- 1Department of Psychological Sciences, Rice University, Houston, TX, United States
- 2Department of Psychiatry and Behavioral Sciences, McGovern Medical School at UTHealth, Houston, TX, United States
- 3Department of Psychological Science, University of California, Irvine, Irvine, CA, United States
- 4Department of Behavioral Sciences, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States
The negative emotions generated following stressful life events can increase one’s risk of depressive symptoms and promote higher levels of perceived stress. The process model of emotion regulation can help distinguish between adaptive and maladaptive emotion regulation strategies to determine who may be at the greatest risk of worse psychological health across the lifespan. Heart rate variability (HRV) may affect these relationships as it indexes aspects of self-regulation, including emotion and behavioral regulation, that enable an individual to dynamically adapt to the changing demands of both internal and external environments. In this study, we expected individual differences in resting vagally mediated HRV to moderate the influence of emotion regulatory strategies among our sample of 267 adults. We found support for the hypothesis that higher vagally mediated HRV buffers against the typical adverse effects of expressive suppression when evaluating depressive symptoms and found weak support when considering perceived stress. There was no evidence for an interaction between cognitive reappraisal and vagally mediated HRV but there was a significant, negative association between cognitive reappraisal and depressive symptoms and perceived stress. Future work may determine if intervening on either emotion regulation strategies or HRV may change these within-persons over time.
Introduction
Stressful life events and their accompanying negative emotions can increase the risk of depressive symptoms, major depressive disorder, and higher levels of perceived stress. Interpersonal stresses, or stress from major interpersonal losses such as death and divorce, are robust predictors of major depressive disorder (Slavich and Irwin, 2014). Even in the context of other interpersonal stresses, spousal bereavement is often considered one of the most stressful possible life events, as widowed spouses have significantly elevated depressive symptoms and perceived stress (Holmes and Rahe, 1967; Stroebe et al., 2007; Fagundes and Wu, 2020). During the first year of bereavement, about half of all widowed spouses will meet the criteria for major depressive disorder (Clayton, 1990; Zisook and Shuchter, 1991; Stroebe et al., 2007). Typically, older adults experience major depressive disorder at lower rates than younger adults, with 15% of community-dwelling older adults meeting the criteria for clinically significant depressive symptoms but not major depressive disorder (Blazer, 2003; Fiske et al., 2009). Compared to younger adults, older adults are better at emotional regulation and less reactive to stress, two important protective factors for depression (Garnefski and Kraaij, 2006; Neupert et al., 2007; Fiske et al., 2009).
Emotion regulation skills play an important role in the relationship between stressful life events and mental health outcomes, such as depression. Using the process model of emotion regulation to study the effects of cognitive reappraisal and expressive suppression can help distinguish between adaptive and maladaptive emotion regulation strategies when facing a stressful life event (Gross, 1998; Gross and John, 2003). Specifically, cognitive reappraisal occurs when an individual alters the meaning of the situation by changing how one thinks about the situation, whereas expressive suppression involves directly changing the experiential, behavioral, or physiological components of an emotional response after response tendencies have begun, such as a facial expression (Gross, 1998). Cognitive reappraisal typically presents more adaptive responses than expressive suppression, but the effects can vary by context (Gross, 1998; Gross and John, 2003). Broadly, previous work indicates that cognitive reappraisal is associated with decreased depressive symptoms and can buffer the relationship between stress and depressive symptoms (Troy et al., 2010; Andreotti et al., 2013), while expressive suppression is associated with increased depressive symptoms, less positive emotion, more negative emotion, feelings of inauthenticity, and sympathetic nervous system responses (Gross and Levenson, 1993; Harris, 2001; Gross and John, 2003; Demaree et al., 2006; Moore et al., 2008; Nezlek and Kuppens, 2008). The discrepancy between these strategies can be traced to differences in the timing of implementation and cognitive effort. Cognitive reappraisal is an antecedent-focused strategy and is implemented before an experienced emotion has initiated changes in behavior and peripheral physiological responses, while expressive suppression, a response-focused strategy, is only implemented after these changes are already underway (Gross and John, 2003). Expressive suppression may also be more cognitively taxing than cognitive reappraisal (Richards and Gross, 2000), which affects an individual’s ability to perform other essential executive functions related to psychological and physiological outcomes during stress, such as increased levels of rumination and experiential avoidance (Moore et al., 2008).
Individual differences in vagally mediated heart rate variability (HRV), a marker of parasympathetic nervous system functioning measured by an individual’s beat-to-beat variability in heart rate, may help explain who has lower levels of stress and depressive symptoms (Thayer and Lane, 2000; Thayer et al., 2009; Fagundes et al., 2018). Importantly, HRV can serve as an index of an individual’s self-regulatory abilities and physiological responses to stressful life events (Porges et al., 1994; Bridgett et al., 2013; Carnevali et al., 2018). Differences in HRV can be used to understand risk and resilience patterns that can impact how individuals adapt to stress (Fagundes et al., 2012; Sbarra and Borelli, 2013). Dynamically adapting to changing environmental demands and stressors is a core component of resilience processes (Kalisch et al., 2017). Those with high HRV may respond more flexibly under stress, while individuals with low HRV may experience adverse outcomes when coping and adapting to stress (Thayer et al., 2021). For example, when discussing an ongoing conflict with one’s partner, men generally experienced more negative affect the more they were suppressing their emotional expression; however, for men with higher vagally mediated HRV, there was no association between suppression and negative affect (Geisler and Schröder-Abé, 2015).
Based on these findings, we expected individual differences in resting vagally mediated HRV to moderate the influence of emotion regulatory strategies in our sample. We hypothesized that higher reported frequencies of expressive suppression would be associated with elevated depressive symptoms (Hypothesis 1a) and perceived stress (Hypothesis 2a) and that these associations with depressive symptoms and perceived stress would be buffered by high vagally mediated HRV (Hypothesis 1b and Hypothesis 2b, respectively). That is, we expected better self-regulation (i.e., high vagally mediated HRV) to be a resilience factor. Conversely, we hypothesized that higher reported frequencies of cognitive reappraisal would be associated with lower depressive symptoms (Hypothesis 3a) and perceived stress (Hypothesis 4a) and that those who reported more frequently using cognitive reappraisal and had high vagally mediated HRV would have the lowest levels of depressive symptoms (Hypothesis 3b) and perceived stress (Hypothesis 4b).
Materials and Methods
Participants
As part of a larger longitudinal-observational study investigating the biological mechanisms underlying greater cardiovascular disease risk during bereavement, our sample includes 267 participants measured across three study visits. The 267 total participants included 167 spousally bereaved participants and 100 control participants (M = 68 years; 68% women; see Table 1 for all sample characteristics). Bereaved participants met inclusion criteria if they had experienced the death of their spouse within 3 months of their first visit, if they had been married to their spouse for at least 3 years, and if they were able to read and write in English. Control participants were age-, sex-, and education-matched to their bereaved counterparts as part of the larger study. Control participants were not excluded based on their relationship status, but they must not have experienced the death of their spouse within the last 5 years. All participants were able to read and write in English. Participants were not included in the study if they had visual or auditory impairments that interfered with their ability to read or hear questionnaires, were pregnant or nursing, had an autoimmune disease, had been divorced in the last year, had experienced the death of a loved one in the last year (excluding spouse for those in the bereaved group), or if they were undergoing cancer treatment. The first visit was approximately 3 months after the death of the bereaved participants’ spouses, the second visit was approximately 4 months after the death of their spouse, and the third visit was approximately 6 months after the death of the spouse; the control participants’ visits were identically spaced.
