- 1School of Aerospace, Transport and Manufacturing Cranfield University, Cranfield, Bedfordshire, United Kingdom
- 2Departamento de Ingeniería, Universidad Loyola Andalucia, Sevilla, Spain
This study investigates how alumina nanoparticles, gum Arabic, and the surfactant 2-ethyl-1-hexanol influence the surface tension of aqueous lithium bromide (LiBr) solutions. Since surface tension, governed by intermolecular attractive forces at the liquid–solid interface, plays a key role in the performance of heat and mass transfer binary fluids, composed of water and LiBr; understanding these effects is critical for optimizing thermal systems. Lithium bromide–based heat transfer fluids were prepared from 55 wt% aqueous LiBr solutions containing alumina nanoparticles (20 nm), gum Arabic as a dispersion stabilizer, and/or 2-ethyl-1-hexanol as a surfactant. Surface tension measurements were conducted over a temperature range of 293–373 K for different fluid compositions: base LiBr solution, LiBr with nanoparticles, LiBr with gum Arabic, and LiBr with both nanoparticles and gum Arabic, with and without surfactant. The base aqueous LiBr solution exhibited surface tension values from 90.6 mN/m at 293 K to 82.7 mN/m at 373 K. The addition of alumina nanoparticles increased the surface tension by an average of 2.5%, whereas gum Arabic decreased it by approximately 2.1%. The introduction of the surfactant 2-ethyl-1-hexanol, regardless of the presence of nanoparticles or gum Arabic, led to a substantial reduction in surface tension of 32%–35%.
1 Introduction
This work focuses on the role of the addition of alumina nanoparticles functionalized with gum Arabic and 2-ethyl-1-hexanol on the surface tension properties of aqueous lithium bromide is analysed. The advancement of nanofluid technology has acquired significant attention for its potential to enhance heat and mass transfer efficiency in various applications. Controlling interfacial thermodynamics—via nanoparticles, layered structures, or surfactants—is key to advancing high-performance materials and fluids in energy-related technologies (Galindo et al., 2010). This is reflected in the number of works that underscore the critical role of nanofluids in advancing thermal engineering and renewable energy technologies. For instance, recent studies have demonstrated the versatility of nanofluids, such as silver nanoparticle-based solutions, as heat transfer fluids in concentrating solar power applications (De los Santos et al., 2024). A recent comprehensive review also highlights the progress and technological importance of nanofluid production for their practical use as heat transfer fluids (Jahnavi and Hegde, 2024). Also, a recent investigation of nano-lithium bromide solutions has revealed their enhanced heat and mass transfer characteristics, particularly in falling film absorption systems (Wang et al., 2024).
Clearly, the heat and mass transfer properties of heat transfer fluids require not only an understanding of their surface tension, but also the study of the addition of additives to the base fluid as catalysts to improve the heat and mass transfer performance (Lee et al., 2009; Cai et al., 2015). The hypothesis that surfaces exert force can explain several interesting phenomena in liquids, such as capillary filling (Dombek et al., 2018). Surface tension results from intermolecular attractive forces in a liquid–air interface. Pure water, for instance, has a high surface tension due to the strong attractive force between the molecules of water resulting from their polarity and ability to form hydrogen bonds. These molecules attract each other strongly, so it takes much tension to pull them apart. The phenomenon is explained as a balance in forces of adhesion and cohesion, with adhesive force being less than cohesion, hence experiencing an inward pull. On the other hand, adding salt (solute) into water (solvent) increases the surface tension of the corresponding solution. When salt such as Lithium Bromide (LiBr) is dissolved in water, it dissociates into positive (Li+) and negative (Br−) ions. These dissociated ions are hydrated by water molecules, thus generating a much stronger force of attraction that increases the net surface tension at the solution-air interface (Kim and Janule, 1994; Lee et al., 2009; Cai et al., 2015).
The molecules of water at the surface are not surrounded by other water molecules as compared to those in bulk of the water. The net inward pull exerted by the bulk molecules on those at the surface creates a spontaneous contraction with free energy at minimum (Vanoss et al., 1981; Hoorfar et al., 2006; Endrino et al., 2008). The effects of intermolecular attractive force at liquid-solid interface is responsible for surface tension (Ravera et al., 2006). In principle, surface tension depends on three independent factors, namely, the nature of the liquid, the surrounding environment, and temperature. Liquids with high surface tension generally have high intermolecular attractive force. A more complicated situation can be observed when liquid encounters solids (Endrino et al., 2008). For instance, when liquid sample drops on alumina, the liquid droplet, the alumina surface and air clearly do not occupy the same angle (Cai et al., 2015). It is not easily understood how alumina nanoparticles (ANPs) surface can exert a drag force (Gunnasegaran et al., 2012; Harikrishnan et al., 2017; Dombek et al., 2018; Liu et al., 2019). In fact, this can be explained by considering its surface energy. The contact angle between the liquid droplet and ANPs that give wettability provides the minimum available energy of the system. If the liquid angle is larger than 90°, it means that the liquid will not wet the ANPs. If the angle is less than 90°, the liquid will wet the ANPs just as water drops will wet soap. Consequently, there are three boundary surface conditions that can be expressed by these forces. This requires applying the continuum equation of fluid mechanics.
1. At the point where two surfaces meet, the energies of the interfaces determine their contact angle.
2.Secondly, the inner surface (i.e., concave side) of a curved liquid surface has a higher pressure that is given by Equation 1, which is known as Young–Laplace equation.
For example, the higher pressure inside a balloon filled with air exerts a force towards small gap. The elastic/potential energy of the surface is converted to kinetic energy.
3. For such liquid droplet, since it has an inner and outer surface, will have a pressure difference equalling twice the surface tension divided by the radius of the liquid droplet as shown in Equation 2.
Where
Surfactants are surface-active agents, as they are so called in some cases. They could be non-ionic, ampholytic, anionic, or cationic, depending on the nature of the charge the molecule carries (Harikrishnan et al., 2017). However, they have one common feature, which they all exhibit, that is having a water-loving end (hydrophilic group) and a water-hating end (hydrophobic group) from their chemical structure (Bhattarai et al., 2021). It was also established that there exists between a liquid–surfactant interface and the bulk liquid equilibrium.
Surfactants can reduce the surface tension of water or a solution because of their ability to replace or position themselves between molecules of the liquid in which they are dissolved. This makes the force of attraction less than what there could be in pure water molecules. The length of the hydrocarbon chain and branch also plays a significant role in the surface activity of a surfactant.
The use of surfactants in absorption system and heat pump is well known and has been studied extensively to improve their efficiency (Ravera et al., 2006; Lonardi and Luke, 2019). Surfactants such as 2-ethyl-1-hexanol (2E1H)–a branched chain alcohol–and 1-Octanol–a straight chain alcohol–are most widely utilised for aqueous LiBr solution for their significant Marangoni convection improvement due to surface tension gradient (Zhou et al., 2002; Cheng et al., 2003; Cai et al., 2015). When a trace amount of 2E1H is added to aqueous LiBr solution, the surface tension of the solution changes on accounts of concentration and temperature only (Cai et al., 2015; Lonardi and Luke, 2019). Surface tension is affected by an increase or decrease in concentration of surfactant or the aqueous solution as well as temperature rise, which is more critical. Consequently, with high thermal energy, surfactant activity also increases thereby decreasing surface tension. However, the influence of temperature having negative or positive effect is primarily a function of the nature of product and its physical property. Negative effect can be simply avoided by adding excess surfactant or diluting such solutions (Defay et al., 1977; Ravera et al., 2006). Non-ionic surfactants have a unique thermal characteristic when added to water. At some temperatures along the way, they cease to solubilise hence form liquid phase with a number of surfactants (Zhou et al., 2002; Kumar et al., 2023). These phases make the solution cloudy, and the temperature at which they occur is referred to as the phase reversal or cloud point. It is possible to alter the cloud point to a suitable operating temperature by adding sufficient additives. The hydrophobic parent chain of non-ionic surfactants usually contains oxygen-containing hydrophilic groups that are covalently bonded together. The solubility of these groups in water brings about hydrogen bonding. As temperature increases, this strong hydrogen bonding declines with decreasing non-ionic surfactant solubility (Cheng et al., 2003).
