Abstract
The clinical significance of benzimidazole-containing drugs has increased in the current study, making them more effective scaffolds. These moieties have attracted strong research interest due to their diverse biological features. To examine their various biological significances, several research synthetic methodologies have recently been established for the synthesis of benzimidazole analogs. The present study aimed to efficiently and quickly synthesize a new series of benzimidazole analogs. Numerous spectroscopic techniques, including 1H-NMR, 13C-NMR, and HREI-MS, were used to confirm the synthesized compounds. To explore the inhibitory activity of the analogs against α-amylase and α-glucosidase, all derivatives (1–17) were assessed for their biological potential. Compared to the reference drug acarbose (IC50 = 8.24 ± 0.08 µM), almost all the derivatives showed promising activity. Among the tested series, analog 2 (IC50 = 1.10 ± 0.10 & 2.10 ± 0.10 µM, respectively) displayed better inhibitory activity. Following a thorough examination of the various substitution effects on the inhibitory capacity of α-amylase and α-glucosidase, the structure-activity relationship (SAR) was determined. We looked at the potential mechanism of how active substances interact with the catalytic cavity of the targeted enzymes in response to the experimental results of the anti-glucosidase and anti-amylase. Molecular docking provided us with information on the interactions that the active substances had with the various amino acid residues of the targeted enzymes for this purpose.
Introduction
The natural metabolism of proteins, lipids, and carbohydrates is impaired by the chronic illness of diabetes mellitus. Additionally, diabetes leads to metabolic changes that cause hyperglycemia (American Diabetes Association, 2013). The prevalence of diabetes has increased globally by 4.4% since 2000 (2.8%), and more than 366 million people will have diabetes worldwide by 2030 (Wild et al., 2004). Sulfonylureas, biguanides, and α-glucosidase inhibitors are only a few of the synthetic oral hypoglycemic drugs used to lower high blood glucose levels. Unfortunately, due to their extended use, the side effects include hypoglycemia, headaches, nausea, and dizziness (Edwin et al., 2006; Chaudhury et al., 2017). Thus, owing to these detrimental impacts, finding novel, effective, and safer drugs is of the utmost importance (Es-Safi et al., 2020). Researchers are currently focusing on heterocyclic compounds due to their potential effectiveness, availability, and generally fewer adverse effects. The present study also evaluates heterocyclic compounds such as benzimidazole-based thiadiazole derivatives. Benzimidazole rings have attracted considerable attention in comparison to other heterocyclic compounds due to their biological significance as the ring is frequently referred to as “privileged”. Researchers are particularly interested in the structure of benzimidazole; The chemistry of their ligands was first described in 1950 (Nofal et al., 2011; Walia et al., 2011). The diverse biological applications of benzimidazole and chemically related substances, such as purine, have also been reported. The key component of the complex, the benzimidazole ring, which is present in vitamin B-12 as 5,6-dimethyl-1-(a-D-ribofuranosyl), was also identified in vitamin B-12 in 1948 (Bonnett, 1963). Metronidazole, thiabendazole, misonidazole, omeprazole, astemizole, clotrimazole, azomycin, cimetidine, and antihistamines are examples of comparable products used in the veterinary, pharmaceutical, and agricultural industries (Al-Muhaimeed, 1997). Drugs containing the benzimidazole moiety have a wide range of pharmacological properties, including bactericidal (Carcanague et al., 2002; Shehab and Mansour, 2021), analgesic (Demirayak et al., 2005; Aghatabay et al., 2007), fungicidal (Solel, 1970; Lezcano et al., 2002), antiviral (Tewari and Mishra, 2006; Tonelli et al., 2008), anti-malarial (Worachartcheewan et al., 2013), HIV-1 infectivity inhibition (Gardiner et al., 1995; Kanwal et al., 2019), AChE and BuChE inhibition (Dinparast et al., 2021; Türkan, 2021), antioxidant (Ayhan-Kilcigil et al., 2004; Usta et al., 2015), and antileishmanial (Mayence et al., 2008) effects. In the field of current medicinal chemistry, researchers have shown that the hybridization of two or more molecules with various bioactive structural motifs is an efficient method for creating novel chemical entities with improved pharmacological properties. In response to the aforementioned findings and ongoing research on the synthesis of novel benzimidazole (Zawawi et al., 2016; Aroua et al., 2021) and thiadiazole (Javid et al., 2018; Khan et al., 2022a) as α-glucosidase agents, we designed and synthesized a series of novel chemical entities together with benzimidazole and thiadiazole structural motifs (Figure 2) to generate potent α-amylase and α-glucosidase agents (Figure 1).
FIGURE 1

Rationale of the current study.
