- Department Functional Surfaces, Leibniz Institute of Surface Engineering (IOM), Leipzig, Germany
Photoconversion of metal-organic precursors to thin film metal oxides using ultraviolet (UV) radiation in oxidative atmosphere is an attractive technology because it can be applied at temperatures <80°C and at ambient pressure. Thus, it enables preparing this class of thin films in a cost-efficient manner on temperature sensitive substrates such as polymer films. In this article, various aspects of research and development in the field of photochemical thin-film fabrication, with particular focus to the application of the produced films as gas permeation barriers for the encapsulation of optoelectronic devices are reviewed. Thereby, it covers investigations on fundamental photochemically initiated reactions for precursor classes containing metal-oxygen and metal-nitrogen bonds, and emphazises the relevance of that understanding for applicative considerations like integration of the single-layer barrier films into relevant encapsulation films. Further perspectives are given concerning integration of additional functionalities like electrical conductivity to the flexible and transparent barrier films.
Introduction
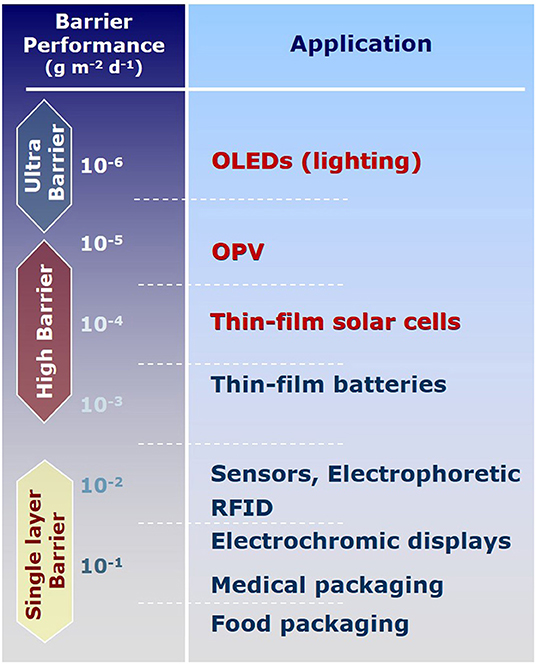
A variety of products, ranging from food to pharmaceuticals or electronic devices, have to be protected from gases present in the ambient atmosphere. Most severe damage usually results from oxygen and water vapor, impairing taste, medical effect, or technical functionality of the protected object. With respect to envisaged storage times or lifespan for this wide range of applications, target permeability for these applications vary in a broad range as shown exemplarily for water vapor transmission rates (WVTR) in Figure 1. Transparent and flexible polymer-based gas barrier materials are commercially available for the packaging of food and pharmaceuticals and have been comprehensively reviewed (Lange and Wyser, 2003). Their target transmission rates are in the order of few cm3 m−2 d−1 bar−1 for oxygen (OTR—oxygen transmission rate), and few g m−2 d−1 for water vapor (WVTR). For those applications, intrinsically high barrier polymers incl. coextruded materials, and nanocomposites are sufficient and cost-efficient solutions are readily available on the market (Wagner, 2010).
The situation is different for flexible stand-alone high barrier films, especially for achieving the more challenging WVTR values below 10−4 g m−2 d−1. A stand-alone film in this review denotes a stack, comprising at least one polymer substrate and one barrier thin film, which is laminated into the flexible product on an industrial scale to introduce a gas permeation barrier. Another promising alternative, which can only be mentioned here, is the use of flexible glass. The availability of a film like this is important for applicants who do not want to integrate the barrier thin film preparation into their production scheme directly. Reasons for that could be the complexity of preparation methods, chemical incompatibility, direct usage of the film as backside for printed electronics as well as deposition or transfer of transparent electrode materials. The integration of stand-alone films can also minimize the risk of damaging the delicate barrier thin film in further processing steps. Even though, interest from industry is extremely high, cost-efficient stand-alone films are not yet commercially available. Ambient pressure coating is a cost-efficient alternative to vacuum-technologies for preparing barrier thin films offering integratability into large-area roll-to-roll processes, like e.g., industrial printing technologies.
Currently, frequently used transparent polymeric substrate films for the development of flexible electronics are polyester films, especially polyethylene terephthalate (PET) and polyethylene naphthalate (PEN). They stand out due to their high tensile strength, chemical and dimensional stability, transparency, reflectivity, gas and aroma barrier properties, and electrical insulation. Even though, a number of amorphous polymer films are commercially available such as polycarbonate, polyethersulfone, and heat resistant polymers like polyimide or halogenated polyolefins, they do not exhibit all the desired properties regarding mechanical stability, solvent resistance, or light transmission properties for above-mentioned applications. Semicrystalline polyester films like PET and partially PEN films are available from various manufacturers (e.g., Mitsubishi, DuPont Teijin Films, Toray) as specifically modified types, differing regarding their surface smoothness, hydrolysis stability, UV resistance under outdoor conditions, and dimensional stability under heat. Since the price for films of PEN is at least 10 times higher than for PET, the latter are preferred substrates for industry even though PEN would show higher intrinsic barrier and thermal stability (permeation coefficients of 2·10−14 vs. 7·10−14 mol m−1 Pa−1 s−1); glass transition temperatures of Tg = 120 vs. 78°C). The extrusion process of these polyester materials results in biaxially oriented films, thus operation at temperatures above Tg can result in dimensional deformation leading to a damage of the top-coated mechanically sensitive metal oxide thin film. Consequently, process temperatures below the Tg are highly desirable for preparing (ultra)high barriers.
Only a few technologies are capable of depositing highly dense metal oxide thin films on polyester substrates at ambient pressure: Sol-gel deposition, atmospheric pressure plasma-enhanced CVD, spatial atomic layer deposition (sALD) and photoconversion of wet deposited precursor films. While sol-gel techniques suffer from long drying times (usually several hours), the three latter approaches show high potential for scale-up on short notice.
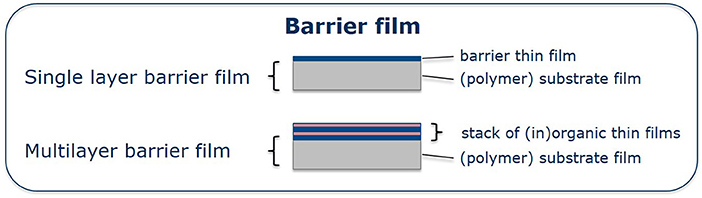
This paper reviews photochemical approaches as non-vacuum based manufacturing methods for the deposition of metal oxide thin films on technically relevant polymeric substrates, with the ultimate goal to use it for flexible and transparent encapsulation of optoelectronic devices. It covers work on the photo-assisted preparation and annealing of metal oxide thin films at temperatures below 80°C, the elucidation of underlying photochemical reactions for the formation of the single barrier thin film, their integration into practically relevant multilayer systems demonstrated in flexible CIGS-based inorganic thin film solar cells and flexible organic solar cells as well as their engineering toward integrated electrode-barriers. It thereby complements to reviews on the above-mentioned vapor phase deposition techniques (AP-PE CVD: Massines et al., 2012; Starostin et al., 2015, sALD: Muñoz-Rojas et al., 2019). For clarity reasons, we use the barrier film terms denoted in Figure 2 throughout the article.
