- 1Faculté de Chirurgie Dentaire, Université de Strasbourg, Strasbourg, France
- 2Institut National de la Santé et de la Recherche Médicale, Unité Mixte de Recherche 1121, Strasbourg, France
The presence of catechol groups in hydrogels, either grafted to the constituting polymers or added in the gel formulation allow to increase the adhesion strength of such hydrogels. In this investigation, we add pyrocatechol (1,2-benzenediol) and pyrogallol (1,2,3-benzenetriol) in gelatin solution to form hydrogels using sodium periodate as an oxidant with the aim to induce interactions between pyrocatechol/pyrogallol and the gelatin chains. The gelation kinetics of the hydrogels as well as their adhesion strength and toughness are evaluated as a function of the concentration in NaIO4 for a constant concentration-10 mM-in pyrocatechol/pyrogallol. It came out that the addition of pyrogallol to gelatin (10% w/v) did not improve the adhesion strength on stainless steel when compared to a pristine gelatin gel. As an interesting finding, the addition of pyrocatechol to gelatin allowed for a major improvement of the adhesive strength between two stainless steel plates and allowed to stabilize the gel up to 50°C. However, the pyrogallol modified gelatin gels displayed no thermal stabilization compared to pristine gelatin. The major differences between pyrocatechol and pyrogallol modified gels are explained on the basis of the electrophilicity of the oxidized polyphenols. In addition, the self-healing behavior of the gelatin based gels was investigated as a function of their composition.
Introduction
In the last years tissue adhesives and glues (Duarte et al., 2012) have attracted major attention because they potentially allow to overcome the intrinsic drawbacks of sutures, namely damage on the neighboring tissues, foreign body reaction, aesthetic reasons and so on. Tissue adhesives have gained much interest during that time because they allow to overcome most of the drawbacks displayed by sutures (Rahimnejad and Zhong, 2017). Inspired by the adhesion mechanism used by living organisms like mussels (Lee et al., 2011) and sandcastle worms, it became a major research topic to modify polymers with catechol groups not only to increase the adhesion to solid substrates but also to increase their cohesion.
Among many examples, nitrodopamine modified 4 arm PEG was mixed with a solution containing Fe3+ cations to reach an Fe/nitrodopamine ratio equal to 3 in order to induce gelation through metal coordination. The obtained gel displayed a self-healing behavior and could be degraded at will through UV illumination to cleave the nitro group (Shafiq et al., 2012). The same 4 arm PEG modified with dopamine and mixed with collagen and hydroxyapatite displayed better adhesion performance under wet conditions than cyanoacrylates (Feng et al., 2017).
Gelatin taking grafted catecholamines was already used as a surgical glue (Fan et al., 2016) and was also modified with 2,3,4-trihydroxybenzaldehyde to get strong adhesion to various materials like iron, ceramics and pigskin (Han et al., 2020). Other biopolymers have been grafted with catechols with the same aim: chitosan (Ryu et al., 2011), hyaluronic acid (Shin et al., 2015) as well as alginates (Lee et al., 2013; Mateescu et al., 2015).
Pyrogallol was also grafted to chitin fibers in order to produce strongly adhering hydrogels (Oh et al., 2015).
On the other hand, very few studies investigated the influence of the blending, without preliminary covalent crosslinking, of a catechol or a catecholamine to a polymer able to gelate on the adhesion performance of the obtained hydrogels (Chen et al., 2016; Nam et al., 2019). The possibility to obtain good adhesion performance by simple blending a polymer able to simultaneously form hydrogels and able to form covalent crosslinks with catechols/catecholamines in the presence of a strong oxidant (Yang et al., 2014) remains hence to be explored. To that aim, we blended gelatin, containing amino end groups and a few L-Lysine side chains with either pyrocatechol or pyrogallol in the presence of NaIO4 as an oxidant (at 10, 20 or 30 mM in the presence of 10 mM of either pyrocatechol or pyrogallol), in order to produce strongly adhesive hydrogels and eventually thermally stable gelatin based low-cost hydrogels. The main interest of our investigation was to compare the influence of an additional hydroxyl group on the aromatic ring, when going from pyrocatechol to pyrogallol (Scheme 1) on the mechanical and thermal performance of the blended hydrogels. Such blends of gelling agents and crosslinking molecules without chemical grafting could have high potential interest in industry. Pyrocatechol and pyrogallol were oxidized with sodium periodate to produce reactive quinones able to react with nucleophilic amino acid side chains (Faure et al., 2013; Yang et al., 2014) allowing hence some eventual crosslinks between gelatin chains and pyrocatechol-pyrogallol based aggregates and hence a marked modification of the gel’s thermomechanical properties.
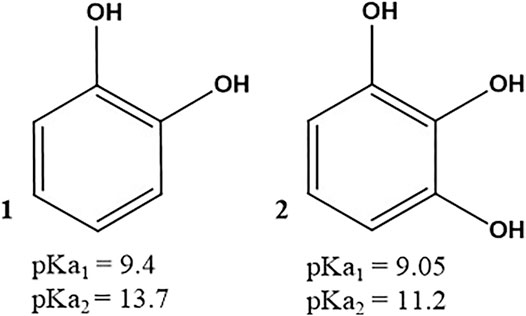
SCHEME 1. Structures of pyrocatechol 1) and pyrogallol 2). The pKa values for each compound are taken from (Slabbert 1977).
In addition, owing to the well-known ability of gelatin to form intermolecular helixes (Djabourov et al., 1988) at the origin of the thermally induced gelation and its ability to undergo self-healing (Sharma et al., 2017), we investigated the influence of either pyrocatechol or pyrogallol to modify this property.
Materials and Methods
Chemicals
All solutions were made from double distilled and deionized water (ρ = 18.2 MΩ cm, Millipore RO system)).
Pyrocatechol (ref. P0381), pyrogallol (C9510), gelatin B (G6650) and sodium periodate (ref. 311448) were purchased from Sigma-Aldrich and used without further purification. The pH of the 50 mM sodium acetate (Merck chemicals) buffer was adjusted to 5.0 using concentrated hydrochloric acid. Pyrocatechol and pyrogallol were dissolved at a concentration of 10 mM in a hot (about 75°C) gelatin solution (10% w/v) as explained in the next paragraph.
