- 1Department of Agricultural and Environmental Sciences - Production Landscape, Agroenergy, Università degli Studi di Milano, Milan, Italy
- 2Department of Bioscience, Università degli Studi di Milano, Milan, Italy
- 3Centre of Applied Studies for the Sustainable Management and Protection of Mountain Areas – CRC Ge.S.Di.Mont., Università degli Studi di Milano, Edolo, Italy
- 4Department of Biology and Biotechnology Università degli Studi di Pavia, Pavia, Italy
Since in late 2019, when the coronavirus 2 (SARS-CoV-2) pathogen of coronavirus disease 2019 (COVID-19) started to spread all over the world, causing the awful global pandemic we are still experiencing, an impressive number of biologists, infectious disease scientists, virologists, pharmacologists, molecular biologists, immunologists, and other researchers working in laboratories of all the advanced countries focused their research on the setting up of biotechnological tools, namely vaccines and monoclonal antibodies, as well as of rational design of drugs for therapeutic approaches. While vaccines have been quickly obtained, no satisfactory anti-Covid-19 preventive, or therapeutic approach has so far been discovered and approved. However, among the possible ways to achieve the goal of COVID-19 prevention or mitigation, there is one route, i.e., the diet, which until now has had little consideration. In fact, in the edible parts of plants supplying our food, there are a fair number of secondary metabolites mainly belonging to the large class of the flavonoids, endowed with antiviral or other health beneficial activities such as immunostimulating or anti-inflammatory action that could play a role in contributing to some extent to prevent or alleviate the viral infection and/or counteract the development of SARS induced by the novel coronavirus. In this review, a number of bioactive phytochemicals, in particular flavonoids, proven to be capable of providing some degree of protection against COVID-19, are browsed, illustrating their beneficial properties and mechanisms of action as well as their distribution in cultivated plant species which supply food for the human diet. Furthermore, room is also given to information regarding the amount in food, the resistance to cooking processes and, as a very important feature, the degree of bioavailability of these compounds. Concluding, remarks and perspectives for future studies aimed at increasing and improving knowledge and the possibility of using this natural complementary therapy to counteract COVID-19 and other viral pathologies are discussed.
Introduction
At the end of 2019, the World Health Organization (WHO) reported numerous cases of low respiratory tract infections in Wuhan (Hubei province, China) caused by a novel virus. The novel virus is a member of the Coronaviridae family and it was identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) due to its high similarity with another previously isolated coronavirus, SARS-CoV (1–4). Due to its spread all over the world, in March 2020, the WHO declared a pandemic (5). Over the recent decades, an increase in diseases caused by new coronaviruses has been reported in humans and animals (6). Among these, SARS-CoV (2002–2003) and MERS-CoV (2012) caused serious health problems and demonstrated the lethality of coronaviruses if they cross the species barrier and subsequently infect humans (7, 8). Currently, the SARS-CoV-2 generated the unprecedented COVID-19 (Coronavirus Disease of 2019) outbreak. This pathogen can affect several tissues at multiple levels in humans: from the cells of nose and throat down to the lung, and also invading the kidneys and the nervous system, where it can lead to severe illness and death (9, 10). The subjects at highest risk of developing severe COVID-19 symptoms are the elderly and those with major chronic diseases, such as diabetes, cancer and hypertension (11). MERS-CoV binds to dipeptidyl-peptidase 4 (DPP4) receptors to infect human cells (6), while both the original SARS-CoV and the novel virus SARS-CoV-2 bind to the angiotensin-converting enzyme 2 (ACE2). However, SARS-CoV-2 has a greater binding affinity to ACE2, presenting an higher infectivity compared to the previous SARS-CoV (12–16). Similarly to SARS-CoV, also in SARS-CoV-2, two open-reading-frames (ORF1a and ORF1b) are translated into two viral enzymes fundamental for virus replication: 3C-like protease (3CLpro) and papain-like protease (PLpro) (17). In this context, an essential role in the infection is played by the spike glycoprotein (S), located on the viral phospholipidic membrane surface (Figure 1). In particular, the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 binds strongly to ACE2 receptors after the activation by two host serine proteases (TMPRSS2 and furin). The entry of the virus into host cells causes an increase of the natural inflammatory response (defined as a cytokine storm), leading to serious problems particularly in the respiratory tract.
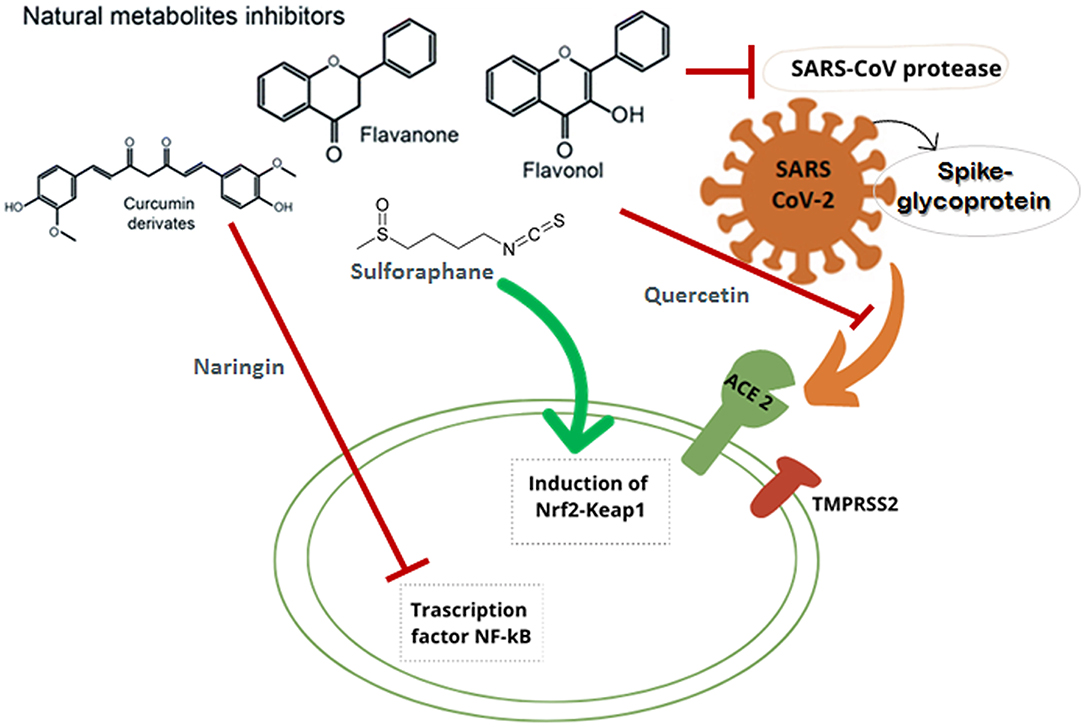
Figure 1. Schematic representation of the main mechanisms of action against SARS CoV-2 of different secondary plant metabolites: flavanones, flavonols, aromatic compounds, and sulfur compounds.
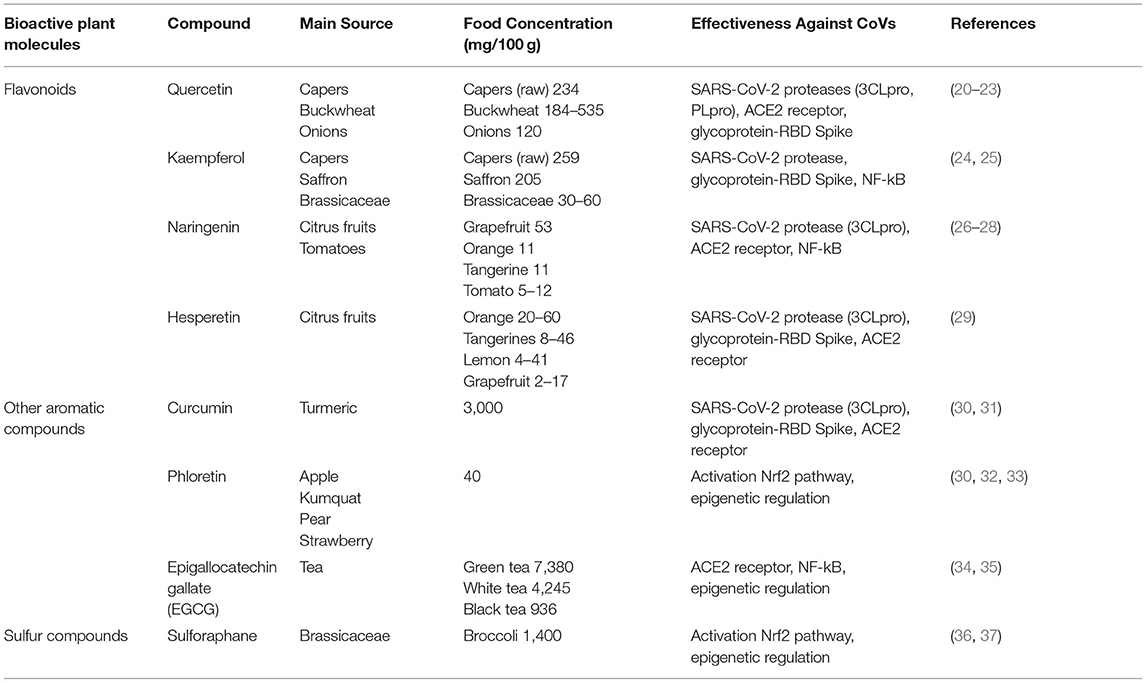
In synergy with therapeutic treatments and vaccines, we propose that the diet might play a significant role to prevent or to mitigate the symptoms of this illness. In fact, it is known that many phytochemicals have great potential in preventing viral infection, modulating immune responses, and decreasing the inflammatory response (18, 19). These natural molecules are present not only in a few medicinal plants, but also in many edible parts (seeds, fruits and vegetables) of cultivated plants which form part of the human diet. Consequently, we have available various “functional foods” that could complement our daily diet, with positive effects on both prevention and reduction of the severity of COVID-19 symptoms (Table 1).
