- 1School of Psychological Sciences, Tel Aviv University, Tel Aviv-Yafo, Israel
- 2School of Behavioral Sciences, The Academic College of Tel Aviv-Yaffo, Tel Aviv-Yafo, Israel
- 3Sagol School of Neuroscience and School of Psychological Sciences, Tel Aviv University, Tel Aviv-Yafo, Israel
- 4Stress, Hope and Cope Laboratory, School of Behavioral Sciences, The Academic College of Tel Aviv-Yaffo, Tel Aviv-Yafo, Israel
Introduction: The stressful pre-operative period exerts a profound impact on psychological, physiological and immunological outcomes. Oncological surgeries, in particular, elicit significantly higher stress responses than most other surgeries. Managing these responses through psychological interventions may improve long-term outcomes. The purpose of the current research was to review studies that have explored pre-operative psychological interventions in cancer patients in order to map the types of current interventions and provide an initial assessment of whether these interventions improved psychological, physiological, and/or immunological indices as well as long-term cancer outcomes.
Methods: A systematic literature search for studies that included pre-operative psychological interventions in oncology patients was conducted, using the databases PubMed and Web of Science. Inclusion criteria included studies pertaining to oncological surgery in adults, study designs that included a clearly defined pre-operative psychological intervention and control group.
Results: We found 44 studies, each using one of the following interventions: psychoeducation, cognitive interventions, relaxation techniques, integrated approaches. All the studies reported improved immediate post-operative psychological, physiological, and/or immunological outcomes. Only a few studies addressed long-term cancer outcomes, and only one reported improved survival.
Conclusions: Research on pre-operative interventions with cancer patients is missing systematic methods. Studies provide varying results, which makes it difficult to compare them and reach reliable conclusions. There is considerable heterogeneity in the literature regarding the specific intervention used, the timing of intervention, the characteristics of the patients studied and the outcome measures. In order to improve research in this field, including the measurement of long-term outcomes, we suggest some steps that should be taken in further research.
Introduction
Surgery as a Stressful Event
Surgery is perceived as one of the most stressful events a person confronts in their life (Gouin and Kiecolt-Glaser, 2011). While anticipating surgery, most people feel emotional distress including fears and concerns regarding: hospitalization, anesthesia and its potential complications, pain, the recovery process, potential disabilities, interactions with hospital staff and roommates, and separation from work, friends, and family members (Johnston, 1980; Gouin and Kiecolt-Glaser, 2011). Pre-operative stress responses tend to emerge approximately 6 days before surgery, escalate 2 days before surgery, and gradually decrease after the operation, partly dissipating within 5–6 days (Johnston, 1980). The return to baseline stress levels usually occurs within several weeks, depending on the type of surgery (Johnston, 1980).
While low to moderate levels of anxiety often facilitate effective coping with the expected surgery (Pritchard, 2009), high levels of anxiety may have negative consequences manifested through physiological, emotional, behavioral, or cognitive perturbations. Physiological responses may include tachycardia, hypertension, the narrowing of peripheral blood vessels, elevated body temperature, and sweating (Scott, 2004; Pritchard, 2009). Additional physiological manifestations include immunological, hematological, metabolic, and hormonal changes; the latter tend to include increased activity of the sympathetic nervous system (SNS) and the hypothalamus-pituitary-adrenal (HPA) axis as well as inflammatory responses (Desborough, 2000). High levels of pre-operative anxiety increase sensory sensitivity, which may lead to a decreased pain threshold, dizziness, and nausea (Carr et al., 2006). Pre-operative anxiety has also been associated with higher doses of intraoperative anesthetics (Maranets and Kain, 1999), higher consumption of post-operative pain medication (Caumo et al., 2002), an extended hospitalization period, a higher rate of post-operative complications (Burg et al., 2003), and chronic post-surgical pain (CPSP; Khan et al., 2016). Emotional responses to surgery include agitation, fear, depression, hopelessness, and anger, which may lead to aggressive behaviors toward the medical staff (Coussens and Werb, 2002; Antoni, 2003). Cognitive and behavioral responses include excessive levels of alertness, negative thinking, and difficulty in concentration, which may lead to difficulties or inability to follow instructions (Antoni, 2003; Pritchard, 2009).
Oncological Surgeries
Oncological surgeries (e.g., breast, prostate and endometrial cancer surgeries) were found to elicit significantly higher pre-operative stress responses than most other surgeries (Lutgendorf et al., 2007; Garssen et al., 2010; Maggi et al., 2019), which is attributed to additional fears regarding tumor recurrence, infertility, functional modifications, body deformation, and mortality (Costanzo et al., 2007; Sciarra et al., 2018; Maggi et al., 2019; Del Giudice et al., 2020). Moreover, some studies have shown that anxiety and distress could emerge even a substantial period of time before surgery, during the biopsy phase, the diagnostic process, the decision making stage, and continue for an extended period, while undergoing various post-operative adjuvant therapies (Coussens and Werb, 2002; Antoni, 2003; Maggi et al., 2019). When discussing oncological surgeries, it is extremely important to assess the influence of surgical stress on the immune system and on long-term outcomes, manifested in tumor progression and metastasis–the main cause of death.
Ample evidence links psychological and physiological stress responses to the dysregulation of the immune system (Bartal et al., 2010; Lutgendorf and Andersen, 2015). High cortisol levels, attributed to increased pre-operative psychological stress (Bartal et al., 2010), and immune system dysfunction, were both reported among patients on the day before surgery (Lutgendorf et al., 2007; Horowitz et al., 2015). Importantly, high levels of cortisol cause immune suppression and are associated with (i) decreased production of Th1 cytokines, such as IFN-γ and IL-12 (Shaashua et al., 2017; Haldar et al., 2018), which were found to be critical regulators of NK cells activity (Antoni, 2003; Horowitz et al., 2015), and (ii) increased production of Th2 cytokines, such as IL-10. In addition, cortisol induces changes in malignant genomes, thus preventing DNA repair and promoting the survival of tumor cells (Flint et al., 2007).
Stress-Inflammatory Responses and Tumor Progression
Having cancer, awaiting surgery, and undergoing the surgical procedure induce stress-inflammatory responses and, specifically, the release of catecholamines (CAs) and prostaglandins (PGs; Antoni and Dhabhar, 2019). CAs and PGs cause immune suppression and the promotion of additional pro-metastatic processes such as the excess release of vascular endothelial growth factor (VEGF) and IL-6 from tumor cells (Horowitz et al., 2015). Moreover, the release of CAs causes changes in the expression level of a large number of genes in both the malignant tissue and its microenvironment, which contribute to cancer growth and metastasis through various known mechanisms (Hiller et al., 2018, 2019). PGs are released by tumor cells in response to stress, CAs, and tissue damage (Antoni and Dhabhar, 2019; Jeney et al., 2019). PGs are potent immunosuppressive factors and promote tumor progression also through their direct effects on the malignant tissue and its microenvironment (Jeney et al., 2019). Overall, the release of CAs and PGs during the pre-operative and entire perioperative period has a direct effect on the malignant tissue and a synergistic immunosuppressive impact that facilitates pro-metastatic processes, as reported in both clinical studies (Flint et al., 2007; Shaashua et al., 2017; Haldar et al., 2018; Antoni and Dhabhar, 2019; Ricon et al., 2019) and translational studies (Sood et al., 2007; Garssen et al., 2010; Neeman and Ben-Eliyahu, 2013; Horowitz et al., 2015).
