- 1School of Medicine, Royal College of Surgeons in Ireland - Medical University of Bahrain, Busaiteen, Bahrain
- 2Data Science Centre, School of Population Health, RCSI, Dublin, Ireland
- 3Research Department, Royal College of Surgeons in Ireland – Medical University of Bahrain, Busaiteen, Bahrain
Aim: Intermittent fasting (IF) has emerged as a beneficial dietary strategy for managing type 2 diabetes mellitus (T2DM), with improvements in certain indicators of body composition and cardiometabolic health. However, limited research compares the different effects of IF in oral hypoglycemic agents (OHAs) versus insulin-treated patients.
Methods: A comprehensive search was performed across PubMed, Scopus, and Cochrane Library (January 2010–January 2025). Randomized controlled trials (RCTs) involving adults (>18-years) with T2DM undergoing IF, time-restricted feeding (TRF), alternate-day fasting (ARF), fasting-mimicking diets (FMD), and Ramadan fasting were included.
Results: In total, 12 studies (n = 1,441 participants) met the inclusion criteria. IF improved glycemic control in both groups, with HbA1c reductions of 0.54% in OHA-users and 2.8% in insulin-users. In the meta-analysis of four eligible trials (n = 280), IF produced a significant pooled reduction in HbA1c (−1.85, 95% CI: −2.86 to −0.84), despite substantial heterogeneity (I2 = 98.1%). In contrast, IF did not produce a significant change in body weight (−1.45 kg, 95% CI: −5.51 to 2.61; I2 = 96.7%). Most studies reported weight loss, with an average body mass index (BMI) reduction of 1.53 kg/m2 in OHA users. Among insulin-users, one study reported a significant reduction in weight for the IF group (−4.77 ± 4.99 kg, p < 0.001).
Conclusion: IF represents an effective adjuvant therapeutic strategy in T2DM and could be widely employed in clinical practice.
Systematic review registration: Identifier CRD42025650065, https://www.crd.york.ac.uk/PROSPERO/view/CRD42025650065.
Introduction
Intermittent fasting (IF), defined as the intentional restriction of food intake during specified periods, has been increasingly promoted as a solution for weight loss, diabetes reversal, and improved cardiovascular health. IF regimens can be divided into four subtypes: time-restricted feeding (TRF, eating within a daily time window), alternate-day fasting (ADF, alternating feeding and fasting days), fast mimicking diet (FMD, mimics the effects of a water-only fast), and periodic fasting (e.g., two non-consecutive fasting days per week) (1). These approaches have been associated with improvements in somatic profiles, glycemic control, and cardio-metabolic parameters in both healthy individuals and those with metabolic diseases (2).
One area of interest is the effect of IF on type 2 diabetes mellitus (T2DM), which affects more than 800 million individuals worldwide and has quadrupled in prevalence since 1990 (3). Healthcare expenditure and treatment complexity continue to rise, with the mean number of daily medications used per patient increasing from 3.7 ± 2.8 medications in 2000 to 5.3 ± 3.2 medications in 2020 (4). Action to address these issues is needed, with lifestyle interventions such as IF being explored as a potential non-pharmacological and inexpensive form of management to improve glycemic control and reduce medication dependence.
Clinical trials have evaluated IF as a primary intervention in controlled settings, limiting its impact and the generalizability of findings to “real world” settings, where patients manage T2DM with a variety of medication regimens and face challenges such as polypharmacy and varying levels of glycemic control. Consequently, there is limited evidence as to how IF performs as an adjuvant therapy in clinical practice, specifically with patients receiving antidiabetic medications.
Even in studies including T2DM patients on medication and IF, there is limited comparative research exploring the impacts of IF on insulin versus oral hypoglycemic agent (OHA) regimes. Previous systematic reviews and meta-analyses have reported that IF can improve several cardiometabolic indicators in adults with T2DM (5, 6). However, these reviews evaluate mixed patient populations and do not differentiate outcomes by antihyperglycemic therapy. No prior review has compared IF responses between insulin and OHA-treated individuals, which is the specific focus of this study. This has clinical importance because, by evaluating how IF interacts with different T2DM medications, an objective interpretation of its effects can be obtained, thus guiding safe integration of fasting into diabetes management strategies.
This systematic review evaluates existing evidence for IF as an add-on strategy in patients with T2DM, comparing the outcomes between insulin-treated versus OHA-treated patients, with a focus on the impact on HbA1c, body weight, and other metabolic indicators.
Materials and methods
This systematic review was conducted following PRISMA guidelines (7). The protocol was registered in the PROSPERO databases (ID: CRD42025650065). The review adhered to PICO (Population, Intervention, Comparison, Outcomes) criteria.
Data sources and search strategy
PubMed, Scopus, and Cochrane Library databases were searched from January 2010 to January 2025 to ensure the inclusion of robust, up-to-date data. The specific search window was chosen because standardized IF protocols only became widely studied after 2010, making earlier studies less reflective of current clinical practice. The search focused on RCTs. Keywords and Medical Subject Heading (MeSH) terms were chosen to include the population and interventions of interest, accounting for variation in terminology of diabetes and fasting across all literature; hence, MeSH terms were “Diabetes Mellitus, Type 2,” “Hypoglycemic Agents,” “Insulin,” “Fasting,” “Caloric Restriction,” and “Time-Restricted Feeding,” as well as relevant keywords “intermittent fasting,” “fasting-mimicking diet,” “alternate-day fasting,” “Ramadan fasting” to account in terminology across databases. The search was limited to English-language published studies, which introduces language bias by omitting relevant studies published in other languages. References from key articles were reviewed to identify any relevant studies not captured in the initial search (Supplementary File 1).
Study selection
Rayyan software (Rayyan Systems Inc., 2025. Web version. Cambridge, MA, USA: Rayyan Systems Inc.) was used for study selection. Duplicates were removed and results filtered according to eligibility criteria.
