Abstract
Cardiovascular diseases represent the number one cause of death globally, with atherosclerosis a major contributor. Despite the clinical need for functional arterial substitutes, success has been limited to arterial replacements of large-caliber vessels (diameter > 6 mm), leaving the bulk of demand unmet. In this respect, one of the most challenging goals in tissue engineering is to design a “bioactive” resorbable scaffold, analogous to the natural extracellular matrix (ECM), able to guide the process of vascular tissue regeneration. Besides adequate mechanical properties to sustain the hemodynamic flow forces, scaffold’s properties should include biocompatibility, controlled biodegradability with non-toxic products, low inflammatory/thrombotic potential, porosity, and a specific combination of molecular signals allowing vascular cells to attach, proliferate and synthesize their own ECM. Different fabrication methods, such as phase separation, self-assembly and electrospinning are currently used to obtain nanofibrous scaffolds with a well-organized architecture and mechanical properties suitable for vascular tissue regeneration. However, several studies have shown that naked scaffolds, although fabricated with biocompatible polymers, represent a poor substrate to be populated by vascular cells. In this respect, surface functionalization with bioactive natural molecules, such as collagen, elastin, fibrinogen, silk fibroin, alginate, chitosan, dextran, glycosaminoglycans (GAGs), and growth factors has proven to be effective. GAGs are complex anionic unbranched heteropolysaccharides that represent major structural and functional ECM components of connective tissues. GAGs are very heterogeneous in terms of type of repeating disaccharide unit, relative molecular mass, charge density, degree and pattern of sulfation, degree of epimerization and physicochemical properties. These molecules participate in a number of vascular events such as the regulation of vascular permeability, lipid metabolism, hemostasis, and thrombosis, but also interact with vascular cells, growth factors, and cytokines to modulate cell adhesion, migration, and proliferation. The primary goal of this review is to perform a critical analysis of the last twenty-years of literature in which GAGs have been used as molecular cues, able to guide the processes leading to correct endothelialization and neo-artery formation, as well as to provide readers with an overall picture of their potential as functional molecules for small-diameter vascular regeneration.
Introduction
Cardiovascular diseases are a group of disorders affecting heart and blood vessels that represent a significant global health problem, being the leading cause of morbidity and mortality in the world. In 2019 acute clinical events, such as heart attack and stroke, mainly caused by the atherosclerosis of coronaries, carotid, and cerebral arteries, have been the cause of 16.2% and 11.6% of the global deaths, respectively (Data from the Global Burden of disease, https://vizhub.healthdata.org/gbd-compare/). Atherosclerosis is a chronic systemic inflammatory condition with a very complex etiology in which both genetic (e.g. hypertension, diabetes, obesity, high blood cholesterol and other lipids) and environmental (e.g. lifestyles including unhealthy diet, physical inactivity, smoking, alcohol abuse) risk factors play a role (Benjamin et al., 2017). Atherosclerosis is characterized by the accumulation of lipids and fibrous elements into the subendothelial space of arteries leading to plaque formation, which, ultimately, could evolve into an acute clinical event due to plaque rupture and thrombosis (Libby et al., 2019). There are many evidences demonstrating that high levels of plasma cholesterol-rich lipoproteins (LDLs) have a causal role in the early events leading to lesion formation (atherogenesis) (Ference et al., 2017). In this respect, it is known that some vascular extracellular matrix (ECM) components mediate their entrapment into the subendothelial space, thus leading to lipids accumulation within the arterial intima.
A major commitment of vascular tissue engineering is to design biomimetic scaffolds that combine the mechanical properties of natural blood vessels with biocompatibility, controlled biodegradability, and a specific combination of molecular signals allowing for vascular regeneration. Despite the clinical need for functional arterial substitutes, success has been limited to arterial replacement of large- and medium-caliber vessels with Dacron (polyethylene terephthalate) and Goretex (expanded polytetrafluoroethylene) synthetic grafts, leaving the bulk of demand unmet. In fact, for the replacement of small-diameter vessels (< 6 mm), such synthetic grafts are often rejected in a few months due to restenosis; hence, autologous veins (e.g. the great saphenous vein) or arteries (e.g. the internal mammary artery, and the radial artery) remain the best choice, despite their limited availability. To overcome these limitations, in the last decades, different tissue engineering approaches have been applied to develop vascular substitutes using natural, synthetic or hybrid materials, some of them reaching the preclinical/clinical stages (Liu et al., 2018).
Several attempts were performed to create completely biological living substitutes, such as collagen- or fibrin-based scaffolds in combination with vascular cells, sometimes with promising results (Peck et al., 2012). The approach of tissue engineering by self-assembly (TESA), allowing for the construction of an engineered blood vessel starting from autologous fibroblasts, obtained from skin biopsy, has been proven to be useful as arterial bypass grafts in long-term animal models (L'Heureux et al., 2006) as well as arteriovenous shunt in hemodialysis patients (L'Heureux et al., 2007). However, besides the high costs of fabrication, the long manufacturing time (i.e. few months) represents a limitation in patients who need rapid intervention. Interesting results were recently obtained by using acellular tissue engineered vessels (A-TEV) (Koobatian et al., 2016; Smith et al., 2019; Smith et al., 2020). This technology relies on decellularized natural matrices functionalized with bioactive molecules, thus providing host cells with a physiological environment. Issues remain about the total removal of xenogenic material and decellularizing agents that could elicit an immune response.
Several synthetic vascular grafts have been developed with the aim to guide vascular cells to attach, proliferate, and synthesize their own ECM, in which the inflammatory/thrombotic nature of the scaffolds could be overcome by coating them with bioactive natural molecules (Pashneh-Tala et al., 2016). Fabrication methods, such as phase separation, self-assembly and electrospinning, have been used to obtain biocompatible nanofibrous scaffolds with a well-organized architecture and mechanical properties suitable for vascular tissue regeneration (Yao et al., 2020). However, several evidences have shown that naked scaffolds for small-diameter blood vessel replacement suffer from low patency rates, are pro-thrombotic, susceptible to infection and do not have growth potential for the pediatric population (Song et al., 2018). Furthermore, they often represent a poor substrate to be populated by vascular cells (Awad et al., 2018). To overcome these issues, variously functionalized bioresorbable scaffolds have been developed as temporary guides for neo-artery formation. In this respect, surface functionalization with drugs or bioactive natural molecules, including ECM components such as collagen, elastin, or glycosaminoglycans (GAGs), and growth factors, has been proven to be effective in reducing thrombotic events, enhancing endothelialization, and further promoting cell proliferation.
Here, the composition of normal blood vessel and some pathophysiological roles of vascular proteoglycans (PGs) and GAGs have been discussed. Besides, the potential of GAGs as functional molecules for vascular tissue regeneration is the main topic of this review. In this respect, we performed an in-depth literature search, using MEDLINE (PubMed), with the aim of providing readers with the main findings of both in vitro and in vivo studies on the different small-diameter vascular devices, functionalized with GAGs, developed in the last 20 years.
Significance of Proteoglycans and Glycosaminoglycans in Vascular Biology
Blood Vessel Wall Structure
Blood vessels share some common features as they all consist of three concentric layers called tunica Intima, facing the vessel lumen, tunica Media and tunica Adventitia. The Intima is composed by a continuous monolayer of endothelial cells anchored to the basement membrane, which is involved in critical events such as blood coagulation, exchange of oxygen and nutrients, vascular tone regulation through mechanosensing (induction of endothelial nitric oxide synthesis by shear stress), inflammation, and immune response (Kruger-Genge et al., 2019). Most of the Intima’s functions are modulated by the endothelial glycocalyx, a dynamic and complex gel-like network on the luminal surface of endothelial cells, consisting of PGs (syndecans, glypicans), glycoproteins, GAGs (hyaluronan, heparan sulfate and chondroitin sulfate) and soluble proteins from both plasma and endothelium. The thickness (approximately 0.5–5.0 μm) and structure of the glycocalyx vary in relation to the vascular bed, blood flow rate, pathophysiological conditions, and they result from a dynamic balance between constant biosynthesis of new glycans and shear-dependent alterations, with a considerable rate of turnover of its components (Machin et al., 2019). Derangement of the endothelial glycocalyx structure plays major roles in several pathological conditions, including cardiovascular disease (Machin et al., 2019), diabetes (Dogne et al., 2018), kidney disease (Jourde-Chiche et al., 2019), sepsis (Uchimido et al., 2019), and trauma (Tuma et al., 2016). Underneath the tunica Intima there is the Media, a thick contractile multilayer of smooth muscle cells that provide support to the vessel as well as participate in regulating both blood flow and pressure. The Adventitia, with its collagenous ECM, is primarily responsible for the tensile strength of blood vessels. The three layers are separated from each other by an internal elastic lamina (between Intima and Media) and an external elastic lamina (between Media and Adventitia), consisting of fibers that provide elasticity to the vessel wall. In this scenario, the ECM in which vascular cells are immersed, mainly composed of collagens, elastin, fibronectin, laminins, glycoproteins, and PGs, not only provides the vascular wall with its mechanical properties, but also plays crucial roles in vascular cells homeostasis and pathogenesis. Therefore, the vascular wall is a very complex multilayer structure, in which different resident and circulating cell types interact with each other and with the ECM. Designing a substitute able to mimic the mechanical properties as well as the physiological functions of the vascular wall represents a challenge for tissue engineering.
Proteoglycans and Glycosaminoglycans of the Vascular Wall
PGs are hydrophilic molecules, which provide the vasculature with viscoelasticity and turgor. These molecules participate in a number of vascular events such as regulation of vascular permeability, lipid metabolism, hemostasis, and thrombosis, as well as interact with vascular cells, growth factors, and cytokines, in order to modify vascular cell adhesion, migration, and proliferation. Some PGs exert specific functions in their soluble forms following cleavage by proteolytic enzymes (Lepedda et al., 2021). The amount of PGs in the artery wall is physiologically low, but increases deeply during the early phases of atherosclerotic lesion formation. Many studies have shown that PGs are involved in retention of cholesterol-rich lipoproteins and other serum components, ECM metabolism, and crosstalk with inflammatory cells that extravasate to the subendothelial space. Readers are referred to the excellent review by Wight TN (Wight, 2018) for a more detailed discussion on this topic.
PGs have common structural features, as they all consist of a core protein covalently linked to one or more GAG chains, primarily responsible for their biological properties (Raman et al., 2005). GAGs are a family of anionic heteropolysaccharides that differ in terms of type of repeating disaccharide units, chains length, charge density, degree of sulfation, and hexuronic acid epimerization, found in connective tissues as well as in biological fluids such as plasma and urine (Vynios et al., 2002). The repeating disaccharide units are composed by a N-acetylated hexosamine (N-acetylgalactosamine or N-acetylglucosamine) and a hexuronic acid (glucuronic acid or its carbon-5 epimer iduronic acid) or galactose (in keratan sulfate). GAGs are key structural and functional components of the ECM of connective tissues, playing numerous biological roles, including embryonic development, ECM assembly and regulation of cell signaling in various physiological and pathological conditions. Indeed, their numerous functions are the result of their large structural heterogeneity, 202 unique disaccharide units have been identified in mammals (Clerc et al., 2019), responsible for the binding of a plethora of proteins including cytokines and chemokines, enzymes and enzyme inhibitors, ECM proteins, and membrane receptors (Kjellen and Lindahl, 2018). A comprehensive GAG-interactome composed of 827 proteins has been recently published (Vallet et al., 2021). Six main classes of GAGs have been described so far: hyaluronan or hyaluronic acid (HA), chondroitin sulfate (CS) and dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), and heparin (Hep) (Figure 1); all GAG classes were found in normal and diseased arteries (Wight, 1980).
FIGURE 1

Structures of the repeating disaccharide units representative of the six GAGs classes [modified from (Vynios et al., 2002)]. Both DS and HS/Hep are co-polymers of two types of disaccharide repeats where glucuronate is variably substituted by its carbon-5 epimer iduronate. Hep has both a higher degree of sulfation and epimerization than HS. Except for HA, sulfation may occur in several positions, thus giving these glycans a characteristic high negative charge. Saccharides are reported as chair conformations. Configurations of the O-glycosidic bonds are reported in red (within disaccharide units) and in green (between adjacent disaccharide units). R1 = SO3−; R2 = COCH3/SO3−.
Besides HA, that is neither sulphated nor covalently linked to a protein core, GAGs together with their protein cores form distinct PG families. PGs can be found in the ECM, in the basement membrane (pericellular PGs), associated with the cell surface (transmembrane, GPI-anchored), or inside the cells (serglycin, the only currently known) (Iozzo and Schaefer, 2015). To date, more than 20 different PGs isoforms have been identified in normal and diseased blood vessels (Wight, 2018). In vascular tissue, versican is the main CS-PG (Yao et al., 1994), whereas DS chains are found linked to decorin and biglycan, two homologous small leucine rich repeat PGs (SLRPs) (Hultgardh-Nilsson et al., 2015). KS is present in fibromodulin and lumican, two more SLRPs (Hultgardh-Nilsson et al., 2015), whereas HS is the main GAG in perlecan (Kinsella and Wight, 2005), syndecans (Gopal, 2020), and glypicans (Filmus et al., 2008). Among them, versican, decorin, biglycan, as well as fibromodulin and lumican, are classified as extracellular PGs, perlecan is a pericellular PG, whereas syndecans and glypicans are localized on the cell surface (Theocharis et al., 2016) (Figure 2).
FIGURE 2

Schematic representation of the main vascular PGs according to their localization. Three main classes are reported: cell-associated, pericellular, and extracellular proteoglycans. Protein moieties are shown as orange backbones (versican G1 domain is highlighted in red).
With the exception of HA, GAGs are polymerized in the Golgi apparatus, starting from a tetrasaccharide linker consisting of xylose–galactose–galactose–uronic acid residues, by the sequential and repetitive addition of constituent monosaccharide residues. Post-synthetic chain modifications, such as epimerization of GlcA to iduronic acid and sulfation at specific positions, occur during polymerization, whereas selective removal of 6-O sulfates by specific sulfatases may occur in the extracellular space. Their structural heterogeneity, in terms of chain length, degree of iduronation, degree and pattern and sulfation, which is further increased by dynamic modifications in response to cellular and environmental stimuli, is so significant that, virtually, there are not two identical glycosaminoglycans in the body.
