- 1Medical Research Center, Chifeng Municipal Hospital, Chifeng, China
- 2Medical Aesthetic Center, Chifeng Municipal Hospital, Chifeng, China
- 3Department of Radiation Therapy, Chifeng Municipal Hospital, Chifeng, China
- 4Basic Medical College, Chifeng Clinical Medical College of lnner Mongolia Medical College, Chifeng, China
- 5Department of Dermatology, Chifeng Municipal Hospital, Chifeng, China
Radiation-induced dermatitis (RID) remains one of the most prevalent and therapeutically challenging complications in cancer radiotherapy, significantly impairing patient quality of life and treatment adherence. In recent years, bioengineered materials have emerged as promising platforms for the prevention and treatment of RID through multifunctional mechanisms. This review systematically summarizes the current landscape of biomaterials applied to radiation-induced skin injury, focusing on the regulation of oxidative stress, inflammatory responses, and regenerative tissue repair. Beyond conventional classifications based on function—such as barrier protection, therapeutic delivery, and tissue reconstruction—we highlight advances in biomaterial design mechanisms. Particular attention is given to surface properties, including roughness, electrical charge, and crosslinking dynamics, which influence immune modulation and cellular behavior at the wound interface. Mechanistic insights are discussed regarding reactive oxygen species-responsive materials, macrophage phenotype regulation, and vascular regeneration in irradiated tissue environments. Comparative analyses with conventional wound dressings, such as alginate-based and silver-containing materials, underscore the superior therapeutic efficacy of biointeractive and stimuli-responsive systems. In addition, emerging technologies including three-dimensional bioprinting, exosome-inspired scaffolds, and multi-responsive drug carriers are critically evaluated for their translational potential. Clinical trials, regulatory pathways, and manufacturing considerations are also discussed to outline future directions for clinical implementation. This review provides a comprehensive and mechanism-driven perspective on next-generation biomaterials for precision treatment of radiation-induced skin damage.
1 Radiation dermatitis: pathogenesis and therapeutic challenges
1.1 Pathophysiological features of radiation-induced skin injury
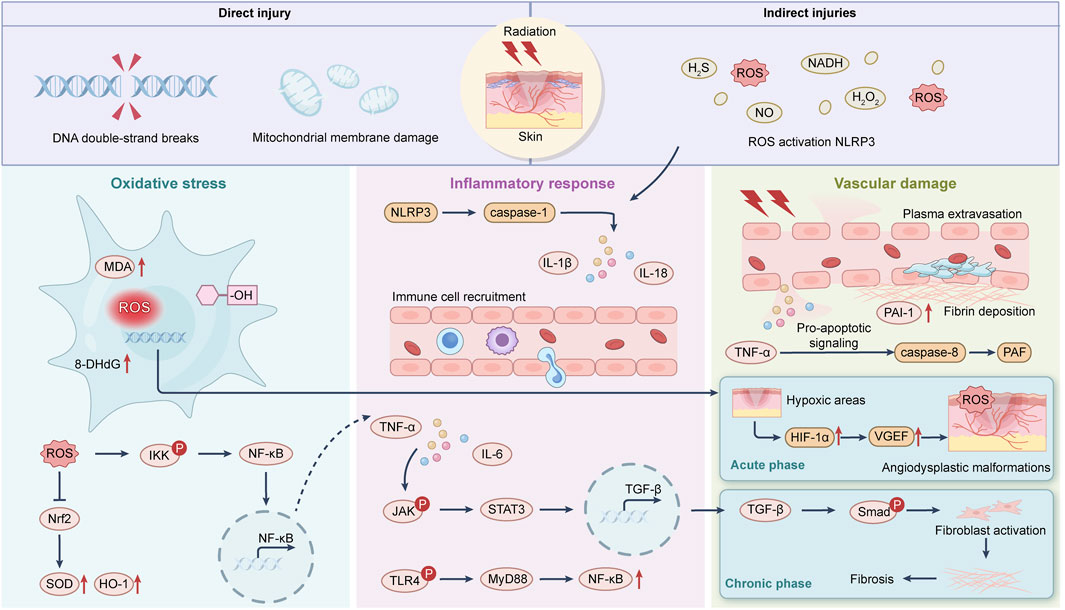
RID (RID) is a frequent and clinically significant complication resulting from therapeutic ionizing radiation exposure. It encompasses a spectrum of skin reactions ranging from transient erythema to chronic ulceration and necrosis. The pathological cascade is primarily driven by overproduction of reactive oxygen species (ROS) and sustained inflammatory responses, leading to cumulative damage across multiple skin compartments (Iacovelli et al., 2020) (Figure 1).
At the molecular level, ionizing radiation causes water radiolysis and mitochondrial dysfunction, generating superoxide anions, hydroxyl radicals, and hydrogen peroxide. These ROS species trigger DNA strand breaks, protein oxidation, and lipid peroxidation, compromising the viability of keratinocytes, fibroblasts, and endothelial cells. DNA damage response mechanisms are activated but often overwhelmed in high-dose or fractionated radiation settings (Pazdrowski et al., 2024).
The oxidative insult initiates a secondary inflammatory cascade. Damaged cells release danger-associated molecular patterns (DAMPs), activating resident macrophages and dendritic cells through Toll-like receptors (TLRs) and NF-κB signaling. This results in upregulation of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and increased matrix metalloproteinase (MMP) activity. These mediators disrupt basement membrane integrity and promote capillary leakage, amplifying tissue damage (Yang et al., 2020).
Vascular damage is another key feature of RID. Radiation induces endothelial cell apoptosis, microvascular thrombosis, and capillary rarefaction, contributing to tissue hypoxia and impaired nutrient exchange. Chronic ischemia exacerbates oxidative stress and impedes recruitment of progenitor cells and immune-regulatory elements necessary for wound resolution (Cui et al., 2024).
In the chronic phase, repeated inflammatory insults and altered fibroblast signaling lead to pathological extracellular matrix (ECM) remodeling and fibrosis (Yarnold and Vozenin Brotons, 2010). The balance between matrix deposition and degradation is disrupted, often resulting in excessive collagen I accumulation, reduced elastin content, and thickened dermal layers. Persistent oxidative stress also depletes epidermal stem cell pools and delays re-epithelialization, rendering wounds refractory to conventional treatments (Straub et al., 2015).
RID involves a complex interplay between oxidative injury, inflammation, vascular dysfunction, and stem cell exhaustion. These pathological events create a hostile microenvironment that hinders natural repair processes, necessitating biomaterial-based interventions tailored to modulate redox states, immune responses, and tissue regeneration.
1.2 Material–cell interaction at the oxidative stress–inflammation axis
Ionizing radiation induces a sustained oxidative environment in cutaneous tissue, marked by elevated ROS levels and imbalanced redox homeostasis. This biochemical shift compromises cell viability and amplifies local inflammation through pro-inflammatory cytokine and DAMP release. Recent bioengineering advances highlight functional biomaterials’ capacity to modulate stress–inflammation pathways via direct cellular interactions (Dong et al., 2020).
Nanoceria-based systems, possessing intrinsic superoxide dismutase (SOD) and catalase-mimetic properties, attenuate intracellular ROS accumulation in irradiated keratinocytes and fibroblasts, reducing DNA double-strand breaks and p53-mediated apoptosis (Celardo et al., 2011). Concurrently, these systems suppress NF-κB nuclear translocation and downregulate TNF-α and IL-1β expression, reducing leukocyte infiltration and edema (Khurana et al., 2018). Similarly, Zn2+-modified hydrogels enhance M2 macrophage polarization by increasing IL-10 secretion and STAT6 activation, shifting immune responses from pro-inflammatory to regenerative phenotypes (Li et al., 2025).
At the cellular interface, electrostatic interactions between biomaterial surfaces and immune cell membranes critically modulate downstream signaling. Materials with tailored surface charges or zwitterionic domains exhibit reduced fibrotic encapsulation and dampen monocyte-derived macrophage activation in ROS-rich environments (Zhang et al., 2013). Modulation of focal adhesion kinase (FAK) and PI3K/Akt signaling via bioactive matrices promotes keratinocyte migration and endothelial survival, supporting re-epithelialization under oxidative stress (Teng et al., 2021).
