- Department of Physics, Simon Fraser University, Burnaby, BC, Canada
Multi-component molecular machines are ubiquitous in biology. We review recent progress on describing their thermodynamic properties using autonomous bipartite Markovian dynamics. The first and second laws can be split into separate versions applicable to each subsystem of a two-component system, illustrating that one can not only resolve energy flows between the subsystems but also information flows quantifying how each subsystem’s dynamics influence the joint system’s entropy balance. Applying the framework to molecular-scale sensors allows one to derive tighter bounds on their energy requirement. Two-component strongly coupled machines can be studied from a unifying perspective quantifying to what extent they operate conventionally by transducing power or like an information engine by generating information flow to rectify thermal fluctuations into output power.
1 Introduction
Livings things are fundamentally out of thermodynamic equilibrium [1]. Maintaining this state requires a constant flow of energy into them accompanied by dissipation of heat into their environment. Quantifying these flows is straightforward for macroscopic systems but much less so on the small scales of molecular machinery. The advent of ever-more-refined experimental equipment capable of probing small-scale thermodynamics has led to the burgeoning field of stochastic thermodynamics [2–5]. Within this theory, energy flows are deduced from the thermally influenced stochastic dynamics of small-scale systems, permitting quantification of heat dissipation and energetic requirements of diverse experimental setups as well as molecular biological machinery.
1.1 Information thermodynamics
Information plays an interesting and, at times, adversarial role in thermodynamics. At the dawn of statistical mechanics, Maxwell illustrated the counterintuitive role of information by arguing that an intelligent demon could separate gas molecules according to their velocity with seemingly no expense of energy, apparently contradicting the second law [6]. Resolving this paradox plagued physicists for a century [6, 7], leading to well-known contributions from Szilard [8], Landauer [9], and Bennett [10] ultimately showing that acquiring, processing, and storing information incurs thermodynamic costs that balance or exceed any benefit gained from it.
Within the theory of stochastic thermodynamics, information has been incorporated in various ways, including measurement and feedback [11–17] performed by an experimenter on a system, and a system interacting with information reservoirs [18–21]; this established information as a proper thermodynamic resource [22] that sets limits on system capabilities similar to work and free energy. Diverse theoretical works [23–29] and experimental realizations [30–48] illustrate information-powered engines.
1.2 Autonomous and complex systems consisting of subsystems
Small-scale information thermodynamics is also relevant for biological systems such as molecular machines and molecular-scale sensors [49, 50]. Understanding living systems at small scales and advancing the design of nanotechnology [51] requires extending thermodynamics beyond conventional contexts: Instead of the scripted experimental manipulation of time-dependent control parameters, living systems are autonomous, driven out of equilibrium by steady-state nonequilibrium boundary conditions.
Moreover, embracing more of the complexity of biology, we seek understanding beyond the interactions of a system with weakly coupled baths, to encompass interactions among strongly coupled subsystems [52–55]. Lacking a clear separation between a measurement that collects information about a system and feedback that acts on this information [56], in autonomous systems it is more practical to differentiate between subsystems: An upstream system that generates information for a downstream system to react to or exploit.
While in the non-autonomous setup apparent second-law violations result from not correctly accounting for non-autonomous interventions by an experimenter [12–17, 28], in autonomous multi-component systems they can be traced back to thermodynamic accounting that ignores the strong coupling [56–59].
In its simplest form such an autonomous setup is realized by a downstream molecular sensor that reacts to an independent upstream signal [60–65]. More complex interactions are realized by two-component strongly coupled molecular machines in which the dynamics of each component is influenced by the other [54, 66, 67] and by assemblies of molecular transport motors that collectively pull cargo [68, 69].
Here we focus on such autonomous systems, collecting results that extend information thermodynamics to contexts lacking explicit external measurement and feedback, and showcase that bipartite Markovian dynamics and information flow are versatile tools to understand the thermodynamics and performance limits of these systems.
1.3 Objectives and organization
Our aims with this review are to:
1. Build on stochastic thermodynamics to give a gentle introduction to the information-flow formalism, deriving all necessary equalities and inequalities and relating the different names and concepts for similar quantities that appear throughout the literature. Section 2 introduces bipartite dynamics and establishes the notation. Section 3 and Section 4 deal with energy and information flows in these bipartite systems in general, while Section 5.1 compares various similar information-flow measures.
2. Collect results valid for biomolecular sensors, for which the information-flow formalism produces a tighter second law. These are contained in Section 5.
3. Address engine setups and show that the information-flow formalism advances understanding of autonomous two-component engines simultaneously as work and information transducers (Section 6).
1.4 Related reviews
The information-flow formalism is firmly rooted in the theory of stochastic thermodynamics. Recent reviews include a comprehensive one by Seifert [2] and reviews by Jarzynski [3] (focusing on non-equilibrium work relations), Van den Broeck and Esposito [4] (explicitly dealing with jump processes), and Ciliberto [70] (on experiments in stochastic thermodynamics). The recent book by Peliti and Pigolotti [5] also gives a pedagogical introduction to the field. Information thermodynamics itself has recently been reviewed by Parrondo et al. [22].
Turning to molecular machinery, the working principles of Brownian motors have been reviewed by Reimann [71]. General aspects of molecular motors can be found in the reviews by Chowdhury [72] and Kolomeisky [73]. Brown and Sivak [74] focus on the transduction of free energy by nanomachines, while reviews by Silverstein [75] and Li and Toyabe [76] specifically treat the efficiencies of molecular motors.
2 Bipartite dynamics
We consider a mesoscopic composite system whose state at time t is denoted by z(t). Due to thermal fluctuations, its dynamics are described by a Markovian stochastic process defined by a Master equation [77, 78]:
where pt(z) is the probability to find the composite system in state z at time t and the transition rates R(z|z′;t) (sometimes also called the generator) encode the jump rates from state z′ to state z. For convenience, we assume a discrete state space; however, all results can easily be translated into continuous state-space dynamics, as we allude to in Section 2.3. If multiple paths between states z′ and z are possible, the RHS in Eq. (1) needs to include a sum over all possible jump paths.
We assume that one can meaningfully divide the state space into distinct parts, e.g., z = {x, y}, where two subsystems X and Y are identified as distinct units interacting with each other. The process z(t) is bipartite if the transition rates can be written as
meaning that transitions cannot happen simultaneously in multiple subsystems. Note that this does not imply that the processes x(t) and y(t) are independent of each other; rather their influence on each other is restricted to modifying the other process’s transition rates.
When the dynamics of the joint system are not bipartite, the dissection of energy and information flows presented in the following is more challenging. Chétrite, et al. have investigated this case [79]. Moreover, information flows for quantum systems (without bipartite structure) have also been analyzed [80]. Here, we exclusively cover classical bipartite systems.
2.1 Paradigmatic examples
Bipartite dynamics should be expected whenever two systems (that possess their own dynamics) are combined such that each fluctuation can be decomposed into independent contributions. The dynamics of systems studied in cellular biology can often be approximated as bipartite.
Two paradigmatic examples that have been well studied are molecular motors (such as Fo−F1 ATP synthase) with strongly coupled interacting sub-components, or cellular sensors that react to a changing external concentration. The joint dynamics of such systems can be decomposed into the distinct fluctuations of each subsystem, each of which is influenced by the other subsystem (in the case of a strongly coupled molecular machine) or into dynamics strongly influenced by an independent process (in the case of a sensor). Figure 1 shows examples and associated simplified state graphs.
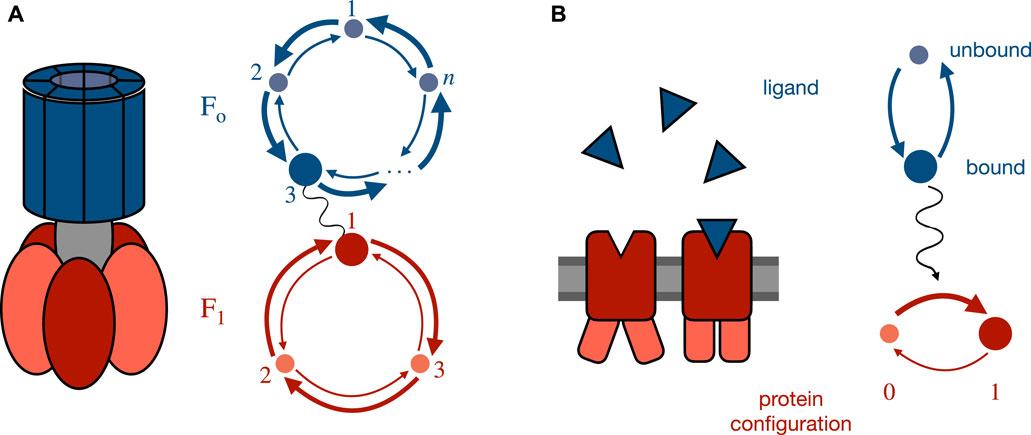
FIGURE 1. Paradigmatic example systems and their simplified state graphs. (A) Simplified model for Fo−F1 ATP synthase. Upstream Fo dynamics are cyclically driven by a proton gradient while downstream F1 dynamics are driven in the opposite direction by ATP hydrolysis [54, 66, 67]. Through their coupling, the joint system can transduce work by driving the downstream system against its natural gradient, thereby converting one chemical fuel into another. (B) Simplified model of a biochemical sensor, e.g., involved in E. coli chemotaxis [60–65]. The upstream signal is the binding state (bound or unbound) of the receptor which is reflected in the downstream protein conformation by modifying its potential-energy landscape and thereby influencing the transition rates between configurations.
