- Department of Stomatology, First Affiliated Hospital of Dalian Medical University, Dalian, China
Zirconia has occupied an increasingly important role in oral clinical applications in recent years. However, how to achieve the ideal bonding effect of zirconia is a significant problem that needs to be solved urgently in oral clinics. Hot etching treatment of zirconia is a hot spot of current research, but it is still unclear about the optimal acid solution and the effect of hot etching on the surface topography and bond strength of zirconia. This study evaluated the effect of hot etching with HF and HCl on the surface topography, roughness, crystalline phase, zirconia/resin cement interfacial evaluation and shear bond strength of zirconia. The results showed that the hot etching groups produced completely different topographical changes on the surface of zirconia than the sandblasting group. Obvious interfacial cracks were observed in the sandblasting group. The HF hot etching group achieved the highest roughness values (78.17 ± 4.94 nm) and the highest shear bond strength (25.09 ± 4.09 MPa). Compared with HCl, hot etching with HF could achieve more uniform and dense porous morphology, greater roughness and shear bond strength. Moreover, there were no prominent zirconia/resin cement interfacial cracks and crystal phase transformations on the surface of zirconia.
1 Introduction
In recent years, zirconia restorations have been widely favored by doctors and patients in oral clinical treatment (Tzanakakis et al., 2020). Optimizing the cement adhesion to zirconia restorations is the key to successful prosthodontic treatment. Especially for restorations like veneers, in order to achieve an ideal clinical effect, it is more dependent on the effect of bonding (Valente et al., 2020). Before bonding to the teeth, conventional all-ceramic restorations were treated with hydrofluoric acid (HF) and silane coupling agents. However, zirconia ceramics do not contain silica components, and surface treatment with hydrofluoric acid and silane coupling agents application of zirconia ceramics is not a well-established method to achieve durable adhesion resin-based materials (Valente et al., 2020). Therefore, achieving the ideal bonding effect of zirconia is a significant problem that urgently needs to be solved in oral clinics (Flores-Ferreyra et al., 2019). Studies have shown that zirconia includes three crystal phases: monoclinic, tetragonal and cubic phases (Sriamporn et al., 2014). When zirconia is under pressure, high temperature or humidity, it may transform from tetragonal to monoclinic (Maroun et al., 2019). However, the tetragonal to monoclinic phase transformation will affect the mechanical strength of zirconia (Allahkarami and Hanan, 2011). So the ideal treatment method for zirconia should avoid transforming its crystal phase (Jiao et al., 2018). At present, some scholars have proposed a scheme for hot etching to treat zirconia. Harb (Harb et al., 2021) indicated that hot etching treatment could improve the bond strength of zirconia restorations. Pin LV (Lv et al., 2015) not only proposed that the shear bond strength between zirconia and resin cement could be significantly increased by hot etching treatment, but also the bond strength of hot etching group was still higher than that of sandblasting group after thermocycling test. However, no consensus has been reached on the optimal type of acid solution for hot etching and the influence of hot etching on the related properties of zirconia (Wei et al., 2021). In this experiment, two acid solutions, HCl and HF, were used to conduct hot etching on zirconia, and then the related properties of zirconia were detected and analyzed. It is hoped to find an acid solution with the best effect on the hot etching of zirconia to provide a reference for future research.
2 Materials and methods
2.1 Specimen preparation
First, put the zirconia discs (ZrO2 ≥ 99.0%,Y2O3 4.5–6.0%, HfO2 ≤ 5.0%, Other oxides ≤1.0%) (Zenostar T, WIELAND, Germany) into the zirconia cutting machine (D500, Guangzhou Yihua Digital Technology Co., Ltd., China) in turn, then cut them according to the operation guide. The cut zirconia ceramic blocks into the zirconia sintering furnace (Vicce K8 Plus, Shenzhen Purong Trading Co., Ltd., China), the ceramic blocks shrink evenly during the sintering process. Then, the zirconia ceramic blocks were ground and trimmed one by one, and 24 pieces of zirconia with a size of 10 × 10 × 2 mm (type I) and 20 pieces of zirconia with a size of 3 × 3 × 2 mm (type II) were obtained. All specimens were uniformly polished on grinding and polishing machine (LABOPOL-25, Struers, Denmark) and successively used 180-, 240-, 500-, 800-, 1000-, 1200-, 1500- and 2000-grit water sandpaper for step-by-step polishing. Then these specimens were placed in a numerically controlled ultrasonic cleaner (KQ-400DE, Kunshan Ultrasound Instrument Co., Ltd., China), cleaned and shaken with deionized water for 15 min, and dried for later use.
2.2 Specimen treatment
All specimens were equally divided into 4 groups, each group including 6 types I specimens and 5 types II specimens, and proceeded according to the following groupings:
Group A. No treatment: No surface treatment procedure was applied.
Group B. Sandblasting: Airborne-particle abrasion with 50 µm Al2O3 particles applied for 15 s at a vertical distance of 10 mm and 0.25 MPa of air pressure.
Group C. Hot etching with HCl: Specimens and a rotor were placed in a reaction kettle filled with hot etching solution (24 ml of methanol, 6 ml of 37% HCl and 0.06 g of FeCl3). Then the reaction kettle was placed in a constant temperature magnetic stirring pot (Zhengzhou Great Wall Technology Industry and Trade Co., Ltd., China), and the treatment was continued for 10 min under the conditions of 100°C and 400 rev/minute.
