- 1Consultant, New York, NY, United States
- 2Consultant, Overland Park, KS, United States
- 3Cybin, Inc., Toronto, ON, Canada
The field of psychedelic medicine is currently developing evidence-based standards and core competencies for training providers who offer psychedelic treatments. This article contributes to this effort by describing the iterative development of the EMBARK training program. This program has been developed by Cybin, Inc. for training session monitors in clinical trials of psychedelic treatments. The EMBARK training program has been used in two successfully completed clinical trials and three ongoing trials. This article details each of the three phases of the EMBARK training program’s development, including their core components, what worked well, lessons learned, and the rationale for any changes made. Innovations included in EMBARKCT, the most recent iteration of the training used in Cybin’s Phase 3 trials, are also discussed. These innovations include the use of artificial intelligence (AI) with expert human oversight to identify the need for additional support and training for session monitors. Finally, recommendations and implications for the field are discussed in this article.
1 Introduction
As psychedelic treatments progress toward regulatory review and potential approval, the question of how to effectively train psychedelic practitioners has received increased attention (Argento et al., 2024; Barber et al., 2024; Brennan and Belser, 2024; Dames et al., 2024a, 2024b; McGuire et al., 2024; Mocanu et al., 2022; Poppe et al., 2025; Rosenbaum et al., 2024; Villiger, 2024; Wilson-Poe et al., 2024). There is a widely recognized need to develop training programs that will meet the anticipated post-approval demand for trained psychedelic practitioners (Knepler et al., 2024).
A key part of addressing this need is the development of training standards, core practitioner competencies, and certification requirements that support safe and efficacious psychedelic care. In the United States and Europe, several groups have begun this process by soliciting perspectives from recognized experts in the field (e.g., Aicher and Gasser, 2024; BrainFutures, 2024; Gardner and Noble, 2024; Jacobs et al., 2024; McGuire et al., 2024; Passie et al., 2025; Perez Rosal et al., 2024; Phelps, 2017). Their work to date has laid important groundwork for creating the agreed-upon training standards for the field.
In addition to considering expert perspectives, the standards and competencies used in the field should have a basis in empirical evidence. One way to facilitate this is by evaluating the training approaches for psychedelic session monitors that have been found to support safety and efficacy in clinical trials. To support this evaluation, the field would benefit from greater transparency about the approaches to training used in successful trials (Brennan et al., 2023).
The current article serves that unmet need by examining the iterative development of the EMBARK psychedelic session monitor training program, which has been used in several successful clinical trials conducted by Cybin, a neuropsychiatric drug development company, and others (Back et al., 2024). This article will present various elements of the EMBARK training program, describe how they have evolved across successive iterations of the training program, and provide the rationale for their evolution. It also describes the challenges encountered, the solutions implemented, and the lessons learned from them. By offering in-depth insights into a program used to train session monitors across several successful trials, this article will provide valuable data that support the development of evidence-based standards for the field.
2 Overview of the EMBARK model
The EMBARK training program is based on the EMBARK model of psychedelic treatment. EMBARK is a trans-drug, transdiagnostic model of psychological support designed for use during psychedelic treatments. It was first developed for Cybin-sponsored clinical trials (Brennan and Belser, 2024; Brennan and Belser, 2022). The model has served as the basis for several trial-specific adaptations of EMBARK that apply a particular drug to a specific clinical indication. For example, the EMBARK approach has been applied to the treatment of major depressive disorder (MDD) using psilocybin-like molecules such as Cybin’s CYB003, a proprietary deuterated psilocin molecule (Brennan and Belser, 2024).
The core elements of the EMBARK model that are present in all adaptations include six clinical domains that comprise its name (Existential-spiritual, Mindfulness, Body aware, Affective-cognitive, Relational, and Keeping momentum) and its four ethical care cornerstones (Trauma-informed care, Culturally competent care, Ethically rigorous care, and Collective care). The six clinical domains represent the wide variety of potentially beneficial treatment experiences a participant may have when given a psychedelic drug in a supportive setting. EMBARK’s multidomain structure recognizes that each participant will have a unique treatment experience and prepares session monitors to conceptualize and support them in a flexible and responsive way. EMBARK’s four ethical care cornerstones reflect the model’s foundational commitment to ethical practice. Guidelines for providers on how to align their practice with each cornerstone are woven into all aspects of each EMBARK adaptation for a specific clinical indication.
Another key feature of EMBARK is that it has been designed to facilitate the incorporation of clinical interventions and established mechanisms of change from non-psychedelic evidence-based treatments (EBTs) and empirically supported treatments (ESTs). Each EMBARK adaptation for a specific clinical indication instructs session monitors to apply concepts from EBTs and ESTs that are most relevant to the content that arises in each participant’s unique treatment experience. This tailoring process is organized by the model’s six clinical domains. For example, a depressed participant having a treatment experience that falls within the “affective-cognitive” domain may be best supported by a cognitive behavioral or emotion focused therapy-based approach, while another having an experience more germane to the “mindfulness” domain may benefit more from an acceptance and commitment therapy-based approach.
