- 1Department of Chemical and Environmental Engineering, University of California, Riverside, Riverside, CA, United States
- 2Materials Science and Engineering Program, University of California, Riverside, Riverside, CA, United States
We demonstrate a new technique to produce lithium sulfide-carbon composite (Li2S-C) cathodes for lithium-sulfur batteries via aerosol spray pyrolysis (ASP) followed by sulfurization. Specifically, lithium carbonate-carbon (Li2CO3-C) composite nanoparticles are first synthesized via ASP from aqueous solutions of sucrose and lithium salts including nitrate (LiNO3), acetate (CH3COOLi), and Li2CO3, respectively. The obtained Li2CO3-C composites are subsequently converted to Li2S-C through sulfurization by reaction to H2S. Electrochemical characterizations show excellent overall capacity and cycle stability of the Li2S-C composites with relatively high areal loading of Li2S and low electrolyte/Li2S ratio. The Li2S-C nanocomposites also demonstrate clear structure-property relationships.
Introduction
Lithium-sulfur (Li-S) batteries are regarded as one of the most promising electrochemical energy storage technologies due to their low cost, environmental benignity, and outstanding theoretical capacity (Wang et al., 2013; Son et al., 2015). However, despite tremendous research and development efforts, there are still a number of challenges hindering their commercialization. Among these key challenges are the polysulfides shuttle effect and high electrolyte/sulfur ratio, which are significantly magnified by the instability of the Li metal anode (Chen J. et al., 2017; Chen S. et al., 2017; Pan et al., 2018; Wu et al., 2018). Therefore, high capacity non-Li anodes, particularly those comprised of silicon-based materials, have been proposed as replacements for Li metal in Li-S batteries (Yang et al., 2010). The use of silicon anode materials would require a pre-lithiated sulfur cathode, i.e., lithium sulfide (Li2S). In recent years, various methods to synthesize Li2S-carbon composite materials have been reported, including high-energy mixing Li2S with carbon (Cai et al., 2012; Jha et al., 2015), chemical lithiation of S-C composites (Hwa et al., 2015), Li2S-C composites synthesis via dissolving and precipitating Li2S in ethanol (Wu et al., 2014a,b,c, 2015, 2016), embedding Li2S in carbon matrix via Li-nitrogen interaction (Guo et al., 2013), reaction between Li metal and carbon disulfide (Tan et al., 2017), converting LiOH to Li2S via sulfurization with H2S (Dressel et al., 2016), and thermal reduction of Li2SO4 by carbon (Yang et al., 2013; Kohl et al., 2015; Li et al., 2015; Yu et al., 2017; Zhang et al., 2017; Ye et al., 2018). In addition, the mechanism studies on Li2S activation and capacity degradation were also reported (Vizintin et al., 2017; Piwko et al., 2018). In this work, we report a new scalable method for synthesizing Li2S-C composites via aerosol spray pyrolysis (ASP) followed by sulfurization.
Materials and Methods
Materials Synthesis
Three lithium salts including lithium nitrate (LiNO3), lithium acetate (CH3COOLi) and lithium carbonate (Li2CO3) were used as the precursors for Li2S with sucrose as the precursor for carbon. Each Li salt was dissolved in deionized water with sucrose at different concentrations as listed in Table S1. The obtained solutions were used in the ASP process.
The ASP system in this study is illustrated in Figure S1. The commercial aerosol generator (TSI, Model 3076) consisting of a nebulizer and a solution reservoir is attached to a diffusion dryer followed by a tubular furnace and a filter collector. The diffusion dryer was composed by two concentric tubes: The outer tube is made of 3-inch inner diameter PVC tubing and the inner tube is made of 0.5-inch diameter steel mesh with the annular space filled with porous silica gel. The aerosol of the precursor solution was generated by the nebulizer and carried through the diffusion dryer by argon gas to desiccate the water content. The resultant dry particles were continuously carried into the tube furnace heated at 850°C to produce the Li2CO3-C nanoparticles, which are collected with a stainless-steel filter down stream outside the tube furnace.
