- 1Department of Physiology and Research Centre of Basic Integrative Medicine, School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Translational Innovation Center, Shenzhen Bay Laboratory, Shenzhen, China
Disulfide bonds are indispensable structural motifs in bioactive peptides, stabilizing conformations which are critical for molecular recognition and biological activity. However, their intrinsic chemical lability under physiological and manufacturing conditions has long presented challenges in peptide drug development. Efforts to address these limitations have yielded a diverse array of disulfide bond surrogates, each with distinct advantages and constraints. Among these, methylene thioacetal linkages have recently emerged as a particularly promising method offering a favorable balance of structural fidelity, synthetic accessibility, and chemical stability. This review summarizes the biological importance and limitations of native disulfide bonds, surveys established strategies for disulfide bond mimicry, and provide a comprehensive summary of research leveraging methylene thioacetal chemistry as an emerging tool in the design of next-generation peptide therapeutics.
1 Introduction
Peptide-based therapeutics have emerged as a rapidly expanding class of drug candidates, offering high target specificity, favorable pharmacokinetic properties and unique ability to modulate complex biological targets (Wang et al., 2022). Advances in peptide synthesis technologies, including solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS), as well as the emerging field of green and sustainable techniques, have enabled the efficient design and production of complex and challenging peptide targets (Ferrazzano et al., 2022; Stefanucci et al., 2025). Recent advances in synthetic methodology, formulation technologies, and delivery systems have revitalized the interest in therapeutic peptides, prospering their clinical relevance across oncology, endocrinology, infectious diseases, and immunotherapy (Anand et al., 2023). Distinguished by their conformationally constrained structures and potent, often exquisitely selective biological activities, disulfide-containing peptides represent a highly privileged class of bioactive molecules which continues to inspire innovative approaches to therapeutic development (Hogg, 2003). These structural features, conferred by disulfide bonds, endow the peptides with the ability to engage challenging biological targets (Erak et al., 2018). Therefore, a versatile class of disulfide-containing peptides, such as hormones and toxins, has been widely explored as valuable pharmacophore templates and starting points in the development of peptide therapeutics (Tyler et al., 2023). Beyond nature’s repertoire, advances in combinatorial and display technologies, such as phage, mRNA, and yeast display, have enabled the generation of artificial disulfide-containing peptides and mini-proteins with tailored binding specificities and improved pharmacokinetic profiles (You et al., 2024). These engineered scaffolds harness the structural advantages of disulfide bonds and expand chemical space beyond linear peptides (Li et al., 2022). Most recently, the artificial intelligence (AI)- or machine learning-driven rational design has emerged as another powerful tool in peptide therapeutics, enabling the de novo generation and optimization of disulfide-containing peptides (Ye et al., 2023).
As the therapeutic landscape for peptides continues to evolve, improving the chemical and metabolic stability of disulfide-containing peptides has emerged as a critical priority in peptide drug development (Al Musaimi et al., 2022). Accordingly, the development of novel, broadly applicable and cost-effective peptide modification methodology has become a central focus in the field (Gori et al., 2017). This review aims to provide a comprehensive framework for understanding the opportunities, challenges and current art associated with disulfide bond surrogate. Particular emphasis is placed on methylene thioacetal linkage which offer exceptional chemical stability, redox inertness and conformational control. Through selected case studies, we highlight the versatility and broad applicability of methylene thioacetal linkage in stabilizing disulfide-containing peptides, positioning it as a compelling platform for next-generation peptide therapeutics.
2 The function and limitations of native disulfides in bioactive peptides
A defining feature of many bioactive peptides is the formation of disulfide bond, a covalent linkage formed between the thiol groups of two cysteine residues (Cys) (Narayan, 2012). These disulfide-containing molecules, spanning native hormones (insulin, salmon calcitonin, human growth factor), venom toxins (ziconotide, spider toxin ProTx2), and plant derived cysteine knot mini-proteins (cyclotides), represent a structurally and functionally diverse class of peptides and mini-proteins exhibiting distinct biological activities, which are often regarded as privileged scaffolds in peptide drug discovery (Figure 1a) (Tyler et al., 2023; Mollica et al., 2014). Several have advanced directly to clinical application, highlighting their substantial therapeutic value and scientific significance (Dang and Sussmuth, 2017).
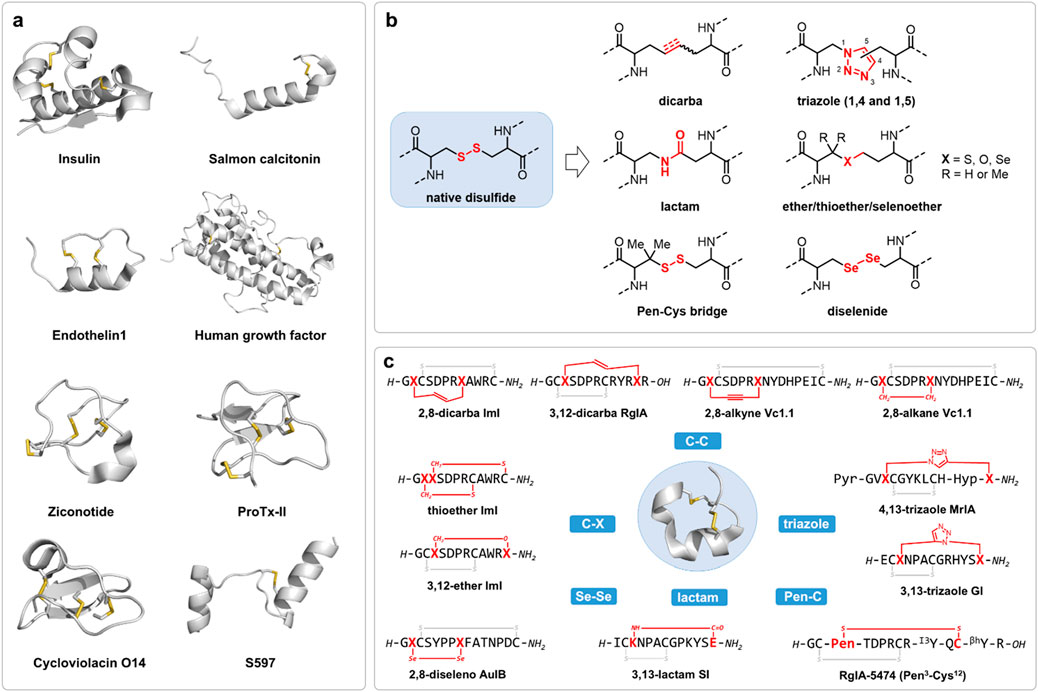
Figure 1. (a) Representative bioactive disulfide-containing peptides. The peptide backbones were represented in cartoon colored in grey and disulfide bonds were represented in stick colored in yellow. Structures generated from PDBs (insulin 1EVR; salmon Calcitonin 7TYN; endothelin1 8XVH; human growth factor 1HGU; ziconotide 7MIX; ProTx2 6N4R; Cycloviolacin O14 2GJ0; S597 8DTL. (b) The development of disulfide bond surrogates and (c) their application in disulfide-rich peptide compounds, exemplified by conotoxins.
