- 1Institut für Theoretische Physik, Technische Universität Braunschweig, Braunschweig, Germany
- 2Max Planck Institute for Solar System Research, Göttingen, Germany
1 Introduction
Diamond nitrogen-vacancy (NV) color centers and other point defects are promising candidates for solid-state qubits, but there are problems with their integration [1, 2]. Recently, a one-by-one irradiation device with the positional accuracy of dopant atoms on the Ångström order has been developed [3–5] or is under development [6–9]. However, the dopant atom
In this paper, we review the cooling prospects of hydrogenated nitrogen
We do not pursue the validity of the transition cycle for cooling, including Rosa’s three fundamental requirements for cooling molecules [10], because the electronic structure of most of the molecules listed here is not sufficiently investigated. We discuss which hydrogenated nitrogen should be the focus of future cooling research to develop precision irradiation.
2 Chemical stability of hydrogenated nitrogens
Hydrogenated nitrogen, namely, hydronitrogen
The following is a comprehensive description of previous research. It makes it clear that knowledge of the electronic structures of hydronitrogens is still insufficient to propose Doppler cooling schemes. The following section gives a concise summary of Tables 1 and 2.
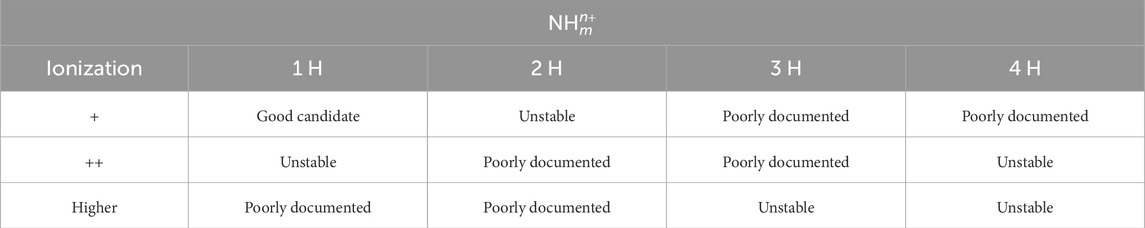
Table 1. Investigation status of the stability of the hydrogenated nitrogen cations
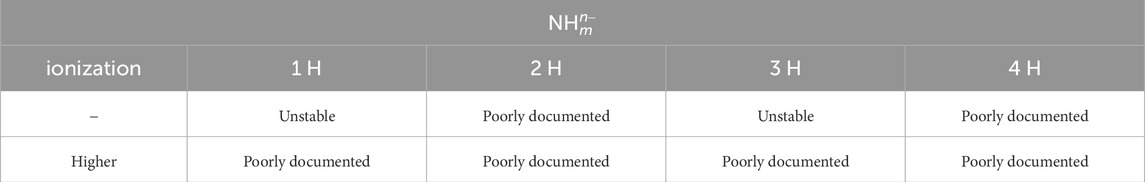
Table 2. Investigation status of the stability of the hydrogenated nitrogen anions
2.1 Monovalent cations
2.1.1
The monovalent cations or monocations
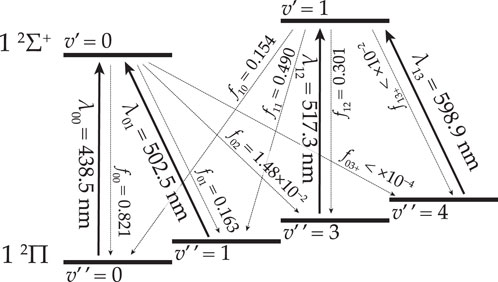
Figure 1. Energy levels of
2.1.2
The amidogen cation
2.1.3
The electronic structure of
2.1.4
We could not find any studies on the electronic excited states of the ammonium ion
2.2 Divalent cations
The cooling feasibility of high-valence ions is rarely noticed. However, high-valence ions are more appropriate for precision irradiation applications because irradiating them can lower the acceleration voltage to achieve the same beam energy.
