- 1Department of Dentistry, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 2Department of Pediatric Orthopaedics, Shenyang Orthopaedic Hospital, Shenyang, China
- 3College of Stomatology, China Medical University, Shenyang, China
- 4Department of Dentistry, The Fourth People’s Hospital of Shenyang, Shenyang, Liaoning, China
Cellulose, as a natural material, serves as an excellent raw material for creating antimicrobial biological materials due to its unique nanostructure for cell scaffolds, customizable mechanical properties, biodegradability, and biocompatibility. The cellulose hydrogel offers exceptional structural adjustability and functional design options, thanks to the abundance of hydroxyl groups on its surface, making it suitable for various applications in tissue engineering, biomedicine carriers, wound dressings, and more. Despite its potential in stomatology, the research progress in this area remains unclear. This review focuses on the performance criteria for ideal cellulose-based hydrogels, including self-healing, adhesion, antibacterial properties, and drug delivery. It also covers preparation methods, repair mechanisms, and applications in biomimetic remineralization for hard tooth tissues, periodontitis, dental body repair, alveolar bone repair, and more. Persistent challenges—including scalable manufacturing processes, cost-effective production of functionalized variants, long-term biological safety assurances, antimicrobial resistance management, and ecological sustainability require resolution. Concurrently, establishing standardized regulatory protocols for clinical translation warrants prioritized efforts. By aligning material innovations with unresolved clinical demands in dental care, this review positions cellulose hydrogels as foundational components for personalized stomatological interventions, accelerating the transition toward precision-oriented dental therapeutics.
1 Introduction
Oral diseases not only affect daily physiological functions of the oral system, such as chewing and swallowing, but are also closely related to systemic illnesses, thereby endangering the quality of life of the patients (González-Moles et al., 2021). It is worth noting that the oral cavity is a complex environment characterized by high moisture, motility, and microbial colonization, making oral diseases not only diverse but also difficult to cure (An et al., 2022; Fan et al., 2016). Oral microorganisms play a crucial role in shaping the oral environment through complex biological signaling systems and interactions with their host (Hutchens et al., 2006). Imbalances in these microorganisms can lead to the production of virulence factors and metabolites, ultimately resulting in conditions such as dental caries and periodontal disease (Chinese Stomatological Association, 2021; Lamont et al., 2018). In recent years, with the broad application of biomaterials, traditional therapeutic methods has been gradually replaced. Among them, hydrogel is one of the most widely used biomaterials due to its good compatibility, easy preparation, and flexibility in design to achieve different ideal functions (Bertsch et al., 2023; Peng et al., 2022). However, wet environment, high motility and oral opportunistic pathogens require hydrogel for more particular properties such as wet adhesion, injectablity, self-healing ability and antibacterial delivery.
Cellulose hydrogels offer distinct advantages over other hydrogel systems, such as gelatin, alginate, and chitosan. Unlike gelatin-based hydrogels, which may degrade too rapidly, cellulose hydrogels exhibit enhanced mechanical stability and resistance to enzymatic degradation. Alginate hydrogels, while biocompatible, often lack the structural integrity required for load-bearing dental applications (Curvello et al., 2019). Chitosan hydrogels demonstrate antibacterial properties but may induce adverse immune responses in certain contexts (Mehrabi et al., 2022). In contrast, cellulose hydrogels, particularly nanofibrillated cellulose (NFC), combine high strength, biodegradability, and minimal immunogenicity, making them superior for precision medicine in stomatology. Moreover, the tunability of cellulose hydrogels through chemical modification (e.g., carboxymethylation or oxidation) allows customization for specific clinical needs, further distinguishing them from conventional hydrogels.
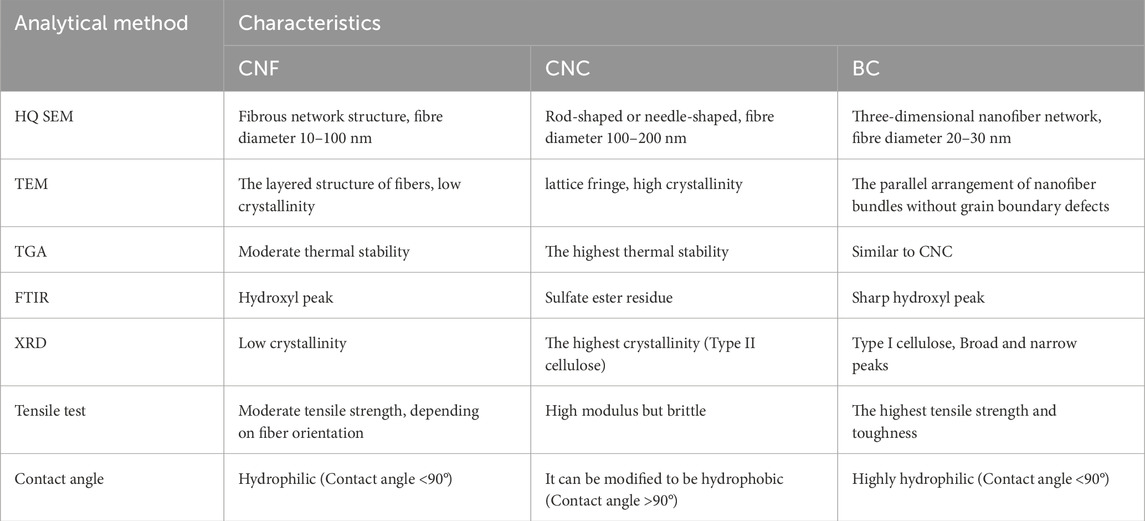
Among various hydrogel materials, cellulose-based hydrogels stand out as particularly promising candidates for oral applications due to their natural abundance, structural versatility, and biocompatibility. Cellulose hydrogels, derived from renewable sources, are classified based on their structural forms and synthesis methods. The primary categories include cellulose nanofibers (CNF) and nanocrystals (CNC), obtained from plant-based materials, and bacterial cellulose (BC), produced microbially. These hydrogels consist of β-1,4-linked glucose units with abundant surface hydroxyl groups, enabling facile chemical modifications (e.g., oxidation, acetylation) to tailor their mechanical, adhesive, and bioactive properties. Such versatility makes them ideal for oral applications, where wet adhesion, antibacterial activity, and biocompatibility are critical (Pereira et al., 2020; Wu et al., 2021). Cellulose can be categorized according to its origin and preparation into cellulose nanofiber (CNF) and cellulose nanocrystal (CNC) derived from wood materials, as well as bacterial cellulose (BC) obtained from bacterial synthesis, etc. (Pereira et al., 2020). CNF is extracted from lignocellulosic biomass materials through physical, chemical, or enzymatic processes, with its mechanical and optical properties influenced by factors like length-diameter ratio and crystallinity; CNC is produced by hydrolyzing CNF and exhibits strong mechanical properties due to its high intermolecular bonding; BC is derived from the metabolic processes of various microorganisms such as fungi, algae, and bacteria (Agrobacterium, Achromobacter, Acetobacter, Salmonella, etc.) (Rahmayetty, 2023). Various modern techniques such as HQ SEM, TEM, TGA, FTIR, XRD, tensile test, contact angle have been used to characterize their performances and their characteristics are listed in Table 1. Both CNF and CNC possess a high surface area and low coefficient of thermal expansion, making them suitable for tissue engineering applications. Moreover, BC not only shares the similar chemical structure as plant cellulose but offers superior properties including high purity, high crystallinity, high water retention, good mechanical properties, and a 3D nanofiber structure (Wu S. et al., 2021). The plentiful hydroxyl groups on the surface of cellulose can be easily modified with biopolymers through processes like oxidation (Curvello et al., 2019), acetylation (Wahid et al., 2021), and phosphorylation (Curvello et al., 2019). Cellulose itself has natural biocompatibility. However, its derivatives for tissue engineering must meet three requirements and can be naturally biodegraded or removed as the wound heals: (i) toxic groups cannot be chemically introduced into the structure of cellulose; (ii) the main chain structure of cellulose shouldn’t be changed; (iii) moderate crystallinity to avoid not to be biodegraded.Therefore cellulose has good compatibility and flexibility in designto reach the complex requirements for particular usage. This makes it a valuable material in tissue engineering and wound dressings, offering advantages such as appropriate mechanical properties, non-immunogenicity, and cost-effectiveness. Additionally, to address the unique moist and bacterial oral environment, researchers have developed a range of oral dressings with wet adhesion and antibacterial properties. Cellulose derivatives thus have been found wide applications in drug delivery systems and various other fields. Cellulose hydrogels have been designed to promote remineralization, osteoinduction properties, and facilitate the regeneration of oral soft and hard tissues based on these strategies (Elgendy et al., 2023; Najafi et al., 2021; Singh et al., 2022).
This article intends to review the application of cellulose hydrogels in stomatology, focusing on their essential properties such as wet adhesion, appropriate mechanical properties, antibacterium and self-healing, as well as their fundamental uses to dicuss their potential application on specific oral conditions such as periodontitis, dental caries, alveolar bone defect, etc (Figure 1). Additionally, the article discusses the advantages of cellulose hydrogels and potential modification strategies.
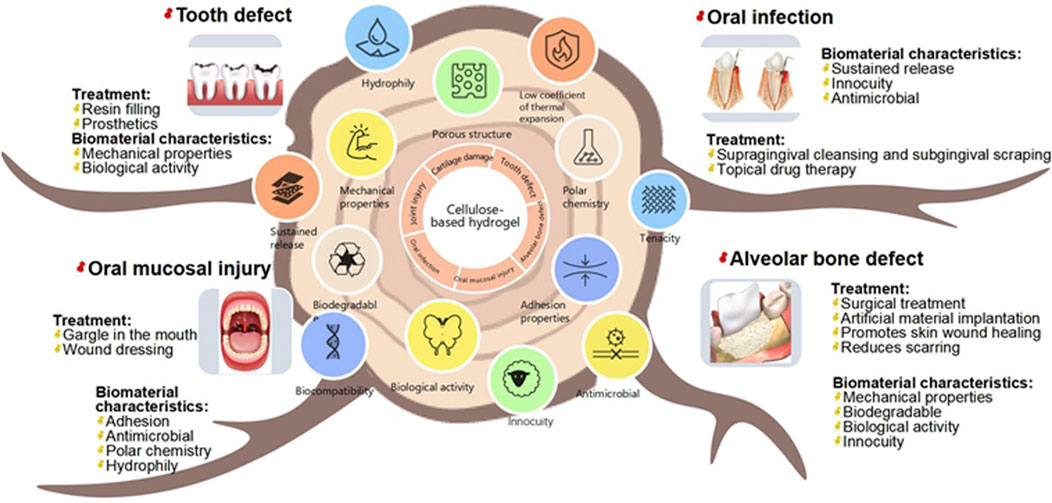
Figure 1. Schematic overview of cellulose hydrogels’ essential properties (wet adhesion, mechanical strength, antibacterial activity, self-healing) and their potential applications in stomatology for treating periodontitis, dental caries, and alveolar bone defects.
2 Performance requirements of cellulose hydrogels for oral tissue repair
An ideal polymer for tissue repair should possess mechanical stability, biocompatibility, bioactivity, and biodegradability (Xu and Hong, 2021). Oral repair materials must meet specific environmental requirements, including adequate strength, wet adhesion, self-healing capabilities, antibacterial properties, and other attributes to enhance the repair and regeneration process of oral soft and hard tissues for improved outcomes (Farshidfar et al., 2023; Montoya et al., 2023).