Nanoparticles, on the other hand, are also known for boosting heat and mass transfer performance of fluids when dispersed in a base fluid owing to their overwhelmingly higher thermal conductivity (Farade et al., 2021; Farade et al., 2025; Karatas and Bicen, 2022; Sah et al., 2024; Aghayari et al., 2015; Harikrishnan et al., 2017; Dombek et al., 2018). They are also said to enhance interfacial convection by reducing the surface tension of the base fluid (Zhu et al., 2010). This suggests that the introduction of some amount of nanoparticles into a base fluid augments the interfacial convection of the fluid by a certain degree. The concentration or volume of nanoparticles dispersed in a base fluid, e.g., the nature of chemical synthesis of a nanofluid, determines the degree of Marangoni convection in the nanofluid flow (Milanova and Kumar, 2008; Karimzadehkhouei et al., 2017). Small amounts of gum Arabic (GA)–a stabilising agent–can be introduced in nanofluid to keep nanoparticles in suspension (Zeng et al., 2006; Aghayari et al., 2015), to prevent settling down. Otherwise, it causes agglomeration due to the high-density nature of the particles. It is reported (Gunnasegaran et al., 2012) that this may reduce surface tension when it forms a solution with nanofluid.
Wen et al. (1991) conducted experimental studies for different concentrations of aqueous LiBr solution with and without 2E1H at different ranges of temperatures. There were some disagreements between the surface tension data reported in literature 25 due to varying methodology and procedure. LiBr was not studied for its surface tension behaviour with and without additives as well as nanoparticles simultaneously across a range of temperatures. In this article, a series of experiments were conducted to investigate the thermal dependence of surface tension in detail for LiBr with and without additives and nanoparticles between temperature ranges of 293 K and 373 K. Particularly, the effect of surface tension on heat and mass transfer rates, is studied by analysing the through measurements of absorption rates and heat transfer coefficients of the synthesized thermofluids.
2 Experimental details
2.1 Materials
In this experimental work, high-purity chemical substances from Sigma-Aldrich, United Kingdom, were purchased to prepare various types of nanofluid samples. The chemical substances include 2-ethyl-1-hexanol 99.60%, gum Arabic from the acacia tree, anhydrous lithium bromide solution 99.9%, ultrapure water, and aluminium oxide nanoparticles 99%, 20 nm particle size (from US Research Nanomaterials, Inc., United States). Instruments used include Mettler balance AE-240, which has a readability of 0.02 mg up to 205 g; Du-Noüy ring model DIN 53915 and ASTM-971; Automatic Surface Tensiometer model DCAT 11 EC, from Data Physics Instruments GmbH, Germany.
2.2 Methods
Different methodology for surface tension measurements reported in the literature includes bubble pressure or maximum bubble pressure, which utilises a surfactant to determine the dynamic surface tension of the liquid (Kim and Janule, 1994). The drop volume or drop weight method is a simple and accurate technique proposed in 1977, which relies on the weight of fluid dropped into a bulk liquid via a capillary tube (Defay et al., 1977; Yildirim et al., 2005). Du Noüy ring, a traditional method, has limitations as direct scale reading could present wrong figures due to ring position and pressure difference between the upper and lower levels (Kim and Janule, 1994). Pendant drop, tensiometer (Krüss, Germany) and Wilhelmy plate for measuring dynamic and static surface tension (Cheng et al., 2003) are other methods to use. A modern methodology for measuring surface tension of nanofluids has been established by Bhuiyan et al. (2015). The method utilised a traditional Du-Noüy ring technique integrated with an automatic surface tensiometer (DCAT 11 EC, Data Physics Instruments GmbH, Germany) (Bhuiyan et al., 2015). In this work, the instruments and methodology used by Bhuiyan et al. (2015) were adopted and are comprehensively explained in detail by the authors. The device has a high-performance electro-dynamic compensation balance of 0.1 mg–210 g ± 0.01 mg, which can read measurements between 1 mN m−1 to 1,000 mN m−1 ± 0.001 mN m−1. It is integrated with a digital thermometer that has a temperature control unit that allows surface tension value measurements at constant temperatures. Every sample was measured five times under atmospheric pressure, and an average of the readings was taken.
2.3 Procedure for LiBr-ANF preparation with additives
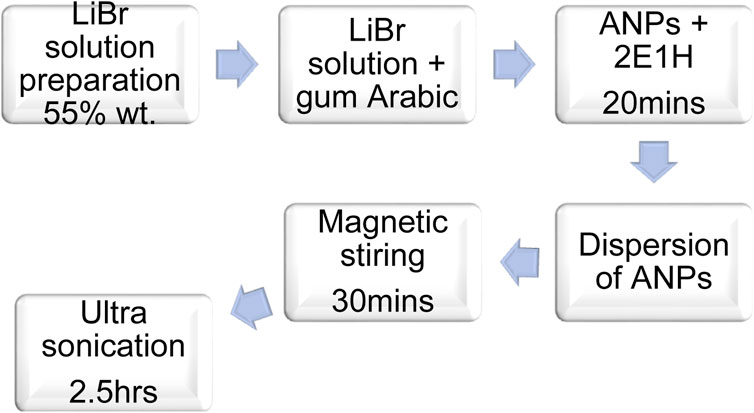
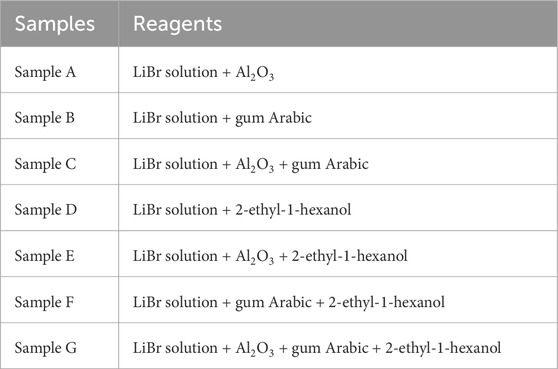
Generally, the nanofluid samples were prepared using the two-step method, but through the particle surface functionalization method developed by Muhammad et al. (2021a) and Muhammad et al. (2021b). The procedure involves functionalization of the nanoparticles with the surfactant, 2E1H and preparing the base fluid with a dispersion stabilizing agent, gum Arabic. Figure 1 outlines the complete procedure. The list of samples prepared for this study is shown in Table 1.
2.4 Procedure for surface tension determination
During the experimental study of lithium bromide-alumina nanofluid (LiBr-ANF) surface tension measurements, various samples were prepared for the test. LiBr was prepared as a base fluid for the nanofluid. Seven different types of samples were synthesized from this initial stock solution of 55 wt% LiBr. Three samples were prepared by adding GA, 2E1H, and GA with 2E1H, respectively. The second set of samples was those of LiBr with alumina nanoparticles (ANPs) in solution. Besides the pure LiBr-ANF, three other samples were also prepared by adding GA, 2E1H and GA with 2E1H. The third set of samples was those used for baseline studies, and they include GA solution, ANF (water as base fluid), and water–2E1H mixture. The concentration of ANPs (20 nm particle size) used was 0.01% by weight, while 2E1H and GA were 150 ppm and 0.01% by weight, respectively. These concentrations are considered best for the nanofluid stability (Schonhorn, 1965; Lipatov and Feinerman, 1979). Each sample was studied at various temperatures from 293 K to 373 K with a 5K interval between each reading. This was carried out with the equipment set up that has an integrated thermometer, allowing for a finite step in the study of surface tension at a constant and specified temperature required. Ultrapure water and pure 2E1H were used as standards for testing equipment accuracy and compared with those in the literature. Finally, each reading was repeated 5 times so that closely repeatable values are averaged and recorded. To simplify the sample labelling, each sample was assigned a name as listed below.