2.2.1
The stability of the dication of the diatomic molecule
where
2.2.2
2.2.3
2.2.4
2.3 Trivalent and higher valence cations
For
2.4 Anions
The polyvalence anion is first discussed. The reports of dianions, that is, divalent anions, are mostly related to large organic molecules, and the relatively small ones are
2.4.1
2.4.2
The ground state of
2.4.3
The stability report of
2.4.4
While
3 Discussion and outlook
The above discussion is summarized in Table 1 for the cations and Table 2 for the anions. The electronic structures of the hydrogenated nitrogens have hardly been investigated. Although there have been previous studies on the cooling potential of
In order to investigate the cooling capability of hydrogenated nitrogens, the energy potential curves of the ground state and the optically transitive excited states should be derived by ab initio calculations. The study of some hydrogenated molecules stagnated for about 30 years, but more recently, calculations using large basis sets have become practically feasible. Ab initio calculations are the first step in the study of Doppler cooling. We strongly emphasize the importance of ab initio calculations of hydrogenated nitrides for integrating solid-state qubits. We encourage quantum chemistry theorists to conduct intensive research on hydrogenated nitrides.
As a next step, absorption, photoelectron, and various active spectra should be obtained over a wide range of wavenumbers for each hydrogenated nitrogen ion. In particular, because the energy levels of the excited state are difficult to match with the calculation results, the spectra must be scanned over a wide range. Obtaining such comprehensive data is less likely to produce immediate scientific results than the effort required for the experiment. Therefore, a cooling investigation driven by engineering and social demands to develop solid-state quantum devices is necessary. As with semiconductor research in the past, research based on engineering and social demands will lead to the development of science.
In addition, a method for analyzing the obtained large-scale spectral data should be developed. Currently, the rovibrational spectra of small molecules are assigned semi-manually using software such as pgopher [91–94]. For extensive data sets, semi-manual assignments are unrealistic. Modern pattern recognition techniques should be applied based on physical understanding. Furthermore, scientific software packages are often developed by individual researchers, and the development is sometimes not stable. pgopher also stopped being updated in 2022 because the author passed away. Standard assignment tools should be systematically developed to analyze large data sets.
The science of molecular cooling must assist in achieving the integration of the NV color centers. As we have discussed, the science of molecular cooling, at least of hydrogenated nitrogen, is not sufficiently advanced. We hope this will be a case where pure science evolves dramatically due to engineering needs.
Author contributions
MI: writing–original draft and writing–review and editing. YN: writing–review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge support by the Open Access Publication Funds of Technische Universität Braunschweig.
Acknowledgments
MI would like to thank K. Chartkunchand for insightful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Atatüre M, Englund D, Vamivakas N, Lee SY, Wrachtrup J. Material platforms for spin-based photonic quantum technologies. Nat Rev Mater (2018) 3:38–51. doi:10.1038/s41578-018-0008-9
