- 1Department of Psychology, Ludwig-Maximilians-University, Munich, Germany
- 2Department of Psychiatry and Psychotherapy, University of Regensburg, Regensburg, Germany
Mentalizing, the ability to attribute mental states to others and oneself, is a cognitive function with high relevance for social interactions. Recent neuroscientific research has increasingly contributed to attempts to decompose this complex social cognitive function into constituting neurocognitive building blocks. Additionally, clinical research that focuses on social cognition to find links between impaired social functioning and neurophysiological deviations has accumulated evidence that mentalizing is affected in most psychiatric disorders. Recently, both lines of research have started to employ transcranial magnetic stimulation: the first to modulate mentalizing in order to specify its neurocognitive components, the latter to treat impaired mentalizing in clinical conditions. This review integrates findings of these two different approaches to draw a more detailed picture of the neurocognitive basis of mentalizing and its deviations in psychiatric disorders. Moreover, we evaluate the effectiveness of hitherto employed stimulation techniques and protocols, paradigms and outcome measures. Based on this overview we highlight new directions for future research on the neurocognitive basis of functional and dysfunctional social cognition.
Introduction
In the middle of the night your neighbor is desperately trying to open your door with his key. Why is he doing that and how will you react? Knowing that he just came back from a birthday party and being aware of his drinking habits, you are able to infer that he probably falsely believes it is his door he is trying to open. Instead of calling the police you might then help him to find his own apartment. This example illustrates how our ability to understand other people's behavior by attributing mental states like beliefs, desires or intentions, also known as Theory of Mind (ToM) reasoning or mentalizing, drives social interactions.
A fast growing body of evidence suggests that mentalizing is affected in most psychiatric disorders, including but not limited to major depressive disorder (MDD; see Schreiter et al., 2013), bipolar disorder (Bora et al., 2005; Van Rheenen and Rossell, 2013), social anxiety (Ribeiro and Fearon, 2010; Samson et al., 2012), borderline personality disorder (Ghiassi et al., 2010; Mier et al., 2013), eating disorders (Schulte-Rüther et al., 2012) and neurodegenerative diseases (Le Bouc et al., 2012; Poletti et al., 2012). Moreover, it has long been hypothesized that social cognitive deficits in autism spectrum disorders (ASD) and schizophrenia result from impaired mentalizing (Brüne and Brüne-Cohrs, 2006; Frith, 2012).
However, this research is still in an early stage. Inconclusive findings (e.g., Arntz et al., 2009; Schreiter et al., 2013) and a heterogeneous conceptualization of impaired social cognition do not yet allow for drawing firm conclusions about the role of impaired mentalizing in psychiatric disorders. To resolve this ambiguity, it has been suggested to focus on the neurocognitive building blocks of mentalizing in order to find links between symptomatic impairment of social interactions and neurophysiological deviations in psychiatric disorders (Frith, 2012; Kennedy and Adolphs, 2012; Happé and Frith, 2014).
Recently, cognitive neuroscientists started to specify these neurocognitive building blocks of mentalizing using transcranial magnetic stimulation (TMS, Hetu et al., 2012). A coil, placed on the skull over the brain area of interest, produces a focal magnetic field which passes through the skull largely undistorted and induces neuronal depolarization in superficial cortical areas. When TMS is applied over specific brain regions in the context of a cognitive task, the interference with behavioral performance enables the study of causal relations between brain activity, cognitive processes, and behavior (Walsh and Cowey, 2000; Robertson et al., 2003; Paus, 2005).
TMS studies on mentalizing almost exclusively use repetitive TMS (rTMS), which is why the current review focuses on this method. A detailed description of recent technical and methodological issues for the application of various TMS protocols in the study of cognition is provided elsewhere (Sandrini et al., 2011). Applying rTMS has both an immediate interrupting effect on neuronal processing in the stimulated area, and a modulatory after-effect, which outlasts the stimulation period by minutes to hours (cf., Eisenegger et al., 2008). The direction of this after-effect (inhibitory/excitatory) depends on stimulation parameters and baseline activity of the stimulated area. Accordingly, rTMS can be applied during the performance of a cognitive task (online) or before task performance (offline). A third possibility is the application of single pulses which interrupt neuronal activity for a short but well-defined period, useful for identifying temporal characteristics of neurocognitive processes.
Parallel to TMS research on functional mentalizing, clinical research began to employ TMS to treat impaired mentalizing in psychiatric disorders. For this purpose, repeated sessions of rTMS have been applied over periods of several weeks for the treatment of mentalizing deficits in ASD and MDD (Enticott et al., 2011, 2014; Berlim et al., 2012).
Here, we integrate and evaluate findings of these separately emerging lines of research to show how TMS can advance our understanding of the neurocognitive basis of mentalizing and its impairment in clinical conditions, specifically in ASD and MDD. Further, Table 1 provides methodological details of hitherto available brain stimulation studies on mentalizing. We suggest that TMS combined with sensitive experimental paradigms is a promising method to specify the neurocognitive architecture of functional and dysfunctional mentalizing, which can be the key to elucidate impaired social functioning in psychiatric disorders.