3 Results and analysis
The experimental results and analysis include those of standard samples such as water, 2E1H, and aqueous LiBr solution, which are available from the literature to ascertain the accuracy of equipment and techniques. In addition, the effects of ANPs and the heat and mass transfer additives–GA and 2E1H–on the surface tension of aqueous LiBr solution were investigated.
3.1 Surface tension of standard samples
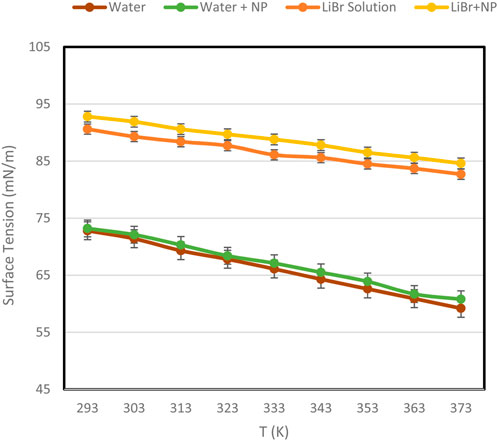
The measurements that were conducted on various samples at different temperatures were focused on system accuracy and deviation of the results and data as obtained from the literature, if any. The results were plotted as shown in Figure 2a, b for 2E1H and ultrapure water, respectively, and compared with data from the literature.
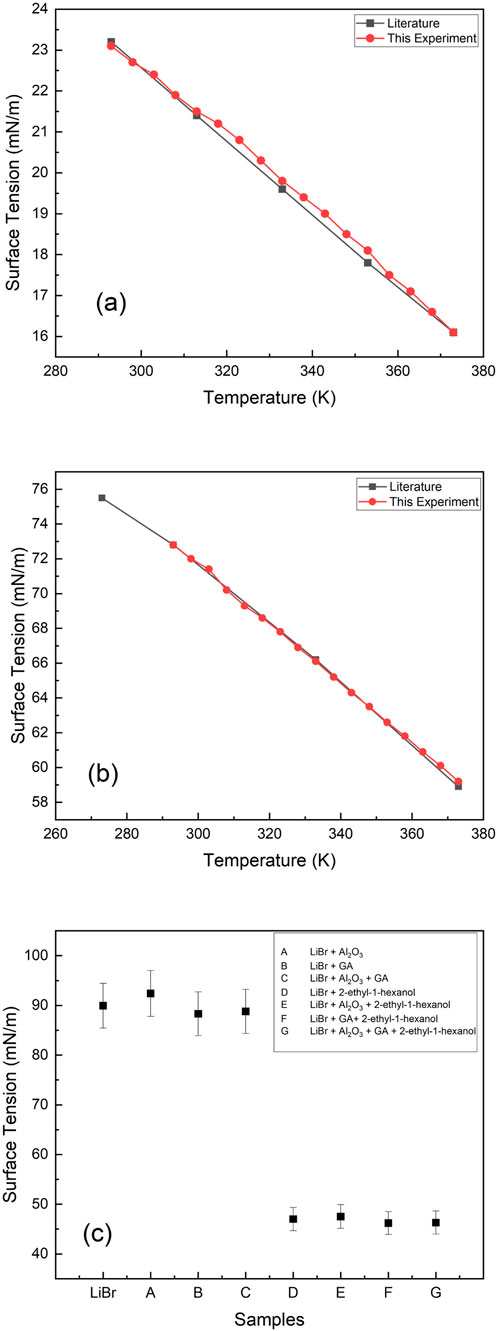
Figure 2. (a,b) Surface tension of 2-ethyl-hexanol and H2O, (c) Surface tension of LiBr-ANF samples at 298 K. All measurements were carried out by Du Noüy ring method with an automatic surface tensiometer.
As it is well known, water has high surface tension, which is higher than many liquids, as the intermolecular attractive force between water molecules results from hydrogen bonding. This type of bonding has a high amount of energy. Consequently, water molecules attract each other so strongly that it needs high tension to pull them apart. The surface tension values of water obtained in this work, Figure 2b, were in conformity with those presented previously (Wen et al., 1991; Gunnasegaran et al., 2012). However, in the case of the alcohol (2E1H) sample, surface tension values in Figure 2a did not completely coincide between 333 K and 353 K when compared to those presented by BASF Petronas chemicals (Wiley, 2002). The maximum standard deviation between this work and literature at these temperatures is+/-0.21. This could be a slight change in the percentage purity of the alcohol. Although in a general context, the standard samples were in agreement with those presented from the literature, which is an indication that the methodology adopted is appropriate for the study.
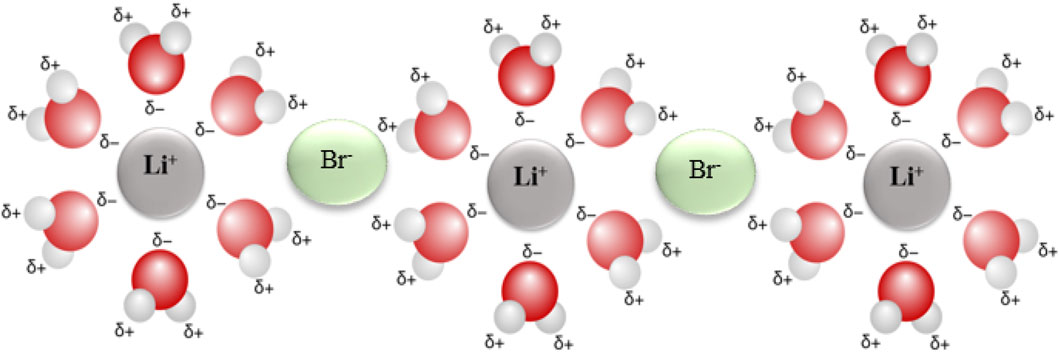
As an ionic salt, LiBr, it dissociates to produce charged (Li+ and Br-) ions when dissolved in water. These ions are hydrated and develop a stronger intermolecular attraction within the solution. Each Li+ ion is surrounded by several oxygen ends of water molecules, while each Br−ion is surrounded by hydrogen ends of the molecule, as shown in Figure 3.
This increases the net intermolecular attractive force between the charged hydrated ions and thus increases the surface tension of the solution. If the concentration of LiBr is increased, the surface tension increases further with an increased attractive force. This implies that interparticulate attraction has increased, and the hydrated charged ions experience a high-tension inward pull towards the bulk solution. The data obtained from the experiments showed higher surface tension in LiBr than in ultrapure water. The data agree with those presented by Cheng et al. (2003) at 296K (90.2 mN/m).
3.2 Effect of alumina nanoparticles
The chemistry of particle adsorption kinetics at the aqueous solution-air interface is a complicated scenario, which depends on several physical properties of both the particle itself and the base fluid such as particle size, wettability, base fluid nature and interfacial shape (Ravera et al., 2006; Zeng et al., 2006). In such a complex binary nanofluid, the morphology of the alumina nanoparticles and the area-to-volume ratio available for effective collision and interaction with the LiBr solution are important (Zhu et al., 2010).