Photo-Assisted Preparation of Metal Oxide Thin Films for Encapsulation of Optoelectronic Devices
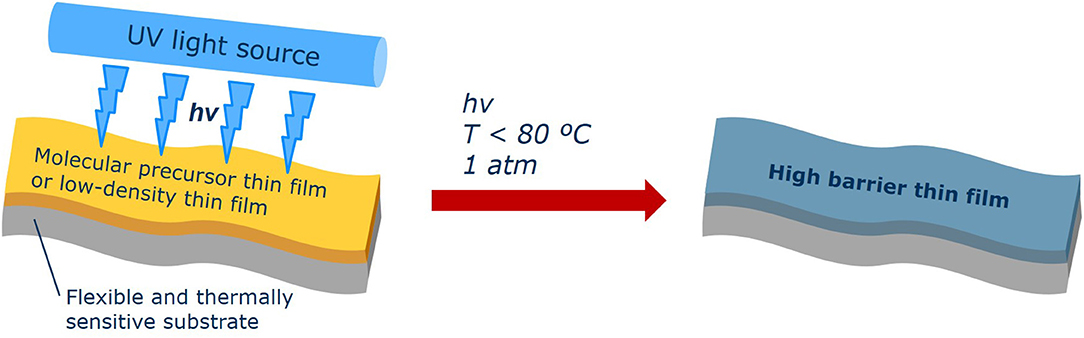
The development of highly efficient excimer lamps has rendered the use of photochemical processes technologically more attractive within the last decades. Excited by a dielectric-barrier discharge, in the gas volume of the lamp tube excited dimers (excimers) of noble gases or heterodimeric complexes (exciplexes) with halogens are formed, respectively (Kogelschatz, 2003). Decomposing immediately after relaxation, these complexes emit narrow-band UV light with high efficiencies at different wavelengths in the ultraviolet or vacuum ultraviolet spectral regions depending on the kind of noble gas and halogen present in the discharge tube. Thus, they offer attractive complements to multispectral low or medium pressure mercury lamps. Commercially available lamps, at least potentially, emit UV light in the energy ranges of about 4.0 eV for XeCl* lamps (corresp. to the emission maximum at λmax = 308 nm), 4.4 eV for XeBr* lamps (283 nm), 5.6 eV for KrCl* lamps (222 nm), and 7.2 eV for lamps (172 nm). They exhibit a high average specific power (up to 1 W cm−2) of UV radiation in the absence of further emission bands in the visible and IR spectral range. Thus, thermal load on the irradiated surface can be minimized compared to mercury lamps, in this way allowing the formation of metal oxide thin films onto polyester films below their glass transition temperature according to Figure 3.
To the best of our knowledge, Onuki and Awazu were the first to publish the water-free photoconversion of wet-chemically coated precursor thin films (tetramethoxysilane) to silicon oxide thin films (Awazu and Onuki, 1996). Since then using this technology, several materials were investigated at laboratory scale. We chose to present studies based on the main precursor classes being metal or half-metal (M) organic compounds containing M-O or M-N bonds. Published examples comprise the preparation of aluminum, iron, titanium, and silicon oxide, which are reviewed in the succeeding chapters. The only precursors investigated containing M-N bonds are silazane based systems; nevertheless, for this case, the highest level of mechanistic understanding has been achieved. Since we consider that a fundamental prerequisite for the transfer of laboratory-scale results to industrial scale roll-to-roll technology, this work is reviewed in an own chapter.
Photoconversion of Molecular Precursor Thin Films Containing M-O Bonds
Awazu and Onuki (1996) reported on the photochemical conversion of tetramethoxysilane films to SiO2 at room temperature, by using a excimer lamp (λmax = 172 nm). Based on FTIR investigations, two major reaction pathways have been postulated which are (i) MeOH formation reacting the precursor with surface silanol surface groups of the silicon wafer substrate and (ii) an intramolecular precursor conversion reaction to SiO2 and Me-O-Me after electronic excitation. In a subsequent work, this approach was also shown to be suitable for room temperature SiO2 thin film formation onto polymethyl methacrylate films using VUV irradiation from a excimer lamp (λmax = 146 nm) (Awazu and Onuki, 1997).
In order to enlarge the scope of metal oxide thin films producible by a purely photochemical conversion of precursor molecules, the VUV-induced (~172 nm) formation of AlxOy layers was investigated using a polymeric aluminum complex of hexanoic acid as a precursor (Wennrich et al., 2013). An almost fully mineralized and homogeneous layer with the composition AlO2.17C0.04 could be obtained applying radiant exposures of 36 J cm−2. The AlxOy was shown by XRD to be amorphous with a slight short-range atom order. Hexanoic acid was found to be the predominant stable gaseous reaction product. In agreement with this, TD-DFT calculations at model compounds confirmed VUV-induced Al–O cleavage as a thermodynamically preferred relaxation pathway. However, the required high radiant exposures are unfavorable for an efficient technical process.
Photochemical conversion of titanium(IV) ethoxide proceeds at lower radiant exposures. About 2 J cm−2 ( excimer irradiation, precursor thickness of 270 nm) are necessary for the mineralization and the nearly complete removal of residual carbon at <35°C (With et al., 2016). Further, it could be demonstrated that the initial kinetics, i.e., the logarithmic apparent rate constant of the precursor film conversion, linearly depends on the initial layer thickness in the range of 270 to 1,060 nm. In other words, the initial kinetics can be characterized by the product of the absorption coefficient and the optical path length (precursor layer thickness). Titanium(IV) ethoxide contains a high portion of organics, which have to be removed during UV conversion. Film thickness decreases nearly by an order of magnitude (e.g., 550 vs. 67 nm) forming a dense metal oxide network with a smooth surface without cracks, wrinkles or macropores.
Interestingly, using ATR-FTIR-spectroscopic monitoring of the reaction, an intermediate structure was observed, suggesting a Ti-(OCO)-ring formation. This idea was supported by DFT calculations, with which energetically favorable reaction pathways for the formation of Ti–(OCO)-ring structures were calculated. Accordingly, different multistep reaction pathways were postulated leading to 4- and 6-membered ring structures, which are exergonic and should be energetically favorable. The formation of, i.e., alkyl acetates as organic gaseous products next to ethanol supports this hypothesis. Using iron(III) tert-butoxide dimer as the molecular precursor, we also found experimental and theoretical evidence for the formation of intermediate Fe-(OCO)-ring species, formed by abstracting a radical from a tert-butoxy group (With et al., 2019b). Formation of this quite stable intermediate species results in high radiant exposures of 24 J cm−2 needed for mineralization. It can be assumed that by an appropriate ligand design the formation of intermediate species can be influenced, thus giving the possibility for tailoring the photochemical reactivity.
From the application point of view, the required radiant exposure for complete mineralization is, besides the temperature, of high importance. The lower the radiant exposure necessary for mineralization, the lower the energy consumption and the higher speeds can be applied in fast roll-to-roll processes. A direct comparison of VUV light-induced conversion of different metal-organic precursors is difficult since their reactivity is strongly influenced by the complexity of the energetically feasible relaxation pathways after molecular excitation as well as by their absorption coefficients and film thicknesses. Therefore, a qualitative comparison for photoconversion of some metal-organic compounds, which are based on FTIR data showing disappearance of C-based vibrations from the films with increasing radiant exposure (Figure 4).
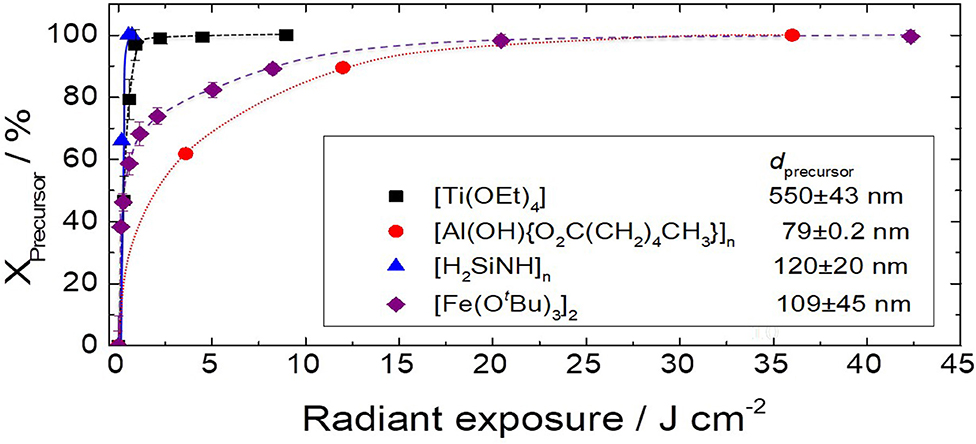
Figure 4. Comparison of VUV-induced (Xe2* excimer irradiation, in a nitrogen atmosphere with 0.25 vol% O2) decomposition of different metal-organic precursor thin films depending on the applied radiant exposure at 1 bar and ≤34 °C (Prager et al., 2007; Wennrich et al., 2013; With et al., 2016, 2019b).
Whereas, the approaches discussed above are devoted to large area coatings, Rim et al. (2014) demonstrated that a direct light patterning at room temperature is possible, by using a photomask for photochemical conversion of heteroleptic metal acetylacetonato compounds (based on In, Ga, Zn, and Sn) to oxides in illuminated areas only, followed by dissolution of areas with unconverted precursor. This yielded a fine patterning with a minimum feature size of ~3 μm. However, a post-treatment at 200–350°C was necessary to remove remaining organics and to densify the oxide network. Although the annealing temperatures are not compatible with low-cost plastic substrates, the example of using a photomask shows the potential of the technology to create finely patterned structures onto polymeric substrates.
