- 1College of Physical Education and Health, Anhui University of Chinese Medicine, Hefei, China
- 2College of Sports Medicine, Wuhan Sports University, Wuhan, China
- 3Institute of Acupuncture and Meridians, Anhui University of Chinese Medicine, Hefei, China
Betaine, a natural compound found in beets, wheat germ, shellfish, and mammalian tissues, plays a crucial role in preventing and treating various chronic diseases. As the global population ages, chronic diseases are posing the primary threat to the health of the elderly, significantly increasing the medical pressure on families and society. Chronic diseases associated with aging involve complex molecular mechanisms and, therefore, developing multipronged interventions is crucial for their prevention and treatment. Although exercise is a primary intervention for preventing and treating chronic diseases, many elderly individuals have motor disabilities. Therefore, researchers are exploring natural products that mimic the therapeutic effects of exercise in individuals who are unable to exercise. Betaine has exhibited significant preventive and therapeutic effects in studies on chronic diseases and is known as an exercise mimetic. A deeper understanding of betaine may help elucidate crucial molecular mechanisms underlying its effects and offer theoretical insights for developing exercise-mimicking foods, supplements, and drugs, which are expected to benefit the human health.
1 Introduction
In 2021, noncommunicable diseases resulted in 1.73 billion disability-adjusted life years (1). Chronic diseases are becoming increasingly common in younger populations. According to the 2025 World Health Statistics, 18 million people aged <70 years died of noncommunicable diseases globally in 2021, accounting for more than half of all deaths in this age group (2). Among Chinese people aged >70 years, the prevalence of multiple chronic diseases is 40% (3). Therefore, prevention and treatment of chronic diseases have become major global public health issues. Although clinicians and researchers have been successful in mitigating the progression of chronic diseases to a certain extent through surgery and drugs, the interventions are associated with high medical costs, reduced quality of life, and increased risk of complications (4). Early prevention and treatment may reduce disease incidence, with exercise playing a crucial role (5). The World Health Organization (6) and American College of Sports Medicine (7) recommend that older adults engage in physical activity at least 2–3 times per week to maintain cardiorespiratory, musculoskeletal, and neuromotor fitness. However, effective exercise is not feasible for individuals who are elderly, bedridden, or suffering from motor function loss. Therefore, researchers in sports medicine are exploring drugs, natural products, and synthetic small molecules that can replace exercise. Compared with drugs, small-molecule agonists, and/or inhibitors, natural ingredients may be safer and more suitable for long-term use and serve as sources of motion mimetics. Betaine, a trimethylglycine abundant in plants, is a primary osmoprotectant and methyl donor that plays a significant role in preventing and treating chronic diseases. It has also been identified as an exercise mimetic. This article summarizes the role of betaine in the prevention and treatment of chronic diseases, providing molecular insights and a theoretical basis for developing functional foods, supplements, and drugs.
2 Betaine
Betaine (C5H11NO2) is a trimethyl derivative of glycine, with a molecular weight of 117.146 g/mol. This stable, nontoxic natural substance, discovered as a byproduct of beet processing, is widely present in microorganisms, plants, and animals. Dietary sources include wheat bran (1,339 mg/100 g), spinach (600–645 mg/100 g), beets (114–297 mg/100 g), shrimp (219 mg/100 g), and whole wheat bread (20 mg/100 g). It is also synthesized by the kidneys and liver (8, 9) (Figure 1A). The median daily betaine intake for the general population is 224.77 mg (10), typically ranging between 100 and 300 mg (11). It is rapidly absorbed through the duodenum, remains unbound to proteins, with its plasma content being 20–70 μmol/L (9, 12) (Figure 1B). Because betaine can pass freely through the kidneys in its unmetabolized form, it is primarily converted into methionine, S-adenosylmethionine (SAM), and dimethylglycine in the body, with only small amounts excreted through urine and sweat. A single dose of betaine (50 mg/kg) in healthy young men reached a peak concentration of 1 mmol/L within 1 h. The elimination half-life of a single dose is approximately 14 h, and <5% of the dose remains after 72 h (13) (Figure 1C). Biologically, betaine functions primarily as a methyl donor in transmethylation and as an osmoprotectant. It participates in various biological processes by gradually removing methyl groups and forming sarcosine and glycine through decomposition and metabolism. As an osmoprotectant, betaine primarily protects cells from osmotic/ionic stress by regulating the concentration and volume of intracellular fluid. Additionally, it exhibits positive regulatory effects in various chronic disease models (14, 15). Betaine regulates cell signals, playing a significant role in disease prevention and treatment.

Figure 1. Schematic depicting the sources, synthesis, and kinetics of betaine. Betaine entering the circulatory system is derived from the consumed food and via synthesis in the liver (A); it is converted by the liver into methyl donors, such as S-sarcosine and glycine (B), as well as osmoprotectants, which participate in transmethylation and cell volume regulation, respectively. Some amount of betaine and its metabolites is excreted from the body through urine and sweat (C).
3 Betaine metabolism
Betaine transfers a methyl group to homocysteine to form methionine via a reaction catalyzed by betaine-homocysteine methyltransferase (BHMT). After losing its methyl group, betaine is converted into dimethylglycine, which undergoes oxidation in the mitochondria. Dimethylglycine dehydrogenase (DMGDH) removes a methyl group to produce sarcosine that enters the electron transport chain, generating reduced nicotinamide adenine dinucleotide/reduced flavin adenine dinucleotides (NADH/FADH2). Sarcosine dehydrogenase (SDH) removes the last methyl group of sarcosine to form glycine, generating NADH/FADH2. Methionine is converted to S-adenosylmethionine (SAM) via the transfer of adenosyl group from adenosine triphosphate (ATP) by methionine adenosyltransferase (MAT). SAM is the primary methyl donor for methylation reactions in the body, providing methyl groups for the modification of DNA, RNA, proteins, phospholipids (phosphatidylcholine), and neurotransmitters. After losing its methyl group, SAM is converted to S-adenosyl-l-homocysteine (SAH)—a potent methyltransferase inhibitor. Increased levels of SAH inhibit methylation reactions, making its rapid metabolism crucial. SAH is hydrolyzed by S-adenosylhomocysteine hydrolase, resulting in the formation of homocysteine and adenosine. Homocysteine can reenter the BHMT pathway and get methylated by betaine to methionine, thereby completing the cycle. If the body is deficient in betaine or folic acid and requires cysteine, homocysteine cannot be remethylated to methionine. Instead, it combines with serine to generate cystathionine through cystathionine β-synthase (CBS), which is then cleaved by cystathionine γ-lyase to generate cysteine and α-ketobutyrate. Cysteine contributes to glutathione and protein synthesis and can be oxidized and decomposed into sulfate for excretion or is converted to taurine (Figure 2). Therefore, the contribution of betaine to the methionine cycle is crucial for maintaining normal cell function, gene expression regulation, antioxidant defense, and numerous biosynthetic pathways.

Figure 2. Metabolism of betaine. In a reaction catalyzed by betaine-homocysteine methyltransferase (BHMT), betaine loses a methyl group to form dimethylglycine, which is subsequently demethylated by dimethylglycine dehydrogenase (DMGDH) to form sarcosine that enters the electron transport chain. Sarcosine dehydrogenase (SDH) removes the last methyl group of sarcosine to form glycine. Homocysteine obtains the methyl group transferred by BHMT-catalyzed betaine to form methionine that is converted into S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT) via transfer of adenosyl group from adenosine triphosphate (ATP). SAM provides methyl groups for the methylation of DNA, RNA, proteins, phospholipids (phosphatidylcholine), and neurotransmitters.
4 Molecular mechanisms underlying the exercise-mimicking effects of betaine
Betaine is involved in a variety of molecular mechanisms in the body. It exhibits similar effects as exercise in delaying aging and promoting health and is, therefore, considered an exercise mimic (16). Exercise is widely advocated as an intervention to promote health and prevent disease; it mainly activates AMPK through energy consumption and is involved in the regulation of multiple network mechanisms (17). Exercise can enhance the antioxidant, anti-inflammatory, and antiapoptotic effects, regulate immunity, activate autophagy, improve the mitochondrial quality, tissue integrity, circadian rhythm, genetics, endocrine system, and gut microbiota, and prevents and delays chronic diseases, such as cognitive decline and skeletal muscle atrophy (18). Betaine can also activate AMPK, reduce oxidative stress, endoplasmic reticulum stress, inflammation, and apoptosis, delay aging and cancer development, and plays a positive regulatory role in maintaining tissue integrity and mitigating chronic diseases (19, 20). Notably, the role of betaine in mimicking exercise is mainly reflected in activating AMPK (20), improving mitochondrial quality (21), regulating autophagy (22), reducing oxidative stress (23), inhibiting inflammation (24), and genetic modification (25). These are also important mechanisms for maintaining health and preventing and treating diseases. These studies have provided insights into the effects of betaine at the molecular level and form a theoretical basis for developing it as a functional food, nutritional supplement, and drug, benefitting human health.
5 Betaine and chronic diseases
Research on the use of betaine in the prevention and treatment of various chronic diseases is ongoing. Below, we provide a review of the literature on the effects of betaine in diseases, such as obesity, diabetes, liver disease, cardiovascular disease, kidney disease, and Alzheimer’s disease, and discuss the underlying mechanisms.
5.1 Betaine for the prevention and treatment of obesity
With industrialization, physical activities performed by humans have considerably decreased, resulting in energy surplus in the body, which leads to obesity—a global public health concern worldwide associated with various chronic diseases. Betaine has been widely studied for the prevention and treatment of obesity. Epidemiologically, people with a higher intake of betaine in their daily diet generally have a lower body weight (10, 26), which may be a consequence of reduced body fat (27). In middle-aged and elderly Chinese men, serum betaine levels were positively associated with lean body mass in the whole body, trunk, and limbs (28). In women, betaine supplementation did not enhance exercise performance but significantly reduced the percentage and mass of body fat (29). These results indicate that the effect of betaine in preventing obesity may not be affected by factors, such as age, total calorie intake, physical activity level, and trunk fat, consistent with the results of relevant studies on Newfoundland residents (30). However, supplementation with exogenous betaine had no significant effect on body weight, composition, or resting energy expenditure (31), indicating that a larger sample size and detailed studies are required for confirmation. However, betaine resistance may exist in obesity and prediabetes, possibly associated with low DMGDH levels that limits betaine metabolism (32). Therefore, the effects of betaine on weight management may require larger sample sizes and detailed studies.
The beneficial effects of betaine on obesity and related metabolic disorders have been confirmed in numerous studies. These effects involve various complementary pathways, such as energy metabolism, inflammation regulation, and gut microecological remodeling. In vivo experiments have demonstrated that betaine activates adenosine monophosphate-activated protein kinase subunit alpha 1 (AMPKα1), upregulating fatty acid oxidation, citric acid cycle, and mitochondrial oxidative phosphorylation, thereby accelerating lipid consumption and limiting weight gain (33, 34) (Figure 3A). In a high-fat, high-sugar diet-induced obese mouse model, betaine intervention significantly enhanced glucose utilization efficiency in the skeletal muscle and liver, lowered fasting blood glucose levels, and alleviated systemic inflammation (35) (Figure 3B). If combined with exercise training, the blood sugar-lowering effect is more significant (36). Betaine facilitates mitochondrial regeneration in white adipose tissue, induces browning of white adipocytes, and inhibits the expression of adipogenic genes. Additionally, it reduces the mRNA levels of proinflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-12 (IL-12), both in muscle tissue in vivo and in adipose tissue cells in vitro, thereby alleviating high-fat diet-induced obesity and insulin resistance (37, 38), consistent with the results of facilitating the reduction of skeletal muscle lipid deposition and enhancing adipose tissue lipolysis (39) (Figure 3C). In vitro studies have confirmed that betaine can inhibit the expression of hypoxia-induced IL-6 and tumor necrosis factor-alpha (TNF-α) mRNAs in human adipocytes, indicating that it directly acts on adipose tissue to reduce obesity-related low-grade inflammation (38). Moreover, it exhibited conservative metabolic-protective effects in lower vertebrate models. After betaine treatment of high-fat-induced obese black sea bream juveniles, the silent information regulator transcript 1/sterol regulatory element-binding protein 1/peroxisome proliferator-activated receptor alpha (SIRT1/SREBP-1/PPARα) axis was activated, anti-inflammatory cytokines transforming growth factor-beta 1 (TGF-β1) and IL-6 were upregulated, whereas proinflammatory signals, such as nuclear factor kappa-light-chain-enhancer of activated b cells (NF-κB), TNF-α, and interleukin-1 beta (IL-1β) were downregulated, confirming its cross-species anti-inflammatory activity (40). However, the conclusions of these population-based studies are inconsistent. Certain randomized controlled trials have demonstrated that betaine reduces the levels of circulating inflammatory markers; however, the differences were not statistically significant. This may be associated with the small sample size, short intervention period, and numerous confounding factors. A large sample size and long-term intervention are required for validation (41). Additionally, betaine exerts antiobesity effects by reshaping the gut microbiota and its metabolites. In a high-fat diet mouse model, betaine facilitated the proliferation of beneficial bacteria, such as Akkermansia muciniphila, Lactobacillus, and Bifidobacterium, and increased the production of acetate and butyrate. Short-chain fatty acids (SCFAs) inhibit lipid synthesis and enhance insulin sensitivity by increasing the DNA methylation of the miR-378a promoter (42, 43) (Figure 3D). A dose-effect study further confirmed that betaine intervention at a dose of 120 mg/kg body weight for 4 weeks was adequate to significantly enhance glycemic and lipid profiles and reduce body weight in mice fed a high-fat diet (44).