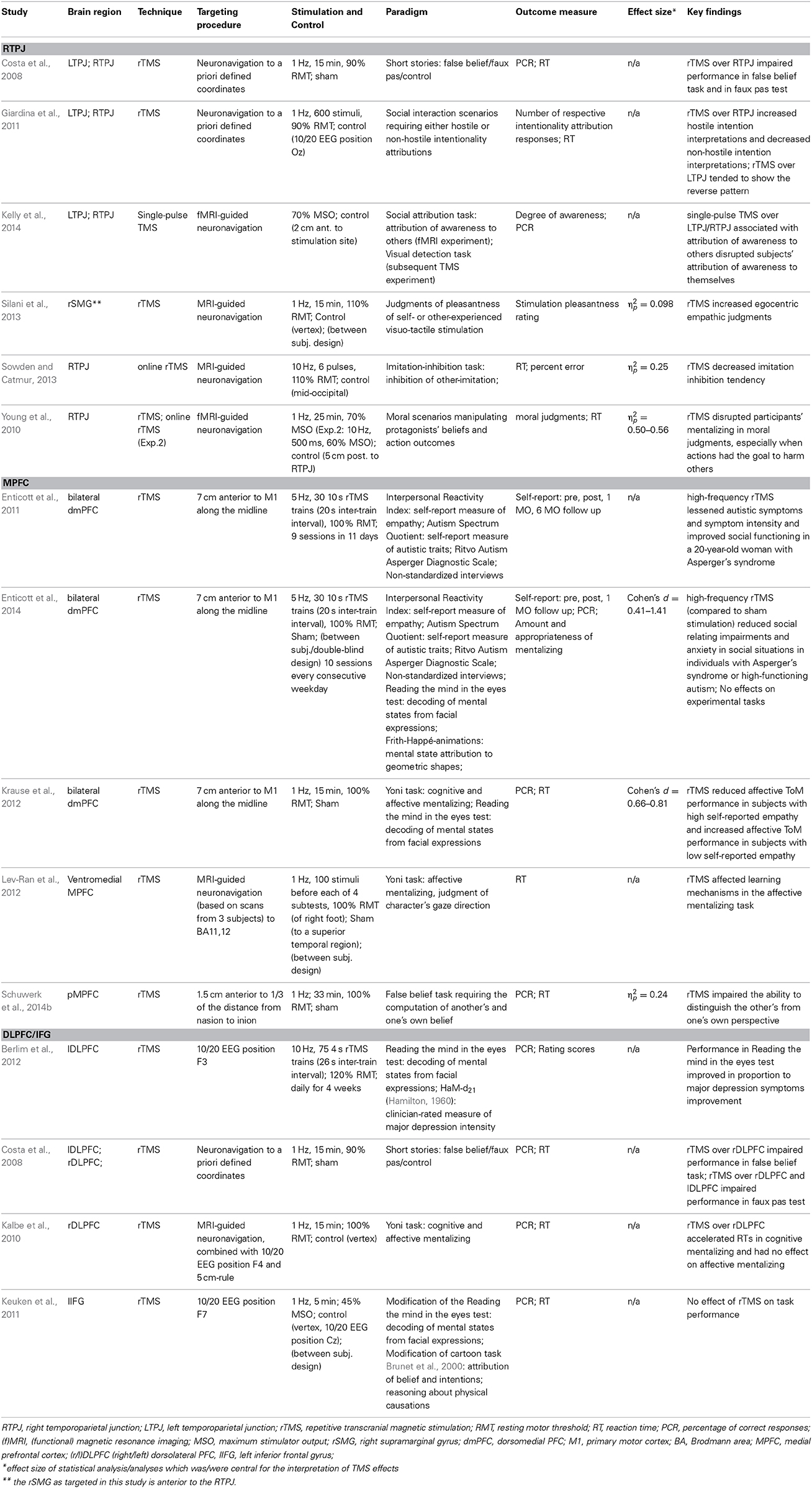
Table 1. Methodological details and key findings of available TMS studies on mentalizing sorted by targeted brain region.
What TMS Reveals About Mentalizing
The Neurocognitive Basis of Mentalizing
Based on a large corpus of findings about the neurophysiological basis of mentalizing (e.g., Van Overwalle, 2009; Mar, 2011), neuroscientific methods are increasingly employed to test specific hypotheses about the neurocognitive processes that constitute mentalizing. Here, we focus on brain regions that appear to be central for mentalizing and have been targeted in TMS studies thus far, namely the temporoparietal junction (TPJ), the dorsolateral prefrontal cortex (DLPFC), the inferior frontal gyrus (IFG), and the medial prefrontal cortex (MPFC; Figure 1).
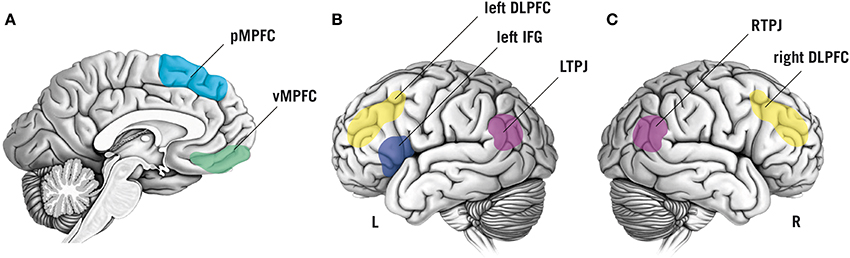
Figure 1. Schematic overview of brain regions that have been targeted by non-invasive brain stimulation to study functional and dysfunctional mentalizing. Color labels are approximate. (A) Sagittal view of the brain showing the posterior medial prefrontal cortex (pMPFC, indicated in light-blue) and the ventromedial prefrontal cortex (vMPFC, in green). (B) Lateral view of the left (L) hemisphere of the brain. Colored regions display the left inferior frontal gyrus (IFG; in dark-blue), the left dorsolateral prefrontal cortex (DLPFC; in yellow), and the left temporoparietal junction (LTPJ; in purple). (C) Lateral view of the right (R) hemisphere: the right DLPFC is displayed in yellow, the right temporoparietal junction (RTPJ) in purple.
