- School of Pharmaceutical Sciences (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Shenzhen, China
Mangrove-derived endophytes are rich in bioactive secondary metabolites with a variety of biological activities. Recently, a fungus Pseudofusicoccum sp. J003 was first isolated by our research group from mangrove species Sonneratia apetala Buch.-Ham. The subsequent chemical investigation of the methanol extract of the culture broth of this strain has led to the isolation of a new sesquiterpenoid named acorenone C (1), two alkaloids (2–3), four phenolic compounds (4–7), and four steroid derivatives (8–11). The new structure of 1 was established by extensive spectroscopic analysis, including 1D, 2D NMR spectroscopy, and HRESIMS. Its absolute configuration was elucidated by experimental ECD and ECD calculation. The in vitro AChE inhibitory, anti-inflammatory, and cytotoxic activities of the selected compounds were evaluated. The results showed that compound 1 showed mild AChE inhibitory activity, with an inhibition rate of 23.34% at the concentration of 50 μM. Compound 9 exerted a significant inhibitory effect against nitric oxide (NO) production in LPS-stimulated RAW 264.7 mouse macrophages, with an inhibition rate of 72.89% at the concentration of 25 μM, better than that of positive control L-NMMA. Compound 9 also displayed obvious inhibition effects on the growth of two human tumor cell lines, HL-60 and SW480 (inhibition rates 98.68 ± 0.97% and 60.40 ± 4.51%, respectively). The antimicrobial activities of the compounds (1–11) against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa were also tested; however, none of them showed antimicrobial activities.
Introduction
The great diversity of creatures in the ocean was found to be a rich reservoir of candidates for drug development (Sigwart et al., 2021). To date, more than 35,000 marine natural products have already been discovered, which have a higher rate of successful drug discovery than other naturally occurring compounds (Lyu et al., 2021; Sigwart et al., 2021). Mangroves are an intertidal wetland ecosystem spreading across low-latitude tropical and subtropical regions, which are found to have potential to control coastal erosion and protect coastal land. The ingredients produced by mangrove plant species may play a role in helping them survive from universally unfavorable factors (Bandaranayake, 2002). Many types of natural products have been identified from mangroves and their endophytes, including heterocyclic compounds, benzofurans, alkaloids, lignin, polysaccharides, fatty acids, lipids, anthocyanins, flavonoids, phenols and quinones, tannins, limonin, terpenoids, steroids, and saponins (Carroll et al., 2020).
Recently, our research group aimed at structurally diverse natural products from the mangroves and their endophytes for pharmaceutical drug discovery. As a small- to medium-sized columnar true mangrove, the plant species Sonneratia apetala Buch.-Ham. is native to South Asia and Southeast Asia and has been cultivated in Guangdong and Hainan provinces, China (Hossain et al., 2016). S. apetala has versatile pharmacological effects, for example, the extracts of barks and leaves of S. apetala exhibited antibacterial, antioxidant, anti-diabetic, and anti-cancer activities (Patra et al., 2015). However, the endophytes of S. apetala were scarcely investigated.
In this work, a fungus Pseudofusicoccum sp. J003 was isolated from the fruit of S. apetala for the first time. Previous studies on the secondary metabolites obtained from the genus Pseudofusicoccum by other research groups revealed the presence of phenolic compounds (Abba et al., 2018), cyclopeptides, and rotenoids (Sobreira et al., 2018). In our study, the chemical investigation into the methanol extract of this strain by repeated column chromatography over silica gel, Sephadex LH-20, RP-C18 silica, and semi-preparative HPLC resulted in the isolation of a new sesquiterpenoid (1) (Figure 1), two alkaloids (2–3), four phenolic compounds (4–7), and four steroid derivatives (8–11). Herein, the isolation, structure determination of isolated compounds, and evaluation of their in vitro anti-inflammatory, antimicrobial, cytotoxic, and AChE inhibitory activities were described.
