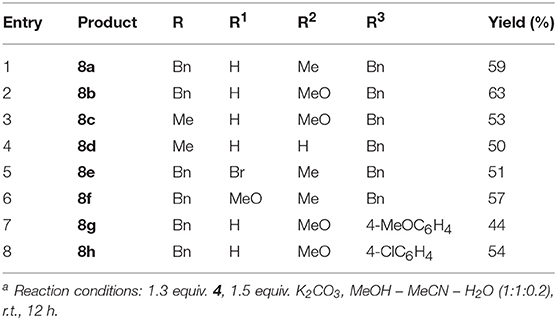
- 1Department of Organic Chemistry, Faculty of Science, Peoples' Friendship University of Russia (RUDN University), Moscow, Russia
- 2Laboratory for Organic & Microwave-Assisted Chemistry (LOMAC), Department of Chemistry, University of Leuven (KU Leuven), Leuven, Belgium
A novel isocyanide-based multicomponent synthesis of alkyl aryl(indol-3-yl)acetimidates has been established. Starting from aryl(indol-3-yl)methylium tetrafluoroborates, aromatic isocyanides and alcohols, the imidates were obtained in moderate to very good yields. Consecutive four-component synthesis of the above mentioned imidates from N-alkylindoles, aromatic aldehydes, aromatic isocyanides and alcohols was also proposed. In addition, it was shown that in the presence of water, aryl(indol-3-yl)methylium tetrafluoroborates reacted with isocyanides to furnish aryl(indol-3-yl)acetamides.
Introduction
Multicomponent reactions (MCRs) serve as a powerful and widely used instrument in organic synthesis (Shiri, 2012; Müller, 2014; Zarganes-Tzitzikas et al., 2015; Zhu et al., 2015; Levi and Müller, 2016). A special place among them is occupied by transformations with the participation of isocyanides, unique reagents where nucleophiles and electrophiles attack the same atom (for a book on isocyanides, see Nenajdenko, 2012; for selected reviews on isocyanide-based MCR, see Dömling and Ugi, 2000; Dömling, 2006; Sadjadi et al., 2016). Common reaction partners in MCR with isocyanides are iminium salts, carbonyl compounds (or oxocarbenium ions), and electron-deficient alkynes (Ugi, 1962; Banfi and Riva, 2005; De Moliner et al., 2011). Several examples of interaction between isocyanides and activated alkenes were reported (Saegusa et al., 1972; Person et al., 1980; Shaabani et al., 2002; Maltsev et al., 2006; Mironov et al., 2006; Jing et al., 2010; Soleimani and Zainali, 2011; Soleimani et al., 2011, 2013). Reactions of isocyanides with α,β-unsaturated imines or the corresponding iminium salts with the possibility of 1,4-addition are still rare (Marchand et al., 1999; Fontaine et al., 2009; Shimizu et al., 2009). Particularly, such imine and iminium salts were used in a formal [4+1] cycloaddition reaction with the formation of a pyrrole ring (Marchand et al., 1999; Fontaine et al., 2009; Kaur et al., 2016). In our opinion, the interaction of isocyanides with α,β-unsaturated imines could well-evolve into a novel multicomponent reaction.
Considering that the indole scaffold is privileged from medicinal chemistry point of view (Barreiro, 2016), we decided to use 3-arylidene-3H-indolium salts as simple vinylogues of iminium ions. Alkylideneindoleninium (3-alkylidene-3H-indolium) ions (I) can be formed as intermediates by cleaving the leaving group from the α-position (“benzylic”) of the substituent at the indole 3-position (Figure 1A) (For reviews, concerning generation and reactivity of alkylideneindolenine intermediates, see Enders et al., 2008; Shaikh et al., 2008; Palmieri et al., 2010; Wang et al., 2014; Zhuo et al., 2014; Jin et al., 2015; Palmieri and Petrini, 2016; Deb et al., 2017; Mei and Shi, 2017). Such intermediates are usually unstable and can react in situ with nucleophiles. This approach has been widely used in the syntheses of various indole derivatives (Enders et al., 2008; Shaikh et al., 2008; Palmieri et al., 2010; Wang et al., 2014; Zhuo et al., 2014; Jin et al., 2015; Palmieri and Petrini, 2016; Deb et al., 2017; Mei and Shi, 2017).
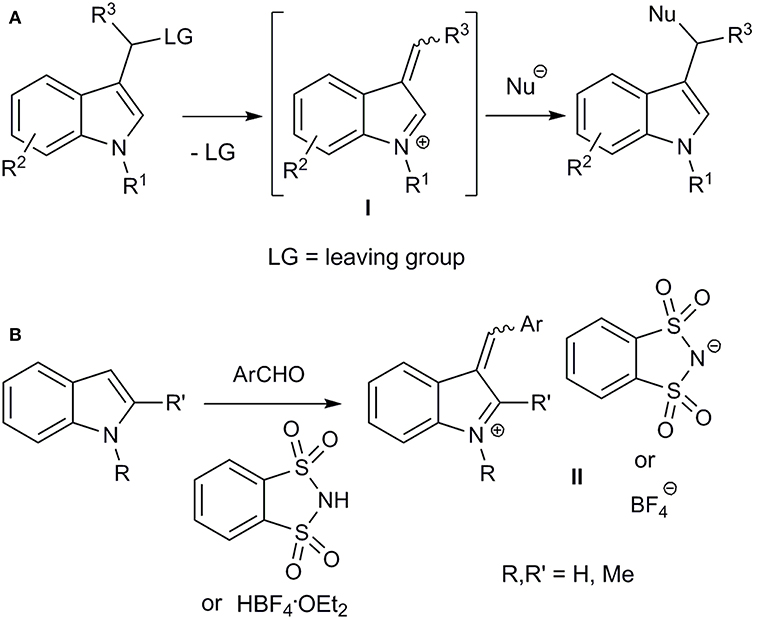
Figure 1. In situ generation of alkylideneindoleninium intermediates (A) and synthesis of stable aryl(indol-3-yl)methylium salts (B).
Moreover, relatively stable 3-arylidene-3H-indolium (aryl(indol-3-yl)methylium) ions II were obtained by acid-catalyzed coupling of indoles and aryl aldehydes, and were isolated and characterized as o-benzenedisulfonimide salts (Figure 1B) (Barbero et al., 2012). The corresponding diarylmethanes were obtained by reduction of these salts (Barbero et al., 2012), and an organocatalytic addition of aliphatic aldehydes to such compounds was developed (Armenise et al., 2015). Recently, an efficient synthesis for a bench-stable aryl(indol-3-yl)methyl tetrafluoroborate has been proposed (Figure 1B) (Barbero et al., 2015; Follet et al., 2015). Lewis acidity of such obtained aryl(indol-3-yl)methylium ions and kinetics of their interaction with different nucleophiles, including allylsilanes, enol silyl ethers, triarylphosphines, pyridines, secondary amines, have been studied (Follet et al., 2015, 2016). It should also be mentioned that the vinylogous iminium character of salts II was confirmed by single-crystal X-ray diffraction analysis (Barbero et al., 2015; Follet et al., 2015).
To the best of our knowledge, reactions of alkylideneindolenines or the corresponding salts with isocyanides have not been published yet. Herein, we report the three-component reaction of 3-arylidenindolium salts with isocyanides and alcohols to form alkyl aryl(indol-3-yl)acetimidates.
Results and Discussion
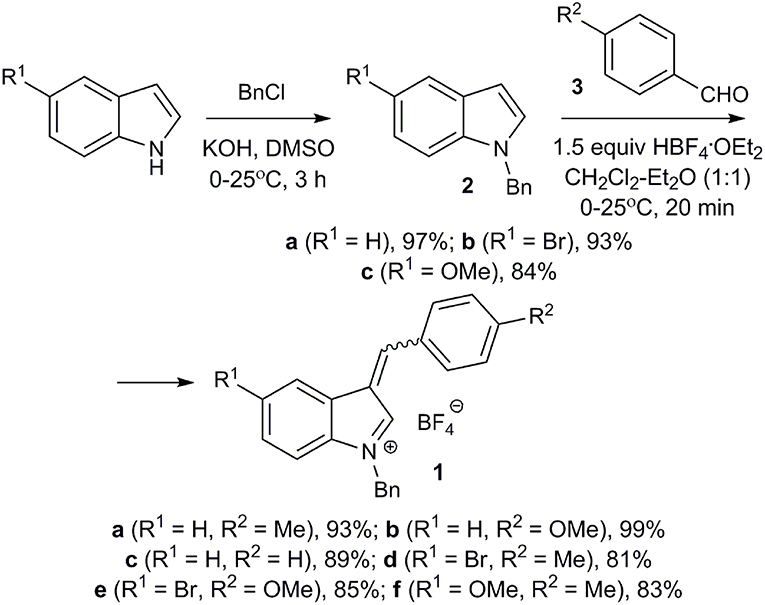
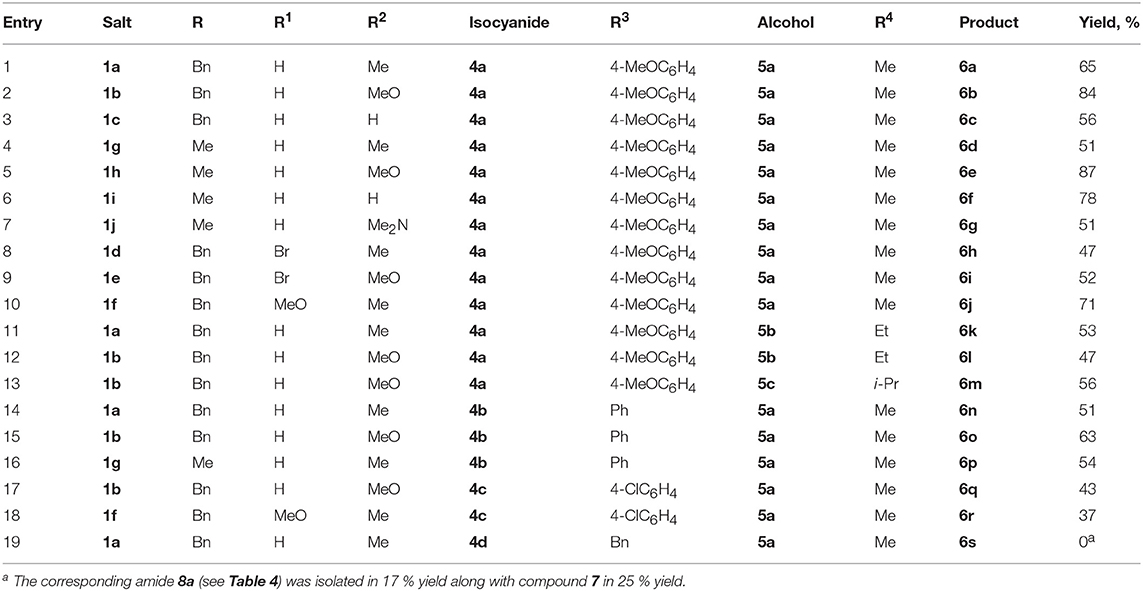
Starting salts 1a-f were obtained by alkylation of the corresponding indoles followed by reaction of N-benzylindoles 2a-c with aromatic aldehydes 3a-c under conditions similar to a previously published procedure (Figure 2) (Follet et al., 2015). Tetrafluoroborates 1a-f containing a benzyl group in the indole 1-position remained unchanged after being stored at room temperature for several weeks and turned out to be more stable than their methyl analogs, whose lability was observed previously (Follet et al., 2015). In solution salts 1a-f exist as a mixture of E- and Z-isomers (for copies of NMR spectra of obtained compounds, see Supplementary Material), what was also noted for 2-unsubstituted derivatives earlier (Follet et al., 2015).
