Abstract
Background: The analysis of eye movements (EM) by eye-tracking has been carried out for several decades to investigate mood regulation, emotional information processing, and psychomotor disturbances in depressive disorders.
Method: A systematic review of all English language PubMed articles using the terms “saccadic eye movements” OR “eye-tracking” AND “depression” OR “bipolar disorders” was conducted using PRISMA guidelines. The aim of this review was to characterize the specific alterations of EM in unipolar and bipolar depression.
Results: Findings regarding psychomotor disturbance showed an increase in reaction time in prosaccade and antisaccade tasks in both unipolar and bipolar disorders. In both disorders, patients have been reported to have an attraction for negative emotions, especially for negative pictures in unipolar and threatening images in bipolar disorder. However, the pattern could change with aging, elderly unipolar patients disengaging key features of sad and neutral stimuli. Methodological limitations generally include small sample sizes with mixed unipolar and bipolar depressed patients.
Conclusion: Eye movement analysis can be used to discriminate patients with depressive disorders from controls, as well as patients with bipolar disorder from patients with unipolar depression. General knowledge concerning psychomotor alterations and affective regulation strategies associated with each disorder can also be gained thanks to the analysis. Future directions for research on eye movement and depression are proposed in this review.
Introduction
The study of eye movements (EM) in psychiatry and psychopathology began in 1908 based on the pioneer research of Allen Ross Diefendorf and Raymond Dodge. These authors were the first to study the ocular reaction in depression, mania, hebephrenic disease, epilepsy, and imbecile populations (Diefendorf and Dodge, 1908). The interest in EM stems from the information we can gain concerning brain functioning and the earliest stages of motor organization (Leigh and Zee, 2006) as well as psychopathology (Helmchen, 1989). Recently, the development of sophisticated eye tracking technologies such as the infra-red limbus or pupil detection method and the camera using the corneal reflection to measure eye movement (Young and Sheena, 1975) facilitated the study of EM in mental disorders. This technique enables to clarify some diagnoses as well as to assess the effect of drugs during the course of a disease and the recoveries or adaptations during treatment. For example, in schizophrenic populations, EM studies have revealed cognitive impairments of inhibition as well as a link between the genetics of physiological traits and smooth pursuit eye movements (SPEM; Matthysse et al., 2004; Gooding and Basso, 2008). In the case of affective disorders, EM studies may help specify the extent of psychomotor symptoms—which are usually reported across the spectrum of depressive disorders (Bennabi et al., 2013)–, and improve the understanding of mood regulation and emotional information processing as well as the prediction of outcome after treatment initiation.
Depression criteria and the use of categorical DSM definitions may lead to difficulties differentiating bipolar from unipolar depression. Some subtle symptoms help distinguish between unipolar and bipolar depression. Among these symptoms, higher rates of psychomotor retardation, greater difficulty to think, more early morning awakening, more morning worsening of mood, and more frequent psychotic symptoms are mainly related to bipolar depression (Mitchell et al., 2008). In spite of these differences in symptoms, a clear-cut distinction between the two pathologies remains currently difficult (Goodwin et al., 2008). Indeed, a patient can experience depressive episodes for several years without experiencing mania or hypomania (Smith and Craddock, 2011). That's why many patients with bipolar depression are often misdiagnosed and thus treated as having unipolar depression, leading to insufficient treatment and poor outcomes (Bowden, 2001, 2010).
It is then of importance to distinguish between unipolar and bipolar depression at an early stage so as to improve care management. A recent pilot study using fMRI and pattern classification to discriminate unipolar and bipolar depression (Grotegerd et al., 2013) during facial emotional picture presentation has given a diagnosis with up to 90% correct classifications. However, this study was conducted in a small sample of subjects and the use of fMRI is not appropriate in routine clinical practice. Depression has also been characterized by a reduction in positive expression recognition coupled with an increase in the recognition of negative emotions when the stimulus is ambiguous (Surguladze et al., 2004). Impairments in emotional information processing have been associated with social dysfunction (Tse and Bond, 2004) and may be implicated in the maintenance of the disease (Fossati et al., 2004). At the physiopathological level, these deficits have been related to structural and functional anomalies, in particular to the prefrontal cortex (Rogers et al., 2004).
On the other hand, modern eye-tracking techniques have been used to characterize both informational processing by establishing the point of gaze, and more basic characteristics of reflexive and voluntary psychomotor activity that can be altered with mood disorders. EM and fixations provide information concerning cortical mechanisms underlying cognitive functions (Hutton, 2008; Henderson et al., 2013); may help to improve the understanding of the pathological mechanisms underlying mood disorders (Leigh and Zee, 2006); and could be a promising behavioral tool to differentiate unipolar and bipolar depression.
Our aim in the following review was to summarize the literature regarding the study of saccadic EM in adult depressive (unipolar and bipolar) population. Firstly, we describe the main eye-tracking paradigms, their characteristics and usefulness. Then, we present studies addressing unipolar and bipolar depression through the use of eye-tracking paradigms. Finally, we suggest perspectives for future research.
Methods
A search of the literature was conducted in accordance with Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA; Moher et al., 2010). Relevant manuscripts were identified in PubMed database in April 2014 using the following keywords: “saccadic eye movements” OR “eye-tracking” AND “depression” OR “bipolar disorders.” The reference lists of the selected manuscripts were scrutinized for additional studies. Searches were limited to human studies reported in English and were eligible for inclusion if they investigated oculomotor performances or emotional information processing with infrared video-oculography or electrooculography in unipolar or bipolar disorders. Articles were included if they contained primary data derived from clinical trials, meta-analysis or case reports. Excluded studies were those addressing mood disorders due to specific disease processes (e.g., Parkinson's disease or dementia), conducted in children or adolescent psychiatric patients or with no abstract available. We initially applied the above eligibility criteria to the citations and abstracts generated by the search. Based on this information, we excluded the publications that did not meet the inclusion criteria.
When an article met the inclusion criteria, or when there was not sufficient information to definitely exclude it, we retrieved the full text. We then reviewed these potentially relevant articles to determine whether the inclusion criteria were really met. Out of the 71 papers with full-text reviewed, a total of 30 articles that did not meet eligibility criteria were excluded. Thus, data were obtained from 41 papers that met our eligibility criteria (Figure 1). The reviewed studies were listed in Tables 1, 2 according to sample, measure and results.
Figure 1
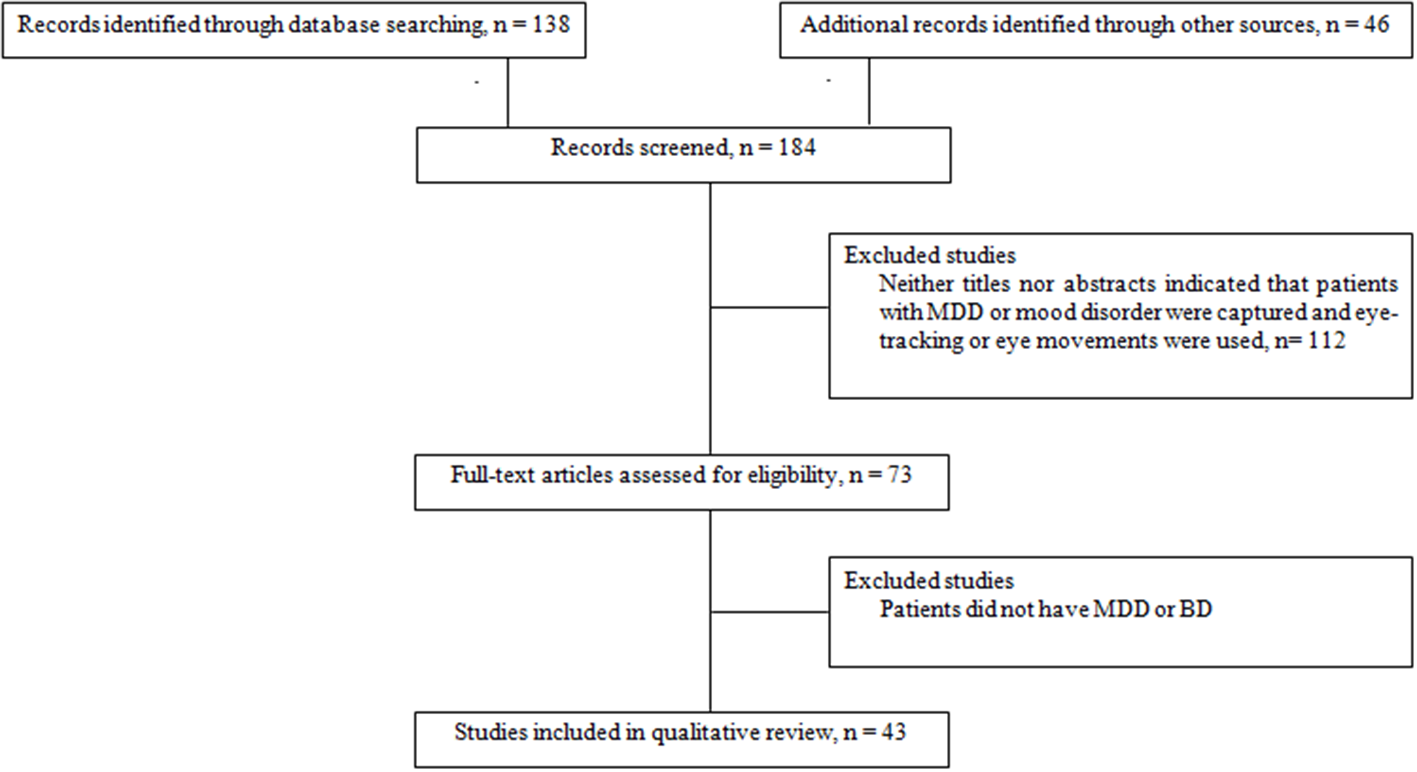
Flow chart of information through the different study phases according to the Preferred Reporting Item for System reviews and Meta-analyses (PRISMA). MDD, Major Depressive Disorder; BD, Bipolar Disorder.