Right TPJ and mental models of self and other
Consistently observed in neuroimaging studies on mentalizing, but little understood, is functional activity of the right TPJ (RTPJ)1. Costa et al. (2008) were the first to show that stimulation of the RTPJ interferes with mentalizing. Applying 1 Hz-rTMS impaired subjects' performance in a false belief task (questions on stories describing false beliefs about an object's location) and in a Faux Pas Test (questions on stories describing protagonists' mental states that led to awkward behavior). Consistently, Giardina et al. (2011) and Young et al. (2010) found that 1 Hz (/10 Hz-online)-rTMS over the RTPJ influenced the use of mental states in reasoning about ethical or unethical behavior of others.
Crucially, TMS can not only tell us that the RTPJ is involved in mentalizing, it also advances our understanding of its underlying function. As outlined below, recent TMS studies provide converging evidence for the idea that RTPJ's function in mentalizing is to handle internal models of one's own and other's mental states and their relation to the environment (Decety and Sommerville, 2003).
Several lines of evidence from neuroimaging and brain stimulation in single cases suggested that the RTPJ integrates multisensory input with internally stored information to form a first-person perspective, i.e., a coherent sense of one's own body situated in and distinguishable from the rest of the world (cf., Blanke et al., 2005; De Ridder et al., 2007; Ionta et al., 2011). This notion has been supported by a study in which RTPJ activity has been disrupted by single-pulse TMS during the rubber hand illusion, resulting in an impaired ability to distinguish between self-relevant (“this is part of my body”) and self-irrelevant (“this is not part of my body”) sensory information (Tsakiris et al., 2008). It was concluded that the RTPJ maintains an internal model of one's own body as a reference for self-relevance evaluations of incoming sensory information.
Heinisch et al. (2011; cf., Heinisch et al., 2012) provided evidence that the RTPJ is also involved in distinguishing self-relevant from other-relevant information. In this study subjects were presented with a picture of another person's face that morphed gradually into a picture of their own face or vice versa. The participants indicated the moment they recognized their own face, and the moment they were sure that they were seeing the face of another person, respectively. The application of 1 Hz-rTMS over the RTPJ biased this self-other discrimination toward self-face recognition at the expense of other-face recognition. Further evidence comes from a control-of-imitation task, in which the participants moved a finger either congruently or incongruently to a simultaneously observed finger movement of another person. The ability to control the tendency to imitate the other's movement was impaired by 10-Hz-online-rTMS (Sowden and Catmur, 2013)2.
In sum, these findings confirm the role of the RTPJ in mentalizing and specify that the RTPJ is critically relevant for simultaneously maintaining mental models of the self and others and their relation to the environment (“how do I perceive the world vs. how does the other perceive the world?”). This may be achieved by integrating sensory information from the environment with internally stored information (Cabeza et al., 2012) and with expectations and predictions about self and other (Koster-Hale and Saxe, 2013). By this we are able to flexibly switch between perspectives, depending on what is required in a certain situation.
DLPFC, IFG, and perspective inhibition
Evidence on the role of the DLPFC in mentalizing is still too sparse to draw firm conclusions about its neurocognitive role in mentalizing (cf., Costa et al., 2008; Kalbe et al., 2010). Further, only little TMS research has focused on the more ventral IFG, a region with much larger evidence on its involvement in mentalizing (Mar, 2011). It has been proposed that the IFG's role in mentalizing is perspective inhibition (Ruby and Decety, 2004; Ramsey et al., 2013). For example, when adopting another's perspective, one's own perspective has to be inhibited and vice versa. While the RTPJ maintains mental models of one's own and another's perspective, the IFG inhibits one of these models during perspective selection. To our knowledge only one study investigated the effect of rTMS over the left IFG (Keuken et al., 2011). In this study a relatively short 1 Hz-rTMS of 5 min had no effect on subsequent performance in two standard mentalizing tasks as compared to control stimulation. More studies employing well-suited tasks and stimulation protocols are required to test the IFG's causal role in mentalizing.
MPFC and decoupling
It was proposed that the MPFC's role in mentalizing is to subserve the decoupling mechanism, i.e., processing another's perspective independently from one's own view on the world (Leslie, 1987, 1994; Frith and Frith, 2003; Gallagher and Frith, 2003; Döhnel et al., 2012). Recent neuroimaging findings showed that during the computation of one's own and another's perspective, the posterior MPFC (pMPFC) is involved in establishing a perspective difference through inhibitory influence on temporoparietal brain regions (Schuwerk et al., 2014a). It seems that while the RTPJ maintains mental models of self and other by integrating internal (memory-based/predicted) and external (sensory) information, inhibitory influence of the pMPFC suppresses processing of external information to enable the decoupled computation of one's own and another's perspective. Consistent with this idea, inhibiting the pMPFC by 1 Hz-rTMS with a double-cone coil impaired the participant's ability to distinguish between one's own and another's perspective in a false belief task (Schuwerk et al., 2014b). In another study, 1 Hz-stimulation of the pMPFC with so-called “deep rTMS” modulated affective ToM performance in dependence of baseline empathic abilities (Krause et al., 2012). Taken together, these findings indicate that the pMPFC can be targeted by specific rTMS techniques and encourage future rTMS research focusing on that area.
