- 1Department of Mechanical Engineering, Tsinghua University, Beijing, China
- 2CCTEG Beijing Tianma Intelligent Control Technology Co., Ltd., Beijing, China
Owing to the great barrier properties, light weight and environmental friendliness, graphene-based anticorrosion coatings are considered to be promising candidates for next-generation anticorrosion materials. Recently, polydopamine has showed considerable research value and application prospects in the field of anticorrosion, and provided new opportunities for chemical modification of graphene for the enhancement of coating performance. However, the applications and mechanisms of polydopamine-modified graphene in anticorrosion coatings have not been systematically summarized. This review focuses on the application and mechanism of graphene and polydopamine-modified graphene in anticorrosion coatings. Firstly, the intrinsic barrier properties and applications of graphene in anticorrosion coatings are summarized, especially in self-healing anticorrosion coatings. Next, the properties and applications of polydopamine in the field of anticorrosion are reviewed and discussed. Moreover, the application and mechanism of polydopamine in graphene-based anticorrosion coatings are systematically reviewed, which mainly includes enhancing the intrinsic barrier properties, improving the dispersibility, modifying with functional materials, and introducing pH-responsive properties. Finally, the critical challenges and future prospects for the development of graphene-based anticorrosion coatings are discussed. This article aims to boost further development of graphene-based anticorrosion coatings to solve the anticorrosion bottleneck of equipment in harsh corrosive environments.
1 Introduction
When the metal is in contact with the surrounding medium, it often fails due to the chemical or electrochemical interaction between them, i.e., corrosion occurs. From the second law of thermodynamics and the Gibbs free energy change of the reaction, all but a few precious metals have a tendency to convert to ions (Li et al., 2023). In other words, metal corrosion is a spontaneous and widespread phenomenon that is involved in almost all areas of industry and human life (Nazari et al., 2022; Cui et al., 2021). As one of the worst corrosive environments, the ocean atmosphere can form a liquid film containing oxygen and salt on metal surfaces, leading to extremely strong corrosion and failure of metal materials. It seriously affects the long-term safe development of infrastructure and engineering equipment in tropical oceans, polar oceans and deep oceans (Yu et al., 2023). Especially with the continuous exploration of oceans, the reliability requirements for marine equipment are getting higher and higher. Due to long-term exposure to the marine corrosive environment containing oxygen, salt spray and ultraviolet rays, it is a big challenge to develop a long-term, effective anticorrosion strategy for high-end equipment in the field of marine engineering.
Metal corrosion is caused by the reaction of metal and external media, so the surface coating is the most direct and effective protective measure to reduce and avoid metal corrosion. Due to the advantages of wide selectivity, wide availability and low cost, organic anticorrosion coatings are the most widely used engineering materials to ensure the safety of marine equipment in harsh corrosive environments. (Zhang F. et al., 2018; Liu et al., 2020; Vesely et al., 2010; Li et al., 2022; Li et al., 2014; Fang et al., 2015; Song et al., 2024). However, the polymer matrix of organic coatings contains large molecular chain gaps and defects such as pores and cracks that are inevitably introduced during the coating process. It means that a pure organic coating can hardly be a perfect physical barrier to corrosive media (Hasani et al., 2018; Cai et al., 2018; Li and Zhou, 2022). In recent years, the emerging graphene-based anticorrosion coatings have the advantages of excellent anticorrosive performance, small coating thickness, light weight, high adhesion and great barrier effects, which is an ideal upgraded alternative to the traditional organic anticorrosion coatings. The barrier effects refer to the ability of the graphene flakes to prevent the penetration of corrosive media like H2O, O2 and Cl−, which improve the anticorrosion performance of organic coatings. In the composition of graphene-based anticorrosion coatings, the addition of graphene as filler is only 0.5%–2%, which can replace 30%–60% of zinc powder filler in traditional organic anticorrosion coatings. Moreover, compared with other 2D materials, graphene has better barrier properties thanks to the special arrangement of carbon atoms in its 2D structure, which will be described in more detail below. Secondly, graphene is only composed of carbon atoms, which gives it the advantage of being more chemically stable and lighter than other 2D materials like transition metal dichalcogenides or MXenes. Third, the industrial mass production of graphene is already available, and the cost of raw materials and processing of graphene has advantages over other 2D materials like h-BN or black phosphorus. Thus, it shows that graphene has unique advantages in anticorrosion performance, reducing costs and environmental protection, making it an ideal filler for improving the performance and reducing costs of anticorrosion coatings (Ollik and Lieder, 2020; Cui et al., 2019; Jin et al., 2022a).
Owing to the advantages of graphene as filler in coatings, the scientific research and engineering application of graphene-based anticorrosion coatings have developed rapidly in recent years. In addition, some emerging graphene modification technologies have also provided new opportunities for the anticorrosion applications of modified graphene coatings, which give the coating new functions such as high-temperature resistance, wear resistance, self-healing properties and self-warning properties. Among them, mussel adhesion protein has been highly valued in the field of surface modification and anticorrosion due to its strong affinity with metal substrates. Polydopamine, the derivative of mussel adhesion protein, has also attracted widespread attention as a new type of modified material and green corrosion inhibitor (Jin et al., 2025). Due to the unique chemical properties of polydopamine, it is expected to make up for the defects of pure graphene-based anticorrosion coatings and accelerate the development and application of high-performance graphene-based anticorrosion coatings.
This review recapitulates the development of graphene-based anticorrosion coatings and the applications of polydopamine in graphene-based anticorrosion coatings. Firstly, the barrier properties of graphene are briefly described. Then, the applications and current problems of graphene in anticorrosion coatings are systematically discussed, especially in self-healing anticorrosion coatings. After that, the properties and the applications of polydopamine in anticorrosion are reviewed and discussed. Finally, the research and the possible anticorrosion mechanism of polydopamine in graphene-based anticorrosion coatings are reviewed and analyzed, as shown in Figure 1. Based on the above, the critical challenges and future prospects for the application of polydopamine in graphene-based coatings as next-generation anticorrosion materials are discussed, aiming to boost further development of modified graphene as a candidate to solve the anticorrosion bottleneck of equipment in harsh corrosive environments such as tropical oceans, polar oceans, deep oceans and deep earth.
Figure 1. Research of polydopamine in graphene-based anticorrosion coatings and applications in harsh corrosive conditions.
2 Development of graphene-based anticorrosion coatings
2.1 Barrier properties of graphene
In 2004, Novoselov et al. from the University of Manchester first used mechanical exfoliation to obtain graphene with a thickness of only 0.35 nm (Novoselov et al., 2004). The study found that graphene is a type of two-dimensional material with only one atomic layer thickness. Its carbon atoms are connected by sp2 orbital hybridization, so the chemical bonds between carbon atoms are highly stable σ bonds (Geim, 2009). Due to its special two-dimensional structure, graphene has great mechanical, electrical, thermal, and optical properties, making it a hot research topic in many fields (Jin et al., 2023; Jin et al., 2022b; Wang et al., 2019; Han et al., 2014). In addition, the excellent barrier property of graphene has gradually attracted attention in the field of anticorrosion. The van der Waals radius of neighboring carbon atoms in graphene is 0.11 nm. The conjugation effect of π-electrons within the surface reduces the diameter of the hexagonal holes to 0.064 nm, which forms a repulsive field to effectively block the penetration of corrosive media such as oxygen and water molecules, as shown in Figure 2a (Berry, 2013). Therefore, graphene is considered as an ideal anticorrosion material due to advantages such as barrier property and chemical stability.
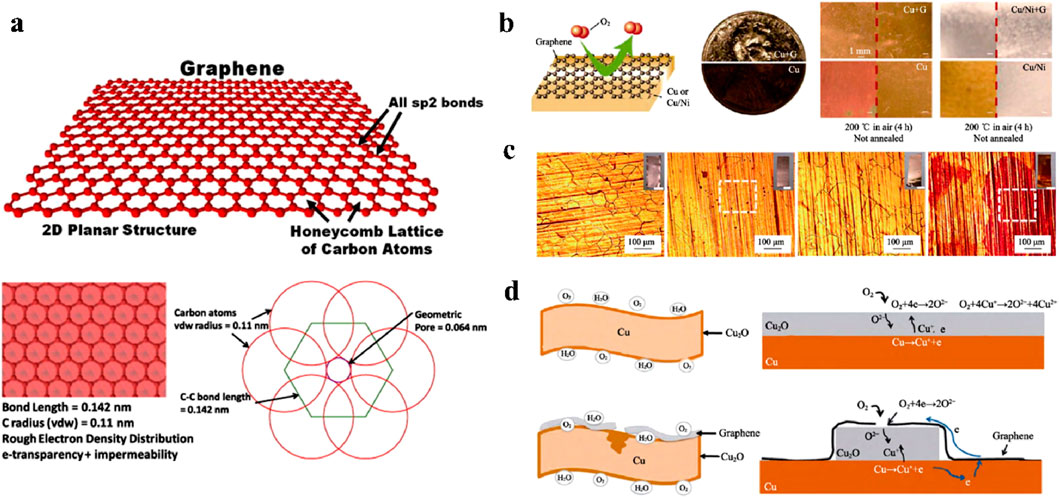
Figure 2. Barrier properties of graphene. (a) Intrinsic impermeability of graphene (Berry, 2013). (b) Barrier properties of graphene film on metal surface (Chen et al., 2011). (c) Graphene film exacerbated the corrosion of copper substrate after 6 months in the atmosphere (Zhou et al., 2013). (d) Electrochemical reaction mechanism of copper corrosion exacerbated by graphene film (Schriver et al., 2013).
Based on the intrinsic barrier properties of graphene, graphene films are directly used for the anticorrosion of metal surfaces (Pan et al., 2024; Li et al., 2022). Chen et al. confirmed the protective performance of graphene films on copper substrates in hydrogen peroxide and high-temperature corrosive environments (Chen et al., 2011). The results showed that graphene had good chemical inertness and high-temperature oxidation resistance. It could block the direct contact between the corrosive medium and the copper substrate, which effectively reduced the oxidation and corrosion rate of copper, as shown in Figure 2b. However, Zhou et al. found that graphene films on metal surfaces may accelerate the corrosion of metal substrates (Zhou et al., 2013). They deposited a layer of graphene film on the copper surface and exposed it to the atmosphere for 6 months. Compared with the pure copper substrate, the copper substrate with graphene film actually suffered more severe corrosion, as shown in Figure 2c. With the deepening of research, Schriver et al. found that intact graphene films could indeed be used as a protective layer to protect the metal substrate in a short period of time (Schriver et al., 2013). However, due to long-term immersion in a corrosive environment, the inevitable defects in graphene films may lead to the penetration of corrosive media such as H2O and O2, causing corrosion of the substrate. Since graphene films are excellent conductors, they can induce electrochemical reactions at the contact interface and promote the corrosion. For copper substrates, as long as there are free electrons at the interface between the graphene film and metallic copper, the free electrons can diffuse from copper to the graphene film, as shown in Figure 2d. This process accelerates the cathode reaction, ultimately leading to the corrosion of copper.
In summary, the intrinsic barrier properties of graphene can indeed inhibit the penetration of corrosive media and provide short-term protection for metals. However, after the long-term immersion in a corrosive environment, defects in the graphene film can allow corrosive media to penetrate into the substrate, causing corrosion of the metal substrate. Due to the conductivity of graphene, it can form a primary battery with the metal substrate, which accelerates the corrosion of the substrate (Cui et al., 2017). Many works have been conducted to improve the intrinsic barrier properties of graphene films by reducing the conductivity of graphene films or avoiding direct contact between graphene films and metal substrates, such as multilayer design strategies (Stoot et al., 2015), atomic layer deposition methods (Hsieh et al., 2014), and heterogeneous atom doping methods (Ren et al., 2018). However, these strategies are not suitable for large-scale anticorrosion applications. Therefore, since pure graphene films are difficult to meet the long-term protection requirement of industrial equipment, the dispersion of graphene powder into organic coatings is a practical way to exert the excellent barrier properties of graphene (Zhao et al., 2024).
