- 1Hainan Key Laboratory of Research and Development of Natural Product From Li Folk Medicine, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
- 2Nanjing Agricultural University, Nanjing, China
- 3East China University of Science and Technology, Shanghai, China
Four picrotoxane-type sesquiterpenes, dendroterpene A–D (1–4), together with four known compounds (5–8), were isolated from the stems of Dendrobium nobile Lindl. Their structures were elucidated by spectroscopic analysis, X-ray diffraction analysis, analysis of the ECD data according to the Klyne's lactone sector rule, and quantum ECD calculation. Compounds 1 and 2 are two new picrotoxane-type sesquiterpenes with a new carbon skeleton containing a formamide group, which may be derived from the previously reported dendrobiumane B skeleton by the C(9)-C(11) carbon bond cleavage. Compounds 3, 5, 6, and 8 exhibited inhibitory activity against α-glycosidase. Compounds 5 and 6 were cytotoxic against SGC-7901, K562, A549, BEL-7402, and Hela cell lines.
Introduction
Dendrobium nobile Lindl, the plant belongs Orchidaceae family, is one of three major plant sources of the traditional Chinese medicine “Shi Hu” in Chinese (edition 2005), which are used as a tonic to nourish the stomach and promote the production of body fluid recorded in the Chinese Pharmacopeia (Jiangsu New Medical College, 1986). The previous reports showed a series of chemical constituents isolated from D. nobile including sesquiterpenes (Zhang et al., 2007a), alkaloids (Liu and Zhao, 2003; Meng et al., 2017), bibenzyls derivatives (Zhang et al., 2007b), and glucosides (Zhao et al., 2001), some of which exhibited antitumor (Zhou et al., 2016), anti-inflammatory (Hwang et al., 2010), and immunomodulatory activities (Zhao et al., 2001).
Picrotoxane-type sesquiterpenes were one of main constituents of D. nobile, which exhibited the angiogenesis effect against sunitinibinduced damage (Meng et al., 2017) and inhibitory activity of nerve growth factor mediated neurite outgrowth (Leon et al., 2019). Our phytochemical investigation on the EtOAc extract of the stems of D. nobile led to the isolation of four new picrotoxane-type sesquiterpenes, dendroterpene A–D (1–4), along with four known compounds, nobilin E (5) (Zhang et al., 2007b), dendrocandin V (6) (Xiao et al., 2017), S(+)-dehydrovomifoliol (7) (Kato et al., 1977) and di-[2-(4-hydroxyphenyl)] ethyl ether (8) (Krysin et al., 2010). Compounds 1 and 2 are two new picrotoxane-type sesquiterpenes with a new carbon skeleton containing a formamide group, which may be derived from the previously reported dendrobiumane B skeleton by the C(9)-C(11) carbon bond cleavage. In this report, the isolation, structure elucidation and bioactivities of these compounds are described.
Materials and Methods
General Experimental Procedures
Optical rotations were measured on a MCP 5100 modular compact polarimeter (Anton Paar, America). IR spectra were taken on a Nicolet 380 FT-IR instrument (Thermo, USA) as KBr discs. NMR spectra were recorded on a Bruker Avance III 500 MHz NMR spectrometer (Bruker, German). ESIMS and HRESIMS were recorded with amaZon SL (Bruker) or Compact QqTOF (Bruker). ECD spectra were measured by APL Chiascan (Applied Photophysics Ltd., England). Semi-preparative HPLC was carried out using a C18 column (Cosmosil-pack, 10 × 250 mm, 5 μm, 4 mL/min, Nacalai Tesque). C18 gel (20–45 μm, Fuji SilysiSa Chemical Co., Ltd., Greenville, NC, UA), Silica gel (60–80, 200–300 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China), Sephadex LH-20 (Merck, Kenilworth, NJ, USA) were used for column chromatograghy. α-glycosidase (Sigma-Aldrich, UA) used for the bioactivity was derived from yeast and its EINECS (EC) unmber is 3.2.1.20.
Plant Material and Extraction
The fresh stems of D. nobile were collected from Shishan town in May 2018, Hainan, China. After drying, the dried stems (13.0 kg) were shattered. Then, the samples were extracted by 95% ethyl alcohol three times and for 5 days each time. The EtOH extract (716.2 g) was dissolved in H2O and extracted three times by petroleum ether (8.0 L), EtOAc (8.0 L), and n-butyl alcohol (8.0 L) successively.
Isolation
The EtOAc extract (87.9 g) was separated into 16 fractions (Fr.1–Fr.16) by a silica gel column (200–300 mesh) using a gradient of petroleum ether-EtOAc (v/v, 20:1– 0:1) and then acetone. Fr.9 (10.0 g) was further separated into 20 fractions (Fr.9-1–Fr.9-20) by ODS column. Fr.9-1 (30.0 mg) was purified by semipreparative HPLC (20% MeOH/H2O) to yield 8 (1.4 mg; tR 12.1 min). Fr.9-6 (112.0 mg) was purified by semipreparative TLC eluting with petroleum ether-acetone (v/v 3:1) to obtain compound 7 (2.7 mg). Fr.9-7 (372.0 mg) was purified by a silica gel column eluting with petroleum ether-CHCl3-MeOH (v/v/v 200:50:1) to yield compound 3 (4.2 mg). Compound 4 (2.0 mg) was obtained by recrystallization from Fr.9-8 (580.3 mg). Fr.9-10 (103.0 mg) was further purified by a silica gel column eluting with petroleum ether-acetone (v/v 8:1) to give 1 (13.0 mg). Fr.10 (9.0 g) was separated by ODS column to get 50 fractions (Fr.10-1–Fr.10-50). Compound 2 (100.2 mg) was obtained by recrystallization from Fr.10-14 (326.0 mg). Fr.10-38 (184.0 mg) was purified by Sephadex LH-20 eluting with MeOH to give 5 (15.0 mg). Fr.11 (9.0 g) was separated into three fractions (Fr.11-1–Fr.11-3) by Sephadex LH-20 eluting with MeOH. Fr.11-3 (80.1 mg) was purified by ODS column eluting with 65% MeOH/H2O to yield compound 6 (20.9 mg).