The synthesized Li2CO3-C composite is placed in an alumina boat in a tubular furnace, followed by purging with argon for an hour. The furnace was then heated to 725°C and maintained at this temperature for 5 h under a flow of 5 vol.% H2S and 95 vol.% argon. After 5 h the flow gas was switched to pure argon and the furnace was cooled naturally to room temperature. The product was collected in an argon-filled glovebox due to the sensitivity of Li2S to moisture.
Materials Characterization
The nitrogen adsorption-desorption isotherms of the produced composite materials were obtained with a surface area and porosity analyzer (Micromeritics ASAP2020). For a particular analysis, approximately 200 mg sample was first degassed at 150°C for 3 h, then the nitrogen adsorption-desorption isotherms were measured from 0 to 1 relative pressure. The surface area was obtained with the Brunauer-Emmett-Teller (BET) method. The crystalline species in the composites were characterized by powder X-Ray diffraction (XRD, PANalytical) with a CuKα source and a scan rate of 0.11° s−1. Kapton tape was used to seal the Li2S-C composites to protect Li2S from reacting with the moisture in ambient environment during measurement. The morphology and microstructure of the composites were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM, Tecnai T12). Carbon content in the Li2CO3-C composites was measured with thermogravimetric analysis (TGA, TA Instruments). The TGA samples were held at 120°C for 30 min to remove the moisture absorbed from environment, followed by heating to 600°C at a rate of 10°C min−1 with an isothermal step in dry air. The carbon contents in Li2CO3-CNitS, Li2CO3-CAceS and Li2CO3-CCarS (Figure S2) are very consistent at 20.7, 22.8, and 21.2 wt.%, respectively. Assuming complete conversion from Li2CO3 to Li2S without carbon loss, the Li2S content in Li2S-CNitS, Li2S-CAceS and Li2S-CCarS can be estimated as 70.4, 67.8, and 69.8 wt.%, respectively. The accurate Li2S content in the Li2S-C composites is determined as follows: 100 mg Li2S-C was thoroughly washed 4 times using 15 mL ethanol each time in the glovebox to remove Li2S. The obtained carbon was weighed after dried at 120°C for 8 h in the glovebox. The Li2S content is 71.3 wt.% in Li2S-CNitS, 69.1 wt.% in Li2S-CAceS, and 71.6 wt.% in Li2S-CCarS, which all agree very well with the estimated values.
Electrode Preparation and Cell Testing
The electrode is composed of 80 wt.% of Li2S-C composite, 10 wt.% of carbon black additive, and 10 wt.% of polystyrene as the binder. Polystyrene was selected as the binder to avoid the use of polar solvents (both protic and aprotic), most of which dissolve Li2S to some extent. Instead, mesitylene (Sigma-Aldrich) was used as the solvent for polystyrene in the electrode slurry. The electrodes were coated on carbon-coated aluminum current collector (MTI Corporation) in the argon-filled glovebox, with the average loading of Li2S-C composite at 2 mg cm−2. The electrodes were dried overnight in argon glovebox at room temperature, followed by drying at 120°C for 4 h. The dried electrodes are assembled into 2032-type coin cells with lithium foil anode (99.9%, Alfa Aesar) and Celgard® 2,500 separator. The electrolyte used in this study is 1M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) solution in a mixture of 1,3-dioxolane (DOL), dimethoxyethane (DME) and 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (Pyr14TFSI) (1:3:1 by vol.) with 1.5 wt.% of LiNO3. The electrolyte to Li2S ratio (μL/mg) was kept at 10 in all coin cells testing. To activate the Li2S-C electrode, the first anodic scan in the cyclic voltammetry (CV) was to 3.9 V vs. Li+/Li, and the anodic limit in the following scans was 2.6 V vs. Li+/Li. Similarly, the first charge was run at a rate of 50 mA g−1 (with respect to Li2S) to a charge cutoff of 3.5 V. Subsequent cycles are run at 117 mA g−1 between 2.6 V and 1.8 V vs. Li+/Li.
