- 1Department of Mathematics, Faculty of Science and Letters, Istanbul Technical University, Istanbul, Turkey
- 2Department of Industrial Engineering, Faculty of Engineering and Natural Sciences, Kadir Has University, Istanbul, Turkey
In the standard Susceptible-Infected-Removed (SIR) and Susceptible-Exposed-Infected-Removed (SEIR) models, the peak of infected individuals coincides with the inflection point of removed individuals. Nevertheless, a survey based on the data of the 2009 H1N1 epidemic in Istanbul, Turkey displayed a time shift between the hospital referrals and fatalities. An analysis of recent COVID-19 data and the records for Spanish flu (1918–1919) and SARS (2002–2004) epidemics confirm this observation. We use multistage SIR and SEIR models to provide an explanation for this time shift. Numerical solutions of these models present strong evidence that the delay between the peak of
1. Introduction
From the early attempts [1–4] to recent studies, epidemic modeling which is applicable in a wide range of fields from informatics [56] to chemistry [7–9] has drawn the attention of researchers in various disciplines. Since the basic compartmental model Susceptible-Infected-Removed (SIR) which is commonly used to model diseases for which the infection confers permanent immunity was introduced by Kermack and McKendrick in 1927 [4]; other compartmental models [10–12] have been developed to model diseases with different structures and dynamics. Especially in recent years, major outbreaks such as avian flu in 2005, swine influenza in 2006, and H1N1 influenza in 2009 have highlighted the need for more effective and reliable models to control the spread of disease and to provide a better knowledge for the prediction of future threats and for the development of stronger containment strategies. In Refs. [13–18], some results on the modeling of different types of epidemic diseases, the solution form of these models, the observation of global stability, and the determination of the final size of the epidemic are obtained. In Ref. [15], global stability criteria are derived for the SEIS model which can be regarded as a model with no immunity, and different SIR models are examined in Refs. [1617]. Moreover, the works [1718] provide some useful results on the final size of the epidemic for SIR models. With the same motivation of these works but rather a different contribution to literature, we use a multistage model [14] in this article to explain the time shift observed in several surveys such as Spanish flu (1918–1919) [19], SARS (2002–2004), the 2009 H1N1 in Istanbul, and recently, COVID-19 [20] (see Figures 1–3).
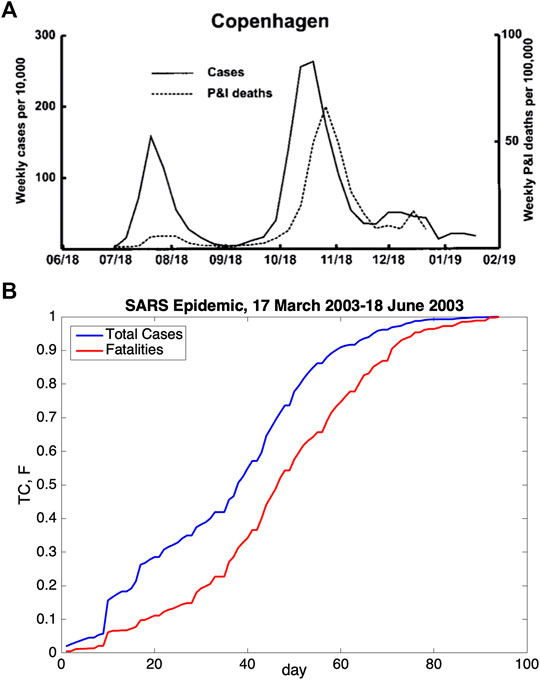
FIGURE 1. (A) Weekly number of influenza cases and respiratory deaths (pneumonia and influenza) in Copenhagen, Denmark, during 1918–1919 are presented. This figure was taken from the study of Andreasen et al. [19] (copyright Andreasen et al. [19]). A shift occurred between the maxima of curves. (B) Daily number of total cases and cumulative number of the fatalities for the 2003 SARS epidemic in the world are shown. The data provided by WHO were used in the generation of this figure (https://www.who.int/csr/sars/country/en/) (last access: September 15, 2020). An explicit shift was also observed for the SARS epidemic.