Disulfide bonds play a central role in stabilizing the three-dimensional structures by constraining conformational flexibility, thereby preserving bioactive peptide conformations essential for molecular recognition, receptor binding, and enzymatic regulation (Fass, 2012). In peptides lacking extensive hydrophobic cores, disulfide bridges are particularly vital in conferring resistance to thermal denaturation and proteolytic degradation. By dictating the precise spatial arrangement of pharmacophoric residues, disulfide linkages enable high-affinity and selective interactions with biological targets (Nagahara, 2011). Moreover, in certain physiological contexts, disulfide bonds serve as redox-sensitive molecular switches, thereby modulating peptide activity in response to changes in cellular oxidative stress or redox signaling (Chiu and Hogg, 2019).
Despite their structural and functional advantages, disulfide-containing peptides face notable limitations that restrict their broader therapeutic applications (Fass, 2012). The inherent redox sensitivity of disulfide bonds renders these peptides vulnerable to reduction in the intracellular environment, leading to premature cleavage and substantial loss of structural integrity and corresponding bioactivity. Disulfide bond scrambling, particularly during peptide synthesis, folding or in vivo circulation, can generate heterogeneous mixtures of isomers with variable or undesired biological activity. In peptides with multiple disulfide bonds, the combinatorial increase in possible regioisomeric arrangements further complicates the synthesis and folding, posing significant challenges for follow-up therapeutic development (Akondi et al., 2014). Furthermore, disulfide-containing peptides often exhibit limited metabolic stability due to susceptibility to enzymatic degradation by thiol-disulfide exchange reactions. Collectively, these drawbacks have spurred efforts to develop chemically robust, redox-stable surrogates and alternative covalent constraints that preserve or enhance the conformational and functional properties conferred by native disulfide bridges.
3 Reported disulfide surrogates in peptide modification
To address the limitations of native disulfide bonds, a broad array of chemical surrogates has been developed, each designed to emulate the structural and conformational constraints imposed by disulfide linkages while providing superior stability under physiological and manufacturing conditions (Figures 1b,c) (Gori et al., 2017).
Dicarba bonds, comprising alkyne, olefin or saturated hydrocarbon linkages, are among the most studied disulfide surrogates (Walensky and Bird, 2014). These chemically non-reducible all-carbon bonds, typically introduced via ring-closing metathesis (RCM) or ring-closing alkyne metathesis (RCAM), serve as effective surrogates for labile disulfide bonds, enhancing both the structural stability and metabolic resilience of peptide therapeutics (Blackwell and Grubbs, 1998). However, although several reported successful applications of dicarba analogues that resulted in improved stability while maintaining the original activity profile, their altered electronic and steric properties may occasionally perturb native-like peptide conformations, leading to reduced bioactivities (Stymiest et al., 2005; Hossain et al., 2009; MacRaild et al., 2009; Ma et al., 2024; Chhabra et al., 2014; Martin-Gago et al., 2014; Chen et al., 2023; Belgi et al., 2021).
Triazole linkages, generated through Cu(I) or Ru(I)-catalyzed azide-alkyne “click” cycloaddition, offer remarkable metabolic stability and synthetic versatility (Angell and Burgess, 2007). Nevertheless, the rigid, planar nature of the triazole moiety may introduce conformational constraints distinct from those imposed by native disulfide bonds, requiring careful optimization in structure-activity relationship studies (White et al., 2020; Williams et al., 2015; Gori et al., 2015; Knuhtsen et al., 2019; Tomassi et al., 2020).
Thioether bridges, formed via alkylation or native chemical ligation, introduce a non-reducible C-S bond that generally preserves native peptide topology (Cui et al., 2021). Although relatively straightforward to install, thioether linkages possess subtle differences in bond length and polarity that can influence peptide folding and biological activity (Dekan et al., 2011). Besides, thioether bonds replacement may lead to reduced hydrophobicity compared with native disulfide bonds. Notably, ether and selenoether bridges are more similar in structure and reactivity with the native disulfides and are oxidation resistant compared with thioether bridges (Zhao et al., 2020; Cui et al., 2021; de Araujo et al., 2014). Recently, a diaminodiacid (DADA) strategy was developed for streamlined and efficient synthesis of thioether replaced analogues (Cui et al., 2013; Zhao et al., 2023a; Zhao et al., 2023b).
Lactam bridges, created through amide bond formation between amine side chains (lysine, ornithine) and acid side chains (glutamic acid, aspartic acid), offering a highly efficient means of stabilizing cyclic or constrained peptides (Houston et al., 1996; Hargittai et al., 2000; Xu et al., 2024; Trotta et al., 2024). However, replacing a disulfide with an amide linkage may alter local hydrogen bonding networks and affect peptide dynamics.
The cysteine-penicillamine (Cys-Pen) bridges introduce steric hindrance via gem-dimethyl substituents on penicillamine, thus enhancing reductive stability while preserving native disulfide bond geometry (Hunt et al., 1993; Gajewiak et al., 2021; Li et al., 2024a; Di Maro et al., 2017). Despite these advantages, their application is constrained by synthetic complexity and potential steric effects on peptide conformation.
Diselenide bonds (Se-Se), formed by substituting cysteine residues with selenocysteine (Sec), have emerged as promising disulfide surrogates due to their unique redox properties and structural similarity to disulfide bonds (Mousa et al., 2017; Muttenthaler et al., 2010). The lower bond dissociation energy and redox potential of diselenide bonds facilitate more efficient oxidative folding pathways, enhancing the foldability and stability of peptides and proteins. For instance, replacing an internal disulfide bridge with a diselenide in human insulin significantly improved its folding efficiency and thermodynamic stability without compromising receptor binding affinity (Arai et al., 2023; Arai et al., 2017).
