Abstract
In this study, cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs) were synthesized in a controllable and one-step way under microwave-assisted conditions. The structure of CCPSF NPs was characterized by SEM images (for morphology and size distribution), TGA (for thermal stability), BET technique (for the specific surface area), FT-IR spectroscopy (for relation group characterization), and XRD patterns (for crystal size). The oxidation of the primary and secondary alcohols to aldehyde and ketone was investigated using synthesized CCPSF NPs under solvent-free microwave-assisted conditions, and high oxidizing activity was observed. In addition to oxidation properties, the anticancer activity of the synthesized CCPSF NPs in breast cancer was evaluated by the MTT method , and significant results were obtained.
1 Introduction
The increase in the world population causes an increase in the consumption of various substances. As we know, the use of traditional methods in the synthesis of compounds leads to environmental pollution. With the progress of science and technology, new and green methods have taken the place of classical methods. Therefore, the use of green methods leads to the reduction of environmental pollution and high productivity. One of the recently developed methods for the synthesis of green materials is the use of nanoparticles (Appa et al., 2019; Singh et al., 2021; Vallinayagam et al., 2021; Venkateswarlu, 2021; Alshahrani et al., 2022; Naidu and Venkateswarlu, 2022).
Nanomaterials with disordered structures such as carbon nanotubes, oxide structures, composites, and metal–organic frameworks (MOFs) have been synthesized and used for various applications (Wu et al., 2013; Athab et al., 2015; Al-Rowaili et al., 2018; Mohanta et al., 2019; Güemes et al., 2020). In the meantime, many applications such as therapeutic activities, gas storage, separation, and catalytic capabilities of MOF compounds have been reported (Chen et al., 2020; Wu et al., 2020). The review of past literature shows that MOF compounds have biological activities such as antitumor activity, antioxidant, DNA cleavage, antimicrobial, and biofilm inhibition activities (Rojas et al., 2014; Gecgel et al., 2022).
The significant porosity, high specific surface area, and small and uniform particle size can be mentioned among the factors that have affected the importance and applications of these compounds (Ding et al., 2019). Co-precipitation methods (Rani et al., 2020), such as sol–gel (Tarzanagh et al., 2019) and hydrothermal (Zhao et al., 2008) methods, are the methods that have been reported for the synthesis of MOF compounds.
It is very essential to use green and environmentally friendly methods to synthesize these compounds. Since 1986, microwave irradiating technology to speed up the process of chemical reactions has been used. In this method, the reaction is performed in a shorter time with high efficiency, and it is a convenient and effective technique for heating the reaction medium (Lee et al., 2004; Mohammadi et al., 2009; Ghalehbandi et al., 2020).
In the synthesis of MOF compounds, the choice of synthesis method is critical and affects the physical and chemical properties of the products. Reviewing the literature shows that the synthesis of these compounds using microwaves can affect their specific surface and improve their properties (Shu et al., 2020; Ma et al., 2021; Mirhosseini et al., 2021).
As mentioned, one of the applications of MOF compounds is to use them as catalysts. In this field, there have been many reports that these compounds have been used to synthesize organic compounds and polymers (Pascanu et al., 2019; Konnerth et al., 2020).
One of the essential developments in the synthesis of organic compounds is the production of carbonyl compounds by oxidizing alcohols (Ghafuri, 2015; Manesh and Nazari, 2015; Alshammari et al., 2017; Al-Khafaji et al., 2018).
Carbonyl-containing organic compounds by the creation of active intermediates are key chemical compounds for the synthesis of advanced chemicals and effective substances (Reis et al., 2010; Bayat et al., 2015; Sun et al., 2017).
Among the reactions that have recently received attention is the oxidation of alcohols using microwave radiation technology. According to the reactions related to the production of carbonyl compounds with the help of oxidation of alcohols, we conclude that there are still many opportunities to develop methods and achieve simpler, gentler, and environmentally friendly strategies (Hashemi et al., 2004; Ghalehbandi et al., 2020; Alameri and Almashhedy, 2021; Lihumis et al., 2022).
Considering the importance of the oxidation reaction of alcohols and the use of efficient catalysts and green methods, in this research, cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs) were synthesized by a microwave synthesis method and used as catalysts in the oxidation of alcohols under microwave conditions. The advantages of this catalyst are that they oxidize type-1 and type-2 alcohols and diols with higher efficiency and less time and can be reused. Continuing investigations on CCPSF NPs, their anticancer properties were evaluated, and they were also introduced as anticancer agents.
2 Experimental Section
2.1 Devices and materials
The SEM images were prepared using a Hitachi S-4800 FESEM. A thermal analyzer, STA 409, at a heating rate of 10°C/min was used to record TGA curves. A Nicolet AVATAR 360 FT-IR spectrophotometer was used to obtain the FT-IR spectrum of compounds. Philips XPERT PRO Cu-Kα radiation was used to obtain the XRD pattern of the compound, and finally, a Bruker FT-NMR Ultra Shield spectrometer (250 and 75 MHz) was used to obtain the 1H and 13C-NMR spectra. For the synthesis of cobalt composite, TAP SONIC (Fanavaran Nano-Meghyas) was used.
An advanced microwave synthesis laboratory station (MicroSYNTH, Milestone Co.) was used for microwave irradiation oxidation of alcohol derivatives.
The solvents and reagents used in this study were prepared by Sigma Aldrich and Merck.
2.2 Synthesis of cobalt composite
A mixture of 0.2 mmol of Co(NO3)2 and 0.6 mmol of pyridine-2,6 dicarboxylic acid in 50 ml of double-distilled water was stirred for 30 min at 70°C. The mixture was placed in an ultrasonicator under a power of 470 W for 20 min at 25°C. Finally, sediment crystals were isolated by centrifugation and nanofiltration, washed several times with acetic acid and water, and dried at room temperature.
2.3 Synthesis of cobalt composite immobilized on polysulfone fibrous network nanoparticles
A mixture of 0.05 g of polysulfone powder and 10 mg of cobalt composite was dissolved in 8 ml of acetic acid. A solution was electrospun at a voltage of 25 kV and a spinning distance of 15 cm, and to eject the solutions from the nozzle tip flow, a rate of 5 ml/h was used.
2.4 Microwave irradiation oxidation of alcohol derivatives by cobalt composite immobilized on polysulfone fibrous network nanoparticles
A mixture of 10 mmol of alcohol derivatives and 1 mg of CCPSF NPs was stirred at room temperature for 5 min, and then the mixture was irradiated. After completion of the reaction (monitored using thin-layer chromatography), the combinations were cooled, and CCPSF NPs were separated by nanofiltration. To reuse the catalyst, after its separation, it was washed several times with double-distilled water and ethanol and dried under vacuum at 100°C. Finally, for a pure product, crude was passed through a short silica gel column with ethyl acetate:ether (1:7) as solvent.
2.4.1 Aldehyde derivatives
1-Octanal (6B); IR (KBr) = 2,999, 2,841, 1725, and 1,487 cm−1; 1H NMR (250 MHz, CDCl3) δ = 9.48 (t, 1H), 2.31–238 (m, 2H), 1.42–1.49 (m, 2H), 1.22–1.27 (m, 8H), and 0.84 (t, 3H); 13C NMR (75 MHz, CDCl3) δ = 191.86, 42.75, 31.66, 30.24, 29.41, and 23.94.
4-Methoxybenzaldehyde (12B); IR (KBr) = 3,021, 2,948, 2,799, 1721, 1,646, 1,499, 1,320, 1,199, and 841 cm−1; 1H NMR (250 MHz, DMSO) δ = 9.91 (s, 1H), 7.65 (d, 2H, J = 8.4 Hz), 7.09 (d, 2H, J = 8.6 Hz), and 3.54 (s, 3H); 13C NMR (75 MHz, CDCl3) δ = 191.21, 131.59, 130.54, 114.79, and 55.26.
2.4.2 Ketone derivatives
2-Methylcyclopentaone (2D); IR (KBr) = 3,431, 2,901, 2,838, 1728, 1,427, 1,254, 1,137, and 876 cm−1; 1H NMR (250 MHz, CDCl3) δ = 2.51 (m, 1H), 2.34 (m, 1H), 2.09 (m, 1H), 1.81 (m, 1H), 1.58 (m, 1H), 1.49 (m, 1H), and 1.19 (m, 1H); 13C NMR (63 MHz, CDCl3) δ = 218.13, 44.01, 37.56, 33.45, 21.94, and 15.46.
Acetophenone (4D); IR (KBr) = 3,140, 3,054, 1701, 1,621, 1,354, 1,246, and 778 cm−1; 1H NMR (250 MHz, CDCl3) δ = 7.39–7.52 (m, 5H) and 2.31(s, 3H); 13C NMR (75 MHz, CDCl3) δ = 193.17, 135.82, 131.45, 127.99, 125.09, and 26.76.
2.4.3 Diketone derivatives
Acetylacetone (1F); IR (KBr) = 3,326, 2,458, 1728, and 1,614 cm−1; 1H NMR (250 MHz, DMSO) δ = 3.51 (s, 2H), 1.96 (s, 6H), and [15.66(O-H), 5.47 (vinyl H in enol form)]; 13C NMR (75 MHz, DMSO-d6) δ = 200.07, 57.95, 31.42, and [191.35, 101.21, 31.17, 22.86, in enol form].
2-Aminoanthraquinone (2F); IR (KBr) = 3,495, 1,676, 1,433, 1,298, 1,251, 1,149, 1,069, and 961, 777 cm−1; 1H NMR (250 MHz, DMSO) δ = 6.51–7.66 (m, 8H) and 2.21 (2H); 13C NMR (75 MHz, DMSO-d6) δ = 189.13, 178.21, 154.66, 135.27, 134.84, 134.18, 131.04, 126.95, 122.43, 118.79, and 110.07.
2.5 Anticancer activity
Anticancer activity studies of CCPSF NPs using the MTT method and previously reported on MCF-7 breast cancer cells were done. The densities of 1.2 × 104 (cells/well) MCF-7 breast cancer cells for 24 and 48 h in concentrations of 5, 10, 20, 40, 80, 120, and 200 mg/ml CCPSF NPs were tested (Moghaddam-Manesh and Hosseinzadegan, 2021).
3 Results and discussion
3.1 Characterization of cobalt composite immobilized on polysulfone fibrous network nanoparticles
Using ultrasonic and electrospinning methods, according to Scheme 1, cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs) were synthesized.
SCHEME 1

