- 13rd Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- 2Department of Physics, Tianjin Renai College, Tianjin, China
- 3College of Modern Industry of Health Management, Jinzhou Medical University, Jinzhou, China
- 4Liaoning Province Key Laboratory of Human Phenome Research, Jinzhou Medical University, Jinzhou, China
This study focuses on a multifunctional fluorescence probe JFT based on the FRET (Fluorescence Resonance Energy Transfer) and TICT (Twisted Intramolecular Charge Transfer) mechanism. JFT combines an electron donor and an acceptor, enabling it to detect sulfite and monitor intracellular viscosity. When reacting with sulfite, its electronic structure changes, turning off FRET and altering fluorescence wavelength and intensity. In different viscosity environments, the rotation of carbon-carbon bonds in the electron acceptor structure of JFT varies, affecting non-radiative transition pathways and fluorescence intensity. Theoretical calculations based on TDDFT reveal the electron distribution changes before and after the reaction with sulfite species, consistent with experimental phenomena. These findings deepen the understanding of the FRET mechanism of fluorescence probes and offer theoretical guidance for the design of more efficient fluorescence probes.
Introduction
Fluorescence probes have emerged as powerful tools in biological and chemical research, enabling real-time and non-invasive monitoring of various analytes and environmental parameters (Peng et al., 2024; Liu et al., 2024a; Liu et al., 2024b; Sun et al., 2019; Liu et al., 2024c). In the context of intracellular studies, simultaneously monitoring the changes in intracellular sulfite concentration and viscosity is of great significance. Sulfite is an important signaling molecule in the body, involved in many physiological and pathological processes, such as vasodilation, neurotransmission, and antioxidant defense (Zhang W. J. et al., 2022; Liu et al., 2023; Liu et al., 2022; Wang et al., 2024; Ren et al., 2024; Li et al., 2023). Abnormal levels of sulfite are associated with various diseases, including cardiovascular diseases, neurodegenerative diseases, and respiratory diseases. Therefore, accurately detecting the concentration of sulfite in cells is crucial for understanding its biological functions and related pathological mechanisms.
On the other hand, intracellular viscosity is a key physical parameter that reflects the micro-environment of cells. It affects many cellular processes, such as protein folding, enzyme activity, membrane fluidity, and intracellular transport. Changes in intracellular viscosity are often related to cell proliferation, differentiation, and apoptosis. Thus, monitoring intracellular viscosity can provide valuable information about cell status and function (Peng et al., 2024; Liu et al., 2024c; Ma et al., 2024; Zhang C. et al., 2022; Arachchige et al., 2024; Wang et al., 2021; Peng et al., 2023).
However, developing a fluorescence probe that can simultaneously monitor these two parameters is challenging. Most traditional fluorescence probes are designed to detect only one analyte. The development of a multifunctional probe requires a careful combination of different sensing mechanisms.
Recently, Wang et al. developed a multifunctional fluorescence probe JFT based on the FRET/TICT mechanism (Wang et al., 2025). This probe effectively combined an electron donor and an acceptor, endowing it with unique properties for detecting sulfite and monitoring viscosity. The FRET property of the probe allowed for sensitive detection of sulfite through changes in fluorescence emission wavelength and intensity. Meanwhile, the rotation of carbon-carbon bonds in the electron acceptor structure of the probe was sensitive to environmental viscosity, enabling viscosity-dependent fluorescence changes. This paper aimed to comprehensively explore the working mechanism of the JFT fluorescence probe, including its response to sulfite and viscosity changes, as well as the underlying structural and electronic changes, which was expected to provide in-depth insights into fluorescence probe design and expand its applications in biological and chemical analysis.
Theoretical methods
The geometric optimization and vibrational frequency analysis on the probe JFT were carried out at the PBE0/def2-TZVPD level with D3 dispersion and GCP correction to remove artificial overbinding effects from BSSE using ORCA 5.0.1 program (Neese, 2022; Su et al., 2013; Laun and Bredow, 2022). To explore the excited-state properties of the molecule, the time-dependent density functional theory (TDDFT) computations were conducted at the CAM-B3LYP/def2-TZVPD level (Shao et al., 2020). The charges of the probe JFT and its reaction product with sulfite were +1 and 0 respectively. DMSO environment under the SMD solvent model was used in calculation which was align with the experimental work. All molecular visualizations were generated via the VMD 1.9.3 software, and the in-depth electronic structure analyses were accomplished by employing the Multiwfn 3.8 (dev) package (Lu and Chen, 2012; Humphrey et al., 1996).