2.2 Applications of graphene in anticorrosion coatings
Graphene can be added to various organic coatings as a filler. It can adapt to different organic coatings and make up for the poor barrier properties of coatings caused by coating defects. Tong et al. studied the effect of graphene structure on the anticorrosion performance of coatings (Tong et al., 2017). Comparing multilayer graphene and few-layer graphene, they found that the anticorrosion performance of few-layer graphene in polyurethane coatings was significantly better than that of multilayer graphene. The results showed that the few-layer graphene had a larger specific surface area and could be better dispersed in the coating to block corrosive media. Qiang et al. studied the effect of different amounts of added graphene on the anticorrosion performance of acrylic resin coatings (Qiang et al., 2020). The results showed that with the increase of addition amount, the anticorrosion performance of the coating first increased and then decreased. Because the flake structure of graphene had a great barrier effect on corrosive media, but it was prone to agglomeration after exceeding an optimal amount of 0.6 wt%, which promoted the formation of coating cracks. Ding et al. further enhanced the barrier properties of graphene by doping boron nitride nanodots and added the composite as a filler to epoxy resin to evaluate the anticorrosion performance of the coating (Ding et al., 2019). The results showed that only 0.1 wt% of graphene with boron nitride nanodots could reduce the corrosion rate of the steel substrate by 280 times and increase the electrochemical impedance modulus by two orders of magnitude, showing excellent anticorrosion performance.
The dispersibility of graphene in the coating is the key to enhancing the barrier properties of the graphene-based coatings (Zhang et al., 2020; Liu et al., 2021). However, due to the strong van der Waals force and π-π interaction between graphene sheets, graphene is prone to stacking and agglomeration in the coating (Jin B. et al., 2021; Wu et al., 2022). The main dispersion methods currently used include physical dispersion methods such as adding surfactants and intercalating agents, and chemical dispersion methods such as modifying and grafting. For the physical dispersion method, Chen et al. used poly(2-butylaniline) as a dispersant to weaken the interaction of graphene sheets by intercalation, which achieved efficient dispersion of graphene in organic solvents, as shown in Figure 3a (Chen et al., 2017). The results showed that the impedance modulus of the composite epoxy coating after enhanced dispersion increased by two orders of magnitude. Qiu et al. used polypyrrole as a dispersant and assisting with ultrasonic dispersion technology to achieve efficient dispersion of graphene in the coating (Qiu et al., 2017). As a result, the graphene composite coating showed a more positive corrosion potential and a higher impedance modulus.
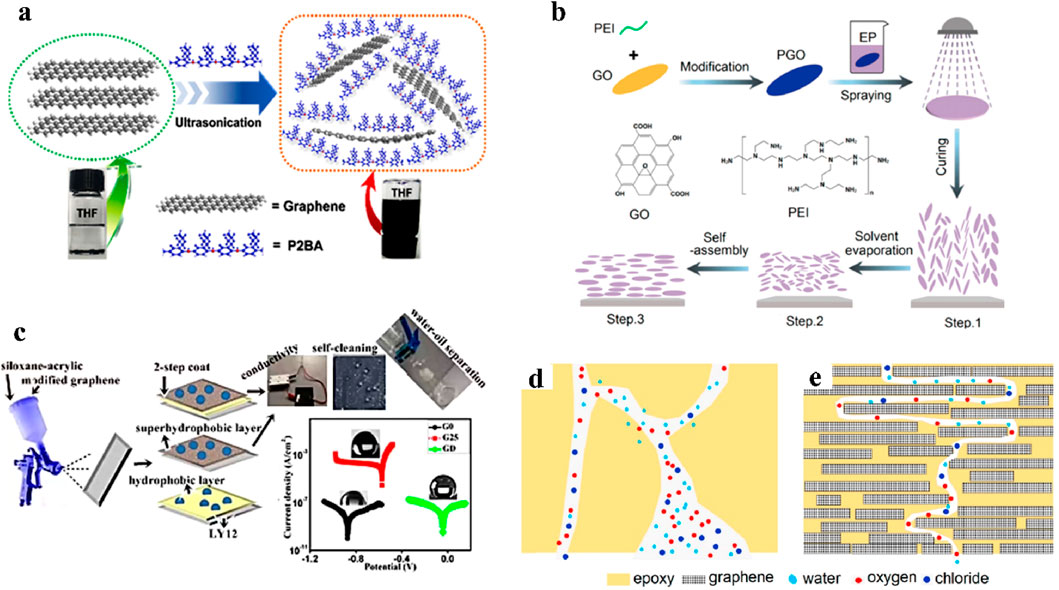
Figure 3. Applications of graphene in anticorrosion coatings. (a) Poly(2-butylaniline) as an intercalating agent for physical dispersion of graphene in organic solvents (Chen et al., 2017). (b) Preparation of uniformly dispersed and oriented graphene-based epoxy coatings by spraying method (Ding et al., 2021). (c) Graphene modified by hydrophobic fluorosilane enhanced superhydrophobic properties (Uzoma et al., 2019). (d) Schematic diagram of corrosive medium penetration into pure organic coatings (Ding et al., 2021). (e) Schematic diagram of corrosive medium penetration into graphene-based organic coatings (Ding et al., 2021).
The chemical dispersion method is based on the principle of “like dissolves like”. Functional groups similar to the matrix groups are grafted onto graphene to enhance its dispersibility in the resin matrix. Dong et al. used KH-570 silane coupling agent to covalently graft graphene oxide and added it to acrylic resin as a filler (Dong and Liu, 2016). The results showed that the modified groups could form covalent bonds with the coating matrix. The modification process not only improved the dispersibility of graphene oxide in the coating, but also enhanced its compatibility with the resin interface. Ye et al. synthesized the graphene oxide modified by polyhedral oligomeric silsesquioxane (POSS-GO) by a one-step condensation reaction. They added the composites to epoxy resin to achieve efficient dispersion of fillers and enhance the barrier properties of the coating (Ye et al., 2019). The results showed that after adding 0.5 wt% of POSS-GO, the impedance modulus of the epoxy coating increased by two orders of magnitude, which was attributed to the great dispersibility of POSS-GO. Ding et al. prepared a graphene-based epoxy coating by spraying oligomer-functionalized graphene oxide, which had the directional arrangement structure of graphene in the coating, as shown in Figure 3b (Ding et al., 2021). The results showed that the impedance modulus of the coating increased by 3 orders of magnitude due to the excellent barrier properties of the uniformly dispersed graphene. In addition to improving the dispersibility of graphene in the coating matrix, chemical modification can also give graphene or graphene-based coatings specific functions to meet the requirements of different applications. Wu et al. proposed a strategy for fluorinated graphene modified by ionic liquid as a filler in coatings (Wu et al., 2020). This work not only achieved stable dispersion of graphene in water-based epoxy resin, but also reduced the conductivity of graphene powder, which inhibited the microcurrent corrosion of the substrate. Uzoma et al. prepared the organic siloxane acrylic resin by acrylic monomer polymerization, and added the graphene modified by hydrophobic fluorosilane as a filler (Uzoma et al., 2019). The results showed that the well-dispersed modified graphene not only improved the anticorrosion and mechanical properties of the coating, but also brought superhydrophobic properties to the coating with a water contact angle of ≥152°, as shown in Figure 3c.
In summary, the anticorrosion performance of graphene is mainly based on its unique two-dimensional conjugated structure. This structure can effectively inhibit the penetration of corrosive media such as water, oxygen, and chloride ions, forming a dense and anti-penetration barrier layer as shown in Figure 3d. The low surface energy and hydrophobicity of graphene also make it difficult for water molecules to penetrate into the coating (Liu et al., 2019). Well-dispersed graphene can be stacked layer by layer in the coating to form a maze-like anticorrosion structure, as shown in Figure 3e (Ding et al., 2021). This “maze effect” can not only fill the defects of the coating matrix, preventing bubbles or cracks in the coating from further expanding, but also increase the diffusion path of the corrosive medium in the coating. The electro-chemical results revealed that the impedance modulus of the graphene-based coatings (∼109 Ω‧cm2) was improved more than by 3 orders than that of pure organic coatings (3.15 × 105 Ω‧cm2). Therefore, dispersing graphene in an organic coating can enhance the barrier properties of the coating, thereby reducing the corrosion rate of the metal substrate.
2.3 Research of graphene in self-healing anticorrosion coatings
Although the barrier structure of graphene in coatings can effectively block the penetration of corrosive media, the effectiveness of the structure must be based on the absence of mechanical damage to the coating. Once the coating is scratched or cracked, the barrier effect of the graphene in the coating will fail. Conventional methods usually remove the damaged coating and replacing it with an intact one. However, it brings new problems such as the poor compatibility between the old and new interfaces, which affects the bonding and mechanical properties of the coating. Therefore, graphene-based anticorrosion coatings with only passive barrier properties are difficult to meet the long-term anticorrosion requirements of high-end equipment. In order to improve the long-term anticorrosion performance of graphene-based anticorrosion coatings and enhance the resistance of coatings to mechanical damage, self-healing anticorrosion coatings with both passive barrier and active healing properties have gradually attracted the attention of researchers (Shchukin and Mohwald, 2013; Cui et al., 2023; Cui et al., 2020).
Graphene-based self-healing anticorrosion coatings use graphene as a carrier platform to load and encapsulate corrosion inhibitors. This not only exerts the barrier properties of graphene in coatings, but also utilizes the corrosion inhibition effect of the corrosion inhibitors to further enhance the corrosion resistance of the coating (Mustehsin et al., 2024; Wu et al., 2024). Even when mechanically damaged, such self-healing coatings can form a protective layer on the metal surface via the release of the corrosion inhibitors on graphene, realizing the healing of the anticorrosion performance of damaged coatings (Zhang M. et al., 2018; Cho et al., 2009; Jia et al., 2020; Yan et al., 2021; Zhong et al., 2021; Cao et al., 2020; Ma et al., 2020). Hao et al. grafted polyaniline onto graphene, and loaded corrosion inhibitor, benzotriazole (BTA), via interactions between the polyaniline and the BTA, as shown in Figure 4a (Hao et al., 2020). The results of the electrochemical measurements showed that the impedance modulus of the composite coating decreased and then increased with immersion time, indicating that the composite coating exhibited the self-healing properties. This was attributed to the barrier effect of graphene and the formation of a protective film on the metal substrate by the released BTA. Ramezanzadeh et al. noncovalently modified the graphene oxide with a corrosion inhibitor, 1H-benzimidazole, and then dispersed the composites into epoxy resin (Kasaeian et al., 2018). The results showed that the composite filler could actively inhibit the corrosion of the substrate, exhibiting excellent self-healing effects and long-term anticorrosion performance. The impedance modulus values of almost greater than 1010 Ω‧cm2 were obtained for this system at long immersion time of 35 days. Ye et al. inserted functionalized carbon dots (CDs) as corrosion inhibitors into the interlayers of graphene via π-π interactions, and then dispersed the CDs-modified graphene into epoxy resin (Ye et al., 2021). The electrochemical results showed that the coating exhibited excellent anticorrosion performance after the addition of CDs-modified graphene. After 50 days of immersion in corrosive environment, the water absorption of the composite coating was only 4.4%.
