- 1Department of Endocrinology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Nursing, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Purpose: To explore the relationship between neuroticism and fear of hypoglycemia (FoH) among patients with type 2 diabetes (T2D), as well as the mediating effects of diabetes distress, anxiety, and cognitive fusion on the relationship between neuroticism and FoH.
Methods: A total of 494 patients with T2D (39.9% females, n = 197) were analyzed using the neuroticism scale of the Eysenck Personality Questionnaire-Revised Short Scale (EPQ-RS), the Fear of Hypoglycemia-15 Scale (FH-15), the Diabetes Distress Scale (DDS), the Self-Rating Anxiety Scale (SAS), and the Cognitive Fusion Questionnaire (CFQ). The bootstrapping method was used to test the separate and parallel mediation models.
Results: FoH was noted in 17.4% (n = 86) of patients. The correlations between neuroticism, diabetes distress, anxiety, cognitive fusion, and FoH were positive. Diabetes distress, anxiety, and cognitive fusion were significant mediators in the association between neuroticism and FoH in both separate and parallel mediation models. In the parallel mediation model, the mediating effect of anxiety was the highest, and the mediating effect of diabetes distress was the lowest, but no significant differences were found in the comparison of these three indirect effects.
Conclusion: This study indicated that neuroticism not only directly affected FoH, but also indirectly influenced FoH via the increase of diabetes distress, anxiety, and cognitive fusion in patients with T2D. The results provide a theoretical basis for the development of intervention programs to ameliorate patients’ FoH directly and indirectly. Healthcare providers should be encouraged to develop appropriate programs based on improving diabetes distress, anxiety, and cognitive fusion to help patients with T2D improve FoH.
Introduction
Hypoglycemia occurs frequently in patients with diabetes treated with insulin and/or insulin secretagogues (Fisher et al., 2018; Johnson-Rabbett and Seaquist, 2019). Approximately 25% of patients with type 2 diabetes (T2D) taking insulin for >5 years have severe hypoglycemic episodes, which is the same as the rate of severe hypoglycemic episodes in adults with type 1 diabetes diagnosed within 5 years (UK Hypoglycaemia Study Group, 2007; Heller et al., 2020). The unpleasant symptoms caused by hypoglycemia may make the patients feel frightened; as a result, the patients may maintain high blood glucose levels to prevent hypoglycemia, which may limit the achievement of glycemic control targets (Amiri et al., 2015). The issue of fear of hypoglycemia (FoH) is of great concern worldwide because of its high prevalence and negative influences. In Japan, the prevalence of FoH in insulin-treated patients with T2D was 27.7% (Sakane et al., 2015). FoH not only influences the glycemic control of patients with T2D but also impairs their quality of life (McCoy et al., 2013), resulting in subsequent negative effects on physical and psychological consequences (Benroubi, 2011). Therefore, FoH is one of the crucial factors affecting the psychological health and self-management of patients with T2D. Globally, the number of patients with diabetes was about 463 million in 2019 and will rise to 578 million by 2030, of which T2D accounts for 90% (Zheng et al., 2018; Saeedi et al., 2019). As the prevalence of diabetes continues to rise and the psychological problems of patients with T2D become increasingly serious (Mukherjee and Chaturvedi, 2019; Saeedi et al., 2019), exploring the potential factors associated with FoH is both critical and important.
Neuroticism, one of the five major domains of personality (Goldberg, 1993), refers to the tendency to experience frequent and strong negative emotions when coping with stress, accompanied by a sense of uncontrollability and an insufficient ability to cope (Barlow et al., 2014). Good emotional regulation ability plays a key role in psychological, social and physical health (Yang et al., 2020). However, individuals with stronger neuroticism are more sensitive to negative information and are more susceptible to mental illness (Yang et al., 2020). Moreover, because of the oversensitivity to internal stimuli, people with high neuroticism tend to exhibit exaggerated physical symptoms (Johnson, 2003; Ozer and Benet-Martinez, 2006). The relationship between neuroticism and fear-related disorders has been explored in previous studies and the results showed a significant positive association between neuroticism and fear-related disorders (Ng et al., 2019; Dursun et al., 2021; Perez-Mengual et al., 2021). More specifically, neuroticism has a positive effect on the patient’s tendency to fear about hypoglycemia (Hepburn et al., 1994). The negative tendency of neuroticism may cause patients to pay more attention to hypoglycemia, perceive uncomfortable symptoms more easily, and experience more FoH. However, personality traits tend to be inflexible and stable (Barlow et al., 2014), so more flexible intervention programs are necessary. To the best of our knowledge, few studies have explored the potential mechanisms between neuroticism and FoH.
The Mediating Role of Diabetes Distress
Diabetes distress refers to the negative emotions or affective experience associated with the challenges of coexistence with diabetes, including different aspects of diabetes management (Fisher et al., 2019; Skinner et al., 2020). Previous studies have found that there is a significant correlation between diabetes distress and FoH (McCarthy et al., 2019; Li et al., 2021). More specifically, the more severe the diabetic distress of the patients, the stronger the FoH. Furthermore, a longitudinal study found that low neuroticism was linked with reduced diabetes distress over the course of a year (Sanatkar et al., 2020), which suggests that diabetes distress may be a specific result of neuroticism. As a result, diabetes distress may be a mediating variable in the relationship between neuroticism and FoH. Based on the findings reported here, we hypothesize (H1) that diabetes distress mediates the relationship between neuroticism and FoH.
The Mediating Role of Anxiety
Anxiety is a natural emotion of human beings when they have proper warning about an anxiety-producing stimulus, yet anxiety becomes a pathological disorder when it exceeds the normal level and cannot be controlled, requires no special external stimuli, and has a wide range of physical, emotional, behavioral and cognitive changes (Joyce and Herbison, 2015). Several previous studies have shown that there is a strong link between anxiety and FoH (Gonder-Frederick et al., 2006; Anderbro et al., 2015; Rechenberg et al., 2017). An anxiety-prone personality is a predictive factor for FoH (Castellano-Guerrero et al., 2018). Moreover, neuroticism is an anxiety-related endophenotype (Gottschalk and Domschke, 2017) that is positively associated with anxiety (Liao et al., 2019). Based on these findings, we can infer that anxiety may be a mediating variable in the relationship between neuroticism and FoH, which to some extent helps us to propose the hypothesis (H2) that anxiety also mediates the effect of neuroticism on FoH.
The Mediating Role of Cognitive Fusion
Cognitive fusion, one of the models of the psychopathology of acceptance and commitment therapy (ACT) (Hayes et al., 2006), is a psychological phenomenon in which people believe in the literal meaning of their thoughts instead of regarding thoughts as short internal states (Thomas and Bardeen, 2020). A study reported that cognitive fusion was positively associated with FoH (Wang, 2019). Furthermore, neuroticism is strongly related to psychological inflexibility (Latzman and Masuda, 2013), and cognitive fusion is a component of psychological inflexibility (Hayes et al., 2012). Therefore, cognitive fusion may be a mediating variable in the relation between neuroticism and FoH. Based on this accumulating evidence, in this study, we hypothesize (H3) that cognitive fusion also mediates the effect of neuroticism on FoH.
Covariates
Factors associated with FoH may influence the relationship between neuroticism and FoH. Female patients have higher FoH scores than male patients (Gjerlow et al., 2014), and older patients are prone to FoH (Anarte et al., 2014; Sakane et al., 2015; Castellano-Guerrero et al., 2018) compared with younger patients. Patients who live alone are also more likely to develop FoH (Sakane et al., 2015). Longer diabetes duration is also linked to greater FoH (Shi et al., 2014). Patients with hypoglycemic episodes in the last year or receiving insulin therapy are at higher risks of FoH (Anarte et al., 2014; Shi et al., 2014). The covariates this study will adjust are age, sex, living alone, duration of diabetes, hypoglycemic episodes in the last year, and duration of insulin use.
Consequently, we adopted separate and parallel mediation models to explore the mediating effects of diabetes distress, anxiety, and cognitive fusion on the relationship between neuroticism and FoH to further clarify the potential mechanisms of neuroticism on FoH, which will provide a theoretical basis for healthcare providers to design intervention programs to improve FoH in patients with T2D.
Materials and Methods
Study Design
A cross-sectional study was conducted from July to December 2020. Participants were recruited from the First Affiliated Hospital of Chongqing Medical University, which is a diabetes center in Chongqing, China. Convenience sampling method was used in this study. Structured questionnaires were administered to eligible patients in the inpatient department face to face. All participants signed the informed consent form before the survey, and the research proposal was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2020-418).
Participants and Procedures
The inclusion criteria were as follows: T2D diagnosed by doctors according to the 1999 World Health Organization criteria, age ≥ 18 years, duration of diabetes ≥ 1 year, and consciousness. The exclusion criteria were as follows: involuntary, pregnancy, condition that was too severe to complete the survey (e.g., headache, palpitations, or dizziness), or a history of mental illness.
For eligible patients, four trained researchers explained the content and aim of the study, and provided the investigation procedure information and the principle of anonymity. Investigations were conducted after the informed consent form was obtained. During the investigation, researchers used uniform instructions to guide and help patients. The researchers collected the questionnaires on the spot upon completion.
Pre-investigation
We used the first 50 samples as a pre-investigation to calculate the internal consistency of the scales. The internal consistency coefficients of the scale of neuroticism, diabetes distress, anxiety, cognitive fusion, and FoH were 0.853, 0.879, 0.735, 0.915, and 0.951, respectively.
Data Collection Tools
Characteristics of Patients
Sociodemographic and clinical characteristics included age, sex, hypoglycemic episodes in the past year, duration of diabetes, duration of insulin use, and living alone, which were also covariates that were adjusted in the model. Psychological constructs included neuroticism, diabetes distress, anxiety, cognitive fusion, and FoH. Hypoglycemia in patients with T2D was defined as a plasma glucose level ≤3.9 mmol/L. The number of hypoglycemic episodes in the past year came from the patients’ blood glucose monitoring diaries or the patients’ self-reports. Living alone was also self-reported by the patients. Age, sex, duration of diabetes, and duration of insulin use were derived from the electronic medical record system.
