et al. Platycodin D inhibits autophagy and increases glioblastoma cell death via LDLR upregulation. Mol Oncol. (2022) 16:250–68. doi: 10.1002/1878-0261.12966
89. El-Baba, C, Baassiri, A, Kiriako, G, Dia, B, Fadlallah, S, Moodad, S, et al. Terpenoids’ anti-cancer effects: focus on autophagy. Apoptosis. (2021) 26:491–511. doi: 10.1007/s10495-021-01684-y
90. Zhao, R, Chen, M, Jiang, Z, Zhao, F, Xi, B, Zhang, X, et al. Platycodin-D induced autophagy in non-small cell lung Cancer cells via PI3K/Akt/mTOR and MAPK signaling pathways. J Cancer. (2015) 6:623–31. doi: 10.7150/jca.11291
91. Li, T, Xu, X, Tang, Z, Wang, Y, Leung, C, Ma, D, et al. Platycodin D induces apoptosis and triggers ERK- and JNK-mediated autophagy in human hepatocellular carcinoma BEL-7402 cells. Acta Pharmacol Sin. (2015) 36:1503–13. doi: 10.1038/aps.2015.99
92. Cao, Z, Zhang, H, Cai, X, Fang, W, Chai, D, Wen, Y, et al. Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell Physiol Biochem. (2017) 43:1803–12. doi: 10.1159/000484066
93. Kim, TW, and Lee, HG. Apigenin induces autophagy and cell death by targeting EZH2 under hypoxia conditions in gastric Cancer cells. Int J Mol Sci. (2021) 22:13455. doi: 10.3390/ijms222413455
94. Kim, SR, Park, EJ, Dusabimana, T, Je, J, Jeong, K, Yun, SP, et al. Platycodon grandiflorus fermented extracts attenuate endotoxin-induced acute liver injury in mice. Nutrients. (2020) 12:2802. doi: 10.3390/nu12092802
95. Al-Amarat, W, Abukhalil, MH, Alruhaimi, RS, Alqhtani, HA, Aldawood, N, Alfwuaires, MA, et al. Upregulation of Nrf2/HO-1 signaling and attenuation of oxidative stress, inflammation, and cell death mediate the protective effect of Apigenin against cyclophosphamide hepatotoxicity. Meta. (2022) 12:648. doi: 10.3390/metabo12070648
96. Lanzhi, H, Yakun, M, HanYanzhonbg, ZZ, Pin, Y, Xiuxiu, S, Xiaohai, X, et al. The protective effect of luteolin on acetaminophen induced L02 liver cell injury. Chin J Chin Mater Med. (2016) 41:4234–9.
97. Wei, H, Guoqing, L, Ji, Z, and Xiaofeng, Z. Hypoglycemic effect of nano-selenium/ Platycodon polysaccharides complex. Stud Trace Elements Health. (2019) 36:1–4.