To provide this type of participant-centered care, the EMBARK training program has aimed to provide session monitors with thoughtful, high-quality training. Its goal has been the same as that of the EMBARK model itself: to prepare session monitors to work ethically and efficaciously with the broad spectrum of emergent phenomena that may arise in psychedelic treatment.
3 Overview of the EMBARK training program
This article will discuss the evolution of the EMBARK training program by describing three phases of its development:
• The original EMBARK training was designed for small groups of session monitors with no prior psychedelic therapy experience.
• The EMBARK crossover training for session monitors who had previously worked on other psychedelic trials or who had completed other psychedelic training programs.
• EMBARK for Clinical Trials (EMBARKCT) was designed to be scalable for multisite Phase 3 trials conducted across several continents in multiple languages.
For each phase, this article will describe the components of the training, discuss what worked well, detail the lessons learned, and provide the rationale for any changes made from a previous phase. The perspective provided therein was developed through discussions among EMBARK team members and outside collaborators who worked on each phase, as well as through other inputs as noted.
4 Original EMBARK training
The first iteration of the EMBARK training was developed to support two trials that began in late 2022 and early 2023. One was an investigator-initiated trial led by Back et al. (2024) at the University of Washington, assessing the efficacy of psilocybin in burnout and depression related to coronavirus disease 2019 (COVID-19) in frontline healthcare workers. The second was a Cybin-sponsored Phase 2b trial assessing the safety and efficacy of CYB003 in the treatment of major depressive disorder (MDD).
The Cybin trial reported strongly positive topline outcome data, with 79% of participants who received two 12-mg doses of CYB003 in remission from MDD at 6-week follow-up, compared to 20% of the control group (n = 32) with full results currently being written up for publication (see Krempien et al., 2024). Dr. Back’s trial (Back et al., 2024) also reported positive outcomes, with a between-group mean difference in Montgomery–Åsberg Depression Rating Scale (MADRS) score reductions of −12.00 (n = 30; p < 0.001).
All session monitors enrolling in the training cohort for the Cybin trial (CYB003-001) were required to undergo an interview with a Cybin EMBARK core faculty member to ensure they had the appropriate experience and fit. Out of 15 qualified applicants, 1 applicant was denied entry into the program due to a lack of transferable skills. The accepted cohort included 14 licensed or license-eligible psychotherapists, a psychiatrist, and a psychiatric nurse practitioner. The cohort trained for the University of Washington trial (Back et al., 2024) included 11 practitioners with diverse professional backgrounds (psychotherapists, medical doctors, physician assistants, and nurse practitioners). No session monitor on either trial had previously worked on a psychedelic clinical trial.
The EMBARK team at Cybin, in collaboration with Dr. Back, developed the structure of the original EMBARK training. It required trainees to complete the following components:
• Self-guided review of the trial-specific EMBARK treatment manual, which included specifications on molecule and indication
• Asynchronous review of 12 video modules, each created by a different subject matter expert, on topics including MDD, working within each of EMBARK’s six clinical domains, and practicing in alignment with each of EMBARK’s four ethical care cornerstones
• Live Q&A sessions with these subject matter experts after watching each video
• Completion of a 3-day in-person training
• This included study molecule and protocol training with a daylong group experiential session in Holotropic Breathwork. During the experiential session, trainees were paired in groups of two. They had a chance to both provide support and be supported in a non-drug-induced non-ordinary state experience that approximates those elicited by classic psychedelic drugs—something that has been suggested as a helpful component of session monitor training (Villiger, 2024).
In addition to the above training, Cybin also provided approximately 8 h of asynchronous clinical trial and International Council for Harmonization-Good Clinical Practice (ICH-GCP) training for each session monitor in efforts to bolster their readiness for clinical trial compliance. A trainee who completed all the aforementioned training components was considered pre-certified as an EMBARK session monitor. Once a pre-certified session monitor began work on a Cybin trial, they were required to do the following during study conduct:
• Complete three educational consultation sessions (either as a dyad or an individual session monitor) with an expert consultant during their work with their first trial participant
• Attend monthly peer consultation meetings as a means to support each session monitor’s learning and development. In these meetings, one session monitor team presented a participant case while the other session monitors asked thoughtful questions, provided suggestions and feedback, and inquired about ways they could provide support to the presenting session monitor team.
Completing the three educational consultation sessions conferred full EMBARK certification onto a session monitor, which was considered a necessary credential to continue working on any current or future Cybin trials (see Figure 1).
4.1 Original training: what worked well
Elements of the first approach to EMBARK training that worked well were determined through soliciting iterative feedback from trained session monitors and continual reflection by program staff. They included:
• Faculty with diverse expertise. Rather than relying on one or a few trainers to deliver training content, training content delivered by multiple faculty members provided session monitors with an opportunity to learn in a manner that approximated a university seminar course or other comprehensive learning contexts.