Hydrogel Preparation
In an 80 ml Teqler laboratory flask, 20 ml of buffer solution was added. The flask was closed and heated to 75°C. It was slowly sprinkled with 2.5 g of gelatin and 2.5 ml of the desired mother solution of either pyrocatechol or pyrogallol (at 100 mM). Finally, to start the oxidation process, 2.5 ml of NaIO4 solution was added. The mother solution of NaIO4 was prepared at a 10-fold higher concentration then the final concentration, which was of either 10, 20, or 30 mM, corresponding to an oxidant/catechol molar ratio equal to 1, 2 or 3 respectively. After homogenization with a magnetic stirrer (450 rpm), 0.6 ml of the mixture for adhesive strength and twice 0.8 ml of mixture for the gelation kinetics and for monitoring of self-healing was deposited on a polished (SiC) and ethanol cleaned stainless steel plate. The temperature of this plate was fixed at (25.0 ± 0.1)°C by means of a Peltier element. The deposition of the pre-gel mixture on the lower stainless steel plate corresponds to the beginning of the gelation process.
Swelling Experiments
The hydrogels prepared according to the previously described procedure and, stored in small polystyrene Petri dishes, were subjected to swelling experiments which could be divided into three separate parts lasting for 24 h each. At the end of each part, the average rates of mass gain was measured, with a minimum of three independent hydrogels per experimental condition. Firstly, hydrogels were immersed in a sodium acetate buffer at pH 5. Secondly, the hydrogels were dried at room temperature on stainless steel plates (in order to prevent excessive shrinkage of the hydrogels). Finally, the hydrogels were again immersed in the buffer. During this step, monitoring of the swelling kinetics was undertaken.
Qualitative Characterization of Self-Healing
The hydrogels prepared in the presence of 30 mM sodium periodate were cut in half and put again immediately in contact with each other while maintaining a pressure of 1 min at the level of the cut. Only slight self-healing of the catechol hydrogel was observed. The gluing operation was repeated and the hydrogels were left as they were in a Petri dish for 1 h, then after re-gluing for 24 h.
Characterization Methods
All rheological experiments were performed with a Kinexus Ultra rheometer (Malvern, United Kingdom) fitted with a 50 N range dynamometer. The gelation kinetics and self-healing measurements were performed in the cone-plate geometry with an upper cone 4 cm in diameter and an angle of 174°. The distance between the apex of the cone and the lower plater was fixed at 150 µm. The adhesion strength experiments were performed in a plate-plate geometry with an upper plate disk 2.0 cm in diameter. All the plates and disks were in stainless steel and were cleaned with soap, hot distilled water and ethanol before each experiment. The gelation kinetics were performed at a constant frequency of 1 Hz, at a constant strain of 1% and at a constant temperature of 25°C. The gelation kinetics was followed during 3 h, data acquisition being performed every 30 s. In certain cases, after completion of the gelation kinetics, a temperature sweep experiment was performed at the same frequency and strain from an initial temperature of 25°C to a final temperature of 50°C at a heating rate of 1.0°C.min−1.
The adhesion strength measurements were performed after 3 h of gelation in the plate-plate geometry, the gap between the lower and the upper plate being fixed at 1.0 mm. A constant compressive force of 1 N was applied during 1 s before retraction of the upper plate at a constant speed of 100 μm s−1. The force was measured every 10 ms up to a separation at which the force was constant and equal to zero corresponding to a complete rupture. A photograph of both plates was then taken to estimate if the rupture was of adhesive (the gel being removed from one of the plates) or cohesive (gel parts being adherent on both plates) nature. The adhesive strength was obtained by dividing the maximal force obtained in such experiments by the area of the upper plate of the rheometer, namely 3.14 cm2 neglecting its roughness. The specific energy of the rupture of the adhesive contact, taking into account the contribution of adhesion and the internal cohesion of the gels was calculated by integrating the area under the peeling curves after transformation of the time into a plate to plate distance (the peeling rate was constant and equal to 100 μm s−1) and normalizing by the area of the upper plate.
In situ self-healing experiments were performed at a constant temperature of 25°C with a constant frequency of 1 Hz. Strain was applied over a range from 10 to 600% depending on the responses of the hydrogel, subject of the experiment. The final applied strain corresponded to the state where the storage modulus became equal to zero. The hydrogel was solicited 3 h after the deposition between the plate and cone, then every hour (eight times in all). Hence 1 h was left between two experiments to allow for self-healing.
The morphology and the roughness of the gelatin + NaIO4 (30 mM), the gelatin + pyrocatechol + NaIO4 (30 mM) and the gelatin + pyrogallol + NaIO4 (30 mM) gels films deposited on glass slides were analyzed by recording topography images in the Intermittent Contact Mode by using a BioScope Catalyst AFM (Bruker Inc., Santa Barbara, United States). The scan assist fluid method was used. The roughness of all the gels was then calculated with the Gwyddion software. The average roughness of the films was determined on image sizes 5 × 5 μm2 in order to see if eventual roughness differences could explain differences in the adhesive behavior of the gels.
UV-vis spectra of hot (about 75°C, to avoid gelation) and freshly prepared pyrogallol + NaIO4, gelatin + Pyrogallol + NaIO4, pyrocatechol + NaIO4 and gelatin + pyrocatechol + NaIO4 solutions were measured with a double beam mc2 spectrophotometer (Safas, Monaco) in quartz cuvettes. The reference cuvette contained the sodium acetate buffer with the corresponding concentration in NaIO4.
Results and Discussion
The gelation kinetics of the gelatin + pyrocatechol + NaIO4 and of the gelatin + pyrogallol + NaIO4 hydrogels, in the presence of variable concentrations of the oxidants, but at constant concentration of pyrocatechol or pyrogallol (10 mM) where investigated at 25°C by means of rheometry (Figure 1). Some experiments were also performed with gelatin (at the same concentration + NaIO4 (Supplementary Figure S1). All these experiments allowed to determine the gelation time (when the storage modulus G′ becomes larger than the loss modulus G”) and the storage modulus of the hydrogels after a given time gelation time at 25°C. It first appears that the gelation kinetics (performed at a constant frequency of 1 Hz and a constant strain of 1%) of the gelatin + pyrogallol + NaIO4 system are continuous curves (Figure 1A) whereas some marked discontinuities (during which G’ tends transiently to zero) are observed for the gelatin + pyrocatechol + NaIO4 systems (Figure 1B). This observation is also made when the gelation kinetics is performed at a higher strain up to 100% but at the same frequency of 1 Hz (Figure 2 in the Supporting Information). Nevertheless, the discontinuities observed during the monitoring of the gelation of the gelatin + pyrocatechol + NaIO4 systems, more frequent for higher concentrations in NaIO4, are not due to a permanent slippage of the hydrogels on the stainless steel plate: they constitute only transient events before restoration of the storage and loss moduli (Figure 1B). We will use our further data to try to explain this observation made for the gelatin + pyrocatechol + NaIO4 hydrogels.