Several foods, particularly fruits and vegetables, are rich in different natural compounds with beneficial effects on human health. In particular, various aromatic and a few sulfur compounds are known for their key roles as antioxidants, antivirals and anti-inflammatories (38). These bioactive phytochemicals may thus alleviate SARS-CoV-2 symptoms, decreasing the inflammatory responses (39, 40).
In this review, we present many natural plant-derived compounds whose intake can be implemented in the human diet and illustrate their antiviral potential or beneficial properties which may counteract COVID-19 progression. Furthermore, within the same species, the varieties characterized by a higher content of these phytochemicals are described. In particular, the focus is on the flavonoids flavonones and flavonols which are reported to be able to significantly counteract coronavirus infection and thus may also play a central role in protection against the novel COVID-19.
Flavonoids
Flavonoids are secondary metabolites synthesized by plants. They are divided into different classes: anthocyanins, flavanols, dihydroflavonols, flavanones, flavones, flavonols, isoflavonoids, chalcones, and dihydrocalcones (18).
The maize flavonoid biosynthesis involves over 20 loci, and historically was the first elucidated plant metabolic pathway, due to the facility with which it enabled work with non-lethal mutants, and corn revealed itself to be the ideal model plant for a variety of different genetic studies.
The pathway starts from the condensation of four molecules of coumaroyl-CoA with 3-malonyl-CoA, which produces naringenin chalcone by the enzyme chalcone synthase, CHS (Figure 2). Naringenin chalcone is subsequently isomerized by chalcone isomerase (CHI) to naringenin, a key intermediate of the biosynthetic pathway. Naringenin is then transformed to dihydrokaempferol, which in turn is the substrate for three enzymes: (1) dihydroflavonol reductase (DFR) which leads to pelargonidin synthesis; (2) flavonol synthase (FLS) that transforms dihydrokaempferol to kaempferol; (3) flavanone 3-hydroxylase (F3′H) that catalyzes the formation of dihydroquercetin. Similarly, dihydroquercetin leads to the synthesis of cyanidin by dihydroflavonol reductase (DFR) or to the formation of quercetin by FLS. F3′H is also a key enzyme for the synthesis of phlobaphenes (41). These red pigments are formed from polymers of luteoforol and apiferol, which in turn derive from eriodictyol and naringenin, through the action of the DFR enzyme. Moreover, eriodictyol can be converted to dihydroquercetin by the activity of F3H (42). All these structural genes are regulated by the presence of two multigene families, c1/pl1/p1 genes, belonging to the family of MYB transcription factors and r1/b1 genes, belonging to MYC transcription factors (43–45). Usually, an active form of each family (acting as dominant) must be present to lead anthocyanin biosynthesis in different plant tissues according to the presence of different alleles.
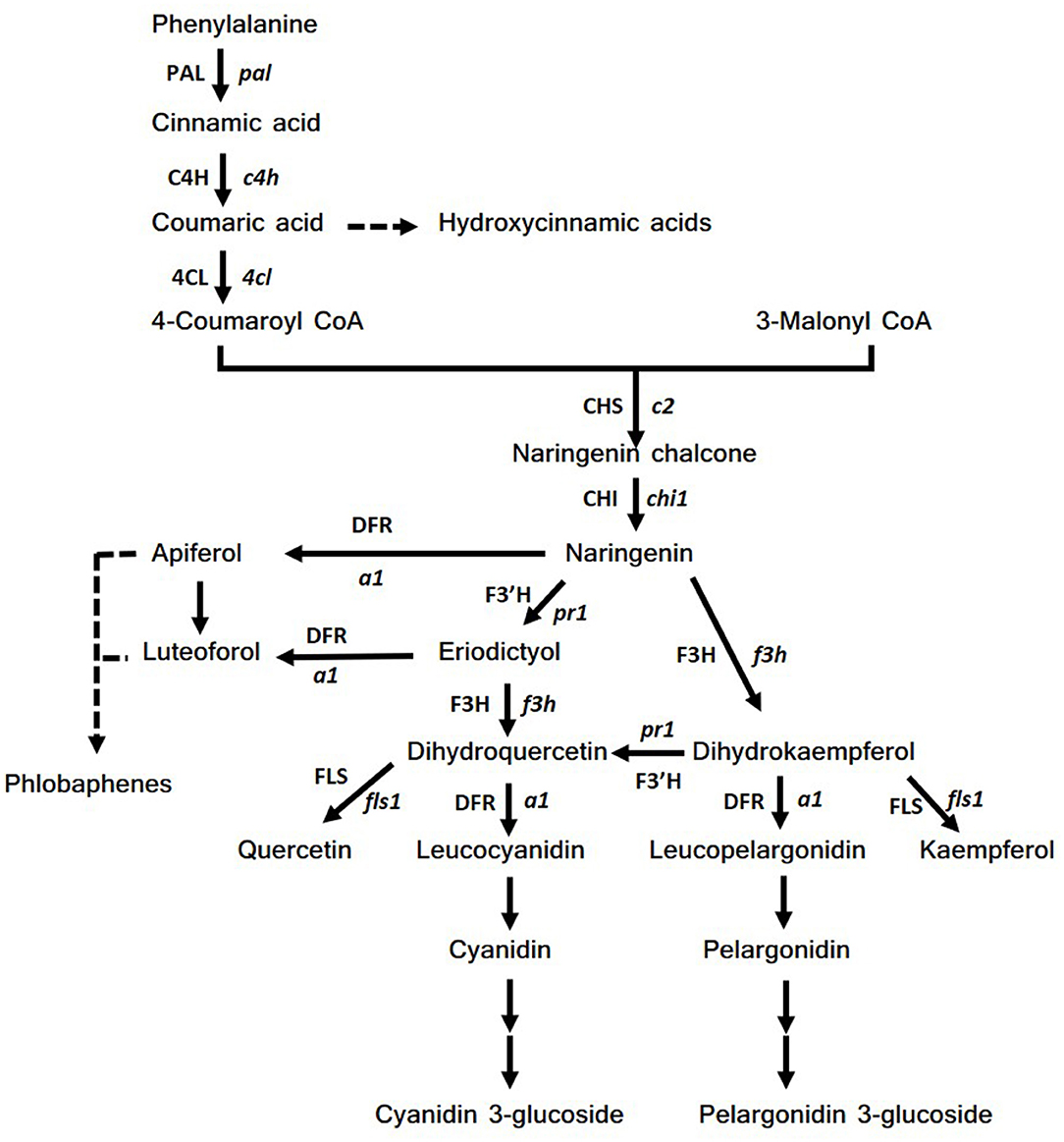
Figure 2. Scheme of flavonoids biosynthetic pathway. Abbreviations of genes (enzymes): pal (PAL), phenylalanine ammonia lyase; c4h (C4H), cinnamic acid 4-hydroxylase; 4cl (4CL), 4-coumarate-CoA ligase; c2 (CHS), chalcone synthase; chi1 (CHI), chalcone isomerase; f3h (F3H), flavanone 3 hydroxylase; pr1 (F3′H), flavonone 3'-hydroxylase; a1 (DFR), dihydroflavonol synthase.
There are various studies that highlight the wide range of biological activities of flavonoids, such as antiviral (46), antioxidant (47), anticancer (48), antimicrobial (49), and anti-inflammatory (50). As antivirals, several flavonoids have been reported to inhibit the targets of SARS and MERS coronaviruses (51) in different ways: blocking the enzymatic activities of viral proteases (3CLpro and PLpro), interfering with spike glycoproteins or suppressing the activity of ACE2 receptors (52, 53), which not only play an important role in cardiovascular diseases, but can be a key factor in viral infections and pneumonia (54). In particular, the hydroxyl group of flavonoids at 7-position appears essential to attack the binding site against 3CLpro and PLpro.
Different studies have focused primarily on the interference of flavonoids with the main viral proteases of SARS and MERS coronaviruses by using tools such as common enzymatic activity measurement, FRET (fluorescence resonance energy transfer) based methods and molecular docking (55–57). 3CLpro and PLpro are both key targets as they process many viral polyproteins that are involved in RNA replication and transcription within host cells (58).
However, the majority of the studies dealing with the health beneficial properties of flavonoids are conducted in vitro on the basis that these compounds show poor stability, low bioavailability, and poor distribution when tested in vivo (52). Among the tools and strategies used to increase these functions, the most promising are the insertion of structural modifications of the molecules (59), the use of absorption enhancers and nanotechnology (60, 61).
In the next step, epidemiological work should focus on clinical trials on COVID-19 patients in order to point out a reduction of the virus multiplication in the patient's body and a decrease in clinical signs (62). Furthermore, the advantage of implementing the diet with flavonoids is related to their high safety profile and lack of major side effects (62).
In this context, several authors have recently suggested kaempferol, quercetin, naringenin, curcumin, catechin, and epicatechin-gallate as recommended compounds found in plants that may act against COVID-19 proteases (18, 19, 26).