Materials and Methods
General Experimental Procedures
The optical rotations, CD, and FT-IR spectra were measured with a Perkin-Elmer 341 polarimeter (PerkinElmer, Waltham, MA, USA), JASCO J-810 spectrometer (Jasco Corporation, Japan), Bruker Vertex 70 FT-IR spectrophotometer (Bruker, Karlsruhe, Germany), respectively. The UV spectrum was recorded using a Waters e2695 spectrophotometer (Waters, Massachusetts, USA) equipped with a DAD and a 1-cm-path length cell. Samples in methanol solution were scanned from 190 to 400 nm in 1-nm steps. The structure characterization of the obtained compound was based on 1D NMR (1H, 13C) and 2D NMR (COSY, HSQC, HMBC, and NOESY) data, recorded on the Bruker AM-400, AM-500, and AM-700 NMR spectrometers (Bruker, Karlsruhe, Germany) with TMS as internal standard, respectively. The detailed parameters for the NMR data of all isolates are provided (see Supporting Information, Supplementary Figures S3–S9; Supplementary Figures S11–S30). Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. HRESIMS data were acquired on a Thermo Fisher LTQ XL LC/MS (Thermo Fisher, Palo Alto, CA, USA). Semi-preparative HPLC was performed on an Agilent 1220 apparatus equipped with a UV detector with a semi-preparative column (RP-C18, 5 μm, 250 × 10 mm, Welch Materials, Inc.). Column chromatography was performed using silica gel (200–300 mesh and 80–120 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China) and SephadexTM LH-20 gel (40–70 μm; Merck, Darmstadt, Germany). Fractions were monitored by TLC (GF254, Qingdao Marine Chemical Co., Ltd., Qingdao), and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH. All solvents were of analytical grade (Guangzhou Chemical Regents Company, Ltd., Guangzhou, China).
Fungal Isolation and Fermentation
The fungal strain Pseudofusicoccum sp. J003 was isolated from the fruit of Sonneratia apetala Buch.-Ham., which was collected at a wetland of Nansha district, Guangzhou, China, in September 2020. The sequence data for this strain have been submitted to the GenBank under accession no. MZ854244. The fungal strain was deposited on 20% aqueous glycerol stock in a −80°C freezer at the School of Pharmaceutical Sciences (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Shenzhen, China. The strain was cultured on potato dextrose agar for 5 days at 28°C. Agar plates, including the strain, were cut into small pieces, and then these pieces were inoculated in a tissue culture bottle (150 × 350 ml) on a solid rice medium (40 g of rice and 35 ml of distilled water) and cultured at room temperature for 30 days.
Extraction and Isolation of Secondary Metabolites
Cultural media were extracted with methanol three times. Methanol was removed by reduced pressure evaporation at 45°C, and the remaining aqueous phase was extracted 4 times with ethyl acetate. The ethyl acetate layer was concentrated under reduced pressure to yield a brown extract (60.0 g). The crude extract was introduced to a silica gel chromatography column (CC) and eluted with petroleum ether/ethyl acetate (35:1→0:1) to obtain seven fractions (Fr. 1–Fr. 7). Fr. 2 (7.3 g) was separated into 7 subfractions (Fr. 2.1–Fr. 2.7) using silica gel CC and eluted with n-hexane/2-propanol. Fr. 2.2 (102.3 mg) was purified by semi-preparative HPLC (100% MeOH, 3.0 ml/min) to yield 4 (21.6 mg, tR 24.5 min). Fr. 2.6 (800.5 mg) was purified by semi-preparative HPLC (45% MeOH/H2O, v/v, 3.0 ml/min) to yield 5 (1.3 mg, tR 8.5 min). Fr. 2.4 (113.0 mg) and Fr. 2.7 (220.7 mg) were separated by repeated CC over silica gel to yield 10 (3.4 mg) and 9 (6.7 mg). Fr. 4 (500.5 mg) was separated with repeated silica gel CC to yield six fractions (Fr. 4.1–Fr. 4.6) and then subjected subfraction Fr. 4.3 (268.3 mg) to a Sephadex LH-20 CC (CHCl2–MeOH, 1:1) to afford three parts (Fr. 4.4a–Fr. 4.4c). Fr. 4.4b (43.2 mg) was purified by semi-preparative HPLC (100% MeOH/H2O, v/v, 3.0 ml/min) to yield 8 (14.0 mg, tR 14.5 min). Fr. 4.4c (55.8 mg) was purified by semi-preparative HPLC (70% MeOH/H2O, v/v, 3.0 ml/min) to yield 1 (2.0 mg, tR 23.1 min). Fr. 5 (18.9 g) was separated with repeated Sephadex LH-20 CC (MeOH) to yield 6 (7.1 mg) and 7 (90.0 mg). Fr. 7 (7.7 g) was separated with repeated silica gel CC and eluted with CH2Cl2/MeOH and n-hexane/2-propanol to yield 2 (22.0 mg), 3 (20.0 mg), and 11 (10.0 mg).
