- 1School of Pharmaceutical and Chemical Engineering, Taizhou University, Taizhou, China
- 2School of Pharmaceutical Science, Zhejiang Chinese Medical University, Hangzhou, China
- 3Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou, China
- 4Institute of Pharmaceutical Science, King's College London, London, United Kingdom
A new series of 3-substituted 4-methylene-quinazolinthiones and 4-methylene-quinazolinones were synthesized in moderate to excellent yield through a simple reaction of 2-aminoacetophenones with isocyanates or isothiocyanates. The reaction shows good tolerance of many important functional groups in the presence of air and water under metal-free conditions. Only water is produced as a coproduct, rendering this “green” methodology a highly versatile and eco-friendly alternative to the existing methods for the construction of the quinazolinone/quinazolinthione framework. We have interpreted the reaction mechanism by use of quantum chemical calculations on the basis of state-of-the-art computational methods SMD-B3LYP-D3(BJ)/BS1//B3LYP/BS1.
Introduction
Quinazolinones and their derivatives occur frequently in natural and synthetic pharmaceutical products. Such compounds act as inhibitors of inducible and neuronal nitric oxide synthases (Camacho et al., 2016), possess anti-melanogenesis activity (Thanigaimalai et al., 2010), inhibit aldosterone synthase (CYP11B) and act as antagonists of platelet activating factor (Walser et al., 1991; Grombein et al., 2015). In recent years, many studies have been directed toward the design of 3,4-dihydroquinazolin-2(1H)-ones for the modulation of cardiotonic contractility (Campbell et al., 1988), inhibition of methionyl-tRNA synthetase (Buckner et al., 2015) and inhibition of HIV-1 Tat-TAR (Zeiger et al., 2014) together with the design of highly selective naked-eye sensors (Mei et al., 2012).
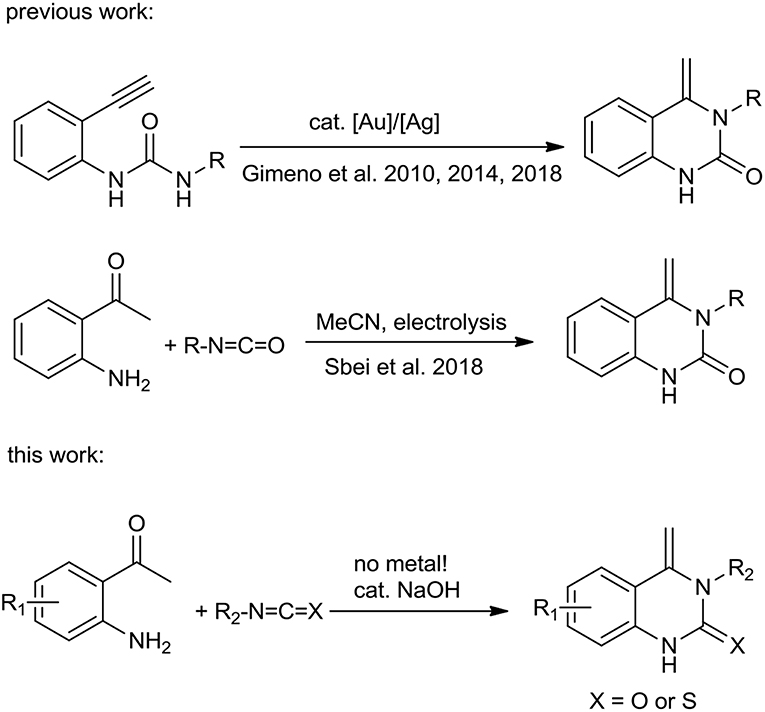
Many 3,4-dihydroquinazolin-2(1H)-one derivatives have been previously synthesized (Huang et al., 2008; Khan et al., 2014, 2015, 2016; Kshirsagar, 2015; Camacho et al., 2016; Maiden and Harrity, 2016; Awad et al., 2018; Zhang et al., 2018; Elkholy et al., 2019; Gatadi et al., 2019; Long et al., 2019; Wang et al., 2019). For example, Wang et al. (2009) reported the synthesis of 4-alkyl-2(1H)-quinazolinones via the cyclization of 1-(2-alkynyl-phenyl)ureas catalyzed by TfOH and Saunthwal et al. (2015) described a green and catalyst-free straightforward tandem synthesis of functionalized tetrahydroquinazolines from 2-aminophenylacrylate. Sawant et al. (2015) developed a microwave-promoted sequential cyclization-Mannich reaction of ketones, o-formyl carbamates and primary amines to form polyfunctionalized 3,4-dihydroquinazolinones. Fukamachi et al. (2010) described a selective construction of 3,4-dihydroquinazoline-2-thiones by reacting 3-(2-isothiocyanatophenyl)propanoic derivatives with primary amines. 3,4-Dihydroquinazoline-2-thiones can also be synthesized from isothiocyanates with 2-aminophenyl acrylates or 2-amino chalcones (Hua et al., 2014; Xie et al., 2016). However, there are very few reports on the production of 4-alkenylquinazolinones and 4-alkenylquinazolinthiones. The first 4-alkenylquinazolinone to be successfully synthesized starting from quinoline-1-carboxamides was achieved in the presence of strong acids (Brack, 1969). Molina et al. (1993) utilized iminophosphorane and 2 equivalents of isocyanate to form 4-methylen-4H-3,1-benzoxazine which upon heating undergoes elimination and rearrangement to furnish 3-substituted 4-methenylquinazolinones. There were only two pioneering methodologies for the synthesis of 4-methylen-3,4-dihydroquinazolin-2-ones, one from 1-(o-alkynylaryl)ureas catalyzed by an Au(I)-complex (Scheme 1, top) (Gimeno et al., 2010, 2014a,b) and the other by reaction of 2-aminoacetophenone with the cyanomethyl anion electrogenerated by acetonitrile reduction at a graphite electrode (Scheme 1, middle) (Sbei et al., 2018). However, these reactions suffer from one or more drawbacks, such as expensive catalysts/ligands, harsh reaction conditions and multi-step preparation of raw materials. Thus, simple and efficient approaches to 4-alkenylquinazolinons and 4-alkenylquinazolinthione synthesis from readily available starting materials remains an important target. Herein, we report the synthesis of 4-alkenylquinazolinones and 4-alkenylquinazolinthiones from readily available materials (Scheme 1, bottom). The process is simple, proceeds under mild conditions in the absence of a catalyst for the synthesis of 4-alkenylquinazolinthiones and with catalytic amounts of NaOH, for the synthesis of 4-alkenylquinazolinones.
Results and Discussion
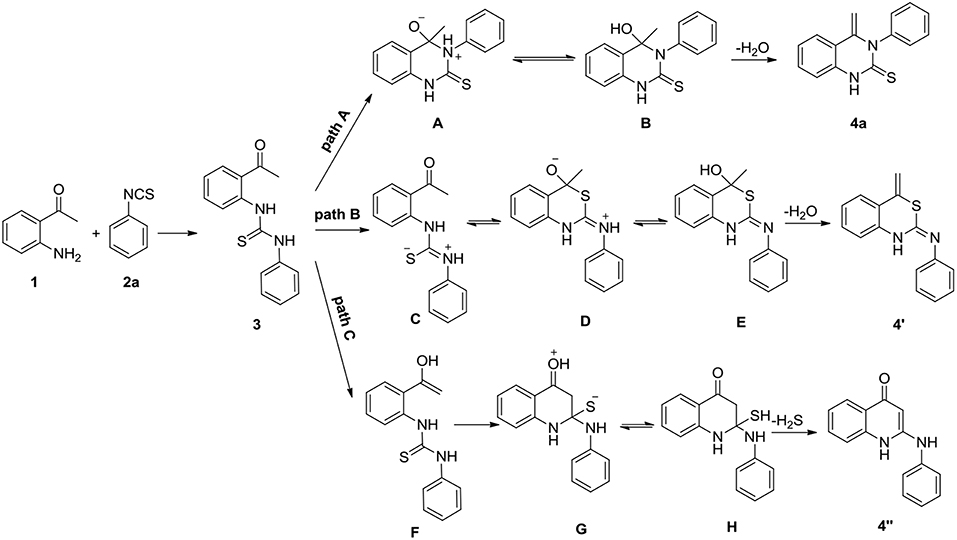
Nucleophilic addition of 2-aminoacetophenones 1 with isothiocyanates 2a results in a formation of 1,3-diarylthiourea 3. In principle, heterocyclization of compound 3 may occur by three different mechanisms (Scheme 2). Firstly, quinazolinthione 4a can result from the nucleophilic attack of N-3 of compound 3 on the carbonyl group of the ketone followed by removal of water (path A). Secondly, compound 3 is transformed to its mesomeric form C and the latter cyclizes through an addition reaction to the carbonyl group of the ketone leading to the formation of benzothiazinimine 4′ by the elimination of one molecule of water (path B). Thirdly, compound 3 is first converted to an enol form and the enol of intermediate F attacks the thiocarbonyl group of thiourea followed by a loss of H2S to afford quinolinone 4′′ (path C).
