Abstract
Tumor immunotherapy mainly relies on activating the immune system to achieve antitumor treatment. However, the present tumor immunotherapy used in the clinic showed low treatment efficacy with high systematic toxicity. To overcome the shortcomings of traditional drugs for immunotherapy, a series of antitumor immunotherapies based on nanomaterials have been developed to enhance the body’s antitumor immune response and reduce systematic toxicity. Due to the noninvasiveness, remote controllability, and high temporal and spatial resolution of light, photocontrolled nanomaterials irradiated by excitation light have been widely used in drug delivery and photocontrolled switching. This review aims to highlight recent advances in antitumor immunotherapy based on photocontrolled nanomaterials. We emphasized the advantages of nanocomposites for antitumor immunotherapy and highlighted the latest progress of antitumor immunotherapy based on photoactivated nanomaterials. Finally, the challenges and future prospects of light-activated nanomaterials in antitumor immunity are discussed.
1 Introduction
Cancer is one of the main causes of human death (Zaimy et al., 2017). Statistics show that there will be an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths worldwide in 2020 (Siegel et al., 2021; Sung et al., 2021). Therefore, the exploration of early diagnosis and effective treatment methods of cancer have attracted much attention. The traditional clinical methods of tumor treatment are mainly surgery, chemotherapy and radiotherapy (Burugu et al., 2017; Hojman et al., 2018), which have defects, such as poor efficacy and high toxicity (Zeng et al., 2021). In recent years, immunotherapy has become a promising method for the treatment of malignant tumors (Riley et al., 2019; Abbott & Ustoyev, 2019; Igarashi & Sasada, 2020; Yang F. et al., 2020). Immunotherapy is the artificial enhancement or suppression of the body’s immune function in the presence of hypo- or hyperfunctioning organisms for the purpose of treating disease. Tumor immunotherapy aims to improve the overall adaptability of the immune system by modulating key immune mechanisms (Topalian, 2017) and redirecting adaptive immune cells to destroy tumor-specific targets (June et al., 2018). To date, a variety of tumor immunotherapy methods have been discovered (Figure 1) (Chauhan et al., 2021; Kumar et al., 2021; Lesch & gill, 2021; Guo et al., 2022), including immune checkpoint blockade (Ribas & Wolchok, 2018; Havel et al., 2019; He & Xu, 2020), cancer vaccines (Duong et al., 2018; DeMaria & Bilusic, 2019; Shemesh et al., 2021), cell therapy (Fry et al., 2018; Mohanty et al., 2019; Wang et al., 2020), immunomodulatory small molecules (Cukier et al., 2017; Berraondo et al., 2019; Gracia, et al., 2019), etc. However, emerging tumor immunotherapy methods still face enormous challenges, such as the low efficacy of targeted drug therapy and the inherent toxicity of immunotherapy drugs, which may lead to severe inflammatory responses and autoimmune diseases (Emens et al., 2017; Kroschinsky et al., 2017). Therefore, it is essential to find a safer and more controllable method for tumor immunotherapy.
FIGURE 1

Tumor immunotherapy. (Reproduced from Chauhan et al., 2021, International Journal of Molecular Science; Guo et al., 2022, Biomaterials).
With the rapid development of nanotechnology, the clinical application of nanomaterials is also increasing (Ulbrich et al., 2016; Zang et al., 2017; Cheng et al., 2021). Due to their special physical and chemical properties, nanoparticles have significant potential therapeutic effects in tumor immunotherapy (Velpurisiva et al., 2017; Park et al., 2018; Irvine & Dane, 2020; Muluh et al., 2021). Tumor immunotherapy mainly relies on efficient drug delivery and targeted tumor therapy. Nanomaterials are used as transport carriers to form stable nanocomplexes through encapsulation or combination, which can improve the efficacy of tumor immunotherapy and reduce drug toxicity (Gonçalves et al., 2020). Furthermore, nanomaterials can enhance the drug delivery efficiency in an active or passive manner (enhanced permeability and retention (EPR) effect) (Liu et al., 2018; Kang et al., 2020; Li et al., 2022), enabling the delivery of drugs, antibodies or other immunotherapeutic agents to preferentially accumulate at the tumor site (Jain & Stylianopoulos, 2010), which can minimize side effects and improve therapeutic efficacy (Duong et al., 2018).
To date, phototherapy has attracted extensive attention in clinical treatment due to its remote controllability, high temporal and spatial resolution, noninvasiveness and high selectivity (Li J. et al., 2019; Wang M. et al., 2019; Zhao et al., 2019). At present, light-responsive nanomaterials mainly include organic materials (photosensitizers, fluorophores and carbon-based nanoparticles) and inorganic-based nanoparticles (quantum dots, upconverting nanoparticles and gold nanoparticles) (Choi & Frangioni, 2010; Son et al., 2019). Light-activated therapy is less invasive, much more precise and safer than traditional treatments such as chemotherapy, surgery and radiation (Riley et al., 2018).
UV light is most commonly used light for photocontrolled drug delivery, release or response owing to its capability to trigger a structural change in light-responsive systems. These photochemical reaction processes then lead to nanoparticle disassembly and the subsequently controllable release of payloads. Most UV light responsive nanomaterials have been modified with photocleavable terminal groups, photocleavable side chains or multiphotocleavable linkers (Yan et al., 2013; Sun et al., 2018), and the most commonly used photocleavable protecting groups are o-nitrobenzyl and coumarin derivatives. According to the photochemical reaction mechanisms, light-induced structural changes are often divided into three major processes: 1) photocleavage of light-responsive units, 2) photoisomerization, and 3) photocrosslinking/-decrosslinking (Zhao et al., 2019). However, light in the ultraviolet‒visible region has poor penetration ability in biological tissues and is harmful to the skin, while near-infrared (NIR) light has deeper tissue penetration and low toxicity, so the application of NIR light to trigger tumor therapy has stronger application potential (Wang et al., 2013; Yan & Li, 2016). The mechanism of converting NIR light irradiation into UV light is discussed in the section on upconversion nanoparticles.
For immune activation triggered by light-activated nanomaterials, the strategies mainly include using light to activate cancer vaccines, chimeric antigen, receptor (CAR)-T-cell therapy, immune checkpoint blockade (ICB) therapy, cytokine therapy, and immune adjuvant therapy (Chu et al., 2021). Phototherapy can enhance the therapeutic effect by amplifying antitumor immunity, reversing the tumor immunosuppressive microenvironment (TIME) and enhancing the effect of immunotherapy by producing an extremely immunogenic tumor microenvironment (TME) (Li H. et al., 2020; Shi et al., 2020). Phototherapy can be combined with immunotherapy to eliminate metastatic tumors. Moreover, when used in combination with conventional immunotherapy, phototherapy can promote the maturation of APCs to initiate immune responses (Jiang et al., 2020).
In recent years, a variety of photocontrolled nanomaterials have been developed, such as gold nanoparticles, carbon nanomaterials, and upconversion nanoparticles (Boyer et al., 2010; Zhang et al., 2016). Here, we mainly review the application of photocontrolled nanomaterials in antitumor immunotherapy.
2 Light-activated nanomaterials for tumor immunotherapy
2.1 Polymer nanomaterial-based antitumor immunity
Polymer-based nanoparticles can serve as excellent carriers for delivering biomolecules, drugs, genes and vaccines to tumor sites in vivo (Wei et al., 2021). Among them, conjugated polymers (CPs) have strong light absorption ability, good stability and biocompatibility in the NIR region. Xuan et al. reported an optogenetic system mediated by conjugated polymer nanoparticles (CPNs), which could activate immunotherapy in situ under NIR irradiation (Fu et al., 2021). Illumination of CPNs with NIR drives the heat shock promoter (HSP70) to trigger gene transcription of the interferon-γ (IFN-γ) cytokine. IFN-γ secreted by tumor cells induces the activation of surrounding tumor-associated macrophages through the IFN-γ-JAK-STA1 signaling pathway, which induces cancer cell killing through immunotherapy (Figure 2A).
FIGURE 2

(A) Schematic illustration of photothermal conjugated polymer nanoparticles (CPN) for remote control cancer immunotherapy (Reproduced from Fu et al., 2021, Advance Materials). (B) Schematic illustration of a microfluidic glass capillary mixer for the synthesis of PORGD NPs. (Reproduced from Wang M. et al., 2019, ACS Applied Materials & Interfaces). (C) Synthetic route and chemical structure of three conjugated polymers. (D) NIR-II fluorescence images of mice with 4T1 tumors at different time points after injection with P1 NPs under 808 nm illumination. (E) Quantitative NIR-II fluorescence signal intensity corresponding to the tumor sites at different time points in (D). (F) Corresponding infrared photothermal images of mice with 4T1 tumors after injection with PBS and P1 NPs under 1,064 nm laser excitation (G) Corresponding temperature changes at the tumor sites. (H) Tumor volumes and (I) weight growth curves of mice with 4T1 tumors treated with the four treatment groups at different time points. (J) Photo of excised tumors after 15 days of therapy. (Reproduced from Chen C. et al., 2021, Journal Materials Chemistry B).
Moreover, CPs can be facilely designed by using molecular engineering to possess certain electrical and optical properties for optimal photothermal therapy (PTT) performance (Tuncel & Demir, 2010; Guo et al., 2017; Qian et al., 2017). Wang et al. first demonstrated the synthesis of conjugated polymer nanoparticles (CP NPs) with a uniform diameter of 52 nm as PTT agents by using a modified nanoprecipitation process (Wang S. et al., 2019). Under 808 nm laser illumination, the thiolated cyclo (Arg-Gly-Asp-D-Phe-Lys (mpa)) peptide (c-RGD)-functionalized CP NPs exhibited high photothermal conversion efficiency, which activated a proinflammatory immune response and induced effective cancer cell death (Figure 2B). Furthermore, studies have found that NIR-Ⅱ light reduces light scattering and photon absorption in biological tissues, so it has better spatial resolution and lower autofluorescence intensity than traditional NIF-FI (700–900 nm) (Zhang D. X. et al., 2019; Hu et al., 2020a; Zhang et al., 2020a; Hu et al., 2020b; Yang J. et al., 2020). Thus, CPs are designed for NIR-Ⅱ because of their changeable chemical structures, adjustable NIR absorption, large Stokes shift, high extinction coefficient, and superior biocompatibility (Lin et al., 2017; Yin et al., 2017; Li T. et al., 2019; Li X. et al., 2019; Cui et al., 2020). Chen et al. designed and developed nanoparticles based on double-acceptor conjugated polymers (P1 NPs) for application in NIR-Ⅱ FI and NIR-Ⅱ PTT (Chen C. et al., 2021) (Figure 2C). The in vivo experiments demonstrated that P1 NPs not only exhibited high accumulation and a high sign-to background ratio (SBR) of vascular imaging at the tumor sites but also showed excellent NIR-II PTT efficiency for tumor treatment (Figures 2D–J).
Tumor cells can escape T-cell-mediated cytotoxicity using the programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) immune checkpoint (Goodman et al., 2017), so blocking the PD-1/PD-L1 checkpoint has been extensively studied in antitumor immunity (Akinleye & Rasool, 2019). Yu et al. designed a synthetic nanoparticulated PD-L antagonist consisting of poly (ethylene glycol)-poly (lactic acid-coglycolic acid) (PEG-PLGA) nanoparticles decorated with a PD-L1 binding peptide. Nanoparticles can accumulate in the tumor site and mediate strong photothermal effects, eliminate primary tumors treated by near infrared radiation and cause strong antitumor immunity by inducing immunogenic cell death (ICD) (Yu et al., 2022).
Semiconducting polymer nanoparticles (SNPs) are transformed from semiconducting polymers (SPs), which are composed of highly π-conjugated backbones (Li W. et al., 2020). Compared with most semiconductor inorganic nanoparticles, SNPs have good biocompatibility and optical properties, as well as excellent optical stability (Jiang B. P. et al., 2019; Li & Pu, 2020; Zhou et al., 2020; Zhen et al., 2021). Zhang et al. reported a semiconducting polymer nano-PROTAC (SPNpro) with phototherapy and activatable protein degrading capabilities for photoimmunometabolic cancer therapy (Zhang et al., 2021). Under NIR light irradiation, SPNpro can generate singlet oxygen to eliminate tumor cells and induce immunogenic cell death (ICD) to enhance tumor immunogenicity. In addition, cathepsin B, a cancer biomarker, can specifically activate the PROTAC of SPNpro, triggering the targeted proteolysis of immunosuppressant indoleamine 2,3-dioxygenase (IDO) in tumors. Sustained IDO degradation blocked the catabolic process of tryptophan (Trp) and promoted the activation of effector T cells (Figures 3A,B).
FIGURE 3

(A) Structure and cathepsin B (CatB)-specific activation mechanism of SPN pro. (B) SPN-mediated activation of two processes of photoimmunometabolic therapy. (Reproduced from Zhang et al., 2021, Nature Communitions). (C) Chemical structures of PFPR, PEG-PLGA, PCPDTBT, and the preparation of SPNI. (D) The process of immune activation mediated by SPNI-based precision photodynamic immunotherapy. (Reproduced from Liu et al., 2022, Advance Materials).
Liu et al. reported an amphipathic semiconductor polymer nanoimmunomodulator (SPNI) that absorbed NIR light to achieve PDT (Zhu et al., 2017) and conjugated with a Toll-like receptor 7 (TLR7) agonist (imiquimod: R837) via an acid-liable Schiff base linker (Figure 3C) (Liu et al., 2022). Introduction of R837 triggers ligation of TLR7 in endosomal membrane localization to promote DC maturation and secretion of proinflammatory cytokines (Lee et al., 2003). Under NIR light, SPNI has the photodynamic effect of direct tumor killing and death of immunogenic cancer cells. The synergistic action of the released immunogenic factor and TLR7 agonist activated by the acidic tumor microenvironment (TME) can be used as a tumor vaccine in situ with strong antitumor activity (Figure 3D) (Liu et al., 2022). Lyu et al. utilized the enzymatic oxidation properties of vinylidene bonds in combination with polymers to synthesize biodegradable semiconductor polymers (DPPV) and convert them into water-soluble nanoparticles (SPNV), which can enhance PA and PTT efficiencies for cancer therapy (Lyu et al., 2018). Wei et al. designed and synthesized a novel diketopyrrolopyrrole polymer nanoparticle [P(AcIID-DPP)], which exhibited strong light absorption and excellent photothermal conversion in the NIR-I to NIR-II optical region. capacity, high biocompatibility and photostability (Wei et al., 2018). In addition, nanoparticles can be efficiently absorbed by cancer cells and thermally ablated under NIR-II laser irradiation, exhibiting excellent anticancer effects. Jiang et al. synthesized an amphiphilic semiconductor polymer (PEG-PCB) that can not only be used as a diagnostic component in NIR fluorescence and PA imaging but also enable effective NIR fluorescence/PA imaging-guided photothermal therapy (Jiang et al., 2017).