Collectively, engineered biomaterials reprogram cell behavior within irradiated dermal niches. By modulating redox signaling, immune cell plasticity, and cell–matrix adhesion dynamics, these materials exert therapeutic control over chronic inflammation and delayed repair in RD.
2 Fundamentals of biomaterials in skin repair
Biomaterials designed for cutaneous wound healing exhibit diverse structural compositions and functional roles, ranging from passive protection to active modulation of the wound microenvironment. Their performance in RID is critically dependent on properties such as biocompatibility, degradation profile, porosity, mechanical compliance, and capacity to interact with cellular and molecular components of irradiated skin (Li et al., 2023).
Natural polymers, particularly collagen, hyaluronic acid, and chitosan, demonstrate high biocompatibility and hydration retention, which are essential for maintaining the moist environment favorable for epithelial migration (Sionkowska, 2021). Crosslinked collagen matrices have been shown to support keratinocyte proliferation and reduce inflammatory cytokine expression in irradiated models, while chitosan derivatives modulate macrophage polarization through surface deacetylation profiles (Dev et al., 2025).
Synthetic polymers, including polyethylene glycol (PEG), polyvinyl alcohol (PVA), and poly (lactic-co-glycolic acid) (PLGA), offer tunable physicochemical properties, enabling precise control over degradation kinetics, drug release, and mechanical strength. PEG-based hydrogels with adjustable crosslinking densities have exhibited controlled swelling behavior and delayed erosion in ROS-rich dermal environments (Wang et al., 2023). Moreover, incorporation of inorganic nanoparticles such as ceria or bioactive glass into polymeric matrices further extends functionality, enabling ROS scavenging and angiogenic activation without inducing cytotoxicity (Kurtuldu et al., 2021).
Hydrogels represent a key class of soft biomaterials in skin repair due to their high water content and ability to serve as drug reservoirs. Radiation-injured tissues benefit from hydrogels’ capacity to modulate interstitial pH and buffer enzymatic degradation (Su et al., 2022). ROS-sensitive linkers and enzymatically cleavable backbones allow responsive drug delivery synchronized with inflammatory fluctuations (Tao and He, 2018). Additionally, hydrogel stiffness has been directly correlated with fibroblast activation and matrix remodeling, suggesting that mechanical tuning is an integral part of biomaterial design for fibrosis-prone wounds (Smithmyer et al., 2014).
Porosity and topological cues also influence cellular infiltration and vascular ingrowth. Micro and nano-structured scaffolds fabricated via electrospinning or 3D bioprinting facilitate aligned cellular migration, enhance capillary morphogenesis, and reduce fibrotic encapsulation in irradiated skin (Cheng et al., 2025). The surface energy and wettability of materials impact protein adsorption and immune cell adhesion, ultimately shaping the inflammatory microenvironment (Protein interactions at material surfaces, 2025).
The fundamental design of wound-repair biomaterials integrates biochemical compatibility, mechanical resilience, and dynamic responsivity. These foundational attributes form the platform upon which multifunctional systems for radiation dermatitis are engineered.
3 Functional applications of advanced biomaterials
3.1 Integrated multifunctionality in next-generation biomaterials
Multifunctionality has emerged as a core design principle in advanced biomaterials for treating radiation-induced skin injury, driven by the recognition that no single therapeutic function is sufficient to reverse the complex, multistage pathogenesis of irradiated wounds (Man et al., 2024). Modern platforms increasingly combine anti-oxidative, anti-inflammatory, angiogenic, and regenerative properties into unified constructs, enabling synchronous modulation of overlapping biological pathways (Tang et al., 2021).
Ceria-based nanogels conjugated with polyethylene glycol and encapsulated anti-inflammatory agents have shown simultaneous ROS scavenging and suppression of NF-κB signaling, resulting in reduced TNF-α expression and improved epithelial thickness in irradiated dermis (Kim et al., 2024; Fu et al., 2024). This convergence of redox buffering and immunomodulation leads to improved wound perfusion and reduced inflammatory persistence. Similarly, exosome-loaded hydrogels have demonstrated the capacity to activate vascular endothelial growth factor (VEGF) signaling while decreasing IL-6 levels, reflecting both angiogenic and anti-inflammatory efficacy (Yang et al., 2025).
From a structural perspective, hydrogel matrices with controlled viscoelasticity and degradability enable the staged release of multiple bioactive molecules in response to wound cues such as pH, ROS, or enzyme levels (Zhang et al., 2025). These responsive systems maintain optimal drug bioavailability while minimizing off-target effects. In parallel, composite systems integrating nanoparticles and polymer networks, such as gelatin-methacrylate hydrogels doped with zinc or cerium, offer mechanical support and microenvironmental modulation tailored to radiation-damaged tissues (Nichol et al., 2010).
Importantly, multifunctional designs also exploit material-cell feedback loops. For example, microenvironment-responsive biomaterials can polarize macrophages toward M2 phenotypes while simultaneously promoting fibroblast migration and extracellular matrix (ECM) remodeling. These cellular programs are essential in restoring barrier integrity and resolving fibrosis in chronic wounds (Guan et al., 2023).
Integrated multifunctionality in biomaterials is not a modular addition of functions but a synergistic strategy to co-regulate inflammation, redox homeostasis, angiogenesis, and matrix dynamics. This systems-level approach is increasingly critical in addressing the biologically layered challenges of radiation-induced skin injury.
3.2 Barrier-forming biomaterials
Barrier-forming biomaterials play a critical role in the early management of radiation-induced skin injury by stabilizing the wound environment and preventing external contamination. These materials form a physical interface that maintains moisture balance, protects underlying tissue from mechanical stress and microbial invasion, and modulates the local inflammatory milieu. In the context of irradiated skin, where epithelial integrity and immune defense are compromised, barrier functionality is essential for preventing secondary damage and facilitating subsequent regeneration (Kobayashi et al., 2019).
Polysaccharide-based systems, such as those derived from alginate or hyaluronic acid, have demonstrated favorable film-forming properties and high water retention, contributing to reduced transepidermal water loss in murine models of radiation dermatitis (Zarei and Hassanzadeh-Tabrizi, 2023; Sepe et al., 2025). Their gel-forming ability in situ supports close contact with irregular wound surfaces, which is particularly important for conforming to irradiated anatomical regions (Asai et al., 2023). Modifications of alginate with divalent cations such as Ca2+ enhance mechanical strength and prolong barrier duration, while simultaneously modulating macrophage response and reducing IL-6 expression (Morrell et al., 2018).
Synthetic barrier materials such as polyurethane (PU) and polyvinyl alcohol (PVA) foams have been explored for their robust mechanical properties and hydrophobicity. PU dressings with microporous surfaces allow vapor transmission while preventing liquid ingress, effectively isolating the wound from external pathogens (Gaaz et al., 2015). These materials also reduce nociceptor activation and erythema severity in fractionated radiation models. However, they often lack intrinsic bioactivity and require functionalization to exert antioxidative or anti-inflammatory effects.
Surface chemistry and topology are increasingly recognized as determinants of barrier performance. Nanostructured coatings and zwitterionic polymers exhibit resistance to protein fouling and bacterial adhesion, reducing the risk of secondary infection and promoting a cleaner wound bed (Joshi et al., 2018). Hydrogels containing amphiphilic copolymers have demonstrated self-healing and mucoadhesive behavior, enabling prolonged residence time on mobile anatomical regions (Wen et al., 2024).
Despite their passive nature, modern barrier materials are being re-engineered toward hybrid functions. Composite barriers incorporating ROS-scavenging nanoparticles or antimicrobial peptides offer both protective and therapeutic actions without compromising structural integrity (ROS, 2025). This convergence of structural shielding and biochemical activity extends the utility of barrier systems beyond initial wound coverage.
3.3 Drug delivery systems
The application of drug delivery systems in RID represents a major advancement in achieving spatially and temporally controlled therapeutic modulation of the wound microenvironment (Ahmadi et al., 2020). Conventional topical agents often suffer from short half-life, poor penetration, and nonspecific biodistribution, which limit their efficacy in chronic irradiated wounds. In contrast, stimuli-responsive delivery platforms enhance therapeutic precision by synchronizing drug release with local biochemical cues such as ROS levels, matrix metalloproteinases (MMPs), or pH gradients (Zheng et al., 2023).