Eysenck Personality Questionnaire-Revised Short Scale
Neuroticism was evaluated by the validated Chinese version of the neuroticism scale of the Eysenck Personality Questionnaire-Revised Short Scale (EPQ-RS) (Qian et al., 2000). The EPQ-RS was developed by Eysenck et al. (1985) and includes 48 items. Its four domains, namely, neuroticism, extraversion, psychoticism, and a lie scale, respectively, include 12 items. Among them, the items in the neuroticism scale were all scored positively (“yes” = 1, “no” = 0), and the total score range of the neuroticism scale was 0–12. The higher the score, the more prominent the neuroticism personality traits. In our study, the internal consistency coefficient was 0.837.
Fear of Hypoglycemia-15 Scale
Fear of hypoglycemia was assessed with the validated Chinese version of the Fear of Hypoglycemia-15 Scale (FH-15) (Liu et al., 2018), which was originally developed by Anarte Ortiz et al. (2011). The FH-15 is a 15-item scale that includes three domains: fear, interference, and avoidance. Each item is rated on 5-point scale, where 1 = “never” to 5 = “every day.” The total score of FH-15 is between 15 and 75. The higher the score, the more serious the fear. In addition, the cutoff score of the Chinese version of the FH-15 is 30.5, which means that patients with a score > 30.5 have FoH. In our study, the internal consistency coefficient was 0.949.
Diabetes Distress Scale
The validated Chinese version of the Diabetes Distress Scale (DDS) was used to evaluate diabetes distress (Yang and Liu, 2010). It was developed by Polonsky et al. (2005). The DDS is a 17-item scale with four domains: emotional burden distress, regimen-related distress, diabetes-related interpersonal distress, and physician-related distress. Each item is assessed with a scale of 1 = “no distress” to 6 = “serious distress”. The total score ranges from 17 to 102. The higher the score was, the more severe the distress was. The internal consistency coefficient was 0.915 in the current sample.
Self-Rating Anxiety Scale
The Self-Rating Anxiety Scale (SAS) was developed by Zung (1971). In the current study, anxiety was measured with the validated Chinese version of the SAS (Tao and Gao, 1994), which has been widely used in China (Wang et al., 2020). The scale includes 20 items, with each item ranging from 1 = “none, or a little of the time” to 4 = “most, or all of the time.” Among them, the 5th, 9th, 13th, 17th, and 19th items have reverse scoring, i.e., 4 = “none, or a little of the time” to 1 = “most, or all of the time.” The raw score (20–80) was converted to a standard score (25–100) via multiplying by 1.25. The higher the score, the greater the anxiety level. The scale showed an internal consistency coefficient of 0.854 in our study.
Cognitive Fusion Questionnaire
Cognitive fusion was assessed with the validated Chinese version of the Cognitive Fusion Questionnaire (CFQ) (Zhang et al., 2014). The CFQ was developed by Gillanders et al. (2010), including two subscales of fusion and defusion, with a total of 13 items. The Chinese version of the CFQ only includes the fusion subscale with 9 items, rated on a 7-point scale ranging from 1 = “never true” to 7 = “always true.” The total score of the Chinese version of the CFQ ranges from 9 to 63. The higher the score, the higher the degree of cognitive fusion. In our study, the internal consistency coefficient was 0.936.
Data Analysis
Statistical analyses were conducted using IBM SPSS Statistics 23.0 and IBM SPSS Amos 23.0. All statistical tests were two-sided, and statistical significance was set as p-value < 0.05. Continuous variables with a normal distribution were expressed as the means ± standard deviation (mean ± SD), and the differences between groups were examined by independent-sample t tests. Continuous variables with a skewed distribution were described as the median (interquartile range), and the Mann–Whitney U test was used to examine the differences between groups. Categorical variables were presented as frequencies (percentages), and the chi-square test and Fisher’s exact test were used to compare the differences between groups. The Cronbach’s alpha coefficient represented the internal consistency of the scales and composite reliability was also calculated. Spearman coefficients and hierarchical regression were used to assess the correlation between variables.
A bootstrapping method was used to test the mediating effects (MacKinnon et al., 2004; MacKinnon, 2008), because such practice allowed the theory to develop (MacCallum and Austin, 2000; Lin et al., 2020). We performed percentile bootstrapping and bias-corrected percentile bootstrapping at a 95% confidence interval (CI) with 5,000 bootstrap samples, and 95% CI was considered statistically significant if zero was not between the lower and upper bound (Hayes, 2009). Diabetes distress, anxiety, and cognitive fusion were regarded as mediating variables. After adjusting age, sex, duration of diabetes, duration of insulin use, hypoglycemic episodes in the past year, and living alone, we calculated the direct, indirect, and total effects, and we also compared the differences among different indirect effects in the parallel mediation model. The model was considered to have a good fit if χ2/df < 3.0, root mean square error of approximation (RMSEA) < 0.06, Tucker-Lewis index (TLI) ≥ 0.95, and confirmatory fit index (CFI) ≥ 0.95 (Hu and Bentler, 1999). In this study, the Mardia’s coefficients for multivariate kurtosis were higher than the cutoff value of 3, indicating that the data deviated from the multivariate normal distribution (Hoi, 2020). Therefore, Bollen–Stine bootstrapping method was performed to evaluate Bollen–Stine p-values and Bollen–Stine χ2 was used to recalculate the fit indices (Bollen and Stine, 1992).
Results
A total of 515 questionnaires were distributed, of which 21 questionnaires were uncompleted. Finally, 494 valid questionnaires were included in the analysis.
Descriptive Analysis
The characteristics of the study participants and a comparison of factors between patients with FoH and patients without FoH are presented in Table 1. The internal consistency coefficients and composite reliability coefficients of the scales are shown in Table 2. The average age of the patients was 60.04 ± 11.71 years, of which 39.9% (n = 197) were female. According to the cutoff score of the Chinese version of the FH-15, patients were divided into FoH group and non-FoH group. The prevalence of FoH in patients with T2D in this study was 17.4% (n = 86). Compared with male patients, female patients had a higher prevalence of FoH (p = 0.035). Patients living alone had a higher rate of FoH than those living with others (p = 0.004). Compared with patients in the non-FoH group, patients in the FoH group showed a longer duration of diabetes (p = 0.029) and duration of insulin use (p < 0.001) and had more hypoglycemic episodes in the past year (p < 0.001). Furthermore, the scores of neuroticism, diabetes distress, anxiety, and cognitive fusion in the FoH group were significantly higher than those in the non-FoH group. No significant difference in age was found between the two groups.
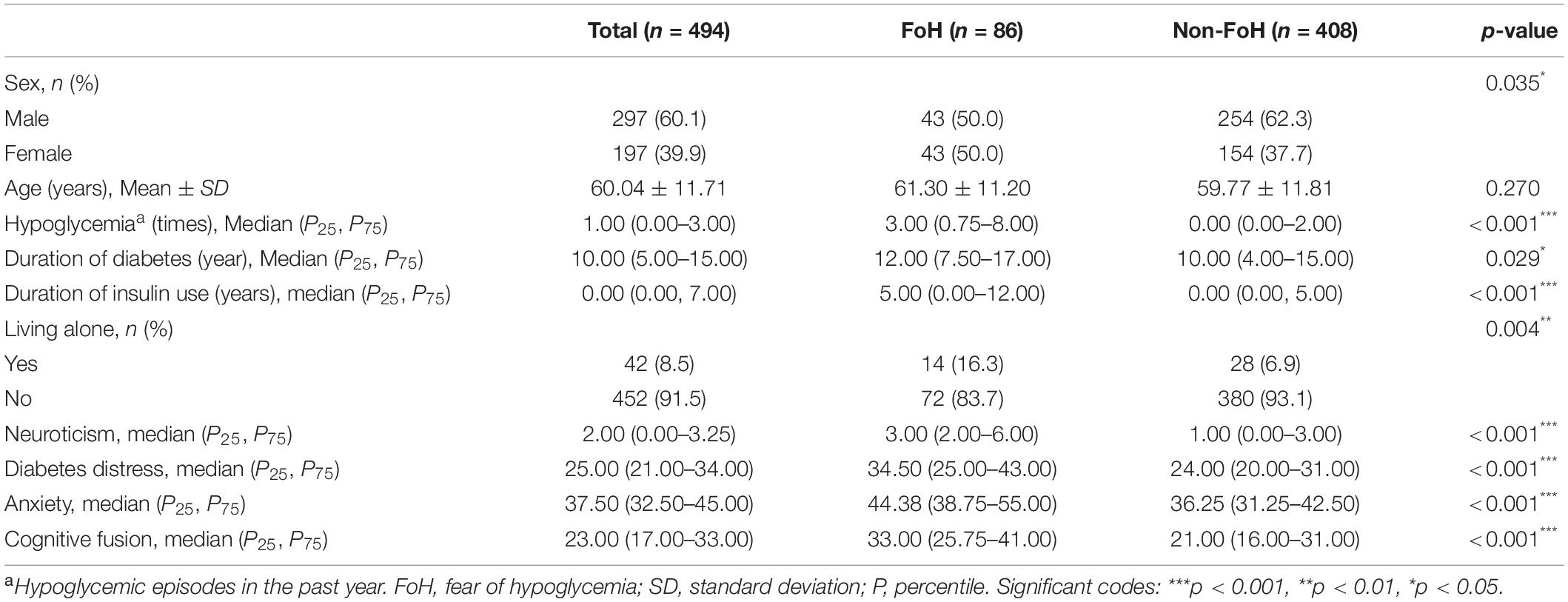
Table 1. Characteristics of the study participants and a comparison of factors between FoH group and non-FoH group.