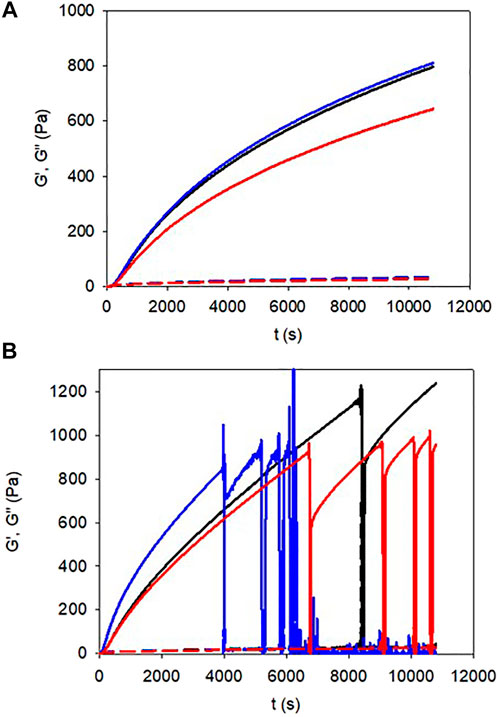
FIGURE 1. gelation kinetics of pyrogallol (A) and pyrocatechol (B) containing gelatin gels for different concentrations in NaIO4: 10 mM (black lines), 20 mM (blue lines), and 30 mM (red lines). The storage moduli curves (G′) correspond to the full lines and the loss moduli curves (G″) correspond to the dashed lines.
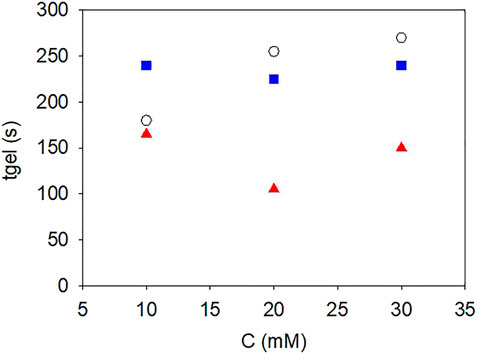
FIGURE 2. Influence of the NaIO4 concentration on the gelation time of gelatin (



It appears that the storage moduli reached for the gelatin + pyrocatechol + NaIO4 hydrogels, 1,000 ± 100 Pa, are significantly higher than those reached for the gelatin + NaIO4 and gelatin + pyrogallol + NaIO4 hydrogels, 750 ± 100 Pa, after 3 h of gelation. Note that in all cases the final storage modulus after 3 h of gelation slightly decreased for the highest concentration in NaIO4 reflecting a probable influence of the strong oxidant on gelatin itself. Similarly, for all the investigated hydrogels, the storage and loss moduli did not reach a steady state value after 3 h, reflecting the non-equilibrium state of the investigated hydrogels in agreement with the acquired knowledge (Harrington and Rao, 1970). Note that we did not investigate gelation durations longer than 3 h which would have required the use of a solvent trap to minimize the influence of water evaporation on the measured results. Concerning the gelation time, it appears that the addition of pyrogallol to gelatin did not modify significantly the gelation time for NaIO4 concentrations between 10 and 30 mM (Figure 2). However, the gelation time decreased markedly when 10 mM pyrocatechol was added to gelatin (Figure 2), suggesting a change in the interactions between gelatin chains favouring their faster association to get a percolation threshold.
The strong influence of pyrocatechol on the mechanical properties of the gelatin based hydrogels was evidenced during peeling experiments performed after 3 h of gelation (in the presence of NaIO4 at 10, 20, or 30 mM) and at a peeling rate of 100 μm s−1 (Figure 3). The maximal force at rupture (Figure 4A) as well as the area under the peeling curve (Figure 4B), proportional to the total work of rupture, increased markedly for the gelatin + pyrocatechol + NaIO4 hydrogels when compared to the gelatin + pyrogallol + NaIO4 and gelatin + NaIO4 hydrogels. In addition, the gelatin + NaIO4 and the gelatin + pyrogallol + NaIO4 hydrogels presented a single minimum in the peeling curves in contrast to the gelatin + pyrocatechol + NaIO4 systems for which multiple rupture events were present on the peeling curves (Figure 3, red curves). All these observations imply that the addition of pyrocatechol to gelatin hydrogels improves markedly either the adhesion of the hydrogel to stainless steel and/or the cohesion of the hydrogel. Observation of the system (seeSupplementary Figure S3) after the end of the peeling tests showed that in the case of the gelatin + pyrogallol + NaIO4 systems, the rupture was either adhesive (one of the steel plates being uncovered) or intermediate whereas for the gelatin + pyrocatechol + NaIO4 systems the rupture mode turned from adhesive to cohesive for NaIO4 concentrations higher than 20 mM after 3 h of gelation (Figure 4B).
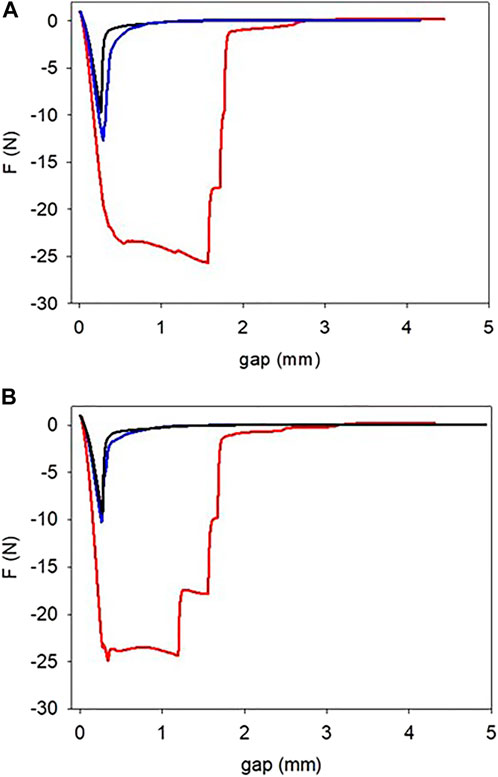
FIGURE 3. Peeling off experiments performed on gelatin (black lines), on gelatin + pyrogallol (blue lines) and gelatin + pyrocatechol (red lines) hydrogels at a peeling speed of 100 μm s−1 in the presence of 20 mM (A) and 30 mM (B) NaIO4. These experiments were performed 3 h after the sol deposition between the two plates of the rheometer, the gelation being allowed at 25°C.