Quercetin
Quercetin is one of the most important flavonoids and belongs to the class of flavonols (Table 1). Quercetin is the aglycone form of several glycoside flavonoids: rutin and quercitin are the most common. In fact, sugars such as glucose, rhamnose, galactose, and rutinose are usually bound to these natural compounds to form glycosides. Quercetin is naturally present in several fruits and vegetables, and also in medicinal herbs (63, 64). Its highest concentration is present in capers (Capparis spinosa L.), which contain 234 mg of flavonol per 100 g of edible portion. Due to its beneficial properties, quercetin is used as a food supplement and can counteract various diseases, acting as antiviral, anticancer, antioxidant, antidiabetic, antiulcer, antiallergy, antihypertensive, anti-inflammatory, and was reported to protect the human body from cardiovascular and gastrointestinal diseases (65). Recently, Solnier et al. have proposed quercetin as a good anti-SARS-CoV-2 candidate (19). In fact, several flavonols showed antiviral activity against coronaviruses (such as SARS-Cov and MERS-CoV) through the inhibition of 3CL and PLpro proteases (51). Since the former SARS-CoV and the new SARS-CoV-2 show high sequence similarity in the spike glycoproteins, flavonols may be also expected to prevent the entry of SARS-CoV-2 into host cells. Moreover, it has been demonstrated that the spike protein of the novel virus binds the ACE2 receptor with higher affinity compared to SARS-CoV (58). Therefore, the inhibition of ACE2 through a competing binding, appears to be a good approach to prevent SARS-CoV-2 infections. In this framework, experimental results have demonstrated that quercetin exerts strong inhibitory effects on ACE2 in vitro, and also in vivo when tested in rats (52–54, 66). Furthermore, the screening of a library of 150 compounds, allowed the identification of quercetin as a potent inhibitor of SARS-CoV-2 3CLpro (67). Taken together, these results suggest that quercetin may prevent the entry of SARS-CoV-2 in the host cell, binding the S protein and inhibiting ACE2 receptors.
Kaempferol
Another important flavonol is kaempferol (Table 1), a secondary metabolite found in a wide variety of edible plants and food-derived products (24), such as kale, common bean, cabbage, broccoli, endive, and leek. The highest level of this compound was found in capers and saffron (259 and 205 mg/100 g, respectively). The glycoside form of kaempferol is astragalin, well-known for its multiple therapeutic properties (68) such as antioxidant (69, 70), anti-inflammatory (71), anticancer (72), neuroprotective (73), and antiviral (25).
Naringenin
A natural compound that belongs to the flavanone class is naringenin (Table 1). Naringenin is present in a wide variety of fruits and vegetables, but the highest concentrations are reported in grapefruit, tangerines, oranges, and tomatoes. Naringenin mainly occurs as glycosides such as naringin or prunin (74). The presence of this flavanone in human diet is relatively high (75), but its bioavailability is limited (nearly 5.81%). Moreover, it appears that the glycosylated form naringin is less bioavailable than the respective aglycone (76). However, in order to solve the problem of naringenin's limited bioavailability, some formulations such as nanoparticles loaded with naringenin have been developed (77). After the absorption via active transport and passive diffusion (78), naringenin attaches to albumin and is finally transported to different organs: brain, liver, kidneys, and heart (79). Like other flavonoids, naringenin was found to be endowed with beneficial strong antioxidant, anti-inflammatory and antiviral properties (80–82). In particular, the antioxidant role of this flavanone was shown to be carried out by eliminating free radicals and preventing DNA oxidative damage (83–85) while the strong anti-inflammatory activity is due to the inhibition of the NF-kB (nuclear factor kappa B) signaling pathway (86) since NF-kB promotes the expression of many fundamental inflammatory proteins (87). The antiviral activity of naringenin was tested against some viruses: HCV, Dengue virus (DENV), Chikungunya virus (CHIKV), and Zika virus (ZIKV) (26). In this context, the beneficial properties and the possible therapeutic effects of naringenin against SARS-CoV-2 have been recently reviewed (26), pointing out that it may exert therapeutic effects against COVID-19 through the inhibition of the main protease 3CLpro and the reduction of ACE2 activity. Moreover, one additional mechanism by which this flavanone can counteract the effects of SARS-CoV-2 infection can be attributed to the attenuation of inflammatory responses.
Hesperetin
Another flavonone similar to naringenin is hesperetin (Table 1), mainly found in the glycoside form (hesperidin) in citrus fruits, where it is particularly abundant in the peel and in the white part of the fruit. Therefore, the consumption of the whole fruit would ensure a greater intake than the juice alone (88, 89). As recently reviewed (90), the content of hesperidin for 100 mL of juice varies according to the fruit: in oranges it ranges from 20 to 60 mg, in lemons from 4 to 41 mg, in tangerines the content is between 8 and 46 mg, while in grapefruit it is lower (2–17 mg). Among all flavonoids, researchers have recently focused the attention on hesperidin because the low binding energy of hesperidin to the spike glycoprotein and to the protease 3CLpro suggests an effective antiviral action (29). In addition, hesperidin is considered an important antioxidant compound (29), able to counteract the damaging effects of oxygen free radicals, triggered by infection and inflammation.
Other Aromatic Compounds
Curcumin
Curcumin is a natural phenolic compound found in turmeric (Curcuma longa L.), a plant native to India and Southeast Asia where curcumin is used as a traditional medicine to treat various disorders. In Europe, this molecule is used as a food dye for its yellow color and it is classified as a food additive. Curcumin is characterized by multiple beneficial properties, acting as anti-inflammatory, antineoplastic, antiangiogenic, but also as an antiviral (influenza virus, hepatitis C virus, HIV), antibacterial (Streptococcus spp., Staphylococcus spp. and Pseudomonas spp.) and antifungal (Candida spp., Aspergillus spp., Cryptococcus spp., and Dermatophytes spp.) natural compound (91). It is active against various human viruses, bacteria and fungi. Nowadays, foods with high curcumin content have been evaluated as SARS-CoV-2 inhibitors (Table 1). Despite its poor bioavailability, some nanoparticle-based approaches have recently been developed (92–94). Furthermore, it was shown that different compounds can increase curcumin bioavailability. In particular, when combined with piperine, the major active component of black pepper, curcumin can increase its bioavailability as much as 20-fold (95).
Phloretin
Another natural phenol is phloretin (Table 1). Phloretin is a dihydrochalcone and phlorizin is its main glucoside. Both compounds are naturally present in apples, kumquat, pear, strawberry, and vegetables (96, 97). Phloretin is a flexible molecule able to efficiently bind biological macromolecules. It is endowed with antiviral as well as anticancer, antifungal, anti-inflammatory, and antibacterial properties, thus conferring important health-beneficial effects (32). Furthermore, this compound can increase the fluidity of membranes and enhance the penetration of administered drugs into cells (98, 99).
EGCG
An additional compound under research for its beneficial properties on human health which may be interesting under the antiviral activity aspect is epigallocatechin gallate (EGCG) (Table 1). EGCG is a type of catechin and it is abundant in green tea (100), while small quantities are also present in onions, plums, and apple skin. EGCG is a strong antioxidant and antitumor molecule and has the potential to prevent and counteract several human diseases with chronic metabolic and inflammatory components, such as diabetes, stroke, obesity, Parkinson's, and Alzheimer's diseases (101–103). Probably due to its ability to interact with DNA methyltransferases (DNMT), ACE-2 and helicase, EPGCG is also an antiviral molecule able to counteract diseases caused by a wide variety of viruses: herpes simplex virus (HSV), human papillomavirus (HPV), adenovirus, hepatitis B and C viruses (HBV and HCV, respectively), dengue virus (DENV), Zika virus (ZIKV), West Nile viruses (WNV), Chikungunya virus (CHIKV), Ebola virus (EBOV), human immunodeficiency virus (HIV), and influenza virus (104–108).
Sulfur Compounds
Sulforaphanes
Sulforaphanes are not phenolic compounds, but possess antiviral potential. They belong to the isothiocyanate group of nitrogen-containing plant secondary metabolites and are classified as sulfur compounds (Table 1). Sulforaphanes are stored as glucoraphanin, their inactive form (109). This natural compound is principally found in cruciferous vegetables (such as broccoli), is used in prevention and support of chronic diseases and is supposed to be involved in human aging (110). Moreover, it has been suggested that sulforaphane, like other natural phytochemicals, may be used in SARS-CoV-2 treatment (36). Cruciferous plants are able to release glucoraphanin, converted by the plant into sulforaphane, which in turn activates Nrf2 (111), an important transcription factor that induces an antiviral action and prevents oxidative stress (112). Nrf2 activity decreases with age, causing the elderly to be more susceptible to oxidative stress-mediated diseases (36).
Strategies to Obtain Flavonol Rich Foods
To our knowledge, caper (Capparis spinosa L.) is one of the edible species capable of accumulating the highest levels of quercetin (quercetin-3-rutinoside, named rutin), a flavonol compound with various curative properties (113, 114). Another plant capable of accumulating high levels of rutin in the seed is buckwheat (Fagopyrum spp.) and in particular Fagopyrum tataricum Gaertn that, compared to Fagopyrum esculentum Moench, is able to accumulate 40–50 × higher amounts of rutin (115, 116). In both cases this flavonol is synthesized via the flavonoid biosynthetic pathways where the main genes are PAL, C4H, 4CL, CHS, CHI, F3′H, F3H, FLS, and UFGT (Figure 2); these genes are very highly conserved among different plants and after the first characterization in maize they were studied in different species such as Arabidopsis, petunia, snapdragon and buckwheat (42, 117, 118). Therefore, it seems that the ability to accumulate large quantities of quercetin is determined by the inactivity of a key gene for anthocyanin biosynthesis, the DFR gene. In fact, the activity of DFR would lead to the synthesis of anthocyanins and/or phlobaphenes by subtracting the common precursor naringenin (Figure 2). As a consequence, both caper and buckwheat are not able to accumulate large amounts of anthocyanins in their tissues, as can also be observed for example in flowers that have colorless or weakly pigmented petals. To strengthen this hypothesis, the mutation in anthocyaninless1 a1 (DFR) maize gene, in a genetic background prone to anthocyanin synthesis, has been reported to cause suppression of anthocyanin production followed by an accumulation of quercetin in the aleurone layer conferring a brownish color (119) (Figure 3). Hence the strategies that can be used to increase the flavanone content in food can be summarized as below:
1) the rediscovery of traditional varieties (landraces) naturally rich in these molecules.