Table 1
| Authors | N | Diagnosis criteria | Age (years) | Treatments | Eye movements tasks | Dependent variables | Results |
|---|---|---|---|---|---|---|---|
| Carvalho et al., 2014 | 20 MDD 47 HC | DSM-IV | 70.4 (9.6) 66.7 (5.5) | ATD BZP AP Anxiolytic | PS AS | RT, gain RT, ER, CF | RT: MDD > NC (p < 0.001); gain: ns RT: MDD > NC (p < 0.001); ER MDD > NC (p < 0.001); gain: ns |
| Chen et al., 2013 | 19 MDD 19 HC | DSM-IV | 28.3 (4.65) 27.9 (4.6) | No medication ATD for the test | FVT | NF, FD, aFD | NF: MDD > HC (p < 0.00001) aFD: MDD > HC (p = 0.039) FD: MDD > HC (p < 0.00001) Cues effect; gaze rate: MDD > HC (p < 0.00001) |
| Malsert et al., 2012b | 2 rcBD 9 HC | DSM-IV | P1:53 P2:60 34 (11) | MS AP ATD | AS PS NS | iER, RT | iER depressive phase: P1 > HC (AS–NS); P2 > HC (AS); iER manic phase: P1 > HC (AS–NS); P2 > HC (AS–NS); iER manic phase > depressive phase–euthymic phase PS correct RT: manic < depressive (p < 0.05) |
| Malsert et al., 2012a | 8 MDD | DSM-IV | 55 (13.3) | rTMS + venlafaxine rTMS + placebo venlafaxine sham rTMS + venlafaxine | AS | RT, ER | Correlation between HDRS–AS RT: Resp > non-Resp (p < 0.03) ER: Resp < non-Resp before treatment (p < 0.024) |
| Suzuki et al., 2009 | 251 Schizo 111 MD 28 ND 250 HC | BPRS | 37.9 (11.3) 44.3 (12.8) 32.7 (10.3) 37.1 (11.3) | ATD | Retention task Comparison task | NF, TESL, MESL, RSS | NF: MD > Schizo (p < 0.01); MD = HC TESL: MD > Schizo (p < 0.01); MD = HC MESL: MD > Schizo (p < 0.01); MD = HC RSS: MD > Schizo (p < 0.01); MD = HC |
| Harris et al., 2009 | 59 Schizo 15 MDD 9 BD 106 HC | DSM-IV | Not specified | No medication | VGS AS | Gain, RT ER, RT | Gain: hypometric saccade (MDD > HC, p < 0.001) RT: ns ER: MDD > HC (p < 0.001); BD > HC (p < 0.05) RT: MDD > all other groups (p < 0.006) |
| Fabisch et al., 2009 | 19 MDD 21 Schizo 21 HC | DSM-IV | 36.8 (12.2) 34.4 (8.3) 37.8 (5.9) | ATD | SPEM VGS | Peak gain, CUS error CUS velocities | Peak gain: Schizo < MDD (p < 0.001) All other variables: ns |
| Winograd-Gurvich et al., 2006a | 9 Mel 10 non-Mel 15 HC | DSM-IV | 47.8 40.8 42.8 | ATD BZP AP thyroid hormones MS | sMGT tsMGT | RT, accuracy, peak velocity, saccade duration, anticipatory, inhibition error RT, primary saccade, peak Velocity, duration RT, primary saccade, peak velocity, duration | RT: mel < non-mel (p < 0.05) All other variables: ns RT: mel < HC (p < 0.05) All other variables: ns All variables: ns |
| Winograd-Gurvich et al., 2006b | 9 Mel 10 non-Mel 15 HC | DSM-IV | 47.8 40.8 42.8 | ATD BZP AP thyroid hormones MS | Self-paced saccade task Oddball task | Intersaccadic interval, accuracy–primary saccade, accuracy–final eye position, peak velocity RT, Accuracy | All variables: ns Accuracy: mel < non-mel (p < 0.05); mel < HC (p < 0.01) RT: ns |
| Crevits et al., 2005 | 11 MDD | DSM-IV | 49.5 (17.1) | ATD Anxiolytic rTMS | VGS PS AS | RT, ER RT, ER RT, ER | All variables: ns All variables: ns RT: After rTMS < Before rTMS (p < 0.01) ER: ns |
| Gooding et al., 2004 | 23 Schizo 10 BD | DSM-IV | 44 (sample) | AP MS ATD Anxiolytic Anti parkinsonian agent | AS Refixation task | Accuracy (%error), Correct RT, Error RT Accuracy (%error) Correct RT | All variable: T1 = T2; ns Correct RT T1 > Error RT T1 (p < 0.05); Correct RT T2 > Error RT T2 (p < 0.05) All variable: T1 = T2; ns |
| Lencer et al., 2004 | 16 Schizo 15 AD (11MDD + 4dBD) 18 OCD 33 HC | DSM-IV | 32.6 (10) 41.9 (11.4) 31.8 (8.8) 31.5 (6.4) | AP ATD Anxiolytic | Foveofugal task Foveopetal task | Initial saccade RT, initial saccade position error, post saccadic velocity gain, steady state velocity gain, gain difference CUS RT, CUS position errors, initial eye Acceleration, pursuit RT, post saccadic velocity gain, steady state velocity gain | Post saccadic velocity gain: AD < HC (p < 0.05) Gain difference: AD > HC (p < 0.05) All other variables: ns Initial eye acceleration: AD > HC (p < 0.05) All other variables: ns |
| Flechtner et al., 2002 | 44 Schizo 34 MDD 42 HC | DSM-III-R | 30.7 (7.2) 46.9 (11) 34.3 (10.9) | Neuroleptic aACH SSRI TCA MS | SPEM | Pursuit gain, CUS Anticipatory saccade, BS, SWJ | All variables: no significance difference between all time for all groups |
| Mahlberg et al., 2001 | 38 Schizo 32 MDD 42 HC | DSM-III-R | 30.3 (6.8) 46.6 (11.1) 34.4 (11) | AP MS ATD Anti-parkinsonian agent | PS Predictive saccade | Peak velocity, RT, Accuracy, Correction Peak velocity, Correction | RT: MDD > HC (p = 0.002) All other variables: ns Correction: MDD > HC (p = 0.0009) Peak velocity: ns |
| Gooding and Tallent, 2001 | 34 Schizo 21 BD 30 HC | DSM-IV | 38.3 (9.2) 39 (9.5) 35 (10.2) | AP MS ATD Anti-parkinsonian agent | AS | RT, ER | ER: BD > HC (p < 0.05) RT: ns |
| Gooding et al., 2000 | 34 Schizo 21 BD 30 HC | DSM-IV | 38.3 (9.2) 39 (9.5) 35 (10.2) | AP MS ATD aACH | Fixation task SPEM | Saccade count, Total saccade Quality score | Saccade count: For all excentricites: ns Total saccade: ns Quality score: ns |
| Sweeney et al., 1999 | 24 HC 26 non-BD 9 BD 12 unmedicated chronic Schizo 20 treatment naive Schizo | DSM-IV | 29 (9) 30 (10.6) 30 (12.4) 31 (7.6) 31 (11.3) | Medication free | Foveopetal task Foveofugal task | Closed loop pursuit gain, % of trials with pursuit before saccade, Pursuit latency Pursuit gain in the first 100 ms, pursuit gain after the frist 100 ms, initial saccade gain | Closed loop pursuit gain: 8°/s: BD < HC (p < 0.01); 16°/s: MDD < HC (p < 0.01), BD < HC (p < 0.01); 24°/s: BD < HC (p < 0.05); 32°/s: ns % of trials with pursuit before saccade: 8°/s: MDD < HC (p < 0.05); 16°/s: MDD < HC (p < 0.05); 24°/s: ns; 32°/s: MDD < HC (p < 0.05) Pursuit latency: for all velocities, ns Pursuit gain in the first 100 ms: MDD < HC (p < 0.05), BD < HC (p < 0.01); 16°/s: MDD < HC (p < 0.05), BD < HC (p < 0.01); 24°/s: MDD < HC (p < 0.05), BD < HC (p < 0.05) Pursuit gain after the first 100 ms: 8°/s: ns; 16°/s: BD < HC (p < 0.05); 24°/s: BD < HC (p < 0.05) Initial saccade gain: 8°/s: BD < HC (p < 0.05); 16°/s: BD < HC (p < 0.05); 24°/s: ns |
| Flechtner et al., 1997 | 34 MDD 43 Schizo 42 HC | DSM-III-R | 46.9 (11) 30.7 (7.2) 34.3 (10.9) | Neuroleptic | SPEM | Pursuit gain, CUS, anticipatory saccade, BUS, SWJ | Pursuit gain: MDD < HC (p = 0.042) CUS: MDD < Schizo, (p = 0.019) All other variables: ns |
| Katsanis et al., 1997 | 33 Schizo 55 Relative schizo 9 MDD 12 BD 38 HC | DSM-III-R | 33.6 (10.9) 43.1 (15.8) 29 (9.4) 32.4 (13.8) 38.6 (14.4) | AP MS Anti-parkinsonian agent BZP aACH | AS | Error rates, RT correct, RT incorrect | ER: BD > HC (p < 0.05) All other variables: ns |
| Tien et al., 1996 | 29 Schizo 26 BD 55 HC | DSM-III-R | 32 (12) 39 (14) 47 (21) | ATD AP MS | Sine task TPR task AS | RMSE RT RT Error rates | Smooth pursuit RMSE: ns; correlation between AS error and RMSE (r = 0.7; p = 0.0002) RT: ns RT: ns ER: ns; BD > HC (p < 0.0001); correlation between BD, WSCT and AS error rates (p = 0.0017) Correlation between BD, SANS, SAPS, BPRS and AS error rates (p = 0.0002) |
| Amador et al., 1995 | 24 MDD 31 Schizo | DSM-III-R | 57 (15.1) 30.6 (7) | BZP | SPEM FT | Target waveform (% abn) Abnormal Fixation | Target waveform: ns Abnormal fixation: MDD < Schizo (p < 0.0008); MDD with psychotic features (n = 10): VF: MDD < Schizo (p < 0.004); target waveform: ns |
| Crawford et al., 1995a | 18 Schizo 18 BD 10 anxD 31 HC | DSM-III-R | 39 (13) 42 (12) 44 (9) 39 (11) | ATD | Reflex saccade AS Remembered saccade Predictive saccade | RT, gain, FEP RT, gain, FEP, distraction errors RT, Gain, FEP, Distraction errors RT, Gain, FEP | All variables: ns RT: BD < HC (p < 0.05) FEP: BD < Schizo (p < 0.001) All other variables: ns Distraction errors: BD < Schizo (p < 0.001) All other variables: ns All variables: ns |
| Crawford et al., 1995b | 40 Schizo +NL 18 Schizo -NL 14 BD +NL 18 BD -NL | DSM-III-R | 39.3 (12.3) 39.4 (13.2) 43.6 (12.1) 42.1 (12.3) | AP Antiparkinsonian agent | Reflex saccade AS Remembered saccade Predictive saccade | RT, gain, FEP RT, Gain, FEP, Distraction errors RT, Gain, FEP, Distraction errors RT, gain, FEP | All variables: ns All variables: ns All variables: ns RT: ns Gain: BD +NL > BD -NL (p < 0.05) FEP: BD +NL > BD -NL (p < 0.05) |
| Malaspina et al., 1994 | 6 dBD 18 MDD 20 HC 30 Schizo | SADS | 57 (15.1) 28.9 (5.6) 30.6 (7) | BZP ECT | SPEM | % abn, large saccades | All variables: ns Improvement of % abn after two sessions of ECT and at 2 month follow-up |
| Sereno and Holzman, 1993 | 16 Schizo 12 AD 14 HC | DSM-III-R | 32.6 29.9 32.3 | Anxiolitic Neuroleptic Antiparkinsonian agent Anti-seizure ATD MS | Saccade task | RT, ER | All variables: ns |
| Gooding et al., 1993 | 26 MDD 31 BD | DSM-III | Not specified | ATD AP MS | SPEM | RMS, rating, intrusive saccade | All variables: no effect of lithium |
| Amador et al., 1991 | 12 BD 30 schizo 20 HC | DSM-III | 40.8 (13.7) 33.5 (6.7) 29.8 (5.8) | AP MS BZP | SPEM | Monitor (% abn), target waveform (% abn) | Monitor: Schizo > BD > HC (p < 0.01) Target waveform: BD > HC (p < 0.001) |
| Abel et al., 1991 | 23 Schizo 16 AD (12 MDD + 4 BD) 21 HC | SADS-L | 37.4 (9) 48.4 (12.4) 37.5 (10.9) | Neuroleptic ATD | SPEM | TWAG, CUS rates, CUS amplitude | CUS rates: 5°/s: AD > HC (p < 0.05); 20°/s: AD < Schizo (p < 0.05) All other variables: ns |
| Iacono et al., 1982 | 25 MDD remitted 24 BD remitted 46 HC | SADS-L | 37.9 (12.9) 36 (12.1) 35 (11.9) | ATD MS | SPEM | RT, RMSE | All variables: ns Greater RMSE for patients with higher frequency of prior episodes of the disorder |
Studies exploring eye movements and fixations in depressive disorders.
aACh, anticholinergic; AD, Affective Disorders; aFD, average Fixation Duration; anxD, anxiety Disorder; AP, Antipsychotic; AS, Antisaccade; ATD, Antidepressant; BD, Bipolar Disorder; BPRS, Brief Psychiatric Rating Scale; BUS, Back-Up Saccades; BZP, Benzodiazepine; CF, Correction Factors; CUS, Catch-Up Saccade; dBD, depressive Bipolar Disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; ECT, Electroconvulsive therapy; ER, Error rate; FD, Fixation Duration; FEP, Final Eye Position; FT, Fixation task; FVT, Free Viewing Task; HC, Healthy Control; iER, inhibition Error Rate; MDD, Major Depressive Disorder; Mel, Melancholic; MESL, Mean Eye Scanning Length; MS, Mood Stabilizer; ND, Neurotic Disorder; NF, Number of Fixation; +NL, with Neuroleptic treatment; -NL, without Neuroleptic treatment; non-Mel, non-Melancholic; non-resp, non-responder; NS, No Saccade; ns, not significant; OCD, Obsessive Compulsive Disorder; PS, Prosaccade; rcBD, rapid cycling Bipolar Disorder; resp, responder; RMS, Root Mean Square; RMSE, Root Mean Square Error; RSS, Response Search Score; RT, Reaction Time; rTMS, repetitive Transcranial Magnetic Stimulation; SADS-L, Schedule for Affective Disorder and Schizophrenia—Lifetime version; SANS, Scale for Assessing Negative Symptoms; SAPS, Scale for Assessing Positive Symptoms; Schizo, Schizophrenia; sMGT, Simple Memory-Guided Task; SPEM, Smooth Pursuit Eye Movements; SSRI, Selective Serotonin Reuptake Inhibitors; SWJ, Square Wave Jerk; TCA, Tricyclic Antidepressant; TESL, Total Eye Scanning Length; TRS, Temporally Random Saccade; tsMGT, two-step Memory-Guided Task; TWAG, Time-Weight Average Gain; VGS, Visually Guided Saccade; WSCT, Wisconsin Card Sorting Test; % abn, percentage abnormalities.
