- 1Department of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Shandong Key Laboratory of Digital Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3Institute of Pharmacy (Institute of TCM Health Industrial Technology), Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Conventional wound dressings can only provide basic protection for wounds and have limited ability to promote wound healing. Therefore, it is of great practical significance to develop new wound dressings with antibacterial, hemostatic, wound healing promotion, and good biocompatibility. Graphene oxide (GO), as a new type of nanomaterial, has received widespread attention in the fields of tissue engineering, bioimaging, biosensing, cancer therapy, and drug delivery. Due to unique physicochemical properties and multifunctionality of GO, polymers integrated with GO have the advantages of excellent antibacterial effect, good biocompatibility, significant wound healing promotion, and intelligent response, thus becoming new wound dressing materials with great potentials. This review systematically summaries the antibacterial, hemostatic, and angiogenic effects and mechanisms of GO. Then the research progress of GO as a core material combined with polymer compounds to form GO-based polymeric wound dressings is focused, followed by an in-depth discussion on their biocompatibility. Finally, this review further prospects the future research direction of GO-based polymeric wound dressings, with a view to providing ideas for their research and application.
1 Introduction
Skin is the largest external organ of the human body and an important defense barrier, playing a vital role in protecting the human skeleton, internal organs and underlying tissues from a wide range of physical and chemical injuries (Taati Moghadam et al., 2020), therefore, repairing damaged skin is of far-reaching significance. Wound healing is a process involving a variety of pathophysiological events usually consisting of three phases: hemostasis and inflammation, new tissue formation (or proliferation) and tissue remodeling (Koehler et al., 2017; Barnum et al., 2020). Wounds are very susceptible to bacterial infections during the healing process, which can delay the healing process and damage the healthy skin tissue surrounding the wound (Skórkowska-Telichowska et al., 2013). The healing of all types of wounds is facing a wide range of challenges due to the complexity of wound healing mechanisms. This complexity arises from four dynamically sequential and intertwined pathophysiological phases: the initial hemostatic phase requires precise coordination of platelet activation, coagulation cascade and inflammatory factor release. The inflammatory phase needs to tightly control the neutrophil/macrophage infiltration intensity and duration. The proliferative phase involves fibroblast differentiation, collagen deposition, and angiogenesis. The final reconstruction phase lasts for months to years as the new blood vessels mature and the keratinocytes stop proliferating and migrating and begin to differentiate, thus completing wound healing. At the same time, the emergence of various drug-resistant bacteria has put wound healing in a more critical situation. Therefore, the selection of appropriate wound dressings and the development of new wound dressings is of great practical significance (Saghazadeh et al., 2018; Wang et al., 2018; Wilkinson and Hardman, 2020).
The concept of wound dressings has been around for a long time and includes various forms such as hydrogels, sponges and membranes. In general, the ideal dressing should fulfil the following characteristics: It prevents loss of water and body fluids; prevents bacterial infection of the wound and fights off invading bacteria; promotes the growth of granulation and epithelial tissues; does not leave scars after wound healing; is soft to the touch, has good breathability and moisture permeability, and retains moisture well; has good biocompatibility; and is comfortable, convenient, easy to prepare, inexpensive, and easily removable (Hamedi et al., 2022; Fiorentini et al., 2023). Currently, most of the materials chosen for wound dressings are polymeric compounds, such as bacterial cellulose, chitosan, sodium alginate (SA), polyvinyl alcohol (PVA), etc. Kamel et al. (2023) prepared a SA/PVA matrix composite scaffold for wound healing. The scaffolds were found to have good thermal stability and controllable biodegradation properties, and formed a homogeneous, regular and porous three-dimensional lamellar structure. In addition, the results of in vitro experiments showed that the scaffold had good biocompatibility and no toxicity reaction to human skin fibroblasts. Sathiyaseelan et al. (2021) prepared a chitosan/PVA matrix composite for wound management. It was found that the composite had a dense and well-connected microporous three-dimensional structure. In addition, the composite maintained excellent biocompatibility while possessing anti-inflammatory activity. The polymeric wound dressings generally have excellent three-dimensional structures, good biocompatibility and degradability, and excellent hemostatic and self-healing properties. However, polymeric wound dressings also face problems such as lack of diverse bioactivities, poor suitability for highly exudative wounds, controversial long-term safety, and low clinical conversion rates (Chattopadhyay and Raines, 2014; Arabpour et al., 2024).
In recent years, graphene oxide (GO) has attracted much attention in the field of wound dressings due to its excellent performance as well as special physicochemical properties. GO is a graphene derivative consisting of a hexagonal carbon network and sp2 and sp3 hybridized carbon by introducing covalent C-O bonds into graphene, which is the same layered planar two-dimensional structure as graphene. Graphene itself has unique and excellent optical, mechanical, and electrical properties (Genorio et al., 2019; Torres et al., 2020), but its application is limited by its poor compatibility with polymers due to its hydrophobicity and its susceptibility to agglomeration (Patil et al., 2021; Gostaviceanu et al., 2024). Compared to graphene, the presence of oxygen-containing groups in the structure of GO, such as -OH, -C-O-C-, -C=O, and -COOH, makes GO highly reactive, good hydrophilicity, and better biocompatibility (Pei et al., 2018; Thebo et al., 2018; Di Crescenzo et al., 2019). In addition, GO is amphiphilic, with its intermediate lamellae being hydrophobic while the edges exhibit hydrophilicity. This hydrophobicity allows GO to load hydrophobic substances through π-π stacking or hydrophobic interactions. At the same time, the hydrophilic edge provides an ideal site for GO functionalization modification and facilitates surface modification, thus creating conditions for binding with other chemical groups.
GO has been widely used in the biomedical industry with its special structure as well as excellent properties (Maiti et al., 2018; Patil et al., 2021). As a wound dressing, GO can exert antibacterial effects in a variety of physical and chemical ways, thus preventing wounds from bacterial infection. At the same time, GO promotes wound healing through its angiogenic properties. When GO is combined with polymeric compounds to form GO-based polymeric wound dressings, it not only exerts the bioactivity of GO, but also has excellent hemostatic properties (Lin et al., 2021; Khan A. et al., 2023). Furthermore, studies have shown that GO-based polymeric wound dressings can significantly reduces the toxicity of GO, demonstrating a high level of safety (Ghulam et al., 2022; Yadav et al., 2022).
This review systematically discusses the antibacterial, hemostatic, and angiogenic effects of GO in the wound healing process. The research progress of GO-based polymeric wound dressings is also reviewed. Finally, this review further prospects the future research direction of GO-based polymeric wound dressings to promote the clinical application of GO-based polymeric wound dressings.
2 Antibacterial effects
Wound infection represents one of the most significant categories of hospital-acquired infections on a global scale. During the wound healing process, if pathogenic bacteria and their associated biofilm formations infect the wound, they can impede the healing process (Ali et al., 2019). Typically, the immune system mounts a natural response to infections at the site of the wound; however, in immunocompromised patients, various bacteria, including Staphylococcus aureus, Acinetobacter baumannii, and Escherichia coli, may disseminate throughout the body, leading to damage in deeper tissues and causing harm to the human body. Furthermore, pathogenic bacteria can enter the wound from other parts of the body, causing damage to adjacent tissues. When these bacteria enter the lymphatic and circulatory systems, sepsis can be triggered, representing the most severe form of clinical infection in patients with wound infections (Zhang et al., 2021). With the increasing prevalence of multidrug-resistant bacteria, the application of numerous antibiotics has become more restricted. In this case, GO has attracted much attention for its excellent antibacterial capacity. GO can produce antibacterial effects through various physical or chemical ways, thus preventing bacterial infection in the wound and accelerating wound healing (Abat et al., 2018).
2.1 Antibacterial effects of GO
2.1.1 Physical mechanism
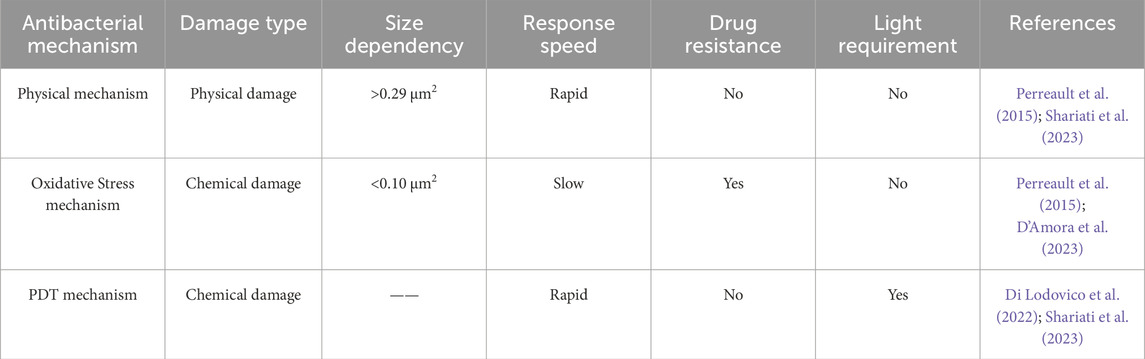
GO can interact with bacterial cells through its sharp edges, which are oriented either parallel or perpendicular to the bacterial cell membranes. Due to van der Waals forces and hydrophobic interactions, the edges of GO become embedded within the phospholipid bilayer, facilitating the spontaneous and rapid insertion of GO into the bacterial cell envelope (Figure 1A). This interaction results in morphological alterations and ruptures of the bacterial cell membrane, leading to the leakage of cellular contents and ultimately, bacterial death (Liu et al., 2011; Mohammed et al., 2020; D’Amora et al., 2023). Moreover, the agglomeration characteristic of GO enables it to adsorb and accumulate bacteria (GO nanosheets>0.29 μm2) (Figures 1B,C), thus inhibiting bacterial adhesion to wounds and the formation of biofilms (Perreault et al., 2015; Shariati et al., 2023).
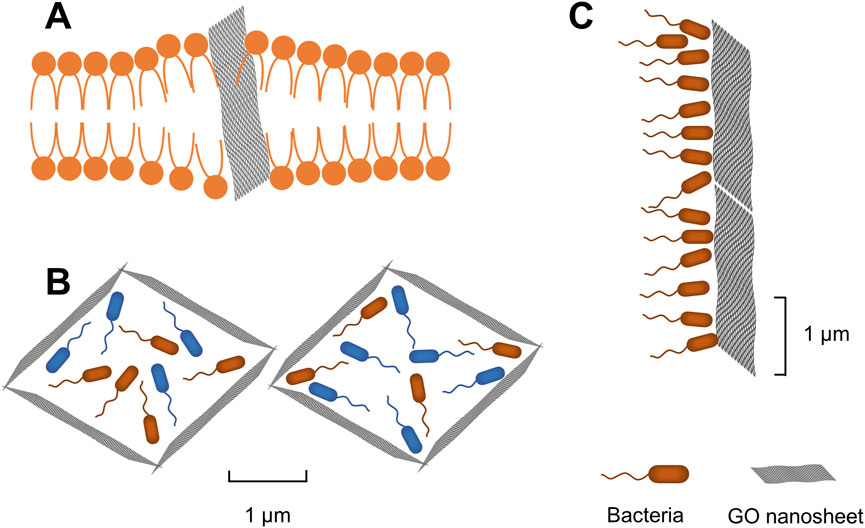
Figure 1. (A) GO nanosheets inserted into bacterial cell membrane. (B) Agglomeration of GO nanosheets to adsorb and encapsulate bacteria. (C) GO nanosheets physically block bacteria.
Pham et al. (2015) discovered that the density of graphene edges substantially influences the antibacterial properties of graphene nanosheets. This effect can result in the formation of pores within the bacterial cell wall, leading to an imbalance in osmotic pressure and, ultimately, cell death. Chen et al. (2014) observed that the encapsulation of bacteria (Pseudomonas syringae and Xanthomonas campestris) and fungi (Fusarium graminearum and Fusarium oxysporum) by GO caused extensive nanosheet aggregation. This aggregation led to local perturbations in the phospholipid bilayer, a reduction in bacterial cell membrane potential, and electrolyte leakage from fungal spores. Di Giulio et al. (2018) stained GO-treated S. aureus as well as Canidia albicans and observed them by light microscopy and atomic force microscopy (AFM), respectively. After 24 h of treatment with GO, Gram staining showed that GO was able to encapsulate S. aureus and Canidia albicans. This result was also confirmed by AFM images, which observed isolated bacteria encapsulated by a layer of GO (Figure 2).