2.2 Notation
To keep the notation concise and unambiguous, we adopt the following conventions:
1. Random variables are denoted with small letters. Occasionally the more explicit notation p(Xt = x) is used to avoid ambiguity.
2. The joint probability of two random variables taking values x and y, respectively, is denoted by p(x, y). The conditional probability of x given y is denoted by p(x|y).
3. Time arguments are dropped for probabilities and transition rates unless distinct times appear in a single expression, as in p(xt, yt′).
4. Total time derivatives are denoted with a dot. The bipartite assumption ensures that rates of change of various quantities can be split into separate contributions due to the X and Y dynamics, respectively. Those individual rates of change are indicated with a dot and the corresponding superscript, i.e.,
5. When no argument is given, symbols represent global quantities, whereas capitalized arguments in square brackets indicate different subsystem-specific quantities, e.g., S ≔ −∑x,yp(x, y) ln p(x, y) is the joint entropy, while S[X] ≔ − ∑x p(x) ln p(x) and S[X|Y] ≔ − ∑xyp(x, y) ln p(x|y) are marginal and conditional entropies, respectively [81, Chap. 2].
2.3 Continuous state spaces
The framework outlined below can also be applied to continuous state spaces. For continuous diffusion processes described by a Fokker-Planck equation (82) this has been done in [58].
For diffusion-type dynamics, Eq. (2) corresponds to the statement that the diffusion matrix must be block-diagonal, such that the Fokker-Planck equation can be written as
for respective subsystem drift coefficients μX and μY and subsystem diffusion coefficients DX and DY. The corresponding coupled Langevin equations (78) are.
where ξX(t) and ξY(t) are independent Gaussian white-noise terms for which
3 Energy flows
As a first step towards a thermodynamic interpretation of the stochastic dynamics described above, we relate stochastic transitions to energy exchanges between the two subsystems and between individual subsystems and the environment as represented by baths/reservoirs of various kinds. For the systems considered here it is safe to assume that all processes are isothermal and that their stochasticity is due to thermal fluctuations.
For systems relaxing to equilibrium the transition rates in Eqs (1) and (2) are related to thermodynamic potentials through the detailed-balance relation. This relation follows from demanding that, in the absence of any driving, the distribution of system states must relax to the equilibrium distribution with no net flux along any transition,
The equilibrium distribution is the Boltzmann distribution
When each system state is a mesostate composed of many microstates, as is common for modeling small biological systems, the potential energy ϵxy must be replaced by a mesostate free energy [83]. The following thermodynamic formalism remains unchanged, however.
The systems we consider here are driven by chemical reactions and external loads and do not obey the detailed-balance relation. Consequently they do not, in general, relax to equilibrium. Conceptually, we could include the state of the other reservoirs (chemical and work reservoirs) into the microstate Z of the system and then describe a non-equilibrium steady state as a very slow relaxation to global equilibrium, driving cyclical processes in the system of interest; however, such a description would be unnecessarily cumbersome. Assuming that these reservoirs are large compared to the system of interest and weakly coupled to it, we split the free energy of the supersystem into contributions from the reservoirs and the system of interest. Then, energy exchanges between all reservoirs and the system of interest are treated in the same way as energy exchanges with a heat bath, giving a local detailed-balance relation [4, 83–85]:
where
3.1 Global energy balance
Armed with the local detailed-balance relation (7), we identify different energy flows in the system. Below, we state the usual conventions of stochastic thermodynamics [2, 4] to identify the different contributions (heat and work) associated with each transition. The average rate
where we assume that the states x and y are consecutively numbered, so that the notation x > x′ indicates a sum over transitions between distinct states, omitting the reverse transitions. Throughout this review all energy flows into the system are positive by convention.
Two types of work can be identified,
and the average rate of mechanical work due to the subsystems’ responses to external forces,
Finally, we identify the rate of change of average internal energy as
With Eqs (7)-(10), we verify the global first law, representing the global energy balance:
which retrospectively justifies identifying the log-ratio of transition rates as heat (8).
3.2 Subsystem-specific energy balances
Due to the bipartite assumption (2), we also find subsystem-specific versions of this balance equation by splitting all energy flows into contributions from the respective subsystems: First the heat flow
splits into subsystem-specific heat flows
Similarly, the chemical work
splits into
where
Finally, the mechanical work
splits into
Moreover, we formally split the change in the joint potential energy
into contributions due to the respective dynamics of each particular subsystem,
where a positive rate indicates that the joint potential energy increases due to the respective subsystem’s dynamics.
We obtain the subsystem-specific first laws as the balances of energy flows into the respective subsystems:
With Eqs (2), (7)–(11), and (13a)–(16c), we verify that the sum of the subsystem-specific first laws in Eqs (17a) and (17b) yields the global first law (12).
3.3 Work done by one subsystem on the other
The subsystem-specific first laws in Eqs (17a) and (17b) stem from a formal argument. Ideally, we would like to identify internal energy flows that the subsystems communicate between each other; i.e., we would like to identify transduced work in the manner of [55]. However, with no clear prescription on how to split the energy landscape into X-, Y-, and interaction components,
the identification of energy flowing from one subsystem to the other remains ambiguous: For example, how much has a change in the X-coordinate changed the potential energy of the X-subsystem and how much has it changed the interaction energy? The ambiguity has already been pointed out in [87], where the authors propose to settle it through physical arguments by identifying a clear interaction term in the Hamiltonian and asking that the splitting leaves constant the average subsystem energy.
We propose a different approach to define an input work into one subsystem. Conventionally work is defined for interactions between a work reservoir (e.g., an experimentalist’s external power source) and a system. Interactions between the work reservoir and the system are mediated by a control parameter influencing the system’s potential-energy landscape. Crucially, there is negligible feedback from the system state to the dynamics of the control parameter. To define work between subsystems, imagine treating subsystem Y as if it were an externally manipulated control parameter influencing the potential-energy landscape of X. Then, the power done by the control parameter Y on system X would be supplied externally and equal the rate of change of internal energy due to the dynamics of the control parameter:
Consequently, we define
as the transduced work from X to Y. Thus, an externally manipulated control parameter could be understood as the limiting case of a negligible back-action from the downstream system to the (possibly deterministic) dynamics of the upstream system. This identification of energy flows communicated between the systems becomes useful when singling out one subsystem that is driven (possibly with feedback) by another one (see Section 6).
Figure 2 summarizes the splitting of the first law presented in this section and illustrates how energy moves between the subsystems.
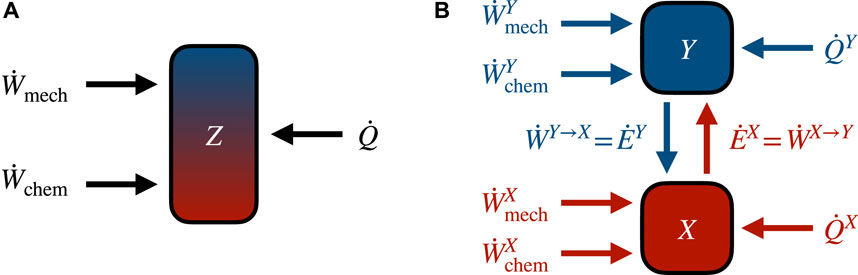
FIGURE 2. Energy flows in autonomous bipartite systems. (A) Global energy flows can be distinguished between work (mechanical and chemical) and heat. Since at steady state the average global internal energy stays constant, average flows of work and heat must cancel. (B) The bipartite assumption (2) allows decomposition of energy flows into contributions from each subsystem. Color and direction of arrows reflect the subsystem-specific first laws (17a) in red and (17b) in blue.
4 Entropy and free-energy balance
As always in thermodynamics, energetics are only half of the picture. We therefore next consider entropic quantities. Together, the rate
4.1 Global entropy balance
Following Ref. [4], we explicitly write the rate of change of system entropy:
where we have used the Master equation (1) in Eq. (21b), the fact that Eq. (21b) sums over every transition twice in Eq. (21c), and the definition of the heat flow (8) in Eq. (21d).
Rearranging the terms gives the global second law:
where
Using the definition of nonequilibrium free energy (20) and the global first law (12), we rewrite the global second law as
4.1.1 Marginal and hidden entropy production
An interesting digression covers related research on inferring total entropy production from the dynamics of only one subsystem. Prominent examples of such systems with hidden degrees of freedom are molecular transport-motor experiments [73, 92] in which only trajectories of an attached cargo are observed while the motor dynamics are hidden. Assessing motor efficiency, however, necessitates a detailed knowledge of the internal motor dynamics. Hence thermodynamic inference [83] is required to infer hidden system properties.
Alongside dynamics on masked Markovian networks [93–102], bipartite systems have been used to model situations in which one sub-component of the full system is hidden. One common strategy consists of mapping the observed dynamics of one subsystem onto a Markov model, which generally produces a lower bound on the total entropy production rate [86] that can subsequently be augmented with any information available about the hidden dynamics [103]. However, the observed process is non-Markovian which results, e.g., in modifications of fluctuation theorems [104–106]. Another approach is to use thermodynamic uncertainty relations [107–111] to produce a bound on the total entropy production using observable currents.
