- 1Beijing Key Laboratory for Catalysis and Separation, Department of Chemical Engineering, College of Materials Science and Engineering, Beijing University of Technology, Beijing, China
- 2Huntsman Advanced Materials, The Woodlands, TX, United States
- 3The Pacific Northwest National Laboratory, The Institute for Integrated Catalysis, Richland, WA, United States
- 4The Department of Chemistry, Texas A&M University-Kingsville, Kingsville, TX, United States
- 5Texas A&M Energy Institute, College Station, TX, United States
- 6Louise Nanoinnovation North American LLC, Corpus Christi, TX, United States
Editorial on the Research Topic
Functionalization of porous materials for sustainable energy applications
Global energy demands are shifting toward a more sustainable future, with the goal of achieving carbon neutrality by 2050. Emerging technologies are driving this transition. The industry, academia, government, non-profit organizations, and the broader community are collaboratively working to reduce greenhouse gas (GHG) emissions and address climate change to ensure a sustainable future. According to the International Energy Agency, in 2022, the production, transportation, and processing of oil and gas resulted in 5.1 billion tons of CO2-equivalent emissions, representing nearly 15% of all energy-related GHG emissions. Moreover, the end-use of oil and gas accounted for an additional 40% of emissions. The IEA’s Net Zero Emissions by 2050 Scenario calls for immediate, collective action from the industry, transportation and other stakeholders to mitigate these emissions. In this effort, the development of energy materials will play a critical role in reducing emissions. Among these, porous materials offer an innovative solution, leveraging their high surface area, adjustable pore sizes, and chemical versatility to address these pressing challenges effectively. By carefully designing their nanostructures, the architecture and properties of these materials can be tailored for specific applications. Key factors such as chemical composition, particle size, pore distribution, and surface area optimization enhance the reactivity and energy conversion efficiency. Additionally, pre- and post-functionalization processes can introduce targeted chemical properties, further improving their performance.
This Research Topic explores recent advancements in energy and materials science through four scholarly papers, showcasing innovative solutions for sustainable energy technologies while providing valuable insights into the unique properties and structure of porous materials (Figure 1). Li et al. present their work on highly defective NiFeV layered triple hydroxides, highlighting enhanced electrocatalytic activity and stability for oxygen evolution reactions (OER). Kovalskii et al. contribute a mini-review on hydrogen storage using hexagonal boron nitride (h-BN) and BN-based materials, offering an insightful overview of these promising materials. Chava et al. discuss their recent achievements in ceramic electrolytes used for improvement of performance of solid-state batteries. Lastly, Li et al. review the properties of porous materials with a focus on shrinkage behavior during the drying process, shedding light on key considerations for material design.
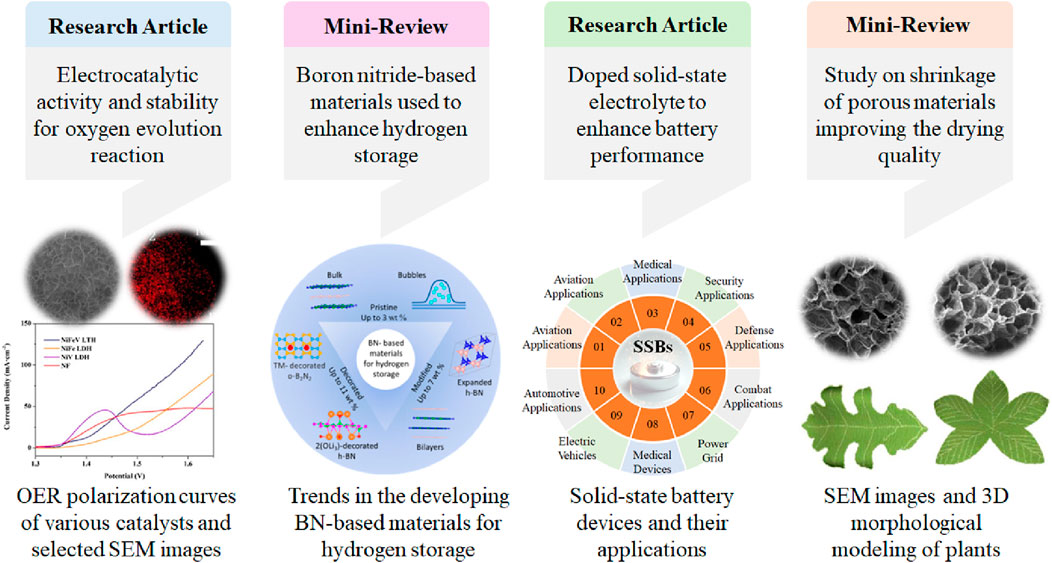
Figure 1. The summary of four papers outlined the recent achievements in oxygen evolution, hydrogen storage, solid-state battery and studying shrinkageproperty of materials.