Results and discussion
Chemistry
The approach taken in the synthesis of thiadiazole derivatives based on benzimidazoles. By treating benzimidazole having an aldehyde group (I) and thiosemicarbazide in methanol and refluxing the reaction mixture for about 3 hours while sodium acetate waspresent, all the synthesized products were obtained. Schiff base was then produced as an intermediate (II). Iodine and potassium carbonate in 1,4-dioxane were then added and refluxed for 5 h to produce a benzimidazole-based thiadiazole containing an amine group (III), which was further mixed in methanol and refluxed for 4 h along with different substituted aldehydes in the presence of acetic acid to obtain benzimidazole-based thiadiazole derivatives (1-17), as shown in Scheme 1. Several spectroscopic methods, including 1H-NMR, 13C-NMR, and HREI-MS, were used to confirm the exact structures of all the synthesized analogues. The proton NMR spectrum of compound 14 indicated that the most de-shielded proton attached to the nitrogen of benzimidazole nitrogen requires a lower applied magnetic field to achieve resonance, resulting in a singlet at chemical shift values of 10.23 ppm. Two more singlets were also observed: one for the (HC = N) proton resonating at a chemical shift of 7.39 ppm and the other for benzimidazole (2-position) proton resonating at a chemical shift value of 7.30 ppm. A doublet of doublets with chemical shift values resonati Qn 1g 1 at 8.50 ppm are created when the proton at position 5 of the benzimidazole ring combines with the ortho-proton next to it to form a doublet, which is further split by its meta-proton. While a proton in the 7-position of benzimidazole appeared as a doublet with a chemical shift value of 7.47 ppm, a proton in the 6-position simultaneously bonded to its surrounding ortho-protons and appeared as a triplet with a chemical shift value of 7.42 ppm. The aromatic ring is further joined to three more protons. Protons at positions 3 and 6 of the aromatic ring form doublets when they couple with neighboring protons, and the proton at position 5 of the aryl ring produces a doublet of doublet that may be seen at chemical shift values of 8.10 ppm.
SCHEME 1

Synthesis of the benzimidazole-based thiadiazole derivatives (1-17).
Spectral analysis
The spectral analyses of the synthesized analogs are included in the Supplementary Information.
In vitro α-amylase and α-glucosidase inhibitory activities
Diabetes mellitus (DM) is a chronic disorder that occurs due to the adverse effects of α-amylase and α-glucosidase enzymes. Various drugs are used to inhibit these enzymes but have risks for serious complications. To overcome these complications, researchers have focused on heterocyclic compounds to synthesize effective inhibitors for the treatment of DM. Among these heterocyclic compounds are benzimidazole-based thiadiazoles. The present study synthesized 17 benzimidazole-based thiadiazole compounds and evaluated their effectiveness against α-amylase and α-glucosidase enzymes. Due to different substituents on the aromatic ring (represented by “R”), these compounds showed a range of inhibitions, with IC50 values ranging from 1.10 ± 0.10 to 24.20 ± 0.40 (α-amylase) and 2.10 ± 0.10 to 26.10 ± 0.10 µM (α-glucosidase).
Structure–activity relationship (SAR)
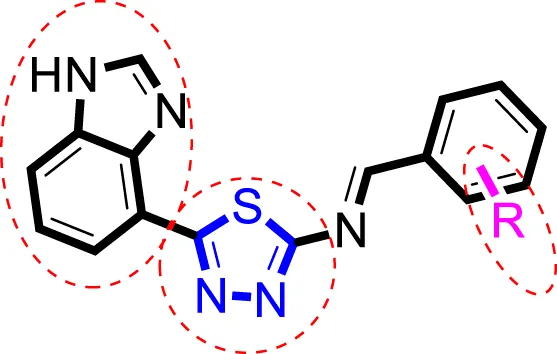
Inhibitory profiles can change depending on the substituent nature, number, and position. Substituent location on the para, ortho, and meta-positions of the aromatic ring and the nature of the substituents determine the functionality of electron-withdrawing groups (EWGs) or electron-donating groups (EDGs). Moreover, the number of substituents indicates the presence of one or more substituents (same or different). The tested analogs were compared by placing the same substituents at different positions of the aromatic ring (Figure 2).
FIGURE 2

General structures of the target compounds showing different structural fragments.
Comparison of analogs with substituted moieties against both α-amylase and α-glucosidase enzymes showed different inhibitory profiles. Nitro-substituted analogs 3 (IC50 = 22.90 ± 0.10 and 24.60 ± 0.20 µM), 4 (IC50 = 6.80 ± 0.20 and 7.40 ± 0.30 µM), 5 (IC50 = 4.80 ± 0.10 and 5.70 ± 0.20 µM), and 14 (IC50 = 8.10 ± 0.30 and 9.30 ± 0.40 µM) were compared with the standard drug acarbose (IC50 = 4.16 ± 0.17 and 5.30 ± 0.12 µM, respectively). Among these nitro group analogs, analog 5 showed remarkable potential, while the others showed good to moderate activity, possibly due to the presence of nitro moiety at the para-position of the aromatic ring, which produces a strong tendency for hydrogen bonding. The remaining analogs showed comparable activity to standard drugs. The lower potential of analog 3 might be due to the presence of the bulky bromo group at the para-position of the ring.