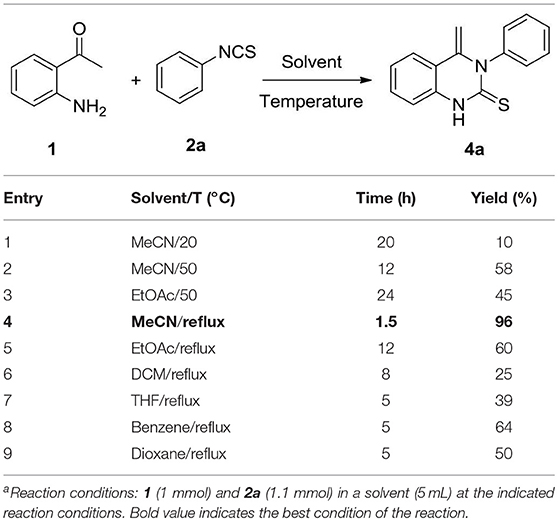
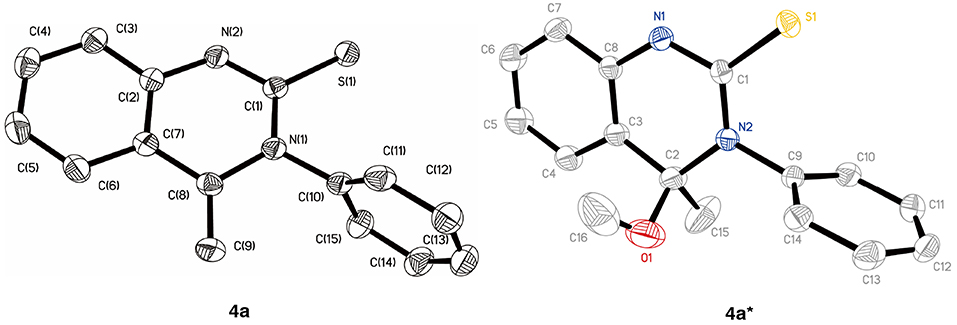
In an initial experiment, the reaction of 2-aminoacetophenone 1 with phenyl isothiocyanate 2a was undertaken in acetonitrile (MeCN) at room temperature. Not surprisingly, the main nucleophilic addition product 3 was isolated (yield: 50%). Apart from this, a small proportion of a new compound was detected (entry 1, Table 1). 1H NMR spectra demonstrated that there are two double peaks at 4.82 (one proton) and 3.69 ppm (one proton), respectively, ruling out the existence of product 4′′. To identify the product structure as being either 4a or 4′, single crystals were isolated and the structure characterized by X-ray crystallographic analysis. It was established that the quinazolinthione 4a was the correct structure (CCDC1906269, Figure 1, left, see Supporting Information for details). However, to our surprise, when crystals of the compound were incubated in methanol, an additional methoxy group was attached to 4-position of the quinazolinthione ring (CCDC1906268, Figure 1, right), indicating that the methylene group of compound 4a is an active site and performs a further coupling reaction. This observation is currently under investigation.
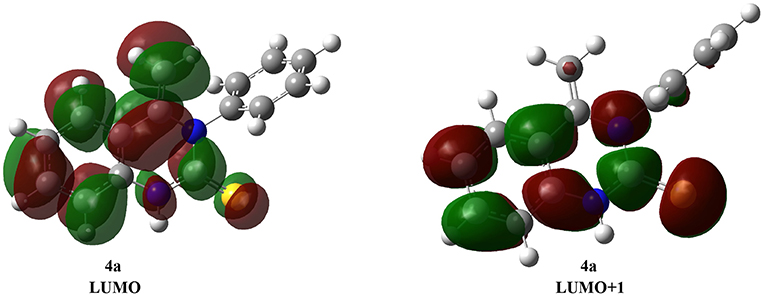
Consistent with Fukui's theory, LUMO (the lowest unoccupied molecular orbital) and ELUMO (the energy of LUMO) are clear, as is the extent of molecular susceptibility toward attack by external electrons (Dhami et al., 1997; Yan et al., 2019). Thus, the carbon-carbon double bond character of the 4a structure is also represented by strong binding interactions in LUMO and LUMO+1 (Figure 2). This calculation provides a realistic description of the nucleophilic attack on the double bond on 4a.
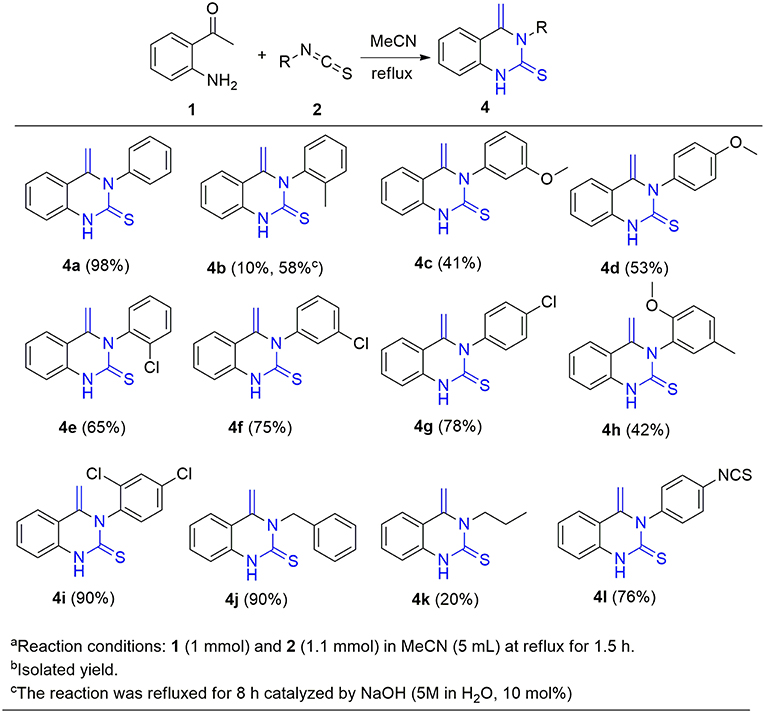
We optimized the reaction conditions in order to obtain the best yield by screening solvents and temperatures. As shown in Table 1, the yield was improved to 58% when the reaction was increased to 50°C (entry 2). In parallel, replacement of acetonitrile with ethyl acetate resulted in lower yield, even with an extended time. The reaction was found to occur with high efficiency at a short reaction time in acetonitrile under reflux conditions (entry 4). Replacement of acetonitrile with other solvents such as ethyl acetate, dichloromethane, THF, benzene and dioxane, resulted in lower yields (entries 5–9). Based on these results, the optimal reaction conditions were selected as 1 (1 mmol) and 2 (1.1 mmol) in MeCN (5 mL) under reflux condition for 1.5 h.
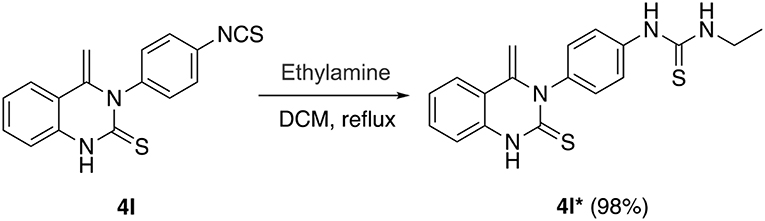
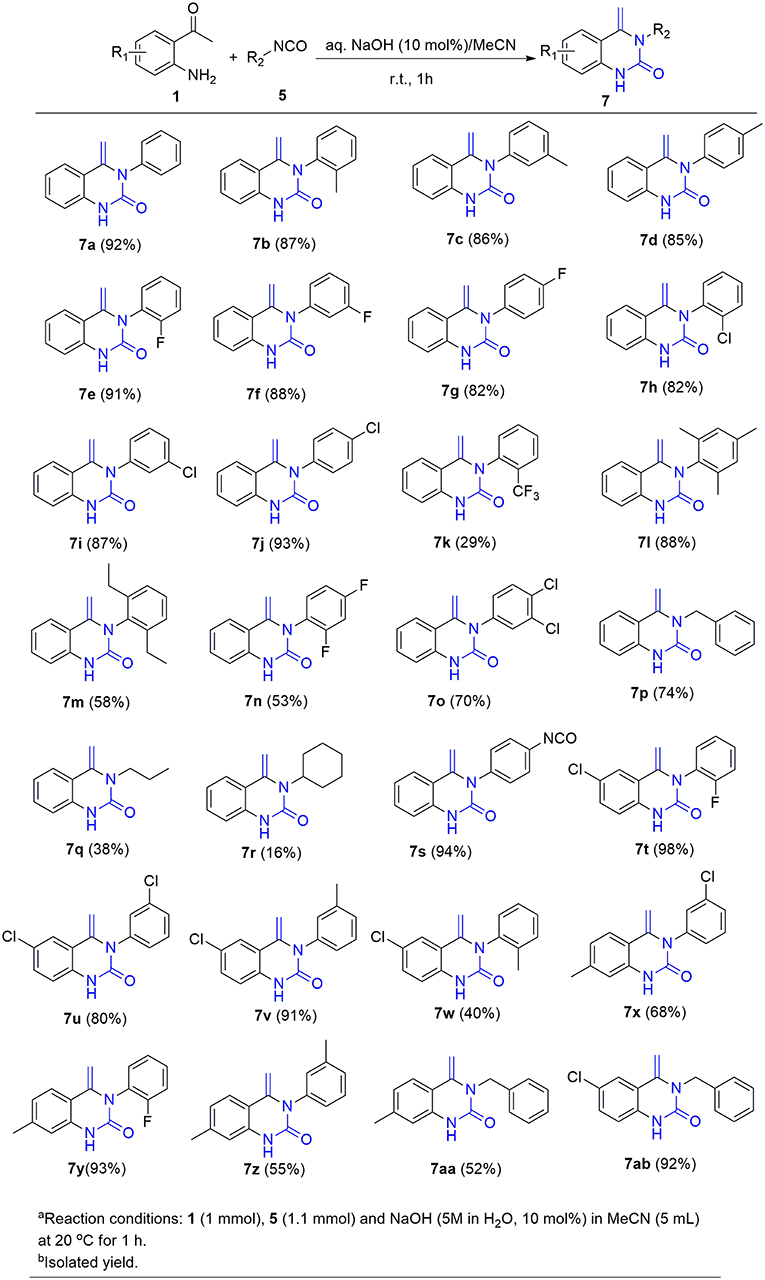
With these optimized reaction conditions in hand, the substrate scope of this methodology was explored (Figure 3). We found that both electron-donating groups (EDG) and electron-withdrawing groups (EWG) on the aromatic ring were well-tolerated by the reaction conditions. However, the yields of the products attached with EDG were significantly lower than those with EWG (4b-4d vs. 4e-4g). In addition, the stereochemistry also influenced the yield. For example, using para- and meta-chloro substituted 2 in the reaction provided good yields of 4 whereas ortho-chloro substituted 2 gave the corresponding product in only a moderate yield (4e-g). The yield sequence followed the order: para- > meta- > ortho-. In similar fashion to mono-substituted analogs, di-substituted isothiocyanates reacted smoothly with 2-aminoacetophenone to furnish the corresponding quinazolinthiones in moderate to high yields (4h-4i). Again, the electronic nature of the aryl moiety on the isothiocyanates had a dramatic effect on the product yield. As a result, 2,4-dichloro substituted 2i gave the corresponding product at 90% yield while the 2-methoxy-5-methyl substituted analog 2h only offered 42% yield of product 4h. To further expand the substrate scope, two aliphatic isothiocyanates (2j, 2k) were also investigated for this cycloaddition reaction. Benzyl isothiocyanate (2j) was found to be smoothly converted to the corresponding product 4j in a high yield, whereas propyl isothiocyanate (2k) only gave 20% yield of the desired 4k. In addition, diisothiocyanate (2l) was also examined for this conversion, but only one isothiocyanate group was involved in the reaction and a mono-cyclized product 4l was obtained with the second isothiocyanate not involved in the reaction, even in the presence of an excess of 2-aminoacetophenone 1 (>2 equiv.). This interesting result provides an opportunity for further diversification and amplification with the entire isothiocyanate group. In this regard, compound 4l was refluxed with ethylamine, a highly nucleophilic amine, to afford compound 4l* with an excellent yield (Scheme 3).
