- 1CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 2College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
- 3Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China
- 4Institute of Tropical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5State Key Laboratory of Applied Microbiology Southern China, Guangdong Provincial Key Laboratory of Microbial Culture Collection and Application, Guangdong Open Laboratory of Applied Microbiology, Guangdong Institute of Microbiology, Guangzhou, China
Three pairs of new spirocyclic alkaloid enantiomers eurotinoids A–C (1–3), as well as a known biogenetically related racemate dihydrocryptoechinulin D (4) were isolated from a marine-derived fungus Eurotium sp. SCSIO F452. Their structures were determined by spectroscopic analyses and electronic circular dichroism (ECD) calculations. Compounds 1 and 2 represent the first two “meta” products from a non-stereoselective [4 + 2] Diels-Alder cycloaddition presumably between an enone group of a diketopiperazine alkaloid and a diene group of a benzaldehyde derivative via a new head-to-tail coupling mode biosynthetically, while 3 and 4 were “ortho” products. Their enantiomers exhibited different antioxidative and cytotoxic activities. The modes of action were investigated by a preliminary molecular docking study.
Introduction
Marine fungi are widely known as a rich source producing structurally diverse and biologically active natural products, which attract people's great interests in pharmaceutical, agrochemical, and food industries (Richards et al., 2012; Martins et al., 2014; Blunt et al., 2018). The fungal genus Eurotium, known as the teleomorph of Aspergillus, has been found to produce diketopiperazine (DKP) alkaloids, prenyl benzaldehyde derivatives, anthraquinones, and so on, which display a wide range of biological activities such as antibacterial, antioxidative, insecticidal, and binding with opioid and cannabinoid receptors activities (Gao et al., 2011; Du et al., 2012, 2017; Gomes et al., 2012; Meng et al., 2015). Motivated by the fascinating secondary metabolites produced by the fungi Eurotium spp., we have launched a chemical investigation on a marine-derived fungus isolated from a South China Sea sediment sample, Eurotium sp. SCSIO F452, and found a series of prenylated DKPs, prenyl benzaldehyde derivatives, especially three pairs of spirocyclic diketopiperazine-anthraquinone enantiomers variecolortins A–C (Wang et al., 2013; Zhong W. et al., 2018; Zhong W.-M. et al., 2018). In order to enrich and improve the chemical diversity of this fungus, we carried out a further chemical study on the Eurotium sp. SCSIO F452, and disclosed three pairs of new spirocyclic alkaloid enantiomers eurotinoids A–C (1–3), as well as a known biogenetically related racemate dihydrocryptoechinulin D (4) (Gao et al., 2012). Structurally, compounds 1–4 feature a cyclohexene core presumably deriving from a key non-stereoselective [4 + 2] Diels-Alder cycloaddition between an enone group of a diketopiperazine alkaloid and a diene group of a benzaldehyde derivative. Interestingly, compounds 1 and 2, 3 and 4 occurred as two pairs of diastereomers. The molecular structures of all the compounds including absolute configurations were unambiguously assigned by comprehensive spectroscopic data and quantum chemical electronic circular dichroism (ECD) calculations. Herein we report the isolation, structure elucidation, plausible biosynthetic pathway, antioxidative and cytotoxic bioactivities, and molecular dockings of 1–4.
Materials and Methods
General Experimental Procedures
Optical rotations were measured with an MCP 500 automatic polarimeter (Anton Paar) with MeCN as solvent. UV spectra were recorded on a UV-2600 spectrometer (Shimadzu). IR spectra were measured on an IR Affinity-1 spectrometer (Shimadzu). 1H, 13C NMR, DEPT, and 2D NMR spectra were recorded on the AVANCE III HD 700 (Bruker). Circular dichroism spectra were measured with a Chirascan circular dichroism spectrometer (Applied Photophysics). HRESIMS spectra data were recorded on a MaXis quadrupole-time-of-flight mass spectrometer. Thin layer chromatography (TLC) was performed on plates precoated with silica gel GF254 (10–40 μm). Column chromatography (CC) was performed over silica gel (200–300 mesh and 300–400 mesh) (Qingdao Marine Chemical Factory) and octadecylsilyl silica gel (ODS) (50 μm, YMC). High performance liquid chromatography was performed on an Agilent 1260 HPLC equipped with a DAD detector, using an ODS column (YMC-pack ODS-A, 10 × 250 mm, 5 μm), two chiral-phase columns (Daicel chiralpak IA and IC, 4.6 × 250 mm, 5 μm). All solvents used in CC and HPLC were of analytical grade (Tianjin Damao Chemical Plant, Tianjin, China) and chromatographic grade (Oceanpak, Sweden), respectively. Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.
Fungal Material
The fungal strain used in this investigation was isolated from a South China Sea sediment sample (17°29.804'N, 110°0.292'E) at a depth of 158 m in May, 2010. It was identified as Eurotium sp. SCSIO F452 according to a molecular biological protocol by DNA amplification and sequencing of the ITS region (deposited in GenBank, accession no. JX481973). The working strain was prepared on potato dextrose agar slants modified with seawater instead of distilled water and stored at 4 °C. A reference culture was maintained at −80 °C in RNAM Centre for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences.
Fermentation and Extraction
The fungus Eurotium sp. SCSIO F452 was cultured and extracted as previously described (Zhong W.-M. et al., 2018).
Isolation and Purification
The EtOAc extract (68 g) was subjected to vacuum liquid chromatography (VLC) on a silica gel column using step gradient elution with petroleum ether (PE)/EtOAc (1:0 to 0:1) and CHCl3/MeOH (1:0 to 0:1) to separate into 18 fractions based on TLC properties. Fr.3 (10 g) was separated by silica gel CC (PE/Acetone 1:0 to 0:1) to obtain 10 subfractions (Frs.3.1–3.10). Then Fr.3.9 (0.8 g) was divided into seven parts (Frs.3.9.1–3.9.7) by ODS CC with a gradient elution of MeOH/H2O (7:3 to 1:0). Fr.3.9.3 (56 mg) was further purified by repeated semi-preparative HPLC (3 mL/min, 59% CH3CN/H2O) to yield 1 (3.1 mg), 2 (1.5 mg), 3 (2.0 mg), and 4 (4.2 mg).