2) the use of classical breeding techniques to specifically drive the accumulation of these molecules.
3) a biotechnological approach such as CRISPR/Cas9 to inactivate the DFR gene in pigmented varieties.
All these methods can be used in synergy to increase the flavonol content in foods.
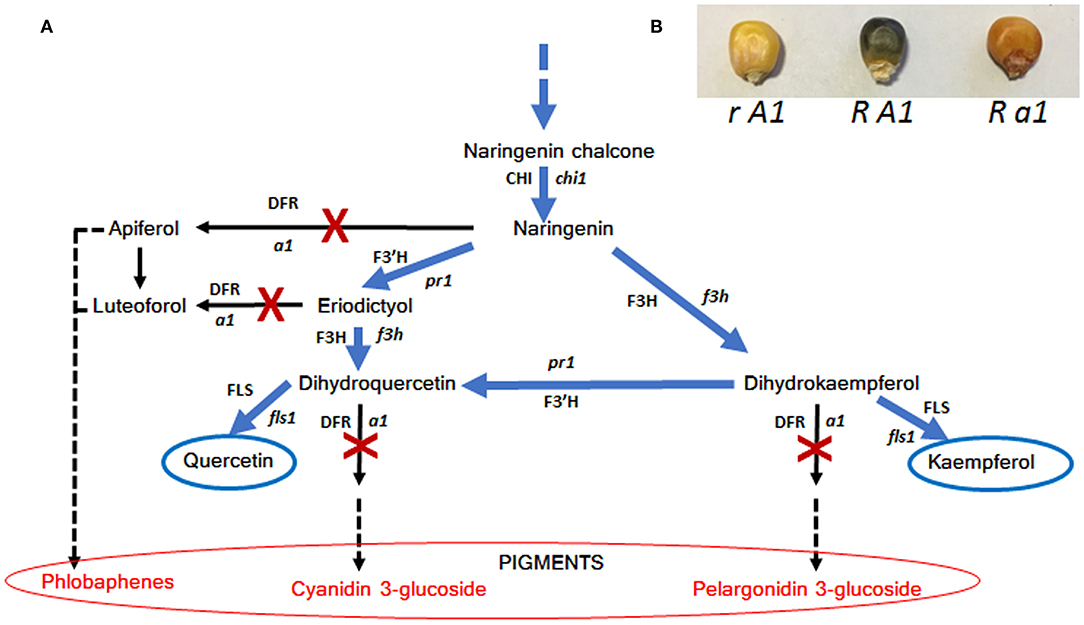
Figure 3. Schematic representation of how plants accumulate quercetin and kaempferol by stopping the activity of DFR (A) and (B) effect of a1 (DFR) mutation on maize seed pigmentation. Genes (enzymes) are abbreviated as follows: c2 (CHS), chalcone synthase; chi1 (CHI), chalcone isomerase; f3h (F3H), flavanone 3-hydroxylase; pr1 (F3′H), flavonone 3′-hydroxylase; a1 (DFR), dihydroflavonol synthase. In (B) R represent red color 1, a regulatory gene conferring anthocyanin pigmentation in the aleurone seed layer.
The species/varieties rich in flavanones such as quercetin or naringenin will be described in the next paragraph. The strategy 1 is the simplest and most user-friendly since it is based on the selection of pre-existing varieties, while the second strategy, concerning breeding techniques, takes several years, and the third, based on the novel NBTs (new breeding techniques), currently has to comply with the same European regulations as those for GMOs (120).
Considering corn as a case study, both traditional and new corn varieties obtained by breeding (121–123) are rich in flavonoids, mainly anthocyanins and phlobaphenes [reviewed by (42, 124, 125)]. These varieties, in addition to being rich in anthocyanin pigments, are able to accumulate discrete quantities of flavonols as previously reported by Lago et al. (124), Cassani et al. (125), and Landoni et al. (126). However, in order to further increase significantly the content of flavonols (in particular quercetin), it would be enough to introduce the a1 mutation by recurrent backcrossing.
Varieties Rich in Flavonoids
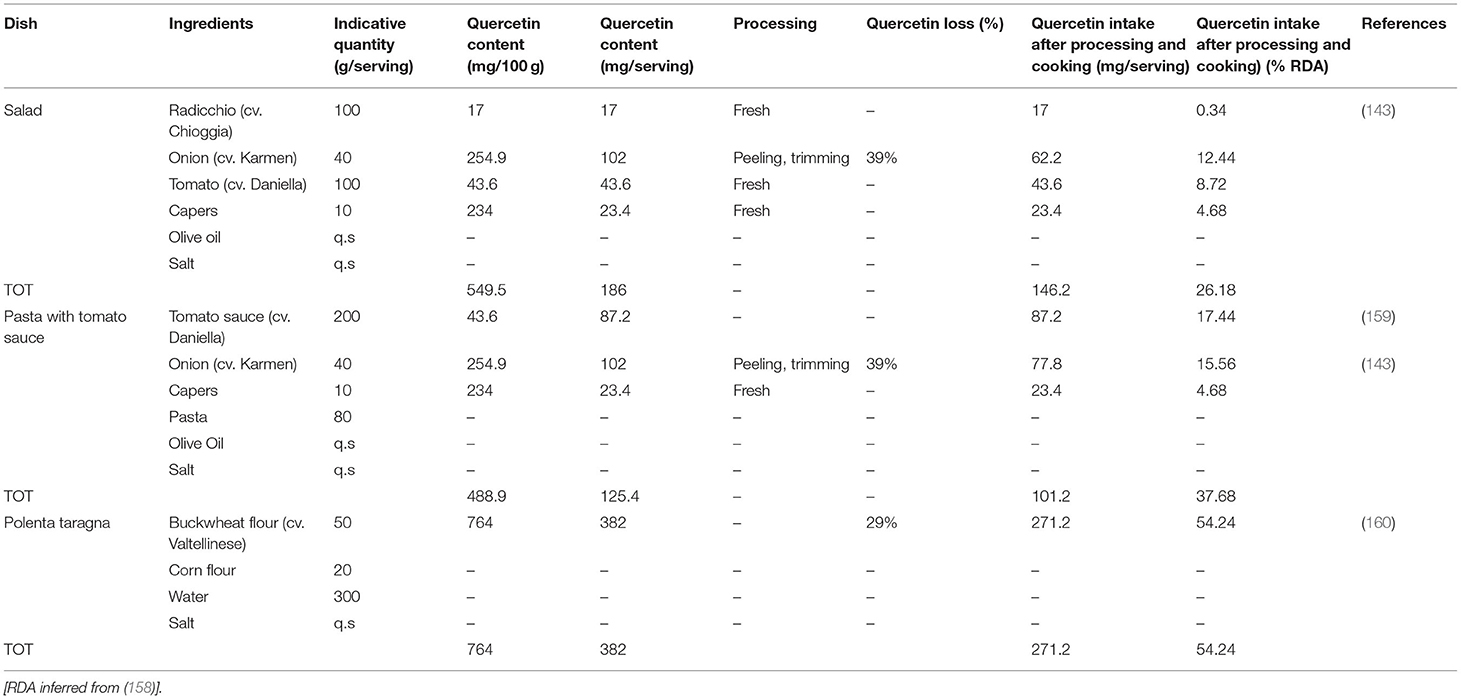
Many cultivated plants are rich in bioactive compounds and mineral elements with potential health benefits (127). A strong variability in the flavonoid content is present among different species and varieties. In Supplementary Table 1 we present different cultivars of fruits and vegetables highlighting the different contents of quercetin, kaempferol, hesperidin and naringenin which have been found in them.
Regarding quercetin, as already mentioned, capers are the richest food, reaching 2,340 mg/kg (20). Onions are also a good source of quercetin: white varieties had a level of 900–1,830 mg/kg, while the red variety Karmen had a content of over 2,500 mg/kg (128). Although pink onions were also rather rich in this compound, the registered level was lower compared to the others. Quercetin is also present in lovage and dill, two aromatic herbs widely used in cooking (129). In Bronte pistachios, quercetin is mainly contained in the skin, so the availability during consumption is relatively low, considering that the peel is only 10% of the whole nut (130). A comparison of the phenolic profiles from available literature data about radicchio (Cichorium intybus) cultivars allowed the selection of three autochthonous varieties (“Verdon da Cortèl,” “Treviso Precoce,” “Chioggia”) cultivated in the Veneto region of Italy. The variability between cultivars ranged from 40 to 250 mg/kg) (131). The most abundant forms of quercetin present in radicchio are quercetin glucuronide (3-O; 7-O) and quercetin-3-O-glucoside. Furthermore, the glycoside form of quercetin (rutin, quercetin-3-O-rutinoside) is present in buckwheat and asparagus. The selected varieties of buckwheat contained rutin in concentrations ranging from 590 to 769 mg/kg (116). A great intraspecific variability was observed in asparagus: the highest rutin values were recorded in green cultivars (119–163 mg/kg), while the lowest in the white (2 mg/kg). Purple varieties had intermediate levels of rutin (15–20 mg/kg) (132).
As to the other flavonol, kaempferol, it is present in its aglycone form in saffron (2,050 mg/kg) (133) and in its glycosylated forms in capers and radicchio (23, 131, 134).
The flavonones hesperetin and naringenin are present in citrus fruits in their glycosylated forms hesperidin and naringin (Supplementary Table 1). They are mainly found in the peel and albedo (white part) of citrus fruits at higher values compared to the juice. In fact, the consumption of fresh fruit allows a greater intake (29). The highest hesperidin levels in 100 mL of juice were recorded in oranges (20–60 mg), but also in mandarins and clementines (8–46 mg) (29). According to Alam et al., the concentration of naringenin in 100 mL of mandarin juice was 300 mg (135). The concentration of this compound in mandarins was 10 times higher compared to grapefruit and over a hundred times higher than that of oranges. In tomatoes, three cultivars appeared to have the highest naringin concentrations: Daniella 12.55 mg/kg, Ramillete 8.14 mg/kg and Canario 8.46 mg/kg. Moreover, these varieties also appeared to have also the highest values of the flavonol quercetin (136).