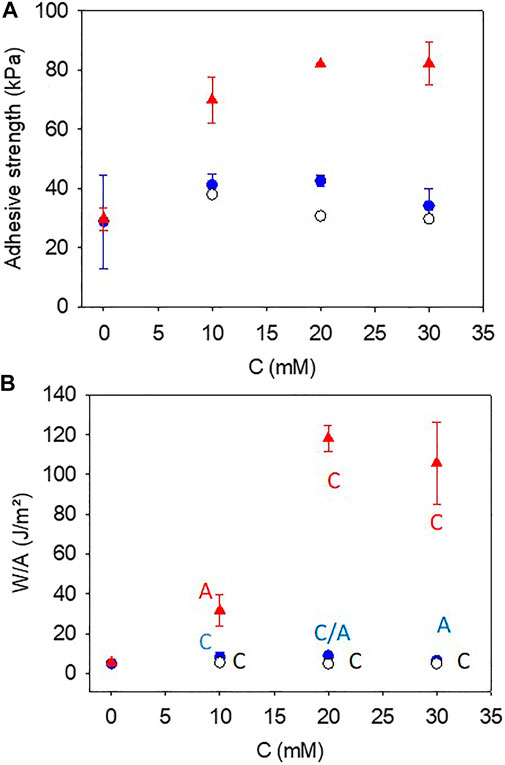
FIGURE 4. Adhesion strength (A) and total energy to rupture the adhesive contact (B) as a function of the concentration in NaIO4 for gelatin + NaIO4 gels (



As expected from the experimental data displayed in Figure 3, both the adhesion strength (Figure 4A) and the total energy (Figure 4B) required to rupture the bonds where markedly higher when the gelatin gel was complemented with 10 mM pyrocatechol. This marked increase in adhesion strength in the presence of pyrocatechol cannot be attributed to morphology or roughness changes because the roughness of all the investigated gels appears similar as found by AFM imaging (Supplementary Figure 4; Supplementary Table S1) the roughness of the steel plates being identical in all cases and conditioned by the polishing step (about 10 µm).
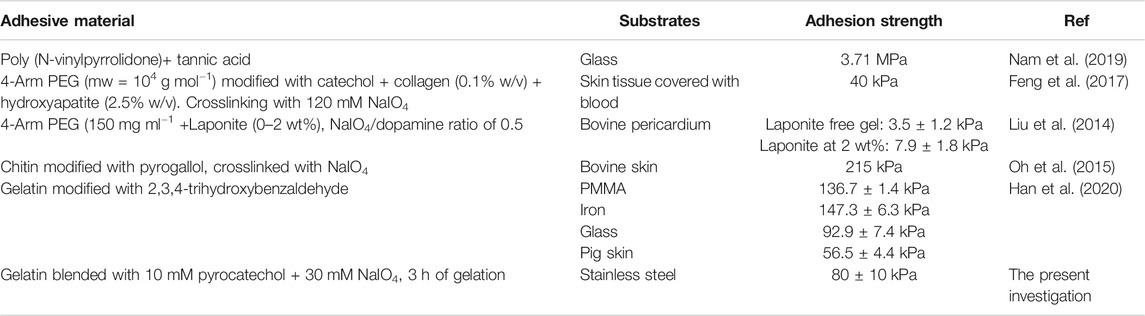
The obtained adhesion strength obtained in the present optimal case, namely for the gelatin + pyrocatechol + NaIO4 30 mM gels (after 3 h of ageing between the gel preparation and the peeling tests) are compared to data from the literature where polymers have been modified with catechols to get robust and underwater adhesive hydrogels in Table 1. It appears that the hydrogels developed in this research, in which the catechol and the gelatin were blended without chemical modification of the polymer prior to gelation, are comparable to other investigated systems. To our opinion, this is a major contribution of the present investigation. Concerning the specific energy to rupture the gel or the gel/steel adhesion, the value obtained herein for the pyrocatechol containing gels in the presence of 30 mM NaIO4 (100 ± 20) J.m−2, are significantly higher than those obtained between glass and a poly (hydroethylmethacrylate) hydrogel modified with various fractions of catechols at the same retraction velocity as herein, namely 100 μm s−1 (Wu et al., 2016).
The addition of pyrocatechol to gelatin and further oxidation with NaIO4 had also the consequence to improve the thermal stability of the obtained hydrogel with respect to its gelatin + NaIO4 and gelatin + pyrogallol + NaIO4 counterparts (Figure 5). Indeed, during a temperature scan performed at 1°C.min−1 the gelatin + pyrocatechol + NaIO4 30 mM hydrogel remained stable up to 50°C whereas the gelatin + NaIO4 30 mM and gelatin + pyrogallol + NaIO4 30 mM hydrogels underwent the gel to sol transition at around 30–32°C (Figure 5A) as expected for gelatin gels (Djabourov et al., 1988). When plotted in the form of G"/G′ vs. temperature (Figure 5B), it appears however that the addition of pyrogallol + NaIO4 to gelatin allows for a small, about 3–4°C, thermal stabilization.
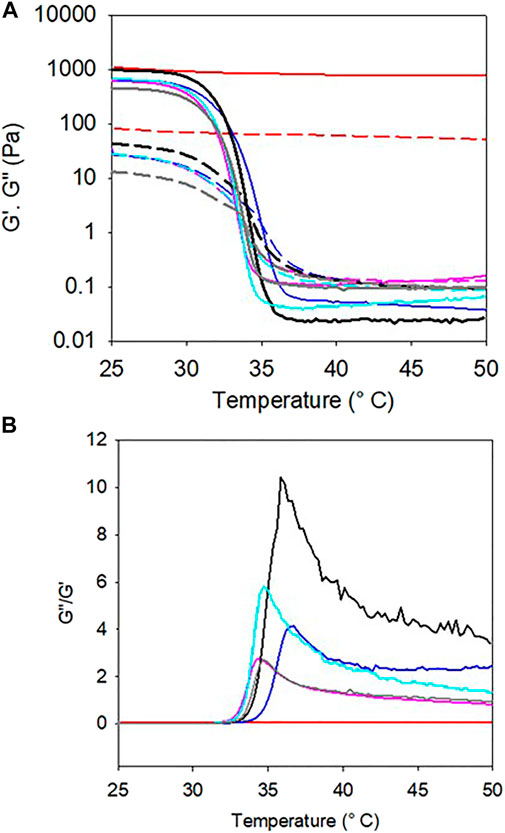
FIGURE 5. A) Evolution of the storage (full lines) and loss (dashed lines) moduli as a function of temperature (scan rate of 1°C.min−1) of gelatin (black), gelatin + 30 mM NaIO4 (gray), gelatin + pyrogallol (light blue), gelatin + pyrogallol + 30 mM NaIO4 (dark blue), gelatin + pyrocatechol (purple) and gelatin + pyrocatechol + 30 mM NaIO4 (red). Gelation was allowed during 3 h in situ between the rheometer plate and cone at 25°C before performing the temperature scans. (B) G”/G′ plots of the experiments displayed in part (A).