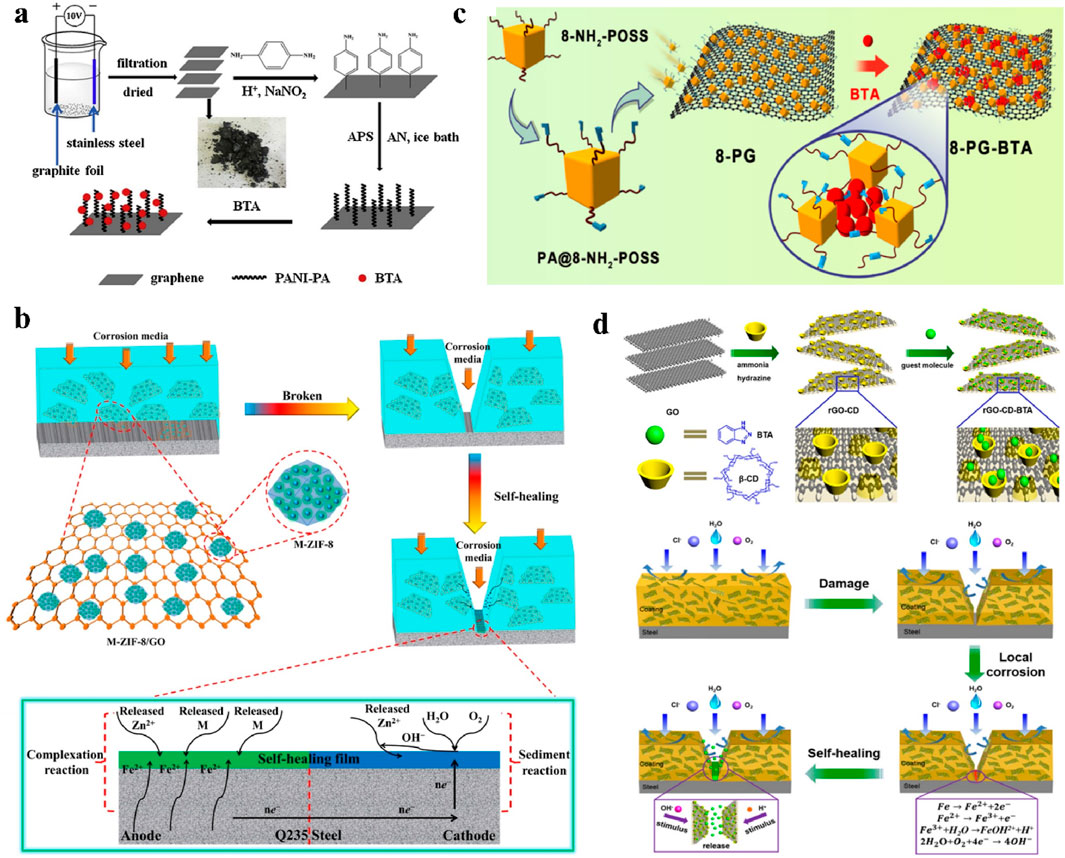
Figure 4. Applications of graphene in self-healing anticorrosion coatings. (a) Preparation of polyaniline-modified graphene loaded with BTA (Hao et al., 2020). (b) Preparation and self-healing mechanism of graphene composite coating based on MOF encapsulated with corrosion inhibitor (Li et al., 2021). (c) Preparation of graphene modified with 8-POSS nanocontainers (Ye et al., 2020). (d) Preparation and self-healing mechanism of graphene modified with β-cyclodextrin supramolecular nanocontainers in coatings (Liu et al., 2018).
One of the main challenges of current graphene-based self-healing anticorrosion coatings is the low loading amount of corrosion inhibitors, resulting from the limited spatial sites and chemical inertness of the graphene platforms (Borisova et al., 2011). Researchers have proposed to load nanocontainers with pore structures on graphene to enhance the loading of corrosion inhibitors. In recent years, many works have made great efforts in designing various kinds of nanocontainers with large capacity, such as hollow or mesoporous nanorods, nanotubes, nanocages, covalent organic frameworks (COFs), and metal-organic frameworks (MOFs) to continuously improve the loading capacity of graphene for corrosion inhibitors. Zhang et al. synthesized COF nanocontainers on graphene oxide sheets for encapsulating the BTA corrosion inhibitor. The loading amount of the composite was 8.53 wt% (Zhang et al., 2022). The results showed that the impedance modulus of the epoxy coating with the composite fillers was still as high as 8.58 × 108 Ω‧cm2 after 60 days of immersion in salt water, which was one order of magnitude higher than that of the coating with the pure graphene oxide. Li et al. encapsulated the corrosion inhibitor of 2-mercaptobenzimidazole into a MOF nanocontainer (ZIF-8) (Li et al., 2021). The nanocontainers were loaded onto graphene oxide with a loading amount of corrosion inhibitors up to 10.21 wt%. The composite filler was then dispersed into epoxy resin to prepare a composite coating with pH-responsive and self-healing properties. The results showed that the composite coating could respond to pH changes caused by metal corrosion, and release the corrosion inhibitor to form a protective film in the corrosion region of the metal substrate, as shown in Figure 4b. The changes of the electrochemical impedance modulus proved the effective self-healing properties of the composite coating. Ye et al. grafted octa-amino polyhedral oligomeric silsesquioxane (8-POSS) onto graphene oxide as a nanocontainer to load the BTA corrosion inhibitor, as shown in Figure 4c (Ye et al., 2020). The 8-POSS was designed with a cage-like structure, which could load more corrosion inhibitor with the loading amount up to 18.6 wt%. The composite coating showed a decreasing trend of conductivity at local defects with the increase of immersion time. Moreover, the corrosion area was significantly reduced, which reflected the self-healing properties of the composite coating. Liu et al. synthesized a novel graphene-based anticorrosion coating via the design of bowl-shaped β-cyclodextrin supramolecular nanocontainers (Liu et al., 2018). The loading amount of corrosion inhibitors was further increased to 24 wt%. The composite coating not only had the barrier properties of graphene, but also could release corrosion inhibitors triggered by the pH change from corrosion as shown in Figure 4d, showing excellent self-healing properties and long-term anticorrosion performance.
In summary, the response and release behaviors of corrosion inhibitors in graphene composites are the keys to the anticorrosion performance of self-healing coatings. The lagging response or uncontrollable release behavior can lead to the untimely interfacial healing and the loss of corrosion inhibitors, which cannot exert the corrosion inhibition effects of the loaded corrosion inhibitors on the metal substrate (Zhou et al., 2020). The response and release characteristics are closely related to the chemical properties of graphene. Therefore, seeking suitable chemical modification strategies for graphene and designing novel modified graphene fillers with more corrosion inhibitors, sensitive response and intelligent release functions are the keys to enhance the performance of graphene-based self-healing anticorrosion coatings.
3 Development of polydopamine in graphene-based anticorrosion coatings
Inspired by marine mussels, the multifunctional chemical properties of polydopamine present new opportunities for the modified graphene-based self-healing anticorrosion coatings (Zhang and Pan, 2019). Polydopamine, a derivative of mussel adhesion protein, is formed by the self-polymerization of dopamine monomer in an alkaline environment. Due to the advantages of adhesion properties, modification properties, metal affinity, and pH-responsive properties, polydopamine has been widely used in the field of anticorrosion as modifier, green corrosion inhibitor, dispersant, and adhesion agent. Moreover, due to the abundance of nitrogen-containing functional groups, polydopamine can also act as an amine curing agent in epoxy resin to enhance the compatibility of fillers with the epoxy coating matrix (Li et al., 2016). Therefore, polydopamine shows considerable research value and application prospects in the field of anticorrosion coatings. It also provides new opportunities for the chemical modification of graphene and the enhancement of the performance of graphene-based self-healing anticorrosion coatings.
3.1 Properties of polydopamine
Marine mussels can adhere to the surfaces of metal, glass and polymers via the mucus secreted by their byssus. This universal adhesion capacity has attracted great attention from researchers in the field of surface coating and functional modification (Ryu et al., 2018; Becherer et al., 2015). The strong adhesion properties are attributed to the adhesion proteins in the mucus secreted by mussel byssus (Anderson et al., 2010; Lee et al., 2006; Lin et al., 2007). These adhesion proteins mainly contain dihydroxyphenylalanine (DOPA) and a small amount of lysine residues (Li et al., 2015). The synergistic effect of the catechol group in DOPA and lysine can replace water molecules on the surface of the substrate, thereby achieving underwater adhesion (Maier et al., 2015; Shin et al., 2021; Qureshi et al., 2021). In 2007, Lee et al. published a pioneering work that applied the adhesion of mussels to the field of surface coating (Lee et al., 2007). They oxidized dopamine under weak alkaline conditions, and the dopamine was self-polymerized on the surfaces of various substrates to form a polydopamine film, as shown in Figure 5a. Dopamine, as a derivative of DOPA, has similar properties to DOPA because it has both the catechol group and the terminal amino residue of lysine (Jin et al., 2024). In that work, they just needed to place the target material in a dopamine alkaline solution at room temperature and immersed for a short time. Dopamine can then spontaneously adsorb on the surface of the target material and form a polydopamine film via covalent and non-covalent self-assembly (Hong et al., 2012). Owing to the convenient modifiability of the hydroxyl and amino groups on the polydopamine film, other functional molecules can be grafted onto the polydopamine film, resulting in functional modification of the substrate (Dreyer et al., 2013). In addition, other studies showed that by introducing other functional molecules during the dopamine polymerization process, a functional polydopamine composite film could be directly formed on the substrate in one step (Kang et al., 2012; Kang et al., 2010; Qiu et al., 2018). Therefore, the surface modification strategy based on polydopamine overcomes the limitations of traditional methods and opens up a new path for rapid modification and multifunctionality of material surfaces, which has attracted great attention in the field of functional coatings and material modification (Barclay et al., 2017; Lee et al., 2019; Zhan et al., 2018).
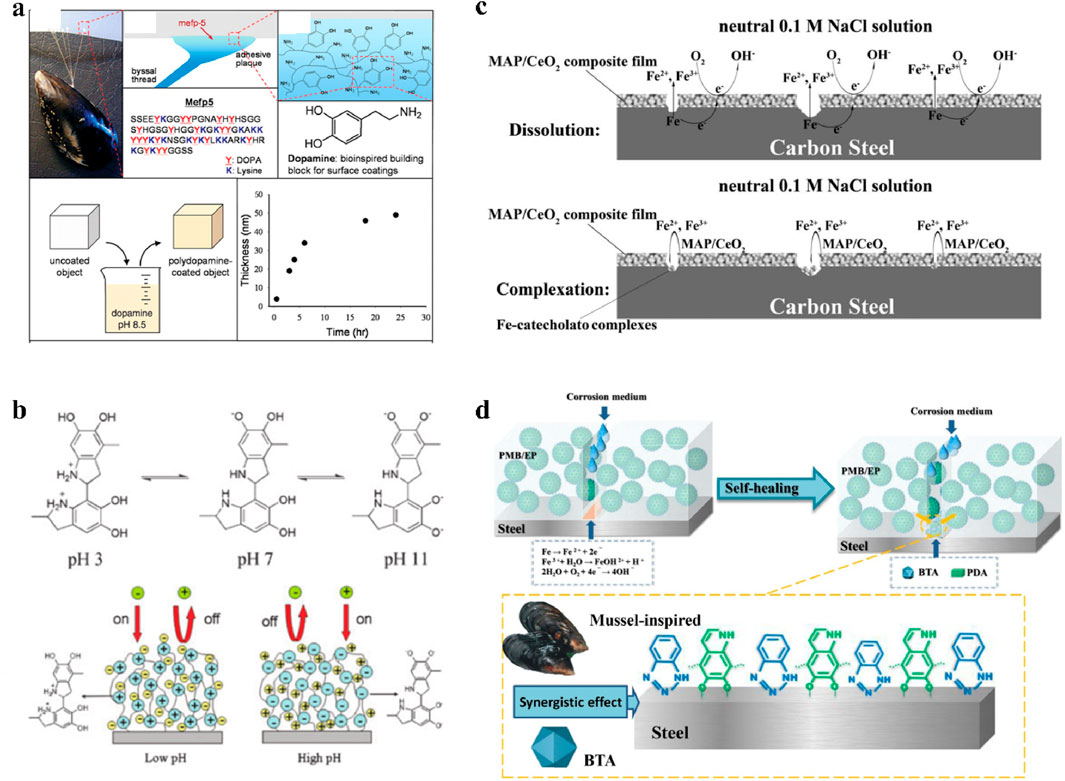
Figure 5. Properties and corrosion inhibition effect of polydopamine. (a) Structure of dopamine and formation of polydopamine (Lee et al., 2007). (b) pH-responsive behaviors of polydopamine (Yu et al., 2010). (c) Self-healing mechanism of Mefp-1/CeO2 composite film (Chen et al., 2016). (d) Anticorrosion mechanism of BTA-loaded polydopamine microcapsules (Cheng et al., 2021).
