- 1Department of Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, China
- 2Department of Laboratory Medicine, Yancheng First Hospital Affiliated of Nanjing University Medical School, The Yancheng Clinical College of Xuzhou Medical University, The First People's Hospital of Yancheng, Yancheng, China
- 3Critical Care Medicine, Jurong Hospital Affiliated to Jiangsu University, Zhenjiang, China
- 4Health Testing Center, Zhenjiang Center for Disease Control and Prevention, Zhenjiang, China
- 5Department of Pulmonary Medicine, Abbassia Chest Hospital, EMOH, Cairo, Egypt
- 6Public Experiment and Service Center, Jiangsu University, Zhenjiang, China
- 7Molecular Medical Research Center, Yancheng Clinical Medical College of Jiangsu University, Yancheng, China
Purpose: Polycystic ovary syndrome (PCOS) is a clinically prevalent endocrine and metabolic disorder characterized by gut microbial disturbances and chronic low-grade inflammatory responses.
Methods: This study explores the therapeutic potential and mechanistic insights of Lactiplantibacillus plantarum NKK20 (LP) in a PCOS murine model established through high-fat diet (HFD) and letrozole co-induction. By integrating multi-omics profiling (16S rRNA sequencing and untargeted metabolomics) with histopathological evaluation, we systematically assessed LP-mediated modulations of gut microbiota composition, metabolic signatures, ovarian function, and intestinal barrier integrity.
Results: The results demonstrated that LP administration effectively counteracted metabolic dysregulation in PCOS mice, mitigating body weight gain, ameliorating lipid abnormalities (reduced total cholesterol, triglycerides, and LDL-C alongside elevated HDL-C), and lowering fasting glucose levels. Hormonally, LP suppressed hyperandrogenism, as evidenced by decreased testosterone, while rebalancing inflammatory mediators through IL-10 upregulation and concomitant reduction of TNF-α, IL-6, IL-1β, and MCP-1. Ovarian histomorphology revealed attenuated follicular cysts and enhanced luteinization. Critically, LP restored intestinal homeostasis by (i) augmenting short-chain fatty acid (SCFA) production—particularly butyrate—(ii) fortifying the gut barrier via increased ZO-1 and occludin expression, and (iii) diminishing circulating endotoxin. Microbial sequencing identified enrichment of Bacteroidetes and Muribaculum following LP treatment. Serum metabolomics further uncovered LP-induced normalization of steroid hormone biosynthesis and glycerophospholipid metabolism, coinciding with elevated anti-inflammatory mediators such as 6a-prostaglandin I1.
Conclusion: Collectively, these findings delineate a novel preventive axis through which LP inhibits PCOS progression — namely, via coordinated “gut microbiota–metabolite–ovarian” crosstalk involving SCFA-mediated barrier restoration, microbial ecology stabilization, and suppression of ovarian inflammatory onset. This work advances the translational rationale for probiotic-based strategies in PCOS prevention.
Introduction
Polycystic ovary syndrome (PCOS), one of the most prevalent endocrine and metabolic disorders among reproductive-aged women, exhibits a global prevalence ranging from 6 to 20% (1, 2). Characterized clinically by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology, this syndrome frequently co-occurs with metabolic disturbances such as insulin resistance and obesity (3, 4). Emerging research has elucidated that PCOS pathogenesis is closely linked to a chronic low-grade inflammatory state triggered by gut microbial dysbiosis (5–7). The conceptualization of the gut-ovary axis has offered transformative insights into disease mechanisms, revealing how gut-derived microbial metabolites (e.g., short-chain fatty acids and bile acids) may modulate ovarian function and systemic glucose-lipid homeostasis through immunoregulatory and endocrine pathways (8–10). Despite these advances, current first-line PCOS treatments—including oral contraceptives and insulin-sensitizing agents—remain limited by inconsistent therapeutic responses and notable adverse effects during prolonged use, particularly beyond six months (11). These clinical constraints have kindled significant interest in developing novel therapeutic strategies targeting the restoration of gut microenvironmental homeostasis as a promising alternative approach (12).
Emerging research has increasingly highlighted the pivotal role of gut microbiota dysbiosis in the pathogenesis of PCOS. Clinical observations consistently demonstrate distinct alterations in the gut microbial ecosystem among PCOS patients, marked by diminished α-diversity indices, an imbalanced Bacteroidetes/Firmicutes ratio, and notably reduced biosynthesis of short-chain fatty acids (SCFAs), particularly butyrate (13, 14). Of translational significance, emerging evidence highlights the therapeutic potential of probiotics to boost gut-derived short-chain fatty acids (SCFAs), particularly butyrate, in PCOS intervention—building on preclinical studies showing that fecal microbiota transplantation (FMT) from healthy donors ameliorates sex hormone dysregulation and reverses polycystic ovarian pathology (15, 16). These benefits are partly mediated by butyrate, a microbial metabolite with pleiotropic effects, including GPCR activation to improve insulin sensitivity, NF-κB suppression to reduce ovarian inflammation, and gut-microbiota-host crosstalk modulation to restore metabolic homeostasis (17–19). However, critical gaps remain in understanding how probiotic-induced SCFA elevation, specifically butyrate, orchestrates these effects, warranting mechanistic studies to optimize microbiota-targeted therapies for PCOS.
Among various microbiota-targeted interventions, probiotics have garnered increasing attention due to their polypharmacological potential, with Lactiplantibacillus plantarum (LP) emerging as a particularly promising candidate (20). This commensal strain, originally isolated from healthy human intestinal microbiota, demonstrates pronounced metabolic-modulating and anti-inflammatory properties (21). Our preliminary investigations with Lactiplantibacillus plantarum NKK20 (LP) revealed its dual capacity to enhance intestinal butyrate production and remodel bile acid metabolism through the farnesoid X receptor (FXR)-dependent pathway, effectively attenuating hyperactivation of the NF-κB/MAPK signaling cascade (21, 22). These mechanistic underpinnings correlate with its demonstrated therapeutic efficacy in preclinical models of metabolic disorders, including non-alcoholic fatty liver disease and diabetic kidney disease (DKD).
Extending beyond these findings, subsequent research has elucidated that LP exerts additional protective effects by (i) activating the Nrf2/HO-1 antioxidant axis to mitigate oxidative damage and (ii) reciprocally modulating gut microbial composition—selectively enriching beneficial taxa (e.g., Akkermansia) while suppressing potential pathobionts like Proteobacteria—thereby reinforcing intestinal barrier integrity (23–25). Crucially, these multimodal actions exhibit striking convergence with core PCOS pathophysiological hallmarks, including insulin resistance and low-grade inflammation (26). We therefore postulate that LP may orchestrate therapeutic effects in PCOS through integrated modulation of the “gut microbiota-metabolite-immune” axis, a hypothesis warranting systematic experimental validation.
Employing an integrative multi-omics approach, this investigation systematically evaluated the therapeutic efficacy and mechanistic insights of LP (L. plantarum NKK20) in a well-characterized PCOS murine model. State-of-the-art high-resolution metabolomics enabled comprehensive profiling of dynamic serum metabolite alterations, while 16S rRNA gene analysis provided multi-dimensional assessments of gut microbiota restructuring through α- and β-diversity metrics, with particular emphasis on phylogenetic shifts at the phylum and genus levels. The experimental design was strategically devised to delineate LP-mediated restoration of gut microbial dysbiosis in PCOS while elucidating its therapeutic modulation of core pathological features via the “microbiota-metabolite-ovary” axis. These findings are anticipated to establish a robust conceptual framework and preclinical evidence base for developing precision microbiota-targeted interventions against PCOS.
Materials and methods
Animal model establishment
Female C57BL/6 mice (8 weeks old) were obtained from Phenok Biosciences (Shanghai, China; Production license No.: SCXK(Hu)2023–0008) and housed in specific pathogen-free (SPF) conditions at the Laboratory Animal Center of Jiangsu University under controlled environmental parameters (22 ± 2 °C, 50–60% humidity, 12/12 h light/dark cycle) with ad libitum access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Jiangsu University (Approval No.: UJS-IACUC-AP-2024030032). The strain L. plantarum NKK20 was provided by the Zhenjiang Tianyi Biotechnology Research Institute (Zhenjiang, China). The animals were randomly assigned to three experimental groups (n = 6/group): normal control (NC), polycystic ovary syndrome (PCOS), and L. plantarum NKK20-treated (LP) groups. While NC group received standard chow, both PCOS and LP groups were maintained on a 60% high-fat diet for 4 weeks to induce metabolic dysfunction (27), followed by a 21-day oral administration of letrozole (1 mg/kg/day, Sigma-Aldrich; suspended in 0.5% carboxmethylcellulose sodium solution) to establish PCOS phenotype (28). Successful model induction was verified through daily vaginal cytology showing prolonged cornification (>10 consecutive days) with disrupted estrous cyclicity alongside hallmark endocrine alterations. Serological analyses confirmed that before the intervention, model mice exhibited significantly elevated serum testosterone (T) levels, and an increased luteinizing hormone to follicle-stimulating hormone (LH/FSH) ratio, compared to the control group fed a regular diet (P<0.05). These parameters collectively confirm the establishment of a PCOS-like hyperandrogenic and anovulatory phenotype prior to LP intervention.