HA is a major constituent of the pericellular matrix and a key player in maintaining endothelial glycocalyx integrity, providing structural support and acting as signaling molecule by binding specific receptors on the cell surface. HA is synthesized by membrane-bound synthases as a high molecular weight GAG (up to 3–4 MDa), consisting of repeating residues of GlcNAc and GlcA linked each other by β-1,3- and β-1,4-glycosidic bonds. The synthesis takes place on the inner side of the plasma membrane and, during HA elongation, chains are extruded through pore-like structures to the cell surface without any further post-synthetic modifications (i.e. sulfation or epimerization) (Moretto et al., 2015). HA accumulates in aged vessels, stimulating vascular smooth muscle cells dedifferentiation and neointima formation (Moretto et al., 2015). Furthermore, during glycocalyx shedding, HA cleavage by hyaluronidase one into pro-inflammatory low molecular weight (<500 kDa) fragments contributes to endothelial derangement (Girish and Kemparaju, 2007; Vigetti et al., 2014).
Nanofibrous Scaffold Design for Vascular Tissue Engineering
A broad range of fabrication technologies are in use for the production of small diameter vascular grafts. In this section, the most used ones will be described, along with the materials used for their manufacture (Figure 3).
FIGURE 3

Main methods for the fabrication of small caliber vascular grafts.
Solvent Casting
Solvent casting is one of the earliest and most widely used techniques. A liquid solution of the polymer of choice for the fabrication of the final vascular graft is poured into a cylindrical mold containing an inner rod. The solution is then solidified and the system is disassembled. If polymers are dissolved in volatile solvents, then the solution is cured and the solvent is left to evaporate. Thermally induced phase separation (TIPS) can be used to introduce porosity to the scaffold, in order to improve cell infiltration. Another strategy to further modulate scaffolds’ porosity is to freeze-dry the polymer solution, by which ice crystals are formed and then sublimated leaving a pore behind. The rate of temperature decrease can influence pore size and pore connectivity. By this approach, scaffolds of silk fibroin loaded with extracellular vesicles isolated from Adipose-derived Mesenchymal Stromal Cells (ADMSC) were fabricated (Cunnane et al., 2020). These grafts presented 100% patency at the early stages of the study (4 weeks), compared to the bare scaffold and scaffold seeded with ADMCS prior to implantation. To improve mechanical strength, the lyophilized silk fibroin scaffolds were reinforced with a layer of electrospun polycaprolactone (PCL) (Gupta et al., 2020).
Porous structures can be formed also by using so-called porogens, by the salt-leaching method. As an example, salt is introduced into the polymer solution, which is then cured and immersed in water to promote the dissolution of the salt, resulting in pore formation (Figure 4). This technique was used to fabricate, for example, elastin-like recombinamer (Fernández-Colino et al., 2019) and fast degrading poly(glycerol sebacate) (PGS) (Wu et al., 2012) scaffolds with promising results. Furthermore, the modification of these scaffolds with PGS derivatives with slower degradation kinetics were shown to improve outcomes in vivo (Fu et al., 2020).
FIGURE 4

Fabrication method of porous tubular scaffolds from elastin-like recombinamers by salt leaching/gas foaming technique and electrospinning [modified from (Fernández-Colino et al., 2019)].
Another strategy to produce porous scaffolds is to use inverted colloid crystals. Microparticles of a material that is immiscible in the polymer of choice are fabricated, tightly packed in the molds, and then fused by annealing, before adding and curing the main polymer. Finally, the microparticles are dissolved, leaving a scaffold with highly interconnected pore network (Joao et al., 2014). While these techniques offer scalability and fast production, they fail to mimic the complexity of the ECM and the microenvironment in which cells are embedded.
Electrospinning
Electrospinning has emerged as one of the most prolific techniques to fabricate vascular grafts, because of its ability to form fibrous scaffolds that replicate the approximate diameter of ECM fibers and scalability. Fibers form on a grounded collecting mandrel, or static flat stage, when a polymer solution is ejected through a charged needle. The fibers obtained with this technique can range from the nano-to the microscale, depending on polymer concentration, flow rate, applied voltage, and air gap between the spinneret and the fabrication target. The rotation speed of the collecting mandrel can be tuned to obtain fibers oriented parallel to each other or circumferentially oriented, in a similar way to the ECM in the media layer (Uttayarat et al., 2010). At low (or no) rotational speeds, the fibers are deposited in a random manner (Figure 5).
FIGURE 5
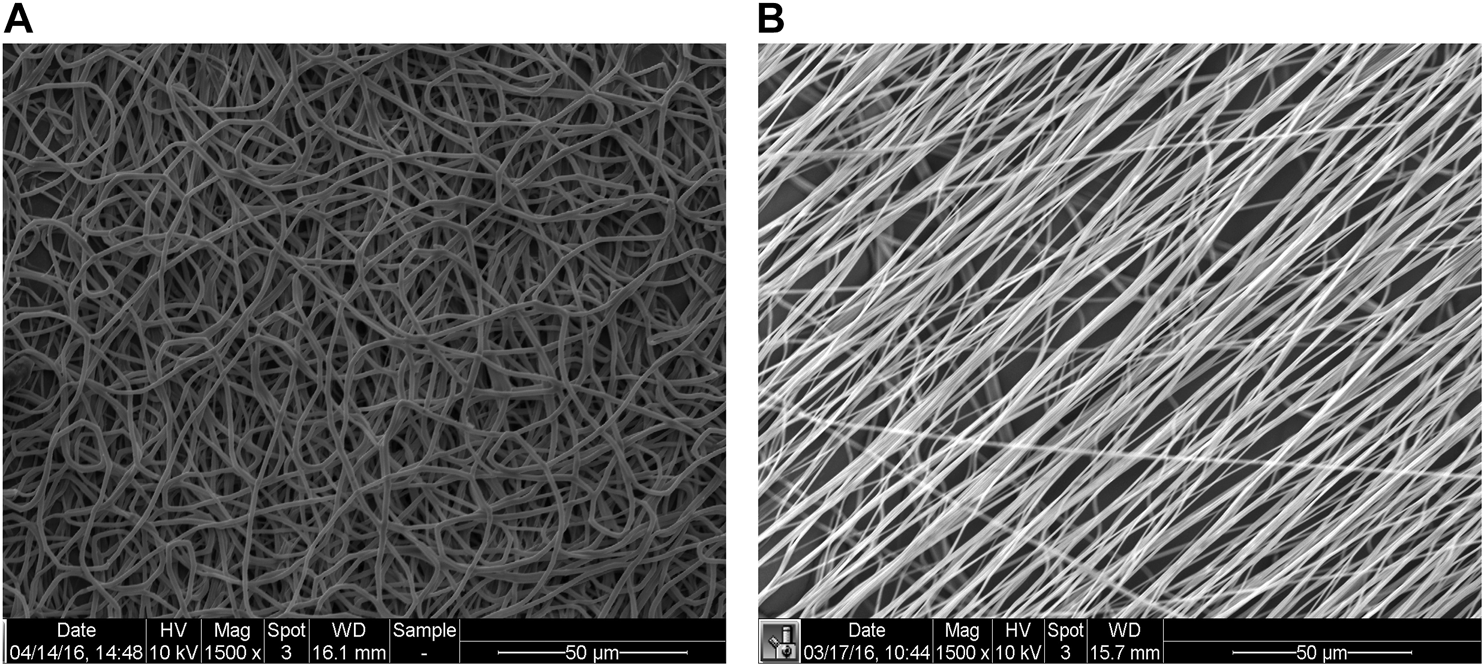
Representative scanning electron microscopy (SEM) images of random (mandrel speed = 500 rpm) and aligned (mandrel speed = 4000 rpm) PCL fibers (panels A and B, respectively) [modified from (Idini et al., 2019)].
Several polymers, either of natural (e.g. fibrinogen, collagens, silk fibroin, chitosan, alginate) or synthetic [e.g. polyurethane, PCL, polylactic acid, poly(propylene carbonate)] origin, have been investigated to produce matrices composed of nanofibers for biomedical applications (Agarwal et al., 2008). Among the former, silk fibroin represents a promising substrate for vascular tissue engineering applications, due to its mechanical properties, biocompatibility and slow degradation (Marelli et al., 2010; Catto et al., 2015; Marcolin et al., 2017).
Multimaterial scaffolds can be fabricated by mixing various polymers into one syringe, by simultaneously applying voltage to different needles or by sequentially spinning different materials. The porosity of scaffolds can be improved by co-electrospinning a polymer, such as PCL, gelatin or PGS, with polyvinyl alcohol, which can be dissolved and washed away after scaffold fabrication (Khosravi et al., 2016; Tan et al., 2016). More complex scaffolds can be obtained by sequential spinning of the same material with different molecular weight (de Valence et al., 2012) or by sequentially spinning different materials, such as PCL, elastin and collagen, to obtain a tri-layered graft (McClure et al., 2010). Often, more bioactive materials have weaker mechanical properties and, thus, synthetic materials have to be added to improve their mechanics. One way to implement this is by co-axial electrospinning in a core-shell manner, in which a strong polymer, such as PCL, is used in the core and a bioactive material, such as collagen, as the shell (Duan et al., 2016). Scaffolds can be mechanically reinforced also by electrospinning an outer layer of PCL, which acts as a sheath. By this approach, Elliott et al. fabricated aligned electrospun fibrin scaffolds with a coverage of PCL, to avoid suture rupture (Elliott et al., 2019).
To improve mechanical properties of electrospun scaffolds, hybrid approaches, combining electrospinning with fused deposition modeling (FDM) (Centola et al., 2010; Wu et al., 2020) or melt electrowriting (MEW) (Brown et al., 2011) were explored too. MEW combines electrospinning and FDM, by applying a voltage to a needle and grounding the collecting surface, mostly using molten polymers and avoiding the use of solvents. MEW has been used both to include reinforcements to electrospun meshes (Jungst et al., 2019) as well as to create standalone scaffolds (Saidy et al., 2020). Other techniques were combined with electrospinning for the fabrication of vascular grafts, including TIPS of thermoplastic polyurethane (Mi et al., 2016) or poly(ester-urethane)urea (Soletti et al., 2010), or braiding and TIPS of thermoplastic polyurethane and silk (Mi et al., 2015).
3D Printing
3D printing relies on the accurate deposition of material in the X-Y plane while also moving, gradually, in the Z direction. 3D printing allows users to input patient-specific data and can recreate the tortuosity and multiple branching of small caliber arteries, such as the coronaries. Techniques such as 2-photon (or multiphoton) polymerization or stereolithography use light to polymerize a resin material, in a bath, by using either a focused laser or a mask, and then illuminating the whole layer. Examples of materials used are methacrylated gelatin (GelMA), methacrylated HA (Thomas et al., 2020), polyethylene glycol diacrylate, polypropylene fumarate (Melchiorri et al., 2016), polyester urethane and poly (caprolactone-co-trimethylenecarbonate) diacrylate resins (Baudis et al., 2011; Kuhnt et al., 2019; Baker et al., 2020), methacrylated poly(ethylene glycol-co-depsipeptide) (Elomaa et al., 2015).
3D bioprinting allows the spatial distribution of different materials and cell populations. By co-axially bioprinting endothelial cells (ECs) in a fugitive ink (such as gelatin or Pluronics) in the inner core and GelMA or decellularized ECM hydrogel with smooth muscle cells (SMCs) on the outer shell and then liquefying the fugitive ink upon temperature switch, a hollow endothelialized structure was obtained (Cui et al., 2019; Gao et al., 2019). This approach was developed after initial work based on co-axial printing of endothelial cells in an alginate/ECM bioink in the shell and a core consisting of Ca-containing Pluoronics fugitive ink (Gao et al., 2018) (Figure 6).
FIGURE 6

3D bioprinting of vasculature using core-shell approach, where endothelial cells are suspended in an alginate-ECM bioink as the shell and the core is a fugitive bioink composed of Pluronic F127 containing Ca2+ ions (CPF127) [modified from (Gao et al., 2018)].
When using soft materials, such as hydrogels, traditional extrusion bioprinting might be unsuitable to create large constructs before they collapse. Strategies have been developed to overcome this limitation, by bioprinting inside a supporting bath consisting of the cross-linker and particles or gels that exhibit shear-thinning properties, which is discarded after printing (Hinton et al., 2015; Senior et al., 2019). These technique modifications have enabled the printing of large-scale cardiovascular constructs at high-resolution (Mirdamadi et al., 2020).
Cellular Self-Assembly
Trying to overcome issues related to both scaffolds’ biodegradation by the cells and undesired immune responses, scaffold-free strategies have been developed. These vascular grafts are produced by taking advantage of the ability of cells to self-assemble and secrete ECM.
Early work by L’Heureux demonstrated that long-term culture of fibroblasts produces an ECM that could be rolled into a tube able to withstand the physiological pulsatile flow (L'Heureux et al., 1998). Further studies showed this approach effective for arterial bypass grafting in long-term animal models (L'Heureux et al., 2006) as well as for arteriovenous shunt in hemodialysis patients (L'Heureux et al., 2007). However, a major downside of this strategy is the long culture period. The research team has recently modified its protocol by making threads from the ECM laid by the fibroblasts, which were knitted into tubes (L’Heureux, 2020; Magnan et al., 2020) (Figure 7). This method allows to produce a fully human tissue graft, with no foreign material.
FIGURE 7

A thread (left) and a woven vessel (right) made from human Cell-assembled Extracellular Matrix [modified from (L’Heureux, 2020)].
Another approach to obtain implantable grafts was to seed SMCs onto fast degrading polyglycolic acid meshes and then to mature these constructs in flow bioreactors (Niklason et al., 1999; Niklason et al., 2001). To speed the process and avoid the need of harvesting autologous cells, the strategy was shifted to decellularizing the constructs once mature (Dahl et al., 2003). Such grafts were implanted in end-stage dialysis patients to treat arteriovenous fistulas (Lawson et al., 2016; Kirkton et al., 2019), as well as in patients with peripheral arterial disease (Gutowski et al., 2020). Exploiting the self-assembly approach, Itoh et al. produced hundreds of cell spheroids from a cell suspension of ECs, SMCs, and fibroblasts, and then placed them onto a needle array using a 3D printer printhead. Once the spheroids had fused into a continuous tissue, the needle array was removed and the construct placed in a perfusion reactor for maturation (Itoh et al., 2015). All these techniques require long culture times in highly controlled facilities, which makes these grafts expensive.