Hydrogel-based carriers remain the most extensively studied systems due to their high water content, biocompatibility, and versatility in encapsulating both hydrophilic and hydrophobic drugs. Crosslinked networks embedded with corticosteroids, anti-TNFα agents, or interleukin inhibitors have demonstrated enhanced retention time and reduced systemic exposure in preclinical radiation models (He et al., 2024). Moreover, hydrogels incorporating disulfide or thioketal linkers undergo cleavage in ROS-rich environments, enabling on-demand release of anti-inflammatory payloads without disturbing intact tissues (Tao and He, 2018).
Nanoparticulate systems, such as micelles, dendrimers, liposomes, and metal-organic frameworks, enable deep tissue penetration and protection of bioactives from premature degradation (Singh et al., 2024). PEGylated cerium oxide nanoparticles not only act as ROS scavengers but also serve as carriers for siRNAs targeting NF-κB signaling, thereby combining gene silencing with antioxidative action (Nelson et al., 2016). Liposomal formulations co-loaded with VEGF and basic fibroblast growth factor (bFGF) have been shown to promote angiogenesis and dermal repair in fractionated irradiation models (Luan et al., 2012).
The emergence of exosome-inspired and cell membrane-coated vesicles introduces bio-interfacing capabilities that mimic native intercellular communication. These platforms exhibit intrinsic tropism to inflamed or hypoxic tissues and deliver microRNAs or immunomodulatory proteins with high specificity (Zhuo et al., 2025). Furthermore, thermoresponsive and self-healing gels allow dynamic adhesion to mobile anatomical regions, preserving sustained local delivery without frequent reapplication (Wang et al., 2022).
By integrating responsive chemistry, biological targeting, and mechanical adaptability, drug delivery systems have transformed from passive depots into active regulators of tissue homeostasis. Their convergence with barrier-forming and regenerative biomaterials represents a frontier for multifunctional composite therapies in radiation dermatitis.
3.4 Regenerative biomaterials
In radiation-induced skin injury, endogenous regenerative capacity is often suppressed due to basal stem cell depletion, disrupted ECM, and microvascular rarefaction (Cui et al., 2024). As a result, regeneration-oriented biomaterials have gained increasing attention for their ability to actively restore tissue integrity by engaging cellular repair programs, modulating growth factor signaling, and supporting neovascularization (Farag, 2023).
Hydrogel scaffolds engineered with ECM-mimetic properties provide a provisional matrix that supports keratinocyte and fibroblast adhesion, migration, and proliferation (Zhu and Marchant, 2011). Gelatin methacrylate (GelMA)-based constructs, when tuned to match physiological stiffness (5–15 kPa), have been shown to upregulate integrin β1 expression and promote re-epithelialization in irradiated wounds (Bupphathong et al., 2022). Furthermore, hybrid scaffolds incorporating collagen, fibrin, and hyaluronic acid restore biomechanical cues while allowing for cell infiltration and matrix remodeling (Egorikhina et al., 2021).
The incorporation of pro-regenerative agents within biomaterials further enhances their therapeutic potential. Controlled release of growth factors, such as VEGF, epidermal growth factor (EGF), and platelet-derived growth factor (PDGF), from nanoporous or thermosensitive carriers stimulates angiogenesis, dermal fibroblast activation, and granulation tissue formation (Nurkesh et al., 2020). Exogenous application of stromal cell-derived exosomes embedded in hydrogel matrices has been reported to elevate miR-21 and miR-126 levels in irradiated dermis, leading to enhanced endothelial sprouting and reduced inflammatory infiltration (Xie et al., 2022).
Beyond soluble factors, regenerative biomaterials also rely on topographic and biochemical cues. Nanoscale surface patterning and ligand-presenting hydrogels enhance mechanotransduction and modulate YAP/TAZ-dependent transcription in epithelial progenitor cells (Francis et al., 2024). These effects restore epithelial polarity and basement membrane integrity, which are often compromised following radiation exposure.
In parallel, vascularization remains a critical bottleneck in successful regeneration. Composite scaffolds incorporating bioactive glass, copper-doped ceramics, or silicon nanoparticles stimulate HIF-1α expression and endothelial tube formation under oxidative stress conditions (Kargozar et al., 2021). These angiogenic effects not only support oxygen delivery but also facilitate clearance of cellular debris and inflammatory mediators.
Regenerative biomaterials are moving toward integrated designs that recapitulate both structural and signaling functions of native skin. Their ability to orchestrate cell–matrix–vascular interactions under hostile post-radiation conditions is central to achieving durable wound resolution.
3.5 Comparative analysis with conventional dressings
Traditional dressings, including silver-impregnated foams, alginate sheets, and petrolatum gauze, remain commonly used in the clinical management of RID due to their availability and basic wound coverage capabilities (Finkelstein et al., 2022). However, these materials are inherently passive and often insufficient in addressing the complex pathophysiology of irradiated skin, particularly in cases involving persistent oxidative stress, inflammatory infiltration, and impaired angiogenesis (Behroozian et al., 2023).
Meta-analyses of silver-containing dressings reveal moderate efficacy in reducing bacterial colonization but negligible effects on inflammatory cytokine profiles or re-epithelialization rates (Jiang et al., 2024). Furthermore, long-term use of silver-based materials has been associated with cytotoxicity toward keratinocytes and fibroblasts, delaying granulation tissue formation and increasing the risk of secondary ulceration (Poon and Burd, 2004). Similarly, alginate dressings offer high absorbency and moisture retention but provide no active modulation of redox balance or immune response (Aderibigbe and Buyana, 2018).
In contrast, advanced biomaterials demonstrate superior multifunctionality by integrating stimuli-responsive delivery, immunomodulation, and structural mimicry. In a murine model of skin injury, cerium oxide-loaded hydrogels significantly reduced erythema scores (by 56%) and accelerated wound closure (by 40%) compared to silver foams over a 10-day treatment window (Huang et al., 2022). Exosome-functionalized matrices showed enhanced epithelial thickness and microvascular density, correlated with upregulation of VEGF and downregulation of TNF-α levels (Su et al., 2021).
Furthermore, dynamic hydrogels with ROS- or pH-cleavable linkers allow real-time drug release in response to injury severity, enabling tight control over therapeutic dosing. These systems outperform conventional dressings not only in histological repair metrics but also in patient-centered outcomes, such as reduced pain scores and improved skin elasticity (Wu et al., 2022).
Clinical studies have increasingly confirmed the therapeutic benefits of advanced biomaterials in managing radiation-induced skin injuries. In a randomized controlled trial conducted by Tayyib, the prophylactic application of Mepitel® film in 60 patients undergoing radiotherapy resulted in a 57% reduction in grade 2 or higher moist desquamation, with an average reduction in healing time of 5.2 days compared to conventional petroleum based gauze dressings (Behroozian et al., 2023). In a prospective trial involving 108 patients with second degree radiation burns demonstrated that multifunctional hydrogel dressings, formulated with antioxidant and anti-inflammatory agents, facilitated epidermal regeneration 43% faster than standard treatments. Patients in the hydrogel group also reported significantly lower pain scores across all radiation dose subgroups (Cui et al., 2025).
In the context of chronic radiation ulcers refractory to standard care, Zasadziński reported that dressings incorporating collagen alginate nanocomposites achieved complete re-epithelialization in 82% of patients within 3 weeks, compared to only 41% in the control group. These results underscore the regenerative advantage of composite biomaterials in treating delayed healing wounds (Zasadziński et al., 2022). Zhou provided compelling translational evidence for hydrogel-based delivery platforms integrating growth factors and cerium oxide nanoparticles. Treated skin biopsies showed significantly reduced levels of pro-inflammatory cytokines, including TNF-α and IL-6, accompanied by improved dermal elasticity and decreased fibrosis, highlighting both molecular and structural benefits (Zhou et al., 2022).