Chiral Separation of Enantiomers
Compounds 1–4 were subjected to chiral-phase HPLC separation on an Agilent 1260 liquid chromatograph system equipped with a DAD detector. Compounds (+)-1 (1.3 mg, tR = 10.825 min) and (–)-1 (1.4 mg, tR = 23.026 min), (+)-3 (0.8 mg, tR = 11.887 min) and (–)-3 (0.8 mg, tR = 17.220 min) were separated by a Daicel chiralpak IC column (250 × 4.6 mm, 5 μm), using an elution mixture of n-hexane/isopropanol (87:13) at a flow rate of 1 mL/min. Compounds (+)-2 (0.6 mg, tR = 6.379 min) and (–)-2 (0.6 mg, tR = 7.053 min) were separated by a Daicel chiralpak IA column (250 × 4.6 mm, 5 μm), using n-hexane/isopropanol (72:28) mixture elution at a flow rate of 0.9 mL/min. Compounds (+)-4 (1.8 mg, tR = 22.237 min) and (–)-4 (1.7 mg, tR = 34.315 min) were separated by a Daicel chiralpak IC column (250 × 4.6 mm, 5 μm), using an elution mixture of n-hexane/isopropanol (90:10) at a flow rate of 1 mL/min.
Computational Details
Molecular Merck force field (MMFF) calculations were done using Spartan'14 program (Wavefunction Inc., Irvine, CA, USA). Density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations were performed with Gaussian09 program package (Frisch et al., 2010). In order to reduce the computational cost, the truncated structures (Figure S5) were used in ECD calculations of compounds (12S,28R,31R)-1′, (12S,28S,31S)-2′, and (12R,28R,31R)-3′, corresponding to innate compounds (12S,28R,31R)-1, (12S,28S,31S)-2, and (12R,28R,31R)-3. For conformational analysis, the conformers generated by a MMFF conformational search in an energy window of 10 kcal/mol were subjected to geometry optimization using the DFT method at the B3LYP/def2-SVP level (Lee et al., 1988; Becke, 1993; Weigend and Ahlrichs, 2005). Frequency calculations were run at the same level to estimate their relative thermal (ΔE) and free energies (ΔG) at 298.15 K. Energies of the low-energy conformers in MeCN were re-calculated at the M06-2X/def2-TZVP level. Solvent (MeCN) effects were taken into account by using polarizable continuum model (IEFPCM). The TDDFT calculations were performed using the hybrid PBE1PBE (Perdew et al., 1996; Adamo and Barone, 1999) and M06-2X (Zhao and Truhlar, 2008) functionals, and the Ahlrichs' basis sets TZVP (Schäfer et al., 1994) and/or def2-SVP. The number of excited states is 36 for each of the compound. The ECD spectra were generated by the program SpecDis (Bruhn et al., 2013) using a Gaussian band shape from dipole-length dipolar and rotational strengths. The equilibrium population of each conformer at 298.15 K was calculated from its ΔG using Boltzmann statistics. The calculated spectra of compounds were generated from the low-energy conformers according to the Boltzmann weighting of each conformer in MeCN solution.
Biological Assays