Polylactic glycolic acid (PLGA) has controlled and sustained-release properties, low toxicity, and good biocompatibility and can be used for drug delivery, cancer imaging and therapy (Clawson et al., 2010; Danhier et al., 2012; Sadat Tabatabaei Mirakabad et al., 2014; Jia et al., 2018). Chen et al. discovered a kind of PLGA-IGG-R837 nanoparticle coated with the photothermal agent indocyanine green (IGG) and TLR7 ligand R837 (Chen et al., 2016). Under NIR light irradiation, PLGA-IGG-R837 ablated tumors by photothermal action and released tumor-associated antigens. Nanoparticle adjuvants loaded with R837 showed vaccine-like function, leading to immune responses. Luo et al. prepared biodegradable PLGA nanoparticles coloaded with hollow gold nanoshells (HAuNS) and anti-PD-1 peptide (APP) (AA@PN). NIR irradiation can not only trigger the release of APP and maintain a long-term immune response in vivo but also enable HAuNS to produce a photothermal effect to ablate tumors. The combined effect of NIR and HAuNS can produce a stronger antitumor effect (Luo et al., 2018).
2.2 Small molecule nanomedicine-based antitumor immunity
Small molecule nanomedicines (SMNs) refer to nanoscale drug delivery systems assembled from small molecule drugs (Ma et al., 2016; Wang Y. et al., 2017; Cheetham et al., 2017). Compared with traditional nanomedicines with complex preparation and possible toxicity of carrier materials, small molecule nanomedicines have been extensively studied (Luo et al., 2016; Li G. et al., 2021). All-drug small-molecule nanomedicines show excellent antitumor effects due to the synergistic effect of different drugs, but they have untraceable and undetermined defects (Xue et al., 2020). Adding photosensitizers to small-molecule nanomedicines can not only achieve light control but also enhance the effect of antitumor immunotherapy in combination with photodynamic therapy or photothermal therapy (Xue et al., 2019).
Li et al. self-assembled small-molecule nanoparticles by the interaction of photosensitizer ICG and epirubicin (EPI) in aqueous solution. ICG-EPI NPs exerted an excellent photothermal effect to ablate tumors under NIR laser irradiation and combined with chemotherapy drugs to further enhance the antitumor effect (Li et al., 2017). Zhang et al. assembled nanoparticles (DINP) using the hydrophobic drugs doxorubicin (DOX) and ICG and coated their surface with ruptured cancer cell membranes to form novel NIR-responsive and highly targeted small-molecule nanoparticles (DOX NPs@ICG@CCCM, DICNPs) (Zhang H. et al., 2018). The cancer cell membrane can enable DICNPs to target the tumor site. After reaching the tumor site, the cancer cell membrane was destroyed under NIR light irradiation to rapidly release DOX and ICG, thereby producing efficient chemical and photothermal effects to achieve antitumor immunotherapy. Zhang et al. assembled amphiphilic amino acids (9-fluorenylmethoxycarbonyl-L-leucine, Fmoc-ll) and photosensitive drugs (Ce6) with metal ions (Mn2+) to form an amino acid-porphyrin-Mn complex nanoplatform (FMCNPs) (Zhang N. et al., 2018). FMCNPs had high drug-loading capacity, good biocompatibility and MRI function and showed excellent tumor accumulation and photodynamic effects under NIR irradiation, which can effectively ablate tumors.
2.3 Porous silicon nanoparticle-based antitumor immunity
Due to its unique optical properties and biodegradability, porous silicon has been widely used in biomedical fields, such as drug delivery, biosensors and imaging (Martín-Palma et al., 2014; Li et al., 2018; Zhang R. et al., 2019; Tieu et al., 2021). Li et al. designed a selective photothermal and weak immunostimulatory nanovaccine based on porous silicon composite nanomaterials (Li J. et al., 2021). Porous silicon nanoparticles (PSiNPs) had a significant immunostimulatory effect on immune cells after special treatment and were coated with the cancer cell membrane (CCM) to obtain the CCM@PSiNPs@Au nanovaccine (Figure 4). Under irradiation with NIR light, immunostimulatory vaccines are released, which can induce the antitumor immune response of the body and control the overproduction of cytokines by immune cells, further enhancing the therapeutic effect of PTT.
FIGURE 4

(A–C) The schematic simulation of (A) the fabrication of PSiNPs, (B) PSiNPs@Au and CCM@(PSiNPs@Au) samples; and (C) the aggregation mechanism of particles induced by CCM coating during their coextrusion. (Reproduced from Li G. et al., 2021, Advance Materials).
NIR dye IR780 is a biodegradable photothermal and imaging agent that can be loaded into mesoporous silica nanoparticles (MSNs) to form biodegradable cores (Jiang et al., 2015; Zhan et al., 2017). Ma et al. coated IR780-loaded MSNs (IMs) with a prefabricated CAR-T membrane to prepare tumor-specific CAR-T membrane-wrapped nanoparticles (CIMSs) (Ma et al., 2020). Experiments in vitro and vivo show that CIMS has stronger tumor targeting and antitumor ability.
MSNPs, with higher intrinsic stability, higher drug loading and larger surface area, can deliver effective concentrations of drugs to tumor sites, which provides a new research direction for targeted drug delivery (Moradipour et al., 2020; Barkat et al., 2021; Ghaferi et al., 2021; Wang et al., 2021; Sheng et al., 2022; Xie et al., 2022), such as the delivery of anti-miR therapeutics (Zhang et al., 2014; Bertucci et al., 2015; Yu et al., 2016; Khatami et al., 2021). Yue et al. developed a multifunctional nanoplatform (MPSNs@R837) formed by mesoporous hexagonal core-shell zinc porphyrin silica nanoparticles (MPSNs) loaded with R837 (Toll-like receptor 7 agonist) (Yue et al., 2022). In the presence of light sources, MPSNs@R837 can effectively destroy primary tumors through PTT and PDT. In addition, the loaded immune adjuvant R837 can be functionalized with tumor-associated antigens, promote the maturation of DCs and trigger a strong immune response (Figure 5A).
FIGURE 5
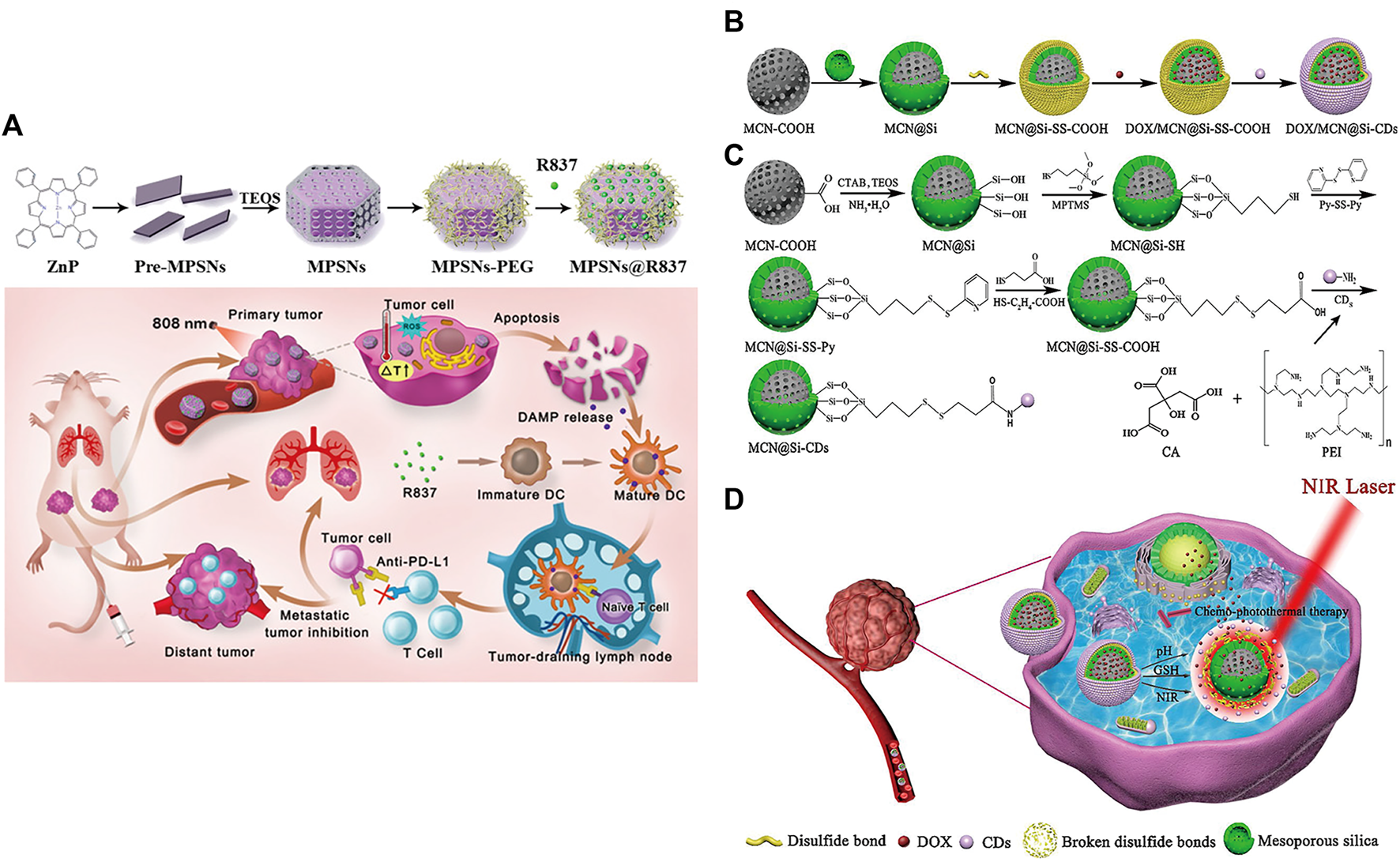
(A) Schematic illustration of the preparation of the core-shell zinc porphyrin nanoplatform (MPSNs@R837) and its use for synergistic antitumor immunity. (Reproduced from Yue et al., 2022, Journal of Nanobiotechnology). (B–D) Synthesis process of (B) DOX/MCN@Si-CDs and (C) MCN@Si-CDs. (D) Schematic illustration of chemo-photothermal synergistic therapy against tumors. (Reproduced from Lu et al., 2020, Colloids and Surfaces B: Biointerfaces).
However, MSNPs suffer from low biocompatibility and dispersibility, premature drug release, and interaction with erythrocyte membranes, leading to hemolysis (Bharti et al., 2015; Zhang et al., 2017). Lu et al. constructed multistimuli-responsive mesoporous silica-coated carbon nanoparticles (DOX/MCN@Si-Cd) with high drug loading capacity and high photothermal conversion efficiency (Lu et al., 2020). The appropriate size of carbon dots (Cd) prevented the premature release of DOX (Hu et al., 2016); DOX was released rapidly at low pH and high glutathione (GSH) concentrations (Cheng et al., 2011; Cui et al., 2012; Duan et al., 2019). Local high temperature generated under NIR radiation can not only directly kill the cells but also accelerate the release of DOX and improve the sensitivity and permeability of cells. The DOX/MCN@Si-Cd compound achieved accurate drug delivery, controlled drug release and synergistic chemo-photothermal antitumor therapy (Figures 5B–D) (Lu et al., 2020).
2.4 Carbon nanomaterial-based antitumor immunity
Carbon nanomaterials (carbon nanotubes, carbon quantum dots, graphene oxide, carbon nanohorns (Karousis et al., 2016), etc.) are widely used in medical research due to their ideal biocompatibility, unique photothermal conversion efficiency and other physiochemical and chemical properties (Jiang Y. et al., 2019; Giordani et al., 2019; Wiehe et al., 2019; Liu et al., 2020; Lee et al., 2021; Sainz-Urruela et al., 2021). Graphene quantum dots (GQDs) have been shown to produce singlet oxygen and other ROS under specific light activation, which is the key to the phototoxicity of PDT (Ge et al., 2014). Zhang et al. proposed a hybrid photosensitizer (GQD-PEG) based on the connection of the original GQDs to polyethylene glycol (PEG), which showed significant ROS generation efficiency and excellent biocompatibility under 560 nm laser irradiation (Zhang et al., 2020b). In addition, GQD-PEG showed a strong ablative effect under irradiation and a significant increase in antitumor immune-associated cytotoxic T lymphocytes (CTLs) and proinflammatory cytokines. Liu et al. found that water-soluble C (60)(OH)(20) nanoparticles have effective antitumor activity in vivo and can increase the production of T helper cell type 1 (Th1) cytokines and decrease the production of Th2 cytokines (Liu et al., 2009).
Single-walled carbon nanotubes (SWNTs) are characterized by strong absorbance in the NIR region (Zhou et al., 2009; Lin et al., 2022) and are able to cross cell membranes without causing cytotoxicity (Porter et al., 2007; Tajabadi, 2019). Some carbon-based nanomaterials can mature DCs and then stimulate an immune response, suggesting that they have potential immunoadjuvant properties in cancer immunotherapy (Wang et al., 2014). Zhou et al. designed a multifunctional SWNT system that can absorb NIR light to destroy tumor cells and carry immune stimulants into tumor cells to enhance tumor immunogenicity (Zhou et al., 2012). However, given the degradability of carbon nanomaterials in vivo (Chong et al., 2015), biodegradable carbon nanotubes or graphene oxide (GO) that have been reported thus far tend to have an inhomogeneous size or morphology, which may lead to uncertain side effects in vivo (Bianco, 2013). Thus, Wang et al. designed a degradable carbon-silica nanocomposite (CSN) with immunoadjuvant properties that could be degraded into small particles (∼5 nm) (Figure 6) (Wang et al., 2020). In vivo, the tumor inhibition efficiency of CSN was above 90% in the 4T1 tumor model and the PDX tumor model.