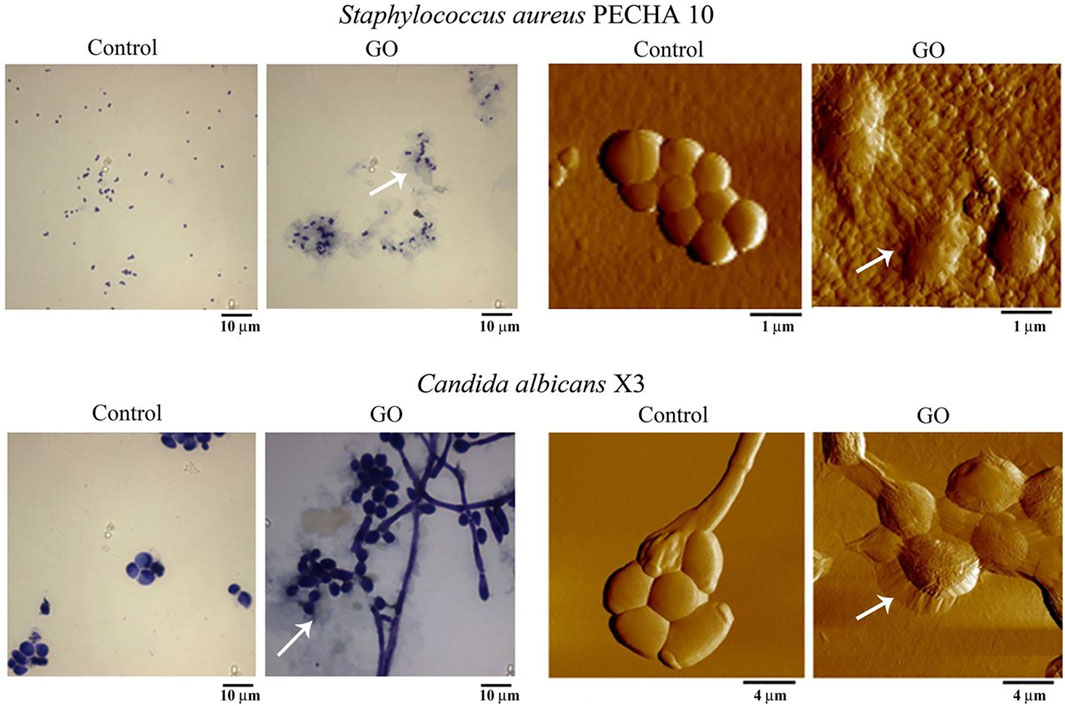
Figure 2. Effect of graphene oxide (GO) at 50 mg/L on planktonic phase of Staphylococcus aureus PECHA 10 and Candida albicans X3. Representative images of control samples and those treated with GO, obtained with Gram staining (columns on the left) and AFM (columns on the right). Arrows indicate GO wrapping microorganisms. Asterisks indicate the GO layer. Reproduced with permission from (Di Giulio et al., 2018). Copyright@ 2018.
2.1.2 Oxidative stress mechanism
Oxidative stress mechanism is considered to be an important antibacterial mechanism of GO (Figure 3). The oxidative stress induced by GO disrupts essential bacterial functions, thereby interfering with bacterial metabolism and ultimately leading to cell death. This mechanism can operate in either a reactive oxygen species (ROS)-dependent or ROS-independent manner. The former mechanism involves the irrational accumulation of intracellular ROS. GO catalyzes the internal production of ROS in bacteria, including hydroxyl radicals (OH−), hydrogen peroxide (H2O2), singlet molecular oxygen (1O2), and superoxide anions (O2−). Accumulation of these ROS leads to bacterial protein inactivation, DNA damage, mitochondrial dysfunction, cell membrane disruption, and lipid peroxidation, resulting in bacterial death. The latter mechanism works through charge transfer between the bacteria and the GO, which directly hijacks electrons in the electron transport chain (ETC), disrupting energy metabolism and leading to bacterial death (Zou et al., 2016; Mohammed et al., 2020; Chen et al., 2021; D’Amora et al., 2023). Perreault et al. (2015) investigated the antibacterial efficacy of E. coli in contact with GO nanosheets of varying sizes over 3 hours. Their findings revealed that the size of GO nanosheet had a significant effect on the antibacterial efficacy. The smaller GO nanosheet (GO nanosheets < 0.10 μm2) exhibited higher antibacterial efficacy. This is attributed to the increased defect density in smaller GO nanosheets, which enhances oxidative stress mechanisms.
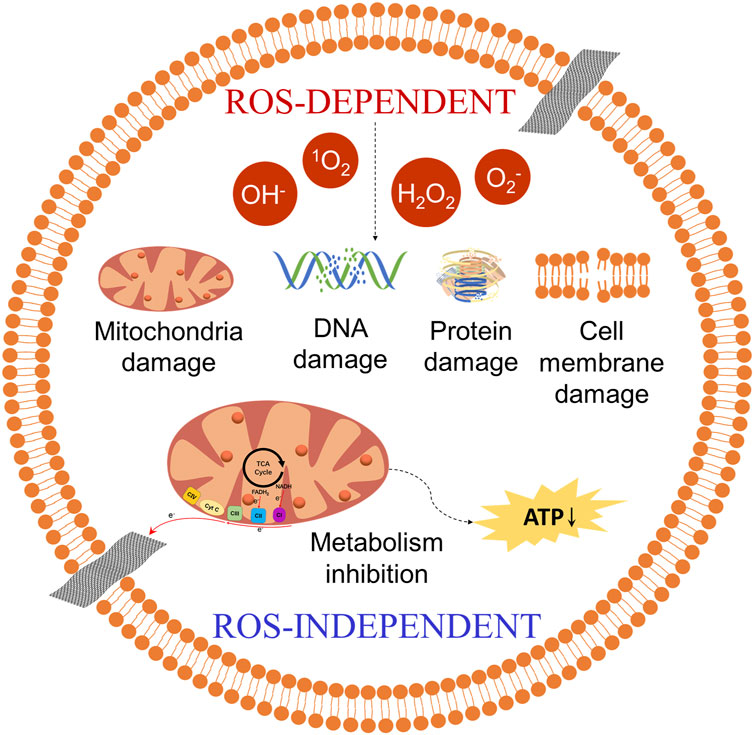
Figure 3. The oxidative stress mechanism of GO. ROS-dependent: GO catalyzes the production of ROS (OH−, 1O2, etc.), causing damage to bacterial DNA, proteins, etc., resulting in bacterial death. ROS-independent: GO directly hijacks electrons in the electron transport chain (ETC), disrupting energy metabolism and leading to bacterial death. CⅠ: NADH dehydrogenase. CⅡ: succinate dehydrogenase. CⅢ: cytochrome bc1 complex. CⅣ: cytochrome c oxidase. Cyt C: cytochrome c. ATP: adenosine triphosphate.
2.1.3 PDT mechanism
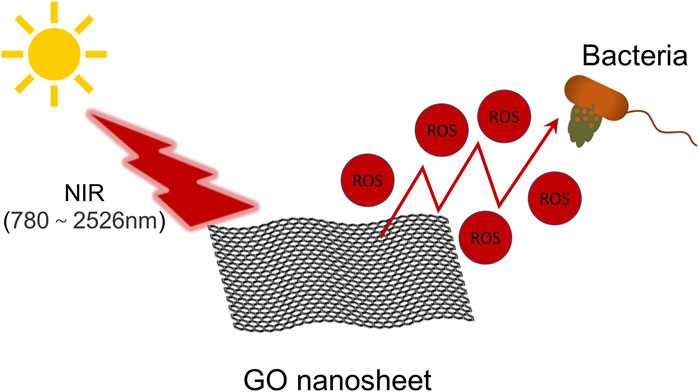
In addition to the aforementioned antibacterial mechanisms, GO can also exhibit antibacterial effects via antibacterial photodynamic therapy (PDT) (Figure 4) (Shariati et al., 2023). PDT induces damage to bacterial cells by absorbing light at specific wavelengths, leading to the generation of ROS, which ultimately result in the demise of pathogenic bacteria. Owing to its extensive surface area, diverse oxygen-containing functional groups, and significant near-infrared (NIR) absorption and photothermal characteristics, GO demonstrates superior photodynamic antibacterial properties (Di Lodovico et al., 2022). Mei et al. (2021) experimentally prepared a zinc tetraaminophthalocyanine-modified GO nanocomposite (Pc-NH2@GO) and found that, when irradiated with light at a wavelength of 680 nm, this composite effectively eradicated bacteria (E. coli and S. aureus). Furthermore, the material disrupts cellular morphology and causes the leakage of cellular contents (Figure 5), thereby eliminating bacteria at the site of wound infections and accelerating the wound healing process. Wei et al. (2022) developed a double-crosslinked cellulose/GO composite hydrogel. This composite hydrogel demonstrates notable antibacterial activity against E. coli (96.5%) and S. aureus (100%) when exposed to NIR light at a wavelength of 808 nm and a power density of 2 W/cm2 for 240 s, attributed to the photothermal properties of GO. Morphological investigations employing scanning electron microscopy demonstrated that the cell membranes of bacteria exposed to composite hydrogel and subsequently irradiated with NIR light exhibited complete disintegration, in stark contrast to the unirradiated E. coli and S. aureus, which maintained their characteristic rod-like and spherical morphologies, respectively. A comparison of different antibacterial mechanisms of GO is presented in Table 1.
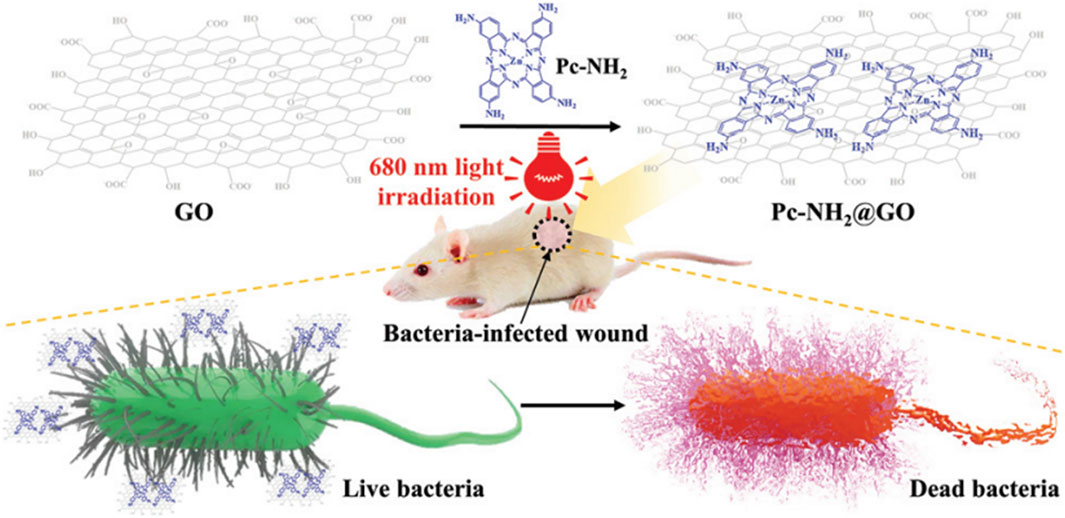
Figure 5. Schematic illustration of the preparation and application of Pc-NH2@GO. Reproduced with permission from (Mei et al., 2021). Copyright@ 2021.
2.2 Antibacterial effects of GO-based polymeric wound dressings
Due to the multiple antibacterial mechanisms of GO, a variety of wound dressings have demonstrated excellent antibacterial effects along with the addition of GO. Mahmoud et al. (2025) prepared a rolled graphene oxide/poly-m-methylaniline (roll-GO/PmMA) core-shell nanocomposite by in situ polymerization technique. The study examined the antibacterial properties of the material using agar well diffusion method. The results showed that the nanocomposites exhibited significant antibacterial activity against Gram-positive (Bacillus subtilis and S. aureus) and Gram-negative (E. coli and Salmonella sp) bacteria. In addition, the inhibition zones increased significantly under light conditions (33 and 18 mm in the case of B. subtilis), indicating that the material possessed good photocatalytic antibacterial performance. Mustafa et al. (2024) prepared polypropylene (PPY) hybrid polymeric membranes by thermally induced phase separation (TIPS) method and introduced rGO for composite modification. The results showed that the PPY/rGO hybrid film exhibited significant inhibition against S. aureus and E. coli. In the agar diffusion experiment, the inhibition zones of the composite reached 8 mm in E. coli and 9 mm in S. aureus. Das et al. (2023) utilized a solvent casting technique incorporating sodium carboxymethyl cellulose (CMC), SA, AgNPs, and GO to fabricate antibacterial nanocomposite films. The research indicated that the mechanical properties of the CMC/SA/Ag-GO nanocomposite films were enhanced as the weight percentage of GO increased. Additionally, the study assessed the antibacterial efficacy of these films. The circle of inhibition diameters of CMC/SA/Ag-GO2% nanocomposite films against E. coli and S. aureus were measured at 21.30 ± 0.70 mm and 18.00 ± 1.00 mm, respectively, indicating that these composites exhibit substantial antibacterial activity.
