- 1Department of Chemical Engineering and Petroleum Industries, Al- Mustaqbal University College, Hillah, Iraq
- 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 3Al-Manara College for Medical Sciences, Maysan, Iraq
- 4Medical Technical College, Al-Farahidi University, Baghdad, Iraq
- 5Department of Medical Laboratories Technology, AL-Nisour University College, Baghdad, Iraq
- 6Department of Anesthesia Techniques, AlNoor University College, Nineveh, Iraq
- 7Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 8Technical Engineering Department College of Technical Engineering, The Islamic University, Najaf, Iraq
- 9College of Medical Technic, Imam Ja’afar Al-Sadiq University, Al-Muthanna, Iraq
Providing green methods for the synthesis of new heterocyclic compounds with biological properties is interesting for scientists. Through the multi-component reaction of aldehyde derivatives, methyl 2-cyanoacetate, and phenylhydrazine using K2CO3, and glycerol as a deep eutectic solvent, new derivatives of 2,3-dihydro-1H-pyrazole-4-carbonitrile were synthesized. Biological evaluation of the synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, including antioxidant activity on DPPH free radical and antimicrobial activity on a wide range of Gram-negative, Gram-positive bacterial, and fungal species, was done. In the antioxidant activity, IC50 value, and in the antimicrobial activity, IZD, MIC, MFC, and MBC parameters were assessed. The structure of the newly synthesized compounds was confirmed using 1H NMR, 13C NMR, and elemental analysis. 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives showed significant antioxidant and antibacterial activity and, in examining the results, a good relationship between the structure and biological activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives were found.
1 Introduction
Excessive accumulation of oxidants in the body leads to oxidative stress, which causes neurological disorders, Alzheimer’s disease, kidney diseases, heart disease, etc. Carotenoids, vitamin C, vitamin E, etc., present in the diet, have antioxidant properties that lead to the neutralization of active oxidants in the body, but in some acute substances, it is necessary to use antioxidant agents (Nocella et al., 2019; Goshtasbi et al., 2022; Kumar et al., 2022).
Other pathogenic factors that affect human health include microbes. Microbes include bacteria, protozoa, viruses, and some fungi. Microbes cause diseases such as pneumonia, bacterial meningitis, and gastroenteritis. These microorganisms are sometimes eliminated by the body, but in some cases, antibiotic drugs are needed to stop them. Most drugs have organic and heterocyclic compounds in their structure (Lees and Aliabadi, 2002; Moser et al., 2019; Rajapaksha et al., 2019; Sarkar et al., 2022).
Celecoxib is a commercial drug that inhibits COX-2. This non-steroidal drug has anti-inflammatory properties. The pyrazole heterocyclic compound forms the core of this drug. Other medicines, such as rimonabant, sildenafil, and fomepizole, have pyrazole in their structure (Ansari, Ali, and Asif, 2017). The heterocyclic compound of pyrazole, with two nitrogens in its structure, is also found abundantly in nature (Kumar et al., 2013). Many synthetic compounds with the biological properties of antioxidant activity, antimicrobial activity, anticancer activity, etc., have been reported in pyrazole derivatives (Kumari, Paliwal, and Chauhan, 2018).
Pyrazoles can be synthesized through multicomponent reactions (MCRs) (Maddila et al., 2017; Becerra, Abonia, and Castillo, 2022). Today, these reactions are widely used for synthesizing organic and heterocyclic compounds based on the laws of green chemistry. In MCRs, three or more reagents react together, leading to the desired product that contains essential parts of all the components used. A reduction in reaction steps, high efficiency, less purification action, and cost-effectiveness are some advantages related to MCRs. The reaction conditions, including suitable solvents and catalysts, are the essential factors in multicomponent reactions (Singh and Chowdhury, 2012; Sharma et al., 2020; Mamaghani and Hossein Nia, 2021; Neto, Rocha, and Rodrigues, 2021).
One of the methods recently noticed in multicomponent reactions involves deep eutectic solvents. Deep eutectic solvents are a mixture of compounds containing hydrogen bond donors and hydrogen bond acceptors or metal salts. Deep eutectic solvents play an essential role in green chemistry. In reactions containing deep eutectic solvents, no catalyst is needed as the solvent also plays the role of a catalyst. They can be used as cheap non-toxic recyclable environmentally friendly materials to synthesize heterocyclic and organic compounds. In organic chemistry and the synthesis of heterocycles, these compounds can play a role as a Lewis and Lowry Bronsted acid and base. (Aryan et al., 2017; Xu et al., 2017; Beyzaei et al., 2018). Several reports of the use of deep eutectic solvents in the synthesis of heterocyclic compounds have been presented. For example, choline chloride and urea have been used as a deep eutectic solvent system in synthesizing 3-amino-1, 2, 4-triazole derivatives. The synthesized compounds in these reports were evaluated biologically, and the tests showed that the synthesized compounds have significant antimicrobial properties (Arab, Beyzaei, and Aryan, 2022). Glucose and urea are another system that has been reported for synthesizing pyrazole derivatives (Aryan et al., 2017; Aryan et al., 2019). Recently, glycerol and K2CO3 (potassium carbonate)/glycerol as a deep eutectic solvent have been used to synthesize heterocycles. For example, the above system was used to synthesize isoxazole derivatives with high efficiency and biological properties, such as antioxidant, antifungal, and antibacterial effects (Beyzaei et al., 2018).