ECD Calculation
The calculations were performed by using the density functional theory (DFT) as carried out in the Gaussian 03 (Frisch et al., 2004). The preliminary conformational distributions search was performed by Sybyl-X 2.0 software. All ground-state geometries were optimized at the B3LYP/6-31G(d) level. Solvent effects of methanol solution were evaluated at the same DFT level by using the SCRF/PCM method (Cammi and Tomasi, 1995). TDDFT at B3LYP/6-31G(d) was employed to calculate the electronic excitation energies and rotational strengths in methanol (Gross et al., 1996).
Characterization of Compounds 1–4
Dendroterpene A (1): Colorless crystals; +149.9 (c 0. 1, MeOH); ECD (MeOH) λmax (Δε) 223 (+2.28); IR (KBr) νmax 3350, 2927, 2856, 1761, 1683, 1118, 1024 cm−1; m.p. 185–186 °C; 1H and 13C NMR (see Table 1); HRESIMS m/z 286.1417 [M + Na]+ (calcd. for C15H21NO3Na: 286.1414).
Dendroterpene B (2): Colorless crystals; +10.0 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 207 (+4.77); IR (KBr) νmax 3414, 2964,2926, 2861, 1764, 1666, 1223, 1021 cm−1; m.p. 200–201°C; 1H and 13C NMR (see Table 1); HRESIMS m/z 302.1376 [M + Na]+ (calcd. for C15H21NO4Na: 302.1363).
Dendroterpene C (3): White powder; +16.0 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 225 (+ 1.16), IR (KBr) νmax 3447, 2928, 1777, 11276, 1111, 1036 cm−1; 1H and 13C NMR (see Table 1); HRESIMS m/z 287.1281 [M + Na]+ (calcd. for C15H20O4Na:287.1254).
Dendroterpene D (4): Colorless crystals; +139.9 (c 0.1, MeOH); IR (KBr) νmax 3414, 2930, 1629, 1108, 1028 cm−1; m.p. 167–168 °C; 1H and 13C NMR (see Table 1); HRESIMS m/z 303.1228 [M + Na]+ (calcd. for C15H20O5 Na: 303.1203).
X-Ray Crystallographic Data of 1, 2, and 4
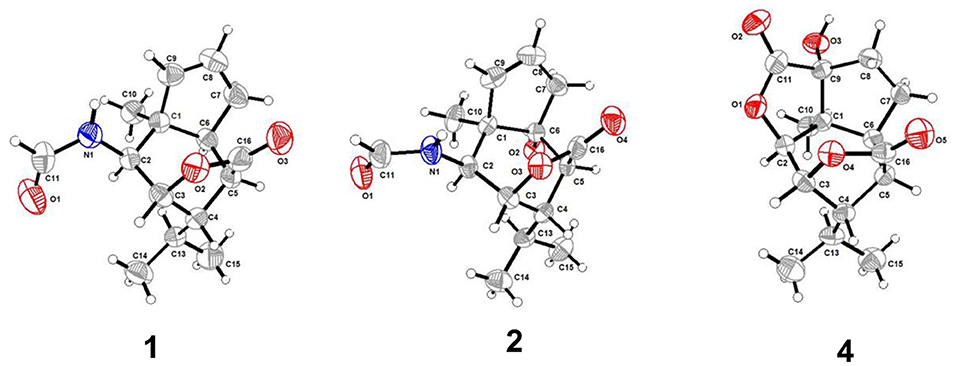
Compound 1 was obtained as colorless crystals with molecular formula of C15H21NO3 from MeOH. Space group P212121, a = 7.7078(5) Å, b = 10.1533 (7) Å, c = 17.8721(12) Å, α = 90.00°, β = 90.00(10)°, γ = 90.00°, V = 1398.66 (16) Å3, Z = 4, Dcalcd = 1.251 g/cm3, μ(CuKα) = 0.7000 mm−1, and F(000) = 568, T = 293(2) K, crystal size 0.32 × 0.20 × 0.12 mm, R1 = 0.0445(I>2sigma(I), wR2 = 0.1054 (all data), Flack parameter = 0.0(4). 3147 reflections measured, 2119 unique (Rint = 0.0213, Rsigma = N/A) which were used in all calculations. The structure was solved by direct methods (SHELXS-97) and expanded using Fourier techniques (SHELXL-97). Crystallographic data (excluding structure factors) for structure 1 in this paper have been deposited in the Cambridge Crystallographic Data Center as supplementary publication number CCDC 1943060.
