- 1Key Laboratory for Thermal Science and Power Engineering of Ministry of Education, Department of Energy and Power Engineering, Tsinghua University, Beijing, China
- 2Hunan Engineering Research Center of Clean and Low-Carbon Energy Technology, School of Energy Science and Engineering, Central South University, Changsha, China
Non-catalytic heterogeneous reactions in fluidized beds involve physical and chemical processes spanning across the atom, surface, grain, particle, and reactor scales. However, a multiscale modeling framework covering all scales has not been fulfilled due to the incomplete coupling strategies. This study develops a multiscale model coupling all five scales. The elementary reaction path is derived from first-principles calculation, which is applied to a dual-site mean-field microkinetics describing the states of active site pairs; bulk-phase ion diffusion is treated by a lumped parameter method considering the asymmetrical effects of different site types. The intrinsic reaction kinetics is coupled with intraparticle gas diffusion and fluidization computed via CFD–DEM; experimental validation is conducted on a micro-fluidized-bed thermogravimetric analyzer measuring the solid conversion. The model is applied to the reduction of CaMn0.375Ti0.5Fe0.125O3−δ by H2 at designed gas concentrations and temperatures, revealing the effects of parameters from all scales on the overall reaction kinetics. The developed multiscale framework can be further adopted in other heterogeneous reactions with determined solid microstructures.
1 Introduction
Non-catalytic heterogeneous reactions between gases and solid crystals are involved in chemical looping (Ishida and Jin, 1994; Lyngfelt et al., 2001), pollutant removal (Ning et al., 2025; Osman et al., 2021), and other chemical systems (André et al., 2016). The solid materials in such systems, including various types of oxygen carriers (Cho et al., 2004) and adsorbents (Abanades et al., 2005; Lupiáñez et al., 2013), are commonly prepared as porous particles, which is especially applicable to fluidized bed reactors. Multiple physical and chemical processes simultaneously occur in the reactor, which leads to sophisticated impacts of design parameters on the reactor’s performance, requiring to be described by a whole process model.
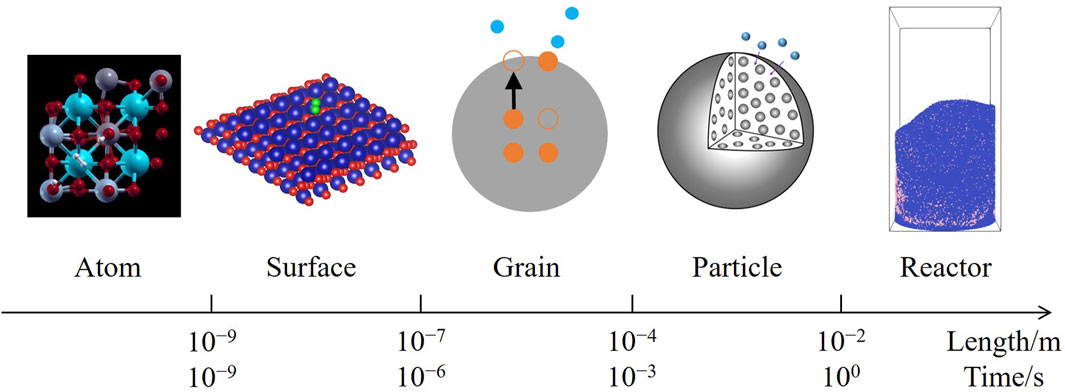
A multiscale modeling framework has been proposed for catalytic reactions (Bruix et al., 2019), spanning from the microscopic atomic behaviors and surface microkinetics to the macroscopic flows; however, this framework does not cover non-catalytic reactions on the aspect of solid conversion, particularly for solids in internal lattices, compared with catalytic reactions during which the solid components remain unchanged. A complete framework for non-catalytic heterogeneous reactions can be constructed by integrating mesoscale mass transfer processes between microkinetics and fluidization, which consists of five scales as Figure 1, including:
1. The atom scale, where solid atoms and gas molecules interact, triggering an elementary reaction;
2. The surface scale, where reaction intermediates are adsorbed onto active sites distributed on the solid surface, according to Langmuir’s adsorption model (Langmuir, 1916);
3. The grain scale, where solid ions migrate between the surface and internal lattices of a grain, converting the solid reactant to the product;
4. The particle scale, where gas molecules diffuse through pores in a particle, before reaching the inner grain surfaces;
5. The reactor scale, where the particles are fluidized by the gas, forming a two-phase flow.
Various validated models for every independent scale are found in the literature. At the atom scale, the reaction paths and rate constants are directly calculated from the material properties with DFT (density functional theory), also known as the first-principles calculation (Bhandari et al., 2020; Fang et al., 2022; Li et al., 2023; Liu T. et al., 2021; Noor et al., 2020; Wang X. et al., 2024; Yan et al., 2020), which is a rigorous theoretical model requiring no empirical parameters. At the surface scale, the microkinetics, evolving the coverages of different species on the active sites, is modeled with mean-field approximation (Madon et al., 2011; Maestri et al., 2008; Thybaut et al., 2011) or kinetic Monte Carlo (Andersen et al., 2019; Reuter, 2016); elementary reactions may occur by single-site mechanisms including adsorption–desorption and Eley–Rideal, or multi-site mechanisms such as dissociation–association and Langmuir–Hinshelwood (Motagamwala and Dumesic, 2021); dual-site mechanisms are possible on a surface with different types of active sites (Van Belleghem et al., 2022; D’Ambrosio et al., 2024). At the grain scale, the solid conversion process can be described by homogeneous bulk diffusion (Arangio et al., 2015; Stearn and Eyring, 1940; Willis and Wilson, 2022), shrinking core model (Ishida and Wen, 1971; Szekely and Evans, 1970), or a more complex product island model (Fang et al., 2011; Li, 2020), based on the structure of the solid reactant. At the particle scale, intraparticle gas diffusion is controlled by the distribution of grains and pores, shaped by either grain models (Dam-Johansen et al., 1991; Gibson III and Harrison, 1980; Szekely and Evans, 1971) or pore models (Bhatia and Perlmutter, 1980; Bhatia and Perlmutter, 1981; He et al., 2013; Petersen, 1957; Sandmann Jr and Zygourakis, 1986). At the reactor scale, numerical simulation is applied to the two-phase flow to predict the fluidization behavior, the gas phase evolved with CFD (computational fluid dynamics), and the dense particle phase modeled with TFM (two-fluid model) (Ishii and Mishima, 1984), MP-PIC (multiphase particle-in-cell) (Andrews and O'Rourke, 1996) or DEM (discrete element method) (Cundall and Strack, 1979; Golshan et al., 2020); as the computational capacity develops, the most detailed and computationally expensive CFD–DEM model has been increasingly adopted, supporting up to 105–108 particles (Golshan et al., 2020).
Despite the variety of abovementioned models, the coupling between adjacent scales, which multiplies the computational costs of both models, has been an obstacle to completing the framework. Existing studies have proposed several coupling strategies for particular scales by simplifying the larger scale to identical sub-processes of the smaller scale. Microscopic coupling, suggested by studies on catalytic microkinetics (Alexopoulos et al., 2016; Chen and Wang, 2024; Jørgensen and Grönbeck, 2016; Rawal et al., 2021; Yin et al., 2020; Yu et al., 2023), assumes that active sites uniformly distribute on the surface, all sites sharing the same reaction paths derived from first-principles calculation; on this basis, the difference among sites is omitted in single-site reactions, while in multi-site reactions, the state of every pair of neighboring sites shall be considered (Razdan and Bhan, 2021). Macroscopic coupling appears in both CFD–DEM studies numerically solving intraparticle diffusion (Hadian et al., 2024), and particle-scale models analytically expressing the reaction rate with the surface gas concentration (Sedghkerdar and Mahinpey, 2015; Wang et al., 2017; Wang et al., 2021; Yang et al., 2016). The above micro- and macroscopic coupling strategies have been generally agreed in the literature; in contrast, mesoscopic coupling, focusing on the relation between microkinetics and solid conversion, has not undergone sufficient research. Some studies have introduced a bulk-phase ion diffusion process, associating the surface coverage of active ions with its bulk concentration (Li, 2022; Li L. et al., 2021; Liu et al., 2022; Wang et al., 2025; Wang Y. et al., 2024); others have described a phase transformation process based on the shrinking core model, which is integrated into the microkinetics (Cai and Li, 2024a; Cai and Li, 2024b; Cai and Li, 2024c).
However, none of above coupling models have accomplished a complete multiscale framework as Figure 1. Studies on microkinetics have not considered the solid conversion processes of a real porous particle; CFD–DEM studies, mainly applied to the combustion of organic solid fuels, whose molecular structures are unclear, have not developed verifiable microkinetics. Consequently, parameters from the absent scales rely on experimental fitting, instead of theoretical calculation or direct measurement. Models with mesoscopic coupling, excluding the reactor-scale, are unable to provide adequate information of the diverse particles; furthermore, only surfaces with a single type of active sites are involved in these studies (Cai and Li, 2024a; Cai and Li, 2024b; Cai and Li, 2024c; Li, 2022; Li T. et al., 2021; Liu et al., 2022; Wang et al., 2025; Wang Y. et al., 2024), while dual-site reaction mechanisms have not been discussed, resulting in a limited scope of application.