The therapeutic intervention commenced concomitantly with high-fat feeding in the LP group, receiving daily probiotic supplementation (1 × 107 CFU L. plantarum NKK20 suspended in PBS). Upon completing the experimental period, euthanasia was performed via intraperitoneal injection of urethane (700 mg/kg, Sigma-Aldrich) following a 6-h fasting period to ensure optimal drug absorption. After injection, mice were individually placed in separate cages lined with soft bedding and monitored continuously for loss of vital signs, including the absence of righting reflex, cessation of heartbeat, and respiratory arrest. To ensure death and minimize potential distress, cervical dislocation was subsequently performed as an adjunctive method. Final confirmation of death was based on the absence of all vital signs, including no detectable heartbeat, irreversible cessation of respiration. All procedures were conducted in accordance with institutional animal welfare guidelines. Biological specimens including serum samples (for hormonal profiling and metabolomics), colonic contents (for 16S rRNA sequencing), and ovarian tissues were collected for subsequent analyses. Fixed ovarian and colonic specimens (4% paraformaldehyde, paraffin-embedded) were subjected to histological and immunohistochemical examinations.
Hormonal and inflammatory marker analyses
Serum concentrations of estradiol (E2), testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits (Meimian Biotechnology, Jiangsu, China) following the manufacturer’s protocols. For ovarian inflammatory markers, total RNA extraction was performed using RNA-easy Isolation Reagent (Vazyme, Nanjing, China), followed by first-strand cDNA synthesis with HiScript III Reverse Transcriptase (+gDNA wiper, Vazyme). Quantitative real-time PCR amplification was conducted on a Bio-Rad CFX96 system (Hercules, CA, USA) under standardized thermal cycling conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s with annealing/extension temperatures optimized for each primer set (sequences provided in Supplementary Table S1). Relative mRNA expression levels were normalized to housekeeping genes and calculated using the comparative threshold cycle (2−ΔΔCt) method.
Analysis of intestinal barrier integrity
Serum endotoxin levels were measured using a chromogenic limulus amebocyte lysate (LAL) assay (Xiamen Bioendo Technology, China; Cat# H245-1). After diluting serum samples 1:10 in endotoxin-free water, thermal pretreatment was performed at 70 °C for 10 min to deactivate interfering substances, followed by centrifugation (Beckman Coulter, Brea, CA, USA. 3,000 × g, 4 °C, 10 min) to obtain clear supernatants. A standard curve ranging from 0.01 to 1.0 EU/mL was prepared as per the manufacturer’s instructions. Subsequently, diluted samples or standards (50 μL) were mixed with an equal volume of LAL reagent in pyrogen-free tubes and incubated at 37 °C for 30 min. The reaction was terminated by adding 100 μL chromogenic substrate (p-nitroaniline), and absorbance was measured at 405 nm to determine endotoxin concentrations, and the mixture was incubated at 37 °C for an additional 6 min to allow for color development. Absorbance was then measured at 405 nm using a microplate reader (BioTek Synergy HT, Agilent, USA). Endotoxin concentrations in the samples were calculated by interpolating their absorbance values against the established standard curve. Intestinal tight junction proteins ZO-1 and Occludin were evaluated via immunohistochemistry (IHC). Colon tissues were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned (4 μm). Following deparaffinization and rehydration, antigen retrieval was conducted in sodium citrate buffer (pH 6.0, 95 °C, 15 min). Endogenous peroxidase activity was quenched by 3% H₂O₂ treatment, followed by blocking with 5% BSA for 30 min at room temperature. Tissue sections were incubated with primary antibodies against ZO-1 (Proteintech, China; 1:200) or Occludin (Proteintech, 1:150) overnight at 4 °C, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime, China; 1:500) for 1 h at room temperature. Diaminobenzidine (DAB, Beyotime, China) was used for chromogenic development, and nuclei were counterstained with hematoxylin before mounting in neutral resin. Image intensity analysis was performed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA; Version 6.0) to quantify protein expression levels.
Gut microbiota 16S rRNA sequencing and bioinformatics analysis
Genomic DNA from murine colonic contents was isolated using a commercial extraction kit (TIANGEN, Beijing, China), followed by targeted amplification and sequencing of the 16S rRNA V3-V4 hypervariable region conducted by Wekemo Tech Group (Shenzhen, China). The raw sequencing data were processed through the cloud-based Wekemo Bioincloud platform (https://www.bioincloud.tech/) for comprehensive bioinformatic evaluation. Microbial community characteristics were assessed through multiple analytical approaches: α-diversity indices (Chao1, Shannon, and Simpson) quantified taxonomic richness and evenness; β-diversity patterns visualized via principal component analysis (PCA) revealed compositional clustering among experimental groups; and hierarchical clustering heatmaps illustrated differential abundance profiles of operational taxonomic units (OTUs) influenced by LP treatment.
Untargeted metabolomics profiling and short-chain fatty acid quantification
For metabolite extraction, 100 μL of serum samples were mixed with 400 μL of ice-cold methanol (chromatography grade, Sigma-Aldrich, St. Louis, MO, USA) containing 0.1% formic acid (chromatography grade, Sigma-Aldrich). The mixture was homogenized by vortexing for 30 s, followed by 10 min of ice-bath ultrasonication. After centrifugation at 12,000 × g for 15 min (4 °C), the supernatants were filtered through 0.22 μm membranes prior to LC–MS analysis performed by Wekemo Tech Group (Shenzhen, China). Metabolic profiling was conducted using an Agilent 1,290 Infinity II UHPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled with a 6,545 Q-TOF mass spectrometer (Agilent Technologies) equipped with an electrospray ionization (ESI) source. Separation was achieved on a HILIC column (2.1 × 100 mm, 1.7 μm) maintained at 40 °C with a 0.3 mL/min flow rate. The mobile phases consisted of aqueous 0.1% formic acid (A) and 0.1% formic acid in acetonitrile (B), employing the following gradient: 5–20% B from 0–2 min, 20–95% B through 2–10 min, 95% B for 10–12 min, with re-equilibration to initial conditions by 15 min. MS parameters included positive/negative ion switching mode, 300 °C drying gas temperature, 10 L/min nebulizer flow, 40 psi capillary pressure, and m/z 50–1,000 scan range with 20/40 eV stepped collision energy for MS/MS fragmentation. Raw LC–MS data were processed using Progenesis QI software (version 3.0; Waters Corporation, Milford, MA, USA) for feature extraction, retention time alignment, and intensity normalization. Metabolites were considered differentially abundant when meeting both VIP > 1 (from OPLS-DA models) and p < 0.05 (Student’s t-test). Compound identification was performed via mass accuracy (<5 ppm) and MS/MS spectral matching against HMDB (Human Metabolome Database, https://hmdb.ca), METLIN (https://metlin.scripps.edu), and LipidMaps databases (Lipid Maps Structure Database, https://www.lipidmaps.org), with critical identifications confirmed using authentic standards. Metabolic pathway enrichment analysis was executed through MetaboAnalyst 5.0 (https://www.metaboanalyst.ca) using KEGG (Kyoto Encyclopedia of Genes and Genomes; https://www.genome.jp/kegg/) reference pathways with FDR < 0.05 significance threshold.
The quantification of short-chain fatty acids (SCFAs) was performed in-house following the approach reported by He et al. (29), with modifications. Briefly, 100 μL of serum was combined with 300 μL of acetonitrile containing 1% formic acid, vortexed for 1 min, and centrifuged (12,000 rpm, 10 min, 4 °C). The supernatant was passed through a 0.22-μm PTFE membrane filter before injection. Chromatographic separation was achieved using an ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm) maintained at 40 °C. The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), delivered at a flow rate of 0.3 mL/min. The gradient elution protocol was as follows: initial 5% B (0 min), ramped to 30% B by 2 min, increased to 95% B at 4 min, held until 5 min, followed by immediate return to 5% B (5.1 min), and re-equilibration until 7 min. The injection volume was 2 μL. Mass spectrometric detection was performed in negative-ion electrospray ionization (ESI) mode with the following parameters: capillary voltage, 2.5 kV; ion source temperature, 150 °C; desolvation temperature, 500 °C; desolvation and cone gas flows, 1,000 and 150 L/h, respectively. Quantification was conducted in multiple reaction monitoring (MRM) mode. Authenticated standards for acetate, propionate, and butyrate (purity≥98%) were obtained from Sigma-Aldrich (USA).
Statistical analysis
Statistical evaluation was conducted using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation. For between-group comparisons, one-way analysis of variance (ANOVA) was applied when data met assumptions of normality and homogeneity of variance, with Tukey’s post-hoc test employed for multiple comparisons. A probability threshold of p < 0.05 was considered statistically significant. Data visualization was performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA) in combination with the Bioincloud online platform (https://bioincloud.tech/).
Results
Body weight, fasting blood glucose and lipid profiles in experimental mice
During the first week of high-fat diet (HFD) feeding, PCOS mice exhibited significantly higher body weight compared to NC animals (p < 0.05), with progressive weight gain observed throughout the study period. Letrozole administration did not alter this weight trajectory in PCOS mice. However, mice treated with LP (LP group) in combination with HFD showed attenuated weight gain relative to PCOS animals by the third week (p < 0.05), maintaining this suppression until study termination (Figure 1A). Terminal metabolic analyses revealed pronounced dyslipidemia in PCOS mice versus NC controls, as evidenced by elevated fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) concentrations, coupled with reduced high-density lipoprotein cholesterol (HDL-C) levels (p < 0.05). Conversely, LP intervention substantially mitigated these alterations, demonstrating lower FBG, TC, TG, and LDL-C alongside increased HDL-C compared to untreated PCOS mice (p < 0.05) (Figures 1B–E).