Figure 3. Molecular mechanisms underlying the effects of betaine in preventing and enhancing obesity. Betaine increases energy expenditure by activating the adenosine monophosphate-activated protein kinase–mitochondrial axis, thereby enhancing mitochondrial oxidative phosphorylation (A); improving hepatic and skeletal muscle glucose utilization, reducing lipomatosis, and improving insulin sensitivity and fasting blood glucose (B); promoting white adipose tissue consumption and mitochondrial regeneration, and inhibiting adipogenesis genes, fat deposition, and inflammation (C); increasing DNA methylation through the gut microbiota-short-chain fatty acid-epigenetic cascade, thereby inhibiting lipid synthesis and enhancing insulin sensitivity (D); and regulating metabolic homeostasis and gut microbiota in the offspring to prevent early-onset obesity and improve metabolic health (E).
The role of betaine is also reflected in maternal and child health. Maternal betaine intake affects obesity risk in the offspring. In experiments on obese rats, betaine supplementation during pregnancy helped the offspring restore normal growth and developmental rhythms while reducing obesity (45). Whether the mother consumes betaine through diet during pregnancy or adds it to breast milk, it can regulate the metabolic state and gut microbiota composition of the offspring, thereby preventing early obesity and facilitating long-term metabolic health (46, 47) (Figure 3E). Additionally, human studies have confirmed that betaine content in the maternal body is associated with the birth weight and abdominal fat mass in babies. In particular, higher maternal betaine levels are associated with healthier birth weight and lower abdominal fat mass in infants (48).
Betaine synergistically enhances obesity and its metabolic complications through various targets via the AMPK-mitochondrial axis, SIRT1/SREBP-1/PPARα signaling, inhibition of inflammation, and the microbiota-SCFA-epigenetic cascade, providing a mechanistic basis for its clinical translation.
5.2 Betaine for the prevention and treatment of diabetes
Diabetes is a common metabolic disease, the onset of which is primarily caused by the combined effects of genetic susceptibility and acquired environmental factors (long-term high-sugar and high-fat diets, sedentary lifestyles, and obesity). The core pathological mechanism is insulin resistance in peripheral tissues and organs (reduced insulin sensitivity of muscles, fat, and liver) and reduced pancreatic β-cell function (insufficient or delayed insulin secretion), resulting in increased blood levels (49). No obvious symptoms might be present in the early stages of the disease; however, as the disease progresses, typical manifestations, such as polydipsia, polyphagia, polyuria, and weight loss occur. If blood sugar is not well controlled for a long time, it gradually damage tissues and organs throughout the body and may induce chronic complications, such as cardiovascular disease, kidney disease, neuropathy, and retinopathy (50).
5.2.1 Type 2 diabetes mellitus
The baseline betaine level in patients with type 2 diabetes mellitus (T2DM) is lower than that in healthy individuals (51); however, hyperglycemia is not the cause of increased betaine excretion (52). Nevertheless, it is significantly correlated with glycated hemoglobin levels (53). This is consistent with the results of another study that used liquid chromatography-tandem mass spectrometry to measure betaine in urine and plasma and a multivariate regression analysis to show that glycated hemoglobin was the strongest determinant of betaine excretion in patients with diabetes (54), although different test results have also been reported (55). Betaine is a marker of diabetes risk in high-risk individuals and has been reported to perform a direct role in regulating metabolic health (56). Lower betaine intake is associated with lower insulin levels, homeostatic model assessment of insulin resistance (HOMA-IR) (57), and an increased risk of other T2DM (58). For example, plasma betaine content in patients with T2DM is negatively associated with the occurrence of microvascular complications (59). In a population-based study in Newfoundland, high serum betaine levels were associated with low lipid levels and insulin resistance (60) (Figure 4A). In adults, higher betaine levels were negatively associated with lower diabetes-related markers, serum insulin concentrations, and HOMA-IR (57). However, no significant association between betaine use and diabetes was observed in some other studies. A follow-up study involving 13,440 participants demonstrated that dietary betaine intake was not associated with T2DM (61). A semi-quantitative food frequency questionnaire survey involving 6,022 participants aged ≥18 years in Tehran did not find any association between dietary betaine intake and T2DM (62). These two studies involved a large number of participants. Because of the numerous factors affecting human health, semi-quantitative food frequency questionnaires may not accurately reflect this phenomenon. Therefore, more rigorous clinical and basic studies are required to verify the relationship between betaine and T2DM.

Figure 4. Molecular mechanisms underlying the effects of betaine in regulating certain tissues and functions in type 2 diabetes mellitus (T2DM), gestational diabetes, and diabetic complications. (A) In T2DM, betaine lowers blood lipids, HbA1c homeostatic model assessment of insulin resistance, serum glucose, and insulin resistance. (B) Betaine reduces the risk of gestational diabetes, mitigates DNA damage in placental and embryonic tissues, and increases the thickness of the placental attachment zone and labyrinth. (C) Betaine reduces retinal Akt/vascular endothelial growth factor/hypoxia-inducible factor 1-alpha and alleviates retinopathy. (D) In the testicular tissue, betaine reduces superoxide dismutase (SOD), malondialdehyde (MDA), and p38 mitogen-activated protein kinase signaling pathways and increases superoxide dismutase (SOD), catalase, and glutathione levels. (E) In the brain tissue, betaine activates phosphatidylinositol 3-kinase/protein kinase B (Akt) and enhances cognitive ability. (F) In the renal tissue, betaine reduces reactive oxygen species (ROS) and malondialdehyde (MDA) levels. (G) Benazepril and its analogs appear to exacerbate betaine loss, leading to elevated plasma homocysteine levels, suggesting that betaine supplementation should be considered. (H) Betaine plays preventive, predictive, diagnostic, and therapeutic roles in diabetes-related diseases.
5.2.2 Gestational diabetes
Gestational diabetes is a glucose metabolism disorder that often occurs during pregnancy. Reduced serum betaine levels are an independent risk factor for gestational diabetes that may be associated with blood sugar regulation and short-term fluctuations (63). Higher dietary betaine intake among pregnant women in China was reported to be inversely associated with the risk of gestational diabetes mellitus in women without a history of childbearing (64). Betaine administration to mice after feeding a high-fat diet for 4 weeks before and during pregnancy to induce gestational diabetes alleviated morphological alterations in the placental junction area and increased the glycogen cell area in pregnant mice. Additionally, in vitro experiments have demonstrated that betaine can alter certain determinants of placental transport during the hyperglycemic response (65). In a streptozotocin (STZ)-induced gestational diabetes model, betaine significantly increased insulin levels, restored normal plasma total homocysteine concentrations, and enhanced insulin resistance and blood lipid status (66). Additionally, betaine could reduce increased DNA damage levels in the placental and embryonic tissues of rats at 14–20 days of gestation, with the best effect achieved when betaine was administered orally at a dose of 100 mg/kg body weight (67) (Figure 4B).
5.2.3 Diabetic complications
Basic research on the effects of betaine on diabetes is increasing, with some studies demonstrating positive results. Betaine can inhibit the activation of protein kinase B (Akt) in the retina of STZ-induced diabetic rats, thereby attenuating the increase in vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-alpha (HIF-1α) expression and inhibiting neovascularization to delay diabetic retinopathy-associated complications (59) (Figure 4C). In the STZ-induced diabetic mouse model, oral administration of betaine reduced the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in the testicular tissue and increased the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), thereby inhibiting the p38 mitogen-activated protein kinase (p38 MAPK) signaling pathway and protecting against blood-testis barrier dysfunction (68) (Figure 4D). Similarly, in STZ-induced diabetic rats, betaine inhibited oxidative stress and inflammation while activating the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway to enhance cognitive impairment (69) (Figure 4E). In a diabetic db/db mouse model, betaine alleviated endoplasmic reticulum and oxidative stress to enhance insulin resistance, hyperlipidemia, and tau protein hyperphosphorylation (70). In STZ-induced male diabetic rats, betaine reduced the increase in the levels of glycosylated hemoglobin in in blood, serum glucose, and lipids, and the pro-oxidative state in the liver and kidney (71) (Figure 4F). Betaine exerts a preventive effect, even in cases of diabetes caused by arsenic poisoning-induced impaired glucose tolerance (72). However, the use of certain drugs may result in the abnormal excretion of betaine from the body. Benazepril esters appear to aggravate betaine loss, resulting in increased plasma homocysteine levels (Figure 4G). Therefore, betaine supplementation should be considered when treating patients with fibric acid drugs (73).
Studies in human subjects and basic experiments show that betaine may have significant potential in the prevention, prediction, diagnosis, and treatment of diabetes and diabetic complications (Figure 4H). Although certain results were inconsistent, more authoritative experiments are required for verification of these findings.
5.3 Betaine for the prevention and treatment of liver disease
As a highly complex chemical factory, the liver plays a crucial role in metabolism and processing of proteins, fats, sugars, vitamins, and hormones. With modern lifestyle, liver disease has become common worldwide. Metabolic fatty liver disease affects 30% of the global population (74); however, no treatment is available for alcoholic liver disease (75). The effects of betaine on the liver have been assessed for decades, and positive effects via various molecular mechanisms have been reported.
5.3.1 Metabolic fatty liver
Metabolic fatty liver disease often coexists with metabolic disorders, such as obesity and metabolic syndrome, and can result in reduced betaine levels in the body (76). Betaine primarily regulates hepatic lipid metabolism by inhibiting lipogenesis and facilitating fatty acid oxidation. In the classic db/db mouse model of metabolic fatty liver, oral administration of betaine can inhibit the interaction between forkhead box protein O6 and peroxisome proliferator-activated receptor gamma (PPARγ), inhibit the expression of primary lipogenic genes, such as fatty acid synthase (FAS) and acetyl-CoA carboxylase, and reduce hepatic lipid accumulation (77, 78). Simultaneously, betaine can significantly increase the expression of genes (carnitine palmitoyltransferase 1 and PPARα) and enhance fatty acid oxidation and lipid transport, thereby alleviating fat accumulation in the liver (79, 80). In a vitamin B6 deficiency-induced hepatic fat deposition model, betaine exhibited a significant inhibitory effect and restored methionine metabolism and very low-density lipoprotein secretion, the mechanisms of which may be associated with the restoration of the phosphatidylethanolamine-to-phosphatidylcholine conversion pathway (81, 82). Epigenetic regulation is another significant regulatory mechanism. For example, in a hen model, betaine inhibited lipogenesis and facilitated lipid decomposition by altering DNA methylation levels or mRNA N6-methyladenosine (m6A) modification in the promoter region of genes, such as sterol regulatory element-binding protein 1, FAS, and stearoyl-CoA desaturase, thereby reducing hepatic triglyceride deposition (83). The addition of demethylase blocked the regulatory effects of betaine on lipid metabolism and mitochondrial content, further confirming that betaine affects RNA methylation (84). Regarding glucose metabolism and whole-body energy balance, betaine can enhance the activity of enzymes associated with glucose uptake, glycogen synthesis, and decomposition in the liver and muscles, indicating its potential to regulate glucose metabolism disorders (35). Additionally, betaine supplementation can increase the levels of fibroblast growth factor 21 (FGF21) in the liver and circulation, enhance white fat oxidation capacity and whole-body energy expenditure, thereby enhancing blood glucose homeostasis and metabolic health (79, 83, 85). Moreover, betaine exhibits antioxidant, anti-inflammatory, and gut microbiota-regulatory functions. In a high fructose-induced rat model, betaine reversed the increased levels of oxidative stress indicators and inflammatory factors and alleviated liver damage (86). In fish, betaine enhanced the structure of the gut microbiota, regulated trimethylamine metabolism and bile acid metabolism, and indirectly alleviated liver fat accumulation induced by a high-carbohydrate diet (87). Notably, the protective effect of betaine is consistent across species and has been verified in various models, such as mice, rats, fish, and poultry, even demonstrating a preventive effect during the embryonic or maternal supplementation stages (80, 83, 88). However, certain studies have indicated that although it facilitates growth in a growing pig model, it does not significantly affect fatty acid oxidation, indicating that the mechanism may be species-specific (89). Betaine regulates liver and systemic metabolism through various pathways and demonstrates broad potential for preventing and alleviating metabolic fatty liver disease. However, its specific mechanism of action varies based on the model and species, and further research is required to clarify this mechanism.