One of the most important advantages of using deep eutectic solvents is their greenness and environmental friendliness. Green chemistry, which has been welcomed by scientists in the last two decades, leads to the safe synthesis of compounds and environmental protection. Deep eutectic solvents fully comply with the rules of green chemistry. For example, it is utterly consistent with safer solvents and auxiliaries (principle 5), less hazardous chemical syntheses (principle 3), prevention (principle 1), etc. (Chen et al., 2020; Ardila-Fierro and Hernández, 2021).
Given that the synthesis and reporting of new compounds with biological properties, as well as the reporting of green and environmentally friendly methods for heterocyclic compounds, are important, in the present study, K2CO3/glycerol was used as an active solvent in synthesizing new 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives. Next, the antioxidant properties of the synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives against DPPH free radicals were investigated. Finally, the inhibitory effects of the synthesized derivatives on a wide range of Gram-positive bacteria, Gram-negative bacteria, and fungal species based on IZD, MIC, MBC, and MFC, using clinical and laboratory standards institute guidelines, were tested.
2 Materials and methods
2.1 Solvents and raw materials
The raw materials and chemical compounds that were used to prepare deep eutectic solvent for synthesizing derivatives, such as K2CO3, glycerol, aldehyde derivatives, ethyl cyanoacetate, and phenylhydrazine, were obtained from Merck and Sigma.
2.2 Devices
The melting points of the compounds were measured using Kruss, KSP1N. PerkinElmer 2400 series II was used for the elemental analysis of derivatives. 1H NMR (250 MHz, CDCl3) and 13C NMR (75 MHz, CDCl3) spectra were obtained using a Bruker Ultra Shield-250. A Unico S2150 spectrophotometer was used to prepare the concentration of bacterial and fungal suspensions and measure the antioxidant properties.
2.3 Synthesis of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
For the synthesis of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, first, the deep eutectic solvent system was prepared. To achieve this, 0.01 mol K2CO3 and 0.04 mol glycerol were stirred for 2 h at 80°C. After a clear solution was observed, the mixture was used as a deep eutectic solvent to synthesize 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives.
To synthesize 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, 1 mmol of aldehyde derivatives, 1 mmol of ethyl cyanoacetate, and 1 mmol of phenylhydrazine were added to 1 g of the synthesized deep eutectic solvent. The mixture was stirred at a temperature of 50°C. The reaction was monitored by thin-layer chromatography. After the completion of the reaction, 5 mL of water/ethanol mixture (1:1) was added, and the sediments obtained were separated and recrystallized in methanol.
2.4 Antioxidant properties of the synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
The DPPH method was used to evaluate the antioxidant activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives. An aliquot of 4 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) 0.004% (w/v) was added to 1 mL of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives at different concentrations (concentrations of 25, 50, 75 and 100 μg/mL of derivatives were prepared). The mixture was stirred for 30 min at room temperature in the dark. Then the absorbance of the mixture was measured at 517 nm. The following formula was used to measure the percentage inhibition of DPPH by 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives (Beyzaei et al., 2018; Hosseinzadegan et al., 2020; Hosseinzadegan, Hazeri, and Maghsoodlou, 2020):
% inhibition = [(Absorption DPPH—Absorption DDPH and sample)/(Absorption DPPH)] × 100.
By using the percentage of inhibition and the concentration of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, the linear equation was obtained, and the IC50 value was calculated (Beyzaei et al., 2018).
2.5 Antimicrobial properties of the synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
The antifungal and antibacterial species examined in this study were obtained from the American Type Culture Collection (ATCC). To investigate antimicrobial activities, the antifungal and antibacterial effects of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives were examined. Clinical and Laboratory Standards Institute guidelines were used in the tests. Guidelines M07-A9, M26-A, M02-A11, M44-A, and M27-A2 were used for measuring the parameters of MIC and MFC (in the evaluation of antibacterial activity) and the parameters of MIC and MBC (in the evaluation of antifungal activity) (Etemadi et al., 2016; Igei et al., 2016; Heidari Majd, Akbarzadeh, and Sargazi, 2017; Moghaddam-Manesh et al., 2020; Abdieva et al., 2022).
3 Results
3.1 Multicomponent synthesis of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives using K2CO3/glycerol as a green deep eutectic solvent
Glycerol and K2CO3 were used as a deep eutectic solvent in the three-component synthesis of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives using aldehyde derivatives, ethyl cyanoacetate, and phenylhydrazine (Scheme 1).
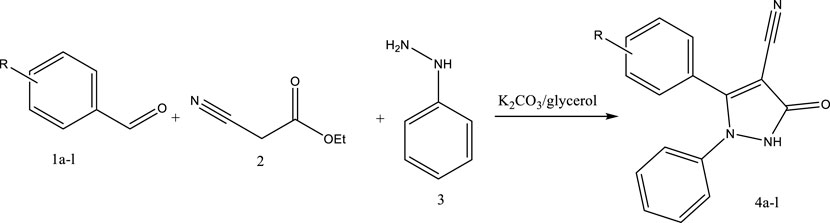
SCHEME 1. Synthesis of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives using aromatic aldehyde derivative K2CO3:glycerol as a green deep eutectic solvent.
At first, the temperature was kept constant at 60°C, and different ratios of K2CO3/glycerol were investigated according to Table 1 (For synthesis 4a). This work aimed to obtain the best ratio of K2CO3 and glycerol for synthesizing 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives under suitable conditions and high efficiency.