98. Liu, Y, Dong, Y, Shen, W, Du, J, Sun, Q, Yang, Y, et al. Platycodon grandiflorus polysaccharide regulates colonic immunity through mesenteric lymphatic circulation to attenuate ulcerative colitis. Chin J Nat Med. (2023) 21:263–78. doi: 10.1016/S1875-5364(23)60435-2
99. Hou, Y, Qi, S, Leng, J, Shen, Q, Tang, S, Zhang, J, et al. Lobetyolin, a Q-marker isolated from Radix Platycodi, exerts protective effects on cisplatin-induced cytotoxicity in HEK293 cells. J Nat Med. (2023) 77:721–34. doi: 10.1007/s11418-023-01714-w
100. Ren, N, Yu, L, Qian, L, Ye, G, Zhu, Z, Yu, J, et al. Exploring the pharmacological mechanism of the effective Chinese medicines against gynecological Cancer based on Meta-analysis combined with network pharmacology analysis. Front Oncol. (2022) 12:817772. doi: 10.3389/fonc.2022.817772
101. Yang, Y, Yuan, L, Liu, W, Lu, D, Meng, F, Yang, Y, et al. Banxia-Shengjiang drug pair inhibits gastric cancer development and progression by improving body immunity. Medicine. (2024) 103:e36303. doi: 10.1097/MD.0000000000036303
102. Huang, M-Y, Jiang, X-M, Xu, Y-L, Yuan, L-W, Chen, Y-C, Cui, G, et al. Platycodin d triggers the extracellular release of programed death Ligand-1 in lung cancer cells. Food Chem Toxicol. (2019) 131:110537. doi: 10.1016/j.fct.2019.05.045
103. Wu, D, Zhang, W, Chen, Y, Ma, H, and Wang, M. Platycodin D inhibits proliferation, migration and induces chemosensitization through inactivation of the NF-κB and JAK2/STAT3 pathways in multiple myeloma cells. Clin Exp Pharmacol Physiol. (2019) 46:1194–200. doi: 10.1111/1440-1681.13145
104. Tang, Z-H, Li, T, Gao, H-W, Sun, W, Chen, X-P, Wang, Y-T, et al. Platycodin D from Platycodonis Radix enhances the anti-proliferative effects of doxorubicin on breast cancer MCF-7 and MDA-MB-231 cells. Chin Med. (2014) 9:16. doi: 10.1186/1749-8546-9-16
105. Zhe, Z, Jiarao, L, Xiaoyong, C, Libing, R, Ling, L, and Genyun, T. Research progress on the mechanism of Saponin compounds in anti-liver cancer. Central Southern Pharm. (2023) 21:1307–14.
106. Weibin, Z, Dancai, F, Zhang Min, Y, Wanlin, DX, Shuhui, X, and Shigu, D. The effect of the aqueous extract of Platycodon grandiflorum and its main component Platycodin D on the pharmacokinetics of cyclophosphamide. J Chin Med Mater. (2016) 39:2633–5. doi: 10.13863/j.issn1001-4454.2016.11.049
107. Jinlin, D, Wanting, D, Zhao Jiao, J, and Aixia, LQ. The effect of apigenin combined with doxorubicin on the proliferation, apoptosis, and cell cycle of HepG2 liver cancer cells. Acta Chin Med Pharmacol. (2021) 49:31–4. doi: 10.19664/j.cnki.1002-2392.210107
108. Ahmed, S, Khan, H, Fratantonio, D, Hasan, MM, Sharifi, S, Fathi, N, et al. Apoptosis induced by luteolin in breast cancer: mechanistic and therapeutic perspectives. Phytomedicine. (2019) 59:152883. doi: 10.1016/j.phymed.2019.152883
109. Sihan, C, Dedong, Z, and Liyun, F. Sensitization effect and mechanism of luteolin on cisplatin induced apoptosis of HepG2 cells in liver cancer. Chin J Clin Pharmacol. (2018) 34:1637–40. doi: 10.13699/j.cnki.1001-6821.2018.14.015
110. Thilakarathna, SH, and Rupasinghe, HPV. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. (2013) 5:3367–87. doi: 10.3390/nu5093367
111. Yu, B, Tai, HC, Xue, W, Lee, LJ, and Lee, RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol. (2010) 27:286–98. doi: 10.3109/09687688.2010.521200
112. Olajubutu, O, Ogundipe, OD, Adebayo, A, and Adesina, SK. Drug delivery strategies for the treatment of pancreatic Cancer. Pharmaceutics. (2023) 15:1318. doi: 10.3390/pharmaceutics15051318