Chen et al. (2020) incorporated polyhexamethylene guanidine (PHMG)-modified graphene oxide (mGO) into a chitosan/PVA (CS/PVA) matrix, significantly enhancing its antibacterial efficacy (Figures 6A–D. The antibacterial efficacy of the composites against both S. aureus and E. coli increased with increasing dose. Among the groups, the composites containing 0.5 wt% mGO showed the best efficacy, its antibacterial efficacy was as high as 80.32% ± 1.42% and 77.33% ± 2.52% against S. aureus and E. coli, respectively (Figures 6E,F). Unnikrishnan et al. (2024) developed a multifunctional GO/silver oxide-PVA/chitosan (GO/Ag2O-PVA/CS) polymeric composite. The study focused on the antibacterial effect of GO in polymeric matrix. Antibacterial experiments showed that PVA-CS alone had limited inhibitory effect on E. coli and S. aureus. However, the addition of 10 wt% GO significantly enhanced the inhibition of the composites against the above bacteria. This enhancement is mainly attributed to GO nanosheets disrupting bacterial cell membranes, ROS generation, and encapsulating bacteria. Kan et al. (2023) prepared a core-shell structured nanocomposite (PVA-PEG-SiO2@PVA-GO) with PVA/polyethylene glycol (PEG)/SiO2 nanoparticles as the core and PVA-GO as the shell, and systematically investigated its antibacterial properties. The results showed that through the synergistic effect of GO and silica nanoparticles, the composites could undergo a hydrogel transformation upon exposure to water, modulating the slow-release behavior of the drugs and significantly enhancing the antibacterial persistence. Staphylococcus aureus was used as a model strain in the antibacterial evaluation. GO polymer core-shell nanofibres (PVA-PEG-SiO2-1x-CHX@PVA-GO) possessed a pronounced bacteriostatic circle, with an antibacterial activity up to 71.92% ± 2.48% (100% with positive control), showing significant antibacterial effect.
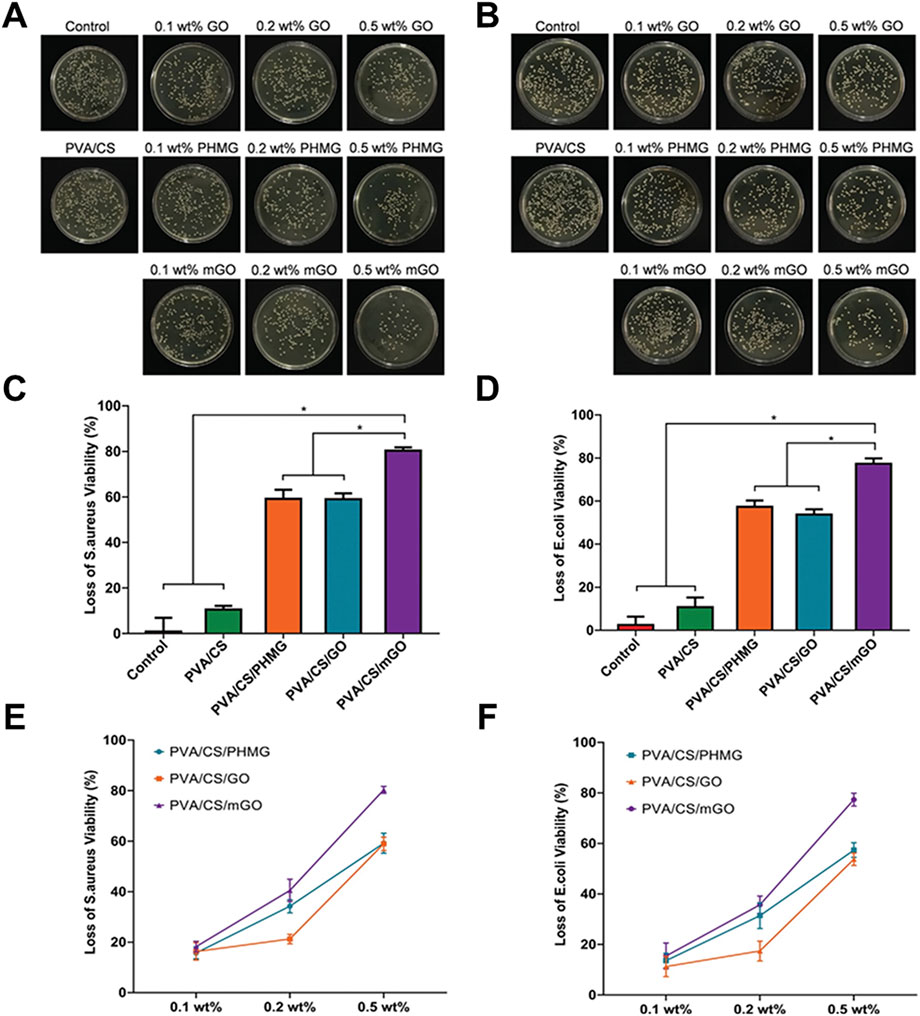
Figure 6. Antibacterial activity of PVA/CS incorporated with different concentrations of GO, PHMG, and mGO. Representative photographs of bacterial colonies formed by (A) S. aureus and (B) E. coli treated with PVA, PVA/CS, PVA/CS/GO, PVA/CS/PHMG, and PVA/CS/mGO for 3 h. The loss of bacterial viability of (C) S. aureus and (D) E. coli after treatment of PVA/CS incorporated with 0.5 wt% GO, PHMG, and mGO. Concentration-dependent antibacterial activity of PVA/CS/PHMG, PVA/CS/GO, and PVA/CS/mGO against (E) S. aureus and (F) E. coli. (*p < 0.05). Reproduced with permission from (Chen et al., 2020). Copyright © 2020. PVA: polyvinyl alcohol. PHMG: polyhexamethylene guanidine. CS: chitosan. mGO: modified graphene oxide.
3 Hemostatic effects
3.1 Hemostatic effects of GO
3.1.1 Activation and aggregation of platelets by GO
Excessive bleeding is a significant cause of mortality among individuals affected by natural disasters, traffic accidents, and traumatic injuries (Chan et al., 2015; Feng et al., 2016; Chen et al., 2019). Consequently, extensive research efforts have been directed towards the development of innovative hemostatic and absorbent agents to enhance hemostatic technologies.
GO exhibits a remarkable ability to activate and aggregate platelets, which fundamentally underpins its hemostatic function. The oxygen-containing functional groups on GO nanosheets can stimulate platelet activation, leading to robust aggregation and initiation of the coagulation cascade (Quan et al., 2015; Guajardo et al., 2021). Studies have shown that GO can trigger the release of intracellular Ca2+ in platelets, enhancing integrin-mediated adhesion and fibrinogen binding (Liang et al., 2018). In vivo models also demonstrate that GO accelerates thrombus formation, and the surface charge intensity of GO is closely correlated with its procoagulant capacity; higher negative surface charge induces stronger platelet activation and clotting responses (Li et al., 2019). These findings provide a theoretical basis for the extensive application of GO in hemostatic materials (Du et al., 2023).
3.1.2 Electrostatic interaction between GO and platelet glycoproteins
GO nanosheets carry abundant negative charges, enabling efficient binding to positively charged glycoproteins (such as GPIIb/IIIa) on the surface of platelets, thus promoting cell aggregation (Quan et al., 2015). This electrostatic attraction further facilitates the accumulation of platelets and erythrocytes on the GO surface, improves the local concentration of coagulation factors, shortens clotting time, and enhances the hemostatic effect (Figueroa et al., 2021). The rough surface texture of GO also favors trapping and adhesion of blood cells, expediting the clotting process (Kenry et al., 2015; Feng and Wang, 2022). In composite wound dressings, GO’s presence enhances interactions with blood components and maximizes its charge-driven procoagulant action (Feng and Wang, 2022).
3.1.3 Enhanced fluid absorption via hydrophilic functional groups
The unique chemical structure of GO confers high hydrophilicity due to its abundance of -OH, -C-O-C-, and -COOH groups (Singh et al., 2012). These functional groups create ideal liquid pathways within the material, making GO-based systems highly effective in absorbing blood and wound exudate (Borges-Vilches et al., 2022; Kanjwal and Ghaferi, 2022). Three-dimensional porous GO constructs show ultrafast uptake of liquid, enabling swift accumulation of blood components at the wound interface to support rapid hemostasis (Guajardo et al., 2021). In addition, GO’s hydrophilic nature reduces tissue adhesion, allows for painless dressing removal, and minimizes secondary wound damage (Aguado-Henche et al., 2022). Incorporating GO into polymer matrices not only strengthens absorption performance but also augments the overall mechanical properties of hemostatic dressings, supporting clinical applications (Feng and Wang, 2022).
3.1.4 Mechanical reinforcement and network enhancement in polymeric dressings
Acting as a nanofiller, GO significantly improves the mechanical strength, elasticity, and cross-linked network density of polymeric hemostatic dressings (Hu et al., 2013; Liang et al., 2019). Hydrogels, sponges, and nanofiber constructs containing GO display enhanced fluid absorption and robust compressive hemostatic capacity (Borges-Vilches et al., 2022). The mechanical integrity provided by GO enables stable physical compression of bleeding sites and, via swelling-induced pressure, further expedites clot formation (Ahuja et al., 2006; Zhang et al., 2019; Feng and Wang, 2022; Du et al., 2023) (Figure 7). Extensive in vitro and in vivo studies demonstrate that GO-reinforced hemostatic materials achieve superior hemostatic speed and efficacy compared to conventional dressings (Homem et al., 2022; Kanjwal and Ghaferi, 2022), revealing promising prospects for clinical translation.
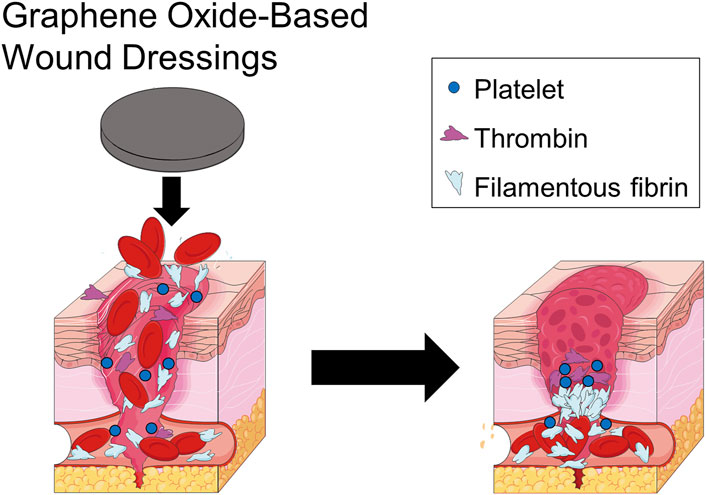
Figure 7. GO-based polymeric wound dressings enrich blood cells, causing them to collect on the wound surface and exert pressure on the wound, promoting blood clotting on the wound surface.
