- 1Key Laboratory of Mesoscopic Chemistry of Ministry of Education, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, China
- 2College of Material Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing, China
- 3School of Petrochemical Engineering, Changzhou University, Changzhou, China
- 4State Key Laboratory of Pollution Control and Resource Reuse, School of Environment, Nanjing University, Nanjing, China
Planar-chiral pillar[5]arenes bearing β-D-galactose substituents on both rims have been successfully synthesized and effectively separated by silica gel chromatography with a high yield. The obtained (Sp)- and (Rp)-β-D-galactose functionalized pillar[5]arenes [(Sp-D)-GP5 and (Rp-D)-GP5] exhibit the Sp and Rp planar chirality. Furthermore, (Sp-D)-GP5 and (Rp-D)-GP5 can not racemize according to dynamic 1H NMR and CD spectra. Notably, GP5 is able to capture a guest molecule (DNS-CPT) to form a host-guest supramolecular amphiphile, which can further self-assemble into chiral nanoparticles with the Sp and Rp planar chirality of (Sp-D)-GP5 and (Rp-D)-GP5 still being retained, suggesting GP5 could be as reliable chiral sources to transfer the Sp and Rp planar chirality.
Introduction
Supramolecular macrocycles, such as cyclodextrins, cucurbiturils, and calixarenes, have played a very important role in supramolecular chemistry (Moghaddam et al., 2011; Zhang and Wang, 2011; Jie et al., 2015; Choi et al., 2017). Compared with these traditional macrocycles, pillar[n]arenes have attracted more attention due to their unique planar chirality (Ogoshi et al., 2011b). The planar chirality of pillar[n]arenes is very useful for chiral molecular recognition, chirality switches, and catalysis because of the outstanding host-guest properties of pillar[n]arenes to capture different guest molecules (Yao et al., 2017; Lee et al., 2018; Park et al., 2019).
As many literatures have presented (Ogoshi et al., 2011a, 2012, 2013a,b, 2016; Kitajima et al., 2014), the planar chirality of pillar[n]arenes is mainly caused by the substitution position of the alkoxy moieties. Ogoshi et al. (2012) and Kitajima et al. (2014) found that all of the synthesized pillar[5]arenes are racemic mixtures and racemization takes place by rotation of units. These racemic mixtures could be divided into eight conformers including diastereomeric conformers: (Sp, Sp, Sp, Sp, Sp), (Rp, Sp, Sp, Sp, Sp), (Rp, Rp, Sp, Sp, Sp), (Rp, Sp, Rp, Sp, Sp) and their antipodal enantiomers: (Rp, Rp, Rp, Rp, Rp), (Sp, Rp, Rp, Rp, Rp), (Sp, Sp, Rp, Rp, Rp), (Sp, Rp, Sp, Rp, Rp). In order to isolate the different pillar[5]arene enantiomers, they have functionalized pillar[5]arene with 10 bulky cyclohexylmethyl groups at both rims to inhibit the rotation of the units (Ogoshi et al., 2011a). Then, two special enantiomers [(Sp, Sp, Sp, Sp, Sp) and (Rp, Rp, Rp, Rp, Rp)] were successfully separated by chiral high performance liquid chromatography (HPLC). The circular dichroism (CD) spectra of the two enantiomers were clearly defined with a complete mirror image, which was defined as (Sp)- and (Rp)-pillar[5]arenes, respectively. Simultaneously, Strutt et al. (2012, 2014) reported the separation of pillararene-based enantiomers by introducing one π-conjugated unit, which expressed good and selective encapsulation of neutral and positively charged electron poor aromatic guests. Moreover, some other researches (Yao et al., 2017; Lee et al., 2018; Park et al., 2019) about planar chirality of pillar[5]arenes have been performed to achieve chiral inversion, chiral transfer and so on. Besides the above mentioned pillar[n]arene derivatives, β-D-galactose-functionalized pillar[5]arene (GP5), as a new-type of sugar modified supramolecular amphiphile, has been widely used in biologically relevant fields for the construction of antibacterial and targeted drug delivery systems (Nierengarten et al., 2013; Yu et al., 2013; Liu et al., 2017; Wu et al., 2017). However, all the results above never revealed the planar chirality of GP5, and there was no report about the investigation of (Sp)- and (Rp)-β-D-galactose-functionalized pillar[5]arene [(Sp-D)-GP5 and (Rp-D)-GP5]. In our previous work (Liu et al., 2017), we have obtained a similar β-D-galactose-based water-soluble pillar[5]arene (GalP5), which showed no planar chirality, because GalP5 possessed one methylene group at the position of β-D-galactose, resulting in the disappearance of planar chirality induced in the progress of functionalized pillar[5]arenes. Herein, we have successfully designed a new β-D-galactose functionalized pillar[5]arene without the presence of methylene group connected with β-D-galactose, and first achieved the separation of diastereoisomers possessing planar chirality by silica gel chromatography to obtain (Sp-D)-GP5 and (Rp-D)-GP5 with a high yield. Their rotational and planar chiral properties were investigated by NMR, UV-Vis and CD measurements, respectively.