5.3.2 Nonalcoholic fatty liver disease
Betaine exhibits multilevel protective effects against nonalcoholic fatty liver disease (NAFLD) through mechanisms involving metabolic regulation, epigenetic modification, signaling pathway regulation, and enhancement of the gut microenvironment. Among these, epigenetic regulation plays a crucial role. As a methyl donor, betaine increases overall methylation levels and regulates abnormal DNA methylation [by regulating cytosine-phosphate-guanine (CpG) methylation in the promoter region of PPARγ and hepcidin antimicrobial peptide genes associated with lipogenesis and iron metabolism]. Additionally, it inhibits the m6A hypomethylation state, thereby regulating gene expression to reduce fatty acid synthesis and increase fat decomposition, which reduces lipid accumulation in the liver and protects it (90–93). Betaine can upregulate BHMT, enhance the production of nicotinamide adenine dinucleotide phosphate, and increase the expression of fat and obesity-related protein (FTO), which reduces m6A levels in the coding sequence region of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) transcript. This upregulates PGC-1α and inhibits lipid accumulation in the liver through the BHMT/FTO/m6A/PGC-1α pathway to alleviate NAFLD (94) (Figure 5A). Betaine activates various primary signaling pathways involved in metabolic regulation. It can inhibit the expression of lipid metabolism-related genes through the FGF10/AMPK pathway, facilitate fatty acid β-oxidation, and alleviate endoplasmic reticulum stress by restoring the expression of liver X receptor alpha and PPARα. Additionally, it enhances the Akt/mechanistic target of rapamycin signaling pathway, activates autophagy, and stimulates insulin receptor substrate 1 to activate downstream signaling pathways, thereby alleviating hepatic steatosis, gluconeogenesis, and inflammatory response (22, 95–97). Betaine exhibits significant antioxidant and anti-inflammatory effects. It enhances the metabolism of sulfur-containing amino acids to enhance antioxidant defense, and reduce oxidative stress, expression of inflammatory factors [TNF-α, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS)], and cell apoptosis through signaling pathways, such as high mobility group box 1/toll-like receptor 4 (TLR4), thereby restoring liver function (98–100). Additionally, betaine enhances mitochondrial function, reduces the number of swollen mitochondria, and increases autophagosome formation, thereby alleviating damage to liver cells (101). Notably, the effects of betaine were cross-organ and cross-generational. Maternal betaine supplementation can enhance high-fat diet-induced NAFLD by regulating the gut microbiota and SCFAs in the offspring (102) (Figure 5B). Moreover, the protective effects of betaine on the liver may involve intergenerational communication between the liver and brain, such as regulating brain phospholipid metabolism (103). Clinical and pathological evidence supports its effectiveness. Patients with NAFLD often have low betaine levels (104), and clinical interventions have demonstrated that oral betaine can significantly reduce serum alanine aminotransferase and aspartate aminotransferase levels and enhance fat degeneration, inflammatory necrosis, and fibrosis (105). Although betaine failed to enhance hepatic steatosis, it prevented the worsening of hepatic steatosis (106). In animals, betaine can effectively reduce the liver triglyceride content, inhibit liver cell swelling and necrosis, and reduce lipid deposition (107, 108). In summary, betaine exhibits significant potential for preventing and alleviating NAFLD through synergistic effects on various targets and pathways.
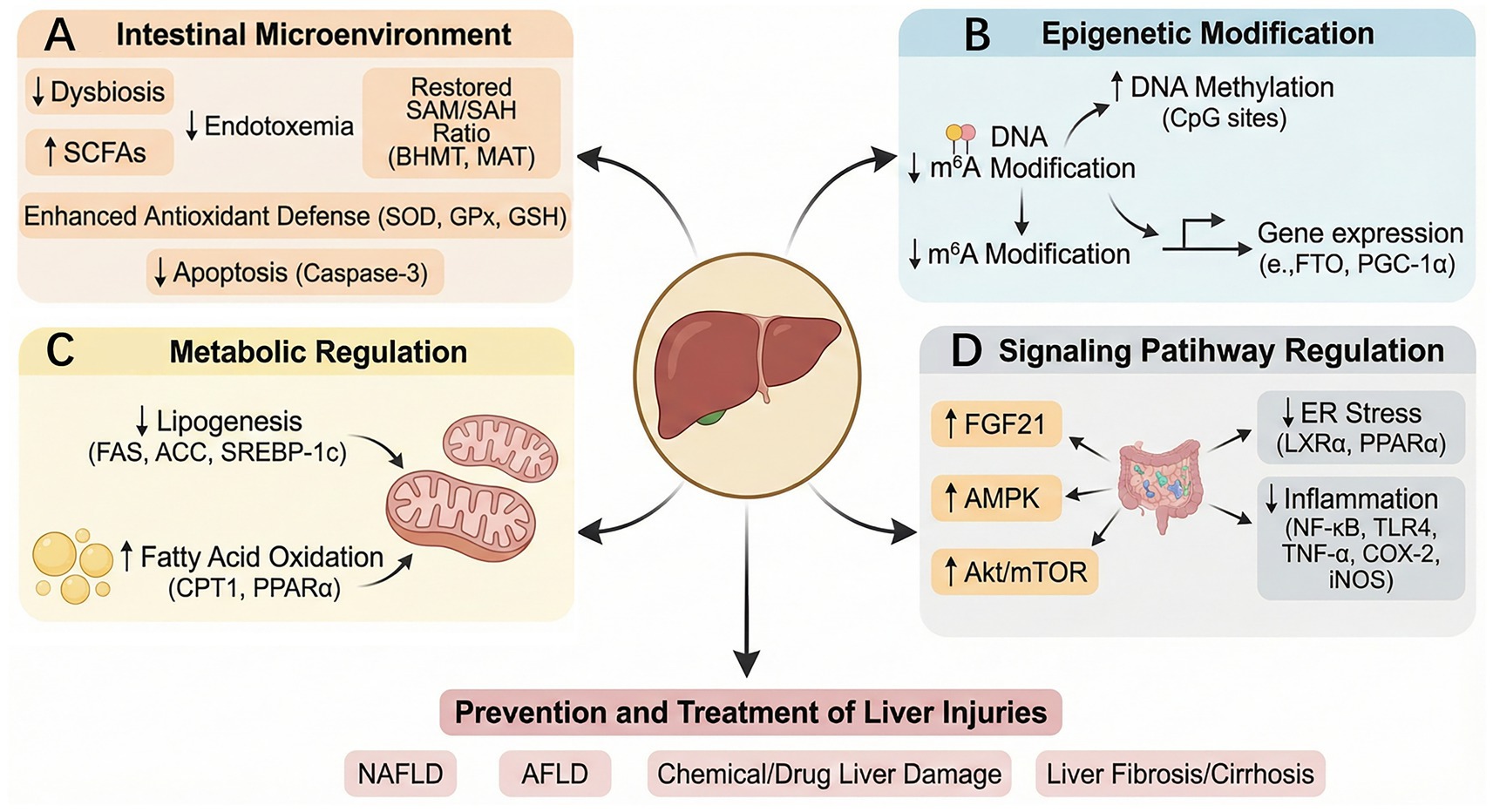
Figure 5. Mechanisms underlying the protective effects of betaine in various liver diseases and injury. Betaine works by improving the gut dysbiosis, increasing short-chain fatty acid levels, reshaping the SAM/SAH ratio, enhancing antioxidant defense, and inhibiting apoptosis (A); regulating the expression of genes such as PGC-1α through m6A methylation of DNA (B); downregulating the levels of genes and proteins related to lipogenesis and upregulating the levels of genes and proteins related to fatty acid oxidation (FAS, ACC, SREBP-1c↓; CPT-1PPARα↑) (C); and participating in the regulation of molecular signaling pathways related to AMPK, Akt/mTOR, endoplasmic reticulum stress, and inflammation (D), thereby preventing and treating liver damage caused by NAFLD, AFLD, liver fibrosis/cirrhosis, and chemical/drug liver damage.
5.3.3 Alcoholic fatty liver disease
Betaine exerts a significant protective effect against ethanol-induced alcoholic liver damage through various pathways. Its core mechanism focuses on restoring methyl metabolic homeostasis, enhancing lipid metabolism, and antagonizing oxidative stress-induced damage. The main role of betaine is to reshape methyl metabolism balance in the liver. Betaine supplementation can enhance hepatic methionine metabolism and SAM levels in the early stages, facilitate choline-histidine methyltransferase activity, and reduce hepatic triglyceride accumulation (109). Additionally, betaine significantly upregulates the expression of BHMT-1, methionine adenosyltransferase-1, and glycine N-methyltransferase, effectively increasing the levels of SAM in the liver and restoring the normal SAM/SAH metabolic ratio (110–114). Normalization of this crucial ratio enhances the activity of phosphoethanolamine methyltransferase, restores normal synthesis of phosphatidylcholine to reduce fat deposition (112), reactivates the repair response mediated by protein L-isoaspartate methyltransferase (111), and attenuates liver damage caused by reduced methylation of the protein phosphatase 2a (PP2A) catalytic subunit (115). Notably, betaine can inhibit alcohol-induced increases in hepatocyte SAH (a potent inhibitor of methylation reactions), increase caspase-3 levels, and reduce DNA content, demonstrating therapeutic potential (116). In regulating lipid metabolism, betaine can directly inhibit primary lipogenesis genes, including diacylglycerol O-acyltransferase 1/2, SREBP-1c, and FAS, while upregulating factors, such as PGC-1α, thereby synergistically enhancing alcohol-induced liver lipid accumulation (117, 118) (Figure 5C). Additionally, betaine exhibits strong antioxidant and anti-inflammatory capabilities, alleviating oxidative damage by reversing GSH depletion, increasing MDA levels, reducing vitamin A content, and inhibiting cytochrome p450 family 2 subfamily E member 1 expression and hydroxytaurine production (113, 119). Simultaneously, betaine can inhibit primary inflammatory signaling pathways, such as TLR4, prevent the upregulation of related proinflammatory factors and signaling molecules (TLR2/4, IL-1β, and signal transducer and activator of transcription 3), and alleviate the inflammatory response (120, 121). Notably, betaine may facilitate adrenaline synthesis by affecting phenylethanolamine N-methyltransferase, thereby indirectly increasing the alcohol metabolism rate and preventing excessive alcohol concentrations (122). The study demonstrated that betaine protects the liver in a dose-dependent manner, with 0.5% betaine in feed sufficient to raise SAM levels that prevent fatty liver disease (114). However, the effects of betaine are selective. For example, in certain models, it did not reverse carbonic anhydrase-III downregulation or reduce the expression of TNF-α and cluster of differentiation 14 mRNA (110, 123), indicating that its mechanism of action is highly specific. In summary, by correcting the imbalance in methyl metabolism, betaine synergistically regulates lipid metabolism, oxidative stress, and inflammatory pathways, forming a multidimensional protective network against alcoholic liver damage.
5.3.4 Other liver damage types
Betaine protects against various types of liver damage, including chemical poisoning, drugs, liver fibrosis, and cirrhosis. Its efficacy is achieved through various mechanisms, including antioxidant, anti-inflammatory, metabolic, and epigenetic regulation.
5.3.4.1 Chemical and drug-induced liver damage
In liver injury models induced by chemical poisons and drugs, such as carbon tetrachloride, diethylnitrosamine, acetaminophen, thioacetamide, and cisplatin, betaine exerts core protective effects primarily by enhancing antioxidant defense and inhibiting inflammation. Betaine can significantly increase the activity of antioxidant enzymes [SOD and glutathione peroxidase (GPx)], restore GSH levels, reduce MDA content, inhibit the expression of primary inflammatory factors (NF-κB, TNF-α, and COX-2), and inhibit lipid deposition and degeneration (124–131) (Figure 5D). Additionally, betaine can protect mitochondrial function by protecting complex II activity and inhibiting apoptosis by inhibiting caspase-3, thereby maintaining hepatocyte structural integrity (21, 126, 131, 132). Betaine may play a protective role against niacin-induced hepatotoxicity by preventing SAM depletion (133).
5.3.4.2 Liver fibrosis and cirrhosis
In a liver fibrosis model induced by a high-fat diet combined with carbon tetrachloride, bile duct ligation, ethanol, and other factors, betaine exerted its effects through the aforementioned antioxidant and anti-inflammatory mechanisms and directly targeted the antifibrosis pathway. Betaine can effectively delay the progression of fibrosis by downregulating TGF-β1, alpha-smooth muscle actin (α-SMA), and collagen type I alpha 1 chain (COL1A1), regulating the balance of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1/-2, and inhibiting the activation of hepatic stellate cells (23, 125, 134–136). Clinical observations have demonstrated that plasma betaine levels in patients with cirrhosis are associated with disease severity and decrease after liver transplantation, indicating their association with disease progression (137).
5.3.4.3 Special types of liver injury caused by radiation, ischemia-reperfusion, and viral hepatitis
In radiation-induced liver injury and liver ischemia-reperfusion models, betaine plays a protective role mainly via its strong antioxidant activity, delaying tissue damage (124, 138). In chronic hepatitis C, betaine exhibits immunomodulatory potential and enhances interferon α antiviral signaling by restoring signal transducer and activator of transcription 1 methylation (139).
5.3.4.4 Transgenerational programming effects and developmental regulation
The protective effects of betaine are characteristics of transgenerational epigenetic programming. Maternal betaine intake (rats and pigs) can programmatically alter liver metabolism, cell proliferation, and the developmental trajectory of offspring—and even the F2 generation—by affecting the DNA methylation pattern of the insulin-like growth factor (IGF) gene family (IGF-1/2) in the liver of the offspring and regulating the expression of glucocorticoid receptor signaling and lipolysis genes (adipose triglyceride lipase and hormone-sensitive lipase) (25, 140–145).
Betaine exhibits multitarget protective effects. Its main function is to provide methyl groups and synergistically regulate various pathways—including antioxidative stress, inflammation suppression, metabolic homeostasis, and epigenetic programming—thereby forming a networked protective system. This has the potential for effective prevention and treatment of various liver injuries, including the chemical, fibrotic, and viral types.
