- 1Center for Affective, Stress and Sleep Disorders (ZASS), University of Basel, Psychiatric Clinics (UPK), Basel, Switzerland
- 2Division of Sport Science and Psychosocial Health, Department of Sport, Exercise and Health, University of Basel, Basel, Switzerland
- 3Substance Abuse Prevention Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 4Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 5Psychiatric Services Solothurn, Solothurn, Switzerland
- 6Psychiatric Clinics (UPK), University of Basel, Basel, Switzerland
- 7Max Planck Institute of Psychiatry, Munich, Germany
- 8Isfahan Neurosciences Research Center, Alzahra Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
- 9Department of Psychology, University of Warwick, Coventry, United Kingdom
Background: Learning is the relatively permanent change of behavior as a result of experience and tightly related to memory and cognition. Learning is particularly important for children. Further, restoring sleep is associated both with improved learning performance and lower cortisol levels as a proxy of the so-called hypothalamus-pituitary-adrenocortical axis activity (HPA-AA). With the present study, we investigated, if and to what extent explicit learning performance was associated with cortisol levels at baseline and under challenge conditions and with objective sleep dimensions among 9-years old children.
Methods: A total of 39 children (mean age = 9.5 years; 39% females) took part in the study. Verbal and figural working and long-term memory were tested before and after the Trier Social Stress Test for Children (TSST-C). Further, children underwent sleep-EEG assessment, and cortisol awakening response (CAR) was assessed.
Results: Higher cortisol levels were associated with lower explicit learning encoding (verbal, but not figural learning). Higher verbal and figural working and long-term memory performance predicted lower cortisol secretion under the TSST-C, along with higher verbal and figural working and long-term memory performance after the TSST-C. Cognitive test performances were not mediated by cortisol secretion under the TSST-C. Cognitive performance, cortisol secretion under challenge (TSST-C) and basal conditions (morning) and sleep patterns were unrelated.
Conclusions: The pattern of results suggests that among a sample of 9-years old children cortisol secretion and stages of memory processes (encoding, storage, retrieval) are associated in a complex and bi-directional way. Further, it appears that cognitive-emotional processes underlying cognitive performance and its evaluation might impact on subsequent cortisol secretion as a proxy of neuroendocrinological response to cognitive-emotional processes. Last, cognitive performance and cortisol secretion under challenge conditions were not related to objective sleep patterns and baseline cortisol secretion.
Introduction
Learning is the relatively permanent change in behavior as a result of own experiences. In humans, complex learning is associated with a broad range of cognitive and emotional processes, which are reflected by neurophysiological changes in synaptic plasticity. Typical steps of learning are encoding, storage, and retrieval of information to and from long-term memory. Further, already Hebb (1955) underlined the importance of arousal for learning, where physiologically, arousal refers to the level of alertness of an organism; psychologically, arousal refers to the tension, ranging from calmness to anxiety. Specifically, Hebb's concept of arousal based on the concept of Yerkes and Dodson (1908), which related arousal to performance. Briefly, the Yerkes and Dodson law claims that (cognitive, behavioral) performance is best at an intermediate level of physiological arousal: while a too low level of arousal (e.g., feeling of being bored; illness; somnolence; fatigue; lack of interest) is related to low performance, a too high level of arousal (e.g., anxiety, panic, state of mania) is also related to poor performance. In this view, above all the state of anxiety and panic is tightly related to an overactivated organism, and such over-activation is neurophysiologically reflected by an increased hypothalamus-pituitary-adrenocortical axis activity (HPA-AA; Miller et al., 2007; Holsboer and Ising, 2010) with cortisol as the ultimate indicator variable.
In the meanwhile, several meta-analyses showed that poor learning performance and increased cortisol levels were associated (Sauro et al., 2003; Het et al., 2005; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016). The underlying concept claims that acute stress, understood as the occurrence of a transient stressor, triggers a cascade of physiologically adaptive changes to cope with the stressor. Among others, a stressor increases the activation of the sympathetic-adrenal-medullary (SAM) axis, the hypothalamic-pituitary-adrenal axis (HPA-A) with the subsequent release of adrenal hormones such as cortisol, and upregulation of the immune system and inflammatory activity (Allen et al., 2014). Such physiological adaptations to cope with the stressor have adverse consequences on cognitive processes, specifically on executive functions (i.e., top-down guidance of behavior toward a specific goal) such as working memory, cognitive flexibility and inhibition (Shields et al., 2016, 2017). To explain such an impairment on cognitive functions, it is claimed that cortisol spikes interrupt typical prefrontal cortical function (Porcelli et al., 2008; Vogel et al., 2016). Henckens et al. (2011, 2012) showed that elevated cortisol levels impaired both working memory and inhibition. Further, blocking cortisol receptors decreased the impairing impact of acute stress on executive functions (Schwabe et al., 2013).
Next, while there is extant evidence of such association between stress, cortisol release and impaired executive functions among adults (Sauro et al., 2003; Het et al., 2005; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016), surprisingly, research on such associations among children is scarce. de Veld et al. (2014) assessed 158 children (mean age: 10.6 years). First, children performed a working memory and delayed retrieval test under control conditions; 1 week later, they repeated the tests under stress conditions, that is: retrieval occurred during the session of psychosocial stress (Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997). Outcome variables were the performance of cognitive tests and the levels of salivary alpha-amylase and saliva cortisol. Results showed that correlations between physiological stress responses and cognitive performance were generally low, that is to say: Alpha-amylase levels and cortisol levels were only modestly associated. Further, de Veld et al. (2014) observed an inverted U-shape association between very low and very high cortisol secretions and poor performance, confirming therefore the law of Yerkes and Dodson (1908) that optimal, but not maximal arousal predicted highest performance. Quesada et al. (2012) assessed 44 children aged 8–10 years, who were randomly assigned either to the control or to the intervention condition. Intervention condition consisted of the TSST-C (Buske-Kirschbaum et al., 1997; see also below). Results showed that compared to children in the control condition, children in the intervention condition had higher cortisol levels, lower mood and a poorer performance in the delayed memory task, while no group differences were found for the working memory performance.