Decellularized Matrices
Decellularized vascular grafts theoretically mimic the content and structure of native arteries more accurately than synthetic constructs. Decellularization consists in removing all cellular content while trying to minimally disrupt the ECM, using physical, chemical and/or biological methods and agents. Arteries from cadaveric donors can be employed (Madden et al., 2004; Olausson et al., 2012). Alternatively, there is the possibility of using xenogenic blood vessels, which should have fewer “availability” problems. Decellularized porcine (Tillman et al., 2012), bovine (Daugs et al., 2017) and ovine (Mancuso et al., 2014) arteries have been used as vascular grafts (Figure 8).
FIGURE 8

Hematoxylin and eosin staining of native bovine pericardium (A) and decellularized bovine pericardium before (B) and after (C) 7 days of culture with bovine fibroblasts. Magnification ×20 [from (Cigliano et al., 2012)].
Decellularized matrices can also be functionalized with bioactive molecules, thus facilitating the repopulation processes as well as reducing adverse events, such as restenosis and thrombosis. In this respect, interesting results were recently obtained by Andreadis’ team that implanted a cell-free small-diameter arterial graft based on small intestinal submucosa (SIS). After coating with Hep-bound vascular endothelial growth factor, implantation in the mouse abdominal aorta and in the sheep carotid artery, showed successful endothelialization. Notably, once integrated, both mechanical properties and vascular contractility of this graft were comparable to native arteries (Koobatian et al., 2016; Smith et al., 2019; Smith et al., 2020).
Although issues were raised on the risk of inflammatory/immune reactions elicited by cell debris, including DNA fragments (Zheng et al., 2005) and alpha-Gal epitopes (Naso et al., 2012), in the last years, decellularized matrices have been widely used in different fields of tissue regeneration. Currently, there is no consensus on the criteria to be used for the decellularization methods (Ji et al., 2019).
Application of Glycosaminoglycans to Vascular Tissue Engineering
Substitution of small-diameter vessels suffers of major problems regarding low-patency rate due to over-proliferation of smooth muscle cells (intimal hyperplasia) and thrombosis due to a lack of a functional endothelium lining the lumen of the graft. Hence, the need to design “smart substitutes” containing the chemical cues able to induce the population of the scaffold by host cells and the formation of a physiological artery wall.
Because of their pleiotropic functions and their physicochemical properties, GAGs have been extensively used in many tissue engineering applications including wound healing, as well as bone, cartilage, muscle, liver and nerve regeneration, as very recently reviewed by Sodhi H and Panitch A (Sodhi and Panitch, 2020). Furthermore, in the last 20 years, many studies have addressed the issues on small-diameter vascular regeneration by combining the most recent fabrication methods with the biofunctionalization with GAGs for the development of effective vascular grafts. We have tried to sum up the main findings of these substantial studies in two tables, according to the typology of GAG used, i.e. high and low molecular weight HA and HA derivatives (HYAFF-11) (Table 1), and Hep (Table 2). Also CS have been employed in a few studies for scaffolds functionalization (see below).
TABLE 1
| References | Scaffold material and manufacturing method | Development level | Main findings |
|---|---|---|---|
| Turner et al. (2004) | HYAFF-11 non-woven scaffolds: Unpressed and pressed felts | In vitro (human saphenous vein endothelial cells) | Pressed felts: Complete endothelialization after 20 days; deposition of a subendothelial matrix containing laminin, fibronectin, type IV and type VIII collagen |
| Remuzzi et al. (2004) | HYAFF-11 non-woven meshes as sheets or 3D tubular scaffolds obtained by wrapping the former around a cylindrical mandrel after 7 days of culture | In vitro (human cell line and primary pig aortic smooth muscle cells) | Tubular scaffolds highly cellularized within the wall thickness but with lower mechanical resistance than porcine coronary arteries |
| Lepidi et al. (2006a) | HYAFF-11 tubes obtained by coagulation of a HYAFF-11/dmso solution around a cylindrical bar (2 mm diameter) in an ethanol bath | In vivo (implantation in the abdominal aorta of 15 rats) | Complete regeneration of a newly formed vascular tube at day 90; no signs of inflammation, stenoses or aneurysms; all animals survived during the 90 days follow up |
| Lepidi et al. (2006b) | HYAFF-11 tubes obtained by coagulation of a HYAFF-11/dmso solution around a cylindrical bar (2 mm diameter) in an ethanol bath | In vivo (implantation in the abdominal aorta of 30 rats) | Complete endothelialization of the tube’s luminal surface; complete vascular wall regeneration 15 days after surgery; complete degradation of the construct 4 months after implantation |
| Arrigoni et al. (2006) | HYAFF-11 non-woven meshes as sheets or 3D tubular scaffolds obtained by wrapping the former around a cylindrical mandrel after 7 days of culture in a medium supplemented with 50 mg/ml of sodium ascorbate | In vitro (primary pig aortic smooth muscle cells) | Sodium ascorbate improved cell proliferation and ECM synthesis as well as mechanical properties of the vascular construct |
| Zavan et al. (2008) | HYAFF-11 tubes obtained by coagulation of a HYAFF-11/dmso solution around a cylindrical bar (4 mm diameter) in an ethanol bath | In vivo (implantation in the carotid artery of 10 pigs) | Confirmation of the potential of hyaluronan-based graft to guide the development of a well-functioning neoartery with organized layers of elastin fibers, 5 months post-surgery; 3 cases of partial or complete occlusion by intimal hyperplasia and graft thrombosis |
| Pandis et al. (2010) | HYAFF-11 tubes obtained by coagulation of a HYAFF-11/dmso solution around a cylindrical bar (2 mm diameter) in an ethanol bath | In vivo (implantation in the vena cava of 15 rats) | Complete vein wall regeneration at day 30; complete reabsorption of the graft 4 months after surgery |
| Pandis et al. (2011) | HYAFF-11 patches (0.1 mm thickness) | In vivo (implantation in the abdominal aorta of 20 rats, rectangular breach of 1 mm × 5 mm) | Almost complete degradation of the scaffold and replacement by a neoartery wall composed of endothelial cells, smooth muscle cells, collagen, and elastin fibers organized in layers, after 16 weeks |
| Du et al. (2011) | Electropun aligned nanofibrous PCL scaffolds functionalized with LMW-HA by using EDC/NHS following aminolysis with 1,6-hexanediamine | In vitro (HUVECs) | The combination of aligned PCL fibers and LMW-HA promotes and guides the formation of a polarized functional endothelium |
| Zhu et al. (2014) | Human-like collagen/hyaluronic acid (HA MW 100,000–110,000 Da) composite disks obtained, at different HLC/HA ratios, by cross-linking with glutaraldehyde followed by freeze-drying | In vitro (human endothelial cells) and in vivo (subcutaneous implantation in 12 mice) | Among the different composites assessed, the 10/1 HLC/HA composite showed higher porosity, better mechanical properties and excellent biocompatibility |
| Yuan et al. (2016) | Highly aligned PLLA/HA (HA MW > 400 KDa) core-shell nanofibers (jet coaxial-electrospinning) crosslinked with glutaraldehyde and hydrochloric acid | In vitro (human umbilical arterial smooth muscle cells) and in vivo (implantation in the carotid artery of 6 rabbits) | Synergistic effect of nanotopographical and biochemical cues in promoting scaffold population by vSMCs and synthesis of elastin. Circumferentially aligned HA/PLLA nanofibers were effective in maintaining patency and promoting vascular regeneration during 6 weeks after surgery |
| Kang et al. (2019) | Electrospun scaffolds of type I collagen glycosilated with HA oligomers by reductive amination (using sodium cyanoborohydride), crosslinked with glutaraldehyde | In vitro (porcine iliac artery endothelial cells) | Endothelial cells proliferation was promoted by HA oligomers and inhibited by high molecular weight HA. The scaffolds had no detectable degree of hemolysis and coagulation |
| Jia et al. (2019) | Electrospun scaffolds of type I collagen glycosilated with HA oligomers using EDC/NHS, crosslinked with glutaraldehyde | In vitro (porcine iliac artery endothelial cells) | Potential of the collagen–HA electrospun nanofibers as the vascular inner-layer scaffold |
Hyaluronan-based vascular constructs for small-caliber artery grafting.
HYAFF-11: 100% benzyl ester hyaluronan-based biomaterial produced by Fidia Advanced Biopolymers (Abano Terme, Italy).
TABLE 2
| References | Scaffold material and manufacturing method | Development level | Main findings |
|---|---|---|---|
| Conklin et al. (2002) | Decellularized porcine carotid artery covalently linked with heparin using EDC | In vivo (implantation in the carotid artery of 2 dogs) | Excellent mechanical properties, antithrombogenicity, and tissue compatibility; effective scaffold population by both smooth muscle cells and endothelial cells within 2 months post-implantation |
| Tiwari et al. (2002) | Poly(carbonate-urea)urethane graft (MyoLink™) functionalized with arginine-glycine-aspartate (RGD) or/and hep | In vitro (HUVECs under pulsatile flow for 6 h) | RGD/Hep functionalization improved cell retention and metabolic activity with respect to native MyoLink |
| Tamura et al. (2003) | Heparinized decellularized porcine carotid artery | In vivo (implantation in the abdominal aorta of a dog model) | Sufficient mechanical properties and successful replacement by the host cells in 18 weeks |
| Conklin et al. (2004) | Decellularized porcine carotid artery, covalently linked with hep using EDC, incubated with basic fibroblast growth factor | In vitro (human microvascular endothelial cells or canine peripheral blood endothelial progenitor cells cultured in static and dynamic conditions) | bFGF coating on the Hep-bound decellularized grafts significantly increases attachment and proliferation of the seeded cells that remain stable under perfusion conditions |
| Zhou et al. (2009) | Decellularized canine carotid artery coated with hep (EDC/NHS) and coated with vascular endothelial growth factor | In vitro (HUVECs) and in vivo (implantation in the carotid artery of 15 dogs) | Complete endothelium regeneration and higher patency rate than the nonmodified scaffold after 6 months implantation |
| Centola et al. (2010) | Electrospun PLLA/Hep scaffolds with an outer layer of PCL by FDM | In vitro (hMSCs) | Drug delivery system with a microenvironment able to induce endothelial differentiation |
| Saitow et al. (2011) | Heparinized silk-based construct | In vitro (human aortic smooth muscle cells) | Stimulation of elastogenesis |
| Ye et al. (2011) | FGF2-loaded electrospun Hep–PCL vascular scaffolds | In vitro (endothelial cells) | In vitro (endothelial cells) |
| Yu et al. (2012) | Electrospun microfibres scaffolds of PLLA-PCL blends functionalized with Hep (EDC/NHS) and heparin-bound stromal cell-derived factor-1α (SDF-1α) | In vivo (implantation in the carotid artery of rats) and in vitro (culturing of explants) | Effective recruitment of endothelial progenitor cells (EPCs) to the luminal surface of the grafts, which differentiated into endothelial cells, and of smooth muscle progenitor cells, which differentiated into smooth muscle cells |
| Wu et al. (2012) | Poly (glycerol sebacate) core surrounded by an electrospun PCL sheath, coated with heparin | In vivo (implantation in the abdominal aorta of 27 rats) | Three months after implantation, the neoarteries resembled native arteries in the following aspects: Regular, strong and synchronous pulsation; a confluent endothelium and contractile smooth muscle layers; expression of elastin, collagen and glycosaminoglycan; and tough and compliant mechanical properties |
| Ye et al. (2012) | Electrospun Hep–PCL nonwoven tubular scaffolds | In vitro (human endothelial cells) and in vivo (implantation in the femoral artery of 2 dogs) | Low protein absorption and good cell biocompatibility; presence of endothelial cells monolayer and extracellular matrix 1 month after surgery |
| Zhou et al. (2012) | Decellularized canine carotid artery coated with Hep (EDC/NHS) cultured with canine endothelial progenitor cells in a custom-made bioreactor | In vivo (implantation in the carotid artery of 20 cell-donor dogs) | Excellent biocompatibility and high patency rate at 3 months post-implantation |
| Lu et al. (2013) | Polyurethane-collagen/Hep-conjugated polycaprolactone double-layer small-diameter vascular graft | In vitro and in vivo (implantation in the femoral artery of dogs) | Good biocompatibility and high patency at 8 weeks after surgery |
| Wang et al. (2013) | Hep-bound P(LLA-CL)/P(LLA-CL) double-layer small-diameter vascular graft | In vitro (endothelial cells from canine femoral vein) and in vivo (implantation in the femoral artery of 20 cell-donor dogs) | Biomechanical properties similar to those of canine femoral arteries; satisfactory endothelialization in vitro |
| Huang et al. (2013) | Hep-bound P(LLA-CL)/P(LLA-CL) double-layer small-diameter vascular graft pre-endothelialized | In vitro (endothelial cells from canine femoral vein) and in vivo (implantation in the femoral artery of 8 cell-donor dogs) | The pre-endothelialization has better mechanical properties and cellular compatibility than the simple heparinization |
| Hoshi et al. (2013) | Heparinized POC-modified ePTFE grafts | In vitro (platelets, primary endothelial cells, blood outgrowth endothelial cells, and smooth muscle cells) | Reduced platelet adhesion and inhibition of blood clotting; support for endothelial cells adhesion, viability, proliferation, NO production, and expression of specific markers. Smooth muscle cells increased expression of α-actin and decreased proliferation |
| Pitarresi et al. (2014) | Electrospun PHEA-eda-g-pla/pcl scaffold functionalized with Hep (EDC/NHS) | In vitro (human vascular endothelial cells, ECV 304) | Effective retention of bFGF and promotion of ecs growth |
| Yao et al. (2014) | Co-electrospun PCL/Chitosan hybrid grafts functionalized with Hep (ionic bonding with chitosan) | In vitro (HUVECs and human SMCs) and in vivo (implantation in the abdominal aorta of 9 rats) | Promotion of HUVECs growth and moderate inhibition of hSMCs proliferation; optimal anti-thrombogenic effects and enhanced in situ endothelialization at 1 month after surgery |
| Wang et al. (2015) | Small-diameter tubular PLLA/PLCL scaffolds obtained by thermally induced phase separation functionalized with Hep (EDC/NHS) | In vitro (pig iliac endothelial cells) and in vivo (subcutaneous implantation in 4 rabbits) | 60% PLCL promising scaffold for engineering small-diameter blood vessel in terms of biomechanical properties; heparinization provided higher hydrophilicity, lower protein adsorption, and better in vitro anticoagulation properties; good cellular attachment, spreading, proliferation, and phenotypic maintenance |