Taken together, these clinical investigations validate that the integration of stimuli responsive drug release, immune modulation, and biophysical tissue mimicry in next-generation biomaterials results in measurable improvements in RID skin injury. These include enhanced epithelial restoration, reduced pain and inflammation, fewer dressing changes, and improved patient reported outcomes, ultimately contributing to better treatment adherence and quality of life.
3.6 Advanced technologies in radiation dermatitis management
Recent advances in materials science and biofabrication have catalyzed a shift from conventional wound dressings toward next-generation technologies that combine architectural fidelity, cellular instruction, and real-time responsiveness. In the treatment of RID, emerging modalities such as 3D bioprinted skin substitutes, exosome-mimetic systems, and intelligent stimuli-responsive materials are positioned to overcome limitations of traditional passive scaffolds by offering spatial precision, molecular targeting, and adaptive bioactivity (Annals of 3D Printed Medicine, 2022).
3D bioprinting enables the fabrication of skin constructs with spatially defined epidermal, dermal, and vascular compartments. Layered hydrogels incorporating keratinocytes and fibroblasts, when deposited with resolution-matched bioinks, have demonstrated enhanced engraftment and faster epithelialization compared to acellular matrices (Shi et al., 2024). Moreover, bioprinted constructs with microchannel networks have been shown to accelerate perfusion and reduce hypoxia in irradiated wounds, restoring redox balance and stem cell viability. Preclinical models suggest that vascularized printed grafts outperform traditional skin substitutes in metrics such as collagen alignment and transepidermal barrier function (Bertassoni et al., 2014).
Exosome-inspired biomaterials represent another frontier, leveraging the endogenous intercellular communication pathways of extracellular vesicles. Engineered hydrogels encapsulating mesenchymal stem cell-derived exosomes have shown significant upregulation of VEGF and miR-126, along with suppression of TNF-α and IL-6 in irradiated dermal tissue (Khayambashi et al., 2021). These systems enable paracrine reprogramming of macrophages and endothelial progenitors without the immunogenic risk of live-cell therapy. Furthermore, surface-engineered vesicles with integrin ligands enhance lesion-specific targeting, improving localization and bioavailability of therapeutic cargo (Approaches to surface engineering of extracellular vesicles, 2021).
Smart-responsive materials further extend the functional scope of biomaterials by synchronizing therapeutic output with dynamic wound environments. Multi-responsive systems, incorporating linkers cleavable by ROS, MMPs, or acidic pH, enable spatiotemporally controlled drug release. For example, dual-sensitive hydrogels have been shown to deliver anti-inflammatory drugs during acute inflammation while releasing angiogenic cues in the remodeling phase (Ruan et al., 2024). Thermosensitive and self-healing polymers additionally support application on anatomically dynamic regions such as joints and neck (Liu et al., 2025).
Despite the promising potential of emerging biomaterial technologies such as 3D bioprinting and exosome-functionalized scaffolds in the treatment of radiation-induced skin injury, several critical barriers hinder their clinical translation. For 3D bioprinting platforms, one of the primary technical challenges lies in maintaining cell viability and spatial resolution during the fabrication of complex skin architectures. Bioink properties such as viscosity, crosslinking kinetics, and biocompatibility must be optimized to support both structural fidelity and vascular integration, particularly in irradiated tissue environments where microvascular networks are often compromised. Moreover, the scalability of bioprinted constructs remains limited, with most current systems confined to laboratory-scale or proof-of-concept models, lacking automated, reproducible manufacturing workflows suitable for clinical-grade production (Lv et al., 2024).
A major concern in the clinical translation of exosome-functionalized scaffolds is their physicochemical and biological stability, both during storage and upon application. Exosomes are inherently fragile nanovesicles that can undergo membrane degradation, aggregation, and loss of cargo functionality when exposed to environmental stressors such as temperature fluctuations, pH shifts, or lyophilization cycles. Studies have shown that storage at −80°C preserves exosomal integrity, but this requirement complicates large-scale clinical deployment due to cold chain logistics.When incorporated into biomaterial scaffolds, interactions between exosomes and the surrounding matrix can significantly influence both release kinetics and structural stability. Factors such as electrostatic binding, non-specific adsorption, or premature diffusion may lead to burst release or uneven spatial distribution, thereby resulting in unpredictable therapeutic outcomes (Fan, 2024). Furthermore, degradation byproducts from hydrogel matrices, particularly acidic compounds generated by natural polymers, can compromise the integrity of exosomal membrane proteins and RNA cargo. The absence of standardized criteria for evaluating exosome scaffold compatibility, including parameters such as surface charge, hydration dynamics, and steric interference, further contributes to inconsistencies across studies.
To overcome these challenges, researchers have investigated stabilization strategies including chemical crosslinking, affinity-based immobilization using CD63 antibody conjugates, and encapsulation within secondary carriers such as liposomes or biodegradable nanoparticles (Hu et al., 2024). However, most of these approaches remain limited to preclinical research, and their influence on in vivo stability, immune clearance, and controlled release profiles has yet to be systematically validated in human models of skin injury.
4 Conclusion and translational outlook
4.1 Summary of mechanistic efficacy and therapeutic potential
The development of biomaterials for radiation-induced skin injury has evolved from monofunctional passive scaffolds toward multifaceted platforms capable of synchronizing biological regulation across oxidative, inflammatory, angiogenic, and regenerative axes. However, systematic evaluation of these materials remains challenging due to the diversity of formulations, heterogeneity in injury models, and variability in outcome metrics. An integrated assessment must consider not only structural and physicochemical parameters but also mechanistic efficacy and translational feasibility.
At the cellular level, anti-oxidative and anti-inflammatory capacities are frequently measured through reductions in intracellular ROS, NF-κB activation, and cytokine levels (e.g., TNF-α, IL-6), alongside macrophage phenotype shifts toward M2 subtypes. In murine models, cerium-doped hydrogels and exosome-laden matrices consistently reduce oxidative markers by >50% and pro-inflammatory cytokines by >40% compared to standard silver dressings (Chemical Engineering, 2024). These immunomodulatory effects are often accompanied by increases in granulation tissue formation, re-epithelialization rates, and epithelial thickness.
Angiogenic performance is quantified through metrics such as microvascular density, endothelial cell viability, and VEGF expression. Materials incorporating VEGF-releasing depots or inorganic proangiogenic dopants. copper, silicon have demonstrated accelerated neovascularization in irradiated dermis, restoring capillary perfusion and oxygenation (Nichols et al., 2013). Furthermore, exosome-loaded matrices enhance endothelial migration and tubulogenesis, suggesting superior support for vascular regeneration compared to protein-only strategies (Xiao et al., 2025).
Matrix remodeling and fibrosis resolution are key indicators of long-term therapeutic value. Biomaterials that support balanced collagen I/III ratios and promote matrix metalloproteinase (MMP) activity without excessive tissue stiffness are preferred. Studies report that MMP-sensitive hydrogels reduce fibrotic area by 35% and restore normal dermal histoarchitecture, as compared to conventional alginate or PVA dressings (Monsalve et al., 2025).
Beyond biological readouts, mechanical adaptability, degradation kinetics, and application feasibility are also critical. Hydrogels with optimized rheological profiles and adhesive properties outperform rigid or brittle scaffolds in conforming to irregular anatomical surfaces (Jastram et al., 2021). Moreover, responsiveness to local stimuli, such as ROS or pH, provides contextual therapeutic control that static formulations lack.
Effective biomaterials for RID must integrate multi-scale design: nanostructural control for cellular targeting, biochemical functionality for immune regulation, and macroscopic compliance for mechanical resilience. Evaluation frameworks that incorporate functional synergy across these dimensions are necessary for benchmarking future innovations.
4.2 Interface engineering and cellular response modulation
The biomaterial, tissue interface constitutes the primary zone of interaction between synthetic constructs and biological systems. In radiation-induced skin injury, where cellular architecture and immune balance are severely disrupted, interface engineering plays a decisive role in modulating cell adhesion, inflammatory resolution, and regenerative signaling (Du et al., 2022). Surface properties, such as topography, charge density, wettability, and stiffness, can dictate host response by influencing protein adsorption, immune cell fate, and epithelial remodeling (Metwally and Stachewicz, 2019).