Replacement of the nitro group by a chloro group at different positions changed the ring activity profiles, possibly due to the nucleophilic characteristic of chlorine, which creates strong interaction with the active sites of enzymes. Analogs 15 (IC50 = 5.40 ± 0.050 and 6.60 ± 0.10 µM) and 17 (IC50 = 2.16 ± 0.50 and 2.30 ± 0.60 µM respectively) showed better inhibitory profiles than nitro-substituted analogs. The change in the activity profile also depends on the number of substituents. Analog 15 bears two chloro groups at the ortho and meta-positions, while analog 17 has two chloro groups at the ortho- and para-positions and a hydroxyl group at the ortho-position. Thus, the number and nature of the substituents also increased the activity in the case of chloro groups. The hydroxyl group increased the analog efficacy through hydrogen binding; thus, analog 17 was much more potent than analog 15 and exhibited a two-fold better activity profile than the standard drug acarbose.
Likewise, fluoro substituents replacing the chloro group further increase the biological potential of analogs that might be smaller in size and can make strong hydrogen bonds with the active sites of enzymes. Among fluoro-substituted analogs 2 (IC50 = 1.10 ± 0.10 and 2.10 ± 0.10 µM), 9 (IC50 = 2.40 ± 0.010 and 3.90 ± 0.10 µM), and 11 (IC50 = 3.18 ± 0.10 and 4.70 ± 0.10 µM, respectively), the differences in inhibitory profiles were due to the position of the fluoro group. Analog 2 was the most potent analog among the series due to both fluoro and hydroxyl moieties (para and ortho-positions, respectively) on the ring leading to the formation of stronger hydrogen bonds with the active sites of enzymes. In addition, the trifluoro-substituted analog 1 (IC50 = 2.20 ± 0.10 and 2.80 ± 0.20 µM, respectively) also showed two-fold better results than acarbose.
Similarly, the methyl/methoxy/and bromo-substituted analogs (3,6-8, 10, 12, 13, and 16) showed at least comparable activity to the standard drug acarbose. The bulky nature of bromine and steric hindrance by the methyl moiety decrease the analog potentials. Varied ranges of inhibitory profiles were exhibited by these analogs (Table 1).
TABLE 1
|
|||
|---|---|---|---|
| S. No. | R | α-Amylase IC50(µM ±SEM) | α-Glucosidase IC50(µM ±SEM) |
| 1 |
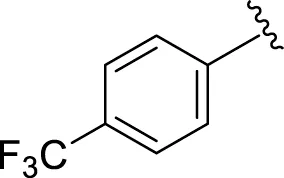
|
2.20 ± 0.10 | 2.80 ± 0.20 |
| 2 |
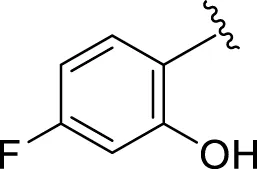
|
1.10 ± 0.10 | 2.10 ± 0.10 |
| 3 |
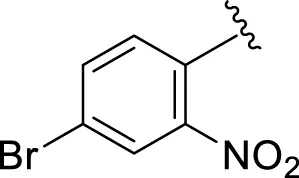
|
22.90 ± 0.10 | 24.60 ± 0.20 |
| 4 |
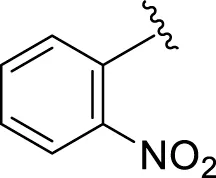
|
6.80 ± 0.20 | 7.40 ± 0.30 |
| 5 |
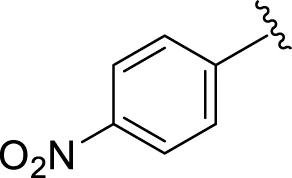