Compound 2 was obtained as colorless crystals with molecular formula of C15H21NO4 from MeOH. Space group P21, a = 7.7610(4) Å, b = 8.8111 (4) Å, c = 10.3388(6) Å, α = 90.00°, β = 104.463(5)°, γ = 90.00°, V = 684.59 (6) Å3, Z = 2, Dcalcd = 1.300 g/cm3, μ(CuKα) = 0.771 mm−1, F(000) = 300, crystal size 0.34 × 0.27 × 0.13 mm, R1 = 0.0390(I>2sigma(I), wR2 = 0.1028 (all data), Flack parameter = 0.0(4). 3304 reflections measured, 2070 unique (Rint = 0.0259, Rsigma = N/A) which were used in all calculations. The structure was solved by direct methods (SHELXS-97) and expanded using Fourier techniques (SHELXL-97). Crystallographic data (excluding structure factors) for structure 2 in this paper have been deposited in the Cambridge Crystallographic Data Center as supplementary publication number CCDC 1943062.
Compound 4 was obtained as colorless crystals with molecular formula of C15H21NO3 from MeOH. Space group P21, a = 7.5120(3) Å, b = 13.6611(7) Å, c = 13.9060(5) Å, α = 90.00°, β = 90.00(10)°, γ = 90.00°, V = 1427.06(11) Å3, Z = 4, Dcalcd = 1.360 mg/m3, μ(CuKα) = 0.842 mm−1, F(000) = 600, crystal size 0.327 × 0.22 × 0.15 mm. R1 = 0.0368(I>2sigma(I), wR2 = 0.0938 (all data), Flack parameter = 0.1(3). 2227 reflections measured, 1628 unique (Rint = 0.0204, Rsigma = N/A) which were used in all calculations. The structure was solved by direct methods (SHELXS-97) and expanded using Fourier techniques (SHELXL-97). Crystallographic data (excluding structure factors) for structure 4 in this paper have been deposited in the Cambridge Crystallographic Data Center as supplementary publication number CCDC 1943065.
Bioassay for α-Glycosidase Inhibitory Activity
The method optimized by Jong et al. (2007) was performed in vitro to test the α-glucosidase inhibitory activity of compounds 1–8. Acarbose was used as positive control.
Bioassay for Cytotoxicity
The MTT method optimized by Mosmann (1983) was performed in vitro to test the cytotoxic activity of compounds 1–8. Cisplatin was used as a positive control.
Results and Discussion
Identification of Compounds 1-4
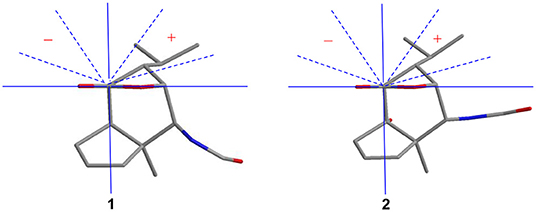
Compound 1 was obtained as colorless crystals with the molecular formula as C15H21NO3, which was determined based on the pseudo-molecular ion peak at m/z 286.1417 [M + Na]+ (calcd. for C15H21NO3Na: 286.1414) in the HRESIMS spectrum. Analysis of its 1D and HSQC NMR spectra (see Supplementary Figures 1, 2, and 4 in the Supplementary Material) revealed two olefinic methines (δC/H 136.6/5.63 and δC/H 130.3/5.51), three methyls (δC/H 27.3/1.04, 21.0/0.93 and 20.1/0.95), one methylene (δC/H 36.9/2.69, 2.35), six sp3 methines (δC/H 84.5/4.34, 52.5/2.02, 51.2/4.24, 46.1/2.28, 42.5/2.33, and 26.4/1.84) with one oxygenated, one quaternary carbon (δC 52.5) and two ester or amide carbonyl groups (δC 180.3 and δC 163.7). The above data and sequential COSY correlations (Figure 2 and Supplementary Figure 3 in the Supplementary Material) from H-2 to H-9, as well as from H-4 to H3-14 and H3-15 through H-13, along with the HMBC correlations (Figure 2 and Supplementary Figure 5 in the Supplementary Material) from H3-10 to C-2, C-6 and C-9, from H-2 to C-6 and C-9, from H-3 and H-6 to C-16, and from H-5 to C-1, revealed a picrotoxane-type sesquiterpene skeleton (Zhao et al., 2003). A detailed comparison of the above data with those of the previously reported dendrobiumane C (Zhao et al., 2003) disclosed high similarity. The differences between them were that the hydroxymethyl group CH2-11 (δC/H 59.8/4.04, 4.27) and the oxymethine group CH-2 (δC/H 73.1/3.67) in dendrobiumane C were replaced by a hydrogen atom H-9 (δH 5.51) and a methine CH-2 (δC/H 51.2/4.24) linked with a formamide group in 1, respectively, as evidence by COSY correlation from H-9 to H-8, along with the HMBC correlations from H-2 to C-9 and C-11, from H-9 to C-6 and C-10, as well as from H3-10 to C-2 and C-6. The ROESY correlations (Figure 3 and Supplementary Figure 6 in the Supplementary Material) from H3-10 to H-2, H-6 and H-13, and from H-2 and H-6 to H-13 suggested that H-2, H-6 and H3-10 were on the same face of the six-member ring, while H-4 was on the face opposite to them. Due to the complex bridge-ring lactone skeleton, the chemical structure models analysis of 1 displayed the only possibility of the relative configurations of C-3 and C-5 is that H-3 and H-5 were on the same face of six-member ring with H-2, H-6, and H3-10 when the relative configurations of C-1, C-2, C-4 and C-6 were confirmed. To support the above structure elucidation and determine the absolute configuration of 1, a single-crystal X-ray diffraction pattern was obtained using the anomalous scattering of Cu Kα radiation (Figure 4), allowing an explicit assignment of the absolute configuration of 1 as 1S, 2S, 3R, 4S, 5R, and 6S. In order to confirm the absolute configuration assignment, Lactone sector rule (Jexkings et al., 1965) based on ECD data was used. The molecule was viewed from the line on the plane of the ester group along the bisectrix of the O–C = O angle, i.e., as shown in Figure 5 for 4S. The functional group at C-4 lying in the back upper right sector was responsible for the positive CD Cotton effect resulted from n–π* transition of lactone, which was well in accordance with the positive Cotton effect at λmax 223 nm of 1 (Supplementary Figure 24 in Supplementary Material). Hence, compound 1, named as dendroterpene A, was determined to be a picrotoxane-type sesquiterpene.