Synthesis of cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs) using ultrasonic and electrospinning methods.
To identify and confirm the structure of cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs), SEM images for morphology and size distribution, TGA for thermal stability, BET technique for specific surface area, FT-IR spectroscopy for relation group characterization, and XRD patterns for the crystal size were used.
SEM images of cobalt composite and CCPSF NPs are given in Figure 1.
FIGURE 1

SEM image of (I) cobalt composite and (II) CCPSF NPs.
Figure 1 shows that the size of the nanoparticles is in the nano range and has the same fiber morphology.
As we know, thermal stability is one of the practical factors in designing MOF nanostructures for application in various fields (Ding et al., 2019). The thermal stability curve of synthesized cobalt composite (I) and CCPSF NPs (II) are displayed in Figure 2. The thermal stability of CCPSF NPs was obtained at around 400°C. The high thermal stability of CCPSF NPs shows their high catalytic ability.
FIGURE 2
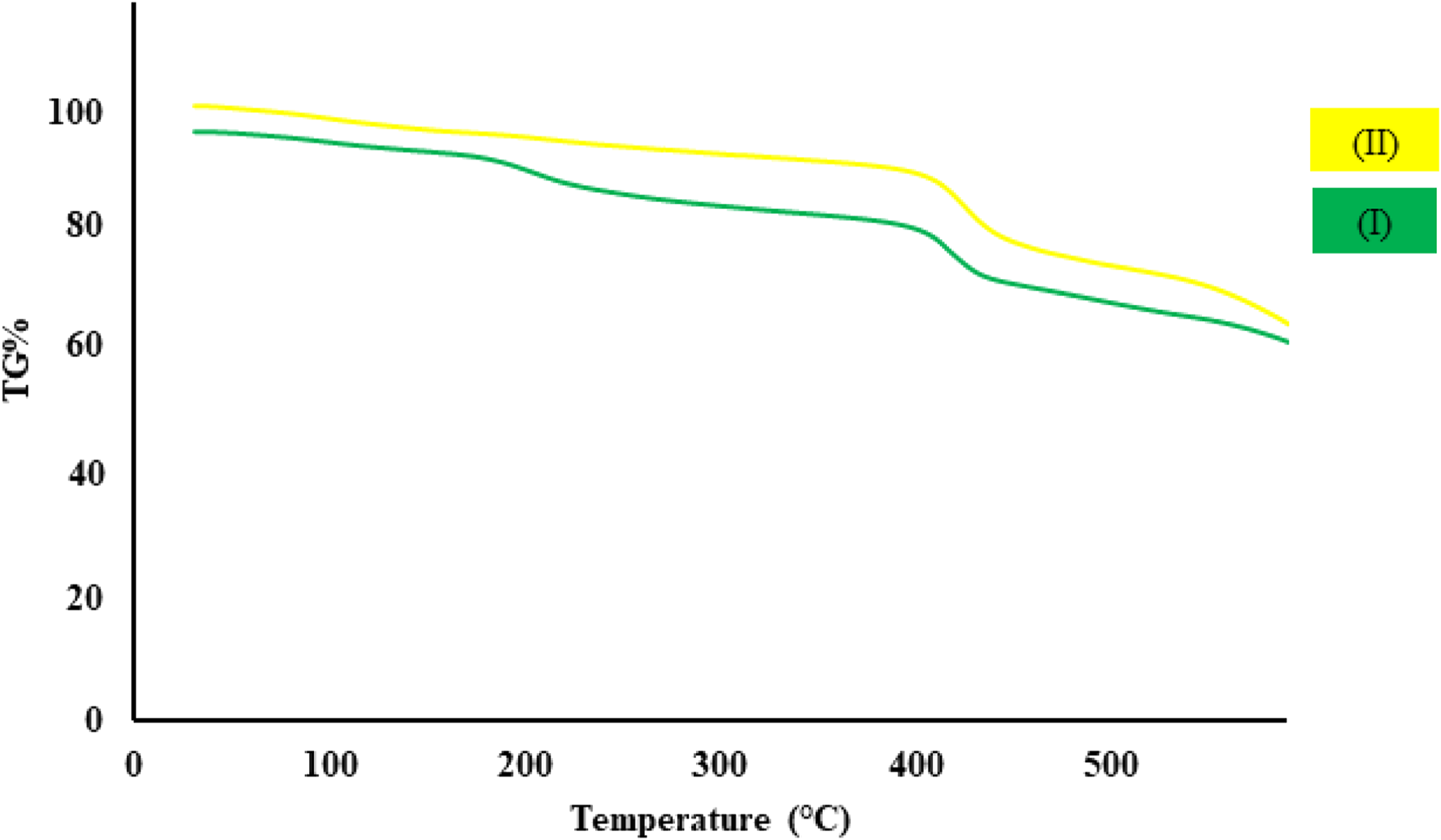
Thermal stability curve of synthesized cobalt composite (I) and CCPSF NPs (II).
The specific surface area of CCPSF NPs by N2 adsorption/desorption isotherms and BET technique 2,450 m2/g was obtained (Figure 3). The specific surface area obtained for CCPSF NPs proves that this compound has a high capability in the contact surface with combinations and use as a catalyst. It also seems that CCPSF NPs with an increased specific contact surface can create an increased contact surface with microbial agents and create a high effect.
FIGURE 3
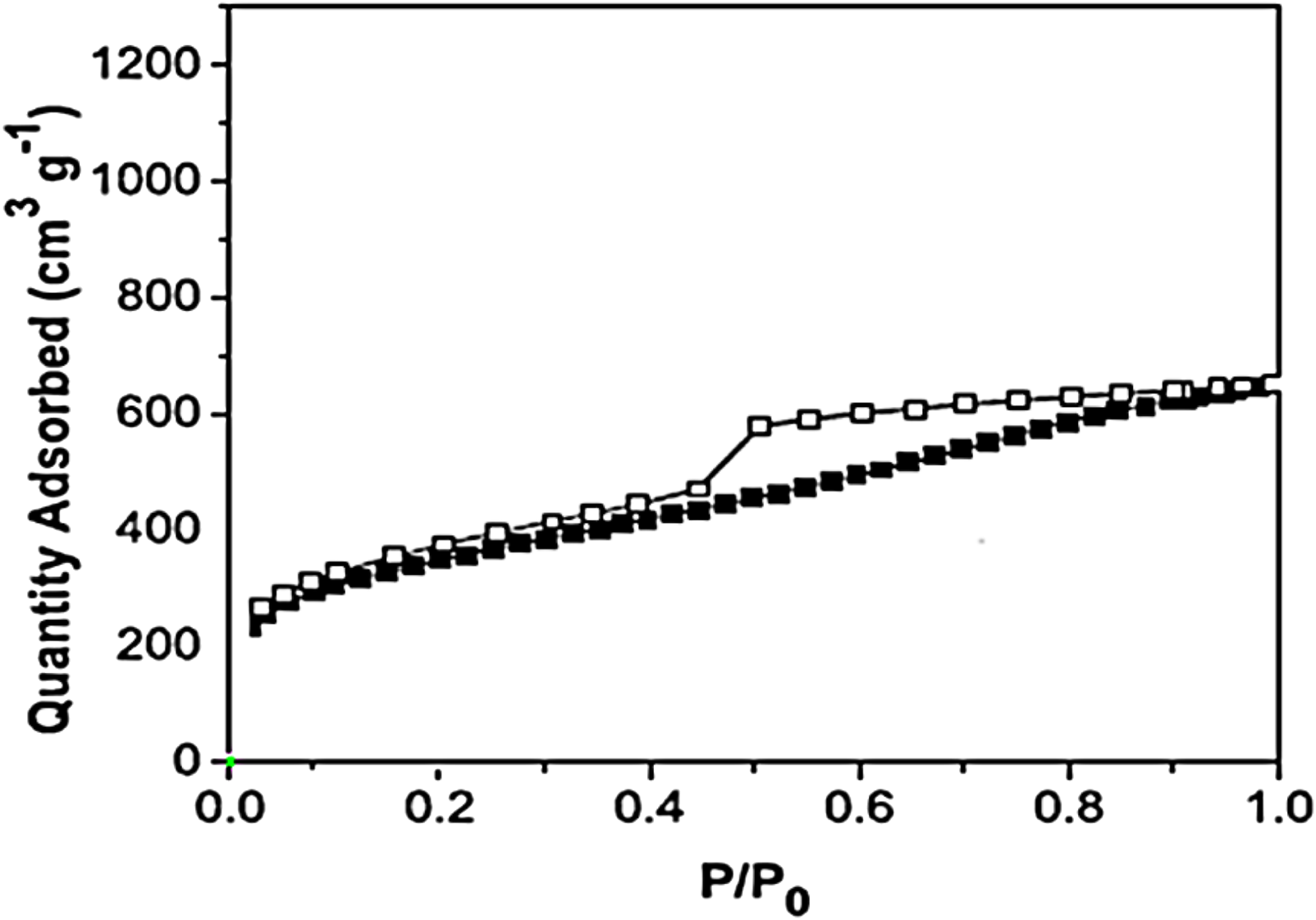
