- 1Department of Neurology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Diagnostic Laboratory for Hematology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Hematologic malignancies pose significant global health burdens, with programmed cell death protein-1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors revolutionizing treatment in subtypes like classical Hodgkin lymphoma (cHL) and primary mediastinal large B-cell lymphoma (PMBCL), achieving high objective response rates (ORR). However, efficacy varies widely, with limited success in multiple myeloma (< 10% ORR) and leukemias, underscoring the need for better predictors beyond tumor-intrinsic biomarkers. This review highlights pre-treatment endocrine–nutritional signatures as key host factors influencing immunotherapy outcomes. Dysregulated hormones (cortisol, thyroid, sex steroids, insulin/insulin-like growth factor-1, adipokines) and nutritional status (vitamin D, zinc, protein-energy malnutrition, iron metabolism) modulate T-cell exhaustion, myeloid suppression, and tumor microenvironment dynamics, often leading to resistance. Evidence from cohorts shows hypercortisolism, hypothyroidism, insulin resistance, vitamin D deficiency, and hypoalbuminemia correlate with inferior ORR, progression-free survival, and overall survival, while thyroid immune-related adverse events and moderate obesity predict benefit. In hematologic contexts, marrow infiltration exacerbates these imbalances, explaining heterogeneous responses. Integrated signatures (e.g., Glasgow Prognostic Score, Prognostic Nutritional Index) offer superior prognostic value, enabling targeted interventions like vitamin D supplementation, metformin, or nutritional support to enhance immune checkpoint inhibitor efficacy. Mechanistic insights reveal convergence on mTOR/IFN-γ pathways and microbiome modulation. Translating these to clinical panels could personalize immunotherapy, addressing gaps in hematologic malignancies literature and improving outcomes in relapsed/refractory settings.
1 Introduction
Hematologic malignancies encompassing lymphomas, leukemias, multiple myeloma (MM), and related disorders represent a major global health challenge, with approximately 1.3 million new cases and 700,000 deaths annually according to GLOBOCAN 2022 estimates, projected to rise substantially by 2050 due to population aging and environmental exposures (1, 2). These cancers disproportionately affect younger populations in low- and middle-income countries and carry high morbidity from disease- and treatment-related immunosuppression (3). Traditional therapies (chemotherapy, targeted agents, hematopoietic stem cell transplantation) have improved survival but plateaued in relapsed/refractory (r/r) settings, prompting a paradigm shift toward immunotherapy, particularly immune checkpoint inhibitors (ICIs) targeting the programmed cell death protein-1 (PD-1)/programmed cell death ligand 1 (PD-L1) axis (4). Since the landmark approvals of nivolumab and pembrolizumab for r/r classical Hodgkin lymphoma (cHL) in 2016–2017, PD-1 blockade has transformed outcomes in select subtypes, achieving objective response rates (ORR) of 70–87% and durable remissions in cHL and primary mediastinal large B-cell lymphoma (PMBCL), with expanding roles in combinations for non-Hodgkin lymphomas and post-transplant relapse (5, 6).
Despite these successes, responses to PD-1/PD-L1 inhibitors remain strikingly heterogeneous across hematologic malignancies: exquisite sensitivity in 9p24.1-altered cHL/PMBCL contrasts with modest activity in T-cell lymphomas (ORR 20–50%), negligible monotherapy efficacy in MM (< 10%), and limited benefit in leukemias outside niche indications (7, 8). Even within responsive diseases, 20–40% of patients exhibit primary resistance or early relapse, highlighting the inadequacy of tumor-centric biomarkers alone to explain variability (9).
This inconsistency has fueled recognition that tumor-intrinsic features (PD-L1 expression, tumor mutational burden, genetic alterations) are insufficient predictors in many hematological contexts, directing attention to host systemic factors that establish a baseline “whole-body immunologic tone,” the integrated metabolic, endocrine, and inflammatory milieu shaping T-cell priming, trafficking, and persistence (10).
Hormones (cortisol, thyroid hormones, sex steroids, insulin/Insulin-like growth factor-1 (IGF-1), adipokines), micronutrients (vitamin D, zinc, selenium), and metabolic state (obesity, sarcopenia, protein-energy status) directly modulate T-cell metabolism, exhaustion, and cytokine networks, influencing whether PD-1/PD-L1 blockade can restore effective antitumor immunity (11, 12). Pre-treatment dysregulation, highly prevalent in hematologic patients due to disease cachexia, marrow infiltration, and prior therapies, correlates with inferior ICI outcomes across cancers, identifying a modifiable determinant of response (13). Assessing these signatures before therapy offers prognostic value and therapeutic opportunity through targeted interventions.
This review synthesizes emerging evidence linking pre-treatment endocrine–nutritional profiles to PD-1/PD-L1 blockade efficacy in hematologic malignancies, emphasizing mechanistic insights, clinical correlations, and translational potential beyond solid tumor-dominated literature.
2 PD-1/PD-L1 blockade in hematologic malignancies: a clinical overview
PD-1/PD-L1 inhibitors have revolutionized relapsed/refractory cHL and PMBCL with high, durable response rates, leading to regulatory approvals for nivolumab and pembrolizumab. Efficacy remains heterogeneous elsewhere: modest in select non-Hodgkin lymphomas [particularly T-cell or Epstein-Barr virus (EBV)-associated], negligible in MM as monotherapy, and emerging but limited in leukemias (mainly post-transplant relapse). Predictive biomarkers such as PD-L1 expression (driven by 9p24.1 alterations in cHL/PMBCL), tumor mutational burden (TMB), and genetic features perform well in cHL but have major limitations in other hematologic settings due to low neoantigen burden, immunosuppressive microenvironments, assay variability, and confounding inflammation.
2.1 Mechanistic basis of immune checkpoint inhibition
The PD-1 receptor on T cells interacts with its ligands PD-L1 (CD274) and PD-L2 (CD273) on tumor cells or antigen-presenting cells, delivering inhibitory signals that induce T-cell exhaustion, energy, and apoptosis mechanisms that tumors exploit for immune evasion (14). PD-1/PD-L1 blockade with monoclonal antibodies (e.g., nivolumab, pembrolizumab targeting PD-1; atezolizumab, durvalumab targeting PD-L1) disrupts this axis, restoring effector T-cell function, cytokine production (IFN-γ), and cytolytic activity (15).
In hematologic malignancies, Reed-Sternberg (RS) cells in cHL and malignant B cells in PMBCL frequently harbor copy number alterations at chromosome 9p24.1 encompassing PD-L1/PD-L2 and JAK2, leading to JAK/STAT-mediated overexpression of PD-L1/PD-L2 and profound dependence on the pathway for survival (9). This genetic addiction renders these tumors exquisitely sensitive to PD-1 blockade, resulting in one of the highest response rates observed across oncology (ORR 69–87%) (5, 16). Additional mechanisms include EBV-driven PD-L1 expression in subsets of lymphomas and leukemias, and chronic antigenic stimulation in the bone marrow niche promoting exhaustion (17).
In contrast to solid tumors, hematologic cancers often exhibit lower somatic mutational burden and fewer neoantigens, yet the amplified PD-L1 expression in specific subtypes overrides this limitation, explaining the outlier success in cHL/PMBCL (18). Preclinical models further demonstrate that PD-1 blockade enhances NK cell activity and reverses myeloid-derived suppressor cell suppression in the marrow microenvironment (19).
2.2 Evidence across major hematologic cancers
Classical Hodgkin lymphoma, PMBCL represents the flagship success of PD-1 blockade in hematologic oncology. Pivotal phase II trials established nivolumab (CheckMate 205) and pembrolizumab (KEYNOTE-087) as standards for r/r disease post-autologous transplant and brentuximab vedotin, with ORR 69–73%, complete response (CR) rates 16–29%, and median duration of response exceeding 16–24 months (16, 20). Long-term follow-up of CheckMate 205 (6–7 years) reported 5-year progression-free survival (PFS) with 72% in transplant-naïve patients and durable remissions beyond 5 years in ∼60% of responders (21). Pembrolizumab demonstrated 5-year OS ∼85% in KEYNOTE-087 updated analyses (22). These agents received FDA accelerated approval in 2016–2017 and full approval thereafter, transforming third-line management and now incorporated earlier (e.g., with AVD chemotherapy in the frontline) with 3-year PFS > 90% in phase III trials (23, 24).
Primary mediastinal large B-cell lymphoma shares the 9p24.1 alteration profile with cHL. Pembrolizumab in KEYNOTE-170 (r/r PMBCL) achieved ORR 45% (CR 13%) with durable responses (median DOR not reached at 3 + years), leading to FDA approval in 2018 (25). Nivolumab has shown similar activity (ORR ∼40–50%) in smaller series (26). PD-1 blockade is now guideline-preferred in r/r PMBCL.
2.2.1 Non-Hodgkin lymphoma
In aggressive B-cell lymphomas, monotherapy yields modest results. Nivolumab in r/r Diffuse Large B-Cell Lymphoma (DLBCL) post-autologous transplant or ineligible for transplant showed ORR 10–36% in non-GCB subtypes, with CR rates < 10% (27). Pembrolizumab in KEYNOTE-013/170 achieved ORR ∼25% in Richter transformation but only 0–10% in standard DLBCL (28). Combination strategies (e.g., nivolumab + rituximab-gemcitabine-oxaliplatin) or with BTK inhibitors have improved ORR to 50–70% in early-phase studies (29, 30). Follicular lymphoma shows even lower activity (ORR < 10%) due to sparse PD-L1 expression and immunosuppressive follicular dendritic networks (31).
2.2.2 T-cell lymphomas exhibit greater heterogeneity
Nivolumab/pembrolizumab monotherapy in r/r PTCL/NK-T-cell lymphoma yields ORR 20–40%, with higher rates in AITL or EBV-associated subtypes (32). Sintilimab and tislelizumab have shown ORR > 50% in relapsed NK/T-cell lymphoma in Asian cohorts (33). Overall, PD-1 blockade has limited single-agent approval in NHL but is increasingly combined with chemotherapy, bispecific antibodies, or lenalidomide in ongoing trials (7).
2.2.3 MM (limited efficacy)
MM has proven largely refractory to PD-1/PD-L1 monotherapy, with ORR consistently < 10% across KEYNOTE and CheckMate trials (34, 35). Phase III trials combining pembrolizumab with lenalidomide-dexamethasone or pomalidomide-dexamethasone (KEYNOTE-183/185) were halted by the FDA in 2017 due to increased mortality in the experimental arms, attributed to excessive immune-related toxicity and lack of efficacy (36). Subsequent trials with nivolumab ± elotuzumab or pomalidomide also failed to show benefit (CheckMate 602, 2024) (37). Emerging data suggest modest activity when sequenced after BCMA-targeted therapies or combined with bispecific antibodies (talquetamab + cetrelimab ORR ∼60–70% in early reports), potentially by overcoming T-cell exhaustion post-BCMA redirection (38, 39).
2.2.4 Leukemias (exceptions and ongoing trials)
Acute leukemias show minimal single-agent activity (ORR < 5% in AML). However, post-allogeneic HCT relapse represents an exception: nivolumab or pembrolizumab can induce durable complete remissions in 30–50% of AML/MDS patients via graft-versus-leukemia enhancement, with mixed chimerism emerging as a predictive factor (40, 41). Hypomethylating agents + PD-1 blockade trials show ORR 20–30% in frontline unfit AML, but no randomized superiority yet (42). Chronic lymphocytic leukemia has negligible responses.
2.3 Current predictive biomarkers and their limitations
PD-L1 expression by IHC (22C3 or 28-8 assays) is the most established biomarker in cHL/PMBCL, where 9p24.1 amplification correlates with near-universal expression and superior ORR/CR rates (> 80–90%) (9, 43). Tumor mutational burden is generally low (< 5 mut/Mb) in hematologic malignancies compared to MSI-high solid tumors, limiting its predictive value outside rare hypermutated cases (44). EBV status (LMP1-driven PD-L1) predicts response in NK/T-cell and PTCL (45).
These biomarkers fail in many hematological settings for several reasons: (i) low neoantigen load reduces baseline T-cell priming; (ii) dominant immunosuppressive marrow microenvironment [myeloid-derived suppressor cells (MDSCs), M2 macrophages, TGF-β] overrides PD-1 blockade; (iii) lack of standardized PD-L1 scoring in liquid tumors and assay discordance; (iv) confounding inflammation elevating PD-L1 without functional significance; (v) rapid resistance via alternative checkpoints (TIM-3, LAG-3, TIGIT) or MHC loss (46, 47). Composite scores incorporating soluble PD-L1, IFN-γ signature, or circulating tumor DNA are under investigation but not yet clinically implemented (48).
3 The immunologic role of endocrine and nutritional systems
The immune system does not operate in isolation but is profoundly shaped by systemic endocrine and nutritional signals that constitute a “whole-body immunologic tone” determining the baseline readiness of antitumor immunity (49, 50). This immuno-endocrine and immuno-nutritional crosstalk is bidirectional: immune activation feeds back to alter hormone secretion and nutrient partitioning, while endocrine/metabolic states reprogram immune cell fate, metabolism, and function (51). In cancer, chronic inflammation and tumor-derived factors frequently dysregulate these axes, fostering T-cell exhaustion, Treg/MDSC expansion, and impaired antigen presentation states that blunt the efficacy of PD-1/PD-L1 blockade (52). Emerging evidence across solid and hematologic malignancies demonstrates that pre-treatment endocrine–nutritional signatures represent modifiable host factors capable of predicting and potentially augmenting checkpoint inhibitor outcomes (10, 53).
3.1 Concept of immuno-endocrine and immuno-nutritional crosstalk
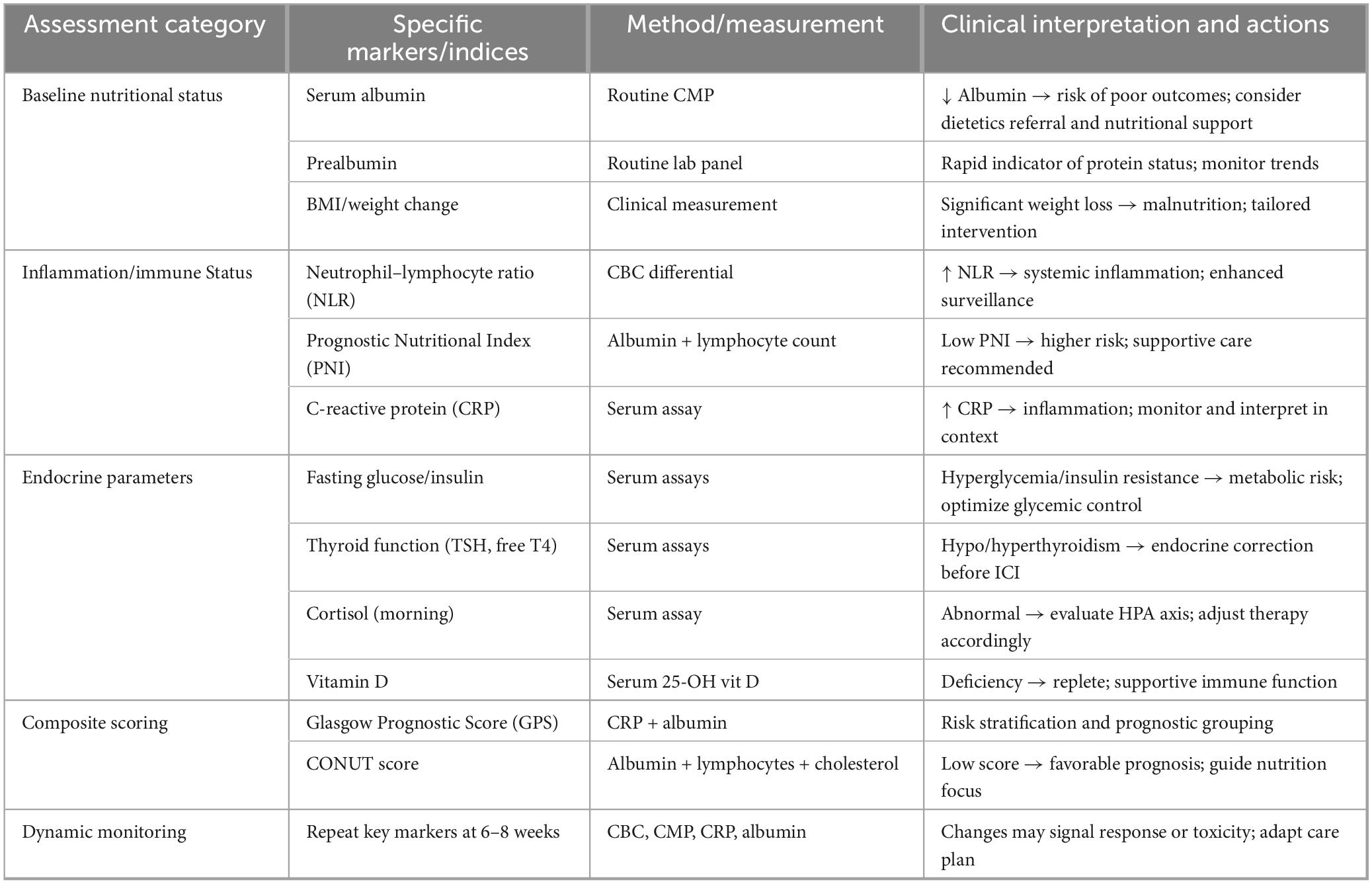
Immune cells express receptors for virtually all hormones, adipokines, and nutrient-sensing pathways (mTOR, AMPK, IGF-1R, VDR, AhR), allowing systemic metabolic cues to orchestrate leukocyte development, trafficking, and effector differentiation (54, 55). Conversely, cytokines (IFN-γ, IL-6, TNF-α) modulate hypothalamic–pituitary axes, adipocyte function, and nutrient transporter expression, creating feedback loops that can either amplify or suppress antitumor responses (50, 56). In the tumor microenvironment (TME), this crosstalk is hijacked: tumors induce chronic low-grade inflammation that elevates cortisol, leptin, and insulin while depleting micronutrients, driving T-cell dysfunction and resistance to PD-1/PD-L1 blockade (57). Preclinical models and clinical cohorts show that correcting these imbalances (e.g., vitamin D repletion, metformin-mediated insulin sensitization) restores CD8+ T-cell metabolism and synergizes with checkpoint inhibition (58, 59). The concept has particular relevance in hematologic malignancies, where marrow infiltration, cachexia, and prior therapies frequently induce profound endocrine–nutritional dysregulation (60). A mechanistic overview of how endocrine and nutritional pathways converge on T-cell immunity is shown in Figure 1.
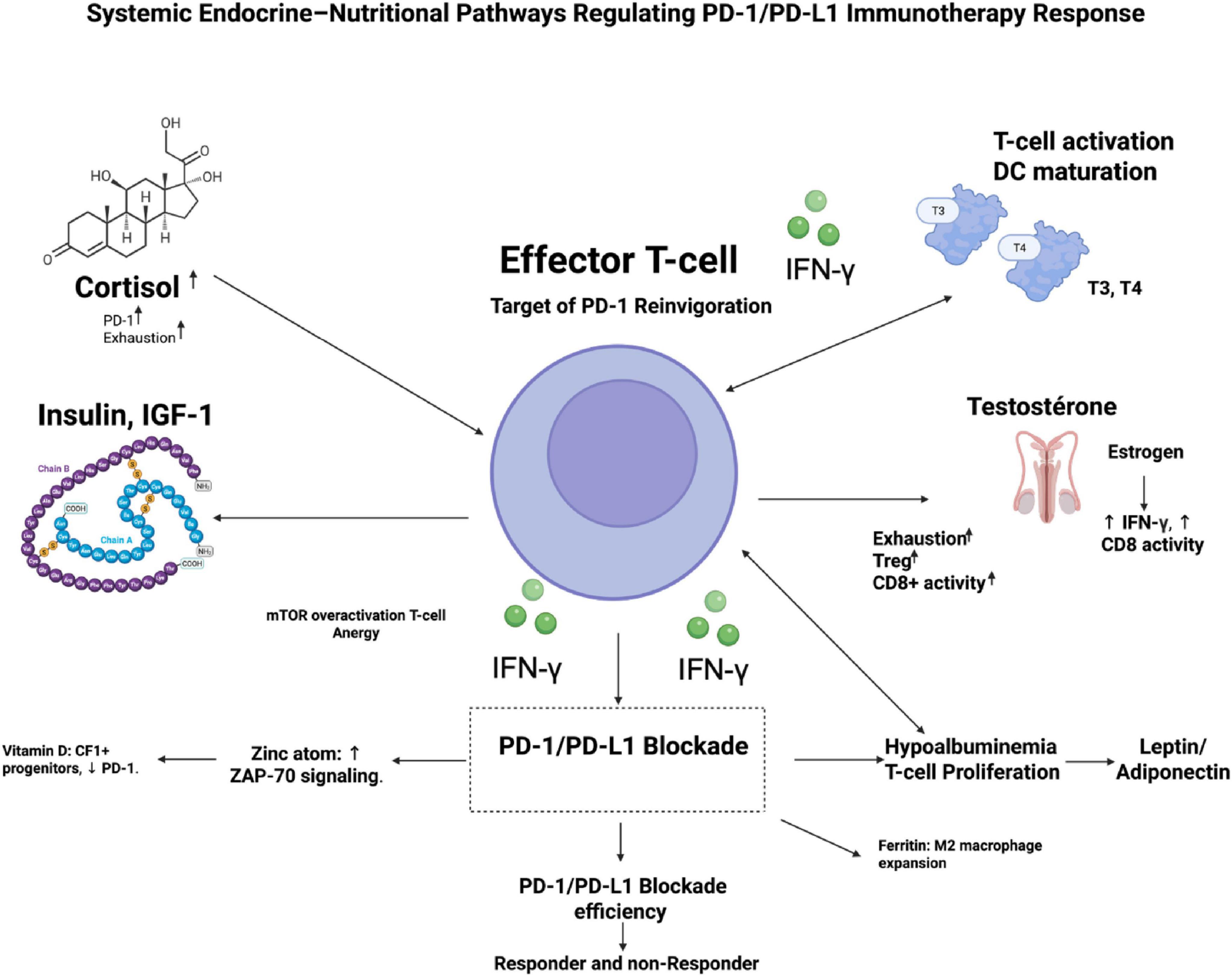
Figure 1. Mechanistic map of endocrine–nutritional pathways regulating PD-1/PD-L1 immunotherapy response. This schematic illustrates how systemic hormonal and nutritional signals converge on CD8+ effector T cells—the primary targets of PD-1 reinvigoration—to shape antitumor immunity. A central T cell displays PD-1 expression with IFN-γ–mediated tumor targeting. Surrounding endocrine regulators include: cortisol (adrenal axis), which drives PD-1 upregulation and T-cell exhaustion; thyroid hormones (T3/T4), enhancing T-cell activation and dendritic-cell maturation; insulin/IGF-1 signaling promoting mTOR overactivation and T-cell energy; and sex hormones, where estrogen supports IFN-γ production and CD8+ T cell cytotoxicity, whereas testosterone increases Treg activity and exhaustion. Nutritional cues include vitamin D maintenance of TCF1+ progenitors and reduced PD-1 levels, zinc enhancement of ZAP-70 signaling, iron overload/ferritin promoting M2 macrophage expansion, hypoalbuminemia impairing T-cell proliferation, and leptin–adiponectin imbalance skewing immune tone. These integrated inputs determine downstream PD-1/PD-L1 blockade efficacy, distinguishing potential responders from non-responders.