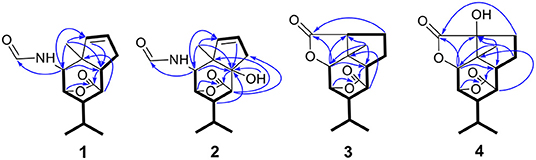
Compound 2 was also isolated as colorless crystals. The HRESIMS (m/z 302.1376 [M + Na]+, calcd. for C15H21NO4Na: 302.1363) indicated a molecular formula of C15H21NO4. The 1D and 2D NMR data of 2 (Table 1 and Figure 2) were very similar to those of 1, except that an oxygenated quaternary carbon C-6 (δC 80.6) in 2 replaced signals corresponding to CH-6 in 1 (δC/H 42.5/2.33). In the HMBC spectrum (Figure 2 and Supplementary Figure 11 in the Supplementary Material) of 2, correlations from H-2, H-4, H-8, H-9, 6-OH, and H3-10 to the oxygenated quaternary carbon C-6, together with the molecular formula, suggested that the H-6 in 1 was oxygenated to a hydroxylated quaternary carbon in 2. The H-2, 6-OH and H3-10 were on the same face of the six-member ring, and the H-4 was on the opposite face in 2, as evidence by the ROESY correlations (Figure 3 and Supplementary Figure 12 in the Supplementary Material) of H3-10 with H-2, H-13 and 6-OH, and of H-2 and 6-OH with H-13. The relative configurations of C-3 and C-5 in 2 were identical with those of 1 according to the chemical structure models analysis of 2.
The above assignment was further confirmed by a single-crystal X-ray diffraction pattern obtained using the anomalous scattering of Cu Kα radiation (Figure 4), which also led to an unambiguous assignment of the absolute configuration of 2 as 1R, 2S, 3R, 4S, 5S, and 6R. The absolute configuration of 2 was also confirmed by Lactone sector rule based on ECD data (Jexkings et al., 1965) (Figure 5 and Supplementary Figure 24 in Supplementary Material). Therefore, compound 2 was also identified as a picrotoxane-type sesquiterpene, and named as dendroterpene B.
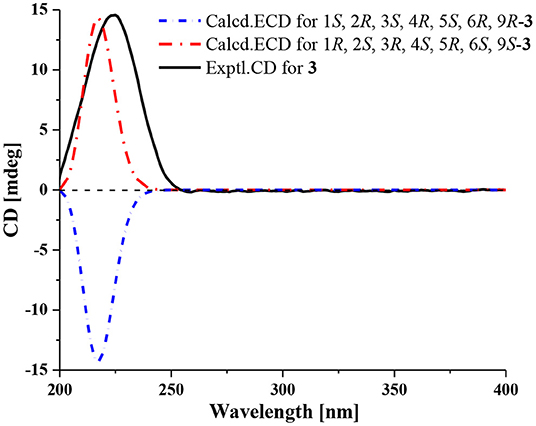
Compound 3 has the molecular formula of C15H20O4 based on HRESIMS (m/z 287.1281 [M + Na]+, calcd for C15H20O4Na:287.1254), suggesting six degrees of unsaturation. 1D NMR and HSQC data displayed three methyls (δC/H 31.3/1.52, 21.4/1.02 and 20.6/1.04), two methylenes, seven methines including two oxygenated ones (δC/H 81.5/4.28 and 77.9/4.75), three quaternary carbons including two ester carbonyl carbons (δC 177.5 and 177.1). The above data of 3 were very similar to those of the previously reported dedrobiumane E (Zhao et al., 2003). Their structural differences were that two oxygenated quaternary carbons C-6 (δC 76.8) and C-9 (δC 83.8) and two oxygenated methine groups CH-7 (δC/H 65.2/4.05) and CH-8 (δC/H 60.1/3.86) in dedrobiumane E were replaced by two sp3 methines (δC/H 43.6/2.20 and δC/H 55.2/2.77) and two sp3 methylenes (δC/H 32.4/2.15 and δC/H 27.8/2.01, 2.14) in 3, as proved by the sequential COSY correlations of H-5/H-6/H-7/H-8/H-9, along with the detail interpretation of the HMBC correlations from H-2 to C-6, C-9, and C-11, from H3-10 to C-2, C-6, and C-9, from H-3 and H-6 to C-16, from H-5 to C-1, as well as from H-8 to C-11 (Figure 2). The ROESY experiments (Figure 3 and Supplementary Figure 18 in the Supplementary Material) displayed the correlations from H3-10 to H-2, H-6, and H-9, as well as the correlations between H-2 and H-13, indicating H-2, H-6, H-9, and H3-10 were on the same face, while H-4 was on the face opposite to them. The relative configurations of C-3 and C-5 in 3 were also determined to be consistent with those of 1 and 2 through the same method. The calculated ECD curve for 3 matched well with the experimental one (Figure 6), assigning the absolute configurations of 3 as 1R, 2S, 3R, 4S, 5R, 6S, and 9S (Wang et al., 2014). Thus, the structure of 3 was established as picrotoxane-type sesquiterpene shown in Figure 1, and named as dendroterpene C.