Results and discussion
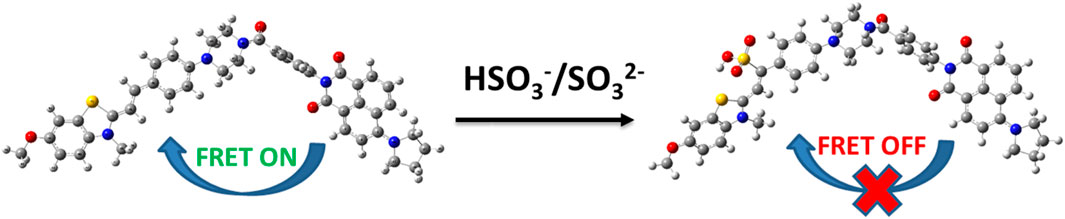
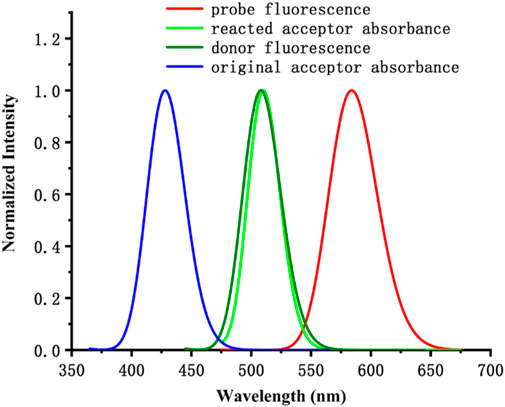
The emission spectrum of the electron donor of probe JFT could well overlap with the absorption spectrum of the acceptor of it, a characteristic that endowed the probe JFT with FRET properties. After the probe reacted with sulfite, the change in its electronic structure could turn off the FRET of probe JFT, thus causing significant changes in its fluorescence emission wavelength and intensity, making it an excellent sulfite detection probe. This mechanism was shown in Scheme 1.
Meanwhile, the electron acceptor structure in probe JFT contained C-C bonds with a high degree of rotational freedom. In a low-viscosity environment, these carbon-carbon bonds could rotate relatively freely, transferring energy among different conformations. This provided some non-radiative transition pathways for probe JFT to de-excite from the excited state to the ground state. When the environmental viscosity increased, the rotation of these carbon-carbon bonds was inhibited, and the non-radiative transition pathways for the probe to de-excite from the excited state to the ground state were cut off, thereby enhancing the corresponding fluorescence intensity. This enabled probe JFT to effectively monitor the intracellular viscosity level.
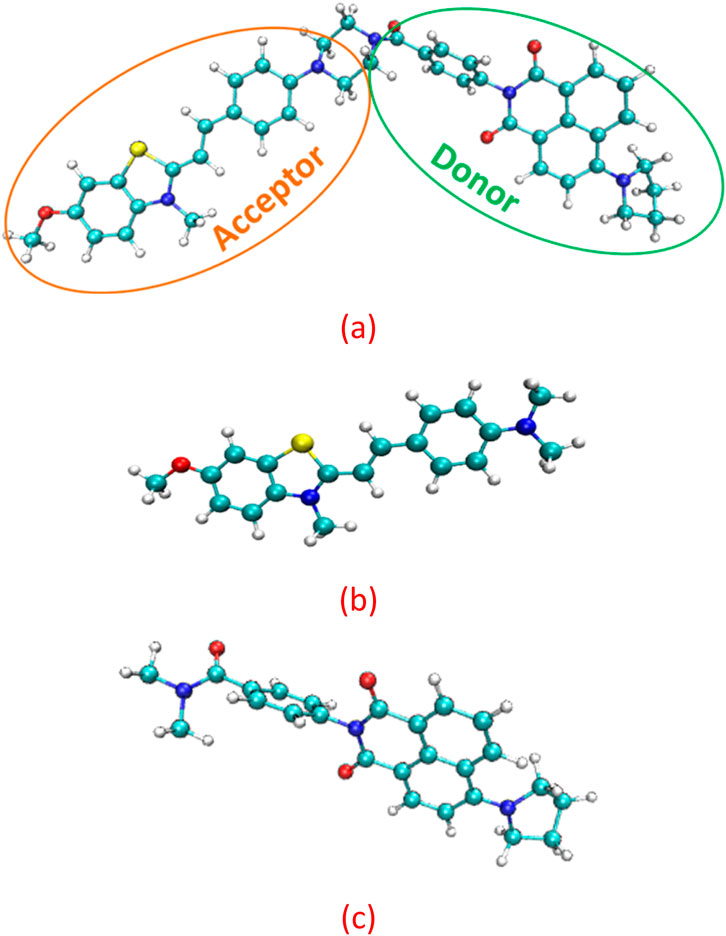
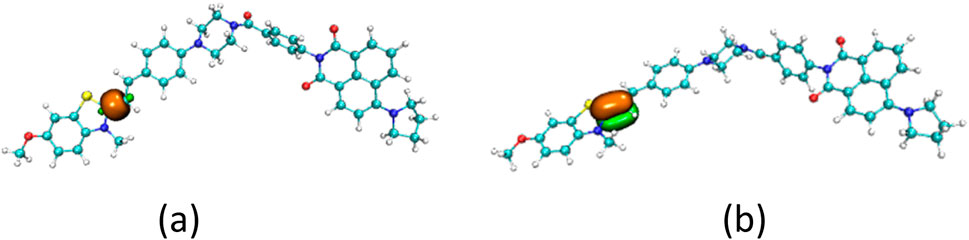
The structures of the JFT fluorescent probe and its electron donor and acceptor parts (same as Scheme 2 in Wang et al. (2025)) were shown in Figure 1. From the structural diagram of the electron acceptor part, it could be seen that the carbon-carbon bonds connecting the N-methylbenzothiazole and dimethylaniline had a large degree of rotational freedom, and the carbon-carbon double bond was also the reaction site