Dishes Rich in Flavonoids: Bioavailability and Cooking Processes
Establishing the bioavailability of bioactive compounds is a fundamental step in determining the effects of phytochemicals on human health (137). From a nutritional point of view, bioavailability is defined as the fraction of a given molecule contained in food that the body can utilize (138, 139). Bioavailability is the result of different processes: digestion, absorption, metabolism and elimination of a compound after food ingestion (137). Flavonoids are first metabolized by phase I and phase II metabolism which take place in the gastrointestinal tract and liver, and subsequently by microbial metabolism in the colon (140). Finally, urinary excretion and plasma concentrations of flavonols in humans could be used in epidemiological studies as biomarkers of intake (141). However, the bioavailability of these compounds varies between individuals and many factors such as age, sex, genotype, but also food composition may affect these metabolic processes (140).
Although some vegetables mentioned in Supplementary Table 1 can be used as fresh products, the majority of flavonoid rich foods require a cooking process before being consumed (142). Such a process can modify the chemical-physical properties of any phytochemical as well as its bioavailability. The first losses of flavonoids may occur in the pre-cooking stages in cases where parts of the product are removed. Onion peeling resulted in a 39% loss of flavonoids and asparagus chopping produced an 18.5% decrease of the rutin content (143, 144). Considerable losses were also recorded in the peeling and dicing of tomatoes (145). Thermal processes (blanching, drying, pasteurization, microwaving etc) also have an impact on the flavonoid content which depends on the magnitude and duration of heating (142). The data reported by Ioannou and Ghoul revealed a different sensitivity to heat treatment for the different flavonoids in aqueous solution. In general, a significant degradation was observed for temperatures above 100°C (142). A higher stability compared to the aglycon form was however observed in rutin (146, 147). During boiling, a fraction of the flavonoids is released into the cooking water causing losses of 20.5% for onions and 43.9% for asparagus (144). Furthermore, onion frying processes diminish flavonoid content (25–33%) (148, 149), while microwaving and steaming do not have a significant effect (149, 150). In contrast, baking increases the total flavonol content in onions (7%) (150). The degradation of flavonoids depends also on other factors such as pH and the presence or absence of oxygen. In fact, the presence of oxygen accelerates the degradation of rutin and quercetin due to the formation of ROS (146, 147).
Ioannou et al. showed the effects of temperature, oxygen and light on naringin content (151). This compound is degraded at temperatures above 100°C, with an oxygen content over 85% or upon exposure to light. In fact, a decrease in the naringin content was recorded by applying 108°C (400 W microwave power). However, by setting the extraction temperature at 80°C, an increase in naringin was observed (151).
A mixture of flavonoids-rich foods is present in the sofrito, widely used in the Mediterranean diet (152). Sofrito is composed of several ingredients rich in phenolic compounds, such as tomatoes, onions and olive oil (153). Naringenin is the main flavonone present in fresh tomatoes and tomato sauces (154, 155) and its content in sofrito is higher compared to tomatoes. By adding 120 g of sofrito to different dishes (such as pasta), a phenolic compound intake of 15–25 mg occurs (152). Adding capers to the sofrito could be a good strategy to increase the content of quercetin and kaempferol. If we consider the possible daily intake of 20 capers (10 g), this will provide from 20 to 80 mg of quercetin (23).
In this context, the varieties richest in these bioactive natural compounds can be implemented in human diet in order to try to protect to some extent from COVID-19.
Considering only quercetin, one of the most abundant dietary flavonoids with a daily average intake of 25–50 g (156), several papers cited in the flavonoid section of this review claimed its physiological effects on inflammation and immune function in murine systems [reviewed by Li et al., (157)]. These effects are linked to a daily intake ranging from 10 to 160 mg per kg. In particular, considering the effect on pro-inflammatory and anti-inflammatory cytokines production, linked to the response to virus infection, the dosage effective on the rat system was reported to be about 10–20 mg/kg (158). From these data we could infer a recommended human daily intake of about 500 mg, considering an average body weight of 65 kg. Several dishes rich in quercetin could fully satisfy this recommended daily intake, with a possible positive effect on human health (Table 2).
Conclusions and Future Perspectives
This survey of the properties of several phytochemicals present in edible organs of many cultivated plants appears to support the concept that people hit all over the world by the COVID-19 pandemic can rely on a very easily usable tool that may contribute to prevent the disease and/or decrease its severe effects which are causing so much lethality. This tool is simply the integration into our diet of the natural foods which selectively implement the daily intake of a few bioactive phytochemicals proven to possess properties which provide some degree of protection against COVID-19. Such natural compounds have in fact been shown to be able to put in place mechanisms of prevention and/or even inhibition of viral infection/replication. The plant derived molecules of anti-Covid interest belong mostly but not exclusively to the chemical class of the flavonoids. In particular, the flavonol quercetin and the flavanones naringenin and hesperidin appear the best candidates to play the role of anti-Covid shelters, particularly because of their ubiquitous spread in many edible fruit and vegetables of large consumption in which they can be found at high levels. Curcumin, a phenolic compound present in Curcuma longa roots is another phytochemical of interest because it is largely extracted and used as a food dye, so it can be easily integrated into the diet. Among other phenolics displaying antiviral activity, phloretin is also to be taken into account as an anti-Covid shelter because it is rather ubiquitous in vegetables and fruits (among them, apples and pears) and epigallocatechin gallate (present mostly in green tea, onion, plum, apple skin) because, besides being endowed with antiviral properties, it displays potential for the mitigation of diseases characterized by a chronic inflammatory component. Finally, the sulfur compounds sulforaphanes, diffused in cruciferous plants such as broccoli, may be antiviral shelters of particular interest because their action relies on the activation of transcription factors which in turn switches on cell mechanisms responsible for antiviral effects. The major future perspectives for enhancing and diffusing the above cited, already known and highly desirable plant-derived biochemical weapons to fight COVID-19, can be summarized in three different strategies and some examples of each of them are reported in the preceding paragraphs. The first one consists in the rather easily doable rediscovery of ancient varieties naturally rich in these molecules; as concerns this approach, it is known that traditional varieties/ecotypes are often richer in phytonutrients than the newly synthesized varieties where the improvement was mainly focused on yield (116, 125, 161). The second strategy is the use of classic genetic improvement techniques to enhance specific accumulation of a given antiviral phytochemical. The third is the use of biotechnological approaches, nowadays available and quite effective, such as CRISPR/Cas9, which are able to activate or, conversely, to inactivate genes involved in the synthesis of specific antiviral phytochemicals leading to the accumulation of specific compounds.
Moreover, in order to modulate and optimize the “functional diet,” it will be necessary to further increase information concerning the actual levels each phytochemical reaches in the blood following intake of food or of nutraceutical preparations endowed with anti-Covid potential. In this regard, in cases in which fruits or vegetables containing the anti-Covid phytochemical must be cooked to be consumed, it will be also necessary to investigate more accurately and extensively the fate of these molecules during the cooking process and determine their absorption rate and extent.
It is probable that in the next decades many other phytochemicals capable of fighting human viral diseases will be found in the edible parts of plants and thoroughly characterized, since much research is at present under way to achieve this goal. Indeed, it seems that nowadays there is an increasing tendency to prefer or juxtapose to the pharmaceutical therapies, preventive or (more rarely) curative treatments based on bioactive nutraceuticals extracted from plants. So, it does not seem impossible that in the future, whenever possible, many diseases will be fought more “naturally” through a more focused and specific education of people's diet.