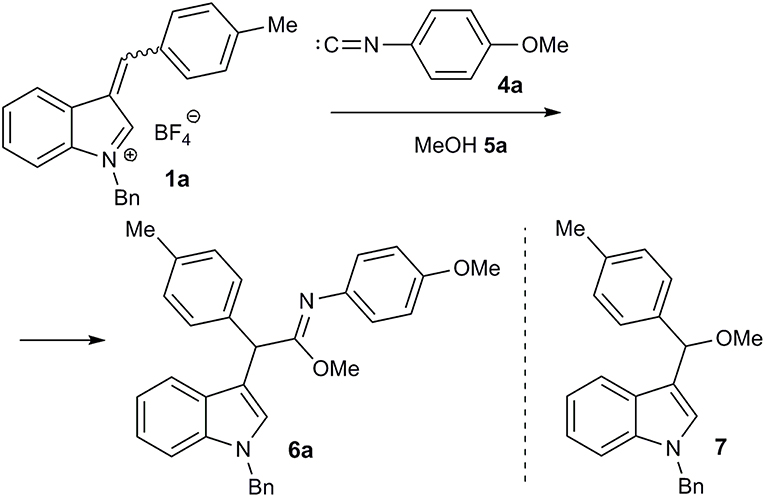
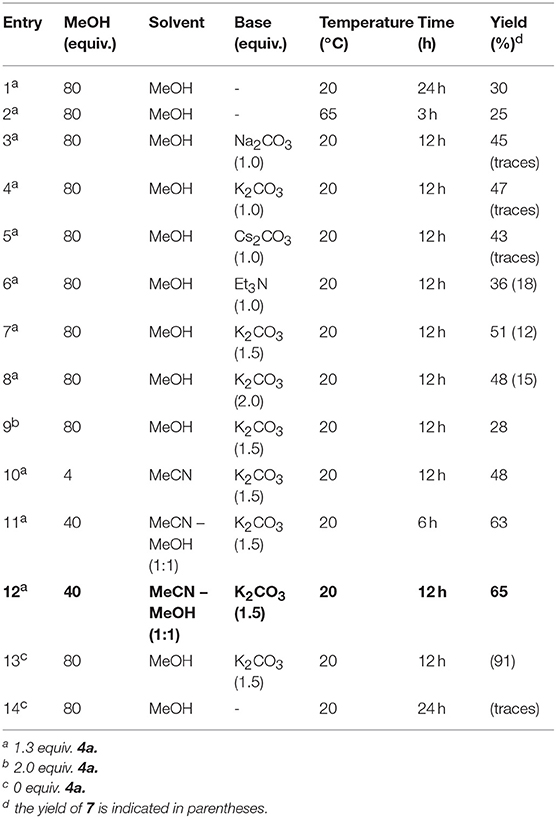
Next, the interaction of salt 1a with p-methoxyphenyl isocyanide (4a) in methanol (5a) was performed at room temperature. After treating the reaction mixture with sodium bicarbonate solution, the imidate 6a was isolated in moderate yield as a result of the expected three component interaction (Figure 3; Table 1, entry 1). This was stable upon chromatography on silica gel and further storage for several weeks. Unfortunately, our attempt to facilitate the reaction by heating was unsuccessful, due to significant tar formation (Table 1, entry 2). The addition of potassium carbonate as a base increased the yield (Table 1, entries 4, 7, 8), but led to the formation of ether 7, resulting from the two-component reaction with methanol. Sodium or cesium carbonate had nearly the same effect, while Et3N was less effective (Table 1, entries 3, 5, 6).
The formation of methoxy derivative 7 was not observed when the reaction was carried out in acetonitrile with 4 equiv of methanol and 1.5 equiv. of K2CO3, (Table 1, entry 10). However, the highest yield of the target product 6a was achieved by using a mixture of equal amounts of methanol and acetonitrile as solvent (Table 1, entry 12).
In these optimized conditions, the reaction was showing significant progress by TLC during the first 3 h. After 6 h, the yield did not change substantially (Table 1, entries 11 and 12). It is worth noting that increasing the amount of isocyanide resulted in a drop of the yield (Table 1, entry 9 vs. 7), whereas, performing the reaction without the addition of isocyanide in the presence of a base led to the formation of two-component reaction product 7. Without the addition of base, this product was only formed in trace amounts (Table 1, entries 13, 14).
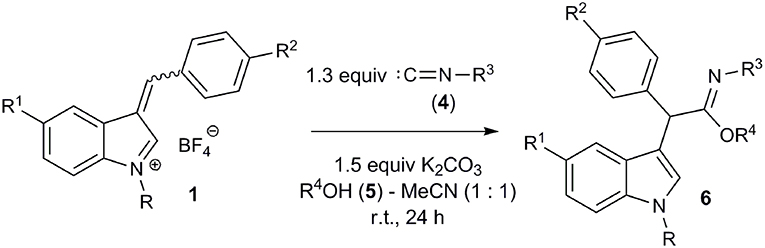
With the optimized conditions in hand, the interaction of salts 1a-f with isocyanides 4a-d and methanol was investigated (Figure 4; Table 2). Moreover, salts 1g-j containing a methyl group at the indole nitrogen atom, which were obtained by a previously described procedure (Follet et al., 2015), were also involved. Reactions of aromatic isocyanides 4a-c led to the corresponding imidates 6 in moderate to good yields. The best results were observed for salts derived from anisaldehyde (Table 2, entries 2, 5). Such results can be explained by stabilization of the 3-arylidene-3H-indolium salts 1b,h with the donor substituent (MeO) and, consequently, by reducing the rate of the starting compound degradation under the reaction conditions, in comparison with more electrophilic salts 1a,c,g,i. At the same time, the stronger electron-donating dimethylamino group notably reduced the electrophilicity of the substrate, which led to lowering the yield of the reaction product with isonitrile 4a (Table 2, entry 7) (for electrophilicity parameters of salts 1j-g, see Follet et al., 2015). Similarly, the presence of an electron-donating methoxy group at the indole 5-position slightly increased the yield of the corresponding imidate 6j (Table 2, entry 1 vs. entry 10). The presence of a bromine atom in the same position decreased the yield (Table 2, entry 8, 9).
Among aromatic isocyanides, the best results were also achieved for isocyanide 4a containing an electron-donating methoxy group. Furthermore, the alcohol component of this MCR could be varied and the reaction was carried out in a mixture of acetonitrile with alcohols 5b,c (Table 2, entries 11-13).
It should be mentioned that, in case of the non-conjugated benzyl isocyanide 4d, it was not possible to isolate the corresponding imidate 6s. Within 12 h, the reaction did not show significant progress according to TLC. After work-up and purification by chromatography on silica gel, amide 8a was isolated in a low yield along with compound 7 (Table 2, entry 19). Apparently, the amide 8a was formed by hydrolysis of the corresponding imidate 6s.
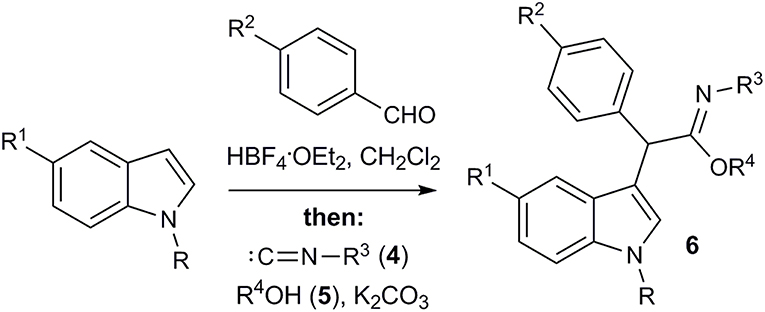
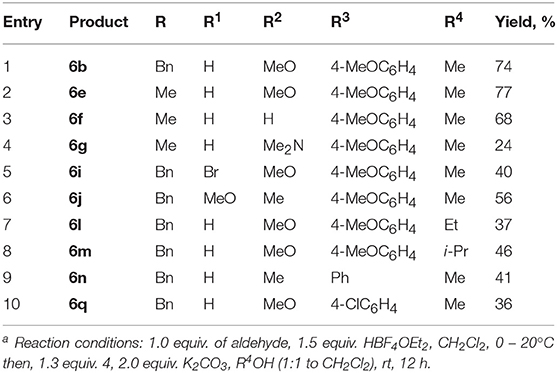
Next, the possibility of imidate synthesis from the indole and the aldehyde by a sequential one-pot four-component process, without isolation of the corresponding 3-arylidene-3H-indolium salt, was investigated (Figure 5; Table 3). After acid-catalyzed condensation of the aldehyde with 1-alkylindole, an excess of K2CO3 and a solution of isocyanide in the appropriate alcohol were added to the reaction mixture. To our great satisfaction, target imidate 6 was obtained in moderate to good yields.
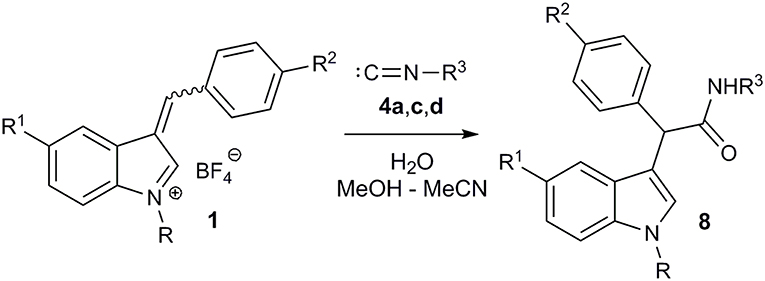
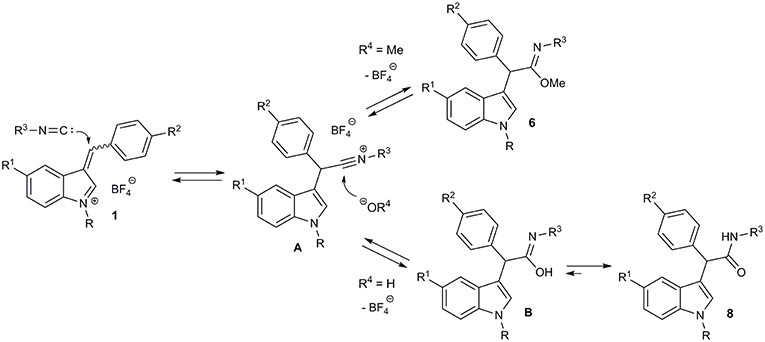
Then we decided to extend our three-component imidate synthesis with the aim to obtain amides 8 (Figure 6; Table 4). As it turned out, addition of 10 volume % of water to the reaction mixture delivered the desired amides. Moreover, under these conditions, the reaction with benzyl isocyanide 4d also proceeded very quickly. A white precipitate of the corresponding amide 8a appeared almost immediately after isocyanide 4d addition. The interaction with aromatic isocyanides 4a,c was slightly slower but also efficient (Table 4, entries 7 and 8). Thus, 8 examples of amides 8 were obtained in a moderate to good yields employing this procedure (Table 4).