• The video content created by these faculty members was later made freely available on www.embarkapproach.com as EMBARK Open Access, an informational training course on psychedelic treatments.
• Early cohort cohesion building in an in-person training. Session monitors benefited from the opportunity to come together in person early in their training and build relationships that would become the foundation for mutual support and collaborative learning throughout the trial.
• Experiential training. The group breathwork experience provided session monitors with a more direct, first-person understanding of the unique needs a participant might have while in a non-ordinary state. It also fostered a deeper sense of interpersonal connectedness among session monitors, which strengthened mutual support throughout the trial.
• Clinical Supervision/Educational consultation. In a follow-up survey, session monitors reported that both the mandated and as-needed consultation sessions were an invaluable support in making clinical judgment calls on challenging cases.
• Peer consultation. In the same follow-up survey, session monitors also reported that meeting regularly with their peers to discuss their work with participants provided a helpful source of support.
• Iterative feedback from session monitors throughout the trial. Session monitors provided continual feedback through formal evaluation forms submitted after each live session and by relating challenges to educational consultants, who then brought these concerns to leadership.
4.2 Original training: lessons learned
The first EMBARK training approach also had limitations that were improved upon in later iterations. These included:
• Budgeting and scalability. Training elements, such as in-person group trainings and mandated educational consultation for all new session monitors, were too resource-intensive for Phase 3 trials being conducted at many sites across several countries.
• Unnecessary session monitor burden. Some of the basic content covered in the EMBARK training modules would likely prove to be duplicative to session monitors with previous training and experience on psychedelic trials by other sponsors.
• Inadequately integrated training content. Although learning from a variety of expert faculty presented benefits, it also made the training content feel somewhat fragmented for some session monitors who wanted the training to give them a more integrated sense of their role. This feedback often contrasted the fragmentation of the training approach with the more well-defined session monitor role delineated in the session monitor manual.
The EMBARK crossover training and EMBARKCT training were developed as responses to these limitations. The specific improvements made to each training will be discussed in the following sections.
5 EMBARK crossover training
A crossover training approach was developed to provide a more focused EMBARK training to session monitors with prior training from a well-established psychedelic facilitation training program by other organizations. Therefore, the crossover training assumes that trainees have a foundational understanding of the role of a psychedelic session monitor and requires that they hold a Certificate of Completion from a reputable psychedelic training program. This abbreviated approach succeeded in reducing duplicative foundational content, thereby alleviating the training burden placed on session monitors. It also succeeded in reducing the total cost of training incurred by the sponsor for ensuring that session monitors had competencies that they had already developed in other training programs.
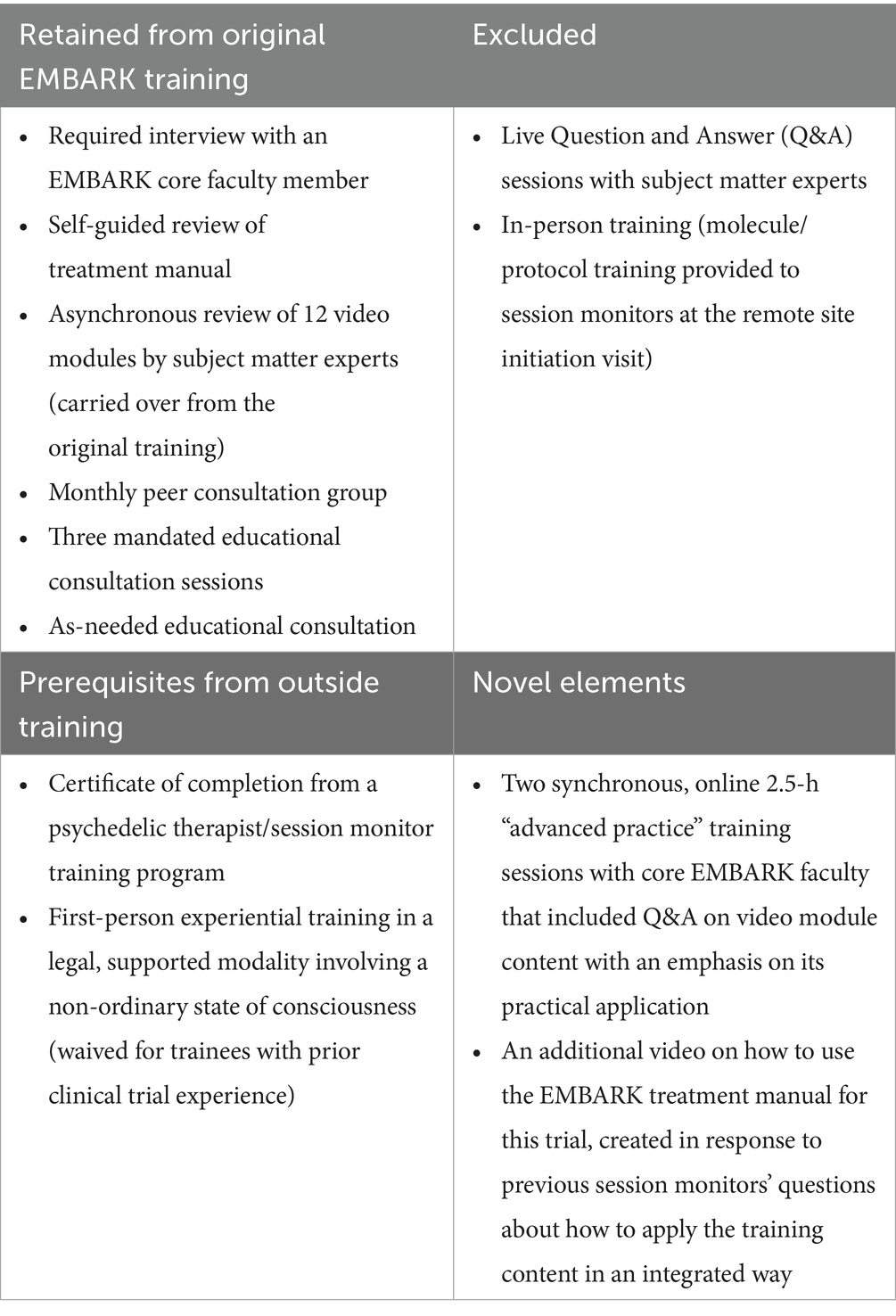
The EMBARK crossover training was first used to train seven session monitors at a second research site that was brought on midway through Cybin’s aforementioned CYB003-MDD Phase 2b trial. This new group of session monitors consisted of six licensed or license-eligible psychotherapists and one licensed psychiatrist. See Table 1 for details on what was retained and excluded from the original EMBARK training.