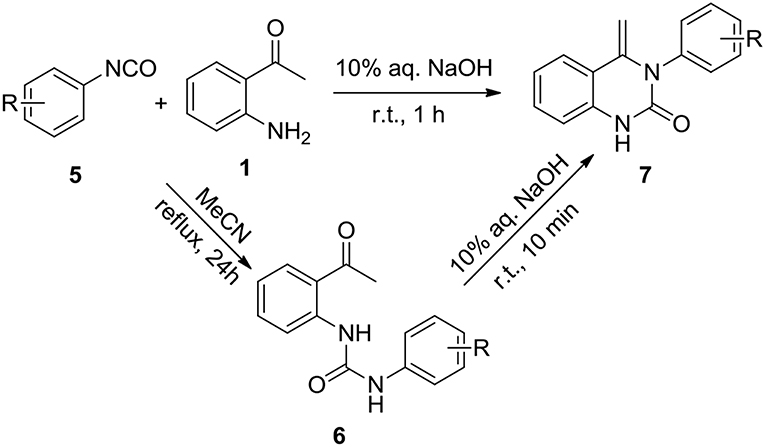
This success led us to further investigate the reaction generality by replacement of isothiocyanates with isocyanates. Under the optimized reaction conditions, the reaction of isocyanate 5 and 2-aminoacetophenone 1 only resulted in the coupling product 6, even after prolonging the reaction time to 24 h. The annulation compound 7 was not detected (Scheme 4). Pleasingly, compound 6 can undergo an intramolecular cyclization to afford the target 4-methylene-quinazolinone 7 in the presence of NaOH (10 mol%). Encouraged by these results, a subsequent study was undertaken to evaluate a one-pot cascade reaction of isocyanates 5 and 2-aminoacetophenone 1. In the presence of a catalytic amount of aqueous NaOH (10 mol%), the reaction progressed smoothly to yield the annulation product 7 even at room temperature. Consequently we modified the conditions for the reaction of 1 with 2, and achieve a significantly improved yield for the production of 4b (58 vs. 10%, Figure 3).
On the basis of this latter result, the scope of substituted isocyanates was explored (Figure 4). Aromatic isocyanates bearing either an EDG or an EWG group were found to undergo the reaction smoothly and the corresponding 4-methylene-quinazolinones were formed in excellent yields (7b-7j). In this case, neither the electronic nature nor position of the functional groups attached to the aromatic ring dramatically influenced the product yield. However, the 2-CF3-substituted isocyanate 5k only gave a moderate yield of the expected product 7k. Compared to the mono-substituted analogs, di-substituted aryl isocyanates were converted into the corresponding quinolinones in relatively low yields (7l-7o). In addition, three aliphatic isocyanates (5p-5r) were investigated for this annulation reaction. It was found that benzyl isocyanate was converted smoothly to afford the corresponding product 7p in a high yield, whereas only moderate yields were achieved for 7q and 7r. The diisocyanate (5s) was also investigated for this conversion. In a similar fashion to the aforementioned reaction with diisothiocyanate (2l), only one isocyanate group was involved in the reaction and an excellent yield of mono-cyclized product 7s was obtained.
The scope of substituted 2-aminoacetophenones 1 was also investigated in this novel cyclization process. 2-Aminoacetophenones bearing either an EWG such as chloro group or an EDG such as methyl group on the aromatic ring, progressed in this reaction smoothly with either aromatic isocyanates or benzyl isocyanate to provide the corresponding quinazolinones in moderate to excellent yield (7t-7ab).
In order to demonstrate the suitability of this new synthetic methodology for industrial use, an increased scale preparation of 7a was investigated. Reaction of substrates 1 and 5a in MeCN (20 mmol, 50 mL) was performed in the presence of NaOH (5 M in H2O, 10 mol%) at room temperature. The corresponding product 7a was afforded in 90% yield, the yield being similar to that of the small-scale reaction (Scheme 5). Moreover, the work-up procedure is simple, as the product precipitated from MeCN on cooling the reaction medium. A pure product was obtained after filtration, without the requirement of further purification.
As indicated in Scheme 2, the heterocyclization of starting materials 1 and 2/5 may in principle lead to at least three different products. To better understand the intramolecular cyclization reaction, we calculated Gibbs free energy of each intermediate and products listed in Scheme 2 by a computational method. Compounds 1 and 2a were selected as the model substrates for this study. The calculation was based on quantum chemical calculations (SMD-B3LYP-D3(BJ)/BS1//B3LYP/BS1). The geometry of transition states (TSs) and compound 3 in Scheme 2 have been fully optimized in vacuum and the TSs were verified to have only one imaginary frequency vibrational mode that connects the reactants and products. The calculated energy values of the intermediates and products, including dispersion interactions are presented in Figure 5. All stationary points were fully optimized and subjected to frequency analyses. The data demonstrates that paths A and C mechanism routes have a negative value of Gibbs free energy for the final product while the path B mechanism route gives a positive value, thus ruling out the path B mechanism. In the route of path A 3 → A → B → 4a (black, Figure 5), there is only one energy barrier at a value of 4.77 kcal/mol. In contrast, there are two high energy barriers (20.53 and 7.19 kcal/mol) in the route of path C 3 → F → G → H → 4″ (blue), rendering the transformation via path C difficult. These results indicate that the route of path A is the most probable pathway for this annulation reaction.
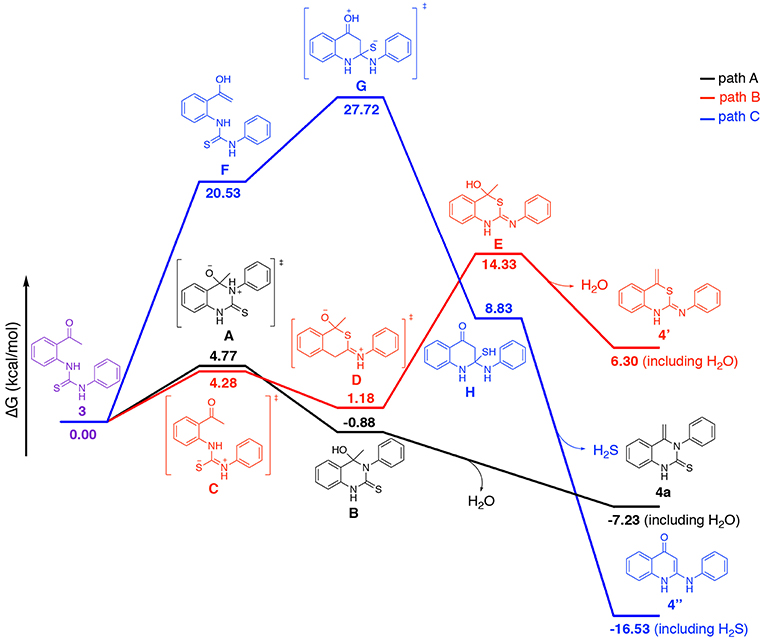
Figure 5. Calculated comparative Gibbs free energy profile for three different mechanism routes. Red, Blue, and Black lines stand for the route of TS and optimized structures Gibbs energy change. Calculated Gibbs free energies relative to 3 at SMD-B3LYP-D3(BJ)/BS1//B3LYP/BS1 [kcal/mol] (see Supporting Information for optimized structures).
Conclusion
In summary, we have presented a novel preparation of 3-substituted 4-methylene-3,4-dihydroquinazoline-2(1H)-thiones/ones from 2-aminoacetophenone and isothiocyanates/isocyanates. The method utilizes commercially available starting materials and is applicable to a large range of compounds. This new synthetic methodology does not require expensive/complicated metal catalysts and the work-up procedure is simple. The novel transformation indicates the feasibility of this pathway in both research and industrial laboratories. Comprehensive quantum chemical calculation studies indicate the probable synthetic route.
Materials and Methods
Experimental and Computational Details
All chemicals were obtained from Aladdin (China) as reagent grade and were used as received. Column chromatography purifications were performed on silica gel 60 (0.04–0.063 mm). Melting points were determined using an Electrothermal WRS-1B Digital Melting Point Apparatus and are uncorrected. 1H-NMR spectra were recorded using a Bruker (400/500 MHz) NMR spectrometer. 13C-NMR spectra were recorded using a Bruker (101/151 MHz) NMR spectrometer. Chemical shifts (δ) are reported in ppm downfield from the internal standard tetramethylsilane (TMS).
Gaussian 09 software (Frisch et al., 2016) packages can implement density functional theory (DFT) conveniently, thus geometry optimizations were carried out with the hybrid B3LYP functional in conjunction with 6-31G++(d,p) basis set for all the atoms of these compounds. Additionally, the geometry optimizations were followed by frequency calculations using the same basis set. Moreover, the effect of dispersion was incorporated using Grimme-D3 approximation during geometry optimizations (Grimme et al., 2010). To further refine the energies, single-point B3LYP calculations including D3 version of Grimme's dispersion with Becke-Johnson damping (D3BJ) (Grimme et al., 2011) were performed with a higher basis set BS1 (BS1 = 6–31++G(d,p) basis set for all atoms). The use of SMD-B3LYP-D3(BJ)/BS1 to calculate compound Gibbs energy was reported in the literature for mechanistic investigation (Markovic et al., 2010; Kleine et al., 2011).
Chemical Synthesis
Synthesis of 4a-4l
A mixture of 2-aminoacetophenone 1 (1 mmol) and isothiocyanatobenzene 2 (1.1 mmol) in acetonitrile (5 ml) was stirred at 82°C for 4 h. After completion, the reaction solvent was reduced to half and upon cooling a white or yellow precipitate was obtained. Recrystallization from ethanol produced white or yellow solid.