Hence, both the adhesion tests and the temperature scans suggest that the oxidation of pyrogallol in gelatin gels has no influence on the thermomechanical properties of the obtained hydrogels whereas the oxidation of pyrocatechol (at the same concentration of 10 mM) has a marked influence in reinforcing and stabilizing the gel from a thermal point of view.
The swelling kinetics of the hydrogels (all oxidized in the presence of 30 mM NaIO4) after drying and rehydration in the sodium acetate buffer are displayed on a double logarithmic scale in Figure 6. Note that the swelling ratio of the gelatin + pyrocatechol + 30 mM NaIO4 gels was systematically lower than that of the other investigated gels and was initially slightly lower than 1. This behavior was reproducible over three experiments and may be related to a significant reduction of the elasticity of the pyrogallol containing gels in the presence of higher concentrations in NaIO4 (seeFigure 1).
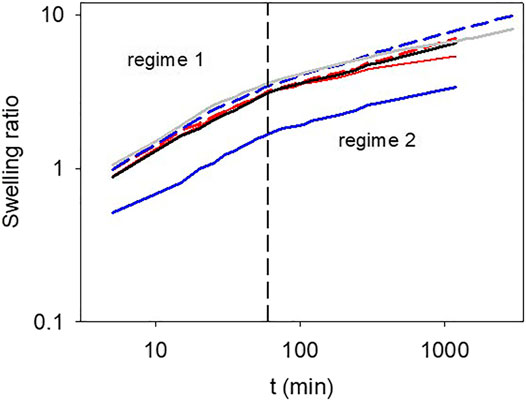
FIGURE 6. Swelling kinetics of the different investigated hydrogels plotted in double logarithmic representation. Black full line: gelatin, gray full line: gelatin + 30 mM NaIO4, blue dashed line: gelatin + pyrogallol, blue full line: gelatin + pyrogallol + 30 mM NaIO4, red dashed line: gelatin + pyrocatechol, red full line: gelatin + pyrocatechol + 30 mM NaIO4. The vertical dashed line represents the transition from the first to the second swelling regime.
In all cases, however, the swelling kinetics appears bimodal with a change in the swelling regime after about 1 h of contact with the buffer. The transition from the fast-swelling regime to the slow one occurs after a time close to 1 h independent on the investigated gel. The fast-swelling regime is characterized by an exponent close to 0.5 in a double log representation (Figure 7) suggesting that it is related to diffusion of water in the gel. The second swelling regime is characterized by an exponent between 0.18 and 0.3 which is markedly dependent on the presence of pyrogallol or pyrocatechol and the presence of the oxidant. Indeed, in the presence of oxidized pyrocatechol, the exponent of the second swelling regime amounts to 0.18 ± 0.02 whereas it amounts to 0.23 ± 0.04 for the gelatin + pyrogallol + NaIO4 30 mM system. This finding suggests, in agreement with the adhesion and thermal stability tests, that the presence of oxidized pyrocatechol markedly modifies the properties of the gelatin based hydrogels. In addition, the presence of NaIO4 slows down the second swelling regime. We attribute this second slow swelling regime to rearrangement of the gelatin chains (Harrington and Rao, 1970).

FIGURE 7. Exponents of the swelling kinetics as obtained from the data given in Figure 6. Full and open symbols correspond to the first and the second swelling regime respectively. Black circles: gelatin, light blue circles: gelatin + pyrogallol, purple circles: gelatin + pyrocatechol. gray triangles: gelatin + 30 mM NaIO4, dark blue triangles: gelatin + pyrogallol + 30 mM NaIO4, red triangles: gelatin + pyrocatechol + 30 mM NaIO4. The errors bars correspond to one standard deviation over two independent experiments.
Taking all the previous results into account, we make the assumption that the presence of oxidized groups on pyrocatechol allows some chemical reactions with nucleophilic side chains of some amino acids present on gelatin, whereas this is not possible with pyrogallol containing gels even if these molecules also undergo oxidation. Some additional experimental evidence of a different chemical behavior of pyrocatechol towards gelatin compared to pyrogallol towards gelatin (in the presence of NaIO4) was provided by UV-vis spectroscopy (Figure 8).
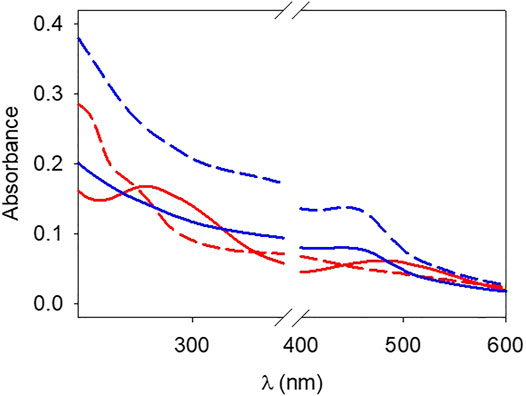
FIGURE 8. UV-vis spectra of hot (about 75 °C) pyrocatechol + NaIO4 (red dashed line), pyrocatechol + NaIO4 + gelatin (red full line), pyrogallol + NaIO4 (blue dashed line) and pyrogallol + NaIO4 + gelatin (blue full line) solutions.
It appears that the oxidation of pyrogallol is unaffected by the presence of gelatin (Figure 8) and displays a broad peak at around 420 nm which can be attributed to purpurogallin (Abrash 1977). However, the spectrum of oxidized pyrocatechol is markedly affected by the presence of gelatin with the appearance of a new peak at around 490–500 nm (Figure 8), not present in the pyrocatechol + NaIO4 solution. These findings are in agreement with the occurrence of a chemical reaction between oxidized pyrocatechol and gelatin and the absence of a reaction between oxidized pyrogallol and gelatin.