Table 2
| Authors | N | Diagnosis criteria | Age (years) | Treatments | Stimulus | Emotion | Dependent variable | Results |
|---|---|---|---|---|---|---|---|---|
| García-Blanco et al., 2014 | 20 dBD 23 eBD 23 mBD 20 HC | DSM-IV | 51.3 (10.2) 40.7 (10.7) 42.4 (12.1) 40.6 (13.4) | Lithium AE AP ATD Anxiolytic | Pictures from IAPS | Sad Threat Positive Neutral | Percent time attending to stimulus, percent fixation per stimulus category, location of the first fixation, mean glance duration | PT: Threatening: (dBD – eBD – mBD > HC; p = 0.007)—Positive (dBD < HC p = 0.03)—Sad: ns; Neutral: ns PF: Threatening: (dBD – eBD – mBD > HC; p = 0.005)—Positive (dBD < HC; p = 0.007)—Sad: ns; Neutral: ns LFF: all BD: Threat and positive > Neutral MGD: all BD = HC in all conditions |
| Wells et al., 2013 | 26 MDD medication free 21 MDD with ATD 47 HC | DSM-IV | 31.3 (8.7) 37.2 (12.8) 33.6 (11.2) | ATD | Images from IAPS | Dysphoric Threat Positive Neutral | MGD, mean NF | MGD: Positive (Medicated > Unmedicated; p < 0.05) NF: Dysphoric (Unmedicated > Medicated; p < 0.05) |
| Sanchez et al., 2013 | 16 MDD 19 HC | DSM-IV | 39.6 (12.7) 37.3 (9.9) | No medication | Pictures from KEDF | Happy Angry Sad | Initial orientation, % of fixation, % fixation time, attentional engagement condition, attentional disengagement condition | Initial orientation: ns Fixation frequency: no group effect Fixation time: Angry (MDD > HC; p < 0.05)—Sad (MDD > HC; p < 0.05) AEC: ns ADC: Sad (MDD > HC; p < 0.05) |
| Armstrong and Olatunji, 2012 | 563 anxD 532 non anxD 162 MDD 257 non-MDD 65 unselect | Not specified | Not specified | Not specified | Faces Pictures Words | Threat Pleasant Dysphoric | Vigilance hypothesis, maintenance of gaze | Vigilance hypothesis: pleasant: depressed < non depressed (p < 0.05); all other emotions: ns Maintenance of gaze: dysphoric: depressed > non depressed (p < 0.01); all other emotions: ns |
| Sears et al., 2011 | 38 ndep 15 pdep 24 dysphoric | DSM-IV | 20.7 (3.4) 21.3 (4.1) 22.6 (3.2) | Not specified | Images from the internet and IAPS | Depression-related Anxiety-related Positive Neutral | Initially fixated images NF Total FT | Initially fixated images: depression-related: pdep > ndep (p < 0.05); all other emotions: ns NF: anxiety-related: pdep > ndep (p < 0.05); positive: pdep < ndep (p < 0.05); all other emotions: ns Total FT: anxiety-related: pdep > ndep (p < 0.05); positive: pdep < ndep (p < 0.05); all other emotions: ns |
| Kellough et al., 2008 | 15 MDD 45 HC | DSM-IV | all: 18.2 (0.9) | Not specified | Images from IAPS | Dysphoric Threat Positive Neutral | Percent time attending to stimulus, percent fixation per stimulus category, location of the first fixation, mean glance duration | PT: Dysphoric (MDD > HC; p = 0.007); Positive (MDD < HC p = 0.03); all other emotion: ns PF: Dysphoric (MDD > HC; p = 0.005); Positive (MDD < HC; p = 0.007); all other emotions: ns LFF: Threat and positive > Neutral MGD: Dep = HC in all conditions |
| Bestelmeyer et al., 2006 | 22 Schizo 19 BD 37 HC | DSM-IV | 40.8 (12.3) 49.2 (10.6) 37.7 (11.1) | AP MS | Pictures from KEDF | Anger Fear Neutral Sadness Disgust Happiness Surprise | Fixation duration, number of fixation, saccade peak velocity, saccade amplitude, saccade duration | Independent picture type: NF, FT, SD: no significant difference between HC and BD SPV for all emotions: BD < HC (p < 0.05) SA for all emotions: BD > HC (p < 0.01) |
| Eizenman et al., 2003 | 8 MDD 9 HC | DSM-IV | 36.9 (9.7) 27 (5.7) | ATD BZP | Images from IAPS | Neutral Loss and Sadness Threat and anxiety | Fixation time, fixation frequencies, mean glance duration | FT: Dysphoric (MDD > HC, p = 0.004); all other emotions: ns Fixation frequencies: all emotions: ns MGD: Dysphoric (MDD > HC, p = 0.023); all other emotions: ns |
| Loughland et al., 2002 | 52 AD (27 MDD + 17BD) 65 Schizo 61 HC | DSM-IV | 36.9 (9.9) 34 (7.8) 25.7 (10.7) | AP MS ATD BZP | Faces from Ekman and Friesen set of monochrome pictures | Neutral Happy Sad | Accuracy, median FT, fixation scanpath length, NF | Seven option accuracy: AD < HC (p < 0.03) Median FT: AD < HC (p < 0.006) Median FT: Happy–sad (AD < schizo; p < 0.01) Fixation scanpath lenght: AD < HC (p < 0.002) Fixation scanpath lenght: Sad (AD < schizo; p < 0.03) NF: AD < HC (p < 0.0001); AD < schizo (p < 0.006) NF Happy (AD > schizo; p < 0.02) Index of fixations to features vs. non features: happy–sad: AD < HC; p < 0.001) |
| Mogg et al., 2000 | 15 MDD 14 GAD 16 HC | Anxiety Disorder Interview schedule | 40.8 (12) 41.5 (16) 36.7 (8.8) | ATD | Pictures (from their own database) | Sad Happy Neutral Threat | Reaction time, direction of initial eye movement | For all variables and all emotions: ns |
Studies exploring emotional exploration by eye movements.
anxD, anxiety Disorder; ADC, Attentional Disengagement Condition; AEC, Attentional Engagement Condition; AP, Antipsychotic; ATD, Antidepressant; BD, Bipolar Disorder; BZP, Benzodiazepine; dBD, depressive Bipolar Disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; eBD, euthymic Bipolar Disorder; FT, Fixation Time; GAD, Generalized anxiety disorder; HC, Healthy Control; IAPS, International Affective Picture System; KEDF, Karolinska Directed Emotional Faces; LFF, Location of the First Fixation; mBD, manic Bipolar Disorder; MDD, Major Depressive Disorder; MGD, Mean Glance Duration; MS, Mood Stabilizer; ndep, never depressed; NF, Number of Fixation; non anxD, no anxiety Disorder; ns, not significant; pdep, previously depressed; PF, Percent Fixation; PT, Percent Time; SA, Saccade Amplitude; Schizo, Schizophrenia; SD, Saccade Duration; SPV, Saccadic Peak Velocity.
Main eye-tracking paradigms
Several eye-tracking paradigms have been developed over the years to probe the behavioral—and underlying brain—processes associated with the various psychopathologies. We reviewed below some of the most commonly used EM tasks in research. Depending on the authors, and due to the lack of standardization, some tasks may involve similar instructions and receive different terminologies (Holmqvist et al., 2011). This is the reason why we sometimes provide the reader with several terminologies.
Prosaccades (PS), visually guided saccades (VGS), and refixation tasks
In PS, VGS, or refixation tasks, a fixation stimulus appears at the center of the screen and a visual target is presented at the peripheral location. In the first version of the PS task, known as the step PS, the central fixation stimulus disappears at the same time as the peripheral target appears. This task assesses the integrity of saccade-generating circuitry and their involvement in the initiation of reflexive saccade. In a second version of PS task, known as the gap PS, a gap-time is added between the disappearance of the central fixation stimulus and the appearance of the peripheral target (Elderkin-Thompson et al., 2009). The gap task is namely used to study the express saccades, which are saccades characterized by short latencies (80–130 ms). In a third version, known as the overlap PS, the central fixation stimulus remains on the screen when the peripheral target appears. The overlap task is used to examine the ability to flexibly disengage and reorient cognitive resources through eye movement.
In these three experimental conditions, subjects are instructed to fix the target at the peripheral location as soon as the latter appears (Figure 2). Typical measures in PS are the latencies, error rates and amplitudes. This task measures the basic EM characteristics of the subject. The cortical structures linked to the PS performances are the superior colliculus (SC), the frontal eye field (FEF), the cerebellum and the parietal cortex (PC; Ettinger et al., 2005; Leigh and Zee, 2006).
Figure 2
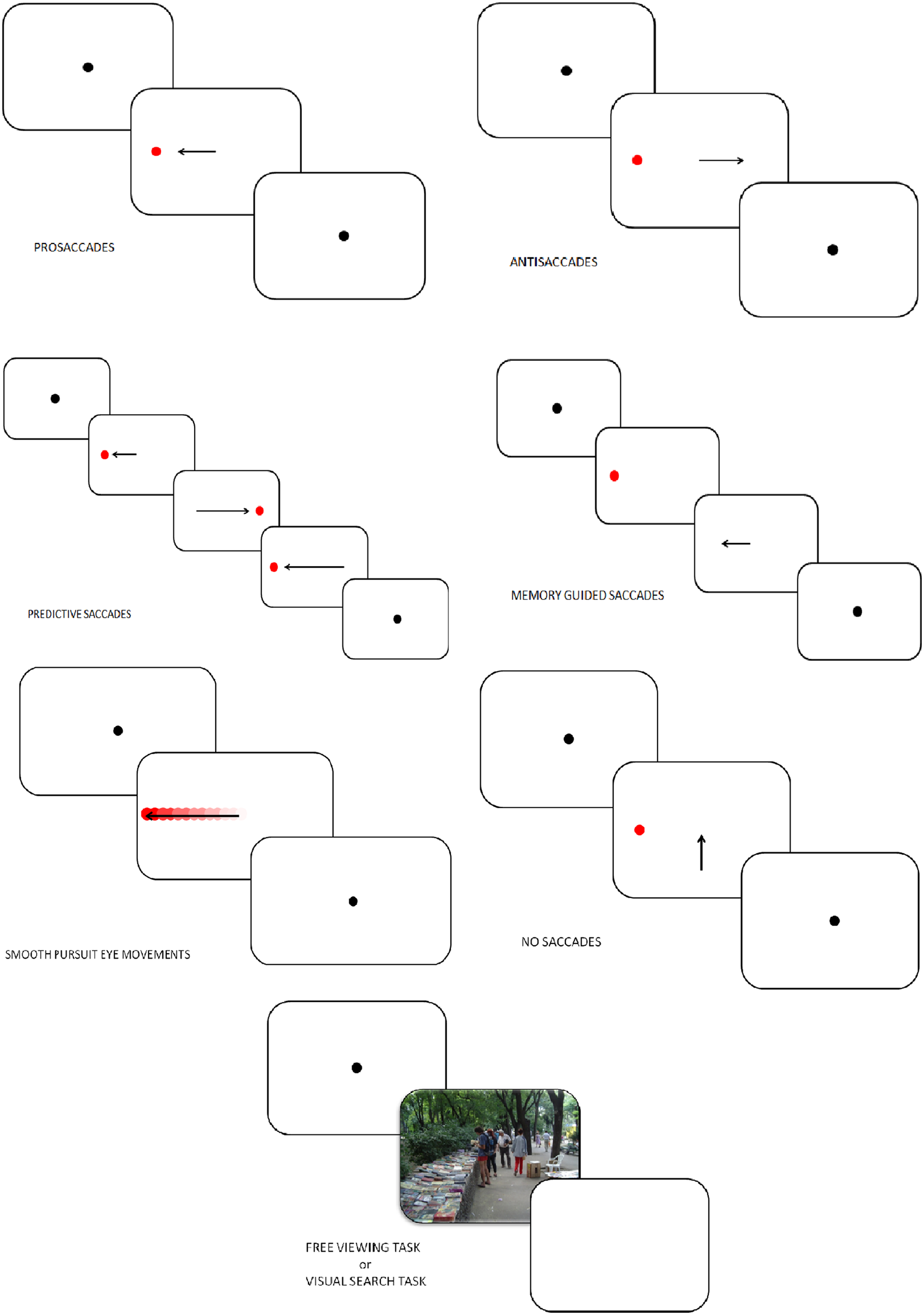
Tasks in which eye-tracking is commonly used (oculomotor, free-viewing, and visual search tasks).