4-Methylene-3-phenyl-3,4-dihydroquinazoline-2(1H)-thione (4a)
Yellow solid, m.p. 217~218°C; 1H NMR (400 MHz, Chloroform-d) δ 9.98 (s, 1H), 7.56 (dd, J = 8.3, 6.8 Hz, 2H), 7.52–7.45 (m, 2H), 7.31–7.26 (m, 3H), 7.24 (d, J = 1.3 Hz, 1H), 7.07 (td, J = 7.7, 1.2 Hz, 1H), 6.91 (dd, J = 8.0, 1.1 Hz, 1H), 4.82 (d, J = 2.6 Hz, 1H), 3.69 (d, J = 2.6 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 174.18, 141.83, 141.66, 133.23, 130.65, 130.07, 128.94, 128.67, 124.40, 123.83, 118.26, 114.84, 90.14. HRMS (ESI): Calc. for C15H12N2S [M + H]+: 253.0794, found 253.2068.
4-Methylene-3-(o-tolyl)-3,4-dihydroquinazoline-2(1H)-thione (4b)
Brown powder, m.p. 214~215°C; 1H NMR (500 MHz, DMSO-d6) δ 11.64 (s, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.40–7.36 (m, 2H), 7.36–7.32 (m, 2H), 7.16–7.11 (m, 2H), 7.09 (dd, J = 7.9, 1.2 Hz, 1H), 4.89 (d, J = 2.1 Hz, 1H), 3.38 (d, J = 2.2 Hz, 1H), 2.13 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 173.38, 140.67, 140.62, 135.83, 134.15, 131.68, 131.29, 129.36, 128.86, 128.10, 124.49, 124.42, 117.65, 115.58, 88.04, 17.16. HRMS (ESI) m/z: Calc. for C16H14N2S [M + H]+: 267.0950, found 257.0933.
3-(3-Methoxyphenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4c)
Yellow powder, m.p. 221~225°C; 1H NMR (500 MHz, DMSO-d6) δ 11.63 (s, 1H), 7.66 (dd, J = 8.1, 1.3 Hz, 1H), 7.47–7.41 (m, 1H), 7.37 (ddd, J = 8.4, 7.3, 1.3 Hz, 1H), 7.14 (dd, J = 8.1, 1.2 Hz, 1H), 7.09 (ddd, J = 8.3, 7.3, 1.2 Hz, 1H), 7.03–6.97 (m, 1H), 6.83–6.79 (m, 2H), 4.92 (d, J = 2.2 Hz, 1H), 3.77 (s, 3H), 3.51 (d, J = 2.2 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 173.95, 160.93, 143.12, 141.84, 134.13, 131.20, 130.93, 124.40, 124.36, 121.71, 118.04, 115.51, 115.30, 114.15, 89.25, 55.81. HRMS (ESI) m/z: Calc. for C16H14N2OS [M + H]+: 283.0900, found 283.0864.
3-(4-Methoxyphenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4d)
White powder, m.p. 212~214°C; 1H NMR (400 MHz, Chloroform-d) δ 9.19 (s, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 7.9 Hz, 1H), 7.22 (d, J = 8.5 Hz, 2H), 7.16–7.04 (m, 3H), 6.87 (s, 1H), 4.85 (d, J = 2.1 Hz, 1H), 3.89 (s, 3H), 3.80 (d, J = 2.1 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 174.47, 159.01, 142.36, 134.93, 134.14, 131.18, 130.57, 124.44, 124.34, 118.05, 115.50, 115.39, 89.18, 55.76. HRMS (ESI) m/z: calc. for C16H14N2OS [M + H]+: 283.0900, found 283.0912.
3-(2-Chlorophenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4e)
Yellow powder, m.p. 203~205°C; 1H NMR (500 MHz, DMSO-d6) δ 11.76 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.65 (dd, J = 7.8, 1.8 Hz, 1H), 7.55–7.45 (m, 2H), 7.44–7.36 (m, 2H), 7.17–7.08 (m, 2H), 4.93 (d, J = 2.6 Hz, 1H), 3.38 (d, J = 2.6 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 173.56, 140.37, 138.85, 133.99, 132.29, 132.00, 131.38, 130.96, 130.54, 129.24, 124.56, 124.52, 117.66, 115.68, 88.17. HRMS (ESI) m/z: calc. for C15H11ClN2S [M + H]+: 287.0404, found 287.0389.
3-(3-Chlorophenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4f)
White powder, m.p. 234~236°C; 1H NMR (400 MHz, DMSO-d6) δ 11.79 (d, J = 2.5 Hz, 1H), 7.69 (t, J = 7.7 Hz, 1H), 7.59 (, J = 7.9, 2.6 Hz, 1H), 7.56–7.49 (m, 1H), 7.46–7.35 (m, 2H), 7.27 (dt, J = 7.8, 1.6 Hz, 1H), 7.21–7.16 (m, 1H), 7.12 (dd, J = 9.8, 5.6 Hz, 1H), 4.96 (d, J = 4.1, 2.2 Hz, 1H), 3.46 (d, J = 2.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 173.93, 143.32, 141.91, 134.21, 134.06, 131.86, 131.30, 129.90, 128.81, 128.77, 124.49, 124.42, 118.03, 115.64, 89.35. HRMS (ESI) m/z: calc. for C15H11ClN2S [M + H]+: 287.0404, found 287.0423.
3-(4-Chlorophenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4g)
White powder, m.p. 240~245°C; 1H NMR (500 MHz, DMSO-d6) δ 11.72 (s, 1H), 7.68 (dd, J = 8.2, 1.4 Hz, 1H), 7.61–7.57 (m, 2H), 7.38 (ddd, J = 8.4, 7.2, 1.3 Hz, 1H), 7.31–7.26 (m, 2H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 7.10 (ddd, J = 8.3, 7.3, 1.3 Hz, 1H), 4.94 (d, J = 2.4 Hz, 1H), 3.45 (d, J = 2.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 174.01, 141.96, 141.00, 134.06, 133.08, 131.74, 131.30, 130.43, 124.49, 124.45, 118.00, 115.61, 89.33. HRMS (ESI) m/z: calc. for C15H11ClN2S [M + H]+: 287.0404, found 287.0367.
3-(2-Methoxy-6-methylphenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4h)
Yellow powder, m.p. 236~237°C; 1H NMR (500 MHz, DMSO-d6) δ 11.54 (s, 1H), 7.64 (dd, J = 8.1, 1.3 Hz, 1H), 7.35 (ddd, J = 8.4, 7.3, 1.3 Hz, 1H), 7.23–7.19 (m, 1H), 7.12 (dd, J = 8.2, 1.2 Hz, 1H), 7.10–7.05 (m, 2H), 6.95 (dd, J = 2.2, 0.8 Hz, 1H), 4.82 (d, J = 2.1 Hz, 1H), 3.71 (s, 3H), 3.50 (d, J = 2.1 Hz, 1H), 2.28 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 174.12, 152.82, 140.82, 134.17, 131.13, 130.83, 130.60, 130.52, 129.80, 124.42, 124.30, 117.86, 115.47, 113.43, 87.98, 56.21, 20.42. HRMS (ESI) m/z: calc. for C17H16N2OS [M + H]+: 297.1056, found 297.1023.
3-(2,4-Dichlorophenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4i)
White powder, m.p. 236~237°C; 1H NMR (500 MHz, DMSO-d6) δ 11.82 (s, 1H), 7.85 (d, J = 2.3 Hz, 1H), 7.71 (dd, J = 8.1, 1.3 Hz, 1H), 7.60 (dd, J = 8.5, 2.4 Hz, 1H), 7.48 (d, J = 8.5 Hz, 1H), 7.39 (ddd, J = 8.4, 7.2, 1.3 Hz, 1H), 7.18–7.13 (m, 1H), 7.11 (dd, J = 8.1, 1.1 Hz, 1H), 4.96 (d, J = 2.7 Hz, 1H), 3.45 (d, J = 2.8 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 173.48, 140.17, 138.00, 134.16, 133.91, 133.65, 133.46, 131.44, 130.64, 129.54, 124.66, 124.55, 117.61, 115.75, 88.33. HRMS (ESI) m/z: calc. for C15H10Cl2N2S [M + H]+: 321.0015, found 321.0023.
3-Benzyl-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4j)
White powder, m.p. 241~242°C (Morgenstern and Richter, 1992, 215~217°C); 1H NMR (500 MHz, DMSO-d6) δ 11.65 (s, 1H), 7.60 (dd, J = 8.1, 1.3 Hz, 1H), 7.37–7.27 (m, 5H), 7.27–7.21 (m, 1H), 7.14 (dd, J = 8.1, 1.2 Hz, 1H), 7.07 (ddd, J = 8.3, 7.3, 1.2 Hz, 1H), 5.68 (s, 2H), 4.98 (d, J = 2.9 Hz, 1H), 4.31 (d, J = 2.9 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 174.87, 138.11, 136.39, 133.73, 131.01, 128.93, 127.28, 126.59, 124.59, 124.27, 118.02, 115.42, 89.39, 52.81. HRMS (ESI) m/z: calc. for C16H14N2S [M + H]+: 267.0950, found 267.0932.
4-Methylene-3-propyl-3,4-dihydroquinazoline-2(1H)-thione (4k)
White powder, m.p. 168~169°C; 1H NMR (500 MHz, DMSO-d6) δ 11.35 (s, 1H), 7.65 (dd, J = 8.3, 1.3 Hz, 1H), 7.33–7.28 (m, 1H), 7.08–7.04 (m, 2H), 5.05 (d, J = 2.9 Hz, 1H), 4.52 (d, J = 2.9 Hz, 1H), 4.25 (s, 2H), 1.78–1.68 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 173.70, 138.33, 133.85, 130.88, 124.37, 124.34, 118.06, 115.18, 87.82, 50.78, 18.16, 11.31. HRMS (ESI) m/z: (M+H+); calc. for C12H14N2S [M + H]+: 219.0950, found 219.0938.
3-(4-Isothiocyanatophenyl)-4-methylene-3,4-dihydroquinazoline-2(1H)-thione (4l)
Yellow powder, m.p. 236~239°C; 1H NMR (500 MHz, DMSO-d6) δ 11.74 (s, 1H), 7.68 (dd, J = 8.1, 1.3 Hz, 1H), 7.61–7.56 (m, 2H), 7.38 (ddd, J = 9.5, 6.7, 1.4 Hz, 1H), 7.35–7.32 (m, 2H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 7.10 (ddd, J = 8.3, 7.2, 1.2 Hz, 1H), 4.94 (d, J = 2.4 Hz, 1H), 3.43 (d, J = 2.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 173.92, 141.93, 141.31, 134.70, 134.05, 131.51, 131.32, 130.13, 128.00, 124.50, 124.45, 118.00, 115.63, 89.35. HRMS (ESI) m/z: calc. for C16H12N3S2 [M + H]+: 310.0467, found 310.0421.