Li et al. have presented a compelling research article that marks an advance in electrocatalysis for the oxygen evolution reaction (OER). Their study focuses on a highly defective NiFeV layered triple hydroxide (LTH) catalyst, synthesized via a one-step hydrothermal process, demonstrating outstanding performance in OER. Compared to conventional catalysts, which often struggle with high overpotentials and stability issues, this innovative NiFeV LTH catalyst exhibits a low overpotential, a slight Tafel slope, and excellent long-term stability under high current densities. Their work represents a significant step forward in pursuing renewable energy technologies. It demonstrates that innovative material design can overcome existing limitations and pave the way for more sustainable and economically viable energy storage and conversion systems. The research reveals that incorporating vanadium plays a pivotal role in optimizing the catalyst’s performance through structural and electrochemical characterizations, including X-ray diffraction, photoelectron spectroscopy, and in situ Raman spectroscopy. In the early stages of the OER, nickel species serve as the primary active sites, facilitating initial reaction steps. Iron and vanadium gradually join the catalytic process as overpotential increases, contributing to robust and efficient oxygen evolution. Notably, the presence of vanadium helps mitigate the dissolution of high-valence nickel species, thereby preventing catalyst degradation and ensuring sustained activity over prolonged operation. This research not only sheds light on the mechanisms that enhance ion transport and surface reconstruction during OER but also highlights the potential for targeted metal ion modifications to improve the performance of LDH-type catalysts. The study’s findings suggest that precise doping strategies and structural optimizations can drive significant improvements in activity and durability. By addressing key challenges in catalyst design with a focus on the sluggish kinetics inherent in the OER’s four-electron transfer process, Dr. Li’s research offers promising avenues for developing next-generation, cost-effective, and high-performance electrocatalysts.
Dr. Kovalskii’s paper, Outstanding Features of Hexagonal Boron Nitride-Based Materials, presents a comprehensive overview of the promise and challenges associated with using h-BN nanomaterials for hydrogen storage. Highlighting a unique blend of high porosity, extended surface area, and exceptional thermal and chemical stability, even up to 1273 K in air, these h-BN materials emerge as attractive alternatives to traditional carbon-based materials. Its strong B–N covalent bonds and inherent structural rigidity suggest versatile applications, particularly in hydrogen storage technology, where chemisorption and physisorption mechanisms have been explored. The review underscores that h-BN-based materials offer reliable performance characteristics, including improved cycling and regeneration compared to conventional systems. However, one significant challenge remains: the lack of cost-effective, scalable production methods to fabricate these nanostructures at an industrial level, alongside efficient post-synthesis modifications to enhance their hydrogen absorption capacity. Kovalskii et al. emphasize that targeted strategies are key to optimizing performance. The approaches include increasing the effective specific surface area, engineering structural defects, tuning interplanar distances, and doping with elements like carbon and oxygen, or functionalizing surfaces with metal clusters. These modifications have shown potential to boost hydrogen uptake within a range of 0.1–9.67 wt%, making the material particularly promising for high-demand applications in automotive energy storage. Both experimental studies and first-principles and density functional theory (DFT) calculations consistently reveal the high potential of h-BN-based materials for effective hydrogen storage. Although theoretical predictions often suggest slightly higher capacities than those measured in laboratory settings, this divergence indicates the need for further experimental investigation to fine-tune material performance. Improved control over hydrogen release and material reuse will also be essential for transforming h-BN from a laboratory curiosity into a commercialization of hydrogen storage system. This work not only elucidates the underlying mechanisms that govern hydrogen sorption in h-BN but also charts a clear course for future research.