3.2 Hemostatic effects of GO-based polymeric wound dressings
Chen et al. (2019) developed a novel Bletilla striata polysaccharide/GO composite sponge (BGCS). The hemostatic efficacy of BGCS was evaluated using both an in vivo rat tail transection model and an in vitro dynamic whole blood coagulation test. In the rat tail transection model, BGCS demonstrated rapid blood absorption upon gentle application to the wound site, forming a stable clot at the interface to effectively arrest hemorrhage. Across six replicate experiments, the mean bleeding cessation time for BGCS was 45.9 ± 4.6 s. For the in vitro dynamic whole blood coagulation assay, it was observed that the group treated with BGCS exhibited a significantly lower absorbance compared to the control group, indicating enhanced blood coagulation. Within the initial 30 s of exposure to whole blood, the absorbance of the BGCS group was markedly reduced relative to the control group, with nearly complete coagulation achieved within this timeframe. Du et al. (2023) developed a novel chitosan/GO composite sponge (CSAG) and systematically evaluated its hemostatic properties. The CSAG series samples achieve different pore structures and surface properties by adjusting the GO content (0%, 10%, 20%, 33%). The samples with high GO content (33 wt%, CSAG33) showed excellent hemostatic and anti-adhesion capabilities in a series of in vitro and in vivo hemostatic experiments. CSAG33 rapidly enriches blood cells and activates both the exogenous and endogenous coagulation cascade pathways, resulting in rapid clot formation and accelerated hemostasis (Figure 8A). In addition, compared to QuikClot® and CSAG0, non-bleeding removal of CSAG33 was achieved due to the higher porosity and surface roughness of CSAG33, as well as weaker electrostatic interactions and hydrogen bonding (Figure 8B). Huang et al. (2025) prepared tranexamic acid-functionalized GO- acellular dermal matrix composite sponges (PGO0.35T1) and systematically evaluated their in vivo hemostatic capacity. The introduction of GO significantly enhanced the sponge porosity and specific surface area. In the rat tail amputation hemostasis test and liver hemostasis test, PGO0.35T1 sponges rapidly absorbed large amounts of blood and formed stable clots. The sponge group had significantly better hemostasis time and blood loss than the gauze and acellular dermal matrix control groups. In particular, in the liver hemostasis test, the hemostasis time was reduced to 76 ± 4 s and blood loss was reduced to 0.19 ± 0.03 g in the PGO0.35T1 group, which was much lower than in the control group.
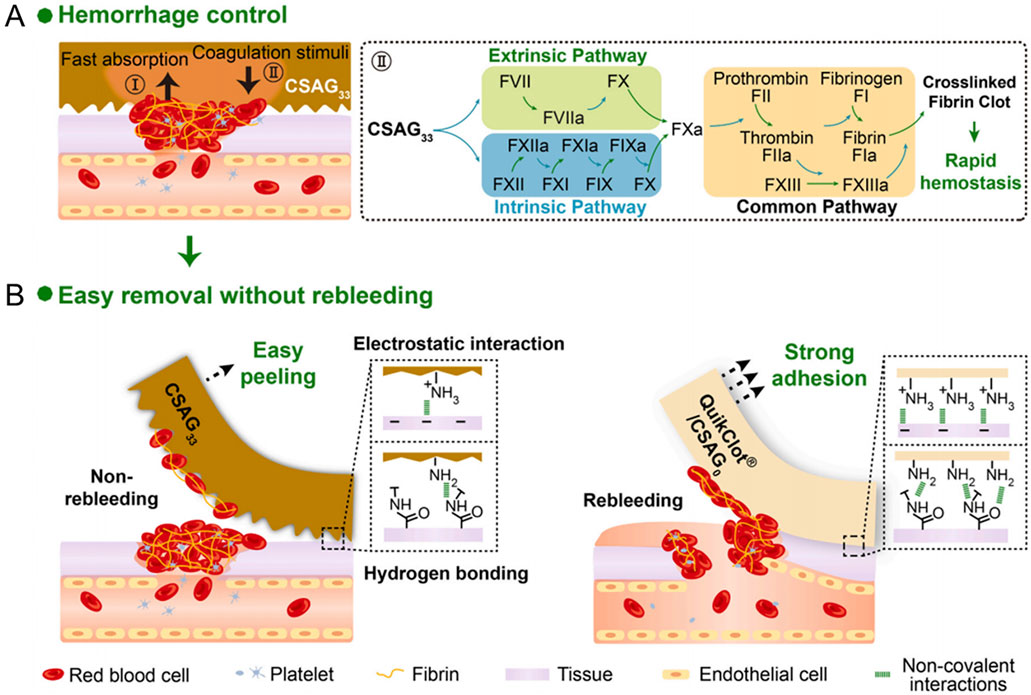
Figure 8. Schematic diagram of hemostasis and anti-adhesion mechanism of CSAG33. (A) First, the CSAG33 rapidly absorbed plasma to enrich blood red blood cells and platelets for the rapid formation of primary clotting clots. Second, the CSAG33 simultaneously activated exogenous and endogenous coagulation cascade pathways to reinforce the initial clotting plug through rich stimuli. (B) Compared to QuikClot® and CSAG0, CSAG33 exhibited weaker electrostatic interactions, hydrogen bonding interactions and higher porosity and surface roughness, which resulting in lower tissue adhesion strength. The removal of CSAG33 was accompanied with the defectdriven detachment of part of the blood scab, which sustained the wound seal and achieved non-rebleeding removal. Reproduced with permission from (Du et al., 2023). Copyright © 2023.
Sun et al. (2024) fabricated a quaternized chitosan-GO composite sponge (Col-QCS-GO) and evaluated its hemostatic efficacy using a rat tail transection model. The results indicated that the Col-QCS-GO sponge exhibited minimal blood loss (<50.0 mg), whereas the saline control group demonstrated substantial blood loss (293.1 ± 23.4 mg) on the filter paper (Figures 9B,D), indicating superior hemostatic performance of the Col-QCS-GO sponge. Furthermore, in vitro hemolysis tests revealed that triton-treated erythrocyte solutions appeared bright red, whereas Col-QCS-GO sponges-treated erythrocyte solutions appeared colorless and transparent (Figure 9A). At the same time, quantitative analysis showed that the hemolysis rate of the Col-QCS-GO sponges was below 5% (Figure 9C), which confirming their favorable safety profile. Du et al. (2025) developed a peptide-immobilized GO/chitosan composite sponge (GCCS-TRAP), which had remarkable rapid hemostatic properties. The study evaluated the hemostatic capacity of GCCS-TRAP through a rat femoral artery hemorrhage model. The results showed that GCCS-TRAP had excellent hemostatic effect. The mean hemostatic time of GCCS-TRAP was 81.3 s and the blood loss was 1.03 g, both of which were significantly better than other hemostatic materials. In vitro coagulation experiments showed that GCCS-TRAP could rapidly absorb blood. The blood clotting index (BCI) of GCCS-TRAP was only 7.1%, indicating that it can induce blood coagulation efficiently.
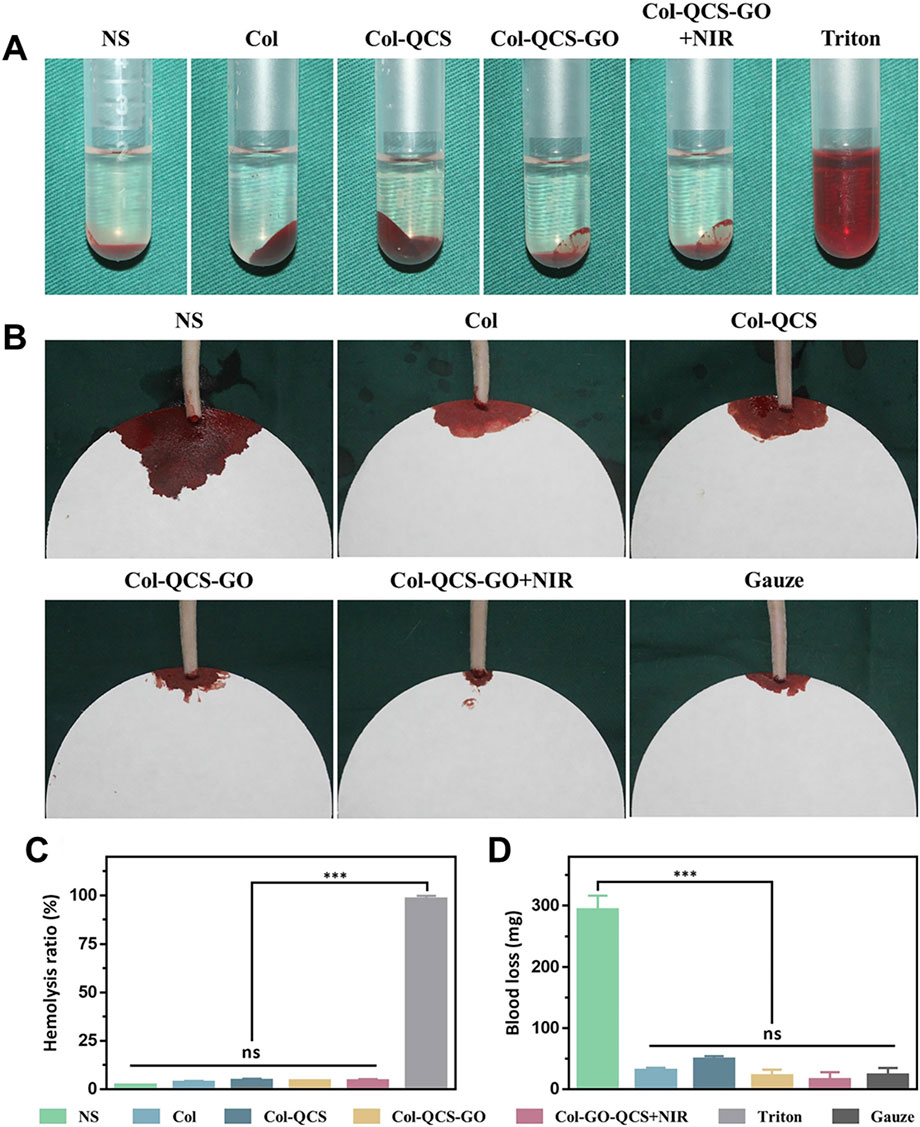
Figure 9. Hemolysis and hemostasis evaluation. (A) Optical images of different samples in red blood cells and (B) on the filter papers after creating an incision in the tail of rats (n = 3). (C) Hemolysis ratios of different samples. (D) Blood loss of different samples in the rat-tail amputation model (n = 3, *** p < 0.001, and ns means not significant). Reproduced with permission from (Sun et al., 2024). Copyright © 2024.
Several studies have confirmed that hemostatic materials incorporating GO can enhance hemostasis and reduce hemostasis time. However, the specific hemostatic mechanism of GO remains unclear, and the hemostatic efficacy of most studied materials often depends on additional drugs, as well as the porous structure and mechanical compression properties of the hemostatic materials. Consequently, further research is required to elucidate the hemostatic mechanism of GO.
4 Angiogenic effects
4.1 Angiogenic effects of GO
The process of human wound healing is a multifaceted mechanism that involves various cellular and tissue components within the body. This process encompasses several distinct phases, including hemostasis, inflammation reduction, antibacterial activity, cellular proliferation, and tissue remodeling (Rodrigues et al., 2019). Research has demonstrated that GO can facilitate angiogenesis, a process also known as neovascularization, which involves multiple signaling pathways. The angiogenic process encompasses the proliferation of endothelial cells in response to growth factors (GFs), followed by cell migration and capillary formation. Angiogenesis is an important part of the wound healing process and promotes wound healing (Hassan et al., 2022; Jiang et al., 2022). Newly formed blood vessels grow from healthy tissue at the wound edges toward the center of the wound, delivering oxygen, nutrients, and immune cells to the wound microenvironment while removing metabolic waste products, providing the foundation for cell proliferation, collagen synthesis, and tissue remodeling (An et al., 2021; Fu et al., 2023). At the same time, angiogenesis is accompanied by an increase in ROS scavenging capacity, thus protecting endothelial cell function and avoiding microangiopathy in a high-glucose environment (An et al., 2021). In addition, angiogenesis accelerates wound tissue re-epithelialization. Neovascular endothelial cells secrete factors such as EGF (Epidermal Growth Factor), which promotes keratinocyte migration and epidermal barrier reconstruction (Veith et al., 2019; An et al., 2021). This process is crucial for wound healing. Upon injury, the body initiates angiogenesis through the activation of endogenous pro-angiogenic factors (e.g., VEGF). These pro-angiogenic GFs are stored within the extracellular matrix (ECM) and in platelets and inflammatory cells that enter the circulation. Genes such as hypoxia-inducible factor (HIF) and cyclooxygenase-2 (COX-2) are upregulated in response to inflammation and hypoxia, thereby regulating the production of these factors. Research has demonstrated that wound healing is intricately linked to the equilibrium between ROS levels and the generation of oxidative stressors (Dunnill et al., 2017). Furthermore, it has been observed that low concentrations of ROS facilitate angiogenesis, while elevated levels of ROS impede this process (Dunnill et al., 2017). Studies have indicated that low doses of GO nanosheets (1–50 ng/mL) exhibit pro-angiogenic activity, potentially attributed to their ability to regulate intracellular ROS production. However, at high doses (>100 ng/mL), GO nanosheets exhibited anti-angiogenic activity, which can be attributed to the elevated levels of ROS (Figure 10) (D’Amora et al., 2023).