Antisaccades (AS)
In AS, the subject looks at a fixation point and a visual target is presented. Subjects are instructed to make a saccade away from the target (Everling and Fischer, 1998; Figure 2). A correct AS involves two mechanisms depending on automatic processes (Theeuwes et al., 1998): the inhibition of reflexive saccade to the onset location and the execution of a voluntary EM to the mirror location of the onset. Consequently, longer latencies and more erroneous saccades characterize AS performances rather than PS. Typical measures in AS are the error rates (reflecting the inhibition failure), and saccadic reaction time. These two measures are linked to cognitive abilities and may help quantify an inhibition deficit (Currie and Ramsden, 1991). The dorsolateral prefrontal cortex (DLPFC; Crevits et al., 2005), the FEF (Gaymard et al., 1998) and the supplementary eye field (SEF; Everling and Munoz, 2000) are more active during AS. Inhibition deficits are generally linked to frontal area dysfunctions.
Predictive saccades, “oddball task,” self-paced saccade
In predictive saccade, “oddball,” and self-paced saccade tasks, subjects are required to keep their eyes fixed on a target that moves regularly back and forth between two known locations (left and right to the center, remaining in each position for some period of time; Figure 2). This task is used to assess participant's ability to adjust their oculomotor response to predictably moving visual stimulus (negative RT, anticipation) and erroneous anticipatory saccades (Bronstein and Kennard, 1987). Moreover, the oddball task also captures the ability to inhibit an expected motor program and reprogram a new saccade to correspond to an “oddball” trajectory. Predictive saccade may help to quantify inhibition of reflexive saccades, performed by the DLPFC.
Memory-guided saccade (MGS)
Participants are instructed to look at a central fixation point. During this fixation period a target appears in the periphery and the participant is not allowed to make a saccade toward the target. The peripheral target disappears, and, after a variable delay period, the subject is required to make a saccade toward the memorized location (Figure 2). Accuracy, number of anticipatory errors and saccade latency are typical measures in MGS. These saccades are used to assess damages of the basal ganglia and regions of the frontal lobe, involved in processes of working memory (Herrera-Guzmán et al., 2009) and seriously affected in depression (Austin et al., 2001). Performances in MGS are also associated to cortical areas such as DLPFC and SEF (Ettinger et al., 2005).
Smooth pursuit eye movements (SPEM), foveofugal, and foveopetal tasks
Participants are instructed to track a target moving between two fixation points with a sinusoidal or constant velocity (Figure 2). Typical measures in SPEM include the pursuit gain (ratio of eye velocity to target velocity), the catch-up saccade (CUS), employed when the gaze position lags behind the target it follows, and therefore temporarily needs to increase velocity to catch up with the target, anticipatory saccades and square wave jerks (SWJ, small pairs of saccade in opposite directions and separated by an intersaccadic interval of 200–450 ms). Analysis of SPEM performances is used to assess the quality of the pursuit system, could therefore indicate cerebellar and basal ganglia disorders, and can be influenced by age, level of attention, and pharmacological treatments (Leigh and Zee, 2006). Two cortical areas play a major role in SPEM: the medial superior temporal area (MST) and the FEF (Nuding et al., 2008).
No saccade (NS) and fixation tasks
Participants have to keep their gaze fixed on a target placed at the center of the screen (Figure 2). After 2 s and only if the participant stared at the fixation dot, a target appears simultaneously in periphery of the screen as distractor. A typical measure in the NS task is the inhibition error, which consists, in this condition, in the movement of the eye. A key cortex area involved in this task is the DLPFC (Pierrot-Deseilligny et al., 2003).
Free viewing tasks (FVT) and visual search tasks (VST)
In FVT, participants are instructed to look at series of images freely, for instance “as if they were watching television.” In VST, subjects are required to explore a complex visual scene to identify specific stimulus features or to compare multiple scenes (Holmqvist et al., 2011; Figure 2). Various parameters can be studied in those tasks: fixation time, fixation number, location of the first fixation, mean glance duration, saccadic amplitude, saccadic duration and peak saccade velocity. Those kinds of task have usually been used to assess attentional biases, and emotion, face, and scene processing.
Results
A summary of eye movement characteristics in the two groups (i.e., patients with unipolar depression and patients with bipolar depression), in each task, is listed below (Table 3).
Table 3
| Task | MDD | BD |
|---|---|---|
| PS, VGS, REFIXATION TASK | ||
| RT | ↗ | – |
| Accuracy | ↘ | – |
| Peak velocity | → | – |
| Correction | → | |
| ANTISACCADE | ||
| RT | ↗ | ↗ |
| ER | ↗ | ↗ |
| Accuracy | – | ↘ |
| PREDICTIVE | ||
| RT | – | → |
| Accuracy | ↘ | → |
| FEP | – | → |
| Correction | ↗ | – |
| SPEM | ||
| RT | – | – |
| Pursuit gain | ↘ | ↘ |
| CUS rates | ↗ | ↗ |
| RMS | – | → |
| Initial eye acceleration | ↗ | ↗ |
| SWJ | → | – |
| BUS | → | – |
| FIXATION TASK | ||
| Saccade count | – | → |
| Total saccade | – | → |
| Inhibition error | – | ↗ |
| MGS | ||
| RT | ↘ | – |
| Peak velocity | → | – |
| Accuracy | → | – |
| NF | → | – |
| Total FD | → | – |
| Average FD | → | – |
Saccadic eye movement's characteristics in unipolar and bipolar depression.
BD, Bipolar Disorder; BUS, Back-Up Saccades; CUS, Catch-up Saccade; ER, Error rates; FD, Fixation Duration; FEP, Final eye position; MDD, Major Depressive Disorder; MGS, Memory Guided Saccade; NF, Number of Fixation; PS, Prosaccade; RMS, Root Mean Square error; RT, Reaction Time; SPEM, Smooth Pursuit Eye Movement; SWJ, Square Wave Jerk; VGS, Visually Guided Saccade; ↗, increase; ↘ decrease; →, not effect; –, not specified.
Prosaccades (PS), visually guided saccades (VGS), and refixation tasks
In 1993, Sereno et al. investigated saccadic performance in affective disorder groups composed by depressed and bipolar patients. They found no difference between affective disorder, schizophrenic and control participants for RT and ER in gap and no-gap conditions. Harris et al. (2009) obtained divergent results with an electro-oculogram (EOG), saccades being more hypometric in unipolar depressed patients than in controls but not between bipolar patient and controls. Mahlberg et al. (2001) explored saccadic EM of 32 patients with MDD using high-resolution infrared oculography. Patients with MDD exhibited longer RT and needed more corrective saccades (saccade to correct the remaining error relative to the target position and the eye position after the main saccade) to reach the target than healthy controls (HC). Crevits et al. (2005) observed no impact of 10 sessions of fast rTMS at a frequency of 10 Hz (50 impulses) on RT and ER. As for antisaccade performances, Gooding et al. (2004) failed to find temporal stability for prosaccade characteristics. Winograd-Gurvich et al. (2006a) explored differences between melancholic, non-melancholic depressed patients and controls in VGS. Melancholic patients were characterized by longer latencies than non-melancholic and controls, and non-melancholic performed the task slower than controls. Malsert et al. (2012b) found very short reaction times in manic phases in patients with rapid cycling BD. Finally, Carvalho et al. (2014) found an increase of RT in patients with MDD compared to controls but the same accuracy in PS.
Antisaccades
In unipolar and bipolar disorders, Harris et al. (2009) found an increase in RT and ER in comparison to HC. In their report on antisaccades performances in bipolar disorder, Crawford et al. (1995a) found an increase in spatial amplitude errors in AS with hypometric saccades compared to controls. In a subsequent report, these authors found no differences between bipolar and schizophrenic patients in terms of latency, gain (corresponding to the ratio of the saccade amplitude divided by the target step amplitude) and distraction errors. Tien et al. (1996) have investigated AS performances in bipolar, schizophrenic and HC, focusing on reaction time and error rates. They found no differences between the three groups concerning RT. However, they observed significantly higher ER in BD than in HC. Based on the same paradigm, other authors obtained concordant results in BD, with no effects of psychotropic medications on performances (Katsanis et al., 1997; Gooding et al., 2000). In 2004, Gooding and collaborators explored test-retest reliabilities on EM performances in bipolar patients, and reported that ER previously described were not temporally stable. More recently, Malsert et al. (2012b) have observed a link between antisaccade performances and clinical scores, suggesting that error rates could be a predictor of treatment response. Moreover, these authors compared antisaccade performances in two patients suffering from rapid cycling bipolar disorder, and found a higher ER in patients during depressive and manic phases, in comparison to HC. Additionally, lower ER characterized depressive and euthymic rather than manic states. In 2014, Carvalho et al. found an increase of RT, ER for MDD patients. However, MDD patients presented a similar correction factor to that observed in controls. This indicated that unipolar patients had not altered abilities for error detection and correction. Crevits et al. (2005) investigated the impact of 10 sessions of rTMS at 10 Hz frequency applied in DLPFC in a cohort of 11 depressed patients with each sessions consisting of 50 train of 5 s duration separated by 30 s pauses, and found a decrease in latency and no effect on ER.
Predictive saccades
In their first study in 1995, Crawford et al. found no differences between BD, schizophrenic patients and HC in predictive saccade performances. In a second study, they investigated the impact of antipsychotic treatments on predictive saccadic performances in BD patients. Patients with BD treated with antipsychotics had more accurate saccades than non-treated BD when the target was visible or temporarily withdraw (Crawford et al., 1995b). In a predictive task, Mahlberg et al. (2001) showed that depressed patients with major depression (as schizophrenic patients) needed more corrections than HC to reach the target. In 2006, Winograd-Gruvich et al. investigated performances of melancholic and non-melancholic depressed patients in an “oddball” task. There were no differences in RT between the two groups of depressed patients, but the melancholic depressed were less accurate than non-melancholic and HC. Melancholia had no effects on performance in self-paced saccade tasks.