Surface topography at the micro- and nanoscale directly alters mechanotransduction pathways and cytoskeletal dynamics in resident skin cells (Warchomicka, 2020). Nanopatterned surfaces fabricated via lithography or electrospinning have been shown to promote aligned keratinocyte migration and suppress fibroblast-mediated contraction in fibrotic models (Talebi et al., 2023). Aligned fibers with diameters below 500 nm reduce YAP/TAZ nuclear localization and TGF-β1 expression, thereby attenuating radiation-induced fibrosis (Wan et al., 2018).
Surface charge and zeta potential influence protein corona formation, immune cell adhesion, and complement activation. Positively charged scaffolds may enhance fibroblast attachment but also increase monocyte activation and pro-inflammatory cytokine secretion (Baldwin et al., 2018). Zwitterionic surfaces or materials functionalized with sulfobetaine exhibit minimal protein fouling and reduced macrophage activation, creating an immune-permissive environment conducive to regeneration (Zhang et al., 2024). Charge tuning is therefore a critical lever in balancing adhesion and immunogenicity.
Hydrophilicity and surface energy affect wound hydration and cellular compatibility. Superhydrophilic coatings promote nutrient exchange and oxygen diffusion, essential for healing under hypoxic post-radiation conditions (Otitoju et al., 2017). Conversely, such as fluorinated coatings, can resist bacterial adhesion but may impair host cell proliferation unless properly biofunctionalized (Li et al., 2020).
Dynamic surface chemistry offers additional control through stimuli-responsive or enzymatically modifiable ligands. ROS-sensitive moieties that degrade under oxidative stress can unmask adhesive peptides or drug depots, enabling spatiotemporal modulation of cell behavior (Gomes et al., 2018). Moreover, biointerfaces engineered with integrin-specific sequences (e.g., RGD, IKVAV) facilitate targeted recruitment of keratinocytes and endothelial progenitors (Tugulu et al., 2017; Patel et al., 2019).
Mechanical interface cues, including stiffness gradients and viscoelastic response, regulate matrix-cell feedback. Interfaces mimicking the native skin modulus (∼10 kPa) support keratinocyte expansion and attenuate myofibroblast differentiation, whereas excessively rigid materials (>100 kPa) activate profibrotic gene programs (Wen et al., 2014). Tunable hydrogels and viscoelastic elastomers provide means to synchronize degradation and cellular mechanosensing during different healing phases (Chaudhuri, 2017).
Collectively, biomaterial interface engineering enables precise orchestration of host response in irradiated skin. By tailoring surface characteristics to the pathophysiological microenvironment, it is possible to redirect inflammation, enhance cellular communication, and promote coordinated tissue repair.
4.3 Clinical translation and application potential
Despite the rapid progression in biomaterial design for radiation-induced skin injury, clinical translation remains limited by regulatory uncertainty, manufacturing scalability, and inconsistent validation metrics. To advance from bench to bedside, next-generation materials must satisfy not only preclinical efficacy but also safety, reproducibility, and integration within radiotherapeutic workflows (Xu et al., 2025).
Currently, most clinical studies on radiation dermatitis still rely on conventional topical agents, including corticosteroids, hyaluronic acid creams, and silver-based dressings. Although several novel formulations have entered early-phase trials, comprehensive data on patient recruitment, stratification by radiation dose, and validated outcome scales remain sparse (Iacovelli et al., 2020). As of 2024, fewer than 10 interventional trials investigating advanced biomaterials for radiation dermatitis are registered on ClinicalTrials.gov, with most limited to observational endpoints and short follow-up periods (CTgov ID: NCT04995328, NCT06831084)
ON101, a hydrogel derived from plant extracts with dual anti-inflammatory and regenerative properties, has demonstrated positive outcomes in a Phase II randomized controlled trial in diabetic ulcers and has now entered a pilot study for radiation-induced skin injury (Man et al., 2024). Preliminary data show reduced erythema and accelerated wound closure, though long-term fibrosis and recurrence outcomes remain unassessed. Similarly, exosome-functionalized hydrogels have entered compassionate-use protocols in East Asia, but face manufacturing and quality control challenges, particularly in cargo consistency and delivery efficacy (Hwang and Lee, 2025).
Regulatory hurdles are amplified by the multifunctionality of emerging platforms. Composites incorporating nanoparticles, live cells, or gene-regulating cargos are frequently classified as combination products, requiring harmonized evaluation across device, drug, and biologic domains. The absence of unified standards for endpoints, such as epithelial closure rate, pain reduction, and fibrosis score, also complicates trial design and cross-study comparison (Souto et al., 2024).
From a translational engineering perspective, material scale-up and sterilization protocols are major barriers. Thermosensitive or shear-thinning materials may degrade during gamma or ethylene oxide sterilization, while batch-to-batch variability in natural polymers complicates regulatory reproducibility (Parvin et al., 2024). Technologies such as lyophilized hydrogel kits and 3D bioprinted matrices with on-demand hydration offer modular solutions compatible with point-of-care use, but require further validation (Hybrid nanosystems, 2021).
To advance clinical readiness, future biomaterial development must incorporate Good Manufacturing Practice (GMP) standards, ensure compatibility with established processing and sterilization methods, and address practical aspects of clinical use. These include dressing change frequency, patient-reported comfort, and comprehensive long-term safety monitoring. With continued refinement and robust validation, next-generation biomaterials hold the potential to significantly improve therapeutic outcomes and redefine standards of care for patients undergoing radiotherapy.
In summary, advanced biomaterials offer substantial promise for the management of radiation-induced dermatitis, with multifunctional platforms such as antioxidant hydrogels, cell-integrated scaffolds, and exosome-based systems addressing the complex biological processes involved in radiation injury.Future research should prioritize multicenter trials, standardized outcome measures, and regulatory convergence to accelerate the pathway from bench to bedside. A sustained focus on real-world functionality, patient quality of life, and long-term safety will be essential to ensure durable impact in clinical oncology practice.