with HSO3−/SO32−. To study the rotational freedom of these carbon-carbon bonds, the compositional characteristics of the carbon-carbon bond in the probe JFT were analyzed through natural adaptive orbital concept and shown in Figure 2. The carbon-carbon bond had a relatively large proportion of easily rotatable σ component (70%), while the non-easily rotatable π component accounted for a relatively small proportion (28%).
To further study the conformational changes of the JFT fluorescence probe brought about by the rotation of these carbon-carbon bonds, the energy scan of the JFT probe’s acceptor based on the rotation of these carbon-carbon bonds was carried out (Figure 3). It can be seen from Figure 3 that when N-methylbenzothiazole and dimethylaniline were in the same plane, the probe was in the lowest-energy conformation. When the carbon-carbon bond rotated to other angles, the probe was in other local low-energy conformations. The energy barriers between these conformations were relatively low. Therefore, in a low-viscosity environment, conversions between different conformations could occur relatively easily, providing a non-radiative energy dissipation path for the de-excitation of the JFT fluorescence probe, resulting in a weak fluorescence intensity of the JFT probe in this state. When the environmental viscosity increased, the rotation of the carbon-carbon bonds in the probe was inhibited. When the JFT probe de-excited from the excited state back to the ground state, the fluorescence radiation path would be highly prioritized, thus causing a significant increase in fluorescence intensity. This conclusion was consistent with the experimental phenomena.
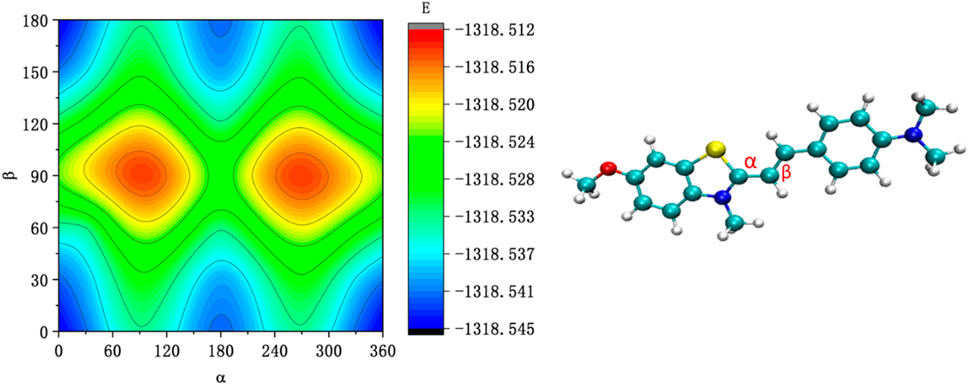
Figure 3. The energy scan of the JFT probe’s acceptor based on the rotation of α and β carbon-carbon bonds.