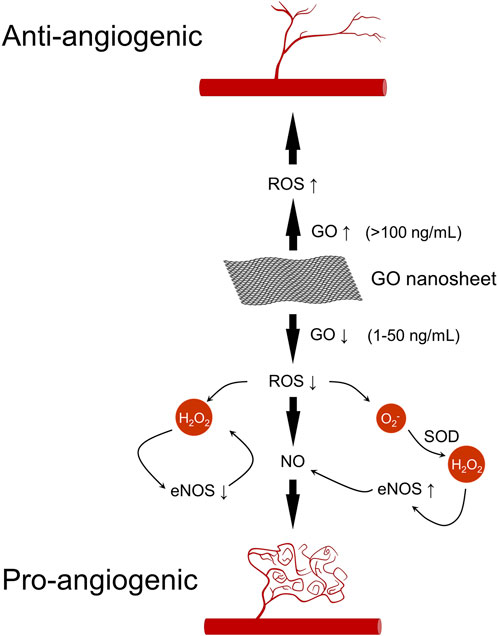
Figure 10. Pro- and anti-angiogenic pathways of GO. Low-dose GO (1–50 ng/mL) promotes angiogenesis through ROS-mediated Akt phosphorylation, eNOS activation, and increased NO production. High-dose GO (>100 ng/mL) inhibits angiogenesis due to excessive ROS accumulation.
The pro-angiogenic effects of GO are primarily associated with increased intracellular ROS and reactive nitrogen species, along with the activation of phosphorylated endothelial nitric oxide synthase (eNOS) and serine/threonine kinase (Akt). Notably, GO may modulate these processes through the regulation of nitric oxide (NO) production (Bretón-Romero and Lamas, 2014). Furthermore, NO production is a critical factor in both physiological and pathological angiogenesis, serving as a key regulator of endothelial cell proliferation, vascular tone, and angiogenesis (Namba et al., 2003). Mukherjee et al. (2015) discovered that GO and rGO induce the intracellular generation of ROS and reactive nitrogen species, along with the activation of phospho-Akt and phospho-eNOS. Specifically, ROS influence the phosphorylation of Akt, while the upregulation of eNOS triggers the activation of the nitric oxide (NO) signaling pathway, leading to an increase in intracellular NO production and subsequently promoting angiogenesis (Figure 10). Given GO’s remarkable antibacterial properties and pro-angiogenic activity, GO-based polymeric wound dressings will have great potential for future applications.
4.2 Angiogenic and wound healing effects of GO-based polymeric wound dressings
Song et al. (2024) nitro-modified GO and constructed a composite hydrogel (BC/PVA/NGO) with bacterial cellulose (BC) and (PVA) as the inner skeleton and an outer layer enriched with modified GO (NGO). BC/PVA/NGO composite hydrogel significantly accelerated the wound healing process in a mouse total skin defects model. BC/PVA/NGO significantly promoted wound healing compared to the control and BC/PVA hydrogel alone groups. After 15 days of treatment, the BC/PVA/NGO hydrogel group showed a high wound closure rate of 99.13%, which was significantly better than the control group and the composite hydrogel group not doped with NGO. Histological (H&E and Masson staining) analysis further confirmed that the NGO hydrogel was effective in reducing inflammatory cell infiltration, promoting collagen deposition and neovascularisation, and enhancing reconstruction of the hair follicle and epidermal layer. Elshahawy et al. (2024) systematically prepared a composite hydrogel with PVA/tragacanth gum (TG) matrix loaded with GO and cinnamon oil (CMO) aimed at promoting wound repair. Cell scratching experiments showed that GO-containing composite hydrogels significantly promoted fibroblast adhesion, migration and proliferation. The composite hydrogel doped with 5% GO promoted cell migration up to 74.05%, much higher than the control. It was shown that the material contributed to cell proliferation and re-epithelialisation, accelerating the wound closure process. Chakraborty et al. (2018) synthesized a porous scaffold by freeze-drying, starting from a polymeric blend of PVA and CMC with different amounts of rGO (0, 0.0025, 0.0005, 0.0075% and 0.01% w/v). The pro-angiogenic features of scaffolds were validated in vivo by using the chick chorioallantoic membrane (CAM) model. Two days after scaffolds implanting on the CAM of a developing chick embryo, angiogenesis was remarkably increased (Figure 11A). Scaffolds with 0.0005, 0.0075% and 0.01% rGO induced a significant increase in the number of blood vessels (Figure 11B), along with an absolute increase in blood vessel thickness up to 51.7% compared with scaffold with 0.005% rGO.
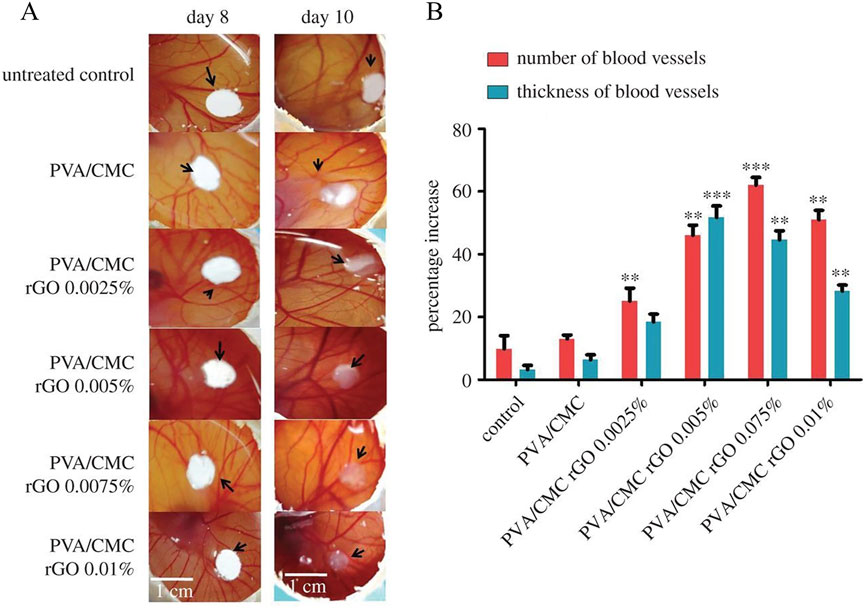
Figure 11. (A) Digital images of the untreated and treated CAM and (B) percentage increase in the average number of blood vessels and average thickness of blood vessels obtained on day 10 of the CAM assay. The values are normalized to that of the untreated control on day 8 (n = 9, **p < 0.01 and ***p < 0.001 versus control). Reproduced with permission from (Chakraborty et al., 2018). Copyright © 2018.
Wang et al. (2021) prepared a composite hydrogel incorporating functionalized GO and chitosan (CS). The whole skin defect experiment confirmed that CS/GO hydrogel could effectively promote wound healing (Figure 12A). Among them, the rats treated with CS/GO hydrogel demonstrated the best wound closure rate at all stages. At day 21, the wound closure rate of CS/GO hydrogel-treated rats reached 92.2%, which was higher than that of chitosan hydrogel-treated rats (90%) (Figure 12B), suggesting that the addition of GO to the hydrogel could improve the wound closure rate and promote wound healing. H&E staining results further confirmed the ability of CS/GO hydrogel to promote wound healing. The spacing between the granulation tissues in the control group was approximately 2,300 μm, whereas in the CS/GO group the spacing was reduced to 600 µm. The magnified image shows a large amount of immature granulation tissue in the control group. However, in the CS/GO group, the collagen fibres were well arranged (Figure 12C). Chen et al. (2025) prepared SA/GO/calcium chloride/cerium nitrate hemostatic powder (SACC-GO) by ball milling method. The wound healing efficacy of SACC-GO was evaluated using a rat total skin defects model. Compared to the control group, wounds treated with SACC-GO exhibited a higher wound healing rate. On day 14, the control group had a 70% wound healing rate, while the SACC-GO group had almost complete wound healing. In addition, H&E staining showed that the epithelial tissue around the wound was well-organized, featuring an increased number of hair follicles, and nearly complete regeneration of dermal tissue, indicating a significant healing effect. Khan et al. (2023b) synthesized a composite hydrogel by blending gelatin and GO-functionalized bacterial cellulose (GO-fBC) with tetraethyl orthosilicate (TEOS). The study revealed that this hydrogel exhibited excellent hemocompatibility, haemostatic properties and antibacterial properties. Furthermore, it was observed that the hydrogel significantly enhanced the viability and proliferation of mouse embryonic fibroblasts (NIH/3T3). With the increase of GO addition, the cell viability and proliferation capacity will be further enhanced. Among them, GBG-4 (hydrogel with 0.04 mg graphene oxide addition) demonstrated the most pronounced effect on fibroblast growth. These findings suggest that the hydrogel possesses potential wound healing capabilities.
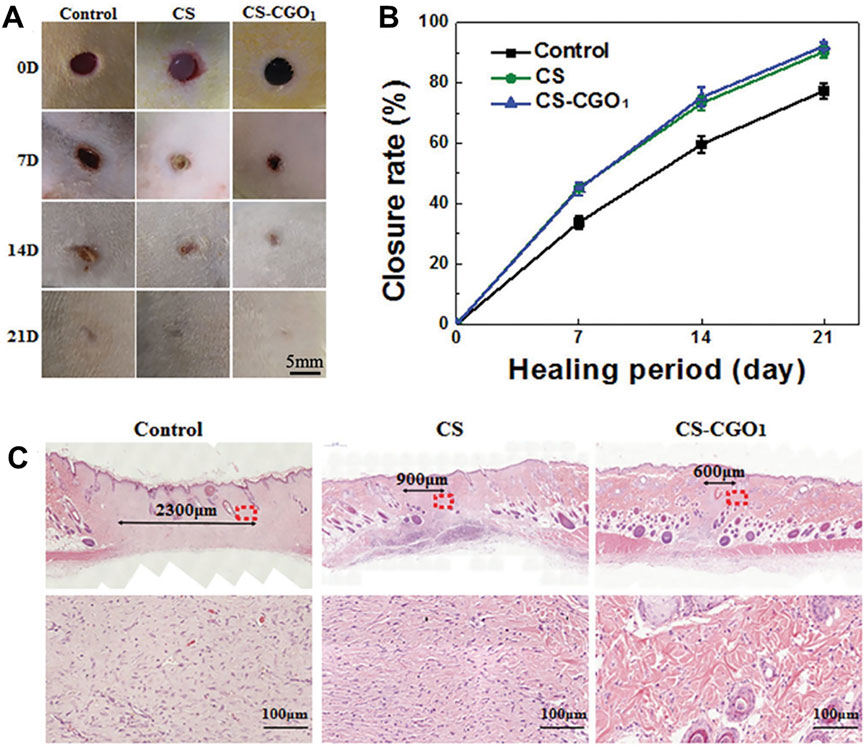
Figure 12. Wound healing by employing CS hydrogel and CS-CGO composite hydrogel. (A) Representative images of wounds treated with CS hydrogel and CS-CGO composite hydrogel at 0, 7, 14, and 21 days. (B) Quantitative analysis of wound closure area for each group. (C) H&E stained histological images of wound tissue from control group, CS hydrogel group, and CS-CGO group on 21 days. Reproduced with permission from (Wang et al., 2021). Copyright © 2021.
GO demonstrates significantly promote angiogenesis through multiple pathways, including modulating cell behavior, inducting angiogenic factor secretion, and adjusting oxidative stress levels. Several animal experiments have also demonstrated that GO-based polymeric wound dressings can significantly improve wound closure rate and accelerate wound healing in animals. Consequently, GO holds considerable promise for applications in wound healing, tissue engineering, and regenerative medicine. However, GO still faces some shortcomings and challenges in wound healing. Although GO has shown its capability to promote angiogenesis and accelerate wound healing by modulating cell behavior and inducing growth factor secretion, its specific molecular mechanisms have not been fully elucidated. Specifically, further research is required to investigate how GO interacts with cell membranes, receptors, or signaling pathways such as VEGF, PI3K/Akt, HIF-1α, among others, as well as to determine whether these interactions exhibit dose-dependent characteristics.