Group D. Hot etching with HF: Specimens and a rotor were placed in a reaction kettle filled with hot etching solution (30 ml of 9.5% HF). Then the reaction kettle was placed in a constant temperature magnetic stirring pot (Zhengzhou Great Wall Technology Industry and Trade Co., Ltd., China), and the treatment was continued for 10 min under the conditions of 100°C and 400 rev/minute.
After the treatment, each group of specimens was placed in a numerically controlled ultrasonic cleaner in turn, ultrasonically oscillated in deionized water for 15 min, and gently dried.
2.3 AFM and SEM evaluation
Three random types I specimens from each group were used for the morphological change and surface roughness analysis using atomic force microscopy (AFM, JPK NanoWizard Ultra Speed, Germany). To analyze the surface roughness of each group of specimens, a silicon AFM probe (k = 3 N/m, f = 75 kHz) was used to perform in tapping mode with a scan size of 3 × 3 µm. AFM data were generated with the JPK Data Processing software. Furthermore, the images of each group of specimens were recorded, and the average was determined as the mean Ra value. Then one type I specimen was randomly selected from each group. Each specimen was sputter-coated with gold and evaluated under a scanning electron microscope (SEM, ZEISS SUPRA 55, Germany) at ×10,000magnifications to assess changes in surface topography.
2.4 XRD evaluation
One type I specimen from each group was randomly selected to determine the crystal structures and phase transformations. Crystalline phase identification of the specimens was carried out by X-ray diffraction (XRD, PANalytical, Empyrean X, the Netherlands) using Cu-Kα radiation operating at 60 kW and 50 mA. Scans were performed over the 2θ range of 20–70° at a scan speed of 0.02°/minute. Finally, each group of XRD patterns was analyzed to conclude.
2.5 Zirconia/resin cement interfacial evaluation
Composite discs (Paradigm MZ100, 3M ESPE, America) were cut into four composite resin blocks with a size of 10 × 10 ×2 mm by a slow-speed diamond saw (IsoMet 1000, BUEHLER, America) under constant water cooling. These composite resin blocks were ground with 180-, 240-, 500-, 800-, 1000-, 1200-, 1500- and 2000-grit water sandpaper, cleaned with deionized water and gently air-dried. One type I specimen was randomly selected from each group, and the specimen was bonded to the resin block according to the operation guide of resin cement (Panavia F, MPD-based resin cement, Kuraray, Japan). Then bonded specimens were stored in a constant temperature water bath (HWS-24, Shanghai Yiheng Scientific Instrument Co., Ltd., China) at 37°C for 24 h. Bonded specimens were sectioned perpendicularly to the interface with a slow-speed diamond saw under constant water cooling. Afterwards, each exposed zirconia/resin cement interface was polished with 180-, 240-, 500-, 800-, 1000-, 1200-, 1500- and 2000-grit water sandpaper. Then these bonded specimens were placed in the numerically controlled ultrasonic cleaner, cleaned and shaken with deionized water for 15 min, and dried. Finally, each bonded specimen was sputter-coated with gold and evaluated under the scanning electron microscope at ×1,000magnifications to assess zirconia/resin cement interfacial properties.
2.6 Shear bond strength test
The upper and mandibular premolars extracted from orthodontics were selected, and no root canal treatment was performed. There are no apparentcracks on the lip surface of the tooth crown, no large-scale caries and defects, no fluoride spots and poor mineralization. The isolated teeth were sequentially embedded in anhydrite and polished underwater sandpaper. Then the enamel with an area of no less than 3 × 3 mm was prepared. Type II specimens from each group were bonded to isolated teeth according to the operation guide of Panavia F resin cement. Then these bonded specimens were stored in the constant temperature water bath at 37°C for 24 h. A universal test machine (Instron, America) was used to complete the shear test of these bonded specimens. The vertical movement speed of the loading head was set to 1 mm/min, and the shear bond strength was calculated.
The tested specimens were placed under a stereomicroscope (NSZ-606, Yongxin, China) in turn to observe the fracture mode. The fracture mode is divided into the following types. When the fracture occurs inside the resin cement, it is a cohesive fracture; when it occurs between the zirconia and resin cement, it is the zirconia-resin interface fracture; Between the enamel and resin cement, it is the enamel-resin interface fracture; if any of the interface fractures and cohesive fractures exist at the same time, it is a mixed fracture.
2.7 Statistical analysis
The statistical analysis of surface roughness and the shear bond strength values were performed with SPSS (IBM SPSS Statistics 26, United States). Data were checked for the normal distribution and homogeneity of variance. One-way analysis of variance and most minor significant difference tests were used to analyze differences in groups (p < 0.05). The final results of XRD were analyzed using MDI Jade 6 combined with Origin 2019 software.
3 Results and discussions
3.1 AFM and SEM images
AFM and SEM images of each group are shown in Figure 1 and Figure 2. The surface of group A is smooth, with only a few traces of water sandpaper polishing. Rough abrasions appear on the surface of group B. The surface of the specimen exhibits a macrorough topography with grooves and scratches with sharp margins. Some scattered crater-like holes can be observed on the surface of group C. The size and shape of these holes are different. In group D, an overall homogenous and finer rough surface can be observed. Compared with group C, the surface of group D shows a more regular and dense porous topography with nanoscale irregular pores. A porous network structure with distinct peak-valley morphology is formed.