Figure 1. Body weight and serum biochemical parameters in experimental mice (n = 6). The figure panels illustrate: (A) Longitudinal body weight measurements, (B) fasting blood glucose (FBG), (C) total cholesterol (TC), (D) triglycerides (TG), (E) low-density lipoprotein cholesterol (LDL-C), and (F) high-density lipoprotein cholesterol (HDL-C). Statistical annotations indicate significant differences at p < 0.05 (a: versus NC group; b: versus PCOS group).
Serum sex hormone levels and ovarian inflammatory/damage markers in mice
Serum sex hormone levels, including estradiol (E2), testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), were quantified by ELISA, with the LH/FSH ratio subsequently calculated (Figures 2A–E). PCOS mice exhibited significantly elevated T, LH, and LH/FSH levels alongside reduced E2 and FSH concentrations compared to NC animals (p < 0.05). LP treatment effectively attenuated these hormonal imbalances, significantly lowering T, LH, and LH/FSH (p < 0.05), though E2 and FSH levels remained statistically unchanged relative to untreated PCOS mice (p > 0.05). Ovarian inflammatory cytokine expression was assessed via qRT-PCR (Figures 2F–J). PCOS animals displayed marked upregulation of proinflammatory mediators (TNF-α, IL-6, IL-1β, MCP-1) and suppression of the anti-inflammatory cytokine IL-10 versus NC controls (p < 0.05). LP administration mitigated this inflammatory response, significantly decreasing proinflammatory cytokine levels while restoring IL-10 expression (p < 0.05).

Figure 2. Serum sex hormone profiles and ovarian inflammatory markers in experimental mice (n = 6). The figure panels display: (A) Testosterone (T) concentrations, (B) estradiol (E2) levels, (C) follicle-stimulating hormone (FSH), (D) luteinizing hormone (LH), (E) LH/FSH ratio, (F) TNF-α/GAPDH, (G) IL-6/GAPDH, (H) IL-1β/GAPDH, (I) MCP-1/GAPDH, and (J) IL-10/GAPDH.
Ovarian histomorphology evaluated by H&E staining (Figure 3) revealed striking structural differences. NC ovaries contained numerous healthy follicles with distinct granulosa cell layering, minimal atresia or vacuolation, normally distributed corpora lutea, and an absence of stromal hyperplasia, fibrosis, or inflammatory infiltrates. PCOS mice exhibited prominent cystic follicles, dilated antral follicles, extensive follicular atresia, sparse corpora lutea, and pronounced stromal cell proliferation. LP intervention partially reversed these pathologies, reducing cystic follicle prevalence, attenuating follicular atresia, promoting nascent corpus luteum formation, and alleviating stromal hyperplasia and inflammatory infiltration.
LP ameliorates intestinal barrier dysfunction and systemic endotoxemia in PCOS mice
PCOS mice exhibited significantly elevated serum endotoxin levels compared to NC controls (p < 0.05), demonstrating that high-fat diet and letrozole induction compromised intestinal permeability and induced endotoxemia. LP administration substantially attenuated this effect, with treated animals showing markedly lower circulating endotoxins than untreated PCOS counterparts (p < 0.05), indicating enhanced intestinal barrier integrity (Figure 4A). Immunohistochemical analysis revealed continuous, sharply defined ZO-1 and occludin staining along colonic mucosal cell borders in NC animals. PCOS mice displayed significantly diminished expression of these tight junction proteins (p < 0.05), exhibiting discontinuous immunoreactivity patterns consistent with epithelial barrier disruption. While LP intervention partially restored ZO-1 and occludin localization compared to PCOS mice, expression levels remained below those observed in NC controls (p < 0.05) (Figures 4B,C).

Figure 4. Serum endotoxin levels and intestinal mucosal expression of ZO-1 and Occludin in mice (n = 3). (A) Serum endotoxin levels; (B) ZO-1 expression; (C) Occludin.
Influence of LP on gut microbiota dysbiosis and restoration of SCFAs production in PCOS mice
Microbial community profiling via 16S rRNA sequencing revealed no significant intergroup differences in α-diversity indices (Shannon, Simpson, and Chao1) among NC, PCOS, and LP-treated mice (Figures 5A–C). However, β-diversity analysis demonstrated distinct clustering of LP-treated samples separate from PCOS controls (Figure 5D), indicating partial microbial community restoration following LP intervention. Taxonomic evaluation identified significant increases in Actinobacteriota and Proteobacteria at the phylum level (Figure 5E), along with elevated Muribaculum and UBA7173 abundance at the genus level in LP-administered mice compared to PCOS counterparts (Figure 5F).
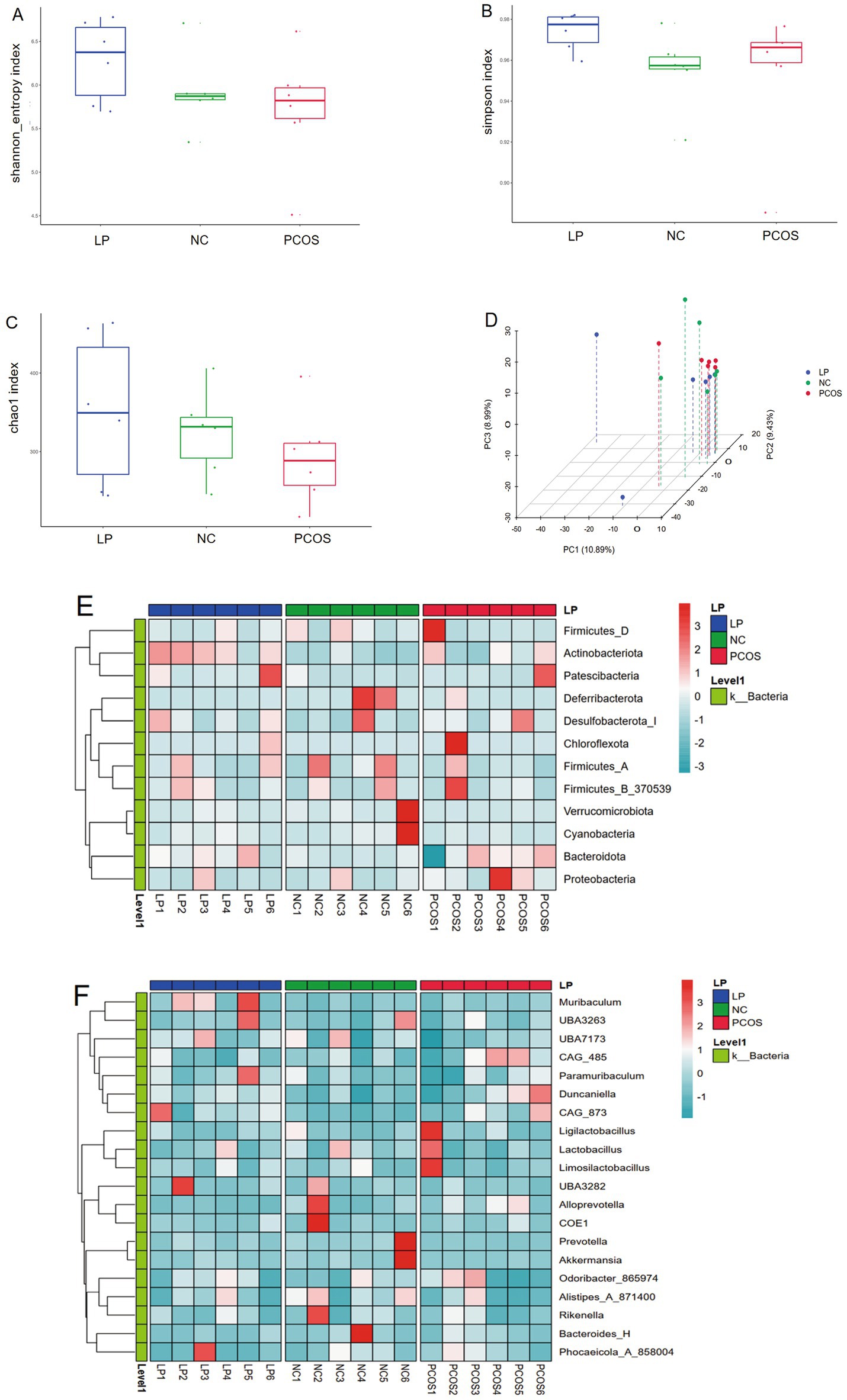
Figure 5. Gut microbiome profiling by 16S rRNA sequencing (n = 6). (A) Shannon diversity index, (B) Simpson diversity metrics, (C) Chao1 richness estimates, (D) Principal coordinate analysis of β-diversity, (E) Heatmap representation of phylum-level taxonomic composition, (F) Genus-level relative abundance heatmap.
Concurrent UPLC-MS/MS quantification of fecal SCFAs demonstrated that PCOS mice exhibited markedly reduced acetate, propionate, and butyrate levels relative to NC controls (p < 0.05), whereas LP treatment significantly upregulated all three SCFAs (p < 0.05), with particularly pronounced effects on butyrate recovery (Figure 6). These findings collectively suggest that the high-fat diet/letrozole-induced PCOS model induces both microbial dysbiosis and SCFA depletion, while LP administration effectively counteracts these metabolic disturbances.