Interestingly, translational studies have indicated that the psychological stress that precedes surgery has an additive or even synergistic deleterious effect on the physiological trauma of surgery, leading to greater immune suppression and tumor progression (Matzner et al., 2019, 2020). Furthermore, prolonged psychological stress can lead to chronic inflammatory processes (Hanahan and Weinberg, 2011), virally induced malignant processes, and defective DNA repair–all processes that foster cancer development and metastasis (Cole et al., 2015). Accordingly, increased stress levels during different stages of cancer were reported to predict early cancer recurrence (Antoni, 2000, 2003; Antoni and Dhabhar, 2019).
It should be noted that the pre-operative period, spanning days to weeks before surgery, was found in cancer patients to induce stress-derived pro-metastatic processes that were attenuated by blocking stress-inflammatory responses (Busetto et al., 2015; Shaashua et al., 2017; Haldar et al., 2018, 2020; Hiller et al., 2019; Ricon et al., 2019).
Interventions Aiming to Reduce Stress
In order to reduce patients’ stress levels, various stress management techniques have been employed as a way of improving psychological, physiological, and immunological indices. However, most research has focused on interventions initiated weeks, months, and 7 years after surgery and thus overlooked the critical period prior to the surgery (e.g., Spiegel et al., 2007; Andersen et al., 2008; Antoni et al., 2012). It has been shown in clinical studies that the pre-operative period presents a “window of opportunity,” during which psychological and physiological stress responses can be moderated, potentially leading to improved immune function and even to reduced risks of cancer recurrence and metastasis (Antoni, 2003; Sood et al., 2007; Garssen et al., 2010; Cole et al., 2015; Horowitz et al., 2015; Shaashua et al., 2017; Ricon et al., 2019; Haldar et al., 2020). In order to map types of current pre-operative psychological interventions with cancer patients and assess whether these interventions improved psychological, physiological, and immunological indices–a scoping review was conducted, since it both identifies and maps the available evidence on a given topic (Munn et al., 2018).
Materials and Methods
We conducted a systematic literature search between February 2019 and August 2021, focusing on studies describing the use of pre-operative psychological interventions among oncological patients. We utilized the electronic databases PubMed and Web of Science and searched for papers in English. Keywords included the following combinations of terms: cancer; pre-operative psychological intervention; psychological preparation before oncological surgery; and presurgical cancer psychological intervention. In addition to this primary literature search, we conducted a manual search in the reference lists of the included papers and in relevant review papers (Montgomery et al., 2002; Hoon et al., 2013; Tsimopoulou et al., 2015; Waller et al., 2015; Kim et al., 2020). Search keywords and corresponding number of retrieved items can be found in Supplementary Material 1. All titles and abstracts of retrieved studies were read by the first author and were discussed with the other authors. Irrelevant studies were excluded, and relevant empirical reports and review paper were comprehensively examined.
Our inclusion criteria included: (1) a planned oncological surgery in adults (age >18 years); (2) an intervention group and appropriate control group; and (3) a defined exclusive pre-operative psychological intervention (see below for types of interventions) delivered by a healthcare provider, including pre and post-operative measures. The full PRISMA checklist for Scoping Reviews (PRISMA-ScR; Tricco et al., 2018) can be found in Supplementary Material 2.
Results
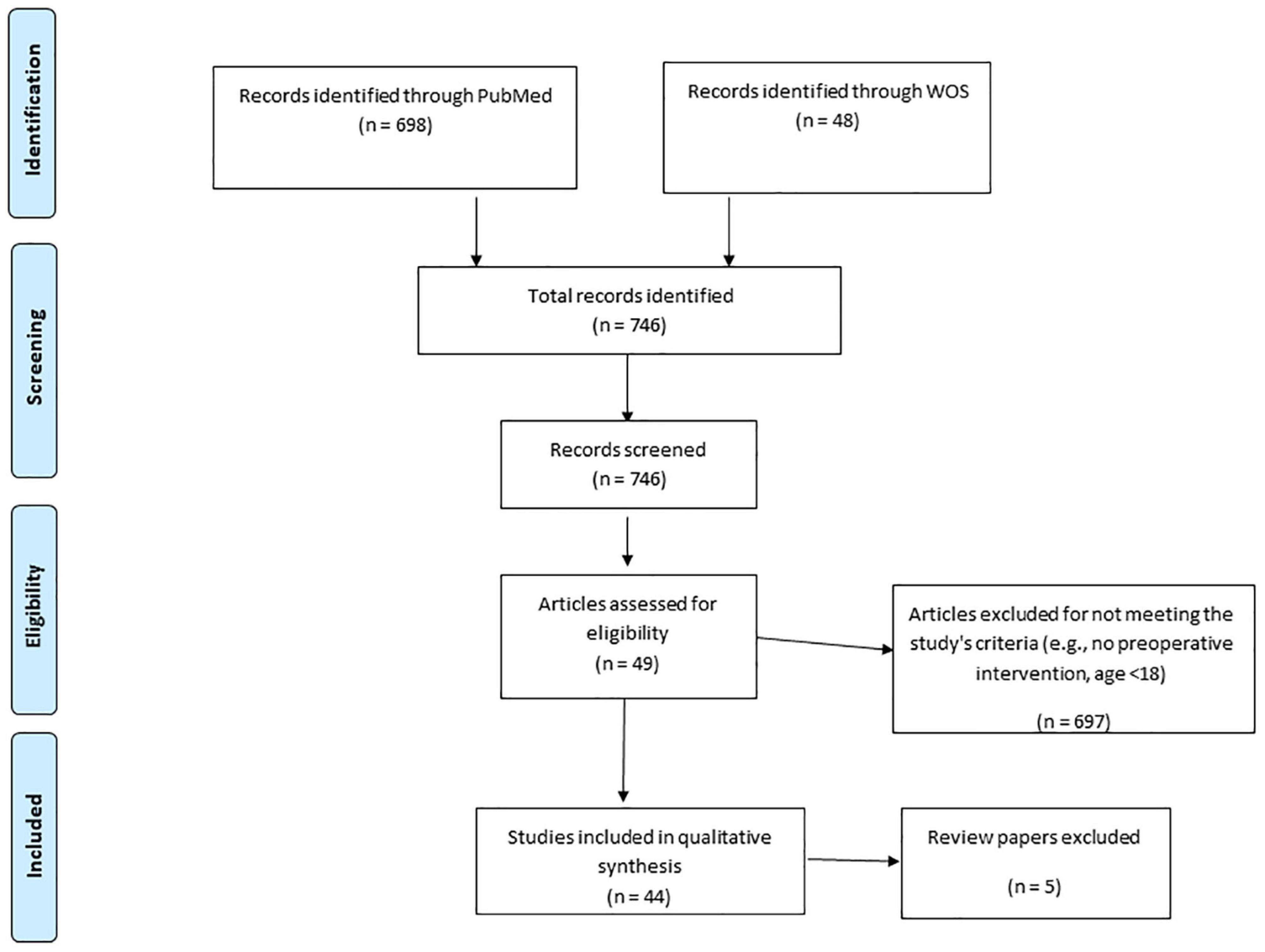
Our literature search yielded 746 papers. Of these papers, only 44 empirical reports met our inclusion criteria [see Figure 1 for a PRISMA-ScR flow diagram (Liberati et al., 2009)]. Charting form Peters et al. (2015) appears in Box 1. Other 5 review papers are discussed but were not included in the PRISMA-ScR flow diagram.