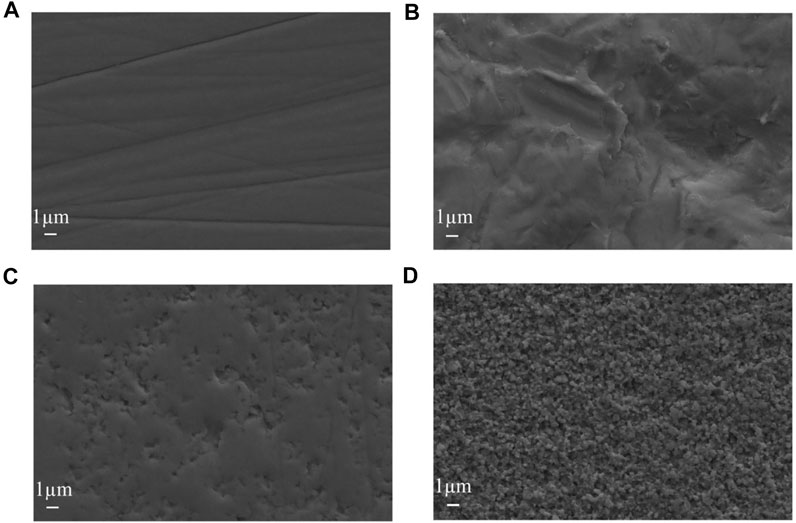
FIGURE 1. SEM observation images of zirconia specimens (×10,000 magnifications). (A) no treatment (B) sandblasting (C) hot etching with HCl (D) hot etching with HF.
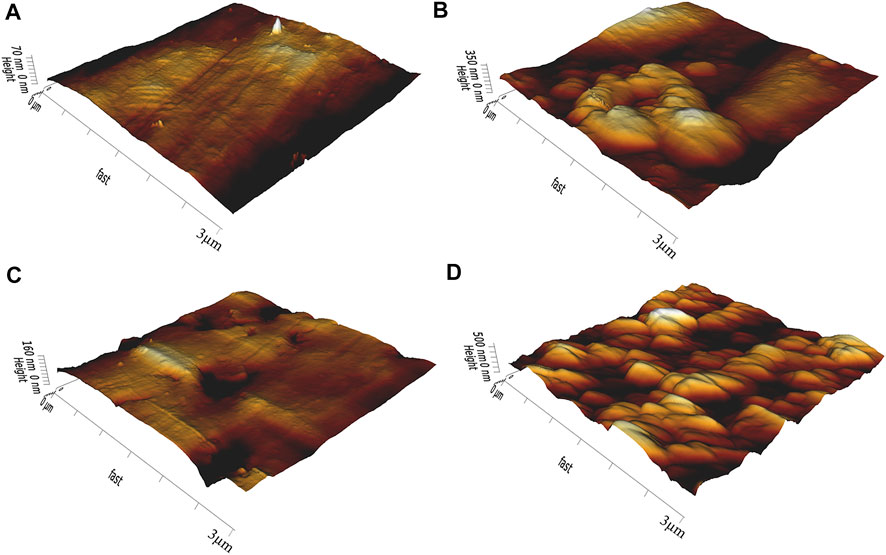
FIGURE 2. AFM observation images of zirconia specimens. (A) no treatment (B) sandblasting (C) hot etching with HCl (D) hot etching with HF.
In 2009, Casucci (Casucci et al., 2009) first tried to use 37% HCl to treat zirconia at 100°C. Moreover, the findings of this study revealed that hot etching might be a feasible method for improving zirconia surface roughness. Similarly, Akay (Akay et al., 2017) found that HCl hot etching for 10 min could increase the shear bond strength of zirconia ceramics. In recent years, many scholars have begun to try to use HF for the hot etching of zirconia. Kang (Kang et al., 2020) treated zirconia with 9.5% HF at 100°Cfor 10 min and found that the bond strength of zirconia in this group was significantly higher than that in other groups. Therefore, 37% HCl and 9.5% HF were selected to treat zirconia in this experiment. Moreover, this study aimed to find the optimal acid solution for the hot etching treatment of zirconia. The images of SEM and AFM show that different treatment methods have different effects on the surface topography of zirconia. During the sandblasting process, countless Al2O3 particles were accelerated to hit the surface of zirconia and then slid in different directions (Hallmann et al., 2016; Barreto et al., 2020). Therefore, various irregular grooves and scratches would be formed on the surface of zirconia (Saade et al., 2019). In the process of HCl hot etching, Fe3+ could destroy the integrity of the zirconia surface and help HCl dissolve the grain structure on the zirconia surface as an oxidizing agent, thereby causing local corrosion of zirconia (El-Korashy and El-Refai, 2014). Therefore, scattered holes could be observed on the surface of zirconia. In contrast, the surface of zirconia treated with HF hot etching shows a more uniform and dense porous topography. This is attributed to HF being more corrosive and permeable than HCl (Han et al., 2020). As the temperature increased, F gradually dissolved zirconia and yttria (Kim et al., 2020). Then reaction products such as fluoride, oxide and hydroxide complexes were produced. When these reaction products were separated from the surface of zirconia, a three-dimensional network structure with dense pores would be formed on the surface of zirconia (Lowalekar and Raghavan, 2004).