Collectively, research on the association of increased stress, higher cortisol secretion and poorer executive functions is abundant among adults (Sauro et al., 2003; Het et al., 2005; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016), whereas research on the same research question among children is scarce (Quesada et al., 2012; de Veld et al., 2014). Accordingly, the first aim of the present study was to investigate, if and if so to what extent psychosocial stress (here: TSST-C) might impact on delayed working memory retrieval among 9-years old children. Second, previous studies (Quesada et al., 2012; de Veld et al., 2014) have focused on verbal learning performances. However, there is extant evidence to show that lexical and graphem information (Sternberg, 2017) are stored as distinguished cognitive information processes. Accordingly, the second aim of the present study was to assess, if and to what extent retrieval of graphem information might be influenced by psychosocial stress (here: TSST-C). To do so, children completed the Rey Osterrieth Complex Figure test (Meyers, 1995).
Last, there is evidence that poor sleep and increased morning cortisol awakening response (CAR) are associated both among children (Hatzinger et al., 2008, 2010, 2012, 2013b, 2014) and adults (Hori et al., 2011; Minkel et al., 2014; van Dalfsen and Markus, 2018). Further, cortisol secretion under basal conditions (that is, CAR) and under stress conditions appeared to be related (Hatzinger et al., 2007; Lemola et al., 2015; Perkinson-Gloor et al., 2015; Maurer et al., 2016). Accordingly, the third aim of the present study was to investigate the associations between sleep patterns and cortisol secretion under baseline (morning cortisol) and challenge (TSST-C) conditions.
The following four hypotheses and one research question were formulated. First, following others (Sauro et al., 2003; Het et al., 2005; Quesada et al., 2012; de Veld et al., 2014; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016; van Dalfsen and Markus, 2018), we assumed that higher cortisol levels would be negatively associated with memory encoding. Second, we expected that a psychosocial stressor impaired retrieval of verbal working memory. Third, following others (Hatzinger et al., 2008, 2010, 2012; Hori et al., 2011; van Dalfsen and Markus, 2018), we assumed that poor sleep and increased morning cortisol secretion were associated. Fourth, following others (Hatzinger et al., 2007; Lemola et al., 2015; Perkinson-Gloor et al., 2015; Maurer et al., 2016), we expected that poor sleep, and cortisol level under baseline (morning cortisol) and under challenge conditions (TSS-C) would be associated. Finally, we examined as exploratory research question, if and to what extent retrieval of working memory performance of figural information (Rey Osterrieth Complex Figure Test) would be associated with cortisol levels as a proxy of psychosocial stress (TSST-C).
To test our hypotheses and to examine our research questions, a sample of 39 children aged about 9 years were assessed in their cognitive performance and cortisol levels before and after a psychosocial stress, along with the objective sleep and cortisol levels in the morning. We had two reasons to assess 9 years old children: First, Hollanders et al. (2017) showed in their systematic review on the cortisol secretion under the TSST-C that mainly children and adolescents at the age of 8, and 10 to 16 years were assessed, while research on 9 years old children was less frequent. Second, the present sample belongs to a larger sample of a long-term study on the associations between psychological functioning, sleep and cortisol secretion; children were assessed at the age of 5, 6, 9, and 14 years, and here, we present data of children at the age of 9 years. Here, children underwent a slightly modified and age-adapted TSST-C protocol, while in previous studies at the age of 5 and 6 years, for want of an appropriate TSST-C, children underwent the MacArthur Story Stem Battery (Hatzinger et al., 2007, 2008, 2010).
Methods
Procedure
Children from a larger long-term study on sleep, behavior and the HPA axis activity among pre-schoolers and children participated in this study (Hatzinger et al., 2007, 2008, 2010, 2012, 2013a,b, 2014; Mikoteit et al., 2012, 2013; Brand et al., 2015; Sadeghi Bahmani et al., 2016). At children's age of about 9 years, children and their parents were informed about the aims of part of the present study. They were assured about anonymous data gathering; then, both children and parents signed the written informed consent. Next, children underwent an in-home sleep-EEG assessment, and morning saliva samples were gathered. In the afternoon of the subsequent day, children underwent the Trier Social Stress Test for Children (TSST-C). Before and after the TSST-C children underwent cognitive testing, while seven saliva samples were gathered from the beginning till the end of the entire testing. The local ethical committee approved the study (Ethik Kommission Basel, Basel, Switzerland), which was performed in accordance of the rules laid down at the Declaration of Helsinki and its later amendments.
While the present results were a part of a larger ongoing study (see above), data were not published in previous papers; thus, the present results are novel.
Sample
A total of 39 children (mean age = 9.5 years, SD = 0.39; 38.4% females) took part in the present study. A brief medical and psychiatric examination (brief interview with parents; medical records and previous study records) ensured that exclusively typically developing and currently somatically and psychologically healthy children took part in the study. Inclusion criteria were: 1. Already participant of the ongoing study (Hatzinger et al., 2007, 2008, 2013a, 2014); 2. willing and able to comply with the study conditions; 3. parents' and children's signed written informed consent. Exclusion criteria were: 1. Current health issues such as flu, injury, psychological or psychiatric issues or similar; 2. intake of mood-, attention- or sleep-altering substances; 3. known skin allergy to electrodes or patches.
Cognitive Testing
Two tests were employed to assess verbal and figural working memory and long-term memory.
For verbal working memory and long-term memory, the Rey Auditory Verbal Learning Test (Schmidt, 1996) was employed. For working memory, a list of 15 words was read, and the child had to reproduce as many words as possible (immediate and free recall; word order is not important). This trial was repeated three times. Next, a word list of 30 words was presented, and the child had to recognize the correct words of the presented list (immediate and cued recall; again, the word order was not important). The delayed free and cued recall was performed after the TSST-C. The outcome measurement is the number of correctly recalled items.
For figural working memory and long-term memory, the Rey Osterrieth Complex Figure Test (Meyers, 1995) was employed. For working memory, a complex figure was presented, and the child had to reproduce the figure as detailed as possible (immediate and free recall). The delayed free recall was performed after the TSST-C. The outcome measurement is the accuracy and the number of correctly drawn elements of the figure.