2. Wan NH, Lu TJ, Chen KC, Walsh MP, Trusheim ME, De Santis L, et al. Large-scale integration of artificial atoms in hybrid photonic circuits. Nature (2020) 583:226–31. doi:10.1038/s41586-020-2441-3
3. Jacob G, Groot-Berning K, Wolf S, Ulm S, Couturier L, Dawkins ST, et al. Transmission microscopy with nanometer resolution using a deterministic single ion source. Phys Rev Lett (2016) 117:043001. doi:10.1103/physrevlett.117.043001
4. Groot-Berning K, Kornher T, Jacob G, Stopp F, Dawkins ST, Kolesov R, et al. Deterministic single-ion implantation of rare-earth ions for nanometer-resolution color-center generation. Phys Rev Lett (2019) 123:106802. doi:10.1103/physrevlett.123.106802
5. Groot-Berning K, Jacob G, Osterkamp C, Jelezko F, Schmidt-Kaler F. Fabrication of 15NV− centers in diamond using a deterministic single ion implanter. New J Phys (2021) 23:063067. doi:10.1088/1367-2630/ac0753
6. Muroo K, Okamoto H, Miyawaki N, Yuri Y. Simulation study of ultrahigh-precision single-ion extraction from a linear Paul trap. Prog Theor Exp Phys (2023) 2023:063G01. doi:10.1093/ptep/ptad071
7. Miyawaki N, Ishii Y, Yuri Y, Narumi K, Muroo K, Ito K, et al. Calculation study of selective ion extraction from ion source with Paul-trap-type laser cooling device. Nucl Instrum Methods Phys Res B (2023) 542:183–7. doi:10.1016/j.nimb.2023.06.015
8. Ishii Y, Ohkubo T, Miyawaki N, Yuri Y, Onoda S, Narumi K, et al. Design of an apertureless two-stage acceleration lens for a single-ion implantation system. Nucl Instrum Methods Phys Res B (2023) 541:200–4. doi:10.1016/j.nimb.2023.05.021
9. Yuri Y, Miyawaki N, Hosaka K, Ishii Y, Hosoya S, Kashiwagi H, et al. Investigating ultralow-emittance nanobeam formation using a coulomb crystal. Prog Theor Exp Phys (2025) 2025:023G01. doi:10.1093/ptep/ptaf019
10. Rosa MD. Laser-cooling molecules: concept, candidates, and supporting hyperfine-resolved measurements of rotational lines in the A-X(0, 0) band of CaH. Eur Phys J D (2004) 31:395–402. doi:10.1140/epjd/e2004-00167-2
11. Nguyen JHV, Viteri CR, Hohenstein EG, Sherrill CD, Brown KR, Odom B. Challenges of laser-cooling molecular ions. New J Phys (2011) 13:063023. doi:10.1088/1367-2630/13/6/063023
12. Qian GR, Niu H, Hu CH, Oganov AR, Zeng Q, Zhou HY. Diverse chemistry of stable hydronitrogens, and implications for planetary and materials sciences. Sci Rep (2016) 6:25947. doi:10.1038/srep25947
13. Rednyk S, Roučka Š, Kovalenko A, Tran TD, Dohnal P, Plašil R, et al. Reaction of NH+, NH+2, and NH+3 ions with H2 at low temperatures: the pathway to ammonia production in the interstellar medium. Astron Astrophys (2019) 625:A74. doi:10.1051/0004-6361/201834149
14. Zhang QQ, Yang CL, Wang MS, Ma XG, Liu WW. Spectroscopic parameters of the low-lying electronic states and laser cooling feasibility of NH+ cation and NH− anion. Spectrochim Acta A (2017) 185:365–70. doi:10.1016/j.saa.2017.06.001
15. Stephens JC, Yamaguchi Y, Sherrill CD, Schaefer HF.
16. Kabbadj Y, Huet T, Uy D, Oka T. Infrared spectroscopy of the amidogen ion, NH2+. J Mol Spectrosc (1996) 175:277–88. doi:10.1006/jmsp.1996.0033
17. Webb AD, Nahler NH, Ashfold MNR. Imaging studies of the photodissociation of NH3+ and ND3+ cations. J Phys Chem A (2009) 113:3773–8. doi:10.1021/jp808854d