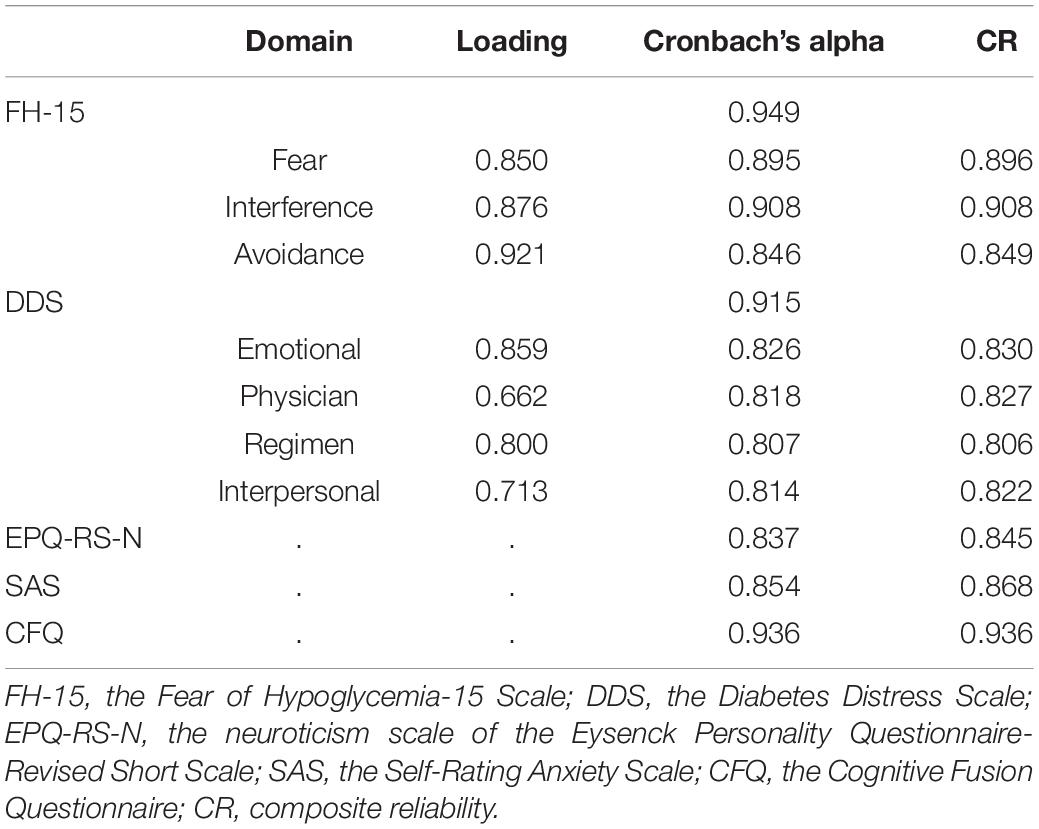
Table 2. The internal consistency coefficients and composite reliability coefficients of the scales.
Bivariate Correlation Analysis
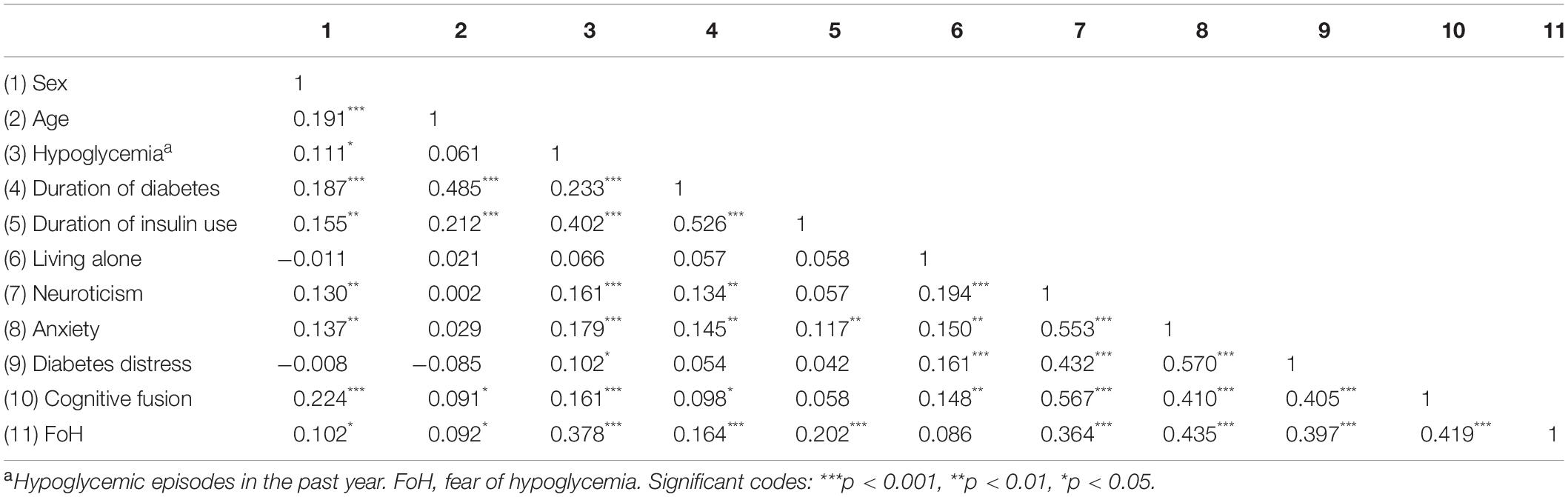
The correlation analysis results of neuroticism, diabetes distress, anxiety, cognitive fusion, and covariates are shown in Table 3. Neuroticism was positively correlated with FoH (r = 0.364, p < 0.001), diabetes distress (r = 0.432, p < 0.001), anxiety (r = 0.553, p < 0.001), and cognitive fusion (r = 0.567, p < 0.001). Diabetes distress (r = 0.397, p < 0.001), anxiety (r = 0.435, p < 0.001), and cognitive fusion (r = 0.419, p < 0.001) were all positively associated with FoH.
Hierarchical Regression Model
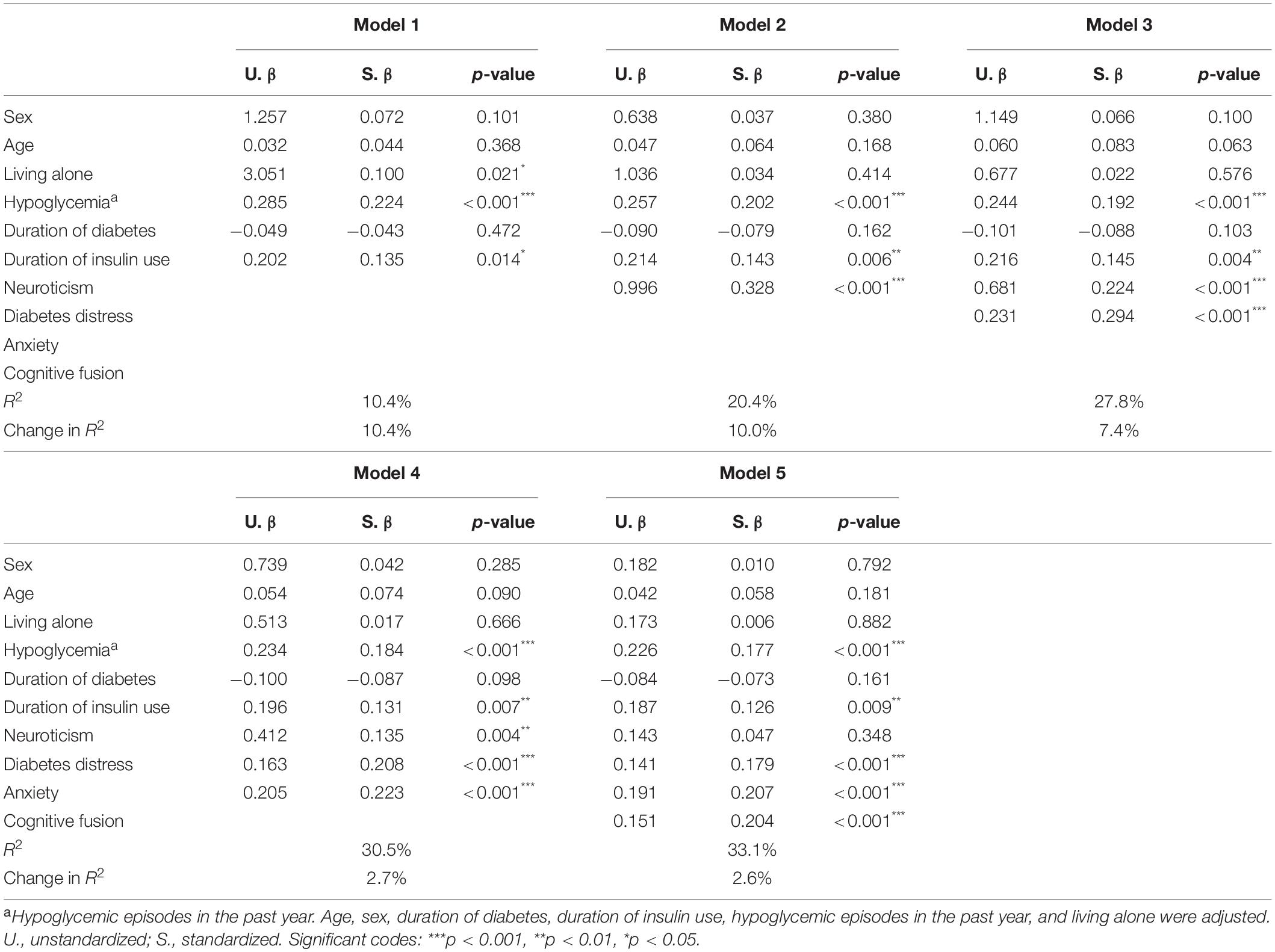
The hierarchical regression models with FoH as the dependent variable and neuroticism, diabetes distress, anxiety, and cognitive fusion as the independent variables are presented in Table 4. In model 2, after adjusting covariates, neuroticism was positively related to FoH (β = 0.328, p < 0.001). In model 5, after adjustment, diabetes distress (β = 0.179, p < 0.001), anxiety (β = 0.207, p < 0.001), and cognitive fusion (β = 0.204, p < 0.001) were positively associated with FoH, while the relationship between neuroticism and FoH (β = 0.047, p = 0.348) was not significant.