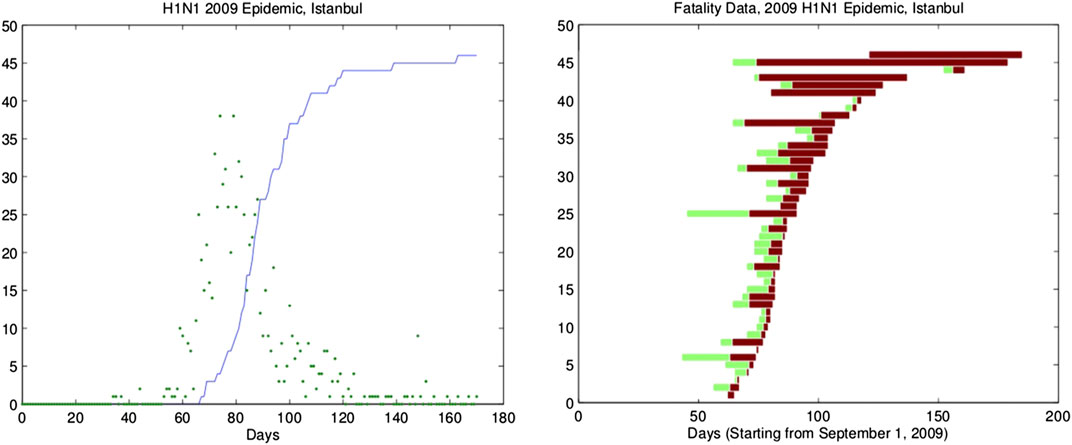
FIGURE 2. Daily number of referrals to hospitals and cumulative number of fatalities for the 2009 H1N1 epidemic in Istanbul, Turkey. The scatter in the number of daily referral to hospitals indicates that the hospitalization rate varies during the epidemic. The asymmetry of the incidence curve is still observable. The fatalities shown here are adjusted to at most 15 days after referral to the hospital (left panel). The duration of symptoms prior to hospitalization (light color) and the duration of hospitalization prior to death (dark color) for the fatalities in Istanbul, Turkey, due to 2009 H1N1 epidemic (right panel).
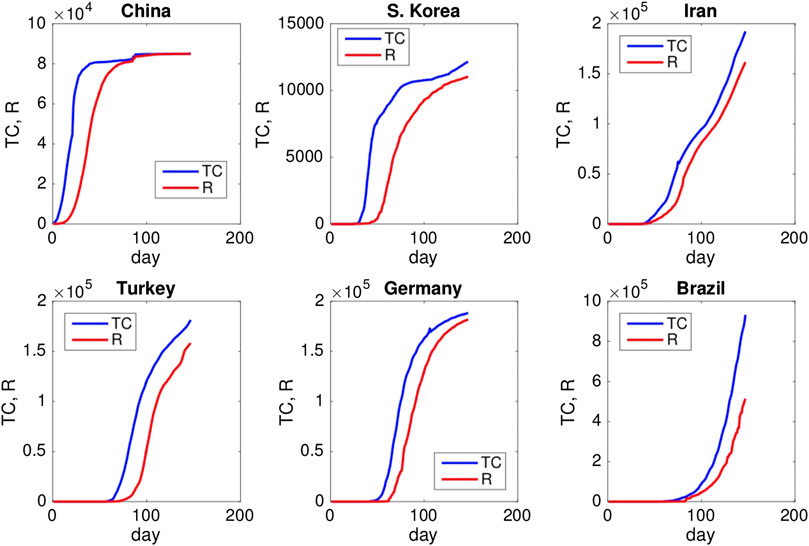
FIGURE 3. Graphs of total cases (TC) and removed individuals (R) for selected countries. The dataset of each country is collected according to published official reports and available at the website http://www.worldometers.info/coronavirus/ (last access: June 28, 2020). Updated data are also available at the website http://epikhas.khas.edu.tr/. The last data in this work were collected on the 16th of June 2020. Data cover the period January 22–June 28, 2020, and in the following, “Day 1” corresponds to January 22, 2020.
In the literature, available observed data that are used for the ordinary differential equation system representing the classical SIR epidemic model is based on the curve of removed individuals. Usually, this curve is obtained by taking into consideration only the fatality data of the epidemic disease, whereas in some research studies, not only the fatality data but also the hospitalization data for the epidemic are taken into account in the modeling process. In Ref. [21], it is shown that there exists a delay between the peak of the hospitalization (infectious) curve and the inflection point of the fatality (removed) curve based on the data collected. The original contribution of this article to the literature is that we explain this time shift by the multidimensional form of SIR and SEIR models and also provide numerical evidence that the expected delay is approximately half of the infectious period of the epidemic disease for both of the multistage systems.
In the second section of this work, we give a brief summary of the classical epidemic SIR and SEIR models and define the multistage form of these models that will be used in further analysis. The graphs obtained by the numerical evaluations of the classical SIR and SEIR models and their multistage forms are also given. Analysis of these graphs confirms that the multistage SIR and SEIR models explain the time shift observed in several surveys. In the third section, the evaluation of delay for different epidemic parameters is presented by using the numerical evaluations of these multistage models. In the fourth section, the distance between the points where successive stages and hence any two stages assume their maximum is found approximately. The last section includes a summary of the results obtained in the previous sections as well as motivations for future analysis.