Acorenone C 1) Colorless oil;
Anti-AChE Assay
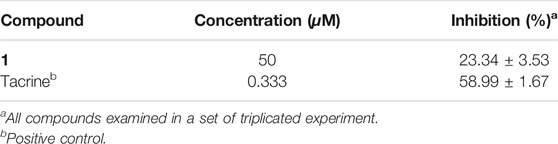
Acetylcholinesterase (AChE) inhibitory activity of the compounds isolated was assayed by the spectrophotometric method with slight modification (Ellman et al., 1961). S-Acetylthiocholine iodide, S-butyrylthiocholine iodide, 5,5′-dithio-bis-(2-nitrobenzoic) acid (DTNB, Ellman’s reagent), and acetylcholinesterase derived from human erythrocytes were purchased from Sigma Chemical. The compounds were dissolved in DMSO. The reaction mixture (totally 200 μL) containing phosphate buffer (pH 8.0), a test compound (50 μM), and acetyl cholinesterase (0.02 U/mL) was incubated for 20 min (37°C). Then the reaction was initiated by the addition of 40 μL of a solution containing DTNB (0.625 mM) and acetylthiocholine iodide (0.625 mM) for AChE inhibitory activity assay, respectively. The hydrolysis of acetylthiocholine was monitored at 405 nm every 30 s for 1 h. Tacrine was used as a positive control with a final concentration of 0.333 μM. All the reactions were performed in triplicate. The percentage inhibition was calculated as follows: % inhibition = (E–S)/E × 100 (E is the activity of the enzyme without the test compound and S is the activity of the enzyme with the test compound).
Anti-Inflammatory Assay
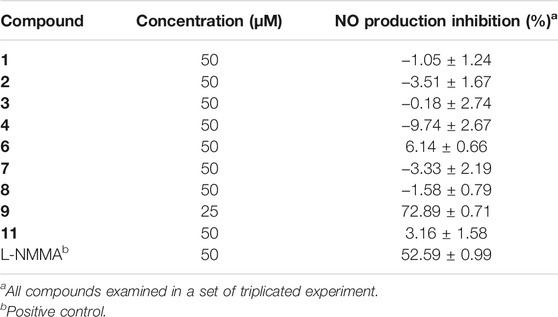
The RAW 264.7 cells (2 × 105 cells/well) were incubated in 96-well culture plates with or without 1 μg/ml LPS (Sigma Chemical Co., USA) for 24 h in the presence or absence of the test compounds. Aliquots of supernatants (50 µL) were then reacted with 100 µL Griess reagent (Sigma Chemical Co., USA). The absorbance was measured at 570 nm by using the Synergy TMHT Microplate Reader (BioTek Instruments Inc., USA). In the study, L-NMMA (Sigma Chemical Co., USA) was used as a positive control. In the remaining medium, an MTT assay was carried out to determine whether the suppressive effect was related to cell viability. The inhibitory rate of NO production = (NO level of blank control – NO level of test samples)/NO level of blank control. The percentage of NO production was evaluated by measuring the amount of nitrate concentration in the supernatants with Griess reagent, as described previously (Wu et al., 2017).
Cytotoxicity Assay
Five human cancer cell lines, including the A549 lung cancer cell line, the HL-60 human myeloid leukemia cell line, the MCF-7 breast cancer cell line, the SMMC-7721 human hepatocarcinoma cell line, and the SW-480 human pancreatic carcinoma were used. Cells were cultured in RPMI-1640 or DMEM medium, supplemented with 10% fetal bovine serum and 5% CO2 at 37°C. The cytotoxicity assay was performed using an MTTS 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium) method in 96-well microplates, as reported previously (Liu et al., 2012), with slight modification. In brief, 100 μL of adherent cells were seeded into each well of the 96-well culture plates and allowed to adhere for 12 h before adding the test compounds, while suspended cells were seeded into wells at a density of 1 × 105 cells/mL just prior to the addition of the test compounds. Each tumor cell line was exposed to the test compound at concentrations of 40 μM in triplicates for 48 h. Wells with DMSO were used as negative controls, and Taxol and DDP were used as positive controls. After treatment of the compounds, cell viability was detected by a microplate reader at λ = 492 nm.
Antimicrobial Assay
Compounds 1–11 were evaluated for their antimicrobial activities against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa. The antimicrobial assay was conducted by the previously described method (Zhang et al., 2019). The sample to be tested was added into a 96-well culture plate, and the maximum concentration of the used compounds was 250 μg/ml. Bacteria liquid was added to each well until the final concentration is 5 × 105 CFU/ml. It was then incubated at 37°C for 24 h, and the OD value at 595 nm was measured by the microplate reader, and the medium blank control was used in the experiment.