Compound 4, colorless crystals, the molecular formula of which was established to be C15H20O5 according to the pseudo-molecular ion peak at m/z 303.1228 [M + Na]+ (calcd. for C15H20O5Na: 303.1203) in the HRESIMS spectrum. The similarity of NMR data (Table 1 and Figure 2) between 3 and 4 indicated that they have the same picrotoxane-type sesquiterpene skeleton. The only structural difference between them was CH-9 (δC/H 55.2/2.77) in 3 was oxygenated to a hydroxylated quaternary carbon (δC 85.1) in 4, as supported by sequential COSY correlations of H-2/H-3/H-4/H-5/H-6/H-7/H-8, together with the HMBC correlations from H-2, H-6, H-7, and H3-10 to C-9. The stereochemical structure of 4 (1S, 2S, 3R, 4S, 5R, 6S, and 9R), was determined by a single-crystal X-ray diffraction pattern obtained using the anomalous scattering of Cu Kα radiation (Figure 4). From a biosynthetic consideration, the absolute configurations of 4 were deduced be identical to those of 1-3. Hence, compound 4 was also identified as a picrotoxane sesquiterpene as shown in Figure 1, named as dendroterpene D.
The Postulated Biosynthesis Pathway of Compounds 1–4
The carbon skeletons of previously reported picrotoxane-type sesquiterpenes are highly conserved.
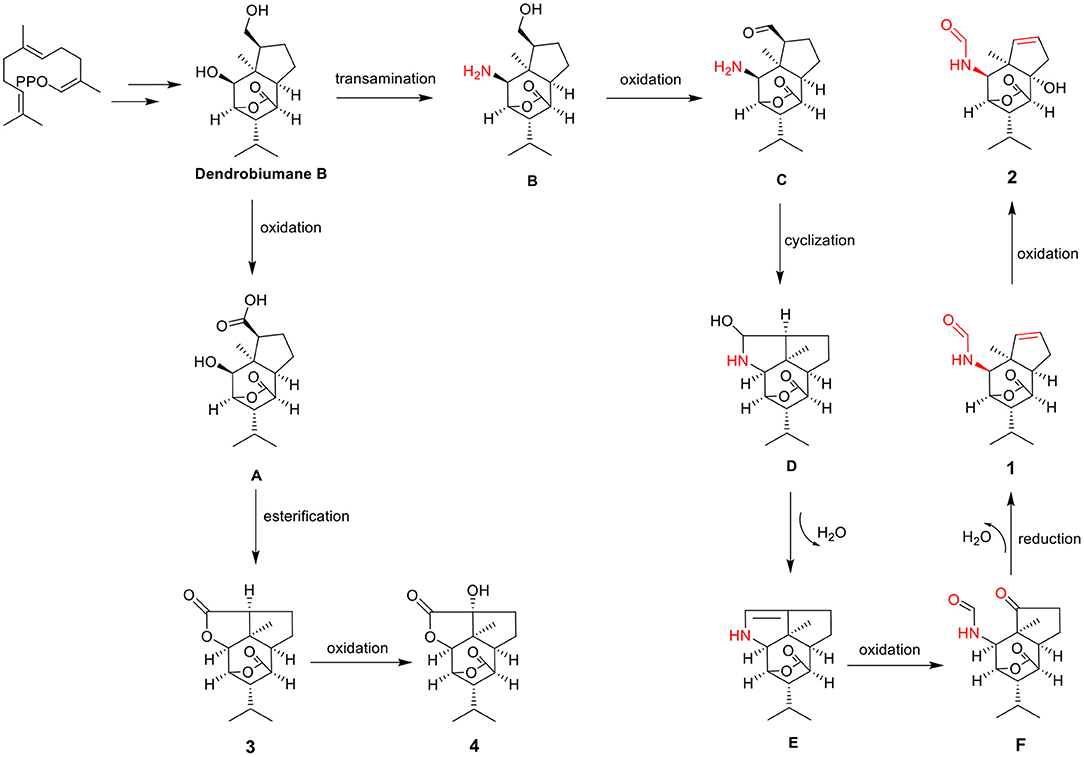
The main skeletal changes are that one is the C-11-O-C-2 ring or the C-11-N-C-2 ring closure exemplified by dendrobiumane E and the other is the C-11-O-C-2 ring or the C-11-N-C-2 ring opening illustrated by dedrobiumane B. In our present study, the discovery of 1 and 2 with a new carbon skeleton containing a formamide group, which was derived from unprecedented carbon bond cleavage pattern prompted us to study its plausible biosynthetic pathways (Figure 7). Dendrobiumane B was assumed to be the biosynthetic precursor of 1–4. Dendrobiumane B underwent oxidation to produce the intermediate (A). A underwent esterification and the C-11-O-C-2 ring closure produced compound 3, which underwent oxidation to afford compound 4. Dendrobiumane B underwent transamination to give the intermediate (B), which underwent oxidation to form intermediate (C). C underwent cyclization to produce intermediate (D), and D underwent dehydration to yield intermediate (E). E underwent oxidation to give intermediate (F), which underwent reduction and dehydration to produce compound 1. Then, 1 underwent oxidation to get 2.