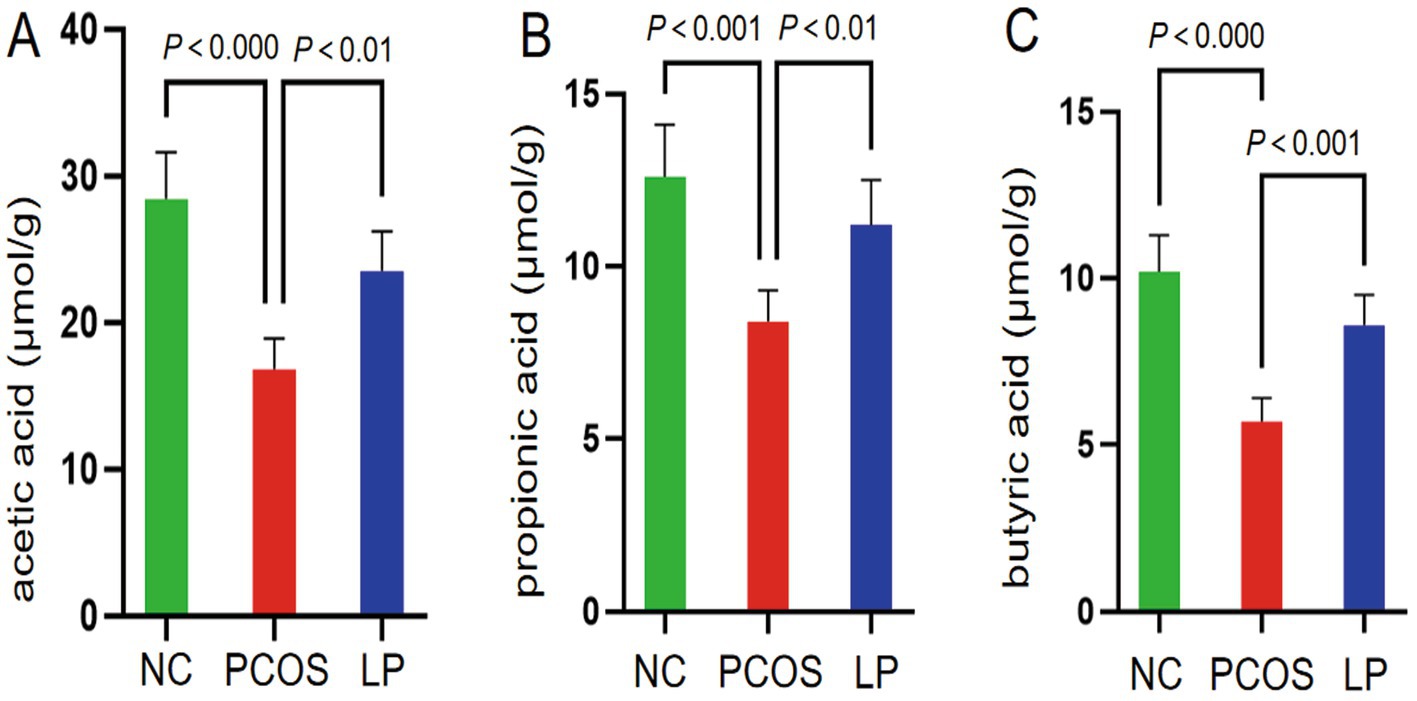
Figure 6. Quantitative analysis of short-chain fatty acid (SCFA) concentrations (μmol/g) in intestinal contents (n = 6). Panels illustrate measured levels of (A) acetic acid, (B) propionic acid, and (C) butyric acid.
Influence of LP on endogenous serum metabolites in PCOS mice
Untargeted serum metabolomics revealed distinct metabolic profiles among the NC, PCOS, and LP-treated groups. Unsupervised PCA analysis demonstrated clear model interpretability, with evident segregation of sample clusters in both ESI + and ESI- modes. Specifically, the PCOS group was distinctly separated from the NC group, confirming successful model establishment. Notably, the LP-treated group exhibited a metabolic profile that shifted away from the PCOS cluster and toward the NC group, visually indicating the restorative effect of LP treatment (Figure 6A). To enhance predictive capacity and identify key metabolites, we performed OPLS-DA modeling. The model showed clear group separation (Figure 6B) and was rigorously validated. Differential metabolites (VIP > 1, p < 0.05) identified by OPLS-DA were subsequently visualized in hierarchically-clustered heatmaps (Figures 6C,D), further detailing the metabolic alterations. LP administration upregulated lipid mediators including SM33:3;20/44:3, LysoPC 20:2, and oleoyl-L-α-lysophosphatidic acid while downregulating SM 8:1;20/38:2 series. Anti-inflammatory effects were evidenced by elevated 6α-prostaglandin 11 with concurrent reductions in Δ17-6-keto prostaglandin F1α and docosatrienoic acid. Hormonal modulation involved increased pyranone derivatives [(2S)-2-(2-hydroxypropan-2-yl)-2H,3H,7H-furo[3,2-g]chromen-7-one] alongside decreased hydrocortisone and 11-ketotestosterone (Figure 7).

Figure 7. Serum metabolomics profiling and differential metabolite analysis (n = 6). Multivariate statistical analyses display: (A) Principal component analysis (PCA) score plot demonstrating group separation, (B) orthogonal projections to latent structures discriminant analysis (OPLS-DA) model visualization, with subsequent hierarchical clustering of significantly altered metabolites shown in heatmaps for (C) ESI positive and (D) ESI negative ionization modes.
Influence of LP treatment on metabolic pathways in PCOS mice
Differential metabolites were subjected to pathway analysis using MetaboAnalyst 5.0, with visualization displaying pathway impact values (x-axis) versus negative log-transformed p-values (y-axis). The enrichment results demonstrated LP’s significant regulatory effects on multiple interconnected metabolic pathways in PCOS mice, particularly involving amino acid metabolism, glycerophospholipid biosynthesis, vitamin metabolism, and steroid hormone synthesis (Figure 8).

Figure 8. Metabolic pathway analysis of serum samples showing LP-mediated modulation in PCOS mice under (A) positive and (B) negative electrospray ionization modes (n = 6).
Discussion
This study systematically investigated the therapeutic potential and mechanistic basis of LP intervention in a PCOS murine model. The results revealed a significant effect of LP administration on the key pathological features of PCOS. Specifically, it was found that LP ameliorated metabolic dysfunction, corrected sex hormone imbalance, and alleviated ovarian pathological alterations. Furthermore, mechanistically, it was demonstrated that the therapeutic effects of LP were linked to its ability to restore gut microbiota homeostasis, enhance the production of short-chain fatty acids, and optimize the host’s systemic metabolic network.
The current study utilized L. plantarum NKK20 strain, isolated from healthy human fecal samples, which exhibits superior butyrogenic capability (21). Extensive evidence suggests that butyrate, as a pivotal gut microbial metabolite, orchestrates host metabolic regulation through two distinct pathways - modulating insulin sensitivity via GPR41/43 receptor activation while concurrently regulating epigenetic modifications through histone deacetylase (HDAC) inhibition (30–32). Our experimental data demonstrated that LP intervention not only elevated intestinal butyrate levels in PCOS mice but also coordinated the reduction of serum testosterone (T), luteinizing hormone (LH), and LH/FSH ratio, accompanied by significant downregulation of ovarian inflammatory mediators, revealing the compound’s potent immunomodulatory properties. Mechanistically, butyrate functions as a natural ligand for peroxisome proliferator-activated receptor γ (PPARγ) (33), suppressing NF-κB signaling pathway to attenuate ovarian expression of pro-inflammatory cytokines including TNF-α, IL-6, IL-1β and MCP-1 (34, 35), while simultaneously potentiating regulatory T cell (Treg) differentiation and IL-10 secretion - findings corroborated by our observation of significantly enhanced IL-10 expression in ovarian tissues following LP administration (36, 37). Furthermore, LP treatment upregulated intestinal tight junction proteins (ZO-1 and Occludin), reinforced gut barrier integrity, and reduced serum endotoxin levels (38, 39), collectively ameliorating PCOS-associated metabolic endotoxemia (40, 41). These comprehensive results delineate a “gut microbiota-butyrate-ovarian inflammation” regulatory cascade that fundamentally underpins LP’s therapeutic efficacy against hyperandrogenism in PCOS pathogenesis.
These findings, together with the present study, delineate a “gut microbiota-butyrate-ovarian inflammation” regulatory cascade that fundamentally underpins LP’s therapeutic efficacy against hyperandrogenism in PCOS pathogenesis. This proposed multi-tissue pathway integrates insights from existing research on gut-brain-ovary axis communication and metabolite-mediated immunomodulation, thereby providing a mechanistic framework that extends beyond isolated metabolic or hormonal corrections. The strength of this study lies in the systemic validation of this cascade, from microbial shift (increased butyrate) to molecular targets (PPARγ/NF-κB/Tregs) and finally to pathological improvement (hyperandrogenism, inflammation). However, the translational implications should be interpreted with consideration of its limitations. The study was conducted in a preclinical murine model, and although butyrate’s role in humans is supported by epidemiological and interventional studies (e.g., 42, 43), the therapeutic efficacy and safety of the specific NKK20 strain in human PCOS require further clinical validation. Additionally, while our data emphasize butyrate, other microbial metabolites or strain-specific effects could contribute to the observed outcomes, warranting future metabolomic and multi-omics investigations to fully disentangle the causal network. Lastly, the interplay between butyrate, other SCFAs, and host genetics in modulating PCOS phenotype remains an open question for personalized therapeutic strategies.
