- 1J. D. Patil Sangludkar College, Daryapur, India
- 2Nanoscience Research Laboratory, Shri Shivaji Science College, Amravati, India
Recently, our modern society demands the portable electronic devices such as mobile phones, laptops, smart watches, etc. Such devices demand light weight, flexible, and low-cost energy storage systems. Among different energy storage systems, supercapacitor has been considered as one of the most potential energy storage systems. This has several significant merits such as high power density, light weight, eco-friendly, etc. The electrode material is the important part of the supercapacitor. Recent studies have shown that there are many new advancement in electrode materials for supercapacitors. In this review, we focused on the recent advancements in the cobalt oxides, manganese oxides, and their composites as an electrode material for supercapacitor.
Introduction
Energy storage has an equal importance as energy production. To face the global challenges, recently, our modern society demands lightweight, flexible, inexpensive, and environmentally friendly energy storage systems (Meng et al., 2010; Chodankar et al., 2015). Battery and supercapacitor are the major energy storage devices. But, slow charge–discharge rate, short life cycles, and high weight of battery limit its applications in portable and wearable devises (Meng et al., 2010). At present, supercapacitors have been receiving a great attention, because of their important features such as high energy density, high power density, light weight, fast charging–discharging rate, secure operation, and long life span (Jayalakshmi and Balasubramanian, 2008; Chodankar et al., 2015). The supercapacitor is also called electrochemical capacitor. This is used in various applications such as hybrid vehicles, power backup, military services, and portable electronic devises like laptops, mobile phones, wrist watches, wearable devised, roll-up displays electronic papers, etc. (Lee et al., 2011; Wang et al., 2012).
Classification of the Supercapacitor
On the basis of charge storage mechanism and material used as the electrode, the supercapacitors are divided into two categories: electrochemical double layer supercapacitors (EDLCs) and pseudocapacitor (Jayalakshmi and Balasubramanian, 2008). In EDLCs, the specific capacitance arises from the non-Faradaic charge storage mechanism between electrode and electrolyte interface (Jayalakshmi and Balasubramanian, 2008; Wang et al., 2012). The materials that have been used as electrode for EDLCs are porous carbon (Kang et al., 2015), SWNT (Liu et al., 2006), MWNT (Huang et al., 2014a), reduce graphine oxide (Zhang and Zhao, 2012), aerogel (Faraji and Ani, 2015), etc. In pseudocapacitor, the specific capacitance arises from Faradaic reaction at the electrode interface. The materials that have been studied as electrode for pseudocapacitors are transition metal oxides and conducting polymers (Wang et al., 2012).
In particular, the specific capacitance of the supercapacitors depends on the surface area and the pore size distribution of the electrode material. Compared with the transition metal oxides and conducting polymers, carbon and its different types have high surface area (3.270 m2g−1) (Kang et al., 2015). However, this high surface area of carbon is not completely accessible for the electrolyte (Faraji and Ani, 2015). To overcome this shortcoming, the composites of carbon with transition metal oxides or conducting polymer have received great attention. These composite are also called hybrid materials. The use of hybrid material as an electrode in supercapacitors result in the third category of supercapacitors called hybrid supercapacitors. In hybrid supercapacitors, the specific capacitance arises from Faradic as well as non-Faradic charge storage mechanism at the electrode and electrolyte interface (Zhang et al., 2013; Pardieu et al., 2015).
Parameters for Supercapacitor
The specific capacitance (Cs) (Fg−1), energy density E (Wh kg−1), power density P (kW kg−1), and retention capacity or coulomb efficiency (η) are the crucial characteristics of the supercapacitor device. The (Cs) (Fg−1) at the single electrode of the device is calculated given by,
where m is the mass (g cm−1) deposited, I(v) is the response current (mA) of the electrode material for unit area, V is the scan rate, Vc−Va is the operational potential window in (V), Va anodic current, and Vc cathodic current. Energy density E (Wh kg−1) and power density P (W kg−1) of supercapacitor are calculated using following relations as,
where Cs is specific capacitance (Fg−1), Vmax and Vmin are the maximum and minimum voltage achieved during charging and discharging process, respectively, in volt (V), and tD is the discharging time (s) for a cycle of the supercapacitor. The retention of specific capacitance is calculated using the relation,
where tC and tD are the charge and discharge time (s), respectively, for a cycle of the supercapacitor (Wang et al., 2010; Dubal et al., 2012).