3.2 Endocrine regulation of antitumor immunity
3.2.1 Hypothalamic–pituitary–adrenal axis and cortisol
Chronic stress activates the HPA axis, elevating glucocorticoids (GCs) that potently suppress antitumor immunity. Endogenous and synthetic GCs upregulate PD-1 expression on CD8+ T cells via GR-mediated transactivation, accelerate exhaustion, impair proliferation, and promote apoptosis (61, 62). In tumor-bearing mice and patients, elevated cortisol or dexamethasone use correlates with reduced CD8+ T-cell infiltration, higher TIM-3/LAG-3 co-expression, and inferior response to PD-1 blockade (63, 64). Mechanistically, GCs inhibit mTORC1 signaling and glucose uptake in T cells while enhancing Treg suppressive function (65). Recent data show that tumor-intrinsic HSD11B1 reactivation of GCs limits IFN-γ signaling and ICI efficacy in melanoma (66).
3.2.2 Thyroid hormones
Triiodothyronine (T3) and thyroxine (T4) enhance T-cell activation, dendritic cell (DC) maturation, and Th1 polarization via thyroid hormone receptor β expressed on immune cells (67). Subclinical or overt hypothyroidism, common in cancer patients, is associated with reduced CD8+ T-cell cytotoxicity and increased Treg frequency (68). Paradoxically, ICI-induced thyroiditis strongly predicts favorable outcomes across malignancies (ORR ↑ 2–3-fold), likely reflecting robust immune activation spilling into autoimmunity (69, 70). Low pre-treatment thyroid stimulating hormone (TSH) or free T4 correlates with poorer PFS/overall survival (OS) on PD-1 blockade, while thyroid hormone supplementation in hypothyroid models restores antitumor immunity (71).
3.2.3 Sex hormones (estrogen, testosterone)
Sex hormones drive marked disparities in immunotherapy outcomes. Estrogen (via ERα/β) enhances CD8+ T cell effector function, DC cross-presentation, and IFN-γ production while reducing PD-1 expression, contributing to superior ICI responses in females in several cancers (72, 73). Conversely, testosterone suppresses Th1 responses, promotes Treg/MDSC accumulation, and upregulates PD-1/CTLA-4; androgen deprivation in prostate cancer models dramatically boosts ICI efficacy (74, 75). Large meta-analyses confirm male sex as an independent negative predictor of PD-1/PD-L1 benefit in prostate cancer/NSCLC, with hormonal aging (declining testosterone/estrogen) further exacerbating immune senescence (76, 77).
3.2.4 Insulin/IGF-1 axis
Hyperinsulinemia and elevated IGF-1 signaling through PI3K/AKT/mTORC1 drives T-cell energy and exhaustion while promoting MDSC and M2 polarization (78). IGF-1R blockade or metformin in preclinical models reverses exhaustion, increases CD8+ T-cell infiltration, and synergizes with PD-1 inhibition (79, 80). Metabolic syndrome and high pre-treatment C-peptide predict inferior outcomes with ICIs, reflecting chronic inflammation and impaired T-cell metabolism (81).
3.2.5 Adipokines (leptin, adiponectin)
Leptin, elevated in obesity, promotes T-cell exhaustion via mTOR activation and PD-1 upregulation while expanding Tregs; leptin-deficient mice show enhanced antitumor immunity (82). Conversely, adiponectin exerts anti-inflammatory effects, enhances CD8+ T-cell function, and correlates with better ICI responses (83). The obesity paradox improved ICI outcomes in overweight patients may partly reflect leptin-driven tonic signaling that paradoxically sustains effector T-cell survival during chronic stimulation (13, 84).
3.3 Nutritional regulation of immunity
3.3.1 Protein status (albumin, prealbumin)
Hypoalbuminemia reflects chronic inflammation (IL-6-driven) and protein-energy malnutrition, impairing T-cell proliferation and cytokine production via reduced mTOR signaling and amino acid availability (85). Low albumin/prealbumin strongly predicts non-response to PD-1 blockade across cancers (86).
3.3.2 Vitamins D, A, B12, folate
Vitamin D (via VDR on T cells/DC) promotes Th1/Tc1 differentiation, inhibits Treg, and enhances PD-L1 blockade efficacy; deficiency correlates with poorer survival and reduced CD8+ T-cell infiltration (12, 87). Preclinical and clinical data show vitamin D repletion overcomes resistance by remodeling the microbiome and boosting IFN-γ signaling (88, 89).
Vitamins A (retinoic acid), B12, and folate are essential for T-cell proliferation and thymic function; deficiencies common in hematologic patients impair DNA synthesis and cytotoxicity (90).
3.3.3 Iron metabolism
Dysregulated iron handling (high ferritin, low transferrin) fuels MDSC and M2 macrophages while starving T cells of iron required for proliferation (91). Anemia and high hepcidin predict inferior ICI outcomes (92).
3.3.4 Zinc, selenium, and trace elements
Zinc is critical for ZAP-70 signaling and NK/CD8+ T-cell cytotoxicity; deficiency increases PD-1+ exhausted T cells (93). Selenium (via selenoproteins) protects against oxidative stress during activation; low levels correlate with Treg expansion and reduced ICI benefit (93, 94).
3.4 Metabolic reprogramming of immune cells
3.4.1 Amino acid metabolism (arginine, tryptophan)
Tumors and MDSCs deplete arginine (via ARG1) and tryptophan (via IDO1), inducing GCN2/mTOR inhibition, T-cell energy, and Treg differentiation (95, 96). IDO1 expression strongly predicts ICI resistance; inhibitors restore effector function in models (97).
3.4.2 Lipid metabolic pathways
Effector T cells rely on fatty acid oxidation; obesity-associated hyperlipidemia paradoxically supports memory formation, but chronic cholesterol overload impairs TCR signaling via ER stress (98).
3.4.3 Gut microbiome as a nutritional mediator
Diet shapes microbiome composition, which systemically regulates ICI efficacy via microbial metabolites (SCFAs, inosine) that enhance DC maturation and CD8+ T-cell infiltration (99, 100). High-fiber diets enrich responder taxa (Akkermansia, Faecalibacterium) and improve ORR/PFS; Western diets deplete them and promote resistance (100, 101). These endocrine–nutritional determinants integrate into a composite immunologic readiness score. Figure 2 illustrates a conceptual framework linking host systemic physiology to response to PD-1/PD-L1 immune checkpoint blockade. The model integrates three interrelated biological axes—endocrine, nutritional, and body composition that collectively shape a composite host fitness score. Key biomarkers within these axes include cortisol and thyroid hormones (endocrine axis), IGF-1, albumin/PNI, micronutrient balance (zinc, selenium, ferritin/iron), and indicators of nutritional status (nutritional axis), as well as BMI and skeletal muscle mass reflecting sarcopenia (body composition axis). These parameters converge to define the System Immunologic Readiness Score, which represents the host’s capacity to mount an effective antitumor immune response. Patients with preserved metabolic and nutritional status, adequate muscle mass, and balanced endocrine signaling are positioned toward higher readiness, characterized by robust CD8+ T-cell metabolism and effective cytokine responses. In contrast, dysregulation across these axes such as sarcopenia, micronutrient deficiency, chronic stress signaling, or iron imbalance correlates with lower immunologic readiness, immune exhaustion, MDSC dominance, and diminished response to PD-1 blockade. Overall, the figure emphasizes that response to immune checkpoint inhibition is not solely tumor-intrinsic but is strongly influenced by systemic host factors, supporting the integration of metabolic, nutritional, and body composition assessments into immunotherapy stratification.
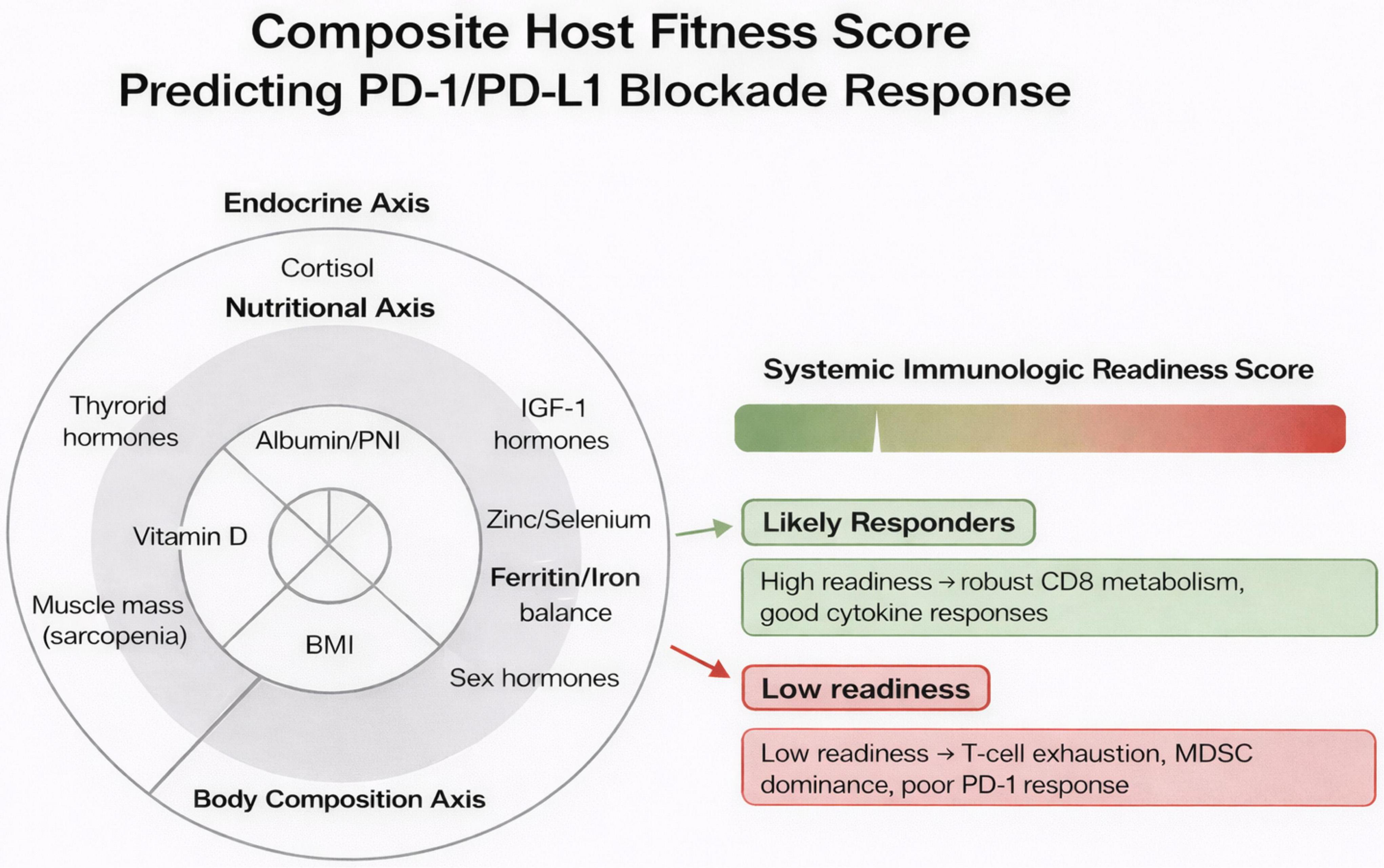
Figure 2. Integrated “immunologic readiness” scoring model for predicting PD-1/PD-L1 blockade response. This conceptual model aggregates endocrine, nutritional, and body composition domains to generate a composite Systemic Immunologic Readiness Score. A three-ring radar-style layout illustrates the contributing axes: (1) Endocrine Axis, encompassing cortisol levels, thyroid hormones, IGF-1, and sex hormones; (2) Nutritional Axis, capturing albumin/PNI, vitamin D, zinc–selenium status, and ferritin/iron balance; and (3) Body Composition Axis, reflecting muscle mass (sarcopenia status), fat mass/leptin activity, and BMI. These parameters integrate into a color-graded scoring meter (green→red) that stratifies patients as likely responders or likely non-responders to PD-1/PD-L1 therapy. Accompanying notes highlight the biological underpinnings: high readiness supports resilient CD8+ T-cell metabolism and strong cytokine production, whereas low readiness is associated with T-cell exhaustion, myeloid-derived suppressor cell (MDSC) dominance, and reduced checkpoint inhibitor efficacy.
4 Evidence linking endocrine abnormalities to checkpoint inhibitor outcomes
Accumulating observational and mechanistic data demonstrate that pre-treatment endocrine abnormalities, particularly dysregulation of the HPA axis, thyroid function, sex hormones, insulin/IGF-1 signaling, vitamin D axis, and adipokine balance, significantly modulate clinical benefit from PD-1/PD-L1 blockade. These host factors influence baseline T-cell fitness, exhaustion state, and tumor microenvironment permissiveness, often explaining part of the heterogeneous responses observed across malignancies. Although most evidence derives from large cohorts in melanoma, NSCLC, and renal cell carcinoma, the immunologic mechanisms are conserved and increasingly corroborated in hematologic cancers (cHL, non-Hodgkin lymphomas, MM), where chronic inflammation and marrow niche effects amplify endocrine-immune crosstalk. Key endocrine biomarkers influencing PD-1/PD-L1 responses are summarized in Table 1.
4.1 Cortisol and stress-axis dysregulation
Chronic activation of the HPA axis and resultant hypercortisolism exerts profound systemic immunosuppression by directly inducing T-cell exhaustion and apoptosis while expanding immunosuppressive myeloid populations (105). Glucocorticoids (GCs) upregulate PD-1, CTLA-4, and TIM-3 on CD8+ T-cells via glucocorticoid receptor-mediated transactivation and transrepression of pro-inflammatory transcription factors (NF-κB, AP-1), simultaneously inhibiting IL-2 and IFN-γ production (61, 62).
Observational findings consistently link baseline GC use (≥ 10 mg prednisone equivalent daily, often for symptom control or comorbidities) with markedly inferior outcomes on PD-1/PD-L1 therapy. A landmark study in NSCLC showed that baseline corticosteroids were associated with reduced ORR (ORR: 8.5% vs. 32.3%), PFS (HR = 1.93), and OS (HR = 2.34) independent of performance status or brain metastases (106). Meta-analyses confirm this detrimental effect across tumor types, with dose- and duration-dependent impairment (107, 108). Endogenous hypercortisolism (e.g., Cushing syndrome or chronic stress-elevated morning cortisol > 500–600 nmol/L) similarly predicts resistance, as shown in preclinical models where tumor-derived GC reactivation via HSD11B1 limits IFN-γ signaling and CD8+ T -cell infiltration (66).
In hematologic malignancies, data are sparser but supportive: real-world cHL cohorts treated with nivolumab/pembrolizumab demonstrate shorter response duration in patients requiring GCs for symptom control or immune-related adverse events (irAEs) (5). Hypophysitis-induced secondary adrenal insufficiency (common irAE, incidence 5–15% with combination CTLA-4/PD-1 blockade) paradoxically does not worsen prognosis when promptly replaced with physiologic hydrocortisone, suggesting that supra-physiologic GC doses, not cortisol deficiency per se, drive immunosuppression (109, 110). The redistribution of circulating memory T cells under hypercortisolism and CXCR4 signaling is illustrated in Figure 3.
Figure 3. Schematic representation of how circulating memory T cells (T_CM and T_EM) redistribute between the spleen, blood, and bone marrow in response to tumor signals and corticosteroid treatment. Increased CXCR4 expression promotes the homing of circulating memory T cells to the bone marrow, where corticosteroids further enhance their retention. The diagram illustrates the movement of these T-cell subsets toward tumor tissue and their dynamic localization within hematopoietic and adipose niches of the bone marrow.
4.2 Thyroid dysfunction and T-cell activation
Thyroid hormones (T3/T4) are essential for T-cell development, proliferation, and Th1 polarization via thyroid receptor expression on lymphocytes and dendritic cells (67). PD-1/PD-L1 inhibitors frequently induce thyroid irAEs (irAE-thyroiditis, incidence 10–40%, highest with combination therapy), manifesting as transient thyrotoxicosis followed by hypothyroidism, reflecting destructive autoimmunity against thyroperoxidase/glutamate decarboxylase antibodies (69).
Multiple large cohorts and meta-analyses (> 10,000 patients) establish that development of thyroid dysfunction is a robust positive predictor of ICI benefit that compared to patients without thyroid irAEs (70, 111, 112). This association holds across melanoma, NSCLC, and renal cancer and appears independent of other irAEs, likely reflecting systemic immune activation spilling into thyroid autoimmunity (113). Mechanistically, ICI-induced thyroiditis correlates with increased CD8+ T-cell reinvigoration, reduced Treg frequency, and lower PD-1 expression on peripheral T cells (114).
Conversely, pre-existing hypothyroidism (often autoimmune Hashimoto’s) is associated with poorer outcomes, possibly via baseline Treg expansion and impaired T-cell priming (68). Subclinical hyperthyroidism or low TSH at baseline may also predict resistance in some series (115). In hematologic cancers, thyroid irAEs occur in 15–25% of cHL patients on PD-1 blockade and similarly predict prolonged responses, while untreated baseline hypothyroidism correlates with early progression in small DLBCL/MM cohorts (22, 31). The cell-specific effects of thyroid hormones on immune activation and differentiation are shown in Figure 4.
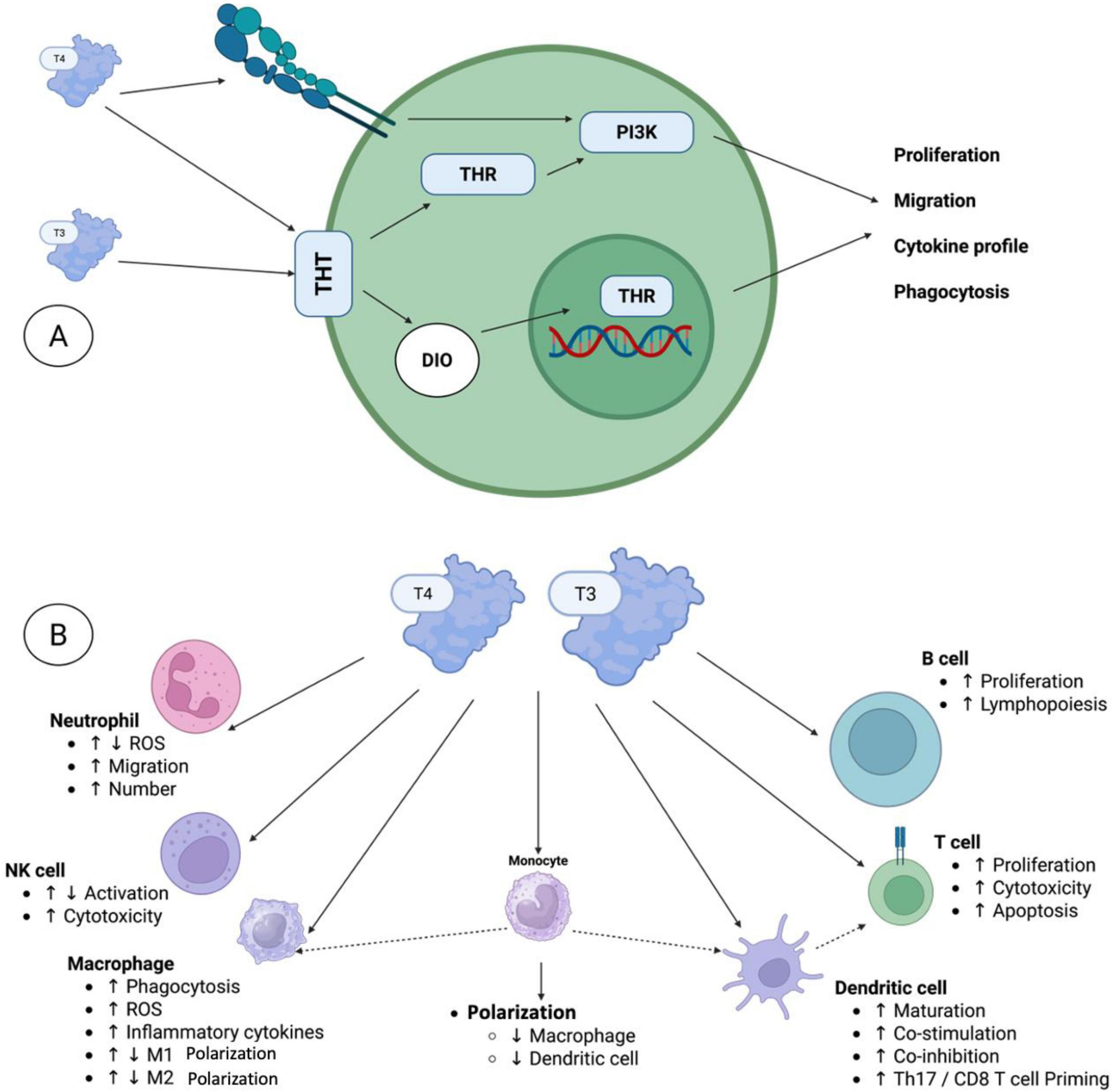
Figure 4. The local effects of thyroid hormones on the immune system. (A) Several immune cell types have been reported to express distinct thyroid hormone transporters (THTs), which mediate the uptake of thyroid hormones (THs) into the cells. Within the cell, deiodinases (DIOs) facilitate the conversion of thyroid hormones (THs), thereby either fostering or restricting TH activation. Intracellular T3 can subsequently bind to thyroid hormone receptors (THRs) in the cytoplasm or nucleus, thereby initiating non-canonical or canonical signaling pathways, respectively. In addition to the non-canonical thyroid hormone receptor action, which involves, among other mechanisms, PI3K signaling pathways, T4 can also bind to integrin αVβ3 on the cell surface, thereby initiating multiple pathways, including PI3K signaling. (B) The local effects of THs were observed in various innate and adaptive immune cells, including neutrophils, natural killer (NK) cells, macrophages, monocytes, dendritic cells, T cells, and B cells. Here, T3 and T4 were characterized as directly governing various functional processes, including activation, differentiation, proliferation, and/or migration. Furthermore, TH signaling in monocytes and dendritic cells indirectly influences the responses of macrophages and dendritic cells, respectively, as well as T cell activity.
