- 1Applied Physiology and Nutrition Research Group, School of Physical Education and Sport, Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil
- 2Center of Lifestyle Medicine, Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil
- 3Laboratory of Assessment and Conditioning in Rheumatology, Hospital das Clínicas HCFMUSP, Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil
- 4Division of Rheumatology, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
Creatine monohydrate supplementation is widely used for enhancing athletic performance and improving clinical outcomes, but concerns regarding its safety persist, particularly relating to risk of cancer, impaired kidney function, dehydration, and gastrointestinal issues. This short review critically evaluates these concerns based on current scientific evidence. Although some theoretical risks, such as creatine’s potential to form carcinogenic compounds, have been discussed, the available research does not support a link between creatine supplementation and cancer. In terms of kidney health, studies consistently show no adverse effects on renal function in healthy individuals, though caution is advised for those with pre-existing kidney conditions and pregnant women, as evidence is lacking for these populations. Claims that creatine leads to dehydration or muscle cramps during exercise are largely unsupported by controlled studies, which demonstrate no significant effects on hydration or thermoregulation; in fact, creatine may reduce the incidence of muscle cramps and assist in maintaining thermoregulatory balance. Gastrointestinal distress is reported in some individuals, particularly at high doses, but such effects are dose-dependent and not universally experienced. Overall, the evidence suggests that creatine monohydrate supplementation is generally safe when used appropriately, with further research needed to understand its impact on specific populations.
1 Introduction
Since its rise in popularity as a performance-enhancing supplement, creatine has been subject to intense scrutiny regarding its safety. Reports of potential adverse effects, including associations with cancer, kidney dysfunction, dehydration, and gastrointestinal issues, have fueled debates among researchers, clinicians, and consumers. However, many of these concerns stem from early anecdotal reports or misinterpretations of physiological mechanisms. This short narrative review critically examines the evidence surrounding these concerns, assessing their validity in light of the latest scientific findings. By addressing both theoretical risks and empirical data, this paper aims to provide a balanced perspective on the safety profile of creatine monohydrate supplementation, particularly in special populations, including pregnant women.
2 Risk of cancer
It has been suggested that creatine may contribute to the formation of chemical compounds, such as heterocyclic amines (HCAs) (1), which are associated with an increased risk of cancer (2). Furthermore, due to its role in cellular metabolism, there is also a current concern that creatine could support cancer progression and metastasis (3). In contrast, creatine and cyclocreatine (its analog) have been shown to delay tumor growth in vitro and animal models and acts as an anticancer agent, working synergistically with chemotherapeutic drugs (4–6). This section will explore the current understanding of both perspectives in this field.
Creatine is a naturally occurring guanidine compound that is endogenously synthesized and/or obtained through the diet, primarily from animal sources (e.g., red meat, fish, and poultry) (7). From an evolutionary perspective, it is noteworthy that creatine has always been a significant component of the human diet, with estimates of prehistoric intake reaching five to ten times the typical levels consumed nowadays (7, 8). It seems then unlikely to imagine that human beings have lost their ability to properly process this nutrient over time. The carcinogenicity of HCAs, which form when creatine combines with glucose and amino acids during high-temperature cooking methods (e.g., grilling and smoking), has been experimentally demonstrated in animal and in vitro studies using much higher amounts of HCAs than those found in cooked/processed meats (9). Evidence from human studies corroborating its carcinogenicity remains scarce.
Pereira et al. (10) have investigated the effects of low- and high-dose creatine monohydrate supplementation (ranging from 2 to 20 g/day) on the production of HCAs in healthy humans using a non-counterbalanced single-blind crossover design. Out of 576 assessments performed on 149 urine samples, only nine exhibited quantifiable levels of HCAs (10). Importantly, six of these samples came from placebo-supplemented participants, whilst only the remaining three were from creatine-supplemented participants, suggesting that creatine supplementation did not stimulate the formation of such mutagens in healthy humans. This conclusion aligns with data from the National Health and Nutrition Examination Survey (NHANES), which indicates that higher dietary creatine intake is associated with a lower risk of cancer or malignancy (11, 12).
Conversely, emerging experimental evidence has linked creatine to cancer progression and metastasis, particularly through the phosphocreatine energy shuttle mediated by creatine kinase in cancer cells (3). Human liver metastases from colorectal and pancreatic cancers have been shown to express higher levels of creatine kinase and SLC6A8, the creatine transporter (13). Using orthotopic mouse models, Zhang et al. observed that creatine-treated mice presented with colorectal and breast cancer metastases through Smad2/3 activation (14), a key mediator of the TGF-β signaling pathway, which regulates cell invasion, immune responses, and microenvironment modification (15). These findings seem to support a potential link between creatine metabolism and cancer progression. On the other hand, creatine supplementation has been demonstrated to enhance the activity of CD8 T cells, which play a central role in mediating and orchestrating immune responses against cancer (16). Moreover, a considerable number of studies have shown that creatine and cyclocreatine can inhibit tumor growth (4–6). This effect is believed to result from the restoration of the creatine-phosphocreatine system in cancer cells, as well as the regulation of acidosis, inflammation, and oxidative stress (17). Some researchers even advocate for creatine’s therapeutic potential in cancer, especially when combined with exercise as an adjunct therapy to ameliorate treatment-related side effects and prevent skeletal muscle loss (18). However, the complexity of the diversity of cancer types, tumors, and experimental models, along with the inherent differences between species, limits our ability to predict creatine’ effects in oncological treatments. Further studies are warranted to better understand these interactions.
Therefore, the claim that creatine intake increases the risk of cancer in humans is not substantiated (Table 1). However, many dietary supplements available on the market may contain unapproved pharmaceutical ingredients, including carcinogenic contaminants (19), so the consumption of creatine supplements with unverified purity should be avoided. Additionally, alternative formulations to creatine monohydrate warrant further investigation.