FIGURE 6
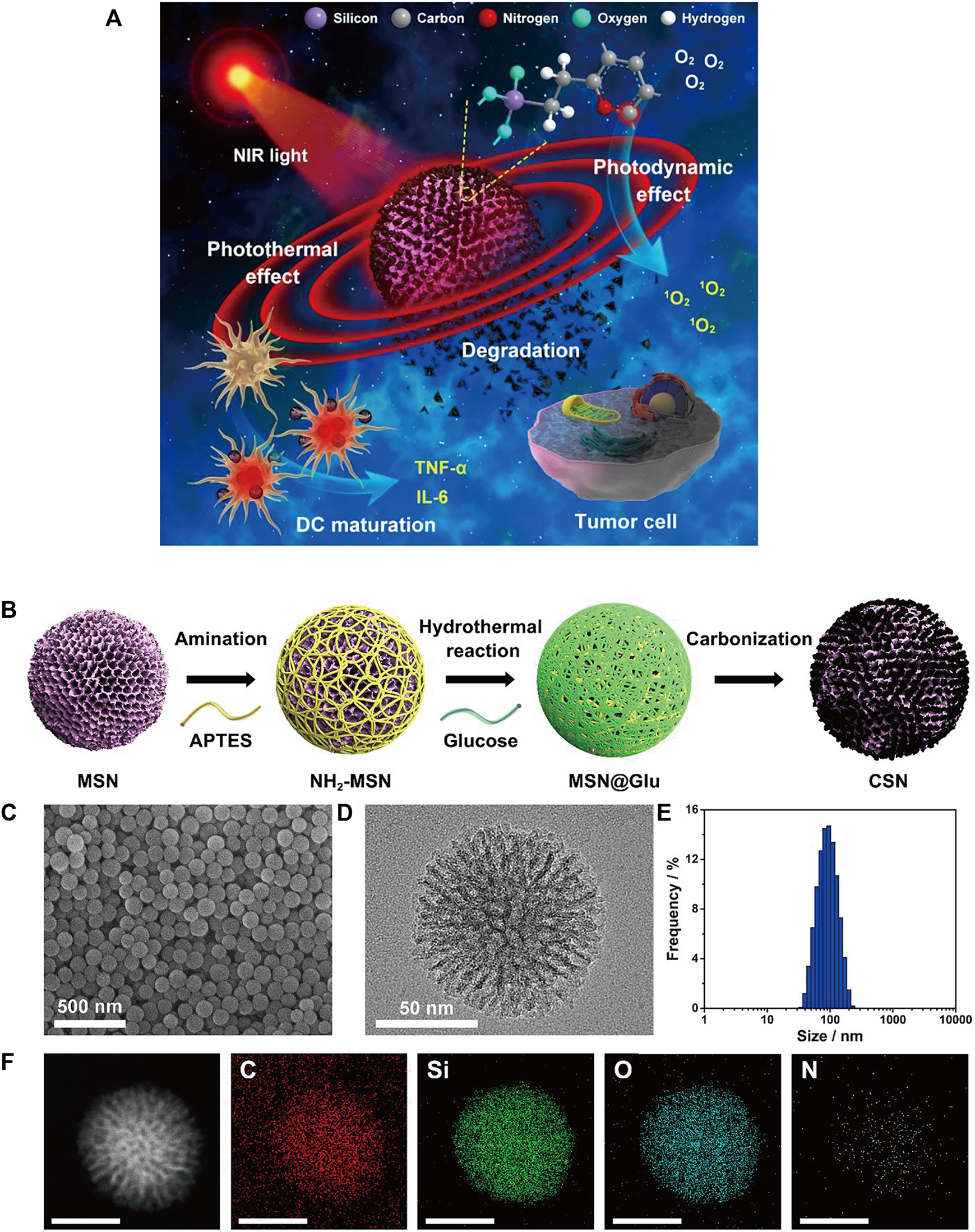
(A) Schematic illustration of degradable CSNs with immunoadjuvant properties for photothermal and photodynamic cancer therapy. (B) Schematic illustration of CSN synthesis. (C) SEM and (D) high-resolution transmission electron microscopy (HR-TEM) images of CSN. (E) Size distribution of CSN. (F) High-angle annular dark-field scanning TEM (HAADF-STEM) image and element mapping of CSN. (Reproduced from Wang et al., 2020, ACS Nano).
GO is considered a promising nanomaterial for NIR drug delivery systems due to its two-dimensional film structure, biocompatibility and near infrared absorption spectroscopy (Daniyal et al., 2020). Tao et al. applied a GO-PEG-PEI nanosystem to efficiently deliver CpG, and its NIR light absorbance can control the immune stimulation activity of CpG ODNs (Tao et al., 2014). Under irradiation with NIR light, the intracellular transport of nanocarriers was accelerated due to PTT, and the immune stimulation response was significantly enhanced. Zhou et al. constructed a nanosystem (rGO/MTX/SB) that loaded the chemotherapy agent mitoxantrone (MTX) and transforming growth factor-β (TGF-β) inhibitor SB-431542 (SB) onto reduced graphene oxide (rGO) (Zhou B. et al., 2021). Under noninvasive NIR light irradiation, MTX-induced ICD effectively activated systemic antitumor immune responses, and SB helped to alter the tumor microenvironment to enhance reduced graphene oxide (rGO) (Figure 7). This synergistic therapy induced superior antitumor immunity, tumor killing and immune processes and triggered effective CTL control of metastasis.
FIGURE 7
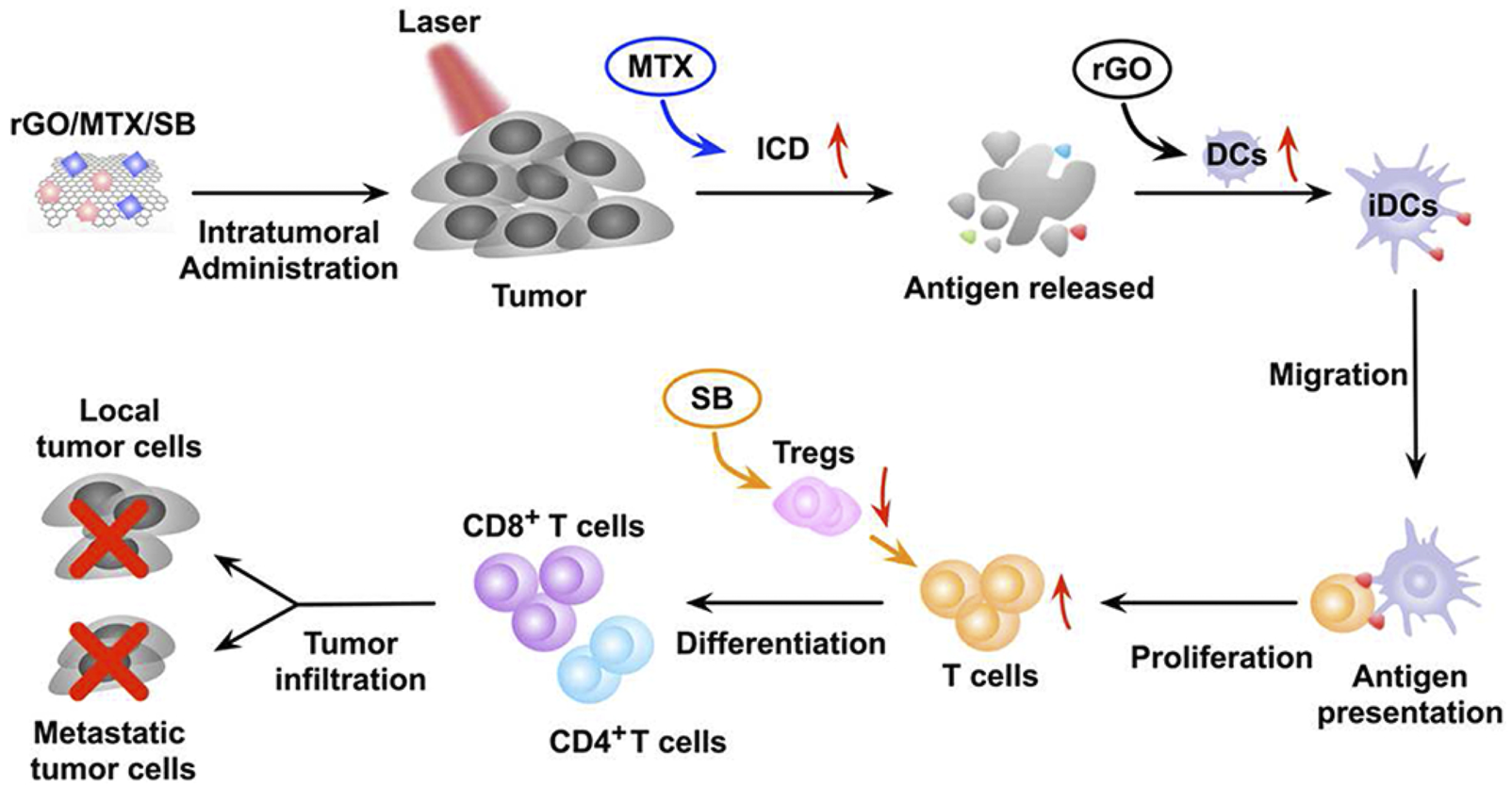
The mechanism of the antitumor immune response induced by rGO/MTX/SB-based PTT. (Reproduced from Zhou B. et al., 2021, Biomaterials).
2.5 Metal nanomaterial-based antitumor immunity
Metal nanomaterials have been widely used in biomedical fields due to their good physicochemical properties (Popescu, et al., 2015; Vimbela, et al., 2017). Among them, gold nanomaterials have the advantages of photocontrol ability, chemical inertness and minimal toxicity (Kohout et al., 2018; Zhou F. et al., 2021) and are widely used in the diagnosis and treatment of tumors (Singh et al., 2018; Ding et al., 2020; Guinart et al., 2020; Essawy et al., 2021). Upconversion nanoparticles are also in the category of metal nanoparticles, but they are a relatively special metal rare earth element that can be used for more effective and safer cancer treatment (Li H. et al., 2020; Liu X. et al., 2021). In addition, there are other metal nanomaterials, such as Pt, Cu and Fe, which are used in cancer therapy due to their unique physicochemical properties.
2.5.1 Gold nanomaterial-based antitumor immunity
Gold nanorods (AuNRs) with tunable and strong NIR absorption are considered one of the most promising drugs for tumor therapy and diagnosis (Lee & Gaharwar, 2020). Yata et al. designed a composite immunostimulatory DNA hydrogel, mixing appropriately designed hexapods with CpG-modified gold nanoparticles to form a composite gold nanoparticle-DNA hydrogel (Yata et al., 2017). Under laser irradiation, the hydrogel released hexapods, effectively stimulating immune cells and releasing proinflammatory cytokines. Ahn et al. reported an AuNP-based therapeutic cancer vaccine carrying endogenous EDB autoantigens (Ahn et al., 2014). Gold nanoparticles can effectively deliver antigens to dendritic cells and induce antigen-specific cytotoxic T lymphocyte responses for effective cancer therapy. Khoobchandani et al. designed a novel nanodrug MGF-AuNP formed by encapsulating mangiferin (MGF) with gold nanoparticles, which can provide an effective immunomodulatory intervention by targeting the tumor microenvironment (Khoobchandani et al., 2021).
The tumor microenvironment is an indispensable part of tumors (Pitt et al., 2016) and is one of the key factors affecting immunotherapy effects (Osipov et al., 2019; Xiao & Yu, 2021). Tian et al. designed a multifunctional nanoparticle (HA-AuNR/M-M2pep NP) to overcome the limitations of the tumor microenvironment on immunotherapy efficiency. It is composed of gold nanorods (HA-AuNR) modified with M2pep melt peptide (M-M2pep) in response to hyaluronic acid (HA) and matrix metalloproteinase-2 (MMP2). Precise PTT can be achieved under NIR light irradiation, triggering tumor immunogenic cell death and antitumor immunity irradiation (Figure 8A). Meanwhile, the nanoparticles release M2pep by cleaving MMP2-sensitive peptide, which can improve the immune activity of the TME and further enhance the antitumor efficacy (Tian et al., 2021).
FIGURE 8

(A) Schematic illustration of enhanced photoimmunotherapy by the combined effect of PTT-induced immune activation and M2-TAM depletion. (Reproduced from Tian et al., 2021, Colloids and Surfaces B: Biointerfaces). (B–D) Schematic illustration of the photothermal genome-editing strategy for cancer immunotherapy. (B) Process of preparation of the ANP/HSP-Cas9 plasmid complex. (C) Illustration of photothermal activation for PD-L1 genome editing in tumor cells. (D) Photoactivable CRISPR/Cas9 strategy reprograms the immunosuppressive tumor environment. (Reproduced from Tang et al., 2021, Advance Materials). (E) Schematic illustration of the combination of photothermal and immunotherapy by Au@Pt-LMDP. (Reproduced from Yang et al., 2019, Journal of Controlled release).
Tang et al. used supramolecular gold nanorods to target and block the immune checkpoint (PD-L1-CRISPR/Cas9), which blocked the gene expression of PD-L1 under NIR light irradiation to improve the transformation of dendritic cells into T cells, promote T-cell infiltration and enhance antitumor immunity in the body (Figures 8B–D) (Tang et al., 2021). In addition, the gold nanorods can produce mild hyperthermia to induce immunogenic cell death after NIR light irradiation and further enhance tumor immunotherapy. Yang et al. reported a Au@Pt-LMDP nanosystem conjugated by Au@Pt with a reasonably designed peptide (LYP-1-PLGVRG-DPPA-1, LMDP) (Yang et al., 2019). The system can effectively eliminate primary tumors through PTT and can also act as a tumor-targeting agent activated by MMPs, releasing D-peptide antagonists of PD-L1 and stimulating the activation of cytotoxic T lymphocytes, thereby inhibiting distant tumor growth and reducing tumor metastasis (Figure 8E).
2.5.2 Upconversion nanoparticle-based antitumor immunity
Upconverted nanoparticles (UCNPs) are a class of lanthanide-doped optical nanocrystals that have broad application prospects in light-controlled tumor therapy owing to their low toxicity, good chemical stability, and good photostability (Qiu et al., 2018; Wen et al., 2018). UCNPs can convert near infrared (NIR) light to UV or visible light via the sequential absorption of two or more low-energy photons, together with their deep penetrating ability, making UCNPs hot materials (Zhou et al., 2015; Wang et al., 2018; Zhou et al., 2018; Liu Y. Q. et al., 2021). Xiang et al. designed UCNPs loaded with dendritic cell (DC) vaccine antigen to label and stimulate DCs to achieve precise tracking and induce antigen-specific immune responses in vivo, thereby exerting antitumor immunity (Xiang et al., 2015). Our team has developed a remote-controlled antitumor immunotherapy device based on UCNPs, constructed by combining UCNPs, immunotherapeutic CpG oligonucleotides (ODN), and complementary ssDNA (PcDNA) containing a photocleavable (PC) bond (Chu et al., 2019). Under irradiation with NIR light, UCNPs can convert NIR light into high-energy UV light, which can photolytically break the PC bond and decompose PcDNA into DNA fragments, thereby releasing CpG ODNs to activate and control the body’s immune activity. Ding et al. reported biodegradable K3ZrF7:Yb/Er UCNPs (ZrNPs) as pyroptosis inducers for cancer immunotherapy (Ding et al., 2021). Sensitizer ions (Yb3+) absorb low-energy infrared radiation and effectively transfer excitation energy to activator ions (Er3+, TM3+, or HO3+), which emit high-energy ultraviolet (UV), visible, and NIR light through a multiphoton process. ZrNP-like ion banks dissolve in cancer cells and release large amounts of K+ and [ZrF7]3− ions, further inducing an increase in oxidative pressure and reactive oxygen species (ROS). In addition, the results confirmed that ZrNPs can increase dendritic cell (DC) maturity and effector memory T-cell frequency, thereby inhibiting tumor growth and metastasis in vivo (Figure 9) (Ding et al., 2021).