Results and Discussion
Planar Chirality of GP5
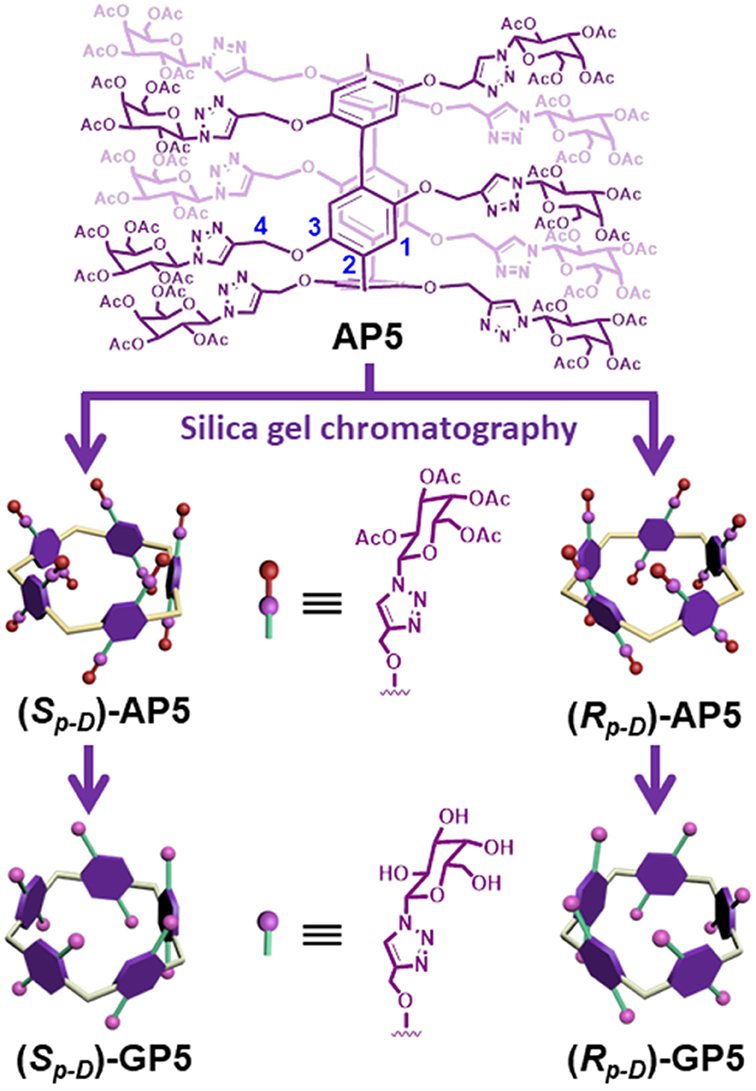
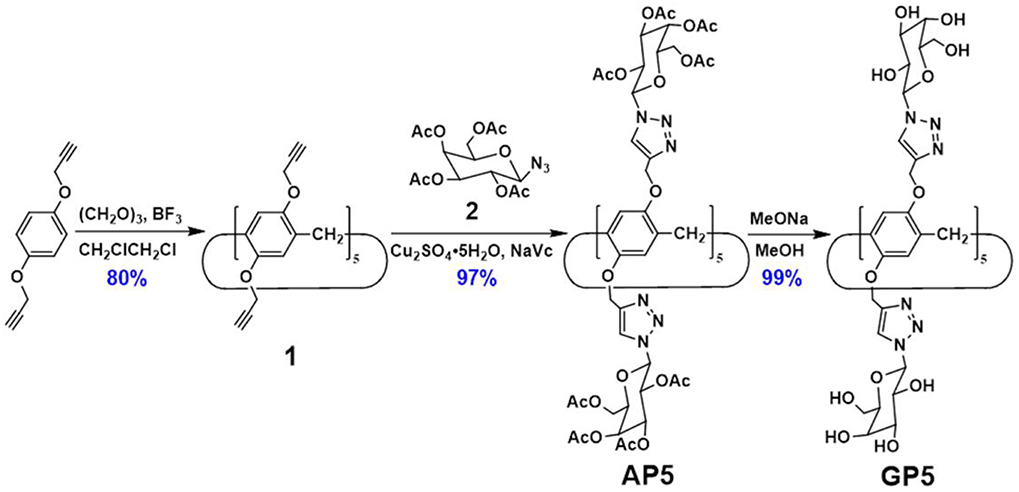
The synthesis of GP5 relies on the copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction, which was used to introduce the bulky β-D-acetylgalactose moieties on both rims of the pillar[5]arene building block. In this way, it can effectively inhibit the rotation of the units and thus achieve the separation of the (Sp)- and (Rp)-β-D-acetyl-galactose pillar[5]arene [(Sp-D)-AP5 and (Rp-D)-AP5] (Figures 1, 9). From the 1H NMR spectrum of AP5, we can clearly find that the resonances of the aromatic protons (H1) show two single peaks, identifying the existence of (Sp-D)-AP5 and (Rp-D)-AP5 (Figure S19). In order to further investigate the planar chirality of AP5, (Sp-D)-AP5 and (Rp-D)-AP5 were successfully obtained by silica gel chromatography with DCM/MeOH = 40:1 as fluent solvent. As shown in Figure S19, every signal of (Sp-D)-AP5 and (Rp-D)-AP5 is different from each other, but corresponding well to the protons of AP5.