Another way to inhibit the rotation of the carbon-carbon bonds in probe JFT was that the composition of the carbon-carbon bonds was changed after the probe reacted with HSO3−/SO32−. The proportion of rotatable σ component versus non-rotatableπ component in the carbon-carbon bond changed from 70%:28%–57%:42% as shown in Figure 4. The addition of the HSO3− group significantly increased the inhibitory effect on the rotation of the carbon-carbon bonds, thereby also causing an enhancement in the fluorescence intensity of probe JFT under optical excitation.
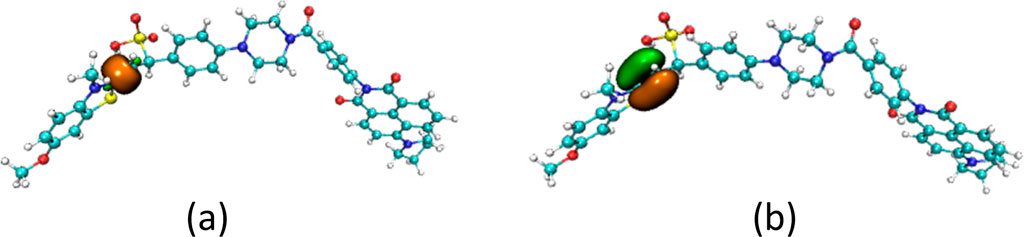
Figure 4. The σ component (a) and π component (b) of the c-c bond in product of JFT probe reacted with HSO3−.
At the same time, the introduction of the HSO3− group changed the electronic structure of the electron acceptor in probe JFT, so that the absorption spectrum of the changed acceptor no longer overlapped with the emission spectrum of its donor, thus cutting off the original FRET channel of the probe, as shown in Figure 5. This structural change caused significant changes in the fluorescence emission wavelength and intensity of probe JFT. The intensity of the original orange fluorescence (582 nm) was significantly weakened, while the intensity of the green fluorescence (530 nm) was significantly enhanced. Experimental results showed that there was a good linear relationship between the ratio I530/I582 and the concentration of HSO3−/SO32− in the environment. Therefore, the fluorescence changed value I530/I582 of probe JFT became an effective indicator for monitoring the concentration of HSO3−/SO32− in the environment.
To further reveal the influence of the electronic structure changes of probe JFT before and after the reaction with HSO3−/SO32− on its fluorescence emission, the electron distribution of probe JFT in the ground state and excited state was studied based on the TDDFT method.
Before the reaction with HSO3−/SO32−, the difference in electron distribution between the ground state S0 and the first excited state S1 of probe JFT showed obvious charge-transfer characteristics, which was consistent with the calculation results of the emission and absorption spectra of the probe’s electron donor and acceptor as mentioned before. These characteristics could be clearly seen from the difference diagram of electron distribution between S0 and S1 as shown in Figure 6, and which atoms participate in the charge-transfer process could be further obtained from the electron-transfer heat map generated through Multiwfn program as shown in Figure 7. When probe JFT reacted with HSO3−/SO32−, due to the change in the geometry and electronic structure of the electron acceptor, the FRET process was interrupted. The difference diagram of electron distribution between S0 and S1 of the product as shown in Figure 8 clearly showed the local excitation characteristics between the two states at this time, and the fluorescence color changed from orange (582 nm) to green (530 nm). Although due to the limitation of accuracy, there was some deviation within the calculated wavelength value from the experimental measurement value, the amplitude and trend of the wavelength change were in good agreement with the experiment. The difference diagrams of electron distribution between S0 and S1 and the electron-transfer heat map (Figure 9) both showed that electron excitation only occurred in the electron donor part of the probe at this time. The calculated results of the fluorescence emission wavelength were consistent with the experiment. The absorption and emission characteristic values of probe JFT before and after the reaction with HSO3−/SO32− and the corresponding donor and acceptor structure () were shown in Tables 1, 2 respectively. These theoretical results deepened our understanding of the FRET mechanism of fluorescence probes and provided theoretical inspiration for designing more efficient fluorescence probes based on these mechanisms in the future.