113. Nsairat, H, Khater, D, Sayed, U, Odeh, F, Al Bawab, A, and Alshaer, W. Liposomes: structure, composition, types, and clinical applications. Heliyon. (2022) 8:e09394. doi: 10.1016/j.heliyon.2022.e09394
114. Kang, C, Wang, J, Li, R, Gong, J, Wang, K, Wang, Y, et al. Smart targeted delivery Systems for Enhancing Antitumor Therapy of active ingredients in traditional Chinese medicine. Molecules. (2023) 28:5955. doi: 10.3390/molecules28165955
115. Sun, Y, Li, B, Cao, Q, Liu, T, and Li, J. Targeting cancer stem cells with polymer nanoparticles for gastrointestinal cancer treatment. Stem Cell Res Ther. (2022) 13:489. doi: 10.1186/s13287-022-03180-9
116. Skopinska-Wisniewska, J, De La Flor, S, and Kozlowska, J. From supramolecular hydrogels to multifunctional carriers for biologically active substances. IJMS. (2021) 22:7402. doi: 10.3390/ijms22147402
117. Wang, Z, Wu, G, Yang, Z, Li, X, Feng, Z, and Zhao, Y. Chitosan/hyaluronic acid/MicroRNA-21 nanoparticle-coated smooth titanium surfaces promote the functionality of human gingival fibroblasts. IJN. (2022) 17:3793–807. doi: 10.2147/IJN.S375180
118. Mohanty, A, Uthaman, S, and Park, I-K. Utilization of polymer-lipid hybrid nanoparticles for targeted anti-Cancer therapy. Molecules. (2020) 25:4377. doi: 10.3390/molecules25194377
119. Xiang, J, Zhao, R, Wang, B, Sun, X, Guo, X, Tan, S, et al. Advanced Nano-carriers for anti-tumor drug loading. Front Oncol. (2021) 11:758143. doi: 10.3389/fonc.2021.758143
120. Kim, J, Gao, C, Guo, P, Sheng, J, and Wang, J. A novel approach to alleviate acetaminophen-induced hepatotoxicity with hybrid balloon flower root-derived exosome-like nanoparticles (BDEs) with silymarin via inhibition of hepatocyte MAPK pathway and apoptosis. Cell Commun Signal. (2024) 22:334. doi: 10.1186/s12964-024-01700-z
121. Cho, E, Mun, S-J, Jeon, M, Kim, HK, Baek, H, Ham, YS, et al. Tumor-targeted liposomes with platycodin D2 promote apoptosis in colorectal cancer. Mater Today Bio. (2023) 22:100745. doi: 10.1016/j.mtbio.2023.100745
122. Cao, X, and Wang, B. Targeted PD-L1 PLGA/liposomes-mediated luteolin therapy for effective liver cancer cell treatment. J Biomater Appl. (2021) 36:843–50. doi: 10.1177/08853282211017701
123. Adel, M, Zahmatkeshan, M, Akbarzadeh, A, Rabiee, N, Ahmadi, S, Keyhanvar, P, et al. Chemotherapeutic effects of Apigenin in breast cancer: preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol Rep (Amst). (2022) 34:e00730. doi: 10.1016/j.btre.2022.e00730
124. Banerjee, K, Banerjee, S, and Mandal, M. Enhanced chemotherapeutic efficacy of apigenin liposomes in colorectal cancer based on flavone-membrane interactions. J Colloid Interface Sci. (2017) 491:98–110. doi: 10.1016/j.jcis.2016.12.025
125. Elsayed, AM, Sherif, NM, Hassan, NS, Althobaiti, F, Hanafy, NAN, and Sahyon, HA. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int J Biol Macromol. (2021) 185:134–52. doi: 10.1016/j.ijbiomac.2021.06.085
126. Mabrouk Zayed, MM, Sahyon, HA, Hanafy, NAN, and El-Kemary, MA. The effect of encapsulated Apigenin nanoparticles on HePG-2 cells through regulation of P53. Pharmaceutics. (2022) 14:1160. doi: 10.3390/pharmaceutics14061160
127. Shi, J, Zhang, R, Wang, Y, Sun, Y, Gu, X, An, Y, et al. Herb–nanoparticle hybrid system for improved Oral delivery efficiency to alleviate breast Cancer lung metastasis. IJN. (2024) 19:7927–44. doi: 10.2147/IJN.S463657
128. Xiaodan, D. The structural characteristics、SeleniumNanoparticles modification and antitumor activity of Platycodon Grandifloruspolysaccharides. [PhD Thesis]. Tianjin: Tianjin University of Science and Technology (2022).