Sleep-EEG Assessment
The in-home sleep-EEG assessment has been extensively described in Hatzinger et al. (Hatzinger et al., 2008, 2012, 2013b; Brand et al., 2015). Children's sleep was recorded with an ambulatory EEG system (Oxford-Medilog 9200; Oxford Medical Diagnostics, Oxford UK). As a standard procedure, four electrodes were placed at C3-A2 and C4-A1 (EEG), two electrodes were placed on the chin for electromyogram (EMG) and two electrodes on the right and left side to register the electrooculogram (EOG). A1 and A2 electrodes were physically linked. As this assessment was children's fourth sleep-EEG assessment (one mock assessment and one full assessment at the age of 5 years, and at the age of 6 years (Hatzinger et al., 2008, 2012, 2013b), a further assessment to avoid any “first night effect” was not necessary anymore. Sleep EEGs were visually analyzed by two qualified raters according to the standard procedures proposed by Rechtschaffen and Kales (Anderer et al., 2007) (inter-rater reliability: kappa = 0.94).
HPA System Activity Under Baseline Conditions (CAR: Cortisol Awakening Response)
Stalder et al. (2016) have shown that the morning cortisol response with strict reference to the time of awakening is a reliable index of basal HPA axis activity, irrespective from time of awakening, sleep duration, physical activity, or morning routines. As described elsewhere (Hatzinger et al., 2008, 2012; Clow et al., 2010; Stalder et al., 2016), and given that children repeatedly participated in the saliva sampling method during the ongoing study, parents just supervised how children took four saliva cortisol samplings in the morning at 0, 10, 20, and 30 min after awakening. Waking time ranged from 6.30 to 7.15 a.m.
HPA System Activity Under Non-Pharmacological Challenge Conditions
The Trier Social Stress Test for Children (TSST-C Buske-Kirschbaum et al., 1997) is a well-established non-pharmacological test to challenge the HPA-axis activity (Gunnar et al., 2009). Briefly, children had to accomplish a thrilling story and to present the end of this story in front of a (mock) jury within an exact time period of 4 min. Further, they were told to speak loudly and clearly in the (mock) microphone, while the entire session was registered on a (mock) camera. No feedback was given, and children were stopped when trespassing the 4-min time frame, or children were told to continue the story, in case they stopped before the 4 min. The next task consisted of mental calculation: Children had to count down loudly from 758 minus 7, and to restart in case of a mistake. As described elsewhere (Buske-Kirschbaum et al., 1997; Gunnar et al., 2009; Stadelmann et al., 2018), the set-up of the TSST-C is such to induce (social) anxiety and uncontrollability, which, by definition, trigger psychosocial stress, which is neuroendocrinologically measured by saliva cortisol samplings. Seven saliva samples were taken as described in Table 1.
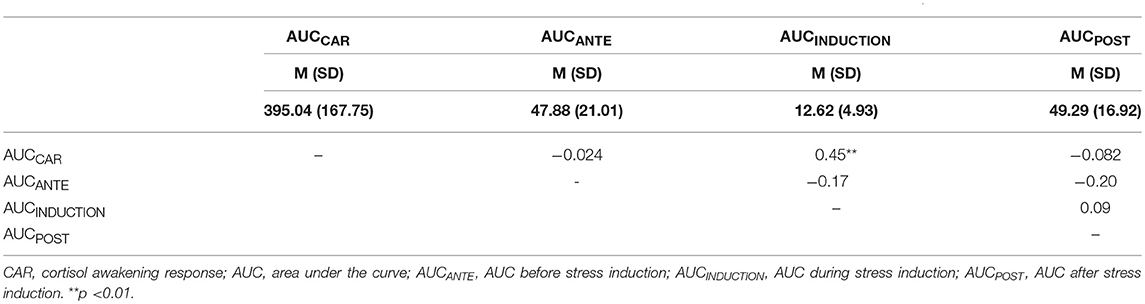
Table 1.1. Correlations (Pearson's Correlation) between the TOTAL AUCCAR (cortisol awakening response), AUCANTE, AUCINDUCTION, and AUCPOST.
Saliva Cortisol Sampling Technique and Cortisol Analysis
As described elsewhere (Hatzinger et al., 2008, 2013a), saliva samples were obtained using the “Salivette” device for quick and hygienic sampling (Sarstedt, Nümbrecht/Germany). This device includes a small cotton swab, on which the subject gently chews for about one min. Thereafter, the swab is transferred into a small plastic tube, the Salivette container, and stored in the freezer. For morning cortisol measurements, saliva sampling was started immediately after awakening without first rinsing the mouth with water. In order to avoid contamination of saliva with food or drinks or with blood caused by micro-injuries in the oral cavity, participants were asked not to eat breakfast or to brush their teeth before sampling was completed. Saliva samples were returned to the laboratory, where samples were centrifuged at 4°C (2,000 rpm, 10 min) and stored at −20°C until assay. Next, free salivary cortisol concentrations were analyzed using a time-resolved immunoassay with fluorometric detection “Coat-A- Count” Cortisol RIA from DPC (Diagnostics Products Corporation; obtained trough H. Biermann GmbH, Bad Nauheim, Germany). Intra- and inter-assay variabilities of this assay were < 2.12 and 2.98%, respectively.
Statistical Analysis
For cortisol secretion, the area-under-the-curve (AUCG) was calculated, using the trapezoidal integration. The baseline cortisol secretion (morning cortisol/CAR; Cortisol Awakening Response) was labeled as AUCCAR. The AUC between the beginning of the examination (see Table 1; Introduction and briefing) and the TSST-C was labeled AUCANTE. The AUC under the TSST-C condition was labeled AUCINDUCTION. The AUC from the end of the TSST-C to the debriefing was labeled AUCPOST.
