- 1Department of Pediatric Dentistry and Orthodontics, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
- 2Saudi Board of Orthodontics and Dentofacial Orthopedics Program, Department of Pediatric Dentistry and Orthodontics, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
- 3Saudi Board of Orthodontics and Dentofacial Orthopedics Program, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia
- 4General dentist, College of Dentistry/ King Saud University, Riyadh, Saudi Arabia
- 5Independent Researcher, Riyadh, Saudi Arabia
- 6Department of Prosthetic Dental Sciences, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
Objective: This study evaluated enamel demineralization, shear bond strength, and failure modes associated with orthodontic adhesives containing silver nanoparticles (AgNPs).
Methods: Forty-eight extracted human premolar teeth were selected and evenly divided into two groups based on the intended assessments, with 28 specimens in each group: Part I focused on enamel demineralization depth, and Part II addressed shear bond strength and failure modes. Specimens were prepared, mounted, and bonded using two adhesive systems: Group A used a conventional adhesive system without AgNPs, while Group B employed a conventional adhesive system mixed with AgNPs. In Part I, specimens underwent artificial demineralization at pH 4.5°C and 37°C for 7 days, followed by buccolingual sectioning. The depth of demineralization was measured using a scanning electron microscope and analyzed with ImageJ software. In Part II, shear bond strength was assessed using a universal testing machine, followed by failure mode evaluation using a stereomicroscope.
Results: Results showed that Group A exhibited a significantly greater demineralization depth compared to Group B (p = 0.000). However, no significant difference was observed between overall location levels (p = 0.093). Additionally, Group B demonstrated significantly higher shear bond strength (p = 0.000) and a more uniform distribution of failure modes compared to Group A.
Conclusions: These findings suggest that incorporating silver nanoparticles into orthodontic adhesives reduces demineralization depth and enhances shear bond strength compared to conventional adhesive systems. In contrast, conventional adhesives were associated with a higher incidence of adhesive mode failure.
1 Introduction
Enamel demineralization [i.e., white spot lesions (WSLs)] can occur during orthodontic therapy, often within the first month after the insertion of orthodontic appliance. WSLs can be detected in up to 96% of orthodontic patients (Tukfekci et al., 2011; Bishara and Ostby, 2008; Borzabadi-Farahani et al., 2014; Chapman et al., 2010; Ren et al., 2014). Maintaining proper oral hygiene becomes challenging when orthodontic appliances are present, which can lead to an increase in the colonization of bacteria such as Lactobacillus acidophilus and Streptococcus mutans. This bacterial growth can lower the pH of the mouth to a critical level of 5.5, promoting the demineralization process and potentially resulting in the formation of WSLs (Khoroushi and Kachuie, 2017).
Maintaining proper oral hygiene is the most effective preventive measure to control biofilm formation and reduce the likelihood of WSL formation (Migliorati et al., 2015). The use of topical fluoride varnish and the inclusion of antibacterial agents in mouthwashes and toothpaste, can further enhance these preventive measures, however, individual compliance with these practices is crucial for their effectiveness (Lopatiene et al., 2016; Cosma LL et al., 2019). More advanced preventive measures may include the use of bioactive glass and Casein Phosphopeptide Amorphous Calcium Phosphate (CPP-ACP) (Taha et al., 2017; Cochrane and Reynolds, 2012; Beerens et al., 2018; De Almeida et al., 2018).
Another common challenge that orthodontists encounter during treatment is bracket detachment, which can occur in approximately 0.6%–28.3% of cases involving light-cured or chemical-cured composite resins (Al-Thomali, 2022). Shear bond strength (SBS) is defined as the maximum stress a material can sustain in a shear loading condition before failing (El Mourad, 2018). This measurement is crucial in orthodontics, as it ensures that brackets remain securely attached to tooth enamel throughout the treatment process.
The success of orthodontic treatments greatly depends on how well adhesives can endure mechanical forces. Failures of these adhesives are typically categorized into three types: adhesive, cohesive, and mixed (Kumar et al., 2005). These failure modes not only influence the durability and effectiveness of orthodontic treatments but also involved in developing new adhesives with greater mechanical strength and better bonding qualities.
The incorporation of nanotechnology into bonding agents has significantly improved adhesive performance and transformed the field. These advanced materials utilize smaller particle sizes, which not only enhance adhesive strength but also provide antibacterial properties, greatly reducing the risk of microbial buildup around brackets (Jung et al., 2008). Metallic nanoparticles are particularly beneficial due to their small size and high surface-to-volume ratio, allowing them to release metal ions that interact with bacterial membranes, resulting in antibacterial effects (Song and Ge 2019; Vimbela et al., 2017).
The integration of silver nanoparticles (AgNPs) into orthodontic adhesive systems provides both antibacterial and antifungal properties. This is primarily attributed to the reactive oxygen species present on the surface of silver nanoparticles, as well as the release of free silver ions that induce cell death and inhibit the growth of Streptococcus mutans (Borzabadi-Farahani et al., 2014; Wang et al., 2016; Tran and Le, 2013). By enhancing the interaction between the adhesive and microorganisms, AgNPs effectively prevent bacterial proliferation at the junction of orthodontic adhesive resins, thereby reducing the likelihood of lesion development (Yassaei et al., 2020; Degrazia et al., 2016; Eslamian et al., 2020).
The shear bond strength of adhesives is affected by the amount of filler they con-tain. Increasing the filler content in adhesives shows improved bonding between the enamel and the bracket (Faltermeier et al., 2007). Previous studies have examined the impact of silver nanoparticles (AgNP) on adhesive bonding and failure, yielding contradictory findings regarding shear bond strength. Some research indicates that adhesives containing AgNP may decrease shear bond strength (Eslamian et al., 2020; Reddy et al., 2016), while other studies suggest that adding AgNP can enhance bond strength by increasing the filler content (Al-Thomali, 2022; Faltermeier et al., 2007).
There are significant differences in the modes of failure observed. Sánchez-Tito and Tay (2024) found that 1% AgNP adhesive caused primary failure at the adhesive-enamel interface; in contrast, failures in the 0.05% AgNP adhesive and the conventional adhe-sive group predominantly took place at the bracket-adhesive interface (Sánchez-Tito and Tay, 2024). Additionally, previous research has found no significant differences in the distribution of the Adhesive Remnant Index (ARI) failure pattern between the modified adhesive and the control group, suggesting a similar SBS effect on the mode of failure (Eslamian et al., 2020).
To the best of our knowledge, there is a significant gap in understanding how incorporating silver nanoparticles (AgNPs) into orthodontic adhesives influences enamel demineralization and essential bonding properties, such as shear bond strength (SBS) and failure modes. Understanding the impact of AgNPs on these factors is crucial for optimizing adhesive performance and enhancing patients’ overall experience and satisfaction with orthodontic treatments. Therefore, it is essential to thoroughly investigate the role of silver nanoparticles in orthodontic adhesive formulations. This study aimed to evaluate the effects of AgNPs-containing orthodontic adhesives on enamel demineralization, SBS, and failure modes. Additionally, this study examined the impact of incorporating AgNPs on the microhardness and surface roughness of the experimental adhesive material. The null hypothesis proposed that there would be no significant differences in enamel demineralization, SBS, or failure mechanisms between orthodontic adhesives with and without silver nanoparticles.