5 Biocompatibility of GO and GO-based polymeric dressings
It has been found that specific concentrations of GO can be toxic to a wide range of organisms such as earthworms, zebrafish, mice, etc., as the use of GO increases the levels of ROS and superoxide dismutase (SOD) in living organisms (Ghulam et al., 2022). Duo et al. (2022) investigated the toxicity of GO to earthworms by exposing earthworms to different concentrations of GO in a filter paper contact test and a soil contact test. The lethal concentration 50 (LC50) of the former earthworms exposed to GO was 2.52 and 2.36 mg/mL at 24 and 48 h, respectively, while the LC50 of the latter earthworms exposed to GO on day 14 was 68.8 g/kg. Histopathology has shown that the skin and gut of earthworms can be severely damaged as GO concentrations increase. Liu et al. (2014) found that GO is significantly toxic to zebrafish embryos, affecting embryo hatching and larval length. Hashemi et al. (2016) analyzed the cytotoxicity of GO on mouse spermatogonial stem cells (SSCs). The results showed that GO significantly increased ROS levels at concentrations of 100 and 400 μg/mL, whereas it had no significant effect at lower concentrations. In addition, (MTT) assay showed significant reduction in the cell number of GO-treated SSCs at high concentrations (100 and 400 μg/mL) compared to untreated SSCs, displaying significant cytotoxicity.
However, reports have shown that the toxicity of GO is significantly improved when it is combined with polymeric compounds to form GO-based polymeric wound dressings for use, demonstrating a high level of safety. This may be an effective means of reducing the toxicity of GO and increasing its range of applications. Khan et al. (2023c) prepared a bioactive hydrogel made of bacterial cellulose (BC), gelatin and GO and explored the hemocompatibility of the hydrogel by in vitro hemolysis assay. The results showed that the hemolysis rate of all hydrogel samples (HGel-1 to HGel-3) was less than 0.5%, which was significantly lower than the clinical safety threshold (5%), suggesting that the hydrogels have excellent hemocompatibility. Ningrum et al. (2023) prepared a hydrogel wound dressing with PVA, Moringa oleifera leaf (MOL) extract and GO. The study assessed the cytotoxicity of hydrogel on 3T3L1 mouse fibroblasts by MTT assay. The results showed that the cell viability of all hydrogel samples ranged from 83%–135% (Figure 13), which was higher than the toxicity threshold of ISO 10993–5:2009. Demenj et al. (2025) prepared a multi-bioactive scaffold based on GO, gelatin and alginate. The study was conducted to evaluate the toxicity of the scaffold using Caenorhabditis elegans assay. The scaffold was co-incubated with C. elegans for 4 days and the survival rate was 100% in all groups, showing that the scaffold had no significant toxicity to C. elegans. In terms of growth rate, there was a slight decrease in body length (about 20%) compared to the control group, but there was no significant abnormality in overall growth and health. González et al. (2024) prepared a biocomposite hydrogel based on collagen with rGO and systematically investigated the biocompatibility of the hydrogel. The effects of hydrogels with different rGO contents (COL/rGO25, COL/rGO50, COL/rGO100) on human dermal fibroblasts (HDF) were evaluated by MTT assay. The results showed that the cell viability of all hydrogel samples was higher than 80%. In addition, the roughness and good water retention of the material surface further promoted cell adhesion and proliferation.
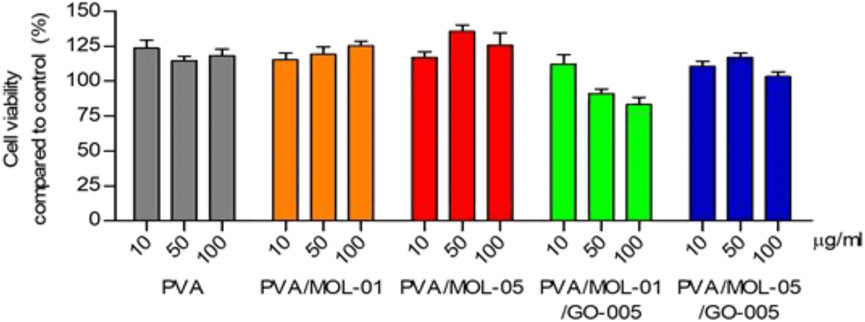
Figure 13. Cytotoxicity assay cultured on the hydrogels after a 24 h incubation compared to control treated with different concentrations. Data represents means ± SD from at least five independent experiments. Reproduced with permission from (Ningrum et al., 2023). Copyright © 2023.
Studies have confirmed that GO-based polymeric wound dressings can improve biocompatibility, but the conformational relationship and the material ratios of the GO-based polymeric wound dressings still need to be further investigated in the future. In addition, novel functionalization strategies (e.g., surface modification, stimulus response, etc.) can be developed to improve the biocompatibility of GO. GO-based polymeric wound dressings are expected to achieve safer and more efficient applications in biomedical fields.
6 Conclusions and future prospects
GO-based polymeric wound dressings exhibit significantly better overall performance than traditional dressings through the innovative combination of GO and polymeric compounds. The superior functionality of these dressings, including high antibacterial efficacy, rapid hemostasis, pro-angiogenesis, and accelerated wound healing, is fundamentally derived from the multiple bioactivities provided by GO as the core functional component (Shariati et al., 2023). GO’s unique physicochemical structure enables it to synergize sterilization through physical destruction, oxidative stress, and PDT effects. Promotes hemostasis through activation of platelets, enrichment of coagulation factors, and enhancement of fluid absorption. Driving angiogenesis by regulating ROS levels and Akt/eNOS signaling pathway. When GO is incorporated into the polymer matrix, its bioactivity is maximized for release. The polymeric compounds not only serves as a carrier to optimize the dispersion and stability of GO, but also synergistically amplifies the efficacy of GO through its three-dimensional porous structure, mechanical enhancement effect and high biocompatibility. In addition, the polymer matrix is key to reducing the potential toxicity of GO (Ghulam et al., 2022; Yadav et al., 2022). While high doses of GO alone may trigger cellular damage, the composite polymer significantly improves the safety of the dressing, such as hemocompatibility and cytotoxicity, by reducing the direct contact of GO with the tissue through physical encapsulation. This synergistic optimization allows GO-based dressings to meet clinical safety thresholds while fulfilling functional requirements.
However, challenges remain in advancing GO-based polymeric wound dressings to the clinic. Although GO-based polymeric wound dressings have demonstrated excellent antibacterial properties, their exact mechanism of action, especially whether it is the same as that of GO alone, remains a key issue that has not been systematically explored. While much of the existing research has focused on the overall antibacterial properties of dressings, little work has been dedicated to investigating the interactions of the components in composite wound dressings and their unique contributions to the antibacterial mechanism. Furthermore, the specific molecular mechanisms by which GO exerts hemostatic, and pro-angiogenic effects in composite wound dressings still need to be explored in greater depth. Such as the dose-dependent effects and precise manner in which GO modulates key signaling pathways (e.g., VEGF, PI3K/Akt, HIF-1α), and its specific targets in the coagulation cascade.
Preparation of GO requires strict control of the degree of oxidation and the number of layers to ensure the stability and consistency of the performance of GO-based polymeric wound dressings. The primary method for synthesizing GO is the Hummers’ method. This method utilizes a strong oxidizing agent (KMnO4, H2SO4, etc.) to react with graphite to introduce oxygen-containing functional groups, ultimately making GO (Chen et al., 2022; Gostaviceanu et al., 2024). GO prepared by the Hummers’ method may suffer from inconsistencies in size, number of layers, and degree of oxidation, which may affect its effectiveness. Furthermore, chemical modifications (such as binding to polymers, proteins, or drugs) of GO are often required to improve their biocompatibility and functionality. However, these functionalization processes involve complex chemical reactions and expensive reagents, potentially increasing production complexity and expenses, as well as introducing new toxicity concerns.
The long-term in vivo biocompatibility, degradation metabolic pathways, and possible chronic effects of GO-based polymeric wound dressings need to be more systematically evaluated. There is a need to establish standardized methods to study the long-term behavior of different sizes, oxidation levels, surface modifications and dispersion states of GO in composite wound dressings and their impact on safety. In addition, animal experiments and clinical translation of GO-based polymeric wound dressings are flawed to some extent. Current studies are mostly based on mouse or rat models (Hao et al., 2023; Yu et al., 2023), whose healing processes differ from those of humans, and such differences may affect the translation of experimental results in a clinical setting, so future studies need to be validated in models that are closer to the clinic. Furthermore, in order to realize the wide application of GO-based polymeric wound dressings in the clinic, future studies should prioritize the optimization of the dosage of GO in the dressings, the long-term safety assessment of the dressings, and the comparative efficacy study with the existing wound dressings. Research in these areas will provide sufficient theoretical support for the widespread use of GO-based polymeric wound dressings in clinical settings in the future.
GO-based polymeric wound dressings represent an important direction for upgrading traditional wound dressings to functionalization and intelligence. GO, as its core active ingredient, is the cornerstone for conferring superior bioactivity to these dressings. The polymer matrix provides an ideal carrier platform for GO, which not only synergistically amplifies its bioactivity, but also improves its biocompatibility and reduces potential risks. Deepening the understanding of the mechanism, optimizing the dressing design and preparation process, and promoting clinical translational research will greatly facilitate the clinical application of GO-based polymeric wound dressings, and provide more efficient and safer treatment options for patients.
Author contributions
SL: Conceptualization, Supervision, Writing – original draft. JW: Investigation, Project administration, Software, Writing – original draft. HZ: Writing – review and editing, Funding acquisition. XZ: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Shandong Provincial Natural Science Foundation, grant number ZR2023QB199, Shandong Provincial Development Plan for Youth Innovation Team in Higher Education Institution, grant number 2023KJ347, Shandong Provincial Medical Health Science and Technology Project, grant number 202213020603, and Scientific Research Funding Project of the Shandong University of Traditional Chinese Medicine, grant number KYZK2024Q09.