4.3 Sex hormones and immunotherapy response
Sex hormones profoundly shape antitumor immunity, with estrogen enhancing CD8+ T-cell effector function, dendritic cell cross-presentation, and IFN-γ signaling via ERα/β, while testosterone suppresses Th1 responses and promotes Treg/MDSC accumulation through androgen receptor signaling (72, 75).
A seminal 2018 meta-analysis of > 20 randomized trials (n > 11,000) found that males derive greater benefit from PD-1/PD-L1 inhibitors than females, a difference most pronounced in monotherapy versus chemotherapy controls (116). Subsequent analyses confirmed male advantage in melanoma, NSCLC, and head and neck cancer, with hazard ratios favoring males by 20–40% (76, 117). Mechanistically, androgen deprivation in prostate cancer models dramatically boosts CD8+ T-cell infiltration and synergizes with PD-1 blockade, while estrogen in females may upregulate alternative checkpoints (LAG-3, TIM-3) (118).
Hormonal aging exacerbates immune senescence: post-menopausal estrogen decline and age-related hypogonadism both correlate with reduced ICI efficacy (73). In hematologic malignancies, male sex is an adverse prognostic factor in some cHL real-world series treated with nivolumab (shorter PFS), consistent with testosterone-driven immunosuppression (119).
4.4 Insulin resistance and metabolic syndrome
Insulin resistance, type 2 diabetes, and metabolic syndrome drive chronic low-grade inflammation (elevated IL-6, TNF-α) that promotes T-cell exhaustion, MDSC expansion, and PD-L1 upregulation on tumor cells (78). Hyperinsulinemia activates PI3K/AKT/mTORC1 in T cells, inducing energy while impairing memory formation (79).
Retrospective studies and meta-analyses show that diabetes at baseline confers a 30–70% increased risk of progression or death on PD-1/PD-L1 therapy (HR 1.3–1.7), with hyperglycemia (> 200 mg/dL) during treatment independently predicting resistance (81, 120). Metabolic syndrome components (obesity + hypertension + dyslipidemia) compound this effect (121). Metformin, by ameliorating hyperinsulinemia and activating AMPK, has shown synergistic effects with ICIs in preclinical models and observational cohorts (improved ORR/PFS in diabetic patients) (122). Data in hematologic cancers are emerging: insulin resistance correlates with inferior responses in myeloma and DLBCL treated with PD-1 combinations (123).
4.5 Vitamin D endocrine axis
Vitamin D (via VDR on immune cells) promotes CD8+ T-cell activation, dendritic cell maturation, chemokine production (CXCL10), and gut microbiome diversity while inhibiting Treg and PD-1 expression (12, 124).
Multiple cohorts demonstrate that vitamin D deficiency (< 20 ng/mL) at baseline is associated with significantly worse ORR, PFS, and OS across ICI-treated cancers (125–127). A 2023 prospective study showed that systematic vitamin D supplementation (≥ 2,000 IU/day) increased ORR from 36 to 56% and median PFS from 5.8 to 11.3 months, with reduced severe irAEs (127). Genetic polymorphisms in VDR and CYP27B1 also predict outcomes (128). In hematologic cohorts, low vitamin D is prevalent (> 60% in lymphoma/MM) and correlates with poorer survival on PD-1-based regimens (129).
4.6 Adipokine imbalance as an immune checkpoint modulator
Adipose tissue secretes adipokines that bidirectionally regulate immunity: leptin promotes T-cell exhaustion via mTOR activation and PD-1 upregulation while expanding Tregs, whereas adiponectin exerts anti-inflammatory, CD8+ T-enhancing effects (82, 83).
Leptin levels are elevated in obesity and directly induce PD-1 on CD8+ T-cells in mouse models and human tumors, accelerating exhaustion; leptin signaling blockade restores ICI efficacy (82, 84). Yet a robust “metabolic obesity paradox” emerges: overweight/obese patients (BMI ≥ 25–30) consistently show superior ORR, PFS, and OS on PD-1/PD-L1 therapy across melanoma, NSCLC, and renal cancer in meta-analyses (> 30,000 patients) (13, 130). Proposed mechanisms include leptin-driven tonic signaling preventing terminal exhaustion, increased CD8+ T-cell infiltration, and altered pharmacokinetics (131). This paradox extends to hematologic malignancies: higher BMI predicts longer response duration in cHL treated with nivolumab/pembrolizumab in real-world series, though sarcopenic obesity negates the benefit (132, 133).
5 Nutritional and micronutrient profiles as predictors of PD-1/PD-L1 outcomes
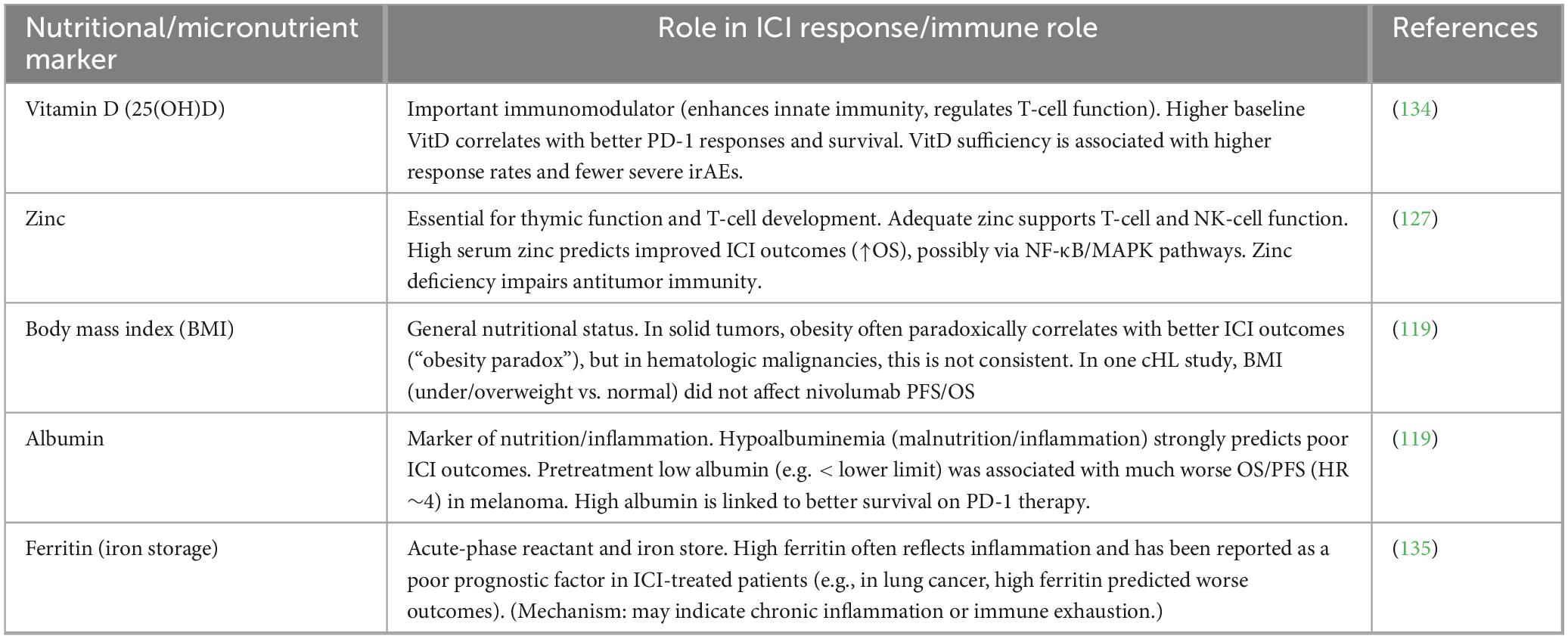
Pre-treatment nutritional status and micronutrient profiles exert a profound influence on the efficacy of PD-1/PD-L1 blockade by directly modulating T-cell metabolism, proliferation, exhaustion state, antigen-presenting cell function, and the composition of the gut microbiome. In hematologic malignancies, where disease-related cachexia, chronic inflammation, marrow infiltration, and prior therapies frequently induce malnutrition, these host factors may be especially relevant to the heterogeneous responses observed with ICIs. While most robust evidence derives from cohorts of patients with solid tumors (melanoma, NSCLC, renal cell carcinoma, and gastrointestinal cancers the underlying immunologic mechanisms are shared and likely apply to lymphomas, MM, and leukemias. Emerging real-world data in cHL and diffuse large B-cell lymphoma (DLBCL) support similar trends. An overview of nutritional and micronutrient markers linked to checkpoint inhibitor efficacy is provided in Table 2.
5.1 Protein-energy malnutrition and survival
Protein-energy malnutrition (PEM) is highly prevalent in hematologic malignancies, affecting 30–60% of patients with aggressive lymphomas and MM, and is characterized by involuntary weight loss, hypoalbuminemia (<35 g/L), low prealbumin (<20 mg/dL), and reduced lean body mass. Multiple large retrospective cohorts have demonstrated that low baseline serum albumin and low Prognostic Nutritional Index (PNI) (PNI = 10 × albumin [g/dL] + 0.005 × lymphocyte count [/mm3]) are independent predictors of inferior objective response rate (ORR, PFS), and OS in patients receiving anti-PD-1/PD-L1 therapy (13, 82, 130, 136).
Mechanistically, hypoalbuminemia reflects chronic systemic inflammation (elevated IL-6, TNF-α) that drives T-cell exhaustion through persistent PD-1 upregulation and reduced mTOR pathway suppression, limiting the amino acid availability required for effector T-cell expansion and memory formation (137). In a multicenter Italian study of > 1 000 patients treated with anti-PD-1/PD-L1 agents, albumin < 35 g/L conferred a hazard ratio of 1.92 for death (82). Similar findings have been reported with the PNI, where PNI < 45 predicted significantly lower ORR (≈15–20% vs. 40–50% in high PNI) and shorter median OS in NSCLC and other cancers (136, 138).
In hematologic malignancies, low albumin is incorporated into established prognostic scores (e.g., International Prognostic Score for Hodgkin lymphoma, R-IPI for DLBCL) and retains prognostic significance in the immunotherapy era. Real-world cohorts of relapsed/refractory cHL treated with nivolumab or pembrolizumab have shown that albumin < 35 g/L is associated with markedly shorter duration of response and increased risk of early progression (139, 140). Prealbumin may be even more sensitive, reflecting acute changes in visceral protein status and predicting non-response in a small series of myeloma patients receiving PD-1-based combinations (141). These data strongly suggest that routine pre-treatment nutritional screening with albumin or PNI could identify patients requiring aggressive supportive care to optimize immunotherapy benefit.
5.2 Iron metabolism, anemia, and immunotherapy response
Anemia affects up to 70% of patients with lymphoma or myeloma at diagnosis and is exacerbated by disease progression or prior therapies. Baseline anemia (Hb < 10–11 g/dL) consistently predicts poorer outcomes with PD-1/PD-L1 blockade across tumor types, with meta-analyses showing hazard ratios of 1.5–2.0 for death (99, 100). High serum ferritin (> 300–500 ng/mL, context-dependent) as an acute-phase reactant reflects systemic inflammation and correlates with resistance to checkpoint inhibition, increased risk of hyperprogressive disease (142, 143).
Mechanistically, elevated hepcidin in inflammatory states sequesters iron in macrophages, depriving T cells of the iron required for proliferation and effector differentiation while simultaneously promoting M2-polarized immunosuppressive macrophages and myeloid-derived suppressor cells (144). In NSCLC cohorts, ferritin > 400 ng/mL was independently associated with reduced OS (HR 1.6–2.1) and lower ORR (145). In hematologic malignancies, hyperferritinemia is a well-known adverse factor in cHL and DLBCL and likely contributes to the immunosuppressive marrow microenvironment that limits PD-1 blockade efficacy in myeloma or leukemia (131). Careful correction of anemia (preferably with transfusion or erythropoietin-sparing approaches) and avoidance of iron overload may represent low-risk interventions to improve immunotherapy outcomes.
5.3 Micronutrients and antioxidant trace elements
Zinc and selenium deficiencies are frequent in hematologic malignancies due to poor intake, malabsorption, and increased utilization. Zinc is essential for thymic function, ZAP-70 signaling, and cytotoxic T-cell activity; low serum zinc levels (< 70 μg/dL) have been associated with significantly lower response rates and shorter PFS/OS in NSCLC patients receiving PD-1 inhibitors (84, 93). Selenium, incorporated into selenoproteins (e.g., GPx-4), protects T cells from oxidative stress during chronic antigen stimulation; low selenium status correlates with higher Treg frequency and reduced effector T-cell function in DLBCL and other cancers (146).
Supplementation studies, though limited, suggest that restoring zinc or selenium levels can enhance CD8+ T-cell cytotoxicity and synergize with PD-1 blockade in preclinical models (92). In DLBCL, low selenium was associated with increased PD-1+ Treg populations, suggesting a direct mechanism of immune evasion that could be reversed by supplementation (93). Routine micronutrient screening and correction may therefore represent a simple, low-cost strategy to augment checkpoint inhibitor efficacy in hematologic patients.
5.4 Diet patterns and metabolomics signatures
Dietary patterns profoundly shape the gut microbiome, which in turn systemically modulates response to PD-1/PD-L1 blockade. High-fiber, plant-rich diets are associated with enrichment of responder-associated taxa (Akkermansia muciniphila, Faecalibacterium prausnitzii, Bifidobacterium spp.) and significantly higher ORR and PFS in melanoma and NSCLC patients treated with anti-PD-1 therapy (147, 148). Conversely, Western-style high-fat/processed food diets correlate with unfavorable microbiome composition and reduced benefit (147). Metabolomic profiling has identified pre-treatment elevations in short-chain fatty acids (butyrate, propionate) and certain amino acid metabolites as predictors of response, reflecting microbial fermentation products that enhance dendritic cell function and T-cell infiltration (132).
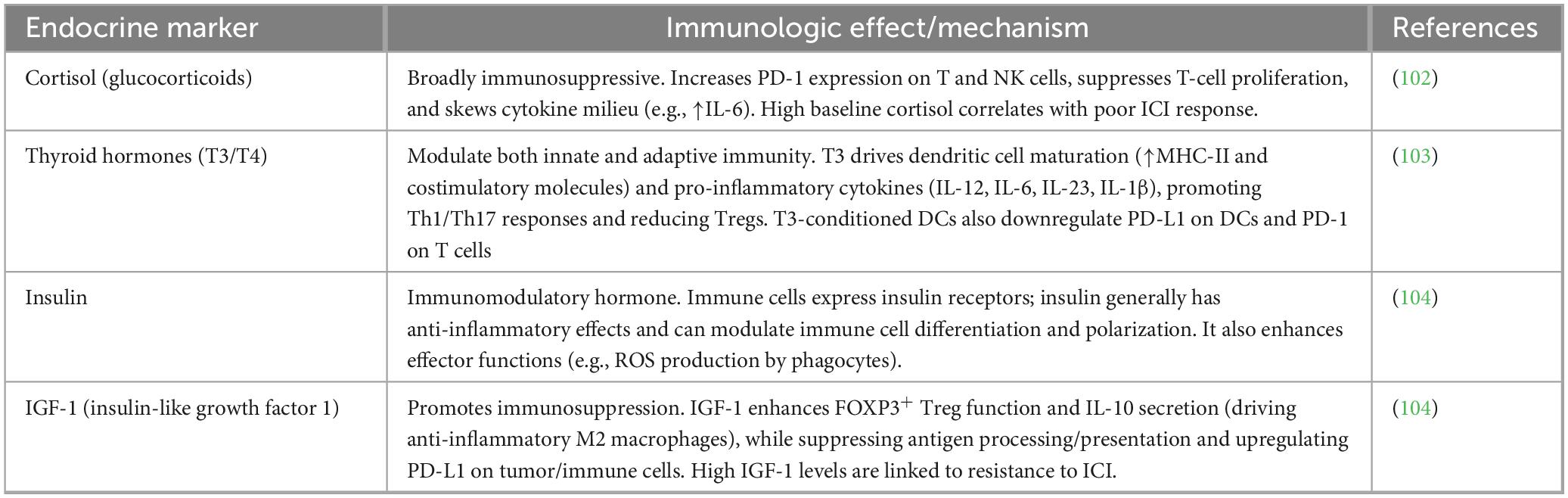
Although direct evidence in hematologic malignancies is limited, dysbiosis is common in lymphoma and myeloma patients (often exacerbated by antibiotics or chemotherapy), and the gut microbiome’s influence on systemic immunity and GVHD after transplant suggests similar relevance for checkpoint inhibitor efficacy (149). Dietary interventions promoting favorable microbiome composition represent a promising adjunctive strategy.
5.5 Obesity, sarcopenia, and body composition analyses
An “obesity paradox” has been repeatedly demonstrated in immunotherapy, with overweight/obese patients (BMI ≥ 25 or ≥ 30 kg/m2) exhibiting superior ORR (up to 2-fold higher), PFS, and OS compared to normal-weight patients in large multicenter cohorts and meta-analyses. The effect is particularly striking in melanoma, NSCLC, and renal cell carcinoma, with hazard ratios for death of 0.6–0.7 in obese patients (150, 151). Proposed mechanisms include increased leptin signaling enhancing CD8+ T-cell function, greater energy reserves supporting prolonged immune activation, and altered adipokine profiles favoring Th1 polarization (152). Preliminary data in classical Hodgkin lymphoma treated with PD-1 inhibitors also suggest a similar trend, with higher BMI associated with improved response duration (153). Representative clinical studies examining endocrine and nutritional predictors of ICI outcomes are summarized in Table 3.
6 Integrated endocrine–nutritional signatures: a new predictive paradigm
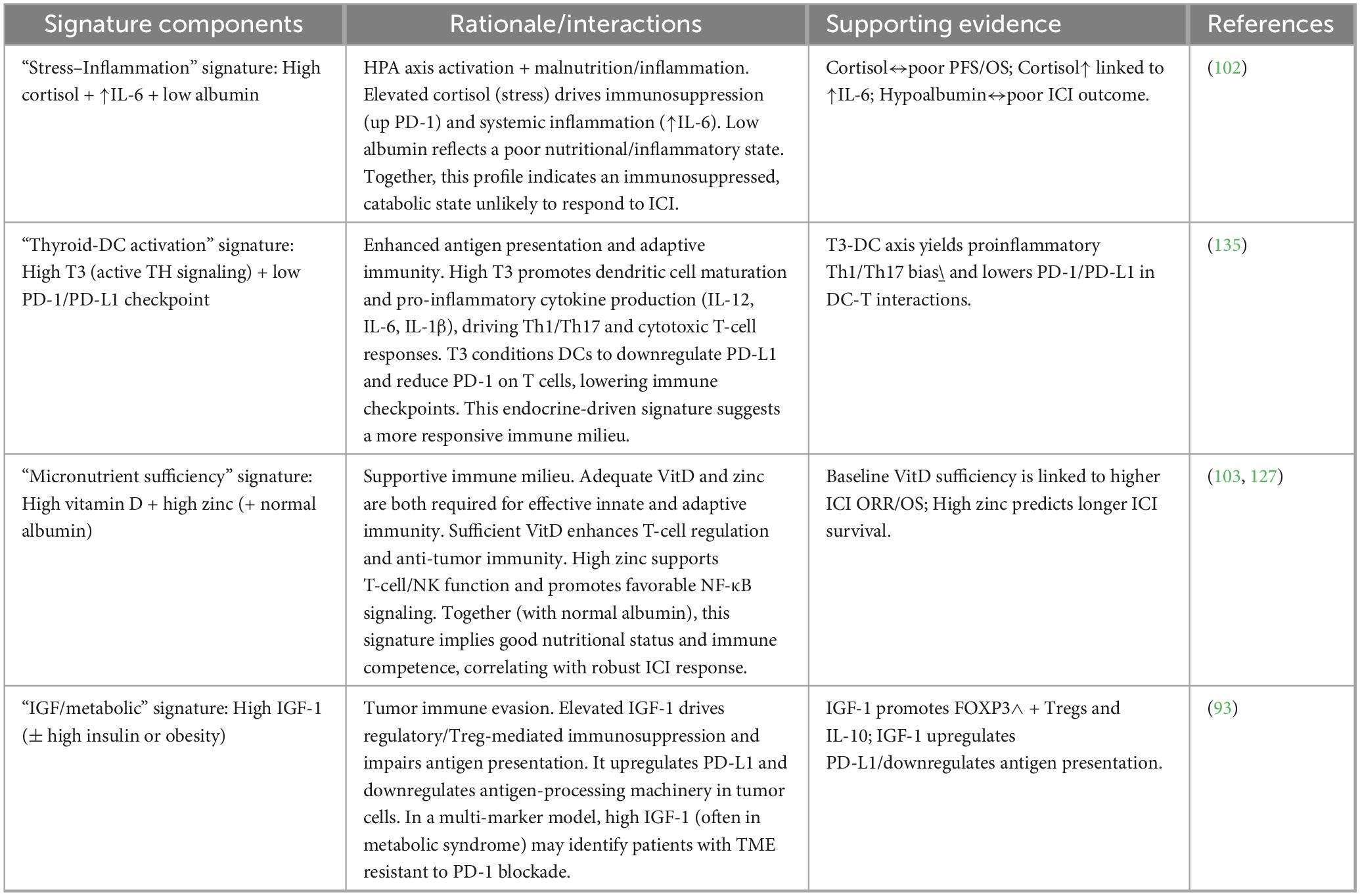
Single biomarkers (e.g., albumin, vitamin D, BMI) frequently fail to reliably predict PD-1/PD-L1 blockade outcomes due to biological redundancy, confounding by disease-related inflammation, and limited capture of multifaceted immuno-endocrine-nutritional crosstalk. Integrated multi-marker signatures combining inflammatory (CRP, NLR), nutritional (albumin, PNI), metabolic (vitamin D, sarcopenia), and adipokine measures offer superior prognostic stratification across cancers treated with ICIs. These composite scores reflect a holistic “systemic immunologic readiness profile” that gauged host capacity to mount and sustain antitumor T-cell responses. Systemic immunologic readiness is the integrated host physiological state determined by endocrine balance, nutritional status, metabolic health, and systemic inflammation that collectively shapes baseline immune competence and the capacity of T cells to be reinvigorated by PD-1/PD-L1 blockade. This concept reflects a whole-body immune context that exists before therapy and modulates treatment responsiveness independently of tumor-intrinsic features. Although most evidence stems from solid tumors, translational data in hematologic malignancies (particularly cHL, diffuse large B-cell lymphoma [DLBCL], and MM) support applicability, with unique marrow-driven metabolic features warranting tailored panels (154). The integrated endocrine–nutritional signatures proposed for hematologic malignancies are summarized in Table 4.