Recent Advances in Cobalt Oxide Supercapacitor
The transition metal oxides have a great scientific significance. These are the basis of a variety of functional materials (Shinde et al., 2015). Among the various supercapacitor electrode materials, transition metal oxides offer high electronegativity, rich redox reactions, low cost, environmental friendliness, and excellent electrochemical performance. Different transition-metal oxides, such as IrO2, RuO2, Co3O4, MnO2, Fe2O3, SnO2, NiO, etc., have been extensively studied as the electrode material for supercapacitor (Luo et al., 2014). Among these, RuO2 has been identified as a dominant candidate because it has high theoretical specific capacitance (1,358 Fg−1), high electrical conductivity (300 S cm−1), and high electrochemical stability (Yu et al., 2013). However, the high cost and toxicity associated with the RuO2 limits its commercial applications (Deng et al., 2014).
Furthermore, the cobalt oxides have received significant interest in recent years because of their low cost, non-toxic, easy synthesis, and environmental friendly nature. The cobalt oxides have high theoretical capacitance (CoO: 4.292 Fg−1, Co2O4: 3.560 Fg−1) (Cheng et al., 2010; He et al., 2012). Additionally, cobalt oxides show excellent electrochemical behavior in alkaline as well as organic electrolyte. These have the ability to interact with the ions of the electrolyte at the surface as well as through the bulk of the material (Vijayakumar et al., 2013). The features of cobalt oxides such as morphology, structures, and dimension can be easily controlled via adjusting the preparative parameters such as, reaction temperature, reaction time, concentration of matrix solution, complexing agent, etc. (Wei et al., 2015a).
An optimize microstructure and controlled morphology of the material will enhance the specific surface area and pore size distribution, which facilitate the electrolyte ion transport in the material (Meher and Rao, 2011). Recently, many new approaches have been successfully in use to synthesize the meso and microporous nanostructure cobalt oxide materials such as hydrothermal method (Meher and Rao, 2011), chemical bath deposition method (Xu et al., 2010), hydrothermal precipitation method (Yu et al., 2009), solvothermal synthesis method (Yang et al., 2013), combustion synthesis method (Deng et al., 2014), microwave-assisted synthesis method (Vijayakumar et al., 2013), etc.
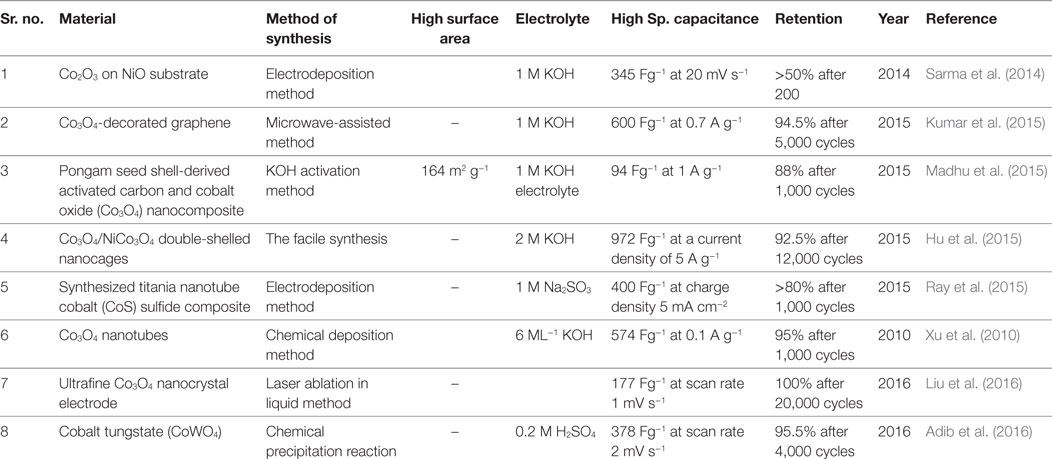
The specific capacitance of the cobalt oxide strongly depends on morphology, surface area, and pore size distribution. Recently, use of new synthesis approaches, surface modifying agents, complexing, and structure directing agent results in high-specific capacitance, which is equal the theoretical specific capacitance cobalt oxide. In this review paper, we have focused the recent advancements in the cobalt oxides and their composites as the electrode material. Table 1 shows the preparation and supercapacitive performance of cobalt oxide and their composites based supercapacitors.