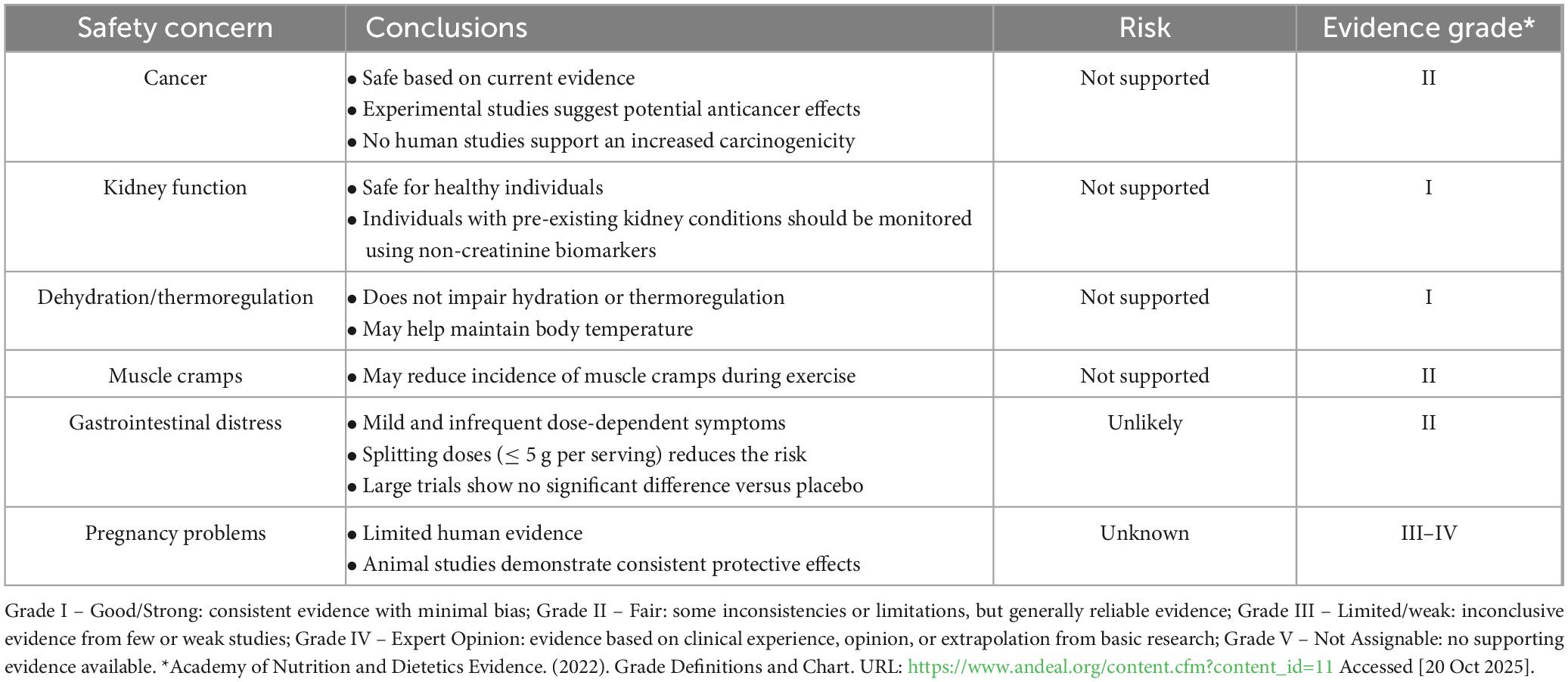
Table 1. Summary of the evidence on the most common safety concerns regarding creatine monohydrate supplementation.
3 Renal dysfunction
Although creatine is one of the most widely used dietary supplements worldwide, benefiting both physically active (e.g., athletes) and clinical populations, some concerns still persist among users, health professionals, and the general public. This section provides a brief overview of the state-of-the-art regarding the effects of creatine supplementation on kidney health [for a comprehensive review, see reference (20)].
Warnings about the potential harm of creatine supplementation on kidney health first emerged in the late 1990’s, driven by case studies and preclinical trials in various animal models (21–30). However, most of these reports rely on retrospective observational data from individuals with preexisting kidney conditions, who engaged in high-intensity and/or high-volume exercise [which can lead to rhabdomyolysis (31)], and/or who abused other substances, including those known to affect renal function (32, 33). Additionally, as previously discussed, findings from animal studies often cannot be extrapolated to humans, as even closely related species may respond differently to creatine intake (34). Therefore, the methodological limitations of these studies compromise their ecological validity.
The concerns about impairments in kidney function are based on the assumptions that creatine intake directly interferes with creatinine (Crn) metabolism. In fact, creatine supplementation increases the body’s total creatine pool, particularly in skeletal muscle, where it is spontaneously (non-enzymatically) and irreversibly degraded into Crn at a rate of approximately 2% per day (1). Consequently, individuals taking creatine supplements may naturally have higher serum Crn levels, without having any impairment in renal function. The problem is that gold-standard methods for assessing renal function are typically restricted to specialized facilities; thus, serum Crn is often used as a proxy for renal function or to estimate glomerular filtration rate (GFR) as a more accessible alternative (35).
In the 1990’s and the mid-2000’s, Jacques Poortmans and colleagues published a series of original studies employing various experimental designs, ranging from single-group pre-to-post investigations to non-randomized, placebo-controlled crossover trials (36–39). Their findings consistently showed no evidence of renal function impairments based on several parameters (including estimated GFR, serum and urinary Crn, urea, proteinuria, and albuminuria) following a wide range of supplementation protocols (1–80 g/day of creatine monohydrate for durations spanning 5 days to 60 months) (36–39). However, it is important to note that participants in all studies were predominantly healthy young men, which limits the generalizability of the findings. In 2011, a narrative review published by the same research group concluded that “the few renal incidents (associated with creatine supplementation) that have been reported remain largely anecdotal” (40). Nonetheless, the authors also warned that creatine supplementation may pose a potential risk for individuals at risk for impaired kidney health (e.g., patients with diabetes, hypertension, reduced GFR, etc.) (40). In contrast, a recent pilot study by Bernales-Delmon et al. examined the effects of 8 weeks of creatine supplementation on physical function and body composition in adults undergoing chronic hemodialysis (41). Except for a statistically significant increase in serum creatinine, no changes were observed in laboratory analytes after supplementation (41). Similarly, Chang et al. reported that administering creatine monohydrate before each dialysis session for 4 weeks caused no adverse effects, except for a slight increase in serum creatinine (42).
We also have published several studies investigating the effects of creatine monohydrate supplementation (from 5 to 20 g/day for up to 24 months) on renal function in various populations, including healthy and diseased individuals at risk for impaired kidney health (10, 43–53). We have found no evidence of renal impairment, even in clinical populations, such as a young man with a single kidney and mild renal insufficiency (48), older adults with type 2 diabetes (49), children and middle-aged adults with diverse rheumatic conditions (50–52), and pre-frail and frail older adults (53). These observations have been based on multiple renal function biomarkers that are independent of Crn metabolism (e.g., cystatin C, serum and urinary electrolytes, proteinuria, and albuminuria), including the 51Cr-EDTA clearance, which is considered a gold-standard technique for measuring GFR.
Therefore, available literature consistently shows that creatine monohydrate is safe when taken at recommended doses, even in clinical populations (Table 1). However, for individuals with (or at risk of) decreased renal function, close monitoring during creatine supplementation is recommended given the limited data. To prevent misdiagnosis (false positives), renal function should be assessed in creatine users preferably using a combination of biomarkers independent of Crn metabolism, such as direct GFR measurements, cystatin C, proteinuria, albuminuria, and/or urinary albumin-to-creatinine ratio.