Results and DISCUSSION
Identification of Compounds
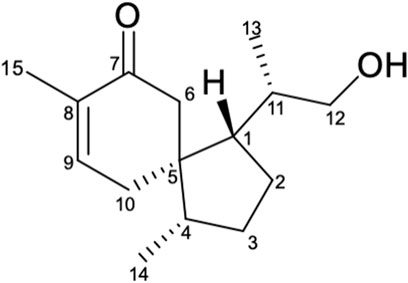
Compound 1 was obtained as colorless oil. Its molecular formula was determined to be C15H24O2 based on the deprotonated ion peak [M + Na]+ at m/z 259.1679 [M + Na]+ (calcd for 259.1669) in the (+)-HRESIMS, indicating 4 degrees of unsaturation. The IR spectrum showed characteristic absorption bands of hydroxyl (3,429 cm−1) and the carbonyl groups (1,662 cm−1). The 13C NMR and DEPT spectra of 1 (Table 1) showed 15 carbon signals, including three methyls, five methylenes (including oxygenated methylene at δc 68.5), three methine groups, two olefinic carbon signals (δc 147.1 and 136.1), an aliphatic quaternary carbon, and a carbonyl carbon (δC 203.2). The 1H NMR spectrum of 1 (Table 1) displayed the presence of an olefinic proton resonated at δH 6.82 (t like, J = 3.9 Hz), two methyl group doublets at 0.91 (d, J = 6.7 Hz) and 0.84 (d, J = 6.8 Hz), and two oxygenated methine protons at δH 3.36 and 3.38. Apart from the two degrees of unsaturation occupied by the carbonyl group and a double bond, the remaining degrees of unsaturation suggested that compound 1 should be a dicyclic sesquiterpenoid (Amandine et al., 2017).
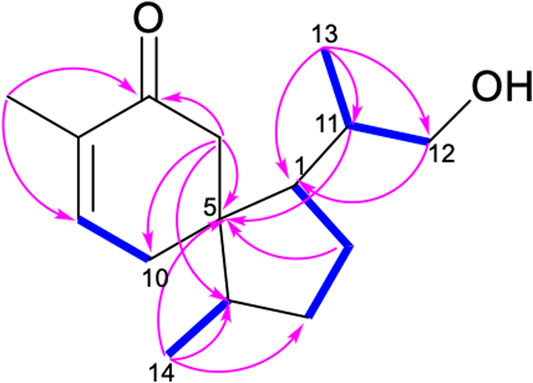
The 1H-1H COSY spectrum of 1 indicated the presence of spin systems of (HO)CH2(12)-CH(11)-CH3(13) and CH(1)-CH2(2)-CH2(3)-CH(4)-CH3(14) (Figure 2). In the HMBC spectrum, the HMBC interactions from H-13 (δH 0.91) and H-12 (δH 3.36 and 3.38) to C-11 (δC 36.3) and C-1 (δC 52.2) revealed the direct C–C linkage from C-11 to C-1 (Figure 2). Subsequently, the HMBC correlations of H-2 and H-11 with C-5 (δC 50.0) and of H-14 with C-3, C-4, and C-5 indicated the presence of a methyl cyclopentane substructure with a 1-propanol substituted at C-1. The spin system of = CH(9)–CH2(10) observed from the 1H–1H COSY spectrum of 1, together with the key HMBC correlations from H3-15 (δH 1.75) to C-7 (δC 203.2)/C-8 (δC 136.1)/C-9 (δC 147.1), from H-6 (δH 2.24 and 2.63) to C-5/C-8/C-10, collaborated with the methyl cyclohexane substructure decorated by an α,β-unsaturated ketone functionality. Based on the aforementioned pieces of evidence, the crucial HMBC correlations from H-6 and H-10 to C-1, C-4, and C-5 and from H-1 and H-4 to C-5, C-6, and C-10 constructed the gross structure of 1, featuring a spiro[4,5]decane scaffold. The planar structure of 1 was thus deduced as shown (Figure 2), resembling the (3S)-1,4-epi-3-hydroxyacorenone (Calva et al., 2017).