Inclusion criteria
• Randomized controlled or noninferiority trials
• Study participants had T2DM and aged ≥18 years
• Study examining effects of intermittent fasting, time-restricted fasting, alternate-day fasting, periodic fasting, or fast-mimicking diet interventions
• Study reported at least one primary outcome, such as changes in HbA1c or body mass index
• Study participants taking either insulin or OHAs, but not both simultaneously
• Study reporting Ramadan
Ramadan was treated as a subtype of intermittent fasting, having an average of 13–16 h of fasting per day
Exclusion criteria
• Non-randomized, observational, case reports, reviews, editorials, abstracts, or protocols
• Participants aged <18 years
• Patients without a confirmed diagnosis of T2DM, or receiving both insulin and OHAs simultaneously
• Use of non-intermittent fasting dietary interventions (e.g., continuous caloric restriction)
• Failure to report relevant outcomes (e.g., HbA1c and fasting glucose)
• Non-English reports
All authors independently screened all citations for title, abstract, and full text. Studies that met the initial inclusion criteria were then assessed through independent full-text screening to confirm eligibility. Discrepancies were discussed collectively to reach a consensus.
The search yielded 9,947 articles. In total, 489 duplicates were removed, 9,450 articles were screened by title, and 9,265 did not meet the eligibility criteria. A total of 185 articles were retrieved for abstract screening, 52 assessed for eligibility, and 12 in total met the inclusion criteria, as shown in the PRISMA flow diagram (Figure 1).

Figure 1. PRISMA flow diagram of study selection. Flowchart illustrating the identification, screening, eligibility, and inclusion process of studies in accordance with PRISMA guidelines. The diagram outlines the number of records identified, screened, excluded (with reasons), and included in the review.
Data extraction and quality assessment
Quantitative and qualitative data were extracted based on multiple categories, including numerical values and patient experience. Three authors independently assessed the Risk of Bias (RoB) in included studies using the Cochrane Collaboration tool. When necessary, disagreements were resolved through discussion with a fourth author.
Data analysis
As a result of substantial heterogeneity in fasting protocols, medication regimens, outcomes, and reporting formats, only four trials contained sufficient data to permit quantitative synthesis (meta-analysis). The remaining studies varied in treatment composition, with two trials not being medication specific and the remaining trials representing insulin or OHA-only cohorts. Hence, meta-analysis by medication type was not feasible.
For the limited number of feasible studies (n = 4), a meta-analysis was conducted to estimate the overall effect of IF on HbA1c and body weight. The analyses for both HbA1c % and weight change were conducted using an inverse-variance random-effects model with τ2 estimated via restricted maximum likelihood. The remaining comparisons for all included studies (n = 12) used a descriptive analytical approach.
In addition, for studies reporting pre- and post-intervention HbA1c, percentage change was calculated to allow comparison across interventions. Mean percentage HbA1c change was then summarized separately for OHA-treated and insulin-treated cohorts; this summary reflects simple averages rather than a pooled statistical estimate. When HbA1c values were reported in mmol/mol, they were converted to percentage units using standard HbA1c conversion equations to maintain consistency across studies. Otherwise, the remaining numerical data were all extracted directly as reported in the RCTs.
Adherence rates were also calculated as the proportion of participants who completed the fasting intervention relative to the number enrolled.
Any reported Standard deviation (SD) values were taken directly from the RCTs; the only SD values calculated were those derived from error bars in graphical figures. All comparisons between insulin- and OHA-treated groups were therefore narrative and descriptive, intended to highlight patterns rather than compute pooled effect estimates.
Primary and secondary outcomes
This review focused on the effectiveness of IF with main outcomes being HbA1c, weight change [e.g., body mass index (BMI)], adverse events, and patient adherence. Secondary outcomes included fasting blood glucose (FBG), medication reliance, and impact on cardiovascular health.
Results
Study range and characteristics
The review included 12 studies with a total of 1,441 participants. Data from each study were extracted as shown in Table 1. Sample sizes ranged from 37 (8) to 343 (9) participants per study, with participants with T2DM aged 18–80 years. The mean duration of studies was 22 weeks. The studies varied in terms of the severity of diabetes in patients, with HbA1c levels ranging from 7 to 10% at baseline. Most participants were overweight, with baseline BMI ranging from 23 to 40 kg/m2.
Key methodological differences included variations in fasting protocols, duration of interventions (12–52 weeks), outcome measures, and type of diabetes medications. Most studies reported HbA1c and weight as primary outcomes; some also assessed lipid profiles, insulin sensitivity, and quality of life (QoL).
The studies included participants from diverse ethnic backgrounds across multiple countries. Studies were conducted in China, India, Thailand, Austria, the Netherlands, Australia, Lebanon, Malaysia, Algeria, South Africa, the UAE, New Zealand, the United Kingdom, and Singapore. Gender distribution varied, with some studies reporting specific numbers: Obermayer et al. (22 women and 24 men) (3), Umphonsathien et al. (29 women, 11 men) (10), and Carter et al. (77 women, 60 men) (11), while others did not specify gender breakdowns.
Meta-analysis (n = 4 studies)
Change in HbA1c%
In a random-effects meta-analysis of four trials (n = 280), the fasting intervention produced a statistically significant reduction in HbA1c compared with controls, based on the difference in change from baseline. The pooled mean difference was −1.85% (95% CI: −2.86 to −0.84; p = 0.0003), indicating that participants in the intervention groups (fasting) experienced greater decreases in HbA1c than those in the control groups. Substantial heterogeneity was present (I2 = 98.1%, p < 0.0001), reflecting considerable between-study variability. Results are shown in Figure 2.
![Forest plot showing four studies comparing fasting and control groups on changes in HbA1c levels. Mean differences (MD) range from -7.40 to -0.75 with confidence intervals (CI). Overall MD is -1.85 with a CI of [-2.86, -0.84]. Studies cited are Che et al. (2021), Obermayer et al. (2023), Yang et al. (2023), and Umphonsanthien et al. (2021). Heterogeneity is high at 98.1%, with a p-value less than 0.0001.](https://www.frontiersin.org/files/Articles/1699384/fnut-13-1699384-HTML/image_m/fnut-13-1699384-g002.jpg)
Figure 2. Forest plot of the effects of intermittent fasting (IF) on HbA1c % change in patients with type 2 diabetes.