Author contributions
CM: Writing – review and editing, Writing – original draft. HZ: Writing – review and editing. CL: Writing – review and editing. WW: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Science and Technology Programm of the Joint Fund of Scientific Research for the Public Hospitals of Inner Mongolia Academy of Medical Sciences (grant number 2024GLLH0989).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aderibigbe, B. A., and Buyana, B. (2018). Alginate in wound dressings. Pharmaceutics 10, 42. doi:10.3390/pharmaceutics10020042
Ahmadi, S., Rabiee, N., Bagherzadeh, M., Elmi, F., Fatahi, Y., Farjadian, F., et al. (2020). Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 34, 100914. doi:10.1016/j.nantod.2020.100914
Annals of 3D Printed Medicine (2022). Perspective: 3D bioprinted skin - engineering the skin for medical applications (2021). Ann. 3D Print. Med 3, 100018. doi:10.1016/j.stlm.2021.100018
Approaches to surface engineering of extracellular vesicles (2021). Approaches to surface engineering of extracellular vesicles (2021). Adv. Drug Deliv. Rev. 173, 416–426. doi:10.1016/j.addr.2021.03.020
Asai, H., Shibata, M., Takenaka, M., Takata, S., Hiroi, K., Ouchi, M., et al. (2023). Micelle-crosslinked hydrogels with stretchable, self-healing, and selectively adhesive properties: random copolymers work as dynamic yet self-sorting domains. Aggregate 4, e316. doi:10.1002/agt2.316
Baldwin, M., Snelling, S., Dakin, S., and Carr, A. (2018). Augmenting endogenous repair of soft tissues with nanofibre scaffolds. J. R. Soc. Interface 15, 20180019. doi:10.1098/rsif.2018.0019
Behroozian, T., Caini, S., van den Hurk, C., Bonomo, P., Chow, E., and Wolf, J. R. (2023). Systematic review and meta-analysis on interventions for radiation dermatitis prevention and management: an overview of the methods. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 31, 261. doi:10.1007/s00520-023-07707-5
Bertassoni, L. E., Cecconi, M., Manoharan, V., Nikkhah, M., Hjortnaes, J., Cristino, A. L., et al. (2014). Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab. Chip 14, 2202–2211. doi:10.1039/C4LC00030G
Bupphathong, S., Quiroz, C., Huang, W., Chung, P.-F., Tao, H.-Y., and Lin, C.-H. (2022). Gelatin methacrylate hydrogel for tissue engineering applications-a review on material modifications. Pharm. (basel Switz.) 15, 171. doi:10.3390/ph15020171
Celardo, I., Pedersen, J. Z., Traversa, E., and Ghibelli, L. (2011). Pharmacological potential of cerium oxide nanoparticles. Nanoscale 3, 1411–1420. doi:10.1039/C0NR00875C
Chaudhuri, O. (2017). Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 5, 1480–1490. doi:10.1039/c7bm00261k
Chemical Engineering Ling, J., Wang, N., and Ouyang, X. (2024). Cerium dioxide nanozyme doped hybrid hydrogel with antioxidant and antibacterial abilities for promoting diabetic wound healing. Chem. Eng. J. 497, 154517. doi:10.1016/j.cej.2024.154517
Cheng, F., Song, D., Li, H., Ravi, S. K., and Tan, S. C. (2025). Recent progress in biomedical scaffold fabricated via electrospinning: design, fabrication and tissue engineering application. Adv. Funct. Mat. 35, 2406950. doi:10.1002/adfm.202406950
Cui, J., Wang, T., Zhang, Y., She, L., and Zhao, Y. C. (2024). Molecular biological mechanisms of radiotherapy-induced skin injury occurrence and treatment. Biomed. Pharmacother. 180, 117470. doi:10.1016/j.biopha.2024.117470
Cui, X., Wang, J., Xu, X., Cao, X., Zhou, Y., and Guo, J. (2025). Progress and application of multifunctional hydrogel in radioactive skin injury. Adv. Mat. Interfaces 12. doi:10.1002/admi.202400976
Dev, A. S., Mohan, N., and Mohan, R. (2025). Chitosan-based composite scaffolds for accelerated epidermal-dermal wound healing. Explor. Biomat-x 2, 101336. doi:10.37349/ebmx.2025.101336
Dong, S., Lyu, X., Yuan, S., Wang, S., Li, W., Chen, Z., et al. (2020). Oxidative stress: a critical hint in ionizing radiation induced pyroptosis. Radiat. Med. Prot. 1, 179–185. doi:10.1016/j.radmp.2020.10.001
Du, J., Katti, D., and Thomas, V. (2022). Interactions between biomaterials and biological tissues and cells, Part I. JOM 74, 3334℃3335. doi:10.1007/s11837-022-05420-y
Egorikhina, M. N., Bronnikova, I. I., Rubtsova, Y. P., Charykova, I. N., Bugrova, M. L., Linkova, D. D., et al. (2021). Aspects of in vitro biodegradation of hybrid fibrin–collagen scaffolds. Polymers 13, 3470. doi:10.3390/polym13203470
Fan, M-H., Pi, J-K., Zou, C-Y., Jiang, Y-L., Li, Q-J., Zhang, X-Z., et al. (2024). Hydrogel-exosome system in tissue engineering: a promising therapeutic strategy. Bioact. Mat. 38, 1–30. doi:10.1016/j.bioactmat.2024.04.007
Farag, M. M. (2023). Recent trends on biomaterials for tissue regeneration applications: review. J. Mater. Sci 58, 527–558. doi:10.1007/s10853-022-08102-x
Finkelstein, S., Kanee, L., Behroozian, T., Wolf, J. R., van den Hurk, C., Chow, E., et al. (2022). Comparison of clinical practice guidelines on radiation dermatitis: a narrative review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 30, 4663–4674. doi:10.1007/s00520-022-06829-6
Francis, E. A., Sarikhani, E., Patel, V., Meganathan, D. P., Sadr, L., Tahir, A., et al. (2024). Nanoscale curvature regulates YAP/TAZ nuclear localization through nuclear deformation and rupture. Adv. Sci. n/a, 2415029. doi:10.1002/advs.202415029
Fu, X., Li, P., Chen, X., Ma, Y., Wang, R., Ji, W., et al. (2024). Ceria nanoparticles: biomedical applications and toxicity. J. Zhejiang Univ. Sci. B 25, 361–388. doi:10.1631/jzus.B2300854
Gaaz, T. S., Sulong, A. B., Akhtar, M. N., Kadhum, A. A. H., Mohamad, A. B., and Al-Amiery, A. A. (2015). Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 20, 22833–22847. doi:10.3390/molecules201219884
Gomes, B., Simões, B., and Mendes, P. (2018). The increasing dynamic, functional complexity of bio-interface materials. Nat. Rev. Chem 2, 0120. doi:10.1038/s41570-018-0120
Guan, Y., Racioppi, L., and Gerecht, S. (2023). Engineering biomaterials to tailor the microenvironment for macrophage–endothelium interactions. Nat. Rev. Mater. 8, 688℃699. doi:10.1038/s41578-023-00591-9
He, H., Zhang, Y., Xie, J., Yang, W., Wang, H., and Zhao, Y. (2024). Acellular dermal matrix hydrogels promote healing of radiation-induced skin injury in a rat model. J. Mater. Chem. B. 12 (21), 4394–4406. doi:10.1039/D4TB00941J
Hu, W., Wang, W., Chen, Z., Chen, Y., and Wang, Z. (2024). Engineered exosomes and composite biomaterials for tissue regeneration. Theranostics 14, 2099–2126. doi:10.7150/thno.93088
Huang, C., Dong, L., Zhao, B., Lu, Y., Huang, S., Yuan, Z., et al. (2022). Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 12, e1094. doi:10.1002/ctm2.1094
Hwang, H. S., and Lee, C. (2025). Exosome-integrated hydrogels for bone tissue engineering. Available online at: https://www.mdpi.com/2310-2861/10/12/762 (Accessed June 12, 2025).
Hybrid nanosystems (2021). Hybrid nanosystems for biomedical applications. Available online at: https://scispace.com/papers/hybrid-nanosystems-for-biomedical-applications-4e7h4y33xq (Accessed June 12, 2025).