| Jiang et al. (2015) | Decellularized rat aortas infused with poly(1,8 octanediol citrate) (POC) and functionalized with Hep (EDC/NHS) | In vitro (platelets, HUVECs and human SMCs) | Reduced platelet adhesion and inhibited whole blood clotting; support for endothelial cell adhesion |
| Dimitrievska et al. (2015) | Click-coated, heparinized, decellularized pig aortic graft | In vitro (platelets, HUVECs) | Reduced platelet adhesion and thrombogenicity; supported endothelial cell adhesion and proliferation |
| Chen et al. (2015) | Electrospun poly(l-lactic acid-co-ε-caprolactone) (P(LLA-CL)) core–shell nanofibers loaded with hep and vascular endothelial growth factor (VEGF) | In vitro (platelets, endothelial progenitor cells) | Effective antithrombotic potential and promotion of endothelial progenitor cells growth |
| Koobatian et al. (2016) | Acellular tissue engineered vessel based on small intestinal submucosa functionalized sequentially with Hep (EDC/NHS) and VEGF | In vivo (implantation into the carotid artery of an ovine model) | Complete endothelialization and formation of a medial layer of circumferentially aligned smooth muscle cells; high elastin and collagen content; impressive mechanical properties and vascular contractility comparable to native arteries |
| Shafiq et al. (2016) | Electrospun poly (l-lactide-co-ε-caprolactone) scaffolds conjugated with Hep and substance p | In vitro (platelets, human bone marrow-derived mesenchymal stem cells) and in vivo (subcutaneous scaffold implantation in 12 rats) | Effective host cell infiltration, neotissue formation, collagen and elastin deposition |
| Duan et al. (2016) | Coaxially electrospun PCL/collagen core–shell nanofibrous scaffolds crosslinked by genipin and functionalized with Hep | In vitro (mouse fibroblast L929 cells, ECs and SMCs) | Good biocompatibility; support for vascular cells attachment and growth on its surface, and for the infiltration of SMCs inside |
| Tan et al. (2016) | Co-electrospun PCL/gelatin/polyvinyl alcohol functionalized with Hep | In vitro (platelets, HUVECs) and in vivo (subcutaneous implantation in rats) | Good mechanical properties; antithrombogenic; enhanced growth of endothelial cells |
| Zhang et al. (2016) | Electrospun PCL/PCL2K-N3 functionalized with alkynyl-Hep | In vitro (rat VSMCs) | Reduced platelet adhesion; inhibition of VSMCs proliferation in a dose-dependent manner and promotion of the transition from synthetic phenotype to contractile one; moderate Hep density induces the formation of a confluent layer of contractile smooth muscle cells |
| Gong et al. (2016) | Decellularized rat aortas coated with electrospun PCL, with a heparinized luminal surface | In vivo (implantation in the abdominal aorta of 12 rats) | Satisfactory patency for up to 6 weeks; successful prevention of the occurrence of vasodilation and aneurysm formation after transplantation and reduced inflammatory cells infiltration |
| Fang et al. (2016) | Electropun fibrous scaffolds of elastic poly (ester urethane)urea with disulfide and amino groups (PUSN) orthogonally functionalized with Hep (EDC/NHS) and endothelial progenitor cells (EPC) recruiting peptide (TPS) | In vitro (platelets, mouse bone marrow-derived EPCs) | Reduced platelet deposition and improved EPCs proliferation |
| Jiang et al. (2016) | Decellularized rat aorta functionalized with CBP-Hep (CBP, collagen binding peptide) | In vitro (platelets, HUVECs) | Reduced platelet binding and whole blood clotting; stabilization of long-term endothelial cell attachment to the lumen of ECM-derived vascular conduits |
| Li et al. (2017) | Polycarbonate polyurethane scaffold treated with NH3 plasma and functionalized with Hep (EDC/NHS) | In vitro (mouse embryonic fibroblasts) and in vivo (implantation in the carotid artery of rabbits) | Improved in vitro anticoagulation and excellent biocompatibility |
| Zamani et al. (2017) | Composite silk-based vascular scaffold functionalized with Hep using hydroxy-iron complexes (HICs) as linkers | In vitro (HUVECs) and in vivo (subcutaneous implantation in 12 rats) | Good biomechanical properties (flexibility, suture retention strength, burst pressure, and compliance); sustained antithrombogenicity, cytocompatibility and nonhemolytic properties |
| Hsieh et al. (2017) | Electrospun poly-l-lactide-co-caprolactone (PLCL) microfiber vascular grafts aminolyzed with plasma treatment or fmoc-peg-diamine insertion for Hep conjugation | In vivo (subcutaneous implantation in rats) | Plasma treatment resulted in significantly higher initial hep density and higher Hep stability on PLCL microfibers than fmoc-peg-diamine treatment as well as better mechanical properties; Hep coating with both methods promoted cell infiltration |
| Braghirolli et al. (2017) | Electrospun polycaprolactone scaffolds functionalized with Hep (EDC/NHS) and absorbed VEGF | In vitro [human EPCs or mesenchymal stem cells (MSCs)] | Mechanical properties compatible with the native arteries; antithrombogenic properties and increased EPC proliferation, favoring the formation of the endothelial layer |
| Qiu et al. (2017) | Electrospun polycarbonate-urethane (PCU) nanofibrous grafts treated with plasma to conjugate Hep via end-point immobilization | In vivo (implantation in a rat common carotid artery anastomosis model) | High patency rate at 2 and 4 weeks; complete endothelialization of the luminal surface with an aligned, well-organized monolayer of endothelial cells, extensive graft integration in terms of vascularization and cell infiltration from the surrounding tissue |
| Henry et al. (2017) | Electrospun PLLA scaffold blended with low MW PCL or low MW PLLA functionalized with Hep (EDC/NHS) and absorbed VEGF | In vivo (implantation in a rat common carotid artery model) | Enhanced endothelium formation and the overall patency of vascular grafts; increased cell infiltration into the electrospun grafts and production of collagen and elastin fibers within the graft wall |
| Lee et al. (2017) | PCL functionalized with Hep–tyramine polymer and a potent anti-neointimal drug (mitogen activated protein kinase II inhibitory peptide; MK2i) | In vitro (platelets, VSMCs) | Enhanced blood compatibility with significantly reduced protein absorption and platelet adhesion; significant inhibitory effects on VSMC migration associated with intimal hyperplasia |
| Hu et al. (2017) | Coaxial-elctrospun scaffolds of poly (l-lactide-co-caprolactone) [P(LLA-CL)]/collagen/elastin with hep and VEGF encapsulated in the core | In vitro (human aortic endothelial cells) and in vivo (implantation into a rabbit infrarenal aortic replacement model) | High attachment efficiency and proliferation; high short-term patency |
| Caracciolo et al. (2017) | Electrospun scaffolds from blends of poly (l-lactic acid) (PLLA) and segmented polyurethane (PHD) functionalized with lysozyme/heparin (EDC/NHS) | In vitro [human adipose-derived stem cells (MSC)] | Inhibition of platelet adhesion and of hemolysis; adhesion and proliferation of human adipose-derived stem cells; antimicrobial activities |
| Cao et al. (2017) | Electrospun PCL scaffolds aminolyzed and functionalized with hep (EDC/NHS) | In vitro (rabbit SMCs) | Induction of SMCs penetration into the scaffold and differentiation into contractile phenotype |
| Jiang et al. (2017) | Rat aorta decellularized vascular graft functionalized with antioxidant poly(1, 8-octamethylene-citrate-co-cysteine) (POCC) and Hep | In vivo (implantation in a rat aorta model) | Grafts displayed antioxidant activity, patency, and minimal intramural cell infiltration with varying degrees of calcification (inversely related to the antioxidant capacity), at 3 months post-surgery |
| Zhou et al. (2018) | Dual layer conduit consisting of collagen I-hyaluronic acid (external layer) and collagen I-Hep (inner layer) crosslinked with EDC | In vitro [fibroblast cell (Cos-7) and human microvascular endothelial cells (HMEC)] | Satisfactory mechanical performance and support for cells adhesion, proliferation and elongation |
| Wan et al. (2018) | Electrospun PCL/keratin nanofibrous mats functionalized with Hep (EDC/NHS) | In vitro (platelets, HUVECs, human umbilical artery smooth muscle cells (HUASMCs)) | Effective antithrombotic potential; induction of NO release, which enhance endothelial cell growth and inhibits smooth muscle cell proliferation and platelet adhesion |
| Xu et al. (2018) | Electrospun PCL scaffolds functionalized with Hep (EDC/NHS) | In vivo (implantation in infrarenal abdominal aorta of 30 rats) | All implanted grafts were patent during the 6 months post-surgery and showed a well-organized neo-tissue with endothelium formation, smooth muscle regeneration, and extracellular matrix formation |
| Norouzi and Shamloo (2019) | Bilayer heparinized vascular graft: Inner layer made by co-electrospinning of PCL and gelatin; outer layer fabricated by freeze-drying of gelatin hydrogel; Hep blending in gelatin solution and emulsion of PCL fibers | In vitro (HUVECs and rat VSMCs) | Improved endothelial cell attachment and decreased amount of activated platelets; mechanical properties similar to the coronary artery |
| Cai et al. (2019) | Decellularized porcine carotid arteries functionalized with Hep (EDC/NHS) | In vivo (subcutaneous implantation in 8 rats) | Improved mechanical properties, reduced inflammatory reaction and slow degradation time; effective inhibition of thrombogenesis |
| Kuang et al. (2019) | Two-layer composite vascular graft: Inner layer made of poly(lactic-co-glycolic acid)/Collagen nanofibers modified by mesoporous silica nanoparticles and grafted with polyethylene glycol and Hep; outer layer made of polyurethane nanofibers | In vitro (HUVECs) and in vivo (implantation into rabbit carotid artery) | Good blood compatibility; absence of inflammatory reaction; regeneration of endothelial cells monolayer and smooth muscle media layer |
| Smith et al. (2019) | Acellular tissue engineered vessel based on small intestinal submucosa functionalized sequentially with Hep (EDC/NHS) and VEGF | In vivo (implantation in abdominal aorta of mice) | Well-demarcated luminal and medial layers resembling those of native arteries; anti-inflammatory action of VEGF on infiltrating monocytes |
| Wang D. et al. (2019) | PLLA/PLGA/PLCL composite scaffolds fabricated by using TIPS, functionalized with Hep (EDC/NHS) and stromal cell-derived factor-1 alpha (SDF-1α) | In vitro (rat EPCs, HUVECs and hVSMCs) | Enhanced anticoagulation of vascular scaffold; acceleration of endothelialization and inhibition of hVSMCs proliferation |
| Jin et al. (2019) | Double-layer vascular scaffold: Inner layer made of electrospun end-group heparinized PCL nano- and microfibers; outer layer made of electrospun PCL | In vitro (HCs and SMCs) and in vivo (implantation into carotid artery of 6 rabbits) | Patency, endothelialization and fine revascularization were observed at 2 months post-implantation; aneurysmal dilatation of the outer layer; no signs of calcification |
| Wang W. et al. (2019) | Electrospun PCL scaffolds functionalized with multiple layers of vascular endothelial growth factor (VEGF) and Hep (repeated electrostatic adsorption self-assembly) crosslinked by genipin | In vitro (platelets, HUVECs) | Early and full release of VEGF to promote rapid endothelialization; gradual but sustained release of Hep for long-term anticoagulation and antithrombogenicity; improved cell viability and rapid endothelialization |
| Shi et al. (2019) | Electrospun PCL/gelatin hybrid vascular grafts functionalized with Hep (EDC/NHS) | In vivo (implantation in abdominal aorta of 18 rats) | Promotion of endothelialization and regulation of smooth muscle regeneration; inhibition of thrombosis |
| Wang et al. (2020) | Electrospun PCL scaffolds functionalized with multiple layers of vascular endothelial growth factor (VEGF), polylysine, and Hep (repeated electrostatic adsorption self-assembly) nanoparticles crosslinked by genipin | In vitro (platelets, HUVECs) | Successful induction of vascular endothelialization and long-term anticoagulation; long-term release of bioactive factors without burst release |
| Smith et al. (2020) | Acellular tissue engineered vessel based on small intestinal submucosa functionalized sequentially with Hep (EDC/NHS) and VEGF | In vivo (implantation into the carotid artery of an ovine model) | Immobilized VEGF captures blood circulating monocytes that differentiate into mature ECs that align in the direction of flow and produce nitric oxide under high shear stress. Highly prevalent circulating MC contribute directly to the endothelialization of acellular vascular grafts under the right chemical and biomechanical cues |
Heparin-functionalized vascular scaffolds for small-caliber artery grafting. Acellular tissue engineered vessels (A-TEVs) (highlighted in gray).
Hyaluronan-Based Vascular Constructs
Since discovery in 1934, HA has been extensively studied, and thanks to its viscoelastic properties, physiological activity, and biocompatibility, it has been used in a wide range of medical fields from orthopedics to cosmetics (Abatangelo et al., 2020). Due to the high-water solubility and the propensity to form very viscous solutions, its use in vascular tissue engineering requires the crosslinking with other natural (e.g. type I collagen) or synthetic materials (e.g. PCL) or its chemical modification to obtain semisynthetic polymers (Table 1). Among the latter, HYAFF-11, an HA derivative obtained by esterification with benzyl alcohol, was proven to be useful for small-diameter vascular grafts fabrication, also in a large animal model. This polymer is biocompatible, bioresorbable, able to interact with polar molecules, and it can be processed to obtain several types of devices such as tubes, membranes, non-woven fabrics, gauzes, and sponges (Vindigni et al., 2009). HYAFF-11 tubes, obtained by coagulation of a HYAFF-11/DMSO solution around a cylindrical bar in an ethanol bath, were successfully implanted in the abdominal aorta of 30 rats (2 mm diameter, 1 cm length) (Lepidi et al., 2006a; Lepidi et al., 2006b), as well as in the carotid artery of 10 pigs (4 mm diameter, 5 cm length) (Zavan et al., 2008), as temporary absorbable guides to promote regeneration of vascular structures. These studies showed the potential of these HA-based grafts to guide the development of a functional neo-artery, consisting of a confluent endothelium lining the luminal surface and a vascular wall with organized layers of elastin fibers. Interesting in vitro results were also obtained with electrospun PCL or collagen type I scaffolds functionalized with either high or low molecular weight HA, showing excellent biocompatibility and the potential to guide the formation of a polarized functional endothelium (Table 1). Recently, Yuan et al. (Yuan et al., 2016) obtained highly aligned HA/PLLA nanofibers in core-shell structure by coaxial stable jet electrospinning, which was shown effective in inducing human umbilical arterial smooth muscle cells elongation, orientation, proliferation, and differentiation toward a contractile phenotype. Circumferentially aligned HA/PLLA nanofibers scaffolds were successfully implanted in the carotid artery of six rabbits, showing high patency and efficacious vascular regeneration, 6 weeks post-surgery (Yuan et al., 2016).