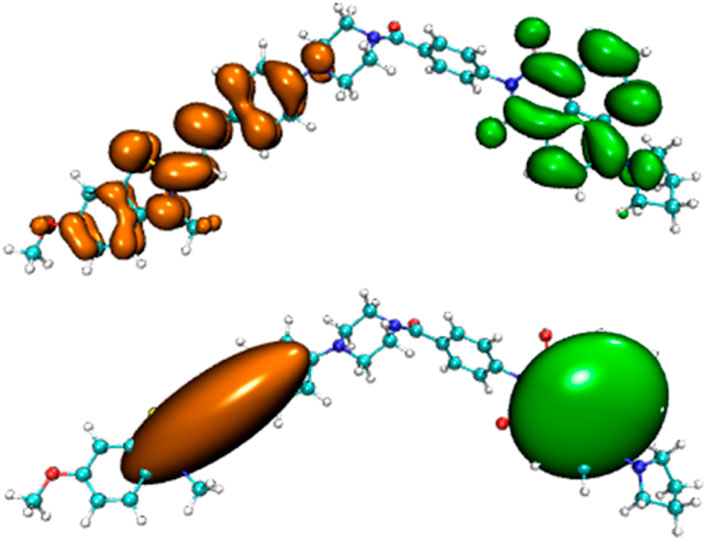
Figure 6. Difference diagram of electron distribution between S0 and S1 of probe JFT (orange:hole, green:electron).
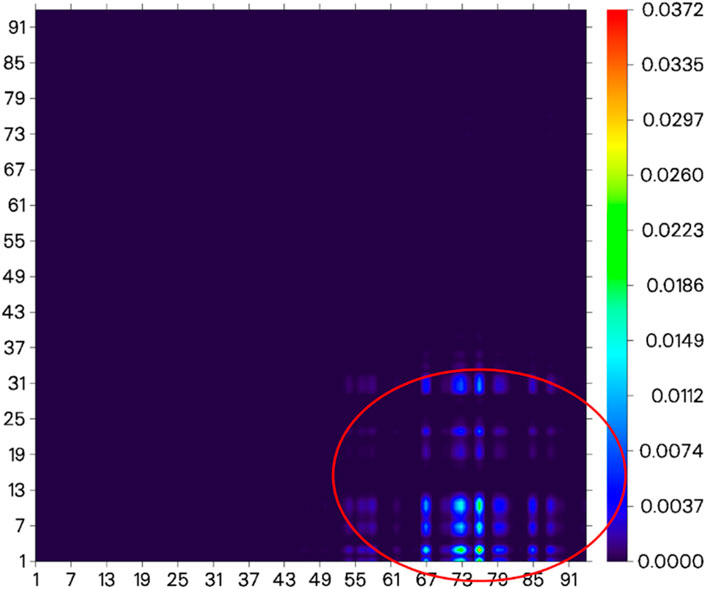
Figure 7. Electron-transfer heat map between S0 and S1 of probe JFT (donor part:1–31 to acceptor part:67–91 as shown in the red circle, the atom number list could be referenced to Supplementary Figures S1, S2).