Smooth pursuit eye movements (SPEM)
In 1991, Abel et al. studied smooth pursuit gain and CUS in affective disorders and schizophrenia. When the constant stimulus velocity was 5°/s, MDD and BD patients had higher CUS rates than HC, whereas for 20°/s velocity, MDD and BD subjects had only fewer CUS errors than schizophrenic patients but not than HC. Amador et al. (1991) observed that manic patients had abnormalities in SPEM in comparison with controls leading to a failure to engage the smooth pursuit system. Another study by Tien et al. (1996) found no difference between BD and control in RMS and RT in a pursuit task. Flechtner et al. (2002) explored SPEM in 34 MDD patients. Patients exhibited lower pursuit gain than HC and lower CUS errors than schizophrenic patients. Sweeney et al. (1999) assessed pursuit EM in foveopetal and foveofugal task in BD, MDD, schizophrenic, and control groups. In tasks based on foveopetal motion, depressed and bipolar patients demonstrated reduced pursuit gain compared to HC. Moreover, MDD patients had more difficulty initiating pursuit before their first CUS than HC. In tasks based on foveofugal motion, MDD, and bipolar patients also exhibited lower open loop (i.e., early period of pursuit) pursuit gain and lower closed loop (i.e., late period of pursuit) pursuit gain than controls. Depressed patients had fewer abnormal visual fixations (SWJ) than schizophrenic patients regardless of psychotic features (Amador et al., 1995). Neuroleptic medication and variation of clinical state in MDD had no impact on SPEM performances (Flechtner et al., 2002). Lencer et al. (2004) found in a pursuit task that MDD and BD patients had higher initial eye acceleration and lower post-saccadic velocity gain (i.e., ratio of eye to target velocity) than HC. These characteristics of pursuit performances led to a higher gain difference in MDD and BD patients than in HC. Fabisch et al. (2009) showed that unipolar depressed patients had also higher peak gain than schizophrenic patients. Iacono et al. (1982) observed that lithium induced a greater number of errors during SPEM in unipolar and bipolar patients. However, Gooding et al. (1993) found no effect of lithium treatment on pursuit performance from the time of initial testing to the time of retest. Furthermore, electroconvulsive therapy (ECT) “transiently disrupted” SPEM but improved pursuit performances after two sessions of ECT and at 2 months follow-up (Malaspina et al., 1994). The authors proposed that SPEM could be a “state marker in severe major depression.”
No saccade and fixation task
In 2000, Gooding et al. found no differences in saccade count regardless of the amplitude of EM, and no differences in the total number of saccades between BD patients, HC and schizophrenic patients, whatever the eccentricities. Studying rapid cycling BD, Malsert et al. (2012b) observed that patients in manic phase had a higher number of NS inhibition errors than those in depressive or euthymic phase. Furthermore, for all phases of BD, patients had a higher percentage of NS inhibition errors than HC.
Memory-guided saccade task
In 2006a, Winograd-Gurvich et al. compared melancholic and non-melancholic depressions in a memory-guided saccade task. A higher RT, a decrease in peak velocity, and an increase in the number of hypometric saccades characterized melancholic patients. Non-melancholic patients only had an increase in peak velocity. Suzuki et al. (2009) explored EM dysfunction using retention and comparison tasks in mood disorders, schizophrenia, neurotic disorders, and in the control population. Mood disorders groups, mainly composed of MDD patients, had a higher number of fixations, total eye scanning length, mean saccade length, and responsive search score (corresponding to the data of EM that occurred for the 5-s period immediately following the question: “Are there any other differences?”) than schizophrenic patients. However, MDD did not differ from healthy subjects. Chen et al. (2013) investigated memory impairment in MDD. These authors found higher fixation number, total and average fixation duration, in MDD than in non-MDD participants. This corresponds to a difficulty in shifting attention and extracting information.
Emotion and oculomotor behaviors
Beyond the analysis of the general dynamics of the oculomotor function, eye-tracking can be employed in order to characterize emotional processing. A detailed table listing emotional exploration characteristics in the two groups is presented in Table 4.
Table 4
| Emotion | MDD | BD |
|---|---|---|
| DYSPHORIC | ||
| MGD | ↗ | – |
| THREATENING | ||
| RT | → | – |
| MGD | → | ↗ |
| NF | – | ↗ |
| SADNESS | ||
| RT | → | – |
| MGD | ↗ | → |
| NF | ↘ | → |
| SPV | – | ↘ |
| SA | – | ↘ |
| Accuracy | ↘ | – |
| FEAR | ||
| SPV | – | ↘ |
| SA | – | ↘ |
| DISGUST | ||
| SPV | – | ↘ |
| SA | – | ↘ |
| ANGER | ||
| MGD | ↗ | – |
| SPV | – | ↘ |
| SA | – | ↘ |
| NF | → | – |
| DEPRESSED-RELATED | ||
| Initially fixated image | ↗ | – |
| ANXIETY-RELATED | ||
| MGD | ↗ | – |
| NF | ↗ | – |
| POSITIVE | ||
| MGD | ↘ | → |
| NF | ↘ | ↘ |
| HAPPINESS | ||
| RT | → | – |
| SPV | – | ↘ |
| SA | – | ↘ |
| Accuracy | ↘ | – |
| NF | ↘ | ↘ |
| SURPRISE | ||
| SPV | – | ↘ |
| SA | – | ↘ |
| NEUTRAL | ||
| RT | → | – |
| MGD | → | → |
| NF | ↘ | ↘ |
| SPV | – | ↘ |
| SA | – | ↘ |
| Accuracy | ↘ | – |
Emotional exploration's characteristics in unipolar and bipolar depression.
BD, Bipolar Disorder; MDD, Major Depressive Disorder; MGD, Mean Glance Duration; NF, Number of Fixation; RT, Reaction Time; SA, Saccadic Amplitude; SPV, Saccadic Peak Velocity; ↗, increase; ↘ decrease; →, no effect; –, not specified.
In 2000, Mogg et al. investigated emotional biases in generalized anxiety disorder (GAD) and MDD, by using EM recording. Examining four different types of emotions (sad, happy, neutral, threatening), depressed patients did not differ from GAD patients or HC for RT and the direction of initial EM. However, Eizenman et al. (2003) found an increase in fixation time and average glance duration on dysphoric images for MDD when compared to HC but not for other emotions (i.e., threat and anxiety, interpersonal attachment and social contact). In BD population, Bestelmeyer et al. (2006) observed an impact of picture type (i.e., landscapes, fractals, faces, noise) rather than social content of the picture on EM. Patients with BD were characterized by lower saccadic peak velocity and lower saccade amplitude compared to HC for all picture types. Using the same paradigms as Eizenman et al. (2003), Kellough et al. (2008) obtained concordant results in MDD. Depressed patients made more and longer fixations on dysphoric content compared to HC, and the opposite was true for positive pictures. However, depressed patients fixed first positive and threat pictures rather than dysphoric or neutral. A study by Sears et al. (2011) explored the effect of history of depression on emotion attention biases. Depressed patients with history of depression had higher number of fixations and higher total fixation time for anxiety related pictures compared to patients with no history of depression. Opposite results were found for positive images. In comparison with patients without depression history, previously depressed patients fixed more often depression-related pictures in comparison with non-previously depressed patients. A recent meta-analysis carried out by Armstrong and Olatunji (2012) summarized the attentional biases in affective disorders. Depressed individuals were characterized by a reduction of initial orientation to pleasant stimuli, compared to non-depressed. However, this meta-analysis did not show any increase in vigilance for threatening stimuli. Regarding the maintenance of gaze, depressed subjects had an increase in attention for dysphoric pictures and a decrease for positive picture. A more recent work of Sanchez et al. (2013), which studied stress in depression, found no effect of emotional valence of picture on initial orientation and fixation frequency. However, depressed patients had longer total fixation time on angry and sad emotional faces than HC. Wells et al. (2013) explored the effect of antidepressant (ATD) medication on emotion perception in MDD. The consumption of ATD led to higher mean gaze duration on positive pictures and fewer fixations on dysphoric images in medicated depressed patients. García-Blanco et al. (2014) investigated visual behaviors at different phases of BD, as well as in HC. In their task, four pictures—three “emotional” (i.e., happy, sad, threatening) and one “neutral”—were simultaneously presented. Compared to HC, only bipolar disorder patients who were in a depressive episode (dBD) had fewer fixations on happy images. BD patients, whatever their episodes, had a higher number of fixations on threatening pictures than HC. Similar results were found for the percentage of fixations. Moreover, all participants had a higher number of first fixations on the happy pictures than on the other ones.
Discussion
Psychomotor disturbance in unipolar and bipolar depression
Eye movement tasks are a useful tool to investigate cognitive and motor functioning, through exploration of both low (i.e., automatic) and high (i.e., resource-demanding) levels of motor control. Varieties of processes such as automatic relocation of visual search, spatial working memory, prediction, and response suppression can be evaluated through eye tracking, and related to mood disorders.
Unipolar depressed patients present psychomotor retardation expressed by an increase in RT in both prosaccade and antisaccade tasks (Mahlberg et al., 2001; Carvalho et al., 2014). These impairments of motor and cognitive features involved in movement production were previously observed in other tasks such as fine motor tasks (Sabbe et al., 1996; Pier et al., 2004), gait analysis (Hausdorff et al., 2004) or while measuring ideational retardation (Smith et al., 1994; Brébion et al., 1995). The alterations of movement production were more pronounced in melancholic depressed patients compared to non-melancholic (Parker et al., 2000; Winograd-Gurvich et al., 2006a) with a decrease of saccade accuracy in melancholic depressed patients. Several studies hypothesized specific alterations of motricity in melancholic depression (Parker et al., 2000; Winograd-Gurvich et al., 2006a).
Patients with BD have also been characterized by an increase in reaction time in prosaccade and antisaccade tasks. These deficits were higher in the depressive phase than in the manic phase. Moreover, dBD and mBD could present an inhibition deficit leading to an increase in the antisaccade error rates (Malsert et al., 2012b) and inhibition errors in the NS task are numerous in mBD (Malsert et al., 2012b). These characteristics may be related to some clinical dimensions of BD such as the production of impulsive processes (Swann, 2010). Indeed, the behavioral disinhibition could represent a core dimension of the manic phase (Swann et al., 2003; Larson et al., 2005) causing inability to shift from a given behavior over time.
Psychopathology of depression and EM: potential neurophysiological foundations
The psychopathology of depression has been associated with an alteration in prefrontal and orbitofrontal cortices (Figure 3). As regards EM control, depressed patients have demonstrated a reduction of performance in visual pursuit tasks. Indeed, they would retain a good perception of visual information, but would have alterations in sensorimotor integration processes that could be responsible for precision alteration found in this task (Van der Linden and Hupet, 1994; Fabisch et al., 2009). A deficit in PS performance could be related to functional alterations affecting cortical structures such as the FEF and the superior colliculus (Schall, 2004). The impairment of deep FEF regions could also account for deficits in the visual pursuit system (Rosano et al., 2002).
Figure 3
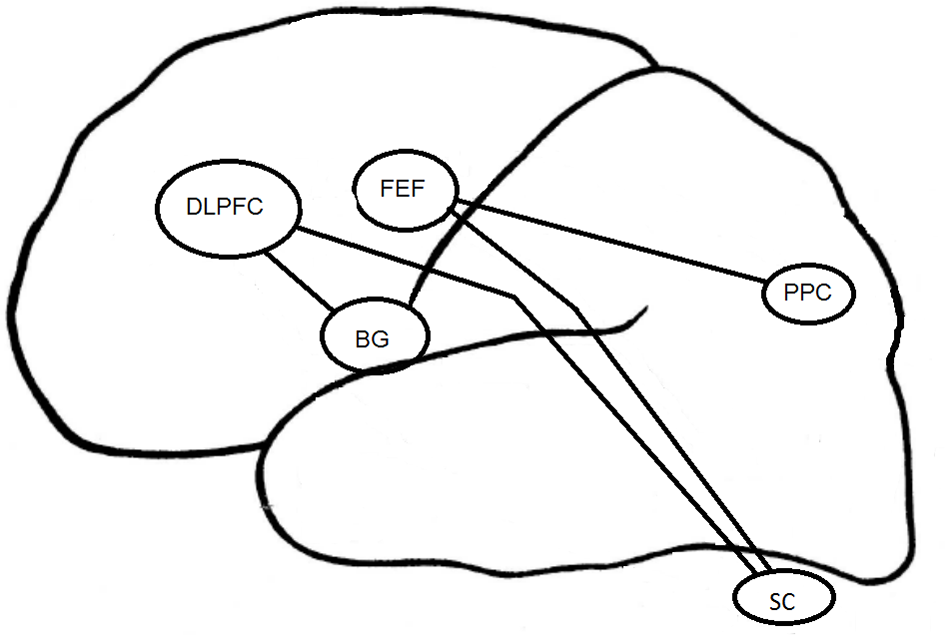
Cortical structures associated to EM in depression. DLPFC, DorsoLateral Prefrontal Cortex; FEF, Frontal Eye Field; BG, Basal Ganglia; PPC, Parietal Posterior Cortex; SC, Superior Colliculus.