Heparin-Based Vascular Constructs
Since its discovery in 1916, Hep has been widely clinically used to treat thrombotic disorders, as it amplifies the inhibitory activity of antithrombin III toward thrombin by facilitating the formation of a ternary complex with factor Xa. Although Hep and HS share the same biosynthetic pathway, the former has higher degree of sulfation (2.6 vs. 0.6 sulfate groups per disaccharide) and epimerization (up to 90 vs. 20% iduronation) (Liu and Linhardt, 2014). Initially known for its anticoagulant properties, many evidences showed Hep’s usefulness as anti-inflammatory agent in the treatment of venous thromboembolism and other vascular diseases. Hep’s pleiotropic roles include the inhibitory activities toward neutrophils functions, endothelial activation and smooth muscle cells proliferation (Yang et al., 2012; Poterucha et al., 2017), well-known key events in atherogenesis and vascular graft failure. Due to its capacity to bind and release growth factors, such as VEGF and bFGF, and to modulate angiogenesis, together with its anti-thrombotic properties, Hep has been used to functionalize many different systems including hydrogels, films, and electrospun fibers (Aslani et al., 2020). Table 2 summarizes the multitude of studies, published in the last 20 years, that exploited the angiogenic regulatory activities of Hep for the development of effective small diameter vascular grafts. Among them, the great majority developed electrospun scaffolds made of exclusively synthetic (e.g. poly-Ɛ-caprolactone, poly-L-lactide-co-caprolactone, poly-L-lactic acid) or hybrid (containing collagen, silk, chitosan, keratin, etc.) mats functionalized with Hep, mainly via chemical crosslinking using N-Ethyl-N′-(3-dimethylaminopropyl) carbodiimide/N-hydroxy succinimide (EDC/NHS). Besides, acellular tissue engineered vessels (A-TEVs) consisting of vascular tissue (mainly carotid artery or aorta segments from pig, dog, or rat) or small intestinal submucosa, undergone a decellularization process and, subsequently, coated with Hep and, in some cases, angiogenic growth factors, have been fabricated.
Most of the reported studies, performed in vitro by culturing either endothelial cells or smooth muscle cells, showed good mechanical properties, enhanced biocompatibility and anti-thrombogenicity of these grafts, and the potential of Hep to promote endothelialization and smooth muscle cells differentiation toward a contractile phenotype. Besides, some interesting results were obtained in vivo with both synthetic grafts and A-TEVs.
Wu et al. (Wu et al., 2012) designed a cell-free fast-degrading elastomeric graft consisting of a PGS core surrounded by a PCL sheath, coated with Hep, which was successfully implanted in the abdominal aorta of 21 rats (porous PCL scaffolds were implanted in six rats as controls). The graft was rapidly substituted by a neo-artery with mechanical and functional properties very similar to those of the native one.
To improve endothelialization and reduce thrombotic events and restenosis, Song’s team developed two methods for optimal surface functionalization of synthetic grafts with Hep. In the first one, electrospun polycarbonate-urethane nanofibrous scaffolds underwent plasma treatment followed by Hep conjugation via end-point immobilization. Grafts were implanted in a rat common carotid artery anastomosis model, showing high patency rate and extensive graft integration (Qiu et al., 2017). In the second one, electrospun scaffolds were obtained using blends of high and low molecular weight elastomeric polymers to improve both functionalization and mechanical properties of the grafts. PLLA scaffolds with 5% low molecular weight PCL were functionalized with Hep, loaded with VEGF and implanted into the carotid artery of rats, evidencing a synergistic effect of these two angiogenic factors on graft patency, endothelialization, and neo-artery formation (Henry et al., 2017).
As above mentioned, Andreadis’ team designed an A-TEV from decellularized SIS, coated it sequentially with Hep and VEGF, and then implanted it in both mouse abdominal aorta and sheep carotid artery (Koobatian et al., 2016; Smith et al., 2019; Smith et al., 2020). They obtained promising results including complete endothelialization and formation of a medial layer of circumferentially aligned smooth muscle cells, high elastin and collagen content, impressive mechanical properties and vascular contractility comparable to native arteries. They showed that VEGF was essential for modulating the inflammatory response of monocytes and the regeneration of a functional artery wall (Smith et al., 2019). Additionally, they demonstrated that VEGF was able to capture circulating monocytes that differentiated into mature endothelial cells, therefore directly contributing to the endothelialization of acellular vascular grafts (Smith et al., 2020).
Chondroitin Sulfate-Based Vascular Constructs
Differently from both HA and Hep, only few in vitro studies dealt with usefulness of CS for functionalization of small-diameter vascular grafts. In particular, Lerouge’s team functionalized poly(ethylene terephthalate) scaffolds with CS (via EDC/NHS) and assessed their functionality culturing HUVECs and VSMCs from rat embryonic thoracic aorta. Overall, these studies showed that CS coating prevented platelet adhesion and activation, while promoting HUVECs growth and resistance to flow-induced shear stress, and survival and inward penetration of VSMCs (Thalla et al., 2014; Savoji et al., 2017). The paucity of these studies could be related with CS implications in atherogenesis. In fact, CS is a major GAG found in both normal vessels and atherosclerotic lesions that participates in apolipoprotein B100 binding, therefore contributing to lipids accumulation into the subendothelial space of arteries. These events are mediated by specific interactions between a proteoglycan-binding site in Apolipoprotein B100, consisting of a few basic amino acids, and the negatively charged CS (Boren et al., 1998), with increasing affinity for over-sulfated chains (Sambandam et al., 1991).
Conclusion
GAGs represent key players in vascular physiology as well as in the pathogenesis of atherosclerosis. A deep knowledge of GAGs structural complexity, which accounts for their numerous functions, still represents a challenge but also a huge potential source of information to pave the way to vascular tissue engineering. In this review, we have provided readers with an overview of the numerous efforts performed in the last 20 years, in the attempt to develop functional resorbable scaffolds for small-diameter vascular regeneration, using GAGs as molecular cues to guide the correct endothelialization of the luminal surface and neo-artery formation. Although many progresses have been obtained both in vitro and in vivo using HA, HA derivatives, or Hep as bioactive molecules, none of the mentioned devices has reached the clinical trial yet, leaving the field open to further studies, including those exploring the effects of specific sulfated saccharide sequences or synthetic glycans related molecules on vascular regeneration.
Statements
Author contributions
AL, GN, JF-P, and LM contributed to manuscript conceptualization and data analysis. All authors contributed to manuscript writing, revision, read, and approved the submitted version.
Funding
This work is supported by the partners of Regenerative Medicine Crossing Borders (RegMed XB), a public-private partnership that uses regenerative medicine strategies to cure common chronic diseases. This collaboration project is financed by the Dutch Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences and Health to stimulate public-private partnerships. AL, GN, and MF thank the University of Sassari for its financial support (Fondo di Ateneo per la Ricerca 2020).
Acknowledgments
AL thanks Regione Autonoma della Sardegna for its financial support (POR-FSE 2014-2020-Asse Prioritario 3 “Istruzione e Formazione”-Obiettivo Tematico: 10, Priorità d’investimento: 10ii, Obiettivo Specifico: 10.5, Azione dell’Accordo di Partenariato 10.5.12-C.U.P. J86C18000270002). GN thanks “Programma Operativo Nazionale (PON) Ricerca e Innovazione 2014-2020—Asse I “Capitale Umano”, Azione I.2—A.I.M. Attraction and International Mobility, Linea 1 Mobilità dei ricercatori, AIM1874325-3, CUP: J54I18000110001” for its financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Abatangelo G. Vindigni V. Avruscio G. Pandis L. Brun P. (2020). Hyaluronic Acid: Redefining its Role. Cells9 (7). 10.3390/cells9071743
2
Agarwal S. Wendorff J. H. Greiner A. (2008). Use of Technique for Biomedical Applications. Polymer49 (26), 5603–5621. 10.1016/j.polymer.2008.09.014
3
Arrigoni C. Camozzi D. Imberti B. Mantero S. Remuzzi A. (2006). The Effect of Sodium Ascorbate on the Mechanical Properties of Hyaluronan-Based Vascular Constructs. Biomaterials27 (4), 623–630. 10.1016/j.biomaterials.2005.06.009
4
Aslani S. Kabiri M. HosseinZadeh S. Hanaee-Ahvaz H. Taherzadeh E. S. Soleimani M. (2020). The Applications of Heparin in Vascular Tissue Engineering. Microvasc. Res.131, 104027. 10.1016/j.mvr.2020.104027
5
Awad N. K. Niu H. Ali U. Morsi Y. S. Lin T. (2018). Electrospun Fibrous Scaffolds for Small-Diameter Blood Vessels: A Review. Membranes (Basel)8 (1), 5. 10.3390/membranes8010015
6
Baker M. Wang R. Damanik F. Kuhnt T. Ippel H. Dijkstra P. et al (2020). Biodegradable Poly(ester) Urethane Acrylate Resins for Digital Light Processing: From Polymer Synthesis to 3D Printed Tissue Engineering Constructs.
7
Baudis S. Nehl F. Ligon S. C. Nigisch A. Bergmeister H. Bernhard D. et al (2011). Elastomeric Degradable Biomaterials by Photopolymerization-Based CAD-CAM for Vascular Tissue Engineering. Biomed. Mater.6 (5), 055003. 10.1088/1748-6041/6/5/055003
8
Benjamin E. J. Blaha M. J. Chiuve S. E. Cushman M. Das S. R. Deo R. et al (2017). Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation135 (10), e146–e603. 10.1161/cir.0000000000000485
9
Borén J. Olin K. Lee I. Chait A. Wight T. N. Innerarity T. L. (1998). Identification of the Principal Proteoglycan-Binding Site in LDL. A Single-point Mutation in Apo-B100 Severely Affects Proteoglycan Interaction without Affecting LDL Receptor Binding. J. Clin. Invest.101 (12), 2658–2664. 10.1172/jci2265
10
Braghirolli D. I. Helfer V. E. Chagastelles P. C. Dalberto T. P. Gamba D. Pranke P. (2017). Electrospun Scaffolds Functionalized with Heparin and Vascular Endothelial Growth Factor Increase the Proliferation of Endothelial Progenitor Cells. Biomed. Mater.12 (2), 025003. 10.1088/1748-605x/aa5bbc
11
Brown T. D. Dalton P. D. Hutmacher D. W. (2011). Direct Writing by Way of Melt Electrospinning. Adv. Mater.23 (47), 5651–5657. 10.1002/adma.201103482
12
Cai Z. Gu Y. Cheng J. Li J. Xu Z. Xing Y. et al (2019). Decellularization, Cross-Linking and Heparin Immobilization of Porcine Carotid Arteries for Tissue Engineering Vascular Grafts. Cell Tissue Bank20 (4), 569–578. 10.1007/s10561-019-09792-5
13
Cao J. Geng X. Wen J. Li Q. Ye L. Zhang A. et al (2017). The Penetration and Phenotype Modulation of Smooth Muscle Cells on Surface Heparin Modified Poly(ɛ-Caprolactone) Vascular Scaffold. J. Biomed. Mater. Res.105 (10), 2806–2815. 10.1002/jbm.a.36144
14
Caracciolo P. C. Rial-Hermida M. I. Montini-Ballarin F. Abraham G. A. Concheiro A. Alvarez-Lorenzo C. (2017). Surface-modified Bioresorbable Electrospun Scaffolds for Improving Hemocompatibility of Vascular Grafts. Mater. Sci. Eng. C.75, 1115–1127. 10.1016/j.msec.2017.02.151
15
Catto V. Farè S. Cattaneo I. Figliuzzi M. Alessandrino A. Freddi G. et al (2015). Small Diameter Electrospun Silk Fibroin Vascular Grafts: Mechanical Properties, In Vitro Biodegradability, and In Vivo Biocompatibility. Mater. Sci. Eng. C.54, 101–111. 10.1016/j.msec.2015.05.003
16
Centola M. Rainer A. Spadaccio C. De Porcellinis S. Genovese J. A. Trombetta M. (2010). Combining Electrospinning and Fused Deposition Modeling for the Fabrication of a Hybrid Vascular Graft. Biofabrication2 (1), 014102. 10.1088/1758-5082/2/1/014102
17