Change in weight (kg)
In a random-effects meta-analysis of four trials (n = 280), the intervention did not significantly change body weight compared with controls, based on the difference in change from baseline. The pooled mean difference was −1.45 kg (95% CI: −5.51 to 2.61; p = 0.48), indicating no clear evidence that the intervention led to greater weight loss than controls. Heterogeneity between studies was substantial (I2 = 96.7%, p < 0.0001), suggesting considerable variability in findings across trials. Results are shown in Figure 3.
![Forest plot showing weight change in fasting versus control groups across four studies. Mean differences (MD) are shown with 95% confidence intervals (CI). Che et al. (2021): MD -2.15, CI [-2.28, -2.02]; Obermayer et al. (2023): MD 4.50, CI [2.35, 6.65]; Yang et al. (2023): MD -5.66, CI [-6.59, -4.73]; Umphonsanthien et al. (2021): MD -2.15, CI [-3.24, -1.06]. Random effects model summary MD is -1.45 with CI [-5.51, 2.61]. Heterogeneity: I-squared 96.7%, tau-squared 16.7601, p < 0.0001.](https://www.frontiersin.org/files/Articles/1699384/fnut-13-1699384-HTML/image_m/fnut-13-1699384-g003.jpg)
Figure 3. Forest plot of the effects of intermittent fasting (IF) on weight (kg) change in patients with type 2 diabetes.
All included studies (n = 12 studies)
HbA1c reduction
Data were extracted from articles reporting baseline values and post-IF values for HbA1c to calculate percentage change (%). Data for OHAs were retrieved from six investigations (8–10, 12–14) and insulin from one investigation (3). Figure 4A shows the HbA1c% change among OHA medication studies.

Figure 4. Effects of intermittent fasting (IF) on glycemic control and body composition in patients with type 2 diabetes. (A) Mean percentage change in HbA1c across individual studies involving patients treated with oral hypoglycemic agents (OHAs). Error bars represent standard deviation (SD) = 0.38. CF, consecutive fasting; NCF, non-consecutive fasting. (B) Comparison of mean percentage change in HbA1c between patients treated with OHAs and those on insulin therapy following IF interventions. Error bars represent SD = 1.59. Data are pooled from eligible studies included in the review. (C) Mean percentage change in body mass index (BMI) following IF in patients treated with OHAs. Error bars represent SD = 1.41. Data are derived from eligible studies.
The results indicate that IF is beneficial for glycemic control in both groups, with a mean HbA1c reduction of 0.54% in OHA-treated and 2.8% in insulin-treated patients (Figure 4B). IF had a greater impact than insulin with a 2.26 ± 1.59% difference.
Three studies investigated the impact of specific OHA medications under IF conditions (Ramadan, FMD), rather than OHAs as a generalized group (9, 13, 14); thus, we compared the HbA1c reduction in metformin, liraglutide, and sulfonylureas. After 33 weeks, 26.7% of patients treated with liraglutide combined with metformin achieved an outcome of HbA1c < 7.0% in comparison to those treated with sulfonylurea plus metformin; 10.3%, with the study reporting a 0.59% decrease in HbA1c in the liraglutide group and a difference of 0.4% in HbA1c reduction between both treatments while fasting (9). Two studies showed no significant difference in HbA1c using sulfonylureas in contrast to liraglutide. From a baseline of 7.7%, the HbA1c only changed (+0.02%) in the sulfonylurea group, indicating no statistical significance compared to 0.3% in the liraglutide group.
Using metformin only, a study reported a decrease in HbA1c values from 52.2 ± 9.3 mmol/mol (6.9 ± 0.8%) at baseline to 49.5 ± 8.2 mmol/mol (6.7 ± 0.8%) at 52 weeks under FMD interventions (13); 0.2% in HbA1c reduction.
Weight change
Both OHA and insulin cohorts demonstrated a net weight decrease for patients undergoing either IF, TRF, ARF, FMD, and Ramadan fasting. For the OHA group, three investigations recorded weight change in terms of BMI (kg/m2) (Figure 4C) (8, 10, 13). The average BMI change for OHA was −1.53 ± 1.41 kg/m2.
Focusing on medication-specific outcomes, sulfonylurea-treated participants experienced a minimal weight increase of 0.34 kg (95% CI: −0.22 to +0.90 kg, p = 0.23) over the 33-week study duration. By contrast, the liraglutide group showed a significant weight loss of 3.94 kg (95% CI: −4.54 to −3.33 kg, p < 0.0001) at 33 weeks, highlighting the efficacy of liraglutide in promoting weight reduction (12). Similarly, participants in the FMD group taking only metformin experienced an average weight loss of 3.6 kg (95% CI: −5.2 to −2.1 kg, p < 0.001) after 12 months (13).
Additionally, two articles reported weight loss of 4.8 ± 3.2 kg in the OHA group (12) and 4.77 ± 4.99 kg in the insulin group, and a decrease in fat mass of 3.5 ± 3.3 kg (BMI not recorded) (3).
Secondary outcomes
Fasting interventions showed promising effects on metabolic and cardiovascular parameters in T2DM patients in both OHA and insulin cohorts. Several studies reported a reduction in FBG levels; one reported a decrease in mean FBG levels for the pre-Ramadan to the post-Ramadan timepoint at the 3-month follow-up from 123.7 ± 27.1 mg/dL to 122.4 ± 14.5 mg/dL to 120.1 ± 27.1 mg/dL (15). FBG reduction was also reflected by a decrease in the Medication Effect Score (MES) in a TRF study; the MES for OHA and insulin decreased by 19% (change = −0.33 ± 0.27). The same study also hypothesized that, over 12 weeks, TRF results in a significant improvement in glucose regulation and insulin sensitivity in patients taking either insulin or OHA (16).
IF in patients treated with OHAs or insulin had no impact on mean blood pressure (fasting versus non-fasting groups, adjusted mean difference −0.32 mmHg; 95% CI: −3.16 to −2.52; p = 0.122) (16). However, the QoL of the fasting group increased by 6.19 ± 6.52, conversely, this decreased by 2.82 ± 3.71, p < 0.05 in the non-fasting group at 12-month follow-up (17).