3.2 Surface roughness (Ra) values
The average surface roughness values (Ra) of each group are shown in Figure 3. Among them, D > C > B > A, the pairwise comparisons are statistically significant (p < 0.05). In this experiment, the zirconia treated by hot etching with two acid solutions was rougher than the sandblasting group, and this experimental finding was consistent with that of Alessio (Casucci et al., 2010). However, the experimental results of You-Jung (Kang et al., 2020) were different. He found that the surface roughness of the zirconia treated with HF hot etching was less than that of the sandblasting group. The reason may be that the concentration of HF selected in this experiment is higher than that of You-Jung’s experiment. In previous studies, some scholars pointed out that the concentration of HF would affect the effect of hot etching on zirconia and found that with the increase of the concentration of HF, the surface roughness would increase accordingly (Kim et al., 2021). Moreover, this finding explains why the two experiments above have different results. However, there is no consensus on the optimal concentration selection of HF, so further exploration is needed (Seo et al., 2022). For two acid solutions, the surface roughness of the HF group was greater than that of the HCl group in this experiment, which was also attributed to the stronger corrosiveness of HF than HCl. At present, there are few comparative studies on the hot etching of zirconia with two acid solutions, so more relevant studies are needed to verify the above findings (Lv et al., 2015).
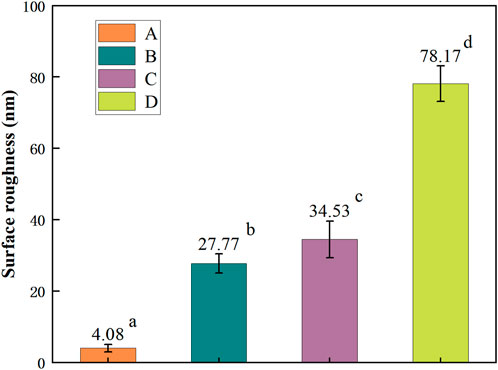
FIGURE 3. Surface roughness (Ra) values of zirconia specimens. Different superscript lowercase letters indicate statistically significant differences (p < 0.05).
Some scholars (Ding et al., 2018) point out that with the increase of roughness, the surface irregularity of zirconia increases, and the contact area between zirconia and resin cement also increases. Numerous resins flow into the pits, which not only help the cured resin cement to lock into the zirconia surface, but also to achieve a larger chemical reaction area. The appropriate increase of the roughness has a positive significance for the excellent bond of zirconia. However, You-Jung (Kang et al., 2020) points out that the relationship between roughness and bond strength is not purely linear. When uniform nano-scale pore structure is formed by hot etching, the roughness is lower, but the bond strength is still high. It can be seen that roughness is not the only factor determining bond strength, ceramic bond surface can not be blindly pursued with ultra-high roughness (Yu et al., 2016). However, there is no conclusion on the best range of roughness for zirconia bonding, it is still necessary to continue to bond strength testing, and then combined with the roughness to be analyzed.
3.3 X-Ray diffraction (XRD)
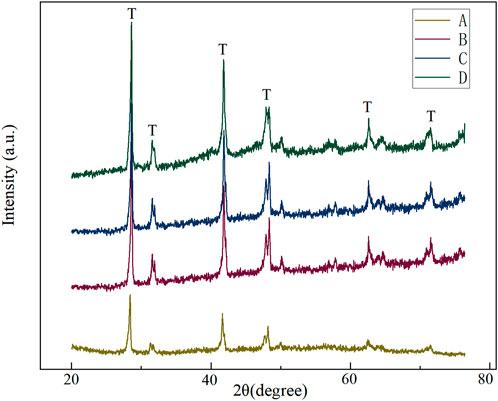
The X-ray diffraction patterns of each group are shown in Figure 4. Zirconia is a metastable ceramic with three crystal phase structures: monoclinic, tetragonal, and cubic phases (Sriamporn et al., 2014). When zirconia is subjected to tension and other factors, the tetragonal to monoclinic phase transformation occurs (Maroun et al., 2019). The mechanical strength of zirconia is adversely affected as the monoclinic phase increases and the tetragonal phase decreases (Allahkarami and Hanan, 2011). In this experiment, all the X-ray diffraction patterns of the Y -TZP surface treatment groups evaluated consisted of tetragonal phase (T) since detectable monoclinic peaks (M) were not observed in all surface treatment group specimens. XRD results show that the crystal structure of the treated zirconia is still stable. The occurrence of phase transformation is closely related to the selection of treatment parameters. Previous studies have proved that too large sandblasting particle size, too high hot etching concentration, and too long acid etching time will cause monoclinic phase transformation (Han et al., 2020). Saade (Saade et al., 2019) also pointed out that the occurrence of phase transformation is directly related to the particle size of sandblasting, and 50 µm causes less monoclinic phase transformation than 100 µm. Similarly, other studies have pointed out that the particle size of 50 μm, the pressure of 0.25 MPa, and the vertical distance of 10 mm are the best choices for Al2O3 sandblasting (Wang, 2019). Therefore, in this experiment, the above parameters were finally selected for the specimen treatment of the sandblasting group, hoping to avoid the occurrence of phase transformation and microcracks to the greatest extent. The XRD results also proved that this choice achieved the desired effect.