Biological Activity
Compounds 1–8 were tested for inhibitory activity against α-glycosidase using the PNPG method and for cytotoxic effects on SGC-7901, K562, A549, BEL-7402, and Hela cell lines using the MTT method (See Supplementary Table 1 in the Supplementary Material). The result showed that compounds 3, 5, 6, and 8 exhibited potent inhibitory activity against α-glycosidase with IC50 values of 0.97, 0.03, 0.68, and 0.30 mM, respectively (Acarbose as positive control with IC50 value of 0.72 mM). Compound 5 displayed weak cytotoxic effects against SGC-7901, K562, A549, BEL-7402, and Hela cell lines with the IC50 values of 17.30, 10.39, 29.03, 20.13, and 22.19 μM, respectively (Cisplatin as positive control with IC50 values of 4.11, 3.08, 1.93, 4.02, and 11.29 μM), in addition, compound 6 showed cytotoxic effects against K562 with the IC50 value of 28.23 μM.
Conclusions
In conclusion, four new picrotoxane-type sesquiterpenes (1–4), together with four known compounds (5–8), were isolated from the stems of D. nobile. Compounds 1 and 2 are two new picrotoxane-type sesquiterpenes with a new carbon skeleton containing a formamide group, which may be derived from the previously reported dendrobiumane B skeleton by the C(9)-C(11) carbon bond cleavage. In the previously report, picrotoxane sesquiterpenes exhibited the angiogenesis effect against sunitinibinduced damage (Meng et al., 2017) and inhibitory activity of nerve growth factor mediated neurite outgrowth (Leon et al., 2019). Compound 3 exhibited inhibitory activity against α-glycosidase with IC50 values of 0.97 mM. However, compound 4 is inactive (IC50 > 1 mM) against α-glycosidase, indicating that the hydroxyl at C-9 may reduce the activity. In addition, the known bibenzyl derivative nobilin E (5) exhibited inhibitory effects on NO production in activated murine macrophage-like cell line RAW 264.7 in the previously report (Zhang et al., 2007b). The bioactivities of the known bibenzyl derivative dendrocandin V (6) have not been reported. In this report, compounds 5 and 6 both exhibited inhibitory activity against α-glycosidase with IC50 values of 0.03 and 0.68 mM, respectively. In addition, Compound 5 displayed weak cytotoxic effects against SGC-7901, K562, A549, BEL-7402 and Hela cell lines with the IC50 values of 17.30, 10.39, 29.03, 20.13, and 22.19 μM, respectively. Compound 6 showed cytotoxic effect against K562 with the IC50 value of 28.23 μM.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
PW, XC, HW, CC, JY, and XX performed the experiments. PW and XC identified the structures. GZ performed ECD calculation. PW, HD, and WM conceived and designed the experiments. SH collected the fresh stems of D. nobile. PW wrote the paper. All authors have approved the final version of the manuscript.
Funding
This research was financially supported by Natural Science Foundation of Hainan (No. 218QN284), Major Technology Project of Hainan Province (No. ZDKJ201805), Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630052016008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00812/full#supplementary-material
These data include 1D and 2D NMR spectra of compounds 1–4, CD spectra of 1 and 2, and cytotoxic and α-glycosidase inhibitory activities of compounds 1–8.
References
Cammi, R., and Tomasi, J. (1995). Remarks on the use of the apparent surface charges (ASC) methods in solvation problems: Iterative versus matrix-inversion procedures and the renormalization of the apparent charges J. Comput. Chem. 16, 1449–1458. doi: 10.1002/jcc.540161202
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2004). Gaussian 03, Revision C.01. Wallingford, CT: Gaussian Inc.
Gross, E. K.U., Dobson, J. F., and Petersilka, M. (1996). Density functional theory of time-dependent phenomena. Dens. Func. Theory II. 81–172. doi: 10.1007/BFb0016643
Hwang, J. S., Lee, S. A., Hong, S. S., Han, X. H, Lee, C., Kang, S. J., Lee, D., et al. (2010). Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg. Med.Chem.Lett. 20, 3785–3787. doi: 10.1016/j.bmcl.2010.04.054
Jexkings, J. P., Klyne, W., and Scope, P. M. (1965). Optical rotatory dispersion. Part XXIV. Lactones. J. Chem. Soc. 7211–7229. doi: 10.1039/jr9650007211
Jiangsu New Medical College (1986). Dictionary of Chinese Herb Medicines. Shanghai: Shanghai Scientific and Technologic Press.