Besides the above-mentioned technologies, other methods constraining target peptides, or referred as peptide stapling techniques by cysteine or lysing conjugations, have provided alternative means for peptide drug designs (Li et al., 2024a; Stefanucci et al., 2017). While these surrogate strategies have advanced peptide drug discovery, many involve trade-offs in synthetic accessibility, conformational fidelity, or pharmacological performance. As such, the search for chemically robust, synthetically tractable, and biologically compatible alternatives remains a central pursuit in peptide therapeutics research.
4 Methylene thioacetal as disulfide surrogate in peptide therapeutics
Methylene thioacetal linkage has attracted growing interest for its favorable balance of chemical robustness, structural mimicry, and synthetic accessibility. In this strategy, the native disulfide bond is inserted with a minimal “one-carbon” unit CH2, preserving the spatial and conformational features of the original S-S bond while imparting resistance to both reductive cleavage and oxidative degradation. Several synthetic strategies have been developed to install the methylene thioacetal bonds, typically involving the selective reduction of native disulfides followed by alkylation of the resulting free thiols with methylene donors (Ueki et al., 1999; Kourra and Cramer, 2016). The most widely adopted method utilizes dihalomethane reagents, such as diiodomethane (CH2I2) and dibromomethane (CH2Br2), under mildly basic or neutral conditions. While methylene thioacetals are isosteric to disulfide bonds, subtle yet important differences in bond geometry and physicochemical properties influence peptide structure and function. Methylene thioacetals feature slightly longer S-S distance (2.9 Å) compared to native disulfide bonds (2.0 Å), and the additional methylene group introduces subtle changes to bond angles and local conformational preferences. This results in a marginally increased rigidity in certain peptide macrocycles and loops, which can be leveraged to enhance receptor binding affinity, proteolytic stability and plasma half-life (Figure 2a).
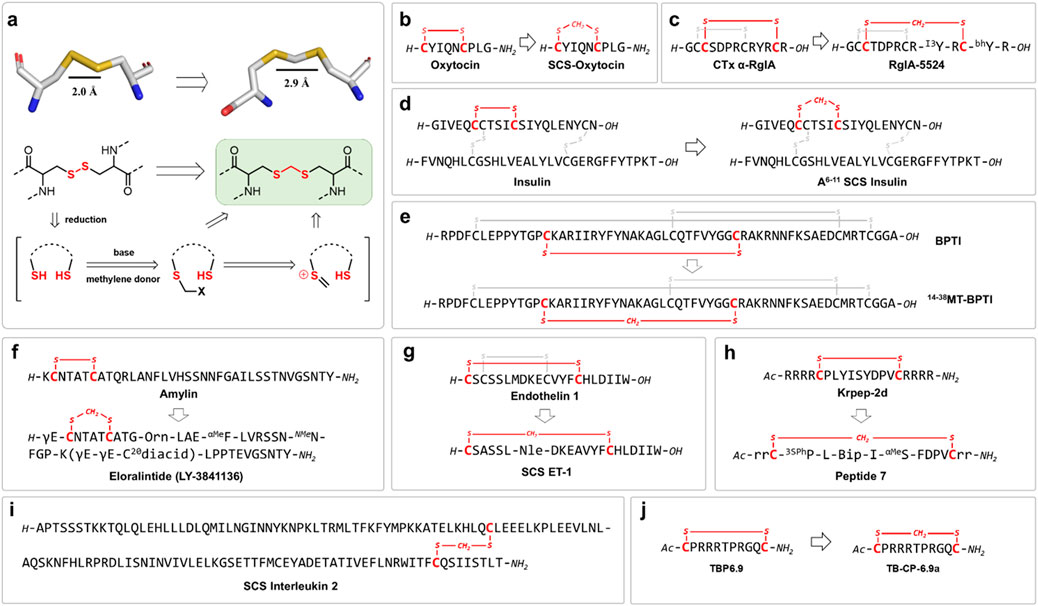
Figure 2. (a) Converting native disulfide into methylene thioacetal linkage by inserting the minimal “one-carbon” unit; Strategic applications of methylene thioacetal bonds as disulfide surrogates in bioactive peptides: (b) oxytocin; (c) conotoxin RgIA; (d) insulin; (e) BPTI; (f) human amylin; (g) human endothelin 1; (h) KRAS inhibitor; (i) interleukin 2 (j) HIV inhibitor.
Investigations have revealed that methylene thioacetal substitution frequently preserves peptide folding and biological activity relative to their native disulfide-containing counterparts, with markedly enhanced serum stability and proteolytic resistance across a range of scaffolds, including conotoxins, anti-tumor peptides and hormone analogues. Notably, several methylene thioacetal modified lead compounds have advanced into clinical evaluation, underscoring the high translational potential of this strategy.
Cramer et al. introduced a mild, biocompatible one-pot methodology for converting native disulfide bonds in peptides into highly stable methylene thioacetal linkages (Kourra and Cramer, 2016). This straightforward and selective post-synthetic transformation occurs under aqueous conditions at ambient temperature, accommodating unprotected peptides with a range of functional groups. As a key application, the authors synthesized a methylene thioacetal-stapled analogue of oxytocin (SCS-OXT), which exhibited a dramatic enhancement in chemical and metabolic stability. In comparison to native oxytocin, SCS-OXT demonstrated complete resistance to reduction in glutathione-rich environments and significantly prolonged half-lives in human serum and at elevated temperatures. Notably, at physiological pH, SCS-OXT’s stability increased approximately 5-fold. Despite the minor structural modification, SCS-OXT retained full agonist activity at the oxytocin receptor, with a nanomolar EC50 in a uterine contraction assay (Figure 2b).