Photoconversion of Silazane Thin Films
Room temperature photoconversion of polysilazanes has been published for the first time in 2004 by Naganuma et al. (2004), describing the effect of VUV irradiation on spin-coated films of perhydropolysilazane using a excimer lamp (λmax = 172 nm). Hydrogen and nitrogen could be effectively removed from the precursor film and silicon oxide was formed by the incorporation of oxygen. The photoconversion process was found to be dependent on the oxygen content in the surrounding gas atmosphere as well as on the applied irradiation dose. A follow-up publication confirmed these first results for deposition on PET films (Naganuma et al., 2013).
Kinetic evaluation of Si-H vibration band intensities at different absorbed UV doses and excitation wavelengths proved the photochemical nature of the Si-H bond scission. For the model molecule H2N-SiH2-NH-SiH3, the excitation energy of 5.63 eV (220 nm) was obtained from density functional theory (DFT) calculations. Employing light sources with different wavelengths below and above that photon energy as well as recorded transmission spectra indicate similar excitation energy for the real precursor structure (Figure 5). Photochemical experiments based on different absorption coefficients of oxygen and ozone at varying wavelengths and concentrations showed, that Si-O bond formation is based on the reaction of photon-induced Si-radicals with molecular oxygen, thus neither externally (ex-situ) produced ozone nor O1(D) alone seem to play a crucial role in the process. Addition of catalysts, e.g., tertiary amines, influences the apparent rate constant of product formation. TD-DFT calculations suggest an excitation of the precursor molecules by UV photons to an excited singlet state (Figure 6). After relaxation to S1 and intersystem crossing, a dissociative triplet state is formed, which energetically preferably undergoes Si-N cleavage. Mass spectrometric measurements verify the formation of ammonia as a side product. Compared to Si-N cleavage, the formation of the Si-O-Si network is slower and dependent on oxygen concentration (Prager et al., 2007). Absorption coefficients of PHPS films in the VUV spectral range were determined. It has been found that penetration depths of 172 nm photons are in the range of 200 nm (Prager et al., 2012). Photoconversion of methylated silazanes was subjected to detailed mechanistic studies (low-temperature ESR studies, laser flash photolysis, FTIR measurements and mass spectrometric detection of gaseous products) coupled with DFT calculations. They support the above mentioned reaction scheme (Figure 7), but exhibit lower WVTR and OTR values compared to the pure inorganic thin films (Prager et al., 2009; Knolle et al., 2010).
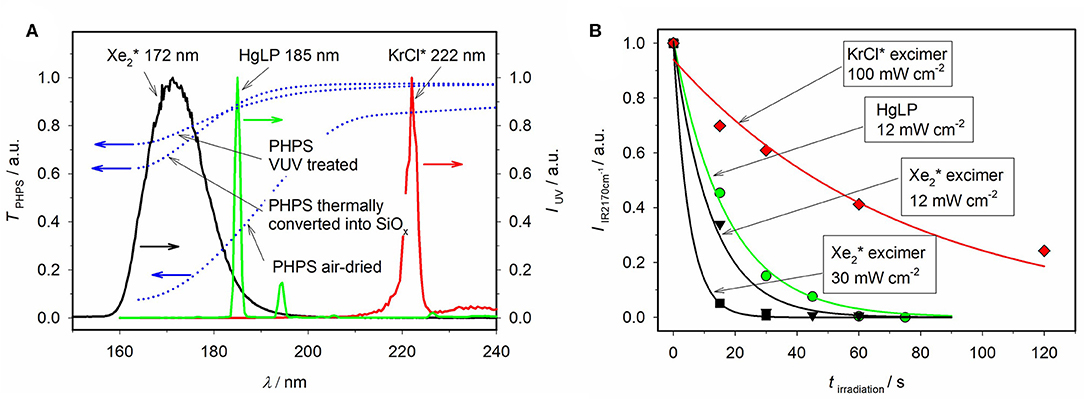
Figure 5. (A) Measured UV-absorption spectra of precursor thin films and excimer-lamp emission spectra. (B) Kinetic evaluation of the intensity of the vibration band of the Si—H bond at 2170 cm−1, adapted from Prager et al. (2007).
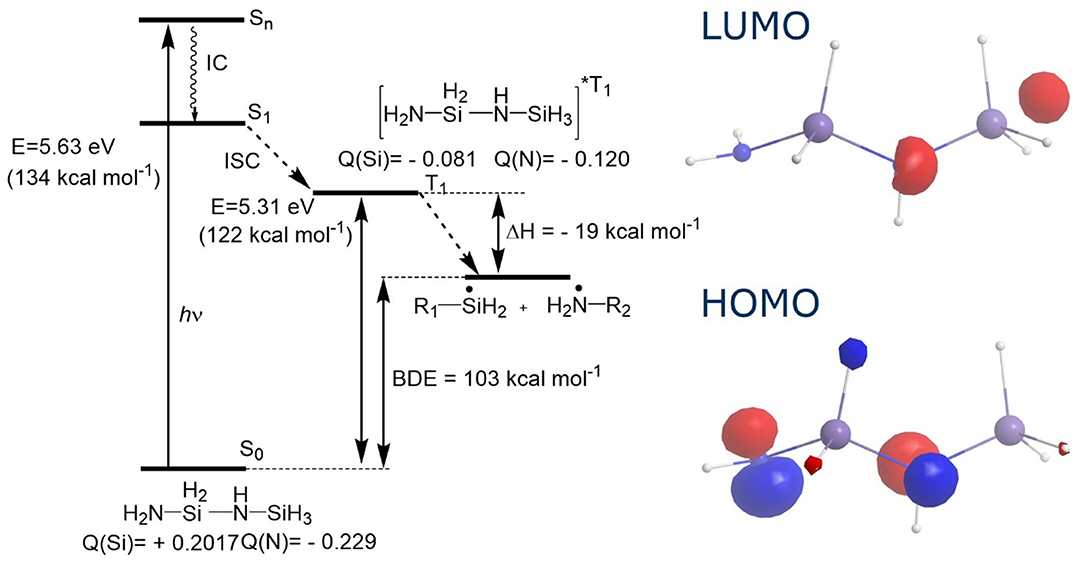
Figure 6. Results on quantum-chemical calculations on the photochemical excitation of model substance H2N-SiH2-NH-SiH3 and subsequent bond scission, adapted from Prager et al. (2007).
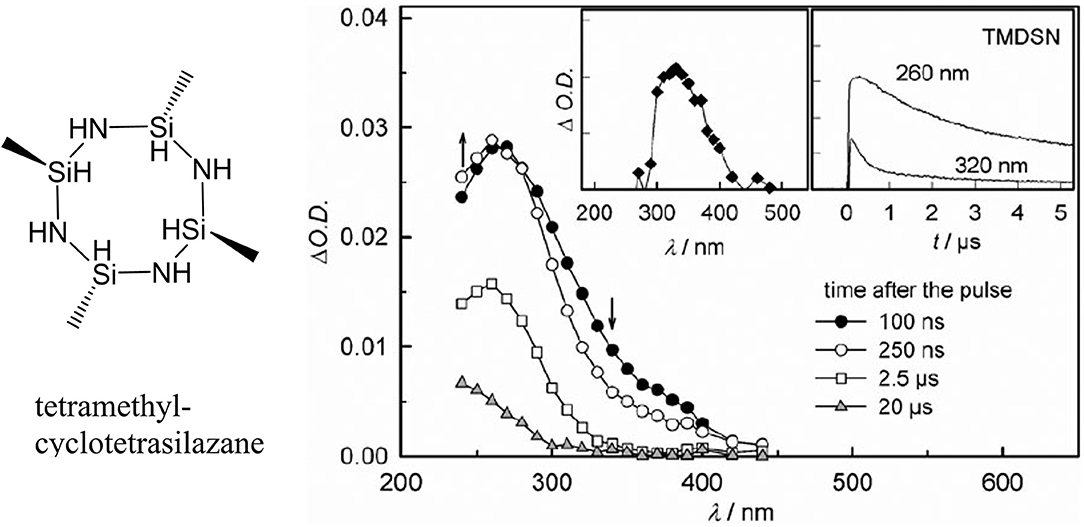
Figure 7. Transient spectra observed after laser flash photolysis of an N2-saturated solution of tetramethylcyclotetrasilazane (molecular structure displayed on left side) (Knolle et al., 2010).