Importantly, the formalism laid out here requires full observability of the dynamics of both subsystems; recent efforts have explored when one can infer the kind of driving mechanism from observations of just one degree of freedom, e.g., the dynamics of probe particles attached to unobserved molecular motors [92, 112].
4.2 Subsystem-specific entropy balance
In analogy to subsystem-specific versions of the first law (17a), (17b) which introduce energy flows between the different subsystems, subsystem-specific versions of the second law introduce an entropic flow between the systems, called information flow.
The bipartite assumption (2) splits the global entropy production (22b) into two nonnegative contributions,
To make contact with the form of the global second law (22a), we identify different contributions to the subsystem-specific entropy productions
where
Importantly, these rates are not the rates of change of marginal entropies S[X] = −∑ x p(x) ln p(x) and S[Y] = −∑yp(y) ln p(y). Rewriting the subsystem-specific entropy productions with these marginal rates leads to the identification of an information flow, as we show in Section 4.3.
Substituting the subsystem-specific first laws (17a), (17b) gives subsystem-specific second laws in terms of work and free energy,
where
While formally appealing, the rate of change of nonequilibrium free energy due to one subsystem’s dynamics has little utility. Often, one only knows the free energy for one subsystem (e.g., by having constructed a potential-energy landscape for one of the subsystems as done in [113] for the F1-component of ATP synthase) or the free energy of one subsystem is unknown or undefined (e.g., for the external environment process in a sensing setup). To this end, we next present other ways of writing (and interpreting) the subsystem-specific second laws.
4.3 Subsystem-specific second laws with information flows
We express the rate
and similarly for
Here, we have identified the remaining terms as the information flows [87],
i.e., the rate of change of mutual information between the subsystems that is due only to the dynamics of one of them. Information flow is positive when the dynamics of the corresponding subsystem increase the mutual information between the two subsystems. In Supplementary Appendix S1 we show that for bipartite Markovian dynamics this definition leads to
i.e., the form used in [57] with which we can verify the equality of Eq. (25a) and Eq. (29a) and similarly of Eq. (25b) and Eq. (29b). Equations (29a), (29b) express the same subsystem-specific entropy productions as Eqs (25a), (25b). The latter contain subsystem-specific changes
where the bipartite assumption (2) ensures that there is no contribution from transitions in which x and y change simultaneously.
The term information flow was first used in the context of non-equilibrium thermodynamics by Allahverdyan et al. [87] and was later taken up by Horowitz and Esposito [57]. Section 5.1 compares information flow with conceptually similar quantities called nostalgia [114] and learning rate [65].
Notice the appealing structure of the subsystem-specific entropy productions in Eqs (29a) and (29b): For interacting subsystems, it is not enough to consider marginal entropy changes and heat flows into one subsystem, because to obtain a non-negative entropy production rate, one needs an additional term due to correlations between the interacting subsystems. Expressed differently: When one explicitly neglects or is unaware of other subsystems strongly coupled to the subsystem of interest, erroneous conclusions about the entropy production are possible, either overestimating it or perhaps even finding it to be negative, leading to a Maxwell-demon-like paradox.
We next present two alternative representations of the same subsystem-specific entropy production that rely on rewriting the rate of change of global entropy
4.3.1 Alternative representation of subsystem-specific entropy production in terms of conditional entropy
In addition to the formulation in Eq. (29a), the subsystem-specific entropy production in Eq. (25a) can also be rewritten in terms of the rate of change
where line (33b) uses the bipartite assumption (2) along with the fact that, due to the log-ratio in Eq. (33b), all terms y ≠ y′ are zero. Line (33e) follows from the definition (31b) of
Comparing with Eq. (25a), which expresses the same subsystem-specific entropy production, we observe a difference in interpretation: If one interprets Y not as a subsystem on equal footing with X but instead as a stochastic control protocol for system X, the subsystem-specific second law in Eq. (34) seems more natural. Such stochastic control protocols arise naturally in the context of sensors, where a changing environment effectively acts as a stochastic protocol [114], and in contexts with measurement-feedback loops where a stochastic measurement of the system state dictates the statistics of the future control protocol [11–15, 115].
4.3.2 Subsystem-specific second law with conditional free energy
In cases where the nonequilibrium free energy of subsystem X is known, we define a conditional nonequilibrium free energy of system X given a control parameter Y as the average energy of X given the particular control-parameter value y less (kBT times) the average entropy of X given the control-parameter value y, all averaged over Y.
Thus, this free energy is averaged over all stochastic control-parameter values.
With the splitting of the first law in Eq. (16a), the subsystem-specific first law in Eq. (17a), and the identification of transduced work
where
4.4 Steady-state flows
At steady state, average energy, entropy, and mutual information are all constant,
i.e., if one subsystem’s dynamics increase the average energy or mutual information, the dynamics of the other must compensate this change accordingly, to ensure constant energy and mutual information at steady state. These relations are especially useful for the dynamics of biological systems which can often be modelled as at steady state.
4.5 Marginal and conditional entropy productions
Note that the subsystem-specific entropy productions
It is possible to define such marginal and conditional entropy productions for bipartite Markov processes. As shown by Crooks and Still [116], the total entropy production
4.6 Tighter second laws and information engines
Historically, the question of how to incorporate information into a thermodynamic theory so as to restore the second law’s validity has attracted much interest. Discussions ranged around the thought experiment of Maxwell’s demon [6, 7], with well-known contributions from Szilard [8], Landauer [9], and Bennett [10]. Within stochastic thermodynamics, Maxwell’s demon has been formalized as a process with (repeated) feedback [11–15] and interactions with an information reservoir (often modeled as a tape of bits) [18–21].
The advent of increasingly refined experimental techniques for microscale manipulation has enhanced the prospect of finding realizations of Maxwell’s thought experiment in real-world molecular machinery, stimulating a formalization of the thermodynamics of information [22]. The bulk of the experimental realizations demonstrating the possibility of information engines utilize some kind of time-dependent external control [32, 34, 37–39, 41, 43–46]. In a recent example, an optically trapped colloidal particle X is ratcheted against gravity without the trap Y transducing any work WY→X to it, thus enabling the complete conversion of heat to actual mechanical output work
The picture of autonomous interacting subsystems does not naturally allow such a clear distinction between measurement and feedback, or between system and tape [56]. Instead, continuous Maxwell demons are identified by current reversals, apparently making heat flow against the direction indicated by the second law [35, 59, 117, 118]. In this context the information-flow formalism produces a bound on apparent second-law violations in one subsystem using an information-theoretic quantity.
We are now in a position to assess the role of information flows in the operation of two-component systems and make contact with Maxwell’s demon. We focus on the specific second law applied to the X-subsystem. Rearranging (34), we obtain:
where the LHS is a conventional expression for the entropy production due to system X (entropy change of the system state X at fixed Y, minus heat flow
Let us distinguish two cases: 1) If
2) If
In Section 5 and Section 6 we discuss both cases in detail.
5 Sensors: External Y-dynamics
The performance limits of biomolecular sensors such as those found in Escherichia coli have gained attention [119–122]. As observed by Berg and Purcell [123], the main challenge faced by sensors tasked with measuring concentrations in the microscopic world is the stochastic nature of their input signal, i.e., the irregular arrival and binding of diffusing ligands; different strategies can improve inference of ligand concentration [124–126].
Sensing has also been studied from an information-thermodynamics perspective, where the main question revolves around the minimum thermodynamic cost to achieve a given sensor accuracy. Maintaining correlation between an internal downstream signalling network and an external varying environment is costly [62, 127–131] and involves erasing and rewriting a memory, analogous to a Maxwell demon [132]. Here, we focus on a high-level characterization of biomolecular sensing that uses bipartite Markov processes.
Specifically, in a sensor setup, the stochastic dynamics of one of the subsystems (the environmental signal) are independent of the other (the sensor). Figure 1B shows an example of a sensor setup inspired by the signaling network involved in E. coli chemotaxis. Let Y be an external process (e.g., whether a ligand is bound to the receptor) that influences the transition rates of the sensor X, but whose transition rates are independent of X:
where in Eq. (39b) we swapped summation indices y ↔ y′ in the first sum, and in Eq. (39c) the term with an underbrace is a relative entropy and hence is nonnegative [81, Chap. 2.3].
Equation (34) thus implies a stronger second-law inequality:
The LHS represents the sensor’s entropy production, which is lower-bounded by an information-theoretic quantity that has various interpretations in the literature. In the following we will build intuition about this quantity and comment on its relation to the sensor’s measuring performance.
5.1 Nostalgia and learning rate
The first inequality in Eq. (40) was originally pointed out by Still, et al. in a discrete-time formalism [114] and for possibly non-Markovian external processes. In that formalism,
A second related quantity is the learning rate ℓx introduced by Barato et al. [65]. Originally defined for systems in steady state, it is exactly the information flow
The learning rate quantifies how the uncertainty in an external signal Y is reduced by the dynamics of X, i.e., how much X learns about Y:
We used the bipartite assumption (2) in the second line. Here
In the special case of a steady state
This motivated Barato et al. to define an informational efficiency [65],
The following series of (in-)equalities sums up the relations between the different measures of information flow:
5.2 Other information-theoretic measures of sensor performance
While information flow bounds sensor dissipation and has intuitive interpretations in terms of predictive power [114] and learning rate [65], other information-theoretic quantities seem more natural to measure sensor performance.