2 Materials and methods
This study was approved by the Institutional Review Board for Health Sciences Colleges Research on Human Subjects, College of Medicine, King Saud University, Riyadh, Saudi Arabia, and the College of Dentistry Research Center, Riyadh, Saudi Arabia (E−23–8,233).
2.1 Specimen selection and preparation
The sample size was determined using the G*Power calculator (version 3.1.9.6, Franz Faul, Universität Kiel, Kiel, Germany). A total sample size of 48 specimens was sufficient at (0.05) type I error rate, 0.15 effect size, and 80% power. The study utilized 48 extracted human premolars for orthodontic purposes were collected, with an inclusion criterion of sound teeth without any crack, restoration, or caries.
All specimens were disinfected in a 10% formalin solution for 1 week at room temperature. They were then mounted on acrylic (methyl methacrylate self-cure resin), ensuring that the buccal surface remained exposed for the bonding of orthodontic brackets.
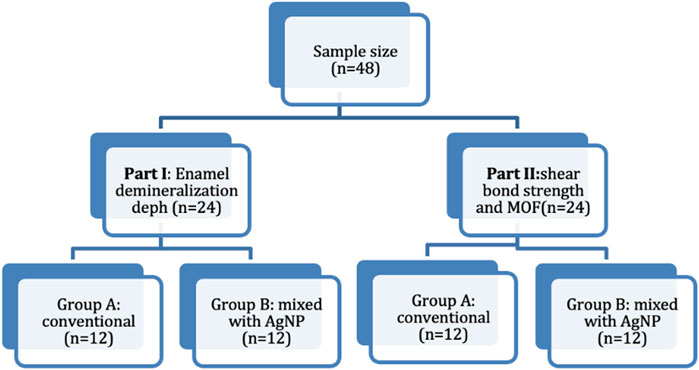
The specimens were simply randomized and evenly divided based on the conducted assessments; Part I: enamel demineralization depth (n = 24) and Part II: shear bond strength and mode of failure (n = 24). Each part was further categorized according to the adhesive system, Group A included a conventional adhesive system without AgNPs (n = 12), and Group B comprised a conventional adhesive system mixed with AgNPs (n = 12). The study grouping is illustrated in Figure 1.
2.2 Silver nanoparticles (AgNP) adhesive preparation
Transbond XT orthodontic bonding agent (Transbond XT, 3M Unitek, CA, United States) was mixed with silver nanoparticles (readymade Ag, 99.99% 50–80 nm, w/-0.2% PVP), Stock#: US1018, CAS#:7,440–22-4, US Research Nanomaterials, Inc., Houston, TX, United States). The mixture was prepared using a ratio of 2% AgNP to 98% Transbond XT. It was processed using a mixer at 3500 RPM for 5 min in a dark room to prevent premature photoactivation of the light-sensitive bonding agent.
2.3 Specimen bonding
- Conventional adhesive system (Group A):
The mid-buccal surfaces of the specimens were etched with 37% phosphoric acid (Unitek Etching Gel, 3M, Monrovia, United States) for 20 s, then rinsed with water. After rinsing, the surfaces were air-dried until a chalky, white surface formed. A bonding agent (Transbond, 3M Unitek, CA, United States) was applied using a disposable brush, followed by a gentle airbrust for 2 s, and light-curing for 15 s, according to the manufacturer’s instructions.
Metal brackets (3M™ Victory Series™ Low Profile Bracket System, Roth 022, with hooks United States), were then placed using a light-cured adhesive (Transbond XT, 3M Unitek, CA, United States) were used. The brackets were positioned and pressed at the center of the mid-buccal surface, parallel to the long axis of the tooth, using a bracket positioning gauge following the manufacturer’s instructions. A lightemitting diode (LED) curing, was then applied for 40 s.
- Conventional adhesive system mixed with AgNP (Group B):
The bonding procedure for this group followed the same steps as Group A but employed the silver nanoparticles-infused adhesive (Transbond XT, 3M Unitek, CA, United States).
2.4 Enamel demineralization depth assessment—Part I
Part I specimens were immersed in 40 mL of an artificial demineralization solution of pH 4.5 at a temperature of 37°C (JSR JSCI-150T General Incubator, Gongju, Korea) for 7 days (Zawawi and Almosa 2023; Buzalaf et al., 2010, Moura et al., 2004,]. The solution was prepared in a 500 mL Erlenmeyer flask, which contained filled with 200 mL of deionized water, 0.166 g of Calcium chloride dihydrate, 0.204 g of disodium phosphate, 25 mL of glacial acetic acid, and 0.154 g of ammonium acetate.
After immersion, brackets were removed using bracket removal pliers (Unitek Debonding Instrument 804–175; 3M, St. Paul, MN, United States), and any excess resin was cleared using a large non-cutting carbide bur with a slow-speed handpiece (FX23 Contra Angle, NSK, Kanuma, Tochigi, Japan).
A low-speed double-sided diamond disk was used for sectioning the teeth buccolingually along the right and left margins of the bracket under continuous water irrigation (0.6-mm ISOMET 2000 Precisions Saw; Buehler, Lake Bluff, IL, United States). The specimens were then subjected to ultrasonic cleaning using a Sonicer machine (Botous Dental, WA, United States).
To enhance image quality, the specimens were gold-coated (1,600 Fine Coat Ion Sputter; JFC, Tokyo, Japan).31 The areas of enamel demineralization in each section were captured using a scanning electron microscope (JEOL 6060 LV Scanning Electron Microscope; JEOL) at an accelerating voltage of 15 kV and magnification of ×100. The depth of enamel demineralization was measured in micrometers using ImageJ software (SMile ViewTM, JEOL Ltd., Tokyo, Japan).
2.5 Shear bond strength assessment and mode of failure evaluation—Part II
All specimens designated for Part II were assessed for shear bond strength in megapascals (MPa) using a universal testing machine (Instron, Illinois Tool Works Inc., Norwood, MA, United States) at a speed of 1 mm per minute (mm/min). The mode of failure was examined using a stereomicroscope (SM80, Swift microscope, Carlsbad, CA, United States) and categorized as adhesive, cohesive, or mixed failures.
Adhesive failure mode occurs at the interface between the adhesive and the tooth or bracket, indicating potential issues such as insufficient bond strength or improper tooth etching. In contrast, cohesive failure mode takes place within the adhesive material or the bracket itself, reflecting the inherent properties of the materials used. Mixed failure mode arises when both adhesive and cohesive factors are involved, suggesting an interplay between the bonding methods and the material characteristics.