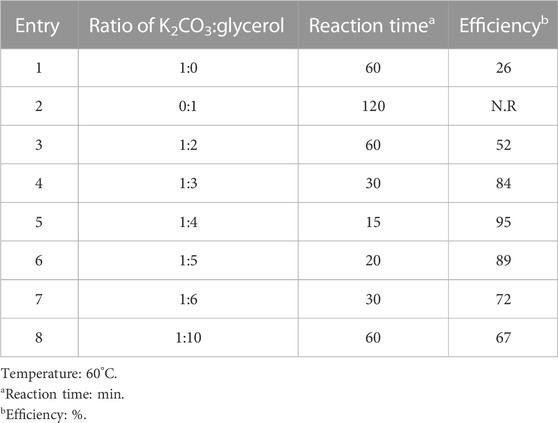
TABLE 1. Optimizing the ratio of solvent components in the synthesis of 5-(4-methoxyphenyl)-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazole-4-carbonitrile (4j).
According to Table 1, the ratio of 1:4, K2CO3 and glycerol, respectively, was the most efficient. As mentioned in previous studies, it has also been proven that a ratio of less than 1:4 of K2CO3:glycerol does not create a homogeneous and transparent mixture (Becerra, Abonia, and Castillo, 2022).
In the next step, the temperature was optimized. For this purpose, the reaction was tested at 50°C, 60°C, 75°C, and 100°C (Table 2). At temperatures below 50°C, owing to the high solvent viscosity, it was not possible to carry out the reaction.
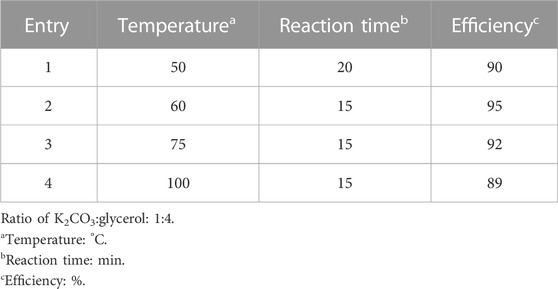
TABLE 2. Temperature optimization in the synthesis of 5-(4-methoxyphenyl)-3-oxo-1-phenyl-2,3-dihydro-1H-pyrazole-4-carbonitrile (4j).
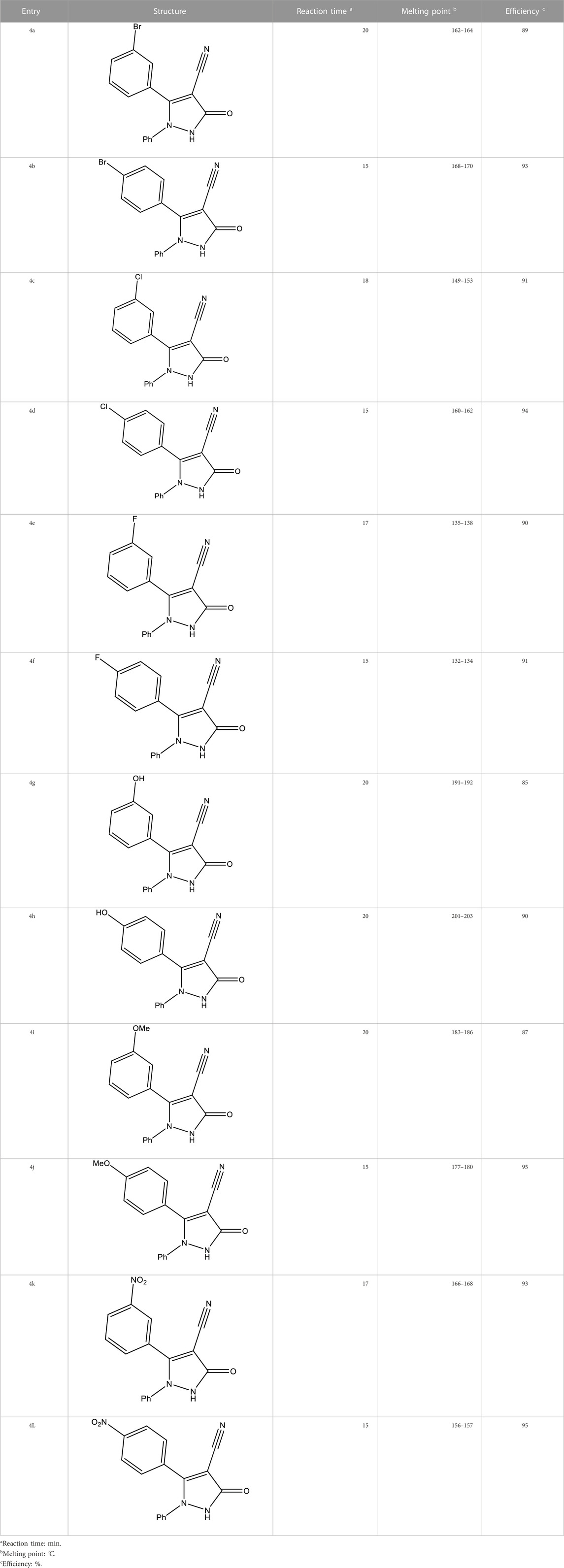
As proven by the results shown in Table 2, the best temperature for the synthesis of 4a was 60 °C, and this temperature was used as the optimal temperature for the synthesis of other 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives. Then, using the obtained optimal conditions, including the ratio of 1:4 K2CO3:glycerol and temperature of 60°C, the 12, 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives shown in Table 3 were synthesized.