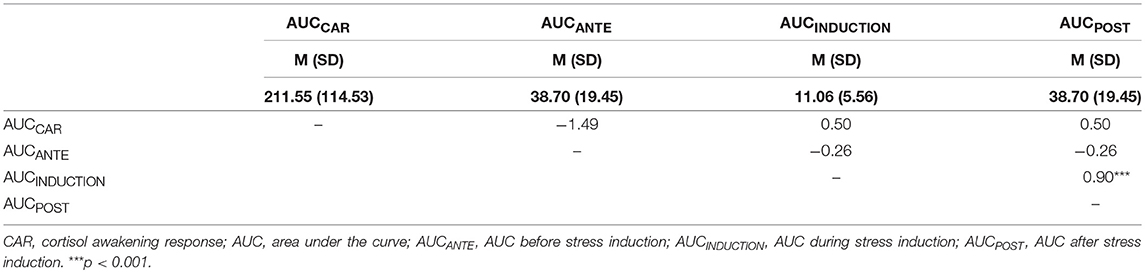
Table 1.2. Correlations (Pearson's Correlation) between the BASAL AUCCAR (cortisol awakening response), AUCANTE, AUCINDUCTION, and AUCPOST.
To compare the cognitive performances before and after the stress challenge, two t-tests for paired samples (verbal; figural) were performed.
A series of Pearson's correlations were performed to calculate the association between AUCs and cognitive performances (immediate retrieval; delayed retrieval; verbal and figural learning). A series of partial correlations were performed between the AUCINDUCTION and cognitive performance after the stress induction (delayed retrieval), controlling for the cognitive performance before the stress induction.
A series of Pearson's correlations were performed to calculate the associations between cortisol secretions under baseline and challenge conditions, sleep parameters, and cognitive performance.
The level of significance was set at alpha ≤ 0.05. All statistical computations were performed with SPSS® 25.0 (IBM Corporation, Armonk NY, USA) for Apple® Mac®.
Results
Verbal and Figural Performance Before and After Stress Induction
Verbal performance (free and cued recall) decreased from immediate recall [M = 10.08, SD = 1.09) to delayed recall (M = 7.85, SD = 1.45; t(38) = 9.72, p = 0.001, d = 1.76].
Figural performance decreased from immediate recall (M = 18.33, SD = 5.38) to delayed recall [M = 17.75, SD = 4.31; t(38) = 4.00, p = 0.001, d = 0.14].
Cortisol at Baseline (CAR), and Under Challenge Conditions (AUCANTE, AUCINDUCTION, AUCPOST)
Table 1 (Tables 1.1–1.3) shows the descriptive and correlational statistical indices of cortisol secretions (AUCCAR, AUCANTE, AUCINDUCTION, AUCPOST). Higher TOTAL AUCCAR levels were associated with higher AUCINDUCTION levels (r = 0.45, p = 0.004). All other correlation coefficients were not significant and therefore trivial. Higher BASAL AUCINDUCTION levels were associated with higher BASAL AUCPOST levels. Higher NETTO AUCANTE levels were associated with lower NETTO AUCINDUCTION levels.
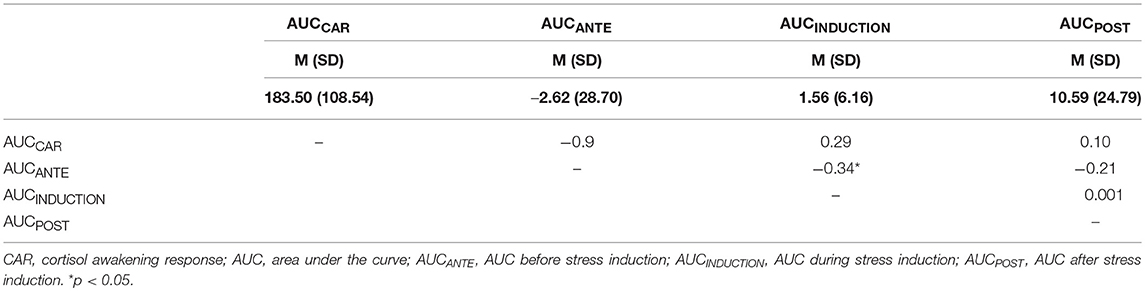
Table 1.3. Correlations (Pearson's Correlation) between the NETTO AUCCAR (cortisol awakening response), AUCANTE, AUCINDUCTION, and AUCPOST.
Associations Between Cortisol Levels and Cognitive Performance
Table 2 reports the correlation coefficients between cortisol levels (AUCs) and cognitive performance.
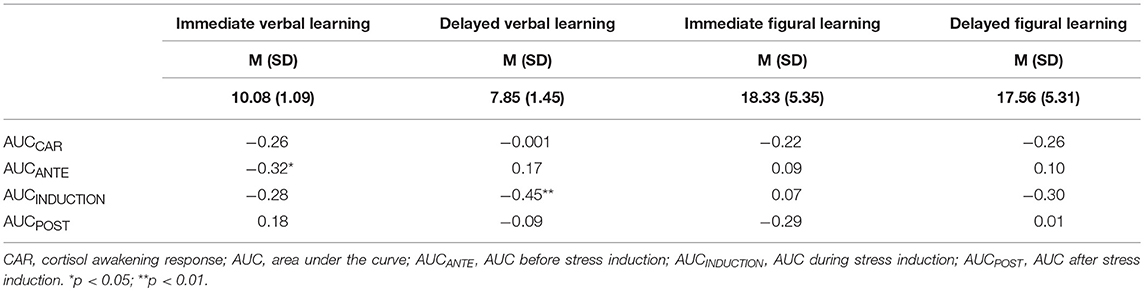
Table 2. Correlation coefficients between cortisol levels (AUCs) and cognitive performance (delayed/immediate verbal/figural learning).
Baseline cortisol levels (AUCCAR) were not statistically significantly associated with cognitive performance (verbal and figural encoding, storage and retrieval; all rs < 0.04, ps > 0.45).
Higher cortisol levels during memory encoding (AUCANTE) were associated with lower immediate free recall performance for verbal (r = −0.32, p < 0.05), but not for figural performance.
A lower memory encoding performance (immediate free recall for verbal, but not for figural encoding) was associated with higher cortisol levels during memory storage (AUCINDUCTION; r = −0.18, p < 0.1) and during memory retrieval (AUCPOST; r = −28, p < 0.05).
A higher cortisol level during memory storage (AUCINDUCTION) was associated with lower delayed cognitive verbal (r = −0.45, p < 0.01), and figural (r = −0.31, p < 0.05) performance.
Cortisol levels during delayed memory retrieval (AUCPOST) were not associated with cognitive verbal and figural performance (encoding, storage, retrieval).