Synthesis of 4l*
A mixture of 4l (1 mmol) and ethylamine (1.1 mmol) in dichloromethane (5 ml) were refluxed for 4 h. After cooling it down to the room temperature, the precipitate was filtered and washed with ethyl acetate. Recrystallization from ethanol produced yellow solid.
1-Ethyl-3-(4-(4-methylene-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)phenyl)thiourea (4l*)
Yellow solid. m.p. 197~202°C; 1H NMR (400 MHz, DMSO-d6) δ 10.33 (s, 1H), 9.69 (s, 1H), 7.94 (s, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 8.4 Hz, 2H), 7.42–7.33 (m, 1H), 7.16–7.07 (m, 3H), 4.93 (d, J = 2.2 Hz, 1H), 3.55 (d, J = 2.1 Hz, 1H), 3.50 (q, J = 6.6 Hz, 2H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 180.40, 174.19, 142.15, 139.56, 137.43, 134.11, 131.21, 129.62, 124.45, 124.38, 121.66, 118.04, 115.51, 89.45, 39.09, 14.61. HRMS (ESI) m/z: calc. for C18H18N4S2 [M + H]+: 355.1046, found 355.1057.
Synthesis of 6a and 6r
A mixture of 2-aminoacetophenone (1 mmol) and isocyanatobenzene/isocyanatocyclohexane (1.1 mmol) in acetonitrile (5 ml) was stirred at 82°C for 4 h. After completion, the reaction solvent was reduced to half and upon cooling a white or yellow precipitate was obtained. Recrystallization from ethanol produced white solid.
1-(2-Acetylphenyl)-3-phenylurea (6a)
White solid. m.p. 218–221°C (Iwamura et al., 1980, 223°C); 1H NMR (400 MHz, Chloroform-d) δ 11.34 (s, 1H), 8.60 (dd, J = 8.6, 1.2 Hz, 1H), 7.85 (dd, J = 8.1, 1.6 Hz, 1H), 7.52 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.48–7.39 (m, 2H), 7.38–7.29 (m, 2H), 7.16–7.08 (m, 1H), 7.03 (ddd, J = 8.3, 7.2, 1.2 Hz, 1H), 6.94 (s, 1H), 2.63 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 203.04, 152.81, 142.43, 137.99, 135.18, 131.68, 129.16, 124.09, 121.23, 120.99, 120.84, 120.19, 28.49. HRMS (ESI) m/z: calc. for C15H13N2O2 [M + H]+:255.1128, found 255.1099.
1-(2-Acetylphenyl)-3-cyclohexylurea (6r)
White solid. m.p. 175–178°C; 1H NMR (500 MHz, DMSO-d6) δ 10.43 (s, 1H), 8.38 (dd, J = 8.6, 1.2 Hz, 1H), 7.94 (dd, J = 8.0, 1.6 Hz, 1H), 7.48 (ddd, J = 8.7, 7.1, 1.6 Hz, 1H), 7.36 (br s, 1H), 6.99 (ddd, J = 8.2, 7.2, 1.2 Hz, 1H), 3.48–3.38 (m, 1H), 2.61 (s, 3H), 1.84–1.75 (m, 2H), 1.74–1.65 (m, 2H), 1.34–1.22 (m, 2H), 1.22–1.00 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ 202.59, 154.47, 142.67, 134.55, 132.40, 121.79, 120.34, 119.91, 48.74, 33.32, 29.16, 25.75, 25.15. HRMS (ESI) m/z: calc. for C15H20N2O2 [M + H]+: 261.1598, found 261.1572.
Synthesis of 7a-7ab
A mixture of 2-aminoacetophenone 1 (1 mmol), isocyanatobenzene 5 (1.1 mmol) and a drop of aqueous NaOH solution (5 M, 10 mol%) in acetonitrile (5 ml) were stirred at room temperature. After completion, the reaction solvent was reduced to half and upon cooling a white or yellow precipitate was obtained. Recrystallization from ethanol produced white solid.
4-Methylene-3-phenyl-3,4-dihydroquinazolin-2(1H)-one (7a)
White solid. m.p. 186~188°C (Sbei et al., 2018, 188~190°C); 1H NMR (400 MHz, Chloroform-d) δ 8.18 (s, 1H), 7.59–7.49 (m, 3H), 7.47–7.40 (m, 1H), 7.35–7.28 (m, 2H), 7.24 (dd, J = 7.9, 1.3 Hz, 1H), 7.06–6.96 (m, 1H), 6.72 (dd, J = 8.1, 1.2 Hz, 1H), 4.74 (d, J = 2.2 Hz, 1H), 3.68 (d, J = 2.2 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 150.88, 143.31, 138.42, 135.16, 130.27, 129.94, 129.19, 128.34, 123.94, 122.73, 117.03, 115.09, 87.43. HRMS (ESI) m/z: calc. for C15H12N2O [M + H]+: 237.1022, found 237.1908.
4-Methylene-3-(o-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7b)
White solid. m.p. 204~207°C; 1H NMR (500 MHz, DMSO-d6) δ 10.30 (s, 1H), 7.68 (dd, J = 8.1, 1.3 Hz, 1H), 7.42–7.37 (m, 1H), 7.37–7.30 (m, 3H), 7.19–7.15 (m, 1H), 7.01 (td, J = 7.8, 1.2 Hz, 1H), 6.96 (dd, J = 8.1, 1.2 Hz, 1H), 4.75 (d, J = 1.8 Hz, 1H), 3.33 (d, J = 1.8 Hz, 1H), 2.11 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 149.27, 142.44, 137.75, 136.56, 136.42, 131.50, 130.90, 129.77, 128.77, 127.89, 124.63, 122.61, 116.29, 115.29, 85.21, 17.19. HRMS (ESI) m/z: calc. for C16H14N2O [M + H]+: 251.1179, found 251.1121.
4-Methylene-3-(m-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7c)
Yellow powder. m.p. 206~208°C; 1H NMR (500 MHz, DMSO-d6) δ 10.27 (s, 1H), 7.65 (dd, J = 8.0, 1.3 Hz, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.31 (ddd, J = 8.4, 6.0, 1.4 Hz, 1H), 7.25–7.22 (m, 1H), 7.09–7.03 (m, 2H), 6.99 (ddd, J = 8.3, 7.2, 1.2 Hz, 1H), 6.95 (dd, J = 8.1, 1.2 Hz, 1H), 4.76 (d, J = 1.8 Hz, 1H), 3.43 (d, J = 1.8 Hz, 1H), 2.35 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 149.71, 143.72, 139.63, 139.04, 136.37, 130.82, 130.16, 129.92, 129.07, 126.71, 124.53, 122.57, 116.59, 115.22, 86.38, 21.24. HRMS (ESI) m/z: calc. for C16H14N2O [M + H]+: 251.1179, found 251.1098.
4-Methylene-3-(p-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7d)
Yellow powder. m.p. 222~224°C; 1H NMR (500 MHz, DMSO-d6) δ 10.26 (s, 1H), 7.66 (dd, J = 8.1, 1.3 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.31 (td, J = 8.0 Hz, 1.2 Hz, 1H), 7.15–7.11 (m, 2H), 7.05 (d, J = 8.3 Hz, 1H), 7.00 (td, J = 8.1 Hz, 1.2 Hz, 1H), 6.94 (d, J = 7.9 Hz, 1H), 4.76 (d, J = 1.8 Hz, 1H), 3.43 (d, J = 1.8 Hz, 1H), 2.37 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 149.77, 143.81, 138.18, 137.74, 136.49, 136.36, 130.83, 130.65, 129.49, 124.56, 122.56, 118.61, 116.58, 115.20, 86.25, 21.21. HRMS (ESI) m/z: calc. for C16H14N2O [M + H]+: 251.1179, found 251.1093.
3-(2-Fluorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7e)
Light yellow powder. m.p. 220~224°C; 1H NMR (500 MHz, DMSO-d6) δ 10.42 (s, 1H), 7.69 (dd, J = 8.1, 1.3 Hz, 1H), 7.55–7.48 (m, 1H), 7.46–7.39 (m, 2H), 7.39–7.31 (m, 2H), 7.03 (ddd, J = 8.3, 7.3, 1.2 Hz, 1H), 6.97 (dd, J = 8.1, 1.3 Hz, 1H), 4.82 (d, J = 2.2 Hz, 1H), 3.48 (dd, J = 2.2, 0.8 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ158.65 (d, J = 248 Hz), 149.25, 142.67, 136.14, 132.07, 131.07, 130.98 (d, J = 7.6 Hz), 126.12 (d, J = 12.6 Hz), 125.97 (d, J = 3.8 Hz), 124.66, 122.85, 117.15 (d, J = 18.9 Hz), 116.21, 115.41, 85.80. HRMS (ESI) m/z: calc. for C15H11FN2O [M + H]+: 255.0928, found 255.0876.
3-(3-Fluorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7f)
Brown powder. m.p. 228~230°C; 1H NMR (600 MHz, DMSO-d6) δ 10.37 (s, 1H), 7.68 (dd, J = 8.1, 1.3 Hz, 1H), 7.60–7.54 (m, 1H), 7.35–7.27 (m, 2H), 7.24 (dt, J = 9.7, 2.2 Hz, 1H), 7.15 (dd, J = 7.8, 1.0 Hz, 1H), 7.01 (ddd, J = 8.3, 7.4, 1.2 Hz, 1H), 6.95 (dd, J = 8.1, 1.2 Hz, 1H), 4.81 (d, J = 2.1 Hz, 1H), 3.44 (d, J = 2.1 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 163.18 (d, J = 245 Hz), 149.52, 143.48, 140.70 (d, J = 9.1 Hz), 136.23, 131.64 (d, J = 9.1 Hz), 130.93, 126.23 (d, J = 3.0 Hz), 124.57, 122.67, 117.32 (d, J = 22.7 Hz), 116.51, 115.56 (d, J = 21 Hz), 115.28, 86.47. HRMS (ESI) m/z: calc. for C15H11FN2O [M + H]+: 255.0928, found 255.0885.
3-(4-Fluorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7g)
Light yellow powder. m.p. 245~247°C (Molina et al., 1993, 136~138°C); 1H NMR (500 MHz, DMSO-d6) δ 10.33 (s, 1H), 7.67 (dd, J = 8.1, 1.3 Hz, 1H), 7.36–7.30 (m, 5H), 7.01 (ddd, J = 8.2, 7.3, 1.2 Hz, 1H), 6.95 (dd, J = 8.1, 1.2 Hz, 1H), 4.79 (d, J = 2.0 Hz, 1H), 3.42 (d, J = 2.0 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 161.80 (d, J = 246 Hz), 149.74, 143.82, 136.29, 135.31 (d, J = 2.5 Hz), 131.97 (d, J = 8.8 Hz), 130.90, 124.58, 122.63, 117.02 (d, J = 22.7 Hz), 116.54, 115.27, 86.38. HRMS (ESI) m/z: calc. for C15H11FN2O [M + H]+: 255.0928, found 255.0943.