Results and Discussion
During ASP synthesis, three aqueous solutions containing sucrose (as carbon precursor) and either lithium nitrate (LiNO3), lithium acetate (CH3COOLi), or lithium carbonate (Li2CO3), denoted as NitS, AceS, and CarS, respectively, were atomized into aerosols with a pressure-enabled atomizer. The aerosols were subsequently carried by argon gas through a diffusion dryer and a tubular furnace for pyrolysis within an inert environment. The powder X-ray diffraction (XRD) patterns in Figure 1A clearly indicate that the obtained composites from all three lithium salts are Li2CO3-C composite with comparable carbon content (20.7 wt.% in Li2CO3-CNitS, 22.8 wt.% in Li2CO3-CAceS and 21.2 wt.% in Li2CO3-CCarS via thermalgravimetric analysis, Figure S2). It is worth noting that sucrose solution without the lithium salts (i.e., precursors of Li2CO3) completely decomposes during the same ASP without any carbon formation. This observation reveals that Li2CO3 serves as the nucleation sites for carbonization of sucrose in ASP (Skrabalak and Suslick, 2006). However, the formation mechanisms of Li2CO3 from these three Li salts are clearly different. For LiNO3, its thermal decomposition is known to proceed according to Reaction 1: (Stern and Weise, 1969)
Based on the XRD evidence of Li2CO3 with the absence of crystalline Li2O, it can be speculated that carbon dioxide (CO2) released from pyrolysis of sucrose further reacts with Li2O to generate Li2CO3 according to Reaction 2:
CH3COOLi undergoes thermal decomposition to generate Li2CO3 and acetone according to Reaction 3: (Roe and Finlay, 1952)
For the CarS precursor, Li2CO3 undergoes precipitation during ASP without decomposition, thus becoming directly embedded into the carbon matrix formed by the carbonization of sucrose.
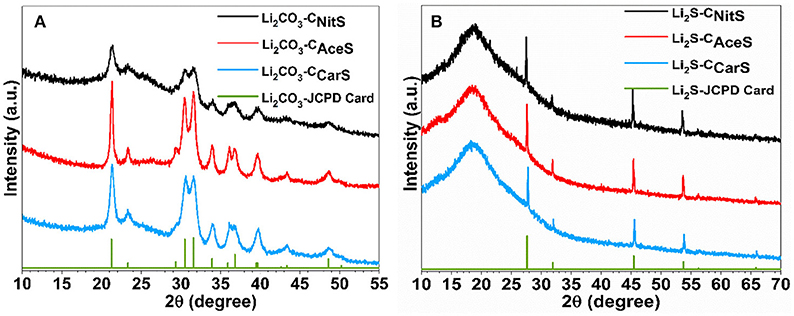
Figure 1. XRD patterns of (A) the Li2CO3-C composites obtained from ASP and (B) the Li2S-C composites after sulfurization.
Although the obtained Li2CO3-C composites have consistent composition and carbon content, they have distinctively different microstructures as displayed by the transmission electron microscopy (TEM) images in Figure 2 (scanning electron microscopy images in Figure S3). The Li2CO3-CNitS nanoparticles in Figure 2A have a hollow-shell structure with irregular-shaped interior voids due to the release of NOx and O2 gases during pyrolysis. The high solubility of LiNO3 in water also contributes to the formation of this hollow structure. When water evaporates during ASP, LiNO3 precipitates at the outer surface of the aerosol droplets following the surface precipitation mechanism (Messing et al., 1993). The microstructure of the Li2CO3-CNitS nanoparticles is further revealed by the TEM image in Figure 2D, after the removal of Li2CO3 using diluted hydrochloric acid (HCl). The carbon matrix of Li2CO3-CNitS has a highly porous structure after Li2CO3 removal, indicating that Li2CO3 occupies the majority of the volume in the Li2CO3-CNitS nanoparticles. The specific surface area of Li2CO3-CNitS before and after Li2CO3 removal obtained from the nitrogen adsorption-desorption isotherms (Figure 3 and Table S2) is consistent with this observation: the specific surface area of Li2CO3-CNitS is significantly increased from 26.8 to 608.2 m2 g−1 after Li2CO3 removal.