Based on these data, we suggest a different reaction mechanism between pyrogallol and pyrocatechol with the amine groups of gelatin in the presence of a strong oxidant like NaIO4 (Scheme 2). It appears that oxidized pyrocatechol has electrophilic sites allowing for the addition of amines whereas oxidized pyrogallol does not contain such electrophilic moieties which could explain its neutral influence in the adhesion tests (Figure 4) and in the thermal stability tests (Figure 5). There are also more resonance structures for the amine-pyrocatechol conjugate as for the amine-pyrogallol conjugate highlighting a greater stability of the former one (Scheme 2).
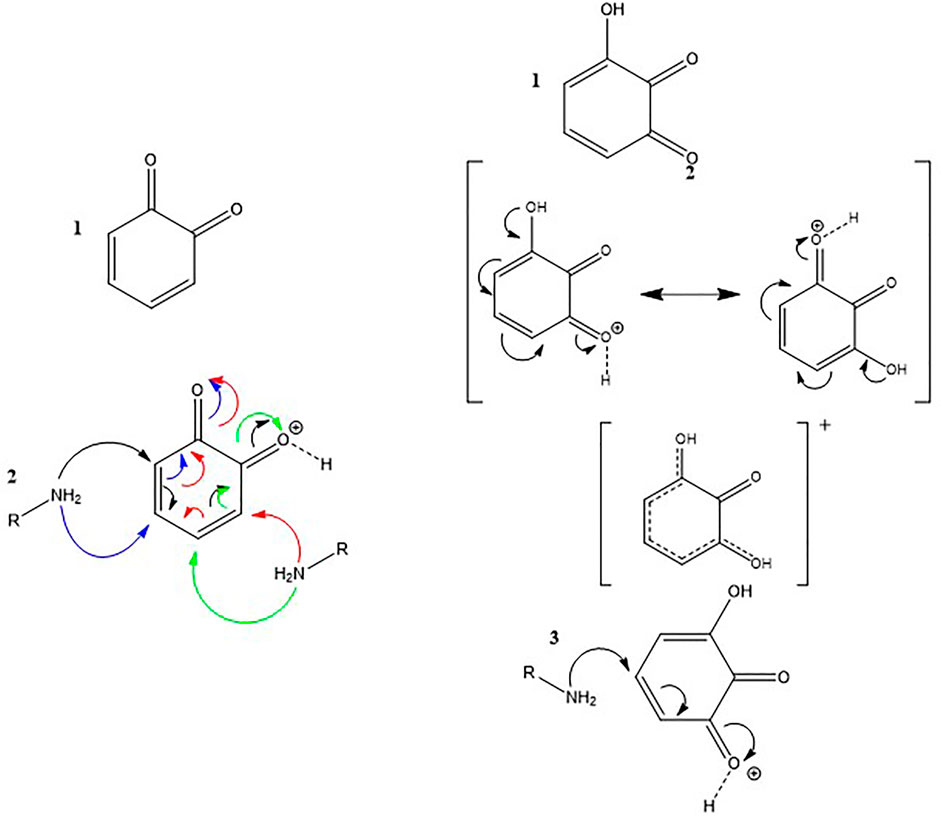
SCHEME 2. Interaction mechanism between amines and oxidized pyrocatechol 1) and oxidized pyrogallol 3). Mesomeric forms of oxidized pyrocatechol and pyrogallol 2).
Gelatin based gels are known to display self-healing behaviors which can be modified by adding some additives like ionomers (Sharma et al., 2017), in addition the presence of catechol and crosslink agents like Fe3+ cations (Krogsgaard et al., 2014; Krogsgaard et al., 2016; Andersen et al., 2018) and control over pH could modify this interesting material property. One could wonder if the formation of covalent bonds between oxidized pyrocatechol and aggregates thereof could decrease the intrinsic self-healing ability of gelatin. Indeed, self-healing requires a dynamic reformation of bonds, and many covalent bonds (with some exceptions like the bonds formed by Diels-Alder reactions) do not allow for such a reversibility. Some typical self-healing experiments are displayed in Supplementary Figure 5. We checked that after having reached a maximal shear modulus, the event corresponding to G’∼0 corresponds indeed to the rupture of the gel and not to a rupture at the gel stainless steel interface. Indeed, after having measured G’∼0 for a shear strain of about 400%, we stopped the experiment and analyzed the resulting gel: it was still adhering to both stainless-steel plates (Supplementary Figure S6). The maximal storage modulus obtained after waiting 1 h between each shear induced rupture are plotted in Figure 9 as a function of the number of rupture–rebonding cycles. It appears that the additions of NaIO4 as a strong oxidant to gelatin (10% w/v) hinders its self-healing ability. The same holds true when pyrogallol is added, confirming our previous findings that this molecule does not contribute to enhance the mechanical properties of gelatin based gels. However, the gelatin + pyrocatechol + NaIO4 30 mM hydrogels have the same self-healing behavior as unmodified gelatin gels suggesting that the addition of pyrocatechol which is oxidized by NaIO4 and is subsequently able to react with amino groups present on side chain of gelatin is able protect gelatin from the degradation by the strong oxidant.
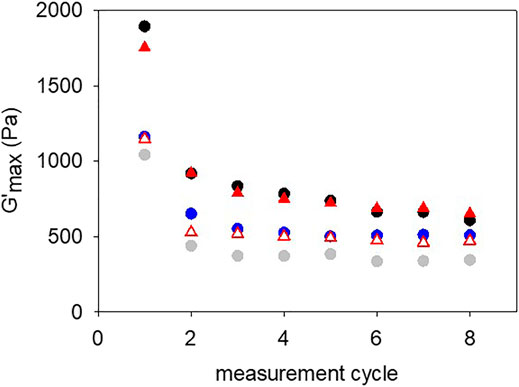
FIGURE 9. Maximal storage modulus reached during the self-healing of the different gelatin gels. After each rupture (seeSupplementary Figure S5) the gels were allowed to self-heal for 1 h before starting the next measurement cycle. Black disks: gelatin, gray disks: gelatin + 30 mM NaIO4, blue disks: gelatin + pyrogallol + 30 mM NaIO4, open red triangles: gelatin + pyrocatechol, red triangles: gelatin + pyrocatechol + 30 mM NaIO4.