2.6 Specimen preparation for microhardness and surface roughness tests
The study evaluated microhardness and surface roughness using twenty adhesive resin discs (10 specimens with Transbond XT orthodontic bonding agent (Transbond XT, 3M Unitek, CA, United States) and 10 specimens with same adhesive mixed with 2% AgNPs). The discs were fabricated using a stainless steel mold, filled incrementally, and covered with a Mylar strip and glass slide. The samples were light-cured using an LED curing unit for 20 s. After polymerization, the discs were polished under running water to maintain consistency and achieve a uniform, smooth surface. This process was used to ensure accurate and reproducible measurements of surface roughness and microhardness.
2.7 Microhardness test
Microhardness testing was conducted using a INNOVATEST microhardness tester (INNOVATEST Europe BV, Bargharenweg 140, 6222 AA Maastricht, Netherlands). To ensure consistent and precise measurement, each specimen was secured in place using a custom polyvinyl siloxane (PVS) putty index (Express™ XT Putty Soft VPS, 3M™, Riyadh, Saudi Arabia), which stabilized the samples throughout the testing process. Microhardness was assessed using a Vickers diamond indenter, applying a load of 100 g for a dwell time of 30 s at each testing point. For every disc, three indentations were performed on the polished surface, spaced 200 μm apart to avoid interference between impressions. The Vickers microhardness (VMH) value for each specimen was calculated as the average of the three measurements, providing a reliable estimate of surface hardness. This protocol was adapted with modifications from previously published methodologies (Al-Angari et al., 2021; Sánchez-Tito and Tay, 2024).
2.8 Surface roughness
Surface roughness (Ra) measurements were carried out using a three-dimensional (3D) optical profilometer (Contour-GT-X®, Bruker Nano Surfaces Division, San Jose, CA, United States), which provides high-resolution, non-contact surface topography analysis. The parameter selected to quantify surface texture was the arithmetic average roughness, expressed in micrometers (µm). This parameter reflects the average vertical deviation of the surface profile from a central mean line, offering a reliable assessment of surface smoothness or roughness. Measurements were performed on all specimens from both experimental groups, with and without silver nanoparticles (AgNPs), to allow for comparative analysis. For each disc, three independent measurements were taken from different locations across the polished surface to account for any surface variability. The mean of these three readings was calculated and recorded as the final Ra value for each specimen, ensuring accuracy and reproducibility in the surface roughness assessment.
2.9 Statistical analyses
The data were analyzed using SPSS software (version 26; IBM, Armonk, NY, United States) at a level of significant p < 0.05. Mean values and standard deviations were calculated to describe each output. The normality and equality of variance were tested using Shapiro-Wilk and Levene’s tests. Two-way ANOVA was conducted to test the interaction between the different adhesive groups and location levels of the enamel demineralization outcome. One-way ANOVA was employed to compare the demineralization depth of different locations within the same group. Independent t-test was used to compare the shear bond strength, mode of failure between the different adhesive groups and for the comparison of the mean values of the VMH and Ra values of the test specimens.
3 Results
3.1 Enamel demineralization depth
Normality and equality of variance were satisfied (p = 0.566). Group A demonstrated the highest mean values for demineralization depth across various sections: Coronal (6.99 ± 1.22), Middle (6.16 ± 0.97), and Cervical (6.69 ± 1.32). In contrast, Group B exhibited the lowest values in these sections: Coronal (4.54 ± 1.18), Middle (5.40 ± 1.60), and Cervical (4.92 ± 1.53).
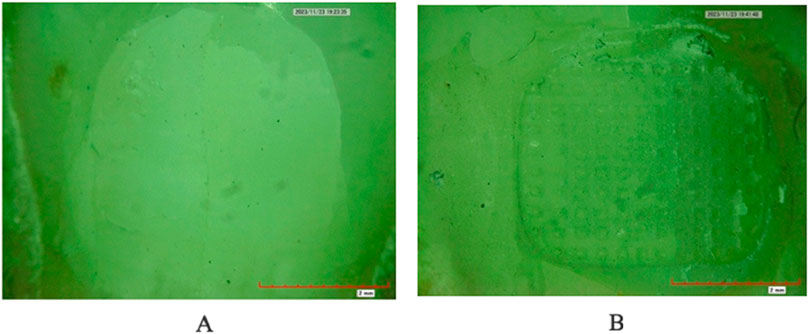
The two-way ANOVA test indicated a significant interaction in demineralization depth between the two groups. Specifically, Group A exhibited a statistically significantly deeper demineralization than Group B (p < 0.05) (Table 1) (Figure 2A). Figure 3 displays SEM images that capture the depth of demineralization for both adhesive systems. There was no significant difference in overall location levels within the same group (Group A: p = 0.236, and Group B: p = 0.361) (Table 2). However, when comparing different adhesive systems within the same section, Group B showed shallower demineralization in the coronal and cervical sections compared to Group A (p < 0.05) (Table 2) (Figure 2B). In contrast, the middle section did not show a significant difference between the two groups (p = 0.173) (Table 2) (Figure 2B).
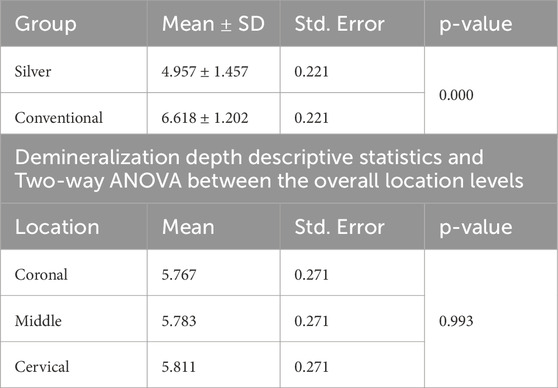
Table 1. Demineralization depth descriptive statistics and Two-way ANOVA between the adhesive systems and the overall location levels.