2.1 Wet adhesion
Bioadhesive materials find widespread application in medical implant technology, wound care, surgical anastomosis, and drug delivery (Deng et al., 2023). Oral bioadhesive agents are often utilized in conjunction with other technologies or dosage forms to enhance oral mucosal delivery of biomacromolecules (Nakipoglu et al., 2023). Moreover, a moist oral environment can impede material fixation (Jia et al., 2024). Thus, there is a pressing need for materials with strong wet adhesion to withstand salivary flushing and moist oral movements.
Hydrogel adhesive patches exhibit strong adhesion to wet surfaces, a critical feature for their clinical applications. Studies have shown that surface modification, such as introducing carboxyl, amine, or catechol groups, combined with non-covalent bonding strategies like hydrogen bonding and ionic interactions, enables effective adhesion to moist tissues (Chakraborty et al., 2023). Polar acid-base interaction forces and van der Waals dispersion forces play a significant role in the adhesion of cellulose to other materials (Liu et al., 2021). The surface free energies of the interface can vary depending on the cellulose source and preparation method. Polar functional groups on the surface of CNC decrease its attraction to lipophilic components significantly (Goldmann, 2021), whereas disordered regions within the CNF chain enhance the accessibility of its surface functional groups and promote better interfacial adhesion (Li B. et al., 2023).
However, when the hydrogel adhesive acts on the surface of wet tissue for a span, hydrone invades into hydrogels influences surface adhesion and mechanical properties, which limits hydrogels’ application. Therefore, some biomimetic hydrogels with wet adhesion has been reported, such as hydrogels inspired by mussel (Cheng et al., 2021; Hou et al., 2023; Xue et al., 2021; Zhang Y. et al., 2021), barnacle (Ni et al., 2022), octopus (Baik et al., 2017; Lee et al., 2022; Chen and Yang, 2017) et al. Marine mussels are well-known for their strong adhesion to various surfaces over time and their adhesion stength to skin can achieve 0.2 mN (Westerman et al., 2023). Mussel-inspired and barnacle-inspired hydrogels adhere to the wet surface by their catechol-derived groups imitating the 3,4-dihydroxy-L-phenylalanine (DOPA). The amino acid L-dopa, specifically its residues tyrosine and two hydroxyl groups, has been identified as a potential enhancer of adhesion (Fichman et al., 2021). It is hypothesized that dopamine (DA)-modified hydrogel exhibits robust adhesion to tissues in the presence of blood or saliva (An et al., 2022). Hou et al. (2023) find that these kinds of hydrogels’ wet adhesion ability much depend on the length of hydrophobic alkyl groups on their framework: the shorter ones disrupt the hydration layer to endow catechol groups to directly interact with wet surface resulting in strong wet adhesion, while the longer ones ofen entangle by strong hydrophobic interaction resulting in augmentation of rigidity and weakness of wet adhesion because they limit the mobility of the catechol and hydrophobic chains. Besides, catecholamine hydogels’ adhesion strength is weakened by self-oxidation, thus limiting their application. Therefore, it has been taken into consideration that to maintain catecholamine groups in these hydrogels should keep their self-oxidation state. Ocutopus-inspired hydrogels are designed by imitating the structure of octopus’ suction cup to physically adhere to wet surface and modifying other chemical groups to enhance their wet adhesion by chemical bongding. Inspired by the predation behavior of blue-ringed octopus, Zhu et al. (2023) (Figure 2a) develop the hydrogel by mimicing the designation of octopus’ suction cup using (Silk-Fp)-poly (N-isopropylacrylamide) (PNIPAm) with its inner wall modified by annic acid (TA). This hydrogel can achive wet adhesion not only by negative air pressure but also by chemical bonding between phenolic hydroxyl groups of TA and tissue proteins. Moreover, PNIPAm endowed the hydrogel with thermal response ability, whose viscosity increased by approximately 10 times with the temperature ranging from 20 °C to 37 °C.
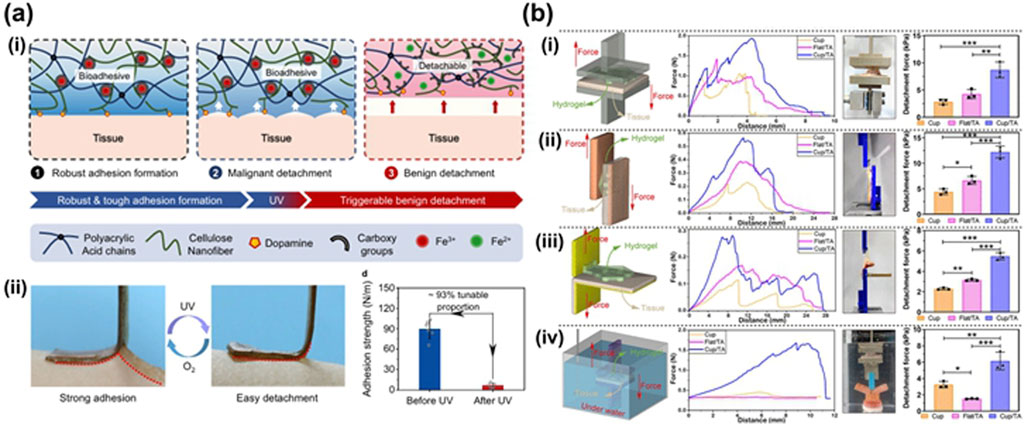
Figure 2. (a) Design of CNF-DA/PAA@Fe3+ dynamic hydrogel based on a light-driven supramolecular network engineering strategy via photo-Fenton-like (P.F.) reaction. (i) Schematic diagrams of a reversible structure of the adhesive and photo-detachable dynamic hydrogel with light-driven supramolecular network. (ii) Photographs of the molecular switch being peeled off from human skins under UV irradiation and air oxidation. And comparison of the adhesive strength of photo-detachable hydrogel before and after UV irradiation. Values represent their means ± SDs from n = 6 independent samples (Huang, 2023). (b) Complete Silk-Fp patch and its adhesive ability. (i) Comparison of the tensile strength of the patches via pull-off tests. (ii) Comparison of the shear strength of the patches via shear tests. (iii) Comparison of the interfacial toughness of the patches via peel tests. (iv) Comparison of the tensile strength of the patches after 1 h’s source underwater via pull-off tests (Zhu et al., 2023).
The hydroxyl groups of cellulose have regulatory properties and can be chemically modified to convert into aldehyde or carboxyl groups. Among them, aldehyde groups can react with amino groups on the skin to form dynamic Schiff bases, which is one of the important strategies for adhesion (Yin et al., 2024). Carboxyl groups, such as modified as carboxymethyl cellulose (CMC), can promote the further functionalization of adhesives, enabling them to incorporate antibacterial properties and facilitate wound healing, thereby expanding the application scope of hydrogels in the biomedical field (Wang et al., 2021a).
2.2 Appropriate mechanical strenth
Cellulose hydrogel’s mechanical strength is directly correlated with its crystallinity, as a higher crystallinity increases the proportion of crystalline area within the fiber (Wang C-H. et al., 2017). A more orderly molecular arrangement enhances the binding force between molecules, leading to higher breaking strength, yield stress, and initial modulus. The abundance of hydroxyl groups on cellulose hydrogel surfaces allows for the formation of electrostatic interactions and hydrogen bonds with other polymers, thereby enhancing its strength (Chen et al., 2023). Alongside crystallinity, fiber orientation is also a crucial factor influencing strength and tensile properties (Li et al., 2022). Althouh CNCs offer broad availability and excellent biocompatibility (Lekshmi et al., 2020), however, their low aspect ratio, limited interaction length, and inadequate shear transfer capability result in subpar mechanical properties (Zhou et al., 2018). Consequently, researchers are focusing on leveraging CNC’s crystallinity and polyhydroxyl structure more effectively to enhance its mechanical properties.
2.3 Self-healing ability
The dynamic reversibility of covalent bonds or non-covalent interactions in the cellulose hydrogel network is crucial for its self-healing properties (Zhang Z. P. et al., 2023; Zhao B. et al., 2023). Some hydrogels exhibit pH-responsive properties under physiological conditions and demonstrate self-healing behavior under acidic conditions, showing significant potential in practical biomedical applications. CNC can transition into a hydrogel state through physical rearrangement in the presence of salt, and its liquid mixture can gel at physiological temperatures when injected directly into the periodontal pocket (Mehrabi et al., 2022). These systems can positively impact the endogenous repair of alveolar bone and may be utilized for periodontal treatment in experimental periodontitis models, such as ligation-induced periodontitis (He X. et al., 2021).
2.4 Drug-delivery performance
The abundance of hydroxyl structures on the surface of cellulose hydrogel facilitate easy chemical modification or loading of antibacterial substances, allowing for targeted binding to various tissue sites due to its adhesive properties (Han et al., 2023). This modified material offers distinct advantages over conventional treatment methods. For example, the intricate non-covalent forces present within and between cellulose molecules pose challenges in dissolving them in conventional solvents (Wang et al., 2021a). Besides, the introduction of nanoparticles not only enhances themechanical strength of hydrogels but also impaits stimulus-response properties, suchas thermotropic properties, thereby enabling controlled diug release (Persano et al., 2025). However, through chemical modification, cellulose can enhance the permeation of hydrophilic drugs and facilitate the targeted release of anti-inflammatory, antibacterial, and other drugs like antibiotics in alkaline conditions (Tang et al., 2022). While a humid setting can enhance drug release, excessive saliva production and flow may lead to premature drug swallowing, resulting in drug loss known as the ‘saliva flushing effect’, thereby impacting drug bioavailability (Hu et al., 2021).
2.5 Biocompatibility and biodegradability
Cellulose derivatives are biocompatible due to their natural origin and non-toxic nature, which aligns with their widespread use in biomedical applications. However, not all cellulose derivatives are biodegradable; biodegradability depends on the degree of modification. For instance, unmodified cellulose is readily biodegradable, whereas oxidized cellulose and CMC may exhibit reduced biodegradability due to structural changes. In the case of cellulose hydrogels, they are fully biodegradable and gradually broken down by enzymatic hydrolysis in dressings, resulting in complete absorption without significant residues. Transitioning to implant applications, cellulose hydrogels function as temporary scaffolds that support tissue integration and are either fully degraded or removed by the body after serving their purpose, depending on their structural integrity. These properties collectively highlight the versatility of cellulose derivatives in advancing precision dental and biomedical therapies.
3 Application of cellulose hydrogel in oral medicine
3.1 Oral infectious diseases
The oral cavity harbors numerous bacteria, with the loose mucous membrane and saliva-rich environment posing challenges to oral tissue repair (Yin Z. et al., 2023). Approximately 700 different types of bacteria can be found in the oral cavity of healthy individuals, with a concentration of 1.5 × 108 CFU mL-1 (Deo and Deshmukh, 2019). The majority of these bacteria, about 94%, belong to Actinomycetes, Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, and Spirochaetes. The oral environment provides an environment conducive to the growth and reproduction of microorganisms by its appropriate temperature, humidity, and slightly acidic saliva (Humphrey and Williamson, 2001). The production of virulence factors and metabolites lead to oral infectious diseases such as dental caries, periodontal disease and mucositis. Dental caries are thought to be a kind of infectious diseases resulted by acid metabolites of dental plaque adhered on the surface of tooth. Dental caries can lead to tooth defect, including enamel and dentin defect, and even pulp infection (Zhang and Yelick, 2021). Periodontitis results from a host response to microbial plaque, which leads to a loss of the connective tissue and alveolar structure and finally tooth lost.