The circular dichroism (CD) and UV-Vis spectra of (Sp-D)-AP5, (Rp-D)-AP5, and AP5 were further investigated to explain the planar chirality of AP5. As expected, two different kinds of CD signals could be observed between (Sp-D)-AP5 and (Rp-D)-AP5, and AP5 showed no obvious signal, which suggested (Sp-D)-AP5 and (Rp-D)-AP5 were mirror images in the planar chirality and they were separated effectively by silica gel chromatography (Figure 2).
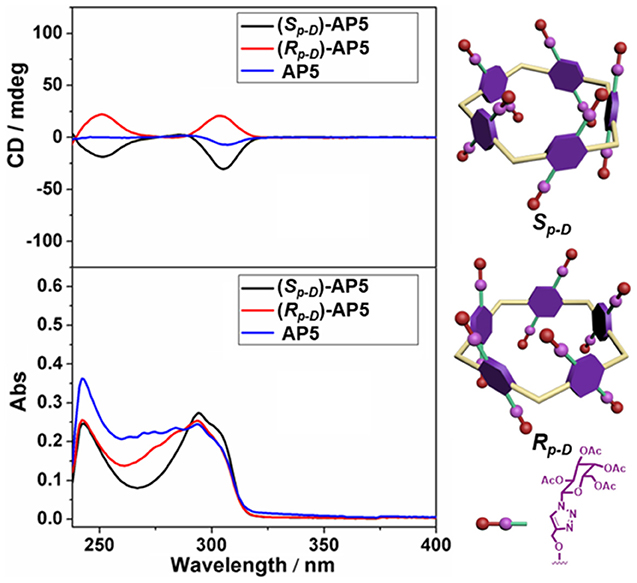
Figure 2. CD and UV-Vis spectra of (Sp-D)-AP5 (8 μM in CHCl3), (Rp-D)-AP5 (8 μM in CHCl3), and AP5 (8 μM in CHCl3).
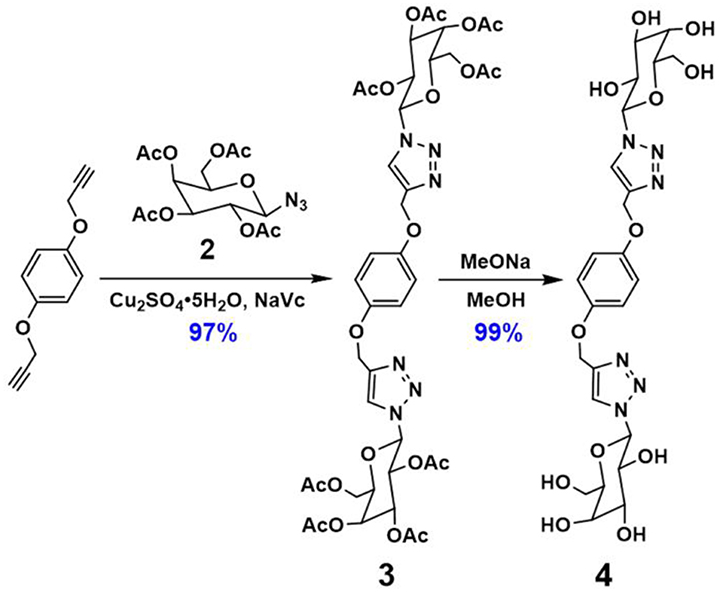
With compounds (Sp-D)-AP5 and (Rp-D)-AP5 in hand, (Sp-D)-GP5 and (Rp-D)-GP5 were successfully obtained by reacting with sodium methoxide solution, respectively. Similar to (Sp-D)-AP5 and (Rp-D)-AP5, 1H NMR and 13C NMR spetra of (Sp-D)-GP5 and (Rp-D)-GP5 are different. However, there is no obvious difference between (Sp-D)-GP5 and (Rp-D)-GP5 in 1H-1H COSY, NOESY, and HSQC spectra. To further investigate the planar chirality of (Sp-D)-GP5 and (Rp-D)- GP5, CD and UV-Vis spectra were performed and two kinds of chiral signals were observed. As shown in Figure 3, the CD signals of (Sp-D)-GP5 and (Rp-D)-GP5 were fully symmetrical, which indicated (Sp-D)-GP5 and (Rp-D)-GP5 were mirror images in the planar chirality. However, no obvious CD signal could be found from GP5, which further confirmed (Sp-D)-GP5 and (Rp-D)-GP5 owned opposite planar chirality. For comparation, a control molecule (compound 4) was synthesized (Scheme S2 and Figure 10) and no CD signal could be observed, which showed the planar chirality of pillar[5]arene was mainly attributed to the cyclization of moiety to form the pillararene backbone.