Jong, A. N., Bhandari, M. R., and Kawabata, J. (2007). α-Glucosidase inhibitors from Devil tree. Food Chem. 103. 1319–1323. doi: 10.1016/j.foodchem.2006.10.043
Kato, T., Tsunakawa, M., Sasaki, N., Aizawa, H., Fujita, K., Kitahara, Y., et al. (1977). Growth and germination inhibitors in rice husks. Phytochemistry 16, 45–48. doi: 10.1016/0031-9422(77)83010-0
Krysin, A. P., Egorova, T. G., and Vasil'ev, V. G. (2010). Thermolysis of 4-(ω- hydroxyl alkyl)-2,6-di-tert-butylphenols. Russ. J. Gen. Chem. 80, 275–283. doi: 10.1134/S1070363210020167
Leon, R. M., Ravi, D., An, J. S., Genio, C. L. D., Rheingold, A. L., Gaur, A.B., et al. (2019). Synthesis of C14-desmethylene corialactone D and discovery of inhibitors of nerve growth factor mediated neurite outgrowth. Org. Lett. 21, 3193–3197. doi: 10.1021/acs.orglett.9b00921
Liu, Q. F., and Zhao, W. M. (2003). A new dendrobine-type alkaloid from Dendrobium nobile. Chinese Chem. Lett. 14, 278–279.
Meng, C. W., He, Y. L., Peng, C., Ding, X. J., Guo, L., and Xiong, L. (2017). Picrotoxane sesquiterpenoids from the stems of Dendrobium nobile and their absolute configurations and angiogenesis effect. Fitoterapia 121, 206–211. doi: 10.1016/j.fitote.2017.07.017
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. doi: 10.1016/0022-1759(83)90303-4
Wang, P., Kong, F. D., Wei, J. J., Wang, Y., Wang, W., Hong, K., et al. (2014). Alkaloids from the mangrove-derived actinomycete Jishengella endophytica 161111. Mar. Drugs 12, 477–490. doi: 10.3390/md12010477
Xiao, S. J., Weng, Q. H., Zhang, M. S., Nie, X. Q., Chen, Y. Z., He, Y. Q., et al. (2017). Dendrocandin V, a new compound separation from Dendrobium nobile and its extraction and separation method. Chinese Patent. 107021947A.
Zhang, X., Liu, H. W., Gao, H., Han, H. Y., Wang, N. L., Wu, H. M., et al. (2007a). Nine New Sesquiterpenes from Dendrobium nobile. Helvetica Chimica Acta 90, 2386–2394. doi: 10.1002/hlca.200790245
Zhang, X., Xu, J. K., Wang, J., Wang, N. L., Kurihara, H., Kitanaka, S., et al. (2007b). Bioactive Bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 70, 24–28. doi: 10.1021/np060449r
Zhao, W., Ye, Q., Tan, X., Jiang, H., Li, X., Chen, K., et al. (2001). Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J. Nat. Prod. 64, 1196–1200. doi: 10.1021/np0102612
Zhao, W. M., Ye, Q. H., Dai, J. Q., Martin, M. T., and Zhu, J. P. (2003). Allo-aromadendrane- and picrotoxane-type sesquiterpenes from Dendrobium moniliforme. Planta. Med. 69, 1136–1140. doi: 10.1055/s-2003-818005
Keywords: Dendrobium nobile Lindl, picrotoxane, sesquiterpenes, α-glycosidase inhibitor, cytotoxicity
Citation: Wang P, Chen X, Wang H, Huang S, Cai C, Yuan J, Zhu G, Xu X, Mei W and Dai H (2019) Four New Picrotoxane-Type Sesquiterpenes From Dendrobium nobile Lindl. Front. Chem. 7:812. doi: 10.3389/fchem.2019.00812
Received: 09 September 2019; Accepted: 11 November 2019;
Published: 29 November 2019.