N2 adsorption/desorption of CCPSF NPs.
Based on the FT-IR spectrum (Figure 4), the peaks of the groups present in the structure of CCPSF NPs were observed. The peak in region 3,300 cm−1 was related to hydroxyl (OH) groups. Stretching peaks of C–H groups were shown near 3,067 cm−1 and 2,919 cm−1. The peak due to carbonyl groups (CO) was near 1,610 cm−1. The peak of C=C was at 1,538 cm−1. The bending peak of C–H was 1,435 cm−1. The S=O groups showed a peak in1370 cm−1. C–O, C–N, and C–S groups showed peaks at 1,206, 1,106, and 810 cm−1, respectively (Moghaddam-Manesh et al., 2020). The absorption due to Co–O was near 710 and 530 cm−1 (Hafeez et al., 2020).
FIGURE 4
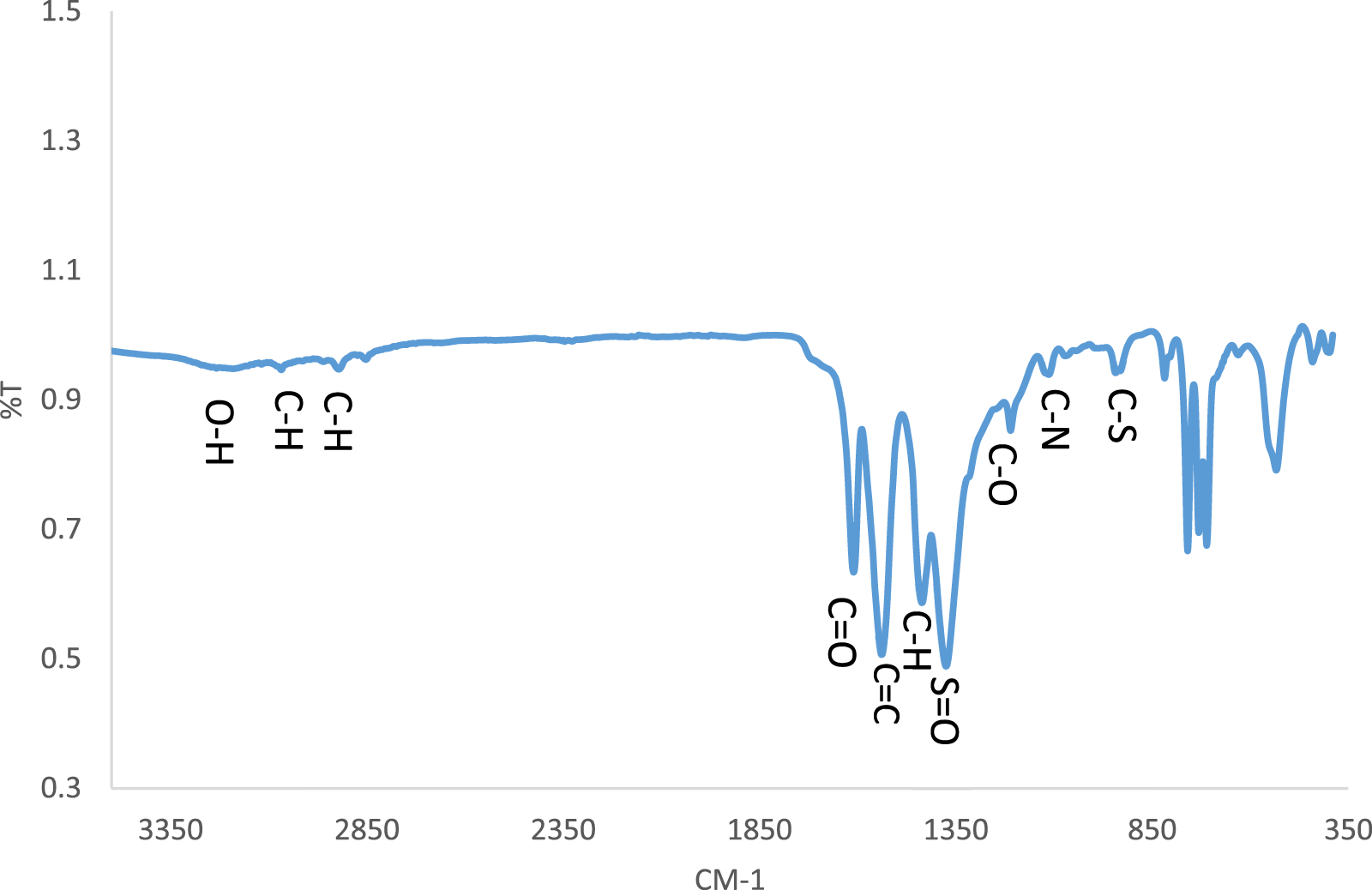
FT-IR spectrum of CCPSF NPs.
XRD patterns of CCPSF NPs are shown in Figure 5. XRD patterns obtained for CCPSF NPs are similar to XRD patterns reported for cobalt nanoparticles (Raza et al., 2016). The crystal size of CCPSF NPs obtained using the Debby Scherrer equation was about 31 nm.
FIGURE 5
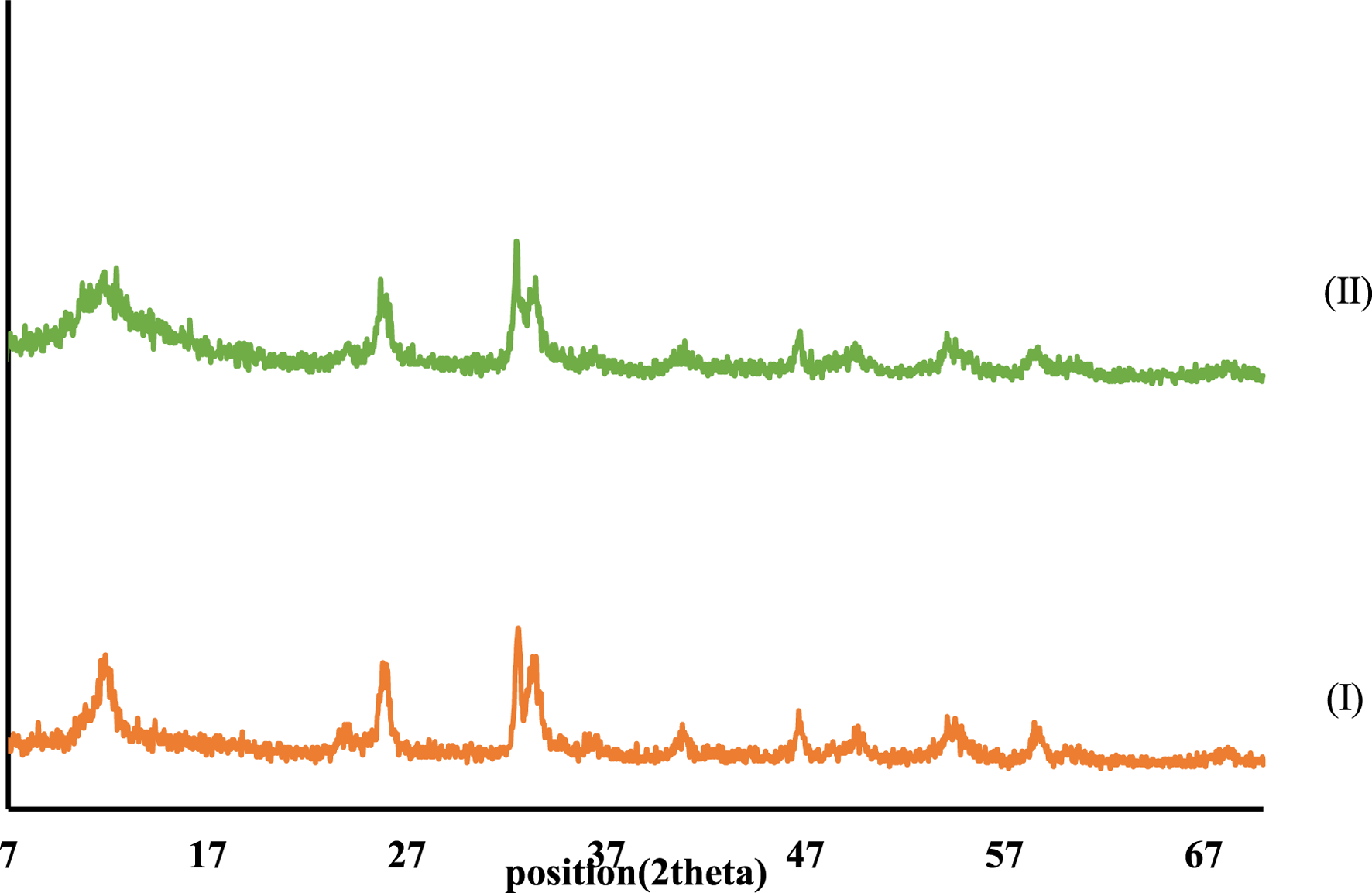
XRD patterns of synthesized cobalt composite (I) and CCPSF NPs (II).
The structure of Figure 6 was consistent for synthesized CCPSF NPs based on the analyses carried out.
FIGURE 6
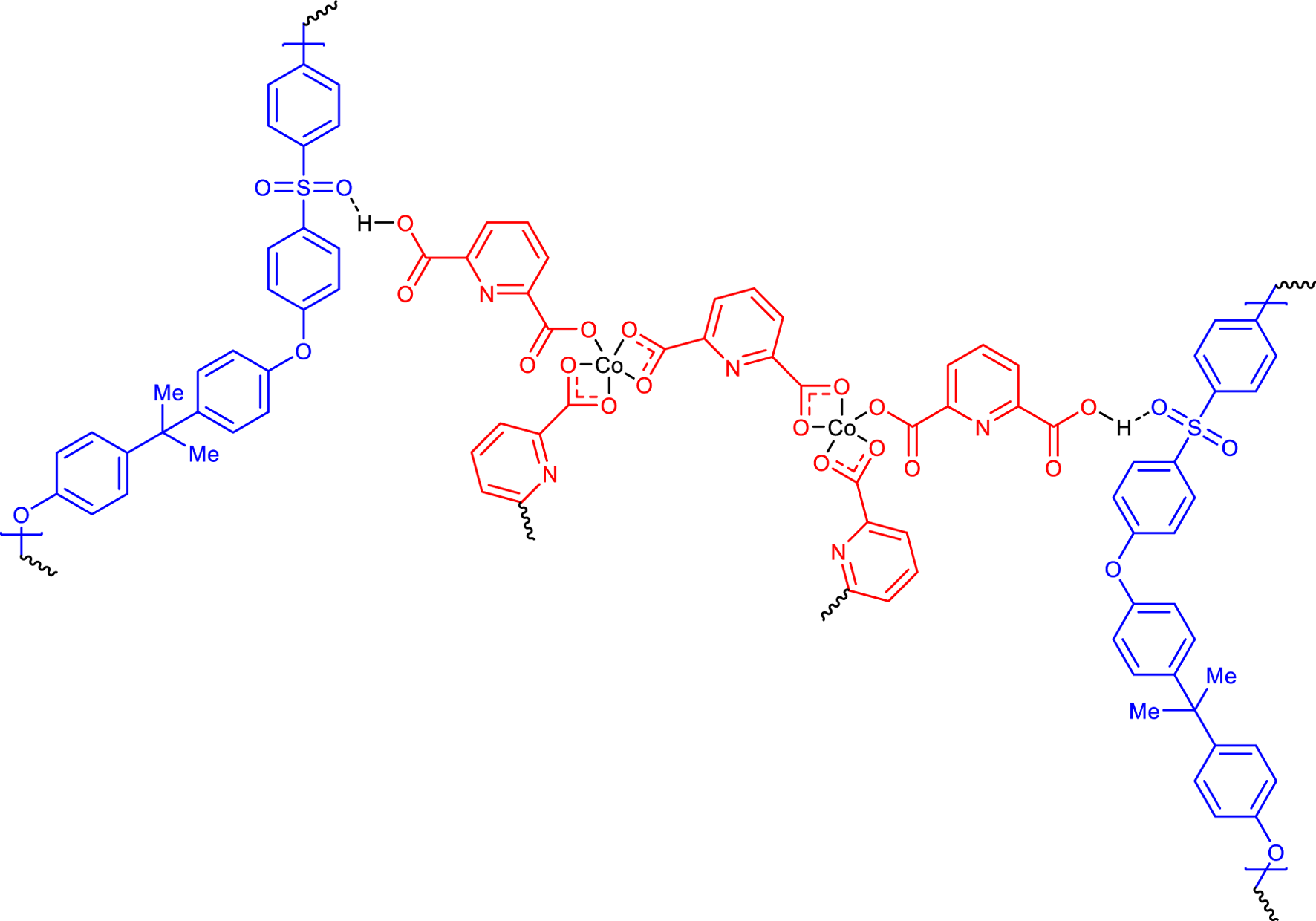
Proposed possible structure for CCPSF NPs.
3.2 Results of microwave irradiation oxidation of alcohol derivatives by cobalt composite immobilized on polysulfone fibrous network nanoparticles as nanocatalyst
In this research, for the oxidation of alcohol derivatives by microwave irradiation and CCPSF NPs as nanocatalysts, first, reaction conditions such as MW power and amount of catalyst for 1-butanol to 1-butanal were optimized (Scheme 2).
SCHEME 2