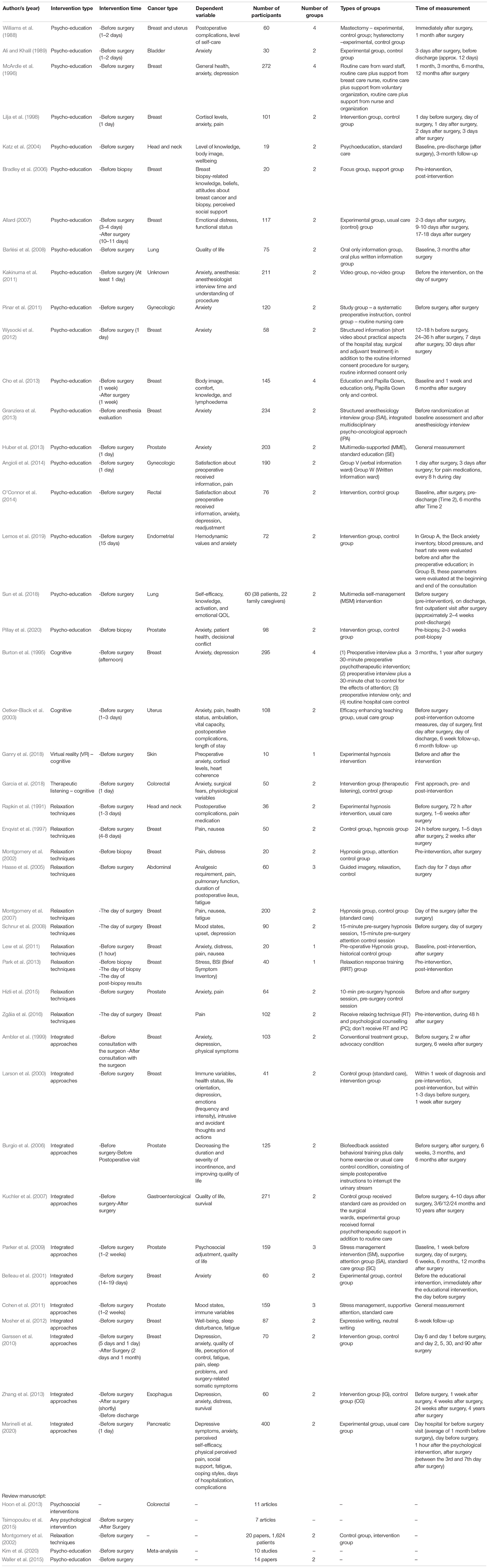
The studies reviewed reported various pre-operative psychological interventions, which we have grouped into four types based on common grouping in the field of psychology: psychoeducation (19 studies); cognitive interventions (4 studies); relaxation techniques (10 studies); and integrated approaches (11 studies). The interventions were employed at different time points and for various oncological surgeries such as breast, lung, prostate, and more. The dependent variables measured in these studies varied as well, including mainly psychological indices such as anxiety and depression; physiological indices such as post-operative complications and level of pain; and immunological indices such as cortisol levels (see Table 1). Hereby the results of the four types of interventions found in our literature review are discussed.
Psychoeducation
Anxiety levels before surgery are influenced by uncertainty regarding the anticipated medical procedures. Psychoeducation aims to provide patients with information about the surgical procedure, treatments, expected side effects, recovery process, functional modifications, etc. via written materials, videos, or by the medical staff. It is often assumed–but remains questionable–that patients feel less anxious when provided with such information. Our review includes 19 studies using psychoeducation interventions. Waller et al. (2015) reviewed the effects of providing information before oncological surgeries in 14 different studies. Their main conclusion was that interventions that include direct personal interaction with an information provider are the most effective: face-to-face interventions showed improvements in levels of anxiety, satisfaction and knowledge, while audio-visual and multimedia interventions advanced satisfaction and knowledge but did not reduce anxiety.
Although most studies in our review demonstrated that providing patients with information before surgery is helpful, some studies showed more complicated outcomes (Miro and Raich, 1999). A possible explanation is that individuals’ personal characteristics affect the need for information and the efficacy of preparation for surgery: specifically, “information seekers” might benefit from information before surgery, while “information avoiders” might exhibit negative effects (Miller, 1987, 1995; Prokop et al., 1991; Miro and Raich, 1999). Similarly, Shelley and Pakenham (2007) reported that a sense of self-efficacy and an external locus of control mediated the effects of pre-operative preparation among a population of cardiac patients: when external control was high, pre-operative preparation helped to reduce stress in patients with high sense of self-efficacy; when external control was low, pre-operative preparation reduced stress levels only among patients with low self-efficacy. Furthermore, it was suggested by Lilja et al. (1998) and Hoon et al. (2013) that extensive information might even have detrimental effects.
Recently, Kim et al. (2020) conducted a meta-analysis on 10 studies that showed that the effects of pre-operative education were greater in younger age groups when delivered using verbal or combined educational methods. In addition, it was found that most interventions were delivered in a single session (e.g., Lilja et al., 1998; Belleau et al., 2001; Barlési et al., 2008; Pinar et al., 2011) often 1 day prior to or on the day of surgery. This may place additional stress on patients who are already highly anxious, reducing the likelihood that the information is processed. Providing information earlier may help patients to take an active role in managing their care and enhance their preparation for the post-operative period.
Overall, these results indicate the need to provide patients with tailored information at the optimal time point in order to create a proper balance between excess and insufficient information. Delivering pre-operative education and matching expectations, can increase cancer patients’ knowledge, satisfaction, coping, and in some cases, reduce anxiety levels, especially when delivered face-to-face.
Cognitive Interventions
Cognitive interventions before surgery focus on the early identification and processing of disruptive pre-operative beliefs and expectations in an attempt to challenge them. Our review includes four studies using cognitive interventions. These studies all deal with patients’ exercise of self-regulation cognitive skills, which were found to reduce stress, fatigue levels, pain intensity, and pain frequency (Prokop et al., 1991; Contrada et al., 2004, 2008). Likewise, identifying the concerns of women on the day before surgery and offering specific coping strategies was found to improve impaired body image (Burton et al., 1995). Oetker-Black et al. (2003) used Bandura’s self-efficacy model (Bandura, 1982) to show that an “efficacy enhancing teaching” intervention before a hysterectomy improved functioning (ambulation, vital capacity, etc.) and reduced post-operative complications including atelectasis, pneumonia, and deep vein thrombosis.
Relaxation Techniques
Various relaxation techniques including meditation and hypnosis are used in order to reduce stress and improve coping. Our review includes 10 studies using relaxation techniques. Several studies have found that using hypnosis prior to breast biopsy or breast cancer surgery reduced the following: (1) the use of anesthetic agents during surgery and painkillers afterward; (2) post-operative side effects such as pain, nausea, vomiting, and fatigue; (3) discomfort, anxiety, depression, and emotional upset after hospital discharge; and (4) reduced use of medication (Enqvist et al., 1997; Montgomery et al., 2007; Schnur et al., 2008; Lew et al., 2011; Hizli et al., 2015; Zgâia et al., 2016). Hypnosis was also shown to have positive effects on the course of the surgery and the duration of post-operative hospitalization (Rapkin et al., 1991). However, relaxation interventions before colorectal cancer surgery were found to have no effect on post-operative clinical indices such as pain intensity or the use of analgesic drugs (Haase et al., 2005).