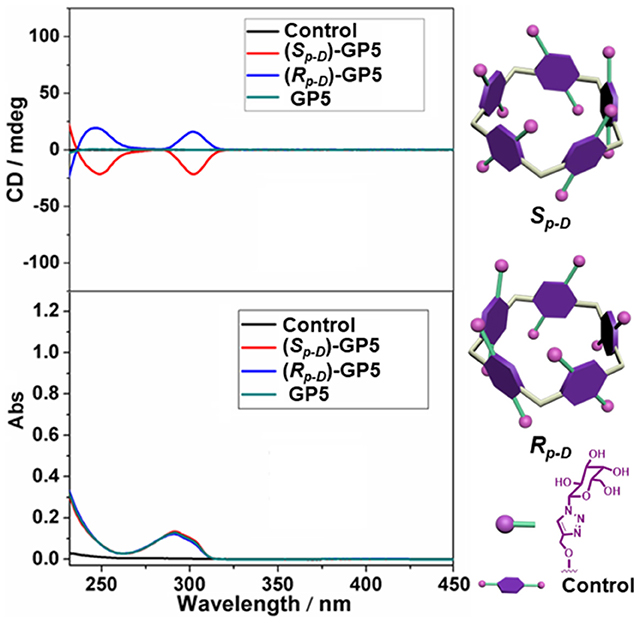
Figure 3. CD and UV-Vis spectra of (Sp-D)-GP5 (8 μM in H2O), (Rp-D)-GP5 (8 μM in H2O), and GP5 (8 μM in H2O), and control molecule (40 μM in H2O).
As we all know, CD spectroscopy is a well-established tool for detecting and tracking the dynamic behavior of molecule and supramolecular chirality. Pillar[5]arene derivatives could show strong CD extrema (CDex) at ca. 310 nm in the absence of any other attached chromophoric groups. According to previous reports (Ogoshi et al., 2012; Yao et al., 2017), the results showed (Sp)-pillar[5]arene derivatives exhibited negative CDex and (Rp)-pillar[5]arene derivatives exhibited positive CDex. Therefore, combining the CD spectra calculated by DFT method (Figure 4 and Figure S21), we deduced the compound with higher retention factor (Rf) value obtained from silica gel chromatography should be the Sp comformer and show negative CDex signal. The compound with lower Rf value was the Rp comformer and positive CDex signal.
Racemization Investigation of (Sp-D)-GP5 and (Rp-D)-GP5
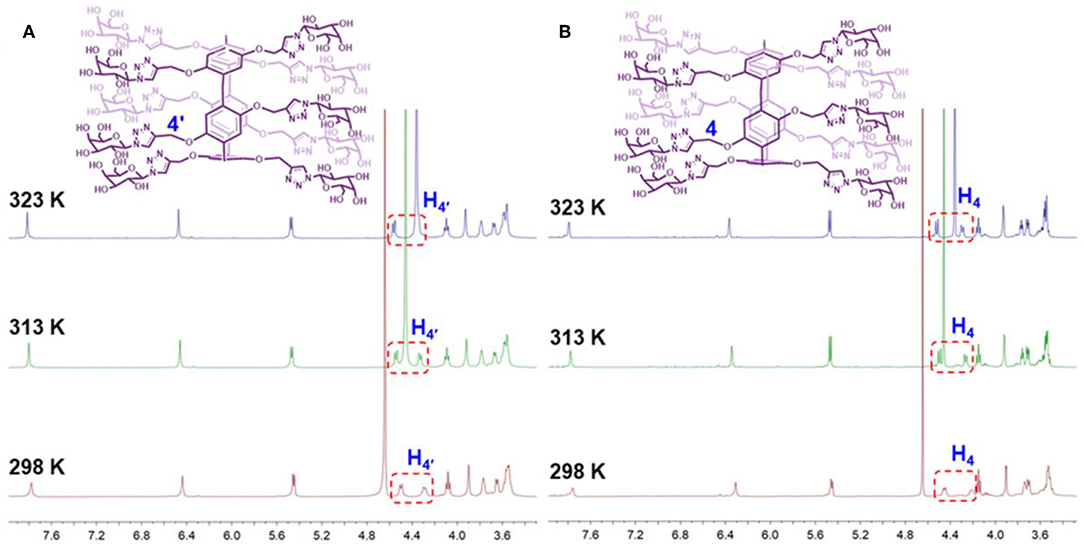
According to previous literatures (Ogoshi et al., 2010a,b, 2011a; Nierengarten et al., 2013), the planar chirality of pillar[5]arene is unstable and will be racemized. In order to explore whether (Sp-D)-GP5 and (Rp-D)-GP5 could exchange with each other, dynamic 1H NMR and CD measurements were further carried out. According to the planar chirality of (Sp-D)-GP5 and (Rp-D)-GP5, the two protons from the methylene moieties adjacent to the O atoms (H4) were different in chemical environment and could split into two groups of double peak in 1:1 integration ratio at 298 K (Figure S20). Thus, the split proton resonances are a useful marker to determine whether the rotation of pillar[5]arenes takes place on the NMR time scale (Ogoshi et al., 2010a,b, 2011b). Moreover, as shown in Figure 5, although the chemical shift of D2O exhibited upfield shift changes due to the weakening of intermolecular hydrogen bonding of D2O with increasing temperature, almost no peak changes for (Sp-D)-GP5 and (Rp-D)-GP5 could be observed (TMS as the reference). More important, the split of and H4 still retained during the progress of heating, indicating (Sp-D)-GP5 and (Rp-D)-GP5 were stable and hardly racemized on the NMR time scale in the measured temperature range.