3.4 Zirconia/resin cement interfacial images
Zirconia/resin cement interfacial SEM images are shown in Figure 5. The top half of the images shows the resin cement and composite resin blocks bonded together, and the bottom half is zirconia. There is a clear boundary between the resin cement and zirconia in group A, and the boundary line is straight. The two materials are independent, and neither tends to protrude into each other. Irregular depressions appear on the surface of zirconia in group B, and the boundary line is no longer straight. There is no close contact between the resin cement and zirconia, and obvious interfacial cracks can be observed (red arrows mark). There are obvious pits on the surface of the zirconia in group C, and the resin cement corresponding to the position of the pits protrudes from the surface and protrudes into the pits. There is a tendency for interlocking between zirconia and resin cement. In group D, we can observe more obvious pits on the surface of zirconia, and the resin cement protrudes into these pits. So a cross-interlocking pattern is formed between the resin cement and the zirconia. The bonding between the resin cement and zirconia is good, the contact is very close, and no cracks are observed in the entire interface.
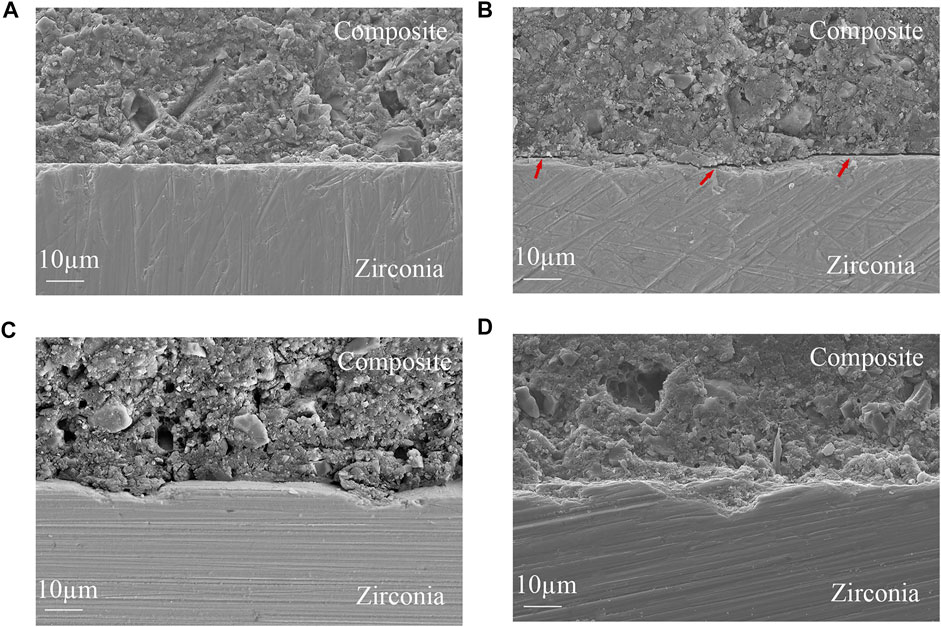
FIGURE 5. Zirconia/resin cement interfacial SEM images (×1,000 magnifications) (A) no treatment (B) sandblasting (C) hot etching with HCl (D) hot etching with HF (Interfacial cracks are visible at the red arrows).
3.5 Shear bond strength
The shear bond strength results of each group are shown in Figure 6. D > C > B > A, the data are statistically significant (p < 0.05). The observed results of fracture modes are summarized in Table 1. The fracture mode images are shown in Figure 7.
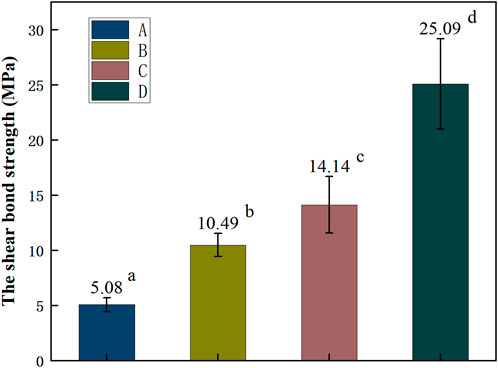
FIGURE 6. Shear bond strength of zirconia specimens. Different superscript lowercase letters indicate statistically significant differences (p < 0.05).
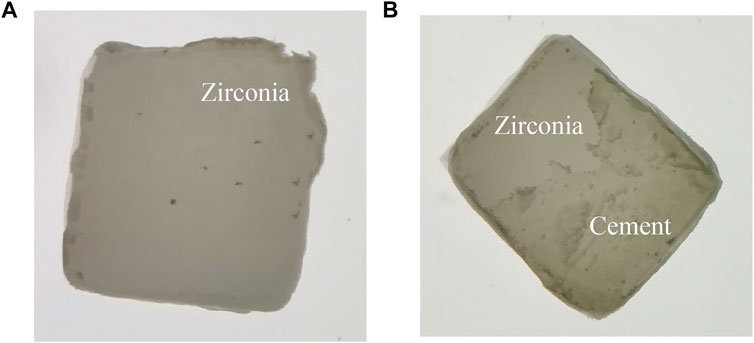
FIGURE 7. Fracture mode images of zirconia specimens. (A) zirconia-resin interface fracture; (B) mixed fracture.