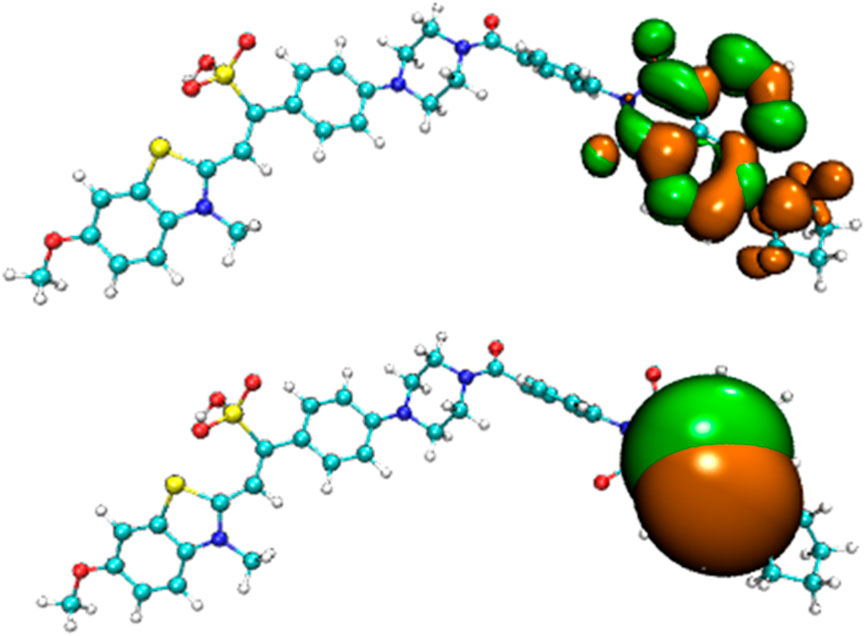
Figure 8. Difference diagram of electron distribution between S0 and S1 of the product of probe JFT reacted with HSO3− (orange:hole, green:electron).
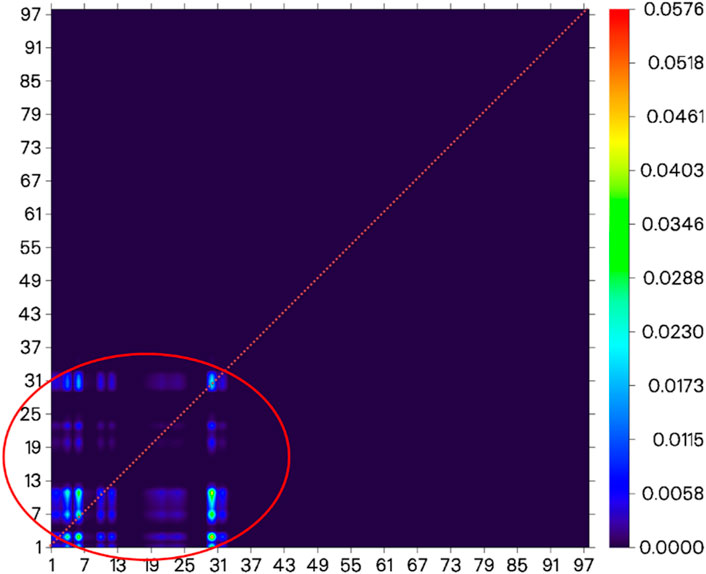
Figure 9. Electron-transfer heat map between S0 and S1 of the product of probe JFT reacted with HSO3− (donor part:1–31 to donor part:1–31 as shown in the red circle, the atom number list could be referenced to Supplementary Figures S1, S2).
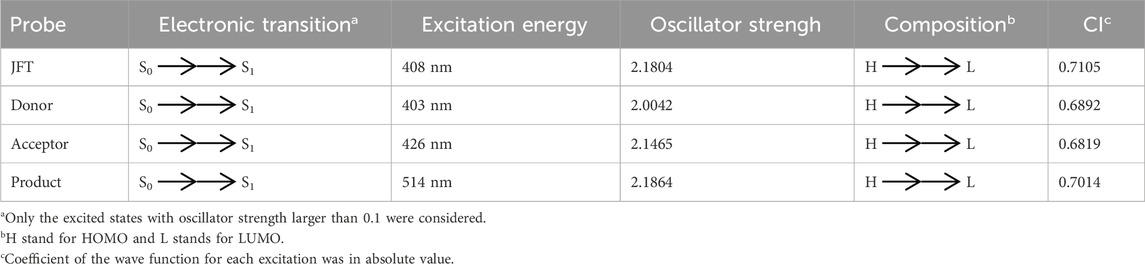
Table 1. The main electron excitation processes in the JFT probe and its product reacting with HSO3−/SO32−.