Subsequently, dynamic CD experiments were investigated, and the results indicated the intensity of (Sp-D)-GP5 and (Rp-D)-GP5 were stable and symmetric, confirming the planar chirality of (Sp-D)-GP5 and (Rp-D)-GP5 was absolutely independent and the racemization of (Sp-D)-GP5 and (Rp-D)-GP5 didn't happen even under higher temperature. Whereas, when more attention was paid to the wavelength from 290 to 310 nm, which was ascribed to π-π* transitions of the aromatic moieties in the pillar[5]arene backbone, both (Sp-D)-GP5 and (Rp-D)-GP5 trended to racemize with increasing temperature (Figure 6 and Figure S22). However, due to the large molecular size of bulky substituent on the rim of GP5, neither (Sp-D)-GP5 nor (Rp-D)-GP5 could racemize actually, which is consistent with the dynamic 1H NMR results (Ogoshi et al., 2011a, 2016). In summary, different from many traditional pillar[5]arene derivatives, the planar chirality of (Sp-D)-GP5 and (Rp-D)-GP5 are very stable and unchangeable, which can be used as a reliable chiral source to induce and transfer the Sp and Rp planar chirality.
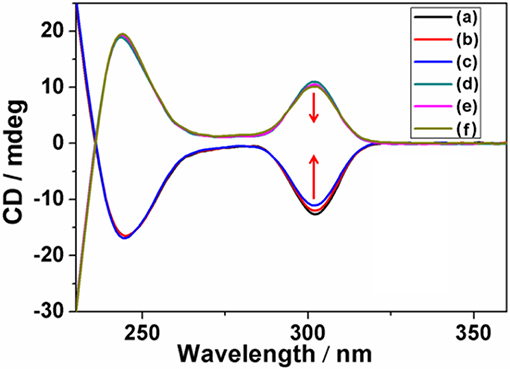
Figure 6. Dynamic CD spectra of (Sp-D)-GP5 [(A–C) 8 μM in H2O] and (Rp-D)-GP5 [(D–F) 8 μM in H2O] at 298, 313, and 323 K, respectively.
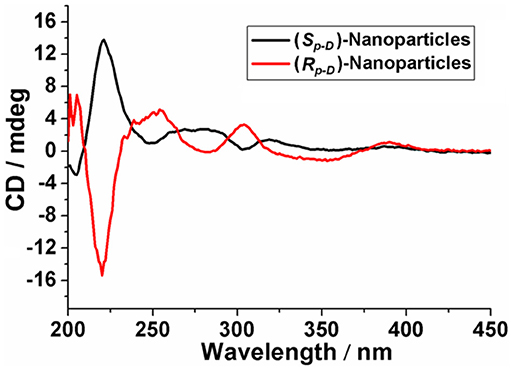
Simultaneously, after dialysis with distilled water, the CD spectra of the (Sp-D)-nanoparticles and (Rp-D)-nanoparticles were obtained, respectively. The results confirmed the planar chirality of these chiral nanoparticles still existed and displayed symmetrical signal, indicating that (Sp-D)-GP5 and (Rp-D)-GP5 could be used as reliable chiral sources to transfer the Sp and Rp planar chirality (Figure 8).
The Construction of Chiral Nanoparticles
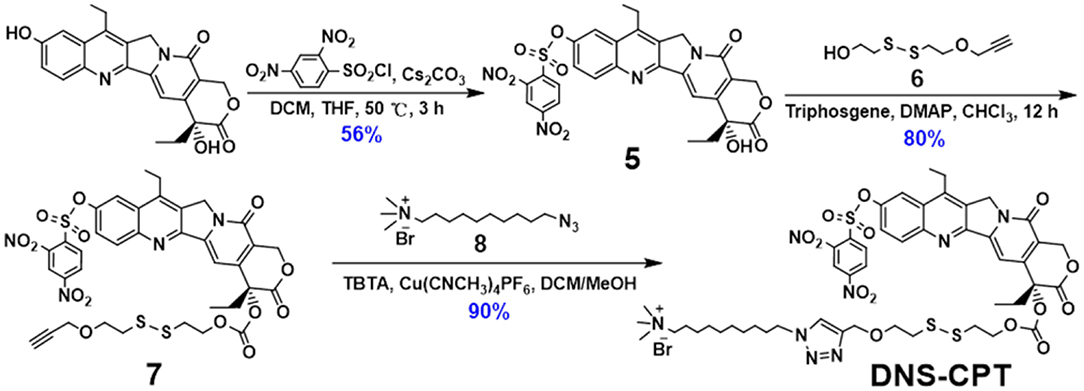
Based on the outstanding host-guest properities of pillar[5]arene, one of our previously reported guest molecule (DNS-CPT) (Sun et al., 2019) was used to investigate the construction of nanoparticles with planar chirality (Figure 11). As shown in Figure 7, when (Sp-D)-GP5 or (Rp-D)-GP5 was added into the DNS-CPT solution, an obvious Tyndall effect could be observed, indicating the formation of large sized aggregates. The diameter of these nanoparticles was confirmed to be 39 and 38 nm by dynamic light scattering (DLS), respectively. The morphology of the nanoparticles was further investigated by transmission electron microscopy (TEM), and the results showed both (Sp-D)- and (Rp-D)-GP5 could form nanoparticles with the presence of the guest molecule DNS-CPT (Figure 7 and Figure S23). Moreover, Zeta potential measurements showed that the obtained nanoparticles possess relatively high positive ζ- potentials (32.85 and 35.93 mV, respectively), suggesting their good stability in solution (Figure S24).