Due to the reducing and weak acidity of phenolic hydroxyl groups, along with the strong coordination ability of catechol for soluble metal ions, dopamine has a strong affinity for metal ions (Ye et al., 2011). Many works have shown that the complexation reaction between dopamine and metal ions such as Cu2+, Fe3+, Ti3+, Ti4+, Mn2+, Mn3+, and Zn2+ can be used to modify metal ions or capture metal ions on the surface of polydopamine films. Due to the bidentate chelation or bridging effect between catechol and oxides such as TiO2, Fe2O3, Fe3O4, ZrO2, MnO2, and SiO2, polydopamine can also be adsorbed on the surface of these oxides (Ye et al., 2011). Therefore, based on the adhesion properties on metal ions and metal oxides, polydopamine is expected to be used as a corrosion inhibitor or passivation film for corrosion protection of metal materials. Moreover, polydopamine with amino groups and phenolic hydroxyl groups can show different charges in different pH environments and has been used to construct pH-responsive biosensors (Jin Z. et al., 2021). Yu et al. studied the permeability selectivity of polydopamine membranes to cations and anions in different pH environments, as shown in Figure 5b (Yu et al., 2010). The results showed that the polydopamine membrane was negatively charged at high pH and exhibited the behavior of repelling anions but passing cations. And it was positively charged at low pH and could pass anions but repel cations. Therefore, this pH-responsive properties of polydopamine are expected to be used to construct a smart self-healing anticorrosion coating that can quickly respond with controllable release of corrosion inhibitors to the pH changes induced by corrosion.
3.2 Applications of polydopamine as corrosion inhibitors
The traditional anticorrosion methods based on chromate corrosion inhibitors are harmful to the natural environment and human health. Mussel adhesion proteins and polydopamine have strong affinity for metal surfaces and can be used as new corrosion inhibitors that are green, efficient, and have self-healing properties (Zhang et al., 2019). Zhang et al. demonstrated that mussel adhesion protein Mefp-1 could effectively protect carbon steels exposed to salt water (Zhang et al., 2011). Under acidic conditions, the corrosion inhibition effect was mainly attributed to the complexation of DOPA with Fe3+. By capturing Fe3+ on the surface of carbon steel, the corrosion reaction of the carbon steel was inhibited, and the effect could last up to 7 days. Under alkaline conditions, DOPA could self-oxidize and form a polydopamine passivation film on the surface of carbon steel to block the corrosive medium. In addition, mussel adhesion protein Mefp-1 has also been used for the surface protection of 304 L stainless steel and aluminum, which can effectively delay the metal dissolution and pitting (Hansen et al., 1995; Hansen and McCafferty, 1996). Sababi et al. utilized the affinity of DOPA for metal ions and deposited a nanocomposite film composed of Mefp-1 and CeO2 nanoparticles on carbon steel to provide great long-term corrosion protection (Sababi et al., 2012). Chen et al. found that the Mefp-1/CeO2 composite film had a self-healing capacity on carbon steel (Chen et al., 2016). The Fe3+ released by corrosion on the steel surface could be captured by the passivation film and continuously form new DOPA-Fe3+ complexes, as shown in Figure 5c. Therefore, the corrosion area could be covered by the new complex, thereby delaying further dissolution and achieving the self-healing effects. Zhang et al. further found that phosphate could synergize with Mefp-1/CeO2 composite films for corrosion protection (Zhang et al., 2013). They could form a double-layer protective film to prevent the invasion of corrosive media, which achieved the industrial anticorrosion application of Mefp-1/CeO2/phosphate composite film in reinforced concrete structures. Cheng et al. prepared a multifunctional Mefp-1/graphene composite film on the surface of carbon steel (Cheng et al., 2020). The anticorrosion effect of the carbon steel surface was enhanced due to the complexation between the corrosion products and Mefp-1. In addition, the physical adsorption between Mefp-1 and graphene enhanced the adhesion between graphene and the substrate, thereby improving the friction reduction and wear resistance of the carbon steel.
In addition to dissolving polydopamine corrosion inhibitors in solutions to exert their corrosion inhibition effects, dispersing them in an organic coating can also achieve great anticorrosion effects (Wang et al., 2025). Cheng et al. prepared polydopamine microcapsules loaded with the BTA corrosion inhibitor, and added the composites to epoxy resin for the corrosion protection of steel substrates (Cheng et al., 2021). BTA released from polydopamine microcapsules could form a passivation film on the steel surface to inhibit the anodic corrosion of the steel surface, as shown in Figure 5d. Moreover, polydopamine could also react with Fe3+ to form a complex and block the invasion of corrosive media. Long-term electrochemical tests proved that the coating had excellent anticorrosion effects. Shchukin et al. coated polydopamine on the surface of mesoporous silica loaded with BTA and added the composite as a filler to the alkyd resin (Qian et al., 2019). Polydopamine could not only control the release of BTA, but also reacted with corrosion products as a corrosion inhibitor to form a passivation film. The results showed that after immersion in salt water for 20 days, the composite coating exhibited excellent anticorrosion performance. Chen et al. prepared a composite (BTA@PDA) consisted of polydopamine (PDA) nanoparticles as the carrier and BTA as the released corrosion inhibitor (Chen et al., 2022a). Based on the pH-dependent changes in charge of amine groups and hydroxyl groups, they further regulated the pH-responsive behaviors of BTA@PDA by controllable group tailoring to enhance the corrosion inhibition effects in coatings. The scratched coatings exhibited an increased impedance modulus due to the dual corrosion inhibition effects of BTA and PDA, triggered by the corrosion-induced pH variation. Moreover, the impedance modulus of the coating maintained at 2.03 × 109 Ω‧cm2 after 21 days of immersion.
3.3 Research of polydopamine in graphene-based anticorrosion coatings
Due to the unique chemical properties of polydopamine, it is expected to make up for the defects of pure graphene-based anticorrosion coatings and accelerate the development and application of multifunctional graphene-based anticorrosion coatings. At present, the research on polydopamine modification in graphene-based anticorrosion coatings mainly includes the following four directions: enhancing the intrinsic barrier properties of graphene, enhancing the dispersibility of graphene in coatings, modifying graphene with other functional materials, and introducing pH-responsive properties to graphene.
3.3.1 Enhancing the intrinsic barrier properties of graphene
Based on the π-π interaction between polydopamine and graphene, polydopamine can fill the defects on the graphene film and thus enhance its barrier properties. Zheng et al. prepared a polydopamine-modified graphene composite film on a copper substrate to reduce the structural defects of the graphene film (Zheng et al., 2021). The work showed that the polydopamine could repair the conjugated structure of graphene through π-π interactions. Meanwhile, the contact between graphene and Cu substrate could be prevented by non-conductive polydopamine, and the galvanic corrosion between graphene and Cu was effectively inhibited. Hence, the synergistic effects of polydopamine-modified graphene effectively improved the anti-permeability performance of the graphene film, with a protection factor of up to 99%, as shown in Figure 6a. Ding et al. applied this strategy to graphene-based epoxy coatings (Ding et al., 2022). They alternately sprayed a polydopamine-modified graphene layer and an epoxy resin layer on the steel surface to obtain a graphene-based anticorrosion coating. In that composite coating, the polydopamine not only reduced the defects of the graphene layer and enhanced its barrier effect, but also bridged the graphene layer and the epoxy resin layer to form an “interlocking” structure. Electrochemical tests showed that the impedance modulus of the composite coating increased by about three orders of magnitude, representing the excellent anticorrosion performance. Chen et al. synthesized an activation-induced ultrathin graphene (UG-BP) with the homogeneous growth of polydopamine nanocontainers encapsulated with BTA (Chen et al., 2023). They found that the polydopamine could assemble on graphene by strong π−π stacking to fill the defects. The conclusion was further proved by the results of electron energy loss spectroscopy. With the structural evolution from the adsorption to the growth of polydopamine, the Iπ*/Iπ*+Iσ* value gradually increased, indicating that polydopamine could grow at the defect sites and enhance the sp2 structure of etched graphene. It meant that the growth of polydopamine on graphene could ensure the integrity of graphene flakes. Thus, the barrier effects of graphene in anticorrosion coatings could be improved by polydopamine, which was confirmed by the enhanced anticorrosion performance of the coating (4.21 × 109 Ω·cm2 over 60 days). These works showed the advantages of PDA modification in enhancing the barrier properties of graphene. The modification process was convenient, low-cost, and green, compared with other defect repair techniques. Therefore, repairing the conjugated structure of graphene by polydopamine was of great significance for the preparation of high-performance graphene-based anticorrosion coatings.
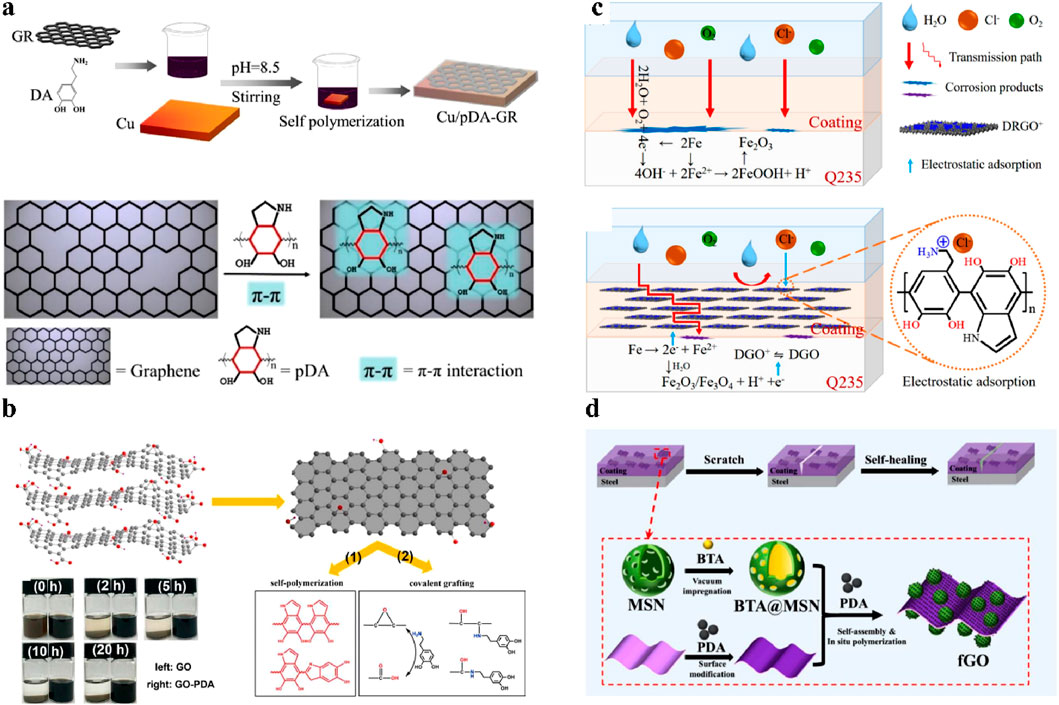
Figure 6. Applications of polydopamine in graphene-based anticorrosion coatings. (a) Methods and mechanisms for repairing structural defects in graphene by polydopamine (Zheng et al., 2021). (b) Preparation of polydopamine-modified graphene oxide and its dispersion stability (Cui et al., 2018). (c) Dispersion stability and oriented alignment of DRGO+ in coatings (Zhu et al., 2020). (d) Preparation of polydopamine-modified graphene composite filler loaded with SiO2 nanocontainers (Ma et al., 2021).