A large number of studies have shown that the combined application of micromechanical interlock and chemical adhesion could effectively improve the bond strength of zirconia (Comino-Garayoa et al., 2021; Babaee et al., 2022). Previous studies have shown that the chemical interaction between the P-OH group of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) and the Zr-OH group on the zirconia surface could effectively improve the bond strength of zirconia (Serichetaphongse et al., 2022). Llerena-Icochea (Llerena-Icochea et al., 2017) proposed that useing resin cement containing 10-MDP can improve the bond strength of zirconia by about 8–72 Mpa. Phosphoric methacrylate ester has also been reported to increase the bond strength of zirconia. However, because the phosphoric methacrylate ester is prone to hydrolysis, its shear bond strength tends to be lower than that of 10-MDP (Lee et al., 2015). At the same time, Panavia F resin cement containing 10-MDP, as one of the representatives of the fifth-generation self-etch adhesive, has been widely used in clinical dental treatment. Therefore, Panavia F resin cement was selected for bonding zirconia in this experiment. The bond strength of group A is the lowest, and all the fracture modes of group A are zirconia-resin interface fractures, which fully shows that chemical adhesion between zirconia and resin cement alone cannot achieve the ideal bonding effect. Group B has obvious interfacial cracks, so the increased surface area of zirconia after roughening treatment is not effectively utilized, and the shear bond strength is lower than that of group C and group D. This finding is consistent with that of Casucci et al. (Casucci et al., 2010; Akay et al., 2017) Some studies have pointed out that small holes can provide better mechanical interlock than grooves that are relatively large and wide (Ramakrishnaiah et al., 2016). So, combined with the different morphological characteristics of the sandblasting group and the hot etching group in this experiment, it can be explained why the hot etching group achieved higher bond strength than the sandblasting group. Comparing two acid solutions, the HF hot etching group achieved higher shear bond strength than the HCl hot etching group. The reason for this result is that the fluoride ion in HF has strong oxidizing power and a small radius, which leads to its strong permeability (Wei et al., 2022). Therefore, the pores formed on the surface of zirconia in the HF group are denser than those in the HCl group. The dense pores facilitate the locking of more cured resin cement on the surface of zirconia and achieve a larger chemical reaction area (Casucci et al., 2011). Finally, with the help of both micromechanical interlock and chemical adhesion, the bonding effect is guaranteed.
The observed results of fracture modes showed that HF hot etching group had less adhesive failure (zirconia-resin interface fracture) than the control and sandblasting group. This result is consistent with Shaymaa’s findings. This observation confirms that sufficient micromechanical retention is important not only for establishing high bond strength but also for impeding adhesive failure (Elsaka, 2013). When there is no sufficient mechanical retention between zirconia and resin cement, the bond is mainly dependent on chemical bond, so the bond effect is not ideal. The fracture mode observed was mainly zirconia-resin interface fracture. There is less resin cement on the surface of zirconia after fracture, which indicates that the bond between zirconia and cement is not good. Therefore, a large area of residual cement can be observed on the fracture surface of zirconia in the HF hot etching group, which further shows that this treatment has a remarkable effect on improving the bond effect between zirconia and resin cement.
Attia (Attia, 2011) has stated that the minimum clinically acceptable bond strength value is approximately 13 MPa. In this experiment, the bonding effect obtained by hot etching can reach this standard, and the zirconia does not produce phase transformation, which proves the development potential of hot etching treatment of zirconia. In future research, the long-term bonding effect of zirconia after hot etching treatment needs to be further explored (Colombo et al., 2020). On the other hand, the acid solution required for the hot etching treatment is cheap, so the cost is easy to control. Moreover, the operation time of hot etching treatment is only 10 min, which can meet the high-efficiency requirements of clinical diagnosis and treatment. All of the above factors will contribute to the future promotion and application of the hot etching treatment method. However, this treatment method also has some disadvantages, including the use of the acid solution is dangerous, the equipment, such as the magnetic stirring pot, is not light and flexible enough, and so far, there is no dedicated hot etching equipment for clinical application. Therefore, it is necessary to continuously improve the flexibility of the equipment and reduce the risk of operation so that the clinical application of hot etching treatment of zirconia can be realized as soon as possible.
4 Conclusion