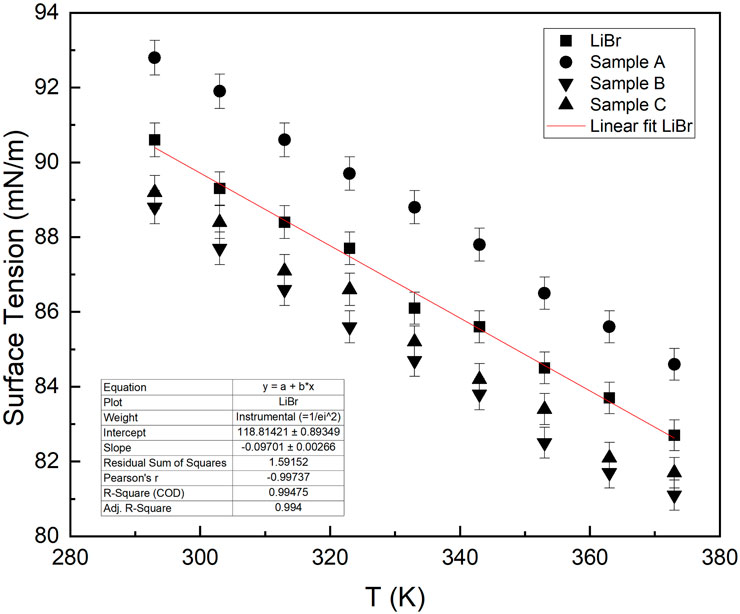
In sample A, when alumina nanoparticles were dispersed in LiBr solution and ultrasonicated, the surface tension of the solution rose as plotted in Figure 4 for the temperature range of 293oK to 373oK. When nanoparticles are dispersed into the base fluid, those particles around the solution surface get closer together because nanofluids have a tendency for agglomeration due to a strong cohesive force of attraction at the solution-air interface, resulting in an increase in surface tension.
Surface tension generally decreases with increasing temperature due to a decrease in cohesive forces as molecular thermal activity increases. The adhesive action/force present within the liquid molecules at the liquid-air interface is due to the environmental influence surrounding the system. Nanoparticles dispersed into a base fluid can also enhance interfacial convection (Zhou et al., 2002); hence, it is expected that addition of ANPs into base fluids such as water reduces the surface tension as suggested by Zhou et al. (2002). Although, an increase in surface tension due to dispersion of nanoparticle are not remarkable because of the build-up of charged ANPs at nanofluid–air interface. The distribution of these charged ANPs was promoted by their fine particle size. The surface tension of the nanofluid with respect to temperature follows the path of the base fluid as shown in Figure 4.
From the data comparison presented in Figure 4, the surface tension of the nanofluids for both H2O-ANF and LiBr-ANF follows a similar trend with change in temperature as that of their base fluids. This is because the addition of ANPs does not affect the chemical properties of their base fluid. However, the relative increase in surface tension by the addition of the ANPs in both cases signifies the role it plays in changing the physical chemistry of the base fluids. This means that nanoparticles can also play a significant role in changing the thermo-physical chemistry, such as thermal conductivity of their base fluid.
3.3 Effect of gum Arabic
When GA was added to LiBr solution in sample B, the surface tension of the solution slightly decreases from that of pure LiBr solution and continues to show a similar trend with increasing temperature as in Figure 5. The decrease in value is a result of the formation of a depletion layer at the interface. The surface tension can be further decreased by adding a higher concentration of GA in the final fluid. Although that is not advisable as it will increase the viscosity of the fluid, which is not desirable in this case. When a portion of high molecular weight hydroxyproline-rich glycoprotein, which is a major constituent of GA, was added to the densely hydrated charged ions (of Li+ and Br−) of LiBr solution, the hydrophobic chain of the hydroxyproline is adsorbed at the interface. On the other hand, the hydroxyl groups (e.g., hydrophilic ends) are dissolved in the bulk solution. Therefore, sample “B” gave rise to a slight reduction in surface tension, which is not more than 2%, resulting from the depletion layer of solute at the interface as depicted in Figure 5. In this case, the concentration of GA was only 0.01 wt%, not high enough to make any significant reduction in the surface tension.
In case of sample C, nanoparticles were initially stabilised, which allowed each molecule to be separated and in suspension in fluid with particle distribution increasing from 20 to 23 particles per millilitre. By the incorporation of GA to sample A, the Van der Waals force of attraction reduces between the molecules, whereas electrosteric repulsive force comes into play and becomes a dominant mechanism. Hence, this reduces the interfacial tension as shown in Figure 5, sample C.
3.4 Effects of 2E1H and combine effect of 2E1H-ANPs and 2E1H-GA in LiBr solution
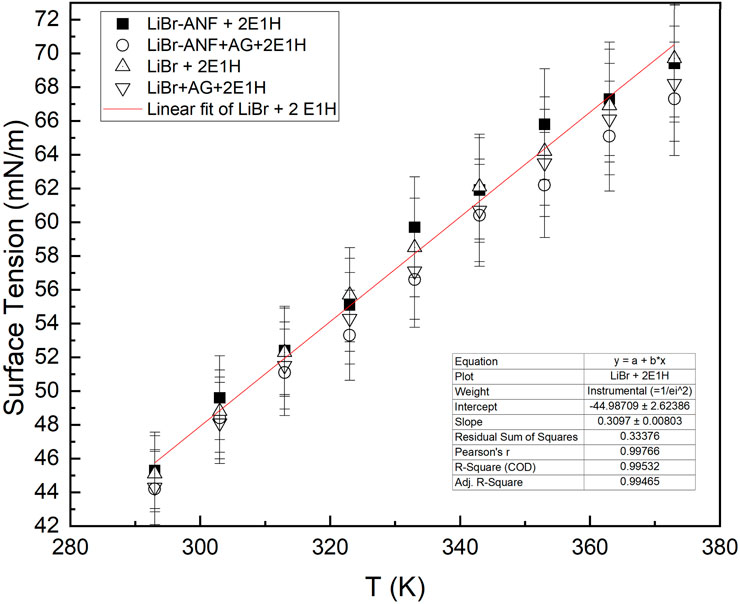
This study is focused on the complex chemistry of the physical interaction among the different interphases while taking into account various combinations of nanoparticles and surfactant, as well as of nanoparticles, GA, and surfactant in aqueous salt solution. Unlike the usual trend of surface tension as discussed in the previous section, the addition of surfactant to the various samples here presents rather an unusual trend, which was also observed by Wen et al. [6]. Wen’s group conducted surface tension analysis for 50 wt% and 60 wt% aqueous LiBr solution using 2E1H and 1-Octanol at various concentrations, and between a temperature range of 298 oK and 323 oK. The surface tension had shown an increase with temperature after an initial significant reduction in values. When surfactants are dissolved in a base fluid, the characteristics of the molecules are determined by a phenomenon referred to as Gibbs adsorption. At the liquid-air interface, this forms a monolayer, resulting in a decrease in interfacial tension (Harikrishnan et al., 2017).
In this trial run, the addition of surfactant in samples D, E, F, and G showed a different trend across the range of temperature from that of samples A, B, C, and pure LiBr solution, which decreases with temperature as shown in Figure 5. The surface tension of nanofluids increasing gradually with temperature as depicted in Figure 6. The results had revealed a significant decrease in surface tension compared to the original aqueous samples prior to addition. There are several reasons for this considerable decrease in surface tension. One of the most significant reasons is associated with the fact that the surfactant can diffuse and be adsorbed at the solution-air interface. While the hydrophilic head of the molecule is dissolved in the solution, the hydrophobic tail is settled at the surface. Hence, this reduces hydrogen-bonding strength to lower surface tension. The gradual rise in surface tension with temperature is due to a decrease in solubility of the surfactant itself. In addition, both surfactant influence on surface tension and surface tension of liquids are temperature dependent. Consequently, with high thermal energy, surfactant activity also increases, thereby decreasing surface tension. However, the influence of temperature having a negative or positive effect on the usual trend of surface tension is primarily a function of the nature of the base fluid and constituents forming the final product. Negative effects can be prevented by adding excess surfactant or diluting such solutions.