2. Standard Epidemic Models and Epidemic Models With Multiple Infectious Stages
The SIR model is commonly used to model diseases for which the removed individuals are assumed to be immune to reinfection. In addition, the total population with constant size is divided into three distinct compartments the size of which change with time t. These compartments are called the susceptible class S, the infective class I, and the removed class R. Healthy individuals with no immunity are members of class S until they are infected with a pathogen and become capable of transmitting the disease to others. They move from the class S into the class I once they are infected and then from I to R once they recover or die. Childhood illnesses like measles or rubella are good examples for the SIR model.
The SIR epidemic model without vital dynamics, that is, the recruitment of new susceptible through birth or immigration as well as the loss through mortality or emigration are ignored, is defined by the following system of nonlinear ordinary differential equations:
where the coefficient β refers to the disease transmission rate and
The standard SIR model ignores a latent phase which is the delay between the time of the acquisition of infection and the onset of infectiousness. In order to define this latent phase, the introduction to the SIR model of an exposed class E whose members are individuals who have been infected with a pathogen but are not yet infectious due to the incubation period of pathogen yields the SEIR model. Chicken pox is suitable for the SEIR model, which is defined by the following system of ordinary differential equations
where
These two models are suitable for mathematical modeling of seasonal diseases, but they fail to reproduce the time shift that was observed in the modeling of the 2009 H1N1 epidemic in Istanbul, Turkey [21], as shown in Figure 2. For COVID-19, a similar time shift is observed between the curves of total cases and removed individuals in China, South Korea, Iran, Turkey, Germany, and Brazil as shown in Figure 3. Publicly accessible data that have been released by the state offices of each country are used for this analysis.
In the literature, a similar time shift is also observed for Spanish flu [19] and SARS epidemics. The graphs for the data of these epidemics are shown in Figure 1. Weekly case and fatality reports for Copenhagen, Denmark, in 1918–1919 are displayed in Figure 1A. In this figure, it can clearly be seen that there is a time shift between the peak of the infectious cases and the peak of fatalities. Similarly, in Figure 1A–B, the time shift is also observable between cumulative cases and cumulative fatalities.
In this study, we use multiple infectivity periods [1314] to explain this delay that is unforeseen in the standard SIR model. The approach of multiple infectious stages consists of replacing the single infectious stage I with
The multistage SIR and SEIR epidemic models are defined by the following systems:
The multistage SIR and SEIR systems with
The linear parts of apparently different infectious stages for
Subsequently, the numerical evaluations of these two systems, SJR and SEJR, defined above will be used for some of the structural comparisons of the classical models and multistage models. A MATLAB® ODE45 solver is used for all the numerical evaluations. In typical cases, initial conditions are chosen so that
and for the SEJR model,