TMS to Study and Treat Dysfunctional Mentalizing?
Recently, researchers began to test the therapeutic use of high-frequency rTMS on dysfunctional mentalizing in ASD and MDD. However, little is known about specifically impaired underlying neurocognitive mechanisms: ASD is characterized by widespread structural and functional brain abnormalities (Philip et al., 2012; Mueller et al., 2013). Among these, a reduced functional connectivity between the MPFC and RTPJ during mentalizing has been observed in individuals with ASD (Castelli et al., 2002; Kana et al., 2009). It can be hypothesized that the decoupled processing of one's own and another's perspective, mediated by inhibitory influence of the pMPFC to the temporoparietal cortex, is impaired in ASD. This is supported by the specific difficulty to attribute false beliefs to other people (Baron-Cohen et al., 1985; Senju et al., 2009), and close to the early hypothesis that a “failure of decoupling” underlies ASD (Leslie, 1987). If this were the case, could high-frequency stimulation of the pMPFC alleviate this mentalizing deficit?
In a double-blind randomized sham-controlled trial, Enticott et al. (2014) tested if high-frequency rTMS over the pMPFC improves impaired social functioning in ASD. Participants with high-functioning autism and Asperger's syndrome received 5 Hz-rTMS of the pMPFC on consecutive days over about 2 weeks. After active treatment as compared to sham treatment, patients reported reduced social relating impairments and anxiety in social situations (Enticott et al., 2014), as well as improved social functioning, including an increased capacity for perspective taking and empathy (Enticott et al., 2011).
Taken together, this fits with the hypothesis that the mentalizing deficit in ASD is related to impaired inhibitory influence of the pMPFC on the RTPJ. However, to provide direct evidence for this idea, future TMS studies have to show that (1) high-frequency TMS increases the connectivity between the pMPFC and RTPJ during mentalizing and (2) improves the ability to establish a perspective difference in a sensitive experimental task.
Clinical research indicates that a mentalizing deficit also plays a role in MDD (Schreiter et al., 2013). Compared to non-depressed controls, individuals with a current depressive episode showed a weaker performance in decoding mental states from facial expressions (Lee et al., 2005). Patients with a currently remitted MDD were impaired in a second-order false belief task (inferring thoughts about thoughts; Inoue et al., 2004) and had a higher risk for relapse 1 year later (Inoue et al., 2006). Also mentalizing-associated brain regions show abnormal functional activity and connectivity in depression (Aan Het Rot et al., 2009; Price and Drevets, 2012).
Is the neurocognitive basis of mentalizing affected in MDD? A prominent symptom of depressed patients is a high self-focus, i.e., a high attentional focus on oneself compared to others (e.g., Flory et al., 2000). An impaired ability to efficiently switch between one's own and another's perspective might be one contributing factor to this predominant self-focus. One's own negatively biased perspective constitutes the reference-point not only in judgments of one's own current and future situation, but also affects how one perceives the rest of the world (cf. the negative triad; Beck, 1972).
Presently, this idea remains speculative. Although a large body of evidence suggests that rTMS of the DLPFC has an antidepressant effect (Lefaucheur et al., 2014), we currently lack evidence on possible links between impaired neurocognitive components of mentalizing and depressive symptoms. To our knowledge, only one study has addressed this issue and found a relation between 10 Hz-rTMS to the left DLPFC, improved performance in a ToM task, and the alleviation of depressive symptoms (Berlim et al., 2012). However, these preliminary findings do not allow for firm conclusions about causal associations of these factors.
In sum, TMS seems to be a valuable tool in the investigation of the relationship between impaired mentalizing, its neuronal correlates, and related psychiatric disorders. At the same time it is definitely premature to claim that TMS constitutes a therapeutic strategy to improve impaired mentalizing. But in the light of accumulating evidence that brain stimulation may enhance (1) cognitive functioning in psychiatric disorders (Demirtas-Tatlidede et al., 2013) and (2) mentalizing in healthy subjects (Santiesteban et al., 2012), it can be regarded as a promising method to tackle dysfunctional mentalizing.
Effectiveness of Employed Stimulation Methods
All previously reported stimulation techniques (online and offline rTMS, single-pulse TMS), brain site localization procedures, and most stimulation protocols produced effects of interest (Table 1). Unfortunately, only half of the TMS studies on mentalizing reviewed here reported effect sizes. In these studies the effect sizes are medium to large. To improve the evaluation of observed findings and facilitate the design of future studies, comprehensive descriptions of all methodological aspects and detailed reporting of results, including effect sizes, are highly desirable.