|
4.80 ± 0.10 | 5.70 ± 0.20 |
| 6 |
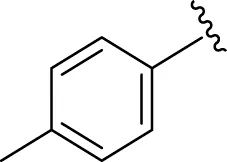
|
13.80 ± 0.20 | 15.30 ± 0.10 |
| 7 |
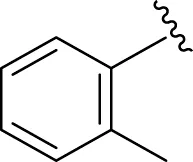
|
18.70 ± 0.20 | 19.90 ± 0.10 |
| 8 |
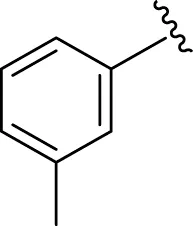
|
19.60 ± 0.20 | 21.60 ± 0.30 |
| 9 |
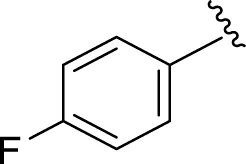
|
2.40 ± 0.010 | 3.90 ± 0.10 |
| 10 |
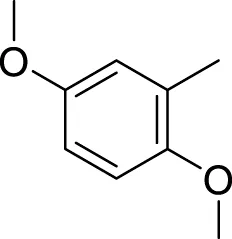
|
14.35 ± 0.50 | 14.50 ± 0.50 |
| 11 |
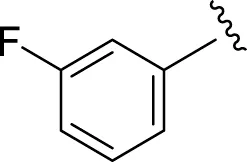
|
3.18 ± 0.10 | 4.70 ± 0.10 |
| 12 |
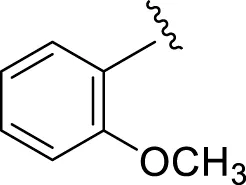
|
14.30 ± 0.20 | 15.40 ± 0.30 |
| 13 |
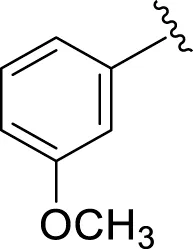
|
15.70 ± 0.30 | 17.20 ± 0.30 |
| 14 |
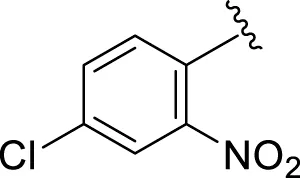
|
8.10 ± 0.30 | 9.30 ± 0.40 |
| 15 |
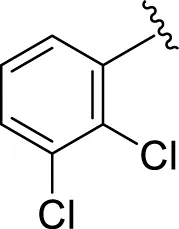
|
5.40 ± 0.050 | 6.60 ± 0.10 |
| 16 |
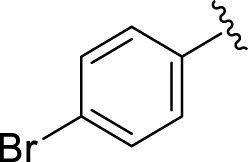
|
24.20 ± 0.40 | 26.10 ± 0.10 |
| 17 |
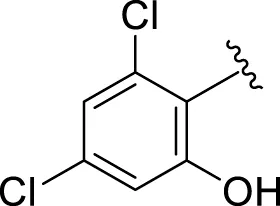
|
2.16 ± 0.50 | 2.30 ± 0.60 |
| Standard drug acarbose | 4.16 ± 0.17 | 5.30 ± 0.12 | |
α-Amylase and α-glucosidase inhibitory activities of benzimidazole-based thiadiazole derivatives (1-17).
Molecular docking studies
Based on the type of attached substituents, which either improve or diminish the interactive qualities, a molecular docking study can disclose the binding interaction of a molecule with the active sites of enzymes. A variety of tools, including Auto Dock Vina (1.5.7), Molecular Operational Environment (MOE-2015), and Discovery Studio Visualizer, were used to conduct molecular docking reQ1s3 earch (DSV-2021) (Kharb et al., 2012; Javid et al., 2018; Khan et al., 2022a; Khan et al., 2022b). Three processes were involved when carrying out the molecular docking studies. The first step protein and ligand, which were both downloaded from the RCSD protein data bank, and reducing energy in MOE. Next, the proteins and ligands were transferred to AutoDock in which polar hydrogen, Kollman, and Gasteiger charges were added and water molecules were removed from the protein. After completion, the PDBQT and the text formats of the protein, ligand, and their X, Y, and Z coordinates were saved, respectively. The final step involved specifying the location of the docking folder and performing a molecular docking investigation using the command prompt. Finally, the binding interaction was examined using DSV (Table 2) as 2D and 3D structures, as shown in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, and Figure 8.
TABLE 2
| Compound | Receptor | Interaction | Distance | Docking score |
|---|---|---|---|---|
| Analog 1(A) against α-amylase | ASP-A-197 | H-B | 4.13A° | −12.7 |