After the CYB003 Phase 2 trial concluded, the session monitors who had undergone this crossover training were brought back to work on another Cybin trial, which is currently ongoing as of the publication of this manuscript. This trial examines the safety and efficacy of CYB004, Cybin’s dimethyltryptamine (DMT) analog, as a possible treatment for generalized anxiety disorder. The additional training required for this trial consisted of two more 2.5-h advanced practice sessions. One session reviewed the EMBARK treatment manual written for this trial, and the other brought in session monitors who worked with another DMT-like molecule in an earlier trial (conducted by Small Pharma) to present on the participant experiences they observed. Educational consultation has not been mandated for the CYB004 Phase 2 trial, though it is still available upon request (see Figure 2).
5.1 Crossover training: what worked well
The EMBARK crossover training demonstrated the following advantages over the original EMBARK training:
• Improved budgeting and scalability. The in-person training components that were replaced with synchronous, remote training sessions were less resource-intensive for the sponsor.
• Reduced session monitor training burden. Session monitors with previous training on psychedelic treatments benefited from reduced training requirements and a reduction of duplicative content.
• Increased focus on practical skill-building. While the original training’s live Q&A sessions focused on understanding the video content, the crossover training’s advanced practice sessions focused more on grasping the specifications of what session monitors were asked to do on this trial. It used vignettes and roleplays to develop skills, rather than engaging in more conceptual discussions about aspects of psychedelic treatments.
• Greater emphasis on the treatment manual. Shifting the focus of the live sessions toward practical skill-building and away from the broad content of the videos also contributed to an increased focus on the specific manualized approach being used in the trial. This helped session monitors develop a clearer sense of their role, which likely improved clinician self-efficacy and treatment fidelity.
5.2 Crossover training: lessons learned
• Failure to more actively integrate session monitors’ prior experience and training. The crossover training could have made a greater effort to understand session monitors’ existing skill sets and understanding of their role at the start of the trial. This would have allowed core faculty to tailor the advanced practice sessions to cover the material that would be most supportive in training session monitors to work within the EMBARK approach.
• Potential lack of camaraderie building. The crossover training was used with a group of session monitors wo had existing relationships carried over from working together at their site on other trials and thus had an established group rapport. However, had this not been the case, the abbreviated design of this training, with its lack of in-person events, may have failed to build a rapport of mutual support and co-learning in a newly formed session monitor group.
• Challenges of requiring first-person experiential training. The process of assessing incoming session monitors’ prior experiential training prompted internal conversations about this requirement. We considered the impact of continuing this requirement into Phase 3 trials, which would span a broader geographical range and include cultures and jurisdictions with varied attitudes toward personal engagement with non-ordinary states. We also considered that not all trainees would be medically cleared to participate in experiential training. We removed this requirement to allow for greater accessibility for the Phase 3 trial EMBARK training.
6 EMBARK for clinical trials (EMBARKCT)
An evolution of EMBARK training was created in preparation for Cybin’s Phase 3 trials with its CYB003 molecule. EMBARKCT (for “clinical trials”) was designed to be a further streamlined training that could be deployed to train session monitors for simultaneous trials at numerous sites across several countries. It was also continually honed so that it could hew closely to advice that the U.S. Food and Drug Administration (FDA) had recently issued for psychedelic research, both in the draft guidance it issued in June 2023 and in its July 2024 advisory committee’s reflections on Lykos’ new drug application for 4-methylenedioxymethamphetamine (MDMA) treatment of PTSD (U.S. Food and Drug Administration, 2023; Wilkinson and Sanacora, 2025).