A critical issue appears to be the employment of sensitive experimental paradigms. Particularly, adaptations of traditional ToM tests, such as the Reading the Mind in the Eyes Test (Baron-Cohen et al., 2001), ToM cartoons (Brunet et al., 2000) or social animations (e.g., Abell et al., 2000), produced heterogeneous results. Several authors stated that their employed tasks might not be sensitive enough to measure TMS-induced effects on behavioral outcome measures (e.g., Krause et al., 2012; Enticott et al., 2014; c.f., Keuken et al., 2011; Lev-Ran et al., 2012). Future studies must carefully design paradigms that allow for detecting TMS effects on reaction times and accuracy rates, the two most prominent outcome measures.
The development of sensitive mentalizing tasks for TMS research will also be critical for the evaluation of rTMS as a therapeutic approach for dysfunctional mentalizing. In a review on the role of social cognition in MDD, Schreiter et al. (2013) pointed out that especially objective measures, i.e., laboratory tasks, seem to be more reliable and sensitive than self-reports, for example.
Future Directions and Conclusions
To date, available TMS studies on mentalizing show that the RTPJ, DLPFC, and MPFC, identified by previous neuroimaging research on ToM, are causally involved in mentalizing. Given the correlational nature of functional magnetic resonance imaging or electroencephalography, this is a critical finding that adds to neuropsychological evidence on the causal role of those brain regions in ToM (e.g., Samson and Michel, 2013). Further, TMS is particularly suited to specify the neurocognitive building blocks of mentalizing, a current issue in ToM research. A major challenge for future research will be to develop sensitive paradigms to detect TMS-induced effects on mentalizing.
Future research should focus on the connectivity between mentalizing-associated brain regions. Both brain functions and stimulation effects are not restricted to specific regions, but have to be conceptualized as network effects. Only if we understand the brain as a network can we learn more about how the interplay of its regions underpins functional and dysfunctional social cognition (e.g., Kennedy and Adolphs, 2012). Although a set of brain regions which were linked to mentalizing by previous research is labeled “ToM network,” little is known about the critical interactions of those regions. One avenue for future research will be the simultaneous stimulation of several brain regions associated with mentalizing while another approach is the combination of brain stimulation and neuroimaging for the assessment of stimulation-induced network effects.
Claiming that TMS can be used as a therapeutic intervention for dysfunctional mentalizing is clearly premature. But, preliminary evidence for its influence on mentalizing in ASD and MDD promises that TMS, combined with sensitive paradigms, will provide insights into the dysfunction of the neurocognitive basis of mentalizing in clinical conditions. In the future, it may be possible to combine TMS with psychotherapeutic interventions in order to tackle impaired social cognition in psychiatric disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank April Moeller for her help with the table and for comments on a previous version of this article.
Footnotes
1. ^Also the left TPJ is involved in mentalizing (e.g., Samson et al., 2004; Schurz et al., 2014). However, because recent theoretical discussions focus on the RTPJ and the TMS studies reviewed here also predominantly targeted the RTPJ, we concentrate on this region in the current article.
2. ^A similar effect was previously shown by Santiesteban et al. (2012) using the same task but a different stimulation method, namely transcranial direct current stimulation. Intriguingly, anodal tDCS (which increases cortical excitability) enhanced the ability to control imitation. Moreover, Santiesteban and colleagues showed that anodal tDCS not only facilitated the self at the expense of the other, but also vice versa. In another task, anodal stimulation facilitated the subjects' ability to inhibit their own perspective in order to adopt the perspective of another person. We point at this finding because of its high theoretical relevance. However, it is difficult to directly compare tDCS and rTMS findings as those two methods substantially differ. We refrain from a detailed discussion of this tDCS study as this falls beyond the scope of this review. For a general overview of both noninvasive brain stimulation methods see Wagner et al. (2007).
References
Aan Het Rot, M., Mathew, S. J., and Charney, D. S. (2009). Neurobiological mechanisms in major depressive disorder. Can. Med. Assoc. J. 180, 305–313. doi: 10.1503/cmaj.080697
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Abell, F., Happè, F., and Frith, U. (2000). Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cogn. Dev. 15, 1–16. doi: 10.1016/s0885-2014(00)00014-9
Arntz, A., Bernstein, D., Oorschot, M., and Schobre, P. (2009). Theory of mind in borderline and cluster-C personality disorder. J. Nerv. Ment. Dis. 197, 801–807. doi: 10.1097/NMD.0b013e3181be78fb
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baron-Cohen, S., Leslie, A. M., and Frith, U. (1985). Does the autistic child have a “theory of mind”? Cognition 21, 37–46. doi: 10.1016/0010-0277(85)90022-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., and Plumb, I. (2001). The “Reading the mind in the eyes” test revised version: a study with normal adults, and adults with asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry 42, 241–251. doi: 10.1111/1469-7610.00715
Beck, A. T. (1972). Depression; Causes and Treatment. Philadelphia, PA: University of Pennsylvania Press.