In OHA-treated patients, lipid profiles showed reductions in total cholesterol from 4.2 ± 1.0 to 3.8 ± 0.8 mmol/L (p = 0.01) and LDL-cholesterol from 2.1 ± 0.8 to 2.0 ± 0.7 mmol/L (p = 0.03) during fasting interventions (8). Triglyceride levels also improved, with one study reporting a decrease from 170.4 mg/dL to 139.3 mg/d (10). Furthermore, one FMD study documented a reduction in MES from 0.7 ± 0.4 to 0.5 ± 0.4 over 12 months; the metformin dose was reduced in 40% of participants and completely stopped in 16% of participants, while among non-fasting patients, only 5% had reduced metformin dosage and 5% stopped the medication (p < 0.001) (13).
In insulin-treated patients, IF resulted in a reduction of 9 ± 10 IU in daily insulin dose, while non-fasting patients, by contrast, had an increase of 4 ± 10 IU (p = 0.008). This study also reported that IF improves cardiovascular risk factors, baseline HDL, and enhanced cholesterol efflux capacity (3). Furthermore, IF provoked elevation in serum apolipoprotein M (ApoM) levels (p = 0.01) (18), which correlated with weight loss and lowering of fasting glucose, suggesting a beneficial impact on cardiovascular health.
Adverse events
Adverse events were generally mild, with hypoglycemia being most common. Most studies reported no increase in severe hypoglycemia events with IF versus non-fasting groups; however, one study reported 7 patients in the IF group presenting with hypoglycemia (n = 51) (11).
Multiple studies reported mild adverse events, mainly gastrointestinal symptoms. One study that included both insulin and OHA medications reported minor adverse outcomes, including fatigue, dizziness, and headaches, during the first week of adaptation (16).
Adverse outcomes differed in OHA versus insulin-treated patients, with a higher incidence of hypoglycemia in insulin-treated groups with consequent insulin dosage reduction required (3).
Two studies reported adverse events specifically in OHA-treated patients. One reported dizziness (n = 12), fatigue (n = 15), nausea (n = 10), and vomiting (n = 4) under FMD intervention during its 12-month duration (n = 43 completed) (13). Another reported headaches (n = 12), dizziness (n = 8), and nausea (n = 6) in the IF group (n = 100) during the intervention’s 6-month duration (12). Insulin-only studies did not report any additional adverse events.
Hypoglycemia incidence varied between OHA treatment groups, with a higher incidence in patients using sulfonylureas versus liraglutide. During Ramadan, 17.8% of patients using sulfonylureas experienced hypoglycemia, compared to 8.6% of those using liraglutide. Documented symptomatic hypoglycemia, such as dizziness or sweating, was also lower (2.0%) in the liraglutide group versus the sulfonylurea group (11.0%). Conversely, mild gastrointestinal symptoms (nausea, vomiting, and diarrhea) were reported in liraglutide users upon initiation of fasting. Major adverse events in the liraglutide group included gastroenteritis (n = 1) (9).
Patient adherence
In total, 8 studies (3, 10–14, 16, 17) reported patient adherence or the number of patients completing the fasting intervention (Table 2). The mean adherence rate was 84.9% in insulin-treated and 84.6% in OHA-treated users, indicating no difference in adherence between groups. Three studies (13, 16, 17) mentioned the cause of dropouts, which were all due to issues with the fasting intervention, such as transportation or lack of contact.

Table 2. Baseline HbA1c, HbA1c change (±SD), medication(s) used, study duration, and adherence across studies investigating IF in patients with type 2 diabetes treated with oral hypoglycemic agents (OHAs) or insulin.
Study quality
The JBI critical appraisal checklist and Cochrane RoB tool were used to evaluate the studies and indicate that the included studies were generally of moderate quality. RoB was assessed across six domains. The bias risk was low for random sequence generation, indicating that randomization was appropriate and well-described. However, allocation concealment varied. Four studies (12, 13, 16, 18) reported adequate methods (e.g., sealed envelopes), while three studies (3, 8, 9) failed to describe or used inadequate concealment, raising concerns of selection bias. Most studies were high risk for participant blinding; for example, medication regimens (e.g., insulin or OHA) were modified during fasting without standardized protocols or analytical adjustments (e.g., intention-to-treat); this likely introduced performance bias, compromising the internal validity of the intervention effects. For outcome blinding, all studies were rated low risk due to the use of objective endpoints (e.g., HbA1c, glucose, weight, and lipids). However, inclusion of subjective outcomes (e.g., adverse effects and quality of life) without clear assessor blinding introduces the possibility of undetected detection bias. For attrition bias, two studies (8, 18) were rated unclear due to insufficient reporting on dropout rates and imputation methods. Studies were low risk for selection reporting as they appeared to report outcomes aligned with study objectives. However, the absence of registered protocols and inconsistencies in outcome reporting (e.g., selective time points) introduce potential bias (see Figure 5).