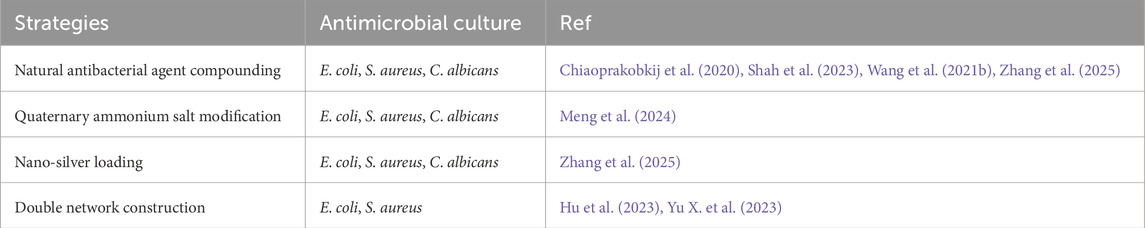
Cellulose itself does not have antibacterial properties. The commonly used modification methods include: i). Quaternary ammonium salt modification method; ii). Natural antibacterial agent compounding method; iii). Dual-network construction method. On this basis, the dual-network construction method can also be adopted to achieve antibacterial performance while endowing cellulose hydrogel with multiple properties such as strong mechanical performance and self-healing. (Table 2).
3.2 Cellulose hydrogels applied to treatment of dental caries
The target of treatment for dental caries is to repair tooth geometric profile and then recover bite. Therefore, strategies of hydrogels applied to treatment of dental caries target to make biomimetic remineralization to repair detected enamel or dentin. The hydroxyl-rich surfaces of cellulose hydrogels play a critical role in facilitating mineral nucleation during enamel and dentin remineralization. These hydroxyl groups act as nucleation sites for calcium phosphate minerals, accelerating the deposition of hydroxyapatite and promoting the repair of hard tissues (Persano et al., 2025).
Biomimetic remineralization of dental hard tissue involves precisely controlling the deposition of inorganic mineral apatite crystals on demineralized dental tissue to restore a similar form and function to natural dental hard tissue (Lei et al., 2024; Zhao H. et al., 2022). Current research suggests that cellulose hydrogels primarily promote remineralization by i) stabilizing calcium and phosphorus ions in solution and inhibiting their premature precipitation (Bussola Tovani et al., 2019). ii) Combined with amorphous calcium phosphate (ACP) to form a nanopolymer, and has a high affinity for the enamel hydroxyapatite (HAP) crystal surface (Liu Q. et al., 2023); iii) provide nucleation sites or mineralization for new crystals template to control the orderly formation of crystals and guide the remineralization of remaining dental tissue (Tang et al., 2024); iv) simulate dentin as a hierarchical structure of tissue repair scaffold (Soares et al., 2018); v) load components related to dental repair, such as: human dental pulp cells (hDPCs) (Qian Y. et al., 2023), HAP (Wu Y. et al., 2023), amorphous fluorinated calcium phosphate (AFCP) (Gao et al., 2024), etc. Furthermore, cellulose derivatives such as carboxymethyl cellulose (CMC) and nanocellulose (NC) interact with oral cells by modulating cell adhesion and proliferation. For example, NC scaffolds have been shown to upregulate osteogenic markers (e.g., OCN and ALP) in human dental pulp stem cells. In terms of biofilm interaction, cellulose hydrogels inhibit bacterial adhesion and biofilm formation, thereby reducing the risk of secondary caries. These mechanisms collectively underscore the potential of cellulose hydrogels as multifunctional biomaterials in dental regeneration (Chakraborty et al., 2023).
The strength of biocomposites, such as bone and crustaceans, can be attributed to their characteristic layered and helical structures of soft and hard phases (Plocher et al., 2021). Biomineralized nanocellulose, with a hierarchical structure and chemistry similar to that of enamel or dentin, is a promising candidate composite material for tooth restoration (Zhao et al., 2024) (Figure 3a). Research on imitating the biomineralization process to enhance the strength of cellulose has gradually gained attention (Yu and Zhu, 2024). Mohammadi et al. (2021) (Figures 4a, 3b) developed a biocomposite material with high strength, stiffness, fracture toughness, and complex shape, inspired by the mantis shrimp toe stick. The material consists of CNCs with long-range helical structures, artificial proteins with cellulose-binding modules (CBMs) and the CMP-1 acidic domain that regulates biomineralization, and apatite. Additionally, they fabricated this biocomposite into dental implant crowns, closely matching the internal quality of natural teeth. This study serves as a valuable reference for large-scale material manufacturing and prototype development in the field of hard tissue bionic repair and bioengineering. Bacterial cellulose is a promising carrier option due to its strong biocompatibility (Tang et al., 2024). In a study by Wang et al. (2024) (Figure 4b), BC/HAP composite hydrogels were created in various concentrations of simulated body fluids (SBF) to mimic the mineralization process and incorporate platelet-rich plasma (PRP), which enhanced the release of growth factors. The scaffold demonstrated outstanding biocompatibility, formation of mineralized nodules, and controlled release abilities in laboratory settings, suggesting significant potential for use in repairing tooth hard tissue.
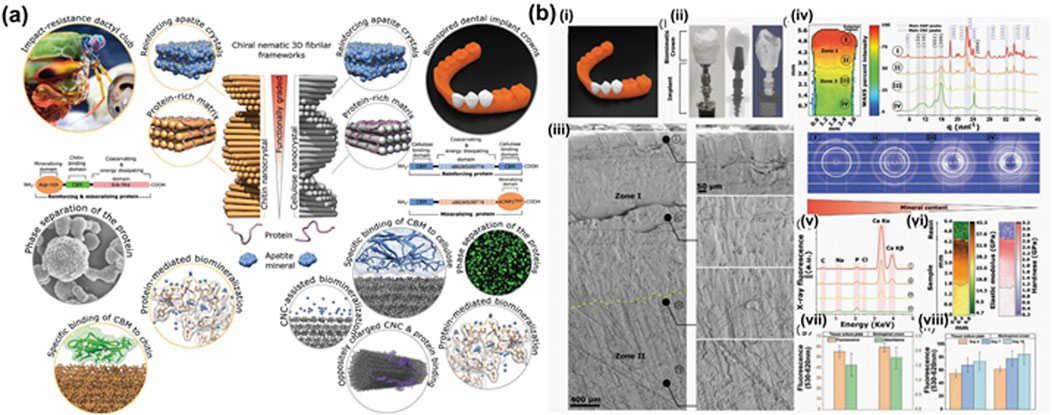
Figure 3. (a) Biologically inspired multiphase nanocomposite with a graded structure that mimics key molecular and architectural features of the mantis shrimp dactyl club (Zhao et al., 2024). (b) Bioinspired dental implant crown design, architecture, and composition. (i) Front view of 3D-printed human lower jaw with second premolar bioinspired crowns in place. (ii) Side view of second premolar implant assembled from titanium screw with the bioinspired crown. The X-ray and µCT 3D tomogram images. (iii) SEM images of cross-sections of the crown indicating distinct microstructural regions. (iv) Synchrotron 2D WAXS mapping corresponding to regions shown in (iii). (v) Synchrotron X-ray fluorescence spectra in different regions shown in (iii). (vi) Large-area (L ≈ 5.5 mm, W ≈ 0.9 mm) 2D nanoindentation map of elastic modulus and hardness for the cross-sectional region of the crown. (vii, viii) AB and LDH assay of adult human dermal fibroblast (AHDFs) cultured for 96 h on a tissue culture plate (TCP) and a bioinspired crown. Cell viability on both scaffolds over a period of 10 d is shown (Mohammadi et al., 2021).
Figure 4. (a) Bioinspired dental implant crown design, architecture, and composition. (i) Front view of 3D-printed human lower jaw with second premolar bioinspired crowns in place. (ii) Side view of second premolar implant assembled from titanium screw with the bioinspired crown. The X-ray and µCT 3D tomogram images. (iii) SEM images of cross-sections of the crown indicating distinct microstructural regions. (iv) Synchrotron 2D WAXS mapping corresponding to regions shown in (iii). v) Synchrotron X-ray fluorescence spectra in different regions shown in (iii). (vi) Large-area (L ≈ 5.5 mm, W ≈ 0.9 mm) 2D nanoindentation map of elastic modulus and hardness for the cross-sectional region of the crown. vii, viii) AB and LDH assay of adult human dermal fibroblast (AHDFs) cultured for 96 h on a tissue culture plate (TCP) and a bioinspired crown. Cell viability on both scaffolds over a period of 10 d is shown (Mohammadi et al., 2021). (b) Schematic showing fabrication of the BC/HAP@PRP hydrogel (Wang et al., 2024).
ACP nanoparticles are crucial precursors for biomimetic mineralization (Zhang et al., 2022a). However, they tend to transition into a crystalline phase in aqueous solutions, highlighting the importance of stabilizing the amorphous state of ACP in remineralization research (Zhang et al., 2022a). In a study by Zhe et al. (2021) (Figure 5b), a mineralized film was developed using hydroxypropyl methylcellulose (HPMC) and polyaspartic acid-stabilized amorphous calcium phosphate (PAsp-ACP) nanoparticles for tooth demineralization and subsequent remineralization. Both in vivo and in vitro experiments demonstrated that the group treated with the mineralized membrane exhibited a significantly enhanced remineralization layer compared to the control group. This effect may be attributed to the abundant hydroxyl, methyl, and methoxy anionic groups in the HPMC gel, which interact with calcium ions to form a stable network structure. Furthermore, the HPMC loaded with PAsp-ACP nanoparticles could create a dry film, effectively stabilizing the nanoparticles. Importantly, this formulation could also induce dentin mineralization in the gel state through synergistic action with PAsp additives.
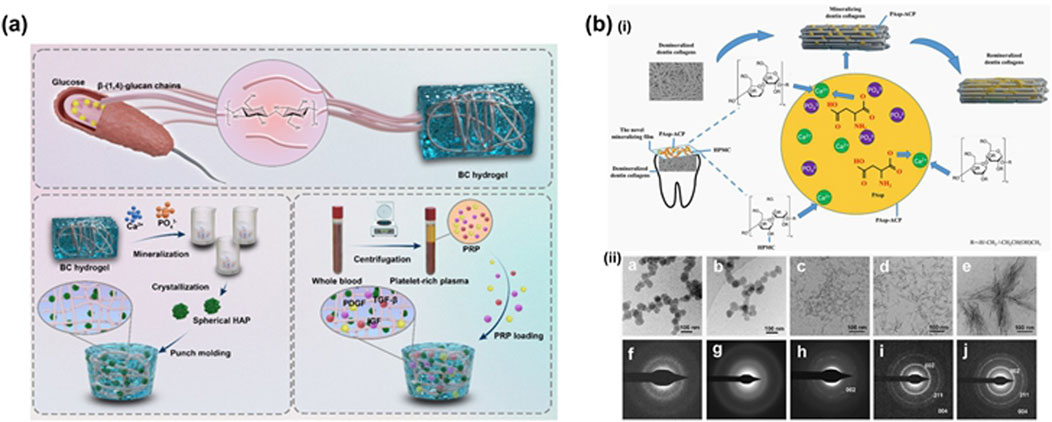
Figure 5. (a) Schematic showing fabrication of the BC/HAP@PRP hydrogel. (Wang et al., 2024). (b) (i) Scheme of the fabrication of mineralized film made of HPMC loaded with PAsp-ACP. (ii) Cryo-TEM images of the mineralizing flm and its SAED pattern at 0 h (a, f), 6 h (b, g), 8 h (c, h), 12 h (d, i) and 24 h (Zhe et al. (2021)).