Berlim, M. T., McGirr, A., Beaulieu, M. M., and Turecki, G. (2012). Theory of mind in subjects with major depressive disorder: is it influenced by repetitive transcranial magnetic stimulation? World J. Biol. Psychiatry 13, 474–479. doi: 10.3109/15622975.2011.615861
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blanke, O., Mohr, C., Michel, C. M., Pascual-Leone, A., Brugger, P., Seeck, M., et al. (2005). Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J. Neurosci. 25, 550–557. doi: 10.1523/JNEUROSCI.2612-04.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bora, E., Vahip, S., Gonul, A. S., Akdeniz, F., Alkan, M., Ogut, M., et al. (2005). Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatr. Scand. 112, 110–116. doi: 10.1111/j.1600-0447.2005.00570.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brüne, M., and Brüne-Cohrs, U. (2006). Theory of mind—evolution, ontogeny, brain mechanisms and psychopathology. Neurosci. Biobehav. Rev. 30, 437–455. doi: 10.1016/j.neubiorev.2005.08.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brunet, E., Sarfati, Y., Hardy-Bayle, M.-C., and Decety, J. (2000). A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 11, 157–166. doi: 10.1006/nimg.1999.0525
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cabeza, R., Ciaramelli, E., and Moscovitch, M. (2012). Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn. Sci. 16, 338–352. doi: 10.1016/j.tics.2012.04.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Castelli, F., Frith, C., Happé, F., and Frith, U. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125, 1839–1849. doi: 10.1093/brain/awf189
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Costa, A., Torriero, S., Oliveri, M., and Caltagirone, C. (2008). Prefrontal and temporo-parietal involvement in taking other's perspective: TMS evidence. Behav. Neurol. 19, 71–74. doi: 10.1155/2008/694632
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Decety, J., and Sommerville, J. A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn. Sci. 7, 527–533. doi: 10.1016/j.tics.2003.10.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Demirtas-Tatlidede, A., Vahabzadeh-Hagh, A. M., and Pascual-Leone, A. (2013). Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 64, 566–578. doi: 10.1016/j.neuropharm.2012.06.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Ridder, D., Van Laere, K., Dupont, P., Menovsky, T., and Van De Heyning, P. (2007). Visualizing out-of-body experience in the brain. N. Engl. J. Med. 357, 1829–1833. doi: 10.1056/NEJMoa070010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Döhnel, K., Schuwerk, T., Meinhardt, J., Sodian, B., Hajak, G., and Sommer, M. (2012). Functional activity of the right temporo-parietal junction and of the medial prefrontal cortex associated with true and false belief reasoning. Neuroimage 60, 1652–1661. doi: 10.1016/j.neuroimage.2012.01.073
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eisenegger, C., Treyer, V., Fehr, E., and Knoch, D. (2008). Time-course of “off-line” prefrontal rTMS effects–a PET study. Neuroimage 42, 379–384. doi: 10.1016/j.neuroimage.2008.04.172
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Enticott, P. G., Fitzgibbon, B. M., Kennedy, H. A., Arnold, S. L., Elliot, D., Peachey, A., et al. (2014). A double-blind, randomized trial of deep Repetitive Transcranial Magnetic Stimulation (rTMS) for autism spectrum disorder. Brain Stimul. 7, 206–211. doi: 10.1016/j.brs.2013.10.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Enticott, P. G., Kennedy, H. A., Zangen, A., and Fitzgerald, P. B. (2011). Deep repetitive transcranial magnetic stimulation associated with improved social functioning in a young woman with an autism spectrum disorder. J. ECT 27, 41–43. doi: 10.1097/YCT.0b013e3181f07948
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Flory, J. D., Räikkönen, K., Matthews, K. A., and Owens, J. F. (2000). Self-focused attention and mood during everyday social interactions. Pers. Soc. Psychol. Bull. 26, 875–883. doi: 10.1177/0146167200269012
Frith, U. (2012). Why we need cognitive explanations of autism. Q. J. Exp. Psychol. 65, 2073–2092. doi: 10.1080/17470218.2012.697178
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frith, U., and Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 358, 459–473. doi: 10.1098/rstb.2002.1218
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gallagher, H. L., and Frith, C. D. (2003). Functional imaging of [‘]theory of mind’. Trends Cogn. Sci. 7, 77–83. doi: 10.1016/s1364-6613(02)00025-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ghiassi, V., Dimaggio, G., and Brüne, M. (2010). Dysfunctions in understanding other minds in borderline personality disorder: a study using cartoon picture stories. Psychother. Res. 20, 657–667. doi: 10.1080/10503307.2010.501040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Giardina, A., Caltagirone, C., and Oliveri, M. (2011). Temporo-parietal junction is involved in attribution of hostile intentionality in social interactions: an rTMS study. Neurosci. Lett. 495, 150–154. doi: 10.1016/j.neulet.2011.03.059
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Happé, F., and Frith, U. (2014). Annual research review: towards a developmental neuroscience of atypical social cognition. J. Child Psychol. Psychiatry 55, 553–577. doi: 10.1111/jcpp.12162
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heinisch, C., Dinse, H. R., Tegenthoff, M., Juckel, G., and Brüne, M. (2011). An rTMS study into self-face recognition using video-morphing technique. Soc. Cogn. Affect. Neurosci. 6, 442–449. doi: 10.1093/scan/nsq062
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heinisch, C., Krüger, M. C., and Brüne, M. (2012). Repetitive transcranial magnetic stimulation over the temporoparietal junction influences distinction of self from famous but not unfamiliar others. Behav. Neurosci. 126, 792–796. doi: 10.1037/a0030581
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hetu, S., Taschereau-Dumouchel, V., and Jackson, P. L. (2012). Stimulating the brain to study social interactions and empathy. Brain Stimul. 5, 95–102. doi: 10.1016/j.brs.2012.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Inoue, Y., Tonooka, Y., Yamada, K., and Kanba, S. (2004). Deficiency of theory of mind in patients with remitted mood disorder. J. Affect. Disord. 82, 403–409. doi: 10.1016/j.jad.2004.04.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Inoue, Y., Yamada, K., and Kanba, S. (2006). Deficit in theory of mind is a risk for relapse of major depression. J. Affect. Disord. 95, 125–127. doi: 10.1016/j.jad.2006.04.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ionta, S., Heydrich, L., Lenggenhager, B., Mouthon, M., Fornari, E., Chapuis, D., et al. (2011). Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 70, 363–374. doi: 10.1016/j.neuron.2011.03.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kalbe, E., Schlegel, M., Sack, A. T., Nowak, D. A., Dafotakis, M., Bangard, C., et al. (2010). Dissociating cognitive from affective theory of mind: a TMS study. Cortex 46, 769–780. doi: 10.1016/j.cortex.2009.07.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kana, R. K., Keller, T. A., Cherkassky, V. L., Minshew, N. J., and Just, M. A. (2009). Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc. Neurosci. 4, 135–152. doi: 10.1080/17470910802198510