Microwave irradiation oxidation of 1-butanol to 1-butanal using CCPSF NPs as nanocatalysts.
The results of the optimization of 1-butanol to 1-butanal as a sample are given in Table 1.
TABLE 1
| Entry | mg catalyst | MW power (W) | Irradiation time (min) | Yield |
|---|---|---|---|---|
| 1 | 1 | — | reflux (12 h) | 61 |
| 2 | 1 | 300 | 10 | 72 |
| 3 | 1 | 400 | 5 | 91 |
| 4 | 1 | 500 | 2 | 95 |
| 5 | 2 | 500 | 2 | 95 |
| 6 | 3 | 500 | 5 | 89 |
| 7 | 4 | 500 | 10 | 85 |
| 8 | 5 | 500 | 20 | 71 |
Optimization conditions in the oxidation of 1-octanol to 1-octanal using CCPSF NPs as nanocatalysts under MW irradiation.
That the bold values indicates the selection of optimal conditions.
The optimal conditions: 1 mg of catalyst (CCPSF NPs) and power of microwave irradiation of 500 W for 2 min were used.
Using CCPSF NPs as nanocatalysts and microwave irradiation, oxidation of primary alcohol derivatives (Scheme 3-I), secondary alcohol derivatives (Scheme 3-II), and diol derivatives (Scheme 3-III) to aldehyde derivatives, ketone derivatives, and diketone derivatives, respectively, was studied.
SCHEME 3

Microwave irradiation oxidation of primary alcohol derivatives (I), secondary alcohol derivatives (II), and diol derivatives (III) using CCPSF NPs as nanocatalysts.
The rest of the primary alcohol derivatives, secondary alcohol derivatives, and diol derivatives studied in this research were oxidized using optimal conditions according to Tables 2–4.
TABLE 2
| Primary alcohol (A) | Aldehyde (B) | Time (min) | Yield (%) | Found M. P. () | Reported M. P. () (Ghalehbandi et al., 2020) | |
|---|---|---|---|---|---|---|
| 1 |

|

|
2 | 91 | Liq. | Liq. |
| 2 |

|

|
3 | 92 | Liq. | Liq. |
| 3 |

|

|
2 | 90 | Liq. | Liq. |
| 4 |

|

|
2 | 91 | Liq. | Liq. |
| 5 |

|

|
2 | 91 | Liq. | Liq. |
| 6 |

|

|
2 | 95 | Liq. | Liq. |
| 7 |

|

|
2 | 100 | Liq. | Liq. |
| 8 |

|

|
2 | 100 | Liq. | Liq. |
| 9 |

|

|
2 | 93 | Liq. | Liq. |
| 10 |

|

|
2 | 98 | 101–103 | 104 |
| 11 |

|

|
2 | 97 | 76–77 | 77 |
| 12 |

|

|
2 | 100 | Liq. | Liq. |
| 13 |

|

|
2 | 100 | Liq. | Liq. |
| 14 |

|

|
2 | 96 | 103–106 | 104–105 |
| 15 |

|

|
2 | 95 | Liq. | Liq. |
| 16 |

|

|
3 | 92 | Liq. | Liq. |
Green oxidation of primary alcohol derivatives to aldehyde derivatives using CCPSF NPs under MW irradiation.
Optimal conditions: 1 mg of CCPSF NPs and power of microwave irradiation 500 (W).
TABLE 3
| Secondary alcohol (C) | Ketone (D) | Time (min) | Yield (%) | Found M. P. () | Reported M. P. () (Ghalehbandi et al., 2020) | |
|---|---|---|---|---|---|---|
| 1 |