The aim of this study is to develop a multiscale model for non-catalytic heterogeneous reactions under the framework of Figure 1, coupling strategies introduced between every two adjacent scales. The atom-scale reaction path is derived from first-principles calculation; the surface-scale mean-field microkinetics describes the coverages of two types of active sites; the grain-scale ion diffusion is coupled with the microkinetics by the lumped parameter method; the particle-scale gas diffusion is treated under a uniform grain model; the reactor-scale fluidization is simulated with CFD–DEM. Experimental validation is conducted on a micro-fluidized-bed thermogravimetric analyzer (MFB–TGA) (Li et al., 2019). Without loss of generality, the dual-site reaction of a perovskite oxygen carrier (CaMn0.375Ti0.5Fe0.125O3−δ, CMTF8341) reduced by H2 is considered. Kinetics of this reaction has been experimentally studied in a prior work (Liu L. et al., 2021), where the material is prepared by spray drying and calcination, sieved between 180 and 250 μm, and tested on MFB–TGA; fast reaction kinetics and good stability have been observed under H2/O2 redox cycles. The gas reactant, H2, offers a special dual-site mechanism as needed, while fewer elementary reactions are involved compared with other reducing agents. Moreover, the perovskite structure allows oxygen anions to undergo homogeneous bulk diffusion, rather than changing the surface structure into another phase. Thus, this reaction is expected to concisely and clearly describe the full modeling framework.
2 Model
The overall reaction of H2 reducing CMTF8341 as Equation 1.
Complete reduction is achieved when
The solid conversion is defined by the real-time solid mass,
which is applicable to a grain, a particle, or all CMTF8341 particles in a reactor. The conversion increases from 0 to 1 during the whole reaction process. The final output of the model is the change of conversion against time in a reactor, which is also measured by the experiment as Section 3.
2.1 Atom scale
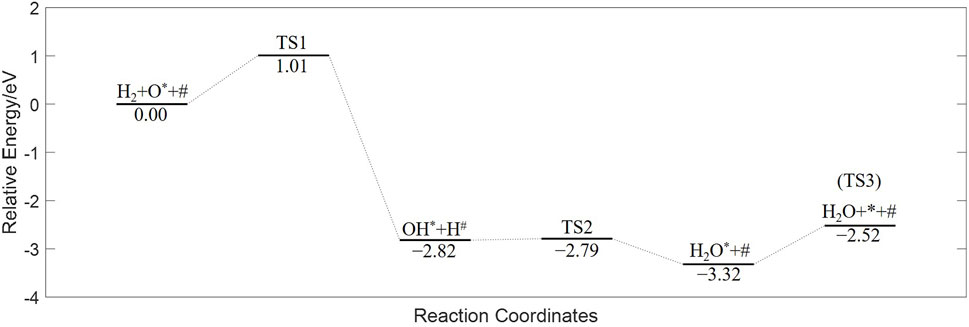
DFT calculation is conducted for H2 reducing fully oxidized CMTF8341. The reaction path is illustrated in Figure 2, consisting of (three elementary steps) as Equations 4–6. First, one H2 molecule undergoes a dissociative adsorption onto the surface; one H atom is combined with an active O atom on a Mn site, forming a hydroxyl (OH) radical; the other H atom is more likely to be placed on a Ca atom, rather than another Mn site. With Mn sites represented by
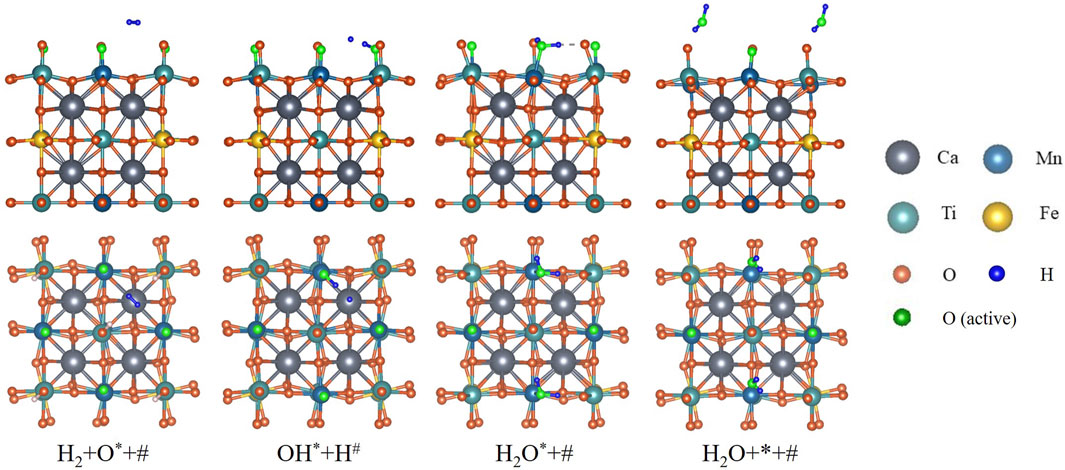
Figure 2. Reaction path of CMTF8341 reduced by H2 from DFT calculation. The H2 molecule undergoes asymmetrical dissociation, re-association, and desorption to become H2O.
Subsequently, the single H atom is associated with the OH radical, forming one H2O molecule on the Mn site.
The last step is the H2O molecule desorbing into the gas phase, leaving a vacant Mn site on the surface.
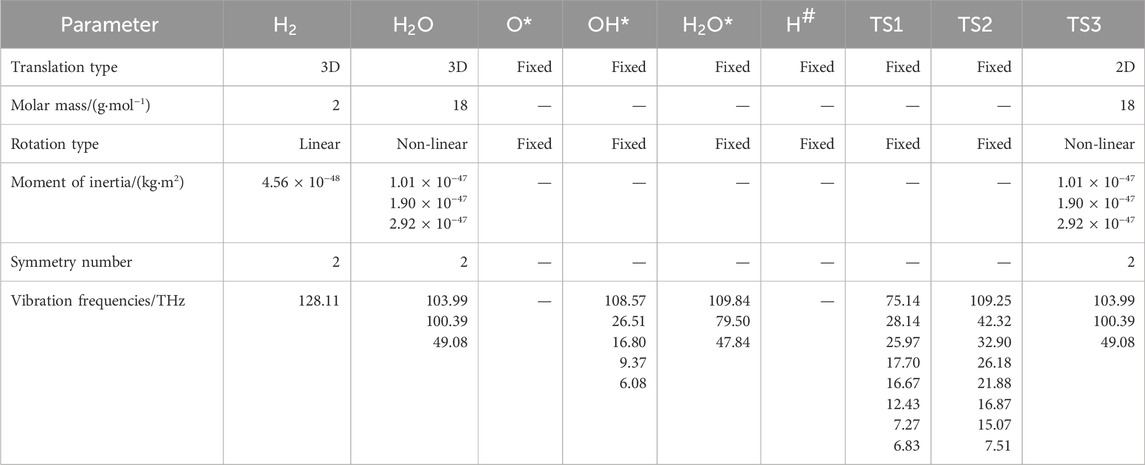
The energy diagram, having undergone zero-point correction, is plotted in Figure 3. The vibration frequencies of the species and transition states, along with other basic parameters, are listed in Table 1.
2.2 Surface scale
2.2.1 Mean-field assumption
The surface of the CMTF8341 crystal is highly periodical, where both Mn sites and Ca sites are evenly distributed. A mean-field assumption is employed in treating the elementary reactions on different sites, which neglects the change of the surface force field caused by different adsorbates, so that all sites are in an identical environment. Consequently, elementary reactions on every site occur along the same paths as Figure 2, while every site shares an equal reacting probability.
Previous studies on single-site reactions, based on the mean-field assumption, have treated the surface species as non-localized independent particle systems (Cai and Li, 2024a; Cai and Li, 2024b; Cai and Li, 2024c; Liu et al., 2022; Wang et al., 2025). A dual-site reaction, however, can only occur on an adjacent pair of sites, rather than every pair; thus, the surface species shall not be treated as completely independent for dual-site reactions. Alternatively, a pair of sites can be regarded independent of other pairs, given that one elementary reaction does not interfere with reactions on other sites.
Consider a CMTF8341 surface, where the numbers of Mn sites and Ca sites are
Assuming that a Mn site is occupied by a surface species
where
2.2.2 Rate equation
The rate of an elementary reaction is derived from the transition state theory. The transition state of the single-site reaction (Equation 6) is formed on a Mn site, so the rate equation is based on the Mn-site coverages, as Equation 9.
where every
As for a dual-site reaction, taking Equation 4 for example, the transition state is formed on a site pair, so the rate is expressed on a site-pair basis, as Equation 10
where
the rate equation is transformed into Equation 12.
The above derivation is the same for Equation 5, resulting in Equation 13.
In brief, the rate constants of dual-site reactions shall be multiplied by the number of neighbors (
2.3 Grain scale
2.3.1 Bulk ion diffusion
The reduction of CMTF8341 is achieved by the removal of oxygen anions (
The ion diffusion process is fast enough in perovskites (Li, 2022), so that the concentration gradient is negligible. A lumped parameter method is employed by treating
The equilibrium of Equation 14 is reached by equalizing the surface and lattice oxygen concentrations, given by Equation 15
where
where
The overall reaction is the sum of elementary reactions Equations 4, 5, 6, 14, as Equation 17
which is equivalent to Equation 1.