FIGURE 9
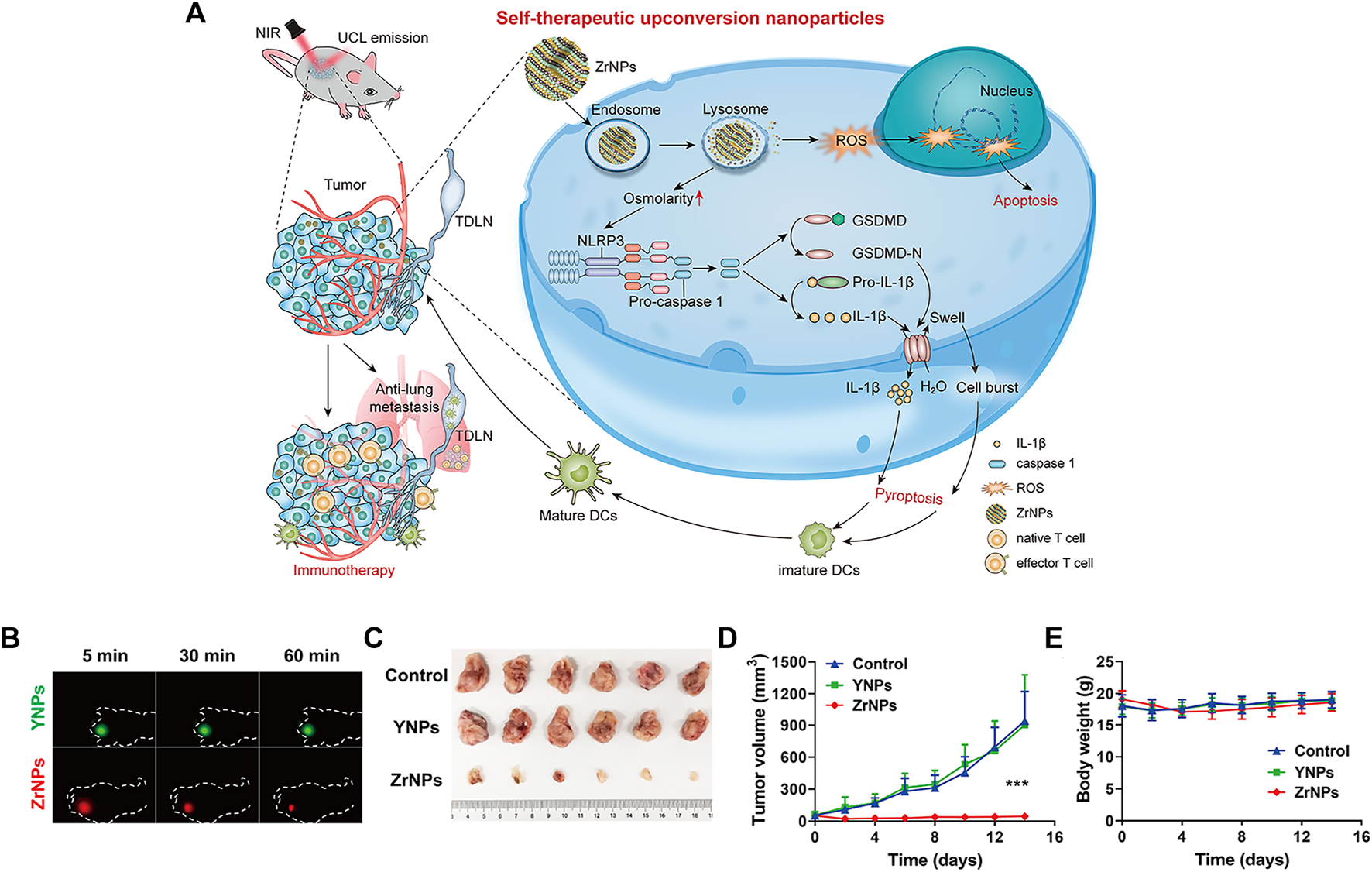
Scheme and therapeutic effects in vivo for antitumor therapy. (A) Schematic illustration of K3ZrF7:Yb/Er upconversion nanoparticles (ZrNPs) to induce pyroptosis for cancer immunotherapy. (B)In vivo UCL images of YNPs or ZrNPs at different time points. (C) Digital photographs of excised tumors. (D) Tumor growth and (E) body weight curves. (Reproduced from Ding et al., 2021, Nano Letters).
Mao et al. reported a nanoscale immune stimulator loaded with the aggregation-induced emission (AIE) photosensitizer TPEBTPy on UCNPs (Figure 10A) (Mao et al., 2020). TPEBTPy with AIE characteristics showed strong fluorescence and ROS generation in the aggregation state (Hu et al., 2018). The combination of TPEBTPy and UCNPs can improve light penetration and have a strong interaction. The nanomaterial enhanced the adaptive immune response to solid tumors by modulating ROS production while simultaneously activating tumor immunogenic cell death (ICD) and dendritic cells to prevent local tumor recurrence and metastasis (Figures 10B–E) (Mao et al., 2020).
FIGURE 10
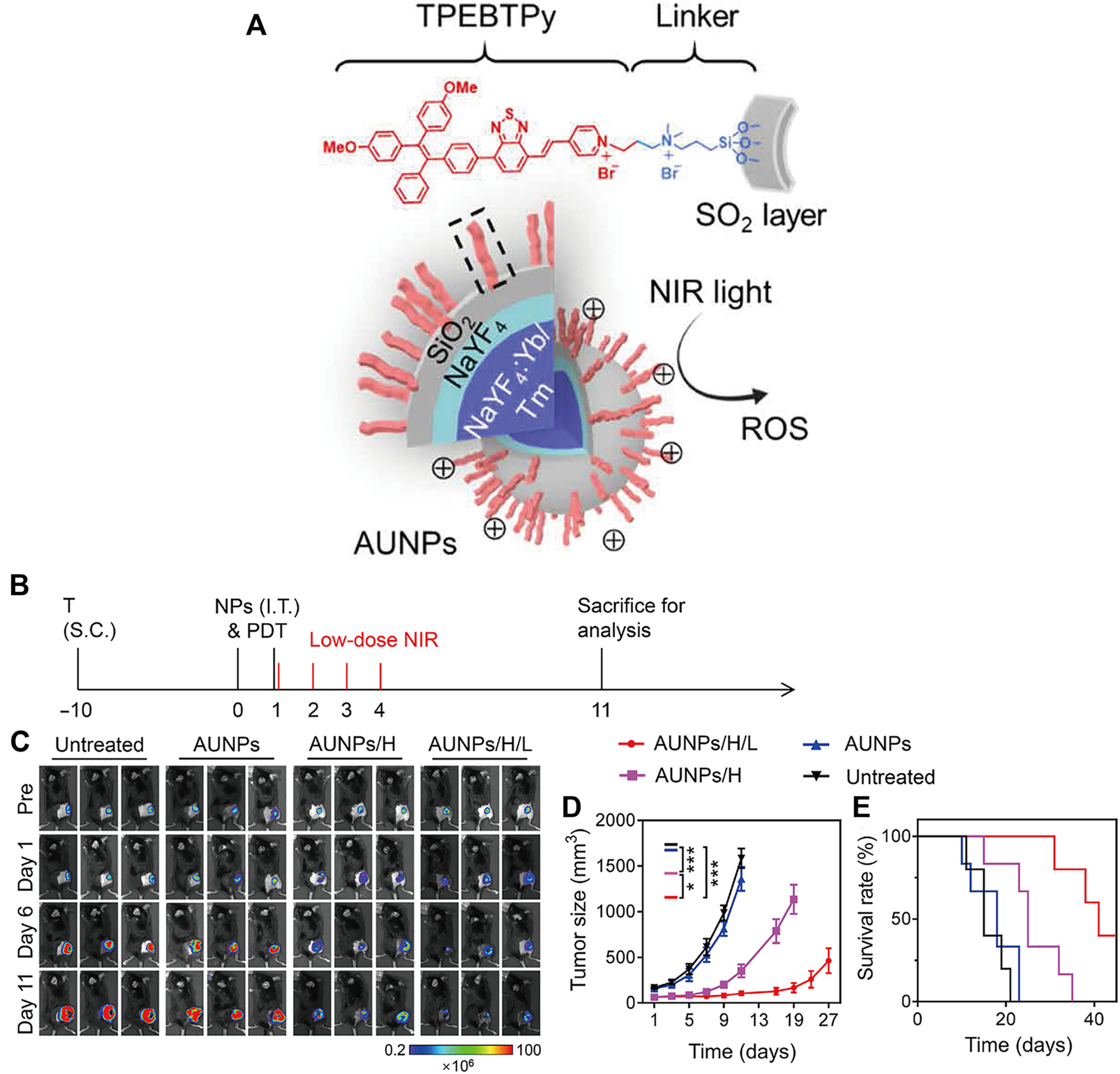
Antitumor immunotherapy with AUNP to inhibit B16F10 tumor growth. (A) Structure of a nanoscale immune stimulator. The dashed box indicates a linked TPEBTPy molecule on the AUNPs. (B) Schematic illustration of the treatment schedule. T, tumor inoculation; S.C., subcutaneous injections. (C) Bioluminescence images of the B16F10 tumor-bearing mice receiving different treatments. (D) Tumor growth curve and (E) survival curve of B16F10 tumor-bearing mice in the control and treated groups (n = 5). (Reproduced from Mao et al., 2020, Science Advances).
Chen et al. reported a tumor-associated macrophage membrane (TAMM) derived from primary tumors, which was coated with a conjugated photosensitizer (NPR@TAMM) on UCNPs (Chen Y. et al., 2021). The TAMM has unique antigen-homing affinity and immune compatibility and can consume CSF1 secreted by tumor cells in the tumor microenvironment (TME), thus blocking the interaction between TAMs and cancer cells. NPR@TAMM-mediated photodynamic immunotherapy transformed macrophage activation from an immunosuppressive M2-like phenotype to a more inflammatory M1-like state and induced immunogenic cell death, thus stimulating antitumor immune efficiency by activating antigen-presenting cells (Figure 11).
FIGURE 11

Schematic illustration of the design and mechanism of tumor-associated macrophage membrane-coated upconverting nanoparticles. (A) Schematic illustration of the preparation of TAMM-coated NPR@TAMMs. (B) Mechanism illustration of TAMM-coated NPR@TAMMs for photodynamic immunotherapy. (Reproduced from Chen Y. et al., 2021, Nano Letters).
However, the therapeutic effect of single immunotherapy is still poor, and synergistic immunotherapy has a better antitumor immune effect (Sang et al., 2019; Guo et al., 2022). As shown in Figure 12A, photothermal therapy (PTT) can induce deep tissue immunogenic cell death and enhance antitumor immunotherapy (Chen et al., 2001; Chen et al., 2020; Li W. et al., 2020). Similarly, photodynamic therapy can induce immunogenic cell death and activate adaptive immune responses to tumor-associated antigens (Figure 12B) (Castano et al., 2006). Therefore, the application of synergistic immunotherapy based on light-controlled nanomaterials has more potential for clinical application.
FIGURE 12

Antitumor immunotherapy based on photothermal therapy and photodynamic therapy. (A) Photothermal therapy increases immunogenic cell death and releases antigens that are delivered to T cells, enhancing the recognition and killing of tumor cells. (Reproduced from Li W. et al., 2020, Frontiers in Immunology). (B) Photodynamic therapy induces the activation of antigen-specific T cells. (Reproduced from Castano et al., 2006, Nature Reviews Cancer).
Xu et al. designed a nanoplatform that combined UCNPs triggered by PDT with checkpoint blockade (Xu et al., 2017). The UCNPs were simultaneously loaded with photosensitizer e6 (Ce6) and toll-like receptor 7 agonist imiquimod (R837) to form UCNP-Ce6-R837, which was then combined with cytotoxic T lymphocyte-associated protein (CTLA-4) checkpoint blocker. The release of tumor-associated antigens through PDT under NIR irradiation also enhances antitumor immune responses with long-term immune memory function (Figure 13A). Wang et al. reported an NIR-triggered antigen nanoplatform for synergistic immunotherapy, which is a combination of lipid molecules (DSPE-PEG-mal), light absorber indocyanine green (ICG) and photosensitizer rose bengal (RB) assembled in UCNPs (Wang Z. et al., 2019). Tumor cells irradiated with NIR can release tumor-derived protein antigen (TDPA), triggering immunogenic cell death. In addition, TAPDs can be captured by the platform to induce tumor-specific immune responses (Figure 13B). Ding et al. prepared upconversion nanoparticles (UCMS) coated with mesoporous silica as an immune adjuvant for antitumor immunotherapy (Ding et al., 2018). UCMS was simultaneously loaded with the photosensitizers merocyanine 540 (MC540), chicken OVA or tumor antigens. NIR light irradiation can activate MC540, which produces ROS and releases TAA to stimulate DCs, resulting in T-cell activation and proliferation and the release of cytokines to kill tumor cells (Figure 13C).
FIGURE 13
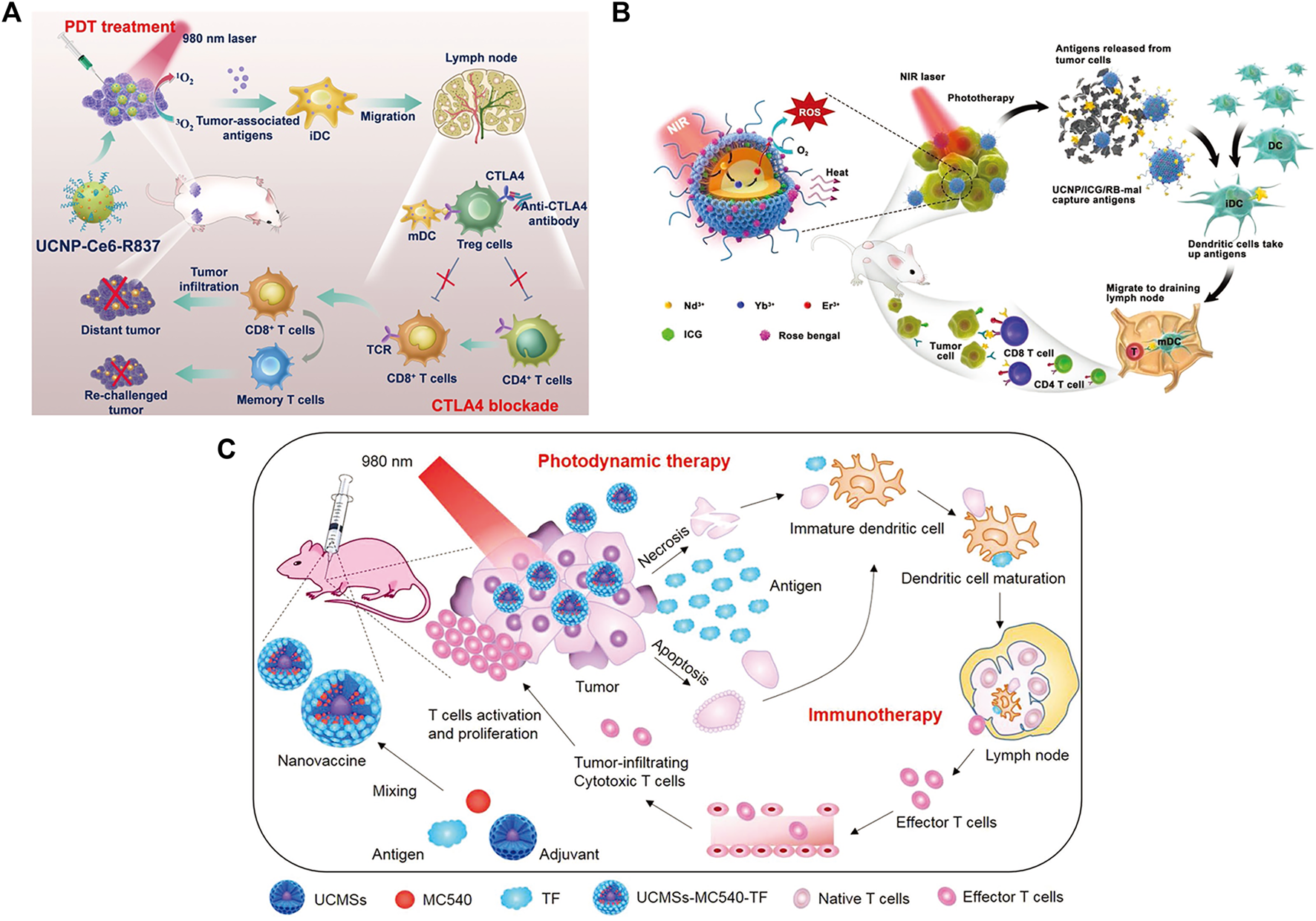
(A) Schematic illustration of NIR-triggered PDT with multitasking UCNPs in combination with checkpoint blockade for immunotherapy of cancer. (Reproduced from Xu et al., 2017, ACS Nano). (B) Schematic illustration of both the fabrication and mechanism of a near-infrared (NIR)-triggered antigen-capturing nanoplatform. (Reproduced from Wang S. et al., 2019, Advance Science). (C) Schematic illustration of the fabrication and mechanism of UCMSs-MC540-TF nanovaccines for PDT and immunotherapy. (Reproduced from Ding et al., 2018, Advance Materials).