6.1 Why single biomarkers are insufficient
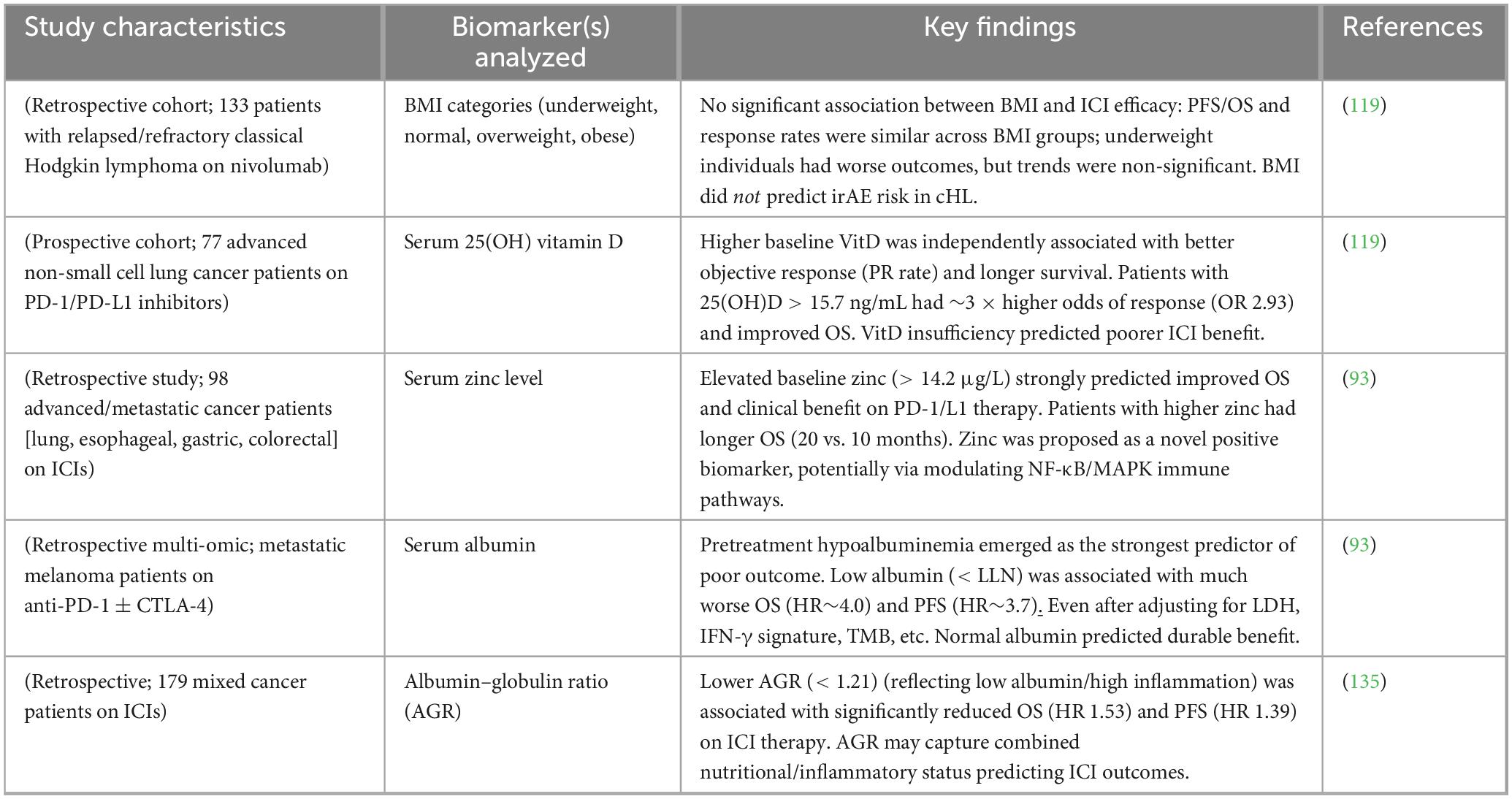
Individual endocrine or nutritional markers demonstrate inconsistent predictive performance for ICI efficacy due to high inter-patient variability, overlapping confounders (comorbidities, steroids, cachexia), and inability to capture synergistic interactions across immuno-metabolic pathways (155, 156). For example, while hypoalbuminemia robustly predicts inferior outcomes, its specificity is limited by non-nutritional causes (hepatic dysfunction, nephrotic syndrome) and failure to account for compensatory mechanisms like adipokine signaling in obesity (85). Similarly, vitamin D deficiency correlates with resistance but shows heterogeneous effect sizes across cohorts, modulated by baseline inflammation or microbiome status (157). BMI alone yields paradoxical results (obesity benefit in many settings) yet ignores muscle quality, where sarcopenia independently drives exhaustion (82). Meta-analyses confirm modest hazard ratios (1.3–1.8) for single markers versus > 2.5–4.0 for composites, underscoring the need for integrated approaches that better reflect the complex “whole-body immunologic tone” governing T-cell fitness (136, 137). Table 5 synthesizes the current clinical evidence linking endocrine and nutritional parameters with PFS and OS in cancer patients receiving ICIs. Collectively, the studies summarized in this table highlight that host metabolic and hormonal status is not merely a background variable but a biologically active determinant of immunotherapy efficacy. Across multiple tumor types and treatment settings, endocrine alterations such as dysregulated thyroid function, cortisol excess, insulin resistance, and sex hormone imbalance emerge as modulators of antitumor immune responses, influencing T-cell activation, exhaustion dynamics, and immune-related adverse event profiles. In parallel, nutritional indices including BMI, sarcopenia, cachexia, serum albumin, PNI, and inflammatory-nutritional composites (e.g., CONUT, GPS, NLR-based scores) consistently correlate with survival outcomes, underscoring the role of systemic energy availability and muscle–immune crosstalk in sustaining effective immune surveillance. Notably, several studies report paradoxical findings such as improved outcomes in overweight patients supporting the concept of an “immunometabolic reserve” that may buffer immune cells against ICI-induced metabolic stress.
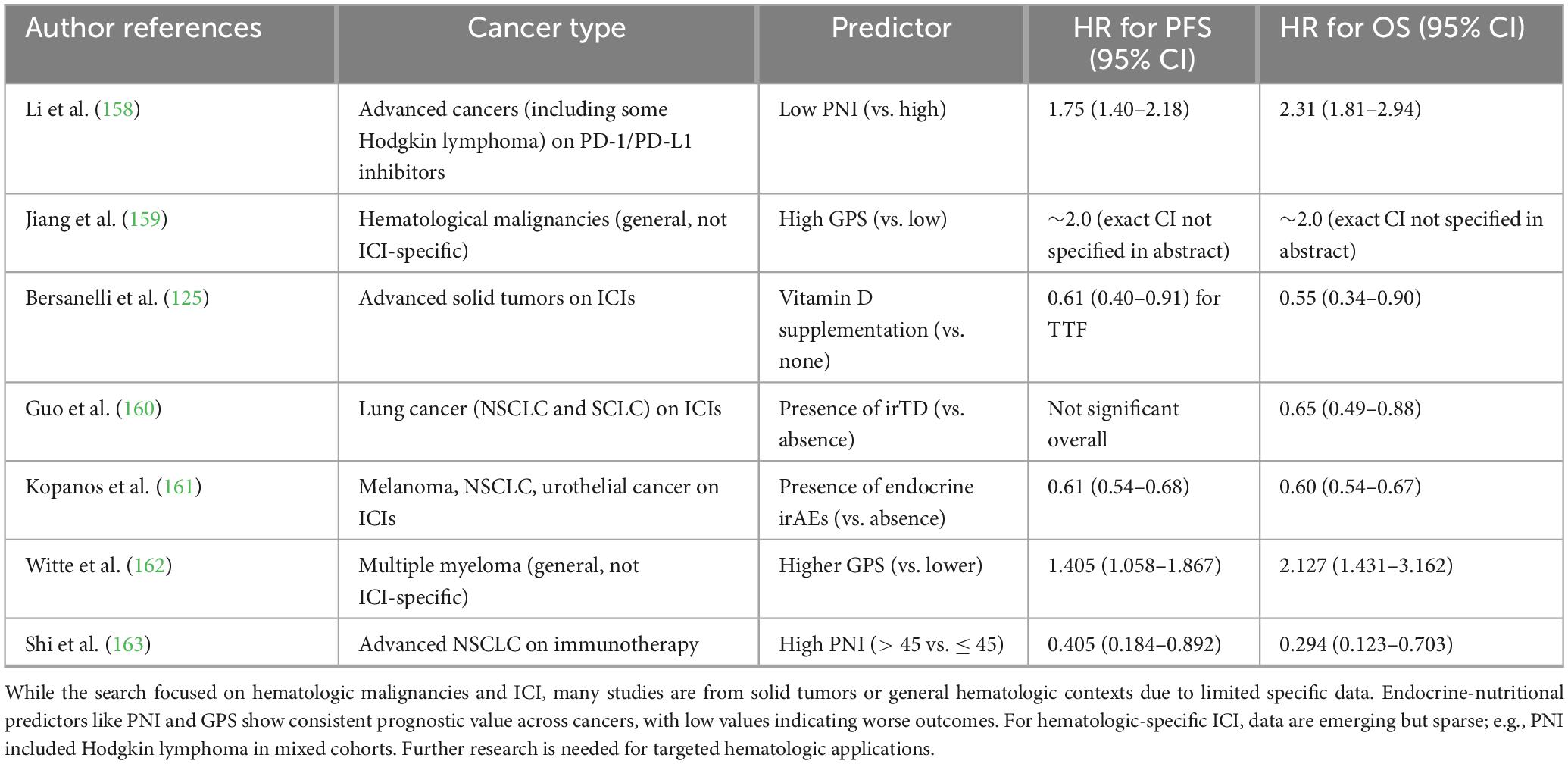
Table 5. Summary of key studies evaluating endocrine-nutritional predictors and their associations with progression-free survival (PFS) and overall survival (OS) in patients treated with immune checkpoint inhibitors (ICIs).
6.2 Concept of a “systemic immunologic readiness profile”
The systemic immunologic readiness profile conceptualizes pre-treatment host status as a composite continuum from “fit” (optimal nutrient reserves, balanced hormones, low inflammation supporting robust CD8+ T cell reinvigoration) to “unfit” (malnutrition, endocrine dysregulation, chronic inflammation enforcing exhaustion and Treg/MDSC dominance) (51, 164). This paradigm shifts focus from tumor-intrinsic features (PD-L1, TMB) to modifiable host factors that determine whether PD-1/PD-L1 blockade can restore effective immunity. Preclinical models demonstrate that combined insults (e.g., cortisol elevation + zinc deficiency + arginine depletion) synergistically impair mTOR signaling and IFN-γ production beyond any single factor (57). Clinically, patients with “fit profiles exhibit higher ORR (50–80% vs. < 20%), longer PFS/OS, and fewer severe irAEs, likely via enhanced T-cell metabolism and reduced alternative checkpoint upregulation (165). In hematologic malignancies, where systemic disease and prior therapies amplify host dysregulation, this profile may explain outlier successes (cHL) versus failures (MM monotherapy) (166).
6.3 Multi-marker signatures studied to date
6.3.1 Albumin + CRP indices
The Glasgow Prognostic Score (GPS) (GPS: CRP > 10 mg/L and albumin < 35 g/L) and modified GPS (mGPS) are among the most validated composites, integrating acute-phase response with visceral protein status. In metastatic cancers treated with ICIs, high GPS/mGPS independently predicts poorer ORR, PFS, and OS across melanoma, NSCLC, and RCC in large cohorts and meta-analyses (167–169). The PNI (PNI = 10 × albumin + 0.005 × lymphocytes) similarly outperforms single markers, with low PNI (< 45) associated with HR 2.0–3.5 for progression in ICI-treated gastric, lung, and mixed cancers (138, 139). Controlling Nutritional Status (CONUT) score adds cholesterol, further refining risk stratification (170).
6.3.2 Vitamin D + inflammatory markers
Combining vitamin D (< 20 ng/mL deficiency) with high CRP, NLR (> 3–5), or GPS enhances predictive accuracy. In NSCLC and melanoma cohorts, deficient vitamin D + elevated inflammation confers the worst outcomes (median PFS < 4 months vs. > 24 months in sufficient/low-inflammation), reflecting impaired DC maturation and microbiome dysbiosis (127, 171). Prospective trials of vitamin D supplementation in high-risk (inflammatory) patients show ORR improvement from ∼35 to ∼55%, supporting dynamic interplay (172).
6.3.3 Sarcopenia + adipokine levels
Body composition analyses reveal sarcopenic obesity (low muscle + high fat) as a high-risk phenotype, combining leptin-driven exhaustion with reduced myokine support for T cells. Meta-analyses (> 5,000 ICI patients) report HR 2.0–3.5 for death in sarcopenic obesity vs. obesity alone (protective HR 0.6–0.7), with leptin/adiponectin ratio emerging as a mechanistic link (173–175). Low adiponectin + sarcopenia predicts severe irAEs and resistance, while high leptin in non-sarcopenic obesity correlates with benefit (83).
6.4 Translating these signatures to hematologic cancers
Hematologic malignancies exhibit unique metabolic vulnerabilities: aggressive lymphomas and myeloma frequently induce protein-energy wasting (30–60% prevalence), hypercatabolism, and marrow adipose tissue remodeling that amplifies MDSC and impair hematopoiesis (19, 176). In cHL, the obesity paradox is pronounced higher BMI/leptin associates with superior duration of response to nivolumab/pembrolizumab, possibly via sustained CD8+ T-cell metabolism overriding 9p24.1-driven evasion (119). DLBCL shows similar trends, with low PNI/GPS predicting early relapse post-ICI combinations (177). MM’s refractory nature to PD-1 monotherapy may stem from profound cachexia, vitamin D deficiency (bone disease), and IGF-1 dysregulation in the marrow niche, fostering T-cell energy (178).
The marrow microenvironment rich in adipocytes, cytokines (IL-6), and hepcidin—amplifies endocrine-nutritional effects: leptin from marrow fat promotes Treg, while iron dysregulation starves T cells (179). Real-world cHL series incorporating PNI report low scores in 40–50% of relapsed patients, correlating with reduced CR rates (139). Emerging data in CAR-T (analogous immune activation) validate CONUT/PNI as predictors in MM, suggesting relevance for ICI combinations (180).
6.5 Potential for composite pre-treatment predictive panels
Composite panels integrating 3–6 markers (e.g., PNI/GPS + vitamin D + sarcopenia index + leptin/adiponectin ratio ± cortisol) could achieve > 80% accuracy in stratifying ICI responders, enabling precision supportive care (nutrition repletion, exercise, metformin, vitamin D) to convert “unfit” to “fit” hosts (180, 181). Machine learning-derived scores (e.g., combining SII + CONUT + BMI) outperform traditional tools in solid tumors and are being validated in lymphoma trials. In hematologic settings, proposed panels might add marrow-specific factors (e.g., ferritin, free light chains) for personalized prediction, potentially augmenting low baseline efficacy in MM or leukemias (182). Prospective trials are essential to standardize cut-offs, timing (circadian variability in cortisol), and interventions.
7 Mechanistic insights: how endocrine and nutritional status alter immune checkpoint response
Endocrine and nutritional status profoundly reprograms immune cell metabolism, signaling, and gene expression, thereby modulating the depth and durability of response to PD-1/PD-L1 blockade. Dysregulated states (hypercortisolism, insulin resistance, leptin excess, vitamin D/zinc deficiency, arginine/tryptophan depletion) converge on pathways that accelerate T-cell exhaustion, suppress antigen presentation, enrich immunosuppressive myeloid cells, and blunt cytokine-driven reinvigoration mechanisms that directly undermine checkpoint inhibitor efficacy (11, 55, 183). Conversely, optimal endocrine–nutritional balance sustains effector T-cell metabolism (glycolysis, OXPHOS, fatty acid oxidation), prevents terminal exhaustion, and enhances IFN-γ-dependent PD-L1 upregulation on tumors, creating a permissive milieu for PD-1/PD-L1 blockade (58, 184). These effects are amplified in hematologic malignancies by the bone marrow niche, where adipocytes, cytokines, and nutrient competition impose additional metabolic constraints (179).
7.1 Impact on T-cell exhaustion, proliferation, and memory
T-cell exhaustion, a hypofunctional state characterized by hierarchical loss of effector cytokines, high co-inhibitory receptor expression (PD-1, TIM-3, LAG-3), and epigenetic remodeling, is metabolically regulated (185). Glucocorticoids, via GR-mediated transactivation, directly upregulate PD-1 and TOX/TOX2 transcription factors while inhibiting mTORC1 and glucose uptake, locking CD8+ T-cells in exhaustion and preventing proliferation/memory formation; blockade of GC signaling restores ICI responsiveness in preclinical models (61, 66).
Leptin, elevated in obesity, activates STAT3/mTORC1 in T cells, leptin-neutralization reinvigorates exhausted T cells and synergizes with PD-1 blockade (82, 84). Hyperinsulinemia/IGF-1 similarly hyperactivates PI3K/AKT/mTORC1, inducing exhaustion while impairing CD8+ memory T precursors; metformin-mediated AMPK activation reverses this and boosts ICI efficacy (186).
Vitamin D/VDR signaling inhibits exhaustion genes (PDCD1, HAVCR2) via direct binding to super-enhancers and promotes stem-like TCF1 + PD-1int progenitors capable of reinvigoration upon PD-1 blockade (12, 124). Zinc deficiency impairs ZAP-70/LCK signaling and IL-2 production, accelerating exhaustion; zinc supplementation restores cytotoxicity and reduces PD-1 expression (187). Arginine and tryptophan availability are critical: tumor/MDSC-derived ARG1/IDO1 deplete these amino acids, activating GCN2/ATF4 stress pathways that upregulate PD-1 and inhibit memory formation; arginine supplementation or IDO inhibitors prevent exhaustion and enhance PD-1 blockade (95, 96). Figure 5 summarizes how endocrine and nutritional factors shape T-cell states and influence PD-1/PD-L1 therapy outcomes.
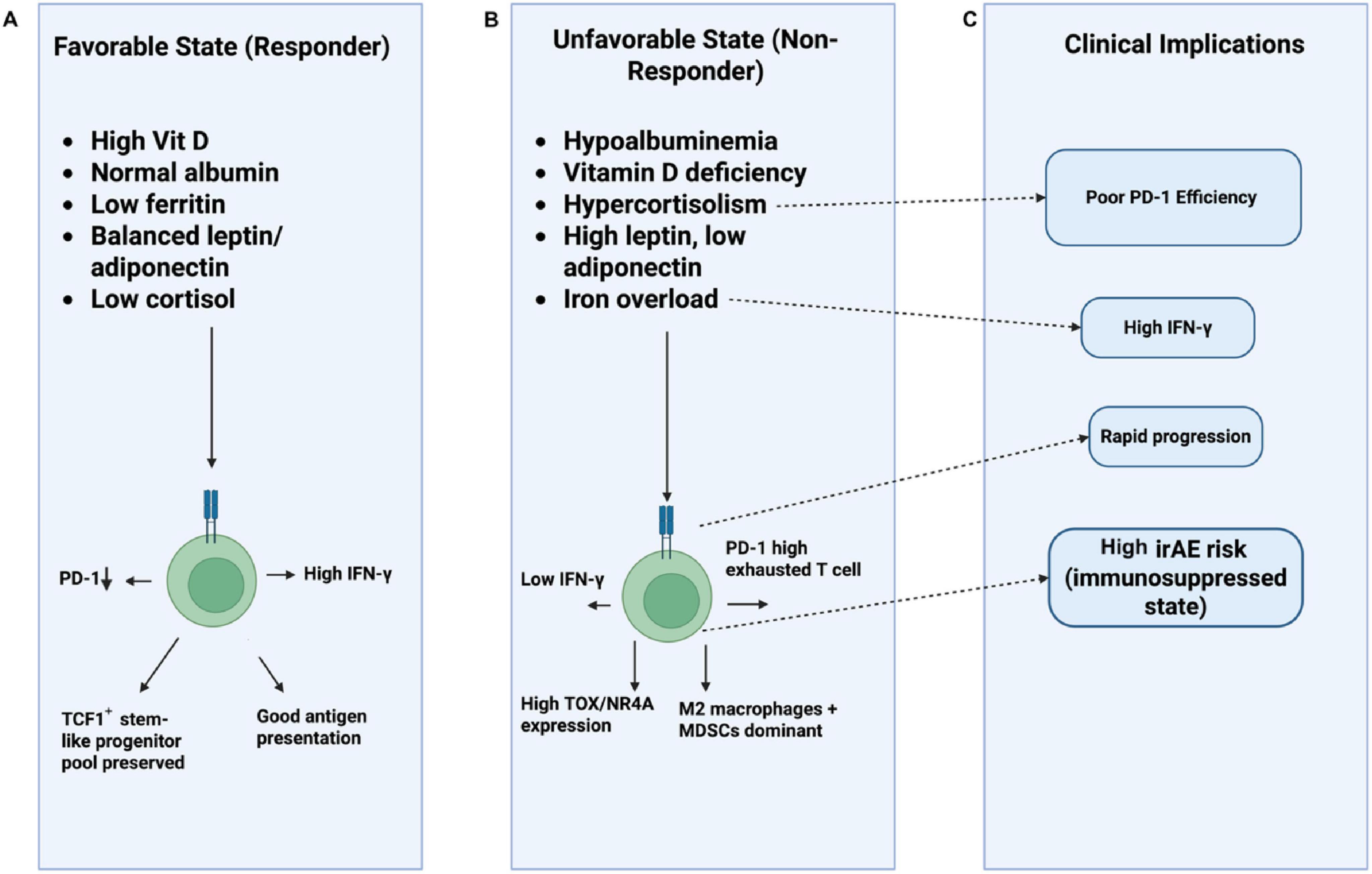
Figure 5. Endocrine-nutritional modulation of T-cell fate and immune checkpoint therapy response. This schematic illustrates how metabolic, endocrine, and nutritional states shape T-cell functionality and influence clinical outcomes during PD-1/PD-L1 checkpoint inhibition. (A) Track A (Favorable State) shows conditions associated with better therapeutic response including sufficient vitamin D, normal albumin, balanced adipokines, low ferritin, and reduced cortisol promoting PD-1low effector activity, preserved TCF1+ progenitor pools, robust IFN-γ production, and efficient antigen presentation. (B) Track B (Unfavorable State) depicts deficiencies and dysregulations such as hypoalbuminemia, vitamin D deficiency, hypercortisolism, adipokine imbalance, and iron overload, which drive PD-1high T-cell exhaustion, elevated TOX/NR4A expression, reduced IFN-γ, and a suppressive myeloid milieu dominated by M2 macrophages and MDSCs. (C) Track C (Clinical Implications) summarizes downstream consequences, linking these states to poor PD-1 inhibitor efficacy, rapid disease progression, and higher incidence of immune-related adverse events due to overall immunosuppression.