2.3.2 Non-catalytic microkinetics
Given the rates of surface reactions Equations 4–6 and ion diffusion Equation 14, the complete microkinetics on Mn sites is expressed as Equation 18
while on Ca sites, there is Equation 19.
For lattice oxygen or vacancies, there is Equation 20.
Note that the ion diffusion rate,
The above equations result in a total of eight species evolved by four elementary reactions. The number of independent variables among the species shall be no more than the number of reactions. One restriction is that the sum of all quantities on one type of sites (or the lattices) shall be constant, as Equation 21
where
Consequently, the state of a grain can be determined by four independent variables (for example,
2.3.3 Quasi-steady approximation
Further simplification is obtained from the partial equilibrium assumption, suggesting that the overall reaction rate is controlled by the slowest step, which is Equation 4 in this case; other reversible elementary reactions, Equations 5, 6, 14, are near equilibrium, as concluded by the results in Section 4.2. However, no analytic solution is obtained from the above assumption. Given that the intermediates,
an approximate solution is derived as Equation 24
where
As only one independent variable (
Substitute
The preceding factor of Equation 26 indicates the effect of ion diffusion compared with surface reaction, hereinafter expressed with the symbol
2.4 Particle scale
The rate equation of a grain involves not only the solid conversion, but also the gas partial pressure at the surface, which is not equal for all grains due to their spatial distribution. Grains at the particle surface obtain the most concentrated gas from the atmosphere, while less gas is received by inner grains as it diffuses through the pores, simultaneously consumed by outer grains. Assuming that the grains are evenly distributed throughout the particle’s volume, a radial gas distribution is governed by an equation considering both reaction and diffusion, as Equation 28
where
The diffusion term is described by the Fick’s Law; the diffusivity shall combine molecular and Knudsen diffusion because the pore radius is smaller than the molecular mean free path length. The particle conversion is the average of all grains, given by Equation 30.
The governing equation, Equation 28, requires numerical solution due to both
where
the Thiele modulus
2.5 Reactor scale
The conversion rate of a particle, as Equation 31, is based on its surface gas partial pressure. Each particle in a fluidized bed moves along a unique trajectory under the fluidization by the inlet gas flow, and is therefore exposed to a gas partial pressure different from other particles. The fluidization behavior is described by the Eulerian–Lagrangian CFD–DEM model in this study, coupling the single-particle reaction kinetics with the particle–fluid motion. The thermal effects are omitted due to the low reduction heat generally observed from various oxygen carriers (García-Labiano et al., 2005; Hallberg et al., 2011), as well as the stable temperature measured during the experiment as Section 3.1.
2.5.1 Particle phase
The particle mass is related to its conversion by Equation 3; consequently, the mass equation of a particle is Equation 33
where
The translational motion of a particle is evolved by Newton’s Second Law as Equations 34, 35
and rotational motion as Equation 36
where
where
where the subscripts “N” and “T” represent the normal and tangential components, respectively. Both components consist of an elastic force proportional to the overlap
2.5.2 Fluid phase
The gas flow in the reactor is regarded incompressible, described by the governing equations of fluid dynamics. As part of the volume is occupied by the dense particle flow, the governing equations shall involve the fluid volume fraction, denoted as
where
The equation of momentum is expressed as Equation 40
where the interphase drag force given by Equation 37 is considered, along with the momentum carried by the transferred mass. The viscosity
3 Experimental and computational setup
3.1 Experiment
Validation experiment is conducted on a micro-fluidized-bed thermogravimetric analyzer (MFB–TGA) (Li et al., 2019). A fluidized bed reactor, 3 cm in diameter and 10 cm in height, is placed on an electronic balance, measuring the real-time solid mass change under fluidization throughout the reaction process. More information is provided in the Supplementary Material.
The measurement accuracy is 1 mg, compared with the maximum mass change of 16 mg. Gas inlet and outlet are connected to the reactor with soft tubes to reduce mass fluctuation introduced by the gas circuit. The temperature is maintained constant by an electric furnace and monitored by a K-type thermocouple, which do not contact the reactor. Consequently, the signal from the electronic balance shall accurately present the total solid mass change in the reactor.
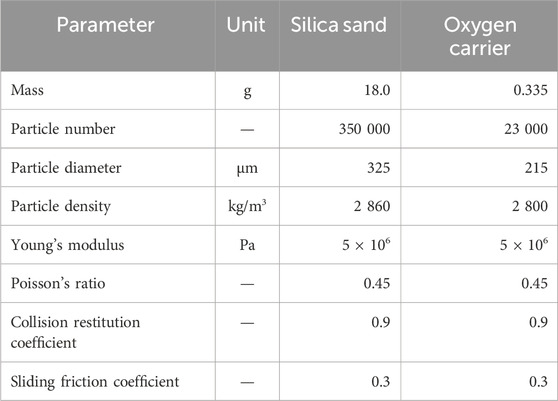
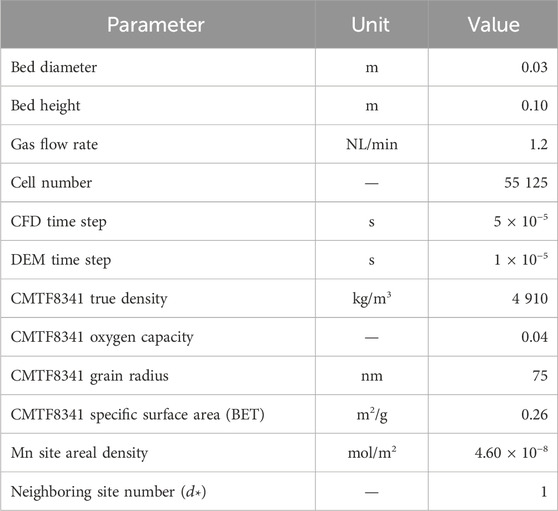
The bed inventory consists of silica sand and oxygen carrier particles, whose fluidization properties are listed in Table 2. A bubbling fluidization regime is observed under a gas flux of 1.2 NL/min. Continuous redox cycles are performed by switching the gas among inert (N2), oxidizing (O2) and reducing (H2) components, under different H2 concentrations (5 vol%, 10 vol% and 20 vol%) and temperatures (750 °C, 800 °C, 850 °C and 900 °C).
3.2 Computation
The simulating conditions in CFD–DEM are identical to those in the MFB–TGA experiment, computational settings and reaction parameters listed in Table 3. Initialization is done by injecting given numbers of sand and fully oxidized oxygen carrier particles, which are immediately packed under gravity. Subsequently, an inert fluidizing gas stream is introduced from the bottom at a given temperature. The gas is switched to a reducing composition after a stable fluidization is achieved, and the reaction simultaneously begins. A mesh-refinement test showing grid-independence is presented in the Supplementary Material. Every case is parallelized into 24 CPU cores and run on a supercomputing center, taking 8 h of real time to progress 1 s of simulation time.
The open source software CFDEM®coupling (Kloss et al., 2012) is selected for CFD–DEM computation, which serves as an interface alternately progressing CFD and DEM time steps. CFD is solved by OpenFOAM® with the PISO algorithm; discretization is accomplished through the finite volume method. Particle motion and collision are solved by LIGGGHTS®, employing a first-order Euler time integration scheme.
The reaction kinetics is not implemented in the original software; instead, it is programmed by the authors into an extended package, whose architecture is shown in Figure 4. The overall reaction is described by a class on the top layer, which outputs the particle conversion rate as Equation 31 to CFDEM®coupling; a list of elementary reactions is recorded as its component. The elementary reaction class calculates the rate constants as Section 2.2.2, relying on the reactant and product species along with the transition state. The species class aggregates submodels calculating the equation of state and partition functions; respective model types are selected for every species. DFT calculation is separately performed on the VASP software (Kresse and Furthmüller, 1996) before CFD–DEM computation.
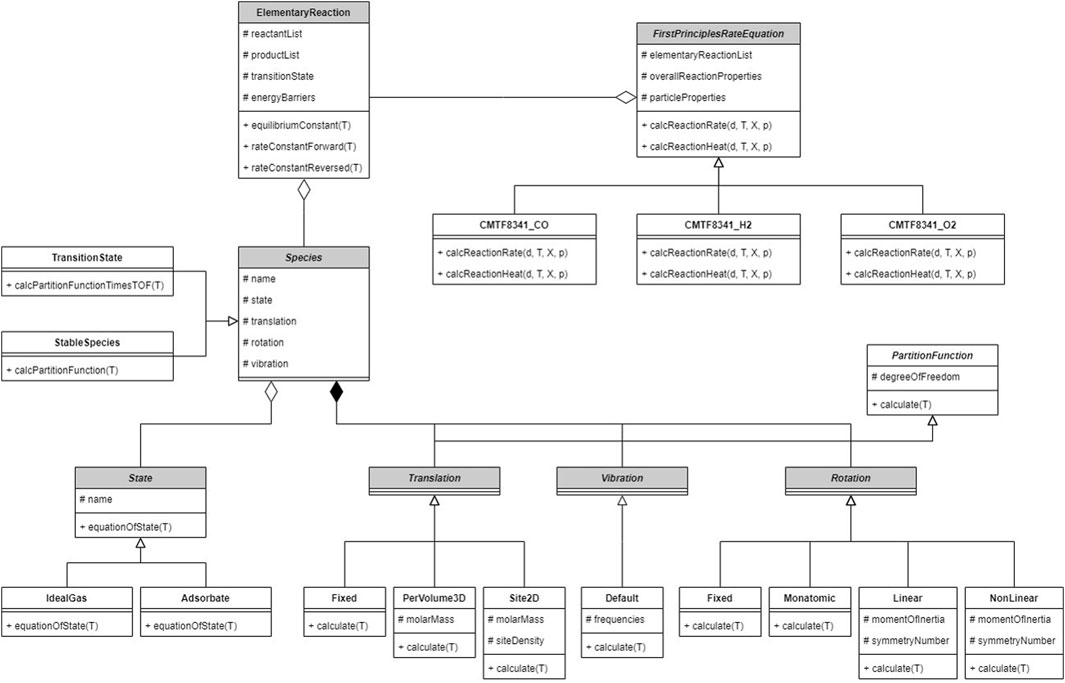
Figure 4. Architecture of the single-particle reaction kinetics program. The abstract class Species is used to construct different species and transition states in the reaction path; statistical-mechanical calculation methods are implemented; elementary and overall reactions are constructed from given species.