Conclusion
The development of the multifunctional fluorescence probe JFT represented a significant advancement in the field of fluorescence detection. Its ability to simultaneously monitor intracellular sulfite concentration and viscosity provided a powerful tool for studying related physiological processes. The combination of the FRET/TICT mechanism allowed the probe to respond sensitively to changes in sulfite levels and environmental viscosity. The clear understanding of the role of carbon-carbon bond rotation in fluorescence intensity regulation, as well as the influence of HSO3−/SO32− on the electronic structure and FRET process, provided a solid theoretical basis for the probe’s performance. The good linear relationship between the fluorescence change value I530/I582 and HSO3−/SO32− concentration demonstrated the probe’s potential for quantitative detection. The TDDFT-based analysis of electron distribution further validated the experimental results and enriched the theoretical understanding of the probe’s working mechanism. Overall, these results not only enhanced our knowledge of the FRET mechanism in fluorescence probes but also offer valuable theoretical inspiration for the future design of more efficient and sensitive fluorescence probes targeting similar biological parameters. However, further research could focus on improving the probe’s accuracy, reducing the deviation between calculated and experimental values, and exploring its application in more complex biological systems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
HH: Data curation, Conceptualization, Formal analysis, Writing – review and editing. BH: Writing – review and editing, Data curation, Conceptualization. YP: Data curation, Writing – original draft, Conceptualization. YL: Conceptualization, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2025.1642191/full#supplementary-material
References
Arachchige, D. L., Dwivedi, S. K., Olowolagba, A. M., Peters, J., Beatty, A. C., Guo, A., et al. (2024). Dynamic insights into mitochondrial function: monitoring viscosity and SO2 levels in living cells. J. Photoch Photobio B 258, 112986. doi:10.1016/j.jphotobiol.2024.112986
Humphrey, W., Dalke, A., and Schulten, K. (1996). VMD: visual molecular dynamics. J. Mol. Graph. 14 (1), 33–38. doi:10.1016/02637855(96)000185
Laun, J., and Bredow, T. (2022). BSSE-corrected consistent Gaussian basis sets of triple-zeta valence with polarization quality of the fifth period for solid-state calculations. J. Comput. Chem. 43 (12), 839–846. doi:10.1002/jcc.26839
Li, Y., Wang, Y., Niu, H., Chen, X., Li, Z., and Wang, Y. (2023). Research progress of sulfur dioxide fluorescent probe targeting mitochondria. Chin. J. Org. Chem. 43 (6), 1952–1962. doi:10.6023/cjoc202210030
Liu, F. T., Jiang, P. F., Wang, Y. P., Zhao, B. X., and Lin, Z. M. (2024a). A ratiometric fluorescent probe based on the FRET platform for the detection of sulfur dioxide derivatives and viscosity. Anal. Chim. Acta 1288, 342184. doi:10.1016/j.aca.2023.342184
Liu, F. T., Wang, Y. P., Jiang, P. F., and Zhao, B. X. (2024c). A FRET-based ratiometric fluorescent probe for sensing bisulfite/sulfite and viscosity and its applications in food, water samples and test strips. Food Chem. 436, 137755. doi:10.1016/j.foodchem.2023.137755
Liu, F. T., Zhai, S. M., Gao, D. F., Yang, S. H., Zhao, B. X., and Lin, Z. M. (2024b). A highly sensitive ratiometric fluorescent probe for detecting HSO3−/SO32− and viscosity change based on FRET/TICT mechanism. Anal. Chim. Acta 1305, 342588. doi:10.1016/j.aca.2024.342588
Liu, P., Liu, Y. L., Huang, H., Bai, G., and Peng, Y. J. (2023). Theoretical investigation on FRET strategy of ratio metric fluorescent probe sensing hydrogen sulfide. Spectrochim Acta A Mol Biomol Spectrosc 289, 122223. doi:10.1016/j.saa.2022.122223
Liu, Y. L., Huang, H., and Peng, Y. J. (2022). Fluorescent probe for simultaneous detection of human serum albumin and sulfite: a theoretical analysis. J. Mol. Struct. 1255, 132441. doi:10.1016/j.molstruc.2022.132441