Iacovelli, N. A., Torrente, Y., Ciuffreda, A., Guardamagna, V. A., Gentili, M., Giacomelli, L., et al. (2020). Topical treatment of radiation-induced dermatitis: current issues and potential solutions. Drugs Context 9, 1–13. doi:10.7573/dic.2020-4-7
Jastram, A., Claus, J., Janmey, P., and Kragl, U. (2021). Rheological properties of hydrogels based on ionic liquids. Polym. Test. 93, 106943. doi:10.1016/j.polymertesting.2020.106943
Jiang, Y., Zhang, Q., Wang, H., Välimäki, M., Zhou, Q., Dai, W., et al. (2024). Effectiveness of silver and iodine dressings on wound healing: a systematic review and meta-analysis. BMJ Open 14, e077902. doi:10.1136/bmjopen-2023-077902
Joshi, M., Adak, B., and Butola, B. S. (2018). Polyurethane nanocomposite based gas barrier films, membranes and coatings: a review on synthesis, characterization and potential applications. Prog. Mater Sci. 97, 230–282. doi:10.1016/j.pmatsci.2018.05.001
Kargozar, S., Mozafar, M., and Ghodrat, S. (2021). Copper-containing bioactive glasses and glass-ceramics: from tissue regeneration to cancer therapeutic strategies (2021). Mat. Sci. Eng. C 121, 111741. doi:10.1016/j.msec.2020.111741
Khayambashi, P., Iyer, J., Pillai, S., Upadhyay, A., Zhang, Y., and Tran, S. D. (2021). Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 22, 684. doi:10.3390/ijms22020684
Khurana, A., Tekula, S., and Godugu, C. (2018). Nanoceria suppresses multiple low doses of streptozotocin-induced type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomed. Lond. Engl. 13, 1905–1922. doi:10.2217/nnm-2018-0085
Kim, Y. G., Lee, Y., Lee, N., Soh, M., Kim, D., and Hyeon, T. (2024). Ceria-based therapeutic antioxidants for biomedical applications. Adv. Mat. 36, 2210819. doi:10.1002/adma.202210819
Kobayashi, T., Naik, S., and Nagao, K. (2019). Choreographing immunity in the skin epithelial barrier. Immunity 50, 552–565. doi:10.1016/j.immuni.2019.02.023
Kurtuldu, F., Kaňková, H., Beltrán, A. M., Liverani, L., Galusek, D., and Boccaccini, A. R. (2021). Anti-inflammatory and antibacterial activities of cerium-containing mesoporous bioactive glass nanoparticles for drug-free biomedical applications. Mat. Today Bio 12, 100150. doi:10.1016/j.mtbio.2021.100150
Li, J., Wu, D., Su, Z., Guo, J., Cui, L., Su, H., et al. (2025). Zinc-induced photocrosslinked konjac glucomannan/glycyrrhizic acid hydrogel promotes skin wound healing in diabetic mice through immune regulation. Carbohydr. Polym. 348, 122780. doi:10.1016/j.carbpol.2024.122780
Li, M., Xia, W., Khoong, Y. M., Huang, L., Huang, X., Liang, H., et al. (2023). Smart and versatile biomaterials for cutaneous wound healing. Biomater. Res. 27, 87. doi:10.1186/s40824-023-00426-2
Li, W., Zhang, H., Li, X., Yu, H., Che, C., Luan, S., et al. (2020). Multifunctional antibacterial materials comprising water dispersible random copolymers containing a fluorinated block and their application in catheters. ACS Appl. Mat. Interfaces 12, 7617–7630. doi:10.1021/acsami.9b22206
Liu, Z., Li, X., Huang, Y., Li, J., Dong, R., Yun, X., et al. (2025). Thermal-responsive microgels incorporated PVA composite hydrogels: integration of two-stage drug release and enhanced self-healing ability for chronic wound treatment. Chem. Eng. J. 506, 159813. doi:10.1016/j.cej.2025.159813
Luan, P., Zhou, H.-H., Zhang, B., Liu, A.-M., Yang, L.-H., Weng, X.-L., et al. (2012). Basic fibroblast growth factor protects C17.2 cells from radiation-induced injury through ERK1/2. CNS Neurosci. Ther. 18, 767–772. doi:10.1111/j.1755-5949.2012.00365.x
Lv, X., Zhao, N., Long, S., Wang, G., Ran, X., Gao, J., et al. (2024). 3D skin bioprinting as promising therapeutic strategy for radiation-associated skin injuries. Wound Repair Regen. 32, 217–228. doi:10.1111/wrr.13181
Man, J., Shen, Y., Song, Y., Yang, K., Pei, P., and Hu, L. (2024). Biomaterials-mediated radiation-induced diseases treatment and radiation protection. J. Control. Release 370, 318–338. doi:10.1016/j.jconrel.2024.04.044
Metwally, S., and Stachewicz, U. (2019). Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mat. Sci. Eng. C 104, 109883. doi:10.1016/j.msec.2019.109883
Monsalve, E., Alvarez, M., and Hosseini, S. (2025). Engineering bioactive synthetic polymers for biomedical applications: a review with emphasis on tissue engineering and controlled release - materials advances. doi:10.1039/D1MA00092F
Morrell, A. E., Brown, G. N., Robinson, S. T., Sattler, R. L., Baik, A. D., Zhen, G., et al. (2018). Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 6, 6–11. doi:10.1038/s41413-018-0007-x
Nelson, B. C., Johnson, M. E., Walker, M. L., Riley, K. R., and Sims, C. M. (2016). Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 5, 15. doi:10.3390/antiox5020015
Nešporová, K., Pavlík, V., Šafránková, B., Vágnerová, H., Odráška, P., and Žídek, O. (2021). Effects of wound dressings containing silver on skin and immune cells - PubMed. Available online at: https://pubmed.ncbi.nlm.nih.gov/32939010/(Accessed June 9, 2025).
Nichol, J. W., Koshy, S. T., Bae, H., Hwang, C. M., Yamanlar, S., and Khademhosseini, A. (2010). Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544. doi:10.1016/j.biomaterials.2010.03.064
Nichols, S. P., Koh, A., Storm, W. L., Shin, J. H., and Schoenfisch, M. H. (2013). Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 113, 2528–2549. doi:10.1021/cr300387j
Nurkesh, A., Jaguparov, A., Jimi, S., and Saparov, A. (2020). Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell. Dev. Biol. 8, 638. doi:10.3389/fcell.2020.00638
Otitoju, T., Ahmad, A., and Ooi, B. (2017). Superhydrophilic (superwetting) surfaces: a review on fabrication and application. J. Ind. Eng. Chem. 47, 19–40. doi:10.1016/j.jiec.2016.12.016
Parvin, N., Joo, S. W., and Mandal, T. K. (2024). Injectable biopolymer-based hydrogels: a next-generation platform for minimally invasive therapeutics. Available online at: https://www.mdpi.com/2310-2861/11/6/383 (Accessed June 12, 2025).
Patel, R., Santhosh, M., Dash, J. K., Karpoormath, R., Jha, A., Kwak, J., et al. (2019). Ile-lys-val-ala-val (IKVAV) peptide for neuronal tissue engineering. Polym. Adv. Technol. 30, 4–12. doi:10.1002/pat.4442
Pazdrowski, J., Gornowicz-Porowska, J., Kaźmierska, J., Krajka-Kuźniak, V., Polanska, A., Masternak, M., et al. (2024). Radiation-induced skin injury in the head and neck region: pathogenesis, clinics, prevention, treatment considerations and proposal for management algorithm. Rep. Pract. Oncol. Radiother. J. Gt. Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 29, 373–390. doi:10.5603/rpor.100775
Poon, V. K. M., and Burd, A. (2004). In vitro cytotoxity of silver: implication for clinical wound care. Burns J. Int. Soc. Burn Inj. 30, 140–147. doi:10.1016/j.burns.2003.09.030
Protein interactions at material surfaces (2025). Protein interactions at material surfaces | SpringerLink. Available online at: https://link.springer.com/chapter/10.1007/978-3-030-49206-9_12 (Accessed June 12, 2025).
ROS (2025). In situ photocrosslinkable hydrogel treats radiation-induced skin injury by ROS elimination and inflammation regulation - PubMed. Available online at: https://pubmed.ncbi.nlm.nih.gov/39413652/(Accessed June 9, 2025).