All previous iterations of the EMBARK training program helped to form the development of the EMBARKCT scaled session monitor training effort for Cybin-sponsored Phase 3 pivotal trials. The EMBARKCT training program retained many of the key components that worked well, while modifying and improving other aspects based on lessons learned.
We ensured that the following elements from prior iterations were retained in EMBARKCT:
• Applicant interviews;
• Content drawn from the diverse subject matter expertise provided by the original EMBARK faculty;
• Educational consultation and peer consultation;
• Improved budgeting and scalability;
• Reduced session monitor training burden;
• Well-integrated training content that emphasizes practical skill-building;
• Consistent integration with training manual content.
Training in EMBARKCT differs from previous iterations of EMBARK training across the following areas:
• Review of applicants’ recorded responses to session vignettes prior to interview. Prior to their first meeting with an EMBARKCT trainer, each applicant views video recordings of treatment events that may be encountered in a clinical trial. They then record a brief response to each video, capturing how they would act as a session monitor in that moment. Their interviewer then reviews their responses prior to the interview, allowing for another layer of assessment of the applicant’s skill set.
• Requirement of prior foundational psychedelic training for applicants. We require that all applicants complete an approved foundational psychedelic treatment training with a duration of at least 6 h from another training program prior to beginning the EMBARKCT training. This allows us to focus the EMBARKCT training on the specifications of the EMBARK approach used on the CYB003-MDD trial, rather than beginning with the basics. Any session monitor without this foundational training is offered enrollment in a 6-h Fluence training course 1 at sponsor expense or another program of their choosing.
• Streamlined session monitor manual. The EMBARK team at Cybin has collaborated with Fluence to develop an updated session monitor manual that presents the treatment approach from the Phase 2 trials in a more focused and accessible way, while making minor adjustments in the approach in response to FDA guidance issued in the interim (Brennan et al., 2024). Session monitor fidelity adherence checklists were also made available to assist them in adhering to the treatment approach.
• Updated Learning Management System (LMS) training content
• Content from both the session monitor manual and the original EMBARK video modules has been woven together into more concise videos that cover the EMBARK domains and care cornerstones.
• Session monitors are required to watch additional Fluence video content on the basics of psilocybin treatment for depressive disorders and review the CYB003 specifications within the manual.
• Session monitors are asked to reflect on and respond in writing to clinical vignettes that raise questions about ethical practice, in addition to a set of questions about how they have navigated ethical issues previously in their clinical practice.
• Session monitors are provided with the fidelity adherence criteria used on the trial, and they are asked to apply these criteria to a series of case demonstration videos as a way of familiarizing themselves with what will be expected of them on the trial.
• Additional roleplay training
• As part of their training, session monitors are paired and required to meet twice to conduct and debrief a total of six 15-min roleplays of different session types conducted by each monitor: pre-dose, dosing, and post-dose. These meetings are recorded and analyzed by Fluence’s AI, which provides feedback on each session monitor’s performance that is discussed in a live session with a trainer.
• The final component of training is a competency assessment in which session monitors meet in a live session with a trainer to complete a final 20-min roleplay. This roleplay is evaluated in real time by a trainer using the study fidelity adherence criteria. The trainer then provides feedback to the session monitor and decides whether to certify them.
• Addition of a streamlined training track for experienced session monitors. A streamlined version of the EMBARKCT program is available to candidates with substantial prior experience and strong demonstrated comprehension of the session monitor role. This assessment is made during the candidate’s initial interview. The streamlined track makes the recorded partner roleplays and trainer feedback session optional, easing scheduling demands for qualified candidates. To ensure readiness, candidates on this track undergo a more extensive competency assessment at the end of the streamlined training (see Figures 3, 4).
6.1 EMBARKCT : incorporation of AI to signal needs for monitor remediation training
Another defining feature of EMBARKCT is the use of AI, not just for safety monitoring and fidelity adherence monitoring, but also to detect the need to either remove a session monitor with a substantiated allegation of misconduct or to provide remediation and educational consultation to session monitors whose work is insufficiently adherent to the treatment approach. All treatment sessions (pre-dose, dosing, and post-dose sessions) are analyzed shortly after they occur by mpathic’s AI-based system, which was trained on the EMBARK treatment approach used in Cybin’s Phase 3 CYB003-MDD trials with data collected from their Phase 2 trial. This system, which involves expert human oversight alongside AI review, offers scalable, unbiased, consistent, and near real-time monitoring of treatment fidelity in the provision of psychological support, effectively closing a gap identified in previous psychedelic trials by the FDA.
Additionally, real-time feedback on adherence facilitates the identification of session monitors who may benefit from additional training or support in their role. Remediation educational consultation is required when either of the following occurs:
• A session monitor’s adherence rating falls below 80% in any phase of treatment (pre-dose, dosing, or post-dose sessions).