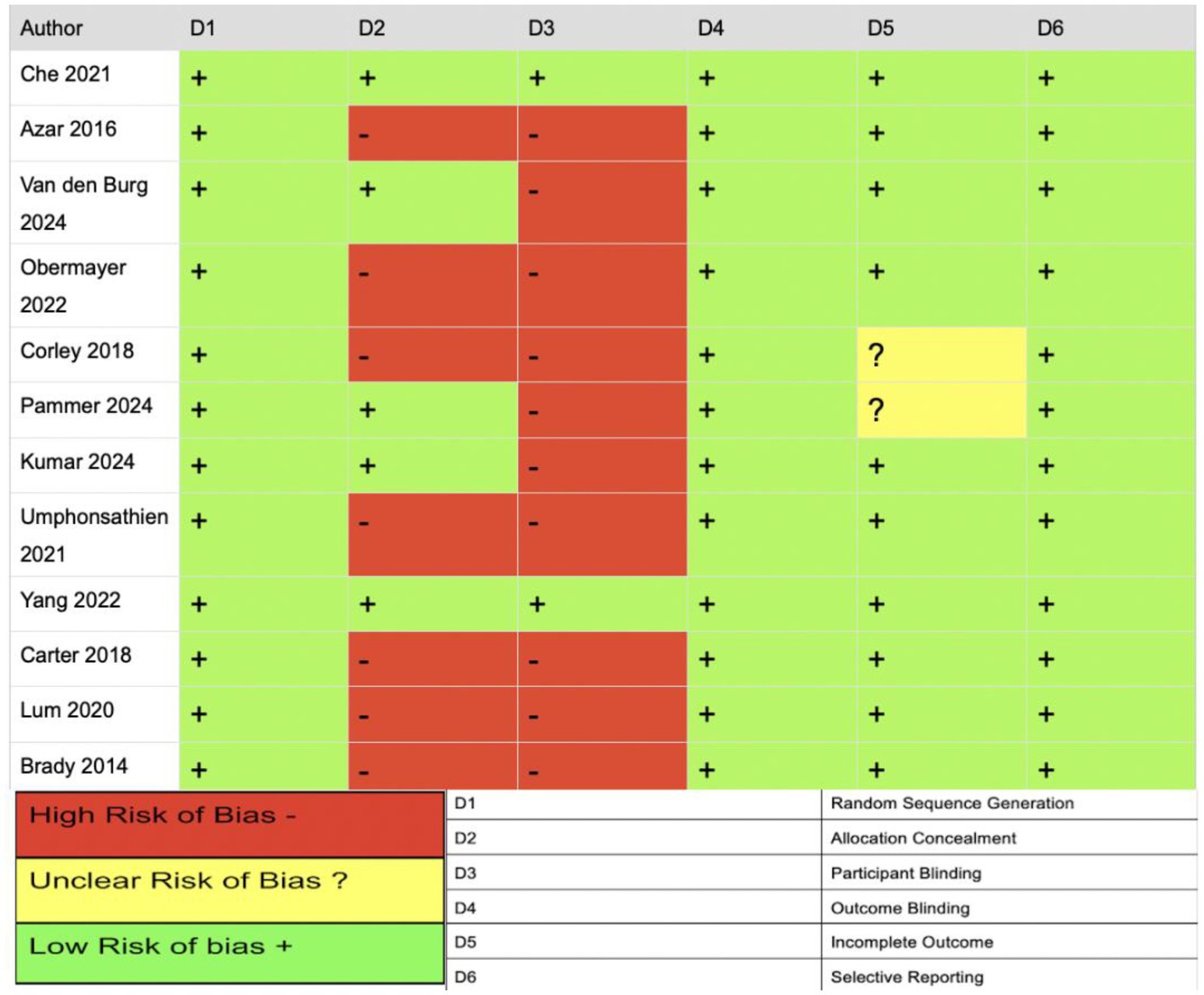
Figure 5. Summary of risk of bias across included studies. Graphical representation of individual risk of bias judgments by domain, using standardized color coding (green = low risk, yellow = unclear risk, red = high risk), assessed via the Cochrane risk of bias tool.
Discussion
The limited meta-analysis that could be undertaken found that intermittent fasting was associated with a significant reduction in HbA1c but did not induce a significant change in body weight when compared with control interventions. Across the four eligible trials, fasting led to a pooled mean decrease of 1.85% in HbA1c, indicating a clinically relevant improvement in glycemic control; however, there was high heterogeneity (I2 = 98.1%), reflecting major differences in fasting protocols, participant characteristics, and treatment regimens across studies. In contrast, the pooled effect on body weight was not statistically significant (loss of 1.45 kg; p = 0.48) with similarly high heterogeneity (I2 = 96.7%), suggesting substantial inconsistency in weight outcomes between trials. Overall, while intermittent fasting may improve HbA1c independently of weight loss, the substantial variability and methodological differences among the included studies indicate that these findings should be interpreted with caution, and firm conclusions about differential effects in insulin- versus OHA-treated populations cannot be drawn due to insufficient data.
Differences in responses to fasting interventions between patients taking OHAs and those taking insulin were found, with a greater decrease in HbA1c in insulin-treated versus OHA-treated patients. However, as only two studies focused on insulin users (3, 18), the data could be skewed or could be consequent upon a higher baseline HbA1c%. For patients using OHAs only, 6 studies (8–10, 12–14) were included, with varying study durations, differences that could have resulted in a lower overall mean reduction. The remaining four studies involved mixed treatment regimens.
When comparing OHA medications, liraglutide combined with metformin demonstrated the greatest HbA1c reduction versus sulfonylurea + metformin and metformin alone over a 52-week period. Liraglutide, a glucagon-like peptide-1 receptor agonist, enhances insulin secretion in accordance with blood glucose, increasing its impact (19). Sulfonylureas, by contrast, stimulate continuous insulin secretion, inducing a moderate HbA1c reduction (20) (greater than metformin, less than liraglutide). Metformin reduces hepatic glucose production, effectively lowering blood glucose but less potently when used as a single therapy (21).
Weight loss and BMI reduction with IF were more apparent in patients taking OHAs, particularly those receiving liraglutide and metformin, agents that more likely enhance weight reduction versus insulin, which counteracts weight loss due to its storage-promoting effects, despite fasting interventions (22, 23). Sulfonylureas, traditionally associated with weight gain, demonstrated no significant weight change during IF (20). These differences underscore the importance of the medication modality in IF.
Fasting interventions are found to improve QoL for patients with T2DM in both OHA and insulin-treated patients, with one article reporting an increase of 6.19 (SD 6.52), p < 0.05 at 12 months (17).
There was a reported decrease in FBG in both insulin and OHA patients (15), which was further shown by the decrease in MES score and medication dosage during fasting (16). Fasting also improves glucose regulation and insulin sensitivity (16) by lowering insulin levels, reducing fat stores and inflammation, and promoting metabolic adaptations that increase efficient glucose handling (24). IF had no impact on blood pressure.
Metabolic and cardiovascular responses differed between insulin and OHA-treated patients, likely due to differences in baseline status and medications. OHA-treated patients exhibited more favorable lipid profile changes, such as reductions in total cholesterol, LDL-cholesterol, and triglycerides, as OHAs tend to enhance lipid metabolism, which is amplified by fasting (24, 25).