Conotoxins are a diverse family of small, disulfide-rich peptides derived from the venom of marine cone snails, evolved to target ion channels, receptors, and transporters with remarkable potency and selectivity (Jin et al., 2019). Their distinct ability to modulate neuronal signaling pathways has made conotoxins invaluable both as pharmacological tools and as leads for therapeutic development. However, the therapeutic exploitation of conotoxins faces significant challenges, chiefly due to their susceptibility to disulfide bond scrambling and oxidative instability under physiological and manufacturing conditions, which can compromise their structural integrity and bioactivity. As such, chemical modifications and alternative bond surrogates are increasingly recognized as necessary strategies to enhance their stability and broaden their clinical applicability. Chou et al. developed α-RgIA analogues incorporating methylene thioacetal bonds to enhance peptide stability and potency against human α9α10 nicotinic acetylcholine receptors (nAChRs), a promising non-opioid target for analgesic drug development (Zheng et al., 2021). The study revealed distinct regioselectivity in disulfide bond replacement: substitution of the disulfide in loop I (Cys2–Cys8) markedly impaired activity, whereas replacement in loop II (Cys3–Cys12) preserved potency. The lead analogue, RgIA-5524, incorporating a methylene thioacetal in loop II, demonstrated potent inhibition of human α9α10 nAChRs (IC50 = 0.9 nM), exceptional selectivity across a wide range of pain-associated ion channels and receptors, and significantly improved serum stability by preventing disulfide scrambling. Notably, in vivo studies showed that RgIA-5524 effectively prevented cold allodynia in a mouse model of oxaliplatin-induced neuropathic pain, an effect absent in α9 knockout mice, confirming robust target engagement. These findings position RgIA-5524 as a promising, stable, and selective α9α10 nAChR antagonist for neuropathic pain treatment. This work underscores how the strategic incorporation of methylene thioacetal surrogates can optimize the therapeutic potential of conotoxin-based, disulfide-rich peptides. However, replacing a disulfide bond in another conotoxin lead candidate Mr1.1 [S4Dap] with a methylene thioacetal, ether by loop I, loop II or both, resulted in complete loss of activity (Li et al., 2024b). This indicates that the use of methylene thioacetal as a disulfide bond surrogate still faces challenges in terms of selectivity and sequence compatibility, even in the same case of α-conotoxins (Figure 2c).
Insulin, a disulfide-rich peptide hormone, plays a crucial role in the regulation of blood glucose levels, with its biological activity and structural integrity critically dependent on three conserved disulfide bonds, including the A6-A11 linkage within the A-chain (Sims et al., 2021). However, insulin’s inherent instability, particularly its propensity to fibrillate and degrade under physiological and stress conditions, presents significant challenges for storage, handling, and therapeutic application (Richter et al., 2023). In response to these issues, Chou et al. engineered a human insulin analogue, SCS-Ins, by replacing the native A6-A11 disulfide bond with a methylene thioacetal surrogate (Zheng et al., 2019). Notably, SCS-Ins exhibited markedly improved resistance to fibrillation compared with native insulin, coupled with enhanced thermal and serum stability, showing superior resistance to degradation under both physiological and elevated temperature conditions. Importantly, SCS-Ins preserved its bioactivity, as confirmed by both in vitro assays and in vivo insulin tolerance tests in mice, where it exhibited comparable efficacy to native insulin. These results highlight the potential of methylene thioacetal incorporation at the A6-A11 position as a promising strategy to enhance the stability of insulin formulations. It is noteworthy that the SCS-Ins represents the limited examples of potent insulin analogues with A6-A11 disulfide replacement. Substitution of this interchain disulfide with either lactam, triazole bridge and dicarba bonds all led to potency deprivation (Figure 2d).
Understanding the process of protein folding is fundamental to deciphering how peptide chains acquire their functional structures and how misfolding can lead to disease (Hidaka and Shimamoto, 2013). Metanis et al. investigated the effect of substituting a native disulfide bond in bovine pancreatic trypsin inhibitor (BPTI) with the methylene thioacetal bridge. Remarkably, replacing the 14–38 disulfide bond preserved the native fold while revealing an alternative folding trajectory not observed in the wild-type protein (Mousa et al., 2018). This discovery highlights the subtle yet critical role individual disulfide bonds play in directing protein folding pathways and demonstrates that the methylene thioacetal substitution can maintain structural integrity while uncovering new mechanistic insights. The study underscores the value of methylene thioacetal as a versatile chemical tool for probing protein folding and stability (Figure 2e).
Amylin, a peptide hormone co-secreted with insulin by pancreas, plays a pivotal role in regulating glucose metabolism and appetite (Hay et al., 2015). The N-terminal disulfide bond in native amylin is crucial for its structural integrity and biological activity. However, amylin is highly prone to aggregation and fibrillation, particularly under physiological conditions, which limits its therapeutic potential (Wilkinson et al., 2023). Eloralintide (LY3841136), a long-acting amylin analogue, was developed to address these challenges, offering enhanced stability and prolonged action compared to native amylin. To improve its stability and mitigate aggregation, Eloralintide incorporates a methylene thioacetal surrogate in place of the native disulfide bonds. This modification significantly enhances resistance to fibrillation and improves its pharmacokinetic profile compared with native amylin. Currently, eloralintide is undergoing clinical evaluation in Phase 1 and Phase 2 trials for the treatment of obesity and overweight conditions, aiming to assess its safety, tolerability, and efficacy, both as a monotherapy and in combination with other agents such as tirzepatide (Figure 2f).
Endothelin-1 (ET-1) is a potent, 21-residue vasoconstrictive peptide that plays a key role in the regulation of vascular tone and cardiovascular homeostasis (Lankhorst et al., 2016). Its biological activity and structural integrity rely on two conserved disulfide bonds, which form a characteristic bicyclic scaffold essential for high-affinity receptor engagement. However, like many disulfide-rich peptides, ET-1 is prone to chemical instability and disulfide scrambling under physiological conditions, which can limit its therapeutic potential. In a recent study, a methylene thioacetal surrogate was introduced to replace one of the native disulfide bonds in ET-1, yielding a chemically stabilized single-loop analogue (Wolf and Beck-Sickinger, 2021). Notably, this modified peptide retained vasoconstrictor potency comparable to native ET-1, demonstrating that selective methylene thioacetal incorporation can preserve biological function while enhancing chemical stability (Figure 2g).