Author Contributions
RP, EN, and ML contributed to conception and design of the study. MG, FC, SS, and ML wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was partially supported by MIND FoodS Hub funded by the European Regional Development Fund under the ROP of the Lombardy Region ERDF 2014–2020—Axis I Strengthen technological research, development and innovation—Action1.b.1.3 Support for co-operative R&D activities to develop new sustainable technologies, products and services—Call Hub.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.661331/full#supplementary-material
References
1. Dai W, Zhang B, Su H, Li J, Zhao Y, Xie X, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. (2020) 368:1331–5. doi: 10.1126/science.abb4489
2. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
3. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. (2020) 579:265–9. doi: 10.1038/s41586-020-2008-3
4. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
5. World Health Organization. Coronavirus Disease (COVID-19) Pandemic. (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed March 25, 2020)
6. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. (2015) 1282:1–23. doi: 10.1007/978-1-4939-2438-7_1
7. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. (2019) 16:1–22. doi: 10.1186/s12985-019-1182-0
8. Zhong NS, Zheng BJ, Li YM, Poon LLM, Xie ZH, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. (2003) 362:1353–8. doi: 10.1016/S0140-6736(03)14630-2
9. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARSCoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
10. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
11. The Lancet. Redefining vulnerability in the era of COVID-19. Lancet. (2020) 395:1089. doi: 10.1016/S0140-6736(20)30757-1
12. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. (2020) 81:537–40. doi: 10.1002/ddr.21656
13. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. (2020) 5:562–9. doi: 10.1038/s41564-020-0688-y
14. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. (2020) 9:231. doi: 10.3390/pathogens9030231
15. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. (2020) 367:1260–3. doi: 10.1126/science.abb2507
16. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. (2020) 63:457–60. doi: 10.1007/s11427-020-1637-5
17. Adedeji AO, Marchand B, Te Velthuis AJW, Snijder EJ, Weiss S, Eoff RL, et al. Mechanism of nucleic acid unwinding by SARS-CoV helicase. PLoS ONE. (2012) 7:e36521. doi: 10.1371/journal.pone.0036521
18. Russo M, Moccia S, Spagnuolo C, Tedesco I, Russo GL. Roles of flavonoids against coronavirus infection. Chem Biol Interact. (2020) 328:109211. doi: 10.1016/j.cbi.2020.109211
19. Solnier J, Fladerer JP. Flavonoids: a complementary approach to conventional therapy of COVID-19? Phytochem Rev. (2020) 1–23. doi: 10.1007/s11101-020-09720-6
20. Derosa G, Maffioli P, D'Angelo A, Di Pierro. F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. (2020) 2020:1–7. doi: 10.1002/ptr.6887
21. Wojdyło A, Nowicka P, Grimalt M, Legua P, Almansa MS, Amorós A, et al. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants. (2019) 8:539. doi: 10.3390/plants8120539
22. Holasova M, Fiedlerova V, Smrcinova H, Orsak M, Lachman J, Vavreinova S. Buckwheat—the source of antioxidant activity in functional foods. Int Food Res J. (2002) 35:207–11. doi: 10.1016/S0963-9969(01)00185-5
23. Inocencio C, Rivera D, Alcaraz F, Tomás-Barberán FA. Flavonoid content of commercial capers (Capparis spinosa, sicula C. and C. orientalis) produced in Mediterranean countries. Eur Food Res Technol. (2000) 212:70–4. doi: 10.1007/s002170000220
24. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem. (2011) 11:298–344. doi: 10.2174/138955711795305335
25. Ahmadian R, Rahimi R, Bahramsoltani R. Kaempferol: an encouraging flavonoid for COVID-19. Bol latinoam Caribe Plantas Med Aromát. (2020) 19:492–5. doi: 10.37360/blacpma.20.19.5.33
26. Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh-Attar MJ. Naringenin, a flavanone with antiviral and anti-inflammatory effects: a promising treatment strategy against COVID-19. Phytother Res. (2020) 34:3137–47. doi: 10.1002/ptr.6781
27. Ranka S, Gee JM, Biro L, Brett G, Saha S, Kroon P, et al. Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake. Eur J Clin Nutr. (2008) 62:1131–8. doi: 10.1038/sj.ejcn.1602827
28. Wilcox LJ, Borradaile NM, Huff MW. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc Drug Rev. (1999) 17:160–78. doi: 10.1111/j.1527-3466.1999.tb00011.x
29. Bellavite P, Donzelli A. Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits. Antioxidants. (2020) 9:742. doi: 10.3390/antiox9080742
30. Haslberger A, Jacob U, Hippe B, Karlic H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: mini review. Funct Food Health Dis. (2020) 10:195–209. doi: 10.31989/ffhd.v10i5.707
31. Liu Z, Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front Cell Dev Biol. (2020) 8:479. doi: 10.3389/fcell.2020.00479
32. Behzad S, Sureda A, Barreca D, Nabavi SF, Rastrelli L, Nabavi SM. Health effects of phloretin: from chemistry to medicine. Phytochem Rev. (2017) 16:527–33. doi: 10.1007/s11101-017-9500-x
33. Zielinska D, Laparra-Llopis JM, Zielinski H, Szawara-Nowak D, Giménez-Bastida JA. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients. (2019) 11:1173. doi: 10.3390/nu11051173
34. Menegazzi M, Campagnari R, Bertoldi M, Crupi R, Di Paola R, Cuzzocrea S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: could such a scenario be helpful to counteract COVID-19? Int J Mol Sci. (2020) 21:5171. doi: 10.3390/ijms21145171
35. Hung WL, Wang S, Sang S, Wan X, Wang Y, Ho CT. Quantification of ascorbyl adducts of epigallocatechin gallate and gallocatechin gallate in bottled tea beverages. Food Chem. (2018) 261:246–52. doi: 10.1016/j.foodchem.2018.04.050
36. Bousquet J, Anto J, Czarlewski W, Haahtela T, Fonseca S, Iaccarino G, et al. Sulforaphane: from death rate heterogeneity in countries to candidate for prevention of severe COVID-19. Authorea. (2020) 14:100498. doi: 10.22541/au.159493397.79345039
37. Thaler R, Maurizi A, Roschger P, Sturmlechner I, Khani F, Spitzer S, et al. Anabolic and antiresorptive modulation of bone homeostasis by the epigenetic modulator sulforaphane, a naturally occurring isothiocyanate. J Biol Chem. (2016) 291:6754–71. doi: 10.1074/jbc.M115.678235
38. Tapas DA, Sakarkar DM, Kakde R. Flavonoids as nutraceuticals: a review. Trop J Pharm Res. (2008) 7:1090–9. doi: 10.4314/tjpr.v7i3.14693
39. Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J. Crohns Colitis. (2020) 14:1334–6. doi: 10.1093/ecco-jcc/jjaa061
40. Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: Combining antiviral and antiinflammatory treatments. Lancet Infect Dis. (2020) 20:400–2. doi: 10.1016/S1473-3099(20)30132-8
41. Sharma M, Chai C, Morohashi K, Grotewold E, Snook ME, Chopra S. Expression of flavonoid 30-hydroxylase is controlled by P1, the regulator of 3-deoxyflavonoid biosynthesis in maize. BMC Plant Biol. (2012) 12:196. doi: 10.1186/1471-2229-12-196
42. Petroni K, Pilu R, Tonelli C. Anthocyanins in corn: a wealth of genes for human health. Planta. (2014) 240:901–911. doi: 10.1007/s00425-014-2131-1
43. Pilu R, Piazza P, Petroni K, Ronchi A, Martin C, Tonelli C. pl-bol3, a complex allele of the anthocyanin regulatory pl1 locus that arose in a naturally occurring maize population. Plant J. (2003) 36:510–21. doi: 10.1046/j.1365-313X.2003.01898.x
44. Pilu R, Bucci A, Casella L, Lago C, Badone FC, Cassani E, et al. A quantitative trait locus involved in maize yield is tightly associated to the r1 gene on the long arm of chromosome 10. Mol Breed. (2012) 30:799–807. doi: 10.1007/s11032-011-9664-9
45. Dooner HK, Robbins TP, Jorgensen RA. Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet. (1991) 25:173–99. doi: 10.1146/annurev.ge.25.120191.001133
46. Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol. (1998) 439:191–225. doi: 10.1007/978-1-4615-5335-9_15
47. D'Amelia V, Aversano R, Chiaiese P, Carputo D. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem Rev. (2018) 17:611–25. doi: 10.1007/s11101-018-9568-y
48. LeJeune TM, Tsui HY, Parsons LB, Miller GE, Whitted C, Lynch, et al. Mechanism of action of two flavone isomers targeting cancer cells with varying cell differentiation status. PLoS ONE. (2015) 10:e0142928. doi: 10.1371/journal.pone.0142928
49. Abreu AC, Coqueiro A, Sultan AR, Lemmens N, Kim HK, Verpoorte R, et al. Looking to nature for a new concept in antimicrobial treatments: isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci Rep. (2017) 7:3777. doi: 10.1038/s41598-017-03716-7
50. Catarino MD, Talhi O, Rabahi A, Silva AMS, Cardoso SM. The antiinflammatory potential of flavonoids. Stud Nat Prod Chem. (2016) 48:65–99. doi: 10.1016/B978-0-444-63602-7.00003-5
51. Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. (2020) 16:1708–17. doi: 10.7150/ijbs.45538
52. Nileeka Balasuriya BW, Vasantha Rupasinghe HP. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct Food Health Dis. (2011) 1:172–88. doi: 10.31989/ffhd.v1i5.132
53. Guerrero L, Castillo J, Quinones M, Garcia-Vallve, ' S, Arola L, Pujadas G, et al. Inhibition of angiotensinconverting enzyme activity by flavonoids: structure-activity relationship studies. PLoS ONE. (2012) 7:e49493. doi: 10.1371/journal.pone.0049493
54. Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. (2006) 54:229–34. doi: 10.1021/jf052263o
55. Chen L, Li J, Luo C, Liu H, Xu W, Chen G, et al. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. (2006) 14:8295–306. doi: 10.1016/j.bmc.2006.09.014
56. Jo S, Kim H, Kim S, Shin DH, Kim M.-S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. (2019) 94:2023–30. doi: 10.1111/cbdd.13604
57. Wang X, Yang Z, Su F, Li J, Boadi EO, Chang YX, et al. Study on structure activity relationship of natural flavonoids against thrombin by molecular docking virtual screening combined with activity evaluation in vitro. Molecules. (2020) 25:422. doi: 10.3390/molecules25020422
58. McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. (2020) 157:104859. doi: 10.1016/j.phrs.2020.104859
59. Srinivas NR. Structurally modified 'dietary flavonoids': are these viable drug candidates for chemoprevention? Curr Clin Pharmacol. (2009) 4:67–70. doi: 10.2174/157488409787236065
60. Ajazuddin SS. Applications of novel drug delivery system for herbal formulations. Fitoterapia. (2010) 81:680–9. doi: 10.1016/j.fitote.2010.05.001