|

|
3 | 91 | Liq. | Liq. |
| 2 |

|

|
4 | 92 | Liq. | Liq. |
| 3 |

|

|
3 | 93 | Liq. | Liq. |
| 4 |

|

|
2 | 100 | Liq. | Liq. |
| 5 |

|

|
2 | 97 | 44–46 | 45 |
Green oxidation of secondary alcohol derivatives to ketone derivatives using CCPSF NPs under MW irradiation.
Optimal conditions: 1-mg of CCPSF NPs and power of microwave irradiation 500 (W).
TABLE 4
| Diol (E) | Diketone (F) | Time (min) | Yield (%) | Found M. P. () | Reported M. P. () (Ghalehbandi et al., 2020) | |
|---|---|---|---|---|---|---|
| 1 |

|

|
3 | 93 | Liq. | Liq. |
| 2 |

|

|
2 | 100 | 282 | 280 |
| 3 |

|

|
3 | 95 | 251–252 | 250–252 |
| 4 |

|

|
4 | 93 | 290–293 | 290 |
Green oxidation of diol derivatives to diketone derivatives using CCPSF NPs under MW irradiation.
Optimal conditions: 1 mg of CCPSF NPs and power of microwave irradiation 500 (W).
The results of Tables 2–4 showed that CCPSF NPs have a high ability to oxidize primary alcohol derivatives, secondary alcohol derivatives, and diol derivatives in optimal conditions with high efficiency.
The main advantage of the oxidation reaction using microwave irradiation and CCPSF NPs as nanocatalysts was that the reaction was carried out in solvent-free microwave-assisted conditions, and the reaction conditions were green.
Another advantage of the CCPSF NPs was their reusability, which was used up to five times (for 1-butanol). The results presented in Figure 7 show that the reaction efficiency did not change much after reuse. For leaching, after the catalyst was reused, a hot filtration test was done, and no enhancement in conversion was noticed in the filtrate.
FIGURE 7
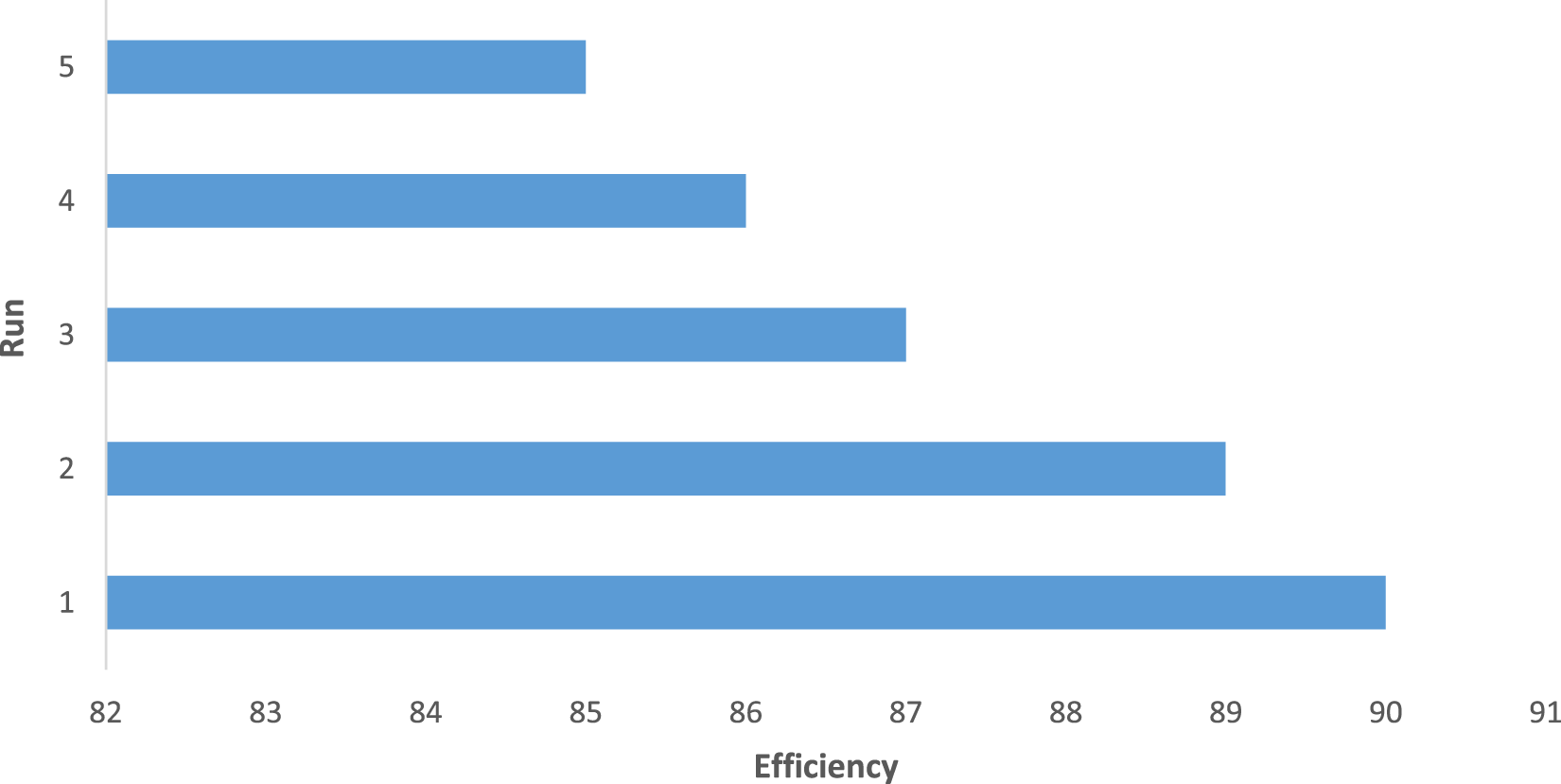
Reusability of CCPSF NPs in the oxidation of benzyl alcohol (row 13 of Table 2)
The proposed mechanism for the oxidation of alcohols using CCPSF NPs as catalyst is presented in Scheme 4.
SCHEME 4