3.3.2 Enhancing the dispersibility of graphene in coatings
The stable dispersion of graphene in coatings is the key to exerting its excellent barrier properties. However, due to the interlayer interaction of graphene, its agglomeration problem in the coating matrix often limits the application of graphene-based anticorrosion coatings. Through polydopamine modification, graphene can obtain abundant functional groups such as hydroxyl and amine groups, so it can be well dispersed in water-based coatings and amine curing agents. Cui et al. enhanced the dispersibility of graphene oxide in waterborne epoxy coatings by introducing hydrophilic functional groups via the π-π interaction and covalent bonding between graphene oxide and polydopamine, as shown in Figure 6b (Cui et al., 2018). The results showed that well-dispersed polydopamine-modified graphene oxide significantly enhanced the anticorrosion performance of the coating. Zhao et al. prepared polydopamine-modified graphene oxide that could be stably dispersed in waterborne polyurethane (Zhao et al., 2019). The results showed that the addition of only 0.5 wt% of modified graphene filler could enhance the anticorrosion performance of waterborne polyurethane. Zhu et al. prepared cationic dopamine-reduced graphene oxide (DRGO+) through the self-polymerization and ionization reaction of dopamine (Zhu et al., 2020). The obtained DRGO+ could be stably dispersed in commercial water-based epoxy emulsion for more than 45 days without precipitation. Due to the presence of NH3+, DRGO+ could be arranged in parallel in the coating under an electric field. This parallel DRGO+ greatly improved the physical barrier properties of the coating via extending the penetration path of corrosive media, as shown in Figure 6c. Electrochemical impedance spectroscopy showed that when the DRGO+ content was 0.5 wt%, the impedance modulus of the coating was one order of magnitude higher than that of the pure epoxy coating, showing excellent anticorrosion performance.
3.3.3 Modifying graphene with other functional materials
The chemical inertness of graphene limits its ability to be composited with other functional materials, which restricts the development of multifunctional graphene-based coatings. Based on the excellent modification capacity of polydopamine, polydopamine-modified graphene composite fillers have been rapidly developed. Huang et al. prepared polydopamine-modified graphene oxide with hexagonal boron nitride (h-BN-rGO@PDA) nanofillers, and dispersed them into polyvinyl butyral coating (Huang et al., 2019). With the rigid h-BN nanosheets attached, h-BN-rGO@PDA nanohybrids became less folding and curling after functionalization, and reveals a superior physical barrier effect to prevent the penetration of corrosive media. Moreover, hexagonal boron nitride weakened the interlayer interaction of graphene oxide, resulting in the great dispersion of h-BN-rGO@PDA in the coating. The results showed that when the mass ratio of hexagonal boron nitride to graphene oxide was 1:1, the impedance modulus of the composite coating (2.07 × 107 Ω‧cm2) was two orders of magnitude higher than that of the blank coating (7.83×105 Ω‧cm2) during the 48 h immersion. Yang et al. modified polyaniline onto graphene oxide by polydopamine to enhance the anticorrosion performance of water-based alkyd varnish (Yang et al., 2019). Owing to the barrier effect of graphene oxide and the hydrophobicity of polyaniline, the composite coating showed excellent anticorrosion performance. Moreover, polydopamine further enhanced the compatibility between the fillers and the coating matrix. Therefore, the impedance modulus increased by more than two orders of magnitude. Zhang et al. prepared a superhydrophobic graphene-based anticorrosion coating based on the great modification capacity of polydopamine (Zhang et al., 2019). They synthesized SiO2 nanoparticles in situ on polydopamine-modified graphene and further modified the composite with silane. The superhydrophobic composite coating was prepared on the steel surface by electrostatic spraying with a contact angle up to 156.3° ± 1.5°. Owing to the uniform adsorption of SiO2 on polydopamine, a unique multi-layer nanostructure was formed on the coating surface, which could maintain its superhydrophobic properties even after being immersed in salt water for 60 days. Ma et al. modified mesoporous SiO2 nanocontainers uniformly onto graphene oxide via polydopamine modification, and loaded BTA in the nanocontainers, as shown in Figure 6d (Ma et al., 2021). The obtained graphene-based epoxy coating showed effective self-healing properties. After immersion in NaCl solution for 30 days, the impedance modulus of the composite coating was two orders of magnitude higher than that of the blank coating.
3.3.4 Introducing pH-responsive properties to graphene
In graphene-based self-healing anticorrosion coatings, the encapsulation and release of corrosion inhibitors by nanocontainers is the key to achieving self-healing properties of the coating. Polydopamine with sensitive pH responsiveness is expected to be used as a switch to achieve smart release of corrosion inhibitors triggered by the corrosion-induced pH variation. Habibiyan et al. prepared polydopamine-modified graphene to capture Zn2+ and added the fillers to epoxy resin (Habibiyan et al., 2020). When corrosion occurred in the damaged area of the coating, polydopamine was able to detect pH variation and release Zn2+. The Zn2+ formed zinc hydroxide compounds in the cathode area, which could reduce the corrosion current on the steel surface. Chen et al. prepared BTA-loaded halloysite nanotubes encapsulated by polydopamine, which were assembled on graphene oxide via π-π interactions (Chen G. et al., 2020). The polydopamine used for encapsulation could be destroyed in an acidic environment to release the BTA in the nanotubes. The results showed that the water-based epoxy resin with the addition of the fillers exhibited excellent barrier properties and self-healing properties. Cheng et al. prepared polydopamine as pH-responsive microcapsules and assembled them on graphene oxide to encapsulate the BTA corrosion inhibitor, as shown in Figure 7a (Cheng et al., 2022). The fillers not only enhanced the barrier properties of the epoxy resin, but also introduced the self-healing properties to the composite coating. The polydopamine could respond quickly to local corrosion areas and released BTA. Even after immersion for 60 days, the impedance modulus of the composite coating remained above 108 Ω‧cm2. Chen et al. prepared a self-healing coating with the enhanced loading of inhibitors enabled by ultra-highly exfoliated graphene and polydopamine modification (Chen et al., 2022b). The work showed that the polydopamine effectively promoted the adsorption of BTA on graphene. The smart self-healing behavior of the coating was triggered by the polydopamine decomposition from corrosion-induced pH variations and the release of BTA. Thus, the coating exhibited an increased impedance modulus (3.31 × 1010 Ω‧cm2) even after 30 days of immersion, owing to the outstanding barrier effect of the ultra-highly exfoliated graphene and the corrosion inhibition effect of PDA/BTA.
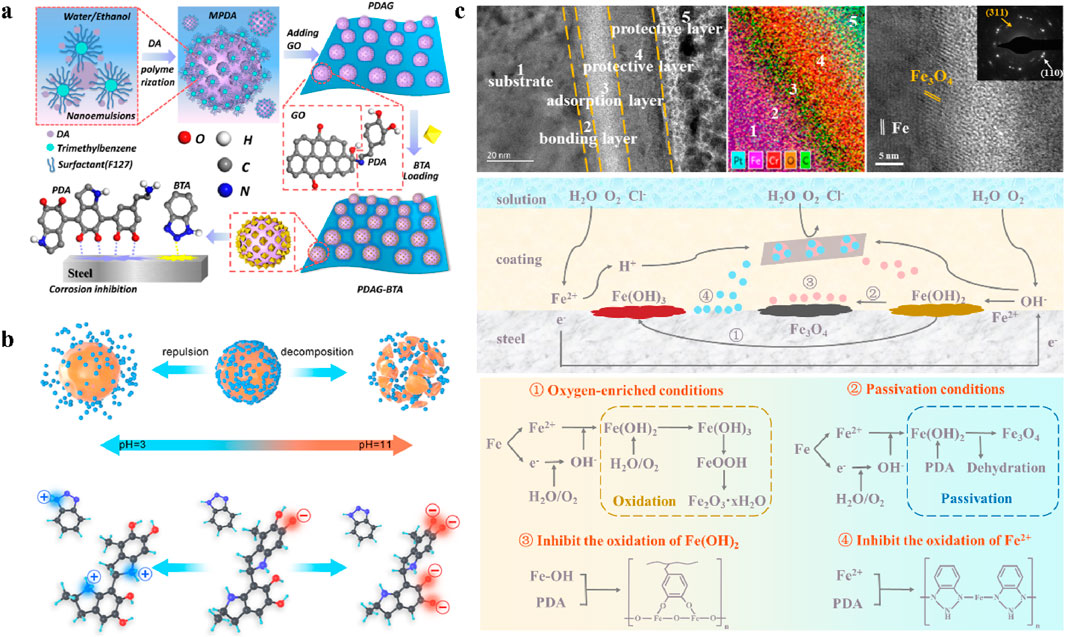
Figure 7. Applications of polydopamine in graphene-based self-healing anticorrosion coatings. (a) Preparation of graphene nanofillers loaded with polydopamine microcapsules (Cheng et al., 2022). (b) Different release mechanisms of PDA encapsulated with BTA in acidic and alkaline environments (Chen et al., 2022a). (c) Microanalysis of the corrosion interface and schematic of anticorrosion mechanism for polydopamine-modified graphene in self-healing anticorrosion coatings (Chen et al., 2023).
The smart pH-responsive mechanism and the interfacial anticorrosion mechanism of polydopamine in self-healing anticorrosion coatings have been revealed in detail. The amine and hydroxyl groups of the polydopamine-modified filler exhibits different charging properties at different pH environments, as shown in Figure 7b. Chen et al. further investigated the intermolecular interaction of polydopamine and encapsulated BTA by density functional theory (Chen et al., 2023). The electrostatic potential map of polydopamine and encapsulated BTA exhibits the ability to get and give electrons in different regions, indicating the pH-sensitive properties in polydopamine-modified fillers. In acidic solutions, both polydopamine and encapsulated BTA are positively charged, so the release process of BTA is mainly driven by electrostatic repulsion. In alkaline solutions, the negatively charged hydroxyl groups will break down polydopamine into oligomers, and release BTA and polydopamine by weakening their π-π interaction. The released polydopamine and BTA can be adsorbed on the corrosion areas of the coating to react with corrosion products. The released polydopamine can react with the interfacial oxides, as shown in Figure 7c. A dehydration process between C−OH of polydopamine and Fe−OH of Fe(OH)2 occurs by the formation of Fe−O−C, which promotes the transformation of the corrosion product from Fe(OH)2 to Fe3O4 (Chen C. et al., 2020). Thus, polydopamine contributes to the inhibition of the formation of Fe2O3-xH2O by depleting Fe−OH of iron oxides. Moreover, polydopamine can form a complex with the interfacial Fe2+, which inhibits the oxidation of Fe2+. Therefore, polydopamine can introduce pH-responsive properties to graphene fillers along with the effective interfacial anticorrosion effects, which is important for the construction of smart self-healing graphene-based anticorrosion coatings.