• A session monitor misses an adherence item deemed critical by study staff (e.g., discussing the possible effects of the study drug).
If a session monitor is found to be non-adherent three times, they are considered for possible removal from the trial. If a session monitor is detected speaking or acting in a way that is harmful to a participant or poses a safety risk, the issue is escalated. This is confirmed with the site PI, and the session monitor is removed from the trial and their EMBARK certification revoked. Additional actions may be taken by the site and/or regulatory or licensing authorities (see Figure 5).
6.2 EMBARKCT : educational and peer consultation processes
All session monitors are encouraged to request educational consultation with an EMBARKCT trainer at any time during study conduct if they have any questions about their work with a participant or about their role generally.
Session monitors also receive required educational consultation when they fail to meet key adherence criteria or drop below 80% in overall treatment adherence. These consultation sessions are structured in a manner that targets the specific treatment component that requires remediation. The session monitor reviews targeted self-study materials on a range of important skills for session monitors to complete prior to meeting with an educational consultant.
Additionally, monthly peer consultation group meetings are incorporated during the study conduct for each research site. Session monitors are encouraged to participate in these meetings as a means to support one another’s learning and development. As in previous iterations of the EMBARK training, these meetings consist of session monitors from each trial site meeting to discuss clinical questions that have arisen in their work with participants.
7 Implications for the field
The following section discusses several key lessons from the development of the various iterations of the EMBARK training program. They are offered in service of the efforts to develop approaches to psychedelic practitioner training that build upon training sessions that have supported treatment efficacy and participant safety in practice. Still, when considering these lessons, it is important to keep in mind that they are simply reflections of those involved in the development of EMBARK training programs and have not been formally tested as hypotheses about what contributes to an effective training program. Additionally, the EMBARKCT program has only recently been deployed and has yet to undergo a completed trial.
Offering post-training educational consultation. EMBARK-trained session monitors have consistently expressed that the opportunity to meet with an educational consultant during their work on a trial was pivotal to their success in their role. Our team thus prioritized finding a way to continue to offer it as we scaled up to Phase 3 trials, which has so far proven possible with the integration of the AI-based referral system discussed earlier, alongside the opportunity to seek optional educational consultation at any time during trial conduct.
Favoring practical education over didactics. EMBARK training has shifted its center away from video-centric learning with content-based Q&A sessions toward roleplays and trainer feedback sessions. This change has given session monitors more opportunity to practice specific skills before using them with participants. It has also provided trainers with more insight into the specific training and support needs of each individual session monitor, allowing for personalized attention.
Enhanced session monitor screening through a rigorous interview and competency assessment process during training. Bringing the right people into training is perhaps the most important factor in predicting its outcomes. EMBARKCT’s multistep onboarding process allows for more engagement with the session monitor and their capacities before they are brought onto the trial.
Setting the right tone when building relationships with session monitors. We have viewed all components of our trainings—from in-person retreats to online roleplay sessions—as a kind of parallel process that models how session monitors are expected to engage with participants. The feedback we have received indicates that session monitors perceived this intention and that it positively impacted their feelings about working on Cybin trials. This emphasis on building relationships with session monitors has also opened the door to session monitors working on multiple Cybin trials, deepening their expertise in EMBARK with each one, with some even becoming trainers and educational consultants within the program.
Synergizing session monitor training with other innovations used in trials. Although the primary reason for the inclusion of mpathic’s AI system was to assess fidelity adherence to the EMBARK model, its secondary function as a referral mechanism for educational consultation demonstrates the value of integrating other trial supports to improve ongoing session monitor training and oversight.
8 Limitations
One important limitation to note is that the EMBARK training has been designed specifically for use in the context of clinical trials, where the goals and constraints differ from those of real-world clinical practice. Training for competence in a trial setting differs from preparing practitioners for independent, long-term clinical work with patients. Caution should be used when applying the findings in this article outside of the controlled conditions of a clinical trial. In a trial context, participant well-being is supported by intensive monitoring, including the AI-based system described in this article. At the same time, this protective function may be served by more extensive provider training in real-world settings.
Also, since this training program has not been developed for post-approval settings, its design should not be used as a model for reducing costs in those settings. While cost and scalability are essential topics for the field, those questions fall outside the scope of the current project and require separate, thoughtful engagement (Marseille et al., 2022; McCrone et al., 2023).
9 Conclusion
As the field of psychedelic treatments develops competencies and standards for the training of practitioners, it would be well-served by learning from the training approaches used in completed trials with efficacious outcomes. The present article offers information on the three phases of the EMBARK training program, as well as perspectives from those involved in their development, for consideration and analysis. In doing so, it aims to create evidence-based psychedelic practitioner training approaches that support the thoughtful and responsible evolution of these promising new treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WB: Writing – review & editing, Writing – original draft. MF: Writing – review & editing. CS: Writing – review & editing. AI: Writing – review & editing. AK: Writing – review & editing, Project administration, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by Cybin IRL Limited, Dublin, Ireland.