Numerical evaluations of the classical SIR and SEIR models are made for specific epidemic parameters, and the results are given in Figure 4. For the evaluations of the classical models, γ and ϵ are fixed as
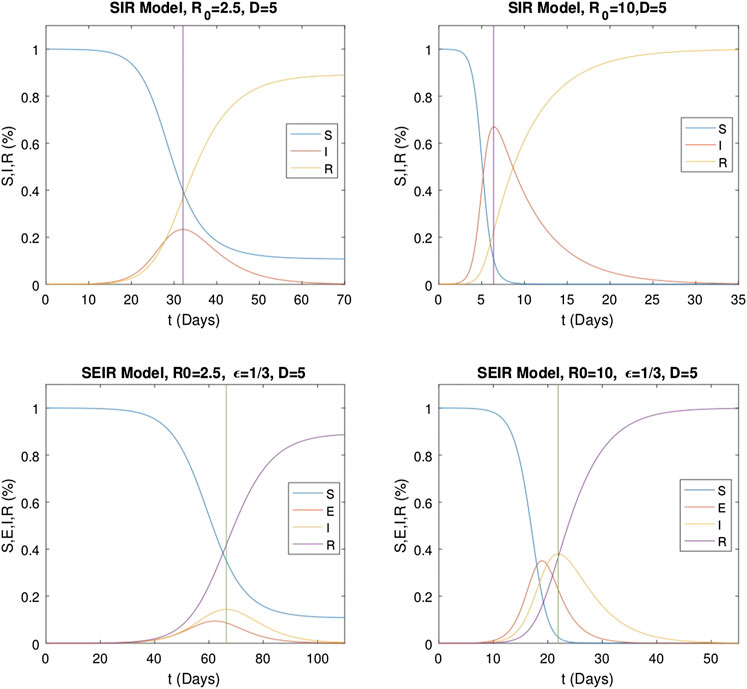
FIGURE 4. Numerical solutions of classical SIR (1) and SEIR (2) models with
Numerical evaluations of the multistage SIR model are repeated for various stage numbers. First,
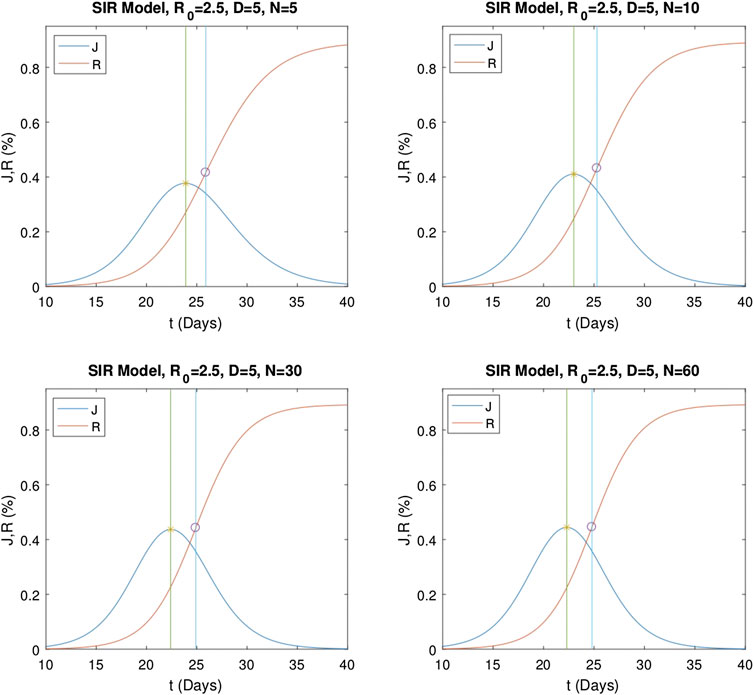
FIGURE 5. Numerical solutions of the multistage SIR model for different stage numbers
Similarly, numerical evaluations of the multistage
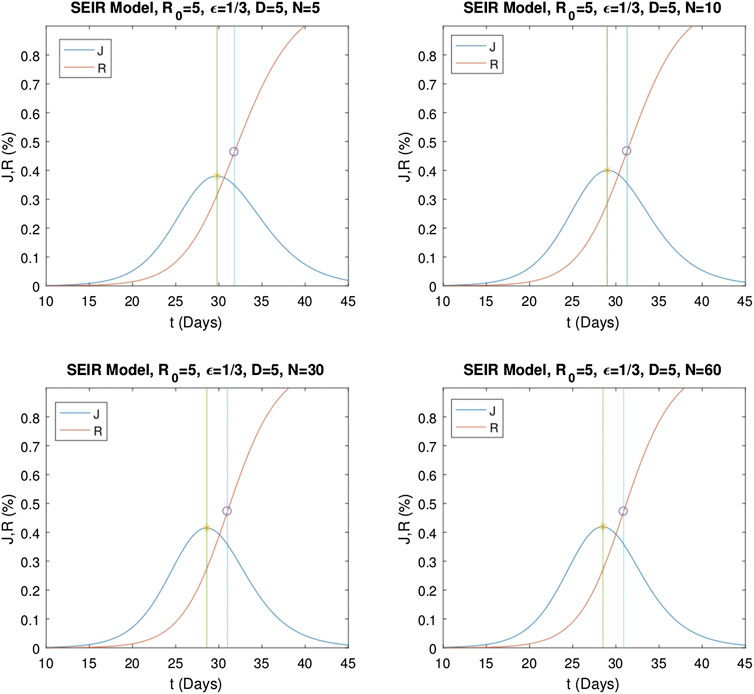
FIGURE 6. Numerical solutions of the multistage SEIR model for different stage numbers
3. Numerical Results for Models With Multiple Infectious Stages
In this section, we investigate the dependency of the delay on the epidemic parameters by numerical evaluations of system Eq. 5. Graphs
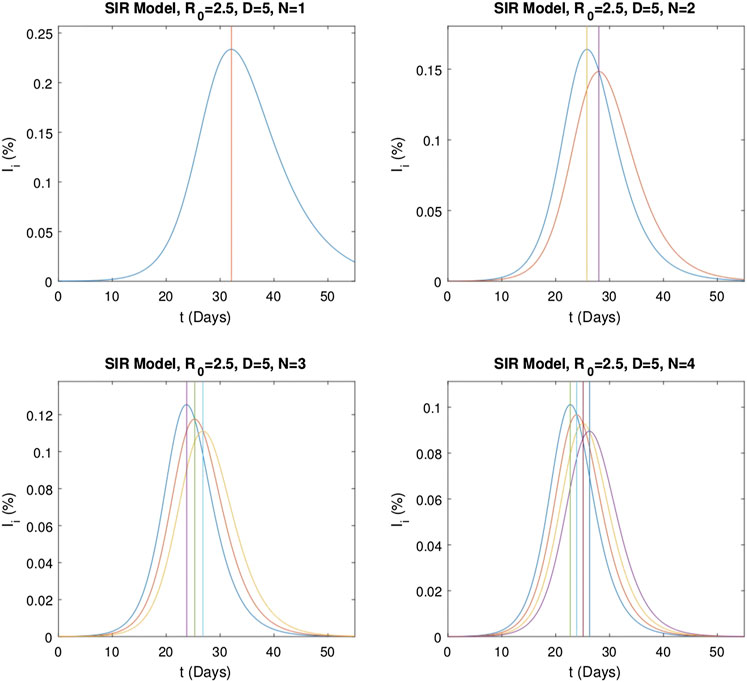
FIGURE 7. Graphs of the independent infectious stages
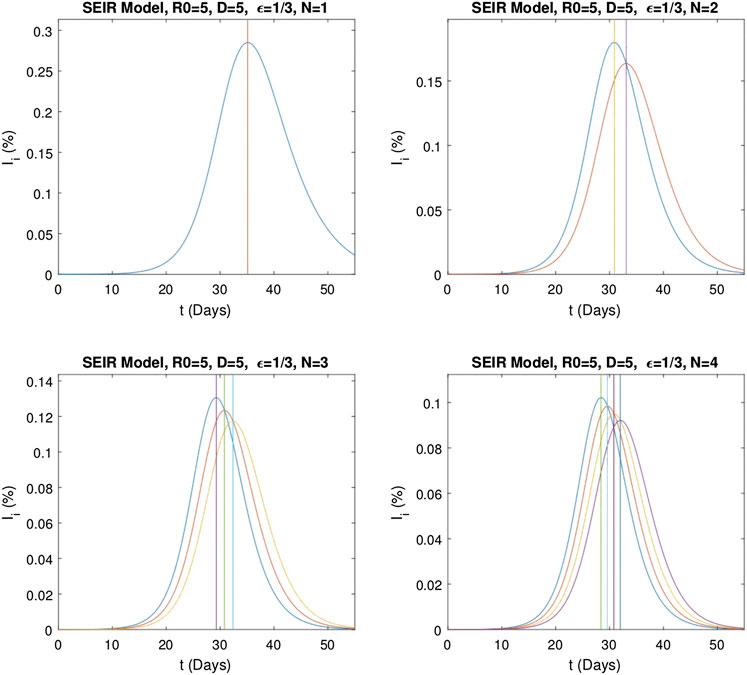
FIGURE 8. Graphs of the independent infectious stages
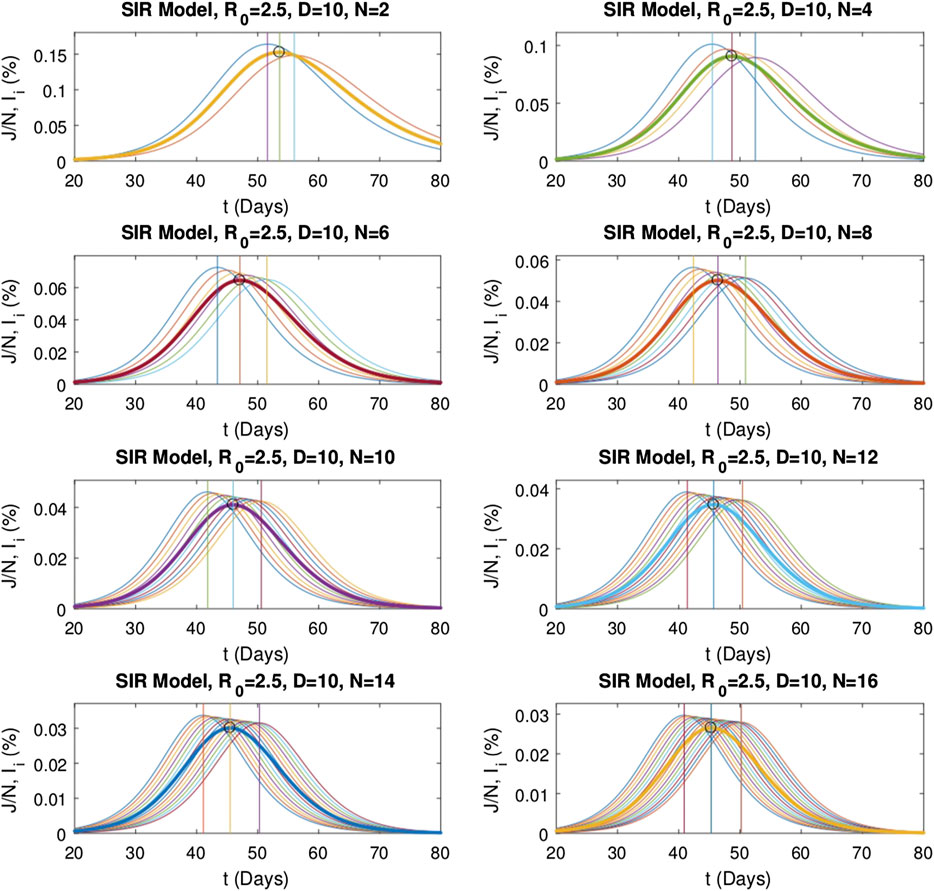
FIGURE 9. Change of the position of maximum of
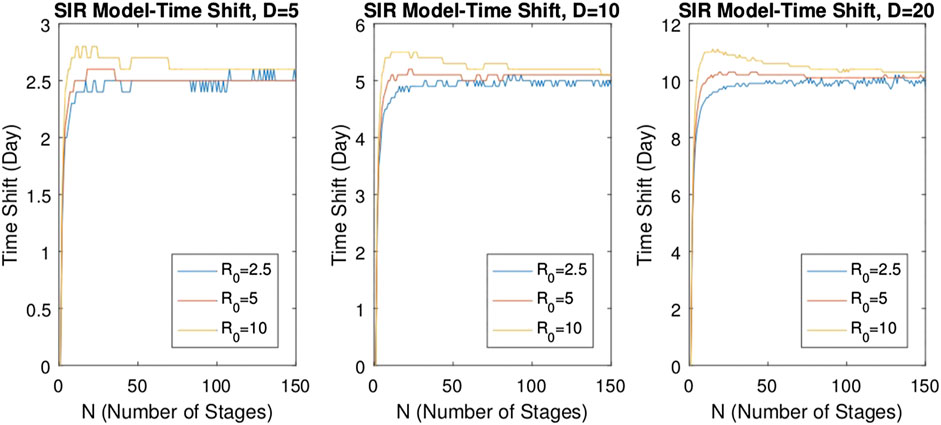
FIGURE 10. Variations of time shift (delay) with respect to the number of stages N for
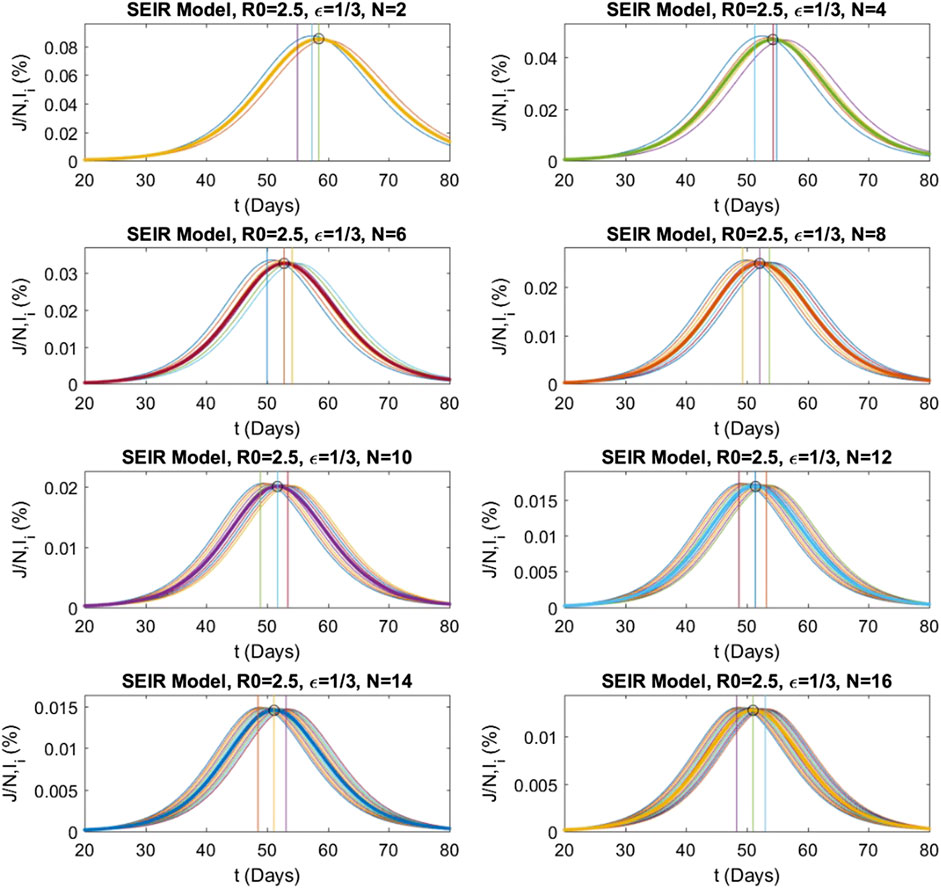
FIGURE 11. Change of the position of maximum of
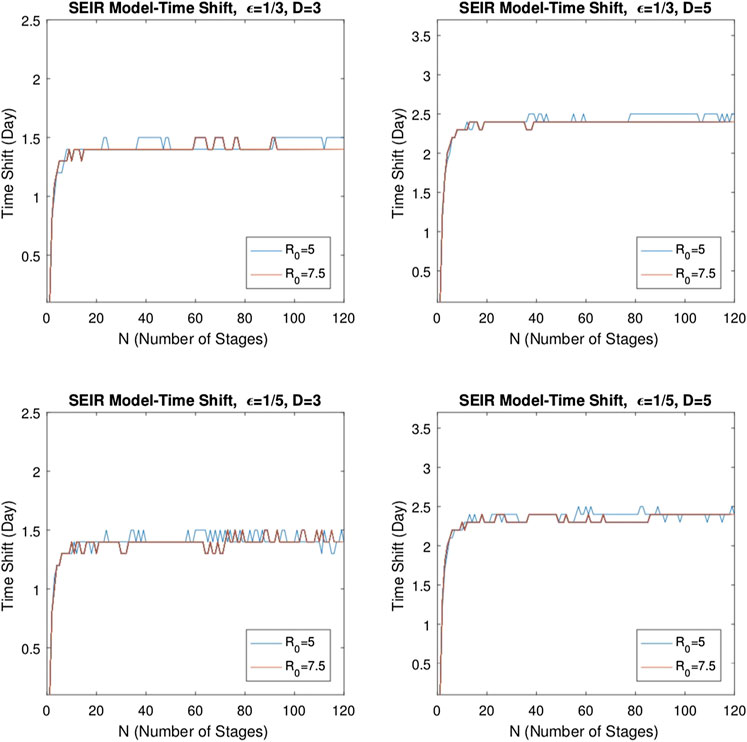
FIGURE 12. Variation of time shift for
For the SIR model, to investigate the effect of the basic reproduction number
The same analysis is repeated for the multistage SEIR model. The system defined by Eq. 6 is solved for the same epidemic parameters and initial conditions as above and with
To illustrate the dependency of the delay on the system parameters of the