In all cases, reduction in value of surface tension is linear but not always uniform across the temperature range. As mentioned earlier, an increase in temperature results in a decrease of surfactant solubility, thereby reducing the impact it has on the intermolecular force of attraction on the interface. In effect, the molecules of the surfactant form more stable molecules of micelles depending on the solubility, which is the major control parameter. Since the solution is ionic in nature, this forms even larger ionic micelles and ultimately lowers the degree of electrostatic interaction and the steric stabilisation among the molecules in the solution.
Another possible explanation for the complex physical mechanisms of nanofluid behaviour can be given on the basis of the adsorption kinetics of the complex binary nanofluid. When the surfactant is added to the solution, it is partially adsorbed at the solution-air interface as postulated by the Gibbs adsorption isotherm (Harikrishnan et al., 2017). This results in a significant decrease in interfacial tension. Temperature is one of the key factors that determines the amount of surfactant surface excess concentration in solution. As the temperature increases, the surface excess concentration decreases, thereby slightly raising the surface tension of the solution, as evident from Figure 6.
When ANPs were added to the solution part of the surfactant, they get adsorbed on a second interface, e.g., the solid-liquid interface. This promotes the stability of the nanofluid. In the combined effect scenario of three constituents, a third layer is incorporated as the surfactant is added in sample G, containing both ANPs and a stabilising agent, gum arabic. GA stabilises ANPs in suspension by forming a protein and sugar polymer layer on them, and the surfactant between the GA and the bulk solution. At the solution-air interface, GA forms a layer between the surfactant and the solution, with surfactant adsorbing at the solution-air interface as illustrated in Figure 7.
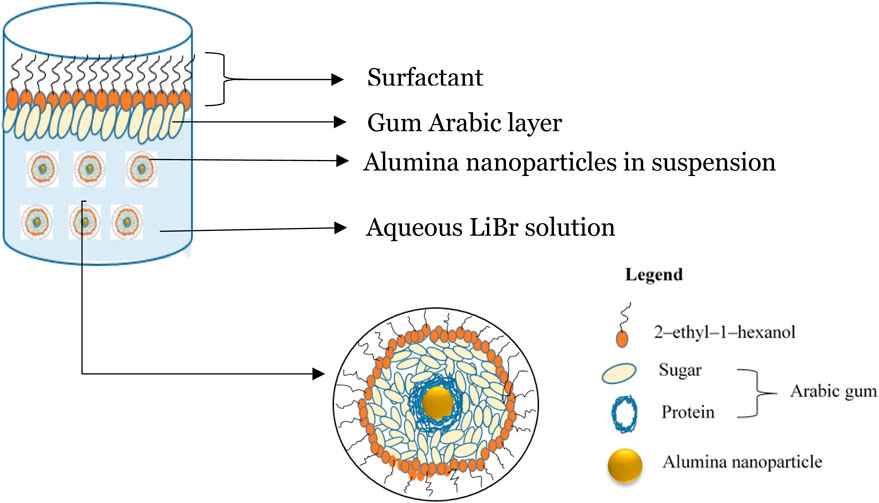
Figure 7. Schematic of combined effect of sample and additives layer formation and their interaction. Aqueous LiBr solution. Alumina nanoparticles in suspension. Gum Arabic layer. Surfactant layer.
As depicted schematically in Figure 7, the final nanofluid sample, subjected to the combined effect, has the lowest possible surface tension across the temperature range of operation. As explained, the addition of GA, a stabilising agent, provides a degree of stability in the nanofluid by enhancing the dispersion of the ANPs throughout the bulk solution. This reduces the Van der Waals forces of attraction between the molecules, imposing electrosteric repulsive interaction, thus preventing the charge build-up effect of ANPs at the nanofluid-air interface. Consequently, along with GA playing the crucial role on the ANPs, the 2E1H is adsorbed at both particle surfaces (and liquid-air interface) to enable this so-called triple effect surface tension reduction phenomenon. In addition, the particle surface area also serves as a carrier for surfactant molecules within the bulk solution.
3.5 Enhancement analysis
The percentage decrease in surface tension of samples presented in Figure 8 shows an increase in surface tension by 2.8%–displaying negative on the % decrease scale–when ANPs were added to LiBr solution (sample A) as discussed earlier. A decrease of 1.8% and 1.3% with the addition of GA to LiBr solution (sample B) and LiBr-ANF (sample C) was recorded at 298K, respectively. These percentages were obtained from surface tension values presented in Figure 2c with respect to aqueous LiBr solution. The addition of surfactant to the samples revealed an excellent technique in controlling surface tension and its degree of reduction. In samples D, E, F, and G, a reduction in surface tension of 47.7%, 47.2%, 48.6%, and 48.5% was achieved with the addition of the surfactant, respectively, at 298 K. The comparison in Figure 8 shows that, irrespective of the addition of ANPs and GA in the solution, the 2E1H addition has a tremendous effect on the surface tension. These percentages of decrease in surface tension shown in Figure 8 were obtained from surface tension values presented in Figure 4 with respect to aqueous LiBr solution. Figure 8 also present percentages of surface tension decrease obtained as a result of addition of additives and nanoparticles plus additives respectively but at different temperatures.
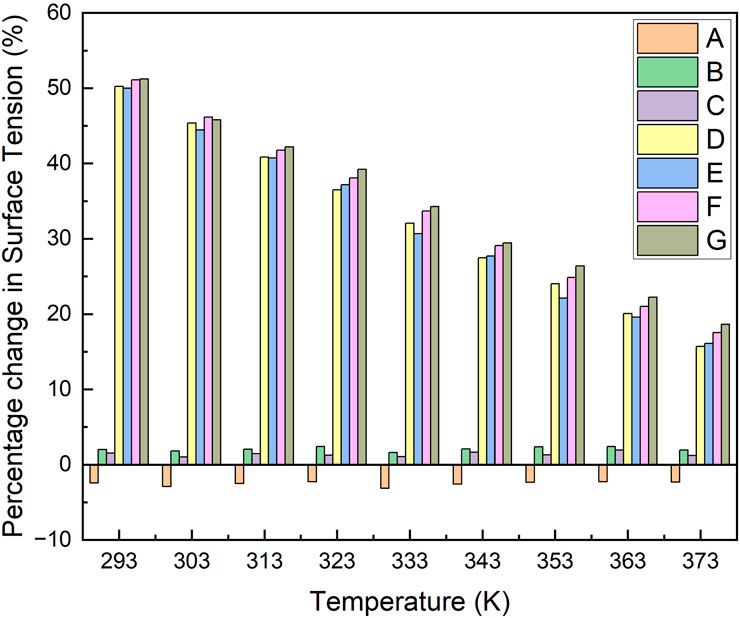
Figure 8. Percentage decrease in surface tension of aqueous LiBr solution due to the addition of ANPs and additives at different temperatures. Additives in LiBr samples: A: Al2O3, B: Gum Arabic, C: Al2O3 and Gum Arabic, D: 2-ethyl-1-hexanol, E: Al2O3 and 2-ethyl-1-hexanol, F: Gum Arabic and 2-ethyl-1-hexanol, G: Al2O3, Gum Arabic and 2-ethyl-1-hexanol.