Partial Correlations Between AUCINDUCTION and Delayed Cognitive Performance (Controlling for Cognitive Performance During Immediate Recall)
A higher cortisol level during memory storage (AUCINDUCTION) was associated with lower delayed cognitive verbal (r = −0.45, p < 0.01), and figural (r = −0.31, p < 0.05) performance. When the cognitive performance during immediate recall (memory encoding) was introduced as co-variate, correlation coefficients collapsed from r = −0.45 and r = −0.31 to r = 0.08 and r = −0.07, suggesting that cognitive performance during encoding, but not cortisol secretion under stress conditions (AUCINDUCTION) predicted cognitive performance during delayed recall.
Sleep Parameters, Cortisol Levels and Cognitive Performance
Table 3 shows the descriptive statistical indices of objective sleep parameters and correlation coefficients between sleep parameters, cortisol levels and cognitive performance.
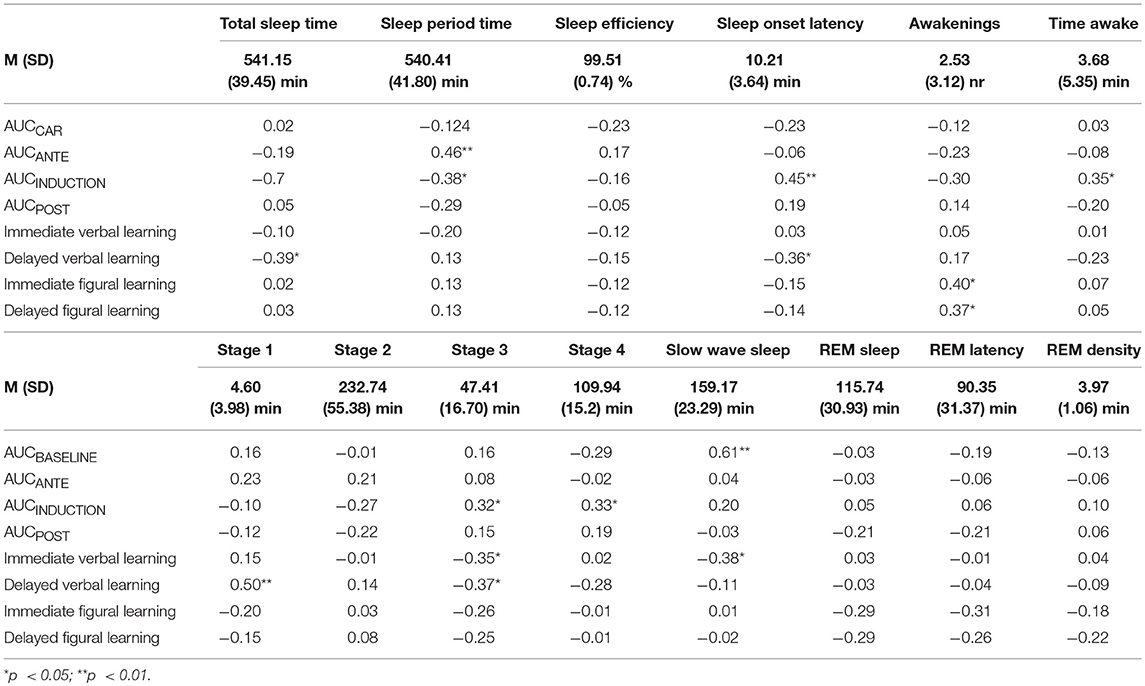
Table 3. Correlation coefficients between sleep parameters, cortisol levels (AUCs) and cognitive performance (immediate/delayed × verbal/figural learning).
With some few exceptions (see Table 3) the general pattern showed no significant associations between sleep patterns, cortisol secretion, and cognitive performance.
Discussion
The key findings of the present study were that among a sample of 9-years old children a higher cortisol secretion was associated with lower cognitive performance during encoding. Further, cognitive performance before and after a stress challenge (Trier Social Stress Test for Children; TSST-C) decreased, though decrease was not affected by cortisol levels during the stress challenge (AUCINDUCTION). Last, objective sleep patterns and both cortisol under baseline (CAR) and challenge conditions (TSST-C) were unrelated. The pattern of results adds to the current literature in an important way, as it revealed a direction of influence neglected so far: The cognitive performance also impacted on neuroendocrinological processes (here: cortisol secretion), while previous studies exclusively focused on the influence of neuroendocrinological processes such as cortisol secretion under challenge conditions on cognitive information elaboration. Thus, in our opinion, we filled a gap in the scientific field, in that we were able to show the bidirectional association between cognitive and neuroendocrinological processes.
More specifically, we hold that with the present study we expanded upon the current literature in the following important ways. First, we performed this study with 9-years old children, and following Hollanders et al. (2017) research on stress-axis reactivity on social stress was mainly performed with 8-years old, or 10- to 16-years old children and adolescents. Second, to the best of our knowledge, no study has investigated the influence of the TSST-C on figural memory performance. Accordingly, we broadened the variety of cognitive outcome variables. Third, similarly, while previous studies mainly focused on the endocrinological response to a social stress condition, we were also interested in assessing the cognitive consequences of such an intervention. Fourth, we were also interested in investigating, if and if so, to what extent the cognitive performance might have had an influence on the stress axis activity, thus reversing the whole paradigm of cause and consequences. As a result, for instance in clinical settings, the bidirectional association between cognitive performance and stress axis activity should be considered as a possible confounder. Accordingly, and to a larger extent, when applying both the TSST-C and the TSST in school settings, clinical settings, and research settings, cognitive processes preceding the stress induction should be carefully monitored. In this view, in a previous study with children with separation anxiety disorder (Brand et al., 2011), we showed that anticipating separation and a social challenge led to higher cortisol secretions already before the stress challenge.
Four hypotheses and one research question were formulated, and each of these is considered now in turn.
With the first hypothesis, we assumed that a higher cortisol secretion would impair verbal working memory encoding, and data did confirm this. Accordingly, the present findings are in accord with a host of previous research (Sauro et al., 2003; Het et al., 2005; Quesada et al., 2012; de Veld et al., 2014; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016). However, we expand upon previous research in two ways: We showed that higher cortisol levels impacted on working memory encoding among about 9-years old children, while the only two available studies in about 9–10 years old children (Quesada et al., 2012; de Veld et al., 2014) investigated the influence of cortisol levels on long-term memory retrieval.