Previously, low yields were observed in formation of imidates with the conjugate addition of alkyl isocyanides to methyl acrylate and acrylonitrile in methanol (Saegusa et al., 1971). Amides were reported to be formed in reaction of dicyanoethylenes with isocyanides (Soleimani et al., 2011). Based on these facts and on the reported reactivity of 3-arylidene-3H-indolium salts (Follet et al., 2015, 2016), as well as on our own experiments, we suggest the following pathway for the multicomponent transformation of 3-arylidene-3H-indolium salts (Figure 7). The conjugate nucleophilic addition of isocyanide to the vinylogous iminium ion 1 leads to nitrilium salt A, which is further attacked by the nucleophile. This could be an alcoholate ion (or the corresponding alcohol). As a result, the imidate 4 is formed. Therefore, the role of the base in this process is to generate a small concentration of alcoholate ion as a strong nucleophile and to bind the released HBF4.
The unsuccessful attempt of the three-component reaction with non-conjugated isocyanide 4d can be explained by the reversibility of the process and the lower stability of the corresponding imidate. In the case that water is present, the hydroxide ion acts as nucleophile leading to the imidic acid B, which is in equilibrium with the corresponding amide 8. This last step makes the entire sequence of reactions almost irreversible.
Conclusions
We have elaborated a three-component reaction of a 3-arylidene-3H-indolium salt, an aromatic isocyanide and an alcohol, leading to a series of alkyl aryl(indol-3-yl)acetimidates with yields up to 87%. We have also established a consecutive four-component synthesis of the above mentioned imidates from a N-alkylindole, an aromatic aldehyde, an aromatic isocyanide and an alcohol. By using aqueous acetonitrile-methanol media we have expanded our method for the synthesis of aryl(indol-3-yl)acetamides. These reactions present a new practical synthetic approach to a series of compounds possessing a privileged indole scaffold and then also extend isocyanide-based MCRs by using vinylogous iminium ions.
Materials and Methods
General
Starting reagents were purchased from commercial sources and were used without any additional purification or were prepared according to literature procedures. 1H and 13C NMR spectra were acquired on a Jeol JNM-ECA 600 spectrometer (with operating frequencies of 600 and 150 MHz, respectively) at room temperature and referenced to the residual signals of the solvent. The solvents used for NMR were DMSO-d6 and CDCl3. Chemical shifts are reported in parts per million (δ/ppm). Coupling constants are reported in Hertz (J/Hz). The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; dd, doublet of doublets and br s, broad singlet. Infrared spectra were measured on an Infralum FT-801 FT/IR instrument. The wavelengths are reported in reciprocal centimeters (νmax /cm−1). Mass spectra were recorded with LCMS-8040 Triple quadrupole liquid chromatograph mass-spectrometer from Shimadzu (ESI) and Kratos MS-30 mass-spectrometer (EI, 70 eV). HRMS spectra were recorded on a Bruker MicrOTOF-Q II. Elemental analysis was performed on an Euro Vector EA-3000 elemental analyzer. The reaction progress was monitored by TLC and the spots were visualized under UV light (254 or 365 nm). Column chromatography was performed using silica gel (230–400 mesh). Melting points were determined on a SMP-10 apparatus and were uncorrected. Solvents were distilled and dried according to standard procedures.
Synthesis of Tetrafluoroborates 1a-f
1-Benzyl-3-(4-Methylbenzylidene)-3H-Indolium Tetrafluoroborate (1a); Typical Procedure
1H-Indole (5.00 g, 42.7 mmol) and benzyl chloride (7.30 ml, 63.4 mmol) were dissolved in 10 ml of dry DMSO and finely ground KOH (3.5 g, 62.5 mmol) was added at 0–5°C in one portion. The reaction mixture was stirred at r.t. for 3 h and then poured into 100 mL of cold water. The product was extracted with ether (2 × 50 mL). The combined organic layers were washed with water (50 mL), brine (30 mL) and dried over anhydrous Na2SO4, after which the solvent was removed in vacuo. The residue was purified by flash chromatography on short column of silica gel (EtOAc – hexane, 20:1) to afford 1-benzyl-3H-indole (2a) (8.6 g, 97%) as white crystals.
1-Benzyl-1H-indole (2a) (1.00 g, 4.82 mmol) and p-tolualdehyde (3a) (4.82 mmol) were dissolved in a mixture of dry CH2Cl2 (5 mL) and Et2O (5 mL). Then HBF4·OEt2 (1.00 ml, 7.35 mmol) was added dropwise at 0–5°C over a period of 2 min. The reaction mixture was allowed to warm to r.t. and stirred for 20 min. The resulting precipitate was filtered, washed thoroughly with Et2O (5 × 20 mL) to give 1-benzyl-3-(4-methylbenzylidene)-3H-indolium tetrafluoroborate.
Yield: 1.78 g (93%); bright orange solid; mp 201–203°C (dec.).
IR (film): 3438, 3127, 1736, 1621, 1587, 1527, 1447, 1369, 1299, 1257, 1186, 1100, 1073, 1048, 813, 761, 711 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 3:1 Z/E diastereomeric mixture): δ = 9.17 (s, 0.67 H), 8.91 (s, 0.33 H), 8.82 (s, 0.67 H), 8.71 (s, 0.33 H), 8.32 (d, J = 8.1 Hz, 0.33 H), 8.01 (d, J = 8.0 Hz, 0.67 H), 7.97 (d, J = 8.1 Hz, 0.67 H), 7.91–7.85 (m, 1.33 H), 7.65–7.52 (m, 3 H), 7.50–7.36 (m, 7 H), 5.70 (s, 1.33 H), 5.60 (s, 0.67 H), 2.54 (s, 1 H), 2.49 (s, 2 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 165.3, 160.6, 159.7, 153.4, 150.6, 149.7, 141.6, 139.9, 134.8, 134.2, 131.7, 131.4, 131.0, 130.95, 130.90, 130.6, 130.1, 129.97, 129.94, 129.86, 129.7, 129.64, 129.60, 129.2, 128.5, 128.45, 128.3, 127.09, 127.07, 125.2, 124.1, 121.2, 115.1, 115, 54.7, 54.2, 22.5, 22.3.
HRMS (TOF ES+): m/z [M – ]+ calcd for C23H20N+: 310.1590; found: 310.1596.
1-Benzyl-3-(4-Methoxylbenzylidene)-3H-Indolium Tetrafluoroborate (1b)
Red solid; yield 1.97 g (99% from 1.00 g 1-benzyl-1H-indole (2a)); mp 214-216°C (dec.).
IR (film): 3130, 2956, 2917, 2847, 1736, 1581, 1556, 1520, 1441, 1370, 1281, 1262, 1173, 1098, 1062, 835, 760, 710, 587 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 2.3:1 Z/E diastereomeric mixture): δ = 9.06 (s, 0.7 H), 8.75 (s, 0.7 H), 8.72 (s, 0.3 H), 8.62 (s, 0.3 H), 8.37 – 8.32 (m, 0.3 H), 8.16 (d, J = 9.0 Hz, 0.6 H), 8.04 (d, J = 8.6 Hz, 1.4 H), 7.99 (d, J = 8.1 Hz, 0.7 H), 7.66 – 7.52 (m, 3 H), 7.48 – 7.34 (m, 5 H), 7.22 – 7.15 (m, 2 H), 5.68 (s, 1.4 H), 5.58 (s, 0.6 H), 4.04 (s, 0.9 H), 4.01 (s, 2.1 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 169.2, 168.3, 164.5, 159.0, 158.6, 150.9, 142.4, 139.5, 138.7, 138.5, 131.9, 131.3, 130.1, 130.0, 129.97, 129.95, 129.90, 129.13, 129.10, 129.0, 128.9, 128.5, 128.1, 127.5, 127.0, 126.9, 125.3, 124.4, 123.7, 120.7, 117.2, 116.3, 114.8, 114.5, 56.6, 56.5, 54.4, 53.9.
HRMS (TOF ES+): m/z [M – ]+ calcd for C23H20NO+: 326.1539; found: 326.1550.
1-Benzyl-3-Benzylidene-3H-indolium Tetrafluoroborate (1c)
Bright orange solid; yield 1.64 g (89% from 1.00 g 1-benzyl-1H-indole (2a)); mp 163-165°C (dec.).
IR (film): 3416, 3115, 1619, 1605, 1587, 1568, 1531, 1447, 1370, 1296, 1253, 1192, 1099, 1050, 826, 759, 710, 678, 599, 570 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 1.7:1 Z/E diastereomeric mixture): δ = 9.15 (s, 0.63 H), 8.93 (s, 0.37 H), 8.90 (s, 0.63 H), 8.74 (s, 0.37 H), 8.29 (d, J = 7.7 Hz, 0.37 H), 8.04 (d, J = 7.7 Hz, 0.63 H), 8.02 (d, J = 8.0 Hz, 0.76 H), 7.92 (d, J = 7.7 Hz, 1.26 H), 7.80 – 7.72 (m, 1 H), 7.69 – 7.56 (m, 5 H), 7.48 – 7.36 (m, 5 H), 5.72 (s, 1.26 H), 5.62 (s, 0.76 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 165.0, 161.4, 159.6, 154.5, 142.9, 140.4, 136.9, 136.4, 134.1, 133.72, 133.70, 133.4, 133.3, 131.03, 131.0, 130.7, 130.6, 130.5, 130.4, 130.3, 130.2, 130.1, 130.03, 130.0, 129.97, 128.7, 128.6, 125.4, 128.2, 125.1, 124.3, 121.6, 115.3, 115.2, 55.0, 54.5.
HRMS (TOF ES+): m/z [M – ]+ calcd for C22H18N+: 296.1434; found: 296.1441.
1-Benzyl-5-Bromo-3-(4-Methylbenzylidene)-3H-Indolium Tetrafluoroborate (1d)
Bright orange solid; yield 1.35 g (81% from 1.00 g 1-benzyl-5-bromo-1H-indole (2b)); mp 220-222°C (dec.).
IR (film): 3126, 1736, 1620, 1587, 1526, 1448, 1424, 1372, 1319, 1259, 1188, 1104, 1051, 802, 772, 743, 714, 653, 629 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 2.3:1 Z/E diastereomeric mixture): δ = 9.13 (s, 0.70 H), 8.85 (s, 0.70 H), 8.83 (s, 0.30 H), 8.76 (s, 0.30 H), 8.49 (d, J = 2.0 Hz, 0.30 H), 8.18 (d, J = 2.0 Hz, 0.70 H), 7.99 (d, J = 8.1 Hz, 0.60 H), 7.90 (d, J = 8.1 Hz, 1.40 H), 7.75 (dd, J = 8.7 Hz, 1.7 Hz, 0.30 H), 7.71 (dd, J = 8.7 Hz, 1.7 Hz, 0.70 H), 7.56 (d, J = 8.1 Hz, 0.60 H), 7.52 (d, J = 8.1 Hz, 1.70 H), 7.50 – 7.42 (m, 3.70 H), 7.42 – 7.36 (m, 2 H), 5.71 (s, 1.40 H), 5.62 (s, 0.60 H), 2.59 (s, 0.90 H), 2.54 (s, 2.10 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 167.1, 161.3, 160.3, 153.2, 152.3, 151.4, 141.6, 138.9, 135.4, 134.8, 133.7, 133.0, 132.2, 131.7, 131.58, 131.57, 131.5, 131.1, 130.7, 130.5, 130.4, 130.24, 130.2, 128.7, 128.4, 128.2, 127.2, 126.1, 124.5, 124.4, 124.2, 117.5, 116.5, 116.4, 55.3, 54.7, 22.4, 22.2.
HRMS (TOF ES+): m/z [M – ]+ calcd for C23H19BrN+: 388.0695; found: 388.0701.
1-Benzyl-5-Bromo-3-(4-Methoxylbenzylidene)-3H-Indolium Tetrafluoroborate (1e)
Red orange solid; yield 1.46 g (85% from 1.00 g 1-benzyl-5-bromo-1H-indole (2b)); mp 226-228°C (dec.).