The details of the experimental procedures for antioxidative and cytotoxic bioassays were similar to those presented in our former paper (Zhong W.-M. et al., 2018).
Molecular Docking Study
The detail procedures of molecular docking study were presented in Supporting Information File.
Results and Discussion
Identification of Compounds 1–3
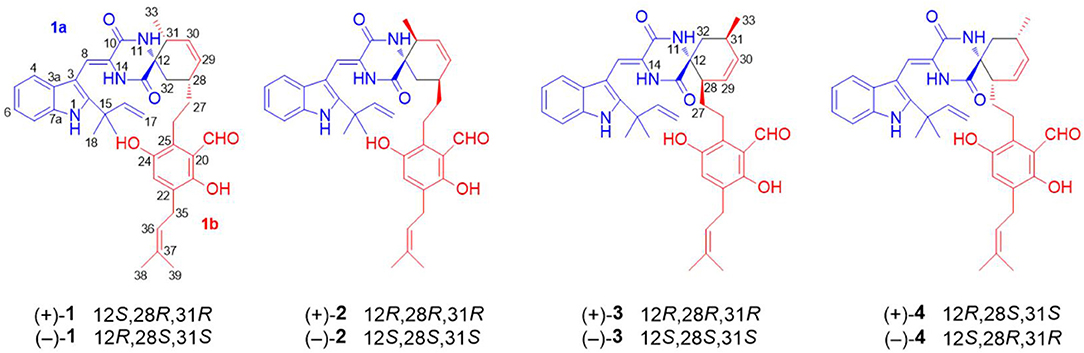
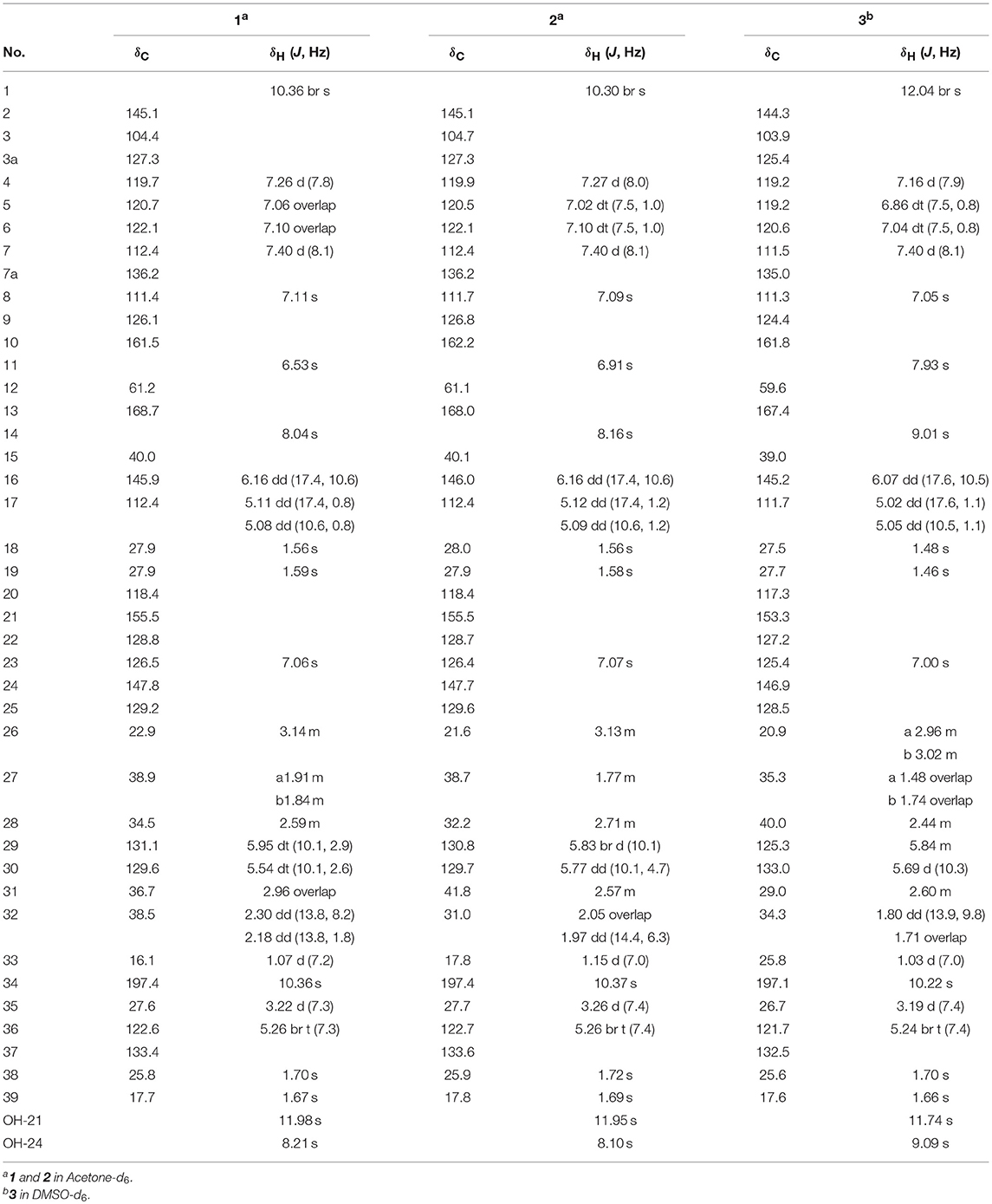
A 30 L fermentation broth of the marine-derived fungus Eurotium sp. SCSIO F452 furnished a crude extract (68 g), which was subsequently separated by repeated chromatographic methods including column chromatography (CC) over silica gel, ODS, and finally semi-preparative HPLC to yield compounds 1–4 (Figure 1). Eurotinoid A (1) was isolated as a yellow solid. Its molecular formula was determined as C38H43N3O5 by the positive HRESIMS (m/z 622.3270 [M + H]+, calcd for 622.3275), indicating 19 degrees of unsaturation. Its IR absorptions suggested the presence of hydroxyl and amine groups (3350, 3337 cm−1) and carbonyl functionalities (1682, 1636 cm−1). The 1H NMR spectrum of 1 (Table 1) recorded in CD3COCD3 showed five methyls at δH 1.07 (d, J = 7.2 Hz), 1.56 (s), 1.59 (s), 1.67 (s), and 1.70 (s), 12 olefinic protons ranging from δH 5.08 to 7.40, and six exchangeable protons at δH 6.53 (s), 8.04 (s), 8.21 (s), 10.36 (2H, overlap), and 11.98 (s). The 13C NMR and DEPT revealed the presence of 38 carbon resonances, including five methyls, five methylenes (one olefinic carbon), 12 methines (10 olefinic carbons), 16 non-protonated carbons (one nitrogenated carbon, three carbonyls, and nine olefinic carbons). By the aid of HSQC spectrum, all proton resonances were unambiguously assigned to their respective carbons except for the exchangeable protons.
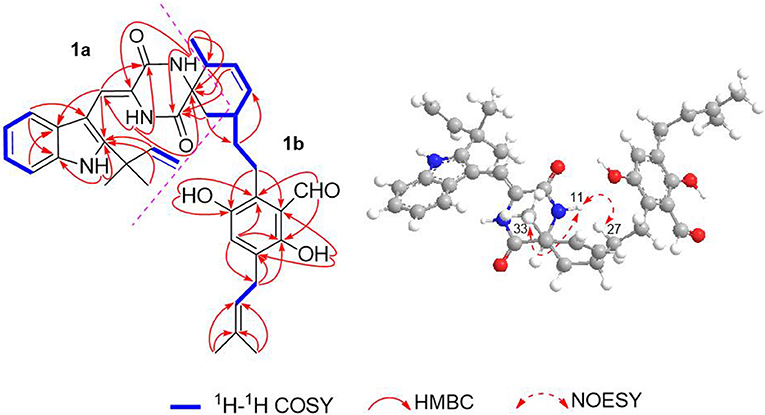
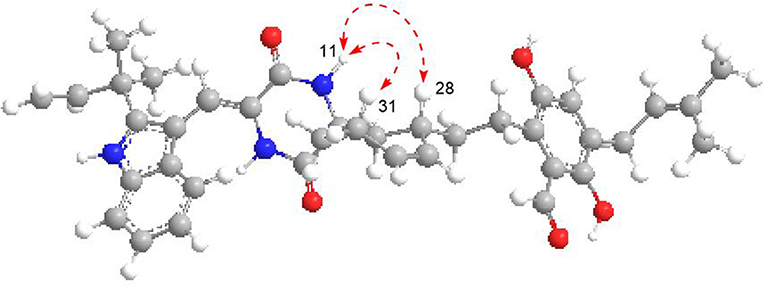
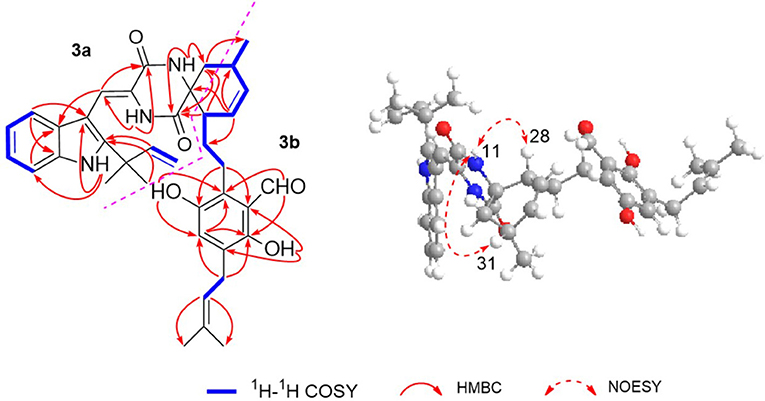
The 1H–1H COZY spectrum of 1 established four spin-spin coupling systems shown in blue bold (Figure 2). The HMBC correlations between H-1 and C-2/C-3/C-7a, H-8 and C-3a/C-10, H-11 and C-9/C-13, H-14 and C-8/C-10/C-12, H-16/H3-18/H3-19, and C-2, H2-32 and C-12/C-13 implied the existence of a C-2 reverse prenylated indole DKP alkaloid unit (1a) in 1. Moreover, the HMBC correlations between H-23 and C-21/C-25/C-35, H2-26 and C-20/C-24/C-28, H2-27 and C-29, H-29 and C-31, H-30 and C-28/C-33, H-34 and C-21/C-25, H2-35 and C-21/C-22/C-37, H3-38/H3-39 and C-36, OH-21 and C-20/C-22, OH-24 and C-24/C-25 supported the presence of a tetrasubstituted benzaldehyde derivative bearing a 3-methyl-2-butenyl and a C7-alkenyl side chain (1b). Extensive analysis of the 1D and 2D data demonstrated that 1 possessing two subunits 1a and 1b, shared partially structural similarities with