| HIS-A-101 | Pi–cation | 5.87A° | ||
| ALA-A-198 | Pi–alkyl | 7.65A° | ||
| TYR-A-62 | Pi–pi stacked | 4.51A° | ||
| TRP-A-59 | Pi–Pi stacked | 5.39A° | ||
| TRP-A-59 | Pi–pi stacked | 6.25A° | ||
| ALA-A-105 | H-B | 3.08A° | ||
| ALA-A-105 | Alkyl | 4.16A° | ||
| VAL-A-107 | Pi–alkyl | 5.00A° | ||
| GLN-A-63 | H-B | 5.22 A° | ||
| Analog 1(B) against α-glucosidase | ILE-A-233 | Pi–alkyl | 4.17A° | −11.6 |
| ASP-A-232 | Pi–anion | 5.13A° | ||
| ALA-A-234 | Pi–alkyl | 4.28A° | ||
| TRP-A-432 | Pi–sigma | 6.84A° | ||
| TRP-A-432 | Pi–alkyl | 6.55A° | ||
| ASP-A-568 | Pi–anion | 5.79A° | ||
| ASP-A-357 | H-F | 5.62A° | ||
| TRP-A-329 | Pi–alkyl | 4.40A° | ||
| PHE-A-601 | Pi–aAlkyl | 6.92A° | ||
| Analog 2(C) against α-amylase | TYR-A-62 | Pi–pi stacked | 4.51A° | −11.4 |
| LEU-A-162 | Pi–alkyl | 7.09A° | ||
| LEU-A-165 | Pi–sigma | 5.20A° | ||
| GLN-A-63 | H-B | 5.01A° | ||
| THR-A-163 | H-B | 4.78A° | ||
| HIS-A-101 | Pi–cation | 7.58A° | ||
| ALA-A-198 | Pi–alkyl | 6.03A° | ||
| ASP-A-197 | H-B | 4.12A° | ||
| Analog 2(D) against α-glucosidase | PHE-A-236 | Pi–pi T-shaped | 6.06A° | −10.2 |
| ALA-A-234 | Pi–alkyl | 4.50A° | ||
| ASP-A-232 | H-B | 5.55A° | ||
| ASP-A-232 | Pi–anion | 5.73A° | ||
| ASP-A-357 | H-F | 5.44A° | ||
| MET-A-470 | Pi–sulfur | 7.33A° | ||
| TRP-A-329 | Pi–pi T-shaped | 6.84A° | ||
| ASP-A-568 | Pi–anion | 5.90A° | ||
| TRP-A-432 | Pi–anion | 5.55A° | ||
| ARG-A-552 | H-B | 6.30A° | ||
| Analog 17(E) against α-amylase | TYR-A-62 | Pi–pi stacked | 4.95A° | −9.8 |
| LEU-A-162 | Pi–alkyl | 7.08A° | ||
| LEU-A-165 | Pi-S | 5.35A° | ||
| GLN-A-63 | H-B | 5.17A° | ||
| TRP-A-59 | Pi-S | 5.40A° | ||
| VAL-A-107 | Pi-R | 5.03A° | ||
| ALA-A-106 | R | 3.62A° | ||
| THR-A-163 | Un-Acc.-Acc | 4.97A° | ||
| HIS-A-101 | Pi–cation | 7.57A° | ||
| ALA-A-198 | Pi-R | 5.99A° | ||
| ASP-A-197 | H-B | 4.09A° | ||
| Analog 17(F) against α-glucosidase | ILE-A-233 | Pi-R | 4.20A° | −8.7 |
| ALA-A-234 | H-B | 4.00A° | ||
| ALA-A-234 | Pi–anion | 4.00A° | ||
| TRP-A-432 | Pi–sigma | 6.98A° | ||
| TRP-A-432 | Pi–alkyl | 6.48A° | ||
| ASP-A-568 | Pi–anion | 5.76A° | ||
| TRP-A-329 | Pi–alkyl | 6.44A° | ||
| ARG-A-552 | H-B | 6,22A° | ||
| ASP-A-232 | H-B | 5.26A° | ||
| ASP-A-232 | Pi–anion | 4.98A° |
Binding interactions of selected molecules, showing the enzyme active residues, distances, and docking scores.
FIGURE 3

Protein–ligand interactions (PLI) of analog-1 against α-amylase.
FIGURE 4

Protein–ligand interactions (PLI) of analog-1 against α-glucosidase.
FIGURE 5
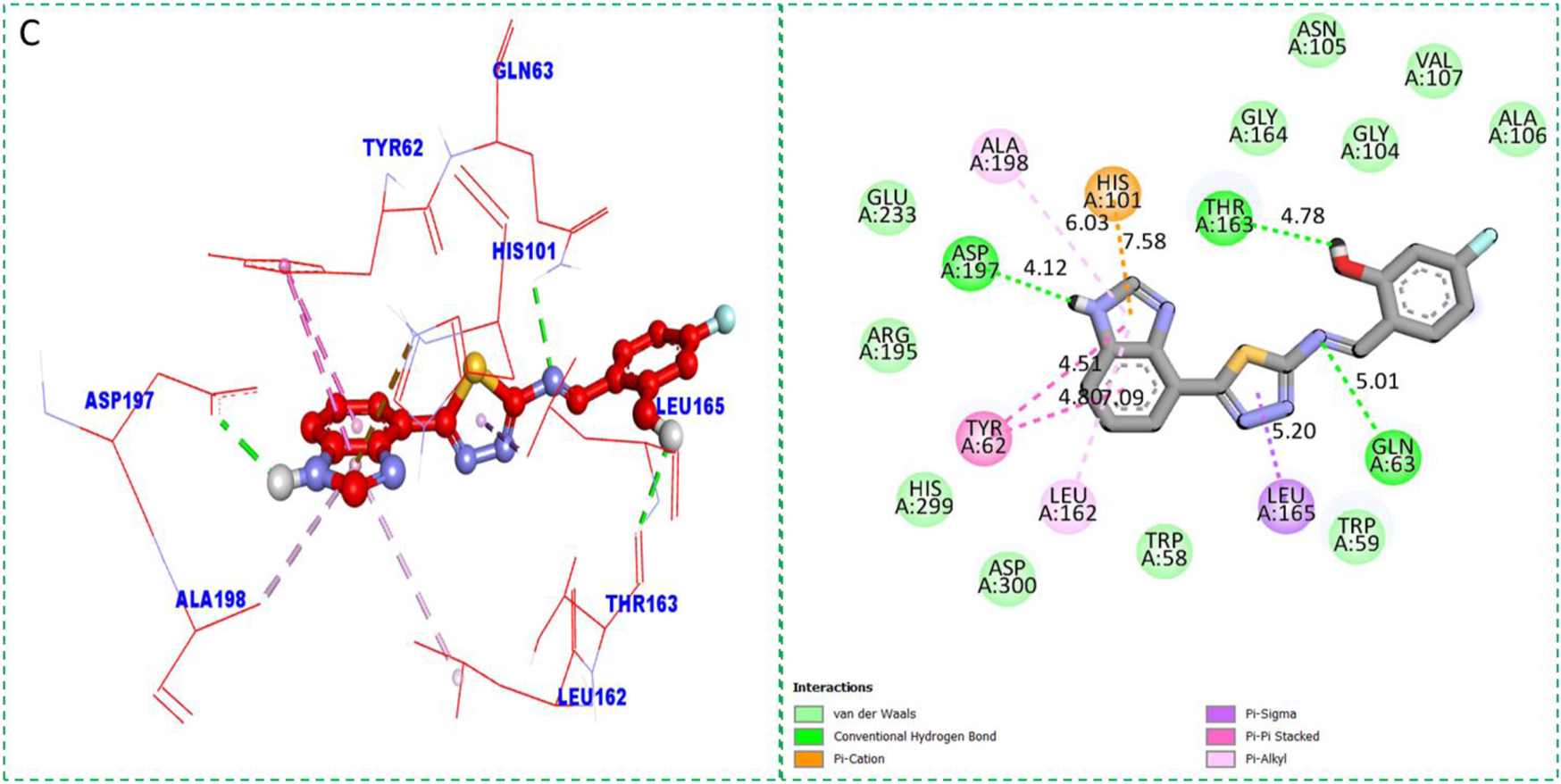
Protein–ligand interactions (PLI) of analog-2 against α-amylase.