FTIR-measurements for photochemically cured PHPS thin films of different thickness as well as time-of-flight secondary ion mass spectrometry (TOF-SIMS) along the depth profile of the cured films nicely shine light on a specific challenge of photochemically produced thin films with gas barrier properties. The gas barrier film is formed top-down and thereby restricts diffusion of the reactants toward deeper layers. This leads to a composite structure with concentration gradients throughout the films depth profile, which has to be considered in technical upscaling processes (Morlier et al., 2014).
An interesting alternative to the production of barrier foils for encapsulation of electronic devices is the direct application of PHPS solutions on device stacks like organic solar cells (Channa et al., 2019) followed by VUV-induced conversion to SiO2. In order to prevent VUV-induced degradation of the underlying photo-active film and to reduce mechanical stress, a protective bi-layer comprising a ZnO-nanoparticle thin film and a UV-curable acrylic resin interlayer were introduced on top of the P3HT film. The best multilayer material was shown to be stable for > 200 h under damp-heat conditions (40°C, 85% r.h.) without losing more than 10% of power conversion efficiency. Stacks from two SiOx-layers (each 170 nm thick) with two UV-curable acrylic interlayers on PET substrate showed an increase of WVTR values (2 × 10−2 g m−2 d−1) by less than 10% after 3,000 bending cycles (bending radius = 3 cm).
Application Aspects of Photochemically Derived Gas Permeation Barriers
In order to reach gas transmission rates below 10−4 g m−2 d−1 as needed for challenging applications like the encapsulation of organic electronics (see Figure 1), all gas permeation pathways within the device need to be considered. The following chapters cover basic principles of gas permeation through different layer stacks as well as strategies for improvement, such as photoannealing or lamination.
Gas Permeation Through Single Barrier Films
Various groups investigated gas permeation mechanisms in simple polymer films or polymer films coated with an inorganic barrier layer (Haas et al., 1999; Greener et al., 2007; Meyer et al., 2010; Schmidt et al., 2012). Gas molecules permeate through amorphous polymeric films by homogeneous diffusion through the bulk material (Paul and DiBenedetto, 1965) depending on diffusion (D) and solubility (S) coefficients of the gas molecules (Schrenk and Alfrey, 1969; Miller and Krochta, 1997), and references therein following the law
with P – permeability coefficient. By depositing dielectric films onto polymer films their gas permeability can be lowered considerably, e.g., by two orders of magnitude on semi-crystalline polymer films (Chatham, 1996; Charton et al., 2006; Gioti et al., 2009). By investigating the transmission rates through metal oxide thin films on polymer substrates it could be shown that permeation of gases like oxygen, nitrogen, hydrogen or noble gases is dominated by transmission through small-sized defects like pinholes or cracks in the nm or μm scale (Tropsha and Harvey, 1997; da Silva Sobrinho et al., 2000; Hanika et al., 2003; Leterrier, 2003; Langowski, 2005; Fahlteich et al., 2009). For water molecules, the reactive diffusion along chemical defects, respectively functionalities, represents a second transmission mechanism, which can dominate the permeation process in case of having low densities of micro or nano pores and pinholes (Tropsha and Harvey, 1997; Henry et al., 1999; Carcia et al., 2010). OH-functionalities, arising from the preparation process, have shown to play a crucial role in aluminum and silicon oxide films (Boehm, 1966; Tropsha and Harvey, 1997; Carcia et al., 2010). Rare examples in the literature include a direct comparison of different binary metal compounds, which point toward superiority of nitridic vs. oxidic barrier materials: Aluminum oxynitride films (Erlat et al., 2001) showed superior water vapor barriers compared to pure aluminum oxide layers. SiN coatings result in higher gas barrier improvement factors (BIFs) than SiOx ones (da Silva Sobrinho et al., 1998, 2000).
Photochemical preparation of inorganic thin films is the only non-vacuum method, in which formation of OH-groups can be avoided already while preparing the film. This leads to thin films with high density compared to other low-temperature processes (Table 1) (Prager et al., 2014; With et al., 2019a). Single layers of photochemically prepared silicon oxide thin films on PET films result in OTR and WVTR values of < 10−2 cm3 m−2 d−1 bar−1 (Channa et al., 2019) and 0.2 g m−2 d−1 (40°C, 90% r.h.; Morlier et al., 2013). Knowledge about the photoconversion mechanism and kinetic data was an inevitable prerequisite for a successful transfer of the process to the pilot-scale (200 mm wide web) (Prager et al., 2014). The gas barrier quality thereby compares to results obtained with single layers of conventional vapor deposition processes (da Silva Sobrinho et al., 1999, 2000; Czeremuszkin et al., 2001; Wuu et al., 2004; Bieder et al., 2005; Wolf et al., 2007).
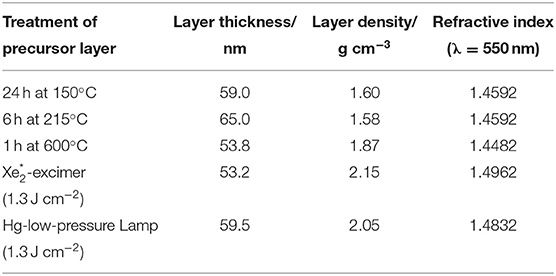
Table 1. Densities and refractive indices of PHPS-layers treated with varying temperatures or VUV-irradiation regimes (Prager et al., 2014).
Optimizing Metal Oxide Barrier Films by Photoannealing
UV-light is capable densifying formed metal oxide films, e.g., by condensation of hydroxyl groups and removal of elemental impurities such as carbon, a process called photoannealing. To induce crystallization even annealing temperatures can be significantly reduced <350°C (Bretos et al., 2018). For oxide films formed at low-temperature, various UV light sources such as UV laser and UV lamps have been investigated. UV-laser irradiation has even been shown to induce crystallization of titanium(IV) oxide (at wavelengths of 6.4 and 4.7 eV) (Imai et al., 1999) zinc(II) oxide (5 eV) (Nagase et al., 2001), and indium(III) oxide thin films (6.4 and 4.7 eV) (Imai et al., 1998). The effect is ascribed to the significant application of energy in a tightly restricted volume, due to which the films melt either partially at the surface or within the full film, and solidify to crystalline phases (Taylor and Fabes, 1992). Industrial applications of UV-laser systems for large-area and R2R processing of metal oxide thin films is still restricted due to high investment and maintenance costs. Thus, usage of UV lamps is more preferable at the moment.
A combination of both, UV-annealing using a 365 nm (3.4 eV) LED lamp together with elevated temperatures of 300°C was able to significantly improve the electrical performance and stability of IGZO films as compared to sole UV- or thermal treatments (Tak et al., 2014). Van de Leest (1995) and Nakajima et al. (2005) concluded a photoannealing at ~100°C with UV-lamps ( and Hg low-pressure lamp; at 7.2, 6.7, and 4.9 eV, respectively) of sol-gel films to be an enhanced condensation reaction between hydroxyl groups. From studies on titanium oxide formation they conclude that in advantage to conventional drying steps in sol-gel methods, UV-irradiation enables the formation of “high-quality” oxide films, because no low-dense polymeric oxide chains are formed, which would inhibit subsequent condensation reactions to a dense 3D network releasing redundant OH-groups. However, photoannealing of titanium oxide thin films is the only example of this technique being successful at temperatures ≤100°C. For silicon oxide thin films a condensation of once produced OH-groups by VUV-light (using and Hg low-pressure lamps) has not been reported so far.
Gas Permeation Through Multilayer Systems
Residual defects in metal oxide thin films produced on an industrial scale are inevitable. In order to meet industrial reproducibility criteria, more than one barrier film has to be coupled within one encapsulation film. Coupling two similar barrier films, the “ideal laminate theory” predicts a maximum improvement of the barrier performance by factor two (Schrenk and Alfrey, 1969):
PL – permeance of the laminate
P1, P2, Pn – permeabilities of the single-barrier films.