IR (film): 3464, 3129, 2841, 1582, 1554, 1520, 1447, 1429, 1375, 1319, 1281, 1178, 1103, 1051, 888, 835, 799, 773, 716, 629, 595, 556 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 3.3:1 Z/E diastereomeric mixture): δ = 9.07 (s, 0.77 H), 8.73 (s, 0.23 H), 8.72 (s, 0.77 H), 8.68 (s, 0.23 H), 8.45 (d, J = 1.5 Hz, 0.23 H), 8.16 (d, J = 9.1 Hz, 0.46 H), ), 8.13 (d, J = 1.5 Hz, 0.77 H), ), 8.08 (d, J = 9.1 Hz, 1.54 H), 7.71 (dd, J = 8.6 Hz, 1.5 Hz, 0.23 H), 7.64 (dd, J = 8.6 Hz, 1.5 Hz, 0.77 H), 7.48 (d, J = 8.6 Hz, 0.23 H), 7.46 – 7.39 (m, 3.77 H), 7.38 – 7.31 (m, 2 H), 7.24 (d, J = 8.9 Hz, 0.46 H), 7.21 (d, J = 8.9 Hz, 0.46 H), 5.67 (s, 1.54 H), 5.57 (s, 0.46 H), 4.07 (s, 0.69 H), 4.04 (s, 2.31 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 170.3, 169.3, 165.8, 159.9, 158.3, 150.6, 141.0, 139.5, 139.1, 138.1, 132.8, 132.0, 131.7, 131.2, 130.7, 130.6, 130.3, 130.1, 130.0, 128.4, 128.2, 128.1, 127.5, 127.1, 127.0, 126.5, 125.5, 123.8, 123.2, 123.0, 117.6, 116.8, 116.0, 115.9, 56.8, 56.7, 54.7, 54.1.
HRMS (TOF ES+): m/z [M – ]+ calcd for C23H19BrNO+: 404.0645; found: 404.0651.
1-Benzyl-5-Methoxy-3-(4-Methylbenzylidene)-3H-Indolium Tetrafluoroborate (1f)
Dark violet solid; yield 1.50 g (83% from 1.00 g 1-benzyl-5-methoxy-1H-indole (2c)); mp 212-214°C (dec.).
IR (film): 3130, 2947, 1619, 1587, 1526, 1482, 1444, 1372, 1298, 1263, 1237, 1189, 1099, 1051, 916, 858, 808, 754, 705, 645 cm−1.
1H NMR (600 MHz, CDCl3+TFA; ~ 2.7:1 Z/E diastereomeric mixture): δ = 8.89 (s, 0.73 H), 8.78 (s, 0.73 H), 8.64 (s, 0.27 H), 8.59 (s, 0.27 H), 7.95 (d, J = 8.1 Hz, 0.54 H), 7.87 (d, J = 2.5 Hz, 0.27 H), ), 7.82 (d, J = 8.1 Hz, 1.46 H), ), 7.54 – 7.51 (m, 1 H), 7.50 – 7.41 (m, 5.73 H), 7.41 – 7.34 (m, 2 H), 7.14 (dd, J = 9.1 Hz, 2.5 Hz, 0.27 H), 7.10 (dd, J = 9.1 Hz, 2.5 Hz, 0.73 H), 5.63 (s, 1.46 H), 5.55 (s, 0.54 H), 3.96 (s, 2.19 H), 3.89 (s, 0.81 H), 2.54 (s, 0.81 H), 2.50 (s, 2.19 H).
13C NMR (150 MHz, CDCl3+TFA): δ = 164.3, 158.9, 158.7, 151.3, 150.4, 149.6, 136.8, 134.6, 134.1, 133.8, 131.8, 131.4, 131.2, 131.0, 130.9, 130.8, 130.7, 130.6, 130.3, 130.2, 130.1, 130.0, 129.3, 128.6, 128.3, 127.3, 127.2, 127.0, 116.8, 116.2, 116.13, 116.09, 110.0, 106.0, 56.44, 56.36, 55.0, 54.4, 22.4, 22.2.
HRMS (TOF ES+): m/z [M – ]+ calcd for C24H22NO+: 340.1696; found: 340.1701.
Synthesis of Imidates 6
Three-Component Synthesis of Imidates 6; General Procedure (GP1)
An isocyanide 4 (0.65 mmol) was dissolved in a mixture of abs. alcohol (1 mL) and MeCN (1 mL). A salt 1 (0.5 mmol) and K2CO3 (0.1 g, 0.75 mmol) were then added. The reaction mixture was stirred at r.t. for 12 h and concentrated in vacuo. The residue was dissolved in EtOAc (50 mL), washed with NaHCO3 (2 × 25 mL), brine (20 mL), and dried over anhydrous Na2SO4. The EtOAc was evaporated in vacuo. The residue was chromatographed on a column with silica gel with EtOAc–hexane.
Consecutive Four-Component Synthesis of Imidates 6; General Procedure (GP2)
N-Alkylindole (0.5 mmol) and aromatic aldehyde (0.5 mmol) were dissolved in dry CH2Cl2 (3 mL). HBF4·OEt2 (0.1 ml, 0.75 mmol) was then added dropwise at 0–5°C over a period of 2 min. The reaction mixture was allowed to warm to r.t. and stirred for additional 20 min. Then K2CO3 (0.14 g, 1.0 mmol), anhydrous alcohol (3 ml) and isocyanide 4 (0.65 mmol) were added. The reaction mixture was stirred at r.t. for 12 h and concentrated in vacuo. The residue was dissolved in EtOAc (50 mL), washed with NaHCO3 (2 × 25 mL), brine (20 mL) and dried over anhydrous Na2SO4. The EtOAc was evaporated in vacuo. The residue was chromatographed on a column with silica gel with EtOAc–hexane.
Methyl 2-(1-Benzyl-1H-Indol-3-yl)-N-(4-Methoxyphenyl)-2-(p-tolyl)acetimidate (6a)
Yield, obtained by following GP1: 154 mg (65%).
Yellowish oil; Rf = 0.47 (EtOAc–hexane, 1:6).
IR (film): 3028, 2925, 2834, 1733, 1661, 1609, 1583, 1506, 1465, 1354, 1303, 1238, 1178, 1032, 909, 831, 740, 696, 636 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.36 – 7.23 (m, 5H), 7.19 – 7.13 (m, 3H), 7.12 – 7.07 (m, 4H), 7.05 – 6.99 (m, 2H), 6.84 (d, J = 9.1 Hz, 2H), 6.74 (d, J = 9.1 Hz, 2H), 5.42 (s, 1H), 5.37 – 5.29 (m, 2H), 3.84 (s, 3H), 3.80 (s,3H), 2.33 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.6, 155.8, 141.8, 137.7, 137.1, 136.7, 136.4, 129.1 (2C), 128.8 (2C), 128.4 (2C), 128.0, 127.7, 127.4, 126.7 (2C), 122.4 (2C), 122.0, 119.7, 119.4, 114.4 (2C), 114.0, 109.9, 55.6, 53.7, 50.1, 42.7, 21.2.
MS (ESI): m/z = 475 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H31N2: 475.2380; found: 475.2375.
Methyl 2-(1-Benzyl-1H-indol-3-yl)-N,2-bis(4-methoxyphenyl)acetimidate (6b)
Yield, obtained by following GP1: 206 mg (84%).
Yield, obtained by following GP2: 181 mg (74%).
Yellowish oil; Rf = 0.33 (EtOAc–hexane, 1:5).
IR (film): 3029, 2997, 2939, 2834, 1734, 1656, 1609, 1583, 1508, 1465, 1301, 1238, 1177, 1104, 1034, 908, 834, 741, 696, 636 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.36 – 7.23 (m, 5H), 7.21 – 7.14 (m, 3H), 7.12 (d, J = 7.0 Hz, 2H), 7.07 – 7.00 (m, 2H), 6.88 – 6.82 (m, 4H), 6.79 – 6.74 (m, 2H), 5.41 (s, 1H), 5.37 – 5.28 (m, 2H), 3.86 (s, 3H), 3.81 (s, 3H), 3.80 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.7, 158.5, 155.8, 141.8, 137.7, 136.78, 132.2, 129.6 (2C), 128.9 (2C), 128.0, 127.7, 127.4, 126.7 (2C), 122.4 (2C), 122.0, 119.7, 119.4, 114.5(2C), 114.3, 113.8 (2C), 109.9, 55.6, 55.3, 53.67, 50.2, 42.4.
MS (ESI): m/z = 491 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H31N2: 491.2329; found: 491.2337.
Methyl 2-(1-Benzyl-1H-Indol-3-yl)-N-(4-Methoxyphenyl)-2-Phenylacetimidate (6c)
Yield, obtained by following GP1: yield 129 mg (56%).
Yellowish oil; Rf = 0.43 (EtOAc–hexane, 1:5).
IR (film): 3058, 3027, 2931, 2833, 2391, 1735, 1662, 1605, 1506, 1466, 1334, 1301, 1238, 1178, 1031, 908, 833, 738, 698, 627 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.32 – 7.29 (m, 2H), 7.28 – 7.25 (m, 5H), 7.24 – 7.22 (m, 3H), 7.15 (dd, J = 8.0, 7.2 Hz, 1H), 7.10 (d, J = 7.6 Hz, 2H), 7.03 – 6.99 (m, 2H), 6.82 (d, J = 8.6 Hz, 2H), 6.73 (d, J = 8.6 Hz, 2H), 5.44 (s, 1H), 5.35 – 5.27 (m, 2H), 3.83 (s, 3H), 3.79 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.4, 155.8, 141.8, 140.2, 137.7, 136.7, 128.9 (2C), 128.5 (2C), 128.4 (2C), 128.1, 127.7, 127.4, 126.8, 126.7 (2C), 122.4 (2C), 122.0, 119.7, 119.4, 114.5 (2C), 113.8, 109.9, 55.6, 53.7, 50.2, 43.1.
MS (ESI): m/z = 461 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2: 461.2224; found: 461.2217.
Methyl N-(4-Methoxyphenyl)-2-(1-Methyl-1H-Indol-3-yl)-2-(p-tolyl)acetimidate (6d)
Yield, obtained by following GP1: yield 101 mg (51%)
Brownish oil; Rf = 0.50 (EtOAc–hexane, 1:5).
IR (film): 3048, 3000, 2941, 2833, 1663, 1505, 1465, 1372, 1237, 1179, 1031, 909, 833, 773, 740, 644 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.29 (d, J = 8.1 Hz, 1H), 7.24 (d, J = 8.1 Hz, 1H), 7.22 – 7.18 (m, 1H), 7.12 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 7.01 (m, 1H), 6.89 (s, 1H), 6.83 (d, J = 8.9 Hz, 2H), 6.73 (d, J = 8.9 Hz, 2H), 5.37 (s, 1H), 3.84 (s, 3H), 3.79 (s,3H), 3.76 (s, 3H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.6, 155.8, 141.8, 137.3, 137.0, 136.3, 129.1 (2C), 128.39, 128.37 (2C), 127.1, 122.4 (2C), 121.7, 119.4, 119.1, 114.4 (2C), 113.4, 109.3, 55.6, 53.7, 42.6, 32.9, 21.2.
MS (ESI): m/z = 399 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C26H27N2: 399.2067; found: 399.2035.
Methyl N,2-bis(4-Methoxyphenyl)-2-(1-Methyl-1H-Indol-3-yl)acetimidate (6e)
Yield, obtained by following GP1: 180 mg (87%).
Yield, obtained by following GP2: 160 mg (77%).