4. Estimation of the Delay
In this section, we use a quadratic approximation to each
Let
for
and then substituting
The multistage SIR model defined by the equations in Eq. 5 suggests that for
Differentiating Eq. 12 yields
By considering the fact that
Substitution of the equation above in Eq. 11 gives the approximate distance formula as follows:
Therefore, the distance between any
Results obtained by the numerical evaluations are compatible with Eq. 14. To observe the distance between the maximum points of the independent infectious stages, solutions of the multistage SIR model with respect to various infectious periods are chosen. In this respect, the basic reproduction numbers
It should be emphasized that Eq. 14 is also valid for the multistage SEIR model. The distance between the maximum points of the independent infectious stages including the E stage is approximately
5. Conclusion
Epidemic data display a time shift between the peaks of infectious cases and fatalities. This time shift is not foreseen by the ordinary differential equations for the SIR and SEIR models since in both of them, the derivative of
In this article, we propose a generalization of these multistage models by allowing the parameters to be unequal in different stages. We showed that within the validity of a quadratic approximation to
We solved the multistage models for a range of epidemic parameters, and we have seen that the solution curves reveal the time shift. While the delay varies for relatively small stage numbers, it is observed that the delay becomes nearly stable as the number of stages increases, and it is independent of the basic reproduction number. This fact supports the validity of the quadratic approximation and shows that the delay phenomenon observed in the infectious diseases defined by the epidemic models SIR and SEIR can be successfully explained by the multistage forms of these models.