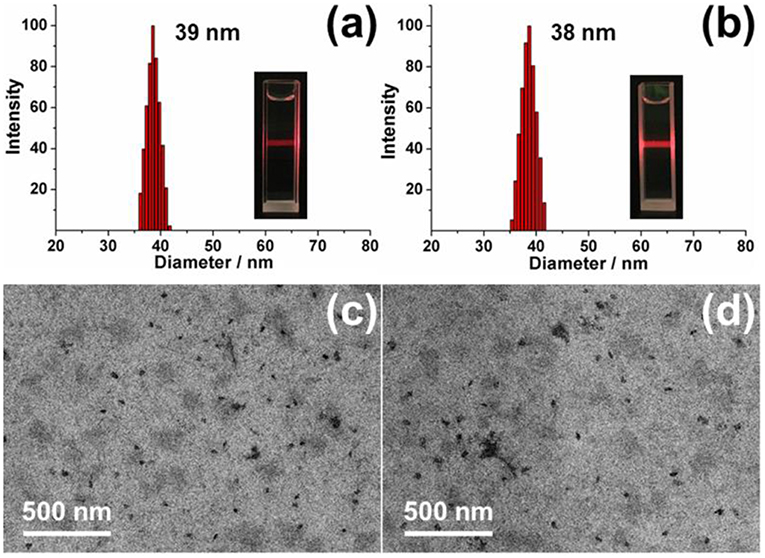
Figure 7. DLS data of chiral nanoparticles: (a) (Sp-D)-nanoparticles. Inset photo: Tyndall effect. (b) (Rp-D)-nanoparticles. Inset photo: Tyndall effect. TEM images of chiral nanoparticles: (c) (Sp-D)-nanoparticles. (d) (Rp-D)-nanoparticles.
Experimental
Synthesis of GP5
As shown in Figure 9, GP5 was synthesized based on the click reaction between compound 1 and 2 to generate compound AP5 successfully. Then, AP5 was reacted with sodium methoxide in methanol for 24 h under an inert atmosphere at ambient temperature. The resulting reaction mixture was filtered and washed with methanol, which gave the target macrocycle GP5 in 99% yield. A combination of 1H, 13C, 1H-1H COSY, NOESY, and HSQC nuclear magnetic resonance spectroscopy (NMR) confirmed (Figures S7–S16) the formation of GP5.
Synthesis of Compound 4
Compound 4 was synthesized based on the click reaction between 1,4-bis(prop-2-yn-1-yloxy)benzene and compound 2 to generate compound 3 successfully. Then, compound 3 was reacted with sodium methoxide in methanol for 24 h under an inert atmosphere at ambient temperature. The resulting reaction mixture was filtered and washed with methanol, which gave the control molecule compound 4 in 99% yield. 1H NMR (Figures S17, S18) confirmed the formation of compounds 3 and 4.
Synthesis of DNS-CPT
DNS-CPT was synthesized and characterized according to our previous work (Sun et al., 2019).
Fourier Transform Infrared Spectrometer (FT-IR) Spectrum
FT-IR experiments of 1,2,3,4,6-penta-o-acetyl-β-D-galactopyranose, compound 1, compound 2, (Sp-D)-AP5, (Rp-D)-AP5, (Sp-D)-GP5, and (Rp-D)-GP5 were carried out to track the functionalization process of pillar[5]arene. As shown in Figure 12, after reaction with trimethylsilyl azide (TMS-N3), a typical N = N = N peak at 2,100 cm−1 could be observed. However, the N = N = N peak disappeared after the click reaction with compound 1, which indicated the 1,2,3,4,6-penta-o-acetyl-β-D-galactopyranose group had been modified to pillar[5]arene to obtain AP5 successfully. Meanwhile, the stretching vibration peak of C = O was detected at 1750 cm−1. Moreover, when the acetyl group of AP5 was removed, the characteristic absorption peak of C = O disappeared and a wide peak of O-H at 3300 cm−1 was observed at the same time, which showed the successful formation of GP5.