3.3 Cellulose hydrogel for periodontitis
Periodontitis is the main cause of tooth loss in all oral bacterial diseases and has become the 11th epidemic disease in the world (Chinese Stomatological Association, 2021). For refractory periodontitis, simple removal of plaque biofilm can not achieve a good therapeutic effect, and local drug treatment must be supplemented (Elgendy et al., 2023). Oral gargle is the main way of oral mucosal damage and antibacterial, but it can not be targeted to treat the recognized pathogenic bacteria of periodontitis (Porphyromonas., Haemophilus., Fusobacterium.), so there is a lack of standard treatment for most patients with periodontitis in clinic, and personalized periodontal medication has become a key problem to be solved urgently in clinic. Currently, there are two primary antibacterial strategies employed in the oral environment: i) incorporating antibiotic molecules or antibacterial proteins/polymers to confer antibacterial properties (Zhang et al., 2022b); ii) utilizing antibacterial patches to create a barrier effect against bacteria (Han et al., 2023). Both strategies necessitate a stable and well-adhesive carrier, with cellulose hydrogels emerging as a preferred antibacterial material due to their exceptional physical and chemical properties (He X. et al., 2021).
An efficient periodontitis treatment system was created by Zhang et al. (2022a) (Figure 6) using a cross-linked cyclodextrin metal-organic framework (COF) suspended in a hydroxyethyl cellulose gel as I2@COF-HEC hydrogel. The outcomes of in vitro experiments showed that COF-HEC hydrogel’s ability to delay iodine release in artificial saliva for up to 5 days. Microcomputed tomography of the alveolar bone morphology in a rat periodontitis model showed that I2@COF-HEC hydrogel reduced pocket depth and alveolar bone resorption in a manner similar to that of the periodontal antibiotic minocycline ointment. I2@COF-HEC hydrogel is a brand-new method of delivering iodine locally and is utilized as a broad-spectrum antibacterial to treat periodontitis.
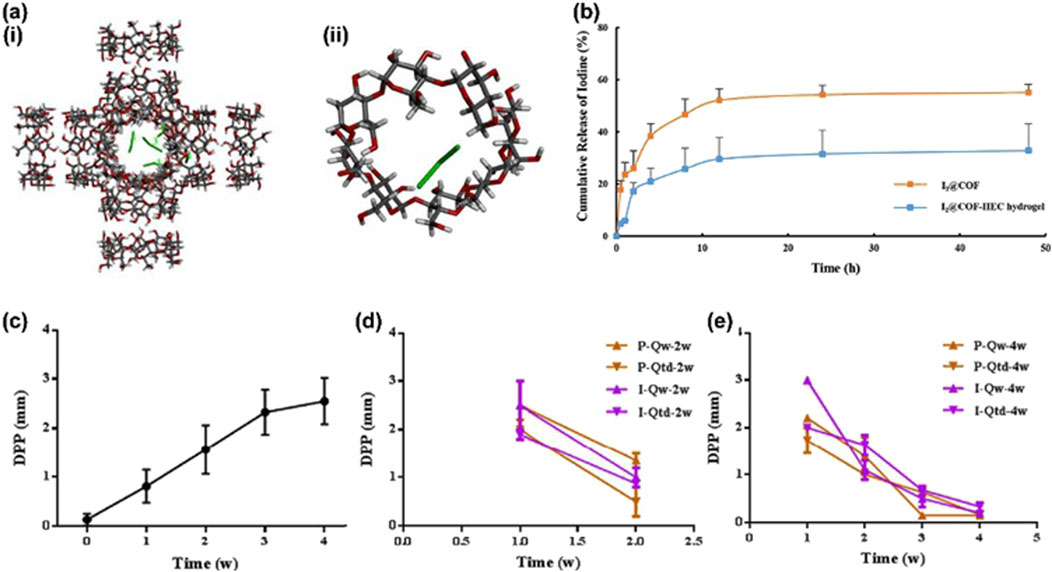
Figure 6. (a) The molecular docking conformation of (i) I3- @COF. (ii) I3- @CD; (b) Cumulative release of iodine from I2@COF particles and I2@COF-HEC hydrogel; (c) Progressive increase in DPP during the periodontitis modelling period; (d) Changes in DPP following two-week dosing of minocycline ointment and I2@COF-HEC hydrogel; (e) Changes in DPP following four-week dosing of minocycline ointment and I2@COF-HEC hydrogel (mean ± SD, n = 3) (Zhang et al., 2022b).
In spite of iodine, curcumin is also an ideal antibacterial. Multifunctional biopolymer composites synthesised by mechanical decomposed BC, gelatin, alginate, curcumin and glycerol are stretchable, transformation sustained and has appropriate stiffness (Chiaoprakobkij et al., 2020). No leakage of curcumin from the membrane was detected during immersion in PBS or synthetic saliva, with liquid absorption ranging from 100% to 700%. Using the model membrane of the pig mucosa, the in vitro adhesion duration ranges from 0.5 to 6 h in the synthetic saliva. It’s not cytotoxic to human gingival fibroblasts and keratinocytes. However, oral cancer cells are strongly inhibited by it. It is possible to further improve these multifunctional films to acquire the necessary features of topical patches for the treatment of oral cancer, periodontitis, and wounds.
3.4 Cellulose materials for maxillofacial trauma
Wound healing necessitates angiogenesis and antimicrobial activity (Chiaoprakobkij et al., 2020; Yu H. et al., 2023). Topical treatment is a promising approach for oral mucositis, and the use of spray film-forming systems can streamline self-administration, deliver anti-inflammatory drugs directly to the affected area, and prolong drug retention at the site of injury. To achieve this, the gelling formulation must be capable of gelling in situ (Guo et al., 2023; Singh et al., 2022; Singh et al., 2023) and rapidly forming a film when sprayed onto damaged oral mucosa. When formulating an optimal wound dressing, key properties to consider include ease of sterilization, promotion of wound debridement, user-friendliness, biodegradability, and non-toxicity (Wu W. et al., 2023).
Cellulose-based hydrogel has been utilized in wound dressings due to its favorable biocompatibility, adjustable mechanical properties, customizable structure, tissue adhesion, anti-infectious properties, and its ability to enhance the tensile properties of scaffolds (Ding et al., 2024). Leveraging its modifiability, these criteria can be effectively addressed. The discussion on the anti-infectious properties of cellulose in this section primarily focuses on its wet adhesion properties. The abundance of hydroxyl groups on cellulose surfaces facilitates easy modification (Zeng et al., 2022). Huang et al. utilized a CNF-enhanced supramolecular network, strong coordination between Fe3+ and polymer chains, and dopamine (DA) adhesion groups to create a CNF-DA/PAA@Fe3+ supramolecular hydrogel (Huang, 2023) (Figure 2b). This hydrogel exhibits reversible tough adhesion and easy photodetachment, attributed to the cellulose nanofiber reinforced network and the coordination between ions and polymer chains. These factors contribute to the dynamic reconstruction of the supramolecular network and the adhesive properties of the hydrogel.
BC is a nanoporous polymer with self-regulated swelling and stable bonding strength in the hydrated state, potentially serving as a bacterial barrier (Zhang et al., 2024) (Figure 4a). Qing-Fang Guan and colleagues developed a lotus biomimetic fiber spiral structure hydrogel using BC fibers, known as biomimetic hydrogel fiber (BHF) (Guan et al., 2021) (Figure 7a). This material exhibits a remarkable toughness of approximately 116.3 MJ m−3, along with high strength, toughness, and stretchability, making it a valuable biofilm material. In a study by Singh et al. (2023) (Figures 4b, 7b), the bacterial barrier properties of bacterial cellulose layered composites and bacterial cellulose-hydrogel (BC-PDz) were compared using Gram-negative Escherichia coli as a model bacterium. The study included an evaluation of light-cured BC-PDz patches. The results, based on measuring colony forming units (CFU) per milliliter of different groups after bacterial penetration, revealed that the pure BC membrane and trans-well groups allowed approximately 0.5–2 × 107 CFU/mL of bacteria to pass through in 24 h. In contrast, the BC-PDz patch group showed results ranging from 0 to 100 CFU/mL. The BC-FD patch demonstrated a 7-log reduction in CFU/mL compared to the control group, indicating its ability to block bacterial passage. BC-PDz was shown to possess unique self-sealing properties, reducing microbial penetration. Therefore, this cellulose hydrogel could potentially leverage its inherent barrier properties for applications aimed at preventing bacterial infections. Singh et al. (2022) developed aqueous composites for delicate epithelial surfaces using fibrotic BC and photoactive bioadhesives. The study demonstrated that these aqueous composites transitioned from a viscous state to an elastic material within 60 s of photoactivation. They exhibited a similar shear modulus to mimic soft tissues like oral mucosa and had adjustable adhesion strength ranging from 3 to 35 kPa on wet substrates. This approach offers a means to efficiently adhere the film to the tissue surface and could potentially be incorporated into the BC hydrogel system for treating mucosal wounds.
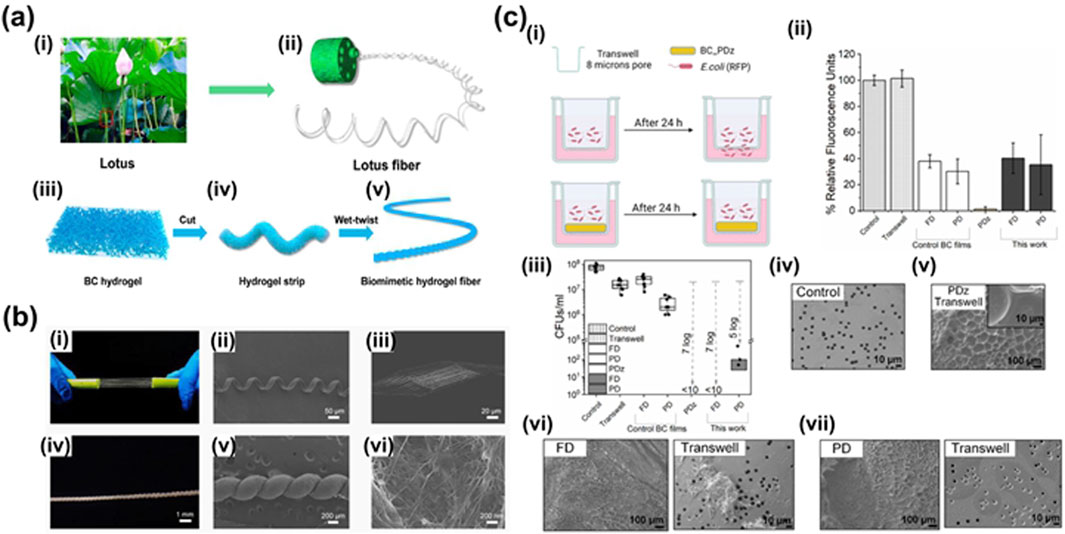
Figure 7. (a) Fabrication and structure analysis of BHF. (i) The stem of the lotus marked by the red square is the place from which the lotus fibers are extracted. (ii) Hierarchical structure of the lotus stem. (iii) Three-dimensional structure of bacterial cellulose hydrogel as the raw material of BHF. (iv) BC hydrogel strip after cutting. (v) BHF after wet-twisting with a lotus-fiber-like spiral structure. (b) Structural characterization and comparison of lotus fibers and BHF (Guan et al., 2021). (c) Double network microcrystalline cellulose hydrogels with high mechanical strength and biocompatibility for cartilage tissue engineering scaffold. (i) Illustration of the setup for bacterial barrier property of photocured BC-PDz patches using Gram negative Escherichia coli as model bacteria; (ii) comparison of % relative fluorescence units observed for different groups owing to bacteria (or fluorescent protein) penetrating through the patches (or controls); (iii) comparison of colony forming units (CFUs) per ml indicating live bacteria penetrated through the matrix after 24 h study duration; SEM micrograph of (iv) control trans-well membrane, (v) tran-swell-PDz interface after the study, (vi) BC-PDz patch (BC-FD)-trans-well interface, (vii) BC-PDz patch (BC-PD)-trans-well interface. BC-FD represents freeze-dried and BC-PD represents press-dried BC films. Data presented as mean ± SD, n = 9, p-values are calculated using two-way ANOVA with Bonferroni post hoc test, *p< 0.05, **p< 0.01, ***p< 0.001 (Yu X. et al., 2021).