5.4 Betaine for the prevention and treatment of cardiovascular disease
Existing studies on the association between betaine and cardiovascular health have yielded inconsistent results. Several large-scale, long-term follow-up studies have demonstrated no significant association between betaine intake and the risk of cardiovascular disease morbidity or mortality in the general adult population (146–149). A study involving 18,076 novel cardiovascular disease events further confirmed the lack of a significant association between the two diseases (148). However, betaine has demonstrated different effects in specific populations or disease contexts. For example, in the Guangdong population of China, higher dietary betaine intake was associated with a lower risk of cardiovascular death (150). In patients with ischemic stroke, betaine helped reduce the risk of recurrent cardiovascular events and enhanced cognitive function (151, 152). In African Americans, dietary betaine intake increased nonlinearly with the risk of coronary heart disease (153). Regarding biomarkers, low-dose betaine supplementation (<4 g/day) can reduce homocysteine levels without causing lipid abnormalities (154), but daily supplementation of ≥4 g may slightly increase total cholesterol levels (155). Betaine levels are inversely associated with blood pressure, particularly in women (156). At the mechanistic level, betaine may exert a cardioprotective effect by inhibiting oxidative stress, inflammation, and fibrosis by regulating the AMPK/nuclear factor erythroid 2-related factor 2 (Nrf2)/TGF-β signaling pathway (24), and enhance pulmonary hypertension and ischemia-reperfusion injury in animal models (Figure 6A). Additionally, the mechanism may be associated with the regulation of Ras homologous family member A/Rho-associated coiled-coil-containing protein kinase pathway (157, 158). Lower plasma betaine levels are associated with an increased risk of heart failure and myocardial infarction (159). In patients with hypertension, a U-shaped relationship was observed between serum betaine levels and the risk of first ischemic stroke (160). Overall, the effects of betaine on the cardiovascular system are heterogeneous across different populations and are dose-dependent. For example, daily supplementation of 4 g for more than 6 weeks may lead to a moderate increase in the levels of plasma total cholesterol (155) and trimethylamine oxide (161), which is detrimental to cardiovascular health.

Figure 6. Mechanisms underlying the effects of betaine in preventing and treating cardiovascular, kidney, and Alzheimer’s diseases. Betaine reduces hypertension and ischemia-reperfusion injury by inhibiting Ras homologous family member A/Rho-associated coiled-coil-containing protein kinase and enhances cardiovascular function by activating the adenosine monophosphate-activated protein kinase/nuclear factor erythroid 2-related factor 2 (Nrf2)/transforming growth factor-beta pathway, thereby reducing oxidative stress, inflammation, and fibrosis (A); reduces oxidative stress, neuroinflammation, and apoptosis by activating Nrf2/heme oxygenase-1, thereby enhancing glomerular function and kidney health (B); enhances cognition and memory by inhibiting amyloid-beta production, oxidative stress, NOD-, LRR-, and pyrin domain-containing protein 3/nuclear factor kappa-light-chain-enhancer of activated B cells pathway, and inflammation, and by activating protein phosphatase 2a to inhibit Tau hyperphosphorylation, thereby enhancing the Alzheimer’s disease (C).
5.5 Betaine for the prevention and treatment of kidney disease
The effects of betaine on kidney health are multifaceted across models and populations; however, these effects are context-dependent. In cats with chronic kidney disease (CKD), betaine supplementation enhances renal health and reverses weight loss, possibly through its effects on one-carbon metabolism, gut microbiota, body composition, and plasma metabolome (162, 163). Similarly, betaine positively modulated plasma and fecal metabolites in a dog model of chronic kidney disease (164). However, population-based studies have demonstrated mixed results. A study of 478 patients with CKD revealed that higher plasma betaine concentrations were a risk factor for adverse renal outcomes (165). In the context of CKD, betaine may also exhibit adverse effects, via multiple molecular mechanisms. For example, the accumulation of TMAO levels in the body promotes renal fibrosis, inflammation, and atherosclerosis, thereby accelerating the progression of CKD (166, 167); high betaine levels may lead to abnormal methylation of profibrotic genes (such as TGF-β and COL1A1), promoting renal interstitial fibrosis (168); and methylation cycle disorders in patients with CKD lead to homocysteine accumulation, whereas abnormal betaine metabolism may exacerbate reactive oxygen species production, promoting renal tubular damage (169). These abnormal changes are likely closely related to renal function, and kidney disease appears to disrupt this process (Table 1). However, plasma betaine levels were significantly associated with the estimated glomerular filtration rate and exhibited good discriminatory ability in distinguishing CKD states (170). In Chinese children and adolescents, betaine intake may reduce the risk of hyperuricemia by enhancing glomerular filtration (171). Mechanistically, numerous animal experiments have revealed the protective potential of betaine, whose effects are primarily reflected in combating oxidative stress, inhibiting inflammatory responses, and regulating primary signaling pathways. For example, in potassium chloride-induced hyperuricemic mice (172), arsenic-exposed mice (173, 174), and sodium fluoride-induced renal injury rat models (175), betaine exerts protective effects by regulating urate transporters, inhibiting oxidative stress, and modulating the Nrf-2/heme oxygenase-1 (HO-1) signaling axis (Figure 6B). In various renal injury models induced by high fructose (176), cadmium (177), cisplatin (178), doxorubicin (179), isoproterenol (180), and carbon tetrachloride (181), betaine protects renal function through various mechanisms, such as reducing lipid deposition, inhibiting inflammasome activation, and inhibiting apoptosis. Additionally, betaine enhances renal pathological damage in diabetic pregnant mice (182) and thioacetamide-induced renal injury mouse models (183).
5.6 Betaine for the prevention and treatment of Alzheimer’s disease
Betaine has demonstrated multipathway protective potential in various experimental models of Alzheimer’s disease (AD). In amyloid-beta (Aβ)-induced AD rat or mouse models, betaine can effectively enhance cognitive dysfunction and memory impairment, and its effects are closely associated with increasing GSH, reducing oxidative markers such as MDA, and activating primary proteins such as SIRT1 (184–186). In a homocysteine-induced cognitive impairment model, an independent risk factor for AD, betaine alleviated cognitive impairment by inhibiting the NLRP3/caspase-1/GSDMD pathway in an m6A-YTHDF2-dependent manner, thereby suppressing microglial pyroptosis (187). At the molecular level, betaine regulates the processing of amyloid precursor proteins by enhancing α-secretase activity and reducing β-secretase activity, thereby reducing Aβ production (188). Additionally, it activates PP2A to reduce Tau protein hyperphosphorylation (189) and inhibits the nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing protein 3/NF-κB signaling pathway to alleviate neuroinflammation (190) (Figure 6C). Moreover, betaine exerts neuroprotective effects by inhibiting the expression of ferroptosis-related factors (acyl-CoA synthetase long-chain family member 4 and transferrin receptor 1) (191). Furthermore, in 3xTg transgenic AD model mice and Caenorhabditis elegans models, betaine enhanced synaptic function and alleviated Aβ toxicity (192, 193). In clinical research, a meta-analysis of 7,009 patients with AD indicated that betaine enhanced the cognitive function and improved the quality of life (194). However, the results of small-scale human trials remain unclear and should be verified with larger samples (195). In summary, betaine may play a significant role in the prevention and intervention of AD through various mechanisms, such as anti-oxidation, anti-inflammation, regulation of Aβ and Tau pathology, and inhibition of ferroptosis.
6 Challenges for the use of betaine in disease prevention and treatment
Based on the current research on betaine use for the prevention and treatment of chronic diseases (196), the vast majority of experimental results from basic research have been positive. However, disease enhancement has rarely been observed in clinical studies. This may be because numerous factors affect the population and studies primarily rely on semi-quantitative questionnaires that limit the ability to accurately assess intake. Additionally, betaine is easily absorbed by the human body and its intake is not problematic. Betaine primarily acts as a methyl donor; however, folic acid, vitamin B12, and choline can also serve this function, which may diminish its unique molecular biological role. The mechanisms of betaine metabolism likely involve methylation, gut microbiota, inflammation, oxidative stress, and apoptosis. Owing to its complexity, betaine may be more suitable for dietary supplementation rather than for targeted drug development. High betaine intake may cause gastrointestinal discomfort, and the long-term safety of high-dose use remains limited, particularly in patients with chronic diseases or in the elderly. During normal supplementation, a daily intake of no more than 6 mg/(kg·bw) of betaine is considered safe (197). High doses (≥3 g/single dose) can cause nausea, diarrhea, and gastrointestinal discomfort, possibly due to increased intestinal water secretion caused by high osmotic pressure. These dose-dependent discomfort symptoms can be alleviated by taking the medication in divided doses or with food. Betaine should be used with caution in individuals with CKD and in those requiring cholesterol control, as it may exacerbate disease progression (166, 198). Current research also indicates that betaine has extremely low toxicity and is not heritable (197, 199). Therefore, large-scale clinical research and deeper mechanistic exploration are required to supplement its specific effects, supplementary dosage, and disease intervention time to maximize its role in preventing and treating chronic diseases.
7 Conclusion
As a primary methyl donor and osmotic pressure regulator, betaine plays a crucial role in the prevention and treatment of various chronic diseases (obesity, diabetes, metabolism-related fatty liver disease, CKD, and Alzheimer’s disease). Its mechanism of action primarily involves inhibiting the inflammatory response, reducing oxidative stress, resisting apoptosis, and regulating gene expression through epigenetic modifications (DNA methylation). Additionally, betaine can significantly alleviate tissue damage and disease processes caused by drug toxicity, viral infection, and chemical exposure. Although preclinical research data on betaine are encouraging, the conclusions drawn from population epidemiological surveys and randomized controlled clinical trials are inconsistent, and most studies have failed to its replicate significant protective effects. This indicates that the therapeutic efficacy of betaine may be affected by various complex factors. However, in this article, we did not compare all the benefits of betaine and exercise and only analyzed the main mechanisms by which exercise affects disease prevention and treatment. Furthermore, it lacks a comprehensive and detailed analysis of the molecular mechanisms of betaine, as well as quantitative data presentation. Future research should introduce advanced technologies, such as multiomics integrated analysis to systematically clarify the precise targets and core regulatory networks of betaine in the human body, reveal the reasons for its variable effects in different individuals, and identify the subgroups most likely to benefit. Therefore, these in-depth studies will provide primary evidence for determining whether betaine has the potential to be a clinically effective nutritional supplement or therapeutic drug and guide its precise application.
Author contributions
YX: Visualization, Writing – original draft. JG: Visualization, Writing – original draft. MW: Conceptualization, Resources, Supervision, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This project was supported by a special support plan of high-level talent introduction of Anhui University of Chinese Medicine (No. DT2500001553).
Acknowledgments
The authors would like to thank Editage for English language editing.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational location1s, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/s0140-6736(24)00757-8
2. World Health Organization. World health statistics 2024: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization (2024).
3. Hu, Y, Wang, Z, He, H, Pan, L, Tu, J, and Shan, G. Prevalence and patterns of multimorbidity in China during 2002–2022: a systematic review and meta-analysis. Ageing Res Rev. (2024) 93:102165. doi: 10.1016/j.arr.2023.102165,
4. Morales-García, D, Rabanal-Llevot, JM, García-Diez, V, Colsa-Gutiérrez, P, Rica, AS, Maseda-Garrido, E, et al. Morbidity and mortality of emergency surgery in octogenarian patient. Cir Cir. (2024) 92:469–74. doi: 10.24875/ciru.23000447,
5. Pedersen, BK, and Saltin, B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. (2015) 25:1–72. doi: 10.1111/sms.12581,