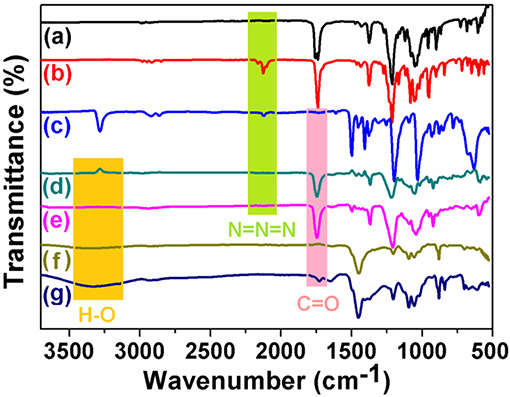
Figure 12. FT-IR spectrum: (a) Penta-O-acetyl-β-D-galactopyranose, (b) Compound 2, (c) Compound 1, (Sp-D)-AP5, (e) (Rp-D)-AP5, (f) (Sp-D)-GP5, and (g) (Rp-D)-GP5, respectively.
Conclusion
In conclusion, we successfully obtained (Sp-D)-AP5, (Rp-D)-AP5, (Sp-D)-GP5, and (Rp-D)-GP5 through silica gel chromatography with a high yield at room temperature. Dynamic CD and 1H NMR experiments revealed the Sp and Rp planar chirality of these pillar[5]arene derivatives (GP5) were very stable and unracemized, which could be used as reliable chiral sources to construct chiral nanoparticles, showing the Sp and Rp planar chirality of GP5 could be transferred by the host-guest interaction based on GP5 and DNS-CPT.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
LW, X-YH, and JJ conceived the project, supervised the study, and revised the manuscript. GS conducted the experiments, wrote the draft manuscript, and prepared the supporting information. LP conducted the TEM experiments. SP conducted the calculation experiments. All authors analyzed and interpreted the data.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 21572101 and 21871136) and the Natural Science Foundation of Jiangsu Province (No. BK20180055).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Yuan Chen and Yukun Shi from our group for the helpful discussion.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00743/full#supplementary-material
References
Choi, H., Cho, K. J., Seo, H., Ahn, J., Liu, J., Lee, S. S., et al. (2017). Transfer and dynamic inversion of coassembled supramolecular chirality through 2D-sheet to rolled-up tubular structure. J. Am. Chem. Soc. 139, 17711–17714. doi: 10.1021/jacs.7b09760
Jie, K., Zhou, Y., Yao, Y., and Huang, F. (2015). Macrocyclic amphiphiles. Chem. Soc. Rev. 44, 3568–3587. doi: 10.1039/C4CS00390J
Kitajima, K., Ogoshi, T., and Yamagishi, T. T. (2014). Diastereoselective synthesis of a [2]catenane from a pillar[5]arene and a pyridinium derivative. Chem. Commun. 50, 2925–2927. doi: 10.1039/c3cc49794a
Lee, E., Ju, H., Park, I. H., Jung, J. H., Ikeda, M., Kuwahara, S., et al. (2018). Pseudo[1]catenane-type pillar[5]thiacrown whose planar chiral inversion is triggered by metal cation and controlled by anion. J. Am. Chem. Soc. 140, 9669–9677. doi: 10.1021/jacs.8b05751
Liu, X., Shao, W., Zheng, Y., Yao, C., Peng, L., Zhang, D., et al. (2017). GSH-Responsive supramolecular nanoparticles constructed by β-D-galactose-modified pillar[5]arene and camptothecin prodrug for targeted anticancer drug delivery. Chem. Commun. 53, 8596–8599. doi: 10.1039/C7CC04932C
Moghaddam, S., Yang, C., Rekharsky, M., Ko, Y. H., Kim, K., Inoue, Y., et al. (2011). New ultrahigh affinity host-guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: thermodynamic analysis and evaluation of M2 affinity calculations. J. Am. Chem. Soc. 133, 3570–3581. doi: 10.1021/ja109904u
Nierengarten, I., Buffet, K., Holler, M., Vincent, S. P., and Nierengarten, J. F. (2013). A mannosylated pillar[5]arene derivative: chiral information transfer and antiadhesive properties against uropathogenic bacteria. Tetrahedron Lett. 54, 2398–2402. doi: 10.1016/j.tetlet.2013.02.100
Ogoshi, T., Akutsu, T., Yamafuji, D., Aoki, T., and Yamagishi, T. A. (2013a). Solvent- and achiral-guest-triggered chiral inversion in a planar chiral pseudo[1]catenane. Angew. Chem. Int. Ed. 52, 8111–8115. doi: 10.1002/anie.201302675
Ogoshi, T., Kitajima, K., Aoki, T., Fujinami, S., Yamagishi, T. A., and Nakamoto, Y. (2010a). Synthesis and conformational characteristics of alkyl-substituted pillar[5]arenes. J. Org. Chem. 75, 3268–3273. doi: 10.1021/jo100273n
Ogoshi, T., Kitajima, K., Aoki, T., Yamagishi, T. A., and Nakamoto, Y. (2010b). Effect of an intramolecular hydrogen bond belt and complexation with the guest on the rotation behavior of phenolic units in pillar[5]arenes. J. Phys. Chem. Lett. 1, 817–821. doi: 10.1021/jz900437r