4 Disturbances in thermoregulation, dehydration and muscle cramps
Another concern about creatine is its putative detrimental effects on thermoregulation and hydration status. Dehydration during exercise triggers a series of physiological changes that can lead to adverse outcomes, including reduced plasma volume, increased cardiac stress (i.e., increased heart rate and reduced stroke volume, accompanied by a reduction in cardiac output), elevated plasma osmolality, increased body temperature, and decreased heat tolerance. These alterations can ultimately compromise athletic performance (54). For years, creatine has been implicated as an allegedly contributing factor leading to the deaths of several football players (55) and wrestlers (56) due to exertional heat stroke.
The physiological rationale underpinning the hypothesis that creatine supplementation could contribute to dehydration and heat stress arises from its osmotic properties. As an osmotically active substance, creatine facilitates water influx into the intracellular space via sodium-dependent creatine transporter. Because this process involves sodium, water is also taken up into the muscle cells, where it helps maintain intracellular osmolality (1), particularly during the initial phase of supplementation. Studies have demonstrated that consuming 20 g/day of creatine for 5 days can result in a 1–3 kg increase in body weight, mostly attributed to total body water (TBW) accumulation (57, 58). Given this osmotic effect, some have hypothesized that creatine may alter fluid balance by promoting intracellular water (ICW) retention, thereby reducing extracellular water (ECW) availability. This shift could, in theory, impair thermoregulation and contribute to an increased incidence of muscle cramps by interfering with the muscle’s contraction-relaxation mechanisms, or by causing dehydration, electrolyte imbalance, and impaired thermoregulation (59). These concerns were overstated after a roundtable report (expert consensus, 2000) from the American College of Sports Medicine (ACSM), which advised caution regarding creatine supplementation in athletes engaged in weight cycling and/or performing strenuous exercise in hot environments, recommending its avoidance under such conditions (60).
However, this proposition is based on very weak evidence, primarily anecdotal reports (from individuals using multiple sports supplements) and speculation that creatine may alter fluid balance or impair thermoregulation. Studies based on self-reports have examined the effects of creatine supplementation among athletes (61–63), particularly football players training in hot environments. Juhn et al. (61) examined 52 male athletes and found that 30% reported diarrhea, 25% muscle cramps, 14% dehydration, and 17% undesired weight gain. Similarly, Greenwood et al. (62) surveyed 219 collegiate athletes, finding that 41% reported creatine use, with 34 athletes experiencing negative effects such as gastrointestinal distress (24%) and muscle cramping (27%). The perceived effects in this study may or may not have been influenced by creatine supplementation, but factors such as training, supplement interactions, nutritional habits, hydration, and heat/humidity could also have affected athletes’ perceptions (62).
Apart from this anecdotal evidence, several controlled studies have examined the effect of creatine supplementation and exercise in hot and/or humid conditions, showing no detrimental effects on thermoregulation and hydration status (64, 65), both in men and woman (66). One of the first studies to examine the effects of creatine under conditions likely to promote dehydration was conducted by Vogel et al. (67). After a 75-min intermittent exercise protocol designed to induce fluid loss, they showed no significant differences between creatine and placebo group in body mass loss or percent change in plasma volume. Regarding hydration measures, multiple studies, but not all (68), have reported increases in TBW and ICW following creatine supplementation, with no difference in sweat rates (69, 70), electrolytes levels (e.g., Na+ and K+) (71), plasma volume (69, 71), or hematocrit (59). In fact, it has been suggested that these increases in TBW and ICW may contribute to maintaining or enhancing thermoregulation. For example, the study by Greenwood et al. (72) suggested that 4 months of creatine supplementation (0.3 g/kg/day for 5 days, followed by 0.03 g/kg/day for 115 days) may reduce the risk of dehydration, cramping, or muscle injury in football players training and competing in hot and humid conditions. Additionally, other physiological measures, such as body temperature and heart rate, appear to remain unchanged with creatine supplementation (67, 71–73). Some authors (59, 74, 75) have also shown that creatine attenuates the rise in body temperature during exercise in the heat.
Therefore, clinical evidence does not support the link between creatine supplementation and the increased risk of heat illness or impaired thermoregulation, even in the presence of pre-existing dehydration. In a double-blind, placebo-controlled, crossover study, Watson et al. (76) investigated the effects of creatine supplementation on heat tolerance in 12 dehydrated men (∼2.0% body mass loss). After undergoing a dehydration protocol, participants exercised for 80 min. The creatine group exhibited better maintenance of plasma volume during the first 20 min of dehydration; however, no significant differences were shown between groups for dehydration, plasma Na+ and K+ concentrations. Aerobic performance, body temperature, and physiological strain index also remained similar between groups.
In summary, although anecdotal reports suggest that creatine may cause side effects in athletes exercising in the heat, a body of evidence from controlled studies shows no negative impact on thermoregulatory or hydration status, including body temperature regulation, dehydration percentage, urinary hydration markers, plasma volume, sweat losses or, muscle cramps (64). On the contrary, by promoting increases in total and intracellular body water, creatine supplementation may help support thermoregulation (76, 77) and potentially lower the incidence of cramping (72), particularly in athletes exposed to hot and humid environments (Table 1).
5 Gastrointestinal distress
While creatine is generally well-tolerated, some reports suggest that supplementation may cause gastrointestinal (GI) distress in certain individuals (78, 79). Epidemiological studies have identified GI issues as the most commonly reported adverse effects, which include diarrhea, stomach discomfort, bloating, and other symptoms. A likely explanation for these adverse effects is creatine’s osmotic effect: when high single doses (> 10 g) are ingested, part of the compound may remain unabsorbed in the intestine, drawing water into the lumen and accelerating transit. As discussed, an anecdotal study of 52 male athletes found that 30% experienced diarrhea (61), while 24% of 219 collegiate athletes reported GI distress (62). It is important to note that, in both studies, athletes exceeded the recommended maintenance dose (i.e., 2–5 g/day), with some of them taking three to four times the recommended dose, reaching 17–20 g of creatine per day. However, scientific evidence supporting these associations remains limited.
Potential GI side effects were assessed in 59 soccer players randomly assigned to one of three groups: two daily doses of 5 g creatine (Cr5), a single 10 g/day dose (Cr10), or placebo (PL) (80). After 28 days, diarrhea (39%), stomach upset (23.8%), and belching (16.9%) were reported across all groups, including placebo. No significant differences in GI distress were observed between Cr5 and PL. Similarly, no group differences were found for abdominal pain, heartburn, nausea, bloating, vomiting, or constipation. However, Cr10 showed a higher incidence of diarrhea than Cr5 (55.6% vs. 28.6%, p < 0.05) and PL (55.6% vs. 35%, p < 0.05). The authors attributed this to higher creatine intake in a single dose, which may leave undissolved creatine in the GI tract, increasing water retention via osmotic activity and leading to diarrhea or loose stools (79). These effects may be minimized by limiting single doses to ≤ 5 g or, if higher daily intake is needed, dividing it into smaller doses (e.g., two daily 5 g doses instead of one 10 g dose).