3-(2-Chlorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7h)
Light yellow powder. m.p. 229~231°C; 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H), 7.71 (ddd, J = 9.3, 7.2, 1.9 Hz, 2H), 7.58–7.44 (m, 3H), 7.36 (ddd, J = 8.3, 7.4, 1.3 Hz, 1H), 7.09–6.98 (m, 2H), 4.82 (d, J = 2.2 Hz, 1H), 3.35 (d, J = 2.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.10, 142.19, 136.30, 136.22, 133.08, 132.16, 131.00, 130.84, 130.55, 129.17, 124.64, 122.75, 116.26, 115.47, 85.50. HRMS (ESI) m/z: calc. for C15H11ClN2O [M + H]+: 271.0633, found 271.0598.
3-(3-Chlorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7i)
White powder. m.p. 227~229°C; 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H), 7.70 (dd, J = 8.1, 1.3 Hz, 1H), 7.63–7.51 (m, 2H), 7.46 (t, J = 2.0 Hz, 1H), 7.39–7.29 (m, 2H), 7.08–6.95 (m, 2H), 4.84 (d, J = 2.1 Hz, 1H), 3.44 (d, J = 2.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.57, 143.58, 140.56, 136.28, 134.12, 131.75, 130.95, 130.12, 128.91, 128.67, 124.58, 122.69, 116.54, 115.36, 86.54. HRMS (ESI) m/z: calc. for C15H11ClN2O [M + H]+: 271.0633, found 271.0569.
3-(4-Chlorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7j)
Light green powder. m.p. 223~225°C (Sbei et al., 2018, 214~217°C); 1H NMR (400 MHz, DMSO-d6) δ 10.42 (s, 1H), 7.70 (dd, J = 8.1, 1.3 Hz, 1H), 7.64–7.57 (m, 2H), 7.40–7.31 (m, 3H), 7.08–6.96 (m, 2H), 4.83 (d, J = 2.1 Hz, 1H), 3.45 (d, J = 2.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.61, 143.63, 138.05, 136.29, 133.00, 131.90, 130.93, 130.27, 124.58, 122.67, 116.54, 115.33, 86.49. HRMS (ESI) m/z: calc. for C15H11ClN2O [M + H]+: 271.0633, found 271.0896.
4-Methylene-3-(2-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-2(1H)-one (7k)
Yellow solid. m.p. 309~312°C; 1H NMR (500 MHz, DMSO-d6) δ 10.42 (s, 1H), 7.91 (dd, J = 7.9, 1.5 Hz, 1H), 7.86 (td, J = 7.8, 1.5 Hz, 1H), 7.70 (td, J = 8.5, 1.4 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.34 (ddd, J = 7.8, 7.3, 1.3 Hz, 1H), 7.02 (ddd, J = 7.8, 7.4, 1.3 Hz, 1H), 6.97 (dd, J = 8.1, 1.3 Hz, 1H), 4.84 (d, J = 2.3 Hz, 1H), 3.26 (d, J = 2.4 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 149.53, 143.52, 136.70, 136.12, 134.73, 133.11, 130.98, 129.79, 128.16 (m), 127.83, 124.87, 124.53, 122.76, 116.34, 115.40, 86.66. HRMS (ESI) m/z: calc. for C16H12F3N2O [M + H]+: 305.0896, found 305.0822.
3-Mesityl-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7l)
White powder. m.p. 230~232°C; 1H NMR (500 MHz, DMSO-d6) δ 10.31 (s, 1H), 7.69 (dd, J = 8.1, 1.3 Hz, 1H), 7.32 (ddd, J = 8.3, 7.3, 1.3 Hz, 1H), 7.00 (d, J = 4.7 Hz, 3H), 6.99–6.95 (m, 1H), 4.70 (d, J = 1.7 Hz, 1H), 3.37 (d, J = 1.6 Hz, 1H), 2.29 (s, 3H), 2.04 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 148.95, 141.08, 137.57, 136.51, 135.94, 133.93, 130.90, 129.66, 124.71, 122.59, 116.03, 115.32, 83.80, 21.07, 17.37. HRMS (ESI) m/z: calc. for C18H18N2O [M + H]+: 279.1492, found 279.1421.
3-(2,6-Diethylphenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7m)
White solid. m.p. 166~168°C; 1H NMR (500 MHz, DMSO-d6) δ 10.33 (s, 1H), 7.69 (dd, J = 8.2, 1.3 Hz, 1H), 7.39–7.29 (m, 2H), 7.25 (d, J = 7.6 Hz, 2H), 7.01 (ddd, J = 8.3, 7.3, 1.2 Hz, 1H), 6.97 (dd, J = 8.1, 1.2 Hz, 1H), 4.74 (d, J = 1.7 Hz, 1H), 3.31 (d, J = 1.8 Hz, 1H), 2.42 (q, J = 7.5 Hz, 4H), 1.12 (t, J = 7.6 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 149.43, 142.12, 141.77, 136.44, 135.36, 132.56, 130.97, 128.88, 127.15, 124.68, 122.66, 120.86, 116.00, 115.32, 84.83, 24.84, 23.79, 15.07, 14.50. HRMS (ESI) m/z: calc. for C19H20N2O [M + H]+: 293.1648, found 293.1606.
3-(2,4-Difluorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7n)
White powder. m.p. 226~228°C; 1H NMR (500 MHz, DMSO-d6) δ 10.46 (s, 1H), 7.70 (dd, J = 8.0, 1.3 Hz, 1H), 7.55–7.47 (m, 2H), 7.34 (ddd, J = 8.4, 7.3, 1.3 Hz, 1H), 7.28–7.22 (m, 1H), 7.03 (ddd, J = 8.2, 7.3, 1.2 Hz, 1H), 6.97 (dd, J = 8.1, 1.2 Hz, 1H), 4.84 (d, J = 2.4 Hz, 1H), 3.52 (d, J = 2.3 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 162.30 (dd, J = 247.5, 11.8 Hz), 158.86 (dd, J = 251.1, 13.2 Hz), 149.26, 142.64, 136.08, 133.28 (dd, J = 9.9, 2.1 Hz), 131.11, 124.69, 122.89, 122.70 (dd, J = 13.4, 4.0 Hz), 116.18, 115.44, 113.07 (dd, J = 22.5, 3.6 Hz), 105.78 (dd, J = 27.1, 24.1 Hz), 85.90. HRMS (ESI) m/z: (M+H+); calc. for C15H10F2N2O [M + H]+: 273.0834, found 273.0789.
3-(3,4-Dichlorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7o)
White powder. m.p. 234~236°C; 1H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H), 7.82 (dd, J = 8.5, 1.2 Hz, 1H), 7.76–7.67 (m, 2H), 7.41–7.31 (m, 2H), 7.09–7.00 (m, 1H), 6.98 (dd, J = 8.1, 1.2 Hz, 1H), 4.85 (d, J = 2.3 Hz, 1H), 3.49 (d, J = 2.3 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.53, 143.45, 139.15, 136.28, 132.36, 132.08, 131.33, 130.97, 130.67, 124.59, 122.71, 116.53, 115.41, 86.71. HRMS (ESI) m/z: calc. for C15H10Cl2N2O [M + H]+: 305.0243, found 305.0185.
3-Benzyl-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7p)
White solid. m.p. 212~214°C; 1H NMR (500 MHz, DMSO-d6) δ 10.28 (s, 1H), 7.62 (d, J = 8.1 Hz, 1H), 7.36–7.19 (m, 6H), 7.00–6.92 (m, 2H), 5.01 (s, 2H), 4.82 (d, J = 2.4 Hz, 1H), 4.12 (d, J = 2.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 150.60, 140.23, 137.50, 136.08, 130.65, 128.92, 127.21, 126.81, 124.40, 122.62, 116.46, 115.12, 85.70, 46.30. HRMS (ESI) m/z: calc. for C16H14N2O [M + H]+: 251.1179, found 251.1085.
4-Methylene-3-propyl-3,4-dihydroquinazolin-2(1H)-one (7q)
Brown powder. m.p. 114~117°C; 1H NMR (500 MHz, DMSO-d6) δ 10.06 (s, 1H), 7.65 (dd, J = 8.2, 1.3 Hz, 1H), 7.26 (ddd, J = 8.4, 7.2, 1.3 Hz, 1H), 6.96 (ddd, J = 8.2, 7.3, 1.3 Hz, 1H), 6.87 (dd, J = 8.1, 1.2 Hz, 1H), 4.87 (d, J = 2.3 Hz, 1H), 4.28 (d, J = 2.4 Hz, 1H), 3.70 (t, J = 7.6 Hz, 2H), 1.65–1.55 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 150.07, 140.25, 136.15, 130.51, 124.45, 122.41, 116.51, 114.90, 84.02, 44.26, 18.91, 11.57. HRMS (ESI) m/z: calc. for C12H14N2S [M + H]+: 203.1179, found 203.1089.
3-Cyclohexyl-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7r)
White powder. m.p. 138~140°C; 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 7.58 (dd, J = 8.0, 1.3 Hz, 1H), 7.25 (td, J = 7.6, 1.3 Hz, 1H), 7.01–6.89 (m, 1H), 6.87 (dd, J = 8.1, 1.2 Hz, 1H), 4.89 (d, J = 2.4 Hz, 1H), 4.53 (d, J = 2.4 Hz, 1H), 3.84 (tt, J = 12.0, 3.6 Hz, 1H), 2.39 (qd, J = 12.5, 3.6 Hz, 2H), 1.82–1.62 (m, 4H), 1.45–1.01 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 150.56, 142.08, 136.27, 130.15, 124.40, 122.27, 118.46, 114.34, 88.15, 58.37, 29.02, 26.42, 25.70. HRMS (ESI) m/z: calc. for C15H18N2O [M + H]+: 243.1492, found 243.1464.