FIGURE 6

Protein–ligand interactions (PLI) of analog-2 against α-glucosidase.
FIGURE 7

Protein–ligand interactions (PLI) of analog-17 against α-amylase.
FIGURE 8

Protein–ligand interactions (PLI) of analog-17 against α-glucosidase.
The synthesized compounds with significant potential against α-amylase and α-glucosidase showed better interactions for the superimposed complex. Bonded functional groups at various positions on the aromatic ring provided analogs with a strong affinity. Trifluoro, nitro, and hydroxyl-containing molecules showed stronger hydrogen bonding. The attached substituents may have contributed to the good-to-poor interactions observed in most of the analogs, although 1, 2, and 17 were the most active analogs, with the highest numbers of interactions. The grid dimension for this docking along the X, Y, and Z-axes were 12.037, −6.834, and 3.446, respectively. The size for all axes (X, Y, and Z) was 20.
Conclusion
Benzimidazole-based thiadiazole analogs (1–17) were produced through a series of reactions using efficient and easy methods. The synthesized scaffolds were characterized using a variety of spectroscopic methods, including 1H-NMR, 13C-NMR, and HREI-MS, and they were tested against the enzymes α-glucosidase and α-amylase. The majority of them were discovered to have good to moderate inhibitory activity, however derivatives 1, 2, 5, 9, 11, and 17 were discovered to have superior activities against both α-amylase and α-glucosidase in comparison to the common medication acarbose. Among the evaluated analogs, analog2 (IC50 = 1.10 ± 0.10 and 2.10 ± 0.10 μM, respectively) was the most potent in the tested series. The α-amylase and α-glucosidase activity of a new class of benzimidazole-based thiadiazole derivatives was discovered. The synthetic derivativesdemonstrated strong correlates with the experimental results in molecular docking investigations. Active substanceswere identified as potential anti-diabetic leads based on their interactions with active site residues and their binding mechanisms. The novel, revolutionary structural hybrids of benzimidazole and thiadiazole moieties are new active leads and attractive possibilities for the development of anti-diabetic drugs due to the level of activity and docking studies they have shown.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SK: Conception, design of study, writing-original draft preparation. SI: Interpret the data, performed major experimental works, writing-original draft preparation and editing. MT: He wrote introduction part in the manuscript, interpreted the data and critical revision. RH: Conception, performed docking analysis, acquisition of data, interpret the data. FR: He wrote the NMR analysis part in the manuscript, interpreted the data and critical revision. MS: Performed structure activity relationship analysis, reviewed original manuscript and critical revision. NA: Visualization of data, docking study, funding acquisition, writing reviewing, and editing. HI: Conception, visualization of data, performed inhibitory activity, funding acquisition. MA: He performed synthesis methodology. AD: reviewed original manuscript and critical revision. HU: design of study, and critical revision. AB: Conception, performed inhibitory analysis, acquisition of data, interpret the data. SA: Funding acquisition, interpreted the data and critical revision. EE: Visualization of data, reviewed original manuscript and critical revision. MR: Interpreted the data and critical revision.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through research groups program under grant number RGP.2/273/44. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R134), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through research groups program under grant number RGP.2/273/44. This research was funded by PrincessNourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R134), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AN declared a past co-authorship with the author EE.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1125915/full#supplementary-material