In line with these findings, a systematic review with meta-analysis on creatine supplementation in female participants found no statistically significant effects of supplementation on GI events when dose regimens were stratified as “maintenance dose only,” “combined dose only,” or “loading dose only” (81). Among the 18 studies analyzed, four reported GI symptoms as the reason for intervention dropout, with similar rates observed in both the creatine and placebo groups (five vs. six participants, respectively) (45, 82, 83). Only one study reported increased adverse GI events when symptoms were combined with muscle cramping (p < 0.05). Yet, no significant difference was observed between groups in the overall number of adverse events reported (83). Similar findings, in both younger (40, 77) and older (84) male or mixed-sex populations were observed, with GI disturbances primarily associated with higher creatine doses exceeding commonly studied dosing protocols, or concurrent supplementation (62, 80, 85). Supporting this, Kreider et al. evaluated 685 randomized controlled trials with over 26,000 participants and found no significant differences in the prevalence or frequency of GI issues between creatine and placebo groups (5.5% vs. 4.2%, p = 0.820) (86). While a slightly higher number of trials reported GI side effects in creatine users, the overall incidence among participants was comparable to placebo, indicating that these effects are uncommon and typically not clinically relevant (86).
It is also speculated that other additives, ingredients, or contaminants generated during industrial production (e.g., sodium sarcosine, cynamide, dicyandiamide, dihydrotriazines creatinine) in some creatine supplements may cause GI discomfort in sensitive individuals (80). This would be a safety issue related to the purity of the supplement, rather than the creatine itself. Moreover, it has been hypothesized that the combination of creatine and caffeine would increase symptoms of GI distress, which might indirectly reduce performance (87, 88). For some individuals, caffeine may increase peristalsis, leading to stomach discomfort and more frequent bowel movements, although caffeine sensitivity is highly individual. In this regard, Vandenberghe et al. (88) reported mild GI distress after 6 days of creatine (0.5 g/kg/day) and creatine (0.5 g/kg/day) plus caffeine (5 mg/kg/day) supplementation in three subjects (∼33%): two during the first 2 days of creatine ingestion and one over the 3 days of creatine plus caffeine supplementation.
While creatine is generally well-tolerated, gastrointestinal upsetting can occur, particularly with high single doses (greater than 10 g) or when combined with other supplements like caffeine. However, evidence linking creatine to GI distress remains limited and inconsistent, with dose regimens and individual variability being key factors (Table 1). To minimize adverse effects, it is recommended to follow dosage protocols and, if needed, split the daily intake into smaller doses (e.g., ≤ 5 g per dose).
6 Creatine supplementation during pregnancy
Pregnancy is a period marked by profound physiological and metabolic adaptations aimed at supporting fetal development and preparing the mother for delivery. Among the nutrients involved in energy metabolism, creatine plays a central role as a high-energy phosphate buffer, maintaining cellular ATP homeostasis. The creatine kinase system is particularly relevant in tissues with high and fluctuating energy demands, such as the uterus, placenta, and developing fetal brain (89, 90). Given these characteristics, creatine supplementation has been investigated as a potential strategy to improve maternal and neonatal outcomes. However, its use during pregnancy raises important safety considerations, as evidence in humans remains limited and inconclusive.
Animal studies provide the bulk of knowledge regarding creatine use in gestation. In rodent and other mammalian models, maternal creatine supplementation has consistently been associated with improved neonatal resilience to hypoxic stress, enhanced survival, and preservation of brain, muscle, and other organ functions (90–94). Importantly, no adverse maternal or fetal effects have been documented in these studies, even when supplementation was initiated early in pregnancy and maintained until term. These findings are biologically plausible, as creatine availability during episodes of oxygen deprivation can sustain mitochondrial ATP production, delay energy failure, and reduce tissue injury. This experimental evidence has led to the hypothesis that creatine supplementation could serve as a prophylactic therapy in pregnancies at risk of intrapartum hypoxia, a major cause of perinatal morbidity and mortality (90). Nevertheless, while preclinical data are promising, interspecies differences in placental transport, creatine metabolism, and developmental trajectories preclude direct extrapolation to humans.
Human evidence remains scarce. A frequently cited case report described the course of a pregnant woman affected by a creatine synthesis disorder (i.e., Arginine:Glycine Amidino-Transferase deficiency) who received creatine supplementation throughout gestation. The pregnancy resulted in the birth of a healthy infant without apparent complications, suggesting safety in that particular context (95). However, the anecdotal nature of this observation, combined with its unique clinical background, prevents generalization to healthy pregnant women. Beyond isolated reports, the current knowledge is derived primarily from observational studies investigating dietary creatine intake and maternal metabolite profiles.
One line of evidence suggests that suboptimal dietary creatine intake may be associated with higher risks of adverse obstetric outcomes. For instance, analyses of maternal dietary patterns indicate that low intake of creatine-rich foods, such as meat and fish, correlates with greater incidence of complications, including low birth weight (96). Observational data indicate that dietary creatine intake below recommended levels may be associated with higher risks of certain obstetric conditions, but the study did not address supplementation safety (96). In addition, there is evidence that maternal plasma creatine concentrations correlate positively with protein intake, reflecting the importance of exogenous dietary sources (97). These associations highlight the relevance of creatine homeostasis during pregnancy but cannot be interpreted as evidence for the safety of supplementation, as observational data are subject to nutritional assessment bias, residual confounding and reverse causation.
Data indicate that plasma creatine concentrations remain relatively stable throughout pregnancy, while urinary excretion declines toward term; guanidinoacetate concentrations, in contrast, fluctuate significantly, suggesting adaptations in endogenous creatine biosynthesis (97). These findings illustrate the complexity of maternal creatine regulation, which appears to adjust throughout pregnancy
Despite the biological rationale and encouraging animal data (90, 91, 93), the absence of randomized controlled trials in humans remains the most significant barrier to recommending creatine supplementation during pregnancy. Regulatory authorities have not established safety categories for creatine in this context, and expert reviews consistently conclude that the evidence base is insufficient. Although creatine monohydrate is considered safe in healthy adults at conventional doses, pregnancy involves unique physiological alterations, including expanded plasma volume, increased renal clearance, hormonal changes, and altered nutrient transfer through the placenta, that could modify both the pharmacokinetics and safety profile of supplementation. Without carefully designed clinical trials, it is impossible to determine whether maternal or fetal risks might emerge from exogenous creatine administration.