Discussion
The aim of this investigation was to improve the adhesive behavior of gelatin without covalent modification of the polymer with adhesion and internal cohesion promoting molecules like catechols but by simply blending gelatin with such molecules. As possible candidates we compared 1,2 benzediol (pyrocatechol) and 1,2,3-benzenetriol (pyrogallol) added at a constant concentration of 10 mM in a gelatin suspension at 10% (w/v). The gelation process was induced after addition of NaIO4 to reach an oxidant/pyrocatechol or oxidant/pyrogallol ratio between 1 and 3 and subsequently reducing the temperature to 25°C. Overall we found that the addition of pyrogallol had no significant contribution to the adhesive behavior of gelatin in opposition to the marked influence of pyrocatechol which allowed to produce gels with a maximal adhesive strength of (80 ± 10) kPa and a total energy before rupture of (100 ± 20) J.m−2 after 3 h of adhesion and an oxidant/pyrocatechol ratio of 3. The adhesive properties increased markedly upon an increase in the NaIO4/pyrocatechol ratio after a constant gelation time. We compared these optimal adhesion data with those obtained with catechol modified polymers (Table 1) and found them of the same order of magnitude but with the advantage that gel preparation was performed in a one pot manner by just blending pyrocatechol with gelatin and subsequent oxidation. Interestingly, the adhesive strength of the gelatin + pyrocatechol gel in wet conditions is comparable to the adhesive strength obtained with cyanoacrylate on pig skin, namely 108 kPa (Feng et al., 2017). Note however that we cannot directly compare our results with the one obtained by Feng et al. because of the different nature of the adhesion substrate. Nevertheless, our results are encouraging and suggest to test the influence of different polyphenols blended with gelatin or other polymers able to undergo gelation and to simultaneously carry nucleophilic groups able to react with quinones in order to crosslink the gel. It should also be noted that in the case of gelatin + pyrocatechol gels, the rupture of the gel after 3 h of gelation was of cohesive nature, offering some further possibilities to reinforce the gel for instance upon the addition of inorganic fillers. Interestingly the gelatin + pyrocatechol gels turned also to remain stable up to 50°C offering the possibility to test their toxicity against cells put in contact with them as well as the possibility to be tested in vivo.
The self-healing behavior of gelatin gels, a well known property of such materials (Sharma et al., 2017) was partially reduced in the presence of NaIO4 as a strong oxidant able by itself to oxidize some amino acid side chains like hydroxyproline and hence to modify the properties of gelatin. The presence of pyrogallol in the gel was not able to restore the initial behavior of the pristine gel in opposition to the addition of pyrocatechol which allowed a full restoration of the self-healing behavior even after oxidation with NaIO4 (Figure 9).
On an experimental basis we found that the addition of pyrocatechol to gelatin + NaIO4 mixture allowed for a change of the absorption spectrum when compared to that of the pyrocatechol + NaIO4 solution. This was in total opposition to the result obtained with pyrogallol where the addition of gelatin to pyrogallol + NaIO4 solution did not produce a change in the peaks present in the optical absorption spectrum (Figure 8). These data, consistent with the influence of pyrocatechol on the thermomechanical properties of gelatin based hydrogels, strongly suggest the possibility for oxidized pyrocatechol to undergo a chemical reaction, namely an addition with amino groups in gelatin. Such a reaction is much more difficult with oxidized pyrogallol which electrophilicity is much lower than that of oxidized pyrocatechol as suggested in Scheme 2.
As an additional observation in this study was the appearance of transient instabilities during the gelation of gelatin + pyrocatechol + NaIO4 mixtures (Supplementary Figures 1, 1B). We believe that these instabilities are related to the formation of strong but brittle interactions between the gels and the steel substrates but which are able to self-heal with the consequence of a further increase in the storage modulus. Since pyrogallol has no influence of the mechanichal behavior of these hydrogels and seems not to interact specifically with gelatin it does not induce such instabilities (Figure 1A).
Since NaIO4 may induce adverse cellular and tissue response, we will replace it by hydrogen peroxide in future studies aiming to test the gelatin based adhesive developped in this study which allowed to demonstrate the importance of pyrocatechol in the desing of strongly adhesive hydrogels prepared in a one step process.
Conclusion
In this investigation, the influence of the addition of pyrocatechol or pyrogallol (both at 10 mM) on the thermomechanical properties of gelatin gels (10% w/v) was investigated after oxidation with NaIO4 at various NaIO4/pyrogallol or NaIO4/pyrocatechol ratios. It was demonstrated that the adhesion of pyrocatechol carrying two adjacent hydroxyl groups on the benzene ring allowed to improve markedly the adhesive properties of the hydrogel whereas the addition of pyrogallol, carrying three adjacent hydroxyl groups on the benzene ring did not contribute at all to the adhesive properties of the gelatin based hydrogels with respect to gelatin + NaIO4. In addition, the pyrocatechol containing hydrogels displayed improved thermal stability in comparison to the gelatin and gelatin + pyrogallol hydrogels (all being put in the presence of 30 mM NaIO4). The probable occurrence of chemical crosslinks between gelatin and pyrocatechol in the presence of NaIO4 was illustrated by UV-vis spectroscopy but also by a slower swelling regime of the gels after an initial and solvent diffusion limited regime common to all the investigated gels.