Suggested mechanism for the oxidation of alcohols using CCPSF NPs as the catalyst.
Some of the recently reported methods for the oxidation of alcohols were using Pd@TiC (Bhaumik et al., 2016), silica-supported DABCO tribromide (Moghaddam et al., 2013), platinum (IV) complex (El-Bendary et al., 2022), and Rh/NAC catalysts (Verma et al., 2017) as catalysts. Table 5 compares some of the reported methods for the oxidation of benzyl alcohol in this study.
TABLE 5
| Entry | Reaction condition | Yield (%) | Time | Ref. |
|---|---|---|---|---|
| 1 | Pd@TiC | 97 | 8 (h) | Bhaumik et al. (2016) |
| 2 | Silica-supported DABCO tribromide | 95 | 1 (h) | Moghaddam et al. (2013) |
| 3 | Rh/NAC catalysts | 50 | 24 (h) | Verma et al. (2017) |
| 4 | This study (CCPSF NPs) | 100 | 2 (min) | — |
Different catalysts recently reported for the oxidation of benzyl alcohol.
Comparing the results proves that CCPSF NPs can oxidize benzyl alcohol with higher efficiency in less time.
3.3 Anticancer activity
The results of the anticancer activity of CCPSF NPs are shown in Figures 8–10.
FIGURE 8
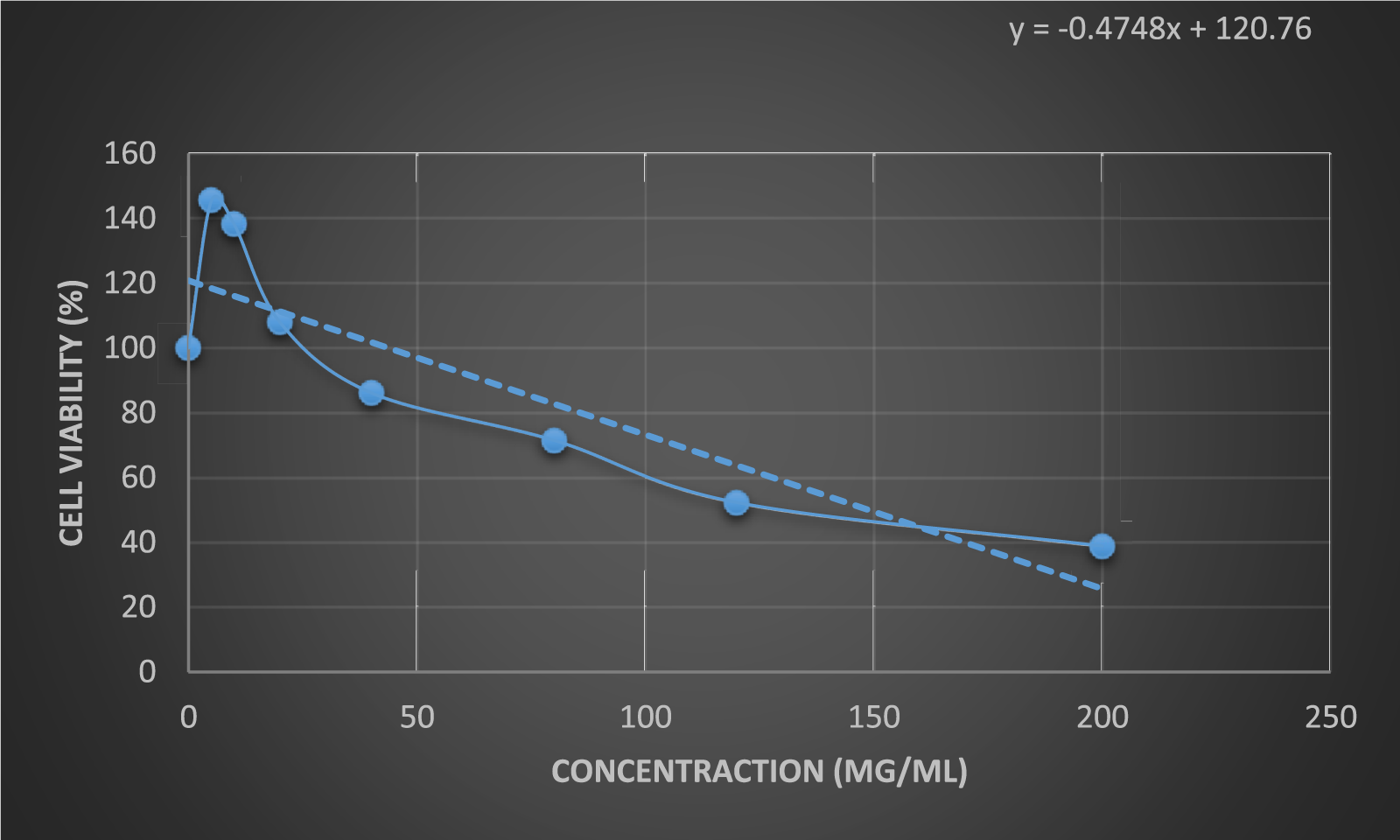
Anticancer activity of CCPSF NPs against MCF-7 breast cancer cells at 24 h. Data represented mean (n = 3) ± SD.
FIGURE 9
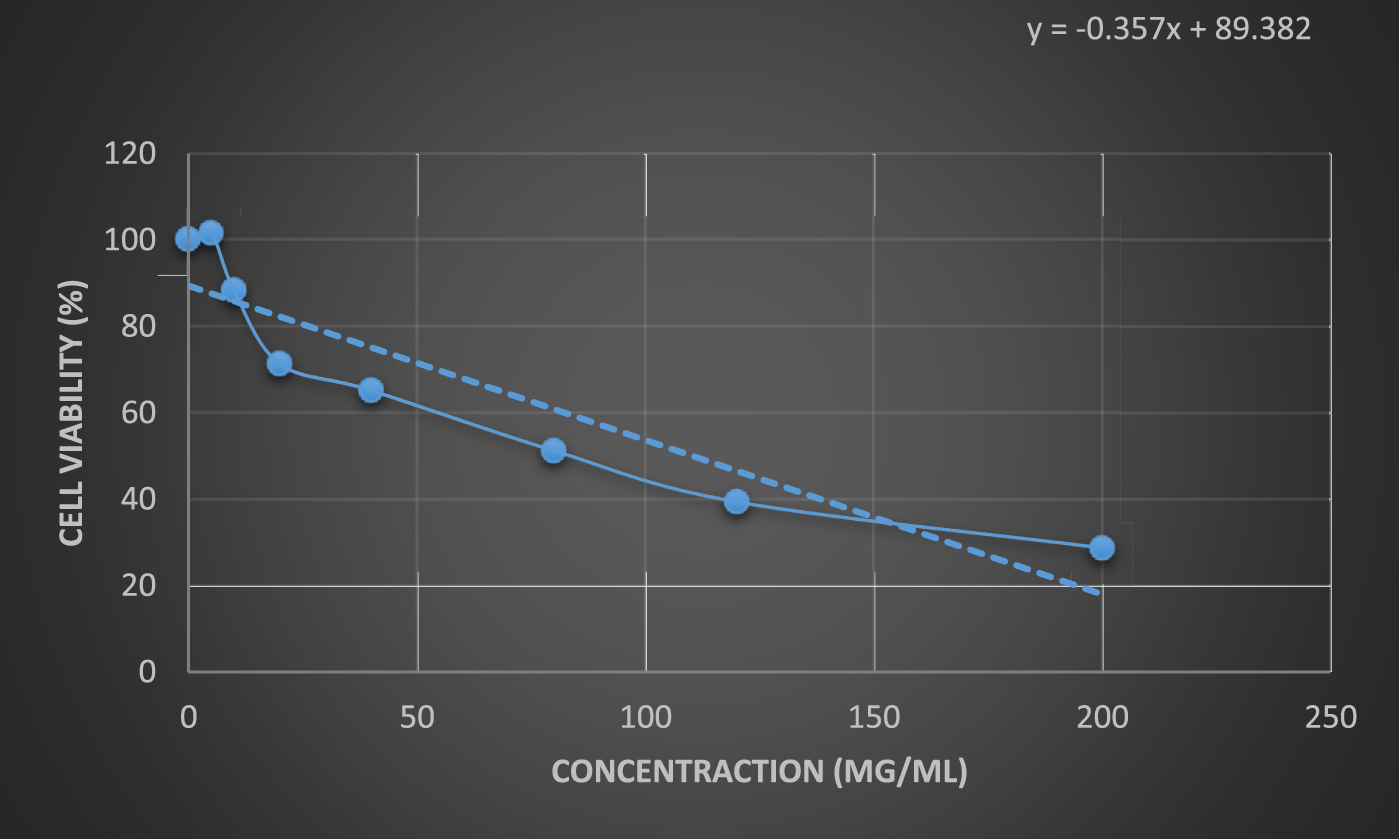
Anticancer activity of CCPSF NPs against MCF-7 breast cancer cells at 48 h. Data represented mean (n = 3) ± SD.
FIGURE 10
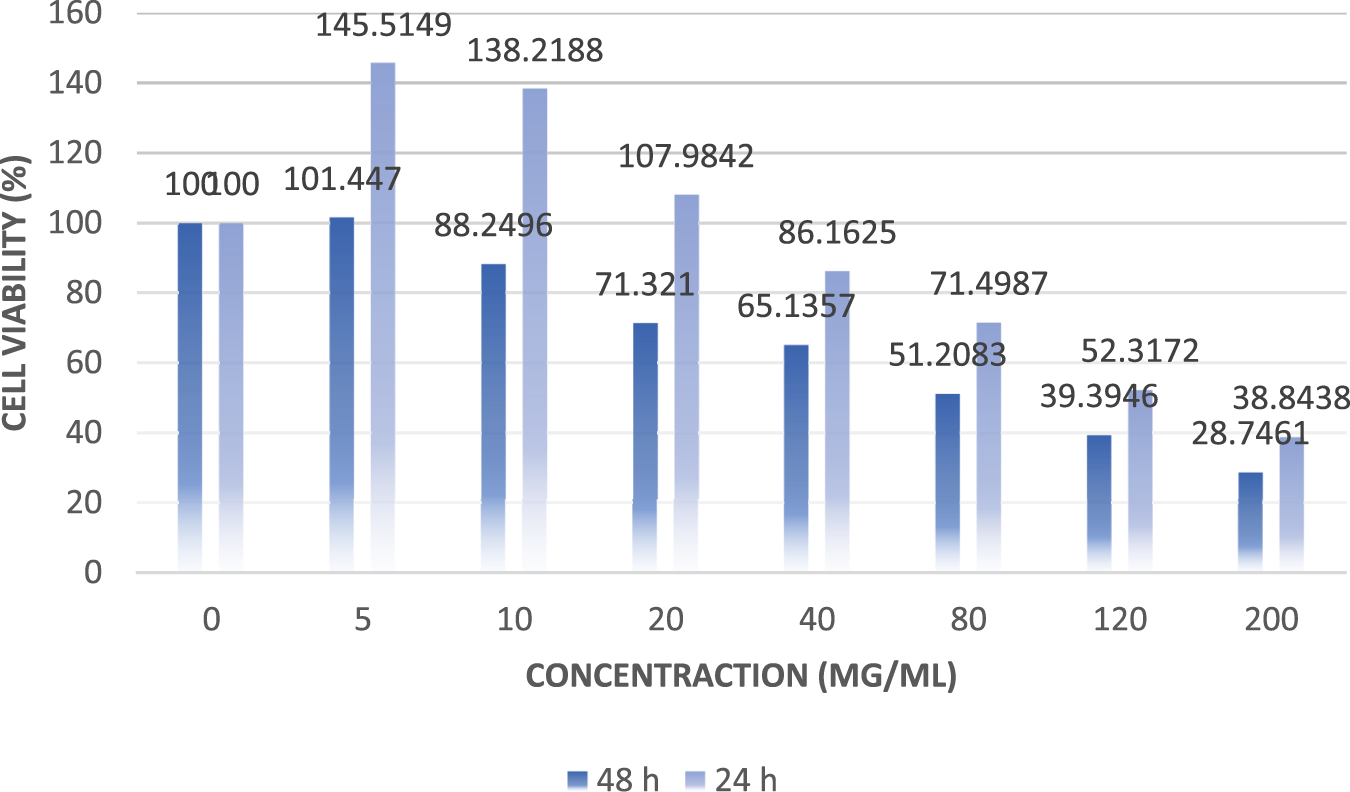
Comparing the results of anticancer activity of CCPSF NPs against MCF-7 breast cancer cells in 24 and 48 h.
The IC50 values of CCPSF NPs at 24 and 48 h, 149.0312 and 110.3137 mg/ml, respectively, were obtained. The cell proliferation and viability were then controlled at a concentration of 200 mg/ml at 24 and 48 h, and 38.9% and 28.8%, respectively, were observed. The comparison of 24 and 48 h is shown in Figure 10.
Based on the obtained results, it can be concluded that the effect of CCPSF NPs on MCF-7 breast cancer cells depends on concentration and time, and the impact increases with increasing concentration and time.
The anticancer activity of CCPSF NPs can be attributed to the presence of polysulfone (with high biological properties) and cobalt in their structure (Mohamad et al., 2019; Alkış et al., 2021; Chen et al., 2021), as well as its high specific surface area, which was created as a result of the appropriate synthesis method.
4 Conclusion
In the present research, using ultrasonic-assisted and electrospinning methods, cobalt composite immobilized on polysulfone fibrous network nanoparticles (CCPSF NPs) were synthesized. After confirming the structure of CCPSF NPs, it was proved that the synthesis method resulted in the synthesis of nanoparticles with a high specific surface area. The high specific surface area of CCPSF NPs made it possible for it to be used as a green, efficient, and reusable nanocatalyst in the oxidation of primary alcohols, secondary alcohols, and diols using microwave irradiation. The obtained results of oxidation of alcohols using CCPSF NPs were higher efficiency and less time required compared to previously reported methods. Among the other capabilities of CCPSF NPs that can be mentioned are their biological properties. In the biological evaluation of nanoparticles, anticancer properties against MCF-7 breast cancer cells were investigated. High effectiveness was observed, which can be attributed to the presence of polysulfone and cobalt in the structure and the high specific surface area of CCPSF NPs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the study and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1015515/full#supplementary-material
References
1
Al-Khafaji Y. F. Al-Lami M. R. Abbas A. S. Al-Ameri A. Mousa A. F. A. (2018). Synthesis and characterization of niobium and tantalum complexes with bidentate ligand and its use in ring opening polymerization of e-caprolactone. Asian J. Chem.30, 460–462. 10.14233/ajchem.2018.21144
2
Al-Rowaili F. N. Jamal A. Ba Shammakh M. S. Rana A. (2018). A review on recent advances for electrochemical reduction of carbon dioxide to methanol using metal–organic framework (MOF) and non-MOF catalysts: Challenges and future prospects. ACS Sustain. Chem. Eng.6, 15895–15914. 10.1021/acssuschemeng.8b03843
3
Alameri A. A. Almashhedy L. A. (2021). The association between adipolin and oxidative stress for diabetic female type II. Ann. Romanian Soc. Cell Biol.25, 1348–1357.
4
Alkış M. E. Keleştemür Ü. Alan Y. Turan N. Buldurun K. (2021). Cobalt and ruthenium complexes with pyrimidine based schiff base: Synthesis, characterization, anticancer activities and electrochemotherapy efficiency. J. Mol. Struct.1226, 129402. 10.1016/j.molstruc.2020.129402
5
Alshahrani S. H. Alameri A. A. Zabibah R. S. Jalil A. T. J. Ahmadi O. Behbudi G. (2022). Screening method synthesis of AgNPs using Petroselinum crispum (parsley) leaf: Spectral analysis of the particles and antibacterial study. J. Mex. Chem. Soc.66. 10.29356/jmcs.v66i4.1803
6
Alshammari H. Alhumaimess M. Alotaibi M. H. Alshammari A. S. (2017). Catalytic activity of bimetallic AuPd alloys supported MgO and MnO2 nanostructures and their role in selective aerobic oxidation of alcohols. J. King Saud Univ. - Sci.29, 561–566. 10.1016/j.jksus.2017.03.003
7