Beyond its established role as a disulfide bond surrogate in natural disulfide-containing peptides, methylene thioacetal has also been effectively applied to the optimization of bioactive macrocyclic peptides and lead compounds identified through library-based screening strategies. In a notable example, Heinis et al. reported the discovery of macrocyclic peptide inhibitors targeting KRAS, a historically challenging oncogenic protein implicated in a wide range of human cancers (Figure 2h). Through phage display-based selection and structure-guided optimization, the team incorporated methylene thioacetal bridges to constrain peptide conformation, significantly improving proteolytic stability while preserving high-affinity binding and enabling cell-active inhibition of KRAS signaling (Lim et al., 2021; Garrigou et al., 2022). Similarly, in efforts to target viral RNA structures, methylene thioacetal was employed in the design of macrocyclic peptide inhibitors against the HIV trans-activation response (TAR) RNA element (Chavali et al., 2020). Co-crystal structures of TAR RNA bound to the lead molecule TB-CP-6.9a revealed key arginine-mediated contacts essential for high-affinity binding, insights that guided the development of cyclic peptides stabilized with methylene thioacetal to maintain bioactive conformations while enhancing chemical and metabolic stability (Figure 2j). Together, these studies underscore the versatility of methylene thioacetal as a valuable tool not only for stabilizing natural peptides but also for advancing the development of structurally defined, pharmacologically robust macrocyclic peptide therapeutics.
Methylene thioacetal has emerged not only as a stabilizing modification for disulfide-rich peptides but also as a biocompatible surrogate for disulfide bonds within larger protein scaffolds. Bode et al. achieved the total chemical synthesis of interleukin-2, and the modified analogue incorporating a methylene thioacetal bridge in place of a native disulfide bond (Murar et al., 2020). Notably, the resulting analogue, compound 14, retained in vitro bioactivity comparable to the recombinant IL-2. This work highlights the broader applicability of methylene thioacetal in the chemical synthesis and stabilization of disulfide-containing proteins, while preserving their functional properties (Figure 2i).
5 Conclusion and outlook
Disulfide-containing peptides represent a valuable class of bioactive molecules with high target selectivity and potent pharmacological activities. However, the inherent instability of disulfide bonds, particularly their susceptibility to reduction and scrambling under physiological and manufacturing conditions, has long posed a barrier to their broader therapeutic application. Methylene thioacetal has emerged as a promising disulfide bond surrogate, offering a chemically robust, non-reducible alternative that preserves the native-like conformation and bioactivity of peptide scaffolds. However, despite these advances, challenges remain regarding sequence-specific compatibility and potential effects on target binding and functional activity.
Moving forward, comprehensive in vivo studies addressing immunogenicity, toxicity, and pharmacodynamics, developing predictive guidelines for sequence compatibility, and integrating these chemistries into modern peptide display and screening platforms will be essential to fully realize the therapeutic potential of this promising strategy. In this landscape, methylene thioacetal stands as a powerful and versatile tool not only for stabilizing natural peptides but also for enabling the next-generation of chemically resilient, bioactive macrocycles in peptide drug discovery.
Author contributions
YZ: Writing – original draft, Formal Analysis, Investigation, Visualization. DW: Formal Analysis, Visualization, Investigation, Writing – review and editing. JX: Funding acquisition, Writing – review and editing, Formal Analysis, Investigation, Visualization. NZ: Formal Analysis, Project administration, Funding acquisition, Conceptualization, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (22307083), the Shenzhen Medical Research Fund (D2403006), the Guangdong Basic and Applied Basic Research Foundation (2023A1515010816), and the Shenzhen Postdoctoral Fellowship.
Acknowledgments
The authors thank Prof. Weijun Shen (Shenzhen Bay Laboratory) and Prof. Xiaochun Xiong (Xuzhou Medical University) for insightful suggestions and discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akondi, K. B., Muttenthaler, M., Dutertre, S., Kaas, Q., Craik, D. J., Lewis, R. J., et al. (2014). Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 114 (11), 5815–5847. doi:10.1021/cr400401e
Al Musaimi, O., Lombardi, L., Williams, D. R., and Albericio, F. (2022). Strategies for improving peptide stability and delivery. Pharm. (Basel) 15 (10), 1283. doi:10.3390/ph15101283
Anand, U., Bandyopadhyay, A., Jha, N. K., Perez de la Lastra, J. M., and Dey, A. (2023). Translational aspect in peptide drug discovery and development: an emerging therapeutic candidate. Biofactors 49 (2), 251–269. doi:10.1002/biof.1913
Angell, Y. L., and Burgess, K. (2007). Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem. Soc. Rev. 36 (10), 1674–1689. doi:10.1039/b701444a
Arai, K., Okumura, M., Lee, Y. H., Katayama, H., Mizutani, K., Lin, Y., et al. (2023). Diselenide-bond replacement of the external disulfide bond of insulin increases its oligomerization leading to sustained activity. Commun. Chem. 6 (1), 258. doi:10.1038/s42004-023-01056-4