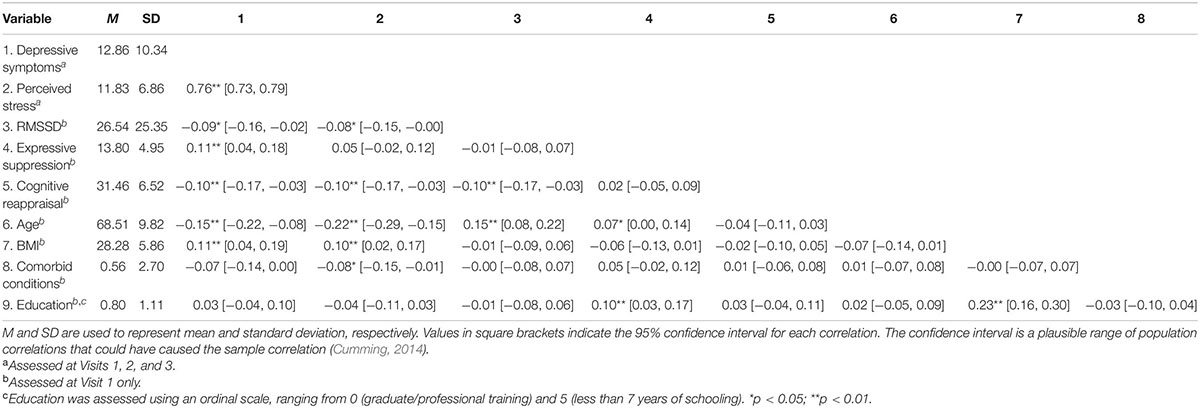
Table 1. Means, standard deviations, and correlations with confidence intervals for key study variables.
Measures
Depressive Symptoms
Participants completed the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) at each visit. The CES-D is a widely utilized measure of depressive symptoms that asks participants to describe the way they have felt in the prior week (e.g., “I felt sad”). Higher scores on this scale indicate greater depressive symptomatology, although the scale can also be used with a cut-score to indicate a clinically significant level of depressive symptoms (Radloff, 1977). Depressive symptoms were measured as a continuous variable by reverse-coding as needed and summing the items, which resulted in good reliability (αV1 = 0.91, αV2 = 0.91, and αV3 = 0.91).
Perceived Stress
Participants completed the 10-item Perceived Stress Scale (PSS; Cohen et al., 1983) at each visit. The PSS asks participants to describe how often they have felt stressed in the last month on a scale of 0 (never) to 4 (very often). An example item reads, “How often have you been upset because of something that happened unexpectedly?” The items were reverse-coded as needed and summed; the scale resulted in good reliability (αV1 = 0.90, αV2 = 0.89, and αV3 = 0.90).
Emotion Regulation
At the baseline visit, participants completed the 10-item Emotion Regulation Questionnaire (ERQ; Gross and John, 2003). The ERQ assesses the frequency of participants’ emotional regulation via reappraisal and expressive suppression on a 7-point Likert scale of 1 (Strongly disagree) to 7 (Strongly agree). The ERQ yields two separate summed scores for cognitive reappraisal (e.g., “I control my emotions by changing the way I think about the situation I’m in”) and expressive suppression (e.g., “I keep my emotions to myself”). Scale items were reverse coded as needed and summed; the resulting cognitive reappraisal scale had good reliability (αV1 = 0.82) and expressive suppression scale had fair reliability (αV1 = 0.69).
Heart Rate Variability
Heart rate variability was measured for 5 continuous minutes at the baseline visit with the Polar s810 wristwatch and the Polar H10 heart rate sensor. The 1,000 Hz sampling rate provides valid and reliable ECG data non-invasively (Müller et al., 2019). All procedures followed the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology (Malik et al., 1996). To prepare HRV data for analysis, we used the KUBIOS HRV Premium analysis software to process the raw interbeat intervals for artifact removal (Tarvainen et al., 2009). Following artifact correction in KUBIOS, each segment was hand-scored to ensure no ectopic beats or other artifacts were present. KUBIOS provides values for vagally mediated (parasympathetic) HRV using the square root of mean successive differences (RMSSD); higher values indicate higher vagally mediated HRV. RMSSD is determined by calculating the differences between consecutive interbeat (RR) intervals before squaring, adding, and averaging the values. Finally, the square root of the values are used as the RMSSD (Stein et al., 1994; Malik, 1996). RMSSD is highly correlated with spectral derived measures of HRV, but is preferred to spectral indices as it is less affected by respiration and other artifacts than spectral indices of HRV (Penttila et al., 2001).
Comorbid Conditions
At the baseline visit, participants completed the 19-item Charlson Comorbidity Index (CCI; Charlson et al., 1994). The CCI is the most common measure of comorbidity. The 19 items are assigned a weight based on their likelihood of influencing 1-year mortality. The CCI was used as a covariate in all adjusted analyses.
Other Covariates
Demographic factors, including bereavement status, age, gender, education, and body mass index (BMI) were included as covariates in all adjusted models. All covariates were assessed at the first visit time point. Bereavement status was dummy coded as 0 (control) or 1 (bereaved). Age and gender were both captured via self-report. Education was assessed using an ordinal scale, ranging from 0 (graduate/professional training) and 5 (less than 7 years of schooling). Finally, BMI was computed from participant height and weight as measured during their first visit in units of kg/m2.
Analytic Method
Preliminary statistical analysis included assessment of normality of distributions and examination for skewness and kurtosis. To satisfy the assumption of normality of residuals we used a square root transformation for depressive symptoms for all analyses involving depressive symptoms. Generalized linear mixed modeling (GLMM), a multilevel regression analytic technique, was used to fit the outcome variables (i.e., depressive symptoms and perceived stress) as a function of the interaction between log RMSSD and emotion regulation strategies (i.e., expressive suppression and cognitive reappraisal), controlling for constituent main effects. Only depressive symptoms and perceived stress were time-varying. The person was the upper level and time was the lower level in all multilevel analyses. We fit each model as an adjusted model with the same set of fixed covariates (months since baseline visit, bereavement status, age, gender, comorbidities, BMI, and education). When we test one component of emotion regulation, we also include the other component of emotion regulation as a covariate (e.g., cognitive reappraisal is a covariate in models focusing on the interaction between expressive suppression and RMSSD). Age, comorbidities, log RMSSD, months since baseline visit, frequency of expressive suppression, and frequency of cognitive reappraisal were centered for analyses. We used a median imputation for missing data on BMI (1.2% of the variable’s total values) and education (0.8% of the variable’s total values).
For each outcome (depressive symptoms; perceived stress), a model comparison approach was used to determine the functional form of change over time (i.e., testing linear, quadratic, and higher-order polynomial effects) and the optimal random effects structure (i.e., evaluating the need to include random intercept and/or slope terms). The Akaike information criterion (AIC) was used to determine the best-fitting model for each outcome. We chose the model based on a significance test between these models and after identifying the lowest AIC value, which indicates the best, most parsimonious fit with the least information lost relative to other models (Bozdogan, 1987; Vrieze, 2012). In each case, the best-fitting model included a random intercept. Further, models for each primary outcome variable demonstrated non-linear change over time, and model comparison indicated that including a linear and a quadratic effect best captured the functional form of these changes. Models including only a linear effect or including higher-order effects (e.g., cubic) effects demonstrated inferior fit.