Chava et al. have delivered a detailed research study on how the increasing demand for efficient and safe energy storage drives rapid advancements in solid-state battery (SSB) technology. These batteries offer higher capacity and improved safety compared to traditional liquid-electrolyte lithium-ion batteries, yet challenges such as lithium dendrite formation and poor solid–solid interfaces continue to hinder their widespread use. Their study addresses these obstacles by enhancing ceramic-based solid-state electrolytes through nanoscale doping with multicomponent halides. Guided by green chemistry principles, the team synthesized Li3InCl6-based electrolytes using an eco-friendly nanoemulsion method with water and natural aloe vera extract. The electrolytes were doped with fluorine (F), cerium (Ce), and molybdenum (Mo), with each dopant offering distinct benefits: The element F improved lattice stability and facilitated Li+ ion mobility; Ce bolstered structural integrity while reducing interfacial resistance; and Mo optimized ionic conductivity, achieving performance levels comparable to commercial liquid electrolytes. The structural and electrochemical analyses revealed that Mo-doped Li3InCl6 crystallizes in a triclinic structure, where modifications in electron density and reduced activation energy barriers lead to enhanced ion transport. Electrochemical impedance spectroscopy confirmed that Mo-doping markedly lowered the charge transfer resistance, indicating more efficient ionic pathways. These results underscore the pivotal role of Mo and other elements in enhancing the electrolyte’s structural stability and overall ionic conductivity. The innovative synthesis approach advances the performance of solid-state electrolytes and aligns with sustainable practices. By employing green chemistry, the research team achieved a 40% reduction in energy consumption and a 75% decrease in hazardous waste generation compared to conventional methods, supporting broader environmental goals. Furthermore, their investigation illuminates the complex interactions among dopants, lattice modifications, ionic occupancy, and structural vacancies. The cyclic voltammetric and Nyquist plot analyses further validate the doped electrolyte’s robust electrochemical performance and stability, confirming its potential for next-generation SSB applications. The study suggests that future research should explore additional doping strategies and integrate advanced computational modeling and machine-learning techniques to optimize electrolyte design further. These efforts are essential for developing sustainable, high-performance energy storage solutions that meet modern demands while addressing critical environmental and resource challenges.
As outlined by Li et al., the shrinkage behavior of porous materials is a critical factor in the drying process, as it profoundly influences both the final product’s texture and the efficiency of energy utilization. This phenomenon is governed by various factors, including the cellular structure, drying conditions, and the material’s glass transition temperature. Advancing our understanding of the drying process necessitates the development of theoretical models that integrate simultaneous heat and mass transfer at the cellular level, as well as simulation tools to analyze morphological changes during drying. This paper examines the key factors influencing shrinkage in porous materials during drying, alongside discussions on modeling approaches, morphological simulations, and considerations for designing drying technologies. These insights aim to guide improvements in product quality and energy efficiency. Drying, a key material preservation process, uses heat to remove moisture, extending shelf life while reducing weight and storage costs. Porous materials undergo shrinkage and deformation during drying, influenced by temperature and moisture migration, impacting energy efficiency, product quality, and equipment design. Higher temperatures increase shrinkage effects. Their study identifies major factors affecting shrinkage behavior, summarizes theoretical and simulation approaches to understand material deformation during drying, and evaluates various industrial drying techniques. Understanding the interplay of these factors during drying is vital for optimizing drying equipment, reducing energy usage, and ensuring the quality and longevity of dried products. The major achievements made by the authors are four folds:
1. Impact of Cellular Structure and Temperature: Shrinkage is strongly influenced by water migration within cellular structures and the mechanical properties of cell walls. Among various drying conditions, temperature plays the most significant role. When drying occurs below the glass transition temperature (Tg), the material assumes a highly viscous state, reducing shrinkage.
2. Multi-Scale Modeling Challenges: Multi-scale models, which span macro-scale and micro-scale perspectives, offer effective tools for analyzing drying processes. However, reducing computational complexity and modeling costs remains a significant challenge in meeting the diverse accuracy requirements of different applications.
3. Simulation Gaps: While computer graphics simulations have primarily focused on plant growth modeling, fewer studies have examined the mechanical deformation of porous materials during drying. Addressing these gaps will enhance our understanding of drying-induced changes.
4. Assessment of Drying Techniques: Different drying methods have unique strengths and weaknesses. Hot air is cost-effective but may deform materials. The microwave is fast yet uneven. Freeze drying preserves structure but is slow. Infrared offers quality control, while vacuum suits heat-sensitive materials.
The editorial team comprises three guest editors and one Research Topic coordinator with diverse expertise spanning academia, industry, and national laboratory. Their collective contributions advance materials design and evaluation, with several members renowned for pioneering applications of these materials in energy system development. Together, they bring a wealth of knowledge and innovation to the field. Dr. Vanadium Li has been serving as a Full Professor in the Department of Chemical Engineering at the Beijing University of Technology since 2018. His research expertise lies in the study of variable valence transition metal elements, focusing on their roles in material processes under high-temperature and wet chemical conditions. Dr. Li specializes in mixed valence oxides, particularly Magnéli phases, and their advanced applications in electro-catalysis and related fields. Dr. Li has an extensive publication record, authoring approximately 50 books and journal articles that have contributed significantly to the advancement of his field. His groundbreaking research has earned him key leadership roles, serving as Principal Investigator (PI) and Co-Principal Investigator (Co-PI) for numerous national and international research projects. Dr. Li’s innovative work in mixed valence oxide systems and catalytic processes continues to push the boundaries of material science, offering practical solutions to challenges in energy conversion, catalysis, and sustainable materials development. His contributions demonstrate a commitment to advancing scientific knowledge and fostering innovation in chemical engineering.