7.2 Effects on antigen-presenting cells and myeloid compartment
Endocrine–nutritional cues reprogram APCs and MDSCs, determining co-stimulatory capacity and immunosuppressive polarization. Vitamin D is a master regulator of DC maturation: 1,25(OH)2D induces tolerogenic phenotype (↑CD14, ↓CD80/86, ↑IL-10) in steady state but enhances cross-presentation and CXCL10 production in inflammatory contexts, promoting CD8+ priming and ICI efficacy (188, 189). Zinc and selenium support lysosomal function and ROS detoxification in DCs, preserving MHC-II expression and co-stimulatory molecule expression; deficiency impairs antigen presentation and favors MDSC expansion (94).
Cortisol and leptin drive M2/macrophage polarization via PPARγ/STAT6 and suppress MHC-II/CD86 on APCs, limiting T-cell priming (65). In obesity, leptin-rich marrow adipocytes secrete IL-6/TGF-β that expand PMN-MDSCs expressing PD-L1 and ARG1, directly inhibiting CD8+ T-cell function (19). Iron dysregulation (high ferritin/hepcidin) promotes MDSC accumulation and M2 polarization while starving T cells; ferroptosis induction in MDSCs enhances ICI responses (179). These myeloid shifts create a “cold” pre-treatment state resistant to PD-1/PD-L1 blockade.
7.3 Modulation of tumor microenvironment in hematologic cancers
The bone marrow TME in lymphomas and myeloma is uniquely sensitive to endocrine–nutritional inputs due to high adipocyte content, chronic IL-6 signaling, and nutrient competition for glucose/amino acids (190). Marrow adipose tissue (MAT) expands dramatically in MM and aggressive lymphomas, secreting leptin, adiponectin (often reduced), and free fatty acids that reprogram resident immune cells (191). Leptin from MAT drives PD-L1 expression on myeloma cells via JAK2/STAT3 and promotes MDSC/Treg infiltration, while adiponectin deficiency removes CD8+-supportive signals (192).
Vitamin D deficiency, prevalent in MM due to bone disease, exacerbates marrow immunosuppression by reducing CXCL10-mediated T-cell trafficking and enhancing RANKL-driven osteoclastogenesis that releases TGF-β (193). Insulin/IGF-1 hyperactivation in obese myeloma patients upregulates PD-L1 on plasma cells and fosters “exhausted-like” marrow-resident T cells with high TOX expression (194). Zinc/selenium depletion—common from poor intake/malabsorption—impairs NK-cell degranulation against RS cells in cHL, contributing to the immunosuppressive niche (93). Thus, unfavorable endocrine–nutritional profiles reinforce marrow as an immune-privileged sanctuary, explaining limited PD-1 monotherapy success in MM vs. cHL (where 9p24.1 amplification overrides some constraints).
7.4 Interplay with cytokine and chemokine networks
Endocrine–nutritional status orchestrates cytokine networks that determine whether PD-1 blockade triggers productive IFN-γ-driven tumor control or futile inflammation. IFN-γ induces PD-L1 on tumor/APCs to enable adaptive resistance; however, chronic IL-6 (driven by obesity, stress, malnutrition) trans-signals STAT3 to upregulate alternative checkpoints (TIM-3, LAG-3) and anti-apoptotic genes, blunting IFN-γ efficacy (97). Cortisol suppresses IFN-γ/IL-2 while elevating IL-10/TGF-β, shifting from Th1 to Treg-polarizing milieu (105).
Vitamin D and short-chain fatty acids (from fiber-rich diets enhance IFN-γ/CXCL9/10/11 production by DCs, promoting CD8+ T-cell trafficking and PD-1 blockade sensitivity (100). Leptin amplifies IL-6/TNF-α loops that sustain exhaustion, whereas adiponectin dampens them (83). Arginine depletion inhibits iNOS-derived NO needed for chemokine receptor expression, impairing T-cell migration; restoration reinvigorates cytokine networks (95).
7.5 Influence on immune-related adverse events
Paradoxically, endocrine–nutritional factors that impair antitumor efficacy often protect against severe irAEs by limiting systemic immune activation. Thyroid irAEs (most common endocrine toxicity, 10–40%) reflect breakthrough autoimmunity: patients developing thyroiditis exhibit higher baseline T-cell reactivity and IFN-γ signatures, translating to both superior tumor control and autoimmunity risk (69, 195). Vitamin D sufficiency correlates with increased irAE incidence (particularly colitis, pneumonitis) but improved survival, likely via enhanced T-cell reinvigoration (125).
Obesity/leptin excess is associated with higher rates of severe irAEs, possibly via tonic mTOR activation, lowering activation threshold (196). Conversely, malnutrition (low albumin, PNI) predicts fewer irAEs but worse oncologic outcomes, reflecting globally suppressed immunity (197). Baseline GC use or endogenous hypercortisolism dramatically reduces irAE risk but abolishes ICI benefit (106). Thus, the same mechanisms driving resistance (exhaustion promotion) often confer irAE protection, while “fit” profiles yield both better tumor responses and manageable toxicity.
8 Clinical applications and future directions
Pre-treatment endocrine–nutritional optimization represents a low-cost, low-toxicity strategy to enhance PD-1/PD-L1 blockade efficacy, with emerging prospective data supporting vitamin D repletion, metformin for insulin resistance, and structured nutritional support. Although most evidence derives from solid tumors, mechanistic overlap and real-world hematologic cohorts suggest translatability, particularly in cachexia-prone lymphomas and myeloma. Integration of simple blood-based composites (e.g., PNI + vitamin D + ferritin) with tumor biomarkers could enable precision supportive care, converting “unfit” hosts into better responders.
8.1 Developing clinically feasible endocrine–nutritional assessments
Routine, low-cost blood tests (albumin, prealbumin, CRP, vitamin D, ferritin, zinc, selenium, morning cortisol, TSH/free T4, fasting glucose/insulin or C-peptide, leptin/adiponectin ratio) combined with CT-based body composition (skeletal muscle index, subcutaneous/visceral fat) provide a feasible “immuno-nutritional panel” implementable in standard oncology practice (183, 198). PNI, CONUT score, and GPS require only CBC and chemistry, achieving high stratification power in ICI cohorts (136). Point-of-care or multiplex assays for adipokines and micronutrients are emerging, with turnaround < 48 h. In hematologic settings, adding serum-free light chains or hepcidin may refine marrow-specific risk (199).
8.2 Can we intervene before therapy?
Prospective trials increasingly support pre-emptive correction of deficiencies to augment ICI outcomes.
8.2.1 Vitamin D correction
Vitamin D deficiency (<20 ng/mL) is correctable with 50,000 IU weekly loading followed by 2,000–4,000 IU daily maintenance. A 2023 prospective cohort (n = 200 + ICI patients) showed systematic supplementation improved ORR from 36 to 56% and median PFS from 5.8 to 11.3 months, with greater CD8+ T-cell reinvigoration and reduced severe irAEs (125). A multicenter analysis confirmed that higher serum 25(OH)D and supplementation independently predicted superior survival in PD-1-treated solid tumors, with similar trends in lymphoma subsets (87).
8.2.2 Iron balance
High ferritin and functional iron deficiency predict resistance via MDSC expansion. Careful IV iron in true deficiency or hepcidin inhibitors (preclinical) may help, but excess iron worsens outcomes; guidelines recommend avoiding overload and monitoring transferrin saturation (200, 201).
8.2.3 Nutrition support
High-protein oral supplementation + resistance exercise reverses sarcopenia and improves PNI in 4–8 weeks. Mediterranean or high-fiber diets enrich responder microbiomes (Akkermansia, Faecalibacterium), with a 2025 phase II trial showing high-fiber intervention increased ORR by 20–30% in ICI patients (100, 202). Enteral/parenteral nutrition in severe malnutrition stabilizes weight and albumin pre-ICI (203).
8.2.4 Hormonal optimization
Metformin in insulin-resistant patients activates AMPK, reduces MDSCs, and potentiates PD-1 blockade; phase II trials in NSCLC/melanoma report ORR ↑20–40% and PFS HR 0.6–0.7 when added to nivolumab/pembrolizumab (59, 204, 205). Thyroid replacement in hypothyroidism and physiologic hydrocortisone in adrenal insufficiency are standard; androgen deprivation in males is under investigation (75).
8.3 Precision supportive care to augment immunotherapy
Risk-stratified supportive algorithms, intensive intervention for high-risk profiles (low PNI + vitamin D deficiency + sarcopenia) could increase population-level ICI benefit by 15–30% at minimal cost (13). Ongoing trials combine metformin + high-protein diet + vitamin D in “unfit” patients starting PD-1 therapy.
8.4 Integration with genomic/immune biomarkers
Composite scores merging host (PNI/GPS + vitamin D) with tumor factors (PD-L1 TPS, TMB, 9p24.1 status in cHL) outperform either alone, achieving > 85% accuracy in predicting durable response in retrospective validation (206, 207). Machine learning models incorporating sarcopenia, leptin, and IFN-γ signature are in development for hematologic ICI combinations (208).
8.5 Proposed algorithm for clinical translation
1. Baseline assessment (day 14 to day 0): blood panel + CT body composition.
2. Risk stratification: low-risk (PNI > 45, vit D > 30, no sarcopenia) → proceed to ICI; high-risk → intervene 4–8 weeks (vitamin D repletion, metformin if HOMA-IR > 2.5, high-protein ONS + exercise, consider short-course nutrition if CONUT ≥ 5.
3. Re-assess panel pre-cycle 1; initiate ICI ± continued support.
4. Monitor response and irAEs; escalate nutrition if progression/cachexia (209).
This pragmatic framework, adaptable to resource settings, warrants prospective validation in phase III adjuvant/neo-adjuvant ICI trials in lymphoma and myeloma. Figure 6 outlines the proposed clinical workflow for endocrine–nutritional optimization before initiating PD-1/PD-L1 therapy. To operationalize the proposed assessment model, we provide a structured framework summarizing endocrine and nutritional biomarkers that can be incorporated into standard pre-treatment evaluation for ICI candidates (Table 6). These markers are chosen based on availability, clinical relevance, and potential to inform risk stratification and supportive interventions.
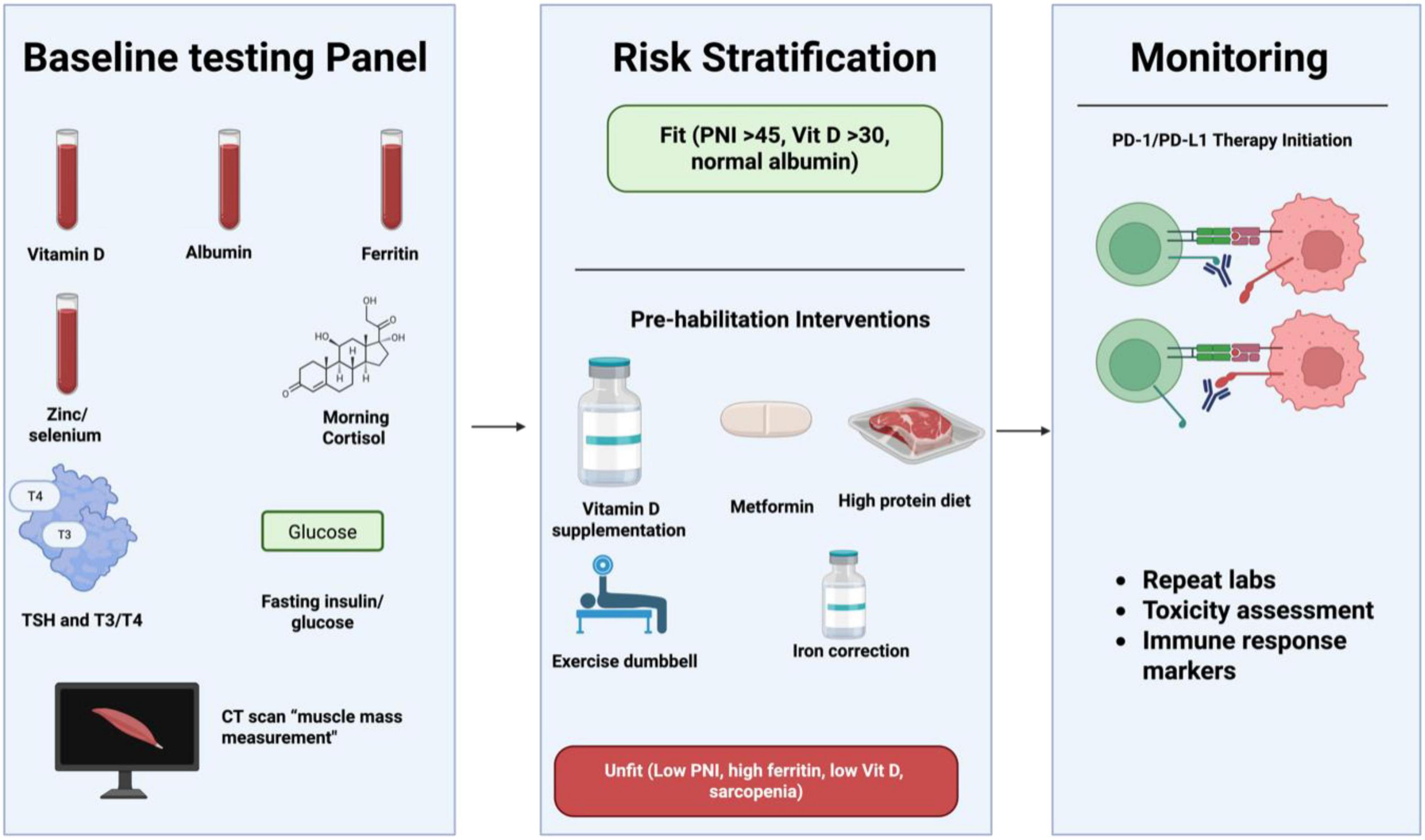
Figure 6. Clinical workflow for endocrine–nutritional optimization before PD-1/PD-L1 immunotherapy. This algorithm summarizes a structured pre-treatment approach designed to enhance immune checkpoint inhibitor efficacy. Step 1 (Baseline Testing Panel): Comprehensive assessment including serum vitamin D, albumin, ferritin, zinc/selenium, morning cortisol, thyroid function (TSH, free T4), fasting insulin–glucose, and imaging-derived muscle mass evaluation. Step 2 (Risk Stratification): Patients are categorized as Fit (e.g., PNI > 45, vitamin D > 30 ng/mL, normal albumin) or Unfit based on markers of malnutrition, inflammation, micronutrient deficiencies, and sarcopenia. Step 3 (Pre-habilitation Interventions): Targeted corrective strategies—vitamin D repletion, metformin use for metabolic optimization, high-protein dietary support, structured exercise, and cautious management of iron overload or deficiency. Step 4 (Therapy Initiation): Commencement of PD-1/PD-L1 blockade with antibody–receptor interaction illustrated. Step 5 (Monitoring): Ongoing evaluation through repeat laboratory testing, toxicity screening, and immunologic response tracking to ensure optimal therapeutic benefit.
Table 6 presents a structured pre-treatment endocrine and nutritional assessment framework designed to optimize patient selection and risk stratification prior to initiation of ICI therapy. This framework integrates routinely accessible hormonal, metabolic, and nutritional parameters to capture baseline host immunometabolic competence, which is increasingly recognized as a determinant of ICI responsiveness and toxicity. Pre-existing endocrine abnormalities such as subclinical thyroid dysfunction, adrenal axis dysregulation, glucose intolerance, and sex hormone imbalance—may predispose patients to altered immune activation thresholds, exaggerated immune-related adverse events, or premature immune exhaustion, thereby influencing both therapeutic efficacy and tolerability. Concurrently, nutritional and body composition metrics including BMI, sarcopenia indices, serum albumin, prealbumin, PNI, and inflammation-linked nutritional scores serve as surrogates for systemic energy reserve, muscle-derived immunomodulatory signaling, and chronic inflammatory burden. By synthesizing these dimensions, the framework outlined in Table 6 moves beyond single biomarker evaluation toward a holistic, host-centered stratification model, enabling identification of patients who may benefit from prehabilitation strategies such as nutritional supplementation, endocrine correction, or metabolic optimization before ICI exposure. Importantly, this approach supports a shift from reactive management of immune-related complications to proactive personalization of immunotherapy, positioning endocrine–nutritional profiling as a pragmatic and scalable tool for improving clinical outcomes.
8.6 Clinical feasibility and implementation considerations
Although endocrine–nutritional signatures show strong biological plausibility and prognostic relevance, their clinical utility depends on feasibility, standardization, and cost-effectiveness. Importantly, many of the proposed biomarkers are already routinely measured in standard oncology practice, facilitating near-term translation without the need for specialized assays (210, 211).
8.6.1 Biomarker availability and cost
Core nutritional and inflammatory markers such as serum albumin, CRP, complete blood count, ferritin, and glucose are inexpensive, widely available, and standardized across laboratories. Composite indices derived from these parameters, including the PNI, GPS, and CONUT score, require no additional testing and can be calculated retrospectively or prospectively at minimal cost. Endocrine parameters such as TSH, free T4, fasting glucose, and insulin are similarly routine and inexpensive, while vitamin D testing is increasingly standardized and cost-effective in many healthcare systems (212). In contrast, certain biomarkers such as leptin/adiponectin ratios, selenium levels, or morning cortisol may be less routinely available and incur higher costs or longer turnaround times. These markers may therefore be best reserved for research settings or high-risk patients, rather than universal screening, until stronger prospective validation is achieved.
8.6.2 Inter-laboratory variability and standardization
Variability in assay platforms and reference ranges represents a key challenge, particularly for biomarkers such as vitamin D, ferritin, cortisol, and adipokines. Circadian variation (e.g., cortisol), acute inflammatory states, and concurrent medications (e.g., corticosteroids, thyroid hormone replacement) can further confound interpretation. To mitigate these issues, standardized timing (e.g., morning sampling for cortisol), repeated baseline measurements, and use of clinically established cut-offs rather than institution-specific percentiles are recommended.
Importantly, composite indices that integrate multiple parameters (e.g., PNI or GPS) are inherently more robust to single-measurement variability and may offer superior reproducibility across institutions compared with single biomarkers.
8.6.3 Clinical workflow integration
From a practical standpoint, endocrine–nutritional assessment can be incorporated into routine pre-immunotherapy evaluation without delaying treatment initiation. Most markers can be obtained within standard pre-treatment laboratory panels, allowing risk stratification within days. Patients identified as “high-risk” based on these profiles may benefit from early supportive interventions (nutritional optimization, vitamin D repletion, metabolic control) without altering oncologic treatment selection (213–215).
8.6.4 Regulatory and translational considerations
Unlike tumor genomic biomarkers, endocrine–nutritional markers do not require regulatory approval as companion diagnostics, lowering barriers to implementation. However, prospective validation studies and harmonized reporting standards are needed before these signatures can be adopted as decision-modifying tools. Future clinical trials incorporating immune checkpoint inhibitors in hematologic malignancies should prospectively collect these parameters to define optimal cut-offs, timing, and intervention strategies. Overall, the low cost, wide availability, and biological relevance of endocrine–nutritional biomarkers make them attractive candidates for real-world clinical application, particularly as adjunctive tools to complement tumor-intrinsic predictors rather than replace them.
8.7 Prospective cohort design, integrative biomarker panels, and AI-based prediction models
Advancing endocrine–nutritional signatures for predicting PD-1/PD-L1 blockade efficacy in hematologic malignancies requires robust prospective cohort designs to overcome retrospective limitations, such as selection bias and incomplete data. Prospective studies enable standardized baseline assessments of hormones (e.g., cortisol, thyroid), nutrients (e.g., vitamin D, zinc), and composites like the PNI, alongside longitudinal monitoring of ORR, PFS, and irAEs. The PROVIDENCE study (2023), a prospective observational trial in advanced cancer patients on ICIs, demonstrated that systematic vitamin D supplementation improved ORR from 36 to 56% and median PFS from 5.8 to 11.3 months, while reducing thyroid irAEs. In hematologic contexts, adapting this approach could involve cohorts of relapsed/refractory cHL or MM patients, stratifying by marrow infiltration and prior therapies, which often induce cachexia and dysregulate the immuno-endocrine axis. Challenges include heterogeneity in disease subtypes and high attrition, but powering for endpoints like 12-month PFS could yield level I evidence, informing guidelines for pre-treatment interventions (125, 216–218).