Apart from reliability analyses conducted in SPSS (IBM Corp, 2016), all analyses were conducted in the R statistical computing environment (R Core Team, 2019). Multilevel analyses used the package nlme (Pinheiro et al., 2020), used ggplot2 (Wickham, 2016) and ggeffects (Lüdecke et al., 2021) for visualization, and apaTables to generate tables (Stanley, 2018).
Results
Descriptive statistics and correlations between key variables can be found in Table 1.
Emotion Regulation, Heart Rate Variability, and Depressive Symptoms
Bereavement Status and Depressive Symptoms
Unsurprisingly, participants in the study who were bereaved (vs. control) reported higher levels of depressive symptoms across the study, b = 1.20, 95% CI [0.88, 1.52], p < 0.001. Although our bereaved participants understandably reported higher depressive symptoms than our control participants, on average, depressive symptoms within both the control group (M = 8.21, SD = 7.77) and the bereaved group (M = 15.60, SD = 10.70) remained below the cut-score (16 or greater; Radloff, 1977) that indicates risk for clinical depression across the 6-month study period. Accordingly, neither the bereaved nor control group would be classified as at risk for clinical depression.
There was neither a reliable interaction between bereavement status and expressive suppression associated with depressive symptoms, b = −0.01, 95% CI [−0.07, 0.05], p = 0.73, nor bereavement status and cognitive reappraisal associated with depressive symptoms, b = −0.00, 95% CI [−0.05, 0.04], p = 0.33, nor bereavement status and log RMSSD associated with depressive symptoms, b = −0.17, 95% CI [−0.59, 0.25], p = 0.44.
Emotion Regulation and Depressive Symptoms
We found support for Hypothesis 1a as there was a reliable, positive association between expressive suppression and depressive symptoms in adjusted models, b = 0.05, 95% CI [0.01, 0.75], p = 0.005, such that those who reported a higher frequency of expressive suppression had elevated depressive symptoms throughout the study compared to those who reported lower frequency of expressive suppression. In these models, we also found support for Hypothesis 3a as there was a reliable, negative association between cognitive reappraisal and depressive symptoms, b = −0.03, 95% CI [−0.05, −0.003], p = 0.031, such that those who reported a greater frequency of cognitive reappraisal had lower depressive symptoms throughout the study.
We analyzed the potential interaction between log RMSSD and frequency of expressive suppression related to depressive symptoms. Here, there was evidence of an interaction between log RMSSD and frequency of expressive suppression associated with depressive symptoms, b = −0.04, 95% CI [−0.08, −0.00], p = 0.042 (see Figure 1; and Table 2 for full model results). Next, we examined simple slope tests and determined that individuals with low HRV (i.e., −1 SD) had a positive relationship between expressive suppression and depressive symptoms (b = 0.08, p < 0.001), but individuals with high HRV (i.e., +1 SD) did not have a significant relationship between expressive suppression and depressive symptoms (b = 0.02, p = 0.48). These findings support Hypothesis 1b and indicate that higher HRV may reduce or eliminate the association between expressive suppression and depressive symptoms.
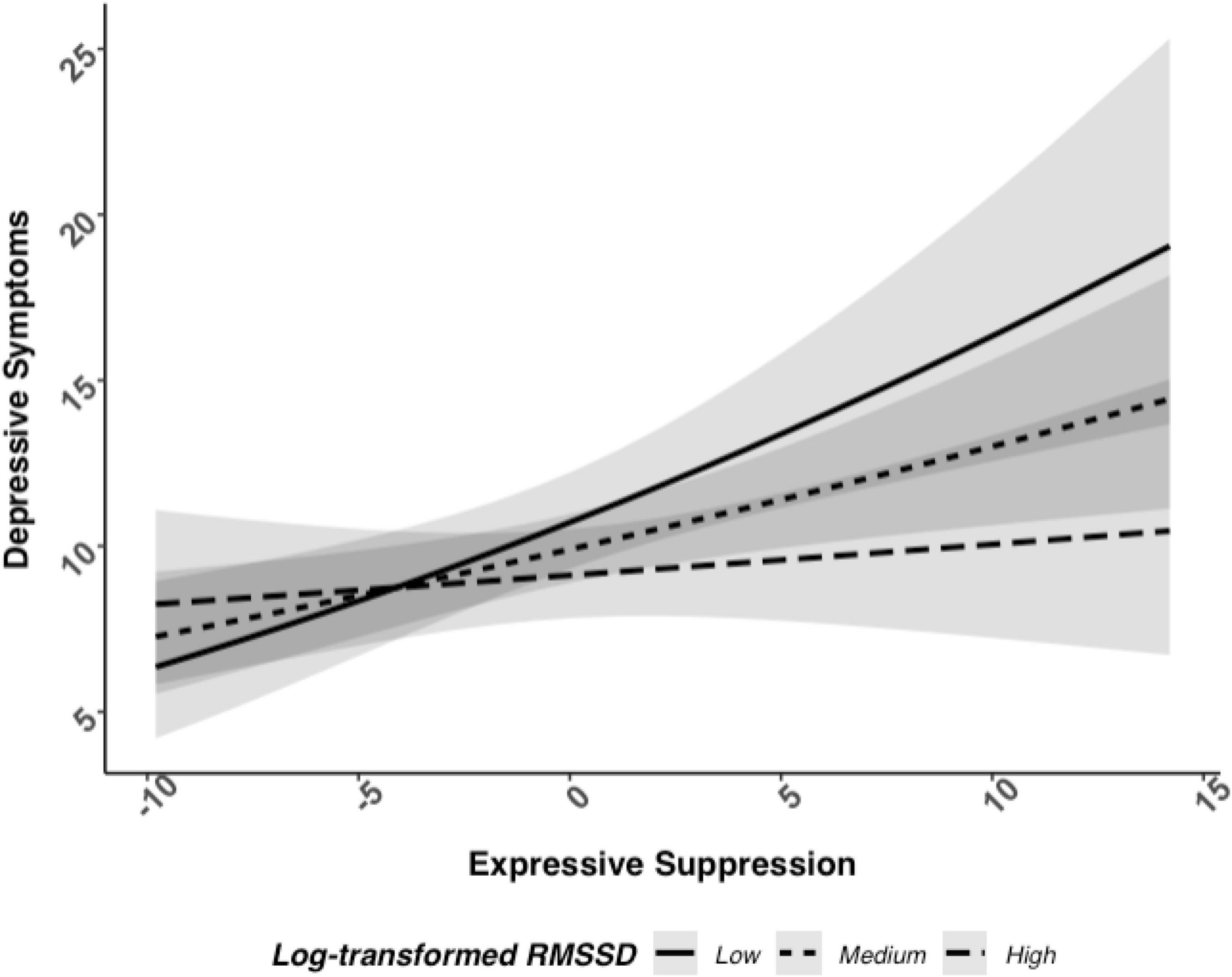
Figure 1. Relationship between log RMSSD, expressive suppression, and depressive symptoms when adjusted with covariates.