Ogoshi, T., Masakai, K., Shiga, R., Kitajima, K., and Yamagishi, T. A. (2011a). Planar-chiral macrocyclic host pillar[5]arene: no rotation of units and isolation of enantiomers by introducing bulky substituents. Org. Lett. 13, 1264–1266. doi: 10.1021/ol200062j
Ogoshi, T., Shiga, R., Yamagishi, T. A., and Nakamoto, Y. (2011b). Planar-chiral pillar[5]arene: chiral switches induced by multiexternal stimulus of temperature, solvents, and addition of achiral guest molecule. J. Org. Chem. 76, 618–622. doi: 10.1021/jo1021508
Ogoshi, T., Yamafuji, D., Akutsu, T., Naito, M., and Yamagishi, T. A. (2013b). Achiral guest-induced chiroptical changes of a planar-chiral pillar[5]arene containing one π-conjugated unit. Chem. Commun. 49, 8782–8784. doi: 10.1039/c3cc44672g
Ogoshi, T., Yamafuji, D., Aoki, T., Kitajima, K., Yamagishi, T. A., Hayashi, Y., et al. (2012). High-yield diastereoselective synthesis of planar chiral [2]- and [3]rotaxanes constructed from per-ethylated pillar[5]arene and pyridinium derivatives. Chem. Eur. J. 18, 7493–7500. doi: 10.1002/chem.201200122
Ogoshi, T., Yamagishi, T. A., and Nakamoto, Y. (2016). Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev. 116, 7937–8002. doi: 10.1021/acs.chemrev.5b00765
Park, J., Choi, Y., Lee, S. S., and Jung, J. (2019). Critical role of achiral guest molecules in planar chirality inversion of alanine-appended pillar[5]arenes. J. Org. Lett. 21, 1232–1236. doi: 10.1021/acs.orglett.9b00277
Strutt, N. L., Fairen-Jimenez, D., Iehl, J., Lalonde, M. B., Snurr, R. Q., Farha, O. K., et al. (2012). Incorporation of an A1/A2-difunctionalized pillar[5]arene into a metal-organic framework. J. Am. Chem. Soc. 134, 17436–17439. doi: 10.1021/ja3082523
Strutt, N. L., Zhang, H., and Stoddart, J. F. (2014). Enantiopure pillar[5]arene active domains within a homochiral metal-organic framework. Chem. Commun. 50, 7455–7458. doi: 10.1039/c4cc02559h
Sun, G., He, Z., Hao, M., Xu, Z., Hu, X.-Y., Zhu, J.-J., et al. (2019). Bifunctional supramolecular prodrug vesicles constructed from camptothecin derivative with water-soluble pillar[5]arene for cancer diagnosis and therapy. Chem. Commun. 55, 10892–10895. doi: 10.1039/c9cc05859a.
Wu, X., Zhang, Y., Lu, Y., Pang, S., Yang, K., Tian, Z., et al. (2017). Synergistic and targeted drug delivery based on nano-CeO2 capped with galactose functionalized pillar[5]arene via host-guest interactions. J. Mater. Chem. B. 5, 3483–3487. doi: 10.1039/C7TB00752C
Yao, J., Wu, W., Liang, W., Feng, Y., Zhou, D., Chruma, J. J., et al. (2017). Temperature-driven planar chirality switching of a pillar[5]arene-based molecular universal joint. Angew. Chem. Int. Ed. 56, 6869–6873. doi: 10.1002/anie.201702542
Yu, G., Ma, Y., Han, C., Yao, Y., Tang, G., Mao, Z., et al. (2013). A sugar-functionalized amphiphilic pillar[5]arene: synthesis, self-assembly in water, and application in bacterial cell agglutination. J. Am. Chem. Soc. 135, 10310–10313. doi: 10.1021/ja405237q
Keywords: supramolecular macrocycles, β-D-galactose-functionalized pillar[5]arenes, planar chirality, self-assembly, nanoparticles
Citation: Sun G, Pu L, Pangannaya S, Xiao T, Hu X-Y, Jiang J and Wang L (2019) β-D-Galactose-Functionalized Pillar[5]arene With Interesting Planar-Chirality for Constructing Chiral Nanoparticles. Front. Chem. 7:743. doi: 10.3389/fchem.2019.00743
Received: 30 August 2019; Accepted: 17 October 2019;
Published: 14 November 2019.
Edited by:
De-Xian Wang, Institute of Chemistry (CAS), ChinaReviewed by:
Min Xue, Zhejiang Sci-Tech University, ChinaYuxin Pei, Northwest A&F University, China
Copyright © 2019 Sun, Pu, Pangannaya, Xiao, Hu, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Yu Hu, aHV4eUBudWFhLmVkdS5jbg==; Juli Jiang, ampsQG5qdS5lZHUuY24=
 Guangping Sun1
Guangping Sun1 Tangxin Xiao
Tangxin Xiao Xiao-Yu Hu
Xiao-Yu Hu Juli Jiang
Juli Jiang Leyong Wang
Leyong Wang