In addition to a theoretical contribution, the existence and the estimation of the time shift between the progression of the infectious cases and the fatalities have a practical importance, in the sense that, in order to take timely actions, the severity of the epidemic should be measured in terms of the increase in the number of infectious cases.
Finally, we note that the importance of the effects of quarantine is realized during the COVID-19 pandemic. In the literature, there are two basic approaches, one of which adds a compartment to the model as in Ref. [22] and the other adds a function
Data Availability Statement
The datasets analysed for this study can be found in the webpages: http://www.worldometers.info/coronavirus/ (last access: 05 November 2020), http://epikhas.khas.edu.tr/ (last access: 05 November 2020) and https://www.who.int/csr/sars/country/en/ (last access: 05 November 2020).
Author Contributions
APD, AHB and SA performed theoretical results; AD, APD, AHB and SA performed computations; AD performed literature survey and APD, AD, AHB and SA wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This manuscript has been released as a pre-print at arxiv.org, arXiv:2004.13178.
References
1. Bernoulli D (1760). Essai d’une nouvelle analyse de la mortalité causée par la petite vérole, et des avantages de l’inoculation pour la prévenir. Mem. Math. Phys. Acad. Roy. Sci., Paris:1–45.
2. Hamer WH. Epidemic disease in England: the evidence of variability and of persistency of type London: Bedford Press (1906). 72 p.