Separate Mediation Analysis With Mediator of Diabetes Distress, Anxiety, and Cognitive Fusion
Mediating Effect of Diabetes Distress
After adjusting covariates, the results showed that neuroticism had a significant effect on FoH (β = 0.332, p < 0.001), and the indirect effect of diabetes distress (β = 0.129, p < 0.001) on the relationship between neuroticism and FoH was significant. The direct effect of neuroticism on FoH was still significant (β = 0.203, p < 0.001) when diabetes distress was added as a mediating variable. The Bollen–Stine adjusted fit indices indicated a good fit: Bollen–Stine bootstrap p-value < 0.01, χ2/df = 1.331, RMSEA = 0.026, CFI = 0.991, TLI = 0.989. The results suggested that diabetes distress was a partial mediator in the association between neuroticism and FoH (see Figure 1 and Table 5).
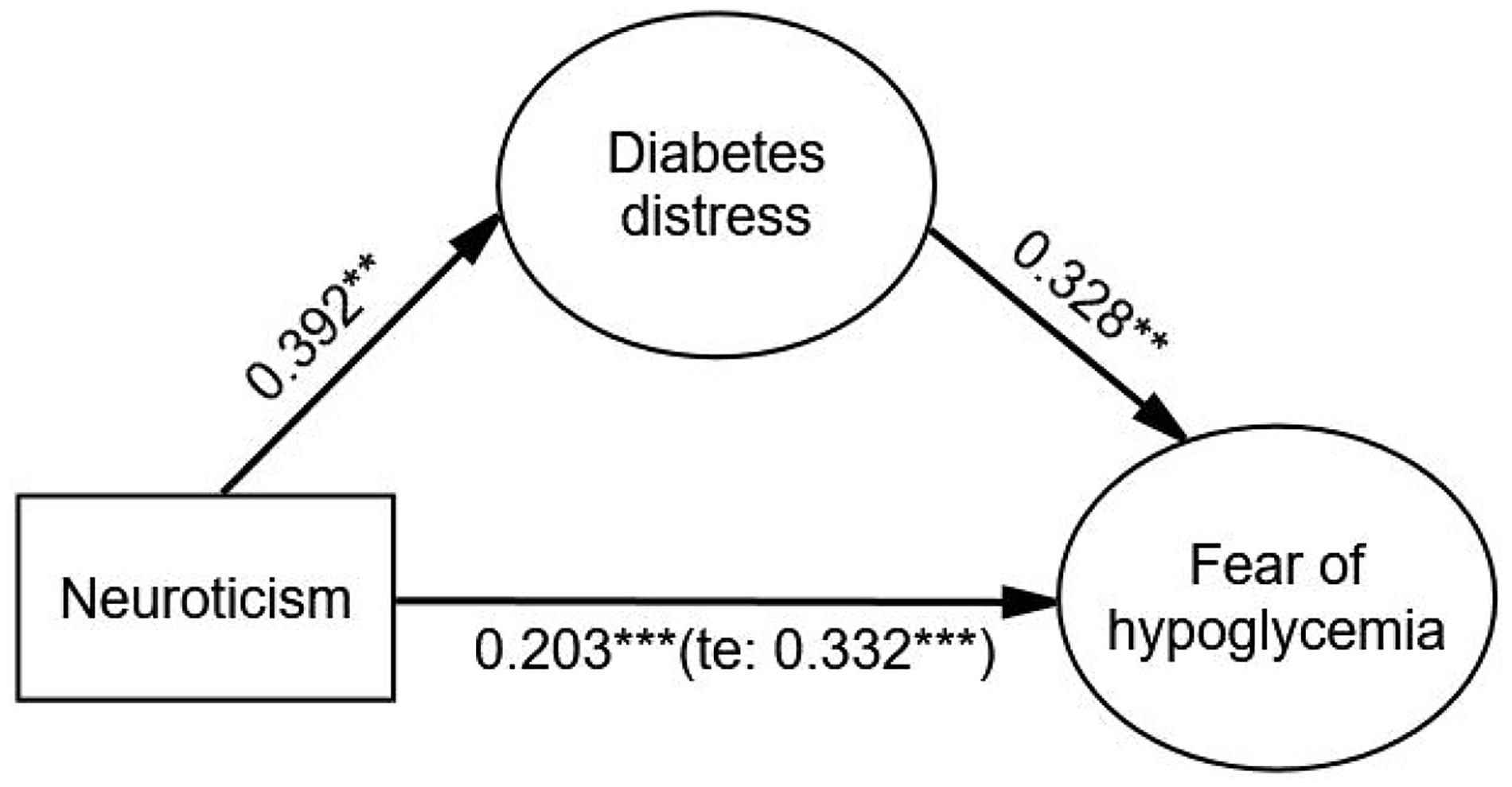
Figure 1. The mediating effect of diabetes distress on the relationship between neuroticism and FoH. Standardized estimating of 5,000 bootstrap sample. Age, sex, duration of diabetes, duration of insulin use, hypoglycemic episodes in the past year, and living alone were controlled. te, total effect. Significant codes: ***p < 0.001, **p < 0.01.
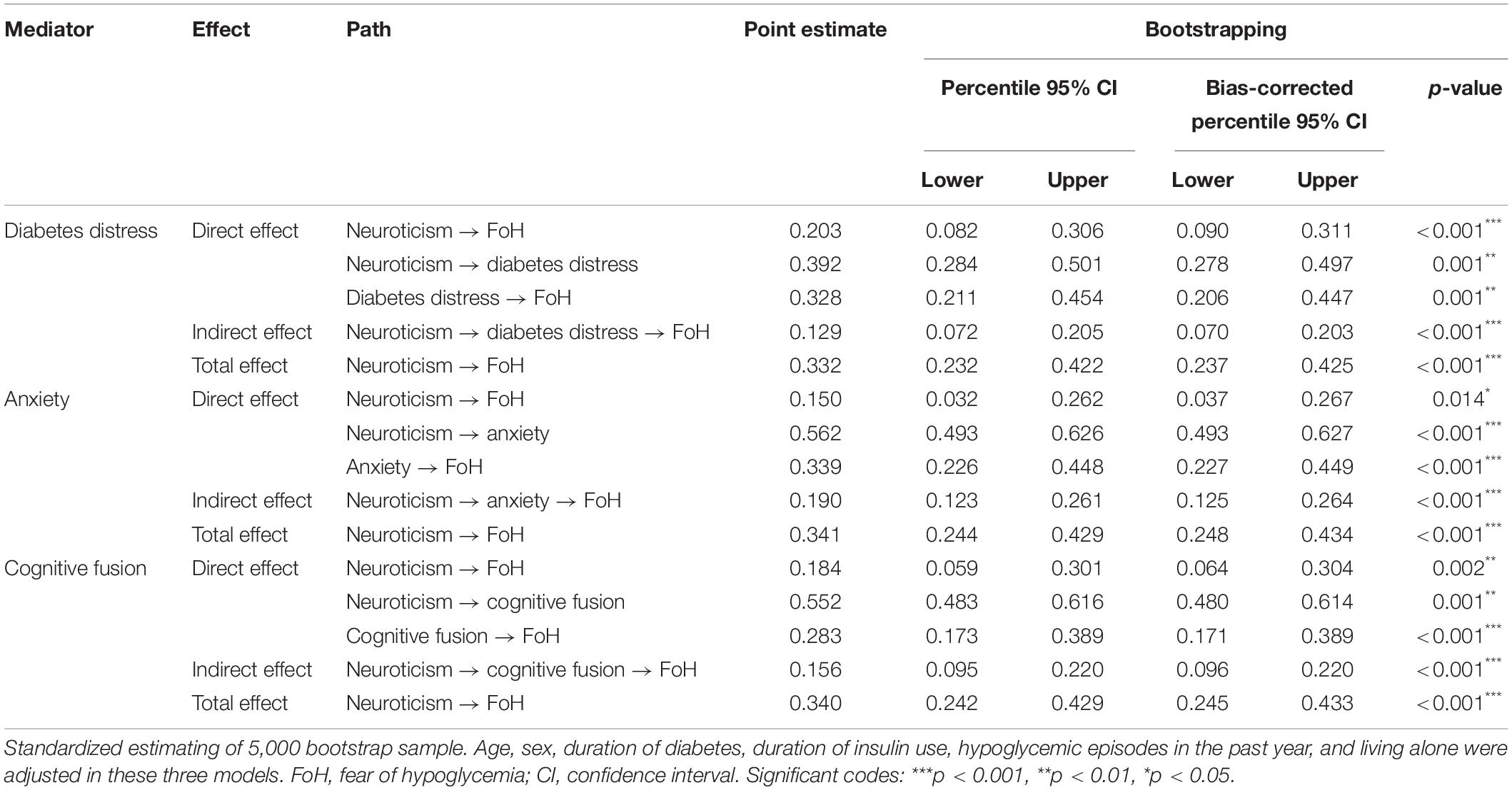
Table 5. Direct, indirect, and total effects of the separate mediation model with mediator of diabetes distress, anxiety, and cognitive fusion.
Mediating Effect of Anxiety
After adjustment, the indirect effect of anxiety (β = 0.190, p < 0.001) on the relationship between neuroticism and FoH was significant, and the direct effect of neuroticism on FoH was weaker but still significant (β = 0.150, p = 0.014) compared with the total effect (β = 0.341, p < 0.001). The Bollen–Stine adjusted fit indices indicated a good fit: Bollen–Stine bootstrap p-value < 0.01, χ2/df = 1.210, RMSEA = 0.021, CFI = 0.995, TLI = 0.994. The results revealed that anxiety was a partial mediator in the relationship between neuroticism and FoH (see Figure 2 and Table 5).