Acceptability and attitude studies provide further insight into the potential translation of this intervention. Many pregnant women would be receptive to creatine supplementation if recommended by their healthcare providers, particularly if framed as a strategy to reduce risks of perinatal hypoxia. At the same time, both patients and professionals emphasize the need for robust safety and efficacy data before clinical adoption (98, 99). This aligns with the broader principle that interventions in pregnancy must adhere to the highest evidentiary standards, given the ethical responsibility to safeguard both maternal and fetal health.
In summary, creatine supplementation during pregnancy remains an investigational concept with promising preclinical support but limited human evidence (Table 1). Animal studies demonstrate consistent protective effects against perinatal hypoxia without adverse outcomes (90). However, these findings cannot substitute for randomized controlled trials, which are essential to evaluate safety, efficacy, and appropriate dosing in pregnant women. Until such studies are conducted, creatine supplementation should not be recommended in clinical practice outside of research protocols. The priority moving forward is to design carefully controlled trials that can clarify whether creatine may serve as a safe and effective adjunct in maternal-fetal medicine, particularly for pregnancies at risk of hypoxic complications (90).
7 Conclusion
This narrative review on over 30 years of accumulated evidence concludes that:
1. When used at recommended doses (5–20 g/day), creatine monohydrate is safe for most populations and has well-documented performance and clinical benefits.
2. Current evidence does not support a link between its supplementation and increased cancer risk nor renal dysfunction in humans.
3. Consumers should be cautious about supplement purity: potential risks stem from contaminants rather than creatine; therefore, only high-quality, third-party tested supplements should be used, and alternative formulations avoided.
4. Individuals with pre-existing kidney conditions should be monitored closely, as evidence is incipient and limited in this population.
5. Assessment of kidney function should use biomarkers independent of Crn (e.g., direct GFR measurements, cystatin C, proteinuria, albuminuria, and/or urinary albumin-to-creatinine ratio).
6. Available evidence does not support claims that creatine increases dehydration, heat illness, or muscle cramps; actually, it may support thermoregulation and reduce the incidence of cramps in some exercise settings.
7. GI distress is generally mild and infrequent, often occurring with high single doses (> 10 g per dose) or co-ingestion with other supplements (e.g., caffeine).
8. Splitting doses into smaller amounts (e.g., ≤ 5 g per dose) can minimize potential GI discomfort.
9. Animal studies demonstrate consistent protective effects against pregnancy problems; however, evidence in humans is considerably limited.
10. Future research should focus on vulnerable populations (e.g., individuals with or at risk of pre-existing kidney disease and pregnant women).
Author contributions
IL: Writing – review & editing, Conceptualization, Writing – original draft, Project administration. MS: Writing – review & editing, Conceptualization, Writing – original draft. HR: Writing – review & editing, Supervision. BG: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financed by the São Paulo Research Foundation (FAPESP), Brazil (Process Numbers #2021/13580-1 and #2024/22058-5 for IL and #2017/13552-2 for BG), and from the National Council for Scientific and Technological Development (CNPq), Brazil (Process #308307/2021-6 for HR). AlzChem provided financial support for the article processing charges (APCs) but had no involvement in the study design, data analysis, interpretation, manuscript writing, or the decision to submit the article for publication.
Conflict of interest
BG received research grants, creatine donation for scientific studies, travel support for participation in scientific conferences, and honorarium for speaking at lectures from AlzChem (a company which manufactures creatine). Additionally, he served as a member of the Scientific Advisory Board for Alzchem. HR received research grants and supplement donations for scientific studies from AlzChem, Natural Alternatives International, DuPont, J.B.S., and NotCompany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. (2000) 80:1107–213. doi: 10.1152/physrev.2000.80.3.1107
2. Layton D, Bogen K, Knize M, Hatch F, Johnson V, Felton J. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. (1995) 16:39–52. doi: 10.1093/carcin/16.1.39
3. Zhang L, Bu P. The two sides of creatine in cancer. Trends Cell Biol. (2022) 32:380–90. doi: 10.1016/j.tcb.2021.11.004
4. Teicher B, Menon K, Northey D, Liu J, Kufe D, Kaddurah-Daouk R. Cyclocreatine in cancer chemotherapy. Cancer Chemother Pharmacol. (1995) 35:411–6. doi: 10.1007/s002800050255
5. Miller E, Evans A, Cohn M. Inhibition of rate of tumor growth by creatine and cyclocreatine. Proc Natl Acad Sci U S A. (1993) 90:3304–8. doi: 10.1073/pnas.90.8.3304
6. Lillie J, O’Keefe M, Valinski H, Hamlin H, Varban M, Kaddurah-Daouk R. Cyclocreatine (1-carboxymethyl-2-iminoimidazolidine) inhibits growth of a broad spectrum of cancer cells derived from solid tumors. Cancer Res. (1993) 53:3172–8.