Integrated Approaches
Integrated therapeutic modalities include a combination of at least two of the above categories of interventions. Our review includes 11 studies using integrated approaches. When such approaches were employed in men before prostate cancer surgery and women before breast cancer surgery, they found: (1) reduced levels of psychological distress (Ambler et al., 1999; Belleau et al., 2001); (2) improved mood states before surgery and throughout the year following surgery (Parker et al., 2009); (3) greater readiness to use mental health services if needed (Mosher et al., 2012); and (4) increased levels of circulating IL12p70 serum, natural killer cell cytotoxicity, and tumor necrosis factor (TNF)-α (Cohen et al., 2011), and decreased interferon gamma production by peripheral mononuclear cells (Larson et al., 2000).
While there are indications that pre-operative interventions contribute to short-term outcomes, there is not enough evidence regarding long-term benefits. The study of long-term cancer outcomes requires long periods of follow-up and a high number of patients to achieve sufficient statistical power (Chida et al., 2008). Attempts to study long-term cancer outcomes have only been conducted in cases where integrated pre-operative interventions were employed. Specifically, among esophageal cancer patients (n = 60), a treatment based on information, psychological support, stress management, and coping strategies was found to shorten duration of hospitalization, reduce the cost of medical care, and increase patients’ satisfaction with their hospital care (Zhang et al., 2013). However, 4 years after surgery, no significant differences were found between the intervention and control groups with regard to survival or health measures, as might be expected in a sample size of only 30 patients per group.
Only two studies directly examined the hypothesis that pre-operative psychological interventions might improve survival. Burton et al. (1995) provided a 30-min pre-operative personalized intervention among breast cancer patients (n = 295) and found reduced post-operative anxiety and distress levels. No impact on recurrence rate was found 1–2 years following surgery. In another study, Kuchler et al. (2007) implemented an individually tailored integrated intervention among 271 randomized gastroenterological cancer patients who underwent surgery between the years 1991–1993 at the University Hospital of Hamburg. Their intervention extended beyond the pre-operative timeframe and included sessions before surgery, during the entire hospitalization, and on hospital discharge. During a 10-year follow-up period, 29 of the 136 treated patients (21%) survived, compared to only 13 of the 135 (9%) control patients. This highly significant favorable effect was maintained when Cox regression analysis included all known covariates (e.g., TNM staging) (Kuchler et al., 2007). We found no other pre-operative studies that reported long-term cancer outcomes.
Discussion
Our review exemplifies the effect of pre-operative psychological interventions among oncological patients on psychological, physiological, and Immunological Indices. However, the research in this field lacks the use of systematic methods and therefore provides varying results, which makes it difficult to compare between them and reach reliable conclusions. Moreover, there is not enough data to assess whether these interventions improve immunological measures and long-term outcomes. Although the existing research indicates that pre-operative interventions are beneficial, there is considerable heterogeneity regarding the specific intervention used, its timing, and the outcome measures (dependent variables).
(1) Interventions
Since research designs use various interventions and it becomes difficult to compare between them, we believe that an intervention adapted protocol might contribute to decrease these limitations. Moreover, a recent research has found specific clusters of stress responses representing interpersonal variation (Jacoby et al., 2021). While planning a pre-surgical intervention, it is worth considering each person’s stress response profile and offering them a tailored intervention.
(2) Time Points
Since there is significant variability regarding time points of interventions, measurements, and follow-up, we wish to emphasize the benefits of developing a protocol which might reduce the variability among these variables.
(3) Dependent Variables
The multiplicity of dependent variables in the studies reviewed does not allow for clear conclusions. A focus on common variables will enable both a comparison and a synthesis of studies’ results. Recent developments in psychoneuroimmunology enable the use of research methods and indices that were not previously available (Slavich, 2020).
(4) Samples
The sample sizes in the studies reviewed were mostly small (10–203, median 71). Six studies had higher participant number (211–400). Therefore, in most studies the statistical power for testing hypotheses regarding long-term cancer outcomes is inadequate. Studies assessing such outcomes may necessitate hundreds of patients per group according to cancer type.
Conclusion
Scoping reviews are designed to provide an overview of the existing evidence base, regardless of quality (Peters et al., 2015). Our review shows that although there are some indications that pre-operative psychological interventions among oncological patients may improve psychological and physiological indices post-operatively, major steps should be taken to improve research in this field. We hope that our findings stimulate specific research questions including the synthesis of effect sizes and incorporation of risk-of-bias assessments. It is possible that lack of sufficient awareness of the importance of the short pre-operative period in determining long-term cancer outcomes has reduced the incentive to intervene at this stage, thus resulting in relatively few reported studies. In addition, interventions during the pre-operative period may be inconvenient to the patient and the medical establishment, thus contributing to the reduced incentive to intervene and conduct research at this point. Nonetheless, the National Comprehensive Cancer Network [NCCN] (2018) has recently acknowledged the importance of psychological interventions before surgery. Their implementation will, we believe, enhance patients’ physiological and psychological health.
The Covid 19 pandemic during 2020–2022 has raised stress levels among most populations, but especially in people who cope with bodily illness and/or are dependent on the medical services. Unfortunately, some populations, such as the elderly and lower-class people, who have limited excess to health services and cannot afford private medicine, are exposed to greater risk and suffering (Chung et al., 2015). Additionally, perioperative routine medical treatments may need reconsideration to accommodate to needed medical procedures and to prevent various risks including disease transaction (Salciccia et al., 2020a,b, 2021). Therefore, future perioperative interventions should take into consideration the impact of such periods on psychological, physiological, and immunological variables.
Limitations
Only psychological interventions alone may lack the capacity to mitigate the stress and inflammatory responses that originate from physiological elements in the perioperative period, such as tissue damage and other physiological perturbations. Therefore, additional interventions, such as medications, should be considered in order to estimate the influence of a combination of psychological and medical interventions on long-term outcomes. Notably, recent biomarker pharmacological studies have indicated a reduction in pro-metastatic malignant potential via pre-operative anti-stress drug interventions. Pharmacological treatments during surgery (Shaashua et al., 2017; Haldar et al., 2018, 2020; Hiller et al., 2019; Knight et al., 2020) may be needed to overcome the impact of tissue damage and other intraoperative procedures that may mask the beneficial effects of stress-reducing psychological interventions. The separate and combined effectiveness of psychological and pharmacological approaches should thus be studied perioperatively.