Carboxymethyl cellulose (CMC) is carboxymethylated by cellulose, which has a rigid chain and thus good mechanical properties. It can be used to prepare hydrogels with good mechanical properties and can also be combined with various chemicals to prepare hydrogels with multiple specific functions. It has many applications in the medical field. Wang et al. (2021) (Figure 8) reported three-dimensional (3D)-printed hydrogels with antibacterial and antioxidant properties as a new type of wound dressing, which can adapt to the shape of wounds and have a more ordered pore structure, and are used for wound repair of large irregular wounds. It is composed by CMC, glycidyl methacrylate (GMA) and ε-Polylysine (ε-PL). PL, a kind of positively charged polypeptide, which also contains -NH2, has been widely reported used as antibacterial material to prepare hydrogel dressings. CMC-GMA and ε-PL-GMA (with GMA grafted onto CMC and ε-PL respectively) were mixed and hydrogels were formed under ultraviolet irradiation of a 3D printer, resulting in printable CP hydrogel dressings. Its 3D porous structure is conducive to tissue growth, cell adhesion and proliferation, as well as oxygen exchange. The degradation time of the material is 6–7 days. It also has good antibacterial properties, biocompatibility and the ability to promote wound repair.

Figure 8. (a) (i) Schematic representations of CP hydrogels obtained by 3D printing; (ii) Internal morphology of the CP41, CP43, and CP45 hydrogels by SEM. (b) (i) DPPH scavenging rate of different concentrations of CP41, CP43, and CP45 hydrogels; The antibacterial efficiency of CP41, CP43, and CP45 hydrogels. (ii) And antibacterial activity (Left: PBS; Right: sample group) (iii) and Live/dead bacterial viability assay of Escherichia coli and S. aureus before and after contact with CP45 hydrogel (Wang et al., 2021a).
3.5 Cellulose materials for alveolar bone defect repair
The repair of jaw and alveolar bone defects poses a significant challenge for dental treatment (Guo et al., 2023; Kang et al., 2021). Various factors such as cleft lip and palate, congenital malformations, trauma, jaw tumors, or tooth extraction can result in bone defects, leading to difficulties in chewing, aesthetic concerns, and language dysfunction (Zhang et al., 2023). The primary objective of bone repair in oral and maxillofacial surgery is to prepare for future tooth or implant restoration, necessitating sufficient bone volume and appropriate bone structure (He et al., 2024). Alveolar bone enhancement is essential for the success of dental implants, with the presence of healthy structural alveolar bone being a key factor for clinical success (Elgali et al., 2017). Barrier materials with strong support properties, osteoconductivity, and osteoinduction potential show promise for such applications (Li Q. et al., 2023; Wu et al., 2024). Guided bone regeneration (GBR) involves using a barrier membrane to direct bone growth, promoting the migration of osteoblasts to the site of bone defect while preventing the interference of other cells (Wu et al., 2024). Infection is a common reason for the failure of GBR technology, highlighting the importance of incorporating antibacterial features into the membranes to enhance treatment success rates (Mu et al., 2021; Qian et al., 2020). Therefore, cellulose hydrogels must possess bone-promoting, antibacterial, and bone-immunomodulatory properties, in addition to acting as a barrier and support structure to ensure the desired bone mass and morphology in new bone formation (Chen et al., 2022; Mao et al., 2023; Wu M. et al., 2023) (Figure 9a).
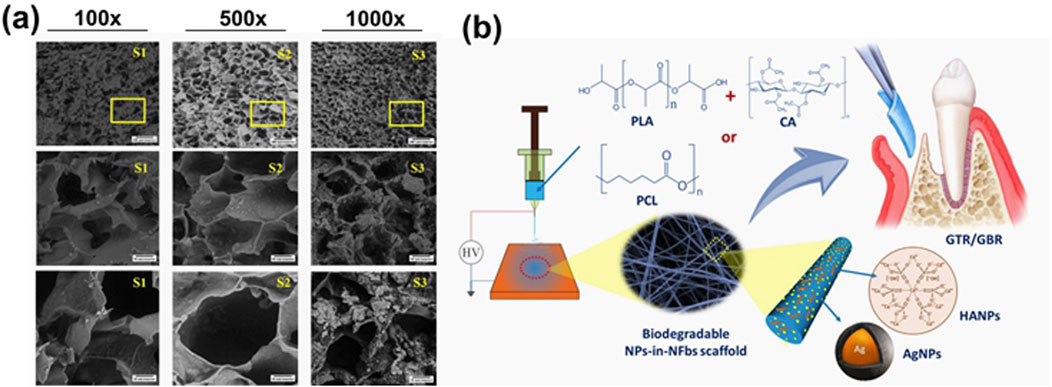
Figure 9. (a) Comparison of porous structure of CH/HPMC/BG/ZnO hydrogel with different concentration of BG 100 mg (S1), 200 mg (S2), 300 mg (S3) respectively at 100× (A), 500× (B) and 1,000× (C) magnification (Wu Y. et al., 2023). (b) A scheme of the development of nanoparticles-in-nanofibrous scaffolds using polylactic acid/cellulose acetate (PLA/CA), silver nanoparticles (AgNP), and hydroxyapatite na-noparticles (HANPs) for GTR/GBR applications with enhanced antibacterial and bone regeneration activity (Abdelaziz et al., 2021).
In order to reduce the risk of bacterial infection during GBR, Abdelaziz et al. (2021) (Figure 9b) fabricated electrospun nanofiber scaffolds using a combination of polylactic acid/cellulose acetate (PLA/CA), silver nanoparticles (AgNP), and hydroxyapatite nanoparticles (HANPs). The addition of HANPs to the scaffold aimed to enhance its antibacterial and bone regeneration properties. In vitro studies demonstrated that nanofibers incorporated with HANPs improved the viability of bone marrow stromal cells (BMSCs) by 41%–51% compared to the control group. Furthermore, all AgNPs-loaded fibers exhibited a significant ability to inhibit bacterial growth, with the antibacterial effect becoming more pronounced over time. Consequently, this nanofiber scaffold shows promising application potential in GBR. Luz et al. (2018) (Figure 10a) capitalized on strontium’s ability to inhibit osteoclastosis, stimulate osteogenesis, and reduce bone resorption by developing a hybrid composite material composed of oxidized bacterial cellulose membrane and strontium apatite. The material demonstrated good biocompatibility, reduced inflammatory responses, and promoted the growth and repair of connective tissue in in vivo experiments. However, its osteoinductive properties did not show significant differences from the control group.
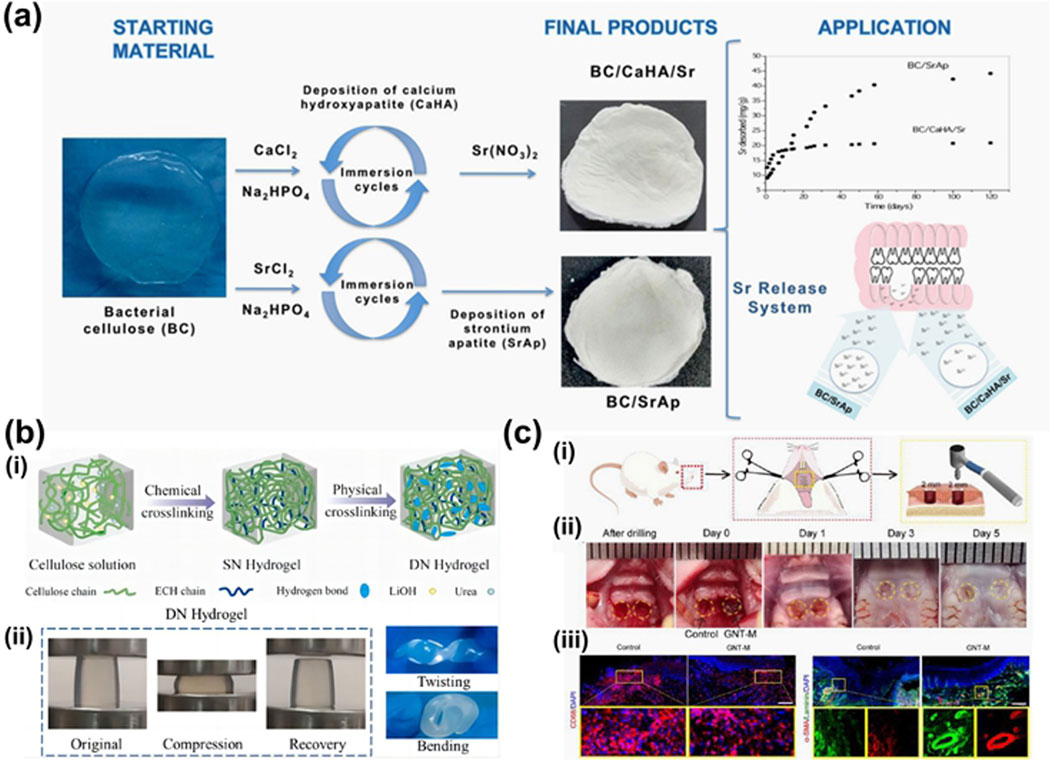
Figure 10. (a) Illustration of BC/SrAp fabrication and application (Luz et al., 2018). (b) Double network microcrystalline cellulose hydrogels with high mechanical strength and biocompatibility for cartilage tissue engineering scaffold. (i) Preparation of SN and DN hydrogel. (ii) Photographs of DN hydrogels under compression, kink, and bending (Yu X. et al., 2021). (c) Therapeutic effect of the GNT hydrogel on full-thickness palatal mucosal defects in rats. (i) Schematic diagram illustrated the surgery to create a bilateral full-thickness hard palatal mucosal defect of 2 mm in diameter in rats. (ii) Macroscopic view of palatal bilateral mucosal wound morphology at different times: control, the left wound; GNT-M, the right wound. (iii) Representative confocal images of macrophages (CD68, red) and nuclei (DAPI, blue) from control and GNT-M groups and their ×4 magnified plots on day 1. Scale bar: 100 μm. (iv) Representative confocal images of α-SMA (red), laminin (green), and nuclei (DAPI, blue) for the control and GNT-M groups and their ×4 magnified plots on day 5. Scale bar: 200 μm (Zhu et al., 2022).