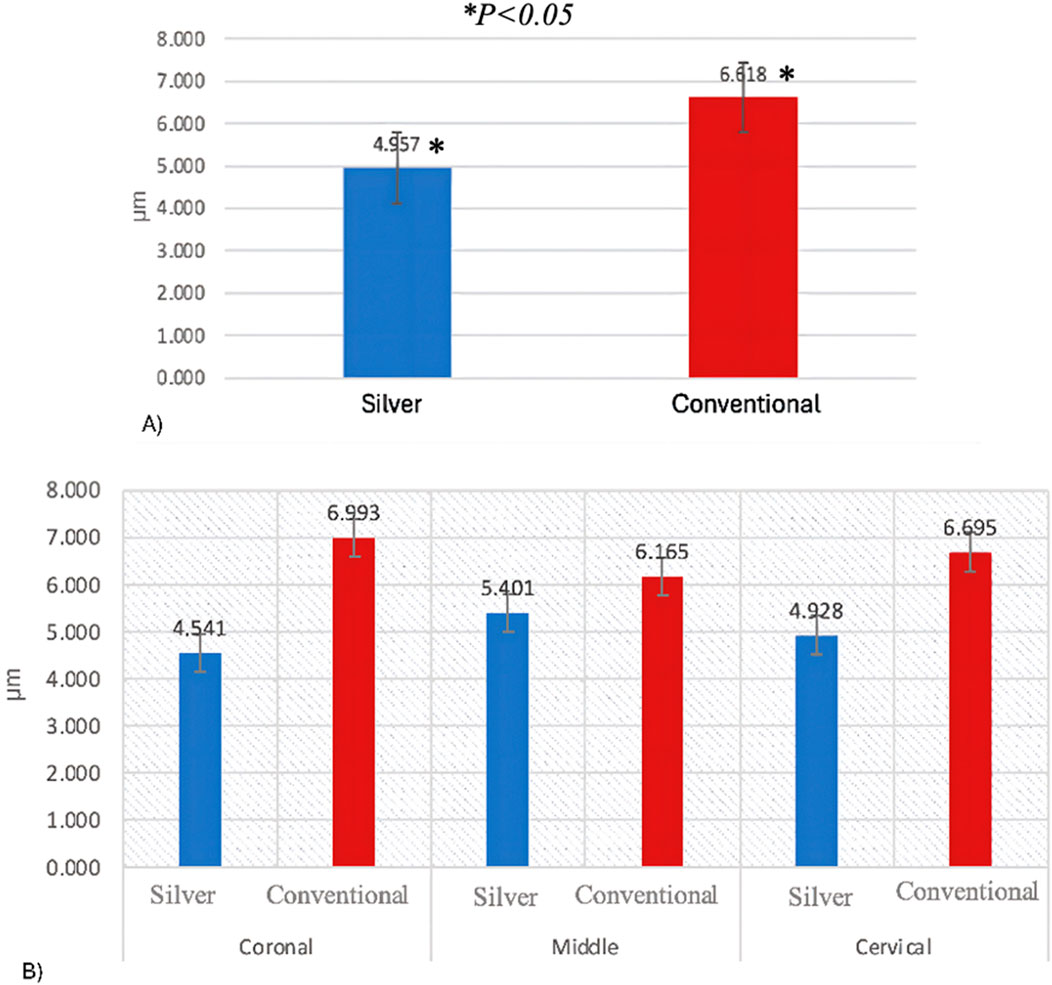
Figure 2. (A) Demineralization depth means according to adhesive system. (B) Demineralization depth means according to location levels.

Figure 3. Enamel Demineralization depth under SEM. (A) Conventional adhesive (B) Silver nanoparticles-infused adhesive.
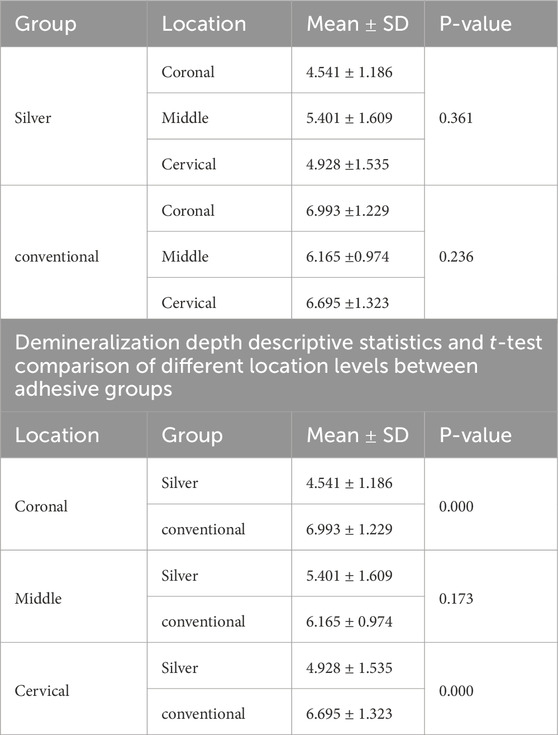
Table 2. Demineralization depth descriptive statistics and One-way ANOVA comparison at different location levels within the same groups.
3.2 Shear bond strength and mode of failure
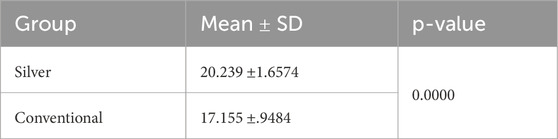
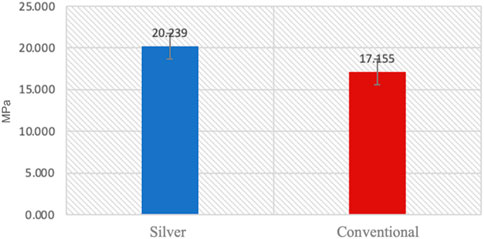
Normality and equality of variance were satisfied (p > 0.05). Group B showed higher mean values compared to Group A, with means of 20.23 ± 1.65 and 17.155 ± 0.94, respectively. The independent t-test indicated that the shear bond strength in Group B was significantly greater than that in Group A (p < 0.05) (Table 3) (Figure 4).
The overall distribution of the mode of failure revealed that 50% of failures were attributed to adhesive failure, 20.8% to cohesive failure, and 29.2% to mixed failure. In Group B the mode of failure was evenly distributed with 33% for adhesive failure, 33% for cohesive failure, and 33% for mixed failure. In contrast, Group A exhibited a different distribution: 66.7% of failures were due to adhesive failure, 8.3% to cohesive failure, and 25% to mixed failure (Table 4). An image of adhesive failure is shown in Figures 5–A, while Figures 5–B displays cohesive failure.
3.3 Microhardness analysis
The results of the microhardness analysis (Table 5) revealed that the incorporation of AgNPs had a significant impact on the VMH of the adhesive specimens. The mean VMH value for the group without AgNPs was 2.94 ± 3.48 MPa, whereas the group with AgNPs exhibited a higher mean value of 3.11 ± 1.99 MPa. Statistical analysis using an independent samples test demonstrated that this increase in microhardness was significant (p = 0.000), indicating that the addition of AgNPs effectively enhanced the surface hardness of the adhesive material.
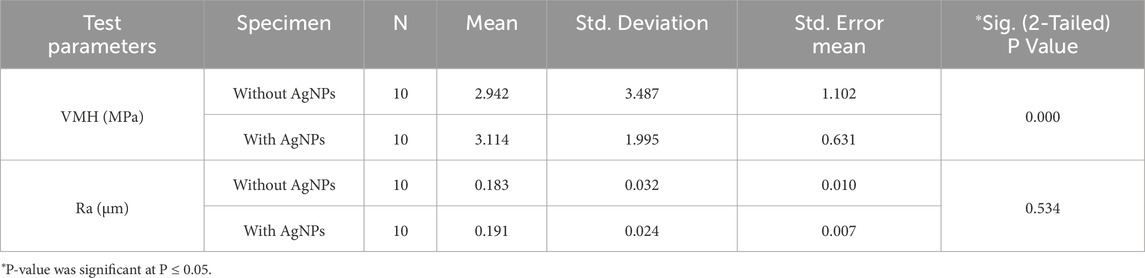
Table 5. Group statistics and comparison of the mean values of the Vickers Micro-Hardness (VMH) and Surface Roughness (Ra) with Independent Samples T-Test for the specimens with or without Silver Nanoparticles (AgNPs).