3-(4-Isocyanatophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7s)
Light yellow powder. m.p. 230~232°C; 1H NMR (500 MHz, DMSO-d6) δ 11.75 (s, 1H), 7.69 (dd, J = 8.1, 1.4 Hz, 1H), 7.57 (t, J = 7.9 Hz, 1H), 7.53–7.49 (m, 1H), 7.43–7.34 (m, 2H), 7.28–7.22 (m, 1H), 7.15 (dd, J = 8.1, 1.3 Hz, 1H), 7.13–7.08 (m, 1H), 4.95 (d, J = 2.5 Hz, 1H), 3.44 (d, J = 2.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 173.93, 143.32, 141.91, 134.18, 134.05, 131.85, 131.30, 129.90, 128.81, 128.79, 124.49, 124.43, 118.03, 115.63, 89.34. HRMS (ESI) m/z: calc. for C16H11N3O2 [M + H]+: 278.0924, found 278.0882.
6-Chloro-3-(2-fluorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7t)
White powder. m.p. 258.9~261.3°C; 1H NMR (400 MHz, DMSO-d6) δ 10.69 (s, 1H), 7.80 (d, J = 2.3 Hz, 1H), 7.56–7.48 (m, 1H), 7.47–7.32 (m, 4H), 6.99 (dd, J = 8.7, 1.9 Hz, 1H), 4.94 (d, J = 2.4Hz, 1H), 3.52 (d, J = 2.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 158.58 (d, J = 249.0 Hz), 149.05, 141.55, 135.32, 132.01, 131.08 (d, J = 8.0 Hz), 130.87, 126.79, 126.01 (d, J = 3.9 Hz), 125.86, 124.15, 117.97, 117.32 (d, J = 9.7 Hz), 117.08, 87.28. HRMS (ESI) m/z: calc. for C16H14FN2O+ [M + H]+: 289.0538, found 289.0552.
6-Chloro-3-(3-chlorophenyl)-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7u)
White powder. m.p. 245.6~247.2°C;1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H), 7.78 (d, J = 2.3 Hz, 1H), 7.61–7.48 (m, 2H), 7.45 (t, J = 1.9 Hz, 1H), 7.38 (dd, J = 8.6, 2.3 Hz, 1H), 7.29 (dt, J = 7.6, 1.7 Hz, 1H), 6.96 (d, J = 8.6 Hz, 1H), 4.94 (d, J = 2.4 Hz, 1H), 3.47 (d, J = 2.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.28, 142.39, 140.29, 135.23, 134.14, 131.78, 130.75, 130.04, 128.82, 128.76, 126.69, 124.10, 118.31, 117.10, 88.14. HRMS (ESI) m/z: calc. for C15H10Cl2N2O [M + H]+: 305.0243, found 305.0229.
6-Chloro-4-methylene-3-(m-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7v)
White powder. m.p. 201.7~202.4°C; 1H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H), 7.74 (d, J = 2.3 Hz, 1H), 7.45–7.32 (m, 2H), 7.24 (d, J = 7.7 Hz, 1H), 7.11–7.02 (m, 2H), 6.96 (d, J = 8.6 Hz, 1H), 4.88 (d, J = 2.2 Hz, 1H), 3.49 (d, J = 2.2 Hz, 1H), 2.35 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 149.44, 142.53, 139.70, 138.79, 135.35, 130.63, 130.08, 129.97, 129.17, 126.63, 126.57, 124.03, 118.34, 117.02, 87.93, 21.23. HRMS (ESI) m/z: calc. for C16H13ClN2O [M + H]+: 287.0795, found 287.0802.
6-Chloro-4-methylene-3-(o-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7w)
White powder. m.p. 221.2~222.9°C; 1H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H), 7.79 (d, J = 2.3 Hz, 1H), 7.42–7.32 (m, 5H), 7.20–7.15 (m, 1H), 6.96 (d, J = 8.6 Hz, 1H), 4.88 (d, J = 2.1 Hz, 1H), 3.36 (d, J = 2.2 Hz, 2H), 2.10 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 148.99, 141.27, 137.48, 136.50, 135.39, 131.55, 130.75, 129.71, 128.89, 127.95, 126.65, 124.17, 118.06, 117.10, 86.86, 17.15. HRMS (ESI) m/z: calc. for C16H13FN2O [M + H]+: 285.0789, found 285.0762.
3-(3-Chlorophenyl)-7-methyl-4-methylene-3,4-dihydroquinazolin-2(1H)-one(7x)
White powder. m.p. 214.8~215.9°C; 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 7.60–7.48 (m, 3H), 7.45–7.39 (m, 1H), 7.27 (dt, J = 7.7, 1.7 Hz, 1H), 6.84 (dd, J = 8.2, 1.7 Hz, 1H), 6.75 (s, 1H), 4.74 (d, J = 2.0 Hz, 1H), 3.35 (d, J = 2.0 Hz, 1H), 2.28 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 149.66, 143.54, 140.75, 140.54, 136.14, 134.08, 131.70, 130.11, 128.90, 128.63, 124.53, 123.75, 115.28, 114.02, 85.57, 21.38. HRMS (ESI) m/z: calc. for C16H13ClN2O [M + H]+: 285.0789, found 285.0762.
3-(2-Fluorophenyl)-7-methyl-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7y)
White powder. m.p. 224.3~224.9°C; 1H NMR (400 MHz, DMSO-d6) δ 10.44 (s, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.55–7.48 (m, 1H), 7.45–7.39 (m, 2H), 7.35 (ddd, J = 7.9, 7.2, 1.4 Hz, 1H), 4.75 (d, J = 2.1 Hz, 1H), 3.43 (d, J = 2.1 Hz, 1H), 2.08 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 156.56 (d, J = 247 Hz), 147.29, 140.58, 138.80, 133.98, 129.98, 128.81 (d, J = 8.0 Hz), 124.08 (d, J = 13.3 Hz), 123.80 (d, J = 3.5 Hz), 122.48, 121.78, 115.02 (d, J = 19.7 Hz), 113.29, 111.62, 82.67, 19.26. HRMS (ESI) m/z: calc. for C16H13FN2O [M + H]+: 269.1085, found 269.1062.
7-Methyl-4-methylene-3-(m-tolyl)-3,4-dihydroquinazolin-2(1H)-one (7z)
Blackish green powder. m.p. 161.3~163.6°C; 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.23 (d, J = 7.7 Hz, 1H), 7.09–7.01 (m, 2H), 6.82 (dd, J = 8.2, 1.7 Hz, 1H), 6.74 (s, 1H), 4.69 (d, J = 1.7 Hz, 1H), 3.36 (d, J = 1.7 Hz, 1H), 2.35 (s, 3H), 2.28 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 149.82, 143.69, 140.61, 139.60, 139.05, 136.26, 130.18, 129.89, 129.04, 126.73, 124.48, 123.64, 115.19, 114.07, 85.39, 21.37, 21.22. HRMS (ESI) m/z: calc. for C17H16N2O [M + H]+: 265.1335, found 265.1358.
3-Benzyl-7-methyl-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7aa)
White powder. m.p. 236.8~238.4°C; 1H NMR (400 MHz, DMSO-d6) δ 10.28 (s, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.32 (t, J = 7.5 Hz, 2H), 7.28–7.19 (m, 3H), 6.79 (dd, J = 8.2, 1.7 Hz, 1H), 6.75 (s, 1H), 4.99 (s, 2H), 4.74 (d, J = 2.3 Hz, 1H), 4.06 (d, J = 2.4 Hz, 1H), 2.26 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 150.72, 140.41, 140.22, 137.58, 135.99, 128.91, 127.18, 126.78, 124.36, 123.68, 115.12, 113.94, 84.68, 46.23, 21.33. HRMS (ESI) m/z: calc. for C17H16N2O [M + H]+: 265.1335, found 265.1326.
3-Benzyl-6-chloro-4-methylene-3,4-dihydroquinazolin-2(1H)-one (7ab)
White powder. m.p. 237.2~238.9°C; 1H NMR (400 MHz, DMSO-d6) δ 10.44 (s, 1H), 7.71 (d, J = 2.2 Hz, 1H), 7.33 (dd, J = 8.8, 6.8 Hz, 3H), 7.27–7.21 (m, 3H), 6.95 (d, J = 8.6 Hz, 1H), 4.99 (s, 2H), 4.93 (d, J = 2.8 Hz, 1H), 4.17 (d, J = 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 150.34, 139.05, 137.27, 135.09, 130.48, 128.95, 127.27, 126.82, 126.62, 123.93, 118.24, 116.97, 87.35, 46.33. HRMS (ESI) m/z: calc. for C16H13ClN2O [M + H]+: 285.0789, found 285.0776.
Data Availability
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00584/full#supplementary-material
Supplementary data (1H, 13C NMR spectrums, computational data, Cartesian coordinates, Crystal Data) associated with this article can be found in the Supporting Information.
References
Awad, M. K., Abdel-Aal, M. F., Atlam, F. M., and Hekal, H. A. (2018). Design, synthesis, molecular modeling, and biological evaluation of novel alpha-aminophosphonates based quinazolinone moiety as potential anticancer agents: DFT, NBO and vibrational studies. J. Mol. Struct. 1173, 128–141. doi: 10.1016/j.molstruc.2018.06.094
Brack, A. (1969). Über 4-Methylen-chinazolone-(2). Eur. Liebigs Ann. Chem. 730, 166–172. doi: 10.1002/jlac.19697300117
Buckner, F. S., Alvarez, X. B., Fan, E., Gillespie, J. B., Hol, W. G. J., Koh, C. Y., et al. (2015). Specific Inhibitors of Methionyl-tRNA Synthetase. U.S. Patent No US2015046357. Washington, DC: World Intellectual Property Organization.
Camacho, M. E., Chayah, M., García, M. E., Fernández-Sáez, N., Arias, F., Gallo, M. A., et al. (2016). Quinazolinones, quinazolinthiones, and quinazolinimines as nitric oxide synthase inhibitors: synthetic study and biological evaluation. Arch. Pharm. 349, 638–650. doi: 10.1002/ardp.201600020
Campbell, H. F., Kuhla, D. E., Studt, W. L., Faith, W. C., and Molino, B. F. (1988). Benzodiazinone-Pyridazinone and Hydroxy-Pyrazolyl Compounds, Cardiotonic Compositions Including the Same, and their Uses. U.S. Patent No US4868300. Doylestown, PA: World Intellectual Property Organization.