7. Brosnan M, Brosnan J. The role of dietary creatine. Amino Acids. (2016) 48:1785–91. doi: 10.1007/s00726-016-2188-1
8. Harris R, Nevill M, Harris D, Fallowfield J, Bogdanis G, Wise J. Absorption of creatine supplied as a drink, in meat or in solid form. J Sports Sci. (2002) 20:147–51. doi: 10.1080/026404102317200855
9. Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. (2004) 95:290–9. doi: 10.1111/j.1349-7006.2004.tb03205.x
10. Pereira R, Dörr F, Pinto E, Solis M, Artioli G, Fernandes A, et al. Can creatine supplementation form carcinogenic heterocyclic amines in humans? J Physiol. (2015) 593:3959–71. doi: 10.1113/JP270861
11. Ostojic S, Grasaas E, Cvejic JJ. Dietary creatine and cancer risk in the US population: NHANES 2017–2020. J Funct Foods. (2023) 108:105733. doi: 10.1016/j.jff.2023.105733
12. Jiang J, Zhao H, Chen J, Du J, Ni W, Zheng B, et al. The association between dietary creatine intake and cancer in U.S. adults: insights from NHANES 2007-2018. Front Nutr. (2024) 11:1460057. doi: 10.3389/fnut.2024.1460057
13. Loo J, Scherl A, Nguyen A, Man F, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. (2015) 160:393–406. doi: 10.1016/j.cell.2014.12.018
14. Zhang L, Zhu Z, Yan H, Wang W, Wu Z, Zhang F, et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. (2021) 33:1111–1123.e4. doi: 10.1016/j.cmet.2021.03.009
16. Di Biase S, Ma X, Wang X, Yu J, Wang Y, Smith D, et al. Creatine uptake regulates CD8 T cell antitumor immunity. J Exp Med. (2019) 216:2869–82. doi: 10.1084/jem.20182044
17. Campos-Ferraz P, Gualano B, das Neves W, Andrade I, Hangai I, Pereira R, et al. Exploratory studies of the potential anti-cancer effects of creatine. Amino Acids. (2016) 48:1993–2001. doi: 10.1007/s00726-016-2180-9
18. Fairman C, Kendall K, Hart N, Taaffe D, Galvão D, Newton R. The potential therapeutic effects of creatine supplementation on body composition and muscle function in cancer. Crit Rev Oncol Hematol. (2019) 133:46–57. doi: 10.1016/j.critrevonc.2018.11.003
19. Tucker J, Fischer T, Upjohn L, Mazzera D, Kumar M. Unapproved pharmaceutical ingredients included in dietary supplements associated with US Food and Drug Administration Warnings. JAMA Netw Open. (2018) 1:e183337. doi: 10.1001/jamanetworkopen.2018.3337
20. Longobardi I, Gualano B, Seguro A, Roschel H. Is it time for a requiem for creatine supplementation-induced kidney failure? A Narrative Review. Nutrients. (2023) 15:1466. doi: 10.3390/nu15061466
21. Pritchard N, Kalra P. Renal dysfunction accompanying oral creatine supplements. Lancet. (1998) 351:1252–3. doi: 10.1016/s0140-6736(05)79319-3
22. Koshy K, Griswold E, Schneeberger E. Interstitial nephritis in a patient taking creatine. N Engl J Med. (1999) 340:814–5. doi: 10.1056/NEJM199903113401017
23. Robinson S. Acute quadriceps compartment syndrome and rhabdomyolysis in a weight lifter using high-dose creatine supplementation. J Am Board Fam Pract. (2000) 13:134–7. doi: 10.3122/15572625-13-2-134
24. Barisic N, Bernert G, Ipsiroglu O, Stromberger C, Müller T, Gruber S, et al. Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy. Neuropediatrics. (2002) 33:157–61. doi: 10.1055/s-2002-33679
25. Révai T, Sápi Z, Benedek S, Kovács A, Kaszás I, Virányi M, et al. Severe nephrotic syndrome in a young man taking anabolic steroid and creatine long term]. Orv Hetil. (2003) 144:2425–7.
26. Thorsteinsdottir B, Grande J, Garovic V. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr. (2006) 16:341–5. doi: 10.1053/j.jrn.2006.04.025
27. Taner B, Aysim O, Abdulkadir U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus. (2011) 4:23–4. doi: 10.1093/ndtplus/sfq177
28. Edmunds J, Jayapalan S, DiMarco N, Saboorian M, Aukema H. Creatine supplementation increases renal disease progression in Han:sprd-cy rats. Am J Kidney Dis. (2001) 37:73–8. doi: 10.1053/ajkd.2001.20590
29. Ferreira L, De Toledo Bergamaschi C, Lazaretti-Castro M, Heilberg I. Effects of creatine supplementation on body composition and renal function in rats. Med Sci Sports Exerc. (2005) 37:1525–9. doi: 10.1249/01.mss.0000177555.94271.44
30. Souza R, Miranda H, Xavier M, Lazo-Osorio R, Gouvea H, Cogo J, et al. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J Sports Sci Med. (2009) 8:672–81.
31. Kim J, Lee J, Kim S, Ryu H, Cha K, Sung D. Exercise-induced rhabdomyolysis mechanisms and prevention: a literature review. J Sport Health Sci. (2016) 5:324–33. doi: 10.1016/j.jshs.2015.01.012
32. Burdmann E, Andoh T, Yu L, Bennett W. Cyclosporine nephrotoxicity. Semin Nephrol. (2003) 23:465–76. doi: 10.1016/s0270-9295(03)00090-1
33. Parente Filho S, Gomes P, Forte G, Lima L, Silva Júnior G, Meneses G, et al. Kidney disease associated with androgenic-anabolic steroids and vitamin supplements abuse: be aware! Nefrologia. (2020) 40:26–31. doi: 10.1016/j.nefro.2019.06.003
34. Tarnopolsky M, Bourgeois J, Snow R, Keys S, Roy B, Kwiecien J, et al. Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am J Physiol Regul Integr Comp Physiol. (2003) 285:R762–9. doi: 10.1152/ajpregu.00270.2003
35. Levey A, Coresh J, Tighiouart H, Greene T, Inker L. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. (2020) 16:51–64. doi: 10.1038/s41581-019-0191-y
36. Poortmans J, Auquier H, Renaut V, Durussel A, Saugy M, Brisson G. Effect of short-term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol. (1997) 76:566–7. doi: 10.1007/s004210050291
37. Poortmans J, Francaux M. Renal dysfunction accompanying oral creatine supplements. Lancet. (1998) 352:234. doi: 10.1016/s0140-6736(05)77836-3
38. Poortmans J, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. (1999) 31:1108–10. doi: 10.1097/00005768-199908000-00005
39. Poortmans J, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med Sci Sports Exerc. (2005) 37:1717–20. doi: 10.1249/01.mss.0000176398.64189.e6
40. Kim H, Kim C, Carpentier A, Poortmans J. Studies on the safety of creatine supplementation. Amino Acids. (2011) 40:1409–18. doi: 10.1007/s00726-011-0878-2
41. Bernales-Delmon W, Schulz S, Guglielmi I, Saravia C, Venegas Y, Joost J, et al. Oral creatine in hemodialysis patients increases physical functional capacity and muscle mass, an open label study. PLoS One. (2025) 20:e0328757. doi: 10.1371/journal.pone.0328757
42. Chang C, Wu C, Yang C, Huang J, Wu M. Creatine monohydrate treatment alleviates muscle cramps associated with haemodialysis. Nephrol Dial Transplant. (2002) 17:1978–81. doi: 10.1093/ndt/17.11.1978
43. Lugaresi R, Leme M, de Salles Painelli V, Murai I, Roschel H, Sapienza M, et al. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J Int Soc Sports Nutr. (2013) 10:26. doi: 10.1186/1550-2783-10-26