Moreover, our review focuses on the pre-operative period. However, there are indications to include the interventions employed during the whole perioperative period (days before and after surgery). Several translational and clinical studies have suggested that the pre-operative period, the surgical procedure, and the immediate post-operative period each have a distinct impact on long-term cancer outcomes. For example, animal studies have clearly indicated the cumulative effects of stress and surgery (including anesthesia, tissue damage, pain, etc.) on cancer progression (Cole et al., 2015; Horowitz et al., 2015; Ricon et al., 2019). Recent clinical studies have provided similar evidences that are based on interim biomarkers and correlative evidence (Shaashua et al., 2017; Haldar et al., 2018, 2020; Hiller et al., 2019; Ricon et al., 2019; Knight et al., 2020). Consequently, it becomes clear that the perioperative period as a whole entails heightened risks for cancer progression but also offers unexploited treatment targets for improving resistance to cancer metastasis (Hiller et al., 2018; Matzner et al., 2019; Ben-Eliyahu, 2003; Benish et al., 2008)–the leading cause of cancer mortality (Mehlen and Puisieux, 2006). We therefore suggest that further research on humans should focus on the impact of interventions conducted during the entire perioperative time frame.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
TH-M: conceptualization, project administration, investigation, writing – original draft, methodology, and writing – review and editing. GN: formal analysis, methodology, investigation, and writing – review and editing. RJ: conceptualization, project administration, investigation, writing – original draft, writing – review and editing, and supervision. SB-E: investigation, writing – original draft, methodology, and writing – review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.839065/full#supplementary-material
References
Allard, N. C. (2007). Day surgery for breast cancer: effects of a psychoeducational telephone intervention on functional status and emotional distress. Oncol. Nurs. Forum 34, 133–141.
Ali, N. S., and Khalil, H. Z. (1989). Effect of psychoeducational intervention on anxiety among Egyptian bladder cancer patients. Cancer Nurs. 12, 236–242.
Ambler, N., Rumsey, N., Harcourt, D., Khan, F., Cawthorn, S., and Barker, J. (1999). Specialist nurse counsellor interventions at the time of diagnosis of breast cancer: comparingadvocacy’with a conventional approach. J. Adv. Nurs. 29, 445–453. doi: 10.1046/j.1365-2648.1999.00902.x
Angioli, R., Plotti, F., Capriglione, S., Aloisi, A., Aloisi, M. E., Luvero, D., et al. (2014). The effects of giving patients verbal or written pre-operative information in gynecologic oncology surgery: a randomized study and the medical-legal point of view. Eur. J. Obstet. Gynecol. Reprod. Biol. 177, 67–71.
Andersen, B. L., Yang, H. C., Farrar, W. B., Golden-Kreutz, D. M., Emery, C. F., Thornton, L. M., et al. (2008). Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer 113, 3450–3458. doi: 10.1002/cncr.23969
Antoni, M. H. (2000). Stress Management Intervention for Women With Breast Cancer. Washington, DC: APA.
Antoni, M. H. (2003). Stress Management Intervention for Women with Breast Cancer: Participant’s Workbook. Washington, DC: APA.
Antoni, M. H., and Dhabhar, F. S. (2019). The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 125, 1417–1431. doi: 10.1002/cncr.31943
Antoni, M. H., Lutgendorf, S. K., Blomberg, B., Carver, C. S., Lechner, S., Diaz, A., et al. (2012). Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiatry 71, 366–372. doi: 10.1016/j.biopsych.2011.10.007
Bandura, A. (1982). Self-efficacy mechanism in human agency. Am. Psychol. 37:122. doi: 10.1037/0003-066x.37.2.122
Barlési, F., Barrau, K., Loundou, A., Doddoli, C., Simeoni, M.-C., Auquier, P., et al. (2008). Impact of information on quality of life and satisfaction of non-small cell lung cancer patients: a randomized study of standardized versus individualized information before thoracic surgery. J. Thoracic Oncol. 3, 1146–1152. doi: 10.1097/JTO.0b013e3181874637
Bartal, I., Melamed, R., Greenfeld, K., Atzil, S., Glasner, A., Domankevich, V., et al. (2010). Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav. Immun. 24, 376–386. doi: 10.1016/j.bbi.2009.02.010
Belleau, F. P., Hagan, L., and Masse, B. (2001). Effects of an educational intervention on the anxiety of women awaiting mastectomies. Can. Oncol. Nurs. J. 11, 172–180.
Ben-Eliyahu, S. (2003). The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav. Immun. 17 (Suppl 1), S27–S36. doi: 10.1016/s0889-1591(02)00063-6
Benish, M., Bartal, I., Goldfarb, Y., Levi, B., Avraham, R., Raz, A., et al. (2008). Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 15, 2042–2052. doi: 10.1245/s10434-008-9890-5
Bradley, P. K., Berry, A., Lang, C., and Myers, R. E. (2006). Getting ready: developing an educational intervention to prepare African American women for breast biopsy. ABNF J. 17, 15–19.
Burg, M. M., Benedetto, M. C., Rosenberg, R., and Soufer, R. (2003). Presurgical depression predicts medical morbidity 6 months after coronary artery bypass graft surgery. Psychosom. Med. 65, 111–118. doi: 10.1097/01.psy.0000038940.33335.09
Burgio, K. L., Goode, P. S., Urban, D. A., Umlauf, M. G., Locher, J. L., Bueschen, A., et al. (2006). Preoperative biofeedback assisted behavioral training to decrease post-prostatectomy incontinence: a randomized, controlled trial. J. Urol. 175, 196–201.
Burton, M. V., Parker, R. W., Farrell, A., Bailey, D., Conneely, J., Booth, S., et al. (1995). A randomized controlled trial of preoperative psychological preparation for mastectomy. PsychoOncology 4, 1–19.
Busetto, G. M., Giovannone, R., Antonini, G., Rossi, A., Del Giudice, F., Tricarico, S., et al. (2015). Short-term pretreatment with a dual 5α-reductase inhibitor before bipolar transurethral resection of the prostate (B-TURP): evaluation of prostate vascularity and decreased surgical blood loss in large prostates. BJU Int. 116, 117–123. doi: 10.1111/bju.12917
Carr, E., Brockbank, K., Allen, S., and Strike, P. (2006). Patterns and frequency of anxiety in women undergoing gynaecological surgery. J. Clin. Nurs. 15, 341–352. doi: 10.1111/j.1365-2702.2006.01285.x
Caumo, W., Hidalgo, M. P., Schmidt, A. P., Iwamoto, C. W., Adamatti, L. C., Bergmann, J., et al. (2002). Effect of pre-operative anxiolysis on postoperative pain response in patients undergoing total abdominal hysterectomy. Anaesthesia 57, 740–746. doi: 10.1046/j.1365-2044.2002.02690.x
Chida, Y., Hamer, M., Wardle, J., and Steptoe, A. (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature clinical practice. Oncology 5, 466–475. doi: 10.1038/ncponc1134
Cho, H. S. M., Davis, G. C., Paek, J. E., Rao, R., Zhao, H., Xie, X. J., et al. (2013). A randomised trial of nursing interventions supporting recovery of the postmastectomy patient. J. Clin. Nurs. 22, 919–929.