All inputs required by the model, including measured properties, DFT results, reaction equations and submodel types, are provided in a text file during runtime; various overall reactions can be constructed by modifying the inputs, which results in different implementations of the abstract classes.
4 Results and discussion
4.1 Solid conversion rate
The conversion of all oxygen carrier particles in the reactor is derived from both computation and experiment as Figure 5. The simulated result represents the average conversion of all particles, corresponding to the experimentally measured conversion of the whole bed inventory. An approximately linear conversion growth is observed in the initial fast stage under every condition, which is transformed to a slow stage as the conversion approaches 0.8. Such phenomenon is affected by the mass transfer resistance of the reactor; as particles near the inlet are converted, more gas is supplied to farther particles, thus compensating the reaction deceleration caused by increased conversion. Therefore, restricting the residence time of particles, to maintain the solid conversion below 0.8, would be beneficial to chemical looping which requires fast redox cycles rather than complete conversion.
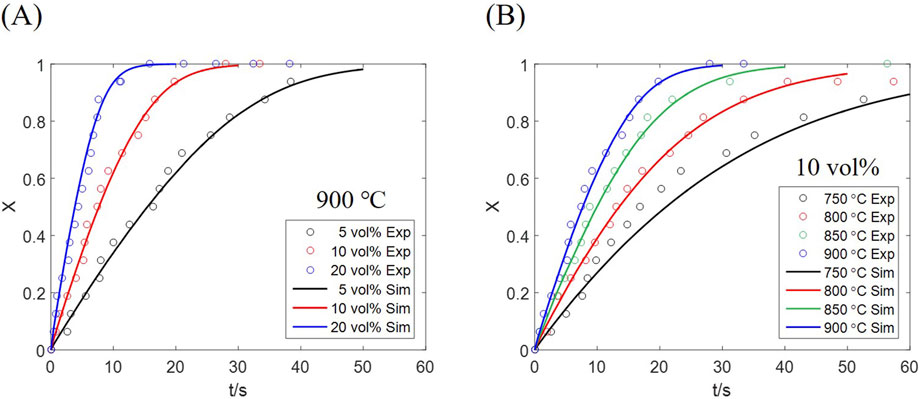
Figure 5. Solid conversion results from computation and experiment at (A) different H2 concentrations, (B) different temperatures.
The conversion curves under different inlet H2 concentrations, at a temperature of 900 °C, are plotted in Figure 5A, which shows that the reaction is accelerated as the inlet concentration increases. An average conversion of 0.8 is reached at ∼8 s for 20 vol% H2, while the time is prolonged to ∼15 s for 10 vol% H2, and ∼30 s for 5 vol% H2. Above results indicate that the fast-stage rate is approximately proportional to the inlet gas concentration, proved by the particle rate equation, Equation 31, where the gas partial pressure appears as an independent factor.
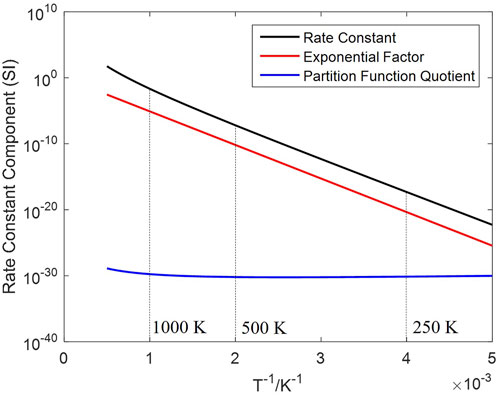
The effect of temperature under an equal H2 concentration (10 vol%) is shown in Figure 5B, a significant rate increase observed at a higher temperature. Temperature has a decisive impact on the rate constant
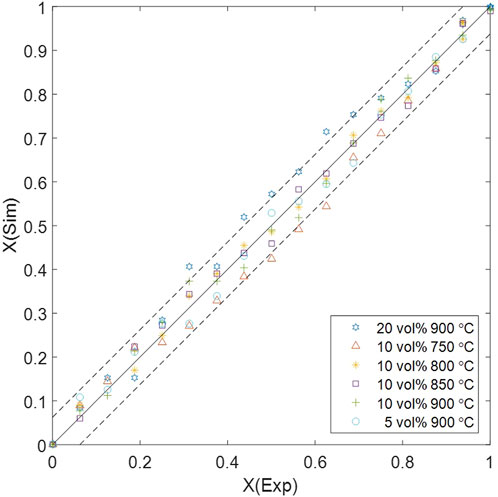
The error margin of solid conversion is determined by the measurement accuracy of MFB–TGA (1 mg), resulting in a solid conversion error of 0.0625. The comparison between computational and experimental results are displayed in Figure 7, showing that the multiscale computation is accurate at most data points. However, exceeded errors are observed under high H2 concentration (20 vol%) or low temperature (750 °C) in the middle of the reaction processes. The concentration-related error is mainly introduced by mass transfer, which has a greater impact when a higher inlet concentration leads to a non-uniform distribution; unresolved CFD–DEM omits the fine structure of the particle surface boundary layer, tending to underestimate the mass transfer resistance (Derksen, 2014; Srinivasakannan et al., 2012). Integrating a surface film model into the particle-scale diffusion is a possible way to show the effect of boundary-layer mass transfer; interparticle gas diffusivity in the dense phase should be more accurately modeled considering the sub-scale velocity distribution, which may need validation by particle-resolved DNS–DEM before application to CFD–DEM (Wang et al., 2023). The temperature-related error depends on the energy barrier
4.2 Microkinetics with bulk diffusion
The areal density of Mn sites (
The microkinetics as Equations 18–20 is originally solved as an ordinary differential equation set; an example condition of 20 vol% H2, 1 vol% H2O and 900 °C is adopted in this section. The coverages of all species on the primary (Mn) site vary as Figures 8A,B. The major components are the reactant (
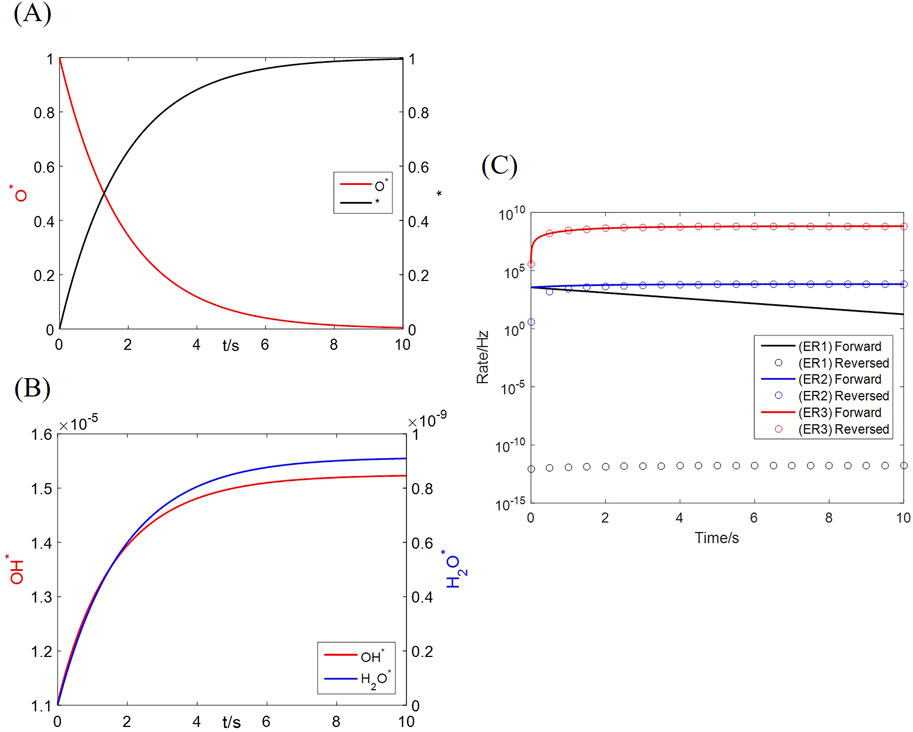
Figure 8. Numerical solution to the dual-site microkinetics with bulk diffusion. (A) Coverages of reactant and product species; (B) Coverages of intermediates; (C) Forward and reversed elementary reaction rates.