61. Zhao J, Yang J, Xie Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int J Pharm. (2019) 570:118642. doi: 10.1016/j.ijpharm.2019.118642
62. Mehany T, Khalifa I, Barakat H, Althwab SA, Alharbi YM, El-Sohaimy S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. (2021) 40:100891. doi: 10.1016/j.fbio.2021.100891
63. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. (2009) 2:270–8. doi: 10.4161/oxim.2.5.9498
64. Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. (2016) 10:84–9. doi: 10.4103/0973-7847.194044
65. Lakhanpal P, Rai DK. Quercetin: A versatile flavonoid. Internet J Med Update. (2007) 2:22–37. doi: 10.4314/ijmu.v2i2.39851
66. Al Shukor N, van Camp J, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, et al. Angiotensinconverting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J Agric Food Chem. (2013) 61:11832–9. doi: 10.1021/jf404641v
67. Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, Ceballos-Laita L, Vega S, Hugh T, et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. (2020) 164:1693–703. doi: 10.1016/j.ijbiomac.2020.07.235
68. Riaz A, Rasul A, Hussain G, Zahoor MK, Jabeen F, Subhani Z, et al. Astragalin: a bioactive phytochemical with potential therapeutic activities. Adv Pharmacol Sci. (2018) 2018:9794625. doi: 10.1155/2018/9794625
69. Kotani M, Matsumoto M, Fujita A, Higa S, Wang W, Suemura M, et al. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin Immunol. (2000) 106:159–66. doi: 10.1067/mai.2000.107194
70. Bitis L, Kultur S, Melikoglu G, Ozsoy N, Can A. Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat Prod Res. (2010) 24:580–9. doi: 10.1080/14786410903075507
71. Kim YH, Choi YJ, Kang MK, Park SH, Antika LD, Lee EJ, et al. Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. J Agric Food Chem. (2017) 65:836–45. doi: 10.1021/acs.jafc.6b05160
72. Burmistrova O, Quintana J, Díaz JG, Estévez F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. Cancer Lett. (2011) 309:71–7. doi: 10.1016/j.canlet.2011.05.018
73. Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am Heart J. (2014) 167:637–45. doi: 10.1016/j.ahj.2014.01.015
74. Felgines C, Texier O, Morand C, Manach C, Scalbert A, Régerat F, et al. Bioavailability of the flavanone naringenin and its glycosides in rats. Am J Physiol Gastrointest Liver Physiol. (2000) 279:1148–54. doi: 10.1152/ajpgi.2000.279.6.G1148
75. Manchope MF, Casagrande R, Verri WA Jr. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. (2017) 8:3766–7. doi: 10.18632/oncotarget.14084
76. Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun. (1999) 265:410–5. doi: 10.1006/bbrc.1999.1695
77. Zobeiri M, Belwal T, Parvizi F, Naseri R, Farzaei MH, Nabavi S, et al. Naringenin and its nano-formulations for fatty liver: cellular modes of action and clinical perspective. Curr Pharm. (2018) 19:196–205. doi: 10.2174/1389201019666180514170122
78. Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. (2007) 61:472–7. doi: 10.1038/sj.ejcn.1602543
79. Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. (2001) 131:235–41. doi: 10.1093/jn/131.2.235
80. Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. (2003) 22:561–8. doi: 10.1016/S0261-5614(03)00059-1
81. Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. (2009) 256:128–34. doi: 10.1016/j.tox.2008.11.012
82. Den Hartogh DJ, Tsiani E. Antidiabetic properties of naringenin: a citrus fruit polyphenol. Biomolecules. (2019) 9:1–21. doi: 10.3390/biom9030099
83. Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. (2010) 90:1238–44. doi: 10.1002/jsfa.3959
84. Da Pozzo E, Costa B, Cavallini C, Testai L, Martelli A, Calderone V, et al. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid Med Cell Longev. (2017) 2017:1–12. doi: 10.1155/2017/9536148
85. Rashmi R, Magesh SB, Ramkumar KM, Suryanarayanan S, SubbaRao MV. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol. (2018) 7:76–84.
86. Jayaraman J, Jesudoss VAS, Menon VP, Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. (2012) 22:568–76. doi: 10.3109/15376516.2012.707255
87. Hernandez-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. (2018) 24:1679–707. doi: 10.3748/wjg.v24.i16.1679
88. Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem. (2006) 70:178–92. doi: 10.1271/bbb.70.178
89. Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of citrus juices. Molecules. (2007) 12:1641–73. doi: 10.3390/12081641
90. Meneguzzo F, Ciriminna R, Zabini F, Pagliaro M. Review of evidence available on hesperidin-rich products as potential tools against COVID-19 and hydrodynamic cavitation-based extraction as a method of increasing their production. Processes. (2020) 8:549. doi: 10.3390/pr8050549
91. Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. (2019) 10:912. doi: 10.3389/fmicb.2019.00912
92. Sindhu K, Rajaram A, Sreeram K, Rajaram R. Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. RSC Adv. (2014) 4:1808–18. doi: 10.1039/C3RA45345F
93. Pietra R. C. C. D. S., Cruz RC, Melo CN, Rodrigues LB, Santos PC, et al. Evaluation of polymeric PLGA nanoparticles conjugated to curcumin for use in aPDT. Braz J Pharm Sci. (2017) 53. doi: 10.1590/s2175-97902017000216043
94. Trigo Gutierrez JK, Zanatta GC, Ortega ALM, Balastegui MIC, Sanit, á PV, Pavarina AC, et al. Encapsulation of curcumin in polymeric nanoparticles for antimicrobial photodynamic therapy. PLoS ONE. (2017) 12:e0187418. doi: 10.1371/journal.pone.0187418
95. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. (1998) 64:353–6. doi: 10.1055/s-2006-957450
96. Gosch C, Halbwirth H, Kuhn J, Miosic S, Stich K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. (2009) 176:223–31. doi: 10.1016/j.plantsci.2008.10.011
97. Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Kumquat (Fortunella japonica Swingle) juice: flavonoid distribution and antioxidant properties. Food Res Int. (2011) 44:2190–7. doi: 10.1016/j.foodres.2010.11.031
98. Rezk BM, Haenen GR, van der Vijgh WJ, Bast A. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem Biophys Res Commun. (2002) 295:9–13. doi: 10.1016/S0006-291X(02)00618-6
99. Nakamura Y, Watanabe S, Miyake N, Kohno H, Osawa T. Dihydrochalcones: evaluation as novel radical scavenging antioxidants. J Agric Food Chem. (2003) 51:3309–12. doi: 10.1021/jf0341060
100. Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea: a review. J Am Coll Nutr. (2006) 25:79–99. doi: 10.1080/07315724.2006.10719518
101. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, antioxidant functions. Crit Rev Food Sci Nutr. (2003) 43:89–143. doi: 10.1080/10408690390826464
102. Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. (2006) 66:2500–5. doi: 10.1158/0008-5472.CAN-05-3636
103. Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. (2008) 13:440–52. doi: 10.2741/2691
104. Kuzuhara T, Iwai Y, Takahashi H, Hatakeyama D, Echigo N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. (2009) 1:RRN1052. doi: 10.1371/currents.RRN1052
105. Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol. (2009) 123:459–65. doi: 10.1016/j.jaci.2008.12.024
106. Ciesek S. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. (2011) 54:1947–55. doi: 10.1002/hep.24610
107. Hartjen P, Frerk S, Hauber I, Matzat V, Thomssen A, Holstermann B, et al. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res Ther. (2012) 9:1–9. doi: 10.1186/1742-6405-9-2
108. Vázquez-Calvo A, de Oya NJ, Martín-Acebes MA, Garcia-Moruno E, Saiz JC. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile Virus, Zika Virus, Dengue Virus. Front Microbiol. (2017) 8:1314. doi: 10.3389/fmicb.2017.01314
109. Quirante-Moya S, Garcia-Ibanez P, Quirante-Moya F, Villano D, Moreno DA. The role of Brassica bioactives on human health: are we studying it the right way? Molecules. (2020) 25:1591. doi: 10.3390/molecules25071591
110. Houghton CA. Sulforaphane: Its “Coming of Age” as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxid Med Cell Longev. (2019) 2019:2716870. doi: 10.1155/2019/2716870
111. Jimenez-Osorio AS, Gonzalez-Reyes S, Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clin Chim Acta. (2015) 448:182–92. doi: 10.1016/j.cca.2015.07.009
112. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. (2018) 29:1727–45. doi: 10.1089/ars.2017.7342
113. Ghafar MFA, Prasad KN, Weng KK, Ismail A. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr J Biotechnol. (2010) 9:326–30.
114. Gull T, Sultana B, Bhatti IA, Jamil A. Antibacterial potential of Capparis spinosa and Capparis decidua extracts. Int J Agric Biol. (2015) 17:727–33. doi: 10.17957/IJAB/14.0007
115. Gupta N, Sharma SK, Rana JC, Chauhan RS. Expression of flavonoid biosynthesis genes vis-à-vis rutin content variation in different growth stages of Fagopyrum species. J Plant Physiol. (2011) 168:2117–23. doi: 10.1016/j.jplph.2011.06.018
116. Giupponi L, Borgonovo G, Panseri S, Giorgi A. Multidisciplinary study of a little known landrace of Fagopyrum tataricum Gaertn. of Valtellina (Italian Alps). Genet Resour Crop Evol. (2019) 66:783–96. doi: 10.1007/s10722-019-00755-z
117. Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. (2001) 126:485–93. doi: 10.1104/pp.126.2.485
118. Koja E, Ohata S, Maruyama Y, Suzuki H, Shimosaka M, Taguchi G. Identification and characterization of a rhamnosyltransferase involved in rutin biosynthesis in Fagopyrum esculentum (common buckwheat). Biosci Biotechnol Biochem. (2018) 82:1790–802. doi: 10.1080/09168451.2018.1491286
119. Reddy AR, Reddy GM. Genetic control of quercetin formation in the aleurone tissue of maize. Genetics. (1975) 81:287–92. doi: 10.1093/genetics/81.2.287
120. Vives-Vallés JA, Collonnier C. The judgment of the CJEU of 25 July 2018 on mutagenesis: interpretation and interim legislative proposal. Front Plant Sci. (2020) 10:1813. doi: 10.3389/fpls.2019.01813
121. Lago C, Landoni M, Cassani E, Doria E, Nielsen E, Pilu R. Study and characterization of a novel functional food: purple popcorn. Mol Breed. (2013) 31:575–85. doi: 10.1007/s11032-012-9816-6
122. Lago C, Cassani E, Zanzi C, Landoni M, Trovato R, Pilu R. Development and study of a maize cultivar rich in anthocyanins: coloured polenta, a new functional food. Plant Breed. (2014) 133:210–7. doi: 10.1111/pbr.12153
123. Lago C, Landoni M, Cassani E, Atanassiu S, Canta-Luppi E, Pilu R. Development and characterization of a coloured sweet corn line as a new functional food. Maydica. (2014) 59:191–200.