The metabolomics analysis revealed a particularly noteworthy negative correlation between butyrate levels and both serum testosterone concentrations and ovarian pro-inflammatory cytokines, suggesting butyrate may serve as the pivotal mediator linking gut microbiota alterations to core PCOS pathologies (18, 42). Previous reports of reduced butyrate-producing bacteria in PCOS patients were supported by our observation that LP intervention significantly increased the abundance of SCFA-producing genera like Muribaculum (43–45). However, while LP treatment robustly elevated intestinal SCFA levels (particularly butyrate), 16S rRNA sequencing demonstrated statistically insignificant changes in other established SCFA producers (Faecalibacterium, Roseburia, Eubacterium) (46, 47), likely reflecting inherent methodological constraints: although powerful for microbial profiling, 16S rRNA sequencing offers limited taxonomic resolution and cannot reliably determine functional metabolic activity (48, 49). Additional technical limitations including PCR amplification bias and sequencing depth may further obscure detection of low-abundance SCFA producers (50, 51). These considerations warrant future investigations employing metagenomic sequencing to achieve higher-resolution functional characterization of microbial communities (52, 53).
Untargeted serum metabolomics analysis demonstrated that LP administration profoundly modulated metabolic profiles of polyunsaturated fatty acids and sphingolipids, with elevated concentrations of specific lysophosphatidylcholine (LPC) species, including LysoPC 20:2 - an arachidonic acid-derived metabolite previously associated with improved insulin sensitivity (54, 55). This bioactive lipid mediates anti-inflammatory effects through PPARγ-mediated suppression of NF-κB signaling, mirroring our observation of elevated LPC O-15:0 and LPC O-18:1 (56–58). The former acts as a key precursor for plasmalogen biosynthesis (endogenous antioxidants) (59, 60), while the latter, an ether-linked phospholipid, contributes to membrane repair and oxidative stress mitigation (61, 62). We postulate that LP-induced butyrate enrichment triggers PPARα activation to upregulate ether lipid synthases (FAR1, AGPS), thereby enhancing production of these cytoprotective lipids (63, 64). Furthermore, LP-mediated elevation of intestinal short-chain fatty acids provides propionate as a potential substrate for LPC 15:0-SN1 biosynthesis, establishing a plausible microbial-lipid axis underlying metabolic improvement in PCOS (65, 66).
Therapeutic administration of LP directly modulated androgen homeostasis by altering key intermediates in steroidogenesis, most notably suppressing the generation of 11-ketotestosterone (11-KT) – a potent bioactive androgen in rodents that mirrors clinical hyperandrogenemia in PCOS patients (67–69). This reduction likely occurs through multiple coordinated mechanisms: (1) enzymatic inhibition of 11β-hydroxysteroid dehydrogenase (11β-HSD1) activity, curtailing the conversion of testosterone to 11-KT (70); (2) upregulated expression of hepatic 3α/β-hydroxysteroid dehydrogenases (3α/β-HSD) that catalyze the inactivation and subsequent excretion of testosterone and 11-KT into androstenedione and etiocholanolone (71, 72); (3) improvement of systemic insulin sensitivity in adipose tissue, thereby reducing insulin resistance-driven overexpression of CYP17A1 and subsequent testosterone overproduction (73); and (4) attenuation of metabolic endotoxemia by restoring gut barrier integrity, consequently decreasing LPS translocation and TLR4/NF-κB-mediated ovarian inflammation that promotes local androgen synthesis (74).
Notably, LP intervention significantly attenuated serum hydrocortisone levels in PCOS mice—a phenomenon potentially attributable to the coordinated modulation of hypothalamic–pituitary–adrenal (HPA) axis hyperactivity commonly observed in insulin-resistant states (75). Elevated circulating cortisol in PCOS may result from hepatic 11β-HSD2 suppression (reducing cortisol-to-cortisone inactivation), chronic low-grade inflammation, and adrenal overactivation (76, 77). We propose that LP exerts multimodal hydrocortisone-lowering effects through: (1) gut-brain axis-mediated downregulation of hypothalamic corticotropin-releasing hormone (CRH), consequently decreasing ACTH-driven adrenal cortisol production; (2) enhanced systemic insulin sensitivity that restores hepatic 11β-HSD2 activity, facilitating cortisol inactivation while simultaneously mitigating visceral adipose tissue inflammation and associated local hydrocortisone synthesis; and (3) improved gut barrier function that reduces endotoxin-mediated TNF-α/IL-1β stimulation of the HPA axis, complemented by propionate-mediated GPR43 signaling that directly suppresses adrenal hydrocortisone hypersecretion.
At the inflammatory level, LP treatment elicited a marked suppression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) alongside augmented IL-10 expression in ovarian tissue—changes that correlated strongly with alterations in specific prostaglandin metabolites in systemic circulation. Particularly noteworthy was the modulation of 6a-PGI1, a 6α-hydroxylated derivative of the anti-inflammatory eicosanoid PGI₂ that arises during COX/PGIS-mediated enzymatic hydroxylation of arachidonic acid (AA) (78). This metabolite exerts pleiotropic benefits through NF-κB pathway inhibition, ultimately mitigating tissue inflammation while improving adipose insulin sensitivity (79). Mechanistically, LP may facilitate 6a-PGI1 biosynthesis via two complementary pathways: (1) secretion of PLA₂-activating factors that enhance AA liberation from intestinal epithelial membranes, thereby increasing PGI₂ precursor availability (80), and (2) NF-κB suppression by SCFAs that potentiates COX activity to further drive 6a-PGI1 production (81, 82). Concurrently, treatment significantly reduced levels of △17-6-keto PGF₁α—a pathogenic metabolite generated from PGF₂α, which contributes to ovarian fibrosis and insulin resistance—through SCFA-mediated NF-κB/PPARγ-dependent downregulation of COX (83, 84). These coordinated modulations suggest that LP’s immunoregulatory effects in PCOS operate via an integrated mechanism involving both direct SCFA-mediated immunomodulation (through dendritic cell/Treg regulation) and indirect prostaglandin pathway remodeling.
Notably, our findings reveal that LP supplementation significantly elevated circulating vitamin A levels in PCOS mice—a metabolic alteration with multifaceted therapeutic implications. Mechanistically, vitamin A exerts protective effects by attenuating ovarian ROS generation, thereby ameliorating oxidative stress and inflammatory damage, while concurrently suppressing testicular androgen biosynthesis through inhibition of CYP17A1 enzymatic activity, ultimately reducing serum free testosterone concentrations (85–87). These observations collectively suggest that LP may function as a vitamin A sensitizer, potentially explaining its efficacy in alleviating hallmark PCOS symptoms. The observed vitamin A deficiency typically associated with PCOS could originate from diet-induced chronic inflammation impairing intestinal absorption (88), a phenomenon potentially reversible through LP’s dual mechanism: (1) restoration of gut barrier integrity to minimize LPS translocation and subsequent systemic inflammation (89), and (2) direct enhancement of vitamin A bioavailability through microbial metabolites that facilitate enterocyte uptake (90).
LP intervention demonstrates superior therapeutic potential compared to current PCOS management strategies. While conventional approaches like metformin primarily target insulin resistance with minimal impact on gut microbiota modulation (91), and oral contraceptives effectively reduce hyperandrogenism at the expense of adverse metabolic effects (92), our study reveals that LP simultaneously addresses the triad of PCOS pathogenesis – metabolic dysfunction, hormonal imbalance, and chronic low-grade inflammation. This multi-pronged therapeutic efficacy highlights the unique advantage of microbial intervention through coordinated modulation of interconnected pathological pathways.
Several study limitations warrant consideration. The inherent biological discrepancies between rodent PCOS models and the heterogeneous clinical manifestations in human patients may affect translational relevance. Furthermore, although we observed significant alterations in SCFA profiles, the tissue-specific bioactivity of these microbial metabolites and their mechanistic association with discrete bacterial taxa require additional mechanistic validation. Dose–response relationships for LP intervention also remain to be systematically characterized. Future investigations should prioritize clinical translation, particularly examining strain colonization kinetics and interindividual variability in therapeutic responses among PCOS patients.
Conclusion
Our investigation provides comprehensive evidence that Lactiplantibacillus plantarum NKK20 (LP) confers significant protection against PCOS pathogenesis by systemically reprogramming the gut-microbiota-metabolite-immune axis. The therapeutic efficacy of LP was evidenced by the amelioration of hallmark PCOS features, including insulin resistance, dyslipidemia, hyperandrogenism (e.g., reduced testosterone and 11-ketotestosterone), and the restoration of ovarian folliculogenesis.
The mechanistic insights gleaned from this multi-omics study reveal that LP’s actions are multifaceted. First, it attenuated gut dysbiosis and significantly elevated levels of SCFAs, with butyrate emerging as a pivotal mediator. This butyrate surge was associated with the reinforcement of the intestinal barrier (upregulation of ZO-1 and Occludin), reduction in endotoxemia, and subsequent suppression of ovarian NF-κB-driven inflammation (TNF-α, IL-6, IL-1β). Second, serum metabolomics uncovered LP’s profound impact on host metabolism, including the upregulation of insulin-sensitizing lysophosphatidylcholines (e.g., LysoPC20:2) and anti-inflammatory lipid mediators like 6α-prostaglandin I1, while downregulating pro-fibrotic metabolites such as Δ17-6-ketoprostaglandin F1α. The modulation of steroidogenesis and vitamin A metabolism further illustrates the hormonal and metabolic refinements induced by LP.