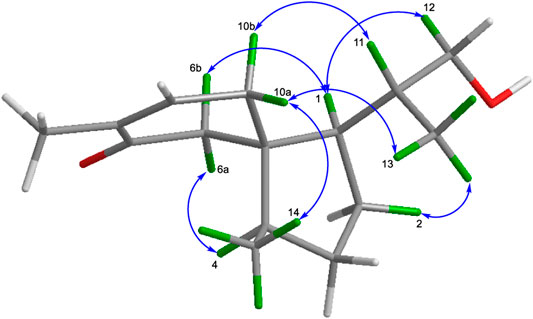
The relative configurations of 1 were elucidated by the observation of its NOESY spectrum. The NOESY correlations of H-10a with H3-13 and H3-14, and Me-13/H-2a revealed that H-10a, H3-13, and H3-14 were co-facial and provisionally assigned to be α-oriented. Accordingly, the NOESY cross-peaks of H-11/H-10b, H-1/H-6b, H-6a/H-4, and H-1/H-12a indicated the β-orientation of H-1, H-4, and H-11. The relative stereochemistries at C-1, C-4, C-5, and C-11 of 1 were thus determined (Figure 3). Therefore, as for the absolute configuration of 1, two possible enantiomers (Figures 4A,B) were presented (Figure 4).
To further determine the absolute stereochemistry of 1, the electronic circular dichroism (ECD) (for detailed procedures, see SI) calculation of the two possible enantiomers (Figures 5A,B; Figures 4, 5) was performed using Gaussian 09 and figured using GaussView 5.0 (Dennington et al., 2009; Frisch et al., 2009). Conformation search via molecular mechanics calculations was conducted in Discovery Studio 3.5 Client, with an MMFF force field with a 20 kcal/mol upper energy limit (Smith and Goodman, 2010). The optimized conformation geometries and thermodynamic parameters of the selected conformations were provided. The predominant conformers were subsequently optimized at the B3LYP/6-31G(d,p) level. The theoretical calculation of ECD was performed using a time-dependent density functional theory (TDDFT) at the B3LYP/6-31G(d,p) level in MeOH with the PCM model. The calculated spectrum of 1b (1S,4S,5R,11S) agreed with the experimental data, showing a negative Cotton effect (CE) at 213 nm and a strong positive CE at 244 nm (Figure 5). Consequently, the structure of 1 was determined to be (1S,4S,5R)-1-((S)-1-hydroxypropan-2-yl)-4,8-dimethylspiro [4.5]dec-8-en-7-one, and a trivial acorenone C was given.
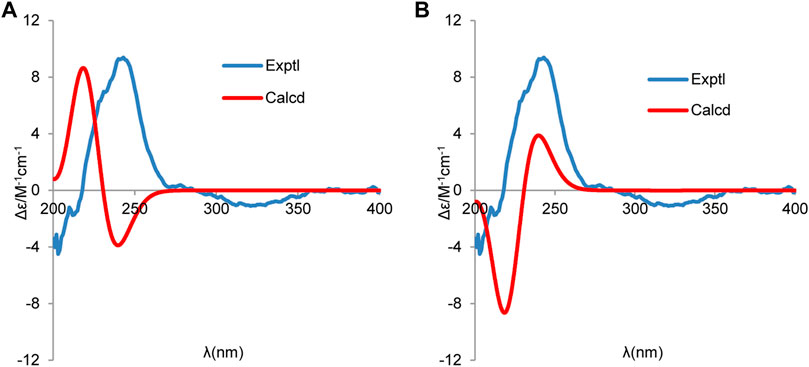
FIGURE 5. Experimental and calculated ECD spectra of (A) (1R,4R,5S,11R) and (B) (1S,4S,5R,11S) (red, calculated at the B3LYP-PCM/6-31G(d,p)//B3LYP/6-31G (d,p) level in CH3OH; blue, experimental in CH3OH).
Ten known compounds, uracil (2) (Xing et al., 2020), cyclo-(L-Pro-L-Tyr) (3) (Jayatilake et al., 1996), bis-(2-ethylhexyl) terephthalate (4) (Firdovsi et al., 2007), 4-hydroxybenzaldehyde (5) (Shataer et al., 2020), 2-phenylethanol (6) (Guerrini et al., 2011), 4-hydroxyphenethyl-alcohol (7) (Wei et al., 2013), estigmast-4-en-6β-ol-3-ona (8) (Correia et al., 2003), ergosterol (9) (Zhang et al., 2002), ergosterol peroxide (10) (Hybelbauerová et al., 2008), and cerevisterol (11) (Kang et al., 2017) were also isolated from Pseudofusicoccum sp. J003. The structures of these compounds (2–11) were elucidated by comparing the spectral data to those reported in the references.