As shown in Tables 3, 6, 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, including 4a, 4e, 4f, 4g, 4h, and 4i, were novel derivatives, and their synthesis is reported for the first time. So far, only one method for synthesizing 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives has been reported. A study of previous research showed that the only method presented for synthesizing the compounds featured in this study is the use of sulfated alumina tungstic acid as a catalyst under reflux conditions in ethanol (Rather, Khan, and Siddiqui, 2020). Among the advantages of using the deep eutectic solvent examined in this research compared with the previously presented method, there is no need for a catalyst, synthesis can be carried out at lower temperatures, and novel 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives can be synthesized.
3.2 Biological activity of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
3.2.1 Antioxidant activity of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
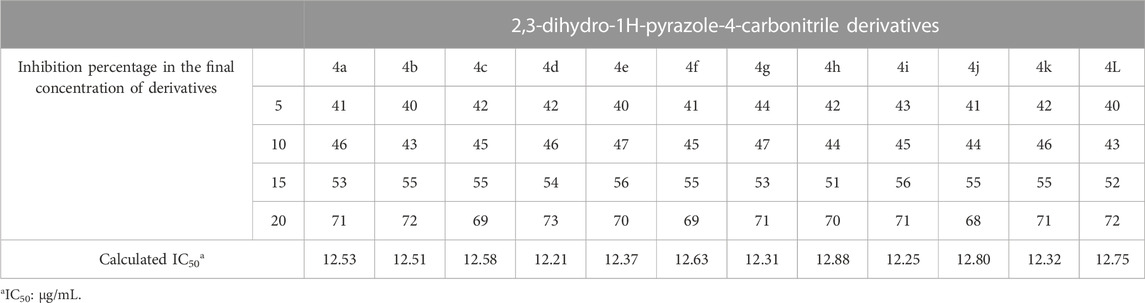
To evaluate the antioxidant activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, the inhibition percentage and IC50 were calculated (Table 4).
Based on the structure of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives, the hydrogen attached to the nitrogen of the pyrrole ring plays a role in the stability of the DPPH free radical. Table 6 shows that the antioxidant properties of the derivatives are very similar to each other. The IC50 calculated for the derivatives was in the range of 12.21–12.88 μg/mL. Therefore, antioxidant effects were not dependent on aldehyde derivatives.