With the second hypothesis, we assumed that a psychosocial stressor (TSST-C) impaired retrieval of verbal working memory, but data did not confirm this. Accordingly, the present findings are at odds with a host of previous research (Sauro et al., 2003; Het et al., 2005; Quesada et al., 2012; de Veld et al., 2014; Shields et al., 2015, 2016, 2017; Starcke and Brand, 2016). To explain this gap, we note the particular study design of the present study, in that also the cognitive performance before the stress induction was taken into account (AUCINDUCTION and delayed verbal recall: without confounder: r = −0.45; with confounder: r = 0.08). Likewise, a deeper introspection of the publications of (Quesada et al., 2012; de Veld et al., 2014) showed, that in these studies, cognitive performances during encoding were statistically not taken into account.
With the third hypothesis, we expected that poor sleep and increased morning cortisol secretion were associated, however, again, data did not support this. Accordingly, the present data are at odds with previous findings (Hatzinger et al., 2008, 2010, 2012; Hori et al., 2011; van Dalfsen and Markus, 2018). To explain this gap between previous research and the present data, we speculate that either the sample size was too low, thus, for these variables, the study was underpowered, or that the sample itself was biased: actually, from the sleep parameters it appears that children's sleep was quite stable (sleep efficiency: 99.5%; sleep onset latency: 10.21 min) and quantitatively and qualitatively high, such to impede statistical variance and therefore the odds of a detecting significant associations with other dimensions.
With the fourth and last hypothesis, we assumed that poor sleep, and high cortisol level under baseline (CAR) and under challenge conditions (TSS-C) would be associated, however, data did not fully confirm this, and again, the present findings are in contrast with previous studies (Hatzinger et al., 2007; Lemola et al., 2015; Perkinson-Gloor et al., 2015; Maurer et al., 2016). However, we also note that those children with higher baseline cortisol levels (AUCCAR) also displayed higher cortisol levels under challenge conditions (TSST-C: AUCBASAL), suggesting that in those children, a generalized over-active HPA-A might be assumed (Hatzinger et al., 2010, 2012, 2013a). In our opinion, the latter observation is also in accord with those studies showing an association between the increased cortisol secretion, the saturation of glucocorticoid-receptors, a negative feedback loop and a decreased cognitive performance (Harris et al., 2013; Finsterwald and Alberini, 2014).
Our final research question examined if and to what extent retrieval of working memory performance of figural information (Rey Osterrieth Complex Figure Test) would be influenced by cortisol levels as a proxy of psychosocial stress (TSST-C). The pattern of results was identical to the results of the verbal working memory task, or simply put: The performance on the figural working memory task before the psychosocial stress predicted the performance on the figural working memory task after the psychosocial stress, while again, the cortisol secretion did not confound this result (that is: stress-related cortisol levels did not influence the delayed figural working memory performance). Further, as shown in Table 3, figural working and long-term memory performance were virtually not associated with cortisol levels, suggesting that information processing of figural information might not rely on those neuronal pathway affected by the HPA-AA.
Despite the new findings, several limitations warn against an overgeneralization of the present results. First, the sample might be considered rather small, and accordingly, a larger sample might have yielded further statistically significant associations. Second, we tested explicit memory performances, whereas the broad range of implicit learning such as skill acquisition, priming, conditioning and non-associative learning (habituation, sensitization) were not assessed. Likewise, it remained unclear if participants with higher cognitive performances used techniques to improving memory such as chunking, imagery, or rehearsal during the learning phase to improve retrieval. Third, it is conceivable that meta-cognitive processes (“what do I think about my thinking?”) and emotional and motivational processes accompanying the learning phase of encoding, storage, and retrieval might have biased two or more dimensions in the same or opposite directions. Fourth, while the Yerkes-Dodson law postulates non-linear associations between neurophysiological arousal and cognitive performance, we did fully rely on linear associations. Fifth, given that 9-years old children are asked to perform and present their efforts in front of their class mates, it is conceivable that the task per se was perceived as too “harmless” and trivial to boost the HPA axis: Actually, performing in front of class mates is part of a young students' task of everyday school life.
Conclusions
While a higher explicit cognitive performance (encoding) leads to a decreased cortisol secretion, the underlying cognitive-emotional and endocrinological processes remain unclear. It appears therefore that thorough and explicit learning reduces neurophysiological arousal; probably, knowing about one's own robust learning performance leads to reduced neurophysiological arousal and higher self-esteem. By constrast, emotions such as anxiety and insecurity might impede successful explicit learning. Further, against expectations, nor the cortisol secretion under baseline and challenge conditions were associated with objective sleep dimensions and cognitive performance. Overall, the pattern of results suggests that the association between cognition, sleep and cortisol secretion appears to be more complex, at least among 9-years old children, and among the present specific sample.
Ethics Statement
This study was carried out in accordance with the rules laid down in the Declaration of Helsinki and its later amendments. The protocol was approved by the local ethics committee of Basel, Switzerland.
Author Contributions
SB, TM, NK, DSB, SaL, MG, SeL, MB, UP, EH-T, and MH: study design, interpretation of the data, writing the draft, and final manuscript. SB, NK, MB, and MH: data gathering. SB, TM, NK, DSB, SaL, MG, SeL, MB, and MH: data analysis. SB, TM, NK, DSB, and MB: integration of the authors' comments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by two unrestricted grants from the Swiss National Science Foundation SNF No. 32-68193.02 (MH) and SNF No. 32-66778.01 (KvK.). We thank R. Hartmann, C. Keppler, L. Kohler, B. Krebs, and P. Walter for their support in data collection. We warmly thank Vladimir Djurdjevic and Marielle König for technical support and data elaboration.