2.5.3 Other metal nanoparticle-based antitumor immunity
Zero-valent iron (ZVI) nanoparticles (NPs) have a strong reduction potential (Zou et al., 2016) and can produce a large number of reactive oxygen species (ROS) (Yang L. X. et al., 2020; He et al., 2022). Hsieh et al. utilized ZVI-NP to enhance phosphorylation-dependent ubiquitination and degradation of nuclear factor E2-related factor 2 (NRF2), resulting in excessive oxidative stress and lipid peroxidation (Hsieh et al., 2021). Furthermore, ZVI-NPs reprogrammed the polarization of tumor-associated macrophages into an antitumor M1 phenotype, increased the cytotoxic function of CD8+ T cells and decreased the proportion of regulatory T cells to enhance antitumor immunity. Cobalt oxide nanoparticles (CoO NPs) are promising tools for delivering antigens to antigen-presenting cells and have induced antitumor immune responses. Chattopadhyay et al. found that CoO NPs modified with N-phosphonylmethyliminodiacetic acid (PMIDA) bound lysate-promoting antigens, which are cancer antigens derived from cancer cell lysis, to form cancer cell lysate antigen-conjugated PMIDA-CoO NPs (Ag-PMIDA-CoO NPs) (Chattopadhyay et al., 2016). The nanoparticles can activate macrophages (M φ) to improve the anticancer immune response, increase serum IFN-γ and TNF-α levels and act as an adjuvant to balance proinflammatory and anti-inflammatory immune responses.
Platinum nanoparticles (Pt NPs) are selectively toxic to cancer cells (Xia et al., 2016; Shoshan et al., 2019) and enable photothermal conversion through NIR irradiation (Yang et al., 2015), leading to targeted hyperthermia (Zhou et al., 2016) and antigen release (Ma et al., 2019). Yu et al. constructed Pt NPs that conjugated PD-L1 inhibitor (BMS-1) to Mal-modified polyethylene glycol (PEG) via thermosensitive bonds (Yu et al., 2021). Under NIR irradiation, Pt NPs ablated tumors by PTT and released BMS-1 to alleviate T-cell depletion and induce effector T cells to infiltrate into tumor tissues and acted as immune adjuvants to stimulate the maturation of DCs. Mal exposed to the surface of nanoparticles captured the antigens released by tumor cells and enhanced antigen internalization and presentation (Figure 14).
FIGURE 14
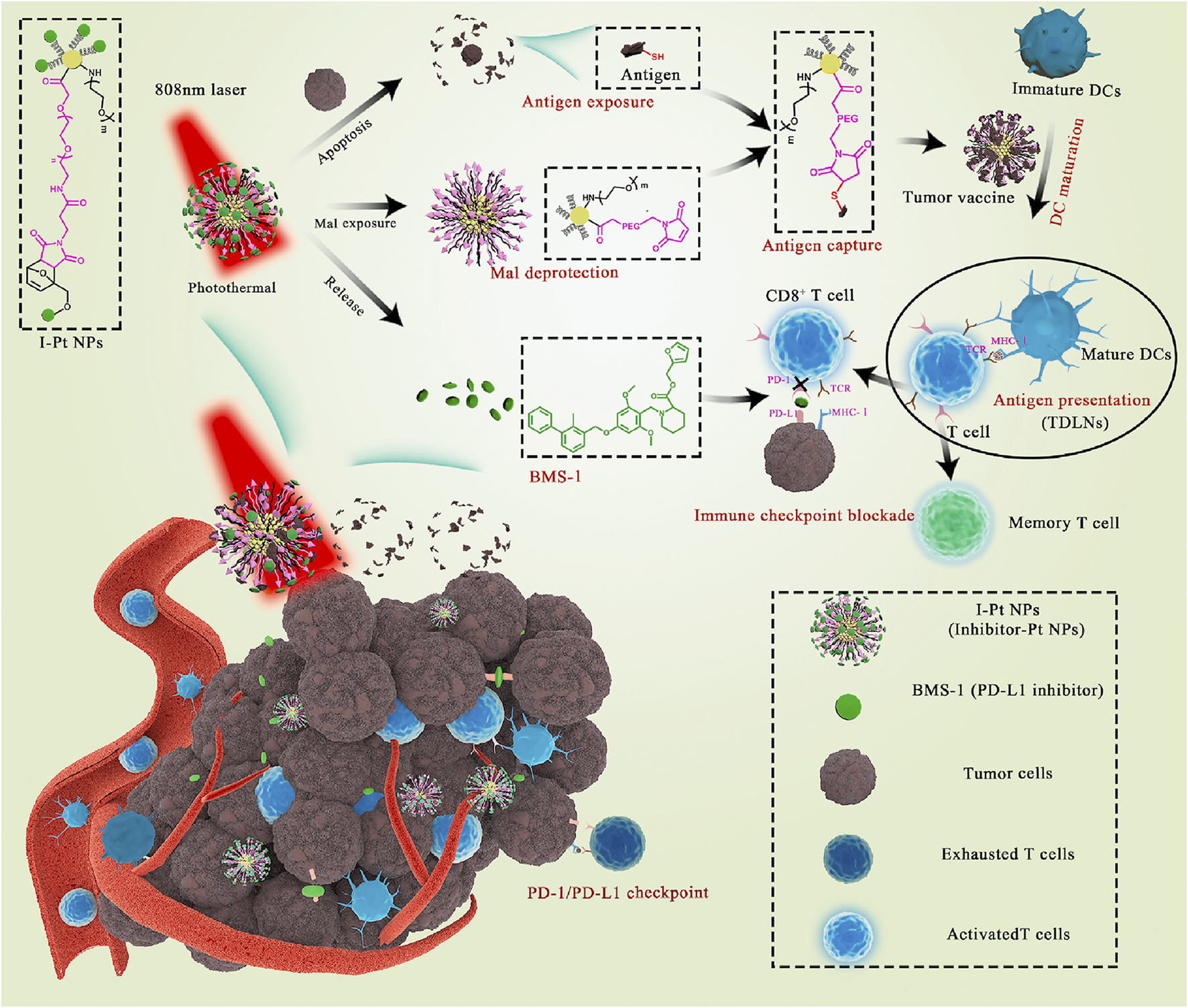
Schematic illustration of Pt NPs conjugated with BMS-1 for NIR-controlled release of inhibitor and exposure to Mal. (Reproduced from Yu et al., 2021, Bioactive Materials).
Hollow copper sulfide nanoparticles (HCuSNPs) are biodegradable photothermal coupling agents that can be excreted from the liver and kidney with low toxicity (Guo et al., 2013). Guo et al. reported a CuS-based transformational nano-CPG system (HCuSNPs-CpG) induced by NIR light (Guo et al., 2014). Upon NIR light irradiation, HCuSNPs-CpG structures were decomposed, reassembled and transformed into chitosan-CPG nanocomplexes, which increased the stability, tumor retention, and internalization of CpG by plasmacytoid dendritic cells and initiated effective systemic antitumor immunity by activating Toll-like receptor 9 signaling (Figure 15).
FIGURE 15

Schematic illustration of the preparation of HCuSNPs-CpG for photothermal immunotherapy. (A) Schematic illustration of the assembly and decomposition of the HCuSNPs-CpG conjugate. “HCuSNPs-Chi” represents chitosan-coated HCuSNPs. “Chi-CpG-NPs” represents chitosan-CpG nanocomplexes. “SCuSNPs” represents small CuS nanoparticles. (B) Schematic illustration of HCuSNPs-CpG-mediated photothermal immunotherapy of both primary treated and distant untreated tumors. (Reproduced from Guo et al., 2014, ACS Nano).
Titanium nanosheets (Ti NSs), as novel and economical two-dimensional nanomaterials, have strong NIR light absorption ability, high photothermal conversion efficiency and good biosafety (Xie et al., 2019; Yuan et al., 2022). However, Ti NSs are prone to oxidation in vivo, and their application in medical materials is also limited. Moreover, polyethylene glycol (PEG) can improve Ti NS stability, increase the retention time of NSs in blood circulation, and enhance the drug delivery capacity of the tumor site (Yang Y. et al., 2020).
In addition, previous studies showed that some transition-metal ions (including Fe3+, Cu2+ and Mn2+) can be bound to the PDA structure by coordination (Li et al., 2016; Wang Z. X. et al., 2017; Ge et al., 2017). Xu et al. prepared Fe (III) chelated PDA nanoparticles with high loading and response to release iron ions, which can improve the light absorption behavior of PDA in the NIR spectrum and endow PDA with better photothermal conversion ability (Xu et al., 2022). The in vivo and in vitro results showed that Fe-PDA could significantly inhibit tumor growth and effectively promote the repolarization of tumor-associated macrophages to the M1 mode compared with PDA. Fe-PDA combined with PTT effectively improved the efficacy of immunotherapy.
A new class of nanophotosensitizers (nPSs) based on nanoscale metal-organic frameworks (nMOFs) have attracted extensive attention in the application of PDT (Shao et al., 2020; Song et al., 2021). Lan et al. reported a novel nanophotosensitizer nanoscale metal-organic framework Fe-TBP, which can overcome tumor hypoxia and enhance the sensitivity of effective PDT, thereby initiating noninflammatory tumors for cancer immunotherapy (Lan et al., 2018). When Fe-TBP is irradiated under anoxic conditions, it can catalyze a cascade reaction to produce O2 through a Fenton-like reaction, and O2 is further converted to singlet oxygen with cytotoxicity by photoexcited porphyrins (O2) to produce PDT effects. In addition, the PDT-induced systemic antitumor response ameliorates α-PD-L1 ICB, leading to the regression of primary and distant tumors through a distant effect.
3 Future and prospects
In summary, this review discusses recent advances in light-activated nanomaterials and their applications in antitumor immunotherapy. With the progress of nanotechnology, the application of nanomaterials in antitumor immunotherapy cannot be ignored. The clinical efficiency of laser treatments is limited by the low penetration of UV, visible light or visible light and makes light-activated imaging or therapy in a dilemma. To achieve deeper tissue penetration ability, near infrared (NIR) light with low energy and long wavelength is a good choice. NIR frequency bands present an optical window for deeper penetration into biological tissue. Materials such as upconversion nanoparticles have the unique capability to efficiently convert NIR light irradiation into UV or visible light via the sequential absorption of two or more low-energy photons. This approach achieves the same goal as UV or visible light with deeper tissue penetration. Despite the described promise, there still exist challenges based on light-activated nanomaterials that need to be overcome to meet the demand in clinics. First, the toxicity of light-activated nanomaterials which is also a general concern for all nanomaterials. To date, research on light-activated nanomaterials has mainly focused on constructing new light-activated activation strategies, and the metabolism and toxicity of materials are not deeply understood. Moreover, most of the models used for nanomaterial exploration are restricted to small animals, and few studies have used large animals. Furthermore, the synthesis standards of nanomaterials, the loading content of drugs, poor solubility in the physiological environment, how to effectively preserve them, etc., as well as industry consensus, are also obstacles to the clinical application of nanomaterials. Since the mechanism of tumorigenesis varies from person to person, a single immunotherapy may not achieve satisfactory antitumor therapeutic effects, and synergistic immunotherapy is becoming an important method of antitumor therapy. It is also a great challenge to combine different therapeutic mechanisms and different materials in the same nanosystem in a rational, compatible and synergistic way to achieve efficient synergistic immunotherapy. In addition, the potential risks of photoactivated nanomaterials in clinical applications, such as systemic toxicity, complexity of clearance, and long-term effects on the human body, must also be considered. The above issues may activate future exploration in the development and improvement of light-activated nanomaterials, providing better opportunities for antitumor immunotherapy for future patients.
Statements
Author contributions
FW and HD wrote the manuscript after discussion with; WX and GS: conceptualization, supervision, validation; ZS and HC supervision, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Funding
This work was supported by the Natural Science Foundation of China (No. 82001946) and Beijing Municipal Natural Science Foundation (No. 7214300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abbott M. Ustoyev Y. (2019). Cancer and the immune system: The history and background of immunotherapy. Semin. Oncol. Nurs.35, 150923. 10.1016/j.soncn.2019.08.002
2
Ahn S. Lee I. H. Kang S. Kim D. Choi M. Saw P. E. et al (2014). Gold nanoparticles displaying tumor-associated self-antigens as a potential vaccine for cancer immunotherapy. Adv. Healthc. Mat.3, 1194–1199. 10.1002/adhm.201300597
3
Akinleye A. Rasool Z. (2019). Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol.12, 92. 10.1186/s13045-019-0779-5
4
Barkat A. Beg S. Panda S. K. S Alharbi K. Rahman M. Ahmed F. J. (2021). Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin. Cancer Biol.69, 365–375. 10.1016/j.semcancer.2019.08.022
5
Berraondo P. Sanmamed M. F. Ochoa M. C. Etxeberria I. Aznar M. A. Pérez-Gracia J. L. et al (2019). Cytokines in clinical cancer immunotherapy. Br. J. Cancer120, 6–15. 10.1038/s41416-018-0328-y
6
Bertucci A. Prasetyanto E. A. Septiadi D. Manicardi A. Brognara E. Gambari R. et al (2015). Combined delivery of temozolomide and anti-miR221 PNA using mesoporous silica nanoparticles induces apoptosis in resistant glioma cells. Small11, 5687–5695. 10.1002/smll.201500540
7
Bharti C. Nagaich U. Pal A. K. Gulati N. (2015). Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig.5, 124–133. 10.4103/2230-973X.160844
8
Bianco A. (2013). Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed.52, 4986–4997. 10.1002/anie.201209099
9
Burugu S. Dancsok A. R. Nielsen T. O. (2017). Emerging targets in cancer immunotherapy. Semin. Cancer Biol.52, 39–52. 10.1016/j.semcancer.2017.10.001
10
Castano A. P. Mroz P. Hamblin M. R. (2006). Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer6, 535–545. 10.1038/nrc1894
11
Chattopadhyay S. Dash S. K. Mandal D. Das B. Tripathy S. Dey A. et al (2016). Metal based nanoparticles as cancer antigen delivery vehicles for macrophage based antitumor vaccine. Vaccine34, 957–967. 10.1016/j.vaccine.2015.12.053
12
Chauhan A. Khan T. Omri A. (2021). Design and encapsulation of immunomodulators onto gold nanoparticles in cancer immunotherapy. Int. J. Mol. Sci.22, 8037. 10.3390/ijms22158037
13
Cheetham A. G. Chakroun R. W. Ma W. Cui H. (2017). Self-assembling prodrugs. Chem. Soc. Rev.46 (21), 6638–6663. 10.1039/c7cs00521k
14
Chen C. Song M. Du Y. Yu Y. Li C. Han Y. et al (2021a). Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett.21, 5522–5531. 10.1021/acs.nanolett.1c00818
15
Chen J. Zeng Z. Huang L. Luo S. Dong J. Zhou F. H. et al (2020). Photothermal therapy technology of metastatic colorectal cancer. Am. J. Transl. Res.12, 3089–3115.