18. Yamaguchi Y, Schaefer HF. A systematic theoretical study of harmonic vibrational frequencies: the ammonium ion NH4+ and other simple molecules. J Chem Phys (1980) 73:2310–8. doi:10.1063/1.440381
19. Schwarz HA. Gas phase infrared spectra of ammoniated ammonium ions. J Chem Phys (1980) 72:284–7. doi:10.1063/1.438892
20. Crofton MW, Oka T. Infrared studies of molecular ions. i. the ν3 band of NH4+. J Chem Phys (1983) 79:3157–8. doi:10.1063/1.446147
21. Schäfer E, Begemann MH, Gudeman CS, Saykally RJ. The ν3 vibrational spectrum of the free ammonium ion (NH4+). J Chem Phys (1983) 79:3159–60. doi:10.1063/1.446148
22. Schäfer E, Saykally RJ, Robiette AG. A high resolution study of the ν3 band of the ammonium ion (NH4+) by velocity modulation laser absorption spectroscopy. J Chem Phys (1984) 80:3969–77. doi:10.1063/1.447279
23. Crofton MW, Oka T. Observation of forbidden transitions of ammonium ion (NH4+) ν3 band and determination of ground state rotational constants. observation of ν3 band allowed transitions of ND4+. J Chem Phys (1987) 86:5983–8. doi:10.1063/1.452484
24. Han H, Song H, Li J, Guo H. Near spectroscopically accurate ab initio potential energy surface for NH4+ and variational calculations of low-lying vibrational levels. J Phys Chem A (2015) 119:3400–6. doi:10.1021/acs.jpca.5b01835
25. Yu HG, Han H, Guo H. Full-dimensional quantum calculations of vibrational levels of NH4+ and isotopomers on an accurate ab initio potential energy surface. J Phys Chem A (2016) 120:2185–93. doi:10.1021/acs.jpca.6b01946
26. Bates DR, Carson TR. Doubly charged diatomic molecular ions. Proc Phys Soc Sec A (1955) 68:1199–202. doi:10.1088/0370-1298/68/12/417
27. Falcinelli S, Rosi M. Production and characterization of molecular dications: experimental and theoretical efforts. Molecules (2020) 25:4157. doi:10.3390/molecules25184157
28. Kramida A, Ralchenko Y. NIST atomic spectra database. NIST Stand reference database (1999) 78. doi:10.18434/T4W30F
29. Koch W, Schwarz H. The NHn2+ (n = 1−4) dications. A theoretical investigation. Int J Mass Spectrom Ion Process. (1986) 68:49–56. doi:10.1016/0168-1176(86)87067-7
30. Boyd R, Singh S, Beynon J. Delayed predissociation and collision-induced processes of the ammonia di-cation NH32+. Chem Phys (1985) 100:297–314. doi:10.1016/0301-0104(85)85013-8
31. Hamdan M, Mazumdar S, Marathe VR, Badrinathan C, Brenton AG, Mathur D. Excited states of XH2+ (X = C, N, O, S) ions: a combined experimental and theoretical study. J Phys B Mol Opt Phys (1988) 21:2571–84. doi:10.1088/0953-4075/21/14/010
32. Proctor CJ, Porter CJ, Ast T, Bolton PD, Beynon JH. Charge stripping reactions of ions formed from methane, ammonia, water and hydrogen sulphide by protonation and by electron impact. Org Mass Spectrom (1981) 16:454–8. doi:10.1002/oms.1210161008
33. Märk TD, Egger F, Cheret M. Ionization of ammonia and deuterated ammonia by electron impact from threshold up to 180 eV. J Chem Phys (1977) 67:3795–802. doi:10.1063/1.435321
34. Hamdan M, Brenton A. Experimentally observed excited states of the dication NH22+. Int J Mass Spectrom Ion Process. (1988) 84:211–4. doi:10.1016/0168-1176(88)83036-2
35. Pope SA, Hillier IH, Guest MF, Kendric J. The structure and stability of the dications, XH2+itn(X = N, O, P, S). Chem Phys Lett (1983) 95:247–9. doi:10.1016/0009-2614(83)87241-8
36. Pope SA, Hillier IH, Guest MF. Structure, stability and energetics of the neutral and singly and doubly ionized first- and second-row hydrides. Faraday Symp Chem Soc (1984) 19:109. doi:10.1039/fs9841900109