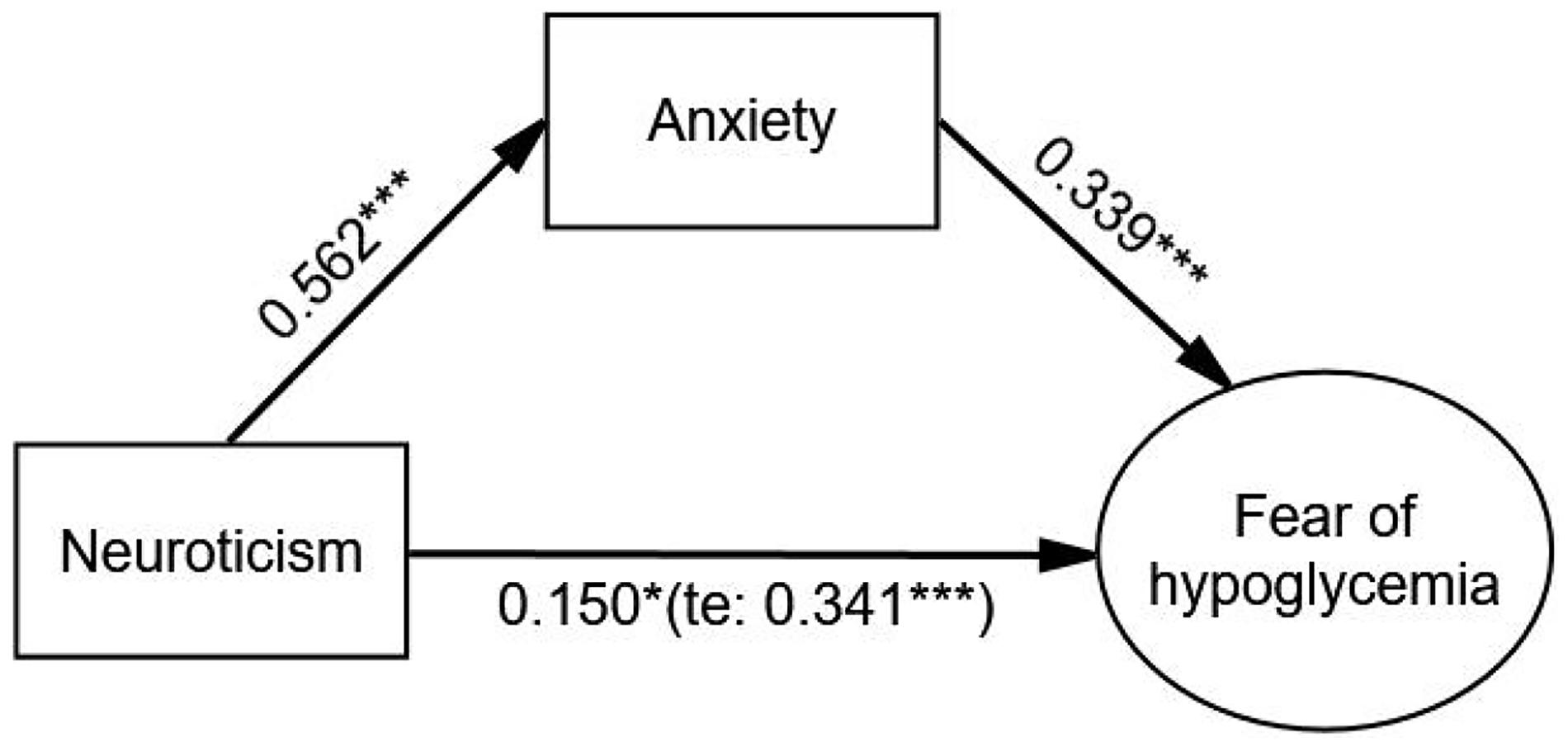
Figure 2. The mediating effect of anxiety on the relationship between neuroticism and FoH. Standardized estimating of 5,000 bootstrap sample. Age, sex, duration of diabetes, duration of insulin use, hypoglycemic episodes in the past year, and living alone were controlled. te, total effect. Significant codes: ***p < 0.001, *p < 0.05.
Mediating Effect of Cognitive Fusion
After adjusting covariates, the relationship between neuroticism and FoH was significantly mediated by cognitive fusion (β = 0.156, p < 0.001). Neuroticism had a significant effect on FoH (β = 0.340, p < 0.001), and the direct effect of neuroticism on FoH was still significant (β = 0.184, p = 0.002) when cognitive fusion was used as a mediating variable. The Bollen–Stine adjusted fit indices indicated a good fit: Bollen–Stine bootstrap p-value < 0.01, χ2/df = 1.189, RMSEA = 0.020, CFI = 0.996, TLI = 0.994. The results showed that cognitive fusion was also a partial mediator in the relationship between neuroticism and FoH (see Figure 3 and Table 5).
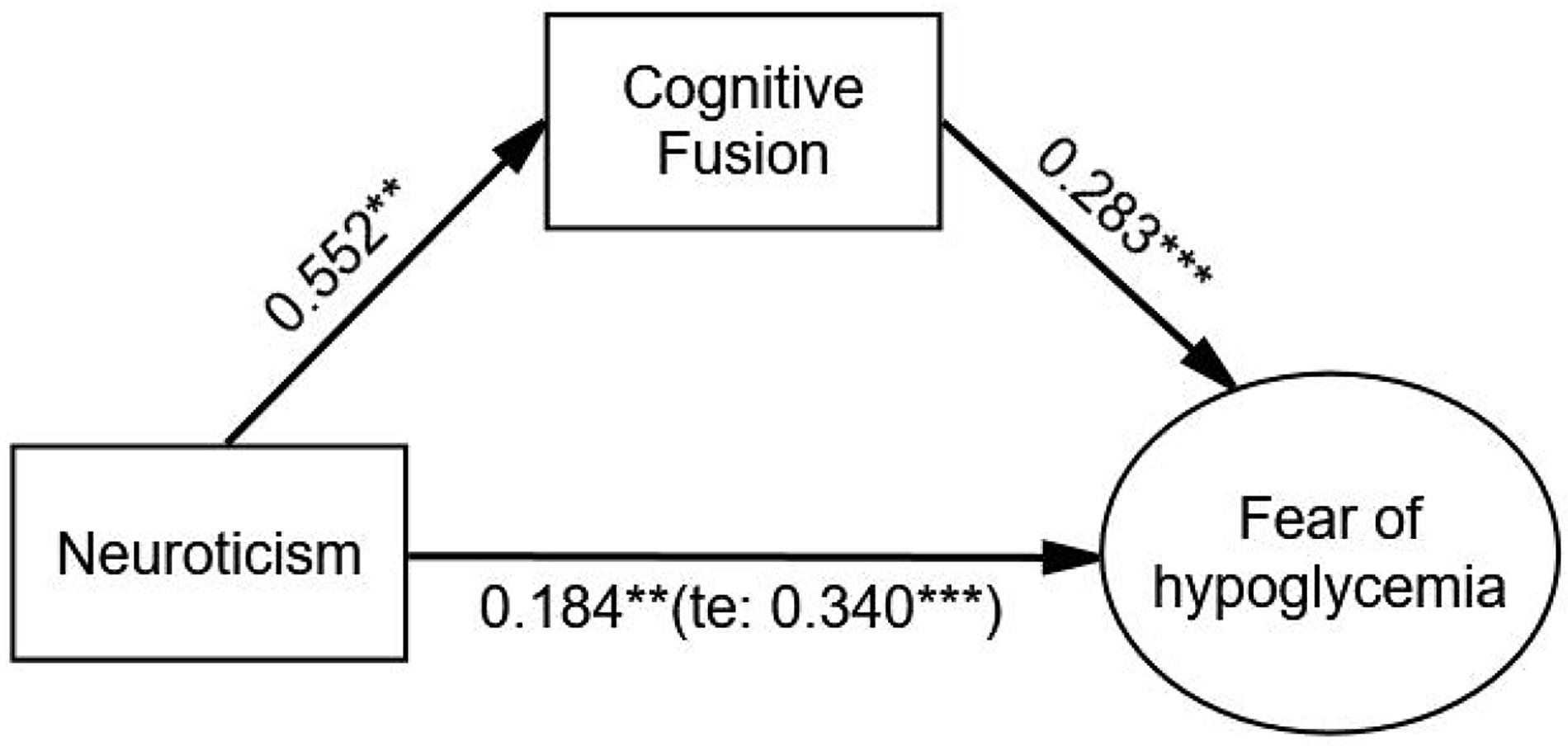
Figure 3. The mediating effect of cognitive fusion on the relationship between neuroticism and FoH. Standardized estimating of 5,000 bootstrap sample. Age, sex, duration of diabetes, duration of insulin use, hypoglycemic episodes in the past year, and living alone were controlled. te, total effect. Significant codes: ***p < 0.001, **p < 0.01.
Parallel Mediation Analysis With Mediator of Diabetes Distress, Anxiety, and Cognitive Fusion
After adjustment, diabetes distress (β = 0.077, p < 0.001), anxiety (β = 0.127, p < 0.001), and cognitive fusion (β = 0.120, p < 0.001) had significant mediating effects on the relationship between neuroticism and FoH. The direct effect of neuroticism on FoH was weaker and not significant (β = 0.031, p = 0.590) compared with the total effect (β = 0.354, p < 0.001). The order of the values of the three indirect effects from large to small were anxiety, cognitive fusion and diabetes distress. There was no significant difference in the comparison of these three indirect effects. The Bollen–Stine adjusted fit indices indicated a good fit: Bollen–Stine bootstrap p-value < 0.01, χ2/df = 1.308, RMSEA = 0.025, CFI = 0.991, TLI = 0.989. The results suggested that diabetes distress, anxiety, and cognitive fusion jointly and fully mediated the association between neuroticism and FoH (see Figure 4 and Table 6).