Arai, K., Takei, T., Okumura, M., Watanabe, S., Amagai, Y., Asahina, Y., et al. (2017). Preparation of selenoinsulin as a long-lasting insulin analogue. Angew. Chem. Int. Ed. 56 (20), 5522–5526. doi:10.1002/anie.201701654
Belgi, A., Burnley, J. V., MacRaild, C. A., Chhabra, S., Elnahriry, K. A., Robinson, S. D., et al. (2021). Alkyne-bridged alpha-conotoxin Vc1.1 potently reverses mechanical allodynia in neuropathic pain models. J. Med. Chem. 64 (6), 3222–3233. doi:10.1021/acs.jmedchem.0c02151
Blackwell, H. E., and Grubbs, R. H. (1998). Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew. Chem. Int. Ed. 37 (23), 3281–3284. doi:10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V
Chavali, S. S., Mali, S. M., Jenkins, J. L., Fasan, R., and Wedekind, J. E. (2020). Co-crystal structures of HIV TAR RNA bound to lab-evolved proteins show key roles for arginine relevant to the design of cyclic peptide TAR inhibitors. J. Biol. Chem. 295 (49), 16470–16486. doi:10.1074/jbc.RA120.015444
Chen, B., Liu, C., Cong, W., Gao, F., Zou, Y., Su, L., et al. (2023). Cyclobutane-bearing restricted anchoring residues enabled geometry-specific hydrocarbon peptide stapling. Chem. Sci. 14 (41), 11499–11506. doi:10.1039/d3sc04279k
Chhabra, S., Belgi, A., Bartels, P., van Lierop, B. J., Robinson, S. D., Kompella, S. N., et al. (2014). Dicarba analogues of alpha-conotoxin RgIA. Structure, stability, and activity at potential pain targets. J. Med. Chem. 57 (23), 9933–9944. doi:10.1021/jm501126u
Chiu, J., and Hogg, P. J. (2019). Allosteric disulfides: sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 294 (8), 2949–5908. doi:10.1074/jbc.REV118.005604
Cui, H. K., Guo, Y., He, Y., Wang, F. L., Chang, H. N., Wang, Y. J., et al. (2013). Diaminodiacid-based solid-phase synthesis of peptide disulfide bond mimics. Angew. Chem. Int. Ed. 52 (36), 9558–9562. doi:10.1002/anie.201302197
Cui, J. B., Wei, X. X., Zhao, R., Zhu, H., Shi, J., Bierer, D., et al. (2021). Chemical synthesis of disulfide surrogate peptides by using beta-carbon dimethyl modified diaminodiacids. Org. Biomol. Chem. 19 (41), 9021–9025. doi:10.1039/d1ob01715b
Dang, T., and Sussmuth, R. D. (2017). Bioactive peptide natural products as lead structures for medicinal use. Acc. Chem. Res. 50 (7), 1566–1576. doi:10.1021/acs.accounts.7b00159
de Araujo, A. D., Mobli, M., Castro, J., Harrington, A. M., Vetter, I., Dekan, Z., et al. (2014). Selenoether oxytocin analogues have analgesic properties in a mouse model of chronic abdominal pain. Nat. Commun. 5, 3165. doi:10.1038/ncomms4165
Dekan, Z., Vetter, I., Daly, N. L., Craik, D. J., Lewis, R. J., and Alewood, P. F. (2011). α-Conotoxin ImI incorporating stable cystathionine bridges maintains full potency and identical three-dimensional structure. J. Am. Chem. Soc. 133 (40), 15866–15869. doi:10.1021/ja206408q
Di Maro, S., Di Leva, F. S., Trotta, A. M., Brancaccio, D., Portella, L., Aurilio, M., et al. (2017). Structure-activity relationships and biological characterization of a novel, potent, and serum stable C-X-C chemokine receptor type 4 (CXCR4) antagonist. J. Med. Chem. 60 (23), 9641–9652. doi:10.1021/acs.jmedchem.7b01062
Erak, M., Bellmann-Sickert, K., Els-Heindl, S., and Beck-Sickinger, A. G. (2018). Peptide chemistry toolbox - transforming natural peptides into peptide therapeutics. Bioorg Med. Chem. 26 (10), 2759–2765. doi:10.1016/j.bmc.2018.01.012
Fass, D. (2012). Disulfide bonding in protein biophysics. Annu. Rev. Biophys. 41, 63–79. doi:10.1146/annurev-biophys-050511-102321
Ferrazzano, L., Catani, M., Cavazzini, A., Martelli, G., Corbisiero, D., Cantelmi, P., et al. (2022). Sustainability in peptide chemistry: current synthesis and purification technologies and future challenges. Green Chem. 24 (3), 975–1020. doi:10.1039/D1GC04387K
Gajewiak, J., Christensen, S. B., Dowell, C., Hararah, F., Fisher, F., Huynh, P. N., et al. (2021). Selective penicillamine substitution enables development of a potent analgesic peptide that acts through a non-opioid-based mechanism. J. Med. Chem. 64 (13), 9271–9278. doi:10.1021/acs.jmedchem.1c00512
Garrigou, M., Sauvagnat, B., Duggal, R., Boo, N., Gopal, P., Johnston, J. M., et al. (2022). Accelerated identification of cell active KRAS inhibitory macrocyclic peptides using mixture libraries and automated ligand identification system (ALIS) technology. J. Med. Chem. 65 (13), 8961–8974. doi:10.1021/acs.jmedchem.2c00154
Gori, A., Gagni, P., and Rinaldi, S. (2017). Disulfide bond mimetics: strategies and challenges. Chem.-Eur. J. 23 (60), 14987–14995. doi:10.1002/chem.201703199
Gori, A., Wang, C. I., Harvey, P. J., Rosengren, K. J., Bhola, R. F., Gelmi, M. L., et al. (2015). Stabilization of the cysteine-rich conotoxin MrIA by using a 1,2,3-triazole as a disulfide bond mimetic. Angew. Chem. Int. Ed. 54 (4), 1361–1364. doi:10.1002/anie.201409678
Hargittai, B., Sole, N. A., Groebe, D. R., Abramson, S. N., and Barany, G. (2000). Chemical syntheses and biological activities of lactam analogues of alpha-conotoxin SI. J. Med. Chem. 43 (25), 4787–4792. doi:10.1021/jm990635c
Hay, D. L., Chen, S., Lutz, T. A., Parkes, D. G., and Roth, J. D. (2015). Amylin: pharmacology, physiology, and clinical potential. Pharmacol. Rev. 67 (3), 564–600. doi:10.1124/pr.115.010629
Hidaka, Y., and Shimamoto, S. (2013). Folding of peptides and proteins: role of disulfide bonds, recent developments. Biomol. Concepts 4 (6), 597–604. doi:10.1515/bmc-2013-0022
Hogg, P. J. (2003). Disulfide bonds as switches for protein function. Trends Biochem. Sci. 28 (4), 210–214. doi:10.1016/S0968-0004(03)00057-4