The reported elevation in ApoM levels in insulin-treated patients suggests an improvement in lipid transport and glucose homeostasis, supporting fasting’s effective role in cardiovascular health. Cardiovascular benefits, including increased HDL-cholesterol, were more pronounced in insulin-treated patients, possibly due to fasting’s role in lowering hyperinsulinemia and inflammation (17).
Insulin secretion and sensitivity are regulated by circadian control and exert significant effects on glucose metabolism; therefore, altered meal timings can further exacerbate glucose intolerance in T2DM patients (26). IF strengthens circadian rhythm alignment, which is a key driver in metabolic improvements and increased insulin sensitivity in T2DM. Fasting creates a consolidated daily feeding window that reinforces the natural day-to-night oscillation of metabolic pathways, restoring synchrony between peripheral clocks in the liver, pancreas, muscle, and adipose tissue. This alignment enhances daytime insulin sensitivity, reduces nocturnal hepatic glucose output, and improves the rhythmic secretion of insulin (27). By re-establishing these circadian patterns, fasting reduces glycemic variability, promotes more efficient glucose handling, and supports better overall metabolic homeostasis in both OHA- and insulin-treated patients.
Adverse events such as vomiting, nausea, headache, diarrhea, and thirst were reported during the first week of IF in both insulin- and OHA-treated patients (16).
Hypoglycemia incidence was higher in insulin-treated versus OHA-treated patients (28). Although no severe episodes occurred, close monitoring and decreased insulin dosage likely mitigated this risk (3). Insulin poses a greater risk of hypoglycemia because it is a direct hormone that immediately stimulates glucose uptake, leading to a steeper drop in blood glucose in comparison to OHAs, which are metabolized more slowly (6).
Although there is a greater risk of hypoglycemia in insulin-treated patients, guidelines are available to increase the safety of fasting. First, insulin dosages should be constantly monitored and eventually decreased, an advantage of IF interventions. Patients should also maintain hydration and balanced nutrition during non-fasting periods, avoid vigorous activity toward the end of the fast, and receive education on recognizing and managing symptoms. Moreover, it is highly recommended to use Self-monitoring of blood glucose (SMBG) devices between 2–5 times a day for T2DM patients to make sure glucose levels are maintained at optimal levels (29).
Adverse events were reported more frequently in OHA-treated patients (12, 13). This is due to the known gastrointestinal side effects of OHAs (dizziness, nausea, vomiting, and fatigue), which could be exacerbated by dietary interventions (30). Furthermore, insulin-treated patients may have also experienced fewer adverse events due to the dose adjustments and closer clinical monitoring to avoid hypoglycemia. Additionally, the limited number of insulin-only studies (3, 18) available reduces the opportunity to detect and report adverse outcomes; thus, the findings should be interpreted cautiously.
Sulfonylureas pose a higher hypoglycemic risk than liraglutide, due to their promotion of continuous insulin secretion regardless of food intake (30). By contrast, liraglutide tends to be hypoglycemia-neutral, lowering hypoglycemia risk and improving glycemic control (31). However, GLP-1 agonists are commonly associated with gastrointestinal side effects, which may be exacerbated by fasting; prolonged periods without food can alter gastric emptying, modify drug absorption, and disrupt fluid balance, thereby increasing the severity of complications, such as the major single adverse event of gastroenteritis reported (30).
Compliance was high for fasting patients across all studies (3, 8, 10–14, 16, 17). The adherence rate was similar (~85%) for OHA and insulin-treated patients. This suggests that IF is a feasible long-term dietary approach for patients with T2DM (13, 16, 17). Furthermore, this emphasizes the safety of fasting for insulin-treated patients, usually perceived as being at higher risk when fasting (3), with careful monitoring and dosage adjustments.
IF is a highly practical intervention for OHA-treated patients. OHA-focused studies (8–10, 12–14) did not require constant monitoring of blood glucose or frequent dosage adjustment versus insulin treatment (3, 18), thereby reducing the burden of management.
Strengths and limitations
Our findings here align with previous systematic reviews, suggesting that IF fasting strategies are beneficial in individuals with T2DM. Other comprehensive reviews (5, 6) have focused upon fasting interventions only, hence concluding that IF alone is not enough for T2DM treatment. Unlike prior studies that examined IF in isolation, our review demonstrates that the effects of fasting on glycemic control vary depending upon the pharmacological regimen, highlighting the role of IF as an adjuvant rather than a stand-alone therapy. This comparative approach fills an unexplored gap in the diabetes management literature.
Furthermore, minimizing heterogeneity in medication regimens (by excluding studies that included patients taking both medications simultaneously) allowed for a clearer determination of IF effects in OHA- versus insulin-treated patients.
By further sub-analysis of the effect of IF on different OHAs (metformin, sulfonylureas, and liraglutide), the potential confounding effect of grouping all OHAs was circumvented.
Methodologically, a strength of this review was the inclusion of RCTs only, ensuring high internal validity and minimization of bias. The generalizability of findings was strengthened by including studies involving ethnically diverse populations. Additionally, our evaluation of both efficacy and safety provides a balanced analysis of the therapeutic potential of IF in T2DM. Finally, the high adherence rate among studies demonstrates the feasibility of IF interventions in both insulin and OHAs-treated populations.
Limitations include the small number of studies available for inclusion, thus limiting the overall reliability and robustness of the review. While IF shows promising improvements in glycemic control and weight among patients with T2DM, the evidence base remains limited, particularly for insulin-treated individuals. Although insulin users appeared to experience a larger average HbA1c reduction (2.8%), this finding is derived from a minimal number of studies, with substantially smaller sample sizes compared with OHA-user cohorts. Moreover, study durations varied significantly, with 5 of the studies lasting only a few weeks (3, 8, 10, 16, 18), thereby providing limited insight into the sustainability and long-term safety of these metabolic improvements in insulin-treated patients. Therefore, despite encouraging initial results, the current evidence for insulin users should be interpreted with caution.