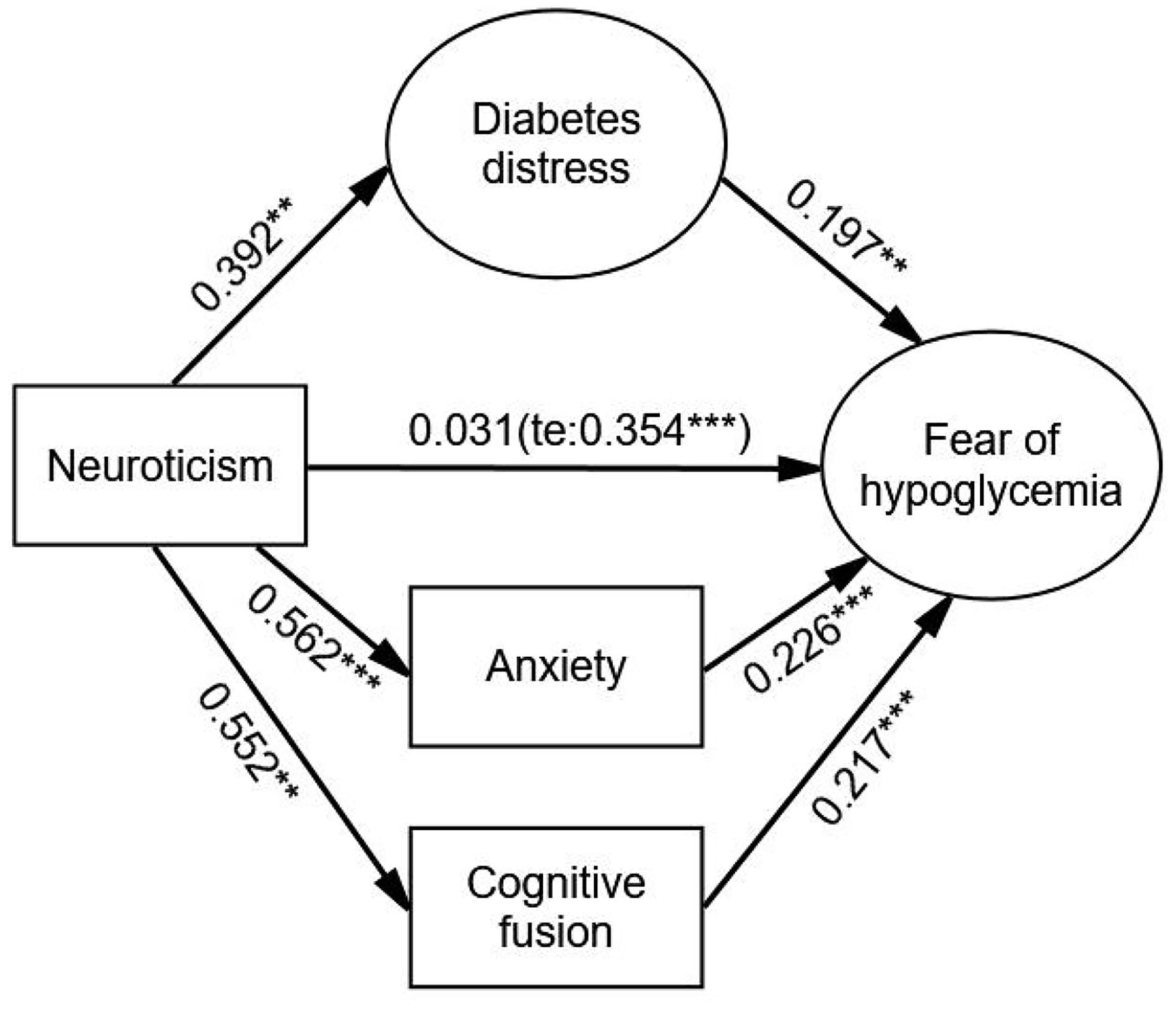
Figure 4. The parallel mediation model with mediator of diabetes distress, anxiety, and cognitive fusion. Standardized estimating of 5,000 bootstrap sample. Age, sex, duration of diabetes, duration of insulin use, hypoglycemic episodes in the past year, and living alone were controlled. te, total effect. Significant codes: ***p < 0.001, **p < 0.01.
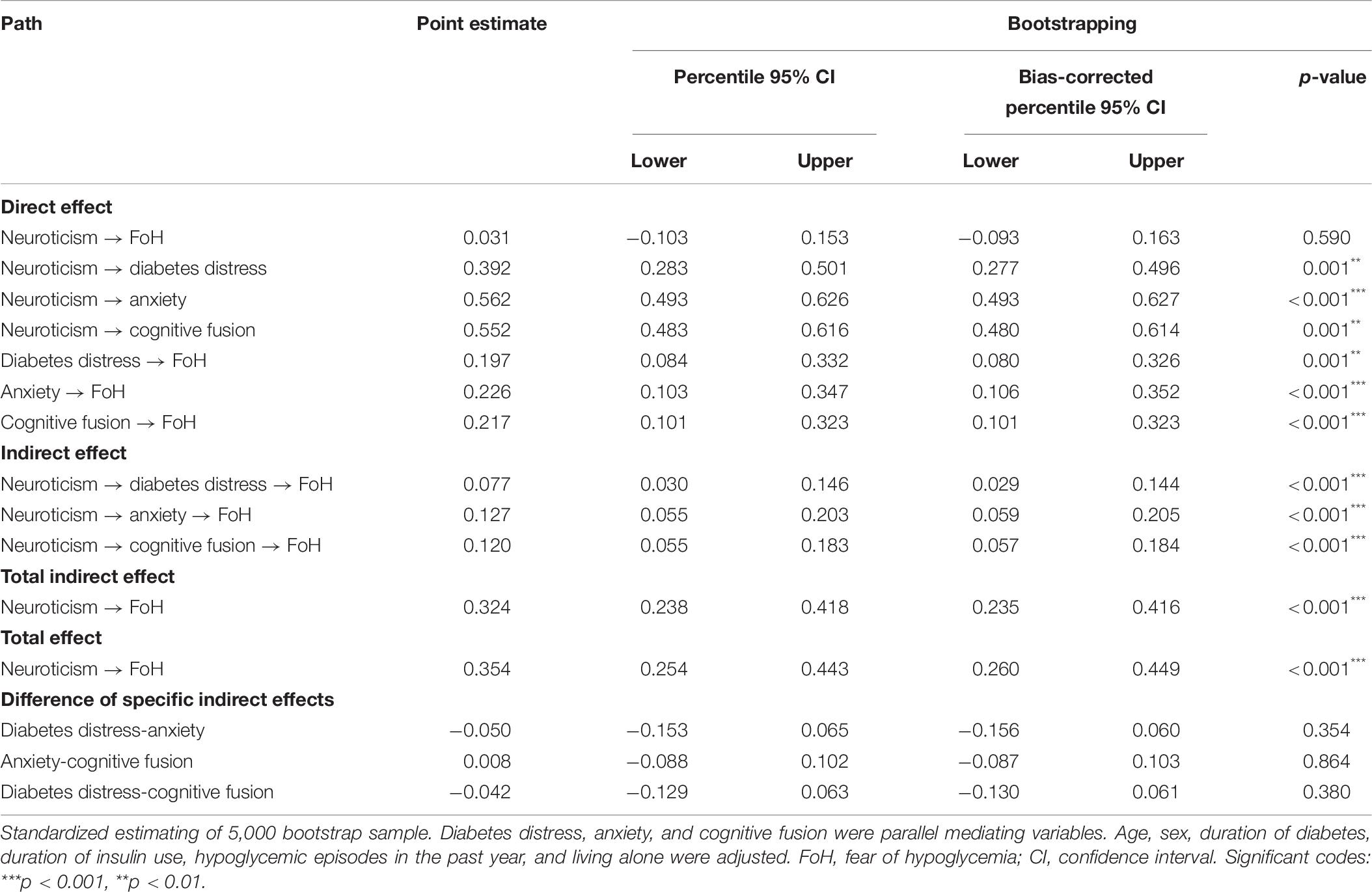
Table 6. Direct, indirect, and total effects of the parallel mediation model with mediator of diabetes distress, anxiety, and cognitive fusion.
Discussion
To our knowledge, this is the first study to perform structural equation model to reveal whether the relationship between neuroticism and FoH is mediated by diabetes distress, anxiety, and cognitive fusion. The separate and parallel mediation analyses conducted by the bootstrapping method supported our research hypotheses. The results of this study substantiated the significant mediating role of diabetes distress, anxiety, and cognitive fusion in the relationship between neuroticism and FoH. Moreover, in the parallel mediation model, these three constructs were full mediators; among them, anxiety had the highest level of indirect effects, followed by cognitive fusion and diabetes distress.
These three mediators were conducive to explaining the mechanism of why neuroticism may be one of the contributors to FoH. First, the results of the current study revealed that diabetes distress mediated the relationship between neuroticism and FoH. In this model, neuroticism was related to a high risk of diabetes distress and diabetes distress was associated with high risks of FoH, which was in line with previous studies (Sanatkar et al., 2020; Li et al., 2021). Patients with diabetes need to adhere to hypoglycemic treatments and a series of management methods, such as diet control, for a long time due to the chronic and long-term nature of diabetes, which can easily give rise to negative emotions in patients (Skinner et al., 2020). Moreover, functional magnetic resonance imaging (fMRI) results showed that neuroticism scores were inversely related to the activation of some brain regions during negative emotion regulation, indicating that neuroticism weakens one’s ability to regulate emotions (Yang et al., 2020). More specifically, patients with high neuroticism are even more unable to regulate negative emotions when coping with diabetes and tend to produce a sense of powerlessness and distress, which may ultimately lead to fear-related disorders such as FoH (Fisher et al., 2019). Therefore, a potential explanation for the positive correlation between neuroticism and FoH is that patients with high levels of neuroticism may develop FoH due to the high risks of diabetes distress. Interventions aimed at improving neuroticism and diabetes distress should be provided for patients with T2D due to the mediating effects of diabetes distress on the relationship between neuroticism and FoH.