4 Conclusion
This article provides an overview of the current utilization of cellulose hydrogels in oral medicine, highlighting the diverse applications and unique characteristics of these materials. Cellulose hydrogels are known for their excellent biocompatibility, sustained release properties, and intricate 3D structure. The abundance of hydroxyl groups on the surface of cellulose enables easy formation of hydrogen bonds and facilitates chemical modifications. By undergoing chemical alterations, cellulose hydrogels can acquire specific properties such as strength, wet adhesion, self-healing abilities, antibacterial effects, and controlled drug delivery capabilities. These versatile hydrogels show promise in treating various oral diseases and in repairing both soft and hard oral tissues. Researchers have developed a range of cellulose hydrogels loaded with bioactive substances like drugs, stem cells, fibroblasts, enamel-forming proteins, and antibacterial agents, with potential applications in dental and alveolar bone repair, periodontal tissue regeneration, and oral mucosa repair. Recent advances in technologies such as 3D printing and nanoengineering provide innovative pathways to optimize cellulose hydrogel design for precision medicine. For instance, multi-material 3D bioprinting enables the fabrication of complex, patient-specific hydrogel scaffolds with spatially controlled drug delivery systems, mimicking native tissue architecture. Similarly, nanoengineering approaches, such as surface modification and nanofiber incorporation, can enhance hydrogel biofunctionality, biocompatibility, and drug release profiles. These cutting-edge techniques not only facilitate personalized treatment strategies but also open new avenues for on-demand, site-specific therapy delivery (Wang et al., 2021b). While cellulose hydrogels have been extensively studied in tissue engineering applications, research on their use in temporomandibular joint cartilage and articular disc repair, as well as in oral malignant tumors, is limited. Furthermore, most of these studies have not progressed to the clinical trial phase yet.
While cellulose hydrogels exhibit promising mechanical properties and biocompatibility, their clinical translation faces significant hurdles. First, their potential toxicity and mechanisms for clearance within the human body remain inadequately explored, necessitating comprehensive in vivo assessments across small (e.g., mice, rats) and large animal models (e.g., monkeys). Additionally, cellulose hydrogels for periodontitis lack specific antibacterial experiments targeting key pathogens such as Porphyromonas, Haemophilus, and Fusobacterium, highlighting the need for more focused research on antibacterial efficacy and bone regeneration mechanisms. Furthermore, material degradation due to enzymatic breakdown in the oral environment can reduce efficacy over time, while safety regulations require extensive biocompatibility testing, including cytotoxicity and genotoxicity assays, potentially delaying market approval. Sterilization protocols may also compromise structural integrity, limiting shelf life. These challenges underscore the importance of interdisciplinary research to develop robust, scalable manufacturing processes and novel modification strategies, with clinical trials serving as a crucial next step for translating cellulose hydrogels into viable clinical solutions.
The unique and excellent biological properties of cellulose hydrogel make it a promising material for applications in oral medicine and biomedicine. As more relevant animal experiments and clinical trial data become available, cellulose hydrogels are expected to be extensively utilized across various biomedical fields. Extensively researched by numerous scholars, cellulose hydrogels have been recognized for their significant application potential in treating periodontitis, repairing dental hard tissue defects, addressing maxillofacial trauma, and repairing jaw bone defects.
Author contributions
JZ: Writing – original draft. RZ: Writing – original draft. XZ: Writing – original draft, Visualization. FD: Writing – original draft, Data curation. YZ: Writing – original draft, Data curation. DL: Writing – original draft, Data curation. SM: Data curation, Writing – original draft. AW: Conceptualization, Writing – review and editing. SL: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Basic Scientific Research Project of Higher Education Institutions of Liaoning Province, grant number 3110024160, and the Medical Education Research Project of Higher Education Institutions of Liaoning Province, grant number 3310024159.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelaziz, D., Hefnawy, A., Al-Wakeel, E., El-Fallal, A., and El-Sherbiny, I. M. (2021). New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 28, 51–62. doi:10.1016/j.jare.2020.06.014
An, H., Gu, Z., Zhou, L., Liu, S., Li, C., Zhang, M., et al. (2022). Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 149, 126–138. doi:10.1016/j.actbio.2022.07.016
Baik, S., Kim, D. W., Park, Y., Lee, T.-J., Ho Bhang, S., and Pang, C. (2017). A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546 (7658), 396–400. doi:10.1038/nature22382
Bertsch, P., Diba, M., Mooney, D. J., and Leeuwenburgh, S. C. G. (2023). Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 123 (2), 834–873. doi:10.1021/acs.chemrev.2c00179
Bussola Tovani, C., Gloter, A., Azaïs, T., Selmane, M., Ramos, A. P., and Nassif, N. (2019). Formation of stable strontium-rich amorphous calcium phosphate: possible effects on bone mineral. Acta Biomater. 92, 315–324. doi:10.1016/j.actbio.2019.05.036
Chakraborty, A., Alexander, S., Luo, W., Al-Salam, N., Van Oirschot, M., Ranganath, S. H., et al. (2023). Engineering multifunctional adhesive hydrogel patches for biomedical applications. Interdiscip. Med. 1 (4), e20230008. doi:10.1002/INMD.20230008
Chen, Y.-C., and Yang, H. (2017). Octopus-inspired assembly of nanosucker arrays for dry/wet adhesion. ACS Nano 11 (6), 5332–5338. doi:10.1021/acsnano.7b00809
Chen, J., Chen, J., Zhu, Z., Sun, T., Liu, M., Lu, L., et al. (2022). Drug-loaded and anisotropic wood-derived hydrogel periosteum with super antibacterial, anti-inflammatory, and osteogenic activities. ACS Appl. Mater. and Interfaces 14 (45), 50485–50498. doi:10.1021/acsami.2c12147
Chen, N., He, X., and Lu, Q. (2023). Highly stretchable, repairable, and tough nanocomposite hydrogel physically cross-linked by hydrophobic interactions and reinforced by surface-grafted hydrophobized cellulose nanocrystals. Macromol. Rapid Commun. 44 (10), e2300053. doi:10.1002/marc.202300053
Cheng, H., Shi, Z., Yue, K., Huang, X., Xu, Y., Gao, C., et al. (2021). Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 124, 219–232. doi:10.1016/j.actbio.2021.02.002
Chiaoprakobkij, N., Suwanmajo, T., Sanchavanakit, N., and Phisalaphong, M. (2020). Curcumin-loaded bacterial cellulose/alginate/gelatin as A multifunctional biopolymer composite film. Mol. Basel, Switz. 25 (17), 3800. doi:10.3390/molecules25173800
Chinese Stomatological Association (2021). Consensus of Chinese stomatological multidisciplinary experts on maintaining periodontal health. Zhonghua Kou Qiang Yi Xue Za Zhi = Zhonghua Kouqiang Yixue Zazhi = Chin. J. Stomatology 56 (2), 127–135. doi:10.3760/cma.j.cn112144-20210112-00013
Curvello, R., Raghuwanshi, V. S., and Garnier, G. (2019). Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 267, 47–61. doi:10.1016/j.cis.2019.03.002
Deng, T., Gao, D., Song, X., Zhou, Z., Zhou, L., Tao, M., et al. (2023). A natural biological adhesive from snail mucus for wound repair. Nat. Commun. 14 (1), 396. doi:10.1038/s41467-023-35907-4
Deo, P. N., and Deshmukh, R. (2019). Oral microbiome: unveiling the fundamentals. J. Oral Maxillofac. Pathology JOMFP 23 (1), 122–128. doi:10.4103/jomfp.JOMFP_304_18
Ding, Y., Zhu, Z., Zhang, X., and Wang, J. (2024). Novel functional dressing materials for intraoral wound care. Adv. Healthc. Mater. 13, e2400912. doi:10.1002/adhm.202400912
Elgali, I., Omar, O., Dahlin, C., and Thomsen, P. (2017). Guided bone regeneration: materials and biological mechanisms revisited. Eur. J. Oral Sci. 125 (5), 315–337. doi:10.1111/eos.12364
Elgendy, H. A., Makky, A. M. A., Elakkad, Y. E., Ismail, R. M., and Younes, N. F. (2023). Syringeable atorvastatin loaded eugenol enriched PEGylated cubosomes in-situ gel for the intra-pocket treatment of periodontitis: statistical optimization and clinical assessment. Drug Deliv. 30 (1), 2162159. doi:10.1080/10717544.2022.2162159
Fan, C., Fu, J., Zhu, W., and Wang, D.-A. (2016). A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 33, 51–63. doi:10.1016/j.actbio.2016.02.003
Farshidfar, N., Assar, S., Amiri, M. A., Sahmeddini, S., Hamedani, S., Zarei, M., et al. (2023). The feasible application of microfluidic tissue/organ-on-a-chip as an impersonator of oral tissues and organs: a direction for future research. Bio-Design Manuf. 6, 478–506. doi:10.1007/s42242-023-00235-5
Fichman, G., Andrews, C., Patel, N. L., and Schneider, J. P. (2021). Antibacterial gel coatings inspired by the cryptic function of a mussel byssal peptide. Adv. Mater. Deerf. Beach, Fla. 33 (40), e2103677. doi:10.1002/adma.202103677
Gao, X., Wang, Z., Yang, H., and Huang, C. (2024). Rapid intrafibrillar mineralization strategy enhances adhesive-dentin interface. J. Dent. Res. 103 (1), 42–50. doi:10.1177/00220345231205492
Goldmann, W. H. (2021). Biosensitive and antibacterial coatings on metallic material for medical applications. Cell Biol. Int. 45 (8), 1624–1632. doi:10.1002/cbin.11604
González-Moles, M. Á., Warnakulasuriya, S., González-Ruiz, I., González-Ruiz, L., Ayén, Á., Lenouvel, D., et al. (2021). Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 27 (4), 813–828. doi:10.1111/odi.13323