Brownish oil; Rf = 0.37 (EtOAc–hexane, 1:5).
IR (film): 3048, 2998, 2943, 2834, 2391, 1737, 1661, 1609, 1583, 1508, 1464, 1440, 1329, 1239, 1178, 1032, 908, 834, 740, 634 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.29 (d, J = 8.1 Hz, 1H), 7.23 (d, J = 8.1 Hz, 1H), 7.22 −7.19 (m, 1H), 7.14 (d, J = 9.1 Hz, 2H), 7.04 – 6.99 (m, 1H), 6.87 (s, 1H), 6.82 (d, J = 8.9 Hz, 2H), 6.80 (d, J = 8.9 Hz, 2H), 6.72 (d, J = 9.1 Hz, 2H), 5.34 (s, 1H), 3.84 (s, 3H), 3.79 (s, 3H), 3.78 (s, 3H), 3.76 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.7, 158.4, 155.7, 141.8, 137.1, 132.4, 129.5 (2C), 128.3, 127.1, 122.4 (2C), 121.7, 119.4, 119.1, 114.4 (2C), 113.7 (2C), 113.6, 109.3, 55.6, 55.3, 53.7, 42.3, 32.9.
MS (ESI): m/z = 415 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C26H27N2: 415.2016; found: 415.2025.
Methyl N-(4-Methoxyphenyl)-2-(1-Methyl-1H-Indol-3-yl)-2-Phenylacetimidate (6f)
Yield, obtained by following GP1: 150 mg (78%).
Yield, obtained by following GP2: 131 mg (68%).
Brownish oil; Rf = 0.50 (EtOAc–hexane, 1:3).
IR (film): 3108, 2996, 2938, 2901, 2834, 1738, 1664, 1604, 1547, 1503, 1439, 1372, 1304, 1239, 1180, 1155, 1105, 1032, 905, 845, 799, 743, 718, 650 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.30 (d, J = 8.2 Hz, 1H), 7.29 – 7.25 (m, 2H), 7.24 – 7.19 (m, 5H), 7.01 (m, 1H), 6.90 (s, 1H), 6.83 (d, J = 8.6 Hz, 2H), 6.73 (d, J = 8.6 Hz, 2H), 5.41 (s, 1H), 3.85 (s, 3H), 3.79 (s, 3H), 3.76 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.4, 155.8, 141.8, 140.3, 137.1, 128.49 (2C), 128.45, 128.4 (2C), 127.1, 126.8, 122.4 (2C), 121.8, 119.4, 119.1, 114.4 (2C), 113.2, 109.3, 55.6, 53.7, 43.0, 32.9.
MS (ESI): m/z = 385 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C25H25N2: 385.1911; found: 385.1911.
Methyl 2-(4-(Dimethylamino)phenyl)-N-(4-Methoxyphenyl) -2-(1-Methyl-1H-indol-3-yl) acetimidate (6g)
Yield, obtained by following GP1: 109 mg (51%).
Yield, obtained by following GP2: 51 mg (24%).
Brownish oil; Rf = 0.44 (EtOAc–hexane, 1:5).
IR (film): 3059, 2927, 2852, 1759, 1703, 1661, 1602, 1512, 1464, 1351, 1237, 1157, 1031, 945, 825, 740, 607 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.29 – 7.26 (m, 2H), 7.22 – 7.18 (m, 1H), 7.12 (d, J = 8.6 Hz, 2H), 6.99 – 7.04 (m, 1H), 6.88 (s, 1H), 6.83 (d, J = 8.6 Hz, 2H), 6.74 (d, J = 8.6 Hz, 2H), 6.73 – 6.66 (m, 2H), 5.31 (s, 1H), 3.83 (s, 3H), 3.79 (s, 3H), 3.75 (s, 3H), 2.93 (s, 6H).
13C NMR (150 MHz, CDCl3): δ = 164.0, 155.8, 141.9, 137.1, 129.5, 129.3, 128.4 (2C), 127.2, 122.5 (2C), 121.8, 121.7, 119.5, 119.0, 114.4 (2C), 114.0, 112.8 (2C), 109.3, 55.6, 53.7, 42.2, 41.0 (2C), 32.9.
MS (ESI): m/z = 428 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C27H30N3: 428.2333; found: 428.2208.
Methyl 2-(1-Benzyl-5-Bromo-1H-Indol -3-yl)-N-(4-Methoxyphenyl)-2-(p-tolyl)acetimidate (6h)
Yield, obtained by following GP1: 130 mg (47%)
Brown oil; Rf = 0.47 (EtOAc–hexane, 1:7).
IR (film): 3030, 2941, 2833, 1733, 1661, 1607, 1505, 1468, 1282, 1237, 1176, 1103, 1033, 909, 874, 828, 793, 765, 732, 697, 641 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.36 (d, J = 2.0 Hz, 1H), 7.34 – 7.27 (m, 3H), 7.21 (dd, J = 8.6, 2.0 Hz, 1H), 7.12 – 7.07 (m, 5H), 7.06 (d, J = 7.7 Hz, 2H), 7.02 (s, 1H), 6.85 (d, J = 9.1 Hz, 2H), 6.71 (d, J = 9.1 Hz, 2H), 5.33 (s, 1H), 5.32 – 5.24 (m, 2H), 3.83 (s, 3H), 3.81 (s, 3H), 2.33 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.3, 155.9, 141.6, 137.2, 136.7, 136.6, 135.3, 129.3 (2C), 129.2, 129.1, 128.9 (2C), 128.3 (2C), 127.9, 126.6 (2C), 124.9, 122.4 (2C), 122.3, 114.5 (2C), 113.6, 112.9, 111.5, 55.6, 53.8, 50.4, 42.5, 21.2.
MS (ESI): m/z = 553 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H30BrN2: 553.1485; found: 553.1505.
Methyl 2-(1-Benzyl-5-Bromo-1H -Indol-3-yl)-N,2-bis(4-Methoxyphenyl)acetimidate (6i)
Yield, obtained by following GP1: 148 mg (52%).
Yield, obtained by following GP2: 114 mg (40%).
Brownish oil; Rf = 0.60 (EtOAc–hexane, 1:5).
IR (film): 3030, 2996, 2943, 2834, 1734, 1662, 1608, 1508, 1467, 1354, 1238, 1176, 1104, 1033, 908, 832, 734, 697, 634 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.37 (d, J = 1.8 Hz, 1H), 7.33 – 7.25 (m, 3H), 7.20 (dd, J = 8.6, 1.8 Hz, 1H), 7.13 (d, J = 8.6 Hz, 2H), 7.08 (d, J = 8.6 Hz, 1H), 7.06 – 7.03 (m, 2H), 7.00 (s, 1H), 6.84 (d, J = 8.6 Hz, 2H), 6.82 (d, J = 9.1 Hz, 2H), 6.71 (d, J = 9.1 Hz, 2H), 5.31 (s, 1H), 5.31 – 5.23 (m, 2H), 3.82 (s, 3H), 3.80 (s, 3H), 3.78 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.3, 158.6, 155.9, 141.6, 137.2, 135.4, 131.7, 129.4 (2C), 129.1, 129.0, 128.9 (2C), 127.9, 126.6 (2C), 124.9, 122.3 (2C), 122.2, 114.5 (2C), 113.9 (2C), 113.8, 112.9, 111.4, 55.6, 55.3, 53.7, 50.4, 42.1.
MS (ESI): m/z = 569 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H30BrN2: 569.1434; found: 569.1412.
Methyl 2-(1-Benzyl-5-Methoxy-1H-Indol-3-yl)-N-(4-Methoxyphenyl)-2-(p-tolyl)acetimidate (6j)
Yield, obtained by following GP1: 179 mg (71%).
Yield, obtained by following GP2: 141 mg (56%).
Brown oil; yield; Rf = 0.56 (EtOAc–hexane, 1:5).
IR (film): 3030, 2994, 2939, 2833, 1734, 1661, 1621, 1578, 1506, 1487, 1452, 1288, 1238, 1176, 1103, 1030, 901, 830, 774, 704 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.31 – 7.25 (m, 3H), 7.15 (d, J = 8.1 Hz, 2H), 7.12 – 7.05 (m, 5H), 6.97 (s, 1H), 6.83 (d, J = 9.1 Hz, 2H), 6.79 (dd, J = 9.1, 2.5 Hz, 1H), 6.75 (d, J = 9.1 Hz, 2H), 6.70 (d, J = 2.5 Hz, 1H), 5.36 (s, 1H), 5.30 – 5.22 (m, 2H), 3.83 (s, 3H), 3.79 (s, 3H), 3.72 (s, 3H), 2.33 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.6, 155.8, 154.0, 141.9, 137.8, 137.80, 137.1, 136.4, 131.9, 129.1 (2C), 128.8 (2C), 128.6, 128.4 (2C), 127.8, 126.6 (2C), 122.5 (2C), 114.4 (2C), 113.5, 112.2, 110.7, 101.2, 55.8, 55.6, 53.7, 50.4, 42.6, 21.2.
MS (ESI): m/z = 505 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C33H33N2: 505.2486; found: 505.2461.
Ethyl 2-(1-Benzyl-1H-Indol-3-yl)-N-(4-Methoxyphenyl)-2-(p-tolyl)acetimidate (6k)
Yield, obtained by following GP1: 129 mg (53%)
Brownish oil; Rf = 0.71 (EtOAc–hexane, 1:5).
IR (film): 3030, 2976, 2946, 2901, 2833, 1732, 1656, 1610, 1505, 1466, 1357, 1334, 1306, 1236, 1178, 1103, 1036, 958, 834, 741, 696 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.34 – 7.23 (m, 5H), 7.17 – 7.13 (m, 3H), 7.11 (d, J = 8.1 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 7.05 (s, 1H), 6.99 – 7.00 (m, 1H), 6.83 (d, J = 9.1 Hz, 2H), 6.73 (d, J = 9.1 Hz, 2H), 5.40 (s, 1H), 5.31 (s, 2H), 4.34 – 4.27 (m, 2H), 3.80 (s, 3H), 2.33 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H).
13C NMR (150 MHz, CDCl3): δ = 162.9, 155.7, 142.1, 137.7, 137.4, 136.7, 136.2, 129.0 (2C), 128.8 (2C), 128.4 (2C), 127.9, 127.7, 127.5, 126.8 (2C), 122.4 (2C), 121.9, 119.8, 119.2, 114.4 (2C), 114.2, 109.7, 61.8, 55.6, 50.1, 42.6, 21.1, 14.2.
MS (ESI): m/z = 489 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C33H33N2: 489.2537; found: 489.2553.
Ethyl 2-(1-Benzyl-1H-Indol-3-yl)-N,2-bis(4-Methoxyphenyl)acetimidate (6l)
Yield, obtained by following GP1: 118 mg (47%).
Yield, obtained by following GP2: 93 mg (37%).
Brownish oil; Rf = 0.71 (EtOAc–hexane, 1:3).
IR (film): 3031, 2930, 2834, 1727, 1658, 1609, 1508, 1465, 1301, 1238, 1177, 1104, 1036, 958, 834, 741, 696, 637 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.32 – 7.28 (m, 2H), 7.27 – 7.25 (m, 2H), 7.23 (d, J = 8.6 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 7.16 – 7.12 (m, 1H), 7.09 (d, J = 8.1 Hz, 2H), 7.02 (s, 1H), 7.02 – 6.98 (m, 1H), 6.81 (d, J = 8.6 Hz, 2H), 6.79 (d, J = 8.6 Hz, 2H), 6.71 (d, J = 8.6 Hz, 2H), 5.35 (s, 1H), 5.30 (s, 2H), 4.32 – 4.21 (m, 2H), 3.79 (s, 3H), 3.77 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.0, 158.4, 155.7, 142.0, 137.7, 136.7, 132.5, 129.5 (2C), 128.8 (2C), 127.8, 127.7, 127.4, 126.7 (2C), 122.4 (2C), 121.9, 119.8, 119.2, 114.4 (2C), 114.3, 113.7 (2C), 109.8, 61.8, 55.6, 55.3, 50.1, 42.3, 14.2.