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kelly, Y. T., Webb, T. W., Meier, J. D., Arcaro, M. J., and Graziano, M. S. (2014). Attributing awareness to oneself and to others. Proc. Natl. Acad. Sci. U.S.A. 11, 5012–5017. doi: 10.1073/pnas.1401201111
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kennedy, D. P., and Adolphs, R. (2012). The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 16, 559–572. doi: 10.1016/j.tics.2012.09.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keuken, M. C., Hardie, A., Dorn, B. T., Dev, S., Paulus, M. P., Jonas, K. J., et al. (2011). The role of the left inferior frontal gyrus in social perception: an rTMS study. Brain Res. 1383, 196–205. doi: 10.1016/j.brainres.2011.01.073
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koster-Hale, J., and Saxe, R. (2013). Theory of mind: a neural prediction problem. Neuron 79, 836–848. doi: 10.1016/j.neuron.2013.08.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Krause, L., Enticott, P. G., Zangen, A., and Fitzgerald, P. B. (2012). The role of medial prefrontal cortex in theory of mind: a deep rTMS study. Behav. Brain Res. 228, 87–90. doi: 10.1016/j.bbr.2011.11.037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Le Bouc, R., Lenfant, P., Delbeuck, X., Ravasi, L., Lebert, F., Semah, F., et al. (2012). My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer's disease. Brain 135, 3026–3038. doi: 10.1093/brain/aws237
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, L., Harkness, K. L., Sabbagh, M. A., and Jacobson, J. A. (2005). Mental state decoding abilities in clinical depression. J. Affect. Disord. 86, 247–258. doi: 10.1016/j.jad.2005.02.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lefaucheur, J.-P., André-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., Benninger, D. H., et al. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206. doi: 10.1016/j.clinph.2014.05.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leslie, A. M. (1987). Pretense and representation: the origins of “Theory of mind.” Psychol. Rev. 94, 412–426.
Leslie, A. M. (1994). Pretending and believing: issues in the theory of ToMM. Cognition 50, 211–238. doi: 10.1016/0010-0277(94)90029-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lev-Ran, S., Shamay-Tsoory, S. G., Zangen, A., and Levkovitz, Y. (2012). Transcranial magnetic stimulation of the ventromedial prefrontal cortex impairs theory of mind learning. Eur. Psychiatry 27, 285–289. doi: 10.1016/j.eurpsy.2010.11.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mar, R. A. (2011). The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 62, 103–134. doi: 10.1146/annurev-psych-120709-145406
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mier, D., Lis, S., Esslinger, C., Sauer, C., Hagenhoff, M., Ulferts, J., et al. (2013). Neuronal correlates of social cognition in borderline personality disorder. Soc. Cogn. Affect. Neurosci. 8, 531–537. doi: 10.1093/scan/nss028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mueller, S., Keeser, D., Samson, A. C., Kirsch, V., Blautzik, J., Grothe, M., et al. (2013). Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS ONE 8:e67329. doi: 10.1371/journal.pone.0067329
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Paus, T. (2005). Inferring causality in brain images: a perturbation approach. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 1109–1114. doi: 10.1098/rstb.2005.1652
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Philip, R. C. M., Dauvermann, M. R., Whalley, H. C., Baynham, K., Lawrie, S. M., and Stanfield, A. C. (2012). A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci. Biobehav. Rev. 36, 901–942. doi: 10.1016/j.neubiorev.2011.10.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poletti, M., Enrici, I., and Adenzato, M. (2012). Cognitive and affective theory of mind in neurodegenerative diseases: neuropsychological, neuroanatomical and neurochemical levels. Neurosci. Biobehav. Rev. 36, 2147–2164. doi: 10.1016/j.neubiorev.2012.07.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Price, J. L., and Drevets, W. C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16, 61–71. doi: 10.1016/j.tics.2011.12.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ramsey, R., Hansen, P., Apperly, I., and Samson, D. (2013). Seeing it my way or your way: frontoparietal brain areas sustain viewpoint-independent perspective selection processes. J. Cogn. Neurosci. 25, 670–684. doi: 10.1162/jocn_a_00345
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ribeiro, L. A., and Fearon, P. (2010). Theory of mind and attentional bias to facial emotional expressions: a preliminary study. Scand. J. Psychol. 51, 285–289. doi: 10.1111/j.1467-9450.2009.00797.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robertson, E. M., Théoret, H., and Pascual-Leone, A. (2003). Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J. Cogn. Neurosci. 15, 948–960. doi: 10.1162/089892903770007344
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ruby, P., and Decety, J. (2004). How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J. Cogn. Neurosci. 16, 988–999. doi: 10.1162/0898929041502661
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Samson, A. C., Lackner, H. K., Weiss, E. M., and Papousek, I. (2012). Perception of other people's mental states affects humor in social anxiety. J. Behav. Ther. Exp. Psychiatry 43, 625–631. doi: 10.1016/j.jbtep.2011.08.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Samson, D., Apperly, I. A., Chiavarino, C., and Humphreys, G. W. (2004). Left temporoparietal junction is necessary for representing someone else's belief. Nat. Neurosci. 7, 499–500. doi: 10.1038/nn1223
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Samson, D., and Michel, C. M. (2013). “Theory of mind: insights from patients with acquired brain damage,” in Understanding Other Minds, eds S. Baron-Cohen, M- Lombardo, and H. Tager-Flusberg (Oxford: University Press), 164–192.