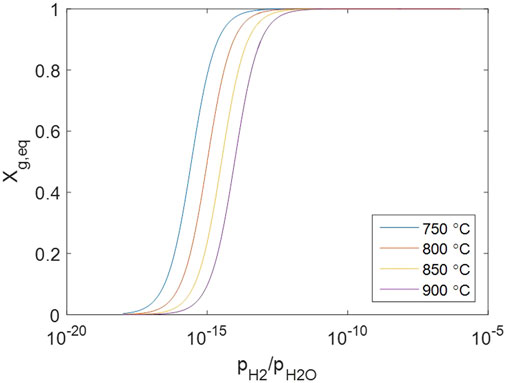
The overall reaction Equation 17 has an equilibrium point when elementary reactions Equations 4, 5, 6, 14 are all equilibrated, as required by the principle of detailed balance. Let Equations 4, 5, 6, have zero net rates in Equations 5, 8 and 9, combined with the ion-diffusion equilibrium as Equation 15, resulting in Equation 41.
Note that the solid concentrations are involved because crystal defects are treated as non-localized systems, which is not the case for perfect crystal species in other common reactions. The equilibrium solid conversion changes against gas concentrations as Figure 9, suggesting that the solid is almost fully converted under normal conditions when both
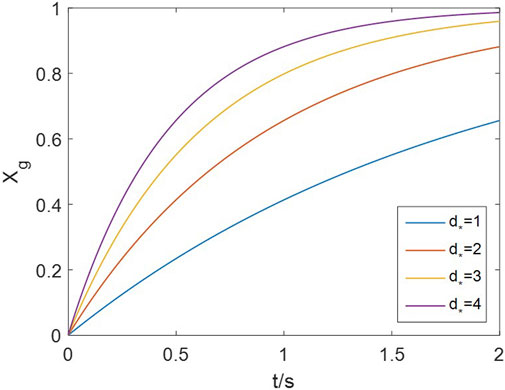
The number of neighboring sites (
4.3 Particle diversity
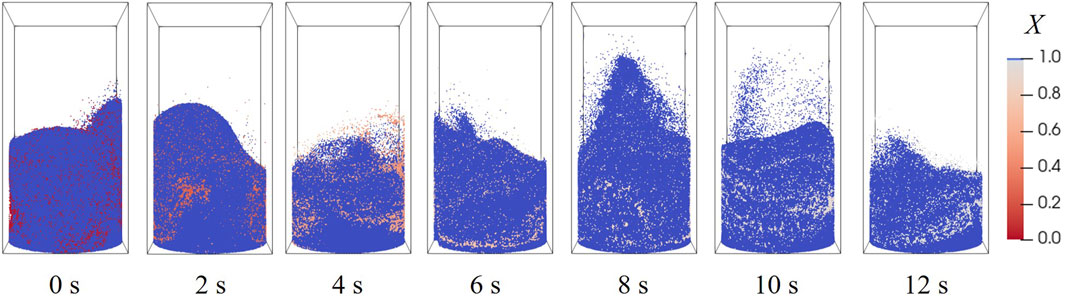
The evolution of particle distribution at 900 °C, 20 vol% H2, is illustrated in Figure 11. The moment of 0 s is the starting point of reaction, when a stable bubbling fluidization has been established. Particles in the center are elevated by the gas stream, and subsequently fall along the walls under gravity; the bed height fluctuates around 3 cm as the void fraction of the bed inventory zone changes. The conversions of oxygen carrier particles are marked by color in Figure 11, where only a minor difference exist among all particles, suggesting that an even mixture is accomplished by fluidization.
Both the computational and experimental results in Section 4.1 display the overall solid conversion, which equals the average of all particles. However, the conversion of a single particle may deviate from the average. The average, maximum and minimum conversions among all particles are plotted in Figure 12A, along with three typical particles, at 20 vol% H2, 900 °C. Particle 3 undergoes an average-rate reaction, while Particle 1 is faster and Particle 2 slower. These particles are exposed to particular H2 concentrations at every moment, determined by their unique trajectories during fluidization. The positions of the three particles, sampled during the initial fast stage of the reaction (0–5 s), are presented in Figures 12B,C. Particle 1 has the lowest average position in height, and Particle 2 the highest, which indicates that Particle 1 is exposed to a higher concentration of H2 than Particles 3 and 2.
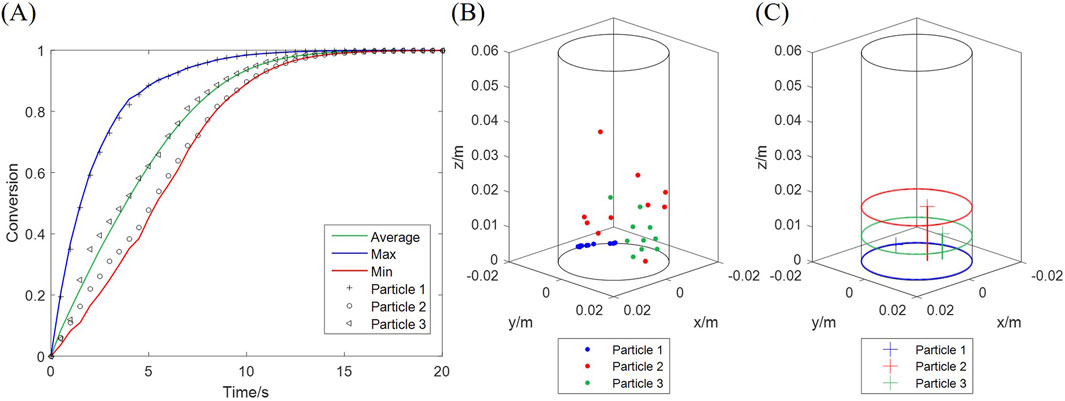
Figure 12. Conversion of three typical particles undergoing fast (particle 1), slow (particle 2) and average (particle 3) reactions. (A) Conversion curves; (B) Positions at different moments; (C) Average positions during the initial 5 s.
A deviation among particle conversions is accumulated during the initial fast stage, with the fastest rate being 2–3 times the slowest rate, which subsequently decreases as the particles are converted. Histograms in Figure 13A display the particle conversion distributions at different average conversions, which all show a normal distribution scheme. Besides, the particle conversion distribution is biased to the lower side in the beginning, which is because most H2 is consumed by the few particles near the inlet, while other particles only obtain a low H2 concentration.
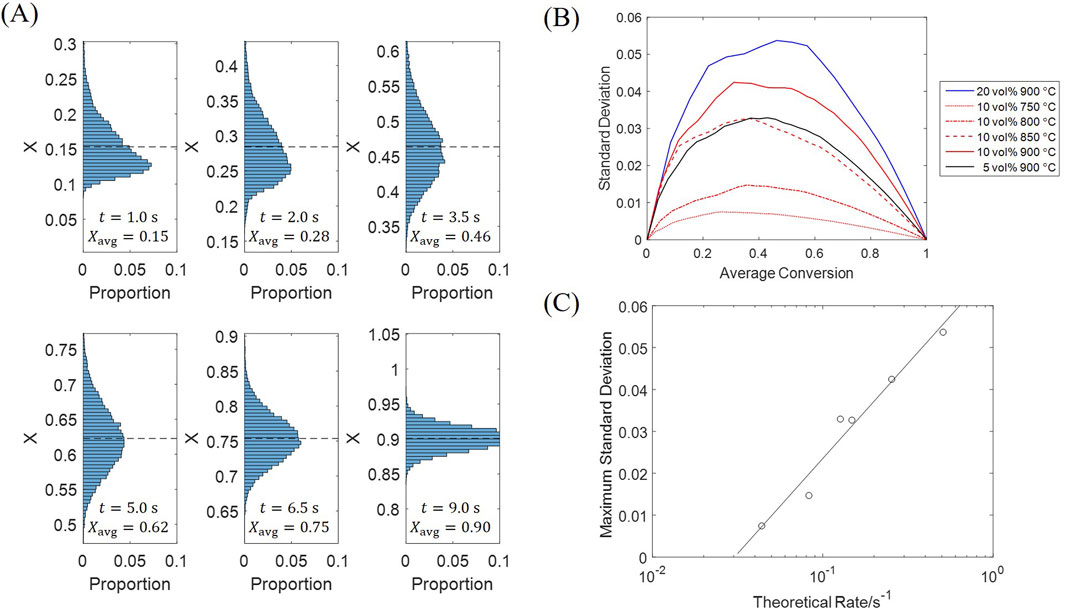
Figure 13. Standard deviation of particle conversion at different moments. (A) Histograms; (B) Standard deviation against average conversion; (C) Maximum standard deviation against theoretical reaction rate.