16
Chen Q. Xu L. Liang C. Wang C. Peng R. Liu Z. (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun.7, 13193. 10.1038/ncomms13193
17
Chen W. R. Singhal A. K. Liu H. Nordquist R. E. (2001). Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res.61, 459–461.
18
Chen Y. Sun B. Jiang X. Yuan Z. Chen S. Sun P. et al (2021b). Double-acceptor conjugated polymers for NIR-II fluorescence imaging and NIR-II photothermal therapy applications. J. Mat. Chem. B9, 1002–1008. 10.1039/d0tb02499f
19
Cheng R. Feng F. Meng F. Deng C. Feijen J. Zhong Z. (2011). Glutathione-responsive nanovehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release152, 2–12. 10.1016/j.jconrel.2011.01.030
20
Cheng Z. Li M. Dey R. Chen Y. (2021). Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol.14, 85. 10.1186/s13045-021-01096-0
21
Choi H. S. Frangioni J. V. (2010). Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging9, 291–310. 10.2310/7290.2010.00031
22
Chong Y. Ge C. Yang Z. Garate J. A. Gu Z. Weber J. K. et al (2015). Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano9, 5713–5724. 10.1021/nn5066606
23
Chu H. Cao T. Dai G. Liu B. Duan H. Kong C. et al (2021). Recent advances in functionalized upconversion nanoparticles for light-activated tumor therapy. RSC Adv.11, 35472–35488. 10.1039/d1ra05638g
24
Chu H. Zhao J. Mi Y. Di Z. Li L. (2019). NIR-light-mediated spatially selective triggering of antitumor immunity via upconversion nanoparticle-based immunodevices. Nat. Commun.10, 2839. 10.1038/s41467-019-10847-0
25
Clawson C. Huang C. T. Futalan D. Seible D. M. Saenz R. Larsson M. et al (2010). Delivery of a peptide via poly(D, L-lactic-co-glycolic) acid nanoparticles enhances its dendritic cell-stimulatory capacity. Nanomedicine6, 651–661. 10.1016/j.nano.2010.03.001
26
Cui D. Li J. Zhao X. Pu K. Zhang R. (2020). Semiconducting polymer nanoreporters for near-infrared chemiluminescence imaging of immunoactivation. Adv. Mat.32, e1906314. 10.1002/adma.201906314
27
Cui Y. Dong H. Cai X. Wang D. Li Y. (2012). Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Appl. Mat. Interfaces4, 3177–3183. 10.1021/am3005225
28
Cukier P. Santini F. C. Scaranti M. Hoff A. O. (2017). Endocrine side effects of cancer immunotherapy. Endocr. Relat. Cancer24, T331–T347. 10.1530/ERC-17-0358
29
Danhier F. Ansorena E. Silva J. M. Coco R. Le Breton A. Préat V. (2012). PLGA-Based nanoparticles: An overview of biomedical applications. J. Control. Release161, 505–522. 10.1016/j.jconrel.2012.01.043
30
Daniyal M. Liu B. Wang W. (2020). Comprehensive review on graphene oxide for use in drug delivery system. Curr. Med. Chem.27, 3665–3685. 10.2174/13816128256661902011296290
31
DeMaria P. J. Bilusic M. (2019). Cancer vaccines. Hematol. Oncol. Clin. North Am.33, 199–214. 10.1016/j.hoc.2018.12.001
32
Ding B. Shao S. Yu C. Teng B. Wang M. Cheng Z. et al (2018). Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv. Mat.30, e1802479. 10.1002/adma.201802479
33
Ding B. Sheng J. Zheng P. Li C. Li D. Cheng Z. et al (2021). Biodegradable upconversion nanoparticles induce pyroptosis for cancer immunotherapy. Nano Lett.21, 8281–8289. 10.1021/acs.nanolett.1c02790
34
Ding Y. Sun Z. Tong Z. Zhang S. Min J. Xu Q. et al (2020). Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics10, 5195–5208. 10.7150/thno.45017
35
Duan Q. Q. Ma Y. Che M. X. Zhang B. Y. Zhang Y. X. Li Y. et al (2019). Fluorescent carbon dots as carriers for intracellular doxorubicin delivery and track. Drug. Deliv. Sci. Technol.49, 527–533. 10.1016/j.jddst.2018.12.015
36
Duong H. T. T. Yin Y. Thambi T. Nguyen T. L. Giang Phan V. H. Lee M. S. et al (2018). Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials185, 13–24. 10.1016/j.biomaterials.2018.09.008
37
Emens L. A. Ascierto P. A. Darcy P. K. Demaria S. Eggermont A. M. M. Redmond W. L. et al (2017). Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer81, 116–129. 10.1016/j.ejca.2017.01.035
38
Essawy M. M. El-Sheikh S. M. Raslan H. S. Ramadan H. S. Kang B. Talaat I. M. et al (2021). Function of gold nanoparticles in oral cancer beyond drug delivery: Implications in cell apoptosis. Oral Dis.27, 251–265. 10.1111/odi.13551
39
Fry T. J. Shah N. N. Orentas R. J. Stetler-Stevenson M. Yuan C. M. Ramakrishna S. et al (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med.24, 20–28. 10.1038/nm.4441
40
Fu X. Huang Y. Zhao H. Zhang E. Shen Q. Di Y. et al (2021). Near-infrared-light remote-controlled activation of cancer immunotherapy using photothermal conjugated polymer nanoparticles. Adv. Mat.33, e2102570. 10.1002/adma.202102570
41
Ge J. Lan M. Zhou B. Liu W. Guo L. Wang H. et al (2014). A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun.5, 4596. 10.1038/ncomms5596
42
Ge R. Lin M. Li X. Liu S. Wang W. Li S. et al (2017). Cu2+-loaded polydopamine nanoparticles for magnetic resonance imaging-guided pH- and near-infrared-light-stimulated thermochemotherapy. ACS Appl. Mat. Interfaces13, 19706–19716. 10.1021/acsami.7b05583
43
Ghaferi M. Koohi Moftakhari Esfahani M. Raza A. Al Harthi S. Ebrahimi Shahmabadi H. Alavi S. E. (2021). Mesoporous silica nanoparticles: Synthesis methods and their therapeutic use-recent advances. J. Drug Target.29, 131–154. 10.1080/1061186X.2020.1812614
44
Giordani S. Camisasca A. Maffeis V. (2019). Carbon nano-onions: A valuable class of carbon nanomaterials in biomedicine. Curr. Med. Chem.26, 6915–6929. 10.2174/0929867326666181126113957
45
Gonçalves M. Mignani S. Rodrigues J. Tomás H. (2020). A glance over doxorubicin based-nanotherapeutics: From proof-of-concept studies to solutions in the market. J. Control. Release317, 347–374. 10.1016/j.jconrel.2019.11.016
46
Goodman A. Patel S. P. Kurzrock R. (2017). PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol.14, 203–220. 10.1038/nrclinonc.2016.168
47
Guinart A. Perry H. L. Wilton-Ely J. D. E. T. Tetley T. D. (2020). Gold nanomaterials in the management of lung cancer. Emerg. Top. Life Sci.4, 627–643. 10.1042/ETLS20200332
48
Guo B. Sheng Z. Hu D. Li A. Xu S. Manghnani P. N. et al (2017). Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano11, 10124–10134. 10.1021/acsnano.7b04685
49
Guo L. Panderi I. Yan D. D. Szulak K. Li Y. Chen Y. T. et al (2013). A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano7, 8780–8793. 10.1021/nn403202w
50
Guo L. Yan D. D. Yang D. Li Y. Wang X. Zalewski O. et al (2014). Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano8, 5670–5681. 10.1021/nn5002112
51
Guo R. Wang S. Zhao L. Zong Q. Li T. Ling G. et al (2022). Engineered nanomaterials for synergistic photoimmunotherapy. Biomaterials282, 121425. 10.1016/j.biomaterials.2022.121425
52
Havel J. J. Chowell D. Chan T. A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer19, 133–150. 10.1038/s41568-019-0116-x
53
He M. F. Li W. Q. Xie Z. H. Yang S. R. He C. S. Xiong Z. K. et al (2022). Peracetic acid activation by mechanochemically sulfidated zero valent iron for micropollutants degradation: Enhancement mechanism and strategy for extending applicability. Water Res.222, 118887. 10.1016/j.watres.2022.118887
54
He X. Xu C. (2020). Immune checkpoint signaling and cancer immunotherapy. Cell Res.30, 660–669. 10.1038/s41422-020-0343-4
55
Hojman P. Gehl J. Christensen J. F. Pedersen B. K. (2018). Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab.27, 10–21. 10.1016/j.cmet.2017.09.015
56
Hsieh C. H. Hsieh H. C. Shih F. S. Wang P. W. Yang L. X. Shieh D. B. et al (2021). An innovative NRF2 nanomodulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics11, 7072–7091. 10.7150/thno.57803
57
Hu C. Liu Y. Chen J. He Q. Gao H. (2016). A simple one-step synthesis of melanin-originated redshift emissive carbonaceous dots for bioimaging. J. Colloid. Interface Sci.480, 85–90. 10.1016/j.jcis.2016.07.007
58
Hu F. Xu S. Liu B. (2018). Photosensitizers with aggregation-induced emission: Materials and biomedical applications. Adv. Mat.30, e1801350. 10.1002/adma.201801350
59
Hu Z. Fang C. Li B. Zhang Z. Cao C. Cai M. et al (2020a). First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng.4, 259–271. 10.1038/s41551-019-0494-0
60
Hu Z. Chen W. H. Tian J. Cheng Z. (2020b). NIRF nanoprobes for cancer molecular imaging: Approaching clinic. Trends Mol. Med.26, 469–482. 10.1016/j.molmed.2020.02.003
61
Igarashi Y. Sasada T. (2020). Cancer vaccines: Toward the next breakthrough in cancer immunotherapy. J. Immunol. Res.2020, 5825401–5825413. 10.1155/2020/5825401
62
Irvine D. J. Dane E. L. (2020). Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol.20, 321–334. 10.1038/s41577-019-0269-6
63
Jain R. K. Stylianopoulos T. (2010). Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol.7, 653–664. 10.1038/nrclinonc.2010.139
64
Jia C. Yang T. Liu Y. Zhu A. Yin F. Wang Y. et al (2018). A novel human papillomavirus 16 L1 pentamer-loaded hybrid particles vaccine system: Influence of size on immune responses. ACS Appl. Mat. Interfaces10, 35745–35759. 10.1021/acsami.8b11556
65
Jiang B. P. Zhou B. Lin Z. Liang H. Shen X. C. (2019a). Recent advances in carbon nanomaterials for cancer phototherapy. Chemistry25, 3993–4004. 10.1002/chem.201804383
66
Jiang C. Cheng H. Yuan A. Tang X. Wu J. Hu Y. (2015). Hydrophobic IR780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomater.14, 61–69. 10.1016/j.actbio.2014.11.041
67
Jiang Q. Wang K. Zhang X. Ouyang B. Liu H. Pang Z. et al (2020). Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small16, e2001704. 10.1002/smll.202001704
68
Jiang Y. Cui D. Fang Y. Zhen X. Upputuri P. K. Pramanik M. et al (2017). Amphiphilic semiconducting polymer as multifunctional nanocarrier for fluorescence/photoacoustic imaging guided chemo-photothermal therapy. Biomaterials145, 168–177. 10.1016/j.biomaterials.2017.08.037
69
Jiang Y. Huang J. Zhen X. Zeng Z. Li J. Xie C. et al (2019b). A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun.10, 2064. 10.1038/s41467-019-10119-x
70
June C. H. O'Connor R. S. Kawalekar O. U. Ghassemi S. Milone M. C. (2018). CAR T-cell immunotherapy for human cancer. Science359, 1361–1365. 10.1126/science.aar6711
71
Kang H. Rho S. Stiles W. R. Hu S. Baek Y. Hwang D. W. et al (2020). Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mat.9, e1901223. 10.1002/adhm.201901223
72
Karousis N. Suarez-Martinez I. Ewels C. P. Tagmatarchis N. (2016). Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev.116, 4850–4883. 10.1021/acs.chemrev.5b00611
73
Khatami F. Matin M. M. Danesh N. M. Bahrami A. R. Abnous K. Taghdisi S. M. et al (2021). Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR-21. Carbohydr. Polym.266, 118111. 10.1016/j.carbpol.2021.118111
74
Khoobchandani M. Khan A. Katti K. K. Thipe V. C. Al-Yasiri A. Y. MohanDoss D. K. D. et al (2021). Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci. Rep.11, 16797. 10.1038/s41598-021-96224-8
75
Kohout C. Santi C. Polito L. (2018). Anisotropic gold nanoparticles in biomedical applications. Int. J. Mol. Sci.19, 3385. 10.3390/ijms19113385
76
Kroschinsky F. Stölzel F. von Bonin. S. Beutel G. Kochanek M. Kiehl M. et al (2017). New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care21, 89. 10.1186/s13054-017-1678-1
77
Kumar A. R. Devan A. R. Nair B. Vinod B. S. Nath L. R. (2021). Harnessing the immune system against cancer: Current immunotherapy approaches and therapeutic targets. Mol. Biol. Rep.48, 8075–8095. 10.1007/s11033-021-06752-9
78
Lan G. Ni K. Xu Z. Veroneau S. S. Song Y. Lin W. (2018). Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc.140, 5670–5673. 10.1021/jacs.8b01072
79
Lee H. P. Gaharwar A. K. (2020). Light-responsive inorganic biomaterials for biomedical applications. Adv. Sci. (Weinh)7, 2000863. 10.1002/advs.202000863
80
Lee J. Chuang T. H. Redecke V. She L. Pitha P. M. Carson D. A. et al (2003). Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A.100, 6646–6651. 10.1073/pnas.0631696100
81
Lee S. H. Rho W. Y. Chang H. Lee J. H. Kim J. Lee S. H. et al (2021). Carbon nanomaterials for biomedical application. Adv. Exp. Med. Biol.1309, 257–276. 10.1007/978-981-33-6158-4_11
82
Lesch S. Gill S. (2021). The promise and perils of immunotherapy. Blood Adv.5, 3709–3725. 10.1182/bloodadvances.2021004453c
83
Li G. Sun B. Li Y. Luo C. He Z. Sun J. (2021a). Small-molecule prodrug nanoassemblies: An emerging nanoplatform for anticancer drug delivery. Small17, e2101460. 10.1002/smll.202101460
84
Li H. Wang X. Huang D. Chen G. (2020a). Recent advances of lanthanide-doped upconversion nanoparticles for biological applications. Nanotechnology31, 072001. 10.1088/1361-6528/ab4f36
85
Li J. Cui D. Jiang Y. Huang J. Cheng P. Pu K. (2019a). Near-infrared photoactivatable semiconducting polymer nanoblockaders for metastasis-inhibited combination cancer therapy. Adv. Mat.31, e1905091. 10.1002/adma.201905091
86
Li J. Huang D. Cheng R. Figueiredo P. Fontana F. Correia A. et al (2021b). Multifunctional biomimetic nanovaccines based on photothermal and weak-immunostimulatory nanoparticulate cores for the immunotherapy of solid tumors. Adv. Mat.34, e2108012. 10.1002/adma.202108012