The increase in RT in MGS task for the melancholic population could result from a change in the FEF and the posterior PC (Winograd-Gurvich et al., 2006a). Non-melancholic depressed patients were not characterized by a psychomotor retardation but rather by an increase in saccadic peak velocity. Brain structures specifically involved in MGS performances are mainly the cerebellum and basal ganglia (Ivry and Keele, 1989; Dreher and Grafman, 2002). The cerebellum is involved in the timing of movement within short time intervals (Clarke et al., 1996), whereas the basal ganglia are involved within longer time intervals (Meck, 1996). All these structures interact with other regions such as the DLPFC (Pierrot-Deseilligny and Burke, 2005).
In the antisaccade task, the mBD patients have more severe inhibition deficits than dBD or euthymic BD patients. The DLPFC seems to be involved in the inhibition of saccades generated by the superior colliculus (Condy et al., 2004; Kaufman et al., 2010). The EM inhibition deficit observed in BD could be linked to previously reported activation deficits in (ventrolateral) prefrontal cortex and impulsivity in BD patients (Jeanningros et al., 2008).
Negative emotion
A basic analysis carried out with eye-tracking in depressed patients concerns the visual processing of emotional information. The majority of the studies included in our review found an attraction for negative emotion and a reduced orientation to positive emotion in depression (Armstrong and Olatunji, 2012). The capacity of emotional information processing would depend on perceptive, attentional, and memory resources (Brosch et al., 2013).
Depression is associated with a greater perceptual focus on negative pictures. Depressed patients tend to maintain their attention on negative images. This process contributes to keep the disorder by rumination of negative information (Joormann and Gotlib, 2007, 2008; Levens and Gotlib, 2010; Horn and Leigh, 2011) and by the inability to inhibit the negative emotional processing (Goeleven et al., 2006; Joormann and Gotlib, 2010). Some studies have highlighted a lack of initial orientation on negative information (Mogg et al., 2006; Kellough et al., 2008; Wisco, 2009; De Raedt and Koster, 2010). According to these studies, depression is characterized by a negative bias in memory encoding processes rather than by a change in early attentional processes (Williams, 1997).
The early attentional bias has been found to be specific to anxiety (vigilance hypothesis) and the attentional maintenance bias, specific to depression (Weierich and Treat, 2008; Peckham et al., 2010). The presence of a negativity bias was also confirmed by studies using emotional picture presentation with a presentation time superior to 10 s (Armstrong and Olatunji, 2012). However, some factors, such as aging, may well moderate those effects. In our very recent study (Noiret et al., 2015), we found specific characteristics of visual fixations and scanning strategies in elderly MDD patients. Older adults with depression have been characterized by a disengagement of their visual fixations from key features of sad and neutral faces (i.e., lower total fixation duration and fewer fixations on emotional regions [eyes and mouth] compared to HC). In this case, positivity effects accompanying emotional processing in aging could account for interactions between aging and depression. In any event, a reversal seems to occur in comparison to what is usually reported in younger depressive patients.
Patients with BD focus more on threatening pictures regardless of the disease phase, but more frequently during the euthymic phase. BD patients who are in a depressive episode, as MDD patients, exhibit a decrease in fixation time on positive pictures and an increase on negative images. García-Blanco et al. (2014) have shown that the bipolar phase effect (depressive–euthymic–manic) had an impact on attention. Patients with dBD were unable to maintain eye contact on a positive picture. This cognitive bias likely alters emotional self-regulation processes and plays a role in the maintenance of the disease. This relative lack of interest in the positive image could be related to an “anhedonic bias.” Similar kinds of cognitive effects were found in MDD and would also be involved in the maintenance of the disease in that population (Fritzsche et al., 2010). In contrast, these effects were not found in mBD. It could mean that, in this situation, an evaluation conflict between negative and positive emotions occurs (Mansell et al., 2007). The presence of a bias toward threatening pictures in the eBD could be associated with an increase in emotional reactivity and with the onset or exacerbation of affective episodes. The bias toward threatening images therefore seems to be a marker of heightened sensitivity to emotional content and possibly a marker of vulnerability to depressive episodes.
Effect of drugs on the basic dynamics of EM
The effects of drugs on EM have been studied for a long time (Wise, 1984). One reason for this is they inform us about drug effects on the central nervous system (Park et al., 2015). Psychotropic drugs can alter the basic EM but also have an impact on oculomotor performances in emotional information processing (Sweeney et al., 1994; Reilly et al., 2008). Most studies evaluating the effect of drugs were conducted in the animal or in healthy human adults. These studies revealed that benzodiazepines cause a reduction in saccade velocity (Ball et al., 1991), increases saccadic RT (Fafrowicz et al., 1995) and ER of AS (Green and King, 1998). Antidepressants have an effect on RT of both PS and AS, as well as on ER of AS (Green et al., 2000). Morrens et al. (2007) have shown an increase in saccadic peak velocity in healthy subjects treated with paroxetine.
In depressed patients, contradictory results have been found. Some studies have shown an effect of antidepressants on oculomotor performances (Green et al., 2000) while other studies have reported no effect of this treatment on RT and ER in depression (Katsanis et al., 1997; Flechtner et al., 2002). Benzodiazepine could cause a decrease in saccadic peak velocity (Green et al., 2000), an increase in saccadic RT in both gap and overlap conditions (Fafrowicz et al., 1995), and antisaccade ER (Green and King, 1998) as well as an alteration of visual pursuit (Van Nechel, 2007). Antipsychotic drugs in depression (Flechtner et al., 2002) would not influence antisaccade velocity, RT and ER. Sweeney et al. (1997) highlighted an increase in saccadic RT and a decrease in saccadic velocity in schizophrenic patients treated by antipsychotics.
Other studies have shown that antidepressants reduce the recognition of negative emotions and increase the recognition of positive emotions in depression (Fu et al., 2004; Harmer et al., 2009; Wells et al., 2013) whereas others did not show any difference (García-Blanco et al., 2014). Difficulties have been encountered while assessing the impact of these drugs, because patients often take multiple treatments. Although the analysis is complex, it seems necessary to develop new studies evaluating the effects of specific drugs on the basic characteristics of EM and emotional processing in depression.
Limitations of the reviewed research
As regards the overall limitation of the research field, the main critical observations are that (i) most sample sizes were relatively small, (ii) there is a great variability in the eye movement parameters studied, (iii) most of the studies included patients treated with psychotropic medication and rigorous control of the medication effects over EM was generally lacking.
Conclusion
EM have been used to identify the characteristics of motor and cognitive alteration in MDD and BD. The psychomotor retardation specificity associated with each disorder helps us distinguish these two populations (Parker et al., 2000). Depressed and bipolar patients have been characterized by an increase in RT. However, melancholic depressed patient have a more important increase in RT than non-melancholic patients. Among BD patients, only those who are in their depressive phase have longer latency. Eye movement studies have also been used to differentiate melancholic from non-melancholic depressed patients (Winograd-Gurvich et al., 2006a). RT in prosaccade and memory-guided saccade task, accuracy in predictive saccade task and peak velocity in memory-guided saccade tasks could be used to discriminate the two populations. The association between the analysis technique of EM and other exploratory methods of motricity (clinical ERD, fine motor tasks, gait analysis, cognitive measure) could also contribute to improve the comprehension of these mechanisms. The analysis of bipolar patients' inhibition capacities through AS performance could have a diagnostic interest to identify the different phases of the disorder at an early stage (depressive–euthymic–manic).
Future eye tracking studies should also further improve the comprehension of physiopathological mechanisms in depression by focusing on the involvement of specific cortical regions (especially, DLPFC and FEF) (Funahashi, 2001). Visual information processing, dependent on genetic factors and brain physiology could constitute a sensitive analysis vector of pathophysiological processes of depression (Arolt et al., 1996; Matthysse et al., 2004). At the therapeutic level, the study of RT changes could be a predictive factor of treatment response as suggested by the studies of Malsert et al. (2012a) and Crevits et al. (2005).
Depression is a mood disorder linked to an alteration of emotional perception that may lead to a reduction of social interaction skills. The analysis of emotional information processing based on eye-tracking technologies could be used to further identify negative biases that may be associated with the reduction of attentional allocation to positive stimuli as a function of episode severity (Sears et al., 2011). Even if the alteration is dependent on the severity of the disease, this impairment seems to be relatively stable over time and is sometimes also present in healthy subjects with increased risk of depression, which suggests endophenotypic characteristics (Bediou et al., 2009). Similarly several studies have reported the presence of altered EM in remitted patients (Joormann and Gotlib, 2010; Malsert et al., 2012b).
To conclude, the results reported in this review highlight the need for additional research efforts in order to account for complex interactions discussed in the preceding sections (e.g., interactions between age and depressive disorders), and to systematically account for medication effects and their potential combinations with the core perceptual-motor effects of each disorder. Moreover, the current state of the literature on EM and depression has permitted us to establish that (i) unipolar depressed patients have been characterized by psychomotor retardation and negative emotional bias, and (ii) bipolar depressed patients present psychomotor retardation, inhibition deficit and are attracted by negative emotions such as threat. All these data are clinically useful for (i) understanding the link between emotion regulation, cognition and mood disorders, (ii) differentiating unipolar and bipolar disorders, and (iii) evaluating therapeutic response.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Statements
Acknowledgments
The authors are grateful to Richard Medeiros, Medical Editor of Medical Editing International, for editing the manuscript. This study was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique: PHRC n°2009-A00942-55). The sponsor has no role in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
AbelL. A.FriedmanL.JesbergerJ.MalkiA.MeltzerH. Y. (1991). Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol. Psychiatry29, 1063–1072. 10.1016/0006-3223(91)90248-K
2
AmadorX. F.MalaspinaD.SackeimH. A.ColemanE. A.KaufmannC. A.HasanA.et al. (1995). Visual fixation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J. Neuropsychiatry Clin. Neurosci.7, 197–206. 10.1176/jnp.7.2.197
3
AmadorX. F.SackeimH. A.MukherjeeS.HalperinR.NeeleyP.MaclinE.et al. (1991). Specificity of smooth pursuit eye movement and visual fixation abnormalities in schizophrenia. Comparison to mania and normal controls. Schizophr. Res.5, 135–144. 10.1016/0920-9964(91)90040-x
4
ArmstrongT.OlatunjiB. O. (2012). Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin. Psychol. Rev.32, 704–723. 10.1016/j.cpr.2012.09.004
5
AroltV.LencerR.NolteA.Müller-MyhsokB.PurmannS.SchürmannM.et al. (1996). Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am. J. Med. Genet.67, 564–579.