3.4 Surface Roughness evaluation
In terms of Ra, the specimens containing AgNPs showed a slight increase in the mean arithmetic roughness (Ra) compared to those without (Table 5). The mean Ra value for the AgNPs-containing group was 0.19 ± 0.02 µm, while the non-AgNPs group had a slightly lower value of 0.18 ± 0.03 µm. Despite this observed difference, statistical analysis indicated that the increase in surface roughness was not statistically significant (p = 0.534). These findings suggest that while AgNPs addition significantly improves microhardness, it does not markedly alter the surface roughness of the adhesive material.
4 Discussion
Enamel demineralization is a common consequence of orthodontic treatment, which can negatively impact individual aesthetics (Althomali YM, 2017; Benkaddour A et al., 2014). It is essential to implement effective strategies to prevent this issue. Silver nanoparticles (AgNPs) have garnered attention in dentistry and orthodontics due to their distinctive properties and potential benefits. Several studies have indicated that AgNPs possess antibacterial properties, which can help reduce bacterial adhesion and the development of demineralization (Eslamian L et al., 2020; Hernandez-Gomora et al., 2017; Zakarzewski et al., 2021). It is essential to ensure that the addition of silver nanoparticles does not compromise the mechanical properties of orthodontic materials, particularly shear bond strength and the mode of failure. Therefore, this study aimed to assess the enamel demineralization, shear bond strength, and failure modes associated with orthodontic adhesives that contain silver nanoparticles (AgNPs).
Previous studies have assessed the SBS and antibacterial behavior of adhesives containing AgNPs (Eslamian et al., 2020; Al-Thomali 2022; Reddy et al., 2016; Blocher et al., 2015). However, this study is unique in that it simultaneously investigates enamel demineralization, SBS, and modes of failure within a single setting. Additionally, it conducted quantitative assessments using SEM to measure enamel lesion depth, providing a more accurate evaluation of demineralization compared to the qualitative methods used in earlier studies. The adhesive’s preparation process allowed for high-speed standardized mixing, ensuring the uniform dispersion of nanoparticles within the adhesive. This uniformity can enhance the mechanical properties and lead to more consistent failure modes. By employing this integrated approach with high methodological rigor, we gain a broader and more clinically relevant understanding of AgNPs in the context of orthodontic adhesives, thereby advancing the field significantly.
The null hypothesis regarding enamel demineralization was rejected, as the study found that silver nanoparticle adhesive resulted in significantly shallower enamel demineralization compared to conventional adhesive systems. These results are consistent with previous research indicating that silver nanoparticles can prevent enamel demineralization by reducing the acid production generated by biofilms (Eslamian L et al., 2020; Zakarzewski et al., 2021; Allaker, 2010; Espinosa-Cristóbal LF et al., 2009; Espinosa-Cristóbal LF et al., 2018; Espinosa-Cristóbal LF et al., 2012; Chou CW et al., 2006). The antibacterial activity of silver nanoparticles against Streptococcus mutans is attributed to their small size and large surface area. Furthermore, the advantages of silver nanoparticles are enhanced by their ability to counterbalance the conventional adhesive nature, which tends to promote the colonization and adherence of microorganisms (Degrazia FW et al., 2016).
This study revealed that the shallow demineralization caused by the silver nanoparticle adhesive was noticeable at the coronal and cervical levels, in contrast to conventional adhesive systems. However, no significant difference in demineralization depth was observed at the middle level between the two types of adhesives. The current literature does not offer a clear understanding of the relationship between the location and the depth of demineralization, which remains to be explored. This finding may be attributed to the fact that the middle part is mostly covered by brackets and is least exposed to the artificial demineralization solution. The artificial demineralization and its limited duration may not accurately reflect the long-term effects of orthodontic adhesives. Additionally, varying the duration of demineralization could either lead to ineffective results or cause excessive erosion of the specimens due to the nature of the chemicals used in the artificial demineralization process. This limitation highlights the need for further studies to evaluate different artificial demineralization protocols that better mimic the clinical long-term effects of orthodontic adhesives.
The null hypothesis regarding shear bond strength was rejected, as a significant increase in shear bond strength was observed in the group using silver nanoparticle adhesive compared to those using conventional adhesives. This enhanced shear bond strength may lead to a reduced risk of leakage, which in turn would result in less leaching of the demineralization solution in an in-vitro setting. Consequently, this contributes to the shallower enamel demineralization observed, particularly at the coronal and cervical levels. Al-Thomali (2022) reported that AgNPs significantly increased SBS when a concentration of 0.05% AgNPs was used, which is lower than the concentration used in the present study. In contrast, Eslamian et al. (2020) found that while AgNPs resulted in a reduced SBS, the strength remained above the recommended range of 5.9–7.8 MPa, with their study utilizing 0.3% AgNPs. Furthermore, another study revealed that conventional adhesives outperform AgNPs in terms of SBS (Reddy et al., 2016). However, another study found no significant impact of AgNPs on SBS (Blocher et al., 2015).
The small particle size and high surface area of AgNPs effectively enhance Van der Waals interactions between positively charged nanoparticles (Hernández-Gómora AE et al., 2017). This helps strengthen the structure of adhesive biomaterials, reduces stress concentrations at the bonding interface by acting as a stress absorber, enhances mechanical properties, and improves the adhesion layer between dental materials and the enamel surface (Chou CW, et al., 2006; Graig RG & Powers JM, 2002). However, the amount and distribution of the nanofiller particles affect the ultimate adhesive properties; the wide variation of w/w AgNPs incorporation ratio could explain the contradictory findings of AgNPs use on SBS in the literature.
The null hypothesis concerning failure modes was rejected. The AgNP-infused adhesive demonstrated a more balanced distribution of failure modes, whereas the conventional adhesive system showed a higher incidence of adhesive failures. Previous studies reported that cohesive failure modes caused surface damage to teeth, with damage rates of 12.9% and 3.6% resulting from the adhesive resin undergoing cohesive resin failure. (Su MZ et al., 2012; Paolone et al., 2023). Another evaluated failure modes of ceramic brackets bonded to enamel found that most brackets failed at the bracket-adhesive interface (Yetkiner and Ozcan, 2014). Studying failure modes is clinically significant, as it helps identify factors related to complete bracket separation, the amount of remaining adhesive, and the integrity of the enamel, all of which contribute to successful bonding procedures (Borzabadi-Farahani et al., 2014; Su et al., 2012; Jakavice et al., 2023). In orthodontics, it is preferable to have adhesive failure occur between the adhesive layer and the enamel surface. This situation helps maintain proper bracket attachment and ensures safer debonding (Yahya NA & Shekh AM, 2019). Cohesive failure that occurs between the adhesive layer and the back surface of the bracket can lead to a higher incidence of enamel damage. The mode of failure can be influenced by several factors, including the type of surface preparation, the adhesive system used, the material of the bracket, and amount of SBS (Paolone et al., 2023; Chen CS et al., 2008). While a higher bond strength may be desirable, a very high SBS can result in cohesive failures during debonding.