Biological Activity
According to the literature, acorenone analogs usually have AChE inhibitory activity (Calva et al., 2017). The AChE inhibition effect of new compound 1 was tested. It exhibited mild inhibitory activity against AChE with an inhibition rate of 23.34% ± 3.53 at the concentration of 50 μM (Table 2). To further test in vitro anti-inflammatory activity, compounds 1–4, 6–9, and 11 were evaluated for their inhibitory activities against LPS-induced nitric oxide (NO) production in RAW 264.7 mouse macrophages, of which compound 9 showed obvious inhibitory activity, with an inhibition rate of 72.89% ± 0.71 at the concentration of 25 μM (Table 3). Since steroid derivatives were reported to have cytotoxic properties against tumor cells (Bok et al., 1999), compounds 8, 9, and 11 were selected to test their cytotoxic activities against five human cancer cell lines, including HL-60, A549, MCF-7, SMMC-7721, and SW480, of which compound 9 inhibited the proliferation of tumor cells HL-60, with an inhibition rate of 98.68% ± 0.97 and SW480 with an inhibition rate of 60.40% ± 4.51 at a concentration of 40 μM, respectively (Table 4). The antimicrobial activity of compounds 1–11 was also evaluated against the bacteria S. aureus, B. subtilis, P. aeruginosa, and E. coli. However, all of them were found to be devoid of significant activity (MIC >250 μg/ml).
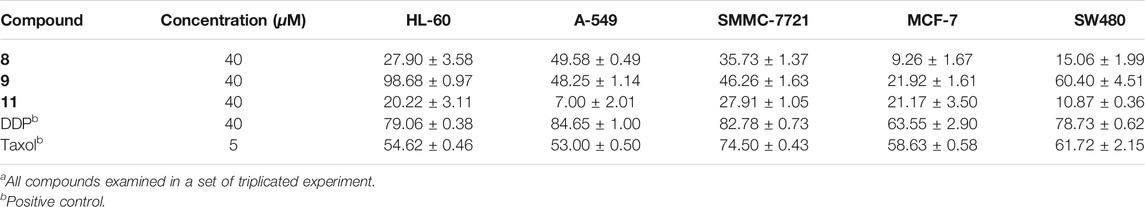
TABLE 4. In vitro cytotoxic activity (cell inhibition (%)) of compounds 8, 9, and 11 against five human tumor cell linesa
Conclusion
Mangroves with significant ecological significance and biodiversity have attracted broad interest from scientific communities. In this research, a new sesquiterpenoid called acorenone C (1), along with ten known compounds (2–11), was identified from the culture medium of an endophyte Pseudofusicoccum sp. J003, a fungus isolated from a mangrove species S. apetala. In addition, compounds 1–6 and 8–11 were identified from the genus Pseudofusicoccum for the first time. Their structures were established by extensive spectroscopic analyses, including 1D, 2D NMR spectroscopy, and HRESIMS, as well as ECD calculation. In the vitro bioassays, compound 1 showed mild AChE inhibitory activity, with an inhibition rate of 23.34% at the concentration of 50 μM. Compound 9 exerted a significant inhibitory effect against nitric oxide (NO) production in LPS-stimulated RAW 264.7 mouse macrophages, with an inhibition rate of 72.89% at the concentration of 25 μM, better than that of positive control L-NMMA. Compound 9 also displayed obvious inhibition effects on the growth of two human tumor cell lines HL-60 and SW480 (inhibition rates of 98.68 ± 0.97% and 60.40 ± 4.51%, respectively). The antimicrobial activities of the compounds (1–11) against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa were also tested; however, none of them showed antimicrobial activities. This work will add new bioactive marine natural products from microbes of mangrove plants.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/ MZ854244
Author Contributions
SJ conducted the main experiments and prepared the manuscript. SJ and WY were responsible for the isolation of compounds. SJ and XD did the antibacterial assay. SJ and XS conducted the anti-AChE assay. SJ, JL, and MW performed the anti-inflammatory assay. YW and XH did the cytotoxicity assay. SJ, XS, and YX identified the structures. XS and YX revised the manuscript. YX initiated the project and oversaw the research. All authors approved the submission of the manuscript for publication.