Second, we found that anxiety was a significant mediator in the relationship between neuroticism and FoH. A previous study found that there was an overlap between the genetic factors that affect the variation of neuroticism among individuals and those that increase anxiety (Hettema et al., 2004). The vulnerability model claims that personality is always formed before the development of mental illness (Kotov et al., 2010). In other words, neuroticism indicates an anxiety-prone personality (Brandes and Bienvenu, 2006). This neuroticism-anxiety orientation is consistent with our results that neuroticism has a positive predictive effect on anxiety. Furthermore, increased anxiety can trigger defensive and maladaptive behaviors in patients, leading to clinical problems (Grecucci et al., 2020). For example, patients with FoH tend to show avoidance behaviors, including cutting the dose of insulin, overeating, and reducing physical activity, etc. As shown in several studies, the relationship between anxiety and FoH was significant (Gonder-Frederick et al., 2006; Anderbro et al., 2015; Rechenberg et al., 2017; Castellano-Guerrero et al., 2018), and a study regarded FoH as one of the anxiety-related syndromes associated with diabetes (Majidi et al., 2015). Consequently, patients with anxiety may be more susceptible to FoH. Another potential explanation for the positive correlation between neuroticism and FoH is that patients with high neuroticism personality traits may be more prone to anxiety, and the negative effects caused by anxiety may further increase one’s risk of FoH. Our results indicate that anxiety is also a useful intervention target for FoH in patients with T2D.
Third, the present study showed that neuroticism indirectly increased FoH through cognitive fusion. Some studies showed a significant correlation between neuroticism and psychological inflexibility (Latzman and Masuda, 2013; Paulus et al., 2016), which was in line with our results that neuroticism was significantly positively related to cognitive fusion. People with neuroticism traits are more likely to perceive negative information, and cognitive fusion makes people susceptible to these negative emotions. Further, we also found that cognitive fusion was significantly related to FoH, which was aligned with previous research (Wang, 2019). This may mean that cognitive fusion prompts patients to automatically extract the literal meaning of negative thinking and produce incorrect self-cognition, thereby promoting or aggravating the fear and worry of hypoglycemia. Consequently, the third potential explanation for the positive correlation between neuroticism and FoH is that patients with high neuroticism may be more susceptible to cognitive fusion, thereby increasing their risks of FoH. These results further indicate that interventions aimed at improving cognitive fusion may contribute to the improvement of FoH.
The main strength of this study is to explore the mediating role of three potential psychological factors containing diabetes distress, anxiety, and cognitive fusion in the relationship between neuroticism and FoH. In terms of clinical implications, this study offers an understanding of the associated factors that may contribute to ameliorate patients’ FoH and provides a theoretical basis for healthcare providers to take measures to directly and indirectly improve FoH via improving neuroticism, diabetes distress, anxiety, and cognitive fusion. The evaluation tools used in this study have sufficient internal consistency coefficients and composite reliability coefficients in this sample, indicating that the results are highly reliable. Despite such strengths, our study also has several limitations. First, the cross-sectional design limits the ability to explain strong causality. Therefore, future studies should adopt longitudinal designs. Second, the study only recruited patients in China. There may be cultural differences among patients in different countries; therefore, future studies need to investigate whether patients in different countries also fit the same mediation model. Third, the questionnaires were all self-reported in nature, which may lead to social desirability bias and memory recall bias. Finally, this study only investigated patients with T2D; thus, future studies should verify whether patients with type 1 diabetes experience the same mediating effects.
Conclusion
This study demonstrates that diabetes distress, anxiety, and cognitive fusion are important mediators in the relationship between neuroticism and FoH in patients with T2D. The results provide a theoretical basis for the development of intervention programs to ameliorate patients’ FoH directly and indirectly. Healthcare providers may gain implications from these findings and focus more on improving neuroticism, diabetes distress, anxiety, and cognitive fusion to ameliorate FoH.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2020-418). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZL proposed the research direction. JH, SD, and SX were responsible for questionnaire development and data collection. JH was responsible for statistical analysis. JH and SD were responsible for writing the manuscript. ZL was responsible for checking the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Nursing Research Fund of Chongqing Medical University (2019hlxk07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Yetao Luo for his statistical guidance and all the nurses of the Endocrinology Department of the First Affiliated Hospital of Chongqing Medical University for their help in the data collection.
References
Amiri, F., Vafa, M., and Gonder-Frederick, L. (2015). Glycemic Control, Self-Efficacy and Fear of Hypoglycemia Among Iranian Children with Type 1 Diabetes. Can. J. Diabetes 39, 302–307. doi: 10.1016/j.jcjd.2014.12.011
Anarte Ortiz, M. T., Caballero, F. F., Ruiz De Adana, M. S., Rondán, R. M., Carreira, M., Domínguez-López, M., et al. (2011). Development of a New Fear of Hypoglycemia Scale FH-15. Psychol. Assess. 23, 398–405. doi: 10.1037/a0021927
Anarte, M. T., Carreira, M., Machado, A., Dominguez, M., Tapia, M. J., Valdes, S., et al. (2014). Identification of risk factors for suffering fear of hypoglycemia in type 1 Diabetes Mellitus patients. Scand. J. Psychol. 55, 554–557. doi: 10.1111/sjop.12158
Anderbro, T., Gonder-Frederick, L., Bolinder, J., Lins, P. E., Wredling, R., Moberg, E., et al. (2015). Fear of hypoglycemia: relationship to hypoglycemic risk and psychological factors. Acta Diabetol. 52, 581–589. doi: 10.1007/s00592-014-0694-8
Barlow, D. H., Ellard, K. K., Sauer-Zavala, S., Bullis, J. R., and Carl, J. R. (2014). The Origins of Neuroticism. Perspect. Psychol. Sci. 9, 481–496. doi: 10.1177/1745691614544528
Benroubi, M. (2011). Fear, guilt feelings and misconceptions: barriers to effective insulin treatment in type 2 diabetes. Diabetes Res. Clin. Pract. 93, S97–S99. doi: 10.1016/s0168-8227(11)70021-3
Bollen, K. A., and Stine, R. A. (1992). Bootstrapping goodness-of-fit measures in structural equation models. Sociol. Methods Res. 21, 205–229.
Brandes, M., and Bienvenu, O. J. (2006). Personality and anxiety disorders. Curr. Psychiatry Rep. 8, 263–269. doi: 10.1007/s11920-006-0061-8
Castellano-Guerrero, A. M., Guerrero, R., Relimpio, F., Losada, F., Mangas, M. A., Pumar, A., et al. (2018). Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol. 55, 943–953. doi: 10.1007/s00592-018-1172-5
Dursun, E., Kizilirmak, A., and Mucuk, S. (2021). The relationship between personality characteristics and fear of childbirth: a descriptive study. Arch. Psychiatr. Nurs. 35, 296–302. doi: 10.1016/j.apnu.2020.09.018
Eysenck, S. B. G., Eysenck, H. J., and Barrett, P. (1985). A revised version of the psychoticism scale. Person. Individ. Diff. 6, 21–29. doi: 10.1016/0191-8869(85)90026-1
Fisher, L., Polonsky, W. H., and Hessler, D. (2019). Addressing diabetes distress in clinical care: a practical guide. Diabet. Med. 36, 803–812. doi: 10.1111/dme.13967
Fisher, S. J., Huang, X., Pawaskar, M., Witt, E. A., Rajpathak, S., Shankar, R. R., et al. (2018). Hypoglycemia in type 2 diabetes: understanding patients’ and physicians’ knowledge and experience. Endocrine 60, 435–444. doi: 10.1007/s12020-018-1545-0
Gillanders, D. T., Bolderston, H., Dempster, M., Bond, F., Campbell, L., Kerr, S., et al. (2010). “The Cognitive fusion questionnaire: further developments in measuring cognitive fusion,” in Conference Presented at the Association for Contextual Behavioral Science, World Congress VIII (Reno: Association for Contextual Behavioral Science).
Gjerlow, E., Bjorgaas, M. R., Nielsen, E. W., Olsen, S. E., and Asvold, B. O. (2014). Fear of hypoglycemia in women and men with type 1 diabetes. Nurs. Res. 63, 143–149. doi: 10.1097/NNR.0000000000000020
Goldberg, L. R. (1993). The structure of phenotypic personality traits. Am. Psychol. 48, 26–34. doi: 10.1037//0003-066x.48.1.26
Gonder-Frederick, L. A., Fisher, C. D., Ritterband, L. M., Cox, D. J., Hou, L., Dasgupta, A. A., et al. (2006). Predictors of fear of hypoglycemia in adolescents with type 1 diabetes and their parents. Pediatr. Diabetes 7, 215–222. doi: 10.1111/j.1399-5448.2006.00182.x
Gottschalk, M. G., and Domschke, K. (2017). Genetics of generalized anxiety disorder and related traits. Dialogues Clin. Neurosci. 19, 159–168. doi: 10.31887/DCNS.2017.19.2/kdomschke
Grecucci, A., Sigirci, H., Lapomarda, G., Amodeo, L., Messina, I., and Frederickson, J. (2020). Anxiety regulation: from affective neuroscience to clinical practice. Brain Sci. 10:846. doi: 10.3390/brainsci10110846
Hayes, A. F. (2009). Beyond baron and kenny: statistical mediation analysis in the new millennium. Commun. Monogr. 76, 408–420. doi: 10.1080/03637750903310360
Hayes, S. C., Luoma, J. B., Bond, F. W., Masuda, A., and Lillis, J. (2006). Acceptance and commitment therapy: model, processes and outcomes. Behav. Res. Ther. 44, 1–25. doi: 10.1016/j.brat.2005.06.006
Hayes, S. C., Strosahl, K. D., and Wilson, K. G. (2012). Acceptance and Commitment Therapy. New York: Guilford.