dihydrocryptoechinulin D (4). However, careful analysis of its 2D NMR data revealed the two subunits were connected by a different coupling mode from 4. The key 1H–1H COZY correlation of H2-32 and H-28, and HMBC correlations between H-11 and C-31, H-31/H2-32 and C-12/C-13, H3-33 and C-12 indicated that 1a and 1b were connected via C-12–C-31 and C-32–C-28 bonds forming a cyclohexene ring, rather than by C-12–C-28 and C-32–C-31 bonds in 4. The diagnostic NOESY correlations (Figure 2) between H-11 and H2-27/H3-33 led to their assignment as α-orientation. The geometry of the Δ8 double bond was elucidated to be Z configuration by the downfield shift of H-8 (δH 7.11, s) due to the deshielding effect of the carbonyl group on the β-vinyl proton, which was also coincident with the lack of NOE effect between H-8 and H-14 (Gao et al., 2012).
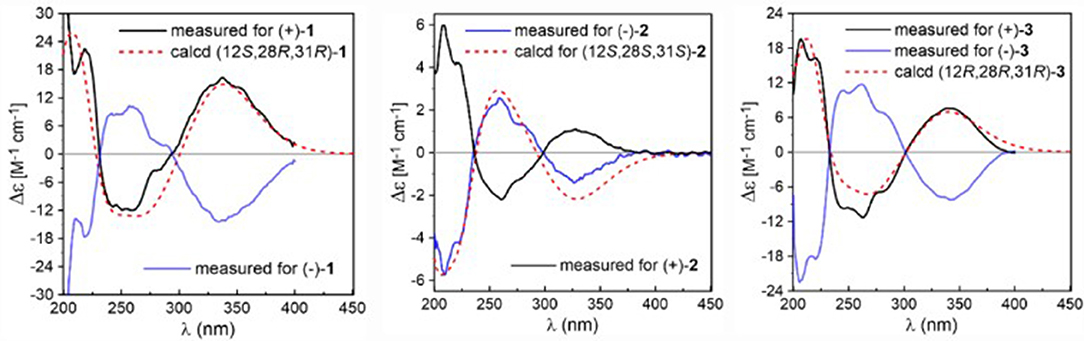
Due to the baseline ECD curve and barely measurable specific rotation, compound 1 was assumed to be a racemic mixture, which was further confirmed by separation of its enantiomers by HPLC using a chiral column (Figure S1). The absolute configurations of individual (+)-1 and (–)-1 were determined by comparison of their calculated ECD spectra with experimental ones. As a result, the calculated ECD curve of 12S,28R,31R-1 displayed good agreement with the experimental one of (+)-1 (Figure 3), which could establish the absolute configurations of 12S,28R,31R for (+)-1, and therefore the 12R,28S,31S for (–)-1 due to their enantiomeric nature.
Eurotinoid B (2) was obtained as a yellow solid. Its molecular formula C38H43N3O5 was established by positive HRESIMS at m/z 644.3099 [M + Na]+ (calcd for 644.3095), which was identical to that of 1. The UV and 1D NMR spectra (Table 1) of 2 highly resembled those of 1, except for some small differences of chemical shifts ascribed to the protons and carbons situated in the cyclohexene ring, indicating 2 was a diastereomer of 1. Further analysis of its 1H–1H COZY, HSQC, and HMBC NMR data confirmed the above elucidation. The NOE correlations (Figure 4) between H-11 and H-28/H-31 led to their assignment as α-orientation, which implied 2 was a C-28 and C-31 isomer of 1. Similar to 1, 2 also occurred as a racemate as justified by the zero specific rotation and baseline ECD curve. Compound 2 was further separated by HPLC using a chiral column to yield a pair of enantiomers (+)-2 and (–)-2 (Figure S2). The absolute configurations of (+)-2 and (–)-2 were also established by comparison of their experimental ECD spectra with calculated one. Consequently, the calculated ECD for 12S,28S,31S-2 showed well consistency with the experimental one of (–)-2 (Figure 3), leading to the unambiguously assignments of 12R,28R,31R for (+)-2 and 12S,28S,31S for (–)-2, respectively.