Chen X. Wang J. An Q. Li D. Liu P. Zhu W. et al (2015). Electrospun Poly(l-Lactic Acid-Co-Ɛ-Caprolactone) Fibers Loaded with Heparin and Vascular Endothelial Growth Factor to Improve Blood Compatibility and Endothelial Progenitor Cell Proliferation. Colloids Surf. B: Biointerfaces128, 106–114. 10.1016/j.colsurfb.2015.02.023
18
Cigliano A. Gandaglia A. Lepedda A. J. Zinellu E. Naso F. Gastaldello A. et al (2012). Fine Structure of Glycosaminoglycans from Fresh and Decellularized Porcine Cardiac Valves and Pericardium. Biochem. Res. Int.2012, 979351. 10.1155/2012/979351
19
Clerc O. Mariethoz J. Rivet A. Lisacek F. Pérez S. Ricard-Blum S. (2019). A Pipeline to Translate Glycosaminoglycan Sequences into 3D Models. Application to the Exploration of Glycosaminoglycan Conformational Space. Glycobiology29 (1), 36–44. 10.1093/glycob/cwy084
20
Conklin B. S. Richter E. R. Kreutziger K. L. Zhong D.-S. Chen C. (2002). Development and Evaluation of a Novel Decellularized Vascular Xenograft. Med. Eng. Phys.24 (3), 173–183. 10.1016/s1350-4533(02)00010-3
21
Conklin B. S. Wu H. Lin P. H. Lumsden A. B. Chen C. (2004). Basic Fibroblast Growth Factor Coating and Endothelial Cell Seeding of a Decellularized Heparin-Coated Vascular Graft. Artif. Organs28 (7), 668–675. 10.1111/j.1525-1594.2004.00062.x
22
Cui H. Zhu W. Huang Y. Liu C. Yu Z. X. Nowicki M. et al (2019). In vitro and In Vivo Evaluation of 3D Bioprinted Small-Diameter Vasculature with Smooth Muscle and Endothelium. Biofabrication12 (1), 015004. 10.1088/1758-5090/ab402c
23
Cunnane E. M. Lorentz K. L. Ramaswamy A. K. Gupta P. Mandal B. B. O’Brien F. J. et al (2020). Extracellular Vesicles Enhance the Remodeling of Cell-free Silk Vascular Scaffolds in Rat Aortae. ACS Appl. Mater. Inter.12 (24), 26955–26965. 10.1021/acsami.0c06609
24
Dahl S. L. M. Koh J. Prabhakar V. Niklason L. E. (2003). Decellularized Native and Engineered Arterial Scaffolds for Transplantation. Cel Transpl.12 (6), 659–666. 10.3727/000000003108747136
25
Daugs A. Hutzler B. Meinke M. Schmitz C. Lehmann N. Markhoff A. et al (2017). Detergent-Based Decellularization of Bovine Carotid Arteries for Vascular Tissue Engineering. Ann. Biomed. Eng.45 (11), 2683–2692. 10.1007/s10439-017-1892-7
26
de Valence S. Tille J.-C. Giliberto J.-P. Mrowczynski W. Gurny R. Walpoth B. H. et al (2012). Advantages of Bilayered Vascular Grafts for Surgical Applicability and Tissue Regeneration. Acta Biomater.8 (11), 3914–3920. 10.1016/j.actbio.2012.06.035
27
Dimitrievska S. Cai C. Weyers A. Balestrini J. L. Lin T. Sundaram S. et al (2015). Click-coated, Heparinized, Decellularized Vascular Grafts. Acta Biomater.13, 177–187. 10.1016/j.actbio.2014.11.015
28
Dogné S. Flamion B. Caron N. (2018). Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications. Atvb38 (7), 1427–1439. 10.1161/atvbaha.118.310839
29
Du F. Zhao W. Zhang M. Mao H. Kong D. Yang J. (2011). The Synergistic Effect of Aligned Nanofibers and Hyaluronic Acid Modification on Endothelial Cell Behavior for Vascular Tissue Engineering. J. Nanosci. Nanotech.11 (8), 6718–6725. 10.1166/jnn.2011.4750
30
Duan N. Geng X. Ye L. Zhang A. Feng Z. Guo L. et al (2016). A Vascular Tissue Engineering Scaffold with Core-Shell Structured Nano-Fibers Formed by Coaxial Electrospinning and its Biocompatibility Evaluation. Biomed. Mater.11 (3), 035007. 10.1088/1748-6041/11/3/035007
31
Elliott M. B. Ginn B. Fukunishi T. Bedja D. Suresh A. Chen T. et al (2019). Regenerative and Durable Small-Diameter Graft as an Arterial Conduit. Proc. Natl. Acad. Sci. USA116 (26), 12710–12719. 10.1073/pnas.1905966116
32
Elomaa L. Pan C.-C. Shanjani Y. Malkovskiy A. Seppälä J. V. Yang Y. (2015). Three-dimensional Fabrication of Cell-Laden Biodegradable Poly(ethylene Glycol-Co-Depsipeptide) Hydrogels by Visible Light Stereolithography. J. Mater. Chem. B3 (42), 8348–8358. 10.1039/c5tb01468a
33
Fang J. Zhang J. Du J. Pan Y. Shi J. Peng Y. et al (2016). Orthogonally Functionalizable Polyurethane with Subsequent Modification with Heparin and Endothelium-Inducing Peptide Aiming for Vascular Reconstruction. ACS Appl. Mater. Inter.8 (23), 14442–14452. 10.1021/acsami.6b04289
34
Ference B. A. Ginsberg H. N. Graham I. Ray K. K. Packard C. J. Bruckert E. et al (2017). Low-density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J.38 (32), 2459–2472. 10.1093/eurheartj/ehx144
35
Fernández-Colino A. Wolf F. Rütten S. Schmitz-Rode T. Rodríguez-Cabello J. C. Jockenhoevel S. et al (2019). Small Caliber Compliant Vascular Grafts Based on Elastin-like Recombinamers for In Situ Tissue Engineering. Front. Bioeng. Biotechnol.7, 340. 10.3389/fbioe.2019.00340
36
Filmus J. Capurro M. Rast J. (2008). Glypicans. Genome Biol.9 (5), 224. 10.1186/gb-2008-9-5-224
37
Fu J. Ding X. Stowell C. E. T. Wu Y.-L. Wang Y. (2020). Slow Degrading Poly(glycerol Sebacate) Derivatives Improve Vascular Graft Remodeling in a Rat Carotid Artery Interposition Model. Biomaterials257, 120251. 10.1016/j.biomaterials.2020.120251
38
Gao G. Kim H. Kim B. S. Kong J. S. Lee J. Y. Park B. W. et al (2019). Tissue-engineering of Vascular Grafts Containing Endothelium and Smooth-Muscle Using Triple-Coaxial Cell Printing. Appl. Phys. Rev.6 (4), 041402. 10.1063/1.5099306
39
Gao G. Park J. Y. Kim B. S. Jang J. Cho D. W. (2018). Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater.7 (23), e1801102. 10.1002/adhm.201801102
40
Girish K. S. Kemparaju K. (2007). The Magic Glue Hyaluronan and its Eraser Hyaluronidase: A Biological Overview. Life Sci.80 (21), 1921–1943. 10.1016/j.lfs.2007.02.037
41
Gong W. Lei D. Li S. Huang P. Qi Q. Sun Y. et al (2016). Hybrid Small-Diameter Vascular Grafts: Anti-expansion Effect of Electrospun Poly ε-caprolactone on Heparin-Coated Decellularized Matrices. Biomaterials76, 359–370. 10.1016/j.biomaterials.2015.10.066
42
Gopal S. (2020). Syndecans in Inflammation at a Glance. Front. Immunol.11, 227. 10.3389/fimmu.2020.00227
43
Gupta P. Lorentz K. L. Haskett D. G. Cunnane E. M. Ramaswamy A. K. Weinbaum J. S. et al (2020). Bioresorbable Silk Grafts for Small Diameter Vascular Tissue Engineering Applications: In vitro and In Vivo Functional Analysis. Acta Biomater.105, 146–158. 10.1016/j.actbio.2020.01.020
44
Gutowski P. Gage S. M. Guziewicz M. Ilzecki M. Kazimierczak A. Kirkton R. D. et al (2020). Arterial Reconstruction with Human Bioengineered Acellular Blood Vessels in Patients with Peripheral Arterial Disease. J. Vasc. Surg.72 (4), 1247–1258. 10.1016/j.jvs.2019.11.056
45
Henry J. J. D. Yu J. Wang A. Lee R. Fang J. Li S. (2017). Engineering the Mechanical and Biological Properties of Nanofibrous Vascular Grafts for In Situ Vascular Tissue Engineering. Biofabrication9 (3), 035007. 10.1088/1758-5090/aa834b
46
Hinton T. J. Jallerat Q. Palchesko R. N. Park J. H. Grodzicki M. S. Shue H. J. et al (2015). Three-dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv.1 (9), e1500758. 10.1126/sciadv.1500758
47
Hoshi R. A. Van Lith R. Jen M. C. Allen J. B. Lapidos K. A. Ameer G. (2013). The Blood and Vascular Cell Compatibility of Heparin-Modified ePTFE Vascular Grafts. Biomaterials34 (1), 30–41. 10.1016/j.biomaterials.2012.09.046
48
Hsieh Y. F. Sahagian K. Huang F. Xu K. Patel S. Li S. (2017). Comparison of Plasma and Chemical Modifications of Poly-L-Lactide-Co-Caprolactone Scaffolds for Heparin Conjugation. Biomed. Mater.12 (6), 065004. 10.1088/1748-605x/aa81aa
49
Hu Y.-T. Pan X.-D. Zheng J. Ma W.-G. Sun L.-Z. (2017). In vitro and In Vivo Evaluation of a Small-Caliber Coaxial Electrospun Vascular Graft Loaded with Heparin and VEGF. Int. J. Surg.44, 244–249. 10.1016/j.ijsu.2017.06.077
50
Huang C. Wang S. Qiu L. Ke Q. Zhai W. Mo X. (2013). Heparin Loading and Pre-endothelialization in Enhancing the Patency Rate of Electrospun Small-Diameter Vascular Grafts in a Canine Model. ACS Appl. Mater. Inter.5 (6), 2220–2226. 10.1021/am400099p
51
Hultgårdh-Nilsson A. Borén J. Chakravarti S. (2015). The Small Leucine-Rich Repeat Proteoglycans in Tissue Repair and Atherosclerosis. J. Intern. Med.278 (5), 447–461. 10.1111/joim.12400
52
Idini M. Wieringa P. Rocchiccioli S. Nieddu G. Ucciferri N. Formato M. et al (2019). Glycosaminoglycan Functionalization of Electrospun Scaffolds Enhances Schwann Cell Activity. Acta Biomater.96, 188–202. 10.1016/j.actbio.2019.06.054
53
Iozzo R. V. Schaefer L. (2015). Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol.42, 11–55. 10.1016/j.matbio.2015.02.003
54
Itoh M. Nakayama K. Noguchi R. Kamohara K. Furukawa K. Uchihashi K. et al (2015). Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS One10 (9), e0136681. 10.1371/journal.pone.0136681
55
Ji Y. Zhou J. Sun T. Tang K. Xiong Z. Ren Z. et al (2019). Diverse Preparation Methods for Small Intestinal Submucosa (SIS): Decellularization, Components, and Structure. J. Biomed. Mater. Res. A.107 (3), 689–697. 10.1002/jbm.a.36582
56
Jia W. Li M. Kang L. Gu G. Guo Z. Chen Z. (2019). Fabrication and Comprehensive Characterization of Biomimetic Extracellular Matrix Electrospun Scaffold for Vascular Tissue Engineering Applications. J. Mater. Sci.54 (15), 10871–10883. 10.1007/s10853-019-03667-6
57
Jiang B. Akgun B. Lam R. C. Ameer G. A. Wertheim J. A. (2015). A Polymer-Extracellular Matrix Composite with Improved Thromboresistance and Recellularization Properties. Acta Biomater.18, 50–58. 10.1016/j.actbio.2015.02.015
58
Jiang B. Suen R. Wang J.-J. Zhang Z. J. Wertheim J. A. Ameer G. A. (2017). Vascular Scaffolds with Enhanced Antioxidant Activity Inhibit Graft Calcification. Biomaterials144, 166–175. 10.1016/j.biomaterials.2017.08.014
59
Jiang B. Suen R. Wertheim J. A. Ameer G. A. (2016). Targeting Heparin to Collagen within Extracellular Matrix Significantly Reduces Thrombogenicity and Improves Endothelialization of Decellularized Tissues. Biomacromolecules17 (12), 3940–3948. 10.1021/acs.biomac.6b01330
60
Jin X. Geng X. Jia L. Xu Z. Ye L. Gu Y. et al (2019). Preparation of Small-Diameter Tissue-Engineered Vascular Grafts Electrospun from Heparin End-Capped PCL and Evaluation in a Rabbit Carotid Artery Replacement Model. Macromol Biosci.19 (8), e1900114. 10.1002/mabi.201900114
61
João C. F. C. Vasconcelos J. M. Silva J. C. Borges J. P. (2014). An Overview of Inverted Colloidal Crystal Systems for Tissue Engineering. Tissue Eng. B: Rev.20 (5), 437–454. 10.1089/ten.teb.2013.0402
62
Jourde-Chiche N. Fakhouri F. Dou L. Bellien J. Burtey S. Frimat M. et al (2019). Endothelium Structure and Function in Kidney Health and Disease. Nat. Rev. Nephrol.15 (2), 87–108. 10.1038/s41581-018-0098-z
63
Jungst T. Pennings I. Schmitz M. Rosenberg A. J. W. P. Groll J. Gawlitta D. (2019). Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts. Adv. Funct. Mater.29 (43), 1905987. 10.1002/adfm.201905987
64
Kang L. Jia W. Li M. Wang Q. Wang C. Liu Y. et al (2019). Hyaluronic Acid Oligosaccharide-Modified Collagen Nanofibers as Vascular Tissue-Engineered Scaffold for Promoting Endothelial Cell Proliferation. Carbohydr. Polym.223, 115106. 10.1016/j.carbpol.2019.115106
65
Khosravi R. Best C. A. Allen R. A. Stowell C. E. T. Onwuka E. Zhuang J. J. et al (2016). Long-Term Functional Efficacy of a Novel Electrospun Poly(Glycerol Sebacate)-Based Arterial Graft in Mice. Ann. Biomed. Eng.44 (8), 2402–2416. 10.1007/s10439-015-1545-7
66
Kinsella M. G. Wight T. N. (2005). Perlecan: An Extracellular Matrix Heparan Sulfate Proteoglycan that Regulates Key Events in Vascular Development and Disease In Chemistry and Biology of Heparin and Heparan Sulfate. GargH. G.LinhardtR. J.HalesC. A. (Amsterdam; Elsevier Science), 607–635. 10.1016/b978-008044859-6/50023-x
67
Kirkton R. D. Santiago-Maysonet M. Lawson J. H. Tente W. E. Dahl S. L. M. Niklason L. E. et al (2019). Bioengineered Human Acellular Vessels Recellularize and Evolve into Living Blood Vessels after Human Implantation. Sci. Transl Med.11 (485), eaau6934. 10.1126/scitranslmed.aau6934
68
Kjellén L. Lindahl U. (2018). Specificity of Glycosaminoglycan-Protein Interactions. Curr. Opin. Struct. Biol.50, 101–108. 10.1016/j.sbi.2017.12.011
69
Koobatian M. T. Row S. Smith Jr R. J. Jr. Koenigsknecht C. Andreadis S. T. Swartz D. D. (2016). Successful Endothelialization and Remodeling of a Cell-free Small-Diameter Arterial Graft in a Large Animal Model. Biomaterials76, 344–358. 10.1016/j.biomaterials.2015.10.020