4 Conclusion and perspectives
Graphene as the anticorrosion filler has the advantages of great barrier properties, light weight and environmental friendliness. Graphene-based anticorrosion coatings have developed as the ideal upgraded alternative for traditional anticorrosion coatings. In order to enhance the long-term anticorrosion performance of graphene-based anticorrosion coatings, the development of self-healing anticorrosion coatings is an effective way, which has important theoretical and application value. In addition, polydopamine has been widely used for chemical modification of graphene due to its excellent modification capacity, pH-responsive properties, and corrosion inhibition effects. Polydopamine-modified graphene nanofillers have shown the advantages in anticorrosion coatings. In particular, a research foundation has been established for the enhancement of graphene dispersion and the introduction of smart pH-responsive properties. This review systematically summarizes the current research progress and application of graphene and polydopamine-modified graphene in the field of anticorrosion coatings. Firstly, the barrier properties of graphene are briefly described. Then, the applications and current problems of graphene in anticorrosion coatings are systematically reviewed, especially in self-healing anticorrosion coatings. Next, the properties and the applications of polydopamine and its derivatives in anticorrosion are discussed. Finally, the applications and the possible anticorrosion mechanism of polydopamine in graphene-based anticorrosion coatings are analyzed.
Despite the remarkable progress in the application of graphene-based anticorrosion coatings, some issues still need to be resolved, which are described as follows:
(1) The microstructure of graphene and the arrangement of graphene in the coating have always been the focus of research. Current commercial graphene fillers are synthesized by different methods and usually have different flake structures. And the microstructure and arrangement of graphene directly affects its barrier properties in coatings. Researchers have investigated the effects of graphene’s microstructure or specific surface area on its barrier properties and the loading content of corrosion inhibitors. In addition, some works have attempted to modulate the arrangement of graphene in the coating through the coating process or an applied electric field. However, there is a lack of systematic research on related works, and the intrinsic mechanism remains unrevealed. Therefore, the systematic research of the optimal preparation method of graphene with ideal microstructure and arrangement is of great significance to enhance the anticorrosion performance of graphene-based anticorrosion coatings.
(2) Substantial research efforts are still required to precisely modulate and optimize the pH-responsive properties of self-healing anticorrosion coatings. The pH-responsive behavior of coatings is closely related to their self-healing properties. Although some research works have been carried out on the pH-responsive properties of polydopamine-modified graphene, the research is limited to the verification of its pH-responsive behavior. In those works, researchers can only passively accept the results of the pH-responsive behavior without mastering the optimization strategy. Some researchers have investigated the charge evolution patterns of polydopamine-modified nanofillers in different pH environments to enable the regulation of their pH-responsive behaviors. However, these related works are still at the research stage of the design of composite fillers. There is still a long way to go to bridge the pH-responsive properties of fillers with the self-healing properties of coatings.
(3) Further systematic investigations are imperative to achieve the quantitative correlation between the loading content of corrosion inhibitors and the anticorrosion performance of the coating. Corrosion inhibitors are fundamental to the self-healing properties of anticorrosion coatings. Therefore, how many corrosion inhibitors need to be encapsulated in the filler? How long does it take for the corrosion inhibitors to be consumed? These are questions that researchers need to think about at the research stage of the design of composite fillers. However, there is a lack of mathematical models to guide the design of fillers. Therefore, the simulation calculation related to the dosage and depletion of corrosion inhibitors will be an important research direction in the future, which will promote the design of optimal fillers and the development of high-performance anticorrosion coatings.
Although numerous efforts are still needed to promote the wide applications of graphene-based anticorrosion coatings, the requirements for sustainable equipment development in harsh corrosive environments are expected to accelerate the utilization of graphene-based anticorrosion coatings from the laboratory to the market. We hope that this review will stimulate further interest in graphene-based materials for the development of next-generation anticorrosion coatings.
Author contributions
GC: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review and editing. ZX: Writing – review and editing, Investigation. YC: Investigation, Writing – review and editing. WZ: Writing – original draft, Writing – review and editing. YX: Writing – original draft, Writing – review and editing. FX: Writing – original draft, Writing – review and editing. YH: Supervision, Writing - review and editing, Methodology. BJ: Funding acquisition, Investigation, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no. 523B2046), and the open research fund of Beijing National Laboratory for Condensed Matter Physics (grant no. 2024BNLCMPKF021).
Conflict of interest
Authors GC, WZ, YX, FX and ZX were employed by CCTEG Beijing Tianma Intelligent Control Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, T., Yu, J., Estrada, A., Hammer, M., Waite, J., and Israelachvili, J. (2010). The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv. Funct. Mat. 20, 4196–4205. doi:10.1002/adfm.201000932
Barclay, T., Hegab, H., Clarke, S., and Ginic-Markovic, M. (2017). Versatile surface modification using polydopamine and related polycatecholamines: chemistry, structure, and applications. Adv. Mat. Inter. 4, 1601192. doi:10.1002/admi.201601192
Becherer, T., Nascimento, M., Sindram, J., Noeske, P., Wei, Q., Haag, R., et al. (2015). Fast and easily applicable glycerol-based spray coating. J. Colloid Interf. Sci. 87, 146–154. doi:10.1016/j.porgcoat.2015.05.003
Berry, V. (2013). Impermeability of graphene and its applications. Carbon 62, 1–10. doi:10.1016/j.carbon.2013.05.052
Borisova, D., Mohwald, H., and Shchukin, D. (2011). Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 5, 1939–1946. doi:10.1021/nn102871v
Cai, W., Wang, J., Quan, X., Zhao, S., and Wang, Z. (2018). Antifouling and anticorrosion properties of one-pot synthesized dedoped bromo-substituted polyaniline and its composite coatings. Surf. Coat. Tech. 334, 7–18. doi:10.1016/j.surfcoat.2017.10.076
Cao, K., Yu, Z., Yin, D., Chen, L., Jiang, Y., and Zhu, L. (2020). Fabrication of BTA-MOF-TEOS-GO nanocomposite to endow coating systems with active inhibition and durable anticorrosion performances. Prog. Org. Coat. 143, 105629. doi:10.1016/j.porgcoat.2020.105629
Chen, C., Qiu, S., Cui, M., Qin, S., Yan, G., Zhao, H., et al. (2017). Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 114, 356–366. doi:10.1016/j.carbon.2016.12.044
Chen, C., Xiao, G., He, Y., Zhong, F., Li, H., Wu, Y., et al. (2020b). Bio-inspired superior barrier self-healing coating: self-assemble of graphene oxide and polydopamine-coated halloysite nanotubes for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 139, 105402. doi:10.1016/j.porgcoat.2019.105402
Chen, C., Zhang, F., Lin, C., and Pan, J. (2016). Corrosion protection and self-healing of a nanocomposite film of mussel adhesive protein and CeO2 nanoparticles on carbon steel. J. Electrochem. Soc. 163, C545–C552. doi:10.1149/2.0521609jes
Chen, G., Jin, B., Li, Y., He, Y., and Luo, J. (2022b). A smart healable anticorrosion coating with enhanced loading of benzotriazole enabled by ultra-highly exfoliated graphene and mussel-inspired chemistry. Carbon 187, 439–450. doi:10.1016/j.carbon.2021.11.048
Chen, G., Jin, B., Li, Y., Zhang, Z., He, Y., and Luo, J. (2022a). Controllable group tailoring enables enhanced pH-responsive behaviors of polydopamine delivery system in smart self-healing anticorrosion coatings. Prog. Org. Coat. 170, 106989. doi:10.1016/j.porgcoat.2022.106989
Chen, G., Jin, B., Zhang, Z., Zhao, J., Li, Y., He, Y., et al. (2023). Engineering active-site-induced homogeneous growth of polydopamine nanocontainers on loading-enhanced ultrathin graphene for smart self-healing anticorrosion coatings. ACS Appl. Mat. Inter. 15, 23679–23689. doi:10.1021/acsami.3c03276
Chen, G., Zhao, J., Chen, K., Liu, S., Zhang, M., He, Y., et al. (2020a). Ultrastable lubricating properties of robust self-repairing tribofilms enabled by in situ-assembled polydopamine nanoparticles. Langmuir 36, 852–861. doi:10.1021/acs.langmuir.9b03214
Chen, S., Brown, L., Levendorf, M., Cai, W., Ju, S., Edgeworth, J., et al. (2011). Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 5, 1321–1327. doi:10.1021/nn103028d
Cheng, J., Chen, S., Zhang, F., Shen, B., Lu, X., and Pan, J. (2020). Corrosion- and wear-resistant composite film of graphene and mussel adhesive proteins on carbon steel. Corros. Sci. 164, 108351. doi:10.1016/j.corsci.2019.108351
Cheng, L., Liu, C., Wu, H., Zhao, H., Mao, F., and Wang, L. (2021). A mussel-inspired delivery system for enhancing self-healing property of epoxy coatings. J. Mat. Sci. Technol. 80, 36–49. doi:10.1016/j.jmst.2020.10.075
Cheng, L., Liu, C., Wu, H., Zhao, H., and Wang, L. (2022). Interfacial assembled mesoporous polydopamine nanoparticles reduced graphene oxide for high performance of waterborne epoxy-based anticorrosive coatings. J. Colloid Interf. Sci. 606, 1572–1585. doi:10.1016/j.jcis.2021.08.150
Cho, S., White, S., and Braun, P. (2009). Self-healing polymer coatings. Adv. Mat. 21, 645–649. doi:10.1002/adma.200802008
Cui, C., Lim, A., and Huang, J. (2017). A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 12, 834–835. doi:10.1038/nnano.2017.187
Cui, G., Bi, Z., Wang, S., Liu, J., Xing, X., Li, Z., et al. (2020). A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 148, 105821. doi:10.1016/j.porgcoat.2020.105821
Cui, G., Bi, Z., Zhang, R., Liu, J., Yu, X., and Li, Z. (2019). A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 373, 104–121. doi:10.1016/j.cej.2019.05.034