Acknowledgments
The authors gratefully acknowledge the postdoctoral program of the Shandong University of Traditional Chinese Medicine, and High-Level Traditional Chinese Medicine Key Disciplines of the State Administration of Traditional Chinese Medicine Pharmaceutics of Traditional Chinese Medicine. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abat, C., Fournier, P.-E., Jimeno, M.-T., Rolain, J.-M., and Raoult, D. (2018). Extremely and pandrug-resistant bacteria extra-deaths: myth or reality. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1687–1697. doi:10.1007/s10096-018-3300-0
Aguado-Henche, S., Escudero, M. L., García-Alonso, M. C., Lozano-Puerto, R. M., and Clemente de Arriba, C. (2022). Biological responses in the blood and organs of rats to intraperitoneal inoculation of graphene and graphene oxide. Mater. (Basel) 15, 2898. doi:10.3390/ma15082898
Ahuja, N., Ostomel, T. A., Rhee, P., Stucky, G. D., Conran, R., Chen, Z., et al. (2006). Testing of modified zeolite hemostatic dressings in a large animal model of lethal groin injury. J. Trauma 61, 1312–1320. doi:10.1097/01.ta.0000240597.42420.8f
Ali, N. H., Amin, M. C. I. M., and Ng, S.-F. (2019). Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J. Biomater. Sci. Polym. Ed. 30, 629–645. doi:10.1080/09205063.2019.1595892
An, Y., Lin, S., Tan, X., Zhu, S., Nie, F., Zhen, Y., et al. (2021). Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 54, e12993. doi:10.1111/cpr.12993
Arabpour, Z., Abedi, F., Salehi, M., Baharnoori, S. M., Soleimani, M., and Djalilian, A. R. (2024). Hydrogel-based skin regeneration. Int. J. Mol. Sci. 25, 1982. doi:10.3390/ijms25041982
Barnum, L., Samandari, M., Schmidt, T. A., and Tamayol, A. (2020). Microneedle arrays for the treatment of chronic wounds. Expert Opin. Drug Deliv. 17, 1767–1780. doi:10.1080/17425247.2020.1819787
Borges-Vilches, J., Aguayo, C., and Fernández, K. (2022). The effect on hemostasis of gelatin-graphene oxide aerogels loaded with grape skin proanthocyanidins: in vitro and in vivo evaluation. Pharmaceutics 14, 1772. doi:10.3390/pharmaceutics14091772
Bretón-Romero, R., and Lamas, S. (2014). Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2, 529–534. doi:10.1016/j.redox.2014.02.005
Chakraborty, S., Ponrasu, T., Chandel, S., Dixit, M., and Muthuvijayan, V. (2018). Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R. Soc. Open Sci. 5, 172017. doi:10.1098/rsos.172017
Chan, L. W., Wang, X., Wei, H., Pozzo, L. D., White, N. J., and Pun, S. H. (2015). A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 7, 277ra29. doi:10.1126/scitranslmed.3010383
Chattopadhyay, S., and Raines, R. T. (2014). Collagen-based biomaterials for wound healing. Biopolymers 101, 821–833. doi:10.1002/bip.22486
Chen, J., Peng, H., Wang, X., Shao, F., Yuan, Z., and Han, H. (2014). Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 6, 1879–1889. doi:10.1039/c3nr04941h
Chen, J., Lv, L., Li, Y., Ren, X., Luo, H., Gao, Y., et al. (2019). Preparation and evaluation of Bletilla striata polysaccharide/graphene oxide composite hemostatic sponge. Int. J. Biol. Macromol. 130, 827–835. doi:10.1016/j.ijbiomac.2019.02.137
Chen, S., Wang, H., Jian, Z., Fei, G., Qian, W., Luo, G., et al. (2020). Novel poly(vinyl alcohol)/chitosan/modified graphene oxide biocomposite for wound dressing application. Macromol. Biosci. 20, e1900385. doi:10.1002/mabi.201900385
Chen, Y., Pandit, S., Rahimi, S., and Mijakovic, I. (2021). Interactions between graphene-based materials and Biological surfaces: a review of underlying molecular mechanisms. Adv. Mater. Inter 8, 2101132. doi:10.1002/admi.202101132
Chen, X., Qu, Z., Liu, Z., and Ren, G. (2022). Mechanism of oxidization of graphite to graphene oxide by the Hummers method. ACS Omega 7, 23503–23510. doi:10.1021/acsomega.2c01963
Chen, A., Wu, L., Wang, K., Qin, L., Zhang, K., Chen, S., et al. (2025). Facile synthesis of rapid hemostatic powder based on sodium alginate for promoting hemostasis and wound healing. Int. J. Biol. Macromol. 308, 142728. doi:10.1016/j.ijbiomac.2025.142728
Das, M., Sethy, C., Kundu, C. N., and Tripathy, J. (2023). Synergetic reinforcing effect of graphene oxide and nanosilver on carboxymethyl cellulose/sodium alginate nanocomposite films: assessment of physicochemical and antibacterial properties. Int. J. Biol. Macromol. 239, 124185. doi:10.1016/j.ijbiomac.2023.124185
Demenj, M., Žabčić, M., Vukomanović, M., Ilić-Tomić, T., Milivojević, D., Tomić, S., et al. (2025). Design of the multi-bioactive graphene-oxide/gelatin/alginate scaffolds as dual ECM-mimetic and specific wound healing phase-target therapeutic concept for advanced wound healing. Pharmaceutics 17, 89. doi:10.3390/pharmaceutics17010089
Di Crescenzo, A., Zara, S., Di Nisio, C., Ettorre, V., Ventrella, A., Zavan, B., et al. (2019). Graphene oxide foils as an osteoinductive stem cell substrate. ACS Appl. Bio Mater 2, 1643–1651. doi:10.1021/acsabm.9b00041
Di Giulio, M., Zappacosta, R., Di Lodovico, S., Di Campli, E., Siani, G., Fontana, A., et al. (2018). Antimicrobial and antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob. Agents Chemother. 62, e00547-18. doi:10.1128/AAC.00547-18
Di Lodovico, S., Diban, F., Di Fermo, P., Petrini, M., Fontana, A., Di Giulio, M., et al. (2022). Antimicrobial combined action of graphene oxide and light emitting diodes for chronic wound management. Int. J. Mol. Sci. 23, 6942. doi:10.3390/ijms23136942
Du, F., A, W., Liu, F., Wu, B., Liu, Y., Zheng, W., et al. (2023). Hydrophilic chitosan/graphene oxide composite sponge for rapid hemostasis and non-rebleeding removal. Carbohydr. Polym. 316, 121058. doi:10.1016/j.carbpol.2023.121058
Du, F., Wang, Y., A, W., Tang, M., Liu, F., Pan, X., et al. (2025). Precision surface-immobilized peptide on graphene/chitosan composite sponge for rapid hemostasis of uncontrolled bleeding. Colloids Surf. B Biointerfaces 253, 114757. doi:10.1016/j.colsurfb.2025.114757
Dunnill, C., Patton, T., Brennan, J., Barrett, J., Dryden, M., Cooke, J., et al. (2017). Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 14, 89–96. doi:10.1111/iwj.12557
Duo, L., Wang, Y., and Zhao, S. (2022). Individual and histopathological responses of the earthworm (Eisenia fetida) to graphene oxide exposure. Ecotoxicol. Environ. Saf. 229, 113076. doi:10.1016/j.ecoenv.2021.113076
D’Amora, U., Dacrory, S., Hasanin, M. S., Longo, A., Soriente, A., Kamel, S., et al. (2023). Advances in the physico-chemical, antimicrobial and angiogenic properties of graphene-oxide/cellulose nanocomposites for wound healing. Pharmaceutics 15, 338. doi:10.3390/pharmaceutics15020338
Elshahawy, M. F., Mohamed, R. D., Ali, A. E.-H., Raafat, A. I., and Ahmed, N. A. (2024). Electron beam irradiation developed cinnamon oil- (polyvinyl alcohol/gum tragacanth)/graphene oxide dressing hydrogels: antimicrobial and healing assessments. Int. J. Biol. Macromol. 277, 134384. doi:10.1016/j.ijbiomac.2024.134384
Feng, W., and Wang, Z. (2022). Biomedical applications of chitosan-graphene oxide nanocomposites. iScience 25, 103629. doi:10.1016/j.isci.2021.103629
Feng, C., Li, J., Wu, G. S., Mu, Y. Z., Kong, M., Jiang, C. Q., et al. (2016). Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS Appl. Mater Interfaces 8, 34234–34243. doi:10.1021/acsami.6b12317
Figueroa, T., Carmona, S., Guajardo, S., Borges, J., Aguayo, C., and Fernández, K. (2021). Synthesis and characterization of graphene oxide chitosan aerogels reinforced with flavan-3-ols as hemostatic agents. Colloids Surf. B Biointerfaces 197, 111398. doi:10.1016/j.colsurfb.2020.111398
Fiorentini, F., Suarato, G., Summa, M., Miele, D., Sandri, G., Bertorelli, R., et al. (2023). Plant-based, hydrogel-like microfibers as an antioxidant platform for skin burn healing. ACS Appl. Bio Mater 6, 3103–3116. doi:10.1021/acsabm.3c00214
Fu, Y.-J., Shi, Y.-F., Wang, L.-Y., Zhao, Y.-F., Wang, R.-K., Li, K., et al. (2023). All-natural Immunomodulatory bioadhesive hydrogel promotes angiogenesis and diabetic wound healing by regulating macrophage heterogeneity. Adv. Sci. (Weinh) 10, e2206771. doi:10.1002/advs.202206771
Genorio, B., Harrison, K. L., Connell, J. G., Dražić, G., Zavadil, K. R., Markovic, N. M., et al. (2019). Tuning the selectivity and activity of electrochemical interfaces with defective graphene oxide and reduced graphene oxide. ACS Appl. Mater Interfaces 11, 34517–34525. doi:10.1021/acsami.9b13391
Ghulam, A. N., Dos Santos, O. A. L., Hazeem, L., Pizzorno Backx, B., Bououdina, M., and Bellucci, S. (2022). Graphene oxide (GO) materials-applications and toxicity on living organisms and environment. J. Funct. Biomater. 13, 77. doi:10.3390/jfb13020077
González, L., Espinoza, V., Tapia, M., Aedo, V., Ruiz, I., Meléndrez, M., et al. (2024). Innovative approach to accelerate wound healing: synthesis and validation of enzymatically cross-linked COL-rGO biocomposite hydrogels. Gels 10, 448. doi:10.3390/gels10070448
Gostaviceanu, A., Gavrilaş, S., Copolovici, L., and Copolovici, D. M. (2024). Graphene-oxide peptide-containing materials for biomedical applications. Int. J. Mol. Sci. 25, 10174. doi:10.3390/ijms251810174
Guajardo, S., Figueroa, T., Borges, J., Aguayo, C., and Fernández, K. (2021). Graphene oxide-gelatin aerogels as wound dressings with improved hemostatic properties. Mater. Today Chem. 20, 100418. doi:10.1016/j.mtchem.2020.100418
Hamedi, H., Moradi, S., Hudson, S. M., Tonelli, A. E., and King, M. W. (2022). Chitosan based bioadhesives for biomedical applications: a review. Carbohydr. Polym. 282, 119100. Not Available. doi:10.1016/j.carbpol.2022.119100
Hao, P.-C., Burnouf, T., Chiang, C.-W., Jheng, P.-R., Szunerits, S., Yang, J.-C., et al. (2023). Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J. Nanobiotechnology 21, 318. doi:10.1186/s12951-023-02068-x
Hashemi, E., Akhavan, O., Shamsara, M., Daliri, M., Dashtizad, M., and Farmany, A. (2016). Synthesis and cyto-genotoxicity evaluation of graphene on mice spermatogonial stem cells. Colloids Surf. B Biointerfaces 146, 770–776. doi:10.1016/j.colsurfb.2016.07.019
Hassan, M. A., Abd El-Aziz, S., Elbadry, H. M., El-Aassar, S. A., and Tamer, T. M. (2022). Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 29, 2978–2988. doi:10.1016/j.sjbs.2022.01.015
Homem, N. C., Miranda, C. S., Teixeira, M. A., Teixeira, M. O., Domingues, J. M., Seibert, D., et al. (2022). Graphene oxide-based platforms for wound dressings and drug delivery systems: a 10 year overview. J. Drug Deliv. Sci. Technol. 78, 103992. doi:10.1016/j.jddst.2022.103992
Hu, H., Zhao, Z., Wan, W., Gogotsi, Y., and Qiu, J. (2013). Ultralight and highly compressible graphene aerogels. Adv. Mater 25, 2219–2223. doi:10.1002/adma.201204530
Huang, C., Chen, L., Liu, X., Chen, Y., Li, X., Wen, Q., et al. (2025). Effect of tranexamic acid-functionalized photothermal hydrothermal treated oxidized graphene sponge on diabetic wound healing: hemostasis, antibacterial, and regeneration. Mater. and Des. 253, 113915. doi:10.1016/j.matdes.2025.113915
Jiang, Y., Zhao, W., Xu, S., Wei, J., López Lasaosa, F., He, Y., et al. (2022). Bioinspired design of mannose-decorated globular lysine dendrimers promotes diabetic wound healing by orchestrating appropriate macrophage polarization. Biomaterials 280, 121323. doi:10.1016/j.biomaterials.2021.121323