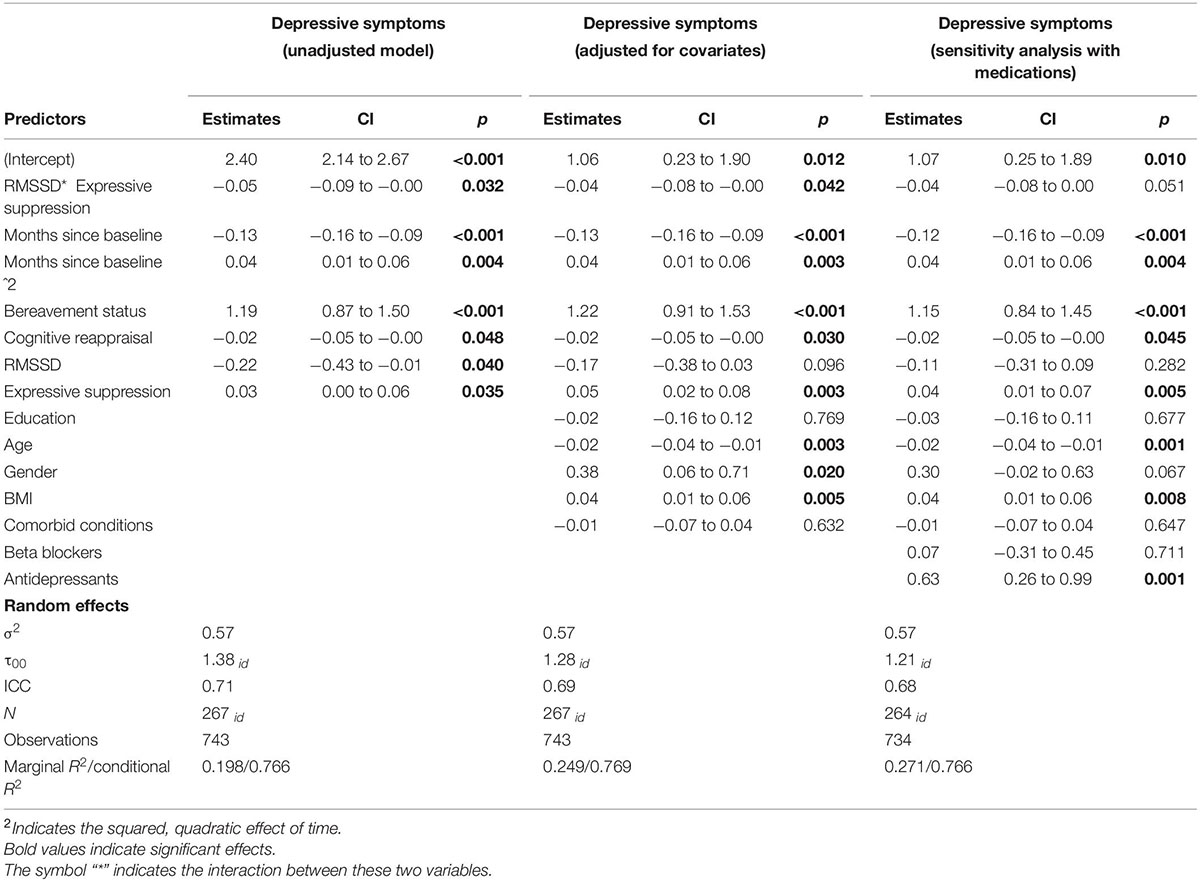
Table 2. Frequency of expressive suppression and RMSSD associated with depressive symptoms over the course of 6 months.
In a sensitivity analysis to test the robustness of this effect among the participants (n = 264) who provided information on their medication use, the interaction between log RMSSD and frequency of expressive suppression associated with depressive symptoms was essentially the same after controlling for antidepressant and beta-blocker use (b = −0.04, 95% CI [−0.08, 0.00], p = 0.051).
There was no evidence for an interaction between cognitive reappraisal and log RMSSD associated with depressive symptoms, b = −0.00, 95% CI [−0.03, 0.03], p = 0.90. Thus, Hypothesis 3b was not supported.
Emotion Regulation, Heart Rate Variability, and Perceived Stress
Bereavement Status and Perceived Stress
Participants in the study who were bereaved (vs. control) reported higher levels of perceived stress across the study, b = 2.33, 95% CI [0.76, 3.90], p = 0.004. There was neither a reliable interaction between bereavement status and expressive suppression associated with perceived stress, b = 0.12, 95% CI [−0.18, 0.41], p = 0.44, nor bereavement status and cognitive reappraisal associated with perceived stress, b = 0.02, 95% CI [−0.20, 0.24], p = 0.88, nor bereavement status and log RMSSD associated with perceived stress, b = −1.08, 95% CI [−3.08, 0.91], p = 0.30.
Emotion Regulation and Perceived Stress
There was support for Hypothesis 2a based on an association between expressive suppression and perceived stress, b = 0.17, 95% CI [0.29, 0.32], p = 0.021; those who reported a greater frequency of expressive suppression had higher perceived stress throughout the study. There was also support for Hypothesis 4a in these models as we identified an association between cognitive reappraisal and perceived stress, b = −0.12, 95% CI [−0.23, −0.02], p = 0.027; those who reported a greater frequency of cognitive reappraisal had lower perceived stress throughout the study.
Next, we analyzed perceived stress to determine if there was an interaction between log RMSSD and frequency of expressive suppression. There was only weak evidence of an interaction between log RMSSD and frequency of expressive suppression associated with perceived stress, which did not reach statistical significance in adjusted models, b = −0.18, 95% CI [−0.38, 0.01], p = 0.069 (see Figure 2; and Table 3 for full model results). The level of statistical significance did not change when controlling for antidepressant and beta-blocker use. Simple slope tests followed the same pattern as depressive symptoms: individuals with low HRV (i.e., −1 SD) had a positive relationship between expressive suppression and perceived stress (b = 0.31, p = 0.004), while individuals with high HRV (i.e., +1 SD) did not have a significant relationship between expressive suppression and perceived stress (b = 0.05, p = 0.64). Thus, Hypothesis 2b was only partially supported.
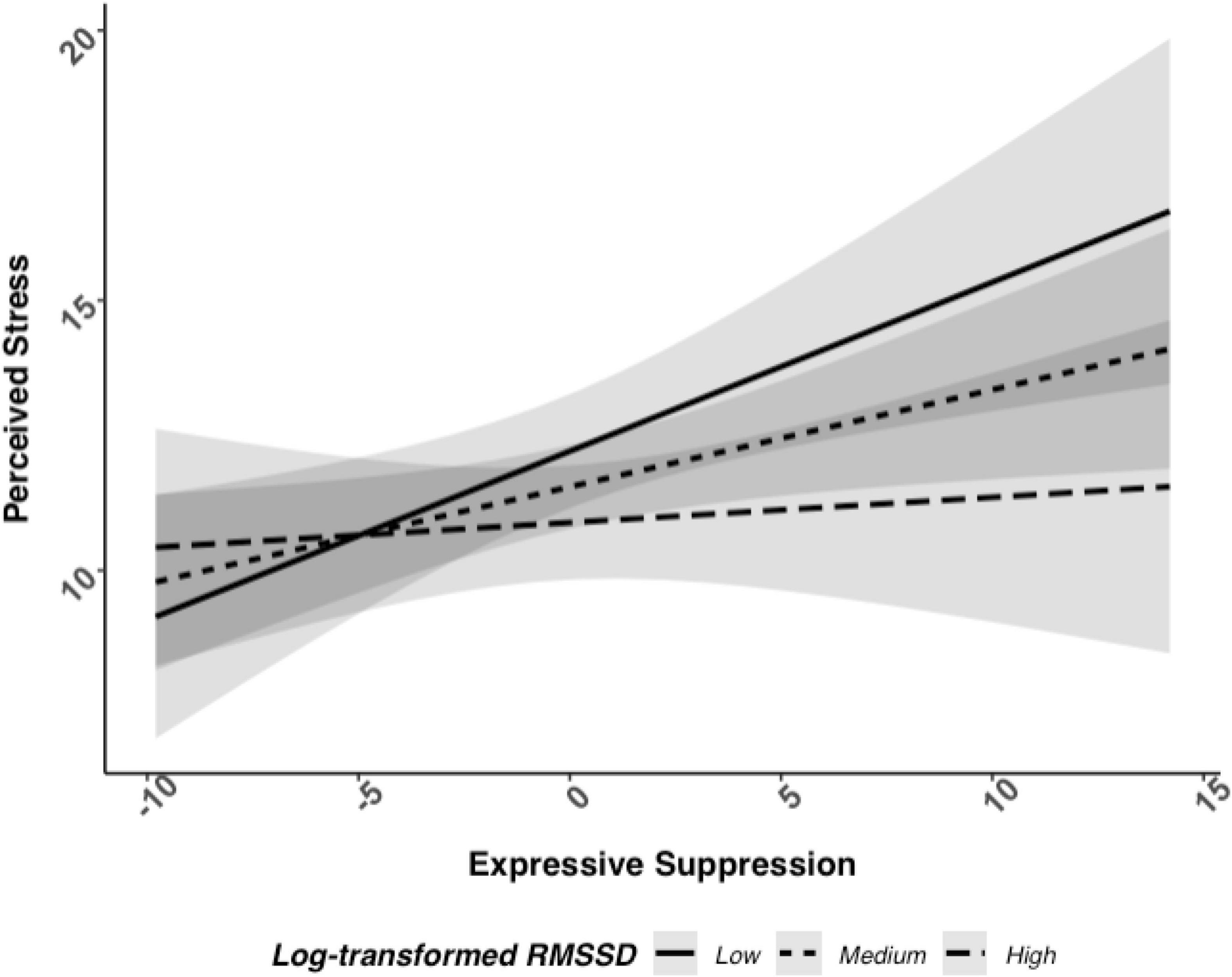
Figure 2. Relationship between log RMSSD, expressive suppression, and perceived stress when adjusted with covariates.