129. Han, L-K, Kimura, Y, Okuda, H, Xu, B-J, and Zheng, Y. Platycodi Radix affects lipid metabolism in mice with high fat diet–induced obesity. J Nutr. (2000) 130:2760–4. doi: 10.1093/jn/130.11.2760
130. Kim, YJ, Kwon, E-Y, Kim, J-W, Lee, Y, Ryu, R, Yun, J, et al. Intervention study on the efficacy and safety of Platycodon grandiflorus ethanol extract in overweight or moderately obese adults: a single-center, randomized, double-blind, placebo-controlled trial. Nutrients. (2019) 11:2445. doi: 10.3390/nu11102445
131. Park, E-J, Jung, AJ, Lee, S-H, Kang, S-K, and Lee, H-J. An 8-week randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of red Platycodon grandiflorus root extract on enhancement of immune function. Phytomedicine. (2021) 93:153811. doi: 10.1016/j.phymed.2021.153811
132. Joint committee for guideline revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. (2018) 15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011
133. Zhang, H, Wang, Y, Mehmood, K, Chang, Y-F, Tang, Z, and Li, Y. Treatment of tibial dyschondroplasia with traditional Chinese medicines: “lesson and future directions”. Poult Sci. (2020) 99:6422–33. doi: 10.1016/j.psj.2020.08.055
134. Lee, K. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol Lett. (2004) 147:271–82. doi: 10.1016/j.toxlet.2003.12.002
135. Zhang, L, Wang, X, Zhang, J, Liu, D, and Bai, G. Ethnopharmacology, phytochemistry, pharmacology and product application of Platycodon grandiflorum: a review. Chin Herbal Med. (2024) 16:327–43. doi: 10.1016/j.chmed.2024.01.005
136. Yin, H, Chunlei, S, Moxaing, L, and Hongyan, Y. Determination of 8 platycodins in platycodon cut crude drug by HPLC-ELSD method. J Yangzhou Univ. (2008) 11:41–4. doi: 10.19411/j.1007-824x.2008.04.011
137. Xing, Y. Study on the extraction and catalytic conversion process of Isabelline Saponin D and Codonanolide from Platycodon grandiflorum. Northeast For Univ. (2023). doi: 10.27009/d.cnki.gdblu.2023.001038
138. Xiulin, Z. Research progress in chemical constituents, biological activities and exploration utilization of Platycodon grandiflorum. China Condiment. (2012) 37:5-8+24
139. Sun Xiaowen, D, Xianjun, XF, Che, X, and Kejian, L. Research Progress on the preparation and biological activity of Platycodon grandiflorum polysaccharides. Modern Food Sci Technol. (2024) 40:338–45. doi: 10.13982/j.mfst.1673-9078.2024.2.0021
140. Qingfen, Z, Zhanjun, L, Xiaoxue, L, Siquan, S, Jiao, L, and Fengjian, Y. Research on the process of extracting PD from Platycodon by homogenization combined with ultrasound-microwave synergistic extraction. For Eng. (2022) 38:110–20.