For example, Tostevin and ten Wolde [134] have calculated the rate of mutual information between a sensor’s input and output; however, Barato et al. [64] have shown that this rate is not bounded by the thermodynamic entropy production rate. (The desired inequality requires both the time-forward trajectory mutual information rate and its time-reversed counterpart [135].)
Another commonly used quantity to infer causation is the (rate of) transfer entropy [136], which in turn is a version of directed information [137, 138] (for a gentle introduction see, e.g. [139], section 15.2.2). Much like information flow, this rate also bounds the sensor’s entropy production rate [133]; however, in general, it represents a looser bound than the information flow. The transfer-entropy rate measures the growth rate of mutual information between the current environmental signal and the sensor’s past trajectory. This motivated Hartich et al. [140] to define sensory capacity as the ratio of learning rate and transfer-entropy rate, measuring the share of total information between environmental signal and the entire sensor’s past that the sensor’s instantaneous state carries. It is maximal if the sensor is an optimal Bayesian filter [141, 142].
Finally, a natural quantity to measure a sensor’s performance is the static mutual information between its state and the environmental signal. Brittain et al. [143] have shown that in simple setups, learning rate and mutual information change in qualitatively similar ways when system parameters are varied; however, in more complex setups with structured environmental processes or feedback from the sensor to the environment, maximizing the learning rate might produce a suboptimal sensor. They rationalize this result by noting that the rate at which the sensor must obtain new information to maintain a given level of static mutual information does not necessarily coincide with the magnitude of that static mutual information.
6 Engine setups: Feedback from X to Y
Here, we consider the more general case of an engine setup in which the two components X and Y cannot be qualitatively distinguished as an external and an internal process; instead, both components X and Y form a joint system. On a formal level, there now is feedback from X to Y, such that (39d) no longer holds in general and it is not possible to make model-independent statements about the direction of information flow. To make contact with analyses of multi-component molecular machines, we present a few conceptual differences between external control by an experimenter and what we call autonomous control by another coupled stochastic system.
6.1 External vs autonomous control
There is a long history of nonequilibrium statistical mechanics motivated by single-molecule experiments. A hallmark of these experiments is dynamical variation by an external apparatus of control parameters such as the position or force of an optical trap [144], magnetic trap [145], or atomic-force microscope [146]. This external control allows an unambiguous identification of work done on a system as the change in internal energy achieved through the variation of control parameters, and heat as the complementary change of internal energy due to the system’s dynamics. Sekimoto [147, 148] has identified heat and work for diffusive dynamics described by a Langevin equation; this identification readily carries over to discrete dynamics [2, 4] and even Hamiltonian dynamics [3].
The notion of a deterministic control-parameter trajectory allows, e.g., the derivation of fluctuation theorems [149–151] and the study of how to optimize such a trajectory to minimize the average work done on the system [152–157] or its fluctuations [158, 159]. Feedback can also be included in the analysis by considering measurements and subsequent modifications to the control-parameter trajectory that depend on measurement outcome [11–15].
However, in biological systems, there is generally no dynamical variation of external control parameters. Instead, these systems are autonomous, and stochastic thermodynamics occurs in the context of relatively constant but out-of-equilibrium “boundary conditions”: a single temperature and a variety of chemical potentials that are mutually inconsistent with a single equilibrium system distribution, thus leading to free-energy transduction [74] when the coupling is sufficiently strong such that not all currents flow in the direction of their driving force. Increasingly, researchers are modeling molecular machines as multi-component systems with internal flows of energy and information. Examples are the molecular motor Fo−F1 ATP synthase [66, 160, 161] that can be modeled using two strongly coupled subsystems [54, 162–166], or molecular motor-cargo collective systems where sometimes hundreds of motors (such as kinesin, dynein [167], and myosin [168]) work in concert [68, 69], leading to different performance trade-offs [169–174].
Nonetheless, multi-component systems can be interpreted as if the dynamics of one component provide a variation of external control parameters to the other. In this context, it can be useful to identify an upstream (more strongly driven by nonequilibrium boundary conditions) system Y and a downstream (more strongly driven by the coupled upstream system than by the nonequilibrium boundary conditions) system X, although the identification of these components may sometimes be ambiguous. This type of autonomous control differs from external control in two important aspects: 1) It is stochastic since the dynamics of the upstream system are itself stochastic; 2) There is feedback from the downstream to the upstream system because the upstream system’s dynamics obey local detailed balance (7). Both aspects can lead to counterintuitive results when one naively applies stochastic energetics to one subsystem that is strongly coupled to others [55].
6.2 Conventional and information engines
Let us more closely examine two-component engines, e.g., the Fo−F1 ATP synthase sketched in Figure 1A. Such a molecular machine can be regarded as a kind of chemical-work transducer using a stronger upstream chemical gradient to drive a downstream chemical reaction against its natural direction [49, 175]. Recently, the coupling characteristics and energy flows in such systems have received attention [54, 55, 67, 166].
It is natural to consider direct energy flows from an input (chemical) reservoir (e.g.,
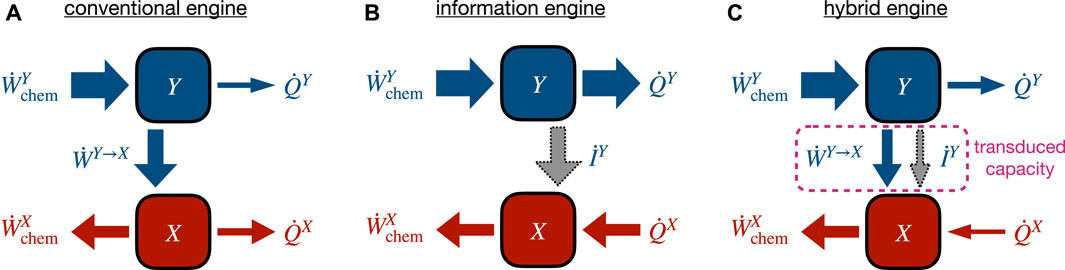
FIGURE 3. Different operational modes of a two-component engine converting chemical input power
6.3 Steady-state transduced capacity
Our discussion indicates that conventional and information engines can be treated with a common framework, as in [49] where a synthetic molecular motor was analyzed, identifying distinct flows of information and energy with which the upstream subsystem drives the downstream subsystem. For concreteness, let Y be the upstream and X be the downstream subsystem. As shown in [67, 176], combining the subsystem-specific second laws at steady state leads to a simultaneous bound on input and output power in terms of an intermediate quantity, called transduced capacity in [67]. Substituting the steady-state identities
Using the subsystem-specific first laws in Eqs (17a) and (17b) and identifying the transduced power in Eq. (19a) gives
This relation suggests that the transduced capacity acts as a bottleneck for the conversion of input to output power. The capacity of this bottleneck consists of two distinct pathways, a conventional energetic component
We expect efficient work transducers to come as close as possible to saturating both inequalities to minimize dissipative losses during their operation. It would be interesting to investigate under which circumstances each of the two pathways leads to the most efficient work transducers and whether real-world biomolecular machinery has evolved to preferentially exploit one over the other.
7 Conclusion, extensions, and outlook
7.1 Summary
In this review we focused on the thermally influenced stochastic dynamics of two-component autonomous systems which are commonly found in biological machinery. We assumed that the dynamics are Markovian and bipartite such that only one subsystem changes its state at a time.
We collected results that show how the bipartite assumption enables the first and second laws of thermodynamics to be split into subsystem-specific versions. The subsystem-specific first laws lead to energy flows between the individual subsystems and the environment and to the transduced power—the energy flow between the subsystems. The subsystem-specific second laws reveal information flows as specific entropic quantities that quantify how the dynamics of a single subsystem change the mutual information shared between the subsystems.
Sensors are a setup to which the formalism applies naturally because an external signal influences the stochastic dynamics of the sensor. Within the framework, the sensor’s dissipation (the energy flow) is bounded by an information-theoretic quantity (the information flow) measuring aspects of the influence of the environmental signal on the sensor.
Studying strongly coupled molecular machines within this framework reveals that the more conventional transduced power (the energy flow) from one subsystem to the other is accompanied by the less conventional information flow, which can be interpreted as a hallmark of information engines. Both flows are capable of supporting energy transduction through the coupled system such that conventional and information engines can be studied from the same perspective.
7.2 More than two subsystems
The question naturally arises whether the information-flow framework can be extended to systems with more than two subsystems. For such systems, Horowitz [58] defined an information flow
However, defining unambiguous directed energy flows as transduced work from one subsystem to another remains challenging for more than two subsystems. Recall that in Section 3.3 we argued that in a bipartite system the dynamics of one subsystem at a fixed state of the other can be interpreted as a control-parameter variation on the fixed subsystem. Hence, any potential-energy changes can be interpreted as work done on the fixed subsystem by the dynamic evolution of the other subsystem. Applying this logic to multipartite systems still permits definition of how much work one subsystem contributes to changing the global potential energy, but not the explicit flow between two subsystems.
Working out conditions under which exact transduced energy flows can be resolved would be an interesting extension and could lead to useful insights for multipartite systems such as energy flows in collections of motors transporting cargoes.
7.3 Optimizing coupled work transducers
In Section 6.3 we illustrated that the sum of transduced power and information flows acts as a kind of bottleneck for the transduction of work in two-component engines. Optimizing a given two-component work transducer and studying which of the two pathways maximize throughput seems like an interesting extension.