Cui, G., Zhang, C., Wang, A., Zhou, X., Liu, J., Li, Z., et al. (2021). Research progress on self-healing polymer/graphene anticorrosion coatings. Prog. Org. Coat. 155, 106231. doi:10.1016/j.porgcoat.2021.106231
Cui, M., Chen, X., Mei, S., and Ren, S. (2023). Bioinspired polydopamine nanosheets for the enhancement in anti-corrosion performance of water-borne epoxy coatings. Chem. Eng. J. 471, 144760. doi:10.1016/j.cej.2023.144760
Cui, M., Ren, S., Zhao, H., Xue, Q., and Wang, L. (2018). Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 335, 255–266. doi:10.1016/j.cej.2017.10.172
Ding, J., Zhao, H., Xu, B., Zhao, X., Su, S., and Yu, H. (2019). Superanticorrosive graphene nanosheets through π deposition of boron nitride nanodots. ACS Sustain. Chem. Eng. 7, 10900–10911. doi:10.1021/acssuschemeng.9b01796
Ding, J., Zhao, H., and Yu, H. (2022). Bio-inspired multifunctional graphene-epoxy anticorrosion coatings by low-defect engineered graphene. ACS Nano 16, 710–720. doi:10.1021/acsnano.1c08228
Ding, J., Zhao, H., Zhou, M., Liu, P., and Yu, H. (2021). Super-anticorrosive inverse nacre-like graphene-epoxy composite coating. Carbon 181, 204–211. doi:10.1016/j.carbon.2021.05.017
Dong, R., and Liu, L. (2016). Preparation and properties of acrylic resin coating modified by functional graphene oxide. Appl. Surf. Sci. 368, 378–387. doi:10.1016/j.apsusc.2016.01.275
Dreyer, D., Miller, D., Freeman, B., Paul, D., and Bielawski, C. (2013). Perspectives on poly(dopamine). Chem. Sci. 4, 3796. doi:10.1039/c3sc51501j
Fang, Y., Wang, D., Jing, X., and Xue, B. (2015). Synthesis and characterization of fluorinated organic-inorganic hybrid coatings on tinplate. J. Appl. Polym. Sci. 132, 42428. doi:10.1002/app.42428
Geim, A. (2009). Graphene: status and prospects. Science 324, 1530–1534. doi:10.1126/science.1158877
Habibiyan, A., Ramezanzadeh, B., Mahdavian, M., Bahlakeh, G., and Kasaeian, M. (2020). Rational assembly of mussel-inspired polydopamine (PDA)-Zn (II) complex nanospheres on graphene oxide framework tailored for robust self-healing anti-corrosion coatings application. Chem. Eng. J. 391, 123630. doi:10.1016/j.cej.2019.123630
Han, S., Wu, D., Li, S., Zhang, F., and Feng, X. (2014). Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv. Mat. 26, 849–864. doi:10.1002/adma.201303115
Hansen, D., Dexter, S., and Waite, J. (1995). The inhibition of corrosion of S30403 stainless-steel by a naturally-occurring catecholic polymer. Corros. Sci. 37, 1423–1441. doi:10.1016/0010-938X(95)00050-T
Hansen, D., and McCafferty, E. (1996). The effect of various naturally occurring metal-binding compounds on the electrochemical behavior of aluminum. J. Electrochem. Soc. 143, 114–119. doi:10.1149/1.1836394
Hao, Y., Zhao, Y., Li, B., Song, L., and Guo, Z. (2020). Self-healing effect of graphene@PANI loaded with benzotriazole for carbon steel. Corros. Sci. 163, 108246. doi:10.1016/j.corsci.2019.108246
Hasani, M., Mahdavian, M., Yari, H., and Ramezanzadeh, B. (2018). Versatile protection of exterior coatings by the aid of graphene oxide nano-sheets; comparison with conventional UV absorbers. Prog. Org. Coat. 116, 90–101. doi:10.1016/j.porgcoat.2017.11.020
Hong, S., Na, Y., Choi, S., Song, I., Kim, W., and Lee, H. (2012). Non-covalent self-assembly and covalent polymerization Co-contribute to polydopamine formation. Adv. Funct. Mat. 22, 4711–4717. doi:10.1002/adfm.201201156
Hsieh, Y., Hofmann, M., Chang, K., Jhu, J., Li, Y., Chen, K., et al. (2014). Complete corrosion inhibition through graphene defect passivation. ACS Nano 8, 443–448. doi:10.1021/nn404756q
Huang, H., Huang, X., Xie, Y., Tian, Y., Jiang, X., and Zhang, X. (2019). Fabrication of h-BN-rGO@PDA nanohybrids for composite coatings with enhanced anticorrosion performance. Prog. Org. Coat. 130, 124–131. doi:10.1016/j.porgcoat.2019.01.059
Jia, Y., Qiu, T., Guo, L., Ye, J., He, L., and Li, X. (2020). Preparation of pH responsive smart nanocontainer via inclusion of inhibitor in graphene/halloysite nanotubes and its application in intelligent anticorrosion protection. Appl. Surf. Sci. 504, 144496. doi:10.1016/j.apsusc.2019.144496
Jin, B., Chen, G., He, Y., Zhang, C., and Luo, J. (2023). Lubrication properties of graphene under harsh working conditions. Mat. Today Adv. 18, 100369. doi:10.1016/j.mtadv.2023.100369
Jin, B., Chen, G., Zhao, J., He, Y., Li, Y., and Luo, J. (2022a). Coupling effect of boundary tribofilm and hydrodynamic film. Cell. Rep. Phys. Sci. 3, 100778. doi:10.1016/j.xcrp.2022.100778
Jin, B., Du, N., Meng, T., Chen, G., Cao, Y., Zhang, H., et al. (2025). High contact stress phase transition structural superlubricity. Carbon 238, 120306. doi:10.1016/j.carbon.2025.120306
Jin, B., Zhang, H., Chen, G., Meng, T., Zhao, J., Zhang, M., et al. (2024). Phase transition structural superlubricity. Matter 7, 3107–3125. doi:10.1016/j.matt.2024.04.044
Jin, B., Zhao, J., Chen, G., He, Y., Huang, Y., and Luo, J. (2021a). In situ synthesis of Mn3O4/graphene nanocomposite and its application as a lubrication additive at high temperatures. Appl. Surf. Sci. 546, 149019. doi:10.1016/j.apsusc.2021.149019
Jin, B., Zhao, J., He, Y., Chen, G., Li, Y., Zhang, C., et al. (2022b). High-quality ultra-flat reduced graphene oxide nanosheets with super-robust lubrication performances. Chem. Eng. J. 438, 135620. doi:10.1016/j.cej.2022.135620
Jin, Z., Yang, L., Shi, S., Wang, T., Duan, G., Liu, X., et al. (2021b). Flexible polydopamine bioelectronics. Adv. Funct. Mat. 31, 2103391. doi:10.1002/adfm.202103391
Kang, S., Hwang, N., Yeom, J., Park, S., Messersmith, P., Choi, I., et al. (2012). One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mat. 22, 2949–2955. doi:10.1002/adfm.201200177
Kang, S., You, I., Cho, W., Shon, H., Lee, T., Choi, I., et al. (2010). One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 49, 9401–9404. doi:10.1002/anie.201004693
Kasaeian, M., Ghasemi, E., Ramezanzadeh, B., Mahdavian, M., and Bahlakeh, G. (2018). Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-Benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM). Corros. Sci. 145, 119–134. doi:10.1016/j.corsci.2018.09.023
Lee, H., Dellatore, S., Miller, W., and Messersmith, P. (2007). Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430. doi:10.1126/science.1147241
Lee, H., Ma, Y., Zhou, F., Hong, S., and Lee, H. (2019). Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 52, 704–713. doi:10.1021/acs.accounts.8b00583
Lee, H., Scherer, N., and Messersmith, P. (2006). Single-molecule mechanics of mussel adhesion. PANS 103, 12999–13003. doi:10.1073/pnas.0605552103
Li, B., Xue, S., Mu, P., and Li, J. (2022). Robust self-healing graphene oxide-based superhydrophobic coatings for efficient corrosion protection of magnesium alloys. ACS Appl. Mat. Inter. 14, 30192–30204. doi:10.1021/acsami.2c06447
Li, G., Schenderlein, M., Men, Y., Möhwald, H., and Shchukin, D. (2014). Monodisperse polymeric core-shell nanocontainers for organic self-healing anticorrosion coatings. Adv. Mat. Interfaces 1, 1300019. doi:10.1002/admi.201300019
Li, H., Qiang, Y., Zhao, W., and Zhang, S. (2021). 2-Mercaptobenzimidazole-inbuilt metal-organic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating. Corros. Sci. 191, 109715. doi:10.1016/j.corsci.2021.109715
Li, L., Yan, B., Yang, J., Chen, L., and Zeng, H. (2015). Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mat. 27, 1294–1299. doi:10.1002/adma.201405166
Li, M., Zhang, B., Chen, L., Kong, J., Wang, Y., and Ba, M. (2023). Spontaneous directed leaching of slippery lubricant in self-stratified marine antifouling coatings for nonporous surfaces. Prog. Org. Coat. 184, 107858. doi:10.1016/j.porgcoat.2023.107858
Li, W., Shang, T., Yang, W., Yang, H., Lin, S., Jia, X., et al. (2016). Effectively exerting the reinforcement of dopamine reduced graphene oxide on epoxy-based composites via strengthened interfacial bonding. ACS Appl. Mat. Inter. 8, 13037–13050. doi:10.1021/acsami.6b02496
Li, X., and Zhou, S. (2022). Epoxy-functionalized Ti3C2 nanosheet for epoxy coatings with prominent anticorrosion performance. Prog. Org. Coat. 162, 106559. doi:10.1016/j.porgcoat.2021.106559
Lin, Q., Gourdon, D., Sun, C., Holten, A., Anderson, T., Waite, J., et al. (2007). Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. PANS 104, 3782–3786. doi:10.1073/pnas.0607852104
Liu, C., Li, J., Jin, Z., Hou, P., Zhao, H., and Wang, L. (2019). Synthesis of graphene-epoxy nanocomposites with the capability to self-heal underwater for materials protection. Compos. Commun. 15, 155–161. doi:10.1016/j.coco.2019.07.011
Liu, C., Zhao, H., Hou, P., Qian, B., Wang, X., Guo, C., et al. (2018). Efficient graphene/cyclodextrin-based nanocontainer: synthesis and host-guest inclusion for self-healing anticorrosion application. ACS Appl. Mat. Inter. 10, 36229–36239. doi:10.1021/acsami.8b11108
Liu, X., Chen, S., Zhang, Y., Liu, M., Emori, W., and Shao, Y. (2021). Preparation of graphene oxide-boron nitride hybrid to reinforce the corrosion protection coating. Corros. Rev. 39, 123–136. doi:10.1515/corrrev-2020-0051
Liu, X., Yue, T., Qi, K., Xia, B., Chen, Z., Qiu, Y., et al. (2020). Probe into metal-organic framework membranes fabricated via versatile polydopamine-assisted approach onto metal surfaces as anticorrosion coatings. Corros. Sci. 177, 108949. doi:10.1016/j.corsci.2020.108949
Ma, Y., Huang, H., Zhou, H., Graham, M., Smith, J., Sheng, X., et al. (2021). Superior anti-corrosion and self-healing bi-functional polymer composite coatings with polydopamine modified mesoporous silica/graphene oxide. J. Mat. Sci. Technol. 95, 95–104. doi:10.1016/j.jmst.2021.04.019
Ma, Y., Zhang, Y., Liu, J., Ge, Y., Yan, X., Sun, Y., et al. (2020). GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating. Mat. Des. 189, 108547. doi:10.1016/j.matdes.2020.108547
Maier, G., Rapp, M., Waite, J., Israelachvili, J., and Butler, A. (2015). Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 349, 628–632. doi:10.1126/science.aab0556
Mustehsin, A., Shi, H., Sharjeel, A., Song, Y., Liu, F., Han, E., et al. (2024). A bi-functional self-healing epoxy composite coating based on coordinated functionalized attapulgite/graphene oxide. Appl. Surf. Sci. 677, 161015. doi:10.1016/j.apsusc.2024.161015
Nazari, M., Zhang, Y., Mahmoodi, A., Xu, G., Yu, J., Wu, J., et al. (2022). Nanocomposite organic coatings for corrosion protection of metals: a review of recent advances. Prog. Org. Coat. 162, 106573. doi:10.1016/j.porgcoat.2021.106573
Novoselov, K., Geim, A., Morozov, S., Jiang, D., Zhang, Y., Dubonos, S., et al. (2004). Electric field effect in atomically thin carbon films. Science 306, 666–669. doi:10.1126/science.1102896