Heller, S. R., Peyrot, M., Oates, S. K., and Taylor, A. D. (2020). Hypoglycemia in patient with type 2 diabetes treated with insulin: it can happen. BMJ Open Diabetes Res. Care 8:e001194. doi: 10.1136/bmjdrc-2020-001194
Hepburn, D. A., Deary, I. J., Macleod, K. M., and Frier, B. M. (1994). Structural equation modeling of symptoms, awareness and fear of hypoglycemia, and personality in patients with insulin-treated diabetes. Diabetes Care 17, 1273–1280. doi: 10.2337/diacare.17.11.1273
Hettema, J. M., Prescott, C. A., and Kendler, K. S. (2004). Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am. J. Psychiatry 161, 1581–1587. doi: 10.1176/appi.ajp.161.9.1581
Hoi, V. N. (2020). Understanding higher education learners’ acceptance and use of mobile devices for language learning: a Rasch-based path modeling approach. Comput. Educ. 146:103761. doi: 10.1016/j.compedu.2019.103761
Hu, L. T., and Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Modeling 6, 1–55. doi: 10.1080/10705519909540118
Johnson, M. (2003). The vulnerability status of neuroticism: over-reporting or genuine complaints? Personal. Individ. Differ. 35, 877–887. doi: 10.1016/s0191-8869(02)00303-3
Johnson-Rabbett, B., and Seaquist, E. R. (2019). Hypoglycemia in diabetes: the dark side of diabetes treatment. A patient-centered review. J. Diabetes 11, 711–718. doi: 10.1111/1753-0407.12933
Joyce, J., and Herbison, G. P. (2015). Reiki for depression and anxiety. Cochrane Database Syst. Rev. 3:CD006833. doi: 10.1002/14651858.CD006833
Kotov, R., Gamez, W., Schmidt, F., and Watson, D. (2010). Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull. 136, 768–821. doi: 10.1037/a0020327
Latzman, R. D., and Masuda, A. (2013). Examining mindfulness and psychological inflexibility within the framework of Big Five personality. Pers. Individ. Differ. 55, 129–134.
Li, S., Fang, L., Lee, A., Hayter, M., Zhang, L., Bi, Y., et al. (2021). The association between diabetes-related distress and fear of hypoglycaemia in patients with type 2 diabetes mellitus: a cross-sectional descriptive study. Nurs. Open 00, 1–10. doi: 10.1002/nop2.800
Liao, A., Walker, R., Carmody, T. J., Cooper, C., Shaw, M. A., Grannemann, B. D., et al. (2019). Anxiety and anhedonia in depression: associations with neuroticism and cognitive control. J. Affect Disord. 245, 1070–1078. doi: 10.1016/j.jad.2018.11.072
Lin, C. Y., Cheung, P., Imani, V., Griffiths, M. D., and Pakpour, A. H. (2020). The mediating effects of eating disorder, food addiction, and insomnia in the association between psychological distress and being overweight among iranian adolescents. Nutrients 12:1371. doi: 10.3390/nu12051371
Liu, Y. Q., Xiong, S. Q., Sang, M., Li, Y. F., Anarte Ortiz, M. T., Xing, Q. L., et al. (2018). Reliability and validity of the Chinese version of the new fear of hypoglycemia scale: FH-15. Int. J. Nurs. Sci. 5, 343–351. doi: 10.1016/j.ijnss.2018.09.008
MacCallum, R. C., and Austin, J. T. (2000). Applications of structural equation modeling in psychological research. Annu. Rev. Psychol. 51, 201–226. doi: 10.1146/annurev.psych.51.1.201
MacKinnon, D. P. (2008). Introduction to Statistical Mediation Analysis. Mahwah: Lawrence Erlbaum Associates.
MacKinnon, D. P., Lockwood, C. M., and Williams, J. (2004). Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav. Res. 39, 99–128.
Majidi, S., Driscoll, K. A., and Raymond, J. K. (2015). Anxiety in children and adolescents with type 1 diabetes. Curr. Diab. Rep. 15:47. doi: 10.1007/s11892-015-0619-0
McCarthy, M. M., Whittemore, R., Gholson, G., and Grey, M. (2019). Diabetes distress, depressive symptoms, and cardiovascular health in adults with type 1 diabetes. Nurs. Res. 68, 445–452. doi: 10.1097/NNR.0000000000000387
McCoy, R. G., Van Houten, H. K., Ziegenfuss, J. Y., Shah, N. D., Wermers, R. A., and Smith, S. A. (2013). Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr. Pract. 19, 792–799. doi: 10.4158/EP12382.OR
Mukherjee, N., and Chaturvedi, S. K. (2019). Depressive symptoms and disorders in type 2 diabetes mellitus. Curr. Opin. Psychiatry 32, 416–421. doi: 10.1097/YCO.0000000000000528
Ng, D. W. L., Kwong, A., Suen, D., Chan, M., Or, A., Ng, S. S., et al. (2019). Fear of cancer recurrence among Chinese cancer survivors: prevalence and associations with metacognition and neuroticism. Psychooncology 28, 1243–1251. doi: 10.1002/pon.5073
Ozer, D. J., and Benet-Martinez, V. (2006). Personality and the prediction of consequential outcomes. Annu. Rev. Psychol. 57, 401–421. doi: 10.1146/annurev.psych.57.102904.190127
Paulus, D. J., Vanwoerden, S., Norton, P. J., and Sharp, C. (2016). Emotion dysregulation, psychological inflexibility, and shame as explanatory factors between neuroticism and depression. J. Affect. Disord. 190, 376–385. doi: 10.1016/j.jad.2015.10.014
Perez-Mengual, N., Aragones-Barbera, I., Moret-Tatay, C., and Moliner-Albero, A. R. (2021). The Relationship of Fear of Death Between Neuroticism and Anxiety During the Covid-19 Pandemic. Front. Psychiatry 12:648498. doi: 10.3389/fpsyt.2021.648498
Polonsky, W. H., Fisher, L., Earles, J., Dudl, R. J., Lees, J., Mullan, J., et al. (2005). Assessing psychosocial distress in diabetes development of the diabetes distress scale. Diabetes Care 28, 626–631. doi: 10.2337/diacare.28.3.626
Qian, M. Y., Wu, G. C., Zhu, R. C., and Zhang, S. (2000). Development of the revised eysenck personality questionnaire short scale for Chinese (EPQ-ESC). Acta Psychol. Sin. 32, 317–323.
Rechenberg, K., Whittemore, R., and Grey, M. (2017). Anxiety in youth with Type 1 diabetes. J. Pediatr. Nurs. 32, 64–71. doi: 10.1016/j.pedn.2016.08.007
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 157:107843. doi: 10.1016/j.diabres.2019.107843
Sakane, N., Kotani, K., Tsuzaki, K., Nishi, M., Takahashi, K., Murata, T., et al. (2015). Fear of hypoglycemia and its determinants in insulin-treated patients with type 2 diabetes mellitus. J. Diabetes Investig. 6, 567–570. doi: 10.1111/jdi.12340
Sanatkar, S., Baldwin, P., Clarke, J., Fletcher, S., Gunn, J., Wilhelm, K., et al. (2020). The influence of personality on trajectories of distress, health and functioning in mild-to-moderately depressed adults with type 2 diabetes. Psychol. Health Med. 25, 296–308. doi: 10.1080/13548506.2019.1668567
Shi, L., Shao, H., Zhao, Y., and Thomas, N. A. (2014). Is hypoglycemia fear independently associated with health-related quality of life? Health Qual. Life Outcomes 12:167. doi: 10.1186/s12955-014-0167-3
Skinner, T. C., Joensen, L., and Parkin, T. (2020). Twenty-five years of diabetes distress research. Diabet. Med. 37, 393–400. doi: 10.1111/dme.14157
Tao, M., and Gao, J. F. (1994). To revise the reliability and validity of the Self-Rating Anxiety Scale (SAS-CR). Chin. J. Nervous Mental Dis. 20, 301–303.
Thomas, K. N., and Bardeen, J. R. (2020). The buffering effect of attentional control on the relationship between cognitive fusion and anxiety. Behav. Res. Ther. 132:103653. doi: 10.1016/j.brat.2020.103653
UK Hypoglycaemia Study Group (2007). Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 50, 1140–1147. doi: 10.1007/s00125-007-0599-y
Wang, M. (2019). Study on The Current Situation of Fear of Hypoglycemia and Its Influencing Factors In Elderly Patients With Type 2 Diabetes Mellitus. Luoyang: Master Henan University.
Wang, Z. H., Yang, H. L., Yang, Y. Q., Liu, D., Li, Z. H., Zhang, X. R., et al. (2020). Prevalence of anxiety and depression symptom, and the demands for psychological knowledge and interventions in college students during COVID-19 epidemic: a large cross-sectional study. J. Affect. Disord. 275, 188–193. doi: 10.1016/j.jad.2020.06.034
Yang, J., Mao, Y., Niu, Y., Wei, D., Wang, X., and Qiu, J. (2020). Individual differences in neuroticism personality trait in emotion regulation. J. Affect. Disord. 265, 468–474. doi: 10.1016/j.jad.2020.01.086
Yang, Q., and Liu, X. Q. (2010). Reliability and Validity of the Diabetes Distress Scale. J. Nurs. 17, 8–10.
Zhang, W. C., Ji, Y., Li, X., Guo, H. N., and Zhu, Z. H. (2014). Reliability and validity of the Chinese version of the cognitive fusion questionnaire. Chin. Mental Health J. 28, 40–44.
Zheng, Y., Ley, S. H., and Hu, F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98. doi: 10.1038/nrendo.2017.151
Keywords: diabetes distress, anxiety, cognitive fusion, fear of hypoglycemia, mediating effect, type 2 diabetes
Citation: Huang J, Ding S, Xiong S and Liu Z (2021) The Mediating Effects of Diabetes Distress, Anxiety, and Cognitive Fusion on the Association Between Neuroticism and Fear of Hypoglycemia in Patients With Type 2 Diabetes. Front. Psychol. 12:697051. doi: 10.3389/fpsyg.2021.697051
Received: 04 May 2021; Accepted: 28 September 2021;
Published: 20 October 2021.
Edited by:
Federica Galli, Sapienza University of Rome, ItalyReviewed by:
Lauren A. Reid, University of South Carolina, United StatesMaria Helena M. Lima, State University of Campinas, Brazil
Copyright © 2021 Huang, Ding, Xiong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiping Liu, bmZtbHpwQDE2My5jb20=
†ORCID: Jing Huang, orcid.org/0000-0002-5740-1767; Shenglan Ding, orcid.org/0000-0002-5739-0092; Shuyuan Xiong, orcid.org/0000-0002-8158-6431; Zhiping Liu, orcid.org/0000-0003-1928-2178
 Jing Huang
Jing Huang Shenglan Ding
Shenglan Ding Shuyuan Xiong
Shuyuan Xiong Zhiping Liu
Zhiping Liu