124. Lago C, Landoni M, Cassani E, Cantaluppi E, Doria E, Nielsen E, et al. Study and characterization of an ancient European flint white maize rich in anthocyanins: Millo Corvo from Galicia. PloS ONE. (2015) 10:e0126521. doi: 10.1371/journal.pone.0126521
125. Cassani E, Puglisi D, Cantaluppi E, Landoni M, Giupponi L, Giorgi A, et al. Genetic studies regarding the control of seed pigmentation of an ancient European pointed maize (Zea mays L.) rich in phlobaphenes: the “Nero Spinoso” from the Camonica valley. Genet Resour Crop Evol. (2017) 64:761–73. doi: 10.1007/s10722-016-0399-7
126. Landoni M, Puglisi D, Cassani E, Borlini G, Brunoldi G, Comaschi C, et al. Phlobaphenes modify pericarp thickness in maize and accumulation of the fumonisin mycotoxins. Sci Rep. (2020) 10:1417. doi: 10.1038/s41598-020-58341-8
127. Guarrera PM, Savo V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J Ethnopharmacol. (2013) 146:659–80. doi: 10.1016/j.jep.2013.01.036
128. Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. (2007) 55:10067–80. doi: 10.1021/jf0712503
129. Justesen U, Knuthsen P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. (2001) 73:245–50. doi: 10.1016/S0308-8146(01)00114-5
130. Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. (2010) 92:1115–22. doi: 10.1016/j.biochi.2010.03.027
131. Tardugno R, Pozzebon M, Beggio M, Del Turco P, Pojana G. Polyphenolic profile of Cichorium intybus L. endemic varieties from the Veneto region of Italy. Food Chem. (2018) 266: 175–82. doi: 10.1016/j.foodchem.2018.05.085
132. Kulczyński B, Kobus-Cisowska J, Kmiecik D, Gramza-Michałowska A, Golczak D, Korczak J. Antiradical capacity and polyphenol composition of asparagus spears varieties cultivated under different sunlight conditions. Acta Sci Pol Technol Aliment. (2016) 15:267–79. doi: 10.17306/J.AFS.2016.3.26
133. Carmona M, Sánchez AM, Ferreres F, Zalacain A, Tomás-Barberán F, Alonso GL. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: comparative study of samples from different geographical origins. Food Chem. (2007) 100:445–50. doi: 10.1016/j.foodchem.2005.09.065
134. Giuffrida D, Salvo F, Ziino M, Toscano G, Dugo G. Initial investigation on some chemical constituents of capers (Capparis spinosa L.) from the island of Salina. Ital. J Food Sci. (2002) 14:25–33.
135. Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. (2014) 5:404–417. doi: 10.3945/an.113.005603
136. Martínez I, Periago MJ, Provan G, Chesson A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicon esculentum). J Sci Food Agric. (2002) 82:323–30. doi: 10.1002/jsfa.1035
137. Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharm. (2013) 75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x
138. Benito P, Miller D. Iron absorption and bioavailability: an updated review. Nutr Res. (1998) 18:581–603. doi: 10.1016/S0271-5317(98)00044-X
139. Fernàndez-Garcìa E, Carvajal-Lérida I, Pérez-Gàlvez A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr Res. (2009) 29:751–60. doi: 10.1016/j.nutres.2009.09.016
140. Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. (2017) 105:10–22. doi: 10.3945/ajcn.116.136051
141. De Vries JH, Hollman PC, Meyboom S, Buysman MN, Zock PL, van Staveren WA, et al. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am J Clin Nutr. (1998) 68:60–5. doi: 10.1093/ajcn/68.1.60
142. Ioannou I, Ghoul M. Biological activities and effects of food processing on flavonoids as phenolic antioxidants. Adv Appl Microbiol. (2012) 101–24. doi: 10.5772/30690
143. Ewald C, Fjelkner-Moding S, Johansson K, Sjoholm I, Akesson B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. (1999) 64:231–5. doi: 10.1016/S0308-8146(98)00136-8
144. Makris DP, Rossiter JT. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): effect on flavonol content and antioxidant status. J Agric Food Chem. (2001) 49:3216–22. doi: 10.1021/jf001497z
145. Valverdú-Queralt A, Medina-Remón A, Andres-Lacueva C, Lamuela-Raventos RM. Changes in phenolic profile and antioxidant activity during production of diced tomatoes. Food Chem. (2011) 126:1700–7. doi: 10.1016/j.foodchem.2010.12.061
146. Makris DP, Rossiter JT. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (Quercetin 3-O-Rhamnosylglucoside) in aqueous model. J Agric Food Chem. (2000) 48:3830–8. doi: 10.1021/jf0001280
147. Buchner N, Krumbein A, Rhon S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun Mass Spectrom. (2006) 20:3229–35. doi: 10.1002/rcm.2720
148. Price KR, Bacon JR, Rhodes MJC. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J Agric Food Chem. (1997) 45:938–42. doi: 10.1021/jf9605916
149. Lee SU, Lee JH, Choi SH, Lee JS, Ohnisi-Kameyama M, Kozukue N, et al. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J Agric Food Chem. (2008) 56:8541–8. doi: 10.1021/jf801009p
150. Lombard K, Peffley E, Geoffriau E, Thompson L, Herring A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J Food Compos Anal. (2005) 18:571–81. doi: 10.1016/j.jfca.2004.03.027
151. Ioannou I, M'hiri N, Chaaban H, Boudhrioua NM, Ghoul M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. J Food Sci Technol. (2018) 53:2754–60. doi: 10.1111/ijfs.13887
152. Storniolo CE, Sacanella I, Mitjavila MT, Lamuela-Raventos RM, Moreno JJ. Bioactive compounds of cooked tomato sauce modulate oxidative stress and arachidonic acid cascade induced by oxidized LDL in macrophage cultures. Nutrients. (2019) 11:1880. doi: 10.3390/nu11081880
153. Valverdú-Queralt A, Rinaldi de Alvarenga JF, Estruch R, Lamuela-Raventos RM. Bioactive compounds present in the Mediterranean sofrito. Food Chem. (2013) 141:3365–72. doi: 10.1016/j.foodchem.2013.06.032
154. Yoshimura M, Sano A, Kamei JI, Obata A. Identification and quantification of metabolites of orally administered naringenin chalcone in rats. J Agric Food Chem. (2009) 57:6432–7. doi: 10.1021/jf901137x
155. Martínez-Huelamo M, Tulipani S, Estruch R, Escribano E, Ilan M, Corella D, et al. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: a pharmacokinetic study. Food Chem. (2015) 173:864–72. doi: 10.1016/j.foodchem.2014.09.156
156. Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. (1995) 33:1061–80. doi: 10.1016/0278-6915(95)00077-1
157. Li M, Zhu X, Tian J, Liu M, Wang G. Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquac Res. (2019) 501:473–81. doi: 10.1016/j.aquaculture.2018.12.011
158. Milenković M, Arsenović-Ranin N, Stojić-Vukanić Z, Bufan B, Vučićević D, Jančić I. Quercetin ameliorates experimental autoimmune myocarditis in rats. Int J Pharm Pharm Sci. (2010) 13:311–9. doi: 10.18433/J3VS3S
159. Bugianesi R, Catasta G, Spigno P, D'Uva A, Maiani G. Naringenin from cooked tomato paste is bioavailable in men. J Nutr. (2002) 132:3349–52. doi: 10.1093/jn/132.11.3349
160. Germ M, Árvay J, Vollmannová A, Tóth T, Golob A, Luthar Z, et al. The temperature threshold for the transformation of rutin to quercetin in Tartary buckwheat dough. Food Chem. (2019) 283:28–31. doi: 10.1016/j.foodchem.2019.01.038
161. Giupponi L, Pilu R, Scarafoni A, Giorgi A. Plant agro-biodiversity needs protection, study and promotion: results of research conducted in Lombardy region (Northern Italy). Biodivers Conserv. (2020) 29:409–30. doi: 10.1007/s10531-019-01889-3
Keywords: flavonol, flavanone, phytochemicals, functional foods, COVID-19, diet
Citation: Ghidoli M, Colombo F, Sangiorgio S, Landoni M, Giupponi L, Nielsen E and Pilu R (2021) Food Containing Bioactive Flavonoids and Other Phenolic or Sulfur Phytochemicals With Antiviral Effect: Can We Design a Promising Diet Against COVID-19? Front. Nutr. 8:661331. doi: 10.3389/fnut.2021.661331
Received: 30 January 2021; Accepted: 17 May 2021;
Published: 17 June 2021.
Edited by:
Elliot Berry, Hebrew University of Jerusalem, IsraelReviewed by:
Chayon Goswami, Bangladesh Agricultural University, BangladeshYoussef Aboussaleh, Ibn Tofail University, Morocco
Copyright © 2021 Ghidoli, Colombo, Sangiorgio, Landoni, Giupponi, Nielsen and Pilu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Pilu, c2FsdmF0b3JlLnBpbHVAdW5pbWkuaXQ=
†These authors have contributed equally to this work
 Martina Ghidoli
Martina Ghidoli Federico Colombo
Federico Colombo Stefano Sangiorgio1
Stefano Sangiorgio1 Michela Landoni
Michela Landoni Luca Giupponi
Luca Giupponi Erik Nielsen
Erik Nielsen Roberto Pilu
Roberto Pilu