A first step towards this goal was accomplished in [67] for a specific model capturing aspects of Fo−F1 ATP synthase. It was found that both transduced power and information flow are required to maximize output power and that maximal power tends to lead to equal subsystem entropy productions
7.4 Application to real-world machinery
Finally, it would be interesting to see the information-flow formalism applied to real-world machinery. This would involve measuring and modeling the dynamics of two components of a biomolecular system, e.g., both units of Fo−F1 ATP synthase, instead of only the dynamics of F1 as is conventionally done in most single-molecule experiments and theory [53, 113, 177–179]. This can be accomplished, e.g., by observing two components of a biomolecular system and explicitly calculating information flow, possibly revealing the ratchet mechanism of a Maxwell’s demon at work. A first step towards this is found in [49] where a synthetic chemical information motor is analyzed: the authors bridge their information-flow analysis to a chemical-reaction analysis [180] and identify regimes in which energy or information is the dominant driving mechanism. Another recent contribution in this direction is [50] where information flow has been calculated explicitly for dimeric molecular motors. Finally, Freitas and Esposito recently suggested [181] a macroscopic Maxwell demon based on CMOS technology and analyzed the information flow between its components [182].
Author contributions
JE and DS conceived the review and wrote and edited the manuscript. Both authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was supported by grant FQXi-IAF19-02 from the Foundational Questions Institute Fund, a donor-advised fund of the Silicon Valley Community Foundation. Additional support was from a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (DS) and a Tier-II Canada Research Chair (DS).
Acknowledgments
We thank Matthew Leighton and John Bechhoefer (SFU Physics) and Mathis Grelier (Grenoble Physics) for helpful conversations and feedback on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphy.2023.1108357/full#supplementary-material
References
2. Seifert U. Stochastic thermodynamics, fluctuation theorems and molecular machines. Rep Prog Phys (2012) 75:126001. doi:10.1088/0034-4885/75/12/126001
3. Jarzynski C. Equalities and inequalities: Irreversibility and the second law of thermodynamics at the nanoscale. Annu Rev Condens Matter (2011) 2:329–51. doi:10.1146/annurev-conmatphys-062910-140506
4. Van den Broeck C, Esposito M. Ensemble and trajectory thermodynamics: A brief introduction. Physica A (2015) 418:6–16. doi:10.1016/j.physa.2014.04.035
5. Peliti L, Pigolotti S. Stochastic thermodynamics: An introduction. Princeton, NJ and Oxford: Princeton University Press (2021).
6. Knott CG. Life and Scientific Work of Peter Guthrie Tait. London: Cambridge University Press (1911). p. 213.
8. Szilard L. Über die Entropieverminderung in einem thermodynamischen System bei Eingriffen intelligenter Wesen. Z Phys (1929) 53:840–856. doi:10.1007/BF01341281
9. Landauer R. Irreversibility and heat generation in the computing process. IBM J Res Dev (1961) 5:183–91. doi:10.1147/rd.53.0183
10. Bennett CH. The thermodynamics of computation—A review. Int J Theor Phys (1982) 21:905–40. doi:10.1007/bf02084158
11. Cao FJ, Feito M. Thermodynamics of feedback controlled systems. Phys Rev E (2009) 79:041118. doi:10.1103/PhysRevE.79.041118
12. Sagawa T, Ueda M. Generalized Jarzynski equality under nonequilibrium feedback control. Phys Rev Lett (2010) 104:090602. doi:10.1103/PhysRevLett.104.090602
13. Ponmurugan M. Generalized detailed fluctuation theorem under nonequilibrium feedback control. Phys Rev E (2010) 82:031129. doi:10.1103/PhysRevE.82.031129
14. Horowitz JM, Vaikuntanathan S. Nonequilibrium detailed fluctuation theorem for repeated discrete feedback. Phys Rev E (2010) 82:061120. doi:10.1103/PhysRevE.82.061120
15. Sagawa T, Ueda M. Nonequilibrium thermodynamics of feedback control. Phys Rev E (2012) 85:021104. doi:10.1103/PhysRevE.85.021104
16. Sagawa T, Ueda M. Fluctuation theorem with information exchange: Role of correlations in stochastic thermodynamics. Phys Rev Lett (2011) 109:180602. doi:10.1103/PhysRevLett.109.180602
17. Sagawa T, Ueda M. Role of mutual information in entropy production under information exchanges. New J Phys (2013) 15:125012. doi:10.1088/1367-2630/15/12/125012
18. Mandal D, Jarzynski C. Work and information processing in a solvable model of Maxwell’s demon. Proc Nat Acad Sci (2012) 109:11641–5. doi:10.1073/pnas.1204263109
19. Mandal D, Quan HT, Jarzynski C. Maxwell’s refrigerator: An exactly solvable model. Phys Rev Lett (2013) 111:030602. doi:10.1103/PhysRevLett.111.030602
20. Barato A, Seifert U. Unifying three perspectives on information processing in stochastic thermodynamics. Phys Rev Lett (2014) 112:090601. doi:10.1103/PhysRevLett.112.090601
21. Barato A, Seifert U. Stochastic thermodynamics with information reservoirs. Phys Rev E (2014) 90:042150. doi:10.1103/PhysRevE.90.042150
22. Parrondo JMR, Horowitz JM, Sagawa T. Thermodynamics of information. Nat Phys (2015) 11:131–9. doi:10.1038/NPHYS3230
23. Bauer M, Abreu D, Seifert U. Efficiency of a Brownian information machine. J Phys A: Math Theo (2012) 45:162001. doi:10.1088/1751-8113/45/16/162001
24. Schmitt RK, Parrondo JMR, Linke H, Johansson J. Molecular motor efficiency is maximized in the presence of both power-stroke and rectification through feedback. New J Phys (2015) 17:065011. doi:10.1088/1367-2630/17/6/065011
25. Bechhoefer J. Hidden Markov models for stochastic thermodynamics. New J Phys (2015) 17:075003. doi:10.1088/1367-2630/17/7/075003
26. Still S. Thermodynamic cost and benefit of memory. Phys Rev Lett (2020) 124:050601. doi:10.1103/PhysRevLett.124.050601
27. Lucero JNE, Ehrich J, Bechhoefer J, Sivak DA. Maximal fluctuation exploitation in Gaussian information engines. Phys Rev E (2021) 104:044122. doi:10.1103/PhysRevE.104.044122
29. Still S, Daimer D. Partially observable Szilard engines. New J Phys (2022) 24:073031. doi:10.1088/1367-2630/ac6b30
30. Serreli V, Lee CF, Kay ER, Leigh DA. A molecular information ratchet. Nature (2007) 445:523–7. doi:10.1038/nature05452
31. Bannerman ST, Price GN, Viering K, Raizen MG. Single-photon cooling at the limit of trap dynamics: Maxwell’s demon near maximum efficiency. New J Phys (2009) 11:063044. doi:10.1088/1367-2630/11/6/063044
32. Toyabe S, Sagawa T, Ueda M, Muneyuki E, Sano M. Experimental demonstration of information-to-energy conversion and validation of the generalized Jarzynski equality. Nat Phys (2010) 6:988–92. doi:10.1038/NPHYS1821
33. Koski JV, Maisi VF, Sagawa T, Pekola JP. Experimental observation of the role of mutual information in the nonequilibrium dynamics of a Maxwell demon. Phys Rev Lett (2014) 113:030601. doi:10.1103/PhysRevLett.113.030601
34. Koski JV, Maisi VF, Pekola JP, Averin DV. Experimental realization of a Szilard engine with a single electron. Proc Natl Acad Sci U.S.A (2014) 111:13786–9. doi:10.1073/pnas.1406966111
35. Koski JV, Kutvonen A, Khaymovich IM, Ala-Nissila T, Pekola JP. On-chip Maxwell’s demon as an information-powered refrigerator. Phys Rev Lett (2015) 115:260602. doi:10.1103/PhysRevLett.115.260602
36. Vidrighin MD, Dahlsten O, Barbieri M, Kim SM, Vedral V, Walmsley IA. Photonic Maxwell’s demon. Phys Rev Lett (2016) 116:050401. doi:10.1103/PhysRevLett.116.050401