6. World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization (2020).
7. Garber, CE, Blissmer, B, Deschenes, MR, Franklin, BA, Lamonte, MJ, Lee, IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb,
8. Day, CR, and Kempson, SA. Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta. (2016) 1860:1098–106. doi: 10.1016/j.bbagen.2016.02.001,
9. Craig, SA. Betaine in human nutrition. Am J Clin Nutr. (2004) 80:539–49. doi: 10.1093/ajcn/80.3.539,
10. Kuerbanjiang, M, Yu, W, Shang, T, Liu, Y, Muheyati, D, Lv, MX, et al. Association between dietary betaine intake and overweight or obesity. Sci Rep. (2024) 14:32031. doi: 10.1038/s41598-024-83646-3,
11. Ueland, PM. Choline and betaine in health and disease. J Inherit Metab Dis. (2011) 34:3–15. doi: 10.1007/s10545-010-9088-4,
12. Hoffman, JR, Ratamess, NA, Kang, J, Rashti, SL, and Faigenbaum, AD. Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr. (2009) 6:7. doi: 10.1186/1550-2783-6-7,
13. Schwahn, BC, Hafner, D, Hohlfeld, T, Balkenhol, N, Laryea, MD, and Wendel, U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br J Clin Pharmacol. (2003) 55:6–13. doi: 10.1046/j.1365-2125.2003.01717.x,
14. Zhao, G, He, F, Wu, C, Li, P, Li, N, Deng, J, et al. Betaine in inflammation: mechanistic aspects and applications. Front Immunol. (2018) 9:1070. doi: 10.3389/fimmu.2018.01070,
15. Zawieja, E, and Chmurzynska, A. Betaine and aging: a narrative review of findings, possible mechanisms, research perspectives, and practical recommendations. Ageing Res Rev. (2025) 104:102634. doi: 10.1016/j.arr.2024.102634,
16. Geng, L, Ping, J, Wu, R, Yan, H, Zhang, H, Zhuang, Y, et al. Systematic profiling reveals betaine as an exercise mimetic for geroprotection. Cell. (2025) 188:5403–5425.e33. doi: 10.1016/j.cell.2025.06.001,
17. Spaulding, HR, and Yan, Z. AMPK and the adaptation to exercise. Annu Rev Physiol. (2022) 84:209–27. doi: 10.1146/annurev-physiol-060721-095517,
18. Qiu, Y, Fernández-García, B, Lehmann, HI, Li, G, Kroemer, G, López-Otín, C, et al. Exercise sustains the hallmarks of health. J Sport Health Sci. (2023) 12:8–35. doi: 10.1016/j.jshs.2022.10.003,
19. Arumugam, MK, Paal, MC, Donohue, TM Jr, Ganesan, M, Osna, NA, and Kharbanda, KK. Beneficial effects of betaine: a comprehensive review. Biology. (2021) 10:456. doi: 10.3390/biology10060456,
20. Cai, D, Yuan, M, Liu, H, Pan, S, Ma, W, Hong, J, et al. Maternal betaine supplementation throughout gestation and lactation modifies hepatic cholesterol metabolic genes in weaning piglets via AMPK/LXR-mediated pathway and histone modification. Nutrients. (2016) 8:646. doi: 10.3390/nu8100646,
21. Heidari, R, Niknahad, H, Sadeghi, A, Mohammadi, H, Ghanbarinejad, V, Ommati, MM, et al. Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed Pharmacother. (2018) 103:75–86. doi: 10.1016/j.biopha.2018.04.010
22. Veskovic, M, Mladenovic, D, Milenkovic, M, Tosic, J, Borozan, S, Gopcevic, K, et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. (2019) 848:39–48. doi: 10.1016/j.ejphar.2019.01.043,
23. Bingül, İ, Başaran-Küçükgergin, C, Aydın, AF, Çoban, J, Doğan-Ekici, I, Doğru-Abbasoğlu, S, et al. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ Toxicol Pharmacol. (2016) 45:170–8. doi: 10.1016/j.etap.2016.05.033,
24. Singh, SK, Yadav, P, Patel, D, Tanwar, SS, Sherawat, A, Khurana, A, et al. Betaine ameliorates doxorubicin-induced cardiomyopathy by inhibiting oxidative stress, inflammation, and fibrosis through the modulation of AMPK/Nrf2/TGF-β expression. Environ Toxicol. (2024) 39:4134–47. doi: 10.1002/tox.24291
25. Cai, D, Jia, Y, Song, H, Sui, S, Lu, J, Jiang, Z, et al. Betaine supplementation in maternal diet modulates the epigenetic regulation of hepatic gluconeogenic genes in neonatal piglets. PLoS One. (2014) 9:e105504. doi: 10.1371/journal.pone.0105504,
26. Gao, X, Wang, Y, Randell, E, Pedram, P, Yi, Y, Gulliver, W, et al. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS One. (2016) 11:e0155403. doi: 10.1371/journal.pone.0155403,
27. Gao, X, Zhang, H, Guo, XF, Li, K, Li, S, and Li, D. Effect of betaine on reducing body fat-a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2019) 11:2480. doi: 10.3390/nu11102480,
28. Huang, BX, Zhu, YY, Tan, XY, Lan, QY, Li, CL, Chen, YM, et al. Serum betaine is inversely associated with low lean mass mainly in men in a Chinese middle-aged and elderly community-dwelling population. Br J Nutr. (2016) 115:2181–8. doi: 10.1017/S0007114516001380,
29. Cholewa, JM, Hudson, A, Cicholski, T, Cervenka, A, Barreno, K, Broom, K, et al. The effects of chronic betaine supplementation on body composition and performance in collegiate females: a double-blind, randomized, placebo controlled trial. J Int Soc Sports Nutr. (2018) 15:37. doi: 10.1186/s12970-018-0243-x,
30. Gao, X, Wang, Y, and Sun, G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition. (2017) 33:28–34. doi: 10.1016/j.nut.2016.08.005,
31. Schwab, U, Törrönen, A, Toppinen, L, Alfthan, G, Saarinen, M, Aro, A, et al. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. (2002) 76:961–7. doi: 10.1093/ajcn/76.5.961
32. Grizales, AM, Patti, ME, Lin, AP, Beckman, JA, Sahni, VA, Cloutier, E, et al. Metabolic effects of betaine: a randomized clinical trial of betaine supplementation in prediabetes. J Clin Endocrinol Metab. (2018) 103:3038–49. doi: 10.1210/jc.2018-00507,
33. Sivanesan, S, Taylor, A, Zhang, J, and Bakovic, M. Betaine and choline improve lipid homeostasis in obesity by participation in mitochondrial oxidative demethylation. Front Nutr. (2018) 5:61. doi: 10.3389/fnut.2018.00061,
34. Zhou, X, Chen, J, Chen, J, Wu, W, Wang, X, and Wang, Y. The beneficial effects of betaine on dysfunctional adipose tissue and N6-methyladenosine mRNA methylation requires the AMP-activated protein kinase α1 subunit. J Nutr Biochem. (2015) 26:1678–84. doi: 10.1016/j.jnutbio.2015.08.014,
35. Xu, G, Pan, H, Fan, L, Zhang, L, Li, J, Cheng, S, et al. Dietary betaine improves glucose metabolism in obese mice. J Nutr. (2024) 154:1309–20. doi: 10.1016/j.tjnut.2024.02.025,
36. Yu, J, Laybutt, DR, Youngson, NA, and Morris, MJ. Concurrent betaine administration enhances exercise-induced improvements to glucose handling in obese mice. Nutr Metab Cardiovasc Dis. (2022) 32:2439–49. doi: 10.1016/j.numecd.2022.08.012,
37. Du, J, Shen, L, Tan, Z, Zhang, P, Zhao, X, Xu, Y, et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. (2018) 10:131. doi: 10.3390/nu10020131,
38. Olli, K, Lahtinen, S, Rautonen, N, and Tiihonen, K. Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br J Nutr. (2013) 109:43–9. doi: 10.1017/s0007114512000888,
39. Szkudelska, K, Chan, M, Okulicz, M, Jasaszwili, M, Lukomska, A, Malek, E, et al. Betaine supplementation to rats alleviates disturbances induced by high-fat diet: pleiotropic effects in model of type 2 diabetes. J Physiol Pharmacol. (2021) 72:72. doi: 10.26402/jpp.2021.5.11,
40. Jin, M, Shen, Y, Pan, T, Zhu, T, Li, X, Xu, F, et al. Dietary betaine mitigates hepatic steatosis and inflammation induced by a high-fat-diet by modulating the Sirt1/Srebp-1/Pparɑ pathway in juvenile black seabream (Acanthopagrus schlegelii). Front Immunol. (2021) 12:694720. doi: 10.3389/fimmu.2021.694720,
41. Xu, J, Nie, Z, Qiu, X, Zhang, J, and Han, S. Effects of betaine supplementation on inflammatory markers: a systematic review and meta-analysis of randomised controlled trials. Int J Food Sci Nutr. (2023) 74:721–9. doi: 10.1080/09637486.2023.2257906,
42. Wang, X, Shang, X, Pan, Y, Fu, Y, Zhu, H, and Yan, S. Betaine alleviates obesity-related metabolic disorders in rats: insights from microbiomes, lipidomics, and transcriptomics. Front Nutr. (2025) 12:1604801. doi: 10.3389/fnut.2025.1604801,
43. Du, J, Zhang, P, Luo, J, Shen, L, Zhang, S, Gu, H, et al. Dietary betaine prevents obesity through gut microbiota-derived microRNA-378a family. Gut Microbes. (2021) 13:1–19. doi: 10.1080/19490976.2020.1862612,
44. Jin, L, Duan, B, Liu, J, Zhang, X, Li, X, Zhu, X, et al. Dietary betaine attenuates obesity: involvement of hepatic microRNAs. Nutrients. (2021) 13:2319–26. doi: 10.1166/sam.2021.4134
45. Joselit, Y, Nanobashvili, K, Jack-Roberts, C, Greenwald, E, Malysheva, OV, Caudill, MA, et al. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr Diabetes. (2018) 8:41. doi: 10.1038/s41387-018-0035-z,
46. Ribo, S, Sánchez-Infantes, D, Martinez-Guino, L, García-Mantrana, I, Ramon-Krauel, M, Tondo, M, et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci Transl Med. (2021) 13:eabb0322. doi: 10.1126/scitranslmed.abb0322,
47. Liu, J, Ding, L, Zhai, X, Wang, D, Xiao, C, Hui, X, et al. Maternal dietary betaine prevents high-fat diet-induced metabolic disorders and gut microbiota alterations in mouse dams and offspring from young to adult. Front Microbiol. (2022) 13:809642. doi: 10.3389/fendo.2022.809642,
48. van Lee, L, Tint, MT, Aris, IM, Quah, PL, Fortier, MV, Lee, YS, et al. Prospective associations of maternal betaine status with offspring weight and body composition at birth: the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr. (2016) 104:1327–33. doi: 10.3945/ajcn.116.138818,
49. Guo, H, Wu, H, and Li, Z. The pathogenesis of diabetes. Int J Mol Sci. (2023) 24:6978. doi: 10.3390/ijms24086978,
50. Zhang, H, and Chen, N. Adropin as an indicator of T2DM and its complications. Food Sci Human Wellness. (2022) 11:1455–63. doi: 10.1016/j.fshw.2022.06.002
51. Liang, J, Hao, J, Qin, Y, Liu, O, Shao, K, Zhao, W, et al. Association of betaine, choline, and TMAO with type 2 diabetes in rural China: a nested case-control study from the Handan Eye Study (HES). Diabetes Metab Syndr Obes. (2025) 18:2537–45. doi: 10.2147/dmso.S522576,
52. Dellow, WJ, Chambers, ST, Barrell, GK, Lever, M, and Robson, RA. Glycine betaine excretion is not directly linked to plasma glucose concentrations in hyperglycaemia. Diabetes Res Clin Pract. (2001) 52:165–9. doi: 10.1016/s0168-8227(01)00237-6,
53. Dellow, WJ, Chambers, ST, Lever, M, Lunt, H, and Robson, RA. Elevated glycine betaine excretion in diabetes mellitus patients is associated with proximal tubular dysfunction and hyperglycemia. Diabetes Res Clin Pract. (1999) 43:91–9. doi: 10.1016/s0168-8227(98)00115-6,
54. Schartum-Hansen, H, Ueland, PM, Pedersen, ER, Meyer, K, Ebbing, M, Bleie, Ø, et al. Assessment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One. (2013) 8:e69454. doi: 10.1371/journal.pone.0069454,
55. Lever, M, Sizeland, PC, Bason, LM, Hayman, CM, Robson, RA, and Chambers, ST. Abnormal glycine betaine content of the blood and urine of diabetic and renal patients. Clin Chim Acta. (1994) 230:69–79. doi: 10.1016/0009-8981(94)90090-6,
56. Walford, GA, Ma, Y, Clish, C, Florez, JC, Wang, TJ, and Gerszten, RE. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. (2016) 65:1424–33. doi: 10.2337/db15-1063,
57. Mlodzik-Czyzewska, MA, Szwengiel, A, and Chmurzynska, A. Betaine and B12 intake, glutathione concentration, and MTHFR, PEMT, and MTHFD1 genotypes are associated with diabetes-related parameters in Polish adults. J Nutr. (2025) 155:111–21. doi: 10.1016/j.tjnut.2024.10.036,
58. Garcia, E, Osté, MCJ, Bennett, DW, Jeyarajah, EJ, Shalaurova, I, Gruppen, EG, et al. High betaine, a trimethylamine N-oxide related metabolite, is prospectively associated with low future risk of type 2 diabetes mellitus in the PREVEND study. J Clin Med. (2019) 8:1813. doi: 10.3390/jcm8111813,
59. van der Vaart, A, de Borst, MH, Bakker, SJL, Connelly, MA, van Dijk, PR, and Dullaart, RPF. Higher betaine is associated with lower incidence of microvascular complications in type 2 diabetes (ZODIAC-61). Eur J Clin Investig. (2023) 53:e13873. doi: 10.1111/eci.13873,
60. Gao, X, Randell, E, Tian, Y, Zhou, H, and Sun, G. Low serum choline and high serum betaine levels are associated with favorable components of metabolic syndrome in Newfoundland population. J Diabetes Complicat. (2019) 33:107398. doi: 10.1016/j.jdiacomp.2019.06.003,
61. Dibaba, DT, Johnson, KC, Kucharska-Newton, AM, Meyer, K, Zeisel, SH, and Bidulescu, A. The association of dietary choline and betaine with the risk of type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. (2020) 43:2840–6. doi: 10.2337/dc20-0733,
62. Hosseini-Esfahani, F, Koochakpoor, G, Golzarand, M, Mirmiran, P, and Azizi, F. Dietary intakes of choline and betaine and incidence of type 2 diabetes: Tehran Lipid and Glucose Study. Metab Syndr Relat Disord. (2023) 21:573–80. doi: 10.1089/met.2023.0096,
63. Zhou, Z, Yao, Y, Sun, Y, Wang, X, Huang, S, Hou, J, et al. Serum betaine and dimethylglycine in mid-pregnancy and the risk of gestational diabetes mellitus: a case-control study. Endocrine. (2024) 85:649–59. doi: 10.1007/s12020-024-03732-4,
64. Lamkin, K, Xu, L, Wang, K, Liu, Y, Yang, K, Wu, H, et al. Choline and betaine intakes during pregnancy in relation to risk of gestational diabetes mellitus among Chinese women. Br J Nutr. (2024) 132:971–8. doi: 10.1017/s0007114524001995,
65. Nanobashvili, K, Jack-Roberts, C, Bretter, R, Jones, N, Axen, K, Saxena, A, et al. Maternal choline and betaine supplementation modifies the placental response to hyperglycemia in mice and human trophoblasts. Nutrients. (2018) 10:1507. doi: 10.3390/nu10101507,
66. Salahi, P, Alirezaei, M, Kheradmand, A, and Rocky, AJ. Betaine: a promising micronutrient in diet intervention for ameliorating maternal blood biochemical alterations in gestational diabetes mellitus. Int J Pept Res Ther. (2020) 26:1177–84. doi: 10.1007/s10989-019-09922-3
67. Zabrodina, VV, Shreder, ED, Shreder, OV, Durnev, AD, and Seredenin, SB. Effect of afobazole and betaine on DNA damage in placental and embryonic tissues of rats with experimental streptozocin diabetes. Bull Exp Biol Med. (2015) 159:757–60. doi: 10.1007/s10517-015-3068-5,