Acknowledgments
The authors wish to acknowledge Dr. Alex Belser for his instrumental contributions during the early development phases of the EMBARK training program, which helped lay the groundwork for the program’s current form. We also extend gratitude to Diane Hollman and Sarah Scheld for their contributions to the EMBARK training program. The authors would also like to acknowledge Fluence and mpathic for their partnership in conducting this work.
Conflict of interest
Cybin, Inc. compensated all authors for their time spent writing this article.
WB and MF are paid consultants at Cybin, Inc. CS is an associate director of clinical development at Cybin, Inc. AI is chief medical officer at Cybin, Inc. AK is vice president of clinical development and head of therapies at Cybin, Inc. In the past two years: WB has also received compensation from Gilgamesh Pharmaceuticals, Fluence, Naropa University, Odyssey PBC, OPEN Foundation, Psychedelics Today, and GuideSite Consulting (where he has also served as an equity-holding member); MF has also received compensation from GuideSite Consulting (where she has also served as an equity-holding member).
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^ The name of the Fluence training is "Introduction to Psychedelic Therapy" and can be found at this URL if more information is required: https://www.fluencetraining.com/content/2892737-1.
References
Aicher, H. D., and Gasser, P. (2024). Treatment recommendations for psychedelic-assisted therapy. Swiss Arch. Neurol. Psychiatry Psychother. doi: 10.4414/sanp.2024.1488043038
Argento, E., Petker, T., Vig, J., Robertson, C., Jaeger, A., Necyk, C., et al. (2024). “This is you teaching you:” exploring providers’ perspectives on experiential learning and enhancing patient safety and outcomes in ketamine-assisted therapy. PLoS One 19:e0306381. doi: 10.1371/journal.pone.0306381
Back, A. L., Freeman-Young, T. K., Morgan, L., Sethi, T., Baker, K. K., Myers, S., et al. (2024). Psilocybin therapy for clinicians with symptoms of depression from frontline care during the COVID-19 pandemic: a randomized clinical trial. JAMA Netw. Open 7:e2449026. doi: 10.1001/jamanetworkopen.2024.49026
Barber, M., Gardner, J., Liknaitzky, P., and Carter, A. (2024). Lost in translation? Qualitative interviews with Australian psychedelic-assisted therapy trial clinicians. Psychol. Psychother. Theory Res. Pract. 97, 626–644. doi: 10.1111/papt.12545
BrainFutures. (2024). Training convening summary. Available online at: https://www.brainfutures.org/wp-content/uploads/2024/06/BrainFutures-Training-Convening-Summary.pdf (accessed May 14, 2025).
Brennan, W., and Belser, A. B. (2022). Models of psychedelic-assisted psychotherapy: a contemporary assessment and an introduction to EMBARK, a transdiagnostic, trans-drug model. Front. Psychol. 13:866018. doi: 10.3389/fpsyg.2022.866018
Brennan, B., and Belser, A. (2024). EMBARK psychedelic therapy for depression: a new approach for the whole person. New York, NY: Oxford University Press.
Brennan, B., Belser, A. B., Field, M., Kelman, A., Benway, T., Molinar, A., et al. (2024). EMBARKCT facilitator manual for CYB003 trials in major depressive disorder (Version 3) : Cybin., Inc.
Brennan, W., Kelman, A. R., and Belser, A. B. (2023). A systematic review of reporting practices in psychedelic clinical trials: psychological support, therapy, and psychosocial interventions. Psychedelic Med. 1, 218–229. doi: 10.1089/psymed.2023.0007
Dames, S., Penn, A., Williams, M., Zamaria, J. A., Rousmaniere, T., and Vaz, A. (2024a). Deliberate practice in psychedelic-assisted therapy : American Psychological Association.
Dames, S., Watler, C., Kryskow, P., Allard, P., Gagnon, M., Taylor, W., et al. (2024b). Psychedelic-assisted therapy training: an argument in support of firsthand experience of nonordinary states of consciousness in the development of competence. Psychedelic Med. 2, 130–137. doi: 10.1089/psymed.2023.0004
Gardner, D., and Noble, S. (2024). A psychedelic-assisted therapy training framework: comprehensive behavioral health learning objectives for practitioners. Available online at: https://www.beckleyacademy.com/a-psychedelic-assisted-therapy-learning-framework/ (accessed May 14, 2025).
Jacobs, E., Earp, B. D., Appelbaum, P. S., Bruce, L., Cassidy, K., Celidwen, Y., et al. (2024). The Hopkins-Oxford psychedelics ethics (HOPE) working group consensus statement. Am. J. Bioeth. 24, 6–12. doi: 10.1080/15265161.2024.2342764
Knepler, E., Smith Richard, I. I., Bautista, M., Blajszczak, E., Rosenbloom, M. R., Gebhardt, M. Z., et al. (2024). Survey on psychedelic therapy curricula in academia. Available online at: https://www.brainfutures.org/wp-content/uploads/2024/12/Survey-on-Psychedelic-Therapy-Curricula-in-Academia_Report.pdf (accessed May 14, 2025).