Ruan, L., Pan, C., Ran, X., Wen, Y., Lang, R., Peng, M., et al. (2024). Dual-delivery temperature-sensitive hydrogel with antimicrobial and anti-inflammatory brevilin a and nitric oxide for wound healing in bacterial infection. Gels 10, 219. doi:10.3390/gels10040219
Sepe, F., Valentino, A., Marcolongo, L., Petillo, O., Conte, R., Margarucci, S., et al. (2025). Marine-derived polysaccharide hydrogels as delivery platforms for natural bioactive compounds. Int. J. Mol. Sci. 26, 764. doi:10.3390/ijms26020764
Shi, B., Zhu, T., Luo, Y., Zhang, X., Yao, J., Cao, X., et al. (2024). Three-dimensional bioprinted cell-adaptive hydrogel with anisotropic micropores for enhancing skin wound healing. Int. J. Biol. Macromol. 280, 136106. doi:10.1016/j.ijbiomac.2024.136106
Singh, K., Singhal, S., Pahwa, S., Arora, V., Sharma, S., Singh, P., et al. (2024). Nanomedicine and drug delivery: a comprehensive review of applications and challenges. Nano-Struct. Nano-Objects 40, 101403. doi:10.1016/j.nanoso.2024.101403
Sionkowska, A. (2021). Collagen blended with natural polymers: recent advances and trends. Prog. Polym. Sci. 122, 101452. doi:10.1016/j.progpolymsci.2021.101452
Smithmyer, M. E., Sawicki, L. A., and Kloxin, A. M. (2014). Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater. Sci. 2, 634–650. doi:10.1039/C3BM60319A
Souto, E. B., Blanco-Llamero, C., Krambeck, K., Kiran, N. S., Yashaswini, C., Postwala, H., et al. (2024). Regulatory insights into nanomedicine and gene vaccine innovation: safety assessment, challenges, and regulatory perspectives. Acta Biomater. 180, 1–17. doi:10.1016/j.actbio.2024.04.010
Straub, J. M., New, J., Hamilton, C. D., Lominska, C., Shnayder, Y., and Thomas, S. M. (2015). Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 141, 1985–1994. doi:10.1007/s00432-015-1974-6
Su, N., Hao, Y., Wang, F., Hou, W., Chen, H., and Luo, Y. (2021). Mesenchymal stromal exosome–functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci. Adv. 7, eabf7207. doi:10.1126/sciadv.abf7207
Su, Y., Cui, H., Yang, C., Li, L., Xu, F., Gao, J., et al. (2022). Hydrogels for the treatment of radiation-induced skin and mucosa damages: an up-to-date overview. Front. Mat. 9. doi:10.3389/fmats.2022.1018815
Talebi, N., Lopes, D., Lopes, J., Macário-Soares, A., Dan, A. K., Ghanbari, R., et al. (2023). Natural polymeric nanofibers in transdermal drug delivery. Appl. Mat. Today 30, 101726. doi:10.1016/j.apmt.2022.101726
Tang, S., Richardson, B. M., and Anseth, K. S. (2021). Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater Sci. 120, 100738. doi:10.1016/j.pmatsci.2020.100738
Tao, W., and He, Z. (2018). ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 13, 101–112. doi:10.1016/j.ajps.2017.11.002
Teng, Y., Fan, Y., Ma, J., Lu, W., Liu, N., Chen, Y., et al. (2021). The PI3K/akt pathway: emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 10, 1219. doi:10.3390/cells10051219
Tugulu, S., Silacci, P., Stergiopulos, N., and Klok, H. (2017). RGD—functionalized polymer brushes as substrates for the integrin specific adhesion of human umbilical vein endothelial cells. Biomaterials 28, 2536–2546. doi:10.1016/j.biomaterials.2007.02.006
Wan, S., Fu, X., Ji, Y., Li, M., Shi, X., and Wang, Y. (2018). FAK- and YAP/TAZ dependent mechanotransduction pathways are required for enhanced immunomodulatory properties of adipose-derived mesenchymal stem cells induced by aligned fibrous scaffolds. Biomaterials 171, 107–117. doi:10.1016/j.biomaterials.2018.04.035
Wang, Y., He, C., Chen, C., Dong, W., Yang, X., Wu, Y., et al. (2022). Thermoresponsive self-healing zwitterionic hydrogel as an in situ gelling wound dressing for rapid wound healing. ACS Appl. Mat. Interfaces 14, 55342–55353. doi:10.1021/acsami.2c15820
Wang, Z., Ye, Q., Yu, S., and Akhavan, B. (2023). Poly ethylene glycol (PEG)-based hydrogels for drug delivery in cancer therapy: a comprehensive review. Adv. Healthc. Mat. 12, 2300105. doi:10.1002/adhm.202300105
Warchomicka, F. (2020). “Surface topographies on the micro and nanoscale of metal alloys for tissue regeneration (2020),” in Nanostructured biomaterials for regenerative medicine (Cambridge, United Kingdom: Woodhead Publishing), 315–336. doi:10.1016/B978-0-08-102594-9.00012-7
Wen, J., Huang, S., Hu, Q., He, W., Wei, Z., Wang, L., et al. (2024). Recent advances in zwitterionic polymers-based non-fouling coating strategies for biomedical applications. Mat. Today Chem. 40, 102232. doi:10.1016/j.mtchem.2024.102232
Wen, J. H., Vincent, L. G., Fuhrmann, A., Choi, Y. S., Hribar, K. C., Taylor-Weiner, H., et al. (2014). Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mat. 13, 979–987. doi:10.1038/nmat4051
Wu, Y., Wang, Y., Long, L., Hu, C., and Kong, Q. (2022). A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 341, 147–165. doi:10.1016/j.jconrel.2021.11.027
Xiao, Y., Zou, D., Liu, J., Dai, F., Zhao, A., and Yang, P. (2025). Dose-responsive effects of endothelial cell-sourced exosomes on vascular cell proliferation and phenotype transition. Biochim. Biophys. Acta (BBA) - Gen. Subj. 1869, 130745. doi:10.1016/j.bbagen.2024.130745
Xie, Y., Guan, Q., Guo, J., Chen, Y., Yin, Y., and Han, X. (2022). Hydrogels for exosome delivery in biomedical applications. Gels 8, 328. doi:10.3390/gels8060328
Xu, Y., Liu, Q., Li, W., Hu, Z., and Shi, C. (2025). Recent advances in the mechanisms, current treatment status, and application of multifunctional biomaterials for radiation-induced skin injury. Theranostics 15, 2700–2719. doi:10.7150/thno.108309
Yang, H., Wang, W., Xiao, J., Yang, R., Feng, L., Xu, H., et al. (2025). ROS-responsive injectable hydrogels loaded with exosomes carrying miR-4500 reverse liver fibrosis. Biomaterials 314, 122887. doi:10.1016/j.biomaterials.2024.122887
Yang, X., Ren, H., Guo, X., Hu, C., and Fu, J. (2020). Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Milano) 12, 23379–23393. doi:10.18632/aging.103932
Yarnold, J., and Vozenin Brotons, M.-C. (2010). Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 97, 149–161. doi:10.1016/j.radonc.2010.09.002
Zarei, N., and Hassanzadeh-Tabrizi, S. A. (2023). Alginate/hyaluronic acid-based systems as a new generation of wound dressings: a review. Int. J. Biol. Macromol. 253, 127249. doi:10.1016/j.ijbiomac.2023.127249
Zasadziński, K., Spałek, M. J., and Rutkowski, P. (2022). Modern dressings in prevention and therapy of acute and chronic radiation dermatitis—a literature review. Pharmaceutics 14, 1204. doi:10.3390/pharmaceutics14061204
Zhang, L., Cao, Z., Bai, T., Carr, L., Ella-Menye, J.-R., Irvin, C., et al. (2013). Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556. doi:10.1038/nbt.2580
Zhang, Q., Deng, N., Dai, Y., Zhan, M., Wu, H., Zeng, J., et al. (2024). Zwitterionic sulfobetaine-based hypercrosslinked hydrophilic materials for bioanalysis. Chem. Eng. J. 494, 153018. doi:10.1016/j.cej.2024.153018
Zhang, Y., Yan, W., Wu, L., Yu, Z., Quan, Y., and Xie, X. (2025). Different exosomes are loaded in hydrogels for the application in the field of tissue repair. Front. Bioeng. Biotechnol. 13, 1545636. doi:10.3389/fbioe.2025.1545636
Zheng, K., Zhu, X., Guo, S., and Zhang, X. (2023). Gamma-ray-responsive drug delivery systems for radiation protection. Chem. Eng. J. 463, 142522. doi:10.1016/j.cej.2023.142522
Zhou, D., Du, M., Luo, H., Ran, F., Zhao, X., Dong, Y., et al. (2022). Multifunctional mesoporous silica-cerium oxide nanozymes facilitate miR129 delivery for high-quality healing of radiation-induced skin injury. J. Nanobiotechnol. 20, 409. doi:10.1186/s12951-022-01620-5
Zhu, J., and Marchant, R. E. (2011). Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 8, 607–626. doi:10.1586/erd.11.27
Keywords: biomaterials, oxidative stress, inflammation modulation, drug delivery systems, tissue regeneration, interface engineering, clinical translation
Citation: Ma C, Zhu H, Liu C and Wang W (2025) Advanced biomaterials for radiation-induced dermatitis: from pathophysiological mechanisms to translational interfaces. Front. Mater. 12:1631141. doi: 10.3389/fmats.2025.1631141
Received: 19 May 2025; Accepted: 30 June 2025;
Published: 10 July 2025.
Edited by:
Wei Sang, Shanxi Medical University, ChinaReviewed by:
Lisi Xie, Sun Yat-sen Memorial Hospital, ChinaCopyright © 2025 Ma, Zhu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxin Wang, NDQxNTA1MjY3QHFxLmNvbQ==
†ORCID: Cheng Ma, orcid.org/0009-0005-3204-0407; Wenxin Wang: orcid.org/0009-0004-0222-4670
 Cheng Ma
Cheng Ma Haijun Zhu2
Haijun Zhu2 Wenxin Wang
Wenxin Wang