37. Camati PA, Peterson JPS, Batalhao TB, Micadei K, Souza AM, Sarthour RS, et al. Experimental rectification of entropy production by Maxwell’s demon in a quantum system. Phys Rev Lett (2016) 117:240502. doi:10.1103/PhysRevLett.117.240502
38. Chida K, Desai S, Nishiguchi K, Fujiwara A. Power generator driven by Maxwell’s demon. Nat Commun (2017) 8:15310. doi:10.1038/ncomms15301
39. Cottet N, Jezouin S, Bretheau L, Campagne-Ibarcq P, Ficheux Q, Anders J, et al. Observing a quantum Maxwell demon at work. Proc Natl Acad Sci USA (2017) 114:7561–4. doi:10.1073/pnas.1704827114
40. Paneru G, Lee DY, Tlusty T, Pak HK. Lossless Brownian information engine. Phys Rev Lett (2018) 120:020601. doi:10.1103/PhysRevLett.120.020601
41. Masuyama Y, Funo K, Murashita Y, Noguchi A, Kono S, Tabuchi Y, et al. Information-to-work conversion by Maxwell’s demon in a superconducting circuit quantum electrodynamical system. Nat Commun (2018) 9:1291. doi:10.1038/s41467-018-03686-y
42. Naghiloo M, Alonso JJ, Rmotio A, Lutz E, Murch KW. Information gain and loss for a quantum Maxwell’s demon. Phys Rev Lett (2018) 121:030604. doi:10.1103/PhysRevLett.121.030604
43. Admon T, Rahav S, Roichman Y. Experimental realization of an information machine with tunable temporal correlations. Phys Rev Lett (2018) 121:180601. doi:10.1103/PhysRevLett.121.180601
44. Paneru G, Lee DY, Park JM, Park JT, Noh JD, Pak HK. Optimal tuning of a Brownian information engine operating in a nonequilibrium steady state. Phys Rev E (2018) 98:052119. doi:10.1103/PhysRevE.98.052119
45. Ribezzi-Crivellari M, Ritort F. Large work extraction and the Landauer limit in a continuous Maxwell demon. Nat Phys (2019) 15:660–4. doi:10.1038/s41567-019-0481-0
46. Paneru G, Dutta S, Sagawa T, Tlusty T, Pak HK. Efficiency fluctuations and noise induced refrigerator-to-heater transition in information engines. Nat Commun (2020) 11:1012–8. doi:10.1038/s41467-020-14823-x
47. Saha TK, Lucero JNE, Ehrich J, Sivak DA, Bechhoefer J. Maximizing power and velocity of an information engine. Proc Natl Acad Sci USA (2021) 118:e2023356118. doi:10.1073/pnas.2023356118
48. Saha TK, Lucero JNE, Ehrich J, Sivak DA, Bechhoefer J. Bayesian information engine that optimally exploits noisy measurements. Phys Rev Lett (2022) 129:130601. doi:10.1103/PhysRevLett.129.130601
49. Amano S, Esposito M, Kreidt E, Leigh DA, Penocchio E, Roberts BMW. Insights from an information thermodynamics analysis of a synthetic molecular motor. Nat Chem (2022) 14:530–7. doi:10.1038/s41557-022-00899-z
50. Takaki R, Mugnai ML, Thirumalai D. Information flow, gating, and energetics in dimeric molecular motors. Proc Nat Acad Sci USA (2022) 119:e2208083119. doi:10.1073/pnas.2208083119
51. Wilson MR, Solà J, Carlone A, Goldup SM, Lebrasseur N, Leigh DA. An autonomous chemically fuelled small-molecule motor. Nature (2016) 534:235–40. doi:10.1038/nature18013
52. Feniouk BA, Cherepanov DA, Junge W, Mulkidjanian AY. Atp-synthase of Rhodobacter capsulatus: Coupling of proton flow through Fo to reactions in F1 under the ATP synthesis and slip conditions. FEBS Lett (1999) 445:409–14. doi:10.1016/S0014-5793(99)00160-X
53. Toyabe S, Watanabe-Nakayama T, Okamoto T, Kudo S, Muneyuki E. Thermodynamic efficiency and mechanochemical coupling of F1-ATPase. Proc Natl Acad Sci USA (2011) 108:17951–6. doi:10.1073/pnas.1106787108
54. Lathouwers E, Lucero JN, Sivak DA. Nonequilibrium energy transduction in stochastic strongly coupled rotary motors. J Phys Chem Lett (2020) 11:5273–8. doi:10.1021/acs.jpclett.0c01055
55. Large SJ, Ehrich J, Sivak DA. Free energy transduction within autonomous systems. Phys Rev E (2021) 103:022140. doi:10.1103/PhysRevE.103.022140
56. Shiraishi N, Ito S, Kawaguchi K, Sagawa T. Role of measurement-feedback separation in autonomous Maxwell’s demons. New J Phys (2015) 17:045012. doi:10.1088/1367-2630/17/4/045012
57. Horowitz JM, Esposito M. Thermodynamics with continuous information flow. Phys Rev X (2014) 4:031015. doi:10.1103/PhysRevX.4.031015
58. Horowitz JM. Multipartite information flow for multiple Maxwell demons. J Stat Mech (2015) 2015:P03006. doi:10.1088/1742-5468/2015/03/P03006
59. Freitas N, Esposito M. Characterizing autonomous Maxwell demons. Phys Rev E (2021) 103:032118. doi:10.1103/PhysRevE.103.032118
60. Barkai N, Leibler S. Robustness in simple biochemical networks. Nature (1997) 387:913–7. doi:10.1038/43199
61. Sourjik V, Wingreen NS. Responding to chemical gradients: Bacterial chemotaxis. Curr Op Cel Bio. (2012) 24:262–8. doi:10.1016/j.ceb.2011.11.008
62. Mehta P, Schwab DJ. Energetic costs of cellular computation. Proc Nat Acad Sci USA (2012) 109:17978–82. doi:10.1073/pnas.1207814109
63. Barato AC, Hartich D, Seifert U. Rate of mutual information between coarse-grained non-markovian variables. J Stat Phys (2013) 153:460–78. doi:10.1007/s10955-013-0834-5
64. Barato AC, Hartich D, Seifert U. Information-theoretic versus thermodynamic entropy production in autonomous sensory networks. Phys Rev E (2013) 87:042104. doi:10.1103/PhysRevE.87.042104
65. Barato AC, Hartich D, Seifert U. Efficiency of cellular information processing. New J Phys (2014) 16:103024. doi:10.1088/1367-2630/16/10/103024
66. Junge W, Nelson N. ATP synthase. Annu Rev Biochem (2015) 84:631–57. doi:10.1146/annurev-biochem-060614-034124
67. Lathouwers E, Sivak DA. Internal energy and information flows mediate input and output power in bipartite molecular machines. Phys Rev E (2022) 105:024136. doi:10.1103/PhysRevE.105.024136
68. Leopold PL, McDowall AW, Pfister KK, Bloom GS, Brady ST. Association of Kinesin with characterized membrane-bounded organelles. Cell Motil. Cytoskeleton (1992) 23:19–33. doi:10.1002/cm.970230104
69. Rastogi K, Puliyakodan MS, Pandey V, Nath S, Elangovan R. Maximum limit to the number of myosin II motors participating in processive sliding of actin. Nat Sci. Rep. (2016) 6:32043. doi:10.1038/srep32043
70. Ciliberto S. Experiments in stochastic thermodynamics: Short history and perspectives. Phys Rev X (2017) 7:021051. doi:10.1103/PhysRevX.7.021051
71. Reimann P. Brownian motors: noisy transport far from equilibrium. Phys Rep (2002) 461:57–265. doi:10.1016/S0370-1573(01)00081-3
72. Chowdhury D. Stochastic mechano-chemical kinetics of molecular motors: A multidisciplinary enterprise from a physicist’s perspective. Phys Rep (2013) 529:1–197. doi:10.1016/j.physrep.2013.03.005
73. Kolomeisky AB. Motor proteins and molecular motors: How to operate machines at the nanoscale. J Phys Condens Matter (2013) 25:463101. doi:10.1088/0953-8984/25/46/463101
74. Brown AI, Sivak DA. Theory of nonequilibrium free energy transduction by molecular machines. Chem Rev (2020) 120:434–59. doi:10.1021/acs.chemrev.9b00254
75. Chowdhury D. An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F1F0 ATP synthases. J Bioenerg Biomembr (2014) 46:229–41. doi:10.1007/s10863-014-9547-y
76. Li CB, Toyabe S. Efficiencies of molecular motors: A comprehensible overview. Biophys Rev (2020) 12:419–23. doi:10.1007/s12551-020-00672-x
77. van Kampen NG. Stochastic processes in Physics and chemistry. 3rd ed. Amsterdam: Elsevier (2007).
79. Chétrite R, Rosinberg ML, Sagawa T, Tarjus G. Information thermodynamics for interacting stochastic systems without bipartite structure. J Stat Mech (2019) 2019:114002. doi:10.1088/1742-5468/ab47fe
80. Ptaszyński K, Esposito M. Thermodynamics of quantum information flows. Phys Rev Lett (2019) 122:150603. doi:10.1103/PhysRevLett.122.150603