MS (ESI): m/z = 505 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C33H33N2: 505.2486; found: 505.2506.
Isopropyl 2-(1-Benzyl-1H-Indol-3-yl)-N,2-bis(4-Methoxyphenyl)acetimidate (6m)
Yield, obtained by following GP1: 145 mg (56%).
Yield, obtained by following GP2: 119 mg (46%).
Brownish oil; yield; Rf = 0.43 (EtOAc–hexane, 1:8).
IR (film): 3056, 2933, 2837, 2736, 1683, 1654, 1600, 1577, 1509, 1465, 1302, 1250, 1160, 1178, 1108, 1030, 976, 834, 742, 698 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.35 – 7.29 (m, 2H), 7.28 – 7.25 (m, 2H), 7.23 (d, J = 8.2 Hz, 1H), 7.15 (d, J = 9.1 Hz, 2H), 7.13 – 7.08 (m, 3H), 7.03 (s, 1H), 7.02 – 6.98 (m, 1H), 6.81 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 8.6 Hz, 2H), 6.70 (d, J = 9.1 Hz, 2H), 5.34 (s, 1H), 5.33 – 5.26 (m, 2H), 5.23 – 5.18 (m, 1H), 3.79 (s, 3H), 3.77 (s, 3H), 1.22 (d, J = 6.2 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H).
13C NMR (150 MHz, CDCl3): δ = 162.1, 158.3, 155.6, 142.2, 137.7, 136.6, 132.7, 129.5 (2C), 128.8 (2C), 127.8, 127.7, 127.5, 126.8 (2C), 126.5, 122.3 (2C), 121.8, 119.8, 119.1, 114.4 (2C), 113.6 (2C), 109.7, 68.0, 55.6, 55.3, 50.1, 42.2, 21.7, 21.6.
MS (ESI): m/z = 519 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C34H35N2: 519.2642; found: 519.2623.
Methyl 2-(1-Benzyl-1H-Indol-3-yl)-N-Phenyl-2-(p-tolyl)acetimidate (6n)
Yield, obtained by following GP1: 113 mg (51%).
Yield, obtained by following GP2: 91 mg (41%).
Orange oil; Rf = 0.41 (EtOAc–hexane, 1:10).
IR (film): 3055, 3028, 2941, 2861, 1665, 1595, 1546, 1511, 1466, 1334, 1249, 1178, 1072, 1017, 908, 834, 740, 695 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.34 – 7.29 (m, 2H), 7.28 – 7.25 (m, 3H), 7.25 – 7.22 (m, 2H), 7.15 – 7.11 (m, 3H), 7.10 – 7.03 (m, 5H), 7.02 – 6.98 (m, 2H), 6.79 (dd, J = 8.4, 1.1 Hz, 2H), 5.35 (s, 1H), 5.34 – 5.27 (m, 2H), 3.84 (s, 3H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.1, 148.6, 137.7, 137.0, 136.7, 136.40, 129.1 (2C), 129.1 (2C), 128.8 (2C), 128.4 (2C), 128.0, 127.6, 127.4, 126.7 (2C), 123.1, 121.9, 121.5 (2C), 119.7, 119.4, 113.9, 109.9, 53.7, 50.1, 42.9, 21.2.
MS (ESI): m/z = 445 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2O+: 445.2274; found: 445.2250.
Methyl 2-(1-Benzyl-1H-Indol-3-yl)-2-(4-Methoxyphenyl)-N-Phenylacetimidate (6o)
Yield, obtained by following GP1: 145 mg (63%).
Orange oil; Rf = 0.66 (EtOAc–hexane, 1:5).
IR (film): 3030, 2928, 2837, 1738, 1664, 1594, 1510, 1465, 1303, 1247, 1175, 1031, 740 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.33 −7.29 (m, 2H), 7.28 – 7.21 (m, 5H), 7.16 – 7.12 (m, 3H), 7.10 – 7.07 (m, 2H), 7.07 – 7.03 (m, 1H), 7.00 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.97 (s, 1H), 6.80 (d, J = 8.6 Hz, 2H), 6.79 (d, J = 8.6 Hz, 2H), 5.34 – 5.26 (m, 3H), 3.83 (s, 3H), 3.78 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.1, 158.5, 148.6, 137.7, 136.8, 132.1, 129.5 (2C), 129.1 (2C), 128.8 (2C), 127.9, 127.6, 127.3, 126.7 (2C), 123.1, 122.0, 121.5 (2C), 119.7, 119.4, 114.2, 113.8 (2C), 109.9, 55.3, 53.7, 50.1, 42.6.
MS (ESI): m/z = 461 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2: 461.2224; found: 461.2239.
Methyl 2-(1-Methyl-1H-Indol-3-y l)-N-Phenyl-2-(p-tolyl)acetimidate (6p)
Yield, obtained by following GP1: 100 mg (54%)
Orange oil; Rf = 0.57 (EtOAc–hexane, 1:5).
IR (film): 3052, 3021, 2952, 2869, 2854, 1737, 1662, 1595, 1511, 1461, 1375, 1330, 1247, 1153, 1023, 900, 740, 698 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.30 – 7.26 (m, 3H), 7.23 – 7.17 (m, 2H), 7.12 (d, J = 8.1 Hz, 2H), 7.10 – 7.04 (m, 3H), 7.00 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.88 (s, 1H), 6.79 (dd, J = 8.4, 1.2 Hz, 2H), 5.32 (s, 1H), 3.85 (s, 3H), 3.76 (s, 3H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.1, 148.6, 137.2, 137.0, 136.4, 129.1 (2C), 129.1 (2C), 128.4, 128.35 (2C), 127.1, 123.1, 121.7, 121.5 (2C), 119.4, 119.1, 113.3, 109.3, 53.8, 42.8, 32.9, 21.2.
MS (ESI): m/z = 369 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C25H25N2O+: 369.1961; found: 369.1961.
Methyl 2-(1-Benzyl-1H-Indol-3-yl)-N-(4-Chlorophenyl)-2-(4-Methoxyphenyl)acetimidate (6q)
Yield, obtained by following GP1: 106 mg (43%).
Yield, obtained by following GP2: 89 mg (36%).
Yellowish oil; yield; Rf = 0.53 (EtOAc–hexane, 1:5).
IR (film): 3028, 2948, 2836, 1736, 1666, 1610, 1510, 1466, 1302, 1242, 1176, 1092, 1031, 908, 835, 739 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.32 – 7.23 (m, 5H), 7.22 (d, J = 8.6 Hz, 2H), 7.19 – 7.12 (m, 3H), 7.08 (d, J = 7.2 Hz, 2H), 7.02 (ddd, J = 7.9, 7.0, 0.9 Hz, 1H), 6.95 (s, 1H), 6.81 (d, J = 9.1 Hz, 2H), 6.71 (d, J = 8.6 Hz, 2H), 5.34 – 5.22 (m, 3H), 3.83 (s, 3H), 3.78 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.1, 158.5, 147.2, 137.7, 136.8, 132.1, 129.5 (2C), 129.1 (2C), 128.8 (2C), 127.9, 127.6, 127.3, 126.7 (2C), 123.1, 121.95, 121.5 (2C), 119.7, 119.4, 114.2, 113.8 (2C), 109.9, 55.3, 53.7, 50.1, 42.6.
MS (ESI): m/z = 495 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H28ClN2: 495.1834; found: 495.1817.
Methyl 2-(1-Benzyl-5-Methoxy-1H-Indol-3-yl)-(4-Chlorophenyl)-2-(p-tolyl)acetimidate (6r)
Yield, obtained by following GP1: 94 mg (37%)
Brownish oil; Rf = 0.49 (EtOAc–hexane, 1:5).
IR (film): 3027, 2992, 2941, 2919, 2833, 1661, 1590, 1511, 1487, 1452, 1398, 1355, 1241, 1213, 1175, 1094, 1028, 903, 869, 829, 796, 732, 704, 643 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.31 – 7.28 (m, 2H), 7.28 – 7.25 (m, 1H), 7.23 (d, J = 8.6 Hz, 2H), 7.14 – 7.05 (m, 7H), 6.95 (s, 1H), 6.80 (dd, J = 8.6, 2.5 Hz, 1H), 6.73 (d, J = 8.6 Hz, 2H), 6.65 (d, J = 2.5 Hz, 1H), 5.29 – 5.22 (m, 3H), 3.83 (s, 3H), 3.72 (s, 3H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 163.7, 154.0, 147.3, 137.7, 136.7, 136.58, 131.9, 129.2 (2C), 129.1 (2C), 128.8 (2C), 128.6, 128.4, 128.3 (2C), 127.7, 127.7, 126.6 (2C), 122.9 (2C), 113.0, 112.3, 110.7, 101.0, 55.8, 53.9, 50.4, 42.9, 21.2.
MS (ESI): m/z = 509 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H30ClN2: 509.1990; found: 509.1962.
1-Benzyl-3-(Methoxy(p-tolyl)Methyl)-1H-Indole (7)
According to GP1 without an isocyanide addition compound 7 (155 mg, 91%) was obtained as an orange oil; Rf = 0.71 (EtOAc–hexane, 1:5).
IR (film): 3029, 2922, 2816, 1717, 1613, 1550, 1511, 1495, 1466, 1453, 1355, 1335, 1306, 1249, 1174, 1084, 1028, 956, 810, 777, 740, 695, 639, 573 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.63 (d, J = 8.1 Hz, 1H), 7.37 (d, J = 8.1 Hz, 2H), 7.29 – 7.23 (m, 3H), 7.22 (d, J = 8.1 Hz, 1H), 7.18 – 7.12 (m, 3H), 7.09 – 7.05 (m, 3H), 6.88 (s, 1H), 5.56 (s, 1H), 5.28 – 5.20 (m, 2H), 3.42 (s, 3H), 2.34 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 138.8, 137.6, 137.161, 129.1, 128.8, 127.6, 127.3, 127.2, 127.1, 126.7, 122.1, 120.2, 119.6, 117.2, 109.9, 79.6, 56.7, 50.1, 21.3.
MS (EI, 70 eV): m/z (%) = 341 (11) [M]+, 310 (63) [M – OCH3]+, 250 (7), 218 (6), 204 (6), 91 [C7H7]+ (100).
Synthesis of Amides 8
General Procedure
A salt 1 (0.5 mmol) and K2CO3 (100 mg, 0.75 mmol) were added to a solution of 0.65 mmol of isonitrile in a mixture of MeOH (1 mL), MeCN (1 mL) and H2O (0.2 mL). The reaction mixture was stirred at room temperature for 12 h. It was then diluted with 40 ml of EtOAc, washed with H2O (2 × 25 mL), brine (20 mL) and dried over anhydrous Na2SO4. The EtOAc was evaporated in vacuo. The residue was chromatographed on a column of silica gel with EtOAc–hexane.
N-Benzyl-2-(1-Benzyl-1H-Indol-3-yl)-2-(p-tolyl)acetamide (8a)
White solid; yield 132 mg (59%); mp 176–178°C; Rf = 0.42 (EtOAc–hexane, 1:3).