6
AustinM. P.MitchellP.GoodwinG. M. (2001). Cognitive deficits in depression: possible implications for functional neuropathology. Br. J. Psychiatry178, 200–206. 10.1192/bjp.178.3.200
7
BallD. M.GlueP.WilsonS.NuttD. J. (1991). Pharmacology of saccadic eye movements in man. 1. Effects of the benzodiazepine receptor ligands midazolam and flumazenil. Psychopharmacology105, 361–367. 10.1007/BF02244431
8
BediouB.SaoudM.HarmerC.Krolak-SalmonP. (2009). L'analyse des visages dans la dépression. L'Évolution Psychiatrique74, 79–91. 10.1016/j.evopsy.2008.12.015
9
BennabiD.VandelP.PapaxanthisC.PozzoT.HaffenE. (2013). Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed Res. Int.2013:158746. 10.1155/2013/158746
10
BestelmeyerP. E.TatlerB. W.PhillipsL. H.FraserG.BensonP. J.St ClairD. (2006). Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr. Res.87, 212–222. 10.1016/j.schres.2006.06.015
11
BowdenC. L. (2001). Strategies to reduce misdiagnosis of bipolar depression. Psychiatr. Serv.52, 51–55. 10.1176/appi.ps.52.1.51
12
BowdenC. L. (2010). Diagnosis, treatment, and recovery maintenance in bipolar depression. J. Clin. Psychiatry71:e01. 10.4088/jcp.8125cc5c
13
BrébionG.SmithM. J.AllilaireJ. F. (1995). Psychometric characteristics of ideational retardation in depressives. Br. J. Clin. Psychol.34(Pt 3), 371–381. 10.1111/j.2044-8260.1995.tb01472.x
14
BronsteinA. M.KennardC. (1987). Predictive eye saccades are different from visually triggered saccades. Vision Res.27, 517–520. 10.1016/0042-6989(87)90037-X
15
BroschT.SchererK. R.GrandjeanD.SanderD. (2013). The impact of emotion on perception, attention, memory, and decision-making. Swiss Med. Wkly.143, w13786. 10.4414/smw.2013.13786
16
CarvalhoN.NoiretN.VandelP.MonninJ.ChopardG.LaurentE. (2014). Saccadic eye movements in depressed elderly patients. PLoS ONE9:e105355. 10.1371/journal.pone.0105355
17
ChenS.ZhouR.CuiH.ChenX. (2013). Deficits in cue detection underlie event-based prospective memory impairment in major depression: an eye tracking study. Psychiatry Res.209, 453–458. 10.1016/j.psychres.2013.01.015
18
ClarkeS.IvryR.GrinbandJ.RobertsS.ShimizuN. (1996). Exploring the domain of the cerebellar timing system, in Advances in Psychology, eds MaríaA. P.JulioA. (Amsterdam: North-Holland), 257–280. 10.1016/s0166-4115(96)80063-x
19
CondyC.Rivaud-PéchouxS.OstendorfF.PlonerC. J.GaymardB. (2004). Neural substrate of antisaccades: role of subcortical structures. Neurology63, 1571–1578. 10.1212/01.WNL.0000142990.44979.5A
20
CrawfordT. J.HaegerB.KennardC.ReveleyM. A.HendersonL. (1995a). Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychol. Med.25, 461–471. 10.1017/S0033291700033389
21
CrawfordT. J.HaegerB.KennardC.ReveleyM. A.HendersonL. (1995b). Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol. Med.25, 473–483. 10.1017/S0033291700033390
22
CrevitsL.Van den AbbeeleD.AudenaertK.GoethalsM.DierickM. (2005). Effect of repetitive transcranial magnetic stimulation on saccades in depression: a pilot study. Psychiatry Res.135, 113–119. 10.1016/j.psychres.2003.10.008
23
CurrieJ.RamsdenB. (1991). Validation of a clinical antisaccadic eye movements test in the assessment of dementia. Arch. Neurol.48, 949. 10.1001/archneur.1991.00530180102024
24
De RaedtR.KosterE. H. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn. Affect. Behav. Neurosci.10, 50–70. 10.3758/CABN.10.1.50
25
DiefendorfA. R.DodgeR. (1908). An experimental study of the ocular reactions of the insane from photographic records. Brain31, 451–489. 10.1093/brain/31.3.451
26
DreherJ. C.GrafmanJ. (2002). The roles of the cerebellum and basal ganglia in timing and error prediction. Eur. J. Neurosci.16, 1609–1619. 10.1046/j.1460-9568.2002.02212.x
27
EizenmanM.YuL. H.GruppL.EizenmanE.EllenbogenM.GemarM.et al. (2003). A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Res.118, 117–128. 10.1016/S0165-1781(03)00068-4
28
Elderkin-ThompsonV.HellemannG.PhamD.KumarA. (2009). Prefrontal brain morphology and executive function in healthy and depressed elderly. Int. J. Geriatr. Psychiatry24, 459–468. 10.1002/gps.2137
29
EttingerU.AntonovaE.CrawfordT. J.MitterschiffthalerM. T.GoswaniS.SharmaT.et al. (2005). Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. Neuroimage24, 487–494. 10.1016/j.neuroimage.2004.08.019
30
EverlingS.FischerB. (1998). The antisaccade: a review of basic research and clinical studies. Neuropsychologia36, 885–899. 10.1016/S0028-3932(98)00020-7
31
EverlingS.MunozD. P. (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci.20, 387–400.
32
FabischK.FitzW.FabischH.Haas-KrammerA.KlugG.ZapotoczkyS.et al. (2009). Sinusoidal smooth pursuit eye tracking at different stimulus frequencies: position error and velocity error before catch-up saccades in schizophrenia and in major depressive disorder. Aust. N.Z. J. Psychiatry43, 855–865. 10.1080/00048670903107542
33
FafrowiczM.UnrugA.MarekT.van LuijtelaarG.NoworolC.CoenenA. (1995). Effects of diazepam and buspirone on reaction time of saccadic eye movements. Neuropsychobiology32, 156–160. 10.1159/000119316
34
FlechtnerK. M.SteinacherB.SauerR.MackertA. (1997). Smooth pursuit eye movements in schizophrenia and affective disorder. Psychol. Med.27, 1411–1419. 10.1017/S0033291797005709
35
FlechtnerK. M.SteinacherB.SauerR.MackertA. (2002). Smooth pursuit eye movements of patients with schizophrenia and affective disorder during clinical treatment. Eur. Arch. Psychiatry Clin. Neurosci.252, 49–53. 10.1007/s004060200011
36
FossatiP.HarveyP. O.Le BastardG.ErgisA. M.JouventR.AllilaireJ. F. (2004). Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J. Psychiatr. Res.38, 137–144. 10.1016/j.jpsychires.2003.08.002
37
FritzscheA.DahmeB.GotlibI. H.JoormannJ.MagnussenH.WatzH.et al. (2010). Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol. Med.40, 815–826. 10.1017/S0033291709990948
38
FuC. H.WilliamsS. C.CleareA. J.BrammerM. J.WalshN. D.KimJ.et al (2004). Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry61, 877–889. 10.1001/archpsyc.61.9.877
39
FunahashiS. (2001). Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci. Res.39, 147–165. 10.1016/S0168-0102(00)00224-8
40
García-BlancoA.SalmerónL.PereaM.LivianosL. (2014). Attentional biases toward emotional images in the different episodes of bipolar disorder: an eye-tracking study. Psychiatry Res.215, 628–633. 10.1016/j.psychres.2013.12.039
41
GaymardB.PlonerC. J.RivaudS.VermerschA. I.Pierrot-DeseillignyC. (1998). Cortical control of saccades. Exp. Brain Res.123, 159–163. 10.1007/s002210050557
42
GoelevenE.De RaedtR.BaertS.KosterE. H. (2006). Deficient inhibition of emotional information in depression. J. Affect. Disord.93, 149–157. 10.1016/j.jad.2006.03.007
43
GoodingD. C.BassoM. A. (2008). The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn.68, 371–390. 10.1016/j.bandc.2008.08.024
44
GoodingD. C.TallentK. A. (2001). The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis.189, 8–16. 10.1097/00005053-200101000-00003
45
GoodingD. C.GrabowskiJ. A.HendershotC. S. (2000). Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Res.97, 119–128. 10.1016/S0165-1781(00)00226-2
46
GoodingD. C.IaconoW. G.KatsanisJ.BeiserM.GroveW. M. (1993). The association between lithium carbonate and smooth pursuit eye tracking among first-episode patients with psychotic affective disorders. Psychophysiology30, 3–9. 10.1111/j.1469-8986.1993.tb03199.x
47
GoodingD. C.MohapatraL.SheaH. B. (2004). Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol. Med.34, 921–932. 10.1017/S003329170300165X
48
GoodwinG. M.AndersonI.ArangoC.BowdenC. L.HenryC.MitchellP. B.et al. (2008). ECNP consensus meeting. Bipolar depression. Nice, March 2007. Eur. Neuropsychopharmacol.18, 535–549. 10.1016/j.euroneuro.2008.03.003
49
GreenJ. F.KingD. J. (1998). The effects of chlorpromazine and lorazepam on abnormal antisaccade and no-saccade distractibility. Biol. Psychiatry44, 709–715. 10.1016/S0006-3223(97)00452-6
50
GreenJ. F.KingD. J.TrimbleK. M. (2000). Antisaccade and smooth pursuit eye movements in healthy subjects receiving sertraline and lorazepam. J. Psychopharmacol.14, 30–36. 10.1177/026988110001400103
51
GrotegerdD.SuslowT.BauerJ.OhrmannP.AroltV.StuhrmannA.et al. (2013). Discriminating unipolar and bipolar depression by means of fMRI and pattern classification: a pilot study. Eur. Arch. Psychiatry Clin. Neurosci.263, 119–131. 10.1007/s00406-012-0329-4
52
HarmerC. J.O'sullivanU.FavaronE.Massey-ChaseR.AyresR.ReineckeA.et al. (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. Am. J. Psychiatry166, 1178–1184. 10.1176/appi.ajp.2009.09020149
53
HarrisM. S.ReillyJ. L.ThaseM. E.KeshavanM. S.SweeneyJ. A. (2009). Response suppression deficits in treatment-naive first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res.170, 150–156. 10.1016/j.psychres.2008.10.031
54
HausdorffJ. M.PengC. K.GoldbergerA. L.StollA. L. (2004). Gait unsteadiness and fall risk in two affective disorders: a preliminary study. BMC Psychiatry4:39. 10.1186/1471-244X-4-39
55
HelmchenH. (1989). Eye movements and psychopathology. Eur. Arch. Psychiatry Neurol. Sci.239, 1–2. 10.1007/BF01739735
56
HendersonJ. M.ShinkarevaS. V.WangJ.LukeS. G.OlejarczykJ. (2013). Predicting cognitive state from eye movements. PLoS ONE8:e64937. 10.1371/journal.pone.0064937
57
Herrera-GuzmánI.Gudayol-FerréE.Herrera-GuzmánD.Guardia-OlmosJ.Hinojosa-CalvoE.Herrera-AbarcaJ. E. (2009). Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J. Psychiatr. Res.43, 855–863. 10.1016/j.jpsychires.2008.10.015
58
HolmqvistK.NyströmM.AnderssonR.DewhurstR.JarodzkaH.van de WeijerJ. (2011). Eye Tracking: A Comprehensive Guide to Methods and Measures. Oxford: OUP.
59
HornA. K.LeighR. J. (2011). The anatomy and physiology of the ocular motor system. Handb. Clin. Neurol.102, 21–69. 10.1016/B978-0-444-52903-9.00008-X
60
HuttonS. B. (2008). Cognitive control of saccadic eye movements. Brain Cogn.68, 327–340. 10.1016/j.bandc.2008.08.021
61
IaconoW. G.PeloquinL. J.LumryA. E.ValentineR. H.TuasonV. B. (1982). Eye tracking in patients with unipolar and bipolar affective disorders in remission. J. Abnorm. Psychol.91, 35–44. 10.1037/0021-843X.91.1.35
62
IvryR. B.KeeleS. W. (1989). Timing functions of the cerebellum. J. Cogn. Neurosci.1, 136–152. 10.1162/jocn.1989.1.2.136
63
JeanningrosR.Mazzola-PomiettoP.KaladjianA. (2008). [Neuroanatomical correlates of impulse control disorders in manic states]. L'Information Psychiatrique84, 121–128. 10.3917/inpsy.8402.0121
64
JoormannJ.GotlibI. H. (2007). Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol.116, 80–85. 10.1037/0021-843X.116.1.80
65
JoormannJ.GotlibI. H. (2008). Updating the contents of working memory in depression: interference from irrelevant negative material. J. Abnorm. Psychol.117, 182–192. 10.1037/0021-843X.117.1.182
66
JoormannJ.GotlibI. H. (2010). Emotion regulation in depression: relation to cognitive inhibition. Cogn. Emot.24, 281–298. 10.1080/02699930903407948
67
KatsanisJ.KortenkampS.IaconoW. G.GroveW. M. (1997). Antisaccade performance in patients with schizophrenia and affective disorder. J. Abnorm. Psychol.106, 468–472. 10.1037/0021-843X.106.3.468
68
KaufmanL. D.PrattJ.LevineB.BlackS. E. (2010). Antisaccades: a probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. J. Alzheimers Dis.19, 781–793. 10.3233/JAD-2010-1275
69
KelloughJ. L.BeeversC. G.EllisA. J.WellsT. T. (2008). Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav. Res. Ther.46, 1238–1243. 10.1016/j.brat.2008.07.004
70
LarsonE. R.ShearP. K.KrikorianR.WelgeJ.StrakowskiS. M. (2005). Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. J. Int. Neuropsychol. Soc.11, 163–172. 10.1017/s1355617705050228
71
LeighR. J.ZeeD. S. (2006). The Neurology of Eye Movements. New York, NY: Oxford University Press.