Krempien, S., Kelman, A., Mahableshwarkar, A., Shenouda, M., Johnson, K., Maass-Robinson, S. A., et al. (2024). CYB003 produces robust and enduring antidepressant effects in patients with major depressive disorder (MDD) [Poster presentation]. 63rd annual meeting of the American College of Neuropsychopharmacology, Phoenix, AZ
Marseille, E., Bertozzi, S., and Kahn, J. G. (2022). The economics of psychedelic-assisted therapies: a research agenda. Front. Psych. 13:1025726. doi: 10.3389/fpsyt.2022.1025726
McCrone, P., Fisher, H., Knight, C., Harding, R., Schlag, A. K., Nutt, D. J., et al. (2023). Cost-effectiveness of psilocybin-assisted therapy for severe depression: exploratory findings from a decision analytic model. Psychol. Med. 53, 7619–7626. doi: 10.1017/s0033291723001411
McGuire, A. L., Cohen, I. G., Sisti, D., Baggott, M., Celidwen, Y., Devenot, N., et al. (2024). Developing an ethics and policy framework for psychedelic clinical care: a consensus statement. JAMA Netw. Open 7:e2414650. doi: 10.1001/jamanetworkopen.2024.14650
Mocanu, V., Mackay, L., Christie, D., and Argento, E. (2022). Safety considerations in the evolving legal landscape of psychedelic-assisted psychotherapy. Subst. Abuse Treat. Prev. Policy 17:37. doi: 10.1186/s13011-022-00468-0
Passie, T., Loizaga-Velder, A., Danforth, A., Grob, C. S., Greer, G. R., Erritzoe, D., et al. (2025). A model training curriculum for psychedelic, psycholytic, and entactogen-assisted psychotherapy. J. Psychopharmacol. :02698811241282759. doi: 10.1177/02698811241282759
Perez Rosal, S. R., La Torre, J. T., Birnkammer, S., Chernoloz, O., Williams, M. T., and Faber, S. C. (2024). Expert recommendations for Germany’s integration of psychedelic-assisted therapy. BMC Med. Educ. 24:1202. doi: 10.1186/s12909-024-06141-3
Phelps, J. (2017). Developing guidelines and competencies for the training of psychedelic therapists. J. Humanist. Psychol. 57, 450–487. doi: 10.1177/0022167817711304
Poppe, C., Villiger, D., Repantis, D., and Trachsel, M. (2025). Mitigating ethical issues in training for psychedelic therapy. Neuroethics 18, 1–13. doi: 10.1007/s12152-025-09596-3
Rosenbaum, D., Hare, C., Hapke, E., Herman, Y., Abbey, S. E., Sisti, D., et al. (2024). Experiential training in psychedelic-assisted therapy: a risk-benefit analysis. Hastings Cent. Rep. 54, 32–46. doi: 10.1002/hast.1602
U.S. Food and Drug Administration. (2023). Psychedelic drugs: Considerations for clinical investigations. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/psychedelic-drugs-considerations-clinical-investigations (accessed May 14, 2025).
Villiger, D. (2024). Personal psychedelic experience of psychedelic therapists during training: should it be required, optional, or prohibited? Int. Rev. Psychiatry 36, 869–878. doi: 10.1080/09540261.2024.2357669
Wilkinson, S. T., and Sanacora, G. (2025). Issues in clinical trial design-lessons from the FDA’S rejection of MDMA. JAMA Psychiatr. 82, 545–546. doi: 10.1001/jamapsychiatry.2025.0442
Wilson-Poe, A., Hoffman, K., Pertl, K., Luoma, J., Bazinet, A., Stauffer, C., et al. (2024). Personal psychedelic experience as a training qualification for facilitators: a thematic analysis of qualitative interviews with psilocybin experts. J. Psychoactive Drugs, 1–8. doi: 10.1080/02791072.2024.2401982
Keywords: psychedelic therapy, psychedelic medicine, psychedelic treatments, training program development, evidence-based training approaches, EMBARK model of psychedelic treatment
Citation: Brennan W, Field M, Schon C, Inamdar A and Kelman A (2025) Psychedelic training program development: lessons from the evolution of the EMBARK program. Front. Psychol. 16:1637887. doi: 10.3389/fpsyg.2025.1637887
Edited by:
Anna Maria Berardi, Université de Lorraine, FranceReviewed by:
Carlton Spotswood, MaineGeneral Health, United StatesCharlie Smith, University of Essex, United Kingdom
Copyright © 2025 Brennan, Field, Schon, Inamdar and Kelman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William Brennan, YmlsbC5icmVubmFuLjMyMTBAZ21haWwuY29t
 William Brennan
William Brennan Melissa Field2
Melissa Field2 Alex Kelman
Alex Kelman