Figure 2. TEM images of (a) Li2CO3-CNitS, (b) Li2CO3-CAceS, (c) Li2CO3-CCarS; TEM images of the carbon matrix of (d) Li2CO3-CNitS, (e) Li2CO3-CAceS, (f) Li2CO3-CCarS after Li2CO3 removed; and TEM images of (g) Li2S-CNitS, (h) Li2S-CAceS, (i) Li2S-CCarS.
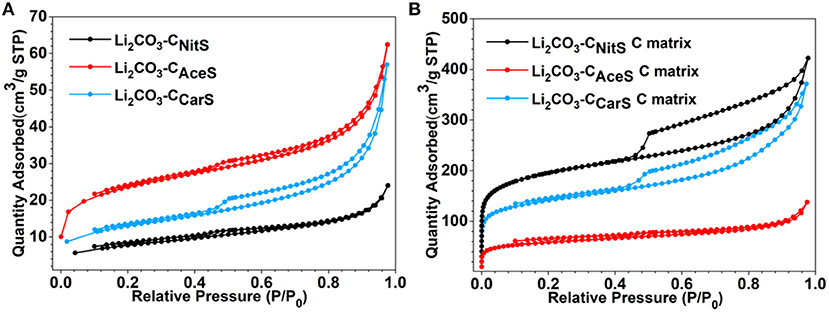
Figure 3. N2 adsorption-desorption isotherms of (A) Li2CO3-C nanoparticles and (B) the carbon matrix after Li2CO3 removal.
On the other hand, Li2CO3-CAceS nanoparticles show a denser spherical structure in Figure 2B. It is worth noting that the AceS precursor solution has a significantly lower sucrose/lithium salt molar ratio at 1:15 compared to 1:1.5 in NitS and 1:1.18 in CarS. Given the 22.8 wt.% carbon content in Li2CO3-CAceS, it is believed the generated acetone during the pyrolysis of CH3COOLi must function as the major source for carbon formation. The TEM image of the carbon matrix after Li2CO3 removal in Figure 2E reveals the distribution of Li2CO3 in the Li2CO3-CAceS nanoparticles is not as uniform as in Li2CO3-CNitS. The carbon matrix has a golf ball-like structure with relatively large pores, previously occupied by Li2CO3, distributed within. The specific surface area of Li2CO3-CAceS is 76.3 m2 g−1, which increases to 184.9 m2 g−1 after Li2CO3 removal. This modest increase of surface area also indicates the relatively larger size of Li2CO3 compared to that of Li2CO3-CNitS.
As shown in Figure 2C, the Li2CO3-CCarS nanoparticles clearly have a different structure resembling crumpled spheres, which is due to the much lower solubility of Li2CO3 in water than those of LiNO3 and CH3COOLi. The concentration of Li2CO3 in the CarS precursor solution is 0.1 M, which is close to saturation (Zou et al., 2013). Therefore, Li2CO3 undergoes fast and uniform precipitation from the aerosol droplets' evaporation in ASP according to the volume precipitation mechanism (Messing et al., 1993). In addition, the ASP of CarS precursor also releases fewer gaseous species without decomposition of Li2CO3. Both factors contribute to better confinement and more uniform distribution of Li2CO3. After Li2CO3 removal, the carbon matrix retains its original structure with apparently higher porosity as shown in Figure 2F. The specific surface area of Li2CO3-CCarS nanoparticles is 43.7 m2 g−1, which increases to 443.6 m2 g−1 after Li2CO3 removal.
The Li2CO3-C nanoparticles obtained via ASP were subsequently reacted with mixed hydrogen sulfide and argon gas (H2S/Ar at 5/95 vol.%) at 725°C to yield the Li2S-C composites according to Reaction 4, confirmed by the XRD patterns shown in Figure 1B.
The TEM images of the Li2S-C composites in Figures 2G–I (scanning electron microscopy images in Figure S4) demonstrate that these nanoparticles sustain their original structures after the conversion to Li2S from Li2CO3.