Guan, Q.-F., Han, Z.-M., Zhu, Y., Xu, W.-L., Yang, H.-B., Ling, Z.-C., et al. (2021). Bio-inspired lotus-fiber-like spiral hydrogel bacterial cellulose fibers. Nano Lett. 21 (2), 952–958. doi:10.1021/acs.nanolett.0c03707
Guo, J., Yao, H., Li, X., Chang, L., Wang, Z., Zhu, W., et al. (2023). Advanced Hydrogel systems for mandibular reconstruction. Bioact. Mater. 21, 175–193. doi:10.1016/j.bioactmat.2022.08.001
Han, Z., Deng, L., Chen, S., Wang, H., and Huang, Y. (2023). Zn2+-Loaded adhesive bacterial cellulose hydrogel with angiogenic and antibacterial abilities for accelerating wound healing. Burns and Trauma 11, tkac048. doi:10.1093/burnst/tkac048
He, X., Li, Z., Li, J., Mishra, D., Ren, Y., Gates, I., et al. (2021). Ultrastretchable, adhesive, and antibacterial hydrogel with robust spinnability for manufacturing strong hydrogel micro/nanofibers. Small Weinheim Der Bergstrasse, Ger. 17 (49), e2103521. doi:10.1002/smll.202103521
He, G., Xian, Y., Lin, H., Yu, C., Chen, L., Chen, Z., et al. (2024). An injectable and coagulation-independent tetra-PEG hydrogel bioadhesive for post-extraction hemostasis and alveolar bone regeneration. Bioact. Mater. 37, 106–118. doi:10.1016/j.bioactmat.2024.03.015
Hou, Y., Li, Y., Li, Y., Li, D., Guo, T., Deng, X., et al. (2023). Tuning water-resistant networks in mussel-inspired hydrogels for robust wet tissue and bioelectronic adhesion. ACS Nano 17 (3), 2745–2760. doi:10.1021/acsnano.2c11053
Hu, S., Pei, X., Duan, L., Zhu, Z., Liu, Y., Chen, J., et al. (2021). A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 12 (1), 1689. doi:10.1038/s41467-021-21989-5
Hu, W., Chen, Z., Chen, X., Feng, K., Hu, T., Huang, B., et al. (2023). Double-network cellulose-based hybrid hydrogels with favourable biocompatibility and antibacterial activity for wound healing. Carbohydr. Polym. 319, 121193. doi:10.1016/j.carbpol.2023.121193
Huang, G. (2023). Research progress of multifunctional nanocellulose-based hydrogels for wound healing. 中国造纸 42 (6), 93–102. doi:10.11980/j.issn.0254-508X.2023.06.013
Humphrey, S. P., and Williamson, R. T. (2001). A review of saliva: normal composition, flow, and function. J. Prosthet. Dent. 85 (2), 162–169. doi:10.1067/mpr.2001.113778
Hutchens, S. A., Benson, R. S., Evans, B. R., O’Neill, H. M., and Rawn, C. J. (2006). Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 27 (26), 4661–4670. doi:10.1016/j.biomaterials.2006.04.032
Jia, B., Zhang, B., Li, J., Qin, J., Huang, Y., Huang, M., et al. (2024). Emerging polymeric materials for treatment of oral diseases: design strategy towards a unique oral environment. Chem. Soc. Rev. 53 (7), 3273–3301. doi:10.1039/d3cs01039b
Kang, J., Zhang, J., Zheng, J., Wang, L., Li, D., and Liu, S. (2021). 3D-printed PEEK implant for mandibular defects repair—A new method. J. Mech. Behav. Biomed. Mater. 116, 104335. doi:10.1016/j.jmbbm.2021.104335
Lamont, R. J., Koo, H., and Hajishengallis, G. (2018). The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16 (12), 745–759. doi:10.1038/s41579-018-0089-x
Lee, Y.-W., Chun, S., Son, D., Hu, X., Schneider, M., and Sitti, M. (2022). A tissue adhesion-controllable and biocompatible small-scale hydrogel adhesive robot. Adv. Mater. Deerf. Beach, Fla. 34 (13), e2109325. doi:10.1002/adma.202109325
Lei, C., Wang, K.-Y., Ma, Y.-X., Hao, D.-X., Zhu, Y.-N., Wan, Q.-Q., et al. (2024). Biomimetic self-maturation mineralization system for enamel repair. Adv. Mater. Deerf. Beach, Fla. 36 (16), e2311659. doi:10.1002/adma.202311659
Lekshmi, G., Sana, S. S., Nguyen, V.-H., Nguyen, T. H. C., Nguyen, C. C., Le, Q. V., et al. (2020). Recent progress in carbon nanotube polymer composites in tissue engineering and regeneration. Int. J. Mol. Sci. 21 (17), 6440. doi:10.3390/ijms21176440
Li, B., Cao, P.-F., Saito, T., and Sokolov, A. P. (2023). Intrinsically self-healing polymers: from mechanistic insight to Current challenges. Chem. Rev. 123 (2), 701–735. doi:10.1021/acs.chemrev.2c00575
Li, L., Wang, W., Sun, J., Chen, Z., Ma, Q., Ke, H., et al. (2022). Improved properties of polyvinyl alcohol films blended with aligned nanocellulose particles induced by a magnetic field. Food Packag. Shelf Life 34, 100985. doi:10.1016/j.fpsl.2022.100985
Li, Q., He, W., Li, W., Luo, S., Zhou, M., Wu, D., et al. (2023). Band-aid-like self-fixed barrier membranes enable superior bone augmentation. Adv. Sci. 10 (16), e2206981. doi:10.1002/advs.202206981
Liu, R., Cui, L., Wang, H., Chen, Q., Guan, Y., and Zhang, Y. (2021). Tough, resilient, adhesive, and anti-freezing hydrogels cross-linked with a macromolecular cross-linker for wearable strain sensors. ACS Appl. Mater. and Interfaces 13 (35), 42052–42062. doi:10.1021/acsami.1c12687
Liu, Q., Zhang, J., Hou, Y., Wang, X., Li, X., Chen, T., et al. (2023). Tough and stretchable all-κ-carrageenan hydrogel based on the cooperative effects between chain conformation transition and stepwise mechanical training. Carbohydr. Polym. 313, 120869. doi:10.1016/j.carbpol.2023.120869
Luz, E. P. C. G., Borges, M. de F., Andrade, F. K., Rosa, M. de F., Infantes-Molina, A., Rodríguez-Castellón, E., et al. (2018). Strontium delivery systems based on bacterial cellulose and hydroxyapatite for guided bone regeneration. Cellulose 25 (11), 6661–6679. doi:10.1007/s10570-018-2008-8
Mao, Y., Zhang, Y., Wang, Y., Zhou, T., Ma, B., and Zhou, P. (2023). A multifunctional nanocomposite hydrogel with controllable release behavior enhances bone regeneration. Regen. Biomater. 10, rbad046. doi:10.1093/rb/rbad046
Mehrabi, A., Karimi, A., Mashayekhan, S., Samadikuchaksaraei, A., and Milan, P. B. (2022). In-situ forming hydrogel based on thiolated chitosan/carboxymethyl cellulose (CMC) containing borate bioactive glass for wound healing. Int. J. Biol. Macromol. 222 (Pt A), 620–635. doi:10.1016/j.ijbiomac.2022.09.177
Meng, S., Borjihan, Q., Xiao, D., Wang, Y., Chen, M., Cheng, C., et al. (2024). Biosynthesis of positively charged bacterial cellulose hydrogel with antibacterial and anti-inflammatory function for efficient wound healing. Int. J. Biol. Macromol. 279 (Pt 3), 135263. doi:10.1016/j.ijbiomac.2024.135263
Mohammadi, P., Gandier, J.-A., Nonappa, null, Wagermaier, W., Miserez, A., and Penttilä, M. (2021). Bioinspired functionally graded composite assembled using cellulose nanocrystals and genetically engineered proteins with controlled biomineralization. Adv. Mater. Deerf. Beach, Fla. 33 (42), e2102658. doi:10.1002/adma.202102658
Montoya, C., Roldan, L., Yu, M., Valliani, S., Ta, C., Yang, M., et al. (2023). Smart dental materials for antimicrobial applications. Bioact. Mater. 24, 1–19. doi:10.1016/j.bioactmat.2022.12.002
Mu, Y., Ma, S., Wei, P., Wang, Y., Jing, W., Zhao, Y., et al. (2021). Multifunctional modification of SIS membrane with chimeric peptides to promote its antibacterial, osteogenic, and healing-promoting abilities for applying to GBR. Adv. Funct. Mater. 31 (31), 2101452. doi:10.1002/adfm.202101452
Najafi, H., Jafari, M., Farahavar, G., Abolmaali, S. S., Azarpira, N., Borandeh, S., et al. (2021). Recent advances in design and applications of biomimetic self-assembled peptide hydrogels for hard tissue regeneration. Bio-Design Manuf. 4 (4), 735–756. doi:10.1007/s42242-021-00149-0
Nakipoglu, M., Tezcaner, A., Contag, C. H., Annabi, N., and Ashammakhi, N. (2023). Bioadhesives with antimicrobial properties. Adv. Mater. 35 (49), e2300840. doi:10.1002/adma.202300840
Ni, Z., Yu, H., Wang, L., Liu, X., Shen, D., Chen, X., et al. (2022). Polyphosphazene and non-catechol-based antibacterial injectable hydrogel for adhesion of wet tissues as wound dressing. Adv. Healthc. Mater. 11 (1), e2101421. doi:10.1002/adhm.202101421
Peng, W., Li, D., Dai, K., Wang, Y., Song, P., Li, H., et al. (2022). Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 208, 400–408. doi:10.1016/j.ijbiomac.2022.03.002
Pereira, A. L. S., Feitosa, J. P. A., Morais, J. P. S., and Rosa, M. de F. (2020). Bacterial cellulose aerogels: influence of oxidation and silanization on mechanical and absorption properties. Carbohydr. Polym. 250, 116927. doi:10.1016/j.carbpol.2020.116927
Persano, F., Lamanna, L., Friuli, M., Stetco, A. C., Barca, A., Verri, T., et al. (2025). Smart temperature-sensitive Injectable methylcellulose-based hydrogels: design and optimization for sustained drug release. VIEW 6 (4), 20250029. doi:10.1002/VIW.20250029
Plocher, J., Mencattelli, L., Narducci, F., and Pinho, S. (2021). Learning from nature: bio-inspiration for damage-tolerant high-performance fibre-reinforced composites. Compos. Sci. Technol. 208, 108669. doi:10.1016/j.compscitech.2021.108669
Qian, W., Feng, Y., He, M., Zhao, W., Qiu, L., and Zhao, C. (2020). A hierarchical janus nanofibrous membrane combining direct osteogenesis and osteoimmunomodulatory functions for advanced bone regeneration. Adv. Funct. Mater. 31, 2008906. doi:10.1002/adfm.202008906
Qian, Y., Gong, J., Lu, K., Hong, Y., Zhu, Z., Zhang, J., et al. (2023). DLP printed hDPSC-loaded GelMA microsphere regenerates dental pulp and repairs spinal cord. Biomaterials 299, 122137. doi:10.1016/j.biomaterials.2023.122137
Rahmayetty, S., and Sulaiman, F. (2023). Wastewater from the arenga starch industry as a potential medium for bacterial cellulose and cellulose acetate production. Polymers 15 (4), 870. doi:10.3390/polym15040870
Shah, S. A., Sohail, M., Karperien, M., Johnbosco, C., Mahmood, A., and Kousar, M. (2023). Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 227, 1203–1220. doi:10.1016/j.ijbiomac.2022.11.307
Singh, J., Steele, T. W. J., and Lim, S. (2022). Fibrillated bacterial cellulose liquid carbene bioadhesives for mimicking and bonding oral cavity surfaces. J. Mater. Chem. B 10 (14), 2570–2583. doi:10.1039/d1tb02044g