4 Effects of surface tension on heat and mass transfer
The heat and mass transfer rates of some samples were previously analysed experimentally by Muhammad et al. (2021b) in the ejector adiabatic absorber and Lee et al. 5 in the falling film absorber. The results reported for the ejector-boosted absorption system are presented in Figure 9. In addition, Equations 4–9 were used to determine the absorption, heat, and mass transfer rates from experimental data as reported by Lee et al. (2009).
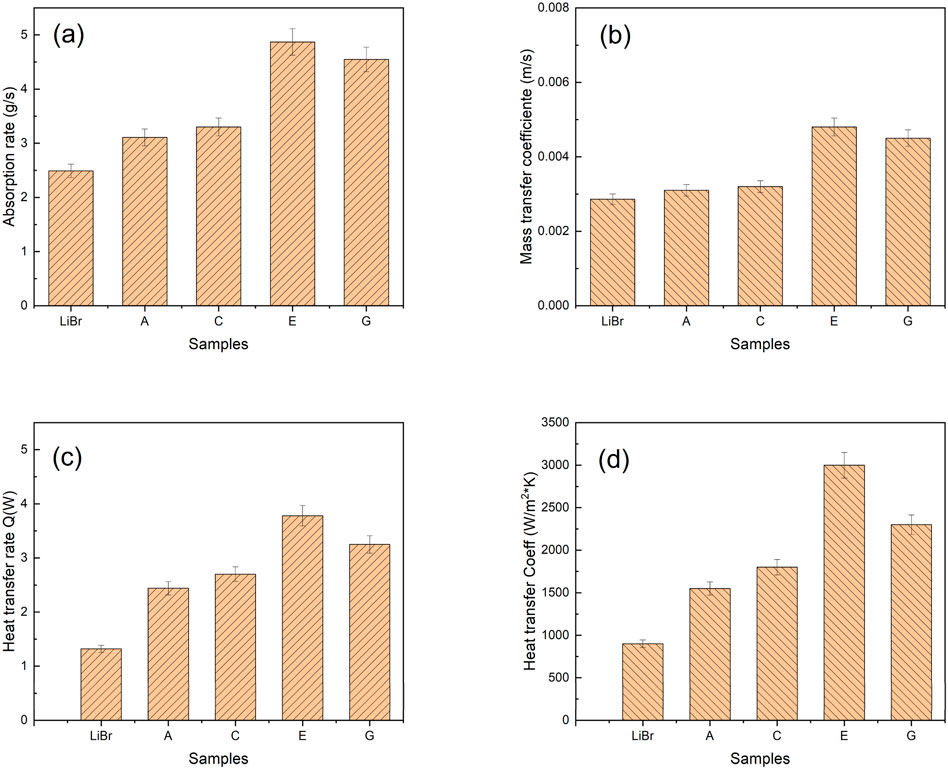
Figure 9. Bar graphs showing comparative mass and heat transfer results at a flow rate of 0.085 kg/s (a) Absorption rate, (b) mass transfer coefficient, (c) heat transfer rate, and (d) heat transfer coefficient. Additives in LiBr samples: A: Al2O3, C: Al2O3 and Gum Arabic, E: Al2O3 and 2-ethyl-1-hexanol, G: Al2O3, Gum Arabic and 2-ethyl-1-hexanol.
Heat transfer equations
Where
And,
This equation can be reframed since, in this case, there is no coolant.
So, for the mass transfer equation we have
Also,
All symbols are defined in the nomenclature section.
Absorption rate and mass transfer coefficient computed from Equation 9 are shown in Figure 9. The addition of heat transfer additives resulted in a threefold increase in heat transfer rate and coefficient in the sample that contained Al2O3and 2-ethyl-1-hexanol.
5 Thermal conductivity analysis of the thermofluid (LiBr-ANF)
Figure 10 show the trend of thermal conductivity and effective thermal conductivity of LiBr-ANF samples at 298 K. The dispersion of ANPs into LiBr solution indicates an enhancement of 8.2% at 298 K, as shown in Figure 10c. When only the 2E1H was introduced into the nanofluid, the enhancement was up to 14.8%. However, this enhancement had slightly diminished to 14.3% with the addition of GA to the nanofluid containing 2E1H. The enhancement when only GA was added to the nanofluid was recorded as 13.5%. GA builds a very thin layer of protein, a constituent of the GA, around the nanoparticles that has an adverse effect, causing a slight reduction in thermal conductivity of the fluid. The thermal conductivity enhancement comparison, as presented in Figure 10c, is quite remarkable. This is because even as thermal conductivity decreases with the introduction of GA into the sample containing surfactant (sample E); the value is still higher than that without the 2E1H (sample C). Furthermore, the sample containing only GA (sample C) also showed an enhancement in value compared to the pure LiBr-ANF (sample B). These two scenarios clearly established that GA performs as a thermal conductivity enhancement additive owing to its nanoparticle dispersion stabilising property.
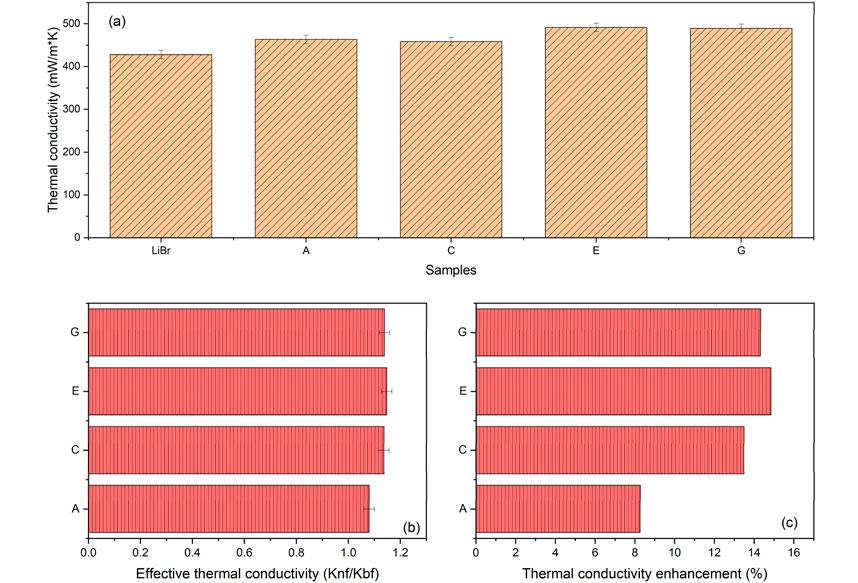
Figure 10. (a,b) Thermal conductivity and thermal conductivity enhancement of LiBr-ANF samples at 298K. Effective thermal conductivity of LiBr-ANF samples at 298K. (c) Thermal conductivity enhancement of aqueous LiBr solution due to adding nanoparticles and additives. A: Al2O3, C: Al2O3 and Gum Arabic, E: Al2O3 and 2-ethyl-1-hexanol, G: Al2O3, Gum Arabic and 2-ethyl-1-hexanol.
Despite the stability provided by the GA, the addition of surfactant is required to improve the thermal conductivity of the nanofluid. This can be explained by the chemical nature of the surfactant, which enables it to modify the surface chemistry of the nanoparticles and their interaction with the base fluid.
6 Dynamic viscosity of the thermofluid (LiBr-ANF)
In Figure 11a, the dynamic viscosity recorded is measured in millipascal-second at 298K, while the percentage enhancement in viscosity as a result of the addition of additives was plotted in Figure 11b for the same temperature. The ultra-sonication of LiBr solution with ANPs resulted to a slight increase in viscosity of the corresponding nanofluid with less than 1%. The introduction of GA into the LiBr-ANF resulted in an increase in viscosity. This is due to its emulsifying property and size transformation of nanoparticles it has caused in the final fluid mixture as depicted earlier in Figure 7. This has resulted in a 4% increase in the viscosity of the final solution. On the other hand, the surfactant has more effect at the interface as explained previously, than on the particles in terms of viscosity. When the surfactant adsorbs at nanofluid-air interface, it increases the shear stress of the nanofluid at the surface and in flow channels, consequently increasing the viscosity by 3.4%. Furthermore, the combined effect of the 2E1H and GA has shown a larger increase in viscosity by up to 9%.