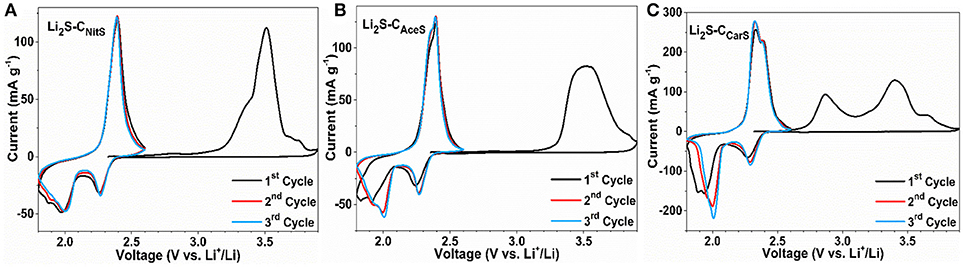
Figure 4 shows the first three CV cycles of the Li2S-C vs. Li counter/reference electrode in two-electrode cells. The cathodic peak in the first delithiation scan of Li2S-CNitS is centered at 3.5 V with a small shoulder at 3.4 V. The Li2S-CAceS composite demonstrates a broader delithiation peak at the same potential. In contrast, Li2S-CCarS shows two distinct cathodic peaks at 2.75 and 3.4 V vs. Li+/Li. The lower cathodic peak of the Li2S-CCarS composite at 2.75 V indicates a lower energy barrier for the delithiation reaction (Zhou et al., 2017). The Li2S-CCarS composite also demonstrates the highest peak current in the consecutive lithiation-delithiation scans. The superior performance of Li2S-CCarS may be reflective of the intimate contact of Li2S and the carbon matrix. Figure 5 displays the representative charge-discharge curves and the cycle stability of the Li2S-C composites. The electrolyte/Li2S ratio is 10:1 (μL/mg), and all Li2S-C composites are first charged to 3.5 V (activation) vs. Li+/Li with a current density of 50 mA g−1. The charge-discharge curves demonstrate similar cycling behavior of these three Li2S-C composites. However, Li2S-CAceS shows the highest charge-discharge hysteresis, which is consistent with the lowest surface area of its carbon matrix. On the other hand, although Li2S-CNitS shows the lowest voltage hysteresis due to the highest surface area of its carbon matrix, its capacity rapidly fades. As a composite with the balanced microstructure, Li2S-CCarS demonstrates the best overall performance: After 200 cycles, Li2S-CCarS can retain a capacity of 540 mAh g−1, superior to 385 mAh g−1 of Li2S-CNitS and 460 mAh g−1 of Li2S-CAceS, indicating the effectiveness of the Li2S-CCarS composite architecture in sequestrating polysulfides. The overall performance demonstrated by Li2S-CCarS, in terms of areal loading, E/Li2S ratio, overall capacity, and cycle stability, is on par with the best performance reported to date (Table S3).
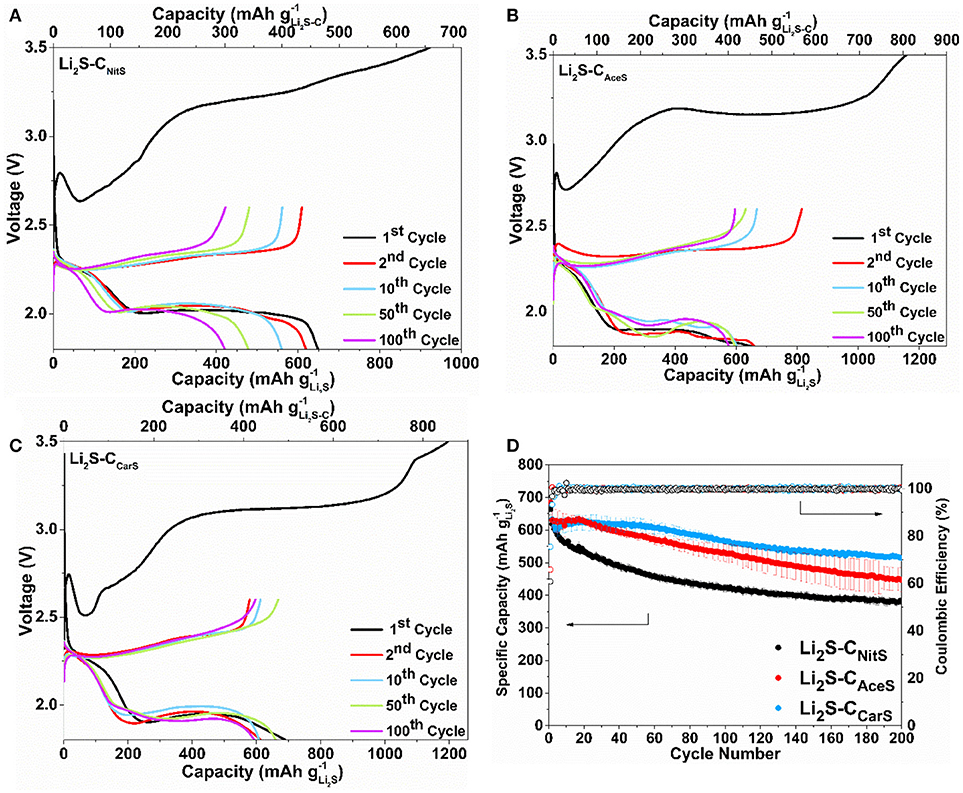
Figure 5. Representative charge-discharge curves of (A) Li2S-CNitS, (B) Li2S-CAceS, (C) Li2S-CCarS at the 1st, 2nd, 10th, 50th, and 100th cycle, and (D) the cycle stability of these composites at 117 mA g-1.
In summary, we examined a new synthetic route for the production of Li2S-C composite materials for Li-S batteries. The combination of aerosol spray pyrolysis and sulfurization has been shown to be a robust method for the conversion of various lithium salts including nitrate, acetate, and carbonate to Li2S-C nanocomposites using sucrose as the carbon precursor. Furthermore, the cycling performance of the Li2S-C composite has been found to be closely correlated to its precursor-derived microstructure. The combination of Li2CO3 and sucrose results in the Li2S-C composite with the best electrochemical performance, which has a non-hollow composite structure with Li2S uniformly embedded in the carbon matrix. The detailed mechanism of aerosol spray pyrolysis and the optimization of the composite's structure and electrochemical performance will be further investigated in our future studies.