Appa R. M. Prasad S. S. Lakshmidevi J. Naidu B. R. Narasimhulu M. Venkateswarlu K. (2019). Palladium‐catalysed room‐temperature Suzuki–Miyaura coupling in water extract of pomegranate ash, a bio‐derived sustainable and renewable medium. Appl. Organomet. Chem.33, e5126. 10.1002/aoc.5126
8
Athab A. Lafta A. Hussein F. (2015). Modification of carbon nanotubes surface using different oxidizing agents. J. Environ. Anal. Chem.2, e112. 10.4172/2380-2391.1000e112
9
Bayat A. Shakourian-Fard M. Ramezanpour S. Hashemi M. M. (2015). A green procedure for direct oxidation of organic halides to aldehydes and ketones catalyzed by a molybdate-based catalyst. New J. Chem.39, 3845–3851. 10.1039/c4nj01886a
10
Bhaumik C. Stein D. Vincendeau S. Poli R. Manoury É. (2016). Oxidation of alcohols by TBHP in the presence of sub-stoichiometric amounts of MnO2. Comptes Rendus Chim.19, 566–570. 10.1016/j.crci.2016.02.012
11
Chen L. Zhang X. Cheng X. Xie Z. Kuang Q. Zheng L. (2020). The function of metal–organic frameworks in the application of MOF-based composites. Nanoscale Adv.2, 2628–2647. 10.1039/d0na00184h
12
Chen Y. Lin B. Qiu Y. (2021). Modification of polysulfone and the biomedical application of modified polysulfone. Int. J. Polym. Mater. Polym. Biomaterials, 1–19. 10.1080/00914037.2021.2006653
13
Ding M. Cai X. Jiang H.-L. (2019). Improving MOF stability: Approaches and applications. Chem. Sci.10, 10209–10230. 10.1039/c9sc03916c
14
El-Bendary M. M. Saleh T. S. Alomari M. M. Ali E. M. Davaasuren B. Jaremko M. et al (2022). Potential anticancer activities and catalytic oxidation efficiency of Platinum (IV) Complex. Molecules27, 4406. 10.3390/molecules27144406
15
Gecgel C. Gonca S. Turabik M. Özdemir S. (2022). An aluminum-based MOF and its amine form as novel biological active materials for antioxidant, DNA cleavage, antimicrobial, and biofilm inhibition activities. Mater. Today Sustain.19, 100204. 10.1016/j.mtsust.2022.100204
16
Ghafuri H. (2015). Transition metal-free oxidation of benzylic alcohols to carbonyl compounds by hydrogen peroxide in the presence of acidic silica gel. Curr. Chem. Lett.4, 27–32. 10.5267/j.ccl.2014.11.001
17
Ghalehbandi S. S. Ghazanfari D. Ahmadi S. A. Sheikhhosseini E. (2020). Solvent-free, microwave assisted oxidation of alcohols with 4-hydroxypyridinium chlorochromate functionalized silica gel. Rev. Roum. Chim.65, 283–289. 10.33224/rrch.2020.65.3.08
18
Güemes A. Fernandez-Lopez A. Pozo A. R. Sierra-Pérez J. (2020). Structural health monitoring for advanced composite structures: A review. J. Compos. Sci.4, 13. 10.3390/jcs4010013
19
Hafeez M. Shaheen R. Akram B. Haq S. Mahsud S. Ali S. et al (2020). Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mat. Res. Express7, 025019. 10.1088/2053-1591/ab70dd
20
Hashemi M. M. Ghazanfari D. Karimi-Jaberi Z. (2004). Oxidation of benzylic methylene compounds to ketones with 4-aminoperoxybenzoic acid supported on silica gel in presence of oxygen or air. Monatsh. Für. Chem./Chem. Mon.135, 185–188. 10.1007/s00706-003-0106-1
21
Konnerth H. Matsagar B. M. Chen S. S. Prechtl M. H. Shieh F.-K. Wu K. C.-W. (2020). Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev.416, 213319. 10.1016/j.ccr.2020.213319
22
Lee J. C. Lee J. Y. Lee S. J. (2004). Efficient oxidation of benzylic alcohols with [hydroxy (tosyloxy) iodo] benzene under microwave irradiation. Tetrahedron Lett.45, 4939–4941. 10.1016/j.tetlet.2004.04.132
23
Lihumis H. S. Alameri A. Zaooli R. Chemistry C. (2022). Design, synthesis of sulfadiazine derivatives bearing some new heterocyclic compounds with study antimicrobial and antioxidant activity. Egypt. J. Chem [Epub ahead of print]. 10.21608/ejchem.2022.131305.5787
24
Ma M. Bi Y. Tong Z. Liu Y. Lyu P. Wang R. et al (2021). Recent progress of MOF-derived porous carbon materials for microwave absorption. RSC Adv.11, 16572–16591. 10.1039/d1ra01880a
25
Manesh A. A. Nazari T. (2015). Silica supported Thallium (III) Nitrate: An effective oxidant for oxidation of alcohols to the carbonyl compounds. J. Chil. Chem. Soc.60, 3001–3004. 10.4067/s0717-97072015000300005
26
Mirhosseini H. Shamspur T. Mostafavi A. Sargazi G. (2021). A novel ultrasonic reverse micelle-assisted electrospun efficient route for Eu-MOF and Eu-MOF/CA composite nanofibers: A high performance photocatalytic treatment for removal of BG pollutant. Environ. Sci. Pollut. Res.28, 4317–4328. 10.1007/s11356-020-10746-8
27
Moghaddam F. M. Masoud N. Foroushani B. K. Saryazdi S. Ghonouei N. Daemi E. (2013). Silica-supported DABCO-tribromide: A recoverable reagent for oxidation of alcohols to the corresponding carbonyl compounds. Sci. Iran.20, 598–602. 10.1016/j.scient.2013.02.015
28
Moghaddam-Manesh M. Hosseinzadegan S. (2021). Introducing new method for the synthesis of polycyclic compounds containing [1, 3] dithiine derivatives, with anticancer and antibacterial activities against common bacterial strains between aquatic and human. J. Heterocycl. Chem.58, 2174–2180. 10.1002/jhet.4345
29
Moghaddam-Manesh M. Sheikhhosseini E. Ghazanfari D. Akhgar M. (2020). Synthesis of novel 2-oxospiro [indoline-3, 4′-[1, 3] dithiine]-5′-carbonitrile derivatives by new spiro [indoline-3, 4′-[1, 3] dithiine]@ Cu (NO3) 2 supported on Fe3O4@ gly@ CE MNPs as efficient catalyst and evaluation of biological activity. Bioorg. Chem.98, 103751. 10.1016/j.bioorg.2020.103751
30