Recent Advances in Manganese Oxide Supercapacitor
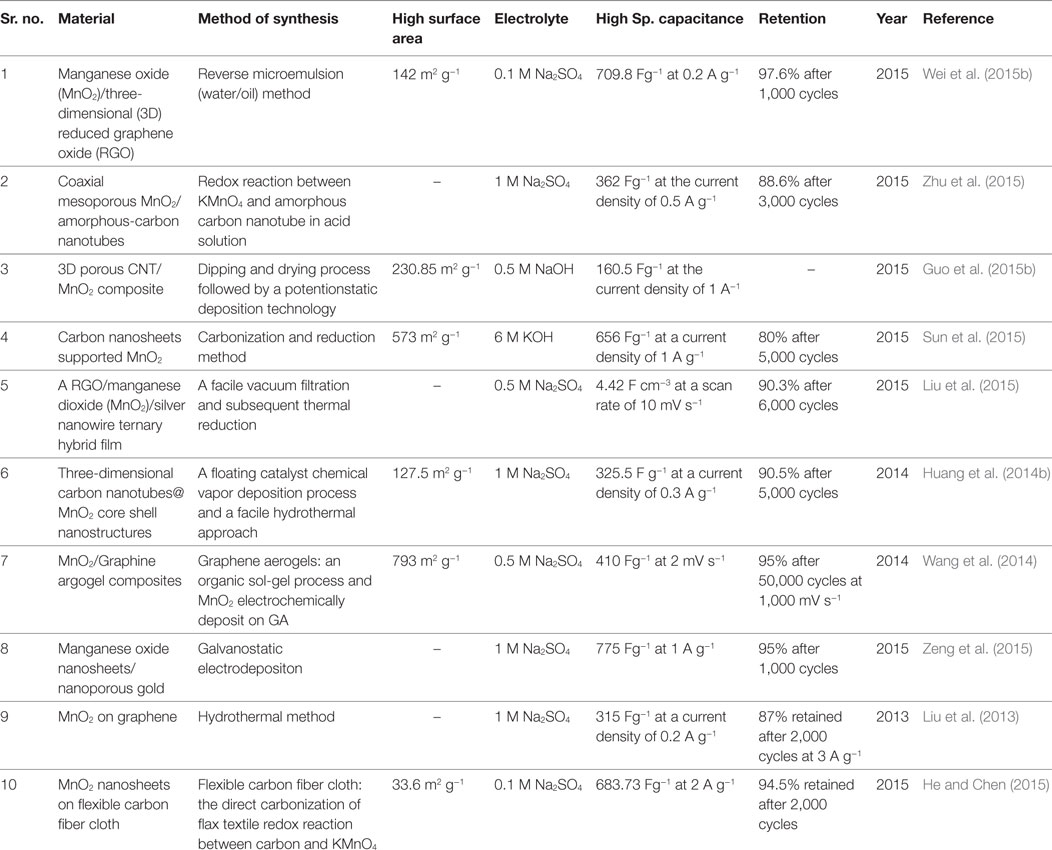
Manganese (Mn) has different oxidation states. Out of these, the most stable oxidation states are Mn (II) and Mn (IV). The Mn (II) forms MnO, on the other hand, Mn (IV) forms MnO2 and Mn2O3. The MnO2 has α, β, γ, and δ -type polymorph (Chen et al., 2014; Salunkhe et al., 2015). The advantages of manganese-based metal oxides include low cost, low toxicity, natural abundance, and environmental friendly in nature (Sui et al., 2015; Wei et al., 2015b). In aqueous and organic electrolyte, the MnO, MnO2, and Mn2O3 can form the different oxidation states. Thus, it results in the high-specific capacitance. The highest reported theoretical specific capacitance of MnO2 is 1.370 Fg−1 (Guo et al., 2015a; Wei et al., 2015b). However, the low electrical conductivity and large volume change during the charge–discharge process result in the unsatisfactory rate performance and cyclic stability. In consequence, this reduces the specific capacitance of the manganese oxides-based supercapacitors (Cabana et al., 2010; Chen et al., 2010). To overcome such hindrances, recently, the researchers have been executing many new strategies, such as use of carbon containing materials for increasing the electrical conductivity and adopt the volume buffers for relaxing internal stresses (Yao et al., 2008; Sui et al., 2015). Manganese oxides have been prepared by various synthesis methods, such as pulse laser deposition method (Xia et al., 2011), hydrothermal method (Zhang et al., 2014), electrochemical synthesis method (Jiang and Kucernak, 2002), redox deposition method (Bordjiba and Bélanger, 2009), successive hydrolysis–condensation method (Sawangphruk and Limtrakul, 2012), etc. Further, the detail of MnO2 synthesis and their supercapacitive performance are shown in Table 2.