81. Cover TM, Thomas JA. Elements of information theory. 2nd ed. Hoboken, NJ: Wiley-Interscience (2006).
83. Seifert U. From stochastic thermodynamics to thermodynamic inference. Annu Rev Condens Matter (2019) 10:171–92. doi:10.1146/annurev-conmatphys-031218-013554
84. Bergmann PG, Lebowitz JL. New approach to nonequilibrium processes. Phys Rev (1955) 99:578–87. doi:10.1103/PhysRev.99.578
85. Maes C. Local detailed balance. Scipost Phys Lect Notes (2021) 32. doi:10.21468/SciPostPhysLectNotes.32
86. Esposito M. Stochastic thermodynamics under coarse graining. Phys Rev E (2012) 85:041125. doi:10.1103/PhysRevE.85.041125
87. Allahverdyan AE, Janzing D, Mahler G. Thermodynamic efficiency of information and heat flow. J Stat Mech (2009) 2009:P09011. doi:10.1088/1742-5468/2009/09/P09011
88. Gaveau B, Schulman LS. A general framework for non-equilibrium phenomena: The master equation and its formal consequences. Phys Lett A (1997) 229:347–53. doi:10.1016/S0375-9601(97)00185-0
89. Gaveau B, Moreau M, Schulman LS. Work and power production in non-equilibrium systems. Phys Lett A (2008) 372:3415–22. doi:10.1016/j.physleta.2008.01.081
90. Esposito M, Van den Broeck C. Second law and Landauer principle far from equilibrium. Europhys Lett (2011) 95:40004. doi:10.1209/0295-5075/95/40004
91. Sivak DA, Crooks GE. Near-equilibrium measurements of nonequilibrium free energy. Phys Rev Lett (2012) 108:150601. doi:10.1103/PhysRevLett.108.150601
92. Zimmerman E, Seifert U. Effective rates from thermodynamically consistent coarse-graining of models for molecular motors with probe particles. Phys Rev E (2015) 91:022709. doi:10.1103/PhysRevE.91.022709
93. Shiraishi N, Sagawa T. Fluctuation theorem for partially masked nonequilibrium dynamics. Phys Rev E (2015) 91:012130. doi:10.1103/physreve.91.012130
94. Polettini M, Esposito M. Effective thermodynamics for a marginal observer. Phys Rev Lett (2017) 119:240601. doi:10.1103/physrevlett.119.240601
95. Bisker G, Polettini M, Gingrich TR, Horowitz JM. Hierarchical bounds on entropy production inferred from partial information. J Stat Mech (2017) 2017:093210. doi:10.1088/1742-5468/aa8c0d
96. Martínez IA, Bisker G, Horowitz JM, Parrondo JMR. Inferring broken detailed balance in the absence of observable currents. Nat Comm (2019) 10:3542. doi:10.1038/s41467-019-11051-w
97. Skinner DJ, Dunkel J. Improved bounds on entropy production in living systems. Proc Nat Acad Sci (2021) 118:e2024300118. doi:10.1073/pnas.2024300118
98. Ehrich J. Tightest bound on hidden entropy production from partially observed dynamics. J Stat Mech (2021) 2021:083214. doi:10.1088/1742-5468/ac150e
99. Skinner DJ, Dunkel J. Estimating entropy production from waiting time distributions. Phys Rev Lett (2021) 127:198101. doi:10.1103/PhysRevLett.127.198101
100. Hartich D, Godec A. Violation of local detailed balance despite a clear time-scale separation. arXiv:2111.14734 (2021).
101. van der Meer J, Ertel B, Seifert U. Thermodynamic inference in partially accessible Markov networks: A unifying perspective from transition-based waiting time distributions. Phys Rev X (2022) 12:031025. doi:10.1103/PhysRevX.12.031025
102. Harunari PE, Dutta A, Polettini M, Roldán E. What to learn from a few visible transitions’ statistics? Phys Rev (2022) X:041026. doi:10.1103/PhysRevX.12.041026
103. Large SJ, Sivak DA. Hidden energy flows in strongly coupled nonequilibrium systems. Europhys Lett (2021) 133:10003. doi:10.1209/0295-5075/133/10003
104. Mehl J, Lander B, Bechinger C, Blickle V, Seifert U. Role of hidden slow degrees of freedom in the fluctuation theorem. Phys Rev Lett (2012) 108:220601. doi:10.1103/PhysRevLett.108.220601
105. Uhl M, Pietzonka P, Seifert U. Fluctuations of apparent entropy production in networks with hidden slow degrees of freedom. J Stat Mech (2018) 2018:023203. doi:10.1088/1742-5468/aaa78b
106. Kahlen M, Ehrich J. Hidden slow degrees of freedom and fluctuation theorems: An analytically solvable model. J Stat Mech (2018) 2018:063204. doi:10.1088/1742-5468/aac2fd
107. Barato AC, Seifert U. Thermodynamic uncertainty relation for biomolecular processes. Phys Rev Lett (2015) 114:158101. doi:10.1103/PhysRevLett.114.158101
108. Gingrich TR, Horowitz JM, Perunov N, England JL. Dissipation bounds all steady-state current fluctuations. Phys Rev Lett (2016) 116:120601. doi:10.1103/PhysRevLett.116.120601
109. Li J, Horowitz JM, Gingrich TR, Fakhri N. Quantifying dissipation using fluctuating currents. Nat Comm (2019) 10:1666. doi:10.1038/s41467-019-09631-x
110. Manikandan SK, Gupta D, Krishnamurthy S. Inferring entropy production from short experiments. Phys Rev Lett (2020) 124:120603. doi:10.1103/PhysRevLett.124.120603
111. Vu TV, Vo VT, Hasegawa Y. Entropy production estimation with optimal current. Phys Rev E (2020) 101:042138. doi:10.1103/PhysRevE.101.042138
112. Pietzonka P, Zimmermann E, Seifert U. Fine-structured large deviations and the fluctuation theorem: Molecular motors and beyond. Europhys Lett (2014) 107:20002. doi:10.1209/0295-5075/107/20002
113. Kawaguchi K, Si S, Sagawa T. Nonequilibrium dissipation-free transport in F1-ATPase and the thermodynamic role of asymmetric allosterism. Biophys J (2014) 106:2450–7. doi:10.1016/j.bpj.2014.04.034
114. Still S, Sivak DA, Bell AJ, Crooks GE. Thermodynamics of prediction. Phys Rev Lett (2012) 109:120604. doi:10.1103/PhysRevLett.109.120604
115. Lahiri S, Rana S, Jayannavar AM. Fluctuation theorems in the presence of information gain and feedback. J Phys A: Math Theor (2012) 45:065002. doi:10.1088/1751-8113/45/6/065002
116. Crooks GE, Still S. Marginal and conditional second laws of thermodynamics. Europhys Lett (2019) 125:40005. doi:10.1209/0295-5075/125/40005
117. Strasberg P, Schaller G, Brandes T, Esposito M. Thermodynamics of a physical model implementing a Maxwell demon. Phys Rev Lett (2013) 110:040601. doi:10.1103/PhysRevLett.110.040601
118. Ciliberto S. Autonomous out-of-equilibrium Maxwell's demon for controlling the energy fluxes produced by thermal fluctuations. Phys Rev E (2020) 102(R):050103. doi:10.1103/PhysRevE.102.050103
119. Bialek W, Setayeshgar S. Physical limits to biochemical signaling. Proc Nat Acad Sci (2005) 102:10040–5. doi:10.1073/pnas.0504321102
120. Tu Y. The nonequilibrium mechanism for ultrasensitivity in a biological switch: Sensing by Maxwell’s demons. Proc Nat Acad Sci (2007) 105:11737–41. doi:10.1073/pnas.0804641105
121. Lan G, Tu Y. Information processing in bacteria: Memory, computation, and statistical physics: A key issues review. Rep Prog Phys (2016) 79:052601. doi:10.1088/0034-4885/79/5/052601
122. Mattingly HH, Kamino K, Machta BB, Emonet T. Escherichia coli chemotaxis is information limited. Nat Phys (2021) 17:1426–31. doi:10.1038/s41567-021-01380-3
123. Berg HC, Purcell EM. Physics of chemoreception. Phys chemoreception. Biophys. J. (1977) 20:193–219. doi:10.1016/S0006-3495(77)85544-6
124. Endres RG, Wingreen NS. Maximum likelihood and the single receptor. Phys Rev Lett (2009) 103:158101. doi:10.1103/PhysRevLett.103.158101
125. Govern CC, ten Wolde PR. Fundamental limits on sensing chemical concentrations with linear biochemical networks. Phys Rev Lett (2012) 109:218103. doi:10.1103/PhysRevLett.109.218103
126. ten Wolde PR, Becker NB, Ouldridge TE, Mugler A. Fundamental limits to cellular sensing. J Stat Phys (2016) 162:1395–424. doi:10.1007/s10955-015-1440-5
127. Lan G, Sartori P, Neumann S, Sourjik V, Tu Y. The energy–speed–accuracy trade-off in sensory adaptation. Nat Phys (2012) 8:422–8. doi:10.1038/nphys2276
128. Govern CC, ten Wolde PR. Optimal resource allocation in cellular sensing systems. Proc Nat Acad Sci (2014) 111:17486–91. doi:10.1073/pnas.1411524111
129. Sartori P, Granger L, Lee CF, Horowitz JM. Thermodynamic costs of information processing in sensory adaptation. Plos Comput Biol (2014) 10:e1003974. doi:10.1371/journal.pcbi.1003974
130. Govern CC, ten Wolde PR. Energy dissipation and noise correlations in biochemical sensing. Phys Rev Lett (2014) 113:258102. doi:10.1103/PhysRevLett.113.258102
131. Bo S, Del Giudice M, Celani A. Thermodynamic limits to information harvesting by sensory systems. J Stat Mech (2015) 2015:P01014. doi:10.1088/1742-5468/2015/01/P01014
132. Ouldrudge TE, Govern CC, ten Wolde PR. Thermodynamics of computational copying in biochemical systems. Phys Rev X (2017) 7:021004. doi:10.1103/PhysRevX.7.021004
133. Hartich D, Barato AC, Seifert U. Stochastic thermodynamics of bipartite systems: Transfer entropy inequalities and a Maxwell’s demon interpretation. J Stat Mech (2014) 2014:P02016. doi:10.1088/1742-5468/2014/02/P02016
134. Tostevin F, ten Wolde PR. Mutual information between input and output trajectories of biochemical networks. Phys Rev Lett (2009) 102:218101. doi:10.1103/PhysRevLett.102.218101
135. Diana G, Esposito M. Mutual entropy production in bipartite systems. J Stat Mech (2014) 2014:P04010. doi:10.1088/1742-5468/2014/04/P04010
136. Schreiber T. Measuring information transfer. Phys Rev Lett (2000) 85:461–4. doi:10.1103/PhysRevLett.85.461
137. Marko H. The bidirectional communication theory - a generalization of information theory. IEEE Trans Comm (1973) 21:1345–51. doi:10.1109/TCOM.1973.1091610
138. Massey JL. Causality, feedback and directed information. In: Proc. 1990 Intl. Symp. on Info. Th. and its Applications; Waikiki, Hawaii (1990). 1.