These findings substantially advance the concept of the gut-ovary axis, moving from correlation to causal mechanism. They position LP not merely as a probiotic but as a potent regulator of a complex host-microbial metabolic network. While this preclinical model offers compelling evidence, translation to the heterogeneous human PCOS population requires further clinical validation. Future research should focus on dose optimization, individual response variability, and the precise delineation of strain-specific mechanisms. Ultimately, this work lays a solid foundation for microbiota-directed interventions, positioning LP supplementation as a promising adjunctive strategy in the multidimensional management of PCOS.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://ngdc.cncb.ac.cn/gsa/browse/CRA036093.
Ethics statement
The animal studies were approved by the Ethics Committee of the Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. XiL: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. WS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. XD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XuL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. AA: Formal analysis, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. KS: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by National Natural Science Foundation of China (Grant No. 32270964); Jiangsu Provincial Social Development Project (Grant No. BE2022779); and Scientific Research Project of Yancheng Municipal Health Commission (YK2024116).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1709581/full#supplementary-material
References
1. Xu, Y, and Qiao, J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. (2022) 2022:9240569. doi: 10.1155/2022/9240569,
2. Rudnicka, E, Suchta, K, Grymowicz, M, Calik-Ksepka, A, Smolarczyk, K, Duszewska, AM, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. (2021) 22:22. doi: 10.3390/ijms22073789,
3. Su, P, Chen, C, and Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J Ovarian Res. (2025) 18:34. doi: 10.1186/s13048-025-01621-6,
4. Han, Y, Wu, H, Sun, S, Zhao, R, Deng, Y, Zeng, S, et al. Effect of high fat diet on disease development of polycystic ovary syndrome and lifestyle intervention strategies. Nutrients. (2023) 15:15. doi: 10.3390/nu15092230,
5. Ricketts, T. General satisfaction and satisfaction with nursing communication on an adult psychiatric ward. J Adv Nurs. (1996) 24:479–87. doi: 10.1046/j.1365-2648.1996.02157.x,
6. Li, P, Shuai, P, Shen, S, Zheng, H, Sun, P, Zhang, R, et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med. (2023) 21:302. doi: 10.1186/s12916-023-02975-8,
7. Babu, A, Devi Rajeswari, V, Ganesh, V, Das, S, Dhanasekaran, S, Usha Rani, G, et al. Gut microbiome and polycystic ovary syndrome: interplay of associated microbial-metabolite pathways and therapeutic strategies. Reprod Sci. (2024) 31:1508–20. doi: 10.1007/s43032-023-01450-2,
8. Liu, M, Peng, R, Tian, C, Shi, J, Ma, J, Shi, R, et al. Effects of the gut microbiota and its metabolite short-chain fatty acids on endometriosis. Front Cell Infect Microbiol. (2024) 14:1373004. doi: 10.3389/fcimb.2024.1373004,
9. Tong, TT, Bai, LB, Yau, LF, Li, JY, Huang, H, and Jiang, ZH. Pharmacological effects of bile acids on polycystic ovary syndrome via the regulation of chemerin. Chin Med. (2025) 20:45. doi: 10.1186/s13020-025-01078-1,
10. Li, M, Zhang, L, Li, X, and Zhao, Y. Impact of short-term ketogenic diet on sex hormones and glucose-lipid metabolism in overweight or obese patients with polycystic ovary syndrome. J Obstet Gynaecol Res. (2025) 51:e16178. doi: 10.1111/jog.16178,
11. Melin, J, Forslund, M, Alesi, S, Piltonen, T, Romualdi, D, Spritzer, PM, et al. Metformin and combined oral contraceptive pills in the management of polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2024) 109:e817–817e836. doi: 10.1210/clinem/dgad465
12. Singh, V, Mahra, K, Jung, D, and Shin, JH. Gut microbes in polycystic ovary syndrome and associated comorbidities; type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and the potential of microbial therapeutics. Probiotics Antimicrob Proteins. (2024) 16:1744–61. doi: 10.1007/s12602-024-10262-y,
13. He, F, and Li, Y. The gut microbial composition in polycystic ovary syndrome with insulin resistance: findings from a normal-weight population. J Ovarian Res. (2021) 14:50. doi: 10.1186/s13048-021-00799-9,
14. da Silva, TR, Marchesan, LB, Rampelotto, PH, Longo, L, de Oliveira, TF, Landberg, R, et al. Gut microbiota and gut-derived metabolites are altered and associated with dietary intake in women with polycystic ovary syndrome. J Ovarian Res. (2024) 17:232. doi: 10.1186/s13048-024-01550-w,
15. Senthilkumar, H, and Arumugam, M. Gut microbiota: a hidden player in polycystic ovary syndrome. J Transl Med. (2025) 23:443. doi: 10.1186/s12967-025-06315-7,
16. Huang, F, Deng, Y, Zhou, M, Tang, R, Zhang, P, and Chen, R. Fecal microbiota transplantation from patients with polycystic ovary syndrome induces metabolic disorders and ovarian dysfunction in germ-free mice. BMC Microbiol. (2024) 24:364. doi: 10.1186/s12866-024-03513-z,
17. Li, G, Lin, J, Zhang, C, Gao, H, Lu, H, Gao, X, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. (2021) 13:1968257. doi: 10.1080/19490976.2021.1968257,
18. Kim, N, and Yang, C. Butyrate as a potential modulator in gynecological disease progression. Nutrients. (2024) 16:4196. doi: 10.3390/nu16234196,
19. He, Y, Wang, Q, Li, X, Wang, G, Zhao, J, Zhang, H, et al. Correction: lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. (2021) 12:4720–1. doi: 10.1039/d1fo90033a,
20. Aljohani, A, Rashwan, N, Vasani, S, Alkhawashki, A, Wu, TT, Lu, X, et al. The health benefits of probiotic lactiplantibacillus plantarum: a systematic review and meta-analysis. Probiotics Antimicrob Proteins. (2024) 17:3358–3377. doi: 10.1007/s12602-024-10287-3,
21. Sun, C, Qiu, C, Zhang, Y, Yan, M, Tan, J, He, J, et al. Lactiplantibacillus plantarum NKK20 alleviates high-fat-diet-induced nonalcoholic fatty liver disease in mice through regulating bile acid anabolism. Molecules. (2023) 28:4042. doi: 10.3390/molecules28104042,
22. Sun, X, Xi, Y, Yan, M, Sun, C, Tang, J, Dong, X, et al. Lactiplantibacillus plantarum NKK20 increases intestinal butyrate production and inhibits type 2 diabetic kidney injury through PI3K/Akt pathway. J Diabetes Res. (2023) 2023:8810106. doi: 10.1155/2023/8810106,
23. Cha, SY, Woo, IK, Cha, YJ, Lee, NK, Jang, HJ, and Paik, HD. Anti-melanogenic and antioxidant activities of Lactiplantibacillus plantarum strains in skin cells via the CREB/MITF and Nrf2/HO-1 pathways. Probiotics Antimicrob Proteins. (2025) 17:3795–810. doi: 10.1007/s12602-025-10671-7,
24. He, Q, Huang, J, Zheng, T, Lin, D, Zhang, H, Li, J, et al. Treatment with mixed probiotics induced, enhanced and diversified modulation of the gut microbiome of healthy rats. FEMS Microbiol Ecol. (2021) 97:97. doi: 10.1093/femsec/fiab151,
25. Allam-Ndoul, B, Pulido-Mateos, EC, Bégin, F, St-Arnaud, G, Tinoco Mar, BA, Mayer, T, et al. Lactiplantibacillus plantarum strengthens the intestinal barrier: involvement of the endocannabinoidome. Am J Physiol Gastrointest Liver Physiol. (2025) 329:G245–245G260. doi: 10.1152/ajpgi.00142.2024
26. Cai, X, Huang, J, Yu, T, Guan, X, Sun, M, Zhao, D, et al. Lactiplantibacillus plantarum BXM2 treatment alleviates disorders induced by a high-fat diet in mice by improving intestinal health and modulating the gut microbiota. Nutrients. (2025) 17:17. doi: 10.3390/nu17030407,
27. Sparks, LM, Xie, H, Koza, RA, Mynatt, R, Hulver, MW, Bray, GA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. (2005) 54:1926–33. doi: 10.2337/diabetes.54.7.1926,
28. Kafali, H, Iriadam, M, Ozardali, I, and Demir, N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. (2004) 35:103–8. doi: 10.1016/j.arcmed.2003.10.005,
29. He, J, Li, X, Yan, M, Chen, X, Sun, C, Tan, J, et al. Inulin reduces kidney damage in type 2 diabetic mice by decreasing inflammation and serum metabolomics. J Diabetes Res. (2024) 2024:1222395. doi: 10.1155/2024/1222395,