Based on the results, the mechanism shown in Scheme 2 is proposed for the antioxidant activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives.
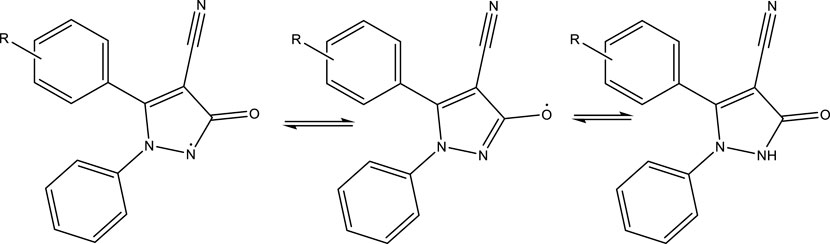
SCHEME 2. Antioxidant activity mechanism of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives and DPPH free radical stability.
3.2.2 Antifungal and antibacterial activity of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives
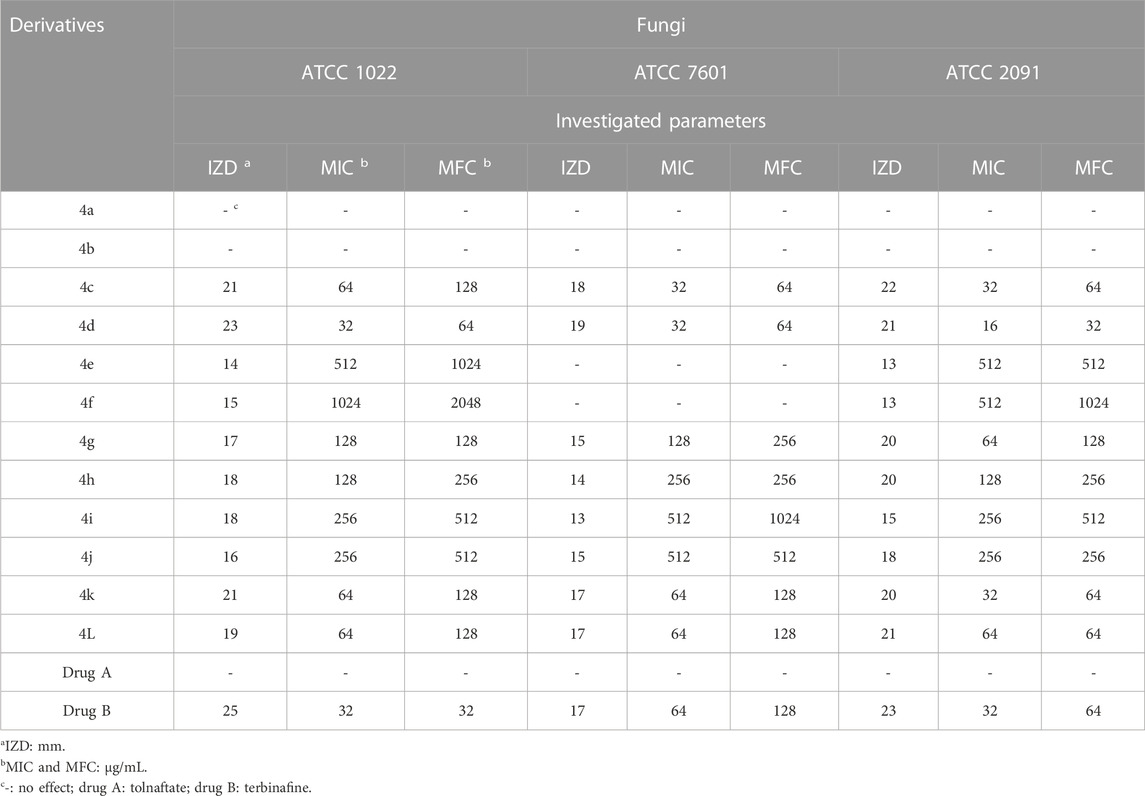
The antimicrobial activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives was studied for three fungal species (Aspergillus fumigatus Fresenius [ATCC 1022], Fusarium oxysporum [ATCC 7601], and Candida albicans [ATCC 2091]), five Gram-positive bacterial species (Rhodococcus equi [ATCC 6939], Bacillus cereus [ATCC 14579], Staphylococcus aureus [ATCC 23235], Streptomyces fradiae [ATCC 10745], and Streptococcus agalactiae [ATCC 13813]), and five Gram negative bacterial species (Proteus mirabilis [ATCC 12453], Acinetobacter baumannii [ATCC 17978], Klebsiella pneumoniae [ATCC 13883], Escherichia coli [ATCC 25922], and Yersinia enterocolitica [ATCC 23715]). The activity results on fungal species and Gram-positive and Gram-negative strains are shown in Tables 5, 6, 7, respectively.
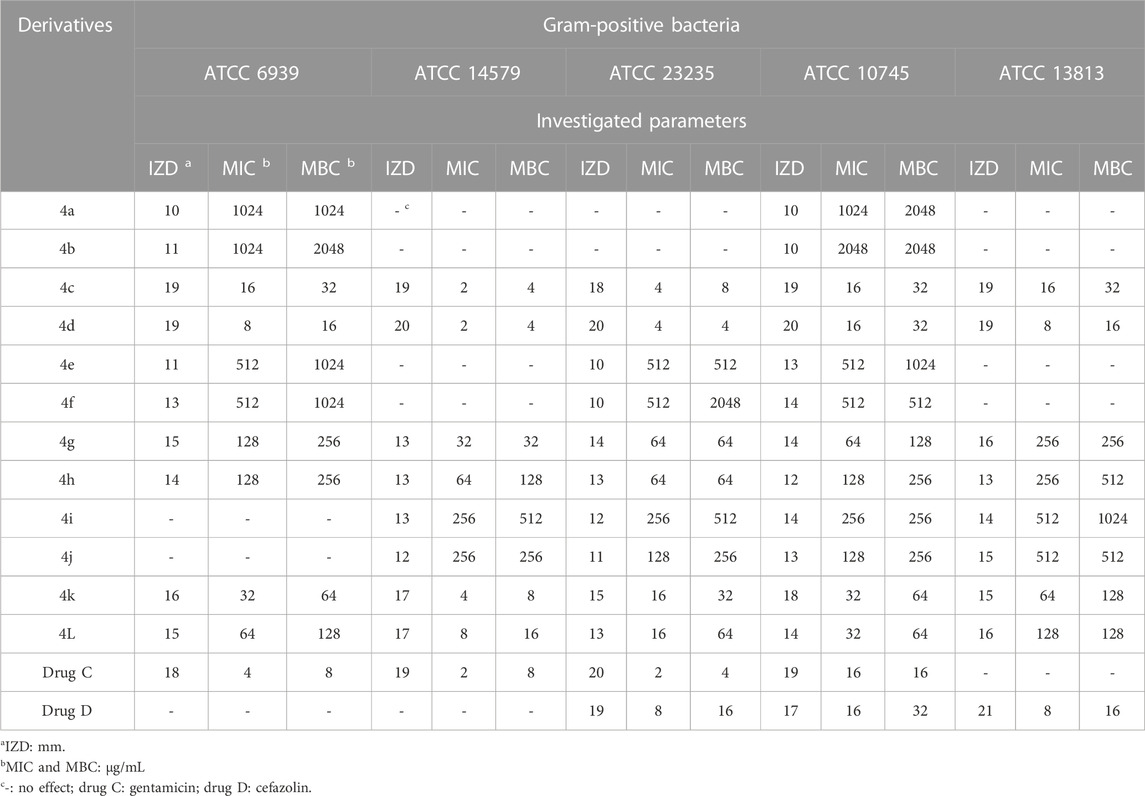
TABLE 6. Antibacterial activity of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives against Gram-positive bacteria.