The main concern with AgNPs is their potential cytotoxicity due to oxidative stress and silver ion release. AgNPs can directly generate free radicals through their active surface and produce hydroxyl radicals when dissolved in acidic endo/lysosomes (Chen HY et al., 2007; He W et al., 2012). The impact of AgNPs on shear bond strength (SBS) and demineralization varies with concentration, underscoring the need for comparative studies to optimize their safety and effectiveness. Cost is another limitation, as AgNP-containing adhesives remain expensive. Preparation methods also influence adhesive quality, curing, stress distribution, and SBS (Chou CW, et al., 2006). The mixture preparation method used in this study offers uniform AgNP distribution, enhancing mechanical strength, reducing shrinkage, and minimizing defects, as demonstrated by high SBS values. Exploring alternative preparation methods, such as heat preparation, may further enhance mechanical properties by increasing AgNP temperatures (Metin-Gursoy G et al., 2017). Additionally, discrepancies in SBS results across studies may result from inconsistent artificial aging protocols, such as water aging at 37°C or thermocycling (Chou CW, et al., 2006; Graig and Powers, 2002). The type of bracket material also affects bonding. AlThomali (2017) found that a 0.05% AgNP adhesive achieved higher SBS for metal brackets compared to ceramic brackets, although conventional adhesives were not tested. Further studies should investigate bonding interactions between different bracket materials and AgNP adhesives.
It is essential to acknowledge the differences between laboratory conditions and the clinical environment, as findings from in vitro studies may not fully translate to clinical settings. Therefore, antibiofilm analysis and also clinical trials are necessary to better understand the practical implications of these results. While silver nanoparticles (AgNPs) have shown promise in preventing enamel demineralization and enhancing shear bond strength (SBS), further research is required to address several unresolved issues. These include evaluating the potential cytotoxicity of AgNPs, their long-term stability within the adhesive matrix, and the overall clinical performance and durability of AgNP-containing orthodontic adhesives.
Incorporating AgNPs into adhesive systems has shown significant effects on microhardness and surface roughness, indicating both mechanical enhancement and potential surface modifications. Chou et al. (2006) noted that embedding AgNPs into polyurethane matrices improved thermal stability and mechanical properties positively (Chou CW, et al., 2006). This supports the hypothesis that AgNPs strengthens the structural network, which likely contributes to increased microhardness in dental adhesives. Similarly, Hernández-Gómora et al. (2017) reported that AgNPs biosynthesized onto orthodontic elastomeric modules enhanced mechanical resistance while also exhibiting antimicrobial activity (Hernández-Gómora AE et al., 2017). The findings of this study demonstrate that the incorporation of silver nanoparticles (AgNPs) into the adhesive resin formulation significantly enhanced its microhardness without causing a statistically significant increase in surface roughness. The observed improvement in VMH may be attributed to the reinforcing effect of AgNPs, which are known to improve the mechanical properties of polymer-based materials by increasing filler content, reducing polymer chain mobility, and promoting better stress distribution within the matrix. This enhancement in surface hardness could potentially lead to greater resistance to wear and mechanical degradation in clinical applications, particularly in stress-bearing areas.
Conversely, although the Ra values showed a slight increase in the presence of AgNPs, this difference was not statistically significant. This suggests that the addition of AgNPs at the tested concentration does not adversely affect the smoothness of the adhesive surface, an important consideration for maintaining aesthetic quality and minimizing plaque accumulation in oral environments. Taken together, these results suggest that AgNP incorporation could be a promising strategy to enhance the mechanical performance of dental adhesives, particularly when increased hardness is desirable. Moreover, the fact that surface roughness remained unaffected supports the potential clinical viability of such formulations, as smooth surface characteristics are essential for both esthetics and biofilm resistance (Holban AM et al., 2021).
The chemical interaction between resin and AgNPs has the potential to induce minor surface chemistry and phase dispersion changes, which would influence the mechanical performance and antimicrobial activity of the adhesive (Chou et al., 2006; Degrazia et al., 2016). Thus, it is essential for future studies to evaluate the effects of AgNPs on the structural, chemical, and phase transition characteristics of adhesive materials. Utilizing techniques such as Fourier Transform Infrared Spectroscopy (FTIR), and X-ray diffraction will be important in these evaluations, to have a better understanding on the changes that AgNPs makes on orthodontic adhesives. Furthermore, future clinical studies that would cover a wider range of variables and more comprehensive assessments is needed.
5 Conclusion
• Orthodontic adhesives infused with silver nanoparticles (AgNPs) demonstrate promising advantages over conventional adhesives, including reduced enamel demineralization and enhanced shear bond strength. However, conventional adhesives show a higher incidence of adhesive mode failure, which may offer safer bracket removal.
• Incorporating AgNPs presents a valuable approach to minimizing enamel demineralization during orthodontic treatment. Nevertheless, further research is essential to determine the optimal AgNP concentration that balances antibacterial efficacy and mechanical stability over the long term.
• The addition of AgNPs significantly improved the microhardness of dental adhesives without affecting surface roughness. These results suggest that AgNPs may enhance the mechanical properties of adhesives while maintaining clinical surface quality.
• Despite their potential benefits, the economic feasibility of AgNP-containing adhesives remains a critical barrier to widespread adoption. Addressing this challenge will be crucial to making this innovative solution more accessible and practical for clinical use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Institutional Review Board for Health Sciences Colleges Research on Human Subjects, College of Medicine, King Saud University, Riyadh, Saudi Arabia, and the College of Dentistry Research Center, Riyadh, Saudi Arabia (E-23-8233).
Author contributions
NiA: Writing - original draft, Conceptualization, Validation, Project administration, Data curation, Supervision. AhA: Methodology, Data curation, Writing - original draft, Investigation. AlA: Software, Visualization, Formal Analysis, Data curation, Writing - original draft, Resources, Funding acquisition, Conceptualization, Project administration, Investigation, Supervision, Validation, Methodology. NdA: Writing - original draft, Investigation, Methodology, Data curation. AbA: Investigation, Data curation, Methodology, Writing - original draft. LA: Data curation, Writing - original draft, Methodology. RZ: Investigation, Writing - original draft, Methodology. SH: Formal Analysis, Software, Funding acquisition, Writing - review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by Ongoing Research Funding Program (ORF-2025-950), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors appreciate the support from Ongoing Research Funding Program (ORF-2025-950), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Angari, S. S., Eckert, G. J., and Sabrah, A. H. A. (2021). Color stability, roughness, and microhardness of enamel and composites submitted to staining/bleaching cycles. Saudi Dent. J. 33, 215–221. doi:10.1016/j.sdentj.2020.08.003
Allaker, R. P. (2010). The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 89 (11), 1175–1186. doi:10.1177/0022034510377794
Al-Thomali, Y. (2022). Shear bond strength of orthodontic brackets after adding silver nanoparticles to a nano-bond adhesive at different thermal cycles and cyclic loading-An in vitro study. J. Orthod. Sci. 11, 28. doi:10.4103/jos.jos18321
Althomali, Y. M. (2017). Comparison of shear bond strength of metal and ceramic orthodontic brackets after adding nanoparticles of silver. Egypt. Dent. J. 63 (1-January), 31–40. doi:10.21608/edj.2017.74368
Beerens, M. W., Ten Cate, J. M., Buijs, M. J., and van der Veen, M. H. (2018). Long-term remineralizing effect of MI Paste Plus on regression of early caries after orthodontic fixed appliance treatment: a 12-month follow-up randomized controlled trial. Eur. J. Orthod. 40 (5), 457. doi:10.1093/ejo/cjx085
Benkaddour, A., Bahije, L., Bahoum, A., and Zaoui, F. (2014). Orthodontics and enamel demineralization: clinical study of risk factors. Int. Orthod. 12 (4), 458–466. doi:10.1016/j.ortho.2014.10.009
Bishara, S. E., and Ostby, A. W. (2008). White spot lesions: formation, prevention, and treatment. Seminars Orthod. 14 (3), 174–182. WB. doi:10.1053/j.sodo.2008.03.002
Blöcher, S., Frankenberger, R., Hellak, A., Schauseil, M., Roggendorf, M. J., and Korbmacher-Steiner, H. M. (2015). Effect on enamel shear bond strength of adding microsilver and nanosilver particles to the primer of an orthodontic adhesive. BMC oral health 15, 1–9.