44. Neves M, Gualano B, Roschel H, Lima F, Lúcia de Sá-Pinto A, Seguro AC, et al. Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women. Appl Physiol Nutr Metab. (2011) 36:419–22. doi: 10.1139/h11-014
45. Lobo D, Tritto A, da Silva L, de Oliveira P, Benatti F, Roschel H, et al. Effects of long-term low-dose dietary creatine supplementation in older women. Exp Gerontol. (2015) 70:97–104. doi: 10.1016/j.exger.2015.07.012
46. Sales L, Pinto A, Rodrigues S, Alvarenga J, Gonçalves N, Sampaio-Barros M, et al. Creatine Supplementation (3 g/d) and Bone Health in Older Women: a 2-Year, Randomized, Placebo-Controlled Trial. J Gerontol A Biol Sci Med Sci. (2020) 75:931–8. doi: 10.1093/gerona/glz162
47. Gualano B, Ugrinowitsch C, Novaes R, Artioli G, Shimizu M, Seguro A, et al. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol. (2008) 103:33–40. doi: 10.1007/s00421-007-0669-3
48. Gualano B, Ferreira D, Sapienza M, Seguro A, Lancha A. Effect of short-term high-dose creatine supplementation on measured GFR in a young man with a single kidney. Am J Kidney Dis. (2010) 55:e7–9. doi: 10.1053/j.ajkd.2009.10.053
49. Gualano B, de Salles Painelli V, Roschel H, Lugaresi R, Dorea E, Artioli G, et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Eur J Appl Physiol. (2011) 111:749–56. doi: 10.1007/s00421-010-1676-3
50. Hayashi A, Solis M, Sapienza M, Otaduy M, de Sá Pinto A, Silva C, et al. Efficacy and safety of creatine supplementation in childhood-onset systemic lupus erythematosus: a randomized, double-blind, placebo-controlled, crossover trial. Lupus. (2014) 23:1500–11. doi: 10.1177/0961203314546017
51. Neves M, Gualano B, Roschel H, Fuller R, Benatti F, Pinto A, et al. Beneficial effect of creatine supplementation in knee osteoarthritis. Med Sci Sports Exerc. (2011) 43:1538–43. doi: 10.1249/MSS.0b013e3182118592
52. Alves C, Santiago B, Lima F, Otaduy M, Calich A, Tritto A, et al. Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res. (2013) 65:1449–59. doi: 10.1002/acr.22020
53. Roschel H, Hayashi A, Fernandes A, Jambassi-Filho J, Hevia-Larraín V, de Capitani M, et al. Supplement-based nutritional strategies to tackle frailty: a multifactorial, double-blind, randomized placebo-controlled trial. Clin Nutr. (2021) 40:4849–58. doi: 10.1016/j.clnu.2021.06.024
54. James L, Mears S. Does dehydration really impair endurance performance? Recent methodological advances helping to clarify an old question. Sports Sci Exchange. (2021) 34:1–6.
55. Kellogg A, Suggs W. Deaths of 3 College Football Players Worry Athletics Officials. (2001) Washington, DC: The Chronicle of Higher Education.
56. Naughton J. NCAA and Federal Agencies Investigate the Death of 3 College Wrestlers. (1998) Washington, DC: The Chronicle of Higher Education.
57. Kraemer W, Volek J. Creatine supplementation. Its role in human performance. Clin Sports Med. (1999) 18:651–66. doi: 10.1016/s0278-5919(05)70174-5
58. Volek J, Duncan N, Mazzetti S, Staron R, Putukian M, Gómez A, et al. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. (1999) 31:1147–56. doi: 10.1097/00005768-199908000-00011
59. Kern M, Podewils L, Vukovich M, Buono M. Physiological response to exercise in the heat following creatine supplementation. J Exerc Physiol Online. (2001) 4:18–27.
60. Terjung R, Clarkson P, Eichner E, Greenhaff P, Hespel P, Israel R, et al. American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. (2000) 32:706–17. doi: 10.1097/00005768-200003000-00024
61. Juhn M, O’Kane J, Vinci D. Oral creatine supplementation in male collegiate athletes: a survey of dosing habits and side effects. J Am Diet Assoc. (1999) 99:593–5. doi: 10.1016/s0002-8223(99)00145-5
62. Greenwood M, Farris J, Kreider R, Greenwood L, Byars A. Creatine supplementation patterns and perceived effects in select division I collegiate athletes. Clin J Sport Med. (2000) 10:191–4. doi: 10.1097/00042752-200007000-00007
63. McGuine T, Sullivan J, Bernhardt D. Creatine supplementation in high school football players. Clin J Sport Med. (2001) 11:247–53. doi: 10.1097/00042752-200110000-00007
64. Lopez R, Casa D, McDermott B, Ganio M, Armstrong L, Maresh C. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J Athl Train. (2009) 44:215–23. doi: 10.4085/1062-6050-44.2.215
65. Rosene J, Matthews T, Mcbride K, Galla A, Haun M, Mcdonald K, et al. The effects of creatine supplementation on thermoregulation and isokinetic muscular performance following acute (3-day) supplementation. J Sports Med Phys Fitness. (2015) 55:1488–96.
66. Rosene J, Whitman S, Fogarty TD. A comparison of thermoregulation with creatine supplementation between the sexes in a thermoneutral environment. J Athl Train. (2004) 39:50–5.
67. Vogel R, Webster M, Erdmann L, Clark R. Creatine supplementation: effect on supramaximal exercise performance at two levels of acute hypohydration. J Strength Condition Res. (2000) 14:214–9.
68. Powers M, Arnold B, Weltman A, Perrin D, Mistry D, Kahler D, et al. Creatine supplementation increases total body water without altering fluid distribution. J Athl Train. (2003) 38:44–50.
69. Weiss B, Powers M. Creatine supplementation does not impair the thermoregulatory response during a bout of exercise in the heat. J Sports Med Phys Fitness. (2006) 46:555–63.
70. Wright G, Grandjean P, Pascoe D. The effects of creatine loading on thermoregulation and intermittent sprint exercise performance in a hot humid environment. J Strength Cond Res. (2007) 21:655–60. doi: 10.1519/R-22186.1
71. Volek J, Mazzetti S, Farquhar W, Barnes B, Gómez A, Kraemer W. Physiological responses to short-term exercise in the heat after creatine loading. Med Sci Sports Exerc. (2001) 33:1101–8. doi: 10.1097/00005768-200107000-00006
72. Greenwood M, Kreider R, Greenwood L, Byars A. Cramping and injury incidence in collegiate football players are reduced by creatine supplementation. J Athl Train. (2003) 38:216–9.
73. Ziegenfuss T, Lowery L, Lemon P. Acute fluid volume changes in men during three days of creatine supplementation. Nutr Exerc. (1998) 1: 1–9.
74. Easton C, Turner S, Pitsiladis Y. Creatine and glycerol hyperhydration in trained subjects before exercise in the heat. Int J Sport Nutr Exerc Metab. (2007) 17:70–91. doi: 10.1123/ijsnem.17.1.70
75. Kilduff L, Georgiades E, James N, Minnion R, Mitchell M, Kingsmore D, et al. The effects of creatine supplementation on cardiovascular, metabolic, and thermoregulatory responses during exercise in the heat in endurance-trained humans. Int J Sport Nutr Exerc Metab. (2004) 14:443–60. doi: 10.1123/ijsnem.14.4.443
76. Watson G, Casa D, Fiala K, Hile A, Roti M, Healey J, et al. Creatine use and exercise heat tolerance in dehydrated men. J Athl Train. (2006) 41:18–29.