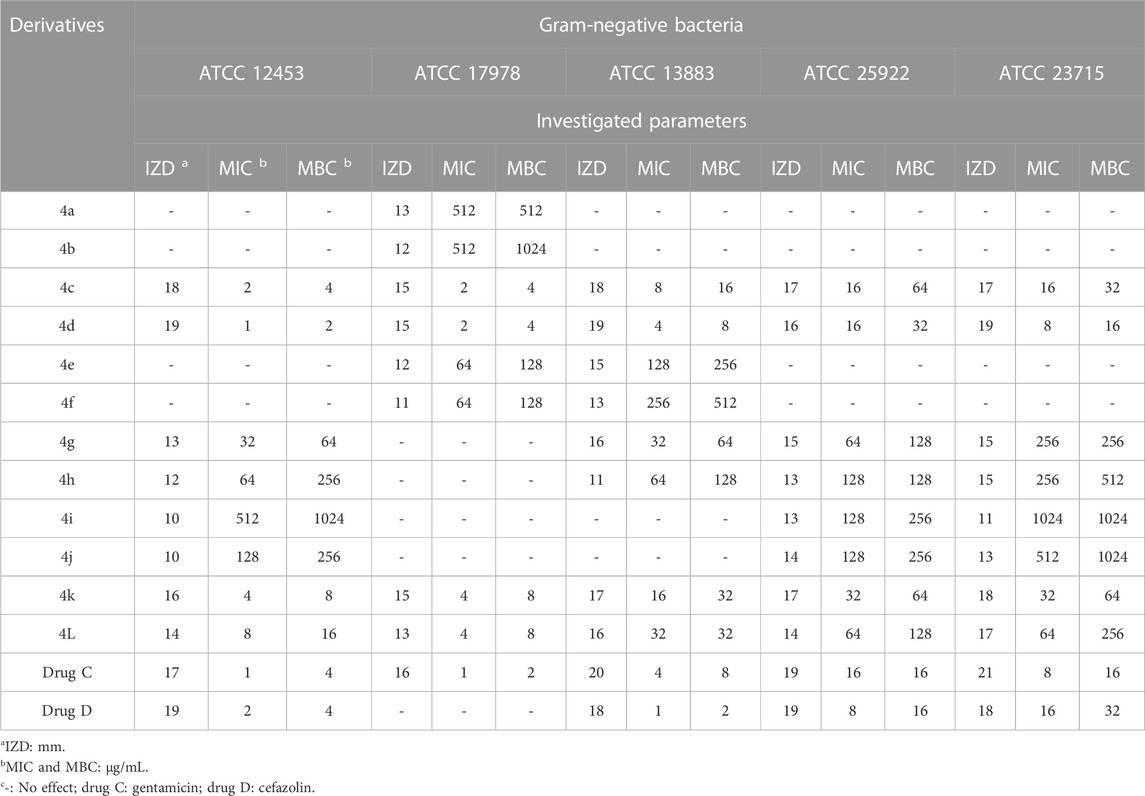
TABLE 7. Antibacterial activity of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives against Gram-negative bacteria.
The order of effect of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives on fungal and bacterial species was as follows:
4d>4c>4k>4l>4g>4h>4j>4i>4e>4f>4a>4b.
From the order of observation, it can be concluded that 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives containing chlorine, nitro, hydroxyl, methoxy, fluorine, and finally, bromine were effective in that order. As mentioned, the highest effectiveness was related to 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives containing chlorine. Chlorine, which has antimicrobial properties (Wei et al., 2019), was introduced here as an agent for the best effect.
As the results of the table show, in the antifungal evaluation, tolnaftate and terbinafine were used as common drugs, and in the antibacterial evaluation, gentamicin and cefazolin were used as standard antibacterial drugs to compare the activity of the 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives. The results proved that all derivatives other than 4a and 4b have higher antifungal activity than standard tolnaftate against ATCC 1022 and ATCC 2091. In addition, derivatives other than 4a, 4b, 4e, and 4f have higher antifungal activity than standard tolnaftate against ATCC 7601. In terms of antibacterial activity, the derivatives, particularly 4d, 4a, 4k, and 4j, had higher effects than gentamicin and cefazolin, which are common drugs.
4 Conclusion
The new 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives were synthesized using K2CO3/glycerol as a deep eutectic solvent. After optimizing the reaction conditions, 12 derivatives of 2,3-dihydro-1H-pyrazole-4-carbonitrile were synthesized with 85%–95% efficiency in 15–20 min. The synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives showed high antioxidant, antifungal, and antimicrobial activity. In the antioxidant activity assessment, the inhibition percentage of derivatives was tested and IC50 values for derivatives between 12.21 and 12.88 μg/mL were calculated. The IZD, MIC, MFC, and MBC were measured to assess antifungal and antibacterial activity. In the antifungal and antibacterial activity of derivatives, MIC values between 4 μg/mL and 2048 μg/mL were obtained. From the biological activity results, we can mention the superior effectiveness of 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives compared with some common drugs. In addition, the logical relationships between the structure of derivatives and their biological properties were observed. Among the novelties of this research, we can mention the synthesis of new 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives in green and easier conditions, compared with the previously presented method, and the bioactivities of the synthesized 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives. Therefore, the above deep eutectic solvent system can be extended to synthesize other heterocyclic compounds with biological properties.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, AZ and IA; methodology, MK; software, MA; validation, RK; formal analysis, AK.; investigation, AZ; resources, EA; data curation, AZ; writing—original draft preparation, MK; writing—review and editing, IA, BR, and AA; visualization, AZ; supervision, MK; project administration, RK; funding acquisition, IA. All authors contributed to the article and approved the submitted version.