141. Hsu, W-C, Ramesh, S, Shibu, MA, Chen, M-C, Wang, T-F, Day, CH, et al. Platycodin D reverses histone deacetylase inhibitor resistance in hepatocellular carcinoma cells by repressing ERK1/2-mediated cofilin-1 phosphorylation. Phytomedicine. (2021) 82:153442. doi: 10.1016/j.phymed.2020.153442
142. Hnit, SST, Yao, M, Xie, C, Bi, L, Wong, M, Liu, T, et al. Apigenin impedes cell cycle progression at G2 phase in prostate cancer cells. Discov Oncol. (2022) 13:44. doi: 10.1007/s12672-022-00505-1
143. Liu, X, Zhao, T, Shi, Z, Hu, C, Li, Q, and Sun, C. Synergism Antiproliferative effects of Apigenin and Naringenin in NSCLC cells. Molecules. (2023) 28:4947. doi: 10.3390/molecules28134947
144. Zhu, J, Han, M, Yang, Y, Feng, R, Hu, Y, and Wang, Y. Exploring the mechanism of Brucea javanica against ovarian cancer based on network pharmacology and the influence of luteolin on the PI3K/AKT pathway. Comb Chem High Throughput Screen. (2023). doi: 10.2174/1386207326666230627114111
145. Zheng, H, Zhu, X, Gong, E, Lv, Y, Li, Y, and Cai, X. Luteolin suppresses lung cancer progression through targeting the circ_0000190/miR-130a-3p/notch-1 signaling pathway. J Chemother. (2023) 35:330–42. doi: 10.1080/1120009X.2022.2102303
146. Wenzong, L, Fan, G, Rui, G, Chen, Z, and Guangfeng, J. Optimization of polysaccharide extracting Technology of Platycodon Grandiflorus with response surface method and its anti-Cancer capacity. J Xi’an Technol Univ. (2015) 35:534–9. doi: 10.16185/j.jxatu.edu.cn.2015.07.004
147. Cheng, L, Zhai, H, Du, J, Zhang, G, and Shi, G. Lobetyolin inhibits cell proliferation and induces cell apoptosis by downregulating ASCT2 in gastric cancer. Cytotechnology. (2023) 75:435–48. doi: 10.1007/s10616-023-00588-w
148. Liu, L, Liu, Z, Yang, L, Wu, X, Zhu, J, Liu, L, et al. Lobetyolin suppressed lung cancer in a mouse model by inhibiting epithelial-mesenchymal transition. Eur J Histochem. (2022) 66:66. doi: 10.4081/ejh.2022.3423
149. Li, W, Liu, Y, Wang, Z, Han, Y, Tian, Y-H, Zhang, G-S, et al. Platycodin D isolated from the aerial parts of Platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. (2015) 6:1418–27. doi: 10.1039/c5fo00094g
150. Hu, J-N, Leng, J, Shen, Q, Liu, Y, Li, X-D, Wang, S-H, et al. Platycodin D suppresses cisplatin-induced cytotoxicity by suppressing ROS-mediated oxidative damage, apoptosis, and inflammation in HEK-293 cells. J Biochem Mol Toxicol. (2021) 35:e22624. doi: 10.1002/jbt.22624
151. Tao, W, Su, Q, Wang, H, Guo, S, Chen, Y, Duan, J, et al. Platycodin d attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int Immunopharmacol. (2015) 27:138–47. doi: 10.1016/j.intimp.2015.05.005
152. Wei, H, Liyan, H, Yunjie, Z, Xiaofeng, Z, Guiseng, L, Xiang, P, et al. Protective effects of nano-selenium/platycodon polysaccharides complex on CCl4-induced liver damage in mice. Sci Technol Food Ind. (2018) 39:308-311+317. doi: 10.13386/j.issn1002-0306.2018.01.056
Keywords: Platycodon grandiflorum, tumor, homology of medicine and food, anti-tumor active ingredients, anti-tumor mechanisms, novel drug delivery systems, preventive healthcare
Citation: Liu W, Jia S, Ma X, Lu D, Du Y, Zhou Z, Yuan L, Yu R and Nan Y (2025) Platycodon grandiflorum, as a medicinal and food homologous plant: a comprehensive review of anti-tumor components, mechanisms, modern applications, and preventive healthcare. Front. Nutr. 12:1674705. doi: 10.3389/fnut.2025.1674705
Edited by:
Tomislav Tosti, University of Belgrade, SerbiaReviewed by:
Sara Milojević, University of Belgrade, SerbiaNikola Mitovic, University of Belgrade, Serbia
Copyright © 2025 Liu, Jia, Ma, Lu, Du, Zhou, Yuan, Yu and Nan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Nan, MjAwODAwMTFAbnhtdS5lZHUuY24=; Rong Yu, MDAyMTY1QGhudWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Wenjing Liu
Wenjing Liu Shumin Jia2
Shumin Jia2 Doudou Lu
Doudou Lu Ziying Zhou
Ziying Zhou Ling Yuan
Ling Yuan Rong Yu
Rong Yu Yi Nan
Yi Nan