References
1
Aghatabay N. M. Somer M. Senel M. Dulger B. Gucin F. (2007). Raman, FT-IR, NMR spectroscopic data and antimicrobial activity of bis [μ2-(benzimidazol-2-yl)-2-ethanethiolato-N, S, S-chloro-palladium (II)] dimer,[(μ2-CH2CH2NHNCC6H4) PdCl] 2· C2H5OH complex. Eur. J. Med. Chem.42, 1069–1075. 10.1016/j.ejmech.2007.01.011
2
Al-Muhaimeed H. (1997). A parallel-group comparison of astemizole and loratadine for the treatment of perennial allergic rhinitis. J. Int. Med. Res.25, 175–181. 10.1177/030006059702500401
3
American Diabetes Association (2013). Diagnosis and classification of diabetes mellitus. Diabetes Care36, S67–S74. 10.2337/dc13-s067
4
Aroua L. M. Almuhaylan H. R. Alminderej F. M. Messaoudi S. Chigurupati S. Al-Mahmoud S. et al (2021). A facile approach synthesis of benzoylaryl benzimidazole as potential α-amylase and α-glucosidase inhibitor with antioxidant activity. Bioorg. Chem.114, 105073. 10.1016/j.bioorg.2021.105073
5
Ayhan-Kilcigil G. Kus C. Çoban T. Can-Eke B. &Iscan M. (2004). Synthesis and antioxidant properties of novel benzimidazole derivatives. J. Enzyme Inhibition Med. Chem.19 (2), 129–135. 10.1080/1475636042000202017
6
Bonnett R. (1963). The chemistry of the vitamin B12 group. Chem. Rev.63 (6), 573–605. 10.1021/cr60226a002
7
Carcanague D. Shue Y.-K. Wuonola M. A. UriaNickelsen M. Joubran C. Abedi J. K. et al (2002). Novel structures derived from 2-[[(2-pyridyl) methyl] thio]-1 h-benzimidazole as anti-helicobacter p ylori agents, Part 2. J. Med. Chem.45, 4300–4309. 10.1021/jm020868v
8
Chaudhury A. Duvoor C. Reddy Dendi V. S. Kraleti S. Chada A. Ravilla R. et al (2017). Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol.8, 6. 10.3389/fendo.2017.00006
9
Demirayak S. karaburun A. C. Kayagil I. Ucucu U. Beis R. (2005). Synthesis and analgesic activities of some 2-(benzazolylacetyl) amino-3-ethoxycarbonylthiophene derivatives. Phosphorous Sulfur SiliconElements180, 1841–1848. 10.1080/104265090889503
10
Dinparast L. Zengin G. &Bahadori M. B. (2021). Cholinesterases inhibitory activity of 1H-benzimidazole derivatives. Biointerface Res. Appl. Chem.11, 10739–10745.
11
Edwin E. Sheeja E. Chaturvedi M. Sharma S. Gupta V. B. (2006). A comparative study on antihyperglycemic activity of fruits and barks of Ficus bengalensis (L.). Adv. Pharmacol. Toxicol.7, 69–71.
12
Es-Safi I. Mechchate H. Amaghnouje A. El Moussaoui A. Cerruti P. Avella M. et al (2020). Marketing and legal status of phytomedicines and food supplements in Morocco. J. Complement. Integr. Med.18, 279–285. 10.1515/jcim-2020-0168
13
Gardiner J. M. Loyns C. R. Burke A. Khan A. (1995). Mahmood, N. Synthesis and HIV-1 inhibition of novel benzimidazole derivatives. Bioorg. Med. Chem. Lett.7, 1251–1254.
14
Javid M. T. Rahim F. Taha M. Rehman H. Nawaz M. wadood A. et al (2018). Synthesis, in vitro α-glucosidase inhibitory potential and molecular docking study of thiadiazole analogs. Bioorg. Chem.78, 201–209. 10.1016/j.bioorg.2018.03.022
15
Kanwal A. Ahmad M. Aslam S. Naqvi S. A. R. &Saif M. J. (2019). Recent advances in antiviral benzimidazole derivatives: A mini review. Pharm. Chem. J.53 (3), 179–187. 10.1007/s11094-019-01976-3
16
Khan A. A. Rahim F. Taha M. Rehman W. Iqbal N. Wadood A. et al (2022). New biologically dynamic hybrid pharmacophore triazinoindole-based-thiadiazole as potent α-glucosidase inhibitors: In vitro and in silico study. Int. J. Biol. Macromol.199, 77–85. 10.1016/j.ijbiomac.2021.12.147
17
Khan S. Ullah H. Rahim F. Nawaz M. Hussain R. Rasheed L. (2022). Synthesis, in vitro α-amylase, α-glucosidase activities and molecular docking study of new benzimidazole bearing thiazolidinone derivatives. J. Mol. Struct.133812.
18
Kharb M. Jat R. K. Parjapati G. Gupta A. (2012). Introduction to molecular docking software technique in medicinal chemistry. Int. J. Drug Res. Technol.2, 189–197.