Within the limitations of this laboratory study, the following conclusions are drawn: The hot etching with HF and HCl would not cause phase transformation of zirconia and would not cause interfacial bond cracks like sandblasting. Compared with HCl, HF hot etching could make zirconia form a more regular and dense porous topography and achieve higher roughness and bond strength. Therefore, HF hot etching is a potential treatment method for zirconia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Methodology and writing- original draft, ZL; Investigation, writing and formal analysis, YL; Conceptualization, review and editing, YJ; Investigation and editing, PL; Investigation,YZ; Resources, FM; Writing, ML; Project administration, ZC; Methodology and supervision, JM; Methodology, resources and supervision, JC; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific Research Project of Liaoning Provincial Department of Education, grant number LZ2020034; Dalian Medical Science Research Program, grant number 2112007.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akay, C., Çakırbay, T., and Şen, M. (2017). Effects of hot chemical etching and 10-metacryloxydecyl dihydrogen phosphate (MDP) monomer on the bond strength of zirconia ceramics to resin-based cements. J. Prosthodont. 26, 419–423. doi:10.1111/jopr.12435
Allahkarami, M., and Hanan, J. C. (2011). Mapping the tetragonal to monoclinic phase transformation in zirconia core dental crowns. Dent. Mat. 27, 1279–1284. doi:10.1016/j.dental.2011.09.004
Attia, A. (2011). Bond strength of three luting agents to zirconia ceramic-influence of surface treatment and thermocycling. J. Appl. Oral Sci. 19, 388–395. doi:10.1590/s1678-77572011005000015
Babaee, H. Y., Neshandar, A. H., Falahchai, M., and Sina, S. (2022). Effect of different surface treatments and orthodontic bracket type on shear bond strength of high-translucent zirconia: An in vitro study. Int. J. Dent. 2022, 1–8. doi:10.1155/2022/9884006
Barreto, S. C., Lima, R. B. W., Aguiar, F. H. B., Santos, C. T. D., Paulillo, L., and Souza, G. (2020). Mechanical properties of aged yttria-stabilized tetragonal zirconia polycrystal after abrasion with different aluminum oxide particles. J. Prosthet. Dent. 124 (11), 599–604. doi:10.1016/j.prosdent.2019.10.027
Casucci, A., Mazzitelli, C., Monticelli, F., Toledano, M., Osorio, R., Osorio, E., et al. (2010). Morphological analysis of three zirconium oxide ceramics: Effect of surface treatments. Dent. Mat. 26, 751–760. doi:10.1016/j.dental.2010.03.020
Casucci, A., Monticelli, F., Goracci, C., Mazzitelli, C., Cantoro, A., Papacchini, F., et al. (2011). Effect of surface pre-treatments on the zirconia ceramic-resin cement microtensile bond strength. Dent. Mat. 27, 1024–1030. doi:10.1016/j.dental.2011.07.002
Casucci, A., Osorio, E., Osorio, R., Monticelli, F., Toledano, M., Mazzitelli, C., et al. (2009). Influence of different surface treatments on surface zirconia frameworks. J. Dent. (Shiraz). 37, 891–897. doi:10.1016/j.jdent.2009.06.013
Colombo, M., Gallo, S., Padovan, S., Chiesa, M., Poggio, C., and Scribante, A. (2020). Influence of different surface pretreatments on shear bond strength of an adhesive resin cement to various zirconia ceramics. Mater. (Basel) 13 (3), 652. doi:10.3390/ma13030652
Comino-Garayoa, R., Peláez, J., Tobar, C., Rodríguez, V., and Suárez, M. J. l. (2021). Adhesion to zirconia: A systematic review of surface pretreatments and resin cements. Materials 14 (11), 2751. doi:10.3390/ma14112751
Ding, Q., Zhang, L., Bao, R., Zheng, G., Sun, Y., and Xie, Q. (2018). Effects of different surface treatments on the cyclic fatigue strength of one-piece CAD/CAM zirconia implants. J. Mech. Behav. Biomed. Mat. 84, 249–257. doi:10.1016/j.jmbbm.2018.05.002
El-Korashy, D. I., and El-Refai, D. A. (2014). Mechanical properties and bonding potential of partially stabilized zirconia treated with different chemomechanical treatments. J. Adhes. Dent. 16, 365–376. doi:10.3290/j.jad.a32069
Elsaka, S. E. (2013). Effect of surface treatments on the bonding strength of self-adhesive resin cements to zirconia ceramics. Quintessence Int. 44 (6), 407. doi:10.3290/j.qi.a29572
Flores-Ferreyra, B. I., Scougall-Vilchis, R. J., Velazquez-Enriquez, U., Garcia-Contreras, R., Aguillon-Sol, L., and Olea-Mejia, O. F. (2019). Effect of airborne-particle abrasion and, acid and alkaline treatments on shear bond strength of dental zirconia. Dent. Mat. J. 38 (2), 182–188. doi:10.4012/dmj.2018-078
Hallmann, L., Ulmer, P., Wille, S., Polonskyi, O., Köbel, S., Trottenberg, T., et al. (2016). Effect of surface treatments on the properties and morphological change of dental zirconia. J. Prosthet. Dent. 115, 341–349. doi:10.1016/j.prosdent.2015.09.007
Han, A., Tsoi, J. K. H., Lung, C. Y. K., and Matinlinna, J. P. l. (2020). An introduction of biological performance of zirconia with different surface characteristics: A review. Dent. Mat. J. 39, 523–530. doi:10.4012/dmj.2019-200
Harb, O., Al-Zordk, W., Özcan, M., and Sakrana, A. A. (2021). Influence of hydrofluoric and nitric acid pre-treatment and type of adhesive cement on retention of zirconia crowns. Mater. (Basel) 14 (4), 960. doi:10.3390/ma14040960
Jiao, Y., Wang, J. D., and Deng, J. P. (2018). Effect of different surface treatments on the crystal structure and properties of zirconia. J. Peking Univ. Heal. Sci. 50 (01), 49–52. doi:10.3969/j.issn.1671-167X.2018.01.008