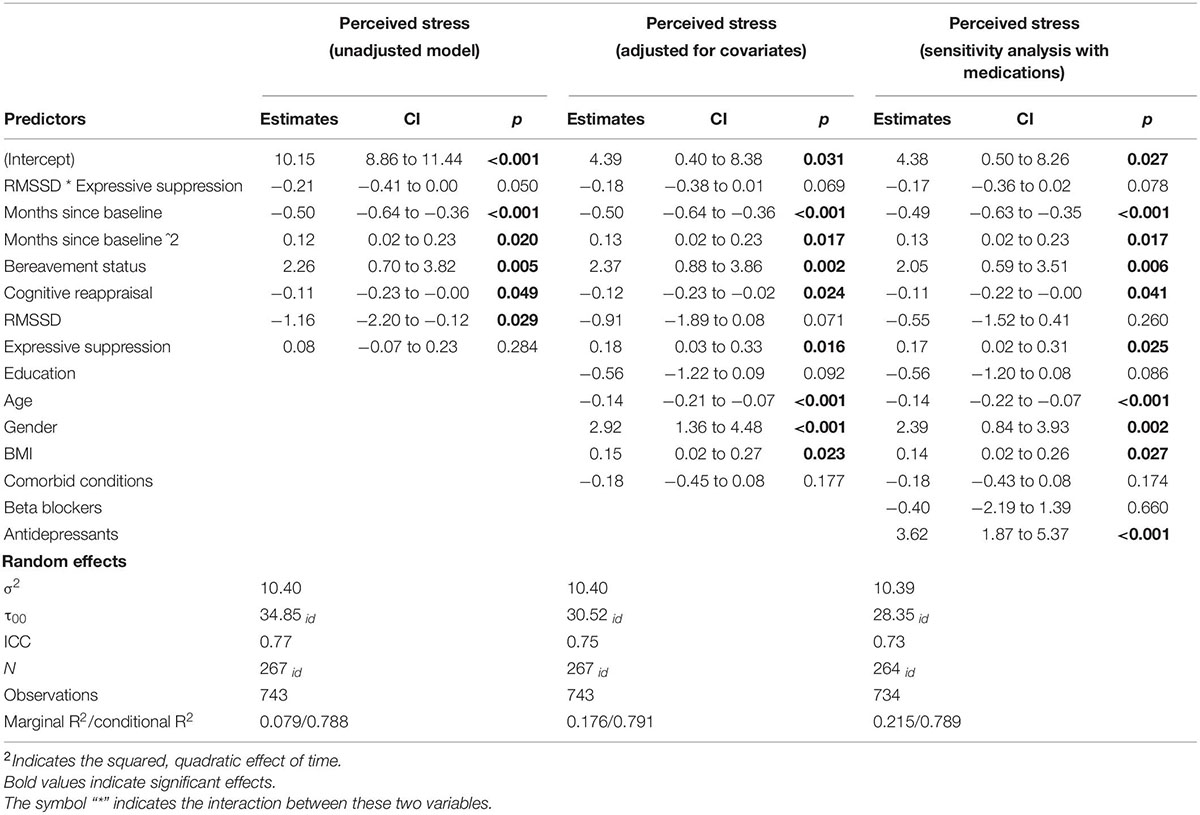
Table 3. Frequency of expressive suppression and RMSSD associated with perceived stress over the course of 6 months.
Next, we determined that there was not a significant interaction between cognitive reappraisal and log RMSSD associated with perceived stress, b = −0.11, 95% CI [−0.26, 0.03], p = 0.14. Thus, Hypothesis 4b was not supported.
Ancillary Analyses
Because this sample is part of a larger study identifying what puts bereaved spouses at greater risk for cardiovascular disease, we also examined whether any of these effects differed based on the bereavement status of the participant in ancillary analyses. Specifically, we examined whether the key interactions of interest may differ based on whether participants were bereaved or not. If there were differences, this could reflect a sensitive period for widow(er)s if higher vagally mediated HRV was not protective, but we made no specific hypotheses regarding group differences. We explored whether there may be a three-way interaction associated with depressive symptoms between bereavement status, emotion regulation strategies, and log RMSSD in ancillary analyses.
There was neither evidence for the interaction between bereavement status, expressive suppression, and log RMSSD, b = −0.35, 95% CI [−0.92, 0.22], p = 0.25, nor for the interaction between bereavement status, cognitive reappraisal, and log RMSSD, b = −0.35, 95% CI [−0.79, 0.10], p = 0.13, associated with depressive symptoms.
We also examined whether there may be a three-way interaction associated with perceived stress between bereavement status, emotion regulation strategies, and log RMSSD in ancillary analyses. Again, there was neither evidence for the interaction between bereavement status, expressive suppression, and log RMSSD, b = −0.15, 95% CI [−0.56, 0.25], p = 0.47, nor for the interaction between bereavement status, cognitive reappraisal, and log RMSSD, b = −0.06, 95% CI [−0.37, 0.25], p = 0.69, associated with perceived stress.
Discussion
Participants who reported using expressive suppression with greater frequency and had higher vagally mediated HRV at rest had lower depressive symptoms severity than participants who also used expressive suppression with greater frequency but had lower vagally mediated HRV. There was also weak evidence for this association between expressive suppression and vagally mediated HRV associated with perceived stress in a similar pattern as predicted depressive symptoms, but this was not statistically significant. These effects did not vary based on whether participants were bereaved or controls, suggesting that higher vagally mediated HRV may buffer against the harmful psychological effects of expressive suppression for adults generally and following a highly stressful life event like spousal bereavement. Broadly, participants who reported using cognitive reappraisal with greater frequency reported lower depressive symptoms and perceived stress levels. However, there was no evidence for an interaction between cognitive reappraisal and vagally mediated HRV associated with depressive symptoms or perceived stress.