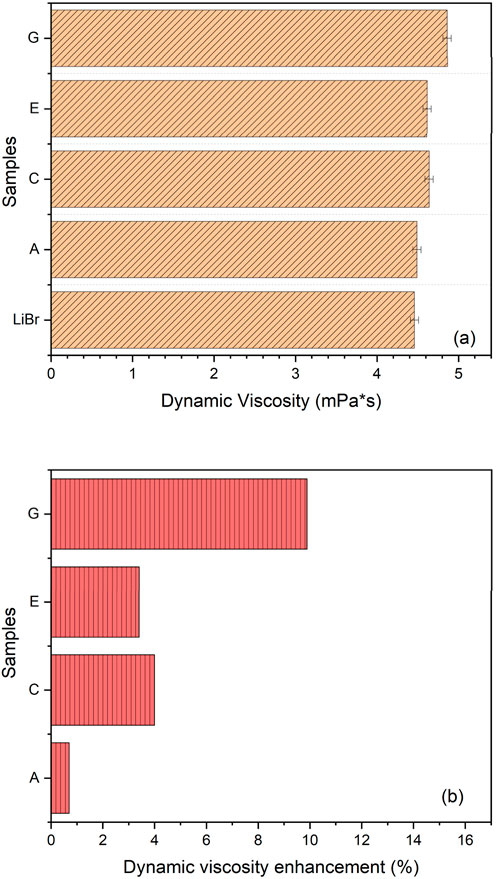
Figure 11. (a) Dynamic viscosity of LiBr-ANF samples (mPa*s) at 298K. (b) Dynamic viscosity enhancement (%). Additives in LiBr samples: A: Al2O3, C: Al2O3 and Gum Arabic, E: Al2O3 and 2-ethyl-1-hexanol, G: Al2O3, Gum Arabic and 2-ethyl-1-hexanol.
7 Conclusion
The analysis of interfacial and surface tension effects in LiBr–ANF demonstrates the crucial role of additives in enhancing heat and mass transfer by controlling surface energy and fluid stability. Dispersing alumina nanoparticles increased surface tension by 2.8% at 298 K, a result of particle agglomeration and strong cohesive forces at the liquid–air interface. In contrast, the triple-additive nanofluid containing ANPs + gum Arabic (GA) + 2-ethyl-1-hexanol (2E1H) (sample G) achieved the lowest surface tension across all tested temperatures, driven by multi-layer adsorption and electrosteric stabilization.
Heat transfer performance was highest in the double-effect fluid (ANPs + 2E1H) rather than the triple-effect sample, as the GA layer surrounding the nanoparticles slightly hindered thermal transport. While GA effectively stabilizes nanoparticles in suspension, its interactions remain complex. Brownian motion is slowed by the increase in dynamic viscosity and changes in particle shape and size induced by GA.
Mass transfer, on the other hand, was greatest in the triple-effect sample because its very low surface tension promotes the Gibbs–Marangoni effect, which enhances interfacial transport regardless of particle motion. Thermal conductivity measurements at 298 K confirmed this hierarchy of enhancement: 8.2% (ANPs only), 13.5% (GA only), 14.8% (2E1H only), and 14.3% (ANPs + GA + 2E1H), showing that the surfactant provides the strongest individual contribution. The resulting surface tension gradients directly increase the absorption rate of the final system.
For practical applications, GA remains essential for long-term nanofluid stability, but its concentration should be kept as low as possible relative to the nanoparticle content to minimize viscosity growth and heat transfer penalties. Overall, this study highlights the fundamental influence of surface tension on the thermal and mass transport properties of LiBr-based nanofluids, providing valuable guidance for designing high-efficiency heat transfer fluids in industrial systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
UM: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. JE: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. JE gratefully acknowledges funding from the Spanish Ministry of Science and Agencia Estatal de Investigación (Projects PID2021-128727OB-I00 and TED2021-132752B-I00).
Acknowledgments
The authors gratefully acknowledge D. Bhattachayya (CU) for his assistance in experimental measurements.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghayari, R., Maddah, H., Ashori, F., Hakiminejad, A., and Aghili, M. (2015). Effect of nanoparticles on heat transfer in mini double-pipe heat exchangers in turbulent flow. Heat And Mass Transf. 51, 301–306. doi:10.1007/s00231-014-1415-0
Bhattarai, A., Rub, M. A., Jaffari, Z. H., Saha, B., Thu, H. T., Alghamdi, Y. G., et al. (2021). Spectroscopic and conductometric analyses of ninhydrin and threonine reaction in double-headed geminis. Ind. Eng. Chem. Res. 60, 14977–14984. doi:10.1021/acs.iecr.1c03184
Bhuiyan, M., Saidur, R., Mostafizur, R., Mahbubul, I., and Amalina, M. (2015). Experimental investigation on surface tension of metal oxide-water nanofluids. Int. Commun. Heat And Mass Transf. 65, 82–88. doi:10.1016/j.icheatmasstransfer.2015.01.002
Cai, W., Kong, W., Wang, Y., Zhu, M., and Wang, X. (2015). Surface tension of lithium bromide aqueous solution/ammonia with additives and nano-particles. J. Of Central South Univ. 22, 1979–1985. doi:10.1007/s11771-015-2718-0
Cheng, W., Chen, Z., Akisawa, A., Hu, P., and Kashiwagi, T. (2003). Theoretical and experimental study on surface tension and dynamic surface tension of aqueous lithium bromide and water with additive. Sci. China Ser. E-Technological Sci. 46, 191–203. doi:10.1360/03ye9021
De los Santos, D., Gallardo, J. J., Carrillo-Berdugo, I., Alcantara, R., and Navas, J. (2024). Efficient nanofluids based on Ag nanoparticles and a linear silicone-based fluid for concentrating solar power. J. Of Mol. Liq. 413, 125898. doi:10.1016/j.molliq.2024.125898
Defay, R., Prigogine, I., and Sanfeld, A. (1977). Surface thermodynamics. J. Colloid Interface Sci. 58, 498–510. doi:10.1016/0021-9797(77)90159-X
Dombek, G., Nadolny, Z., and Marcinkowska, A. (2018). Effects of nanoparticles materials on heat transfer in electro-insulating liquids. Appl. Sciences-Basel 8, 2538. doi:10.3390/app8122538
Endrino, J., Marco, J., Allen, M., Poolcharuansin, P., Phani, A., Albella, J., et al. (2008). Functionalization of hydrogen-free diamond-like carbon films using open-air dielectric barrier discharge atmospheric plasma treatments. Appl. Surf. Sci. 254, 5323–5328. doi:10.1016/j.apsusc.2008.02.065
Farade, R. A., Wahab, N. I. A., Mansour, D.-E. A., Azis, N. B., Jasni, J.Bt., Veerasamy, V., et al. (2021). The effect of interfacial zone due to nanoparticle–surfactant interaction on dielectric properties of vegetable oil based nanofluids. IEEE Access 9, 107033–107045. doi:10.1109/ACCESS.2021.3098758
Farade, R. A., Mansour, D.-E. A., Wahab, N. I. A., Ariffin, M. K. A., Emara, R. F., Emara, M. M., et al. (2025). Deeper insight into thermal conductivity, size, sedimentation, band gap, and moisture effect of nanoparticles on thermo-dielectric performance of nanofluids. J. Mol. Liq. 434, 127986. doi:10.1016/j.molliq.2025.127986
Galindo, R. E., Endrino, J. L., Martínez, R., and Albella, J. M. (2010). Improving the oxidation resistance of AlCrN coatings by tailoring chromium out-diffusion. Spectrochim. Acta Part B At. Spectrosc. 65, 950–958. doi:10.1016/j.sab.2010.09.005