77. Kreider R, Melton C, Rasmussen C, Greenwood M, Lancaster S, Cantler E, et al. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem. (2003) 244:95–104.
78. Francaux M, Poortmans J. Side effects of creatine supplementation in athletes. Int J Sports Physiol Perform. (2006) 1:311–23. doi: 10.1123/ijspp.1.4.311
79. Williams M, Branch J. Creatine supplementation and exercise performance: an update. J Am Coll Nutr. (1998) 17:216–34. doi: 10.1080/07315724.1998.10718751
80. Ostojic S, Ahmetovic Z. Gastrointestinal distress after creatine supplementation in athletes: Are side effects dose dependent? Res Sports Med. (2008) 16:15–22. doi: 10.1080/15438620701693280
81. de Guingand D, Palmer K, Snow R, Davies-Tuck M, Ellery S. Risk of adverse outcomes in females taking oral creatine monohydrate: a systematic review and meta-analysis. Nutrients. (2020) 12:1780. doi: 10.3390/nu12061780
82. Lyoo I, Yoon S, Kim T, Hwang J, Kim J, Won W, et al. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry. (2012) 169:937–45. doi: 10.1176/appi.ajp.2012.12010009
83. Chilibeck P, Candow D, Landeryou T, Kaviani M, Paus-Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc. (2015) 47:1587–95. doi: 10.1249/MSS.0000000000000571
84. Groeneveld G, Beijer C, Veldink J, Kalmijn S, Wokke J, van den Berg L. Few adverse effects of long-term creatine supplementation in a placebo-controlled trial. Int J Sports Med. (2005) 26:307–13. doi: 10.1055/s-2004-817917
85. Hersch S, Schifitto G, Oakes D, Bredlau A, Meyers C, Nahin R, et al. The CREST-E study of creatine for Huntington disease: a randomized controlled trial. Neurology. (2017) 89:594–601. doi: 10.1212/WNL.0000000000004209
86. Kreider R, Gonzalez D, Hines K, Gil A, Bonilla D. Safety of creatine supplementation: analysis of the prevalence of reported side effects in clinical trials and adverse event reports. J Int Soc Sports Nutr. (2025) 22(sup1):2488937. doi: 10.1080/
87. Hespel P, Op’t Eijnde B, Van Leemputte M. Opposite actions of caffeine and creatine on muscle relaxation time in humans. J Appl Physiol. (2002) 92:513–8. doi: 10.1152/japplphysiol.00255.2001
88. Vandenberghe K, Gillis N, Van Leemputte M, Van Hecke P, Vanstapel F, Hespel P. Caffeine counteracts the ergogenic action of muscle creatine loading. J Appl Physiol. (1996) 80:452–7. doi: 10.1152/jappl.1996.80.2.452
89. Muccini A, Tran N, de Guingand D, Philip M, Della Gatta P, Galinsky R, et al. Creatine metabolism in female reproduction, pregnancy and newborn health. Nutrients. (2021) 13:490. doi: 10.3390/nu13020490
90. Dickinson H, Ellery S, Ireland Z, LaRosa D, Snow R, Walker D. Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth. (2014) 14:150. doi: 10.1186/1471-2393-14-150
91. Ellery S, LaRosa D, Kett M, Della Gatta P, Snow R, Walker D, et al. Dietary creatine supplementation during pregnancy: a study on the effects of creatine supplementation on creatine homeostasis and renal excretory function in spiny mice. Amino Acids. (2016) 48:1819–30. doi: 10.1007/s00726-015-2150-7
92. LaRosa D, Ellery S, Snow R, Walker D, Dickinson H. Maternal creatine supplementation during pregnancy prevents acute and long-term deficits in skeletal muscle after birth asphyxia: a study of structure and function of hind limb muscle in the spiny mouse. Pediatr Res. (2016) 80:852–60. doi: 10.1038/pr.2016.153
93. Cannata D, Ireland Z, Dickinson H, Snow R, Russell A, West J, et al. Maternal creatine supplementation from mid-pregnancy protects the diaphragm of the newborn spiny mouse from intrapartum hypoxia-induced damage. Pediatr Res. (2010) 68:393–8. doi: 10.1203/PDR.0b013e3181f1c048
94. Tran N, Muccini A, Snow R, Nitsos I, Hale N, Walker D, et al. The physiological effects of creatine supplementation in fetal sheep before, during, and after umbilical cord occlusion and global hypoxia. J Appl Physiol. (2021) 131:1088–99. doi: 10.1152/japplphysiol.00092.2021
95. Alessandrì M, Strigini F, Cioni G, Battini R. Increased creatine demand during pregnancy in Arginine: glycine Amidino-Transferase deficiency: a case report. BMC Pregnancy Childbirth. (2020) 20:506. doi: 10.1186/s12884-020-03192-4
96. Ostojic S, Stea T, Ellery S, Smith-Ryan A. Association between dietary intake of creatine and female reproductive health: evidence from NHANES 2017-2020. Food Sci Nutr. (2024) 12:4893–8. doi: 10.1002/fsn3.4135
97. de Guingand D, Palmer K, Callahan D, Snow R, Davies-Tuck M, Ellery S. Creatine and pregnancy outcomes: a prospective cohort study of creatine metabolism in low-risk pregnant females. Am J Clin Nutr. (2024) 119:838–49. doi: 10.1016/j.ajcnut.2023.11.006
98. Deborah D, Jade B, Stacey E, Kirsten P. Exploring pregnant women’s knowledge and attitudes toward dietary/nutritional supplementation in pregnancy. Does it match with health professionals’ current clinical practice? J Paediatr Child Health. (2019) 55(S1):72. doi: 10.1111/jpc.14410_52
Keywords: dietary supplements, nutrition, sports, creatinine, adverse effects, pregnancy, malignancy
Citation: Longobardi I, Solis MY, Roschel H and Gualano B (2025) A short review of the most common safety concerns regarding creatine ingestion. Front. Nutr. 12:1682746. doi: 10.3389/fnut.2025.1682746
Received: 09 August 2025; Accepted: 31 October 2025;
Published: 01 December 2025.
Edited by:
Daniel Moore, University of Toronto, CanadaReviewed by:
Scott Forbes, Brandon University, CanadaEric Williamson, University of Toronto, Canada
Copyright © 2025 Longobardi, Solis, Roschel and Gualano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno Gualano, Z3VhbGFub0B1c3AuYnI=
†These authors have contributed equally to this work and share first authorship
 Igor Longobardi
Igor Longobardi Marina Yazigi Solis1,2†
Marina Yazigi Solis1,2† Hamilton Roschel
Hamilton Roschel Bruno Gualano
Bruno Gualano