140. Hartich D, Barato AC, Seifert U. Sensory capacity: An information theoretical measure of the performance of a sensor. Phys Rev E (2016) 93:022116. doi:10.1103/physreve.93.022116
141. Horowitz JM, Sandberg H. Second-law-like inequalities with information and their interpretations. New J Phys (2014) 16:125007. doi:10.1088/1367-2630/16/12/125007
142. Särkkä S. Bayesian filtering and smoothing. New York: Cambridge University Press (2013). doi:10.1017/cbo9781139344203
143. Brittain RA, Jones NS, Ouldridge TE. What we learn from the learning rate. J Stat Mech (2017) 2017:063502. doi:10.1088/1742-5468/aa71d4
144. Bustamane CJ, Chemla YR, Liu S, Wang MD. Optical tweezers in single-molecule biophysics. Nat Rev Methods Primers (2021) 1:25. doi:10.1038/s43586-021-00021-6
145. Megli A, Praly E, Ding F, Allemand JF, Bensimon D, Croquette V. Single DNA/protein studies with magnetic traps. Curr Op Struct Bio (2009) 19:615–22. doi:10.1016/j.sbi.2009.08.005
146. Neumann K, Nagy A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods (1008) 5:491–505. doi:10.1038/nmeth.1218
147. Sekimoto K. Langevin equation and thermodynamics. Prog Theo Phys Suppl (1998) 130:17–27. doi:10.1143/PTPS.130.17
149. Jarzynski C. Nonequilibrium equality for free energy differences. Phys Rev Lett (1997) 78:2690–3. doi:10.1103/PhysRevLett.78.2690
150. Crooks GE. Entropy production fluctuation theorem and the nonequilibrium work relation for free energy differences. Phys Rev E (1999) 60:2721–6. doi:10.1103/PhysRevE.60.2721
151. Seifert U. Entropy production along a stochastic trajectory and an integral fluctuation theorem. Phys Rev Lett (2005) 95:040602. doi:10.1103/PhysRevLett.95.040602
152. Schmiedl T, Seifert U. Optimal finite-time processes in stochastic thermodynamics. Phys Rev Lett (2007) 98:108301. doi:10.1103/PhysRevLett.98.108301
153. Then H, Engel A. Computing the optimal protocol for finite-time processes in stochastic thermodynamics. Phys Rev E (2008) 77:041105. doi:10.1103/PhysRevE.77.041105
154. Sivak DA, Crooks GE. Thermodynamic metrics and optimal paths. Phys Rev Lett (2012) 108:190602. doi:10.1103/PhysRevLett.108.190602
155. Zulkowski PR, Sivak DA, Crooks GE, DeWeese MR. Geometry of thermodynamic control. Phys Rev E (2012) 86:041148. doi:10.1103/PhysRevE.86.041148
156. Martínez IA, Petrosyan A, Guéry-Odelin D, Trizac E, Ciliberto S. Engineered swift equilibration of a brownian particle. Nat Phys (2016) 12:843–6. doi:10.1038/nphys3758
157. Tafoya S, Large SJ, Liu S, Bustamante C, Sivak DA. Using a system’s equilibrium behavior to reduce its energy dissipation in nonequilibrium processes. Proc Nat Acad Sci (2019) 116:5920–4. doi:10.1073/pnas.1817778116
158. Solon AP, Horowitz JM. Phase transition in protocols minimizing work fluctuations. Phys Rev Lett (2018) 120:180605. doi:10.1103/PhysRevLett.120.180605
159. Blaber S, Sivak DA. Skewed thermodynamic geometry and optimal free energy estimation. J Chem Phys (2020) 153:244119. doi:10.1063/5.0033405
160. Boyer PD. The ATP synthase - a splendid molecular machine. Annu Rev Biochem (1997) 66:717–49. doi:10.1146/annurev.biochem.66.1.717
161. Yoshida M, Muneyuki E, Hisabori T. ATP synthase a marvellous rotary engine of the cell. Nat Rev Mol Cel Biol. (2001) 2:669–77. doi:10.1038/35089509
162. Xing J, Liao JC, Oster G. Making ATP. Proc Nat Acad Sci (2005) 102:16539–46. doi:10.1073/pnas.0507207102
163. Golubeva N, Imparato A, Peliti L. Efficiency of molecular machines with continuous phase space. Europhys Lett (2012) 97:60005. doi:10.1209/0295-5075/97/60005
164. Ai G, Liu P, Ge H. Torque-coupled thermodynamic model for FoF1-ATPase. Phys Rev E (2017) 95:052413. doi:10.1103/PhysRevE.95.052413
165. Fogedby HC, Imparato A. A minimal model of an autonomous thermal motor. Europhys Lett (2017) 119:50007. doi:10.1209/0295-5075/119/50007
166. Suñé M, Imparato A. Efficiency fluctuations in steady-state machines. J Phys A: Mathm Theor (2019) 52:045003. doi:10.1088/1751-8121/aaf2f8
167. Encalada SE, Szpankowski L, Xia C, Goldstein L. Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell (2011) 144:551–65. doi:10.1016/j.cell.2011.01.021
168. Cooke R. Actomyosin interaction in striated muscle. Phys Rev (1997) 77:671–97. doi:10.1152/physrev.1997.77.3.671
169. Klumpp S, Lipowsky R. Cooperative cargo transport by several molecular motors. Proc Nat Acad Sci USA (2005) 102:17284–9. doi:10.1073/pnas.0507363102
170. Bhat D, Gopalakrishnan M. Transport of organelles by elastically coupled motor proteins. Eur Phus J E (2016) 39:71. doi:10.1140/epje/i2016-16071-0
171. Bhat D, Gopalakrishnan M. Stall force of a cargo driven by n interacting motor proteins. Europhys Lett (2017) 117:28004. doi:10.1209/0295-5075/117/28004
172. Wagoner JA, Dill KA. Evolution of mechanical cooperativity among myosin II motors. Proc Nat Acad Sci USA (2021) 118:e2101871118. doi:10.1073/pnas.2101871118
173. Leighton MP, Sivak DA. Performance scaling and trade-offs for collective motor-driven transport. New J Phys (2022) 24:013009. doi:10.1088/1367-2630/ac3db7
174. Leighton MP, Sivak DA. Dynamic and thermodynamic bounds for collective motor-driven transport. Phys Rev Lett (2022) 129:118102. doi:10.1103/PhysRevLett.129.118102
175. Wachtel A, Rao R, Esposito M. Free-energy transduction in chemical reaction networks: From enzymes to metabolism. arXiv:2202.01316 (2022).
176. Barato AC, Seifert U. Thermodynamic cost of external control. New J Phys (2017) 19:073021. doi:10.1088/1367-2630/aa77d0
177. Yasuda R, Noji H, Yoshida M, Kinosita K, Itoh H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-Atpase. Nature (2001) 410:898–904. doi:10.1038/35073513
178. Toyabe S, Okamoto T, Watanabe-Nakayama T, Taketani H, Kudo S, Muneyuki E. Nonequilibrium energetics of a single F1-ATPase molecule. Phys Rev Lett (2010) 104:198103. doi:10.1103/PhysRevLett.104.198103
179. Hayashi R, Sasaki K, Nakamura S, Kudo S, Inoue Y, Noji H, et al. Giant acceleration of diffusion observed in a single-molecule experiment on F1−ATPase. Phys Rev Lett (2015) 114:248101. doi:10.1103/PhysRevLett.114.248101
180. Penocchio E, Avanzini F, Esposito M. Information thermodynamics for deterministic chemical reaction networks. J Chem Phys (2022) 157:034110. doi:10.1063/5.0094849
181. Freitas N, Esposito M. Maxwell demon that can work at macroscopic scales. Phys Rev Lett (2022) 129:120602. doi:10.1103/PhysRevLett.129.120602
Keywords: information thermodynamics, stochastic thermodynamics, nonequilibrium statistical mechanics, molecular motor, biochemical sensor, entropy production
Citation: Ehrich J and Sivak DA (2023) Energy and information flows in autonomous systems. Front. Phys. 11:1108357. doi: 10.3389/fphy.2023.1108357
Received: 25 November 2022; Accepted: 17 February 2023;
Published: 06 April 2023.
Edited by:
Deepak Bhat, VIT University, IndiaReviewed by:
Emanuele Penocchio, Northwestern University, United StatesBernardo A. Mello, University of Brasilia, Brazil
Nahuel Freitas, University of Buenos Aires, Argentina
Copyright © 2023 Ehrich and Sivak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jannik Ehrich, amVocmljaEBzZnUuY2E=; David A. Sivak, ZHNpdmFrQHNmdS5jYQ==
 Jannik Ehrich
Jannik Ehrich David A. Sivak
David A. Sivak