68. Jiang, YP, Yang, JM, Ye, RJ, Liu, N, Zhang, WJ, Ma, L, et al. Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomed Pharmacother. (2019) 120:109474. doi: 10.1016/j.biopha.2019.109474,
69. Huang, B, Hu, X, Hu, J, Chen, Z, and Zhao, H. Betaine alleviates cognitive deficits in diabetic rats via PI3K/Akt signaling pathway regulation. Dement Geriatr Cogn Disord. (2020) 49:270–8. doi: 10.1159/000508624
70. Jung, GY, Won, SB, Kim, J, Jeon, S, Han, A, and Kwon, YH. Betaine alleviates hypertriglycemia and tau hyperphosphorylation in db/db mice. Toxicol Res. (2013) 29:7–14. doi: 10.5487/tr.2013.29.1.007,
71. Evran, B, Aydın, AF, Uğuralp, B, Sar, M, Doğru-Abbasoğlu, S, and Uysal, M. Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats. Turk J Biol. (2018) 43:343–51. doi: 10.1515/tjb-2016-0183
72. Esfahani, PP, Mahdavinia, M, Khorsandi, L, Rezaei, M, Nikravesh, H, and Khodayar, MJ. Betaine protects against sodium arsenite-induced diabetes and hepatotoxicity in mice. Environ Sci Pollut Res Int. (2023) 30:10880–9. doi: 10.1007/s11356-022-22941-w,
73. Lever, M, George, PM, Slow, S, Elmslie, JL, Scott, RS, Richards, AM, et al. Fibrates may cause an abnormal urinary betaine loss which is associated with elevations in plasma homocysteine. Cardiovasc Drugs Ther. (2009) 23:395–401. doi: 10.1007/s10557-009-6188-1,
74. Saeed, H, Díaz, LA, Gil-Gómez, A, Burton, J, Bajaj, JS, Romero-Gomez, M, et al. Microbiome-centered therapies for the management of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. (2025) 31:S94–S111. doi: 10.3350/cmh.2024.0811,
75. Xiao, J, Wang, F, Yuan, Y, Gao, J, Xiao, L, Yan, C, et al. Epidemiology of liver diseases: global disease burden and forecasted research trends. Sci China Life Sci. (2025) 68:541–57. doi: 10.1007/s11427-024-2722-2,
76. Muzsik-Kazimierska, A, Szwengiel, A, Nikrandt, G, and Chmurzynska, A. Lower plasma glutathione, choline, and betaine concentrations are associated with fatty liver in postmenopausal women. Nutr Res. (2022) 101:23–30. doi: 10.1016/j.nutres.2022.02.004,
77. Park, MH, and Kim, DH. Betaine suppresses lipid accumulation in liver: inhibition of FoxO6 and PPARγ interaction. J Med Food. (2024) 27:1231–42. doi: 10.1089/jmf.2024.k.0150,
78. Kim, DH, Lee, B, Kim, MJ, Park, MH, An, HJ, Lee, EK, et al. Molecular mechanism of betaine on hepatic lipid metabolism: inhibition of forkhead box O1 (FoxO1) binding to peroxisome proliferator-activated receptor gamma (PPARγ). J Agric Food Chem. (2016) 64:6819–25. doi: 10.1021/acs.jafc.6b02644,
79. Xu, L, Huang, D, Hu, Q, Wu, J, Wang, Y, and Feng, J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr. (2015) 113:1835–43. doi: 10.1017/s0007114515001130,
80. Adjoumani, JY, Wang, K, Zhou, M, Liu, W, and Zhang, D. Effect of dietary betaine on growth performance, antioxidant capacity and lipid metabolism in blunt snout bream fed a high-fat diet. Fish Physiol Biochem. (2017) 43:1733–45. doi: 10.1007/s10695-017-0405-9,
81. Kitagawa, E, Yamamoto, T, Fujishita, M, Ota, Y, Yamamoto, K, Nakagawa, T, et al. Choline and betaine ameliorate liver lipid accumulation induced by vitamin B(6) deficiency in rats. Biosci Biotechnol Biochem. (2017) 81:316–22. doi: 10.1080/09168451.2016.1240604,
82. Kitagawa, E, Ota, Y, Hasegawa, M, Nakagawa, T, and Hayakawa, T. Accumulation of liver lipids induced by vitamin B(6) deficiency was effectively ameliorated by choline and, to a lesser extent, betaine. J Nutr Sci Vitaminol. (2019) 65:94–101. doi: 10.3177/jnsv.65.94,
83. Yang, Y, Jin, X, Lu, D, Wu, Y, Meng, F, Zhang, M, et al. Betaine decreases hepatic lipid deposition through DNA 5 mC and RNA m(6)A methylation-mediated regulation of fatty acid metabolic genes expression in laying hens. Poult Sci. (2025) 104:105496. doi: 10.1016/j.psj.2025.105496,
84. Zhang, L, Qi, Y, ALuo, Z, Liu, S, Zhang, Z, and Zhou, L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. (2019) 10:216–23. doi: 10.1039/c8fo02004c,
85. Chen, X, Luo, J, Yang, L, Guo, Y, Fan, Y, Liu, J, et al. miR-143-mediated responses to betaine supplement repress lipogenesis and hepatic gluconeogenesis by targeting MAT1a and MAPK11. J Agric Food Chem. (2022) 70:7981–92. doi: 10.1021/acs.jafc.2c02940,
86. Giriş, M, Doğru-Abbasoğlu, S, Soluk-Tekkeşin, M, Olgaç, V, and Uysal, M. Effect of betaine treatment on the regression of existing hepatic triglyceride accumulation and oxidative stress in rats fed on high fructose diet. Gen Physiol Biophys. (2018) 37:563–70. doi: 10.4149/gpb_2018005
87. Wang, F, Xu, J, Jakovlić, I, Wang, WM, and Zhao, YH. Dietary betaine reduces liver lipid accumulation via improvement of bile acid and trimethylamine-N-oxide metabolism in blunt-snout bream. Food Funct. (2019) 10:6675–89. doi: 10.1039/c9fo01853k,
88. Hu, Y, Sun, Q, Liu, J, Jia, Y, Cai, D, Idriss, AA, et al. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci Rep. (2017) 7:40251. doi: 10.1038/srep40251,
89. Wray-Cahen, D, Fernández-Fígares, I, Virtanen, E, Steele, NC, and Caperna, TJ. Betaine improves growth, but does not induce whole body or hepatic palmitate oxidation in swine (Sus scrofa domestica). Comp Biochem Physiol A. (2004) 137:131–40. doi: 10.1016/j.cbpb.2003.09.015,
90. Wang, LJ, Zhang, HW, Zhou, JY, Liu, Y, Yang, Y, Chen, XL, et al. Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J Nutr Biochem. (2014) 25:329–36. doi: 10.1016/j.jnutbio.2013.11.007,
91. Li, Y, Jiang, W, Feng, Y, Wu, L, Jia, Y, and Zhao, R. Betaine alleviates high-fat diet-induced disruption of hepatic lipid and iron homeostasis in mice. Int J Mol Sci. (2022) 23:6263. doi: 10.3390/ijms23116263,
92. Zhao, N, Yang, S, Jia, Y, Sun, B, He, B, and Zhao, R. Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J Nutr Biochem. (2018) 54:105–12. doi: 10.1016/j.jnutbio.2017.12.003,
93. Chen, J, Zhou, X, Wu, W, Wang, X, and Wang, Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J Physiol Biochem. (2015) 71:405–13. doi: 10.1007/s13105-015-0420-1,
94. Liu, J, Liu, Y, Chen, Y, Liu, Y, Huang, C, Luo, Y, et al. Betaine alleviates nonalcoholic fatty liver disease (NAFLD) via a manner involving BHMT/FTO/m(6)A/ PGC1α signaling. J Nutr Biochem. (2024) 134:109738. doi: 10.1016/j.jnutbio.2024.109738,
95. Chen, W, Zhang, X, Xu, M, Jiang, L, Zhou, M, Liu, W, et al. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE(−/−) mice. Eur J Nutr. (2021) 60:1655–68. doi: 10.1007/s00394-020-02362-6,
96. Ge, CX, Yu, R, Xu, MX, Li, PQ, Fan, CY, Li, JM, et al. Betaine prevented fructose-induced NAFLD by regulating LXRα/PPARα pathway and alleviating ER stress in rats. Eur J Pharmacol. (2016) 770:154–64. doi: 10.1016/j.ejphar.2015.11.043,
97. Kathirvel, E, Morgan, K, Nandgiri, G, Sandoval, BC, Caudill, MA, Bottiglieri, T, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. (2010) 299:G1068–77. doi: 10.1152/ajpgi.00249.2010,
98. Zhang, W, Wang, LW, Wang, LK, Li, X, Zhang, H, Luo, LP, et al. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci. (2013) 58:3198–206. doi: 10.1007/s10620-013-2775-x,
99. Jung, YS, Kim, SJ, Kwon, DY, Ahn, CW, Kim, YS, Choi, DW, et al. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol. (2013) 62:292–8. doi: 10.1016/j.fct.2013.08.049,
100. Kwon, DY, Jung, YS, Kim, SJ, Park, HK, Park, JH, and Kim, YC. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr. (2009) 139:63–8. doi: 10.3945/jn.108.094771,
101. Vesković, M, Labudović-Borović, M, Mladenović, D, Jadžić, J, Jorgačević, B, Vukićević, D, et al. Effect of betaine supplementation on liver tissue and ultrastructural changes in methionine-choline-deficient diet-induced NAFLD. Microsc Microanal. (2020) 26:997–1006. doi: 10.1017/s1431927620024265,
102. Sun, L, Tan, X, Liang, X, Chen, H, Ou, Q, Wu, Q, et al. Maternal betaine supplementation mitigates maternal high fat diet-induced NAFLD in offspring mice through gut microbiota. Nutrients. (2023) 15:284. doi: 10.3390/nu15020284,
103. Abu Ahmad, N, Raizman, M, Weizmann, N, Wasek, B, Arning, E, Bottiglieri, T, et al. Betaine attenuates pathology by stimulating lipid oxidation in liver and regulating phospholipid metabolism in brain of methionine-choline-deficient rats. FASEB J. (2019) 33:9334–49. doi: 10.1096/fj.201802683R,
104. Sookoian, S, Puri, P, Castaño, GO, Scian, R, Mirshahi, F, Sanyal, AJ, et al. Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int. (2017) 37:611–9. doi: 10.1111/liv.13249,
105. Abdelmalek, MF, Angulo, P, Jorgensen, RA, Sylvestre, PB, and Lindor, KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. (2001) 96:2711–7. doi: 10.1111/j.1572-0241.2001.04129.x,
106. Abdelmalek, MF, Sanderson, SO, Angulo, P, Soldevila-Pico, C, Liu, C, Peter, J, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. (2009) 50:1818–26. doi: 10.1002/hep.23239,
107. Vesković, M, Labudović-Borović, M, Zaletel, I, Rakočević, J, Mladenović, D, Jorgačević, B, et al. The effects of betaine on the nuclear fractal dimension, chromatin texture, and proliferative activity in hepatocytes in mouse model of nonalcoholic fatty liver disease. Microsc Microanal. (2018) 24:132–8. doi: 10.1017/s1431927617012806,
108. Kawakami, S, Han, KH, Nakamura, Y, Shimada, K, Kitano, T, Aritsuka, T, et al. Effects of dietary supplementation with betaine on a nonalcoholic steatohepatitis (NASH) mouse model. J Nutr Sci Vitaminol. (2012) 58:371–5. doi: 10.3177/jnsv.58.371,
109. Barak, AJ, Beckenhauer, HC, and Tuma, DJ. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol. (1996) 13:483–6. doi: 10.1016/0741-8329(96)00040-7,
110. Kharbanda, KK, Vigneswara, V, McVicker, BL, Newlaczyl, AU, Bailey, K, Tuma, D, et al. Proteomics reveal a concerted upregulation of methionine metabolic pathway enzymes, and downregulation of carbonic anhydrase-III, in betaine supplemented ethanol-fed rats. Biochem Biophys Res Commun. (2009) 381:523–7. doi: 10.1016/j.bbrc.2009.02.082,
111. Kharbanda, KK, Mailliard, ME, Baldwin, CR, Sorrell, MF, and Tuma, DJ. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: effects on protein-L-isoaspartyl methyltransferase activity. J Hepatol. (2007) 46:1119–25. doi: 10.1016/j.jhep.2007.01.026,
112. Kharbanda, KK, Mailliard, ME, Baldwin, CR, Beckenhauer, HC, Sorrell, MF, and Tuma, DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. (2007) 46:314–21. doi: 10.1016/j.jhep.2006.08.024,
113. Kim, SJ, Jung, YS, Kwon, DY, and Kim, YC. Alleviation of acute ethanol-induced liver injury and impaired metabolomics of S-containing substances by betaine supplementation. Biochem Biophys Res Commun. (2008) 368:893–8. doi: 10.1016/j.bbrc.2008.02.003,
114. Barak, AJ, Beckenhauer, HC, and Tuma, DJ. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol. (1994) 11:501–3. doi: 10.1016/0741-8329(94)90075-2
115. Dou, X, Xia, Y, Chen, J, Qian, Y, Li, S, Zhang, X, et al. Rectification of impaired adipose tissue methylation status and lipolytic response contributes to hepatoprotective effect of betaine in a mouse model of alcoholic liver disease. Br J Pharmacol. (2014) 171:4073–86. doi: 10.1111/bph.12765,
116. Kharbanda, KK, Rogers, DD 2nd, Mailliard, ME, Siford, GL, Barak, AJ, Beckenhauer, HC, et al. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol. (2005) 70:1883–90. doi: 10.1016/j.bcp.2005.09.021,
117. Yang, W, Huang, L, Gao, J, Wen, S, Tai, Y, Chen, M, et al. Betaine attenuates chronic alcohol-induced fatty liver by broadly regulating hepatic lipid metabolism. Mol Med Rep. (2017) 16:5225–34. doi: 10.3892/mmr.2017.7295,
118. Varatharajalu, R, Garige, M, Leckey, LC, Arellanes-Robledo, J, Reyes-Gordillo, K, Shah, R, et al. Adverse signaling of scavenger receptor class B1 and PGC1s in alcoholic hepatosteatosis and steatohepatitis and protection by betaine in rat. Am J Pathol. (2014) 184:2035–44. doi: 10.1016/j.ajpath.2014.03.005,
119. Kanbak, G, Inal, M, and Bayçu, C. Ethanol-induced hepatotoxicity and protective effect of betaine. Cell Biochem Funct. (2001) 19:281–5. doi: 10.1002/cbf.926,
120. Li, J, Li, XM, Caudill, M, Malysheva, O, Bardag-Gorce, F, Oliva, J, et al. Betaine feeding prevents the blood alcohol cycle in rats fed alcohol continuously for 1 month using the rat intragastric tube feeding model. Exp Mol Pathol. (2011) 91:540–7. doi: 10.1016/j.yexmp.2011.05.009,
121. Shi, QZ, Wang, LW, Zhang, W, and Gong, ZJ. Betaine inhibits toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J Gastroenterol. (2010) 16:897–903. doi: 10.3748/wjg.v16.i7.897,
122. French, SW. How to prevent alcoholic liver disease. Exp Mol Pathol. (2015) 98:304–7. doi: 10.1016/j.yexmp.2015.03.007,
123. Ji, C, and Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. (2003) 124:1488–99. doi: 10.1016/s0016-5085(03)00276-2,
124. Shedid, SM, Abdel-Magied, N, and Saada, HN. Role of betaine in liver injury induced by the exposure to ionizing radiation. Environ Toxicol. (2019) 34:123–30. doi: 10.1002/tox.22664,
125. Kim, SK, Seo, JM, Chae, YR, Jung, YS, Park, JH, and Kim, YC. Alleviation of dimethylnitrosamine-induced liver injury and fibrosis by betaine supplementation in rats. Chem Biol Interact. (2009) 177:204–11. doi: 10.1016/j.cbi.2008.09.021,
126. Khodayar, MJ, Kalantari, H, Khorsandi, L, Rashno, M, and Zeidooni, L. Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomed Pharmacother. (2018) 103:1436–45. doi: 10.1016/j.biopha.2018.04.154
127. Khodayar, MJ, Kalantari, H, Khorsandi, L, Rashno, M, and Zeidooni, L. Upregulation of Nrf2-related cytoprotective genes expression by acetaminophen-induced acute hepatotoxicity in mice and the protective role of betaine. Hum Exp Toxicol. (2020) 39:948–59. doi: 10.1177/0960327120905962,
128. Junnila, M, Barak, AJ, Beckenhauer, HC, and Rahko, T. Betaine reduces hepatic lipidosis induced by carbon tetrachloride in Sprague-Dawley rats. Vet Hum Toxicol. (1998) 40:263–6.