Chung, B. I., Leow, J. J., Gelpi-Hammerschmidt, F., Wang, Y., Del Giudice, F., De, S., et al. (2015). Racial disparities in postoperative complications after radical nephrectomy: a population-based analysis. Urology 85, 1411–1416. doi: 10.1016/j.urology.2015.03.001
Cohen, L., Parker, P. A., Vence, L., Savary, C., Kentor, D., Pettaway, C., et al. (2011). Presurgical stress management improves postoperative immune function in men with prostate cancer undergoing radical prostatectomy. Psychosom. Med. 73, 218–225. doi: 10.1097/PSY.0b013e31820a1c26
Cole, S. W., Nagaraja, A. S., Lutgendorf, S. K., Green, P. A., and Sood, A. K. (2015). Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 15:563. doi: 10.1038/nrc3978
Contrada, R. J., Boulifard, D. A., Hekler, E. B., Idler, E. L., Spruill, T. M., Labouvie, E. W., et al. (2008). Psychosocial factors in heart surgery: presurgical vulnerability and postsurgical recovery. Health Psychol. 27:309. doi: 10.1037/0278-6133.27.3.309
Contrada, R. J., Goyal, T. M., Cather, C., Rafalson, L., Idler, E. L., and Krause, T. J. (2004). Psychosocial factors in outcomes of heart surgery: the impact of religious involvement and depressive symptoms. Health Psychol. 23:227. doi: 10.1037/0278-6133.23.3.227
Costanzo, E. S., Lutgendorf, S. K., Mattes, M. L., Trehan, S., Robinson, C. B., Tewfik, F., et al. (2007). Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br. J. Cancer 97, 1625–1631. doi: 10.1038/sj.bjc.6604091
Del Giudice, F., Kasman, A. M., Ferro, M., Sciarra, A., De Berardinis, E., Belladelli, F., et al. (2020). Clinical correlation among male infertility and overall male health: a systematic review of the literature. Invest. Clin. Urol. 61:355. doi: 10.4111/icu.2020.61.4.355
Desborough, J. P. (2000). The stress response to trauma and surgery. Br. J. Anaesth. 85, 109–117. doi: 10.1093/bja/85.1.109
Enqvist, B., Bjorklund, C., Engman, M., and Jakobsson, J. (1997). Preoperative hypnosis reduces postoperative vomiting after surgery of the breasts. A prospective, randomized and blinded study. Acta Anaesthesiol. Scand. 41, 1028–1032. doi: 10.1111/j.1399-6576.1997.tb04831.x
Flint, M. S., Baum, A., Chambers, W. H., and Jenkins, F. J. (2007). Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32, 470–479. doi: 10.1016/j.psyneuen.2007.02.013
Ganry, L., Hersant, B., Sidahmed-Mezi, M., Dhonneur, G., and Meningaud, J. (2018). Using virtual reality to control preoperative anxiety in ambulatory surgery patients: a pilot study in maxillofacial and plastic surgery. J. Stomatol. Oral Maxillofac. Surg. 119, 257–261.
Garcia, A. C. M., Simão-Miranda, T. P., Carvalho, A. M. P., Elias, P. C. L., Pereira, M. D. G., and Carvalho, E. C. (2018). The effect of therapeutic listening on anxiety and fear among surgical patients: randomized controlled trial. Rev. Lat. Am. Enfermagem 26:e3027.
Garssen, B., Boomsma, M. F., and Beelen, R. H. (2010). Psychological factors in immunomodulation induced by cancer surgery: a review. Biol. Psychol. 85, 1–13. doi: 10.1016/j.biopsycho.2010.05.006
Gouin, J.-P., and Kiecolt-Glaser, J. K. (2011). The impact of psychological stress on wound healing: methods and mechanisms. Immunol. Allergy Clin. 31, 81–93. doi: 10.1016/j.iac.2010.09.010
Granziera, E., Guglieri, I., Del Bianco, P., Capovilla, E., Dona’, B., Ciccarese, A. A., et al. (2013). A multidisciplinary approach to improve preoperative understanding and reduce anxiety: a randomised study. Eur. J. Anaesthesiol. 30, 734–742.
Haase, O., Schwenk, W., Hermann, C., and Müller, J. (2005). Guided imagery and relaxation in conventional colorectal resections: a randomized, controlled, partially blinded trial. Dis. Colon Rectum 48, 1955–1963. doi: 10.1007/s10350-005-0114-9
Haldar, R., Ricon-Becker, I., Radin, A., Gutman, M., Cole, S. W., Zmora, O., et al. (2020). Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer 126, 3991–4001. doi: 10.1002/cncr.32950
Haldar, R., Shaashua, L., Lavon, H., Lyons, Y. A., Zmora, O., Sharon, E., et al. (2018). Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav. Immun. 73, 294–309. doi: 10.1016/j.bbi.2018.05.014
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674.
Hiller, J. G., Cole, S. W., Crone, E. M., Byrne, D. J., Shackleford, D. M., Pang, J.-M. B., et al. (2019). Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a Phase II randomized trial. Clin. Cancer Res. 26, 1803–1811. doi: 10.1158/1078-0432.CCR-19-2641
Hiller, J. G., Perry, N. J., Poulogiannis, G., Riedel, B., and Sloan, E. K. (2018). Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15:205. doi: 10.1038/nrclinonc.2017.194
Hizli, F., Ozcan, O., Selvi, I., Eraslan, P., Kosus, A., Bas, O., et al. (2015). The effects of hypnotherapy during transrectal ultrasound-guided prostate needle biopsy for pain and anxiety. Int. Urol. Nephrol. 47, 1773–1777. doi: 10.1007/s11255-015-1111-0
Hoon, L. S., Sally, C. W. C., and Hong-Gu, H. (2013). Effect of psychosocial interventions on outcomes of patients with colorectal cancer: a review of the literature. Eur. J. Oncol. Nurs. 17, 883–891. doi: 10.1016/j.ejon.2013.05.001
Horowitz, M., Neeman, E., Sharon, E., and Ben-Eliyahu, S. (2015). Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 12, 213–226. doi: 10.1038/nrclinonc.2014.224
Huber, J., Ihrig, A., Yass, M., Bruckner, T., Peters, T., Huber, C. G., et al. (2013). Multimedia support for improving preoperative patient education: a randomized controlled trial using the example of radical prostatectomy. Ann. Surg. Oncol. 20, 15–23.
Jacoby, R., Greenfeld Barsky, K., Porat, T., Harel, S., Hanalis Miller, T., and Goldzweig, G. (2021). Individual stress response patterns: preliminary findings and possible implications. PLoS One 16:e0255889. doi: 10.1371/journal.pone.0255889
Jeney, A., Lapis, K., Kopper, L., Pal, K., Timar, F., Institoris, L., et al. (2019). “Tumor progression and prostaglandins,”in Hemostasis and Cancer, 1nd Edn. <gnm>L.</gnm> <snm>Muszbek</snm> Boca Raton, FL: CRC Press. doi: 10.1007/s10555-007-9096-5
Kakinuma, A., Nagatani, H., Otake, H., Mizuno, J., and Nakata, Y. (2011). The effects of short interactive animation video information on preanesthetic anxiety, knowledge, and interview time: a randomized controlled trial. Anesth. Analg. 112, 1314–1318. doi: 10.1213/ANE.0b013e31820f8c18
Katz, M. R., Irish, J. C., and Devins, G. M. (2004). Development and pilot testing of a psychoeducational intervention for oral cancer patients. Psychooncology 13, 642–653.