Funding
This research was financially supported by the National Natural Science Foundation of China (Nos. 31770379 and 21977120), the Key Basic Research Programme of the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20200109142215045), the Hundred Talents Program of Sun Yat-sen University (No. 75110-18841218). We express our sincere thanks to Xiaonian Li and Jianchao Chen for their helpful assistance in NMR measurement.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.780304/full#supplementary-material
References
Abba, C., Eze, M. P., Nwachukwu, C., Okoye, F., Eboka, C., Eze, P., et al. (2018). Phenolic Compounds from Endophytic Pseudofusicoccum Sp. Isolated from Annona Muricata. Tjnpr 2, 332–337. doi:10.26538/tjnpr/v2i7.6
André, A., Wojtowicz, N., Touré, K., Stien, D., and Eparvier, V. (2017). New Acorane Sesquiterpenes Isolated from the Endophytic Fungus Colletotrichum Gloeosporioides SNB-GSS07. Tetrahedron Lett. 58, 1269–1272. doi:10.1016/j.tetlet.2017.02.024
Bandaranayake, W. M. (2002). Bioactivities, Bioactive Compounds and Chemical Constituents of Mangrove Plants. Wetl. Ecol. Manag. 10, 421–452. doi:10.1023/A:1021397624349
Bok, J. W., Lermer, L., Chilton, J., Klingeman, H. G., and Towers, G. H. N. (1999). Antitumor Sterols from the Mycelia of Cordyceps Sinensis. Phytochemistry 51 (7), 891–898. doi:10.1016/s0031-9422(99)00128-4
Calva, J., Bec, N., Gilardoni, G., Larroque, C., Cartuche, L., Bicchi, C., et al. (2017). Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton Dissecta (Benth.) J.F. Macbr. Pharmaceuticals 10, 84. doi:10.3390/ph10040084
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2020). Marine Natural Products. Nat. Prod. Rep. 37, 175–223. doi:10.1039/C9NP00069K
Correia, S. d. J., David, J. P., and David, J. M. (2003). Constituintes das cascas de Tapirira guianensis (Anacardiaceae). Quím. Nova 26, 36–38. doi:10.1590/S0100-40422003000100008
Dennington, R., Keith, T., and Millam, J. (2009). Gauss View. Version 5. Shawnee Mission: Semichem Inc.
Ellman, G. L., Courtney, K. D., Andres, V., and Featherstone, R. M. (1961). A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 7, 88–95. doi:10.1016/0006-2952(61)90145-9
Firdovsi, S. T., Yagoub, M., and Parvin, A. E. (2007). Transesterification Reaction of Dimethyl Terephthalate by 2-Ethylhexanol in the Presence of Heterogeneous Catalysts under Solvent-free Condition. Chin. J. Chem. 25, 246–249. doi:10.1002/cjoc.200790049
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2009). Gaussian 09. Wallingford, CT: Gaussian, Inc.
Guerrini, A., Rossi, D., Paganetto, G., Tognolini, M., Muzzoli, M., Romagnoli, C., et al. (2011). Chemical Characterization (GC/MS and NMR Fingerprinting) and Bioactivities of South-African Pelargonium Capitatum (L.) L' Her. (Geraniaceae) Essential Oil. Chem. Biodiversity 8, 624–642. doi:10.1002/cbdv.201000045
Hossain, S. J., Iftekharuzzaman, M., Haque, M. A., Saha, B., Moniruzzaman, M., Rahman, M. M., et al. (2016). Nutrient Compositions, Antioxidant Activity, and Common Phenolics ofSonneratia apetala(Buch.-Ham.) Fruit. Int. J. Food Prop. 19, 1080–1092. doi:10.1080/10942912.2015.1055361
Hybelbauerová, S., Sejbal, J., Dračínský, M., Hahnová, A., and Koutek, B. (2008). Chemical Constituents ofStereum Subtomentosum and Two Other Birch-Associated Basidiomycetes: An Interspecies Comparative Study. C&B 5 (5), 743–750. doi:10.1002/cbdv.200890070
Jayatilake, G. S., Thornton, M. P., Leonard, A. C., Grimwade, J. E., and Baker, B. J. (1996). Metabolites from an Antarctic Sponge-Associated Bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 59, 293–296. doi:10.1021/np960095b