In terms of risk of bias assessment, high risk in multiple domains (allocation concealment, lack of participant blinding, and deviations from intended interventions) limits the reliability of the data and highlights the need for future studies to implement more rigorous risk mitigation strategies.
Another limitation was the substantial heterogeneity in the types of IF protocols used across the studies, which included TRF, ADF, FMD, and Ramadan fasting, as well as differences in fasting duration, caloric restriction, and fasting frequency; grouping all studies under a single “IF” umbrella without stratified analysis assumes homogeneity of physiological impact, which represents an inherent weakness. The heterogeneity in this review is driven by the differences in IF modalities and intervention durations. For instance, TRF primarily improves insulin sensitivity, whilst ADF and FMD induce deeper ketogenesis and greater caloric deficits, potentially producing larger reductions in weight and HbA1c (32). Ramadan fasting, however, involves prolonged daily fasting with nocturnal re-feeding, which attenuates metabolic benefits due to compensatory caloric intake and altered sleep patterns (15). As a result, pooling all interventions caused the variability in outcomes to increase.
Conclusion
In conclusion, this review demonstrates that IF is beneficial in T2DM patients treated with both OHAs and insulin. Though there remains a need for more robust, high-quality, long-term RCTs to confirm and optimize the effect of IF, the current findings are still clinically relevant. They underscore the potential for medical healthcare professionals to consider IF as a conservative, cost-effective strategy for patient-centered management of T2DM. The benefits of fasting include improved glycemic control, increased insulin sensitivity, and reductions in adverse metabolic parameters in both insulin and OHA-treated patients. Here, we conclude that OHA-treated patients derive benefit with minimal risks. Similarly, insulin-treated patients derive benefits from fasting with few instances of hypoglycemia; however, caution and close monitoring are essential in this patient group during fasting periods.
As current evidence is limited by heterogeneity in IF protocols (type, duration, and baseline patient characteristics), future studies should focus on identifying the most effective regimes for T2DM patient subgroups.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JQ: Visualization, Methodology, Formal analysis, Writing – review & editing, Investigation, Writing – original draft. NB: Writing – review & editing, Methodology, Writing – original draft, Formal analysis, Visualization, Investigation. NI: Writing – review & editing, Writing – original draft, Methodology, Investigation, Visualization, Formal analysis. SK: Writing – original draft, Formal analysis, Visualization, Methodology, Writing – review & editing, Investigation. LD: Formal analysis, Writing – original draft, Writing – review & editing. AB: Writing – review & editing, Supervision, Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2026.1699384/full#supplementary-material
Abbreviations
IF, Intermittent fasting; TRF, Time-restricted feeding; ADF, Alternate-day fasting; RCTs, Randomized controlled trials; T2DM, Type 2 diabetes mellitus; OHA, Oral hypoglycemic agents; MeSH terms, Medical Subject Heading terms; FMD, Fast-mimicking diet; BMI, Body mass index; RoB, Risk of Bias; QoL, Quality of Life; SD, Standard deviation; FBG, Fasting blood glucose; MES, Medication Effect Score; ApoM, Apolipoprotein M; CF, Consecutive Fasting; NCF, Non-consecutive Fasting; GLP1-RA, glucagon like peptide-1 agonist; DPP4i, dipeptidyl peptidase-4 inhibitors.
References
1. Sutton, EF, Beyl, R, Early, KS, Cefalu, WT, Ravussin, E, and Peterson, CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010,
2. Xiaoyu, W, Yuxin, X, and Li, L. The effects of different intermittent fasting regimens in people with type 2 diabetes: a network meta-analysis. Front Nutr. (2024) 11:1325894. doi: 10.3389/fnut.2024.1325894,
3. Obermayer, A, Tripolt, NJ, Pferschy, PN, Kojzar, H, Aziz, F, Müller, A, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)-a randomized controlled trial. Diabetes Care. (2023) 46:463–8. doi: 10.2337/dc22-1622,
4. Bashir, MI, Pathan, MF, Raza, SA, Ahmad, J, Khan, AKA, Ishtiaq, O, et al. Role of oral hypoglycemic agents in the management of type 2 diabetes mellitus during Ramadan. Indian J Endocrinol Metab. (2012) 16:503–7. doi: 10.4103/2230-8210.97994,
5. Sharma, SK, Mudgal, SK, Kalra, S, Gaur, R, Thakur, K, and Agarwal, R. Effect of intermittent fasting on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Eur Endocrinol. (2023) 19:25–32. doi: 10.17925/EE.2023.19.1.25,
6. Khalafi, M, Habibi Maleki, A, Symonds, ME, Rosenkranz, SK, Rohani, H, and Ehsanifar, M. The effects of intermittent fasting on body composition and cardiometabolic health in adults with prediabetes or type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. (2024) 26:3830–41. doi: 10.1111/dom.15730
7. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71,
8. Corley, BT, Carroll, RW, Hall, RM, Weatherall, M, Parry-Strong, A, and Krebs, JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. (2018) 35:588–94. doi: 10.1111/dme.13595,
9. Azar, ST, Echtay, A, Wan Bebakar, WM, Al Araj, S, Berrah, A, Omar, M, et al. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA-Ramadan): a randomized trial. Diabetes Obes Metab. (2016) 18:1025–33. doi: 10.1111/dom.12733,
10. Umphonsathien, M, Rattanasian, P, Lokattachariya, S, Suansawang, W, Boonyasuppayakorn, K, and Khovidhunkit, W. Effects of intermittent very-low calorie diet on glycemic control and cardiovascular risk factors in obese patients with type 2 diabetes mellitus: a randomized controlled trial. J Diabetes Investig. (2021) 13:156–66. doi: 10.1111/jdi.13619,