Eurotinoid C (3) was isolated as a yellow solid. Its molecular formula C38H43N3O5 was determined on the basis of positive HRESIMS at m/z 622.3282 [M + H]+ (calcd for 622.3275), corresponding to an index of hydrogen deficiency of 19, which was identical to that of 4. Its UV and IR spectra displayed typical hydroxyl and amine groups, and carbonyl functionalities. The 1H and 13C NMR spectra (Table 1) of 3 exhibited characteristic resonances of that of compound 4. Further analysis of its 2D NMR spectra data allowed us to establish two fragments 3a and 3b with their coupling manner similar to 4, different from 1 and 2. The key 1H–1H COZY (Figure 5) correlation between H2-32 and H-31, and HMBC correlations between H-11 and C-12/C-13/C-32, H2-27 and C-12/C-29, H-28 and C-12/C-13/C-30/C-32, H-29 and C-12/C-31, H2-32 and C-12/C-13/C-28/C-30/C-33 clearly supported the above elucidation. Interestingly, the planar structure of 3 was defined to be identical to dihydrocryptoechinulin D (4), indicating 3 was a diastereomer of 4. The above elucidation was verified by the key NOE correlations (Figure 5) between H-11 and H-28/H-31 in 3, which defined its relative configuration as 12R*,28R*,31R*, revealing 3 was a C-28 and C-31 isomer of 4. Similarly to 4, 3 was also a racemic mixture. The absolute configurations of individual (+)-3 and (–)-3 were determined by comparison of their experimental and calculated ECD spectra. As a result, the calculated ECD curve for 12R,28R,31R-3 displayed good agreement with the experimental one of (+)-3 (Figure S3). Thus, the absolute configurations of (+)-3 and (–)-3 were unambiguously assigned as 12R,28R,31R and 12S,28S,31S, respectively.
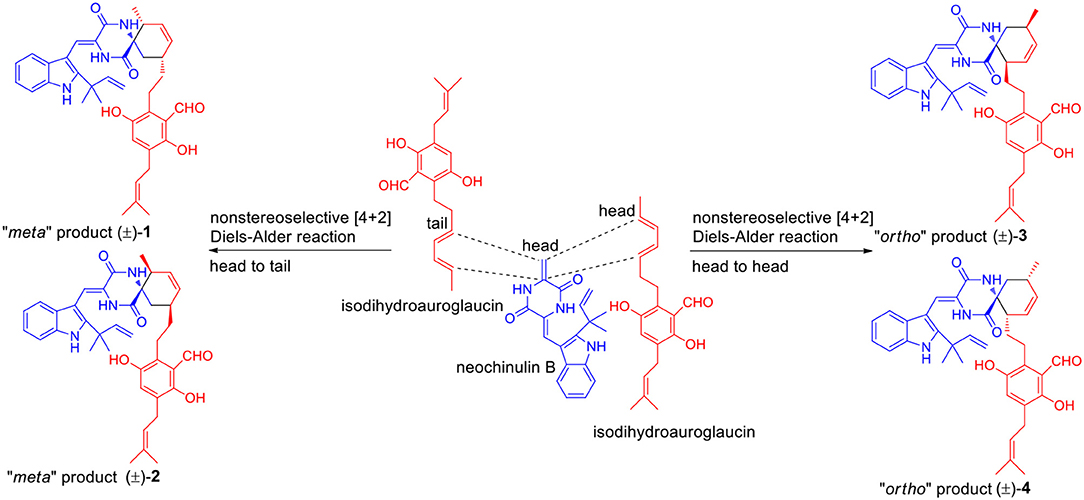
The Diels-Alder (DA) reaction is classified as a [4 + 2] cycloaddition in the pericyclic reaction involving a 1,3-diene and a dienophile to afford a six-membered ring with four contiguous stereocenters (Minami and Oikawa, 2016). Hundreds of natural products (NP) containing carbocycles or heterocycles are potentially biosynthesized by DA reaction. With the advancements in whole-genome sequencing and searching tools of biosynthetic gene clusters of secondary metabolites, some Diels-Alderases, such as SpnF, PyrE3, Pyrl4, AbyU, and PvhB, have been identified and substantiated to be of great importance in the biosynthesis of NPs (Takao et al., 2005; Kim et al., 2011; Fage et al., 2015; Tian et al., 2015; Byrne et al., 2016; Tan et al., 2019). Besides, some NPs like spirotriscoumarins A and B (Tang et al., 2016), artemisians A–D (Xue et al., 2017), and alpininoids A–E (Liu et al., 2018) are proposed to be biosynthesized by spontaneous DA cycloaddition. A plausible biogenetic route of 1–4 is proposed in Scheme 1. The enone group of neoechinulin B, an indole DKP alkaloid, was supposed to be the dienophile to couple with the diene group of a benzaldehyde derivative, isodihydroauroglaucin, forming the four pairs of enantiomers 1–4 through a key non-stereoselective [4 + 2] Diels-Alder cycloaddition. To be specific, the dienophile undergone a DA reaction with the diene by a head-to-tail approach to produce 1 and 2, and by a head-to-head mode to yield 3 and 4, respectively. Notably, neoechinulin B and isodihydroauroglaucin both have been isolated from this fungus in previous chemical study (Wang et al., 2013; Zhong W.-M. et al., 2018), which supported the biosynthetic hypothesis. To the best of our knowledge, only four such kind of spirocyclic alkaloids have been reported, namely, cryptoechinuline B (Gatti, 1976), cryptoechinuline D (Gatti, 1976), dihydrocryptoechinulin D (Gao et al., 2012), and effusin A (Gao et al., 2012), all isolated from fungi. Structurally, the former three reported compounds, together with compound 3 were all tend to be “ortho” products, while 1 and 2 were represented as the first two “meta” products belonging to this kind of spirocyclic alkaloids.