70
Kruger-Genge A. Blocki A. Franke R. P. Jung F. (2019). Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci.20 (18), 4411. 10.3390/ijms20184411
71
Kuang H. Yang S. Wang Y. He Y. Ye K. Hu J. et al (2019). Electrospun Bilayer Composite Vascular Graft with an Inner Layer Modified by Polyethylene Glycol and Haparin to Regenerate the Blood Vessel. J. Biomed. Nanotechnol15 (1), 77–84. 10.1166/jbn.2019.2666
72
Kuhnt T. Marroquín García R. Camarero-Espinosa S. Dias A. ten Cate A. T. van Blitterswijk C. A. et al (2019). Poly(caprolactone-co-trimethylenecarbonate) Urethane Acrylate Resins for Digital Light Processing of Bioresorbable Tissue Engineering Implants. Biomater. Sci.7 (12), 4984–4989. 10.1039/c9bm01042d
73
L'Heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T. N. et al (2006). Human Tissue-Engineered Blood Vessels for Adult Arterial Revascularization. Nat. Med.12 (3), 361–365. 10.1038/nm1364
74
L'Heureux N. McAllister T. N. de la Fuente L. M. (2007). Tissue-engineered Blood Vessel for Adult Arterial Revascularization. N. Engl. J. Med.357 (14), 1451–1453. 10.1056/nejmc071536
75
L'Heureux N. Pâquet S. Labbé R. Germain L. Auger F. A. (1998). A Completely Biological Tissue‐engineered Human Blood Vessel. FASEB j.12 (1), 47–56. 10.1096/fsb2fasebj.12.1.47
76
Lawson J. H. Glickman M. H. Ilzecki M. Jakimowicz T. Jaroszynski A. Peden E. K. et al (2016). Bioengineered Human Acellular Vessels for Dialysis Access in Patients with End-Stage Renal Disease: Two Phase 2 Single-Arm Trials. The Lancet387 (10032), 2026–2034. 10.1016/s0140-6736(16)00557-2
77
Lee Y. Le Thi P. Seon G. M. Ryu S. B. Brophy C. M. Kim Y. et al (2017). Heparin-functionalized Polymer Graft Surface Eluting MK2 Inhibitory Peptide to Improve Hemocompatibility and Anti-neointimal Activity. J. Controlled Release266, 321–330. 10.1016/j.jconrel.2017.10.002
78
Lepedda A. J. Nieddu G. Piperigkou Z. Kyriakopoulou K. Karamanos N. Formato M. (2021). Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin. Thromb. Hemost.47 (3), 295–307. 10.1055/s-0041-1725063
79
Lepidi S. Abatangelo G. Vindigni V. Deriu G. P. Zavan B. Tonello C. et al (2006a). In vivo regeneration of Small‐diameter (2 Mm) Arteries Using a Polymer Scaffold. FASEB j.20 (1), 103–105. 10.1096/fj.05-4802fje
80
Lepidi S. Grego F. Vindigni V. Zavan B. Tonello C. Deriu G. P. et al (2006b). Hyaluronan Biodegradable Scaffold for Small-Caliber Artery Grafting: Preliminary Results in an Animal Model. Eur. J. Vasc. Endovascular Surg.32 (4), 411–417. 10.1016/j.ejvs.2006.02.012
81
L’Heureux N. (2020). Cell-assembled Extracellular Matrix (CAM) as a Biomaterial for Building Vascular Grafts. FASEB J.34 (S1), 1. 10.1096/fsb2.v34.S1
82
Li Q. Mu L. Zhang F. Mo Z. Jin C. Qi W. (2017). Manufacture and Property Research of Heparin Grafted Electrospinning PCU Artificial Vascular Scaffolds. Mater. Sci. Eng. C78, 854–861. 10.1016/j.msec.2017.04.148
83
Libby P. Buring J. E. Badimon L. Hansson G. K. Deanfield J. Bittencourt M. S. et al (2019). Atherosclerosis. Nat. Rev. Dis. Primers5 (1), 56. 10.1038/s41572-019-0106-z
84
Liu J. Linhardt R. J. (2014). Chemoenzymatic Synthesis of Heparan Sulfate and Heparin. Nat. Prod. Rep.31 (12), 1676–1685. 10.1039/c4np00076e
85
Liu R. H. Ong C. S. Fukunishi T. Ong K. Hibino N. (2018). Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B: Rev.24 (2), 133–143. 10.1089/ten.teb.2017.0350
86
Lu G. Cui S. J. Geng X. Ye L. Chen B. Feng Z. G. et al (2013). Design and Preparation of Polyurethane-Collagen/heparin-Conjugated Polycaprolactone Double-Layer Bionic Small-Diameter Vascular Graft and its Preliminary Animal Tests. Chin. Med. J. (Engl)126 (7), 1310–1316.
87
Machin D. R. Phuong T. T. Donato A. J. (2019). The Role of the Endothelial Glycocalyx in Advanced Age and Cardiovascular Disease. Curr. Opin. Pharmacol.45, 66–71. 10.1016/j.coph.2019.04.011
88
Madden R. L. Lipkowitz G. S. Browne B. J. Kurbanov A. (2004). Experience with Cryopreserved Cadaveric Femoral Vein Allografts Used for Hemodialysis Access. Ann. Vasc. Surg.18 (4), 453–458. 10.1007/s10016-004-0055-0
89
Magnan L. Labrunie G. Fénelon M. Dusserre N. Foulc M.-P. Lafourcade M. et al (2020). Human Textiles: A Cell-Synthesized yarn as a Truly "bio" Material for Tissue Engineering Applications. Acta Biomater.105, 111–120. 10.1016/j.actbio.2020.01.037
90
Mancuso L. Gualerzi A. Boschetti F. Loy F. Cao G. (2014). Decellularized Ovine Arteries as Small-Diameter Vascular Grafts. Biomed. Mater.9 (4), 045011. 10.1088/1748-6041/9/4/045011
91
Marcolin C. Draghi L. Tanzi M. Fare S. (2017). Electrospun Silk Fibroin-Gelatin Composite Tubular Matrices as Scaffolds for Small Diameter Blood Vessel Regeneration. J. Mater. Sci. Mater. Med.28 (5), 80. 10.1007/s10856-017-5884-9
92
Marelli B. Alessandrino A. Farè S. Freddi G. Mantovani D. Tanzi M. C. (2010). Compliant Electrospun Silk Fibroin Tubes for Small Vessel Bypass Grafting. Acta Biomater.6 (10), 4019–4026. 10.1016/j.actbio.2010.05.008
93
McClure M. J. Sell S. A. Simpson D. G. Walpoth B. H. Bowlin G. L. (2010). A Three-Layered Electrospun Matrix to Mimic Native Arterial Architecture Using Polycaprolactone, Elastin, and Collagen: a Preliminary Study. Acta Biomater.6 (7), 2422–2433. 10.1016/j.actbio.2009.12.029
94
Melchiorri A. J. Hibino N. Best C. A. Yi T. Lee Y. U. Kraynak C. A. et al (2016). 3D-Printed Biodegradable Polymeric Vascular Grafts. Adv. Healthc. Mater.5 (3), 319–325. 10.1002/adhm.201500725
95
Mi H.-Y. Jing X. McNulty J. Salick M. R. Peng X.-F. Turng L.-S. (2016). Approaches to Fabricating Multiple-Layered Vascular Scaffolds Using Hybrid Electrospinning and Thermally Induced Phase Separation Methods. Ind. Eng. Chem. Res.55 (4), 882–892. 10.1021/acs.iecr.5b03462
96
Mi H.-Y. Jing X. Yu E. McNulty J. Peng X.-F. Turng L.-S. (2015). Fabrication of Triple-Layered Vascular Scaffolds by Combining Electrospinning, Braiding, and Thermally Induced Phase Separation. Mater. Lett.161, 305–308. 10.1016/j.matlet.2015.08.119
97
Mirdamadi E. Tashman J. W. Shiwarski D. J. Palchesko R. N. Feinberg A. W. (2020). FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater. Sci. Eng.6 (11), 6453–6459. 10.1021/acsbiomaterials.0c01133
98
Moretto P. Karousou E. Viola M. Caon I. D'Angelo M. L. De Luca G. et al (2015). Regulation of Hyaluronan Synthesis in Vascular Diseases and Diabetes. J. Diabetes Res.2015, 167283. 10.1155/2015/167283
99
Naso F. Gandaglia A. Iop L. Spina M. Gerosa G. (2012). Alpha-Gal Detectors in Xenotransplantation Research: a Word of Caution. Xenotransplantation19 (4), 215–220. 10.1111/j.1399-3089.2012.00714.x
100
Niklason L. E. Abbott W. Gao J. Klagges B. Hirschi K. K. Ulubayram K. et al (2001). Morphologic and Mechanical Characteristics of Engineered Bovine Arteries. J. Vasc. Surg.33 (3), 628–638. 10.1067/mva.2001.111747
101
Niklason L. E. Gao J. Abbott W. M. Hirschi K. K. Houser S. Marini R. et al (1999). Functional Arteries Grown In Vitro. Science284 (5413), 489–493. 10.1126/science.284.5413.489
102
Norouzi S. K. Shamloo A. (2019). Bilayered Heparinized Vascular Graft Fabricated by Combining Electrospinning and Freeze Drying Methods. Mater. Sci. Eng. C94, 1067–1076. 10.1016/j.msec.2018.10.016
103
Olausson M. Patil P. B. Kuna V. K. Chougule P. Hernandez N. Methe K. et al (2012). Transplantation of an Allogeneic Vein Bioengineered with Autologous Stem Cells: a Proof-Of-Concept Study. The Lancet380 (9838), 230–237. 10.1016/s0140-6736(12)60633-3
104
Pandis L. Zavan B. Abatangelo G. Lepidi S. Cortivo R. Vindigni V. (2010). Hyaluronan-based Scaffold for In Vivo Regeneration of the Rat Vena Cava: Preliminary Results in an Animal Model. J. Biomed. Mater. Res. A.93 (4), 1289–1296. 10.1002/jbm.a.32626
105
Pandis L. Zavan B. Bassetto F. Ferroni L. Iacobellis L. Abatangelo G. et al (2011). Hyaluronic Acid Biodegradable Material for Reconstruction of Vascular Wall: a Preliminary Study in Rats. Microsurgery31 (2), 138–145. 10.1002/micr.20856
106
Pashneh-Tala S. MacNeil S. Claeyssens F. (2016). The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B: Rev.22 (1), 68–100. 10.1089/ten.teb.2015.0100
107
Peck M. Gebhart D. Dusserre N. McAllister T. N. L'Heureux N. (2012). The Evolution of Vascular Tissue Engineering and Current State of the Art. Cells Tissues Organs195 (1-2), 144–158. 10.1159/000331406
108
Pitarresi G. Fiorica C. Palumbo F. S. Rigogliuso S. Ghersi G. Giammona G. (2014). Heparin Functionalized Polyaspartamide/polyester Scaffold for Potential Blood Vessel Regeneration. J. Biomed. Mater. Res.102 (5), 1334–1341. 10.1002/jbm.a.34818
109
Poterucha T. J. Libby P. Goldhaber S. Z. (2017). More Than an Anticoagulant: Do Heparins Have Direct Anti-inflammatory Effects?Thromb. Haemost.117 (3), 437–444. 10.1160/th16-08-0620
110
Qiu X. Lee B. L.-P. Ning X. Murthy N. Dong N. Li S. (2017). End-point Immobilization of Heparin on Plasma-Treated Surface of Electrospun Polycarbonate-Urethane Vascular Graft. Acta Biomater.51, 138–147. 10.1016/j.actbio.2017.01.012
111
Raman R. Sasisekharan V. Sasisekharan R. (2005). Structural Insights into Biological Roles of Protein-Glycosaminoglycan Interactions. Chem. Biol.12 (3), 267–277. 10.1016/j.chembiol.2004.11.020
112
Remuzzi A. Mantero S. Colombo M. Morigi M. Binda E. Camozzi D. et al (2004). Vascular Smooth Muscle Cells on Hyaluronic Acid: Culture and Mechanical Characterization of an Engineered Vascular Construct. Tissue Eng.10 (5-6), 699–710. 10.1089/1076327041348347
113
Saidy N. T. Shabab T. Bas O. Rojas-Gonzalez D. M. Menne M. Henry T. et al (2020). Melt Electrowriting of Complex 3D Anatomically Relevant Scaffolds. Front. Bioeng. Biotechnol.8, 793. 10.3389/fbioe.2020.00793
114
Saitow C. Kaplan D. L. Castellot J. J. Jr. (2011). Heparin Stimulates Elastogenesis: Application to Silk-Based Vascular Grafts. Matrix Biol.30 (5-6), 346–355. 10.1016/j.matbio.2011.04.005
115
Sambandam T. Baker J. R. Christner J. E. Ekborg S. L. (1991). Specificity of the Low Density Lipoprotein-Glycosaminoglycan Interaction. Arterioscler Thromb.11 (3), 561–568. 10.1161/01.atv.11.3.561
116
Savoji H. Maire M. Lequoy P. Liberelle B. De Crescenzo G. Ajji A. et al (2017). Combining Electrospun Fiber Mats and Bioactive Coatings for Vascular Graft Prostheses. Biomacromolecules18 (1), 303–310. 10.1021/acs.biomac.6b01770
117
Senior J. J. Cooke M. E. Grover L. M. Smith A. M. (2019). Fabrication of Complex Hydrogel Structures Using Suspended Layer Additive Manufacturing (SLAM). Adv. Funct. Mater.29 (49), 1904845. 10.1002/adfm.201904845
118
Shafiq M. Jung Y. Kim S. H. (2016). Covalent Immobilization of Stem Cell Inducing/recruiting Factor and Heparin on Cell-free Small-Diameter Vascular Graft for Acceleratedin Situtissue Regeneration. J. Biomed. Mater. Res.104 (6), 1352–1371. 10.1002/jbm.a.35666
119
Shi J. Chen S. Wang L. Zhang X. Gao J. Jiang L. et al (2019). Rapid Endothelialization and Controlled Smooth Muscle Regeneration by Electrospun Heparin‐loaded Polycaprolactone/gelatin Hybrid Vascular Grafts. J. Biomed. Mater. Res.107 (6), 2040–2049. 10.1002/jbm.b.34295
120
Smith R. J. Jr. Nasiri B. Kann J. Yergeau D. Bard J. E. Swartz D. D. et al (2020). Endothelialization of Arterial Vascular Grafts by Circulating Monocytes. Nat. Commun.11 (1), 1622. 10.1038/s41467-020-15361-2
121
Smith R. J. Jr. Yi T. Nasiri B. Breuer C. K. Andreadis S. T. (2019). Implantation of VEGF‐functionalized Cell‐free Vascular Grafts: Regenerative and Immunological Response. FASEB j.33 (4), 5089–5100. 10.1096/fj.201801856r
122
Sodhi H. Panitch A. (2020). Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules11 (1). 10.3390/biom11010029
123
Soletti L. Hong Y. Guan J. Stankus J. J. El-Kurdi M. S. Wagner W. R. et al (2010). A Bilayered Elastomeric Scaffold for Tissue Engineering of Small Diameter Vascular Grafts. Acta Biomater.6 (1), 110–122. 10.1016/j.actbio.2009.06.026
124
Song H.-H. G. Rumma R. T. Ozaki C. K. Edelman E. R. Chen C. S. (2018). Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell22 (3), 340–354. 10.1016/j.stem.2018.02.009
125
Tamura N. Nakamura T. Terai H. Iwakura A. Nomura S. Shimizu Y. et al (2003). A New Acellular Vascular Prosthesis as a Scaffold for Host Tissue Regeneration. Int. J. Artif. Organs26 (9), 783–792.