Generally, we found support for the notion that higher vagally mediated HRV is a resilience factor that protects against the typical adverse effects of expressive suppression. In our sample, vagally mediated HRV was protective when examining depressive symptoms and followed a similar pattern, though the interaction overall was not significant, for perceived stress. These results are consistent with findings from other research groups that higher vagally mediated HRV reduces or eliminates effects of expressive suppression on negative affect during conflict (Geisler and Schröder-Abé, 2015), enables people to spontaneously suppress negative facial expressions in an adaptive and socially appropriate manner when exposed to a negative film clip (Pu et al., 2010), and protects against depressive symptoms for those with emotion regulation difficulties (Fantini-Hauwel et al., 2020). Although meta-analytic results indicate only a small correlation between self-regulation and HRV (Holzman and Bridgett, 2017), these results may be interpreted as an interplay between this bio-marker of one’s self-regulatory ability and the frequency participants report using expressive suppression. It will be important to determine if these relationships can be modified within-persons; future work may examine whether interventions seeking to increase vagally mediated HRV also successfully reduce the impact of expressive suppression on psychological well-being.
As our sample primarily consists of older adults, our findings indicate the relationship between emotion regulation strategies and psychological well-being in older adulthood. Positive emotional experiences are more important to older adults than younger adults (Carstensen et al., 2003) and older adults may be more likely to engage in emotion regulation strategies other than cognitive reappraisal and expressive suppression (e.g., situation selection; Urry and Gross, 2010). Despite the potentially low base rate of older adults using cognitive reappraisal and expressive suppression, we found that cognitive reappraisal is inversely related to perceived stress and depressive symptoms. This finding aligns with other work showing that positive reappraisal is negatively associated with depressive symptoms and may be especially effective for older adults (Garnefski and Kraaij, 2006; Shiota and Levenson, 2009). Older adults and younger adults are equally able to implement expressive suppression (Kunzmann et al., 2005; Shiota and Levenson, 2009), although this strategy was directly related to increased perceived stress and depressive symptoms in our results. In addition, we found that expressive suppression and HRV interacted such that people with low HRV experienced higher depressive symptoms when they employed more expressive suppression, while people with high HRV had no such experience. Broadly, these findings are situated in the context of our sample as older adults are less likely to utilize expression suppression than younger adults (John and Gross, 2004; Kunzmann et al., 2005; Phillips et al., 2008).
Limitations and Future Directions
Despite the strengths of this study, there are some limitations that could be avenues for future research. The present findings are correlational; hence, causality cannot be established by the current data. Identifying which variable (expressive suppression or HRV) is truly the moderator of this relationship is another limitation of this study – more fine-grained longitudinal analyses of changes in expressive suppression frequency and/or HRV over time would benefit the development of interventions. Although we consider HRV to be an important resilience factor, we used a single baseline measurement of HRV as an indication of how flexibly one can respond to the changing demands of their environment, which is limiting because it does not capture the process of adapting over time in response to a specific stressor (Kalisch et al., 2017). We also did not assess HPA axis activity or fronto-amygdala circuitry that may be involved in these relationships. Because expressive suppression and cognitive reappraisal are associated with different neural networks and differing levels of amygdala attenuation (Vanderhasselt et al., 2013; Dörfel et al., 2014; Lopez et al., 2018), future work may examine how variation in amygdala-frontal connectivity during emotion regulation impacts changes in daily cortisol levels (Urry et al., 2006; Hakamata et al., 2017). Assessing these parameters would provide a more holistic view of the relative influence of each of these systems on depressive symptoms and perceived stress.
Additionally, the operationalization of emotion regulation has some limitations. The ERQ currently measures self-reported cognitive reappraisal and expressive suppression frequency, not actual efficacy in implementation. This study assumes that self-reported cognitive reappraisal frequency is a proxy for emotion regulation ability. This assumption can be made because those who implement cognitive reappraisal more frequently have shown to be better at cognitive reappraisal since they are naturally incorporating it more into their daily lives (Gross and John, 2003). Because emotion regulation tactics can be implemented automatically and unconsciously, there are limitations in assessing frequency in a self-report measure. Future experimental studies will help address these limitations by providing information on emotion regulation ability and strategy frequency. Further, the ERQ only measures the frequency of two emotion regulation strategies, but other strategies as categorized by Gross (1998) may also promote positive health outcomes (Sheppes et al., 2011). Cognitive reappraisal has also been defined by two different tactics of reinterpretation and distancing, which have been differentially effective given the intensity of the perceived stress (McRae et al., 2012). Future work should explore these same relationships with other emotion regulation strategies and tactics longitudinally. These relationships were measured in older adulthood and may not generalize to other developmental stages. Finally, the present study included a largely non-Hispanic White sample; thus, future studies should investigate these associations in more diverse samples.
The present study found evidence that high HRV buffers the negative effects of expressive suppression on depressive symptoms and perceived stress over 6 months. Thus, vagal tone, a proxy for trait-level self-regulation, may help reduce the impact of more frequently using less adaptive emotion regulation strategies. While correlational, the present results support HRV as a candidate biological mechanism through which individuals regulate their depressive symptoms and perceived stress, aligning with prior findings (Segerstrom and Nes, 2007; Thayer et al., 2012). Because early-life stress may alter these relationships, future work may examine neurodevelopment, vagally mediated HRV, and emotion regulation longitudinally throughout childhood and emerging adulthood. These results motivate future longitudinal, experimental work to determine if receiving training to improve HRV may be causally related to changes in stress and depressive symptoms during bereavement or for non-bereaved adults. For example, interventions such as vagus nerve stimulation or cognitive behavioral therapy (Carney et al., 2000; Bonaz et al., 2021) may help alleviate the influence of greater expressive suppression frequency on psychological well-being by increasing one’s vagally mediated HRV. Broadly, this finding aligns with a previous meta-analysis of 24 studies that showed HRV biofeedback training is associated with improvements in stress and anxiety (Goessl et al., 2017). Increased knowledge of potentially adaptive regulatory options may help people prepare to cope with current and future stressful circumstances.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Rice University, Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CF and JT developed the parent study concept and study design. CF oversaw all aspects of data collection and manuscript preparation. JT oversaw the heart rate variability analysis for the parent study, which includes the data presented here. RB and MC began the investigation for this specific manuscript and performed the data analysis and interpretation under the supervision of CF. RS aided in data analysis and interpretation throughout the revision process. RB, MC, JP, and ED drafted and edited the manuscript. EW-C, AL, MM, JT, RS, and CF provided critical revisions. All authors approved the final version of the manuscript for submission.
Funding
This research reported in this publication was supported by the National Heart, Lung, and Blood Institute (PI: CF, 5R01HL127260-05; PI: AL, F32HL146064-02) and the National Institute on Aging (PI: MC, 5F31AG069439-02) of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Patricia Morales and Kristi English for study management, as well as Levi Saucedo and Kyle Murdock for data management. Finally, we are extremely grateful to the study participants who devoted their time and effort to participate in this study.