Edited by:
Tao Wang, Tianjin University of Traditional Chinese Medicine, ChinaReviewed by:
Jian Guang Luo, China Pharmaceutical University, ChinaCaijuan Zheng, Hainan Normal University, China
Shuang-Gang Ma, China Academy of Chinese Medical Sciences, China
Copyright © 2019 Wang, Chen, Wang, Huang, Cai, Yuan, Zhu, Xu, Mei and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenli Mei, bWVpd2VubGlAaXRiYi5vcmcuY24=; Haofu Dai, ZGFpaGFvZnVAaXRiYi5vcmcuY24=
 Pei Wang
Pei Wang Xin Chen
Xin Chen Hao Wang
Hao Wang Shengzhuo Huang1
Shengzhuo Huang1 Caihong Cai
Caihong Cai Jingzhe Yuan
Jingzhe Yuan Xinglian Xu
Xinglian Xu Wenli Mei
Wenli Mei Haofu Dai
Haofu Dai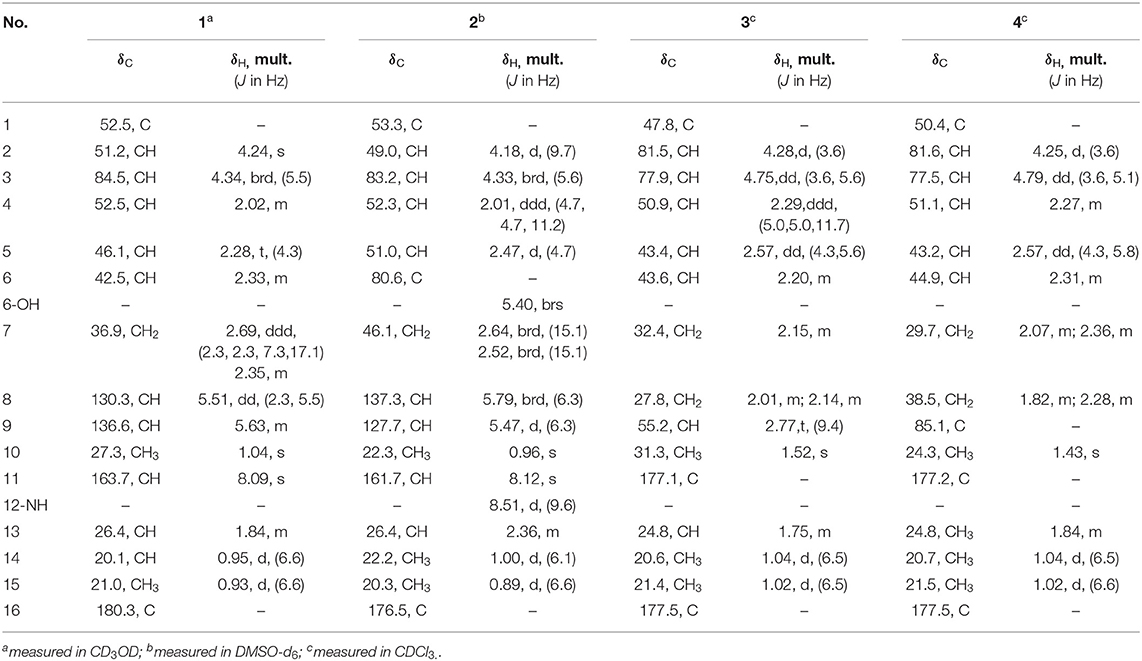
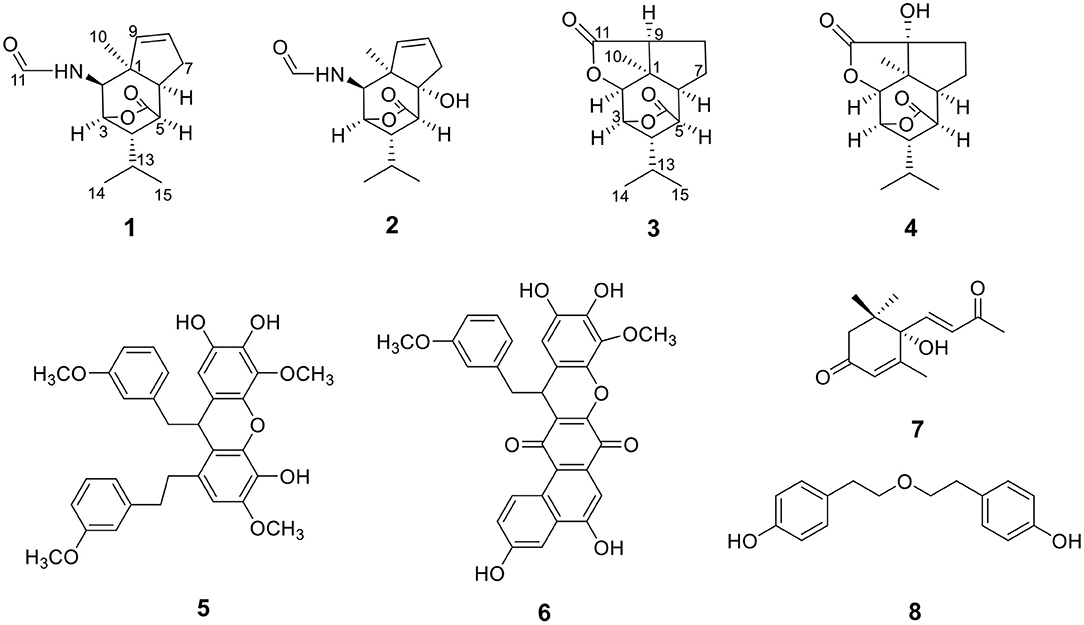
 ) and 1H–1H COSY (−) correlations of compounds 1–4.
) and 1H–1H COSY (−) correlations of compounds 1–4.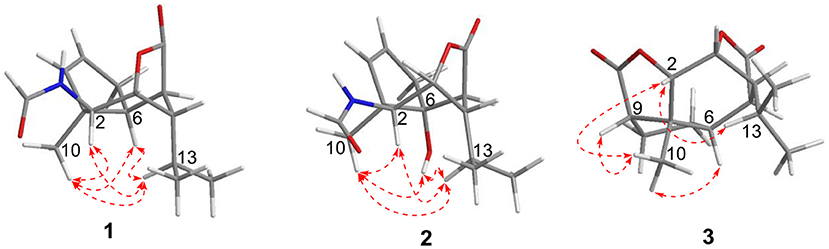
 ) correlations of compounds 1–3.
) correlations of compounds 1–3.