Gunnasegaran, P., Shuaib, N., Jalal, M., and Sandhita, E. (2012). “Application of nanofluids in heat transfer enhancement of compact heat exchanger,” in Universiti tenaga nasional. Editors K. Noorsal, and A. Masrom, 408–425. doi:10.1063/1.4769160
Harikrishnan, A., Dhar, P., Agnihotri, P., Gedupudi, S., and Das, S. (2017). Effects of interplay of nanoparticles, surfactants and base fluid on the surface tension of nanocolloids. Eur. Phys. J. E 40, 53. doi:10.1140/epje/i2017-11541-5
Hoorfar, M., Kurz, M., Policova, Z., Hair, M., and Neumann, A. (2006). Do polysaccharides such as dextran and their monomers really increase the surface tension of water? Langmuir 22, 52–56. doi:10.1021/la0512805
Jahnavi, K. R., and Hegde, G. S. (2024). A review on nano fluid production, mathematical modelling and applications. J. Of Nanofluids 13, 269–305. doi:10.1166/jon.2024.2138
Karatas, M., and Bicen, Y. (2022). Nanoparticles for next-generation transformer insulating fluids: a review. Renew. Sustain. Energy Rev. 167, 112645. doi:10.1016/j.rser.2022.112645
Karimzadehkhouei, M., Shojaeian, M., Sendur, K., Mengüç, M., and Kosar, A. (2017). The effect of nanoparticle type and nanoparticle mass fraction on heat transfer enhancement in pool boiling. Int. J. Of Heat And Mass Transf. 109, 157–166. doi:10.1016/j.ijheatmasstransfer.2017.01.116
Kim, K., and Janule, V. (1994). Dynamic surface-tension of aqueous lithium bromide with 2-Ethyl-1-Hexanol. Int. Commun. Heat And Mass Transf. 21, 839–848. doi:10.1016/0735-1933(94)90037-X
Kumar, D., Khan, J. M., Posa, M., Pulikkal, A. K., Saha, B., and Bhattarai, A. (2023). Effect of Quaternary ammonium gemini surfactant solution on the rate constant of the ninhydrin–lysine reaction. Ind. Eng. Chem. Res. 62, 15897–15906. doi:10.1021/acs.iecr.3c02499
Lee, J., Kim, H., Kim, M., Koo, J., and Kang, Y. (2009). The effect of additives and nanoparticles on falling film absorption performance of binary nanofluids (H2O/LiBr + nanoparticles). J. Of Nanosci. And Nanotechnol. 9, 7456–7460. doi:10.1166/jnn.2009.1790
Lipatov, Y., and Feinerman, A. (1979). Surface-tension and surface free-energy of polymers. Adv. Colloid And Interface Sci. 11, 195–233. doi:10.1016/0001-8686(79)80007-X
Liu, L., Stetsyuk, V., Kubiak, K., Yap, Y., Goharzadeh, A., and Chai, J. (2019). Nanoparticles for convective heat transfer enhancement: heat transfer coefficient and the effects of particle size and zeta potential. Chem. Eng. Commun. 206, 761–771. doi:10.1080/00986445.2018.1525364
Lonardi, F., and Luke, A. (2019). Adsorption mechanisms of alcoholic additives in water at low pressure - an experimental study. Int. J. Of Refrigeration-Revue Int. Du Froid 105, 33–40. doi:10.1016/j.ijrefrig.2018.12.001
Milanova, D., and Kumar, R. (2008). Heat transfer behavior of silica nanoparticles in pool boiling experiment. J. Of Heat Transfer-Transactions Of Asme 130, 042401. doi:10.1115/1.2787020
Muhammad, U., Bhattacharyya, D., Endrino, J., and Fereres, S. (2021a). Preparation of binary nanofluid with heat transfer additives by particle surface functionalisation. Emergent Mater. 4, 1649–1664. doi:10.1007/s42247-021-00260-z
Muhammad, U., Bhattacharyya, D., Endrino, J., and Fereres, S. (2021b). The effects of ejector adiabatic absorber on heat and mass transfer of binary nanofluid with heat transfer additives. Emergent Mater. 4, 1665–1678. doi:10.1007/s42247-021-00287-2
Ravera, F., Santini, E., Loglio, G., Ferrari, M., and Liggieri, L. (2006). Effect of nanoparticles on the interfacial properties of liquid/liquid and liquid/air surface layers. J. Of Phys. Chem. B 110, 19543–19551. doi:10.1021/jp0636468
Sah, M. K., Thakuri, B. S., Pant, J., Gardas, R. L., and Bhattarai, A. (2024). The multifaceted perspective on the role of green synthesis of nanoparticles in promoting a sustainable green economy. Sustain. Chem. 5, 40–59. doi:10.3390/suschem5020004
Schonhorn, H. (1965). Surface free energy of polymers. J. Of Phys. Chem. 69, 1084–1085. doi:10.1021/j100887a517
Wiley, V. C. H. (2002). Ullmann’s encyclopedia of industrial chemistry. Weinheim, Germany: Wiley-VCH. doi:10.1002/14356007
Vanoss, C., Absolom, D., Neumann, A., and Zingg, W. (1981). Determination of the surface tension of proteins I. Surface tension of native serum proteins in aqueous media. Biochimica Biophysica Acta 670, 64–73. doi:10.1016/0005-2795(81)90049-0
Wang, G., Li, J., Yan, G., Xu, R., Xie, G., and Lue, X. (2024). Numerical investigation on heat and mass transfer characteristics of inclined plate falling film absorption with nano-lithium bromide solution. Appl. Therm. Eng. 239, 122124. doi:10.1016/j.applthermaleng.2023.122124
Wen, Y., Bjurstrom, H., and Setterwall, F. (1991). Surface-tension of lithium bromide solutions with heat-transfer additives. J. Of Chem. And Eng. Data 36, 96–98. doi:10.1021/je00001a029
Yildirim, O., Xu, Q., and Basaran, O. (2005). Analysis of the drop weight method. Phys. Of Fluids 17, 062107. doi:10.1063/1.1938227
Zeng, C., Bissig, H., and Dinsmore, A. (2006). Particles on droplets: from fundamental physics to novel materials. Solid State Commun. 139, 547–556. doi:10.1016/j.ssc.2006.06.001
Zhou, X., Yuan, Z., and Herold, K. (2002). Phase distribution of the surfactant, 2-ethyl-hexanol in aqueous lithium bromide. Hvac&R Res. 8, 371–381. doi:10.1080/10789669.2002.10391296
Keywords: 2-ethyl-1-hexanol, alumina-nanofluid, gum Arabic, H2O/LiBr, interfacial-tension, surfactant, thermo-fluid
Citation: Muhammad UA and Endrino JL (2025) The effect of surface functionalization of nanoparticles with additives on surface tension of binary nanofluid. Front. Mater. 12:1639560. doi: 10.3389/fmats.2025.1639560
Received: 02 June 2025; Accepted: 30 September 2025;
Published: 17 October 2025.
Edited by:
Jagpreet Singh, Rayat Bahra University, IndiaReviewed by:
Ajaya Bhattarai, Tribhuvan University, NepalRizwan Farade, Putra Malaysia University, Malaysia
Copyright © 2025 Muhammad and Endrino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. L. Endrino, amxlbmRyaW5vQHVsb3lvbGEuZXM=
 U. A. Muhammad1
U. A. Muhammad1 J. L. Endrino
J. L. Endrino