Khan, J. S., Devereaux, P. J., LeManach, Y., and Busse, J. W. (2016). Patient coping and expectations about recovery predict the development of chronic post-surgical pain after traumatic tibial fracture repair. Br. J. Anaesth. 117, 365–370. doi: 10.1093/bja/aew225
Kim, N., Yang, J., Lee, K. S., and Shin, I. S. (2020). The effects of preoperative education for patients with cancer: a systematic review and meta-analysis. Cancer Nurs. 44, E715–E726. doi: 10.1097/NCC.0000000000000871
Knight, J. M., Rizzo, J. D., Hari, P., Pasquini, M. C., Giles, K. E., D’Souza, A., et al. (2020). Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 4, 467–476. doi: 10.1182/bloodadvances.2019000765
Kuchler, T., Bestmann, B., Rappat, S., Henne-Bruns, D., and Wood-Dauphinee, S. (2007). Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J. Clin. Oncol. 25, 2702–2708. doi: 10.1200/JCO.2006.08.2883
Larson, M. R., Duberstein, P. R., Talbot, N. L., Caldwell, C., and Moynihan, J. A. (2000). A presurgical psychosocial intervention for breast cancer patients. psychological distress and the immune response. J. Psychosom. Res. 48, 187–194. doi: 10.1016/s0022-3999(99)00110-5
Lemos, M. F., Lemos-Neto, S. V., Barrucand, L., Verçosa, N., and Tibirica, E. (2019). Preoperative education reduces preoperative anxiety in cancer patients undergoing surgery: usefulness of the self-reported Beck anxiety inventory. Rev. Bras. Anestesiol. 69, 1–6.
Lew, M. W., Kravits, K., Garberoglio, C., and Williams, A. C. (2011). Use of preoperative hypnosis to reduce postoperative pain and anesthesia-related side effects. Int. J. Clin. Exp. Hypn. 59, 406–423. doi: 10.1080/00207144.2011.594737
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 62, e1–e34.
Lilja, R. S., Rydén, S., and Fridlund, B. (1998). Effects of extended preoperative information on perioperative stress: an anaesthetic nurse intervention for patients with breast cancer and total hip replacement. Intensive Crit. Care Nurs. 14, 276–282. doi: 10.1016/s0964-3397(98)80688-5
Lutgendorf, S. K., and Andersen, B. L. (2015). Biobehavioral approaches to cancer progression and survival: mechanisms and interventions. Am. Psychol. 70, 186–197. doi: 10.1037/a0035730
Lutgendorf, S. K., Lang, E. V., Berbaum, K. S., Russell, D., Berbaum, M. L., Logan, H., et al. (2007). Effects of age on responsiveness to adjunct hypnotic analgesia during invasive medical procedures. Psychosom. Med. 69, 191–199. doi: 10.1097/PSY.0b013e31803133ea
Maggi, M., Gentilucci, A., Salciccia, S., Gatto, A., Gentile, V., Colarieti, A., et al. (2019). Psychological impact of different primary treatments for prostate cancer: a critical analysis. Andrologia 51:e13157. doi: 10.1111/and.13157
Maranets, I., and Kain, Z. N. (1999). Preoperative anxiety and intraoperative anesthetic requirements. Anesth. Analg. 89, 1346–1351. doi: 10.1213/00000539-199912000-00003
Marinelli, V., Danzi, O. P., Mazzi, M. A., Secchettin, E., Tuveri, M., Bonamini, D., et al. (2020). PREPARE: PreoPerative Anxiety REduction. One-year feasibility RCT on a brief psychological intervention for pancreatic cancer patients prior to major surgery. Front. Psychol. 11:362. doi: 10.3389/fpsyg.2020.00362
Matzner, P., Sandbank, E., Neeman, E., Zmora, O., Gottumukkala, V., and Ben-Eliyahu, S. (2020). Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 17, 313–326. doi: 10.1038/s41571-019-0319-9
Matzner, P., Sorski, L., Haldar, R., Shaashua, L., Benbenishty, A., Lavon, H., et al. (2019). Deleterious synergistic effects of distress and surgery on cancer metastasis: abolishment through an integrated perioperative immune-stimulating stress-inflammatory-reducing intervention. Brain Behav. Immun. 80, 170–178. doi: 10.1016/j.bbi.2019.03.005
McArdle, J. M., George, W. D., McArdle, C. S., Smith, D. C., Moodie, A. R., Hughson, A. V., et al. (1996). Psychological support for patients undergoing breast cancer surgery: a randomised study. BMJ 312, 813–816.
Mehlen, P., and Puisieux, A. (2006). Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458. doi: 10.1038/nrc1886
Miller, S. M. (1987). Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J. Pers. Soc. Psychol. 52, 345–353. doi: 10.1037//0022-3514.52.2.345
Miller, S. M. (1995). Monitoring versus blunting styles of coping with cancer influence the information patients want and need about their disease, implications for cancer screening and management. Cancer 76, 167–177. doi: 10.1002/1097-0142(19950715)76:2<167::aid-cncr2820760203>3.0.co;2-k
Miro, J., and Raich, R. M. (1999). Effects of a brief and economical intervention in preparing patients for surgery: does coping style matter? Pain 83, 471–475. doi: 10.1016/S0304-3959(99)00151-7
Montgomery, G. H., Bovbjerg, D. H., Schnur, J. B., David, D., Goldfarb, A., Weltz, C. R., et al. (2007). A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J. Natl. Cancer Inst. 99, 1304–1312. doi: 10.1093/jnci/djm106
Montgomery, G. H., David, D., Winkel, G., Silverstein, J. H., and Bovbjerg, D. H. (2002). The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth. Analg. 94, 1639–1645.
Mosher, C. E., Duhamel, K. N., Lam, J., Dickler, M., Li, Y., Massie, M. J., et al. (2012). Randomised trial of expressive writing for distressed metastatic breast cancer patients. Psychol. Health 27, 88–100. doi: 10.1080/08870446.2010.551212
Munn, Z., Peters, M. D., Stern, C., Tufanaru, C., McArthur, A., and Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18:143. doi: 10.1186/s12874-018-0611-x
National Comprehensive Cancer Network [NCCN] (2018). NCCN clinical practice guidelines: breast cancer, version 4.2017. Bristoln, CT: NCCNetwork.
Neeman, E., and Ben-Eliyahu, S. (2013). Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 30, S32–S40. doi: 10.1016/j.bbi.2012.03.006
O’Connor, G., Coates, V., and O’Neill, S. (2014). Randomised controlled trial of a tailored information pack for patients undergoing surgery and treatment for rectal cancer. Eur. J. Oncol. Nurs. 18, 183–191.
Oetker-Black, S. L., Jones, S., Estok, P., Ryan, M., Gale, N., and Parker, C. (2003). Preoperative teaching and hysterectomy outcomes. AORN J. 121, 1215–1231. doi: 10.1016/s0001-2092(06)60983-6
Park, E. R., Traeger, L., Willett, J., Gerade, B., Webster, A., Rastegar, S., et al. (2013). A relaxation response training for women undergoing breast biopsy: exploring integrated care. Breast 22, 799–805.
Parker, P. A., Pettaway, C. A., Babaian, R. J., Pisters, L. L., Miles, B., Fortier, A., et al. (2009). The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. J. Clin. Oncol. 27, 3169–3176. doi: 10.1200/JCO.2007.16.0036
Peters, M. D., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., and Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 13, 141–146.
Pillay, B., Moon, D., Meyer, D., Crowe, H., Mann, S., Howard, N., et al. (2020). Exploring the impact of providing men with information about potential prostate cancer treatment options prior to receiving biopsy results. Support. Care Cancer 28, 507–514.