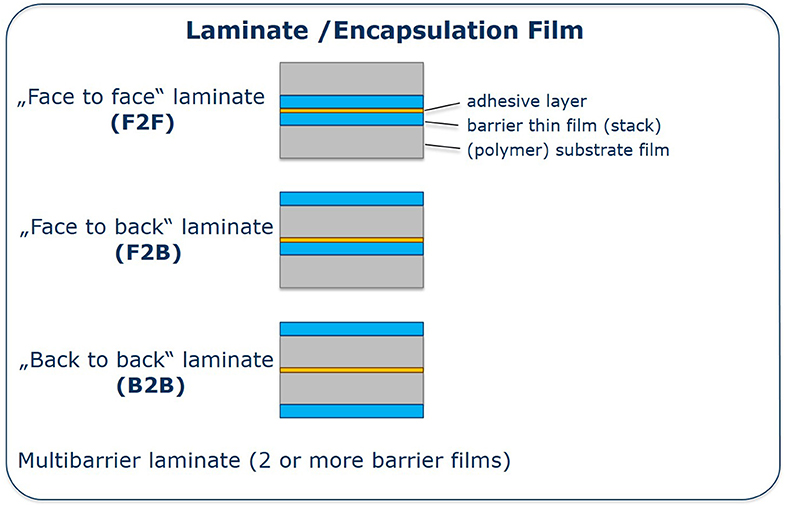
To this effect, one simple way enhancing gas barriers is the coupling of some single barrier films by lamination. Lamination can be carried out in different orientations resulting in three different laminate types as denoted in Figure 8. Each of them possesses different advantages and disadvantages: In the F2F case, both mechanically sensitive barrier layers are innately protected by both substrate films. Both laminate surfaces have a more hydrophilic character, e.g., pending OH groups, and UV curable epoxide or hybrid formulations were investigated and applied for bonding of inorganic layers (Schmidt et al., 2012). The F2B orientation minimizes gas permeation directly at the outside of the barrier film, avoiding dissolution of water vapor inside the polymer film. In addition to that B2B separates the substrate film (polymer films can show high uptake of water vapor) from the sensitive electronic device inside. Depending on the orientation of the laminated films, the lamination adhesive has to be adapted to the surfaces. In the case of B2B two hydrophobic surfaces have to be laminated whereas the F2B case requires lamination of different surfaces.
Mechanical decoupling of several brittle inorganic thin films allows lower bending radii and have been shown to exhibit long term bending stability; only a moderate increase of WVTR values by less than 10% was observed after 3,000 bending cycles (Channa et al., 2019). The position of the gas barrier film within the functional film stack becomes crucial for bending stability of optoelectronic devices. Han et al. applied non-linear finite element analysis to identify the neutral axis as preferred position for the inorganic barrier and adjusted its height within real stacks by introducing organic buffer layers (Han et al., 2016). Planarization of the polymeric substrate film with organic or hybrid materials coatings can improve the quality of the barrier thin film within the multilayer stack (Affinito et al., 1996; Amberg-Schwab et al., 2010). Incorporation of water-scavenging particles (Solovyov, 2006) or high-aspect ratio particles (Feldman, 2013; Choudalakis and Gotsis, 2014; Cui et al., 2015, 2016) into lamination adhesives, respectively organic interlayers opens further potential to enhance barrier properties of laminated barrier films. An increase of lag-time is provided, which might lead to high-performance for products with lower life-time requirements. Pre-drying of the barrier laminates to release water from the polymeric films is a technological approach that should be implemented by the end-user of the encapsulation film.
On the other hand and according to the above-mentioned law, in defect-free multilayer barrier stacks, the film with lowest permeability dominates the steady-state transmission rates, usually being the inorganic thin film (Graff et al., 2004; Kiese et al., 2019). To that effect and in order to reach higher BIFs for single-barrier films and consequently also in multilayer stacks, very promising results have been reported by sealing of defects by direct wet or gas phase overcoating with additional inorganic thin films. Exemplary, sealing of a PVD-SiOx-coated barrier film with a photochemically prepared SiOx-film produced in a continuous roll-to-roll process (200 mm wide) on polyester substrates exhibits gas transmission rates (H2O, O2) between 5 × 10−3 and 1 × 10−2 g m−2 d−1 or cm3 m−2 d−1 bar−1, respectively, compared to 0.5 g m−2 d−1 of the base barrier film. Stacks of alternating organic and inorganic layers, wherein one inorganic layer comprises of two directly stacked inorganic single layers leads to the most efficient and reliable design principle for high-barrier films at the current state of knowledge. This way, a sealing effect and a mechanical decoupling can operate as a combined advantage in multilayer stacks. WVTR values ≈ 10−4 g m−2 d−1 can be obtained, and encapsulated CIGS-based thin-film solar cells were found to be stable in industrial climate testing (damp-heat exposure, thermo-cycles, UV exposure, and wetting) usually accepted as verification for a 20 years lifetime (Meyer et al., 2010; Morlier et al., 2013; Prager et al., 2014).
In contrast to the lamination of layers with defined compositions, Sun et al. (2019) recently presented an approach in which first a polydimethylsiloxane (PDMS) and then a PHPS layer were coated alternating. Subsequent VUV irradiation not only converts the PHPS layer to silica but also partially, provided with a depth gradient, the PDMS into SiO2. Thus, organic/inorganic layer structures with gradual transitions of the composition in the intermediate layer between PDMS and silica were created, for which WVTR values <10−4 g m−2 d−1 were measured and with which OLEDs could be encapsulated with shown long-term stability.
Characterization of Gas Permeation
Recent developments in flexible (organic) electronic devices and wearables require flexible stand-alone encapsulation materials with high and ultra barrier properties against water vapor transmission, to protect the sensitive active materials from degradation and to ensure their long-term stability. In order to detect the very low quantities of permeants, if possible in due course during or after the barrier film production, the metrology must also evolve. Several approaches can be used for the measurement of water vapor permeation through films. Practical relevance has been reached by Ca-mirror tests, coulometric electrochemical devices, radioactive tracer methods, mass spectrometry, spectroscopic methods, pressure and thermal conductivity measurement, as described elsewhere (Nisato et al., 2014; Jeong et al., 2020). The above-mentioned methods are summarized in Table 2 including their measurement sensitivities as well as typical test conditions. For most methods the WVTR measurement vessel is divided in two chambers by the barrier film, with a water vapor containing atmosphere on one side. The water vapor permeation through the sample can be done by equal pressure or by pressure difference methods (e.g., mass spectrometric methods). Besides the determination of permeation rates, the optical Ca-mirror test enables an investigation of defect amount and distribution.
An experimental comparison between spectrometric and coulometric methods as well as different types of Ca-mirror tests is given by Nisato et al. (2014). The tests, carried out in different laboratories, included different measurement techniques as well as test cell geometries, evaluation in different environmental conditions, and on different barrier materials, resulting in some misunderstandings and inconsistencies. Especially environmental conditions, such as temperature and relative humidity significantly impact the permeation rates through thin films. If this is considered, different methods can result in comparable values for the gas transmission rates (Jarvis et al., 2017 #105).
Measurement of ultra barrier film materials is challenging, since measurement time, e.g., by isostatic carrier gas systems (e.g., commercial MOCON, Inc. systems), can take up to several months to reach steady state transmission rates. Kiese et al. (2017, 2019) presented a modification of the classical two-chamber method in that way that the water is collected before being transported to the measuring thermal conductivity sensor. This results in a higher sensitivity of the method, delivering permeation values which are in satisfying agreement with the values measured by the commercially available MOCON system, but in a shorter timescale. The large temperature range of 23–80°C and the available humidity range from 15 to 90% RH enables Arrhenius plots to be created. That way, WVTR values can be measured at higher temperatures in a time-saving manner and values can be calculated for lower temperatures. A combination of the time-dependent measurement with finite element calculations can further shorten the measuring times from months to weeks by a prediction of the transmission rates.
Investigations by Schulze et al. showed that the substitution of water vapor by helium leads to significant shorter measuring times for inorganically modified ETFE (ethylene tetrafluoroethylene copolymer) and represents an interesting alternative for fast determination of permeation values (Schulze et al., 2017). However, correlations of He-transmission rates with WVTR values depending on composition and structure of the barrier films and, probably, on the specific technological manufacturing conditions is not published yet.
Perspectives
Within the last two decades, fundamental aspects of photoconversion reactions for preparing metal oxide thin films could be unraveled by studies of initial photochemical reaction mechanisms. Application relevant systems have been investigated in detail with a focus on using this process as a low-cost roll-to-roll technology preparing transparent gas permeation barriers on temperature-sensitive polyester films. Industry-relevant gas barrier results were obtained by using these fundamental principles to develop the technology on the pilot plant scale. Various aspects of barrier preparation and optimization on one hand, as well as the integration of single layer barrier films into application relevant encapsulation films, on the other hand, were successfully implemented technologically.