The standard deviation reaches its maximum at an average conversion of 0.3–0.5, under all tested conditions as Figure 13B; a greater standard deviation is observed for a condition with a faster reaction rate (given by Figure 5). The maximum standard deviation can be selected as a quantitative indicator of particle diversity, which is plotted against the theoretical reaction rate in Figure 13C. The theoretical reaction rate is calculated from Equation 31, when the particle has a conversion of zero and obtains an inlet gas concentration, acting as a superior limit which leads to a rate between 0% and 100% of this limit for every single particle. Results show that the maximum standard deviation is approximately linear against the logarithm of theoretical rate. As the fluidization process provides the spatial H2 concentration distribution with a similar pattern under all conditions, consequently, a greater deviation is formed at a faster theoretical rate. Such diversity of particles decelerates the overall reaction, because more gas is involved in a slower reaction stage with the highly-converted particles; the utilization efficiency of the solid material is thus affected, which shall be compensated in the system design.
4.4 Intraparticle gas diffusion
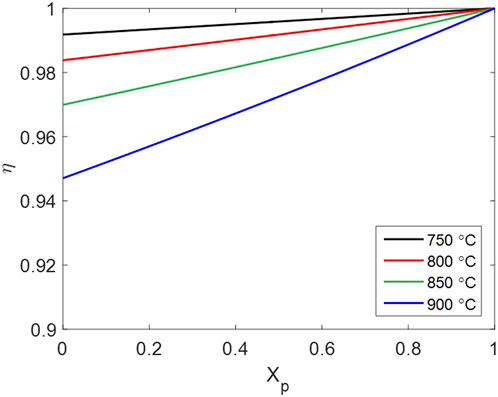
The influence of intraparticle gas diffusion is represented by the effectiveness factor
4.5 Overall reaction order
The overall rate equation derived from quasi-steady approximation, Equation 26, is first-order for the solid species, as the solid conversion appears as a factor of
where the H2 molecule is dissociated on two identical sites (
which significantly varies from the first-order rate under dual-site dissociation Equation 4.
Experimental validation in Section 4.1 has agreed with the first-order rate equation, along with the dual-site dissociation mechanism. Dual-site microkinetics lead to asymmetrical impacts on bulk diffusion, which only occurs on primary sites (Mn) rather than secondary sites (Ca). Therefore, the atom-scale reaction mechanism may have crucial impacts on the mesoscale mass transfer and macroscopic kinetics.
4.6 Computational cost for scale-up systems
The heavy computational cost has been an obstacle to scaling-up CFD–DEM simulation to industrial-scale reactors. Parallel computing accelerates computation by independently handling local interactions. A scale-up test is conducted based on the setup as Section 3.2, by varying the number of processors between 2 and 48, thus changing the number of particles per processor. The real time cost per second of simulation time is plotted in Figure 15. An approximately proportional acceleration is obtained in lowly-parallelized cases. However, extra cost is introduced by interprocess communication; the computational speed thus remains stable and subsequently slows down as more processors are employed.
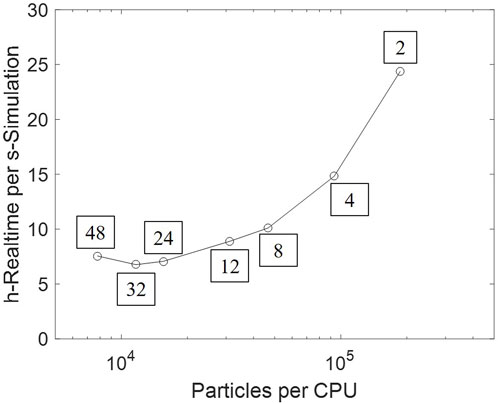
Figure 15. Computational time varying against the number of particles per processor. The processor number is tagged beside each data point.
Therefore, further acceleration approaches shall provide essential improvements, for either computation or the DEM model itself. Graphical processing units (GPU), which are especially applicable to batches of simple computation, can be used to handle the massive particle collision (Lu, 2022). Existing investigation on modeling improvements include: (1) the coarse-grain model, which significantly reduces particles in the system, by combining multiple particles into a parcel (Sakai et al., 2014); (2) machine learning algorithms, which replaces direct force calculation with a prediction–correction updating (Lu et al., 2021). These modeling treatments have partially sacrificed the accuracy of fluidization; however, given the minor effect of particle diversity (as Section 4.3), such simplification would be acceptable, provided that the fluidization regime is maintained.
5 Conclusion
A multiscale model is developed regarding the whole process of non-catalytic heterogeneous reactions in a fluidized bed. Physical and chemical processes spanning across various scales are involved, including the atom-scale elementary reaction path, the surface-scale dual-site microkinetics, the grain-scale asymmetrical bulk diffusion, the particle-scale gas diffusion, and the reactor-scale fluidization behavior. The model is applied to the reduction of CMTF8341 by H2 and validated by the MFB–TGA experiment, revealing the effects of inlet gas concentration and temperature on the macroscopic reaction kinetics.
The prediction of overall reaction rate strongly relies on the reaction path from DFT calculation, whose results suggest a dual-site dissociation for H2 molecules, resulting in a first-order overall rate equation compared with the second-order symmetrical dissociation mechanism. A mean-field microkinetics regarding active site pairs is established, every site connected to an equal number of neighbors on the periodical surface, forming the same number of equivalent reaction paths. Furthermore, the dual-site microkinetics is coupled with bulk diffusion in an asymmetrical approach, where the primary site exchanges ions with inner lattices, while the secondary site only accommodates intermediates to assist surface reactions. The neighboring-site number is currently a hypothetic parameter; scanning transmission electron microscopic (STEM) and scanning tunneling microscopic (STM) observation are potential ways to justify corresponding assumptions.
The rigorous theoretical model is based on physical parameters instead of experimental fitting, thus reducing the experimental costs when applied to other reaction systems. An extensible software package is developed for mesoscopic models and integrated into open source CFD–DEM software; generalization to other heterogeneous reactions can be accomplished by inputting DFT results along with elementary reaction equations and instrumentally measured properties. This work is conducted on a lab-scale reactor, while the computational cost is an obstacle to scaling the model to industrial-scale systems; potential methods including coarse-graining models and GPU acceleration may be involved in relevant future studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RW: Data curation, Methodology, Formal Analysis, Investigation, Software, Conceptualization, Writing – original draft. ZL: Writing – review and editing, Funding acquisition, Conceptualization, Supervision. LL: Validation, Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (No. 2024YFB4105904).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2025.1656180/full#supplementary-material
References
Abanades, J. C., Anthony, E. J., Wang, J., and Oakey, J. E. (2005). Fluidized bed combustion systems integrating CO2 capture with CaO. Environ. Sci. Technol. 39 (8), 2861–2866. doi:10.1021/es0496221
Alexopoulos, K., John, M., Van der Borght, K., Galvita, V., Reyniers, M. F., and Marin, G. B. (2016). DFT-based microkinetic modeling of ethanol dehydration in H-ZSM-5. J. Catal. 339, 173–185. doi:10.1016/j.jcat.2016.04.020
Andersen, M., Panosetti, C., and Reuter, K. (2019). A practical guide to surface kinetic Monte Carlo simulations. Front. Chem. 7, 202. doi:10.3389/fchem.2019.00202
André, L., Abanades, S., and Flamant, G. (2016). Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sust. Energy Rev. 64, 703–715. doi:10.1016/j.rser.2016.06.043
Andrews, M. J., and O'Rourke, P. J. (1996). The multiphase particle-in-cell (MP-PIC) method for dense particulate flows. Int. J. Multiph. Flow. 22 (2), 379–402. doi:10.1016/0301-9322(95)00072-0
Arangio, A. M., Slade, J. H., Berkemeier, T., Pöschl, U., Knopf, D. A., and Shiraiwa, M. (2015). Multiphase chemical kinetics of OH radical uptake by molecular organic markers of biomass burning aerosols: humidity and temperature dependence, surface reaction, and bulk diffusion. J. Phys. Chem. A 119 (19), 4533–4544. doi:10.1021/jp510489z
Bhandari, S., Rangarajan, S., Maravelias, C. T., Dumesic, J. A., and Mavrikakis, M. (2020). Reaction mechanism of vapor-phase formic acid decomposition over platinum catalysts: DFT, reaction kinetics experiments, and microkinetic modeling. ACS Catal. 10 (7), 4112–4126. doi:10.1021/acscatal.9b05424
Bhatia, S. K., and Perlmutter, D. D. (1980). A random pore model for fluid-solid reactions: I. isothermal, kinetic control. AIChE J. 26, 379–386. doi:10.1002/aic.690260308
Bhatia, S. K., and Perlmutter, D. D. (1981). A random pore model for fluid-solid reactions: II. diffusion and transport effects. AIChE J. 27, 247–254. doi:10.1002/aic.690270211
Bruix, A., Margraf, J. T., Andersen, M., and Reuter, K. (2019). First-principles-based multiscale modelling of heterogeneous catalysis. Nat. Catal. 2 (8), 659–670. doi:10.1038/s41929-019-0298-3
Cai, J., and Li, Z. (2024a). First principle-based rate equation theory for the carbonation kinetics of CaO with CO2 in calcium looping. Chem. Eng. J. 479, 147484. doi:10.1016/j.cej.2023.147484
Cai, J., and Li, Z. (2024b). First principle-based rate equation theory for the calcination kinetics of CaCO3 in calcium looping. Energy fuels. 38 (9), 8145–8156. doi:10.1021/acs.energyfuels.4c00877