IR (film): 3299, 3087, 3029, 2949, 2920, 2874, 1645, 1612, 1532, 1496, 1433, 1356, 1334, 1222, 1030, 807, 768, 745, 696, 649 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.44 (d, J = 8.1 1H), 7.27 – 7.21 (m, 9H), 7.17 – 7.11 (m, 5H), 7.07 – 7.03 (m, 3H), 6.95 (d, J = 0.8 Hz, 1H), 6.12 (dd, J = 6.0, 5.7 Hz, 1H), 5.23 (s, 2H), 5.15 (s, 1H), 4.50 (dd, J = 15.1, 6.0 Hz, 1H), 4.41 (dd, J = 15.1, 5.7 Hz, 1H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 172.5, 138.4, 137.5, 137.0, 136.9, 136.5, 129.5 (2C), 128.8 (2C), 128.7 (2C), 128.5 (2C), 127.9, 127.7 (2C), 127.7, 127.5, 127.4, 126.7 (2C), 122.4, 119.8, 119.5, 114.0, 110.1, 50.9, 50.2, 43.8, 21.2.
MS (ESI): m/z = 445 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2O+: 445.2274; found: 445.2301.
N-Benzyl-2-(1-Benzyl-1H-Indol-3-yl)-2-(4-Methoxyphenyl)acetamide (8b)
White solid; yield 144 mg (63%); mp 177–179°C; Rf = 0.41 (EtOAc–hexane, 1:3).
IR (film): 3328, 3062, 3028, 2930, 2837, 1735, 1642, 1610, 1510, 1466, 1354, 1303, 1243, 1179, 1029, 734, 698 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.42 (d, J = 8.1, 1H), 7.28 (d, J = 8.6 Hz, 2H), 7.26 – 7.21 (m, 7H), 7.19 – 7.13 (m, 3H), 7.07 – 7.02 (m, 3H), 6.95 (s, 1H), 6.85 (d, J = 8.6 Hz, 2H), 6.12 (dd, J = 6.1, 5.6 Hz, 1H), 5.24 (s, 2H), 5.13 (s, 1H), 4.50 (dd, J = 14.9, 6.1 Hz, 1H), 4.42 (dd, J = 14.9, 5.6 Hz, 1H), 3.78 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 172.6, 158.8, 138.4, 137.4, 137.0, 131.63, 129.7 (2C), 128.9 (2C), 128.7 (2C), 127.9, 127.7 (2C), 127.7, 127.4, 127.4, 126.7 (2C), 122.4, 119.8, 119.5, 114.2 (2C), 114.1, 110.1, 55.4, 50.5, 50.2, 43.8.
MS (ESI): m/z = 461 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2: 461.2224; found: 461.2243.
N-Benzyl-2-(4-Methoxyphenyl)-2-(1-Methyl-1H-Indol-3-yl)acetamide (8c)
White solid; yield 101 mg (53%); mp 207–209°C; Rf = 0.26 (EtOAc–hexane, 1:3).
IR (film): 3280, 3088, 3006, 2920, 2835, 1647, 1607, 1557, 1507, 1473, 1419, 1342, 1248, 1223, 1174, 1031, 753 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.42 (d, J = 7.6, Hz, 1H), 7.30 – 7.20 (m, 7H), 7.18 −7.14 (m, 2H), 7.07 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.86 (d, J = 9.1 Hz, 2H), 6.76 (s, 1H), 6.19 (dd, J = 6.1, 5.6 Hz, 1H), 5.12 (s, 1H), 4.51 (dd, J = 14.9, 6.1 Hz, 1H), 4.41 (dd, J = 14.9, 5.6 Hz, 1H), 3.79 (s, 3H), 3.70 (s, 3H)
13C NMR (150 MHz, CDCl3): δ = 173.0, 158.8, 138.3, 137.4, 131.6, 129.7 (2C), 128.7 (2C), 128.5, 127.8 (2C), 127.5, 127.1, 122.2, 119.5, 119.3, 114.2 (2C), 113.3, 109.5, 55.4, 50.3, 43.8, 32.9.
MS (ESI): m/z = 385 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C25H24N2: 385.1911; found: 385.1919.
N-Benzyl-2-(1-Methyl-1H-indol-3-yl)-2-Phenylacetamide (8d)
White solid; yield 88 mg (50%); mp 146–148°C; Rf = 0.29 (EtOAc–hexane, 1:3).
IR (film): 3256, 3050, 2940, 2098, 1736, 1634, 1542, 1475, 1451, 1372, 1335, 1216, 1152, 1111, 1029, 990, 745, 699, 584 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.44 (d, J = 8.1 Hz, 1H), 7.38 – 7.36 (m, 2H), 7.35 – 7.31 (m, 2H), 7.30 – 7.21 (m, 6H), 7.19 – 7.15 (m, 2H), 7.08 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.77 (d, J = 1.0 Hz, 1H), 6.18 (dd, J = 6.1, 5.7 Hz, 1H), 5.16 (s, J = 6.8 Hz, 1H), 4.52 (dd, J = 15.5, 6.1 Hz, 1H), 4.42 (dd, J = 15.1, 5.7 Hz, 1H), 3.70 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 172.4, 139.7, 138.4, 137.3, 128.8 (2C), 128.7 (2C), 128.6 (2C), 128.6, 127.8 (2C), 127.5, 127.2, 127.1, 122.2, 119.6, 119.3, 113.1, 109.5, 51.2, 43.8, 32.9.
MS (ESI): m/z = 355 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C24H23N2O+: 355.1805; found: 355.1808.
N-Benzyl-2-(1-Benzyl-5-Bromo-1H-Indol-3-yl)-2-(p-tolyl)acetamide (8e)
White solid; yield 132 mg (51%); mp 184–186°C; Rf = 0.15 (EtOAc–hexane, 1:5).
IR (film): 3250, 3068, 3029, 2923, 1645, 1560, 1511, 1469, 1356, 1234, 1173, 1021, 869, 817, 802, 733, 697 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.55 (d, J = 2.0 Hz, 1H), 7.30 – 7.25 (m, 8H), 7.22 (dd, J = 8.6, 2.0 Hz, 1H) 7.17 (d, J = 7.1 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 8.6 Hz, 1H), 7.01 (dd, J = 7.6, 1.9 Hz, 2H), 7.00 (s, 1H), 6.07 – 6.02 (m, 1H), 5.21 (s, 2H), 5.05 (s, 1H), 4.52 – 4.43 (m, 2H), 2.32 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 172.1, 138.2, 137.2, 137.0, 136.0, 135.6, 129.7 (2C), 129.2, 129.1, 128.9 (2C), 128.8 (2C), 128.4 (2C), 127.9, 127.7 (2C), 127.5, 126.6 (2C), 125.2, 122.0, 113.5, 113.2, 111.6, 50.6, 50.5, 43.9, 21.2.
MS (ESI): m/z = 523 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H28BrN2O+: 523.1380; found: 523.1398.
N-Benzyl-2-(1-Benzyl-5-Methoxy-1H-Indol-3-yl)-2-(p-tolyl)acetamide (8f)
White solid; yield 135 mg (57%); mp 168–170°C; Rf = 0.13 (EtOAc–hexane, 1:5).
IR (film): 3283, 3031, 2923, 1649, 1622, 1489, 1453, 1352, 1228, 1171, 1032, 918, 822, 794, 742, 699 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.26 (d, J = 8.1 Hz, 2H), 7.25 – 7.21 (m, 6H), 7.16 (dd, J = 7.6, 2.0 Hz, 2H), 7.13 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 8.6 Hz, 1H), 6.99 – 7.04 (m, 2H), 6.87 (s, 1H), 6.86 (d, J = 2.4 Hz, 1H), 6.80 (dd, J = 8.9, 2.4 Hz, 1H), 6.19 (dd, J = 6.1, 5.8 Hz, 1H), 5.19 (s, 2H), 5.11 (s, 1H), 4.51 (dd, J = 15.1, 6.1 Hz, 1H), 4.43 (dd, J = 15.1, 5.8 Hz, 1H), 3.72 (s, 3H), 2.33 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 172.5, 154.3, 138.4, 137.5, 136.9, 136.5, 132.2, 129.5 (2C), 128.8 (2C), 128.7 (2C), 128.6, 128.5 (2C), 127.8, 127.7 (2C), 127.7, 127.4, 126.6 (2C), 113.5, 112.7, 111.0, 101.0, 55.9, 50.9, 50.4, 43.8, 21.2.
MS (ESI): m/z = 475 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C32H31N2: 475.2380; found: 475.2399.
2-(1-Benzyl-1H-Indol-3-yl)-N,2-bis(4-Methoxyphenyl)acetamide (8g)
White solid; yield 105 mg (44%); mp 208–210°C; Rf = 0.57 (EtOAc–hexane, 1:3).
IR (film): 3287, 3255, 3196, 3134, 3058, 3024, 2962, 2835, 1655, 1606, 1546, 1509, 1469, 1410, 1346, 1303, 1251, 1171, 1031, 969, 833, 794, 746, 730, 700 cm−1.
1H NMR (600 MHz, DMSO-d6): δ = 10.20 (s, 1H), 7.53 (d, J = 9.1 Hz, 2H), 7.44 (s, 1H), 7.43 – 7.34 (m, 4H), 7.32 – 7.27 (m, 2H), 7.25 – 7.21 (m, 1H), 7.18 (d, J = 7.1 Hz, 2H), 7.09 – 7.04 (m, 1H), 6.99 – 6.95 (m, 1H), 6.90 – 6.84 (m, 4H), 5.41 (s, 2H), 5.26 (s, 1H), 3.71 (s, 3H) 3.70 (s, 3H).
13C NMR (150 MHz, DMSO-d6): δ = 170.2, 158.1, 155.2, 138.4, 136.1, 132.4, 132.3, 129.3 (2C), 128.5 (2C), 127.4, 127.3, 127.1, 127.0 (2C), 121.4, 120.7 (2C), 118.84, 118.82, 113.9 (2C), 113.7, 113.6 (2C), 110.2, 55.2, 55.0, 49.0, 48.6.
MS (ESI): m/z = 477 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C31H29N2: 477.2173; found: 477.2193.
2-(1-Benzyl-1H-Indol-3-yl)-N-(4-Chlorophenyl)-2-(4-Methoxyphenyl)acetamide (8h)
White solid; yield 132 mg (54%); mp 179–181°C; Rf = 0.30 (EtOAc–hexane, 1:4).
IR (film): 3282, 3061, 3030, 2928, 1658, 1593, 1510, 1493, 1467, 1397, 1299, 1248, 1173, 1093, 1030, 824, 786, 739, 697 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.65 (s, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 9.0 Hz, 2H), 7.34 – 7.25 (m, 6H), 7.20 (d, J = 8.6 Hz, 2H), 7.19 – 7.16 (m, 1H), 7.10 – 7.06 (m, 3H), 7.01 (s, 1H), 6.87 (d, J = 8.6 Hz, 2H), 5.27 (s, 2H), 5.21 (s, 1H), 3.78 (s, 3H).
13C NMR (150 MHz, CDCl3): δ = 171.0, 159.0, 137.3, 137.1, 136.4, 131.1, 129.7 (2C), 129.4, 129.0 (2C), 128.9 (2C), 128.0, 127.8, 127.2, 126.8 (2C), 122.6, 121.2 (2C), 120.1, 119.3, 114.4 (2C), 113.6, 110.3, 55.4, 51.3, 50.3.
MS (ESI): m/z = 481 [M + H]+
HRMS (TOF ES+): m/z [M + H]+ calcd for C30H25ClN2: 481.1677; found: 481.1683.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
NG and HN were responsible for designing and performing the experiments. NG, HN, AG and AV discussed the evolution of the project and revised the manuscript together. LV and EV directed the project and wrote the publication.