129. Ahn, M, Park, JS, Chae, S, Kim, S, Moon, C, Hyun, JW, et al. Hepatoprotective effects of Lycium chinense miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem. (2014) 116:1104–12. doi: 10.1016/j.acthis.2014.05.004,
130. Kamel, NA, Bashir, DW, El-Leithy, EMM, Tohamy, AF, Rashad, MM, Ali, GE, et al. Polyethylene terephthalate nanoplastics caused hepatotoxicity in mice can be prevented by betaine: molecular and immunohistochemical insights. J Biochem Mol Toxicol. (2024) 38:e70088. doi: 10.1002/jbt.70088,
131. Hagar, H, Husain, S, Fadda, LM, Attia, NM, Attia, MMA, and Ali, HM. Inhibition of NF-κB and the oxidative stress-dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats. Pharmacol Rep. (2019) 71:1025–33. doi: 10.1016/j.pharep.2019.06.003,
132. Junnila, M, Rahko, T, Sukura, A, and Lindberg, LA. Reduction of carbon tetrachloride-induced hepatotoxic effects by oral administration of betaine in male Han-Wistar rats: a morphometric histological study. Vet Pathol. (2000) 37:231–8. doi: 10.1354/vp.37-3-231,
133. McCarty, MF. Co-administration of equimolar doses of betaine may alleviate the hepatotoxic risk associated with niacin therapy. Med Hypotheses. (2000) 55:189–94. doi: 10.1054/mehy.1999.1011,
134. Bingül, İ, Aydın, AF, Başaran-Küçükgergin, C, Doğan-Ekici, I, Çoban, J, Doğru-Abbasoğlu, S, et al. High-fat diet plus carbon tetrachloride-induced liver fibrosis is alleviated by betaine treatment in rats. Int Immunopharmacol. (2016) 39:199–207. doi: 10.1016/j.intimp.2016.07.028,
135. Erman, F, Balkan, J, Cevikbaş, U, Koçak-Toker, N, and Uysal, M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. (2004) 27:199–205. doi: 10.1007/s00726-004-0105-5,
136. Murakami, T, Nagamura, Y, and Hirano, K. The recovering effect of betaine on carbon tetrachloride-induced liver injury. J Nutr Sci Vitaminol. (1998) 44:249–55. doi: 10.3177/jnsv.44.249,
137. van den Berg, EH, Flores-Guerrero, JL, Garcia, E, Connelly, MA, de Meijer, VE, Bakker, SJL, et al. High plasma levels of betaine, a trimethylamine N-oxide-related metabolite, are associated with the severity of cirrhosis. Liver Int. (2023) 43:424–33. doi: 10.1111/liv.15310,
138. Váli, L, Stefanovits-Bányai, E, Szentmihályi, K, Fébel, H, Sárdi, E, Lugasi, A, et al. Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrition. (2007) 23:172–8. doi: 10.1016/j.nut.2006.11.004,
139. Duong, FH, Christen, V, Filipowicz, M, and Heim, MH. S-adenosylmethionine and betaine correct hepatitis C virus induced inhibition of interferon signaling in vitro. Hepatology. (2006) 43:796–806. doi: 10.1002/hep.21116,
140. Ma, S, Wang, Y, Chen, L, Wang, W, Zhuang, X, Liu, Y, et al. Parental betaine supplementation promotes gosling growth with epigenetic modulation of IGF gene family in the liver. J Anim Sci. (2024) 102:skae065. doi: 10.1093/jas/skae065,
141. Yang, S, Zhao, N, Sun, B, Yang, Y, Hu, Y, and Zhao, R. Grandmaternal betaine supplementation enhances hepatic IGF2 expression in F2 rat offspring through modification of promoter DNA methylation. J Sci Food Agric. (2020) 100:1486–94. doi: 10.1002/jsfa.10156,
142. Cai, D, Wang, J, Jia, Y, Liu, H, Yuan, M, Dong, H, et al. Gestational dietary betaine supplementation suppresses hepatic expression of lipogenic genes in neonatal piglets through epigenetic and glucocorticoid receptor-dependent mechanisms. Biochim Biophys Acta. (2016) 1861:41–50. doi: 10.1016/j.bbalip.2015.10.002,
143. Cai, D, Yuan, M, Jia, Y, Liu, H, Hu, Y, and Zhao, R. Maternal gestational betaine supplementation-mediated suppression of hepatic cyclin D2 and presenilin1 gene in newborn piglets is associated with epigenetic regulation of the STAT3-dependent pathway. J Nutr Biochem. (2015) 26:1622–31. doi: 10.1016/j.jnutbio.2015.08.007,
144. Zhao, N, Yang, S, Hu, Y, Dong, H, and Zhao, R. Maternal betaine supplementation in rats induces intergenerational changes in hepatic IGF-1 expression and DNA methylation. Mol Nutr Food Res. (2017) 61:1600940. doi: 10.1002/mnfr.201600940,
145. Zhao, N, Yang, S, Sun, B, Feng, Y, and Zhao, R. Maternal betaine protects rat offspring from glucocorticoid-induced activation of lipolytic genes in adipose tissue through modification of DNA methylation. Eur J Nutr. (2020) 59:1707–16. doi: 10.1007/s00394-019-02025-1,
146. Golzarand, M, Mirmiran, P, and Azizi, F. Association between dietary choline and betaine intake and 10.6-year cardiovascular disease in adults. Nutr J. (2022) 21:1. doi: 10.1186/s12937-021-00755-9,
147. Nagata, C, Wada, K, Tamura, T, Konishi, K, Kawachi, T, Tsuji, M, et al. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. (2015) 145:1787–92. doi: 10.3945/jn.114.209296,
148. Meyer, KA, and Shea, JW. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients. (2017) 9:711. doi: 10.3390/nu9070711,
149. Bidulescu, A, Chambless, LE, Siega-Riz, AM, Zeisel, SH, and Heiss, G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. (2007) 7:20. doi: 10.1186/1471-2261-7-20,
150. Liu, S, Wang, D, Li, B, Li, K, Dai, X, Cheng, L, et al. Dietary betaine intake and risk of mortality in patients with coronary artery disease: the prospective Guangdong coronary artery disease cohort. Br J Nutr. (2023) 130:10–9. doi: 10.1017/s0007114522002975
151. Zhong, C, Miao, M, Che, B, Du, J, Wang, A, Peng, H, et al. Plasma choline and betaine and risks of cardiovascular events and recurrent stroke after ischemic stroke. Am J Clin Nutr. (2021) 114:1351–9. doi: 10.1093/ajcn/nqab199
152. Zhong, C, Lu, Z, Che, B, Qian, S, Zheng, X, Wang, A, et al. Choline pathway nutrients and metabolites and cognitive impairment after acute ischemic stroke. Stroke. (2021) 52:887–95. doi: 10.1161/strokeaha.120.031903,
153. Millard, HR, Musani, SK, Dibaba, DT, Talegawkar, SA, Taylor, HA, Tucker, KL, et al. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson heart study. Eur J Nutr. (2018) 57:51–60. doi: 10.1007/s00394-016-1296-8,
154. Ashtary-Larky, D, Bagheri, R, Ghanavati, M, Asbaghi, O, Tinsley, GM, Mombaini, D, et al. Effects of betaine supplementation on cardiovascular markers: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2022) 62:6516–33. doi: 10.1080/10408398.2021.1902938,
155. Zawieja, EE, Zawieja, B, and Chmurzynska, A. Betaine supplementation moderately increases total cholesterol levels: a systematic review and meta-analysis. J Diet Suppl. (2021) 18:105–17. doi: 10.1080/19390211.2019.1699223,
156. Wang, L, Zhao, M, Liu, W, Li, X, Chu, H, Bai, Y, et al. Association of betaine with blood pressure in dialysis patients. J Clin Hypertens. (2018) 20:388–93. doi: 10.1111/jch.13190,
157. Lv, Y, Ma, P, Wang, J, Xu, Q, Fan, J, Yan, L, et al. Betaine alleviates right ventricular failure via regulation of rho A/ROCK signaling pathway in rats with pulmonary arterial hypertension. Eur J Pharmacol. (2021) 910:174311. doi: 10.1016/j.ejphar.2021.174311,
158. Li, Q, Qu, M, Wang, N, Wang, L, Fan, G, and Yang, C. Betaine protects rats against ischemia/reperfusion injury-induced brain damage. J Neurophysiol. (2022) 127:444–51. doi: 10.1152/jn.00400.2021,
159. Lever, M, George, PM, Elmslie, JL, Atkinson, W, Slow, S, Molyneux, SL, et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. (2012) 7:e37883. doi: 10.1371/journal.pone.0037883,
160. Xie, L, Zhao, BX, Luo, J, Li, Y, Zhu, F, Li, GF, et al. A U-shaped association between serum betaine and incident risk of first ischemic stroke in hypertensive patients. Clin Nutr. (2020) 39:2517–24. doi: 10.1016/j.clnu.2019.11.011,
161. Wang, Z, Tang, WH, Buffa, JA, Fu, X, Britt, EB, Koeth, RA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. (2014) 35:904–10. doi: 10.1093/eurheartj/ehu002,
162. Hall, JA, Jewell, DE, and Ephraim, E. Feeding cats with chronic kidney disease food supplemented with betaine and prebiotics increases total body mass and reduces uremic toxins. PLoS One. (2022) 17:e0268624. doi: 10.1371/journal.pone.0268624,
163. Ephraim, E, and Jewell, DE. Betaine and soluble fiber improve body composition and plasma metabolites in cats with chronic kidney disease. Front Biosci. (2023) 15:8. doi: 10.31083/j.fbe1502008,
164. Ephraim, E, and Jewell, DE. Effect of added dietary betaine and soluble fiber on metabolites and fecal microbiome in dogs with early renal disease. Metabolites. (2020) 10:370. doi: 10.3390/metabo10090370,
165. Obeid, R, Awwad, H, Heine, GH, Emrich, IE, Fliser, D, Zawada, AM, et al. Plasma concentrations of trimethylamine-N-oxide, choline, and betaine in patients with moderate to advanced chronic kidney disease and their relation to cardiovascular and renal outcomes. J Ren Nutr. (2024) 34:530–8. doi: 10.1053/j.jrn.2024.03.009,
166. Tang, WH, Wang, Z, Kennedy, DJ, Wu, Y, Buffa, JA, Agatisa-Boyle, B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. (2015) 116:448–55. doi: 10.1161/CIRCRESAHA.116.305360,
167. Missailidis, C, Hällqvist, J, Qureshi, AR, Barany, P, Heimbürger, O, Lindholm, B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. (2016) 11:e0141738. doi: 10.1371/journal.pone.0141738,
168. van Guldener, C, Stam, F, and Stehouwer, CD. Hyperhomocysteinaemia in chronic kidney disease: focus on transmethylation. Clin Chem Lab Med. (2005) 43:1026–31. doi: 10.1515/cclm.2005.180
169. Zeisel, SH, and Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. (2017) 37:157–81. doi: 10.1146/annurev-nutr-071816-064732,
170. Guo, F, Dai, Q, Zeng, X, Liu, Y, Tan, Z, Zhang, H, et al. Renal function is associated with plasma trimethylamine-N-oxide, choline, L-carnitine and betaine: a pilot study. Int Urol Nephrol. (2021) 53:539–51. doi: 10.1007/s11255-020-02632-6,
171. Li, C, Li, J, Diao, Z, Chen, L, Yu, S, Yu, L, et al. Associations of dietary choline intake and kidney function with hyperuricemia in Chinese children and adolescents: a cross-sectional study. EClinicalMedicine. (2025) 79:103012. doi: 10.1016/j.eclinm.2024.103012,
172. Liu, YL, Pan, Y, Wang, X, Fan, CY, Zhu, Q, Li, JM, et al. Betaine reduces serum uric acid levels and improves kidney function in hyperuricemic mice. Planta Med. (2014) 80:39–47. doi: 10.1055/s-0033-1360127,