Integrative biomarker panels enhance predictive accuracy by combining endocrine (e.g., insulin/IGF-1), nutritional (e.g., albumin, ferritin), inflammatory (e.g., CRP/neutrophil-lymphocyte ratio), and body composition metrics (e.g., sarcopenia via CT). Panels like the Systemic Immune-Inflammation Index predict poorer OS/PFS in ICI-treated cancers, reflecting T-cell exhaustion and myeloid suppression. In hematologic malignancies, the Systemic Immunologic Readiness Score integrating GPS, PNI, and microbiome data stratifies “fit” vs. “unfit” patients, with high scores correlating to superior ORR in cHL. Preclinical models validate synergy: hypercortisolism plus hypoalbuminemia triples resistance via mTOR/IFN-γ inhibition. Hematology-tailored panels could incorporate hepcidin for iron dysregulation in MM, enabling targeted therapies like metformin or nutritional support to boost efficacy (219–223). AI-based prediction models revolutionize personalization by integrating multi-omics (genomics, metabolomics) with endocrine–nutritional data. Machine learning algorithms, such as random forests, predict ICI response with > 85% accuracy using routine blood parameters in pan-cancer cohorts. In 2025 updates, AI models for PD-1/PD-L1 efficacy in hematologic malignancies incorporate PD-L1 expression estimation from H&E slides, forecasting PFS in NSCLC analogs adaptable to lymphomas. Federated learning across registries (e.g., LYSA) handles real-time inputs like cortisol rhythms, identifying non-responders for adjunctives. Ethical issues like bias require validation in trials (e.g., NCT05352750), but these models could optimize r/r settings, addressing gaps in tumor-centric biomarkers (224–228).
8.8 Future directions and global applicability
Future studies should address ethnic and geographic variability in pre-treatment endocrine–nutritional signatures to enhance the global applicability of these predictive biomarkers for PD-1/PD-L1 blockade. Baseline levels of key components such as vitamin D, iron stores, thyroid hormones, cortisol, and nutritional indices vary substantially across populations due to differences in latitude, sunlight exposure, dietary patterns, genetic polymorphisms, socioeconomic factors, and healthcare access. For example, vitamin D deficiency is highly prevalent in populations residing at higher latitudes and among individuals with increased skin pigmentation, cultural sun-avoidance practices, or limited dietary fortification, potentially influencing immune competence and immunotherapy responsiveness. Similarly, regional differences in iron deficiency or overload, micronutrient availability (e.g., zinc and selenium), and sarcopenia prevalence may differentially shape systemic immunologic readiness across ethnic groups. Prospective, multi-ethnic cohorts are therefore needed to define population-specific reference ranges and thresholds for these signatures, as well as to determine whether their predictive value is consistent across diverse hematologic malignancies and treatment settings. Incorporating geographic, lifestyle, and genetic modifiers into predictive models may enable more accurate patient stratification and support the development of tailored nutritional or endocrine interventions to optimize immunotherapy outcomes worldwide.
9 Challenges
Translating endocrine–nutritional signatures into reliable predictors of PD-1/PD-L1 blockade outcomes faces substantial hurdles that currently limit clinical adoption (229). Disease heterogeneity poses a primary challenge: hematologic malignancies exhibit profound inter- and intra-patient variability in metabolic phenotypes, e.g., Reed-Sternberg cells in classical Hodgkin lymphoma drive unique leptin-rich microenvironments, while MM features IGF-1-dominated marrow niches, making universal cut-offs elusive and requiring disease-specific validation (190). Confounders further complicate interpretation: concomitant corticosteroids (used in 20–40% of patients for symptom palliation or irAE management) potently suppress T-cell function and confound inflammatory/nutritional indices, with baseline use independently worsening PFS/OS (HR 1.5–2.5) yet often unavoidable in lymphoproliferative disorders (106, 108). Cancer cachexia, affecting 50–80% of advanced patients, drives hypoalbuminemia and sarcopenia through IL-6-mediated hypercatabolism rather than pure malnutrition, masking true immunologic readiness (168). Comorbidities (diabetes, obesity, autoimmune disease) introduce bidirectional bias, as insulin resistance promotes exhaustion while obesity paradoxically protects via leptin-sensitive subsets (81, 230).
9.1 Knowledge gaps
Endocrine markers display marked circadian variability, with cortisol peaks at 06:00–08:00 and nadirs at midnight, with flattened rhythms prognostic of poorer survival in multiple cancers necessitating standardized morning sampling that is rarely enforced in retrospective cohorts (231, 232). The evidence base remains predominantly observational, with few prospective interventional trials: vitamin D repletion studies show promise but are limited by small size and lack of randomization, while nutritional prehabilitation lacks phase III data in ICI settings (233, 234). Finally, the absence of consensus cut-offs hampers comparability. Albumin thresholds range 30–35 g/L, PNI 40–50, vitamin D 20–30 ng/mL across studies reflecting population-specific inflammation burdens and assay differences (235). These gaps underscore the need for large, prospective, biomarker-driven trials incorporating serial sampling, confounder adjustment, and harmonized thresholds to establish causative links and enable routine clinical use (236, 237).
10 Limitations
While this review synthesizes current evidence on endocrine–nutritional signatures and their relevance in hematologic malignancies, several limitations should be acknowledged.
10.1 Heterogeneity of evidence
The mechanistic frameworks we describe draw from studies with diverse designs, patient populations, and endpoints. Biomarker associations identified in retrospective cohorts may not fully capture causal relationships and may reflect underlying confounders. Moreover, different studies employ varying assay platforms, cut-offs, and composite index definitions, which complicates direct comparisons and quantitative synthesis.
10.2 Small and selective cohorts
Several mechanistic insights and prognostic associations derive from small or single-institution cohorts. These studies may be underpowered to detect modest effects, and findings may not generalize across broader demographic or disease subgroups. The limited sample sizes also restrict our ability to evaluate interactions among biomarkers, treatment regimens, and clinical outcomes.
10.3 Speculative biological pathways
Although many endocrine–nutritional pathways have plausible roles in modulating immune function and treatment response, some proposed mechanisms remain speculative due to incomplete experimental validation. For example, the interplay between adipokines, systemic metabolism, and antitumor immunity has strong preclinical support but requires further confirmation in longitudinal clinical studies with standardized sampling.
10.4 Measurement variability and timing
As discussed above, biomarker measurements may vary with circadian rhythms, acute illness, and assay methodology. Most published studies do not report standardized timing or repeated measures, which could influence observed associations. This limitation is particularly relevant for hormones with diurnal variation or for markers influenced by concurrent medications (e.g., corticosteroids).
The manuscript’s reliance on retrospective cohorts introduces significant biases, including selection bias from non-randomized patient inclusion, confounding by unmeasured variables (e.g., comorbidities or prior therapies), and incomplete data on endocrine-nutritional profiles, potentially overestimating associations with PD-1/PD-L1 outcomes. For instance, real-world studies in hematologic malignancies often lack standardized biomarker assessments, leading to inconsistent findings on predictors like hypoalbuminemia or vitamin D deficiency. Small sample sizes further compound issues, limiting statistical power and increasing the risk of spurious results or type II errors, as seen in early-phase ICI trials where cohorts under 100 patients fail to detect subgroup effects in diverse hematologic subtypes. Overrepresentation of Asian populations in cited studies (e.g., higher EGFR mutation rates influencing immunotherapy responses) may reduce generalizability to Western cohorts, where genetic and environmental factors differ, potentially skewing efficacy estimates for endocrine signatures (238–242). Future research should prioritize prospective, multicenter trials with larger, diverse cohorts to mitigate biases and validate biomarkers like PNI or GPS. Strategies include integrating multi-omics (e.g., metabolomics with nutritional data), machine learning for predictive models, and global collaborations to ensure ethnic representation, enhancing translational applicability in hematologic settings (226, 243–245).
10.5 Future directions to mitigate limitations
Prospective, multicenter studies with standardized protocols are needed to validate promising signatures and to clarify causal mechanisms. Harmonized reporting standards and collaborative consortia will facilitate meta-analyses that overcome individual cohort limitations. Integration of mechanistic studies with clinical outcomes will strengthen the biological rationale and translational potential of endocrine–nutritional markers. By acknowledging these constraints, our review aims to present a balanced interpretation of current evidence while highlighting opportunities for future research.
11 Conclusion
In summary, pre-treatment endocrine–nutritional signatures emerge as pivotal, modifiable predictors of PD-1/PD-L1 blockade efficacy in hematologic malignancies, extending beyond tumor-centric biomarkers. Dysregulations in cortisol, thyroid hormones, sex steroids, insulin/IGF-1, adipokines, vitamin D, zinc, and protein status converge to impair T-cell reinvigoration, antigen presentation, and marrow microenvironment permissiveness, driving heterogeneous responses. Integrated multi-marker panels, such as GPS or PNI combined with vitamin D and sarcopenia indices, provide robust prognostic stratification, with potential to guide personalized interventions like hormone modulation, micronutrient repletion, metformin, or dietary optimization. While evidence is strongest in cHL and PMBCL, applicability to refractory MM and leukemias warrants prospective trials to validate signatures, standardize cut-offs, and test adjunctive strategies. By addressing these host factors, we can convert immunologically “unfit” patients to responders, enhancing durable remissions and reducing resistance in this challenging landscape. Future research should incorporate machine learning for dynamic profiling and microbiome integration to fully harness endocrine–nutritional crosstalk for immunotherapy success.
Author contributions
NH: Data curation, Formal analysis, Writing – review & editing, Methodology, Software, Writing – original draft, Investigation. YG: Funding acquisition, Supervision, Conceptualization, Project administration, Methodology, Resources, Writing – original draft, Writing – review & editing, Investigation, Visualization.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by Future Plan for Traditional Chinese Medicine development of Science and Technology of Shanghai Municipal Hospital of Traditional Chinese Medicine (WL-HBMS-2021009K).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel R, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Batista J, Birmann B, Epstein M. Epidemiology of hematologic malignancies. Pathology and epidemiology of cancer. Berlin: Springer (2016). p. 543–69.
4. Ansell S. Hodgkin lymphoma: 2025 update on diagnosis, risk-stratification, and management. Am J Hematol. (2024) 99:2367–78. doi: 10.1002/ajh.27470
5. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. (2018) 36:1675–84. doi: 10.1200/JCO.2017.77.0412
6. Ansell S, Bröckelmann P, von Keudell G, Lee H, Santoro A, Zinzani P, et al. Nivolumab for relapsed/refractory classical Hodgkin lymphoma: 5-year survival from the pivotal phase 2 CheckMate 205 study. Blood Adv. (2023) 7:6266–74. doi: 10.1182/bloodadvances.2023010334
7. Li X, Gao S, Shan C, Zhang Q, Tan Y, Yu X, et al. Advances in PD-1/PD-L1 pathway inhibitors in the treatment of thyroid cancer: mechanisms and clinical therapeutic perspectives. Front Immunol. (2025) 16:1643421. doi: 10.3389/fimmu.2025.1643421
8. Dimopoulos M, Leleu X, Moreau P, Richardson P, Liberati A, Harrison S, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: icaria-mm subgroup analysis. Leukemia. (2021) 35:562–72. doi: 10.1038/s41375-020-0868-z
9. Roemer M, Advani R, Ligon A, Natkunam Y, Redd R, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J Clin Oncol. (2016) 34:2690–7. doi: 10.1200/JCO.2016.66.4482
10. Ge Y, Liu X, Xu Y, Su Y, Li Y, Wang L. Combined systemic immune-inflammatory index and prognostic nutritional index predicts the efficacy and prognosis of ES-SCLC patients receiving PD-L1 inhibitors combined with first-line chemotherapy. Front Oncol. (2024) 14:1485849. doi: 10.3389/fonc.2024.1485849
11. Sun C, Wang A, Zhou Y, Chen P, Wang X, Huang J, et al. Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer. Nat Commun. (2023) 14:2692. doi: 10.1038/s41467-023-38360-5
12. Fraga M, Yáñez M, Sherman M, Llerena F, Hernandez M, Nourdin G, et al. Immunomodulation of t helper cells by tumor microenvironment in oral cancer is associated with CCR8 expression and rapid membrane Vitamin D signaling pathway. Front Immunol. (2021) 12:643298. doi: 10.3389/fimmu.2021.643298
13. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. (2019) 7:57. doi: 10.1186/s40425-019-0527-y
14. Pardoll D. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
15. Chen D, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
16. Armand P, Shipp M, Ribrag V, Michot J, Zinzani P, Kuruvilla J, et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. (2016) 34:3733–9. doi: 10.1200/JCO.2016.67.3467
17. Ansell S, Lesokhin A, Borrello I, Halwani A, Scott E, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. (2015) 372:311–9. doi: 10.1056/NEJMoa1411087
18. Green M, Monti S, Rodig S, Juszczynski P, Currie T, O’Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. (2010) 116:3268–77. doi: 10.1182/blood-2010-05-282780
19. Vari F, Arpon D, Keane C, Hertzberg M, Talaulikar D, Jain S, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. (2018) 131:1809–19. doi: 10.1182/blood-2017-07-796342
20. Chen R, Zinzani P, Fanale M, Armand P, Johnson N, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. (2017) 35:2125–32. doi: 10.1200/JCO.2016.72.1316
21. Ansell S, Bröckelmann P, von Keudell G, Lee H, Santoro A, Zinzani P, et al. HL-398: five-year overall survival from the CheckMate 205 study of nivolumab for relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL). Clin Lymphoma Myeloma Leukemia. (2021) 21:S373–4.
22. Armand P, Zinzani P, Lee H, Johnson N, Brice P, Radford J, et al. Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood. (2023) 142:878–86. doi: 10.1182/blood.2022019386
23. Domingo-Domènech E, Ramchandren R, Rueda A, Trněný M, Feldman T, Lee H, et al. S821 Nivolumab plus doxorubicin, vinblastine and dacarbazine for newly diagnosed advanced-stage classical hodgkin lymphoma: 2-year extended follow-up from cohort d of the phase 2 checkmate 205 study. HemaSphere. (2019) 3:363.
24. Cheson B, Bartlett N, LaPlant B, Lee H, Advani R, Christian B, et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial. Lancet Haematol. (2020) 7:e808–15. doi: 10.1016/S2352-3026(20)30275-1
25. Zinzani P, Thieblemont C, Melnichenko V, Bouabdallah K, Walewski J, Majlis A, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma: final analysis of KEYNOTE-170. Blood. (2021) 142:141–5. doi: 10.1182/blood.2022019340
26. Zinzani P, Santoro A, Gritti G, Brice P, Barr P, Kuruvilla J, et al. Nivolumab combined with brentuximab vedotin for R/R primary mediastinal large B-cell lymphoma: a 3-year follow-up. Blood Adv. (2023) 7:5272–80. doi: 10.1182/bloodadvances.2023010254
27. Ansell S, Minnema M, Johnson P, Timmerman J, Armand P, Shipp M, et al. Nivolumab for relapsed/refractory diffuse large B-Cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. (2019) 37:481–9. doi: 10.1200/JCO.18.00766
28. Armand P, Chen Y, Redd R, Joyce R, Bsat J, Jeter E, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. (2019) 134:22–9. doi: 10.1182/blood.2019000215
29. Driessen J, de Wit F, Herrera A, Zinzani P, LaCasce A, Cole P, et al. Brentuximab vedotin and chemotherapy in relapsed/refractory Hodgkin lymphoma: a propensity score-matched analysis. Blood Adv. (2024) 8:2740–52. doi: 10.1182/bloodadvances.2023012145
30. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat J, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. (2020) 7:e511–22. doi: 10.1016/S2352-3026(20)30120-4
31. Nastoupil L, Chin C, Westin J, Fowler N, Samaniego F, Cheng X, et al. Safety and activity of pembrolizumab in combination with rituximab in relapsed or refractory follicular lymphoma. Blood Adv. (2022) 6:1143–51. doi: 10.1182/bloodadvances.2021006240
32. Advani R, Moskowitz A, Bartlett N, Vose J, Ramchandren R, Feldman T, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. (2021) 138:427–38. doi: 10.1182/blood.2020009178
33. Tao R, Fan L, Song Y, Hu Y, Zhang W, Wang Y, et al. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: a multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct Target Ther. (2021) 6:365. doi: 10.1038/s41392-021-00768-0
34. Lesokhin A, Ansell S, Armand P, Scott E, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. (2016) 34:2698–704. doi: 10.1200/JCO.2015.65.9789
35. Nishihori T, Hoffman J, Huff A, Kapoor G, Eleftheriadou I, Zajic S, et al. Safety and efficacy of letetresgene autoleucel alone or with pembrolizumab for relapsed/refractory multiple myeloma. Blood Adv. (2023) 7:1168–77. doi: 10.1182/bloodadvances.2022008460
36. Mateos M, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. (2019) 6:e459–69. doi: 10.1016/S2352-3026(19)30110-3
37. Oriol A, Hájek R, Spicka I, Sandhu I, Cohen Y, Gatt M, et al. Nivolumab, pomalidomide, and elotuzumab combination regimens for treatment of relapsed and refractory multiple myeloma: results from the phase 3 CheckMate 602 study. Clin Lymphoma Myeloma Leuk. (2024) 24:703–14. doi: 10.1016/j.clml.2024.05.014
38. Cohen Y, Magen H, Gatt M, Sebag M, Kim K, Min C, et al. Talquetamab plus teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. (2025) 392:138–49. doi: 10.1056/NEJMoa2406536
39. Caraccio C, Krishna S, Phillips D, Schürch C. Bispecific antibodies for multiple myeloma: a review of targets, drugs, clinical trials, and future directions. Front Immunol. (2020) 11:501. doi: 10.3389/fimmu.2020.00501
40. Magenau J, Frame D, Riwes M, Maciejewski J, Anand S, Pawarode A, et al. PD-1 inhibition for relapse after allogeneic transplantation in acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. (2025) 9:3878–86. doi: 10.1182/bloodadvances.2024015200
41. Kong Y, Zhang J, Claxton D, Ehmann W, Rybka W, Zhu L, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. (2015) 5:e330. doi: 10.1038/bcj.2015.58
42. Shimony S, Bewersdorf J, Shallis R, Liu Y, Schaefer E, Zeidan A, et al. Hypomethylating agents plus venetoclax compared with intensive induction chemotherapy regimens in molecularly defined secondary AML. Leukemia. (2024) 38:762–8. doi: 10.1038/s41375-024-02175-0
43. Roemer M, Redd R, Cader F, Pak C, Abdelrahman S, Ouyang J, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic hodgkin lymphoma. J Clin Oncol. (2018) 36:942–50. doi: 10.1200/JCO.2017.77.3994
44. Jeong A, Trando A, Thomas S, Riviere P, Sakowski P, Sokol E, et al. Higher tumor mutational burden and PD-L1 expression correlate with shorter survival in hematologic malignancies. Ther Adv Med Oncol. (2024) 16:17588359241273053. doi: 10.1177/17588359241273053
45. Li X, Zhang W. Expression of PD-L1 in EBV-associated malignancies. Int Immunopharmacol. (2021) 95:107553. doi: 10.1016/j.intimp.2021.107553
46. Pianko M, Liu Y, Bagchi S, Lesokhin A. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig. (2017) 4:32. doi: 10.21037/sci.2017.03.04
47. Jain M, Kuruvilla J. Anti-PD-1 antibodies as a therapeutic strategy in classical hodgkin lymphoma. Drugs. (2017) 77:1645–55. doi: 10.1007/s40265-017-0796-z
48. Wang Y, Jiang C, Zhou H, Han R. Transforming cancer immunotherapy: integration of distinct immune-based approaches as redefined dual immunotherapy with potential third-sensitizer. Exp Hematol Oncol. (2025) 14:114. doi: 10.1186/s40164-025-00705-9
49. Chereshnev V, Yushkov B. Towards the question of the theory neuro-immuno-endocrine system. Russian J Immunol. (2025). doi: 10.46235/1028-7221-17074-TTQ
50. Besedovsky H, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. (1996) 17:64–102. doi: 10.1210/edrv-17-1-64
51. Kaymak I, Williams K, Cantor J, Jones R. Immunometabolic interplay in the tumor microenvironment. Cancer Cell. (2021) 39:28–37. doi: 10.1016/j.ccell.2020.09.004
53. Zhao P, Zhao T, Yu L, Ma W, Liu W, Zhang C. The risk of endocrine immune-related adverse events induced by PD-1 inhibitors in cancer patients: a systematic review and meta-analysis. Front Oncol. (2024) 14:1381250. doi: 10.3389/fonc.2024.1381250
54. Csaba G. The immuno-endocrine system: hormones, receptors and endocrine function of immune cells. The packed-transport theory. Adv Neuroimmune Biol. (2011) 1:71–85. doi: 10.3233/NIB-2011-007
55. Hotamisligil G. Inflammation, metaflammation and immunometabolic disorders. Nature. (2017) 542:177–85. doi: 10.1038/nature21363
56. Straub R. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. (2014) 16:203. doi: 10.1186/ar4484