37. Wong MW, Radom L. Multiply charged isoelectronic analogs of cyclopropenyl/propargyl cation: cyclic or open chain? J Am Chem Soc (1989) 111:6976–83. doi:10.1021/ja00200a012
38. Gu JP, Wang ZX, Huang MB. Electronic states of the dication NH22+. Int J Mass Spectrom Ion Process. (1992) 120:157–61. doi:10.1016/0168-1176(92)80058-9
39. Mann MM, Hustrulid A, Tate JT. The ionization and dissociation of water vapor and ammonia by electron impact. Phys Rev (1940) 58:340–7. doi:10.1103/physrev.58.340
40. Dorman FH, Morrison JD. Double and triple ionization in molecules induced by electron impact. J Chem Phys (1961) 35:575–81. doi:10.1063/1.1731972
41. Locht R, Momigny J. The double ionization of ammonia. its dissociation into the doubly ionized fragment N2+. Chem Phys Lett (1987) 138:391–6. doi:10.1016/0009-2614(87)80527-4
42. Leyh B, Hoxha A. Reaction window in the single-electron capture by ammonia dications. Chem Phys (1995) 192:65–77. doi:10.1016/0301-0104(94)00365-h
43. Wei L, Chen S, Zhang Y, Wang B, Yu W, Ren B, et al. Dissociation of NH32+ induced by collision of 300 eV electrons with NH3. Eur Phys J D (2020) 74:133. doi:10.1140/epjd/e2020-10094-7
44. Winkoun D, Dujardin G. Fragmentation of doubly charged ammonia cations NH3++ studied by the photoion-photoion coincidence (PIPICO) method. Z Phys D Atoms Mol Clusters (1986) 4:57–64. doi:10.1007/bf01432498
45. Piancastelli MN, Cauletti C, Adam MY. Angle-resolved photoelectron spectroscopic study of the outer- and inner-valence shells of NH3 in the 20–80 eV photon energy range. J Chem Phys (1987) 87:1982–6. doi:10.1063/1.453171
46. Stankiewicz M, Hatherly PA, Frasinski LJ, Codling K, Holland DMP. The double photoionisation of NH3 using the triple coincidence (PEPIPICO) technique. J Phys B: Mol Opt Phys (1989) 22:21–31. doi:10.1088/0953-4075/22/1/006
47. Zhao YJ, Shan XB, Sheng LS, Wang ZY, Zhang J, Yu CR. Synchrotron radiation vuv double photoionization of some small molecules. Chin Phys B (2011) 20:043201. doi:10.1088/1674-1056/20/4/043201
48. Larsen KA, Rescigno TN, Streeter ZL, Iskandar W, Heck S, Gatton A, et al. Mechanisms and dynamics of the NH2+ + H+ and NH+ + H+ + H fragmentation channels upon single-photon double ionization of NH3. J Phys B Mol Opt Phys (2020) 53:244003. doi:10.1088/1361-6455/abc3aa
49. Larsen KA, Rescigno TN, Severt T, Streeter ZL, Iskandar W, Heck S, et al. Photoelectron and fragmentation dynamics of the H+ + H+ dissociative channel in NH3 following direct single-photon double ionization. Phys Rev Res (2020) 2:043056. doi:10.1103/physrevresearch.2.043056
50. Samson JAR, Haddad GN, Kilcoyne LD. Absorption and dissociative photoionization cross sections of NH3 from 80 to 1120 Å. J Chem Phys (1987) 87:6416–22. doi:10.1063/1.453472
51. Locht R, Davister M, Denzer W, Jochims H, Baumgärtel H. About the double ionization of ammonia and carbon dioxide. a comparison between photoionization and electron impact. Chem Phys (1989) 138:433–40. doi:10.1016/0301-0104(89)87149-6
52. Eland JH. Double photoionisation spectra of methane, ammonia and water. Chem Phys (2006) 323:391–6. doi:10.1016/j.chemphys.2005.09.047
53. Shaw R, Jen J, Thomas T. Auger spectrum of ammonia. J Electron Spectrosc Relat Phenom (1977) 11:91–100. doi:10.1016/0368-2048(77)85050-0
54. White J, Rye R, Houston J. Experimental auger electron spectrum of ammonia. Chem Phys Lett (1977) 46:146–50. doi:10.1016/0009-2614(77)85183-x