126
Tan Z. Wang H. Gao X. Liu T. Tan Y. (2016). Composite Vascular Grafts with High Cell Infiltration by Co-electrospinning. Mater. Sci. Eng. C67, 369–377. 10.1016/j.msec.2016.05.067
127
Thalla P. K. Fadlallah H. Liberelle B. Lequoy P. De Crescenzo G. Merhi Y. et al (2014). Chondroitin Sulfate Coatings Display Low Platelet but High Endothelial Cell Adhesive Properties Favorable for Vascular Implants. Biomacromolecules15 (7), 2512–2520. 10.1021/bm5003762
128
Theocharis A. D. Skandalis S. S. Gialeli C. Karamanos N. K. (2016). Extracellular Matrix Structure. Adv. Drug Deliv. Rev.97, 4–27. 10.1016/j.addr.2015.11.001
129
Thomas A. Orellano I. Lam T. Noichl B. Geiger M.-A. Amler A.-K. et al (2020). Vascular Bioprinting with Enzymatically Degradable Bioinks via Multi-Material Projection-Based Stereolithography. Acta Biomater.117, 121–132. 10.1016/j.actbio.2020.09.033
130
Tillman B. W. Yazdani S. K. Neff L. P. Corriere M. A. Christ G. J. Soker S. et al (2012). Bioengineered Vascular Access Maintains Structural Integrity in Response to Arteriovenous Flow and Repeated Needle Puncture. J. Vasc. Surg.56 (3), 783–793. 10.1016/j.jvs.2012.02.030
131
Tiwari A. Salacinski H. J. Punshon G. Hamilton G. Seifalian A. M. (2002). Development of a Hybrid Cardiovascular Graft Using a Tissue Engineering Approach 1. FASEB j.16 (8), 791–796. 10.1096/fj.01-0826com
132
Tuma M. Canestrini S. Alwahab Z. Marshall J. (2016). Trauma and Endothelial Glycocalyx. Shock46 (4), 352–357. 10.1097/shk.0000000000000635
133
Turner N. J. Kielty C. M. Walker M. G. Canfield A. E. (2004). A Novel Hyaluronan-Based Biomaterial (Hyaff-11) as a Scaffold for Endothelial Cells in Tissue Engineered Vascular Grafts. Biomaterials25 (28), 5955–5964. 10.1016/j.biomaterials.2004.02.002
134
Uchimido R. Schmidt E. P. Shapiro N. I. (2019). The Glycocalyx: a Novel Diagnostic and Therapeutic Target in Sepsis. Crit. Care23 (1), 16. 10.1186/s13054-018-2292-6
135
Uttayarat P. Perets A. Li M. Pimton P. Stachelek S. J. Alferiev I. et al (2010). Micropatterning of Three-Dimensional Electrospun Polyurethane Vascular Grafts. Acta Biomater.6 (11), 4229–4237. 10.1016/j.actbio.2010.06.008
136
Vallet S. D. Clerc O. Ricard-Blum S. (2021). Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem.69 (2), 93–104. 10.1369/0022155420946403
137
Vigetti D. Karousou E. Viola M. Deleonibus S. De Luca G. Passi A. (2014). Hyaluronan: Biosynthesis and Signaling. Biochim. Biophys. Acta (Bba) - Gen. Subjects1840 (8), 2452–2459. 10.1016/j.bbagen.2014.02.001
138
Vindigni V. Cortivo R. Iacobellis L. Abatangelo G. Zavan B. (2009). Hyaluronan Benzyl Ester as a Scaffold for Tissue Engineering. Ijms10 (7), 2972–2985. 10.3390/ijms10072972
139
Vynios D. H. Karamanos N. K. Tsiganos C. P. (2002). Advances in Analysis of Glycosaminoglycans: its Application for the Assessment of Physiological and Pathological States of Connective Tissues. J. Chromatogr. B Analyt Technol. Biomed. Life Sci.781 (1-2), 21–38. 10.1016/s1570-0232(02)00498-1
140
Wan X. Wang Y. Jin X. Li P. Yuan J. Shen J. (2018). Heparinized PCL/keratin Mats for Vascular Tissue Engineering Scaffold with Potential of Catalytic Nitric Oxide Generation. J. Biomater. Sci. Polym. Edition29 (14), 1785–1798. 10.1080/09205063.2018.1504192
141
Wang D. Wang X. Li X. Jiang L. Chang Z. Li Q. (2020). Biologically Responsive, Long-Term Release Nanocoating on an Electrospun Scaffold for Vascular Endothelialization and Anticoagulation. Mater. Sci. Eng. C107, 110212. 10.1016/j.msec.2019.110212
142
Wang D. Wang X. Zhang Z. Wang L. Li X. Xu Y. et al (2019). Programmed Release of Multimodal, Cross-Linked Vascular Endothelial Growth Factor and Heparin Layers on Electrospun Polycaprolactone Vascular Grafts. ACS Appl. Mater. Inter.11 (35), 32533–32542. 10.1021/acsami.9b10621
143
Wang S. Mo X. Jiang B. J. Gao C. J. Qiu H. S. Zhuang Y. G. et al (2013). Fabrication of Small-Diameter Vascular Scaffolds by Heparin-Bonded P(LLA-CL) Composite Nanofibers to Improve Graft Patency. Ijn8, 2131–2139. 10.2147/ijn.s44956
144
Wang W. Hu J. He C. Nie W. Feng W. Qiu K. et al (2015). Heparinized PLLA/PLCL Nanofibrous Scaffold for Potential Engineering of Small‐diameter Blood Vessel: Tunable Elasticity and Anticoagulation Property. J. Biomed. Mater. Res.103 (5), 1784–1797. 10.1002/jbm.a.35315
145
Wang W. Liu D. Li D. Du H. Zhang J. You Z. et al (2019). Nanofibrous Vascular Scaffold Prepared from Miscible Polymer Blend with Heparin/stromal Cell-Derived Factor-1 Alpha for Enhancing Anticoagulation and Endothelialization. Colloids Surf. B: Biointerfaces181, 963–972. 10.1016/j.colsurfb.2019.06.065
146
Wight T. N. (1980). Vessel Proteoglycans and Thrombogenesis. Prog. Hemost. Thromb.5, 1–39.
147
Wight T. N. (2018). A Role for Proteoglycans in Vascular Disease. Matrix Biol.71-72, 396–420. 10.1016/j.matbio.2018.02.019
148
Wu D. J. van Dongen K. Szymczyk W. Besseling P. J. Cardinaels R. M. Marchioli G. et al (2020). Optimization of Anti-kinking Designs for Vascular Grafts Based on Supramolecular Materials. Front. Mater.7 (220). 10.3389/fmats.2020.00220
149
Wu W. Allen R. A. Wang Y. (2012). Fast-degrading Elastomer Enables Rapid Remodeling of a Cell-free Synthetic Graft into a Neoartery. Nat. Med.18 (7), 1148–1153. 10.1038/nm.2821
150
Xu Z. Gu Y. Li J. Feng Z. Guo L. Tong Z. et al (2018). Vascular Remodeling Process of Heparin-Conjugated Poly(ε-Caprolactone) Scaffold in a Rat Abdominal Aorta Replacement Model. J. Vasc. Res.55 (6), 338–349. 10.1159/000494509
151
Yang Z. Tu Q. Wang J. Huang N. (2012). The Role of Heparin Binding Surfaces in the Direction of Endothelial and Smooth Muscle Cell Fate and Re-endothelialization. Biomaterials33 (28), 6615–6625. 10.1016/j.biomaterials.2012.06.055
152
Yao L. Y. Moody C. Schönherr E. Wight T. N. Sandell L. J. (1994). Identification of the Proteoglycan Versican in Aorta and Smooth Muscle Cells by DNA Sequence Analysis, In Situ Hybridization and Immunohistochemistry. Matrix Biol.14 (3), 213–225. 10.1016/0945-053x(94)90185-6
153
Yao T. Baker M. B. Moroni L. (2020). Strategies to Improve Nanofibrous Scaffolds for Vascular Tissue Engineering. Nanomaterials (Basel)10 (5). 10.3390/nano10050887
154
Yao Y. Wang J. Cui Y. Xu R. Wang Z. Zhang J. et al (2014). Effect of Sustained Heparin Release from PCL/chitosan Hybrid Small-Diameter Vascular Grafts on Anti-thrombogenic Property and Endothelialization. Acta Biomater.10 (6), 2739–2749. 10.1016/j.actbio.2014.02.042
155
Ye L. Wu X. Duan H. Y. Geng X. Chen B. Gu Y. Q. et al (2012). The In Vitro and In Vivo Biocompatibility Evaluation of Heparin-Poly(ε‐caprolactone) Conjugate for Vascular Tissue Engineering Scaffolds. J. Biomed. Mater. Res.100A (12), 3251–3258. 10.1002/jbm.a.34270
156
Ye L. Wu X. Mu Q. Chen B. Duan Y. Geng X. et al (2011). Heparin-Conjugated PCL Scaffolds Fabricated by Electrospinning and Loaded with Fibroblast Growth Factor 2. J. Biomater. Sci. Polym. Ed.22 (1-3), 389–406. 10.1163/092050610x487710
157
Yu J. Wang A. Tang Z. Henry J. Li-Ping Lee B. Zhu Y. et al (2012). The Effect of Stromal Cell-Derived Factor-1α/heparin Coating of Biodegradable Vascular Grafts on the Recruitment of Both Endothelial and Smooth Muscle Progenitor Cells for Accelerated Regeneration. Biomaterials33 (32), 8062–8074. 10.1016/j.biomaterials.2012.07.042
158
Yuan H. Qin J. Xie J. Li B. Yu Z. Peng Z. et al (2016). Highly Aligned Core-Shell Structured Nanofibers for Promoting Phenotypic Expression of vSMCs for Vascular Regeneration. Nanoscale8 (36), 16307–16322. 10.1039/c6nr05075a
159
Zamani M. Khafaji M. Naji M. Vossoughi M. Alemzadeh I. Haghighipour N. (2017). A Biomimetic Heparinized Composite Silk-Based Vascular Scaffold with Sustained Antithrombogenicity. Sci. Rep.7 (1), 4455. 10.1038/s41598-017-04510-1
160
Zavan B. Vindigni V. Lepidi S. Iacopetti I. Avruscio G. Abatangelo G. et al (2008). Neoarteries Grown In Vivo Using a Tissue‐engineered Hyaluronan‐based Scaffold. FASEB j.22 (8), 2853–2861. 10.1096/fj.08-107284
161
Zhang J. Wang J. Wei Y. Gao C. Chen X. Kong W. et al (2016). ECM-mimetic Heparin Glycosamioglycan-Functionalized Surface Favors Constructing Functional Vascular Smooth Muscle Tissue In Vitro. Colloids Surf. B: Biointerfaces146, 280–288. 10.1016/j.colsurfb.2016.06.023
162
Zheng M. H. Chen J. Kirilak Y. Willers C. Xu J. Wood D. (2005). Porcine Small Intestine Submucosa (SIS) Is Not an Acellular Collagenous Matrix and Contains Porcine DNA: Possible Implications in Human Implantation. J. Biomed. Mater. Res.73B (1), 61–67. 10.1002/jbm.b.30170
163
Zhou J. Ying H. Wang M. Su D. Lu G. Chen J. (2018). Dual Layer Collagen-GAG Conduit that Mimic Vascular Scaffold and Promote Blood Vessel Cells Adhesion, Proliferation and Elongation. Mater. Sci. Eng. C92, 447–452. 10.1016/j.msec.2018.06.072
164
Zhou M. Liu Z. Liu C. Jiang X. Wei Z. Qiao W. et al (2012). Tissue Engineering of Small-Diameter Vascular Grafts by Endothelial Progenitor Cells Seeding Heparin-Coated Decellularized Scaffolds. J. Biomed. Mater. Res.100B (1), 111–120. 10.1002/jbm.b.31928
165
Zhou M. Liu Z. Wei Z. Liu C. Qiao T. Ran F. et al (2009). Development and Validation of Small-Diameter Vascular Tissue from a Decellularized Scaffold Coated with Heparin and Vascular Endothelial Growth Factor. Artif. Organs33 (3), 230–239. 10.1111/j.1525-1594.2009.00713.x
166
Zhu C. Fan D. Wang Y. (2014). Human-like Collagen/hyaluronic Acid 3D Scaffolds for Vascular Tissue Engineering. Mater. Sci. Eng. C34, 393–401. 10.1016/j.msec.2013.09.044
Summary
Keywords
glycosaminoglycans, scaffolds, tissue engineering, vascular regeneration, vascular disease
Citation
Lepedda AJ, Nieddu G, Formato M, Baker MB, Fernández-Pérez J and Moroni L (2021) Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 9:680836. doi: 10.3389/fchem.2021.680836
Received
15 March 2021
Accepted
03 May 2021
Published
18 May 2021
Volume
9 - 2021
Edited by
Laura Russo, Università di Milano-Bicocca, Italy
Reviewed by
MariaCristina Tanzi, Politecnico di Milano, Italy
Iman Shabani, Amirkabir University of Technology, Iran
Updates
Copyright
© 2021 Lepedda, Nieddu, Formato, Baker, Fernández-Pérez and Moroni.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Moroni, l.moroni@maastrichtuniversity.nl
This article was submitted to Chemical Biology, a section of the journal Frontiers in Chemistry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.