Borzabadi-Farahani, A., Borzabadi, E., and Lynch, E. (2014). Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol. Scand. 72 (6), 413. doi:10.3109/00016357.2013.859728
Buzalaf, M. A. R., Hannas, A., Magalhães, R. A. C., Rios, D., Honório, H. M., and Delbem, A. C. (2010). pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J. Appl. Oral Sci. 18, 316–334. doi:10.1590/s1678-77572010000400002
Chapman, J. A., Roberts, W. E., Eckert, G. J., Kula, K. S., and González-Cabezas, C. (2010). Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 138 (2), 188–194. doi:10.1016/j.ajodo.2008.10.019
Chen, C. S., Hsu, M. L., Chang, K. D., Kuang, S. H., Chen, P. T., and Gung, Y. W. (2008). Failure analysis: enamel fracture after debonding orthodontic brackets. Angle Orthod. 78 (6), 1071–1077. doi:10.2319/091907-449.1
Chen, H. Y., Su, M. Z., Chang, H. F., Chen, Y. J., Lan, W. H., and Lin, C. P. (2007). Effects of different debonding techniques on the debonding forces and failure modes of ceramic brackets in simulated clinical set-ups. Am. J. Orthod. Dentofac. Orthop. 132 (5), 680–686. doi:10.1016/j.ajodo.2006.01.035
Chou, C. W., Hsu, S. H., Chang, H., Tseng, S. M., and Lin, H. R. (2006). Enhanced thermal and mechanical properties and biostability of polyurethane containing silver nanoparticles. Polym. Degrad. Stab. 91 (5), 1017–1024. doi:10.1016/j.polymdegradstab.2005.08.001
Cochrane, N. J., and Reynolds, E. (2012). “Calcium phosphopeptides—mechanisms of action and evidence for clinical efficacy,”Adv. Dent. Res., 24, 41–47. doi:10.1177/0022034512454294
Cochrane, N. J., Saranathan, S., Cai, F., Cross, K. J., and Reynolds, E. C. (2008). Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 42 (2), 88. doi:10.1159/000113161
Cosma, L. L., Şuhani, R. D., Mesaroş, A., and Badea, M. E. (2019). “Current treatment modalities of orthodontically induced white spot lesions and their outcome–a literature review,” Med. Pharm. Rep., 92, 25–30. doi:10.15386/cjmed-1090
De Almeida, C. M., Da Rosa, W. L. O., Meereis, C. T. W., de Almeida, S. M., Ribeiro, J. S., da Silva, A. F., et al. (2018). Efficacy of antimicrobial agents incorporated in orthodontic bonding systems: a systematic review and meta-analysis. J. Orthod. 45 (2), 79. doi:10.1080/14653125.2018.1443872
Degrazia, F. W., Leitune, V. C. B., Garcia, I. M., Arthur, R. A., Samuel, S. M. W., and Collares, F. M. (2016). Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral Sci. 24, 404–410. doi:10.1590/1678-775720160154
El Mourad, A. M. (2018). Assessment of bonding effectiveness of adhesive materials to tooth structure using bond strength test methods: a review of literature. Open Dent. J. 12, 664–678. doi:10.2174/1745017901814010664
Eslamian, L., Borzabadi-Farahani, A., Karimi, S., Saadat, S., and Badiee, M. R. (2020). Evaluation of the shear bond strength and antibacterial activity of orthodontic adhesive containing silver nanoparticle, an in-vitro study. Nanomaterials 10 (8), 1466. doi:10.3390/nano10081466
Espinosa-Cristóbal, L. F., López-Ruiz, N., Cabada-Tarín, D., Reyes-López, S. Y., Zaragoza-Contreras, A., Constandse-Cortéz, D., et al. (2018). Antiadherence and antimicrobial properties of silver nanoparticles against Streptococcus mutans on brackets and wires used for orthodontic treatments. J. Nanomater 2018, 1–11. doi:10.1155/2018/9248527
Espinosa-Cristóbal, L. F., Martínez-Castañón, G. A., Martínez-Martínez, R. E., Loyola-Rodríguez, J. P., Patiño-Marín, N., Reyes-Macías, J. F., et al. (2009). Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater Lett. 63 (29), 2603–2606. doi:10.1016/j.matlet.2009.09.01
Espinosa-Cristóbal, L. F., Martínez-Castañón, G. A., Martínez-Martínez, R. E., Loyola-Rodríguez, J., Patiño-Marín, N., Reyes-Macías, J. F., et al. (2012). Antimicrobial sensibility of Streptococcus mutans serotypes to silver nanoparticles. Mater Sci. Eng. C 32 (4), 896–901. doi:10.1016/j.msec.2012.02.009
Faltermeier, A., Rosentritt, M., Faltermeier, R., Reicheneder, C., and Müssig, D. (2007). Influence of filler level on the bond strength of orthodontic adhesives. Angle Orthod. 77 (3), 494–498. doi:10.2319/0003-3219(2007)077[0494:IOFLOT]2.0.CO;2
He, W., Zhou, Y. T., Wamer, W. G., Boudreau, M. D., and Yin, J. J. (2012). Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 33 (30), 7547–7555. doi:10.1016/j.biomaterials.2012.06.076
Hernández-Gómora, A. E., Lara-Carrillo, E., Robles-Navarro, J. B., Scougall-Vilchis, R. J., Hernández-López, S., Medina-Solís, C. E., et al. (2017). Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules 22 (9), 1407. doi:10.3390/molecules22091407
Holban, A. M., Farcasiu, C., Andrei, O. C., Grumezescu, A. M., and Farcasiu, A. T. (2021). Surface modification to modulate microbial biofilms-applications in dental medicine. Mater. (Basel) 14 (22), 6994. doi:10.3390/ma14226994
Jakavičė, R., Kubiliūtė, K., and Smailienė, D. (2023). Bracket bond failures: incidence and association with different risk factors—a retrospective study. Int. J. Environ. Res. Public Health 20, 4452. doi:10.3390/ijerph20054452
Jung, W. K., Koo, H. C., Kim, K. W., Shin, S., Kim, S. H., and Park, Y. H. (2008). Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74 (7), 2171–2178. doi:10.1128/AEM.02001-07
Khoroushi, M., and Kachuie, M. (2017). “Prevention and treatment of white spot lesions in orthodontic patients,”Contemp. Clin. Dent., 8, 11. doi:10.4103/ccd.ccd_216_17