Sandrini, M., Umilta, C., and Rusconi, E. (2011). The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci. Biobehav. Rev. 35, 516–536. doi: 10.1016/j.neubiorev.2010.06.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Santiesteban, I., Banissy, M. J., Catmur, C., and Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Curr. Biol. 22, 2274–2277. doi: 10.1016/j.cub.2012.10.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schreiter, S., Pijnenborg, G. H., and Aan Het Rot, M. (2013). Empathy in adults with clinical or subclinical depressive symptoms. J. Affect. Disord. 150, 1–16. doi: 10.1016/j.jad.2013.03.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schulte-Rüther, M., Mainz, V., Fink, G. R., Herpertz-Dahlmann, B., and Konrad, K. (2012). Theory of mind and the brain in Anorexia nervosa: relation to treatment outcome. J. Am. Acad. Child Adolesc. Psychiatry 51, 832–841.e811. doi: 10.1016/j.jaac.2012.06.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schurz, M., Radua, J., Aichhorn, M., Richlan, F., and Perner, J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schuwerk, T., Döhnel, K., Sodian, B., Keck, I. R., Rupprecht, R., and Sommer, M. (2014a). Functional activity and effective connectivity of the posterior medial prefrontal cortex during processing of incongruent mental states. Hum. Brain Mapp. 35, 2950–2965. doi: 10.1002/hbm.22377
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schuwerk, T., Schecklmann, M., Langguth, B., Döhnel, K., Sodian, B., and Sommer, M. (2014b). Inhibiting the posterior medial prefrontal cortex by rTMS decreases the discrepancy between self and other in theory of mind reasoning. Behav. Brain Res. 274, 312–318. doi: 10.1016/j.bbr.2014.08.031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Senju, A., Southgate, V., White, S., and Frith, U. (2009). Mindblind eyes: an absence of spontaneous theory of mind in asperger syndrome. Science 325, 883–885. doi: 10.1126/science.1176170
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Silani, G., Lamm, C., Ruff, C. C., and Singer, T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 33, 15466–15476. doi: 10.1523/JNEUROSCI.1488-13.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sowden, S., and Catmur, C. (2013). The role of the right temporoparietal junction in the control of imitation. Cereb. Cortex. doi: 10.1093/cercor/bht306. [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsakiris, M., Costantini, M., and Haggard, P. (2008). The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia 46, 3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30, 829–858. doi: 10.1002/hbm.20547
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Rheenen, T. E., and Rossell, S. L. (2013). Picture sequencing task performance indicates theory of mind deficit in bipolar disorder. J. Affect. Disord. 151, 1132–1134. doi: 10.1016/j.jad.2013.07.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wagner, T., Valero-Cabre, A., and Pascual-Leone, A. (2007). Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 9, 527–565. doi: 10.1146/annurev.bioeng.9.061206.133100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Walsh, V., and Cowey, A. (2000). Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 1, 73–79. doi: 10.1038/35036239
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Young, L., Camprodon, J. A., Hauser, M., Pascual-Leone, A., and Saxe, R. (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc. Natl. Acad. Sci. U.S.A. 107, 6753–6758. doi: 10.1073/pnas.0914826107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: transcranial magnetic stimulation, social cognition, mentalizing, theory of mind, autism spectrum disorders, major depressive disorder, psychiatric disorders
Citation: Schuwerk T, Langguth B and Sommer M (2014) Modulating functional and dysfunctional mentalizing by transcranial magnetic stimulation. Front. Psychol. 5:1309. doi: 10.3389/fpsyg.2014.01309
Received: 23 August 2014; Accepted: 28 October 2014;
Published online: 18 November 2014.
Edited by:
Olga Lucía Gamboa Arana, Goethe University Frankfurt am Main, GermanyReviewed by:
Vera Moliadze, Schleswig-Holstein University Hospital (UK-SH), GermanyOlga Lucía Gamboa Arana, Goethe University Frankfurt am Main, Germany
Copyright © 2014 Schuwerk, Langguth and Sommer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monika Sommer, Department of Psychiatry and Psychotherapy, University of Regensburg, Germany, Universitätsstr. 84, 93053 Regensburg, Germany e-mail:bW9uaWthLnNvbW1lckBtZWRiby5kZQ==
 Tobias Schuwerk
Tobias Schuwerk Berthold Langguth
Berthold Langguth Monika Sommer
Monika Sommer