Conclusion and Future Prospective
Recently, cobalt- and manganese-based metal oxide as the electrode materials for supercapacitor have been receiving the great attention. From the recent reports, it has concluded that,
(1) Advanced chemical method such as hydrothermal, pulse laser deposition, reverse microemulsion, microwave-assisted, etc., has been assisted to synthesize cobalt- and manganese-based metal oxide material.
(2) The specific capacitance of the cobalt oxide- and manganese-based metal oxide supercapacitor strongly depends on morphology, surface area, and pore-size distribution.
(3) In most of the reports, the composites of cobalt oxide or manganese oxide with carbon material, i.e., hybrid materials are used as an electrode for supercapacitor. Moreover, this results in high-specific capacitance.
(4) In addition, the increase in conductivity of the cobalt oxide and manganese oxides is projected if this material and carbon material are combined. This makes the application of cobalt oxide and manganese oxides in high energy applications. As a result, the proposed material cobalt oxides and manganese oxide are a promising material for flexible, portable high-rate hybrid supercapacitor, and has plenty room for advancements.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work is financially supported by University Grant Commission (UGC) New Delhi, India under Minor Research Project (File No.47-763/13/WRO).
References
Adib, K., Rahimi-Nasrabadi, M., Rezvani, Z., Pourmortazavi, S. M., Ahmadi, F., Naderi, H. R., et al. (2016). Facile chemical synthesis of cobalt tungstates nanoparticles as high performance supercapacitor. J. Mater. Sci. Mater. Electron. 27, 4541. doi: 10.1007/s10854-016-4329-4
Bordjiba, T., and Bélanger, D. (2009). Direct redox deposition of manganese oxide on multiscaled carbon nanotube/microfiber carbon electrode for electrochemical capacitor. J. Electrochem. Soc. 156, A378–A384. doi:10.1149/1.3090012
Cabana, J., Monconduit, L., Larcher, D., and Palacin, M. R. (2010). Beyond intercalation-based Li-Ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. Weinheim 22, 35. doi:10.1002/adma.201000717
Chen, I. L., Chen, T. Y., Wei, Y. C., Hu, C. C., and Lin, T. L. (2014). Capacitive performance enhancements of RuO2 nanocrystals through manipulation of preferential orientation growth originated from the synergy of Pluronic F127 trapping and annealing. Nanoscale 6, 2861–2871. doi:10.1039/c3nr04479c
Chen, S., Zhu, J., Wu, X., Han, Q., and Wang, X. (2010). Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 4, 2822–2830. doi:10.1021/nn901311t
Cheng, H., Lu, Z. G., Deng, J. Q., Chung, C. Y., Zhang, K., and Li, Y. Y. (2010). A facile method to improve the high rate capability of Co3O4 nanowire array electrodes. Nano Res. 3, 895–901. doi:10.1007/s12274-010-0063-z
Chodankar, N. R., Dubal, D. P., and Gund, G. S. (2015). Flexible all-solid-state MnO2 thin films based symmetric supercapacitors. Electrochim. Acta 165, 338–347. doi:10.1016/j.electacta.2015.02.246
Deng, J., Kang, L., Bai, G., Li, Y., Li, P., Liu, X., et al. (2014). Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta 132, 127–135. doi:10.1016/j.electacta.2014.03.158
Dubal, D. P., Kim, W. B., and Lokhande, C. D. (2012). Galvanostatically deposited Fe: MnO2 electrodes for supercapacitor application. J. Phys. Chem. Solids 73, 18–24. doi:10.1016/j.jpcs.2011.09.005
Faraji, S., and Ani, F. N. (2015). The development supercapacitor from activated carbon by electroless plating – a review. Renew. Sustain. Energ. Rev. 42, 823–834. doi:10.1016/j.rser.2014.10.068