Nevertheless, a lot of questions arise, which wait to be investigated by fundamental and applied research and development. The formulation of general principles for underlying photochemical mechanisms would help for a rational design of other thin film systems. One could think of other binary metal compounds or even combining different metals in variable composition. Application as catalytically active or conductive components of thin film stacks seems promising.
Further challenges also arise from the worldwide problem of accumulation of polymer residues in ecosystems. Recent solutions for the production of degradable polymers, like e.g., polylactide or cellulose nanofibrils, exhibit lower thermal stability than conventional ones, at present. This creates the need for further reducing production temperatures for thin films and rises considerable challenges in technology and precursor design.
Just as much as indium tin oxide layers on glass substrates are used as transparent conductive barrier substrates for rigid optoelectronic devices, integration of electrical conductivity into a transparent flexible gas barrier film would be of interest to the flexible electronics community. Minimizing stack heights and production costs are the main upcoming challenges. Thinking about carbon based materials, various barrier/electrode combinations are conceivable, and thus, this area of research and development is highly active both in industry and publicly funded research organizations. Industrially relevant options comprise indium tin oxide, silver nanowire meshes, carbon-based materials or PEDOT:PSS. It is to be seen, for which system integration into technically relevant products will be successful. Problems like adhesion, conductivity and cost-efficient industrial-scale production promise exciting topics for research and development toward functional gas barriers in the upcoming decades.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Affinito, J. D., Gross, M. E., Coronado, C. A., Graft, G. L., Greenwell, I. N., et al. (1996). A new method for fabricating transparent barrier layers. Thin Solid Films 290–291, 63–67. doi: 10.1016/S0040-6090(96)09202-4
Amberg-Schwab, S., Noller, K., Weber, U., and Miesbauer, O. (2010). Production of High-Barrier Multilayer Composites by Coating Substrates With Thin Hybrid Layer Arranged Between Inorganic Layers, WO 2010069958 A1. Munich: European Patent Organisation.
Awazu, K., and Onuki, H. (1996). Photoinduced synthesis of amorphous SiO2 with tetramethoxysilane. Appl. Phys. Lett. 69, 482–484. doi: 10.1063/1.118147
Awazu, K., and Onuki, H. (1997). Photo-induced synthesis of amorphous SiO2 film from tetramethoxy-silane on polymethylmethacrylate at room temperature. J. Non Cryst. Solids 215, 176–181. doi: 10.1016/S0022-3093(97)00047-1
Bieder, A., Gruniger, A., and von Rohr, R. (2005). Deposition of SiOx diffusion barriers on flexible packaging materials by PECVD. Surf. Coat. Technol. 200, 928–931. doi: 10.1016/j.surfcoat.2005.02.004
Boehm, H.-P. (1966). Funktionelle Gruppen an Festkörper-Oberflächen. Angew. Chem. 78, 617–652. doi: 10.1002/ange.19660781202
Bretos, I., Jiménez, R., Ricote, J., and Calzada, M. L. (2018). Low-temperature crystallization of solution-derived metal oxide thin films assisted by chemical processes. Chem. Soc. Rev. 47, 291–308. doi: 10.1039/C6CS00917D
Carcia, P. F., McLean, R. S., and Reilly, M. H. (2010). Permeation measurements and modeling of highly defective Al2O3 thin films grown by atomic layer deposition on polymers. Appl. Phys. Lett. 97:221901. doi: 10.1063/1.3519476
Channa, I. A., Distler, A., Zaiser, M., Brabec, C. J., and Egelhaaf, H.-J. (2019). Thin film encapsulation of organic solar cells by direct deposition of polysilazanes from solution. Adv. Energy Mater. 9:1900598. doi: 10.1002/aenm.201900598
Charton, C., Schiller, N., Fahland, M., Hollander, A., Wedel, A., and Noller, K. (2006). Development of high barrier films on flexible polymer substrates. Thin Solid Films 502, 99–103. doi: 10.1016/j.tsf.2005.07.253
Chatham, H. (1996). Oxygen diffusion barrier properties of transparent oxide coatings on polymeric substrates. Surf. Coat. Technol. 78, 1–9. doi: 10.1016/0257-8972(95)02420-4
Choi, J. H., Kim, Y. M., Park, Y. W., Huh, J. W., Ju, B. K., Kim, I. S., et al. (2007). Evaluation of gas permeation barrier properties using electrical measurements of calcium degradation. Rev. Sci. Instrum. 78:064701. doi: 10.1063/1.2747168
Choudalakis, G., and Gotsis, A. D. (2014). “Recent Developments in the permeability of polymer clay nanocomposites,” in Handbook of Polymernanocomposites. Processing, Performance and Application: Volume A: Layered Silicates, eds. J. K. Pandey, K. R. Reddy, A. K. Mohanty, and M. Misra (Berlin; Heidelberg: Springer), 415–451.
Cros, S., Firon, M., Lenfant, S., Trouslard, P., and Beck, L. (2006). Study of thin calcium electrode degradation by ion beam analysis. Nucl. Instrum. Methods Phys. Res. Sect. B 251, 257–260. doi: 10.1016/j.nimb.2006.06.014
Cui, Y., Kumar, S., Rao Kona, B., and van Houcke, D. (2015). Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 5, 63669–63690. doi: 10.1039/C5RA10333A
Cui, Y., Kundalwal, S. I., and Kumar, S. (2016). Gas barrier performance of graphene/polymer nanocomposites. Carbon 98, 313–333. doi: 10.1016/j.carbon.2015.11.018
Czeremuszkin, G., Latrèche, M., Wertheimer, M. R., and da Silva Sobrinho, A. S. (2001). Ultrathin silicon-compound barrier coatings for polymeric packaging materials: an industrial perspective. Plasmas Polym. 6, 107–120. doi: 10.1023/A:1011308919705
da Silva Sobrinho, A. S., Czeremuszkin, G., Latrèche, M., Dennler, G., and Wertheimer, M. R. (1999). A study of defects in ultra-thin transparent coatings on polymers. Surf. Coat. Technol. 116–119, 1204–1210. doi: 10.1016/S0257-8972(99)00152-8
da Silva Sobrinho, A. S., Czeremuszkin, G., Latrèche, M., and Wertheimer, M. R. (2000). Defect-permeation correlation for ultrathin transparent barrier coatings on polymers. J. Vacuum Sci. Technol. A 18, 149–157. doi: 10.1116/1.582156
da Silva Sobrinho, A. S., Latrèche, M., Czeremuszkin, G., Klemberg-Sapieha, J. E., and Wertheimer, M. R. (1998). Transparent barrier coatings on polyethylene terephthalate by single- and dual-frequency plasma-enhanced chemical vapor deposition J. Vacuum Sci. Technol. A 16, 3190–3198. doi: 10.1116/1.581519
Dameron, A. A., Davidson, S. D., Burton, B. B., Carcia, P. F., McLean, R. S., and George, S. M. (2008). Gas diffusion barriers on polymers using multilayers fabricated by Al2O3 and rapid SiO2 atomic layer deposition. J. Phys. Chem. C 112, 4573–4580. doi: 10.1021/jp076866
Erlat, A. G., Henry, B. M., Ingram, J. J., Mountain, D. B., McGuigan, A., Howson, R. P., et al. (2001). Characterisation of aluminium oxynitride gas barrier films. Thin Solid Films 388, 78–86. doi: 10.1016/S0040-6090(01)00836-7
Fahlteich, J., Fahland, M., Schönberger, W., and Schiller, N. (2009). Permeation barrier properties of thin oxide films on flexible polymer substrates. Thin Solid Films 517, 3075–3080. doi: 10.1016/j.tsf.2008.11.089
Feldman, D. (2013). Polymer nanocomposite barriers. J. Macromol. Sci. Part A 50, 441–448. doi: 10.1080/10601325.2013.768440
Gioti, M., Logothetidis, S., Schroeder, J., and Steiniger, G. (2009). Real-time evaluation of thickness, optical properties and stoichiometry of SiOx gas barrier coatings on polymers. Thin Solid Films 517, 6230–6233. doi: 10.1016/j.tsf.2009.02.102