4. Kermack WO, McKendrick AG (1927). A contribution to the mathematical theory of epidemics. Proc R Soc Lond–Ser A Contain Pap a Math Phys Character 115:700–21.
5. Miller JC. A note on a paper by erik volz: sir dynamics in random networks. J Math Biol (2011). 62:349–58. doi:10.1007/s00285-010-0337-9
6. Miller JC, Volz EM. Incorporating disease and population structure into models of sir disease in contact networks. PLoS One (2013). 8:e69162. doi:10.1371/journal.pone.0069162
7. Bilge AH, Pekcan Ö, Gürol MV. Application of epidemic models to phase transitions Phase Transitions (2012). 85:1009–17. doi:10.1080/01411594.2012.672648
8. Bilge AH, Pekcan O, Kara S, Ogrenci AS. Epidemic models for phase transitions: application to a physical gel. Phase Transitions (2017). 90:905–13. doi:10.1080/01411594.2017.1286487
9. Bilge AH, Ogrenci AS, Pekcan O. Mathematical models for phase transitions in biogels. Mod Phys Lett B (2019). 33:1950111. doi:10.1142/s0217984919501112
10. Hethcote HW, van den Driessche P. An sis epidemic model with variable population size and a delay. J Math Biol (1995). 34:177–94. doi:10.1007/bf00178772
11. Hethcote HW, van den Driessche P. Two sis epidemiologic models with delays. J Math Biol (2000). 40:3–26. doi:10.1007/s002850050003
12. Cooke KL, Van Den Driessche P. Analysis of an seirs epidemic model with two delays. J Math Biol (1996). 35:240–60. doi:10.1007/s002850050051
13. Iggidr A, Mbang J, Sallet G, Tewa J-J. Multi-compartment models. Discrete Contin. Dyn. Syst (2007). 506–19.
14. Lloyd AL. Realistic distributions of infectious periods in epidemic models: changing patterns of persistence and dynamics. Theor Popul Biol (2001). 60:59–71. doi:10.1006/tpbi.2001.1525
15. Bame N, Bowong S, Mbang J, Sallet G, Tewa J-J. Global stability analysis for SEIS models with n latent classes. Math Biosci Eng (2008). 5:20–33. doi:10.3934/mbe.2008.5.20
16. Guo H, Li MY, Shuai Z. Global dynamics of a general class of multistage models for infectious diseases. SIAM J Appl Math (2012). 72:261–79. doi:10.1137/110827028
17. Sherborne N, Blyuss KB, Kiss IZ. Dynamics of multi-stage infections on networks. Bull Math Biol (2015). 77:1909–33. doi:10.1007/s11538-015-0109-1
18. Brauer F. A simple model for behaviour change in epidemics. BMC Public Health (2011). 11:1–5. doi:10.1186/1471-2458-11-s1-s3
19. Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in copenhagen: implications for pandemic control strategies. J Infect Dis (2008). 197:270–8. doi:10.1086/524065
20. Ahmetolan S, Bilge AH, Demirci A, Peker-Dobie A, Ergonul O. What can we estimate from fatality and infectious case data using the susceptible-infected-removed (sir) model? a case study of covid-19 pandemic. Front Med (2020). 7:570. doi:10.3389/fmed.2020.556366
21. Bilge AH, Samanlioglu F, Ergonul O. On the uniqueness of epidemic models fitting a normalized curve of removed individuals. J Math Biol (2015). 71:767–94. doi:10.1007/s00285-014-0838-z
22. Piqueira JRC, Batistela CM. Considering quarantine in the sira malware propagation model. Math Probl Eng (2019). 2019:1–8. doi:10.1155/2019/6467104
Keywords: COVID-19, epidemic models, multistage Susceptible-Infected-Removed model, multistage Susceptible-Exposed-Infected-Removed model, time shift
Citation: Peker-Dobie A, Demirci A, Bilge AH and Ahmetolan S (2020) On the Time Shift Phenomena in Epidemic Models. Front. Phys. 8:578455. doi: 10.3389/fphy.2020.578455
Received: 30 June 2020; Accepted: 12 October 2020;
Published: 18 November 2020.
Edited by:
Aristides Moustakas, University of Crete, GreeceReviewed by:
Jose Roberto Castilho Piqueira, University of São Paulo, BrazilIbrahim Halil Aslan, Batman University, Turkey
Changjing Zhuge, Beijing University of Technology, China
Copyright © 2020 Peker-Dobie, Demirci, Bilge and Ahmetolan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Demirci, ZGVtaXJjaWFsQGl0dS5lZHUudHI=
 Ayse Peker-Dobie1,2
Ayse Peker-Dobie1,2 Ali Demirci
Ali Demirci Ayse Humeyra Bilge
Ayse Humeyra Bilge Semra Ahmetolan
Semra Ahmetolan