Funding
We thank the Russian Science Foundation (grant 18-73-10099) for the financial support of this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00345/full#supplementary-material
References
Armenise, N., Dughera, S., Gualandi, A., Mengozzi, L., Barbero, M., and Cozzi, P. G. (2015). Organocatalyzed asymmetric alkylation of stable aryl or heteroaryl(3-indolyl)methylium o-benzenedisulfonimides. Asian J. Org. Chem. 4, 337–345. doi: 10.1002/ajoc.201402275
Banfi, L., and Riva, R. (2005). “The passerini reaction,” in Organic Reactions, ed L. E. Overman (New York, NY: Wiley), 1–140. doi: 10.1002/0471264180.or065.01
Barbero, M., Buscaino, R., Cadamuro, S., Dughera, S., Gualandi, A., Marabello, D., et al. (2015). Synthesis of bench-stable diarylmethylium tetrafluoroborates. J. Org. Chem. 80, 4791–4796. doi: 10.1021/acs.joc.5b00418
Barbero, M., Cadamuro, S., Cauda, F., Dughera, S., Gervasio, G., and Venturello, P. (2012). Preparation and characterization of aryl or heteroaryl(3-indolyl)methylium o-benzenedisulfonimides. J. Org. Chem. 77, 4278–4287. doi: 10.1021/jo300099y
Barreiro, E. J. (2016). Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation. Cambridge: RSC Publishing.
De Moliner, F. D., Banfi, L., Riva, R., and Basso, A. (2011). Beyond Ugi and Passerini reactions: multicomponent approaches based on isocyanides and alkynes as an efficient tool for diversity oriented synthesis. Comb. Chem. High Throughput Screen. 14, 782–810. doi: 10.2174/138620711796957099
Deb, M. L., Borpatra, P. J., Saikia, P. J., and Baruah, P. K. (2017). Base-promoted three-component one-pot approach to 3-(α,α-Diarylmethyl)indoles via arylation of 3-indolylalcohols. Synthesis 49, 1401–1409. doi: 10.1055/s-0036-15880098
Dömling, A. (2006). Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106, 17–89. doi: 10.1021/cr0505728
Dömling, A., and Ugi, I. (2000). Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39, 3169–3210. doi: 10.1002/1521-773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Enders, D., Narine, A. A., Toulgoat, F., and Bisschops, T. (2008). Asymmetric bronsted acid catalyzed isoindoline synthesis: enhancement of enantiomeric ratio by stereoablative kinetic resolution. Angew. Chem. Int. Ed. 47, 5661–5665. doi: 10.1002/anie.200801354
Follet, E., Berionni, G., Mayer, P., and Mayr, H. (2015). Structure and reactivity of indolylmethylium ions: scope and limitations in synthetic applications. J. Org. Chem. 80, 8643–8656. doi: 10.1021/acs.joc.5b01298
Follet, E., Mayer, P., and Mayr, H. (2016). Lewis acidities of Indol-3-ylmethylium ions and intrinsic barriers of their reactions with phosphines and pyridines. Eur. J. Org. Chem. 23, 4050–4058. doi: 10.1002/ejoc.201600572
Fontaine, P., Masson, G., and Zhu, J. (2009). Synthesis of pyrroles by consecutive multicomponent reaction/[4 + 1] cycloaddition of α-iminonitriles with isocyanides. Org. Lett. 11, 1555–1558. doi: 10.1021/ol9001619
Jin, L., Jing, L., Luo, G., Wang, R., Yin, M., Wu, C., et al. (2015). 3-(1-Arylsulfonylalkyl)-7-azaindoles as precursors of vinylogous imine intermediates for hetero-Michael addition reactions. Tetrahedron 71, 4039–4046. doi: 10.1016/j.tet.2015.04.062
Jing, L., Wei, J., Zhou, L., Huang, Z., Li, Z., Wu, D., et al. (2010). Highly enantioselective michael addition of malononitrile to vinylogous imine intermediates generated in situ from arylsulfonyl indoles. Chem. Eur. J. 16, 10955–10958. doi: 10.1002/chem.201001662
Kaur, T., Wadhwa, P., Bagchi, S., and Sharma, A. (2016). Isocyanide based [4+1] cycloaddition reactions: An indispensable tool in multi-component reactions (MCRs). Chem. Commun. 52, 6958–6976. doi: 10.1039/c6cc01562j
Levi, L., and Müller, T. J. (2016). Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 45, 2825–2846. doi: 10.1039/c5cs00805k
Maltsev, S. S., Mironov, M. A., and Bakulev, V. A. (2006). Synthesis of cyclopentene derivatives by the cyclooligomerization of isocyanides with substituted benzylidenemalononitriles. Mendeleev Commun. 16, 201–202. doi: 10.1070/MC2006v016n04ABEH002354
Marchand, E., Morel, G., and Sinbandhit, S. (1999). A new access to 2-(alkylamino)- and 2-(arylamino)pyrroles by addition of isocyanides to protonated 1-azabutadienes. Eur. J. Org. Chem. 7, 1729–1738. doi: 10.1002/(SICI)1099-0690(199907)1999:7<1729::AID-EJOC1729>3.0.CO;2-S
Mei, G.J., and Shi, F. (2017). Indolylmethanols as Reactants in Catalytic Asymmetric Reactions. J. Org. Chem. 82, 7695–7707. doi: 10.1021/acs.joc.7b01458
Mironov, M. A., Ivantsova, M. N., and Mokrushin, V. S. (2006). A novel isocyanide-based multicomponent reaction: an easy access to substituted propionamides and succinimides. Synlett. 615–617. doi: 10.1055/s-2006-933104
Nenajdenko, V. G. (2012). Isocyanide Chemistry: Applications in Synthesis and Material Science. Weinheim: Wiley-VCH.
Palmieri, A., and Petrini, M. (2016). Sulfonyl azoles in the synthesis of 3-functionalized azole derivatives. Chem. Rec. 16, 1353–1379. doi: 10.1002/tcr.201500291
Palmieri, A., Petrini, M., and Shaikh, R. R. (2010). Synthesis of 3-substituted indoles via reactive alkylideneindolenine intermediates. Org. Biomol. Chem. 8, 1259–1270. doi: 10.1039/b919891a
Person, H., Del Aguila Pardo, M., and Foucaud, A. (1980). Reaction des isonitriles avec les β-nitrostyrenes β-substitues. Nouvelle synthese des hydroxy-1 indoles. Tetrahedron Lett. 21, 281-284. doi: 10.1016/S0040-4039(00)71189-0
Sadjadi, S., Heravi, M. M., and Nazari, N. (2016). Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv. 6, 53203–53272. doi: 10.1039/c6ra02143c
Saegusa, T., Ito, Y., Tomita, S., Kinoshita, H., and Taka-Ishi, N. (1971). Reaction of isocyanide with α,β-unsaturated carbonyl and nitrile compounds. Tetrahedron 27, 27–31. doi: 10.1016/S0040-4020(01)92394-4
Saegusa, T., Kobayashi, S., Ito, Y., and Morino, I. (1972). Reaction of isonitrile with nitroalkene. Tetrahedron 28, 3389–3392. doi: 10.1016/0040-4020(72)88100-6
Shaabani, A., Teimouri, M. B., and Bijanzadeh, H. R. (2002). One-pot three component condensation reaction in water: an efficient and improved procedure for the synthesis of furo[2,3-d]pyrimidine-2,4(1H,3H)-diones. Tetrahedron Lett. 43, 9151–9154. doi: 10.1016/S0040-4039(02)02260-8
Shaikh, R. R., Mazzanti, A., Petrini, M., Bartoli, G., and Melchiorre, P. (2008). Proline-catalyzed asymmetric formal α-alkylation of aldehydes via vinylogous iminium ion intermediates generated from arylsulfonyl indoles. Angew. Chem. Int. Ed. 47, 8707–8710. doi: 10.1002/anie.200803947
Shimizu, M., Hachiya, I., and Mizota, I. (2009). Conjugated imines and iminium salts as versatile acceptors of nucleophiles. Chem. Commun. 8, 874–889. doi: 10.1039/b814930e
Shiri, M. (2012). Indoles in multicomponent processes (MCPs). Chem. Rev. 112, 3508–3549. doi: 10.1021/cr2003954
Soleimani, E., and Zainali, M. (2011). Isocyanide-based multicomponent reactions: synthesis of alkyl-2-(1-(alkylcarbamoyl)-2,2-dicyanoethyl)benzoate and isochromeno[3,4-b] pyrrole derivatives. J. Org. Chem. 76, 10306–10311. doi: 10.1021/jo201908f
Soleimani, E., Zainali, M., Ghasemi, N., and Notash, B. (2013). Isocyanide-based multicomponent reactions: Synthesis of 2-(1-(alkylcarbamoyl)-2,2-dicyanoethyl)-N-alkylbenzamide and 1,7-diazaspiro[4,4]nonane-2,4-dione derivatives. Tetrahedron 69, 9832–9838. doi: 10.1016/j.tet.2013.09.011
Soleimani, E., Zainali, M., and Samadi, S. (2011). Isocyanide-based multicomponent reactions: Synthesis of 3,3-dicyano-N-alkyl-2-arylpropanamide derivatives. Tetrahedron Lett. 52, 4186–4188. doi: 10.1016/j.tetlet.2011.06.013
Ugi, I. (1962), The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. Engl. 1, 8–21. doi: 10.1002/anie.196200081
Wang, L., Chen, Y., and Xiao, J. (2014). Alkylideneindoleninium ions and alkylideneindolenines: Key intermediates for the asymmetric synthesis of 3-indolyl derivatives. Asian J. Org. Chem. 3, 1036–1052. doi: 10.1002/ajoc.201402093
Zarganes-Tzitzikas, T., Chandgude, A. L., and Dömling, A. (2015). Multicomponent reactions, union of MCRS and beyond. Chem. Rec. 15, 981–996. doi: 10.1002/tcr.201500201
Zhu, J., Wang, Q., and Wang, M.-X. (2015). Multicomponent Reactions in Organic Synthesis. Weinheim: Wiley-VCH.
Keywords: multicomponent reactions, isocyanides, indoleninium ions, aryl(indol-3-yl)methylium ions, imidates
Citation: Golantsov NE, Nguyen HM, Golubenkova AS, Varlamov AV, Van der Eycken EV and Voskressensky LG (2019) Three-Component Reaction of 3-Arylidene-3H-Indolium Salts, Isocyanides, and Alcohols. Front. Chem. 7:345. doi: 10.3389/fchem.2019.00345
Received: 27 February 2019; Accepted: 26 April 2019;
Published: 16 May 2019.
Edited by:
Alexander Dömling, University of Groningen, NetherlandsReviewed by:
Andrea Gualandi, University of Bologna, ItalyDiogo Seibert Lüdtke, Federal University of Rio Grande do Sul, Brazil
Copyright © 2019 Golantsov, Nguyen, Golubenkova, Varlamov, Van der Eycken and Voskressensky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erik V. Van der Eycken, ZXJpay52YW5kZXJleWNrZW5Aa3VsZXV2ZW4uYmU=
Leonid G. Voskressensky, bHZvc2tyZXNzZW5za3lAc2NpLnBmdS5lZHUucnU=
 Nikita E. Golantsov
Nikita E. Golantsov Hung M. Nguyen
Hung M. Nguyen Alexandra S. Golubenkova
Alexandra S. Golubenkova Alexey V. Varlamov1
Alexey V. Varlamov1 Leonid G. Voskressensky
Leonid G. Voskressensky