Hossain, M. A., Rosengren, K. J., Zhang, S., Bathgate, R. A., Tregear, G. W., van Lierop, B. J., et al. (2009). Solid phase synthesis and structural analysis of novel A-chain dicarba analogs of human relaxin-3 (INSL7) that exhibit full biological activity. Org. Biomol. Chem. 7 (8), 1547–1553. doi:10.1039/b821882j
Houston, M. E., Campbell, A. P., Lix, B., Kay, C. M., Sykes, B. D., and Hodges, R. S. (1996). Lactam bridge stabilization of alpha-helices: the role of hydrophobicity in controlling dimeric versus monomeric alpha-helices. Biochemistry 35 (31), 10041–10050. doi:10.1021/bi952757m
Hunt, J. T., Lee, V. G., Liu, E. C., Moreland, S., McMullen, D., Webb, M. L., et al. (1993). Control of peptide disulfide regioisomer formation by mixed cysteine-penicillamine bridges. Application to endothelin-1. Int. J. Pept. Protein Res. 42 (3), 249–258. doi:10.1111/j.1399-3011.1993.tb00139.x
Jin, A. H., Muttenthaler, M., Dutertre, S., Himaya, S. W. A., Kaas, Q., Craik, D. J., et al. (2019). Conotoxins: chemistry and biology. Chem. Rev. 119 (21), 11510–11549. doi:10.1021/acs.chemrev.9b00207
Knuhtsen, A., Whitmore, C., McWhinnie, F. S., McDougall, L., Whiting, R., Smith, B. O., et al. (2019). α-Conotoxin GI triazole-peptidomimetics: potent and stable blockers of a human acetylcholine receptor. Chem. Sci. 10 (6), 1671–1676. doi:10.1039/C8SC04198A
Kourra, C., and Cramer, N. (2016). Converting disulfide bridges in native peptides to stable methylene thioacetals. Chem. Sci. 7 (12), 7007–7012. doi:10.1039/c6sc02285e
Lankhorst, S., Danser, A. H., and van den Meiracker, A. H. (2016). Endothelin-1 and antiangiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310 (3), R230–R234. doi:10.1152/ajpregu.00373.2015
Li, T., Tae, H. S., Chen, S., Li, X., Liang, J., Pan, T., et al. (2024a). Development of an intravenously stable disulfide-rich peptide for the treatment of chemotherapy-induced neuropathic pain. J. Med. Chem. 67 (21), 18741–18752. doi:10.1021/acs.jmedchem.4c00974
Li, X., Craven, T. W., and Levine, P. M. (2022). Cyclic peptide screening methods for preclinical drug discovery. J. Med. Chem. 65 (18), 11913–11926. doi:10.1021/acs.jmedchem.2c01077
Li, Y., Wu, M., Fu, Y., Xue, J., Yuan, F., Qu, T., et al. (2024b). Therapeutic stapled peptides: efficacy and molecular targets. Pharmacol. Res. 203, 107137. doi:10.1016/j.phrs.2024.107137
Lim, S., Boyer, N., Boo, N., Huang, C., Venkatachalam, G., Angela Juang, Y. C., et al. (2021). Discovery of cell active macrocyclic peptides with on-target inhibition of KRAS signaling. Chem. Sci. 12 (48), 15975–15987. doi:10.1039/d1sc05187c
Ma, B., Liu, D., Zheng, M., Wang, Z., Zhang, D., Jian, Y., et al. (2024). Development of a double-stapled peptide stabilizing both alpha-helix and beta-sheet structures for degrading transcription factor AR-V7. JACS Au 4 (2), 816–827. doi:10.1021/jacsau.3c00795
MacRaild, C. A., Illesinghe, J., van Lierop, B. J., Townsend, A. L., Chebib, M., Livett, B. G., et al. (2009). Structure and activity of (2,8)-dicarba-(3,12)-cystino alpha-ImI, an alpha-conotoxin containing a nonreducible cystine analogue. J. Med. Chem. 52 (3), 755–762. doi:10.1021/jm8011504
Martin-Gago, P., Ramon, R., Aragon, E., Fernandez-Carneado, J., Martin-Malpartida, P., Verdaguer, X., et al. (2014). A tetradecapeptide somatostatin dicarba-analog: synthesis, structural impact and biological activity. Bioorg Med. Chem. Lett. 24 (1), 103–107. doi:10.1016/j.bmcl.2013.11.065
Mollica, A., Carotenuto, A., Novellino, E., Limatola, A., Costante, R., Pinnen, F., et al. (2014). Novel cyclic biphalin analogue with improved antinociceptive properties. ACS Med. Chem. Lett. 5 (9), 1032–1036. doi:10.1021/ml500241n
Mousa, R., Lansky, S., Shoham, G., and Metanis, N. (2018). BPTI folding revisited: switching a disulfide into methylene thioacetal reveals a previously hidden path. Chem. Sci. 9 (21), 4814–4820. doi:10.1039/c8sc01110a
Mousa, R., Notis Dardashti, R., and Metanis, N. (2017). Selenium and selenocysteine in protein chemistry. Angew. Chem. Int. Ed. Engl. 56 (50), 15818–15827. doi:10.1002/anie.201706876
Murar, C. E., Ninomiya, M., Shimura, S., Karakus, U., Boyman, O., and Bode, J. W. (2020). Chemical synthesis of interleukin-2 and disulfide stabilizing analogues. Angew. Chem. Int. Ed. 59 (22), 8425–8429. doi:10.1002/anie.201916053
Muttenthaler, M., Nevin, S. T., Grishin, A. A., Ngo, S. T., Choy, P. T., Daly, N. L., et al. (2010). Solving the alpha-conotoxin folding problem: efficient selenium-directed on-resin generation of more potent and stable nicotinic acetylcholine receptor antagonists. J. Am. Chem. Soc. 132 (10), 3514–3522. doi:10.1021/ja910602h
Nagahara, N. (2011). Intermolecular disulfide bond to modulate protein function as a redox-sensing switch. Amino Acids 41 (1), 59–72. doi:10.1007/s00726-010-0508-4
Narayan, M. (2012). Disulfide bonds: protein folding and subcellular protein trafficking. FEBS J. 279 (13), 2272–2282. doi:10.1111/j.1742-4658.2012.08636.x
Richter, B., Bongaerts, B., and Metzendorf, M. I. (2023). Thermal stability and storage of human insulin. Cochrane Database Syst. Rev. 11 (11), CD015385. doi:10.1002/14651858.CD015385.pub2
Sims, E. K., Carr, A. L. J., Oram, R. A., DiMeglio, L. A., and Evans-Molina, C. (2021). 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat. Med. 27 (7), 1154–1164. doi:10.1038/s41591-021-01418-2
Stefanucci, A., Carotenuto, A., Macedonio, G., Novellino, E., Pieretti, S., Marzoli, F., et al. (2017). Cyclic biphalin analogues incorporating a xylene bridge: synthesis, characterization, and biological profile. ACS Med. Chem. Lett. 8 (8), 858–863. doi:10.1021/acsmedchemlett.7b00210