Lu, T., and Chen, F. W. (2012). Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33 (5), 580–592. doi:10.1002/jcc.22885
Ma, J. Y., Zhao, M. T., Qin, C., Kong, X. T., Xie, H., Zhang, X. S., et al. (2024). An oxidizer-resistant fluorescence probe for detecting bisulfite and viscosity in biosystems. Dyes Pigments 229, 112321. doi:10.1016/j.dyepig.2024.112321
Neese, F. (2022). Software update: the ORCA program system-Version 5.0. Wires Comput. Mol. Sci. 12 (5). doi:10.1002/wcms.1606
Peng, Y. J., Huang, H., Liu, Y. L., and Zhao, X. Y. (2023). Theoretical insights into a near-infrared fluorescent probe NI-VIS based on the organic molecule for monitoring intracellular viscosity. Molecules 28 (16), 6105. doi:10.3390/molecules28166105
Peng, Z. X., Zhang, D., Yang, H., Zhou, Z., Wang, F. Y., Wang, Z., et al. (2024). Mitochondria-targeted fluorescent probe for simultaneously imaging viscosity and sulfite in inflammation models. Analyst 149 (12), 3356–3362. doi:10.1039/d4an00467a
Ren, J., Zhang, W., Gao, X., Song, B., and Yuan, J. (2024). Unveiling the negative correlation of formaldehyde and hydrogen sulfide in Parkinson's disease models using a dual-responsive ruthenium(II) complex probe. Chem. Eng. J. 486, 150349. doi:10.1016/j.cej.2024.150349
Shao, Y. H., Mei, Y., Sundholm, D., and Kaila, V. R. I. (2020). Benchmarking the performance of time-dependent density functional theory methods on biochromophores. J. Chem. Theory Comput. 16 (1), 587–600. doi:10.1021/acs.jctc.9b00823
Su, N. Q., Adamo, C., and Xu, X. (2013). A comparison of geometric parameters from PBE-based doubly hybrid density functionals PBE0-DH, PBE0-2, and xDH-PBE0. J. Chem. Phys. 139 (17), 174106. doi:10.1063/1.4827024
Sun, C., Cao, W. F., Zhang, W., Zhang, L. L., Feng, Y., Fang, M., et al. (2019). Design of a ratiometric two-photon fluorescent probe for dual-response of mitochondrial SO2 derivatives and viscosity in cells and in vivo. Dyes Pigments 171, 107709. doi:10.1016/j.dyepig.2019.107709
Wang, J. F., Cui, X. L., Lun, S. H., Yang, D., Gao, C., Zhang, K. Y., et al. (2025). A FRET/TICT based multifunctional fluorescent probe for the monitoring of SO2 derivatives and viscosity in living cells and real samples. Spectrochim Acta A Mol Biomol Spectrosc 325, 125074. doi:10.1016/j.saa.2024.125074
Wang, X., Tang, H., and Huang, X. H. (2021). Water-soluble fluorescent probes for bisulfite and viscosity imaging in living cells: pyrene vs. anthracene. Spectrochim Acta A Mol Biomol Spectrosc 260, 119902. doi:10.1016/j.saa.2021.119902
Wang, X., Zhou, X., Li, C., Qu, C., Shi, Y., Li, C.-J., et al. (2024). Integrative analysis of whole genome bisulfite and transcriptome sequencing reveals the effect of sodium butyrate on DNA methylation in the differentiation of bovine skeletal muscle satellite cells. Genomics 116 (6), 110959. doi:10.1016/j.ygeno.2024.110959
Zhang, C., Li, J. R., Chen, X. L., Hu, Y. J., and Huang, X. H. (2022). Rational design of ratiometric fluorescent probes for bisulfite and viscosity detection in mitochondria without spectral crosstalk. Dyes Pigments 207, 110666. doi:10.1016/j.dyepig.2022.110666
Keywords: multifunctional, fluorescent probe, sulfite, intracellular viscosity, theoretical investigation
Citation: Han H, Han B, Peng Y and Liu Y (2025) Decoding JFT: a multifunctional fluorescence probe for sulfite and viscosity insights. Front. Chem. 13:1642191. doi: 10.3389/fchem.2025.1642191
Received: 06 June 2025; Accepted: 07 July 2025;
Published: 23 July 2025.
Edited by:
Jose Carlos Corchado, University of Extremadura, SpainReviewed by:
Krishanthi C. Weerasinghe, Siena Heights University, United StatesJuan Manuel Garrido-Zoido, University of Extremadura, Spain
Copyright © 2025 Han, Han, Peng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjin Peng, cGVuZ3lqQGp6bXUuZWR1LmNu; Yuling Liu, bGl1eWxAanptdS5lZHUuY24=
 Hua Han1
Hua Han1 Yongjin Peng
Yongjin Peng