Cai, J., and Li, Z. (2024c). First principles-based kinetic analysis of Ca(OH)2 dehydration in thermochemical energy storage system. J. Energy Storage 97, 112759. doi:10.1016/j.est.2024.112759
Chen, Y. C., and Wang, G. C. (2024). Structure sensitivity of the water–gas shift reaction over Cu/CeO2: a combination of DFT, MF-MKM, and kMC. J. Phys. Chem. C 128 (24), 9926–9939. doi:10.1021/acs.jpcc.4c01539
Cho, P., Mattisson, T., and Lyngfelt, A. (2004). Comparison of iron-nickel-copper-and manganese-based oxygen carriers for chemical-looping combustion. Fuel 83 (9), 1215–1225. doi:10.1016/j.fuel.2003.11.013
Christensen, R., Hansen, H. A., and Vegge, T. (2015). Identifying systematic DFT errors in catalytic reactions. Catal. Sci. Technol. 5, 4946–4949. doi:10.1039/C5CY01332A
Cundall, P. A., and Strack, O. D. L. (1979). A discrete numerical model for granular assemblies. Géotechnique 29 (1), 47–65. doi:10.1680/geot.1979.29.1.47
Dam-Johansen, K., Hansen, P. F. B., and Østergaard, K. (1991). High-temperature reaction between sulphur dioxide and limestone—III. A grain-micrograin model and its verification. Chem. Eng. Sci. 46 (3), 847–853. doi:10.1016/0009-2509(91)80191-Z
Derksen, J. J. (2014). Simulations of solid–liquid mass transfer in fixed and fluidized beds. Chem. Eng. J. 255, 233–244. doi:10.1016/j.cej.2014.06.067
D’Ambrosio, A., Bertino, A., Todaro, S., Santoro, M., Cannilla, C., Frusteri, F., et al. (2024). Kinetic modeling of the direct dimethyl ether (DME) synthesis over hybrid multi-site catalysts. Catalysts 14 (1), 61. doi:10.3390/catal14010061
Fang, F., Li, Z. S., Cai, N. S., Tang, X. Y., and Yang, H. T. (2011). AFM investigation of solid product layers of MgSO4 generated on MgO surfaces for the reaction of MgO with SO2 and O2. Chem. Eng. Sci. 66 (6), 1142–1149. doi:10.1016/j.ces.2010.12.014
Fang, D., Hou, S., Ye, Y., Jin, Q., He, F., and Xie, J. (2022). Insight into highly efficient FeOx catalysts for the selective catalytic reduction of NOx by NH3: experimental and DFT study. Appl. Surf. Sci. 599, 153998. doi:10.1016/j.apsusc.2022.153998
García-Labiano, F., de Diego, L. F., Adánez, J., Abad, A., and Gayán, P. (2005). Temperature variations in the oxygen carrier particles during their reduction and oxidation in a chemical-looping combustion system. Chem. Eng. Sci. 60, 851–862. doi:10.1016/j.ces.2004.09.049
Gibson III, J. B., and Harrison, D. P. (1980). The reaction between hydrogen sulfide and spherical pellets of zinc oxide. Ind. Eng. Chem. Process Des. Dev. 19 (2), 231–237. doi:10.1021/i260074a005
Golshan, S., Sotudeh-Gharebagh, R., Zarghami, R., Mostoufi, N., Blais, B., and Kuipers, J. A. M. (2020). Review and implementation of CFD-DEM applied to chemical process systems. Chem. Eng. Sci. 221, 115646. doi:10.1016/j.ces.2020.115646
Hadian, M., de Munck, M. J. A., Buist, K. A., Bos, A. N. R., and Kuipers, J. A. M. (2024). Modeling of a catalytic fluidized bed reactor via coupled CFD-DEM with MGM: from intra-particle scale to reactor scale. Chem. Eng. Sci. 284, 119473. doi:10.1016/j.ces.2023.119473
Hallberg, P., Leion, H., and Lyngfelt, A. (2011). A method for determination of reaction enthalpy of oxygen carriers for chemical looping combustion – application to ilmenite. Thermochim. Acta 524, 62–67. doi:10.1016/j.tca.2011.06.015
He, W., Liu, Y., He, R., Ito, T., Suda, T., Fujimori, T., et al. (2013). Combustion rate for char with fractal pore characteristics. Combust. Sci. Technol. 185 (11), 1624–1643. doi:10.1080/00102202.2013.822370
Ishida, M., and Jin, H. (1994). A new advanced power-generation system using chemical-looping combustion. Energy 19 (4), 415–422. doi:10.1016/0360-5442(94)90120-1
Ishida, M., and Wen, C. Y. (1971). Comparison of zone-reaction model and unreacted-core shrinking model in solid–gas reactions—I Isothermal analysis. Chem. Eng. Sci. 26 (7), 1031–1041. doi:10.1016/0009-2509(71)80017-9
Ishii, M., and Mishima, K. (1984). Two-fluid model and hydrodynamic constitutive relations. Nucl. Eng. Des. 82 (2–3), 107–126. doi:10.1016/0029-5493(84)90207-3
Jørgensen, M., and Grönbeck, H. (2016). First-principles microkinetic modeling of methane oxidation over Pd(100) and Pd(111). ACS Catal. 6 (10), 6730–6738. doi:10.1021/acscatal.6b01752
Kim, M. C., Sim, E., and Burke, K. (2013). Understanding and reducing errors in density functional calculations. Phys. Rev. Lett. 111, 073003. doi:10.1103/PhysRevLett.111.073003
Kloss, C., Goniva, C., Hager, A., Amberger, S., and Pirker, S. (2012). Models, algorithms and validation for opensource DEM and CFD-DEM. Prog. Comput. Fluid Dy. 12 (2–3), 140–152. doi:10.1504/pcfd.2012.047457
Koch, D. L., and Hill, R. J. (2001). Inertial effects in suspension and porous-media flows. Annu. Rev. Fluid Mech. 33, 619–647. doi:10.1146/annurev.fluid.33.1.619
Kresse, G., and Furthmüller, J. (1996). Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186. doi:10.1103/PhysRevB.54.11169
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 38 (11), 2221–2295. doi:10.1021/ja02268a002
Li, Z. (2020). General rate equation theory for gas–solid reaction kinetics and its application to CaO carbonation. Chem. Eng. Sci. 227, 115902. doi:10.1016/j.ces.2020.115902
Li, Z. (2022). First-principles-based microkinetic rate equation theory for oxygen carrier reduction in chemical looping. Chem. Eng. Sci. 247, 117042. doi:10.1016/j.ces.2021.117042
Li, J., and Li, Z. (2024). First principle based rate equation (1pRE) for reduction kinetics of Fe2O3 with syngas in chemical looping. Proc. Combust. Inst. 40 (1–4), 105363. doi:10.1016/j.proci.2024.105363
Li, Y., Wang, H., Li, W., Li, Z., and Cai, N. (2019). CO2 gasification of a lignite char in microfluidized bed thermogravimetric analysis for chemical looping combustion and chemical looping with oxygen uncoupling. Energy fuels. 33 (1), 449–459. doi:10.1021/acs.energyfuels.8b02909
Li, Z., Cai, J., and Liu, L. (2021). A first-principles microkinetic rate equation theory for heterogeneous reactions: application to reduction of Fe2O3 in chemical looping. Ind. Eng. Chem. Res. 60 (43), 15514–15524. doi:10.1021/acs.iecr.1c03214
Li, Z., Zhang, W., Chen, Z., Ren, Z., Ning, S., and Li, M. (2023). A kinetics mechanism of NOX formation and reduction based on density functional theory. Sci. Total Environ. 867, 161519. doi:10.1016/j.scitotenv.2023.161519
Liu, L., Wang, H., Cai, J., Cai, N., and Li, Z. (2022). First-principles-based multiscale modelling of heterogeneous CoO oxidation kinetics in high-temperature thermochemical energy storage. Fuel Process. Technol. 228, 107164. doi:10.1016/j.fuproc.2022.107164
Liu, L., Li, Z., Li, Z., Larring, Y., Li, Y., and Cai, N. (2021). Fast redox kinetics of a perovskite oxygen carrier measured using micro-fluidized bed thermogravimetric analysis. Proc. Combust. Inst. 38, 5259–5269. doi:10.1016/j.proci.2020.06.160
Liu, T., Yang, R., Zhang, G., Wu, W., Yang, Z., Lin, R., et al. (2021). Mechanism of selective catalytic reduction of NOx with NH3 over CeO2-TiO2: insight from in-situ DRIFTS and DFT calculations. Appl. Surf. Sci. 568, 150764. doi:10.1016/j.apsusc.2021.150764
Lu, L. (2022). GPU accelerated MFiX-DEM simulations of granular and multiphase flows. Particuology 62, 14–24. doi:10.1016/j.partic.2021.08.001
Lu, L., Gao, X., Dietiker, J. F., Shahnam, M., and Rogers, W. A. (2021). Machine learning accelerated discrete element modeling of granular flows. Chem. Eng. Sci. 245, 116832. doi:10.1016/j.ces.2021.116832
Lupiáñez, C., Guedea, I., Bolea, I., Díez, L. I., and Romeo, L. M. (2013). Experimental study of SO2 and NOx emissions in fluidized bed oxy-fuel combustion. Fuel Process. Technol. 106, 587–594. doi:10.1016/j.fuproc.2012.09.030
Lyngfelt, A., Leckner, B., and Mattisson, T. (2001). A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 56 (10), 3101–3113. doi:10.1016/S0009-2509(01)00007-0
Madon, R. J., Braden, D., Kandoi, S., Nagel, P., Mavrikakis, M., and Dumesic, J. A. (2011). Microkinetic analysis and mechanism of the water gas shift reaction over copper catalysts. J. Catal. 281 (1), 1–11. doi:10.1016/j.jcat.2011.03.008
Maestri, M., Vlachos, D. G., Beretta, A., Groppi, G., and Tronconi, E. (2008). Steam and dry reforming of methane on Rh: microkinetic analysis and hierarchy of kinetic models. J. Catal. 259 (2), 211–222. doi:10.1016/j.jcat.2008.08.008
Motagamwala, A. H., and Dumesic, J. A. (2021). Microkinetic modeling: a tool for rational catalyst design. Chem. Rev. 121 (2), 1049–1076. doi:10.1021/acs.chemrev.0c00394
Ning, H., Tang, R., Li, C., Gu, X., Gong, Z., Zhu, C., et al. (2025). Recent advances in process and materials for dry desulfurization of industrial flue gas: an overview. Sep. Purif. Technol. 353, 128425. doi:10.1016/j.seppur.2024.128425
Noor, T., Qi, Y., and Chen, D. (2020). Hydrogen dependence of the reaction mechanism and kinetics of water gas shift reaction on Ni catalyst: experimental and DFT study. App. Catal. B Environ. 264, 118430. doi:10.1016/j.apcatb.2019.118430
Nørskov, J. K., Studt, F., Abild-Pedersen, F., and Bligaard, T. (2014). Fundamental concepts in heterogeneous catalysis. Hoboken, New Jersey: John Wiley and Sons, Inc.