Kang, X., Xu, Y., Chen, G., and Wen, L. (2017). Study on Screening of Endophytic Fungi from Cyclosorus Parasiticus for Antibacterial Activity and Secondary Metabolites from the Active Fungus Pestalotiopsis Sp. CYC38. J. Guangdong Pharm. Univ. 33, 1–5. doi:10.16809/j.cnki.2096-3653.2016102401
Liu, X., Xue, Y.-B., Dong, K., Li, X.-N., Li, Y., Pu, J.-X., et al. (2012). Three New Ent-Kaurane Diterpenoids from Isodon Rubescens and Their Cytotoxicities. Chin. J. Nat. Medicines 10, 464–470. doi:10.1016/S1875-5364(12)60088-0
Lyu, C., Chen, T., Qiang, B., Liu, N., Wang, H., Zhang, L., et al. (2021). CMNPD: a Comprehensive marine Natural Products Database towards Facilitating Drug Discovery from the Ocean. Nucleic Acids Res. 49, D509–D515. doi:10.1093/nar/gkaa763
Patra, J. K., Das, S. K., and Thatoi, H. (2015). Phytochemical Profiling and Bioactivity of a Mangrove Plant, Sonneratia Apetala, from Odisha Coast of India. Chin. J. Integr. Med. 21, 274–285. doi:10.1007/s11655-014-1854-y
Shataer, D., Abdulla, R., Ma, Q. L., Liu, G. Y., and Aisa, H. A. (2020). Chemical Composition of Extract of Corylus Avellana Shells. Chem. Nat. Compd. 56, 338–340. doi:10.1007/s10600-020-03024-z
Sigwart, J. D., Blasiak, R., Jaspars, M., Jouffray, J.-B., and Tasdemir, D. (2021). Unlocking the Potential of marine Biodiscovery. Nat. Prod. Rep. 38, 1235–1242. doi:10.1039/d0np00067a
Smith, S. G., and Goodman, J. M. (2010). Assigning Stereochemistry to Single Diastereoisomers by Giao Nmr Calculation: The Dp4 Probability. J. Am. Chem. Soc. 132, 12946–12959. doi:10.1021/ja105035r
Sobreira, A. C. M., Pinto, F. d. C. L., Florêncio, K. G. D., Wilke, D. V., Staats, C. C., Streit, R. d. A. S., et al. (2018). Endophytic Fungus Pseudofusicoccum Stromaticum Produces Cyclopeptides and Plant-Related Bioactive Rotenoids. RSC Adv. 8, 35575–35586. doi:10.1039/c8ra06824k
Wei, S.-S., He, J.-B., and Yan, Y.-M. (2013). Studies on the Chemical Constituents of Tenodera sinensis Saussure Egg. J. Int. Pharm. Res. 32, 257–258. doi:10.13506/j.cnki.jpr.2013.05.013
Wu, Y., Xie, S.-S., Hu, Z.-X., Wu, Z.-D., Guo, Y., Zhang, J.-W., et al. (2017). Triterpenoids from Whole Plants of Phyllanthus Urinaria. Chin. Herbal Medicines 9, 193–196. doi:10.1016/S1674-6384(17)60095-9
Xing, Z., Wu, X., Zhao, J., Zhao, X., Zhu, X., Wang, Y., et al. (2020). Isolation and Identification of Induced Systemic Resistance Determinants from Bacillus Simplex Sneb545 against Heterodera glycines. Sci. Rep. 10, 11586. doi:10.1038/s41598-020-68548-4
Zhang, N., Shi, Z., Guo, Y., Xie, S., Qiao, Y., Li, X.-N., et al. (2019). The Absolute Configurations of Hyperilongenols A-C: Rare 12,13-Seco-Spirocyclic Polycyclic Polyprenylated Acylphloroglucinols with Enolizable β,β′-tricarbonyl Systems from Hypericum Longistylum Oliv. Org. Chem. Front. 6, 1491–1502. doi:10.1039/C9QO00245F
Keywords: Pseudofusicoccum sp., Sonneratia apetala Buch.-Ham., sesquiterpenoid, anti-inflammation, acetylcholinesterase
Citation: Jia S, Su X, Yan W, Wu M, Wu Y, Lu J, He X, Ding X and Xue Y (2021) Acorenone C: A New Spiro-Sesquiterpene from a Mangrove-Associated Fungus, Pseudofusicoccum sp. J003. Front. Chem. 9:780304. doi: 10.3389/fchem.2021.780304
Received: 20 September 2021; Accepted: 25 October 2021;
Published: 25 November 2021.
Edited by:
Khaled A. Shaaban, University of Kentucky, United StatesReviewed by:
Mohamed Shaaban, National Research Center, EgyptVivekanandan Subramanian, University of Kentucky, United States
Copyright © 2021 Jia, Su, Yan, Wu, Wu, Lu, He, Ding and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongbo Xue, eHVleWJAbWFpbC5zeXN1LmVkdS5jbg==
 Shujie Jia
Shujie Jia Xiangdong Su
Xiangdong Su Xin Ding
Xin Ding Yongbo Xue
Yongbo Xue