Guo, W. H., Liu, T. J., Jiang, P., and Zhang, Z. J. (2015a). Free-standing porous manganese dioxide/graphene composite films for high performance supercapacitors. J. Colloid Interface Sci. 437, 304–310. doi:10.1016/j.jcis.2014.08.060
Guo, C., Li, H., Zhang, X., Huo, H., and Xu, C. (2015b). 3D porous CNT/MnO2 composite electrode for high-performance enzymeless glucose detection and supercapacitor application. Sens. Actuators B Chem. 206, 407–414. doi:10.1016/j.snb.2014.09.058
He, G., Li, J., Chen, H., Shi, J., Sun, X., Chen, S., et al. (2012). Hydrothermal preparation of Co3O4@ graphene nanocomposite for supercapacitor with enhanced capacitive performance. Mater. Lett. 82, 61. doi:10.1016/j.matlet.2012.05.048
He, S., and Chen, W. (2015). Application of biomass-derived flexible carbon cloth coated with MnO2 nanosheets in supercapacitors. J. Power Sources 294, 150–158. doi:10.1016/j.jpowsour.2015.06.051
Hu, H., Guan, B., Xia, B., and Lou, X. W. (2015). Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 137, 5590–5595. doi:10.1021/jacs.5b02465
Huang, K. J., Wang, L., Zhang, J. Z., Wang, L. L., and Mo, Y. P. (2014a). One-step preparation of layered molybdenum disulfide/multi-walled carbon nanotube composites for enhanced performance supercapacitor. Energy 67, 234–240. doi:10.1016/j.energy.2013.12.051
Huang, M., Zhang, Y., Li, F., Zhang, L., Wen, Z., and Liu, Q. (2014b). Facile synthesis of hierarchical Co3O4@MnO2 core-shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 252, 98–106. doi:10.1016/j.jpowsour.2013.12.030
Jayalakshmi, M., and Balasubramanian, K. (2008). Simple capacitor to supercapacitor – an overview. Int. J. Electrochem. Sci. 11, 1196–1217.
Jiang, J., and Kucernak, A. (2002). Electrochemical supercapacitor material based on manganese oxide: preparation and characterization. Electrochim. Acta 47, 2381–2386. doi:10.1016/S0013-4686(02)00031-2
Kang, D., Liu, Q., Gu, J., Su, Y., Zhang, W., and Zhang, D. (2015). “Egg-box”-assisted fabrication of porous carbon with small mesopores for high-rate electric double layer capacitors. ACS Nano 9, 11225–11233. doi:10.1021/acsnano.5b04821
Kumar, R., Singh, R. K., Dubey, P. K., Singh, D. P., and Yadav, R. M. (2015). Self-assembled hierarchical formation of conjugated 3D cobalt oxide nanobead–CNT–graphene nanostructure using microwaves for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 7, 15042–15051. doi:10.1021/acsami.5b04336
Lee, S. W., Gallant, B. M., Byon, H. R., Hammond, P. T., and Shao-Horn, Y. (2011). Nanostructured carbon-based electrodes: bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors. Energy Environ. Sci. 4, 1972–1985. doi:10.1039/c0ee00642d
Liu, W., Lu, C., Wang, X., Tay, R. Y., and Tay, B. K. (2015). High-performance microsupercapacitors based on two-dimensional graphene/manganese dioxide/silver nanowire ternary hybrid film. ACS Nano 9, 1528–1542. doi:10.1021/nn5060442
Liu, X. Y., Gao, Y. Q., and Yang, G. W. (2016). A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 8, 4227–4235. doi:10.1039/C5NR09145D
Liu, Y., Yan, D., Zhuo, R., Li, S., Wu, Z., Wang, J., et al. (2013). Design, hydrothermal synthesis and electrochemical properties of porous birnessite-type manganese dioxide nanosheets on graphene as a hybrid material for supercapacitors. J. Power Sources 242, 78–85. doi:10.1016/j.jpowsour.2013.05.062
Liu, T., Kumar, S., Inventors; Georgia Tech Research Corporation, Assignee. (2006). Supercapacitor Having Electrode Material Comprising Single-Wall Carbon Nanotubes and Process for Making the Same. United States patent US 7,061,749.