87
Li J. Pu K. (2020). Semiconducting polymer nanomaterials as near-infrared photoactivatable protherapeutics for cancer. Acc. Chem. Res.53, 752–762. 10.1021/acs.accounts.9b00569
88
Li Q. Shi Z. Zhang F. Zeng W. Zhu D. Mei L. (2022). Symphony of nanomaterials and immunotherapy based on the cancer-immunity cycle. Acta Pharm. Sin. B12, 107–134. 10.1016/j.apsb.2021.05.031
89
Li T. Li C. Ruan Z. Xu P. Yang X. Yuan P. et al (2019b). Polypeptide-conjugated second near-infrared organic fluorophore for image-guided photothermal therapy. ACS Nano13, 3691–3702. 10.1021/acsnano.9b00452
90
Li W. Liu Z. Fontana F. Ding Y. Liu D. Hirvonen J. T. et al (2018). Tailoring porous silicon for biomedical applications: From drug delivery to cancer immunotherapy. Adv. Mat.30, e1703740. 10.1002/adma.201703740
91
Li W. Peng A. Wu H. Quan Y. Li Y. Lu L. et al (2020b). Anti-cancer nanomedicines: A revolution of tumor immunotherapy. Front. Immunol.11, 601497. 10.3389/fimmu.2020.601497
92
Li X. Liu L. Li S. Wan Y. Chen J. X. Tian S. et al (2019c). Biodegradable π-conjugated oligomer nanoparticles with high photothermal conversion efficiency for cancer theranostics. ACS Nano13, 12901–12911. 10.1021/acsnano.9b05383
93
Li Y. Liu G. Ma J. Lin J. Lin H. Su G. et al (2017). Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. J. Control. Release258, 95–107. 10.1016/j.jconrel.2017.05.011
94
Li Y. Xie Y. Wang Z. Zang N. Carniato F. Huang Y. et al (2016). Structure and function of iron-loaded synthetic melanin. ACS Nano10, 10186–10194. 10.1021/acsnano.6b05502
95
Li Z. Zhu L. Sun H. Shen Y. Hu D. Wu W. et al (2020c). Fluorine assembly nanocluster breaks the shackles of immunosuppression to turn the cold tumor hot. Proc. Natl. Acad. Sci. U. S. A.117, 32962–32969. 10.1073/pnas.2011297117
96
Lin H. Gao S. Dai C. Chen Y. Shi J. (2017). A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc.139, 16235–16247. 10.1021/jacs.7b07818
97
Lin N. S. Kitamura M. Saito M. Hirayama K. Ide Y. Umemura K. (2022). Distinguishing antioxidant molecules with near-infrared photoluminescence of DNA-wrapped single-walled carbon nanotubes. ACS Omega7, 28896–28903. 10.1021/acsomega.2c02038
98
Liu J. He S. Luo Y. Zhang Y. Du X. Xu C. et al (2022). Tumor-microenvironment-activatable polymer nano-immunomodulator for precision cancer photoimmunotherapy. Adv. Mat.34, e2106654. 10.1002/adma.202106654
99
Liu J. Li R. Yang B. (2020). Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci.6, 2179–2195. 10.1021/acscentsci.0c01306
100
Liu J. Liang H. Li M. Luo Z. Zhang J. Guo X. et al (2018). Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials157, 107–124. 10.1016/j.biomaterials.2017.12.003
101
Liu X. Chen Z. H. Zhang H. Fan Y. Zhang F. (2021a). Independent luminescent lifetime and intensity tuning of upconversion nanoparticles by gradient doping for multiplexed encoding. Angew. Chem. Int. Ed.60, 7041–7045. 10.1002/anie.202015273
102
Liu Y. Jiao F. Qiu Y. Li W. Qu Y. Tian C. et al (2009). Immunostimulatory properties and enhanced TNF- alpha mediated cellular immunity for tumor therapy by C60(OH)20 nanoparticles. Nanotechnology20, 415102. 10.1088/0957-4484/20/41/415102
103
Liu Y. Q. Qin L. Y. Li H. J. Wang Y. X. Zhang R. Shi J. M. et al (2021b). Application of lanthanide-doped upconversion nanoparticles for cancer treatment: A review. Nanomedicine16, 2207–2242. 10.2217/nnm-2021-0214
104
Lu H. Zhao Q. Wang X. Mao Y. Chen C. Gao Y. et al (2020). Multistimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids Surf. B Biointerfaces190, 110941. 10.1016/j.colsurfb.2020.110941
105
Luo C. Sun J. Sun B. Liu D. Miao L. Goodwin T. J. et al (2016). Facile fabrication of tumor redox-sensitive nanoassemblies of small-molecule oleate prodrug as potent chemotherapeutic nanomedicine. Small12, 6353–6362. 10.1002/smll.201601597
106
Luo L. Yang J. Zhu C. Jiang M. Guo X. Li W. et al (2018). Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J. Control. Release278, 87–99. 10.1016/j.jconrel.2018.04.002
107
Lyu Y. Zeng J. Jiang Y. Zhen X. Wang T. Qiu S. et al (2018). Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Nano12, 1801–1810. 10.1021/acsnano.7b08616
108
Ma W. Cheetham A. G. Cui H. (2016). Building nanostructures with drugs. Nano Today11, 13–30. 10.1016/j.nantod.2015.11.003
109
Ma W. Zhu D. Li J. Chen X. Xie W. Jiang X. et al (2020). Coating biomimetic nanoparticles with chimeric antigen receptor T-cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics10, 1281–1295. 10.7150/thno.40291
110
Ma Y. Zhang Y. Li X. Zhao Y. Li M. Jiang W. et al (2019). Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano13, 11967–11980. 10.1021/acsnano.9b06040
111
Mao D. Hu F. Yi Z. Kenry, Xu S. Yan S. et al (2020). AIEgen-coupled upconversion nanoparticles eradicate solid tumors through dual-mode ROS activation. Sci. Adv.6, eabb2712. 10.1126/sciadv.abb2712
112
Martín-Palma R. J. Hernández-Montelongo J. Torres-Costa V. Manso-Silván M. Muñoz-Noval Á. (2014). Nanostructured porous silicon-mediated drug delivery. Expert Opin. Drug Deliv.11, 1273–1283. 10.1517/17425247.2014.919254
113
Mohanty R. Chowdhury C. R. Arega S. Sen P. Ganguly P. Ganguly N. (2019). CAR T cell therapy: A new era for cancer treatment (review). Oncol. Rep.42, 2183–2195. 10.3892/or.2019.7335
114
Moradipour M. Chase E. K. Khan M. A. Asare S. O. Lynn B. C. Rankin S. E. et al (2020). Interaction of lignin-derived dimer and eugenol-functionalized silica nanoparticles with supported lipid bilayers. Colloids Surf. B Biointerfaces191, 111028. 10.1016/j.colsurfb.2020.111028
115
Muluh T. A. Chen Z. Li Y. Xiong K. Jin J. Fu S. et al (2021). Enhancing cancer immunotherapy treatment goals by using nanoparticle delivery system. Int. J. Nanomedicine16, 2389–2404. 10.2147/IJN.S295300
116
Osipov A. Saung M. T. Zheng L. Murphy A. G. (2019). Small molecule immunomodulation: The tumor microenvironment and overcoming immune escape. J. Immunother. Cancer7, 224. 10.1186/s40425-019-0667-0
117
Park W. Heo Y. J. Han D. K. (2018). New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res.22, 24. 10.1186/s40824-018-0133-y
118
Pitt J. M. Marabelle A. Eggermont A. Soria J. C. Kroemer G. Zitvogel L. (2016). Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol.27, 1482–1492. 10.1093/annonc/mdw168
119
Popescu R. C. Fufă M. O. Grumezescu A. M. (2015). Metal-based nanosystems for diagnosis. Rom. J. Morphol. Embryol.56, 635–649.
120
Porter A. E. Gass M. Muller K. Skepper J. N. Midgley P. A. Welland M. (2007). Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol.2, 713–717. 10.1038/nnano.2007.347
121
Qian C. G. Chen Y. L. Feng P. J. Xiao X. Z. Dong M. Yu J. C. et al (2017). Conjugated polymer nanomaterials for theranostics. Acta Pharmacol. Sin.38, 764–781. 10.1038/aps.2017.42
122
Qiu H. Tan M. Ohulchanskyy T. Y. Lovell J. F. Chen G. (2018). Recent progress in upconversion photodynamic therapy. Nanomater. (Basel).8, 344. 10.3390/nano8050344
123
Ribas A. Wolchok J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science359, 1350–1355. 10.1126/science.aar4060
124
Riley R. S. June C. H. Langer R. Mitchell M. J. (2019). Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov.18, 175–196. 10.1038/s41573-018-0006-z
125
Riley R. S. O'Sullivan R. K. Potocny A. M. Rosenthal J. Day E. S. (2018). Evaluating nanoshells and a potent biladiene photosensitizer for dual photothermal and photodynamic therapy of triple negative breast cancer cells. Nanomater. (Basel).8, 658. 10.3390/nano8090658
126
Sadat Tabatabaei Mirakabad F. Nejati-Koshki K. Akbarzadeh A. Yamchi M. R. Milani M. Zarghami N. et al (2014). PLGA-based nanoparticles as cancer drug delivery systems. Asian pac. J. Cancer Prev.15, 517–535. 10.7314/apjcp.2014.15.2.517
127
Sainz-Urruela C. Vera-López S. San Andrés M. P. Díez-Pascual A. M. (2021). Graphene-based sensors for the detection of bioactive compounds: A review. Int. J. Mol. Sci.22, 3316. 10.3390/ijms22073316
128
Sang W. Zhang Z. Dai Y. Chen X. (2019). Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev.48, 3771–3810. 10.1039/c8cs00896e
129
Shao Y. Liu B. Di Z. Zhang G. Sun L. D. Li L. et al (2020). Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. J. Am. Chem. Soc.142, 3939–3946. 10.1021/jacs.9b12788
130
Shemesh C. S. Hsu J. C. Hosseini I. Shen B. Q. Rotte A. Twomey P. et al (2021). Personalized cancer vaccines: Clinical landscape, challenges, and opportunities. Mol. Ther.29, 555–570. 10.1016/j.ymthe.2020.09.038
131
Sheng G. Tian N. Duan H. J. Sun Z. G. Chu H. Q. (2022). Advances in therapeutic nanodrug delivery systems for infectious lung diseases: A review. Acta Mat. Med.1, 343–364. 10.15212/AMM-2022-0019
132
Shi C. Li M. Zhang Z. Yao Q. Shao K. Xu F. et al (2020). Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials233, 119755. 10.1016/j.biomaterials.2020.119755
133
Shoshan M. S. Vonderach T. Hattendorf B. Wennemers H. (2019). Peptide-coated platinum nanoparticles with selective toxicity against liver cancer cells. Angew. Chem. Int. Ed.58, 4901–4905. 10.1002/anie.201813149
134
Siegel R. L. Miller K. D. Fuchs H. E. Jemal A. (2021). Cancer statistics, 2021. CA Cancer J. Clin.71, 7–33. 10.3322/caac.21654
135
Singh P. Pandit S. Mokkapati V. R. S. S. Garg A. Ravikumar V. Mijakovic I. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci.19, 1979. 10.3390/ijms19071979
136
Son J. Yi G. Yoo J. Park C. Koo H. Choi H. S. (2019). Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev.138, 133–147. 10.1016/j.addr.2018.10.002
137
Song Y. Wang L. Xie Z. (2021). Metal-organic frameworks for photodynamic therapy: Emerging synergistic cancer therapy. Biotechnol. J.16, e1900382. 10.1002/biot.201900382
138
Sun J. Birnbaum W. Anderski J. Picker M. T. Mulac D. Langer K. et al (2018). Use of light-degradable aliphatic polycarbonate nanoparticles as drug carrier for photosensitizer. Biomacromolecules19, 4677–4690. 10.1021/acs.biomac.8b01446
139
Sung H. Ferlay J. Siegel R. L. Laversanne M. Soerjomataram I. Jemal A. et al (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660
140
Tajabadi M. (2019). Application of carbon nanotubes in breast cancer therapy. Drug Res.10.1055/a-0945-1469
141
Tang H. Xu X. Chen Y. Xin H. Wan T. Li B. et al (2021). Reprogramming the tumor microenvironment through second-near-infrared-window photothermal genome editing of PD-L1 mediated by supramolecular gold nanorods for enhanced cancer immunotherapy. Adv. Mat.33, e2006003. 10.1002/adma.202006003
142
Tao Y. Ju E. Ren J. Qu X. (2014). Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials35, 9963–9971. 10.1016/j.biomaterials.2014.08.036
143
Tian D. Qin F. Zhao H. Zhang C. Wang H. Liu N. et al (2021). Bio-Responsive nanoparticle for tumor targeting and enhanced photo-immunotherapy. Colloids Surf. B Biointerfaces202, 111681. 10.1016/j.colsurfb.2021.111681
144
Tieu T. Wojnilowicz M. Huda P. Thurecht K. J. Thissen H. Voelcker N. H. et al (2021). Nanobody-displaying porous silicon nanoparticles for the co-delivery of siRNA and doxorubicin. Biomater. Sci.9, 133–147. 10.1039/d0bm01335h
145
Topalian S. L. (2017). Targeting immune checkpoints in cancer therapy. JAMA318, 1647–1648. 10.1001/jama.2017.14155
146
Tuncel D. Demir H. V. (2010). Conjugated polymer nanoparticles. Nanoscale2, 484–494. 10.1039/b9nr00374f
147
Ulbrich K. Holá K. Šubr V. Bakandritsos A. Tuček J. Zbořil R. (2016). Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev.116, 5338–5431. 10.1021/acs.chemrev.5b00589
148
Velpurisiva P. Gad A. Piel B. Jadia R. Rai P. (2017). Nanoparticle design strategies for effective cancer immunotherapy. J. Biomed. (Syd).2, 64–77. 10.7150/jbm.18877
149
Vimbela G. V. Ngo S. M. Fraze C. Yang L. Stout D. A. (2017). Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomedicine12, 3941–3965. 10.2147/IJN.S134526
150
Wang C. Xu L. Liang C. Xiang J. Peng R. Liu Z. (2014). Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mat.26, 8154–8162. 10.1002/adma.201402996
151
Wang H. Pan X. Wang X. Wang W. Huang Z. Gu K. et al (2020). Degradable carbon-silica nanocomposite with immunoadjuvant property for dual-modality photothermal/photodynamic therapy. ACS Nano14, 2847–2859. 10.1021/acsnano.9b06168
152
Wang M. Song J. Zhou F. Hoover A. R. Murray C. Zhou B. et al (2019a). NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv. Sci. (Weinh).6, 1802157. 10.1002/advs.201802157
153
Wang S. Wu W. Manghnani P. Xu S. Wang Y. Goh C. C. et al (2019b). Polymerization-enhanced two-photon photosensitization for precise photodynamic therapy. ACS Nano13, 3095–3105. 10.1021/acsnano.8b08398
154
Wang X. Zhong X. Li J. Liu Z. Cheng L. (2021). Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev.50, 8669–8742. 10.1039/d0cs00461h
155
Wang Y. Cheetham A. G. Angacian G. Su H. Xie L. Cui H. (2017a). Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev.110-111, 112–126. 10.1016/j.addr.2016.06.015