Biological Evaluation of Compounds (±)-1–(±)-4
We examined (+)-1, (–)-1, (+)-2, (–)-2, (+)-3, (–)-3, (+)-4, and (–)-4 for their antioxidative activities against DPPH and cytotoxic activities against SF-268 and HepG2 cell lines in vitro with the SRB method (Zhong W.-M. et al., 2018). All the compounds showed significant radical scavenging activities against DPPH with IC50 values ranging from 3.7 to 24.9 μM, which were more potent than that of the positive control ascorbic acid (Vc) (28.4 μM) (Table 2). In addition, compound (+)-4 showed moderate cytotoxicities against SF-268 and HepG2 cell lines with IC50 values of 51.7 ± 2.8, 49.9 ± 2.0 μM, and those of (–)-4 were 97.3 ± 1.8, 98.7 ± 1.0 μM, respectively (Table 2). Interestingly, according to the bioactivity assay results, (+)-enantiomers exhibited more potent activities than corresponding (–)-enantiomers, indicating that the stereochemistry of the compounds could contribute to their biological activities, which were widely found in the enantiomers of substances, such as R-/S-thalidomide, R-/S-warfarin, R-/S-ehlorpheniramine, and R-/S-ketamine (Dou et al., 2014). To investigate their bioactive mechanism, 23 and 22 proteins, belonging to five common types of antioxidative targets and six common types of cytotoxic targets respectively, were screened using molecular docking technique. The results (Figure 6 and Figure S7) showed that lipoxygenase (LOX) was the potential antioxidative target for compounds (±)-1–(±)-4, and epidermal growth factor receptor (EGFR) was the potential cytotoxic target for compounds (±)-4, because the trends of detected bioactivities of those compounds and the calculated binding forces between these compounds and proteins of 5FNO (a member of LOX; PDB ID, and so forth) and 4RJ3 (EGFR) were consistent. Furthermore, electrostatic potential contact (EPC) might play critical roles in stabilizing the complexes of the targets and corresponding compounds. Specifically, each isomer of (+)-1–(+)-4 had stronger EPC with the bioactive pocket of 5FNO (a type of LOX; PDB ID, and so forth) than corresponding (–)-1–(–)-4. Similarly, (+)-4 possessed tenser EPC with the bioactive pocket of 4RJ3 (EGFR) than (–)-4. It revealed proper configurations of the compounds were important for bioactivities.
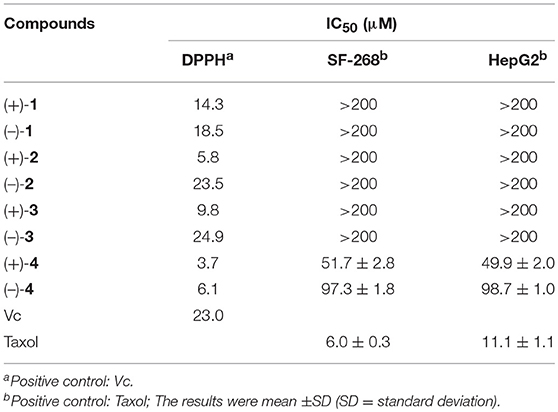
Table 2. Antioxidative activities against DPPH and cytotoxic activities against two tumor cell lines of compounds (±)-1–(±)-4.
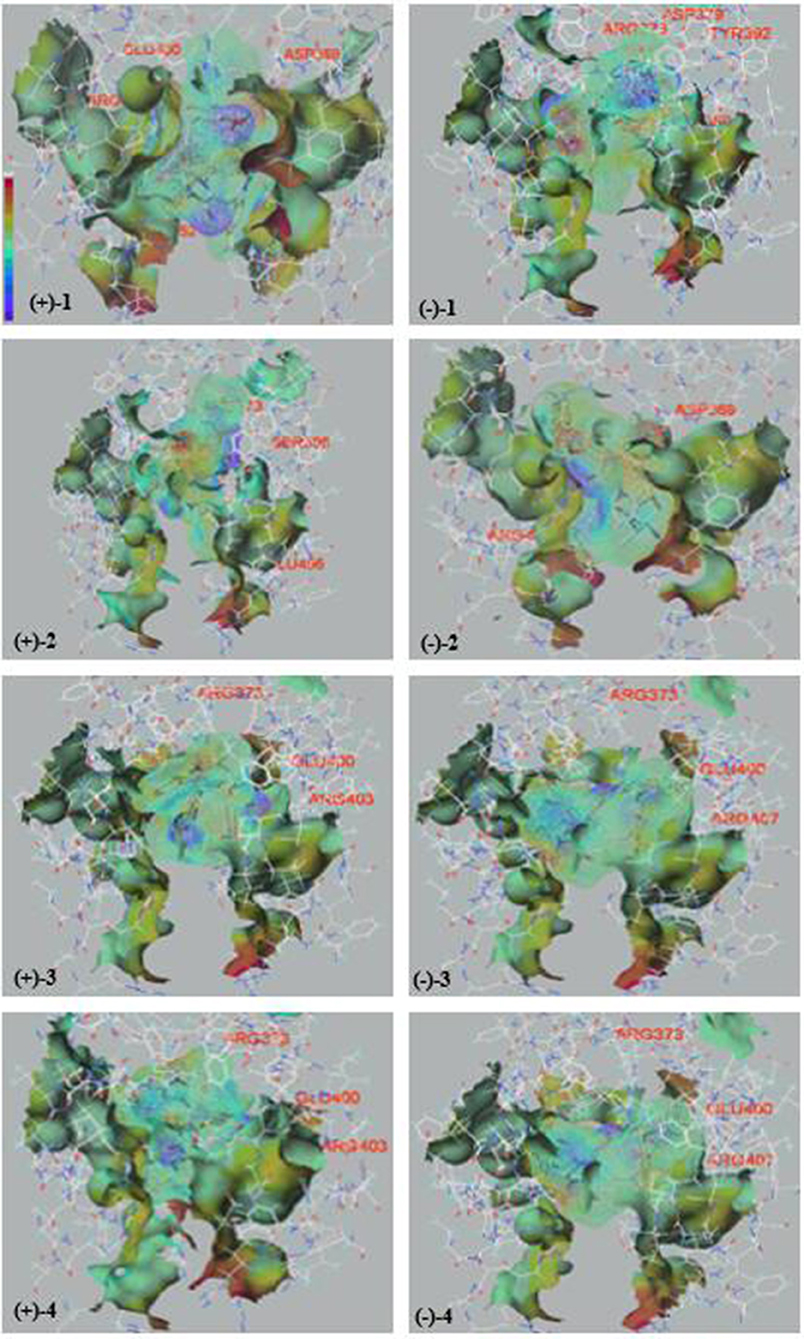
Figure 6. The electrostatic potential and hydrogen-bonds of compounds (±)-1–(±)-4 and the bioactive pockets of 5FNO. (Purple represented stronger electrostatic potential; red represented the residues to form hydrogen-bonds).