Author Contributions
NH completed most of the experiments. JS, JZ, and CF helped with the experiments and data analysis. JG designed the experiments. All authors co-wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the financial support from Power Energy Solutions Inc.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00476/full#supplementary-material
References
Cai, K., Song, M., Cairns, J. E., and Zhang, Y. (2012). Nanostructured Li2S-C composites as cathode material for high-energy Lithium/Sulfur batteries. Nano Lett. 12, 6474–6479. doi: 10.1021/nl303965a
Chen, J., Henderson, W. A., Pan, H., Perdue, R. B., Cao, R., Hu, J. Z., et al. (2017). Improving Lithium–Sulfur battery performance under lean electrolyte through nanoscale confinement in soft swellable gels. Nano Lett. 17, 3061–3067. doi: 10.1021/acs.nanolett.7b00417
Chen, S., Gao, Y., Yu, Z., Gordin, L. M., Song, J., and Wang, D. (2017). High capacity of Lithium-Sulfur batteries at low electrolyte/Sulfur ratio enabled by an Organosulfide containing electrolyte. Nano Energy 31, 418–423. doi: 10.1016/j.nanoen.2016.11.057
Dressel, B. C., Jha, H., Eberle, A., Gasteiger, A. H., and Fassler, F. T. (2016). Electrochemical performance of Lithium-Sulfur batteries based on a Sulfur cathode obtained by H2S gas treatment of a Lithium salt. J. Power Sour. 307, 844–848. doi: 10.1016/j.jpowsour.2015.12.140
Guo, J., Yang, Z., Yu, Y., Abruña, H. D., and Archer, L. A. (2013). Lithium–Sulfur battery cathode enabled by Lithium–Nitrile interaction. J. Am. Chem. Soc. 135, 763–767. doi: 10.1021/ja309435f
Hwa, Y., Zhao, J., and Cairns, E. J (2015) Lithium Sulfide (Li2S)/Graphene Oxide nanospheres with conformal carbon coating as a high-rate, long-life cathode for Li/S cells. Nano Lett. 15, 3479–3486. doi: 10.1021/acs.nanolett.5b00820
Jha, H., Buchberger, I., Cui, X., Meini, S., and Gasteiger, H. A. (2015). Li-S batteries with Li2S cathodes and Si/C anodes. J. Electochem. Soc. 162, 1829–1835. doi: 10.1149/2.0681509jes
Kohl, M., Bruckner, J., Bauer, I., Althues, H., and Kaskel, S. (2015). Synthesis of highly electrochemically active Li2S nanoparticles for Lithium–Sulfur-batteries. J. Mater. Chem. A 3, 16307–16312. doi: 10.1039/C5TA04504E
Li, Z., Zhang, S., Zhang, C., Ueno, K., Yasuda, T., Tatara, R., et al. (2015). One-pot pyrolysis of Lithium Sulfate and graphene nanoplatelet aggregates: in situ formed Li2S/ graphene composite for Lithium–Sulfur batteries. Nanoscale 7, 14385–14392. doi: 10.1039/C5NR03201F
Messing, G., Zhang, S., and Jayanthi, G. (1993). Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 76, 2707–2726.