Kamel, S., Dacrory, S., Hesemann, P., Bettache, N., Ali, L. M. A., Postel, L., et al. (2023). Wound dressings based on sodium alginate-polyvinyl alcohol-moringa oleifera extracts. Pharmaceutics 15, 1270. doi:10.3390/pharmaceutics15041270
Kan, Y., Bondareva, J. V., Statnik, E. S., Koudan, E. V., Ippolitov, E. V., Podporin, M. S., et al. (2023). Hydrogel-inducing graphene-oxide-derived core-shell fiber composite for antibacterial wound dressing. Int. J. Mol. Sci. 24, 6255. doi:10.3390/ijms24076255
Kanjwal, M. A., and Ghaferi, A. A. (2022). Graphene incorporated electrospun nanofiber for electrochemical sensing and biomedical applications: a critical review. Sensors (Basel) 22, 8661. doi:10.3390/s22228661
Kenry, , Loh, K. P., and Lim, C. T. (2015). Molecular hemocompatibility of graphene oxide and its implication for antithrombotic applications. Small 11, 5105–5117. doi:10.1002/smll.201500841
Khan, A., Rehman, W., Alanazi, M. M., Khan, Y., Rasheed, L., Saboor, A., et al. (2023a). Development of novel multifunctional electroactive, self-healing, and tissue adhesive scaffold to accelerate cutaneous wound healing and hemostatic materials. ACS Omega 8, 39110–39134. doi:10.1021/acsomega.3c04135
Khan, M. U. A., Stojanović, G. M., Hassan, R., Anand, T. J. S., Al-Ejji, M., and Hasan, A. (2023b). Role of graphene oxide in bacterial cellulose-gelatin hydrogels for wound dressing applications. ACS Omega 8, 15909–15919. doi:10.1021/acsomega.2c07279
Khan, M. U. A., Stojanović, G. M., Rehman, R. A., Moradi, A.-R., Rizwan, M., Ashammakhi, N., et al. (2023c). Graphene oxide-functionalized bacterial cellulose-gelatin hydrogel with curcumin release and kinetics: in vitro biological evaluation. ACS Omega 8, 40024–40035. doi:10.1021/acsomega.2c06825
Koehler, J., Wallmeyer, L., Hedtrich, S., Goepferich, A. M., and Brandl, F. P. (2017). pH-modulating poly(ethylene glycol)/alginate hydrogel dressings for the treatment of chronic wounds. Macromol. Biosci. 17, 1600369. doi:10.1002/mabi.201600369
Li, G., Liang, Y., Xu, C., Sun, H., Tao, L., Wei, Y., et al. (2019). Polydopamine reinforced hemostasis of a graphene oxide sponge via enhanced platelet stimulation. Colloids Surfaces B Biointerfaces 174, 35–41. doi:10.1016/j.colsurfb.2018.10.074
Liang, Y., Xu, C., Li, G., Liu, T., Liang, J. F., and Wang, X. (2018). Graphene-kaolin composite sponge for rapid and riskless hemostasis. Colloids Surf. B Biointerfaces 169, 168–175. doi:10.1016/j.colsurfb.2018.05.016
Liang, Y., Zhao, X., Hu, T., Chen, B., Yin, Z., Ma, P. X., et al. (2019). Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 15, e1900046. doi:10.1002/smll.201900046
Lin, X., Shen, Y., and Wang, L. (2021). Multi-scale photoacoustic assessment of wound healing using chitosan–graphene oxide hemostatic sponge. Nanomater. (Basel) 11, 2879. doi:10.3390/nano11112879
Liu, S., Zeng, T. H., Hofmann, M., Burcombe, E., Wei, J., Jiang, R., et al. (2011). Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5, 6971–6980. doi:10.1021/nn202451x
Liu, X. T., Mu, X. Y., Wu, X. L., Meng, L. X., Guan, W. B., Ma, Y. Q., et al. (2014). Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 27, 676–683. doi:10.3967/bes2014.103
Mahmoud, A. M., Alghuthaymi, M. A., Shaban, M., and Rabia, M. (2025). A promising eco-friendly and cost-effective photocatalytic rolled graphene oxide/poly(m-methylaniline) core-shell nanocomposite for antimicrobial action. Biotechnol. Appl. Biochem. 72, 43–57. doi:10.1002/bab.2645
Maiti, D., Tong, X., Mou, X., and Yang, K. (2018). Carbon-based nanomaterials for biomedical applications: a recent study. Front. Pharmacol. 9, 1401. doi:10.3389/fphar.2018.01401
Mei, L., Shi, Y., Miao, Z., Cao, F., Hu, K., Lin, C., et al. (2021). Photo-initiated enhanced antibacterial therapy using a non-covalent functionalized graphene oxide nanoplatform. Dalton Trans. 50, 8404–8412. doi:10.1039/D1DT00642H
Mohammed, H., Kumar, A., Bekyarova, E., Al-Hadeethi, Y., Zhang, X., Chen, M., et al. (2020). Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front. Bioeng. Biotechnol. 8, 465. doi:10.3389/fbioe.2020.00465
Mukherjee, S., Sriram, P., Barui, A. K., Nethi, S. K., Veeriah, V., Chatterjee, S., et al. (2015). Graphene oxides show angiogenic properties. Adv. Healthc. Mater 4, 1722–1732. doi:10.1002/adhm.201500155
Mustafa, K., Iqbal, N., Ahmad, S., Iqbal, S., Rezakazemi, M., Verpoort, F., et al. (2024). Highly efficient aramid fiber supported polypropylene membranes modified with reduced graphene oxide based metallic nanocomposites: antimicrobial and antiviral capabilities. RSC Adv. 14, 16421–16431. doi:10.1039/d4ra00724g
Namba, T., Koike, H., Murakami, K., Aoki, M., Makino, H., Hashiya, N., et al. (2003). Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation 108, 2250–2257. doi:10.1161/01.cir.0000093190.53478.78
Ningrum, D. R., Hanif, W., Mardhian, D. F., and Asri, L. A. T. W. (2023). In vitro biocompatibility of hydrogel polyvinyl alcohol/moringa oleifera leaf extract/graphene oxide for wound dressing. Polym. (Basel) 15, 468. doi:10.3390/polym15020468
Patil, T. V., Patel, D. K., Dutta, S. D., Ganguly, K., and Lim, K.-T. (2021). Graphene oxide-based stimuli-responsive platforms for biomedical applications. Molecules 26, 2797. doi:10.3390/molecules26092797
Pei, S., Wei, Q., Huang, K., Cheng, H.-M., and Ren, W. (2018). Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 9, 145. doi:10.1038/s41467-017-02479-z
Perreault, F., de Faria, A. F., Nejati, S., and Elimelech, M. (2015). Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9, 7226–7236. doi:10.1021/acsnano.5b02067
Pham, V. T. H., Truong, V. K., Quinn, M. D. J., Notley, S. M., Guo, Y., Baulin, V. A., et al. (2015). Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 9, 8458–8467. doi:10.1021/acsnano.5b03368
Quan, K., Li, G., Luan, D., Yuan, Q., Tao, L., and Wang, X. (2015). Black hemostatic sponge based on facile prepared cross-linked graphene. Colloids Surfaces B Biointerfaces 132, 27–33. doi:10.1016/j.colsurfb.2015.04.067
Rodrigues, M., Kosaric, N., Bonham, C. A., and Gurtner, G. C. (2019). Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706. doi:10.1152/physrev.00067.2017
Saghazadeh, S., Rinoldi, C., Schot, M., Kashaf, S. S., Sharifi, F., Jalilian, E., et al. (2018). Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 127, 138–166. doi:10.1016/j.addr.2018.04.008
Sathiyaseelan, A., Saravanakumar, K., Mariadoss, A. V. A., and Wang, M.-H. (2021). Antimicrobial and wound healing properties of FeO fabricated chitosan/PVA nanocomposite sponge. Antibiot. (Basel) 10, 524. doi:10.3390/antibiotics10050524
Shariati, A., Hosseini, S. M., Chegini, Z., Seifalian, A., and Arabestani, M. R. (2023). Graphene-based materials for inhibition of wound infection and accelerating wound healing. Biomed. and Pharmacother. 158, 114184. doi:10.1016/j.biopha.2022.114184
Singh, S. K., Singh, M. K., Kulkarni, P. P., Sonkar, V. K., Grácio, J. J. A., and Dash, D. (2012). Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano 6, 2731–2740. doi:10.1021/nn300172t
Skórkowska-Telichowska, K., Czemplik, M., Kulma, A., and Szopa, J. (2013). The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol 68, e117–e126. doi:10.1016/j.jaad.2011.06.028
Song, S., Liu, X., Ding, L., Liu, Z., Abubaker, M. A., Xu, Y., et al. (2024). A bacterial cellulose/polyvinyl alcohol/nitro graphene oxide double layer network hydrogel efficiency antibacterial and promotes wound healing. Int. J. Biol. Macromol. 269, 131957. doi:10.1016/j.ijbiomac.2024.131957
Sun, Z., Hu, K., Wang, T., Chen, X., Meng, N., Peng, X., et al. (2024). Enhanced physiochemical, antibacterial, and hemostatic performance of collagen-quaternized chitosan-graphene oxide sponges for promoting infectious wound healing. Int. J. Biol. Macromol. 266, 131277. doi:10.1016/j.ijbiomac.2024.131277
Taati Moghadam, M., Khoshbayan, A., Chegini, Z., Farahani, I., Shariati, A., and Shariati, A. (2020). Bacteriophages, a new therapeutic solution for inhibiting multidrug-resistant bacteria causing wound infection: lesson from animal models and clinical trials. Drug Des. Devel Ther. 14, 1867–1883. doi:10.2147/dddt.s251171
Thebo, K. H., Qian, X., Zhang, Q., Chen, L., Cheng, H.-M., and Ren, W. (2018). Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 9, 1486. doi:10.1038/s41467-018-03919-0
Torres, J., Liu, Y., So, S., Yi, H., Park, S., Lee, J.-K., et al. (2020). Effects of surface modifications to single and multilayer graphene temperature coefficient of resistance. ACS Appl. Mater Interfaces 12, 48890–48898. doi:10.1021/acsami.0c09621
Unnikrishnan, G., Muthuswamy, S., Kolanthai, E., Megha, M., Thomas, J., Haris, M., et al. (2024). Synthesis and analysis of multifunctional graphene oxide/Ag2O-PVA/chitosan hybrid polymeric composite for wound healing applications. Int. J. Biol. Macromol. 277, 134301. doi:10.1016/j.ijbiomac.2024.134301
Veith, A. P., Henderson, K., Spencer, A., Sligar, A. D., and Baker, A. B. (2019). Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 146, 97–125. doi:10.1016/j.addr.2018.09.010
Wang, P.-H., Huang, B.-S., Horng, H.-C., Yeh, C.-C., and Chen, Y.-J. (2018). Wound healing. J. Chin. Med. Assoc. 81, 94–101. doi:10.1016/j.jcma.2017.11.002
Wang, Y., Liu, S., and Yu, W. (2021). Functionalized graphene oxide-reinforced chitosan hydrogel as biomimetic dressing for wound healing. Macromol. Biosci. 21, e2000432. doi:10.1002/mabi.202000432
Wei, P., Wang, L., Xie, F., and Cai, J. (2022). Strong and tough cellulose–graphene oxide composite hydrogels by multi-modulus components strategy as photothermal antibacterial platform. Chem. Eng. J. 431, 133964. doi:10.1016/j.cej.2021.133964
Wilkinson, H. N., and Hardman, M. J. (2020). Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 10, 200223. doi:10.1098/rsob.200223
Yadav, S., Singh Raman, A. P., Meena, H., Goswami, A. G., Bhawna, null, Kumar, V., et al. (2022). An update on graphene oxide: applications and toxicity. ACS Omega 7, 35387–35445. doi:10.1021/acsomega.2c03171
Yu, Q., Shen, C., Wang, X., Wang, Z., Liu, L., and Zhang, J. (2023). Graphene oxide/gelatin nanofibrous scaffolds loaded with N-acetyl cysteine for promoting wound healing. Int. J. Nanomedicine 18, 563–578. doi:10.2147/IJN.S392782
Zhang, Y., Guan, J., Wu, J., Ding, S., Yang, J., Zhang, J., et al. (2019). N-alkylated chitosan/graphene oxide porous sponge for rapid and effective hemostasis in emergency situations. Carbohydr. Polym. 219, 405–413. doi:10.1016/j.carbpol.2019.05.028
Zhang, M., Wang, D., Ji, N., Lee, S., Wang, G., Zheng, Y., et al. (2021). Bioinspired design of sericin/chitosan/Ag@MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polym. (Basel) 13, 2812. doi:10.3390/polym13162812
Keywords: graphene oxide, wound dressing, antibacterial, hemostasis, angiogenesis
Citation: Li S, Wang J, Zhang H and Zhang X (2025) Advances in graphene oxide-based polymeric wound dressings for wound healing. Front. Mater. 12:1635502. doi: 10.3389/fmats.2025.1635502
Received: 26 May 2025; Accepted: 21 August 2025;
Published: 04 September 2025.
Edited by:
Bolívar Damasceno, State University of Paraíba, BrazilReviewed by:
Abhimanyu Sharma, Intel, United StatesAirlla Cavalcanti, State University of Paraíba, Brazil
Copyright © 2025 Li, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zhang, NjAyMzAwODNAc2R1dGNtLmVkdS5jbg==; Hua Zhang, emhvbmd5aXlhbzc3QDEyNi5jb20=
 Shifeng Li
Shifeng Li Jiao Wang1
Jiao Wang1 Xin Zhang
Xin Zhang