Physico-Chemical Constants of Compounds 1–3
Eurotinoid A (1): yellow solid; [α] = 0 (c 0.05, CH3CN); UV (CH3CN) λmax (log ε) 203 (4.7), 275 (4.2), 342 (4.2) nm; IR vmax 3350, 3337, 3314, 2926, 2855, 1682, 1636, 1429, 1207, 1142, 745, 725 cm−1; HRESIMS m/z 622.3270 [M + H]+ (calcd for 622.3275). 1H and 13C NMR see Table 1.
(+)-1: yellow solid; [α] = +200.4 (c 0.05, CH3CN); ECD (CH3CN) λmax (Δε) 218 (+11.2), 260 (−6.0), 338 (+8.2) nm.
(–)-1: yellow solid; [α] = −196.8 (c 0.05, CH3CN); ECD (CH3CN) λmax (Δε) 218 (−8.8), 260 (+5.1), 338 (−7.1) nm.
Eurotinoid B (2): yellow solid; [α] = 0 (c 0.05, CH3CN); UV (CH3CN) λmax (log ε) 225 (5.1), 276 (4.6), 334 (4.5) nm; HRESIMS m/z 644.3099 [M + Na]+ (calcd for 644.3095). 1H and 13C NMR see Table 1.
(+)-2: yellow solid; [α] = +193.3 (c 0.015, CH3CN); ECD (CH3CN) λmax (Δε) 209 (+15.9), 260 (−5.9), 328 (+2.9) nm.
(–)-2: yellow solid; [α] = −200.0 (c 0.015, CH3CN); ECD (CH3CN) λmax (Δε) 209 (−15.3), 260 (+6.7), 326 (−3.8) nm.
Eurotinoid C (3): yellow solid; [α] = 0 (c 0.05, CH3CN); UV (CH3CN) λmax (log ε) 203 (4.9), 274 (4.4), 347 (4.3) nm; IR vmax 3364, 2918, 2851, 1715, 1670, 1636, 1541, 1614, 1456, 1412, 1375, 743 cm−1; HRESIMS m/z 622.3282 [M + H]+ (calcd for 622.3275). 1H and 13C NMR see Table 1.
(+)-3: yellow solid; [α] = +212.4 (c 0.025, CH3CN); ECD (CH3CN) λmax (Δε) 207 (+36.8), 263 (−22.3), 339 (+14.3) nm.
(–)-3: yellow solid; [α] = −218.4 (c 0.025, CH3CN); ECD (CH3CN) λmax (Δε) 207 (−41.9), 262 (+22.2), 339 (−15.1) nm.
Conclusions
In conclusion, three pairs of new spirocyclic alkaloid enantiomers eurotinoids A–C (1–3), as well as a known biogenetically related racemate dihydrocryptoechinulin D (4), were isolated from a marine-derived fungus Eurotium sp. SCSIO F452. Their structures including absolute configurations were determined by extensive spectroscopic data and ECD calculations. Compounds 1 and 2 were represented as the first two “meta” products biosynthetically from a non-stereoselective [4 + 2] Diels-Alder cycloaddition presumably between an enone group of a diketopiperazine alkaloid and a diene group of a benzaldehyde derivative via a new head-to-tail coupling mode biosynthetically. All the enantiomers were evaluated antioxidative and cytotoxic activities. A preliminary molecular docking study provided an inside perspective of the action of their different biological activities.
Author Contributions
WZho performed the isolation, purification, characterization and evaluation the antioxidative activities of all the compounds, and prepared the manuscript. JW contributed to the structure elucidation and revised the manuscript. XW performed the ECD calculations. TF and XH performed the molecular docking experiments. YC and WZha contributed to the determination of cytotoxic activities. QZ and ZH contributed to the isolation of the compounds and determination of antioxidative activities. SZ, LL, and FW designed and supervised the research and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 41476136, 41776169, 41230962), the National Key Research and Development Program of China (2017YFC0506300), the Guangdong Province Science and Technology Plan Project (2015B090904003, 2016A020222010), the Pearl River S&T Nova Program of Guangzhou (No. 201710010136), and the Science and Technology Program of Guangzhou (201607020018). We gratefully acknowledge support from the Guangzhou Branch of the Supercomputing Center of the Chinese Academy of Sciences and the analytical facilities in SCSIO.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00350/full#supplementary-material
References
Adamo, C., and Barone, V. (1999). Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6169. doi: 10.1063/1.478522
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. doi: 10.1063/1.464913
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2018). Marine natural products. Nat. Prod. Rep. 35, 8–53. doi: 10.1039/c7np00052a
Bruhn, T., Schaumlöffel, A., Hemberger, Y., and Bringmann, G. (2013). SpecDis: quantifying the comparison of calculated and experimental eectronic circular dichroism spectra. Chirality 25, 243–249. doi: 10.1002/chir.22138
Byrne, M. J., Lees, N. R., Han, L. C., van der Kamp, M. W., Mulholland, A. J., Stach, J. E., et al. (2016). The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail. J. Am. Chem. Soc. 138, 6095–6098. doi: 10.1021/jacs.6b00232
Dou, M., Di, L., Zhou, L. L., Yan, Y. M., Wang, X. L., Zhou, F. J., et al. (2014). Cochlearols A and B, polycyclic meroterpenoids from the fungus Ganoderma cochlear that have renoprotective activities. Org. Lett. 16, 6064–6067. doi: 10.1021/ol502806j
Du, F. Y., Li, X., Li, X. M., Zhu, L. W., and Wang, B. G. (2017). Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 15, 1–10. doi: 10.3390/md15020024
Du, F. Y., Li, X. M., Li, C. S., Shang, Z., and Wang, B. G. (2012). Cristatumins A-D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 22, 4650–4653. doi: 10.1016/j.bmcl.2012.05.088
Fage, C. D., Isiorho, E. A., Liu, Y., Wagner, D. T., Liu, H., and Keatinge-Clay, A. T. (2015). The structure of SpnF, a standalone enzyme that catalyzes [4 + 2] cycloaddition. Nat. Chem. Biol. 11, 256–258. doi: 10.1038/nchembio.1768
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2010). Gaussian 09, Revision C.01. Wallingford, CT: Gaussian, Inc.