Funding
The authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Research Group Project under grant number RGP.02/260/44.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2023.1196583/full#supplementary-material
References
Abdieva, G. A., Patra, I., Al-Qargholi, B., Shahryari, T., Singh Chauhan, N. P., and Moghaddam-Manesh, M. (2022). An efficient ultrasound-assisted synthesis of Cu/Zn hybrid MOF nanostructures with high microbial strain performance. Front. Bioeng. Biotechnol. 10, 861580. doi:10.3389/fbioe.2022.861580
Ansari, A., Ali, A., Asif, M., and Shamsuzzaman, S. (2017). Review: Biologically active pyrazole derivatives. New J. Chem. 41 (1), 16–41. doi:10.1039/c6nj03181a
Arab, M., Hamid, B., and Aryan, R. (2022). One-pot synthesis of 3-amino-1, 2, 4-triazoles using choline chloride-urea and their antibacterial activities. ChemistrySelect 7 (38), e202203291. doi:10.1002/slct.202203291
Ardila-Fierro Karen, J., and Hernández, J. G. (2021). Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 14 (10), 2145–2162. doi:10.1002/cssc.202100478
Aryan, R., Hamid, B., Nojavan, M., Pirani, F., Samareh Delarami, H., and Sanchooli, M. (2019). Expedient multicomponent synthesis of a small library of some novel highly substituted pyrido [2, 3-d] pyrimidine derivatives mediated and promoted by deep eutectic solvent and in vitro and quantum mechanical study of their antibacterial and antifungal activities. Mol. Divers. 23, 93–105. doi:10.1007/s11030-018-9859-7
Aryan, R., Hamid, B., Nojavan, M., and Rezaei, M. (2017). Novel biocompatible glucose-based deep eutectic solvent as recyclable medium and promoter for expedient multicomponent green synthesis of diverse three and four substituted pyrazole-4-carbonitrile derivatives. Res. Chem. Intermed. 43 (8), 4731–4744. doi:10.1007/s11164-017-2908-5
Becerra, D., Rodrigo, A., and Juan-Carlos, C. (2022). Recent applications of the multicomponent synthesis for bioactive pyrazole derivatives. Molecules 27 (15), 4723. doi:10.3390/molecules27154723
Beyzaei, H., Kamali Deljoo, M., Aryan, R., Ghasemi, B., Mehdi Zahedi, M., and Moghaddam-Manesh, M. (2018). Green multicomponent synthesis, antimicrobial and antioxidant evaluation of novel 5-amino-isoxazole-4-carbonitriles. Chem. Central J. 12 (1), 114–118. doi:10.1186/s13065-018-0488-0
Chen, T. L., Kim, H., Pan, S. Y., Po-Chih, T., Lin, Y. P., and Pen-Chi, C. (2020). Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 716, 136998. doi:10.1016/j.scitotenv.2020.136998
Etemadi, Y., Ali, S., Eshghi, H., Akbarzadeh, M., Saadat, K., Mozafari, S., et al. (2016). Synthesis, characterisation, and in vitro antibacterial evaluation of a new class of 2-substituted-4-methyl-7, 8-dihydro-5H-pyrimido [4, 5-d] thiazolo [3, 2-a] pyrimidines. J. Chem. Res. 40 (10), 600–603. doi:10.3184/174751916x14737838285904
Goshtasbi, H., Parvin Samadi, P., Ali, M., Barar, J., Castejon, A. M., Omidian, H., et al. (2022). Impacts of oxidants and antioxidants on the emergence and progression of Alzheimer's disease. Neurochem. Int. 153, 105268. doi:10.1016/j.neuint.2021.105268
Hosseinzadegan, S., Hazeri, N., and Maghsoodlou, M. T. (2020a). Synthesis and evaluation of antimicrobial and antioxidant activity of novel 7-Aryl-6H, 7H-benzo [f] chromeno [4, 3-b] chromen-6-one by MgO nanoparticle as green catalyst. J. Heterocycl. Chem. 57 (2), 621–626. doi:10.1002/jhet.3796
Hosseinzadegan, S., Hazeri, N., Malek Taher, M., Moghaddam-Manesh, M., and Shirzaei, M. (2020b). Synthesis and evaluation of biological activity of novel chromeno [4, 3-b] quinolin-6-one derivatives by SO 3 H-tryptamine supported on Fe 3 O 4@ SiO 2@ CPS as recyclable and bioactive magnetic nanocatalyst. J. Iran. Chem. Soc. 17 (12), 3271–3284. doi:10.1007/s13738-020-01990-3
Igei, M., Bakavoli, M., Ali, S., Ebrahimpour, Z., Hamid, A., Hamid, B., et al. (2016). Synthesis of some new pyrimido [4, 5-e] tetrazolo [5, 1-b] [1, 3, 4] thiadiazine derivatives via an S–N type Smiles rearrangement and their antibacterial evaluation. J. Chem. Res. 40 (10), 628–632. doi:10.3184/174751916x14742893137631
Kumar, P., Osahon, O. B., Vides, D. B., Hanania, N., Minard, C. G., and Sekhar, R. V. (2022). Severe glutathione deficiency, oxidative stress and oxidant damage in adults hospitalized with COVID-19: Implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants 11 (1), 50. doi:10.3390/antiox11010050
Kumar, Vinod, Kaur, Kamalneet, Gupta, Girish Kumar, and Sharma, Anil Kumar (2013). Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 69, 735–753. doi:10.1016/j.ejmech.2013.08.053