Mohamad A. D. M. Abualreish M. Abu-Dief A. M. (2019). Antimicrobial and anticancer activities of cobalt (III)-hydrazone complexes: Solubilities and chemical potentials of transfer in different organic co-solvent-water mixtures. J. Mol. Liq.290, 111162. 10.1016/j.molliq.2019.111162
31
Mohammadi M. K. Ghammamy S. Imanieh H. (2009). Microwave‐assisted oxidation of alcohols and polyarenes with cetyltrimethylammonium bromochromate. Chin. J. Chem.27, 1501–1504. 10.1002/cjoc.200990252
32
Mohanta D. Patnaik S. Sood S. Das N. (2019). Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Analysis9, 293–300. 10.1016/j.jpha.2019.04.003
33
Naidu B. R. Venkateswarlu K. (2022). Wepa: A reusable waste biomass-derived catalyst for external oxidant/metal-free quinoxaline synthesis via tandem condensation–cyclization–oxidation of α-hydroxy ketones. Green Chem.24, 6215–6223. 10.1039/d2gc02386e
34
Pascanu V. GonzáLez Miera G. Inge A. K. Martín-Matute B. (2019). Metal–organic frameworks as catalysts for organic synthesis: A critical perspective. J. Am. Chem. Soc.141, 7223–7234. 10.1021/jacs.9b00733
35
Rani L. Kaushal J. Srivastav A. L. Mahajan P. (2020). A critical review on recent developments in MOF adsorbents for the elimination of toxic heavy metals from aqueous solutions. Environ. Sci. Pollut. Res.27, 44771–44796. 10.1007/s11356-020-10738-8
36
Raza M. A. Kanwal Z. Riaz S. Naseem S. (2016). “Synthesis, characterization and antibacterial properties of nano-sized cobalt particles,” in Proceedings of the 2016 world congress on advances in civil, enviromental, and materials research (ACEM16) (Korea: Jeju Province).
37
Reis D. C. Pinto M. C. Souza-Fagundes E. M. Wardell S. M. Wardell J. L. Beraldo H. (2010). Antimony (III) complexes with 2-benzoylpyridine-derived thiosemicarbazones: Cytotoxicity against human leukemia cell lines. Eur. J. Med. Chem.45, 3904–3910. 10.1016/j.ejmech.2010.05.044
38
Rojas S. Quartapelle-Procopio E. Carmona F. Romero M. Navarro J. Barea E. (2014). Biophysical characterisation, antitumor activity and MOF encapsulation of a half-sandwich ruthenium (II) mitoxantronato system. J. Mat. Chem. B2, 2473–2477. 10.1039/c3tb21455a
39
Shu J.-C. Yang X.-Y. Zhang X.-R. Huang X.-Y. Cao M.-S. Li L. et al (2020). Tailoring MOF-based materials to tune electromagnetic property for great microwave absorbers and devices. Carbon162, 157–171. 10.1016/j.carbon.2020.02.047
40
Singh R. Singh T. Hassan M. Larroche C. (2021). Biofuels from inulin-rich feedstocks: A comprehensive review. Bioresour. Technol.346, 126606. 10.1016/j.biortech.2021.126606
41
Sun A.-H. Pan J. Han S.-D. Xue X.-Y. Wei Q. Li J.-H. et al (2017). In situ ligand modification strategy for the construction of one-two-and three-dimensional heterometallic iodides. Inorg. Chem.56, 13785–13793. 10.1021/acs.inorgchem.7b01807
42
Tarzanagh Y. J. Seifzadeh D. Rajabalizadeh Z. Habibi-Yangjeh A. Khodayari A. Sohrabnezhad S. (2019). Sol-gel/MOF nanocomposite for effective protection of 2024 aluminum alloy against corrosion. Surf. Coatings Technol.380, 125038. 10.1016/j.surfcoat.2019.125038
43
Vallinayagam S. Rajendran K. Lakkaboyana S. K. Soontarapa K. Remya R. Sharma V. K. et al (2021). Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J. Environ. Chem. Eng.9, 106553. 10.1016/j.jece.2021.106553
44
Venkateswarlu K. (2021). Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett.19, 3887–3950. 10.1007/s10311-021-01253-4
45
Verma S. Baig R. N. Nadagouda M. N. Varma R. S. (2017). Aerobic oxidation of alcohols in visible light on Pd-grafted Ti cluster. Tetrahedron73, 5577–5580. 10.1016/j.tet.2016.07.070
46
Wu C. Feng F. Xie Y. (2013). Design of vanadium oxide structures with controllable electrical properties for energy applications. Chem. Soc. Rev.42, 5157–5183. 10.1039/c3cs35508j
47
Wu T. Liu X. Liu Y. Cheng M. Liu Z. Zeng G. et al (2020). Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev.403, 213097. 10.1016/j.ccr.2019.213097
48
Zhao H. Qu Z.-R. Ye H.-Y. Xiong R.-G. (2008). In situ hydrothermal synthesis of tetrazole coordination polymers with interesting physical properties. Chem. Soc. Rev.37, 84–100. 10.1039/b616738c
Summary
Keywords
green chemistry, nanocatalyst, oxidation of alcohols, microwave-assisted conditions, anticancer activity, cobalt composite immobilized on polysulfone
Citation
Ramírez-Coronel AA, Mezan SO, Patra I, Sivaraman R, Riadi Y, Khakberdiev S, Lafta HA, Abosaooda M, Turki Jalil A and Fakri Mustafa Y (2022) A green chemistry approach for oxidation of alcohols using novel bioactive cobalt composite immobilized on polysulfone fibrous network nanoparticles as a catalyst. Front. Chem. 10:1015515. doi: 10.3389/fchem.2022.1015515
Received
09 August 2022
Accepted
12 October 2022
Published
20 December 2022
Volume
10 - 2022
Edited by
Tamer S. Saleh, National Research Centre, Egypt
Reviewed by
Zhengping Dong, Lanzhou University, China
Katta Venkateswarlu, Yogi Vemana University, India
Updates
Copyright
© 2022 Ramírez-Coronel, Mezan, Patra, Sivaraman, Riadi, Khakberdiev, Lafta, Abosaooda, Turki Jalil and Fakri Mustafa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasser Fakri Mustafa, Dr.yassermustafa@uomosul.edu.iq; Abduladheem Turki Jalil, abedalazeem799@gmail.com
This article was submitted to Green and Sustainable Chemistry, a section of the journal Frontiers in Chemistry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.