19
Lezcano M. Al-Soufi W. Novo M. Rodriguez-Nunez E. Tato J. V. (2002). Complexation of several benzimidazole-type fungicides with α-and β-cyclodextrins. J. Agric. Food. Chem.50, 108–112. 10.1021/jf010927y
20
Li Z. Gu J. Zhuang H. Kang L. Zhao X. Guo Q. (2015). Adaptive molecular docking method based on information entropy genetic algorithm. App. Soft Comput.26, 299–302. 10.1016/j.asoc.2014.10.008
21
Mayence A. Pietka A. Collins M. S. Cushion M. T. Tekwani B. L. Huang T. L. et al (2008). Novel bisbenzimidazoles with antileishmanial effectiveness. Bioorg. Med. Chem. Lett.18 (8), 2658–2661. 10.1016/j.bmcl.2008.03.020
22
Nofal Z. M. Soliman E. A. Abd El-Karim S. S. El Zahar M. I. Srour A. M. Sethumadhavan S. et al (2011). Novel benzimidazole derivatives as expected anticancer agents. Acta Pol. Pharm.68 (4), 519–534.
23
Rao C. M. M. P. Naidu N. Priya J. Rao K. P. C. Ranjith K. Shobha S. et al (2021). Molecular docking and dynamic simulations of benzimidazoles with beta-tubulins. Bioinformation17 (3), 404. 10.6026/97320630017404
24
Shehab O. R. Mansour A. M. (2021). Exploring electronic structure, and substituent effect of some biologically active benzimidazole derivatives: Experimental insights and DFT calculations. J. Mol. Struct.1223, 128996. 10.1016/j.molstruc.2020.128996
25
Solel Z. (1970). The systemic fungicidal effect of benzimidazole derivatives and thiophanate against Cercospora leaf spot of sugar beet. Phytopathology60 (8), 1186–1190. 10.1094/phyto-60-1186
26
Tewari A. K. Mishra A. (2006). Synthesis and antiviral activities of N-substituted-2-substituted-benzimidazole derivatives. Indian J. Chem. Sect.45, 489–493.
27
Tonelli M. Paglietti G. Boido V. Sparatore F. Marongiu F. Marongiu E. et al (2008). Antiviral activity of benzimidazole derivatives. I. Antiviral activity of 1‐substituted‐2‐[(benzotriazol‐1/2‐yl) methyl] benzimidazoles. Chem. Biodivers.5 (11), 2386–2401. 10.1002/cbdv.200890203
28
Türkan F. (2021). Investigation of the toxicological and inhibitory effects of some benzimidazole agents on acetylcholinesterase and butyrylcholinesterase enzymes. Archives Physiology Biochem.127 (2), 97–101. 10.1080/13813455.2019.1618341
29
Usta A. Yılmaz F. Kapucu G. Baltas N. &Mentese E. (2015). Synthesis of some new benzimidazole derivatives with their antioxidant activities. Lett. Org. Chem.12 (4), 227–232. 10.2174/1570178612666150203233804
30
Walia R. Hedaitullah M. Naaz S. F. Iqbal K. Lamba H. S. (2011). Benzimidazole derivatives-an overview. Int. J. Res. Pharm. Chem.1 (3), 565–574.
31
Wild S. Roglic G. Green A. Sicree R. King H. (2004). Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care27, 2569–1053. 10.2337/diacare.27.10.2569-a
32
Worachartcheewan A. Nantasenamat C. Isarankura-Na-Ayudhya C. &Prachayasittikul V. (2013). QSAR study of amidino bis-benzimidazole derivatives as potent anti-malarial agents against Plasmodium falciparum. Chem. Pap.67 (11), 1462–1473. 10.2478/s11696-013-0398-5
33
Zawawi N. K. N. A. Taha M. Ahmat N. Wadood A. Ismail N. H. Rahim F. et al (2016). Benzimidazole derivatives as new α-glucosidase inhibitors and in silico studies. Bioorg. Chem.64, 29–36. 10.1016/j.bioorg.2015.11.006
Summary
Keywords
synthesis, benzimidazole, thiadiazol, α-amylase, α-glucosidase, SAR and molecular docking, α-glucosidase
Citation
Khan S, Iqbal S, Taha M, Hussain R, Rahim F, Shah M, Awwad NS, Ibrahium HA, Alahmdi MI, Dera AA, Ullah H, Bahadur A, Aljazzar SO, Elkaeed EB and Rauf M (2023) Synthesis, in vitro biological assessment, and molecular docking study of benzimidazole-based thiadiazole derivatives as dual inhibitors of α-amylase and α-glucosidase. Front. Chem. 11:1125915. doi: 10.3389/fchem.2023.1125915
Received
16 December 2022
Accepted
07 March 2023
Published
05 May 2023
Volume
11 - 2023
Edited by
Alessio Nocentini, University of Florence, Italy
Reviewed by
Afzal Basha Shaik, Jawaharlal Nehru Technological University, India
Mohamed Hamdy El-Naggar, University of Sharjah, United Arab Emirates
Updates
Copyright
© 2023 Khan, Iqbal, Taha, Hussain, Rahim, Shah, Awwad, Ibrahium, Alahmdi, Dera, Ullah, Bahadur, Aljazzar, Elkaeed and Rauf.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahid Iqbal, shahid.i14@yahoo.com; Ali Bahadur, abahadur@wku.edu.cn; Eslam B. Elkaeed, ikaeed@mcst.edu.sa
This article was submitted to Medicinal and Pharmaceutical Chemistry, a section of the journal Frontiers in Chemistry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.