Pan, H., Han, S. K., Engelhard, H. M., Cao, R., Chen, J., Zhang, J., et al. (2018). Addressing passivation in Lithium–Sulfur battery under lean electrolyte condition. Adv. Funct. Mater. 28:1707234. doi: 10.1002/adfm.201707234.
Piwko, M., Weller, C., Hippauf, F., Dorfler, S., Althues, H., and Kaskel, S. (2018). Symmetric Lithium Sulfide-Sulfur cells: a method to study degradation mechanisms of cathode, separator and electrolyte concepts for Lithium-Sulfur batteries. J. Electrochem. Soc. 165, A1084–A1091. doi: 10.1149/2.1131805jes
Roe, A., and Finlay, J. B. (1952). The isotope effect II, pyrolysis of Lithium Acetate 1-C14. J. Am. Chem. Soc. 74, 2442–2443.
Skrabalak, S. E., and Suslick, K. S. (2006). Porous carbon powders prepared by ultrasonic spray pyrolysis. J. Am. Chem. Soc. 128, 12642–12643. doi: 10.1021/ja064899h
Son, Y., Son, Y., Lee, J., Jang, J., and Cho, J. (2015). Recent advances in Lithium Sulfide cathode materials and their use in Lithium Sulfur batteries. Adv. Energy Mat. 5:1500110. doi: 10.1002/aenm.201500110
Stern, K. H., and Weise, E. L. (1969). High Temperature Properties and Decomposition of Inorganic Salts; Part 2. Carbonates. Washington, DC: United States Dept of Commerce.
Tan, G., Xu, R., Xing, Z., Yuan, Y., Lu, J., Wen, J., et al. (2017). Burning lithium in CS2 for high-performing compact Li2 S–graphene nanocapsules for Li–S batteries. Nat. Energy 2:17090. doi: 10.1038/nenergy.2017.90
Vizintin, A., Chabanne, L., Tchernychova, E., Arcon, I., Stievano, L., Aquilanti, G., et al. (2017). The mechanism of Li2S activation in Lithium-sulfur batteries: can we avoid the Polysulfide formation? J. Power Sour. 344, 208–217. doi: 10.1016/j.jpowsour.2017.01.112
Wang, D., Zeng, Q., Zhou, G., Yin, L., Li, F., Cheng, H., et al. (2013). Carbon-Sulfur composites for Li-S batteries: status and prospects. J. Mat. Chem. A 1, 9382–9394. doi: 10.1039/C3TA11045A
Wu, F., Kim, H., Magasinski, A., Lee, J., Lin, H., and Yushin, G. (2014a). Harnessing steric separation of freshly nucleated Li2S nanoparticles for bottom-up assembly of high-performance cathodes for Lithium-Sulfur and Lithium-Ion batteries. Adv. Energy Mater. 4:1400196. doi: 10.1002/aenm.201400196.
Wu, F., Lee, J., Magasinski, A., Kim, H., and Yushin, G. (2014b). Solution-based processing of graphene-Li2S composite cathodes for Lithium-Ion and Lithium-Sulfur batteries. Part. Part. Syst. Charact. 31, 639–644. doi: 10.1002/ppsc.201300358.