55. Camilloni R, Stefani G, Giardini-Guidoni A. The measured auger electron spectrum of ammonia vapour. Chem Phys Lett (1977) 50:213–7. doi:10.1016/0009-2614(77)80166-8
56. Appell J, Horsley JA. Electronic states of doubly ionized ammonia. J Chem Phys (1974) 60:3445–8. doi:10.1063/1.1681557
57. Griffiths WJ, Harris FM. An experimental determination of the energy of the first triplet doubly-ionized state of ammonia. Rapid Commun Mass Spectrom (1990) 4:366–8. doi:10.1002/rcm.1290041003
58. Langford M, Harris F, Fournier P, Fournier J. Determination of singlet- and triplet-state energies of the doubly ionized ammonia molecule by double-charge-transfer spectroscopy. Int J Mass Spectrom Ion Process. (1992) 116:53–69. doi:10.1016/0168-1176(92)80019-w
59. Joshi BD. Study of BeH3−, BH3, CH3+, NH3++, and OH33+ by one-center-expansion, self-consistent-field method. J Chem Phys (1967) 46:875–87. doi:10.1063/1.1840821
60. Økland MT, Fægri K, Manne R. Calculated auger emission spectrum of ammonia. Chem Phys Lett (1976) 40:185–8. doi:10.1016/0009-2614(76)85055-5
61. Jennison DR. Initial-state relaxation effects in molecular auger spectra. Phys Rev A (1981) 23:1215–22. doi:10.1103/physreva.23.1215
62. Tarantelli F, Tarantelli A, Sgamellotti A, Schirmer J, Cederbaum L. On the doubly ionized states of ammonia. Chem Phys Lett (1985) 117:577–82. doi:10.1016/0009-2614(85)80305-5
63. Brammer R. A computational study of the x-ray satellite spectrum of NH3. J Chem Phys (1987) 87:1153–61. doi:10.1063/1.453295
64. Sironi M, Cooper DL, Gerratt J, Raimondi M. Spin-coupled VB study of the di-cations of methane, ammonia and water. Mol Phys (1988) 65:251–9. doi:10.1080/00268978800101001
65. Mitra A, Mahapatra US, Majumder D, Sinha D. Multiple solutions of coupled cluster equations: an application to molecular auger spectra. J Phys Chem A (1998) 102:7277–85. doi:10.1021/jp972116w
66. Ida T, Ortiz JV. Second-order, two-electron dyson propagator theory: comparisons for vertical double ionization potentials. J Chem Phys (2008) 129:084105. doi:10.1063/1.2973533
67. Streit L, Custodio R. The auger spectra and the calculation of double-ionization potentials for H2O and NH3 using the diffusion quantum Monte Carlo method. Chem Phys Lett (2009) 482:148–52. doi:10.1016/j.cplett.2009.09.084
68. Wolff W, Luna H, Montenegro EC, Rodrigues Junior LC. Multiple fragmentation mechanisms in ammonia: collisions with protons in the intermediate velocity regime. Phys Rev A (2020) 102:052821. doi:10.1103/physreva.102.052821
69. Rasul G, Prakash GKS, Olah GA. XH42+ Dications and search for XH43+ Trications (X = N, P, and As). J Phys Chem A (1998) 102:8457–9. doi:10.1021/jp980981q
70. Bhatt P, Sairam T, Kumar A, Kumar H, Safvan CP. Formation of H2+ and H3+ in energetic highly-charged-ion collisions with NH3. Phys Rev A (2017) 96:022710. doi:10.1103/physreva.96.022710
71. Scheller MK, Cederbaum LS. Stability of MX32- ions in the gas phase and when do ionic molecules have large ionization potentials. J Chem Phys (1993) 99:441–55. doi:10.1063/1.465768
72. Zhao XL, Litherland AE. Observation of LIF32−. Phys Rev A (2005) 71:064501. doi:10.1103/physreva.71.064501
73. Weikert HG, Cederbaum LS. Free doubly negative tetrahalides. J Chem Phys (1993) 99:8877–91. doi:10.1063/1.465556
74. Middleton R, Klein J. Experimental verification of the existence of the gas-phase dianions BeF42− and MgF42−. Phys Rev A (1999) 60:3515–21. doi:10.1103/physreva.60.3515