References
Andreotti, C., Thigpen, J. E., Dunn, M. J., Watson, K., Potts, J., Reising, M. M., et al. (2013). Cognitive reappraisal and secondary control coping: associations with working memory, positive and negative affect, and symptoms of anxiety/depression. Anxiety Stress Coping 26, 20–35. doi: 10.1080/10615806.2011.631526
Blazer, D. G. (2003). Depression in Late Life: Review and Commentary. J. Gerontol. Series A 58, 249–65. doi: 10.1093/gerona/58.3.M249
Bonaz, B., Sinniger, V., and Pellissier, S. (2021). Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases. Front. Neurosci. 15:650971. doi: 10.3389/fnins.2021.650971
Bozdogan, H. (1987). Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika 52, 345–370. doi: 10.1007/BF02294361
Bridgett, D. J., Oddi, K. B., Laake, L. M., Murdock, K. W., and Bachmann, M. N. (2013). Integrating and differentiating aspects of self-regulation: effortful control, executive functioning, and links to negative affectivity. Emotion 13, 47–63. doi: 10.1037/a0029536
Carnevali, L., Koenig, J., Sgoifo, A., and Ottaviani, C. (2018). Autonomic and Brain Morphological Predictors of Stress Resilience. Front. Neurosci. 12:228. doi: 10.3389/fnins.2018.00228
Carney, R. M., Freedland, K. E., Stein, P. K., Skala, J. A., Hoffman, P., and Jaffe, A. S. (2000). Change in Heart Rate and Heart Rate Variability During Treatment for Depression in Patients With Coronary Heart Disease. Psychosomat. Med. 62, 639–647. doi: 10.1097/00006842-200009000-00007
Carstensen, L. L., Fung, H. H., and Charles, S. T. (2003). Socioemotional Selectivity Theory and the Regulation of Emotion in the Second Half of Life. Motivat. Emot 27, 103–123. doi: 10.1023/A:1024569803230
Charlson, M., Szatrowski, T. P., Peterson, J., and Gold, J. (1994). Validation of a combined comorbidity index. J. Clin. Epidemiol. 47, 1245–1251. doi: 10.1016/0895-4356(94)90129-5
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396.
Cumming, G. (2014). The new statistics: why and how. Psychol. Sci. 25, 7–29. doi: 10.1177/0956797613504966
Demaree, H. A., Schmeichel, B. J., Robinson, J. L., Pu, J., Everhart, D. E., and Berntson, G. G. (2006). Up- and down-regulating facial disgust: affective, vagal, sympathetic, and respiratory consequences. Biol. Psychol. 71, 90–99. doi: 10.1016/j.biopsycho.2005.02.006
Dörfel, D., Lamke, J.-P., Hummel, F., Wagner, U., Erk, S., and Walter, H. (2014). Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: a comparative fMRI investigation. Neuroimage 101, 298–309. doi: 10.1016/j.neuroimage.2014.06.051
Fagundes, C. P., Diamond, L. M., and Allen, K. P. (2012). Adolescent attachment insecurity and parasympathetic functioning predict future loss adjustment. Personal. Soc. Psychol. Bull. 38, 821–832. doi: 10.1177/0146167212437429
Fagundes, C. P., Murdock, K. W., LeRoy, A., Baameur, F., Thayer, J. F., and Heijnen, C. (2018). Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: mechanisms underlying cardiovascular risk? Psychoneuroendocrinology 93, 65–71. doi: 10.1016/j.psyneuen.2018.04.010
Fagundes, C. P., and Wu, E. L. (2020). Matters of the Heart: Grief, Morbidity, and Mortality. Curr. Dir. Psychol. Sci. 29, 235–241. doi: 10.1177/0963721420917698
Fantini-Hauwel, C., Batselé, E., Gois, C., and Noel, X. (2020). Emotion Regulation Difficulties Are Not Always Associated With Negative Outcomes on Women: The Buffer Effect of HRV. Front. Psychol. 11:697. doi: 10.3389/fpsyg.2020.00697
Fiske, A., Wetherell, J. L., and Gatz, M. (2009). Depression in Older Adults. Annu. Rev. Clin. Psychol. 5, 363–389. doi: 10.1146/annurev.clinpsy.032408.153621
Garnefski, N., and Kraaij, V. (2006). Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Personal. Individ. Differ. 40, 1659–1669. doi: 10.1016/j.paid.2005.12.009
Geisler, F. C. M., and Schröder-Abé, M. (2015). Is emotion suppression beneficial or harmful? It depends on self-regulatory strength. Motiv. Emot. 39, 553–562. doi: 10.1007/s11031-014-9467-5
Goessl, V. C., Curtiss, J. E., and Hofmann, S. G. (2017). The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol. Med. 47, 2578–2586. doi: 10.1017/S0033291717001003
Gross, J. J. (1998). The Emerging Field of Emotion Regulation: an Integrative Review. Rev. Gen. Psychol. 2, 271–299.
Gross, J. J., and John, O. P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Personal. Soc. Psychol. 85, 348–362. doi: 10.1037/0022-3514.85.2.348
Gross, J. J., and Levenson, R. W. (1993). Emotional suppression: physiology, self-report, and expressive behavior. J. Personal. Soc. Psychol. 64, 970–986. doi: 10.1037/0022-3514.64.6.970
Hakamata, Y., Komi, S., Moriguchi, Y., Izawa, S., Motomura, Y., Sato, E., et al. (2017). Amygdala-centred functional connectivity affects daily cortisol concentrations: a putative link with anxiety. Sci. Rep. 7:8313. doi: 10.1038/s41598-017-08918-7
Harris, C. R. (2001). Cardiovascular responses of embarrassment and effects of emotional suppression in a social setting. J. Personal. Soc. Psychol. 81, 886–897. doi: 10.1037/0022-3514.81.5.886
Holmes, T. H., and Rahe, R. H. (1967). The Social Readjustment Rating Scale. J. Psychosomat. Res. 11, 213–218. doi: 10.1016/0022-3999(67)90010-4
Holzman, J. B., and Bridgett, D. J. (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci. Biobehav. Rev. 74, 233–255. doi: 10.1016/j.neubiorev.2016.12.032
John, O. P., and Gross, J. J. (2004). Healthy and Unhealthy Emotion Regulation: Personality Processes, Individual Differences, and Life Span Development. J. Personal. 72, 1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x
Kalisch, R., Baker, D. G., Basten, U., Boks, M. P., Bonanno, G. A., Brummelman, E., et al. (2017). The resilience framework as a strategy to combat stress-related disorders. Nat. Hum. Behav. 1, 784–790. doi: 10.1038/s41562-017-0200-8
Kunzmann, U., Kupperbusch, C. S., and Levenson, R. W. (2005). Behavioral Inhibition and Amplification During Emotional Arousal: A Comparison of Two Age Groups. Psychol. Aging 20, 144–158. doi: 10.1037/0882-7974.20.1.144
Lopez, R. B., Denny, B. T., and Fagundes, C. P. (2018). Neural mechanisms of emotion regulation and their role in endocrine and immune functioning: a review with implications for treatment of affective disorders. Neurosci. Biobehav. Rev. 95, 508–514. doi: 10.1016/j.neubiorev.2018.10.019
Lüdecke, D. (2021). sjPlot: Data Visualization for Statistics in Social Science. Available online at: https://cran.r-project.org/web/packages/sjPlot/index.html
Malik, M. (1996). Heart Rate Variability. Annals Noninvasive Electrocardiol. 1, 151–181. doi: 10.1111/j.1542-474X.1996.tb00275.x
Malik, M., Bigger, J. T., Camm, A. J., Kleiger, R. E., Malliani, A., Moss, A. J., et al. (1996). Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043.