Kumar, M., Sequeira, P. S., Peter, S., and Bhat, G. K. (2005). Sterilisation of extracted human teeth for educational use. Indian J. Med. Microbiol. 23 (4), 256–258. doi:10.1016/s0255-0857(21)02532-9
Lopatiene, K., Borisovaite, M., and Lapenaite, E. (2016). Prevention and treatment of white spot lesions during and after treatment with fixed orthodontic appliances: a systematic literature review. J. oral and Maxillofac. Res. 7 (2), e1. doi:10.5037/jomr.2016.7201
Metin-Gursoy, G., Taner, L., and Akca, G. (2017). Nanosilver coated orthodontic brackets: in vivo antibacterial properties and ion release. Eur. J. Orthod. 39 (1), 9–16. doi:10.1093/ejo/cjv097
Migliorati, M., Isaia, L., Cassaro, A., Rivetti, A., Silvestrini-Biavati, F., Gastaldo, L., et al. (2015). Efficacy of professional hygiene and prophylaxis on preventing plaque increase in orthodontic patients with multibracket appliances: a systematic review. Eur. J. Orthod. 37 (3), 297–307. doi:10.1093/ejo/cju044
Moura, J. S., Rodrigues, L. K. A., Del Bel Cury, A. A., Lima, EMCX, and Garcia, RMCR (2004). Influence of storage solution on enamel demineralization submitted to pH cycling. J. Appl. Oral Sci. 12, 205–208. doi:10.1590/s1678-77572004000300008
Paolone, G., Mandurino, M., Baldani, S., Paolone, M. G., Goracci, C., Scolavino, S., et al. (2023). Quantitative volumetric enamel loss after orthodontic debracketing/debonding and clean-up procedures: a systematic review. Appl. Sci. 13 (9), 5369. doi:10.3390/app13095369
Reddy, A. K., Kambalyal, P. B., Patil, S. R., Vankhre, M., Khan, M. Y. A., and Kumar, T. R. (2016). Comparative evaluation and influence on shear bond strength of incorporating silver, zinc oxide, and titanium dioxide nanoparticles in orthodontic adhesive. J. Orthod. Sci. 5 (4), 127. doi:10.4103/2278-0203.192115
Ren, Y., Jongsma, M. A., Mei, L., van der Mei, H. C., and Busscher, H. J. (2014). “Orthodontic treatment with fixed appliances and biofilm formation—a potential public health threat?,” Clin. oral Investig., 18, 1711–1718. doi:10.1007/s00784-014-1240-3
Sánchez-Tito, M., and Tay, L. Y. (2024). Effect of the addition of silver nanoparticles on the mechanical properties of an orthodontic adhesive. Saudi Dent. J. 36 (2), 359–363. doi:10.1016/j.sdentj.2023.11.021
Song, W., and Ge, S. (2019). Application of antimicrobial nanoparticles in dentistry. Molecules 24 (6), 1033. doi:10.3390/molecules24061033
Su, M. Z., Lai, E. H., Chang, J. Z., Chen, H. J., Chang, F. H., Chiang, Y. C., et al. (2012). Effect of simulated debracketing on enamel damage. J. Formos. Med. Assoc. 111 (10), 560–566. doi:10.1016/j.jfma.2011.12.008
Taha, A. A., Patel, M. P., Hill, R. G., and Fleming, P. S. (2017). The effect of bioactive glasses on enamel remineralization: a systematic review. J. Dent. 67, 9. doi:10.1016/j.jdent.2017.09.007
Tran, Q. H., and Le, A. T. (2013). Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 4 (3), 033001. doi:10.1088/2043-6262/4/3/033001
Tufekci, E., Dixon, J. S., Gunsolley, J. C., and Lindauer, S. J. (2011). Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 81 (2), 206. doi:10.2319/051710-262.1
Vimbela, G. V., Ngo, S. M., Fraze, C., Yang, L., and Stout, D. A. (2017). Antibacterial properties and toxicity from metallic nanomaterials. Int. J. nanomedicine 12, 3941–3965. doi:10.2147/ijn.s134526
Wang, M., Marepally, S. K., Vemula, P. K., and Xu, C. (2016). “Inorganic nanoparticles for transdermal drug delivery and topical application,”Nanosci. Dermatol, 57–72. doi:10.1016/b978-0-12-802926-8.00005-7
Yahya, N. A., and Shekh, A. M. (2019). Shear bond strength and failure mode of different dental adhesive systems. Ann. Dent. Univ. Malaya 26, 1–7.
Yassaei, S., Nasr, A., Zandi, H., and Motallaei, M. N. (2020). Comparison of antibacterial effects of orthodontic composites containing different nanoparticles on Streptococcus mutans at different times. Dent. Press J. Orthod. 25, 52–60. doi:10.1590/2177-6709.25.2.052-060.oar
Yetkiner, E., and Özcan, M. (2014). Adhesive strength of metal brackets on existing composite, amalgam and restoration-enamel complex following air-abrasion protocols. Int. J. Adhes. Adhes. 54, 200–205.
Zakrzewski, W., Dobrzynski, M., Dobrzynski, W., Zawadzka-Knefel, A., Janecki, M., Kurek, K., et al. (2021). Nanomaterials application in orthodontics. Nanomaterials 11 (2), 337. doi:10.3390/nano11020337
Keywords: orthodontic adhesive, silver nanoparticles, shear bond strength, mode of failure, enamel demineralization
Citation: Almosa N, Alsaleh A, Alnowaiser A, Alhogail N, Alsuliman A, Alswailem L, Zawawi R and Habib SR (2025) Assessment of enamel demineralization, shear bond strength, and failure mode following the use of orthodontic adhesive containing silver nanoparticles: an in-vitro study. Front. Mater. 12:1598515. doi: 10.3389/fmats.2025.1598515
Received: 23 March 2025; Accepted: 12 May 2025;
Published: 05 June 2025.
Edited by:
Gaurav Bhanjana, Guru Jambheshwar University of Science and Technology, IndiaReviewed by:
Jyotsana Mehta, Guru Jambheshwar University of Science and Technology Hisar, IndiaBhavani Patel, Medline Industries, LP, United States
Copyright © 2025 Almosa, Alsaleh, Alnowaiser, Alhogail, Alsuliman, Alswailem, Zawawi and Habib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Rashid Habib, c3loYWJpYkBrc3UuZWR1LnNh
 Naif Almosa1
Naif Almosa1 Rahaf Zawawi
Rahaf Zawawi Syed Rashid Habib
Syed Rashid Habib