Stefanucci, A., Santoro, F., D'Ingiullo, S., Marinaccio, L., Procino, E., Learte-Aymami, S., et al. (2025). Development of linear β-turn inducers containing peptides as arc mimetics with DNA topological and sequence selectivity. Eur. J. Med. Chem. 289, 117423. doi:10.1016/j.ejmech.2025.117423
Stymiest, J. L., Mitchell, B. F., Wong, S., and Vederas, J. C. (2005). Synthesis of oxytocin analogues with replacement of sulfur by carbon gives potent antagonists with increased stability. J. Org. Chem. 70 (20), 7799–7809. doi:10.1021/jo050539l
Tomassi, S., Trotta, A. M., Ierano, C., Merlino, F., Messere, A., Rea, G., et al. (2020). Disulfide bond replacement with 1,4- and 1,5-disubstituted [1,2,3]-Triazole on C-X-C chemokine receptor type 4 (CXCR4) peptide ligands: small changes that make big differences. Chem.-Eur. J. 26 (44), 10113–10125. doi:10.1002/chem.202002468
Trotta, A. M., Mazzarella, V., Roggia, M., D'Aniello, A., Del Bene, A., Vetrei, C., et al. (2024). Comprehensive structural investigation of a potent and selective CXCR4 antagonist via crosslink modification. Eur. J. Med. Chem. 279, 116911. doi:10.1016/j.ejmech.2024.116911
Tyler, T. J., Durek, T., and Craik, D. J. (2023). Native and engineered cyclic disulfide-rich peptides as drug leads. Molecules 28 (7), 3189. doi:10.3390/molecules28073189
Ueki, M., Ikeo, T., Iwadate, M., Asakura, T., Williamson, M. P., and Slaninova, J. (1999). Solid phase synthesis and biological activities of [Arg8]-vasopressin methylenedithioether. Bioorg Med. Chem. Lett. 9 (13), 1767–1772. doi:10.1016/s0960-894x(99)00269-3
Walensky, L. D., and Bird, G. H. (2014). Hydrocarbon-stapled peptides: principles, practice, and progress. J. Med. Chem. 57 (15), 6275–6288. doi:10.1021/jm4011675
Wang, L., Wang, N., Zhang, W., Cheng, X., Yan, Z., Shao, G., et al. (2022). Therapeutic peptides: current applications and future directions. Signal Transduct. Target Ther. 7 (1), 48. doi:10.1038/s41392-022-00904-4
White, A. M., de Veer, S. J., Wu, G., Harvey, P. J., Yap, K., King, G. J., et al. (2020). Application and structural analysis of triazole-bridged disulfide mimetics in cyclic peptides. Angew. Chem. Int. Ed. 59 (28), 11273–11277. doi:10.1002/anie.202003435
Wilkinson, M., Xu, Y., Thacker, D., Taylor, A. I. P., Fisher, D. G., Gallardo, R. U., et al. (2023). Structural evolution of fibril polymorphs during amyloid assembly. Cell 186 (26), 5798–5811 e26. doi:10.1016/j.cell.2023.11.025
Williams, G. M., Lee, K., Li, X., Cooper, G. J., and Brimble, M. A. (2015). Replacement of the CysA7-CysB7 disulfide bond with a 1,2,3-triazole linker causes unfolding in insulin glargine. Org. Biomol. Chem. 13 (13), 4059–4063. doi:10.1039/c5ob00160a
Wolf, P., and Beck-Sickinger, A. G. (2021). The ring size of monocyclic ET-1 controls selectivity and signaling efficiency at both endothelin receptor subtypes. J. Pept. Sci. 27 (7), e3325. doi:10.1002/psc.3325
Xu, R., Jap, E., Gubbins, B., Hagemeyer, C. E., and Karas, J. A. (2024). Semisynthesis of A6-A11 lactam insulin. J. Pept. Sci. 30 (2), e3542. doi:10.1002/psc.3542
Ye, J., Li, A., Zheng, H., Yang, B., and Lu, Y. (2023). Machine learning advances in predicting peptide/protein-protein interactions based on sequence information for lead peptides discovery. Adv. Biol. (Weinh). 7 (6), e2200232. doi:10.1002/adbi.202200232
You, S., McIntyre, G., and Passioura, T. (2024). The coming of age of cyclic peptide drugs: an update on discovery technologies. Expert Opin. Drug Discov. 19 (8), 961–973. doi:10.1080/17460441.2024.2367024
Zhao, R., Shi, P., Chen, J., Sun, S., Chen, J., Cui, J., et al. (2020). Chemical synthesis and biological activity of peptides incorporating an ether bridge as a surrogate for a disulfide bond. Chem. Sci. 11 (30), 7927–7932. doi:10.1039/d0sc02374d
Zhao, R., Shi, P., Cui, J. B., Shi, C., Wei, X. X., Luo, J., et al. (2023a). Single-shot solid-phase synthesis of full-length H2 relaxin disulfide surrogates. Angew. Chem. Int. Ed. 62 (6), e202216365. doi:10.1002/anie.202216365
Zhao, R., Shi, P., Wei, X. X., Xia, Z., Shi, C., and Shi, J. (2023b). Synthesis of A11(cys)-B11(cys) disulfide surrogates of H2 relaxin through an intermolecular native chemical ligation-assisted diaminodiacid strategy. Org. Lett. 25 (35), 6544–6548. doi:10.1021/acs.orglett.3c02381
Zheng, N., Christensen, S. B., Dowell, C., Purushottam, L., Skalicky, J. J., McIntosh, J. M., et al. (2021). Discovery of methylene thioacetal-incorporated α-RgIA analogues as potent and stable antagonists of the human α9α10 nicotinic acetylcholine receptor for the treatment of neuropathic pain. J. Med. Chem. 64 (13), 9513–9524. doi:10.1021/acs.jmedchem.1c00802
Keywords: disulfide surrogate, methylene thioacetal bond, stability and bioactivity, peptide drug discovery, peptide synthesis
Citation: Zhou Y, Wang D, Xu J and Zheng N (2025) Strategic applications of methylene thioacetal bonds as disulfide surrogates in peptide drug discovery. Front. Chem. 13:1637329. doi: 10.3389/fchem.2025.1637329
Received: 29 May 2025; Accepted: 20 June 2025;
Published: 27 June 2025.
Edited by:
Alfonso Carotenuto, University of Naples Federico II, ItalyReviewed by:
Salvatore Di. Maro, Second University of Naples, ItalyAzzurra Stefanucci, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2025 Zhou, Wang, Xu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Zheng, emhlbmduYW5Ac3pibC5hYy5jbg==
†These authors have contributed equally to this work
 Yaqi Zhou1†
Yaqi Zhou1† Dongyuan Wang
Dongyuan Wang Jiean Xu
Jiean Xu Nan Zheng
Nan Zheng