72
LencerR.TrillenbergP.Trillenberg-KreckerK.JunghannsK.KordonA.BroocksA.et al. (2004). Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol. Med.34, 451–460. 10.1017/S0033291703001314
73
LevensS. M.GotlibI. H. (2010). Updating positive and negative stimuli in working memory in depression. J. Exp. Psychol. Gen.139, 654–664. 10.1037/a0020283
74
LoughlandC. M.WilliamsL. M.GordonE. (2002). Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction?Biol. Psychiatry52, 338–348. 10.1016/S0006-3223(02)01356-2
75
MahlbergR.SteinacherB.MackertA.FlechtnerK. M. (2001). Basic parameters of saccadic eye movements–differences between unmedicated schizophrenia and affective disorder patients. Eur. Arch. Psychiatry Clin. Neurosci.251, 205–210. 10.1007/s004060170028
76
MalaspinaD.AmadorX. F.ColemanE. A.MayrT. L.FriedmanJ. H.SackeimH. A. (1994). Smooth pursuit eye movement abnormality in severe major depression: effects of ECT and clinical recovery. J. Neuropsychiatry Clin. Neurosci.6, 36–42. 10.1176/jnp.6.1.36
77
MalsertJ.GuyaderN.ChauvinA.PolosanM.PouletE.SzekelyD.et al. (2012a). Antisaccades as a follow-up tool in major depressive disorder therapies: a pilot study. Psychiatry Res.200, 1051–1053. 10.1016/j.psychres.2012.05.007
78
MalsertJ.GuyaderN.ChauvinA.PolosanM.SzekelyD.BougerolT.et al. (2012b). Saccadic performance and cortical excitability as trait-markers and state-markers in rapid cycling bipolar disorder: a two-case follow-up study. Front. Psychiatry3:112. 10.3389/fpsyt.2012.00112
79
MansellW.MorrisonA. P.ReidG.LowensI.TaiS. (2007). The interpretation of, and responses to, changes in internal states: an integrative cognitive model of mood swings and bipolar disorders. Behav. Cogn. Psychother.35, 515–539. 10.1017/s1352465807003827
80
MatthysseS.HolzmanP. S.GusellaJ. F.LevyD. L.HarteC. B.JørgensenA.et al. (2004). Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am. J. Med. Genet. B, Neuropsychiatr. Genet.128B, 30–36. 10.1002/ajmg.b.30030
81
MeckW. H. (1996). Neuropharmacology of timing and time perception. Brain Res. Cogn. Brain Res.3, 227–242. 10.1016/0926-6410(96)00009-2
82
MitchellP. B.GoodwinG. M.JohnsonG. F.HirschfeldR. M. (2008). Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord.10(1 Pt 2), 144–152. 10.1111/j.1399-5618.2007.00559.x
83
MoggK.BradburyK. E.BradleyB. P. (2006). Interpretation of ambiguous information in clinical depression. Behav. Res. Ther.44, 1411–1419. 10.1016/j.brat.2005.10.008
84
MoggK.MillarN.BradleyB. P. (2000). Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J. Abnorm. Psychol.109, 695–704. 10.1037/0021-843X.109.4.695
85
MoherD.LiberatiA.TetzlaffJ.AltmanD. G.GroupP. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg.8, 336–341. 10.1016/j.ijsu.2010.02.007
86
MorrensM.WezenbergE.VerkesR. J.HulstijnW.RuigtG. S.SabbeB. G. (2007). Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J. Clin. Psychopharmacol.27, 15–21. 10.1097/jcp.0b013e31802dfff0
87
NoiretN.CarvalhoN.LaurentÉ.VulliezL.BennabiD.ChopardG.et al. (2015). Visual scanning behavior during processing of emotional faces in older adults with major depression. Aging Ment. Health19, 264–273. 10.1080/13607863.2014.926473
88
NudingU.OnoS.MustariM. J.BüttnerU.GlasauerS. (2008). A theory of the dual pathways for smooth pursuit based on dynamic gain control. J. Neurophysiol.99, 2798–2808. 10.1152/jn.90237.2008
89
ParkK. M.ShinK. J.HaS. Y.ParkJ.KimS. E.KimH. C.et al. (2015). Can the adverse effects of antiepileptic drugs be detected in saccadic eye movements?Seizure25, 33–36. 10.1016/j.seizure.2014.12.003
90
ParkerG.RoyK.WilhelmK.MitchellP.Hadzi-PavlovicD. (2000). The nature of bipolar depression: implications for the definition of melancholia. J. Affect. Disord.59, 217–224. 10.1016/S0165-0327(99)00144-5
91
PeckhamA. D.McHughR. K.OttoM. W. (2010). A meta-analysis of the magnitude of biased attention in depression. Depress. Anxiety27, 1135–1142. 10.1002/da.20755
92
PierM. P.HulstijnW.SabbeB. G. (2004). Differential patterns of psychomotor functioning in unmedicated melancholic and nonmelancholic depressed patients. J. Psychiatr. Res.38, 425–435. 10.1016/j.jpsychires.2003.11.008
93
Pierrot-DeseillignyC.MüriR. M.PlonerC. J.GaymardB.DemeretS.Rivaud-PechouxS. (2003). Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain126, 1460–1473. 10.1093/brain/awg148
94
Pierrot-DeseillignyE.BurkeD. C. (2005). The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders.New York, NY: Cambridge University Press. 10.1017/cbo9780511545047
95
ReillyJ. L.LencerR.BishopJ. R.KeedyS.SweeneyJ. A. (2008). Pharmacological treatment effects on eye movement control. Brain Cogn.68, 415–435. 10.1016/j.bandc.2008.08.026
96
RogersM. A.KasaiK.KojiM.FukudaR.IwanamiA.NakagomeK.et al. (2004). Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci. Res.50, 1–11. 10.1016/j.neures.2004.05.003
97
RosanoC.KriskyC. M.WellingJ. S.EddyW. F.LunaB.ThulbornK. R.et al. (2002). Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb. Cortex12, 107–115. 10.1093/cercor/12.2.107
98
SabbeB.HulstijnW.Van HoofJ.ZitmanF. (1996). Fine motor retardation and depression. J. Psychiatr. Res.30, 295–306. 10.1016/0022-3956(96)00014-3
99
SanchezA.VazquezC.MarkerC.LeMoultJ.JoormannJ. (2013). Attentional disengagement predicts stress recovery in depression: an eye-tracking study. J. Abnorm. Psychol.122, 303–313. 10.1037/a0031529
100
SchallJ. D. (2004). On the role of frontal eye field in guiding attention and saccades. Vision Res.44, 1453–1467. 10.1016/j.visres.2003.10.025
101
SearsC.NewmanK.FerenceJ.ThomasC. (2011). Attention to emotional images in previously depressed individuals: an eye-tracking study. Cognit. Ther. Res.35, 517–528. 10.1007/s10608-011-9396-5
102
SerenoA. B.HolzmanP. S. (1993). Express saccades and smooth pursuit eye movement function in schizophrenic, affective disorder, and normal subjects. J. Cogn. Neurosci.5, 303–316. 10.1162/jocn.1993.5.3.303
103
SmithD. J.CraddockN. (2011). Unipolar and bipolar depression: different of the same?Br. J. Psychiatry199, 272–274. 10.1192/bjp.bp.111.092726
104
SmithM. J.BrébionG.BanquetJ. P.AllilaireJ. F. (1994). Experimental evidence for two dimensions of cognitive disorders in depressives. J. Psychiatr. Res.28, 401–411. 10.1016/0022-3956(94)90021-3
105
SurguladzeS. A.YoungA. W.SeniorC.BrébionG.TravisM. J.PhillipsM. L. (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology18, 212–218. 10.1037/0894-4105.18.2.212
106
SuzukiM.TakahashiS.MatsushimaE.TsunodaM.KurachiM.OkadaT.et al. (2009). Exploratory eye movement dysfunction as a discriminator for schizophrenia: a large sample study using a newly developed digital computerized system. Eur. Arch. Psychiatry Clin. Neurosci.259, 186–194. 10.1007/s00406-008-0850-7
107
SwannA. C. (2010). Mechanisms of impulsivity in bipolar disorder and related illness. Epidemiol. Psichiatr. Soc.19, 120–130. 10.1017/S1121189X00000828
108
SwannA. C.PazzagliaP.NichollsA.DoughertyD. M.MoellerF. G. (2003). Impulsivity and phase of illness in bipolar disorder. J. Affect. Disord.73, 105–111. 10.1016/S0165-0327(02)00328-2
109
SweeneyJ. A.BauerK. S.KeshavanM. S.HaasG. L.SchoolerN. R.KrobothP. D. (1997). Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology16, 217–228. 10.1016/S0893-133X(96)00195-9
110
SweeneyJ. A.ClementzB. A.HaasG. L.EscobarM. D.DrakeK.FrancesA. J. (1994). Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J. Abnorm. Psychol.103, 222–230.
111
SweeneyJ. A.LunaB.HaasG. L.KeshavanM. S.MannJ. J.ThaseM. E. (1999). Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol. Psycchiatry46, 671–680.
112
TheeuwesJ.KramerA. F.HahnS.IrwinD. E. (1998). Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol. Sci.9, 379–385. 10.1111/1467-9280.00071
113
TienA. Y.RossD. E.PearlsonG.StraussM. E. (1996). Eye movements and psychopathology in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis.184, 331–338. 10.1097/00005053-199606000-00001
114
TseW. S.BondA. J. (2004). The impact of depression on social skills. J. Nerv. Ment. Dis.192, 260–268. 10.1097/01.nmd.0000120884.60002.2b
115
Van der LindenM.HupetM. (1994). Le Vieillissement Cognitif. Paris: Presses Universitaires de France - PUF.
116
Van NechelC. (2007). Les anomalies oculomotrices dues aux médicaments. Bull. Soc. Ophtalmol.304, 179–184.
117
WeierichM. R.TreatT. A. (2008). Theories and measurement of visual attentional processing in anxiety. Cogn. Emot.22, 985–1018. 10.1080/02699930701597601
118
WellsT. T.ClerkinE. M.EllisA. J.BeeversC. G. (2013). Effect of antidepressant medication use on emotional information processing in major depression. Am. J. Psychiatry.171, 195–200. 10.1176/appi.ajp.2013.12091243
119
WilliamsJ. M. G. (1997). Cognitive Psychology and Emotional Disorders. Chichester: John Wiley and Sons.
120
Winograd-GurvichC.Georgiou-KaristianisN.FitzgeraldP. B.MillistL.WhiteO. B. (2006a). Ocular motor differences between melancholic and non-melancholic depression. J. Affect. Disord.93, 193–203. 10.1016/j.jad.2006.03.018
121
Winograd-GurvichC.Georgiou-KaristianisN.FitzgeraldP. B.MillistL.WhiteO. B. (2006b). Self-paced and reprogrammed saccades: differences between melancholic and non-melancholic depression. Neurosci. Res.56, 253–260. 10.1016/j.neures.2006.07.003
122
WiscoB. E. (2009). Depressive cognition: self-reference and depth of processing. Clin. Psychol. Rev.29, 382–392. 10.1016/j.cpr.2009.03.003
123
WiseS. P. (1984). Saccadic eye movements in response to drug action in the midbrain. Trends Neurosci.7, 357–358. 10.1016/S0166-2236(84)80049-1
124
YoungL. R.SheenaD. (1975). Eye-movement measurement techniques. Am. Psychol.30, 315–330. 10.1037/0003-066X.30.3.315
Summary
Keywords
unipolar depression, bipolar depression, eye movement, saccade, emotion
Citation
Carvalho N, Laurent E, Noiret N, Chopard G, Haffen E, Bennabi D and Vandel P (2015) Eye Movement in Unipolar and Bipolar Depression: A Systematic Review of the Literature. Front. Psychol. 6:1809. doi: 10.3389/fpsyg.2015.01809
Received
02 May 2015
Accepted
09 November 2015
Published
15 December 2015
Volume
6 - 2015
Edited by
John Monterosso, University of Southern California, USA
Reviewed by
Philip B. Mitchell, University of New South Wales, Australia; Breno Satler Diniz, Federal University of Minas Gerais, Brazil
Updates
Copyright
© 2015 Carvalho, Laurent, Noiret, Chopard, Haffen, Bennabi and Vandel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas Carvalho nic.carvalh@gmail.com;
This article was submitted to Psychopathology, a section of the journal Frontiers in Psychology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.