Kang, Y. J., Shin, Y., and Kim, J. H. (2020). Effect of low-concentration hydrofluoric acid etching on shear bond strength and biaxial flexural strength after thermocycling. Mater. (Basel) 13 (6), 1409. doi:10.3390/ma13061409
Kim, H. E., Lim, M. J., Yu, M. K., and Lee, K. W. l. (2020). Changes in bond strength and topography for Y-tzp etched with hydrofluoric acid depending on concentration and temperature conditions. Medicina 56 (11), 568. doi:10.3390/medicina56110568
Kim, H. K., Yoo, K. W., Kim, S. J., and Jung, C. H. (2021). Phase transformations and subsurface changes in three dental zirconia grades after sandblasting with various Al2O3 particle sizes. Mater. (Basel) 14 (18), 5321. doi:10.3390/ma14185321
Lee, S. E., Bae, J. H., Choi, J. W., Jeon, Y. C., Jeong, C. M., Yoon, M. J., et al. (2015). Comparative shear-bond strength of six dental self-adhesive resin cements to zirconia. Materials 8 (06), 3306–3315. doi:10.3390/ma8063306
Llerena-Icochea, A. E., Costa, R. M., Borges, A., Bombonatti, J., and Furuse, A. Y. (2017). Bonding polycrystalline zirconia with 10-MDP-containing adhesives. Oper. Dent. 42, 335–341. doi:10.2341/16-156-L
Lowalekar, V., and Raghavan, S. (2004). Etching of zirconium oxide, hafnium oxide, and hafnium silicates in dilute hydrofluoric acid solutions. J. Mat. Res. 19, 1149–1156. doi:10.1557/JMR.2004.0149
Lv, P., Yang, X., and Jiang, T. (2015). Influence of hot-etching surface treatment on zirconia/resin shear bond strength. Mater. (Basel) 8, 8087–8096. doi:10.3390/ma8125409
Maroun, E. V., Guimarães, J. G. A., Miranda, W. G., Netto, L., Elias, A. B., and Silva, E. M. (2019). Bond strength stability of self-adhesive resin cement to etched vitrified yttria-stabilized tetragonal zirconia polycrystal ceramic after thermomechanical cycling. Oper. Dent. 44 (5), 545–555. doi:10.2341/18-131-L
Ramakrishnaiah, R., Alkheraif, A. A., Divakar, D. D., Matinlinna, J. P., and Vallittu, P. K. (2016). The effect of hydrofluoric acid etching duration on the surface micromorphology, roughness, and wettability of dental ceramics. Int. J. Mol. Sci. 17 (6), 822. doi:10.3390/ijms17060822
Saade, J., Skienhe, H., Ounsi, H., Matinlinna, J. P., and Salameh, Z. l. (2019). Effect of different combinations of surface treatment on adhesion of resin composite to zirconia. Clin. Cosmet. Investig. Dent. 11, 119–129. doi:10.2147/CCIDE.S204986
Seo, S. H., Kim, J. E., Nam, N. E., and Moon, H. S. (2022). Effect of air abrasion, acid etching, and aging on the shear bond strength with resin cement to 3Y-TZP zirconia. J. Mech. Behav. Biomed. Mat. 134, 105348. doi:10.1016/j.jmbbm.2022.105348
Serichetaphongse, P., Chitsutheesiri, S., and Chengprapakorn, W. (2022). Comparison of the shear bond strength of composite resins with zirconia and titanium using different resin cements. J. Prosthodont. Res. 66 (1), 109–116. doi:10.2186/jpr.JPR_D_20_00299
Sriamporn, T., Thamrongananskul, N., Busabok, C., Poolthong, S., Uo, M., and Tagami, J. (2014). Dental zirconia can be etched by hydrofluoric acid. Dent. Mat. J. 33 (1), 79–85. doi:10.4012/dmj.2013-243
Tzanakakis, E., Kontonasaki, E., Voyiatzis, G., Andrikopoulos, K., and Tzoutzas, I. (2020). Surface characterization of monolithic zirconia submitted to different surface treatments applying optical interferometry and Raman spectrometry. Dent. Mat. J. 39, 111–117. doi:10.4012/dmj.2018-358
Valente, F., Mavriqi, L., and Traini, T. (2020). Effects of 10-MDP based primer on shear bond strength between zirconia and new experimental resin cement. Mater. (Basel) 13 (1), 235. doi:10.3390/ma13010235
Wang, C. (2019). Research progress on surface treatment methods before bonding of zirconia ceramics. Adhesion 40 (03), 33–36+54.
Wei, M., Zhu, H., Liang, Z. R., Jiang, Y. L., and Chen, J. F. (2022). Effect of different hot acid solution etching treatment on the bonding strength and the flexural strength of zirconia. J. Pract. Stomatology 5, 1–5. doi:10.3969/j.issn.1001-3733.2022.04.005
Wei, M., Zhu, H., Liang, Z. R., Jiang, Y. L., and Chen, J. F. (2021). The effect of different concentrations of hydrofluoric acid on the bonding strength of zirconia. J. Pract. Stomatology 37 (06), 758–761. doi:10.3969/j.issn.1001-3733.2021.06.006
Keywords: zirconia ceramic, hot etching, surface topography, surface roughness, bond strength
Citation: Liang Z, Liu Y, Jiang Y, Liu P, Zhang Y, Meng F, Liu M, Cui Z, Ma J and Chen J (2022) Effect of hot etching with HF on the surface topography and bond strength of zirconia. Front. Mater. 9:1008704. doi: 10.3389/fmats.2022.1008704
Received: 01 August 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Jianxun Ding, Changchun Institute of Applied Chemistry (CAS), ChinaReviewed by:
Lei Sui, Tianjin Medical University, ChinaXufeng Dong, Dalian University of Technology, China
Ling Ren, Institute of Metal Research (CAS), China
Qiang Wang, China Medical University, China
Copyright © 2022 Liang, Liu, Jiang, Liu, Zhang, Meng, Liu, Cui, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbao Ma, bWFqaW5iYW9AbWUuY29t; Jianfeng Chen, Y2hlbmpmMTIzNDU2NzhAMTYzLmNvbQ==
†These authors contributed equally to this work and shared the first authorship.
 Zhuoran Liang†
Zhuoran Liang† Jianfeng Chen
Jianfeng Chen