57. Takenaka M, Gabriely G, Rothhammer V, Mascanfroni I, Wheeler M, Chao C, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. (2019) 22:729–40. doi: 10.1038/s41593-019-0370-y
58. Luoma A, Suo S, Wang Y, Gunasti L, Porter C, Nabilsi N, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. (2022) 185:2918–35.e29. doi: 10.1016/j.cell.2022.06.018
59. Amato L, De Rosa C, Di Guida G, Sepe F, Ariano A, Capaldo S, et al. Addition of metformin to anti-PD-1/PD-L1 drugs activates anti-tumor immune response in peripheral immune cells of NSCLC patients. Cell Death Dis. (2025) 16:286. doi: 10.1038/s41419-025-07636-7
60. Zhang Z, Liu S, Zhang B, Qiao L, Zhang Y, Zhang Y. T cell dysfunction and exhaustion in cancer. Front Cell Dev Biol. (2020) 8:17. doi: 10.3389/fcell.2020.00017
61. Acharya N, Madi A, Zhang H, Klapholz M, Escobar G, Dulberg S, et al. Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity. (2020) 53:658–71.e6. doi: 10.1016/j.immuni.2020.08.005
62. Maeda N, Maruhashi T, Sugiura D, Shimizu K, Okazaki I, Okazaki T. Glucocorticoids potentiate the inhibitory capacity of programmed cell death 1 by up-regulating its expression on T cells. J Biol Chem. (2019) 294:19896–906. doi: 10.1074/jbc.RA119.010379
63. Maxwell R, Luksik A, Garzon-Muvdi T, Hung A, Kim E, Wu A, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. (2018) 7:e1500108. doi: 10.1080/2162402X.2018.1500108
64. Tokunaga A, Sugiyama D, Maeda Y, Warner A, Panageas K, Ito S, et al. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med. (2019) 216:2701–13. doi: 10.1084/jem.20190738
65. Giles A, Hutchinson M, Sonnemann H, Jung J, Fecci P, Ratnam N, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. (2018) 6:51. doi: 10.1186/s40425-018-0371-5
66. Martins Nascentes Melo L, Herrera-Rios D, Hinze D, Löffek S, Oezel I, Turiello R, et al. Glucocorticoid activation by HSD11B1 limits T cell-driven interferon signaling and response to PD-1 blockade in melanoma. J Immunother Cancer. (2023) 11:e004150. doi: 10.1136/jitc-2021-004150
67. De Vito P, Incerpi S, Pedersen J, Luly P, Davis F, Davis P. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. (2011) 21:879–90. doi: 10.1089/thy.2010.0429
68. Clark A, Dotson C, Elson A, Voigt A, Boehm U, Meyerhof W, et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J. (2015) 29:164–72. doi: 10.1096/fj.14-262246
69. Muir C, Clifton-Bligh R, Long G, Scolyer R, Lo S, Carlino M, et al. Thyroid Immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:e3704–13. doi: 10.1210/clinem/dgab263
70. García-Goñi M, Vázquez Gutiérrez B, Sanmamed M, Martín-Algarra S, Luis Pérez-Gracia J, Olmedo M, et al. Thyroid dysfunction caused by immune checkpoint inhibitors improves cancer outcomes. Endocr Relat Cancer. (2024) 31:e240064. doi: 10.1530/ERC-24-0064
71. Thuillier P, Joly C, Alavi Z, Crouzeix G, Descourt R, Quere G, et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother. (2021) 70:2023–33. doi: 10.1007/s00262-020-02802-6
72. Özdemir B, Dotto G. Sex hormones and anticancer immunity. Clin Cancer Res. (2019) 25:4603–10. doi: 10.1158/1078-0432.CCR-19-0137
73. Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. (2019) 111:772–81. doi: 10.1093/jnci/djz094
74. Hawley J, Obradovic A, Dallos M, Lim E, Runcie K, Ager C, et al. Anti-PD-1 immunotherapy with androgen deprivation therapy induces robust immune infiltration in metastatic castration-sensitive prostate cancer. Cancer Cell. (2023) 41:1972–88.e5. doi: 10.1016/j.ccell.2023.10.006
75. Guan X, Polesso F, Wang C, Sehrawat A, Hawkins R, Murray S, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. (2022) 606:791–6. doi: 10.1038/s41586-022-04522-6
76. Wallis C, Klaassen Z, Bhindi B, Goldberg H, Chandrasekar T, Farrell A, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naïve prostate cancer: a systematic review and network meta-analysis. Eur Urol. (2018) 73:834–44. doi: 10.1016/j.eururo.2017.10.002
77. Rodriguez-Lara V, Soca-Chafre G, Avila-Costa M, Whaley J, Rodriguez-Cid J, Ordoñez-Librado J, et al. Role of sex and sex hormones in PD-L1 expression in NSCLC: clinical and therapeutic implications. Front Oncol. (2023) 13:1210297. doi: 10.3389/fonc.2023.1210297
78. Pellegrino M, Secli V, D’Amico S, Petrilli L, Caforio M, Folgiero V, et al. Manipulating the tumor immune microenvironment to improve cancer immunotherapy: igf1r, a promising target. Front Immunol. (2024) 15:1356321. doi: 10.3389/fimmu.2024.1356321
79. Cao H, Dong W, Qu X, Shen H, Xu J, Zhu L, et al. Metformin enhances the therapy effects of Anti-IGF-1R mAb figitumumab to NSCLC. Sci Rep. (2016) 6:31072. doi: 10.1038/srep31072
80. Wu Z, Zhang C, Najafi M. Targeting of the tumor immune microenvironment by metformin. J Cell Commun Signal. (2022) 16:333–48. doi: 10.1007/s12079-021-00648-w
81. Cortellini A, D’Alessio A, Cleary S, Buti S, Bersanelli M, Bordi P, et al. Type 2 diabetes mellitus and efficacy outcomes from immune checkpoint blockade in patients with cancer. Clin Cancer Res. (2023) 29:2714–24. doi: 10.1158/1078-0432.CCR-22-3116
82. Wang Z, Aguilar E, Luna J, Dunai C, Khuat L, Le C, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. (2019) 25:141–51. doi: 10.1038/s41591-018-0221-5
83. Braun L, Giesler S, Andrieux G, Riemer R, Talvard-Balland N, Duquesne S, et al. Adiponectin reduces immune checkpoint inhibitor-induced inflammation without blocking anti-tumor immunity. Cancer Cell. (2025) 43:269–91.e19. doi: 10.1016/j.ccell.2025.01.004
84. Bader J, Wolf M, Lupica-Tondo G, Madden M, Reinfeld B, Arner E, et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature. (2024) 630:968–75. doi: 10.1038/s41586-024-07529-3
85. Gupta D, Lis C. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. doi: 10.1186/1475-2891-9-69
86. Dai M, Wu W. Prognostic role of C-reactive protein to albumin ratio in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Front Oncol. (2023) 13:1148786. doi: 10.3389/fonc.2023.1148786
87. Yang Q, Shu C, Li H, Xie X, Wu H, Zhou Y, et al. Higher serum vitamin D concentration and supplementation were associated with improved survival outcomes and treatment response in cancer patients receiving immunotherapy: a systematic review and meta-analysis. Nutr Res. (2025) 141:82–95. doi: 10.1016/j.nutres.2025.08.003
88. Franco F, McCoy K. Microbes and vitamin D aid immunotherapy. Science. (2024) 384:384–5. doi: 10.1126/science.adp1309
89. Li K, Lu E, Wang Q, Xu R, Yuan W, Wu R, et al. Serum vitamin D deficiency is associated with increased risk of γδ T cell exhaustion in HBV-infected patients. Immunology. (2024) 171:31–44. doi: 10.1111/imm.13696
90. Maggini S, Wintergerst E, Beveridge S, Hornig D. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. (2007) 98:S29–35. doi: 10.1017/S0007114507832971
91. Sacco A, Battaglia A, Botta C, Aversa I, Mancuso S, Costanzo F, et al. Iron metabolism in the tumor microenvironment-implications for anti-cancer immune response. Cells. (2021) 10:303. doi: 10.3390/cells10020303
92. Yang Y, Li Y, Chen Z. Impact of low serum iron on treatment outcome of PD-1 inhibitors in advanced gastric cancer. BMC Cancer. (2023) 23:1095. doi: 10.1186/s12885-023-11620-9
93. Wang J, Wang W, Liu B, Zhao R, Zhao J, Jiang F, et al. Serum zinc as a biomarker to predict the efficacy of immune checkpoint inhibitors in cancers. PLoS One. (2025) 20:e0326057. doi: 10.1371/journal.pone.0326057
94. Avery J, Hoffmann P. Selenium, selenoproteins, and immunity. Nutrients. (2018) 10:1203. doi: 10.3390/nu10091203
95. Geiger R, Rieckmann J, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T Cell metabolism and enhances survival and anti-tumor activity. Cell. (2016) 167:829–42.e13. doi: 10.1016/j.cell.2016.09.031
96. Munn D, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. (2016) 37:193–207. doi: 10.1016/j.it.2016.01.002
97. Spranger S, Gajewski T. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. (2018) 18:139–47. doi: 10.1038/nrc.2017.117
98. Turbitt W, Buchta Rosean C, Weber K, Norian L. Obesity and CD8 T cell metabolism: implications for anti-tumor immunity and cancer immunotherapy outcomes. Immunol Rev. (2020) 295:203–19. doi: 10.1111/imr.12849
99. Gopalakrishnan V, Spencer C, Nezi L, Reuben A, Andrews M, Karpinets T, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
100. Spencer C, McQuade J, Gopalakrishnan V, McCulloch J, Vetizou M, Cogdill A, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. (2021) 374:1632–40. doi: 10.1126/science.aaz7015
101. Routy B, Le Chatelier E, Derosa L, Duong C, Alou M, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
102. Cui Y, Han X, Liu H, Xie Q, Guan Y, Yin B, et al. Impact of endogenous glucocorticoid on response to immune checkpoint blockade in patients with advanced cancer. Front Immunol. (2023) 14:1081790. doi: 10.3389/fimmu.2023.1081790
103. Montesinos M, Pellizas C. Thyroid hormone action on innate immunity. Front Endocrinol. (2019) 10:350. doi: 10.3389/fendo.2019.00350
104. van Niekerk G, Christowitz C, Conradie D, Engelbrecht A. Insulin as an immunomodulatory hormone. Cytokine Growth Factor Rev. (2020) 52:34–44. doi: 10.1016/j.cytogfr.2019.11.006
105. Cain D, Cidlowski J. Immune regulation by glucocorticoids. Nat Rev Immunol. (2017) 17:233–47. doi: 10.1038/nri.2017.1
106. Arbour K, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. (2018) 36:2872–8. doi: 10.1200/JCO.2018.79.0006
107. Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo E, Ruggieri L, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers. (2020) 12:546. doi: 10.3390/cancers12030546
108. Ricciuti B, Dahlberg S, Adeni A, Sholl L, Nishino M, Awad M. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. (2019) 37:1927–34. doi: 10.1200/JCO.19.00189
109. Faje A, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. (2018) 124:3706–14. doi: 10.1002/cncr.31629
110. Di Dalmazi G, Ippolito S, Lupi I, Caturegli P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab. (2019) 14:381–98. doi: 10.1080/17446651.2019.1701434
111. Cheung Y, Wang W, McGregor B, Hamnvik O. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. (2022) 71:1795–812. doi: 10.1007/s00262-021-03128-7
112. Basak E, van der Meer J, Hurkmans D, Schreurs M, Oomen-de Hoop E, van der Veldt A, et al. Overt Thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. (2020) 30:966–73. doi: 10.1089/thy.2019.0726
113. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with Anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860
114. Kim H, Kim M, Lee S, Park S, Kim Y, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. (2018) 7:e1375642. doi: 10.1080/2162402X.2017.1375642
115. Zhai Y, Ye X, Hu F, Xu J, Guo X, Zhuang Y, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. (2019) 7:286. doi: 10.1186/s40425-019-0754-2
116. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. (2018) 19:737–46. doi: 10.1016/S1470-2045(18)30261-4
117. Mondal M, Lahiri A, Vundavilli H, Del Priore G, Reeves N, Datta A. A computational study of efficient combinations of FDA-approved drugs and dietary supplements in endometrial cancer. Piscataway, NJ: IEEE (2024).
118. Robert C, Lebbé C, Lesimple T, Lundström E, Nicolas V, Gavillet B, et al. Phase I study of androgen deprivation therapy in combination with anti-PD-1 in melanoma patients pretreated with anti-PD-1. Clin Cancer Res. (2023) 29:858–65. doi: 10.1158/1078-0432.CCR-22-2812
119. De Filippi R, Morabito F, Santoro A, Tripepi G, D’Alò F, Rigacci L, et al. Body mass index is not associated with survival outcomes and immune-related adverse events in patients with Hodgkin lymphoma treated with the immune checkpoint inhibitor nivolumab. J Transl Med. (2021) 19:489. doi: 10.1186/s12967-021-03134-4
120. Arecco A, Petolicchio C, Pastorino A, Tanda E, Vera L, Boschetti M, et al. Cemiplimab and diabetic ketoacidosis: a case report of a rare endocrinopathy associated with immune checkpoint inhibitors. Front Endocrinol. (2025) 16:1550702. doi: 10.3389/fendo.2025.1550702
121. Bersanelli M, Verzoni E, Cortellini A, Giusti R, Calvetti L, Ermacora P, et al. Impact of influenza vaccination on survival of patients with advanced cancer receiving immune checkpoint inhibitors (INVIDIa-2): final results of the multicentre, prospective, observational study. EClinicalMedicine. (2023) 61:102044. doi: 10.1016/j.eclinm.2023.102044
122. Huang C, Chang M, Chen Y, Chen T, Chen C, Cheng W. Insulin-like growth factors inhibit dendritic cell-mediated anti-tumor immunity through regulating ERK1/2 phosphorylation and p38 dephosphorylation. Cancer Lett. (2015) 359:117–26. doi: 10.1016/j.canlet.2015.01.007
123. Liu J, Li X, Li Y, Gong Q, Luo K. Metformin-based nanomedicines for reprogramming tumor immune microenvironment. Theranostics. (2025) 15:993–1016. doi: 10.7150/thno.104872
124. Egan H, Treacy O, Lynch K, Leonard N, O’Malley G, Reidy E, et al. Targeting stromal cell sialylation reverses T cell-mediated immunosuppression in the tumor microenvironment. Cell Rep. (2023) 42:112475. doi: 10.1016/j.celrep.2023.112475
125. Bersanelli M, Cortellini A, Leonetti A, Parisi A, Tiseo M, Bordi P, et al. Systematic vitamin D supplementation is associated with improved outcomes and reduced thyroid adverse events in patients with cancer treated with immune checkpoint inhibitors: results from the prospective PROVIDENCE study. Cancer Immunol Immunother. (2023) 72:3707–16. doi: 10.1007/s00262-023-03522-3
126. Kosenko E, Tikhonova L, Alilova G, Montoliu C. Erythrocytes functionality in SARS-CoV-2 infection: potential link with Alzheimer’s disease. Int J Mol Sci. (2023) 24:5739. doi: 10.3390/ijms24065739
127. You W, Liu X, Tang H, Lu B, Zhou Q, Li Y, et al. Vitamin D status is associated with immune checkpoint inhibitor efficacy and immune-related adverse event severity in lung cancer patients: a prospective cohort study. J Immunother. (2023) 46:236–43. doi: 10.1097/CJI.0000000000000469
128. Luo J, Chen H, Ma F, Xiao C, Sun B, Liu Y, et al. Vitamin D metabolism pathway polymorphisms are associated with efficacy and safety in patients under anti-PD-1 inhibitor therapy. Front Immunol. (2022) 13:937476. doi: 10.3389/fimmu.2022.937476
129. Bittenbring J, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M, et al. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. (2014) 32:3242–8. doi: 10.1200/JCO.2013.53.4537
130. Kichenadasse G, Miners J, Mangoni A, Rowland A, Hopkins A, Sorich M. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241
131. Alden S, Charmsaz S, Li H, Tsai H, Danilova L, Munjal K, et al. Pan-tumor analysis to investigate the obesity paradox in immune checkpoint blockade. J Immunother Cancer. (2025) 13:e009734. doi: 10.1136/jitc-2024-009734
132. Hemadri A, Lin H, Lin Y, Rose A, Sander C, Najjar Y, et al. Association of baseline body mass index (BMI) with response and survival in patients (Pts) with advanced melanoma (MEL) receiving PD-1 inhibitors. Alexandira, VA: American Society of Clinical Oncology (2019).
133. Lee J, Hwang S, Jee B, Kim J, Lee J, Chung J, et al. Fat loss in patients with metastatic clear cell renal cell carcinoma treated with immune checkpoint inhibitors. Int J Mol Sci. (2023) 24:3994. doi: 10.3390/ijms24043994
134. Nandakumar A, Barberis A, Kim J, Lang C, Mills J, Rieunier G, et al. IGF-1 regulates cancer cell immune evasion in prostate cancer. Sci Rep. (2025) 15:38422. doi: 10.1038/s41598-025-22288-5
135. Leek L, Notohardjo J, de Joode K, Velker E, Haanen J, Suijkerbuijk K, et al. Multi-omic analysis identifies hypoalbuminemia as independent biomarker of poor outcome upon PD-1 blockade in metastatic melanoma. Sci Rep. (2024) 14:11244. doi: 10.1038/s41598-024-61150-y
136. Ni L, Huang J, Ding J, Kou J, Shao T, Li J, et al. Prognostic nutritional index predicts response and prognosis in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Nutr. (2022) 9:823087. doi: 10.3389/fnut.2022.823087
137. Guven D, Sahin T, Erul E, Rizzo A, Ricci A, Aksoy S, et al. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Mol Biosci. (2022) 9:1039121. doi: 10.3389/fmolb.2022.1039121
138. Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao R, et al. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front Nutr. (2022) 9:1038118. doi: 10.3389/fnut.2022.1038118
139. Wang L, Long X, Zhu Y, Luo A, Yang M. Association of prognostic nutritional index with long-term survival in lung cancer receiving immune checkpoint inhibitors: a meta-analysis. Medicine (Baltimore). (2024) 103:e41087. doi: 10.1097/MD.0000000000041087
140. Zarogoulidis P, Hohenforst-Schmidt W, Huang H, Zhou J, Wang Q, Wang X, et al. Intratumoral treatment with chemotherapy and immunotherapy for NSCLC with EBUS-TBNA 19G. J Cancer. (2021) 12:2560–9. doi: 10.7150/jca.55322
141. Zhang Y, Jin J, Tang M, Li P, Zhou L, Du Y, et al. Prognostic nutritional index predicts outcome of PD-L1 negative and MSS advanced cancer treated with PD-1 inhibitors. Biomed Res Int. (2022) 2022:6743126. doi: 10.1155/2022/6743126
142. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. (2018) 359:104–8. doi: 10.1126/science.aao3290
143. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of sarcopenia with and efficacy of anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. J Clin Med. (2019) 8:450. doi: 10.3390/jcm8040450
144. Lv W, Jin J, Xu Z, Luo H, Guo Y, Wang X, et al. lncMGPF is a novel positive regulator of muscle growth and regeneration. J Cachexia Sarcopenia Muscle. (2020) 11:1723–46. doi: 10.1002/jcsm.12623
145. Deng H, Chen Z, Qiu X, Zhu D, Tang X, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: a comprehensive systematic review and meta-analysis. Nutrition. (2021) 90:111345. doi: 10.1016/j.nut.2021.111345
146. Torigoe S, Schutt C, Yamasaki S. Immune discrimination of the environmental spectrum through C-type lectin receptors. Int Immunol. (2021) 33:847–51. doi: 10.1093/intimm/dxab074
147. Sehgal K. Hyperprogression in patients with cancer receiving immune checkpoint inhibitors. JAMA Netw Open. (2021) 4:e211839. doi: 10.1001/jamanetworkopen.2021.1839
148. Chen Y, Hu J, Bu F, Zhang H, Fei K, Zhang P. Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: a systematic review and meta-analysis. BMC Cancer. (2020) 20:707. doi: 10.1186/s12885-020-07206-4
149. Lee S, Kim J, Song W, Sung H, Jeon H, Jeong B, et al. Prognostic role of pre-treatment body composition parameters in patients undergoing first-line immunotherapy for metastatic renal cell carcinoma. Cancer Manag Res. (2024) 16:1091–101. doi: 10.2147/CMAR.S476150
150. Golban C, Susa S, Varga N, Ivan C, Schirta P, Schirta N, et al. The impact of body composition on outcomes in NSCLC patients treated with immune checkpoint inhibitors: a systematic review. Cancers. (2025) 17:2765. doi: 10.3390/cancers17172765
151. Fang Q, Yu J, Li W, Luo J, Deng Q, Chen B, et al. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin Exp Pharmacol Physiol. (2023) 50:178–90. doi: 10.1111/1440-1681.13740
152. Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu B, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2023) 14:1219929. doi: 10.3389/fimmu.2023.1219929
153. Nabel C, Gourgue F, Vander Heiden M. Macrophages dig into the obesity paradox in cancer. Immunity. (2024) 57:1731–3. doi: 10.1016/j.immuni.2024.07.013
154. Alishvandi A, Aram C, Shahrivar F, Kesharwani P, Sahebkar A. Pyroptosis in cancer therapy: a double-edged sword for immune activation and tumor progression. Mol Cancer. (2025) 24:297. doi: 10.1186/s12943-025-02506-4
155. Lee B, Ordovás J, Parks E, Anderson C, Barabási A, Clinton S, et al. Research gaps and opportunities in precision nutrition: an NIH workshop report. Am J Clin Nutr. (2022) 116:1877–900. doi: 10.1093/ajcn/nqac237
156. Xu C, Hao M, Zai X, Song J, Huang Y, Gui S, et al. A new perspective on gut-lung axis affected through resident microbiome and their implications on immune response in respiratory diseases. Arch Microbiol. (2024) 206:107. doi: 10.1007/s00203-024-03843-6
157. Yoshida M, Matsuoka Y, Mitsuyuki S, Yonetani N, Kawai J, Kondo T, et al. Early prediction of cytokine release syndrome by measuring phosphate and magnesium levels following chimeric antigen receptor T cell therapy. Blood Cell Ther. (2023) 6:129–34. doi: 10.31547/bct-2023-021
158. Li P, Lai Y, Tian L, Zhou Q. The prognostic value of prognostic nutritional index in advanced cancer receiving PD-1/L1 inhibitors: a meta-analysis. Cancer Med. (2022) 11:3048–56. doi: 10.1002/cam4.4668
159. Jiang L, Jin W. Prognostic value of Glasgow prognostic score in hematological malignancies: a systematic review and meta-analysis. Int J Hematol. (2025) 121:450–61. doi: 10.1007/s12185-025-03935-z
160. Guo G, Jing Z, Dou W, Wang G, Dang J, Li Y, et al. Immune-related thyroid dysfunction as a positive prognostic factor for patients with lung cancer in China: a real-world retrospective study. Front Immunol. (2024) 15:1495460. doi: 10.3389/fimmu.2024.1495460
161. Kopanos S, Filippatos C, Rousakis P, Kostopoulos I, Baxevanis C, Tentolouris A, et al. Prognostic significance of endocrine-related adverse events in patients with melanoma, non-small cell lung cancer and urothelial cancer after treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers. (2025) 17:3675. doi: 10.3390/cancers17223675
162. Witte H, Bonorden B, Riecke A, Biersack H, Steinestel K, Merz H, et al. The glasgow prognostic score at diagnosis is a predictor of clinical outcome in patients with multiple myeloma undergoing autologous haematopoietic stem cell transplantation. Cancers. (2020) 12:921. doi: 10.3390/cancers12040921
163. Shi Y, Liu X, Liu J, Zhang D, Liu X, Yue Y, et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non-small cell lung cancer patients who received immunotherapy-based treatments. Transl Lung Cancer Res. (2021) 10:4477–93. doi: 10.21037/tlcr-21-710
164. Tian B, Yang Y, Yang C, Yan L, Ding Z, Liu H, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy. (2022) 14:1481–96. doi: 10.2217/imt-2022-0133
165. Marrano N, Caporusso M, Ganini C, Borraccino A, Cignarelli A, Perrini S, et al. Risk factors for endocrinological immune-related adverse events in patients with renal cell carcinoma treated with immune checkpoint inhibitors. J Endocrinol Invest. (2025): doi: 10.1007/s40618-025-02732-z Online ahead of print.