30. Zheng, M, Yang, X, Wu, Q, Gong, Y, Pang, N, Ge, X, et al. Butyrate attenuates hepatic steatosis induced by a high-fat and fiber-deficient diet via the hepatic GPR41/43-CaMKII/HDAC1-CREB pathway. Mol Nutr Food Res. (2023) 67:e2200597. doi: 10.1002/mnfr.202200597,
31. Pedersen, SS, Ingerslev, LR, Olsen, M, Prause, M, and Billestrup, N. Butyrate functions as a histone deacetylase inhibitor to protect pancreatic beta cells from IL-1β-induced dysfunction. FEBS J. (2024) 291:566–83. doi: 10.1111/febs.17005,
32. Kibbie, JJ, Dillon, SM, Thompson, TA, Purba, CM, McCarter, MD, and Wilson, CC. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology. (2021) 226:152126. doi: 10.1016/j.imbio.2021.152126,
33. Wen, S, He, L, Zhong, Z, Zhao, R, Weng, S, Mi, H, et al. Stigmasterol restores the balance of Treg/Th17 cells by activating the butyrate-PPARγ axis in colitis. Front Immunol. (2021) 12:741934. doi: 10.3389/fimmu.2021.741934,
34. Marion-Letellier, R, Déchelotte, P, Iacucci, M, and Ghosh, S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. (2009) 58:586–93. doi: 10.1136/gut.2008.162859,
35. Sun, B, Jia, Y, Hong, J, Sun, Q, Gao, S, Hu, Y, et al. Sodium butyrate ameliorates high-fat-diet-induced non-alcoholic fatty liver disease through peroxisome proliferator-activated receptor α-mediated activation of β oxidation and suppression of inflammation. J Agric Food Chem. (2018) 66:7633–42. doi: 10.1021/acs.jafc.8b01189,
36. Hu, M, Alashkar Alhamwe, B, Santner-Nanan, B, Miethe, S, Harb, H, Renz, H, et al. Short-chain fatty acids augment differentiation and function of human induced regulatory T cells. Int J Mol Sci. (2022) 23:23. doi: 10.3390/ijms23105740,
37. Shi, Z, Li, M, Zhang, C, Li, H, Zhang, Y, Zhang, L, et al. Butyrate-producing Faecalibacterium prausnitzii suppresses natural killer/T-cell lymphoma by dampening the JAK-STAT pathway. Gut. (2025) 74:557–70. doi: 10.1136/gutjnl-2024-333530,
38. Nascimento, JC, Matheus, VA, Oliveira, RB, Tada, S, and Collares-Buzato, CB. High-fat diet induces disruption of the tight junction-mediated paracellular barrier in the proximal small intestine before the onset of type 2 diabetes and endotoxemia. Dig Dis Sci. (2021) 66:3359–74. doi: 10.1007/s10620-020-06664-x,
39. Zheng, J, Ahmad, AA, Yang, Y, Liang, Z, Shen, W, Feng, M, et al. Lactobacillus rhamnosus CY12 enhances intestinal barrier function by regulating tight junction protein expression, oxidative stress, and inflammation response in lipopolysaccharide-induced Caco-2 cells. Int J Mol Sci. (2022) 23:23. doi: 10.3390/ijms231911162,
40. Rose, EC, Odle, J, Blikslager, AT, and Ziegler, AL. Probiotics, prebiotics and epithelial tight junctions: a promising approach to modulate intestinal barrier function. Int J Mol Sci. (2021) 22:22. doi: 10.3390/ijms22136729,
41. Yang, Q, Wan, Q, and Wang, Z. Curcumin mitigates polycystic ovary syndrome in mice by suppressing TLR4/MyD88/NF-κB signaling pathway activation and reducing intestinal mucosal permeability. Sci Rep. (2024) 14:29848. doi: 10.1038/s41598-024-81034-5,
42. Liu, C, Fan, P, Dai, J, Ding, Z, Yi, Y, Zhan, X, et al. Integrated microbiome and metabolome analysis reveals that zishen qingre lishi huayu recipe regulates gut microbiota and butyrate metabolism to ameliorate polycystic ovary syndrome. Microb Pathog. (2025) 204:107533. doi: 10.1016/j.micpath.2025.107533,
43. He, Y, Shi, L, Qi, Y, Wang, Q, Zhao, J, Zhang, H, et al. Butylated starch alleviates polycystic ovary syndrome by stimulating the secretion of peptide tyrosine-tyrosine and regulating faecal microbiota. Carbohydr Polym. (2022) 287:119304. doi: 10.1016/j.carbpol.2022.119304,
44. Salehi, S, Allahverdy, J, Pourjafar, H, Sarabandi, K, and Jafari, SM. Gut microbiota and polycystic ovary syndrome (PCOS): understanding the pathogenesis and the role of probiotics as a therapeutic strategy. Probiotics Antimicrob Proteins. (2024) 16:1553–65. doi: 10.1007/s12602-024-10223-5,
45. Song, Y, Shen, H, Liu, T, Pan, B, De Alwis, S, Zhang, W, et al. Effects of three different mannans on obesity and gut microbiota in high-fat diet-fed C57BL/6J mice. Food Funct. (2021) 12:4606–20. doi: 10.1039/d0fo03331f,
46. Fusco, W, Lorenzo, MB, Cintoni, M, Porcari, S, Rinninella, E, Kaitsas, F, et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. (2023) 15:2211. doi: 10.3390/nu15092211,
47. Ghosh, S, and Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch Microbiol. (2021) 203:5281–308. doi: 10.1007/s00203-021-02516-y,
48. Matchado, MS, Rühlemann, M, Reitmeier, S, Kacprowski, T, Frost, F, Haller, D, et al. On the limits of 16S rRNA gene-based metagenome prediction and functional profiling. Microb Genom. (2024) 10:10. doi: 10.1099/mgen.0.001203,
49. Zheng, M, Wen, L, He, C, Chen, X, Si, L, Li, H, et al. Sequencing-guided re-estimation and promotion of cultivability for environmental bacteria. Nat Commun. (2024) 15:9051. doi: 10.1038/s41467-024-53446-4,
50. Shirazi, S, Meyer, RS, and Shapiro, B. Revisiting the effect of PCR replication and sequencing depth on biodiversity metrics in environmental DNA metabarcoding. Ecol Evol. (2021) 11:15766–79. doi: 10.1002/ece3.8239,
51. Shi, H, Zhou, Y, Jia, E, Pan, M, Bai, Y, and Ge, Q. Bias in RNA-seq library preparation: current challenges and solutions. Biomed Res Int. (2021) 2021:6647597. doi: 10.1155/2021/6647597,
52. Purushothaman, S, Meola, M, and Egli, A. Combination of whole genome sequencing and metagenomics for microbiological diagnostics. Int J Mol Sci. (2022) 23:23. doi: 10.3390/ijms23179834,
53. Han, Y, He, J, Li, M, Peng, Y, Jiang, H, Zhao, J, et al. Unlocking the potential of metagenomics with the PacBio high-fidelity sequencing technology. Microorganisms. (2024) 12:12. doi: 10.3390/microorganisms12122482,
54. Zhong, Z, Hu, Z, Zhou, W, Qin, X, and Tan, S. The bone marrow lipidomics of mice reveal sex-related differences. Biomed Chromatogr. (2024) 38:e5875. doi: 10.1002/bmc.5875,
55. Duchez, AC, Fauteux-Daniel, S, Sut, C, Ebermeyer, T, Heestermans, M, Arthaud, CA, et al. Bioactive lipids as biomarkers of adverse reactions associated with apheresis platelet concentrate transfusion. Front Immunol. (2023) 14:1031968. doi: 10.3389/fimmu.2023.1031968,
56. An, B, Shin, CH, Kwon, JW, Tran, NL, Kim, AH, Jeong, H, et al. M1 macrophage-derived exosomal microRNA-29c-3p suppresses aggressiveness of melanoma cells via ENPP2. Cancer Cell Int. (2024) 24:325. doi: 10.1186/s12935-024-03512-0,
57. Mohapatra, A, Amarjeet, S, Pancholi, B, Babu, R, Abate, SK, and Garabadu, D. Lysophosphatidylcholine induces ceramide synthase-2 expression in primary culture model of astrocytopathy: potential therapeutic target in multiple sclerosis. Mol Biol Rep. (2025) 52:166. doi: 10.1007/s11033-025-10252-5
58. Wang, X, Xie, Z, Zhang, J, Chen, Y, Li, Q, Yang, Q, et al. Interaction between lipid metabolism and macrophage polarization in atherosclerosis. iScience. (2025) 28:112168. doi: 10.1016/j.isci.2025.112168,
59. Takić, M, Jovanović, V, Marković, S, Miladinović, Z, Jadranin, M, Krstić, G, et al. Plasma and serum LC-MS lipidomic fingerprints of bipolar disorder and schizophrenia. Int J Mol Sci. (2025) 26:26. doi: 10.3390/ijms26136134,
60. Feng, X, Li, J, Zhang, L, Rao, Z, Feng, S, Wang, Y, et al. Integrated lipidomic and metabolomics analysis revealing the effects of frozen storage duration on pork lipids. Meta. (2022) 12:12. doi: 10.3390/metabo12100977,
61. Mishra, S, Kell, P, Scherrer, D, Dietzen, DJ, Vite, CH, Berry-Kravis, E, et al. Accumulation of alkyl-lysophosphatidylcholines in Niemann-pick disease type C1. J Lipid Res. (2024) 65:100600. doi: 10.1016/j.jlr.2024.100600,
62. Kasperova, BJ, Mraz, M, Svoboda, P, Hlavacek, D, Kratochvilova, H, Modos, I, et al. Sodium-glucose cotransporter 2 inhibitors induce anti-inflammatory and anti-ferroptotic shift in epicardial adipose tissue of subjects with severe heart failure. Cardiovasc Diabetol. (2024) 23:223. doi: 10.1186/s12933-024-02298-9,