156
Wang Y. F. Liu G. Y. Sun L. D. Xiao J. W. Zhou J. C. Yan C. H. (2013). Nd (3+)-sensitized upconversion nanophosphors: Efficient in vivo bioimaging probes with minimized heating effect. ACS Nano7, 7200–7206. 10.1021/nn402601d
157
Wang Y. Zheng K. Song S. Fan D. Zhang H. Liu X. (2018). Remote manipulation of upconversion luminescence. Chem. Soc. Rev.47, 6473–6485. 10.1039/c8cs00124c
158
Wang Z. Cao Y. J. (2020). Adoptive cell therapy targeting neoantigens: A frontier for cancer research. Front. Immunol.11, 176. 10.3389/fimmu.2020.00176
159
Wang Z. Guo B. Middha E. Huang Z. Hu Q. Fu Z. et al (2019c). Microfluidics-prepared uniform conjugated polymer nanoparticles for photo-triggered immune microenvironment modulation and cancer therapy. ACS Appl. Mat. Interfaces11, 11167–11176. 10.1021/acsami.8b22579
160
Wang Z. X. Li Y. Huang Y. Parent Y. Ditri L. R. Zang T. et al (2017b). Tunable, metal-loaded polydopamine nanoparticles analyzed by magnetometry. Chem. Mat.29, 8195–8201. 10.1021/acs.chemmater.7b02262
161
Wei J. Liu Y. Yu J. Chen L. Luo M. Yang L. et al (2021). Conjugated polymers: Optical toolbox for bioimaging and cancer therapy. Small17, e2103127. 10.1002/smll.202103127
162
Wei Z. Wu M. Lan S. Li J. Zhang X. Zhang D. et al (2018). Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. (Camb)54, 13599–13602. 10.1039/c8cc07583b
163
Wen S. Zhou J. Zheng K. Bednarkiewicz A. Liu X. Jin D. (2018). Advances in highly doped upconversion nanoparticles. Nat. Commun.9, 2415. 10.1038/s41467-018-04813-5
164
Wiehe A. O'Brien J. M. Senge M. O. (2019). Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci.18, 2565–2612. 10.1039/c9pp00211a
165
Xia H. Li F. Hu X. Park W. Wang S. Jang Y. et al (2016). pH-sensitive Pt nanocluster assembly overcomes cisplatin resistance and heterogeneous stemness of hepatocellular carcinoma. ACS Cent. Sci.2, 802–811. 10.1021/acscentsci.6b00197
166
Xiang J. Xu L. Gong H. Zhu W. Wang C. Xu J. et al (2015). Antigen-loaded upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano9, 6401–6411. 10.1021/acsnano.5b02014
167
Xiao Y. Yu D. (2021). Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther.221, 107753. 10.1016/j.pharmthera.2020.107753
168
Xie D. Jiang Y. Li K. Yang X. Zhang Y. (2022). Pickering emulsions stabilized by mesoporous nanoparticles with different morphologies in combination with DTAB. ACS Omega7, 29153–29160. 10.1021/acsomega.2c03215
169
Xie Z. Chen S. Duo Y. Zhu Y. Fan T. Zou Q. et al (2019). Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl. Mat. Interfaces11, 22129–22140. 10.1021/acsami.9b04628
170
Xu J. Xu L. Wang C. Yang R. Zhuang Q. Han X. et al (2017). Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano11, 4463–4474. 10.1021/acsnano.7b00715
171
Xu N. Hu A. Pu X. Li J. Wang X. Wang J. et al (2022). Fe (III)-chelated polydopamine nanoparticles for synergistic tumor therapies of enhanced photothermal ablation and antitumor immune activation. ACS Appl. Mat. Interfaces14, 15894–15910. 10.1021/acsami.1c24066
172
Xue X. Lindstrom A. Li Y. (2019). Porphyrin-based nanomedicines for cancer treatment. Bioconjug. Chem.30, 1585–1603. 10.1021/acs.bioconjchem.9b00231
173
Xue X. Lindstrom A. Qu H. Li Y. (2020). Recent advances on small-molecule nanomedicines for cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.12, e1607. 10.1002/wnan.1607
174
Yan L. Li X. (2016). Biodegradable stimuli-responsive polymeric micelles for treatment of malignancy. Curr. Pharm. Biotechnol.17, 227–236. 10.2174/138920101703160206142821
175
Yan Q. Han D. Zhao Y. (2013). Main-chain photoresponsive polymers with controlled location of light-cleavable units: From synthetic strategies to structural engineering. Polym. Chem.4, 5026–5037. 10.1039/c3py00804e
176
Yang F. Shi K. Jia Y. P. Hao Y. Peng J. R. Qian Z. Y. (2020a). Advanced biomaterials for cancer immunotherapy. Acta Pharmacol. Sin.41, 911–927. 10.1038/s41401-020-0372-z
177
Yang H. He L. Q. Hu Y. W. Lu X. Li G. R. Liu B. et al (2015). Quantitative detection of photothermal and photoelectrocatalytic effects induced by SPR from Au@Pt nanoparticles. Angew. Chem. Int. Ed.54, 11462–11466. 10.1002/anie.201505985
178
Yang J. Zhang X. Liu C. Wang Z. Cui W. Feng C. et al (2020b). Biologically modified nanoparticles as theranostic bionanomaterials. Prog. Mat. Sci.118, 100768. 10.1016/J.PMATSCI.2020.100768
179
Yang L. X. Wu Y. N. Wang P. W. Huang K. J. Su W. C. Shieh D. B. (2020c). Silver-coated zero-valent iron nanoparticles enhance cancer therapy in mice through lysosome-dependent dual programed cell death pathways: Triggering simultaneous apoptosis and autophagy only in cancerous cells. J. Mat. Chem. B8, 4122–4131. 10.1039/c9tb01477b
180
Yang Q. Peng J. Shi K. Xiao Y. Liu Q. Han R. et al (2019). Rationally designed peptide-conjugated gold/platinum nanosystem with active tumor-targeting for enhancing tumor photothermal-immunotherapy. J. Control. Release308, 29–43. 10.1016/j.jconrel.2019.06.031
181
Yang Y. Fan X. Li L. Yang Y. Nuernisha A. Xue D. et al (2020d). Semiconducting polymer nanoparticles as theranostic system for near-infrared-II fluorescence imaging and photothermal therapy under safe laser fluence. ACS Nano14, 2509–2521. 10.1021/acsnano.0c00043
182
Yata T. Takahashi Y. Tan M. Nakatsuji H. Ohtsuki S. Murakami T. et al (2017). DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials146, 136–145. 10.1016/j.biomaterials.2017.09.014
183
Yin C. Zhen X. Zhao H. Tang Y. Ji Y. Lyu Y. et al (2017). Amphiphilic semiconducting oligomer for near-infrared photoacoustic and fluorescence imaging. ACS Appl. Mat. Interfaces9, 12332–12339. 10.1021/acsami.7b02014
184
Yu C. Qian L. Ge J. Fu J. Yuan P. Yao S. C. et al (2016). Cell-penetrating poly(disulfide) assisted intracellular delivery of mesoporous silica nanoparticles for inhibition of miR-21 function and detection of subsequent therapeutic effects. Angew. Chem. Int. Ed.55, 9272–9276. 10.1002/anie.201602188
185
Yu J. Liu S. Wang Y. He X. Zhang Q. Qi Y. et al (2021). Synergistic enhancement of immunological responses triggered by hyperthermia sensitive Pt NPs via NIR laser to inhibit cancer relapse and metastasis. Bioact. Mat.7, 389–400. 10.1016/j.bioactmat.2021.05.030
186
Yu Y. Li J. Song B. Ma Z. Zhang Y. Sun H. et al (2022). Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials280, 121312. 10.1016/j.biomaterials.2021.121312
187
Yuan X. Zhu Y. Li S. Wu Y. Wang Z. Gao R. et al (2022). Titanium nanosheet as robust and biosafe drug carrier for combined photochemo cancer therapy. J. Nanobiotechnology20, 154. 10.1186/s12951-022-01374-0
188
Yue J. Mei Q. Wang P. Miao P. Dong W. F. Li L. (2022). Light-triggered multifunctional nanoplatform for efficient cancer photoimmunotherapy. J. Nanobiotechnology20, 181. 10.1186/s12951-022-01388-8
189
Zaimy M. A. Saffarzadeh N. Mohammadi A. Pourghadamyari H. Izadi P. Sarli A. et al (2017). New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther.24, 233–243. 10.1038/cgt.2017.16
190
Zang X. Zhao X. Hu H. Qiao M. Deng Y. Chen D. (2017). Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm.115, 243–256. 10.1016/j.ejpb.2017.03.013
191
Zeng Y. Xiang Y. Sheng R. Tomás H. Rodrigues J. Gu Z. et al (2021). Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mat.6, 3358–3382. 10.1016/j.bioactmat.2021.03.008
192
Zhan Y. Cao X. Li Y. Tian J. Liang J. Chen X. et al (2017). Silica cross-linked micellar core-shell nanoparticles encapsulating IR-780 with strong bright and good biocompatibility for optical imaging in vivo. J. Biomed. Nanotechnol.13, 144–154. 10.1166/jbn.2017.2332
193
Zhang C. Zeng Z. Cui D. He S. Jiang Y. Li J. et al (2021). Semiconducting polymer nano-PROTACs for activatable photoimmunometabolic cancer therapy. Nat. Commun.12, 2934. 10.1038/s41467-021-23194-w
194
Zhang D. X. Esser L. Vasani R. B. Thissen H. Voelcker N. H. (2019a). Porous silicon nanomaterials: Recent advances in surface engineering for controlled drug-delivery applications. Nanomedicine14, 3213–3230. 10.2217/nnm-2019-0167
195
Zhang H. Liu K. Li S. Xin X. Yuan S. Ma G. et al (2018a). Self-assembled minimalist multifunctional theranostic nanoplatform for magnetic resonance imaging-guided tumor photodynamic therapy. ACS Nano12, 8266–8276. 10.1021/acsnano.8b03529
196
Zhang M. Liu J. Kuang Y. Li Q. Zheng D. W. Song Q. et al (2017). Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int. J. Biol. Macromol.98, 691–700. 10.1016/j.ijbiomac.2017.01.136
197
Zhang N. Li M. Sun X. Jia H. Liu W. (2018b). NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials159, 25–36. 10.1016/j.biomaterials.2018.01.007
198
Zhang P. Cheng F. Zhou R. Cao J. Li J. Burda C. et al (2014). DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microRNA-responsive controlled drug delivery. Angew. Chem. Int. Ed.53, 2371–2375. 10.1002/anie.201308920
199
Zhang R. Xu Y. Zhang Y. Kim H. S. Sharma A. Gao J. et al (2019b). Rational design of a multifunctional molecular dye for dual-modal NIR-II/photoacoustic imaging and photothermal therapy. Chem. Sci.10, 8348–8353. 10.1039/c9sc03504d
200
Zhang W. Wang F. Wang Y. Wang J. Yu Y. Guo S. et al (2016). pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. J. Control. Release232, 9–19. 10.1016/j.jconrel.2016.04.001
201
Zhang X. An L. Tian Q. Lin J. Yang S. (2020a). Tumor microenvironment-activated NIR-II reagents for tumor imaging and therapy. J. Mat. Chem. B8, 4738–4747. 10.1039/d0tb00030b
202
Zhang X. Li H. Yi C. Chen G. Li Y. Zhou Y. et al (2020b). Host immune response triggered by graphene quantum-dot-mediated photodynamic therapy for oral squamous cell carcinoma. Int. J. Nanomedicine15, 9627–9638. 10.2147/IJN.S276153
203
Zhao W. Zhao Y. Wang Q. Liu T. Sun J. Zhang R. (2019). Remote light-responsive nanocarriers for controlled drug delivery: Advances and perspectives. Small15, 1903060. 10.1002/smll.201903060
204
Zhen X. Pu K. Jiang X. (2021). Photoacoustic imaging and photothermal therapy of semiconducting polymer nanoparticles: Signal amplification and second near-infrared construction. Small17, e2004723. 10.1002/smll.202004723
205
Zhou B. Guo X. Yang N. Huang Z. Huang L. Fang Z. et al (2021a). Surface engineering strategies of gold nanomaterials and their applications in biomedicine and detection. J. Mat. Chem. B9, 5583–5598. 10.1039/d1tb00181g
206
Zhou B. Shi B. Jin D. Liu X. (2015). Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol.10, 924–936. 10.1038/nnano.2015.251
207
Zhou F. Wang M. Luo T. Qu J. Chen W. R. (2021b). Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials265, 120421. 10.1016/j.biomaterials.2020.120421
208
Zhou F. Wu S. Song S. Chen W. R. Resasco D. E. Xing D. (2012). Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials33, 3235–3242. 10.1016/j.biomaterials.2011.12.029
209
Zhou F. Xing D. Ou Z. Wu B. Resasco D. E. Chen W. R. (2009). Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt.14, 021009. 10.1117/1.3078803
210
Zhou L. Fan Y. Wang R. Li X. Fan L. Zhang F. (2018). High-capacity upconversion wavelength and lifetime binary encoding for multiplexed biodetection. Angew. Chem. Int. Ed.57, 12824–12829. 10.1002/anie.201808209
211
Zhou W. Chen Y. Zhang Y. Xin X. Li R. Xie C. et al (2020). Iodine-rich semiconducting polymer nanoparticles for CT/fluorescence dual-modal imaging-guided enhanced photodynamic therapy. Small16, e1905641. 10.1002/smll.201905641
212
Zhou Z. Wang Y. Yan Y. Zhang Q. Cheng Y. (2016). Dendrimer-templated ultrasmall and multifunctional photothermal agents for efficient tumor ablation. ACS Nano10, 4863–4872. 10.1021/acsnano.6b02058
213
Zhu H. Fang Y. Miao Q. Qi X. Ding D. Chen P. et al (2017). Regulating near-infrared photodynamic properties of semiconducting polymer nanotheranostics for optimized cancer therapy. ACS Nano11, 8998–9009. 10.1021/acsnano.7b03507
214
Zou Y. Wang X. Khan A. Wang P. Liu Y. Alsaedi A. et al (2016). Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol.50, 7290–7304. 10.1021/acs.est.6b01897
Summary
Keywords
antitumor immunity, light-activated, nanomaterials, immunotherapy, synergistic therapy
Citation
Wang F, Duan H, Xu W, Sheng G, Sun Z and Chu H (2022) Light-activated nanomaterials for tumor immunotherapy. Front. Chem. 10:1031811. doi: 10.3389/fchem.2022.1031811
Received
30 August 2022
Accepted
20 September 2022
Published
07 October 2022
Volume
10 - 2022
Edited by
Peisheng Zhang, Hunan University of Science and Technology, China
Reviewed by
Zhigang Xu, Southwest University, China
Bowen Li, National University of Singapore, Singapore
Jingchao Li, Donghua University, China
Updates
Copyright
© 2022 Wang, Duan, Xu, Sheng, Sun and Chu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqian Chu, chuhongqian@bjxkyy.cn
†These authors have contributed equally to this work and share first authorship
This article was submitted to Chemical Biology, a section of the journal Frontiers in Chemistry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.