166. Li F, Feng Y, Yin Z, Wang Y. Mitochondrial metabolism in T-cell exhaustion. Int J Mol Sci. (2025) 26:7400. doi: 10.3390/ijms26157400
167. Brown J, Liu Y, Shabto J, Martini D, Ravindranathan D, Hitron E, et al. Modified Glasgow Prognostic Score associated with survival in metastatic renal cell carcinoma treated with immune checkpoint inhibitors. J Immunother Cancer. (2021) 9:e002851. doi: 10.1136/jitc-2021-002851
168. Madeddu C, Busquets S, Donisi C, Lai E, Pretta A, López-Soriano F, et al. Effect of cancer-related cachexia and associated changes in nutritional status, inflammatory status, and muscle mass on immunotherapy efficacy and survival in patients with advanced non-small cell lung cancer. Cancers. (2023) 15:1076. doi: 10.3390/cancers15041076
169. Wasamoto S, Imai H, Tsuda T, Nagai Y, Minemura H, Yamada Y, et al. Pretreatment glasgow prognostic score predicts survival among patients administered first-line atezolizumab plus carboplatin and etoposide for small cell lung cancer. Front Oncol. (2023) 12:1080729. doi: 10.3389/fonc.2022.1080729
170. Wang Y, Jiang Y, Luo Y, Lin X, Song M, Li J, et al. Prognostic nutritional index with postoperative complications and 2-year mortality in hip fracture patients: an observational cohort study. Int J Surg. (2023) 109:3395–406. doi: 10.1097/JS9.0000000000000614
171. Jin X, Ma X, Zhao D, Yang L, Ma N. Immune microenvironment and therapeutic progress of recurrent hepatocellular carcinoma after liver transplantation. Transl Oncol. (2023) 28:101603. doi: 10.1016/j.tranon.2022.101603
172. Galus Ł, Michalak M, Lorenz M, Stoińska-Swiniarek R, Tusień Małecka D, Galus A, et al. Vitamin D supplementation increases objective response rate and prolongs progression-free time in patients with advanced melanoma undergoing anti-PD-1 therapy. Cancer. (2023) 129:2047–55. doi: 10.1002/cncr.34718
173. Aslan V, Kılıç A, Sütcüoğlu O, Eraslan E, Bayrak A, Öksüzoğlu B, et al. Cachexia index in predicting outcomes among patients receiving immune checkpoint inhibitor treatment for metastatic renal cell carcinoma. Urol Oncol. (2022) 40:494.e1-10. doi: 10.1016/j.urolonc.2022.07.018
174. Jung J, Heo Y, Park S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: a real-world pan-tumor analysis. J Immunother Cancer. (2023) 11:e006454. doi: 10.1136/jitc-2022-006454
175. Hahn A, Tidwell R, Pilie P, Yu Y, Liu J, Surasi D, et al. Body composition as a determinant of the therapeutic index with androgen signaling inhibition. Prostate Cancer Prostatic Dis. (2025) 28:802–8. doi: 10.1038/s41391-024-00870-8
176. Ganjoo S, Gupta P, Corbali H, Nanez S, Riad T, Duong L, et al. The role of tumor metabolism in modulating T-Cell activity and in optimizing immunotherapy. Front Immunol. (2023) 14:1172931. doi: 10.3389/fimmu.2023.1172931
177. Go S, Kim H, Kang M, Park S, Lee G. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B-cell lymphoma. BMC Cancer. (2020) 20:439. doi: 10.1186/s12885-020-06921-2
178. Zhou X, Lu Y, Xia J, Mao J, Wang J, Guo H. Association between baseline controlling nutritional status score and clinical outcomes of patients with multiple myeloma. Cancer Biomark. (2021) 32:65–71. doi: 10.3233/CBM-210073
179. Liang W, Ferrara N. Iron metabolism in the tumor microenvironment: contributions of innate immune cells. Front Immunol. (2021) 11:626812. doi: 10.3389/fimmu.2020.626812
180. Rejeski K, Cordas Dos Santos DM, Parker NH, Bücklein VL, Winkelmann M, Jhaveri KS, et al. Influence of adipose tissue distribution, sarcopenia, and nutritional status on clinical outcomes after CD19 CAR T-cell therapy. Cancer Immunol Res. (2023) 11:707–19. doi: 10.1158/2326-6066.CIR-22-0487
181. Wang X, Jiang H, Liang M, Cai D, Ai S, Hu Q, et al. A Multi-marker model based on serum IL-10 predicts response to conversion immunochemotherapy in gastric cancer patients: a retrospective cohort study. BMC Gastroenterol. (2025) 25:621. doi: 10.1186/s12876-025-04136-y
182. van de Donk N, Moreau P, San-Miguel J, Mateos M, Dimopoulos M, Zweegman S, et al. Optimising T-cell immunotherapy in patients with multiple myeloma: practical considerations from the European Myeloma Network. Lancet Haematol. (2025) 12:e635–49. doi: 10.1016/S2352-3026(25)00117-6
183. Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. (2019) 10:2965. doi: 10.3389/fimmu.2019.02965
184. Pascual G, Benitah S. Lipids in the tumor microenvironment: immune modulation and metastasis. Front Oncol. (2024) 14:1435480. doi: 10.3389/fonc.2024.1435480
185. Blank C, Haining W, Held W, Hogan P, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. (2019) 19:665–74. doi: 10.1038/s41577-019-0221-9
186. Yang H, Wu C, Powell J, Lu K. Manipulation of metabolic pathways and its consequences for anti-tumor immunity: a clinical perspective. Int J Mol Sci. (2020) 21:4030. doi: 10.3390/ijms21114030
187. Mu S, Yang H, Wang S, Tong A, Ding R, Wang J, et al. Zinc-based nanomaterials in cancer therapy: mechanisms, applications, and future directions. Theranostics. (2025) 15:7841–71. doi: 10.7150/thno.117773
188. Zitvogel L, Fidelle M, Kroemer G. Long-distance microbial mechanisms impacting cancer immunosurveillance. Immunity. (2024) 57:2013–29. doi: 10.1016/j.immuni.2024.07.020
189. Chiriva-Internati M, Grizzi F, Monari M, Taverna G, Figueroa J, Daoyan W, et al. Immunomodulatory role of vitamin D and emerging immunotherapies in hepatocellular carcinoma. Front Nutr. (2025) 12:1611829. doi: 10.3389/fnut.2025.1611829
190. Morris E, Edwards C. Bone marrow adiposity and multiple myeloma. Bone. (2019) 118:42–6. doi: 10.1016/j.bone.2018.03.011
191. Diedrich J, Cole C, Pianko M, Colacino J, Bernard J. Non-toxicological role of Aryl hydrocarbon receptor in obesity-associated multiple myeloma cell growth and survival. Cancers. (2023) 15:5255. doi: 10.3390/cancers15215255
192. Yu W, Cao D, Li Q, Mei H, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. (2016) 7:86075–86. doi: 10.18632/oncotarget.13342
193. Pezeshki S, Asnafi A, Khosravi A, Shahjahani M, Azizidoost S, Shahrabi S. Vitamin D and its receptor polymorphisms: new possible prognostic biomarkers in leukemias. Oncol Rev. (2018) 12:366. doi: 10.4081/oncol.2018.366
194. Wang C, Wang W, Wang M, Deng J, Sun C, Hu Y, et al. Different evasion strategies in multiple myeloma. Front Immunol. (2024) 15:1346211. doi: 10.3389/fimmu.2024.1346211
195. Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. (2020) 30:177–84. doi: 10.1089/thy.2019.0250
196. Guzman-Prado Y, Ben Shimol J, Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunol Immunother. (2021) 70:89–100. doi: 10.1007/s00262-020-02663-z
197. Egoryan G, Zimmet A, Yu M, Pozdol J, Subramanian A, Reddy S, et al. A novel intersection: cytomegalovirus gastritis following cemiplimab and talimogene laherparepvec in a patient with advanced cutaneous squamous cell carcinoma. Clin Case Rep. (2024) 12:e9632. doi: 10.1002/ccr3.9632
198. Dvir K, Giordano S, Leone J. Immunotherapy in breast cancer. Int J Mol Sci. (2024) 25:7517. doi: 10.3390/ijms25147517
199. Riedl C, Bormann D, Steinmaurer A, Novak A, Testa G, Poldlehner E, et al. Inflammation alters myeloid cell and oligodendroglial iron-handling in multiple sclerosis. Acta Neuropathol Commun. (2025) 13:124. doi: 10.1186/s40478-025-02020-0
200. Luo J, Li L, Wang H, Zhang X, He F, Shi M, et al. Analysis of therapeutic effects and influencing factors of ICIs in lung-cancer patients. Clin Transl Oncol. (2025) 27:2597–604. doi: 10.1007/s12094-024-03767-z
201. Zhang Y, Han Y, Li W, Xu R, Ju H. Tumor iron homeostasis and immune regulation. Trends Pharmacol Sci. (2024) 45:145–56. doi: 10.1016/j.tips.2023.12.003
202. Ojo O, Ojo O, Zand N, Wang X. The effect of dietary fibre on gut microbiota, lipid profile, and inflammatory markers in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients. (2021) 13:1805. doi: 10.3390/nu13061805
203. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. (2017) 36:11–48. doi: 10.1016/j.clnu.2016.07.015
204. Akce M, Farran B, Switchenko J, Rupji M, Kang S, Khalil L, et al. Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J Immunother Cancer. (2023) 11:e007235. doi: 10.1136/jitc-2023-007235
205. Scharping N, Menk A, Whetstone R, Zeng X, Delgoffe G. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. (2017) 5:9–16. doi: 10.1158/2326-6066.CIR-16-0103
206. Lim K, Tippu Z, Corrie P, Hubank M, Larkin J, Lawley T, et al. MANIFEST: multiomic platform for cancer immunotherapy. Cancer Discov. (2025) 15:878–83. doi: 10.1158/2159-8290.CD-25-0099
207. Lin X, Zong C, Zhang Z, Fang W, Xu P. Progresses in biomarkers for cancer immunotherapy. MedComm. (2023) 4:e387. doi: 10.1002/mco2.387
208. Wang B, Zhang J, Shi Y, Wang Y. Clinical significance of the combined systemic immune-inflammatory index and prognostic nutritional index in predicting the prognosis of patients with extensive-stage small-cell lung cancer receiving immune-combination chemotherapy. BMC Cancer. (2024) 24:1574. doi: 10.1186/s12885-024-13343-x
209. Vitale E, Rizzo A, Maistrello L, Cauli O, Mollica V, Marques Monteiro F, et al. Decreased appetite in cancer patients treated with immune checkpoint inhibitors (ICIs): the MOUSEION-012 systematic review and meta-analysis. Clin Nutr ESPEN. (2025) 70:591–603. doi: 10.1016/j.clnesp.2025.10.031
210. Sun D, Hu Y, Li X, Peng J, Dai Z, Wang S. Unlocking the full potential of memory T cells in adoptive T cell therapy for hematologic malignancies. Int Immunopharmacol. (2025) 144:113392. doi: 10.1016/j.intimp.2024.113392
211. Feng C, Wang Y, Xu J, Zheng Y, Zhou W, Wang Y, et al. Precisely tailoring molecular structure of doxorubicin prodrugs to enable stable nanoassembly, rapid activation, and potent antitumor effect. Pharmaceutics. (2024) 16:1582. doi: 10.3390/pharmaceutics16121582
212. Yan Q, Qin Q, Zhang S, Chen F, Ru Y, Zhong Y, et al. Glial cell nutrient sensing: mechanisms of nutrients regulating Alzheimer’s pathogenesis and precision intervention. Crit Rev Food Sci Nutr. (2025): doi: 10.1080/10408398.2025.2568606 Online ahead of print.
213. Firuzpour F, Barancheshmeh M, Ziarani F, Karami L, Aram C. The HER2 target for designing novel multi-peptide vaccine against breast cancer using immunoinformatics and molecular dynamic simulation. Biochem Biophys Rep. (2025) 43:102135. doi: 10.1016/j.bbrep.2025.102135
214. Firuzpour F, Saleki K, Aram C, Rezaei N. Nanocarriers in glioblastoma treatment: a neuroimmunological perspective. Rev Neurosci. (2025) 36:431–53. doi: 10.1515/revneuro-2024-0097
215. Aram C, Firuzpour F, Barancheshmeh M, Kamali M. Unveiling the translational and therapeutic potential of small interfering RNA molecules in combating SARS-CoV-2: a review. Int J Biol Macromol. (2025) 318:145203. doi: 10.1016/j.ijbiomac.2025.145203
216. Makuku R, Khalili N, Razi S, Keshavarz-Fathi M, Rezaei N. Current and future perspectives of PD-1/PDL-1 blockade in cancer immunotherapy. J Immunol Res. (2021) 2021:6661406. doi: 10.1155/2021/6661406
217. Zervanos D, Galatou E, Miliotou A, Theodoroula N, Grigoriadis N, Vizirianakis I. Assessing the pharmacological and pharmacogenomic data of PD-1/PD-L1 inhibitors to enhance cancer immunotherapy outcomes in the clinical setting. Future Pharmacol. (2025) 5:43. doi: 10.3390/futurepharmacol5030043
218. Liu J, Chen M, Li S, Cai L, Ma L, Yang Q, et al. Biomarkers in the early stage of PD-1 inhibitor treatment have shown superior predictive capabilities for immune-related thyroid dysfunction. Front Immunol. (2024) 15:1458488. doi: 10.3389/fimmu.2024.1458488
219. Puntambekar M, Shery N, Parokkaran I, Al-Hamas M. Predictive biomarkers in cancer immunotherapy: a narrative review across selected solid tumors. Cureus. (2025) 17:e88647. doi: 10.7759/cureus.88647
220. Kou J, Huang J, Li J, Wu Z, Ni L. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and meta-analysis. Clin Exp Med. (2023) 23:3895–905. doi: 10.1007/s10238-023-01035-y
221. Xiao G, Tanzhu G, Gao X, Li L, Liu Z, Xia X, et al. An immune scoring system predicts prognosis and immune characteristics in lung adenocarcinoma brain metastases by RNA sequencing. Acta Neuropathol Commun. (2024) 12:181. doi: 10.1186/s40478-024-01895-9
222. Mokhtari R, Sambi M, Shekari F, Satari K, Ghafoury R, Ashayeri N, et al. A comprehensive oncological biomarker framework guiding precision medicine. Biomolecules. (2025) 15:1304. doi: 10.3390/biom15091304
223. Rucevic M, Mehta A, Horneaus K, Sprecher E, Schneider A, Jenkins R, et al. 152 Immuno-oncology protein biomarker panels provide greater biological insights and improved patient stratification. BMJ Specialist J. (2023) 11:172. doi: 10.1136/jitc-2023-SITC2023.0152
224. Yang W, Chen C, Ouyang Q, Han R, Sun P, Chen H. Machine learning models for predicting of PD-1 treatment efficacy in pan-cancer patients based on routine hematologic and biochemical parameters. Cancer Cell Int. (2024) 24:258. doi: 10.1186/s12935-024-03439-6
225. Oisakede E, Akinro O, Bello O, Analikwu C, Egbon E, Olawade D. Predictive models for immune checkpoint inhibitor response in cancer: a review of current approaches and future directions. Crit Rev Oncol Hematol. (2025) 216:104980. doi: 10.1016/j.critrevonc.2025.104980
226. Yoo S, Fitzgerald C, Cho B, Fitzgerald B, Han C, Koh E, et al. Prediction of checkpoint inhibitor immunotherapy efficacy for cancer using routine blood tests and clinical data. Nat Med. (2025) 31:869–80. doi: 10.1038/s41591-024-03398-5
227. Park S, Yang S, Lee S, Joo S, Park T, Kim D, et al. Machine-learning parsimonious prediction model for diagnostic screening of severe hematological adverse events in cancer patients treated with PD-1/PD-L1 inhibitors: retrospective observational study by using the common data model. Diagnostics. (2025) 15:226. doi: 10.3390/diagnostics15020226
228. Firuzpour F, Heydari M, Aram C, Alishvandi A. The role of artificial intelligence in enhancing breast cancer screening and diagnosis: a review of current advances. Bioimpacts. (2025) 15:30984. doi: 10.34172/bi.30984
229. Li X, Qu J, Teng X, Zhuang H, Dai Y, Yang Z, et al. The emerging role of super-enhancers as therapeutic targets in the digestive system tumors. Int J Biol Sci. (2023) 19:1036–48. doi: 10.7150/ijbs.78535
230. Luo G, Zhou Z, Cao Z, Huang C, Li C, Li X, et al. M2 macrophage-derived exosomes induce angiogenesis and increase skin flap survival through HIF1AN/HIF-1α/VEGFA control. Arch Biochem Biophys. (2024) 751:109822. doi: 10.1016/j.abb.2023.109822
231. Sephton S, Sapolsky R, Kraemer H, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. (2000) 92:994–1000. doi: 10.1093/jnci/92.12.994
232. Savvidis C, Kallistrou E, Kouroglou E, Dionysopoulou S, Gavriiloglou G, Ragia D, et al. Circadian rhythm disruption and endocrine-related tumors. World J Clin Oncol. (2024) 15:818–34. doi: 10.5306/wjco.v15.i7.818
233. Huang Y. Multi-Omics and Mendelian Randomization Studies Unveiling Genetic and Molecular Mechanisms of Cancer Risk and Prognosis. England: The University of Manchester (2024).
234. Caccialanza R, Cereda E, Agustoni F, Klersy C, Casirati A, Montagna E, et al. Multicentre, randomised, open-label, parallel-group, clinical phase II study to evaluate immunonutrition in improving efficacy of immunotherapy in patients with metastatic non-small cell lung cancer, undergoing systematic nutritional counseling. BMC Cancer. (2022) 22:1212. doi: 10.1186/s12885-022-10296-x
235. Széles Á, Kubik A, Váncsa S, Grünwald V, Hadaschik B, Ács N, et al. Prognostic and predictive value of pre-treatment blood-based inflammatory biomarkers in patients with urothelial carcinoma treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2025) 16:1554048. doi: 10.3389/fimmu.2025.1554048
236. Wang J, Luo J, Rotili D, Mai A, Steegborn C, Xu S, et al. SIRT6 protects against lipopolysaccharide-induced inflammation in human pulmonary lung microvascular endothelial cells. Inflammation. (2024) 47:323–32. doi: 10.1007/s10753-023-01911-5
237. Wen Y, Liu Q, Zeng H, Lyu L, He X, Zhang X, et al. Age-specific reference intervals for plasma amino acids and their associations with nutrient intake in the Chinese pediatric population. Imeta. (2025) 4:e70051. doi: 10.1002/imt2.70051
238. Huang G, Liu S, Dong J, Xi X, Kong R, Li W, et al. PD-1 inhibitor-based adverse events in solid tumors: a retrospective real-world study. Front Pharmacol. (2022) 13:974376. doi: 10.3389/fphar.2022.974376
239. Ladjevardi C, Skribek M, Koliadi A, Rydén V, El-Naggar A, Digkas E, et al. Differences in immune-related toxicity between PD-1 and PD-L1 inhibitors: a retrospective cohort study in patients with advanced cancer. Cancer Immunol Immunother. (2024) 74:14. doi: 10.1007/s00262-024-03869-1
240. Framke T, Beutel G, Ganser A, Koch A, Großhennig A. Randomization in phase II trials: no exemption based on sample size. Br J Clin Pharmacol. (2025) 91:2750–4. doi: 10.1002/bcp.70167
241. Lee J, Sun J, Lee S, Ahn J, Park K, Ahn M. Are there any ethnic differences in the efficacy and safety of immune checkpoint inhibitors for treatment of lung cancer? J Thorac Dis. (2020) 12:3796–803. doi: 10.21037/jtd.2019.08.29
242. Cheung A, Palapattu E, Pompa I, Aldrighetti C, Niemierko A, Willers H, et al. Racial and ethnic disparities in a real-world precision oncology data registry. NPJ Precis Oncol. (2023) 7:7. doi: 10.1038/s41698-023-00351-6
243. Nityashree K, Rachitha P, Hanchinmane S, Raghavendra V. Advancing precision medicine: uncovering biomarkers and strategies to mitigate immune-related adverse events in immune checkpoint inhibitors therapy. Toxicol Rep. (2025) 14:102035. doi: 10.1016/j.toxrep.2025.102035
244. Monteiro J, Fernandes A, Tato D, Moreira E, Ribeiro R, Reguengo H, et al. Optimizing Anti-PD1 immunotherapy: an overview of pharmacokinetics, biomarkers, and therapeutic drug monitoring. Cancers. (2025) 17:3262. doi: 10.3390/cancers17193262
Keywords: endocrine signatures, hematologic malignancies, immune checkpoint inhibitors, immunotherapy outcomes, nutritional biomarkers, PD-1/PD-L1 blockade
Citation: Huang N and Guan Y (2026) Pre-treatment endocrine–nutritional signatures predict clinical benefit from PD-1/PD-L1 blockade in hematologic malignancies. Front. Nutr. 12:1753660. doi: 10.3389/fnut.2025.1753660
Received: 25 November 2025; Revised: 25 December 2025; Accepted: 26 December 2025;
Published: 05 February 2026.
Edited by:
Wenhong Deng, Renmin Hospital of Wuhan University, ChinaReviewed by:
Zhanzhan Li, Central South University, ChinaChong Jin, Taizhou Central Hospital, China
Copyright © 2026 Huang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guan Yu, Z3lfc2NpQDE2My5jb20=
 Ningjing Huang1
Ningjing Huang1 Yu Guan
Yu Guan