11. Carter, S, Clifton, PM, and Keogh, JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. (2018) 1:e180756. doi: 10.1001/jamanetworkopen.2018.0756,
12. Kumar, A. Impact of intermittent fasting on glycemic control and cardiovascular risk factors in type 2 diabetes: a randomized controlled trial. Int J Life Sci Biotechnol Pharma Res (2024). Available online at: https://www.ijlbpr.com/htmlissue.php?id=4194 (Accessed June 29, 2025)
13. van den Burg, EL, Schoonakker, MP, van Peet, PG, van den Akker-van Marle, EM, Lamb, HJ, Longo, VD, et al. Integration of a fasting-mimicking diet programme in primary care for type 2 diabetes reduces the need for medication and improves glycaemic control: a 12-month randomised controlled trial. Diabetologia. (2024) 67:1245–59. doi: 10.1007/s00125-024-06137-0,
14. Brady, EM, Davies, MJ, Gray, LJ, Saeed, MA, Smith, D, Hanif, W, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the treat 4 Ramadan trial. Diabetes Obes Metab. (2014) 16:527–36. doi: 10.1111/dom.12249,
15. Lum, ZK, Khoo, ZR, Toh, WYS, Kamaldeen, SAK, Shakoor, A, Tsou, KYK, et al. Efficacy and safety of use of the fasting algorithm for Singaporeans with type 2 diabetes (FAST) during Ramadan: a prospective, multicenter, randomized controlled trial. Ann Fam Med. (2020) 18:139–47. doi: 10.1370/afm.2500,
16. Che, T, Yan, C, Tian, D, Zhang, X, Liu, X, and Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab. (2021) 18:88. doi: 10.1186/s12986-021-00613-9,
17. Yang, X, Zhou, J, Shao, H, Huang, B, Kang, X, Wu, R, et al. Effect of an intermittent calorie-restricted diet on type 2 diabetes remission: a randomized controlled trial. J Clin Endocrinol Metab. (2022) 108:1415–24. doi: 10.1210/clinem/dgac661,
18. Pammer, A, Obermayer, A, Stadler, JT, Pferschy, PN, Tripolt, NJ, Habisch, H, et al. Effects of dietary interventions and intermittent fasting on HDL function in obese individuals with T2DM: a randomized controlled trial. Cardiovasc Diabetol. (2024) 23:339. doi: 10.1186/s12933-024-02426-5,
19. Collins, L, and Costello, RA. Glucagon-like peptide-1 receptor agonists. Treasure Island (FL): StatPearls Publishing (2025).
20. Costello, RA, Nicolas, S, and Shivkumar, A. Sulfonylureas. Treasure Island (FL): StatPearls Publishing (2025).
21. Dutta, S, Shah, RB, Singhal, S, Dutta, SB, Bansal, S, Sinha, S, et al. Metformin: a review of potential mechanism and therapeutic utility beyond diabetes. Drug Des Devel Ther. (2023) 17:1907–32. doi: 10.2147/DDDT.S409373,
22. Keskin, L, and Yaprak, B. Comparison of the effect of liraglutide and metformin therapy on the disease regulation and weight loss in obese patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. (2022) 26:6813–20. doi: 10.26355/eurrev_202209_29783,
23. Russell-Jones, D, and Khan, R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab. (2007) 9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x,
24. Fink, J, Tanaka, M, and Horie, S. Effects of fasting on metabolic hormones and functions: a narrative review. Juntendō Iji Zasshi. (2024) 70:348–59. doi: 10.14789/jmj.JMJ24-0012-R,
25. Gillani, SW, Ghayedi, N, Roosta, P, Seddigh, P, and Nasiri, O. Effect of metformin on lipid profiles of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Pharm Bioallied Sci. (2021) 13:76–82. doi: 10.4103/jpbs.JPBS_370_20,
26. Henry, CJ, Kaur, B, and Quek, RYC. Chrononutrition in the management of diabetes. Nutr Diabetes. (2020) 10:6. doi: 10.1038/s41387-020-0109-6,
27. Longo, VD, and Panda, S. Fasting, circadian rhythms, and time restricted feeding in healthy lifespan. Cell Metab. (2016) 23:1048–59. doi: 10.1016/j.cmet.2016.06.001,
28. Seaquist, ER, Anderson, J, Childs, B, Cryer, P, Dagogo-Jack, S, Fish, L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. (2013) 36:1384–95. doi: 10.2337/dc12-2480,
29. Bajaj, HS, Abouhassan, T, Ahsan, MR, Arnaout, A, Hassanein, M, Houlden, RL, et al. Diabetes Canada position statement for people with types 1 and 2 diabetes who fast during Ramadan. Can J Diabetes. (2019) 43:3–12. doi: 10.1016/j.jcjd.2018.04.007,
30. Ganesan, K, Rana, MBM, and Sultan, S. Oral hypoglycemic medications. Treasure Island (FL): StatPearls Publishing (2025).
31. Yousef, CC, Thomas, A, Matar, MA, Ghandoura, L, Aldossary, I, Almuhanna, SM, et al. Liraglutide effects on glycemic control and weight in patients with type 2 diabetes mellitus: a real-world, observational study and brief narrative review. Diabetes Res Clin Pract. (2021) 177:108871. doi: 10.1016/j.diabres.2021.108871,
Keywords: fasting-mimicking diet, glycemic control, insulin, intermittent fasting, oral hypoglycemic agents, time-restricted feeding, type 2 diabetes mellitus, weight loss
Citation: Al Qudah J, Beni ND, Ibrahim N, Khader S, Dempsey L and Butler AE (2026) Effects of intermittent fasting on HbA1c and weight in insulin versus oral hypoglycemic therapy-treated patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Nutr. 13:1699384. doi: 10.3389/fnut.2026.1699384
Edited by:
Falak Zeb, University of Sharjah, United Arab EmiratesReviewed by:
Krzysztof Czaja, Ross University School of Veterinary Medicine, Saint Kitts and NevisShaokang Wang, Southeast University, China
Copyright © 2026 Al Qudah, Beni, Ibrahim, Khader, Dempsey and Butler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra E. Butler, YWViOTEwMTFAZ21haWwuY29t; YWJ1dGxlckByY3NpLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jana Al Qudah
Jana Al Qudah Newsha Davoudian Beni
Newsha Davoudian Beni Nooran Ibrahim
Nooran Ibrahim Salma Khader
Salma Khader Laura Dempsey2
Laura Dempsey2 Alexandra E. Butler
Alexandra E. Butler