Kumari, Simpal, Sarvesh, K. Paliwal, and Chauhan, Rajani (2018). An improved protocol for the synthesis of chalcones containing pyrazole with potential antimicrobial and antioxidant activity. Curr. Bioact. Compd. 14 (1), 39–47. doi:10.2174/1573407212666161101152735
Lees, P., and Fariborz Shojaee, A. (2002). Rational dosing of antimicrobial drugs: Animals versus humans. Int. J. Antimicrob. agents 19 (4), 269–284. doi:10.1016/s0924-8579(02)00025-0
Maddila, Suresh, SreekanthaJonnalagadda, B., KranthiGangu, K., and Maddila, Surya N. (2017). Recent advances in the synthesis of pyrazole derivatives using multicomponent reactions. Curr. Org. Synth. 14 (5), 634–653. doi:10.2174/1570179414666161208164731
Majd, Heidari, Mostafa Akbarzadeh, Abolfazl, and Azam, Sargazi (2017). Evaluation of host–guest system to enhance the tamoxifen efficiency. Artif. cells, nanomedicine, Biotechnol. 45 (3), 441–447. doi:10.3109/21691401.2016.1160916
Mamaghani, Manouchehr, and Roghayeh Hossein, Nia (2021). A review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl. Aromat. Compd. 41 (2), 223–291. doi:10.1080/10406638.2019.1584576
Moghaddam-Manesh, Mohammadreza, Ghazanfari, Dadkhoda, Sheikhhosseini, Enayatollah, and Akhgar, Mohammadreza (2020). Synthesis, characterization and antimicrobial evaluation of novel 6'-Amino-spiro [indeno [1, 2-b] quinoxaline [1, 3] dithiine]-5'-carbonitrile derivatives. Acta Chim. Slov. 67 (1), 276–282. doi:10.17344/acsi.2019.5437
Moser, Claus, Christian Johann, Lerche, Kim, Thomsen, Tom, Hartvig, Schierbeck, Jens, Peter Østrup, Jensen, et al. (2019). Antibiotic therapy as personalized medicine–general considerations and complicating factors. Apmis 127 (5), 361–371. doi:10.1111/apm.12951
Neto, Brenno A. D., Rocha, Rafael O., and Rodrigues, Marcelo O. (2021). Catalytic approaches to multicomponent reactions: A critical review and perspectives on the roles of catalysis. Molecules 27 (1), 132. doi:10.3390/molecules27010132
Nocella, Cristina, Cammisotto, Vittoria, Pigozzi, Fabio, Borrione, Paolo, Fossati, Chiara, D’Amico, Alessandra, et al. (2019). Impairment between oxidant and antioxidant systems: Short-and long-term implications for athletes’ health. Nutrients 11 (6), 1353. doi:10.3390/nu11061353
Rajapaksha, P., Elbourne, Aaron, Gangadoo, Sheeana, Brown, R., Cozzolino, Daniel, and Chapman, James (2019). A review of methods for the detection of pathogenic microorganisms. Analyst 144 (2), 396–411. doi:10.1039/c8an01488d
Rather Abdullah, Ryhan, Khan, Mohd Umar, and Siddiqui, Zeba N. (2020). Sulphated alumina tungstic acid (SATA): A highly efficient and novel heterogeneous mesostructured catalyst for the synthesis of pyrazole carbonitrile derivatives and evaluation of green metrics. RSC Adv. 10 (2), 818–827. doi:10.1039/c9ra09013d
Sarkar, Sougata, Srivastava, Vartika, Samrat Samajhdar, Shambo, Pattanayak, Chaitali, and Tripathi, Santanu (2022). Rational use of antibiotics: An area of concern. J. Young Pharm. 14 (2), 165–168. doi:10.5530/jyp.2022.14.31
Sharma, Upendra K., Ranjan, Prabhat, ErikVan der Eycken, V., and You, Shu-Li (2020). Sequential and direct multicomponent reaction (MCR)-based dearomatization strategies. Chem. Soc. Rev. 49 (23), 8721–8748. doi:10.1039/d0cs00128g
Singh, Maya Shankar, and Chowdhury, Sushobhan (2012). Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. Rsc Adv. 2 (11), 4547–4592. doi:10.1039/c2ra01056a
Wei, Lijie, Tan, Wenqiang, Wang, Gang, Li, Qing, Dong, Fang, and Guo, Zhanyong (2019). The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 226, 115256. doi:10.1016/j.carbpol.2019.115256
Keywords: green chemistry, deep eutectic solvent, pyrazoles, antioxidant activity, antimicrobial activity
Citation: Zwain AA, Ahmad I, Khalaf Jebur Ali R, Kahtan M, Khdyair Hamad A, Abdulgader Hassan E, Asiri M, Ridha BM and Alsalamy A (2023) Synthesis, antioxidant, and antimicrobial evaluation of novel 2,3-dihydro-1H-pyrazole-4-carbonitrile derivatives using K2CO3/glycerol as a green deep eutectic solvent. Front. Mater. 10:1196583. doi: 10.3389/fmats.2023.1196583
Received: 30 March 2023; Accepted: 16 June 2023;
Published: 06 July 2023.
Edited by:
Ceren Karaman, Akdeniz University, TürkiyeReviewed by:
Mahmoud Tolba, The New Valley University, EgyptFariba Heidarizadeh, Shahid Chamran University of Ahvaz, Iran
Copyright © 2023 Zwain, Ahmad, Khalaf Jebur Ali, Kahtan, Khdyair Hamad, Abdulgader Hassan, Asiri, Ridha and Alsalamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali A. Zwain, YWxpLmFiYmFzQG11c3RhcWJhbC1jb2xsZWdlLmVkdS5pcQ==
 Ali A. Zwain
Ali A. Zwain Irfan Ahmad2
Irfan Ahmad2