Osman, A. I., Hefny, M., Abdel Maksoud, M. I. A., Elgarahy, A. M., and Rooney, D. W. (2021). Recent advances in carbon capture storage and utilisation technologies: a review. Environ. Chem. Lett. 19, 797–849. doi:10.1007/s10311-020-01133-3
Petersen, E. E. (1957). Reaction of porous solids. AIChE J. 3 (4), 443–448. doi:10.1002/aic.690030405
Rawal, T. B., Le, D., Hooshmand, Z., and Rahman, T. S. (2021). Toward alcohol synthesis from CO hydrogenation on Cu(111)-supported MoS2–predictions from DFT+KMC. J. Chem. Phys. 154, 174701. doi:10.1063/5.0047835
Razdan, N. K., and Bhan, A. (2021). Catalytic site ensembles: a context to reexamine the Langmuir-Hinshelwood kinetic description. J. Catal. 404, 726–744. doi:10.1016/j.jcat.2021.09.016
Reuter, K. (2016). Ab initio thermodynamics and first-principles microkinetics for surface catalysis. Catal. Lett. 146 (3), 541–563. doi:10.1007/s10562-015-1684-3
Sakai, M., Abe, M., Shigeto, Y., Mizutani, S., Takahashi, H., Viré, A., et al. (2014). Verification and validation of a coarse grain model of the DEM in a bubbling fluidized bed. Chem. Eng. J. 244, 33–43. doi:10.1016/j.cej.2014.01.029
Sandmann Jr, C. W., and Zygourakis, K. (1986). Evolution of pore structure during gas-solid reactions: discrete models. Chem. Eng. Sci. 41 (4), 733–739. doi:10.1016/0009-2509(86)87152-4
Sedghkerdar, M. H., and Mahinpey, N. (2015). A modified grain model in studying the CO2 capture process with a calcium-based sorbent: a semianalytical approach. Ind. Eng. Chem. Res. 54 (3), 869–877. doi:10.1021/ie503989n
Srinivasakannan, C., Al Shoibi, A., and Balasubramanian, N. (2012). Combined resistance bubbling bed model for drying of solids in fluidized beds. Heat. Mass Transf. 48, 621–625. doi:10.1007/s00231-011-0909-2
Stearn, A. E., and Eyring, H. (1940). Absolute rates of solid reactions: diffusion. J. Phys. Chem. 44 (8), 955–980. doi:10.1021/j150404a001
Szekely, J., and Evans, J. W. (1970). A structural model for gas-solid reactions with a moving boundary. Chem. Eng. Sci. 25 (6), 1091–1107. doi:10.1016/0009-2509(70)85053-9
Szekely, J., and Evans, J. W. (1971). A structural model for gas-solid reactions with a moving boundary-II. Chem. Eng. Sci. 26 (11), 1901–1913. doi:10.1016/0009-2509(71)86033-5
Thybaut, J. W., Sun, J., Olivier, L., Van Veen, A. C., Mirodatos, C., and Marin, G. B. (2011). Catalyst design based on microkinetic models: oxidative coupling of methane. Catal. Today 159 (1), 29–36. doi:10.1016/j.cattod.2010.09.002
Van Belleghem, J., Yang, J., Janssens, P., Poissonnier, J., Chen, D., Marin, G. B., et al. (2022). Microkinetic model validation for Fischer-Tropsch synthesis at methanation conditions based on steady state isotopic transient kinetic analysis. J. Ind. Eng. Chem. 105, 191–209. doi:10.1016/j.jiec.2021.09.017
Wang, H., Li, Z., Fan, X., and Cai, N. (2017). Rate-equation-based grain model for the carbonation of CaO with CO2. Energy fuels. 31 (12), 14018–14032. doi:10.1021/acs.energyfuels.7b02751
Wang, H., Li, Z., Li, Y., and Cai, N. (2021). Reduced-order model for CaO carbonation kinetics measured using micro-fluidized bed thermogravimetric analysis. Chem. Eng. Sci. 229, 116039. doi:10.1016/j.ces.2020.116039
Wang, S., Hu, C., Luo, K., Yu, J., and Fan, J. (2023). Multi-scale numerical simulation of fluidized beds: model applicability assessment. Particuology 80, 11–41. doi:10.1016/j.partic.2022.11.011
Wang, R., Li, Z., and Liu, L. (2025). Multiscale modeling for the reduction kinetics of a perovskite oxygen carrier based on quantum chemistry and CFD–DEM. Carbon Capture Sci. Technol. 14, 100357. doi:10.1016/j.ccst.2024.100357
Wang, X., Wang, W., Xiong, W., Jiang, X., Ouyang, T., Bai, Y., et al. (2024). A DFT study of the mechanism of NH3-SCR NOx reduction over Mn-doped and Mn–Ti co-doped CoAl2O4 catalysts. J. Mat. Chem. C 12, 5073–5082. doi:10.1039/D4TC00239C
Wang, Y., Liu, H., Duan, Q., and Li, Z. (2024). Understanding the negative Apparent activation energy for Cu2O and CoO oxidation kinetics at high temperature near equilibrium. Catalysts 14 (11), 832. doi:10.3390/catal14110832
Willis, M. D., and Wilson, K. R. (2022). Coupled interfacial and bulk kinetics govern the timescales of multiphase ozonolysis reactions. J. Phys. Chem. A 126 (30), 4991–5010. doi:10.1021/acs.jpca.2c03059
Yan, X., Li, Y., Zhao, J., and Wang, Z. (2020). Density functional theory study on CO2 adsorption by Ce-promoted CaO in the presence of steam. Energy fuels. 34 (5), 6197–6208. doi:10.1021/acs.energyfuels.0c00972
Yang, W., Cloete, S., Morud, J., and Amini, S. (2016). An effective reaction rate model for gas-solid reactions with high intra-particle diffusion resistance. Int. J. Chem. React. Eng. 14 (1), 331–342. doi:10.1515/ijcre-2015-0127
Yin, P., Yang, Y. S., Chen, L. F., Xu, M., Chen, C. Y., Zhao, X. J., et al. (2020). DFT study on the mechanism of the water gas shift reaction over NixPy catalysts: the role of P. J. Phys. Chem. C 124 (12), 6598–6610. doi:10.1021/acs.jpcc.9b11142
Yu, Y., Ji, P., Zhang, W., Yang, K., and Zhang, M. (2023). Theoretical study coupling DFT calculations and kMC simulation of CO methanation on Ni(111) and Ni3Fe(111). New J. Chem. 47, 17923–17936. doi:10.1039/D3NJ03516F
Glossary
Keywords: multiscale model, first-principles calculation, dual-site microkinetics, bulk diffusion, CFD-DEM, oxygen carrier, chemical looping
Citation: Wang R, Li Z and Liu L (2025) Multiscale reaction model coupling dual-site microkinetics with bulk diffusion and CFD–DEM for a perovskite oxygen carrier. Front. Chem. 13:1656180. doi: 10.3389/fchem.2025.1656180
Received: 29 June 2025; Accepted: 14 August 2025;
Published: 04 September 2025.
Edited by:
Kun Zhao, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Jin-Woo Kim, Seoul National University, Republic of KoreaFrancesco Orsini, Polytechnic University of Turin, Italy
Copyright © 2025 Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenshan Li, bGl6c0B0c2luZ2h1YS5lZHUuY24=
 Ruiwen Wang
Ruiwen Wang Zhenshan Li1*
Zhenshan Li1* Lei Liu
Lei Liu