Luo, Y., Zhang, H., Guo, D., Ma, J., Li, Q., Chen, L., et al. (2014). Porous NiCo2O4-reduced graphene oxide (rGO) composite with superior capacitance retention for supercapacitors. Electrochim. Acta 132, 332–337. doi:10.1016/j.electacta.2014.03.179
Madhu, R., Veeramani, V., Chen, S. M., Manikandan, A., Lo, A. Y., and Chueh, Y. L. (2015). Honeycomb-like porous carbon-cobalt oxide nanocomposite for high-performance enzymeless glucose sensor and supercapacitor applications. ACS Appl. Mater. Interfaces 7, 15812–15820. doi:10.1021/acsami.5b04132
Meher, S. K., and Rao, G. R. (2011). Effect of microwave on the nanowire morphology, optical, magnetic, and pseudocapacitance behavior of Co3O4. J. Phys. Chem. C 115, 25543–25556. doi:10.1021/jp209165v
Meng, C., Liu, C., Chen, L., Hu, C., and Fan, S. (2010). Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett. 10, 4025–4031. doi:10.1021/nl1019672
Pardieu, E., Pronkin, S., Dolci, M., Dintzer, T., Pichon, B. P., Begin, D., et al. (2015). Hybrid layer-by-layer composites based on a conducting polyelectrolyte and Fe3O4 nanostructures grafted onto graphene for supercapacitor application. J. Mater. Chem. A 3, 22877–22885. doi:10.1039/C5TA05132K
Ray, R. S., Sarma, B., Jurovitzki, A. L., and Misra, M. (2015). Fabrication and characterization of titania nanotube/cobalt sulfide supercapacitor electrode in various electrolytes. Chem. Eng. J. 260, 671–683. doi:10.1016/j.cej.2014.07.031
Salunkhe, R. R., Ahn, H., Kim, J. H., and Yamauchi, Y. (2015). Rational design of coaxial structured carbon nanotube–manganese oxide (CNT–MnO2) for energy storage application. Nanotechnology 26, 204004. doi:10.1088/0957-4484/26/20/204004
Sarma, B., Ray, R. S., Mohanty, S. K., and Misra, M. (2014). Synergistic enhancement in the capacitance of nickel and cobalt based mixed oxide supercapacitor prepared by electrodeposition. Appl. Surf. Sci. 300, 29–36. doi:10.1016/j.apsusc.2014.01.186
Sawangphruk, M., and Limtrakul, J. (2012). Effects of pore diameters on the pseudocapacitive property of three-dimensionally ordered macroporous manganese oxide electrodes. Mater. Lett. 68, 230–233. doi:10.1016/j.matlet.2011.10.096
Shinde, S. K., Dubal, D. P., Ghodake, G. S., Gomez-Romero, P., Kim, S., and Fulari, V. J. (2015). Hierarchical 3D-flower-like CuO nanostructure on copper foil for supercapacitors. RSC Adv. 5, 30478–30484. doi:10.1039/C4RA11164H
Sui, Z. Y., Wang, C., Shu, K., Yang, Q. S., Ge, Y., Wallace, G. G., et al. (2015). Manganese dioxide-anchored three-dimensional nitrogen-doped graphene hybrid aerogels as excellent anode materials for lithium ion batteries. J. Mater. Chem. A 3, 10403–10412. doi:10.1039/C5TA05759K
Sun, K., Wang, H., Peng, H., Wu, Y., Ma, G., and Lei, Z. (2015). Manganese oxide nanorods supported on orange peel-based carbon nanosheets for high performance supercapacitors. Int. J. Electrochem. Sci. 10, 2000–2013.