References
Allen, A. P., Kennedy, P. J., Cryan, J. F., Dinan, T. G., and Clarke, G. (2014). Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci. Biobehav. Rev. 38, 94–124. doi: 10.1016/j.neubiorev.2013.11.005
Anderer, P., Gruber, G., Parapatics, S., and Dorffner, G. (2007). Automatic sleep classification according to Rechtschaffen and Kales. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 3994–3997. doi: 10.1109/IEMBS.2007.4353209
Brand, S., Hatzinger, M., Stadler, C., Bolten, M., von Wyl, A., Perren, S., et al. (2015). Does objectively assessed sleep at five years predict sleep and psychological functioning at 14 years?- Hmm, yes and no! J. Psychiatr. Res. 60, 148–155. doi: 10.1016/j.jpsychires.2014.10.007
Brand, S., Wilhelm, F. H., Kossowsky, J., Holsboer-Trachsler, E., and Schneider, S. (2011). Children suffering from separation anxiety disorder (SAD) show increased HPA axis activity compared to healthy controls. J. Psychiatr. Res. 45, 452–459. doi: 10.1016/j.jpsychires.2010.08.014
Buske-Kirschbaum, A., Jobst, S., Wustmans, A., Kirschbaum, C., Rauh, W., and Hellhammer, D. (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 59, 419–426. doi: 10.1097/00006842-199707000-00012
Clow, A., Hucklebridge, F., Stalder, T., Evans, P., and Thorn, L. (2010). The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 35, 97–103. doi: 10.1016/j.neubiorev.2009.12.011
de Veld, D. M., Riksen-Walraven, J. M., and de Weerth, C. (2014). Acute psychosocial stress and children's memory. Stress 17, 305–313. doi: 10.3109/10253890.2014.919446
Finsterwald, C., and Alberini, C. M. (2014). Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 112, 17–29. doi: 10.1016/j.nlm.2013.09.017
Gunnar, M. R., Talge, N. M., and Herrera, A. (2009). Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology 34, 953–967. doi: 10.1016/j.psyneuen.2009.02.010
Harris, A. P., Holmes, M. C., de Kloet, E. R., Chapman, K. E., and Seckl, J. R. (2013). Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology 38, 648–658. doi: 10.1016/j.psyneuen.2012.08.007
Hatzinger, M., Brand, S., Perren, S., Stadelmann, S., von Wyl, A., von Klitzing, K., et al. (2008). Electroencephalographic sleep profiles and hypothalamic-pituitary-adrenocortical (HPA)-activity in kindergarten children: early indication of poor sleep quality associated with increased cortisol secretion. J. Psychiatr. Res. 42, 532–543. doi: 10.1016/j.jpsychires.2007.05.010
Hatzinger, M., Brand, S., Perren, S., Stadelmann, S., von Wyl, A., von Klitzing, K., et al. (2010). Sleep actigraphy pattern and behavioral/emotional difficulties in kindergarten children: association with hypothalamic-pituitary-adrenocortical (HPA) activity. J. Psychiatr. Res. 44, 253–261. doi: 10.1016/j.jpsychires.2009.08.012
Hatzinger, M., Brand, S., Perren, S., von Wyl, A., Stadelmann, S., von Klitzing, K., et al. (2012). Pre-schoolers suffering from psychiatric disorders show increased cortisol secretion and poor sleep compared to healthy controls. J. Psychiatr. Res. 46, 590–599. doi: 10.1016/j.jpsychires.2012.01.018
Hatzinger, M., Brand, S., Perren, S., Von Wyl, A., Stadelmann, S., von Klitzing, K., et al. (2013a). In pre-school children, cortisol secretion remains stable over 12 months and is related to psychological functioning and gender. J. Psychiatr. Res. 47, 1409–1416. doi: 10.1016/j.jpsychires.2013.05.030
Hatzinger, M., Brand, S., Perren, S., Von Wyl, A., Stadelmann, S., von Klitzing, K., et al. (2013b). In pre-school children, sleep objectively assessed via sleep-EEGs remains stable over 12 months and is related to psychological functioning, but not to cortisol secretion. J. Psychiatr. Res. 47, 1809–1814. doi: 10.1016/j.jpsychires.2013.08.007
Hatzinger, M., Brand, S., Perren, S., Von Wyl, A., Stadelmann, S., von Klitzing, K., et al. (2014). In pre-school children, sleep objectively assessed via actigraphy remains stable over 12 months and is related to psychological functioning, but not to cortisol secretion. J. Psychiatr. Res. 55, 22–28. doi: 10.1016/j.jpsychires.2014.04.008
Hatzinger, M., Brand, S., Perren, S., von Wyl, A., von Klitzing, K., and Holsboer-Trachsler, E. (2007). Hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: importance of gender and associations with behavioral/emotional difficulties. J. Psychiatr. Res. 41, 861–870. doi: 10.1016/j.jpsychires.2006.07.012
Henckens, M. J., van Wingen, G. A., Joëls, M., and Fernández, G. (2011). Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc. Natl. Acad. Sci. U.S.A. 108, 5801–5806. doi: 10.1073/pnas.1019128108
Henckens, M. J., van Wingen, G. A., Joëls, M., and Fernández, G. (2012). Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Front. Integr. Neurosci. 6:66. doi: 10.3389/fnint.2012.00066
Het, S., Ramlow, G., and Wolf, O. T. (2005). A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30, 771–784. doi: 10.1016/j.psyneuen.2005.03.005
Hollanders, J. J., van der Voorn, B., Rotteveel, J., and Finken, M. J. J. (2017). Is HPA axis reactivity in childhood gender-specific? A systematic review. Biol. Sex Differ. 8:23. doi: 10.1186/s13293-017-0144-8
Holsboer, F., and Ising, M. (2010). Stress hormone regulation: biological role and translation into therapy. Annu. Rev. Psychol. 61, 81–109, C101–111. doi: 10.1146/annurev.psych.093008.100321
Hori, H., Teraishi, T., Sasayama, D., Ozeki, Y., Matsuo, J., Kawamoto, Y., et al. (2011). Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J. Psychiatr. Res. 45, 1257–1263. doi: 10.1016/j.jpsychires.2011.04.001
Lemola, S., Perkinson-Gloor, N., Hagmann-von Arx, P., Brand, S., Holsboer-Trachsler, E., Grob, A., et al. (2015). Morning cortisol secretion in school-age children is related to the sleep pattern of the preceding night. Psychoneuroendocrinology 52, 297–301. doi: 10.1016/j.psyneuen.2014.12.007
Maurer, N., Perkinson-Gloor, N., Stalder, T., Hagmann-von Arx, P., Brand, S., Holsboer-Trachsler, E., et al. (2016). Salivary and hair glucocorticoids and sleep in very preterm children during school age. Psychoneuroendocrinology 72, 166–174. doi: 10.1016/j.psyneuen.2016.07.003
Meyers, J. E. M. (1995). Rey Complex Figure Test and Recognition Trial: Professional Manual. Odessa: Psychological Assessment Resources.