McRae, K., Ciesielski, B., and Gross, J. J. (2012). Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion 12, 250–255. doi: 10.1037/a0026351
Moore, S. A., Zoellner, L. A., and Mollenholt, N. (2008). Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav. Res. Ther. 46, 993–1000. doi: 10.1016/j.brat.2008.05.001
Müller, A. M., Wang, N. X., Yao, J., Tan, C. S., Low, I. C. C., Lim, N., et al. (2019). Heart Rate Measures From Wrist-Worn Activity Trackers in a Laboratory and Free-Living Setting: Validation Study. JMIR Mhealth Uhealth 7:e14120. doi: 10.2196/14120
Neupert, S. D., Almeida, D. M., and Charles, S. T. (2007). Age Differences in Reactivity to Daily Stressors: The Role of Personal Control. J. Gerontol. Series B 62, 216–225. doi: 10.1093/geronb/62.4.P216
Nezlek, J. B., and Kuppens, P. (2008). Regulating Positive and Negative Emotions in Daily Life. J. Personal. 76, 561–580. doi: 10.1111/j.1467-6494.2008.00496.x
Penttila, J., Helminen, A., Jartti, T., Kuusela, T., Huikuri, H. V., Tulppo, M. P., et al. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin. Physiol. 21, 365–376. doi: 10.1046/j.1365-2281.2001.00337.x
Phillips, L. H., Henry, J. D., Hosie, J. A., and Milne, A. B. (2008). Effective Regulation of the Experience and Expression of Negative Affect in Old Age. J. Gerontol. Series B 63, 138–145. doi: 10.1093/geronb/63.3.P138
Pinheiro, J., Bates, D., DebRoy, S., Sakar., D. and R Core Team (2020). nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-152. Available online at: https://CRAN.R-project.org/package=nlme
Porges, S. W., Doussard−Roosevelt, J. A., and Maiti, A. K. (1994). VAGAL TONE AND THE PHYSIOLOGICAL REGULATION OF EMOTION. Monogr. Soc. Res. Child Dev. 59, 167–186. doi: 10.1111/j.1540-5834.1994.tb01283.x
Pu, J., Schmeichel, B. J., and Demaree, H. A. (2010). Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biol. Psychol. 84, 531–540. doi: 10.1016/j.biopsycho.2009.07.006
Radloff, L. S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Measure. 1, 385–401.
R Core Team (2019). R: A Language and Environment for Statistical Computing [Computer Software]. Vienna: R Foundation for Statistical Computing.
Richards, J. M., and Gross, J. J. (2000). Emotion regulation and memory: the cognitive costs of keeping one’s cool. J. Pers. Soc. Psychol. 79, 410–424. doi: 10.1037//0022-3514.79.3.410
Sbarra, D. A., and Borelli, J. L. (2013). Heart rate variability moderates the association between attachment avoidance and self-concept reorganization following marital separation. Int. J. Psychophysiol. 88, 253–260. doi: 10.1016/j.ijpsycho.2012.04.004
Segerstrom, S. C., and Nes, L. S. (2007). Heart Rate Variability Reflects Self-Regulatory Strength, Effort, and Fatigue. Psychol. Sci. 18, 275–281. doi: 10.1111/j.1467-9280.2007.01888.x
Sheppes, G., Scheibe, S., Suri, G., and Gross, J. J. (2011). Emotion-Regulation Choice. Psychol. Sci. 22, 1391–1396. doi: 10.1177/0956797611418350
Shiota, M. N., and Levenson, R. W. (2009). Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychol. Aging 24, 890–900. doi: 10.1037/a0017896
Slavich, G. M., and Irwin, M. R. (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815.
Stanley, D. (2018). apaTables: Create American Psychological Association (APA) Style Tables (R Package Version 2.0.5) [Computer software]. Available online at: https://CRAN.R-project.org/package=apaTables
Stein, P. K., Bosner, M. S., Kleiger, R. E., and Conger, B. M. (1994). Heart rate variability: a measure of cardiac autonomic tone. Am. Heart J. 127, 1376–1381. doi: 10.1016/0002-8703(94)90059-0
Stroebe, M., Schut, H., and Stroebe, W. (2007). Health outcomes of bereavement. Lancet 370, 1960–1973. doi: 10.1016/S0140-6736(07)61816-9
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-aho, P. O., and Karjalainen, P. A. (2009). “Kubios HRV—A Software for Advanced Heart Rate Variability Analysis,” in 4th European Conference of the International Federation for Medical and Biological Engineering. IFMBE Proceedings, eds J. Vander Sloten, P. Verdonck, M. Nyssen, and J. Haueisen (Berlin: Springer), 1022–1025.
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., and Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
Thayer, J. F., Hansen, A. L., Saus-Rose, E., and Johnsen, B. H. (2009). Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Ann. Behav. Med. 37, 141–153. doi: 10.1007/s12160-009-9101-z
Thayer, J. F., and Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 61, 201–216. doi: 10.1016/S0165-0327(00)00338-4
Thayer, J. F., Mather, M., and Koenig, J. (2021). Stress and aging: a neurovisceral integration perspective. Psychophysiology 58:e13804. doi: 10.1111/psyp.13804
Troy, A. S., Wilhelm, F. H., Shallcross, A. J., and Mauss, I. B. (2010). Seeing the Silver Lining: Cognitive Reappraisal Ability Moderates the Relationship Between Stress and Depressive Symptoms. Emotion 10, 783–795. doi: 10.1037/a0020262
Urry, H. L., and Gross, J. J. (2010). Emotion Regulation in Older Age. Curr. Dir. Psychol. Sci. 19, 352–357. doi: 10.1177/0963721410388395
Urry, H. L., van Reekum, C. M., Johnstone, T., Kalin, N. H., Thurow, M. E., Schaefer, H. S., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 26, 4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006
Vanderhasselt, M.-A., Baeken, C., Van Schuerbeek, P., Luypaert, R., and De Raedt, R. (2013). Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: an event related fMRI study. Biol. Psychol. 92, 433–439. doi: 10.1016/j.biopsycho.2012.03.005
Vrieze, S. I. (2012). Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 17, 228–243. doi: 10.1037/a0027127
Keywords: emotion regulation, heart rate variability (HRV), resilience, depression, perceived stress
Citation: Brown RL, Chen MA, Paoletti J, Dicker EE, Wu-Chung EL, LeRoy AS, Majd M, Suchting R, Thayer JF and Fagundes CP (2022) Emotion Regulation, Parasympathetic Function, and Psychological Well-Being. Front. Psychol. 13:879166. doi: 10.3389/fpsyg.2022.879166
Received: 18 February 2022; Accepted: 23 May 2022;
Published: 03 August 2022.
Edited by:
Darren J. Edwards, Swansea University, United KingdomReviewed by:
Ilya Yaroslavsky, Cleveland State University, United StatesBiao Sang, East China Normal University, China
Bruno Bonaz, Centre Hospitalier Universitaire de Grenoble, France
Copyright © 2022 Brown, Chen, Paoletti, Dicker, Wu-Chung, LeRoy, Majd, Suchting, Thayer and Fagundes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher P. Fagundes, Y2hyaXN0b3BoZXIuZmFndW5kZXNAcmljZS5lZHU=
 Ryan L. Brown
Ryan L. Brown Michelle A. Chen
Michelle A. Chen Jensine Paoletti
Jensine Paoletti Eva E. Dicker
Eva E. Dicker E. Lydia Wu-Chung
E. Lydia Wu-Chung Angie S. LeRoy
Angie S. LeRoy Marzieh Majd
Marzieh Majd Robert Suchting2
Robert Suchting2 Julian F. Thayer
Julian F. Thayer Christopher P. Fagundes
Christopher P. Fagundes