Pinar, G., Kurt, A., and Gungor, T. (2011). The efficacy of preopoerative instruction in reducing anxiety following gyneoncological surgery: a case control study. World J. Surg. Oncol. 9:38. doi: 10.1186/1477-7819-9-38
Pritchard, M. J. (2009). Identifying and assessing anxiety in pre-operative patients. Nurs. Stand. 23, 35–40. doi: 10.7748/ns.23.51.35.s46
Prokop, A., Tubergen, D., and Pichlmaier, H. (1991). [Surgical therapy of colorectal cancer]. Der. Internist. 32, 335–347.
Rapkin, D. A., Straubing, M., and Holroyd, J. C. (1991). Guided imagery, hypnosis and recovery from head and neck cancer surgery: an exploratory study. Int. J. Clin. Exp. Hypn. 39, 215–226. doi: 10.1080/00207149108409637
Ricon, I., Hanalis-Miller, T., Haldar, R., Jacoby, R., and Ben-Eliyahu, S. (2019). Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling. Cancer 125, 45–56. doi: 10.1002/cncr.31594
Salciccia, S., Del Giudice, F., Eisenberg, M. L., Mastroianni, C. M., De Berardinis, E., Ricciuti, G. P., et al. (2021). Testosterone target therapy: focus on immune response, controversies and clinical implications in patients with COVID-19 infection. Therap. Adv. Endocrinol. Metab. 12:20420188211010105. doi: 10.1177/20420188211010105
Salciccia, S., Del Giudice, F., Eisenberg, M. L., Mastroianni, C. M., De Berardinis, E., Ricciuti, G. P., et al. (2020a). Androgen-deprivation therapy and SARS-Cov-2 infection: the potential double-face role of testosterone. Therap. Adv. Endocrinol. Metab. 11:2042018820969019. doi: 10.1177/2042018820969019
Salciccia, S., Del Giudice, F., Gentile, V., Mastroianni, C. M., Pasculli, P., Di Lascio, G., et al. (2020b). Interplay between male testosterone levels and the risk for subsequent invasive respiratory assistance among COVID-19 patients at hospital admission. Endocrine 70, 206–210. doi: 10.1007/s12020-020-02515-x
Schnur, J. B., Bovbjerg, D. H., David, D., Tatrow, K., Goldfarb, A. B., Silverstein, J. H., et al. (2008). Hypnosis decreases presurgical distress in excisional breast biopsy patients. Anesth. Analg. 106, 440–444. doi: 10.1213/ane.0b013e31815edb13
Sciarra, A., Gentilucci, A., Salciccia, S., Von Heland, M., Ricciuti, G. P., Marzio, V., et al. (2018). Psychological and functional effect of different primary treatments for prostate cancer: a comparative prospective analysis. Urol. Oncol. 62, 340.e7–340.e21. doi: 10.1016/j.urolonc.2018.03.022
Scott, A. (2004). Managing anxiety in ICU patients: the role of pre-operative information provision. Nurs. Crit. Care 9, 72–79. doi: 10.1111/j.1478-5153.2004.00053.x
Shaashua, L., Shabat-Simon, M., Haldar, R., Matzner, P., Zmora, O., Shabtai, M., et al. (2017). Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 23, 4651–4661. doi: 10.1158/1078-0432.CCR-17-0152
Shelley, M., and Pakenham, K. (2007). The effects of preoperative preparation on postoperative outcomes: the moderating role of control appraisals. Health Psychol. 26, 183–191. doi: 10.1037/0278-6133.26.2.183
Slavich, G. M. (2020). “Psychoneuroimmunology of stress and mental health,” in The Oxford Handbook of Stress and Mental Health, eds K. Harkness and E. P. Hayden (New York, NY: Oxford University Press), 519–546.
Sood, A. K., Lutgendorf, S. K., and Cole, S. W. (2007). “Neuroendocrine regulation of cancer progression: biological mechanisms and clinical relevance,” in Psychoneuroimmunology, Vol. IV, eds R. Ader, N. Cohen, and D. Felten (San Diego, CA: Elsevier), 233–250.
Spiegel, D., Butler, L. D., Giese-Davis, J., Koopman, C., Miller, E., DiMiceli, S., et al. (2007). Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer 110, 1130–1138.
Sun, V., Kim, J. Y., Raz, D. J., Chang, W., Erhunmwunsee, L., Uranga, C., et al. (2018). Preparing cancer patients and family caregivers for lung surgery: development of a multimedia self-management intervention. J. Cancer Educ. 33, 557–563.
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. An. Intern. Med. 169, 467–473.
Tsimopoulou, I., Pasquali, S., Howard, R., Desai, A., Gourevitch, D., Tolosa, I., et al. (2015). Psychological prehabilitation before cancer surgery: a systematic review. Ann. Surg. Oncol. 22, 4117–4123. doi: 10.1245/s10434-015-4550-z
Waller, A., Forshaw, K., Bryant, J., Carey, M., Boyes, A., and Sanson-Fisher, R. (2015). Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ. Couns. 98, 1540–1549. doi: 10.1016/j.pec.2015.05.008
Williams, P., Valderrama, D. M., Gloria, M. D., Pascoguin, L. G., Saavedra, L. D., De la Rama, D. T., et al. (1988). Effects of preparation for mastectomy/hysterectomy on women’s post-operative self-care behaviors. Int. J. Nurs. Stud. 25, 191–206. doi: 10.1016/0020-7489(88)90046-6
Wysocki, W., Mitus, J., Komorowski, A., and Karolewski, K. (2012). Impact of preoperative information on anxiety and disease-related knowledge in women undergoing mastectomy for breast cancer: a randomized clinical trial. Acta Chir. Belg. 112, 111–115.
Zgâia, A., Pop, F., Achimaş-Cadariu, P., Vlad, C., Rogobete, A., Lisencu, C., et al. (2016). The impact of relaxation technique and pre-operative psychological counselling on pain, analgesic consumption and psychological symptoms on patients scheduled for breast cancer surgery-a randomized clinical study. J. Evid. Based Psychother. 16, 205–220.
Keywords: stress, surgery, pre-operative, psychological intervention, cancer, oncology
Citation: Hanalis-Miller T, Nudelman G, Ben-Eliyahu S and Jacoby R (2022) The Effect of Pre-operative Psychological Interventions on Psychological, Physiological, and Immunological Indices in Oncology Patients: A Scoping Review. Front. Psychol. 13:839065. doi: 10.3389/fpsyg.2022.839065
Received: 19 December 2021; Accepted: 22 February 2022;
Published: 14 April 2022.
Edited by:
Francesco Del Giudice, Sapienza University of Rome, ItalyReviewed by:
Matteo Ferro, European Institute of Oncology (IEO), ItalyEttore De Berardinis, Sapienza University of Rome, Italy
Copyright © 2022 Hanalis-Miller, Nudelman, Ben-Eliyahu and Jacoby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsipi Hanalis-Miller, VHNpcGkyNUBob3RtYWlsLmNvbQ==
 Tsipi Hanalis-Miller
Tsipi Hanalis-Miller Gabriel Nudelman
Gabriel Nudelman Shamgar Ben-Eliyahu
Shamgar Ben-Eliyahu Rebecca Jacoby
Rebecca Jacoby