173. Norouzzadeh, M, Kalantar, H, Khorsandi, L, Mohtadi, S, and Khodayar, MJ. Betaine ameliorates arsenic-induced kidney injury in mice by mitigating oxidative stress-mediated inflammation. Arch Biochem Biophys. (2024) 758:110076. doi: 10.1016/j.abb.2024.110076,
174. Sharma, S, Kaur, T, Sharma, AK, Singh, B, Pathak, D, Yadav, HN, et al. Betaine attenuates sodium arsenite-induced renal dysfunction in rats. Drug Chem Toxicol. (2022) 45:2488–95. doi: 10.1080/01480545.2021.1959699,
175. Owumi, S, Agbarogi, H, Oluwawibe, BJ, Otunla, MT, Anifowose, MM, and Arunsi, UO. Modulation of the Nrf-2 and HO-1 signalling axis is associated with betaine's abatement of fluoride-induced hepatorenal toxicities in rats. Naunyn Schmiedeberg's Arch Pharmacol. (2024) 397:7725–45. doi: 10.1007/s00210-024-03133-4,
176. Fan, CY, Wang, MX, Ge, CX, Wang, X, Li, JM, and Kong, LD. Betaine supplementation protects against high-fructose-induced renal injury in rats. J Nutr Biochem. (2014) 25:353–62. doi: 10.1016/j.jnutbio.2013.11.010,
177. Hagar, H, and Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol. (2014) 37:803–11. doi: 10.1016/j.etap.2014.02.013,
178. Hagar, H, Medany, AE, Salam, R, Medany, GE, and Nayal, OA. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol. (2015) 67:133–41. doi: 10.1016/j.etp.2014.11.001,
179. Patel, D, Yadav, P, Singh, SK, Tanwar, SS, Sehrawat, A, Khurana, A, et al. Betaine alleviates doxorubicin-induced nephrotoxicity by preventing oxidative insults, inflammation, and fibrosis through the modulation of Nrf2/HO-1/NLRP3 and TGF-β expression. J Biochem Mol Toxicol. (2024) 38:e23559. doi: 10.1002/jbt.23559,
180. Ghartavol, MM, Gholizadeh-Ghaleh Aziz, S, Babaei, G, Hossein Farjah, G, and Hassan Khadem Ansari, M. The protective impact of betaine on the tissue structure and renal function in isoproterenol-induced myocardial infarction in rat. Mol Genet Genomic Med. (2019) 7:e00579. doi: 10.1002/mgg3.579,
181. Ozturk, F, Ucar, M, Ozturk, IC, Vardi, N, and Batcioglu, K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology. (2003) 62:353–6. doi: 10.1016/s0090-4295(03)00255-3,
182. Salahi, P, Rocky, A, Dezfoulian, O, Azizi, A, and Alirezaei, M. Betaine alleviated hepatic and renal injury in diabetic pregnant rats: biochemical and histopathological evidences. J Diabetes Metab Disord. (2020) 19:859–67. doi: 10.1007/s40200-020-00572-7,
183. Jorgačević, B, Stanković, S, Filipović, J, Samardžić, J, Vučević, D, and Radosavljević, T. Betaine modulating MIF-mediated oxidative stress, inflammation and fibrogenesis in thioacetamide-induced nephrotoxicity. Curr Med Chem. (2022) 29:5254–67. doi: 10.2174/0929867329666220408102856,
184. Alipourfard, F, Shajiee, H, Nazari-Serenjeh, F, Hojati, V, and Alirezaie, M. Betaine attenuates oxidative stress and cognitive dysfunction in an amyloid β-induced rat model of Alzheimer’s disease. Res Pharm Sci. (2023) 18:270–8. doi: 10.4103/1735-5362.371583,
185. Ibi, D, Kondo, S, Ohmi, A, Kojima, Y, Nakasai, G, Takaba, R, et al. Preventive effect of betaine against cognitive impairments in amyloid β peptide-injected mice through sirtuin1 in hippocampus. Neurochem Res. (2022) 47:2333–44. doi: 10.1007/s11064-022-03622-z,
186. Ibi, D, Tsuchihashi, A, Nomura, T, and Hiramatsu, M. Involvement of GAT2/BGT-1 in the preventive effects of betaine on cognitive impairment and brain oxidative stress in amyloid β peptide-injected mice. Eur J Pharmacol. (2019) 842:57–63. doi: 10.1016/j.ejphar.2018.10.037
187. Yang, ZJ, Huang, SY, Zhong, KY, Huang, WG, Huang, ZH, He, TT, et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m(6)A-YTHDF2-dependent manner. Redox Biol. (2024) 69:103026. doi: 10.1016/j.redox.2024.103026,
188. Liu, XP, Qian, X, Xie, Y, Qi, Y, Peng, MF, Zhan, BC, et al. Betaine suppressed Aβ generation by altering amyloid precursor protein processing. Neurol Sci. (2014) 35:1009–13. doi: 10.1007/s10072-014-1630-y,
189. Chai, GS, Jiang, X, Ni, ZF, Ma, ZW, Xie, AJ, Cheng, XS, et al. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem. (2013) 124:388–96. doi: 10.1111/jnc.12094,
190. Zhang, Y, and Jia, J. Betaine mitigates amyloid-β-associated neuroinflammation by suppressing the NLRP3 and NF-κB signaling pathways in microglial cells. J Alzheimer Dis. (2023) 94:S9–S19. doi: 10.3233/jad-230064,
191. Hacioglu, C, Kar, F, Ozbayer, C, and Gundogdu, AC. Ex vivo investigation of betaine and boric acid function as preprotective agents on rat synaptosomes to be treated with Aβ (1-42). Environ Toxicol. (2024) 39:2138–49. doi: 10.1002/tox.24098,
192. Ibi, D, Hirashima, K, Kojima, Y, Sumiya, K, Kondo, S, Yamamoto, M, et al. Preventive effects of continuous betaine intake on cognitive impairment and aberrant gene expression in hippocampus of 3xTg mouse model of Alzheimer’s disease. J Alzheimer's Dis. (2021) 79:639–52. doi: 10.3233/jad-200972,
193. Leiteritz, A, Dilberger, B, Wenzel, U, and Fitzenberger, E. Betaine reduces β-amyloid-induced paralysis through activation of cystathionine-β-synthase in an Alzheimer model of Caenorhabditis elegans. Genes Nutr. (2018) 13:21. doi: 10.1186/s12263-018-0611-9,
194. Kocatürk, RR, Temizyürek, A, Özcan, Ö, Ergüzel, TT, Karahan, M, Konuk, M, et al. Effect of nutritional supports on malnutrition, cognition, function and biomarkers of Alzheimer's disease: a systematic review. Int J Neurosci. (2023) 133:1355–73. doi: 10.1080/00207454.2022.2079506,
195. Knopman, D, and Patterson, M. An open-label, 24-week pilot study of the methyl donor betaine in Alzheimer disease patients. Alzheimer Dis Assoc Disord. (2001) 15:162–5. doi: 10.1097/00002093-200107000-00008,
196. Yang, ZJ, Huang, SY, Cheng, J, Zeng, JW, Wusiman, M, Li, HB, et al. Betaine: a comprehensive review on dietary sources, health benefits, mechanisms of action, and application. Food Chem. (2025) 463:140993. doi: 10.1016/j.foodchem.2024.140993
197. Turck, D, Castenmiller, J, De Henauw, S, Hirsch-Ernst, KI, Kearney, J, Maciuk, A, et al. Safety of betaine as a novel food pursuant to regulation (EU) 2015/2283. EFSA J. (2019) 17:e05658. doi: 10.2903/j.efsa.2019.5658,
198. Lau, CE, Siskos, AP, Maitre, L, Robinson, O, Athersuch, TJ, Want, EJ, et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. (2018) 16:202. doi: 10.1186/s12916-018-1190-8
199. Hayes, KC, Pronczuk, A, Cook, MW, and Robbins, MC. Betaine in sub-acute and sub-chronic rat studies. Food Chem Toxicol. (2003) 41:1685–700. doi: 10.1016/s0278-6915(03)00196-0,
200. Sun, J, Wen, S, Zhou, J, and Ding, S. Association between malnutrition and hyperhomocysteine in Alzheimer’s disease patients and diet intervention of betaine. J Clin Lab Anal. (2017) 31:e22090. doi: 10.1002/jcla.22090,
201. Miao, M, Du, J, Che, B, Guo, Y, Zhang, J, Ju, Z, et al. Circulating choline pathway nutrients and depression after ischemic stroke. Eur J Neurol. (2022) 29:459–68. doi: 10.1111/ene.15133,
202. Imbard, A, Toumazi, A, Magréault, S, Garcia-Segarra, N, Schlemmer, D, Kaguelidou, F, et al. Efficacy and pharmacokinetics of betaine in CBS and cblC deficiencies: a cross-over randomized controlled trial. Orphanet J Rare Dis. (2022) 17:417. doi: 10.1186/s13023-022-02567-4,
203. Filipowicz, M, Bernsmeier, C, Terracciano, L, Duong, FH, and Heim, MH. S-adenosyl-methionine and betaine improve early virological response in chronic hepatitis C patients with previous nonresponse. PLoS One. (2010) 5:e15492. doi: 10.1371/journal.pone.0015492,
204. van Guldener, C, Janssen, MJ, de Meer, K, Donker, AJ, and Stehouwer, CD. Effect of folic acid and betaine on fasting and postmethionine-loading plasma homocysteine and methionine levels in chronic haemodialysis patients. J Intern Med. (1999) 245:175–83. doi: 10.1046/j.1365-2796.1999.00430.x
205. Atkinson, W, Elmslie, J, Lever, M, Chambers, ST, and George, PM. Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am J Clin Nutr. (2008) 87:577–85. doi: 10.1093/ajcn/87.3.577,
206. Schartum-Hansen, H, Pedersen, ER, Svingen, GF, Ueland, PM, Seifert, R, Ebbing, M, et al. Plasma choline, smoking, and long-term prognosis in patients with stable angina pectoris. Eur J Prev Cardiol. (2015) 22:606–14. doi: 10.1177/2047487314524867,
207. Holm, PI, Bleie, Ø, Ueland, PM, Lien, EA, Refsum, H, Nordrehaug, JE, et al. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arterioscler Thromb Vasc Biol. (2004) 24:301–7. doi: 10.1161/01.Atv.0000114569.54976.31,
208. Moat, SJ, Madhavan, A, Taylor, SY, Payne, N, Allen, R, Stabler, SP, et al. High- but not low-dose folic acid improves endothelial function in coronary artery disease. Eur J Clin Investig. (2006) 36:850–9. doi: 10.1111/j.1365-2362.2006.01735.x
209. Teixeira Araújo, G, Domenici, F, Elias, J Jr, and Vannucchi, H. Betaine: a potential agent for the treatment of hepatopathy associated with short bowel syndrome. Nutr Hosp. (2014) 29:1366–71. doi: 10.3305/nh.2014.29.6.7378,
210. Gahl, WA, Bernardini, I, Chen, S, Kurtz, D, and Horvath, K. The effect of oral betaine on vertebral body bone density in pyridoxine-non-responsive homocystinuria. J Inherit Metab Dis. (1988) 11:291–8. doi: 10.1007/bf01800372,
Keywords: betaine, exercise mimetics, obesity, diabetes, liver disease, cardiovascular diseases, kidney disease, Alzheimer’s disease
Citation: Xu Y, Gao J, Wang M and Zhang H (2026) Exercise-mimicking effects of betaine in chronic disease prevention and management. Front. Nutr. 12:1762908. doi: 10.3389/fnut.2025.1762908
Edited by:
Simin Feng, Zhejiang University of Technology, ChinaReviewed by:
Zhi-Jun Yang, Guangdong Academy of Agricultural Sciences, ChinaYaír Adonaí Sánchez Nuño, University of Guadalajara, Mexico
Copyright © 2026 Xu, Gao, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Wang, d2FuZ21pZ25odWkyOTAzMzVAMTYzLmNvbQ==; Hu Zhang, aHV6aGFuZzA3MDNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuhui Xu1†
Yuhui Xu1† Jianhong Gao
Jianhong Gao Hu Zhang
Hu Zhang