75. Behera S, Jena P. Stability and spectroscopic properties of singly and doubly charged anions. J Phys Chem A (2012) 116:5604–17. doi:10.1021/jp210095q
76. Neumark DM, Lykke KR, Andersen T, Lineberger WC. Infrared spectrum and autodetachment dynamics of NH−. J Chem Phys (1985) 83:4364–73. doi:10.1063/1.449052
77. Wickham-Jones CT, Ervin KM, Ellison GB, Lineberger WC. NH2 electron affinity. J Chem Phys (1989) 91:2762–3. doi:10.1063/1.456994
78. Kleingeld JC, Ingemann S, Jalonen JE, Nibbering NMM. Formation of the NH4− ion in the gas phase. J Am Chem Soc (1983) 105:2474–5. doi:10.1021/ja00346a061
79. Coe JV, Snodgrass JT, Freidhoff CB, McHugh KM, Bowen KH. J Chem Phys (1985) 83:3169–70. doi:10.1063/1.449223
80. Snodgrass JT, Coe JV, Freidhoff CB, McHugh KM, Bowen KH. Faraday Discuss Chem Soc (1988) 86:241–56. doi:10.1039/dc9888600241
81. Xu SJ, Nilles JM, Hendricks JH, Lyapustina SA, Bowen KH. J Chem Phys (2002) 117:5742–7. doi:10.1063/1.1499491
82. Hu Q, Song H, Johnson CJ, Li J, Guo H, Continetti RE. Imaging a multidimensional multichannel potential energy surface: photodetachment of H−(NH3) and NH4−. J Chem Phys (2016) 144:244311. doi:10.1063/1.4954187
83. Herzberg G. Rydberg spectra of triatomic hydrogen and of the ammonium radical. Faraday Discuss Chem Soc (1981) 71:165. doi:10.1039/dc9817100165
84. Cardy H, Larrieu C, Dargelos A. An ab initio study of the tetrahedral NH4− ion. Chem Phys Lett (1986) 131:507–12. doi:10.1016/0009-2614(86)80573-5
85. Ortiz JV. Vertical and adiabatic ionization energies of NH4− isomers via electron propagator theory and many body perturbation theory calculations with large basis sets. J Chem Phys (1987) 87:3557–62. doi:10.1063/1.453000
86. Gutowski M, Simons J, Hernandez R, Taylor HL. “dougle-rydberg” molecular anions. J Phys Chem (1988) 92:6179–82. doi:10.1021/j100333a004
87. Ortiz JV. Structures and properties of double-rydberg anions. J Phys Chem (1990) 94:4762–3. doi:10.1021/j100375a002
88. Simons J, Gutowski M. Double-rydberg molecular anions. Chem Rev (1991) 91:669–77. doi:10.1021/cr00005a002
89. Matsunaga N, Gordon MS. A theoretical study of NH4− and PH4−. J Phys Chem (1995) 99:12773–80. doi:10.1021/j100034a014
90. Ortiz JV. A double rydberg anion with a hydrogen bond and a solvated double rydberg anion: interpretation of the photoelectron spectrum of N2H7−. J Chem Phys (2002) 117:5748–56. doi:10.1063/1.1499492
91. Western CM. PGOPHER: a program for simulating rotational, vibrational and electronic spectra. J Quant Spectrosc Radiat Transf (2017) 186:221–42. doi:10.1016/j.jqsrt.2016.04.010
92. Western CM, Billinghurst BE. Automatic assignment and fitting of spectra with PGOPHER. Phys Chem Chem Phys (2017) 19:10222–6. doi:10.1039/c7cp00266a
Keywords: synthesizing quantum material, qubit integration, NV color center, laser cooling, Paul trap, ion micro beam, dissociation, autodetachment
Citation: Iizawa M and Narita Y (2025) The cooling prospect of hydrogenated nitrogen ions for quantum defect integration. Front. Phys. 13:1527062. doi: 10.3389/fphy.2025.1527062
Received: 12 November 2024; Accepted: 21 March 2025;
Published: 18 July 2025.
Edited by:
Mario Siciliani de Cumis, Italian Space Agency (ASI), ItalyReviewed by:
Somnath Bhowmick, The Cyprus Institute, CyprusCopyright © 2025 Iizawa and Narita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masatomi Iizawa, bWFzYXRvbWkuaWl6YXdhQHR1LWJyYXVuc2Nod2VpZy5kZQ==
 Masatomi Iizawa
Masatomi Iizawa Yasuhito Narita
Yasuhito Narita