Singh, J., Steele, T. W. J., and Lim, S. (2023). Bacterial cellulose adhesive patches designed for soft mucosal interfaces. Biomater. Adv. 144, 213174. doi:10.1016/j.bioadv.2022.213174
Soares, D. G., Zhang, Z., Mohamed, F., Eyster, T. W., de Souza Costa, C. A., and Ma, P. X. (2018). Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 68, 190–203. doi:10.1016/j.actbio.2017.12.037
Tang, K. Y., Heng, J. Z. X., Chai, C. H. T., Chan, C. Y., Low, B. Q. L., Chong, S. M. E., et al. (2022). Modified bacterial cellulose for biomedical applications. Chem. Asian J. 17 (19), e202200598. doi:10.1002/asia.202200598
Tang, Z., Chen, Z., Wang, D., Shan, S., Jin, W., Sun, K., et al. (2024). Peptide amphiphile-mediated assembly and fusion of anisotropic amorphous particles for enamel remineralization. Adv. Funct. Mater. 34 (21), 2306900. doi:10.1002/adfm.202306900
Wahid, F., Huang, L.-H., Zhao, X.-Q., Li, W.-C., Wang, Y.-Y., Jia, S.-R., et al. (2021). Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 53, 107856. doi:10.1016/j.biotechadv.2021.107856
Wang, C.-H., Lin, Y.-T., and Lin, Y.-T. J. (2017). A survey of natal and neonatal teeth in newborn infants. J. Formos. Med. Assoc. 116 (3), 193–196. doi:10.1016/j.jfma.2016.03.009
Wang, X., Cui, L., Fan, S., Li, X., and Liu, Y. (2021a). Biodegradable poly(butylene adipate-co-terephthalate) antibacterial nanocomposites reinforced with MgO nanoparticles. Polymers 13 (4), 507. doi:10.3390/polym13040507
Wang, X., Qi, J., Zhang, W., Pu, Y., Yang, R., Wang, P., et al. (2021b). 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 187, 91–104. doi:10.1016/j.ijbiomac.2021.07.115
Wang, X., Yang, X., Xiao, X., Li, X., Chen, C., and Sun, D. (2024). Biomimetic design of platelet-rich plasma controlled release bacterial cellulose/hydroxyapatite composite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 269, 132124. doi:10.1016/j.ijbiomac.2024.132124
Wenze, C., Keyin., L., Xiaoyu, L., Jing, W., Lu, C., Zihan, Y., et al. (2024). Harmonizing thickness and permeability in bone tissue engineering: a novel silk fibroin membrane inspired by spider silk dynamics. Adv. Mater. 36 (13), 2310697. doi:10.1002/adma.202310697
Westerman, C. R., McGill, B. C., and Wilker, J. J. (2023). Sustainably sourced components to generate high-strength adhesives. Nature 621 (7978), 306–311. doi:10.1038/s41586-023-06335-7
Wu, S., Gong, Y., Liu, S., Pei, Y., and Luo, X. (2021). Functionalized phosphorylated cellulose microspheres: design, characterization and ciprofloxacin loading and releasing properties. Carbohydr. Polym. 254, 117421. doi:10.1016/j.carbpol.2020.117421
Wu, S., Luo, S., Cen, Z., Li, Q., Li, L., Li, W., et al. (2024). All-in-one porous membrane enables full protection in guided bone regeneration. Nat. Commun. 15 (1), 119. doi:10.1038/s41467-023-43476-9
Wu, M., Liu, H., Zhu, Y., Chen, F., Chen, Z., Guo, L., et al. (2023). Mild photothermal-stimulation based on injectable and photocurable hydrogels orchestrates immunomodulation and osteogenesis for high-performance bone regeneration. Small 19 (28), e2300111. doi:10.1002/smll.202300111
Wu, W., Lu, Z., Lu, C., Sun, X., Ni, B., Cölfen, H., et al. (2023). Bioinspired stabilization of amorphous calcium carbonate by carboxylated nanocellulose enables mechanically robust, healable, and sensing biocomposites. ACS Nano 17 (7), 6664–6674. doi:10.1021/acsnano.2c12385
Wu, Y., Wang, Y., Zheng, C., Hu, C., Yang, L., Kong, Q., et al. (2023). A versatile glycopeptide hydrogel promotes chronic refractory wound healing through bacterial elimination, sustained oxygenation, immunoregulation, and neovascularization. Adv. Funct. Mater. 33 (49), 2305992. doi:10.1002/adfm.202305992
Xu, H., and Hong, X. (2021). Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 15, 250–271. doi:10.1016/j.bioactmat.2021.11.029
Xue, B., Gu, J., Li, L., Yu, W., Yin, S., Qin, M., et al. (2021). Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 12 (1), 7156. doi:10.1038/s41467-021-27529-5
Yin, Z., Liu, Y., Anniwaer, A., You, Y., Guo, J., Tang, Y., et al. (2023). Rational designs of biomaterials for combating oral biofilm infections. Adv. Mater. 37, e2305633. doi:10.1002/adma.202305633
Yin, S., Duan, M., Fellner, M., Wang, Z., Lv, C., Zang, J., et al. (2024). pH/glucose dual-responsive protein-based hydrogels with enhanced adhesive and antibacterial properties for diabetic wound healing. Food Innovation Adv. 3 (4), 332–343. doi:10.48130/fia-0024-0032
Yu, H.-P., and Zhu, Y.-J. (2024). Guidelines derived from biomineralized tissues for design and construction of high-performance biomimetic materials: from weak to strong. Chem. Soc. Rev. 53 (9), 4490–4606. doi:10.1039/d2cs00513a
Yu, H., Li, Y., Pan, Y., Wang, H., Wang, W., Ren, X., et al. (2023). Multifunctional porous poly (L-lactic acid) nanofiber membranes with enhanced anti-inflammation, angiogenesis and antibacterial properties for diabetic wound healing. J. Nanobiotechnology 21 (1), 110. doi:10.1186/s12951-023-01847-w
Yu, X., Li, X., Kan, L., Pan, P., Wang, X., Liu, W., et al. (2023). Double network microcrystalline cellulose hydrogels with high mechanical strength and biocompatibility for cartilage tissue engineering scaffold. Int. J. Biol. Macromol. 238, 124113. doi:10.1016/j.ijbiomac.2023.124113
Zeng, Q., Qi, X., Shi, G., Zhang, M., and Haick, H. (2022). Wound dressing: from nanomaterials to diagnostic dressings and healing evaluations. ACS Nano 16 (2), 1708–1733. doi:10.1021/acsnano.1c08411
Zhang, W., and Yelick, P. C. (2021). Tooth repair and regeneration: potential of dental stem cells. Trends Mol. Med. 27 (5), 501–511. doi:10.1016/j.molmed.2021.02.005
Zhang, B., Yin, X., Zhang, F., Hong, Y., Qiu, Y., Yang, X., et al. (2023). Customized bioceramic scaffolds and metal meshes for challenging large-size mandibular bone defect regeneration and repair. Regen. Biomater. 10, rbad057. doi:10.1093/rb/rbad057
Zhang, Y., Pon, N., Awaji, A., and Rowan, S. J. (2021). Squid beak inspired cross-linked cellulose nanocrystal composites. Biomacromolecules 22 (1), 201–212. doi:10.1021/acs.biomac.0c01051
Zhang, L., Wan, C., Su, J., Zhang, C., Wei, S., Tian, W., et al. (2022a). A dual-crosslinked self-healing and antibacterial nanocellulose hydrogel for monitoring of human motions. Mater. and Des. 215, 110464. doi:10.1016/j.matdes.2022.110464
Zhang, L., Zhang, Y., Yu, T., Peng, L., Sun, Q., and Han, B. (2022b). Engineered fabrication of enamel-mimetic materials. Engineering 14, 113–123. doi:10.1016/j.eng.2021.02.027
Zhang, L., Chen, L., Wang, S., Wang, S., Wang, D., Yu, L., et al. (2024). Cellulose nanofiber-mediated manifold dynamic synergy enabling adhesive and photo-detachable hydrogel for self-powered E-skin. Nat. Commun. 15 (1), 3859. doi:10.1038/s41467-024-47986-y
Zhang, S., Gatsi, B., Yao, X., Jin, Y., and Amhal, H. (2025). Cellulose nanofiber-reinforced antimicrobial and antioxidant multifunctional hydrogel with self-healing, adhesion for enhanced wound healing. Carbohydr. Polym. 352, 123189. doi:10.1016/j.carbpol.2024.123189
Zhang, Z. P., Rong, M. Z., and Zhang, M. Q. (2023). Self-healable functional polymers and polymer-based composites. Prog. Polym. Sci. 144, 101724. doi:10.1016/j.progpolymsci.2023.101724
Zhao, X., Chen, Z., Zhuo, H., Zhong, C., Shi, G., Liu, T., et al. (2024). Ultra-strong and transparent biomimetic nanocomposite through orientation effects and in situ biomineralization. Adv. Funct. Mater. 34 (1), 2310094. doi:10.1002/adfm.202310094
Zhao, H., Liu, S., Lu, J., Yang, X., Yang, Z., Li, F., et al. (2022). Natural tooth enamel and its analogs. Cell Rep. Phys. Sci. 3 (7), 100945. doi:10.1016/j.xcrp.2022.100945
Zhao, P., Chen, W., Feng, Z., Liu, Y., Liu, P., Xie, Y., et al. (2022). Electrospun nanofibers for periodontal treatment: a recent progress. Int. J. Nanomedicine 17, 4137–4162. doi:10.2147/IJN.S370340
Zhe, W., Zihuai, Z., Jiayan, F., Leiqing, Z., Zhixin, Z., Wu, Z., et al. (2021). Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization. J. Nanobiotechnology 19 (1), 385. doi:10.1186/s12951-021-01133-7
Zhou, L., Li, N., Shu, J., Liu, Y., Wang, K., Cui, X., et al. (2018). One-pot preparation of carboxylated cellulose nanocrystals and their liquid crystalline behaviors. ACS Sustain. Chem. and Eng. 6 (9), 12403–12410. doi:10.1021/acssuschemeng.8b02926
Zhu, Z., Wang, J., Pei, X., Chen, J., Wei, X., Liu, Y., et al. (2023). Blue-ringed octopus-inspired microneedle patch for robust tissue surface adhesion and active injection drug delivery. Sci. Adv. 9 (25), eadh2213. doi:10.1126/sciadv.adh2213
Keywords: cellulose, hydrogel, stomatology, biomaterials, tissue engineering
Citation: Zhang J, Zhao R, Zhang X, Dong F, Zhang Y, Liu D, Meng S, Wang A and Liu S (2025) Engineered cellulose hydrogels: multifunctional platforms for innovative stomatological therapies and precision medicine. Front. Mater. 12:1647024. doi: 10.3389/fmats.2025.1647024
Received: 14 June 2025; Accepted: 06 October 2025;
Published: 05 November 2025.
Edited by:
Luiz Fernando Romanholo Ferreira, Catholic University of Brasilia (UCB), BrazilReviewed by:
Xiaolei Li, University of Pennsylvania, United StatesMohammad El-Nablaway, Mansoura University, Egypt
Deyaa Abol-Fotouh, City of Scientific Research and Technological Applications Advanced Technology and New Materials Research Institute, Egypt
Copyright © 2025 Zhang, Zhao, Zhang, Dong, Zhang, Liu, Meng, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai Wang, d2FuZ2FpMTAxMUAxNjMuY29t; Siyu Liu, amVzc2lldzE2QHllYWgubmV0
†These authors have contributed equally to this work and share first author
 Junyang Zhang
Junyang Zhang Rui Zhao2†
Rui Zhao2† Shuyu Meng
Shuyu Meng Ai Wang
Ai Wang