Vijayakumar, S., Ponnalagi, A. K., Nagamuthu, S., and Muralidharan, G. (2013). Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim. Acta 106, 500–505. doi:10.1016/j.electacta.2013.05.121
Wang, C. C., Chen, H. C., and Lu, S. Y. (2014). Manganese oxide/graphene aerogel composites as an outstanding supercapacitor electrode material. Chem. A Eur. J. 20, 517–523. doi:10.1002/chem.201303483
Wang, G., Lu, X., Ling, Y., Zhai, T., Wang, H., Tong, Y., et al. (2012). LiCl/PVA gel electrolyte stabilizes vanadium oxide nanowire electrodes for pseudocapacitors. ACS Nano 6, 10296–10302. doi:10.1021/nn304178b
Wang, H. Q., Li, Z. S., Huang, Y. G., Li, Q. Y., and Wang, X. Y. (2010). A novel hybrid supercapacitor based on spherical activated carbon and spherical MnO2 in a non-aqueous electrolyte. J. Mater. Chem. 20, 3883–3889. doi:10.1039/c000339e
Wei, H., He, C., Liu, J., Gu, H., Wang, Y., Yan, X., et al. (2015a). Electropolymerized polypyrrole nanocomposites with cobalt oxide coated on carbon paper for electrochemical energy storage. Polymer 67, 192–199. doi:10.1016/j.polymer.2015.04.064
Wei, B., Wang, L., Miao, Q., Yuan, Y., Dong, P., Vajtai, R., et al. (2015b). Fabrication of manganese oxide/three-dimensional reduced graphene oxide composites as the supercapacitors by a reverse microemulsion method. Carbon N. Y. 85, 249–260. doi:10.1016/j.carbon.2014.12.063
Xia, X. H., Tu, J. P., Zhang, Y. Q., Mai, Y. J., Wang, X. L., Gu, C. D., et al. (2011). Three-dimentional porous nano-Ni/Co(OH)2 nanoflake composite film: a pseudocapacitive material with superior performance. J. Phys. Chem. C 115, 22662–22668. doi:10.1021/jp208113j
Xu, J., Gao, L., Cao, J., Wang, W., and Chen, Z. (2010). Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 56, 732–736. doi:10.1016/j.electacta.2010.09.092
Yang, W., Gao, Z., Ma, J., Wang, J., Wang, B., and Liu, L. (2013). Effects of solvent on the morphology of nanostructured Co3O4 and its application for high-performance supercapacitors. Electrochim. Acta 112, 378–385. doi:10.1016/j.electacta.2013.08.056
Yao, W. L., Wang, J. L., Yang, J., and Du, G. D. (2008). Novel carbon nanofiber-cobalt oxide composites for lithium storage with large capacity and high reversibility. J. Power Sources 176, 369–372. doi:10.1016/j.jpowsour.2007.10.073
Yu, G., Xie, X., Pan, L., Bao, Z., and Cui, Y. (2013). Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2, 213–234. doi:10.1016/j.nanoen.2012.10.006
Yu, Z. J., Dai, Y., and Chen, W. (2009). Synthesis of Co2O4 microspheres by hydrothermal-precipitation for electrochemical supercapacitors. Adv. Mater. Res. 66, 280–283. doi:10.4028/www.scientific.net/AMR.66.280
Zeng, Z., Zhou, H., Long, X., Guo, E., and Wang, X. (2015). Electrodeposition of hierarchical manganese oxide on metal nanoparticles decorated nanoporous gold with enhanced supercapacitor performance. J. Alloys Comp. 632, 376–385. doi:10.1016/j.jallcom.2015.01.240
Zhang, F., Zhang, T., Yang, X., Zhang, L., Leng, K., Huang, Y., et al. (2013). A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 6, 1623–1632. doi:10.1039/c3ee40509e
Zhang, J., and Zhao, X. S. (2012). Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 116, 5420–5426. doi:10.1021/jp211474e
Zhang, X., Sun, X., Zhang, H., Li, C., and Ma, Y. (2014). Comparative performance of birnessite-type MnO2 nanoplates and octahedral molecular sieve (OMS-5) nanobelts of manganese dioxide as electrode materials for supercapacitor application. Electrochim. Acta 132, 315–322. doi:10.1016/j.electacta.2014.03.176
Keywords: hybrid supercapacitor, cobalt oxide, manganese oxide, specific capacitance, specific surface area
Citation: Uke SJ, Akhare VP, Bambole DR, Bodade AB and Chaudhari GN (2017) Recent Advancements in the Cobalt Oxides, Manganese Oxides, and Their Composite As an Electrode Material for Supercapacitor: A Review. Front. Mater. 4:21. doi: 10.3389/fmats.2017.00021
Received: 10 February 2017; Accepted: 30 June 2017;
Published: 02 August 2017
Edited by:
Sravendra Rana, University of Petroleum and Energy Studies, IndiaReviewed by:
Zhengjun Zhang, Tsinghua University, ChinaHongchang Pang, Dalian University of Technology (DUT), China
Copyright: © 2017 Uke, Akhare, Bambole, Bodade and Chaudhari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Santosh J. Uke, c2FudG9zaF91a2VAcmVkaWZmbWFpbC5jb20=
 Santosh J. Uke
Santosh J. Uke Vijay P. Akhare2
Vijay P. Akhare2