Dhami, S., Rumbles, G., MacRobert, A. J., and Phillips, D. (1997). Comparative photophysical study of disulfonated aluminum phthalocyanine in unilamellar vesicles and leukemic K562 cells. Photochem. Photobiol. 65, 85–90. doi: 10.1111/j.1751-1097.1997.tb01881.x
Elkholy, A. E., Rizk, S. A., and Rashad, A. M. (2019). Enhancing lubricating oil properties using novel quinazolinone derivatives: DFT study and molecular dynamics simulation. J. Mol. Struct. 1175, 788–796. doi: 10.1016/j.molstruc.2018.08.045
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2016). Gaussian 09, Revision C.01. Wallingford, CT: Gaussian, Inc.
Fukamachi, S., Konishi, H., and Kobayashi, K. (2010). Synthesis of 2-(2-dialkylamino-4H-3,1-benzothiazin-4-yl)acetic acid derivatives and 2-(2-thioxo-1,2,3,4-tetrahydroquinazolin-4-yl)acetic acid derivatives. Synthesis-Stuttgart 10, 1593–1598. doi: 10.1002/chin.201039169
Gatadi, S., Lakshmi, T. V., and Nanduri, S. (2019). 4(3H)-Quinazolinone derivatives: promising antibacterial drug leads. Eur. J. Med. Chem. 170, 157–172. doi: 10.1016/j.ejmech.2019.03.018
Gimeno, A., Cuenca, A. B., Medio-Simon, M., and Asensio, G. (2014a). Gold(I)- catalyzed reactions of 1-(ortho-Alkynylaryl)ureas: highly selective heterocyclization and synthesis of mixed N,O-acetals. Adv. Synth. Catal. 356, 229–236. doi: 10.1002/adsc.201300730
Gimeno, A., Cuenca, A. B., Suárez-Pantiga, S., Ramirez de Arellano, C., Medio-Simón, M., and Asensio, G. (2014b). Competitive gold-activation modes in terminal alkynes: an experimental and mechanistic study. Chem. Eur. J. 20, 683–688. doi: 10.1002/chem.201304087
Gimeno, A., Medio-Simón, M., Ramirez de Arellano, C., Asensio, G., and Cuenca, A. B. (2010). NHC-stabilized gold(I) complexes: suitable catalysts for 6-exo-dig heterocyclization of 1-(o-ethynylaryl)ureas. Org. Lett. 12, 1900–1903. doi: 10.1021/ol100595s
Grimme, S., Antony, J., Ehrlich, S., and Krieg, H. (2010). A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132:154104. doi: 10.1063/1.3382344
Grimme, S., Ehrlich, S., and Goerigk, L. (2011). Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465. doi: 10.1002/jcc.21759
Grombein, C. M., Hu, Q., Rau, S., Zimmer, C., and Hartmann, R. W. (2015). Heteroatom insertion into 3,4-dihydro-1H-quinolin-2-ones leads to potent and selective inhibitors of human and rat aldosterone synthase. Eur. J. Med. Chem. 90, 788–796. doi: 10.1016/j.ejmech.2014.12.022
Hua, L., Yao, Z., Xu, F., and Shen, Q. (2014). Chemoselective reactions under solvent-free conditions: lanthanide-catalyzed syntheses of 2-amino-3,1-benzothiazines and 3,4-dihydroquinazoline-2-thiones. RSC Adv. 4, 3113–3120. doi: 10.1039/C3RA44829K
Huang, C., Fu, Y., Fu, H., Jiang, Y., and Zhao, Y. (2008). Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. Chem. Commun. 47, 6333–6335. doi: 10.1039/b814011a
Iwamura, H., Fujita, T., Koyama, S., Koshimizu, K., and Kumazawa, Z. (1980). Quantitative structure-activity relationship of cytokinin-active adenine and urea derivatives. Phytochemistry 19, 1309–1319. doi: 10.1016/S0031-9422(00)82070-1
Khan, I., Ibrar, A., Abbas, N., and Saeed, A. (2014). Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur. J. Med. Chem. 76, 193–244. doi: 10.1016/j.ejmech.2014.02.005
Khan, I., Ibrar, A., Ahmed, W., and Saeed, A. (2015). Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur. J. Med. Chem. 90, 124–169. doi: 10.1016/j.ejmech.2014.10.084
Khan, I., Zaib, S., Batool, S., Abbas, N., Ashraf, Z., Iqbal, J., et al. (2016). Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: an update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 24, 2361–2381. doi: 10.1016/j.bmc.2016.03.031
Kleine, T., Fröhlich, R., Wibbeling, B., and Würthwein, E., U. (2011). Cyclization reactions of 3,4-diazaheptatrienyl metal compounds. pyridines from an anionic analogue of the fischer indole synthesis: experiment and theory. J. Org. Chem. 76, 4591–4599. doi: 10.1021/jo200487v
Kshirsagar, U. A. (2015). Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 13, 9336–9352. doi: 10.1039/C5OB01379H
Long, S., Resende, D. I. S. P., Kijjoa, A., Silva, A. M. S., Fernandes, R., Xavier, C. P. R., et al. (2019). Synthesis of new proteomimetic quinazolinone alkaloids and evaluation of their neuroprotective and antitumor effects. Molecules 24:534. doi: 10.3390/molecules24030534
Maiden, T. M., and Harrity, J. P. (2016). Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 14, 8014–8025. doi: 10.1039/C6OB01402J
Markovic, Z. S., Markovic, J. M. D., and Dolicanin, C. B. (2010). Mechanistic pathways for the reaction of quercetin with hydroperoxy radical. Theor. Chem. Acc. 127, 69–80. doi: 10.1007/s00214-009-0706-x
Mei, Q., Wang, L., Tian, B., Yan, F., Zhang, B., Huang, W., et al. (2012). A highly selective and naked-eye sensor for Hg2+ based on quinazoline-4(3H)-thione. New J. Chem. 36, 1879–1883. doi: 10.1039/c2nj40400a
Molina, P., Conesa, C., Alías, A., Arques, A., Velasco, M. D., Llamas-Saiz, A. L., et al. (1993). Preparation and synthetic applications of iminophosphoranes derived from o-substituted arylazides: preparation of pyrazolo[1,2-b]indazole, 4H-3,1-benzoxazine and quinoline derivatives. Crystal structure of 2-[2-(4-methoxybenzoylamino)phenyl]-4-methylq. Cheminformatics 49, 7599–7612. doi: 10.1016/S0040-4020(01)87234-3
Morgenstern, O., and Richter, P. H. (1992). Chemistry and biological-activity of 1,3,4-benzotriazepines. 2. Die Pharm. 47, 655–677.
Saunthwal, R. K., Patel, M., Tiwari, R. K., Parang, K., and Verma, A. K. (2015). On water: catalyst-free chemoselective synthesis of highly functionalized tetrahydroquinazolines from 2-aminophenylacrylate. Green Chem. 17, 1434–1441. doi: 10.1039/C4GC02154A
Sawant, R. T., Stevens, M. Y., and Odell, L. R. (2015). Rapid access to polyfunctionalized 3,4-dihydroquinazolinones through a sequential N-acyliminium ion mannich reaction cascade. Eur. J. Med. Chem. 35, 7743–7755. doi: 10.1002/ejoc.201501178
Sbei, N., Batanero, B., Barba, F., Haouas, B., Benkhoud, M. L., and Barba, I. (2018). Facile preparation of 3-substituted 2-quinazolinones via electrogenerated base. Tetrahedron 74, 2068–2072. doi: 10.1016/j.tet.2018.03.010
Thanigaimalai, P., Sharma, V. K., Lee, K.-C., Yun, C.-Y., Kim, Y., and Jung, S.-H. (2010). Refinement of the pharmacophore of 3,4-dihydroquinazoline-2(1H)-thiones for their anti-melanogenesis activity. Bioorg. Med. Chem. Lett. 20, 4771–4773. doi: 10.1016/j.bmcl.2010.06.123
Walser, A., Flynn, T., Mason, C., Crowley, H., Maresca, C., Yaremko, B., et al. (1991). Triazolobenzo- and triazolothienodiazepines as potent antagonists of platelet activating factor. J. Med. Chem. 34, 1209–1221. doi: 10.1021/jm00107a048
Wang, H., Liu, L., Wang, Y., Peng, C., Zhang, J., and Zhu, Q. (2009). An efficient synthesis of 4-alkyl-2(1H)-quinazolinones and 4-alkyl-2-chloroquinazolines from 1-(2-alkynylphenyl)ureas. Tetrahedron Lett. 50, 6841–6843. doi: 10.1016/j.tetlet.2009.09.130
Wang, H.-X., Liu, H.-Y., Li, W., Zhang, S., Wu, Z., Li, X., et al. (2019). Design, synthesis, antiproliferative and antibacterial evaluation of quinazolinone derivatives. Med. Chem. Res. 28, 203–214. doi: 10.1007/s00044-018-2276-8
Xie, T., Xiao, Y., Zhao, S., Hu, X.-Q., and Xu, P.-F. (2016). Catalyst-free chemoselective synthesis of 3,4-dihydroquinazoline-2-thiones and 2-imino[1,3]benzothiazines. J. Org. Chem. 81, 10499–10505. doi: 10.1021/acs.joc.6b01232
Yan, H., Hider, R. C., and Ma, Y. (2019). Synthesis, characterisation and quantum chemical studies of a new series of iron chelatable fluorescent sensors. Mol. Phys. 117, 661–671. doi: 10.1080/00268976.2018.1533147
Zeiger, M., Stark, S., Kalden, E., Ackermann, B., Ferner, J., Scheffer, U., et al. (2014). Fragment based search for small molecule inhibitors of HIV-1 Tat-TAR. Bioorg. Med. Chem. Lett. 24, 5576–5580. doi: 10.1016/j.bmcl.2014.11.004
Keywords: cyclization, quinazolinone, quinazolinthione, quantum chemical calculations, SMD-B3LYP
Citation: Yan H, Xiao X-Q, Hider RC and Ma Y (2019) A Simple Metal-Free Cyclization for the Synthesis of 4-Methylene-3-Substituted Quinazolinone and Quinazolinthione Derivatives: Experiment and Theory. Front. Chem. 7:584. doi: 10.3389/fchem.2019.00584
Received: 21 June 2019; Accepted: 02 August 2019;
Published: 16 August 2019.
Edited by:
Guigen Li, Texas Tech University, United StatesCopyright © 2019 Yan, Xiao, Hider and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmin Ma, eW9uZ21pbi5tYUB0emMuZWR1LmNu
In Memoriam: This paper is dedicated to the memory of Prof. Yongmin Zhang (1932-2019).
 Huihui Yan
Huihui Yan Xu-Qiong Xiao3
Xu-Qiong Xiao3 Yongmin Ma
Yongmin Ma