Dr. Ayrat Gizzatov is a distinguished leader in sustainable energy innovation and materials science, currently serving as Senior Manager of R&D Innovation at Huntsman Corporation. In this role, he leads initiatives in methane pyrolysis to produce hydrogen and carbon nanotubes. Prior to joining Huntsman, Dr. Gizzatov was a Senior Researcher at the Aramco Research Center in Boston, focusing on enhanced oil recovery, CO2 sequestration, and alternative energy carriers. His research during this period included the development of nanofluids for enhanced oil recovery and CO2 sequestration techniques. Dr. Gizzatov has also made significant contributions to the field of medical contrast agents, particularly in the development of magnetic nanoconstructs for tumor imaging and graphene-based materials for improved MRI relaxivity. An active member of the American Chemical Society’s Division of Energy and Fuels (ENFL), Dr. Gizzatov has organized various symposia on alternative energy carriers and contributed to the division’s initiatives. He served as Chair of the Communication and Technology Committee for the Energy and Fuels Division of the American Chemical Society, demonstrating leadership and dedication to advancing the division’s mission.
Dr. Honghong “Crystal” Shi is a Staff Scientist at the Institute for Integrated Catalysis (IIC) at the Pacific Northwest National Laboratory (PNNL). She is an exceptional young scientist whose innovative work in chemical engineering is driving advancements in catalysis and sustainable processes. She earned her Ph.D. in Chemical Engineering with honors from the University of Kansas in December 2019, demonstrating academic excellence and pioneering research contributions. Her research spans diverse and impactful areas, including experimental studies on single-atom catalysts (SACs) for hydrogenation and aldol-condensation reactions, collaborative X-ray absorption spectroscopy (XAS) investigations with the Advanced Photon Source (APS), and the development of innovative reaction systems utilizing liquid-phase CO2 for the ozonolysis of aromatic compounds. Additionally, she has explored material characterizations and kinetic studies of Pt-based bimetallic catalysts for the oxidative conversion of biomass-derived sugars and polyols, alongside thermochemical conversion studies and heating value modeling of municipal solid wastes. Recognized for her contributions to science, Dr. Shi has been awarded numerous accolades, including the first Place Poster Award at the Pacific Coast Catalysis Society Meeting (PCCS) in 2022 and the Young Scientist Presenter Award at the 17th International Congress on Catalysis (ICC) in 2020. Her publication record spans a variety of high-impact journals, showcasing her dedication to advancing knowledge and innovation in chemical engineering and catalysis.
Dr. J. Louise Liu is a Full Professor at Texas A&M University-Kingsville and an affiliated faculty member of the Texas A&M Energy Institute. Her research focuses on designing and characterizing nanostructured materials for sustainable energy and forensic science applications. Her team has developed graphene-ceramic cathodic materials, solid electrolytes for lithium-ion batteries, and achieved record-breaking power densities for proton exchange membrane fuel cells. Pioneering the use of metal-organic frameworks in disinfection, her lab has also created advanced blood simulants for crime scene reconstruction and bio-compatible fingerprint extraction agents, improving efficiency by 40% over commercial alternatives. Dr. Liu has produced over 160 works, including peer-reviewed papers, books, patents, and more than 180 presentations. A co-founder of a small business, Louise NanoInnovation North American, LLC, she brings over 20 years of advanced R&D experience, having led multi-million-dollar projects and developed innovative energy solutions. An expert in R&D management, she integrates safety analysis, process improvement, and workforce training into her efforts. Recognized as a Fellow by the American Chemical Society, Royal Society of Chemistry, and Linnean Society, Dr. Liu has received numerous awards, including the Distinguished Texas Scientist, The IUPAC Distinguished Women in Chemistry or Chemical Engineering Award in 2021.
Author contributions
VL: Investigation, Writing – review and editing. AG: Data curation, Writing – review and editing. HS: Writing – review and editing. JL: Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author AG was employed by Huntsman Advanced Materials. Author JL was employed by Louise Nanoinnovation North American LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: porous materials, energy materials, multi-scale models, drying techniques, emerging energy technologies
Citation: Li V, Gizzatov A, Shi H and Liu JL (2025) Editorial: Functionalization of porous materials for sustainable energy applications. Front. Mater. 12:1604024. doi: 10.3389/fmats.2025.1604024
Received: 01 April 2025; Accepted: 07 April 2025;
Published: 08 May 2025.
Edited and reviewed by:
Liming Dai, University of New South Wales, AustraliaCopyright © 2025 Li, Gizzatov, Shi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingbo Louise Liu, amluZ2JvLmxpdUB0YW11ay5lZHU=
 Vanadium Li1
Vanadium Li1 Ayrat Gizzatov
Ayrat Gizzatov Jingbo Louise Liu
Jingbo Louise Liu