The major interest of this work was to highlight the possibility to obtain strongly adhesive gelatin based hydrogels by blending the targeted molecule with gelatin without preliminary chemical grafting. However, this approach is not extensible to all polyphenols as shown here in the case of pyrogallol which displayed no influence at all on the thermomechanical properties of the gelatin based hydrogels. A future goal will be to target other polyphenols able to react with gelatin, 1,2-dihydroxyphenols being the more likely molecules and to reinforce its mechanical-cohesive and adhesive properties. For practical applications as biological glues, it will also be mandatory to select experimental conditions able to produce strongly adhesive gels in a time duration much shorter than 3 h. In addition, the influence of metallic cations like Fe3+ on the adhesive properties of the same hydrogels should be compared to the influence of NaIO4.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
FB and YA performed the experiments. FB and VB designed the experiments. YA and VB contributed to the interpretation and the paper writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2021.671451/full#supplementary-material
References
Abrash, H. I. (1977). The Interaction of Oxidation Products of Pyrogallol with Poly-L-Lysine. A Model Oxidative Beer Haze. Carlsberg Res. Commun. 42, 27–33. doi:10.1007/bf02906707
Andersen, A., Krogsgaard, M., and Birkedal, H. (2018). Mussel-Inspired Self-Healing Double-Cross-Linked Hydrogels by Controlled Combination of Metal Coordination and Covalent Cross-Linking. Biomacromolecules 19, 1402–1409. doi:10.1021/acs.biomac.7b01249 |
Chen, Y.-N., Peng, L., Liu, T., Wang, Y., Shi, S., and Wang, H. (2016). Poly(vinyl Alcohol)-Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Inter. 8, 27199–27206. doi:10.1021/acsami.6b08374 |
Djabourov, M., Leblond, J., and Papon, P. (1988). Gelation of Aqueous Gelatin Solutions. I. Structural Investigation. J. Phys. France 49, 319–332. doi:10.1051/jphys:01988004902031900
Duarte, A. P., Coelho, J. F., Bordado, J. C., Cidade, M. T., and Gil, M. H. (2012). Surgical Adhesives: Systematic Review of the Main Types and Development Forecast. Prog. Polym. Sci. 37, 1031–1050. doi:10.1016/j.progpolymsci.2011.12.003
Fan, C., Fu, J., Zhu, W., and Wang, D.-A. (2016). A Mussel-Inspired Double-Crosslinked Tissue Adhesive Intended for Internal Medical Use. Acta Biomater. 33, 51–63. doi:10.1016/j.actbio.2016.02.003 |
Faure, E., Falentin-Daudré, C., Jérôme, C., Lyskawa, J., Fournier, D., Woisel, P., et al. (2013). Catechols as Versatile Platforms in Polymer Chemistry. Prog. Polym. Sci. 38, 236–270. doi:10.1016/j.progpolymsci.2012.06.004
Feng, J., Ton, X.-A., Zhao, S., Paez, J., and del Campo, A. (2017). Mechanically Reinforced Catechol-Containing Hydrogels with Improved Tissue Gluing Performance. Biomimetics 2, 23. doi:10.3390/biomimetics2040023 |
Han, N., Xu, Z., Cui, C., Li, Y., Zhang, D., Xiao, M., et al. (2020). A Fe3+-Crosslinked Pyrogallol-Tethered Gelatin Adhesive Hydrogel with Antibacterial Activity for Wound Healing. Biomater. Sci. 8, 3164–3172. doi:10.1039/d0bm00188k |
Harrington, W. F., and Rao, N. V. (1970). Collagen Structure in Solution. I. Kinetics of Helix Regeneration in Single-Chain Gelatins. Biochemistry 9, 3714–3724. doi:10.1021/bi00821a010 |
Krogsgaard, M., Andersen, A., and Birkedal, H. (2014). Gels and Threads: Mussel-Inspired One-Pot Route to Advanced Responsive Materials. Chem. Commun. 50, 13278–13281. doi:10.1039/c4cc05293e |
Krogsgaard, M., Nue, V., and Birkedal, H. (2016). Mussel-inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 22, 844–857. doi:10.1002/chem.201503380 |
Lee, B. P., Messersmith, P. B., Israelachvili, J. N., and Waite, J. H. (2011). Mussel-inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 41, 99–132. doi:10.1146/annurev-matsci-062910-100429 |
Lee, C., Shin, J., Lee, J. S., Byun, E., Ryu, J. H., Um, S. H., et al. (2013). Bioinspired, Calcium-free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 14, 2004–2013. doi:10.1021/bm400352d |
Liu, Y., Meng, H., Konst, S., Sarmiento, R., Rajachar, R., and Lee, B. P. (2014). Injectable Dopamine-Modified Poly(Ethylene Glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Inter. 6, 16982–16992. doi:10.1021/am504566v
Mateescu, M., Baixe, S., Garnier, T., Jierry, L., Ball, V., Haikel, Y., et al. (2015). Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. Plos One 10, e0145143. doi:10.1371/journal.pone.0145143 |
Nam, H. G., Nam, M. G., Yoo, P. J., and Kim, J.-H. (2019). Hydrogen Bonding-Based Strongly Adhesive Coacervate Hydrogels Synthesized Using poly(N-Vinylpyrrolidone) and Tannic Acid. Soft Matter 15, 785–791. doi:10.1039/c8sm02144a |
Oh, D. X., Kim, S., Lee, D., and Hwang, D. S. (2015). Tunicate-mimetic Nanofibrous Hydrogel Adhesive with Improved Wet Adhesion. Acta Biomater. 20, 104–112. doi:10.1016/j.actbio.2015.03.031 |
Rahimnejad, M., and Zhong, W. (2017). Mussel-inspired Hydrogel Tissue Adhesives for Wound Closure. RSC Adv. 7, 47380–47396. doi:10.1039/c7ra06743g
Ryu, J. H., Lee, Y., Kong, W. H., Kim, T. G., Park, T. G., Lee, H., et al. (2011). Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 12, 2653–2659. doi:10.1021/bm200464x |
Shafiq, Z., Cui, J., Pastor-Pérez, L., San Miguel, V., Gropeanu, R. A., Serrano, C., et al. (2012). Bioinspired Underwater Bonding and Debonding on Demand. Angew. Chem. Int. Ed. 51, 4332–4335. doi:10.1002/anie.201108629 |
Sharma, A., Rawat, K., Solanki, P. R., and Bohidar, H. B. (2017). Self-healing Gelatin Ionogels. Int. J. Biol. Macromolecules 95, 603–607. doi:10.1016/j.ijbiomac.2016.11.103
Shin, J., Lee, J. S., Lee, C., Park, H.-J., Yang, K., Jin, Y., et al. (2015). Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 25, 3814–3824. doi:10.1002/adfm.201500006
Slabbert, N. P. (1977). Ionisation of Some Flavanols and Dihydroflavonols. Tetrahedron 33, 821–824. doi:10.1016/0040-4020(77)80200-7
Wu, H., Sariola, V., Zhao, J., Ding, H., Sitti, M., and Bettinger, C. J. (2016). Composition-dependent Underwater Adhesion of Catechol-Bearing Hydrogels. Polym. Int. 65, 1355–1359. doi:10.1002/pi.5246
Keywords: adhesion, gelatin, catechol, pyrogallol, sodium periodate, biomietic gels
Citation: Back F, Ball V and Arntz Y (2021) Influence of the NaIO4 Concentration on the Gelation and the Adhesive Strength of Pyrocatechol/Pyrogallol Containing Gelatin Hydrogels. Front. Mater. 8:671451. doi: 10.3389/fmats.2021.671451
Received: 23 February 2021; Accepted: 26 April 2021;
Published: 13 May 2021.
Edited by:
Alexey A. Sokol, University College London, United KingdomReviewed by:
Daisuke Kawaguchi, Kyushu University, JapanKatsuhiro Yamamoto, Nagoya Institute of Technology, Japan
Copyright © 2021 Back, Ball and Arntz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincent Ball, dmJhbGxAdW5pc3RyYS5mcg==
 Florence Back1
Florence Back1 Vincent Ball
Vincent Ball Youri Arntz
Youri Arntz