Wu, F., Lee, J., Zhao, E., Zhang, B., and Yushin, G. (2016). Graphene–Li2S–carbon nanocomposite for Lithium–Sulfur batteries. ACS Nano 10, 1333–1340. doi: 10.1021/acsnano.5b06716
Wu, F., Lee, J. E., Fan, F., Nitta, N., Kim, H., Zhu, T., et al. (2015). A hierarchical particle–shell architecture for long-term cycle stability of Li2S cathodes. Adv. Mater. 27, 5579–5586. doi: 10.1002/adma.201502289.
Wu, F., Magasinski, A., and Yushin, G. (2014c). Nanoporous Li2S and MWCNT-linked Li2S powder cathodes for Lithium-Sulfur and Lithium-ion battery chemistries. J. Mater. Chem. A 2, 6064–6070. doi: 10.1039/C3TA14161F
Wu, S. D., Shi, F., Zhou, G., Zu, C., Liu, C., Liu, K., et al. (2018). Quantitative investigation of Polysulfide adsorption capability of candidate materials for Li-S batteries. Energy Storage Mater. 13, 241–246. doi: 10.1016/j.ensm.2018.01.020
Yang, Y., McDowell, M. T., Jackson, A., Cha, J. J., Hong, S. S., and Cui, Y. (2010). New nanostructured Li2S/Silicon rechargeable battery with high specific energy. Nano Lett. 10, 1486–1491. doi: 10.1021/nl100504q
Yang, Z., Guo, J., Das, K. S., Yu, Y., Zhou, Z., Abruna, D. H., et al. (2013). In situ synthesis of Lithium Sulfide–carbon composites as cathode materials for rechargeable Lithium batteries. J. Mater. Chem. A 1, 1433–1440. doi: 10.1039/C2TA00779G
Ye, F., Noh, H., Lee, J., Lee, H., and Kim, H. (2018). Li2S/carbon nanocomposite strips from a low-temperature conversion of Li2SO4 as high-performance Lithium-Sulfur cathodes. J. Mater. Chem. A 6, 6617–6624. doi: 10.1039/C8TA00515J
Yu, M., Wang, Z., Wang, Y., Dong, Y., and Qiu, J. (2017). Freestanding flexible Li2S paper electrode with high mass and capacity loading for high-energy Li-S batteries. Adv. Energy Mater. 7:1700018. doi: 10.1002/aenm.201700018.
Zhang, J., Shi, Y., Ding, Y., Peng, L., Zhang, W., and Yu, G. (2017). A conductive molecular framework derived Li2S/N,P-codoped carbon cathode for advanced Lithium–Sulfur Batteries. Adv. Energy Mater. 7:1602876. doi: 10.1002/aenm.201602876.
Zhou, G., Tian, H., Jin, Y., Tao, X., Liu, B., Zhang, R., et al. (2017). Catalytic oxidation of Li2S on the surface of metal Sulfides for Li–S batteries. Proc. Natl. Acad. Sci. U.S.A. 114, 840–845. doi: 10.1073/pnas.1615837114
Keywords: aerosol spray pyrolysis, nanocomposites, lithium-sulfur batteries, lithium sulfide, sulfurization
Citation: Hart N, Shi J, Zhang J, Fu C and Guo J (2018) Lithium Sulfide–Carbon Composites via Aerosol Spray Pyrolysis as Cathode Materials for Lithium–Sulfur Batteries. Front. Chem. 6:476. doi: 10.3389/fchem.2018.00476
Received: 20 July 2018; Accepted: 20 September 2018;
Published: 09 October 2018.
Edited by:
Fan Zhang, Fudan University, ChinaReviewed by:
Piercarlo Mustarelli, University of Pavia, ItalyYuan Yang, Columbia University, United States
Copyright © 2018 Hart, Shi, Zhang, Fu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juchen Guo, amd1b0BlbmdyLnVjci5lZHU=
 Noam Hart
Noam Hart Jiayan Shi
Jiayan Shi Jian Zhang2
Jian Zhang2 Juchen Guo
Juchen Guo