Ollik, K., and Lieder, M. (2020). Review of the application of graphene-based coatings as anticorrosion layers. Coatings 10, 883. doi:10.3390/coatings10090883
Pan, C., He, J., Zhu, J., Li, S., Li, W., Yang, W., et al. (2024). Corrosion control by carbon-based nanomaterials: a review. ACS Appl. Nano Mat. 7, 2515–2528. doi:10.1021/acsanm.3c05547
Qian, B., Zheng, Z., Michailids, M., Fleck, N., Bilton, M., Song, Y., et al. (2019). Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection. ACS Appl. Mat. Inter. 11, 10283–10291. doi:10.1021/acsami.8b21197
Qiang, Y., Li, H., and Lan, X. (2020). Self-assembling anchored film basing on two tetrazole derivatives for application to protect copper in sulfuric acid environment. J. Mat. Sci. Technol. 52, 63–71. doi:10.1016/j.jmst.2020.04.005
Qiu, S., Li, W., Zheng, W., Zhao, H., and Wang, L. (2017). Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl Solution. ACS Appl. Mat. Inter. 9, 34294–34304. doi:10.1021/acsami.7b08325
Qiu, W., Yang, H., and Xu, Z. (2018). Dopamine-assisted co-deposition: an emerging and promising strategy for surface modification. Adv. Colloid Interfac. 256, 111–125. doi:10.1016/j.cis.2018.04.011
Qureshi, D., Goffredo, S., Kim, Y., Han, Y., Guo, M., Ryu, S., et al. (2021). Why mussel byssal plaques are tiny yet strong in attachment. Matter 5, 710–724. doi:10.1016/j.matt.2021.12.001
Ren, S., Cui, M., Li, W., Pu, J., Xue, Q., and Wang, L. (2018). N-doping of graphene: toward long-term corrosion protection of Cu. J. Mat. Chem. A 6, 24136–24148. doi:10.1039/c8ta05421e
Ryu, J., Messersmith, P., and Lee, H. (2018). Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mat. Inter. 10, 7523–7540. doi:10.1021/acsami.7b19865
Sababi, M., Zhang, F., Krivosheeva, O., Forslund, M., Pan, J., Claesson, P., et al. (2012). Thin composite films of mussel adhesive proteins and ceria nanoparticles on carbon steel for corrosion protection. J. Electrochem. Soc. 159, C364–C371. doi:10.1149/2.061208jes
Schriver, M., Regan, W., Gannett, W., Zaniewski, A., Crommie, M., and Zettl, A. (2013). Graphene as a long-term metal oxidation barrier: worse than nothing. ACS Nano 7, 5763–5768. doi:10.1021/nn4014356
Shchukin, D., and Mohwald, H. (2013). A coat of many functions. Science 341, 1458–1459. doi:10.1126/science.1242895
Shin, M., Park, Y., Jin, S., Jung, Y., and Cha, h. (2021). Two faces of amine–catechol pair synergy in underwater cation−π interactions. Chem. Mat. 33, 3196–3206. doi:10.1021/acs.chemmater.1c00079
Song, Y., Wang, X., Tang, J., and Li, G. (2024). Dynamic polymer/metal-organic framework hybrid microcapsules for self-healing anticorrosion coatings. ACS Appl. Mat. Interfaces 16, 68478–68486. doi:10.1021/acsami.4c16670
Stoot, A., Camilli, L., Spiegelhauer, S., Yu, F., and Boggild, P. (2015). Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 293, 846–851. doi:10.1016/j.jpowsour.2015.06.009
Tong, Y., Bohm, S., and Song, M. (2017). The capability of graphene on improving the electrical conductivity and anti-corrosion properties of Polyurethane coatings. Appl. Surf. Sci. 424, 72–81. doi:10.1016/j.apsusc.2017.02.081
Uzoma, P., Liu, F., Xu, L., Zhang, Z., Han, E., Ke, W., et al. (2019). Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 127, 239–251. doi:10.1016/j.porgcoat.2018.11.018
Vesely, D., Kalendova, A., and Kalenda, P. (2010). A study of diatomite and calcined kaoline properties in anticorrosion protective coatings. Prog. Org. Coat. 68, 173–179. doi:10.1016/j.porgcoat.2010.02.007
Wang, J., Jin, X., Li, C., Wang, W., Wu, H., and Guo, S. (2019). Graphene and graphene derivatives toughening polymers: toward high toughness and strength. Chem. Eng. J. 370, 831–854. doi:10.1016/j.cej.2019.03.229
Wang, L., Zang, J., Liu, T., Sun, F., Wang, Q., Wang, Y., et al. (2025). An active self-healing and UV-resistant anticorrosion coating based on loading-enhanced nanocontainers tailored for Q235 carbon steel protection. Prog. Org. Coat. 201, 109128. doi:10.1016/j.porgcoat.2025.109128
Wu, D., Su, Q., Chen, L., Cui, H., Zhao, Z., Wu, Y., et al. (2022). Achieving high anti-wear and corrosion protection performance of phenoxy-resin coatings based on reinforcing with functional graphene oxide. Appl. Surf. Sci. 601, 154156. doi:10.1016/j.apsusc.2022.154156
Wu, W., Zhao, G., Chu, L., Wu, J., Miao, K., Shen, L., et al. (2024). Janus GO/BTA/PMMA microcapsules for biobased self-healing anticorrosion coatings with ultrahigh performance. ACS Appl. Mat. Interfaces 16, 53033–53041. doi:10.1021/acsami.4c13505
Wu, Y., Zhao, W., Qiang, Y., Chen, Z., Wang, L., Gao, X., et al. (2020). π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: synthesis and anti-corrosion application. Carbon 159, 292–302. doi:10.1016/j.carbon.2019.12.047
Yan, H., Fan, X., Cai, M., Song, S., and Zhu, M. (2021). Amino-functionalized Ti3C2Tx loading ZIF-8 nanocontainer@benzotriazole as multifunctional composite filler towards self-healing epoxy coating. J. Colloid Interf. Sci. 602, 131–145. doi:10.1016/j.jcis.2021.06.004
Yang, N., Yang, T., Wang, W., Chen, H., and Li, W. (2019). Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 377, 142–151. doi:10.1016/j.jhazmat.2019.05.063
Ye, Q., Zhou, F., and Liu, W. (2011). Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 40, 4244. doi:10.1039/c1cs15026j
Ye, Y., Chen, H., Zou, Y., Ye, Y., and Zhao, H. (2020). Corrosion protective mechanism of smart graphene-based self-healing coating on carbon steel. Corros. Sci. 174, 108825. doi:10.1016/j.corsci.2020.108825
Ye, Y., Chen, H., Zou, Y., and Zhao, H. (2021). Study on self-healing and corrosion resistance behaviors of functionalized carbon dot-intercalated graphene-based waterborne epoxy coating. J. Mat. Sci. Technol. 67, 226–236. doi:10.1016/j.jmst.2020.06.023
Ye, Y., Zhang, D., Li, J., Liu, T., Pu, J., Zhao, H., et al. (2019). One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 147, 9–21. doi:10.1016/j.corsci.2018.10.034
Yu, B., Liu, J., Liu, S., and Zhou, F. (2010). Pdop layer exhibiting zwitterionicity: a simple electrochemical interface for governing ion permeability. Chem. Commun. 46, 5900. doi:10.1039/c0cc00596g
Yu, J., An, R., Ren, J., Wang, P., Hu, H., and Liu, S. (2023). Hydrophobic and anticorrosive properties of secondary doped polyaniline-modified basalt flake composite epoxy coatings. China Surf. Eng. 37, 364–376. doi:10.11933/j.issn.1007-9289.20231217001
Zhan, Y., Wan, X., He, S., Yang, Q., and He, Y. (2018). Design of durable and efficient poly(arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 333, 132–145. doi:10.1016/j.cej.2017.09.147
Zhang, C., Li, W., Liu, C., Zhang, C., Cao, L., Kong, D., et al. (2022). Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid Interf. Sci. 608, 1025–1039. doi:10.1016/j.jcis.2021.10.024
Zhang, F., Brinck, T., Brandner, B., Claesson, P., Dedinaite, A., and Pan, J. (2013). In situ confocal Raman micro-spectroscopy and electrochemical studies of mussel adhesive protein and ceria composite film on carbon steel in salt solutions. Electrochim. Acta 107, 276–291. doi:10.1016/j.electacta.2013.05.078
Zhang, F., Ju, P., Pan, M., Zhang, D., Huang, Y., Li, G., et al. (2018a). Self-healing mechanisms in smart protective coatings: a review. Corros. Sci. 144, 74–88. doi:10.1016/j.corsci.2018.08.005
Zhang, F., and Pan, J. (2019). Recent development of corrosion protection strategy based on mussel adhesive protein. Front. Mat. 6, 207. doi:10.3389/fmats.2019.00207
Zhang, F., Pan, J., and Claesson, P. (2011). Electrochemical and AFM studies of mussel adhesive protein (Mefp-1) as corrosion inhibitor for carbon steel. Electrochim. Acta 56, 1636–1645. doi:10.1016/j.electacta.2010.10.033
Zhang, H., Zhang, N., and Fang, F. (2020). Electrodeposition of nickel/graphene oxide particle composite coatings: effect of surfactants on graphene oxide dispersion and coating performance. J. Electrochem. Soc. 167, 162501. doi:10.1149/1945-7111/abcc32
Zhang, M., Ma, L., Wan, L., Sun, Y., and Liu, Y. (2018b). Insights into the use of metal-organic framework as high-performance anticorrosion coatings. ACS Appl. Mat. Interfaces 10, 2259–2263. doi:10.1021/acsami.7b18713
Zhang, X., Liu, Z., Li, Y., Wang, C., Zhu, Y., Wang, H., et al. (2019). Robust superhydrophobic epoxy composite coating prepared by dual interfacial enhancement. Chem. Eng. J. 371, 276–285. doi:10.1016/j.cej.2019.04.040
Zhao, M., Zhang, Z., Shi, W., Li, Y., Xue, C., Hu, Y., et al. (2024). Enhanced copper anticorrosion from Janus-doped bilayer graphene. Nat. Commun. 14, 7447. doi:10.1038/s41467-023-43357-1
Zhao, Z., Guo, L., Feng, L., Lu, H., Xu, Y., Wang, J., et al. (2019). Polydopamine functionalized graphene oxide nanocomposites reinforced the corrosion protection and adhesion properties of waterborne polyurethane coatings. Eur. Polym. J. 120, 109249. doi:10.1016/j.eurpolymj.2019.109249
Zheng, Z., Xiao, L., Huang, P., and Wang, F. (2021). Polydopamine improved anticorrosion of graphene on copper: inhibiting galvanic corrosion and healing structure defects. Appl. Mat. Today 24, 101069. doi:10.1016/j.apmt.2021.101069
Zhong, F., He, Y., Wang, P., Chen, C., Li, H., Zhang, C., et al. (2021). Novel graphene/hollow polyaniline carrier with high loading of benzotriazole improves barrier and long-term self-healing properties of nanocomposite coatings. Prog. Org. Coat. 151, 106086. doi:10.1016/j.porgcoat.2020.106086
Zhou, C., Li, Z., Li, J., Yuan, T., Chen, B., Ma, X., et al. (2020). Epoxy composite coating with excellent anticorrosion and self-healing performances based on multifunctional zeolitic imidazolate framework derived nanocontainers. Chem. Eng. J. 385, 123835. doi:10.1016/j.cej.2019.123835
Zhou, F., Li, Z., Shenoy, G., Li, L., and Liu, H. (2013). Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano 7, 6939–6947. doi:10.1021/nn402150t
Keywords: graphene, polydopamine, anticorrosion, self-healing, coating
Citation: Chen G, Xiao Z, Cao Y, Zhao W, Xu Y, Xiao F, He Y and Jin B (2025) Application and mechanism of graphene and polydopamine-modified graphene in anticorrosion coatings. Front. Mater. 12:1586574. doi: 10.3389/fmats.2025.1586574
Received: 03 March 2025; Accepted: 26 May 2025;
Published: 19 June 2025.
Edited by:
Peide Ye, Purdue University, United StatesReviewed by:
Lakshmi Narayanan Mosur Saravana Murthy, Intel, United StatesWang Yong, Qingdao University of Technology, China
Xiushuo Zhang, Tsinghua University, China
Copyright © 2025 Chen, Xiao, Cao, Zhao, Xu, Xiao, He and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao Jin, amluYkBtYWlsLnRzaW5naHVhLmVkdS5jbg==; Yongyong He, aGV5eUBtYWlsLnRzaW5naHVhLmVkdS5jbg==
 Guangyan Chen1,2
Guangyan Chen1,2 Yuwei Cao
Yuwei Cao Bao Jin
Bao Jin