Mikoteit, T., Brand, S., Beck, J., Perren, S., von Wyl, A., von Klitzing, K., et al. (2012). Visually detected NREM Stage 2 sleep spindles in kindergarten children are associated with stress challenge and coping strategies. World J Biol Psychiatry 13, 259–268. doi: 10.3109/15622975.2011.562241
Mikoteit, T., Brand, S., Beck, J., Perren, S., Von Wyl, A., Von Klitzing, K., et al. (2013). Visually detected NREM Stage 2 sleep spindles in kindergarten children are associated with current and future emotional and behavioural characteristics. J. Sleep Res. 22, 129–136. doi: 10.1111/j.1365-2869.2012.01058.x
Miller, G. E., Chen, E., and Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 133, 25–45. doi: 10.1037/0033-2909.133.1.25
Minkel, J., Moreta, M., Muto, J., Htaik, O., Jones, C., Basner, M., et al. (2014). Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 33, 1430–1434. doi: 10.1037/a0034219
Perkinson-Gloor, N., Hagmann-von Arx, P., Brand, S., Holsboer-Trachsler, E., Grob, A., Weber, P., et al. (2015). The role of sleep and the hypothalamic-pituitary-adrenal axis for behavioral and emotional problems in very preterm children during middle childhood. J. Psychiatr. Res. 60, 141–147. doi: 10.1016/j.jpsychires.2014.10.005
Porcelli, A. J., Cruz, D., Wenberg, K., Patterson, M. D., Biswal, B. B., and Rypma, B. (2008). The effects of acute stress on human prefrontal working memory systems. Physiol. Behav. 95, 282–289. doi: 10.1016/j.physbeh.2008.04.027
Quesada, A. A., Wiemers, U. S., Schoofs, D., and Wolf, O. T. (2012). Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology 37, 125–136. doi: 10.1016/j.psyneuen.2011.05.013
Sadeghi Bahmani, D., Hatzinger, M., Gerber, M., Lemola, S., Clough, P. J., Perren, S., et al. (2016). The origins of mental toughness – prosocial behavior and low internalizing and externalizing problems at age 5 predict higher mental toughness scores at age 14. Frontiers in Psychology, 7:1221. doi: 10.3389/fpsyg.2016.01221
Sauro, M. D., Jorgensen, R. S., and Pedlow, C. T. (2003). Stress, glucocorticoids, and memory: a meta-analytic review. Stress 6, 235–245. doi: 10.1080/10253890310001616482
Schmidt, M. (1996). Rey Auditory and Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services.
Schwabe, L., Tegenthoff, M., Höffken, O., and Wolf, O. T. (2013). Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol. Psychiatry 74, 801–808. doi: 10.1016/j.biopsych.2013.06.001
Shields, G. S., Bonner, J. C., and Moons, W. G. (2015). Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology 58, 91–103. doi: 10.1016/j.psyneuen.2015.04.017
Shields, G. S., Sazma, M. A., McCullough, A. M., and Yonelinas, A. P. (2017). The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol. Bull. 143, 636–675. doi: 10.1037/bul0000100
Shields, G. S., Sazma, M. A., and Yonelinas, A. P. (2016). The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 68, 651–668. doi: 10.1016/j.neubiorev.2016.06.038
Stadelmann, S., Jaeger, S., Matuschek, T., Bae, Y. J., von Klitzing, K., Klein, A. M., et al. (2018). Endocrinological and subjective stress responses in children with depressive, anxiety, or externalizing disorders. Dev. Psychopathol. 30, 605–622. doi: 10.1017/S0954579417001146
Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., et al. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. doi: 10.1016/j.psyneuen.2015.10.010
Starcke, K., and Brand, M. (2016). Effects of stress on decisions under uncertainty: a meta-analysis. Psychol. Bull. 142, 909–933. doi: 10.1037/bul0000060
van Dalfsen, J. H., and Markus, C. R. (2018). The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: a systematic review. Sleep Med. Rev. 39, 187–194. doi: 10.1016/j.smrv.2017.10.002
Vogel, S., Fernández, G., Joëls, M., and Schwabe, L. (2016). Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn. Sci. 20, 192–203. doi: 10.1016/j.tics.2015.12.003
Keywords: Trier Social Stress Test for Children, learning, sleep, cortisol secretion, figural learning, bidirectionality, 9 years-old children
Citation: Brand S, Mikoteit T, Kalak N, Sadeghi Bahmani D, Lemola S, Gerber M, Ludyga S, Bossard M, Pühse U, Holsboer-Trachsler E and Hatzinger M (2018) Cortisol Impacted on Explicit Learning Encoding, but Not on Storage and Retrieval, and Was Not Associated With Sleep Patterns—Results From the Trier Social Stress Test for Children (TSST-C) Among 9-Years Old Children. Front. Psychol. 9:2240. doi: 10.3389/fpsyg.2018.02240
Received: 21 September 2018; Accepted: 29 October 2018;
Published: 21 November 2018.
Edited by:
Roumen Kirov, Institute of Neurobiology (BAS), BulgariaReviewed by:
Sam Cortese, University of Southampton, United KingdomKostas A. Papageorgiou, Queen's University Belfast, United Kingdom
Copyright © 2018 Brand, Mikoteit, Kalak, Sadeghi Bahmani, Lemola, Gerber, Ludyga, Bossard, Pühse, Holsboer-Trachsler and Hatzinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serge Brand, c2VyZ2UuYnJhbmRAdXBrLmNo
 Serge Brand
Serge Brand Thorsten Mikoteit1,5,6,7
Thorsten Mikoteit1,5,6,7 Markus Gerber
Markus Gerber Sebastian Ludyga
Sebastian Ludyga Madleina Bossard
Madleina Bossard Uwe Pühse
Uwe Pühse