63. Ferdinandusse, S, McWalter, K, Te Brinke, H, IJlst, L, Mooijer, PM, Ruiter, J, et al. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet Med. (2021) 23:740–50. doi: 10.1038/s41436-020-01027-3,
64. Papin, M, Fontaine, D, Goupille, C, Figiel, S, Domingo, I, Pinault, M, et al. Endogenous ether lipids differentially promote tumor aggressiveness by regulating the SK3 channel. J Lipid Res. (2024) 65:100544. doi: 10.1016/j.jlr.2024.100544,
65. Liu, T, Zhang, M, Xie, Q, Gu, J, Zeng, S, and Huang, D. Unveiling the antiobesity mechanism of sweet potato extract by microbiome, transcriptome, and metabolome analyses in mice. J Agric Food Chem. (2025) 73:7807–21. doi: 10.1021/acs.jafc.4c13173,
66. Gouda, H, Ji, Y, Rath, S, Watkins, D, Rosenblatt, D, Mootha, V, et al. Differential utilization of vitamin B12-dependent and independent pathways for propionate metabolism across human cells. J Biol Chem. (2024) 300:107662. doi: 10.1016/j.jbc.2024.107662,
67. Handelsman, DJ, Cooper, ER, and Heather, AK. Bioactivity of 11 keto and hydroxy androgens in yeast and mammalian host cells. J Steroid Biochem Mol Biol. (2022) 218:106049. doi: 10.1016/j.jsbmb.2021.106049,
68. Dahmani, C, Caron, P, Simonyan, D, Lacombe, L, Aprikian, A, Saad, F, et al. High 11-Ketotestosterone linked to shorter time to castration resistance in recurrent nonmetastatic prostate cancer. J Urol. (2025) 213:283–94. doi: 10.1097/JU.0000000000004333,
69. Storbeck, KH, and O'Reilly, MW. The clinical and biochemical significance of 11-oxygenated androgens in human health and disease. Eur J Endocrinol. (2023) 188:R98–98R109. doi: 10.1093/ejendo/lvad047,
70. Fujisawa, Y, Sano, S, Murai, Y, Sakaguchi, K, Masunaga, Y, Kinjo, K, et al. Production of 11-ketotestosterone in childhood adrenal tumors with virilization or peripheral precocious puberty: dominant expression of 11β-hydroxysteroid dehydrogenase type 2. J Steroid Biochem Mol Biol. (2025) 251:106747. doi: 10.1016/j.jsbmb.2025.106747,
71. Yazawa, T, Sato, T, Nemoto, T, Nagata, S, Imamichi, Y, Kitano, T, et al. 11-Ketotestosterone is a major androgen produced in porcine adrenal glands and testes. J Steroid Biochem Mol Biol. (2021) 210:105847. doi: 10.1016/j.jsbmb.2021.105847,
72. Piper, T, Fußhöller, G, and Thevis, M. Employing 11-Ketotestosterone as a target analyte for adrenosterone (11OXO) administration in doping controls. Meta. (2024) 14:14. doi: 10.3390/metabo14030141,
73. Walzer, D, Turcu, AF, Jha, S, Abel, BS, Auchus, RJ, Merke, DP, et al. Excess 11-oxygenated androgens in women with severe insulin resistance are mediated by adrenal insulin receptor signaling. J Clin Endocrinol Metab. (2022) 107:2626–35. doi: 10.1210/clinem/dgac365,
74. Mukherjee, AG, Wanjari, UR, Kannampuzha, S, Murali, R, Namachivayam, A, Ganesan, R, et al. The implication of mechanistic approaches and the role of the microbiome in polycystic ovary syndrome (PCOS): a review. Meta. (2023) 13:13. doi: 10.3390/metabo13010129,
75. Guo, X, Zhang, D, Pang, H, Wang, Z, Gao, L, Wang, Y, et al. Safety of withholding perioperative hydrocortisone for patients with pituitary adenomas with an intact hypothalamus-pituitary-adrenal axis: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2242221. doi: 10.1001/jamanetworkopen.2022.42221,
76. Dobre, MZ, Virgolici, B, and Cioarcă-Nedelcu, R. Lipid hormones at the intersection of metabolic imbalances and endocrine disorders. Curr Issues Mol Biol. (2025) 47:47. doi: 10.3390/cimb47070565,
77. Cao, J, Wang, Y, Wang, S, Shen, Y, Li, W, Wei, Z, et al. Expression of key steroidogenic enzymes in human placenta and associated adverse pregnancy outcomes. Matern Fetal Med. (2023) 5:163–72. doi: 10.1097/FM9.0000000000000167,
78. Dai, C, and Khalil, RA. Calcium signaling dynamics in vascular cells and their dysregulation in vascular disease. Biomolecules. (2025) 15:15. doi: 10.3390/biom15060892,
79. Sokołowska, P, Bleibel, L, Owczarek, J, and Wiktorowska-Owczarek, A. PPARγ, NF-κB and the UPR pathway as new molecular targets in the anti-inflammatory actions of NSAIDs: novel applications in cancers and central nervous system diseases. Adv Clin Exp Med. (2024) 33:1007–22. doi: 10.17219/acem/174243,
80. Chaiyabutr, N, Vasaruchapong, T, Laoungbua, P, Khow, O, Chanhome, L, and Sitprija, V. Phospholipase A2 from Daboia siamensis venom induces acute kidney injury: involvement of ion channels in an isolated perfused rabbit kidney model. J Venom Anim Toxins Incl Trop Dis. (2025) 31:e20250016. doi: 10.1590/1678-9199-JVATITD-2025-0016,
81. Yang, Q, Burkardt, AC, Sunkara, LT, Xiao, K, and Zhang, G. Natural cyclooxygenase-2 inhibitors synergize with butyrate to augment chicken host defense peptide gene expression. Front Immunol. (2022) 13:819222. doi: 10.3389/fimmu.2022.819222,
82. Tański, W, Świątoniowska-Lonc, N, Tabin, M, and Jankowska-Polańska, B. The relationship between fatty acids and the development, course and treatment of rheumatoid arthritis. Nutrients. (2022) 14:14. doi: 10.3390/nu14051030,
83. Davis, TL, Whitesell, JD, Cantlon, JD, Clay, CM, and Nett, TM. Does a nonclassical signaling mechanism underlie an increase of estradiol-mediated gonadotropin-releasing hormone receptor binding in ovine pituitary cells. Biol Reprod. (2011) 85:770–8. doi: 10.1095/biolreprod.111.091926,
84. Karmiol, S, Remick, DG, Kunkel, SL, and Phan, SH. Regulation of rat pulmonary endothelial cell interleukin-6 production by bleomycin: effects of cellular fatty acid composition. Am J Respir Cell Mol Biol. (1993) 9:628–36. doi: 10.1165/ajrcmb/9.6.628,
85. Masjedi, F, Keshtgar, S, Zal, F, Talaei-Khozani, T, Sameti, S, Fallahi, S, et al. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol. (2020) 197:105521. doi: 10.1016/j.jsbmb.2019.105521,
86. Ye, L, Su, ZJ, and Ge, RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. (2011) 16:9983–10001. doi: 10.3390/molecules16129983,
87. Oskarsson, A, Spatafora, C, Tringali, C, and Andersson, ÅO. Inhibition of CYP17A1 activity by resveratrol, piceatannol, and synthetic resveratrol analogs. Prostate. (2014) 74:839–51. doi: 10.1002/pros.22801,
88. Potrykus, M, Czaja-Stolc, S, Stankiewicz, M, Kaska, Ł, and Małgorzewicz, S. Intestinal microbiota as a contributor to chronic inflammation and its potential modifications. Nutrients. (2021) 13:13. doi: 10.3390/nu13113839,
89. Kong, C, Yang, M, Yue, N, Zhang, Y, Tian, C, Wei, D, et al. Restore intestinal barrier integrity: An approach for inflammatory bowel disease therapy. J Inflamm Res. (2024) 17:5389–413. doi: 10.2147/JIR.S470520,
90. Barone, M, D'Amico, F, Brigidi, P, and Turroni, S. Gut microbiome-micronutrient interaction: the key to controlling the bioavailability of minerals and vitamins. Biofactors. (2022) 48:307–14. doi: 10.1002/biof.1835,
91. Zhang, Q, and Hu, N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2020) 13:5003–14. doi: 10.2147/DMSO.S286430,
Keywords: gut microbiota, gut-ovary axis, Lactiplantibacillus plantarum NKK20, metabolomics, polycystic ovary syndrome, short-chain fatty acids
Citation: Xu H, Liu X, Sun W, Dong X, Liu X, Xie Y, He J, Ali A, Chen M, Wu L, Ma J and Shao K (2026) Multi-omics elucidation of Lactiplantibacillus plantarum NKK20 in preventing PCOS via the gut-ovary axis: SCFAs-mediated microbiota-metabolite-immune crosstalk. Front. Nutr. 12:1709581. doi: 10.3389/fnut.2025.1709581
Edited by:
Fatih Ozogul, Çukurova University, TürkiyeReviewed by:
Semra Çiçek, Atatürk University, TürkiyeVeeramani Karuppuchamy, The Ohio State University, United States
Dilber Ayhan, The Scientific and Technological Research Council of Türkiye, Türkiye
Copyright © 2026 Xu, Liu, Sun, Dong, Liu, Xie, He, Ali, Chen, Wu, Ma and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wu, d2x1anNAdWpzLmVkdS5jbg==; Jie Ma, anNkeG1hamllQHVqcy5lZHUuY24=; Keke Shao, a2VrZTg3ODkwMzk0QDE2My5jb20=
†These authors have contributed equally to this work
 Hao Xu
Hao Xu Xinyu Liu1†
Xinyu Liu1† Wen Sun
Wen Sun Xueyun Dong
Xueyun Dong Xuehui Liu
Xuehui Liu Jiayuan He
Jiayuan He Asmaa Ali
Asmaa Ali Liang Wu
Liang Wu Jie Ma
Jie Ma Keke Shao
Keke Shao