Graff, G. L., Williford, R. E., and Burrows, P. E. (2004). Mechanisms of vapor permeation through multilayer barrier films: lag time versus equilibrium permeation. J. Appl. Phys. 96, 1840–1849. doi: 10.1063/1.1768610
Greener, J., Ng, K. C., Vaeth, K. M., and Smith, T. M. (2007). Moisture permeability through multilayered barrier films as applied to flexible OLED display. J. Appl. Polym. Sci. 106, 3534–3542. doi: 10.1002/app.26863
Haas, K. H., Amberg-Schwab, S., Rose, K., and Schottner, G. (1999). Functionalized coatings based on inorganic–organic polymers (ORMOCER®s) and their combination with vapor deposited inorganic thin films. Surf. Coat. Technol. 111, 72–79. doi: 10.1016/S0257-8972(98)00711-7
Han, Y. C., Jeong, E. G., Kim, H., Kwon, S., Im, H.-G., Bae, B.-S., et al. (2016). Reliable thin-film encapsulation of flexible OLEDs and enhancing their bending characteristics through mechanical analysis. RSC Adv. 6, 40835–40843. doi: 10.1039/C6RA06571F
Hanika, M., Langowski, H. C., Moosheimer, U., and Peukert, W. (2003). Inorganic layers on polymeric films – influence of defects and morphology on barrier properties. Chem. Eng. Technol. 26, 605–614. doi: 10.1002/ceat.200390093
Henry, B. M., Dinelli, F., Zhao, K. Y., Grovenor, C. R. M., Kolosov, O. V., Briggs, G. A. D., et al. (1999). A microstructural study of transparent metal oxide gas barrier films. Thin Solid Films 355–356, 500–505. doi: 10.1016/S0040-6090(99)00461-7
Hülsmann, P., Philipp, D., and Köhl, M. (2009). Measuring temperature-dependent water vapor and gas permeation through high barrier films. Rev. Sci. Instrum. 80:113901. doi: 10.1063/1.3250866
Imai, H., Hirashima, H., and Awazu, K. (1999). Alternative modification methods for sol-gel coatings of silica, titania and silica-titania using ultraviolet irradiation and water vapor. Thin Solid Films 351, 91–94. doi: 10.1016/S0040-6090(98)01784-2
Imai, H., Tominaga, A., Hirashima, H., Toki, M., and Aizawa, M. (1998). Ultraviolet-laser-induced crystallization of sol-gel derived indium oxide films. J Sol Gel Sci. Technol. 13, 991–994. doi: 10.1023/A:1008604213960
Jarvis, K. L., Evans, P. J., Cooling, N. A., Vaughan, B., Habsuda, J., Belcher, W. J., et al. (2017). Comparing three techniques to determine the water vapour transmission rates of polymers and barrier films. Surf. Interfaces 9, 182–188. doi: 10.1016/j.surfin.2017.09.009
Jeong, E. G., Kwon, J. H., Kang, K. S., Jeong, S. Y., and Choi, K. C. (2020). A review of highly reliable flexible encapsulation technologies towards rollable and foldable OLEDs. J. Inform. Display 21, 19–32. doi: 10.1080/15980316.2019.1688694
Kiese, S., Kücükpinar, E., Miesbauer, O., and Langowski, H.-C. (2019). Time-dependent water vapor permeation through multilayer barrier films: empirical versus theoretical results. Thin Solid Films 672, 199–205. doi: 10.1016/j.tsf.2019.01.001
Kiese, S., Uucukpinar, E. K., Reinelt, M., Miesbauer, O., Ewender, J., and Langowski, H. C. (2017). A systematic approach for the accurate and rapid measurement of water vapor transmission through ultra-high barrier films. Rev. Sci. Instrum. 88:025108. doi: 10.1063/1.4974952
Knolle, W., Wennrich, L., Naumov, S., Czihal, K., Prager, L., Decker, D., et al. (2010). 222 nm Photo-induced radical reactions in silazanes. A combined laser photolysis, EPR, GC-MS and QC Study. Phys. Chem. Chem. Phys. 12, 2380–2391. doi: 10.1039/B918814B
Kogelschatz, U. (2003). Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 23, 1–46. doi: 10.1023/A:1022470901385
Kumar, R. S., Auch, M., Ou, E., Ewald, G., and Jin, C. S. (2002). Low moisture permeation measurement through polymer substrates for organic light emitting devices. Thin Solid Films 417, 120–126. doi: 10.1016/S0040-6090(02)00584-9
Lange, J., and Wyser, Y. (2003). Recent innovations in barrier technologies for plastic packaging—a review. Packag. Technol. Sci. 16, 149–158. doi: 10.1002/pts.621
Langowski, H.-C. (2005). Stofftransport durch polymere und anorganische Schichten. Vakuum Forschung Praxis 17, 6–13. doi: 10.1002/vipr.200500242
Leterrier, Y. (2003). Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 48, 1–55. doi: 10.1016/S0079-6425(02)00002-6
Massines, F., Sarra-Bournet, C., Fanelli, F., Naudé, N., and Gherardi, N. (2012). Atmospheric pressure low temperature direct plasma technology: status and challenges for thin film deposition. Plasma Process. Polym. 9, 1041–1073. doi: 10.1002/ppap.201200029
Meyer, J., Schmidt, H., Kowalsky, W., Riedl, T., and Kahn, A. (2010). The origin of low water vapor transmission rates through Al2O3/ZrO2 nanolaminate gas-diffusion barriers grown by atomic layer deposition. Appl. Phys. Lett. 96:243308. doi: 10.1063/1.3455324
Miller, K. S., and Krochta, J. M. (1997). Oxygen and aroma barrier properties of edible films: a review. Trends Food Sci. Technol. 8, 228–237. doi: 10.1016/S0924-2244(97)01051-0
MOCON (2014). AQUATRAN Model 2: High Sensitivity Coulometric Water Vapor Transmission Rate Test System: Brochure.
MOCON (2020). Available online at: https://www.ametekmocon.com/products/permeationanalyzers/wvtr-permeation-analyzers/ (accessed March 6, 2020) [Online].
Morlier, A., Cros, S., Garandet, J.-P., and Alberola, N. (2013). Gas barrier properties of solution processed composite multilayer structures for organic solar cells encapsulation. Solar Energy Mater. Solar Cells 115, 93–99. doi: 10.1016/j.solmat.2013.03.033
Morlier, A., Cros, S., Garandet, J. P., and Alberola, N. (2014). Structural properties of ultraviolet cured polysilazane gas barrier layers on polymer substrates. Thin Solid Films 550, 85–89. doi: 10.1016/j.tsf.2013.10.140
Muñoz-Rojas, D., Maindron, T., Esteve, A., Piallat, F., Kools, J. C. S., and Decams, J. M. (2019). Speeding up the unique assets of atomic layer deposition. Mater. Today Chem. 12, 96–120. doi: 10.1016/j.mtchem.2018.11.013
Naganuma, Y., Horiuchi, T., Kato, C., and Tanaka, S. (2013). Low-temperature synthesis of silica coating on a poly(ethylene terephthalate) film from perhydropolysilazane using vacuum ultraviolet light irradiation. Surf. Coat. Technol. 225, 40–46. doi: 10.1016/j.surfcoat.2013.03.014
Naganuma, Y., Tanaka, S., Kato, C., and Shindo, T. (2004). Formation of silica coatings from perhydropolysilazane using vacuum ultraviolet excimer lamp. J. Ceram. Soc. of Jpn. 112, 599–603. doi: 10.2109/jcersj.112.599
Nagase, T., Ooie, T., Makita, Y., Kasaishi, S., Nakatsuka, M., and Mizutani, N. (2001). Morphology, structure and photoluminescence properties of zinc oxide films prepared by excimer laser irradiation of sol-gel-derived precursors. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 40, 6296–6303. doi: 10.1143/Jjap.40.6296
Nakajima, A., Hayashi, N., Taniguchi, Y., Kameshima, Y., and Okada, K. (2005). Effect of vacuum ultraviolet light illumination on the crystallization of sol–gel-derived titanium dioxide precursor films. Surf. Coat. Technol. 192, 112–116. doi: 10.1016/j.surfcoat.2004.04.034
Nisato, G., Klumbies, H., Fahlteich, J., Müller-Meskamp, L., van de Weijer, P., Bouten, P., et al. (2014). Experimental comparison of high-performance water vapor permeation measurement methods. Org. Electron. 15, 3746–3755. doi: 10.1016/j.orgel.2014.10.014
Paul, D. R., and DiBenedetto, A. T. (1965). Diffusion in amorphous polymers. J. Polym. Sci. Part C Polym. Sympos. 10, 17–44. doi: 10.1002/polc.5070100105.