Gao, H., Liu, W., Zhu, T., Mo, X., Mandi, A., Kurtan, T., et al. (2012). Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 10, 9501–9506. doi: 10.1039/c2ob26757h
Gao, J., Leon, F., Radwan, M. M., Dale, O. R., Husni, A. S., Manly, S. P., et al. (2011). Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens. J. Nat. Prod. 74, 1636–1639. doi: 10.1021/np200147c
Gatti, G. (1976). Structure determination of two extractives from Aspergillus amstelodami by nuclear magnetic resonance spectroscopy. J. Chem. Soc. Chem. Commun. 12, 435–436. doi: 10.1039/C39760000435
Gomes, N. M., Dethoup, T., Singburaudom, N., Gales, L., Silva, A. M. S., and Kijjoa, A. (2012). Eurocristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 5, 717–720. doi: 10.1016/j.phytol.2012.07.010
Kim, H. J., Ruszczycky, M. W., Choi, S., Liu, Y., and Liu, H. (2011). Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 473, 109–112. doi: 10.1038/nature09981
Lee, C., Yang, W., and Parr, R. G. (1988). Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789. doi: 10.1103/PhysRevB.37.785
Liu, H., Wu, Z. L., Huang, X. J., Peng, Y., Huang, X., Shi, L., et al. (2018). Evaluation of diarylheptanoid-terpene adduct enantiomers from Alpinia officinarum for neuroprotective activities. J. Nat. Prod. 81, 162–170. doi: 10.1021/acs.jnatprod.7b00803
Martins, A., Vieira, H., Gaspar, H., and Santos, S. (2014). Marketed marine natural products in the pharmaceutical and cosmeceutical industries: tips for success. Mar. Drugs 12, 1066–1101. doi: 10.3390/md12021066
Meng, L. H., Du, F. Y., Li, X. M., Pedpradab, P., Xu, G. M., and Wang, B. G. (2015). Rubrumazines A-C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 78, 909–913. doi: 10.1021/np5007839
Minami, A., and Oikawa, H. (2016). Recent advances of Diels-Alderases involved in natural product biosynthesis. J. Antibiot. 69, 500–506. doi: 10.1038/ja.2016.67
Perdew, J. P., Burke, K., and Ernzerhof, M. (1996). Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868. doi: 10.1103/PhysRevLett.77.3865
Richards, T. A., Jones, M. D., Leonard, G., and Bass, D. (2012). Marine fungi: their ecology and molecular diversity. Annu. Rev. Mar. Sci. 4, 495–522.doi: 10.1146/annurev-marine-120710-100802
Schäfer, A., Huber, C., and Ahlrichs, R. (1994). Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. Chem. Phys. 100, 5829–5835. doi: 10.1063/1.467146
Takao, K., Munakata, R., and Tadano, K. (2005). Recent advances in natural product synthesis by using intramolecular Diels–Alder reactions. Chem. Rev. 105, 4779–4807. doi: 10.1021/cr040632u
Tan, D., Jamieson, C. S., Ohashi, M., Tang, M. C., Houk, K. N., and Tang, Y. (2019). Genome-mined Diels-Alderase catalyzes formation of the cis-octahydrodecalins of varicidin A and B. J. Am. Chem. Soc. 141, 769–773. doi: 10.1021/jacs.8b12010
Tang, Z. H., Liu, Y. B., Ma, S. G., Li, L., Li, Y., Jiang, J. D., et al. (2016). Antiviral spirotriscoumarins A and B: two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from toddalia asiatica. Org. Lett. 18, 5146–5149. doi: 10.1021/acs.orglett.6b02572
Tian, Z., Sun, P., Yan, Y., Wu, Z., Zheng, Q., Zhou, S., et al. (2015). An enzymatic [4+2] cyclization cascade creates the pentacyclic core of pyrroindomycins. Nat. Chem. Biol. 11, 259–265. doi: 10.1038/nchembio.1769
Wang, F. Z., Huang, Z., Shi, X. F., Chen, Y. C., Zhang, W. M., Tian, X. P., et al. (2013). Analysis of secondary metabolites produced by Eurotium sp. SCSIO F452 isolated from the South China Sea sediment. Zhongguo Haiyang Yaowu 32, 7–12. doi: 10.13400/j.cnki.cjmd.2013.01.002
Weigend, F., and Ahlrichs, R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305. doi: 10.1039/b508541a
Xue, G. M., Han, H., Chen, C., Li, L. N., Wang, X. B., Yang, M. H., et al. (2017). Artemisians A-D, diseco-guaianolide involved heterodimeric [4 + 2] adducts from Artemisia argyi. Org. Lett. 19, 5410–5413. doi: 10.1021/acs.orglett.7b02681
Zhao, Y., and Truhlar, D. G. (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. 120, 215–241. doi: 10.1007/s00214-007-0310-x
Zhong, W., Wang, J., Wei, X., Chen, Y., Fu, T., Xiang, Y., et al. (2018). Variecolortins A-C, three pairs of spirocyclic diketopiperazine enantiomers from the marine-derived fungus Eurotium sp. SCSIO F452. Org. Lett. 20, 4593–4596. doi: 10.1021/acs.orglett.8b01880
Keywords: Eurotium sp. SCSIO F452, spirocyclic alkaloid enantiomers, antioxidative and cytotoxic activities, Diels-Alder cycloaddition, molecular docking
Citation: Zhong W, Wang J, Wei X, Fu T, Chen Y, Zeng Q, Huang Z, Huang X, Zhang W, Zhang S, Long L and Wang F (2019) Three Pairs of New Spirocyclic Alkaloid Enantiomers From the Marine-Derived Fungus Eurotium sp. SCSIO F452. Front. Chem. 7:350. doi: 10.3389/fchem.2019.00350
Received: 14 September 2018; Accepted: 29 April 2019;
Published: 20 May 2019.
Edited by:
Laurent G. Désaubry, Centre National de la Recherche Scientifique (CNRS), FranceCopyright © 2019 Zhong, Wang, Wei, Fu, Chen, Zeng, Huang, Huang, Zhang, Zhang, Long and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Long, bG9uZ2xqQHNjc2lvLmFjLmNu
Fazuo Wang, d2FuZ2ZhenVvQHNjc2lvLmFjLmNu
 Weimao Zhong
Weimao Zhong Junfeng Wang
Junfeng Wang Xiaoyi Wei3
Xiaoyi Wei3 Lijuan Long
Lijuan Long