Prager, L., Dierdorf, A., Liebe, H., Naumov, S., Stojanović, S., Heller, R., et al. (2007). Conversion of perhydropolysilazane into a SiOx network triggered by vacuum ultraviolet irradiation: access to flexible, transparent barrier coatings. Chem. Eur. J. 13, 8522–8529. doi: 10.1002/chem.200700351
Prager, L., Helmstedt, U., Herrnberger, H., Kahle, O., Kita, F., Münch, M., et al. (2014). Photochemical approach to high-barrier films for the encapsulation of flexible laminary electronic devices. Thin Solid Films 570, 87–95. doi: 10.1016/j.tsf.2014.09.014
Prager, L., Wennrich, L., Heller, R., Knolle, W., Naumov, S., Prager, A., et al. (2009). Vacuum-UV Irradiation-Based Formation of Methyl-Si-O-Si Networks from Poly(1,1-Dimethylsilazane-co-1-methylsilazane). Chem. Eur. J. 15, 675–683. doi: 10.1002/chem.200801659
Prager, L., Wennrich, L., Knolle, W., Naumov, S., and Prager, A. (2012). Absorption of acrylates and polysilazanes in the far UVC and the VUV regions. Mater. Chem. Phys. 134, 235–242. doi: 10.1016/j.matchemphys.2012.02.058
Rim, Y. S., Chen, H., Liu, Y., Bae, S.-H., Kim, H. J., and Yang, Y. (2014). Direct light pattern integration of low-temperature solution-processed all-oxide flexible electronics. ACS Nano 8, 9680–9686. doi: 10.1021/nn504420r
Schmidt, M., Rodler, N., Miesbauer, O., Rojahn, M., Vogel, T., Dörfler, R., et al. (2012). Adhesion and barrier performance of novel barrier adhesives used in multilayered high-barrier laminates. J. Adhes. Sci. Technol. 26, 2405–2436. doi: 10.1163/156856111X599535
Schrenk, W. J., and Alfrey, T. Jr. (1969). Some physical properties of multilayered films. Polym. Eng. Sci. 9, 393–399. doi: 10.1002/pen.760090604
Schubert, S., Klumbies, H., Müller-Meskamp, L., and Leo, K. (2011). Electrical calcium test for moisture barrier evaluation for organic devices. Rev. Sci. Instrum. 82:094101. doi: 10.1063/1.3633956
Schulze, S.-H., Ehrich, C., Meitzner, R., and Pander, M. (2017). Helium permeation as a fast quality indicator for barrier properties of solar cell encapsulates. Prog. Photovolt. Res. Appl. 25, 1051–1058. doi: 10.1002/pip.2922
SEMPA (2020). Available online at: https://www.hibarsens.de (accessed March 6, 2020) [Online].
Solovyov, S. E. (2006). Reactivity of gas barrier membranes filled with reactive particulates. J. Phys. Chem. B 110, 17977–17986. doi: 10.1021/jp0630929
Starostin, S. A., Creatore, M., Bouwstra, J. B., van de Sanden, M. C. M., and de Vries, H. W. (2015). Towards roll-to-roll deposition of high quality moisture barrier films on polymers by atmospheric pressure plasma assisted process. Plasma Process. Polym. 12, 545–554. doi: 10.1002/ppap.201400194
Sun, L., Uemura, K., Takahashi, T., Yoshida, T., and Suzuri, Y. (2019). Interfacial engineering in solution processing of silicon-based hybrid multilayer for high performance thin film encapsulation. ACS Appl. Mater. Interfaces 11, 43425–43432. doi: 10.1021/acsami.9b14994
SYSTECH (2020). Available online at: https://www.systechillinois.com/en/products/7000-water-vapor-permeation-analyser (accessed March 6, 2020) [Online].
Tak, Y. J., Yoon, D. H., Yoon, S., Choi, U. H., Sabri, M. M., Ahn, B. D., et al. (2014). Enhanced electrical characteristics and stability via simultaneous ultraviolet and thermal treatment of passivated amorphous In-Ga-Zn-O thin-film transistors. ACS Appl. Mater. Interfaces 6, 6399–6405. doi: 10.1021/am405818x
Taylor, D. J., and Fabes, B. D. (1992). Laser processing of sol-gel coatings. J. Non Cryst. Solids 147, 457–462. doi: 10.1016/S0022-3093(05)80658-1
Technolox (2020). Available online at: http://www.technolox.com (accessed March 6, 2020) [Online].
Tropsha, Y. G., and Harvey, N. G. (1997). Activated rate theory treatment of oxygen and water transport through silicon oxide/poly(ethylene terephthalate) composite barrier structures. J. Phys. Chem. B 101, 2259–2266. doi: 10.1021/jp9629856
Van de Leest, R. E. (1995). UV photo-annealing of thin sol-gel films. Appl. Surf. Sci. 86, 278–285. doi: 10.1016/0169-4332(94)00398-X
Wennrich, L., Khalil, H., Bundesmann, C., Decker, U., Gerlach, J. W., Helmstedt, U., et al. (2013). Photochemical preparation of aluminium oxide layers via vacuum ultraviolet irradiation of a polymeric hexanoato aluminium complex. Mater. Chem. Phys. 137, 1046–1052. doi: 10.1016/j.matchemphys.2012.11.026
With, P. C, Lehnert, J., Seifert, L., Dietrich, S., Krautscheid, H., Naumov, S., et al. (2019a). “Low Temperature Surface Reaction with VUV Photons - Insight into the Conversion of Iron(III) tert-butoxide to Iron(III) Oxide Thin Films,” in 14. ThGOT - Thementage Grenz- und Oberflächentechnik / 6. Optik - Kolloquium (Zeulenroda).
With, P. C., Helmstedt, U., Naumov, S., Sobottka, A., Prager, A., Decker, U., et al. (2016). Low-temperature photochemical conversion of organometallic precursor layers to titanium(IV) oxide thin films. Chem. Mater. 28, 7715–7724. doi: 10.1021/acs.chemmater.6b02757
With, P. C., Lehnert, J., Seifert, L., Dietrich, S., Krautscheid, H., Naumov, S., et al. (2019b). Photochemical low-temperature synthesis of iron(III) oxide thin films. Appl. Surf. Sci. 493, 525–532. doi: 10.1016/j.apsusc.2019.06.272
Wolf, R., Wandel, K., and Boeffel, C. (2007). Moisture barrier films deposited on PET by ICPECVD of SiNx. Plasma Process. Polym. 4, S185–S189. doi: 10.1002/ppap.200730608
Wuu, D. S., Lo, W. C., Chang, L. S., and Horng, R. H. (2004). Properties of SiO2-like barrier layers on polyethersulfone substrates by low-temperature plasma-enhanced chemical vapor deposition. Thin Solid Films 468, 105–108. doi: 10.1016/j.tsf.2004.04.031
Yoshida, H., Ebina, T., Arai, K., Kobata, T., Ishii, R., Aizawa, T., et al. (2017). Development of water vapor transmission rate measuring device using a quadrupole mass spectrometer and standard gas barrier films down to the 10–6 g m−2 day−1 level. Rev. Sci. Instrum. 88:043301. doi: 10.1063/1.4980074
Keywords: UV photoconversion, wet coating, metal oxide, thin films, gas permeation barriers, polymer substrates, flexible electronics, encapsulation
Citation: With PC, Helmstedt U and Prager L (2020) Flexible Transparent Barrier Applications of Oxide Thin Films Prepared by Photochemical Conversion at Low Temperature and Ambient Pressure. Front. Mater. 7:200. doi: 10.3389/fmats.2020.00200
Received: 10 December 2019; Accepted: 29 May 2020;
Published: 23 July 2020.
Edited by:
Yves Leterrier, École Polytechnique Fédérale de Lausanne, SwitzerlandReviewed by:
Sung Min Cho, Sungkyunkwan University, South KoreaSubimal Majee, Research Institutes of Sweden (RISE), Sweden
Copyright © 2020 With, Helmstedt and Prager. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick C. With, UGF0cmljay53aXRoQGlvbS1sZWlwemlnLmRl
 Patrick C. With
Patrick C. With Ulrike Helmstedt
Ulrike Helmstedt Lutz Prager
Lutz Prager